| PROSPECTUS SUPPLEMENT |
Filed
Pursuant to Rule 424(b)(5) |
| (To Prospectus
dated May 1, 2023) |
Registration
No. 333-271385 |

50,500,000 Ordinary Shares Represented by 505,000
American Depositary Shares
Pre-Funded Warrants to Purchase up to 82,833,400
Ordinary Shares Represented by 828,334 American Depositary Shares
Up to 82,833,400 Ordinary Shares Represented
by 828,334 American Depositary Shares Underlying the Pre-Funded Warrants
We
are offering to an institutional investor pursuant to this prospectus supplement and the accompanying prospectus (i) 50,500,000
ordinary shares represented by 505,000 American Depositary Shares, or ADSs, at an offering price of $2.85 (or €2.53) per ADS, and
(ii) pre-funded warrants to purchase up to 82,833,400 ordinary shares represented by 828,334 ADSs, at an offering price of $2.84
(or €2.52) per pre-funded warrant. Each ADS represents the right to receive 100 ordinary shares. See “Description of American
Depositary Shares” and “Description of Share Capital” in the accompanying prospectus for more information. Each of
the pre-funded warrants will be exercisable for one ADS. The pre-funded warrants will have an exercise price of $0.01 (or €0.01)
per ADS, will be immediately exercisable and may be exercised at any time upon issuance and will expire 10 years following the date of
issuance. We are also offering the ordinary shares represented by ADSs issuable from time to time upon exercise of the pre-funded warrants
being offered by this prospectus supplement and accompanying prospectus.
In a concurrent private placement, we are also
issuing to the investor, for each ADS or pre-funded warrant issued in this offering, an unregistered warrant, or ordinary warrant, to
purchase up to 100 ordinary shares represented by one ADS at an exercise price of $3.00 (or €2.66) per ADS, resulting in our issuance
of ordinary warrants to purchase up to an aggregate of 133,333,400 ordinary shares represented by 1,333,334 ADSs. The ordinary warrants
are exercisable at any time upon issuance and will expire three years following the date of issuance. In the concurrent private placement,
the unregistered ordinary warrants, the unregistered ADSs issuable upon the exercise of the ordinary warrants and the ordinary shares
represented by such ADSs are being offered pursuant to the exemption provided in Section 4(a)(2) under the Securities Act of
1933, as amended, or the Securities Act, and/or Regulation D promulgated thereunder, and they are not being offered pursuant to this
prospectus supplement and the accompanying prospectus.
The
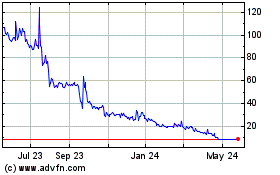
ADSs are listed on the Nasdaq Capital Market under the symbol “BPTS.” On July 18, 2023, the closing price of the ADSs
on Nasdaq was $3.11 per ADS. Our ordinary shares also trade on the Euronext Growth Paris, or Euronext, under the symbol “ALBPS.”
On July 18, 2023, the last reported sale price of our ordinary shares on Euronext was €0.0253 (or $0.0285) per share (translated
solely for convenience into dollars at an exchange rate of €1.00-$1.1255, the reference exchange rate of the European Central Bank
on July 18, 2023). There is no established trading market for the registered pre-funded warrants or the unregistered ordinary warrants,
and we do not intend to list the pre-funded warrants or ordinary warrants on any securities exchange or nationally recognized trading
system.
The aggregate market value of
our outstanding voting and non-voting common equity held by non-affiliates as of July 18, 2023, based on the closing price of the
ADSs on Nasdaq on July 18, 2023, as calculated in accordance with General Instruction I.B.5. of Form F-3, was approximately
$13.8 million. During the prior 12 calendar month period that ends on, and includes, the date of this prospectus supplement (excluding
this offering), we have not sold any securities pursuant to General Instruction I.B.5 of Form F-3.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page S-10 of this prospectus
supplement and on page 8 of the accompanying prospectus for a discussion of certain factors you should consider before investing
in our securities.
Neither the U.S. Securities
and Exchange Commission nor any state or other foreign securities commission has approved or disapproved of these securities or determined
if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
We have retained H.C. Wainwright &
Co., LLC, or the placement agent, to act as our exclusive placement agent in connection with the offering. The placement agent has agreed
to use its reasonable best efforts to sell the securities offered by this prospectus supplement and the accompanying prospectus, but
the placement agent has no obligation to purchase or sell any of such securities or to arrange for the purchase or sale of any specific
number or dollar amount of such securities. We have agreed to pay the placement agent fees set forth in the table below.
| |
|
Per ADS |
|
|
Per Pre-Funded
Warrant |
|
|
Total(2) |
|
| Offering price |
|
$ |
2.85 |
|
|
$ |
2.85 |
|
|
$ |
3,800,002 |
|
| Placement agent’s fees(1) |
|
$ |
0.19 |
|
|
$ |
0.19 |
|
|
$ |
266,000 |
|
| Proceeds, before expenses, to us |
|
$ |
2.66 |
|
|
$ |
2.66 |
|
|
$ |
3,534,002 |
|
| (1) |
We will pay the placement agent
a cash fee equal to 7.0% of the aggregate gross proceeds from this offering. We have also agreed to pay the placement agent a management
fee equal to 1.0% of the aggregate gross proceeds and certain of its expenses, as well as certain closing costs, including reimbursement
of the out-of-pocket costs of the escrow agent or clearing agent, as applicable. Please refer to the section entitled “Plan
of Distribution” on page S-28 of this prospectus supplement for additional information with respect to the compensation
payable to the placement agent. |
| |
|
| (2) |
The amount of the offering proceeds to us, including the net proceeds,
presented in this table gives effect to the exercise of the pre-funded warrants and does not give effect to the sale or exercise,
if any, of the unregistered ordinary warrants. |
We expect to deliver the securities
being offered pursuant to this prospectus supplement and accompanying prospectus supplement on or about July 21, 2023, subject to
satisfaction of customary closing conditions.
H.C. Wainwright & Co.
The
date of this prospectus supplement is July 18, 2023.
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
ABOUT THIS PROSPECTUS
SUPPLEMENT
This
prospectus supplement and the accompanying prospectus are part of an effective “shelf” registration statement that we filed
with the U.S. Securities and Exchange Commission, or the SEC, on April 21, 2023 and which was declared effective by the SEC on May 1,
2023. This prospectus supplement provides you with the specific details regarding this offering. The accompanying prospectus provides
you with more general information, some of which does not apply to this offering. You should rely only on the information contained or
incorporated by reference in this prospectus supplement, the accompanying prospectus or any free writing prospectus that we have authorized
for use in connection with this offering. To the extent information in this prospectus supplement is inconsistent with the accompanying
prospectus or any of the documents incorporated by reference into this prospectus supplement and the accompanying prospectus, you should
rely on this prospectus supplement. You should read and consider the information in both this prospectus supplement and the accompanying
prospectus together with the additional information described under the headings “Where You Can Find More Information” and
“Incorporation by Reference.”
Unless otherwise indicated,
all references in this prospectus supplement or the accompanying prospectus to “Biophytis,” “the company,” “our
company,” “we,” “us” and “our” refer to Biophytis S.A. and our consolidated subsidiaries.
This
prospectus supplement and the accompanying prospectus contain summaries of certain provisions contained in some of the documents described
herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety
by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by
reference as exhibits to the registration statement of which this prospectus supplement is a part, and you may obtain copies of those
documents as described below under the heading “Where You Can Find More Information.”
Neither
we, the placement agent, nor any agent, underwriter or dealer has authorized any person to give any information or to make any representation
other than those contained or incorporated by reference in this prospectus supplement, the accompanying prospectus or any related free
writing prospectus prepared by or on behalf of us or to which we have referred you. This prospectus supplement, the accompanying prospectus
or any related free writing prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other
than the registered securities to which they relate, nor do this prospectus supplement, the accompanying prospectus or any related free
writing prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to
whom it is unlawful to make such offer or solicitation in such jurisdiction. If anyone provides you with different or inconsistent information,
you should not rely on it. We and the placement agent take no responsibility for, and can provide no assurance as to the reliability
of, any other information that others may give you.
The
information contained in this prospectus supplement, the accompanying prospectus and any accompanying free writing prospectus is accurate
only as of the date of this prospectus supplement, the accompanying prospectus and any such accompanying free writing prospectus, regardless
of the time of delivery of this prospectus supplement, the accompanying prospectus, any such accompanying free writing prospectus or
of any sale of our securities. You should not assume that the information contained in this prospectus supplement, the accompanying prospectus
or any related free writing prospectus is accurate on any date subsequent to the date set forth on the front of the document or that
any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference,
even though this prospectus supplement, the accompanying prospectus or any related free writing prospectus is delivered, or securities
are sold, on a later date. Our business, financial condition, results of operations and prospects may have changed since those dates.
You should read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement
and the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection with this offering, in
their entirety before making an investment decision.
On March 30, 2023, we effected
a change in the ratio of the ADSs to ordinary shares from one ADS representing 10 ordinary shares to a new ratio of one ADS representing
100 ordinary shares. For ADS holders, the ratio change had the same effect as a one-for-ten reverse ADS split. All ADS and related warrant
information presented in this prospectus have been retroactively adjusted to reflect the reduced number of ADSs and the increase in the
ADS price which resulted from this action. Unless otherwise indicated, in this prospectus, fractional ADSs have been rounded to the nearest
whole number.
For investors outside the
United States: We have not done anything that would permit the offering or possession or distribution of this prospectus supplement and
the accompanying prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside
the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about,
and observe any restrictions relating to, the offering of the securities described herein and the distribution of this prospectus supplement
and the accompanying prospectus outside the United States.
We are incorporated in France,
and a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the SEC, we
are currently eligible for treatment as a “foreign private issuer.” As a foreign private issuer, we will not be required
to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic registrants whose securities
are registered under the Securities Exchange Act of 1934, as amended.
Presentation of Financial Information
Our audited consolidated
financial statements have been prepared in accordance with International Financial Reporting Standards as issued by the International
Accounting Standards Board. Our consolidated financial statements are presented in euros, and unless otherwise specified, all monetary
amounts are in euros. For the convenience of the reader, we have translated some of our financial information into U.S. dollars. Unless
otherwise indicated, these translations were made at the reference exchange rate of the European Central Bank (€1.00 = $1.0666 at
December 31, 2022 and €1.00 = $1.1255 at July 18, 2023). Such U.S. dollar amounts are not necessarily indicative of the
amounts of U.S. dollars that could actually have been purchased upon exchange of euros at the dates indicated. None of the consolidated
financial statements incorporated by reference into this prospectus supplement were prepared in accordance with generally accepted accounting
principles in the United States.
All references in this
prospectus supplement and the accompanying prospectus to “$,” “US$,” “U.S.$,” “U.S. dollars,”
“dollars” and “USD” mean U.S. dollars and all references to “€” and “euros” mean
euros, unless otherwise noted.
Trademarks
This
prospectus supplement may contain references to our trademarks and to trademarks belonging to other entities. Solely for convenience,
trademarks and trade names referred to in this annual report, including logos, artwork and other visual displays, may appear without
the ® or TM symbols, but such references are not intended to indicate,
in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor
to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply
a relationship with, or endorsement or sponsorship of us by, any other company.
SPECIAL NOTE REGARDING
FORWARD LOOKING STATEMENTS
This prospectus includes certain
forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”),
and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), concerning our business, operations
and financial performance and condition as well as our plans, objectives and expectations for our business operations and financial performance
and condition. Any statements that are not of historical facts may be deemed to be forward-looking statements. You can identify these
forward-looking statements by words such as “believes,” “estimates,” “anticipates,” “expects,”
“plans,” “intends,” “may,” “could,” “might,” “will,” “should,”
“aims,” or other similar expressions that convey uncertainty of future events or outcomes. Forward-looking statements
include, but are not limited to, statements about:
| |
· |
the timing, progress and results
of clinical trials for our drug candidates, including statements regarding the timing of initiation and completion of clinical trials,
dosing of subjects and the period during which the results of the clinical trials will become available; |
| |
· |
the potential impact of COVID-19
on our clinical trials and our operations generally; |
| |
· |
the timing, scope or likelihood
of regulatory filings and approvals for our drug candidates; |
| |
· |
our ability to successfully commercialize
our drug candidates; |
| |
· |
potential benefits of the clinical
development and commercial experience of our management team; |
| |
· |
our ability to effectively market
any drug candidates that receive regulatory approval, emergency use authorization, or conditional marketing authorization on our
own or through third parties; |
| |
· |
our commercialization, marketing
and manufacturing capabilities and strategy; |
| |
· |
our expectation regarding the safety
and efficacy of our drug candidates; |
| |
· |
the potential clinical utility and
benefits of our drug candidates; |
| |
· |
our ability to advance our drug
candidates through various stages of development, especially through pivotal safety and efficacy trials; |
| |
· |
the likelihood of success and difficulty
in ensuring success of clinical investigations; |
| |
· |
our estimates regarding the potential
market opportunity for our drug candidates; |
| |
· |
developments and projections relating
to our competitors or our industry; |
| |
· |
our ability to become profitable; |
| |
· |
our estimates regarding expenses,
future revenue, capital requirements and needs for additional financing; |
| |
· |
our ability to secure additional
financing when needed on acceptable terms; |
| |
· |
the impact of government laws and
regulations in the United States, France and foreign countries; |
| |
· |
the implementation of our business
model, strategic plans for our business, drug candidates and technology; |
| |
· |
our intellectual property position; |
| |
· |
our ability to rely on orphan drug
designation for market exclusivity; |
| |
· |
our ability to attract or retain
key employees, advisors or consultants; and |
| |
· |
whether we are classified as a passive
foreign investment company for current and future periods. |
By their nature, forward-looking
statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on economic
circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe
that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that forward-looking
statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that are in
some cases beyond our control. All of our forward-looking statements are subject to risks and uncertainties that may cause our actual
results to differ materially from our expectations.
Any forward-looking statements
that we make in this prospectus speak only as of the date of such statement, and we undertake no obligation to update such statements
to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events. Comparisons
of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless
expressed as such, and should only be viewed as historical data. You should, however, review the factors and risks we describe in the
reports we will file from time to time with the SEC after the date of this prospectus. See “Where You Can Find More Information.”
You
should also read carefully the factors described in the “Risk Factors” section of this prospectus, in “Item 3. Key
Information—D. Risk Factors” in our Annual Report on Form 20-F for the year ended December 31, 2022,
as amended, and in the other documents that we file with the SEC after the date of this prospectus that are incorporated by reference
into this prospectus to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements.
As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate.
Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties
in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person
that we will achieve our objectives and plans in any specified timeframe, or at all.
PROSPECTUS SUPPLEMENT
SUMMARY
This summary highlights selected
information contained elsewhere in this prospectus supplement and accompanying prospectus that we consider important. This summary does
not contain all of the information you should consider before investing in our securities. You should read this summary together with
the entire prospectus supplement and accompanying prospectus, including the risks we describe under “Risk Factors” and our
consolidated financial statements and the related notes before making an investment in our securities.
Overview
We are a clinical-stage biotechnology
company focused on the development of therapeutics that are aimed at slowing the degenerative processes associated with aging and improving
functional outcomes for patients suffering from age-related diseases, including severe respiratory failure in patients suffering from
COVID-19. Our goal is to become a leader in the emerging field of aging science by delivering life-changing therapies to the growing
number of patients in need. To accomplish this goal, we have assembled an experienced and skilled group of industry professionals, scientists,
clinicians and key opinion leaders from leading industry and academic institutions around the world.
A number of degenerative diseases
associated with aging have been characterized in the last century, including sarcopenia and age-related macular degeneration (AMD). The
pathophysiology of these and many other age-related diseases is not yet well understood, and effective treatment options are lacking.
The global population of people over the age of 60 is expected to double from approximately 962 million in 2017 to 2.1 billion
by 2050, according to estimates from the United Nations’ World Population Prospects: the 2017 Revision. We believe that the need
for effective therapeutics for age-related diseases will continue to grow throughout the 21st century. In addition, healthcare
costs, including costs associated with treatments and long-term care for age-related diseases associated with this demographic shift,
are expected to rise proportionally, as effective treatment options are currently lacking. We believe that developing treatments to slow
disease progression and reduce the risk of severe disability associated with age-related diseases is of the utmost importance.
As we age, our physical, respiratory,
visual and cognitive performances gradually decline due, in part, to the cumulative deleterious effect of multiple biological and environmental
stresses, including current and emerging viral infections, to which we are exposed during our lifetime. The functional decline can be
much faster in some individuals as a consequence of, among other things, the degenerative processes affecting specific cells, tissues
and organs. Through evolution, cells, tissues and organisms have developed natural means or pathways to counteract and balance the effects
of the many stresses they face. This natural ability to compensate for stress and remain functional, called biological resilience, degrades
over time. The decline in biological resilience contributes to the acceleration of these degenerative processes and the impairment of
functional performances, which, in turn, can lead to severe disability, reduced health-span and ultimately death. This occurs as we age,
but can occur at a younger age, when genetic mutations exist, or in the case of infection and inflammation.
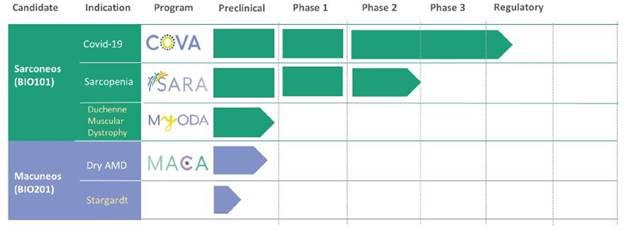
Our lead drug candidate, Sarconeos
(BIO101), is a plant-derived pharmaceutical-grade purified 20-hydroxyecdysone that is an orally administered small molecule.
The initial indication we are
seeking approval for is sarcopenia, an age-related degeneration of skeletal muscle, which is characterized by a loss of muscle mass,
strength and function in elderly people (adults 65 years of age and older) leading to reduced mobility, or mobility disability, and increased
risk of adverse health events and hospitalization, and potential death resulting from falls, fractures, and physical disability. There
is currently no approved medication for sarcopenia, which is present in the elderly (greater than 65 years old) with an estimated prevalence
range between six to 22% worldwide.
Sarconeos (BIO101) is also being
developed to treat patients with severe respiratory manifestations of COVID-19. Our therapeutic approach is aimed at targeting and activating
key biological resilience pathways that can protect against and counteract the effects of the multiple biological and environmental stresses,
including inflammatory, oxidative, metabolic and viral stresses that lead to age-related diseases. We have conducted the COVA study,
a global, multicenter, double-blind, placebo-controlled, group-sequential, and adaptive two-part Phase 2-3 study, in patients with SARS-CoV-2
pneumonia. Final results from this study were released on February 2, 2023. The study met its pre-defined primary endpoint demonstrating
a statistically significant difference between Sarconeos (BIO101) and placebo in the proportion of patients with respiratory failure
or early death at day 28, representing a relative reduction of risk of 44% (p=0.043, Cochran-Mantel-Haenszel test). Moreover, the analysis
of time to respiratory failure or early death had shown significant differences over 28 days in the Kaplan Meier curves for Sarconeos
(BIO101) versus placebo (p=0.022). The pre-specified analysis of time to death over the complete follow-up period over 90 days showed
that mortality rate with Sarconeos (BIO101) was reduced compared to placebo in the ITT population (p=0.083) and in the PP population
(p=0.038).
Most people infected with the
COVID-19 virus and its variants experience mild to moderate respiratory illness and recover without requiring special treatment. Older
people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease and cancer are
more likely to develop serious illness and to be at risk of respiratory failure. Based on the positive data from our COVA Phase 2-3 study,
we initiated the Early Access Program regulatory path in France in March 2023. We intend to renew the expanded access program to
treat hospitalized patients with severe COVID-19 symptoms that are mechanically ventilated with Sarconeos (BIO101) in Brazil as we did
initially received approval for such a program in January 2022. We also continue to prepare conditional marketing authorization
applications in Europe and in the US due to the health emergency.
We are also developing Sarconeos
(BIO101) for Duchenne muscular dystrophy, or DMD, a rare genetic neuromuscular disease in male children and young adults, which is characterized
by an accelerated degeneration of muscle and is responsible for a loss of mobility, respiratory failure and cardiomyopathy, leading to
premature death. There is currently no cure and limited treatment options for DMD, which affects approximately 2.8 out of 100,000 people
worldwide (approximately 20,000 new cases annually worldwide), based on our estimates from publicly available information.
Our second drug candidate, Macuneos
(BIO201), is an orally administered small molecule in development for the treatment of retinopathies. It is a plant-derived pharmaceutical-grade
purified norbixin. We have completed preclinical cellular and animal studies of Macuneos (BIO201) for the treatment of retinopathies.
While we are still in the early stages of development, we believe that the results from our preclinical studies support continued investigation
into whether Macuneos (BIO201) may stimulate biological resilience and protect the retina against phototoxic damage that leads to vision
loss. The initial indication we plan to seek approval for is dry AMD, a common eye disorder among people over the age of 50 that affects
central vision, impairing functions such as reading, driving, and facial recognition, and has a major impact on quality of life and the
ability to live independently. There are currently no approved treatments for dry AMD. Based on our estimates from publicly available
information, AMD affects approximately 8.5% of the global population (ages 45 to 85) and is expected to increase over time as the population
ages.
We are also exploring Macuneos
(BIO201) as a potential treatment for Stargardt disease, which shares many of the characteristics of dry AMD. Stargardt disease is the
most common form of inherited macular degeneration that typically develops in childhood and leads to vision loss and, in some cases,
blindness.
We hold exclusive commercialization
rights through licenses for each of our drug candidates. We currently plan to develop our drug candidates through clinical PoC (typically
Phase 2), and then seek licensing and/or partnership opportunities for further clinical development through regulatory approval and commercialization.
We have developed our lead clinical
drug candidate Sarconeos (BIO101), preclinical drug candidate Macuneos (BIO201), and a preclinical pipeline of life-cycle extension products,
consisting of BIO103 and BIO203, through a drug discovery platform in collaboration with Sorbonne University in Paris, France based on
work with medicinal plants. Plants are major sources of small molecules, called secondary metabolites, which they produce as a defense
mechanism to various environmental stresses, including attack from predatory and pathogenic species (e.g., insects, bacteria and
fungi). Our drug discovery platform is based on a reverse pharmacology approach that tests a collection of bioactive secondary metabolites
along with chemical analogs that we have synthesized in phenotypic screens of various age-related diseases. Our long-term goal is to
advance the field of aging science with the continued discovery and development of new drug candidates that treat age-related diseases
by stimulating biological resilience pathways that are involved in the aging process and/or age-related diseases.
We have assembled an executive
team of scientific, clinical, and business leaders with broad expertise in biotechnology and clinical drug development (see “Item
6.A Directors, Senior Management and Employees” in our Annual Report on Form 20-F for the year ended December 31, 2022,
as amended, for more information on our directors and senior management).
Our Clinical Pipeline
We are developing a portfolio
of programs targeting biological resilience pathways that slow the degenerative processes associated with aging and improve functional
outcomes for patients suffering from age-related diseases. Our current pipeline of drug candidates is illustrated below.
Recent Developments
Corporate Matters
On March 30, 2023, we effected
a change to the ratio of the ADSs to ordinary shares from one ADS representing 10 ordinary shares to one ADS representing 100 ordinary
shares.
On April 17, 2023, our
board of directors, acting upon delegation of the shareholder meeting held on the same day, reduced the share capital from EUR 62 283
310,20 to EUR 3 114 165,51 by way of cancellation of losses. The capital reduction was effected by reducing the par value of the ordinary
shares from EUR 0.20 to EUR 0.01.
New Partnership with Seqens
On
July 18, 2023, we issued a press release announcing that we have entered into a master agreement with Seqens for the production
of the active compound in Sarconeos (BIO101). Seqens will produce the active ingredient in Sarconeos (BIO101) in France at its Villeneuve
La Garenne plant, near Paris.
Company Information
We were incorporated as a société
anonyme under the laws of France on September 27, 2006. We are registered at the Paris Registre du Commerce et des Sociétés
under the number 492 002 225. Our principal executive offices are located at Sorbonne University-BC 9, Bâtiment A 4ème
étage, 4 place Jussieu 75005 Paris, France and our telephone number is +33 1 44 27 23 00. Our website address is www.biophytis.com.
Our agent for service of process in the United States is Puglisi & Associates, 850 Library Avenue, Suite 204, Newark, Delaware
19711. The reference to our website is an inactive textual reference only and the information contained in, or that can be accessed through,
our website is not a part of this prospectus and should not be considered a part of this prospectus or any supplement to this prospectus.
Our ordinary shares are listed
on Euronext Growth Paris (Ticker: ALBPS - ISIN: FR0012816825). The ADSs (American Depositary Shares) are listed on the Nasdaq Capital
Market (Ticker: BPTS – ISIN: US09076G1040) since February 10, 2021.
The Offering
The following summary contains
basic information about our securities and the offering and is not intended to be complete. It does not contain all the information that
may be important to you. For a more complete understanding of the ADSs, you should read the section of the accompanying prospectus entitled
“Description of American Depositary Shares.”
| Issuer |
|
Biophytis
S.A. |
| |
|
| ADSs
we are offering |
|
50,500,000
ordinary shares represented by 505,000 ADSs. |
| |
|
|
| Offering
price per ADS |
|
$2.85
(or €2.53) per ADS. |
| |
|
| Pre-funded warrants
we are offering |
|
Pre-funded
warrants to purchase up to 82,833,400 ordinary shares represented by 828,334 ADSs. The pre-funded warrants will have an exercise
price of $0.01 (or €0.01) per ADS, will be immediately exercisable and may be exercised at any time upon issuance and
will expire 10 years following the date of issuance. This prospectus supplement also relates to the offering of the ordinary shares
represented by ADSs issuable upon exercise of the pre-funded warrants. |
| |
|
|
| Offering price per pre-funded
warrant |
|
$2.84
(or €2.52) per pre-funded warrant. |
| |
|
|
| Concurrent Private Placement |
|
In a
concurrent private placement, we are also issuing to the investors, for each ADS or pre-funded warrant issued in this offering, an
ordinary warrant to purchase up to 100 ordinary shares represented by one ADS, with an exercise price of $3.00 (or €2.66) per
ADS, resulting in our issuance of ordinary warrants to purchase up to an aggregate of 133,333,400 ordinary shares represented by
1,333,334 ADSs. The unregistered ordinary warrants are exercisable at any time upon issuance and will expire three years following
the date of issuance. The unregistered ordinary warrants, the ordinary shares issuable upon the exercise of the ordinary warrants
and the ADSs representing such ordinary shares are being offered pursuant to the exemption provided in Section 4(a)(2) under
the Securities Act and/or Regulation D promulgated thereunder, and they are not being offered pursuant to this prospectus supplement
and the accompanying prospectus. |
| |
|
|
| Use
of Proceeds |
|
We estimate
the net proceeds from this offering and the sale of the unregistered ordinary warrants in the concurrent private placement will be
approximately $3.1 million (or €2.8 million), based upon an offering price of $2.85 per ADS and $2.84 per pre-funded
warrant, after deducting placement agent fees and estimated offering expenses payable by us, and assuming full exercise of the pre-funded
warrants to be issued in this offering and no exercise of any unregistered ordinary warrants to be issued to the investors in the
concurrent private placement. We currently intend to use the net proceeds from this offering for funding research and development
and clinical trials and for other working capital and general corporate purposes. See “Use of Proceeds” on page S-14
of this prospectus supplement. |
| |
|
|
| Ordinary shares to be outstanding
after this offering |
|
592,689,660
ordinary shares, assuming the full exercise of the pre-funded warrants sold hereunder and no exercise of any unregistered
ordinary warrants to be issued in the concurrent private placement. |
| |
|
|
| Risk
factors |
|
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page S-10 of this prospectus
supplement and on page 8 of the accompanying prospectus, for a discussion of certain factors you should consider before investing
in our securities. |
| Listings |
|
The
ADSs representing our ordinary shares are listed on the Nasdaq Capital Market under the symbol “BPTS.” Our ordinary shares
currently trade on the Euronext Growth Paris under the symbol “ALBPS.” We do not intend to list the pre-funded warrants
or the ordinary warrants on any securities exchange or nationally recognized trading system. |
| |
|
|
| Depositary |
|
The Bank
of New York Mellon. |
The
number of shares to be outstanding after this offering is based on 509,856,260 ordinary shares outstanding as of July 18,
2023 and excludes as of such date the following:
| |
· |
10,077,791
ordinary shares issuable upon the exercise of outstanding warrants at a weighted-average
exercise price of €0.370 (or $0.416) per ordinary share (based on a USD/EUR exchange
rate of 1.1255 as of July 18, 2023); |
| |
|
|
| |
· |
157,418,339 ordinary
shares issuable upon conversion of convertible bonds held by Atlas Capital at the 10-day VWAP of €0.02541 (or $0.0286) as of
July 18, 2023 (based on a USD/EUR exchange rate of 1.1255 as of July 18, 2023); |
| |
|
|
| |
· |
3,472,222 ordinary shares
issuable upon conversion of convertible bonds held by Kreos Capital at the contractual price of €0.648
(or $0.729) as of July 18, 2023 (based on a USD/EUR exchange rate of 1.1255 as of July 18, 2023); and |
| |
· |
18,904,159 ordinary
shares issuable upon the vesting of free shares granted to certain officers and employees. |
Unless otherwise indicated,
all information in this prospectus supplement assumes no exercise of the outstanding warrants described above, or any of the unregistered
ordinary warrants to be issued in the concurrent private placement, and unless otherwise indicated, gives retroactive effect to the adjustment
to the ratio of ADSs to ordinary shares from one ADS representing 10 ordinary shares to one ADS representing 100 ordinary shares, which
was effected on March 30, 2023, and the reduction of the nominal value of our ordinary shares from EUR 0.20 to EUR 0.01, which became
effective on April 17, 2023.
RISK FACTORS
An investment in our securities involves significant
risks. Before making an investment in our securities, you should carefully read all of the information contained in this prospectus supplement,
the accompanying prospectus and in the documents incorporated by reference herein. For a discussion of risk factors that you should carefully
consider before deciding to purchase any of our securities, please review the additional risk factors disclosed below, the information
under the heading “Risk Factors” in the accompanying prospectus and the section entitled “Risk Factors” contained
in our annual
report on Form 20-F for the year ended December 31, 2022 filed with the SEC on April 19, 2023, as
amended on July 3, 2023. In addition, please read “About this Prospectus Supplement” and “Special Note Regarding
Forward-Looking Statements” in this prospectus supplement, where we describe additional uncertainties associated with our business
and the forward-looking statements included or incorporated by reference in this prospectus supplement and the accompanying prospectus.
Please note that additional risks not currently known to us or that we currently deem immaterial also may adversely affect our business,
operations results of operations, financial condition and prospects.
Risks Relating to the ADSs and this Offering
Since we have broad discretion in how we use
the proceeds from this offering, we may use the proceeds in ways with which you disagree.
We currently intend to use the
net proceeds from this offering for funding research and development and clinical trials and for other working capital and general corporate
purposes. Accordingly, our management will have significant flexibility in applying the net proceeds of this offering. You will be relying
on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your
investment decision, to assess whether the proceeds are being used in ways with which you would agree. It is possible that the net proceeds
will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively
could have a material adverse effect on our business, financial condition, operating results and cash flow.
You will experience immediate dilution
in book value of any ADSs you purchase.
Because the price per
ADS being offered is substantially higher than our net tangible book value per ADS, you will suffer substantial dilution in the net tangible
book value of any ADSs you purchase in this offering. After giving effect to the sale by us of (i) an aggregate of 50,500,000 ordinary
shares represented by 505,000 ADSs at the offering price of $2.85 (or €2.53) per ADS in this offering, (ii) the pre-funded
warrants to purchase up to 82,833,400 ordinary shares represented by 828,334 ADSs at the offering price of $2.84 (or €2.52) per
pre-funded warrant in this offering (assuming full exercise of the pre-funded warrants sold in this offering), and (iii) the unregistered
ordinary warrants to purchase up to 133,333,400 ordinary shares represented by 1,333,334 ADSs, with an exercise price of $3.00 (or €2.66)
per ADS in the concurrent private placement (assuming no exercise of any unregistered ordinary warrants to be issued to the investors
in the concurrent private placement), the as adjusted net tangible book value of the ADSs would have been approximately -€1.8 million
(or -$1.7 million), or -€0.48 (or -$0.47) per ADS, as of December 31, 2022 (an increase in net tangible book value of approximately
€1.44 (or $1.58) per ADS to our existing shareholders). If you purchase ADSs in this offering, you will suffer immediate and substantial
dilution of approximately €1.23 (or $1.31) per ADS, after deducting the placement agent fees and estimated offering expenses payable
by us. See “Dilution” on page S-16 for a more detailed discussion of the dilution you will incur in connection with
this offering.
A substantial number of ADSs may be sold in
this offering, which could cause the price of the ADSs or ordinary shares to decline.
In this offering we will sell
50,500,000 ordinary shares represented by 505,000 ADSs and 82,833,400 ordinary shares represented by 828,334 ADSs underlying pre-funded
warrants. In addition, in a concurrent private placement, we are selling to the investors unregistered ordinary warrants to purchase
up to an aggregate of 133,333,400 ordinary shares represented by 1,333,334 ADSs at an exercise price of $3.00 (or €2.66) per ADS.
This sale and any future sales of a substantial number of ADSs or ordinary shares in the public market, or the perception that such sales
may occur, could adversely affect the price of the ADSs on Nasdaq or our ordinary shares on the Euronext Growth Paris. We cannot predict
the effect, if any, that market sales of the ADSs or ordinary shares or the availability of the ADSs or ordinary shares for sale will
have on the market price of the ADSs or our ordinary shares.
Issuance of additional equity securities may
adversely affect the market price of the ADSs or ordinary shares.
We are currently authorized to issue 3,696,282,189
ordinary shares. As of July 18, 2023, we had 509,856,260 ordinary shares issued and outstanding. As of July 18, 2023, we also
had warrants to purchase 10,077,791 ordinary shares outstanding and convertible bonds exercisable into 160,890,561 ordinary shares outstanding
based on Biophytis closing share price on Euronext Growth as of July 4, 2023.
To the extent that ADSs or ordinary
shares are issued or warrants are exercised, holders of the ADSs and our ordinary shares will experience dilution. In addition, in the
event of any future issuances of equity securities or securities convertible into or exchangeable for ADSs or ordinary shares, holders
of the ADSs or our ordinary shares may experience dilution. We also consider from time to time various strategic alternatives that could
involve issuances of additional ADSs or ordinary shares, including but not limited to acquisitions and business combinations, but do
not currently have any definitive plans to enter into any of these transactions.
We have been notified by The
Nasdaq Stock Market LLC (“Nasdaq”) of our failure to comply with certain continued listing requirements and, if we are unable
to regain compliance with all applicable continued listing requirements and standards of Nasdaq, the ADSs could be delisted from Nasdaq.
The
ADSs are currently listed on Nasdaq. In order to maintain that listing, we must satisfy minimum financial
and other continued listing requirements and standards, including those regarding minimum stockholders’ equity, minimum share price,
and certain corporate governance requirements.
On April 24,
2023, we received notice from the Listing Qualifications Staff of Nasdaq indicating that we were not in compliance with the Minimum Stockholders’
Equity Requirement, because our stockholders’ equity of (€1,911,000) as reported in our Annual Report on Form 20-F
for the year ended December 31, 2022 was below the required minimum of $2.5 million, and because, as of April 24, 2023, we
did not meet the alternative compliance standards, relating to the market value of listed securities of $35 million or net income from
continuing operations of $500,000 in the most recently completed fiscal year or in two of the last three most recently completed fiscal
years. The letter has no immediate effect on the listing of the ADSs, and they will continue to trade on Nasdaq under the symbol “BPTS”.
On June 15,
2023, we submitted to Nasdaq a plan to regain compliance with the Minimum Stockholders’ Equity Requirement. If Nasdaq accepts
our plan, Nasdaq may grant an extension of up to 180 calendar days from the date of the notice, or until October 21, 2023, for us
to demonstrate compliance with Rule 5550(b)(1). If Nasdaq does not accept our plan, or if Nasdaq does not grant an extension until,
and we do not regain compliance by, October 21, 2023, or if we fail to satisfy another Nasdaq requirement for continued listing
such as the Bid Price Requirement, Nasdaq could provide notice that our securities will become subject to delisting. In such event, Nasdaq
rules permit us to request a hearing before an independent Hearings Panel, which has the authority to grant us an additional extension
of time of up to 180 calendar days to regain compliance.
We do not intend to apply for any listing
of the pre-funded warrants or the ordinary warrants on any exchange or nationally recognized trading system, and we do not expect a market
for the pre-funded warrants or the ordinary warrants to develop.
We do not intend to apply for
any listing of either the pre-funded warrants or the ordinary warrants on Nasdaq or any other securities exchange or nationally recognized
trading system, and we do not expect a market to develop for the pre-funded warrants or the ordinary warrants. Without an active market,
the liquidity of the pre-funded warrants and the ordinary warrants will be limited. Further, the existence of the pre-funded warrants
and the ordinary warrants may act to reduce both the trading volume and the trading price of the ADSs.
Except as otherwise provided in the pre-funded
warrants and the ordinary warrants, holders of pre-funded warrants issued in this offering and ordinary warrants issued in the concurrent
private placement will have no rights as stockholders of our ordinary shares until such holders exercise their pre-funded warrants and/or
ordinary warrants.
The pre-funded warrants offered in this offering
and the ordinary warrants offered in the concurrent private placement do not confer any rights of ordinary share ownership on their holders,
such as voting rights or the right to receive dividends, but rather merely represent the right to acquire the ADSs at a fixed price.
Specifically, a holder of a pre-funded warrant may exercise the right to acquire ordinary shares represented by an ADS and pay a nominal
exercise price of $0.01 (or €0.01) at any time and a holder of an ordinary warrant may exercise the right to acquire ordinary shares
represented by an ADS at an exercise price of $3.00 (or €2.66) per ADS at any time during the three years following the date of
issuance. Upon exercise of the pre-funded warrants and/or the ordinary warrants, the holders thereof will be entitled to exercise the
rights of a holder of ordinary shares only as to matters for which the record date occurs after the exercise date.
We will not receive any meaningful amount
of additional funds upon the exercise of the pre-funded warrants.
Each pre-funded warrant will
be exercisable by means of payment of the nominal cash purchase price upon exercise or by means of a “cashless exercise”
according to a formula set forth in the pre-funded warrant. Accordingly, we will not receive any meaningful additional funds upon the
exercise of the pre-funded warrants.
We have no plans to pay dividends on our ordinary
shares, and you may not receive funds without selling the ADSs or ordinary shares.
We have not declared or paid
any cash dividends on our ordinary shares, nor do we expect to pay any cash dividends on our ordinary shares for the foreseeable future.
We currently intend to retain any additional future earnings to finance our operations and growth and for future stock repurchases and,
therefore, we have no plans to pay cash dividends on our ordinary shares at this time. Any future determination to pay cash dividends
on our ordinary shares will be at the discretion of our board of directors and will be dependent on our earnings, financial condition,
operating results, capital requirements, any contractual restrictions, and other factors that our board of directors deems relevant.
Accordingly, you may have to sell some or all of the ADSs or ordinary shares in order to generate cash from your investment. You may
not receive a gain on your investment when you sell the ADSs or ordinary shares and you may lose the entire amount of your investment.
The market price of our ordinary shares and
ADSs is subject to fluctuation, which could result in substantial losses by our investors.
The stock market in general
and the market price of our ordinary shares on the Euronext Growth Paris and the ADSs representing our ordinary shares on Nasdaq is subject
to fluctuation, and changes in our share price may be unrelated to our operating performance. The market price of our ordinary shares
and ADSs are and will be subject to a number of factors, including:
| · | actual
or anticipated fluctuations in our financial condition and operating results; |
| · | actual
or anticipated changes in our growth rate relative to our competitors; |
| · | developments
concerning regulatory submissions, regulatory approvals or intellectual property rights; |
| · | competition
from existing products or new products that may emerge; |
| · | announcements
by us or our competitors of significant acquisitions, strategic partnerships, joint ventures,
collaborations, or capital commitments; |
| · | failure
to meet or exceed financial estimates and projections of the investment community or that
we provide to the public; |
| · | issuance
of new or updated research or reports by securities analysts; |
| · | fluctuations
in the valuation of companies perceived by investors to be comparable to us; |
| · | price
and volume fluctuations attributable to inconsistent trading volume levels of our securities; |
| · | additions
or departures of key management or scientific personnel; |
| · | lawsuits
threatened or filed against us, disputes or other developments related to proprietary rights,
including patents, litigation matters, and our ability to obtain patent protection for our
technologies; |
| · | changes
to coverage policies or reimbursement levels by commercial third-party payors and government
payors and any announcements relating to coverage policies or reimbursement levels; |
| · | announcement
or expectation of additional debt or equity financing efforts; |
| · | sales
of ADSs representing our ordinary shares or ordinary shares by us, our insiders or our other
holders; and |
| · | general
economic and market conditions. |
These
and other market and industry factors may cause the market price and demand for our securities to fluctuate substantially, regardless
of our actual operating performance, which may limit or prevent investors from readily selling their securities and may otherwise negatively
affect the liquidity of the trading market for our securities.
Additionally, market prices
for securities of biotechnology and pharmaceutical companies historically have been very volatile. The market for these securities has
from time to time experienced significant price and volume fluctuations for reasons unrelated to the operating performance of any one
company. In the past, following periods of market volatility, shareholders have often instituted securities class action litigation.
If we were involved in securities litigation, it could have a substantial cost and divert resources and attention of management from
our business, even if we are successful.
There can be no assurance that we will not
be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in 2023 or in any subsequent year. If we are a
PFIC, there may be negative tax consequences for U.S. taxpayers that are holders of our ordinary shares, ADSs and warrants.
We will be treated as a PFIC
for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of our gross income is “passive
income” or (ii) on average at least 50% of our assets by value produce passive income or are held for the production of passive
income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains
from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income
also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining
whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly
or indirectly, at least a 25% interest (by value) is taken into account.
Based on our analysis of our
income, assets, and operations, we do not believe that we were a PFIC for 2022. Because the PFIC determination is highly fact intensive,
there can be no assurance that we will not be a PFIC in 2023 or in any other taxable year. If we were to be characterized as a PFIC for
U.S. federal income tax purposes in any taxable year during which a U.S. shareholder owns our ordinary shares, ADSs and warrants, then
“excess distributions” to such U.S. shareholder, and any gain realized on the sale or other disposition of our ordinary shares,
the ADS and warrants, as applicable, will be subject to special rules. Under these rules: (i) the excess distribution or gain would
be allocated ratably over the U.S. shareholder’s holding period for the ordinary shares (or ADSs or warrants) as the case may be);
(ii) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we
were a PFIC would be taxed as ordinary income; and (iii) the amount allocated to each of the other taxable years would be subject
to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed
deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. Certain of the adverse
consequences of PFIC status can be mitigated if a U.S. shareholder makes an election to treat us as a “qualified electing fund,”
or QEF, or makes a “mark-to-market” election. A QEF election is unavailable with respect to our warrants, and a mark-to-market
election is unavailable with respect to our warrants. In addition, if the U.S. Internal Revenue Service, or IRS, determines that we are
a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. shareholder to make
a timely QEF or mark-to-market election. U.S. shareholders who hold our ordinary shares, ADSs and warrants during a period when we are
a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. shareholders
who made a timely QEF or mark-to-market election (to the extent available). A U.S. shareholder can make a QEF election by completing
the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Upon request, we intend to annually
furnish U.S. shareholders with information needed in order to complete IRS Form 8621 (which form would be required to be filed with
the IRS on an annual basis by the U.S. shareholder) and to make and maintain a valid QEF election for any year in which we or any of
our subsidiaries that we control is a PFIC.
USE OF PROCEEDS
We estimate the net proceeds
from this offering and the sale of the unregistered ordinary warrants in the concurrent private placement will be approximately $3.1
million (or €2.8 million), based upon an offering price of $2.85 (or €2.53) per ADS and $2.84 (or €2.52) per pre-funded
warrant in this offering, after deducting placement agent fees and estimated offering expenses payable by us, and assuming full exercise
of the pre-funded warrants to be issued in this offering and no exercise of any ordinary warrants. We do not know when the ordinary warrants
that are issued to the investors in the concurrent private placement will be exercised, if at all.
We currently intend to use the
net proceeds from this offering for funding research and development and clinical trials and for other working capital and general corporate
purposes. As a result, our management will retain broad discretion in the allocation and use of the net proceeds of this offering, and
the investors will be relying on the judgment of our management with regard to the use of these net proceeds.
Pending application of the net
proceeds for the purposes as described above, we expect to invest the net proceeds in short-term, interest-bearing securities, investment
grade securities, certificates of deposit or direct or guaranteed obligations of the U.S. government.
CAPITALIZATION
The following table sets forth
our capitalization:
| |
· |
on an actual basis as of December 31,
2022; and |
| |
· |
on an as adjusted
basis, giving additional effect to the sale of (i) an aggregate of 50,500,000 ordinary shares represented by 505,000
ADSs at the offering price of $2.85 (or €2.53) per ADS in this offering, (ii) the pre-funded warrants to purchase up to
82,833,400 ordinary shares represented by 828,334 ADSs at the offering price of $2.84 (or €2.52) per pre-funded warrant in this
offering, with an exercise price of $0.01 (or €0.01) per ADS (assuming full exercise of the pre-funded warrants sold in this
offering), and (iii) the unregistered ordinary warrants to purchase up to 133,333,400 ordinary shares represented by 1,333,334
ADSs, with an exercise price of $3.00 (or €2.66) per ADS in the concurrent private placement (assuming no exercise of any unregistered
ordinary warrants to be issued to the investors in the concurrent private placement), after deducting the placement agent fees and
estimated offering expenses payable by us, resulting in net proceeds of approximately $3.1 million (or €2.8 million). |
The as adjusted amounts shown
below are unaudited and represent management’s estimate. The information in this table should be read in conjunction with and is
qualified by reference to the financial statements and notes thereto and other financial information incorporated by reference into this
prospectus supplement.
| | |
As of
December 31, 2022 | |
| | |
Actual | | |
As Adjusted | |
| | |
€ | | |
$ | | |
€ | | |
$ | |
| | |
| | |
| | |
| | |
| |
| | |
(in thousands) | |
| Cash
and cash equivalents | |
| 11,053 | | |
| 11,789 | | |
| 17,403 | | |
| 18,948 | |
| Non-current
financial liabilities | |
| 4,367 | | |
| 4,658 | | |
| 4,367 | | |
| 4,658 | |
| Current financial
liabilities | |
| 10,213 | | |
| 10,893 | | |
| 10,213 | | |
| 10,893 | |
| Shareholders’
equity | |
| | | |
| | | |
| | | |
| | |
| Ordinary shares,
€0.20 nominal value: 238,297,642 shares issued and outstanding, actual; and 504,964,442 shares issued and outstanding, as adjusted | |
| 47,660 | | |
| 50,834 | | |
| 50,327 | | |
| 53,767 | |
| Premiums related to
the share capital | |
| (1,588 | ) | |
| (1,694 | ) | |
| 2,095 | | |
| 2,532 | |
| Treasury shares | |
| (21 | ) | |
| (22 | ) | |
| (21 | ) | |
| (22 | ) |
| Foreign currency translation
adjustment | |
| (25 | ) | |
| (27 | ) | |
| (25 | ) | |
| (27 | ) |
| Accumulated deficit—attributable
to our shareholders | |
| (23,689 | ) | |
| (25,267 | ) | |
| (23,689 | ) | |
| (25,267 | ) |
| Net loss—attributable
to our shareholders | |
| (24,216 | ) | |
| (25,829 | ) | |
| (24,216 | ) | |
| (25,829 | ) |
| Non-controlling
interests | |
| (32 | ) | |
| (34 | ) | |
| (32 | ) | |
| (34 | ) |
| Total
shareholders’ equity (deficit) | |
| (1,911 | ) | |
| (2,038 | ) | |
| 4
439 | | |
| 5
120 | |
| Total
capitalization | |
| 12,669 | | |
| 13,513 | | |
| 19
019 | | |
| 20
671 | |
The above table is based on
238,297,642 ordinary shares outstanding as of December 31, 2022 and excludes as of such date the following:
| |
· |
10,083,754 ordinary
shares issuable upon the exercise of outstanding warrants at a weighted-average exercise price of €0.376 (or $0.401) per ordinary
share; |
| |
|
|
| |
· |
285,000,000 ordinary
shares issuable upon conversion of convertible bonds held by Atlas Capital at the closing price of €0.020 (or $0.0213) per ordinary
share; and |
| |
|
|
| |
· |
3,472,222 ordinary shares
issuable upon conversion of convertible bonds held by Kreos Capital at the contractual price of €0.648 (or $0.691) per
ordinary share. |
Unless otherwise indicated,
all information in this prospectus supplement assumes no exercise of the outstanding warrants described above, or any of the unregistered
ordinary warrants to be issued in the concurrent private placement, and unless otherwise indicated, gives retroactive effect to the adjustment
to the ratio of ADSs to ordinary shares from one ADS representing ten (10) ordinary shares to one ADS representing one hundred (100)
ordinary shares effected on March 30, 2023.
DILUTION
If you invest in our securities,
your ownership interest will be diluted to the extent of the difference between the offering price per share and the net tangible book
value per share after this offering. We calculate net tangible book value per ADS by dividing the net tangible book value, which is tangible
assets less total liabilities, by the number of outstanding ordinary shares as represented by ADSs.
Our net tangible book value
as of December 31, 2022, was approximately €4.6 million (or $4.9 million), or approximately $0.20 (or €0.19) per ADS.
Net tangible book value per ADS represents the amount of our total tangible assets less total liabilities divided by the total number
of ADSs outstanding as of December 31, 2022.
After giving further effect to the sales of (i) an
aggregate of 50,500,000 ordinary shares represented by 505,000 ADSs at the offering price of $2.85 (or €2.53) per ADS in this offering,
(ii) the pre-funded warrants to purchase up to 82,833,400 ordinary shares represented by 828,334 ADSs at the offering price of $2.84
(or €2.52) per pre-funded warrant in this offering, with an exercise price of $0.01 (or €0.01) per ADS (assuming full exercise
of the pre-funded warrants sold in this offering), and (iii) the unregistered ordinary warrants to purchase up to 133,333,400 ordinary
shares represented by 1,333,334 ADSs, with an exercise price of $3.00 (or €2.66) per ADS, in the concurrent private placement (assuming
no exercise of any such unregistered ordinary warrants), our as adjusted net tangible book value as of December 31, 2022 would have
been -€1.8 million (or -$1.7 million), or -€0.48 (or -$0.47) per ADS. This represents an immediate increase in the net tangible
book value of €1.44 (or $1.58) per ADS to our existing shareholders and an immediate and substantial dilution in net tangible book
value of €1.23 (or $1.31) per ADS to the new investor. The following table illustrates this per share dilution:
| |
|
As
of December 31, 2022 |
|
| |
|
Per
Ordinary
Share |
|
|
Per
ADS |
|
| |
|
(€) |
|
|
($) |
|
|
(€) |
|
|
($) |
|
| Offering price per ordinary share or ADS |
|
|
0.025 |
|
|
|
0.029 |
|
|
|
2.53 |
|
|
|
2.85 |
|
| Historical net
tangible book value per ordinary share or ADS |
|
|
(0.019) |
|
|
|
(0.020) |
|
|
|
(1.92) |
|
|
|
(2.04) |
|
| Increase
in net tangible book value per ordinary share or ADS attributable to investors purchasing ADSs in the offering |
|
|
0.014 |
|
|
|
0.015 |
|
|
|
1.44 |
|
|
|
1.58 |
|
| As adjusted net tangible book value per ordinary share
or ADS after the offering |
|
|
(0.005) |
|
|
|
0.005 |
|
|
|
(0.48) |
|
|
|
(0.47) |
|
| Dilution per ordinary share or ADS to the new investor
in this offering |
|
|
(0.012) |
|
|
|
(0.013) |
|
|
|
(1.23) |
|
|
|
(1.31) |
|
The above table is based on
238,297,642 ordinary shares outstanding as of December 31, 2022 and excludes as of such date the following:
| |
· |
10,083,754 ordinary
shares issuable upon the exercise of outstanding warrants at a weighted-average exercise price of €0.376 (or $0.401) per ordinary
share; |
| |
|
|
| |
· |
285,000,000 ordinary
shares issuable upon conversion of convertible bonds held by Atlas Capital at the closing price of €0.020 (or $0.0213) per ordinary
share; and |
| |
· |
3,472,222 ordinary shares
issuable upon conversion of convertible bonds held by Kreos Capital at the contractual price of €0.648 (or $0.691) per
ordinary share. |
The above illustration of dilution
per share to the investors participating in this offering assumes no exercise of outstanding warrants to purchase ADSs or ordinary shares.
The exercise of outstanding warrants having an exercise price less than the offering price will increase dilution to the new investors.
DESCRIPTION OF THE SECURITIES
WE ARE OFFERING
The following description
of our share capital summarizes certain provisions of our articles of association. Such summaries do not purport to be complete and are
subject to, and are qualified in their entirety by reference to, all of the provisions of our articles of association, a copy of which
has been filed as an exhibit to the registration statement of which this prospectus supplement forms a part.
We
are offering (i) 50,500,000 ordinary shares represented by 505,000 ADSs, at an offering price of $2.85 (or €2.53) per ADS,
and (ii) pre-funded warrants to purchase up to 82,833,400 ordinary shares represented by 828,334 ADSs, at an offering price of $2.84
(or €2.52) per pre-funded warrant. Each ADS represents 100 ordinary shares. Each of the pre-funded warrant will be exercisable for
one ADS. The pre-funded warrants will have an exercise price of $0.01 (or €0.01) per ADS, will be immediately exercisable and
may be exercised at any time upon issuance and will expire 10 years following the date of issuance. We are also offering the ordinary
shares represented by ADSs issuable from time to time upon exercise of the pre-funded warrants being offered by this prospectus supplement
and accompanying prospectus.
American Depositary Shares
See
“Description of American Depositary Shares” in the accompanying prospectus for a description of the material terms of the
ADSs.
Ordinary Shares
See
“Description of Share Capital—Key Provisions of Our Articles of Association and French Law Affecting Our Ordinary Shares”
in the accompanying prospectus for a description of the material terms of our ordinary shares.
General
As
of December 31, 2022, our outstanding share capital consisted of €47,659,528.20 divided into 238,297,642 fully subscribed and
paid up shares, with a nominal value of €0.20 per share. As of July 18, 2023, our outstanding share capital consisted of €4,593,562.60
divided into 459,356,260 fully subscribed and paid up shares, with a nominal value of €0.01 per share. As of July 18, 2023,
we also hold 816,008 ordinary shares in treasury with a book value of €16,484 and a face value of €15,912.
Under
French law, our by-laws set forth only our issued and outstanding share capital as of the date of the by-laws. Our fully diluted share
capital represents all issued and outstanding shares, as well as all potential shares which may be issued upon exercise of outstanding
employee and non-employee warrants, as approved by our shareholders and granted by our board of directors.
| Reconciliation
of the Number of Ordinary Shares Outstanding through July 18, 2023 |
|
|
|
|
| Shares
outstanding at December 31, 2021 |
|
|
135,953,657 |
|
| Number
of shares issued in connection with the conversion of convertible bonds |
|
|
93,189,046 |
|
| Number
of shares issued in connection with the definitive acquisition of free shares |
|
|
9,131,979 |
|
| Number
of shares issued in connection with the exercise of warrants |
|
|
22,960 |
|
| Shares
outstanding as of December 31, 2022 |
|
|
238,297,642 |
|
| Number
of shares issued in connection with the conversion of convertible bonds |
|
|
115,755,884 |
|
| Number
of shares issued in connection with a private placement |
|
|
103,717,811 |
|
| Number
of shares issued in connection with the definitive acquisition of free shares |
|
|
1,578,960 |
|
| Number
of shares issued in connection with the exercise of warrants |
|
|
5,963 |
|
| Number
of shares outstanding as of July 18, 2023 |
|
|
459,356,260 |
|
Shareholder Authorizations Regarding Share
Capital
At
a combined general meeting of shareholders held on April 17, 2023, our board of directors received the following authorizations
from shareholders:
Fourth Resolution
of the combined general meeting of shareholders held on April 17, 2023
The Shareholders, deciding in
accordance with the quorum and majority required for extraordinary shareholders' meetings, having reviewed the Board of Directors' report
and the Statutory Auditors' special report, in accordance with the provisions of articles L. 225-129 et seq. of the French commercial
Code, in particular articles L. 225-129-2, L. 225-135, L. 225-138, L. 22-10-49, L. 228-91 to L. 228-93,
After having acknowledged that
the share capital is fully paid up,
Delegate to the Board of Directors
its authority, with the option of sub-delegation to the Chief Executive Officer, which may include an option for the latter to sub-delegate,
in order to decide, on one or more occurrences in France or abroad, in the proportions, at the times and in the manner deemed appropriate,
in euros or in a foreign currency, or in any other monetary unit established by reference to several currencies, on the issuance, on
the French and / or international market, with waiver of preferential subscription rights, ordinary shares of the Company (including
American Depositary Shares) and/or any other securities giving access, immediately or in the future, at any time or on a fixed date,
to the share capital of the Company (whether new or existing shares), or of companies that directly or indirectly own more than half
of its share capital or companies in which it directly or indirectly owns more than half of the share capital, and/or giving the right
to the allocation of debt securities, by subscription either in cash or by offsetting receivables, conversion, exchange, redemption,
presentation of a warrant or in any other manner, the securities representing debts that may be issued with or without guarantee, in
such forms, rates and conditions deemed appropriate, by the Board of Directors,
It being specified that issuance
of preference shares and all securities giving access to preference shares are excluded from this delegation,
Decide, in the event of use
of this delegation by the Board of Directors, to set the following limits on the amounts of authorized issuances:
| · | the
total nominal amount of the share capital increases that may be completed, immediately and/or
in the future, pursuant to this delegation, may not exceed EUR thirty-eight million (EUR
38,000,000) or the equivalent in any other currency or monetary unit established by reference
to several currencies on the date of the issuance, it being understood that this amount shall
be deducted from the limit of the overall cap on share capital increases set in the 10th
resolution of this Shareholders’ Meeting, to this cap shall be added, where appropriate,
the par value of any ordinary shares to be issued, in the event of new financial transactions,
to preserve, in accordance with legal and regulatory provisions and, where applicable, contractual
provisions providing for other cases of adjustment, the rights of holders of securities giving
access to the share capital, subscription options or rights to free allocation of shares, |
| · | the
total nominal amount of debt securities giving access to the share capital of the Company
or of companies which directly or indirectly own more than half of its share capital or of
the companies in which it directly or indirectly owns more than half of the share capital,
or giving the right to a debt security by subscription either in cash or by offsetting receivables,
conversion, exchange, redemption, presentation of a warrant or in any other manner, which
may be issued pursuant to this delegation may not exceed EUR forty million (EUR 40,000,000)
or the equivalent in any other currency or monetary unit established by reference to several
currencies on the date of issuance, it being understood that: |
| o | this
amount will be deducted on the limit of the overall cap on capital increases set in the 10th
resolution of the present Shareholders’ Meeting, |
| o | this
cap will be increased, if applicable, by any redemption premium above par, |
| o | this
cap does not apply to debt securities whose issuance would be decided or authorized by the
Board of Directors in accordance with Article L. 228-40 of the French commercial Code, |
Acknowledge and decide, as necessary,
that this delegation of authority automatically entails, to the benefit of the holders of securities giving immediate or future access
to the Company's share capital, the express waiver by the shareholders of their preferential subscription right to the shares to which
these securities give right, in accordance with the provisions of article L. 225-132 of the French commercial Code,
Decide that this delegation
of authority is granted to the Board of Directors for a period of eighteen (18) months from this meeting, beyond it shall be considered
null and void if the Board of Directors does not use it. This delegation terminates any previous authorization with the same purpose,
to the extent of the unused part of it.
Decide to waive shareholders'
preferential subscription rights to shares, securities and debt securities that may be issued under this delegation of authority, to
the benefit of the following categories of beneficiaries:
| · | any
natural person who wishes to invest in a company in order to benefit from an income tax reduction
(in accordance with the provisions of Article 199 terdecies-0 A of the CGI) or any other
equivalent tax measure under equivalent foreign law in the jurisdiction in which the individual
who wishes to invest would be a tax resident, for a minimum individual subscription amount
in the Company of EUR 10,000 per transaction (subject to the Company's eligibility for such
tax measures), |
| · | any
company which normally invests in small and medium-sized enterprises and which wishes to
invest in a company in order to enable its shareholders or partners to benefit from a reduction
of income tax (in accordance with the provisions of Article 199 terdecies-0 A of the
CGI) or any other equivalent tax measure under equivalent foreign law in the jurisdiction
in which the shareholders or partners are tax residents, for a minimum individual subscription
amount in the Company of EUR 20,000 per transaction (subject to the Company's eligibility
for these tax measures), |
| · | investment
funds investing on a regular basis in small and medium-sized companies and wishing to invest
in a company in order to enable the subscribers of their shares to benefit from a reduction
of income tax (in accordance with the provisions of article 199 terdecies-0 A of the CGI)
or any other equivalent tax measure under equivalent foreign law in the jurisdiction in which
subscribers are tax residents, for a minimum individual subscription amount in the Company
of EUR 20,000 per transaction (subject to the Company's eligibility for these tax measures), |
| · | French
or foreign companies, investment companies and investment funds, collective investment undertakings,
bodies, institutions or entities in any form, investing mainly in so-called growth companies
(i.e. unlisted or whose market capitalization does not exceed 500 million euros) whatever
they may be, including mutual funds in innovation ("FCPI"), venture capital mutual
funds ("FCPR") and local investment funds ("FIP"), with their head office
or management company in the European Union, the United States, China or Japan, for a minimum
individual subscription amount of EUR 50.000 (including issue premium), |
| · | any
legal or natural person under French or foreign law involved in the healthcare, biotechnology
and/or pharmaceutical sectors that has concluded or is about to conclude with the Company
a scientific and/or industrial and/or commercial partnership agreement of substantial impact
for the Company activity, and/or any holder of the titles of a legal person under French
law or foreign law active in the healthcare, biotechnology and/or pharmaceutical sectors
that has agreed to transfer its securities of this legal entity to the Company as part of
or not a scientific and/or industrial and/or commercial partnership agreement of substantial
scope for the Company's business, |
| · | industrial
or commercial companies, investment companies and investment funds, collective investment
undertakings, bodies, institutions or entities, regardless of their form, French or foreign,
regularly investing in the health sector, the biotechnological and/or pharmaceutical sector,
for a minimum individual subscription amount of EUR 20,000 (including the issuance premium), |
| · | French
or foreign companies, investment companies and investment funds, collective investment undertakings,
bodies, institutions or entities in any form, that may invest in French companies listed
on the Euronext, Euronext Access or Euronext Growth markets or on any other regulated and/or
regulated market and that specialise in structured bond issuance for small or medium-sized
companies, |
| · | any
financial institution, public body, development bank, French or European sovereign fund or
any institution attached to the European Union wishing to grant funds to small and medium-sized
companies and whose investment conditions may include all or part of an investment in equity
and/or in the form of securities giving immediate or future access to share capital, |
| · | managers,
directors and/or executive employees of the Company wishing to invest concurrently with beneficiaries
mentioned by the above categories, |
| · | creditors
holding liquid, certain and payable debts, on the Company who have expressed their wish to
have their debt converted into the Company's securities and for which the Board of Directors
of the Company would consider it appropriate to compensate their debt with the Company's
securities (being specified, for all intents and purposes, that any trust put in place by
the Company in connection with the restructuring or redemption of its debts and any price
receivable in connection with an acquisition of securities by the Company fall in the scope
of this category), and |
| · | French
or foreign investment service providers who can guarantee such a transaction, in accordance
with the provisions of article L. 411-2 of the French monetary and financial Code for French
investors (qualified investors within the meaning of article 2 of the Regulation (EU) No. 2017/1129
of 14 June 2017 and a limited circle of investors within the meaning of Article D.
411-4 of the French monetary and financial Code) and equivalent provisions for foreign investors. |
It being understood that the
Board of Directors shall determine the precise list of beneficiaries of such issuances of ordinary shares and/or reserved securities,
within such categories of beneficiaries as well as the number of shares to be allocated to each of them,
Decide that:
| · | for
share capital increases, the issuance price of the new shares will be set by the Board of
Directors and must be at least equal to 75% of the volume-weighted average of the fifteen
(15), ten (10) or five (5) last trading sessions (according to the Board's assessment)
preceding the day on which it is set, |
| · | for
securities giving access to the share capital, including independent warrants, the issuance
price shall be set by the Board of Directors in such a way that the sums immediately received
by the Company upon issuance of the concerned securities, increased by the sums likely to
be received subsequently by the Company for each share attached to and/or underlying the
securities issued, are at least equal to the lowest price between the following three amounts: |
| o | 75%
of the volume-weighted average of the prices for the last fifteen (15), ten (10) or
five (5) last trading sessions (according to the Board's assessment) preceding the day
on which it is set, or |
| o | 75%
of the volume-weighted average of the prices for the last fifteen (15), ten (10) or
five (5) last trading days (according to the Board's assessment) preceding the date
of the conversion, redemption and conversion into shares of each equity offering access to
the share capital, or |
| o | 75%
of the lowest of the stock market prices over the last fifteen (15) trading days preceding
the date of conversion, redemption and transformation into shares of each security giving
access to the share capital, |
and the conversion, redemption and transformation
into shares of each security giving access to the share capital shall, taking into account the nominal value of the said security, be
made into a number of shares such that the amount received by the Company, for each share, is at least equal to the lowest price between
the three amounts referred to above,
| · | for
securities giving access to the share capital, including independent warrants, the issuance
price of shares likely to result from their exercise, conversion or exchange may, as the
case may be, be set, at the discretion of the Board of Directors, by reference to a calculation
formula defined by it and applicable after issuance of such securities (for instance, when
they are exercised, converted or exchanged) in which case the above maximum discount may
be assessed, if the Board of Directors considers it appropriate for the Company, on the date
of application of the said formula (and not on the date of fixing the issuance price). |
Decide that the new shares issued
in connection with the share capital increases shall be fully assimilated to the existing shares and subject to all provisions of the
articles of association and decisions of the shareholders' meetings,
Specify that the transactions
referred to in this resolution may be completed at any time, including during a public offering period of the Company's shares, in compliance
with applicable legal and regulatory provisions,
Decide that the Board of Directors
shall have all powers, with the option of sub-delegation to the Chief Executive Officer, which may include the latter's right to sub-delegate,
whether or not to implement this delegation, as well as the right to postpone it, as the case may be, under the legal conditions and
within the limits and conditions specified above, in order, in particular, to:
| · | decide
on the share capital increase and determine securities to be issued, decide on issuance of
securities giving access to debt securities and, more generally, decide on issuances within
the framework of this delegation, |
| · | decide
the amount of share capital increase and, more generally, the amount of issuance in the event
of issuance of securities giving access to debt securities, the date and terms of share capital
increase and the issuances, |
| · | set
issuance price as well as the amount of premium that may, as the case may be, be requested
at issuance, within the limits set by this resolution, |
| · | determine
the nature and characteristics of ordinary shares and/or securities to be issued, decide
in addition, in the case of bonds or other debt securities giving access to the Company's
share capital and/or giving entitlement to the allocation of debt securities, whether or
not they are subordinated (and, as the case may be, their subordination rank in accordance
with the provisions of article L. 228-97 of the French commercial Code), set their interest
rates (in particular fixed or variable rate or zero or indexed coupon), their duration (fixed
or indefinite) as well as the other terms and conditions of the issuance (including the granting
of guarantees or sureties) and their amortisation, these securities could be associated with
warrants giving the right to the allocation, acquisition or subscription of bonds or other
debt securities or take the form of complex bonds as defined by the stock exchange authorities, |
| · | modify,
during the term of the concerned securities, the terms and conditions referred to above,
in compliance with the applicable formalities, |
| · | decide,
in the event that subscriptions have not absorbed the entire issuance, to limit the issuance
amount to the received subscription amount, provided that this amount reaches at least three-quarters
of the decided issuance, |
| · | determine
the method of payment of the ordinary shares or securities giving access to the share capital
to be issued, |
| · | determine,
if applicable, the terms and conditions for the exercise of the rights attached to the ordinary
shares or securities to be issued and, in particular, determine the date, even retroactively,
from which the new shares (i.e. any potential underlying securities) will bear dividend rights,
determine the terms and conditions for the exercise of the rights, if any, to conversion,
exchange, redemption, including by delivery of assets of the Company such as shares or securities
already issued by the Company, as well as any other terms and conditions of the issuance's
completion, |
| · | provide
for the possibility of suspending the exercise of the rights attached to these securities
in accordance with legal and regulatory provisions for a maximum period of three (3) months, |
| · | at
its sole initiative, charge the issuance costs against the amount of the related premiums
and deduct from this amount the necessary sums to increase the legal reserve to one-tenth
of the new share capital after each share capital increase ensured, |
| · | determine
and make any adjustments to take into account the impact of transactions on the Company's
share capital, in particular in the event of a change in the par value of the share, a share
capital increase by incorporation of reserves, a free allocation of shares, a stock split
or reverse stock split, the distribution of reserves or of all other assets, the amortization
of share capital, or any other transaction affecting company’s net equity, and determine
the terms and conditions under which the Company will be insured, as the case may be, the
preservation of the holders of securities giving access to the share capital, |
| · | acknowledge
the completion of each share capital increase and make the corresponding amendments to the
articles of association, |
| · | take
all the measures and decisions and carry out all formalities required for the listing of
the securities thus issued on the Euronext Growth market of Euronext Paris and on any other
market on which the Company's shares are then listed, |
| · | in
general, enter into any agreement, in particular for preserving the potential rights of any
holders of securities giving immediate or future entitlement to a part of the share capital,
take any measures and carry out any formalities required for the issuance, registration and
financial service of the securities issued pursuant to this delegation as well as for the
exercise of the rights attached thereto, carry out any formalities and declarations, and
request any authorisations that may prove necessary to the successful completion of this
issuance and, in general, do what is necessary. |
Acknowledge
that the final terms and conditions of the transactions completed pursuant to this delegation will be the subject of an additional report,
in accordance with the requirements of articles L. 225-129-5 and R. 225-116 of the French commercial Code, which the Board of Directors
will establish when it uses this delegation of authority granted to it by this Shareholders' Meeting. The Statutory Auditors will also
prepare a supplementary report on this occurrence
Eight Resolution
of the combined general meeting of shareholders held on April 17, 2023
The Shareholders, deciding in
accordance with the quorum and majority conditions required for shareholders’ extraordinary meetings, having reviewed the Board
of Directors' report, in accordance with the provisions of articles L. 225-135-1 and R. 225-118 of the French commercial Code,
After having acknowledged that
the share capital is fully paid up,
Authorizes
the Board of Directors, with the option to sub-delegate to the Chief Executive Officer may include an option for the latter to
sub-delegate, (i) to increase the number of securities issued for each issue decided for the purpose of covering possible over-allocations
and to stabilize prices in the context of an issuance, with or without preferential subscription right, of ordinary shares (particularly
in the form of American Depositary Shares) and/or any other securities that provide immediate or future access, at any time or fixed
date, to the Company's share capital, (whether new or existing shares) or companies which directly or indirectly own more than half of
its capital or the company in which it directly or indirectly owns more than half of the share capital, or giving right to a debt by
subscription either in cash or by offsetting receivables, conversion, exchange, redemption, presentation of a warrant or otherwise, under
the delegations of authority conferred under the 2nd, 3rd, 4th, 5th, 6th and 7th
resolutions and (ii) to proceed with the corresponding issue, at the same price as the original issue and within a cap of
a 15% limit on the original issue, Decide that this delegation of authority is granted to the Board of Directors for a period of twenty-six
(26) months from the date of this Shareholders’ Meeting, beyond which it shall be considered null and void if the Board of Directors
does not use it. This delegation terminates any previous authorisation having the same purpose,
Decide that this authorization
must be implemented within thirty (30) days of the closing of the concerned initial issuance subscription, if the Board of Directors
has not made use of it within this thirty (30) day period, it shall be considered null and void in respect of the concerned issuance,
Decide that the nominal amount
of the corresponding issuance, immediately and/or in the future, pursuant to this authorization, shall be deducted from the limit of
the overall cap on share capital increases set in the 10th resolution of the present Shareholders’ Meeting.
History of Securities Issuances
From January 1, 2020 through December 31,
2022, the following events have changed the number and classes of our issued and outstanding shares:
| Month/Year | |
Transaction type | |
Number of
ordinary shares issued | |
| January 2020 | |
Conversion of convertible bonds held by Negma | |
| 1,700,000 | |
| February 2020 | |
Conversion of convertible bonds held by Negma | |
| 1,200,000 | |
| February 2020 | |
Share issuance | |
| 12,394,071 | |
| March 2020 | |
Conversion of convertible bonds held by Negma | |
| 500,000 | |
| May 2020 | |
Exercise of warrants | |
| 1,680,000 | |
| May 2020 | |
Exercise of warrants | |
| 80,145 | |
| June 2020 | |
Exercise of warrants | |
| 744,124 | |
| June 2020 | |
Private placement | |
| 2,050,000 | |
| June 2020 | |
Exercise of warrants | |
| 192,328 | |
| June 2020 | |
Exercise of warrants | |
| 694,444 | |
| June 2020 | |
Exercise of warrants | |
| 330,924 | |
| June 2020 | |
Conversion of convertible bonds held by Atlas | |
| 3,188,272 | |
| June 2020 | |
Share issuance | |
| 6,060,606 | |
| June 2020 | |
Exercise of warrants | |
| 52,727 | |
| July 2020 | |
Exercise of warrants | |
| 4,083 | |
| July 2020 | |
Conversion of convertible bonds held by Atlas | |
| 1,541,459 | |
| July 2020 | |
Private placement | |
| 9,563,732 | |
| July 2020 | |
Exercise of warrants | |
| 35,889 | |
| August 2020 | |
Exercise of warrants | |
| 22,831 | |
| August 2020 | |
Exercise of employee warrants | |
| 2,152 | |
| August 2020 | |
Conversion of convertible bonds held by Atlas | |
| 1,207,174 | |
| September 2020 | |
Exercise of warrant | |
| 19,574 | |
| September 2020 | |
Exercise of warrants | |
| 3,652 | |
| September 2020 | |
Exercise of warrants | |
| 1,406 | |
| September 2020 | |
Conversion of convertible bonds held by Atlas | |
| 7,806,116 | |
| October 2020 | |
Private placement | |
| 21,276,596 | |
| November 2020 | |
Conversion of convertible bonds held by Atlas | |
| 3,435,662 | |
| November 2020 | |
Exercise of warrants | |
| 34,115 | |
| December 2020 | |
Exercise of warrants | |
| 18,042 | |
| December 2020 | |
Exercise of warrants | |
| 34,083 | |
| January 2021 | |
Exercise of warrants | |
| 606,246 | |
| January 2021 | |
Exercise of employee warrants | |
| 313,417 | |
| January 2021 | |
Exercise of warrants | |
| 29,876 | |
| February 2021 | |
Exercise of warrants | |
| 206,092 | |
| February 2021 | |
Share issuance | |
| 12,000,000 | |
| February 2021 | |
Exercise of warrants | |
| 141,242 | |
| March 2021 | |
Exercise of warrants | |
| 585,936 | |
| April 2021 | |
Exercise of warrants | |
| 25,764 | |
| May 2021 | |
Exercise of warrants | |
| 47,265 | |
| Month/Year | |
Transaction type | |
Number of
ordinary shares issued | |
| May 2021 | |
Exercise of employee warrants | |
| 4,303 | |
| June 2021 | |
Exercise of warrants | |
| 6,796 | |
| June 2021 | |
Exercise of employee warrants | |
| 50,424 | |
| June 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,199,868 | |
| July 2021 | |
Exercise of warrants | |
| 605,632 | |
| July 2021 | |
Exercise of employee warrants | |
| 103,946 | |
| July 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,386,214 | |
| August 2021 | |
Share issuance | |
| 4,950,000 | |
| August 2021 | |
Exercise of warrants | |
| 1,793 | |
| August 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,159,648 | |
| September 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,444,685 | |
| September 2021 | |
Exercise of warrants | |
| 5,439 | |
| September 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,005,222 | |
| October 2021 | |
Conversion of convertible bonds held by Atlas | |
| 2,305,638 | |
| October 2021 | |
Exercise of warrants | |
| 3,722 | |
| November 2021 | |
Exercise of warrants | |
| 1,217 | |
| November 2021 | |
Conversion of convertible bonds held by Atlas | |
| 1,000,416 | |
| December 2021 | |
Exercise of warrants | |
| 453 | |
| December 2021 | |
Conversion of convertible bonds held by Atlas | |
| 3,001,070 | |
| December 2021 | |
Conversion of convertible bonds held by Atlas | |
| 2,362,346 | |
| December 2021 | |
Conversion of convertible bonds held by Atlas | |
| 590,586 | |
| January 2022 | |
Exercise of warrants | |
| 2,404 | |
| January 2022 | |
Conversion of convertible bonds held by Atlas | |
| 1,499,243 | |
| February 2022 | |
Exercise of warrants | |
| 141 | |
| February 2022 | |
Conversion of convertible bonds held by Atlas | |
| 3,550,550 | |
| March 2022 | |
Exercise of warrants | |
| 4,281 | |
| March 2022 | |
Conversion of convertible bonds held by Atlas | |
| 3,518,524 | |
| April 2022 | |
Conversion of convertible bonds held by Atlas | |
| 2,014,623 | |
| April 2022 | |
Exercise of warrants | |
| 2,535 | |
| April 2022 | |
Conversion of convertible bonds held by Atlas | |
| 4,832,504 | |
| May 2022 | |
Exercise of warrants | |
| 3,002 | |
| June 2022 | |
Conversion of convertible bonds held by Atlas | |
| 10,559,824 | |
| June 2022 | |
Exercise of warrants | |
| 5,241 | |
| June 2022 | |
Conversion of convertible bonds held by Atlas | |
| 2,795,821 | |
| July 2022 | |
Exercise of employee warrants | |
| 6,455 | |
| July 2022 | |
Exercise of warrant | |
| 665 | |
| August 2022 | |
Conversion of convertible bonds held by Atlas | |
| 10,164,735 | |
| September 2022 | |
Exercise of warrants | |
| 582 | |
| September 2022 | |
Conversion of convertible bonds held by Atlas | |
| 9,090,908 | |
| September 2022 | |
Conversion of convertible bonds held by Atlas | |
| 10,964,912 | |
| September 2022 | |
Attribution of free shares | |
| 6,631,068 | |
| October 2022 | |
Conversion of convertible bonds held by Atlas | |
| 1,623,376 | |
| October 2022 | |
Conversion of convertible bonds held by Atlas | |
| 1,666,666 | |
| November 2022 | |
Conversion of convertible bonds held by Atlas | |
| 10,563,380 | |
| November 2022 | |
Conversion of convertible bonds held by Atlas | |
| 1,976,284 | |
| November 2022 | |
Conversion of convertible bonds held by Atlas | |
| 2,012,072 | |
| December 2022 | |
Attribution of free shares | |
| 2,500,911 | |
Listing
The ADSs, each representing 100 of our ordinary
shares (or a right to receive 100 ordinary shares), are listed on the Nasdaq Capital Market under the symbol “BPTS.” Our
ordinary shares are currently listed on Euronext Growth Paris under the symbol “ALBPS.”
Transfer Agent and Registrar
The transfer agent and registrar for the ADSs
is Computershare, Inc. Our share register is currently maintained by Uptevia, 89-91, rue Gabriel Péri, 92120 Montrouge, France,
registered under n°439 430 976. The share register reflects only record owners of our ordinary shares. Holders of ADSs will not be
treated as one of our shareholders and their names will therefore not be entered in our share register. The depositary, the custodian
or their nominees will be the holder of the shares underlying the ADSs. Holders of the ADSs have a right to receive the ordinary shares
underlying the ADSs.
Pre-Funded Warrants
The following is a summary of
the material terms and provisions of the pre-funded warrants that are being offered hereby. This summary is subject to and qualified
in its entirety by the form of pre-funded warrants, which has been provided to the investors in this offering and which will be filed
with the SEC as an exhibit to our Report on Form 6-K in connection with this offering and incorporated by reference into the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
warrant for a complete description of the terms and conditions of the pre-funded warrants.
Form.
The pre-warrants will be issued in dematerialised (dématérialisé) registered form (au nominati). The
pre-funded warrants are freely negotiable and will be detachable from the ordinary shares upon issue. Title to the pre-funded warrants
held by the holders thereof will be established and evidenced in accordance with Article L.211-3 and R.211-1 of the
French Code monétaire et financier, or the French Monetary and Financial Code, by book-entries (inscription en compte).
No physical document of title will be issued with respect to the pre-funded warrants.
Exercise Price.
The exercise price to the investors for the 100 ordinary shares issuable upon exercise of a pre-funded warrant is €0.0001, equating
to €0.0001 per ordinary share (corresponding to $0.01 per pre-funded warrant and $0.01 per ADS). The exercise price and/or the number
of ordinary shares issuable upon exercise of the pre-funded warrants will be subject to adjustment from time to time according to mandatory
legal requirements imposed by the French Code de Commerce, or the French Commercial Code, and in particular by articles L. 228-98 to
L. 228-101 (with the exception of the provisions of Articles L. 228-99 1°) and L. 228-99 2°)) and
articles R. 228-90 to R. 228-92 of the French Commercial Code.
Exercisability.
The pre-funded warrants are exercisable at any time after their original issuance until 5:00 p.m. Paris time on July 21, 2033.
Each pre-funded warrant will be exercisable for 100 ordinary shares. The pre-funded warrants will be exercisable, at the option of each
holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of ordinary
shares purchased upon such exercise.
Limitations
on Exercisability. A holder (together with its affiliates and other attribution parties) may not exercise any portion
of a pre-funded warrant to the extent that immediately prior to or after giving effect to such exercise the holder would own more than
9.99% of our outstanding ordinary shares immediately after exercise, which percentage may be changed at the holder’s election to
a higher or lower percentage not in excess of 9.99% upon 61 days’ notice to us subject to the terms of the pre-funded warrants.
Suspension
of Exercisability. In the event of a capital increase, absorption, merger, spin-off or issuance of new ordinary
shares or securities giving access to the share capital, or any other financial transaction involving a preferential subscription right
or reserving a priority subscription period for the benefit of the our shareholders, we may suspend exercises of the pre-funded warrants
for a period that may not exceed the shorter of three months or any other required (rather than recommended) period set by the applicable
regulations. Our decision to suspend the ability to exercise the pre-funded warrants will be published (to the extent that such publication
is required under French law or any other form of communication compliant with applicable regulations) in the Bulletin des annonces
légales obligatoires and pursuant to the terms and conditions of the pre-funded warrants. The notice will be published
at least seven calendar days (so long as required by French law) before the suspension becomes effective and will indicate the dates
on which the suspension exercise of the pre-funded warrants will begin and end.
Adjustments
to Exercise Ratio and Exercise Price; Fundamental Transactions. The pre-funded warrants are considered securities giving
access to the share capital of the company within the meaning of Article L. 228-91 et seq. of the French Commercial
Code. In accordance with the provisions of Article R. 228-92 of the French Commercial Code, if we decide to issue new
ordinary shares or securities giving access to the capital with preferential subscription rights limited to our shareholders, to distribute
reserves (in cash or in kind) and share premiums or to change the distribution of our profits by creating preference shares, or to otherwise
carry out certain specified transactions, we will inform (as long as the current regulation so requires) the holders of pre-funded warrants
via an announcement in the Bulletin des Annonces Légales Obligatoires and pursuant to the notice terms of the
pre-funded warrants. If our company is absorbed by another company or merges or consolidates with (fusions) one or several other
companies to participate in the incorporation of a new entity, or proceeds with a split (scission), the holders of pre-funded
warrants will exercise their rights in the entity(ies) benefiting of the contributions in accordance with the provisions of Article L. 228-101 of
the French Commercial Code. Certain specified transactions may result in an adjustment to the exercise ratio and exercise price as specified
in the terms and conditions of the pre-funded warrants.
Fractional
Shares. No fractional ordinary shares will be issued upon the exercise of the pre-funded warrants, including fractional interests
in ADSs. Rather, the number of ordinary shares to be issued will be rounded, at the holder’s election, either up (in which case
the holder will pay us an amount equal to the value of the additional fraction of a share) or down (in which case we will make a cash
payment to the holder in an amount equal to the product of the exercise price and the remaining fractional share).
Fundamental
Transactions.In the event of any fundamental transaction, as described in the terms and conditions of the pre-funded warrants
and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange
offer, or reclassification of our ordinary shares, then upon any subsequent exercise of a pre-funded warrant, the holder will have the
right to receive as alternative consideration, for each ordinary share that would have been issuable upon such exercise immediately prior
to the occurrence of such fundamental transaction, the number of common shares of the successor or acquiring corporation or of our company,
if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of
the number of ordinary shares for which the pre-funded warrant is exercisable immediately prior to such event.
Transferability.
The pre-funded warrants will, upon issuance, solely be inscribed in our books. Title to the pre-funded warrants will be evidenced by
entries in our books, and transfer of the pre-funded warrants may only be effected through registration of the transfer in our books.
None of the pre-funded warrants will be inscribed in the books of nor admitted to the operations of Euroclear France.
Exchange
Listing. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
The ADSs representing the ordinary shares issuable upon exercise of the pre-funded warrants are currently listed on the Nasdaq Capital
Market.
Rights
as a Stockholder. Under French law, the holders of the pre-funded warrants, as a collective group, constitute a legal entity,
or the Masse, to defend their common interests. The Masse will be governed by the provisions of the French Commercial Code
(with the exception of the provisions of Article L.228-48 thereof), subject to the following provisions:
| · | The Masse will
be a separate legal entity by virtue of Article L.228-103 of the French Commercial
Code, acting in part through a representative and in part through a holders’ general
meeting; and |
| · | The Masse alone,
to the exclusion of all individual holders of the pre-funded warrants, will exercise the
common rights, actions and benefits which now or in the future may accrue with respect to
the pre-funded warrants. |
PRIVATE
PLACEMENT OF ORDINARY WARRANTS
Concurrently with the
sale of ADSs and pre-funded warrants in this offering, we will issue and sell to the investors, for each ADS or pre-funded warrant issued
in the offering, one unregistered ordinary warrant to purchase up to 100 ordinary shares represented by one ADS, with an exercise price
of $3.00 (or €2.66) per ADS, resulting in our issuance of ordinary warrants to purchase up to an aggregate of 133,433,400 ordinary
shares represented by 1,333,334 ADSs. The following is a summary of the material terms and provisions of the ordinary warrants being
offered in the concurrent private placement. This summary is subject to and qualified in its entirety by the form of ordinary warrant,
which has been provided to the investors and which will be filed with the SEC as an exhibit to our Report on Form 6-K in connection
with this offering and incorporated by reference into the registration statement of which this prospectus forms a part.
The
unregistered ordinary warrants and the ADSs and ordinary shares issuable upon exercise of the ordinary warrants will be issued and sold
without registration under the Securities Act, or state securities laws, in reliance on the exemptions provided by Section 4(a)(2) of
the Securities Act and/or Regulation D promulgated thereunder and in reliance on similar exemptions under applicable state laws. Accordingly,
the investors may exercise the unregistered ordinary warrants and sell the underlying ADSs and ordinary shares only pursuant to an effective
registration statement under the Securities Act covering the resale of those securities or pursuant to an applicable exemption from registration
under the Securities Act.
Form.
The ordinary warrants will be issued in dematerialised (dématérialisé) registered form (au nominatif).
The ordinary warrants are freely negotiable and will be detachable from the ordinary shares upon issue. Title to the ordinary warrants
held by the holders thereof will be established and evidenced in accordance with Article L.211-3 and R.211-1 of the
French Monetary and Financial Code, by book-entries (inscription en compte). No physical document of title will be issued with
respect to the ordinary warrants.
Exercise Price.
The exercise price to the investors for the 100 ordinary shares issuable upon exercise of an ordinary warrant is €0.026655, equating
to €0.026655 per ordinary share (corresponding to $3.00 per ordinary warrant and $3.00 per ADS). The exercise price and/or the number
of ordinary shares issuable upon exercise of the ordinary warrants will be subject to adjustment from time to time according to mandatory
legal requirements imposed by the French Commercial Code, and in particular by articles L. 228-98 to L. 228-101 (with
the exception of the provisions of Articles L. 228-99 1°) and L. 228-99 2°)) and articles R. 228-90 to
R. 228-92 of the French Commercial Code.
Exercisability.
The ordinary warrants are exercisable at any time after their original issuance until 5:00 p.m. Paris time on July 21, 2026.
Each ordinary warrant will be exercisable for 100 ordinary shares. The ordinary warrants will be exercisable, at the option of each holder,
in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of ordinary shares
purchased upon such exercise.
Limitations
on Exercisability. A holder (together with its affiliates and other attribution parties) may not exercise any portion
of an ordinary warrant to the extent that immediately prior to or after giving effect to such exercise the holder would own more than
4.99% (or, at the election of the purchaser, 9.99%) of our outstanding ordinary shares immediately after exercise, which percentage may
be changed at the holder’s election to a higher or lower percentage not in excess of 9.99% upon 61 days’ notice to us subject
to the terms of the ordinary warrants.
Suspension
of Exercisability. In the event of a capital increase, absorption, merger, spin-off or issuance of new ordinary
shares or securities giving access to the share capital, or any other financial transaction involving a preferential subscription right
or reserving a priority subscription period for the benefit of our shareholders, we may suspend exercises of the ordinary warrants for
a period that may not exceed three months or any other period set by the applicable regulations. In the event of any suspension related
to (i) an issuance of new ordinary shares or securities giving access to the share capital, or any other financial transaction involving
a preferential subscription right or reserving a priority subscription period for the benefit of our shareholders, or (ii) a stock-split
or a reverse stock-split, the exercise period will be automatically extended for the same duration as the period of suspension. Our decision
to suspend the ability to exercise the ordinary warrants will be published (to the extent that such publication is required under French
law or any other form of communication compliant with applicable regulations) in the Bulletin des annonces légales obligatoires and
pursuant to the terms and conditions of the ordinary warrants. The notice will be published at least seven calendar days (so long as
required by French law) before the suspension becomes effective and will indicate the dates on which the suspension exercise of the ordinary
warrants will begin and end.
Adjustments
to Exercise Ratio and Exercise Price; Fundamental Transactions. The ordinary warrants are considered securities giving
access to the share capital of the company within the meaning of Article L. 228-91 et seq. of the French Commercial
Code. In accordance with the provisions of Article R. 228-92 of the French Commercial Code, if we decide to issue new
ordinary shares or securities giving access to the capital with preferential subscription rights limited to our shareholders, to distribute
reserves (in cash or in kind) and share premiums or to change the distribution of our profits by creating preference shares, or to otherwise
carry out certain specified transactions, we will inform (as long as the current regulation so requires) the holders of ordinary warrants
via an announcement in the Bulletin des Annonces Légales Obligatoires and pursuant to the notice terms of the
ordinary warrants. If our company is absorbed by another company or merges or consolidates with (fusions) one or several other
companies to participate in the incorporation of a new entity, or proceeds with a split (scission), the holders of ordinary warrants
will exercise their rights in the entity(ies) benefiting of the contributions in accordance with the provisions of Article L. 228-101 of
the French Commercial Code. Certain specified transactions may result in an adjustment to the exercise ratio and exercise price as specified
in the terms and conditions of the ordinary warrants.
Fractional
Shares. No fractional ordinary shares will be issued upon the exercise of the ordinary warrants, including fractional interests
in ADSs. Rather, the number of ordinary shares to be issued will be rounded, at the holder’s election, either up (in which case
the holder will pay us an amount equal to the value of the additional fraction of a share) or down (in which case we will make a cash
payment to the holder in an amount equal to the product of the exercise price and the remaining fractional share).
Fundamental
Transactions. In the event of any fundamental transaction, as described in the terms and conditions of the ordinary warrants
and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange
offer, or reclassification of our ordinary shares, then upon any subsequent exercise of an ordinary warrant, the holder will have the
right to receive as alternative consideration, for each ordinary share that would have been issuable upon such exercise immediately prior
to the occurrence of such fundamental transaction, the number of common shares of the successor or acquiring corporation or of our company,
if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of
the number of ordinary shares for which the ordinary warrant is exercisable immediately prior to such event.
Additionally,
in the event of any fundamental transaction, at the holder’s option, our company or any successor entity will purchase the ordinary
warrant from the holder by paying an amount of cash equal to the Black Scholes Value (as defined in the terms and conditions of the ordinary
warrants) of the remaining unexercised portion of the ordinary warrant on the date of the consummation of such fundamental transaction.
Transferability.
The ordinary warrants will, upon issuance, solely be inscribed in our books. Title to the ordinary warrants will be evidenced by entries
in our books, and transfer of the ordinary warrants may only be effected through registration of the transfer in our books. None of the
ordinary warrants will be inscribed in the books of nor admitted to the operations of Euroclear France.
Exchange
Listing. We do not intend to list the ordinary warrants on any securities exchange or nationally recognized trading system.
The ADSs representing the ordinary shares issuable upon exercise of the ordinary warrants are currently listed on the Nasdaq Capital
Market.
Rights
as a Stockholder. Under French law, the holders of the ordinary warrants, as a collective group, constitute a legal entity,
or the Masse, to defend their common interests. The Masse will be governed by the provisions of the French Commercial Code
(with the exception of the provisions of Article L.228-48 thereof), subject to the following provisions:
| · | The Masse will
be a separate legal entity by virtue of Article L.228-103 of the French Commercial
Code, acting in part through a representative and in part through a holders’ general
meeting; and |
| · | The Masse alone,
to the exclusion of all individual holders of the ordinary warrants, will exercise the common
rights, actions and benefits which now or in the future may accrue with respect to the ordinary
warrants. |
Registration Rights
Pursuant
to the terms of the investor subscription agreements, we are required to file a resale registration statement on Form F-1, or the
Registration Statement, with the SEC to register for resale the ordinary shares represented by ADSs issuable upon exercise of the unregistered
ordinary warrants, within 30 days of the signing date of the investor subscription agreement, or the Signing Date, and to have such Registration
Statement declared effective within 60 days after the Signing Date in the event the Registration Statement is not reviewed by the SEC,
or 90 days of the Signing Date in the event the Registration Statement is reviewed by the SEC in full.
PLAN OF DISTRIBUTION
Pursuant
to a placement agency agreement dated July 18, 2023, we have engaged H.C. Wainwright & Co., LLC, or the placement agent,
to act as our exclusive placement agent in connection with this offering of the ADSs representing our ordinary shares pursuant to this
prospectus supplement and accompanying prospectus. Under the terms of the placement agency agreement, the placement agent has agreed
to be our exclusive placement agent, on a reasonable best efforts basis, in connection with the issuance and sale by us of our securities
in this takedown from our shelf registration statement. The terms of this offering were subject to market conditions and negotiations
between us, the placement agent and prospective investors. The placement agency agreement does not give rise to any commitment by the
placement agent to purchase any of our securities, and the placement agent will have no authority to bind us by virtue of the placement
agency agreement. Further, the placement agent does not guarantee that it will be able to raise new capital in any prospective offering
and is not purchasing or selling any securities, nor is required to arrange for the purchase and sale of any specific number or dollar
amount of securities other than the use its reasonable “best efforts” to arrange for the sale of securities by us. Therefore,
we may not sell the entire amount of securities being offered. The placement agent may engage one or more sub-agents or selected dealers
to assist with the offering.
We
have entered into a subscription agreement directly with each investor in connection with this offering, and we will only sell our securities
offered hereby to investors who have entered into a subscription agreement.
We
expect to deliver the securities being offered pursuant to this prospectus supplement and the accompanying prospectus on or about July 21,
2023, subject to satisfaction of certain customary closing conditions.
We
will pay the placement agent a cash fee equal to 7.0% of the aggregate gross proceeds of this offering and the concurrent private placement.
We will also pay the placement agent a management fee equal to 1.0% of the gross proceeds raised in this offering and the concurrent
private placement, a non-accountable expense allowance of €40,000 in connection with this offering and the concurrent private placement,
up to €150,000 for fees and expenses of legal counsel and other out-of-pocket expenses, and closing costs. We estimate our total expenses
associated with the offering, excluding placement agent fees and expenses, will be approximately $122,550.
The
following table sets forth the per ADS and total cash placement agent’s fees we will pay to the placement agent in connection with
the sale of the ADSs and pre-funded warrants pursuant to this prospectus supplement and the accompanying prospectus:
| |
|
Per ADS |
|
|
Per Pre-Funded
Warrant |
|
|
Total |
|
| Offering price |
|
$ |
2.85 |
|
|
$ |
2.85 |
|
|
$ |
3,800,002 |
|
| Placement agent’s fees |
|
$ |
0.19 |
|
|
$ |
0.19 |
|
|
$ |
266,000 |
|
| Proceeds, before expenses, to us |
|
$ |
2.66 |
|
|
$ |
2.66 |
|
|
$ |
3,534,002 |
|
After
deducting those fees and expenses due to the placement agent and our other estimated offering expenses, we expect the net proceeds from
this offering and the sale of the unregistered ordinary warrants in the concurrent private placement to be approximately $3.1 million
(or €2.8 million), assuming full exercise of the pre-funded warrants.
Right of First Refusal
For a period of 15-months following the consummation of this offering or any other private or public offering during
the term of our engagement of the underwriter, if we or any of our subsidiaries decides to raise funds by means of a public offering (including
an at-the-market facility) or a private placement or any other capital-raising financing of equity or equity-linked, the placement agent
(or any affiliate designated by the placement agent) shall have the right to act as sole book running manager, sole underwriter or sole
placement agent for such financing.
Tail Financing Payments
We have also agreed to pay the underwriter a tail fee equal to the cash compensation
in this offering, if any investor, who was contacted or introduced to us by the placement agent during the term of its engagement, provides
us with capital in any public or private offering or other financing or capital raising transaction during the 12-month period following
the termination or expiration of our engagement agreement with the placement agent.
Lock-up Restrictions
Pursuant
to the placement agency agreement, we have agreed that, for a period commencing on the date of this prospectus and ending on the date
that is the 90th day immediately following the date of this prospectus, we will not, without the placement agent’s prior
written consent, directly or indirectly, issue, enter into an agreement to issue or announce the issuance or proposed issuance of the
ADSs, ordinary shares or ordinary shares equivalents, subject to certain customary exceptions.
Indemnification
We
have agreed to indemnify the placement agent and specified other persons against certain civil liabilities, including liabilities under
the Securities Act, and the Securities Exchange Act of 1934, as amended, or the Exchange Act, and to contribute to payments that the
placement agent may be required to make in respect of such liabilities.
Regulation M
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by it, and any profit realized on the resale of the securities sold by it while acting as principal, might be deemed to be underwriting
discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements
of the Securities Act and the Securities Exchange Act of 1934, as amended, or Exchange Act, including, without limitation, Rule 415(a)(4) under
the Securities Act, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of
purchases and sales of ADSs, ordinary shares and warrants by the placement agent acting as principal. Under these rules and regulations,
the placement agent:
| · | may
not engage in any stabilization activity in connection with our securities; and |
| · | may
not bid for or purchase any of our securities, or attempt to induce any person to purchase
any of our securities, other than as permitted under the Exchange Act, until it has completed
its participation in the distribution in the securities offered by this prospectus supplement. |
Other Relationships
The
placement agent and its affiliates may have provided us and our affiliates in the past, and may provide from time to time in the future,
certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course
of their business, for which they have received, and may continue to receive, customary fees and commissions. In addition, from time
to time, the placement agent and their affiliates may effect transactions for their own account or the account of customers, and hold
on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
The placement agent acted as our sole book-running manager in our initial public offering in 2021, for which it received compensation.
The foregoing description of
the placement agency agreement is only a summary, does not purport to be complete and is qualified in its entirety by reference to such,
a copy of which will be attached as an exhibit to our Report on Form 6-K being filed with the SEC in connection with this offering.
The depositary for the ADSs
to be issued in this offering is The Bank of New York Mellon.
EXPERTS
The consolidated financial statements
of Biophytis S.A. as of December 31, 2021 and for each of the two years in the period ended December 31, 2021 incorporated
by reference in this prospectus supplement and accompanying prospectus have been audited by Ernst & Young et Autres, independent
registered public accounting firm, as set forth in their report thereon included therein, and incorporated herein by reference. Such
consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm
as experts in accounting and auditing.
The offices of Ernst &
Young et Autres are located at 1/2 place des Saisons, 92400 Courbevoie, Paris La Défense 1, France.
The consolidated financial statements
of Biophytis S.A. as of December 31, 2022, and for the year then ended, have been incorporated by reference herein in reliance upon
the report of KPMG S.A., independent registered public accounting firm, incorporated by reference herein, and upon the authority of said
firm as experts in accounting and auditing.
The offices of KPMG S.A. are
located at 2, avenue Gambetta, CS 60055, 92066 Paris-la-Défense, France.
LEGAL MATTERS
Reed Smith, LLP, New York, New
York has passed upon certain legal matters regarding the securities offered hereby under U.S. law, and Reed Smith LLP, Paris, France,
has passed upon certain legal matters regarding the securities offered hereby under French law.
WHERE YOU CAN FIND MORE
INFORMATION
We have filed with the SEC a
registration statement on Form F-3 under the Securities Act, including amendments and relevant exhibits and schedules, covering
the underlying securities offered hereby. This prospectus, which constitutes a part of the registration statement on Form F-3, summarizes
material provisions of contracts and other documents that we refer to in the prospectus. Since this prospectus does not contain all of
the information contained in the registration statement, you should read the registration statement and its exhibits and schedules for
further information with respect to us and the securities offered hereby.
We file annual reports on Form 20-F
and other information with the SEC and furnish reports on Form 6-K to the SEC. We are not required to disclose certain other information
that is required from U.S. domestic issuers. Also, as a foreign private issuer, we are exempt from the rules of the Exchange Act
prescribing the furnishing of proxy statements to shareholders and our directors, senior management and principal shareholders are exempt
from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
As a foreign private issuer,
we are also exempt from the requirements of Regulation FD (Fair Disclosure) which, generally, are meant to ensure that select groups
of investors are not privy to specific information about an issuer before other investors. We are, however, still subject to the anti-fraud
and anti-manipulation rules of the SEC, such as Rule 10b-5. Since many of the disclosure obligations required of us as a foreign
private issuer are different than those required by other U.S. domestic reporting companies, our shareholders, potential shareholders
and the investing public in general should not expect to receive information about us in the same amount and at the same time as information
is received from, or provided by, other U.S. domestic reporting companies. We are liable for violations of the rules and regulations
of the SEC which do apply to us as a foreign private issuer.
Our
SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. You may also
access our SEC filings at our website www.biophytis.com. Our website and the information contained on, or that can be accessed
through, our website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should
not rely on our website or any such information in making your decision whether to purchase our securities.
INCORPORATION BY REFERENCE
The SEC allows us to incorporate
by reference the information we file with it, which means that we can disclose important information to you by referring you to another
document that we have filed separately with the SEC. You should read the information incorporated by reference because it is an important
part of this prospectus. We incorporate by reference the following information or documents that we have filed with the SEC:
All annual reports on Form 20-F
and any amendment thereto and any report on Form 6-K (or portion thereof) that expressly indicates it is being incorporated by reference
in this prospectus, in each case, that we file with or furnish to the SEC prior to the termination or completion of the offering under
this prospectus (including all such reports or documents we may file with or furnish to the SEC on or after the date on which the registration
statement of which this prospectus is a part is first filed with the SEC and prior to the effectiveness of the registration statement),
will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing or furnishing
of such reports and documents. Unless expressly incorporated by reference, nothing in this prospectus shall be deemed to incorporate
by reference information furnished to, but not filed with, the SEC.
Any statement contained in any
document incorporated by reference herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent
that a statement contained in this prospectus or any prospectus supplement modifies or supersedes such statement. Any statement so modified
or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
All of the documents that are
incorporated by reference are available at the website maintained by the SEC at http://www.sec.gov. In addition, copies of
all documents incorporated by reference in this prospectus, other than exhibits to those documents unless such exhibits are specifically
incorporated by reference in this prospectus, will be provided at no cost to each person, including any beneficial owner, to whom a copy
of this prospectus is delivered on the written or oral request of that person made to: Biophytis S.A., Sorbonne University-BC 9, Bâtiment
A 4ème étage, 4 place Jussieu 75005 Paris, France, Attention: Chief Executive Officer.
EXPENSES
The following table sets forth
estimated costs and expenses, other than any placement agent fees and expenses, we expect to incur in connection with the offering.
| Legal fees and expenses |
|
$ |
112,550 |
|
| Accounting fees and expenses |
|
$ |
20,000 |
|
| Total |
|
$ |
132,550 |
|
$100,000,000
Ordinary Shares
Preferential
Shares
Warrants
Debt Securities
Units

Biophytis S.A.
We may, from time to time in one or more offerings, offer and
sell, together or separately, ordinary shares, which may be represented by American Depositary Shares (“ADSs”), preferential
shares, which may be represented by ADSs, warrants, debt securities or any combination thereof in units as described in this prospectus.
Any ADS will represent a specified number of ordinary shares or preferential shares. The preferential shares may be convertible into
or exchangeable for ordinary shares, the warrants may be exercisable for ordinary shares, preferential shares or debt securities and
the debt securities may be convertible into or exchangeable for ordinary shares or preferential shares or other debt securities.
This prospectus provides a general description of
the securities we may offer. We will provide the specific terms of the securities offered in one or more supplements to this prospectus.
We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus
supplement and any related free writing prospectus may add, update or change information contained in this prospectus. You should read
carefully this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as the documents incorporated
or deemed to be incorporated by reference, before you invest in any of our securities. This prospectus may not be used to offer or sell
any securities unless accompanied by the applicable prospectus supplement.
ADSs, each representing 100 of our ordinary shares (or a right to
receive 100 ordinary shares), are listed on the Nasdaq Capital Market under the symbol “BPTS.” The last reported sale price
of the ADSs on the Nasdaq Capital Market on April 20, 2023 was $3.88 per ADS.
As of April 21, 2023, the aggregate market value of our outstanding
ordinary shares held by non-affiliates, or public float, was approximately $18,824,730, based on 311,416,551 of our ordinary shares outstanding,
of which approximately 299,280,298 shares are held by non-affiliates, and a per share price of approximately $0.0629, which represents
one one-hundredth of $6.29, which was the price of our ADSs on March 6, 2023, and which was the highest reported closing sale price of
our ADSs on The Nasdaq Capital Market, the principal market for our common equity, in the 60 days prior to April 21, 2023. We have
not offered any securities pursuant to General Instruction I.B.5 of Form F-3 during the prior 12 calendar month period that ends
on and includes the date of this prospectus. Pursuant to General Instruction I.B.5. of Form F-3, in no event will we sell securities
registered on this registration statement in a public primary offering with a value exceeding more than one-third of our public float
in any 12-month period so long as our public float remains below $75 million.
We may offer and sell our securities to or through one or more agents,
underwriters, dealers or other third parties or directly to one or more purchasers on a continuous or delayed basis. If agents, underwriters
or dealers are used to sell our securities, we will name them and describe their compensation in a prospectus supplement. The price to
the public of our securities and the net proceeds we expect to receive from the sale of such securities will also be set forth in a prospectus
supplement.
Investing
in our securities involves a high degree of risk. Before buying any of our securities, you should carefully read the discussion of material
risks of investing in our securities. Please see the section entitled “Risk Factors”
beginning on page 8 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation
to the contrary is a criminal offense.
The
date of this prospectus is May 1 , 2023
TABLE OF CONTENTS
We are responsible for the information contained and incorporated
by reference in this prospectus, in any accompanying prospectus supplement, and in any related free writing prospectus we prepare or
authorize. We have not authorized anyone to give you any other information, and we take no responsibility for any other information that
others may give you. If you are in a jurisdiction where offers to sell, or solicitations of offers to purchase, the securities offered
by this documentation are unlawful, or if you are a person to whom it is unlawful to direct these types of activities, then the offer
presented in this document does not extend to you. The information contained in this document speaks only as of the date of this document,
unless the information specifically indicates that another date applies. Our business, financial condition, results of operations and
prospects may have changed since those dates.
ABOUT THIS PROSPECTUS
This prospectus is part of a shelf registration statement that we
have filed with the U.S. Securities and Exchange Commission (the “SEC”). Under this shelf registration, we may offer our
ordinary shares or preferential shares, which may be in the form of ADSs, warrants to purchase ordinary shares, preferential shares or
debt securities, debt securities, or any combination thereof in the form of units, from time to time in one or more offerings.
This prospectus only provides you with a general description of the
securities that we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement
that will contain more specific information about the specific terms of the offering. If any such securities are to be listed or quoted
on a securities exchange or quotation system, the applicable prospectus supplement will say so. We may also authorize one or more free
writing prospectuses to be provided to you that may contain material information relating to these offerings. This prospectus may not
be used to sell our securities unless accompanied by a prospectus supplement. Each such prospectus supplement and any free writing prospectus
that we may authorize to be provided to you may also add, update or change information contained in this prospectus or in documents incorporated
by reference into this prospectus. We urge you to carefully read this prospectus, any applicable prospectus supplement and any related
free writing prospectus, together with the information incorporated herein by reference as described under the headings “Where
You Can Find More Information” and “Incorporation by Reference” before you invest in our securities.
We have not authorized anyone to provide you with additional information
or information different from that contained in or incorporated by reference in this prospectus, any applicable prospectus supplement
and any related free writing prospectus filed with the SEC. We take no responsibility for, and can provide no assurances as to the reliability
of, any information not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus that
we may authorize to be provided to you. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances
and in jurisdictions where offers and sales of the securities are legally permitted.
The information contained in this prospectus, any applicable prospectus
supplement or any related free writing prospectus we file is accurate only as of the date on the front of the document and any information
incorporated by reference in such document is accurate only as of the date of the document incorporated by reference, regardless of the
time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus, or any sale of a security.
Our business, financial condition, results of operations and prospects may have changed since that date. We will update this prospectus
to the extent required by law.
This prospectus contains summaries of certain provisions contained
in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries
are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be
filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may
obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
We further note that the representations, warranties and covenants
made by us in any agreement that is filed as an exhibit to the registration statement of which this prospectus is a part were made solely
for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such
agreement rather than establishing matters of fact. The information in the exhibits should not be read alone and instead should be read
in conjunction with the information in this prospectus and other filings that we make with the SEC. Moreover, such representations, warranties
or covenants were accurate only as of the date they were made. Accordingly, such representations, warranties and covenants should not
be relied on as accurately representing the current state of our affairs.
Unless otherwise indicated or the context otherwise requires, references
in this prospectus to “Biophytis,” “the Company,” “we,” “us,” and “our” refer
to Biophytis S.A. and its consolidated subsidiaries. All references in this prospectus to “$,” “U.S. dollars,”
“dollars” and “USD” mean U.S. dollars and all references to “€” and “euros” mean
euros, unless otherwise noted.
Unless otherwise mentioned or unless the context requires otherwise,
throughout this prospectus, any applicable prospectus supplement and any related free writing prospectus, the words “Biophytis,”
“we,” “us,” “our,” “the company,” “our company” or similar references refer
to Biophytis S.A. and its consolidated subsidiaries; and the term “securities” refers collectively to our ordinary shares,
which may be in the form of ADSs, preferential shares, which may be in the form of ADSs, warrants to purchase ordinary shares, preferential
shares or debt securities, debt securities, or any combination of the foregoing securities in units.
MARKET
AND INDUSTRY DATA
Unless otherwise indicated, information contained
in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position,
market opportunity and market size estimates, is based on information from independent industry analysts, third-party sources and management
estimates. Management estimates are derived from publicly available information released by independent industry analysts and third-party
sources, as well as data from our internal research, and are based on assumptions made by us based on such data and our knowledge of
such industry and market, which we believe to be reasonable. Although we are responsible for all of the disclosures contained in this
prospectus, we have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic
assumptions relied upon therein. In addition, while we believe the market opportunity information included in this prospectus is generally
reliable and is based on reasonable assumptions, such data involves risks and uncertainties, including those discussed under the heading
"Risk Factors."
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus includes certain forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of
the Securities Exchange Act of 1934, as amended (the “Exchange Act”), concerning our business, operations and financial performance
and condition as well as our plans, objectives and expectations for our business operations and financial performance and condition.
Any statements that are not of historical facts may be deemed to be forward-looking statements. You can identify these forward-looking
statements by words such as “believes,” “estimates,” “anticipates,” “expects,” “plans,”
“intends,” “may,” “could,” “might,” “will,” “should,” “aims,”
or other similar expressions that convey uncertainty of future events or outcomes. Forward-looking statements include, but are not limited
to, statements about:
| · | the timing, progress
and results of clinical trials for our drug candidates, including statements regarding the
timing of initiation and completion of clinical trials, dosing of subjects and the period
during which the results of the clinical trials will become available; |
| · | the potential impact
of COVID-19 on our clinical trials and our operations generally; |
| · | the timing, scope
or likelihood of regulatory filings and approvals for our drug candidates; |
| · | our ability to successfully
commercialize our drug candidates; |
| · | potential benefits
of the clinical development and commercial experience of our management team; |
| · | our ability to effectively
market any drug candidates that receive regulatory approval, emergency use authorization,
or conditional marketing authorization on our own or through third parties; |
| · | our commercialization,
marketing and manufacturing capabilities and strategy; |
| · | our expectation regarding
the safety and efficacy of our drug candidates; |
| · | the potential clinical
utility and benefits of our drug candidates; |
| · | our ability to advance
our drug candidates through various stages of development, especially through pivotal safety
and efficacy trials; |
| · | the likelihood of
success and difficulty in ensuring success of clinical investigations; |
| · | our estimates regarding
the potential market opportunity for our drug candidates; |
| · | developments and projections
relating to our competitors or our industry; |
| · | our ability to become
profitable; |
| · | our estimates regarding
expenses, future revenue, capital requirements and needs for additional financing; |
| · | our ability to secure
additional financing when needed on acceptable terms; |
| · | the impact of government
laws and regulations in the United States, France and foreign countries; |
| · | the implementation
of our business model, strategic plans for our business, drug candidates and technology; |
| · | our intellectual property
position; |
| · | our ability to rely
on orphan drug designation for market exclusivity; |
| · | our ability to attract
or retain key employees, advisors or consultants; and |
| · | whether we are classified
as a passive foreign investment company for current and future periods. |
By their nature, forward-looking statements involve risks and uncertainties
because they relate to events, competitive dynamics and industry change, and depend on economic circumstances that may or may not occur
in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each
forward-looking statement contained in this prospectus, we caution you that forward-looking statements are not guarantees of future performance
and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. All of our forward-looking
statements are subject to risks and uncertainties that may cause our actual results to differ materially from our expectations.
Any forward-looking statements that we make in this prospectus speak
only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after
the date of this prospectus or to reflect the occurrence of unanticipated events. Comparisons of results for current and any prior periods
are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed
as historical data. You should, however, review the factors and risks we describe in the reports we will file from time to time with
the SEC after the date of this prospectus. See “Where You Can Find More Information.”
You should also read carefully the factors described in the “Risk
Factors” section of this prospectus, in “Item 3. Key Information—D. Risk Factors” in our Annual
Report on Form 20-F for the year ended December 31, 2022 and in the other documents that we file with the SEC after the
date of this prospectus that are incorporated by reference into this prospectus to better understand the risks and uncertainties inherent
in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking
statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy
may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements
as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or
at all.
ABOUT THE COMPANY
Our Company
We are a clinical-stage biotechnology company
focused on the development of therapeutics that are aimed at slowing the degenerative processes associated with aging and improving functional
outcomes for patients suffering from age-related diseases, including severe respiratory failure in patients suffering from COVID-19.
Our goal is to become a leader in the emerging field of aging science by delivering life-changing therapies to the growing number of
patients in need. To accomplish this goal, we have assembled an experienced and skilled group of industry professionals, scientists,
clinicians and key opinion leaders from leading industry and academic institutions around the world.
A number of degenerative diseases associated with
aging have been characterized in the last century, including sarcopenia and age-related macular degeneration (AMD). The pathophysiology
of these and many other age-related diseases is not yet well understood, and effective treatment options are lacking. The global population
of people over the age of 60 is expected to double from approximately 962 million in 2017 to 2.1 billion by 2050, according
to estimates from the United Nations’ World Population Prospects: the 2017 Revision. We believe that the need for effective therapeutics
for age-related diseases will continue to grow throughout the 21st century. In addition, healthcare costs, including
costs associated with treatments and long-term care for age-related diseases associated with this demographic shift, are expected to
rise proportionally, as effective treatment options are currently lacking. We believe that developing treatments to slow disease progression
and reduce the risk of severe disability associated with age-related diseases is of the utmost importance.
As we age, our physical, respiratory, visual and
cognitive performances gradually decline due, in part, to the cumulative deleterious effect of multiple biological and environmental
stresses, including current and emerging viral infections, to which we are exposed during our lifetime. The functional decline can be
much faster in some individuals as a consequence of, among other things, the degenerative processes affecting specific cells, tissues
and organs. Through evolution, cells, tissues and organisms have developed natural means or pathways to counteract and balance the effects
of the many stresses they face. This natural ability to compensate for stress and remain functional, called biological resilience, degrades
over time. The decline in biological resilience contributes to the acceleration of these degenerative processes and the impairment of
functional performances, which, in turn, can lead to severe disability, reduced health-span and ultimately death. This occurs as we age,
but can occur at a younger age, when genetic mutations exist, or in the case of infection and inflammation.
Our lead drug candidate, Sarconeos (BIO101), is
a plant-derived pharmaceutical-grade purified 20-hydroxyecdysone that is an orally administered small molecule.
The initial indication we are seeking approval
for is sarcopenia, an age-related degeneration of skeletal muscle, which is characterized by a loss of muscle mass, strength and function
in elderly people (adults 65 years of age and older) leading to reduced mobility, or mobility disability, and increased risk of adverse
health events and hospitalization, and potential death resulting from falls, fractures, and physical disability. There is currently no
approved medication for sarcopenia, which is present in the elderly (greater than 65 years old) with an estimated prevalence range between
six to 22% worldwide.
Sarconeos (BIO101) is also being developed to
treat patients with severe respiratory manifestations of COVID-19. Our therapeutic approach is aimed at targeting and activating key
biological resilience pathways that can protect against and counteract the effects of the multiple biological and environmental stresses,
including inflammatory, oxidative, metabolic and viral stresses that lead to age-related diseases. We have conducted the COVA study,
a global, multicenter, double-blind, placebo-controlled, group-sequential, and adaptive two-part Phase 2-3 study, in patients with SARS-CoV-2
pneumonia. Final results from this study were released on February 2, 2023. The study met its pre-defined primary endpoint demonstrating
a statistically significant difference between Sarconeos (BIO101) and placebo in the proportion of patients with respiratory failure
or early death at day 28, representing a relative reduction of risk of 44% (p=0.043, Cochran-Mantel-Haenszel test). Moreover, the analysis
of time to respiratory failure or early death had shown significant differences over 28 days in the Kaplan Meier curves for Sarconeos
(BIO101) versus placebo (p=0.022). The pre-specified analysis of time to death over the complete follow-up period over 90 days showed
that mortality rate with Sarconeos (BIO101) was reduced compared to placebo in the ITT population (p=0.083) and in the PP population
(p=0.038).
Most people infected with the COVID-19 virus and
its variants experience mild to moderate respiratory illness and recover without requiring special treatment. Older people, and those
with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease and cancer are more likely to develop
serious illness and to be at risk of respiratory failure. Based on the positive data from our COVA Phase 2-3 study, we initiated the
Early Access Program regulatory path in France in March 2023. We intend to renew the expanded access program to treat hospitalized
patients with severe COVID-19 symptoms that are mechanically ventilated with Sarconeos (BIO101) in Brazil as we did initially received
approval for such a program in January 2022. We also continue to prepare conditional marketing authorization applications in Europe
and in the US due to the health emergency
We are also developing Sarconeos (BIO101) for
Duchenne muscular dystrophy (“DMD”), a rare genetic neuromuscular disease in male children and young adults, which is characterized
by an accelerated degeneration of muscle and is responsible for a loss of mobility, respiratory failure and cardiomyopathy, leading to
premature death. There is currently no cure and limited treatment options for DMD, which affects approximately 2.8 out of 100,000 people
worldwide (approximately 20,000 new cases annually worldwide), based on our estimates from publicly available information.
Our second drug candidate, Macuneos (BIO201),
is an orally administered small molecule in development for the treatment of retinopathies. It is a plant-derived pharmaceutical-grade
purified norbixin. We have completed preclinical cellular and animal studies of Macuneos (BIO201) for the treatment of retinopathies.
While we are still in the early stages of development, we believe that the results from our preclinical studies support continued investigation
into whether Macuneos (BIO201) may stimulate biological resilience and protect the retina against phototoxic damage that leads to vision
loss. The initial indication we plan to seek approval for is dry AMD, a common eye disorder among people over the age of 50 that affects
central vision, impairing functions such as reading, driving, and facial recognition, and has a major impact on quality of life and the
ability to live independently. There are currently no approved treatments for dry AMD. Based on our estimates from publicly available
information, AMD affects approximately 8.5% of the global population (ages 45 to 85) and is expected to increase over time as the population
ages.
We are also exploring Macuneos (BIO201) as a potential
treatment for Stargardt disease, which shares many of the characteristics of dry AMD. Stargardt disease is the most common form of inherited
macular degeneration that typically develops in childhood and leads to vision loss and, in some cases, blindness.
We hold exclusive commercialization rights through
licenses for each of our drug candidates. We currently plan to develop our drug candidates through clinical PoC (typically Phase 2),
and then seek licensing and/or partnership opportunities for further clinical development through regulatory approval and commercialization.
We have developed our lead clinical drug candidate
Sarconeos (BIO101), preclinical drug candidate Macuneos (BIO201), and a preclinical pipeline of life-cycle extension products, consisting
of BIO103 and BIO203, through a drug discovery platform in collaboration with Sorbonne University in Paris, France based on work with
medicinal plants. Plants are major sources of small molecules, called secondary metabolites, which they produce as a defense mechanism
to various environmental stresses, including attack from predatory and pathogenic species (e.g., insects, bacteria and fungi).
Our drug discovery platform is based on a reverse pharmacology approach that tests a collection of bioactive secondary metabolites along
with chemical analogs that we have synthesized in phenotypic screens of various age-related diseases. Our long-term goal is to advance
the field of aging science with the continued discovery and development of new drug candidates that treat age-related diseases by stimulating
biological resilience pathways that are involved in the aging process and/or age-related diseases.
We have assembled an executive team of scientific,
clinical, and business leaders with broad expertise in biotechnology and clinical drug development (see Item 6.A for more information
on our directors and senior management).
Our Clinical Pipeline
We are developing a portfolio of programs targeting
biological resilience pathways that slow the degenerative processes associated with aging and improve functional outcomes for patients
suffering from age-related diseases. Our current pipeline of drug candidates is illustrated below.
Recent Developments
On
March 30, 2023, we effected a change to the ratio of our ADSs to ordinary shares from one ADS representing 10 ordinary shares
to one ADS representing 100 ordinary shares.
On April 17 ; 2023, the board of directors, acting upon delegation
of the shareholder meeting held on the same day, reduced the share capital from EUR 62,283,310.20 to EUR 3,114,165.51 by way of cancellation
of losses. The capital reduction was effected by reducing the par value of the ordinary shares from EUR 0.20 to EUR 0.01.
Company Information
We were incorporated as a société anonyme under
the laws of France on September 27, 2006. We are registered at the Paris Registre du Commerce et des Sociétés under
the number 492 002 225. Our principal executive offices are located at Sorbonne University-BC 9, Bâtiment A 4ème étage,
4 place Jussieu 75005 Paris, France and our telephone number is +33 1 44 27 23 00. Our website address is www.biophytis.com. Our
agent for service of process in the United States is Puglisi & Associates, 850 Library Avenue, Suite 204, Newark, Delaware
19711. The reference to our website is an inactive textual reference only and the information contained in, or that can be accessed through,
our website is not a part of this prospectus and should not be considered a part of this prospectus or any supplement to this prospectus.
Our ordinary shares are listed on Euronext Growth Paris (Ticker: ALBPS
- ISIN: FR0012816825). The ADSs (American Depositary Shares) are listed on the Nasdaq Capital Market (Ticker: BPTS – ISIN: US09076G1040)
since February 10, 2021.
RISK FACTORS
Investing in our securities involves a high degree of risk. You should
carefully consider the risks described in “Item 3. Key Information—D. Risk Factors” in our Annual
Report on Form 20-F for the fiscal year ended December 31, 2022, which is incorporated herein by reference, and other documents
we file with the SEC that are incorporated by reference in this prospectus and any applicable prospectus supplement, before making an
investment decision. Each of the risks described could materially adversely affect our business, financial condition or results of operations,
or the trading price of our securities. In such case, you could lose all or a portion of your original investment. See “Where You
Can Find More Information.”
OFFER STATISTICS AND
EXPECTED TIMETABLE
We may from time to time, offer and sell any combination
of the securities described in this prospectus up to a total dollar amount of $100,000,000 in one or more offerings. The securities offered
under this prospectus may be offered separately, together, or in separate series, and in amounts, at prices, and on terms to be determined
at the time of sale. We will keep the registration statement of which this prospectus is a part effective until such time as all of the
securities covered by this prospectus have been disposed of pursuant to and in accordance with such registration statement.
USE OF PROCEEDS
Unless
otherwise set forth in a prospectus supplement, we currently intend to use the net proceeds of any offering of securities for working
capital and other general corporate purposes. Accordingly, we will have significant discretion in the use of any net proceeds. We may
provide additional information on the use of the net proceeds from the sale of the offered securities in an applicable prospectus supplement
relating to the offered securities.
DESCRIPTION OF SHARE
CAPITAL
The following description of our share capital
summarizes certain provisions of our articles of associations. Such summaries do not purport to be complete and are subject to, and are
qualified in their entirety by reference to, all of the provisions of our articles of association, copies of which have been filed with
the SEC. Holders of ADSs will be able to exercise their rights with respect to the ordinary shares underlying the ADSs only in accordance
with the terms of the deposit agreement. See “Description of American Depositary Shares” for more information.
General
As of April 21, our outstanding share capital consisted of a
total of 311,416,551 issued and fully paid ordinary shares, with nominal value €0.01 per share. We have no preferred shares outstanding.
Under French law, our articles of association set forth only our issued
and outstanding share capital as of the date of the articles of association. Our fully diluted share capital represents all issued and
outstanding shares, as well as all potential shares which may be issued upon exercise of outstanding employee warrants, employee share
options and non-employee warrants, as granted by our board of directors.
We are entitled under French law to issue preferred shares but our
articles of association do not currently specify specific characteristics or rights attached to any specific category of preferred shares,
which would be determined by the extraordinary general meeting convened for such purpose.
Key Provisions of Our Articles of Association and French Law Affecting
Our Ordinary Shares
The
description below reflects the terms of our articles of association, and summarizes the material rights of holders of our ordinary shares
under French law. This is only a summary and is not intended to be exhaustive. For further information, please refer to the full version
of our articles of association, which are included Exhibit 1.1 to the annual report on Form 20-F of which this description
is also an exhibit.
Corporate
Purpose (Article 2 of the Articles of Association)
Our corporate purpose in France and
abroad includes:
| · | the
creation, operation, leasing, lease management of all operating assets, factories, institutions,
the taking of stakes in any company, as well as all attached or connected commercial, financial,
industrial, securities and property operations, relating directly or indirectly to the activity
of research production, distribution and marketing of any product and service beneficial
to human or animal health; |
| · | the
research and development of drug candidates and nutraceuticals, particularly in the field
of age-related diseases; and |
| · | all
financial, commercial, industrial, civil, securities or property operations, which may be
associated directly or indirectly, in whole or in part, with one or other of the purposes
specified above or any other similar or related purpose. |
Directors
Quorum and Voting (Article 17
of the Articles of Association). The board of directors may only deliberate validly if at least half of the
directors are present or considered to be present, subject to the adjustments made by the internal regulations (règlement intérieur)
in the event of use of videoconferencing or another means of telecommunication.
Unless otherwise provided in the articles of association and subject
to the adjustments made by the internal regulations in the event of use of videoconferencing or other means of telecommunications, decisions
are taken by majority of votes of members who are present or represented or regarded as present. In the event of a tied vote, the Chairman
of the session will have the deciding vote.
For the calculation of the quorum and majority, directors participating
in the board meeting by videoconference or telecommunications media will be regarded as present under the conditions defined by the internal
regulations of the board of directors. However, the effective presence or presence by representation will be necessary for all board
of director decisions regarding the drafting of the annual financial statements and consolidated accounts and the drawing up of the management
report and the report on the management of the group, as well as for decisions regarding the dismissal of the Chairman of the board of
directors, the CEO and, as the case may be, the Deputy CEO.
Directors' Voting Powers on Proposal,
Arrangement or Contract in which any Director Is Materially Interested (Article 21 of the Articles of Association). Except
for those relating to current operations concluded under normal conditions, any agreement entered into, directly or indirectly through
an intermediary, between the Company and any of our directors, CEO, deputy CEOs or with a shareholder holding more than 10% of the voting
rights of the Company, or in the case of a corporate shareholder, the company which controls it, will be subject to prior authorization
by the board of directors.
Agreements between the Company and another company will also be subject
to prior authorization, if the CEO, one of the deputy CEOs or a director of the Company is the owner, partner with unlimited liability,
manager, director, member of the supervisory board or, in general, a director of the Company.
Directors (other than legal entities) are forbidden from taking out
loans in any form from the Company, to be granted current account or overdraft by it, or arranging for the Company to guarantee or endorse
any commitments with regard to third parties.
Directors' Compensation (Article 20
of the Articles of Association). The General Meeting may allocate to the directors, as remuneration for their
activities, by way of attendance fees, a fixed annual sum, which this meeting will determine without being bound by previous decisions.
The amount of the same shall be attributed to operating expenses.
The board of directors will freely distribute among its members the
global overall amounts allocated to directors in the form of attendance fees; it may notably allocate to the directors who are members
of study committees, a higher share than that of the other directors.
The board of directors may allocate exceptional remuneration for assignments
or mandates entrusted to the directors. The board of directors may authorize the reimbursement of travel costs and expenses incurred
by the directors in the interest of the Company.
Board of Directors' Borrowing
Powers. There are currently no limits imposed on the amounts of loans or borrowings that the board
of directors may approve.
Directors' Age Limits. There
are currently no age limits imposed for service on our board of directors. The Chairman of the board of directors must be under 75. The
number of directors aging above 75 shall not be greater than one third of the total number of directors.
Directors' Share Ownership Requirements. None.
Rights, Preferences and Restrictions Attaching to Ordinary Shares
Dividends (Article 34 of
the By-laws). We may only distribute dividends out of our "distributable profits," plus
any amounts held in our reserves that the shareholders decide to make available for distribution, other than those reserves that are
specifically required by law.
"Distributable profits" consists of (a) the
profits for the last closed financial period increased by (b) any retained earnings, less (c) losses carried forward increased
by (d) amounts to be placed in reserve pursuant to the law or the articles of association.
Legal Reserve. Pursuant
to French law, we must allocate 5% of our unconsolidated net profit for each year to our legal reserve fund before dividends may be paid
with respect to that year. Funds must be allocated until the amount in the legal reserve is equal to 10% of the aggregate par value of
the issued and outstanding share capital. This restriction on the payment of dividends also applies to our French subsidiary on an unconsolidated
basis.
Approval of Dividends. Pursuant
to French law, our board of directors may propose a dividend for approval by the shareholders at the annual ordinary general meeting.
Upon recommendation of our board of directors, our shareholders may
decide to allocate all or part of any distributable profits to special or general reserves, to carry them forward to the next fiscal
year as retained earnings or to allocate them to the shareholders as dividends. However, dividends may not be distributed when our net
assets are or would become as a result of such distribution lower than the amount of the share capital plus the amount of the legal reserves
which, under French law, may not be distributed to shareholders.
Our board of directors may distribute interim dividends after the
end of the fiscal year but before the approval of the financial statements for the relevant fiscal year when the interim balance sheet,
established during such year and certified by an auditor, reflects that we have earned distributable profits since the close of the last
financial year, after recognizing the necessary depreciation and provisions and after deducting prior losses, if any, and the sums to
be allocated to reserves, as required by law or the by-laws, and including any retained earnings. The amount of such interim dividends
may not exceed the amount of the profit so defined.
Distribution of Dividends. Dividends
are distributed to shareholders pro rata according to their respective holdings of shares. In the case of interim dividends, distributions
are made to shareholders on the date set by our board of directors during the meeting in which the distribution of interim dividends
is approved. The actual dividend payment date is decided by the shareholders at an ordinary general shareholders' meeting or by our board
of directors in the absence of such a decision by the shareholders. Shareholders that own shares on the actual payment date are entitled
to the dividend.
Dividends may be paid in cash or, if the shareholders' meeting so
decides, in kind, provided that all shareholders receive a whole number of assets of the same nature paid in lieu of cash.
Timing of Payment. Pursuant
to French law, dividends must be paid within a maximum of nine months after the close of the relevant fiscal year, unless extended by
court order. Dividends not claimed within five years after the payment date shall be deemed to expire and revert to the French state.
Voting Rights (Article 14
of the Articles of Association). The voting rights attached to ordinary shares or dividend shares is
proportional to the amount of capital they represent. Each share is entitled to one vote.
A double voting right has been established for all registered and
fully paid-up shares registered in the name of the same beneficiary for at least two years.
Under French law, treasury shares or shares held by entities controlled
by us are not entitled to voting rights and do not count for quorum purposes.
Rights to Share in Our Profit. Each
share entitles its holder to a portion of the corporate profits and assets proportional to the amount of share capital represented thereby.
Rights to Share in the Surplus
in the Event of Liquidation. If we are liquidated, any assets remaining after payment of the debts,
liquidation expenses and all of the remaining obligations will first be used to repay in full the par value of our shares. Any surplus
will be distributed pro rata among shareholders in proportion to the number of shares respectively held by them, taking into account,
where applicable, of the rights attached to shares of different classes.
Repurchase and Redemption of Shares. Under
French law, we may acquire our own shares for the following purposes only:
| · | to
decrease our share capital, provided that such a decision is not driven by losses and that
a purchase offer is made to all shareholders on a pro rata basis, with the approval
of the shareholders at an extraordinary general meeting; in this case, the shares repurchased
must be cancelled within one month from the expiry of the purchase offer; |
| · | to
provide shares for distribution to employees or managers under a profit-sharing, free share
or share option plan; in this case the shares repurchased must be distributed within 12 months
from their repurchase failing which they must be cancelled; |
| · | to
meet obligations arising from debt securities that are exchangeable into equity instruments;
or |
| · | under
a buy-back program to be authorized by the shareholders in accordance with the provisions
of Article L. 22-10-62 of the French Commercial Code and in accordance with the general
regulations of, and market practices accepted by the Financial Markets Authority (AMF). This
authorization may only be given for a period not exceeding eighteen months. |
Under The Market Abuse Regulation (MAR) and in accordance with the
General Regulations of the AMF (Réglement Général de l'AMF), a corporation shall report to the competent
authority of the trading value on which the shares have been admitted to trading or are traded, no later than by the end of the seventh
daily market session following the date of the execution of the transaction, all the transactions relating to the buy-back program, in
a detailed form and in an aggregated form.
No such repurchase of shares may result in us holding, directly or
through a person acting on our behalf, more than 10% of our issued share capital. Shares repurchased by us continue to be deemed "issued"
under French law but are not entitled to dividends or voting rights so long as we hold them directly or indirectly, and we may not exercise
the preemptive rights attached to them.
Sinking Fund Provisions. Our
articles of association do not provide for any sinking fund provisions.
Liability to Further Capital Calls. Shareholders
are liable for corporate liabilities only up to the par value of the shares they hold; they are not liable to further capital calls.
Requirements for Holdings Exceeding
Certain Percentages. None except as described below under "—Form, Holding and Transfer
of Shares—Ownership of Shares by Non-French Persons".
Actions Necessary to Modify Shareholders' Rights
Shareholders' rights may be modified as allowed by French law. Only
the extraordinary shareholders' meeting is authorized to amend any and all provisions of our articles of association. It may not, however,
increase shareholder commitments without the prior approval of each shareholder.
Special Voting Rights of Warrant Holders
Under French law, the holders of warrants of the same class (i.e., warrants
that were issued at the same time and with the same rights), including founders' warrants, are entitled to vote as a separate class at
a general meeting of that class of warrant holders under certain circumstances, principally in connection with any proposed modification
of the terms and conditions of the class of warrants or any proposed issuance of preferred shares or any modification of the rights of
any outstanding class or series of preferred shares.
Rules for Admission to and Calling Annual Shareholders'
Meetings and Extraordinary Shareholders' Meetings
Access to, Participation in and
Voting Rights at Shareholders' Meetings (Articles 27 &28 of the Articles of Association). Shareholders'
meetings are composed of all shareholders. Each shareholder has the right to attend the meetings and participate in the discussions (1) personally,
or (2) by granting proxy to any individual or legal entity of his choosing; or (3) by sending a proxy to the company without
indication of the mandate, or (4) by voting by correspondence, or (5) by videoconference or another means of telecommunication
in accordance with applicable laws that allow identification. For any proxy given by a shareholder without indication of the mandate,
the chairman of the general meeting shall cast a vote in favor of the adoption of the draft resolutions presented or approved by the
board of directors and a vote against the adoption of all other draft resolutions. The board of directors organizes, in accordance with
legal and regulatory requirements, the participation and vote of the shareholders at the meeting, assuring, in particular, the effectiveness
of the means of identification.
Participation in shareholders' general meetings, in any form whatsoever,
is subject to registration or registration of shares under the conditions and time limits provided for applicable laws.
The final date for returning voting ballots by correspondence is set
by the board of directors and disclosed in the notice of meeting published in the French Journal of Mandatory Statutory Notices (BALO).
This date cannot be earlier than three days prior to the meeting.
The shareholder having voted by correspondence will no longer be able
to participate directly in the meeting or to be represented. In the case of returning the proxy form and the voting by correspondence
form, the proxy form is taken into account, subject to the votes cast in the voting by correspondence form.
Any shareholder may be represented at meetings by any individual or
legal entity of his choosing, by means of a proxy form which is addressed to him by us (1) at his request, addressed to us by any
means. This request must be received at the registered office at least five days before the date of the meeting; or (2) at our initiative.
The proxy is only valid for a single meeting or for successive meetings
convened with the same agenda. It can also be granted for two meetings, one ordinary, the other extraordinary, held on the same day or
within a period of 15 days.
Any shareholder may vote by correspondence by means of a voting form,
which is sent by us (1) upon request, addressed in writing (this request must be received at the registered office at least six
days before the date of the meeting); or (2) at our initiative; or (3) in appendix to a proxy voting form under the conditions
provided for by current laws and requirements. In any case this voting form is available on our website at least 21 days before
the date of the meeting.
The voting by correspondence form addressed by a shareholder is only
valid for a single meeting or for successive meetings convened with the same agenda.
Notice of Annual Shareholders'
Meetings. Shareholders' meetings are convened by our board of directors, or, failing that, by the
statutory auditors, or by a court appointed agent or liquidator in certain circumstances. Meetings are held at our registered offices
or at any other location indicated in the convening notice. A convening notice is published in the French Journal of Mandatory Statutory
Notices (Bulletin des Annonces Légales Obligatoires (BALO)) at least 35 days prior to a meeting, as well as on our
website at least 21 days prior to the meeting. In addition to the particulars relative to the company, it indicates, notably, the
meeting's agenda and the draft resolutions that will be presented. The requests for recording of issues or draft resolutions on the agenda
must be addressed to the company under the conditions provided for in the current legislation.
Subject to special legal provisions, the meeting notice is sent out
at least 15 days prior to the date of the meeting, by means of a notice inserted both in a legal announcement bulletin of the registered
office department and in the French Journal of Mandatory Statutory Notices (BALO). Further, the holders of registered shares for at least
a month at the time of the latest of the insertions of the notice of meeting shall be summoned individually, by regular letter (or by
registered letter if they request it and include an advance of expenses) sent to their last known address. This notice may also be transmitted
by electronic means of telecommunication, in lieu of any such mailing, to any shareholder requesting it beforehand by registered letter
with acknowledgment of receipt in accordance with legal and regulatory requirements, specifying his e-mail address. The latter may at
any time expressly request by registered letter to the Company with acknowledgment of receipt that the aforementioned means of telecommunication
should be replaced in the future by a mailing.
The convening notice must also indicate the conditions under which
the shareholders may vote by correspondence and the places and conditions in which they can obtain voting forms by mail.
The convening notice may be addressed, where appropriate, with a proxy
form and a voting by correspondence form, under the conditions specified in our bylaws, or with a voting by correspondence form alone,
under the conditions specified in our bylaws. When the shareholders' meeting cannot deliberate due to the lack of the required quorum,
the second meeting must be called at least ten days in advance in the same manner as used for the first notice.
Agenda and Conduct of Annual Shareholders'
Meetings. The agenda of the shareholders' meeting shall appear in the notice to convene the meeting and
is set by the author of the notice. The shareholders' meeting may only deliberate on the items on the agenda except for the removal of
directors and the appointment of their successors which may be put to vote by any shareholder during any shareholders' meeting. One or
more shareholders representing a percentage of share capital required by French law, and acting in accordance with legal requirements
and within applicable time limits, may request the inclusion of items or proposed resolutions on the agenda.
Shareholders' meetings shall be chaired by the Chairman of the board
of directors or, in his or her absence, the meeting itself shall elect a Chairman. Vote counting shall be performed by the two members
of the meeting who are present and accept such duties, who represent, either on their own behalf or as proxies, the greatest number of
votes.
Ordinary Shareholders' Meeting. Ordinary
shareholders' meetings are those meetings called to make any and all decisions that do not amend our by-laws. An ordinary meeting shall
be convened at least once a year within six months of the end of each fiscal year in order to approve the annual and consolidated accounts
for the relevant fiscal year or, in case of postponement, within the period established by court order. Upon first notice, the meeting
may validly deliberate only if the shareholders present or represented by proxy or voting by mail, by videoconference or by means of
telecommunication (to the extent the board of directors authorizes it when convening the shareholders) represent at least one-fifth of
the shares entitled to vote. Upon second notice, no quorum is required. Decisions are made by a majority of the votes held by the shareholders
present, or represented by proxy, or voting by mail, by videoconference or by means of telecommunications (to the extent the board of
directors authorizes it when convening the shareholders). Pursuant to the French Law n° 2019-744, dated July 19, 2019, abstention
from voting, blank votes or null votes by those present or those represented by proxy or voting by email are no longer counted as votes
against the resolution submitted to a shareholder vote at any type of meeting.
Extraordinary Shareholders' Meeting. Only
an extraordinary shareholders' meeting is authorized to amend our by-laws. It may not, however, increase shareholder commitments without
the approval of each shareholder. Subject to the legal provisions governing share capital increases from reserves, profits or share premiums,
the resolutions of the extraordinary meeting shall be valid only if the shareholders present, represented by proxy or voting by mail,
by videoconference or by means of telecommunication (to the extent the board of directors authorizes it when convening the shareholders)
represent at least one-fourth of all shares entitled to vote upon first notice, or one-fifth upon second notice. If the latter quorum
is not reached, the second meeting may be postponed to a date no later than two months after the date for which it was initially called.
Decisions are made by a two-thirds majority of the votes held by the shareholders present, represented by proxy, or voting by mail, by
videoconference or by means of telecommunication (to the extent the board of directors authorizes it when convening the shareholders).
Pursuant to the French Law n° 2019-744, dated July 19, 2019, abstention from voting, blank votes or null votes by those present
or those represented by proxy or voting by email are no longer counted as votes against the resolution submitted to a shareholder vote
at any type of meeting.
Mechanisms for Delaying, Deferring
or Preventing a Change in Control of the Company
Provisions contained in our Articles of Association and/or French
corporate law could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our shareholders.
In addition, provisions of our bylaws impose various procedural and other requirements, which could make it more difficult for shareholders
to effect certain corporate actions. These provisions include the following:
| · | under
French law, the owner of 90% of voting rights of a public company listed on a regulated market
in a Member State of the European Union or in a state party to the European Economic Area,
or EEA, Agreement, including France, has the right to force out minority shareholders following
a tender offer made to all shareholders; |
| · | under
French law, a non-resident of France as well as any French entity controlled by non-French
residents may have to file an administrative notice with French authorities in connection
with a direct or indirect investment in us, as defined by administrative rulings; |
| · | a
merger (i.e., in a French law context, a stock for stock exchange following which
our company would be dissolved into the acquiring entity and our shareholders would become
shareholders of the acquiring entity) of our company into a company incorporated in the European
Union would require the approval of our board of directors as well as a two-thirds majority
of the votes held by the shareholders present, represented by proxy or voting by mail at
the relevant meeting; |
| · | under
French law, a cash merger is treated as a share purchase and would require the consent of
each participating shareholder; |
| · | our
shareholders have granted and may grant in the future our board of directors broad authorizations
to increase our share capital or to issue additional ordinary shares or other securities,
such as warrants, to our shareholders, the public or qualified investors, including as a
possible defense following the launching of a tender offer for our shares; |
| · | our
shareholders have preferential subscription rights on a pro rata basis on the issuance by
us of any additional securities for cash or a set-off of cash debts, which rights may only
be waived by the extraordinary general meeting (by a two-thirds majority vote) of our shareholders
or on an individual basis by each shareholder; |
| · | our
board of directors has the right to appoint directors to fill a vacancy created by the resignation
or death of a director, for the remaining duration of such director's term of office, provided
that prior to such decision of the board of directors, the number of directors remaining
in office exceeds the minimum required by law and by the bylaws, and subject to the subsequent
approval by the shareholders of such appointment at the next shareholders' meeting, which
prevents shareholders from having the sole right to fill vacancies on our board of directors; |
| · | our
board of directors can be convened by our chairman (directly or upon request of our managing
director), or, when no board meeting has been held for more than three consecutive months,
by directors representing at least one third of the total number of directors; |
| · | our
board of directors meetings can only be regularly held if at least half of the directors
attend either physically or by way of videoconference or teleconference enabling the directors'
identification and ensuring their effective participation in the board's decisions; |
| · | our
shares are nominative or bearer, if the legislation so permits, according to the shareholder's
choice; |
| · | under
French law, certain investments in any entity governed by French law relating to certain
strategic industries (such as research and development in biotechnologies and activities
relating to public health) and activities by individuals or entities not French, not resident
in France or controlled by entities not French or not resident in France are subject to prior
authorization of the Ministry of Economy; |
| · | approval
of at least a majority of the votes held by shareholders present, represented by a proxy,
or voting by mail at the relevant ordinary shareholders' general meeting is required to remove
directors with or without cause; |
| · | advance
notice is required for nominations to the board of directors or for proposing matters to
be acted upon at a shareholders' meeting, except that a vote to remove and replace a director
can be proposed at any shareholders' meeting without notice; |
| · | our
bylaws can be changed in accordance with applicable laws; |
| · | the
crossing of certain thresholds has to be disclosed and can impose certain obligations; |
| · | transfers
of shares shall comply with applicable insider trading rules and regulations and, in
particular, with the Market Abuse Directive and Regulation dated April 16, 2014; and |
| · | pursuant
to French law, our bylaws, including the sections relating to the number of directors and
election and removal of a director from office, may only be modified by a resolution adopted
by two-thirds of the votes of our shareholders present, represented by a proxy or voting
by mail at the meeting. |
Declaration of Crossing of
Ownership Thresholds
Set forth below is a summary of certain provisions of our articles
of association and of the French Commercial Code applicable to us. This summary is not intended to be a complete description of applicable
rules under French law.
Our articles of association provide that any individual or legal entity
coming to directly or indirectly own, alone or in concert, a number of shares representing a fraction of our capital or voting rights
equal to 5%, 10%, 15%, 20%, 25%, 30%, 33.33%, 50%, 66.66%, 90% or 95% inform us of the total number of shares and voting rights and of
securities giving access to the capital or voting rights that it owns immediately or over time, within a period of four trading days
from the crossing of the said holding thresholds.
This obligation also applies under the same conditions when crossing
each of the above-mentioned thresholds in a downward direction.
In case of failure to declare, shares or voting rights exceeding the
fraction that should have been declared are deprived of voting rights at General Meetings of Shareholders for any meeting that would
be held until the expiry of a period of two years from the date of regularization of the notification in accordance with Article L.
233-14 of the French Commercial Code, if the failure to declare has been determined.
These requirements are without prejudice to the threshold crossing
declarations provided for under French law which impose a declaration to us and to the AMF upon crossing of the following thresholds
no later than the Fourth trading day following the crossing: 50% and 95% of the capital or voting rights.
Further, and subject to certain exemptions, any shareholder crossing,
alone or acting in concert, the 50% threshold shall file a mandatory public tender offer.
Changes in Share Capital
Increases in Share Capital. Pursuant
to French law, our share capital may be increased only with shareholders' approval at an extraordinary general shareholders' meeting
following the recommendation of our board of directors. The shareholders may delegate to our board of directors either the authority
(délégation de compétence) or the power (délégation de pouvoir) to carry out any increase
in share capital.
Increases in our share capital may be effected by:
| · | issuing
additional shares; |
| · | increasing
the par value of existing shares; |
| · | creating
a new class of equity securities; and |
| · | exercising
the rights attached to securities giving access to the share capital. |
Increases in share capital by issuing additional securities may be
effected through one or a combination of the following:
| · | in
consideration for cash; |
| · | in
consideration for assets contributed in kind; |
| · | through
an exchange offer; |
| · | by
conversion of previously issued debt instruments; |
| · | by
capitalization of profits, reserves or share premium; and |
| · | subject
to certain conditions, by way of offset against debt incurred by us. |
Decisions to increase the share capital through the capitalization
of reserves, profits and/or share premium require shareholders' approval at an extraordinary general shareholders' meeting, acting under
the quorum and majority requirements applicable to ordinary shareholders' meetings. Increases effected by an increase in the par value
of shares require unanimous approval of the shareholders, unless effected by capitalization of reserves, profits or share premium. All
other capital increases require shareholders' approval at an extraordinary general shareholders' meeting acting under the regular quorum
and majority requirements for such meetings.
Reduction in Share Capital. Pursuant
to French law, any reduction in our share capital requires shareholders' approval at an extraordinary general shareholders' meeting following
the recommendation of our board of directors. The share capital may be reduced either by decreasing the par value of the outstanding
shares or by reducing the number of outstanding shares. The number of outstanding shares may be reduced by the repurchase and cancellation
of shares. Holders of each class of shares must be treated equally unless each affected shareholder agrees otherwise.
Preferential Subscription Right. According
to French law, if we issue additional securities for cash, current shareholders will have preferential subscription rights to these securities
on a pro rata basis. Preferential subscription rights entitle the individual or entity that holds them to subscribe pro rata
based on the number of shares held by them to the issuance of any securities increasing, or that may result in an increase of, our
share capital by means of a cash payment or a set-off of cash debts. The preferential subscription rights are transferable during the
subscription period relating to a particular offering. Since October 1, 2016, preferential subscription rights may only be exercised
two business days prior to the day on which the subscription is opened until the second business day prior to its closing. Thus, the
preferential subscription rights are transferable during the same period as their period of exercise. In accordance with French law,
the period of exercise shall be no less than five business days.
The preferential subscription rights with respect to any particular
offering may be waived at an extraordinary general meeting by a two-thirds vote of our shareholders or individually by each shareholder.
Our board of directors and our independent auditors are required by French law to present reports to the shareholders' meeting that specifically
address any proposal to waive the preferential subscription rights.
Our current shareholders waived their preferential subscription rights
with respect to this offering at an extraordinary general shareholders' general meeting held on June 21, 2022.
In the future, to the extent permitted under French law, we may seek
shareholder approval to waive preferential subscription rights at an extraordinary general shareholders' meeting in order to authorize
the board of directors to issue additional shares and/or other securities convertible or exchangeable into shares.
Form, Holding and Transfer of Shares
Form of Shares. The
shares are nominative or bearer, if the legislation so permits, according to the shareholder's choice.
Further, in accordance with applicable laws, we may request at any
time from the central depository responsible for holding our Shares, the information referred to in Article L. 228-2 of the French
Commercial Code. Thus, we are, in particular and at any time, entitled to request the name and year of birth or, in the case of a legal
entity, the name and the year of incorporation, nationality and address of holders of securities conferring immediate or long-term voting
rights at its General Meetings of Shareholders and the amount of securities owned by each of them and, where applicable, the restrictions
that the securities could be affected by.
Holding of Shares. In
accordance with French law concerning the "dematerialization" of securities, the ownership rights of shareholders are represented
by book entries instead of share certificates. Shares issued are registered in individual accounts opened by us or any authorized intermediary,
in the name of each shareholder and kept according to the terms and conditions laid down by the legal and regulatory provisions.
Ownership of Shares by Non-French
Persons. Neither French law nor our articles of association limit the right of non-residents of
France or non-French persons to own or, where applicable, to vote our securities. However, (a) any non-French citizen, (b) any
French citizen not residing in France, (c) any non-French entity or (d) any French entity controlled by one of the aforementioned
persons or entities may have to file a declaration for statistical purposes with the Bank of France (Banque de France) within twenty
working days following the date of certain direct foreign investments in us, including any purchase of our ADSs. In particular, such
filings are required in connection with investments exceeding €15,000,000 that lead to the acquisition of at least 10% of our share
capital or voting rights or cross such 10% threshold. Violation of this filing requirement may be sanctioned by five years of imprisonment
and a fine of up to twice the amount of the relevant investment. This amount may be increased fivefold if the violation is made by a
legal entity.
Moreover, under French law, certain investments in any entity governed
by a French law relating to certain strategic industries (such as research and development in biotechnologies and activities relating
to public health) and activities by individuals or entities not French, not resident in France or controlled by entities not French or
not resident in France are subject to prior authorization of the Ministry of Economy.
Assignment and Transfer of Shares. Shares
are freely negotiable, subject to applicable legal and regulatory provisions. French law notably provides for standstill obligations
and prohibition of insider trading.
Registration Rights
None of our security holders possess registration rights.
Differences in Corporate Law
The laws applicable to French sociétés anonymes
differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between
the provisions of the French Commercial Code applicable to us and the Delaware General Corporation Law relating to shareholders' rights
and protections.
| |
|
France |
|
Delaware |
| Number of Directors |
|
Under French law, a société anonyme
must have at least three and may have up to 18 directors. The number of directors is fixed by or in the manner provided in the
by-laws. |
|
Under Delaware law, a corporation must have at least
one director and the number of directors shall be fixed by or in the manner provided in the by-laws. |
| |
|
|
|
|
| Director Qualifications |
|
Under French law, a corporation may prescribe qualifications
for directors under its by-laws. In addition, under French law, members of a board of directors of a corporation may be legal entities
(with the exception of the Chairman of the board of directors), and such legal entities may designate an individual to represent
them and to act on their behalf at meetings of the board of directors. |
|
Under Delaware law, a corporation may prescribe qualifications for directors under its certificate of incorporation or by-laws.
|
| |
|
|
|
|
| Removal of Directors |
|
Under French law, directors may be removed from office,
with or without cause, at any shareholders' meeting without notice or justification, by a simple majority vote of the shareholders
present and voting at the meeting in person or by proxy. |
|
Under Delaware law, unless otherwise provided in the
certificate of incorporation, directors may be removed from office, with or without cause, by a majority stockholder vote, though
in the case of a corporation whose board is classified, stockholders may effect such removal only for cause. |
| |
|
|
|
|
| Vacancies on the Board of Directors |
|
Under French law, vacancies on the board of directors
resulting from death or a resignation, provided that at least three directors remain in office, may be filled by a majority of the
remaining directors pending ratification by the shareholders by the next shareholders' meeting. |
|
Under Delaware law, vacancies on a corporation's board
of directors, including those caused by an increase in the number of directors, may be filled by a majority of the remaining directors. |
| |
|
|
|
|
| Annual General Meeting |
|
Under French law, the annual general meeting of shareholders
shall be held at such place, on such date and at such time as decided each year by the board of directors and notified to the shareholders
in the convening notice of the annual meeting, within six months after the close of the relevant fiscal year unless such period is
extended by court order. |
|
Under Delaware law, the annual meeting of stockholders
shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as
provided in the certificate of incorporation or by the by-laws. |
| |
|
|
|
|
| General Meeting |
|
Under French law, general meetings of the shareholders
may be called by the board of directors or, failing that, by the statutory auditors, or by a court appointed agent or liquidator
in certain circumstances, or by the majority shareholder in capital or voting rights following a public tender offer or exchange
offer or the transfer of a controlling block on the date decided by the board of directors or the relevant person. |
|
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons
as may be authorized by the certificate of incorporation or by the by-laws. |
| |
|
France |
|
Delaware |
| Notice of General Meetings |
|
A convening notice is published in the French Journal
of Mandatory Statutory Notices (BALO) at least 35 days prior to a meeting and made available on the website of the company at
least 21 days prior to the meeting. Subject to special legal provisions, the meeting notice is sent out at least 15 days
prior to the date of the meeting, by means of a notice inserted both in a legal announcement bulletin of the registered office department
and in the French Journal of Mandatory Statutory Notices (BALO). Further, the holders of registered shares for at least a month at
the time of the latest of the insertions of the notice of meeting shall be summoned individually, by regular letter (or by registered
letter if they request it and include an advance of expenses) sent to their last known address. This notice may also be transmitted
by electronic means of telecommunication, in lieu of any such mailing, to any shareholder requesting it beforehand by registered
letter with acknowledgment of receipt in accordance with legal and regulatory requirements, specifying his e-mail address. |
|
Under Delaware law, written notice of any meeting of
the stockholders must be given to each stockholder entitled to vote at the meeting not less than 10 nor more than 60 days before
the date of the meeting and shall specify the place, date, hour, and purpose or purposes of the meeting. |
| |
|
|
|
|
| |
|
The convening notice must also indicate the conditions
under which the shareholders may vote by correspondence and the places and conditions in which they can obtain voting forms by mail. |
|
|
| |
|
|
|
|
| |
|
The notice must specify the name of the company, its
legal form, share capital, registered office address, registration number with the French Registry of Commerce and Companies (registre
du commerce et des sociétés), the place, date, hour and agenda of the meeting and its nature (ordinary and/or extraordinary
meeting). |
|
|
| |
|
|
|
|
| Proxy |
|
Each shareholder has the right to attend the meetings
and participate in the discussions (i) personally, or (ii) by granting proxy to any individual or legal entity of his choosing;
or (iii) by sending a proxy to the company without indication of the mandate, or (iv) by voting by correspondence, or (v) by
videoconference or another means of telecommunication in accordance with applicable laws that allow identification. |
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by
proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period.
|
| |
|
France |
|
Delaware |
| |
|
The proxy is only valid for a single meeting or for
successive meetings convened with the same agenda. It can also be granted for two meetings, one ordinary, the other extraordinary,
held on the same day or within a period of 15 days. |
|
|
| |
|
|
|
|
| Shareholder action by written consent |
|
Under French law, shareholders' action by written consent is not permitted in a société anonyme.
|
|
Under Delaware law, a corporation's certificate of
incorporation (1) may permit stockholders to act by written consent if such action is signed by all stockholders, (2) may
permit stockholders to act by written consent signed by stockholders having the minimum number of votes that would be necessary to
take such action at a meeting or (3) may prohibit actions by written consent. |
| |
|
|
|
|
| Preemptive Rights |
|
Under French law, in case of issuance of additional
shares or other securities for cash or set-off against cash debts, the existing shareholders have preferential subscription rights
to these securities on a pro rata basis unless such rights are waived by a two-thirds majority of the votes held by the shareholders
present at the extraordinary meeting deciding or authorizing the capital increase, voting in person or represented by proxy or voting
by mail. In case such rights are not waived by the extraordinary general meeting, each stockholder may individually either exercise,
assign or not exercise its preferential rights. |
|
Under Delaware law, unless otherwise provided in a
corporation's certificate of incorporation, a stockholder does not, by operation of law, possess preemptive rights to subscribe to
additional issuances of the corporation's stock. |
| |
|
|
|
|
| Sources of Dividends |
|
Under French law, dividends may only be paid by a French
société anonyme out of "distributable profits," plus any distributable reserves and "distributable
premium" that the shareholders decide to make available for distribution, other than those reserves that are specifically
required by law. "Distributable profits" consist of the unconsolidated net profits of the relevant corporation for
each fiscal year, as increased or reduced by any profit or loss carried forward from prior years. |
|
Under Delaware law, dividends may be paid by a Delaware
corporation either out of (1) surplus or (2) in case there is no surplus, out of its net profits for the fiscal year in
which the dividend is declared and/or the preceding fiscal year, except when the capital is diminished by depreciation in the value
of its property, or by losses, or otherwise, to an amount less than the aggregate amount of capital represented by issued and outstanding
stock having a preference on the distribution of assets. |
| |
|
France |
|
Delaware |
| |
|
"Distributable premium"
refers to the contribution paid by the stockholders in addition to the par value of their shares for their subscription that the
stockholders decide to make available for distribution. |
|
|
| |
|
|
|
|
| |
|
Except in case of a share capital reduction,
no distribution can be made to the stockholders when the net equity is, or would become, lower than the amount of the share capital
plus the reserves which cannot be distributed in accordance with the law or the by- laws. Since October 1, 2016, preferential
subscription rights may only be exercised two business days prior to the day on which the subscription is opened until the second
business day prior to its closing. Thus, the preferential subscription rights are transferable during the same period as their period
of exercise. In accordance with French law, the period of exercise shall be no less than 5 business days. |
|
|
| |
|
|
|
|
| Repurchase of Shares |
|
Under French law, a corporation may acquire its own shares. Such acquisition may be challenged on the ground of market
abuse regulations. However, the Market Abuse Regulation 596/2014 of April 16, 2014 (MAR) provides for safe harbor exemptions
when the acquisition is made for the following purposes only: |
|
Under Delaware law, a corporation may generally redeem or repurchase shares of its stock unless the capital of the corporation
is impaired or such redemption or repurchase would impair the capital of the corporation.
|
| |
|
|
|
|
| |
|
• |
to decrease its share capital, provided that such
decision is not driven by losses and that a purchase offer is made to all shareholders on a pro rata basis, with the approval of
the shareholders at the extraordinary general meeting deciding the capital reduction; |
|
|
| |
|
|
|
|
|
| |
|
• |
with a view to distributing within one year of their repurchase the
relevant shares to employees or managers under a profit- sharing, free share or share option plan; |
|
|
| |
|
|
|
|
|
| |
|
• |
to meet obligations arising from debt securities that are exchangeable
into equity instruments; or |
|
|
| |
|
|
|
|
|
| |
|
• |
under a buy-back program to be authorized by the shareholders in accordance
with the provisions of Article L. 22-10-62 of the French Commercial Code and in accordance with the general regulations of the
Financial Markets Authority (AMF). |
|
|
| |
|
France |
|
Delaware |
| |
|
A simple exemption is provided when the acquisition
is made under a buy-back program to be authorized by the shareholders in accordance with the provisions of Article L. 22-10-62
of the French Commercial Code and in accordance with the General Regulations of the Financial Markets Authority (AMF). |
|
|
| |
|
|
|
|
| |
|
A simple exemption is provided when the acquisition
is made under a buy-back program to be authorized by the shareholders in accordance with the provisions of Article L. 22-10-62
of the French Commercial Code and in accordance with the General Regulations of the Financial Markets Authority (AMF). |
|
|
| |
|
|
|
|
| |
|
No such repurchase of shares may result in the company
holding, directly or through a person acting on its behalf, more than 10% of its issued share capital. |
|
|
| |
|
|
|
|
| Liability of Directors and Officers |
|
Under French law, the directors and the officers are individually
or jointly and severally liable, as the case may be, to the company or to third parties, either for breaches of the laws or regulations
applicable to société anonyme, or for breaches of the Articles of Association, or for misconduct in their management.
In addition, French law provides for cases of criminal liability of the directors and officers. The by-laws may not include any provisions
limiting the liability of directors.
|
|
Under Delaware law, a corporation's certificate
of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its
stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of
a director for: |
| |
|
|
|
| |
|
|
• |
any breach of the director's duty of loyalty to the
corporation or its stockholders; |
| |
|
|
|
|
| |
|
|
• |
acts or omissions not in good faith or that involve
intentional misconduct or a knowing violation of law; |
| |
|
|
|
|
| |
|
|
• |
intentional or negligent payment of unlawful dividends
or stock purchases or redemptions; or |
| |
|
|
|
|
|
| |
|
|
|
• |
any transaction from which the director derives an
improper personal benefit. |
| |
|
France |
|
Delaware |
| Voting Rights |
|
French law provides that, unless otherwise
provided in the by-laws, each shareholder is entitled to one vote for each share of capital stock held by such shareholder. |
|
Delaware law provides that, unless otherwise
provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such
stockholder. |
| |
|
|
|
|
| Shareholder Vote on Certain Transactions |
|
Generally, under French law,
completion of a merger, dissolution, sale, lease or exchange of all or substantially all of a corporation's assets requires: |
|
Generally, under Delaware law, unless the certificate of incorporation
provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or
substantially all of a corporation's assets or dissolution requires: |
| |
|
|
| |
|
|
|
|
| |
|
· |
the approval of the board of directors; and |
|
| |
|
|
|
|
|
| |
|
· |
approval by a two-thirds
majority of the votes held by the shareholders present, represented by proxy or voting by mail at the relevant meeting. |
|
· |
the approval of the board of directors;
and |
| |
|
|
|
|
|
| |
|
|
|
· |
approval by the vote of the holders of a majority of
the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the
votes of the outstanding stock of a corporation entitled to vote on the matter. |
| Dissent or Dissenters
Appraisal Rights |
|
French law does not provide
for any such right but provides that a merger is subject to shareholders' approval by a two-thirds majority vote as stated above. |
|
Under Delaware
law, a holder of shares of any class or series has the right, in specified circumstances, to dissent from a merger or consolidation
by demanding payment in cash for the stockholder's shares equal to the fair value of those shares, as determined by the Delaware
Chancery Court in an action timely brought by the corporation or a dissenting stockholder. |
| |
|
|
|
|
| |
|
|
|
Delaware law
grants these appraisal rights only in the case of mergers or consolidations and not in the case of a sale or transfer of assets or
a purchase of assets for stock. Further, no appraisal rights are available for shares of any class or series that is listed on a
national securities exchange or held of record by more than 2,000 stockholders, unless the agreement of merger or consolidation requires
the holders to accept for their shares anything other than: |
| |
|
|
|
|
| |
|
|
|
• |
shares of stock
of the surviving corporation; |
| |
|
|
|
|
|
| |
|
|
|
• |
shares of stock of another
corporation that are either listed on a national securities exchange or held of record by more than 2,000 stockholders; |
| |
|
France |
|
Delaware |
| |
|
|
|
• |
cash in lieu of fractional shares
of the stock described in the two preceding bullet points; or |
| |
|
|
|
|
|
| |
|
|
|
• |
any combination of the above. |
| |
|
|
|
|
| |
|
|
|
In addition, appraisal rights are not available
to holders of shares of the surviving corporation in specified mergers that do not require the vote of the stockholders of the surviving
corporation. |
| |
|
|
|
|
| Standard of Conduct for Directors |
|
French law does not contain specific provisions setting
forth the standard of conduct of a director. However, directors have a duty to act without self-interest, on a well-informed basis
and they cannot make any decision against a corporation's corporate interest (intérêt social). |
|
Delaware law does not contain specific
provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined
by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well- informed basis
and in a manner they reasonably believe to be in the best interest of the stockholders. |
| |
|
|
|
|
| Shareholder Suits |
|
The plaintiff must remain a shareholder through the duration of
the legal action.
There is no other case where shareholders may initiate a derivative
action to enforce a right of a corporation.
A shareholder may alternatively or cumulatively bring individual
legal action against the directors, provided he has suffered distinct damages from those suffered by the corporation. In this case,
any damages awarded by the court are paid to the relevant shareholder.
|
|
Under Delaware law, a stockholder may initiate
a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
|
| |
|
|
|
| |
|
|
• |
state that the plaintiff was a stockholder at the time
of the transaction of which the plaintiff complains or that the plaintiff's shares thereafter devolved on the plaintiff by operation
of law; and |
| |
|
|
|
| |
|
|
• |
allege with particularity the efforts made by the plaintiff
to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff's failure to obtain the action; or |
| |
|
|
|
|
| |
|
|
|
• |
state the reasons for not making the effort. |
| |
|
|
|
|
| |
|
|
|
• |
Additionally, the plaintiff must remain a stockholder
through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware
Court of Chancery. |
| |
|
|
|
|
| Amendment of Certificate of Incorporation |
|
Under French law, any modification of the
information reflected on the certificate of incorporation at the time of registration (i.e. legal form, registered office, share
capital, year-end, company's name, directors, statutory auditors) must be filed with the French Registry of Commerce and Companies. |
|
Under Delaware law, generally a corporation
may amend its certificate of incorporation if: |
| |
|
|
|
| |
|
|
• |
its board of directors has adopted a resolution setting
forth the amendment proposed and declared its advisability; and |
| |
|
France |
|
Delaware |
| |
|
|
|
• |
the amendment
is adopted by the affirmative votes of a majority (or greater percentage as may be specified by the corporation) of the outstanding
shares entitled to vote on the amendment and a majority (or greater percentage as may be specified by the corporation) of the outstanding
shares of each class or series of stock, if any, entitled to vote on the amendment as a class or series. |
| |
|
|
|
|
| Amendment of By-laws |
|
Under French law, only
the extraordinary shareholders' meeting is authorized to adopt or amend the by-laws. |
|
Under Delaware
law, the stockholders entitled to vote have the power to adopt, amend or repeal by-laws. A corporation may also confer, in its certificate
of incorporation, that power upon the board of directors. |
Legal Name; Formation;
Fiscal Year; Registered Office
Our legal and commercial name is Biophytis S.A. We were incorporated
as a société par actions simplifiée under the laws of the French Republic on September 27, 2006 for
a period of 99 years expiring on September 26, 2105, unless dissolved in advance or extended. The Company was transformed into
a société anonyme on May 22, 2015. We are registered at the Paris Commerce and Companies Register under the
number 492 002 225. Our principal executive offices are located at Sorbonne University—BC 9, Bâtiment A 4ème
étage, 4 place Jussieu 75005 Paris, France and our telephone number is +33 1 44 27 23 00. Our registered office is 14, Avenue
de l'Opéra, Paris, France. Our website address is www.biophytis.com. Our agent for service of process in the United States
is Puglisi & Associates. Our fiscal year ends December 31.
Listing
ADSs, each representing 10 of our ordinary
shares (or a right to receive 10 ordinary shares), are listed on the Nasdaq Capital Market under the symbol “BPTS.” Our
ordinary shares are currently listed on Euronext Growth Paris under the symbol "ALBPS."
Transfer Agent and Registrar
The transfer agent and registrar for the ADSs is Computershare, Inc.
Our share register is currently maintained by Uptevia, 89-91, rue Gabriel Péri, 92120 Montrouge, France, registered under n°439
430 976. The share register reflects only record owners of our ordinary shares. Holders of ADSs will not be treated as one of our shareholders
and their names will therefore not be entered in our share register. The depositary, the custodian or their nominees will be the holder
of the shares underlying the ADSs. Holders of the ADSs have a right to receive the ordinary shares underlying the ADSs.
DESCRIPTION OF PREFERENTIAL
SHARES
We may issue preferential shares (including preferential shares represented
by ADSs). The particular terms of each issue or series of preferential shares will be described in the related prospectus supplement.
This description will include, where applicable, a description of:
| · | the title and nominal
value of the preferential shares; |
| · | the number of preferential
shares we are offering; |
| · | the liquidation preference
per preferential share, if any; |
| · | the issue price per
preferential share (or if applicable, the calculation formula of the issue price per preferential
share); |
| · | whether preferential
subscription rights will be issued to existing shareholders; |
| · | the dividend rate
per preferential share, dividend period and payment dates and method of calculation for dividends; |
| · | whether dividends
will be cumulative or non-cumulative and, if cumulative, the date from which dividends will
accumulate; |
| · | our right, if any,
to defer payment of dividends and the maximum length of any such deferral period; |
| · | the relative ranking
and preferences of the preferential shares as to dividend rights (preferred dividend if any)
and rights if we liquidate, dissolve or wind up the Company; |
| · | the procedures for
any auction and remarketing, if any; |
| · | the provisions for
redemption or repurchase, if applicable, and any restrictions on our ability to exercise
those redemption and repurchase rights; |
| · | any listing of the
preferential shares on any securities exchange or market; |
| · | whether the preferential
shares will be convertible into our ordinary shares (including in the form of ADSs) or preferential
shares of another category, and, if applicable, conditions of an automatic conversion into
ordinary shares (including in the form of ADSs), if any, the conversion period, the conversion
price, or how such price will be calculated, and under what circumstances it may be adjusted; |
| · | voting rights, if
any, of the preferential shares; |
| · | preemption rights,
if any; |
| · | other restrictions
on transfer, sale or assignment, if any; |
| · | whether interests
in the preferential shares will be represented by ADSs; |
| · | a discussion of any
material or special U.S. federal and French income tax considerations applicable to the preferential
shares; |
| · | any limitations on
issuances of any class or series of preferential shares ranking senior to or on a parity
with the series of preferential shares being issued as to dividend rights and rights if we
liquidate, dissolve or wind up our affairs; |
| · | any rights attached
to the preferential shares regarding the corporate governance of our company, which may include,
for example representation rights to the board of directors; and |
| · | any other specific
terms, rights, preferences, privileges, qualifications or restrictions of the preferential
shares. |
Prior to issuing preferential shares, we must convene an extraordinary
shareholders meeting at which shareholders would determine the terms and conditions of the preferential shares, decide the issuance of
the preferential shares or delegate authority to the board of directors to decide the issuance and vote to modify the articles of association
in order to include the characteristics and particular rights of the preferential shares.
The extraordinary shareholders meeting would also decide the maximum
aggregate amount of share capital increases which we may carry out by issuing preferential shares, which may not exceed a specified amount
of gross issue proceeds to be determined.
The issuance of preferential shares could adversely affect the voting
power of holders of ordinary shares and ADSs and reduce the likelihood that holders of ordinary shares and ADSs will receive dividend
payments and payments upon liquidation. The issuance could have the effect of decreasing the market price of our ADSs. The issuance of
preferential shares also could have the effect of delaying, deterring or preventing a change in control of our company.
DESCRIPTION OF WARRANTS
We may issue warrants for the purchase of our ordinary shares (including
ordinary shares represented by ADSs), preferential shares (including preferential shares represented by ADSs), debt securities, or any
combination of the foregoing. Each warrant will entitle the holder to purchase the number of ordinary shares (including ordinary shares
represented by ADSs), preferential shares (including preferential shares represented by ADSs), debt securities, or combination thereof,
as the case may be, at the exercise price and in the manner specified in the prospectus supplement relating to such warrants. Warrants
may be exercised at any time up to the date and time specified in the applicable warrant agreement and set forth in the applicable prospectus
supplement.
Warrants will be issued under one or more warrant agreements to be
entered into between the Company and one or more purchasers of such warrants or a bank or trust company acting as warrant agent. The
material terms and provisions of such warrants to be issued and a description of the material provisions of the applicable warrant agreement
will be set forth in the applicable prospectus supplement. The form of warrant agreement that will be entered into with respect to a
particular offering of warrants will be filed as an exhibit to a post-effective amendment to, or incorporated by reference into, the
registration statement of which this prospectus forms a part.
The applicable prospectus supplement will describe the terms of any
warrants in respect of which this prospectus and such prospectus supplement is being delivered, which terms may include the following
if applicable to those warrants:
| · | the title and aggregate
number of the warrants; |
| · | the price or prices
at which such warrants will be issued; |
| · | the currency or currency
unit in which the warrants are denominated; |
| · | if the warrants are
for the purchase of ordinary shares, the number of ordinary shares that may be purchased
upon exercise of each warrant; the price, or the manner of determining the price, at which
the ordinary shares may be purchased upon the exercise of the warrants; |
| · | if the warrants are
for the purchase of preferential shares, the designation and terms of the series of preferential
shares, and the number of such preferential shares, purchasable upon exercise of the warrants;
the price, or the manner of determining the price, at which the preferential shares may be
purchased upon exercise of the warrants; |
| · | if the warrants are
for the purchase of debt securities, the exercise price for the debt securities, the amount
of debt securities to be received upon exercise, and a description of the series of debt
securities; |
| · | the price at which
the securities purchasable upon exercise of such warrants may be purchased; |
| · | if other than cash,
the manner in which the exercise price of the warrants may be paid; and any maximum or minimum
number of warrants that may be exercisable at any one time; |
| · | the time or times
at which, or period or periods during which, the warrants may be exercised and the expiration
date of the warrants; |
| · | the terms of any right
of the Company to redeem the warrants; |
| · | the terms of any right
of the Company to accelerate the exercise of the warrants upon the occurrence of certain
events; |
| · | whether the warrants
will be sold with any other securities, and the date, if any, on and after which the warrants
and the other related securities will be separately transferable; |
| · | whether the warrants
will be issued in registered or bearer form and information with respect to book-entry procedures,
if any; |
| · | a discussion of certain
material tax, accounting and other special considerations, procedures and limitations relating
to the warrants; and |
| · | any other terms of
the warrants, including terms, procedures and limitations relating to the exchange and exercise
of such warrants. |
DESCRIPTION OF DEBT
SECURITIES
We may offer debt securities. The following description of debt securities
sets forth the material terms and provisions of the debt securities to which any prospectus supplement may relate. Our debt securities
would be issued under an indenture between us and a trustee. The debt securities we may offer may be convertible into common stock or
other securities. The indenture, a form of which is included as an exhibit to the registration statement of which this prospectus is
a part, will be executed at the time we issue any debt securities. Any supplemental indentures will be filed with the SEC on a Form 6-K
or by a post-effective amendment to the registration statement of which this prospectus is a part.
The particular terms of the debt securities offered by any prospectus
supplement, and the extent to which the general provisions described below may apply to the offered debt securities, will be described
in the applicable prospectus supplement. The indenture will be qualified under the Trust Indenture Act of 1939, as amended (the “Trust
Indenture Act”). The terms of the debt securities will include those stated in the indenture and those made part of the indenture
by reference to the Trust Indenture Act.
Because the following summaries of the material terms and provisions
of the indenture and the related debt securities are not complete, you should refer to the form of the indenture and the debt securities
for complete information on some of the terms and provisions of the indenture, including definitions of some of the terms used below,
and the debt securities.
General
The provisions of the indenture do not limit the aggregate principal
amount of debt securities which may be issued thereunder. Unless otherwise provided in a prospectus supplement, the debt securities will
be our direct, unsecured and unsubordinated general obligations and will have the same rank in liquidation as all of our other unsecured
and unsubordinated debt. The debt securities may be convertible into common stock or other securities if specified in the applicable
prospectus supplement.
Payments
We may issue debt securities from time to time in one or more series.
The provisions of the indenture allow us to “reopen” a previous issue of a series of debt securities and issue additional
debt securities of that series. The debt securities may be denominated and payable in U.S. dollars or other currencies. We may also issue
debt securities from time to time with the principal amount or interest payable on any relevant payment date to be determined by reference
to one or more currency exchange rates, securities or baskets of securities, commodity prices or indices. Holders of these types of debt
securities will receive payments of principal or interest that depend upon the value of the applicable currency, security or basket of
securities, commodity or index on the relevant payment dates.
Debt securities may bear interest at a fixed rate, which may be zero,
a floating rate, or a rate which varies during the lifetime of the debt security. Debt securities bearing no interest or interest at
a rate that at the time of issuance is below the prevailing market rate may be sold at a discount below their stated principal amount.
Terms Specified in the Applicable Prospectus Supplement
The applicable prospectus supplement will contain, where applicable,
the following terms of, and other information relating to, any offered debt securities:
| · | the specific designation; |
| · | any limit on the aggregate
principal amount of the debt securities, their purchase price and denomination; |
| · | the currency in which
the debt securities are denominated and/or in which principal, premium, if any, and/or interest,
if any, is payable; |
| · | the interest rate
or rates or the method by which the calculation agent will determine the interest rate or
rates, if any; |
| · | the interest payment
dates, if any; |
| · | the place or places
for payment of the principal of and any premium and/or interest on the debt securities; |
| · | any repayment, redemption,
prepayment or sinking fund provisions, including any redemption notice provisions; |
| · | whether we will issue
the debt securities in registered form or bearer form or both and, if we are offering debt
securities in bearer form, any restrictions applicable to the exchange of one form for another
and to the offer, sale and delivery of those debt securities in bearer form; |
| · | whether we will issue
the debt securities in definitive form and under what terms and conditions; |
| · | the terms on which
holders of the debt securities may convert or exchange these securities into or for common
stock or other securities, any specific terms relating to the adjustment of the conversion
or exchange feature and the period during which the holders may make the conversion or exchange; |
| · | information as to
the methods for determining the amount of principal or interest payable on any date and/or
the currencies, securities or baskets of securities, commodities or indices to which the
amount payable on that date is linked; |
| · | any agents for the
debt securities, including trustees, depositaries, authenticating or paying agents, transfer
agents or registrars; |
| · | whether and under
what circumstances we will pay additional amounts on debt securities for any tax, assessment
or governmental charge withheld or deducted and, if so, whether we will have the option to
redeem those debt securities rather than pay the additional amounts; |
| · | any material United
States federal income tax or other income tax consequences, including, but not limited to: |
| o | tax
considerations applicable to any discounted debt securities or to debt securities issued
at par that are treated as having been issued at a discount for United States federal income
tax purposes; and |
| o | tax
considerations applicable to any debt securities denominated and payable in non-United States
currencies; |
| · | whether certain payments
on the debt securities will be guaranteed under a financial insurance guarantee policy and
the terms of that guarantee; |
| · | whether the debt securities
will be secured; |
| · | any applicable selling
restrictions; and |
| · | any other specific
terms of the debt securities, including any modifications to or additional events of default,
covenants or modified or eliminated acceleration rights, and any terms required by or advisable
under applicable laws or regulations. |
Some of the debt securities may be issued as original issue discount
securities. Original issue discount securities bear no interest or bear interest at below-market rates and may be sold at a discount
below their stated principal amount. The applicable prospectus supplement will contain information relating to income tax, accounting,
and other special considerations applicable to original issue discount securities.
Registration and Transfer of Debt Securities
Holders may present debt securities for exchange, and holders of registered
debt securities may present these securities for transfer, in the manner, at the places and subject to the restrictions stated in the
debt securities and described in the applicable prospectus supplement. We will provide these services without charge except for any tax
or other governmental charge payable in connection with these services and subject to any limitations or requirements provided in the
indenture or the supplemental indenture or issuer order under which that series of debt securities is issued. Holders may transfer debt
securities in bearer form and/or the related coupons, if any, by delivery to the transferee. If any of the securities are held in global
form, the procedures for transfer of interests in those securities will depend upon the procedures of the depositary for those global
securities.
Events of Default
The indenture provides holders of debt securities with remedies if
we fail to perform specific obligations, such as making payments on the debt securities, or if we become bankrupt. Holders should review
these provisions and understand which actions trigger an event of default and which actions do not. The indenture permits the issuance
of debt securities in one or more series, and, in many cases, whether an event of default has occurred is determined on a series-by-series
basis.
An event of default is defined under the indenture, with respect to
any series of debt securities issued under the indenture, as any one or more of the following events, subject to modification in a supplemental
indenture, each of which we refer to in this prospectus as an event of default, having occurred and be continuing:
| · | default is made for
more than 30 days in the payment of interest, premium or principal in respect of the securities; |
| · | we fail to perform
or observe any of our other obligations under the securities and this failure has continued
for the period of 60 days next following the service on us of notice requiring the same to
be remedied; |
| · | our bankruptcy, insolvency
or reorganization under any applicable bankruptcy, insolvency or insolvency related reorganization
law; |
| · | an order is made or
an effective resolution is passed for the winding up or liquidation of us; or |
| · | any other event of
default provided in the supplemental indenture or issuer order, if any, under which that
series of debt securities is issued. |
Acceleration of Debt Securities Upon an Event of Default
The indenture provides that, unless otherwise set forth in a supplemental
indenture:
| · | if an event of default
occurs due to the default in payment of principal of, or any premium or interest on, any
series of debt securities issued under the indenture, or due to the default in the performance
or breach of any other covenant or warranty of us applicable to that series of debt securities
but not applicable to all outstanding debt securities issued under the indenture occurs and
is continuing, either the trustee or the holders of not less than 25% in aggregate principal
amount of the outstanding debt securities of each affected series, voting as one class, by
notice in writing to us may declare the principal of and accrued interest on the debt securities
of such affected series (but not any other debt securities issued under the indenture) to
be due and payable immediately; |
| · | if an event of default
occurs due to specified events of bankruptcy, insolvency or reorganization of us, the principal
of all debt securities and interest accrued on the debt securities to be due and payable
immediately; and |
| · | if an event of default
due to a default in the performance of any other of the covenants or agreements in the indenture
applicable to all outstanding debt securities issued under the indenture occurs and is continuing,
either the trustee or the holders of not less than 25% in aggregate principal amount of all
outstanding debt securities issued under the indenture for which any applicable supplemental
indenture does not prevent acceleration under the relevant circumstances, voting as one class,
by notice in writing to us may declare the principal of all debt securities and interest
accrued on the debt securities to be due and payable immediately. |
Annulment of Acceleration and Waiver of Defaults
In some circumstances, if any and all events of default under the
indenture, other than the non-payment of the principal of the securities that has become due as a result of an acceleration, have been
cured, waived or
otherwise remedied, then the holders of a majority in aggregate principal
amount of all series of outstanding debt securities affected, voting as one class, may annul past declarations of acceleration or waive
past defaults of the debt securities.
Indemnification of Trustee for Actions Taken on Your Behalf
The indenture provides that the trustee shall not be liable with respect
to any action taken or omitted to be taken by it in good faith in accordance with the direction of the holders of debt securities issued
under the indenture relating to the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising
any trust or power conferred upon the trustee. In addition, the indenture contains a provision entitling the trustee, subject to the
duty of the trustee to act with the required standard of care during a default, to be indemnified to its satisfaction by the holders
of debt securities issued under the indenture before proceeding to exercise any right or power at the request of holders. Subject to
these provisions and specified other limitations, the holders of a majority in aggregate principal amount of each series of outstanding
debt securities of each affected series, voting as one class, may direct the time, method and place of conducting any proceeding for
any remedy available to the trustee, or exercising any trust or power conferred on the trustee.
Limitation on Actions by You as an Individual Holder
The indenture provides that no individual holder of debt securities
may institute any action against us under the indenture, except actions for payment of overdue principal and interest, unless the following
actions have occurred:
| · | the holder must have
previously given written notice to the trustee of the continuing default; |
| · | the holders of not
less than 25% in aggregate principal amount of the outstanding debt securities of each affected
series, treated as one class, must have: |
| o | requested
the trustee to institute that action; and |
| o | offered
the trustee indemnity satisfactory to it; |
| · | the trustee must have
failed to institute that action within 60 days after receipt of the request referred to above;
and |
| · | the holders of a majority
in principal amount of the outstanding debt securities of each affected series, voting as
one class, must not have given directions to the trustee inconsistent with those of the holders
referred to above. |
The indenture contains a covenant that we will file annually with
the trustee a certificate of no default or a certificate specifying any default that exists.
Discharge, Defeasance and Covenant Defeasance
We have the ability to eliminate most or all of our obligations on
any series of debt securities prior to maturity if we comply with the following provisions:
Discharge
of Indenture. We may discharge all of our obligations, other than as to transfers and exchanges, under the indenture
after we have:
| · | paid or caused to
be paid the principal of and interest on all of the outstanding debt securities in accordance
with their terms; |
| · | delivered to the trustee
for cancellation all of the outstanding debt securities; or |
| · | irrevocably deposited
with the trustee cash or, in the case of a series of debt securities payable only in U.S.
dollars, U.S. government obligations in trust for the benefit of the holders of any series
of debt securities issued under the indenture that have either become due and payable, or
are by their terms due and payable, or are scheduled for redemption, within one year, in
an amount certified to be sufficient to pay on each date that they become due and payable,
the principal of and interest on, and any mandatory sinking fund payments for, those debt
securities. However, the deposit of cash or U.S. government obligations for the benefit of
holders of a series of debt securities that are due and payable, or are scheduled for redemption,
within one year will discharge obligations under the indenture relating only to that series
of debt securities. |
Defeasance
of a Series of Securities at Any Time. We may also discharge all of our obligations, other than as to transfers
and exchanges, under any series of debt securities at any time, which we refer to as defeasance in this prospectus. We may be released
with respect to any outstanding series of debt securities from the obligations imposed by any covenants and elect not to comply with
those covenants without creating an event of default. Discharge under those procedures is called covenant defeasance.
Defeasance or covenant defeasance may be effected
only if, among other things:
| · | we irrevocably deposit
with the trustee cash or, in the case of debt securities payable only in U.S. dollars, U.S.
government obligations, as trust funds in an amount certified to be sufficient to pay on
each date that they become due and payable, the principal of and interest on, and any mandatory
sinking fund payments for, all outstanding debt securities of the series being defeased;
and |
| · | we deliver to the
trustee an opinion of counsel to the effect that: |
| o | the
holders of the series of debt securities being defeased will not recognize income, gain or
loss for United States federal income tax purposes as a result of the defeasance or covenant
defeasance; |
| o | the
defeasance or covenant defeasance will not otherwise alter those holders’ United States
federal income tax treatment of principal and interest payments on the series of debt securities
being defeased; and |
| o | in
the case of a defeasance, this opinion must be based on a ruling of the Internal Revenue
Service or a change in United States federal income tax law occurring after the date of this
prospectus, since that result would not occur under current tax law. |
Modification of the Indenture
Modification
without Consent of Holders. We and the trustee may enter into supplemental indentures without the consent of the
holders of debt securities issued under the indenture to:
| · | secure any debt securities; |
| · | evidence the assumption
by a successor corporation of our obligations; |
| · | add covenants for
the protection of the holders of debt securities; |
| · | cure any ambiguity
or correct any inconsistency; |
| · | establish the forms
or terms of debt securities of any series; or |
| · | evidence the acceptance
of appointment by a successor trustee. |
Modification
with Consent of Holders. We and the trustee, with the consent of the holders of not less than a majority in aggregate
principal amount of each affected series of outstanding debt securities, voting as one class, may add any provisions to, or change in
any manner or eliminate any of the provisions of, the indenture or modify in any manner the rights of the holders of those debt securities.
However, we and the trustee may not make any of the following changes to any outstanding debt security without the consent of each holder
that would be affected by the change:
| · | extend the final maturity
of the security; |
| · | reduce the principal
amount; |
| · | reduce the rate or
extend the time of payment of interest; |
| · | reduce any amount
payable on redemption; |
| · | change the currency
in which the principal, including any amount of original issue discount, premium, or interest
on the security is payable; |
| · | modify or amend the
provisions for conversion of any currency into another currency; |
| · | reduce the amount
of any original issue discount security payable upon acceleration or provable in bankruptcy; |
| · | alter the terms on
which holders of the debt securities may convert or exchange debt securities for common stock
or other securities, other than in accordance with the antidilution provisions or other similar
adjustment provisions included in the terms of the debt securities; |
| · | impair the right of
any holder to institute suit for the enforcement of any payment on any debt security when
due; or |
| · | reduce the percentage
of debt securities the consent of whose holders is required for modification of the indenture. |
Form of Debt Security
Each debt security will be represented either by a certificate issued
in definitive form to a particular investor or by one or more global securities representing the entire issuance of securities. Both
certificated securities in definitive form and global securities may be issued either:
| · | in registered form,
where our obligation runs to the holder of the security named on the face of the security;
or |
| · | in bearer form, where
our obligation runs to the bearer of the security. |
Definitive securities name you or your nominee as the owner of the
security, other than definitive bearer securities, which name the bearer as owner, and in order to transfer or exchange these securities
or to receive payments other than interest or other interim payments, you or your nominee must physically deliver the securities to the
trustee, registrar, paying agent or other agent, as applicable.
Global securities name a depositary or its nominee as the owner of
the debt securities represented by these global securities, other than global bearer securities, which name the bearer as owner. The
depositary maintains a computerized system that will reflect each investor’s beneficial ownership of the securities through an
account maintained by the investor with its broker/dealer, bank, trust company or other representative, as we explain more fully below.
Global Securities
Registered
Global Securities. We may issue the debt securities in the form of one or more fully registered global securities
that will be deposited with a depositary or its nominee identified in the applicable prospectus supplement and registered in the name
of that depositary or nominee. In those cases, one or more registered global securities will be issued in a denomination or aggregate
denominations equal to the portion of the aggregate principal or face amount of the securities to be represented by registered global
securities. Unless and until it is exchanged in whole for securities in definitive registered form, a registered global security may
not be transferred except as a whole by and among the depositary for the registered global security, the nominees of the depositary or
any successors of the depositary or those nominees. If not described below, any specific terms of the depositary arrangement with respect
to any securities to be represented by a registered global security will be described in the prospectus supplement relating to those
securities. We anticipate that the following provisions will apply to all depositary arrangements:
Ownership of beneficial interests in a registered global security
will be limited to persons, called participants, that have accounts with the depositary or persons that may hold interests through participants.
Upon the issuance of a registered global security, the depositary will credit, on its book-entry registration and transfer system, the
participants’ accounts with the respective principal or face amounts of the securities beneficially owned by the participants.
Any dealers, underwriters or selling agents participating in the distribution of the securities will designate the accounts to be credited.
Ownership of beneficial interests in a registered global security will be shown on, and the transfer of ownership interests will be effected
only through, records maintained by the depositary, with respect to interests of participants, and on the records of participants, with
respect to interests of persons holding through participants. The laws of some jurisdictions may require that some purchasers of securities
take physical delivery of these securities in definitive form. These laws may impair your ability to own, transfer or pledge beneficial
interests in registered global securities. So long as the depositary, or its nominee, is the registered owner of a registered global
security, that depositary or its nominee, as the case may be, will be considered the sole owner or holder of the securities represented
by the registered global security for all purposes under the indenture.
Except as described below, owners of beneficial interests in a registered
global security will not be entitled to have the securities represented by the registered global security registered in their names,
will not receive or be entitled to receive physical delivery of the securities in definitive form and will not be considered the owners
or holders of the securities under the indenture. Accordingly, each person owning a beneficial interest in a registered global security
must rely on the procedures of the depositary for that registered global security and, if that person is not a participant, on the procedures
of the participant through which the person owns its interest, to exercise any rights of a holder under the indenture. We understand
that under existing industry practices, if we request any action of holders or if an owner of a beneficial interest in a registered global
security desires to give or take any action that a holder is entitled to give or take under the indenture, the depositary for the registered
global security would authorize the participants holding the relevant beneficial interests to give or take that action, and the participants
would authorize beneficial owners owning through them to give or take that action or would otherwise act upon the instructions of beneficial
owners holding through them.
Principal, premium, if any, and interest payments on debt securities
represented by a registered global security registered in the name of a depositary or its nominee will be made to the depositary or its
nominee, as the case may be, as the registered owner of the registered global security. None of us, the trustee or any other agent of
us or agent of the trustee will have any responsibility or liability for any aspect of the records relating to payments made on account
of beneficial ownership interests in the registered global security or for maintaining, supervising or reviewing any records relating
to those beneficial ownership interests. We expect that the depositary for any of the securities represented by a registered global security,
upon receipt of any payment of principal, premium, interest or other distribution of underlying securities or other property to holders
on that registered global security, will immediately credit participants’ accounts in amounts proportionate to their respective
beneficial interests in that registered global security as shown on the records of the depositary. We also expect that payments by participants
to owners of beneficial interests in a registered global security held through participants will be governed by standing customer instructions
and customary practices, as is now the case with the securities held for the accounts of customers in bearer form or registered in “street
name”, and will be the responsibility of those participants.
If the depositary for any of these securities represented by a registered
global security is at any time unwilling or unable to continue as depositary or ceases to be a clearing agency registered under the Exchange
Act, and a successor depositary registered as a clearing agency under the Exchange Act is not appointed by us within 90 days, we will
issue securities in definitive form in exchange for the registered global security that had been held by the depositary. In addition,
we may, at any time and in our sole discretion, decide not to have any of the securities represented by one or more registered global
securities. If we make that decision, we will issue securities in definitive form in exchange for all of the registered global security
or securities representing those securities. Any securities issued in definitive form in exchange for a registered global security will
be registered in the name or names that the depositary gives to the relevant trustee or other relevant agent of ours or theirs. It is
expected that the depositary’s instructions will be based upon directions received by the depositary from participants with respect
to ownership of beneficial interests in the registered global security that had been held by the depositary.
Bearer
Global Securities. The securities may also be issued in the form of one or more bearer global securities that will
be deposited with a common depositary for the Euroclear System and Clearstream Banking, société anonyme or
with a nominee for the depositary identified in the prospectus supplement relating to those securities. The specific terms and procedures,
including the specific terms of the depositary arrangement, with respect to any securities to be represented by a bearer global security
will be described in the prospectus supplement relating to those securities.
New York Law to Govern
The indenture and the debt securities will be governed by the laws
of the State of New York.
DESCRIPTION OF UNITS
We may issue units consisting of ordinary shares
(including ordinary shares represented by ADSs), preferential share (including preferential shares represented by ADSs), warrants or
debt securities, in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included
in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each security included in the unit. The unit
agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately,
at any time or at any time before a specified date.
We will describe in the applicable prospectus
supplement the terms of the series of units being offered, including:
| · | the
designation and terms of the units and of the securities comprising the units, including
whether and under what circumstances those securities may be held or transferred separately; |
| · | any
provisions of the governing unit agreement; and |
| · | any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of
the securities comprising the units. |
The provisions described in this section, as well
as those set forth in any prospectus supplement or as described under “Description of Capital Stock,” “Description
of Preferential Shares,” “Description of Warrants, and “Description of Debt Securities” will apply to each unit,
as applicable, and to any ordinary shares, preferential shares, warrants or debt securities included in each unit, as applicable.
Unit Agent
The name and address of the unit agent for any
units we offer will be set forth in the applicable prospectus supplement.
Issuance in Series
We may issue units in such amounts and in such
numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent
under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder of any unit.
A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility
in case of any default by us under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings
at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the related unit agent or the holder
of any other unit, enforce by appropriate legal action its rights as holder under any security included in the unit.
DESCRIPTION OF AMERICAN
DEPOSITARY SHARES
The Bank of New York Mellon, as depositary, registers and delivers
our ordinary share ADSs. Each ADS represents 10 ordinary shares (or a right to receive 10 ordinary shares) deposited with Societe Generale,
as custodian for the depositary in France. Each ADS will also represent any other securities, cash or other property which may be held
by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred
to as the deposited securities. The depositary's office at which the ADSs will be administered and its principal executive office are
located at 240 Greenwich Street, New York, New York 10286.
An ADS holder may hold ADSs either (A) directly (i) by having
an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in
the ADS holder’s name, or (ii) by having uncertificated ADSs registered in the ADS holder’s name, or (B) indirectly
by holding a security entitlement in ADSs through the ADS holder’s broker or other financial institution that is a direct or indirect
participant in The Depository Trust Company, also called DTC. If an ADS holder hold ADSs directly, he or she is a registered ADS holder.
If an investor holds the ADSs indirectly, the investor must rely on the procedures of his or her broker or other financial institution
to assert the rights of ADS holders described in this section.
Registered holders of uncertificated ADSs will receive statements
from the depositary confirming their holdings.
ADS holders will not be treated as shareholders and ADS holders will
not have shareholder rights. French law governs shareholder rights. The depositary will be the holder of the shares underlying the ADSs.
As a registered holder of ADSs, the ADS holders will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders
and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the
depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit
agreement with The Bank of New York Mellon.
Dividends and Other Distributions
How will ADS holders receive dividends and other distributions
on the shares?
The depositary has agreed to pay or distribute to ADS holders the
cash dividends or other distributions it or the custodian receives on shares or other deposited securities, upon payment or deduction
of its fees and expenses. ADS holders will receive these distributions in proportion to the number of shares the ADSs represent.
Distributions of Cash
The depositary will convert any cash dividend or other cash distribution
we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States.
If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to
distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot
convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable
for any interest.
Before making a distribution, any withholding taxes, or other governmental
charges that must be paid will be deducted. The depositary will distribute only whole U.S. dollars and cents and will round fractional
cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency,
ADS holders may lose some of the value of the distribution.
Distributions of Shares
The depositary may, and will if the company so requests in writing,
distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute
whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute
the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will
also represent the new shares. The depositary may sell a portion of the distributed shares (or ADSs representing those shares) sufficient
to pay its fees and expenses in connection with that distribution.
Distribution of Rights
If we offer holders of our securities any rights to subscribe for
additional shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders, (ii) distribute
those rights to ADS holders or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction
or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse.
In that case, ADS holders will receive no value for them. The depositary will exercise or distribute rights only if we ask it
to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase
the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares,
to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict
the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders,
and the securities distributed may be subject to restrictions on transfer.
Other Distributions
The depositary will send to ADS holders anything else we distribute
on deposited securities by any means it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary
has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it
may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary
is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it
is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its
fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute
securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful
or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities
under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything
else to ADS holders. This means that ADS holders may not receive the distributions we make on our shares or any value for them if
it is illegal or impractical for us to make them available to ADS holders.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares
or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as
stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and
will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
ADS holders may surrender ADSs to the depositary for the purpose of
withdrawal. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the
depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder
designates at the office of the custodian. Or, at the ADS holder’s request, risk and expense, the depositary will deliver the deposited
securities at its office, if feasible. However, the depositary is not required to accept surrender of ADSs to the extent it would require
delivery of a fraction of a deposited share or other security. The depositary may charge ADS holders a fee and its expenses for instructing
the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and
uncertificated ADSs?
ADS holders may surrender their ADR to the depositary for the purpose
of exchanging their ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming
that the ADS holder is the registered holder of uncertificated ADSs. Upon receipt by the depositary of a proper instruction from a registered
holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver
to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do ADS holders vote?
ADS holders may instruct the depositary how to vote the number of
deposited shares their ADSs represent. If we request the depositary to solicit ADS holders’ voting instructions (and we are not
required to do so), the depositary will notify the ADS holders of a shareholders' meeting and send or make voting materials available
to them. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote.
For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical,
subject to the laws of France and the provisions of our articles of association or similar documents, to vote or to have its agents vote
the shares or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit voting instructions
from our ADS holders, ADS holders may still send voting instructions, and, in that case, the depositary may try to vote as instructed,
but it is not required to do so.
Except by instructing the depositary
as described above, ADS holders won't be able to exercise voting rights unless they surrender their ADSs and withdraw the shares. However,
ADS holders may not know about the meeting enough in advance to withdraw the shares. In any event, the depositary will not
exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed or as described in the
following sentence. If (i) we asked the depositary to solicit voting instructions at least 45 days before the meeting date,
(ii) the depositary does not receive voting instructions from the ADS holder by the specified date and (iii) we confirm in
writing to the depositary that:
| · | we
wish to receive a proxy to vote uninstructed shares; |
| · | we
reasonably do not know of any substantial shareholder opposition to a particular question;
and |
| · | the
particular question is not materially adverse to the interests of shareholders, |
the depositary will consider the ADS holder to have instructed it
to give, and it will give, a discretionary proxy to a person designated by us to vote the number of deposited securities represented
by the ADS holder’s ADSs as to that question.
We cannot assure ADS holders that they will receive the voting materials
in time to ensure that they can instruct the depositary to vote their shares. In addition, the depositary and its agents are not responsible
for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may
not be able to exercise voting rights and there may be nothing they can do if their shares are not voted as requested.
In order to give ADS holders a reasonable opportunity to instruct
the depositary as to the exercise of voting rights relating to deposited securities, if we request the depositary to act, we agree to
give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 45 days in advance of
the meeting date.
Fees and Expenses
| Persons
depositing or withdrawing shares or ADS holders must pay: |
|
For:
|
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuances resulting from a distribution
of shares or rights or other property
Cancellation of ADSs for the purpose of withdrawal, including
if the deposit agreement terminates |
| |
|
|
| $.05 (or less) per ADS |
|
Any cash distribution to ADS holders |
| |
|
|
| A fee equivalent to the fee that would be payable if
securities distributed to ADS holder had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of
deposited securities (including rights) that are distributed by the depositary to ADS holders |
| |
|
|
| $.05 (or less) per ADS per calendar year |
|
Depositary services |
| |
|
|
| Registration or transfer fees |
|
Transfer and registration of shares on our share register
to or from the name of the depositary or its agent when the ADS holder deposits or withdraws shares |
| |
|
|
| Expenses of the depositary |
|
Cable (including SWIFT) and facsimile transmissions (when expressly
provided in the deposit agreement)
Converting foreign currency to U.S. dollars |
| |
|
|
| Taxes and other governmental charges the depositary
or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
| |
|
|
| Any charges incurred by the depositary or its agents
for servicing the deposited securities |
|
As necessary |
The depositary collects its fees for delivery and surrender of ADSs
directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them.
The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling
a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from
cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The
depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other
property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting
services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse
us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services
provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit
agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated
with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates,
or the custodian or we may convert currency and pay U.S. dollars to the depository. Where the depository converts currency itself or
through any of its affiliates, the depository acts as principal for its own account and not as agent, advisor, broker or fiduciary on
behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account.
The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under
the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own
account. The depositary makes no representation that the exchange rate used or obtained by it or its affiliates in any currency conversion
under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate
will be determined will be the most favorable to ADS holders, subject to the depositary's obligation under to act without negligence
or bad faith. The methodology used to determine exchange rates used in currency made by the depositary is available upon request. Where
the custodian converts currency, the custodian has no obligation to obtain the most favorable rate that could be obtained at the time
or to ensure that the method by which that rate will be determined will be the most favorable to ADS holders, and the depositary makes
no representation that the rate is the most favorable rate and will not be liable for any direct or indirect losses associated with the
rate. In certain instances, the depositary may receive dividends or other distributions in U.S. dollars that represent the proceeds of
a conversion of foreign currency or translation from foreign currency at a rate that was obtained or determined by us and, in such cases,
the depositary will not engage in, or be responsible for, any foreign currency transactions and neither it nor we make any representation
that the rate obtained or determined by us is the most favorable rate and neither it nor we will be liable for any direct or indirect
losses associated with the rate.
Payment of Taxes
ADS holders will be responsible for any taxes or other governmental
charges payable on the ADSs or on the deposited securities represented by any of the ADSs. The depositary may refuse to register any
transfer of the ADSs or allow ADS holders to withdraw the deposited securities represented by the ADSs until those taxes or other charges
are paid. It may apply payments owed to ADS holders or sell deposited securities represented by the ADSs to pay any taxes owed and the
ADS holder will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number
of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the
taxes.
Tender and Exchange Offers; Redemption, Replacement or Cancellation
of Deposited Securities
The depositary will not tender deposited securities in any voluntary
tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary
may establish.
If deposited securities are redeemed for cash in a transaction that
is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number
of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division,
combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited
securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary
will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides, after
consultation with the company to the extent practicable, it would not be lawful and practical to hold the replacement securities because
those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities
and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary
will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or
ask ADS holders to surrender their outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if
the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary
may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and
the ADRs without the ADS holders’ consent for any reason. If an amendment adds or increases fees or charges, except for taxes and
other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or
prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary
notifies ADS holders of the amendment. At the time an amendment becomes effective, ADS holders are considered, by continuing to hold
ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement
if we instruct it to do so. The depositary may initiate termination of the deposit agreement if:
| · | 60 days
have passed since the depositary told us it wants to resign but a successor depositary has
not been appointed and accepted its appointment; |
| · | we
delist our shares from an exchange on which they were listed and do not list the shares on
another exchange; |
| · | we
appear to be insolvent or enter insolvency proceedings; |
| · | all
or substantially all the value of the deposited securities has been distributed either in
cash or in the form of securities; |
| · | there
are no deposited securities underlying the ADSs or the underlying deposited securities have
become apparently worthless; or |
| · | there
has been a replacement of deposited securities. |
If the deposit agreement will terminate, the depositary will notify
ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited
securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the
deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not
surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After the termination date and before the depositary sells, ADS holders
can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender
for the purpose of withdrawing deposited securities or reverse previously accepted surrenders of that kind if it would interfere with
the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited
securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination
date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited
securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement
except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary;
Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations
of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| · | are
only obligated to take the actions specifically set forth in the deposit agreement without
negligence or bad faith, and the depositary will not be a fiduciary or have any fiduciary
duty to holders of ADSs; |
| · | are
not liable if we are or it is prevented or delayed by law or by events or circumstances beyond
our or its control from performing our or its obligations under the deposit agreement; |
| · | are
not liable if we or it exercises discretion permitted under the deposit agreement; |
| · | are
not liable for the inability of any holder of ADSs to benefit from any distribution on deposited
securities that is not made available to holders of ADSs under the terms of the deposit agreement,
or for any special, consequential or punitive damages for any breach of the terms of the
deposit agreement; |
| · | have
no obligation to become involved in a lawsuit or other proceeding related to the ADSs or
the deposit agreement on the ADS holder’s behalf or on behalf of any other person; |
| · | may
rely upon any documents we believe or it believes in good faith to be genuine and to have
been signed or presented by the proper person; |
| · | are
not liable for the acts or omissions of any securities depository, clearing agency or settlement
system; and |
| · | the
depositary has no duty to make any determination or provide any information as to our tax
status, or any liability for any tax consequences that may be incurred by ADS holders as
a result of owning or holding ADSs or be liable for the inability or failure of an ADS holder
to obtain the benefit of a foreign tax credit, reduced rate of withholding or refund of amounts
withheld in respect of tax or any other tax benefit. In the deposit agreement, we and the
depositary agree to indemnify each other under certain circumstances. |
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs,
make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
| · | payment
of stock transfer or other taxes or other governmental charges and transfer or registration
fees charged by third parties for the transfer of any shares or other deposited securities; |
| · | satisfactory
proof of the identity and genuineness of any signature or other information it deems necessary;
and |
| · | compliance
with regulations it may establish, from time to time, consistent with the deposit agreement,
including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of
ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable
to do so.
Right to Receive the Shares Underlying ADSs
ADS holders have the right to cancel the ADSs and withdraw the underlying
shares at any time except:
| · | when
temporary delays arise because: (i) the depositary has closed its transfer books or
we have closed our transfer books; (ii) the transfer of shares is blocked to permit
voting at a shareholders' meeting; or (iii) we are paying a dividend on our shares; |
| · | when
the ADS holder owes money to pay fees, taxes and similar charges; or |
| · | when
it is necessary to prohibit withdrawals in order to comply with any laws or governmental
regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision
of the deposit agreement.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge
that the Direct Registration System, also referred to as DRS, and Profile Modification System, also referred to as Profile, will apply
to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertifiated ADSs and holding
of security entitlements in ADSs through DTC and a DTC participant. Profile is a feature of DRS that allows a DTC participant, claiming
to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC
or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization
from the ADS holder to register the transfer.
In connection with an in accordance with the arrangements and procedures
relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant
that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph
above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code).
In the deposit agreement, the parties agree that the depositary's reliance on and compliance with instructions received by the depositary
through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of
the depositary.
Shareholder communications; inspection
of register of holders of ADSs
The depositary will make available for ADS holders’ inspection
at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders
of deposited securities. The depositary will send ADS holders copies of those communications or otherwise make those communications available
to them if they ask it to. ADS holders have a right to inspect the register of holders of ADSs, but not for the purpose of contacting
those holders about a matter unrelated to our business or the ADSs.
Jury Trial Waiver
The deposit agreement provides that, to the extent permitted by law,
ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our
shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws. If we or the depositary opposed
a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable in the facts and circumstances
of that case in accordance with applicable case law. By agreeing to the jury trial waiver provision in the deposit agreement, ADS holders
will not be deemed to have waived our compliance with or the depositary's compliance with the federal securities laws and the rules and
regulations promulgated thereunder.
PLAN OF DISTRIBUTION
We may offer and sell the securities in one
or more of the following ways (or in any combination) from time to time:
| · | through underwriters
or dealers; |
| · | directly to a limited
number of purchasers or to a single purchaser; |
| · | in “at the market
offerings,” within the meaning of Rule 415(a)(4) of the Securities Act, to
or through a market maker or into an existing trading market on an exchange or otherwise; |
| · | through any other
method permitted by applicable law and described in the applicable prospectus supplement. |
The prospectus supplement will state the
terms of the offering of the securities, including:
| · | the name or names
of any underwriters, dealers or agents; |
| · | the purchase price
of such securities and the proceeds to be received by us, if any; |
| · | any underwriting discounts
or agency fees and other items constituting underwriters’ or agents’ compensation; |
| · | any public offering
price; |
| · | any discounts or concessions
allowed or reallowed or paid to dealers; and |
| · | any securities exchanges
on which the securities may be listed. |
Any public offering price and any discounts
or concessions allowed or reallowed or paid to dealers may be changed from time to time.
If underwriters are used in the sale, the
securities will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions,
including:
| · | negotiated transactions; |
| · | at a fixed public
offering price or prices, which may be changed; |
| · | at market prices prevailing
at the time of sale; |
| · | at prices related
to prevailing market prices; or |
Unless otherwise stated in a prospectus supplement,
the obligations of the underwriters to purchase any securities will be conditioned on customary closing conditions and the underwriters
will be obligated to purchase all of such series of securities, if any are purchased.
The securities may be sold through agents
from time to time. The prospectus supplement will name any agent involved in the offer or sale of the securities and any commissions
paid to them. Generally, any agent will be acting on a commercially reasonable efforts basis for the period of its appointment.
Sales to or through one or more underwriters
or agents in at-the-market offerings will be made pursuant to the terms of a distribution agreement with the underwriters or
agents. Such underwriters or agents may act on an agency basis or on a principal basis. During the term of any such agreement, shares
may be sold on a daily basis on any stock exchange, market or trading facility on which the ordinary shares are traded, in privately
negotiated transactions or otherwise as agreed with the underwriters or agents. The distribution agreement will provide that any ordinary
share sold will be sold at negotiated prices or at prices related to the then prevailing market prices for our ordinary shares. Therefore,
exact figures regarding proceeds that will be raised or commissions to be paid cannot be determined at this time and will be described
in a prospectus supplement. Pursuant to the terms of the distribution agreement, we may also agree to sell, and the relevant underwriters
or agents may agree to solicit offers to purchase, blocks of our ordinary shares or other securities. The terms of each such distribution
agreement will be described in a prospectus supplement.
We may authorize underwriters, dealers or
agents to solicit offers by certain purchasers to purchase the securities at the public offering price set forth in the prospectus supplement
pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. The contracts will be subject
only to those conditions set forth in the prospectus supplement, and the prospectus supplement will set forth any commissions paid for
solicitation of these contracts.
Underwriters and agents may be entitled under
agreements entered into with us to indemnification by us against certain civil liabilities, including liabilities under the Securities
Act, or to contribution with respect to payments which the underwriters or agents may be required to make.
The prospectus supplement may also set forth
whether or not underwriters may over-allot or effect transactions that stabilize, maintain or otherwise affect the market price of the
securities at levels above those that might otherwise prevail in the open market, including, for example, by entering stabilizing bids,
effecting syndicate covering transactions or imposing penalty bids.
Underwriters and agents may be customers
of, engage in transactions with, or perform services for us and our affiliates in the ordinary course of business.
Each series of securities will be a new issue
of securities and will have no established trading market, other than our ordinary shares, which are listed on Nasdaq. Any underwriters
to whom securities are sold for public offering and sale may make a market in the securities, but such underwriters will not be obligated
to do so and may discontinue any market making at any time without notice. The securities, other than our ordinary shares, may or may
not be listed on a national securities exchange.
LIMITATIONS AFFECTING
SHAREHOLDERS OF A FRENCH COMPANY
Ownership
of ADSs or Ordinary Shares by Non-French Residents
Neither the French Commercial Code nor our
bylaws presently impose any restrictions on the rights of non-French residents or non-French shareholders to own and vote shares. However,
any person who comes to hold or ceases to hold a number of shares representing a fraction equal to 5%, 10%, 15%, 20%, 25%, 30%, 33.33%,
50%, 66.66%, 90% or 95% of our share capital or voting rights must inform us at the latest before the close of business on the fourth
trading day following the threshold crossing. In addition, any person who comes to hold or ceases to hold a fraction equal to 50% or
95% of our share capital or voting rights is required to inform the French Stock Exchange Authority (Autorité des marchés
financiers).
Moreover, the French Monetary and Financial
Code (CMF) provides for statistical reporting requirements. Transactions by which non-French residents acquire at least 10% of
our share capital or voting rights, or cross the 10% threshold, of a French resident company, are considered as foreign direct investments
in France and are subject to statistical reporting requirements (Articles R. 152-1; R.152-3 and R. 152-11 of the CMF). When the
investments exceeds €15,000,000, companies must declare foreign transactions directly to the Banque de France within
20 business days following the date of certain direct foreign investments in us, including any purchase of ADSs. Failure to comply with
such statistical reporting requirement may be sanctioned by five years imprisonment and a fine of a maximum amount equal to twice the
amount which should have been reported, in accordance with Article L 165-1 of the CMF. This amount may be increased fivefold if
the violation is made by a legal entity.
Certain foreign investments in companies
incorporated under French law are subject to the prior authorization of the French Minister of the Economy, where all or a part of the
target's business and activity relate to a strategic section, such as (i) national defense; (ii) essential infrastructure and
services related to energy, transportation, public health, telecommunications, etc.; and (iii) research and development activities
related to critical technologies including cybersecurity, artificial intelligence, robotics, semiconductors, etc and biotechnology since
order of April 27, 2020 relating to foreign investment in France which completed the list of "critical technologies" protected
under Article R. 151-3 of the CMF. As a consequence, crossing directly or indirectly, alone or in concert, for non-EU or non-EEA
investors, the threshold of 25% of the voting rights of an entity under French law active in the R&D biotechnology sector is an investment
subject to prior authorization by the French Minister of Economy (Article R.151-2 of the CMF). Additionally, in the context of the
COVID-19 pandemic, the French government has replaced the 25% threshold of voting rights by a 10% threshold of voting rights for non-EU
and non-EEA investments in French listed companies at least until December 31, 2023 (decree not published yet). In the absence of
such authorization, the relevant investment shall be deemed null and void. The relevant investor may be found criminally liable and may
be sanctioned with a fine not to exceed the greater of the following amounts: (i) twice the amount of the relevant investment, (ii) 10%
of the annual turnover before tax of the target company or (iii) €5,000,000 for a company.
Foreign
Exchange Controls
Under current French foreign exchange control
regulations there are no limitations on the amount of cash payments that we may remit to residents of foreign countries. Laws and regulations
concerning foreign exchange controls do, however, require that all payments or transfers of funds made by a French resident to a non-resident
such as dividend payments be handled by an accredited intermediary. All registered banks and substantially all credit institutions in
France are accredited intermediaries.
Availability
of Preferential Subscription Rights
Our shareholders will have the preferential
subscription rights described under "Description of Share Capital—Key Provisions of Our Bylaws and French Law Affecting Our
Ordinary Shares—Changes in Share Capital—Preferential Subscription Right." Under French law, shareholders have preferential
rights to subscribe for cash (including by way of set-off against receivables held by the subscriber against the company) issues of new
shares or other securities giving rights to acquire additional shares on a pro rata basis. Holders of our securities in the United States
(which may be in the form of shares or ADSs) may not be able to exercise preferential subscription rights for their securities unless
a registration statement under the Securities Act is effective with respect to such rights or an exemption from the registration requirements
imposed by the Securities Act is available. We may, from time to time, issue new shares or other securities giving rights to acquire
additional shares (such as warrants) at a time when no registration statement is in effect and no Securities Act exemption is available.
If so, holders of our securities in the United States will be unable to exercise any preferential subscription rights and their interests
will be diluted. We are under no obligation to file any registration statement in connection with any issuance of new shares or other
securities. We intend to evaluate at the time of any rights offering the costs and potential liabilities associated with registering
the rights, as well as the indirect benefits to us of enabling the exercise by holders of shares and holders of ADSs in the United States
of the subscription rights, and any other factors we consider appropriate at the time, and then to make a decision as to whether to register
the rights. We cannot assure you that we will file a registration statement.
For holders of our ordinary shares represented
by ADSs, the depositary may make these rights or other distributions available to ADS holders. If the depositary does not make the rights
available to ADS holders and determines that it is impractical to sell the rights, it may allow these rights to lapse. In that case ADS
holders will receive no value for them. The section of this prospectus titled "Description of American Depositary Shares—Dividends
and Other Distributions" explains in detail the depositary's responsibility in connection with a rights offering. See also "Risk
Factors—The right as a holder of ADSs to participate in any future preferential subscription rights or to elect to receive dividends
in shares may be limited, which may cause dilution to the holdings of purchasers of ADSs in the offering."
ENFORCEABILITY OF CIVIL
LIABILITIES
We are a société anonyme,
or S.A., organized under the laws of France. The majority of our directors and officers are citizens and residents of countries other
than the United States, and the majority of our assets are located outside of the United States. Accordingly, U.S. investors may find
it difficult and may be unable:
| · | to effect service
of process upon or obtain jurisdiction over our company or our officers and directors in
U.S. courts in actions predicated on the civil liability provisions of the U.S. federal securities
laws; |
| · | to enforce, either
inside or outside the United States, judgments obtained in U.S. or non-U.S. courts
in actions predicated upon the civil liability provisions of the U.S. federal securities
laws against us or our officers and directors; |
| · | to bring an original
action in a French court to enforce liabilities based upon the U.S. federal securities laws
against us or our officers or directors; and/or |
| · | to enforce against
us or our directors in non-U.S. courts, including French courts, judgments of U.S.
courts predicated upon the civil liability provisions of the U.S. federal securities laws. |
Nevertheless, a final judgment for the payment of money rendered by
any federal or state court in the United States based on civil liability, whether or not predicated solely upon the U.S. federal securities
laws, would be recognized and enforced in France provided that a French judge considers that this judgment meets the French legal requirements
concerning the recognition and the enforcement of foreign judgments and is capable of being immediately enforced in the United States.
In the absence of a bilateral international convention, a French court is therefore likely to grant the enforcement of a foreign judgment
without a review of the merits of the underlying claim, only if (1) the U.S. federal or state court has jurisdiction, (2) that
judgment does not contravene international public order and public policy of France and (3) that judgement is not tainted with fraud
..
We have been informed by Reed Smith LLP,
our counsel, that there is doubt as to enforceability in France, either in original actions or in actions for enforcement of judgments
of U.S. courts, of civil liabilities predicated in the U.S. federal securities laws.
In addition, actions in the United States
under the U.S. federal securities laws could be affected under certain circumstances by the French law No. 68-678 of July 26,
1968 as amended by French Law No. 80-538 of July 16, 1980, which may preclude or restrict the obtaining of evidence
in France or from French persons in connection with those actions. Each of the foregoing statements also applies to our auditors.
LEGAL MATTERS
Certain legal matters with respect to the validity of certain of the
offered securities will be passed upon for us by Reed Smith LLP, Paris, France and Reed Smith LLP, New York, New York. If the validity
of any securities is also passed upon by counsel for the underwriters of an offering of those securities, that counsel will be named
in the prospectus supplement relating to that offering.
EXPERTS
The consolidated financial statements of Biophytis S.A. as of December
31, 2021 and for each of the two years in the period ended December 31, 2021 appearing in Biophytis S.A.’s Annual Report on Form 20-F
for the year ended December 31, 2022, have been audited by Ernst & Young et Autres, independent registered public accounting
firm, as set forth in their report thereon included therein, and incorporated herein by reference. Such consolidated financial statements
are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The offices of Ernst & Young et Autres are located at 1/2
place des Saisons, 92400 Courbevoie, Paris La Défense 1, France.
The consolidated financial statements of Biophytis S.A. as of December
31, 2022, and for the year then ended, have been incorporated by reference herein in reliance upon the report of KPMG S.A., independent
registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and
auditing.
The offices of KPMG S.A. are located at 2, avenue Gambetta, CS 60055,
92066 Paris-la-Défense, France.
WHERE YOU CAN FIND
MORE INFORMATION
We have filed with the SEC a registration statement on Form F-3
under the Securities Act, including amendments and relevant exhibits and schedules, covering the underlying securities offered hereby.
This prospectus, which constitutes a part of the registration statement on Form F-3, summarizes material provisions of contracts
and other documents that we refer to in the prospectus. Since this prospectus does not contain all of the information contained in the
registration statement, you should read the registration statement and its exhibits and schedules for further information with respect
to us and the securities offered hereby.
We file annual reports on Form 20-F and other information with
the SEC and furnish reports on Form 6-K to the SEC. We are not required to disclose certain other information that is required from
U.S. domestic issuers. Also, as a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing
of proxy statements to shareholders and our directors, senior management and principal shareholders are exempt from the reporting and
short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
As a foreign private issuer, we are also exempt from the requirements
of Regulation FD (Fair Disclosure) which, generally, are meant to ensure that select groups of investors are not privy to specific information
about an issuer before other investors. We are, however, still subject to the anti-fraud and anti-manipulation rules of the SEC,
such as Rule 10b-5. Since many of the disclosure obligations required of us as a foreign private issuer are different than those
required by other U.S. domestic reporting companies, our shareholders, potential shareholders and the investing public in general should
not expect to receive information about us in the same amount and at the same time as information is received from, or provided by, other
U.S. domestic reporting companies. We are liable for violations of the rules and regulations of the SEC which do apply to us as
a foreign private issuer.
Our SEC filings are available to the public
over the Internet at the SEC’s website at http://www.sec.gov. You may also access our SEC filings at our website www.biophytis.com.
Our website and the information contained on, or that can be accessed through, our website will not be deemed to be incorporated by reference
in, and are not considered part of, this prospectus. You should not rely on our website or any such information in making your decision
whether to purchase our securities.
INCORPORATION OF CERTAIN
INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference the information we file
with it, which means that we can disclose important information to you by referring you to another document that we have filed separately
with the SEC. You should read the information incorporated by reference because it is an important part of this prospectus. We incorporate
by reference the following information or documents that we have filed with the SEC:
| · | Reports of Foreign
Private Issuers on Form 6-K furnished to the SEC on January 31,
2023 (interim financial report, excluding the Statement by the person responsible for
the half-year financial report and the Statutory Auditors' limited review report on the half-year
condensed consolidated financial statements prepared in accordance with IFRS standards as
adopted in the European Union) and February 2,
2023 (excluding the statement of management); and |
All annual reports on Form 20-F and any amendment thereto and
any report on Form 6-K (or portion thereof) that expressly indicates it is being incorporated by reference in this prospectus, in
each case, that we file with or furnish to the SEC prior to the termination or completion of the offering under this prospectus (including
all such reports or documents we may file with or furnish to the SEC on or after the date on which the registration statement of which
this prospectus is a part is first filed with the SEC and prior to the effectiveness of the registration statement), will also be incorporated
by reference into this prospectus and deemed to be part of this prospectus from the date of the filing or furnishing of such reports
and documents. Unless expressly incorporated by reference, nothing in this prospectus shall be deemed to incorporate by reference information
furnished to, but not filed with, the SEC.
Any statement contained in any document incorporated by reference
herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this
prospectus or any prospectus supplement modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed,
except as so modified or superseded, to constitute a part of this prospectus.
All of the documents that are incorporated by reference are available
at the website maintained by the SEC at http://www.sec.gov. In addition, copies of all documents incorporated by reference in
this prospectus, other than exhibits to those documents unless such exhibits are specifically incorporated by reference in this prospectus,
will be provided at no cost to each person, including any beneficial owner, to whom a copy of this prospectus is delivered on the written
or oral request of that person made to: Biophytis S.A., Sorbonne University-BC 9, Bâtiment A 4ème étage, 4 place
Jussieu 75005 Paris, France, Attention: Chief Executive Officer.

50,500,000 Ordinary Shares Represented by 505,000
American Depositary Shares
Pre-Funded Warrants to Purchase up to 82,833,400
Ordinary Shares
Represented by 828,334 American Depositary
Shares
Up to 82,833,400 Ordinary Shares Represented
by 828,334 American Depositary Shares
Underlying the Pre-Funded Warrants
Prospectus Supplement
July 18, 2023
H.C. Wainwright & Co.
Exhibit 107
The prospectus supplement to which this Exhibit is attached is a final
prospectus for the related offering of equity securities. The maximum aggregate offering price of the offering is $3,800,002.
Biophytis (NASDAQ:BPTS)
Historical Stock Chart
From Feb 2025 to Mar 2025
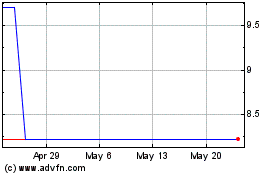
Biophytis (NASDAQ:BPTS)
Historical Stock Chart
From Mar 2024 to Mar 2025