SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-163
UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For
the month of November 2023
Alterity
Therapeutics Limited
(Name
of Registrant)
Level 14, 350 Collins Street,
Melbourne, Victoria 3000 Australia
(Address
of Principal Executive Office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
This
Form 6-K is being incorporated by reference into our Registration Statement on Form S-8 (Files No. 333-251073, 333-248980
and 333-228671) and our
Registration Statements on Form F-3 (Files No. 333-274816, 333-251647, 333-231417
and 333-250076)
ALTERITY
THERAPEUTICS LIMITED
(a
development stage enterprise)
The
following exhibits are submitted:
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
Alterity Therapeutics Limited |
| |
|
| |
By: |
/s/ Geoffrey P. Kempler |
| |
|
Geoffrey P. Kempler |
| |
|
Chairman |
Date:
November 22, 2023
2
Exhibit 99.1

Alterity Therapeutics (NASDAQ:ATHE, ASX:ATH) D av i d S ta ml e r , M D CEO No ve mb e r 2023

Forward Looking Statements This presentation may contain some statements that may be considered “Forward - Looking Statements”, within the meaning of the US Securities Laws . Thus, any forward - looking statement relating to financial projections or other statements relating to the Company’s plans, objectives, expectations or intentions involve risks and uncertainties that may cause actual results to differ materially . For a discussion of such risks and uncertainties as they relate to us, please refer to our 2023 Form 20 - F, filed with US Securities and Exchange Commission, in particular Item 3 , Section D, titled “Risk Factors . ’’ 2

Alterity is dedicated to creating an alternate future for people living with neurodegenerative diseases. Alterity means the state of being different Our goal is to modify the course of disease We’re here to disrupt the trajectory of illness and improve quality of life 3

• Developing disease modifying therapies • ATH434: Novel drug candidate targeting proteins implicated in neurodegeneration of Parkinson’s disease and related disorders • First indication: Multiple System Atrophy (MSA), a parkinsonian disorder with no approved treatment • Orphan Drug designation for MSA in the US and EU • Phase 2 program ongoing • Randomized, double blind study in early - stage MSA • Biomarker trial in more advanced MSA • Strong patent portfolio • Significant R&D experience including 3 neurology drug approvals by FDA 4 Investment Highlights

Experienced Leadership Team with Multiple F DA A pp r o va l s i n Ne u r ology 5 Margaret Bradbury, Ph.D. VP, Nonclinical Development Auspex/Teva | Neurocrine | Merck • Auspex - led strategic planning and program management in Huntington Disease chorea from IND through NDA filing • Teva - led non - clinical development of several neuroscience programs Kathryn Andrews, CPA Chief Financial Officer Antisense Therapeutics | Rio Tinto | Consultant • Extensive experience advising private and public CFOs, mainly in the biotechnology sector • Prior CFO and Company Secretary of Antisense Therapeutics Limited • 15+ years in finance and accounting roles at Rio Tinto Limited and BP Australia Limited David Stamler, M.D. Chief Executive Officer Auspex/Teva | Abbott | Prestwick Xenoport | Fujisawa • 3 FD A App r ova ls in Neu r o l ogy • Fo rm e r C MO, Auspex • VP, Clinical Development & Therapeutic Head, Movement Disorders, Teva Pharmaceuticals • Part of Teva’s US$3.5 billion acqui s i t io n o f Auspe x i n 2015 • Led development of AUSTEDO ® (deutetrabenazine) for treatment of Huntington disease and Tardive dyskinesia, both approved in 2017 Cynthia Wong, M.P.H . Senior Director, Clinical Operations Auspex/Teva | Nextwave | Astex | Intermune | Impax Labs • Clinical Operations leadership at Auspex/Teva. • Led clinical trial activities for the registration study of AUSTEDO ® in Huntington Disease chorea. • Prior, led Phase 1 - 3 studies, including registration studies for marketing approval for Quillichew ER, Esbriet and Infergen.
Parkinsonian Disorders: A Significant Unmet Need 6 Current therapies treat the symptoms and NOT the underlying pathology of disease P A R KINS O N ’ S DISEASE MS A PSP PARKINSONIAN DISORDERS • Parkinsonism is a syndrome of motor symptoms that includes slowed movement, stiffness and tremor • Parkinson’s disease most common cause • Major source of disability • Parkinsonian disorders include Multiple system atrophy (MSA) and Progressive supranuclear palsy (PSP) • Prominent non - motor symptoms • Limited response to available treatments
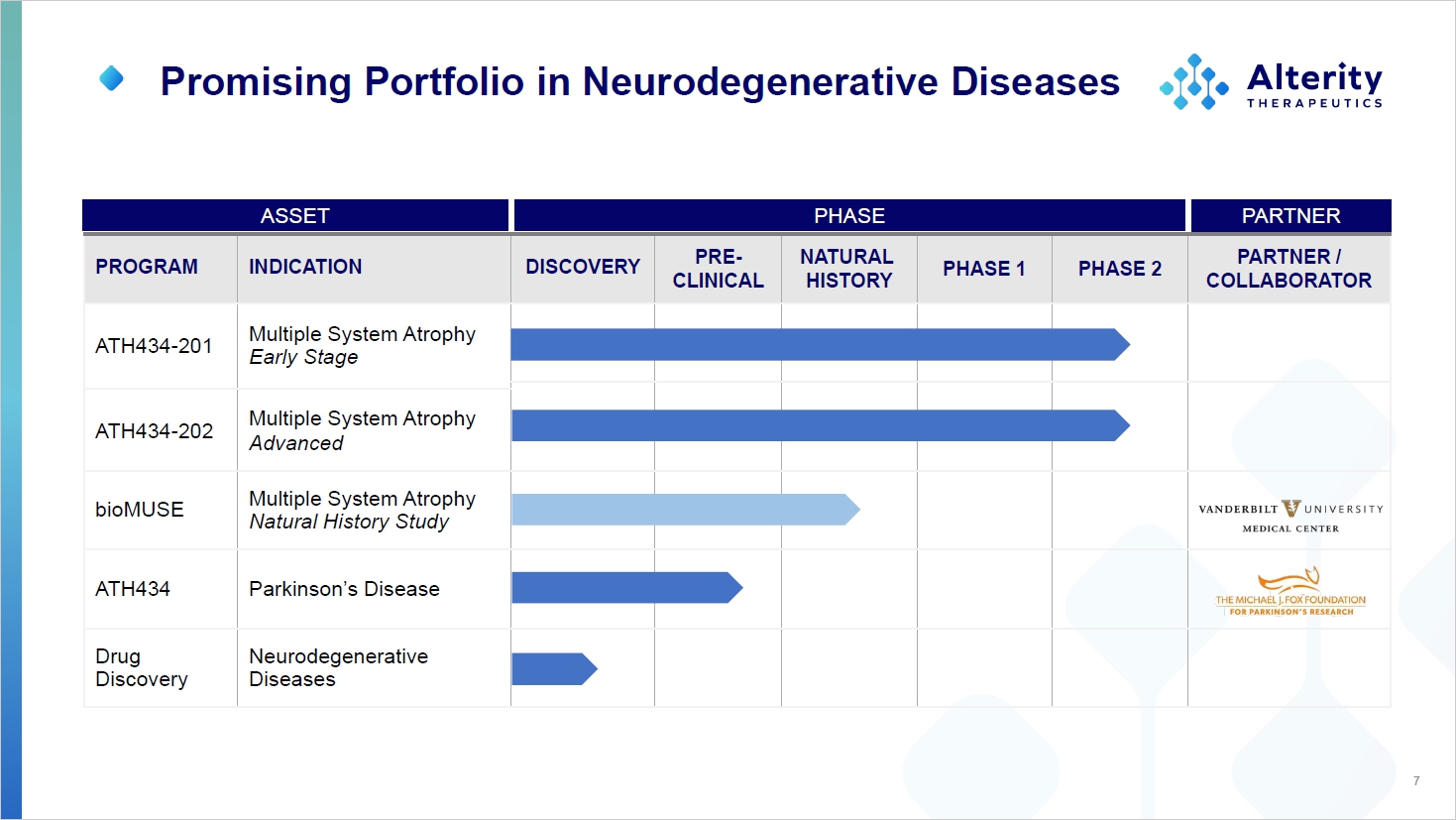
P A R TNER 7 PARTNER PHASE ASSET PARTNER / COLLABOR A T OR PHASE 2 PHASE 1 N A TURAL HISTORY PRE - CL I N I CAL DISCOVERY INDICATION PROGRAM M ultiple S ystem A trophy Early Stage ATH434 - 201 M ultiple S ystem A trophy Advanced ATH434 - 202 M ultiple S ystem A trophy Natural History Study bioMUSE Parkinson’s Disease ATH434 N eurod egener a t ive Diseases Drug D iscovery Promising Portfolio in Neurodegenerative Diseases

The Role of Alpha - Synuclein and Iron in Parkinsonian Disorders 8
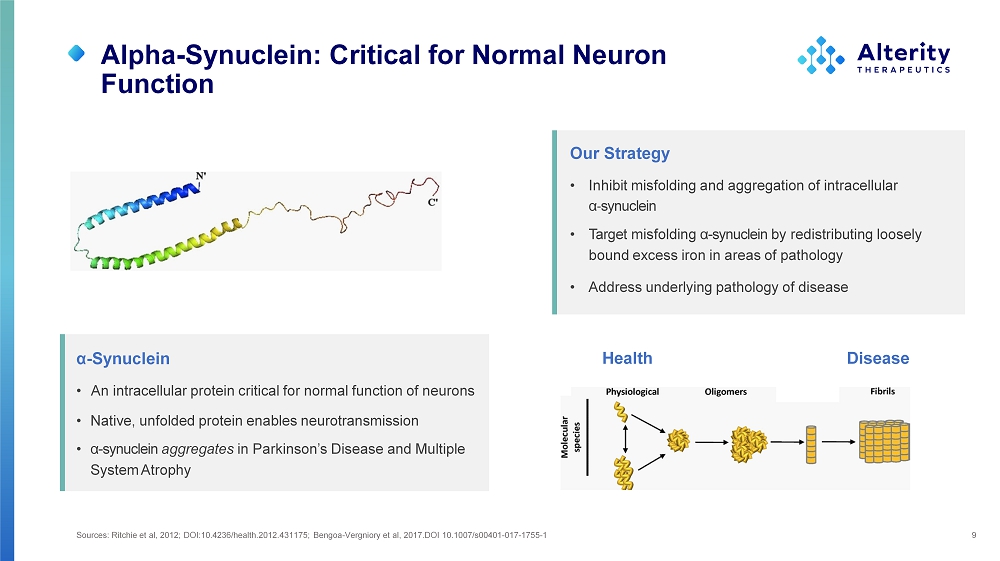
Alpha - Synuclein: Critical for Normal Neuron Function Sources: Ritchie et al, 2012; DOI:10.4236/health.2012.431175; Bengoa - Vergniory et al, 2017.DOI 10.1007/s00401 - 017 - 1755 - 1 9 α - Synuclein • An intracellular protein critical for normal function of neurons • Native, unfolded protein enables neurotransmission • α - synuclein aggregates in Parkinson’s Disease and Multiple System Atrophy Our Strategy • Inhibit misfolding and aggregation of intracellular α - synuclein • Target misfolding α - synuclein by redistributing loosely bound excess iron in areas of pathology • Address underlying pathology of disease H eal th D isease
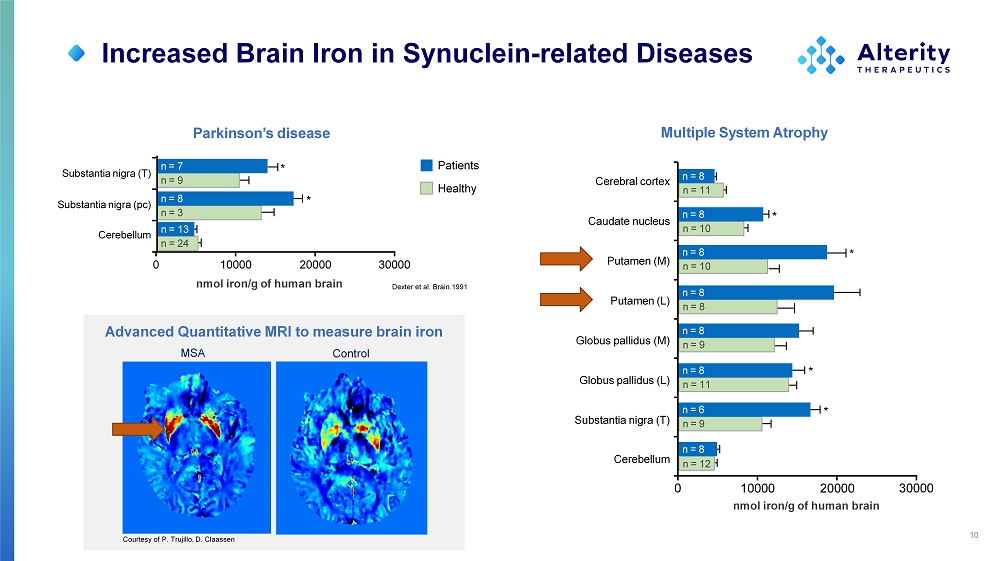
Increased Brain Iron in Synuclein - related Diseases 10 Dexter et al. Brain.1991 Parkinson’s disease Multiple System Atrophy Courtesy of P. Trujillo, D. Claassen n = 24 n = 13 n = 9 n = 7 * n = 3 n = 8 * 10000 20000 nmol iron/g of human brain 0 30000 Cerebral cortex Caudate nucleus Putamen (M) Putamen (L) Globus pallidus (M) Globus pallidus (L) Substantia nigra (T) Cerebellum n = 11 n = 8 n = 8 n = 8 n = 9 n = 8 n = 11 n = 8 * n = 9 n = 8 n = 12 n = 6 * 0 30000 n = 10 n = 8 * n = 10 n = 8 * 10000 20000 nmol iron/g of human brain Patients Healthy Substantia nigra (T) Substantia nigra (pc) Cerebellum Advanced Quantitative MRI to measure brain iron MSA Control
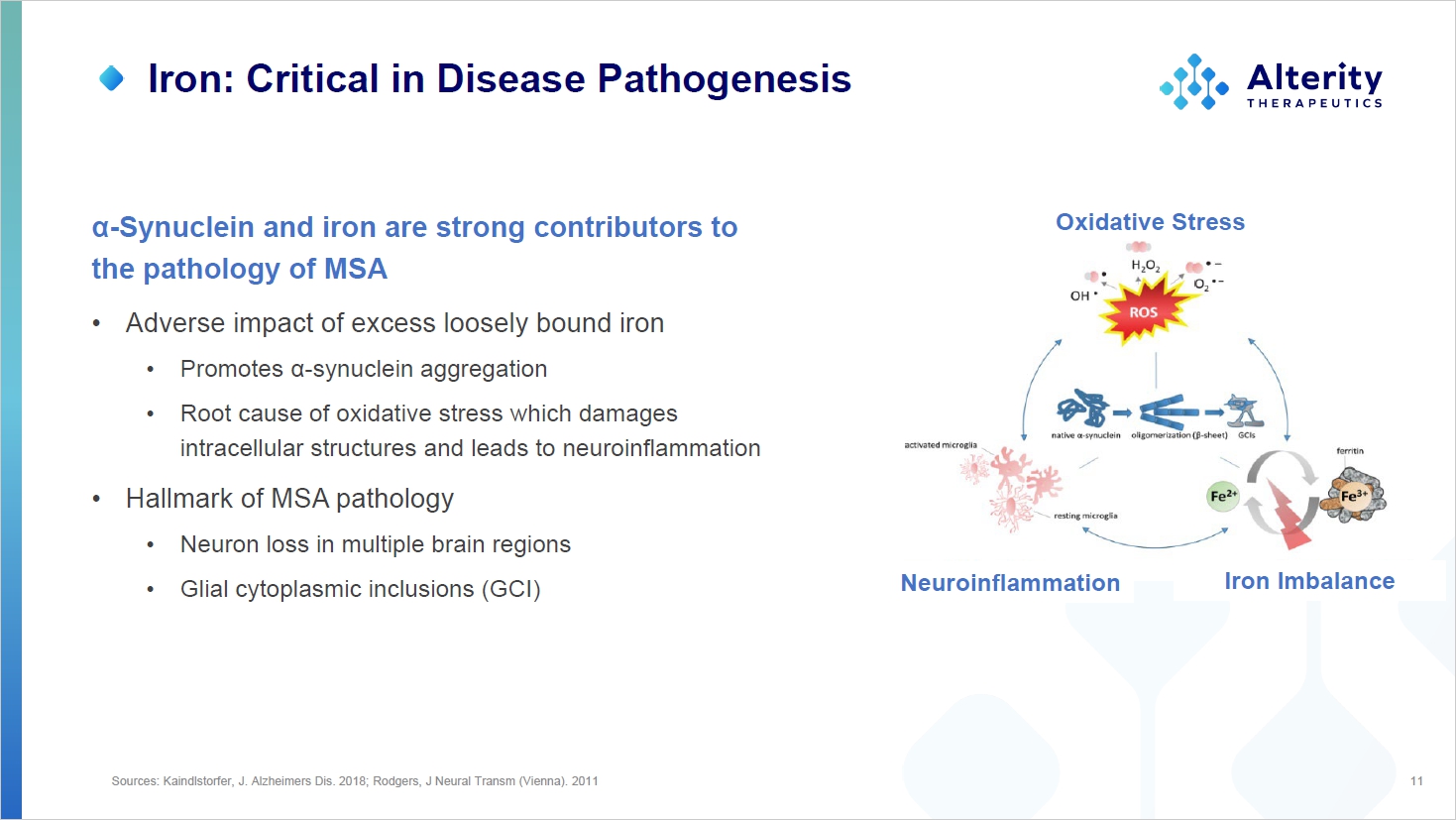
Sources: Kaindlstorfer, J. Alzheimers Dis. 2018; Rodgers, J Neural Transm (Vienna). 2011 11 Oxidative Stress Neuroinflammation Iron Imbalance Iron: Critical in Disease Pathogenesis α - Synuclein and iron are strong contributors to the pathology of MSA • Adverse impact of excess loosely bound iron • Promotes α - synuclein aggregation • Root cause of oxidative stress which damages intracellular structures and leads to neuroinflammation • Hallmark of MSA pathology • Neuron loss in multiple brain regions • Glial cytoplasmic inclusions (GCI)

Approach: Address Underlying Pathology of Disease 12 Redistribute excess iron in the Central Nervous System Reduce α - synuclein aggregation and Oxidative stress Rescue neuronal function Potential Disease Modifying Therapy for MSA

ATH434: Disease Modifying Drug Candidate 13
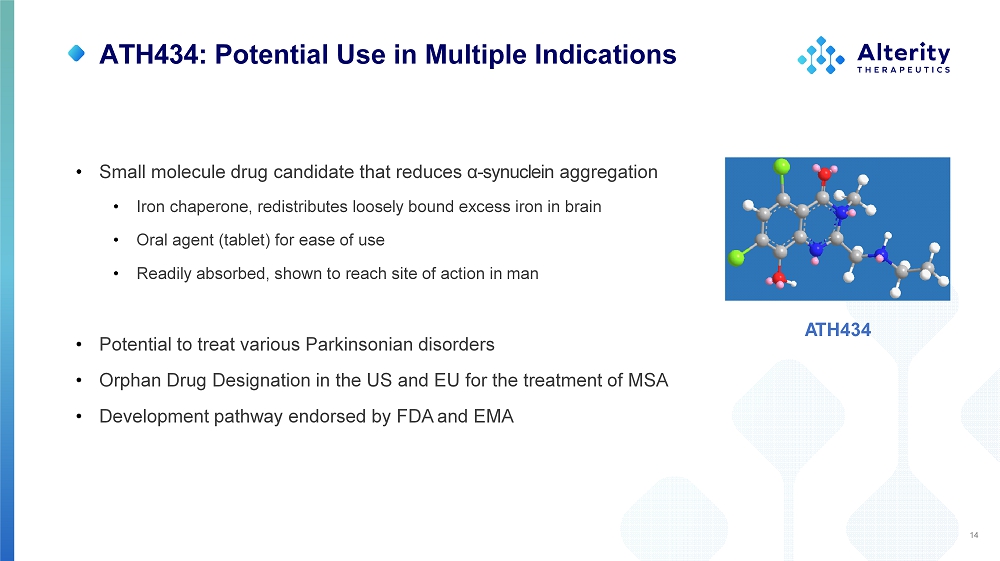
ATH434: Potential Use in Multiple Indications 14 A T H434 • Small molecule drug candidate that reduces α - synuclein aggregation • Iron chaperone, redistributes loosely bound excess iron in brain • Oral agent (tablet) for ease of use • Readily absorbed, shown to reach site of action in man • Potential to treat various Parkinsonian disorders • Orphan Drug Designation in the US and EU for the treatment of MSA • Development pathway endorsed by FDA and EMA
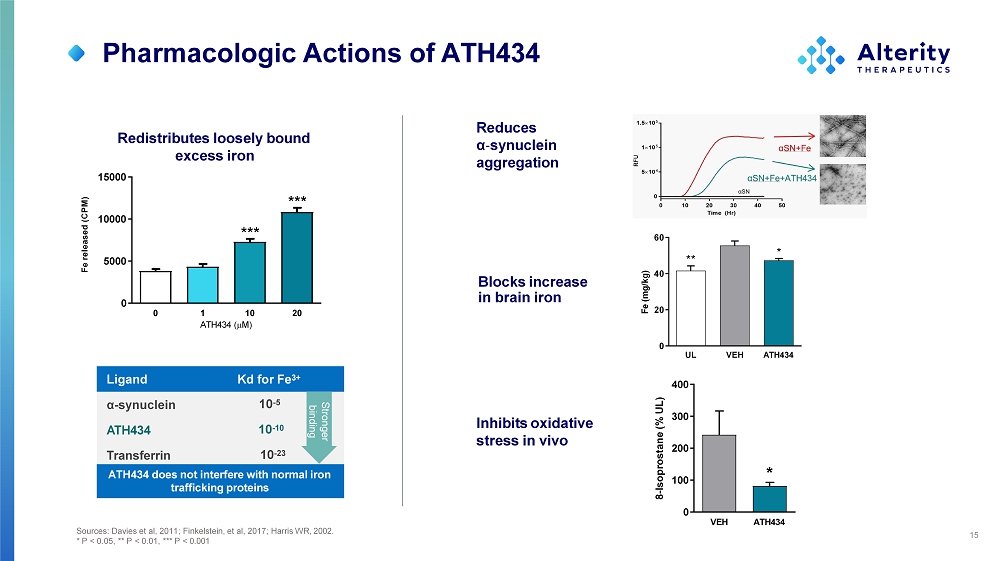
15 Reduces α - synuclein a gg re g a t i o n Blocks increase in brain iron Inhibits oxidative stress in vivo 0 1 10 A T H 4 3 4 ( M ) 20 0 50 0 0 100 0 0 150 0 0 Fe released (CPM) *** *** Redistributes loosely bound excess iron L i gand Kd for Fe 3+ α - synuclein ATH434 Transferrin 10 - 5 10 - 10 10 - 23 ATH434 does not interfere with normal iron trafficking proteins UL V E H A TH434 0 20 40 60 F e ( m g /k g) * ** St ron g e r binding V EH ATH4 3 4 0 1 00 2 00 3 00 4 00 8 - Isoprostane (% UL) * Sources: Davies et al, 2011; Finkelstein, et al, 2017; Harris WR, 2002. * P < 0.05, ** P < 0.01, *** P < 0.001 Pharmacologic Actions of ATH434
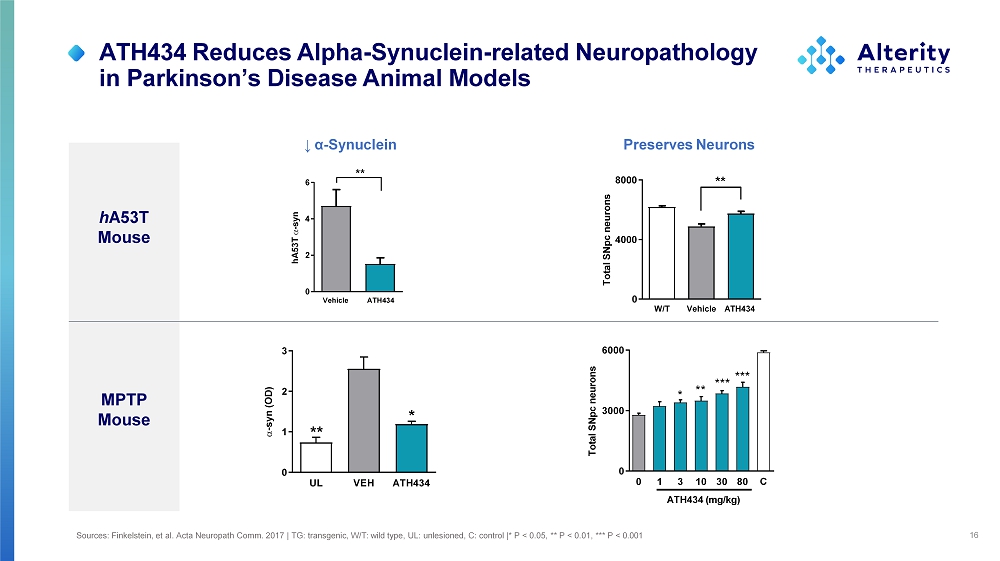
MPTP Mouse hA53T - syn V e h icl e ATH 4 3 4 0 2 4 6 ** ↓ α - Synuclein h A53T Mouse Preserves Neurons Total SNpc neurons W /T Vehicle ATH434 0 40 0 0 80 0 0 ** UL VEH ATH434 0 1 2 3 ** * - syn (OD) 0 1 3 10 30 80 C 0 30 0 0 60 0 0 Total SNpc neurons ATH434 (mg/kg) *** *** ** * ATH434 Reduces Alpha - Synuclein - related Neuropathology in Parkinson’s Disease Animal Models 16 Sources: Finkelstein, et al. Acta Neuropath Comm. 2017 | TG: transgenic, W/T: wild type, UL: unlesioned, C: control |* P < 0.05, ** P < 0.01, *** P < 0.001
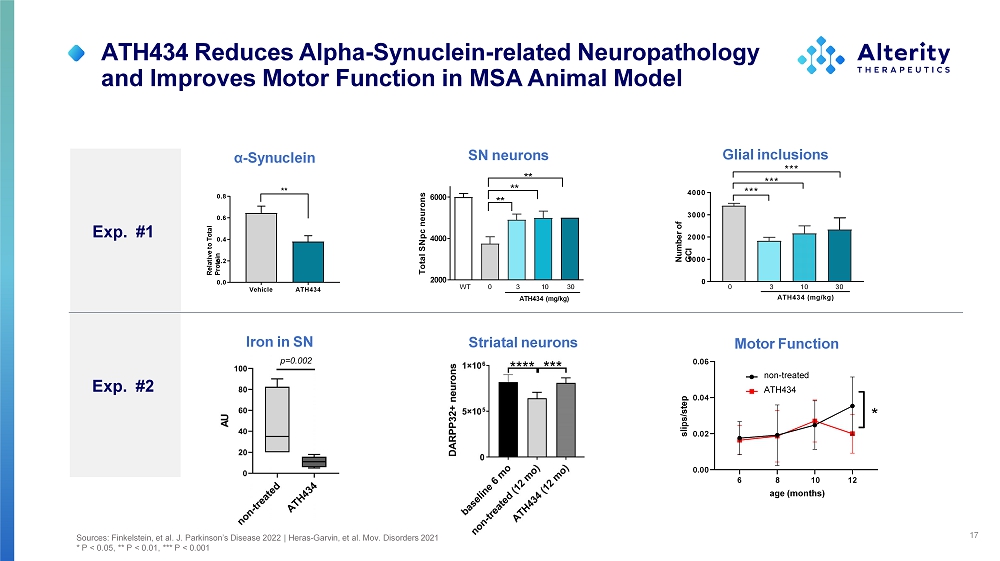
ATH434 Reduces Alpha - Synuclein - related Neuropathology and Improves Motor Function in MSA Animal Model 17 Vehicle A TH434 0 . 0 0 . 2 0 . 4 0 . 6 0 . 8 Re l a t i v e t o To t a l P r o t e i n ** α - S y nu clein SN neurons 20 0 0 40 0 0 60 0 0 Total SNpc neurons ** ** ** 0 3 10 30 WT A T H 43 4 ( m g /k g ) Glial inclusions 0 10 0 0 20 0 0 30 0 0 40 0 0 Numb e r o f G C I *** *** *** 0 3 10 30 ATH434 (mg/kg) Exp. #1 Striatal neurons Iron in SN Exp. #2 Sources: Finkelstein, et al. J. Parkinson’s Disease 2022 | Heras - Garvin, et al. Mov. Disorders 2021 * P < 0.05, ** P < 0.01, *** P < 0.001 Motor Function 6 12 0 . 0 0 0 . 0 2 0 . 0 4 0 . 0 6 8 10 age (months) sli p s /s t e p n o n - t r eat ed ATH434 *

Multiple System Atrophy Clinical Development Program 18
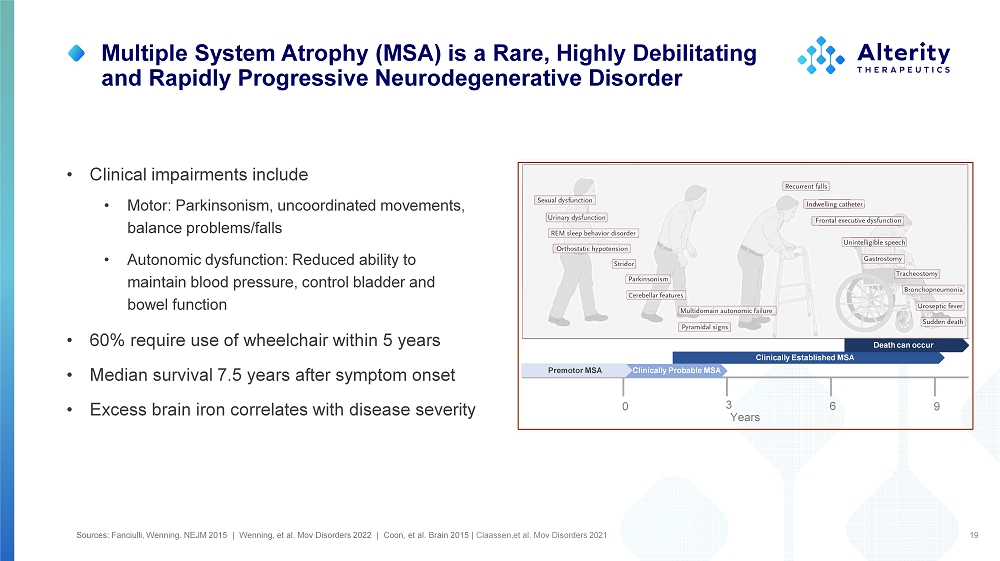
Multiple System Atrophy (MSA) is a Rare, Highly Debilitating and Rapidly Progressive Neurodegenerative Disorder 19 • Clinical impairments include • Motor: Parkinsonism, uncoordinated movements, balance problems/falls • Autonomic dysfunction: Reduced ability to maintain blood pressure, control bladder and bowel function • 60% require use of wheelchair within 5 years • Median survival 7.5 years after symptom onset • Excess brain iron correlates with disease severity 0 6 9 3 Years P re mo t o r MSA Clinically Established MSA Sources: Fanciulli, Wenning. NEJM 2015 | Wenning, et al. Mov Disorders 2022 | Coon, et al. Brain 2015 | Claassen,et al. Mov Disorders 2021 Death can occur Clinically Probable MSA
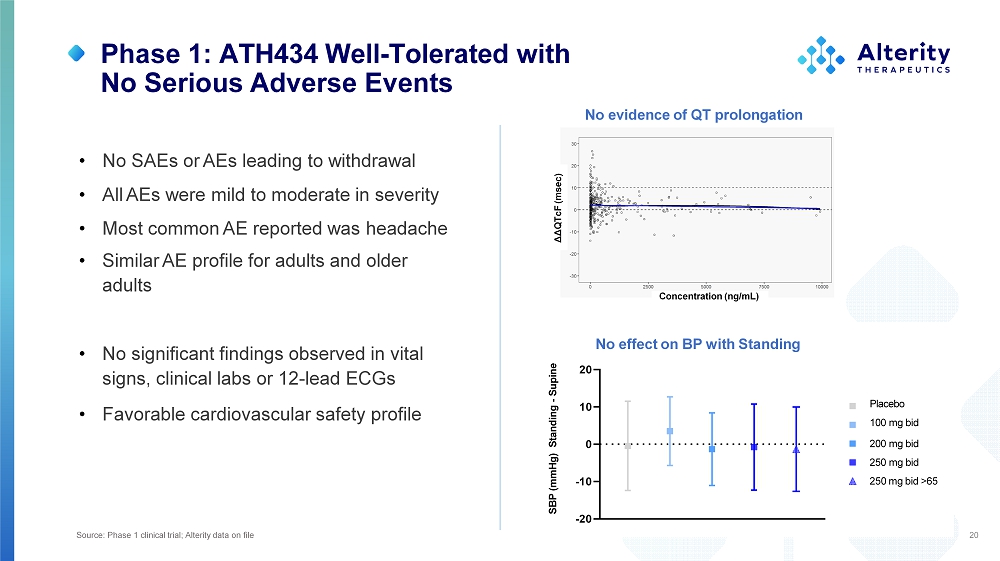
Phase 1: ATH434 Well - Tolerated with No Serious Adverse Events • No SAEs or AEs leading to withdrawal • All AEs were mild to moderate in severity • Most common AE reported was headache • Similar AE profile for adults and older adults • No significant findings observed in vital signs, clinical labs or 12 - lead ECGs • Favorable cardiovascular safety profile 20 - 2 0 - 1 0 0 10 20 SBP (mmHg) Standing - Supine Placebo 10 0 m g b i d 20 0 m g b i d 25 0 m g b i d 250 mg bid >65 ΔΔQTcF (msec) Concentration (ng/mL) No evidence of QT prolongation No effect on BP with Standing Source: Phase 1 clinical trial; Alterity data on file
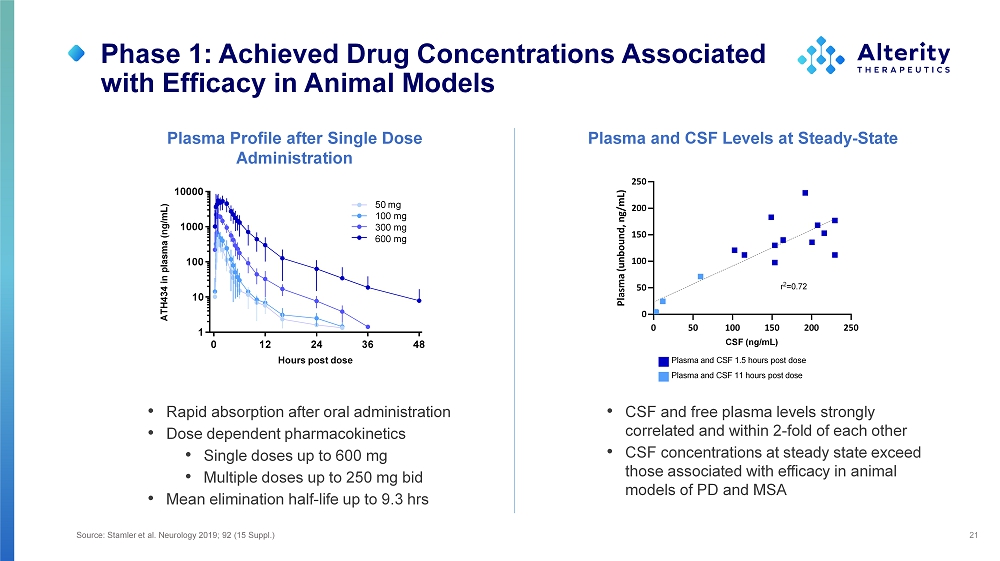
Phase 1: Achieved Drug Concentrations Associated with Efficacy in Animal Models 21 Plasma Profile after Single Dose Plasma and CSF Levels at Steady - State Administration • Rapid absorption after oral administration • Dose dependent pharmacokinetics • Single doses up to 600 mg • Multiple doses up to 250 mg bid • Mean elimination half - life up to 9.3 hrs 0 12 36 48 1 10 100 1000 10000 24 Hours post dose ATH434 in plasma (ng/mL) 50 mg 10 0 m g 30 0 m g 60 0 m g 0 5 0 2 5 0 1 0 0 1 5 0 2 0 0 CSF (ng/mL) 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 Plasma (unbound, ng/mL) r 2 =0.72 Plasma and CSF 1.5 hours post dose Plasma and CSF 11 hours post dose • CSF and free plasma levels strongly correlated and within 2 - fold of each other • CSF concentrations at steady state exceed those associated with efficacy in animal models of PD and MSA Source: Stamler et al. Neurology 2019; 92 (15 Suppl.)
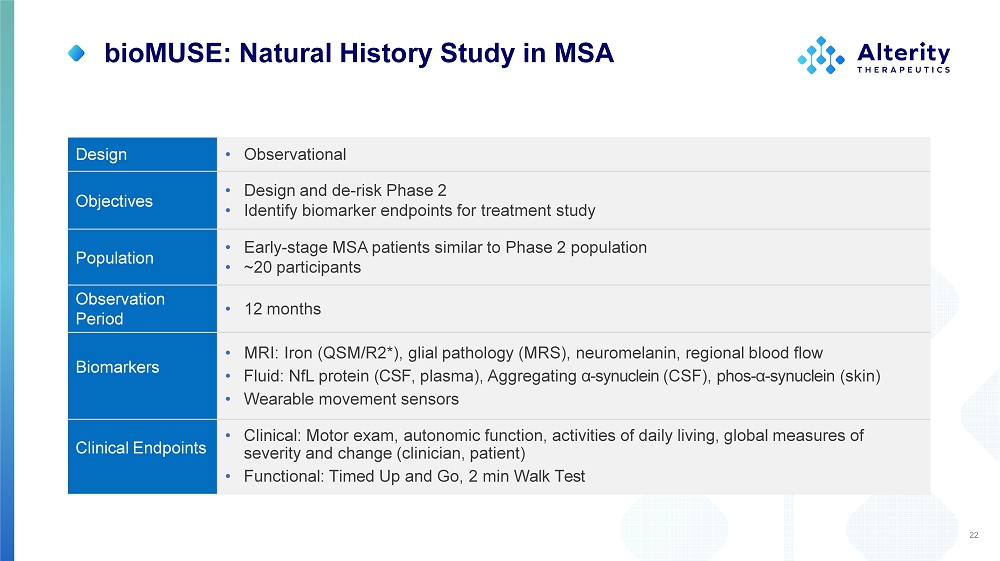
bioMUSE: Natural History Study in MSA • Observational Design • Design and de - risk Phase 2 • Identify biomarker endpoints for treatment study Objectives • Early - stage MSA patients similar to Phase 2 population • ~20 participants Population • 12 months O b s e rv ation Period • MRI: Iron (QSM/R2*), glial pathology (MRS), neuromelanin, regional blood flow • Fluid: NfL protein (CSF, plasma), Aggregating α - synuclein (CSF), phos - α - synuclein (skin) • Wearable movement sensors Biomarkers • Clinical: Motor exam, autonomic function, activities of daily living, global measures of severity and change (clinician, patient) • Functional: Timed Up and Go, 2 min Walk Test Clinical Endpoints 22
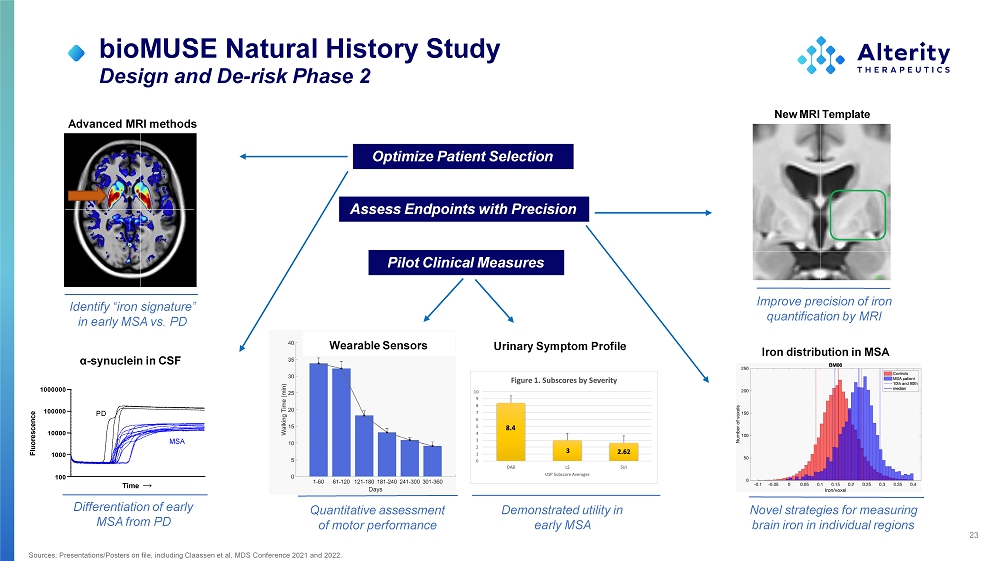
23 Sources: Presentations/Posters on file, including Claassen et al, MDS Conference 2021 and 2022. bioMUSE Natural History Study Design and De - risk Phase 2 Optimize Patient Selection Pilot Clinical Measures Assess Endpoints with Precision Identify “iron signature” in early MSA vs. PD Advanced MRI methods 1000 100 10000 1 00000 1 000000 Time → Fluorescence PD MS A α - synuclein in CSF Differentiation of early MSA from PD New MRI Template Improve precision of iron quantification by MRI Novel strategies for measuring brain iron in individual regions Iron distribution in MSA Quantitative assessment of motor performance Wearable Sensors Urinary Symptom Profile Demonstrated utility in early MSA
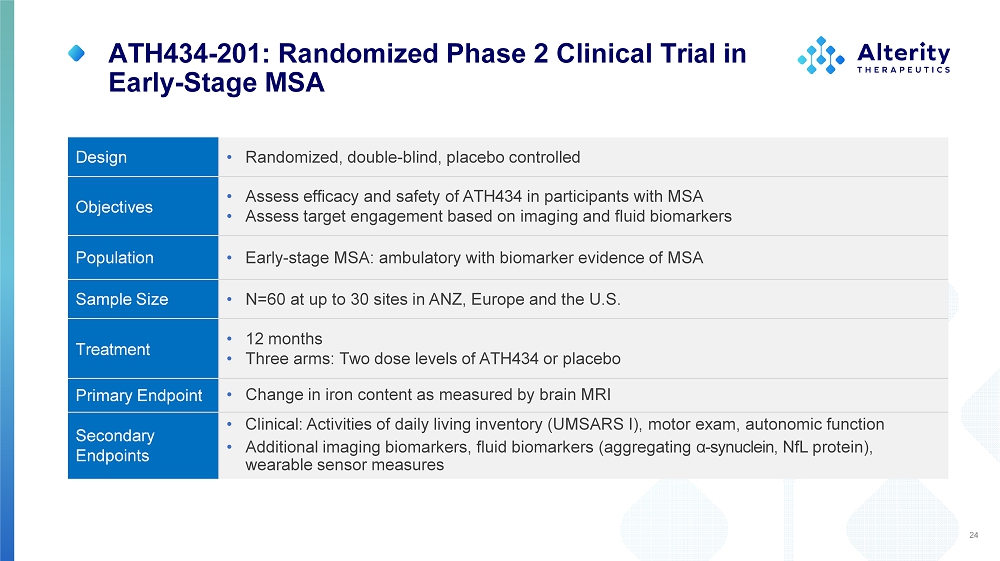
ATH434 - 201: Randomized Phase 2 Clinical Trial in Early - Stage MSA • Randomized, double - blind, placebo controlled Design • Assess efficacy and safety of ATH434 in participants with MSA • Assess target engagement based on imaging and fluid biomarkers Objectives • Early - stage MSA: ambulatory with biomarker evidence of MSA Population • N=60 at up to 30 sites in ANZ, Europe and the U.S. Sample Size • 12 months • Three arms: Two dose levels of ATH434 or placebo Treatment • Change in iron content as measured by brain MRI Primary Endpoint • Clinical: Activities of daily living inventory (UMSARS I), motor exam, autonomic function • Additional imaging biomarkers, fluid biomarkers (aggregating α - synuclein, NfL protein), wearable sensor measures Se c onda ry Endpoints 24

ATH434 - 201 Phase 2 Design and Primary Endpoint Source: Claassen, et al. Mov Disorders 2021 25 Primary Endpoint: Change in Brain Iron on MRI R² = 0.63 0. 30 0. 25 0. 20 0. 15 0. 10 0. 05 0. 00 Iron Content (GPe) 0 20 40 60 80 Uni f ie d M S A Ra t in g S c ale BioMUSE Natural History Study Demonstrates Brain iron correlates with disease severity in MSA
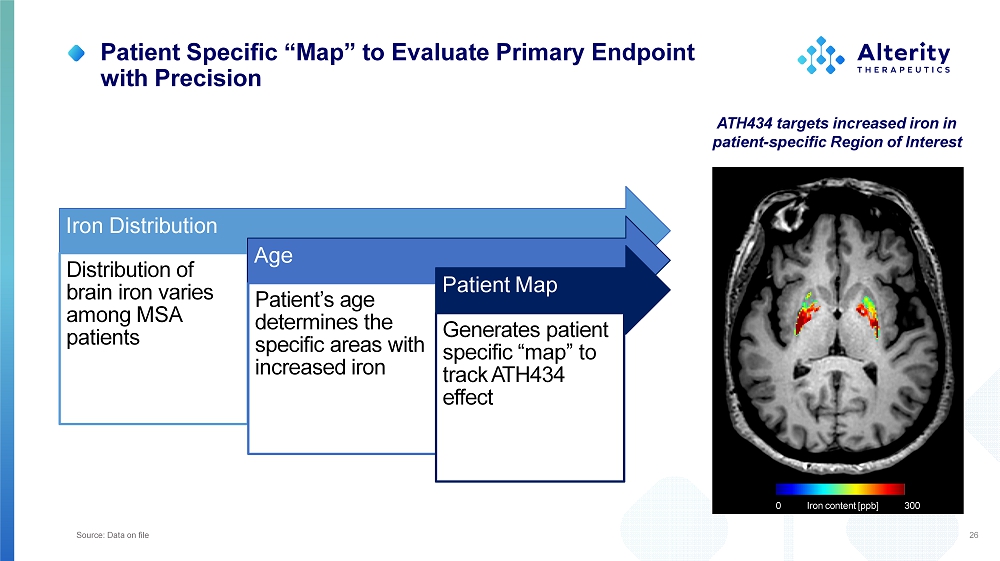
Patient Specific “Map” to Evaluate Primary Endpoint with Precision Iron Distribution Distribution of brai n iro n varies among MSA patients A ge Patient’s age determines the specifi c area s wit h increased iron Patient Map Generate s patient specific “map” to t ra c k A TH434 effect 26 ATH434 targets increased iron in patient - specific Region of Interest 0 300 Iron content [ppb] Source: Data on file
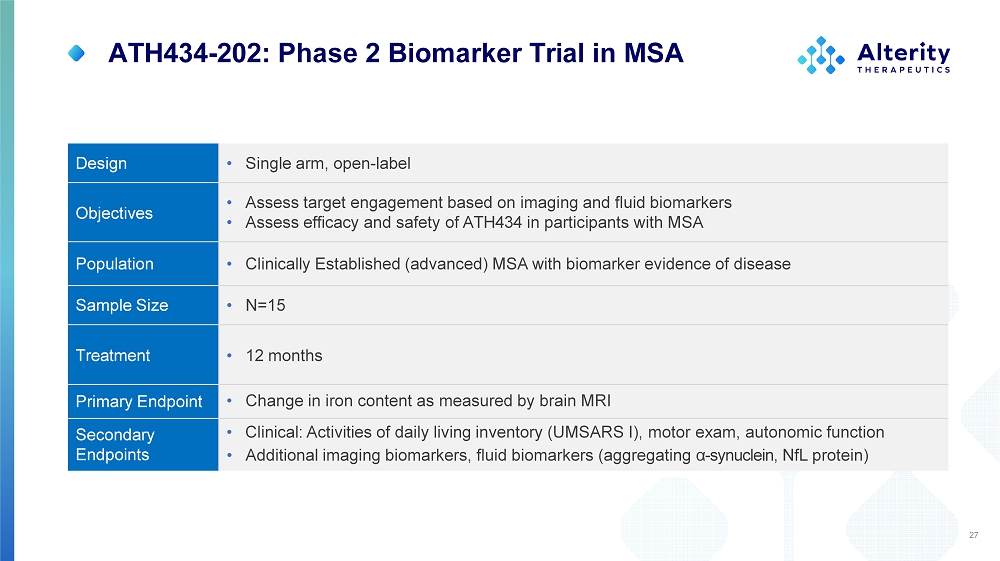
ATH434 - 202: Phase 2 Biomarker Trial in MSA • Single arm, open - label Design • Assess target engagement based on imaging and fluid biomarkers • Assess efficacy and safety of ATH434 in participants with MSA Objectives • Clinically Established (advanced) MSA with biomarker evidence of disease Population • N=15 Sample Size • 12 months Treatment • Change in iron content as measured by brain MRI Primary Endpoint • Clinical: Activities of daily living inventory (UMSARS I), motor exam, autonomic function • Additional imaging biomarkers, fluid biomarkers (aggregating α - synuclein, NfL protein) Se c onda ry Endpoints 27
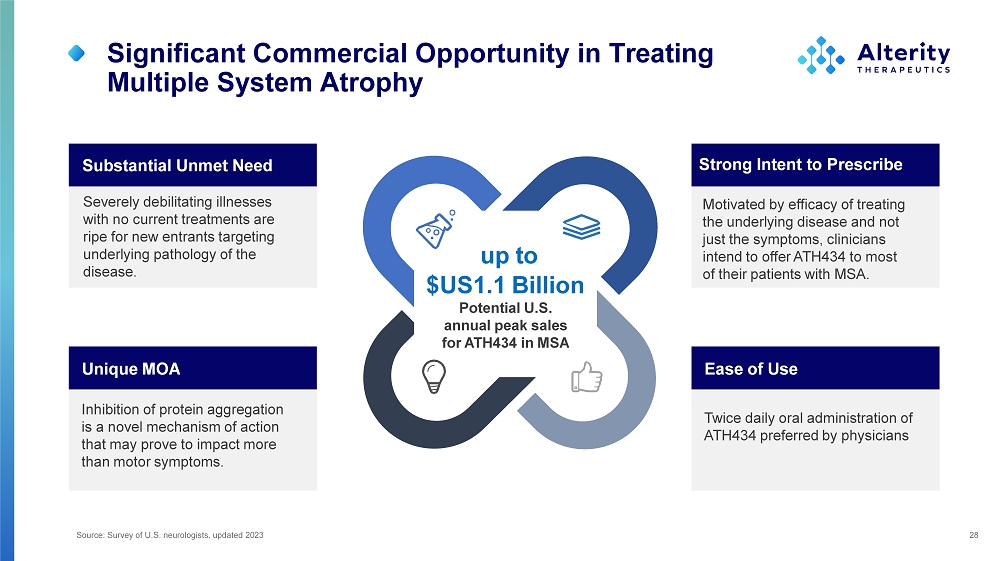
Significant Commercial Opportunity in Treating Multiple System Atrophy 28 Severely debilitating illnesses with no current treatments are ripe for new entrants targeting underlying pathology of the disease. Substantial Unmet Need Motivated by efficacy of treating the underlying disease and not just the symptoms, clinicians intend to offer ATH434 to most of their patients with MSA. Strong Intent to Prescribe Twice daily oral administration of ATH434 preferred by physicians Ease of Use Inhibition of protein aggregation is a novel mechanism of action that may prove to impact more than motor symptoms. Unique MOA up to $US1.1 Billion Potential U.S. annual peak sales for ATH434 in MSA Source: Survey of U.S. neurologists, updated 2023

Alterity: Poised for Progress 29 • Targeting Orphan disease with no approved treatments • T w o Pha s e 2 c lini c a l t rial s ongoi n g • D oub l e - b li n d tr i a l enro l l i n g g l oba ll y • B i omarke r tr i a l enro lli n g i n U .S . • bioMUSE Natural History Study de - risking Phase 2 • Development team with multiple FDA approvals • Drug discovery generating patentable compounds as next generation therapies • Ca s h balan c e o f $16 . 7 M A U D a s o f 3 0 Sep t 202 3 Clinical Milestones A TH 434 - 20 1 P ha s e 2 D oub l e - Bli n d T ri al x Q 1 2023 : Fir s t P a ti en t s D o s e d i n U.S . an d E u r ope x Q 2 2023 : Fir s t P a ti en t D o s e d i n A u s tr a li a x Q 3 2023 : DM C r e c ommend s c on ti nu i ng tri a l a s p l anned x O c t obe r 2023 : Cl o s e d S c r een i ng x N o v embe r 2023 : C omp l e t e E n r o llment • Q 4 2024 : St ud y C omp l e t e A TH 434 - 20 2 P ha s e 2 Bi oma r k e r T ri al x Q 2 2023 : I n iti a t e T ri al • H 1 2024 : Pr e limi na r y D a t a MS A N a t u r a l Hi s t o r y St udy x Q 2 2023 : Di agno s ti c Pr e c i s i o n D a t a • Q 4 2023 : Pr e s en t ne w b i oma r k e r da t a
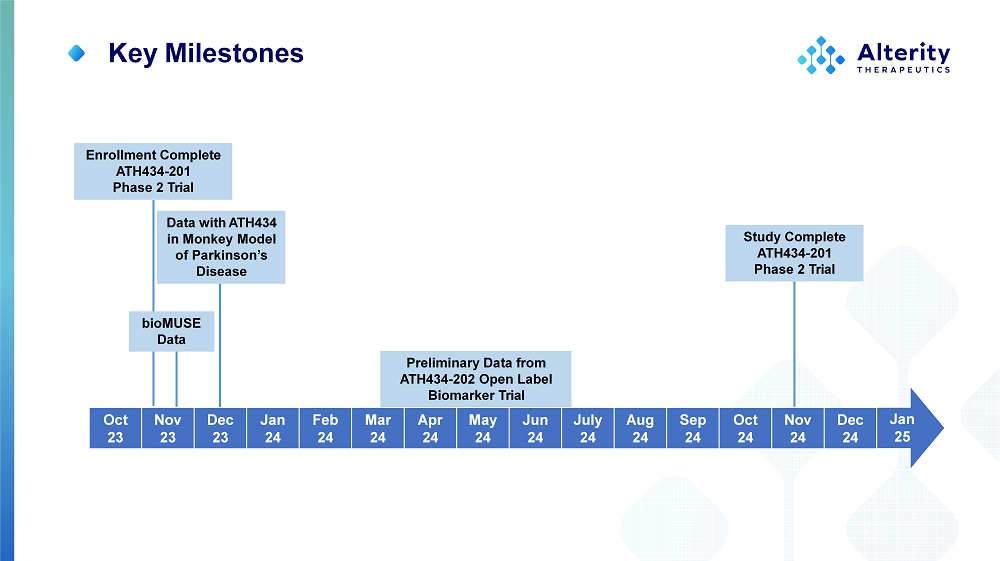
Preliminary Data from ATH434 - 202 Open Label Biomarker Trial Study Complete ATH434 - 201 Phase 2 Trial Jan 25 D ec 24 No v 24 O ct 24 S ep 24 Au g 24 J u ly 24 J u n 24 M ay 24 Ap r 24 Mar 24 F eb 24 Jan 24 D ec 23 No v 23 O ct 23 Key Milestones Enrollment Complete ATH434 - 201 Phase 2 Trial Data with ATH434 in Monkey Model of Parkinson’s Disease b i oMUSE Data

Alterity Therapeutics (PK) (USOTC:PRNAF)
Historical Stock Chart
From Apr 2024 to May 2024

Alterity Therapeutics (PK) (USOTC:PRNAF)
Historical Stock Chart
From May 2023 to May 2024
