0001421204
true
S-1/A
0001421204
2021-01-01
2021-09-30
0001421204
2020-12-31
0001421204
2019-12-31
0001421204
us-gaap:SeriesAPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesAPreferredStockMember
2019-12-31
0001421204
us-gaap:SeriesBPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesBPreferredStockMember
2019-12-31
0001421204
us-gaap:SeriesCPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesCPreferredStockMember
2019-12-31
0001421204
us-gaap:SeriesDPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesDPreferredStockMember
2019-12-31
0001421204
us-gaap:SeriesEPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesEPreferredStockMember
2019-12-31
0001421204
us-gaap:SeriesFPreferredStockMember
2020-12-31
0001421204
us-gaap:SeriesFPreferredStockMember
2019-12-31
0001421204
2020-01-01
2020-12-31
0001421204
2019-01-01
2019-12-31
0001421204
us-gaap:PreferredStockMember
2018-12-31
0001421204
us-gaap:CommonStockMember
2018-12-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2018-12-31
0001421204
us-gaap:RetainedEarningsMember
2018-12-31
0001421204
2018-12-31
0001421204
us-gaap:PreferredStockMember
2019-01-01
2019-12-31
0001421204
us-gaap:CommonStockMember
2019-01-01
2019-12-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2019-01-01
2019-12-31
0001421204
us-gaap:RetainedEarningsMember
2019-01-01
2019-12-31
0001421204
us-gaap:PreferredStockMember
2019-12-31
0001421204
us-gaap:CommonStockMember
2019-12-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2019-12-31
0001421204
us-gaap:RetainedEarningsMember
2019-12-31
0001421204
us-gaap:PreferredStockMember
2020-01-01
2020-12-31
0001421204
us-gaap:CommonStockMember
2020-01-01
2020-12-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-01-01
2020-12-31
0001421204
us-gaap:RetainedEarningsMember
2020-01-01
2020-12-31
0001421204
us-gaap:PreferredStockMember
2020-12-31
0001421204
us-gaap:CommonStockMember
2020-12-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-12-31
0001421204
us-gaap:RetainedEarningsMember
2020-12-31
0001421204
us-gaap:IntellectualPropertyMember
2020-01-01
2020-12-31
0001421204
2020-03-31
0001421204
us-gaap:CashMember
2021-01-31
0001421204
us-gaap:CashMember
2020-10-31
0001421204
nspx:RidgewayMember
2020-10-05
0001421204
nspx:SeriesFConvertiblePreferredStockMember
nspx:RidgewayMember
2020-10-05
0001421204
nspx:SeriesFConvertiblePreferredStockMember
nspx:RidgewayMember
2020-01-01
2020-12-31
0001421204
2020-10-31
0001421204
2021-01-31
0001421204
2020-06-01
2020-06-10
0001421204
us-gaap:EmployeeStockOptionMember
2020-01-01
2020-12-31
0001421204
us-gaap:EmployeeStockOptionMember
2019-01-01
2019-12-31
0001421204
us-gaap:WarrantMember
2020-01-01
2020-12-31
0001421204
us-gaap:WarrantMember
2019-01-01
2019-12-31
0001421204
us-gaap:ConvertibleDebtMember
2020-01-01
2020-12-31
0001421204
us-gaap:ConvertibleDebtMember
2019-01-01
2019-12-31
0001421204
us-gaap:ConvertiblePreferredStockMember
2020-01-01
2020-12-31
0001421204
us-gaap:ConvertiblePreferredStockMember
2019-01-01
2019-12-31
0001421204
us-gaap:FairValueInputsLevel1Member
2020-12-31
0001421204
us-gaap:FairValueInputsLevel2Member
2020-12-31
0001421204
us-gaap:FairValueInputsLevel3Member
2020-12-31
0001421204
us-gaap:FairValueInputsLevel1Member
2019-12-31
0001421204
us-gaap:FairValueInputsLevel2Member
2019-12-31
0001421204
us-gaap:FairValueInputsLevel3Member
2019-12-31
0001421204
us-gaap:MeasurementInputPriceVolatilityMember
srt:MinimumMember
2020-12-31
0001421204
us-gaap:MeasurementInputPriceVolatilityMember
srt:MaximumMember
2020-12-31
0001421204
us-gaap:MeasurementInputExpectedTermMember
srt:MinimumMember
2020-12-31
0001421204
us-gaap:MeasurementInputExpectedTermMember
srt:MaximumMember
2020-12-31
0001421204
us-gaap:MeasurementInputRiskFreeInterestRateMember
srt:MinimumMember
2020-12-31
0001421204
us-gaap:MeasurementInputRiskFreeInterestRateMember
srt:MaximumMember
2020-12-31
0001421204
us-gaap:MeasurementInputExpectedDividendRateMember
2020-12-31
0001421204
us-gaap:SeriesFPreferredStockMember
2020-10-06
0001421204
nspx:SeriesE0ConvertiblePreferredStockMember
2020-05-01
2020-05-02
0001421204
nspx:SeriesE0ConvertiblePreferredStockMember
2020-05-02
0001421204
us-gaap:SeriesEPreferredStockMember
2020-01-01
2020-12-31
0001421204
us-gaap:SeriesDPreferredStockMember
2018-12-31
0001421204
nspx:SeriesDConvertiblePreferredStockMember
2019-01-01
2019-01-31
0001421204
us-gaap:SeriesDPreferredStockMember
2020-01-01
2020-12-31
0001421204
nspx:SeriesA0ConvertiblePreferredStockMember
2015-01-01
2015-12-31
0001421204
nspx:SeriesA0ConvertiblePreferredStockMember
2015-12-31
0001421204
nspx:SeriesB0ConvertiblePreferredStockMember
2016-01-01
2016-12-31
0001421204
nspx:SeriesB0ConvertiblePreferredStockMember
2016-12-31
0001421204
nspx:SeriesC0ConvertiblePreferredStockMember
2017-03-01
2017-04-30
0001421204
nspx:SeriesC0ConvertiblePreferredStockMember
2017-04-30
0001421204
nspx:SeriesA0ConvertiblePreferredStockMember
2020-01-01
2020-12-31
0001421204
2020-11-26
0001421204
2020-11-27
0001421204
2019-09-10
2019-09-17
0001421204
nspx:RidgewayTerminationAgreementMember
2020-10-01
2020-10-31
0001421204
us-gaap:CommonStockMember
2020-01-01
2020-12-31
0001421204
us-gaap:CommonStockMember
nspx:PrincipalMember
2020-01-01
2020-12-31
0001421204
us-gaap:CommonStockMember
2019-01-01
2019-12-31
0001421204
us-gaap:CommonStockMember
nspx:PrincipalMember
2019-01-01
2019-12-31
0001421204
nspx:ExecutiveCompensationPlan2007Member
2020-12-31
0001421204
nspx:ExecutiveCompensationPlan2009Member
2020-01-01
2020-12-31
0001421204
nspx:ExecutiveCompensationPlan2007Member
us-gaap:CommonStockMember
2020-12-31
0001421204
nspx:ExecutiveCompensationPlan2009Member
2020-12-31
0001421204
nspx:InducementAwardStockOptionPlanMember
2020-12-31
0001421204
nspx:InducementAwardStockOptionPlanMember
2020-01-01
2020-12-31
0001421204
nspx:EquityCompensationPlan2017Member
2020-12-31
0001421204
us-gaap:EmployeeStockOptionMember
2018-12-31
0001421204
us-gaap:EmployeeStockOptionMember
2019-12-31
0001421204
us-gaap:EmployeeStockOptionMember
2020-12-31
0001421204
us-gaap:WarrantMember
2018-12-31
0001421204
us-gaap:WarrantMember
2019-12-31
0001421204
us-gaap:WarrantMember
2020-12-31
0001421204
us-gaap:SeriesCPreferredStockMember
srt:MinimumMember
2020-12-31
0001421204
us-gaap:SeriesCPreferredStockMember
srt:MaximumMember
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:ConsultantMember
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:ConsultantMember
2020-01-01
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:Financing2016Member
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:Financing2016Member
2020-01-01
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:Financing2017Member
2020-12-31
0001421204
us-gaap:WarrantMember
nspx:Financing2017Member
2020-01-01
2020-12-31
0001421204
nspx:October2020DebenturesMember
2020-10-01
2020-10-23
0001421204
nspx:October2020DebenturesMember
2020-10-23
0001421204
nspx:October2020DebenturesMember
2020-12-31
0001421204
nspx:October2020DebenturesMember
2020-01-01
2020-12-31
0001421204
nspx:March2020DebenturesMember
2020-03-01
2020-03-06
0001421204
nspx:March2020DebenturesMember
2020-12-31
0001421204
nspx:March2020DebenturesMember
2020-01-01
2020-12-31
0001421204
nspx:SabbyVolatilityWarrantMasterFundLtdMember
2020-12-31
0001421204
nspx:SabbyVolatilityWarrantMasterFundLtdMember
2020-01-01
2020-12-31
0001421204
nspx:SabbyVolatilityWarrantMasterFundLtdMember
us-gaap:ExtendedMaturityMember
2020-01-01
2020-12-31
0001421204
nspx:October2019DebenturesMember
2020-01-01
2020-12-31
0001421204
nspx:SecuritiesPurchaseAgreementMember
2019-06-22
2019-07-16
0001421204
nspx:SecuritiesPurchaseAgreementMember
2019-07-16
0001421204
us-gaap:ConvertibleNotesPayableMember
2018-12-03
2018-12-13
0001421204
us-gaap:ConvertibleNotesPayableMember
us-gaap:ExtendedMaturityMember
2018-12-03
2018-12-13
0001421204
nspx:SecuritiesPurchaseAgreementMember
2018-06-22
2018-07-03
0001421204
nspx:SecuritiesPurchaseAgreementMember
2018-07-03
0001421204
us-gaap:ExtendedMaturityMember
2018-07-01
2018-07-31
0001421204
nspx:ExchangeAgreementMember
nspx:InvestorsMember
2017-09-12
0001421204
nspx:SeriesAConvertiblePreferredStockMember
2017-09-12
0001421204
nspx:SeriesBConvertiblePreferredStockMember
2017-09-12
0001421204
nspx:ExchangeAgreementMember
2017-09-05
2017-09-12
0001421204
nspx:ExchangeAgreementMember
2017-09-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeniorConvertibleDebenturesMember
2021-01-01
2021-01-12
0001421204
us-gaap:SubsequentEventMember
nspx:InvestorsMember
us-gaap:CashMember
2021-01-12
0001421204
us-gaap:ConvertibleDebtMember
us-gaap:SubsequentEventMember
2021-01-01
2021-01-12
0001421204
us-gaap:SubsequentEventMember
nspx:ClaireThomMember
2021-01-01
2021-03-01
0001421204
us-gaap:SubsequentEventMember
nspx:ScottOgilvieMember
2021-01-01
2021-03-01
0001421204
us-gaap:SubsequentEventMember
2021-10-01
2021-10-05
0001421204
2021-09-30
0001421204
us-gaap:SeriesAPreferredStockMember
2021-09-30
0001421204
us-gaap:SeriesBPreferredStockMember
2021-09-30
0001421204
us-gaap:SeriesCPreferredStockMember
2021-09-30
0001421204
us-gaap:SeriesDPreferredStockMember
2021-09-30
0001421204
us-gaap:SeriesEPreferredStockMember
2021-09-30
0001421204
us-gaap:SeriesFPreferredStockMember
2021-09-30
0001421204
2021-07-01
2021-09-30
0001421204
2020-07-01
2020-09-30
0001421204
2020-01-01
2020-09-30
0001421204
us-gaap:PreferredStockMember
2020-01-01
2020-03-31
0001421204
us-gaap:CommonStockMember
2020-01-01
2020-03-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-01-01
2020-03-31
0001421204
us-gaap:RetainedEarningsMember
2020-01-01
2020-03-31
0001421204
2020-01-01
2020-03-31
0001421204
us-gaap:PreferredStockMember
2020-03-31
0001421204
us-gaap:CommonStockMember
2020-03-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-03-31
0001421204
us-gaap:RetainedEarningsMember
2020-03-31
0001421204
us-gaap:PreferredStockMember
2020-04-01
2020-06-30
0001421204
us-gaap:CommonStockMember
2020-04-01
2020-06-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-04-01
2020-06-30
0001421204
us-gaap:RetainedEarningsMember
2020-04-01
2020-06-30
0001421204
2020-04-01
2020-06-30
0001421204
us-gaap:PreferredStockMember
2020-06-30
0001421204
us-gaap:CommonStockMember
2020-06-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-06-30
0001421204
us-gaap:RetainedEarningsMember
2020-06-30
0001421204
2020-06-30
0001421204
us-gaap:PreferredStockMember
2020-07-01
2020-09-30
0001421204
us-gaap:CommonStockMember
2020-07-01
2020-09-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-07-01
2020-09-30
0001421204
us-gaap:RetainedEarningsMember
2020-07-01
2020-09-30
0001421204
us-gaap:PreferredStockMember
2020-09-30
0001421204
us-gaap:CommonStockMember
2020-09-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2020-09-30
0001421204
us-gaap:RetainedEarningsMember
2020-09-30
0001421204
2020-09-30
0001421204
us-gaap:PreferredStockMember
2021-01-01
2021-03-31
0001421204
us-gaap:CommonStockMember
2021-01-01
2021-03-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-01-01
2021-03-31
0001421204
us-gaap:RetainedEarningsMember
2021-01-01
2021-03-31
0001421204
2021-01-01
2021-03-31
0001421204
us-gaap:PreferredStockMember
2021-03-31
0001421204
us-gaap:CommonStockMember
2021-03-31
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-03-31
0001421204
us-gaap:RetainedEarningsMember
2021-03-31
0001421204
2021-03-31
0001421204
us-gaap:PreferredStockMember
2021-04-01
2021-06-30
0001421204
us-gaap:CommonStockMember
2021-04-01
2021-06-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-04-01
2021-06-30
0001421204
us-gaap:RetainedEarningsMember
2021-04-01
2021-06-30
0001421204
2021-04-01
2021-06-30
0001421204
us-gaap:PreferredStockMember
2021-06-30
0001421204
us-gaap:CommonStockMember
2021-06-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0001421204
us-gaap:RetainedEarningsMember
2021-06-30
0001421204
2021-06-30
0001421204
us-gaap:PreferredStockMember
2021-07-01
2021-09-30
0001421204
us-gaap:CommonStockMember
2021-07-01
2021-09-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-07-01
2021-09-30
0001421204
us-gaap:RetainedEarningsMember
2021-07-01
2021-09-30
0001421204
us-gaap:PreferredStockMember
2021-09-30
0001421204
us-gaap:CommonStockMember
2021-09-30
0001421204
us-gaap:AdditionalPaidInCapitalMember
2021-09-30
0001421204
us-gaap:RetainedEarningsMember
2021-09-30
0001421204
us-gaap:IntellectualPropertyMember
2021-01-01
2021-09-30
0001421204
us-gaap:CashMember
2021-06-30
0001421204
nspx:SeriesFConvertiblePreferredStockMember
nspx:RidgewayMember
2021-01-01
2021-09-30
0001421204
us-gaap:EmployeeStockOptionMember
2021-07-01
2021-09-30
0001421204
us-gaap:EmployeeStockOptionMember
2021-01-01
2021-09-30
0001421204
us-gaap:EmployeeStockOptionMember
2020-07-01
2020-09-30
0001421204
us-gaap:EmployeeStockOptionMember
2020-01-01
2020-09-30
0001421204
us-gaap:WarrantMember
2021-07-01
2021-09-30
0001421204
us-gaap:WarrantMember
2021-01-01
2021-09-30
0001421204
us-gaap:WarrantMember
2020-07-01
2020-09-30
0001421204
us-gaap:WarrantMember
2020-01-01
2020-09-30
0001421204
us-gaap:ConvertiblePreferredStockMember
2021-07-01
2021-09-30
0001421204
us-gaap:ConvertiblePreferredStockMember
2021-01-01
2021-09-30
0001421204
us-gaap:ConvertiblePreferredStockMember
2020-07-01
2020-09-30
0001421204
us-gaap:ConvertiblePreferredStockMember
2020-01-01
2020-09-30
0001421204
us-gaap:FairValueInputsLevel1Member
2021-09-30
0001421204
us-gaap:FairValueInputsLevel2Member
2021-09-30
0001421204
us-gaap:FairValueInputsLevel3Member
2021-09-30
0001421204
srt:MinimumMember
us-gaap:MeasurementInputPriceVolatilityMember
2021-09-30
0001421204
srt:MaximumMember
us-gaap:MeasurementInputPriceVolatilityMember
2021-09-30
0001421204
us-gaap:MeasurementInputExpectedTermMember
srt:MinimumMember
2021-09-30
0001421204
us-gaap:MeasurementInputExpectedTermMember
srt:MaximumMember
2021-09-30
0001421204
srt:MinimumMember
us-gaap:MeasurementInputRiskFreeInterestRateMember
2021-09-30
0001421204
srt:MaximumMember
us-gaap:MeasurementInputRiskFreeInterestRateMember
2021-09-30
0001421204
us-gaap:MeasurementInputExpectedTermMember
2021-01-01
2021-09-30
0001421204
us-gaap:CommonStockMember
2021-01-01
2021-09-30
0001421204
us-gaap:CommonStockMember
nspx:PrincipalMember
2021-01-01
2021-09-30
0001421204
us-gaap:CommonStockMember
2021-07-01
2021-09-30
0001421204
us-gaap:CommonStockMember
2020-01-01
2020-09-30
0001421204
us-gaap:CommonStockMember
nspx:PrincipalMember
2020-01-01
2020-09-30
0001421204
us-gaap:CommonStockMember
2020-07-01
2020-09-30
0001421204
nspx:June2021DebentureMember
2021-06-01
2021-06-18
0001421204
nspx:June2021DebentureMember
2021-06-18
0001421204
nspx:June2021DebentureMember
2021-07-01
2021-09-30
0001421204
nspx:June2021DebentureMember
2021-01-01
2021-09-30
0001421204
nspx:June2021DebentureMember
2021-09-30
0001421204
nspx:January2021DebentureMember
2021-01-01
2021-01-12
0001421204
nspx:January2021DebentureMember
2021-01-01
2021-09-30
0001421204
nspx:January2021DebentureMember
2021-09-30
0001421204
nspx:January2021DebentureMember
2021-01-12
0001421204
nspx:January2021DebentureMember
2021-07-01
2021-09-30
0001421204
nspx:October2020DebenturesMember
2021-01-01
2021-09-30
0001421204
nspx:October2020DebenturesMember
2021-09-30
0001421204
nspx:October2020DebenturesMember
2021-07-01
2021-09-30
0001421204
nspx:November2019DebenturesMember
2021-09-30
0001421204
nspx:November2019DebenturesMember
2021-01-01
2021-09-30
0001421204
nspx:October2019DebenturesMember
2021-01-01
2021-09-30
0001421204
nspx:July2019DebenturesMember
2019-07-01
2019-07-16
0001421204
nspx:December2018DebenturesMember
2018-12-01
2018-12-13
0001421204
nspx:ExchangeAgreementMember
2021-01-01
2021-09-30
0001421204
nspx:ExchangeAgreementMember
2021-09-30
0001421204
us-gaap:SubsequentEventMember
us-gaap:CommonStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesAConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesBConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesCConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesDConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesEConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
nspx:SeriesFConvertiblePreferredStockMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
2021-10-01
2021-10-12
0001421204
us-gaap:SubsequentEventMember
2021-10-12
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
As
filed with the Securities and Exchange Commission on December 3, 2021
Registration
No. 333-255618
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
PRE-EFFECTIVE
AMENDMENT NO. 1
TO
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF 1933
REBUS HOLDINGS, INC.
(Exact
name of registrant as specified in its charter)
Delaware
(State
or jurisdiction of incorporation or organization)
2834
(Primary
Standard Industrial
20-0438951
(I.R.S.
Employer Identification No.)
2629 Townsgate Road, Suite 215
Westlake Village, CA 91361
(818)
597-7552
FAX
(805) 553-9783
(Address,
including zip code, and telephone number,
including
area code, of registrant’s principal executive offices)
Agent
for Service:
Cogency
Global, Inc.
850
New Burton Road, Suite 201
Dover,
DE 19904
(800)
483-1140
(Name,
address, including zip code, and telephone number,
including
area code, of agent for service)
Copy
to:
Raul
Silvestre
Silvestre
Law Group, P.C.
2629
Townsgate Road, Suite 215
Westlake
Village, CA 91361
(818)
597-7552
Fax
(805) 553-9783
From
time to time after this registration statement becomes effective.
Approximate
date of commencement of proposed sale to the public
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
☐
|
|
Accelerated filer
|
☐
|
|
Non-accelerated filer
|
☒
|
|
Smaller reporting company
|
☒
|
|
|
|
|
Emerging growth company
|
☐
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards pursuant to Section 7(a)(2)(B) of the Securities act. ☐
CALCULATION
OF REGISTRATION FEES
|
Title of Each Class of Securities
to be Registered
|
|
Amount
to be
Registered(1)
|
|
|
Proposed
Maximum
Offering
Price Per
Share
|
|
|
Proposed
Maximum
Aggregate
Offering
Price
|
|
|
Amount
of
Registration
Fee(4)
|
|
|
Senior
Convertible Debenture, Common Stock underlying Senior Convertible Debenture, par value $0.0001
|
|
|
5,806,864
|
(2)
|
|
$
|
0.06225
|
(3)
|
|
$
|
361,477.29
|
|
|
$
|
33.51
|
|
|
Total
|
|
|
5,806,864
|
|
|
|
|
|
|
$
|
361,477.29
|
|
|
$
|
33.51
|
|
|
|
(1)
|
Pursuant
to SEC Rule 416, also covers additional common shares that may be offered to prevent dilution
as a result of stock splits or stock dividends.
|
|
|
(2)
|
Number
of shares is based on a bona fide estimate based on 85% the volume weighted adjusted price
of the Senior Convertible Debentures calculated on October 25, 2021. 85% of the volume weighted
adjusted price was $0.086105
|
|
(3)
|
Fee
based on the high and low prices of the Registrant’s Common Stock on November 30, 2021.
|
|
(4)
|
$1,481
was previously paid in connection with the filing of the initial registration statement. No remaining balance is required to be paid
hereunder.
|
THE
REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE
REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE
IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933, AS AMENDED, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE
ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SUCH SECTION 8(a), MAY DETERMINE.
SUBJECT
TO COMPLETION, DATED NOVEMBER 24, 2021
The
information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does
it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PROSPECTUS
5,806,864
Shares
Common
Stock
This
prospectus relates to the resale of 5,806,864 shares of our common stock, by the selling stockholder(s) identified in the selling stockholders
tables beginning on page 18 of this prospectus (“Selling Stockholder(s)”). We will not receive any proceeds from the sale
of these shares by the Selling Stockholders.
The
Selling Stockholders will sell their shares of common stock at $0.08 per share until our shares are quoted on the OTCQB, OTCQX or listed
on a national securities exchange, and thereafter, at fixed prices, at prevailing market prices at the time of the sale, at varying
prices determined at the time of sale, or at negotiated prices, including, without limitation, in one or more transactions that may take
place by ordinary brokerage transactions, privately-negotiated transactions or through sales to one or more underwriters or broker-dealers
for resale. We provide more information about how a Selling Stockholder may sell its shares of common stock in the section titled “Plan
of Distribution” on page 20.
Information
regarding the Selling Stockholders is provided under the “Selling Stockholders” section of this prospectus.
Our
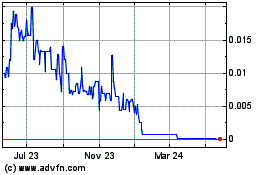
common stock is quoted on the Pink Sheets of the OTC Markets Group Inc., under the symbol “RBSH”. On November 18, 2021, the
closing price of our common stock was $0.081 per share. You are urged to obtain current market quotations of our common stock before
purchasing any of the shares being offered for sale pursuant to this prospectus.
Our
principal executive offices are located at 2629 Townsgate Road #215, Westlake Village, California 91361, telephone number 818-597-7552.
Investing
in our common stock is highly speculative and involves a high degree of risk. You should consider carefully the risks and uncertainties
in the section entitled “Risk Factors” beginning on page 4 of this prospectus before investing in our common stock.
The
date of this prospectus is _______, 2021
TABLE
OF CONTENTS
Please
read this prospectus carefully. It describes our business, our financial condition and our results of operations. We have prepared this
prospectus so that you will have the information necessary to make an informed investment decision.
You
may rely only on the information contained in this prospectus. We have not, authorized anyone to provide information or to make representations
not contained in this prospectus. This prospectus is neither an offer to sell, nor a solicitation of an offer to buy, these securities
in any jurisdiction where an offer or solicitation would be unlawful. Neither the delivery of this prospectus, nor any sale made under
this prospectus, means that the information contained in this prospectus is correct as of any time after the date of this prospectus.
This prospectus may be used only where it is legal to offer and sell these securities.
USE
OF MARKET AND INDUSTRY DATA
This
prospectus includes market and industry data that has been obtained from third party sources, including industry publications, as well
as industry data prepared by our management on the basis of its knowledge of and experience in the industries in which we operate (including
our management’s estimates and assumptions relating to such industries based on that knowledge). Management’s knowledge of
such industries has been developed through its experience and participation in these industries. While our management believes the third-party
sources referred to in this prospectus are reliable, neither we nor our management have independently verified any of the data from such
sources referred to in this prospectus or ascertained the underlying economic assumptions relied upon by such sources. Internally prepared
and third-party market forecasts, in particular, are estimates only and may be inaccurate, especially over long periods of time. In addition,
the underwriters have not independently verified any of the industry data prepared by management or ascertained the underlying estimates
and assumptions relied upon by management. Furthermore, references in this prospectus to any publications, reports, surveys or articles
prepared by third parties should not be construed as depicting the complete findings of the entire publication, report, survey or article.
The information in any such publication, report, survey or article is not incorporated by reference in this prospectus.
ADVISEMENT
We
urge you to read this entire prospectus carefully, including the” Risk Factors” section and the financial statements and
related notes included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the Securities
and Exchange Commission (“SEC”) as well all subsequent reports we file with the SEC. As used in this prospectus, unless the
context otherwise requires, the words “we,” “us,” “our,” “the Company,” “Rebus,”
“Rebus Holdings,” and “Registrant” refer to Rebus Holdings, Inc. (fka Inspyr Therapeutics, Inc.). Also, any reference
to “common stock” or “common shares” refers to our $0.0001 par value common stock. Also, any reference to “preferred
stock” or “preferred shares” refers to our $0.0001 par value Series A preferred stock, our $0.0001 par value series
B preferred stock our $0.0001 par value Series C preferred stock, our $0.0001 Series D preferred stock, our $0.0001 series E preferred
stock, and our $0.0001 Series F Preferred Stock. The information contained herein is current as of the date of this prospectus, unless
another date is specified. All references to common stock, share and per share amounts have been retroactively restated to reflect the
1:75 reverse stock split that became effective on October 12, 2021 as if it had taken place as of the beginning of the earliest period
presented.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
Prospectus includes “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended,
or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements relate
to our business development plans, clinical trials, regulatory reviews, timing, strategies, expectations, anticipated expense levels,
business prospects and positioning with respect to the market for our proposed products, business outlook, technology spending and various
other matters (including contingent liabilities and obligations and changes in accounting policies, standards and interpretations), express
our current intentions, beliefs, expectations, strategies or predictions, as well as historical information. Such forward-looking statements
involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements, to
be materially different from anticipated results, performance or achievements expressed or implied by such forward-looking statements.
When used in this Prospectus, statements that are not statements of current or historical fact may be deemed to be forward-looking statements.
Without limiting the foregoing, the words “plan,” “intend,” “may,” “will,” “expect,”
“believe,” “could,” “anticipate,” “estimate,” or “continue” or similar expressions
or other variations or comparable terminology are intended to identify such forward-looking statements. Although we believe that the
assumptions on which the forward-looking statements contained herein are based are reasonable, any of those assumptions could prove to
be inaccurate given the inherent uncertainties as to the occurrence or nonoccurrence of future events. These statements are not guarantees
of future performance and involve risks and uncertainties that are difficult to predict. Our future operating results are dependent upon
many factors which are outside our control. You should not place undue reliance on forward-looking statements. Forward-looking statements
may not be realized due to a variety of factors, including, without limitation, our ability to:
|
|
●
|
continue
to increase our corporate operations;
|
|
|
●
|
attract,
build and retain a senior management team;
|
|
|
●
|
manage
our business given continuing operating losses and negative cash flows;
|
|
|
●
|
obtain
sufficient capital or a strategic business arrangement to fund our operations and expansion
plans;
|
|
|
●
|
build
the infrastructure necessary to support the growth of our business;
|
|
|
●
|
manage
competitive factors and developments beyond our control;
|
|
|
●
|
manage
scientific and medical developments which may be beyond our control;
|
|
|
●
|
manage
the governmental regulation of our business including state, federal and international laws;
|
|
|
●
|
maintain
and protect our intellectual property;
|
|
|
●
|
obtain
patents based on our current and/or future patent applications;
|
|
|
●
|
obtain
and maintain other rights to technology required or desirable to conduct or expand our business;
|
|
|
●
|
achieve
any potential strategic benefits of licensing transactions, collaborations, acquisitions,
or in-licensing of new technologies, if any;
|
|
|
●
|
successfully
integrate the assets previously licensed to Ridgeway Therapeutics, Inc. pursuant to the termination
of such license in October 2020; and
|
|
|
●
|
manage
any other factors discussed in the “Risk Factors” section, and elsewhere in this
Prospectus.
|
All
forward-looking statements attributable to us are expressly qualified in their entirety by these and other factors. We undertake no obligation
to update or revise these forward-looking statements, whether to reflect events or circumstances after the date initially filed or published,
to reflect the occurrence of unanticipated events or otherwise, except to the extent required by federal securities laws. The risks discussed
in this Prospectus should be considered in evaluating our business and future prospects.
RISK
FACTORS
Investing
in our common stock involves a high degree of risk. You should carefully consider the following risk factors and all other information
contained in this prospectus in evaluating our common stock. If any of the following events were to occur, our business, financial condition
or results of operations could be materially and adversely affected. In these circumstances, the market price of our common stock could
decline, and you could lose your entire investment.
Risks
Related to our Financial Position, Need to Raise Additional Capital, and Series F Preferred Stock
We
were forced to curtail our operations due to a lack of operating capital and we will not be able to continue as a going concern if we
do not obtain additional financing.
Since
our inception, we have funded our operations through the sale of our securities. Our cash balances at December 31, 2020 were approximately
$404,000. Despite raising $1,000,000 in gross proceeds through the sale of convertible debentures consisting of (i) $500,000 in January
2021 and (ii) $500,000 (for cash) in June 2021, our ability to continue as a going concern is still wholly dependent upon obtaining sufficient
capital to fund our operations. We have no committed sources of additional capital and our access to capital funding is always uncertain.
Accordingly, despite our ability to secure capital in the past, we cannot assure you that we will be able to secure additional capital
through financing transactions, including issuance of debt, or through other means such as the licensing of our technology or grants.
In the event that we are not able to secure additional funding, we may be forced to curtail operations, delay or stop ongoing clinical
trials, cease operations altogether or file for bankruptcy.
Our
auditors have expressed substantial doubt about our ability to continue as a going concern.
Our
auditors’ report on our December 31, 2020 consolidated financial statements expressed an opinion that our capital resources as
of the date of their audit report were not sufficient to sustain operations or complete our planned activities for the upcoming year
unless we raised additional funds. Our current cash level raises substantial doubt about our ability to continue as a going concern past
the second quarter of 2022. If we do not obtain additional funds by such time, we may no longer be able to continue as a going concern
and will cease operation which means that our shareholders will lose their entire investment.
If
we do not raise sufficient capital, we may lose rights to certain intellectual property which is the basis of our lead product candidates.
In
October 2020, pursuant to the cancellation of a license agreement, we reacquired the rights to US Patent 9,593,118, which covers both
A2B and dual A2A/A2B antagonists. The intellectual property contained in US Patent 9,593,118 is the
basis of our lead product candidates. As a condition to the cancelation of the license, we are required to raise an aggregate of $5 million
prior to October of 2023. If we are unable to raise such capital, the license cancelation will be revoked and the license will reinstated in exchange for the return of the Common Shares and Series
F Preferred Stock. In such event, we will lose all rights to the technology which forms the basis of our lead product candidate which
will have a material adverse effect on our business and prospects.
Our
shareholders will experience substantial dilution upon the conversion of our Series F Preferred Stock.
On
October 5, 2020, we reacquired the rights to certain intellectual property that is the basis of our lead proposed product. In exchange
for the cancelation of the prior license, which resulted in our reacquisition of such technology, we issued 8,000 shares of Series F
Preferred Stock. The 8,000 shares of Series F Preferred Stock are convertible into an aggregate of 80% of our issued and outstanding
Common Stock immediately prior to conversion. Upon conversion, our shareholders will experience substantial dilution.
Risks
Relating to Our Stage of Development and Business
If
we are unable to successfully attract and retain a new management team and secure additional members and employees, our business could
be harmed.
On
June 16, 2021, Michael Cain, our interim chief executive officer and principal accounting officer resigned as an officer and as a member
of the Board of Directors. On August 16, 2021, we appointed Raul Silvestre as interim chief executive officer and principal accounting
officer. We will need to augment senior management as well as engage additional personnel to execute our business plan and grow our business.
Our success depends largely on the development and execution of our business strategy by our management team. The recent transitions
in our executive team may be disruptive to our business, and if we are unable to manage an orderly transition, our business may be adversely
affected. Additionally, since our management team consists of only one individual, Mr. Silvestre, the loss of Mr. Silvestre would likely
harm our ability to implement our business strategy and respond to the rapidly changing market conditions in which we operate. There
may be a limited number of persons with the requisite skills to serve in these positions, and we cannot assure you that we would be able
to identify or employ such qualified personnel on acceptable terms, if at all. Additionally, we cannot assure you that management will
succeed in working together as a team. In the event that we are unsuccessful, our business and prospects could be harmed.
We
are an early-stage company, have no product revenues, are not profitable and may never be profitable.
From
inception through September 30, 2021, we have raised approximately $39.1 million through the sale of our securities and exercise of outstanding
warrants. During this same period, we have recorded an accumulated deficit of approximately $64.3 million. Our net losses for the two
most recent fiscal years ended December 31, 2020 and 2019 were $6,295,000 and $934,000, respectively. Our increase in net losses is primarily
the result of a loss from the change in fair value of our derivative instruments and the cost attributable to the termination of our
license with Ridgeway Therapeutic, Inc., partially offset by an increase in gains from conversion of debt and a decrease in interest
expense. None of our products in development have received approval from the United States Food and Drug Administration or FDA, or other
regulatory authorities; we have no sales and have never generated revenues nor do we expect to for the foreseeable future. We have currently
curtailed our pre-clinical and clinical trials related to mipsagargin and are currently focusing our efforts on the development of our
adenosine receptor modulators. We expect to incur significant operating losses for the foreseeable future as we continue the research,
pre-clinical and clinical development of our product candidates as well as the possible in-licensing of additional clinical and pre-clinical
assets. Accordingly, we will need additional capital to fund our continuing operations and any expansion plans. Since we do not generate
any revenue, the most likely sources of such additional capital include the sale of our securities, a strategic licensing collaboration
transaction or joint venture involving the rights to one or more of our product candidates, or from grants. To the extent that we raise
additional capital by issuing equity securities, our stockholders are likely to experience dilution with regard to their percentage ownership
of the company, which may be significant. If we raise additional funds through collaborations or licensing arrangements, we may be required
to relinquish some or all the rights to our technologies, product candidates, or grant licenses on terms that are not favorable to us.
If we raise additional capital by incurring debt, we could incur significant interest expense and become subject to covenants that could
affect the manner in which we conduct our business, including securing such debt obligations with our assets.
Our
product candidates are at various stages of early development and significant financial resources are required to develop commercially
viable products and obtain regulatory approval to market and sell such products. We will need to devote significantly more research and
development efforts, financial resources and personnel to develop commercially viable products and obtain regulatory approvals. We may
encounter hurdles and unexpected issues as we proceed in the development of our other product candidates. While initial data from our
research appear promising, the outcome of the pre-clinical and development work is uncertain and future trials may ultimately be unsuccessful.
If we fail to develop and successfully commercialize our product candidates, our business may be materially harmed and could fail.
We
have a limited operating history as a company and may not be able to effectively operate our business.
Our
limited staff and operating history mean that there is a high degree of uncertainty regarding our ability to:
|
|
●
|
develop
and commercialize our technologies and proposed products;
|
|
|
●
|
obtain
regulatory approval to commence the marketing of our products;
|
|
|
●
|
identify,
hire and retain the needed personnel to implement our business plan;
|
|
|
●
|
achieve
market acceptance or insurance reimbursement for any of our proposed products, if successfully
developed; or
|
|
|
●
|
respond
to competition.
|
No
assurances can be given as to exactly when, if at all, we will be able to fully develop, and take the necessary steps to derive any revenues
from our proposed product candidates.
We
rely on technologies that we may not be able to commercially develop, which will prevent us from generating revenues, operating profitably
or providing investors any return on their investment.
We
have refocused our development on our adenosine receptor modulator technologies and our ability to generate revenue and operate profitably
will depend on us being able to develop these technologies for human applications. We cannot guarantee that the results obtained in clinical
evaluation of our therapies will be sufficient to warrant approval by the FDA for clinical use. Even if our therapies are approved for
use by the FDA, there is no guarantee that they will exhibit an enhanced efficacy relative to competing products such that they will
be adopted by the medical community. Without significant adoption by the medical community our product candidates will have limited commercial
potential which will likely result in the loss of your entire investment.
Inability
to complete pre-clinical and clinical testing and trials will impair the viability of the Company.
We
are in the development stage and have not yet applied for approval by the FDA to conduct clinical trials. Even if we successfully file
an IND application and receive clearance from the FDA to commence trials, the outcome of pre-clinical, clinical and product testing of
our product candidates is uncertain, and if we are unable to satisfactorily complete such testing, or if such testing yields unsatisfactory
results, we will be unable to commercially produce our proposed products. Before obtaining regulatory approvals for the commercial sale
of any potential human products, our product candidates will be subjected to extensive pre-clinical and clinical testing to demonstrate
their safety and efficacy in humans. No assurances can be given that the clinical trials of our product candidates, or those of licensees
or collaborators, will demonstrate the safety and efficacy of such product candidates at all, or to the extent necessary to obtain appropriate
regulatory approvals, or that the testing of such product candidates will be completed in a timely manner, if at all, or without significant
increases in costs, program delays or both, all of which could harm our ability to generate revenues. In addition, our product candidates
may not prove to be more effective for treating disease than current therapies. Accordingly, we may have to delay or abandon efforts
to research, develop or obtain regulatory approval to market our product candidates. Many companies involved in biotechnology research
and development have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. The failure
to adequately demonstrate the safety and efficacy of a therapeutic product under development could delay or prevent regulatory approval
of the product and could harm our ability to generate revenues, operate profitably or produce any return on an investment in our company.
Raising
capital may be difficult as a result of our history of losses and limited operating history in our current stage of development.
When
making investment decisions, investors typically look at a company’s management, earnings and historical performance in evaluating
the risks and operations of the business and the business’s future prospects. Our history of losses, new senior management team
and relatively limited operating history in our current stage of development makes such evaluation, as well as any estimation of our
future performance, substantially more difficult. As a result, investors may be unwilling to invest in us or on terms or conditions which
are acceptable. If we are unable to secure additional financing, we may need to materially scale back our business plan and/or operations
or cease operations altogether.
A
pandemic, epidemic or outbreak of an infectious disease in the markets in which we operate or that otherwise impacts our facilities or
advisors could adversely impact our business.
If
a pandemic, epidemic, or outbreak of an infectious disease including the recent outbreak of respiratory illness caused by a novel coronavirus
(COVID-19) or other public health crisis were to affect our facilities or those of our suppliers, our business could be adversely affected.
A pandemic typically results in social distancing, travel bans and quarantine, and this may limit access to our facilities, management,
support staff and professional advisors. These factors, in turn, may not only materially impact our operations and financial condition,
but our overall ability to react timely to mitigate the impact of this event. Also, it may hamper our efforts to comply with our filing
obligations with the Securities and Exchange Commission.
Business
or economic disruptions or global health concerns could seriously harm our development efforts and increase our costs and expenses.
Broad-based
business or economic disruptions could adversely affect our ongoing or planned research and development activities. For example, in November
2019 an outbreak of a novel strain of coronavirus originated in Wuhan, China, and has since spread around the world, including to the
United States. To date, this outbreak has already resulted in extended shutdowns of many businesses around the world, including in the
United States. Global health concerns, such as coronavirus, could also result in social, economic, and labor instability in the countries
in which we or the third parties with whom we engage operate. We cannot presently predict the scope, severity and longevity of any potential
business shutdowns or disruptions, but if we or any of the third parties with whom we engage or plan to engage, including the suppliers,
clinical trial sites, regulators and other third parties with whom we conduct business or plan to conduct business, were to experience
shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could
be materially and negatively impacted. It is also possible that global health concerns such as this one could disproportionately impact
the hospitals and clinical sites in which we conduct or plan to conduct any of our clinical trials, which could have a material adverse
effect on our business and our results of operation and financial condition.
Risks
Related to Commercialization
The
market for our proposed products is rapidly changing and competitive.
The
pharmaceutical and biotechnology industries are subject to rapid and substantial technological change and innovation. Developments by
others may render our proposed products noncompetitive or obsolete, or we may be unable to keep pace with technological developments
and other market factors. Competition from pharmaceutical and biotechnology companies, universities, governmental entities and others
diversifying into the field is intense and is expected to increase.
As
a pre-revenue company, our resources are limited, and we may experience challenges inherent in the early development of novel therapeutics.
Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competition.
Some of these technologies may have an entirely different approach or means of accomplishing similar therapeutic efforts compared to
our proposed products. Our competitors may develop therapies that are safer, more effective and less costly than our proposed products
and therefore, present a serious competitive threat to us.
The
acceptance of therapies that are alternatives to ours may limit market acceptance of our proposed products, even if commercialized. Many
of our targeted diseases and conditions can also be treated by other medications and treatments. These treatments may be widely accepted
in medical communities and have a longer history of use. The established use of other competing therapies may limit the potential for
our proposed products, even if commercialized.
Our
proposed products may not be accepted by the healthcare community.
Our
proposed products, if approved for marketing, may not achieve market acceptance by the healthcare community since hospitals, physicians,
patients, or the medical community in general may decide not to utilize them. We are attempting to develop products that are likely to
be first approved for marketing as a treatment for late-stage cancer where there is no truly effective standard of care. If approved
for use in late-stage cancer, our proposed products might then be evaluated in earlier stages where they could represent a substantial
departure from established treatment methods and would most likely compete with a number of more conventional drugs and therapies which
are manufactured and marketed by major pharmaceutical companies. It is too early in the development cycle of our proposed products for
us to predict our major competitors. The degree of market acceptance of our products, if developed, will depend on a number of factors,
including but not limited to:
|
|
●
|
our
ability to demonstrate the clinical efficacy and safety of our proposed products to the medical
community;
|
|
|
●
|
our
ability to create products that are superior to alternative products;
|
|
|
●
|
our
ability to establish in the medical community the potential advantage of our treatments over
alternative treatment methods; and
|
|
|
●
|
the
reimbursement policies of government and third-party payors.
|
If
the healthcare community does not accept our products, our business could be materially harmed.
Our
potential competitors in the biotechnology and pharmaceutical industries have significantly greater resources than we have.
We
compete against numerous companies, many of which have substantially greater resources than we have. Several such competitors have research
programs and/or efforts to treat the same diseases we target. Companies that may compete with us have substantially greater financial,
research, manufacturing and marketing resources than we do. As a result, such competitors may find it easier to compete in our industry
and bring competing products to market.
Risks
Related to the Development and Manufacturing of Our Product Candidates
We
intend to rely exclusively upon third-party FDA-regulated manufacturers and suppliers for our proposed products.
We
currently have no internal manufacturing capability and intend to rely exclusively on FDA-approved licensees, strategic partners or third-party
contract manufacturers or suppliers for the foreseeable future. Because manufacturing facilities are subject to regulatory oversight
and inspection, the failure of any of our third-party FDA regulated manufactures or suppliers to comply with regulatory requirements
could result in material manufacturing delays and product shortages, which could delay or otherwise negatively impact our clinical trials
and product development plans. Should we be forced to manufacture our proposed products, we cannot give any assurance that we would be
able to develop internal manufacturing capabilities or secure third-party suppliers for raw materials. In the event that we seek third
party suppliers or alternative manufacturers, they may require us to purchase a minimum amount of materials or could require other unfavorable
terms. Any such event could materially impact our business prospects and could delay the development of our proposed products. Moreover,
we cannot give any assurance that the contract manufacturers or suppliers that we select will be able to supply our products in a timely
or cost-effective manner or in accordance with applicable regulatory requirements or our own specifications.
We
may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize our
product candidates.
As
needed, we plan to rely heavily on third party collaborators, partners, licensees, clinical research organizations, clinical investigators,
vendors or other third parties to support our research and development efforts and to conduct clinical trials for our product candidates.
We cannot guarantee that we will be able to successfully negotiate agreements for, or maintain relationships with, these third parties
on a commercially reasonable basis, if at all. Additionally, to commercialize our proposed products, we intend to rely on third party
licensees or the outright sale of our proposed products to pharmaceutical partner(s). If we fail to establish or maintain such third-party
relationships as anticipated, our business could be adversely affected.
We
are dependent upon third parties to develop our product candidates, and such parties are, to some extent, outside of our control.
We
depend and plan to depend upon independent contract research organizations, investigators, and collaborators, such as universities and
medical institutions, to conduct our pre-clinical and clinical studies. These individuals and/or entities are not our employees and we
cannot control the amount or timing of resources that they devote to our programs. These third parties may not assign as great a priority
to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. If these third parties fail
to devote sufficient time and resources to our programs, or if their performance is substandard, the development of our drug candidates
and corresponding FDA approval could be delayed or fail entirely.
Our
therapeutic compounds may not be able to be manufactured profitably on a large enough scale to support commercialization.
To
date, our therapeutic compounds have only been manufactured at a scale which is adequate to supply our research activities and early-stage
clinical trials. There can be no assurance that the procedures currently used to manufacture our therapeutic compounds will work at a
scale which is adequate for commercial needs. In the event our therapeutic compounds cannot be manufactured in sufficient quantities
for commercialization, our future prospects could be significantly impacted, and our financial prospects would be materially harmed.
Risks
Relating to our Intellectual Property
Our
competitive position is dependent on our intellectual property and we may not be able to withstand challenges to our intellectual property
rights.
We
rely on our intellectual property, including our issued and applied for U.S. and foreign patents as the foundation of our business. If
our intellectual property rights are challenged, no assurances can be given that our patents or licenses would survive claims alleging
invalidity or infringement on other patents and/or licenses. In addition, disputes may arise regarding inventorship of our intellectual
property. It is possible that our intellectual property may be infringing upon existing patents that we are not currently unaware of.
As the number of participants in the marketplace grows, the possibility of patent infringement claims against us increases. It is difficult,
if not impossible, to determine how such disputes would be resolved. Furthermore, because of the substantial amount of discovery required
in connection with patent litigation, there is a risk that some of our confidential information could be required to be publicly disclosed.
Any litigation claims against us may cause us to incur substantial costs and could place a significant strain upon our financial resources,
divert the attention of management or restrict our core business or result in the public disclosure of confidential information.
We
may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights
and we may be unable to protect our rights to, or use of, our technology.
Some
or all of our patent applications may not issue as patents, or the claims of any issued patents may not afford meaningful protection
for our technologies or products. In addition, patents issued to us or our licensors, if any, may be challenged and subsequently narrowed,
invalidated or circumvented. Patent litigation is widespread in the biotechnology industry and could harm our business. Litigation might
be necessary to protect our patent position or to determine the scope and validity of third-party proprietary rights. If we choose to
go to court to stop someone else from using the inventions claimed in our patents, that individual or company would have the right to
ask the court to rule that such patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive,
and we may not have the required resources to pursue such litigation or to protect our patent rights. In addition, there is a risk that
the court might decide that these patents are not valid and that we do not have the right to stop the other party from using the inventions.
There is also the risk that, even if the validity of these patents is upheld, the court could refuse to stop the other party on the ground
that such other party’s activities do not infringe on our rights contained in these patents.
Furthermore,
a third party may claim that we are using inventions covered by their patent rights and may go to court to stop us from engaging in our
normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could materially
increase our operating expenses and divert the attention of managerial and technical personnel. There is a risk that a court would decide
that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In addition,
there is a risk that a court would order us to pay the other party damages for having violated the other party’s patents. The biotechnology
industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover
various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation
is not always uniform.
Because
some patent applications in the United States may be maintained in secrecy until the patents are issued, patent applications in the United
States and many foreign jurisdictions are typically not published until eighteen months after filing, and publications in the scientific
literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered
by our issued patents or our pending applications or that we were the first to invent the technology. Our competitors may have filed,
and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over
our patent applications and could further require us to obtain rights to issued patents covering such technologies.
If
another party has filed a United States patent application on inventions similar to ours, we may have to participate in an interference
or other proceeding in the U.S. Patent and Trademark Office, or the PTO, or a court to determine priority of invention in the United
States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in
a loss of our United States patent position with respect to such inventions.
Some
of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially
greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material
adverse effect on our ability to raise the capital necessary to continue our operations.
Obtaining
and maintaining our patent protection depends upon compliance with various procedural, documentary, fee payment and other requirements
imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The
PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other
provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent
application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might
be able to enter the market earlier than would otherwise have been the case.
We
may not be able to adequately protect our intellectual property.
We
rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets
are difficult to protect, and we cannot be certain that others do not develop the same or similar technologies on their own. Additionally,
research with regard to our technologies has been performed in countries outside of the United States, and we also anticipate conducting
joint ventures, collaborations and future clinical trials outside the US. The laws in some of these countries may not provide protection
for our trade secrets and intellectual property. We have taken steps, including entering into confidentiality agreements with our employees,
consultants, service providers, and potential strategic partners to protect our trade secrets and unpatented know-how. These agreements
generally require that the other party keep confidential and not disclose to third parties all confidential information developed by
the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements
from these parties which provide that inventions conceived by the party in the course of rendering services to us are our property. However,
these agreements may not be honored, including in foreign countries in which we conduct research, and may not effectively assign intellectual
property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive
and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade
secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We
may be subject to claims that our employees or consultants have wrongfully used or disclosed alleged trade secrets of their former employers.
As
is common in the biotechnology and pharmaceutical industries, we may employ and hire individuals and/or entities who were previously
employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against
us are currently pending, we may be subject to claims that these individuals, entities or that we have inadvertently or otherwise used
or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against
these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction
to management.
Risks
Relating to Marketing Approval and Government Regulations
Data
obtained from clinical trials are susceptible to varying interpretations and may not be sufficient to support approval of our proposed
products by the FDA.
The
design of our potential clinical trials will be based on many assumptions about the expected effect of our product candidates and if
those assumptions are incorrect, our potential clinical trials may not produce statistically significant results. Preliminary results
may not be confirmed on full analysis of the detailed results of early clinical trials. Data already obtained, or in the future obtained,
from pre-clinical studies and clinical trials do not necessarily predict the results that may be obtained from later trials. Moreover,
pre-clinical and clinical data are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. A
number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials,
even after promising results in earlier trials. The failure to adequately demonstrate the safety and effectiveness of a proposed formulation
or product under development could delay or prevent regulatory clearance of the potential drug. Our products may not prove to be safe
and effective in clinical trials and may not meet all regulatory requirements needed to receive regulatory approval. While data from
our completed trials appear promising, the outcome of the current trials is uncertain, and these trials or future trials may ultimately
be unsuccessful. Our clinical trials may among other things, not demonstrate sufficient levels of safety and efficacy necessary to obtain
the requisite regulatory approvals for our drugs, and thus our proposed drugs may not be approved for marketing.
Our
proposed products may not receive FDA or other regulatory approvals.
The
FDA and comparable government agencies in foreign countries impose substantial regulations on the manufacture and marketing of pharmaceutical
products through expensive, lengthy and detailed laboratory, pre-clinical and clinical testing procedures, sampling activities and other
costly and time-consuming procedures. Satisfaction of these regulations typically takes several years or more and varies substantially
based upon the type, complexity and novelty of the proposed product. Our proposed products are subject to extensive regulation and/or
acceptance by numerous governmental authorities in the United States, including the FDA, and authorities in other countries. Most of
our proposed products will require governmental approval before they can be commercialized. Our failure to receive the regulatory approvals
in the United States or foreign countries will materially impact our business.
Our
proposed products may not have favorable results in clinical trials or receive regulatory approval.
Encouraging
results from our studies to date should not be relied upon as evidence that our planned pre-clinical and clinical trials will ultimately
be successful, or our products approved for marketing. Even though the results of our studies to date may seem promising in certain respects,
we will be required to demonstrate through further pre-clinical and clinical trials that our product candidates are safe and effective
for use in a diverse population before we can seek regulatory approvals for their commercial sale. There is typically an extremely high
rate of attrition from the failure of product candidates as they proceed through clinical trials. If any product candidate fails to demonstrate
sufficient safety and efficacy in any clinical trial, then we could experience potentially significant delays in, or be required to abandon,
development of that product candidate. While initial data from our preliminary studies appear promising, the outcome of any clinical
trials is uncertain and such trials or future trials may ultimately be unsuccessful.
If
users of our proposed products are unable to obtain adequate reimbursement from third-party payors, market acceptance of our proposed
products may be limited, and we may not achieve revenues or profits.
The
continuing efforts of governments, insurance companies, health maintenance organizations and other payers of healthcare costs to contain
or reduce costs of health care may affect our future revenues and profitability as well as the future revenues and profitability of our
potential customers, suppliers and collaborative partners in addition to the availability of capital. In other words, our ability to
commercialize our proposed products depends in large part on the extent to which appropriate reimbursement levels for the cost of our
proposed formulations, products and related treatments are obtained by the health care providers of these products and treatments. At
this time, we cannot predict the precise impact that recently adopted or future laws will have on these reimbursement levels.
We
may be unable to comply with our reporting and other requirements under federal securities laws.
The
Sarbanes-Oxley Act of 2002, as well as related new rules and regulations implemented by the United States Securities and Exchange Commission,
or SEC, and the Public Company Accounting Oversight Board, require changes in the corporate governance practices and financial reporting
standards for public companies. These laws, rules and regulations, including compliance with Section 404 of the Sarbanes-Oxley Act of
2002 relating to internal control over financial reporting, would be expected to materially increase the Company’s legal and financial
compliance costs and make some activities more time-consuming and more burdensome. Presently we qualify as a non-accelerated filer. Accordingly,
we are exempt from the requirements of Section 404(b) and our independent registered public accounting firm is not required to audit
the design and operating effectiveness of our internal controls and management’s assessment of the design and the operating effectiveness
of such internal controls. In the event that we become an accelerated filer, we will be required to expend substantial capital in connection
with compliance.
We
do not have effective internal controls over our financial reporting.
Because
of our limited resources, management has concluded that our internal control over financial reporting may not be effective in providing
reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes
in accordance with U.S. generally accepted accounting principles. Effective internal controls over financial reporting and disclosure
controls and procedures are necessary for us to provide reliable financial and other reports and effectively prevent fraud. If we cannot
provide reliable financial or SEC reports or prevent fraud, investors may lose confidence in our SEC reports, our operating results and
the trading price of our common stock could suffer materially, and we may become subject to litigation.
Compliance
with changing regulation of corporate governance and public disclosure may result in additional expenses and will divert time and attention
away from revenue generating activities.
Changing
laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and
related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with
accessing the public markets and public reporting. Our management team invests significant time and financial resources to comply with
both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion
of management time and attention from developing our business to compliance activities which could have an adverse effect on our business.
Risks
Relating to our Securities
Our
common stock price may be particularly volatile because of our stage of development and business.
The
market prices for the securities of biotechnology and pharmaceutical companies in general, and early-stage drug development companies
in particular, such as ours, have been highly volatile and may continue to be highly volatile in the future. The following may have a
significant impact on the market price of our common stock:
|
|
●
|
our
ability to retain and augment our current management team and workforce, which currently
consists of only one employee, our chief executive officer;
|
|
|
●
|
the
development status of our drug candidates, particularly the results of our clinical trials;
|
|
|
●
|
market
conditions or trends related to the biotechnology and pharmaceutical industries, or the market
in general;
|
|
|
●
|
announcements
of technological innovations, new commercial products, or other material events by our competitors
or us;
|
|
|
●
|
disputes
or other developments concerning our proprietary rights;
|
|
|
●
|
changes
in, or failure to meet, securities analysts’ or investors’ expectations of our
financial and developmental performance;
|
|
|
●
|
additions
or departures of key personnel;
|
|
|
●
|
loss
of any strategic relationship;
|
|
|
●
|
discussions
of our business, products, financial performance, prospects, or stock price by the financial
and scientific press and online investor communities such as chat rooms;
|
|
|
●
|
industry
developments, including, without limitation, changes in healthcare policies or practices
or third-party reimbursement policies;
|
|
|
●
|
public
concern as to, and legislative action with respect to, testing or other research areas of
biopharmaceutical and pharmaceutical companies, the pricing and availability of prescription
drugs, or the safety of drugs;
|
|
|
●
|
regulatory
developments in the United States or foreign countries; and
|
|
|
●
|
economic,
political and other external factors.
|
Broad
market fluctuations may cause the market price of our common stock to decline substantially. Additionally, fluctuations in the trading
price or liquidity of our common stock may materially and adversely affect, among other things, the interest of investors to purchase
our common stock on the open market and, generally, our ability to raise capital.
Our
board of directors has broad discretion to issue additional securities, in the event that we have adequate authorized capital to issue
such securities.
We
are authorized under our certificate of incorporation to issue up to 1,000,000,000 shares of common stock and 30,000,000 “blank
check” shares of preferred stock. Shares of our blank check preferred stock provide the board of directors with broad authority
to determine voting, dividend, conversion, and other rights. As of October 31, 2021, we have issued and outstanding 10,309,212 shares
of common stock. We have also issued 1,853 shares of Series A 0% Convertible Preferred Stock, of which 133.8125 are outstanding, 1,000
shares of Series B 0% Convertible Preferred Stock, of which 71 are outstanding, 290.43148 shares of Series C 0% Convertible Preferred
Stock, that are all outstanding, 5,000 shares of Series D 0% Convertible Preferred Stock, all of which are outstanding, 5,000 shares
of Series E 0% Convertible Preferred Stock, all of which are outstanding, and 8,000 shares of Series F 0% Convertible Preferred Stock,
all of which are outstanding. Accordingly, we are entitled to issue 989,690,788 shares of common stock, and 29,981,505 additional shares
of “blank check” preferred stock. Our board may generally issue those common and preferred shares, or convertible securities
to purchase those shares, without further approval by our shareholders. Any additional preferred shares we may issue could have such
rights, preferences, privileges, and restrictions as may be designated from time-to-time by our board, including preferential dividend
rights, voting rights, conversion rights, redemption rights and liquidation provisions.
It
is likely that we will issue a large amount of additional securities to raise capital in order to further our business plans. It is also
likely that we will issue a large amount of additional securities to directors, officers, employees and consultants as compensatory grants
in connection with their services, both in the form of stand-alone grants or under our various stock plans. Any issuances could be made
at a price that reflects a discount to, or a premium from, the then-current market price of our common stock. These issuances would dilute
the percentage ownership interest of our current shareholders, which would have the effect of reducing your influence on matters on which
our stockholders vote, and might dilute the net tangible book value per share of our common stock.
Future
sales of our common stock could cause our stock price to fall.
Transactions
that result in a large amount of newly issued shares become readily tradable, or other events that cause current stockholders to sell
shares, could place downward pressure on the trading price of our common stock. In addition, the lack of a robust trading market may
require a stockholder who desires to sell a large number of shares of common stock to sell the shares in increments over time to mitigate
any adverse impact of the sales on the market price of our stock. If our stockholders sell, or the market perceives that our stockholders
intend to sell for various reasons, substantial amounts of our common stock in the public market, including shares issued upon the exercise
of outstanding options or warrants, the market price of our common stock could fall. Sales of a substantial number of shares of our common
stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable
or appropriate. We may become involved in securities class action litigation that could divert management’s attention and harm
our business.
As
of October 31, 2021, we had 1,000,000,000 shares of common stock authorized and 10,309,212 shares outstanding, 1,853 shares of Series
A 0% Convertible Preferred Stock issued and 133.8125 Series A 0% Convertible Preferred Stock outstanding, 1,000 shares of Series B 0%
Convertible Preferred Stock issued and 71 Series B 0% Convertible Preferred Stock outstanding, 290.43148 shares of Series C 0% Convertible
Preferred Stock issued and outstanding, 5,000 shares of Series D 0% Convertible Preferred Stock issued and outstanding, 5,000 shares
of Series E 0% Convertible Preferred Stock issued and outstanding, and 8,000 shares of Series F 0% Convertible Preferred Stock issued
and outstanding. We additionally have issued an aggregate of $5,591,048 of senior convertible debentures and convertible notes that are
convertible into common stock at any time, of which $810,072 is outstanding. Substantially all of the common shares and common shares
underlying the Series A 0% Convertible Preferred, Series B 0% Convertible Preferred, Series C 0% Convertible Preferred, Series D 0% Convertible
Preferred, Series E 0% convertible Preferred, and Series F 0% Convertible Preferred are available for public sale, subject in some cases
to volume and other limitations or delivery of a prospectus. As of October 31, 2021, we were obligated to reserve for issuance (i) 5
shares of our common stock issuable upon the conversion of 133.8125 shares of Series A 0% Convertible Preferred Stock including an additional
number of common shares we are contractually obligated to reserve pursuant to our December 2015 offering; (ii) 189,334 shares of our
common stock issuable upon the conversion of 71 shares of Series B 0% Convertible Preferred Stock including an additional number of common
shares we are contractually obligated to reserve pursuant to our December 2016 offering; (iii) 678 shares of our common stock issuable
upon the conversion of 290.43148 shares of Series C 0% Convertible Preferred Stock including an additional number of common shares we
are contractually obligated to reserve pursuant to our March 2017 offering, (iv) 18 shares of common stock issuable upon the conversion
of 5,000 shares of Series D 0% Convertible Preferred Stock, (v) 222 shares of common stock issuable upon the conversion of 5,000 shares
of Series E 0% Convertible Preferred Stock, (vi) an indeterminate number of shares of common stock issuable upon the conversion of 8,000
shares of Series F 0% Convertible Preferred Stock (such amount will equal 80% of the common stock post conversion), (vii) 102 shares
of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $5,032.78 per share, including
an additional number of common shares we are contractually obligated to reserve pursuant to our December 2015 offering, December 2016
offering and March 2017 offering, (viii) 9,202,880 shares of our common stock issuable upon conversion of our outstanding convertible
notes/debentures. Subject to applicable vesting requirements and holding periods, upon conversion or exercise of the outstanding convertible
notes and warrants, the underlying shares may be resold into the public market. We cannot predict if future issuances or sales of our
common stock, or the availability of our common stock for sale, would harm the market price of our common stock or our ability to raise
capital.
The
market for our common stock has historically been illiquid and our investors may be unable to sell their shares.
Our
common stock has historically traded with limited volume on the pink sheets of the OTC Markets Group Inc. Accordingly, although there
has been an increased public market for our common stock, it is still has historically been relatively illiquid compared to that of a
seasoned issuer. Prior to making an investment in our securities, you should consider the historically limited market for our common
stock. No assurances can be given that the trading volume of our common stock will increase or remain the same.
We
have not paid cash dividends in the past and do not expect to pay cash dividends in the foreseeable future.
We
have never paid cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable
future. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if the
market price of our common stock appreciates.
Provisions
of Delaware law and executive employment agreements may prevent or delay a change of control, which could depress the trading price of
our common stock.
We
are subject to the Delaware anti-takeover laws regulating corporate takeovers. These anti-takeover laws prevent Delaware corporations
from engaging in a merger or sale of more than 10% of its assets with any stockholder, including all affiliates and associates of the
stockholder, who owns 15% or more of the corporation’s outstanding voting stock, for three years following the date that the stockholder
acquired 15% or more of the corporation’s assets unless:
|
|
●
|
the
Board of Directors approved the transaction in which the stockholder acquired 15% or more
of the corporation’s assets;
|
|
|
●
|
after
the transaction in which the stockholder acquired 15% or more of the corporation’s
assets, the stockholder owned at least 85% of the corporation’s outstanding voting
stock, excluding shares owned by directors, officers and employee stock plans in which employee
participants do not have the right to determine confidentially whether shares held under
the plan will be tendered in a tender or exchange offer; or
|
|
|
●
|
on
or after this date, the merger or sale is approved by the Board of Directors and the holders
of at least two-thirds of the outstanding voting stock that is not owned by the stockholder.
|
A
Delaware corporation may opt out of the Delaware anti-takeover laws if its certificate of incorporation or bylaws so provides. We have
not opted out of the provisions of the anti-takeover laws. As such, these laws could prohibit or delay mergers or other takeover or change
of control transactions and may discourage attempts by other companies to acquire us.
In
addition, employment agreements with certain executive officers provide for the payment of severance and accelerated vesting of options
and restricted stock in the event of termination following a change of control. These provisions could have the effect of discouraging
potential takeover attempts even if it would be beneficial to shareholders.
Our
certificate of incorporation and bylaws contain provisions that could discourage a third-party from acquiring us.
Our
certificate of incorporation and bylaws, as applicable, among other things (i) provide our board with the ability to alter the bylaws
without stockholder approval and (ii) provide that vacancies on our board of directors may be filled by a majority of directors in office.
These provisions, while designed to reduce vulnerability to an unsolicited acquisition proposal, and to discourage certain tactics used
in proxy fights, may negatively impact a third-party’s decision to acquire us even if it would be beneficial to shareholders.
If
securities or industry analysts do not publish research or reports or if they publish unfavorable research or reports, an active market
for our common stock may not develop and the price of our common stock could decline.
We
are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment
community that generate or influence sales volume. Even if we come to the attention of such persons, they may be reluctant to follow
or recommend an unproven company such as ours until such time as we became more seasoned and viable. Generally, the trading market for
a company’s securities depends in part on the research and reports that securities or industry analysts publish. We currently have
limited research coverage by securities and industry analysts. As a consequence, there may be periods of time when trading activity in
our shares is minimal or non-existent, as compared to a seasoned issuer with significant research coverage. We cannot give you any assurance
that a broader or more active public trading market for our common stock will develop or if developed, will be sustained, or that current
trading levels could be sustained or not diminish. In addition, in the event any analysts downgrades our securities, the price of our
shares would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest
in the purchase of our securities could decrease, which could cause the price of our common stock and its trading volume, if any, to
decline.
Our
common stock is considered a “penny stock,” and is subject to additional sale and trading regulations that may make it more
difficult to sell.
Our
common stock is considered a “penny stock.” The principal result or effect of being designated a penny stock is that securities
broker-dealers participating in sales of our common stock are subject to the penny stock regulations set forth in Rules 15g-2 through
15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential
investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document
at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires
broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock
to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial
situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in
penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable
of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which
the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor,
confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance
with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third
parties or to otherwise dispose of them in the market or otherwise.
The
number of brokerage firms depositing and transacting trades for penny stock companies is very limited.
Currently,
our Common Stock is traded on the OTC Markets Pink Tier. Many traditional brokerage firms and on-line brokerages refuse to accept for
deposit and trade any penny stocks generally. For those that do, the time, effort and costs associated with depositing common stock in
companies such as ours which has recently had sub-penny bid and ask are onerous, time consuming and costly. This may present material
concerns and obstacles to those persons beneficially owning our common stock in certificate or book entry form, and wish to deposit same
into a brokerage account.
USE
OF PROCEEDS
We
will not receive any of the proceeds from the sale of the shares by any of the Selling Stockholders.
DETERMINATION
OF OFFERING PRICE
The
Selling Stockholders will offer their shares at in the manner described in the section of this Prospectus entitled “Plan of
Distribution.”
SELLING
STOCKHOLDERS
This
prospectus relates to the offering and sale, from time to time, of up to 5,806,864 shares of our Common Stock issuable to the selling
stockholders named in the tables below (“Selling Stockholders”), upon the conversion of $500,000 in principal of outstanding
senior convertible debentures issued on June 18, 2021.
June
18, 2021 Senior Convertible Debenture Offering
On
June 18, 2021, the Company sold $600,000 of senior convertible debentures (“June 2021 Debentures”) for (i) $500,000 in cash
to an existing institutional investor of the Company and (ii) $100,000 in cancellation of outstanding obligations. We are only registering
hereunder the shares of Common Stock underlying he $500,000 of June 2021 Debentures that were sold for cash
The
June 2021 Debentures (i) are non-interest bearing, (ii) have a maturity date of June 18, 2022, (iii) are convertible into shares of common
stock (“Common Stock”) of the Company at the election of the holder at any time, subject to a beneficial ownership limitation
of 4.99% (or 9.99% for the institutional investor), and (iv) has a conversion price equal to the lesser of $24.75 and 85% of the lowest
Volume Weighted Average Price (VWAP) during the five (5) Trading Days immediately prior to the conversion date, subject to adjustment,
as described therein.
The
June 2021 Debenture also contains provisions providing for an adjustment in the event of stock splits or dividends, and fundamental transactions.
The holders also have the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the June 2021
Debentures contain anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then applicable
conversion price until such time that the June 2021 Debentures are no longer outstanding. Additionally, the Company has the option to
redeem some or all of the June 2021 Debenture for cash upon notice of twenty (20) trading days provided certain conditions are met by
the Company as more fully described in the June 2021 Debentures.
Without
the approval of the holders of 67% of the then outstanding June 2021 Debentures, the Company may not (i) amend its charter documents
in any manner that adversely affects the rights of any January 2021 Debenture holder, (ii) repay or repurchase or acquire shares of its
Common Stock, (iii) repay, repurchase, or acquire certain indebtedness, or (iv) pay cash dividends or distributions on any equity securities
of the Company.
Selling
Stockholders
The
Common Stock being offered by the Selling Stockholder is those issuable to the Selling Stockholder, upon conversion of the June 2021
Debentures. For additional information regarding the issuances of those shares of Common Stock upon conversion of the June 2021 Debentures,
see the description entitled “June 18, 2021 Senior Convertible Debenture Offering” contained above. We are registering the
shares of Common Stock underlying the $500,000 June 2021 Debenture issued to the institutional investor for cash in order to permit the
Selling Stockholder to offer the shares for resale from time to time. Except for (i) the ownership of the shares of Common Stock issuable
pursuant to the conversion of the June 2021 Debentures, (ii) the ownership of shares of common stock issued to the Selling Shareholder
pursuant to the conversion of outstanding debentures previously held by the Selling Stockholder, (iii) the ownership of other outstanding
debentures convertible into common stock held by the Selling Stockholders that were purchased for cash or pursuant to the cancellation
of outstanding indebtedness, the Selling Stockholder have not had any material relationship with us within the past three years.
The
table below lists the Selling Stockholder and other information regarding the beneficial ownership of the shares of Common Stock by the
Selling Stockholder. The second column lists the number of shares of Common Stock beneficially owned by such Selling Stockholder, based
on its ownership of the shares of Common Stock (or Common Shares underlying convertible securities), as of October 31, 2021, assuming
conversion of outstanding convertible securities held by the Selling Stockholder on that date, without regard to any limitations on conversions
or exercises.
Pursuant
to the terms of the June 2021 Debentures, the Selling Stockholder may not convert the securities to the extent the Selling Stockholder,
together with its affiliates and attribution parties, would beneficially own a number of shares of common stock which would exceed 9.99%
of our then outstanding Common Stock following such conversion, excluding for purposes of such determination shares of Common Stock issuable
upon conversion of debentures that have not yet been converted. The Selling Stockholder may sell all, some or none of their shares in
this offering. See “Plan of Distribution.”
|
|
|
Common
Shares
Owned Before Sale(1)
|
|
|
Shares
|
|
|
Common
Shares
Owned After Sale(2)
|
|
|
|
|
Held
Outright
|
|
|
Convertible
Securities
|
|
|
Amount
|
|
|
%
of
class
|
|
|
being
registered
|
|
|
Amount
|
|
|
%
of
Class
|
|
|
Sabby
Volatility Warrant Master Fund, Ltd.(3)
|
|
|
1
|
|
|
|
5,807,712
|
|
|
|
5,807,713
|
|
|
|
36.03
|
%
|
|
|
5,806,864
|
|
|
|
849
|
|
|
|
*
|
|
|
TOTAL
|
|
|
1
|
|
|
|
5,807,712
|
|
|
|
5,807,713
|
|
|
|
36.03
|
%
|
|
|
5,806,864
|
|
|
|
849
|
|
|
|
*
|
|
|
|
*
|
Represents
less than 1%
|
|
|
**
|
Unless
otherwise stated, the individual(s) with voting and dispositive control of securities offered
on behalf of trusts or custodial accounts is the individual or entity referenced in the name
of such accounts.
|
|
|
(1)
|
Pursuant
to Rules 13d-3 and 13d-5 of the Exchange Act, beneficial ownership includes any common shares
as to which a shareholder has sole or shared voting power or investment power, and also any
common shares which the shareholder has the right to acquire within 60 days, including upon
exercise of common shares purchase options or warrants. There were 10,309,212 common shares
outstanding as of October 31, 2021.
|
|
|
(2)
|
Includes
the sale of all common shares registered herein.
|
|
|
(3)
|
The
shares being registered include up to 5,806,864 common shares issuable upon conversion of
$500,000 in principal of June 2021 Debentures sold on June 18, 2021, subject to a 9.99% beneficial
ownership limitation. Sabby Management, LLC serves as the investment manager of the selling
stockholder, Sabby Volatility Warrant Master Fund, Ltd. and Sabby Healthcare Master Fund,
Ltd. Sabby Healthcare Master Fund additionally owns (i) 1 common share and (ii) $72.25 in
senior convertible debentures issued on September 12, 2017, convertible as of 10/31/2021
into approximately 839 shares and (iii) 9 shares underlying outstanding warrants, none of
which are being registered hereunder. Hal Mintz is the manager of Sabby Management, LLC.
Each of Sabby Management, LLC and Hal Mintz disclaims beneficial ownership over the securities
covered by the Form S-1 except to the extent of its pecuniary interest therein.
|
PLAN OF DISTRIBUTION
Each Selling
Stockholder of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all
of their securities covered hereby on the principal trading market or any other stock exchange, market or trading facility on which the
securities are traded or in private transactions. These sales may only be at $0.08 per share until our shares of Common Stock are quoted
on the OTCQX, OTCQB or listed on a national securities exchange, and thereafter, through public transactions or private transactions
at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following methods when selling securities:
|
|
●
|
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
●
|
block
trades in which the broker-dealer will attempt to sell the securities as agent but may position
and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
●
|
an
exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
●
|
privately
negotiated transactions;
|
|
|
●
|
settlement
of short sales;
|
|
|
●
|
in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified
number of such securities at a stipulated price per security;
|
|
|
●
|
through
the writing or settlement of options or other hedging transactions, whether through an options
exchange or otherwise;
|
|
|
●
|
a
combination of any such methods of sale; or
|
|
|
●
|
any
other method permitted pursuant to applicable law.
|
The
Selling Stockholders may also sell securities under Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”),
if available, rather than under this prospectus.
Broker-dealers
engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or
markdown in compliance with FINRA IM-2440.
In
connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the
common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders
and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
There
can be no assurance that any Selling Stockholder will sell any or all of the shares of common stock registered pursuant to
the registration statement, of which this prospectus forms a part.
The Selling
Stockholders and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and
the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of
purchases and sales of any of the shares of common stock by the Selling Stockholders and any other participating person. Regulation
M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making
activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common
stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
DESCRIPTION
OF SECURITIES
General
As
of October 31, 2021, our authorized capital stock consisted of:
|
|
●
|
1,000,000,000
shares of common stock, par value $0.0001;
|
|
|
●
|
1,853
shares of Series A 0% convertible preferred stock, par value $0.0001
|
|
|
●
|
1,000
shares of Series B 0% convertible preferred stock, par value $0.0001;
|
|
|
●
|
290.43148
shares of Series C 0% convertible preferred stock, par value $0.0001;
|
|
|
●
|
5,000
shares of Series D 0% convertible preferred stock, par value $0.0001;
|
|
|
●
|
5,000
shares of Series E 0% convertible preferred stock, par value $0.0001;
|
|
|
●
|
8,000
shares of Series F 0% convertible preferred stock, par value $0.0001; and
|
|
|
●
|
29,981,505
shares of “blank check” preferred stock, par value $0.0001.
|
As
of October 31, 2021, we had (i) 10,309,212 shares of common stock issued and outstanding, (ii) 1,853 shares of series A 0% convertible
preferred stock were issued and 133.8125 are outstanding, (iii) 1,000 shares of Series B 0% convertible preferred stock issued and 71
are outstanding, (iv) 290.43148 shares of Series C 0% convertible preferred stock issued and outstanding, (v) 5,000 shares of Series
D 0% convertible preferred stock are issued and outstanding, (vi) 5,000 shares of Series E 0% convertible preferred stock are issued
and outstanding, and (vii) 8,000 shares of Series F 0% convertible preferred stock are issued and outstanding. All of our currently issued
and outstanding shares of capital stock were validly issued, fully paid and non-assessable under the Delaware General Corporate Law or
DGCL.
Set
forth below is a summary description of all of the material terms of securities being registered hereunder, including the outstanding
senior convertible debentures that are convertible into common shares being registered hereunder. This description is qualified in its
entirety by reference to our amended and restated certificate of incorporation, bylaws and form of convertible securities, each of which
is filed as an exhibit to the registration statement, of which this prospectus forms a part.
Common
Stock
The
holders of our common stock are entitled to one vote per share on each matter submitted to a vote at a meeting of our stockholders, except
to the extent that the voting rights of our shares of any class or series of stock are determined and specified as greater or lesser
than one vote per share in the manner provided by our certificate of incorporation. Our stockholders have no pre-emptive rights to acquire
additional shares of our common stock or other securities. Our common stock is not subject to redemption rights and carries no subscription
or conversion rights. In the event of liquidation of our company, the shares of our common stock are entitled to share equally in corporate
assets after satisfaction of all liabilities and any preferences to outstanding preferred stock of the Company. All shares of our common
stock now outstanding are fully paid and non-assessable. Our bylaws authorize the board of directors to declare dividends on our outstanding
shares.
Senior
Convertible Debentures
On
June 18, 2021, the Company sold $600,000 of senior convertible debentures consisting of (i) $500,000 sold for cash to an existing institutional
investor and (ii) $100,000 sold for the cancellation of outstanding obligations (“June 2021 Debentures”).
The
June 2021 Debentures (i) are non-interest bearing, (ii) have a maturity date of June 18, 2022, (iii) are convertible into shares of common
stock of the Company at the election of the holder at any time, subject to a beneficial ownership limitation of 4.99% subject to increase
to 9.99% on 61 days’ notice (or 9.99% for the institutional investor / Selling Stockholder), and (iv) has a conversion price equal
to the lesser of $24.75 and 85% of the lowest Volume Weighted Average Price (VWAP) during the five (5) Trading Days immediately prior
to the conversion date, subject to adjustment, as described therein.
The
June 2021 Debentures also contains provisions providing for an adjustment in the event of stock splits or dividends, and fundamental
transactions. The holder also has the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the
June 2021 Debentures contain anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then
applicable conversion price until such time that the June 2021 Debenture are no longer outstanding. Additionally, the Company has the
option to redeem some or all of the June 2021 Debentures for cash upon notice of twenty (20) trading days provided certain conditions
are met by the Company as more fully described in the June 2021 Debentures.
Without
the approval of the holders of 67% of the outstanding principal the June 2021 Debentures, the Company may not (i) amend its charter documents
in any manner that adversely affects the rights of any January 2021 Debenture holder, (ii) repay or repurchase or acquire shares of its
Common Stock, (iii) repay, repurchase, or acquire certain indebtedness, or (iv) pay cash dividends or distributions on any equity securities
of the Company.
For
purposes of calculating the number of common shares to register upon conversion of $500,000 of the June 2021 Debentures, we have utilized
85% of the VWAP on October 25, 2021, or $0.086105.
Delaware
Anti-Takeover Law and Charter and Bylaws Provisions
We
are subject to Section 203 of the Delaware General Corporation Law. This provision generally prohibits a Delaware corporation from engaging
in any business combination with any interested stockholder for a period of three years following the date the stockholder became an
interested stockholder, unless:
|
|
●
|
prior
to such date, the board of directors approved either the business combination or the transaction
that resulted in the stockholder becoming an interested stockholder;
|
|
|
●
|
upon
consummation of the transaction that resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding
at the time the transaction commenced, excluding for purposes of determining the number of
shares outstanding those shares owned by persons who are directors and also officers and
by employee stock plans in which employee participants do not have the right to determine
confidentially whether shares held subject to the plan will be tendered in a tender or exchange
offer; or
|
|
|
●
|
on
or subsequent to such date, the business combination is approved by the board of directors
and authorized at an annual meeting or special meeting of stockholders and not by written
consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that
is not owned by the interested stockholder.
|
Section
203 defines a business combination to include:
|
|
●
|
any
merger or consolidation involving the corporation and the interested stockholder;
|
|
|
●
|
any
sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation
involving the interested stockholder;
|
|
|
●
|
subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation
of any stock of the corporation to the interested stockholder;
|
|
|
●
|
any
transaction involving the corporation that has the effect of increasing the proportionate
share of the stock of any class or series of the corporation beneficially owned by the interested
stockholder; or
|
|
|
●
|
the
receipt by the interested stockholder of the benefit of any loans, advances, guarantees,
pledges or other financial benefits provided by or through the corporation.
|
In
general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding
voting stock of a corporation, or an affiliate or associate of the corporation and was the owner of 15% or more of the outstanding voting
stock of a corporation at any time within three years prior to the time of determination of interested stockholder status, and any entity
or person affiliated with or controlling or controlled by such entity or person.
Our
certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals
or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. In
particular, the certificate of incorporation and bylaws, as applicable, among other things:
|
|
●
|
provide
our board of directors with the ability to alter its bylaws without stockholder approval;
and
|
|
|
●
|
provide
that vacancies on our board of directors may be filled by a majority of directors in office,
although less than a quorum.
|
Such
provisions may have the effect of discouraging a third-party from acquiring us, even if doing so would be beneficial to our stockholders.
These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and
in the policies formulated by them, and to discourage some types of transactions that may involve an actual or threatened change in control
of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage some
tactics that may be used in proxy fights. We believe that the benefits of increased protection of our potential ability to negotiate
with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company outweigh the disadvantages of discouraging
such proposals because, among other things, negotiation of such proposals could result in an improvement of their terms.
However,
these provisions could have the effect of discouraging others from making tender offers for our shares that could result from actual
or rumored takeover attempts. These provisions also may have the effect of preventing changes in our management.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is American Stock Transfer & Trust Company. The address of American Stock Transfer
& Trust Company is 59 Maiden Lane, New York, New York, 10038 and the phone number is (718) 921-8201. We act as the transfer agent
and registrar for our all classes of our preferred stock.
DESCRIPTION
OF BUSINESS
Overview
Rebus
Holdings, Inc. (fka Inspyr Therapeutics, Inc.) is a pharmaceutical company focused on the research and development of novel targeted
precision therapeutics for the treatment of cancer. Our approach utilizes our proprietary delivery technology to better enhance immuno-modulation
for improved therapeutic outcomes. Our potential first-in-class immune-oncology lead asset, RT-AR001, an adenosine A2B receptor
antagonist, is differentiated by its intratumoral delivery of nano- or microparticle formulations that allows for better tumor infiltration.
The adenosine A2 Receptor is one of many T-cell surface immune checkpoint proteins. Our patented portfolio of adenosine receptor
antagonists provides flexibility to optimize treatment based on the specific adenosine targets found in each type of cancer.
Adenosine
Receptor Modulators
The
adenosine receptor modulators include A2B and dual A2A/A2B antagonists, that have broad development
applicability including indications within immuno-oncology. Very high concentrations of adenosine are produced in the tumor microenvironment
which prevents the host’s own immune cells from attacking the tumor. Adenosine receptor antagonists as single-agents and in combination
with other existing immuno-oncology agents may overcome this immunosuppression and boost the host immune response leading to enhanced
anti-tumor activity as well as inhibition of metastasis. Preclinical data has shown effects with our drug candidates in animal models
utilizing a novel platform delivery system. While we believe that the data from our nonclinical studies appear encouraging, the outcome
of our ongoing or future studies may ultimately be unsuccessful.
Rebus
HJoldings / Ridgeway Licensing Agreement
Pursuant
to our recent termination of license with Ridgeway Therapeutics, Inc., we reacquired the rights to certain intellectual property, discussed
above, and are currently focusing on a pipeline of small molecule adenosine receptor modulators. In October 2020, pursuant to the cancellation
of a license agreement whereby we previously licensed US Patent 9,593,118, we reacquired the exclusive right to such patent that covers
both A2B and dual A2A/A2B antagonists. Accordingly, going forward our major focus will be: (i) further
characterization of the anti-cancer activity of our unique pipeline delivery platform containing A2B and dual A2A/A2B
antagonists, leading to selection of a clinical candidate or candidates for an Investigative New Drug or IND enabling studies;
and (ii) licensing and/or partnering our delivery platform and the A2B and dual A2A/A2B antagonists
for further development.
Our ability to execute
the business plan is contingent upon our ability to raise the necessary funds. During March 2020, we sold $250,000 of debt securities
for cash, in October 2020, we sold $500,000 of debt securities for cash, in January 2021, we sold $500,000 of debt securities for cash,
and in June 2021, we sold $600,000 of debt securities for $500,000 in cash and $100,000 in cancellation of outstanding obligations. We
are currently using such funds to maintain our SEC reporting requirements, pay outstanding invoices to our independent registered accounting
firm, legal fees, and to retain consultants and other personnel in preparation for an Investigational New Drug Application (“IND”)
filing related to our unique delivery platform and portfolio of adenosine A2R antagonists for the treatment of certain solid
tumors. Should we fail to further raise sufficient funds to execute our business plan, our priority would be to maintain our intellectual
property portfolio and seek business development opportunities with potential development partners and/or acquirors.
Pre-Revenue
We
are a pre-revenue, early-stage company that has not achieved profitability, and has no product revenues. Additionally, we have no approved
products for sale.
Going
Concern
Our
auditors’ report on our December 31, 2020 consolidated financial statements expressed an opinion that our capital resources as
of the date of their Audit Report were not sufficient to sustain operations or complete our planned activities for the upcoming year
unless we raised additional funds. Notwithstanding our financings in (i) March 2020 where we raised $250,000, (ii) October 2020 where
we raised $500,000, (iii) January 2021 where we raised $500,000, and (iv) June 2021 where we raised $500,000, our current cash level
raises substantial doubt about our ability to continue as a going concern past the second quarter of 2022. If we do not obtain additional
funds by such time, we may no longer be able to continue as a going concern and will cease operation which means that our shareholders
will lose their entire investment.
Recent
Developments
|
|
●
|
Effective October 12,
2021, we (i) completed a 1-for-75 Reverse Stock Split and (ii) a holding company reorganization whereby we changed our name to Rebus
Holdings, Inc.
|
|
|
|
|
|
|
●
|
On August 16, 2021,
we appointed Raul Silvestre, Esq. as (i) our interim chief executive officer and principal accounting officer and (ii) as a member
of the Board of Directors.
|
|
|
|
|
|
|
●
|
On June 18, 2021, we
completed the private placement of $600,000 of non-interest bearing senior convertible debentures consisting of (i) $500,000 purchased
in cash and (ii) $100,000 purchased pursuant to the cancellation of outstanding obligations.
|
|
|
●
|
On
January 12, 2021, we completed the private placement of $500,000 of non-interest bearing
senior convertible debentures
|
|
|
●
|
On
October 23, 2020, we completed the private placement of $600,000 of non-interest bearing
senior convertible debentures in exchange for $500,000 in cash and the cancellation of $100,000
in obligations.
|
|
|
●
|
On
October 6, 2020, our stockholders approved an increase in our authorized shares of Common
Stock from one hundred fifty million (150,000,000) to one billion (1,000,000,000) shares,
as well as authorizing a reverse stock split of our Common Stock at the discretion of the
Board of not less than 1-for-2 and not greater than 1-for-200 at any time prior to October
5, 2021.
|
|
|
●
|
On
October 5, 2020, in exchange for the issuance of (i) 866,667 shares of Common Stock and (ii)
8,000 shares of Series F 0% Convertible Preferred Stock, we entered into an agreement to
terminate an outstanding license agreement with Ridgeway Therapeutics, Inc. whereby we had
previously licensed certain immune-oncology delivery technologies for the treatment of cancer
to Ridgeway Therapeutics (“License Termination”). As a result of the License
Termination, the Company announced on October 8, 2020 that it would be refocusing its efforts
on a novel-immuno-oncology delivery technology targeting adenosine receptor antagonists for
the treatment of cancer.
|
|
|
●
|
On
March 6, 2020, we completed the private placement of $250,000 of non-interest bearing senior
convertible debentures.
|
Product
Development of Adenosine Receptor Modulators
As
a result of the License Termination, the Company has refocused its business plan on the research and development of its lead asset, RT-AR001,
an adenosine A2 receptor antagonist, which is differentiated by its intratumoral delivery of nano- or microparticle formulations
that allows for better tumor infiltration.
Adenosine
is an extracellular signaling molecule that regulates multiple aspects of tissue function and specifically plays a role in immunity and
inflammation. The adenosine A2 receptor is one of many T-cell surface immune checkpoint proteins. High levels of adenosine
in the tumor microenvironment are produced and, therefore, adenosine signaling, mediated through the A2A and A2B receptors,
suppresses the host immune response to the tumor cells.
As
such, our portfolio of adenosine receptor antagonists has broad applicability as potential immuno-oncology (IO) therapeutic agents
in multiple solid tumor types both as a potential single agent and in combination with other IO agents, in addition to traditional
cytotoxic chemotherapy. We are actively seeking licensing opportunities and/or partners to further develop our unique platform
delivery system of A2B and dual A2A/A2B receptor antagonists. Our current product development plan
for adenosine receptor antagonists contemplates the following major initiatives, subject to the Company receiving sufficient
funds:
|
|
●
|
Continue
development of anti-cancer agents with partner company, Ridgeway Therapeutics, Inc.
|
|
|
●
|
Further
characterization of our platform delivery system and existing agents in preclinical studies,
and towards an investigational new drug (IND) application.
|
|
|
●
|
Support
ongoing licensing / partnership activities.
|
Pre-IND
and IND
The Company is currently
pursuing an IND filing related to our unique delivery platform and portfolio of adenosine A2R antagonists for the treatment
of certain solid tumors, and is preparing its pre-IND application, for its lead asset, RT-AR001, an adenosine A2A receptor
antagonist. The company plans to provide an update on RT-AR001’s clinical development in the second half of 2021.
Our
Technology
We
have what we believe to be a robust intellectual property portfolio covering proprietary A2A agonists (LNC-001, see below),
A2B antagonists (LNC-002, see below), and dual A2A/A2B antagonists (LNC-003, see below). We also have
a substantial catalogue of synthesized compounds, specifically A2A agonists and A2B antagonists that require further
characterization and testing for potential clinical candidates. We believe that our proprietary dual A2A/A2B antagonists
have great potential and should be further explored.
Patents
and Proprietary Rights
Our
success will likely depend upon our ability to preserve our proprietary technologies and operate without infringing the proprietary rights
of other parties. However, we may rely on certain proprietary technologies and know-how that are not patentable or that we determine
to keep as trade secrets. We protect our proprietary information, in part, using confidentiality agreements with our employees, consultants,
significant scientific collaborators, and sponsored researchers that generally provide that all inventions conceived by the individual
in the course of rendering services to us shall be our exclusive property.
The
intellectual property underlying our technology is covered by certain patents and patent applications previously owned by Lewis and Clark
Pharmaceuticals, Inc. (LNC) and now fully owned by the Company. All of the LNC intellectual property has been assigned to the Company.
We solely own all of our patents and patent applications for adenosine receptor modulators, which include three patent estates,
one for A2A agonists, the second for A2B antagonists, and the third for dual A2A/A2B antagonists.
Ownership of these patent estates came from our purchase (in exchange for 7,122,172 shares of our common stock) of Lewis and
Clark Pharmaceuticals, Inc. (LNC) on July 31, 2017. The purchase of LNC also included all know-how, pre-clinical data, and
development data that relate to and form the basis of our technology. Under the purchase agreement, we are sole owners of the technology
and patent estates and are not required to make any other future payments, including fees or other reimbursements, milestones, or royalties,
to LNC.
|
FILE
#
|
|
COUNTRY
|
|
STATUS
|
|
APPLICATION
#
|
|
DATE
FILED
|
|
PATENT
#
|
|
GRANT
DATE
|
|
LNC-001-US
|
|
United States of America
|
|
Issued
|
|
13/956,111
|
|
Jul 31, 2013
|
|
9067963
|
|
Jun 30, 2015
|
|
LNC-001-US-CNT1
|
|
United States of America
|
|
Issued
|
|
14/752,861
|
|
Jun 27, 2015
|
|
9822141
|
|
Nov 21, 2017
|
|
LNC-002-AU
|
|
Australia
|
|
Issued
|
|
2016246068
|
|
Apr 8, 2016
|
|
2016246068
|
|
10-Dec-20
|
|
LNC-002-BE
|
|
Belgium
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-BR
|
|
Brazil
|
|
Pending
|
|
BR 11 2017 021386-9
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-CH
|
|
Switzerland
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-CN
|
|
China
|
|
Issued
|
|
201680026835.1
|
|
Apr 8, 2016
|
|
ZL 20160026835
|
|
Jan 8, 2021
|
|
LNC-002-CZ
|
|
Czech Republic
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-DE
|
|
Germany
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-DK
|
|
Denmark
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-EA
|
|
Eurasian Patent Office
|
|
Issued
|
|
201792156
|
|
Apr 8, 2016
|
|
36954
|
|
Jan 19, 2021
|
|
LNC-002-EP
|
|
European Patent Office
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-ES
|
|
Spain
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-FR
|
|
France
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-GB
|
|
United Kingdom
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-IE
|
|
Ireland
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-IL
|
|
Israel
|
|
Pending
|
|
254902
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-IN
|
|
India
|
|
Pending
|
|
201727039305
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-IT
|
|
Italy
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-JP
|
|
Japan
|
|
Issued
|
|
2018-504080
|
|
Apr 8, 2016
|
|
6738405
|
|
Jul 21, 2020
|
|
LNC-002-KR
|
|
Republic of Korea
|
|
Pending
|
|
10-2017-7031978
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-MX
|
|
Mexico
|
|
Pending
|
|
MX/a2017/012783
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-NL
|
|
Netherlands
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-NZ
|
|
New Zealand
|
|
Pending
|
|
736705
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-PL
|
|
Poland
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-PT
|
|
Portugal
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-SE
|
|
Sweden
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-SG
|
|
Singapore
|
|
Pending
|
|
11201707753X
|
|
Apr 8, 2016
|
|
|
|
|
|
LNC-002-TR
|
|
Turkey
|
|
Issued
|
|
16777436.3
|
|
Apr 8, 2016
|
|
3280417
|
|
Jul 29, 2020
|
|
LNC-002-US
|
|
United States of America
|
|
Issued
|
|
15/094,903
|
|
Apr 8, 2016
|
|
9593118
|
|
Mar 14, 2017
|
|
LNC-002-ZA
|
|
South Africa
|
|
Issued
|
|
2017/07248
|
|
Apr 8, 2016
|
|
201707248
|
|
Oct 31, 2018
|
|
LNC-003-P3
|
|
United States of America
|
|
Pending
|
|
63/000,286
|
|
Mar 26, 2020
|
|
|
|
|
|
LNC-003-PCT
|
|
PCT
|
|
Pending
|
|
PCT/US21/15087
|
|
Jan 26, 2021
|
|
|
|
|
When
appropriate, we will continue to seek patent protection for inventions in our core technologies and in ancillary technologies that support
our core technologies or which we otherwise believe will provide us with a competitive advantage. We will accomplish this by filing and
maintaining patent applications for discoveries we make, either alone or in collaboration with scientific collaborators and strategic
partners. Typically, we plan to file patent applications in the United States and, for LNC-003, worldwide. In addition, we plan to obtain
licenses or options to acquire licenses to patent filings from other individuals and organizations that we anticipate could be useful
in advancing our research, development, and commercialization initiatives and our strategic business interest.
Development
Strategy
We anticipate that under
the planning and direction of key personnel, to outsource all our nonclinical development and manufacturing, and the majority of our
clinical development activities to contract research organizations (CROs) and contract manufacturing organizations (CMOs). Our contract
CROs and CMOs are required to comply with federal, state and United States Food and Drug Administration or FDA regulations including
Good Manufacturing Practices (cGMP), Good Clinical Practices (GCP), and Good Lab Practices (GLP).
We intend
to conduct further characterization and testing of our A2A antagonists to select a candidate for pre-clinical and clinical
trials in an oncology indication. This oncology work is expected to be run in conjunction with and oversight from Ridgeway Therapeutics,
Inc. for the selection of an anti-cancer agent.
Commercialization
Strategy
We
intend to (i) license or sell the underlying technology of our therapeutics to third parties during or after our clinical trials, (ii)
seek a corporate partner for further development, or (iii) continue developing our drug candidates ourselves. It is expected that such
third parties would then continue to develop, market, sell, and distribute any resulting products. As part of our overall strategic plan,
we are exploring our options and actively seeking to engage in a collaborative, strategic and/or licensing arrangement with another pharmaceutical
company. If we enter into any such transaction, we may be required to give up certain rights to our technology and control over its future
development.
Intellectual
Property
We
regard the protection of patents and other intellectual property rights that we own or license as critical to our business and competitive
position. To protect our intellectual property, we rely on patent, trade secret, and copyright law, as well as confidentiality, nondisclosure,
assignment of invention and other contractual arrangements with our officers, directors, employees, consultants, investigators, clinical
trial sites, contractors, collaborators and other third parties to whom we disclose confidential information. Our policy is to pursue
patent applications on inventions and discoveries that we believe are commercially important to the development and growth of our business.
We solely own or have exclusive licenses to our patents and patent applications.
Our
pipeline currently includes a substantial catalogue of synthesized compounds, specifically A2A agonists and A2B antagonists
that require further characterization and testing for potential clinical candidates. Our proprietary dual A2A/A2B antagonists
have great potential and need to be further explored.
Our
intellectual property estate, shown above, has twenty-four (24) issued patents in twenty-two (22) different jurisdictions and ten (10)
currently pending applications. With appropriate funding and upon further research into our dual A2A/A2B antagonists,
we intend to file regular US and foreign applications to enable worldwide protection of these antagonists.
When
appropriate and funding permitting, we plan to continue to seek patent protection for inventions in our core technologies and in ancillary
technologies that support our core technologies or which we otherwise believe would provide us with a competitive advantage. We expect
to be able to accomplish this by filing and maintaining patent applications for discoveries we make, either alone or in collaboration
with scientific collaborators and strategic partners. Typically, we plan to file patent applications in the United States as well as
foreign countries, where applicable. In addition, we may obtain licenses or options to acquire licenses to patent filings from other
individuals and organizations that we anticipate could be useful in advancing our research, development and commercialization initiatives
and our strategic business interest.
Manufacturing
and Supply
We
do not plan to develop company-owned or company-operated manufacturing facilities. We historically have and we plan to in the future,
outsource all drug manufacturing to contract manufacturers that are required to operate in compliance with cGMP. We may also seek to
refine the current manufacturing process in order to achieve improvements in efficiency, costs, purity and the like as well as address
different drug formulations to achieve improvements in stability and/or drug delivery.
Governmental
Regulations
FDA
Approval Process
Prior
to commencement of clinical studies involving humans, preclinical testing of new pharmaceutical products is generally conducted on animals
in the laboratory to evaluate the potential efficacy and safety of the product candidate. The results of these studies are submitted
to the FDA as part of an IND application, which must become effective before clinical testing in humans can begin. Typically, human clinical
evaluation involves a time-consuming and costly three-phase process. In Phase I, clinical trials are conducted with a small number of
people to assess safety, tolerability and to evaluate the pattern of drug distribution within the body. In Phase II, clinical trials
are conducted with groups of patients afflicted with a specific disease in order to determine preliminary efficacy, optimal dosages and
expanded evidence of safety. (In some cases, an initial trial is conducted in diseased patients to assess both preliminary efficacy and
preliminary safety, in which case it is referred to as a Phase I/II trial.) In Phase III, large-scale, multi-center, comparative trials
are conducted with patients afflicted with a target disease in order to provide enough data to demonstrate the efficacy and safety required
by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate,
alter, suspend, or terminate the testing based upon the data which have been accumulated to that point and its assessment of the risk/benefit
ratio to the patient. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuing
process.
The
results of the preclinical and clinical testing on non-biologic drugs and certain diagnostic drugs are submitted to the FDA in the form
of a New Drug Application (NDA) for approval prior to commencement of commercial sales. In responding to an NDA submission, the FDA may
grant marketing approval, may request additional information, may deny the application if it determines that the application does not
provide an adequate basis for approval, and may also refuse to review an application that has been submitted if it determines that the
application does not provide an adequate basis for filing and review. There can be no assurance that approvals would be granted on a
timely basis, if at all, for any of our proposed products.
Orphan
Drugs
Under
the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally
defined as a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested
before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use
are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory
review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular
disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product,
for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug
for the same orphan indication, except in limited circumstances, such as a showing of clinical superiority to the product with orphan
drug exclusivity in that it is shown to be safer, more effective or makes a major contribution to patient care. Orphan drug exclusivity
does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease
or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application
user fee.
Asia,
European and Other Regulatory Approval
Whether
or not FDA approval has been obtained, approval of a product by comparable regulatory authorities in Europe and other countries is necessary
prior to commencement of marketing the product in such countries. The regulatory authorities in each country may impose their own requirements
and may refuse to grant an approval, or may require additional data before granting it, even though the relevant product has been approved
by the FDA or another authority. As with the FDA, the regulatory authorities in the European Union (EU), countries located in Asia, and
other developed regions have lengthy approval processes for pharmaceutical products. The process for gaining approval in particular countries
and regions varies, but generally follows a similar sequence to that described for FDA approval. In Europe, the European Committee for
Proprietary Medicinal Products provides a mechanism for EU-member states to exchange information on all aspects of product licensing.
The EU has established a European agency for the evaluation of medical products, with both a centralized community procedure and a decentralized
procedure, the latter being based on the principle of licensing within one member country followed by mutual recognition by the other
member countries. In China, the CFDA functions as the counterpart to the FDA in the United States and is responsible for overseeing
drug approvals in China and its territories.
Reimbursement
and Health Care Cost Control
Reimbursement
for the costs of treatments and products such as ours from government health administration authorities, private health insurers and
others, both in the United States and abroad, is a key element in the success of new health care products. Significant uncertainty often
exists as to the reimbursement status of newly approved health care products. The revenue and profitability of some health care-related
companies have been affected by the continuing efforts of governmental and third-party payors to contain or reduce the cost of health
care through various means. Payors are increasingly attempting to limit both coverage and the levels of reimbursement for new therapeutic
products approved for marketing by the FDA, and are refusing, in some cases, to provide any coverage for uses of approved products for
disease indications for which the FDA has not granted marketing approval. In certain foreign markets, pricing or profitability of prescription
pharmaceuticals is subject to government control.
In
the United States, there have been a number of federal and state proposals to implement government control over health care costs. The
U.S. Patient Protection and Affordance Care Act and the Health Care and Education Reconciliation Act were signed into law in March 2010.
A number of provisions of those laws require further rulemaking action by governmental agencies to implement. The laws change access
to health care products and services and create new fees for the pharmaceutical and medical device industries. Future rulemaking could
increase rebates, reduce prices or the rate of price increases for health care products and services, or require additional reporting
and disclosure. The laws also include new authorization to the FDA to approve companies to market biosimilar products within the United
States, although to date FDA rulemaking under this legislation has been limited. We cannot predict the timing or impact of any such future
rulemaking on our business.
Other
Regulations
We
are also subject to various U.S. federal, state, local and international laws, regulations and recommendations relating to safe working
conditions, laboratory and manufacturing practices and the use and disposal of hazardous or potentially hazardous substances, including
radioactive compounds and infectious disease agents, used in connection with our business. Additionally, we are subject to regulations
and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, and Securities and Exchange
Commission regulations. We cannot accurately predict the extent of government regulation which might result from future legislation or
administrative action.
Employees
As
of October 31, 2021, we employed only Mr. Silvestre, on a part time basis, as our interim chief executive officer who is our only employee.
In addition, we contract with a limited number of consultants to assist in activities related to our operations.
Corporate
History
We
were incorporated in the State of Delaware in November 2003. In August of 2016, we changed our name from GenSpera, Inc. to Inspyr Therapeutics,
Inc. In October 2021, we completed a holding company reorganization structure, changing our name to Rebus Holdings, Inc. Our principal
office is located in Westlake Village, California. Since our inception, we have invested a substantial portion of our efforts and financial
resources in the development of mipsagargin (G-202). In July of 2017, we acquired Lewis and Clark Pharmaceuticals and licensed certain
assets to Ridgeway Therapeutics for further development. Upon the termination of such license in October 2020, we resumed operations
focusing our efforts on our Adenosine Receptor Modulators. On October 12, 2021, we completed a 1:75 reverse stock split of our common
stock. We have generated no revenues from the sale of our product candidates and have experienced substantial net operating losses.
Where
to Find More Information
We
make our public filings with the SEC, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports
on Form 8-K and all exhibits and amendments to these reports. These materials are available on the SEC’s web site, www.sec.gov.
You
may also read and copy any materials you file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE., Washington,
DC 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may obtain information on the operation of the Public Reference
Room by calling the SEC at 1–800–SEC–0330. Additionally, the SEC maintains an Internet site that contains reports,
proxy and information statements, and other information regarding issuers that file electronically with the SEC. The Internet site is
located at www.sec.gov. Alternatively, you may obtain copies of these filings, including exhibits, by writing or telephoning us at:
REBUS
HOLDINGS, INC.
2629
Townsgate Road #215
Westlake
Village, CA 91361
Attn:
Chief Executive Officer
Tel:
(818) 597-7552
PROPERTIES
Our
executive offices are located at 2629 Townsgate Road, Suite 215, Westlake Village, CA 91361. At present our employee and consultants
work virtually from around the country. We currently pay no money for these facilities. There is no affiliation between us or any of
our principals or agents and our landlords or any of their principals or agents.
LEGAL
PROCEEDINGS
None.
MARKET
PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND
RELATED STOCKHOLDER MATTERS
Market
Information
Our
common shares are quoted on the pink sheets of the OCT Markets under the symbol RBSH. Any over-the-counter market quotations
reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
Holders
As
of October 31, 2021, we had approximately 155 record holders of our common stock.
Dividend
Policy
We
have never declared or paid any cash dividends on our capital stock and we do not currently anticipate declaring or paying cash dividends
on our capital stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance the operation
and expansion of our business. Any future determination relating to our dividend policy will be made at the discretion of our board of
directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects,
contractual restrictions and covenants, applicable law and other factors that our board of directors may deem relevant. If we do not
pay dividends, a return on your investment will occur only if the market price of our common stock appreciates.
Securities
authorized for issuance under equity compensation plans
The
following table sets forth information as of December 31, 2020 with respect to our compensation plans under which equity securities may
be issued.
|
|
|
(a)
|
|
|
(b)
|
|
|
(c)
|
|
|
|
|
Number
of Securities
to be Issued
upon Exercise of
Outstanding
Options, Warrants
and Rights
|
|
|
Weighted-Average
Exercise Price of
Outstanding
Options,
Warrants and
Rights
|
|
|
Number
of Securities
Remaining Available for
Future Issuance under
Equity Compensation Plans
(Excluding Securities
Reflected in Column (a))
|
|
|
Equity
compensation plans approved by security holders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N/A
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
compensation plans not approved by security holders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N/A
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
Our
2007 Equity Compensation Plan, 2009 Executive Compensation Plan, Inducement Award Stock Option
Plan and 2017 Equity Compensation Plan have all expired or been terminated by the Board.
|
2007
Equity Compensation Plan
Our
2007 Equity Compensation Plan (“2007 Plan”) was administered by our board or any of its committees. The purposes of the 2007
Plan was to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive
to Employees, Directors and Consultants, and to promote the success of our business. The issuance of awards under our 2007 Plan was at
the discretion of the administrator, which had the authority to determine the persons to whom any awards shall be granted and the terms,
conditions and restrictions applicable to any award. Under our 2007 Plan, we were able to grant stock options, restricted stock, stock
appreciation rights, restricted stock units, performance units, performance shares and other stock based awards. Our 2007 Plan authorized
the issuance of up to 67 shares (pre-October 2021 reverse split) of common stock for the foregoing awards per fiscal year with an aggregate
of 267 shares (pre-October 2021 reverse split) of common stock available for issuance under the 2007 Plan. As of December 31, 2020, we
had granted awards under the 2007 Plan equal to approximately 241 shares (pre-October 2021 reverse split) of our common stock, and 202
shares (pre-October 2021 reverse split) have been cancelled or forfeited. In the event of a change in control, awards under the 2007
Plan will become fully vested unless such awards are assumed or substituted by the successor corporation.
2009
Executive Compensation Plan
Our
2009 Executive Compensation Plan, as amended (“2009 Plan”) was administered by our Board or any of its committees. The purpose
of our 2009 Plan was to advance the interests of the Company and our stockholders by attracting, retaining and rewarding persons performing
services for us and to motivate such persons to contribute to our growth and profitability. The issuance of awards under our 2009 Plan
was at the discretion of the administrator, which had the authority to determine the persons to whom any awards shall be granted and
the terms, conditions and restrictions applicable to any award. Under our 2009 Plan, we were able to grant stock options, restricted
stock, stock appreciation rights, restricted stock units, performance units, performance shares and other stock-based awards. As of December
31, 2020, our 2009 Plan authorized the issuance of up to 267 shares (pre-October 2021 reverse split) of our common stock for the foregoing
awards, and we have granted awards under the plan equal to approximately 220 common shares (pre-October 2021 reverse split), and 184
shares (pre-October 2021 reverse split) had been cancelled or forfeited.
Inducement
Award Stock Option Plan
Our
Inducement Award Stock Option Plan (“Inducement Plan”) was administered by our board or our compensation committee. The Plan
is intended to be used in connection with the recruiting and inducement of senior management and employees. The issuance of wards under
the Inducement Plan is at the discretion of the administrator which has the authority to determine the persons to whom any awards shall
be granted and the terms, conditions and restrictions applicable to any award. The Company did not seek approval of the Plan by our stockholders.
Pursuant to the Inducement Plan, the Company was able to grant stock options for up to a total of 400 shares (pre-October 2021 reverse
split) of common stock to new employees of the Company. As of December 31, 2020, 282 grants (pre-October 2021 reverse split) have been
made pursuant to the Plan, and 130 shares (pre-October 2021 reverse split) have been cancelled or forfeited.
Inspyr
Therapeutics 2017 Equity Compensation Plan
Our
2017 Equity Compensation Plan (“2017 Plan”) was administered by our board or any of its committees. The purposes of the 2017
Plan was to attract and retain the best available personnel for positions of substantial responsibility, to provide additional incentive
to Employees, Directors and Consultants, and to promote the success of our business. The issuance of awards under our 2017 Plan was at
the discretion of the administrator, which had the authority to determine the persons to whom any awards shall be granted and the terms,
conditions and restrictions applicable to any award. Under our 2017 Plan, we were able to grant stock options, restricted stock, stock
appreciation rights, restricted stock units, performance units, performance shares and other stock based awards. Our 2017 Plan authorized
the issuance of up to 2,667 shares (pre-October 2021 reverse split) of common stock for the foregoing awards. As of December 31, 2020,
we had granted no awards under the 2017 Plan, and no shares had been cancelled or forfeited.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The
following Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements
regarding our business development plans, pre-clinical and clinical studies, regulatory reviews, timing, strategies, expectations, anticipated
expenses levels, business prospects and positioning with respect to market, demographic and pricing trends, business outlook, technology
spending and various other matters (including contingent liabilities and obligations and changes in accounting policies, standards and
interpretations) and express our current intentions, beliefs, expectations, strategies or predictions. These forward-looking statements
are based on a number of assumptions and currently available information and are subject to a number of risks and uncertainties. Our
actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors, including
those set forth under “Special Note Regarding Forward-Looking Statements” and under “Risk Factors” and elsewhere
in this annual report. The following discussion should be read in conjunction with our financial statements and related notes thereto
included elsewhere in this annual report.
Our
Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is provided in addition
to the accompanying financial statements and notes to assist readers in understanding our results of operations, financial condition,
and cash flows. MD&A is organized as follows:
|
|
●
|
Company
Overview – Discussion of our business plan and strategy in order to provide context
for the remainder of MD&A.
|
|
|
●
|
Critical
Accounting Policies – Accounting policies that we believe are important to understanding
the assumptions and judgments incorporated in our reported financial results and forecasts.
|
|
|
●
|
Results
of Operations – Analysis of our financial results comparing the year ended December
31, 2020 to the year ended December 31, 2019.
|
|
|
●
|
Liquidity
and Capital Resources – Liquidity discussion of our financial condition and potential
sources of liquidity.
|
Company
Overview
Business
Rebus
Holdings, Inc is a pharmaceutical company focused on the research and development of novel targeted precision therapeutics for the treatment
of cancer. Our approach utilizes our proprietary delivery technology to better enhance immuno-modulation for improved therapeutic outcomes.
Our potential first-in-class immune-oncology lead asset, RT-AR001, an adenosine A2B receptor antagonist, is differentiated
by its intratumoral delivery of nano- or microparticle formulations that allows for better tumor infiltration. The adenosine A2
Receptor is one of many T-cell surface immune checkpoint proteins. Our patented portfolio of adenosine receptor antagonists provides
flexibility to optimize treatment based on the specific adenosine targets found in each type of cancer.
Adenosine
Receptor Modulators
The
adenosine receptor modulators include A2B and dual A2A/A2B antagonists, that have broad development
applicability including indications within immuno-oncology. Very high concentrations of adenosine are produced in the tumor microenvironment
which prevents the host’s own immune cells from attacking the tumor. Adenosine receptor antagonists as single-agents and in combination
with other existing immuno-oncology agents may overcome this immunosuppression, and boost the host immune response leading to enhanced
anti-tumor activity as well as inhibition of metastasis. Preclinical data has shown effects with our drug candidates in animal models
utilizing a novel platform delivery system. While we believe that the data from our nonclinical studies appear encouraging, the outcome
of our ongoing or future studies may ultimately be unsuccessful.
Inspyr
/ Ridgeway Licensing Agreement
Pursuant
to our recent termination of license with Ridgeway Therapeutics, Inc., we reacquired the rights to certain intellectual property, discussed
above, and are currently focusing on a pipeline of small molecule adenosine receptor modulators. In October 2020, pursuant to the cancellation
of a license agreement whereby we previously licensed US Patent 9,593,118, we reacquired the exclusive right to such patent that covers
both A2B and dual A2A/A2B antagonists. Accordingly, going forward our major focus will be to: (i) further
characterization of the anti-cancer activity of our unique pipeline delivery platform containing A2B and dual A2A/A2B
antagonists, leading to selection of a clinical candidate or candidates for an Investigative New Drug or IND enabling studies;
and (ii) licensing and/or partnering our delivery platform and the A2B and dual A2A/A2B antagonists
for further development.
During
March 2020, we sold $250,000 of debt securities for cash, in October 2020, we sold $500,000 of debt securities for cash, in January
2021, we sold $500,000 of debt securities for cash and in June 2021, we sold $500,000 of debt securities for cash. We are currently using such funds to maintain our SEC reporting requirements,
pay outstanding invoices to our independent registered accounting firm, legal fees, and to retain consultants and other personnel in
preparation for an IND filing related to our unique delivery platform and portfolio of adenosine A2R antagonists for the
treatment of certain solid tumors. Should we fail to further raise sufficient funds to execute our business plan, our priority would
be to maintain our intellectual property portfolio and seek business development opportunities with potential development partners
and/or acquirors.
Pre-Revenue
We
are a pre-revenue, early-stage company that has not achieved profitability, and has no product revenues. Additionally, we have no approved
products for sale.
Recent
Developments
|
|
●
|
On
June 18, 2021, we completed the private placement of $600,000 of non-interest bearing senior convertible debentures consisting of
(i) $500,000 purchased in cash and (ii) $100,000 purchased pursuant to the cancellation of outstanding obligations.
|
|
|
●
|
On
January 12, 2021, we completed the private placement of $500,000 of non-interest bearing
senior convertible debentures.
|
|
|
●
|
On
October 23, 2020, we completed the private placement of $600,000 of non-interest bearing
senior convertible debentures in exchange for $500,000 in cash and the cancellation of $100,000
in obligations
|
|
|
●
|
On
October 6, 2020, our stockholders approved an increase in our authorized shares of Common
Stock from one hundred fifty million (150,000,000) to one billion (1,000,000,000) shares,
as well as authorizing a reverse stock split of our Common Stock at the discretion of the
Board of not less than 1-for-2 and not greater than 1-for-200 at any time prior to October
5, 2021.
|
|
|
●
|
On
October 5, 2020, in exchange for the issuance of (i) 866,667 shares of Common Stock and (ii)
8,000 shares of Series F 0% Convertible Preferred Stock, we entered into an agreement to
terminate an outstanding license agreement with Ridgeway Therapeutics, Inc. whereby we had
previously licensed certain immune-oncology delivery technologies for the treatment of cancer
to Ridgeway Therapeutics (“License Termination”). As a result of the License
Termination, the Company announced on October 8, 2020 that it would be refocusing its efforts
on a novel-immuno-oncology delivery technology targeting adenosine receptor antagonists for
the treatment of cancer.
|
|
|
●
|
On
March 6, 2020, we completed the private placement of $250,000 of non-interest bearing senior
convertible debentures.
|
Financial
To
date, we have devoted substantially all of our efforts and financial resources to the development of our proposed drug candidates. We
have not received FDA approval to market, distribute or sell any products. We have recently begun working on developing IND approved
studies for our adenosine receptor technology platform.
Since
our inception in 2003, we have generated no revenue from product sales and have funded our operations principally through the private
and public sales of our equity securities. We have never been profitable and as of September 30, 2021 we had an accumulated deficit of approximately
$64.3 million. We expect to continue to incur significant operating losses for the foreseeable future as we continue the development
of our product candidates and advance them through clinical trials.
Our
cash balances at December 31, 2020 were approximately $404,000 representing 100% of total assets. In October 2020,
we completed a private placement of $500,000 in cash of our debt securities, in January 2021, we completed a private placement of $500,000
in cash of our debt securities, and in June 2021, we completed another private placement of $500,000 in cash of our debt securities.
Based on our current expected level of operating expenditures and current cash balance as of the date of this report, we expect to be
able to fund our operations into the second quarter of 2022. This period could be shortened if there are any significant increases in
spending that were not anticipated or other unforeseen events.
We
anticipate raising additional cash through the private or public sales of equity or debt securities, collaborative arrangements, licensing
agreements or a combination thereof, to continue to fund our operations and the development of our product candidates. There is no assurance
that any such collaborative arrangement will be entered into or that financing will be available to us when needed in order to allow
us to continue our operations, or if available, on terms acceptable to us. If we do not raise sufficient funds in a timely manner, we
may be forced to curtail operations, delay or stop our ongoing pre-clinical studies and potential clinical trials, cease operations altogether,
or file for bankruptcy. We currently do not have commitments for future funding from any source.
Going
Concern
Our
auditors’ report on our December 31, 2020 consolidated financial statements expressed an opinion that our capital resources as
of the date of their Audit Report were not sufficient to sustain operations or complete our planned activities for the upcoming year
unless we raised additional funds. During February of 2018, we curtailed our operations due to our lack of cash, but upon the cancellation
of the Ridgeway license, we resumed preclinical development. Notwithstanding our recent financings in January of 2021 whereby we raised
$500,000 and June of 2021 whereby we raised an additional $500,000, our current cash level raises substantial doubt about our ability
to continue as a going concern. If we do not obtain additional funds, we may no longer be able to continue as a going concern and will
cease operation which means that our shareholders will lose their entire investment.
Critical
Accounting Policies
We
have prepared our financial statements in conformity with accounting principles generally accepted in the United States, which requires
management to make significant judgments and estimates that affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. We base
these significant judgments and estimates on historical experience and other applicable assumptions we believe to be reasonable based
upon information presently available. These estimates may change as new events occur, as additional information is obtained and as our
operating environment changes. These changes have historically been minor and have been included in the financial statements as soon
as they became known. Actual results could materially differ from our estimates under different assumptions, judgments or conditions.
All
of our significant accounting policies are discussed in Note 3, Summary of Critical Accounting Policies and Use of Estimates, to our
financial statements, included elsewhere in this annual report. We have identified the following as our critical accounting policies
and estimates, which are defined as those that are reflective of significant judgments and uncertainties, are the most pervasive and
important to the presentation of our financial condition and results of operations and could potentially result in materially different
results under different assumptions, judgments or conditions.
We
believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of
our financial statements:
Use
of Estimates - The preparation of financial statements in conformity with generally accepted accounting principles requires management
to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying disclosures. Significant
estimates include the fair value of derivative instruments, stock-based compensation, recognition of clinical trial costs and other accrued
liabilities. Actual results may differ from those estimates.
Derivative
Liability - The Company has financial instruments that are considered derivatives or contain embedded features subject to derivative
accounting. Embedded derivatives are valued separately from the host instrument and are recognized as derivative liabilities in the Company’s
balance sheet. The Company measures these instruments at their estimated fair value and recognizes changes in their estimated fair value
in results of operations during the period of change. The Company values its derivative liabilities using the Black-Scholes option valuation
model. The resulting liability is valued at each reporting date and the change in the liability is reflected as change in derivative
liability in the statement of operations.
Fair
Value of Financial Instruments - Derivative liabilities consist of certain of our preferred stock and warrants with anti-dilution
provisions, and are valued using option pricing models which incorporate the Company’s stock price, volatility, U.S. risk-free
rate, dividend rate, and estimated life.
Recent
Accounting Pronouncements
With
the exception of those discussed below, there have not been any recent changes in accounting pronouncements and Accounting Standards
Update (ASU) issued by the Financial Accounting Standards Board (FASB) during the year ended December 31, 2020 that are of significance
or potential significance to the Company.
In
December 2019, the FASB issued ASU 2019-12, “Simplifying the Accounting for Income Taxes.” This guidance, among other provisions,
eliminates certain exceptions to existing guidance related to the approach for intraperiod tax allocation, the methodology for calculating
income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. This guidance also requires
an entity to reflect the effect of an enacted change in tax laws or rates in its effective income tax rate in the first interim period
that includes the enactment date of the new legislation, aligning the timing of recognition of the effects from enacted tax law changes
on the effective income tax rate with the effects on deferred income tax assets and liabilities. Under existing guidance, an entity recognizes
the effects of the enacted tax law change on the effective income tax rate in the period that includes the effective date of the tax
law. ASU 2019-12 is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. We do
not expect that the adoption of this standard will have a material impact on the Company’s consolidated financial statements.
Result
of Operations
Year
Ended December 31, 2020 Compared to the Year Ended December 31, 2019
Our
results of operations have varied significantly from year to year and quarter to quarter and may vary significantly in the future. We
did not have revenue during the years ending December 31, 2020 and 2019. We do not anticipate generating any revenues during 2021. Net
loss for 2020 and 2019 were $6,295,000 and $934,000, respectively, resulting from the operational activities described below.
Operating
Expenses
Operating
expense totaled $2.5 million and $0.6 million during 2020 and 2019, respectively. The increase in operating expenses is the result of
the following factors.
|
|
|
Year Ended
|
|
|
Change in
2020
|
|
|
|
|
December
31,
|
|
|
Versus
2019
|
|
|
|
|
2020
|
|
|
2019
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in thousands)
|
|
|
|
|
|
|
|
|
Operating
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research
and development
|
|
$
|
18
|
|
|
$
|
44
|
|
|
$
|
(26
|
)
|
|
|
(60
|
)%
|
|
License
termination cost
|
|
|
1,969
|
|
|
|
—
|
|
|
|
1,969
|
|
|
|
100
|
%
|
|
General
and administrative
|
|
|
494
|
|
|
|
565
|
|
|
|
(71
|
)
|
|
|
(13
|
)%
|
|
Total
operating expense
|
|
$
|
2,481
|
|
|
$
|
609
|
|
|
$
|
1,872
|
|
|
|
307
|
%
|
Research
and Development
Research
and development expenses totaled $18,000 and $44,000 for the years ended 2020 and 2019, respectively. The decrease of $26,000, or 60%,
in 2020 compared to 2019 was primarily due to the termination of storage costs related to Mipsagargin, which was being developed prior
to the curtailment of operations in February of 2018, partially offset by the engagement of consultants to develop the adenosine A2R
antagonists and in preparation for an IND filing.
Our
future research and development expenses will consist primarily of expenditures related to consultants and other personnel and costs
required to develop the adenosine A2R antagonists and in preparation for an IND filing related to our unique delivery platform and portfolio
of adenosine A2R antagonists for the treatment of certain solid tumors.
License
Termination Cost
License
termination cost relates to the termination of a licensing agreement previously entered into on August 3, 2018, as more fully described
elsewhere in this filing. We incurred noncash expense of approximately $1,944,000 related to the issuance of 866,667 shares of common
stock and 8,000 shares of Series F 0% Convertible Preferred Stock. Additionally, we have assumed certain expenses and costs of approximately
$25,000.
General
and Administrative
General
and administrative expenses totaled $0.5 million and $0.57 million during 2020 and 2019, respectively. The decrease of approximately
$0.07 million, or 13%, in 2020 compared to 2019 was primarily the result of a decrease in professional fees.
Year
Ended December 31, 2020 Compared to the Year Ended December 31, 2019
Other
Income (Expense)
Other
income (expense) totaled approximately $3.7 million and $0.3 million of expense for 2020 and 2019, respectively.
|
|
|
Year Ended
|
|
|
Change
in
|
|
|
|
|
December
31,
|
|
|
2020
Versus 2019
|
|
|
|
|
2020
|
|
|
2019
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in thousands)
|
|
|
|
|
|
|
|
|
Gain
(loss) on change in fair value of derivative liability
|
|
$
|
(3,846
|
)
|
|
$
|
327
|
|
|
$
|
(4,173
|
)
|
|
|
(1,276
|
)%
|
|
Gain
on conversion of debt
|
|
|
334
|
|
|
|
125
|
|
|
|
209
|
|
|
|
167
|
%
|
|
Interest
(expense), net
|
|
|
(302
|
)
|
|
|
(777
|
)
|
|
|
475
|
|
|
|
61
|
%
|
|
Total other income (expense)
|
|
$
|
(3,814
|
)
|
|
$
|
(325
|
)
|
|
$
|
(3,489
|
)
|
|
|
(1,074
|
)%
|
Loss
on change in fair value of derivative liability
As
a result of a change in the fair value of our derivative liability, we realized loss of $3.8 million and gain of $0.3 million during
the years ended December 31, 2020 and 2019, respectively. The change in the fair value of our derivative liability was the result of
our convertible debentures and notes issued in September 2017, July 2018, December 2018, July 2019, October 2019, November 2019, March
2020 and October 2020, where we issued convertible notes with variable conversion rates, and to the issuance of our Series F preferred
stock in October 2020, which is convertible into a variable number of shares of common stock. Refer to Note 7 in our Consolidated Financial
Statements for further discussion on our derivative liability.
Gain
on conversion of debt
There
was a gain on conversion of debt of approximately $0.3 million during the year ended December 31, 2020, with a gain of approximately
$0.1 million during the year ended December 31, 2019. Gain on conversion of debt results from the difference between the fair value of
common stock issued upon conversion and the carrying amount of the debt converted.
Interest
income (expense)
We
had $0.3 million net interest expense in 2020, compared to $0.8 million of expense in 2019. The decrease of $0.5 million was attributable
to a decrease in the cost associated with derivative instruments issued with a value in excess of proceeds received.
Three
Months Ended September 30, 2021 Compared to Three Months Ended September 30, 2020
Our
results of operations have varied significantly from year to year and quarter to quarter and may vary significantly in the future. We
did not have revenue during the three months ended September 30, 2021 and 2020, and we do not anticipate generating any revenues during
2021. Net income for the three months ending September 30, 2021 was approximately $6.1 million and net income for the three months ended
September 30, 2020 was approximately $2.1 million, resulting from the operational activities described below.
Operating
Expenses
Operating
expense totaled approximately $0.2 million and $0.1 million during the three months ended September 30, 2021 and 2020, respectively.
The increase in operating expenses is the result of the following factors.
|
|
|
Three
months ended
September 30,
|
|
|
Change
in
2021 versus 2020
|
|
|
|
|
2021
|
|
|
2020
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in
thousands)
|
|
|
|
|
|
|
|
|
Operating
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research
and development
|
|
$
|
97
|
|
|
$
|
11
|
|
|
$
|
86
|
|
|
|
782
|
%
|
|
General
and administrative
|
|
|
118
|
|
|
|
126
|
|
|
|
(8
|
)
|
|
|
6
|
%
|
|
Total
operating expenses
|
|
$
|
215
|
|
|
$
|
137
|
|
|
$
|
78
|
|
|
|
57
|
%
|
Research
and Development Expenses
Research
and development expenses totaled approximately $0.1 million and $0.01 million for the three months ended September 30, 2021 and 2020,
respectively.
Our
current research and development expenses currently consist primarily of consulting fees and development expense related to development
of the adenosine A2R antagonists and preparation for an IND filing.
General
and Administrative
General
and administrative expenses totaled approximately $0.1 million and $0.1 million for the three months ended September 30, 2021 and 2020,
respectively. The decrease of approximately $0.01 million, or 6%, for the three months ended September 30, 2021 compared to the same
period in 2020, was primarily due to decreased director compensation.
Our
general and administrative expenses currently consist primarily of expenditures related to legal, accounting and tax, other professional
services, and general operating expenses.
Other
Income (Expense)
Other
income (expense) totaled approximately $6.3 million of income and $2.2 million of income for the three months ended September 30, 2021
and 2020, respectively.
|
|
|
Three
Months Ended
September 30,
|
|
|
Change
in
2021 Versus 2020
|
|
|
|
|
2021
|
|
|
2020
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in
thousands)
|
|
|
|
|
|
|
|
|
Gain
(loss) on change in fair value of derivative liability
|
|
$
|
6,660
|
|
|
$
|
2,024
|
|
|
$
|
4,636
|
|
|
|
229
|
%
|
|
Gain
(loss) on conversion of debt
|
|
|
(48
|
)
|
|
|
240
|
|
|
|
(288
|
)
|
|
|
120
|
%
|
|
Interest
(expense), net
|
|
|
(272
|
)
|
|
|
(21
|
)
|
|
|
(251
|
)
|
|
|
1,195
|
%
|
|
Total
other (expense)
|
|
$
|
6,340
|
|
|
$
|
2,243
|
|
|
$
|
4,097
|
|
|
|
183
|
%
|
Gain
(loss) on change in fair value of derivative liability
As
a result of a change in the fair value of our derivative liability, we realized gain of $6.7 million and $2.0 million during the three
months ended September 30, 2021 and 2020, respectively. The change in the fair value of our derivative liability was the result of our
convertible debentures and notes issued in September 2017, October 2020, January 2021 and June 2021, where we issued convertible notes
with variable conversion rates, and to the issuance of our Series F preferred stock in October 2020, which is convertible into a variable
number of shares of common stock. Refer to Note 6 in our unaudited condensed consolidated financial statements for further discussion
on our derivative liability.
Gain
on conversion of debt
There
was a loss on conversion of debentures of approximately $0.05 million during the three months ended September 30, 2021, with gain of
approximately $0.2 million during the three months ended September 30, 2020. Gain on conversion of debt results from the difference between
the fair value of common stock issued upon conversion and the carrying amount of the debt converted.
Interest
income (expense)
We
had net interest expense of $0.3 million in the three months ended September 30, 2021 compared to expense of $0.02 million for the three
months ended September 30, 2020. The increase of $0.3 million was attributable an increase in the cost associated with derivative instruments
issued with a value in excess of proceeds received.
Nine
Months Ended September 30, 2021 Compared to Nine Months Ended September 30, 2020
Our
results of operations have varied significantly from year to year and quarter to quarter and may vary significantly in the future. We
did not have revenue during the nine months ended September 30, 2021 and 2020, and we do not anticipate generating any revenues during
2021. Net income for the nine months ended September 30, 2021 was approximately $2.6 million and net income for the nine months ended
September 30, 2020 was approximately $0.2 million, resulting from the operational activities described below.
Operating
Expenses
Operating
expense totaled approximately $0.6 million and $0.4 million during the nine months ended September 30, 2021 and 2020, respectively. The
increase in operating expenses is the result of the following factors.
|
|
|
Nine
months ended
September 30,
|
|
|
Change
in
2021 versus 2020
|
|
|
|
|
2021
|
|
|
2020
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in
thousands)
|
|
|
|
|
|
|
|
|
Operating
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research
and development
|
|
$
|
190
|
|
|
$
|
33
|
|
|
$
|
157
|
|
|
|
476
|
%
|
|
General
and administrative
|
|
|
371
|
|
|
|
344
|
|
|
|
27
|
|
|
|
8
|
%
|
|
Total
operating expenses
|
|
$
|
561
|
|
|
$
|
377
|
|
|
$
|
184
|
|
|
|
49
|
%
|
Research
and Development Expenses
Research
and development expenses totaled approximately $0.2 million and $0.03 million for the nine months ended September 30, 2021 and 2020,
respectively.
Our
current research and development expenses currently consist primarily of consulting fees and development expense related to development
of the adenosine A2R antagonists and preparation for an IND filing.
General
and Administrative
General
and administrative expenses totaled approximately $0.4 million and $0.3 million for the nine months ended September 30, 2021 and 2020,
respectively. The increase of approximately $0.03 million, or 8%, for the nine months ended September 30, 2021 compared to the same period
in 2020, was primarily due to increased professional fees partially offset by a reduction in director compensation.
Our
general and administrative expenses currently consist primarily of expenditures related to legal, accounting and tax, other professional
services, and general operating expenses.
Other
Income (Expense)
Other
income (expense) totaled approximately $3.2 million and $0.6 million of income for the nine months ended September 30, 2021 and 2020,
respectively.
|
|
|
Nine
Months Ended
September 30,
|
|
|
Change
in
2021 Versus 2020
|
|
|
|
|
2021
|
|
|
2020
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in
thousands)
|
|
|
|
|
|
|
|
|
Loss
on change in fair value of derivative liability
|
|
$
|
3,001
|
|
|
$
|
353
|
|
|
$
|
2,648
|
|
|
|
750
|
%
|
|
Gain
on conversion of debt
|
|
|
1,130
|
|
|
|
398
|
|
|
|
732
|
|
|
|
184
|
%
|
|
Interest
(expense), net
|
|
|
(924
|
)
|
|
|
(169
|
)
|
|
|
(755
|
)
|
|
|
447
|
%
|
|
Total
other (expense)
|
|
$
|
3,207
|
|
|
$
|
582
|
|
|
$
|
2,625
|
|
|
|
451
|
%
|
Gain
(loss) on change in fair value of derivative liability
As
a result of a change in the fair value of our derivative liability, we realized income of $3.0 million and $0.4 million during the nine
months ended September 30, 2021 and 2020, respectively. The change in the fair value of our derivative liability was the result of our
convertible debentures and notes issued in September 2017, July 2018, December 2018, July 2019, October 2019, November 2019, March 2020,
October 2020, January 2021 and June 2021, where we issued convertible notes with variable conversion rates, and to the issuance of our
Series F preferred stock in October 2020, which is convertible into a variable number of shares of common stock. Refer to Note 6 in our
unaudited condensed consolidated financial statements for further discussion on our derivative liability.
Gain
on conversion of debt
There
was a gain on conversion of debentures of approximately $1.1 million during the nine months ended September 30, 2021, compared to a gain
of $0.4 million during the nine months ended September 30, 2020. Gain on conversion of debt results from the difference between the fair
value of common stock issued upon conversion and the carrying amount of the debt converted.
Interest
income (expense)
We
had net interest expense of $0.9 million in the nine months ended September 30, 2021 compared to expense of $0.2 million for the nine
months ended September 30, 2020. The increase of $0.8 million was attributable an increase in the cost associated with derivative instruments
issued with a value in excess of proceeds received.
Liquidity
and Capital Resources
We
have incurred losses since our inception in 2003 as a result of significant expenditures on operations, research and development and
the lack of any approved products to generate revenue. We have an accumulated deficit of $64.3 million as of September 30, 2021 and anticipate
that we will continue to incur additional losses for the foreseeable future. To date, we have funded our operations through the private
sale of our equity securities, convertible debentures, and exercise of options and warrants, resulting in gross proceeds of approximately
$39.1 million. Cash at September 30, 2021 was approximately $995,000.
Our
auditors’ report on our December 31, 2020 financial statements expressed an opinion that our capital resources as of the date of
their audit report were not sufficient to sustain operations or complete our planned activities for the upcoming year unless we raised
additional funds. Based on our current level of expected operating expenditures, we expect to be able to fund our operations into second
quarter of 2022. This assumes that we spend minimally on general operations and only continue conducting our ongoing pre-clinical studies,
and that we do not encounter any unexpected events or other circumstances that could shorten this time period. If we do not obtain additional
funds by such time, we may no longer be able to continue as a going concern and will cease operation which means that our shareholders
will lose their entire investment.
We
are actively seeking sources of financing to fund our continued operations and research and development programs. To raise additional
capital, we may sell equity or debt securities, or enter into collaborative, strategic and/or licensing transactions. There can be no
assurance that we will be able to complete any financing transaction in a timely manner or on acceptable terms or otherwise. If we are
not able to raise additional cash, we may be forced to further delay, curtail, or cease development of our product candidates, or cease
operations altogether.
|
|
|
Nine
months ended
September 30,
|
|
|
Change
in
2021 versus 2020
|
|
|
|
|
2021
|
|
|
2020
|
|
|
$
|
|
|
%
|
|
|
|
|
(amount in
thousands)
|
|
|
|
|
|
|
|
|
Cash
at beginning of period
|
|
$
|
404
|
|
|
$
|
23
|
|
|
$
|
381
|
|
|
|
1,657
|
%
|
|
Net
cash used in operating activities
|
|
|
(409
|
)
|
|
|
(245
|
)
|
|
|
(164
|
)
|
|
|
67
|
%
|
|
Net
cash provided by investing activities
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
%
|
|
Net
cash provided by financing activities
|
|
|
1,000
|
|
|
|
255
|
|
|
|
745
|
|
|
|
292
|
%
|
|
Cash
and restricted cash at end of period
|
|
$
|
995
|
|
|
$
|
33
|
|
|
$
|
962
|
|
|
|
2,915
|
%
|
Cash
totaled approximately $1.0 million and $0.03 million as of September 30, 2021 and 2020, respectively. The increase of approximately $1.0
million at September 30, 2021 compared to the same period in 2020 was primarily attributable to cash available from current and prior
year private placements offset by an increase in cash used in operations.
Net
Cash Used in Operating Activities
Net
cash used in operating activities was approximately $0.4 million and $0.2 million for the nine months ended September 30, 2021 and 2020,
respectively. Cash used for operations increased by approximately $0.2 million, or 67%, during the nine months ended September 30, 2021,
compared to the same period in 2020. The increase in cash used was primarily attributable to an increase in our net loss (after adjusting
for noncash items) of approximately $0.2 million.
Net
Cash Provided by Investing Activities
There
was no cash provided by or used in investing activities for the nine months ended September 30, 2021 and 2020.
Net
Cash Provided by Financing Activities
There
was $1,000,000 cash provided by financing activities for the nine months ended September 30, 2021, compared to $255,000 cash provided
by financing activities for the nine months ended September 30, 2020. In 2021, we received proceeds of $1,000,000 from the sale of convertible
debentures and in 2020 we received proceeds of $250,000 from the sale of convertible debentures and $5,000 from the sale of preferred
stock.
OUR
MANAGEMENT
Directors,
Executive Officers and Significant Employees
The
names of our directors and executive officers and their ages, positions, and biographies as of June 30, 2021, are set forth below. Our
executive officers are appointed by, and serve at the discretion of the Board. There are no family relationships among any of our directors
or executive officers. All directors hold office until the next annual meeting of shareholders or until their respective successors are
elected, except in the case of death, resignation, or removal. On June 16, 2021,Michael Cain resigned as chief executive officer, principal
accounting officer and as a member of the Board. The Company is currently searching for a qualified replacement principal executive officer
and principal accounting officer.
|
Name
|
|
Position
|
|
Age
|
|
Position
Since
|
|
Executive
Directors
|
|
|
|
|
|
|
|
Raul
Silvestre
|
|
Chief
Executive Officer, Chief Financial Officer, President and Director
|
|
50
|
|
8/2021
|
|
Independent
Directors
|
|
|
|
|
|
|
|
Scott
V. Ogilvie
|
|
Director
|
|
66
|
|
03/2008
|
|
Claire
Thom, Pharm.D.
|
|
Director
|
|
65
|
|
10/2016
|
Raul
Silvestre, Esq., has been general outside counsel to Rebus Holdings since 2008 and the managing partner of the Silvestre Law
Group, P.C. since 1999. While serving as counsel to the company, Mr. Silvestre has been responsible for managing and overseeing all legal
aspects of the company including capital raising transaction and corporate governance and has been instrumental in the reacquisition
of the technology which is the basis of our lead proposed product. Mr. Silvestre’s legal career has focus on corporate and securities
law where he has assisted both private and public companies in capital raising, merger and acquisition transactions as well as general
corporate matters, recapitalizations and restructurings. Mr. Silvestre has also been an active investor, manager, and principal in, public
and private entities with an emphasis in the biotechnology and pharmaceutical industries. Mr. Silvestre received a B.S. with an emphasis
in Finance from the University of Southern California and his Juris Doctorate from Pepperdine School of Law. In evaluating Mr. Silvestre’s
specific experience, qualifications, attributes, and skills in connection with his appointment to our Board, we took into account his
prior service to the company, corporate legal experience, knowledge of capital markets, and experience in publicly traded biotechnology
and drug development companies.
Scott
V. Ogilvie has served as a director on our board since February 2008. Mr. Ogilvie is currently the President of AFIN International,
Inc., a private equity/business advisory firm, which he founded in 2006. Prior to December 31, 2009, he was CEO of Gulf Enterprises International,
Ltd, a company that brings strategic partners, expertise and investment capital to the Middle East and North Africa. He held this position
since August 2006. Mr. Ogilvie previously served as Chief Operating Officer of CIC Group, Inc., an investment manager, a position he
held from 2001 to 2007. He began his career as a corporate and securities lawyer with Hill, Farrer & Burrill, and has extensive public
and private corporate management and board experience in finance, real estate, and technology companies. Mr. Ogilvie currently serves
on the board of directors of Neuralstem, Inc. (NASDAQ: CUR) and Research Solutions, Inc. (OTCQB: RSSS). Mr. Ogilvie also served on the
board of directors of Preferred Voice Inc. (OTCQB: PRFV), Innovative Card Technologies, Inc. (OTCBB: INVC) and National Healthcare Exchange,
Inc. (OTCBB: NHXS). In evaluating Mr. Ogilvie’s specific experience, qualifications, attributes and skills in connection with his
appointment to our board, we took into account his prior work in both public and private organizations regarding corporate finance, securities
and compliance and international business development.
Claire
Thom, PharmD has served as a director on our board since October 2016. Dr. Thom has two decades of experience in the pharmaceutical
industry, with responsibilities including drug development, new product planning, and marketing. Most recently, from July 2013 until
June 2016, Dr. Thom was the Senior Vice President Global Therapeutic Head for Oncology at Astellas Pharma (TOKYO: ALPMY). At Astellas,
she developed and supervised the implementation of the company’s oncology strategy. In addition, she was appointed to serve
on the Board of Directors for Agensys, a fully-owned subsidiary of Astellas. Prior to her roles at Astellas, Dr. Thom served as
Senior Vice President of Portfolio Management, Drug Development Management and Strategic Business Operations at Millennium Pharmaceuticals,
the Takeda Oncology Company, (TOKYO: TKPYY) from August 2008 until January 2013. Prior to her assignment at Millennium, she held several
positions of increasing responsibility at Takeda to become the company’s Oncology Franchise Leader. Earlier, she worked at G.D.
Searle and began her career as a clinical pharmacist. Ms. Thom was awarded a Doctor of Pharmacy and a Bachelor of Pharmacy, both with
honors, from the University of Illinois. In evaluating Dr. Thom’s specific experience, qualifications, attributes and skills in
connection with her appointment to our board, we took into account her knowledge of scientific matters affecting our business and her
understanding of our industry.
Family
Relationships
There
are no family relationships between any director, executive officer, or person nominated or chosen by the registrant to become a director
or executive officer.
CORPORATE
GOVERNANCE
Independent
Directors
For
purposes of determining independence, the Company has adopted the definition of independence as contained in NASDAQ Market Place Rule
5605(a)(2). Pursuant to the definition, the Company has determined that Dr. Thom and Mr. Ogilvie qualify as independent.
Committees
The
board of directors has established three standing committees: (1) an Audit Committee, (2) a Nominating and Corporate Governance Committee,
and (3) a Leadership Development and Compensation Committee. Each of the committees operates under a written charter adopted by the board
of directors. A copy of each respective committee’s charter can be viewed as Exhibits 99.01, 99.02 and 99.03 to this Annual Report.
The
table below identifies the Board’s standing committees and committee membership as of October 31, 2021:
|
Director
|
|
Independent
|
|
Audit
Committee
|
|
Nominating
and
Corporate
Governance
Committee
|
|
Leadership
Development
and
Compensation
Committee
|
|
|
Scott Ogilvie
|
|
Yes
|
|
Chair
|
|
Chair
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claire Thom, PharmD
|
|
Yes
|
|
Member
|
|
—
|
|
Chair
|
|
Each
member of the Audit Committee, the Compensation Committee, and the Nominating and Corporate Governance Committee is considered independent
under the NASDAQ Market Place Rules.
Audit
Committee
The
main function of our Audit Committee, which was established in accordance with Section 3(a)(58)(A) of the Exchange Act, is to oversee
our accounting and financial reporting processes, internal systems of control, independent auditor relationships and the audits of our
financial statements. This committee’s responsibilities include:
|
|
●
|
Selecting
and hiring our independent auditors.
|
|
|
●
|
Evaluating
the qualifications, independence and performance of our independent auditors.
|
|
|
●
|
Approving
the audit and non-audit services to be performed by our independent auditors.
|
|
|
●
|
Reviewing
the design, implementation, adequacy and effectiveness of our internal controls and our critical
accounting policies.
|
|
|
●
|
Overseeing
and monitoring the integrity of our financial statements and our compliance with legal and
regulatory requirements as they relate to financial statements or accounting matters.
|
|
|
●
|
Reviewing with management
any earnings announcements and other public announcements regarding our results of operations.
|
|
|
●
|
Reviewing
regulatory filings with management and our auditors.
|
|
|
●
|
Preparing
any report the SEC requires for inclusion in our annual proxy statement.
|
|
|
●
|
The
Audit Committee will review and approve all related party transactions.
|
Our
Audit Committee is currently comprised of Scott V. Ogilvie and Claire Thom, each of whom is a non-employee member of our board of directors.
Our board of directors has determined that each of the directors serving on our Audit Committee is independent within the meaning of
the rules of the SEC and rule 5605(a)(2) of the Marketplace Rules of NASDAQ. Additionally, our board has determined that Scott V. Ogilvie
is an audit committee financial expert as defined under the rules of the SEC. A copy of the charter is contained in Exhibit 99.01 to
this Registration Statement.
Nominating
and Corporate Governance Committee
Our
Nominating and Corporate Governance Committee’s purpose is to assist our board of directors in identifying individuals qualified
to become members of our board of directors consistent with criteria set by our board of directors and to develop our corporate governance
principles. This committee’s responsibilities include:
|
|
●
|
Evaluating
the composition, size, organization and governance of our board of directors and its committees,
determining future requirements, and making recommendations regarding future planning, the
appointment of directors to our committees and selection of chairs of these committees.
|
|
|
●
|
Reviewing
and recommending to our board of directors, director independence determinations made with
respect to continuing and prospective directors.
|
|
|
●
|
Establishing
a policy for considering stockholder nominees for election to our board of directors.
|
|
|
●
|
Recommending
ways to enhance communications and relations with our stockholders.
|
|
|
●
|
Evaluating
and recommending candidates for election to our board of directors.
|
|
|
●
|
Overseeing
our board of directors’ performance and self-evaluation process and developing continuing
education programs for our directors.
|
|
|
●
|
Evaluating
and recommending to the board of directors, termination of service of individual members
of the board of directors as appropriate, in accordance with governance principles, for cause
or for other proper reasons.
|
|
|
●
|
Making
regular written reports to the board of directors.
|
|
|
●
|
Reviewing
and reexamining the committee’s charter and making recommendations to the board of
directors regarding any proposed changes.
|
|
|
●
|
Reviewing
annually the committee’s own performance against responsibilities outlined in its charter
and as otherwise established by the board of directors.
|
Our
Nominating and Corporate Governance Committee is currently comprised of Scott V. Ogilvie, a non-employee member of our board of directors.
Our board of directors has determined that Mr. Ogilvie is independent as defined in rule 5605(a)(2) of the Marketplace Rules of NASDAQ.
The charter of the Nominating and Corporate Governance Committee is contained in Exhibit 99.03 of this Registration Statement.
Leadership
Development and Compensation Committee
The
purpose of our Leadership Development and Compensation Committee is to oversee our compensation programs. The committee may form and
delegate authority to subcommittees or, with respect to compensation for employees and consultants who are not executive officers for
purposes of Section 16 of the Exchange Act, to our officers, in either instance as the committee determines appropriate. The committee’s
responsibilities include:
|
|
●
|
Reviewing
and approving our general compensation strategy.
|
|
|
●
|
Establishing
annual and long-term performance goals for our CEO and other executive officers.
|
|
|
●
|
Conducting
and reviewing with the board of directors an annual evaluation of the performance of the
CEO and other executive officers.
|
|
|
●
|
Evaluating
the competitiveness of the compensation of the CEO and the other executive officers.
|
|
|
●
|
Reviewing
and making recommendations to the board of directors regarding the salary, bonuses, equity
awards, perquisites and other compensation and benefit plans for the CEO.
|
|
|
●
|
Reviewing
and approving all salaries, bonuses, equity awards, perquisites and other compensation and
benefit plans for our other executive officers.
|
|
|
●
|
Reviewing
and approving the terms of any offer letters, employment agreements, termination agreements
or arrangements, change-in-control agreements, indemnification agreements and other material
agreements between the company and our executive officers.
|
|
|
●
|
Acting
as the administering committee for our stock and bonus plans and for any equity or cash compensation
arrangements that we may adopt from time to time.
|
|
|
●
|
Providing
oversight for our overall compensation plans and benefit programs, monitoring trends in executive
and overall compensation and making recommendations to the board of directors with respect
to improvements to such plans and programs or the adoption of new plans and programs.
|
|
|
●
|
Reviewing
and approving compensation programs as well as salaries, fees, bonuses and equity awards
for non-employee members of the board of directors.
|
|
|
●
|
Reviewing
plans for the development, retention and succession of our executive officers.
|
|
|
●
|
Reviewing
executive education and development programs.
|
|
|
●
|
Monitoring
total equity usage for compensation and establishing appropriate equity dilution levels.
|
|
|
●
|
Reporting
regularly to the board of directors on the committee’s activities.
|
|
|
●
|
Reviewing
and discussing with management the required annual compensation discussion and analysis disclosure,
if any, regarding named executive officer compensation and, based on this review and discussions,
making a recommendation to include in our annual public filings.
|
|
|
●
|
Preparing
and approving any required committee report to be included in our annual public filings.
|
|
|
●
|
Performing
a review, at least annually, of the performance of the committee and its members and reporting
to the board of directors on the results of this review.
|
|
|
●
|
Investigating
any matter brought to its attention, with full access to all our books, records, facilities
and employees and obtaining advice, reports or opinions from internal or external counsel
and expert advisors in order to help it perform its responsibilities.
|
Our
Leadership Development and Compensation Committee is currently comprised of Claire Thom, who is a non-employee member of our board of
directors. Dr. Thom is an “outside” director as defined in Section 162(m) of the Internal Revenue Code of 1986, as amended
(the “Code”), and a “non-employee” director within the meaning of Rule 16b-3 of the Exchange Act. Our board of
directors has determined that Dr. Thom is independent as defined in rule 5605(a)(2) of the Marketplace Rules of NASDAQ. A copy of the
charter is contained in Exhibit 99.02 to this Registration Statement.
Section
16(a) Beneficial Ownership Reporting Compliance
Section
16(a) of the Exchange Act requires the Company’s officers and directors, and persons who own more than ten percent of a registered
class of the Company’s equity securities, to file reports of securities ownership and changes in such ownership with the SEC. Officers,
directors and greater than ten percent shareholders also are required by SEC rules to furnish the Company with copies of all Section
16(a) forms they file.
Based
solely upon a review of the copies of such forms furnished to the Company, and on written representations from the reporting persons,
the Company believes that all Section 16(a) filing requirements applicable to the Company’s directors and officers were timely
met during 2020.
Limitation
on Liability and Indemnification of Directors and Officers
Our
certificate of incorporation states that, to the fullest extent permitted by the Delaware General Corporate Law, or the DGCL, no director
shall be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as director; provided, however,
that this provision eliminating personal liability of a director shall not eliminate or limit the liability of a director (i) for any
breach of the director’s duty of loyalty to us or our stockholders, (ii) for acts or omissions not in good faith or which involve
intentional misconduct or a knowing violation of law, (iii) under section 174 of the DGCL, or (iv) for any transaction from which the
director derived an improper personal benefit.
Section 174
of the DGCL provides, among other things, that a director who willfully and negligently approves of an unlawful payment of dividends
or an unlawful stock purchase or redemption may be held liable for such actions. A director who was either absent when the unlawful actions
were approved or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books
containing the minutes of the meetings of the board of directors at the time the action occurred or immediately after the absent director
receives notice of the unlawful acts.
Our
certificate of incorporation and bylaws provide that we will indemnify our directors and officers and may indemnify our employees or
agents to the fullest extent permitted by law against liabilities and expenses incurred in connection with litigation in which they may
be involved because of their offices or positions with us. However, nothing in our certificate of incorporation or bylaws protects or
indemnifies a director, officer, employee or agent against any liability to which that person would otherwise be subject by reason of
willful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of that person’s office
or position. To the extent that a director has been successful in defending any proceeding brought against him, the Delaware General
Corporation Law provides that the director shall be indemnified against reasonable expenses incurred by him in connection with the proceeding.
Diversity
of Board of Directors
We
do not have a formal policy with regard to the consideration of diversity in identifying director nominees, but the Nominating and Corporate
Governance Committee strives to nominate Directors with a variety of complementary skills so that, as a group, the board of directors
will possess the appropriate talent, skills, and expertise to oversee our businesses.
Code
of Ethics
We
have adopted a “Code of Ethics” that applies to our principal executive officer, principal financial officer, principal accounting
officer or controller, or persons performing similar functions. A copy of our code is attached to this Registration Statement as Exhibit
14.01.
EXECUTIVE COMPENSATION
The
following table provides disclosure concerning all compensation paid for services to us in all capacities for our fiscal years ended
December 31, 2020 and 2019 provided by (i) each person serving as our principal executive officer, or PEO, or acting in a similar capacity
during our fiscal year ended December 31, 2020 (ii) our most highly compensated executive officers other than our PEO who were serving
as executive officers on December 31, 2020 and whose total compensation exceeded $100,000 (collectively with the PEO referred to as the
“named executive officers” in this Executive Compensation section); and (iii) our Principal Financial Officer.
|
Name
& Principal Position
|
|
Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards
($)
|
|
|
Non-Equity
Incentive Plan
Compensation
($)
|
|
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
|
|
All
Other
Compensation
($)
|
|
|
Total
($)
|
|
|
Christopher
Lowe,
|
|
2020
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Chief
Executive Officer and Chief Financial Officer
|
|
2019
|
|
|
—
|
(1)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael
Cain,
|
|
2020
|
|
|
—
|
(2)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Chief
Executive Officer and Chief Financial Officer (3)
|
|
2019
|
|
|
—
|
(2)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Raul
Silvestre
|
|
2020
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Chief
Executive Officer and Chief Financial Officer (4)
|
|
2019
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1)
|
No
salary was paid or accrued for Mr. Lowe in 2019. Effective July 15, 2019, Mr. Lowe resigned
as chief executive officer and as a member of the Board.
|
|
|
(2)
|
Mr.
Cain became our chief executive officer and principal accounting officer effective July 26,
2019. Mr. Cain did not receive any compensation for his services.
|
|
|
(3)
|
Mr. Cain resigned as
principal executive and accounting officer, and as a member of the Board effective June 16, 2021.
|
|
|
(4)
|
Mr. Silvestre was appointed
as principal executive and accounting officer, and as a member of the Board effective August 16, 2021.
|
Outstanding
Executive Equity Awards at Fiscal Year-End 2020
None.
Employment
Agreements and Change in Control
Raul
Silvestre
Raul
Silvestre was appointed chief executive officer and chief financial officer / principal accounting officer of the Company effective August
16, 2021. He was also appointed as a member of the Board of Directors. Mr. Silvestre does not have an employment agreement covering his
services and has not received any compensation for services as chief executive officer or chief financial officer Mr. Silvestre is not
subject to any compensation arrangements in the event that he is terminated or resigns as an officer for any reason.
Michael
Cain
Michael
Cain was appointed chief executive officer and chief financial officer / principal accounting officer of the Company effective July 26,
2019. Effective June 16, 2021, Mr. Cain resigned from all positions with the Company and as a member of the Board of Directors. During
his tenure, the Company did not have any employment agreement covering Mr. Cain’s services and he did not receive any compensation
as our chief executive officer or chief financial officer.
Christopher
Lowe
Employment
Agreement
We
employed Christopher Lowe as our Chief Executive Officer and Chief Financial Officer pursuant to a written contract that until such time
that either the Company or Mr. Lowe terminates the agreement. Mr. Lowe resigned his employment with the Company effective July 15, 2019.
Mr. Lowe received a base salary of $316,250, of which we deducted $41,250 during the first year and pay such amount to a third party
as a placement fee for Mr. Lowe’s employment. As a result, Mr. Lowe received a net base salary of $275,000 for his first year of
employment.
Mr.
Lowe’s employment agreement provided for severance in the event Company terminates Mr. Lowe’s employment without Cause or
Mr. Lowe resigned with Good Reason, as each term is defined in the employment agreement, provided certain company funding requirements
were met. Pursuant to Mr. Lowe’s resignation, no severance was paid pursuant to the terms of his employment agreement.
Equity
Compensation Plans
For
information related to our equity compensation plans for which our officers and directors are issued securities from, please see Securities
authorized for issuance under equity compensation plans beginning on page 33 of this Registration Statement.
Director
Compensation
|
Name
|
|
Fees Earned
or Paid in
Cash
($)
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards
($)
|
|
|
Non-Equity
Incentive
Plan Compensation
($)
|
|
|
Non-Qualified
Deferred
Compensation Earnings
($)
|
|
|
All
Other
Compensation
($)
|
|
|
Total
($)
|
|
|
Scott Ogilvie
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30,000
|
(1)
|
|
|
30,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Claire Thom
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
20,000
|
(2)
|
|
|
20,000
|
|
|
|
(1)
|
On
March 1, 2021, we entered into an agreement with Mr. Ogilvie whereby each of the parties entered into a mutual release and we agreed
to pay Mr. Ogilvie an aggregate of $60,000, of which $30,000 was due and payable on November 13, 2020 with the remaining $30,000
due and payable on February 18, 2021. In exchange for such payments, Mr. Ogilvie agreed to waive $231,167 in outstanding director
fees owed to Mr. Ogilvie from March 31, 2017 through March 31, 2021. Further, pursuant to the agreement, we agreed to issue a common
stock purchase option with a Black Scholes’ value of $60,000 to Mr. Ogilvie immediately prior to the announcing approval from
the FDA to commence our first Phase 1 clinical trial (this has not yet occurred). The option will have an exercise price equal to
the closing price of the Company’s common stock on the day preceding the announcement and a term of 10 years.
|
|
|
(2)
|
On
March 1, 2021, we entered into an agreement with Dr. Thom whereby each of the parties entered into a mutual release and we agreed
to pay Dr. Thom an aggregate of $40,000, of which $20,000 was due and payable on November 13, 2020 with the remaining $20,000 due
and payable on February 18, 2021. In exchange for such payments, Dr. Thom agreed to waive $204,500 in outstanding director fees owed
to Dr. Thom from March 31, 2017 through March 31, 2021. Further, pursuant to the agreement, we agreed to issue a common stock purchase
option with a Black Scholes’ value of $40,000 to Dr. Thom immediately prior to the announcing approval from the FDA to commence
our first Phase 1 clinical trial (this has not yet occurred). The option will have an exercise price equal to the closing price of
the Company’s common stock on the day preceding the announcement and a term of 10 years.
|
Current
Amended Non-Employee Director Compensation Policy
Effective
April 1, 2021, the Board of Directors amended its employee director compensation policy (“Amended Director Policy”). Pursuant
to the Amended Director Policy, each Board member will receive $5,000 in cash per quarter of service on the Board of Directors.
PRINCIPAL
STOCKHOLDERS
Security
Ownership of Certain Beneficial Owners and Management
The
following table sets forth, as of October 31, 2021, information regarding beneficial ownership of our capital stock by:
|
|
●
|
each
person, or group of affiliated persons, known by us to be the beneficial owner of 5% or more
of any class of our voting securities;
|
|
|
●
|
each
of our current directors and nominees;
|
|
|
●
|
each
of our current named executive officers; and
|
|
|
●
|
all
current directors and named executive officers as a group.
|
Beneficial
ownership is determined according to the rules of the SEC. Beneficial ownership means that a person has or shares voting or investment
power of a security and includes any securities that person or group has the right to acquire within 60 days after the measurement date.
This table is based on information supplied by officers, directors and principal stockholders. Except as otherwise indicated, we believe
that each of the beneficial owners of the common stock listed below, based on the information such beneficial owner has given to us,
has sole investment and voting power with respect to such beneficial owner’s shares, except where community property laws may apply.
|
Name
and Address of Beneficial Owner(1)
|
|
Shares
|
|
|
Common Stock Shares
Underlying
Convertible
Securities(2)
|
|
|
Total
|
|
|
Percent
of
Class(2)
|
|
|
Directors
and named Executive Officers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Raul
Silvestre (3)
|
|
|
1
|
|
|
|
541,448
|
|
|
|
541,449
|
|
|
|
4.99
|
%
|
|
Michael
Cain +
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
*
|
|
|
Christopher
Lowe @
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
*
|
|
|
Scott
Ogilvie
|
|
|
1
|
|
|
|
—
|
|
|
|
1—
|
|
|
|
*
|
|
|
Claire
Thom
|
|
|
1
|
|
|
|
—
|
|
|
|
1—
|
|
|
|
*
|
|
|
All
directors and executive officers as a group (5 persons)
|
|
|
3
|
|
|
|
541,448
|
|
|
|
541,451
|
|
|
|
4.99
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5%
Shareholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sabby
Volatility Warrant Master Fund, Ltd. (4)
|
|
|
1
|
|
|
|
1,144,195
|
|
|
|
1,144,195
|
(5)
|
|
|
9.99
|
%
|
|
Ridgeway
Therapeutics, Inc. (6)
|
|
|
866,667
|
|
|
|
|
(7)
|
|
|
866,667
|
(7)
|
|
|
8.41
|
%
|
|
|
*
|
Less than one percent.
|
|
|
+
|
Mr. Cain resigned as
an officer and director of the Company effective June 16, 2021.
|
|
|
@
|
Mr. Lowe resigned as
an officer and director of the Company effective July 15, 2019.
|
|
|
(1)
|
Except
as otherwise indicated, the persons named in this table have sole voting and investment power
with respect to all shares of common stock shown as beneficially owned by them, subject to
community property laws where applicable and to the information contained in the footnotes
to this table. Unless otherwise indicated, the address of the beneficial owner is 2629 Townsgate
Road #215, Westlake Village, CA 91361.
|
|
|
(2)
|
Pursuant
to Rules 13d-3 and 13d-5 of the Exchange Act, beneficial ownership includes any shares as
to which a shareholder has sole or shared voting power or investment power, and also any
shares which the shareholder has the right to acquire within 60 days, including upon exercise
of common shares purchase options or warrants. There were 10,309,212 shares of common stock
issued and outstanding as of October 31, 2021.
|
|
|
(3)
|
All
share amounts underlying convertible securities represent debentures held by Silvester Law Group, P.C, of which Mr. Silvestre serves
as its managing director. Excludes 2,640,986 shares of common stock underlying debentures held by Silvestre Law Group, P.C.
|
|
|
(4)
|
89
Nexus Way, Camana Bay, Grand Cayman Ky1-9007, Cayman Islands.
|
|
|
(5)
|
Excludes
4,663,508 common shares underlying outstanding debentures and 9 common shares underlying outstanding warrants.
|
|
|
(6)
|
4085
Campbell Ave. #150, Menlo Park, CA 94025. Colin Hislop has voting and dispositive control
with respect to the securities.
|
|
|
(7)
|
Ridgeway
Therapeutics additionally holds 8,000 shares of our Series F Convertible Preferred Stock
that converts into Common Stock. The 8,000 shares of Series F Convertible Preferred Stock
are convertible into an aggregate of 80% of the issued and outstanding Common Stock post-conversion
on the conversion date. The Series F Preferred Stock votes on an as converted to Common Stock
basis. Additionally, upon the termination, conversion or otherwise extinguishment of certain
of our outstanding convertible debentures, the Series F Preferred Stock will automatically
convert into Common Stock.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Related
Party Transactions
Information
regarding disclosure of an employment relationship or transaction involving an executive officer and any related compensation solely
resulting from that employment relationship or transaction is incorporated by reference from the section of this annual report entitled
“Executive Compensation.”
Information
regarding disclosure of compensation to a director is incorporated by reference from the section of this annual report entitled “Director
Compensation.”
Summarized
below are certain transactions and business relationships between the Company and persons who are or were an executive officer, director
or holder of more than five percent of any class of our securities (at the time of the transaction) since January 1, 2019:
Related
Party Transactions
|
|
●
|
We
have entered into an indemnification agreement with each of our directors and executive officers.
The indemnification agreements and our certificate of incorporation and bylaws require us
to indemnify our directors and executive officers to the fullest extent permitted by Delaware
law. The indemnification agreements are substantially similar to those entered into with
our executive officers and as a more fully described in the section of this annual report
entitled “Employment Agreements and Change in Control.”
|
|
|
●
|
On
March 1, 2021, we entered into a settlement and release agreement with Claire Thom, one of
our independent directors for the settlement of past due director fees and the mutual release
of all claims. Pursuant to the agreement, Dr. Thom agreed to waive $204,500 in outstanding
director fees in exchange for the following: (i) the payment of $40,000 (of which $20,000
was paid in November 2020 and $20,000 in February 2021) and (ii) immediately prior to the
announcement that the Company has received approval from the FDA to commence its first Phase
1 clinical trial after March 1, 2021, a common stock purchase option with a Black Scholes’
value of $40,000, having an exercise price equal to the closing price on the day preceding
the announcement, and a term of 10 years. We also agreed to amend our non-employee director
compensation policy such that on April 1, 2021 each director will be entitled to receive
a quarterly Board fee of $5,000 in cash.
|
|
|
●
|
On
March 1, 2021, we entered into a settlement and release agreement with Scott Ogilvie, one
of our independent directors for the settlement of all past due director fees and the mutual
release of all claims. Pursuant to the agreement, Mr. Ogilvie agreed to waive $231,167 in
outstanding director fees in exchange for the following: (i) the payment of $60,000 (of which
$30,000 was paid in November 2020 and $30,000 in February 2021) and (ii) immediately prior
to the announcement that the Company has received approval from the FDA to commence its first
Phase 1 clinical trial after March 1, 2021, a common stock purchase option with a Black Scholes’
value of $40,000, having an exercise price equal to the closing price on the day preceding
the announcement, and a term of 10 years. We also agreed to amend our non-employee director
compensation policy such that on April 1, 2021 each director will be entitled to receive
a quarterly Board fee of $5,000 in cash.
|
INDEMNIFICATION
OF DIRECTORS AND OFFICERS
The
Corporation Laws of the State of Delaware and the Company’s Bylaws provide for indemnification of the Company’s Directors
for expenses actually and necessarily incurred by them in connection with the defense of any action, suit or proceeding in which they,
or any of them, are made parties, or a party, by reason of having been Director(s) or Officer(s) of the corporation, or of such other
corporation, except, in relation to matter as to which any such Director or Officer or former Director or Officer or person shall be
adjudged in such action, suit or proceeding to be liable for negligence or misconduct in the performance of duty. Furthermore, the personal
liability of the Directors is limited as provided in the Company’s Articles of Incorporation.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling
the Company pursuant to the foregoing provisions, the Securities and Exchange Commission’s position is that such indemnification
is against public policy as expressed in the Securities Act of 1933 and is therefore unenforceable.
EXPERTS
The
consolidated financial statements as of and for the years ended December 31, 2020, and 2019 included in this prospectus and in the registration
statement of which it forms a part, have been so included in reliance on the reports of Liggett & Webb, P.A., our independent registered
public accounting firm, appearing elsewhere in this prospectus and the registration statement of which it forms a part, given on the
authority of said firms as experts in auditing and accounting.
INTERESTS
OF NAMED EXPERTS AND COUNSEL
The
validity of our securities offered and to be issued by this prospectus will be passed upon for us by Silvestre Law Group, P.C. of Westlake
Village, CA. The Silvestre Law Group, P.C. or its various principals and/or affiliates, own approximately $290,000 in principal of non-interest
bearing senior convertible debentures.
WHERE
YOU CAN FIND MORE INFORMATION
We
make our public filings with the SEC, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports
on Form 8-K and all exhibits and amendments to these reports. These materials are available on the SEC’s web site, www.sec.gov.
You
may also read and copy any materials you file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE., Washington,
DC 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may obtain information on the operation of the Public Reference
Room by calling the SEC at 1–800–SEC–0330. Additionally, the SEC maintains an Internet site that contains reports,
proxy and information statements, and other information regarding issuers that file electronically with the SEC. The Internet site is
located at www.sec.gov. Alternatively, you may obtain copies of these filings, including exhibits, by writing or telephoning us at:
REBUS
HOLDINGS
2629
Townsgate Road #215
Westlake
Village, CA 91361
Attn:
Chief Executive Officer
Tel:
(818) 597-7552
This
prospectus is part of a registration statement on Form S-1 that we filed with the SEC. Certain information in the registration statement
has been omitted from this prospectus in accordance with the rules and regulations of the SEC. We have also filed exhibits and schedules
with the registration statement that are excluded from this prospectus. For further information you may:
|
|
●
|
read
a copy of the registration statement, including the exhibits and schedules, without charge
at the SEC’s public reference rooms; or
|
|
|
●
|
obtain
a copy from the SEC upon payment of the fees prescribed by the SEC.
|
REBUS
HOLDINGS, INC.
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
PART
I
FINANCIAL
INFORMATION
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Stockholders and Board of Directors of:
Inspyr
Therapeutics, Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of Inspyr Therapeutics, Inc. and Subsidiary (the “Company”) as
of December 31, 2020 and 2019, the related consolidated statements of losses, changes in stockholders’ deficit and cash flows for
each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as “the consolidated
financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial
position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years
in the period ended December 31, 2020 in conformity with accounting principles generally accepted in the United States of America.
Explanatory
Paragraph – Going Concern
The
accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed
in Note 2 to the consolidated financial statements, the Company has incurred losses since inception and has generated no revenues. The
Company has a working capital deficit and an accumulated deficit. These factors raise substantial doubt about the Company’s ability
to continue as a going concern. Management’s plans in regard to these matters are described in Note 2. The consolidated financial
statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis
for Opinion
These
consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion
on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public
Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company
in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission
and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. Accordingly,
we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence
regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles
used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We
believe that our audits provide a reasonable basis for our opinion.
Critical
Audit Matters
The
critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements
that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are
material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The
communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole,
and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the
accounts or disclosures to which they relate.
Valuation
of Derivative Liabilities
As
described in Note 3 to the consolidated financial statements, the Company measures fair value of derivative
liabilities at fair value using level three inputs. To determine fair value of derivative liabilities, the Company determines the appropriate
valuation methodology and assumptions, including unobservable inputs. The derivative liabilities are measured at fair value using a Black-Scholes
valuation model that uses significant assumptions, including the Company’s stock price, historical volatility of the Company’s
shares, risk-free interest rate and probability of conversion occurrence through maturity.
Auditing
management’s estimate for the fair value of derivative liabilities was
complex and highly judgmental as it involved our assessment of the significant assumptions used by management because
the fair value calculations were sensitive to changes in assumptions described above, and certain inputs used in the determination of
fair values were based on unobservable data, including, but not limited to, the historical volatility and probability of conversion.
To
test the fair value of derivative liabilities, we performed audit procedures that included, among others, evaluating the methodologies
used in the valuation model and the significant assumptions used by the Company.
/s/
Liggett & Webb, P.A.
LIGGETT
& WEBB, P.A.
Certified
Public Accountants
We
have served as the Company’s auditor since 2014.
Boynton
Beach, Florida
March
31, 2021, except for footnote 3 “Reverse Stock Split,”, footnote 9 “Reverse Stock Split”
and
footnote
15 “Reverse Stock Split,” as to which the date is December 3, 2021
INSPYR
THERAPEUTICS, INC.
CONSOLIDATED
BALANCE SHEETS
(in
thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
404
|
|
|
$
|
4
|
|
|
Restricted
cash
|
|
|
-
|
|
|
|
19
|
|
|
Total
current assets
|
|
|
404
|
|
|
|
23
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
assets
|
|
$
|
404
|
|
|
$
|
23
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts
payable
|
|
$
|
2,261
|
|
|
$
|
2,269
|
|
|
Accrued
expenses
|
|
|
1,940
|
|
|
|
1,866
|
|
|
Convertible
debentures, net of unamortized discount of $488 and $281
|
|
|
1,878
|
|
|
|
2,826
|
|
|
Derivative
liability
|
|
|
6,828
|
|
|
|
1,785
|
|
|
Total
current liabilities
|
|
|
12,907
|
|
|
|
8,746
|
|
|
Total
liabilities
|
|
|
12,907
|
|
|
|
8,746
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments
and contingencies (Note 8)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
deficit:
|
|
|
|
|
|
|
|
|
|
Convertible
preferred stock, undesignated, par value $.0001 per share; 29,978,846 shares authorized, no shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series A, par value $.0001 per share; 1,854 shares authorized, 134 shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series B, par value $.0001 per share; 1,000 shares authorized, 71 shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series C, par value $.0001 per share; 300 shares authorized, 290 shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series D, par value $.0001 per share; 5,000 shares authorized, 5,000 and 5,000 shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series E, par value $.0001 per share; 5,000 shares authorized, 5,000 and no shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible
preferred stock Series F, par value $.0001 per share; 8,000 shares authorized, 8,000 and no shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Common
stock, par value $.0001 per share; 1,000,000,000 shares authorized, 2,478,848 and 12,160 shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Additional
paid-in capital
|
|
|
54,472
|
|
|
|
51,957
|
|
|
Accumulated
deficit
|
|
|
(66,975
|
)
|
|
|
(60,680
|
)
|
|
Total
stockholders’ deficit
|
|
|
(12,503
|
)
|
|
|
(8,723
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total
liabilities and stockholders’ deficit
|
|
$
|
404
|
|
|
$
|
23
|
|
The
accompanying notes are an integral part of these audited consolidated financial statements.
INSPYR
THERAPEUTICS, INC.
CONSOLIDATED
STATEMENTS OF LOSSES
(in
thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
Years
Ended
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Operating
expenses:
|
|
|
|
|
|
|
|
|
|
Research
and development
|
|
$
|
18
|
|
|
$
|
44
|
|
|
License
termination cost
|
|
|
1,969
|
|
|
|
-
|
|
|
General
and administrative
|
|
|
494
|
|
|
|
565
|
|
|
Total
operating expenses
|
|
|
2,481
|
|
|
|
609
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
from operations
|
|
|
(2,481
|
)
|
|
|
(609
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other
income (expense):
|
|
|
|
|
|
|
|
|
|
Gain
(loss) on change in fair value of derivative liability
|
|
|
(3,846
|
)
|
|
|
327
|
|
|
Gain
on conversion of debt
|
|
|
334
|
|
|
|
125
|
|
|
Interest
(expense), net
|
|
|
(302
|
)
|
|
|
(777
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Loss
before provision for income taxes
|
|
|
(6,295
|
)
|
|
|
(934
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Provision
for income taxes
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
(6,295
|
)
|
|
|
(934
|
)
|
|
Deemed
dividend
|
|
|
(64
|
)
|
|
|
(54
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss attributable to common shareholders
|
|
$
|
(6,359
|
)
|
|
$
|
(988
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss per common share, basic and diluted
|
|
$
|
(10.93
|
)
|
|
$
|
(141.69
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average shares outstanding
|
|
|
581,760
|
|
|
|
6,973
|
|
The
accompanying notes are an integral part of these audited consolidated financial statements.
INSPYR
THERAPEUTICS, INC.
CONSOLIDATED
STATEMENT OF STOCKHOLDERS’ DEFICIT
FOR
THE YEARS ENDED DECEMBER 31, 2020 AND 2019
(in
thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible
Preferred Stock
|
|
|
Common
Stock
|
|
|
Additional
Paid-in
|
|
|
Accumulated
|
|
|
Stockholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Deficit
|
|
|
Balance,
December 31, 2018
|
|
|
495
|
|
|
$
|
-
|
|
|
|
5,375
|
|
|
$
|
-
|
|
|
$
|
51,479
|
|
|
$
|
(59,746
|
)
|
|
$
|
(8,267
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale
of preferred stock
|
|
|
5,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5
|
|
|
|
-
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion
of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
6,785
|
|
|
|
-
|
|
|
|
473
|
|
|
|
-
|
|
|
|
473
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(934
|
)
|
|
|
(934
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2019
|
|
|
5,495
|
|
|
|
-
|
|
|
|
12,160
|
|
|
|
-
|
|
|
|
51,957
|
|
|
|
(60,680
|
)
|
|
|
(8,723
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale
of preferred stock
|
|
|
5,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5
|
|
|
|
-
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance
of preferred stock for license termination
|
|
|
8,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance
of common stock for license termination
|
|
|
-
|
|
|
|
-
|
|
|
|
866,667
|
|
|
|
-
|
|
|
|
267
|
|
|
|
-
|
|
|
|
267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion
of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
1,600,021
|
|
|
|
-
|
|
|
|
2,243
|
|
|
|
-
|
|
|
|
2,243
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(6,295
|
)
|
|
|
(6,295
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2020
|
|
|
18,495
|
|
|
$
|
-
|
|
|
|
2,478,848
|
|
|
$
|
-
|
|
|
$
|
54,472
|
|
|
$
|
(66,975
|
)
|
|
$
|
(12,503
|
)
|
The
accompanying notes are an integral part of these audited consolidated financial statements.
INSPYR
THERAPEUTICS, INC.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
(in
thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
Years
Ended
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Cash flows
from operating activities:
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
$
|
(6,295
|
)
|
|
$
|
(934
|
)
|
|
Adjustments
to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
and amortization
|
|
|
-
|
|
|
|
36
|
|
|
Common
and preferred stock issued for license termination
|
|
|
1,944
|
|
|
|
-
|
|
|
(Gain)
loss on change in fair value of derivative liability
|
|
|
3,846
|
|
|
|
(327
|
)
|
|
Gain on
conversion of debt
|
|
|
(334
|
)
|
|
|
(125
|
)
|
|
Amortization
of debt discount
|
|
|
280
|
|
|
|
281
|
|
|
Finance
cost
|
|
|
20
|
|
|
|
494
|
|
|
Increase
in operating liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts
payable and accrued expenses
|
|
|
165
|
|
|
|
262
|
|
|
Cash
used in operating activities
|
|
|
(374
|
)
|
|
|
(313
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows
from investing activities:
|
|
|
|
|
|
|
|
|
|
Cash
provided by investing activities
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows
from financing activities:
|
|
|
|
|
|
|
|
|
|
Proceeds
from convertible notes
|
|
|
750
|
|
|
|
-
|
|
|
Proceeds
from sale of preferred stock
|
|
|
5
|
|
|
|
5
|
|
|
Cash
provided by financing activities
|
|
|
755
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase
(decrease) in cash and restricted cash
|
|
|
381
|
|
|
|
(308
|
)
|
|
Cash
and restricted cash, beginning of year
|
|
|
23
|
|
|
|
331
|
|
|
Cash
and restricted cash, end of year
|
|
$
|
404
|
|
|
$
|
23
|
|
The
accompanying notes are an integral part of these audited consolidated financial statements.
INSPYR
THERAPEUTICS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER
31, 2020 AND 2019
NOTE
1 – BACKGROUND
Inspyr
Therapeutics, Inc. (“we”, “us”, “our company”, “our”, “Inspyr” or the “Company”)
was formed under the laws of the State of Delaware in November 2003, and has its principal office in Westlake Village, California. We
are focused on the research and development of novel targeted precision therapeutics for the treatment of cancer.
Our
approach utilizes our proprietary delivery technology to better enhance immuno-modulation for improved therapeutic outcomes. Our potential
first-in-class immune-oncology lead asset, RT-AR001, an adenosine receptor A2B antagonist, is differentiated by its novel microparticle
formulation that allows for better tumor infiltration and enhanced outcomes when administered intra-tumorally. Our patented portfolio
of adenosine receptor antagonists provides flexibility to optimize treatment based on the specific targets found in each type of cancer.
The
adenosine receptor modulators include A 2B antagonists, dual A 2A /A 2B antagonists, and A 2A antagonists that have broad development
applicability including indications within immuno-oncology and inflammation. Adenosine is implicated in immunosuppression in the tumor
microenvironment. Adenosine receptor antagonists may boost the host immune response against the tumor as a single-agent and in combination
with other existing immuno-oncology agents leading to enhanced tumor killing and inhibition of metastasis. Adenosine also has anti-inflammatory
properties in the acute and chronic setting. Adenosine receptor antagonists may promote a decreased inflammatory response and can potentially
treat a broad range of inflammatory and autoimmune based diseases and conditions (e.g., rheumatoid arthritis, joint injury, Crohn’s
disease, psoriasis) as well as improve wound healing and decrease pain.
Pursuant
to our recent termination of license with Ridgeway Therapeutics, Inc. (“Ridgeway”), we reacquired the rights to certain intellectual
property, discussed above, and are currently focusing on a pipeline of small molecule adenosine receptor modulators. In October 2020,
pursuant to the cancellation of a license agreement whereby we previously licensed US Patent 9,593,118, we reacquired the exclusive right
to such patent that covers both A2B and dual A2A/A2B antagonists. Accordingly, going forward our major focus will be to: (i) further
characterization of the anti-cancer activity of our unique pipeline delivery platform containing A2B and dual A2A/A2B antagonists, leading
to selection of a clinical candidate or candidates for an Investigative New Drug or IND enabling studies; and (ii) licensing and/or partnering
our delivery platform and the A2B and dual A2A/A2B antagonists for further development.
Our
ability to execute the business plan is contingent upon our ability to raise the necessary funds. During March 2020, we sold approximately
$250,000 of debt securities and in October 2020, we sold $500,000 of debt securities for cash. In January 2021, we sold an additional
$500,000 of debt securities for cash (see Note 15). We are currently using such funds to maintain our SEC reporting requirements, pay
legal accounting and other professional fees, and to retain consultants and other personnel to develop the adenosine A2R antagonists
and in preparation for an IND filing related to our unique delivery platform and portfolio of adenosine A2R antagonists for the treatment
of certain solid tumors. Should we fail to further raise sufficient funds to execute our business plan, our priority would be to maintain
our intellectual property portfolio and seek business development opportunities with potential development partners and/or acquirors.
Termination
of License Agreement
On
October 5, 2020, the Company entered into an agreement with Ridgeway Therapeutics, Inc. (“Termination Agreement”) whereby
the parties terminated the licensing agreement previously entered into on August 3, 2018 (“Licensing Agreement”), whereby
the Company had previously licensed certain technologies related to targeting adenosine receptor antagonists for the treatment of cancer
(the “Licensed Assets”). As a result of the Termination Agreement, the Company reacquired full ownership and worldwide rights
to all of the Licensed Assets as well as any improvements made thereto.
In
exchange for entering into the Termination Agreement, the Company issued to Ridgeway: (i)
866,667 shares (“Common Shares”) of the Company’s common stock, (“Common
Stock”), and (ii) 8,000 shares of Series F 0% Convertible Preferred Stock (“Series
F Preferred Stock”). Additionally, we have agreed to pay certain expenses and costs
of Ridgeway’s aggregating approximately $25,000.
The
Company has filed a certificate of designation (“COD”) with the Secretary of State of the State of Delaware that contains
the rights, preferences, and privileges of the Series F Preferred Stock. Pursuant to the COD, each share of Series F Preferred Stock
has a stated value of $10.00 per share and is convertible into Common Stock at any time at the election of the holder. In the aggregate,
all of the Series F Preferred Stock issued to Ridgeway is convertible into such number of shares of Common Stock equal to eighty percent
(80%) of the issued and outstanding shares of Common Stock, post-conversion, on the conversion date (taking into effect any forward or
reverse stock splits or consolidations). The Series F Preferred Stock votes on an as if converted to common stock basis. Additionally,
upon the Company’s outstanding Convertible Debentures (as such term is defined in the COD) being terminated, converted, or otherwise
extinguished, the Series F Preferred Stock will automatically convert into Common Stock.
Pursuant
to the Termination Agreement, in the event that the Company is unable to secure equity financing resulting in aggregate gross proceeds
to the Company of at least $5,000,000 by October 5, 2023, or in the event that the Company ceases its operations, then the Termination
Agreement will be deemed terminated and the Licensing Agreement will be reinstated in exchange for the return of the Common Shares and
Series F Preferred Stock.
As
a result of the issuance of the Common Shares and Series F Preferred Stock, Ridgeway Therapeutics became the owner of approximately 54.14%
of the Company’s issued and outstanding Common Stock. Furthermore, by virtue of the issuance of the Series F Preferred Stock, Ridgeway
will vote on an as if converted to common stock basis which shall be equal to eighty percent (80%) of the issued and outstanding Common
Stock post-conversion. Accordingly, the board of directors of the Company has determined that a change in control of the registrant has
occurred. The Company did not have a prior relationship with Ridgeway, or any of its principals, except pursuant to the terms contained
in the Termination Agreement and its previous relationship under the Licensing Agreement.
NOTE
2 – MANAGEMENT’S PLANS TO CONTINUE AS A GOING CONCERN
Basis
of Presentation
The
opinion of our independent registered accounting firm on our consolidated financial statements contains explanatory going concern
language. We have prepared our consolidated financial statements on the basis that we will continue as a going concern, which
contemplates the realization of assets and satisfaction of liabilities in the normal course of business. We have incurred losses
from operations since inception, we have a working capital deficit of $12.5
million and we have an accumulated deficit of $67
million as of December 31, 2020. We anticipate incurring additional losses for the foreseeable future until such time, if ever,
that we can generate significant sales from our therapeutic product candidates which are currently in development or we enter into
cash flow positive business development transactions.
To
date, we have generated no sales or revenues, have incurred significant losses and expect to incur significant additional losses as we
advance our product candidates through development. Consequently, our operations are subject to all the risks inherent in the establishment
of a pre-revenue business enterprise as well as those risks associated with a company engaged in the research and development of pharmaceutical
compounds.
Our
cash balances at December 31, 2020 were approximately $404,000, representing 100% of our total assets. We had curtailed substantially
all operations in February 2018. Based on our current expected level of operating expenditures, and including approximately $250,000
that we raised in March 2020, $500,000 that we raised in October 2020 and $500,000 that we raised in January 2021 (see Note 15), pursuant
to the sale of our senior convertible debentures, we expect to be able to fund our operations into the second quarter of 2022. We will
require additional cash to fund and continue our operations beyond that point. This period could be shortened if there are any unanticipated
increases in planned spending on development programs or other unforeseen events. We anticipate raising additional funds through collaborative
arrangements, licensing agreements, public or private sales of debt or equity securities, or some combination thereof. There is no assurance
that any such arrangement will be entered into or that financing will be available when needed in order to allow us to continue our operations,
or if available, on terms favorable or acceptable to us.
In
the event additional financing is not obtained, we may pursue cost cutting measures as well as explore the sale of assets to generate
additional funds. If we are required to significantly reduce operating expenses and delay, reduce the scope of, or eliminate any of our
development programs or clinical trials, these events could have a material adverse effect on our business, results of operations, and
financial condition. These factors raise substantial doubt about our ability to continue as a going concern. The consolidated financial
statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and
classification of liabilities that might be necessary should we be unable to continue as a going concern.
Our
current cash level raises substantial doubt about our ability to continue as a going concern past the second quarter of 2022. If we do
not obtain additional funds by such time, we may no longer be able to continue as a going concern and will cease operation which means
that our shareholders will lose their entire investment.
NOTE
3 – SUMMARY OF CRITICAL ACCOUNTING POLICIES AND USE OF ESTIMATES
Principles
of Consolidation
The
consolidated financial statements include the accounts of the parent company, Inspyr Therapeutics, Inc., and its wholly-owned subsidiary,
Lewis & Clark Pharmaceuticals, Inc. All significant intercompany accounts and transactions have been eliminated.
Reverse
Stock Split and Increase in Authorized Shares
On
September 1, 2021, the Board of Directors approved a one-for-seventy-five (1-for-75) reverse stock split of the Company’s Common
Stock (“Reverse Stock Split”). The Reverse Stock Split became effective with the Secretary of State of Delaware as of 4:59
p.m. Eastern Time on October 5, 2021, and the Company began trading on a post Reverse Stock Split basis at the market open on October
12, 2021. As a result of the Reverse Stock Split, each of the holders of the Company’s Common Stock received one (1) new share
of Common Stock for every seventy-five (75) shares such shareholder held immediately prior. No fractional shares were issued as
a result of the Reverse Stock Split. Any fractional shares that would have otherwise resulted from the Reverse Stock Split will be rounded
up to the next whole number of shares. The Reverse Stock Split also affected the Company’s outstanding stock options, warrants
and other exercisable or convertible instruments and resulted in the shares underlying such instruments being reduced and the exercise
price being increased proportionately to the Reverse Stock Split ratio.
On
June 10, 2020, the Company’s Board of Directors approved a one-for-thirty (1-for-30) reverse stock split of the Company’s
common stock (“Reverse Stock Split”). Pursuant to the Reverse Stock Split, the Company filed an amended and restated certificate
of incorporation with the Secretary of State of Delaware to effect the Reverse Stock Split effective as of 5:00 p.m. Eastern Time on
June 26, 2020 (“Effective Time”). Accordingly, at the Effective Time, each of the Company’s common stock shareholders
received one new share of common stock for every thirty shares such shareholder held immediately prior to the Effective Time. The Reverse
Stock Split also affected the Company’s outstanding stock options, warrants and other exercisable or convertible instruments and
resulted in the shares underlying such instruments being reduced and the exercise price being increased proportionately to the Reverse
Stock Split ratio.
On
September 17, 2019, the Company’s Board of Directors approved a one-for-twenty five (1-for-25) reverse stock split of the Company’s
common stock. In furtherance of the Reverse Stock Split, the Company has filed an amended and restated certificate of incorporation with
the Secretary of State of Delaware to effect the Reverse Stock Split effective as of 5:00 p.m. Eastern Time on September 30, 2019 (“Effective
Time”). Accordingly, at the Effective Time, each of the Company’s common stock shareholders received one new share of common
stock for every twenty-five shares such shareholder held immediately prior to the Effective Time. The Reverse Stock Split will also affected
the Company’s outstanding stock options, warrants and other exercisable or convertible instruments and resulted in the shares underlying
such instruments being reduced and the exercise/conversion price being increased proportionately to the Reverse Stock Split ratio.
All
share and per share data has been retroactively adjusted in the accompanying consolidated financial statements and footnotes for all
periods presented to reflect the effects of the October 5, 2021, June 26, 2020 and September 30, 2019 amendments.
Pursuant
to a joint written consent of the board of directors and a majority of the voting power of the Company’s stockholders, the Company’s
shareholders approved amending and restated the Company’s Certificate of Incorporation to (i) increase the Company’s authorized
Common Stock from 150,000,000 shares to 1,000,000,000 shares and to (ii) increase or decrease (but
not below the number of shares of such class outstanding) the number of authorized shares of the class of Common Stock or Preferred Stock
by the affirmative vote of the holders of a majority in voting power of the outstanding shares of capital stock of the Company irrespective
of the provisions of Section 242(b)(2) of the Delaware General Corporation Law.
The
Company filed the Amended and Restated Certificate of Incorporation with Delaware’s Secretary of State reflecting the foregoing
changes with an effective date and time of November 27, 2020.
Use
of Estimates
The
preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates
and assumptions that affect the amounts reported in the financial statements and accompanying disclosures. Significant estimates include
the fair value of derivative instruments, stock-based compensation, recognition of clinical trial costs and other accrued liabilities.
Actual results may differ from those estimates.
Research
and Development
Research
and development costs are charged to expense as incurred. Our research and development expenses consist primarily of expenditures for
toxicology and other studies, manufacturing, clinical trials, compensation and consulting costs.
We
incurred research and development expenses of $0.02 million and $0.04 million for the years ended December 31, 2020 and 2019, respectively.
Cash
Equivalents
For
purposes of the statements of cash flows, we consider all highly liquid debt instruments purchased with a maturity date of three months
or less to be cash equivalents. We maintain our cash in bank deposit accounts which, at times, may exceed applicable government mandate
insurance limits. We have not experienced any losses in our accounts. We did not have any cash equivalents at December 31, 2020 or 2019.
Restricted
Cash
Restricted
cash consisted of funds held in trust for the Company. The use of these funds was restricted to: (i) the payment of professional fees
in connection with bringing the Company’s filings current, and (ii) the payment of vendors associated with the issuance and trading
of the Company’s securities, such as transfer agent fees and fees payable to the OTCQB and FINRA. There were no restrictions on
the use of cash at December 31, 2020.
Concentrations
of Credit Risk
Financial
instruments and related items, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and
cash equivalents. The Company places its cash and temporary cash investments with credit quality institutions. At times, such investments
may exceed applicable government mandated insurance limits. Cash and restricted cash was $0.4 million and $0.02 million at December 31,
2020 and 2019, respectively. As of December 31, 2020 and 2019, there was no cash over the federally insured limit.
Intangible
Assets
Intangible
assets consist of licensed technology, patents, and patent applications (see Note 5). The assets associated with licensed technology
are recorded at cost and are being amortized on the straight line basis over their estimated useful lives of twelve to seventeen years.
Office
and Lab Equipment
Equipment
is stated at cost less accumulated depreciation. Depreciation is calculated on the straight line basis over the estimated useful lives
of the assets of three to seven years. Expenditures for repair and maintenance which do not materially extend the useful lives of property
and equipment are charged to expense. When property or equipment is sold or otherwise disposed of, the cost and related accumulated depreciation
are removed from the respective accounts with the resulting gain or loss reflected in operations. Management periodically reviews the
carrying value of its equipment for impairment.
Depreciation
expense was approximately $0 and $2,000 for the years ended December 31, 2020 and 2019, respectively.
Loss
per Share
Basic
loss per share is calculated by dividing net loss and net loss attributable to common shareholders by the weighted average number of
common shares outstanding for the period. Basic and diluted loss per share are the same, in that any potential common stock equivalents
would have the effect of being anti-dilutive in the computation of net loss per share.
The
following potentially dilutive securities have been excluded from the computations of weighted average shares outstanding as of December
31, 2020 and 2019, as they would be anti-dilutive:
|
Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share
|
|
|
|
|
|
|
|
|
|
|
|
Year
Ended
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Shares
underlying options outstanding
|
|
|
16
|
|
|
|
28
|
|
|
Shares
underlying warrants outstanding
|
|
|
53
|
|
|
|
65
|
|
|
Shares
underlying convertible notes outstanding
|
|
|
6,949,870
|
|
|
|
284,943
|
|
|
Shares
underlying convertible preferred stock outstanding
|
|
|
9,995,250
|
|
|
|
3,517
|
|
|
|
|
|
16,945,189
|
|
|
|
288,553
|
|
Derivative
Liability
The
Company has financial instruments that are considered derivatives or contain embedded features subject to derivative accounting. Embedded
derivatives are valued separately from the host instrument and are recognized as derivative liabilities in the Company’s balance
sheet. The Company measures these instruments at their estimated fair value and recognizes changes in their estimated fair value in results
of operations during the period of change. The Company values its derivative liabilities using the Black-Scholes option valuation model.
The resulting liability is valued at each reporting date and the change in the liability is reflected as change in derivative liability
in the statement of operations.
Fair
Value of Financial Instruments
Our
short-term financial instruments, including cash, accounts payable and other liabilities, consist primarily of instruments with maturities
of one year or less when acquired. We believe that the fair values of our current assets and current liabilities approximate their reported
carrying amounts.
The
derivative liabilities consist of our convertible notes and Series F preferred stock with variable conversion features. The Company uses
the Black-Scholes option-pricing model to value its derivative liabilities which incorporate the Company’s stock price, volatility,
U.S. risk-free interest rate, dividend rate, and estimated life.
Fair
Value Measurements
The
U.S. GAAP Valuation Hierarchy establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure fair value.
This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets
for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs
that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full
term of the financial instrument. Level 3 inputs are unobservable inputs based on our own assumptions used to measure assets and liabilities
at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input
that is significant to the fair value measurement.
The
Company has recorded a derivative liability for its convertible notes and preferred stock with variable conversion features as of December
31, 2020 and 2019. The tables below summarize the fair values of our financial liabilities as of December 31, 2020 and 2019 (in thousands):
|
Schedule of fair values of financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair
Value at
December 31,
|
|
|
Fair
Value Measurement Using
|
|
|
|
|
2020
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Convertible notes
|
|
$
|
2,705
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
2,705
|
|
|
Preferred
stock
|
|
|
4,123
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,123
|
|
|
Derivative
liability
|
|
$
|
6,828
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
6,828
|
|
|
|
|
Fair
Value at
December 31,
|
|
|
Fair
Value Measurement Using
|
|
|
|
|
2019
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Derivative
liability – convertible notes
|
|
$
|
1,785
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,785
|
|
The
reconciliation of the derivative liability measured at fair value on a recurring basis using unobservable inputs (Level 3) is as follows
(in thousands):
|
Schedule of derivative liability measured at fair value on a recurring basis using unobservable inputs (Level 3)
|
|
|
|
|
|
|
|
|
|
|
|
Year
ended
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Balance
at beginning of year
|
|
$
|
1,785
|
|
|
$
|
2,134
|
|
|
Additions
to derivative instruments
|
|
|
2,465
|
|
|
|
243
|
|
|
Reclassification
on conversion
|
|
|
(1,268
|
)
|
|
|
(265
|
)
|
|
Loss
(gain) on change in fair value of derivative liability
|
|
|
3,846
|
|
|
|
(327
|
)
|
|
Balance
at end of year
|
|
$
|
6,828
|
|
|
$
|
1,785
|
|
Income
Taxes
Income
taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences
attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective
tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected
to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred
tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. A valuation
allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. The ultimate
realization of deferred tax assets is dependent upon the generation of future taxable income and the reversal of deferred tax liabilities
during the period in which the related temporary difference becomes deductible.
Stock-Based
Compensation
We
account for our stock-based compensation under ASC 718 “Compensation – Stock Compensation” using the fair value based
method. Under this method, compensation cost is measured at the grant date based on the value of the award and is recognized over the
shorter of the service period or the vesting period of the stock-based compensation. This guidance establishes standards for the accounting
for transactions in which an entity exchanges it equity instruments for goods or services. It also addresses transactions in which an
entity incurs liabilities in exchange for goods or services that are based on the fair value of the entity’s equity instruments
or that may be settled by the issuance of those equity instruments. The Company estimates the fair value of each stock option at the
grant date by using the Black-Scholes option pricing model. Determining the fair value of stock-based compensation at the grant date
under this model requires judgment, including estimating volatility, employee stock option exercise behaviors and forfeiture rates. The
assumptions used in calculating the fair value of stock-based compensation represent the Company’s best estimates, but these estimates
involve inherent uncertainties and the application of management judgment.
Through
December 31, 2018 we used the fair value method for equity instruments granted to non-employees and used the Black-Scholes model for
measuring the fair value of options. The stock based fair value compensation is determined as of the date of the grant or the date at
which the performance of the services is completed (measurement date) and is recognized over the vesting periods.
On
January 1, 2019, the Company adopted ASU 2018-07, which substantially aligns stock-based compensation for employees and non-employees
and accounts for non-employee share-based awards in accordance with the measurement and recognition criteria of ASC 718. The Company
used the modified prospective method of adoption. There was no cumulative effect of the adoption of ASC 718.
Recent
Accounting Pronouncements
With
the exception of those discussed below, there have not been any recent changes in accounting pronouncements and Accounting Standards
Update (ASU) issued by the Financial Accounting Standards Board (FASB) during the year ended December 31, 2020 that are of significance
or potential significance to the Company.
In
December 2019, the FASB issued ASU 2019-12, “Simplifying the Accounting for Income Taxes.” This guidance, among other provisions,
eliminates certain exceptions to existing guidance related to the approach for intraperiod tax allocation, the methodology for calculating
income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. This guidance also requires
an entity to reflect the effect of an enacted change in tax laws or rates in its effective income tax rate in the first interim period
that includes the enactment date of the new legislation, aligning the timing of recognition of the effects from enacted tax law changes
on the effective income tax rate with the effects on deferred income tax assets and liabilities. Under existing guidance, an entity recognizes
the effects of the enacted tax law change on the effective income tax rate in the period that includes the effective date of the tax
law. ASU 2019-12 is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. We do
not expect that the adoption of this standard will have a material impact on the Company’s consolidated financial statements.
NOTE
4 – SUPPLEMENTAL CASH FLOW INFORMATION
The
following table contains additional information for the periods reported (in thousands).
|
Schedule of additional information of cash flow
|
|
|
|
|
|
|
|
|
|
|
|
Year
Ended
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Non-cash
financial activities:
|
|
|
|
|
|
|
|
|
|
Common
stock issued on conversion of notes payable and derivative liability
|
|
$
|
2,243
|
|
|
$
|
473
|
|
|
Debentures
converted to common stock
|
|
|
1,310
|
|
|
|
331
|
|
|
Derivative
liability extinguished upon conversion of notes payable
|
|
|
1,268
|
|
|
|
265
|
|
|
Derivative
liability issued
|
|
|
2,465
|
|
|
|
243
|
|
|
Accounts
payable paid through issuance of debentures
|
|
|
100
|
|
|
|
—
|
|
There
was no cash paid for interest and income taxes for the years ended December 31, 2020 and 2019.
NOTE
5 – INTELLECTUAL PROPERTY
We
solely own or have exclusive licenses to all of our patents and patent applications. Between 2008 and 2011, we entered into license and
assignment agreements with Johns Hopkins University (JHU), the University of Copenhagen (UC) and certain co-inventors (Assignee Co-Founders),
in which we paid $212,000 in cash and common stock. As a result of these payments and pursuant to the agreements, we acquired worldwide,
exclusive, fully paid up rights in know-how, pre-clinical data, development data and certain patent portfolios that relate to, and form
the basis of, our technology. Under these agreements, we are not required to make any other future payments, including fees or other
reimbursements, milestones, or royalties, to JHU, UC, or the Assignee Co-Founders.
Amortization
expense recorded during the year ended December 31, 2019 was approximately $33,000. Intangibles have been fully amortized at December
31, 2019.
NOTE
6 – ACCRUED EXPENSES
Accrued
expenses consist of the following (in thousands):
|
Schedule of accrued expenses
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Accrued compensation
and benefits
|
|
$
|
1,326
|
|
|
$
|
1,326
|
|
|
Accrued research and development
|
|
|
233
|
|
|
|
233
|
|
|
Accrued other
|
|
|
381
|
|
|
|
307
|
|
|
Total accrued expenses
|
|
$
|
1,940
|
|
|
$
|
1,866
|
|
NOTE
7 – DERIVATIVE LIABILITY
We
account for equity-linked financial instruments, such as our convertible preferred stock, convertible debentures and our common stock
warrants as either equity instruments or derivative liabilities depending on the specific terms of the respective agreement. Equity-linked
financial instruments are accounted for as derivative liabilities, in accordance with ASC Topic 815 – Derivatives and Hedging,
if the instrument allows for cash settlement or issuance of a variable number of shares. We classify derivative liabilities on the balance
sheet at fair value, and changes in fair value during the periods presented in the statement of operations, which is revalued at each
balance sheet date subsequent to the initial issuance of the stock warrant.
We
have issued convertible debentures and preferred stock which contain variable conversion features, anti-dilution protection and other
conversion price adjustment provisions. As a result, the Company assessed its outstanding equity-linked financial instruments and concluded
that the convertible notes and preferred stock are subject to derivative accounting. The fair value of the conversion feature is classified
as a liability in the consolidated financial statements, with the change in fair value during the periods presented recorded in the consolidated
statement of losses.
During
the years ended December 31, 2020 and 2019, we recorded loss of approximately $3.8 million and gain of approximately $0.3 million, respectively,
related to the change in fair value of the derivative liabilities during the periods. For purpose of determining the fair market value
of the derivative liability, the Company used Black Scholes option valuation model. The significant assumptions used in the Black Scholes
valuations of the derivatives at December 31, 2020 are as follows:
|
Schedule of black scholes valuations of derivatives
|
|
|
|
|
|
|
2020
|
|
|
Volatility
|
|
343%
- 367%
|
|
|
Expected
term (years)
|
|
3
- 12 months
|
|
|
Risk-free
interest rate
|
|
0.09%
– 0.095%
|
|
|
Dividend
yield
|
|
None
|
|
As
of December 31, 2020 and 2019, the derivative liability recognized in the financial statements was approximately $6.8 million and $1.8
million, respectively.
NOTE
8 – COMMITMENTS AND CONTINGENCIES
Operating
Leases
Inspyr
currently does not have any ongoing leases for office space. It has availability to office space on an as needed basis. Its employees
work on a remote basis.
There
was no rent expense for the years ended December 31, 2020 and 2019, respectively.
Legal
Matters
The
Company is subject at times to legal proceedings and claims, which arise in the ordinary course of its business. Although occasional
adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material
adverse effect on its financial position, results of operations or liquidity.
COVID-19
Uncertainty
On
March 11, 2020, the World Health Organization declared a pandemic related to the rapidly spreading coronavirus (COVID-19) outbreak, which
has led to a global health emergency. The extent of the public-health impact of the outbreak is currently unknown and rapidly evolving,
and the related health crisis could adversely affect the global economy, resulting in an economic downturn. Any disruption of the Company’s
facilities or those of our suppliers could likely adversely impact the Company’s operations. At this time, there is significant
uncertainty relating to the potential effect of the novel coronavirus on our business.
NOTE
9 – CAPITAL STOCK AND STOCKHOLDERS’ EQUITY
Preferred
Stock
As
of December 31, 2020, there were outstanding 134 133.8
shares of Series A Preferred Stock, 71 shares
of Series B Preferred Stock, 290.4
shares of Series C Preferred Stock, 5,000 shares
of Series D Preferred Stock, 5,000 shares
of Series E Preferred Stock and 8,000 shares
of Series F Preferred Stock.
On
October 6, 2020, the Company has filed a certificate of designation (“COD”) with the Secretary of State of the State of Delaware
that contains the rights, preferences, and privileges of the Series F Preferred Stock. Pursuant to the COD, each share of Series F Preferred
Stock has a stated value of $10.00 per share and is convertible into Common Stock at any time at the election of the holder. We issued
all 8,000 shares of the Series F stock to Ridgeway Therapeutics, Inc. in connection with the Termination Agreement described in Note
1. In the aggregate, all of the Series F Preferred Stock issued to Ridgeway is convertible into such number of shares of Common Stock
equal to eighty percent (80%) of the issued and outstanding shares of Common Stock, post-conversion, on the conversion date (taking into
effect any forward or reverse stock splits or consolidations). The Series F Preferred Stock votes on an as if converted to common stock
basis. Additionally, upon the Company’s outstanding Convertible Debentures (as such term is defined in the COD) being terminated,
converted, or otherwise extinguished, the Series F Preferred Stock will automatically convert into Common Stock.
On
May 2, 2020, we sold 5,000 shares of Series E 0% Convertible Preferred Stock to an accredited investor at a price per share of $1.00
for aggregate gross proceeds of $5,000. Each share of Series E Preferred Stock has stated value of $1.00. The Series E Preferred Stock
is convertible, at any time after the Original Issue Date at the option of the Holder into that number of shares of Common Stock (Subject
to the limitations set forth in Section 6(d) of the certificate of designation of the Series E Preferred Stock), determined by dividing
the stated value by the then in effect conversion price. As of December 31, 2020, the conversion price is $22.50 per share.
With
respect to a vote of stockholders to approve a reverse split of the Common Stock to occur no later than December 31, 2022 only, each
share of Series E Preferred Stock held by a holder, as such, is entitled to 1,333 votes. On any matter presented to the stockholders
of the Company for their action or consideration at any meeting of stockholders of the Company (or by written consent of stockholders
in lieu of meeting), each holder of outstanding shares of Series E Preferred Stock shall be entitled to cast the number of votes equal
to the number of whole shares of Common Stock into which the shares of Series E Preferred Stock held by such holder are convertible as
of the record date for determining stockholders entitled to vote on such matter. Except as provided by law or by the other provisions
of the certificate of incorporation, holders of the Series E Preferred Stock shall vote together with the holders of Common Stock as
a single class.
During
December 2018, we designated 5,000 shares of preferred stock as Series D 0% Convertible Preferred Stock (the “Series D Preferred
Stock”). Each share of Preferred Stock has a par value of $0.0001 per share and a stated value equal to $1.00 (the “Stated
Value”). During January 2019, we issued the 5,000 shares of Series D Convertible Preferred Stock for proceeds of $5,000. Each share
of Series D Preferred Stock shall be convertible, at any time and from time to time from and after the Original Issue Date at the option
of the Holder thereof, into that number of shares of Common Stock (subject to the limitations set forth in Section 6(d)) determined by
dividing the Stated Value of such share of Series D Preferred Stock by the Conversion Price. As of December 31, 2020, the Conversion
Price is $281.25 per share of Series D Preferred Stock.
With
respect to a vote of stockholders to approve a reverse split of the Common Stock to occur no later than December 31, 2019, only, each
share of Series D Preferred Stock held by a Holder, as such, was entitled to 400 votes. On any matter presented to the stockholders of
the Corporation for their action or consideration at any meeting of stockholders of the Corporation (or by written consent of stockholders
in lieu of meeting), each holder of outstanding shares of Series D Preferred Stock shall be entitled to cast the number of votes equal
to the number of whole shares of Common Stock into which the shares of Series D Preferred Stock held by such holder are convertible as
of the record date for determining stockholders entitled to vote on such matter. Except as provided by law or by the other provisions
of the certificate of incorporation, holders of the Series D Preferred Stock shall vote together with the holders of Common Stock as
a single class.
In
December 2015, we issued 1,853 shares of our Series A 0% Convertible Preferred Stock (the “Series A Preferred Stock”), with
a stated value of $1,000 per share and the common shares are issuable pursuant to conversion of the Series A Preferred Stock at a conversion
price of $29,812.50 per share as of December 31, 2020, subject to a 9.99% beneficial ownership limitation and subject to adjustment pursuant
to stock splits and dividends. The Series A Preferred Stock was subject to adjustment pursuant to anti-dilution protection for subsequent
equity sales for a period of 18 months from the effective date of the registration statement registering the shares underlying the Series
A Preferred Stock, or until July 29, 2017.
In
December 2016, we issued 1,000 shares of our Series B 0% Convertible Preferred Stock (the “Series B Preferred Stock”), with
a stated value of $1,000 per share and the common shares are issuable pursuant to conversion of the Series B Preferred Stock at a conversion
price of $0.75 per share as of December 31, 2020, subject to beneficial ownership limitations and subject to adjustment pursuant to stock
splits and dividends, and subject to adjustment pursuant to anti-dilution protection for subsequent equity sales and other conversion
price adjustments.
In
March and April 2017, we issued 290.43148 shares of Series C 0% Convertible Preferred Stock (the “Series C Preferred Stock”).
The Series C Preferred Stock has a stated value of $1,000 and common shares are issuable pursuant to conversion of the Series C Preferred
Stock at a conversion price of $1,125.00 per share for 200 shares of series C preferred stock and a conversion price of $562.50 per shares
for 90.43418 shares of our Series C preferred stock as of December 31, 2020, subject to certain beneficial ownership limitations and
subject to adjustment pursuant to stock splits and dividends, and pursuant to anti-dilution protection for subsequent equity sales for
a period of twelve (12) months from the issuance of the Series C Preferred Stock.
As
a result of past equity financings and conversions of debentures, the conversion prices of (i) our Series A Preferred Stock has been
reduced to $29,812.50 per share at December 31, 2020, (ii) our Series B Preferred Stock has been reduced to $0.75 per share at December
31, 2020, (iii) 200 shares of our Series C preferred stock has been reduced to $1,125.00 per share at December 31, 2020, (iv) 90.43418
shares of our Series C Preferred Stock has been reduced to $562.50 per share at December 31, 2020.
As
a result of the reductions of the conversion prices of our preferred stock, we have recorded a deemed dividend of approximately $64,000
and $54,000 during the years ended December 31, 2020 and 2019, respectively.
Common
Stock
Increase
in Authorized Shares
Pursuant
to a joint written consent of the board of directors and a majority of the voting power of the Company’s stockholders, the Company’s
shareholders approved amending and restated the Company’s Certificate of Incorporation to (i) increase the Company’s authorized
Common Stock from 150,000,000 shares to 1,000,000,000 shares and to (ii) increase or decrease (but
not below the number of shares of such class outstanding) the number of authorized shares of the class of Common Stock or Preferred Stock
by the affirmative vote of the holders of a majority in voting power of the outstanding shares of capital stock of the Company irrespective
of the provisions of Section 242(b)(2) of the Delaware General Corporation Law.
The
Company filed the Amended and Restated Certificate of Incorporation with Delaware’s Secretary of State reflecting the foregoing
changes with an effective date and time of November 27, 2020.
Reverse
Stock Splits
On September
1, 2021, the Board of Directors approved a one-for-seventy-five (1-for-75) reverse stock split of the Company’s Common Stock (“Reverse
Stock Split”). The Reverse Stock Split became effective with the Secretary of State of Delaware as of 4:59 p.m. Eastern Time on
October 5, 2021, and the Company began trading on a post Reverse Stock Split basis at the market open on October 12, 2021. As a result
of the Reverse Stock Split, each of the holders of the Company’s Common Stock received one (1) new share of Common Stock for
every seventy-five (75) shares such shareholder held immediately prior. No fractional shares were issued as a result of the Reverse
Stock Split. Any fractional shares that would have otherwise resulted from the Reverse Stock Split will be rounded up to the next whole
number of shares. The Reverse Stock Split also affected the Company’s outstanding stock options, warrants and other exercisable
or convertible instruments and resulted in the shares underlying such instruments being reduced and the exercise price being increased
proportionately to the Reverse Stock Split ratio.
On
June 10, 2020, the Company’s Board of Directors approved a one-for-thirty (1-for-30) reverse stock split of the Company’s
common stock (“Reverse Stock Split”). Pursuant to the Reverse Stock Split, the Company filed an amended and restated certificate
of incorporation with the Secretary of State of Delaware to effect the Reverse Stock Split effective as of 5:00 p.m. Eastern Time on
June 26, 2020 (“Effective Time”). Accordingly, at the Effective Time, each of the Company’s common stock shareholders
received one new share of common stock for every thirty shares such shareholder held immediately prior to the Effective Time. The Reverse
Stock Split also affected the Company’s outstanding stock options, warrants and other exercisable or convertible instruments and
resulted in the shares underlying such instruments being reduced and the exercise price being increased proportionately to the Reverse
Stock Split ratio.
On
September 17, 2019, the Company’s Board of Directors approved a one-for-twenty five (1-for-25) reverse stock split of the Company’s
common stock. In furtherance of the Reverse Stock Split, the Company has filed an amended and restated certificate of incorporation with
the Secretary of State of Delaware to effect the Reverse Stock Split effective as of 5:00 p.m. Eastern Time on September 30, 2019 (“Effective
Time”). Accordingly, at the Effective Time, each of the Company’s common stock shareholders received one new share of common
stock for every twenty-five shares such shareholder held immediately prior to the Effective Time. The Reverse Stock Split will also affected
the Company’s outstanding stock options, warrants and other exercisable or convertible instruments and resulted in the shares underlying
such instruments being reduced and the exercise/conversion price being increased proportionately to the Reverse Stock Split ratio.
All
share and per share data has been retroactively adjusted in the accompanying consolidated financial statements and footnotes for all
periods presented to reflect the effects of the October 5, 2021, June 26, 2020 and September 30, 2019 amendments.
Common
Stock Activity
In
October 2020, we issued 866,667 shares of common stock with a fair value of $266,500 in connection with the Ridgeway Termination Agreement
(see Note 1).
During
the year ended December 31, 2020, we issued a total of 1,600,021 shares of common stock, valued at $2,243,628, upon the conversion of
$1,310,068 principal amount of our convertible debentures. We recorded gain on conversion of debt of $334,206 during the year ended December
31, 2020.
During
the year ended December 31, 2019, we issued a total of 6,785 shares of common stock, valued at $471,583, upon the conversion of $331,415
principal amount of our convertible debentures. We recorded gain on conversion of debt of $125,089 during the year ended December 31,
2019.
NOTE
10 – STOCK OPTIONS
Deferred
Compensation Plan
In
July of 2011, we adopted Executive Deferred Compensation Plan (the Deferred Plan). The Deferred Plan is intended to comply with the requirements
of Section 409A of the Internal Revenue Code of 1986, as amended (the Code). The Deferred Plan is intended to be an unfunded “top
hat” plan which is maintained primarily to provide deferred compensation benefits for a select group of our “management or
highly compensated employees” within the meaning of Sections 201, 301, and 401 of the Employee Retirement Income Security Act of
1974, as amended (ERISA), and to therefore be exempt from the provisions of Parts 2, 3, and 4 of Title I of ERISA. The Deferred Plan
is intended to help build a supplemental source of savings and retirement income through pre-tax deferrals of eligible compensation,
which may include cash, option and stock bonus awards, discretionary cash, option and stock awards and/or any other payments which may
be designated by the Deferred Plan administrator, as eligible, for deferral under the Deferred Plan from time to time. As administered,
the Deferred Plan is used to defer compensation of stock awards granted under our other equity compensation plans and does not by its
terms approve any grants or awards.
Inspyr’s
Compensation Plans
The
Company’s 2007 Equity Compensation Plan (2007 Plan), 2009 Executive Compensation Plan (2009 Plan), 2017 Equity Compensation Plan
(2017 Plan), and the Inducement Award Stock Option Plan (Inducement Plan) (together, the Plans) provide for the awarding of stock grants,
nonqualified and incentive stock options, restricted stock units, performance units or other stock-based awards to officers, directors,
employees and consultants of the Company. The purpose of the Plans is to advance the interests of Inspyr and our stockholders by attracting,
retaining and rewarding persons performing services for us and to motivate such persons to contribute to our growth and profitability.
Our Plans are administered by a committee of non-employee directors (the Committee). The Committee determines: who shall be granted awards;
the vesting periods; the exercise price; and any other terms deemed appropriate for any award.
Our
2007 Plan is administered by our board or any of its committees. The purposes of the 2007 Plan are to attract and retain the best available
personnel for positions of substantial responsibility, to provide additional incentive to Employees, Directors and Consultants, and to
promote the success of our business. The issuance of awards under our 2007 Plan is at the discretion of the administrator, which has
the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable to any
award. Under our 2007 Plan, we may grant stock options, restricted stock, stock appreciation rights, restricted stock units, performance
units, performance shares and other stock based awards. Our 2007 Plan authorizes the issuance of up to 67 shares (pre-October 2021 reverse
split) of common stock for the foregoing awards per fiscal year with an aggregate of 267 shares (pre-October 2021 reverse split) of common
stock available for issuance under the 2007 Plan. As of December 31, 2020, we have granted awards under the 2007 Plan equal to approximately
241 (pre-October 2021 reverse split) shares of our common stock, and 202 shares (pre-October 2021 reverse split) have been cancelled
or forfeited. Accordingly, there are 228 shares (pre-October 2021 reverse split) of common stock available for future awards under the
2007 Plan. In the event of a change in control, awards under the 2007 Plan will become fully vested unless such awards are assumed or
substituted by the successor corporation.
Our
2009 Plan, as amended is administered by our Board or any of its committees. The purpose of our 2009 Plan is to advance the interests
of the Company and our stockholders by attracting, retaining and rewarding persons performing services for us and to motivate such persons
to contribute to our growth and profitability. The issuance of awards under our 2009 Plan is at the discretion of the administrator,
which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable
to any award. Under our 2009 Plan, we may grant stock options, restricted stock, stock appreciation rights, restricted stock units, performance
units, performance shares and other stock-based awards. As of December 31, 2020, our 2009 Plan authorizes the issuance of up to 267 shares
(pre-October 2021 reverse split) of our common stock for the foregoing awards, and we have granted awards under the plan equal to approximately
220 common shares (pre-October 2021 reverse split), and 184 shares (pre-October 2021 reverse split) have been cancelled or forfeited.
Accordingly, there are 231 shares (pre-October 2021 reverse split) of common stock available for future awards under the 2009 Plan.
Our
Inducement Plan is administered by our board or our compensation committee. The Plan is intended to be used in connection with the recruiting
and inducement of senior management and employees. The issuance of wards under the Inducement Plan is at the discretion of the administrator
which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable
to any award. The Company did not seek approval of the Plan by our stockholders. Pursuant to the Inducement Plan, the Company may grant
stock options for up to a total of 400 shares (pre-October 2021 reverse split) of common stock to new employees of the Company. As of
December 31, 2020, 282 grants (pre-October 2021 reverse split) have been made pursuant to the Plan, and 130 shares (pre-October 2021
reverse split) have been cancelled or forfeited. Accordingly, there are 248 shares (pre-October 2021 reverse split) of common stock available
for future awards under the Inducement Plan.
Our
2017 Plan is administered by our Board or any of its committees. The purpose of our 2017 Plan is to attract and retain the best available
personnel for positions of substantial responsibility, to provide additional incentive to employees, directors and consultants, and to
promote the success of the Company’s business. The issuance of awards under our 2017 Plan is at the discretion of the administrator,
which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions and restrictions applicable
to any award. Under our 2017 Plan, we may grant stock options, restricted stock, stock appreciation rights, restricted stock units, performance
units, performance shares and other stock-based awards. As of December 31, 2020, our 2017 Plan authorizes the issuance of up to 2,667
shares (pre-October 2021 reverse split) of our common stock for the foregoing awards, and we have not granted any awards under the plan.
Accordingly, there are 2,667 shares (pre-October 2021 reverse split) of common stock available for future awards under the 2017 Plan.
The
Company has not recorded any stock-based compensation expense related to the issuance of stock option awards during the years ended December
31, 2020 and 2019. As of December 31, 2020, there was no unrecognized compensation cost related to non-vested stock options.
The
following table summarizes stock option activity for the years ended December 31, 2020 and 2019:
|
Schedule of stock option activity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
of
shares
|
|
|
Weighted-
average exercise price
|
|
|
Weighted-
average remaining contractual term (in years)
|
|
|
Outstanding
at December 31, 2018
|
|
|
34
|
|
|
$
|
772,114
|
|
|
|
|
|
|
Granted
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
Forfeited
|
|
|
(6
|
)
|
|
$
|
381,797
|
|
|
|
|
|
|
Outstanding
at December 31, 2019
|
|
|
28
|
|
|
$
|
855,753
|
|
|
|
2.9
|
|
|
Granted
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
Forfeited
|
|
|
(12
|
)
|
|
$
|
1,522,968
|
|
|
|
|
|
|
Outstanding
at December 31, 2020
|
|
|
16
|
|
|
$
|
355,342
|
|
|
|
2.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable
at December 31, 2020
|
|
|
16
|
|
|
$
|
355,342
|
|
|
|
2.4
|
|
No
options were issued or exercised during the years ended December 31, 2020 and 2019. The options had no intrinsic value at December 31,
2020.
NOTE
11 – WARRANTS
Transactions
involving our warrants are summarized as follows:
|
Schedule of transactions involving of warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
of
shares
|
|
|
Weighted-
average
exercise price
|
|
|
Weighted-
average
remaining contractual term (in years)
|
|
|
Outstanding
at December 31, 2018
|
|
|
129
|
|
|
$
|
861.515
|
|
|
|
|
|
|
Granted
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
Forfeited
|
|
|
(46
|
)
|
|
$
|
2,008,492
|
|
|
|
|
|
|
Outstanding
at December 31, 2019
|
|
|
83
|
|
|
$
|
225,842
|
|
|
|
1.8
|
|
|
Granted
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
Forfeited
|
|
|
(12
|
)
|
|
$
|
787,500
|
|
|
|
|
|
|
Outstanding
at December 31, 2020
|
|
|
53
|
|
|
$
|
5,033
|
|
|
|
1.0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable
at December 31, 2020
|
|
|
53
|
|
|
$
|
5,033
|
|
|
|
1.0
|
|
No
warrants were issued or exercised during the years ended December 31, 2020 and 2019. The warrants had no intrinsic value at December
31, 2020.
As
a result of recent equity financings and conversions of debentures, the exercise prices of the warrants issued in conjunction with our
Series B preferred stock have been reduced to $22.50 and the warrants issued in conjunction with our Series C preferred stock have also
been reduced to $562.50 - $1,125.00 at December 31, 2020.
The
following table summarizes outstanding common stock purchase warrants as of December 31, 2020:
|
Schedule of outstanding common stock purchase warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
shares
|
|
|
Weighted-
average
exercise price
|
|
|
Expiration
|
|
|
Issued
to consultants
|
|
|
1
|
|
|
$
|
244,688
|
|
|
August 2023
|
|
|
Issued
pursuant to 2016 financings
|
|
|
30
|
|
|
$
|
23
|
|
|
December 2021
|
|
|
Issued
pursuant to 2017 financings
|
|
|
22
|
|
|
$
|
972
|
|
|
March
2022 through April 2022
|
|
|
|
|
|
53
|
|
|
|
|
|
|
|
|
NOTE
12 – CONVERTIBLE DEBENTURES AND NOTES
Extension
of Outstanding Debentures until December 31, 2020
Effective
March 6, 2020, Sabby Healthcare Master Fund, Ltd and Sabby Volatility Warrant Master Fund, Ltd. waived certain events of default under
debentures and notes issued in our December 2018 note offering, July 2018 debenture offering and September 2017 debenture offering (collectively,
the “Debenture Offerings”) and extended the maturity date of such debentures until July 16, 2020. Effective July 16, 2020
the maturity dates of all of the debentures was extended to December 31, 2020. Effective December 31, 2020, the maturity date of all
debentures that matured during 2020 were extended to June 30, 2021.
Conversion
Price Adjustment Agreement
On
November 25, 2020, the Company entered into a conversion price adjustment agreement (the “Agreement”) with Sabby Healthcare
Master Fund, Ltd. and Sabby Volatility Warrant Master Fund, Ltd. (collectively, “Sabby”). Pursuant to the Agreement, approximately
$2.4 million in outstanding senior convertible debentures held by Sabby were amended such that their conversion prices into common stock
of the Company are equal to the lesser of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price during the five trading
days immediately prior to the date of conversion.
October
2020 Debentures
On
October 23, 2020, the Company sold an aggregate of $600,000 of senior convertible debentures (“Debentures”) for (i) $500,000
in cash and (ii) $100,000 in cancellation of outstanding indebtedness to existing accredited and institutional investors (the “Investors”)
of the Company.
The
Debentures (i) are non-interest bearing, (ii) have a maturity date of October 23, 2021, (iii) are convertible into shares of common stock
(“Common Stock”) of the Company at the election of the Investors at any time, subject to a beneficial ownership limitation
of 9.99%, and (iv) have a conversion price equal to the lesser of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price
during the five trading days immediately prior to the date of conversion.
The
Debentures also contain provisions providing for an adjustment in the event of stock splits or dividends, and fundamental transactions.
The Investors also have the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the Debentures
contain anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then applicable conversion
price until such time that the Debentures are no longer outstanding. Additionally, the Company has the option to redeem some or all of
the Debentures for cash upon notice of twenty (20) trading days provided certain conditions are met by the Company as more fully described
in the Debentures.
Without
the approval of the Debenture holders holding at least 67% of the then outstanding principal amount of the Debentures, the Company may
not (i) amend its charter documents in any manner that adversely affects the rights of any Investor, (ii) repay or repurchase or acquire
shares of its Common Stock, (iii) repay, repurchase, or acquire certain indebtedness, or (iv) pay cash dividends or distributions on
any equity securities of the Company.
We
recorded an initial derivative liability of $619,627 related to the fair value of the derivative liability associated with the debentures.
We recorded debt discount of $600,000, which will be amortized to interest expense over the term of the debentures, and we charged $19,627
to interest expense upon issue. We have amortized $112,500 of discount to interest expense at December 31, 2020.
March
2020 Debentures
On
March 6, 2020, the Company sold an aggregate of $250,000 of senior convertible debentures (the “March 2020 Debentures”) for
cash to existing accredited institutional investors of the Company (the “March 2020 Offering”). The March 2020 Debentures
issued (i) are non-interest bearing, (ii) have a maturity date of July 16, 2020 and (iii) are convertible into shares of common stock
of the Company at the election of the Investor at any time, subject to a beneficial ownership limitation of 9.99%. The March Debentures
have a conversion price equal to the lesser of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price during the five trading
days immediately prior to the date of conversion. The maturity date of the debentures has been extended to June 30, 2021.
The
March 2020 Debentures also contain provisions providing for an adjustment in the event of stock splits or dividends, and fundamental
transactions. The investors will also have the right to participate in subsequent rights offerings and pro rata distributions. Additionally,
the March 2020 Debentures contain anti-dilution protection in the event of subsequent equity sales at a price that is lower than the
then applicable conversion price until such time that the March 2020 Debentures are no longer outstanding. Additionally, the Company
has the option to redeem some or all of the March 2020 Debentures for cash upon notice of twenty (20) trading days provided certain conditions
are met by the Company as more fully described in the debentures.
Furthermore,
without the approval of the debenture holders holding at least 67% of the then outstanding principal amount of the March Debentures,
the Company may not (i) amend its charter documents in any manner that adversely affects the rights of any investor, (ii) repay or repurchase
or acquire shares of its common stock, (iii) repay, repurchase, or acquire certain indebtedness, or (iv) pay cash dividends or distributions
on any equity securities of the Company.
We
recorded debt discount of $167,080 related to the fair value of the derivative liability associated with the debentures at the date of
issuance. This discount has been fully amortized to interest expense at December 31, 2020.
November
2019 Debentures
Sabby
Volatility Warrant Master Fund, Ltd. has paid certain of our accounts payable in the amount of $26,235. We issued $26,235 in new debentures
with substantially the same terms as those issued in our Debenture Offerings. The debentures were issued in November 2019. The debentures
originally matured November 20, 2020. The maturity date of the debentures has been extended to June 30, 2021.
October
2019 Debentures
Effective
September 30 2019, Sabby Healthcare Master Fund, Ltd and Sabby Volatility Warrant Master Fund, Ltd. waived certain events of default
under debentures and notes issued in our Debenture Offerings and extended the maturity date of such debentures until March 31, 2020 in
exchange for the issuance of $96,000 in new debentures with substantially the same terms as those issued in our Debenture Offerings.
The debentures were issued in October 2019. The debentures originally matured on October 1, 2020. The maturity date of the debentures
has been extended to June 30, 2021.
July
2019 Debentures
On
July 16, 2019, we entered into securities purchase agreements with certain institutional investors. Pursuant to the securities purchase
agreement, we issued an aggregate of $154,000 of senior convertible debentures (the “July 2019 Debentures”) in exchange for
the extension of the maturity date of our December 2018 convertible notes and certain of our July 2018 and September 2017 convertible
debentures, and the waiver of certain default provisions of our July 2018 and September 2017 convertible debentures. We charged $154,000
to finance cost at the date of issuance.
The
July 2019 Debentures (i) are non-interest bearing, (ii) have a maturity date one (1) year from the date of issuance and (iii) are convertible
into shares of our common stock at the election of the investor at any time, subject to a beneficial ownership limitation of 4.99% which
may be increased to 9.99% by the investor upon 61 days’ notice. The July 2019 Debentures have a conversion price equal to the lesser
of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price during the five trading days immediately prior to the date of
conversion. The July 2019 Debentures also contain provisions providing for an adjustment in the event of stock splits or dividends, and
fundamental transactions. The investors will also have the right to participate in subsequent rights offerings and pro rata distributions.
Additionally, the July 2019 Debentures contain anti-dilution protection in the event of subsequent equity sales at a price that is lower
than the then applicable conversion price until such time that the July 2019 Debentures are no longer outstanding. Additionally, the
Company has the option to redeem some or all of the July 2019 Debentures for cash upon notice of twenty (20) trading days provided certain
conditions are met by the Company as more fully described in the July 2019 Debentures. The maturity date of the debentures has been extended
to June 30, 2021.
Furthermore,
without the approval of the Investors holding at least 67% of the then outstanding principal amount of the July 2019 Debentures, the
Company may not (i) amend its charter documents in any manner that adversely affects the rights of any Investor, (ii) repay or repurchase
or acquire shares of its Common Stock, (iii) repay, repurchase, or acquire certain indebtedness, or (iv) pay cash dividends or distributions
on any equity securities of the Company. The Company is also obligated under the Securities Purchase Agreement to pay investors, as partial
liquidated damages, a fee of 2.0% of each Investor’s initial principal amount of such Investor’s July 2019 Debenture in cash
upon our failure to have current public information available beginning six (6) months after the issuance date of the Debentures.
December
2018 Debentures
On
December 13, 2018 we issued an aggregate of $25,000 in convertible promissory notes (“Notes”) for cash proceeds of $25,000.
The Notes will mature on the earlier of (i) June 30, 2019 or (ii) such time as we raise capital in exchange for the sale of securities
(“Maturity Date”) and bear interest at 10% per year, payable on the Maturity Date. Pursuant to the terms of the Notes, the
Notes may be converted into shares of common stock upon an Event of Default (as such term is defined in the Notes) or upon the Maturity
Date at the election of the holder at a price per share equal to 75% of the lowest trade price of our common stock on the trading day
immediately prior to the date such exchange is exercised by the holder. The maturity date of the debentures has been extended to June
30, 2021.
July
2018 Debentures
On
July 3, 2018, we entered into securities purchase agreements with certain institutional investors. Pursuant to the securities purchase
agreement, we sold an aggregate of $515,000 of senior convertible debentures (“July 2018 Debentures”) consisting of $500,000
in cash and the cancellation of $15,000 of obligations of the Company. Pursuant to the terms of the securities purchase agreement, we
issued $515,000 in principal amount of July 2018 Debentures. The July 2018 Debentures have substantially the same terms as the July 2019
Debentures. The maturity date of the debentures has been extended to June 30, 2021.
September
2017 Debentures
On
September 12, 2017 we entered into an exchange agreement (“Exchange Agreement”) with certain holders of our Series A 0%
Convertible Preferred Stock (“Series A Shares”) and Series B 0% Convertible Preferred Stock (“Series B
Shares”). Pursuant to the terms of the Exchange Agreement, we issued to the investors approximately $2.5
million in principal amount of senior convertible debentures (the “September 2017 Debentures”) in exchange for
1,614.8125 1,615 Series A Shares with a stated value of approximately $1.6 million
and 890
Series B Shares with a stated value of approximately $0.9
million.
On
September 12, 2017, we sold an aggregate of $320,000 of our September 2017 Debentures. The sale consisted of $250,000 in cash and the
cancellation of $70,000 of obligations of the Company.
The
September 2017 Debentures have substantially the same terms as the July 2019 Debentures. The maturity date of the debentures has been
extended to June 30, 2021.
As
a result of a buy-in failure to deliver certain shares pursuant to a debenture conversion, the Company incurred penalties of $24,551,
as provided for in the debenture; such amount reduced the gain on our conversion of debt during the year ended December 31, 2020.
NOTE
13 — LICENSE TERMINATION COST
As
described in Note 1, on October 5, 2020, we entered into an agreement with Ridgeway Therapeutics, Inc. whereby the parties terminated
the Licensing Agreement previously entered into on August 3, 2018.
In
exchange for entering into the Termination Agreement, the Company issued to Ridgeway 866,667 shares of common stock and 8,000 shares
of Series F 0% Convertible Preferred Stock Additionally, we have agreed to pay certain expenses and costs of Ridgeway’s aggregating
approximately $25,000.
We
valued the 866,667 shares of common stock at $266,500, based on the fair value of the stock on the date of the agreement, which has been
charged to license termination cost. Additionally, since the Series F preferred stock contains a variable conversion feature, we recorded
a derivative value associated with the preferred stock of $1,677,901, which has been charged to license termination cost.
NOTE
14 — INCOME TAXES
The
Company had, subject to limitation, $42 million of net operating loss carryforwards at December 31, 2020, of which $39.9 million will
expire at various dates through 2037. In addition, the Company has research and development tax credits of approximately $458,000 at
December 31, 2020 available to offset future taxable income, which will expire from 2028 through 2037. We have provided a 100% valuation
allowance for the deferred tax benefits resulting from the net operating loss carryover and our tax credits due to our lack of earnings
history. In addressing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion
or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation
of future taxable income during the periods in which those temporary differences are deductible. The valuation allowance increased by
approximately $226,000 and $241,000 for the years ended December 31, 2020 and 2019, respectively. Significant components of deferred
tax assets and liabilities are as follows (in thousands):
|
Schedule of deferred tax assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
Net operating loss carryover
|
|
$
|
9,064
|
|
|
$
|
8,838
|
|
|
Stock-based compensation
|
|
|
1,920
|
|
|
|
1,920
|
|
|
Accrued compensation
|
|
|
334
|
|
|
|
334
|
|
|
Other
|
|
|
30
|
|
|
|
30
|
|
|
Tax credits
|
|
|
458
|
|
|
|
458
|
|
|
Total deferred tax assets
|
|
|
11,806
|
|
|
|
11,580
|
|
|
Less: valuation allowance
|
|
|
(11,806
|
)
|
|
|
(11,580
|
)
|
|
Net deferred tax assets
|
|
$
|
—
|
|
|
$
|
—
|
|
The
actual tax benefit differs from the expected tax benefit for the years ended December 31, 2020 and 2019 (computed by applying the U.S.
Federal Corporate tax rate of 21% to income before taxes) are as follows:
|
Schedule of actual tax benefit differs from the expected tax benefit
|
|
|
|
|
|
|
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Statutory federal income tax rate
|
|
|
(21.0
|
)%
|
|
|
(21.0
|
)%
|
|
State income taxes, net of federal benefits
|
|
|
(7.0
|
)%
|
|
|
(7.0
|
)%
|
|
Non-deductible items
|
|
|
24.4
|
%
|
|
|
2.2
|
%
|
|
Valuation allowance
|
|
|
3.6
|
%
|
|
|
25.8
|
%
|
|
Effective income tax
rate
|
|
|
—
|
%
|
|
|
—
|
%
|
The
Company’s tax returns for the previous three years remain open for audit by the respective tax jurisdictions.
NOTE
15 – SUBSEQUENT EVENTS
On
January 12, 2021, we sold $500,000 of senior convertible debentures (“Debenture”) for (i) $500,000 for cash to an existing
institutional investor (the “Investor”) of the Company. The Debenture (i) is non-interest bearing, (ii) has a maturity date
of January 12, 2022, (iii) is convertible into shares of common stock (“Common Stock”) of the Company at the election of
the Investor at any time, subject to a beneficial ownership limitation of 9.99%, and (iv) has a conversion price equal to the lesser
of $24.75 and 85% of the lowest Volume Weighted Average Price (VWAP) during the five (5) Trading Days immediately prior to the conversion
date, subject to adjustment, as described therein.
The
Debenture also contains provisions providing for an adjustment in the event of stock splits or dividends, and fundamental transactions.
The Investor also has the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the Debentures
contains anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then applicable conversion
price until such time that the Debenture is no longer outstanding. Additionally, the Company has the option to redeem some or all of
the Debenture for cash upon notice of twenty (20) trading days provided certain conditions are met by the Company as more fully described
in the Debenture.
During
2021 we issued 4,248,864 shares of common stock upon conversion of $1,964,500 principal amount of our convertible debentures.
On
March 1, 2021, we entered into a settlement and release agreement with Claire Thom, one of our independent directors for the settlement
of past due director fees and the mutual release of all claims. Pursuant to the agreement, Dr. Thom agreed to waive $204,500 in outstanding
director fees in exchange for the following: (i) the payment of $40,000 (of which $20,000 was paid in November 2020 and $20,000 in February
2021) and (ii) immediately prior to the announcement that the Company has received approval from the FDA to commence its first Phase
1 clinical trial after March 1, 2021, a common stock purchase option with a Black Scholes’ value of $40,000, having an exercise
price equal to the closing price on the day preceding the announcement, and a term of 10 years. We also agreed to amend our non-employee
director compensation policy such that on April 1, 2021 each director will be entitled to receive a quarterly Board fee of $5,000 in
cash.
On
March 1, 2021, we entered into a settlement and release agreement with Scott Ogilvie, one of our independent directors for the settlement
of all past due director fees and the mutual release of all claims. Pursuant to the agreement, Mr. Ogilvie agreed to waive $231,167 in
outstanding director fees in exchange for the following: (i) the payment of $60,000 (of which $30,000 was paid in November 2020 and $30,000
in February 2021) and (ii) immediately prior to the announcement that the Company has received approval from the FDA to commence its
first Phase 1 clinical trial after March 1, 2021, a common stock purchase option with a Black Scholes’ value of $40,000, having
an exercise price equal to the closing price on the day preceding the announcement, and a term of 10 years. We also agreed to amend our
non-employee director compensation policy such that on April 1, 2021 each director will be entitled to receive a quarterly Board fee
of $5,000 in cash.
Reverse
Stock Split
On
October 5, 2021 the Company effectuated a reverse split of the Company’s issued and outstanding common stock at an exchange ratio
of 1-for-75, effective on that date. All share and per share data in the accompanying consolidated financial statements and footnotes
has been retroactively adjusted to reflect the effects of the reverse stock split.
REBUS
HOLDINGS, INC.
(FKA
INSPYR THERAPEUTICS, INC.)
CONDENSED
CONSOLIDATED BALANCE SHEETS
(in
thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
|
|
|
December 31,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
995
|
|
|
$
|
404
|
|
|
Total current assets
|
|
|
995
|
|
|
|
404
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
995
|
|
|
$
|
404
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,931
|
|
|
$
|
2,261
|
|
|
Accrued expenses
|
|
|
1,986
|
|
|
|
1,940
|
|
|
Convertible debentures, net of unamortized discount of
$458
and $488
|
|
|
440
|
|
|
|
1,878
|
|
|
Derivative liability
|
|
|
1,958
|
|
|
|
6,828
|
|
|
Total current liabilities
|
|
|
6,315
|
|
|
|
12,907
|
|
|
Total liabilities
|
|
|
6,315
|
|
|
|
12,907
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 7)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ deficit:
|
|
|
|
|
|
|
|
|
|
Convertible preferred stock, undesignated,
par value $.0001
per share; 29,978,846
shares authorized, no
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
A, par value $.0001
per share; 1,854
shares authorized, 134
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
B, par value $.0001
per share; 1,000
shares authorized, 71
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
C, par value $.0001
per share; 300
shares authorized, 290
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
D, par value $.0001
per share; 5,000
shares authorized, 5,000
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
E, par value $.0001
per share; 5,000
shares authorized, 5,000
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Convertible preferred stock Series
F, par value $.0001
per share; 8,000
shares authorized, 8,000
shares issued and outstanding, respectively
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, par value $.0001
per share; 1,000,000,000
shares authorized, 9,579,879
and 2,478,848
shares issued and outstanding, respectively
|
|
|
1
|
|
|
|
-
|
|
|
Additional paid-in capital
|
|
|
59,008
|
|
|
|
54,472
|
|
|
Accumulated deficit
|
|
|
(64,329
|
)
|
|
|
(66,975
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ deficit
|
|
|
(5,320
|
)
|
|
|
(12,503
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’
deficit
|
|
$
|
995
|
|
|
$
|
404
|
|
The accompanying notes
are an integral part of these unaudited condensed consolidated financial statements.
REBUS HOLDINGS, INC.
(FKA INSPYR THERAPEUTICS, INC.)
CONDENSED CONSOLIDATED STATEMENTS OF INCOME
(unaudited)
(in thousands, except share and per share
data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three
Months Ended
September 30,
|
|
|
Nine
Months Ended
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
97
|
|
|
$
|
11
|
|
|
$
|
190
|
|
|
$
|
33
|
|
|
General and administrative
|
|
|
118
|
|
|
|
126
|
|
|
|
371
|
|
|
|
344
|
|
|
Total operating expenses
|
|
|
215
|
|
|
|
137
|
|
|
|
561
|
|
|
|
377
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(215
|
)
|
|
|
(137
|
)
|
|
|
(561
|
)
|
|
|
(377
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on change in fair value of derivative liability
|
|
|
6,660
|
|
|
|
2,024
|
|
|
|
3,001
|
|
|
|
353
|
|
|
Gain on conversion of debt
|
|
|
(48
|
)
|
|
|
240
|
|
|
|
1,130
|
|
|
|
398
|
|
|
Interest expense, net
|
|
|
(272
|
)
|
|
|
(21
|
)
|
|
|
(924
|
)
|
|
|
(169
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before provision for income taxes
|
|
|
6,125
|
|
|
|
2,106
|
|
|
|
2,646
|
|
|
|
205
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
6,125
|
|
|
$
|
2,106
|
|
|
$
|
2,646
|
|
|
$
|
205
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per common share,
basic
|
|
$
|
0.81
|
|
|
$
|
11.84
|
|
|
$
|
0.39
|
|
|
$
|
2.25
|
|
|
Net income (loss) per common share,
diluted
|
|
$
|
(0.01
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.03
|
)
|
|
$
|
(0.05
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding, basic
|
|
|
7,612,241
|
|
|
|
181,774
|
|
|
|
6,740,889
|
|
|
|
94,764
|
|
|
Weighted average shares outstanding, diluted
|
|
|
38,536,773
|
|
|
|
7,230,566
|
|
|
|
20,928,472
|
|
|
|
7,143,556
|
|
The accompanying notes
are an integral part of these unaudited condensed consolidated financial statements.
REBUS HOLDINGS, INC.
(FKA INSPYR THERAPEUTICS, INC.)
CONDENSED CONSOLIDATED STATEMENTS
OF STOCKHOLDERS’ DEFICIT
FOR THE THREE AND NINE MONTHS
ENDED SEPTEMBER 30, 2021 AND 2020
(unaudited)
(in thousands, except share and
per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock
|
|
|
Common
Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
Stockholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Deficit
|
|
|
Balance, December
31, 2019
|
|
|
5,495
|
|
|
$
|
-
|
|
|
|
12,160
|
|
|
$
|
-
|
|
|
$
|
51,957
|
|
|
$
|
(60,680
|
)
|
|
$
|
(8,723
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
58,378
|
|
|
|
-
|
|
|
|
730
|
|
|
|
-
|
|
|
|
730
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(726
|
)
|
|
|
(726
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2020 (unaudited)
|
|
|
5,495
|
|
|
|
-
|
|
|
|
70,538
|
|
|
|
-
|
|
|
|
52,687
|
|
|
|
(61,406
|
)
|
|
|
(8,719
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale of preferred
stock
|
|
|
5,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5
|
|
|
|
-
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,175
|
)
|
|
|
(1,175
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2020 (unaudited)
|
|
|
10,495
|
|
|
|
-
|
|
|
|
70,538
|
|
|
|
-
|
|
|
|
52,692
|
|
|
|
(62,581
|
)
|
|
|
(9,889
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
473,186
|
|
|
|
-
|
|
|
|
570
|
|
|
|
-
|
|
|
|
570
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
income
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,106
|
|
|
|
2,106
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
September 30, 2020 (unaudited)
|
|
|
10,495
|
|
|
$
|
-
|
|
|
|
543,724
|
|
|
$
|
-
|
|
|
$
|
53,262
|
|
|
$
|
(60,475
|
)
|
|
$
|
(7,213
|
)
|
|
|
|
Convertible
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock
|
|
|
Common
Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
Stockholders’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Deficit
|
|
|
Balance, December
31, 2020
|
|
|
18,495
|
|
|
$
|
-
|
|
|
|
2,478,848
|
|
|
$
|
-
|
|
|
$
|
54,472
|
|
|
$
|
(66,975
|
)
|
|
$
|
(12,503
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
4,248,864
|
|
|
|
1
|
|
|
|
3,462
|
|
|
|
-
|
|
|
|
3,463
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Director
compensation waived
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
336
|
|
|
|
-
|
|
|
|
336
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(20,635
|
)
|
|
|
(20,635
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2021 (unaudited)
|
|
|
18,495
|
|
|
|
-
|
|
|
|
6,727,712
|
|
|
|
1
|
|
|
|
58,270
|
|
|
|
(87,610
|
)
|
|
|
(29,339
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
378,167
|
|
|
|
-
|
|
|
|
232
|
|
|
|
-
|
|
|
|
232
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
income
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
17,156
|
|
|
|
17,156
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2021 (unaudited)
|
|
|
18,495
|
|
|
|
-
|
|
|
|
7,105,879
|
|
|
|
1
|
|
|
|
58,502
|
|
|
|
(70,454
|
)
|
|
|
(11,951
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes
|
|
|
-
|
|
|
|
-
|
|
|
|
2,474,000
|
|
|
|
-
|
|
|
|
506
|
|
|
|
-
|
|
|
|
506
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
income
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
6,125
|
|
|
|
6,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
September 30, 2021 (unaudited)
|
|
|
18,495
|
|
|
$
|
-
|
|
|
|
9,579,879
|
|
|
$
|
1
|
|
|
$
|
59,008
|
|
|
$
|
(64,329
|
)
|
|
$
|
(5,320
|
)
|
The accompanying notes
are an integral part of these unaudited condensed consolidated financial statements.
REBUS HOLDINGS, INC.
(FKA INSPYR THERAPEUTICS, INC.)
CONDENSED CONSOLIDATED STATEMENTS OF CASH
FLOWS
(unaudited)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months
Ended
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
2,646
|
|
|
$
|
205
|
|
|
Adjustments to reconcile net income (loss) to net cash
used in operating activities:
|
|
|
|
|
|
|
|
|
|
Gain on change in fair value of derivative liability
|
|
|
(3,001
|
)
|
|
|
(353
|
)
|
|
Gain on conversion of debt
|
|
|
(1,130
|
)
|
|
|
(398
|
)
|
|
Amortization of debt discount
|
|
|
924
|
|
|
|
167
|
|
|
Increase in operating liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
|
|
152
|
|
|
|
134
|
|
|
Cash used in operating activities
|
|
|
(409
|
)
|
|
|
(245
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Cash used in investing activities
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
Proceeds from convertible notes
|
|
|
1,000
|
|
|
|
250
|
|
|
Proceeds from sale of preferred stock
|
|
|
-
|
|
|
|
5
|
|
|
Cash provided by financing activities
|
|
|
1,000
|
|
|
|
255
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and restricted cash
|
|
|
591
|
|
|
|
10
|
|
|
Cash and restricted cash, beginning
of period
|
|
|
404
|
|
|
|
23
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and restricted cash, end of
period
|
|
$
|
995
|
|
|
$
|
33
|
|
The accompanying notes
are an integral part of these unaudited condensed consolidated financial statements.
REBUS
HOLDINGS, INC.
(FKA
INSPYR THERAPEUTICS, INC.)
NOTES
TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER
30, 2021 AND 2020
NOTE
1 – BACKGROUND
Rebus
Holdings, Inc. (“we”, “us”, “our company”, “our”, “Rebus,” “Rebus Holdings,”
or the “Company”) (formerly known as Inspyr Therapeutics, Inc., see Note 10 Subsequent Events) was formed under the laws
of the State of Delaware in November 2003, and has its principal office in Westlake Village, California. We are focused on the research
and development of novel targeted precision therapeutics for the treatment of cancer.
Our
approach utilizes our proprietary delivery technology to better enhance immuno-modulation for improved therapeutic outcomes. Our potential
first-in-class immune-oncology lead asset, RT-AR001, an adenosine receptor A2B antagonist, is differentiated by its novel microparticle
formulation that allows for better tumor infiltration and enhanced outcomes when administered intra-tumorally. Our patented portfolio
of adenosine receptor antagonists provides flexibility to optimize treatment based on the specific targets found in each type of cancer.
The
adenosine receptor modulators include A 2B antagonists, dual A 2A /A 2B antagonists, and A 2A antagonists that have broad development
applicability including indications within immuno-oncology and inflammation. Adenosine is implicated in immunosuppression in the tumor
microenvironment. Adenosine receptor antagonists may boost the host immune response against the tumor as a single-agent and in combination
with other existing immuno-oncology agents leading to enhanced tumor killing and inhibition of metastasis. Adenosine also has anti-inflammatory
properties in the acute and chronic setting. Adenosine receptor antagonists may promote a decreased inflammatory response and can potentially
treat a broad range of inflammatory and autoimmune based diseases and conditions (e.g., rheumatoid arthritis, joint injury, Crohn’s
disease, psoriasis) as well as improve wound healing and decrease pain.
Pursuant
to our recent termination of license with Ridgeway Therapeutics, Inc., a Delaware Corporation (“Ridgeway”), we reacquired
the rights to certain intellectual property, discussed above, and are currently focusing on a pipeline of small molecule adenosine receptor
modulators. In
October 2020, pursuant to the cancellation of a license agreement whereby we previously licensed US Patent 9,593,118, we reacquired the
exclusive right to such patent that covers both A2B and dual A2A/A2B antagonists. Accordingly, going forward our major focus will be
to: (i) further characterization of the anti-cancer activity of our unique pipeline delivery platform containing A2B and dual A2A/A2B
antagonists, leading to selection of a clinical candidate or candidates for an Investigative New Drug or IND enabling studies; and (ii)
licensing and/or partnering our delivery platform and the A2B and dual A2A/A2B antagonists for further development.
Our
ability to execute the business plan is contingent upon our ability to raise the necessary funds. During March 2020, we sold approximately
$250,000
of debt securities and in October 2020,
we sold $500,000
of debt securities for cash. In January
2021, we sold an additional $500,000
of debt securities for cash and in June
2021, we sold an additional $500,000
of debt securities for cash. We are currently
using such funds to maintain our SEC reporting requirements, pay legal accounting and other professional fees, and to retain consultants
and other personnel to develop the adenosine A2R antagonists and in preparation for an IND filing related to our unique delivery platform
and portfolio of adenosine A2R antagonists for the treatment of certain solid tumors. Should we fail to further raise sufficient funds
to execute our business plan, our priority would be to maintain our intellectual property portfolio and seek business development opportunities
with potential development partners and/or acquirors.
Reverse
Stock Split
As
further described in Note 10, Subsequent Events, pursuant to a written consent, Rebus’ shareholders approved a proposal authorizing
the Board of Directors to effect a reverse stock split of the Company’s common stock (“Common Stock”).
On September 1, 2021, the Board of Directors
approved a one-for-seventy-five (1-for-75) reverse stock split of the Company’s Common Stock (“Reverse Stock Split”).
The Reverse Stock Split became effective with the Secretary of State of Delaware as of 4:59 p.m. Eastern Time on October 5, 2021, and
the Company began trading on a post Reverse Stock Split basis at the market open on October 12, 2021. As a result of the Reverse Stock
Split, each of the holders of the Company’s Common Stock received one (1) new share of Common Stock for every seventy-five
(75) shares such shareholder held immediately prior. No fractional shares were issued as a result of the Reverse Stock Split. Any
fractional shares that would have otherwise resulted from the Reverse Stock Split will be rounded up to the next whole number of shares.
The Reverse Stock Split also affected the Company’s outstanding stock options, warrants and other exercisable or convertible instruments
and resulted in the shares underlying such instruments being reduced and the exercise price being increased proportionately to the Reverse
Stock Split ratio. All share and per share data have been retroactively restated in the accompanying consolidated financial statements
and footnotes for all periods presented to reflect the effects of the Reverse Stock Split.
Termination
of License Agreement
On
October 5, 2020, the Company entered into an agreement with Ridgeway(“Termination Agreement”) whereby the parties terminated
the licensing agreement previously entered into on August 3, 2018 (“Licensing Agreement”), The Company had previously licensed
certain technologies related to targeting adenosine receptor antagonists for the treatment of cancer (the “Licensed Assets”).
As a result of the Termination Agreement, the Company reacquired full ownership and worldwide rights to all of the Licensed Assets as
well as any improvements made thereto.
In
exchange for entering into the Termination Agreement, the Company issued to Ridgeway: (i) 866,667 shares Common Stock, and
(ii) 8,000 shares of Series F 0% Convertible Preferred Stock (“Series F Preferred Stock”). Additionally, the Company paid
expenses and costs of Ridgeway’s aggregating approximately $25,000.
Pursuant
to a Certificate of Designation of the Series F Preferred Stock, each share of Series F Preferred Stock has a stated value of $10.00
per share and is convertible into Common Stock at any time at the election of the holder. In the aggregate, all of the Series F Preferred
Stock issued to Ridgeway is convertible into such number of shares of Common Stock equal to eighty percent (80%) of the issued and outstanding
shares of Common Stock, post-conversion, on the conversion date (taking into effect any forward or reverse stock splits or consolidations).
The Series F Preferred Stock votes on an as if converted to Common Stock basis. Additionally, upon the Company’s outstanding Convertible
Debentures (as such term is defined in the Certificate of Designation) being terminated, converted, or otherwise extinguished, the Series
F Preferred Stock will automatically convert into Common Stock.
Pursuant
to the Termination Agreement, in the event that the Company is unable to secure equity financing resulting in aggregate gross proceeds
to the Company of at least $5,000,000
by October 5, 2023, or in the event that
the Company ceases its operations, then the Termination Agreement will be deemed terminated and the Licensing Agreement will be reinstated
in exchange for the return of the Common Shares and Series F Preferred Stock issued to Ridgeway.
As
a result of the issuance of the Common Shares and Series F Preferred Stock, Ridgeway became the owner of approximately 54.14%
of the Company’s issued and outstanding Common Stock. Furthermore, by virtue of the issuance of the Series F Preferred Stock, Ridgeway
will vote on an as if converted to common stock basis which shall be equal to eighty percent (80%) of the issued and outstanding Common
Stock post-conversion. Accordingly, the Board of Directors determined that a change in control of the registrant has occurred. The Company
did not have a prior relationship with Ridgeway, or any of its principals, except pursuant to the terms contained in the Termination
Agreement and its previous relationship under the Licensing Agreement.
NOTE
2 – MANAGEMENT’S PLANS TO CONTINUE AS A GOING
CONCERN
Basis
of Presentation
We
have prepared our unaudited condensed consolidated financial statements on the basis that we will continue as a going concern, which
contemplates the realization of assets and satisfaction of liabilities in the normal course of business. We have incurred losses from
operations since inception, we have a working capital deficit of $5.3
million and we have an accumulated deficit
of $64.3
million as of September 30, 2021. We anticipate
incurring additional losses for the foreseeable future until such time, if ever, that we can generate significant sales from our therapeutic
product candidates which are currently in development, or we enter into cash flow positive business development transactions.
To
date, we have generated no sales or revenues, have incurred significant losses and expect to incur significant additional losses as we
advance our product candidates through development. Consequently, our operations are subject to all the risks inherent in the establishment
of a pre-revenue business enterprise as well as those risks associated with a company engaged in the research and development of pharmaceutical
compounds.
Our
cash balances at September 30, 2021 were approximately $995,000,
representing 100%
of our total assets. Based on our current expected level of operating expenditures, and including $500,000
that we raised in January 2021 and $500,000
that we raised in June 2021, pursuant
to the sale of our senior convertible debentures, we expect to be able to fund our operations into the second quarter of 2022. We will
require additional cash to fund and continue our operations beyond that point. This period could be shortened if there are any unanticipated
increases in planned spending on development programs or other unforeseen events. We anticipate raising additional funds through public
or private sales of debt or equity securities, or some combination thereof. There is no assurance that any such financing will be available
when needed in order to allow us to continue our operations, or if available, on terms favorable or acceptable to us.
In
the event additional financing is not obtained, we may pursue cost cutting measures as well as explore the sale of assets to generate
additional funds. If we are required to significantly reduce operating expenses and delay, reduce the scope of, or eliminate any of our
development programs or clinical trials, these events could have a material adverse effect on our business, results of operations, and
financial condition. These factors raise substantial doubt about our ability to continue as a going concern. The consolidated financial
statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and
classification of liabilities that might be necessary should we be unable to continue as a going concern.
Our
current cash level raises substantial doubt about our ability to continue as a going concern past the second quarter of 2022. If we do
not obtain additional funds by such time, we may no longer be able to continue as a going concern and will cease operation which means
that our shareholders will lose their entire investment.
NOTE
3 – SUMMARY OF CRITICAL ACCOUNTING POLICIES AND USE
OF ESTIMATES
Basis
of Presentation
The
accompanying condensed consolidated financial statements are unaudited. The unaudited interim consolidated financial statements have
been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and pursuant to the
rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and note disclosures normally
included in annual financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to those rules and regulations,
although the Company believes that the disclosures made are adequate to make the information not misleading.
These
interim consolidated financial statements for the three and nine months ended September 30, 2021 and 2020 are unaudited; however, in
the opinion of management, such statements include all adjustments (consisting of normal recurring accruals) necessary to present fairly
the financial position, results of operations and cash flows of the Company for the periods presented. The results for the three and
nine months ended September 30, 2021 are not necessarily indicative of the results to be expected for the year ending December 31, 2021
or for any future period. All references to September 30, 2021 and 2020 financials in these footnotes refer to unaudited consolidated
financial statements as of those dates.
These
unaudited condensed consolidated financial statements should be read in conjunction with our audited financial statements and the notes
thereto for the year ended December 31, 2020, included in the Company’s annual report on Form 10-K filed with the SEC on March
31, 2021.
The
consolidated balance sheet as of December 31, 2020 has been derived from the audited consolidated financial statements at such date but
do not include all disclosures required by the accounting principles generally accepted in the United States of America.
Principles
of Consolidation
The
consolidated financial statements include the accounts of the parent company, Rebus Holdings, Inc., (fka Inspyr Therapeutics, Inc.) and
its wholly-owned subsidiary, Lewis & Clark Pharmaceuticals, Inc. All significant intercompany accounts and transactions have been
eliminated.
Use
of Estimates
The
preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates
and assumptions that affect the amounts reported in the financial statements and accompanying disclosures. Significant estimates include
the fair value of derivative instruments, stock-based compensation, recognition of clinical trial costs and other accrued liabilities.
Actual results may differ from those estimates.
Research
and Development
Research
and development costs are charged to expense as incurred. Our research and development expenses consist primarily of expenditures for
pre-clinical research, toxicology and other studies, manufacturing, clinical trials, compensation and consulting costs associated therewith.
We
incurred research and development expenses of approximately $97,000
and $11,000
for the three months ended September 30,
2021 and 2020, respectively. We incurred research and development expenses of approximately $190,000
and $33,000
for the nine months ended September 30,
2021 and 2020, respectively.
Cash
Equivalents
For
purposes of the statements of cash flows, we consider all highly liquid debt instruments purchased with a maturity date of three months
or less to be cash equivalents. We maintain our cash in bank deposit accounts which, at times, may exceed applicable government mandate
insurance limits. We have not experienced any losses in our accounts. We did not
have any cash equivalents at September 30, 2021 or December 31, 2020.
Concentrations
of Credit Risk
Financial
instruments and related items, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and
cash equivalents. The Company places its cash and temporary cash investments with credit quality institutions. At times, such investments
may exceed applicable government mandated insurance limits. Cash was $1.0
million and $0.4
million at September 30, 2021 and December
31, 2020, respectively. As of September 30, 2021 and December 31, 2020, there was no cash over the federally insured limit.
Loss
per Share
Basic
loss per share is calculated by dividing net loss and net loss attributable to common shareholders by the weighted average number of
common shares outstanding for the period. Basic and diluted loss per share are the same, in that any potential common stock equivalents
would have the effect of being anti-dilutive in the computation of net loss per share.
The
following potentially dilutive securities have been excluded from the computations of diluted weighted average shares outstanding for
the three and nine months ended September 30, 2021 and 2020, as they would be anti-dilutive:
|
Schedule of Anti dilutive Securities Excluded from
Computation of Earnings Per Share
|
|
|
|
|
|
|
|
|
|
|
|
Three
and Nine Months
Ended
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Shares underlying options outstanding
|
|
|
9
|
|
|
|
9
|
|
|
Shares underlying warrants outstanding
|
|
|
53
|
|
|
|
53
|
|
|
Shares underlying convertible preferred stock outstanding
|
|
|
95,250
|
|
|
|
3,739
|
|
|
|
|
|
95,312
|
|
|
|
3,801
|
|
Diluted
loss per share for the three and nine months ended September 30, 2021 and 2020 is calculated as follows:
|
Diluted loss per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months
ended
|
|
|
Three months
ended
|
|
|
Nine months
ended
|
|
|
Nine months
ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
September 30,
|
|
|
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
2021
|
|
|
2020
|
|
|
Net income attributable to common shareholders
|
|
$
|
6,125
|
|
|
$
|
2,106
|
|
|
$
|
2,646
|
|
|
$
|
205
|
|
|
Income attributable to convertible instruments
|
|
|
(6,612
|
)
|
|
|
(2,264
|
)
|
|
|
(4,131
|
)
|
|
|
(751
|
)
|
|
Expense attributable to convertible instruments
|
|
|
272
|
|
|
|
21
|
|
|
|
924
|
|
|
|
169
|
|
|
Diluted loss attributable to common shareholders
|
|
$
|
(215
|
)
|
|
$
|
(137
|
)
|
|
$
|
(561
|
)
|
|
$
|
(377
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic shares outstanding
|
|
|
7,612,241
|
|
|
|
181,774
|
|
|
|
6,740,889
|
|
|
|
94,764
|
|
|
Dilutive convertible instruments
|
|
|
30,924,532
|
|
|
|
7,048,792
|
|
|
|
14,187,583
|
|
|
|
7,048,792
|
|
|
Diluted shares outstanding
|
|
|
38,536,773
|
|
|
|
7,230,566
|
|
|
|
20,928,472
|
|
|
|
7,143,556
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted loss per share
|
|
$
|
(0.01
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.03
|
)
|
|
$
|
(0.05
|
)
|
Derivative
Liability
The
Company has financial instruments that are considered derivatives or contain embedded features subject to derivative accounting. Embedded
derivatives are valued separately from the host instrument and are recognized as derivative liabilities in the Company’s balance
sheet. The Company measures these instruments at their estimated fair value and recognizes changes in their estimated fair value in results
of operations during the period of change. The Company values its derivative liabilities using the Black-Scholes option valuation model.
The resulting liability is valued at each reporting date and the change in the liability is reflected as change in derivative liability
in the statement of operations.
Fair
Value of Financial Instruments
Our
short-term financial instruments, including cash, accounts payable and other liabilities, consist primarily of instruments with maturities
of one year or less when acquired. We believe that the fair values of our current assets and current liabilities approximate their reported
carrying amounts.
The
derivative liabilities consist of our convertible notes and Series F preferred stock with variable conversion features. The Company uses
the Black-Scholes option-pricing model to value its derivative liabilities which incorporate the Company’s stock price, volatility,
U.S. risk-free interest rate, dividend rate, and estimated life.
Fair
Value Measurements
The
U.S. GAAP Valuation Hierarchy establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure fair value.
This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets
for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs
that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full
term of the financial instrument. Level 3 inputs are unobservable inputs based on our own assumptions used to measure assets and liabilities
at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input
that is significant to the fair value measurement.
The
Company has recorded a derivative liability for its convertible notes and preferred stock with variable conversion features as of September
30, 2021 and 2020. The tables below summarize the fair values of our financial liabilities as of September 30, 2021 and December 31,
2020 (in thousands):
|
Schedule of fair values of financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value at
September 30,
|
|
|
Fair Value Measurement Using
|
|
|
|
|
2021
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes
|
|
$
|
542
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
542
|
|
|
Preferred stock
|
|
|
1,416
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,416
|
|
|
Derivative liability
|
|
$
|
1,958
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,958
|
|
|
|
|
Fair Value at
December 31,
|
|
|
Fair Value Measurement Using
|
|
|
|
|
2020
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes
|
|
$
|
2,705
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
2,705
|
|
|
Preferred stock
|
|
|
4,123
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,123
|
|
|
Derivative liability
|
|
$
|
6,828
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
6,828
|
|
The
reconciliation of the derivative liability measured at fair value on a recurring basis using unobservable inputs (Level 3) is as follows
(in thousands):
|
Schedule of derivative liability measured at fair
value on a recurring basis using unobservable inputs (Level 3)
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months ended
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
|
|
Balance at beginning of period
|
|
$
|
6,828
|
|
|
$
|
1,785
|
|
|
Additions to derivative instruments
|
|
|
1,354
|
|
|
|
167
|
|
|
Reclassification on conversion
|
|
|
(3,223
|
)
|
|
|
(766
|
)
|
|
Gain on change in fair value of derivative liability
|
|
|
(3,001
|
)
|
|
|
(353
|
)
|
|
Balance at end of period
|
|
$
|
1,958
|
|
|
$
|
833
|
|
Recent
Accounting Pronouncements
With
the exception of those discussed below, there have not been any recent changes in accounting pronouncements and Accounting Standards
Update (ASU) issued by the Financial Accounting Standards Board (FASB) during the nine months ended September 30, 2021 that are of significance
or potential significance to the Company.
In
December 2019, the FASB issued ASU 2019-12, “Simplifying the Accounting for Income Taxes.” This guidance, among other provisions,
eliminates certain exceptions to existing guidance related to the approach for intraperiod tax allocation, the methodology for calculating
income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. This guidance also requires
an entity to reflect the effect of an enacted change in tax laws or rates in its effective income tax rate in the first interim period
that includes the enactment date of the new legislation, aligning the timing of recognition of the effects from enacted tax law changes
on the effective income tax rate with the effects on deferred income tax assets and liabilities. Under existing guidance, an entity recognizes
the effects of the enacted tax law change on the effective income tax rate in the period that includes the effective date of the tax
law. ASU 2019-12 is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The adoption
of this standard did not have a material impact on the Company’s consolidated financial statements.
NOTE
4 – SUPPLEMENTAL CASH FLOW INFORMATION
The
following table contains additional information for the periods reported (in thousands).
|
Schedule of additional information of cash flow
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30,
|
|
|
|
|
2021
|
|
|
2020
|
|
|
Non-cash financial activities:
|
|
|
|
|
|
|
|
|
|
Common stock issued on conversion of notes payable and derivative liability
|
|
$
|
4,202
|
|
|
$
|
1,300
|
|
|
Debentures converted to common stock
|
|
|
2,568
|
|
|
|
933
|
|
|
Derivative liability extinguished upon conversion of notes payable
|
|
|
3,223
|
|
|
|
766
|
|
|
Derivative liability issued
|
|
|
1,354
|
|
|
|
167
|
|
|
Accounts payable paid through issuance of debentures
|
|
|
100
|
|
|
|
—
|
|
|
Accrued directors fees forgiven and credited to paid in capital
|
|
|
336
|
|
|
|
—
|
|
There
was no cash paid for interest and income taxes for the nine months ended September 30, 2021 and 2020.
NOTE
5 – ACCRUED EXPENSES
Accrued
expenses consist of the following (in thousands):
|
Schedule of accrued expenses
|
|
|
|
|
|
|
|
|
|
|
|
September
30,
2021
|
|
|
December 31,
2020
|
|
|
Accrued compensation and benefits
|
|
$
|
1,326
|
|
|
$
|
1,326
|
|
|
Accrued research and development
|
|
|
233
|
|
|
|
233
|
|
|
Accrued other
|
|
|
427
|
|
|
|
381
|
|
|
Total accrued
expenses
|
|
$
|
1,986
|
|
|
$
|
1,940
|
|
NOTE
6 – DERIVATIVE LIABILITY
We
account for equity-linked financial instruments, such as our convertible preferred stock, convertible debentures and our common stock
warrants as either equity instruments or derivative liabilities depending on the specific terms of the respective agreement. Equity-linked
financial instruments are accounted for as derivative liabilities, in accordance with ASC Topic 815 – Derivatives and Hedging,
if the instrument allows for cash settlement or issuance of a variable number of shares. We classify derivative liabilities on the balance
sheet at fair value, and changes in fair value during the periods presented in the statement of operations, which is revalued at each
balance sheet date subsequent to the initial issuance of the stock warrant.
We
have issued convertible debentures and preferred stock which contain variable conversion features, anti-dilution protection and other
conversion price adjustment provisions. As a result, the Company assessed its outstanding equity-linked financial instruments and concluded
that the convertible notes and preferred stock are subject to derivative accounting. The fair value of the conversion feature is classified
as a liability in the consolidated financial statements, with the change in fair value during the periods presented recorded in the consolidated
statement of losses.
During
the three months ended September 30, 2021 and 2020, we recorded income of approximately $6.7
million and $2.0
million, respectively, related to the
change in fair value of the derivative liabilities during the periods. During the nine months ended September 30, 2021 and 2020, we recorded
income of approximately $3.0
million and $0.4
million, respectively. For purpose of
determining the fair market value of the derivative liability, the Company used Black Scholes option valuation model. The significant
assumptions used in the Black Scholes valuations of the derivatives at September 30, 2021 are as follows:
|
Schedule of valuations of derivatives
|
|
|
|
|
|
Volatility
|
|
|
121%
- 182
|
%
|
|
Expected
term (years)
|
|
|
3
- 9
months
|
|
|
Risk-free
interest rate
|
|
|
0.04%
– 0.05
|
%
|
|
Dividend
yield
|
|
|
None
|
|
As
of September 30, 2021 and December 31, 2020, the derivative liability recognized in the financial statements was approximately $2.0
million and $6.8
million, respectively.
NOTE
7 – COMMITMENTS AND CONTINGENCIES
Operating
Leases
the
Company currently does not have any ongoing leases for office space. It has availability to office space on an as needed basis. Its employees
work on a remote basis.
There
was no
rent expense for the three and nine months
ended September 30, 2021 and 2020.
Legal
Matters
The
Company is subject at times to legal proceedings and claims, which arise in the ordinary course of its business. Although occasional
adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material
adverse effect on its financial position, results of operations or liquidity.
NOTE
8 – CAPITAL STOCK AND STOCKHOLDERS’ EQUITY
Preferred
Stock
As
of September 30, 2021, there were outstanding (i) 133.8134
shares of Series A Preferred Stock, (ii)
71
shares of Series B Preferred Stock, (iii)
290.4
shares of Series C Preferred Stock, (iv)
5,000
shares of Series D Preferred Stock, (v)
5,000
shares of Series E Preferred Stock, and
(vi) 8,000
shares of Series F Preferred Stock.
Pursuant
to a Certificate of Designation filed with the Delaware Secretary of State on October 6, 2020, each share of Series F Preferred Stock
has a stated value of $10.00 per share and is convertible into Common Stock at any time at the election of the holder. We issued all
8,000
shares of the Series F stock to Ridgeway
Therapeutics, Inc. in connection with the Termination Agreement described in Note 1. In the aggregate, all of the Series F Preferred
Stock issued to Ridgeway is convertible into such number of shares of Common Stock equal to eighty percent (80%) of the issued and outstanding
shares of Common Stock, post-conversion, on the conversion date (taking into effect any forward or reverse stock splits or consolidations).
The Series F Preferred Stock votes on an as if converted to common stock basis. Additionally, upon the Company’s outstanding Convertible
Debentures (as such term is defined in the Certificate of Designation) being terminated, converted, or otherwise extinguished, the Series
F Preferred Stock will automatically convert into Common Stock.
As
a result of past equity financings and conversions of debentures, the conversion prices of (i) our Series A Preferred Stock has been
reduced to $29,812.50 per share at September 30, 2021, (ii) our Series B Preferred Stock has been reduced to $0.75 per share at September
30, 2021, (iii) 200 shares of our Series C preferred stock has been reduced to $1,125.00 per share at September 30, 2021, (iv) 90.43418
shares of our Series C Preferred Stock has been reduced to $562.50 per share at September 30, 2021.
Common
Stock
During
the nine months ended September 30, 2021, we issued a total of 7,101,031
shares of common stock, valued at $4,201,812,
upon the conversion of $2,568,048
principal amount of our convertible debentures.
We recorded loss on conversion of debt of $47,872
and gain on conversion of debt of $1,129,632
during the three and nine months ended
September 30, 2021, respectively.
During
the three months ended March 31, 2021, we entered into settlement and release agreements with two of our independent directors for the
settlement of past due director fees and the mutual release of all claims. Pursuant to the agreements, the directors agreed to waive
an aggregate of $435,667
in outstanding director fees in exchange
for the following: (i) the
aggregate payment of $100,000 (of which $50,000 was paid in November 2020 and $50,000 in February 2021) and (ii) immediately prior to
the announcement that the Company has received approval from the FDA to commence its first Phase 1 clinical trial after March 1, 2021
(which has yet to occur), common stock purchase options with an aggregate Black Scholes’ value of $80,000, having an exercise price
equal to the closing price on the day preceding the announcement, and a term of 10 years. The difference between the amount waived of
$435,667 and the cash paid of $100,000 has been credited to paid in capital during the three months ended March 31, 2021.
During
the nine months ended September 30, 2020, we issued a total of 531,564
shares of common stock, valued at $1,300,653,
upon the conversion of $932,935
principal amount of our convertible debentures.
We recorded gain on conversion of debt of $240,356
and $398,323
during the three and nine months ended
September 30, 2020, respectively.
NOTE
9 – CONVERTIBLE DEBENTURES AND NOTES
June
2021 Debenture
On
June 18, 2021, the Company sold an aggregate of $600,000
of senior convertible debentures (“June
Debentures”) for (i) $500,000
in cash and (ii) $100,000
in cancellation of outstanding indebtedness
to existing accredited and institutional investors of the Company. The June Debentures (i) are non-interest bearing, (ii) have a maturity
date of June
18, 2022, (iii) are convertible
into shares of Common Stock at the election of the holders at any time, subject to a beneficial ownership limitation of 9.99%,
and (iv) have a conversion
price equal to the lesser of $24.75 and 85% of the lowest Volume Weighted Average Price (VWAP) during the five (5) trading days immediately
prior to the conversion date, subject to adjustment, as described therein.
The
June Debentures also contain provisions providing for an adjustment in the event of stock splits or dividends, and fundamental transactions.
The investors also have the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the June Debentures
contains anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then applicable conversion
price until such time that the June Debentures are no longer outstanding. Additionally, the Company has the option to redeem some or
all of the June Debentures for cash upon notice of twenty (20) trading days provided certain conditions are met by the Company as more
fully described in the June Debentures.
We
recorded an initial derivative liability of $644,457
related to the fair value of the derivative
liability associated with the June Debentures. We recorded debt discount of $600,000,
which will be amortized to interest expense over the term of the June Debentures, and we charged $44,457
to interest expense upon issue. We have
amortized $155,000
and $175,000
of discount to interest expense during
the three and nine months ended September 30, 2021, respectively. Unamortized discount at September 30, 2021 was $425,000.
January
2021 Debenture
On
January 12, 2021, we sold a $500,000
senior convertible debenture (“January
Debenture”) for (i) $500,000
for cash to an existing institutional
investor of the Company. The January Debenture (i) is non-interest bearing, (ii) has a maturity date of January
12, 2022, (iii) is convertible
into shares of Common Stock at the election of the holder at any time, subject to a beneficial ownership limitation of 9.99%,
and (iv) has a conversion
price equal to the lesser of $24.75 and 85% of the lowest VWAP during the five (5) trading days immediately prior to the conversion date,
subject to adjustment, as described therein.
The
January Debenture also contains provisions providing for an adjustment in the event of stock splits or dividends, and fundamental transactions.
The investor also has the right to participate in subsequent rights offerings and pro rata distributions. Additionally, the January Debentures
contains anti-dilution protection in the event of subsequent equity sales at a price that is lower than the then applicable conversion
price until such time that the January Debenture is no longer outstanding. Additionally, the Company has the option to redeem some or
all of the January Debenture for cash upon notice of twenty (20) trading days provided certain conditions are met by the Company as more
fully described in the January Debenture.
During
the nine months ended September 30, 2021, $412,480
of January Debenture has been converted
to Common Stock and $87,520
remained outstanding at September 30,
2021.
We
recorded an initial derivative liability of $709,835
related to the fair value of the derivative
liability associated with the January debenture. We recorded debt discount of $500,000,
which will be amortized to interest expense over the term of the January debenture, and we charged $209,835
to interest expense upon issue. We have
amortized $94,404
and $326,253
of discount to interest expense during
the three and nine months ended September 30, 2021, respectively, and $148,842
has been charged off against gain upon
the conversion of the October Debentures during the three and nine months ended September 30, 2021. Unamortized discount at September
30, 2021 was $24,905.
October
2020 Debentures
On
October 23, 2020, the Company sold an aggregate of $600,000
of senior convertible debentures (“October
Debentures”) for (i) $500,000
in cash and (ii) $100,000
in cancellation of outstanding indebtedness
to existing accredited and institutional investors of the Company.
The
October Debentures (i) are non-interest bearing, (ii) have a maturity date of October
23, 2021, (iii) are convertible
into shares of Common Stock at the election of the holders at any time, subject to a beneficial ownership limitation of 9.99%,
and (iv) have a conversion
price equal to the lesser of (i) $24.75 and (ii) 85% of the lowest VWAP during the five trading days immediately prior to the date of
conversion.
During
the nine months ended September 30, 2021, $500,000
of October Debentures have been converted
to common stock and $100,000
remains outstanding at September 30, 2021.
We
had recorded debt discount of $600,000
related to the October Debentures, which
will be amortized to interest expense over the term of the October Debentures. We have amortized $23,551
and $168,539
of discount to interest expense during
the three and nine months ended September 30, 2021, and $0
and $311,111
has been charged off against gain upon
the conversion of the October Debentures during the three and nine months ended September 30, 2021, respectively. Unamortized discount
at September 30, 2021 was $7,850.
March
2020 Debentures
On
March 6, 2020, the Company sold an aggregate of $250,000
of senior convertible debentures (the
“March Debentures”) for cash to existing accredited institutional investors of the Company. The March Debentures (i) are
non-interest bearing, (ii) have a maturity date of July
16, 2020 and (iii) are
convertible into shares of Common Stock at the election of the holder at any time, subject to a beneficial ownership limitation of 9.99%.
The March Debentures
have a conversion price equal to the lesser of (i) $24.75 and (ii) 85% of the lowest VWAP during the five trading days immediately prior
to the date of conversion. The maturity date of the debentures was extended to June 30, 2021.
The
March Debentures were fully converted to Common Stock during the three months ended March 31, 2021.
November
2019 Debentures
An
existing institutional investor has paid certain of our accounts payable in the amount of $26,235.
We issued $26,235
in new debentures with substantially the
same terms as our October Debenture. The debentures were issued in November 2019. The debentures originally matured November 20, 2020.
The maturity date of the debentures was extended to June 30, 2021.
The
debentures were fully converted to Common Stock during the three months ended March 31, 2021.
October
2019 Debentures
Effective
September 30 2019, debenture holders waived certain events of default under debentures and notes issued in our debenture offerings and
extended the maturity date of such debentures until March 31, 2020 in exchange for the issuance of $96,000
in new debentures with substantially the
same terms as our October Debentures. The debentures were issued in October 2019. The debentures originally matured on October 1, 2020.
The maturity date of the debentures was extended to June 30, 2021.
The
debentures were fully converted to Common Stock during the three months ended March 31, 2021.
July
2019 Debentures
On
July 16, 2019, we entered into securities purchase agreements with certain institutional investors. Pursuant to the securities purchase
agreement, we issued an aggregate of $154,000
of senior convertible debentures (the
“July 2019 Debentures”) in exchange for the extension of the maturity date of our December 2018 convertible notes and certain
of our July 2018 and September 2017 convertible debentures, and the waiver of certain default provisions of our July 2018 and September
2017 convertible debentures. The maturity date of the debentures was extended to June 30, 2021.
The
July 2019 Debentures were fully converted to Common Stock during the three months ended March 31, 2021.
December
2018 Notes
On
December 13, 2018, we issued an aggregate of $25,000
in convertible promissory notes for cash
proceeds of $25,000.
The notes mature on the earlier of (i) June 30, 2019 or (ii) such time as we raise capital in exchange for the sale of securities and
bear interest at 10% per year, payable on the maturity date. Pursuant to the terms of the notes, they may be converted into shares of
Common Stock upon an event of default (as such term is defined in the notes) or upon the maturity date at the election of the holder
at a price per share equal to 75% of the lowest trade price of our Common Stock on the trading day immediately prior to the date such
exchange is exercised by the holder. The maturity date of the notes was extended to June 30, 2021.
The
notes were fully converted to Common Stock during the three months ended March 31, 2021.
July
2018 Debentures
On
July 3, 2018, we entered into securities purchase agreements with certain institutional investors. Pursuant to the securities purchase
agreement, we sold an aggregate of $515,000
of senior convertible debentures (“July
2018 Debentures”) consisting of $500,000
in cash and the cancellation of $15,000
of obligations of the Company. Pursuant
to the terms of the securities purchase agreement, we issued $515,000
in principal amount of July 2018 Debentures.
The July 2018 Debentures have substantially the same terms as the October Debentures. The maturity date of the debentures was extended
to June 30, 2021.
The
July 2018 Debentures were fully converted to common stock during the three months ended March 31, 2021.
September
2017 Debentures
On
September 12, 2017, we entered into an exchange agreement with certain holders of our Series A 0% Convertible Preferred Stock (“Series
A Shares”) and Series B 0% Convertible Preferred Stock (“Series B Shares”). Pursuant to the terms of the agreement,
we issued to the investors approximately $2.5
million in principal amount of senior
convertible debentures (the “September 2017 Debentures”) in exchange for 1,614.8125 Series A Shares with a stated value of
approximately $1.6
million and 890
Series B Shares with a stated value of
approximately $0.9
million. On September 12, 2017, we sold
an aggregate of $320,000
of our September 2017 Debentures. The
sale consisted of $250,000
in cash and the cancellation of $70,000
of obligations of the Company. The maturity
date of the debentures was extended to December 31, 2021.
During
the nine months ended September 30, 2021, $589,334
of debenture have been converted to common
stock and $110,072
remains outstanding at September 30, 2021.
NOTE
10 – SUBSEQUENT EVENTS
Adoption
of Agreement and Plan of Merger and Consummation of Reorganization
On
September 28, 2021 the Company implemented a holding company reorganization through an Agreement and Plan of Merger (the “Merger
Agreement”), which became effective with the Financial Industry Regulatory Authority (“FINRA”) on October 11, 2021
at 5:00 p.m. Eastern Time (the “Effective Time”). The Merger Agreement was entered into by and among Inspyr Therapeutics,
Inc., Rebus Holdings, Inc., and Rebus Sub, Inc., a wholly-owned subsidiary of Rebus Holdings which has resulted in Rebus becoming the
direct parent company of Inspyr Therapeutics and replacing Inspyr Therapeutics as the public company trading on the OTC Markets (“OTC”)
(the “Reorganization”). Further, the Company will begin trading under the symbol RBSH in twenty (20) trading days from October
12, 2021.
Pursuant
to the Merger Agreement, Rebus Sub has merged with Inspyr Therapeutics pursuant to the filing of a certificate of merger with Inspyr
Therapeutics surviving as a direct, wholly-owned subsidiary of Rebus Holdings (the “Merger”). At the Effective Time of the
Merger:
|
|
(i)
|
Each
outstanding share of Inspyr Therapeutics Common Stock, par value $0.0001 per share (“Inspyr Common Stock”), automatically
converted into one share of Common Stock of Rebus Holdings, having the same designation, rights, powers, and preferences, and qualifications,
limitations, and restrictions as a share of Inspyr Therapeutics Common Stock immediately prior to the Reorganization;
|
|
|
(ii)
|
Each
outstanding share of Inspyr Series A Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series A Stock”),
automatically converted into one share of Series A Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series A Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series A Stock immediately prior to the Reorganization;
|
|
|
(iii)
|
Each
outstanding share of Inspyr Series B Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series B Stock”),
automatically converted into one share of Series B Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series B Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series B Stock immediately prior to the Reorganization;
|
|
|
(iv)
|
Each
outstanding share of Inspyr Series C Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series C Stock”),
automatically converted into one share of Series C Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series C Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series C Stock immediately prior to the Reorganization;
|
|
|
(v)
|
Each
outstanding share of Inspyr Series D Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series D Stock”),
automatically converted into one share of Series D Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series D Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series D Stock immediately prior to the Reorganization;
|
|
|
(vi)
|
Each
outstanding share of Inspyr Series E Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series E Stock”),
automatically converted into one share of Series E Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series E Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series E Stock immediately prior to the Reorganization; and
|
|
|
(vii)
|
Each
outstanding share of Inspyr Series F Convertible Preferred Stock, par value $0.0001 per share (“Inspyr Series F Stock”),
automatically converted into one share of Series F Convertible Preferred Stock par value $0.0001 per share, of Rebus Holdings (“Rebus
Series F Stock”), having the same designation, rights, powers, and preferences, and qualifications, limitations, and restrictions
as a share of Inspyr Series F Stock immediately prior to the Reorganization.
|
Accordingly,
upon consummation of the Reorganization (and the Reverse Stock Split as defined below), Inspyr stockholders automatically became stockholders
of Rebus Holdings, on a one-for-one basis, with the same number and approximate ownership percentage of shares of the same
class as they held in Inspyr immediately prior to the Effective Time. The Reorganization is intended to be a tax-free transaction
for U.S. federal income tax purposes for Inspyr stockholders.
The
Reorganization has been conducted pursuant to Section 251(g) of the General Corporation Law of the State of Delaware, which provides
for the formation of a holding company without a vote of the stockholders of the constituent corporation. The conversion of stock occurred
automatically without an exchange of stock certificates. In addition, at the Effective Time:
|
|
●
|
Each
outstanding and unexpired option to purchase Inspyr Common Stock automatically converted into one share of Rebus Holdings Common
Stock;
|
|
|
●
|
Each
outstanding warrant to purchase Inspyr Common Stock (“Inspyr Warrant”), whether or not vested, automatically converted
into and become a warrant to purchase Rebus Holdings Common Stock (“Rebus Warrant”) and Rebus Holdings assumed each Inspyr
Warrant in accordance with the terms of each Inspyr Warrant, and such Rebus Warrant has the same number of shares, the same exercise
price (subject to adjustments), the same restrictions on exercise, and any other provisions contained in the Inspyr Warrants; and
|
|
|
●
|
Each
outstanding convertible debt instrument of Inspyr Therapeutics, including but not limited to, promissory notes or debentures that
are convertible into Inspyr Common Stock (“Inspyr Convertible Notes”) automatically converted into, assumed, and became
the convertible debt instruments of Rebus Holdings (“Rebus Convertible Notes”).
|
As
a result of the Reorganization, Rebus Holdings became the successor issuer to Inspyr Therapeutics pursuant to Rule 12g-3(a) of
the Exchange Act, and as a result, shares of Rebus Holdings Common Stock are deemed registered under Section 12(g) of the Exchange
Act as the Common Stock of the successor issuer.
Reverse
Stock Split
The
1-for-75
Reverse Stock Split became
effective with the Secretary of State of Delaware as of 4:59 p.m. Eastern Time on October 5, 2021, and the Company began trading on a
post Reverse Stock Split basis at the market open on October 12, 2021. As a result of the Reverse Stock Split, each of the holders of
the Company’s Common Stock received one (1) new share of Common Stock for every seventy-five (75) shares such shareholder
held immediately prior. The Reverse Stock Split also affected the Company’s outstanding stock options, warrants and other exercisable
or convertible instruments and resulted in the shares underlying such instruments being reduced and the exercise price being increased
proportionately to the Reverse Stock Split ratio. All share and per share data has been retroactively restated in the accompanying consolidated
financial statements and footnotes for all periods presented to reflect the effects of the Reverse Stock Split.
As
a result, effective on October 12, 2021 (and just prior to the completion of the Reorganization), each of the holders of Inspyr Common
Stock received one (1) new share of Inspyr Common Stock for every seventy-five (75) shares such shareholder held immediately
prior to the Reverse Split Effective Time. The Reverse Stock Split also affected the Company’s outstanding stock options, warrants
and other exercisable or convertible instruments and resulted in the shares underlying such instruments being reduced and the exercise
price being increased proportionately to the Reverse Stock Split ratio. No fractional shares were issued as a result of the Reverse Stock
Split. Any fractional shares that would have otherwise resulted from the Reverse Stock Split will be rounded up to the next whole number
of shares.
As
a result of the Reverse Stock Split, the number of issued and outstanding shares of Common Stock was adjusted from 772,902,289 shares
to 10,309,212
shares. Additionally, pursuant to the
terms of their Certificates of Designation, each Series of Inspyr preferred stock had the conversion price at which shares of such applicable
preferred stock may be converted into shares of Inspyr Common Stock proportionately adjusted to reflect the Reverse Stock Split. Additionally,
all outstanding options, warrants and convertible debt of Inspyr were adjusted proportionately pursuant to the Reverse Stock Split.
As
a result of the Reorganization, each shareholder received such number of shares of Rebus Holdings Common Stock and Rebus Holdings Preferred
Stock as the shareholder would have held of Inspyr immediately following the Reverse Stock Split.
Post
Reverse Stock Split and Reorganization Information
The
Company began trading on post Reverse Stock Split and Reorganization basis on the Pink Sheets of the OTC Markets Group on October 12,
2021 under the same symbol NSPX. In twenty (20) trading days from October 12, 2021, the Company will begin trading under the symbol RBSH
.
The
officers and members of the Board of Inspyr became the officers and members of the board of directors of the Rebus Holdings.
Pursuant
to the Reorganization, Rebus Holdings has, on a consolidated basis, the same assets, businesses, and operations as Inspyr Therapeutics
had immediately prior to the Reorganization.
Issuance
of Common Stock upon Conversion of Debentures
The
Company issued 729,333
shares of Common Stock pursuant to the
conversion of $87,520
of our outstanding debentures.
REBUS
HOLDINGS, INC.
5,806,864
Shares
of Common Stock
Prospectus
,
2021
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item 13. Other
Expenses of Issuance and Distribution.
The
following table sets forth the estimated costs and expenses in connection with the sale and distribution of the securities being registered,
other than placement agent fees. All expenses incurred will be paid by the Company. All of the amounts shown are estimates other than
the Securities and Exchange Commission, or SEC, registration fees.
|
|
|
To
be
Paid by the
Registrant
|
|
|
SEC
registration fees
|
|
$
|
33.51
|
|
|
Legal
fees and expenses
|
|
$
|
60,000
|
|
|
Accounting
fees and expenses
|
|
$
|
10,000
|
|
|
Printing
and engraving expenses
|
|
$
|
1,000
|
|
|
Transfer
agent’s fees
|
|
$
|
500
|
|
|
Miscellaneous
fees and expenses
|
|
$
|
1,000
|
|
|
Total
|
|
$
|
72,533.51
|
|
Item 14. Indemnification
of Directors and Officers.
Section 102
of the Delaware General Corporation Law, as amended, or DGCL, allows a corporation to eliminate the personal liability of directors to
a corporation or its stockholders for monetary damages for a breach of a fiduciary duty as a director, except where the director (i)
breached his duty of loyalty, (ii) failed to act in good faith, (iii) engaged in intentional misconduct or knowingly violated a law,
(iv) authorized the payment of a dividend or approved a stock repurchase or redemption in violation of Delaware corporate law or (v)
obtained an improper personal benefit.
Our
certificate of incorporation states that, to the fullest extent permitted by the DGCL, no director shall be personally liable to us or
our stockholders for monetary damages for breach of fiduciary duty as director; provided, however, that this provision eliminating personal
liability of a director shall not eliminate or limit the liability of a director (i) for any breach of the director’s duty of loyalty
to us or our stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation
of law, (iii) under section 174 of the DGCL, or (iv) for any transaction from which the director derived an improper personal benefit.
Section 145
of the DGCL provides, among other things, that a corporation may indemnify any person who was or is a party or is threatened to be made
a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of the corporation)
by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the
corporation’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other
enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably
incurred by the person in connection with the action, suit or proceeding. The power to indemnify applies if (i) such person is successful
on the merits or otherwise in defense of any action, suit or proceeding or (ii) such person acted in good faith and in a manner
he or she reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action
or proceeding, had no reasonable cause to believe his conduct was unlawful. The power to indemnify applies to actions brought by or in
the right of the corporation as well, but only to the extent of defense expenses (including attorneys’ fees) actually and reasonably
incurred and not to any satisfaction of judgment or settlement of the claim itself, and with the further limitation that in such actions
no indemnification shall be made in the event of any adjudication of negligence or misconduct in the performance of his duties to the
corporation, unless a court believes that in light of all the circumstances indemnification should apply.
Section 174
of the DGCL provides, among other things, that a director who willfully and negligently approves of an unlawful payment of dividends
or an unlawful stock purchase or redemption may be held liable for such actions. A director who was either absent when the unlawful actions
were approved or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books
containing the minutes of the meetings of the board of directors at the time the action occurred or immediately after the absent director
receives notice of the unlawful acts.
Our
bylaws provide that we shall, to the fullest extent authorized by the DGCL, indemnify any person who was or is made a party or threatened
to be made a party to or is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by
reason of the fact that he or she, or a person of whom he or she is the legal representative, is or was our director or officer or is
or was serving at our request as a director or officer of another corporation, or as a controlling person of a partnership, joint venture,
trust or other enterprise, including service with respect to employee benefit plans, whether the basis of such proceeding is alleged
action in an official capacity as a director or officer, or in any other capacity while serving as a director or officer, against all
expenses, liability or loss reasonably incurred or suffered by such person in connection with such action, suit or proceeding.
Our
bylaws also provide that we may enter into one or more agreements with any director, officer, employee or agent of ours, or any person
serving at our request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise,
including employee benefit plans, that provides for indemnification rights equivalent to or, if our board of directors so determines,
greater than, those provided for in such bylaws. As a general policy, we enter into indemnification agreements with our officers and
directors.
Item 15.
Recent Sales of Unregistered Securities.
The
following information is given with regard to unregistered securities sold during the preceding three years including the dates and amounts
of securities sold, the persons or class of persons to whom we sold the securities, the consideration received in connection with such
sales and, if the securities were issued or sold other than for cash, the description of the transaction and the type and amount of consideration
received. The descriptions contained below are a summary and qualified by the agreements, if applicable, included as Exhibits to this
Registration Statement. The following securities were issued in private offerings pursuant to the exemption from registration contained
in Section 4(2) of the Securities Act of 1933, as amended, or the Securities Act and the rules promulgated thereunder. All share amounts
and prices reflect the 1-for-75 reverse stock split that was effective October 12, 2021 and include adjustment for any prior reverse
stock splits that may have occurred subsequent the issuances described hereunder.
|
|
●
|
During
January 2019, we issued 5,000 shares of Series D Convertible Preferred Stock for proceeds of $5,000. The Series D Convertible Preferred
Stock is convertible into common stock at a conversion price of $281.25 per share.
|
|
|
●
|
Between
January 1, 2019 and March 31, 2019, Debenture holders converted an aggregate of $204,221 into approximately 1,163 shares of common
stock at per share conversion prices ranging from $118.125 to $258.75.
|
|
|
●
|
In
July 2019, we issued an aggregate of $154,000 in senior convertible debentures (“July
2019 Debentures”) to certain accredited investors in exchange for the extending the
maturity dates of all of our existing debentures until September 30, 2019, and waiving certain
potential defaults related to registration rights related to shares underlying such debentures.
The July 2019 Debentures, as amended had materially the same terms as the March 2020 Debentures
described below. As of the date hereof, they have been fully converted into common stock.
|
|
|
●
|
On
October 1, 2019, we issued an aggregate of $96,000 in senior convertible debentures (“October
2019 Debentures”) to certain accredited investors in exchange for the extending the
maturity dates of all of our existing debentures until March 31, 2019. The October 2019 Debentures,
as amended had materially the same terms as the March 2020 Debentures described below. As
of the date hereof, they have been fully converted into common stock.
|
|
|
●
|
In
November 2019, in exchange for the payment of a payable, we issued an aggregate of approximately
$26,000 in senior convertible debentures to an accredited investor. The terms of these debentures,
as amended, are materially the same as the March 2020 Debentures described below. As of the
date hereof, they have been fully converted into common stock.
|
|
|
●
|
Between
October 1, 2019 and December 31, 2019, debenture holders converted an aggregate of $127,193 into approximately 5,622 shares of common
stock at per share conversion prices ranging from $15.525 to $118.35.
|
|
|
●
|
Between
January 1, 2020 and March 31, 2020, debenture holders converted an aggregate of $451,662 into approximately 58,379 shares of common
stock at per share conversion prices ranging from $0.1575 to $35.25.
|
|
|
●
|
On
March 6, 2020, we issued an aggregate of $250,000 in senior convertible debentures (“March 2020 Debentures”) to certain
accredited investors for cash. At issuance, the conversion price of the March 2020 Debentures was equal to the lesser of (i)
$24.75 and (ii) 85% of the lesser of (a) the volume weighted average price on the trading day immediately preceding a conversion
date and (b) the volume weighted average price on a conversion date. Pursuant to an amendment to the March 2020 Debentures and all
other outstanding convertible debentures owned by certain shareholders, the conversion price was amended such that the conversion
price is equal to the lesser of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price during the five trading days
immediately prior to the date of conversion. As of the date of this Registration Statement, the March 2020 Debentures have been fully
converted into common stock.
|
|
|
●
|
On
May 2, 2020, we sold 5,000 shares of Series E 0% Convertible Preferred Stock to an accredited investor at a price per share of $1.00
for aggregate gross proceeds of $5,000. The Series E Preferred Stock are convertible into shares of Common Stock at a conversion
price of $22.50 per share.
|
|
|
●
|
Between
July 1, 2020 and August 12, 2020, debenture holders converted an aggregate of $80,906 into approximately 21,138 shares of common
stock at per share conversion prices ranging from $2.3625 to $6.45.
|
|
|
●
|
On
October 5, 2020, we entered into an agreement with Ridgeway Therapeutics (“Ridgeway”) to terminate an outstanding license
agreement previously entered into with Ridgeway (“Termination Agreement”). Pursuant to the Termination Agreement, as
consideration, we issued Ridgeway (i) 866,667 shares of our Common Stock and (ii) 8,000 shares of Series F 0% Convertible Preferred
Stock. The 8,000 shares of Series F Preferred Stock are convertible into an aggregate of eighty percent (80%) of the issued and outstanding
Common Stock post-conversion on the conversion date. The Series F Preferred Stock votes on an as converted to Common Stock basis.
Additionally, upon the termination, conversion, or otherwise extinguishment of certain or our outstanding convertible debentures,
the Series F Preferred Stock will automatically convert into Common Stock.
|
|
|
●
|
On
October 23, 2020, we issued an aggregate of $600,000 in senior convertible debentures (“October 2020 Debentures”) to
certain accredited investors for $500,000 cash and the cancellation of $100,000 in outstanding obligations. The October 2020
Debentures have the same terms as the March 2020 Debentures, as amended, and mature on October 23, 2021. As of the date hereof, they
have been fully converted into common stock.
|
|
|
●
|
Between
August 17, 2020 and October 9, 2020, debenture holders converted an aggregate of $525,290 into approximately 1,045,504 shares of
common stock at per share conversion prices ranging from $0.195 to $1.425.
|
|
|
●
|
Between
November 30, 2020 and December 31, 2020, debenture holders converted an aggregate of $252,210 into approximately 475,000 shares of
common stock at per share conversion prices ranging from $0.3375 to $0.6675.
|
|
|
●
|
Between
January 1, 2021 and March 15, 2021, debenture holders converted an aggregate of $1,964,500 into 4,248,864 shares of common stock
at per share conversion prices ranging from $0.3375 to $0.5475.
|
|
|
●
|
On
January 12, 2021 we issued an aggregate of $500,000 in senior convertible debentures (“January 2021 Debentures”) to certain
accredited investors for cash. The January 2021 Debentures mature on January 12, 2022. The conversion price of the January 2021 Debentures
is equal to the lesser of (i) $24.75 and (ii) 85% of the lowest volume-weighted average price during the five trading days immediately
prior to the date of conversion. As of the date of this report, all of January 2021 Debentures have been converted into common stock
of the Company.
|
|
|
●
|
Between March 16, 2021
and June 30, 2021, debenture holders converted an aggregate of $191,068 into 378,167 shares of common stock at per share conversion
prices ranging from $0.3975 to $0.7275.
|
|
|
|
|
|
|
●
|
On June 18, 2021, we
issued an aggregate of $600,000 in senior convertible debentures (“June 2021 Debentures”) to certain accredited investors
for $500,000 cash and $100,000 in cancellation of obligations. The June 2021 Debentures have the same terms as the January 2021 Debentures
and mature on June 18, 2022.
|
|
|
●
|
Between July 1, 2021
and August 2, 2021, debenture holders converted an aggregate of $58,000 into 133,333 shares of common stock at per share conversion
prices ranging from $0.315 to $0.3375.
|
|
|
|
|
|
|
●
|
Between August 3, 2021
and September 30, 2021, debenture holders converted an aggregate of $354,480 into 2,340,667 shares of common stock at per share conversion
prices ranging from $0.12 to $0.37.
|
|
|
●
|
Between
October 1, 2021 and October 31, 2021, debenture holders converted an aggregate of $87,520 into 729,333 shares of common stock at
a per share conversion price of $0.12.
|
Item 16.
Exhibits.
See
Exhibit Index beginning on page 56 of this registration statement.
Item 17.
Undertakings.
Insofar
as indemnification by the Registrant for liabilities arising under the Securities Act may be permitted to directors, officers and controlling
persons of the Registrant pursuant to the provisions referenced in Item 15 of this registration statement or otherwise, the Registrant
has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act, and
is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant
of expenses incurred or paid by a director, officer, or controlling person of the Registrant in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder,
the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act
and will be governed by the final adjudication of such issue.
The
undersigned registrant hereby undertakes:
(II)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(II)
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) (§230.424(b) of this chapter) if, in
the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth
in the “Calculation of Registration Fee” table in the effective registration statement.
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement;
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
(4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(II)
If the registrant is relying on Rule 430B (§230.430B of this chapter):
(II)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) (§230.424(b)(3) of this chapter) shall be deemed to be part of
the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) (§230.424(b)(2), (b)(5), or (b)(7) of this chapter)
as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x)
(§230.415(a)(1)(i), (vii), or (x) of this chapter) for the purpose of providing the information required by section 10(a) of the
Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form
of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in
the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such
date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement
to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement
or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of
the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify
any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such
document immediately prior to such effective date; or
(5)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities:
The
undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold
to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser:
(II)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424 (§230.424 of this chapter);
(ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant;
(iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and
(iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6)
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed
as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant
to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time
it was declared effective.
(7)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of
prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Raul Silvestre, acting alone, with
full power of substitution and resubstitution and full power to act, his true and lawful attorney-in-fact and agent, with full power
of substitution and resubstitution for him and in his name, place and stead, in any and all capacities, to sign any and all amendments
to this Registration Statement, and all documents in connection therewith (including all post-effective amendments and any subsequent
registration statement filed pursuant to Rule 462(b) under the Securities Act), with the SEC, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises,
as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in
the capacities indicated on December 3, 2021.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/
RAUL SILVESTRE
|
|
Chief
Executive Officer, Chief Financial Officer and Director
|
|
December 3, 2021
|
|
Raul
Silvestre
|
|
(Principal
Executive Officer)
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/
SCOTT OGILVIE
|
|
Director
|
|
December 3, 2021
|
|
Scott
Ogilvie
|
|
|
|
|
|
|
|
|
|
|
|
/s/
CLAIRE THOM
|
|
Director
|
|
December 3, 2021
|
|
Claire
Thom, Pharm.D.
|
|
|
|
|
INDEX
TO EXHIBITS
|
|
|
|
|
|
|
Incorporated by Reference
|
|
Exhibit
No.
|
|
Description
|
|
Filed
Herewith
|
|
Form
|
|
Exhibit
No.
|
|
File
No.
|
|
Filing
Date
|
|
2.01
|
|
Form
of agreement and Plan of Merger among Inspyr Therapeutics, Inc., Rebus Holdings, Inc., and Rebus
Sub, Inc. dated September 28, 2021.
|
|
|
|
8-K
|
|
3.01
|
|
000-55331
|
|
10/4/21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.01(i)
|
|
Amended
and Restated Certificate of Incorporation dated September 4, 2013
|
|
|
|
8-K
|
|
3.01
|
|
333-153829
|
|
9/6/13
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.02(i)
|
|
Amendment
to the Amended and Restated Certificate of Incorporation, effective August 1, 2016
|
|
|
|
8-K
|
|
3.01
|
|
333-153829
|
|
8/2/16
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.03(i)
|
|
Amended
and Restated Certificate of Incorporation dated October 21, 2016
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
11/10/16
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.04(i)
|
|
Amended
and Restated Certificate of Incorporation, effective September 30, 2019
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
9/30/19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.05(i)
|
|
Amended
and Restated Certificate of Incorporation, effective June 26, 2020
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
6/29/20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.06(ii)
|
|
Amended
and Restated Bylaws
|
|
|
|
8-K
|
|
3.02
|
|
333-153829
|
|
1/11/10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.07(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series A 0% Convertible Preferred Stock
|
|
|
|
8-K
|
|
3.01
|
|
000-55331
|
|
12/23/15
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.08(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series B 0% Convertible Preferred Stock
|
|
|
|
8-K
|
|
3.01
|
|
000-55331
|
|
12/12/16
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.09(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series C 0% Convertible Preferred Stock
|
|
|
|
8-K
|
|
3.01
|
|
000-55331
|
|
3/20/17
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.10(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series D 0% Convertible Preferred Stock
|
|
|
|
10-K
|
|
3.08(i)
|
|
000-55331
|
|
4/26/19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.11(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series E 0% Convertible Preferred Stock
|
|
|
|
10-K
|
|
3.10(i)
|
|
000-55331
|
|
5/14/20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.12(i)
|
|
Certificate
of Designation of Preferences, Rights and Limitations of Series F 0% Convertible Preferred Stock
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
10/8/20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.13(i)
|
|
Amended
and Restated Certificate of Incorporation Effective November 27, 2020
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
11/27/20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.14(i)
|
|
Amended
and Restated Certificate of Incorporation of Inspyr Therapeutics, Inc. effecting 1-for-75 Reverse Stock Split
|
|
|
|
8-K
|
|
3.01(i)
|
|
000-55331
|
|
10/4/21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.15(i)
|
|
Certificate
of Merger between Inspyr Therapeutics, Inc. and Rebus Sub, Inc.
|
|
|
|
8-K
|
|
3.02(i)
|
|
000-55331
|
|
10/4/21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.16(ii)
|
|
Rebus
Holdings Bylaws dated November 11, 2021
|
|
|
|
10-Q
|
|
3.16(ii)
|
|
000-55331
|
|
11/12/21
|
|
|
**
|
Management
contracts or compensation plans or arrangements in which directors or executive officers
are eligible to participate.
|
Rebus (CE) (USOTC:RBSH)
Historical Stock Chart
From Dec 2024 to Jan 2025
Rebus (CE) (USOTC:RBSH)
Historical Stock Chart
From Jan 2024 to Jan 2025