UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16
OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of February 2024
Commission File Number: 001-39458
Medicenna Therapeutics Corp.
(Translation of registrant's name into English)
2 Bloor St. W., 7th Floor
Toronto, Ontario M4W 3E2, Canada
(Address of principal executive office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F [ X ] Form 40-F [ ]
Other
Events
The
information in this Report on Form 6-K and in the attached Exhibits 99.1 and 99.2 shall be deemed to be incorporated by
reference into the Company’s registration statements on Forms F-10 (File No. 333-238905), Form S-8 (No. 333-240225) and Form
F-3 (No. 333-269868), and related prospectuses, as such registration statements and prospectuses may be amended from time to time, and
to be a part thereof from the date on which this report is filed, to the extent not superseded by documents or reports subsequently filed
or furnished.
The
information in the attached Exhibits 99.3, 99.4 and 99.5 is being furnished and shall not be deemed “filed” for the
purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject
to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing made by the Company under the Securities Act
of 1933, as amended, or the Exchange Act, except as otherwise set forth herein or as shall be expressly set forth by specific reference
in such a filing.
EXHIBIT
INDEX
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf
by the undersigned, thereunto duly authorized.
| |
MEDICENNA THERAPEUTICS CORP. |
| |
|
|
|
| |
|
|
|
| |
|
|
|
| Date: February 14, 2024 |
By: |
/s/ Fahar Merchant |
|
| |
Name: |
Fahar Merchant |
| |
Title: |
President and Chief Executive Officer |
Exhibit 99.1

Interim condensed consolidated financial statements of
Medicenna Therapeutics Corp.
(Expressed in Canadian Dollars)
For the three and nine months ended December 31, 2023
Medicenna Therapeutics Corp.
Interim Condensed Consolidated Statements of Financial Position
(Expressed in thousands of Canadian Dollars, except for share
and per share amounts)
(Unaudited)
| | |
December 31, | | |
March 31, | |
| | |
2023 | | |
2023 | |
| Assets | |
$ | | |
$ | |
| | |
| | |
| |
| Current assets | |
| | | |
| | |
| Cash and cash equivalents | |
| 21,758 | | |
| 33,596 | |
| Prepaids and deposits | |
| 1,049 | | |
| 1,934 | |
| Other receivables | |
| 403 | | |
| 855 | |
| | |
| 23,210 | | |
| 36,385 | |
| | |
| | | |
| | |
| Intangible assets (Note 8) | |
| 58 | | |
| 61 | |
| | |
| 23,268 | | |
| 36,446 | |
| | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
| 4,026 | | |
| 3,800 | |
| | |
| 4,026 | | |
| 3,800 | |
| | |
| | | |
| | |
| Warrant derivative (Note 6) | |
| 613 | | |
| 3,160 | |
| | |
| 4,639 | | |
| 6,960 | |
| Shareholders' Equity | |
| | | |
| | |
| | |
| | | |
| | |
| Common shares (Note 3) | |
| 100,924 | | |
| 100,924 | |
| Contributed surplus (Notes 4 and 5) | |
| 10,208 | | |
| 9,486 | |
| Accumulated other comprehensive income | |
| 40 | | |
| 57 | |
| Deficit | |
| (92,543 | ) | |
| (80,981 | ) |
| | |
| 18,629 | | |
| 29,486 | |
| | |
| 23,268 | | |
| 36,446 | |
Nature of business and going concern (Note 1)
Subsequent event (Note 11)
The accompanying notes are an integral part of these interim condensed consolidated
financial statements.
Medicenna Therapeutics Corp.
Interim Condensed Consolidated Statements of Loss and Comprehensive
Loss
(Expressed in thousands of Canadian Dollars, except for share and per share amounts)
(Unaudited)
| | |
3 months ended December 31, 2023 | | |
3 months ended December 31, 2022 | | |
9 months ended December 31, 2023 | | |
9 months ended December 31, 2022 | |
| | |
$ | | |
$ | | |
$ | | |
$ | |
| Operating expenses | |
| | | |
| | | |
| | | |
| | |
| General and administration (Note 10) | |
| 1,786 | | |
| 1,976 | | |
| 5,736 | | |
| 6,266 | |
| Research and development (Note 10) | |
| 2,991 | | |
| 2,945 | | |
| 8,937 | | |
| 7,718 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total operating expenses | |
| 4,777 | | |
| 4,921 | | |
| 14,673 | | |
| 13,984 | |
| | |
| | | |
| | | |
| | | |
| | |
| Finance income | |
| (282 | ) | |
| (340 | ) | |
| (970 | ) | |
| (532 | ) |
| Change in fair value of warrant derivative (Note 6) | |
| 160 | | |
| (3,747 | ) | |
| (2,547 | ) | |
| (5,547 | ) |
| Foreign exchange loss (gain) | |
| 322 | | |
| 307 | | |
| 406 | | |
| (1,713 | ) |
| | |
| 200 | | |
| (3,780 | ) | |
| (3,111 | ) | |
| (7,792 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss for the period | |
| (4,977 | ) | |
| (1,141 | ) | |
| (11,562 | ) | |
| (6,192 | ) |
| Cumulative translation adjustment | |
| 11 | | |
| (23 | ) | |
| (17 | ) | |
| (37 | ) |
| Comprehensive loss for the period | |
| (4,966 | ) | |
| (1,164 | ) | |
| (11,579 | ) | |
| (6,229 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted loss per share for the period | |
| (0.07 | ) | |
| (0.02 | ) | |
| (0.17 | ) | |
| (0.10 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding
(Note 3) | |
| 69,637,469 | | |
| 69,637,469 | | |
| 69,637,469 | | |
| 63,132,537 | |
The accompanying notes are an integral part of these interim condensed
consolidated financial statements.
Medicenna Therapeutics Corp.
Interim Condensed Consolidated Statements of Cash Flows
(Expressed in thousands of Canadian Dollars)
(Unaudited)
| | |
Nine months ended December 31, 2023 | | |
Nine months ended December 31, 2022 | |
| | |
$ | | |
$ | |
| Operating activities | |
| | | |
| | |
| Net loss for the period | |
| (11,562 | ) | |
| (6,192 | ) |
| Items not involving cash | |
| | | |
| | |
| Depreciation | |
| 3 | | |
| 3 | |
| Stock based compensation | |
| 722 | | |
| 1,177 | |
| Unrealized foreign exchange | |
| 406 | | |
| (1,072 | ) |
| Accrued interest | |
| - | | |
| (122 | ) |
| Change in fair value of warrant derivative (Note 6) | |
| (2,547 | ) | |
| (5,547 | ) |
| Changes in non-cash working capital | |
| | | |
| | |
| Other receivables and deposits | |
| 1,338 | | |
| 1,019 | |
| Accounts payable and accrued liabilities | |
| 225 | | |
| 368 | |
| | |
| (11,415 | ) | |
| (10,366 | ) |
| Financing activities | |
| | | |
| | |
| Issuance of share capital on ATM, net of issuance costs (Note 3) | |
| - | | |
| 848 | |
| Issuance of share capital and warrants in public offering, net of issuance costs (Note 3 | |
| - | | |
| 23,912 | |
| Warrant amendment (Note 4) | |
| - | | |
| 189 | |
| | |
| - | | |
| 24,949 | |
| | |
| | | |
| | |
| Effect of foreign exchange on cash and cash equivalents | |
| (423 | ) | |
| 1,035 | |
| Net (decrease) increase in cash and cash equivalents | |
| (11,415 | ) | |
| 15,618 | |
| Cash and cash equivalents, beginning of period | |
| 33,596 | | |
| 20,535 | |
| Cash and cash equivalents, end of period | |
| 21,758 | | |
| 36,153 | |
The accompanying notes are an integral part of these interim
condensed consolidated financial statements.
Medicenna Therapeutics Corp.
Interim Condensed Consolidated Statements of Changes in Shareholders’
Equity
(Expressed in thousands of Canadian Dollars, except for share and
per share amounts)
(Unaudited)
| | |
| Common
Shares | | |
| Common
Shares | | |
| Contributed
surplus | | |
| Accumulated
other
comprehensive
income | | |
| Deficit | | |
Total |
| | |
| # | | |
| $ | | |
| $ | | |
| $ | | |
| $ | | |
$ |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, March 31, 2022 | |
| 55,647,479 | | |
| 83,671 | | |
| 7,926 | | |
| 171 | | |
| (70,933 | ) | |
20,835 |
| Stock based compensation | |
| - | | |
| - | | |
| 1,177 | | |
| - | | |
| - | | |
1,177 |
| Issued on ATM financing (Note 3) | |
| 656,656 | | |
| 848 | | |
| - | | |
| - | | |
| - | | |
848 |
| Issued pursuant to public offering, net of warrant derivative (Note 3) | |
| 13,333,334 | | |
| 16,405 | | |
| - | | |
| - | | |
| - | | |
16,405 |
| Warrant amendment (Note 4) | |
| - | | |
| - | | |
| 189 | | |
| - | | |
| - | | |
189 |
| Cumulative translation adjustment | |
| - | | |
| - | | |
| - | | |
| (37 | ) | |
| - | | |
(37) |
| Net loss for the period | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,192 | ) | |
(6,192) |
| Balance, December 31, 2022 | |
| 69,637,469 | | |
| 100,924 | | |
| 9,292 | | |
| 134 | | |
| (77,125 | ) | |
33,225 |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
|
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
|
| Balance, March 31, 2023 | |
| 69,637,469 | | |
| 100,924 | | |
| 9,486 | | |
| 57 | | |
| (80,981 | ) | |
29,486 |
| Stock based compensation | |
| - | | |
| - | | |
| 722 | | |
| - | | |
| - | | |
722 |
| Cumulative translation adjustment | |
| - | | |
| - | | |
| - | | |
| (17 | ) | |
| - | | |
(17) |
| Net loss for the period | |
| - | | |
| - | | |
| - | | |
| - | | |
| (11,562 | ) | |
(11,562) |
| Balance, December 31, 2023 | |
| 69,637,469 | | |
| 100,924 | | |
| 10,208 | | |
| 40 | | |
| (92,543 | ) | |
18,629 |
The accompanying notes are an integral part of these interim
condensed consolidated financial statements.
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
| 1. | Nature of business and going concern |
The Company's principal business activity is the development
and commercialization of IL-2, IL-4 and IL-13 Superkines and Empowered Superkines for the treatment of cancer, inflammation and immune-mediated
diseases. Medicenna has four wholly owned subsidiaries, Medicenna Therapeutics Inc. (“MTI”) (British Columbia), Medicenna
Biopharma Inc. (“MBI”) (Delaware), Medicenna Biopharma Inc. (“MBIBC”) (British Columbia) and Medicenna Australia
PTY Ltd (“MAL”) (Australia). Medicenna is traded on the Toronto Stock Exchange under the symbol ‘’MDNA”.
As at December 31, 2023, the head and registered office is
located at 2 Bloor St W, 7th Floor, Toronto, Ontario, Canada.
Since inception, the Company has devoted its resources to
funding R&D programs, including securing intellectual property rights and licenses, conducting discovery research, manufacturing drug
supplies, initiating preclinical and clinical studies, submitting regulatory dossiers and providing administrative support to R&D
activities, which has resulted in an accumulated deficit of $92.5 million as of December 31, 2023. With current finance income only consisting
of interest earned on excess cash, cash equivalents and marketable securities, losses are expected to continue while the Company’s
R&D programs are advanced.
The Company currently does not
earn any revenues from our product candidates and are therefore considered to be in the development stage. As required, the Company will
continue to finance its operations through the sale of equity or pursue non-dilutive funding sources available to the Company in the future.
The continuation of our research and development activities for bizaxofusp (formerly MDNA55), MDNA11 and the BiSKITsä
platform and the commercialization of bizaxofusp is dependent upon our ability to successfully finance and complete our research and development
programs through a combination of equity financing and revenues from strategic partners. There is no guarantee of future financing and
that our research and development activities associated with bizaxofusp, MDNA11 and the BiSKITs platform will be successful, which may
require a change in plans of the Company. The Company has no current sources of revenue from strategic partners.
The ability of the Corporation to continue as a going concern
is dependent upon raising additional financing through equity and non-dilutive funding and partnerships. There can be no assurance that
the Corporation will have sufficient capital to fund its ongoing operations and develop or commercialize any products without future financings.
These material uncertainties cast substantial doubt as to the Corporation’s ability to meet its obligations as they come due and,
accordingly, the appropriateness of the use of accounting principles applicable to a going concern. There can be no assurance that additional
financing will be available on acceptable terms or at all. If the Corporation is unable to obtain additional financing when required,
the Corporation may have to substantially reduce or eliminate planned expenditures or the Corporation may be unable to continue operations.
The Corporation's ability to continue as a going concern is dependent upon its ability to fund its research and development programs and
defend its patent rights. These interim condensed consolidated financial statements do not reflect the adjustments to the carrying values
of assets and liabilities and the reported expenses and statements of financial position classifications that would be necessary if the
Corporation were unable to realize its assets and settle its liabilities as a going concern in the normal course of operations. Such adjustments
could be material.
| 2. | Basis of presentation and significant accounting policies |
| a) | Statement of compliance |
These interim condensed consolidated financial statements
have been prepared in accordance with International Accounting Standards (“IAS”) 34 ‘Interim Financial Reporting’
(IAS 34) using accounting policies consistent with International Financial Reporting Standards (“IFRS”) as issued by the International
Accounting Standards Board (“IASB”) and the Interpretations of the International Financial Reporting and Interpretations Committee
(“IFRIC”).
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
| 2. | Basis of presentation and significant accounting policies cont’d |
The interim condensed consolidated financial statements
do not include all the information and disclosures required in the annual financial statements and should be read in conjunction with
the Company’s audited financial statements for the year ended March 31, 2023. The interim condensed consolidated financial statements
were approved by the Company’s Board of Directors and authorized for issue on February 14, 2024.
| b) | Functional and presentation currency |
The functional currency of an entity and its subsidiary
is the currency of the primary economic environment in which the entity operates. The functional currency of the parent company is the
Canadian dollar and the functional currency of MBI is the US dollar, the functional currency of MTI and MBI BC is the Canadian dollar,
the functional currency of MAL is the Australian dollar, and the presentation currency of the parent company is the Canadian dollar.
| c) | Significant accounting judgments, estimates and assumptions |
The preparation of these unaudited interim condensed consolidated
financial statements in accordance with IFRS requires management to make judgments, estimates and assumptions that affect the application
of accounting policies and reported amounts of assets and liabilities at the date of the unaudited condensed consolidated interim financial
statements and reported amounts of revenues and expenses during the reporting period. Actual outcomes could differ from these estimates.
The unaudited interim condensed consolidated financial
statements include estimates, which, by their nature, are uncertain. The impacts of such estimates are pervasive throughout the unaudited
interim condensed consolidated financial statements and may require accounting adjustments based on future occurrences. The estimates
and underlying assumptions are reviewed on a regular basis. Revisions to accounting estimates are recognized in the period in which the
estimate is revised and in any future periods affected.
The accompanying unaudited interim condensed consolidated
financial statements are prepared in accordance with IFRS and follow the same accounting policies and methods of application as the audited
consolidated financial statements of the Company for the year ended March 31, 2023. They do not include all of the information and disclosures
required by IFRS for annual financial statements. In the opinion of management, all adjustments considered necessary for fair presentation
have been included in these unaudited condensed consolidated interim financial statements. Operating results for the nine
months ended December 31, 2023 are not necessarily indicative of the results that
may be expected for the full year ended March 31, 2024. For further information, see the Company’s audited consolidated financial
statements including notes thereto for the year ended March 31, 2023.
Authorized
Unlimited common shares
Equity Issuances
August 2022 Public Offering
On August 10, 2022, pursuant to an underwritten public
offering, 13,333,334 units were sold at a purchase price of US$1.50 per unit for gross proceeds of US$20.0 million ($25.6 million). Each
unit included one common share with a fair value of US$1.06 and one common share purchase warrant with a fair value of US$0.44 (see Note
6). Each common share purchase warrant entitles the holder to purchase one common share at an exercise price of US$1.85 until August 9,
2027.
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
The Company incurred transaction costs of $2.2 million
(US$1.7 million) of which $1.6 million (US$1.2 million) were allocated to share issue costs and $0.6 million (US$0.5 million) were allocated
to operating expenses, based on their relative fair values.
At-The-Market Facility
On February 17, 2023, the Company entered into a sales agreement with Oppenheimer & Co. Inc., acting
as sales agent (the “2023 ATM Agreement”), pursuant to which the Company may, from time to time sell, through at-the-market
offerings on the Nasdaq such number of Common Shares as would have an aggregate offering price of up to US$10.0 million (the “2023
ATM Facility”). During the nine months ended December 31, 2023, the Company did not issue any Common Shares pursuant to the
2023 ATM Facility. Further to the Nasdaq delisting in November 2023, the 2023 ATM Agreement was terminated.
On December 30, 2020, the Company entered into a sales
agreement with SVB Leerink acting as sales agent, pursuant to which the Company may, from time to time sell, through at-the-market (“ATM”)
on the NASDAQ such number of common shares as would have an aggregate offering price of up to US$25.0 million (the ATM Offering), which
expired December 30, 2022. During the nine months ended December 31, 2022, the Company issued 656,656 common shares for gross proceeds
of US$0.8 million at an average price of US$1.20. The company received; net of commissions US$0.7 million. In total, the Company incurred
share issuance costs (including commissions) of US$0.1 million.
Calculation of loss per share
Loss per common share is calculated using the weighted average number of common
shares outstanding. For the periods ended December 31, 2023 and 2022, the calculation was as follows:
| | |
Three months ended
December 31, | | |
Nine months ended
December 31, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Common shares issued and outstanding, beginning of year | |
| 69,637,469 | | |
| 69,637,469 | | |
| 69,637,469 | | |
| 55,647,479 | |
| ATM issuances | |
| - | | |
| - | | |
| - | | |
| 600,210 | |
| Shares issued on 2022 Public Offering | |
| - | | |
| - | | |
| - | | |
| 6,884,848 | |
| Weighted average shares outstanding, end of period | |
| 69,637,469 | | |
| 69,637,469 | | |
| 69,637,469 | | |
| 63,132,537 | |
The effect of any potential exercise
of the Company’s stock options and warrants outstanding during the period has been excluded from the calculation of diluted loss
per common share as it would be anti- dilutive.
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
Warrant continuity:
| | |
Warrants
# | | |
Weighted average exercise price
$ | |
| Balance – March 31, 2022 | |
| 2,964,542 | | |
| 1.51 | |
| Warrants issued in the 2022 Public Offering | |
| 13,333,334 | | |
| 2.39 | |
| Warrants expired during the period | |
| (112,490 | ) | |
| 1.75 | |
| Balance – March 31, 2023 | |
| 16,185,386 | | |
| 2.23 | |
| Warrants expired during the period | |
| (200,000 | ) | |
| 1.20 | |
| Balance – December 31, 2023 | |
| 15,985,386 | | |
| 2.25 | |
On December 21, 2023, 200,000 warrants held by insiders
of the Company expired.
At December 31, 2023, warrants were outstanding and exercisable,
enabling holders to acquire common shares as follows:
| Expiry Date | |
Exercise Price | | |
Warrants | |
| | |
$ | | |
# | |
| July 31, 2024 | |
| 1.20 | | |
| 1,103,000 | |
| October 17, 2024 | |
| 1.75 | | |
| 1,549,052 | |
| August 9, 2027 | |
| US 1.85 | | |
| 13,333,334 | |
| | |
| | | |
| 15,985,386 | |
On July 5, 2023, the warrants issued on October 17, 2019,
in correlation with a public offering, were due to expire on July 17, 2023 and were extended to October 17, 2024.
On December 18, 2023, the warrants
issued on December 21, 2018 were due to expire on December 21, 2023 and were extended to July 31, 2024.
During the nine months
ended December 31, 2023, the Company granted 3,384,000 stock options at an average exercise price of $0.39 per share. Of these granted,
2,655,000 options were granted to the Company’s officers and employees and vest 50% after one year, 25% after two years and
25% after three years, and have a ten-year life. Another 250,000 options were granted to advisors of the Company and vest 50%
upon issuance and 50% over 1 year and have a five-year life. Finally, 479,000 options were granted to the Board and advisors of the
Company and vest 50% upon issuance and 50% after 1 year and have a five-year life.
During the
nine months ended December 31, 2022, the Company granted 1,290,713 stock options at an average exercise price of $1.44 per share. 997,608
of the options were granted to the Company’s officers and employees and vest 1/3 after one year, 1/3 after two years and 1/3 after
three years, and have a ten-year life; and 293,105 options were granted to Directors of the Company at a price of $1.45 and vest 50% upon
issuance and 50% after 1 year and have a five-year life.
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
Stock option transactions for the periods
ended December 31, 2023 and 2022 are set forth below:
| | |
Number of options | | |
Weighted average exercise price | |
| | |
| | |
| |
| Balance outstanding at March 31, 2022 | |
| 4,464,640 | | |
| 2.00 | |
| Granted | |
| 1,290,713 | | |
| 1.44 | |
| Expired | |
| (100,000 | ) | |
| 2.88 | |
| Forfeited | |
| (45,000 | ) | |
| 3.86 | |
| Balance outstanding at March 31, 2023 | |
| 5,610,353 | | |
$ | 1.84 | |
| Granted | |
| 3,384,000 | | |
| 0.39 | |
| Forfeited | |
| (1,937,596 | ) | |
| (1.06 | ) |
| Balance outstanding at December 31, 2023 | |
| 7,056,757 | | |
$ | 1.34 | |
The
following assumptions were used in the Black-Scholes option-pricing model to determine the fair value of stock options granted during
the period:
| | |
December 31,
2023 | | |
March 31,
2023 | |
| Exercise price | |
| $0.38 - $0.67 | | |
| $1.36-1.45 | |
| Grant date share price | |
| $0.38 - $0.67 | | |
| $1.36-1.45 | |
| Risk free interest rate | |
| 5 | % | |
| 5.10 | % |
| Expected life of options | |
| 5-10 years | | |
| 5 years | |
| Expected volatility | |
| 86%-90% | | |
| 90 | % |
| Expected dividend yield | |
| - | | |
| - | |
| Forfeiture rate | |
| 0% - 15% | | |
| 0% - 15% | |
| Weighted average fair value of options granted during the period | |
$ | 0.28 | | |
$ | 1.04 | |
The following table summarizes information
about stock options outstanding at December 31, 2023:
| | |
Options outstanding | | |
Options exercisable | |
| Exercise price range | |
Number | | |
Weighted average exercise price | | |
Weighted average remaining contractual life (years) | | |
Number | | |
Weighted average exercise price | |
| $ | |
# | | |
$ | | |
| | |
# | | |
$ | |
| | |
| | |
| | |
| | |
| | |
| |
| 0.38-1.99 | |
| 4,995,851 | | |
| 0.92 | | |
| 6.30 | | |
| 2,576,720 | | |
| 1.11 | |
| 2.00-2.99 | |
| 1,379,000 | | |
| 2.03 | | |
| 3.27 | | |
| 1,379,000 | | |
| 2.03 | |
| 3.00-5.19 | |
| 681,906 | | |
| 3.76 | | |
| 5.93 | | |
| 535,263 | | |
| 3.79 | |
| | |
| 7,056,757 | | |
| 1.34 | | |
| 5.92 | | |
| 4,490,983 | | |
| 1.71 | |
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
On August 10, 2022, pursuant to an underwritten public
offering, 13,333,334 units were sold at a purchase price of US$1.50 per unit for gross proceeds of US$20.0 million ($25.6 million). Each
unit included one common share and one common share purchase warrant. Each common share purchase warrant entitles the holder to purchase
one common share at an exercise price of US$1.85 until August 9, 2027. The Company incurred transaction costs of $2.2 million (US$1.7
million) of which $1.6 million (US$1.2 million) were allocated to share issue costs and $0.6 million (US$0.5 million) were allocated to
operating expenses, based on their relative fair values.
Under IFRS 9 Financial Instruments and IAS 32 Financial
Instruments: Presentation, warrants with an exercise price denominated in a currency that differs from an entity's functional currency
are treated as a derivative measured at fair value with subsequent changes in fair value accounted for through the consolidated statement
of loss. The Company’s warrants have an exercise price of US$1.85 and therefore meet this requirement. Consequently, the value of
these warrants is presented as a non-current liability on the interim consolidated statement of financial position. Upon exercise, the
recorded liability is to be reallocated to share capital with the proceeds from the exercise. In the case that these warrants expire,
the related liability is reversed through the interim condensed consolidated statement of loss and comprehensive loss.
Estimating the fair value for the warrant derivative
requires determining an appropriate valuation model which is dependent on the terms and conditions of the issuance. This estimate also
requires determining the most appropriate inputs to the valuation model, including the expected life of the warrant derivative, expected
share price volatility and expected dividend.
The Company uses historical data to estimate the expected
dividend yield and expected volatility of our stock in determining the fair value of the warrants. The risk-free interest rate is based
on U.S. Department of Treasury benchmark treasury yield rates in effect at the time of valuation and the expected life of the warrants
represents the estimated length of time the warrants are expected to remain outstanding.
The
following table summarizes the key assumptions used in the Black-Scholes valuation of the warrant derivative at December, 2023:
| | |
December 31,
2023 | | |
March 31,
2023 | |
| | |
| | |
| |
| Fair value of warrants | |
$ | 0.046 | | |
$ | 0.24 | |
| Underlying share price | |
$ | 0.42 | | |
$ | 0.88 | |
| Risk free interest rate | |
| 5.00 | % | |
| 5.10 | % |
| Expected hold period to exercise | |
| 1.75 years | | |
| 2.5 years | |
| Expected share price volatility | |
| 96 | % | |
| 85 | % |
| Expected dividend yield | |
| Nil | | |
| Nil | |
A reconciliation of the change in fair value of the warrant
derivative is as follows:
| | |
$ | |
| Balance September 30, 2022 | |
| 5,707 | |
| Change in fair value of warrant derivative | |
| (2,588 | ) |
| Foreign exchange loss | |
| 41 | |
| Balance March 31, 2023 | |
| 3,160 | |
| Change in fair value of warrant derivative | |
| (2,254 | ) |
| Foreign exchange loss | |
| (293 | ) |
| Balance December 31, 2023 | |
| 613 | |
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
CPRIT assistance
In February 2015, the Company was awarded a grant by
the Cancer Prevention Research Institute of Texas (“CPRIT”). Under the terms of the grant, the Company is required to pay
a royalty to CPRIT, comprised of 3-5% of revenues on net sales of MDNA55 until aggregate royalty payments equal 400% of the grant funds
received at which time the ongoing royalty will be 0.5% of revenues. At this time the royalty is not probable and therefore no liability
has been recorded. In addition, the Company must maintain a presence in Texas for three years following completion of the grant.
Intellectual property
The Company has entered into various license agreements
with respect to accessing patented technology. In order to maintain these agreements, the Company is obligated to pay certain costs
based on timing or certain milestones within the agreements, the timing of which is uncertain. These costs include ongoing license
fees, patent prosecution and maintenance costs, royalty, and other milestone payments.
As at December 31, 2023, the Company estimates that
project milestones of approximately US$1.1 million will be due in the next five years.
| 9. | Related party disclosures and key compensation |
| (a) | Key management personnel |
Key management personnel, which consists of the Company’s
officers (President and Chief Executive Officer, Chief Development Officer, former Chief Financial Officers, and former Chief Business
Officer) and directors, earned the following compensation for the following periods:
| | |
Three months ended
December 31, | | |
Nine months ended
December 31, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| Salaries and wages | |
| 505 | | |
| 331 | | |
| 1,482 | | |
| 836 | |
| Board fees | |
| 76 | | |
| 95 | | |
| 253 | | |
| 247 | |
| Stock option expense | |
| 119 | | |
| 261 | | |
| 546 | | |
| 972 | |
| | |
| 700 | | |
| 687 | | |
| 2,281 | | |
| 2,055 | |
| (b) | Amounts payable to related parties and key management personnel |
As at December 31, 2023, the Company had trade and other
payables in the normal course of business, owing to directors and officers of $0.2 million (2022 - $0.1 million) related to board fees
and accrued vacation.
Medicenna Therapeutics Corp.
Notes to the interim condensed consolidated financial statements (unaudited)
For the Three and Nine months ended December 31, 2023 and 2022
(Tabular amounts expressed in thousands of Canadian Dollars, except for share and per share amounts)
| 10. | Components of Expenses |
| | |
Three months ended
December 31, | | |
Nine months ended
December 31, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
$ | | |
$ | | |
$ | | |
$ | |
| General and Administration Expenses | |
| | | |
| | | |
| | | |
| | |
| Depreciation expense (non-cash) | |
| 2 | | |
| 2 | | |
| 4 | | |
| 4 | |
| Stock based compensation (non-cash) | |
| 62 | | |
| 183 | | |
| 323 | | |
| 724 | |
| Facilities and operations | |
| 227 | | |
| 149 | | |
| 603 | | |
| 422 | |
| Public company expenses | |
| 1,030 | | |
| 1,213 | | |
| 3,454 | | |
| 3,587 | |
| Transaction costs, warrant derivative (Note 6) | |
| - | | |
| - | | |
| - | | |
| 652 | |
| Salaries and benefits | |
| 465 | | |
| 429 | | |
| 1,356 | | |
| 877 | |
| | |
| 1,786 | | |
| 1,976 | | |
| 5,736 | | |
| 6,266 | |
| | |
Three months ended
December 31, | | |
Nine months ended
December 31, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
$ | | |
$ | | |
$ | | |
$ | |
| Research and Development Expenses | |
| | | |
| | | |
| | | |
| | |
| Chemistry, manufacturing, and controls | |
| 257 | | |
| 146 | | |
| 1,028 | | |
| 686 | |
| Regulatory | |
| 27 | | |
| 15 | | |
| 77 | | |
| 52 | |
| Discovery and pre-clinical | |
| 216 | | |
| 295 | | |
| 1,102 | | |
| 1,166 | |
| Clinical | |
| 1,454 | | |
| 1,221 | | |
| 3,545 | | |
| 2,752 | |
| Salaries and benefits | |
| 606 | | |
| 780 | | |
| 1,760 | | |
| 1,839 | |
| Licensing, patent, legal fees and royalties | |
| 208 | | |
| 303 | | |
| 1,065 | | |
| 739 | |
| Stock based compensation (non-cash) | |
| 97 | | |
| 156 | | |
| 399 | | |
| 452 | |
| Research and development tax credits | |
| - | | |
| - | | |
| (200 | ) | |
| - | |
| Other research and development expenses | |
| 126 | | |
| 29 | | |
| 161 | | |
| 32 | |
| | |
| 2,991 | | |
| 2,945 | | |
| 8,937 | | |
| 7,718 | |
Effective February 5, 2024, the Company entered into an
agreement with a vendor to provide investor relations services to the Company. Pursuant to the agreement, in addition to monthly cash
compensation of $1,000 per month, the Company will issue 1,000,000 common share purchase options with a strike price of $0.75 per share
and term of 5 years. The options will vest in tranches over a period of sixteen months.
12
Exhibit 99.2

Management’s Discussion and Analysis
For the Three and Nine Months Ended
December 31, 2023
DATE OF REPORT: February 14, 2024
MANAGEMENT’S DISCUSSION AND ANALYSIS
The following management’s discussion and analysis (“MD&A”)
has been prepared as at February 14, 2024 for the three and nine months ended December 31, 2023 and should be read in conjunction with
the unaudited interim condensed consolidated financial statements of Medicenna Therapeutics Corp. for the three and nine months ended
December 31, 2023, and the audited annual consolidated financial statements and accompanying notes for the year ended March 31, 2023 (the
“Annual Financial Statements”), which have been prepared in accordance with International Financial Reporting Standards (“IFRS”)
as issued by the International Accounting Standards Board (“IASB”). Our IFRS accounting policies are set out in note 2 of
the Annual Financial Statements and all dollar amounts are expressed in Canadian dollars unless otherwise noted.
All references in this MD&A to “the Company”, “Medicenna”,
“we”, “us”, or “our” and similar expressions refer to Medicenna Therapeutics Corp. and the subsidiaries
through which it conducts its business, unless otherwise indicated.
FORWARD-LOOKING STATEMENTS
This MD&A contains forward-looking statements within the meaning
of applicable securities laws. Forward-looking statements are neither historical facts nor assurances of future performance. Instead,
they are based on current beliefs, expectations, or assumptions regarding the future of the business, future plans and strategies, operational
results and other future conditions of the Company. These statements involve known and unknown risks, uncertainties and other factors
which may cause the actual results, performance or achievements of the Company, or industry results, to be materially different from any
future results, performance or achievements expressed or implied by such forward-looking statements. All statements contained herein other
than statements of historical fact regarding the prospects of the Company’s industry or its prospects, plans, financial position
or business strategy may constitute forward-looking statements and can generally be identified by the use of forward-looking words, such
as “seek”, “plan”, “expect”, “is expected”, “continue”, “predict”,
“potential”, “budget”, “scheduled”, “estimate”, “forecast”, “contemplate”,
“intend”, “anticipate”, or “believe” or variations (including negative variations) of such words and
phrases, or statements that certain actions, events or results “may”, “could”, “would”, “should”,
“might”, “shall” or “will” be taken, occur or be achieved and similar expressions are generally intended
to identify forward-looking statements.
By their very nature, forward-looking statements involve inherent risks and
uncertainties, both general and specific, and risks exist that predictions, forecasts, projections and other forward-looking statements
will not be achieved. The Company cautions readers not to place undue reliance on these statements as a number of important factors could
cause the actual results to differ materially from the beliefs, plans, objectives, expectations, anticipations, estimates and intentions
expressed in such forward-looking statements. There can be no assurance that such statements will prove to be accurate and actual results
and future events could differ materially from those anticipated in such statements. Risks, uncertainties and other factors which may
cause the actual results, performance or achievements of the Company, as applicable, to be materially different from any future results,
performance or achievements expressed or implied by such forward-looking information and statements include, but are not limited to, the
risks described under the heading “Risks and Uncertainties” in this MD&A, the Company’s annual report on Form 20-F
for the fiscal year ended March 31, 2023 (the “Annual Report on Form 20-F”) filed with the U.S. Securities and Exchange Commission
on June 27, 2023.
Forward-looking statements in this MD&A include, but are not limited
to:
- the therapeutic potential, clinical development and related milestones of
the Company’s Superkines and Empowered Superkines including MDNA11, the BiSKITsTM platform, the T-MASKTM platform
and bizaxofusp (formerly MDNA55);
- the timely completion of the milestones related to the MDNA11 ABILITY Study
(as defined below);
- the impact of the delay on clinical data;
- the clinical trial collaboration and supply agreement (“CTCSA”)
with Merck (known as MSD outside the United States and Canada);
- statements related to the potential extensions of the term of patents;
- a potential strategic partnership to facilitate bizaxofusp’s further
development and commercialization; and
- the use of proceeds from public equity offerings and the necessity for the
Company to have recourse to such public equity offerings.
Although the Company has attempted to identify important factors that
could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other
factors that cause actions, events or results to differ from those anticipated, estimated or intended, including the following:
- the lack of product revenue and inability to continue operations and research
and development without sufficient funding;
- the Company’s requirements for, and our ability to obtain, future funding on favorable terms or
at all;
- the Company’s history of losses and expectations of future losses;
- the Company’s inability to complete development of or the inability
to commercialize (if approved)
- the Company’s product candidates, which are in the early stages of development;
- the expense, length and uncertainty of clinical drug development programs;
- the inability to achieve publicly announced milestones according to schedule,
or at all;
- the risk that competitors may develop and market products that are more effective
that the Company’s product candidates or that the products developed by competitors may render the Company’s product candidates
obsolete or uncompetitive;
- the Company’s inability to secure a partnership for bizaxofusp (formerly
MDNA55);
- the costs and uncertainty associated with extensive government regulation;
- the potential negative results from clinical trials or studies, adverse safety
events or toxicities involving the Company’s products used alone or in combination with other products of collaborators;
- the Company’s ability to manage the unique risks and uncertainties related
to developing biologics which could have a negative impact on future results of operations;
- the risk that preliminary and interim data from our clinical trials that we
may announce or publish from time to time may change as patient data are further examined, audited or verified and more patient data become
available;
- the value of the “Fast Track” designation granted to bizaxofusp
and that it may not actually lead to a faster development or regulatory review or approval process and could be withdrawn by the United
States Food and Drug Administration (“FDA”);
- the unfavorable pharmacokinetic (“PK”) or pharmacodynamic (“PD”)
properties of MDNA11 and MDNA19 used alone or in combination with other products of collaborators;
- the potential of the pre-clinical products of the Company;
- the risk of product liability claims;
- the Company’s inability to enroll subjects in clinical trials or complete
clinical trials on a timely basis;
- the failure of our product candidates to receive the marketing approval or
market acceptance necessary for commercial success or to maintain any ongoing regulatory requirements it may be subject to;
- the potential for environmental exposure to hazardous or radioactive materials
that are used in the Company’s discovery and development process;
- the disruption in the availability of key components for ongoing clinical
studies that could delay clinical studies, product testing and regulatory approval of the Company’s product candidates;
- the Company’s reliance on third parties for the planning, conduct and
monitoring of preclinical and clinical trials and for the manufacture of drug product;
- the Company’s reliance on contract manufacturers over whom the Company
has limited control;
- the loss of license rights due to breach of license agreements;
- the conditions and restrictions of the Cancer Prevention and Research Institute
of Texas (“CPRIT”) grant;
- the ability to protect the Company’s intellectual property and proprietary
technology;
- the ability for the Company to obtain patent’s term extensions;
- the potential involvement in intellectual property litigation;
- the risk that third parties on whom we rely for product development may not
adequately protect the Company’s trade secrets;
- the risk of product liability claims;
- the limitations surrounding intellectual property rights;
- the volatility in the price of our common shares (“Common Shares”);
- the dilution of investor’s voting power and reductions in earnings per
share owing to future issuances of equity or the conversion of securities into Common Shares;
- the fact that future profits will likely be used for the continued growth
of the Company’s business and not for the payment of dividends;
- the Company’s treatment as a passive foreign investment company and
potential adverse U.S. federal income tax consequences associated with such treatment;
- the difficulty U.S. investors may face in bringing actions against the Company
for violations of U.S. federal or state securities laws and challenges in enforcing the judgments of U.S. courts against the Company and
its directors and executive officers;
- the Company’s status as a foreign private issuer under applicable U.S.
securities laws;
- the potential for the Company to lose its status as a foreign private issuer;
- changes in government regulations that could impact our business and operations;
- failure to comply with the U.S. Foreign Corrupt Practices Act, the Canadian
Corruption of Foreign Public Officials Act and other global corruption and anti-bribery laws;
- failure to comply with healthcare laws;
- the ability of the Company’s significant shareholders to assert a material
influence over the Company’s operations and governance;
- the adverse impact of factors outside our control, such as global health pandemics,
natural disasters, geopolitical conflict and macroeconomic challenges;
- the Company’s ability to successfully manage its growth;
- the failure of any acquired business, product, service or alliance to yield
expected benefits;
- the Company’s dependence upon certain key personnel, the loss of whom
could adversely affect our ability to achieve our business objectives;
- changes in government regulations that could impact our business and operations;
- foreign currency exchange risks relating to the relative value of the United
States dollar;
- the failure of our disclosure controls and procedures to detect all errors
or prevent all incidences of fraud;
- the failure to maintain an effective system of internal controls;
- the vulnerability of the computer and information systems of the Company,
its consultants and contractors, and third parties on which the Company relies, to security breaches or failure; and
- the pursuit of opportunities for further research and development or additional
business opportunities.
The forward-looking information in this MD&A does not include a full assessment
or reflection of the negative effect of adverse economic conditions, including a potential recession, and related inflationary cost pressures,
higher interest rates, financial and capital market volatility and labor challenges; the negative effect of adverse conditions associated
with the continued evolution of the COVID-19 pandemic and geopolitical events; a declining level of business and consumer spending; regulatory
initiatives, proceedings and decisions, government consultations and government positions that affect us and influence our business; and
the efforts of the Company to mitigate such conditions or events.
All forward-looking statements reflect the Company’s beliefs and
assumptions based on information available at the time the assumption was made.
Although the forward-looking statements contained in this MD&A are
based upon what the Company’s management believes to be reasonable assumptions, the Company cannot assure readers that actual results
will be consistent with these forward-looking statements.
Any forward-looking statements represent the Company’s estimates
only as of the date of this MD&A and should not be relied upon as representing the Company’s estimates as of any subsequent
date. The Company undertakes no obligation to update any forward-looking statement or statements to reflect events or circumstances after
the date on which such statement is made or to reflect the occurrence of unanticipated events, except as may be required by securities
laws.
COMPANY OVERVIEW
Medicenna Therapeutics is a clinical-stage immunotherapy company developing
engineered cytokines, called Superkines, designed to improve the specificity, function and safety profile of unmodified interleukins (“IL”).
Medicenna’s Superkine Platform transforms Superkines into multi-functional therapies that modulate, dampen, amplify or fine-tune
the immune system. Medicenna’s mission is to harness the power of directed evolution to develop novel immunotherapies that have
the potential to revolutionize the treatment landscape in oncology and other immune-related diseases.
Medicenna owns diverse platforms licensed from Stanford
University (“Stanford”) to develop a pipeline of Superkine candidates: IL-2 agonists, IL-2 antagonists and partial agonists
of IL-2. Additional assets from Stanford also include several super-agonists of IL-4 and IL-13 and dual IL-4/IL-13 antagonists. These
Superkines can be developed either on their own as short or long-acting therapeutics or fused with cell-killing proteins to generate Empowered
Superkines that precisely deliver potent payloads to cancer cells without harming adjacent healthy cells. Superkines can also be fused
with a large variety of proteins, antibodies, checkpoint inhibitors, and even other Superkines to incorporate two synergistic therapeutic
activities into one molecule, creating novel Bi-specific SuperKine ImmunoTherapies and Targeted
Metalloprotease Activated SuperKines, referred to by Medicenna as BiSKITsTM and T-MASKTM,
respectively.
Medicenna’s most advanced candidate is MDNA55, or bizaxofusp,
for the treatment of recurrent glioblastoma (“rGBM”), the most common and uniformly fatal form of brain cancer. Bizaxofusp
is a fusion of a circularly permuted version of IL-4, fused to a potent fragment of the bacterial toxin, Pseudomonas exotoxin (“PE”),
and is designed to preferentially target tumor cells that over-express the interleukin 4 receptor (“IL-4R”). Bizaxofusp has
successfully completed a Phase 2b trial for rGBM and holds FastTrack and Orphan Drug status from the U.S. Food and Drug Administration
(“FDA”) and FDA/European Medicines Agency (“EMA”), respectively. In addition, FDA has recommended Medicenna to
proceed with a novel, practical and cost-effective Phase 3 clinical trial for approval of bizaxofusp, where a majority of patients enrolled
in the control arm will comprise of clinical data compiled from cancer registries of matched rGBM patients previously treated with other
approved therapies (External Control Arm). Medicenna plans to further develop bizaxofusp with a potential partner.
Our second clinical program is MDNA11, a next-generation long-acting
“beta-enhanced, not-alpha” IL-2 super-agonist. MDNA11 comprises a molecule of human albumin that accumulates in tumors and
augments MDNA11’s half-life. MDNA11 is currently being evaluated in the ABILITY-1 (“A Beta-only IL-2 ImmunoTherapY”)
Phase 1/2 study in patients with melanoma and other solid cancers. The ABILITY-1 study is a global, multi-center, open-label clinical
trial that will assess the safety, tolerability and anti-tumor activity of MDNA11 as a monotherapy or in combination with pembrolizumab
(Keytruda®) under a clinical collaboration with Merck. MDNA11 has successfully completed Phase 1 dose-escalation portion of the study
with a favourable safety profile and demonstrated early signs of efficacy in the monotherapy setting. The monotherapy recommended dose
for expansion (“RDE”) for MDNA11 has been established and enrollment in the phase 2 dose-expansion portion of the ABILITY-1
trial is currently underway. In addition, Medicenna has also commenced the dose-escalation portion of the trial in combination with Merck’s
pembrolizumab, the world’s biggest selling therapeutic.
Our earlier stage candidates from the BiSKITsTM and T-MASKTM
platforms are in pre-clinical development and are expected to enter first in human clinical trials in 2025.
RECENT ACHIEVEMENTS & HIGHLIGHTS
The following are the achievements and highlights for
the three months ended December 31, 2023 through to the date hereof:
| · | On February 14, 2024, the Company reported promising clinical data from the
on-going monotherapy escalation and expansion arms of the ABILITY-1 study. In addition to previously announced tumor response data, a
third patient in the study has also shown a partial response. Amongst 13 patients, all having previously failed or resistant to immune
checkpoint inhibitors (“ICI”), receiving high doses of MDNA11 (³ 60 mg/kg)
with tumor types being evaluated in the monotherapy expansion cohort, the response rate, clinical benefit rate, and tumor control rate
increased to 23% (3 partial responses), 46% (3 PRs and 3 patients with stable disease for ³
24 weeks), and 69% (3 PRs and 6 SDs), respectively, with concomitant shrinkage of target lesions in all patients with stable disease. |
| · | On February 14, 2024, the Company announced that the Board of Directors approved the transition of Dr.
Humphrey Gardner from CMO to consultant. Dr. Arash Yavari, Chair of Medicenna’s Development Advisory Committee, will lead the clinical
activities as Director of Clinical Strategy. |
| · | On February 14, 2024, the Company announced that the Board of Directors approved the appointment of David
Hyman, CA, CBV as CFO of the Company. Mr. Hyman is an experienced financial professional with over 25 years of experience spanning public
practice, capital markets, private equity and industry. For the past five years, Mr. Hyman has provided fractional and full time CFO services
to multiple public and private companies, including two early-stage pharmaceutical companies. |
| · | On February 13, 2024, the Company announced that it had dosed the first patient in the combination arm
of the ABILITY-1 clinical trial, evaluating potential synergistic effect of MDNA11 when administered with KEYTRUDAÒ
(pembrolizumab). The study will evaluate the safety, tolerability, recommended combination dose for expansion (“cRDE”) and
therapeutic activity of MDNA11 when combined with pembrolizumab in the dose-escalation and dose-expansion arms of the clinical trial. |
| · | On January 12, 2024, the Company announced that its Board of Directors approved the appointment of MNP
LP as the auditor of the Company. |
| · | On January 9, 2024, the Company announced the initiation of enrollment in the combination arm of the Phase
1/2 ABILITY study evaluating MDNA11 with KEYTRUDA®. The combination portion of the study is designed to evaluate the potential
for a synergistic effect of MDNA11 with KEYTRUDA® in patients with advanced solid tumors. |
| · | On December 19, 2023, the Company announced the commencement of trading on the OTCQB Venture Market in the
United States. |
| · | On November 17, 2023, the Company announced that a poster presentation and an oral summary highlighting
longer term follow up results from the Phase 2b clinical trial of bizaxofusp, the Company’s first-in-class IL-4R targeted therapy
for the treatment of patients with rGBM, was presented by Dr. Steven Brem, M.D. (Medical Director, Centre for Precision Surgery, Abramson
Cancer Center, Perelman School of Medicine, University of Pennsylvania) at the 2023 Annual Meeting of the Society for Neuro-Oncology (“SNO”)
held in Vancouver, Canada. |
| · | On November 6, 2023, Medicenna announced encouraging single-agent activity from the dose escalation and
evaluation portion of the ABILITY-1 study in advanced cancer patients receiving doses ³
60 µg/kg of MDNA11 (N = 15) who had previously failed immune check-point inhibitor therapies. The results included ongoing partial
responses with 100% and 70% reduction of target lesions in pancreatic and melanoma cancer patients, respectively, in addition to durable
stable disease in 3 melanoma patients (> 20 to 80 weeks). This data was presented at the 38th Annual Meeting of the Society
for Immunotherapy of Cancer (“SITC”) held in San Diego. See Research & Development Update – MDNA11 for clinical
updates. |
| · | On November 3, 2023, the Company presented preclinical data on its first-in-class IL-13Ra2
targeted candidate, MDNA113, from its T-MASK platform, which delivers a masked bispecific anti-PD1-IL2 Superkine to IL-13Ra2
expressing tumors (annual incidence of over 2 million)1 where it is activated by cancer specific enzymes. This data was presented
at the 38th Annual Meeting of the SITC held in San Diego. See Research & Development Update – T-MASK Platform
for research updates. |
| · | On November 2, 2023, the trading of the Company’s common shares on the Nasdaq Capital Market
(“Nasdaq”) was suspended as a result of the Company’s failure to comply with the US$1.00 per share minimum bid price
requirement. Form 25-NSE was filed with the United States Securities and Exchange Commission, which removed the Company’s securities
from listing and registration on Nasdaq (the “Nasdaq Delisting”). As of December 19, 2023, the Company’s common shares
continue to trade on the Toronto Stock Exchange and on the OTCQB in the United States, as noted above. |
| · | On October 27, 2023, Medicenna announced that it was delisted from the Nasdaq as the Company did not meet
the listing requirements, that it is reducing its presence in the US to conserve cash, and that Jeff Caravella, Chief Financial Officer,
and Brent Meadows, Chief Business Officer, departed the Company, effective October 26, 2023. |
| · | On October 25, 2023, Medicenna announced dosing of the first patient in the Phase 2 monotherapy dose expansion
portion of the ABILITY-1 Study. |
| · | On October 3, 2023, new preclinical data characterizing MDNA223, an anti-PD1-IL-2 BiSKITÔ
(Bifunctional SuperKine for ImmunoTherapy), including its synergy when combined with STING agonists were presented
at the 2023 AACR Special Conference in Cancer Research: Tumor Immunology and Immunotherapy, held in Toronto, Canada. See Research &
Development Update – BiSKITs Platform for research updates. |
FINANCING UPDATE
Nine months ended December 31, 2023
2023 At-The-Market Facility
On February 17, 2023, the Company entered into a sales agreement with Oppenheimer
& Co. Inc., acting as sales agent (the “2023 ATM Agreement”), pursuant to which the Company may, from time to time sell,
through at-the-market offerings on the Nasdaq such number of Common Shares as would have an aggregate offering price of up to US$10.0
million (the “2023 ATM Facility”). During the nine months ended December 31, 2023, the Company did not issue any Common Shares
pursuant to the 2023 ATM Facility. Further to the Nasdaq delisting in November 2023, the 2023 ATM Agreement was terminated.
Warrants
During the nine months ended December 31, 2023, no warrants were exercised.
On July 5, 2023, the warrants issued on October 17, 2019, in correlation with
a public offering, were due to expire on July 17, 2023 and were extended to October 17, 2024.
On December 18, 2023, the warrants issued on December 21, 2018 were due to
expire on December 21, 2023 and were extended to July 31, 2024.
________________________
1 https://www.wcrf.org/cancer-trends/worldwide-cancer-data/
RESEARCH & DEVELOPMENT UPDATE
Our Pipeline of Superkines
Bizaxofusp (formerly named MDNA55) for the treatment of recurrent Glioblastoma (“rGBM”)
Glioblastoma (“GBM”) is one of the most complex, deadly,
and treatment-resistant cancers. It is expected that in the US and Canada, there will be at least 15,000 new diagnoses of GBM with more
than 10,000 individuals succumbing to the disease within one year. Five-year survival rate for GBM patients is only 6.9 percent; these
survival rates and mortality statistics have remained virtually unchanged for decades.
Despite first being identified in the scientific literature in the 1920’s,
there have only been four drugs and one device ever approved by the USFDA specifically for the treatment of GBM. Unfortunately, none have
succeeded in significantly extending patient lives beyond a few extra months for newly diagnosed GBM and a few extra weeks for patients
with rGBM. GBM is also one of the more expensive cancers to treat, often leaving patients and families with major financial hardship in
addition to the burden of the disease. Given the limitation of all current therapeutics, development of novel approaches for treating
GBM and rGBM remains a great unmet need.
Bizaxofusp is a genetically engineered fusion of a circularly permuted
version of IL-4 to a potent catalytic component of the bacterial toxin, Pseudomonas exotoxin, which effectively arrests protein synthesis
leading to cell death. The IL-4 component is engineered and designed to preferentially target tumor cells that over-express the interleukin
4 receptor (“IL-4R”). The drug is delivered only once locally into the tumor, using a minimally invasive technique, bypassing
the blood-brain barrier. Bizaxofusp holds a FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively.
Bizaxofusp has successfully completed a Phase 2b (N=44) trial for nonresectable
rGBM where it demonstrated compelling Overall Survival (“OS”) benefit versus Standard of Care (“SOC”). The Phase
2b clinical trial was conducted in a multi-center, open-label, single-arm study in patients with first or second recurrence or progression
of GBM after surgery or radiotherapy ± adjuvant therapy or other experimental therapies. Results were published in June 2023 issue
of NeuroOncology (doi: 10.1093/neuonc/noac285).
Phase 2b Results Published as the Cover Story in Neuro-Oncology

A separate analysis collected rGBM survival and prognostic data from
eligibility matched 81 patients who had contemporaneously received treatment at major clinical centres using current SOC. These data from
patient registries were used to establish a matched External Control Arm (“ECA”). Blinded survival data from propensity matched
ECA (established by matching with bizaxofusp-treated population based on 11 different prognostic factors using propensity scoring methods)
were then used as a control arm versus survival data from the Phase 2b bizaxofusp trial. Survival follow-up has concluded, and final study
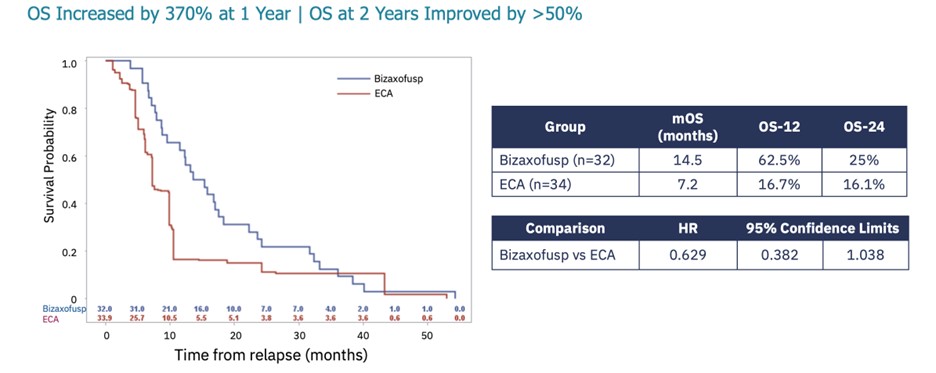
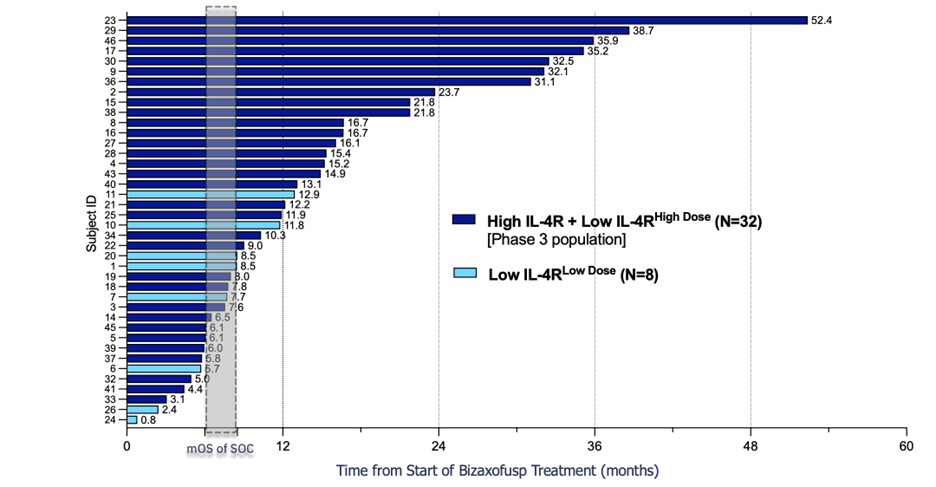
results were presented at the 28th Annual Meeting of the Society of NeuroOncology in Vancouver on November 16-19, 2023. Key
findings from the presentation are shown in the figure below and include:
| · | Bizaxofusp doubled mOS irrespective of IL-4R expression (IL-4R high + IL-4R low treated with high dose
bizaxofusp; proposed Phase 3 population) compared to propensity matched ECA (14.5 months vs. 7.2 months). |
| · | Compared to propensity matched ECA, bizaxofusp increase OS by 370% at year
1 and by more than 50% at year 2. |
Bizaxofusp Doubles Overall Survival in Phase 3 Population vs ECA
Bizaxofusp Shows Compelling Overall Survival Benefit Over Standard
or Care
Following the End of Phase 2 (EOP2) meeting, the FDA endorsed an innovative
open-label hybrid Phase 3 registration trial that allows use of a substantial number of patients (two-thirds) from a propensity matched
ECA to support marketing authorization of bizaxofusp for rGBM (see the graphic below).
Phase 3 Trial with Hybrid External Control Arm (“ECA”)
to be Conducted in Collaboration with Potential Partner
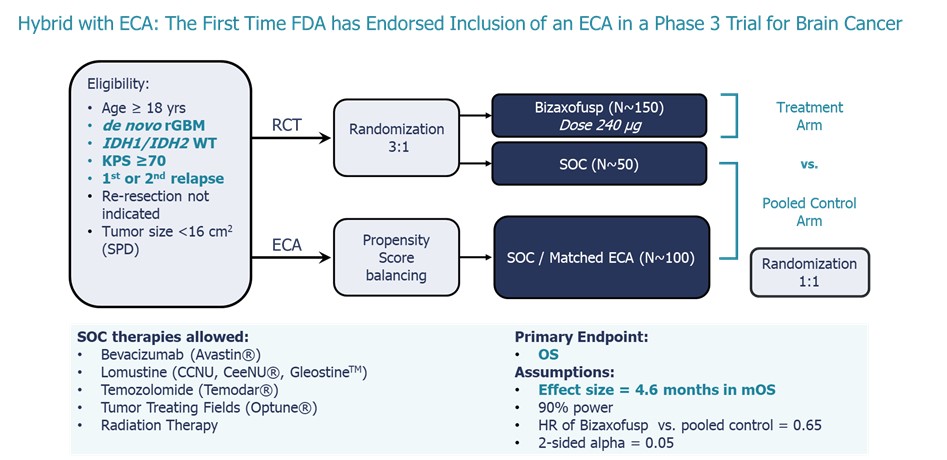
In order to add additional value to the bizaxofusp program Medicenna
is planning to seek Breakthrough Therapy Designation from the FDA and is also seeking alignment from the EMA for the FDA-endorsed Phase
3 trial design in the coming months.
Medicenna is pursuing strategic partnerships to assist with additional
clinical development of bizaxofusp, as well as preparing the program for commercialization and its subsequent launch in various countries
where marketing authorization has been granted. Medicenna estimates that the total costs of completion of a pivotal registrational trial,
associated regulatory and manufacturing activities and preparing bizaxofusp for commercial launch to be approximately $60 to $80 million
USD.
Through confidential primary market research conducted for the Company, bizaxofusp
has a market potential of more than US$800M annually for GBM alone. In addition, metastatic IL-4R positive brain tumors account for ~US$4B
market.
MDNA11
MDNA11 is a long-acting albumin-fusion, beta-enhanced
not-alpha IL-2 super agonist designed to preferentially activate anti-cancer immune cells (CD8+ T and NK) over immunesuppressive (pro-cancer)
Tregs. Fusion with human albumin augments MDNA11’s half-life and promote its accumulation in tumors. MDNA11 is currently being evaluated
in the ABILITY Phase 1/2 study in patients with melanoma and other solid cancers. The ABILITY Study is a global, multi-center, open-label
study that assesses the safety, tolerability and anti-tumor activity of MDNA11 as monotherapy or in combination with pembrolizumab (Keytruda®).
The figure below describes the ABILITY Phase 1/2 study.
ABILITY Phase 1/2 Study Schema: MDNA11 Monotherapy
and in Combination with Pembrolizumab
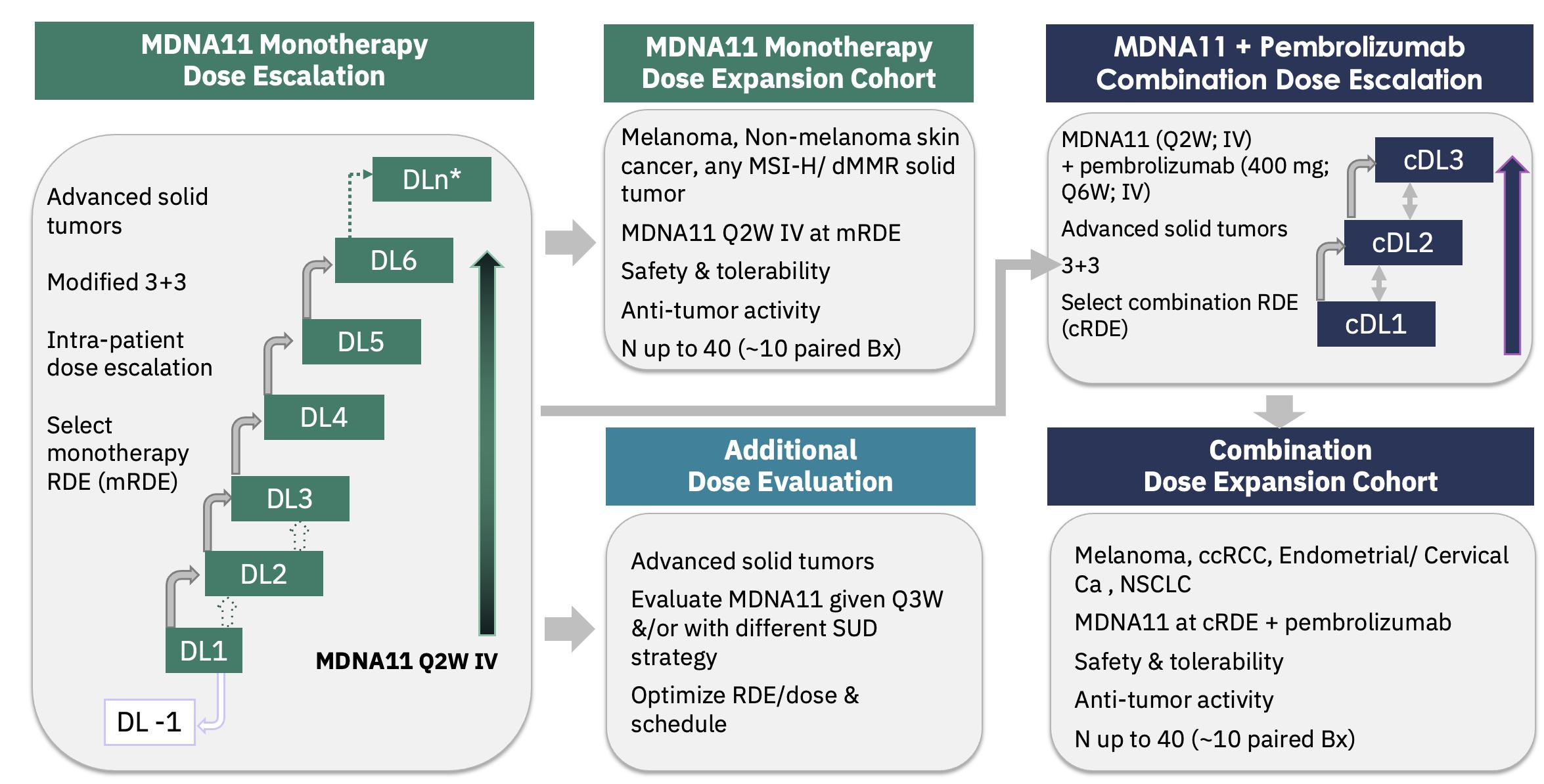
On August 9, 2023, Medicenna held a R&D
day webcast where Dr. Arash Yavari, Chair of the Company’s Development Advisory Committee (“DAC”), presented a clinical
update on the monotherapy dose escalation portion of the ABILITY Study. Key findings from the dose escalation portion of the ABILITY Study
include:
| · | Favorable safety profile: MDNA11 was generally well tolerated across cohorts, with
majority of adverse events (AEs) being grade 1 or 2, with no grade 4 or 5 AEs. |
| · | Promising single-agent activity and durable tumor control: Several patients exhibited
encouraging evidence of single-agent activity with clinical benefit observed in 7 of 19 evaluable patients (37%). |
| · | Confirmed partial response to single-agent MDNA11 in a highly aggressive tumor type: A
patient in cohort 4 (60mg/kg dose) with metastatic pancreatic ductal adenocarcinoma (PDAC), who
had failed to respond to multiple prior systemic therapies, continues to show tumor shrinkage of all metastatic lesions in the liver after
each successive scan. The most recent scan showed an 80% decrease in total tumor size with complete regression of 2 out of 3 lesions.
This patient continues on study treatment with MDNA11. |
| · | Prolonged stable disease in metastatic melanoma progressed on prior immune checkpoint inhibition: A
patient in cohort 2 (commenced on 10 mg/kg dose and subsequently increased to 30, 60 and 90 mg/kg),
having failed prior immunotherapy, experienced stable disease for 84 weeks. |
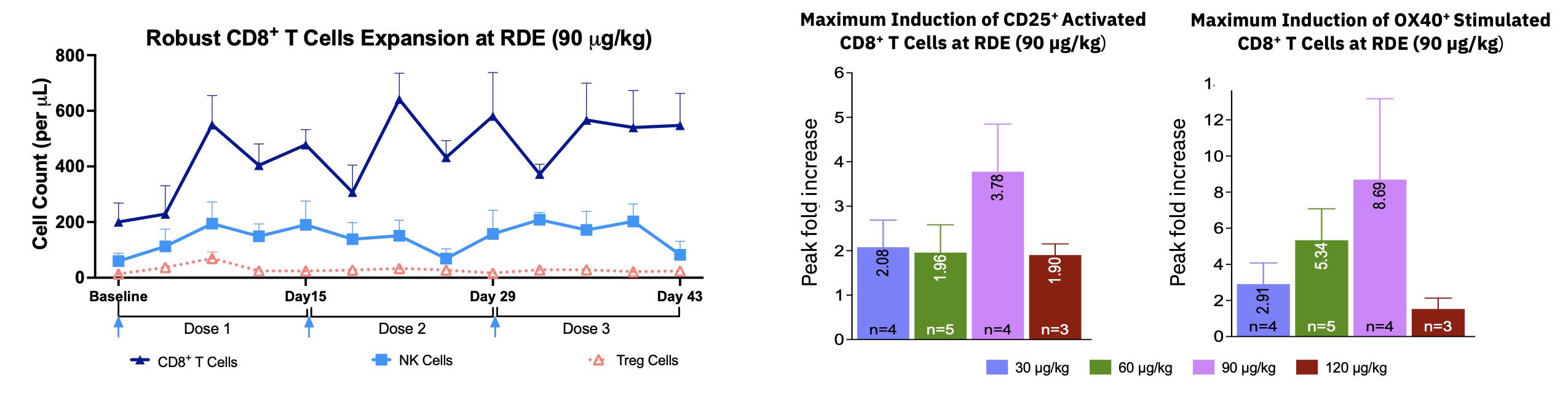
| · | Pharmacodynamic data on effector anti-tumor immune cells continue to support the mechanistic rationale
for MDNA11’s promising anti-tumor activity, with MDNA11 inducing robust expansion of a population of potent activated CD8+T
cells and increasing NK cells, but with limited expansion of Tregs which can suppress anti-tumor immunity. |
Based on the totality of the dose escalation data,
a Recommended Dose for Expansion (“RDE”) of 90 mg/kg given every other week
by IV infusion has been chosen for the monotherapy expansion phase of the trial.
Selection of specific cancers for evaluation in the
monotherapy dose expansion phase was determined based on clinical data available from the ABILITY-1 Study, discussions with Medicenna’s Clinical
Advisory Board (“CAB”) and other expert KOLs, and an understanding of the immunobiology of the selected tumor types and
the potential for MDNA11 monotherapy in the post-checkpoint inhibitor setting. The following tumor types will be recruited in
the dose expansion phase of the study:
| · | Melanoma (Cutaneous, Mucosal or Acral). |
| · | Non-Melanoma Skin Cancers (cSCC, BCC, MCC). |
| · | Microsatellite Instability-High (MSI-H) or deficient DNA mismatch repair (dMMR) cancers. This population
was selected to determine if the response achieved in the PDAC patient may have been due to the MSI-H profile. The PDAC patient unequivocally
progressed on pembrolizumab, which is approved for MSI-H cancers. |
On October 25, 2023, Medicenna reported that the first patient was dosed in
the monotherapy dose expansion portion of the study. Preliminary data from the monotherapy dose expansion part of the study are expected
to be presented in the first half of calendar 2024.
Enrolment for combination dose escalation with KEYTRUDAÒ
commenced end of calendar 2023 with the first patient for combination dose escalation being dosed on February 7, 2024.
At the 38th Annual Meeting of the SITC held in San Diego from November
1-5, 2023, Medicenna presented further updates from the Phase 1/2 ABILITY Study. Key clinical data are summarized and shown graphically
below:
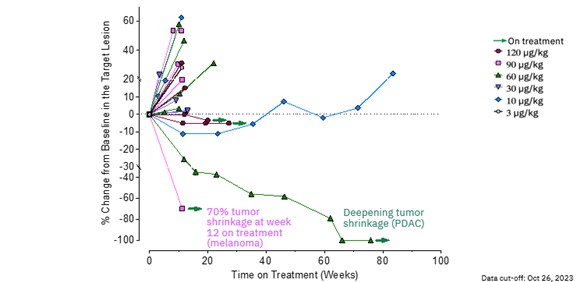
| · | MDNA11 continues to demonstrate encouraging single-agent activity from the dose escalation and evaluation
portion of the ABILITY-1 study, including ongoing partial response with 100% reduction of target and non-target lesions in one pancreatic
cancer patient and a second patient with metastatic melanoma demonstrating a 70% reduction of target lesion at week 12. |
| · | MDNA11 showed durable stable disease in 3 melanoma patients for longer than 5 months, with one patient
showing durable stable disease for 18 months, with concomitant shrinkage of target lesions, following prior failure with immune check-point
therapies. |
| · | At dose levels above 60 mg/kg, MDNA11 achieved a disease control
rate of 33.3% (2 PRs and 3 durable SDs in 15 patients) irrespective of tumor type. |
| · | MDNA11 is generally well tolerated with no dose-limiting toxicities and vascular leak syndrome reported
in any of the monotherapy dose escalation cohorts. |
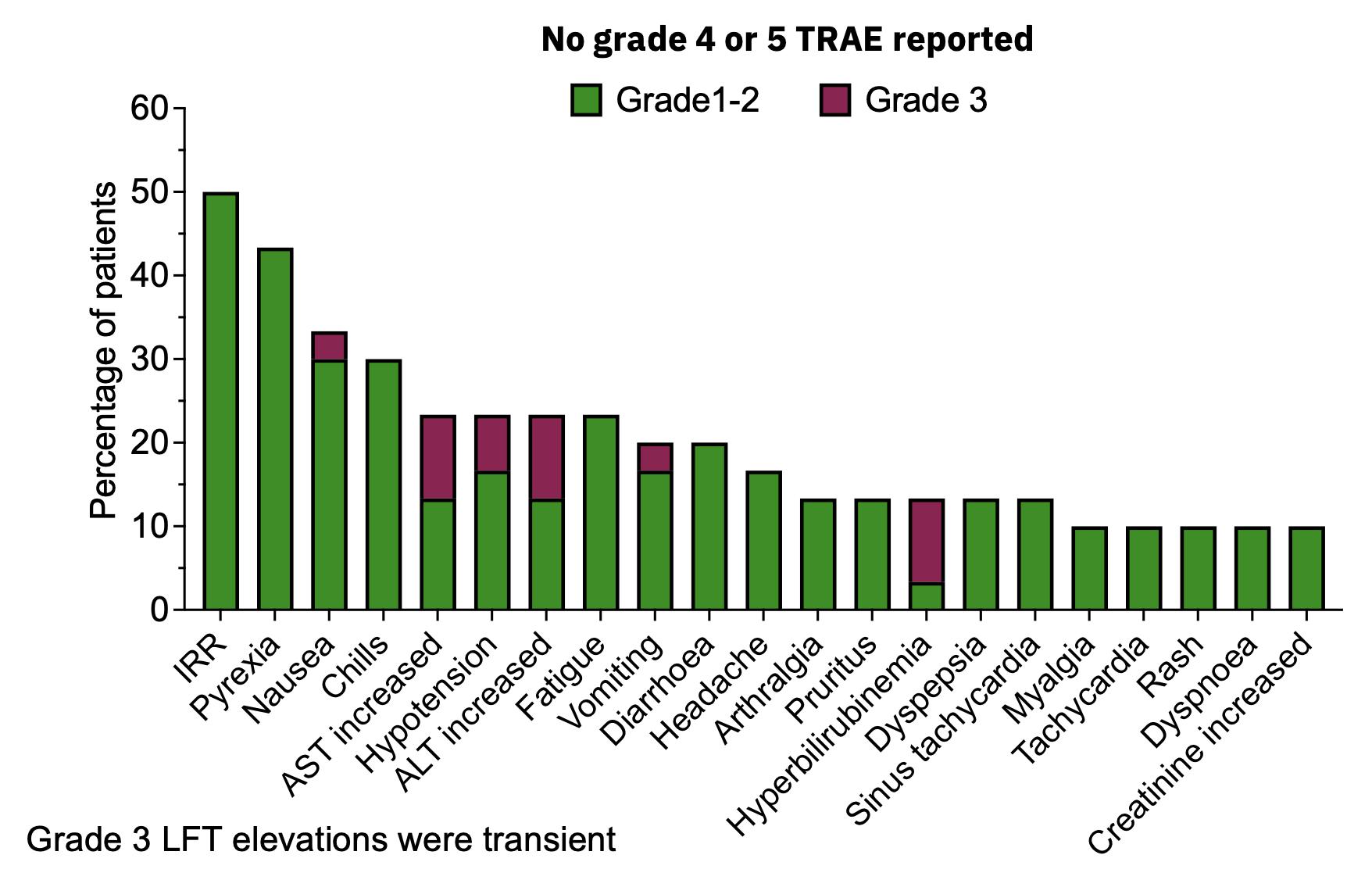
| · | Vast majority (95.6%) of treatment related adverse events were grade 1-2 severity and resolved within
48 hours; grade 3 TRAEs mainly constituted transient LFT elevations; no grade 4 or 5 events were reported. |
| · | Pharmacodynamic response showed robust expansion and activation (CD25 and OX40) of CD8+ T cells with some
expansion of NK cells and limited increase in number of immune-suppressive Treg cells, in all dose cohorts and particularly at 90 mg/kg. |
| · | Target dose of 90 mg/kg (following 2 step-up doses of 30 and
60 mg/kg) Q2W by IV infusion was chosen as the Recommended Dose for Expansion (RDE) in the monotherapy
expansion portion of the ABILITY study. |
2 Partial Responses (PR) and 3 Durable Stable Diseases (SD) with Single-agent MDNA11 (Data cut-off
as of October 26, 2023) – Additional, updated data are presented below
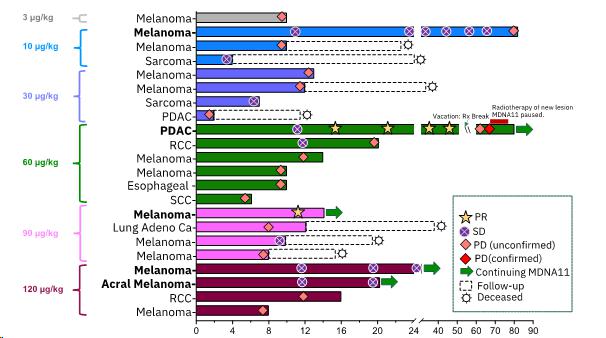
Deep Shrinkage of Tumor Lesions with Single-agent MDNA11
MDNA11 has Highly Favorable Safety Profile: No Dose Limiting Toxicities
Robust Expansion and Activation of Anti-Cancer Effector Cells but Not Immune
Suppressive Tregs
Following the SITC presentation, additional follow-up data from MDNA11 monotherapy
further confirms single agent activity. Key updates are shown in the below figures and include:
| · | PDAC PR patient continues to show complete resolution of all baseline lesions. |
| · | Melanoma PR patient shows further deepening of tumor shrinkage. |
| · | A new melanoma patient with iPR (PR as per iRECIST) showed pseudo-progression (at week 8) with a iPR (at
week 12). Additional data will be presented at medical conferences in H1 2024. |
| · | Amongst 13 patients, all having previously failed or resistant to immune checkpoint inhibitors (“ICI”),
receiving high doses of MDNA11 (³60 mg/kg) and
with tumor types being evaluated in the monotherapy expansion cohort, the response rate, clinical benefit rate, and tumor control rate
increased to 23% (3 partial responses), 46% (3 PRs and 3 patients with stable disease for ³24
weeks), and 69% (3 PRs and 6 SDs), respectively, with concomitant shrinkage of target lesions in all patients with stable disease. |
Compelling Single-Agent Activity in Heavily Pre-treated Patients with Advanced
Solid Tumors (Data cut-off as of February 5th)
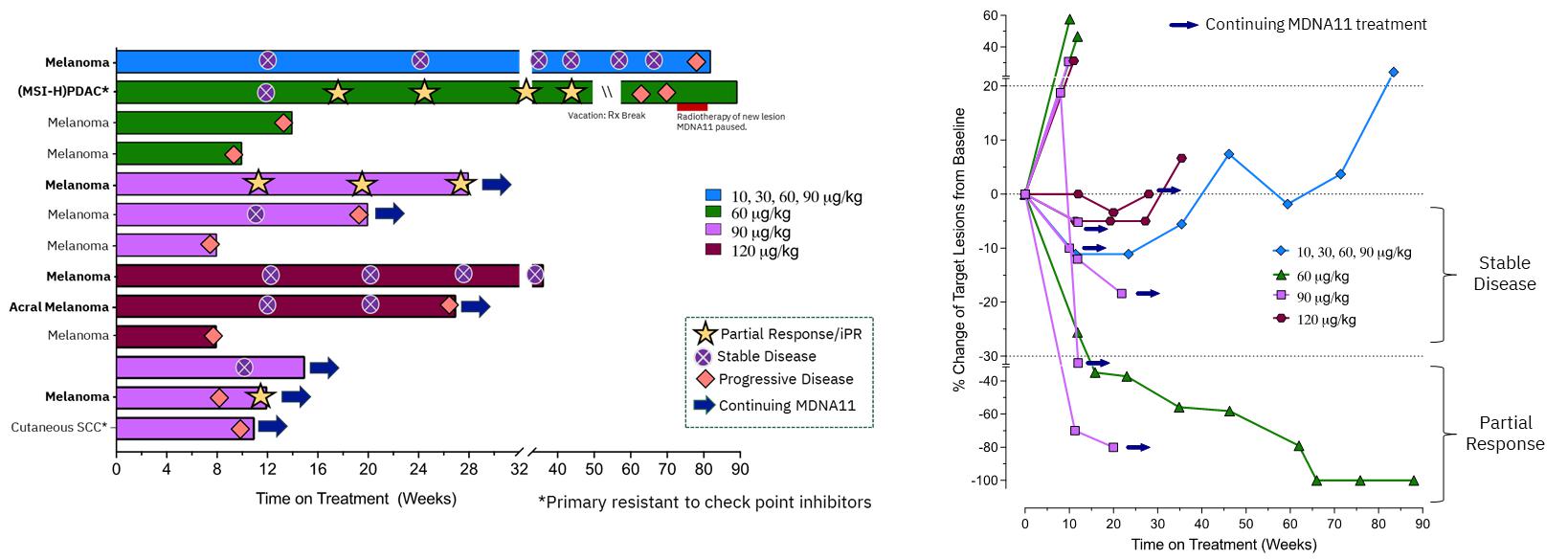
On February 13, 2024, Medicenna reported that the first patient was dosed in
the combination dose escalation with KEYTRUDAÒ. Preliminary data from the combination
escalation part of the study are expected to be presented in the first half of calendar 2024.
In addition to general clinical expenses, which are distributed amongst the
various clinical projects, $12.4 million is currently estimated to be allocated to the Phase 2 monotherapy dose expansion and Phase 2
combination trial with KEYTRUDAÒ.
IL-4 and IL-13 Superkines in
preclinical development
Medicenna’s IL-4 and IL-13 Superkines, licensed from Stanford,
are engineered cytokines which possess enhanced affinity and selectivity for either the Type 1 or Type 2 IL-4 receptors or dedicated IL13
receptors such as IL13Ra2. This selectivity is achieved through mutations of the IL-4 or IL-13
cytokines to enhance affinity for binding to specific IL4R or IL13R subunits. Additional mutations have also been engineered to modulate
their bioactivity, resulting in Superkines with enhanced signalling (super-agonists) or the ability to block signalling (super-antagonists).
MDNA413 : An IL-4/IL-13 Dual Super-Antagonist
One promising IL-13 Superkine antagonist is MDNA413. Compared to wild-type
IL-13, MDNA413 has been engineered to have a 2,000-fold higher selectivity for the Type 2 IL4R and potently blocks IL-4 and IL-13 signalling
(Moraga et al., 2015). Blocking of Type 2 IL4R by MDNA413 may be relevant not only for targeting solid tumors that overexpress this receptor,
but also the Th2-biased tumor microenvironment, which shields cancer from the immune system.
We believe that MDNA413’s ability to block IL-4/IL-13 signalling
has the potential to address a significant unmet medical need for effective therapies against immunologically cold tumors which are often
resistant to checkpoint inhibitors and other immunotherapeutic agents due to their immunosuppressive tumor microenvironment (“TME”).
MDNA132 and MDNA213: High Affinity Cancer-Specific Targeting Ligands
Another promising IL-13 Superkine is MDNA132, and its variant, MDNA213.
Unlike MDNA413, MDNA132 and MDNA213 are IL-13 ligands that have been engineered to increase affinity for IL13Ra2
overexpressed on certain solid tumors while exhibiting sharply decreased affinity for IL13Ra1.
Medicenna believes MDNA132 and MDNA213 have superior targeting compared to other IL-13 variants in development and is an attractively
differentiated tumor targeting domain for (a) cell-based immunotherapies (such as those using chimeric antigen receptors or CARs); (b)
potent payloads used in antibody-drug conjugates (“ADC”); (c) targeted fusion toxins or (d) radiopharmaceuticals. Development
timelines for MDNA132 and MDNA213 have yet to be established. MDNA132 and/or MDNA213 are also being evaluated as potential fusion proteins
in our BiSKITsTM and the T-MASKTM platforms.
On April 17, 2023, we presented new preclinical data
characterizing the Interleukin 13 (“IL-13”) Superkines, MDNA132 and MDNA213, and a series of next generation IL-13 Superkine
therapies, at the AACR Annual Meeting, which took place in Orlando, Florida, from April 14 to 19, 2023. The AACR poster included data
demonstrating that both MDNA132 and MDNA213 exhibit highly selective binding to the IL-13 decoy receptor (IL-13Rα2) and, in a murine
model, selectively accumulate in the TME for several days. MDNA132 and MDNA213 exhibit high affinity and selectivity for the IL13Rα2,
which is overexpressed in various tumors such as pancreatic, prostate, bladder, colorectal, breast and lung cancer but minimally expressed
in healthy tissues. A fusion of MDNA213 with a toxin selectively caused cell death in IL-13Rα2 expressing cancer cells in vitro
and tumors in vivo. High expression of IL13Rα2 in these tumors is generally associated with more aggressive cancer and poor survival
outcomes.
BiSKITsTM (Bi-functional
SuperKine ImmunoTherapies) Platform
Our BiSKITsTM
platform allows us to develop designer Superkines by fusing them to other proteins, checkpoint inhibitors, antibodies or cytokines to
our IL-2, IL-4 and/or IL-13 Superkines in order to combine two distinct and yet synergistic mechanisms of action into one molecule: a BiSKITTM.
MDNA223 is a fusion of Medicenna’s IL-2 Superkine
with an anti-PD1 antibody, designed to maximize anti-tumor response by concurrently facilitating IL-2R pathway stimulation and PD1 checkpoint
blockade on the same effector immune cell as described in the figure below.
Cis-binding Facilitates IL-2R Activation and PD-1
Blockade on the Same CD8+ T Cell to Maximize Anti-Cancer Response
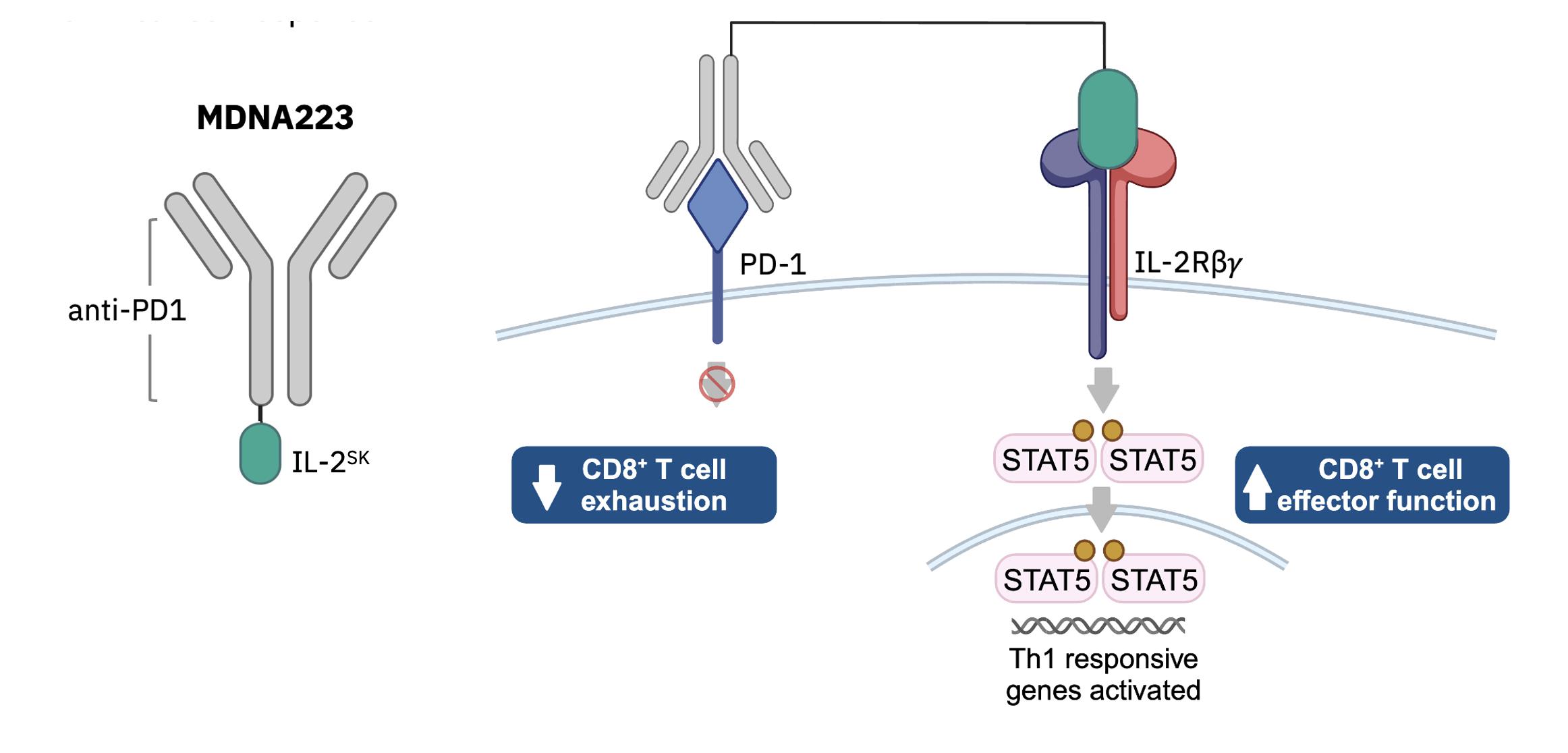
On October 3, 2023, we presented a poster at the AACR
Special Conference in Cancer Research: Tumor Immunology and Immunotherapy with preclinical data demonstrating that the MDNA223
BiSKIT:
| · | Showed enhanced IL-2Rbeta selectivity and no binding to IL-2Ralpha, leading to preferential stimulation
of CD8+ T cells over Tregs in human PBMCs. |
| · | Retained high affinity to PD-1, generating potent blockade of PD-1/PD-L1 mediated exhaustion of T cells. |
| · | Induced durable proliferation and expansion of CD8+ T cells in the periphery, and enhanced tumor infiltration
of functionally active CD8+ T cells. |
| · | Demonstrated superior efficacy and survival benefit in multiple syngeneic tumor models, including “cold”
tumors compared to co-administration (combination) of anti-PD1 and IL-2 super-agonist (MDNA19) (see figure below). |
| · | Synergized with agonist of the STING (Stimulator of Interferon Genes) pathway to enhance
tumor inhibition and promote an abscopal effect as demonstrated by shrinkage of the untreated tumor on opposite flank. |
| · | Enhanced tumor response while being well-tolerated in a step-up dosing setting. |
| · | The sum of encouraging preclinical data on MDNA223 highlights the potential of Medicenna’s BiSKIT
platform to broadly deliver effective therapy to otherwise challenging-to-treat ‘cold’ tumors. |
MDNA223 Shows Superior Efficacy to Combination of MDNA19 (IL-2 agonist)
+ anti-PD-1
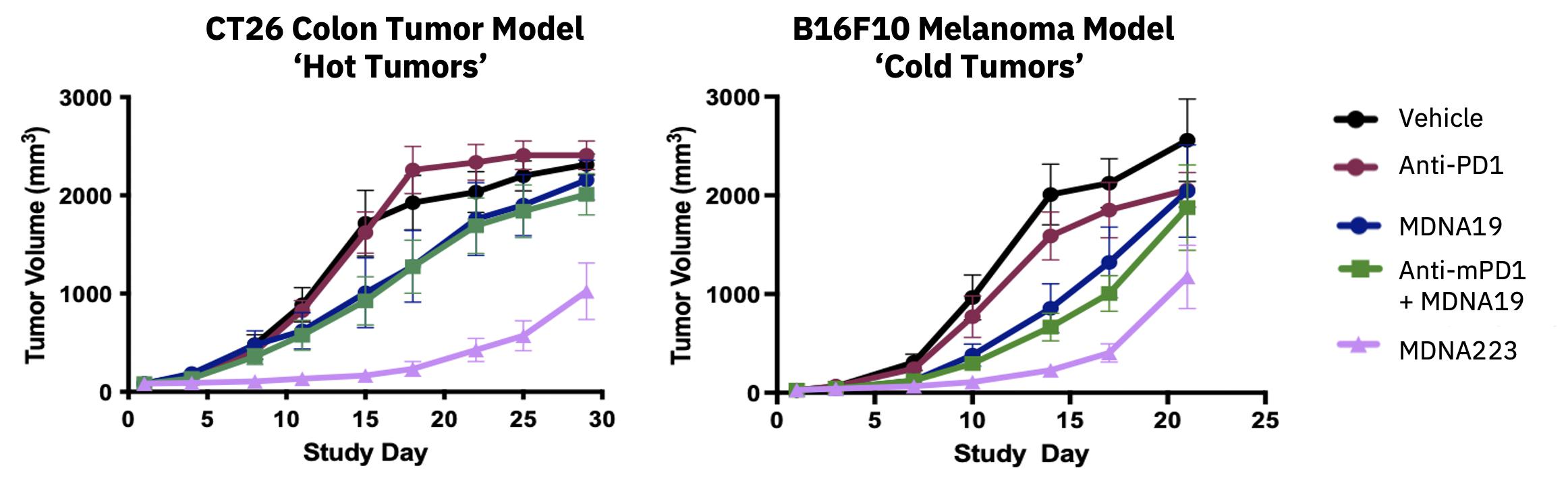
Medicenna is currently screening and optimizing
a variety of IL-2/IL-4/IL-13 Superkines as part of our BiSKITÔ
platform. As preparing, submitting, and advancing applications for regulatory approval, developing drugs and drug product and clinical
trials are sometimes complex, costly, and time-consuming processes, an estimate of the future costs is not reasonable at this time.
T-MASKÔ
(Targeted Metallo/protease Activated SuperKine) Platform
Our T-MASKÔ
platform involves fusion of a dual IL-13 tumor targeting/masking domain to an IL-2 superkine or IL-2 BiSKITÔ
via a matrix metalloprotease (“MMP”) sensitive linker (“PSL”), to provide the following unique features:
| · | Tunable blockade of IL-2R agonism to reduce peripheral immune stimulation for enhanced tolerability. |
| · | Tumor targeting to IL-13Ra2 highly expressed in a broad range
of cancer indications but not normal tissues. |
| · | Cleavage and release of IL-13 tumor-targeting/masking domain by MMPs restores IL-2R agonism within the
tumor microenvironment (TME). |
Proposed mechanism of action of the
T-MASKÔ platform is shown in the figure below for an IL-2 BiSKIT (MDNA223; Anti-PD1-IL-2SK)
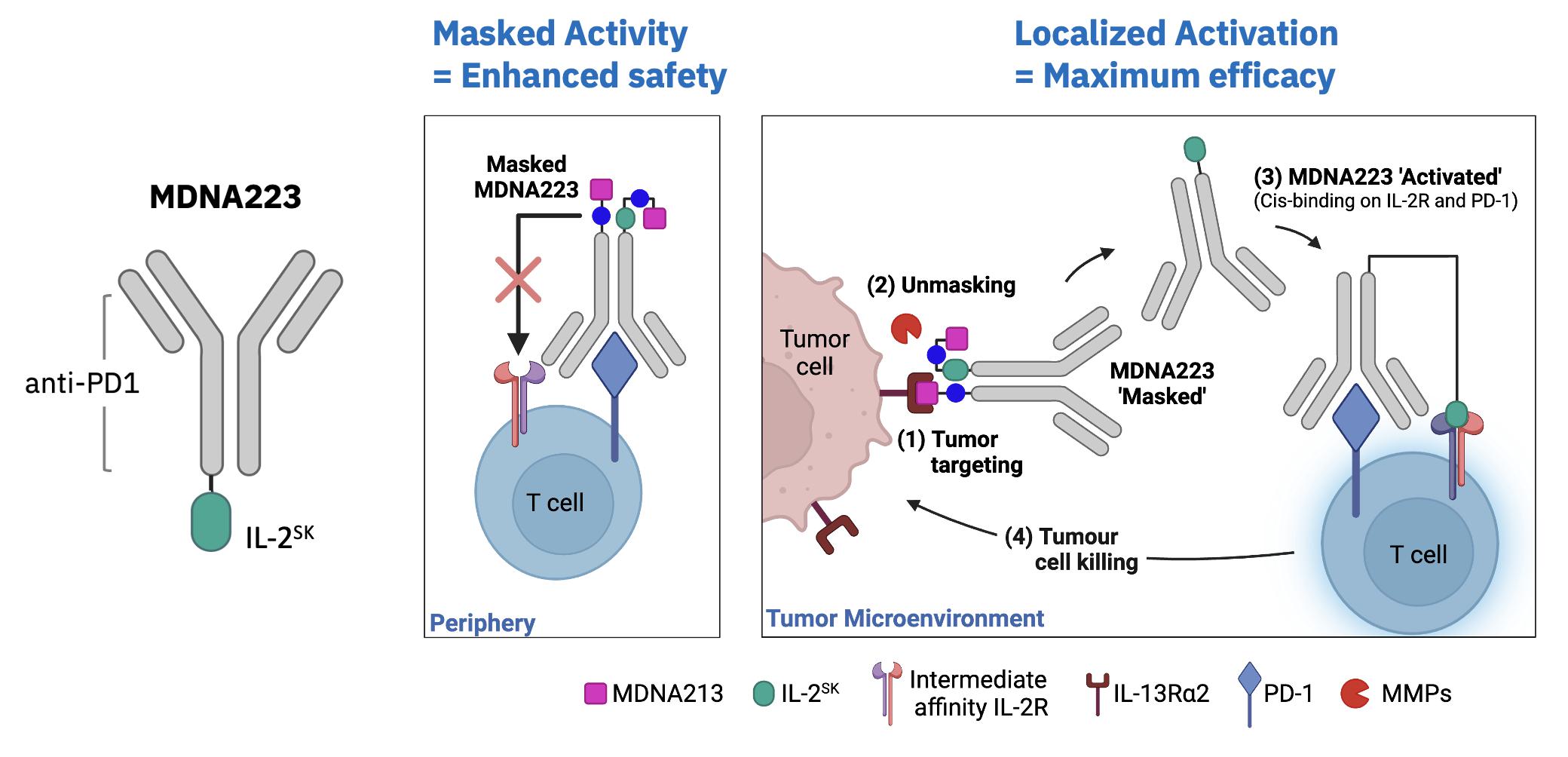
At the 38th Annual Meeting
of the SITC held in San Diego from November 1-5, 2023, Medicenna presented preclinical data on characterization of its T-MASKÔ
platform, by evaluating T-MASKÔ versions of long-acting IL-2 superkine and MDNA223, an
anti-PD1-IL-2 BiSKITÔ. Key features of the platform and preclinical data presented in the
conference are summarized below:
| · | The IL-13 superkine acts as an effective dual tumor targeting and masking domain to selectively deliver
potent immune modulators to the tumor microenvironment where they are activated by cancer specific enzymes. |
| · | IL-13Ra2 is highly expressed in a broad range of aggressive tumors
at an annual rate of more than 2 million cases worldwide and include some of the most immunologically “cold” tumors.2 |
| · | MDNA113 is a first-in-class IL-13Ra2 targeted therapy that delivers
a masked anti-PD1-IL-2 bi-functional superkine to the tumor microenvironment. |
| · | MDNA113 showed reduced potency in IL-2 signaling assays but activity was restored upon MMP cleavage to
remove the IL-13 Mask. |
| · | Masking had no effect on blockade of PD1/PD-L1 immune suppressive signaling in vitro. |
| · | Systemic administration of MDNA113 in mice showed reduced peripheral immune cell expansion compared to
non-masked version. |
| · | MDNA113 is as effective as its non-masked version at inhibiting tumor growth in mouse cancer models; an
uncleavable version of MDNA113 has reduced efficacy as expected (see figure below). |
| · | The level of masking is tunable and avoids complete blockade of the immune modulator thereby retaining
good tolerability while achieving adequate immune stimulation during transit to the tumor micro-environment. |
_________________________________
2 Sharma A., et al. Journal for ImmunoTherapy for Cancer.
2023; 11 (Suppl 1) https://doi.org/10.1136/jitc-2023-SITC2023.1071
Effective Tumor Inhibition by MDNA113 Requires Activation by MMP Cleavage
Within TME
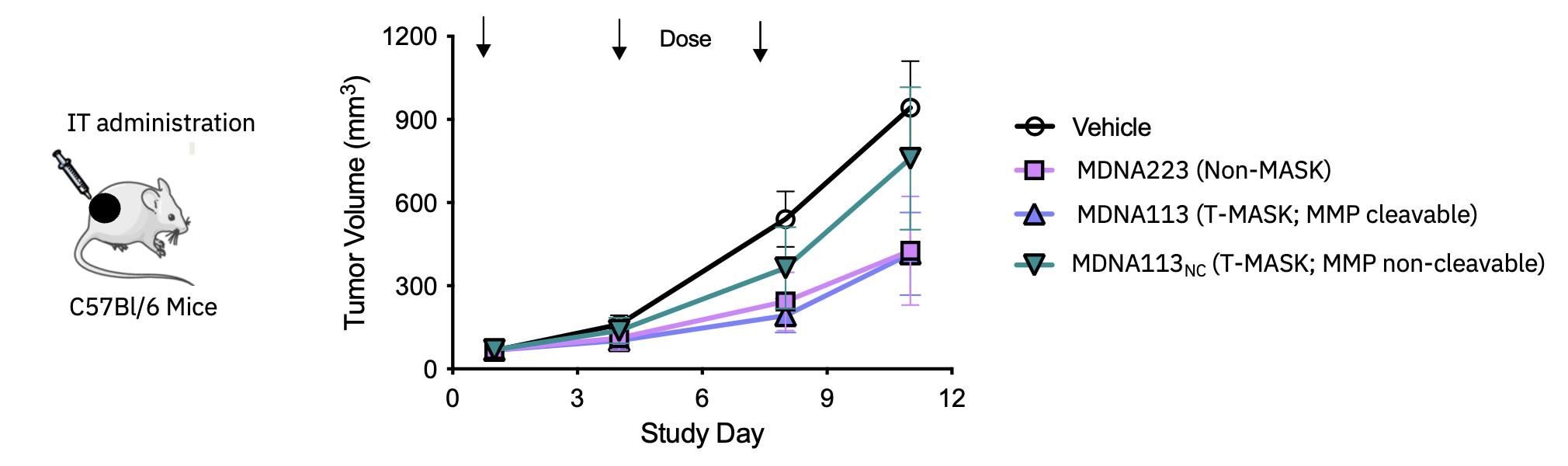
Medicenna is currently screening and optimizing a
variety of IL-2/IL-4/IL-13 Superkines as part of our BiSKITsTM and T-MASKTM
platforms. Additional funding will be necessary to advance one or more of these product candidates into clinical trials. As preparing,
submitting, and advancing applications for regulatory approval, developing drugs and drug product and clinical trials are sometimes complex,
costly, and time-consuming processes, an estimate of the future costs is not reasonable at this time.
SELECTED FINANCIAL INFORMATION
All tabular amounts below are presented in thousands of Canadian dollars, except for per
share amounts.
| | |
| Three
months ended
December 31,
| | |
| Nine
months ended
December 31,
| |
| |
| 2023 | | |
| 2022 | | |
| 2023 | | |
| 2022 | |
| Statements of loss and comprehensive loss data: | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| General and administration | |
| 1,786 | | |
| 1,976 | | |
| 5,736 | | |
| 6,266 | |
| Research and development | |
| 2,991 | | |
| 2,945 | | |
| 8,937 | | |
| 7,718 | |
| Total operating expenses | |
| 4,777 | | |
| 4,921 | | |
| 14,673 | | |
| 13,984 | |
| | |
| | | |
| | | |
| | | |
| | |
| Finance income | |
| (282 | ) | |
| (340 | ) | |
| (970 | ) | |
| (532 | ) |
| Change in fair value of warrant derivative | |
| 160 | | |
| (3,747 | ) | |
| (2,547 | ) | |
| (5,547 | ) |
| Foreign exchange loss (gain) | |
| 322 | | |
| 307 | | |
| 406 | | |
| (1,713 | ) |
| | |
| 200 | | |
| (3,780 | ) | |
| (3,111 | ) | |
| (7,792 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net Loss | |
| (4,977 | ) | |
| (1,141 | ) | |
| (11,562 | ) | |
| (6,192 | ) |
| Basic and diluted loss per share | |
| (0.07 | ) | |
| (0.02 | ) | |
| (0.17 | ) | |
| (0.10 | ) |
| | |
| As of | |
| | |
| December 31, 2023 | | |
| March 31, 2023 | |
| Statement of financial position: | |
| | | |
| | |
| | |
| $ | | |
| $ | |
| Cash | |
| 21,758 | | |
| 33,596 | |
| Total assets | |
| 23,268 | | |
| 36,446 | |
| Total liabilities | |
| 4,639 | | |
| 6,960 | |
| Working capital | |
| 19,184 | | |
| 32,585 | |
| Accumulated deficit | |
| (92,543 | ) | |
| (80,981 | ) |
| Total shareholder's equity | |
| 18,629 | | |
| 29,486 | |
The Company has not generated revenue in any of the previous fiscal
years, other than income from interest earned on cash and cash equivalents.
For the three and nine months ended December 31, 2023, the Company reported
a net loss of $5.0 million ($0.07 loss per share), and $11.6 million ($0.17 loss per share), compared to a net loss of $1.1 million ($0.02
loss per share) and $6.2 million ($0.10 loss per share), respectively for the three and nine months ended December 31, 2022. The increase
in net loss for the three and nine month period ended December 31, 2023, compared with the period ended December 31, 2022, was primarily
related to the non-cash change in fair value of warrant derivate. Variances within the general and administrative and research and development
categories are discussed specifically below.
RESULTS OF OPERATIONS FOR THE THREE AND NINE MONTHS ENDED DECEMBER 31, 2023
Research and Development (“R&D”) Expenses
| | |
| Three months ended
December 31, | | |
| Nine months ended
December 31, | |
| | |
| 2023 | | |
| 2022 | | |
| 2023 | | |
| 2022 | |
| | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| Chemistry, manufacturing, and controls | |
| 257 | | |
| 146 | | |
| 1,028 | | |
| 686 | |
| Regulatory | |
| 27 | | |
| 15 | | |
| 77 | | |
| 52 | |
| Discovery and pre-clinical | |
| 216 | | |
| 295 | | |
| 1,102 | | |
| 1,166 | |
| Clinical | |
| 1,454 | | |
| 1,221 | | |
| 3,545 | | |
| 2,752 | |
| Salaries and benefits | |
| 606 | | |
| 780 | | |
| 1,760 | | |
| 1,839 | |
| Licensing, patent legal fees and royalties | |
| 208 | | |
| 303 | | |
| 1,065 | | |
| 739 | |
| Stock based compensation | |
| 97 | | |
| 156 | | |
| 399 | | |
| 452 | |
| Research and development tax credits | |
| — | | |
| — | | |
| (200 | ) | |
| — | |
| Other research and development expenses | |
| 126 | | |
| 29 | | |
| 161 | | |
| 32 | |
| | |
| 2,991 | | |
| 2,945 | | |
| 8,937 | | |
| 7,718 | |
R&D expenses of $3.0 million and $8.9 million were incurred during
the three and nine months ended December 31, 2023, compared with $2.9 million and $7.7 million incurred during the three and nine months
ended December 31, 2022.
The increase in R&D expenses in the three and nine months ended December
31, 2023, compared to the three and nine months ended December 31, 2022, is primarily attributable to:
| · | increased clinical costs during the nine months ended December 31, 2023, relative to the prior year due
to the expansion of the MDNA11 ABILITY-1 Study to new clinical sites in US and South Korea. |
| · | increased manufacturing costs during the nine months ended December 31, 2023, relative to the prior year
due to one-time labeling and packaging costs of Keytruda for use in the combination portion of MDNA11 ABILITY-1 study. |
| · | increase in licensing, patent legal fees and royalties during the nine months ended December 31, 2023
relative to the prior year due to the timing of royalty and milestone payments. |
| · | The above increases for the nine month period ending December 31, 2023 related to the prior comparative
period were partially offset by tax credits related to filed SRED claims. |
General and Administrative (“G&A”) Expenses
| | |
| Three months ended
December 31,
| | |
| Nine months ended
December 31,
| |
| | |
| 2023 | | |
| 2022 | | |
| 2023 | | |
| 2022 | |
| | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| Public company expenses | |
| 1,030 | | |
| 1,213 | | |
| 4,001 | | |
| 3,587 | |
| Salaries and benefits | |
| 465 | | |
| 429 | | |
| 805 | | |
| 877 | |
| Facilities and operations | |
| 227 | | |
| 149 | | |
| 603 | | |
| 422 | |
| Stock based compensation | |
| 62 | | |
| 183 | | |
| 323 | | |
| 724 | |
| Transaction costs, warrant derivative | |
| — | | |
| — | | |
| — | | |
| 652 | |
| Depreciation expense | |
| 2 | | |
| 2 | | |
| 4 | | |
| 4 | |
| | |
| 1,786 | | |
| 1,976 | | |
| 5,736 | | |
| 6,266 | |
G&A expenses of $1.8 million and $5.7 million were incurred during
the three and nine months ended December 31, 2023, compared with $2.0 million and $6.3 million during the three and nine months ended
December 31, 2022.
G&A expenses in the three and nine month period ended December 31,
2023 decreased relative to the comparative three and nine month periods ended December 31, 2022 primarily due to a reduction in directors
and officers liability insurance premiums.
SUMMARY OF QUARTERLY FINANCIAL RESULTS:
| |
Dec. 31
2023 |
Sep. 30
2023 |
Jun. 30
2023 |
Mar. 31
2023 |
Dec. 31
2022 |
Sep. 30
2022 |
Jun. 30
2022 |
Mar. 31
2022 |
| |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
$ |
| Revenue |
- |
- |
- |
- |
- |
- |
- |
- |
| General and administration |
1,786 |
2,303 |
1,647 |
1,385 |
1,976 |
1,719 |
1,919 |
1,936 |
| Research and development |
2,991 |
3,134 |
2,812 |
1,586 |
2,945 |
2,362 |
2,411 |
1,191 |
| Change in fair value of warrant derivative |
160 |
(960) |
(1,747) |
1,200 |
(3,747) |
(1,800) |
- |
- |
| Net loss |
(4,977) |
(3,723) |
(2,862) |
(3,856) |
(1,141) |
(896) |
(4,155) |
(3,206) |
| Basic and diluted loss per share |
(0.07) |
(0.05) |
(0.04) |
(0.06) |
(0.02) |
(0.01) |
(0.07) |
(0.06) |
| Total assets |
23,268 |
27,743 |
31,546 |
36,446 |
38,174 |
42,560 |
20,140
|
23,456
|
| Total liabilities |
4,026 |
4,306 |
4,646 |
6,960 |
4,949 |
8,644 |
2,147 |
2,621 |
R&D expenses have remained relatively constant over the last three
quarters as there have been no significant changes in the Company’s clinical programs. Refundable tax credits of $0.7 million contributed
to a decrease in R&D expenses during the quarter ended March 31, 2022, and during the quarter ended March 31, 2023. R&D expenses
increased in the quarter ended December 31, 2022, the quarter ended June 30, 2023, and quarter ended September 30, 2023, due to timing
of activity in the MDNA11 ABILITY Study.
G&A expenses have remained relatively consistent quarter over quarter
other than the quarter ended September 30, 2023, which reflects non-recurring legal and recruitment costs related to personnel additions
during the quarter.
There was a non-cash change in the fair value of the warrant derivative
(gain) loss from the quarter ended September 30, 2022 onwards. The fair value of the warrant derivative will fluctuate quarterly due to
volatility of share price, expected dividend yield and expected risk-free interest rate.
LIQUIDITY AND CAPITAL RESOURCES
Since inception, the Company has devoted its resources to funding R&D
programs, including securing intellectual property rights and licenses, conducting discovery research, manufacturing drug supplies, initiating
preclinical and clinical studies, submitting regulatory dossiers, and providing administrative support to R&D activities, which has
resulted in an accumulated deficit of $92.5 million as of December 31, 2023. With current revenues only consisting of interest earned
on excess cash, cash equivalents and marketable securities, losses are expected to continue while the Company’s R&D programs
are advanced.
The Company does not earn any revenue from our product candidates and
is therefore considered to be in the development stage. As required, the Company will continue to finance its operations through the issuance
of equity or pursue non-dilutive funding sources. The continuation of our research and development activities for bizaxofusp, MDNA11,
the BiSKITsTM and T-MASKTM platforms and the commercialization of bizaxofusp is dependent upon the ability to successfully
finance and complete our research and development programs through a combination of equity financing and revenues from strategic partners.
The Company currently has no sources of revenue from strategic partners.
Management has forecasted that the Company’s current level of cash will
be sufficient to execute its current planned expenditures into Q2 of calendar 2025. The Company has the ability to reduce or eliminate
planned expenditures to extend its operating runway if it is unable to obtain additional financing when required. The Company’s
ability to continue as a going concern beyond the first quarter of 2025 is dependent on its ability to secure additional financing.
These circumstances cast substantial doubt as to the ability of the
Company to continue as a going concern and, hence, the appropriateness of the use of accounting principles applicable to a going concern.
The following table summarizes the Corporation’s cash flows for
the periods indicated:
| | |
| Nine Months ended
December 31,
| |
| | |
| 2023 | | |
| 2022 | |
| Net cash (used in) provided by: | |
| | | |
| | |
| Operating activities | |
| (11,415 | ) | |
| (10,366 | ) |
| Financing activities | |
| — | | |
| 24,949 | |
| Net increase (decrease) in cash and cash equivalents | |
| (11,415 | ) | |
| 14,583 | |
Cash utilized in operating activities for the nine months ended December
31, 2023 was $11.4 million, compared to the nine months ended December 31, 2022 of $10.4 million. The increase in cash utilized in the
nine months ended December 31, 2023, compared to the nine months ended December 31, 2022 is primarily as a result of increased research
and development expenses and changes in working capital.
Cash provided by financing activities for the nine months ended December
31, 2022 is comprised of an underwritten public offering completed on August 10, 2022. Total proceeds from the August 2022 Public Offering,
net of issuance costs was $23.9 million. The remaining cash provided by financing activities was from the 2020 ATM Facility. There was
no cash generated from financing activities during the nine months ended December 31, 2023.
CASH POSITION
At December 31, 2023, the Company had a cash and cash equivalents balance
of $21.8 million, compared to $33.6 million at March 31, 2023. The Company invests cash in excess of current operational requirements
in highly rated and liquid instruments. Working capital on December 31, 2023 was $19.1 million (March 31, 2023 - $32.6 million). These
funds are expected to provide the Company with sufficient capital to execute its current planned expenditures through the key milestones
associated with the ABILITY Study and into calendar Q2 2025 based on its current plans and projections.
The Company does not expect to generate positive cash flow from operations
for the foreseeable future due to additional R&D expenses, including expenses related to drug discovery, preclinical testing, clinical
trials, chemistry, manufacturing and controls and operating expenses associated with supporting these activities. It is expected that
negative cash flow from operations will continue until such time, if ever, that we receive marketing authorization to commercialize any
of our product candidates under development and/or royalty or milestone revenue from any such products should they exceed our expenses.
CONTRACTUAL OBLIGATIONS
CPRIT Assistance
In February 2015, the Company received notice that it had been awarded
a grant by the CPRIT whereby the Company was eligible to receive up to US$14.1 million on eligible expenditures over a three-year period
related to the development of the Company’s Phase 2b clinical program for bizaxofusp. As of March 31, 2022, all of the US$14.1 million
had been received and the grant with CPRIT was complete.
Under the terms of the grant, the Company is required to pay a royalty
to CPRIT, comprised of 3-5% of revenues on net sales of bizaxofusp until aggregate royalty payments equal 400% of the grant funds received
at which time the ongoing royalty will be 0.5% of revenues. At this time the royalty is not probable and therefore no liability has been
recorded. In addition, the Company must maintain a presence in Texas for three years following completion of the grant.
Refundable tax credits
In July 2023, the Company received $0.7 million through our Australian
R&D incentive program relating to the year ended March 31, 2023 (March 31, 2022: $0.7 million). The amount receivable was recorded
as a reduction in research and development expenses in the year ended March 31, 2023.
In September 2023, the Company received $0.1 million through the Canadian
scientific research and experimental development (SR&ED) relating to the year ended March 31, 2021. The Company received an additional
$0.2 million of SR&ED funding related to the year ended March 31, 2022 in January 2024. Amounts received for SRED are recorded as
a reduction in research and development expenses.
Intellectual Property
The Company has entered into various license agreements with respect
to accessing patented technology. In order to maintain these agreements, the Company is obligated to pay ongoing license fees, patent
prosecution and maintenance costs, royalty and other milestone payments, the timing of which is uncertain. As of December 31, 2023, the
Company is obligated to pay the following:
| · | Given the current development plans and expected timelines of the Company, it is assumed that project
milestones of US$1.2 million will be due in the next five years; and |
| · | As part of these license agreements, the Company has committed to make certain royalty payments based
on net sales to the FDA, NIH and Stanford. |
The Company also maintains a robust portfolio of intellectual property
assets that encompasses a diverse range of patents covering novel drug candidates, formulations, and therapeutic methods. Notably, the
Company was granted three new patents that further bolster the IP portfolio. These assets are integral to strategic initiatives, providing
exclusivity and market differentiation for groundbreaking treatments. The ongoing investment in IP development and enforcement reflects
the commitment to sustaining the leadership position and delivering value to shareholders and patients alike.
TRANSACTIONS WITH RELATED PARTIES
Key management personnel, which consists of the Company’s officers
(President and Chief Executive Officer, Chief Medical Officer, Chief Development Officer, former Chief Financial Officers, and former
Chief Business Officer) and directors, earned the following compensation for the following periods:
| | |
| Three months ended
December 31,
| | |
| Nine months ended
December 31,
|
| | |
| 2023 | | |
| 2022 | | |
| 2023 | | |
| 2022 | |
| | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| Salaries and wages | |
| 505 | | |
| 331 | | |
| 1,482 | | |
| 836 | |
| Board fees | |
| 76 | | |
| 95 | | |
| 253 | | |
| 247 | |
| Stock option expense | |
| 119 | | |
| 261 | | |
| 546 | | |
| 972 | |
| | |
| 700 | | |
| 687 | | |
| 2,281 | | |
| 2,055 | |
As at December 31, 2023, the Company had trade and other payables in
the normal course of business, owing to directors and officers of $0.2 million (2022 - $0.1 million) related to board fees and accrued
vacation.
OFF-BALANCE SHEET ARRANGEMENTS
The Company has no material undisclosed off-balance sheet arrangements
that have, or are reasonably likely to have, a current or future effect on our results of operations, financial condition, revenues or
expenses, liquidity, capital expenditures or capital resources that is material to investors.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The significant accounting policies of the Company are described in note 2
of the Annual Financial Statements and available on SEDAR+ at www.sedarplus.ca and included in the Annual Report on Form 20-F filed on
EDGAR at www.sec.gov.
Estimates and assumptions are continually evaluated and are based on
historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
The determination of estimates requires the exercise of judgement based on various assumptions and other factors such as historical experience
and current and expected economic conditions. Actual results could differ from those estimates. Critical judgements in applying the Company’s
accounting policies are detailed in the Annual Financial Statements, filed on SEDAR+ at www.sedarplus.ca and included in the Annual Report
on Form 20-F filed on EDGAR at www.sec.gov.
FINANCIAL INSTRUMENTS
Financial instruments are defined as a contractual right or obligation
to receive or deliver cash on another financial asset. The Company recognizes financial instruments based on their classification. Depending
on the financial instrument’s classification, changes in subsequent measurements are recognized in net loss or other comprehensive
loss.
A description of the financial instruments, their fair value and risk
management is included in notes 2, 5 and 6 of the Annual Financial Statements, filed on SEDAR+ at www.sedarplus.ca and included in the
Annual Report on Form 20-F filed on EDGAR at www.sec.gov/edgar.
2020 Public Offering and USE OF
PROCEEDS
The following table provides an update on the anticipated use of proceeds
raised as part of the 2020 public offering of Common Shares (the “2020 Public Offering”), which initially closed on March
17, 2020, along with amounts actually expended. Following completion of the 2020 Public Offering, Medicenna selected MDNA11 as its lead
IL-2 candidate over MDNA19 to progress to the clinic and, as such, proceeds from the 2020 Public Offering, which were initially allocated
to the development of MDNA19, have been re-directed to the development of MDNA11 in the same proportions. As of December 31, 2023, the
following expenditures had been incurred (in thousands of Canadian dollars):
| Item |
Amount to Spend
($) |
Spent to Date
($) |
Adjustments
($) |
Remaining to Spend
($) |
| Preclinical development |
3,300 |
3,300 |
- |
- |
| Manufacturing of clinical batch |
4,400 |
4,400 |
- |
- |
| Clinical development |
13,150 |
13,150 |
- |
- |
| General corporate and working capital purposes |
11,350 |
11,350 |
- |
- |
| Total |
32,200 |
32,200 |
- |
- |
2022 Public Offering and USE OF
PROCEEDS
The following table provides an update on the anticipated use of proceeds
raised under an underwritten public offering of units, with each unit consisting of one Common Share and one Common Share purchase warrant,
on August 11, 2022 (the “2022 Public Offering”), along with amounts actually spent. As of December 31, 2023, the following
expenditures had been incurred (in thousands of US dollars):
| Item |
Amount to Spend
($USD) |
Spent to Date
($USD) |
Adjustments
($USD) |
Remaining to Spend
($USD) |
| Phase 1/2 MDNA11 ABILITY Study |
8,000 |
722 |
- |
7,278 |
| General corporate purposes and pre-clinical development of a BiSKIT candidate |
8,000 |
2,128 |
- |
8,872 |
| Total |
16,000 |
2,850 |
- |
13,150 |
RISKS AND UNCERTAINTIES
The Company is an immunotherapy company that operates in a highly competitive
industry that is dependent on a number of factors that include the Company’s capacity to raise additional funding on reasonable
terms when necessary, secure partnerships for the development of its product candidates, obtain necessary regulatory approvals and achieve
market acceptance, face disruption in availability of key components for ongoing clinical studies, obtain positive results from pre-clinical
and clinical studies, successfully develop existing and new products, hire and retain skilled staff and key personnel, rely on third-party
providers, protect its intellectual property and face litigation risk in connection thereof. An investment in the Common Shares is subject
to a number of risks and uncertainties, including the risks related to the Company being a foreign private issuer.
In addition, the Company may, from time to time, announce or publish preliminary
or interim data from its clinical trials. Preliminary and interim data remains subject to audit and verification procedures that may result
in the final data being materially different from the preliminary or interim data. Preliminary and interim results of a clinical trial
are not necessarily predictive of final results. There can be no assurance that favorable interim or preliminary data will result in favorable
final data. Preliminary and interim data are subject to the risk that one or more of the clinical outcomes may materially change as patient
enrollment continues, patient data are further examined and reviewed, more patient data become available, and the Company prepares and
issues its final clinical study report. As a result, preliminary and interim data should be viewed with caution until the final, complete
data are available. Material adverse changes in the final data compared to the preliminary or interim data could significantly harm the
Company’s business, prospects, financial condition and results of operations.
An investor should carefully consider these risks, as well as the risks described
in the Company’s Annual Report on Form 20-F, as well as the other information filed with the securities regulators before investing
in the Common Shares. If any of such described risks occur, or if others occur, the Company’s business, financial condition and
the results of operations could be seriously harmed, and investors could lose all or part of their investment.
There are important risks which management believes could impact the Company’s
business. For information on risks and uncertainties, please also refer to the “Risk Factors” section of the Company’s
most recent Annual Report on Form 20-F filed on SEDAR+ at www.sedarplus.ca and EDGAR at www.sec.gov/edgar.
DISCLOSURE CONTROLS AND INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company has implemented a system of internal controls that it believes
adequately protects the assets of the Company and is appropriate for the nature of its business and the size of its operations. The internal
control system was designed to provide reasonable assurance that all transactions are accurately recorded, that transactions are recorded
as necessary to permit preparation of financial statements in accordance with IFRS and that our assets are safeguarded.
These internal controls include disclosure controls and procedures designed
to ensure that information required to be disclosed by the Company is accumulated and communicated as appropriate to allow timely decisions
regarding required disclosure.
Internal control over financial reporting means a process designed by
or under the supervision of the Chief Executive Officer and the Vice President of Finance to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as issued
by the IASB.
The internal controls are not expected to prevent and detect all misstatements
due to error or fraud. There were no changes in our internal control over financial reporting that occurred during the three and nine
months ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over
financial reporting.
As of December 31, 2023, the Company’s management assessed the
effectiveness of our internal control over financial reporting and disclosure controls and procedures using the Committee of Sponsoring
Organizations of the Treadway Commission’s 2013 framework. Based on their evaluation, the Chief Executive Officer and the Vice President
of Finance have concluded that these controls and procedures are effective.
OUTSTANDING SHARE DATA
As at the date of this report, the Company has the following securities
outstanding:
| |
|
| |
Number |
| Common Shares |
69,637,469 |
| Warrants |
16,185,386 |
| Stock options |
8,056,757 |
| Total |
92,561,979 |
For a detailed summary of the outstanding securities convertible into,
exercisable or exchangeable for voting or equity securities of Medicenna as at March 31, 2023, refer to notes 9, 10, and 11 of the Annual
Financial Statements of the Company.
ADDITIONAL INFORMATION
Additional information relating to the Company, including the Company’s
Annual Report on Form 20-F, is available under the Company’s profile on SEDAR+ at www.sedarplus.ca and EDGAR at www.sec.gov, respectively.
27
Exhibit 99.3
FORM 52-109F2
CERTIFICATION OF INTERIM FILINGS
FULL CERTIFICATE
I, Fahar Merchant, Chairman, President and Chief Executive Officer of Medicenna Therapeutics Corp. (“Medicenna”) certify the
following:
1. Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”)
of Medicenna (the “issuer”) for the interim period ended December 31, 2023.
2. No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain
any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement
not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings.
3. Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together
with the other financial information included in the interim filings fairly present in all material respects the financial condition,
financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings.
4. Responsibility: The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining
disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National
Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer.
5. Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying
officer(s) and I have, as at the end of the period covered by the interim filings
| A. | designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that |
| I. | material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings
are being prepared; and |
| II. | information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it
under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation;
and |
| B. | designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial
reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. |
5.1 Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s
ICFR is Internal Control – Integrated Framework (COSO 2013 Framework) issued by the Committee of Sponsoring Organizations of the
Treadway Commission.
5.2 ICFR – material weakness relating to design: N/A
5.3 Limitation on scope of design: N/A
6. Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that
occurred during the period beginning on October 1, 2023 and ended on December 31, 2023 that has materially affected, or is reasonably
likely to materially affect, the issuer’s ICFR.
Date: February 14, 2024
“Fahar Merchant”
Fahar Merchant
Chairman, President and Chief Executive Officer
Exhibit 99.4
FORM 52-109F2
CERTIFICATION OF INTERIM FILINGS
FULL CERTIFICATE
I, David Hyman, Chief Financial Officer of Medicenna Therapeutics Corp. (“Medicenna”) certify the following:
1. Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”)
of Medicenna (the “issuer”) for the interim period ended December 31, 2023.
2. No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain
any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement
not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings.
3. Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together
with the other financial information included in the interim filings fairly present in all material respects the financial condition,
financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings.
4. Responsibility: The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining
disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National
Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer.
5. Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying
officer(s) and I have, as at the end of the period covered by the interim filings
| A. | designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that |
| I. | material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings
are being prepared; and |
| II. | information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it
under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation;
and |
| B. | designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial
reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. |
5.1 Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s
ICFR is Internal Control – Integrated Framework (COSO 2013 Framework) issued by the Committee of Sponsoring Organizations of the
Treadway Commission.
5.2 ICFR – material weakness relating to design: N/A
5.3 Limitation on scope of design: N/A
6. Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that
occurred during the period beginning on July 1, 2023 and ended on September 30, 2023 that has materially affected, or is reasonably likely
to materially affect, the issuer’s ICFR.
Date: February 14, 2024
“David Hyman”
David Hyman
Chief Financial Officer
EXHIBIT 99.1
Medicenna Therapeutics Reports Third Quarter Fiscal 2024 Financial Results and Corporate Update
With the Response Rate increasing to 23%, MDNA11 continues to show compelling single-agent activity in the ABILITY-1 study amongst high-dose phase-2 eligible patients (N=13) that failed checkpoint inhibitor therapies, while maintaining an acceptable safety profile
Tumor shrinkage was also observed in all high-dose phase-2 eligible patients with stable disease (SD). In addition to a new PR, the clinical benefit rate (PR plus SD for over 24 weeks) and tumor control rate (PR plus all SD) increased to 46% and 69%, respectively
First patient was dosed in Phase 1 combination escalation portion of the ABILITY Study evaluating MDNA11 with KEYTRUDA® (pembrolizumab)
Additional monotherapy data updates and preliminary combination escalation and expansion data to be shared in H1 and H2 of 2024
Cash runway extended through multiple data readouts and into Q2 of calendar 2025
TORONTO and HOUSTON, Feb. 14, 2024 (GLOBE NEWSWIRE) -- Medicenna Therapeutics Corp. (“Medicenna” or the “Company”)(TSX: MDNA, OTCQB: MDNAF), a clinical-stage immunotherapy company focused on the development of Superkines, today announced financial results and corporate highlights for the third quarter of fiscal 2024, ended December 31, 2023, and outlined its strategic outlook for 2024.
“We are off to a solid start in 2024 with further validation of single-agent anti-tumor activity of our IL-2 super-agonist, MDNA11, in the ABILITY-1 study,” said Dr. Fahar Merchant, President and CEO of Medicenna. “We are encouraged by another PR, increasing the total number of PRs to 3 in patients with prior failure from immune checkpoint therapy. With response rates above 20% at this early stage of the trial in high-dose phase-2 eligible patients, we believe that MDNA11’s differentiated mechanism demonstrates its clinical superiority to competing IL-2 programs. This is especially gratifying in view of the desirable safety, pharmacokinetic and pharmacodynamic characteristics observed to date. We look forward to reporting additional monotherapy dose expansion and combination escalation and expansion data from the ABILITY-1 study during H1 and H2 2024.”
Dr. Merchant continued, “We believe 2024 will be a transformational year for Medicenna as we continue advancing our mid- and late-stage MDNA11 and bizaxofusp assets for patients battling difficult to treat cancers. The global multi-arm Phase 1/2 ABILITY-1 study is expanding to various cancer centers in the US, Canada, Australia, South Korea and Europe evaluating MDNA11 as a single-agent and in combination with KEYTRUDA® in patients with advanced solid tumors. For bizaxofusp, we plan to seek Breakthrough Therapy Designation from the FDA, in addition to securing alignment with the EMA for the Phase 3 trial design supported by the FDA. These endeavors will further enhance potential partnership activities for bizaxofusp. We are excited for the year ahead and are increasingly confident about the significant potential of our evolutionary cytokines and delivering on our mission to bring revolutionary medicines to patients.”
PROGRAM AND BUSINESS UPDATE:
Highlights for the three months ended December 31, 2023, along with recent developments include:
MDNA11
- New additional iPR in the monotherapy dose expansion portion of the MDNA11 ABILITY-1 Study. Today, the Company reports promising clinical data from the on-going monotherapy escalation and expansion arms of the ABILITY-1 study. In addition to previously announced tumor response data, a third patient in the study has also shown a partial response (PR). A melanoma patient with iPR (PR as per iRECIST) showed pseudo-progression (at week 8) with a PR (at week 12).
- Response rate exceeds 20% in immune checkpoint failed patients. Amongst 13 patients, all having previously failed or resistant to immune checkpoint inhibitors ("ICI"), receiving high doses of MDNA11 (≥60 µg/kg) with tumor types being evaluated in the monotherapy expansion cohort, the response rate, clinical benefit rate and tumor control rate increased to 23% (3 partial responses), 46% (3 PRs and 3 patients with SD for ≥ 24 weeks) and 69% (3 PRs and 6 SDs), respectively, with shrinkage of target lesions in all patients with SD.
- Dosed first patient in the combination arm of the ABILITY-1 study. On February 13, 2024, the Company announced that it had dosed the first patient in the combination arm of the ABILITY-1 clinical trial, evaluating potential synergistic effect of MDNA11 when administered with KEYTRUDA® (pembrolizumab). The study will evaluate the safety, tolerability, recommended combination dose for expansion ("cRDE") and therapeutic activity of MDNA11 when combined with KEYTRUDA® in the dose-escalation and dose-expansion arms of the clinical trial.
- Presented positive MDNA11 monotherapy data at SITC 2023. On November 6, 2023, Medicenna announced encouraging single-agent activity from the dose escalation and evaluation portion of the ABILITY-1 study in advanced cancer patients receiving MDNA11 at doses of ≥60 µg/kg (N = 15) who had previously failed ICI therapies. The results included ongoing partial responses with 100% and 70% reduction of target lesions in pancreatic and melanoma cancer patients, respectively, in addition to durable stable disease in 3 melanoma patients (> 20 to 80 weeks). This data was presented at the 38th Annual Meeting of the Society for Immunotherapy of Cancer ("SITC") held in San Diego.
- On October 25, 2023, Medicenna announced dosing of the first patient in the Phase 2 monotherapy dose expansion portion of the ABILITY-1 Study.
Bizaxofusp (MDNA55)
- Single treatment with bizaxofusp increased median overall survival (mOS) by 100%. On November 17, 2023, the Company announced a poster presentation and an oral summary at the Annual Meeting of the Society of Neuro-Oncology (SNO) highlighting longer term follow up results from the Phase 2b clinical trial of bizaxofusp in patients with recurrent glioblastoma (rGBM). The data demonstrated that a single treatment with bizaxofusp increased median overall survival (mOS) by 100% (14.5 vs. 7.2 months) when compared to a propensity matched external control arm (ECA) irrespective of IL-4R (interleukin-4 receptor) expression and defined as the Phase 3 population. Overall survival (OS) for bizaxofusp-treated patients increased by 370% at Year 1 (62.5% vs 16.7%) and by more than 50% at Year 2 (25% vs 16.1%) when compared to the ECA. No systemic or clinically significant laboratory abnormalities were reported. Treatment-related adverse events were primarily neurological or aggravation of pre-existing neurological deficits due to rGBM.
- Potential for Breakthrough Therapy Designation (BTD) for bizaxofusp. With compelling longer term survival benefit with bizaxofusp in rGBM patients, as presented at the SNO meeting held in November 2023, Medicenna will seek to apply for BTD with the FDA.
- Seek alignment with the European Medicines Agency (“EMA”) for the Phase 3 registration trial of bizaxofusp incorporating an ECA and developed with the support of FDA. The proposed Phase 3 trial design incorporating a hybrid external control arm has been supported by the FDA. Medicenna is currently working toward securing alignment with the EMA thereby enabling data from a single Phase 3 registrational trial being sufficient to file for approval in the EU and USA.
Other Pipeline Programs
- On November 3, 2023, the Company presented preclinical data on its first-in-class IL-13Rα2 targeted candidate, MDNA113, from its T-MASKTM platform, which specifically delivers a masked bispecific anti-PD1-IL2 Superkine to IL-13Rα2 expressing tumors (affecting over 2 million cancer patients annually) where it is activated by cancer specific enzymes. This data was presented at the 38th Annual Meeting of the SITC held in San Diego.
- On October 3, 2023, new preclinical data characterizing MDNA223, an anti-PD1-IL-2 BiSKIT (Bifunctional SuperKine for ImmunoTherapy), including its synergy when combined with STING agonists were presented at the 2023 AACR Special Conference in Cancer Research: Tumor Immunology and Immunotherapy, held in Toronto, Canada.
Corporate Highlights
- Transition of CMO to consultant role - Dr. Humphrey Gardner has transitioned from Chief Medical Officer to a consulting role. Dr. Arash Yavari, Chair of Medicenna’s Development Advisory Committee, will henceforth lead the clinical activities as Director of Clinical Strategy.
- Appointment of new CFO – David Hyman has been appointed as Chief Financial Officer of the Company. David Hyman, CA, CBV is an experienced financial professional with over 25 years of experience spanning public practice, capital markets, private equity and industry. For the past five years, Mr. Hyman has provided fractional and full time CFO services to multiple public and private companies, including two early-stage pharmaceutical companies.
- Appointment of new Auditor – On January 12, 2024, the Company announced that its Board of Directors approved the appointment of MNP LP as the auditor of the Company.
- OTCQB Listing - On December 19, 2023, the Company announced the commencement of trading on the OTCQB Venture Market in the United States.
- NASDAQ delisting - On October 27, 2023, Medicenna announced that it was delisted from the Nasdaq as the Company did not meet the listing requirements and that it was reducing its presence in the US to conserve cash. The Company's common shares continue to trade on the Toronto Stock Exchange.
Expected Upcoming Milestones
- Preliminary monotherapy and dose expansion data of MDNA11’s ABILITY-1 study, to be presented at medical conferences in H1 and H2 of 2024.
- Clinical update from the combination arm of the ABILITY-1 study evaluating MDNA11 in with KEYTRUDA® expected in H1 and H2 of 2024.
Financial Results
As of December 31, 2023, cash and cash equivalents were $21.8 million, compared to $25.7 million on September 30, 2023.
Net loss for the quarter ended December 31, 2023, was $5.0 million or ($0.07) per share compared to a net loss of $1.1 million or ($0.02) per share for the quarter ended December 31, 2022. The increase was primarily a result of a reduction in the non-cash gain related to the change in valuation of the Company’s derivative warrant liability.
Research and development expenses of $3.0 million were incurred during the quarter ended December 31, 2023, compared with $2.9 million incurred in the quarter ended December 31, 2022. Higher clinical costs associated with the MDNA11 ABILITY-1 study relative to the prior year quarter were mostly offset by reductions in stock-based compensation and licensing fees.
General and administrative expenses of $1.8 million were incurred during the quarter ended December 31, 2023, compared with $2.0 million during the quarter ended December 31, 2022. The decrease in general and administrative expenses in the current quarter is primarily related to a reduction in directors’ and officers’ liability insurance premiums.
Medicenna’s interim consolidated financial statements for the three and nine months ended December 31, 2023, and the related management’s discussion and analysis (MD&A) are available on SEDAR+ at www.sedarplus.ca and EDGAR.
About Medicenna Therapeutics
Medicenna is a clinical-stage immunotherapy company focused on developing novel, highly selective versions of IL-2, IL-4 and IL-13 Superkines and first-in-class empowered superkines. Medicenna’s long-acting IL-2 Superkine, MDNA11, is a next-generation IL-2 with superior affinity toward CD122 (IL-2 receptor beta) and no CD25 (IL-2 receptor alpha) binding, thereby preferentially stimulating cancer-killing effector T cells and NK cells. Medicenna’s IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55), has been studied in 5 clinical trials enrolling over 130 patients, including a Phase 2b trial for recurrent GBM, the most common and uniformly fatal form of brain cancer. Bizaxofusp has obtained FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively. Medicenna’s early-stage BiSKITs™ (Bifunctional SuperKine ImmunoTherapies) and the T-MASK™ (Targeted Metalloprotease Activated SuperKine) programs are designed to enhance the ability of Superkines to treat immunologically “cold” tumors.
For more information, please visit www.medicenna.com, and follow us on Twitter and LinkedIn.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of applicable securities laws that relate to the future operations of the Company, plans and projections and other statements that are not historical facts, including, without limitation, statements on the Company’s cash runway, clinical development activities, clinical potential, safety profiles and upcoming milestones and data reporting, including with respect to MDNA11, the ABILITY study and its expansion, bizaxofusp (MDNA55), MDNA113, MDNA223, partnership activities and opportunities and strategic outlook. Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expect”, “believe”, “seek”, “potentially” and similar expressions. and are subject to risks and uncertainties. There can be no assurance that such statements will prove to be accurate and actual results and future events could differ materially from those anticipated in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the risks detailed in the latest Annual Report on Form 20-F of the Company and in other filings made by the Company with the applicable securities regulators from time to time in Canada.
The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management, may prove to be incorrect and actual results may differ materially from those anticipated. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date hereof and except as required by law, we do not intend and do not assume any obligation to update or revise publicly any of the included forward-looking statements.
Investor and Media Contact:
Christina Cameron
Investor Relations, Medicenna Therapeutics
ir@medicenna.com
(647) 953-0673
Medicenna Therapeutics (NASDAQ:MDNA)
Historical Stock Chart
From Jan 2025 to Feb 2025
Medicenna Therapeutics (NASDAQ:MDNA)
Historical Stock Chart
From Feb 2024 to Feb 2025