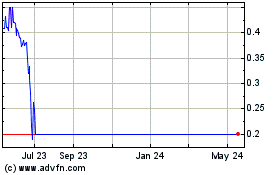
Nymox Delisting from NASDAQ
July 05 2023 - 5:11PM

Nymox Pharmaceutical Corporation (NASDAQ: NYMX) (the “Company”)
today received a Nasdaq Hearing Delist Decision, noting that Nymox
has not regained the required $1.00 share price within the 6-month
extension period granted by Nasdaq, and hence the Company's shares
will be suspended from trading on NASDAQ at the open of business on
July 7, 2023. Nymox shares will be moved to the OTC market. The
mechanics of trading the stock remain the same, as do the Company's
business fundamentals.
Nymox is in the process of submitting
applications for the approval to market the Company's first in
class drug NYMOZARFEX to treat the symptoms of benign prostatic
hyperplasia (BPH). BPH is one of the most common conditions
affecting middle aged and elderly men throughout the world. BPH can
be devastating to men who suffer from the condition. Current
treatments are associated with numerous intolerable side effects
including sexual problems, such as impotence and retrograde
ejaculation. Medications for BPH have been associated with prostate
cancer, depression, gynecomastia and other adverse effects. The
majority of men stop taking the available medications due to these
and other problems. Surgery is often needed for advanced BPH.
Surgery is usually effective but it is not without risks, the
discomforts of surgery, and BPH surgery has side effects such as
permanent retrograde ejaculation for many patients.
Nymozarfex was submitted to the Danish
authorities in December 2022 and the application was accepted for
review in early 2023 and is currently undergoing review. The
Company announced that another submission was expected to be
submitted for approval in another jurisdiction in the second
quarter of 2023. It is expected to be submitted in the near future
and the Company will provide the appropriate update when that
occurs.
Dr. Paul Averback, CEO of Nymox said, "This is a
key time for the Company, with more than one important marketing
application being submitted. We are grateful to our loyal
shareholders for their patience and we wish to assure our
supporters that we are doing our best to deliver important
milestones in our business development, in as reasonable a time
horizon as possible. It took a long time to get to this point but
we believe enhanced fundamental value has been developed -- and we
intend to continue to do everything in our power to deliver the
goods to the public and to our stakeholders."
About NYMOZARFEX (TM) (Fexapotide)
NYMOZARFEX (TM) is given in an in-office
procedure that is administered in a few minutes without need of
anesthesia or analgesia. The drug has been tested in clinical
trials involving overall more than 1750 BPH patients with over 1600
injections administered including over 1200 Fexapotide
administrations. Fexapotide has led to significant long-term
improvements and has shown an excellent safety profile without the
side effects normally associated with existing BPH treatments.
For more information please contact
info@nymox.com or 800-936-9669.
Forward Looking Statements
To the extent that statements contained in this
press release are not descriptions of historical facts regarding
Nymox, they are forward-looking statements reflecting the current
beliefs and expectations of management made pursuant to the safe
harbor provisions of the Private Securities Litigation Reform Act
of 1995, including statements regarding the need for new options to
treat BPH and prostate cancer, the potential of Fexapotide to treat
BPH and prostate cancer and the estimated timing of further
developments for Fexapotide. Such forward-looking statements
involve substantial risks and uncertainties that could cause our
clinical development program, future results, performance or
achievements to differ significantly from those expressed or
implied by the forward-looking statements. Such risks and
uncertainties include, among others, the uncertainties inherent in
the clinical drug development process, including the regulatory
approval process, the timing of Nymox's regulatory filings, Nymox's
substantial dependence on Fexapotide, Nymox's commercialization
plans and efforts and other matters that could affect the
availability or commercial potential of Fexapotide. Nymox
undertakes no obligation to update or revise any forward looking
statements. For a further description of the risks and
uncertainties that could cause actual results to differ from those
expressed in these forward-looking statements, as well as risks
relating to the business of Nymox in general, see Nymox's current
and future reports filed with the U.S. Securities and Exchange
Commission, including its Annual Report on Form 20-F for the year
ended December 31, 2022, and its Quarterly Reports.
For Further
Information Contact:Nymox
Pharmaceutical Corporation 1-800-93NYMOXwww.nymox.com
Nymox Pharmaceutical (NASDAQ:NYMX)
Historical Stock Chart
From Dec 2024 to Jan 2025
Nymox Pharmaceutical (NASDAQ:NYMX)
Historical Stock Chart
From Jan 2024 to Jan 2025