Blueprint Medicines Gets FDA Approval for Ayvakit
May 22 2023 - 4:27PM
Dow Jones News
By Denny Jacob
Blueprint Medicines on Monday said the Food and Drug
Administration approved ayvakit as a treatment for adults with
indolent systemic mastocytosis, a hematologic disorder that can
have debilitating symptoms across multiple organs.
The precision therapy company said ayvakit has been FDA approved
for the treatment of advanced systemic mastocytosis since June
2021.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
May 22, 2023 17:12 ET (21:12 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
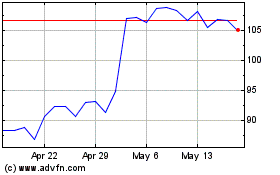
Blueprint Medicines (NASDAQ:BPMC)
Historical Stock Chart
From Jun 2024 to Jul 2024
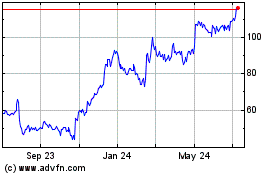
Blueprint Medicines (NASDAQ:BPMC)
Historical Stock Chart
From Jul 2023 to Jul 2024