LumiraDx Gets FDA Emergency Authorization for Dual Flu-COVID Test
February 06 2023 - 7:59AM
Dow Jones News
By Dean Seal
LumiraDx Ltd. said it received emergency use authorization from
the U.S. Food and Drug Administration for its multiplex test that
can detect and differentiate strains of influenza and Covid-19.
The London-based company said Monday morning that it would
immediately commence commercial shipping of the test, which can
simultaneously identify influenza A, influenza B and Covid-19
infections within 20 minutes or less.
LumiraDx launched the test in the European Union last June and
will now begin its commercialization efforts in the U.S. and
U.K.
"With the limited number of direct amplification multi-analyte
tests available, we believe that this test will become a
fundamental tool for the detection and differentiation of
SARS-CoV-2 from influenza," said Sanjay Malkani, president of
LumiraDx's molecular-diagnostics business.
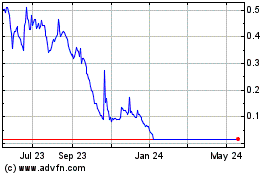
Shares rose 6.7%, to $1.28, in premarket trading Monday.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
February 06, 2023 08:44 ET (13:44 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
LumiraDx (NASDAQ:LMDX)
Historical Stock Chart
From May 2024 to Jun 2024
LumiraDx (NASDAQ:LMDX)
Historical Stock Chart
From Jun 2023 to Jun 2024