TIDMHIK
RNS Number : 7772H
Hikma Pharmaceuticals Plc
06 August 2021
London, 6 August 2021 - Hikma Pharmaceuticals PLC ('Hikma' or
'Group'), the multinational pharmaceutical company, today reports
its interim results for the six months ended 30 June 2021.
Siggi Olafsson, Chief Executive Officer of Hikma, said:
"Once again, we have benefited from the resilience of our
portfolio and our flexible manufacturing footprint. Our strong
performance included solid year-over-year increases in revenue and
operating profit, underscoring our ability to generate positive
results in challenging market conditions. We are continuing to
benefit from investments we have made to build our pipeline of new
medicines and our progress in the first half underpins our improved
outlook for the full year. Looking ahead, our clear strategy,
strong pipeline and agility give us the confidence to drive
continued growth and deliver increased value to all our
stakeholders."
Highlights:
Reported results (statutory) Constant currency(1)
$ million H1 2021 H1 2020 Change change
---------- -------- -------
Revenue 1,216 1,132 7% 7%
Operating profit 326 297 10% 15%
Profit attributable to shareholders 248 212 17% 26%
Cashflow from operating activities 224 292 (23)% -
Basic earnings per share (cents)(2) 107.4 87.6 23% 32%
Interim dividend per share (cents)(2) 18.0 16.0 13% -
--------------------------------------- ---------- -------- ------- ---------------------
Core results(3) (underlying) Constant currency(1)
$ million H1 2021 H1 2020 Change change
------------------------------- ---------- -------- ------- ---------------------
Core revenue 1,216 1,132 7% 7%
Core operating profit 309 284 9% 15%
Core profit attributable to
shareholders 223 205 9% 18%
Core basic earnings per share
(cents)(2) 96.5 85.3 13% 23%
------------------------------- ---------- -------- ------- ---------------------
Strong first half performance
-- Core Group revenue grew 7% driven by a strong performance in
Generics and Branded and the resilience of our Injectables
business
-- Core operating profit grew 9% to $309 million and core
operating margin reached 25%
-- Generated good cashflow from operating activities of $224
million. The reduction compared to the first half of 2020 reflects
the timing of tax payments
-- Maintained low leverage with net debt(4) to core EBITDA(5) of
0.9x at 30 June 2021 (31 December 2020 0.9x)
Continued revenue growth in all three businesses
-- Generics delivered strong revenue growth and significant
margin improvement reflecting good demand for new and recently
launched products, a more favourable product mix and lower
operating expenses
-- Global Injectables revenue grew modestly, following the
exceptionally strong performance in H1 2020, reflecting the
resilience of our product portfolio. Injectables operating profit
declined in line with expectations, primarily due to a shift in
product mix
-- Branded achieved double digit revenue growth and improved
margins, driven by a strong performance across Tier 1 markets
Further progress increasing availability of important
medicines
-- Resumed launch of generic Advair Diskus(R) in April
-- Received approval for and launched 8mg Naloxone nasal spray,
demonstrating ability to bring complex generic products to
customers and patients
-- Launched 9 injectable products in the US, with further
launches expected in the second half
-- Launched our first locally manufactured oral oncology product
in Algeria
Improved FY outlook driven by upgraded guidance for Generics
-- Injectables - we continue to expect revenue growth in the
mid-single digits, with core operating margin in the range of 37%
to 38%
-- Generics - we now expect revenue to be in the range of $810
million to $830 million and core operating margin to be in the
range of 22% to 24%
-- Branded - we continue to expect revenue to grow in the
mid-single digits in constant currency
Further information:
A recording of the presentation will be available on the
Company's website at www.hikma.com from 8:00am BST. Hikma will also
host a webinar with a live Q&A for analysts and investors at
9:30am BST, and a recording will be available on the Company's
website later that day.
Please register your attendance in advance by clicking here:
https://hikma.zoom.us/webinar/register/WN_wugtG0VQTwm8c5b5FM8e5A .
For further information please contact Tiina Lugmayer -
tlugmayer@hikma.com .
Hikma (Investors):
Susan Ringdal
EVP, Strategic Planning and Global +44 (0)20 7399 2760/ +44 (0)7776
Affairs 477050
Guy Featherstone +44 (0)20 3892 4389/ +44 (0)7795
Senior Investor Relations Manager 896738
Layan Kalisse +44 (0)20 7399 2788/ +44 (0)7970
Investor Relations Analyst 709912
Teneo (Press):
Charles Armitstead / Claire Scicluna +44 (0)7703 330 269/ +44 (0)7385 395 028
About Hikma:
Hikma helps put better health within reach every day for
millions of people around the world. For more than 40 years, we've
been creating high-quality medicines and making them accessible to
the people who need them. Headquartered in the UK, we are a global
company with a local presence across the United States (US), the
Middle East and North Africa (MENA) and Europe, and we use our
unique insight and expertise to transform cutting-edge science into
innovative solutions that transform people's lives. We're committed
to our customers, and the people they care for, and by thinking
creatively and acting practically, we provide them with a broad
range of branded and non-branded generic medicines. Together, our
8,600 colleagues are helping to shape a healthier world that
enriches all our communities. We are a leading licensing partner,
and through our venture capital arm, are helping bring innovative
health technologies to people around the world. For more
information, please visit: www.hikma.com
Hikma Pharmaceuticals PLC (LSE: HIK) (NASDAQ Dubai: HIK) (OTC:
HKMPY) (LEI:549300BNS685UXH4JI75) (rated BBB-/stable S&P and
Ba1/stable Moody's)
STRATEGIC REVIEW
During the first half of 2021, we made excellent progress
ensuring our customers have the products they need, delivering on
our purpose of putting better health within reach, every day. We
achieved this through our strategy of delivering more from our
foundation, building a portfolio that anticipates future health
needs, and inspiring and enabling our people.
Our broad and growing portfolio is supporting a strong
performance
The breadth of Hikma's portfolio and our flexible manufacturing
facilities have enabled us to respond quickly to evolving demand
since the beginning of the pandemic.
In Injectables, our portfolio in the US of 119 products and an
incremental contribution from new launches has enabled us to
partially offset lower demand for products used in the treatment of
COVID-19 when compared with the first half of 2020. We have also
benefited from our presence in Europe and MENA, where we have seen
good demand for our growing portfolio and manufacturing
capabilities.
In Generics we have been adding to, and diversifying our
portfolio, and these newer products have been a key contributor to
our profitable growth. We are working closely with our customers
and leveraging our improved service levels to help drive demand and
offset the effects of a more competitive environment on certain
products in our base portfolio.
In our Branded business we have seen a strong revenue and profit
performance in each of our Tier 1 markets, Algeria, Saudi Arabia
and Egypt. Our presence across the rest of our 18 MENA markets and
our broad portfolio made up of both own-brand generics and
in-licenced innovative products is further providing a foundation
to our growth.
In Algeria, we are benefitting from our strengthened commercial
capabilities and broad product portfolio as well as our established
local presence, which enables us to take market opportunities when
they arise. In Saudi Arabia, we saw good demand across a range of
products and benefitted from the flexibility of our commercial and
manufacturing operations. In Egypt, we have continued to see demand
for some COVID-19 related products, as well as a good performance
from the broader portfolio. We have also seen a strong performance
in Sudan where volumes are up significantly, compared to the first
half of 2020, when this market faced severe disruptions.
Delivering new products to patients and anticipating future
needs
Maintaining a high potential and differentiated portfolio of new
products is essential for delivering sustainable growth. We
continue to expand our portfolio with successful new launches
across our three businesses and this has been a driver of our
strong financial performance in the first half, particularly in
Generics. Through our focus on R&D and business development, we
also continued to build our product pipeline.
The Generics business launched 4 products in the first half.
This included the launch of generic Advair Diskus(R) in April and
we are pleased with how our sales are progressing. We also
announced the approval and launch of our KLOXXADO(TM) (naloxone
hydrochloride) nasal spray 8mg, an important new medicine for the
treatment of opioid overdose.
Our Injectables business launched a total of 44 products
globally, including 9 in the US, building on our track record of
consistently delivering launches to deliver incremental growth.
In our Branded business we launched 39 products across our
markets and are seeing a return to normal conditions following
disruptions to our product launches in 2020 due to the pandemic.
Our sales force has returned to in person operations, whilst
maintaining some virtual interaction.
We also continue to build our extensive network of global
partners to increase patients' access to differentiated medicines.
We expanded our licencing agreement with Melinta in the MENA
region, adding two novel anti-infective injectable products. We
also announced the licencing of Combogesic(R) IV in the US from AFT
Pharmaceuticals, expanding our pipeline of non-opioid pain
management treatments. Through these efforts, we are ensuring that
we build a portfolio of products that meets the evolving needs of
healthcare professionals and patients.
Making a positive impact
Our strong performance in the first half would not have been
possible without the dedication of our employees. We are focussed
on the wellbeing of our employees and have worked to ensure all our
colleagues are informed and connected, particularly during the
pandemic. We continue to focus on diversity, equity and inclusion
in our workplace, with a sub-division of our executive committee
dedicated to this area and employee-led affinity groups. We are
working to ensure we are an inclusive and inspiring place to
work.
As well as focusing on our people, we remained committed to the
communities in which we operate. From a social perspective, Hikma's
purpose of putting better health within reach, every day, covers
not only our core business of supplying medicines, but also our
support of local communities. This has continued in the first half
of 2021 including, but not limited to: working with the UNHCR to
provide 40 four-year tertiary education scholarships for refugees
in Jordan, Egypt and Algeria, executing on our new US medicine
donation program to consistently and frequently donate medicines to
vulnerable populations in need, continuing our support of US food
banks after donating 600,000 meals in 2020 and recommitting to our
partnership with Save the Children.
We recognise that our ambition of helping to shape a healthier
world goes beyond our social impact. We are committed to our
environmental responsibility and making our operations more energy
efficient. We continue to work to fully understand our
environmental impact, and also to develop targets to ensure we are
on the path to reduce emissions in line with the goals of the Paris
Agreement on limiting the effects of climate change. We look
forward to updating all our stakeholders on this work in due
course.
Outlook
Our strategy continues to deliver results and we are pleased
with the strategic progress and financial performance we achieved
in the first half. We are confident that our broad and diversified
portfolio and our extensive and flexible manufacturing capabilities
will enable us to drive further growth in the second half, while
also continuing to manage the evolving challenges of the COVID-19
pandemic.
We now expect Generics revenue to be in the range of $810
million to $830 million for the full year, up from $770 million to
$810 million, and core operating margin to be in the range of 22%
to 24%, up from around 20%. This improved outlook reflects a strong
performance from recently launched products.
Our full year expectations for our Injectables and Branded
business, remain the same as previously guided.
We expect Injectables revenue to grow in the mid-single digits
and core operating margin to be in the range of 37% to 38%.
We expect Branded revenue to grow in the mid-single digits in
constant currency.
We expect Group net finance expense to be around $50 million in
2021 and the core effective tax rate to be around 22% to 23%. We
expect Group capital expenditure to be around $140 million to $160
million.
BUSINESS AND FINANCIAL REVIEW
The business and financial review set out below summarises the
performance of the Group and our three main business segments,
Injectables, Generics and Branded, for the six months ended 30 June
2021.
Group
$ million Constant
currency
H1 2021 H1 2020 Change change
Revenue 1,216 1,132 7% 7%
--------- -------- -------- ----------
Core revenue 1,216 1,132 7% 7%
--------- -------- -------- ----------
Gross profit(6) 616 577 7% 7%
--------- -------- -------- ----------
Core gross profit 616 577 7% 7%
--------- -------- -------- ----------
Core gross margin 50.7% 51.0% (0.3)pp (0.2)pp
--------- -------- -------- ----------
Operating profit 326 297 10% 15%
--------- -------- -------- ----------
Core operating profit 309 284 9% 15%
--------- -------- -------- ----------
Core operating margin 25.4% 25.1% 0.3pp 1.8pp
--------- -------- -------- ----------
EBITDA 358 328 9% 14%
--------- -------- -------- ----------
Core EBITDA 358 328 9% 14%
--------- -------- -------- ----------
Group revenue grew by 7% reflecting a good performance from all
three businesses, particularly in Generics and Branded. Gross
margin reduced slightly, with a reduction in Injectables offsetting
an improving margin in Branded and Generics.
Group operating expenses were $290 million (H1 2020: $280
million). Excluding adjustments related to the amortisation of
intangible assets (other than software) of $29 million (H1 2020:
$21 million) and net income from exceptional items of $46 million
(H1 2020: $34 million), Group core operating expenses were $307
million (H1 2020: $293 million).
Selling, general and administrative (SG&A) expenses were
$261 million (H1 2020: $251 million). Excluding the amortisation of
intangible assets (other than software), core SG&A expenses
were $232 million (H1 2020: $229 million), up slightly reflecting
good control of costs.
Core and reported research and development (R&D) expenses
were $59 million (H1 2020: $62 million). The slight decrease
reflects the timing of spend, with higher investment in R&D
expected in the second half of the year.
Other net operating income was $30 million (H1 2020: $31
million). Excluding other adjustments,(7) core other net operating
expenses were $16 million (H1 2020: $(4) million), which primarily
comprised foreign exchange-related costs related to the devaluation
of the Sudanese pound.
The improvements in core operating profit by 9% and core
operating margin to 25.4% were primarily driven by the strong
performances of Generics and Branded.
Group core revenue by business segment
$ million H1 2021 H1 2020
Injectables 492 41% 485 43%
------ ---- ------ ----
Generics 400 33% 369 33%
------ ---- ------ ----
Branded 319 26% 275 24%
------ ---- ------ ----
Others 5 0% 3 0%
------ ---- ------ ----
Total 1,216 1,132
------ ---- ------ ----
Group core revenue by region
$ million H1 2021 H1 2020
US 718 59% 716 63%
------ ---- ------ ----
MENA 396 33% 351 31%
------ ---- ------ ----
Europe and ROW 102 8% 65 6%
------ ---- ------ ----
Total 1,216 1,132
------ ---- ------ ----
Injectables
$ million Constant
currency
H1 2021 H1 2020 Change change
Revenue 492 485 1% 0.2%
-------- -------- -------- ----------
Core revenue 492 485 1% 0.2%
-------- -------- -------- ----------
Gross profit 273 287 (5)% (5)%
-------- -------- -------- ----------
Core gross profit 273 287 (5)% (5)%
-------- -------- -------- ----------
Core gross margin 55.5% 59.2% (3.7)pp (3.0)pp
-------- -------- -------- ----------
Operating profit 175 192 (9)% (5)%
-------- -------- -------- ----------
Core operating profit 187 204 (8)% (5)%
-------- -------- -------- ----------
Core operating margin 38.0% 42.1% (4.1)pp (2.2)pp
-------- -------- -------- ----------
Following a very strong performance in the first half of 2020
across all of our Injectables markets, Injectables revenue
increased slightly in the first half of 2021, reflecting our
resilient and broad global Injectables portfolio and the breadth,
quality and flexibility of our manufacturing facilities.
US Injectables revenue was down 8% to $318 million (H1 2020:
$347 million), reflecting reduced demand for COVID-19 related
products, increased competition on certain other products and the
slow return of elective surgeries, which were only partially offset
by the contribution from recent product launches. We continue to
expect to deliver growth in US Injectables for the full year.
The decline in the US was more than offset by an increase in
European Injectables revenue, up 54% to $97 million (H1 2020: $63
million). In constant currency, European Injectables revenue
increased by 41%, reflecting an exceptionally strong contribution
from contract manufacturing and good demand across our portfolio of
own products, including new launches.
In MENA, Injectables revenue was $77 million, up 3% (H1 2020:
$75 million), or up 1% in constant currency. We were pleased to see
demand remain steady across our MENA Injectables portfolio,
especially given the exceptionally strong performance of this
business in the first half of 2020.
Injectables core gross profit and margin declined in the first
half reflecting the shift in product mix in the US. Injectables
operating expenses were broadly flat, reflecting good control of
costs, with slightly lower investment in R&D offset by the
adverse impact of foreign exchange movements, primarily related to
the Sudanese pound. The decline in Injectables core operating
profit, which excludes the amortisation of intangible assets (other
than software) and exceptional items(8) , was therefore driven by
the reduced gross margin.
During H1 2021, the Injectables business launched 9 products in
the US, 15 in MENA and 20 in Europe. We submitted 56 filings to
regulatory authorities across all markets. We further developed our
portfolio through new licensing agreements.
We continue to expect our Injectables business to deliver
revenue growth in the mid-single digits and core operating margin
to be in the range of 37% to 38%.
Generics
$ million H1 2021 H1 2020 Change
Revenue 400 369 8%
-------- -------- -------
Core revenue 400 369 8%
-------- -------- -------
Gross profit 188 161 17%
-------- -------- -------
Core gross profit 188 161 17%
-------- -------- -------
Gross margin 47.0% 43.6% 3.4pp
-------- -------- -------
Operating profit 134 102 31%
-------- -------- -------
Core operating profit 100 72 39%
-------- -------- -------
Core operating margin 25.0% 19.5% 5.5pp
-------- -------- -------
The strong growth in Generics revenue, up 8%, was driven by new
launches, as well as continued good contributions from products
launched in 2020. This more than offset the effects of competition
on certain products.
Generics gross profit growth and gross margin expansion to 25.0%
was primarily a result of a change in product mix driven by a good
performance from our recent launches.
Generics core operating profit, which excludes the amortisation
of intangible assets (other than software) and impairment reversal
adjustments,(9) increased primarily due to the improved gross
margin, as well as the timing of R&D spend, which we expect
will increase in the second half of the year.
During H1 2021, we launched 4 products from our R&D
pipeline.
To reflect the strong performance in the first half and our
positive outlook for the rest of the year, we are increasing our
guidance and now expect Generics revenue to be in the range of $810
million to $830 million and core operating margin to be around 22%
to 24% for the full year.
Branded
$ million Constant
currency
H1 2021 H1 2020 Change change
Revenue 319 275 16% 17%
-------- -------- ------- ----------
Core revenue 319 275 16% 17%
-------- -------- ------- ----------
Gross profit 153 128 20% 20%
-------- -------- ------- ----------
Core gross profit 153 128 20% 20%
-------- -------- ------- ----------
Gross margin 48.0% 46.5% 1.5pp 1.2pp
-------- -------- ------- ----------
Operating profit 59 46 28% 52%
-------- -------- ------- ----------
Core operating profit 64 51 25% 47%
-------- -------- ------- ----------
Core operating margin 20.1% 18.5% 1.6pp 4.7pp
-------- -------- ------- ----------
The Branded business achieved a very strong performance in the
first half, with revenue up 16%, driven by good growth in each of
our Tier 1 markets, Algeria, Saudi Arabia and Egypt. We also saw a
good performance in Sudan, where sales have recovered following
disruptions in the first half of 2020, and growth across our other
MENA markets. During the period, we benefited from good demand for
chronic medications and an increase in sales of anti-infectives, as
well as a pull-forward in demand for certain products.
Branded gross profit and margin improved ahead of revenue,
reflecting an improved product mix.
Branded core operating profit, which excludes the amortisation
of intangibles (other than software) and exceptional items,(10)
grew strongly, primarily reflecting the improvement in gross
profit, which more than offset the negative impact of foreign
exchange related to the devaluation of the Sudanese pound.
During H1 2021, the Branded business launched 39 products and
submitted 67 filings to regulatory authorities. Revenue from
in-licensed products represented 41% of Branded revenue (H1 2020:
46%).
We continue to expect full year Branded revenue growth of
mid-single digits in constant currency, and for revenue to be more
evenly spread across the year when compared with 2020.
Other businesses
Other businesses primarily comprise Arab Medical Containers
(AMC), a manufacturer of plastic specialised medicinal sterile
containers and International Pharmaceuticals Research Centre
(IPRC), which conducts bio-equivalency studies. These businesses
contributed revenue of $5 million (H1 2020: $3 million), reflecting
an improvement from H1 2020 when IPRC temporarily closed due to
COVID-19 restrictions. These other businesses made $1 million
operating profit in the period (H1 2020: $0 million).
Research and development
Our investment in R&D and business development is core to
our strategy and enables us to continue expanding the Group's
product portfolio. During H1 2021, we had 87 new launches and
received 112 approvals.
H1 2021 submissions(11) H1 2021 approvals(11) H1 2021 launches(11)
Injectables 56 55 44
------------------------ ---------------------- ---------------------
US 0 7 9
------------------------ ---------------------- ---------------------
MENA 29 33 15
------------------------ ---------------------- ---------------------
Europe 27 15 20
------------------------ ---------------------- ---------------------
Generics 2 3 4
------------------------ ---------------------- ---------------------
Branded 67 54 39
------------------------ ---------------------- ---------------------
Total 125 112 87
------------------------ ---------------------- ---------------------
To ensure the continuous development of our product pipeline, we
submitted 125 regulatory filings.
Net finance expense
Reported net finance expense was $7 million (H1 2020: $23
million) which reflects non-cash net income of $17 million
resulting from the remeasurement and unwinding of the contingent
consideration related to the Generics business. Core net finance
expense was $24 million (H1 2020: $19 million) primarily due to a
drop in finance income as a result of the low interest rate
environment and a lower cash balance when compared with 30 June
2020.
We continue to expect core net finance expense to be around $50
million in 2021.
Profit before tax
Reported profit before tax was $319 million (H1 2020: $274
million). Core profit before tax was $285 million (H1 2020: $265
million), reflecting a good performance from our three business
segments, particularly Generics and Branded.
Tax
The Group incurred a tax expense of $71 million (H1 2020: $62
million). Excluding the tax impact of exceptional items, the Group
core tax expense was $62 million in H1 2021 (H1 2020: $60 million).
The core effective tax rate for H1 2021 was 21.8% (H1 2020: 22.6%).
We continue to expect the Group's core effective tax rate to be
around 22% to 23% for the full year.
Profit attributable to shareholders
Profit attributable to shareholders was $248 million (H1 2020:
$212 million). Excluding the amortisation of intangible assets
(other than software) and exceptional items(12) , core profit
attributable to shareholders increased by 8.8% to $223 million (H1
2020: $205 million).
Earnings per share
Constant
currency
H1 2021 H1 2020 Change change
Basic earnings per share (cents) 107.4 87.6 23% 32%
-------- -------- ------- ----------
Core basic earnings per share
(cents) 96.5 85.3 13% 23%
-------- -------- ------- ----------
Diluted earnings per share (cents) 106.9 87.2 23% 32%
-------- -------- ------- ----------
Core diluted earnings per share
(cents) 96.1 84.8 13% 23%
-------- -------- ------- ----------
Weighted average number of Ordinary
Shares for the purposes of basic
earnings ('m) 231 242 - -
-------- -------- ------- ----------
Weighted average number of Ordinary
Shares for the purposes of diluted
earnings ('m) 232 243 - -
-------- -------- ------- ----------
The increase in earnings per share reflects the strong
performance of the Group and the value for shareholders created by
the Group's buy back of 12.8 million ordinary shares in the first
half of 2020.
Dividend
The Board is recommending an interim dividend of 18 cents per
share (approximately 13 pence per share) (H1 2020: 16 cents per
share). The interim dividend will be paid on 20 September 2021 to
eligible shareholders on the register at the close of business on
20 August 2021.
Net cash flow, working capital and net debt
The Group generated operating cash flow of $224 million (H1
2020: $292 million). This reduction is primarily driven by an
increase in income tax paid, due in part to our growing US
business, as well as the timing of tax payments - the 2020 US Cares
Act resulted in the movement of our H1 2020 tax payments to H2
2020.
Group working capital days were up 39 days to 266 days,
primarily driven by a reduction in payable days when compared with
the first half of 2020. When compared to the position on December
31 2020, working capital days are up two days. Over the course of
2020, due to the COVID-19 pandemic, we made a strategic decision to
increase inventory levels to ensure continuity of supply for our
customers, which increased trade payable levels, while
simultaneously we saw our sales mix result in reduced receivable
collection periods. As the impact of the pandemic has begun to
ease, we have started returning to more normalised inventory levels
as well as receivable and payable days.
Cash capital expenditure was $65 million (H1 2020: $66 million).
In the US, $26 million was spent upgrading equipment, expanding
packaging areas and adding new technologies for our Generics and
Injectables businesses. In MENA, $29 million was spent
strengthening and expanding manufacturing and warehousing
capabilities. In Europe, we spent $10 million, expanding our
facilities and enhancing capabilities in Portugal. We continue to
expect Group capital expenditure to be around $140 million to $160
million in 2021.
The Group's total debt remained constant at $932 million at 30
June 2021 (31 December 2020: $932 million).
The Group's cash balance was $326 million (31 December 2020:
$327 million). The Group's net debt was $606 million at 30 June
2021 (31 December 2020: $605 million).(13) We continue to have a
very strong balance sheet with a net debt to core EBITDA ratio of
0.9x.
Balance sheet
Net assets at 30 June 2021 were $2,303 million (31 December
2020: $2,148 million). Net current assets increased to $995 million
(31 December 2020: $894 million).
Responsibility statement
We confirm that to the best of our knowledge:
These interim financial statements for the six months ended 30
June 2021 have been prepared in accordance with the Disclosure
Guidance and Transparency Rules sourcebook of the UK's Financial
Conduct Authority and with UK adopted IAS 34, 'Interim financial
reporting', and as issued by the International Accounting Standards
Board (IASB). The interim financial statements should be read in
conjunction with the annual consolidated financial statements for
the year ended 31 December 2020.
The interim results announcement includes a fair review of the
information required by:
a) DTR 4.2.7R of the Disclosure and Transparency Rules, being an
indication of important events that have occurred during the first
six months of the financial year and their impact on the condensed
set of financial statements; and a description of the principal
risks and uncertainties for the remaining six months of the
financial year; and
b) DTR 4.2.8R of the Disclosure and Transparency Rules, being
related party transactions that have taken place in the first six
months of the current financial year and that have materially
affected the financial position or performance of the enterprise
during that period; and any changes in the related party
transactions described in the last annual report that could do
so.
By order of the Board
Sigurdur Olafsson Khalid Nabilsi
Chief Executive Officer Chief Financial Officer
5 August 2021 5 August 2021
The Board
The Board of Directors that served during all or part of the
six-month period to 30 June 2021 and their respective
responsibilities can be found on the Leadership team section of
www.hikma.com .
Cautionary statement
This interim results announcement has been prepared solely to
provide additional information to the shareholders of Hikma and
should not be relied on by any other party or for any other
purpose.
Definitions
We use a number of non-IFRS measures to report and monitor the
performance of our business. Management uses these adjusted numbers
internally to measure our progress and for setting performance
targets. We also present these numbers, alongside our reported
results, to external audiences to help them understand the
underlying performance of our business. Our core numbers may be
calculated differently to other companies.
Adjusted measures are not substitutable for IFRS results and
should not be considered superior to results presented in
accordance with IFRS.
Core results
Reported results represent the Group's overall performance.
However, these results can include one-off or non-cash items which
are excluded when assessing the underlying performance of the
Group. To provide a more complete picture of the Group's
performance to external audiences, we provide, alongside our
reported results, core results, which are a non-IFRS measure. Our
core results exclude the exceptional items and other adjustments
set out in Note 5.
Group operating profit H1 2021 H1 2020
$million $million
Core operating profit 309 284
---------- ----------
Jordan warehouse fire incident - 1
---------- ----------
MENA severance and restructuring costs - (1)
---------- ----------
Intangible assets amortisation other
than software (29) (21)
---------- ----------
Impairment reversal of product related
intangibles 46 34
---------- ----------
Reported operating profit 326 297
---------- ----------
Constant currency
As the majority of our business is conducted in the US, we
present our results in US dollars. For both our Branded and
Injectable businesses, a proportion of their sales are denominated
in a currency other than the US dollar. In order to illustrate the
underlying performance of these businesses, we include information
on our results in constant currency.
Constant currency numbers in H1 2021 represent reported H1 2021
numbers translated using H1 2020 exchange rates, excluding price
increases in the business resulting from the devaluation of
currencies and excluding the impact from hyperinflation accounting.
Lebanon and Sudan are considered hyperinflationary economies,
therefore the spot exchange rate as at 30 June 2021 was used to
translate the results of these operations into US dollars.
EBITDA
EBITDA is earnings before interest, tax, depreciation,
amortisation, impairment and exceptional and other items.
EBITDA
$ million H1 2021 H1 2020
Reported operating profit 326 297
-------- --------
Depreciation 44 38
-------- --------
Amortisation 34 26
-------- --------
Impairment charges/reversals (46) (33)
-------- --------
EBITDA 358 328
-------- --------
Exceptional items:
-------- --------
Jordan warehouse fire incident - (1)
-------- --------
MENA severance and restructuring
costs - 1
-------- --------
Core EBITDA 358 328
-------- --------
Working capital days
We believe Group working capital days provides a useful measure
of the Group's working capital management and liquidity. Group
working capital days are calculated as Group receivable days plus
Group inventory days, less Group payable days. Group receivable
days are calculated as Group trade receivables x 365, divided by
trailing 12 months Group revenue. Group inventory days are
calculated as Group inventory x 365 divided by 12 months Group cost
of sales. Group payable days are calculated as Group trade payables
x 365, divided by 12 months Group cost of sales.
Group net debt
We believe Group net debt is a useful measure of the strength of
the Group financial position. Group net debt is calculated as Group
total debt less Group total cash. Group total debt excludes
co-development agreements and acquired contingent liabilities.
Group net debt
$ million Jun-21 Dec-20
Short-term financial debts (172) (158)
------- -------
Short-term leases liabilities (9) (10)
------- -------
Long-term financial debts (676) (692)
------- -------
Long-term leases liabilities (75) (72)
------- -------
Total debt (932) (932)
------- -------
Cash, cash equivalents and restricted
cash 326 327
------- -------
Net debt (606) (605)
------- -------
Forward looking statements
This announcement contains certain statements which are, or may
be deemed to be, "forward looking statements" which are prospective
in nature with respect to Hikma's expectations and plans, strategy,
management objectives, future developments and performance, costs,
revenues and other trend information. All statements other than
statements of historical fact may be forward-looking statements.
Often, but not always, forward-looking statements can be identified
by the use of forward looking words such as "intends", "believes",
"anticipates", "expects", "estimates", "forecasts", "targets",
"aims", "budget", "scheduled" or words or terms of similar
substance or the negative thereof, as well as variations of such
words and phrases or statements that certain actions, events or
results "may", "could", "should", "would", "might" or "will" be
taken, occur or be achieved.
By their nature, forward looking statements are based on current
expectations and projections about future events and are therefore
subject to assumptions, risks and uncertainties that are beyond
Hikma's ability to control or estimate precisely and which could
cause actual results or events to differ materially from those
expressed or implied by the forward looking statements. Where
included, such statements have been made by or on behalf of Hikma
in good faith based upon the knowledge and information available to
the Directors on the date of this announcement. Accordingly, no
assurance can be given that any particular expectation will be met
and Hikma's shareholders are cautioned not to place undue reliance
on the forward-looking statements. Forward looking statements
contained in this announcement regarding past trends or activities
should not be taken as a representation that such trends or
activities will continue in the future.
Other than in accordance with its legal or regulatory
obligations (including under the Market Abuse Regulation ((EU) No.
596/2014) and the UK Listing Rules and the Disclosure Guidance and
Transparency Rules of the Financial Conduct Authority), Hikma does
not undertake to update the forward looking statements contained in
this announcement to reflect any changes in events, conditions or
circumstances on which any such statement is based or to correct
any inaccuracies which may become apparent in such forward looking
statements. Except as expressly provided in this announcement, no
forward looking or other statements have been reviewed by the
auditors of Hikma. All subsequent oral or written forward looking
statements attributable to Hikma or any of its members, directors,
officers or employees or any person acting on their behalf are
expressly qualified in their entirety by the cautionary statement
above. Past share performance cannot be relied on as a guide to
future performance. Nothing in this announcement should be
construed as a profit forecast.
Neither the content of Hikma's website nor any other website
accessible by hyperlinks from Hikma's website are incorporated in,
or form part of, this announcement.
Principal risks and uncertainties
The Group faces risks from a range of sources that could have a
material impact on our financial results and ability to trade in
the future. The principal risks are determined via robust
assessment considering our risk context by the Board of Directors
with input from executive management. The principal risks for the
company have not materially changed in the last six months . They
are described below and in more detail in the 2020 annual report on
pages 55 - 58. They are not expected to change significantly in the
second six months of the financial year.
The Board recognises that certain risk factors that influence
the principal risks are outside of the control of management. The
Board is satisfied that the principal risks are being managed
appropriately and consistently with the risk appetite. The set of
principal risks should not be considered as an exhaustive list of
all the risks the Group faces. In addition to the Principal risks,
new and emerging risks are monitored as part of our risk management
framework, including climate-related risks. We continue to prepare
for alignment of our public disclosures with the Taskforce on
Climate-related Financial Disclosures (TCFD) recommendations.
Principal risks What does the risk cover?
Industry dynamics The commercial viability of the industry and business
model we operate may change significantly as a result
of political action, economic factors, societal pressures,
regulatory interventions or changes to participants
in the value chain of the industry.
----------------------------------------------------------------
Product pipeline Selecting, developing and registering new products
that meet market needs and are aligned with Hikma's
strategy to provide a continuous source of future growth.
----------------------------------------------------------------
Organisational Developing, maintaining and adapting organisational
development structures, management processes and controls, and
talent pipeline to enable effective delivery by the
business in the face of rapid and constant internal
and external change.
----------------------------------------------------------------
Reputation Building and maintaining trusted and successful partnerships
with our stakeholders relies on developing and sustaining
our reputation as one of our most valuable assets.
----------------------------------------------------------------
Ethics and compliance Maintaining a culture underpinned by ethical decision
making, with appropriate internal controls to ensure
staff and third parties comply with our Code of Conduct,
associated policies and procedures, as well as all
applicable legislation.
----------------------------------------------------------------
Information and Ensuring the integrity, confidentiality, availability
cyber security, and resilience of data, securing information stored
technology and and/or processed internally or externally from cyber
infrastructure and non-cyber threats, maintaining and developing technology
systems that enable business processes, and ensuring
infrastructure supports the organisation effectively.
----------------------------------------------------------------
Legal, regulatory Complying with laws and regulations, and their application.
and intellectual Managing litigation, governmental investigations, sanctions,
property contractual terms and conditions and adapting to their
changes while preserving shareholder value, business
integrity and reputation.
----------------------------------------------------------------
Inorganic growth Identifying, accurately pricing and realising expected
benefits from acquisitions or divestments, licensing,
or other business development activities.
----------------------------------------------------------------
Active pharmaceutical Maintaining availability of supply, quality and competitiveness
ingredient (API) of API purchases and ensuring proper understanding
and third-party and control of third-party risks.
risk management
----------------------------------------------------------------
Crisis response Preparedness, response, continuity and recovery from
and business disruptive events, such as natural catastrophe, economic
continuity turmoil, operational issues, pandemic, political crisis,
and regulatory intervention.
----------------------------------------------------------------
Product quality Maintaining compliance with current Good Practices
and safety for Manufacturing (cGMP), Laboratory (cGLP), Distribution
(cGDP) and Pharmacovigilance (cGVP) by staff, and ensuring
compliance is maintained by all relevant third parties
involved in these processes.
----------------------------------------------------------------
Financial control Effectively managing income, expenditure, assets and
and reporting liabilities, liquidity, exchange rates, tax uncertainty,
debtor and associated activities, and in reporting
accurately, in a timely manner and in compliance with
statutory requirements and accounting standards.
----------------------------------------------------------------
1 Constant currency numbers in H1 2021 represent reported H1
2021 numbers translated using H1 2020 exchange rates, excluding
price increases in the business resulting from the devaluation of
currencies and excluding the impact from hyperinflation accounting.
Lebanon and Sudan are considered hyperinflationary economies,
therefore the spot exchange rate as at 30 June 2021 was used to
translate the results of these operations into US dollars
2 In June 2020, Hikma purchased 12.8 million ordinary shares
from Boehringer Ingelheim, which are being held in treasury.
(3) Core results throughout the document are presented to show
the underlying performance of the Group, excluding the exceptional
items and other adjustments set out in Note 5. Core results are a
non-IFRS measure and a reconciliation to reported IFRS measures is
provided on page 14
4 Group net debt is calculated as Group total debt less Group
total cash, including restricted cash. Group net debt is a non-IFRS
measure. See page 15 for a reconciliation of Group net debt to
reported IFRS figures
5 Core EBITDA is earnings before interest, tax, depreciation,
amortisation, impairment and exceptional and other items. EBITDA is
a non-IFRS measure, see page 15 for a reconciliation to reported
IFRS results. For the purposes of the leverage calculation, core
EBITDA is calculated for trailing twelve months ended 30 June
2021
6 Beginning in full year 2020, inventory related provisions are
reported under the cost of sales line item and are shown here for
both H1 2021 and H1 2020 comparatives. In the H1 2020 financial
statements, inventory related provisions were included in other
operating income/(expenses). The reason for reclassification is to
be in line with industry practice. The effect of the adjustment on
the operating profit is shown in Note 2.
7 Other adjustments comprises a $46 million impairment reversal
in respect of generic Advair Diskus(R) intangible asset as a result
of launching the product. Refer to Note 5 for further
information
8 Exceptional items comprised amortisation of intangible assets
other than software, of $12 million. Refer to Note 5 for further
information
(9) Adjustment comprised a $46 million impairment reversal in
respect of generic Advair Diskus(R) intangible asset as a result of
launching the product and amortisation of intangible assets other
than software, of $12 million. Refer to Note 5 for further
information
1 (0) Exceptional items comprised amortisation of intangible
assets other than software, of $5 million. Refer to Note 5 for
further information
1 (1) New products submitted, approved and launched by country
in H1 2021
1(2) Excluding $17 million of exceptionals included in operating
profit and the $17 million net effect related to unwinding and
remeasurement of contingent consideration and other financial
liability and $9 million tax effect
1 (3) Group net debt is a non-IFRS measure that includes long
and short-term financial debts (Note 16), lease liabilities, net of
cash and cash equivalents and collateralised and restricted cash.
Group net debt excludes co-development and earnout payments,
acquired contingent liabilities and contingent consideration (Note
15). See page 15 for a reconciliation of Group net debt to reported
IFRS results
Independent review report to Hikma Pharmaceuticals PLC
Report on the condensed consolidated interim financial
statements
Our conclusion
We have reviewed Hikma Pharmaceuticals PLC's condensed
consolidated interim financial statements (the "interim financial
statements") in the interim results press release of Hikma
Pharmaceuticals PLC for the 6 month period ended 30 June 2021 (the
"period").
Based on our review, nothing has come to our attention that
causes us to believe that the interim financial statements are not
prepared, in all material respects, in accordance with UK adopted
International Accounting Standard 34, 'Interim Financial Reporting'
and the Disclosure Guidance and Transparency Rules sourcebook of
the United Kingdom's Financial Conduct Authority.
What we have reviewed
The interim financial statements comprise:
-- the condensed consolidated interim balance sheet as at 30 June 2021;
-- the condensed consolidated interim income statement and
condensed consolidated interim statement of comprehensive income
for the period then ended;
-- the condensed consolidated interim cash flow statement for the period then ended;
-- the condensed consolidated interim statement of changes in
equity for the period then ended; and
-- the explanatory notes to the interim financial statements.
The interim financial statements included in the interim results
press release of Hikma Pharmaceuticals PLC have been prepared in
accordance with UK adopted International Accounting Standard 34,
'Interim Financial Reporting' and the Disclosure Guidance and
Transparency Rules sourcebook of the United Kingdom's Financial
Conduct Authority.
Responsibilities for the interim financial statements and the
review
Our responsibilities and those of the directors
The interim results press release, including the interim
financial statements, is the responsibility of, and has been
approved by the directors. The directors are responsible for
preparing the interim results press release in accordance with the
Disclosure Guidance and Transparency Rules sourcebook of the United
Kingdom's Financial Conduct Authority.
Our responsibility is to express a conclusion on the interim
financial statements in the interim results press release based on
our review. This report, including the conclusion, has been
prepared for and only for the company for the purpose of complying
with the Disclosure Guidance and Transparency Rules sourcebook of
the United Kingdom's Financial Conduct Authority and for no other
purpose. We do not, in giving this conclusion, accept or assume
responsibility for any other purpose or to any other person to whom
this report is shown or into whose hands it may come save where
expressly agreed by our prior consent in writing.
What a review of interim financial statements involves
We conducted our review in accordance with International
Standard on Review Engagements (UK and Ireland) 2410, 'Review of
Interim Financial Information Performed by the Independent Auditor
of the Entity' issued by the Auditing Practices Board for use in
the United Kingdom. A review of interim financial information
consists of making enquiries, primarily of persons responsible for
financial and accounting matters, and applying analytical and other
review procedures.
A review is substantially less in scope than an audit conducted
in accordance with International Standards on Auditing (UK) and,
consequently, does not enable us to obtain assurance that we would
become aware of all significant matters that might be identified in
an audit. Accordingly, we do not express an audit opinion.
We have read the other information contained in the interim
results press release and considered whether it contains any
apparent misstatements or material inconsistencies with the
information in the interim financial statements.
PricewaterhouseCoopers LLP
Chartered Accountants
London
5 August 2021
Hikma Pharmaceuticals PLC
Condensed consolidated interim income statement
H1 2021 H1 2021 H1 2021 H1 2020 H1 2020 H1 2020
Core Exceptional items Reported results Core Exceptional items Reported results
results and other results and other
adjustments adjustments
(Note 5) (Note 5)
Note $m (Unaudited) $m (Unaudited) $m (Unaudited) $m (Unaudited) $m (Unaudited) $m (Unaudited)
------------------- ------------------- ------------------- ----------------- ------------------- -----------------
Revenue 3 1,216 - 1,216 1,132 - 1,132
Cost of sales(1) (600) - (600) (555) - (555)
------------------- -----------------
Gross profit 616 - 616 577 - 577
------------------- ------------------- ------------------- ----------------- ------------------- -----------------
Selling, general
and administrative
expenses (232) (29) (261) (229) (22) (251)
Net impairment
reversals on
financial assets - - - 2 - 2
Research and
development
expenses (59) - (59) (62) - (62)
Other operating
income/(expenses),
net(1) (16) 46 30 (4) 35 31
------------------- ------------------- ------------------- ----------------- ------------------- -----------------
Total operating
(expenses)/income (307) 17 (290) (293) 13 (280)
Operating profit 4 309 17 326 284 13 297
Finance income 1 29 30 5 - 5
Finance expense (25) (12) (37) (24) (4) (28)
------------------- ------------------- ------------------- ----------------- ------------------- -----------------
Profit before tax 285 34 319 265 9 274
Tax 6 (62) (9) (71) (60) (2) (62)
Profit for the
half-year 223 25 248 205 7 212
=================== =================== =================== ================= =================== =================
Attributable to:
Non-controlling - - -
interests - - -
Equity holders of
the parent 223 25 248 205 7 212
------------------- ------------------- ------------------- ----------------- ------------------- -----------------
223 25 248 205 7 212
=================== =================== =================== ================= =================== =================
Earnings per share
(cents)
Basic 96.5 107.4 85.3 87.6
Diluted 96.1 106.9 84.8 87.2
1.Inventory related provisions have been reclassified under the
cost of sales line item to align with industry practice. Previously
the costs were reflected in other operating income/(expenses), net
and hence the H1 2020 numbers have consequently been restated. See
Note 2 for more details
On this page and throughout this financial information 'H1 2021'
refers to the six months ended 30 June 2021. 'H1 2020' refers to
the six months ended 30 June 2020.
Hikma Pharmaceuticals PLC
Condensed consolidated interim statement of comprehensive
income
H1 2021 H1 2020
Reported Reported
results results
$m (Unaudited) $m (Unaudited)
Profit for the half-year 248 212
Other Comprehensive Income
Items that may subsequently be reclassified
to the consolidated income statement,
net of tax:
Currency translation loss and hyperinflation
movement (31) (13)
Total comprehensive income for the half-year 217 199
==================== ===================
Attributable to:
Non-controlling interests - (1)
Equity holders of the parent 217 200
-------------------- -------------------
217 199
==================== ===================
Hikma Pharmaceuticals PLC
Condensed consolidated interim balance sheet
30 June 31 December
2021 2020
Note $m $m
(Unaudited) (Audited)
------------------- --------------------------
Non-current assets
Goodwill 286 289
Other intangible assets 8 620 587
Property, plant and equipment 1,001 1,009
Right-of-use assets 60 59
Investment in joint ventures 10 9
Deferred tax assets 208 221
Financial and other non-current assets 9 40 39
2,225 2,213
------------------- --------------------------
Current assets
Inventories 10 736 757
Income tax receivable 49 36
Trade and other receivables 11 809 756
Collateralised and restricted cash 4 4
Cash and cash equivalents 12 322 323
Other current assets 13 35 46
1,955 1,922
------------------- --------------------------
Total assets 4,180 4,135
=================== ==========================
Current liabilities
Short-term financial debts 16 172 158
Lease liabilities 9 10
Trade and other payables 14 386 470
Income tax payable 63 72
Other provisions 29 28
Other current liabilities 15 301 290
960 1,028
------------------- --------------------------
Net current assets 995 894
------------------- --------------------------
Non-current liabilities
Long-term financial debts 16 676 692
Lease liabilities 75 72
Deferred tax liabilities 21 31
Other non-current liabilities 17 145 164
917 959
------------------- --------------------------
Total liabilities 1,877 1,987
=================== ==========================
Net assets 2,303 2,148
=================== ==========================
Equity
Share capital 41 41
Share premium 282 282
Other reserves (65) (80)
Retained earnings 2,032 1,892
------------------- --------------------------
Equity attributable to equity holders of the parent 2,290 2,135
Non-controlling interests 13 13
------------------- --------------------------
Total equity 2,303 2,148
=================== ==========================
Hikma Pharmaceuticals PLC
Condensed consolidated interim statement of changes in
equity
Merger Translation Own Total Retained Share Share Equity Non-controlling Total
and reserve shares other earnings capital premium attributable interests equity
revaluation reserves to
reserves equity
shareholders
of
the
parent
$m $m $m $m $m $m $m $m $m $m
------------ ------------ ------- --------- --------- -------- -------- ------------- ---------------- -------
Balance at 1 January
2020 57 (235) (1) (179) 1,973 41 282 2,117 12 2,129
Reclassification(1) - - 1 1 (1) - - - - -
------------ ------------ ------- --------- --------- -------- -------- ------------- ---------------- -------
Balance at 1 January
2020 as adjusted 57 (235) - (178) 1,972 41 282 2,117 12 2,129
Profit for the
half-year(2) 34 - - 34 178 - - 212 - 212
Currency translation
loss - (12) - (12) - - - (12) (1) (13)
Total comprehensive
income for the
half-year 34 (12) - 22 178 - - 200 (1) 199
------------ ------------ ------- --------- --------- -------- -------- ------------- ---------------- -------
Total transactions
with owners,
recognised
directly in equity
Cost of
equity-settled
employee share
scheme - - - - 14 - - 14 - 14
Dividends paid (Note
7) - - - - (73) - - (73) (1) (74)
Share buyback - - - - (367) - - (367) - (367)
Current income tax
arising from Share
buyback - - - - (1) - - (1) - (1)
Balance at 30 June
2020 (unaudited) 91 (247) - (156) 1,723 41 282 1,890 10 1,900
============ ============ ======= ========= ========= ======== ======== ============= ================ =======
Balance at 31
December 2020
(audited) and 1
January 2021 119 (199) - (80) 1,892 41 282 2,135 13 2,148
Profit for the
half-year(2) 46 - - 46 202 - - 248 - 248
Currency translation
loss and
hyperinflation
movement - (31) - (31) - - - (31) - (31)
------------ ------------ ------- --------- --------- -------- -------- ------------- ---------------- -------
Total comprehensive
income for the
half-year 46 (31) - 15 202 - - 217 - 217
------------ ------------ ------- --------- --------- -------- -------- ------------- ---------------- -------
Total transactions
with owners,
recognised
directly in equity
Cost of
equity-settled
employee share
scheme - - - - 16 - - 16 - 16
Dividends paid (Note
7) - - - - (78) - - (78) - (78)
Balance at 30 June
2021 (unaudited) 165 (230) - (65) 2,032 41 282 2,290 13 2,303
============ ============ ======= ========= ========= ======== ======== ============= ================ =======
1.Beginning in 2020, own shares are deducted from retained
earnings
2.An impairment reversal of $46 million has been allocated from
retained earnings to the merger and revaluation reserves in
relation to generic Advair Diskus(R) cash generating unit (CGU)
(Note 5 and 8)
In H1 2020, $34 million impairment reversal has been allocated
from retained earnings to the merger and revaluation reserves in
relation to the Generics segment (Year ended 2020: net impairment
reversal of $62 million)
Hikma Pharmaceuticals PLC
Condensed consolidated interim cash flow statement
H1 H1
2021 2020
Note $m (Unaudited) $m (Unaudited)
----------------- ------------------
Cash flows from operating activities
Cash generated from operations 18 312 311
Income taxes paid (88) (19)
Net cash inflow from operating activities 224 292
Cash flow from investing activities
Purchases of property, plant and equipment (65) (66)
Purchase of intangible assets (29) (35)
Additions of investments at FVTOCI (1) (3)
Proceeds from investment divestiture 1 2
Interest income received 1 5
Investment related amounts held in escrow
account - (3)
Payments of acquired contingent liability (11) -
Net cash outflow from investing activities (104) (100)
Cash flow from financing activities
Increase in collateralised and restricted
cash - 1
Proceeds from issue of long-term financial
debts 3 700
Repayment of long-term financial debts (21) (507)
Proceeds from short-term borrowings 219 156
Repayment of short-term borrowings (202) (101)
Repayment of lease liabilities (7) (7)
Dividends paid (78) (73)
Dividends paid to non-controlling shareholders
of subsidiaries - (1)
Interest and bank charges paid (25) (24)
Share buyback - (371)
Commitment fees received related to share
buyback - 7
Payment to co-development and earnout
payment agreement (1) -
Net cash outflow from financing activities (112) (220)
Net increase/(decrease) in cash and cash
equivalents 8 (28)
Cash and cash equivalents at beginning
of the half-year 323 442
Foreign exchange translation movements (9) (1)
Cash and cash equivalents at end of the
half-year 12 322 413
================= ==================
Hikma Pharmaceuticals PLC
Notes to the condensed consolidated interim financial
statements
1. General information
Hikma Pharmaceuticals PLC is a public limited liability company
incorporated and domiciled in England and Wales under the Companies
Act 2006. The registered office address is 1 New Burlington Place,
London W1S 2HR, UK.
The Group's principal activities are the development,
manufacturing, marketing and selling of a broad range of generic,
branded and in-licensed pharmaceuticals products in solid,
semi-solid, liquid and injectable final dosage forms.
2. Accounting policies
The unaudited condensed consolidated interim financial
statements (financial statements) for the six months ended 30 June
2021 have been prepared on the basis of policies set out in the
2020 annual report.
Reclassification of H1 2020 interim financial statements
Beginning in H2 2020, inventory related provisions were reported
under the cost of sales line item. In H1 2020 interim financial
statements, inventory related provisions were included in other
operating income/(expenses), net line item. The reason for
reclassification is to be in line with industry practice. The
effect of the adjustment on the operating profit was as
follows:
H1 2020 Adjustment Adjusted
results H1 2020
as Reported
previously results
reported (Unaudited)
(Unaudited)
$m $m $m
Cost of sales (530) (25) (555)
------------------------------------ ------------- ----------- -------------
Gross profit 602 (25) 577
------------------------------------ ------------- ----------- -------------
Other operating income/(expenses),
net (305) 25 (280)
------------------------------------ ------------- ----------- -------------
Operating Profit 297 - 297
------------------------------------ ------------- ----------- -------------
Basis of preparation
The currency used in the presentation of the accompanying
financial statements is the US dollar ($) as most of the Group's
business is conducted in US dollars.
These interim financial statements for the six months ended 30
June 2021 have been prepared in accordance with the Disclosure
Guidance and Transparency Rules sourcebook of the UK's Financial
Conduct Authority with UK adopted IAS 34, 'Interim financial
reporting', and as issued by the International Accounting Standards
Board (IASB). The financial statements should be read in
conjunction with the annual consolidated financial statements for
the year ended 31 December 2020, which have been prepared in
accordance with:
(i) International Financial Reporting Standards ('IFRS') in
conformity with the requirements of the Companies Act 2006 the
applicable legal requirements of the Companies Act 2006. In
addition to complying with IFRS in conformity with the requirements
of the Companies Act 2006, the consolidated financial statements
also comply with IFRS adopted pursuant to Regulation (EC) No
1606/2002 as it applies in the European Union.
(ii) IFRS as issued by the International Accounting Standards
Board (IASB).
The financial information does not constitute statutory accounts
as defined in section 435 of the Companies Act 2006. A copy of the
statutory accounts for 2020 have been delivered to the Registrar of
Companies. The auditors' report on those accounts was unqualified,
did not draw attention to any matters by way of emphasis and did
not contain any statement under Section 498 (2) or (3) of the
Companies Act 2006. These interim financial statements have been
reviewed, not audited.
In the year to 31 December 2021 the annual financial statements
will be prepared in accordance with IFRS as adopted by the UK
Endorsement Board which is a change in basis of preparation that is
required by UK company law for the purposes of financial reporting
as a result of the UK's exit from the EU on 31 January 2020 and the
cessation of the transition period on 31 December 2020. This change
does not constitute a change in accounting policy but rather a
change in framework which is required to ground the use of IFRS in
company law. There is no impact on recognition, measurement or
disclosure between the two frameworks in the period reported.
New standards interpretations and amendments adopted by the
Group
The accounting policies adopted in the preparation of the
financial statements are consistent with those followed in the
preparation of the Group's annual consolidated financial statements
for the year ended 31 December 2020.
The Group has not early adopted any standard, interpretation or
amendment that has been issued but is not yet effective.
- Interest Rate Benchmark Reform - Phase 2: Amendments to IFRS
9, IAS 39, IFRS 7, IFRS 4 and IFRS 16
The amendments provide temporary reliefs which address the
financial reporting effects when an interbank offered rate (IBOR)
is replaced with an alternative nearly risk-free interest rate
(RFR).
The amendments include the following practical expedient:
A practical expedient to require contractual changes, or changes
to cash flows that are directly required by the reform, to be
treated as changes to a floating interest rate, equivalent to a
movement in a market rate of interest.
These amendments had no significant impact on the interim
condensed consolidated financial statements of the Group. The Group
intends to use the practical expedients in future periods if they
become applicable.
Going concern
The Directors have considered the going concern position of the
Group at 30 June 2021. The Directors believe that the Group is well
diversified due to its geographic spread, product diversity and
large customer and supplier base. The Group's business activity,
together with the factors likely to affect its future development,
performance and position are set out in the Interim Results Press
Release. The Interim Results Press Release also includes a summary
of the financial position, cash flow and borrowing facilities.
At 30 June 2021 the Group had undrawn long term committed
banking facilities of $1,101 million. The Group's total debt at 30
June 2021 was $932 million while the Group's cash, cash equivalent
and
collateralised and restricted cash at 30 June 2021 balance was
$326 million making the net debt(1) $607 million. The Group's net
debt(1) to trailing core EBITDA ratio was 0.9x at 30 June 2021.
Taking into account the Group's current position and its principal
risks for a period longer than twelve months, a going concern
analysis has been prepared using realistic scenarios applying a
severe but plausible downside considering
the principal risks facing the business including delays to the
pipeline, lower sales of newly launched products, increased price
erosion impacting existing products, and disruption in certain MENA
markets , which shows sufficient liquidity headroom. Therefore, the
Directors believe that the Group and its subsidiaries are
adequately placed to manage its business and financing risks
successfully, despite the current uncertain economic and political
outlook. Having reassessed the principal risks, the Directors
considered it appropriate to adopt the going concern basis of
accounting in preparing the interim financial information.
Notwithstanding the fact that financial covenants have been
suspended for as long as the Group retains its investment grade
status from two Rating Agencies(2) , the Group was in compliance on
30 June and expects to remain in compliance with those covenants in
the period to 31 December 2022 even in the severe but plausible
downside scenario. As at 30 June 2021 the Group investment grade
rating is affirmed by S&P and Fitch.
1. Group net debt is a non-IFRS measure that includes long and
short-term financial debts (Note 16), lease liabilities, net of
cash and cash equivalents and collateralised and restricted cash.
Group net debt excludes co-development and earnout payments,
acquired contingent liabilities and contingent consideration (Notes
15 and 17)
2. Rating agencies: means each of Fitch, Moody's and S&P or
any of their affiliates or successors
3. Revenue from contracts with customers
Business and geographical markets
The following table provides an analysis of the Group's reported
sales by segment and geographical market, irrespective of the
origin of the goods/services:
Branded Injectables Generics Others Total
H1 2021 (unaudited) $m $m $m $m $m
------------- ------------------ ------------- ------------- -------------
United States - 318 400 - 718
Middle East and North
Africa 316 77 - 3 396
Europe and Rest of the
World 3 95 - 2 100
United Kingdom - 2 - - 2
319 492 400 5 1,216
============= ================== ============= ============= =============
Branded Injectables Generics Others Total
H1 2020 (unaudited) $m $m $m $m $m
------------- ------------------ ------------- ------------- -------------
United States - 347 369 - 716
Middle East and North
Africa 273 75 - 3 351
Europe and Rest of the
World 2 61 - - 63
United Kingdom - 2 - - 2
275 485 369 3 1,132
============= ================== ============= ============= =============
The top selling markets are shown below:
H1 2021 H1 2020
$m $m
--------------- ---------------
(Unaudited) (Unaudited)
--------------- ---------------
United States 718 716
Saudi Arabia 114 113
Egypt 66 58
898 887
=============== ===============
In H1 2021, included in revenue arising from the Generics and
Injectables segments are sales the Group made to two (H1 2020:
three) wholesalers in the US of $335 million (H1 2020: $412
million)
Each of these customers accounted for greater than 10% of
Group's revenue in the period on an individual basis.
4. Business segments
For management reporting purposes, the Group is organised into
three principal operating divisions - Injectables, Generics and
Branded. These divisions are the basis on which the Group reports
its segmental information.
Core operating profit, defined as 'segment result', is the
principal measure used in the decision-making and resource
allocation process of the chief operating decision maker, who is
the Group's Chief Executive Officer.
Information regarding the Group's operating segments is reported
below:
H1 2021 H1 2021 H1 2021 H1 2020 H1 2020 H1 2020
Core Exceptional Reported Core Exceptional Reported
results items and results results items and results
(Unaudited) other (Unaudited) (Unaudited) other (Unaudited)
adjustments adjustments
(Note 5) (Note 5)
Injectables (Unaudited) (Unaudited)
$m $m $m $m $m $m
------------- ------------- -------------- -------------- ------------- --------------
Revenue 492 - 492 485 - 485
Cost of
sales(1) (219) - (219) (198) - (198)
------------- -------------- ------------- --------------
Gross profit 273 - 273 287 - 287
------------- ------------- -------------- -------------- ------------- --------------
Total
operating
expenses(1) (86) (12) (98) (83) (12) (95)
------------- ------------- -------------- -------------- ------------- --------------
Segment
result 187 (12) 175 204 (12) 192
============= ============= ============== ============== ============= ==============
H1 2021 H1 2021 H1 2021 H1 2020 H1 2020 H1 2020
Core Exceptional Reported Core Exceptional Reported
results items and results results items and results
(Unaudited) other (Unaudited) (Unaudited) other (Unaudited)
adjustments adjustments
(Note 5) (Note 5)
Generics (Unaudited) (Unaudited)
$m $m $m $m $m $m
------------- ------------- -------------- -------------- ------------- --------------
Revenue 400 - 400 369 - 369
Cost of
sales(1) (212) - (212) (208) - (208)
------------- -------------- -------------- ------------- --------------
Gross profit 188 - 188 161 - 161
------------- ------------- -------------- -------------- ------------- --------------
Total
operating
expenses(1) (88) 34 (54) (89) 30 (59)
------------- ------------- -------------- -------------- ------------- --------------
Segment
result 100 34 134 72 30 102
============= ============= ============== ============== ============= ==============
H1 2021 H1 2021 H1 2021 H1 2020 H1 2020 H1 2020
Core Exceptional Reported Core Exceptional Reported
results items and results results items and results
(Unaudited) other (Unaudited) (Unaudited) other (Unaudited)
adjustments adjustments
(Note 5) (Note 5)
Branded (Unaudited) (Unaudited)
$m $m $m $m $m $m
------------- ------------- -------------- -------------- ------------- --------------
Revenue 319 - 319 275 - 275
Cost of
sales(1) (166) - (166) (147) - (147)
------------- ------------- -------------- -------------- ------------- --------------
Gross profit 153 - 153 128 - 128
------------- ------------- -------------- -------------- ------------- --------------
Total
operating
expenses(1) (89) (5) (94) (77) (5) (82)
------------- ------------- -------------- -------------- ------------- --------------
Segment
result 64 (5) 59 51 (5) 46
============= ============= ============== ============== ============= ==============
1.Inventory related provisions have been reclassified under the
cost of sales line item to align with industry practice. Previously
the costs were reflected in other operating income/(expenses), net
and hence the H1 2020 numbers have consequently been restated. See
Note 2 for more details
H1 2021 H1 2021 H1 2021 Reported H1 2020 H1 2020 H1 2020 Reported
Core Exceptional results Core Exceptional results (Unaudited)
results items and (Unaudited) results items and
(Unaudited) other (Unaudited) other
adjustments adjustments
(Note 5) (Note 5)
Others(1) (Unaudited) (Unaudited)
$m $m $m $m $m $m
------------------ ------------ ------------------ ------------------- ------------ --------------------
Revenue 5 - 5 3 - 3
Cost of
sales (3) - (3) (2) - (2)
------------------ ------------ ------------------ ------------------- ------------ --------------------
Gross
profit 2 - 2 1 - 1
------------------ ------------ ------------------ ------------------- ------------ --------------------
Total
operating
expenses (1) - (1) (1) - (1)
------------------ ------------ ------------------ ------------------- ------------ --------------------
Segment
result 1 - 1 - - -
================== ============ ================== =================== ============ ====================
1 . Others mainly comprises Arab Medical Containers LLC and
International Pharmaceutical Research Center LLC
H1 2021 H1 2021 H1 2021 H1 2020 H1 2020 H1 2020
Core Exceptional items Reported Core Exceptional Reported
results and other results results items and results
(Unaudited) adjustments (Unaudited) (Unaudited) other (Unaudited)
(Note 5) (Unaudited) adjustments
(Note 5)
Group (Unaudited)
$m $m $m $m $m $m
------------ --------------------- ------------ ------------ ------------ -------------------
Segment result 352 17 369 327 13 340
--------------------- ------------ ------------ ------------ -------------------
Unallocated
expenses(2) (43) - (43) (43) - (43)
------------ --------------------- ------------ ------------ -------------------
Operating profit 309 17 326 284 13 297
------------ --------------------- ------------ ------------ ------------ -------------------
Finance income 1 29 30 5 - 5
Finance expense (25) (12) (37) (24) (4) (28)
Profit before tax 285 34 319 265 9 274
------------ --------------------- ------------ ------------ ------------ -------------------
Tax (62) (9) (71) (60) (2) (62)
Profit for the
half-year 223 25 248 205 7 212
============ ===================== ============ ============ ============ ===================
Attributable to:
Non-controlling
interests - - - - - -
Equity holders of
the parent 223 25 248 205 7 212
------------ --------------------- ------------ ------------ ------------ -------------------
223 25 248 205 7 212
============ ===================== ============ ============ ============ ===================
2. Unallocated corporate expenses mainly comprise employee costs
and third-party professional fees
5. Exceptional items and other adjustments
Exceptional items and other adjustments are disclosed separately
in the condensed consolidated income statement to assist in the
understanding of the Group's core performance.
H1 2021 Generics Injectables Branded Others Unallocated Total
$m $m $m $m $m $m
------------ -------------- ----------- ------- --------------- -------------
Exceptional - - - - - -
Items
------------ -------------- ----------- ------- --------------- -------------
Other
adjustments
Impairment
reversal of
product
related Other operating
intangibles (expense)/income 46 - - - - 46
Intangible
assets
amortisation
other than
software SG&A (12) (12) (5) - - (29)
Re measurement
of contingent
consideration Finance income - - - - 29 29
Unwinding and
remeasurement
of contingent
consideration
and other
financial
liability Finance expense - - - - (12) (12)
------------ -------------- ----------- ------- ---------------
Exceptional items and other
adjustments included in profit
before tax 34 (12) (5) - 17 34
------------ -------------- ----------- ------- --------------- -------------
Tax effect Tax (9)
-------------
Impact on profit for the half-year 25
=============
Other adjustments:
- $46 million impairment reversal in respect of generic Advair
Diskus(R) intangible asset as a result of launching the product
(Note 8) following FDA approval in April 2021 of an amendment
submitted to its Abbreviated New Drug Application in January
2021.
- Intangible assets amortisation other than software of $29 million.
- Remeasurement of contingent consideration finance income
represents the income resulting from the valuation of the
liabilities associated with the future contingent payments in
respect of contingent consideration recognised through business
combinations (Notes 15 and 17).
- Unwinding and remeasurement of contingent consideration and
other financial liability finance expense represents the expense
resulting from the unwinding and the valuation of the liabilities
associated with the future contingent payments in respect of
contingent consideration recognised through business combinations
and the financial liability in relation to the co-development
earnout payment agreement (Notes 15 and 17).
The tax effect represents the tax effect on pre-tax other
adjustments which is calculated based on the applicable tax rate in
each jurisdiction.
H1 2020 Generics Injectables Branded Others Unallocated Total
$m $m $m $m $m $m
------------ -------------- ----------- -------- ---------------- -------------
Exceptional
Items
MENA severance
and
restructuring
costs SG&A - - (1) - - (1)
Jordan Other
warehouse operating
fire incident (expense)/income - - 1 - - 1
Exceptional - - - - - -
Items
------------ -------------- ----------- -------- ---------------- -------------
Other
adjustments
Impairment
reversal of
product Other
related operating
intangibles (expense)/income 34 - - - - 34
Intangible
assets
amortisation
other than
software SG&A (4) (12) (5) - - (21)
Unwinding and
remeasurement
of contingent
consideration
and other
financial Finance
liability expense - - - - (4) (4)
------------ -------------- ----------- -------- ----------------
Exceptional items and other
adjustments included in profit
before tax 30 (12) (5) - (4) 9
------------ -------------- ----------- -------- ---------------- -------------
Tax effect Tax (2)
-------------
Impact on profit for the half-year 7
=============
In H1 2020, exceptional items related to the following:
- Jordan warehouse fire incident: In H1 2020, Hikma received $1
million of insurance compensation related to a fire incident which
took place in 2019 at one of Hikma's Jordan facilities. This is
included in other operating (expenses)/income. Since the incident
occurred, Hikma received $15 million for insurance
compensation.
- MENA severance and restructuring costs: of $1 million related
to one-off organisational restructuring in MENA that started in
2019 and are included in selling, general and administrative
expenses (SG&A). The total spent on this since 2019 is $10
million.
In H1 2020 other adjustments related to the following :
- $34 million impairment reversal in respect of specific product
related intangibles in the Generics segment which reflects a
better-than-expected performance of certain marketed products (Note
8). This is included in other operating (expenses)/income.
- Intangible assets amortisation other than software of $21 million.
- Remeasurement of contingent consideration and other financial
liability represents the net difference resulting from the
valuation and unwinding of the liabilities associated with the
future contingent payments in respect of contingent consideration
recognised through business combinations and the financial
liability in relation to the co-development earnout payment
agreement (Notes 15 and 17).
The tax effect represents the tax effect on pre-tax exceptional
items and other adjustments which is calculated based on the
applicable tax rate in each jurisdiction.
6. Tax
The Group incurred a tax expense of $71 million (H1 2020: $62
million). The reported effective tax rate for H1 2021 is 22.3% (H1
2020: 22.6%), representing the best estimate of the average annual
effective tax rate expected for the full year on a legal entity
basis, applied to the pre-tax income for H1 2021 and adjusted for
the tax effect of any discrete items recorded in the same
period.
The application of tax law and practice is subject to some
uncertainty and amounts are provided where the likelihood of a cash
outflow is probable.
The effective tax rate is higher than the UK tax rate of 19%
which is primarily driven by the earnings mix being concentrated in
jurisdictions with higher tax rates such as the United States.
7. Dividends
H1 2021 H1 2020
$m $m
------------ ------------
(Unaudited) (Unaudited)
------------ ------------
Amounts recognised as distributions to equity holders in the period:
Final dividend for the year ended 31 December 2020 of 34 cents (2019: 30 cents) per
share 78 73
78 73
============ ============
The proposed interim dividend for the H1 2021 is 18 cents (H1
2020: 16 cents) per share.
The proposed interim dividend will be paid on 20 September 2021
to eligible shareholders on the register at the close of business
on 20 August 2021.
Based on the number of shares in issue at 30 June 2021 of
231,432,099 the unrecognised liability is $42 million.
8. Other intangible assets
During the period, the Group performed a review of its CGUs and
other intangibles assets, considering whether any indicators of
impairment or impairment reversal existed at 30 June 2021 in the
context of IAS 36.
As per the Group policy, the launching of generic Advair
Diskus(R) following FDA approval in April 2021 of an amendment
submitted to its Abbreviated New Drug Application in January 2021
was considered as an indicator for an impairment reversal
assessment. As a result the Group evaluated the generic Advair
Diskus(R) CGU recoverable value based on a probability weighted
average of different possible cash flows which resulted in a
reversal of impairment of $46 million. Details relating to the
recoverable value evaluation are as follows:
Key assumptions
Probability weighted average of different possible sales growth rates,
informed by conversion
rates from the branded products and competitor entries
Profit margins and profit margin growth rates
Useful life
Discount rates
---------------------------------------- ---------------------------------------------------------------------
Period of specific projected cash flows 5 years
---------------------------------------- ---------------------------------------------------------------------
Useful life 11 years
---------------------------------------- ---------------------------------------------------------------------
Post-tax discount rate 7.10%
----------------------------------------- ---------------------------------------------------------------------
The Group performed sensitivity analysis over the valuation of
the generic Advair Diskus(R) CGU assuming a 10% reduction/increase
in the projected cash flows, which resulted in a reduction/increase
of $16 million on the CGU value.
No other indicators of impairment or impairment reversal were
identified.
Other intangible assets increased by $33 million (H1 2020:
increased by $18 million) during the period. This was as a result
of the $46 million impairment reversal (H1 2020: net impairment
reversal of $33 million) and $21 million of additions (H1 2020: $11
million), offset by amortisation of $34 million (H1 2020: $26
million).
9. Financial and other non-current assets
30 June 31 December
2021 2020
$m $m
------------ ------------
(Unaudited) (Audited)
------------ ------------
Investments at FVTOCI 26 25
Others 14 14
------------ ------------
40 39
============ ============
Investments at FVTOCI include investments in nine venture-backed
start-up companies through the Group's venture capital arm, Hikma
International Ventures and Developments LLC and Hikma Ventures
Limited. During H1 2021, the venture arm increased investment in
two existing ventures, invested in a new company and sold one
investment. These investments are unlisted shares without readily
determinable fair values that fall under level 3 valuation (Note
19), their value is measured at cost minus any impairment, and
adjusted for observable price changes in orderly transactions for
the identical or a similar investment from the same issuer.
Others mainly represent long term receivables and a sublease
arrangement in US.
10. Inventories
During H1 2021, the Group wrote down $29 million (H1 2020: $25
million) of inventories. This expense is included in cost of sales
in the condensed consolidated interim income statement.
11. Trade and other receivables
30 June 31 December
2021 2020
$m $m
------------------ ------------------
(Unaudited) (Audited)
------------------ ------------------
Trade receivables 693 662
Prepayments 78 58
VAT and sales tax recoverable 36 35
Employee advances 2 1
809 756
================== ==================
The fair value of receivables is estimated to be not
significantly different from the respective carrying amounts.
12. Cash and cash equivalents
30 June 31 December
2021 2020
$m $m
--------------- -------------------
(Unaudited) (Audited)
--------------- -------------------
Cash at banks and on hand 243 85
Time deposits 77 203
Money market deposits 2 35
322 323
=============== ===================
Cash and cash equivalents include highly liquid investments with
maturities of three months or less which are convertible to known
amounts of cash and are subject to insignificant risk of changes in
value.
13. Other current assets
30 June 31 December
2021 2020
$m $m
------------------- -------------------
(Unaudited) (Audited)
------------------- -------------------
Investment at FVTPL 24 24
Others 11 22
35 46
=================== ===================
Investments at FVTPL represents the agreement the Group entered
into with an asset management firm in 2015 to manage a $20 million
portfolio of underlying debt instruments. The investment comprises
a portfolio of assets that are managed by an asset manager and is
measured at fair value; any changes in fair value go through the
condensed consolidated income statement. These assets are
classified as level 1 valuation (Note 19) as they are based on
quoted prices in active markets.
Others balance at 30 June 2021, mainly represent compensation
due from suppliers in relation to inventory price adjustment. At 31
December 2020, the balance includes an insurance compensation
receivable of $10 million and revenue contract asset of $3
million.
14. Trade and other payables
30 June 31 December
2021 2020
$m $m
------------------ ------------------
(Unaudited) (Audited)
------------------ ------------------
Trade payables 226 279
Accrued expenses 145 175
Other payables 15 16
------------------ ------------------
386 470
================== ==================
The fair value of payables is estimated to be not significantly
different from the respective carrying amounts.
15. Other current liabilities
30 June 31 December
2021 2020
$m $m
------------------- -------------------
(Unaudited) (Audited)
------------------- -------------------
Contract liabilities 183 162
Co-development and earnout payment (Note 17 and 19) 1 2
Acquired contingent liability (Note 17) 15 18
Contingent consideration (Note 17 and 19) 14 13
Indirect rebates and other allowances 71 74
Others 17 21
301 290
=================== ===================
Contract liabilities: the Group allows customers to return
products within a specified period prior to and subsequent to the
expiration date. In addition, free goods are issued to customers as
sale incentives, reimbursement of agreed upon expenses incurred by
the customer or as compensation for expired or returned goods.
Indirect rebates and other allowances: mainly represents rebates
granted to healthcare authorities and other parties under
contractual arrangements with certain indirect customers.
16. Financial debts
Short-term financial debts
30 June 31 December
2021 2020
$m $m
------------ -------------------
(Unaudited) (Audited)
------------ -------------------
Bank overdrafts 8 3
Import and export financing(1) 94 67
Short-term loans 30 47
Current portion of long-term loans 40 41
172 158
============ ===================
1.Import and export financing represents short-term financing
for the ordinary trading activities of the Group.
Long-term financial debts
30 June 31 December
2021 2020
$m $m
-------------------- --------------------
(Unaudited) (Audited)
-------------------- --------------------
Long-term loans 224 242
Long-term borrowings (Eurobond) 492 491
Less: current portion of long-term loans (40) (41)
Long-term financial loans 676 692
==================== ====================
Breakdown by maturity:
Within one year 40 41
In the second year 53 48
In the third year 38 44
In the fourth year 35 36
In the fifth year 517 522
In the sixth year 22 21
Thereafter 11 21
716 733
==================== ====================
The loans are held at amortised cost.
Major arrangements entered into by the Group:
a) A syndicated revolving credit facility of $1,175 million was
entered into on 27 October 2015. From the $1,175 million, $175
million matured on 24 December 2019, $130 million matured in
January 2021 and the remaining $870 million was renewed until
December 2023. At 30 June 2021 the facility has an outstanding
balance of $nil (2020: $nil) and a $870 million unused available
limit (2020: $1,000 million). The facility can be used for general
corporate purposes
b) A ten-year $150 million loan from the International Finance
Corporation was entered into on 21 December 2017. There was full
utilisation of the loan in April 2020. Quarterly equal repayments
of the long-term loan has commenced on 15 March 2021 with
outstanding balance of $139 million (fair value of $138 million).
The loan was used for general corporate purposes. The facility
matures on 15 December 2027
c) Hikma issued a $500 million (carrying value of $492 million,
and fair value of $522 million) 3.25%, five-year Eurobond on 9 July
2020 with a rating of (BBB-/Ba1) which is due in July 2025. The
proceeds of the issuance were used for general corporate
purposes
d) An eight-year $200 million loan from the International
Finance Corporation and Managed Co-lending Portfolio program was
entered into on 26 October 2020. There was no utilisation of the
loan as of 30 June 2021. The facility matures on 15 September 2028
and can be used for general corporate purposes
Interbank Offered Rates (IBORs) Reform
As at 30 June 2021, approximately 4.3% ($41 million) of the
Group's utilised debt portfolio as well as $1,197 million of the
Group's unutilised debt facilities, have USD LIBOR as the benchmark
interest rate. The unutilised debt facilities relates mainly to the
Group's syndicated revolving credit facility of $870 million and
the IFC loan of $200 million. The Group has not identified any
other significant IBOR exposures that are expected to be impacted
by IBOR reform.
The Group is monitoring the market developments surrounding the
IBOR reform. To date the Group have identified the need to amend
the credit facilities, which reference USD LIBOR, in order that
they reference an alternative reference rate once USD LIBOR is
discontinued.
17. Other non-current liabilities
30 June 31 December
2021 2020
$m $m
------------ ------------
(Unaudited) (Audited)
------------ ------------
Contingent consideration (Note 15 and 19) 58 76
Acquired contingent liability (Note 15) 72 80
Co-development and earnout payment (Note 15 and 19) 3 3
Others 12 5
------------ ------------
145 164
============ ============
Contingent consideration and acquired contingent liability
represent contractual liabilities to make payments to third parties
in the form of milestone payments that depend on the achievement of
certain US FDA approval milestones, and royalty payments based on
future sales of certain products.
18. Cash generated from operating activities
H1 H1
2021 2020
$m (Unaudited) $m (Unaudited)
----------------- ------------------
Profit before tax 319 274
Adjustments for:
Depreciation, amortisation, impairment charges/reversal
and write-down of:
Property, plant and equipment 39 34
Intangible assets (12) (7)
Right-of-use of assets 5 4
Movement in provisions 1 1
Cost of equity-settled employee share scheme 16 14
Finance income (30) (5)
Interest and bank charges 37 27
Foreign exchange loss and net monetary hyperinflation
impact 16 4
Changes in working capital:
Change in trade and other receivables (63) 53
Change in other current assets 11 1
Change in inventories 11 (111)
Change in trade and other payables (60) (15)
Change in other current liabilities 22 55
Change in other non-current liabilities - (18)
Cash flows from operating activities 312 311
================= ==================
19. Fair value of financial assets and liabilities
The fair value of financial assets and liabilities is included
at the amount at which the instrument could be exchanged in a
current transaction between willing parties, other than in a forced
or liquidation sale.
The following financial assets/liabilities are presented at
their carrying values which approximates to their fair values:
-- Cash at bank and on hand, time deposits and collateralised
and restricted cash - due to the short-term maturities of these
financial instruments and given that generally they have negligible
credit risk, management considers their carrying amounts to be not
significantly different from their fair values
-- Short-term loans and overdrafts approximates to their fair
values because of the short maturity of these instruments
-- Long-term loans-loans with variable rates are re-priced in
response to any changes in market rates and so management considers
their carrying values to be not significantly different from their
fair values
-- Loans with fixed rates relate mainly to:
- $500 million (carrying value of $492 million, and fair value
of $522 million) Eurobond accounted for at amortised cost. The fair
value is determined with reference to a quoted price in an active
market as at the balance sheet date (Note 16).
- A ten-year $150 million loan from the International Finance
Corporation with outstanding balance of $139 million (fair value of
$138 million). Fair value is estimated by discounting future cash
flows using the current rates at which similar loans would be made
to borrowers with similar credit ratings and for the same remaining
maturities of such loans.
-- Receivables and payables - the fair values of receivables and
payables are estimated to not be significantly different from the
respective carrying amounts
Management classifies items that are recognised at fair value
based on the level of the inputs used in their fair value
determinations as described below:
-- Level 1 : Quoted prices in active markets for identical assets or liabilities
-- Level 2 : Inputs that are observable for the asset or liability
-- Level 3 : Inputs that are not based on observable market data
Financial assets and liabilities that fall under Level 1
are:
-- Investments at FVTPL which amounted to $24 million (Note 13)
-- Money market deposits (Note 12)
Financial assets and liabilities that fall under Level 3
are:
-- Co-development and earnout payment liabilities (Notes 15 and 17)
-- Contingent consideration liability resulting from the
acquisition of the Columbus business (Note 15 and 17)
-- Investment at FVTOCI (Note 9)
The following table presents the changes in Level 3 items for H1
2021 and the year ended 31 December 2020:
Financial asset Financial liability
$m $m
Balance at 1 January 2020 18 178
------------------------------------------------------------ ---------------- --------------------
Settled - (61)
Remeasurement of contingent consideration and other
financial liability recognised in finance income - (38)
Unwinding of contingent consideration and other financial
liability recognised in finance expense - 15
Additions 5 -
Change in investments at FVTOCI 2 -
Balance at 31 December 2020 25 94
============================================================ ---------------- --------------------
Settled - (1)
Remeasurement of contingent consideration recognised
in finance income - (29)
Unwinding and remeasurement of contingent consideration
and other financial liability recognised in finance
expense - 12
Additions 1 -
Balance at 30 June 2021 26 76
============================================================ ================ ====================
The critical areas of estimates in relation to the contingent
consideration are the probabilities assigned to reaching the
success-based milestones and management's estimate of future sales
(Note 15 and 17).
If the future sales were 5% higher or lower, the fair value of
the contingent consideration will increase/decrease by $5 million
(Note 15 and 17).
If the probability assigned to reaching the success-based
milestones were 5% higher or lower, the fair value of the
contingent consideration will increase/decrease by $1 million (Note
15 and 17).
20. Contingent liabilities
Guarantees and letters of credit
A contingent liability existed at the balance sheet date in
respect of external guarantees and letters of credit totalling $39
million (31 December 2020: $41 million) arising in the normal
course of business. No provision for these liabilities has been
made in these financial statements.
A contingent liability existed at the balance sheet date for a
potential stamp duty obligation of $7 million (31 December 2020: $8
million) that may arise for a repayment of a loan by intercompany
guarantors. It's not probable that the repayment will be made by
the intercompany guarantors.
Legal proceedings
The Group is involved in a number of legal proceedings in the
ordinary course of its business, including actual or threatened
litigation and actual or potential government investigations
relating to employment matters, product liability, commercial
disputes, pricing, sales and marketing practices, infringement of
IP rights, the validity of certain patents and competition
laws.
Most of the claims involve highly complex issues. Often these
issues are subject to substantial uncertainties and, therefore, the
probability of a loss, if any, being sustained and/or an estimate
of the amount of any loss is difficult to ascertain. It is the
Group's policy to accrue for amounts related to these legal matters
if it is probable that a liability has been incurred and an amount
is reasonably estimable.
- In 2018, the Group received a civil investigative demand from
the US Department of Justice requesting information related to
products, pricing and related communications. In 2017, the Group
received a subpoena from a US state attorney general and a subpoena
from the US Department of Justice. Hikma denies having engaged in
any conduct that would give rise to liability with respect to these
demands but is cooperating with all such demands. At this point,
management does not believe sufficient evidence exists to make any
provision for this.
- Starting in 2016, several complaints have been filed in the
United States on behalf of putative classes of direct and indirect
purchasers of generic drug products, as well as several individual
direct purchasers opt-out plaintiffs (including two products).
These complaints, which allege that the defendants engaged in
conspiracies to fix, increase, maintain and/or stabilise the prices
of the generic drug products named, have been brought against Hikma
and various other defendants. The plaintiffs generally seek damages
and injunctive relief under federal antitrust law and damages under
various state laws. Hikma denies having engaged in conduct that
would give rise to liability with respect to these civil suits and
is vigorously pursuing defense of these cases. At this point,
management does not believe sufficient evidence exists to make any
provision for this.
- Starting in June 2020, several complaints have been filed in
the United States on behalf of putative classes of direct and
indirect purchasers of Xyrem(R) against Hikma and other defendants.
These complaints allege that Jazz Pharmaceuticals PLC and its
subsidiaries entered into unlawful reverse payment agreements with
each of the defendants, including Hikma, in settling patent
infringement litigation over Xyrem(R). The plaintiffs in these
lawsuits seek treble damages and a permanent injunction. Hikma
denies having engaged in conduct that would give rise to liability
with respect to these lawsuits and is vigorously pursuing defence
of these cases. At this point, management does not believe
sufficient evidence exists to make any provision for this.
- Numerous complaints have been filed with respect to Hikma's
sales and distribution of opioid products. Those complaints now
total approximately 677 in number. These lawsuits have been filed
against distributors, branded pharmaceuticals manufacturers,
pharmacies, hospitals, generic pharmaceuticals manufacturers,
individuals, and other defendants by a number of cities, counties,
states, other governmental agencies and private plaintiffs in both
state and federal courts. Most of the federal cases have been
consolidated into a multidistrict litigation in the Northern
District of Ohio. These cases assert in general that the defendants
allegedly engaged in improper marketing and distribution of opioids
and that defendants failed to develop and implement systems
sufficient to identify suspicious orders of opioid products and
prevent the abuse and diversion of such products. Plaintiffs seek a
variety of remedies, including restitution, civil penalties,
disgorgement of profits, treble damages, attorneys' fees and
injunctive relief. Hikma denies having engaged in conduct that
would give rise to liability with respect to these civil suits and
is vigorously pursuing defense of these cases. At this point,
management does not believe sufficient evidence exists to make any
provision for this.
- In October 2020, Hikma received a voluntary request for
information from the US Federal Trade Commission requesting
information related to its investigation into whether Amarin
Pharma, Inc. has engaged in, or is engaging in, anticompetitive
practices or unfair methods of competition relating to the drug
Vascepa(R). In October 2020, Hikma also received a subpoena duces
tecum from the State of New York, Office of the Attorney General,
seeking information relevant and material to an investigation
related to Amarin Pharma, Inc. Hikma is cooperating with all such
demands.
Tax
In April 2019, the European Commission released its decision
that certain tax exemptions offered by the UK authorities could
constitute State Aid and where this is the case, the relevant tax
will need to be paid to the UK tax authorities. The UK Government
has subsequently appealed against this decision. In common with
other UK headquartered international companies whose arrangements
were in line with current UK CFC legislation, Hikma could have been
affected by the outcome of this decision and had estimated the
maximum potential liability to be approximately $2.4 million.
Following discussions and correspondence with HMRC on whether
certain tax exemptions offered by the UK authorities could
constitute State Aid pursuant to the April 2019 European Union
decision, the UK tax authority has confirmed that Hikma is not a
beneficiary of State Aid in accordance with the European
Commission's decision and the UK's Controlled Foreign Company
legislation. Hikma is awaiting formal letters of confirmation. As
HMRC will not seek to impose charging notices in relation to State
Aid, Hikma believes this no longer represents a contingent
liability.
21. Related party balances and transactions
No significant transactions between the Group and its associates
and other related parties were undertaken during the half-year. Any
transactions between the Company and its subsidiaries have been
eliminated on consolidation.
, the news service of the London Stock Exchange. RNS is approved by
the Financial Conduct Authority to act as a Primary Information
Provider in the United Kingdom. Terms and conditions relating to
the use and distribution of this information may apply. For further
information, please contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
IR BIGDIXGGDGBU
(END) Dow Jones Newswires
August 06, 2021 02:00 ET (06:00 GMT)
Hikma Pharmaceuticals (LSE:HIK)
Historical Stock Chart
From Mar 2024 to Apr 2024
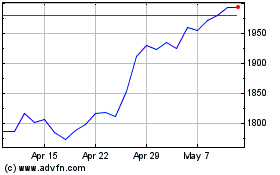
Hikma Pharmaceuticals (LSE:HIK)
Historical Stock Chart
From Apr 2023 to Apr 2024