Pfizer Reports Positive Phase 3 Results for Antibiotic Combination
June 01 2023 - 9:54AM
Dow Jones News
By Colin Kellaher
Pfizer on Thursday reported positive results from a pair of
Phase 3 studies evaluating the efficacy, safety and tolerability of
the novel investigational antibiotic combination
aztreonam-avibactam, or ATM-AVI.
The New York drugmaker, which is studying the combination in the
treatment of serious bacterial infections due to
antibiotic-resistant Gram-negative bacteria, said the study data
support that ATM-AVI is effective and well-tolerated, with no new
safety findings and a similar safety profile to aztreonam
alone.
Pfizer said it expects data from studies to form the basis for
planned regulatory filings in the U.S., U.K., European Union and
China in the second half of the year. Pfizer holds the global
rights to commercialize ATM-AVI outside of the U.S. and Canada,
where development partner AbbVie holds the rights.
"We believe these data demonstrate that ATM-AVI, if approved,
could be an important treatment option for patients with
life-threatening bacterial infections that are resistant to almost
all currently available antibiotics," Pfizer said.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
June 01, 2023 10:39 ET (14:39 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
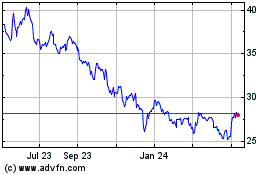
Pfizer (NYSE:PFE)
Historical Stock Chart
From Mar 2024 to Apr 2024
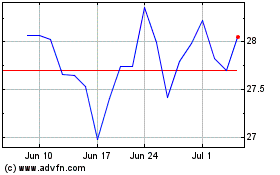
Pfizer (NYSE:PFE)
Historical Stock Chart
From Apr 2023 to Apr 2024