Tactile Medical Receives PDAC Approval for Nimbl™ Lymphedema Platform
September 16 2024 - 7:00AM

Tactile Systems Technology, Inc. (“Tactile Medical”; the “Company”)
(Nasdaq: TCMD), a medical technology company providing therapies
for people with chronic disorders, today announced that the
Pricing, Data Analysis, and Coding (PDAC) contractor for the
Centers for Medicare & Medicaid Services (CMS) has approved the
use of Healthcare Common Procedure Coding System (HCPCS) code E0651
for billing the Durable Medical Equipment Medicare Administrative
Contractors for Nimbl™, the Company’s next-generation pneumatic
compression platform. PDAC approval is subsequent to the receipt of
510(k) clearance from the U.S. Food and Drug Administration (FDA)
in June 2024. The Company will make Nimbl commercially available in
the United States in the coming weeks.
Nimbl is the next generation of the Company’s
basic lymphedema compression solution and is indicated for the
treatment of lymphedema, chronic edema, venous insufficiency, and
wound healing. The device features several key patient-friendly
enhancements over prior generations, including a 40% and 68%
reduction in size and weight, respectively, making it more portable
and ideal for active lifestyles. Nimbl offers connectivity to the
Company’s Kylee™ digital application, enabling patients to actively
track their therapy progress and share results with their care
team.
“The receipt of PDAC approval for Nimbl comes
earlier than expected, and we are pleased that CMS recognizes the
potential health and quality-of-life enhancing benefits this device
offers for Medicare patients struggling with lymphedema and chronic
venous insufficiency,” said Sheri Dodd, Chief Executive Officer at
Tactile Medical. “Nimbl’s new sleeker design and technical
advancements reflect our commitment to meaningful product
innovation that seeks to meet the patient wherever they are in the
treatment pathway. I am proud of the work our team has accomplished
in developing this new platform, which I believe will elevate
lymphedema therapy and increase patient acceptance and adherence.
We look forward to Nimbl’s upcoming full commercial launch in the
weeks ahead.”
About Tactile Systems Technology, Inc. (DBA Tactile
Medical)
Tactile Medical is a leader in developing and
marketing at-home therapies for people suffering from underserved,
chronic conditions including lymphedema, lipedema, chronic venous
insufficiency and chronic pulmonary disease by helping them live
better and care for themselves at home. Tactile Medical
collaborates with clinicians to expand clinical evidence, raise
awareness, increase access to care, reduce overall healthcare costs
and improve the quality of life for tens of thousands of patients
each year.
Investor Inquiries:Sam BentzingerGilmartin
Groupinvestorrelations@tactilemedical.com
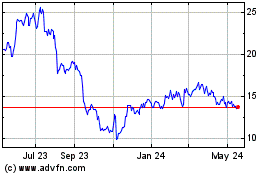
Tactile Systems Technology (NASDAQ:TCMD)
Historical Stock Chart
From Oct 2024 to Nov 2024
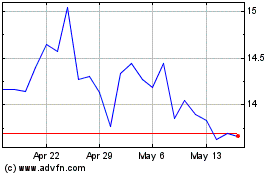
Tactile Systems Technology (NASDAQ:TCMD)
Historical Stock Chart
From Nov 2023 to Nov 2024