TIDMBPCR
RNS Number : 7778L
BioPharma Credit PLC
15 September 2021
BIOPHARMA CREDIT PLC
(THE "COMPANY")
HALF-YEARLY REPORT FOR THE PERIODED 30 JUNE 2021
NAV Remains Resilient with an Attractive Pipeline for Deploying
Capital Resources
BioPharma Credit PLC (LSE: BPCR), a specialist life sciences
debt investment trust, is pleased to present the Half-Yearly Report
of the Company for the period ended 30 June 2021.
The full Half-Yearly Report and Financials Statements can be
accessed via the Company's website at www.bpcruk.com or by
contacting the Company Secretary by telephone on 01392 477500.
INVESTMENT HIGHLIGHTS
-- The Company invested $150m in a senior secured loan to
LumiraDX Investment Ltd, a UK based diagnostics enterprise on 24
March 2021
-- Total income for the first half of 2021 was a robust $58m (H1 2020: $59m)
-- Attractive additional liquidity during the period totalled
$124m including valuable early pre-payments at significant IRRs,
principally:
o $97m from payments from the Sebela loan including a final
prepayment premium totalling $1.5m. The final payment was made on
30 June 2021 and the Company realized an IRR of 11.2% on this
investment
o Scheduled amortisation payments from the Collegium loan
o BMS purchased payments
CORPORATE HIGHLIGHTS
-- On 10 September 2021, the Company distributed a circular to
shareholders for its proposed admission to trade on the premium
segment of the main market of the London Stock Exchange
o Main market movement would provide the Company with the
potential benefits of index inclusion greater access to capital and
potential greater liquidity
-- The Company has decided that a Continuation Resolution will
also be brought forward from the required five year mark within its
articles of association and will instead be held at a General
Meeting on 30 September 2021:
o This will give investors greater certainty as to the Company's
longer term existence in the context of the proposed migration to
the Premium Segment
o The Directors will be voting unanimously in favour of
continuation and would encourage shareholders to do likewise
-- On 10 September 2021 the Company was pleased to be able to
refinance its revolving credit facility with JPMorgan Chase Bank on
even more favourable terms. Key terms to the amendment include:
o Reduction in the committed Revolving Credit Facility ("RCF")
from US$200 million to US$50 million together with changes in the
accordion feature allowing for an increase in the RCF to US$100
million and up to US$200 million in term loans
o Extension of the maturity date to 22 June 2024
o A reduction in the margin payable under the RCF from 4.00% to
2.75%
-- The Company appointed PwC as its statutory auditor for one
further year at a general meeting held on 24 June 2021
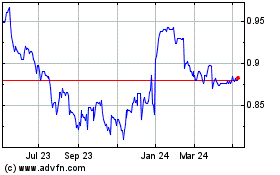
ORDINARY SHARES Assets
as at 30 June 2021 as at 30 June 2021
Share price Net assets
$0.09660 $1,371.1m
(31 December 2020: $0.9960) (31 December 2020: $1,378.9m)
NAV per Share Shares in issue
$0.9980 1,373.9m
(31 December 2020: $1.0037) (31 December 2019: 1,373.9m)
Discount to NAV per Share Target dividend
3.2% 7 cents per annum
(31 December 2020: 0.8%) (31 December 2020: 7 cents per annum)
Net income per share Leverage
$0.0338 0%
(30 June 2020: $0.0337) (31 December 2020: 0%)
PORTFOLIO COMPOSITION
($ in millions) As at 30 June 2021 As at 31 December
2020
Sarepta Therapeutics senior secured
loan 350 350
LumiraDx senior secured loan 150 -
BMS purchased payments 149 160
Collegium Therapeutics senior
secured loan 113 134
Epizyme senior secured loan 110 110
Sebela senior secured loan - 92
Global Blood Therapeutics senior
secured loan 83 83
BioDelivery Sciences senior secured
loan 80 80
Optinose senior secured note
and warrants 72 72
Akebia senior secured loan 50 50
BioDelivery Sciences equity 10 11
Cash and cash equivalents 212 250
Other net assets (8) (13)
------------------------------------- ------------------- ------------------
Total net assets 1,371 1,379
------------------------------------- ------------------- ------------------
Pedro Gonzalez de Cosio, CEO and co-founder of Pharmakon
Advisors L. P., the Investment Manager of BioPharma Credit PLC,
said:
"We are pleased to present another robust set of financial
results. The Company's portfolio of carefully underwritten loans to
companies in the life sciences sector are of outstanding quality
and the NAV remains unmoved by equity market volatility. In our
view, the Company therefore represents an alternative income
investment uncorrelated to equity market movements of compelling,
proven credentials.
"We are also pleased to be announcing a proposed migration to
the main market of the London Stock Exchange which we believe will
have significant benefits for the Company including potential index
inclusion, greater access to capital and potential increased
liquidity. In line with best in class corporate governance
practice, the Company will also bring forward the Continuation
Resolution within its articles of association to provide
shareholders with an opportunity to vote on the future of the
Company at this key milestone.
"The Company is evaluating a number of investment opportunities
and our market remains robust and relatively unchanged since IPO
given the high barriers to entry and our own strong position in the
sector. The Company's capital resources represent an outstanding
opportunity for us to fund growth and diversify our pipeline and we
look forward to updating shareholders as this progresses."
Results presentation
As announced on 25 August 2021, a management presentation for
analysts will be delivered via a conference call facility at 2:00pm
BST on the day of results. To request dial-in details please RSVP
biopharmacredit@buchanan.uk.com .
Enquiries
Buchanan
David Rydell / Mark Court / Jamie Hooper / Henry Wilson
+44 (0) 20 7466 5000
biopharmacredit@buchanan.uk.com
Notes to Editors
BioPharma Credit PLC is London's only specialist debt investor
to the life sciences industry and joined the LSE in March 2017. The
Company seeks to provide long-term shareholder returns, principally
in the form of sustainable income distributions from exposure to
the life sciences industry. The Company seeks to achieve this
objective primarily through investments in debt assets secured by
royalties or other cash flows derived from the sales of approved
life sciences products.
CHAIRMAN'S STATEMENT
DURING THE FIRST HALF OF 2021, THE COMPANY INCREASED ITS
DIVERSIFICATION THROUGH THE $150 MILLION INVESTMENT IN
LUMIRADX.
During the first half of 2021, the Company, through its wholly
owned subsidiary, BPCR Limited Partnership, invested $150 million
in a senior secured loan to LumiraDx Investment Limited, a UK based
diagnostics enterprise. Including assets and liabilities from its
financing subsidiary, BPCR Limited Partnership, the Company ended
the period with total net assets of $1,371 million, comprising
$1,167 million of investments, $212 million of cash and $8 million
of other net liabilities.
The Company and its subsidiaries saw $124 million increased
liquidity from the early repayment of the Sebela loan and the
scheduled amortisation payments from the Collegium loan and the BMS
purchased payments.
In 2020, the Company entered into a $200 million revolving
credit facility with JPMorgan Chase Bank through its wholly owned
subsidiary, BPCR Limited Partnership. On 10 September 2021, the
Company was able to negotiate and amend the revolving credit
facility on more favorable terms. The key terms to the amendment
include a reduction in the committed Revolving Credit Facility
("RCF") from $200 million to $50 million together with changes in
the accordion feature allowing for an increase in the RCF to $100
million and up to $200 million in term loans, extension of the
maturity date to 22 June 2024 and a reduction in the margin payable
under the RCF from 4.00 per cent. to 2.75 per cent.
On 10 September 2021, the Company distributed a circular to
shareholders for its proposed admission to trade on the premium
segment of the main market of the London Stock Exchange. The
circular outlines the proposed amendments to the existing Articles
in connection with Admission, Adoption of the Investment Policy and
a Continuation Resolution and amendments to the Existing Articles
in connection therewith. The Company believes that the benefits
associated with the migration include potential index inclusion,
greater access to capital, potential increased liquidity and
expanded analyst coverage.
Under the existing articles of the Company, a Continuation
Resolution is required to be held at the first annual general
meeting following the fifth anniversary of the Company's IPO and at
every third annual general meeting thereafter. However, the
Directors believe that it is beneficial to the Company for the
first Continuation Resolution to be held earlier, at the General
Meeting on 30 September 2021 so as to give investors greater
certainty as to the Company's longer term existence in the context
of the proposed migration to the Premium Segment. The Directors
will be voting in favour of continuation and would encourage
shareholders to do likewise in the belief that the Company has a
successful long-term investment programme.
Audit Tender
As previously announced to shareholders, the Company undertook a
competitive tender process in relation to the statutory audit of
the Company in March 2021, at the end of which it was agreed that
Ernst & Young ("EY") would conduct the statutory audit of the
Company for the year ending 31 December 2021. Regrettably, EY
informed the Board that, due to the tax work carried out by its
U.S. offices for the Company for previous financial years up to the
financial year ending on 31 December 2020, it was unable to accept
the proposed appointment as auditor to the Company for the year to
31 December 2021. Consequently, the Board agreed that
PricewaterhouseCoopers LLP ("PwC") should be appointed as auditor
to the Company for one further year. The appointment of PwC was
approved by shareholders at the general meeting held on 24 June
2021.
Shareholder Returns
The total income for the first half of 2021 was $58 million,
down from the $59 million reported during the first half of 2020.
On 30 June 2021, the Company's Ordinary Shares closed at $0.9660,
below the closing price on 31 December 2020 of $0.9960. Net Asset
Value ("NAV") per Ordinary Share decreased over the same timeframe
by $0.0057 from $1.0037 to $0.9980.
The Company made two dividend payments over the period totaling
$0.0379 per share, referencing net income for the quarters ending
31 December 2020 and 31 March 2021.
The Company was therefore able to maintain its record of paying
a dividend of at least 1.75 cents per share in every quarter since
that ending 30 June 2018.
The COVID-19 pandemic is continuing to have effects on
restrictions to the movement of people and disruption to business
operations. Despite the challenging environment, the Company and
its service providers have performed well and the portfolio
continues to be resilient. The recent progress made on national
vaccination programmes is encouraging; however uncertainty remains
and the Board continues to monitor the situation with Pharmakon
Advisors, our investment manager. Our investment manager believes
that the COVID-19 pandemic has not had a material impact on the
credit quality of the Company's loans. We will continue to monitor
the situation and will inform shareholders of any material changes
to this assessment.
Outlook
The Investment Manager continues to develop a pipeline of
additional potential investments and, as a consequence, we expect
to be evaluating a number of potential alternatives to fund future
growth and further diversify our portfolio. On behalf of the Board,
I should like to express our thanks to Pharmakon for their
continued achievements on behalf of the Company in 2021 and to our
shareholders for their continued support.
Harry Hyman
Chairman
14 September 2021
INVESTMENT MANAGER'S REPORT
Pharmakon is pleased to present an update on the Company's
portfolio and investment outlook. The Company's existing portfolio
investments continue to perform well .
Pharmakon's engagement during the period with potential
counterparties resulted in the execution of a new investment
totaling $150 million. During the period, the Company announced the
repayment of the Sebela loan, with payments during the first six
months of 2021 totaling $97 million, including a prepayment premium
totaling $1.5 million.
LumiraDx
On 24 March 2021, the Company and BioPharma-V, a private fund
also investing in life sciences debt managed by Pharmakon Advisors,
entered into a definitive senior secured loan agreement for $300
million with LumiraDx Investment Limited and LumiraDx Group Limited
(collectively "LumiraDx").
The Company and its subsidiaries funded $150 million of the $300
million loan on 29 March 2021.
The loan will mature in March 2024 and will bear interest at
8.00 per cent. per annum along with an additional consideration of
2.50 per cent. of the loan amount paid upon funding and an
additional 1.50 per cent. of the loan payable at maturity. The
Company and its subsidiaries will also be receiving warrants as
part of the financing.
LumiraDx is a UK based, next-generation Point of Care, or POC,
diagnostic company addressing the current limitations of legacy POC
systems by bringing performance comparable to a central lab to the
POC in minutes, on a single instrument for a broad menu of tests
with a low cost of ownership. To date, LumiraDx has developed and
launched four diagnostic tests for use with its platform: a
SARS-CoV-2 ("COVID-19") antigen test commercially available under
an Emergency Use Authorization in the United States, and a
Conformité Européenne (CE) Mark in the European Economic Area, as
well as a SARS-CoV-2 antibody test, an International Normalized
Ratio, or INR, test, and a D-Dimer test, all of which are CE
Marked.
LumiraDx has also used its technology to develop two rapid
COVID-19 reagent testing kits for use on open molecular systems,
LumiraDx SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete, both
of which obtained Emergency Use Authorization by the FDA.
Total loan amount Company commitment
$300m $150m
Investment type Date invested
Secured loan 23 March 2021
Maturity
March 2024
Collegium
On 7 February 2020, the Company and BioPharma-V entered into a
definitive senior secured term loan agreement for $200 million with
Collegium Pharmaceutical, Inc. (Nasdaq: COLL), a biopharmaceutical
company focused on developing and commercialising new medicines for
responsible pain management ("Collegium").
The Company and its subsidiaries funded $165 million of the $200
million loan on 13 February 2020.
The loan will mature in February 2024 and bears interest at
three-month LIBOR plus 7.50 per cent. per annum subject to a 2.00
per cent. LIBOR floor with a one-time additional consideration of
2.50 per cent. of the loan amount paid upon funding. The loan
amortises quarterly and had a remaining balance of $113 million as
of 30 June 2021.
Collegium currently markets Xtampza(R) ER, an abuse-deterrent,
extended- release, oral formulation of oxycodone and Nucynta(R)
(tapentadol), a centrally acting synthetic analgesic.
Total loan amount Company commitment
$200m $165m
Investment type Date invested
Secured loan 7 February 2020
Maturity
February 2024
GBT
On 18 December 2019, the Company and BioPharma-V entered into a
definitive senior secured term loan agreement for up to $150
million with Global Blood Therapeutics (Nasdaq: GBT), a
biopharmaceutical company focused on innovative treatments that
provide hope to underserved patient communities ("GBT").
GBT drew down $75 million at closing and an additional $75
million on 20 November 2020.
The Company and its subsidiaries funded $41 million of each
tranche for a total investment of $83 million. The loan will mature
in December 2025 and bears interest at three-month LIBOR plus 7.00
per cent. per annum subject to a 2.00 per cent. floor along with a
one-time additional consideration of 1.50 per cent. of the total
loan amount paid upon funding and an additional 2.00 per cent.
payable upon the repayment of the loan.
GBT manufactures and sells Oxbryta TM (voxelotor) for the
treatment of sickle cell disease in adults and pediatric patients
12 years of age and older.
Total loan amount Company commitment
$150m $83m
Investment type Date invested
Secured loan 17 December 2019
Maturity
December 2025
Sarepta
On 13 December 2019, the Company and BioPharma-V entered into a
definitive senior secured term loan agreement for up to $500
million with Sarepta Therapeutics (Nasdaq: SRPT), a fully
integrated biopharmaceutical company focused on precision genetic
medicine ("Sarepta").
On 24 September 2020 the Sarepta loan agreement was amended and
the loan amount was increased to $550 million. Sarepta drew down
the first $250 million tranche at closing and an additional $300
million on 2 November 2020.
The Company and its subsidiaries funded $175 million of each
tranche for a total investment of $350 million. The first tranche
will mature in December 2023 and the second tranche in December
2024. The loan bears interest at 8.5 per cent. per annum along with
a one-time additional consideration of 1.75 per cent. of the first
tranche and 2.95 per cent. of the second tranche paid upon funding
and an additional 2 per cent. payable upon the repayment of the
loan.
Sarepta currently markets Exondys 51 (eteplirsen), Vyondys 53
(golodirsen) and Amondys (casimersen) in the US for the treatment
of Duchenne muscular dystrophy (DMD).
Total loan amount Company commitment
$500m $350m
Investment type Date invested
Secured loan 13 December 2019
Maturity
December 2024
Akebia
On 11 November 2019, the Company and BioPharma-V entered into a
definitive senior secured term loan agreement for up to $100
million with Akebia (Nasdaq: AKBA), a fully integrated
biopharmaceutical company focused on the development and
commercialisation of therapeutics for people living with kidney
disease ("Akebia").
Akebia drew down $80 million at closing and an additional $20
million on 10 December 2020.
The Company and its subsidiaries funded $40 million of the $80
million first tranche and $10 million of the second tranche.
The loan will mature in November 2024 and bears interest at
LIBOR plus 7.5 per cent. per annum along with a one-time additional
consideration of 2 per cent. of the total loan amount paid upon
funding. Akebia currently markets Auryxia(R) (ferric citrate) which
is approved in the US for hyperphosphatemia (elevated phosphorus
levels in blood serum) in adult patients with chronic kidney
disease (CKD) on dialysis and iron deficiency anaemia in adult
patients with CKD not on dialysis.
Total loan amount Company commitment
$100m $50m
Investment type Date invested
Secured loan 25 November 2019
Maturity
December 2024
Epizyme
On 4 November 2019, the Company and BioPharma-V entered into a
definitive senior secured term loan agreement for up to $70 million
with Epizyme (Nasdaq: EPZM), a late-stage biopharmaceutical company
developing novel epigenetic therapies for cancer.
On 3 November 2020 the Epizyme loan agreement was amended and
the loan amount was increased to $220 million. Epizyme drew down
$25 million at closing and an additional $195 million during
2020.
The Company and its subsidiaries funded a total of $110 million
of the Epizyme loan. The loan will mature in November 2024 and
bears interest at LIBOR plus 7.75 per cent. per annum along with a
one-time additional consideration of 2 per cent. of the total loan
amount paid upon funding. Epizyme's lead product, TAZVERIK
(tazemetostat), is a first-in-class, oral EZH2 inhibitor that
received FDA approval for epithelioid sarcoma on 23 January 2020
and follicular lymphoma on 18 June 2020.
Total loan amount Company commitment
$220m $110m
Investment type Date invested
Secured loan 18 November 2019
Maturity
November 2026
Optinose
On 12 September 2019, the Company and BioPharma-V entered into a
definitive senior secured note purchase agreement for the issuance
and sale of senior secured notes in an aggregate original principal
amount of up to US$150 million by OptiNose US, a wholly-owned
subsidiary of OptiNose (Nasdaq: OPTN), a commercial-stage specialty
pharmaceutical company. Optinose drew a total of US$130 million in
three tranches: $80 million on 12 September 2019, $30 million on 13
February 2020 and $20 million on 1 December 2020. There are no
additional funding commitments.
On 2 March 2021, the sales covenants in the notes were reduced
by 16 per cent. for 2021 and 3 per cent. thereafter to allow for
slower growth due to the temporary impact of COVID 19 from reduced
patient visits. The revised covenant for 2021 of $80 million still
represents growth of 65 per cent. from 2020.
The Company and its subsidiaries funded a total $72 million
across all tranches and was allocated 445,696 warrants. The notes
mature in September 2024 and bear interest at 10.75 per cent. per
annum along with a one-time additional consideration of 0.75 per
cent. of the aggregate original principal amount of senior secured
notes which the Company was committed to purchase under the
facility and 810,357 warrants exercisable into common stock of
OptiNose.
OptiNose's leading product, XHANCE(R) (fluticasone propionate),
is a nasal spray approved by the U.S. Food and Drug Administration
(FDA) in September 2017 for the treatment of nasal polyps in
patients 18 years or older. XHANCE(R) utilises a novel and
proprietary exhalation delivery system to deliver the drug high and
deep into the sinuses, targeting areas traditional intranasal
sprays are not able to reach.
Total loan amount Company commitment
$130m $72m
Investment type Date invested
Secured loan 12 September 2019
Maturity
September 2024
BioDelivery Sciences
On 23 May 2019, the Company entered into a senior secured loan
agreement for up to $80 million with BioDelivery Sciences
International (Nasdaq: BDSI), a commercial-stage specialty
pharmaceutical company ("BDSI"). BDSI utilises its novel and
proprietary BioErodible MucoAdhesive (BEMA(R)) technology, to
develop and commercialize new applications of proven therapies
aimed at addressing important unmet medical needs. In addition, the
Company acquired 5,000,000 BDSI shares at $5.00 each for a total
cost of $25 million in a public offering that took place on 11
April 2019.
The first tranche of the loan for $60 million was funded on 28
May 2019 and the second $20 million tranche was funded on 22 May
2020. The loan will mature in May 2025 and bears interest at LIBOR
plus 7.5 per cent., along with 2 per cent. additional consideration
paid at closing. The Company sold 46 per cent. of its BDSI shares
during 2019 at an average price of $6.5. BDSI shares closed at
$3.58 on 30 June 2021 September 2021.
BDSI's leading products include BELBUCA(R) (buprenorphine buccal
film) and Symproic(R) (naldemedine).
Total loan amount Equity
$80m $25m
Investment type Date invested
Secured loan 28 May 2019
Company commitment Maturity
$105m May 2025
Bristol-Myers Squibb, Inc.
On 8 December 2017, the Company's wholly-owned subsidiary
entered into a purchase, sale and assignment agreement with a
wholly-owned subsidiary of Royalty Pharma Investments ("RPI"), an
affiliate of the Investment Manager, for the purchase of a 50 per
cent. interest in a stream of payments (the "Purchased Payments")
acquired by RPI's subsidiary from Bristol-Myers Squibb (NYSE: BMY)
through a purchase agreement dated 14 November 2017.
As a result of the arrangements, RPI's subsidiary and the
Company's subsidiary are each entitled to the benefit of 50 per
cent. of the Purchased Payments under identical economic terms. The
Purchased Payments are linked to tiered worldwide sales of Onglyza
and Farxiga, diabetes agents marketed by AstraZeneca, and related
products. The Company was expected to fund $140 million to $165
million during 2018 and 2019, determined by product sales over that
period, and will receive payments from 2020 through 2025. The
Purchased Payments are expected to generate attractive
risk-adjusted returns in the high single digits per annum. As of 30
June 2021, the Company funded all of the Purchased Payments based
on sales from 1 January 2018 to 31 December 2019 for a total of
$162 million.
Sebela
On 1 May 2018, the Company was lead arranger of a $316 million
senior secured term loan for Sebela BT Holdings Inc. ("Sebela"), a
subsidiary of Sebela Pharmaceuticals. The Company committed to a
$194 million investment, with the remaining $122 million balance
coming from co-investors.
The five-year senior secured loan began amortising in the third
quarter of 2018 and was due to fully mature in December 2022. The
loan bore interest at LIBOR (un-capped) plus a single digit spread
and included additional consideration.
Sebela is a private specialty pharmaceutical company focused on
gastrointestinal medicines, dermatology, and women's health. The
Company received its final loan payment on 30 June 2021 with
payments during the first six months of 2021 totaling $97 million,
including a prepayment premium totaling $1.5 million. The Company
earned a 11.2 per cent. internal rate of return on its Sebela
investment.
Investment Outlook
The life sciences industry is expected to continue to have
substantial capital needs during the coming years as the number of
products undergoing clinical trials continues to grow. All else
being equal, companies seeking to raise capital are generally more
receptive to straight debt financing alternatives at times when
equity markets are soft, increasing the number and size of
fixed-income investment opportunities for the Company, and will be
more inclined to issue equity or convertible bonds at times when
equity markets are strong. A good indicator of the life sciences
equity market is the New York Stock Exchange Biotechnology Index
("BTK Index"). While there was substantial volatility during the
period, the BTK index grew 3 per cent. during the period, compared
to 13 per cent. during the first six months of 2020. Global equity
issuance by life sciences companies during the period was $59
billion, a 6 per cent. decrease from the $63 billion issued during
the first six months of 2020. We anticipate a slowdown in equity
issuance coupled with greater appetite for fixed income as a source
of capital during the remainder of 2021.
Acquisition financing is an important driver of capital needs in
the life sciences industry in general and a source of investment
opportunities. An active M&A market helps drive opportunities
for investors such as the Company, as acquiring companies need
capital to fund acquisitions. Global life sciences M&A volume
during the period was $111 billion, a 516 per cent. increase from
the $18 billion witnessed during the first six months of 2020,
driven mainly by an increase in M&A activity globally as a
result of COVID-19 pandemic restrictions easing. We are encouraged
by the number of M&A opportunities that are starting to build
up which should lead to a more active market in the near term.
Despite the challenging environment due to the COVID-19
pandemic, we continue to carefully track and monitor the Company's
operations and its service providers, and we have not experienced
any technical or operational difficulties during the pandemic.
COVID-19 continues to cause major disruptions across the globe
however we have confidence in the performance of our loans and
there has not been a material impact on the credit quality of the
Company's investments. We will continue to monitor the pandemic and
will inform investors of any material changes to this
assessment.
Global transition away from USD LIBOR has been postponed to July
2023. As of today, major financial institutions continue to use USD
LIBOR as a reference for USD loans and other financial instruments
and will be permitted to continue to do so until January 2022. The
industry received further clarification from the Alternative
Reference Rates Committee (ARRC) following the July 2021 meeting.
The recommendation for most new instruments that reference USD
LIBOR is to transition away from LIBOR to Secured Overnight
Financing Rate (SOFR). The Company has five loans with coupons that
reference 3 Month USD LIBOR, they all include language that
describes how a new reference rate will be used in the absence of
USD LIBOR and all have a 2.00 per cent. LIBOR floor. As of 9
September 2021, the 3 Month USD LIBOR rate was 0.11 per cent.,
significantly below the floors in the five loans. The Investment
Manager will continue to monitor news on the transition and will
take steps in accordance with industry standards.
We continue to see a robust pipeline of investment opportunities
and expect it to continue to grow as new products are approved. We
remain focused on our mission of creating the premier dedicated
provider of debt capital to the life sciences industry while
generating attractive returns and sustainable income to investors.
Further, Pharmakon remains confident of our ability to deliver
attractive returns that will enable the Company to continue to pay
its target dividend yield to its investors.
Pedro Gonzalez de Cosio
Co-founder and CEO, Pharmakon
14 September 2021
Case study - LumiraDx
Industry leading innovator, providing simple, accessible and
affordable point of care testing.
LumiraDx, a Next-Generation Point of Care Diagnostics Testing
Company, was founded in 2014 by a group of entrepreneurs with a
successful track record in building and scaling diagnostics and
health IT businesses. These included Medisense, Inverness Medical
and Alere. Having worked together for more than a decade, this team
brings significant expertise in, developing, manufacturing and
commercializing industry-leading point of care (POC) diagnostic
Platforms, using cloud-based platforms to integrate health system
networks and transfer patient data and using data to develop
supported self-care plans to improve individuals' health and
system-wide outcomes.
LumiraDx's innovative diagnostic testing Platform has been in
development since 2014 and is designed to offer a broad menu of
tests with lab-comparable performance at a low cost and with
results in less than 12 minutes at the point of care. High
sensitivity COVID-19 antigen tests currently being deployed
globally in partnership with governments, health systems, retail
chains and global health foundations are meeting the urgent global
health needs. The LumiraDx SARS-CoV-2 Ag Test combines performance
with speed, without compromising a high sensitivity of
detection.
In common with other viruses, SARS-CoV-2 carries the risk of
mutation and becoming more diverse with new variants of the virus
occurring over time. While some new variants may emerge and
disappear, or be clinically inconsequential, others emerge and
dominate and have been detected globally during this pandemic.
Government regulators in the US, UK and other countries have
instituted guidelines that tests must meet to be able to detect the
emerging variants that are driving many new COVID-19 cases
globally.
The LumiraDx SARS-CoV-2 Antigen test is well positioned to
detect these existing and new variants. Unlike polymerase chain
reaction (PCR), the LumiraDx SARS- CoV-2 Ag Test uses antibodies,
not nucleic acid based- primers, to capture SARS-CoV-2 nucleocapsid
antigen, not the spike protein. Antibodies typically recognize 8-15
amino acid target sequences which are equivalent to 24-45
nucleotide sequences. Thus, single nucleic acid point mutations are
not likely to affect the performance of the LumiraDx SARS-CoV-2 Ag
Test. Furthermore, mutations outside of the nucleocapsid viral
coding region (ex Spike protein) are also unlikely to affect the
assay performance.
LumiraDx has a pipeline of 30 plus assays across common health
conditions including infectious disease, cardiovascular disease,
diabetes, and coagulation disorders to address a $50 plus billion
global market opportunity with the LumiraDx Platform.
STATEMENT OF DIRECTORS' RESPONSIBILITIES
INTERIM MANAGEMENT REPORT
The important events that have occurred during the period under
review, the key factors influencing the financial statements and
the principal factors that could impact the remaining six months of
the financial year are set out in the Chairman's Statement and the
Investment Manager's report above.
The Directors and the Investment Manager have considered the
adverse impact of potential changes in law, regulation and taxation
and the matter of foreign exchange risk.
The Directors have considered the principal risks facing the
Company and there have not been any material changes to the
principal risks and uncertainties and approach to mitigating these
risks since the publication of the Annual Report and Financial
Statements for the year ended 31 December 2020, and expect that,
for the remainder of the year ending 31 December 2021, these will
continue to be as set out on pages 19 to 25 of that report.
Risks faced by the Company include, but are not limited to:
-- Failure to achieve target returns;
-- The success of the Company depends on the ability and expertise of the Investment Manager;
-- The Company may from time to time commit to make future
investments that exceed the Company's current liquidity;
-- The Investment Manager's ability to source and advise appropriately on investments;
-- There can be no assurance that the Board will be able to find
a replacement investment manager if the Investment Manager
resigns;
-- Concentration in the Company's portfolio may affect the
Company's ability to achieve its investment objective;
-- Life sciences products are subject to intense competition and various other risks;
-- Investments in debt obligations are subject to credit and interest rate risks;
-- Counterparty risk;
-- Sales of life sciences products are subject to regulatory
actions that could harm the Company's ability to make distributions
to investors;
-- Net asset values published will be estimates only and may
differ materially from actual results;
-- Changes in taxation legislation or practice may adversely
affect the Company and the tax treatment for shareholders investing
in the Company;
-- COVID-19 may affect the Company's ability to continue operations; and
-- Changes to accounting regulation may require the Company to
make a change in accounting policy that could have a material
impact on its reported results including its net asset value, net
income and distributable reserves.
GOING CONCERN
The financial statements continue to be prepared on a going
concern basis. The Directors have reviewed areas of potential
financial risk and cash flow forecasts.
No material uncertainties have been detected which would
influence the Company's ability to continue as a going concern 12
months from the date of this report. Accordingly, the Board of
Directors continue to adopt the going concern basis in preparing
the financial statements. The important events that have occurred
during the period under review, the key factors influencing the
financial statements and the principal factors that could impact
the remaining six months of the financial year are set out in the
Chairman's statement and the Investment Manager's report above.
DIRECTORS' RESPONSIBILITY STATEMENT
The Directors confirm that to the best of their knowledge:
-- this set of condensed financial statements has been prepared
in accordance with UK adopted International Accounting Standard
("IAS") 34, 'Interim Financial Reporting', as adopted by the
European Union ("EU"); and gives a true and fair view of the
assets, liabilities, financial position and profit of the Company;
and
-- this Half-Yearly Report includes a fair review of the
information required by:
(a) DTR 4.2.7R of the Disclosure Guidance and Transparency
Rules, being an indication of important events that have occurred
during the first six months of the financial year and their impact
on the condensed set of financial statements; and a description of
the principal risks and uncertainties for the remaining six months
of the year; and
(b) DTR 4.2.8R of the Disclosure Guidance and Transparency
Rules, being related party transactions that have taken place
during the first six months of the financial year and that have
materially affected the financial position or performance of the
Company during that period; and any changes in the related party
transactions that could do so.
This Half-Yearly Report was approved by the Board of Directors
on 14 September 2021 and the above responsibility statement was
signed on its behalf by Harry Hyman, Chairman.
On behalf of the Board
Harry Hyman
Chairman
14 September 2021
CONDENSED STATEMENT OF COMPREHENSIVE INCOME
For the period ended 30 June 2021
(In $000s except per share amounts)
Period ended 30 June Period ended 30 June 2020
2021 (Unaudited) (Unaudited)
------------------------------- ------------------------------
Note Revenue Capital Total Revenue Capital Total
-------------------- ----- -------- ---------- --------- ---------- -------- --------
Income
Investment
income 3 74,679 - 74,679 45,793 - 45,793
Other income 3 12 - 12 1,033 - 1,033
Net (losses)/gains
on investments
at fair
value 7 - (22,702) (22,702) - 1,524 1,524
Net currency
exchange
gains/ (losses) - 1 1 - (37) (37)
-------------------- -----
Total income 74,691* (22,701) 51,990 46,826** 1,487 48,313
Expenses
Management
fee 4 (6,866) - (6,866) (6,872) - (6,872)
Directors'
fees 4 (198) - (198) (198) - (198)
Other expenses 4 (679) - (679) (877) - (877)
-------------------- -----
Total expenses (7,743) - (7,743) (7,947) - (7,947)
-------------------- ----- -------- ---------- --------- ---------- -------- --------
Return on
ordinary
activities
after finance
costs and
before taxation 66,948 (22,701) 44,247 38,879 1,487 40,366
Taxation
on ordinary
activities 5 - - - - - -
-------------------- -----
Return on
ordinary
activities
after finance
costs and
taxation 66,948 (22,701) 44,247 38,879 1,487 40,366
-------------------- ----- -------- ---------- --------- ---------- -------- --------
Net revenue
and capital
return per
ordinary
share (basic
and diluted) 11 $0.0487 $(0.0165) $0.0322 $0.0283 $0.0011 $0.0294
* Includes $17 million from prior year income and current year
expenses from its financing subsidiary, BPCR Limited Partnership.
Total recorded income for the first six months of 2021, was $58
million. Please see note 3 for full details.
** Total income for the first six months of 2020 was $59
million, which includes $12 million relating to the change in fair
value of its subsidiary, BPCR Limited Partnership. Please see note
3 for full details.
The total column of this statement is the Company's Condensed
Statement of Comprehensive Income prepared in accordance with IFRS.
The supplementary revenue and capital columns are presented for
information purposes as recommended by the Statement of Recommended
Practice ("SORP") issued by the Association of Investment Companies
("AIC").
All items in the above Statement derive from continuing
operations.
There is no other comprehensive income, and therefore the return
on ordinary activities after finance costs and taxation is also the
total comprehensive income.
The notes below form part of these financial statements.
CONDENSED STATEMENT OF CHANGES IN EQUITY
For the period ended 30 June 2021
(In $000s)
Total equity
Share Special attributable
-------------------------
to shareholders
Share premium distributable Capital Revenue of the
For the period ended 30
June 2021 (unaudited) Note capital account reserve* reserve** reserve* Company
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
Net assets attributable
to shareholders at 1
January
2021 13,739 607,125 730,492 20,014 7,545 1,378,915
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
Return on ordinary
activities
after finance costs and
taxation - - - (22,701) 66,948 44,247
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
Dividends paid to
Ordinary
Shareholders 6 - - (2,262) - (49,810) (52,072)
Net assets attributable
to shareholders at 30
June
2021 13,739 607,125 728,230 (2,687) 24,683 1,371,090
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
Total equity
Share Special attributable
-------------------------
to shareholders
Share premium distributable Capital Revenue of the
For the period ended 30
June 2020 (unaudited) Note capital account reserve* Reserve** reserve* Company
------------------------- -------- -------- -------------- ---------- --------- -------------------------
Net assets attributable
to shareholders at 1
January
2020 13,739 607,125 730,631 10,552 41,689 1,403,736
------------------------- -------- -------- -------------- ---------- --------- -------------------------
Return on ordinary
activities
after finance costs and
taxation - - - 1,487 38,879 40,366
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
Dividends paid to
Ordinary
Shareholders 6 - - - - (65,674) (65,674)
------------------------- -----
Net assets attributable
to shareholders at 30
June
2020 13,739 607,125 730,631 12,039 14,894 1,378,428
------------------------- ----- -------- -------- -------------- ---------- --------- -------------------------
* The special distributable reserve and revenue reserves can be
distributed in the form of a dividend.
** The negative capital reserve at 30 June 2021 is due to
unrealised depreciation on BPCR LP - see note 7. The capital
reserve can be used to repurchase treasury shares. It cannot be
used for distributions.
The notes below form part of these financial statements.
CONDENSED STATEMENT OF FINANCIAL POSITION
As of 30 June 2021
(In $000s except per share amounts)
30 June 2021
(Unaudited) 31 December
Note 2020 (Audited)
--------------------------------------- ----- -------------- ----------------
Non-current assets
Investments at fair value through
profit or loss 7 1,226,058 1,194,831
--------------------------------------- -----
1,226,058 1,194,831
Current assets
Trade and other receivables 8 26,376 208
Cash and cash equivalents 9 122,478 193,269
--------------------------------------- -----
148,854 193,477
--------------------------------------- ----- -------------- ----------------
Total assets 1,374,912 1,388,308
--------------------------------------- -----
Current liabilities
--------------------------------------- ----- -------------- ----------------
Trade and other payables 10 3,822 9,393
Total current liabilities 3,822 9,393
Total assets less current liabilities 1,371,090 1,378,915
--------------------------------------- ----- -------------- ----------------
Net assets 1,371,090 1,378,915
--------------------------------------- ----- -------------- ----------------
Represented by:
Share capital 13 13,739 13,739
Share premium account 607,125 607,125
Special distributable reserve 728,230 730,492
Capital reserve (2,687) 20,014
Revenue reserve 24,683 7,545
--------------------------------------- -----
Total equity attributable to
shareholders of the Company 1,371,090 1,378,915
--------------------------------------- ----- -------------- ----------------
Net asset value per Ordinary
Share (basic and diluted) 12 $0.9980 $1.0037
--------------------------------------- ----- -------------- ----------------
The financial statements of BioPharma Credit PLC registered
number 10443190 were approved and authorised for issue by the Board
of Directors on 14 September 2021 and signed on its behalf by:
Harry Hyman
Chairman
14 September 2021
The notes below form part of these financial statements.
CONDENSED CASH FLOW STATEMENT
For the period ended 30 June 2021
(In $000s)
30 June 2021 30 June 2020
-----------------------------------------
Note (Unaudited) (Unaudited)
----------------------------------------- ----- ------------- -------------
Cash flows from operating activities
Investment income received 48,472 37,888
Other income received 150 1,458
Investment management fee paid (6,870) (6,774)
Performance fee paid (5,473) (20,968)
Net amounts paid on behalf of
BPCR Limited Partnership - 26,241
Other expenses paid (1,070) (1,039)
Cash generated from operations 15 35,209 36,806
Net cash flow generated from
operating activities 35,209 36,806
----------------------------------------- ----- ------------- -------------
Cash flow from investing activities
Purchase of investments* (146,250) (225,736)
Redemptions of investments** - 8,308
Sales of investments** 92,321 10,753
----------------------------------------- ----- ------------- -------------
Net cash flow used in investing
activities (53,929) (206,675)
----------------------------------------- ----- ------------- -------------
Cash flow from financing activities
Dividends paid to Ordinary shareholders 6 (52,072) (65,674)
Net cash flow used in financing
activities (52,072) (65,674)
----------------------------------------- ----- ------------- -------------
Decrease in cash and cash equivalents
for the period (70,792) (235,543)
----------------------------------------- ----- ------------- -------------
Cash and cash equivalents at
start of period 9 193,269 296,638
Revaluation of foreign currency
balances 1 (37)
----------------------------------------- ----- ------------- -------------
Cash and cash equivalents at
end of period 9 122,478 61,058
----------------------------------------- ----- ------------- -------------
*2020 Purchases of investments includes Collegium, Optinose
Tranche B, Epizyme Tranche B and BDSI Tranche B fundings before
assets were transferred in kind to the financing subsidiary, BPCR
LP, on 22 May 2020. These payments do not include investments made
by BPCR LP.
** BPCR LP investments not included.
The notes below form part of these financial statements.
NOTES TO THE FINANCIAL STATEMENTS
For the period ended 30 June 2021
1. GENERAL INFORMATION
BioPharma Credit PLC is a closed-ended investment company
incorporated and domiciled in England and Wales on 24 October 2016
with registered number 10443190. The registered office of the
Company is Beaufort House, 51 New North Road, Exeter, EX4 4EP. On 6
February 2017 the Company changed its name from PRECIS (2772)
PLC.
The Company carries on the business as an investment trust
company within the meaning of Sections 1158/1159 of the Corporation
Tax Act 2010.
The Company's Investment Manager is Pharmakon Advisors L.P.
("Pharmakon"). Pharmakon is a limited partnership established under
the laws of the State of Delaware. It is registered as an
investment adviser with the Securities and Exchange Commission
("SEC") under the United States Investment Advisers Act of 1940, as
amended.
Pharmakon is authorised as an Alternative Investment Fund
Manager ("AIFM") under the Alternative Investment Fund Managers
Directive ("AIFMD"). Pharmakon has, with the consent of the
Directors, delegated certain administrative duties to Link
Alternative Fund Administrators Limited ("Link").
2. ACCOUNTING POLICIES
A) Basis of preparation
The Company's condensed half-year financial statements covers
the period from 1 January 2021 to 30 June 2021 and have been
prepared in conformity with UK adopted International Accounting
Standard 34 'Interim Financial Reporting'. They do not include all
financial information required for full annual financial statements
and have been prepared using the accounting policies adopted in the
audited financial statements for the year ended 31 December 2020.
The Company's annual financial statements were prepared in
conformity with IFRS, which comprise standards and interpretations
approved by the International Accounting Standards Board ("IASB"),
and as applied in accordance with the Disclosure Guidance
Transparency Rules sourcebook of the Financial Conduct Authority
(FCA) and the AIC SORP (issued in April 2021) for the financial
statements of investment trust companies and venture capital
trusts, except to any extent where it is not consistent with the
requirements of IFRS. The financial statements have adopted the
following accounting policies in their preparation, which remain
consistent with the accounting policies adopted in the audited
financial statements for the year ended 31 December 2020.
The financial statements are presented in US dollars, being the
functional currency of the Company. The financial statements have
been prepared on a going concern basis under historical cost
convention, except for the measurement at fair value of investments
measured at fair value through profit or loss.
The Company's condensed half-year information contained in this
Half-Yearly Report does not constitute full statutory accounts as
defined in Section 435 of the Companies Act 2006. The financial
information for the periods ended 30 June 2021 and 30 June 2020 are
not financial years and have not been audited. The information for
the year ended 31 December 2020 has been extracted from the latest
published financial statements, which have been delivered to the
Registrar of Companies. The Auditor's Report on those financial
statements contained no qualification or statement under Section
498 of the Companies Act 2006.
ASSESSMENT AS AN INVESTMENT ENTITY
Entities that meet the definition of an investment entity within
IFRS 10 'Consolidated Financial Statements' are required to measure
their subsidiaries at fair value through profit or loss rather than
consolidate the entities. The criteria which define an investment
entity are as follows:
-- an entity that obtains funds from one or more investors for
the purpose of providing those investors with investment
services;
-- an entity that commits to its investors that its business
purpose is to invest funds solely for returns from capital
appreciation, investment income or both; and
-- an entity that measures and evaluates the performance of
substantially all of its investments on a fair value basis.
The Directors have concluded that the Company meets the
characteristics of an investment entity, in that it has more than
one investor and its investors are not related parties; holds a
portfolio of investments, predominantly in the form of loans which
generates returns through interest income. All investments,
including its subsidiaries, BPCR Ongdapa Limited and BPCR Limited
Partnership, are reported at fair value to the extent allowed by
IFRS.
B) PRESENTATION OF CONDENSED STATEMENT OF COMPREHENSIVE
INCOME
In order to better reflect the activities of an investment trust
company and in accordance with guidance issued by the AIC,
supplementary information which analyses the Condensed Statement of
Comprehensive Income between items of a revenue and capital nature
has been prepared alongside the Income Statement.
C) SEGMENTAL REPORTING
The Directors are of the opinion that the Company has one
operating and reportable segment being the investment in debt
assets secured by royalties or other cash flows derived from the
sales of approved life sciences products.
D) INVESTMENTS AT FAIR VALUE THROUGH PROFIT OR LOSS
The principal activity of the Company is to invest in
interest-bearing debt assets with a contractual right to future
cash flows derived from royalties or sales of approved life
sciences products. In accordance with IFRS, the financial assets
are measured at fair value through profit or loss. They are
accounted for on their trade date at fair value, which is
equivalent to the cost of the investment. The fair value of the
asset reflects any contractual amortising balance and accrued
interest.
The fair value hierarchy consists of the following three
levels:
-- Level 1 - Quoted market price for identical instruments in
active markets
-- Level 2 - Valuation techniques using observable inputs
-- Level 3 - Valuation techniques using significant unobservable
inputs
Listed level 1 investments where a financial instrument is
active are priced by quoted market prices.
Level 2 investments may be valued using market data obtained
from external, independent sources. The data used could include
quoted prices for similar assets and liabilities in active markets,
prices for identical or similar assets and liabilities in inactive
markets, or models with observable inputs.
For unlisted level 3 investments where the market for a
financial instrument is not active, fair value is established using
valuation techniques in accordance with the International Private
Equity and Venture Capital Valuation ("IPEV") Guidelines (issued in
December 2018), which may include recent arm's length market
transactions between knowledgeable, willing parties, if available,
reference to the current fair value of another instrument that is
substantially the same, discounted cash flow analysis and option
pricing models. Where there is a valuation technique commonly used
by market participants to price the instrument and that technique
has proved reliable from estimates of prices obtained in actual
market transactions, that technique is utilised.
Unlisted investments often require the manager to make estimates
and judgements and apply assumptions or subjective judgement to
future events and other matters that may affect fair value. For
unlisted investments valued using a discounted cash flow analysis,
the key judgements are the size of the market, pricing, projected
sales of the product at trade date and future growth and other
factors that will support the repayment of a senior secured or
royalty debt instrument.
Changes in the fair value of investments held at fair value
through profit or loss, and gains or losses on disposal, are
recognised in the Statement of Comprehensive Income as gains or
losses from investments held at fair value through profit or loss.
Transaction costs incurred on the purchase and disposal of
investments are included within the cost or deducted from the
proceeds of the investments. All purchases and sales are accounted
for on trade date.
E) FOREIGN CURRENCY
Transactions denominated in currencies other than US dollars are
recorded at the rates of exchange prevailing on the date of the
transaction. Items which are denominated in foreign currencies are
translated at the rates prevailing on the balance sheet date. Any
gain or loss arising from a change in exchange rate subsequent to
the date of the transaction is included as an exchange gain or loss
in the Condensed Statement of Comprehensive Income.
F) INCOME
There are five main sources of revenue for the Company: interest
income, income from subsidiaries, royalty revenue, make-whole and
prepayment income, dividends and paydown fees.
Interest income is recognised when it is probable that the
economic benefits will flow to the Company. Interest is accrued on
a time basis, by reference to the principal outstanding and the
effective interest rate that is applicable. Accrued interest is
included within trade and other receivables on the Condensed
Statement of Financial Position.
The Company recognises accrued income for investments that it
holds directly. The Company also holds an investment in BPCR
Limited Partnership, its wholly owned subsidiary which it measures
at fair value through profit or loss rather than consolidate. BPCR
Limited Partnership also recognises accrued income for investments
it holds directly. When the accrued income is recorded at the
Partnership, the Company recognises the income in capital within
the Condensed Statement of Comprehensive Income. When the Company's
right to receive the income is established, funds are transferred
from the Partnership to the Company and income is transferred to
revenue within the Statement of Comprehensive Income.
Royalty revenue is recognised on an accrual basis in accordance
with the substance of the relevant agreement (provided that it is
probable that the economic benefits will flow to the Company and
the amount of revenue can be measured reliably). Royalty
arrangements that are based on production, sales and other measures
are recognised by reference to the underlying arrangement.
Make-whole and prepayment income is recognised when payments are
received by the Company and is recorded to revenue within the
Condensed Statement of Comprehensive Income.
Dividends are receivable on equity shares and recognised on the
ex-dividend date. Where no ex- dividend date is quoted, dividends
are recognised when the Company's right to receive payment is
established. Dividends from investments in unquoted shares and
securities are recognised when they become receivable.
Some investments include additional consideration in the form of
structuring fees, which are paid on completion of the transaction.
As the investments are classified as level 3 in the fair value
hierarchy, there is no observable evidence of the fair value of the
investments excluding the fees, therefore the fees should be
included in the day one fair value of the investments. From 1
January 2020, such fees are included in the fair value of the
investment and released to the Condensed Statement of Comprehensive
Income over the life of the investment. Prior to this date they
were recognised as a gain in the Statement of Comprehensive Income
at the funding date. We consider incorporating the fees in the fair
value gains and losses over the life of the loans to be more
reflective of the period over which the benefit is received. The
impact of this change is immaterial to both the current and prior
period. These fees are allocated to revenue within the Condensed
Statement of Comprehensive Income.
Bank interest and other interest receivable are accounted for on
an accruals basis.
G) DIVIDS PAID TO SHAREHOLDERS
The Company intends to pay dividends in US Dollars on a
quarterly basis, however, shareholders can elect to have dividends
paid in sterling. The Company may, where the Directors consider it
appropriate, use the reserve created by the cancellation of its
share premium account to pay dividends.
The Company intends to comply with the requirements for
maintaining investment trust status for the purposes of section
1158 of the Corporation Tax Act 2010 (as amended) regarding
distributable income. As such, the Company will distribute amounts
such that it does not retain in respect of an accounting period an
amount greater than 15 per cent. of its income (as calculated for
UK tax purposes) for that period.
H) EXPENSES
All expenses are accounted for on an accruals basis. Expenses,
including investment management fees, performance fees and finance
costs, are charged through the revenue account except as
follows:
-- expenses which are incidental to the acquisition or disposal
of an investment are treated as capital costs and separately
identified and disclosed in Note 4; and
-- expenses of a capital nature are accounted for through the
capital account.
The performance fee is considered to be an annual fee and is
only recognised at the end of each performance period. It is
calculated in accordance with the details in Note 4(b) below. Any
performance fee triggered, whether payable or deferred, is
recognised in the Condensed Statement of Comprehensive Income.
Where a performance fee is payable within the next twelve months,
it is treated as a current liability in the Condensed Statement of
Financial Position. Where a performance fee is deferred by more
than twelve months, it is treated as a non-current liability in the
Condensed Statement of Financial Position. It becomes payable to
the Investment Manager at the end of the first performance period
in respect to which the compounding condition is satisfied.
I) TRADE AND OTHER RECEIVABLES
Trade and other receivables are recognised and carried at
amortised cost as the Company collects contractual interest
payments from its borrowers. An allowance for estimated
unrecoverable amounts are measured and recognised where necessary.
The Company assesses, on a forward-looking basis, the expected
losses associated with its trade and other receivables.
J) CASH AND CASH EQUIVALENTS
Cash and cash equivalents are defined as cash in hand, demand
deposits, and short-term, highly liquid investments readily
convertible to known amounts of cash and subject to insignificant
risk of changes in value.
Cash and cash equivalents includes interest and income from
money market funds.
K) TRADE AND OTHER PAYABLES
Trade and other payables are recognised and carried at amortised
cost, do not carry any interest and are short-term in nature.
L) TAXATION
The Company may, if it so chooses, designate as an 'interest
distribution' all or part of the amount it distributes to
shareholders as dividends, to the extent that it has 'qualifying
interest income' for the accounting period. Were the Company to
designate any dividend it pays in this manner, it should be able to
deduct such interest distributions from its income in calculating
its taxable profit for the relevant accounting period. The Company
intends to elect for the 'streaming' regime to apply to the
dividend payments it makes to the extent that it has such
'qualifying interest income'. Shareholders in receipt of such a
dividend will be treated, for UK tax purposes, as though they had
received a payment of interest, which results in a reduction of the
corporation tax payable by the Company.
Tax on the profit or loss for the period comprises current and
deferred tax. Corporation tax is recognised in the Condensed
Statement of Comprehensive Income.
Current tax is the expected tax payable on the taxable income
for the period, using tax rates enacted or substantively enacted at
the balance sheet date and any adjustment to tax payable in respect
of previous periods. The tax effect of different items of
expenditure is allocated between revenue and capital on the same
basis as the particular item to which it relates, using the
Company's marginal method of tax, as applied to those items
allocated to revenue, for the accounting period.
Deferred tax is provided, using the liability method, on all
temporary differences at the balance sheet date between the tax
basis of assets and liabilities and their carrying amount for
financial reporting purposes. Deferred tax liabilities are measured
at the tax rates that are expected to apply to the period when the
liability is settled, based on tax rates (and tax laws) that have
been enacted or substantively enacted at the balance sheet
date.
M) SHARE CAPITAL AND RESERVES
The share capital represents the nominal value of the Company's
ordinary shares.
The share premium account represents the excess over nominal
value of the fair value of consideration received for the Company's
ordinary shares, net of expenses of the share issue. This reserve
cannot be distributed.
The special distributable reserve was created on 29 June 2017 to
enable the Company to buy back its own shares and pay dividends out
of such distributable reserve, in each case when the Directors
consider it appropriate to do so, and for other corporate
purposes.
The capital reserve represents realised and unrealised capital
and exchange gains and losses on the disposal and revaluation of
investments and of foreign currency items. The realised capital
reserve can be used for the repurchase of shares. This reserve
cannot be distributed.
The revenue reserve represents retained profits from the income
derived from holding investment assets less the costs and interest
on cash balances associated with running the Company. This reserve
can be distributed.
N) CRITICAL ACCOUNTING ESTIMATES AND ASSUMPTIONS
The preparation of these financial statements in conformity with
IFRS requires the Directors to make accounting estimates which will
not always equal the actual results. The Directors also need to
exercise judgement in applying the Company's accounting
policies.
This note provides an overview of the areas that involve a
higher degree of judgement or complexity and of items which are
more likely to be materially adjusted due to estimates and
judgements included in other notes, together with information about
the basis of calculation for each line in the financial
statements.
In particular estimates are made in determining the fair
valuation of unquoted investments for which there is no observable
market and may cause material adjustments to the carrying value of
those investments.
Determining fair value of investments with unobservable market
inputs is an area involving management estimates, requiring
assessment as to whether the value of assets can be supported by
the net present value of future cash flows derived from such assets
using cash flow projections which have been discounted at an
appropriate rate. In calculating the net present value of the
future cash flows, certain critical assumptions are required to be
made including management's expectations of short and long term
growth rates in product sales and the selection of discount rates
to reflect the risks involved. These are valued in accordance with
Note 2(d) above and using the valuation techniques described in
Note 7 below.
Also, estimates including cash flow projections, discount rates
and growth rates in product sales are made when determining any
deferred performance fee; this may be affected by future changes in
the Company's portfolio and other assets and liabilities.
Any deferred performance fee is calculated in accordance with
Note 4(b) below and is recognised in accordance with Note 2(h)
above.
These estimates are reviewed on an ongoing basis. Revisions to
these estimates are also reviewed on an ongoing basis. Revisions
are recognised prospectively.
O) NEW ACCOUNTING STANDARDS EFFECTIVE 1 JANUARY 2021
Amendment to IFRS 3 'Business Combinations'
The Directors have considered the implications of the amendments
to IFRS 3 and are of the opinion that the Company's subsidiaries
are already measured at fair value. Therefore, there has been no
impact on the current and comparative financial statements for
this
accounting standard.
Definition of Material (Amendments to IAS 1 and IAS 8)
The Directors have considered the implications of the amendments
to IAS 1 and IAS 8 and are of the opinion that there is no impact
to the Company. Therefore, there has been no impact on the current
and comparative financial statements for this accounting
standard.
P) ACCOUNTING STANDARDS NOT YET EFFECTIVE
The IASB and International Financial Reporting Interpretations
Committee ("IFRIC") have issued and endorsed the following
standards and interpretations, applicable to the Company, which are
not yet effective for the period ended 30 June 2021 and have
therefore not been applied in preparing these financial
statements.
The Directors do not expect that the adoption of the standards
and interpretations will have a material impact on the financial
statements.
Other future development includes the IASB undertaking a
comprehensive review of existing IFRSs. The Company will consider
the financial impact of these new standards as they are
finalised.
3. INCOME
Period Period
ended ended
30 June 30 June
2021 2020
$000 $000
---------------------------------------- -------- -------------
Income from investments
US unfranked investment income from 70,901 -
BPCR LP
US unfranked investment income from
BPCR Ongdapa - 3,440
US fixed interest investment income 136 19,633
US floating interest investment income 2,978 21,826
Paydown fee* - 427
Prepayment premium** 1,474 -
Additional consideration received*** - 467
---------------------------------------- -------- -------------
74,679 45,793
Other income
Interest income from liquidity/money
market funds 12 1,033
12 1,033
Total income 74,691 46,826
---------------------------------------- -------- -------------
* In 2020 the Company's senior secured term loans to Sarepta and
GBT included paydown fees of $357,000 and $70,000.
** In 2021 the Company's senior secured term loan to Sebela
included a prepayment premium of $1,474,000, which was paid upon
the loan repayment and recognised as income in the year.
*** In 2020 the Company's senior secured term loan to Collegium
included additional consideration in the form of structuring fees
of $4,125,000 which was paid upon the completion of the transaction
and $467,000 of this amount recognised as income in the period.
The below table reconciles the total income with the total
income of BioPharma Credit PLC and BPCR Limited Partnership as
disclosed in the chairman's statement above.
Period Period
ended ended
30 June 30 June
2021 2020
$000 $000
Total income of BioPharma Credit PLC 74,691 46,826
Prior year income paid to Biopharma (20,484) -
Credit PLC from BPRC Limited Partnership
Undistributed income due to Biopharma
Credit PLC from BPCR Limited Partnership - 12,009
Expenses incurred by BPCR Limited
Partnership 3,961 585
Total income of BioPharma Credit PLC
and BPCR Limited Partnership 58,168 59,420
4. FEES AND EXPENSES
Expenses
Period ended 30 June Period ended 30 June 2020
2021
Revenue Capital Total Revenue Capital Total
------------------------
GBP000 $000 $000 GBP000 $000 $000
------------------------ -------- -------- ------ ---------- --------- -------
Management fee
(note 4a) 6,866 - 6,866 6,872 - 6,872
------------------------ -------- -------- ------ ---------- --------- -------
Directors' fees
(note 4c) 198 - 198 198 - 198
------------------------ -------- -------- ------ ---------- --------- -------
Other operating
expenses
Company Secretarial
fee 45 - 45 42 - 42
Administration
fee 64 - 64 56 - 56
Legal & professional
fees 57 - 57 263 - 263
Public relations
fees 100 - 100 105 - 105
Director's and
Officer's Liability
Insurance 92 - 92 65 - 65
Auditor's remuneration
- statutory audit 138 - 138 130 - 130
Auditor's remuneration
- other audit-related
services - interim
review 53 - 53 37 - 37
Auditor's remuneration
- other audit-related
services - Agreed
upon procedures 15 - 15 9 - 9
VAT (47) - (47) 17 - 17
Other expenses 162 - 162 153 - 153
------------------------ -------- -------- ------ ---------- --------- -------
679 - 679 877 - 877
------------------------ -------- -------- ------ ---------- --------- -------
Total expenses 7,743 - 7,743 7,947 - 7,947
------------------------ -------- -------- ------ ---------- --------- -------
A) INVESTMENT MANAGEMENT FEE
With effect from the Initial Admission, the Investment Manager
is entitled to a management fee ("Management Fee") calculated on
the following basis: (1/12 of 1 per cent of the NAV on the last
business day of the month in respect of which the Management Fee is
to be paid (calculated before deducting any accrued Management Fee
in respect of such month)) minus (1/12 of $100,000).
The Management Fee payable in respect of any quarter will be
reduced by an amount equal to the Company's pro rata share of any
transaction fees, topping fees, break-up fees, investment banking
fees, closing fees, consulting fees or other similar fees which the
Investment Manager (or an affiliate) receives in connection with
transactions involving investments of the Company ("Transaction
Fees"). The Company's pro rata share of any Transaction Fees will
be in proportion to the Company's economic interest in the
investment(s) to which such Transaction Fees relate.
B) PERFORMANCE FEE
Subject to: (i) the NAV attributable to the Ordinary Shares as
at the end of a performance period representing a minimum of 6 per
cent. annualised rate of return annualised on the Company's IPO
gross proceeds (adjusted for dividends, share issues and buybacks
as appropriate), (ii) the total return on the NAV attributable to
the Ordinary Shares (adjusted for dividends, share issues and
buybacks as appropriate) exceeding 6 per cent. over such
performance period, and (iii) a high watermark, the Investment
Manager will be entitled to receive a performance fee equal to the
lesser of: (a) 50 per cent. of the total return above 6 per cent.;
and (b) 10 per cent. of the total return over such performance
period provided always that the amount of any performance fee
payable to the Investment Manager will be reduced to the extent
necessary to ensure that after account is taken of such fee,
condition (iii) above remains satisfied.
Where the Investment Manager is not entitled to a performance
fee solely because condition (i) has not been satisfied, such fee
will be deferred and paid in a subsequent performance period in
which such condition is satisfied. Where condition (i) is satisfied
in a performance period but the payment of a performance fee (or
any deferred performance fee from previous performance periods) in
full would result in that condition failing, the Investment Manager
shall be entitled to such a portion of such fee that does not
result in the failure of the condition (i) above and the balance
would be deferred to a future performance period.
Any performance fee (whether deferred or otherwise) shall be
paid as soon as practicable after the end of the relevant
performance period and, in any event, within 15 business days of
the publication of the Company's audited annual financial
statements relating to such period.
Where the payment of performance fee (or any deferred
performance fee from previous performance periods) in full would
result in the failure of condition (i) above, the Investment
Manager shall only be entitled to 50 per cent. of such fee that
does not result in the failure of condition (i) with the balance
being deferred to a future performance period.
If, during the last month of a performance period, the Shares
have, on average, traded at a discount of 1 per cent. or more to
the NAV per Share (calculated by comparing the middle market
quotation of the Shares at the end of each business day in the
month to the prevailing published NAV per Share (exclusive of any
dividend declared) as at the end of such business day and averaging
this comparative figure over the month), the Investment Manager
shall (or shall procure that its Associate does) apply 50 per cent.
of any Performance Fee paid by the Company to the Investment
Manager (or its Associate) in respect of that performance period
(net of all taxes and charges applicable to such portion of the
Performance Fee) to make market acquisitions of Shares (the
"Performance Shares") as soon as practicable following the payment
of the Performance Fee by the Company to the Investment Manager (or
its Associate) and at least until such time as the Shares have, on
average, traded at discount of less than 1 per cent. to the NAV per
Share over a period of five business days (calculated by comparing
the middle market quotation of the Shares at the end of each such
business day to the prevailing published NAV per Share (exclusive
of any dividend declared) and averaging this comparative figure
over the period of five business days). The Investment Manager's
obligation:
1) shall not apply to the extent that the acquisition of the
Performance Shares would require the Investment Manager to make a
mandatory bid under Rule 9 of the Takeover Code; and
2) shall expire at the end of the performance period which
immediately follows the performance period to which the obligation
relates.
The Performance Fee for a performance period shall be paid as
soon as practicable after the end of the relevant performance
period and, in any event, within three calendar months of the end
of such performance period.
The below table shows the accrued and payable
performance fee.
As at As at
30 June 30 June At 31 December
2021 2020 2020
$000 $000 $000
Accrued performance
fee - - 4,909
Performance fee payable - - 5,473
Performance fee deferred - 564 -
During the period a performance fee of $5,473,000 was paid to
Pharmakon.
The Performance Fee for a performance period shall be paid as
soon as practicable after the end of the relevant performance
period and, in any event, within three calendar months of the end
of such performance period.
C) DIRECTORS
Each of the Directors is entitled to receive a fee from the
Company at such rate as may be determined in accordance with the
Articles. The Directors' remuneration is $70,000 per annum for each
Director other than:
-- the Chairman, who receives an additional $30,000 per annum;
and
-- the Chairman of the Audit and Risk Committee, who receives an
additional $15,000 per annum.
5. TAXATION ON ORDINARY ACTIVITIES
It is the intention of the Directors to conduct the affairs of
the Company so as to satisfy the conditions for approval of the
Company by HMRC as an investment trust under Section 1158 of the
Corporation Tax Act 2010 (as amended) and pursuant to regulations
made under Section 1159 of the Corporation Tax Act 2010. As an
investment trust, the Company is exempt from corporation tax on
capital gains.
The current taxation charge for the period is different from the
standard rate of corporation tax in the UK of 19.00 per cent., The
effective tax rate was 0.00 per cent. The differences are explained
below.
Period ended 30 June Period ended 30 June
2021 2020
Revenue Capital Total Revenue Capital Total
$000 $000 $000 $000 $000 $000
-------------------------- --------- --------- --------- -------- -------- --------
Total return on ordinary
activities before
taxation 66,948 (22,701) 44,247 38,879 1,487 40,366
-------------------------- --------- --------- --------- -------- -------- --------
Theoretical tax at
UK Corporation tax
rate of 19.00% (30
June 2020: 19.00%)* 12,720 (4,313) 8,407 7,387 283 7,670
Effects of:
Capital items that
are not taxable - 4,313 4,313 - (283) (283)
Tax deductible interest
distributions (12,720) - (12,720) (7,387) - (7,387)
-------------------------- --------- --------- --------- -------- -------- --------
Total tax charge - - - - - -
-------------------------- --------- --------- --------- -------- -------- --------
* The theoretical tax rate is calculated using a blended tax
rate over the period.
At 30 June 2021, the Company had no unprovided deferred tax
liabilities. At that date, based on current estimates and including
the accumulation of net allowable losses, the Company had no
unrelieved losses.
Deferred tax is not provided on capital gains and losses arising
on the revaluation or disposal of investments because the Company
meets (and intends to continue to meet for the foreseeable future
to meet) the conditions for approval as an Investment Trust
company.
6. DIVIDS
Dividends paid in respect of the period under review:
Period ended 30 June 2021 Period ended 30 June 2020
Revenue Capital Total Revenue Capital Total
$000 $000 $000 $000 $000 $000
------------------------------------------------------ ---------- --------- ------- ---------- --------- -------
In respect of the current period
First interim dividend of $0.0175 per Ordinary share
(2020: $0.0175 per Ordinary share) 21,782 2,262 24,044 24,044 - 24,044
In respect of the previous year ended 31 December
2020 (31 December 2019):
Special dividend of $0.0029 per Ordinary share (2020:
$0.0128 per Ordinary share) 3,984 - 3,984 17,586 - 17,586
Fourth interim dividend of $0.0175 per Ordinary share
(2020: $0.0175 per Ordinary share) 24,044 - 24,044 24,044 - 24,044
49,810 2,262 52,072 65,674 - 65,674
------------------------------------------------------ ---------- --------- ------- ---------- --------- -------
7. INVESTMENTS AT FAIR VALUE THROUGH PROFIT AND LOSS
As at As at
-----------------------------------------------
30 June 31 December
-----------------------------------------------
2021 2020
$000 $000
----------------------------------------------- ---------- ------------
Investment portfolio summary
Listed investments at fair value through
profit and loss 9,649 11,320
Unlisted investments in subsidiaries measured
at fair value through profit and loss 1,216,328 1,090,887
Unlisted fixed interest investments at
fair value through profit and loss 81 303
Unlisted floating interest investments
at fair value through profit and loss - 92,321
1,226,058 1,194,831
----------------------------------------------- ---------- ------------
Period ended 30 June 2021
Unlisted Unlisted
investments Unlisted fixed floating
------------------------------
Listed in interest interest
------------------------------
investments subsidiaries investments investments Total
$000 $000 $000 $000 $000
------------------------------ ------------ ------------- --------------- ------------ ----------
Investment portfolio
summary
Opening cost at beginning
of period 13,544 1,070,139 1,238 92,321 1,177,242
Opening unrealised
(depreciation)/appreciation
at beginning of period (2,224) 20,748 (935) - 17,589
------------------------------ ------------ ------------- --------------- ------------ ----------
Opening fair value
at beginning of period 11,320 1,090,887 303 92,321 1,194,831
------------------------------ ------------ ------------- --------------- ------------ ----------
Movements in the period:
Purchases at cost - 146,250 - - 146,250
Redemption and sales
proceeds - - - (92,321) (92,321)
Change in unrealised
depreciation (1,671) (20,809) (222) - (22,702)
------------------------------ ------------ ------------- --------------- ------------ ----------
Closing fair value
at the end of the
period 9,649 1,216,328 81 - 1,226,058
------------------------------ ------------ ------------- --------------- ------------ ----------
Closing cost at end
of period 13,544 1,216,389 1,238 - 1,231,171
Closing unrealised
depreciation at end
of period (3,895) (61) (1,157) - (5,113)
------------------------------ ------------ ------------- --------------- ------------ ----------
Closing fair value
at the end of the
period 9,649 1,216,328 81 - 1,226,058
------------------------------ ------------ ------------- --------------- ------------ ----------
Period
Period ended ended
30 June 30 June
2021 2020
--------------------------- ------------- ---------
$000 $000
Realised gains on sale of
investments - (647)
Unrealised appreciation/
(depreciation) (22,702) 2,171
----------------------------- ------------- ---------
(22,702) 1,524
There were no transaction costs for the acquisition or disposal
of investments in any of the relevant periods.
The Company is required to classify fair value measurements
using a fair value hierarchy that reflects the significance of the
inputs used in making the measurements. The fair value hierarchy
consists of the following three levels:
-- Level 1 - Quoted prices (unadjusted) in active markets for identical assets or liabilities.
-- Level 2 - Inputs other than quoted prices included within
Level 1 that are observable for the asset or liability, either
directly (that is, as prices) or indirectly (that is, derived from
prices).
-- Level 3 - Inputs for the asset or liability that are not
based on observable market data (unobservable inputs).
The level of the fair value hierarchy, within which the fair
value measurement is categorised, is determined on the basis of the
lowest level input that is significant to the fair value of the
investment.
As at 30 June 2021
Level 1 Level 2 Level 3 Total
$000 $000 $000 $000
---------------------------- -------- -------- ---------- ----------
Investment portfolio
summary
Listed investments
at fair value through
profit and loss 9,649 - - 9,649
Unlisted investments
in subsidiaries at
fair value through
profit and loss - - 1,216,328 1,216,328
Unlisted fixed interest
investments at fair
value through profit
and loss - 81 - 81
---------------------------- -------- -------- ---------- ----------
Unlisted floating interest
investments at fair
value through profit
and loss - - - -
---------------------------- -------- -------- ---------- ----------
9,649 81 1,216,328 1,226,058
Liquidity/money market
funds 122,353 - - 122,353
---------------------------- -------- -------- ---------- ----------
Total 132,022 81 1,216,328 1,348,411
---------------------------- -------- -------- ---------- ----------
As at 31 December 2020
Level 1 Level 2 Level 3 Total
$000 $000 $000 $000
---------------------------- -------- -------- ---------- ----------
Investment portfolio
summary
---------------------------- -------- -------- ---------- ----------
Listed investments at
fair value through profit
and loss 11,320 - - 11,320
Unlisted investments
in subsidiaries measured
at fair value through
profit and loss - - 1,090,887 1,090,887
Unlisted fixed interest
investments at fair
value through profit
and loss - 303 - 303
Unlisted floating interest
investments at fair
value through profit
and loss - - 92,321 92,321
---------------------------- -------- -------- ---------- ----------
11,320 303 1,183,208 1,194,831
Liquidity/money market
funds 181,542 - - 181,532
---------------------------- -------- -------- ---------- ----------
Total 192,852 303 1,183,208 1,376,363
---------------------------- -------- -------- ---------- ----------
A reconciliation of fair value measurements in Level 3 is set
out below.
LEVEL 3 FINANCIAL ASSETS AT FAIR VALUE THROUGH PROFIT OR
LOSS
Period ended 30 June 2021
Unlisted
----------------------
floating
----------------------
Unlisted
investments interest investments Total
---------------------- ------------- --------------------- ----------
$000 $000 $000
---------------------- ------------- --------------------- ----------
Opening balance 1,090,887 92,321 1,183,208
Investments in
subsidiaries 146,250 - 146,250
Redemptions* - (92,321) (92,321)
Change in unrealised
appreciation (20,809) - (20,809)
Closing balance
at 30 June 2021 1,216,328 - 1,216,328
---------------------- ------------- --------------------- ----------
* Redemptions are the proceeds received from the repayment of
investments.
There were no transfers between levels during the period.
VALUATION TECHNIQUES
Unrealised gains and losses recorded on Level 1 financial
instruments are reported in net gains on investments at fair value
on the Condensed Statement of Comprehensive Income. The fund
administrator utilises quoted prices in active markets that they
have access to and the Investment Manager verifies the quoted
prices on Bloomberg.
Unrealised gains and losses recorded on Level 2 and 3 financial
instruments are reported in net gains on investments at fair value
on the Condensed Statement of Comprehensive Income. Level 2 and
Level 3 financial instruments are fair valued using inputs that
reflect management's best estimate of what market participants
would use in pricing the assets or liabilities at the measurement
date. Consideration is given to the risk inherent in the valuation
techniques and the risk inherent in the inputs of the model.
Level 3 financial instruments are fair valued using a discounted
cash flow methodology. For capped royalty investments, discount
rates are applied to the consensus forecasts or the manager's
forecast for sales of the underlying products to determine fair
value. The significant unobservable input used in the fair value
measurement of the Company's level 3 investments is the discount
rate used to discount future cash flows from borrowers.
Significant increases (decreases) in the discount rate would
result in a significantly lower (higher) fair value measurement.
The Investment Manager believes 100 basis points is an appropriate
threshold for determining a reasonably possible change in fair
value.
Investments held in subsidiaries, namely BPCR LP, are based on
the fair value of the investments held in those entities.
The Company's unlisted investments, including those of it's
wholly owned subsidiary BPCR LP, are all classified as Level 3
investments. The fair values of the unlisted investments have been
determined principally by reference to discounted cash flows. The
significant unobservable input used is detailed below:
As at 30 June 2021
Fair value
at Level Fair value Fair value
3 financial sensitivity sensitivity
assets at to a 100bps to a 100bps
fair value decrease increase
through profit Valuation Unobservable Discount in the discount in the discount
Assets or loss technique input rate rate rate
$000 $000 $000
---------------- ---------------- --------------- ------------- --------- ----------------- ------------------
Assets held
by BPCR LP*
Discounted
cash Discount
Akebia 50,000 flow rate 11.5% 50,847 49,178
Discounted
cash Discount
BDSI 80,000 flow rate 11.3% 81,423 78,620
Discounted
cash Discount
BMS 149,329 flow rate 10.5% 152,118 146,637
Other net 59,561 Amortised cost - - - -
assets of
BPCR LP
Discounted
cash Discount
Collegium 113,438 flow rate 12.4% 114,586 112,317
Discounted
cash Discount
Epizyme 110,000 flow rate 11.1% 113,201 106,933
Discounted
Global Blood cash Discount
Therapeutics 82,500 flow rate 11.1% 84,362 80,702
Lumira Discounted
Investment cash Discount
Limited 150,000 flow rate 9.9% 153,106 146,988
Discounted
OptiNose cash Discount
US 71,500 flow rate 13.3% 72,676 70,356
Discounted
Sarepta cash Discount
Therapeutics 350,000 flow rate 10.7% 357,509 342,732
1,216,328 1,179,828 1,134,463
*The Company holds an investment in BPCR Limited Partnership,
its wholly owned subsidiary, which it measures at fair value
through profit or loss rather than consolidate.
As at 31 December 2020
-------------------------------------------------------------------------- -------------------
Fair value Fair value Fair value
at Level 3 sensitivity sensitivity
financial to a 100bps to a 100bps
assets at fair decrease increase
value through Valuation Unobservable Discount in the discount in the discount
Assets profit or loss technique input rate rate rate
--------------------- ---------------- ----------- ------------- --------- ----------------- -------------------
$000 $000 $000
--------------------- ---------------- ----------- ------------- --------- ----------------- -------------------
Discounted Discount
Sebela 92,321 cash flow rate 12.4% 92,860 91,789
Assets held by BPCR LP*
Discounted Discount
Akebia 50,000 cash flow rate 11.1% 51,035 48,999
Discounted Discount
BDSI 80,000 cash flow rate 11.1% 81,717 78,341
Discounted Discount
BMS 160,180 cash flow rate 9.4% 163,586 150,906
Other net
assets of Amortised
BPCR LP 52,644 cost - - - -
Discounted Discount
Collegium 134,063 cash flow rate 12.0% 135,668 132,500
Discounted Discount
Epizyme 110,000 cash flow rate 11.0% 113,546 106,615
Global Blood Discounted Discount
Therapeutics 82,500 cash flow rate 13.9% 84,158 80,894
Discounted Discount
OptiNose US 71,500 cash flow rate 12.8% 72,932 70,112
Discounted Discount
Sarepta Therapeutics 350,000 cash flow rate 10.4% 358,804 341,514
1,183,208 1,154,306 1,101,670
--------------------- ---------------- ----------- ------------- --------- ----------------- -------------------
* The Company holds an investment in BPCR Limited Partnership,
its wholly owned subsidiary, which it measures at fair value
through profit or loss rather than consolidate.
8. TRADE AND OTHER RECEIVABLES
As at As at
--------------------------------------------------
30 June 31 December
--------------------------------------------------
2021 2020
$000 $000
-------------------------------------------------- -------- ------------
Unlisted income receivable from BPCR LP 26,071 -
Interest accrued on liquidity/money market funds 1 3
Other debtors 304 205
-------------------------------------------------- -------- ------------
26,376 208
-------------------------------------------------- -------- ------------
9. CASH AND CASH EQUIVALENTS
As at As at
------------------------------
30 June 31 December
------------------------------
2021 2020
$000 $000
------------------------------ -------- ------------
Cash at bank 125 11,737
Liquidity/money market funds 122,353 181,532
122,478 193,269
------------------------------ -------- ------------
10. TRADE AND OTHER PAYABLES
As at As at
30 June 31 December
2021 2020
$000 $000
------------------------- -------- ------------
Current liabilities
Performance fee payable - 5,473
Management fees accrual 3,427 3,431
Accruals 395 489
3,822 9,393
------------------------- -------- ------------
11. RETURN PER ORDINARY SHARE
Revenue return per ordinary share is based on the net revenue
after taxation of $66,948,000 (30 June 2020: $38,879,000) and
1,373,872,373 (30 June 2020: 1,373,932,067) ordinary shares, being
the weighted average number of ordinary shares for the period.
Capital return per ordinary share is based on net capital loss
for the period of $22,701,000 (30 June 2020: profit of $1,487,000)
and on 1,373,872,373 (30 June 2020:1,373,932,067) ordinary shares,
being the weighted average number of ordinary shares for the
period.
Basic and diluted return per share are the same as there are no
arrangements which could have a dilutive effect on the Company's
ordinary shares.
12. NET ASSET VALUE PER ORDINARY SHARE
The basic total net assets per ordinary share is based on the
net assets attributable to equity shareholders at 30 June 2021 of
$1,371,090,000 (30 June 2020: $1,378,428,000 and 31 December 2020:
$1,378,915,000) and ordinary shares of 1,373,872,373 (30 June 2020:
1,373,932,067 and 31 December 2020: 1,373,872,373), being the
number of ordinary shares in issue at 30 June 2021.
There is no dilution effect and therefore there is no difference
between the diluted total net assets per ordinary share and the
basic total net assets per ordinary share.
13. SHARE CAPITAL
Period ended Year ended
30 June 2021 31 December 2020
Number of Number of
---------------------------
shares $000 shares $000
--------------------------- -------------- ------- -------------- -------
Issued and fully paid:
Ordinary shares of $0.01:
Balance at beginning
of the period 1,373,932,067 13,739 1,373,932,067 13,739
Balance at end of the
period 1,373,932,067 13,739 1,373,932,067 13,739
--------------------------- -------------- ------- -------------- -------
Total voting rights at 30 June 2021 were 1,373,872,373 (31
December 2020: 1,373,872,373). During the previous year 59,694
shares were bought back for treasury. The balance of treasury
shares on 30 June 2021 was 59,694 (31 December 2020: 59,694).
14. SUBSIDIARY
The Company formed a wholly-owned subsidiary, BPCR Ongdapa
Limited ("BPCR Ongdapa"), incorporated in Ireland on 5 October 2017
for the purpose of entering into a purchase, sale and assignment
agreement with a wholly-owned subsidiary of Royalty Pharma for the
purchase of a 50 per cent. interest in a stream of payments
acquired by Royalty Pharma from Bristol-Myers Squibb ("BMS"). In
accordance with IFRS 10, the Company is exempt from consolidating a
controlled investee as an investment entity. Therefore, the
Company's investment in BPCR Ongdapa is recognised at fair value
through profit or loss. The registered address for BPCR Ongdapa is
BPCR Ongdapa Limited, 2 Grand Canal Square, Grand Canal Harbour,
Dublin, Ireland. The aggregate amount of its capital reserves as at
30 June 2021 is $1 (30 June 2020: $1 and 31 December 2020: $1) and
the profit and loss for the period ended 30 June 2021 is $159,669
(30 June 2020: $nil and 31 December 2020: $445,582).
The Company formed a wholly-owned subsidiary, BPCR Limited
Partnership (BPCR LP), incorporated in England and Wales on 27
March 2020 for the purpose of entering into a three year $200
million revolving credit facility with JPMorgan Chase Bank. BPCR
Limited Partnership has its registered office at 51 New North Road,
Exeter, United Kingdom, EX4 4EP and received an initial
contribution of GBP1.00 at formation from the Company, its sole
Limited Partner. In accordance with IFRS 10, the Company is
exempted from consolidating a controlled investee as it is an
investment entity. Therefore, the Company's investment in BPCR
Limited Partnership will be recognised at fair value through profit
or loss.
The Company is not exempt from consolidating the the financial
statements of BPCR GP under IFRS 10, however the highly immaterial
(nil) balance of BPCR GP would produce accounts with almost
identical balances to the Company. Furthermore with reference to
the Companies Act, section 405 (2) "A subsidiary undertaking may be
excluded from consolidation if its inclusion is not material for
the purpose of giving a true and fair view". The registered address
for BPCR GP Limited is BPCR GP Limited, 51 New North Road, Exeter,
United Kingdom, EX4 4EP. The aggregate amount of its capital
reserves as at 30 June 2021 is $nil (2020: $nil) and the profit and
loss for the period to 30 June 2021 is $nil (2020: $nil).
The Company formed two wholly-owned structured subsidiaries,
BPCR Ongdapa Ltd. and BPCR Limited Partnership to assist in meeting
its investment objectives and enter into a revolving credit
facility. The Company generates returns and retains the ownership
risks in the investments retained in the subsidiaries. These
entities meet the definition of an investment entity within IFRS 10
and the Company is required to measure these subsidiaries at fair
value through profit and loss rather than consolidate. The maximum
exposure to loss for the unconsolidated structured subsidiaries is
the fair value and any accrued unpaid fees.
15. RECONCILIATION OF TOTAL RETURN FOR THE PERIOD BEFORE
TAXATION TO CASH
GENERATED FROM OPERATIONS
Period ended Period ended
-------------------------------------------------
30 June 2021 30 June 2020
-------------------------------------------------
$000 $000
------------------------------------------------- ------------- -------------
Total return for the period before taxation 44,247 40,366
Capital (losses/(gains) 22,701 (1,487)
(Increase)/decrease in trade receivables (26,168) 12,983
(Decrease)/increase in trade payables (5,571) 6,702
Non-cash movement for additional consideration* - (400)
Other assets transferred to BPCR LP** - (21,358)
------------------------------------------------- ------------- -------------
Cash generated from operations 35,209 36,806
------------------------------------------------- ------------- -------------
* In 2020 the Company's senior secured term loan of $20,000,000
to BDSI included additional consideration of $400,000. This reduced
the value of the payment made.
** On 22 May 2020, the Company transferred the full carrying
amount of several investments to its newly incorporated,
wholly-owned subsidiary BPCR LP in return for an investment in BPCP
LP of the same amount $1,048,000,000. The balance on the transfer
line of $21,358,000 relates to accrued income which is subsequently
reflected in the fair value of BPCR LP, previously disclosed as
part of trade and other receivables in the Company and expenses
paid on the behalf of BPCR LP.
ANALYSIS OF NET CASH AND NET DEBT
Net cash
At 1 January Exchange At 30 June
2021 Cash flow movement 2021
$000 $000 $000 $000
Cash and cash equivalents 193,269 (70,792) 1 122,478
--------------------------- ------------- ---------- --------- -----------
At 1 January Exchange At 30 June
2020 Cash flow movement 2020
$000 $000 $000 $000
Cash and cash equivalents 296,638 (235,543) (37) 61,058
--------------------------- ------------- ---------- --------- -----------
16. FINANCIAL INSTRUMENTS
The Company's financial instruments include its investment
portfolio, cash balances, trade receivables and trade payables that
arise directly from its operations. Adherence to the Company's
investment policy is key in managing risk. Refer to the Strategic
Overview on pages 14 to 26 of the Company's annual financial
statements for the year ended 31 December 2020 for a full
description of the Company's investment objective and policy.
The Investment Manager monitors the financial risks affecting
the Company on an ongoing basis and the Directors regularly receive
financial information, which is used to identify and monitor risk.
All risks are actively reviewed and monitored by the Board. Details
of the Company's principal risks can be found in the Strategic
Report on pages 19 to 26 of the Company's annual financial
statements for the year ended 31 December 2020.
The main risks arising from the Company's financial instruments
are:
A) market risk, including price risk, currency risk and interest
rate risk;
B) liquidity risk; and
C) credit risk.
(A) Market risk
Market risk is the risk of loss arising from movements in
observable market variables. The fair value of future cash flows of
a financial instrument held by the Company may fluctuate because of
changes in market prices. The Investment Manager assesses the
exposure to market risk when making each investment decision and
these risks are monitored by the Investment Manager on a regular
basis and the Board at quarterly meetings with the Investment
Manager.
Market price risk
The Company is exposed to price risk arising from its
investments whose future prices are uncertain. The Company's
exposure to price risk comprises movements in the value of the
Company's investments. See Note 7 above for investments that fall
into Level 3 of the fair value hierarchy and refer to the
description of valuation policies in Note 2(d). The nature of the
Company's investments, with a high proportion of the portfolio
invested in unlisted debt instruments, means that the investments
are valued by the Company after consideration of the most recent
available information from the underlying investments. The
Company's portfolio is diversified among counterparties and by the
sectors in which the underlying companies operate, minimising the
impact of any negative industry-specific trends.
The table below analyses the effect of a 10 per cent. change in
the fair value of investments. The Investment Manager believes 10
per cent. is the appropriate threshold for determining whether a
material change in market value has occurred.
As at 30 June 2021 As at 30 June 2020 At 31 December 2020
10 per 10 per 10 per
cent. Increase/ cent. Increase/ cent. Increase/
decrease decrease decrease
in market in market in market
Fair value value Fair value value Fair value value
$000 $000 $000 $000 $000 $000
---------------------- ----------- ----------------- ----------- ----------------- ----------- -----------------
Biodelivery
Sciences
International
Equity 9,649 965 11,751 1,175 11,320 1,132
Convertible
bonds - - 4,376 438 - -
Lexicon Senior
Secured Loan - - 124,500 12,450 - -
OptiNose
US warrants 81 8 1,296 130 303 30
Sebela Senior
Secured Loan - - 122,013 12,201 92,321 9,232
Assets held
by BPCR LP
Akebia 50,000 5,000 40,000 4,000 50,000 5,000
Amicus Senior
Secured Loan - - 150,000 15,000 - -
Biodelivery
Sciences
International
Loan 80,000 8,000 80,000 8,000 80,000 8,000
BMS Purchased
Payments
(BPCR Ongdapa) 149,329 14,933 162,032 16,203 160,180 16,018
Collegium 113,438 11,344 154,687 15,469 134,063 13,407
Epizyme 110,000 11,000 35,000 3,500 110,000 11,000
Global Blood
Therapeutics 82,500 8,250 41,250 4,125 82,500 8,250
Lumira Investment
Limited 150,000 15,000 - - - -
Optinose
US Note 71,500 7,150 60,500 6,050 71,500 7,150
Optinose
US Equity - - - - 101 10
Other Assets
of BPCR LP 59,561 5,956 33,679 3,368 52,543 5,254
Novocure
Senior Secured
Loan - - 150,000 15,000 - -
Sarepta Therapeutics 350,000 35,000 175,000 17,500 350,000 35,000
---------------------- ----------- ----------------- ----------- -----------------
1,226,058 122,606 1,346,084 134,609 1,194,831 119,483
---------------------- ----------- ----------------- ----------- ----------------- ----------- -----------------
The Board manages the risks inherent in the investment portfolio
by ensuring full and timely reporting of relevant information from
the Investment Manager. Investment performance and exposure are
reviewed at each Board meeting.
Currency risk
Currency risk is the risk that fair values of future cash flows
of a financial instrument fluctuate because of changes in foreign
exchange rates.
At 30 June 2021, the Company held cash balances in GBP Sterling
of GBP81,000 ($112,000) (30 June 2020: GBP20,000 ($24,000) and 31
December 2020: GBP72,000 ($99,000)) and in Euro of EUR7,000
($8,000) (30 June 2020: EUR10,000 ($11,000) and 31 December 2020:
EUR10,000 ($12,000)) .
The currency exposures (including non-financial assets) of the
Company as at 30 June 2021:
Other net
-----------
assets/
-----------
Cash Investments (liabilities) Total
$000 $000 $000 $000
----------- -------- ------------ -------------- ----------
Sterling 112 - (40) 72
Euro 8 - - 8
US Dollar 122,358 1,226,068 22,594 1,371,010
----------- -------- ------------ -------------- ----------
122,478 1,226,058 22,554 1,371,090
----------- -------- ------------ -------------- ----------
The currency exposures (including non-financial assets) of the
Company as at 30 June 2020:
Other net
-----------
assets/
Cash Investments (liabilities) Total
$000 $000 $000 $000
----------- ------- ------------ -------------- ----------
Sterling 24 - (33) (9)
Euro 11 - - 11
US Dollar 61,023 1,346,084 (28,681) 1,378,426
----------- ------- ------------ -------------- ----------
61,058 1,346,084 (28,714) 1,378,428
----------- ------- ------------ -------------- ----------
The currency exposures (including non-financial assets) of the
Company as at 31 December 2020:
Other net
-----------
assets/
Cash Investments (liabilities) Total
$000 $000 $000 $000
----------- -------- ------------ -------------- ----------
Sterling 99 - (209) (110)
Euro 12 - - 12
US Dollar 193,158 1,194,831 (8,976) 1,379,013
----------- -------- ------------ -------------- ----------
193,269 1,194,831 (9,185) 1,378,915
----------- -------- ------------ -------------- ----------
A 10 per cent. increase in the Sterling exchange rate would have
increased net assets by $16,000 (30 June 2020: $21,000 and 31
December 2020: $20,000). A 10 per cent. increase in the Euro
exchange rate would have increased net assets by $1,000 (30 June
2020: $1,000 and 31 December 2020: $1,000). A 10 per cent decrease
would have decreased net assets by the same amount (30 June 2020:
same and 31 December 2020: same).
Interest rate risk
Interest rate risk is the risk that fair value of future cash
flows of a financial instrument will fluctuate because of changes
in market interest rates. Interest rate movements may potentially
affect future cash flows from:
-- investments in fixed interest rate securities and unquoted
loans; and
-- the level of income receivable on cash deposits and liquidity
funds.
The Lexicon, Novocure, OptiNose US, Sarepta Therapeutics and the
convertible bond have a fixed interest rate and therefore are not
subject to interest rate risk. The below table shows the percentage
of the Company's net assets they represent.
As at As at
30 June 30 June At 31 December
2021 2020 2020
% of Company % of Company % of Company
Net Assets Net Assets Net Assets
Sarepta Therapeutics 25,53 12.70 25.38
OptiNose US 5.21 4.39 5.19
Novocure Senior Secured Loan - 10.88 -
Lexicon Senior Secured Loan - 9.03 -
Convertible bonds - 0.32 -
The BMS Purchased Payments, Collegium, Amicus, Sebela, BDSI, Global
Blood Therapeutics, Akebia, Lumira and Epizyme loans and cash and
cash equivalents, including investments in liquidity funds, have
a floating rate of interest. The below table shows the percentage
of the Company's net assets they represent.
As at As at
30 June 30 June At 31 December
2021 2020 2020
% of Company % of Company % of Company
Net Assets Net Assets Net Assets
Lumira Investment Limited 10.94 - -
BMS Purchased Payments (BPCR Ongdapa) 10.89 11.75 11.62
Collegium 8.27 11.22 9.72
Epizyme 8.02 2.54 7.98
Global Blood Therapeutics 6.02 2.99 5.98
Biodelivery Sciences International Loan 5.83 5.80 5.80
Akebia 3.65 2.90 3.63
Amicus Senior Secured Loan - 10.88 -
Sebela Senior Secured Loan - 8.85 6.70
Cash and cash equivalents* 8.93 4.43 14.02
* Cash and cash equivalents represents the Company only and does
not include cash held by BPCR LP.
(B) Liquidity risk
This is the risk that the Company will encounter difficulty in
meeting obligations associated with financial liabilities. At 30
June 2021, the Company had cash and cash equivalents, including
investments in liquidity/money market funds with balances of
$122,478,000 (30 June 2020: $61,058,000 and 31 December 2020:
$193,269,000) and maximum unfunded commitments of $nil (30 June
2020: $248,250,000 and 31 December 2020: $nil).
The Company maintains sufficient liquid investments through its
cash and cash equivalents to pay accounts payable, accrued expenses
and ongoing expenses of the Company. Liquidity risk is manageable
through a number of options, including the Company's ability to
issue debt and/or equity and by selling all or a portion of an
investment in the secondary market. On 22 May 2020, the Company
entered into a $200 million revolving credit facility with JPMorgan
Chase Bank. This facility will increase the Company's flexibility
in relation to funding new lending opportunities and provide
liquidity for funding outstanding obligations. As of 30 June 2021,
the outstanding balance on the credit facility was $nil.
(C) Credit risk
This is the risk the Company's trade and other receivables will
not meet their obligations to the Company. While the Company will
often seek to be a secured lender for each debt asset, there is no
guarantee that the relevant borrower will repay the loan or that
the collateral will be sufficient to satisfy the amount owed. All
of the Company's investments are senior secured investments as
detailed in the Investment Manager's Report.
The Investment Manager performs a robust credit risk analysis
during the investment process for all new investments and
constantly monitors the collateral on its outstanding senior
secured loans as to minimise the credit risk to the Company of
default. The credit risk of the senior secured loans will increase
significantly after initial recognition when borrowers are not
making principal and interest payments as agreed. The fair value of
the senior secured loan will be adjusted, either partially or in
full, when there is no realistic prospect of recovery and the
amount of the change in fair value has been determined by the
Investment Manager. Subsequent recoveries of amounts previously
adjusted will decrease the amount of the fair value loss recorded.
Changes to a counterparty's risk profile are monitored by the
Investment Manager on a regular basis and discussed with the Board
at quarterly meetings.
The Company's maximum exposure to credit risk at any given time
is the fair value of its investment portfolio. At 30 June 2021, the
Company's maximum exposure to credit risk was $1,226,058,000 (30
June 2020: $1,346,084,000 and 31 December 2020: $1,194,831,000).
The Company's concentration of credit risk by counterparty can be
found in the Investment Manager's Report.
Capital management
The Company's primary objectives in relation to the management
of capital are:
-- to ensure its ability to continue as a going concern;
-- to ensure that the Company conducts its affairs to enable it
to continue to meet the criteria to qualify as an investment trust;
and
-- to maximise the long-term shareholder returns in the form of
sustainable income distributions through an appropriate balance of
equity capital and debt.
The Company is subject to externally imposed capital
requirements:
-- as a public company, the Company has a minimum share capital
of GBP50,000.
The Company has complied with all the above requirements during
this financial period.
17. RELATED PARTY TRANSACTIONS
The amount incurred in respect of management fees during the
period to 30 June 2021 was $6,866,000 (30 June 2020: $6,872,000),
of which $3,427,000 (30 June 2020: $3,427,000) was outstanding at
30 June 2021. The amount due to the Investment Manager for
performance fees at 30 June 2021 was $nil (31 December 2020:
$5,473,000).
The amount incurred in respect of Directors' fees during the
period to 30 June 2021 was $198,000 (30 June 2020: $198,000) of
which $nil was outstanding at 30 June 2021 (30 June 2020:
$nil).
The Shared Services Agreement was entered into by and between RP
Management, LLC, an affiliate of Pharmakon Advisors, L.P., and the
Investment Manager on 30 November 2016 and deemed effective as of 1
January 2016. Under the terms of the Shared Services Agreement, the
Investment Manager will have access to the expertise of certain
Royalty Pharma employees, including its research, legal and
compliance, and finance teams.
BPCR Limited Partnership and its General Partner, BPCR GP
Limited, are related entities of the Company, as they are
wholly-owned subsidiaries and formed for the purpose of entering
into a new credit facility. On 22 May 2020, several investments
totaling $1,070,139,000 were transferred to BPCR LP from the
Company. In the period to 30 June 2021, the Company recorded income
of $49,281,000 (30 June 2020: $12,009,000) and the outstanding
balance on 30 June 2021 was $1,216,328,000 (30 June 2020:
$1,082,148,000). BPCR GP Limited had an outstanding balance as at
30 June 2021 of $nil (30 June 2020: $nil).
On 24 March 2021, the Company and BioPharma Credit Investments V
(Master) LP ("BioPharma V"), a fund managed by the Investment
Manager, entered into a definitive senior secured term loan
agreement for $300,000,000 with LumiraDx Group Limited
("LumiraDx"). The Company's share of the transaction was
$150,000,000 and the Company funded the term loan on 29 March 2021.
The loan will mature in March 2024 and will bear interest at 8.00
per cent. per annum along with a one-time additional consideration
of 2.50 per cent. of the loan amount payable upon funding plus an
additional 1.50 per cent. of the loan payable at maturity. In the
first half of 2021, the BPCR LP recorded interest of $3,133,000 (30
June 2020: $nil). The outstanding balance as at 30 June 2021 was
$150,000,000 (30 June 2020: $nil).
On 7 February 2020, the Company and BioPharma V entered into a
definitive senior secured term loan agreement for $200,000,000 with
Collegium Pharmaceutical, Inc. (Nasdaq: COLL). The Company's share
of the transaction was $165,000,000 and the Company funded the term
loan on 13 February 2020. The loan will mature in January 2024 and
will bear interest at 3-month LIBOR plus 7.50 per cent. per annum
subject to a 2.00 per cent. floor along with a one-time additional
consideration of 2.50 per cent. of the loan amount which was paid
at funding. In the first half of 2021, BPCR LP recorded interest of
$6,156,000 (30 June 2020: $2,864,000). The outstanding balance as
at 30 June 2021 was $113,437,500 (30 June 2020: $154,687,000).
On 18 December 2019, the Company and BioPharma V entered into a
definitive senior secured term loan agreement with Global Blood
Therapeutics (Nasdaq: GBT). GBT drew down $75,000,000 at closing on
20 December 2019 and $75,000,000 of the second tranche on 20
November 2020. The Company funded $41,250,000 of each tranche for a
total investment of $82,500,000 and BioPharma V invested the
remaining $67,500,000. The loan will mature in December 2025 and
will bear interest at three-month LIBOR plus 7.00 per cent. per
annum subject to a 2.00 per cent. floor along with a one-time
additional consideration of 1.50 per cent. of the total loan amount
payable upon funding and an additional 2.00 per cent. payable upon
the repayment of the loan. In the first half of 2021, BPCR LP
recorded interest of $3,733,000 (30 June 2020: $4,354,000). The
outstanding balance as at 30 June 2021 was $82,500,000 (30 June
2020: $41,250,000).
On 13 December 2019, the Company and BioPharma V entered into a
definitive senior secured term loan agreement for up to
$500,000,000 with Sarepta Therapeutics (Nasdaq: SRPT). On 24
September 2020 the Sarepta loan agreement was amended and the loan
amount was increased to $550,000,000. Sarepta drew down the first
$250,000,000 tranche on 20 December 2019 and the second
$300,000,000 tranche on 2 November 2020. The Company funded
$175,000,000 of each tranche for a total investment of $350,000,000
and BioPharma V invested the remaining $200,000,000. The first
tranche will mature in December 2023 and the second tranche in
December 2024. The loan will bear interest at 8.50 per cent. per
annum along with a one-time additional consideration of 1.75 per
cent. of the first tranche and 2.95 per cent. of the second tranche
payable upon funding and an additional 2.00 per cent. payable upon
the repayment of the loan. In the first half of 2021, BPCR LP
recorded interest of $14,958,000 (30 June 2020: $5,909,000). The
outstanding balance as at 31 December 2020 was $350,000,000 (30
June 2019: $175,000,000).
On 11 November 2019, the Company and BioPharma V entered into a
definitive senior secured term loan agreement for up to
$100,000,000 with Akebia (Nasdaq: AKBA). Akebia drew down the first
$80,000,000 on 25 November 2019 and the second $20,000,000 tranche
on 10 December 2020. The Company invested $40,000,000 and
$10,000,000 of the first and second tranche, respectively. The loan
will mature in November 2024 and will bear interest at LIBOR plus
7.50 per cent. per annum along with a one-time additional
consideration of 2.00 per cent. of the total loan amount. In the
first half of 2021, BPCR LP recorded interest of $2,388,000 (30
June 2020: $1,509,000). The outstanding balance as at 30 June 2021
was $50,000,000 (30 June 2020: $40,000,000).
On 4 November 2019, the Company and BioPharma V entered into a
definitive senior secured term loan agreement for up to $70,000,000
with Epizyme (Nasdaq: EPZM). On 3 November 2020, the Epizyme loan
agreement was amended and the loan amount was increased to
$220,000,000. Epizyme drew down the $25,000,000 on 18 November 2019
and an additional $195,000,000 during 2020. The Company funded a
total of $110,000,000 of the Epizyme loan. The first three tranches
of the loan will mature in November 2024 and the fourth tranche
will mature in November 2026. The loan will bear interest at LIBOR
plus 7.75 per cent. per annum along with a one-time additional
consideration of 2.00 per cent. of the total loan amount. On 4
November 2019, Royalty Pharma, an affiliate of Pharmakon Advisors,
announced an agreement to purchase future royalties on tazemetostat
net sales outside of Japan owned by Eisai Co. for $330,000,000 and
a separate $100,000,000 equity investment directly in Epizyme.
Pablo Legorreta, a principal of Pharmakon and RP management was
named to the Epizyme board of directors. In the first half of 2021,
BPCR LP recorded interest of $5,392,000 (30 June 2020: $677,000).
The outstanding balance as at 30 June 2021 was $110,000,000 (30
June 2020: $35,000,000).
On 12 September 2019, the Company and BioPharma V, entered into
a definitive senior secured note purchase agreement for the
issuance and sale of senior secured notes in an aggregate original
principal amount of up to $150,000,000 by OptiNose US. OptiNose US
is a wholly-owned subsidiary of OptiNose (Nasdaq: OPTN), a
commercial-stage specialty pharmaceutical company. Optinose drew a
total of $130,000,000 in three tranches: $80,000,000 on 12
September 2019, $30,000,000 on 13 February 2020 and $20,000,000 on
1 December 2020. There are no further funding commitments. The
notes mature in September 2024 and bear interest at 10.75% per
annum along with a one-time additional consideration of 0.75% of
the aggregate original principal amount of senior secured notes
which the Company and BioPharma-V are committed to purchase under
the facility and 810,357 warrants exercisable into common stock of
OptiNose. The Company funded a total $71,500,000 across all
tranches and was allocated 445,696 warrants. In the first half of
2021, BPCR LP recorded interest of $3,865,000 (30 June 2020:
$2,372,000). The outstanding balance as at 30 June 2021 of the
outstanding notes was $71,500,000 (30 June 2020: $60,500,000).
On 7 February 2018, the Company entered into a senior secured
term loan agreement for $150,000,000 with Novocure Limited (NASDAQ:
NVCR) ("Novocure"). The $150,000,000 loan was originally scheduled
to mature in February 2023 and bore interest at 9.0 per cent. per
annum. Novocure used $100,000,000 of the net proceeds to entirely
prepay the $100,000,000, 10.0 per cent. coupon loan made by
BioPharma III Holdings, LP ("BioPharma III") in 2015 that was
scheduled to mature in 2020. The Company was a limited partner in
BioPharma III and therefore received a distribution of
approximately $46,000,000 from BioPharma III as a result of the
prepayment from Novocure. In the period to 30 June 2021, BPCR LP
recorded interest of $nil (30 June 2020: $5,363,000). On 18 August
2020, NovoCure Limited repaid their loan and the Company received a
payment of $154,838,000 million comprised of $150,000,000 million
in principal, $1,838,000 million in accrued interest, and
$3,000,000 million in prepayment fees. The outstanding balance as
at 30 June 2021 was $nil (30 June 2020: $150,000,000).
On 8 December 2017, the Company's wholly-owned subsidiary BPCR
Ongdapa entered into a purchase, sale and assignment agreement with
RPI Acquisitions (Ireland) Limited ("RPI Acquisitions"), an
affiliate of Royalty Pharma, for the purchase of a 50 per cent.
interest in a stream of Purchased Payments acquired by RPI
Acquisitions from Bristol-Myers Squibb through a purchase agreement
dated 14 November 2017. As a result of the arrangements, RPI's
subsidiary and the Company's subsidiary are each entitled to the
benefit of 50 per cent. of the Purchased Payments under identical
economic terms. The Purchased Payments are linked to tiered
worldwide sales of Onglyza and Farxiga, diabetes agents marketed by
AstraZeneca, and related products. The Company was expected to fund
$140,000,000 to $165,000,000 between 2018 and 2020, determined by
product sales and will receive payments from 2020 through 2025
estimated to yield a return in the high single-digits per annum.
The Company advanced $nil to RPI Acquisitions in the first half of
2021 (30 June 2020: $12,136,000) for the Purchased Payments. In the
first half of 2021, BPCR LP recorded interest of $7,060,000 (30
June 2020: $3,440,000).
On 4 December 2017, the Company and BioPharma Credit Investments
IV, S.àr.L. ("BioPharma IV"), a fund managed by the Investment
Manager, entered into a definitive term loan agreement for up to
$200,000,000 with Lexicon Pharmaceuticals (NASDAQ: LXRX), a fully
integrated biopharmaceutical company ("Lexicon"). The loan was
secured by substantially all of Lexicon's assets, including its
rights to Xermelo(R) and sotagliflozin. The $200,000,000 loan was
available in two tranches, each maturing in December 2022 and
bearing interest at 9.0 per cent. per annum. The first $150,000,000
was available immediately and an additional tranche of $50,000,000
was not drawn down. The Company funded $124,500,000 of the first
tranche on 18 December 2017 and Lexicon has not drawn the second
tranche. In the first half of 2021, BPCR LP recorded interest of
$nil (30 June 2020: $5,665,000). On 8 September 2020, Lexicon
repaid the $150,000,000 loan. The Company received a payment of
$132,300,000 million on its $124,500,000 share of the loan,
including the make-whole and prepayment premium totalling
$5,600,000. The
outstanding balance as at 30 June 2021 was $nil (30 June 2020:
$124,500,000).
BioPharma III, BioPharma IV, and RPI Acquisitions are related
entities of the Company due to a principal of the Investment
Manager having significant influence over each of these
entities.
18. CONTINGENCIES, GUARANTEES AND FINANCIAL COMMITMENTS
At 30 June 2021, there were no outstanding commitments (30 June
2020: $248,250,000 and 31 December 2020: $nil) in respect of
investments (see Note 17 for further details).
19. SUBSEQUENT EVENTS
On 10 September 2021, the company distributed a circular to
shareholders for its proposed admission to trade on the premium
segment of the main market of the London Stock Exchange.
On 10 September 2021, the Company was able to negotiate and
amend the original $200 million revolving credit facility with
JPMorgan Chase Bank through its wholly owned subsidiary, BPCR
Limited Partnership, on more favorable terms. The key terms to the
amendment include a reduction in the committed Revolving Credit
Facility ("RCF") from $200 million to $50 million together with
changes in the accordion feature allowing for an increase in the
RCF to $100 million and up to $200 million in term loans, extension
of the maturity date to 22 June 2024 and a reduction in the margin
payable under the RCF from 4.00 per cent. to 2.75 per cent.
GLOSSARY OF TERMS AND ALTERNATIVE PERFORMANCE MEASURES (APM)
NET INCOME PER ORDINARY SHARE
Net income per share is the net revenue for the year divided by
the number of ordinary shares outstanding.
NAV PER ORDINARY SHARE
Net Asset Value (NAV) is the value of total assets less
liabilities. The NAV per share is calculated by dividing this
amount by the number of ordinary shares outstanding.
PREMIUM (DISCOUNT) TO NAV PER ORDINARY SHARE
As stock markets and share prices vary, an investment trust's
share price is rarely the same as its NAV. When the share price is
lower than the NAV per share it is said to be trading at a
discount. The size of the discount is calculated by subtracting the
share price from the NAV per share and it is usually expressed as a
percentage of the NAV per share. If the share price is higher than
the NAV per share, it is said to be trading at a premium.
RETURN PER ORDINARY SHARE
Revenue return per Ordinary share is based on the net revenue
after taxation divided by the weighted average number of Ordinary
Shares for the year. Capital return per Ordinary Share is based on
net capital gains divided by weighted average number of Ordinary
Shares for the year.
ONGOING CHARGES
Ongoing charges are the Company's expenses expressed (excluding
and including performance fee) as a percentage of its average
monthly net assets and follows the AIC recommended methodology.
Ongoing charges are different to total expenses as not all expenses
are considered to be operational and recurring.
DIRECTORS, ADVISERS AND OTHER SERVICE PROVIDERS
DIRECTORS
Harry Hyman (Chairman)
Duncan Budge (Senior Independent Director)
Colin Bond
Stephanie Léouzon
Rolf Soderstrom
INVESTMENT MANAGER AND AIFM
Pharmakon Advisors L.P.
110 East 59th Street #3300
New York, NY 10022
USA
ADMINISTRATOR
Link Alternative Fund Administrators Limited
Beaufort House
51 New North Road
Exeter
EX4 4EP
COMPANY SECRETARY AND REGISTERED OFFICE
Link Company Matters Limited
Beaufort House
51 New North Road
Exeter
EX4 4EP
Tel: 01392 477500
COMPANY WEBSITE
www.bpcruk.com
CUSTODIAN
Bank of New York Mellon
One Canada Square
London
E14 5AL
FINANCIAL AND STRATEGIC COMMUNICATIONS
Buchanan Communications Limited
107 Cheapside
London
EC2V 6DN
INDEPENDENT AUDITOR
Pricewaterhouse Coopers LLP
7 More London Riverside
London
SE1 2RT
JOINT BROKERS
J.P. Morgan Cazenove
25 Bank Street
London
E14 5JP
Goldman Sachs International
Peterborough Court
133 Fleet Street
London
EC4A 2BB
LEGAL ADVISER
Herbert Smith Freehills LLP
Exchange House
Primrose Street
London
EC2A 2EG
REGISTRAR
Link Group
10(th) Floor
Central Square
29 Wellington Street
Leeds
LS1 4DL
TISE SPONSOR
Carey Commercial Limited
1st and 2nd Floors
Elizabeth House
Les Ruettes Brayes
St Peter Port
Guernsey
GY1 1EW
COMPANY INFORMATION
The Company is a closed-ended investment company incorporated on
24 October 2016. The Ordinary Shares were admitted to trading on
the Specialist Fund Segment of the Main Market of the LSE and TISE
on 27 March 2017.
The Company intends to carry on business as an investment trust
within the meaning of Chapter 4 of Part 24 of the Corporation Tax
Act 2010 and an investment company within the meaning of Section
833 of the Companies Act 2006.
INVESTMENT OBJECTIVE
The Company aims to generate long-term shareholder returns,
predominantly in the form of sustainable income distributions from
exposure to the life sciences industry.
SUMMARY OF INVESTMENT POLICY
The Company will seek to achieve its investment objective
primarily through investments in debt assets secured by royalties
or other cash flows derived from sales of approved life sciences
products. Subject to certain restrictions and limitations, the
Company may also invest in unsecured debt and equity issued by
companies in the life sciences industry.
The Investment Manager will select investment opportunities
based upon in-depth, rigorous analysis of the life sciences
products backing an investment as well as the legal structure of
the investment. A key component of this process is to examine
future sales potential of the relevant product which is affected by
several factors, including but not limited to; clinical utility,
competition, patent estate, pricing, reimbursement (insurance
coverage), marketer strength, track record of safety, physician
adoption and sales history.
The Company will seek to build a diversified portfolio by
investing across a range of different forms of assets issued by a
variety of borrowers. In particular, no more than 30 per cent. of
the Company's gross assets will be exposed to any single
borrower.
SHAREHOLDER INFORMATION
KEY DATES
March Annual results announced
Payment of fourth interim dividend
June Annual General Meeting
Company's half-year end
Payment of first interim dividend
September Half-yearly results announced
Payment of second interim dividend
December Company's year end
Payment of third interim dividend
FREQUENCY OF NAV PUBLICATION
The Company's NAV is released to the LSE and TISE on a monthly
basis and is published on the Company's website.
ANNUAL AND HALF-YEARLY REPORT
Copies of the Company's Annual and Half-yearly Reports, stock
exchange announcements and further information on the Company can
be obtained from the Company's website www.bpcruk.com.
IDENTIFICATION CODES
SEDOL: BDGKMY2
ISIN: GB00BDGKMY29
TICKER: BPCR
LEI: 213800AV55PYXAS7SY24
CONTACTING THE COMPANY
Shareholder queries are welcomed by the Company. While any
queries regarding your shareholding should be directed to the
Registrar, shareholders who wish to raise any other matters with
the Company may do so using the following contact details:
Company Secretary - biopharmacreditplc@linkgroup.co.uk
Chairman - chairman@bpcruk.com
Senior Independent Director - sid@bpcruk.com
Neither the contents of the Company's website nor the contents
of any website accessible from hyperlinks on the Company's website
(or any other website) is incorporated into, or forms part of this
announcement.
This information is provided by RNS, the news service of the
London Stock Exchange. RNS is approved by the Financial Conduct
Authority to act as a Primary Information Provider in the United
Kingdom. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
IR GPURGBUPGGMC
(END) Dow Jones Newswires
September 15, 2021 02:00 ET (06:00 GMT)
Biopharma Credit (LSE:BPCR)
Historical Stock Chart
From Mar 2024 to Apr 2024
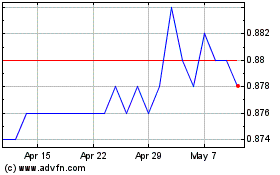
Biopharma Credit (LSE:BPCR)
Historical Stock Chart
From Apr 2023 to Apr 2024