European market clearance for the NeuroLF® System
– a pioneering dedicated brain PET imaging system. This MDR
approval (CE Mark) follows recent FDA clearance which makes NeuroLF
the first device of its kind to obtain regulatory approval in the
US and in Europe.
ZURICH, Oct. 3, 2024
/PRNewswire/ -- Positrigo, a Swiss based company developing nuclear
medical imaging devices to advance functional brain imaging, has
achieved another major milestone with the CE Mark in Europe for its dedicated brain Positron
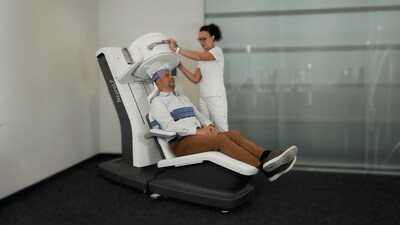
Emission Tomography (PET) system NeuroLF®. This
innovative imaging device is ultra-compact and assists in
diagnosing and monitoring of brain related disorders like
Alzheimer's disease, Brain Tumors, Epilepsy, Parkinson's disease
and others.
"The CE mark of the NeuroLF system is another important
milestone in the development of this dedicated brain PET system and
represents a significant advance in the availability of brain PET
imaging, allowing nuclear medicine physicians in Europe to offer a dedicated imaging modality
to diagnose and monitor patients with brain related disorders",
says Prof. Dr. med. Osama
Sabri, Director and Chairman of the Department of
Nuclear Medicine at the University of Leipzig Medical Center.
Indeed, the NeuroLF system is much smaller and more affordable
compared to the currently available clinical hybrid PET systems
with a much larger footprint and weight and always combined with
either MRI or CT. Dr. Jannis
Fischer, co-founder and CEO of Positrigo states: "At
Positrigo, we seek to push the limits by developing and
commercializing new types of medical imaging technologies. The CE
mark of the NeuroLF system is testament to this, now offering
healthcare professionals the potential to improve the diagnosis for
people living with brain related disorders like Alzheimer's disease
(AD) while setting a new standard in brain PET imaging."
Increased Demand for Brain PET in Europe
The European Association of Nuclear Medicine (EANM) Neuroimaging
Committee believes that amyloid PET is now at a historical turning
point, where it has paved the way for early AD diagnosis. These
experts expect that amyloid PET will be more widely used in the
near future to justify the initiation and to monitor the effect of
disease-modifying therapies on biological grounds (1). The Nuclear
Medicine community should therefore be prepared to adjust its
capacities to the increased demand for amyloid PET radiotracers,
PET imaging infrastructure, and training for image reading and
reporting in the near future. According to some estimates, the need
for amyloid PET may increase by a factor of 20 (2). Thanks to the
European market clearance of NeuroLF, Positrigo is now able to
offer a solution which can improve the PET imaging
infrastructure.
Commercialization in Europe
with selected partners
Positrigo plans to sell its novel technology in Europe by leveraging a network of established
companies in selected countries. "Due to the fragmented healthcare
system in Europe with different
country specific purchase mechanisms and healthcare reimbursement
systems, it is most efficient to partner with local companies in
selected countries," explains Dr. Stefan
Bircher, CCO at Positrigo. "This way we believe to
achieve fast commercial traction and hope to be be able to offer
best in class after sales support at the same time".
The NeuroLF system is the first device of its kind which
received market clearance in Europe and in the US. These two market
approvals are important milestones for commercial opportunities in
other parts of the world.
About Positrigo:
Positrigo is a pioneer in nuclear medical imaging technologies.
Headquartered in Zurich,
Switzerland, the medical device company was founded in 2018
as a spin-off of ETH Zurich. Positrigo's technology, development,
clinical testing and commercialization has been supported by
various private investors, the Swiss government and the European
Innovation Council. NeuroLF – the company's first device – is an
ultra-compact brain Positron Emission Tomography (PET) scanner
which has applications in the assessment of causes of dementias,
such as Alzheimer's disease and other brain related disorders.
Learn more at http://www.positrigo.com.
Media Contact:
Dr. Stefan Bircher
Phone: +41 77 510 7262
References:
1 FDA approval of lecanemab: the real start of
widespread amyloid PET use? — the EANM Neuroimaging Committee
perspective | European Journal of Nuclear Medicine and Molecular
Imaging (springer.com)
2 The approval of a
disease-modifying treatment for Alzheimer's disease: impact and
consequences for the nuclear medicine community | European Journal
of Nuclear Medicine and Molecular Imaging (springer.com)
Photo -
https://mma.prnewswire.com/media/2521781/Positrigo_NeuroLF_System.jpg
Logo -
https://mma.prnewswire.com/media/2521782/Positrigo_Logo.jpg
 View original content to download
multimedia:https://www.prnewswire.co.uk/news-releases/positrigo-secures-ce-mark-in-europe-for-its-pioneering-dedicated-brain-pet-system-302265617.html
View original content to download
multimedia:https://www.prnewswire.co.uk/news-releases/positrigo-secures-ce-mark-in-europe-for-its-pioneering-dedicated-brain-pet-system-302265617.html