iRhythm Technologies Expands Global Reach with the Launch of Zio®
Cardiac Arrhythmia Monitoring Service in Austria, the Netherlands,
Switzerland, and Spain
- Zio long-term continuous
monitoring (LTCM) service consists of a prescription only patch ECG
monitoring device (Zio monitor) worn for up to 14 days, the ZEUS
(Zio ECG Utilization Software) System (iRhythm’s advanced AI
algorithm), and a comprehensive end-of-wear report reviewed and
curated by certified cardiographic technicians.
- The next-generation Zio monitor LTCM provides up to 14 days
of continuous, uninterrupted, patch-based ECG monitoring, marking a
significant advancement in these countries, compared to older,
commonly used 24- to 48-hour (Holter) monitoring
technology1.
- Zio LTCM is associated with a greater diagnostic yield of
specified arrhythmias, the lowest likelihood of retesting and the
lowest incremental acute care utilization, as compared to Holter
monitoring
services1,2,3,4.
- Zio products and services are backed by over 100
peer-reviewed clinical research and scientific
papers5.
SAN FRANCISCO, Aug. 29, 2024 (GLOBE NEWSWIRE) -- iRhythm
Technologies, Inc. (NASDAQ:IRTC), a leading digital health care
company focused on creating trusted solutions that detect, predict,
and prevent disease, today announced the commercial launch of its
Zio monitor and Zio long-term continuous (LTCM) ambulatory ECG
monitoring service in Austria, the Netherlands, Spain, and
Switzerland. The Zio monitor and ZEUS (Zio ECG Utilization
Software) system received the European Union’s CE Marking Under
Medical Device Regulation (EU MDR) in December 2023. The Zio LTCM
service provides up to 14 days of continuous, uninterrupted ECG
monitoring, marking a significant advancement in these countries,
compared to older, commonly used 24- to 48-hour Holter monitoring
services1.
“Expanding access to our innovative Zio cardiac arrhythmia
monitoring system and services for millions more people in Europe
is an important milestone for iRhythm’s patient-first mission to
positively impact people across the globe,” said Quentin Blackford,
iRhythm President and CEO. “Our commitment extends to broadening
our highly innovative digital healthcare solutions, while ensuring
they are cost-effective and accessible worldwide.”
Advancing Cardiac Care in Europe
Zio LTCM service offers a range of distinct advantages that set it
apart as a leading solution for cardiac monitoring in Europe:
- Up to 14 days of continuous, uninterrupted ECG monitoring
- 99% patient compliance with prescribed wear
times6,7 and 99% analyzable
time6,
- Highest diagnostic yield of specified arrhythmias, lowest
likelihood of retesting, and lowest incremental acute care
utilization compared to traditionally used 24- or 48-hour Holter
monitoring services1-4.
Additionally, Zio monitor demonstrates:
- Improved form-factor over the previous generation for a better
patient wear experience and is 23%
thinner8,9, 62%
lighter10, 72% smaller10and weighs 10
grams
- Patient-centric design that is breathable, waterproof
housing10,11, increased adhesive
area, a symptom button for easy patient interaction, and no
maintenance or battery changes are required12
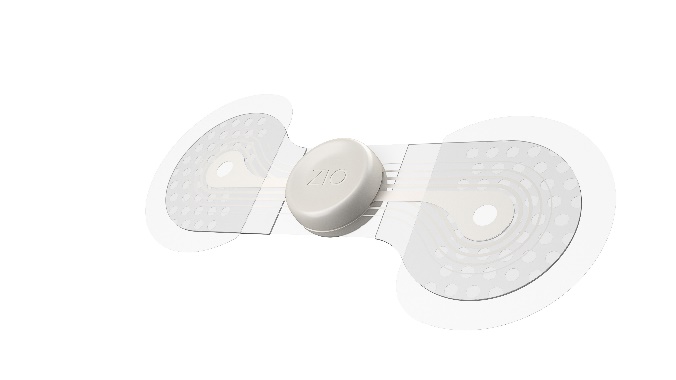
Zio® monitor by
iRhythm Technologies
Clinically Proven Performance
The value of the Zio service has been demonstrated in over 100
original scientific research manuscripts5, including the
recent real-world Cardiac
Ambulatory Monitor
EvaLuation of
Outcomes and Time to Events
(CAMELOT) study, published in the American Heart Journal.
The CAMELOT study, a US retrospective data analysis of 287,789
Medicare patients, found that the Zio LTCM service, is associated
with the highest diagnostic yield of specified arrhythmias, lowest
likelihood of retesting, and the lowest incremental of acute care
utilization compared to all other monitoring services, including
other long-term continuous monitoring services, in the
study1-4.
iRhythm’s advanced CE-marked and FDA-cleared AI has a
deep-learned algorithm clinically proven to be as accurate as
expert cardiologists,13,14 The
deep learning approach can classify a broad range of distinct
arrhythmias with high diagnostic performance similar to that of
cardiologists13.
“The global burden of arrhythmias continues to rise, with atrial
fibrillation (AF) alone affecting 1.5-2% of Europeans and with a
lifetime risk of 1 in 4,” said Mintu Turakhia, iRhythm Chief
Medical and Scientific Officer and EVP, Product Innovation. “At the
same time, we are also seeing a greater need for post-treatment
monitoring, with catheter ablation strongly on the rise in Europe
and worldwide. Zio monitor is an evidence-based solution that
provides 2 weeks of uninterrupted monitoring from a single patch in
places where only 1-2 days of monitoring is available, often from
bulkier technologies that don’t leverage AI like we do. We believe
the introduction of the Zio service in these countries will
positively disrupt the monitoring paradigm to align clinical
practice with evidence and improve outcomes.”
Cardiac Arrhythmias and Prevalence
A cardiac arrhythmia is a condition in which the heart beats too
quickly, too slowly or irregularly due to abnormal electrical
impulses15. If undetected and untreated, some
arrhythmias can damage the heart, brain, or other organs, and lead
to an increased risk of stroke and death16. Early
detection and treatment of arrhythmias can prevent adverse outcomes
and improve quality of life17.
As the prevalence of arrhythmias continues to rise across
Europe18, atrial fibrillation—the most common cardiac
arrhythmia—now affects 1.5-2% of European adults, with projections
indicating a surge to 9.5% in individuals over 65 by
206019. The recent CAMELOT study has demonstrated that
Zio LTCM service offers the highest diagnostic yield for specified
arrythmias, the lowest likelihood of retesting, and the most
significant reduction in acute care utilization as compared to
Holter monitoring services which are traditionally utilized in
Europe1-4. This positions Zio LTCM service as a
significant advancement in cardiac arrhythmia monitoring for the
region.
"The introduction of the Zio monitor to European countries can
help physicians better identify, diagnose, and manage cardiac
arrhythmias faster, as speed to diagnosis is especially critical
for cardiac care," said José L. Merino, MD, PhD, FEHRA, and Chief
of the Arrhythmia and Robotic EP Unit, Hospital Universitario La
Paz in Madrid, who took part in a Zio market evaluation in Spain.
"The Zio service enhances clinical workflows and boosts
productivity, enabling more precise and timely diagnoses, which
ultimately alleviates pressure on healthcare services. And Zio
monitor is really convenient for the patient.”
“The availability of a long-term cardiac arrhythmia monitoring
solution that is friendly to wear closes an important clinical gap
for patients in Europe," said Patrick Badertscher, Professor and
Senior Physician, Cardiac Electrophysiology, and Michael Kühne,
Professor of Cardiology at the University Hospital Basel both who
took part in a Zio market evaluation in Switzerland. “We will be
able to streamline clinical processes with highly accurate
diagnoses allowing for more effective treatment decisions. And when
it comes to the monitoring device, many patients express a
preference for Zio."
Availability
Zio monitor will begin shipping in Austria, the Netherlands,
Switzerland, and Spain in September, with widespread availability
in these countries anticipated in 2025. Zio monitor, Zio XT and Zio
AT (mobile cardiac telemetry) services are currently available in
the U.S., and Zio XT service is available in the UK. To learn more
about Zio monitor, go to info.irhythmtech.com/.
About iRhythm Technologies, Inc.
iRhythm is a leading digital health care company that creates
trusted solutions that detect, predict, and prevent disease.
Combining wearable biosensors and cloud-based data analytics with
powerful proprietary algorithms, iRhythm distills data from
millions of heartbeats into clinically actionable information.
Through a relentless focus on patient care, iRhythm’s vision is to
deliver better data, better insights, and better health for all. To
learn more about iRhythm, go to iRhythmtech.com.
Investor Relations Contact
Stephanie Zhadkevich
investors@irhythmtech.com
Media Contact
Kassandra Perry
irhythm@highwirepr.com
1 Reynolds et al. Comparative effectiveness and
healthcare utilization for ambulatory cardiac monitoring strategies
in Medicare beneficiaries. Am Heart J. 2024;269:25–34. Accessed
January 2, 2024. https://doi.org/10.1016/j.ahj.2023.12.002
2A specified arrhythmia refers to an arrhythmia
encounter diagnosis as per Hierarchical Condition Categories (HCC)
96.
3Based on previous generation Zio XT device data. Zio
monitor utilizes the same operating principles and ECG algorithm.
Additional data on file.
4Zio LTCM service refers to Zio XT and Zio monitor
service.
5 Data on file. iRhythm Technologies, 2023:
https://www.irhythmtech.com/providers/evidence/list-of-clinical-articles
6 Based on the US data
7 Zio service provides continuous, uninterrupted
recording and a comprehensive end-of-wear report
8 Data on file. iRhythm Technologies, 2023.
9 Compared to previous generation.
10 Data on file. iRhythm Technologies, 2017, 2023.
11 The Zio monitor patch should not be submerged in
water. During a bath, keep the device above water. Please refer to
the Zio monitor labeling instructions or Patient Guide for the full
set of details.
12 Zio monitor Instructions for Use. iRhythm
Technologies, 2023.
14 Zio has the only FDA-cleared, deep learned algorithm
that’s as accurate as human, expert-level interpretation:
https://www.irhythmtech.com/providers/zio-service/ai
15 American Heart Association (2024) What is an
arrhythmia?
https://www.heart.org/en/health-topics/arrhythmia/about-arrhythmia
(Accessed 8-2-24)
16 National Heart Lung and Blood Institute, 2022. What
is an arrhythmia? https://www.nhlbi.nih.gov/health/arrhythmias
(Accessed 8-2-24)
17 Rillig et al. Early Rhythm Control in Patients With
Atrial Fibrillation and High Comorbidity Burden. Circulation.
2022;146(11):836-847.
doi:https://doi.org/10.1161/circulationaha.122.060274
18Mensah, AH. et al. Global Burden of Cardiovascular
Diseases and Risks, 1990-2022. Journal of the American College of
Cardiology, 2023.
19Linz, D. et. Al. Atrial fibrillation: epidemiology,
screening and digital health. The Lancet Regional Health, 2024.
A photo accompanying this announcement is available
at https://www.globenewswire.com/NewsRoom/AttachmentNg/57909a3f-08ca-4a8a-859d-e0bfe63b59d9


Irhythm Technologies (LSE:0A7L)
Historical Stock Chart
From Nov 2024 to Dec 2024
Irhythm Technologies (LSE:0A7L)
Historical Stock Chart
From Dec 2023 to Dec 2024