false000137469000013746902024-05-092024-05-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): May 09, 2024 |
Larimar Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-36510 |
20-3857670 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
Three Bala Plaza East |
|
Bala Cynwyd, Pennsylvania |
|
19004 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (844) 511-9056 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
|
LRMR |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On May 9, 2024, Larimar Therapeutics, Inc. (the “Company”) announced its financial results and operational highlights for the first quarter ended March 31, 2024. A copy of the press release is being furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished pursuant to this Item 2.02, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
On May 9, 2024, the Company posted on its website an updated slide presentation, which is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference. Representatives of the Company will use the presentation in various meetings with investors, analysts and other parties from time to time.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Below is a list of exhibits included with this Current Report on Form 8-K.
* Furnished herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Larimar Therapeutics, Inc. |
|
|
|
|
Date: |
May 9, 2024 |
By: |
/s/ Carole S. Ben-Maimon, M.D. |
|
|
|
Name: Carole S. Ben-Maimon, M.D.
Title: President and Chief Executive Officer |
Exhibit 99.1

Larimar Therapeutics Reports First Quarter 2024 Operating and Financial Results
•First patient dosed in open label extension (OLE) study with 25 mg daily dosing of nomlabofusp; interim data on track for Q4 2024
•Positive top-line Phase 2 dose exploration study data demonstrated nomlabofusp was generally well-tolerated with dose-dependent increases in tissue frataxin levels, reinforcing therapeutic potential
•Biologics License Application (“BLA”) submission targeted for 2H 2025; discussions initiated with Food and Drug Administration (“FDA”) on potential to pursue accelerated approval pathway
•Successful $161.8 million financing strengthens cash, cash equivalents, and marketable securities to $239 million as of March 31, 2024, extending projected cash runway into 2026
Bala Cynwyd, PA, May 9, 2024 – Larimar Therapeutics, Inc. (“Larimar”) (Nasdaq: LRMR), a clinical-stage biotechnology company focused on developing treatments for complex rare diseases, today reported its first quarter 2024 operating and financial results.
“We have started 2024 off strong, achieving critical milestones that support late-stage advancement of our nomlabofusp clinical program. Positive Phase 2 dose exploration study data demonstrated that nomlabofusp appears to be generally well-tolerated, and observed dose dependent increases in tissue frataxin levels that have the potential to address the underlying frataxin deficiency that is the root cause of Friedreich’s ataxia (FA). In March, we dosed the first patient in our OLE study and continue to enroll patients and activate additional sites. We are on track to report interim data in the fourth quarter of the year which will inform on the long-term safety and tissue frataxin levels of nomlabofusp,” said Carole Ben-Maimon, MD, President, and Chief Executive Officer of Larimar. “Together, these datasets will help support our BLA submission which we are targeting for the second half of 2025. We are continuing our regulatory discussions with the FDA on the potential use of frataxin as a novel surrogate endpoint to support accelerated approval and are planning for a global double-blind placebo-controlled confirmatory study which we expect to initiate prior to BLA submission. With our recent capital infusion and runway extended through key registrational catalysts, we are well positioned to further advance the first potential therapy to increase frataxin levels in patients with FA.”
Recent Highlights
•In February 2024, Larimar announced positive top-line data and successful completion of its four-week, placebo-controlled Phase 2 dose exploration study of nomlabofusp (CTI-1601) in patients with FA. Nomlabofusp was generally well-tolerated throughout the four-week treatment periods, had a predictable pharmacokinetic profile, and led to dose-dependent increases in frataxin in skin and buccal cells after daily dosing for 14 days followed by every other day dosing until day 28 in the 25 mg and 50 mg cohorts. Increases in frataxin levels in skin cells were seen in all treated patients, and in buccal cells for the majority of patients. At Day 14, all patients (with quantifiable levels at baseline and Day 14) treated with 50 mg of nomlabofusp achieved frataxin levels in skin cells greater than 33% of the average level observed in healthy volunteers, with 3 patients achieving levels greater than 50% of the average healthy volunteer level.
•In February 2024, Larimar announced the Company had initiated discussions with the FDA on use of tissue frataxin levels as a potential novel surrogate endpoint. Larimar received FDA acknowledgement that frataxin deficiency appears to be critical to the pathogenic mechanism of FA, and that there continues to be an unmet need for treatments that address the underlying disease pathophysiology. The Company intends to pursue an accelerated approval using FXN levels, supportive pharmacodynamics and clinical information, and safety data from the OLE study,
along with additional nonclinical pharmacology information needed to support the novel surrogate endpoint approach, with a BLA submission targeted for the second half of 2025.
•In February 2024, Larimar raised net proceeds of approximately $161.8 million through a public offering of common stock.
•In March 2024, the first patient was dosed in the OLE study evaluating daily subcutaneous injections of 25 mg of nomlabofusp self-administered or administered by a caregiver. Participants who completed treatment in the Phase 2 dose exploration study, or who previously completed a prior clinical trial of nomlabofusp, are potentially eligible to screen for the OLE study. The OLE study will evaluate the safety and tolerability, pharmacokinetics, and frataxin levels in peripheral tissues as well as other exploratory pharmacodynamic markers (lipid profiles and gene expression data) following long-term subcutaneous administration of nomlabofusp. Dose escalation in the OLE study is contingent on the FDA’s review of data from the 50 mg cohort of the Phase 2 study and available data from the OLE study, due to the continued partial clinical hold. Interim data is expected in the fourth quarter of 2024. In addition, clinical assessments collected during the study will be compared to data from a matched control arm derived from participants in the Friedreich’s Ataxia Clinical Outcomes Measures Study (FACOMS) database.
•In March 2024, Larimar began to build its commercial team with the appointment of Frank Nazzario, RPh, as Vice President of Commercial. Mr. Nazzario brings nearly 30 years of leadership experience in drug launches for rare diseases. Most recently, he served as Senior Vice President of Sales at BioCryst Pharmaceuticals. Previously, he held commercial leadership roles at Spark Therapeutics where he led the commercialization of Luxturna®, the first FDA-approved gene therapy for an inherited retinal disorder, and at ViroPharma, Inc., where he led the launch of Cinryze®, the first approved biologic for Hereditary Angioedema.
First Quarter 2024 Financial Results
As of March 31, 2024, the Company had cash, cash equivalents and marketable securities totaling $239 million. In February 2024, we raised approximately $161.8 million in net proceeds through a public offering of common stock.
The Company reported a net loss for the first quarter of 2024 of $14.7 million, or $0.27 per share, compared to a net loss of $6.5 million, or $0.15 per share, for the first quarter of 2023.
Research and development expenses for the first quarter of 2024 were $12.9 million, compared to $4.6 million for the first quarter of 2023. The increase in research and development expenses was primarily driven by an increase of $5.7 million in nomlabofusp manufacturing costs, an increase of $1.0 million in clinical costs primarily associated with the initiation of the OLE study, an increase of $1.0 million in personnel expense driven by increasing headcount, an increase of $0.3 million in consulting fees and an increase of $0.2 million in stock compensation expense.
General and administrative expenses were $3.8 million in the first quarter of 2024, compared to $3.1 million in the first quarter of 2023. The increase in general and administrative expenses was primarily driven by an increase of $0.2 million in personnel expense, an increase of $0.2 million in legal fees, and an increase of $0.1 million in stock compensation expense.
About Larimar Therapeutics
Larimar Therapeutics, Inc. (Nasdaq: LRMR), is a clinical-stage biotechnology company focused on developing treatments for complex rare diseases. Larimar’s lead compound, nomlabofusp, is being developed as a potential treatment for Friedreich's ataxia. Larimar also plans to use its intracellular delivery platform to design other fusion proteins to target additional rare diseases characterized by deficiencies in intracellular bioactive compounds. For more information, please visit: https://larimartx.com.
Forward-Looking Statements
This press release contains forward-looking statements that are based on Larimar’s management’s beliefs and assumptions and on information currently available to management. All statements contained in this release other than statements of historical fact are forward-looking statements, including but not limited to statements regarding Larimar’s ability to develop and commercialize nomlabofusp (also known as CTI-1601) and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials, interactions with the FDA and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical milestones and continued interactions with the FDA; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later clinical trials, and assessments; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this press release represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law.
Investor Contact:
Joyce Allaire
LifeSci Advisors
jallaire@lifesciadvisors.com
(212) 915-2569
Company Contact:
Michael Celano
Chief Financial Officer
mcelano@larimartx.com
(484) 414-2715
Larimar Therapeutics, Inc.
Condensed Consolidated Balance Sheet
(unaudited)
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
110,125 |
|
$ |
26,749 |
Short-term marketable securities |
|
|
117,171 |
|
|
60,041 |
Prepaid expenses and other current assets |
|
|
3,657 |
|
|
3,385 |
Total current assets |
|
|
230,953 |
|
|
90,175 |
Long-term marketable securities |
|
|
11,711 |
|
|
— |
Property and equipment, net |
|
|
604 |
|
|
684 |
Operating lease right-of-use assets |
|
|
2,920 |
|
|
3,078 |
Restricted cash |
|
|
1,339 |
|
|
1,339 |
Other assets |
|
|
678 |
|
|
659 |
Total assets |
|
$ |
248,205 |
|
$ |
95,935 |
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
1,918 |
|
$ |
1,283 |
Accrued expenses |
|
|
10,098 |
|
|
7,386 |
Operating lease liabilities, current |
|
|
825 |
|
|
837 |
Total current liabilities |
|
|
12,841 |
|
|
9,506 |
Operating lease liabilities |
|
|
4,520 |
|
|
4,709 |
Total liabilities |
|
|
17,361 |
|
|
14,215 |
Commitments and contingencies (See Note 8) |
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
Preferred stock; $0.001 par value per share; 5,000,000 shares authorized as of March 31, 2024 and December 31, 2023; no shares issued and outstanding as of March 31, 2024 and December 31, 2023 |
|
|
— |
|
|
— |
Common stock, $0.001 par value per share; 115,000,000 shares authorized as of
March 31, 2024 and December 31, 2023; 63,800,017 and 43,909,069 shares issued
and outstanding as of March 31, 2024 and December 31, 2023, respectively |
|
|
64 |
|
|
43 |
Additional paid-in capital |
|
|
434,013 |
|
|
270,150 |
Accumulated deficit |
|
|
(203,208) |
|
|
(188,554) |
Accumulated other comprehensive gain (loss) |
|
|
(25) |
|
|
81 |
Total stockholders’ equity |
|
|
230,844 |
|
|
81,720 |
Total liabilities and stockholders’ equity |
|
$ |
248,205 |
|
$ |
95,935 |
Larimar Therapeutics, Inc.
Condensed Consolidated Statements of Operations
(In thousands, except share and per share data)
(unaudited)
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
|
|
2024 |
|
|
2023 |
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
$ |
12,939 |
|
$ |
4,562 |
General and administrative |
|
|
3,795 |
|
|
3,075 |
Total operating expenses |
|
|
16,734 |
|
|
7,637 |
Loss from operations |
|
|
(16,734) |
|
|
(7,637) |
Other income, net |
|
|
2,080 |
|
|
1,111 |
Net loss |
|
|
(14,654) |
|
|
(6,526) |
Net loss per share, basic and diluted |
|
$ |
(0.27) |
|
$ |
(0.15) |
Weighted average common shares outstanding, basic and diluted |
|
|
53,553,707 |
|
|
43,897,603 |
Comprehensive loss: |
|
|
|
|
|
|
Net loss |
|
$ |
(14,654) |
|
$ |
(6,526) |
Other comprehensive gain (loss): |
|
|
|
|
|
|
Unrealized gain (loss) on marketable securities |
|
|
(106) |
|
|
31 |
Total other comprehensive gain (loss) |
|
|
(106) |
|
|
31 |
Total comprehensive loss |
|
$ |
(14,760) |
|
$ |
(6,495) |

May 2024 Larimar Therapeutics Corporate Deck Exhibit 99.2

This presentation contains forward-looking statements that are based on the beliefs and assumptions of Larimar Therapeutics, Inc. ( “Company”) and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize nomlabofusp (CTI-1601) and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical milestones and continued interactions with the FDA; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later clinical trials, and assessments; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law. Forward-Looking Statements
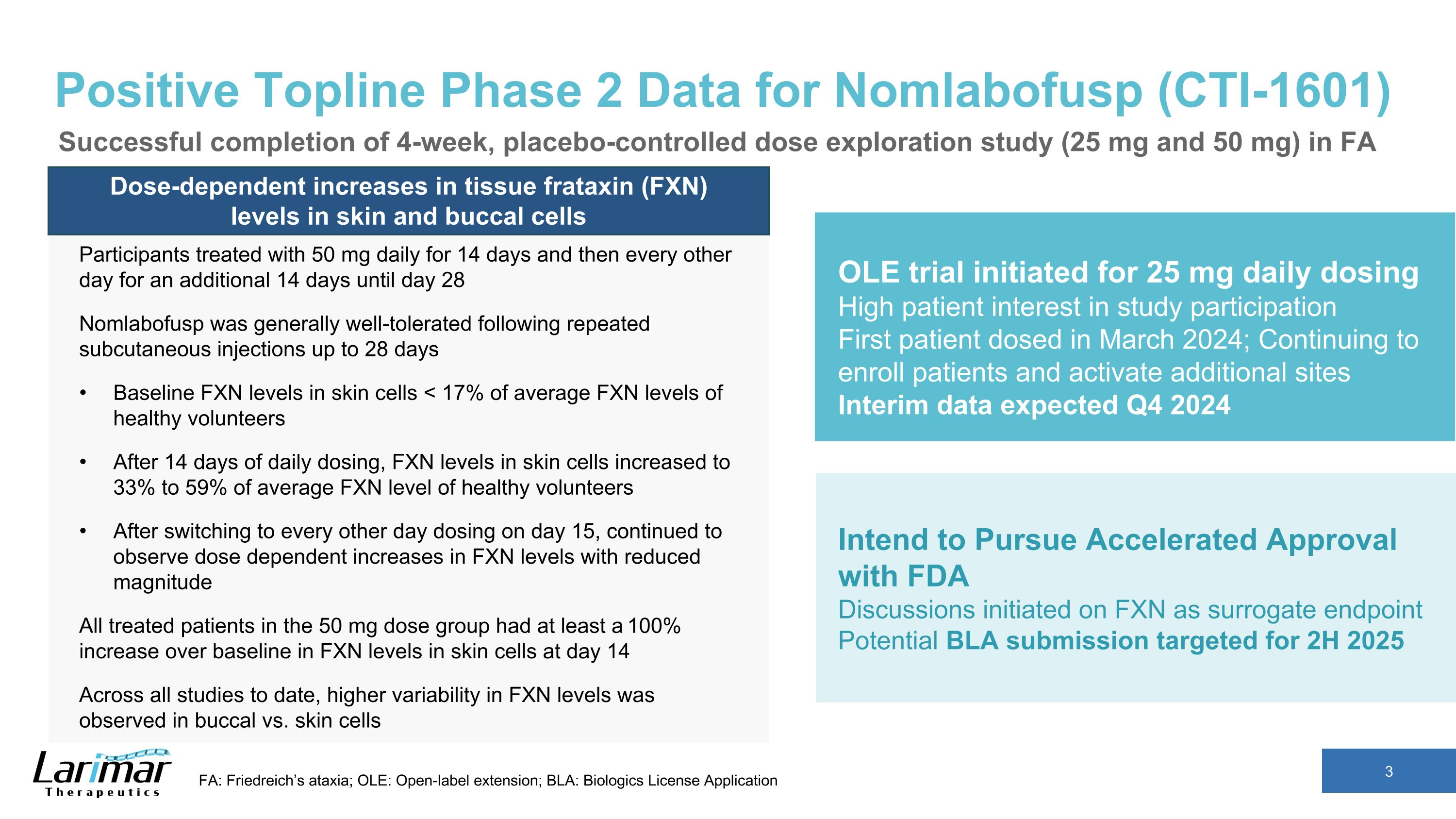
Positive Topline Phase 2 Data for Nomlabofusp (CTI-1601) Dose-dependent increases in tissue frataxin (FXN) levels in skin and buccal cells Participants treated with 50 mg daily for 14 days and then every other day for an additional 14 days until day 28 Nomlabofusp was generally well-tolerated following repeated subcutaneous injections up to 28 days Baseline FXN levels in skin cells < 17% of average FXN levels of healthy volunteers After 14 days of daily dosing, FXN levels in skin cells increased to 33% to 59% of average FXN level of healthy volunteers After switching to every other day dosing on day 15, continued to observe dose dependent increases in FXN levels with reduced magnitude All treated patients in the 50 mg dose group had at least a 100% increase over baseline in FXN levels in skin cells at day 14 Across all studies to date, higher variability in FXN levels was observed in buccal vs. skin cells FA: Friedreich’s ataxia; OLE: Open-label extension; BLA: Biologics License Application OLE trial initiated for 25 mg daily dosing High patient interest in study participation First patient dosed in March 2024; Continuing to enroll patients and activate additional sites Interim data expected Q4 2024 Intend to Pursue Accelerated Approval with FDA Discussions initiated on FXN as surrogate endpoint Potential BLA submission targeted for 2H 2025 Successful completion of 4-week, placebo-controlled dose exploration study (25 mg and 50 mg) in FA
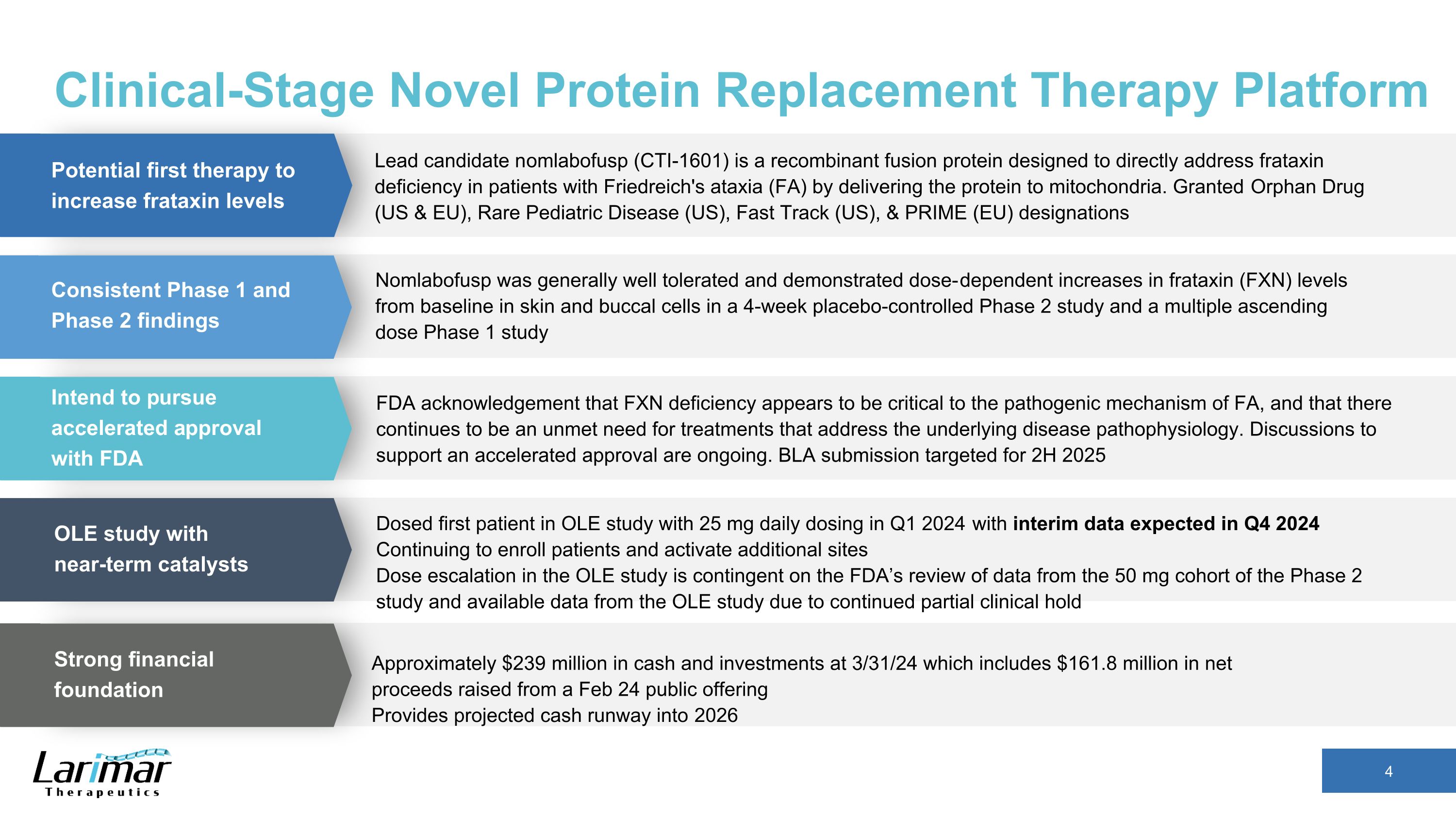
Clinical-Stage Novel Protein Replacement Therapy Platform Lead candidate nomlabofusp (CTI-1601) is a recombinant fusion protein designed to directly address frataxin deficiency in patients with Friedreich's ataxia (FA) by delivering the protein to mitochondria. Granted Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), & PRIME (EU) designations Nomlabofusp was generally well tolerated and demonstrated dose-dependent increases in frataxin (FXN) levels from baseline in skin and buccal cells in a 4-week placebo-controlled Phase 2 study and a multiple ascending dose Phase 1 study Dosed first patient in OLE study with 25 mg daily dosing in Q1 2024 with interim data expected in Q4 2024 Continuing to enroll patients and activate additional sites Dose escalation in the OLE study is contingent on the FDA’s review of data from the 50 mg cohort of the Phase 2 study and available data from the OLE study due to continued partial clinical hold Approximately $239 million in cash and investments at 3/31/24 which includes $161.8 million in net proceeds raised from a Feb 24 public offering Provides projected cash runway into 2026 Potential first therapy to increase frataxin levels Consistent Phase 1 and Phase 2 findings Intend to pursue accelerated approval with FDA OLE study with near-term catalysts Strong financial foundation FDA acknowledgement that FXN deficiency appears to be critical to the pathogenic mechanism of FA, and that there continues to be an unmet need for treatments that address the underlying disease pathophysiology. Discussions to support an accelerated approval are ongoing. BLA submission targeted for 2H 2025
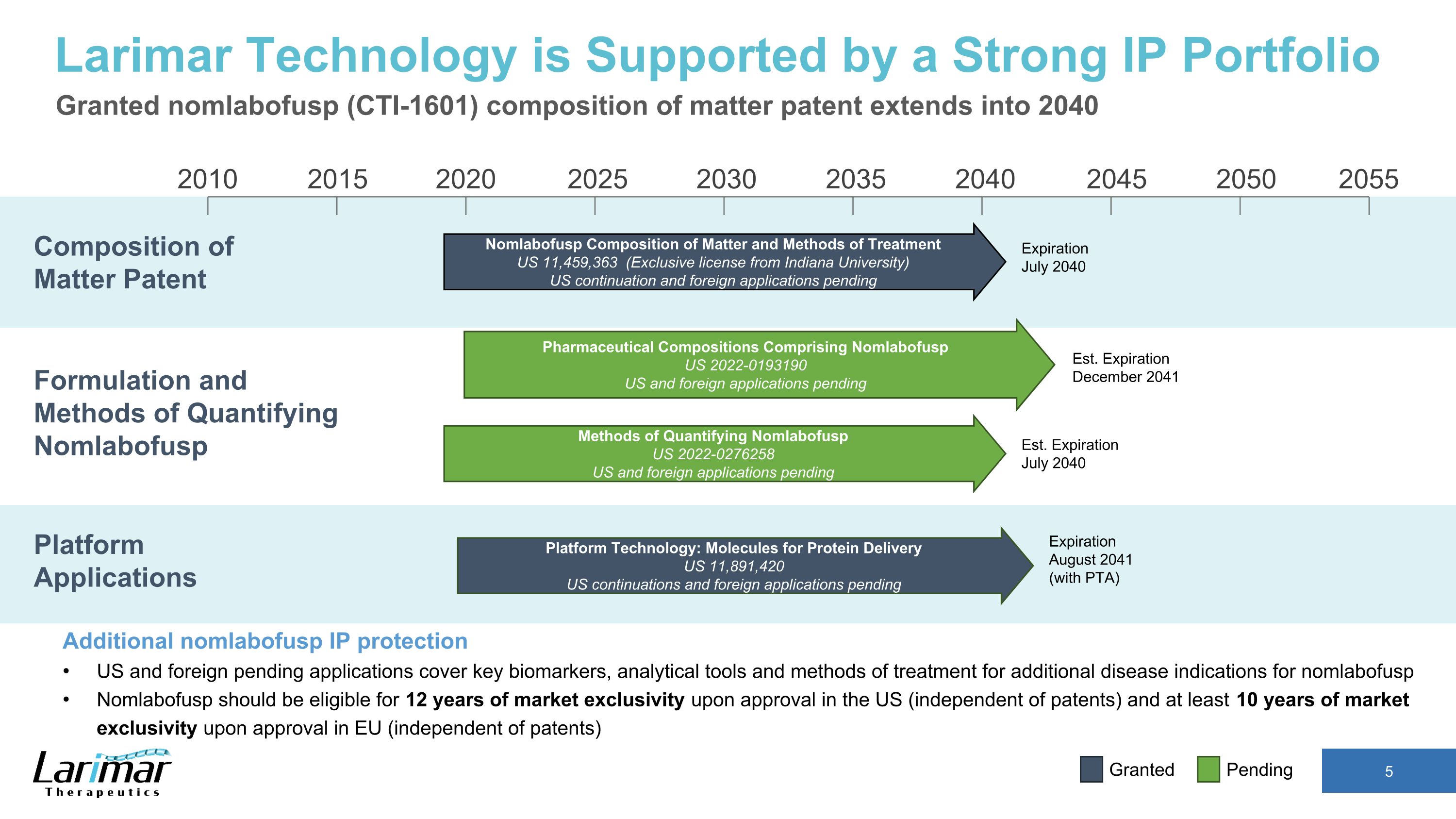
2015 2020 2030 2035 2040 2045 2050 2055 2010 2025 Nomlabofusp Composition of Matter and Methods of Treatment US 11,459,363 (Exclusive license from Indiana University) US continuation and foreign applications pending Expiration July 2040 Composition of Matter Patent Larimar Technology is Supported by a Strong IP Portfolio Granted nomlabofusp (CTI-1601) composition of matter patent extends into 2040 Additional nomlabofusp IP protection US and foreign pending applications cover key biomarkers, analytical tools and methods of treatment for additional disease indications for nomlabofusp Nomlabofusp should be eligible for 12 years of market exclusivity upon approval in the US (independent of patents) and at least 10 years of market exclusivity upon approval in EU (independent of patents) Platform Applications Formulation and Methods of Quantifying Nomlabofusp Platform Technology: Molecules for Protein Delivery US 11,891,420 US continuations and foreign applications pending Pharmaceutical Compositions Comprising Nomlabofusp US 2022-0193190 US and foreign applications pending Methods of Quantifying Nomlabofusp US 2022-0276258 US and foreign applications pending Est. Expiration December 2041 Est. Expiration July 2040 Expiration August 2041 (with PTA) Pending Granted

Friedreich’s Ataxia (FA): A rare and progressive disease 6 * E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. Most patients with FA only produce ~20-40% of normal frataxin levels depending on the tissue, sampling technique, and assay considered* Genetic defect on both alleles lowers frataxin levels Progressive disease Initial symptoms include unsteady posture and frequent falling, and patients are eventually confined to a wheelchair�Life expectancy of 30-50 years with an early death usually caused by heart disease Affects ~20,000 patients globally ~5,000 patients in the U.S., with most remaining patients in the EU�~70% of patients present before age 14 No approved therapies increase frataxin levels Only treatment approved for FA does not address frataxin deficiency
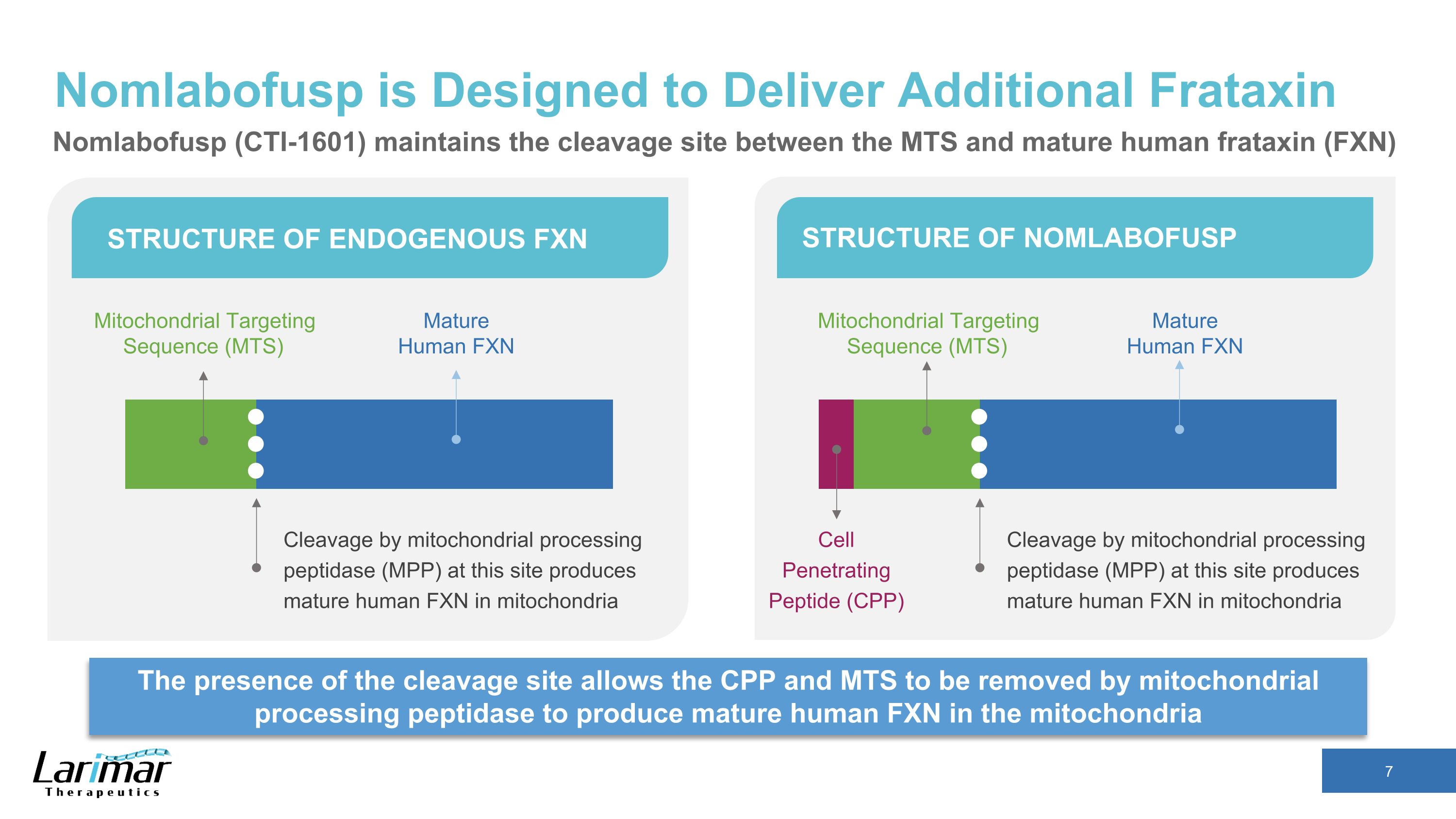
Nomlabofusp is Designed to Deliver Additional Frataxin The presence of the cleavage site allows the CPP and MTS to be removed by mitochondrial processing peptidase to produce mature human FXN in the mitochondria STRUCTURE OF ENDOGENOUS FXN STRUCTURE OF NOMLABOFUSP Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mitochondrial Targeting Sequence (MTS) Mature Human FXN Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mature Human FXN Cell Penetrating Peptide (CPP) Mitochondrial Targeting Sequence (MTS) Nomlabofusp (CTI-1601) maintains the cleavage site between the MTS and mature human frataxin (FXN)
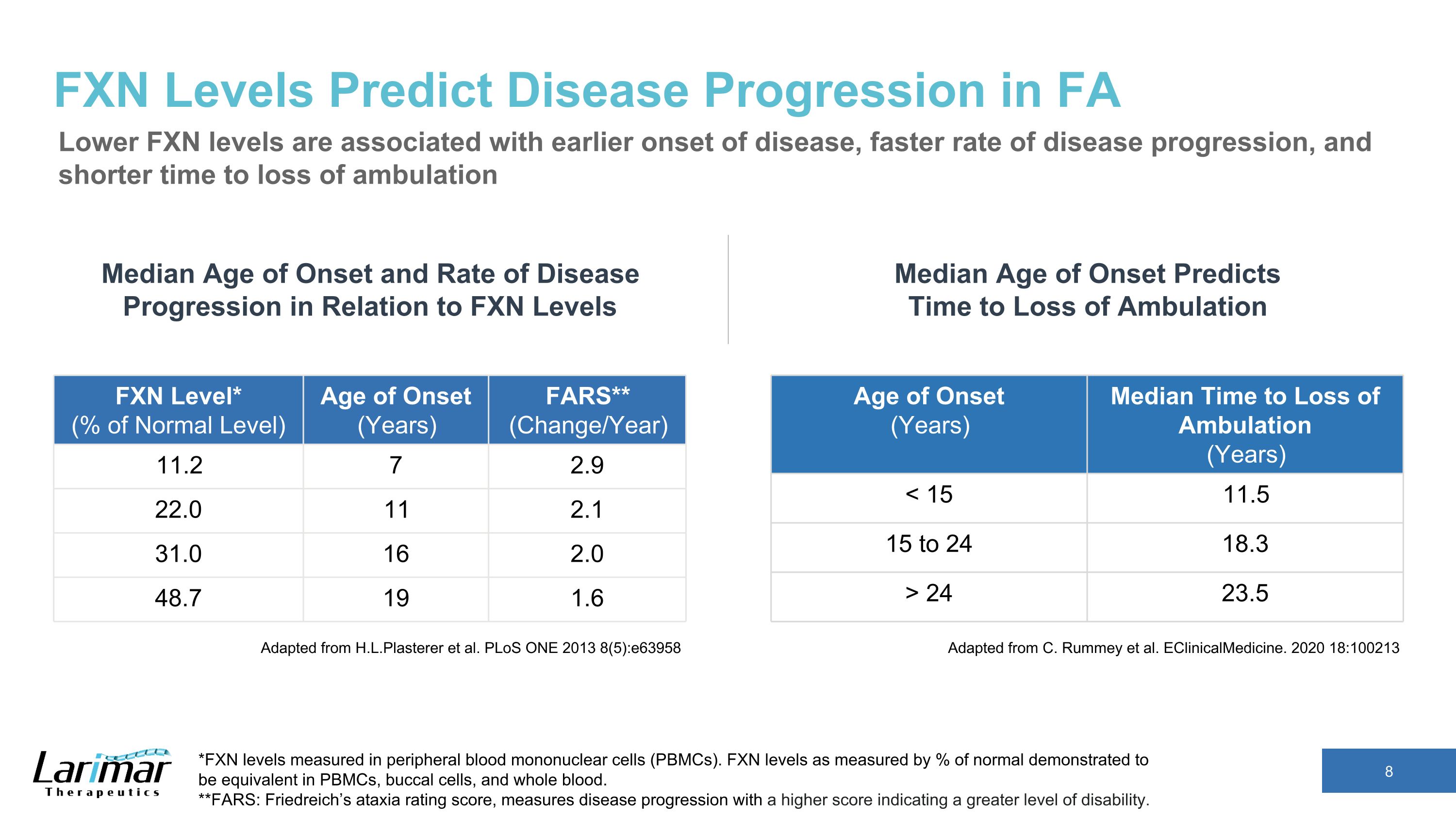
FXN Levels Predict Disease Progression in FA Lower FXN levels are associated with earlier onset of disease, faster rate of disease progression, and shorter time to loss of ambulation Adapted from H.L.Plasterer et al. PLoS ONE 2013 8(5):e63958 Age of Onset (Years) Median Time to Loss of Ambulation (Years) < 15 11.5 15 to 24 18.3 > 24 23.5 Median Age of Onset and Rate of Disease Progression in Relation to FXN Levels *FXN levels measured in peripheral blood mononuclear cells (PBMCs). FXN levels as measured by % of normal demonstrated to be equivalent in PBMCs, buccal cells, and whole blood. **FARS: Friedreich’s ataxia rating score, measures disease progression with a higher score indicating a greater level of disability. FXN Level* (% of Normal Level) Age of Onset (Years) FARS** (Change/Year) 11.2 7 2.9 22.0 11 2.1 31.0 16 2.0 48.7 19 1.6 Adapted from C. Rummey et al. EClinicalMedicine. 2020 18:100213 Median Age of Onset Predicts Time to Loss of Ambulation
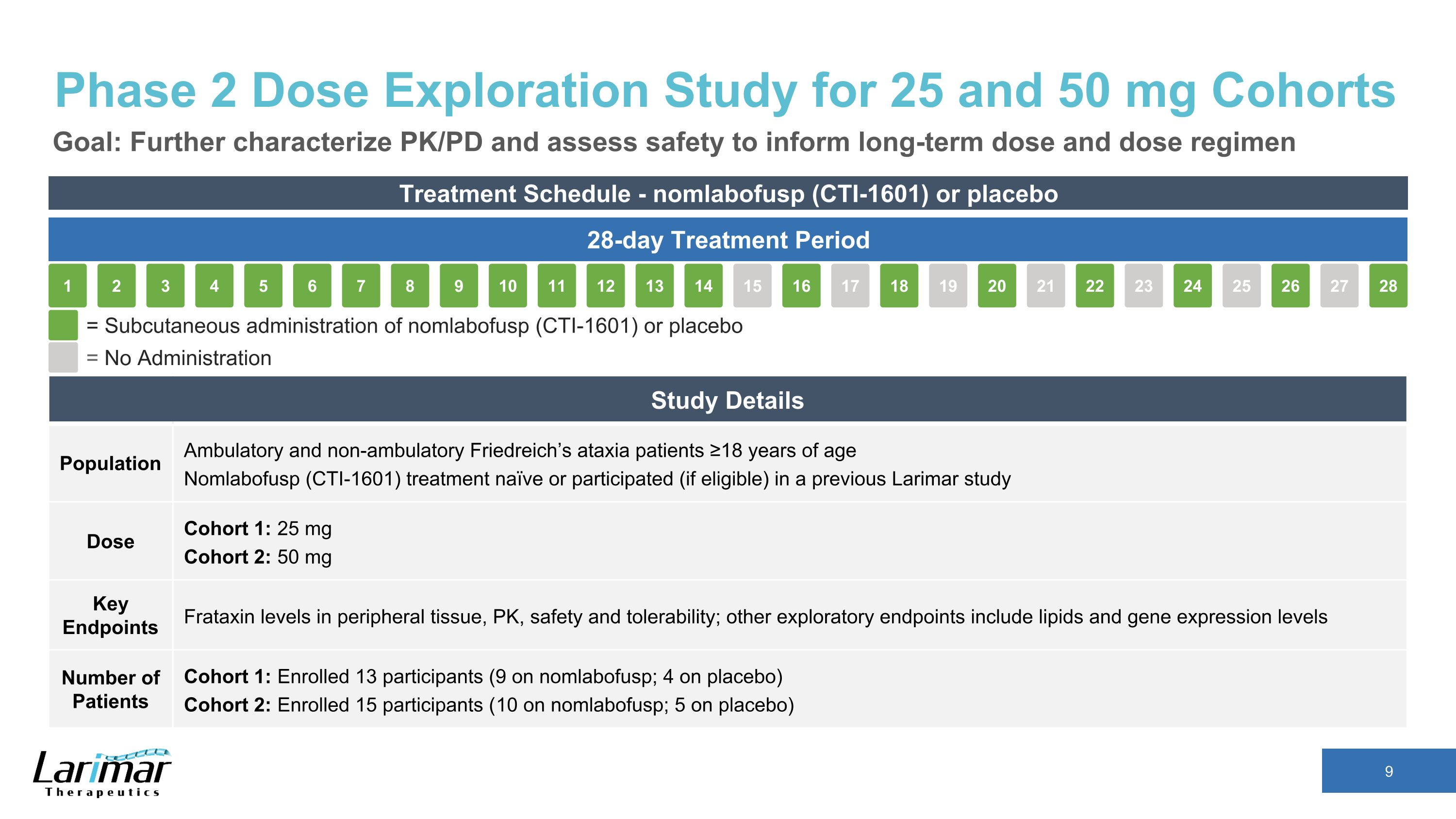
Phase 2 Dose Exploration Study for 25 and 50 mg Cohorts Goal: Further characterize PK/PD and assess safety to inform long-term dose and dose regimen Treatment Schedule - nomlabofusp (CTI-1601) or placebo 28-day Treatment Period 16 17 18 19 15 20 21 22 23 24 25 26 27 28 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Subcutaneous administration of nomlabofusp (CTI-1601) or placebo = No Administration Study Details Population Ambulatory and non-ambulatory Friedreich’s ataxia patients ≥18 years of age Nomlabofusp (CTI-1601) treatment naïve or participated (if eligible) in a previous Larimar study Dose Cohort 1: 25 mg Cohort 2: 50 mg Key Endpoints Frataxin levels in peripheral tissue, PK, safety and tolerability; other exploratory endpoints include lipids and gene expression levels Number of Patients Cohort 1: Enrolled 13 participants (9 on nomlabofusp; 4 on placebo) Cohort 2: Enrolled 15 participants (10 on nomlabofusp; 5 on placebo)
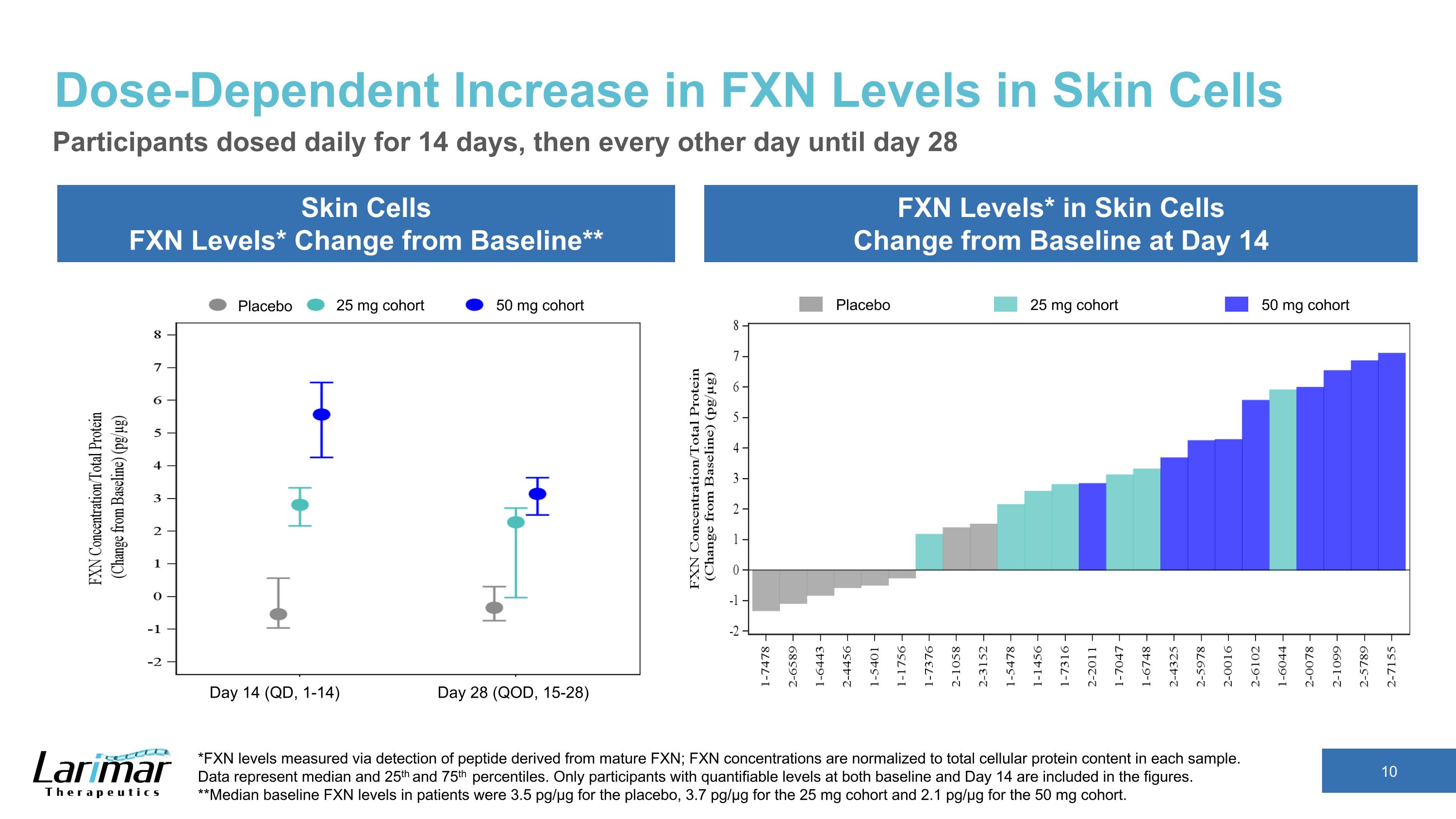
Dose-Dependent Increase in FXN Levels in Skin Cells Skin Cells FXN Levels* Change from Baseline** FXN Levels* in Skin Cells Change from Baseline at Day 14 Participants dosed daily for 14 days, then every other day until day 28 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample.�Data represent median and 25th and 75th percentiles. Only participants with quantifiable levels at both baseline and Day 14 are included in the figures. **Median baseline FXN levels in patients were 3.5 pg/µg for the placebo, 3.7 pg/µg for the 25 mg cohort and 2.1 pg/µg for the 50 mg cohort. Placebo 25 mg cohort 50 mg cohort Placebo 25 mg cohort 50 mg cohort Day 14 (QD, 1-14) Day 28 (QOD, 15-28)
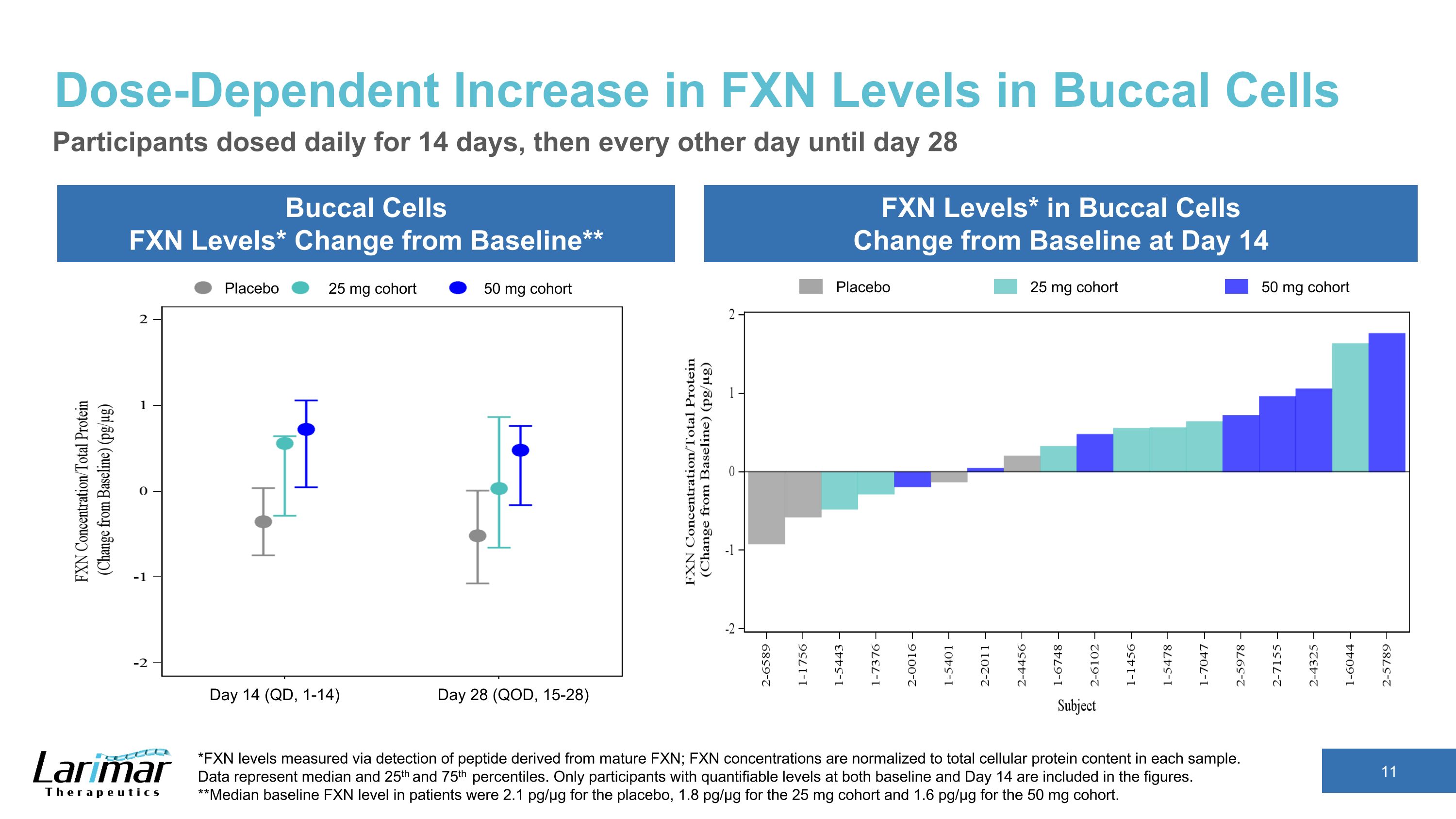
Dose-Dependent Increase in FXN Levels in Buccal Cells Buccal Cells FXN Levels* Change from Baseline** FXN Levels* in Buccal Cells Change from Baseline at Day 14 Participants dosed daily for 14 days, then every other day until day 28 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample. �Data represent median and 25th and 75th percentiles. Only participants with quantifiable levels at both baseline and Day 14 are included in the figures. **Median baseline FXN level in patients were 2.1 pg/µg for the placebo, 1.8 pg/µg for the 25 mg cohort and 1.6 pg/µg for the 50 mg cohort. Placebo 25 mg cohort 50 mg cohort Placebo 25 mg cohort 50 mg cohort Day 14 (QD, 1-14) Day 28 (QOD, 15-28)
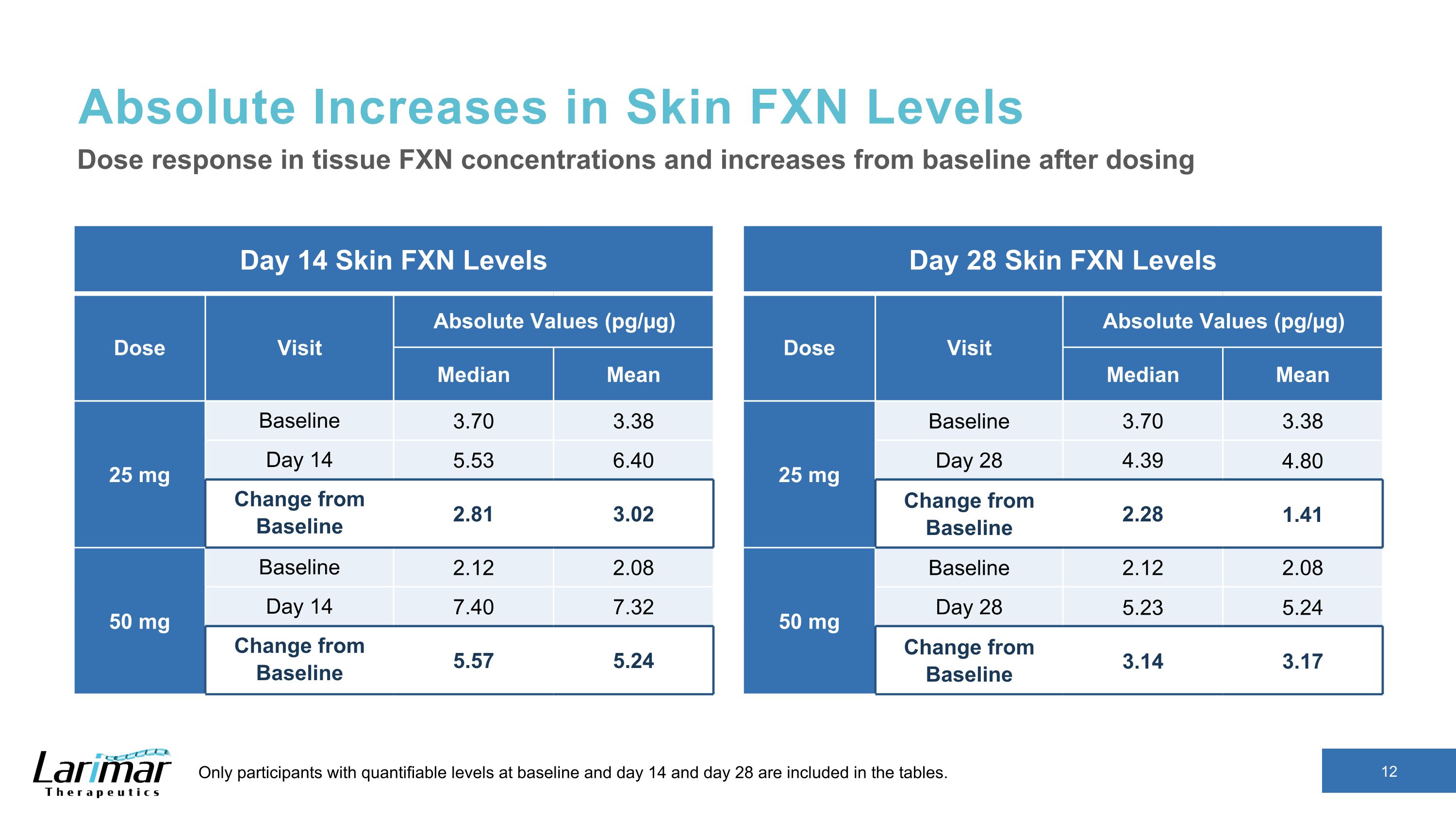
Absolute Increases in Skin FXN Levels Day 14 Skin FXN Levels Dose Visit Absolute Values (pg/µg) Median Mean 25 mg Baseline 3.70 3.38 Day 14 5.53 6.40 Change from Baseline 2.81 3.02 50 mg Baseline 2.12 2.08 Day 14 7.40 7.32 Change from Baseline 5.57 5.24 Dose response in tissue FXN concentrations and increases from baseline after dosing Only participants with quantifiable levels at baseline and day 14 and day 28 are included in the tables. Day 28 Skin FXN Levels Dose Visit Absolute Values (pg/µg) Median Mean 25 mg Baseline 3.70 3.38 Day 28 4.39 4.80 Change from Baseline 2.28 1.41 50 mg Baseline 2.12 2.08 Day 28 5.23 5.24 Change from Baseline 3.14 3.17
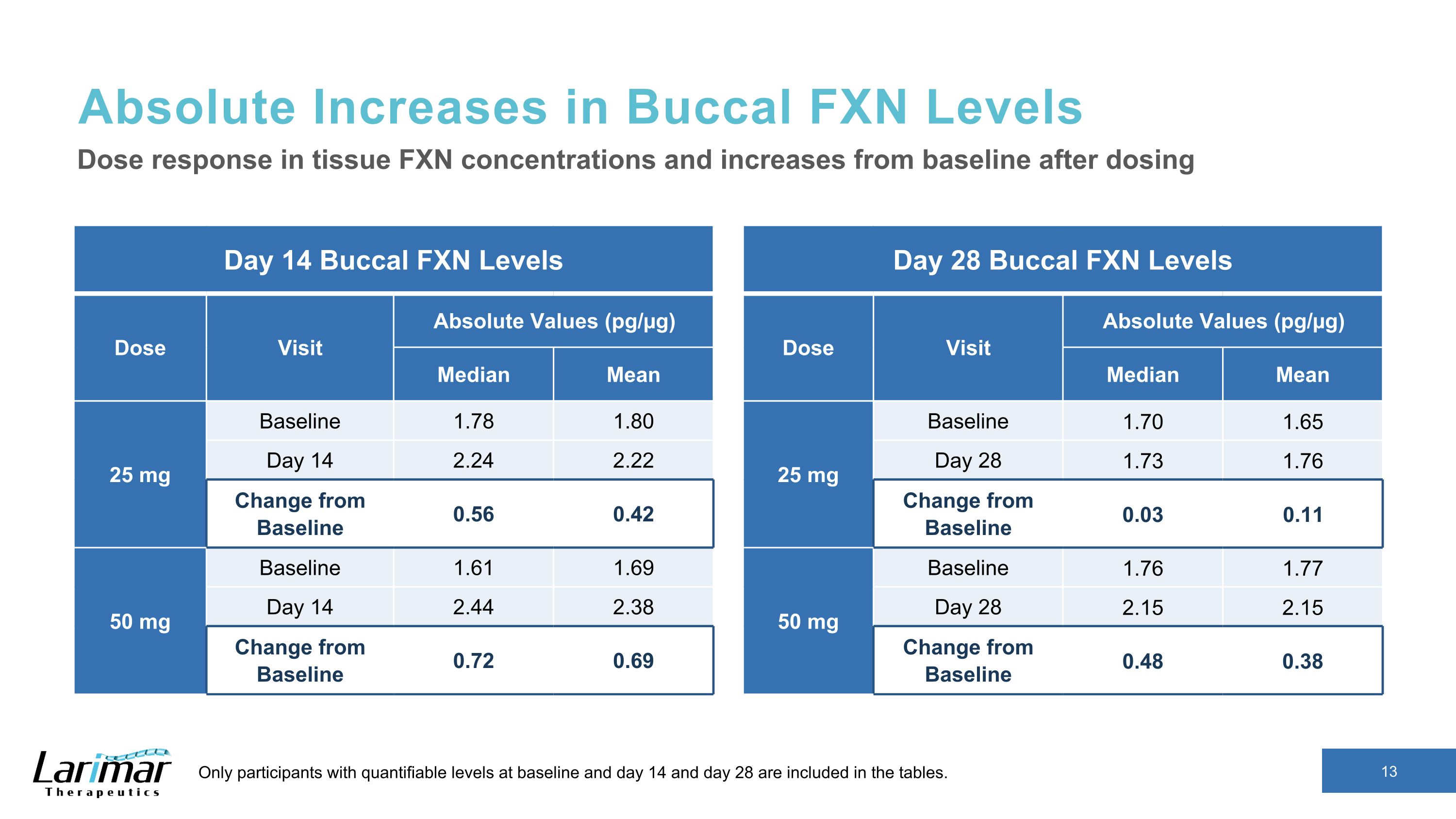
Absolute Increases in Buccal FXN Levels Day 14 Buccal FXN Levels Dose Visit Absolute Values (pg/µg) Median Mean 25 mg Baseline 1.78 1.80 Day 14 2.24 2.22 Change from Baseline 0.56 0.42 50 mg Baseline 1.61 1.69 Day 14 2.44 2.38 Change from Baseline 0.72 0.69 Dose response in tissue FXN concentrations and increases from baseline after dosing Only participants with quantifiable levels at baseline and day 14 and day 28 are included in the tables. Day 28 Buccal FXN Levels Dose Visit Absolute Values (pg/µg) Median Mean 25 mg Baseline 1.70 1.65 Day 28 1.73 1.76 Change from Baseline 0.03 0.11 50 mg Baseline 1.76 1.77 Day 28 2.15 2.15 Change from Baseline 0.48 0.38
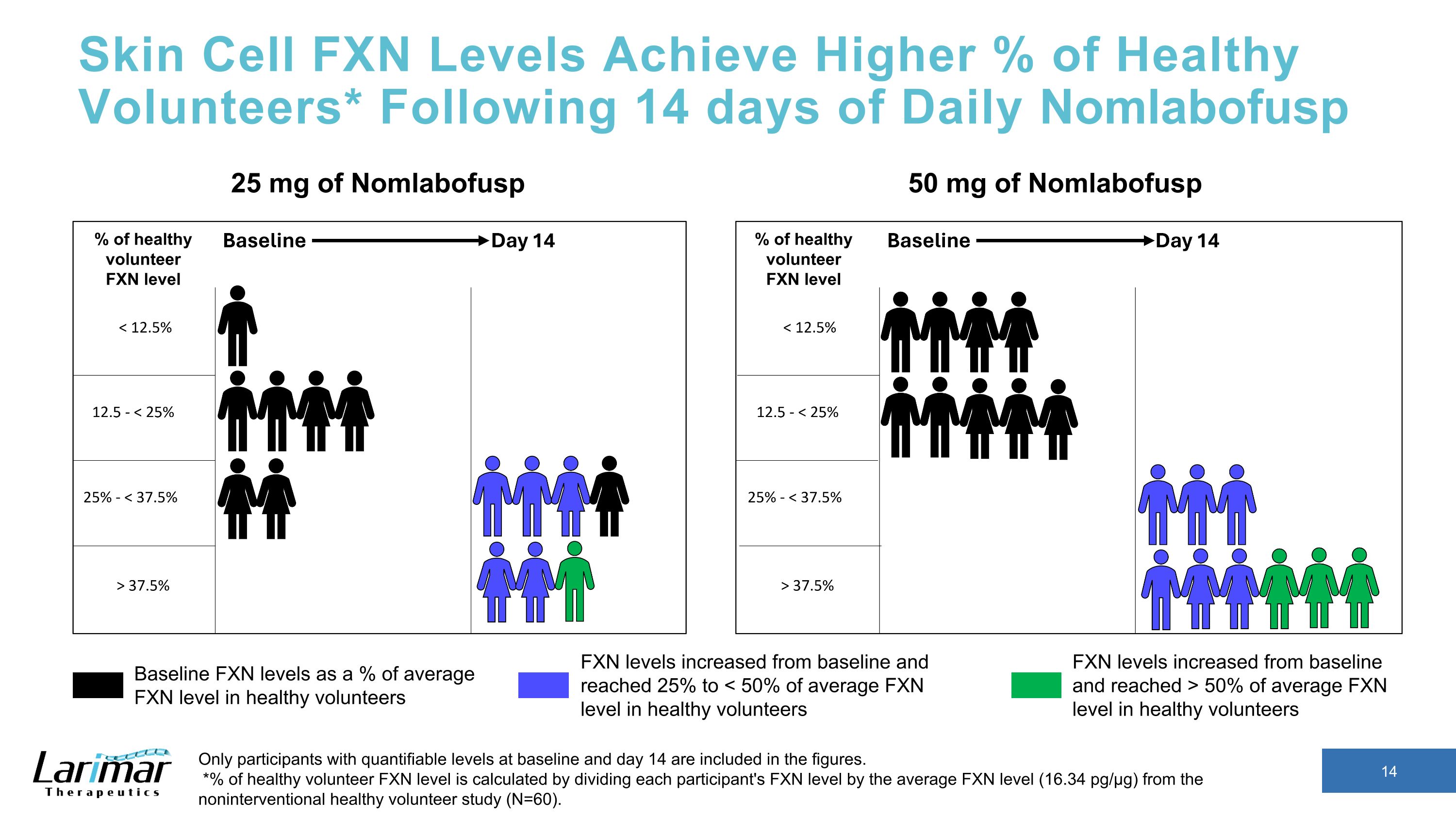
Skin Cell FXN Levels Achieve Higher % of Healthy Volunteers* Following 14 days of Daily Nomlabofusp Only participants with quantifiable levels at baseline and day 14 are included in the figures. *% of healthy volunteer FXN level is calculated by dividing each participant's FXN level by the average FXN level (16.34 pg/µg) from the noninterventional healthy volunteer study (N=60). 25 mg of Nomlabofusp 50 mg of Nomlabofusp Baseline FXN levels as a % of average FXN level in healthy volunteers FXN levels increased from baseline and reached > 50% of average FXN level in healthy volunteers FXN levels increased from baseline and reached 25% to < 50% of average FXN level in healthy volunteers % of healthy volunteer FXN level % of healthy volunteer FXN level
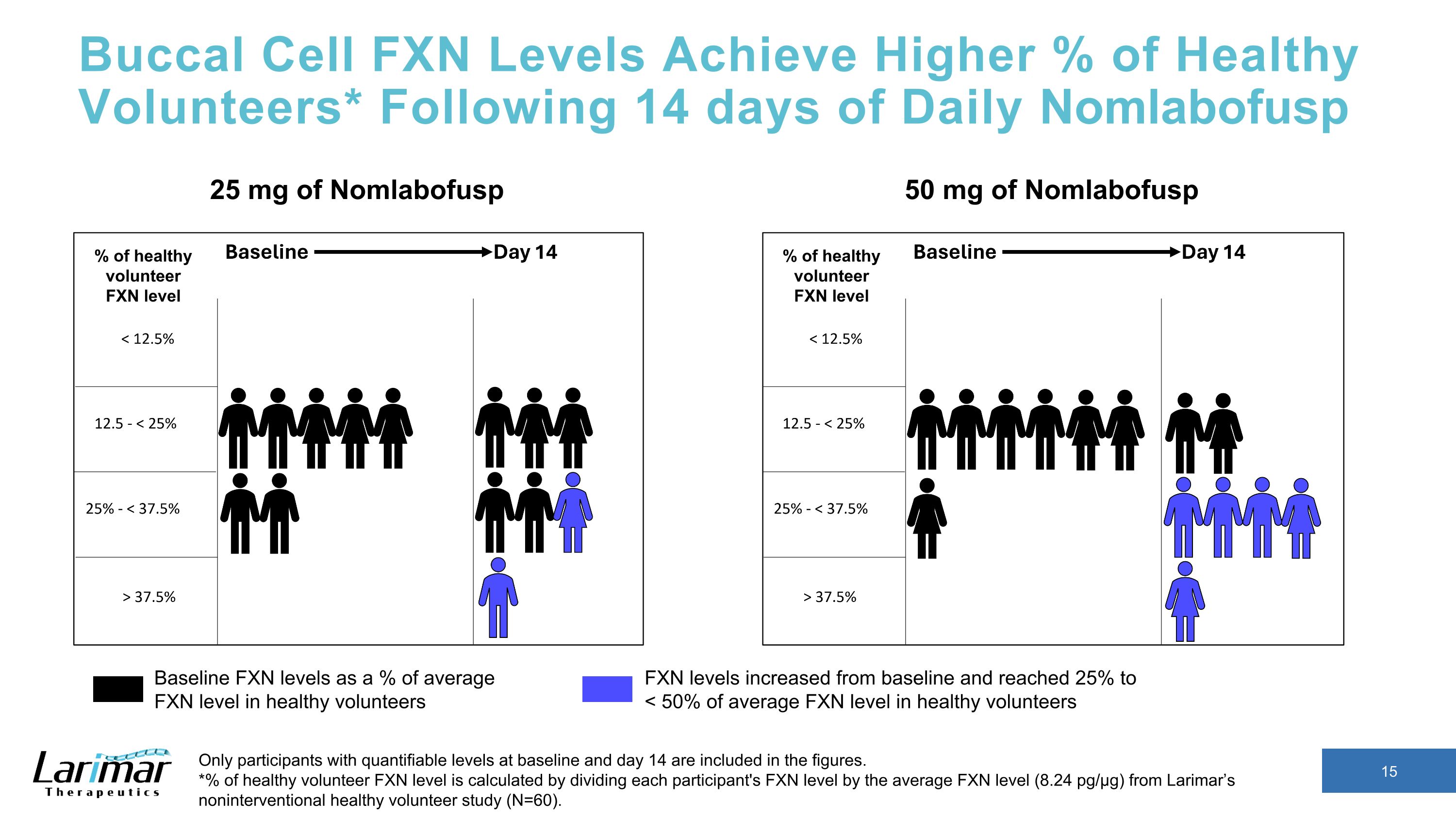
Buccal Cell FXN Levels Achieve Higher % of Healthy Volunteers* Following 14 days of Daily Nomlabofusp Only participants with quantifiable levels at baseline and day 14 are included in the figures. *% of healthy volunteer FXN level is calculated by dividing each participant's FXN level by the average FXN level (8.24 pg/µg) from Larimar’s noninterventional healthy volunteer study (N=60). 50 mg of Nomlabofusp Baseline FXN levels as a % of average FXN level in healthy volunteers FXN levels increased from baseline and reached 25% to �< 50% of average FXN level in healthy volunteers 25 mg of Nomlabofusp % of healthy volunteer FXN level % of healthy volunteer FXN level
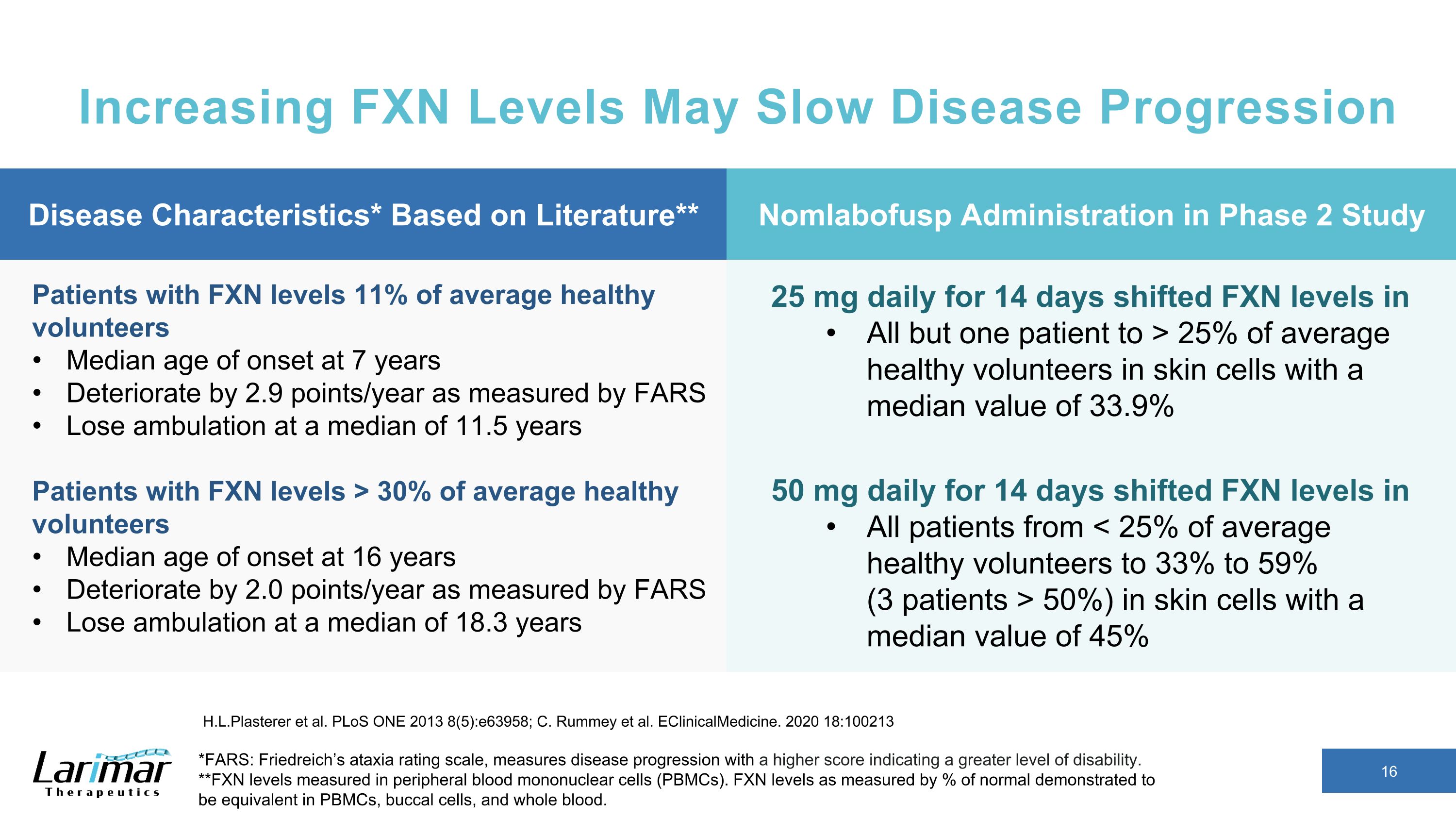
Increasing FXN Levels May Slow Disease Progression *FARS: Friedreich’s ataxia rating scale, measures disease progression with a higher score indicating a greater level of disability. **FXN levels measured in peripheral blood mononuclear cells (PBMCs). FXN levels as measured by % of normal demonstrated to be equivalent in PBMCs, buccal cells, and whole blood. H.L.Plasterer et al. PLoS ONE 2013 8(5):e63958; C. Rummey et al. EClinicalMedicine. 2020 18:100213 Patients with FXN levels 11% of average healthy volunteers Median age of onset at 7 years Deteriorate by 2.9 points/year as measured by FARS Lose ambulation at a median of 11.5 years Patients with FXN levels > 30% of average healthy volunteers Median age of onset at 16 years Deteriorate by 2.0 points/year as measured by FARS Lose ambulation at a median of 18.3 years 25 mg daily for 14 days shifted FXN levels in All but one patient to > 25% of average healthy volunteers in skin cells with a median value of 33.9% 50 mg daily for 14 days shifted FXN levels in All patients from < 25% of average healthy volunteers to 33% to 59% �(3 patients > 50%) in skin cells with a median value of 45% Disease Characteristics* Based on Literature** Nomlabofusp Administration in Phase 2 Study
Nomlabofusp: Predictable Pharmacokinetics Quick absorption after subcutaneous administration 1 2 3 Dose-proportional increases in exposure observed cc Pharmacokinetic profile consistent with Phase 1 studies
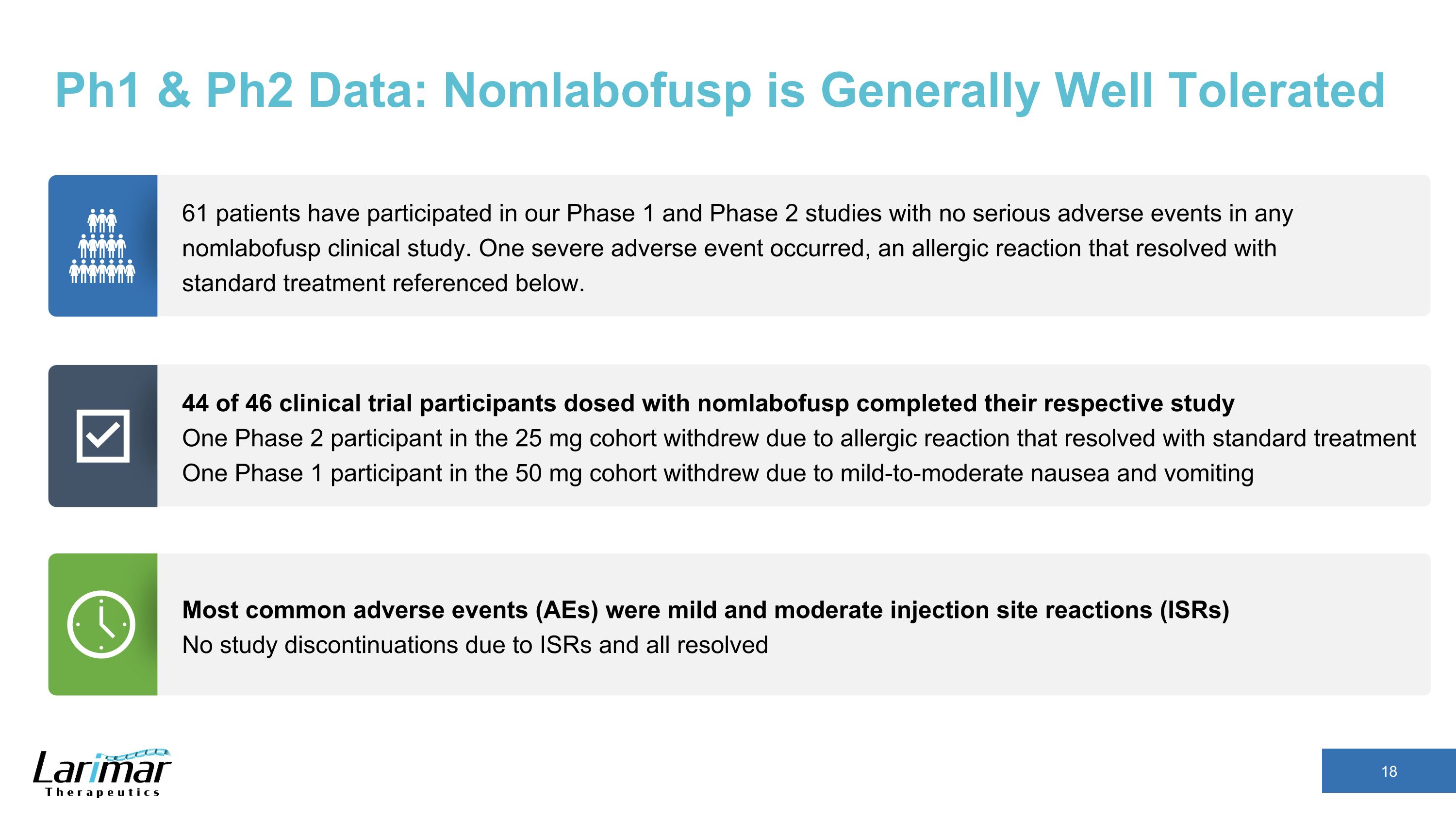
Ph1 & Ph2 Data: Nomlabofusp is Generally Well Tolerated 44 of 46 clinical trial participants dosed with nomlabofusp completed their respective study One Phase 2 participant in the 25 mg cohort withdrew due to allergic reaction that resolved with standard treatment One Phase 1 participant in the 50 mg cohort withdrew due to mild-to-moderate nausea and vomiting 61 patients have participated in our Phase 1 and Phase 2 studies with no serious adverse events in any nomlabofusp clinical study. One severe adverse event occurred, an allergic reaction that resolved with standard treatment referenced below. Most common adverse events (AEs) were mild and moderate injection site reactions (ISRs) No study discontinuations due to ISRs and all resolved
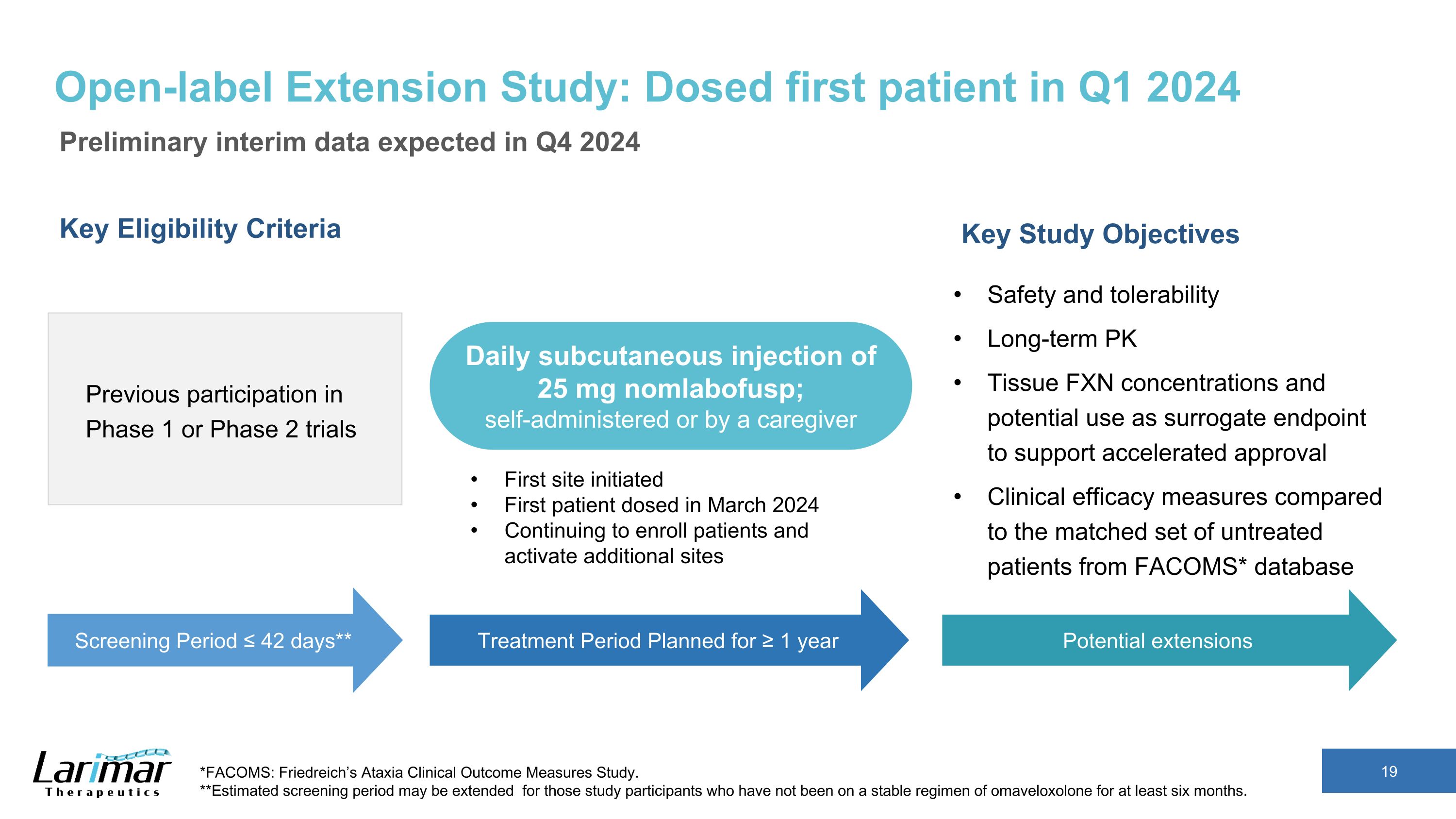
Open-label Extension Study: Dosed first patient in Q1 2024 Preliminary interim data expected in Q4 2024 Key Eligibility Criteria Previous participation in Phase 1 or Phase 2 trials Key Study Objectives Safety and tolerability Long-term PK Tissue FXN concentrations and potential use as surrogate endpoint to support accelerated approval Clinical efficacy measures compared to the matched set of untreated patients from FACOMS* database *FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study. **Estimated screening period may be extended for those study participants who have not been on a stable regimen of omaveloxolone for at least six months. Screening Period ≤ 42 days** Treatment Period Planned for ≥ 1 year Daily subcutaneous injection of 25 mg nomlabofusp; self-administered or by a caregiver First site initiated First patient dosed in March 2024 Continuing to enroll patients and activate additional sites Potential extensions
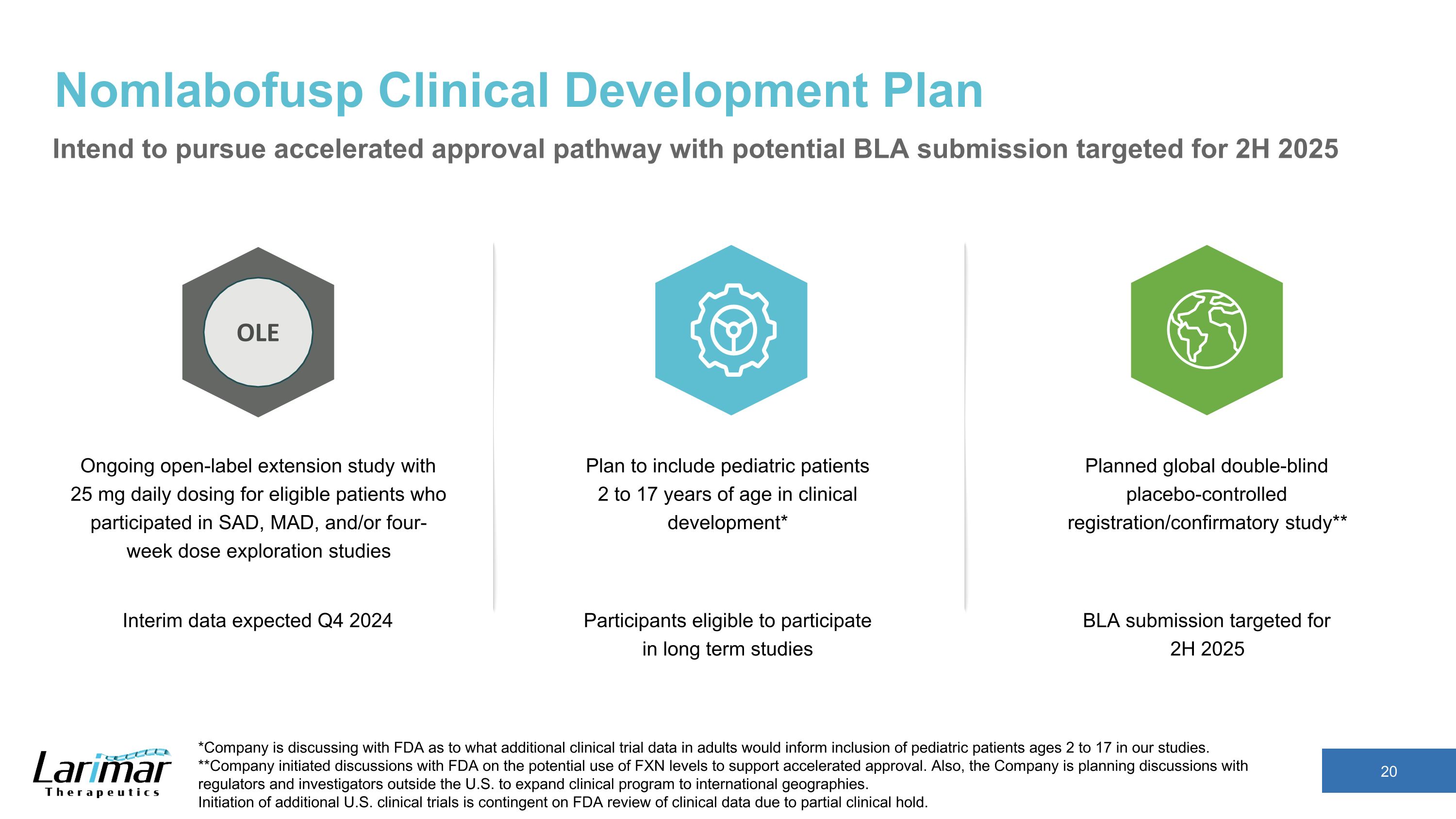
Nomlabofusp Clinical Development Plan Planned global double-blind placebo-controlled registration/confirmatory study** Plan to include pediatric patients 2 to 17 years of age in clinical development* Intend to pursue accelerated approval pathway with potential BLA submission targeted for 2H 2025 *Company is discussing with FDA as to what additional clinical trial data in adults would inform inclusion of pediatric patients ages 2 to 17 in our studies. **Company initiated discussions with FDA on the potential use of FXN levels to support accelerated approval. Also, the Company is planning discussions with regulators and investigators outside the U.S. to expand clinical program to international geographies. Initiation of additional U.S. clinical trials is contingent on FDA review of clinical data due to partial clinical hold. Ongoing open-label extension study with 25 mg daily dosing for eligible patients who participated in SAD, MAD, and/or four-week dose exploration studies OLE BLA submission targeted for �2H 2025 Participants eligible to participate in long term studies Interim data expected Q4 2024
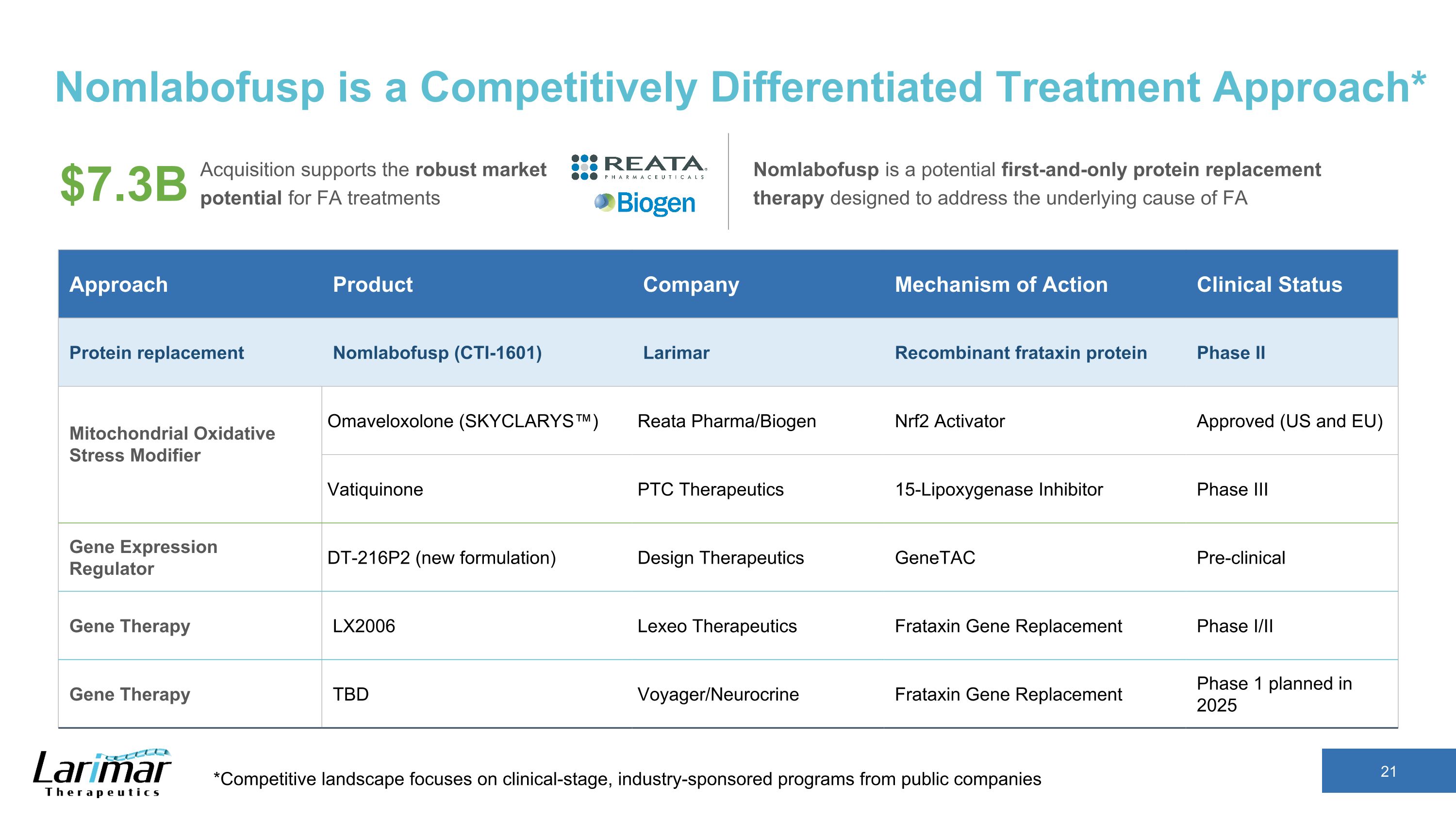
Nomlabofusp is a Competitively Differentiated Treatment Approach* *Competitive landscape focuses on clinical-stage, industry-sponsored programs from public companies Acquisition supports the robust market potential for FA treatments Nomlabofusp is a potential first-and-only protein replacement therapy designed to address the underlying cause of FA $7.3B Approach Product Company Mechanism of Action Clinical Status Protein replacement Nomlabofusp (CTI-1601) Larimar Recombinant frataxin protein Phase II Mitochondrial Oxidative Stress Modifier Omaveloxolone (SKYCLARYS™) Reata Pharma/Biogen Nrf2 Activator Approved (US and EU) Vatiquinone PTC Therapeutics 15-Lipoxygenase Inhibitor Phase III Gene Expression Regulator DT-216P2 (new formulation) Design Therapeutics GeneTAC Pre-clinical Gene Therapy LX2006 Lexeo Therapeutics Frataxin Gene Replacement Phase I/II Gene Therapy TBD Voyager/Neurocrine Frataxin Gene Replacement Phase 1 planned in 2025
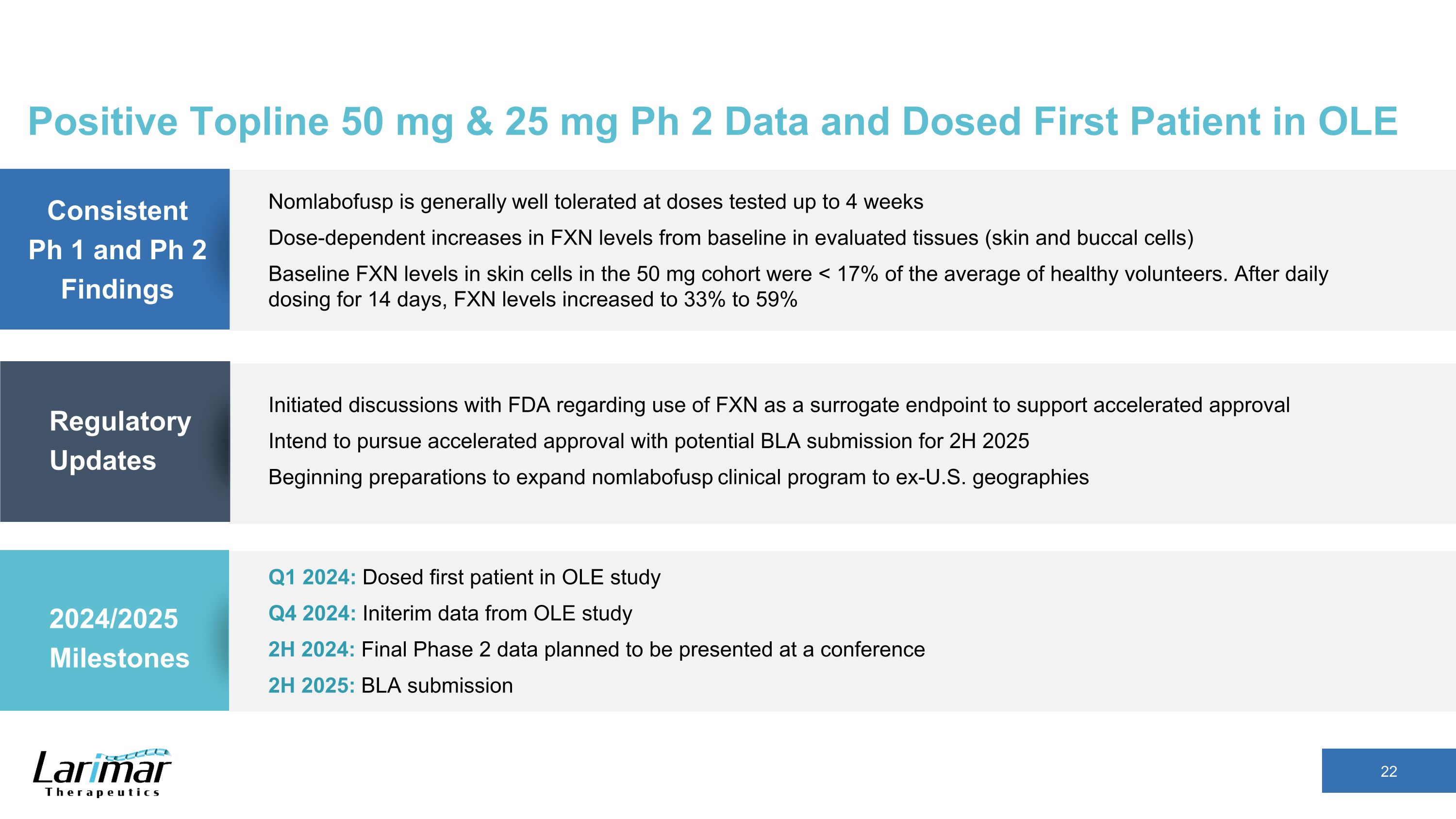
Regulatory Updates Q1 2024: Dosed first patient in OLE study Q4 2024: Initerim data from OLE study 2H 2024: Final Phase 2 data planned to be presented at a conference 2H 2025: BLA submission 2024/2025 Milestones Consistent Ph 1 and Ph 2 Findings Initiated discussions with FDA regarding use of FXN as a surrogate endpoint to support accelerated approval Intend to pursue accelerated approval with potential BLA submission for 2H 2025 Beginning preparations to expand nomlabofusp clinical program to ex-U.S. geographies Nomlabofusp is generally well tolerated at doses tested up to 4 weeks Dose-dependent increases in FXN levels from baseline in evaluated tissues (skin and buccal cells) Baseline FXN levels in skin cells in the 50 mg cohort were < 17% of the average of healthy volunteers. After daily dosing for 14 days, FXN levels increased to 33% to 59% Positive Topline 50 mg & 25 mg Ph 2 Data and Dosed First Patient in OLE
Clinical-Stage Novel Protein Replacement Therapy Platform Lead candidate nomlabofusp (CTI-1601) is a recombinant fusion protein designed to directly address frataxin deficiency in patients with Friedreich's ataxia (FA) by delivering the protein to mitochondria. Granted Orphan Drug (US & EU), Rare Pediatric Disease (US), Fast Track (US), & PRIME (EU) designations Nomlabofusp was generally well tolerated and demonstrated dose-dependent increases in frataxin (FXN) levels from baseline in skin and buccal cells in a 4-week placebo-controlled Phase 2 study and a multiple ascending dose Phase 1 study Dosed first patient in OLE study with 25 mg daily dosing in Q1 2024 with interim data expected in Q4 2024 Continuing to enroll patients and activate additional sites Dose escalation in the OLE study is contingent on the FDA’s review of data from the 50 mg cohort of the Phase 2 study and available data from the OLE study due to continued partial clinical hold Approximately $239 million in cash and investments at 3/31/24 which includes $161.8 million in net proceeds raised from a Feb 24 public offering Provides projected cash runway into 2026 Potential first therapy to increase frataxin levels Consistent Phase 1 and Phase 2 findings Intend to pursue accelerated approval with FDA OLE study with near-term catalysts Strong financial foundation FDA acknowledgement that FXN deficiency appears to be critical to the pathogenic mechanism of FA, and that there continues to be an unmet need for treatments that address the underlying disease pathophysiology. Discussions to support an accelerated approval are ongoing. BLA submission targeted for 2H 2025

THANK YOU Larimar Therapeutics May 2024 Corporate Deck

Appendix Larimar Therapeutics

Scientific Advisory Board Co-founder of Chondrial Therapeutics, which became Larimar Therapeutics, Inc. Professor of Pediatrics at Indiana University School of Medicine Mark Payne, MD Executive Director of the Mitochondrial Medicine Frontier Program at The Children’s Hospital of Philadelphia (CHOP) Professor in the Division of Human Genetics, Department of Pediatrics at University of Pennsylvania Perelman School of Medicine Marni J. Falk, MD Medical Director and Division Chief of the University of California San Francisco (UCSF) Movement Disorders and Neuromodulation Center. Carlin and Ellen Wiegner Endowed Professor of Neurology Jill Ostrem, MD Giovanni Manfredi, MD, PhD Finbar and Marianne Kenny Professor in Clinical and Research Neurology at Weill Cornell Medicine. Professor of Neuroscience at Weill Cornell Medicine.

Company has strong relationship with Friedreich’s Ataxia Research Alliance (FARA) National, non-profit organization dedicated to the pursuit of scientific research leading to treatments and a cure for FA FARA provides industry with several key items Assistance with patient recruitment and education Access to Global Patient Registry with demographic and clinical information on more than 1,000 FA patients Sponsored a Patient-Focused Drug Development Meeting in 2017 resulting in a publication titled “The Voice of the Patient” Strong Relationship with FARA

Mitochondrial Localization and Preclinical Data
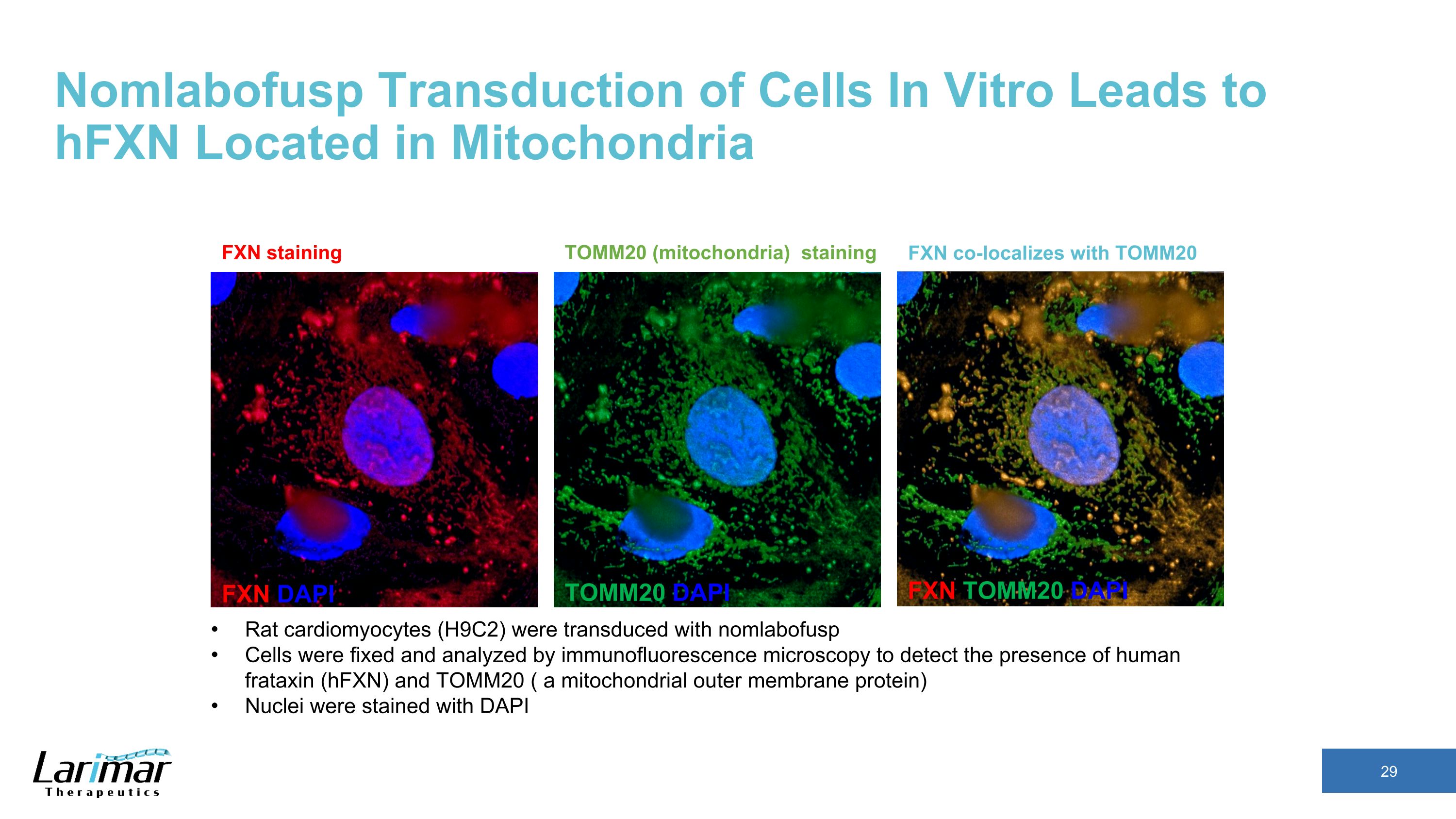
Nomlabofusp Transduction of Cells In Vitro Leads to �hFXN Located in Mitochondria FXN DAPI TOMM20 DAPI FXN TOMM20 DAPI FXN co-localizes with TOMM20 FXN staining TOMM20 (mitochondria) staining Rat cardiomyocytes (H9C2) were transduced with nomlabofusp Cells were fixed and analyzed by immunofluorescence microscopy to detect the presence of human frataxin (hFXN) and TOMM20 ( a mitochondrial outer membrane protein) Nuclei were stained with DAPI
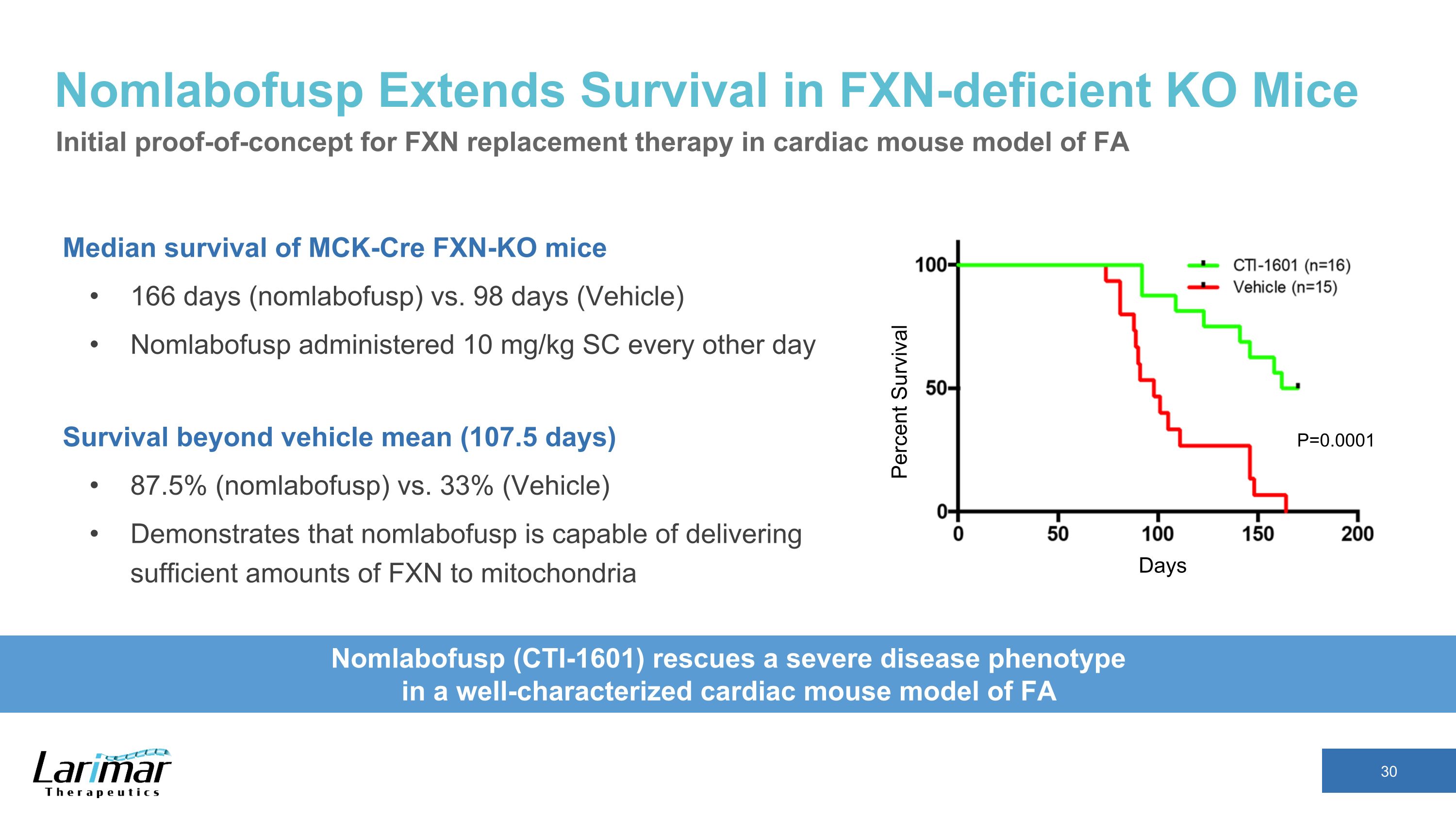
Nomlabofusp Extends Survival in FXN-deficient KO Mice Median survival of MCK-Cre FXN-KO mice 166 days (nomlabofusp) vs. 98 days (Vehicle) Nomlabofusp administered 10 mg/kg SC every other day Survival beyond vehicle mean (107.5 days) 87.5% (nomlabofusp) vs. 33% (Vehicle) Demonstrates that nomlabofusp is capable of delivering sufficient amounts of FXN to mitochondria Days Percent Survival Nomlabofusp (CTI-1601) rescues a severe disease phenotype �in a well-characterized cardiac mouse model of FA P=0.0001 Initial proof-of-concept for FXN replacement therapy in cardiac mouse model of FA

Nomlabofusp Prevents Development of �Ataxic Gait in Neurologic KO Mouse Model hFXN replacement with nomlabofusp prevents development of ataxic gait Nomlabofusp-treated mice survive longer than untreated mice Human frataxin present in brain, dorsal root ganglia and spinal cord demonstrating central nervous system penetration In-Vivo Efficacy Data in Pvalb-Cre FXN-KO Mouse Model Single dose level: 10 mg/kg nomlabofusp or vehicle given intraperitoneally three times per week
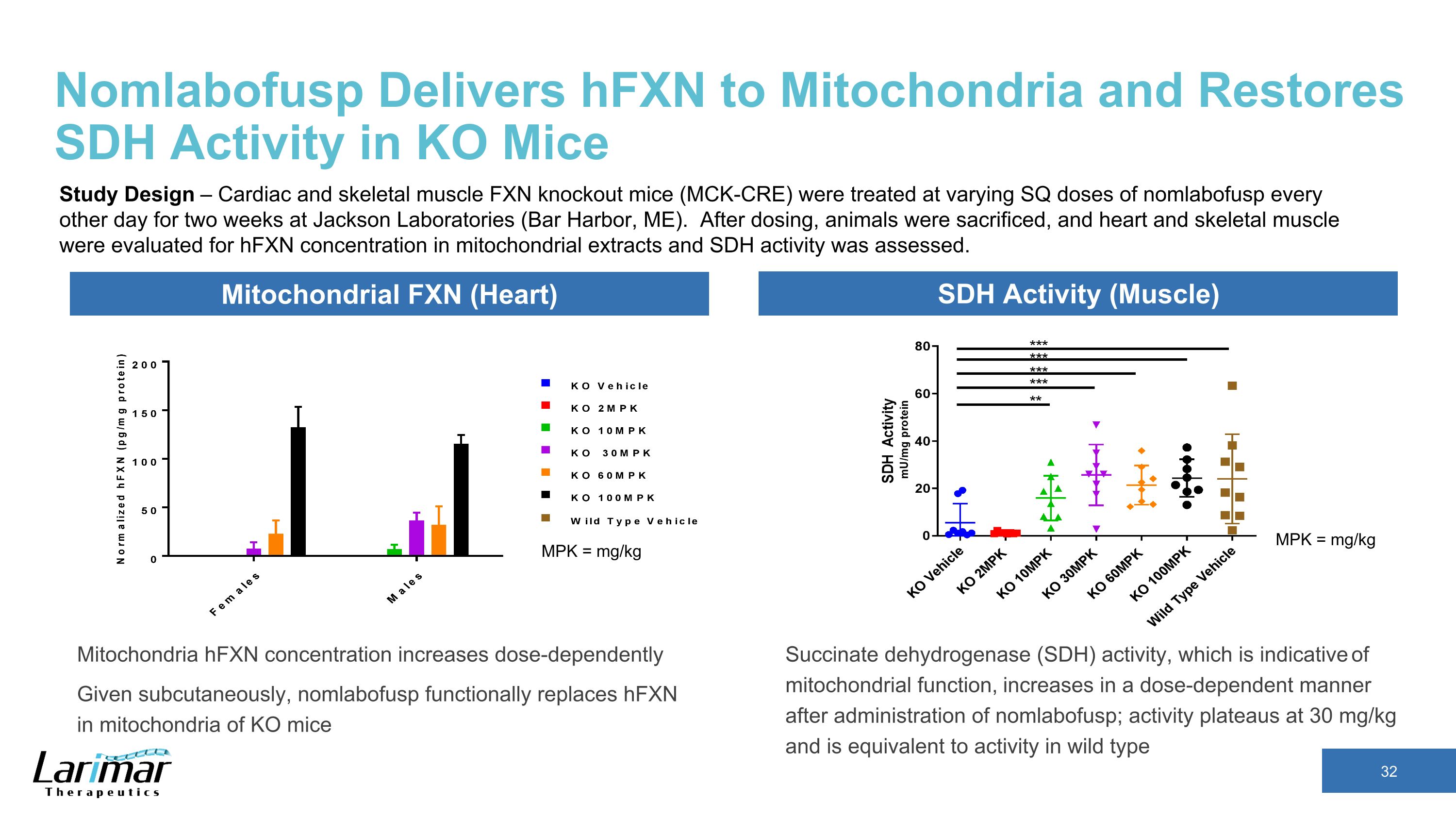
Nomlabofusp Delivers hFXN to Mitochondria and Restores SDH Activity in KO Mice Mitochondria hFXN concentration increases dose-dependently Given subcutaneously, nomlabofusp functionally replaces hFXN in mitochondria of KO mice MPK = mg/kg MPK = mg/kg Mitochondrial FXN (Heart) SDH Activity (Muscle) Succinate dehydrogenase (SDH) activity, which is indicative of mitochondrial function, increases in a dose-dependent manner after administration of nomlabofusp; activity plateaus at 30 mg/kg and is equivalent to activity in wild type Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at varying SQ doses of nomlabofusp every other day for two weeks at Jackson Laboratories (Bar Harbor, ME). After dosing, animals were sacrificed, and heart and skeletal muscle were evaluated for hFXN concentration in mitochondrial extracts and SDH activity was assessed.

Nomlabofusp Prevents Left Ventricle Dilation in KO Mice Left ventricular (LV) volume increases in systole in untreated mice by 8 weeks (after 4 weeks of dosing with vehicle), but remains similar to wildtype when treated with nomlabofusp (10 mg/kg every other day) Diameter (mm) Age in Weeks Age in Weeks Volume (μL) KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Left Ventricle Internal Diameter (Systole) Left Ventricle Volume (Systole) Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at 10 mg/kg every other day at Jackson Laboratories (Bar Harbor, ME). Echocardiograms were performed pre-dose and post dose. Nomlabofusp-treated mice have similar LV volume as wild type; echocardiogram shows significant differences between vehicle and nomlabofusp treated (10 mg/kg every other day) KO mice
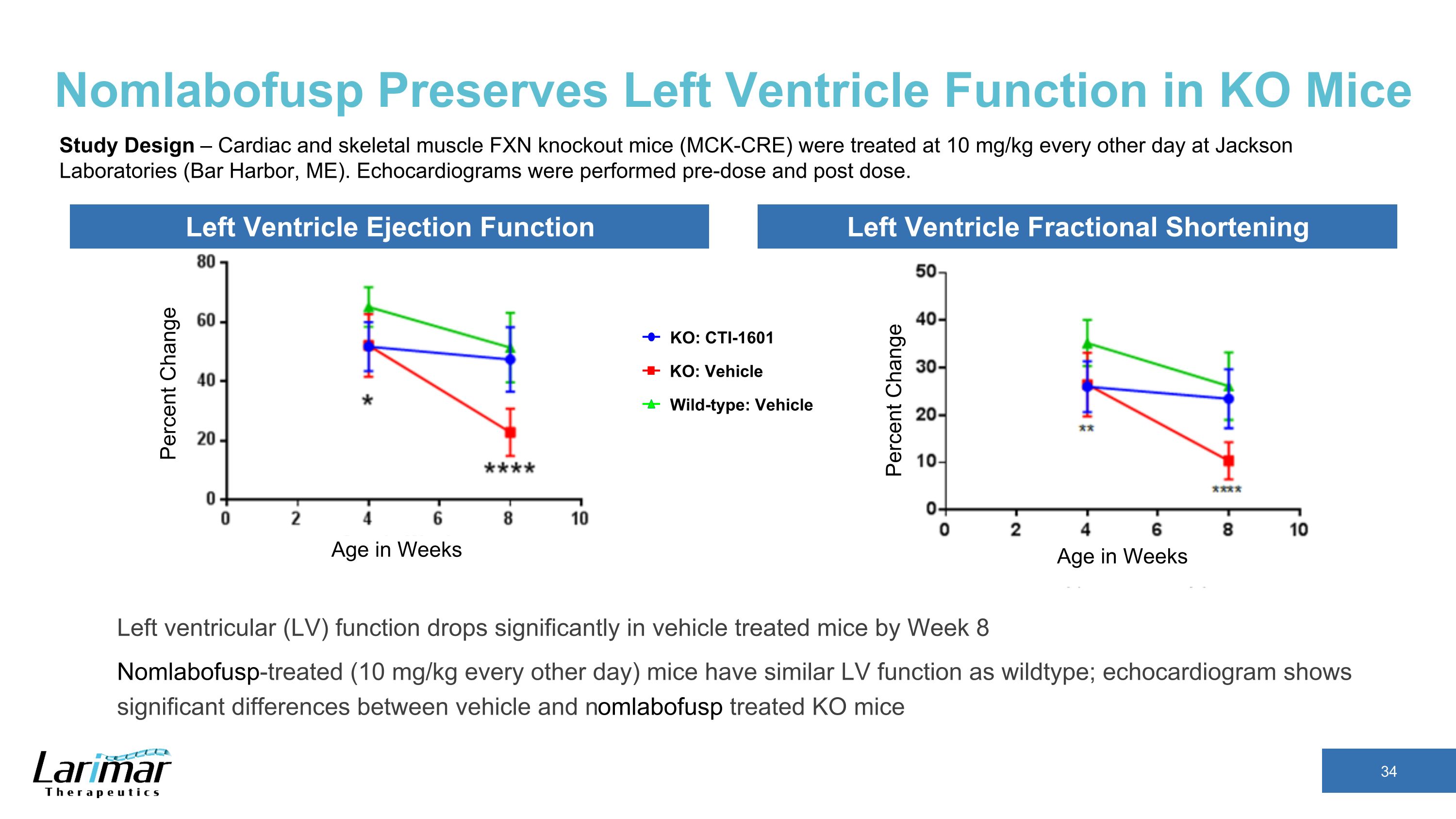
Nomlabofusp Preserves Left Ventricle Function in KO Mice Percent Change Age in Weeks Left Ventricle Ejection Function Left Ventricle Fractional Shortening Percent Change Age in Weeks KO: CTI-1601 Wild-type: Vehicle KO: Vehicle Study Design – Cardiac and skeletal muscle FXN knockout mice (MCK-CRE) were treated at 10 mg/kg every other day at Jackson Laboratories (Bar Harbor, ME). Echocardiograms were performed pre-dose and post dose. Left ventricular (LV) function drops significantly in vehicle treated mice by Week 8 Nomlabofusp-treated (10 mg/kg every other day) mice have similar LV function as wildtype; echocardiogram shows significant differences between vehicle and nomlabofusp treated KO mice

Phase 1 Clinical Data
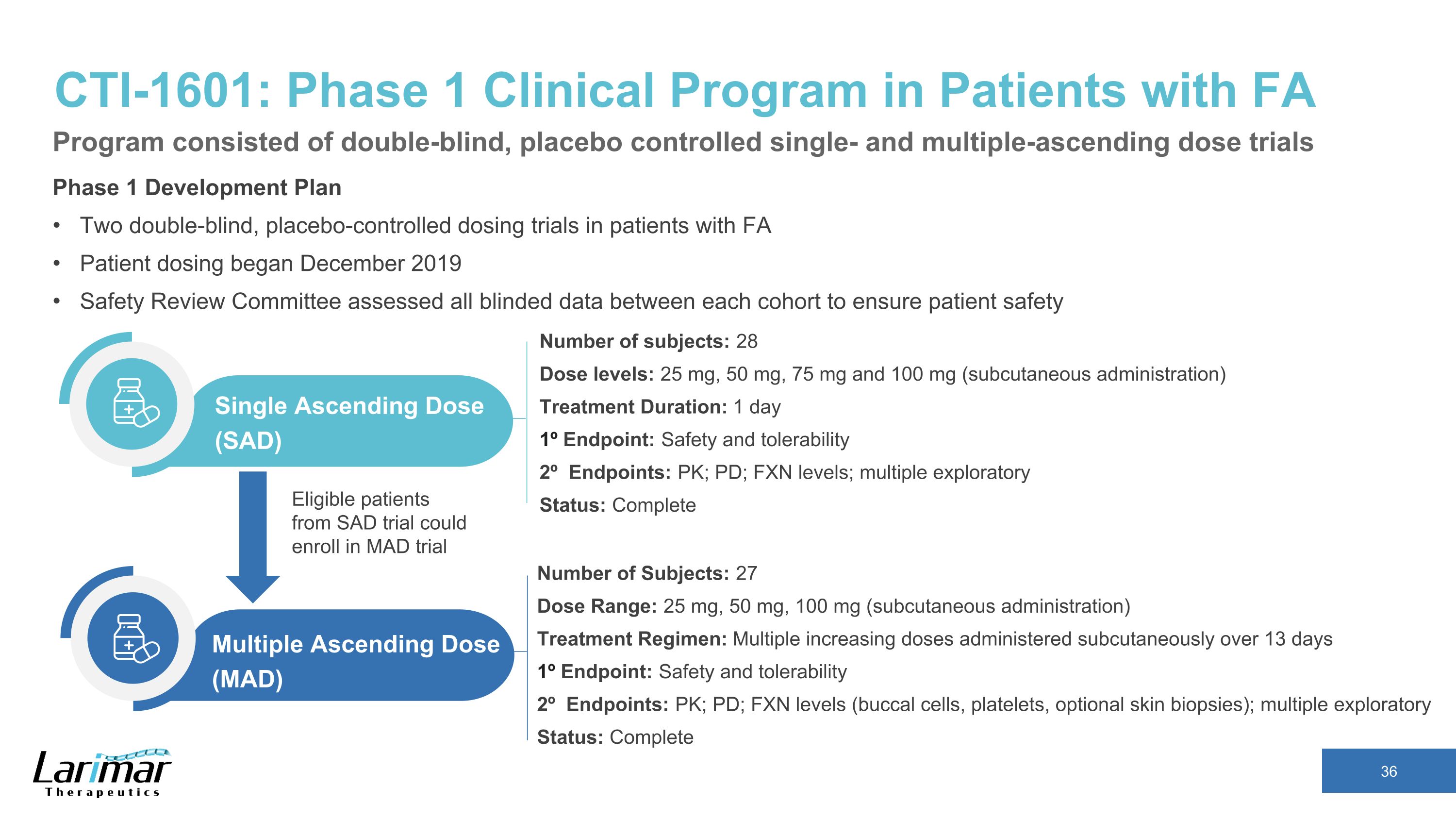
CTI-1601: Phase 1 Clinical Program in Patients with FA Phase 1 Development Plan Two double-blind, placebo-controlled dosing trials in patients with FA Patient dosing began December 2019 Safety Review Committee assessed all blinded data between each cohort to ensure patient safety Number of subjects: 28 Dose levels: 25 mg, 50 mg, 75 mg and 100 mg (subcutaneous administration) Treatment Duration: 1 day 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels; multiple exploratory Status: Complete Single Ascending Dose (SAD) Number of Subjects: 27 Dose Range: 25 mg, 50 mg, 100 mg (subcutaneous administration) Treatment Regimen: Multiple increasing doses administered subcutaneously over 13 days 1º Endpoint: Safety and tolerability 2º Endpoints: PK; PD; FXN levels (buccal cells, platelets, optional skin biopsies); multiple exploratory Status: Complete Multiple Ascending Dose (MAD) Eligible patients from SAD trial could enroll in MAD trial Program consisted of double-blind, placebo controlled single- and multiple-ascending dose trials
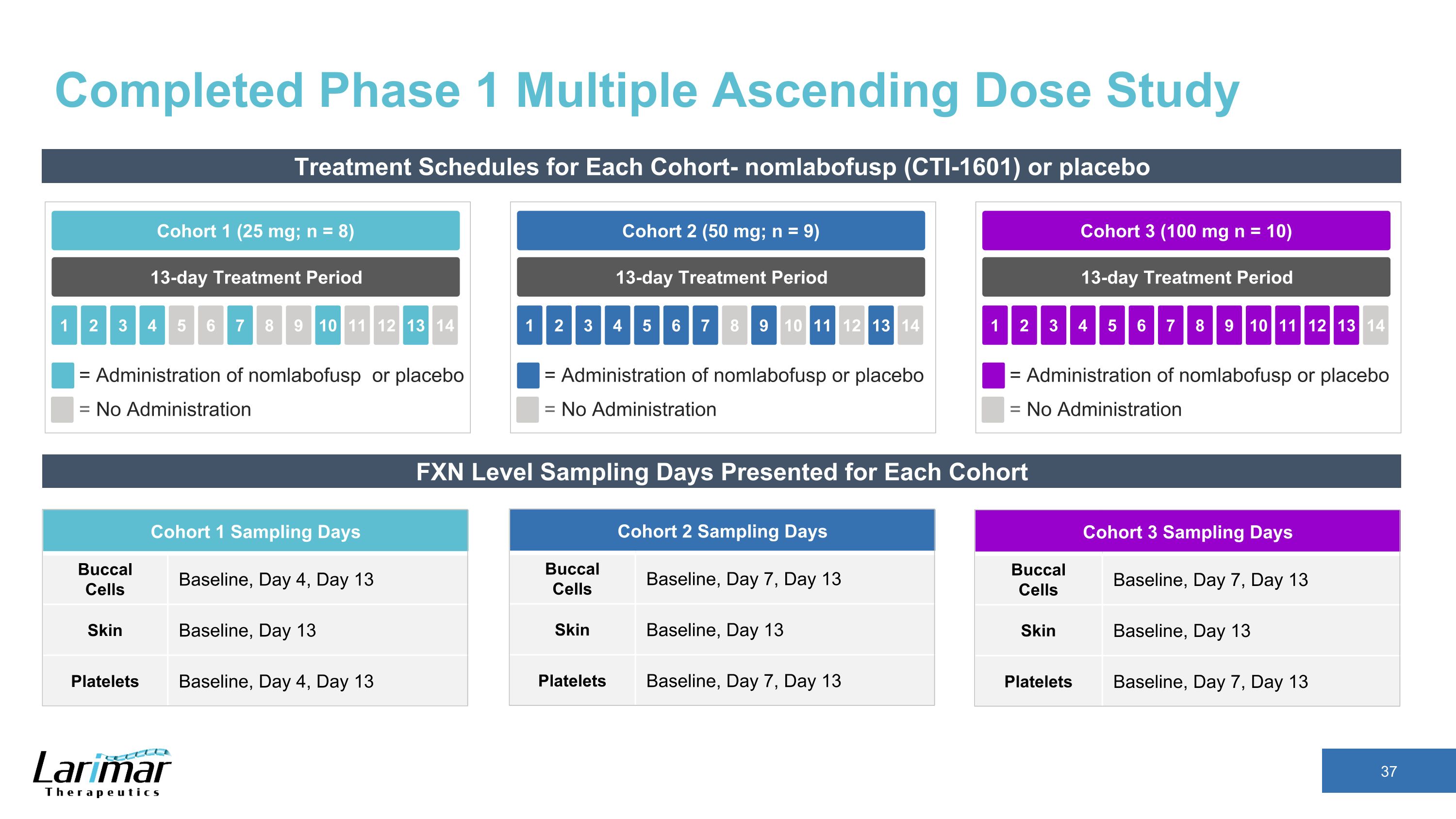
Completed Phase 1 Multiple Ascending Dose Study Treatment Schedules for Each Cohort- nomlabofusp (CTI-1601) or placebo 13-day Treatment Period Cohort 2 (50 mg; n = 9) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration 13-day Treatment Period Cohort 1 (25 mg; n = 8) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration 13-day Treatment Period Cohort 3 (100 mg n = 10) 2 3 4 5 1 6 7 8 9 10 11 12 13 14 = Administration of nomlabofusp or placebo = No Administration FXN Level Sampling Days Presented for Each Cohort Cohort 1 Sampling Days Buccal Cells Baseline, Day 4, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 4, Day 13 Cohort 2 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13 Cohort 3 Sampling Days Buccal Cells Baseline, Day 7, Day 13 Skin Baseline, Day 13 Platelets Baseline, Day 7, Day 13
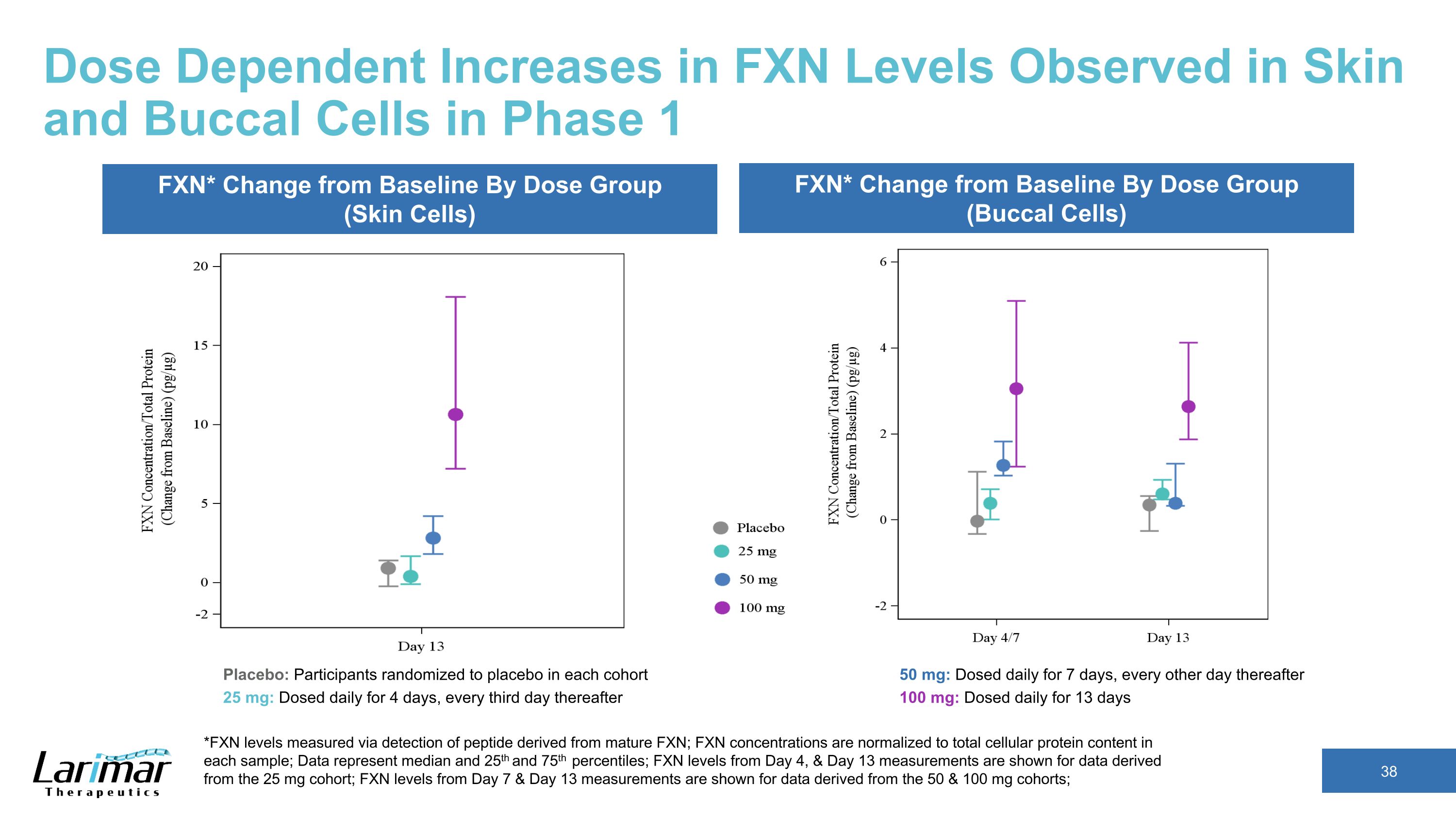
Dose Dependent Increases in FXN Levels Observed in Skin and Buccal Cells in Phase 1 *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample; Data represent median and 25th and 75th percentiles; FXN levels from Day 4, & Day 13 measurements are shown for data derived from the 25 mg cohort; FXN levels from Day 7 & Day 13 measurements are shown for data derived from the 50 & 100 mg cohorts; FXN* Change from Baseline By Dose Group (Skin Cells) FXN* Change from Baseline By Dose Group (Buccal Cells) Placebo: Participants randomized to placebo in each cohort 25 mg: Dosed daily for 4 days, every third day thereafter 50 mg: Dosed daily for 7 days, every other day thereafter 100 mg: Dosed daily for 13 days
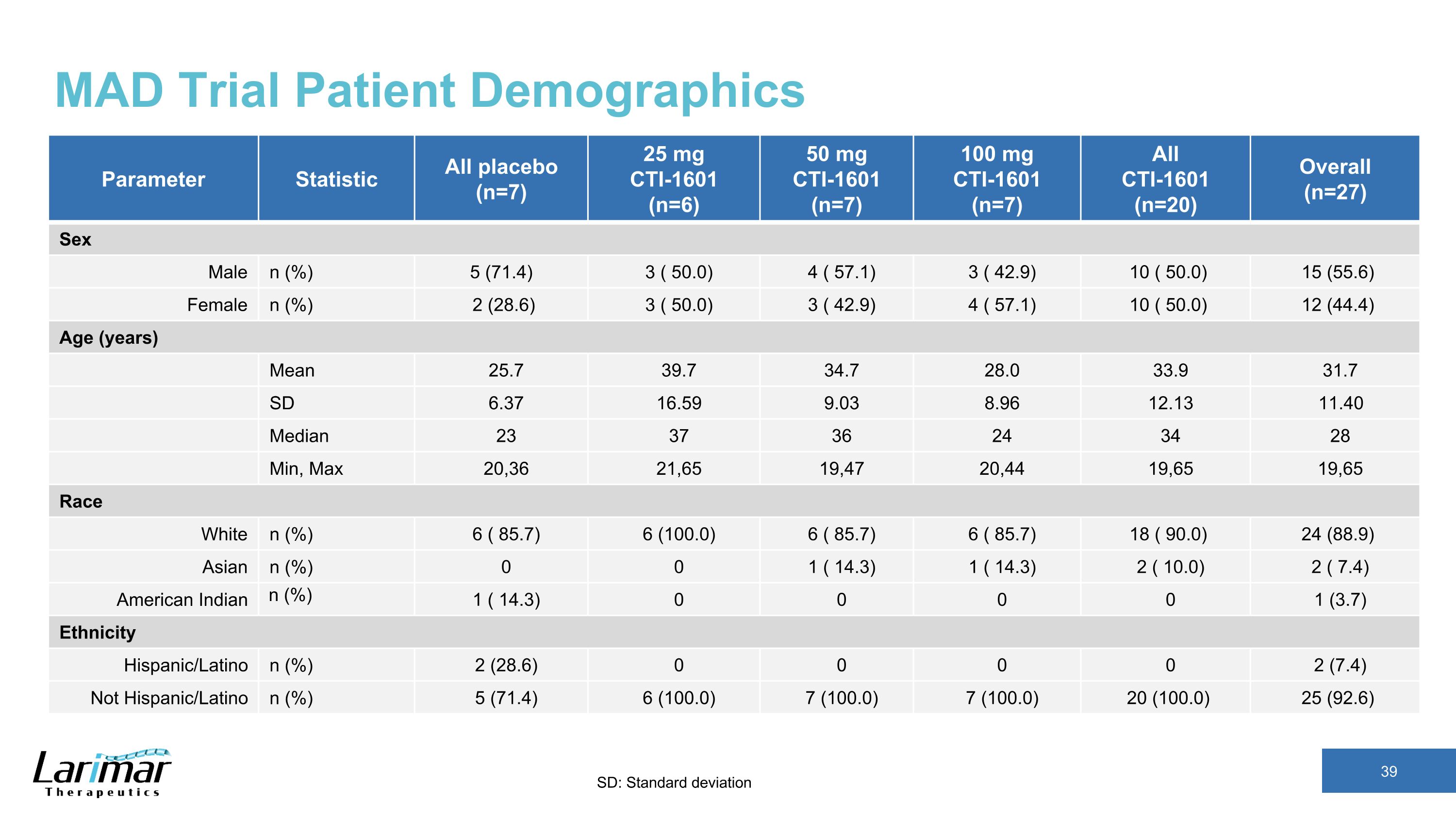
MAD Trial Patient Demographics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Sex Male n (%) 5 (71.4) 3 ( 50.0) 4 ( 57.1) 3 ( 42.9) 10 ( 50.0) 15 (55.6) Female n (%) 2 (28.6) 3 ( 50.0) 3 ( 42.9) 4 ( 57.1) 10 ( 50.0) 12 (44.4) Age (years) Mean 25.7 39.7 34.7 28.0 33.9 31.7 SD 6.37 16.59 9.03 8.96 12.13 11.40 Median 23 37 36 24 34 28 Min, Max 20,36 21,65 19,47 20,44 19,65 19,65 Race White n (%) 6 ( 85.7) 6 (100.0) 6 ( 85.7) 6 ( 85.7) 18 ( 90.0) 24 (88.9) Asian n (%) 0 0 1 ( 14.3) 1 ( 14.3) 2 ( 10.0) 2 ( 7.4) American Indian n (%) 1 ( 14.3) 0 0 0 0 1 (3.7) Ethnicity Hispanic/Latino n (%) 2 (28.6) 0 0 0 0 2 (7.4) Not Hispanic/Latino n (%) 5 (71.4) 6 (100.0) 7 (100.0) 7 (100.0) 20 (100.0) 25 (92.6) SD: Standard deviation
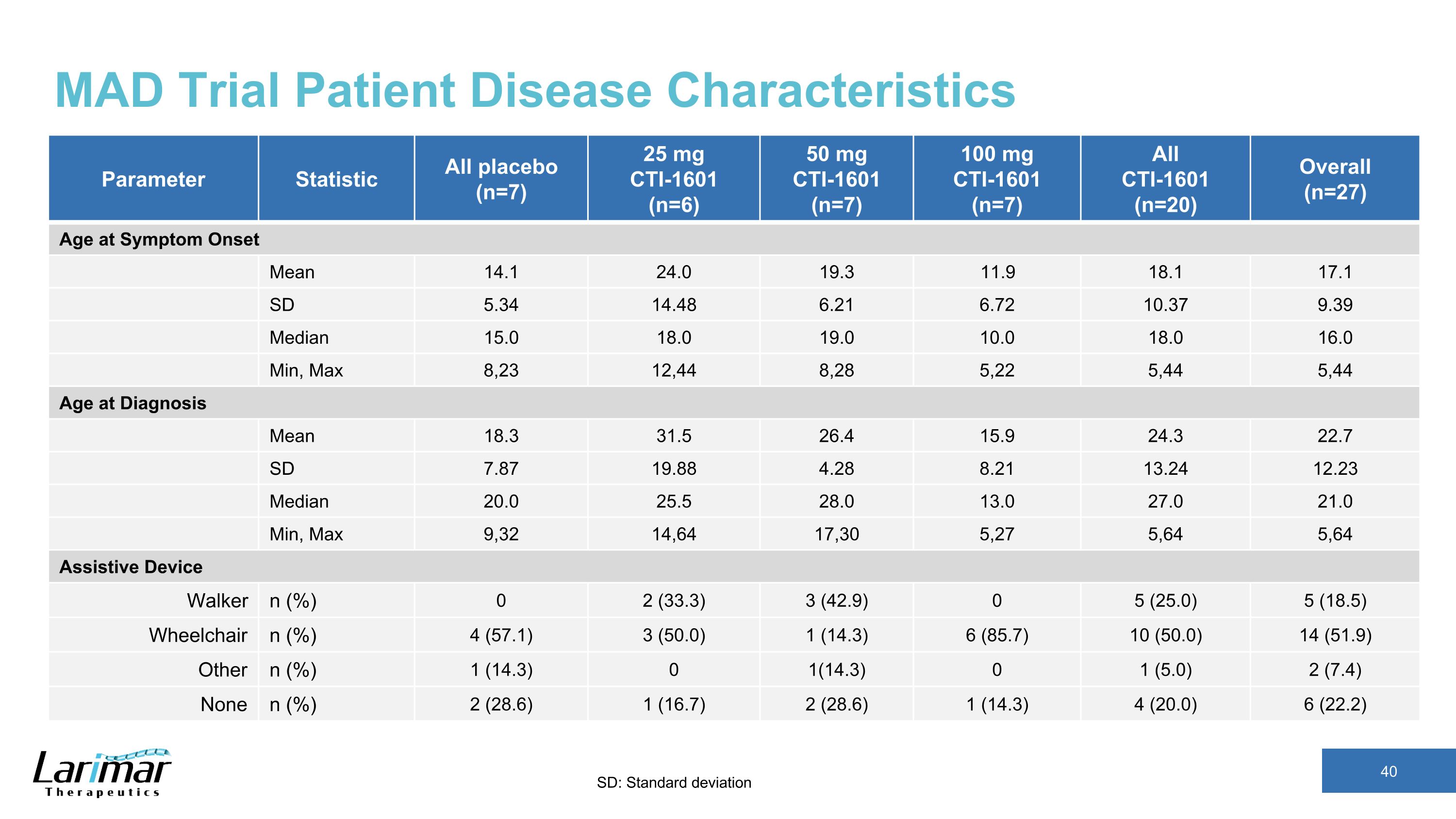
MAD Trial Patient Disease Characteristics Parameter Statistic All placebo (n=7) 25 mg CTI-1601 (n=6) 50 mg CTI-1601 (n=7) 100 mg CTI-1601 (n=7) All CTI-1601 (n=20) Overall (n=27) Age at Symptom Onset Mean 14.1 24.0 19.3 11.9 18.1 17.1 SD 5.34 14.48 6.21 6.72 10.37 9.39 Median 15.0 18.0 19.0 10.0 18.0 16.0 Min, Max 8,23 12,44 8,28 5,22 5,44 5,44 Age at Diagnosis Mean 18.3 31.5 26.4 15.9 24.3 22.7 SD 7.87 19.88 4.28 8.21 13.24 12.23 Median 20.0 25.5 28.0 13.0 27.0 21.0 Min, Max 9,32 14,64 17,30 5,27 5,64 5,64 Assistive Device Walker n (%) 0 2 (33.3) 3 (42.9) 0 5 (25.0) 5 (18.5) Wheelchair n (%) 4 (57.1) 3 (50.0) 1 (14.3) 6 (85.7) 10 (50.0) 14 (51.9) Other n (%) 1 (14.3) 0 1(14.3) 0 1 (5.0) 2 (7.4) None n (%) 2 (28.6) 1 (16.7) 2 (28.6) 1 (14.3) 4 (20.0) 6 (22.2) SD: Standard deviation
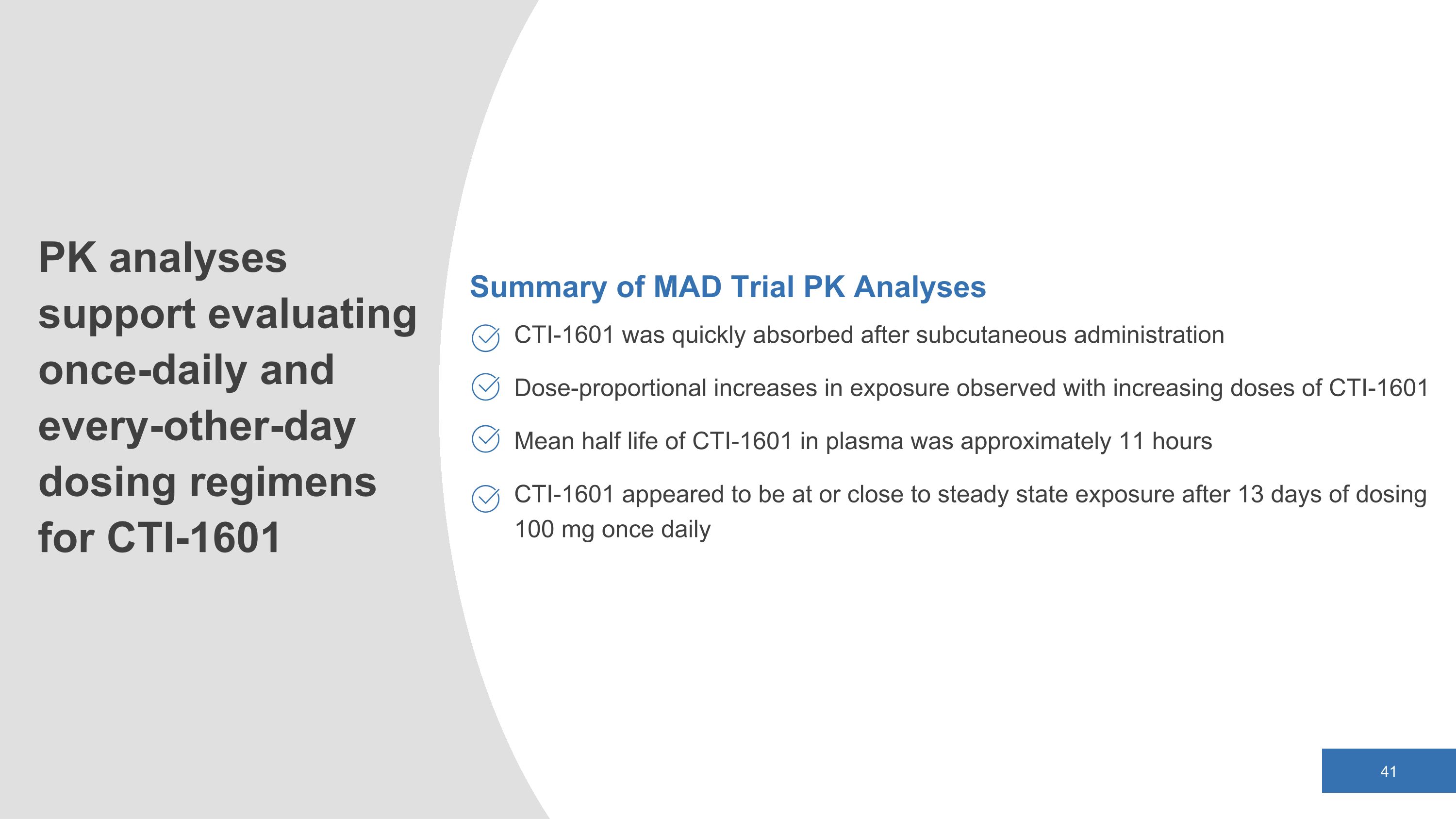
Summary of MAD Trial PK Analyses CTI-1601 was quickly absorbed after subcutaneous administration Dose-proportional increases in exposure observed with increasing doses of CTI-1601 Mean half life of CTI-1601 in plasma was approximately 11 hours CTI-1601 appeared to be at or close to steady state exposure after 13 days of dosing 100 mg once daily PK analyses support evaluating once-daily and every-other-day dosing regimens for CTI-1601

Phase 2 Demographic/ Disease Characteristics Data
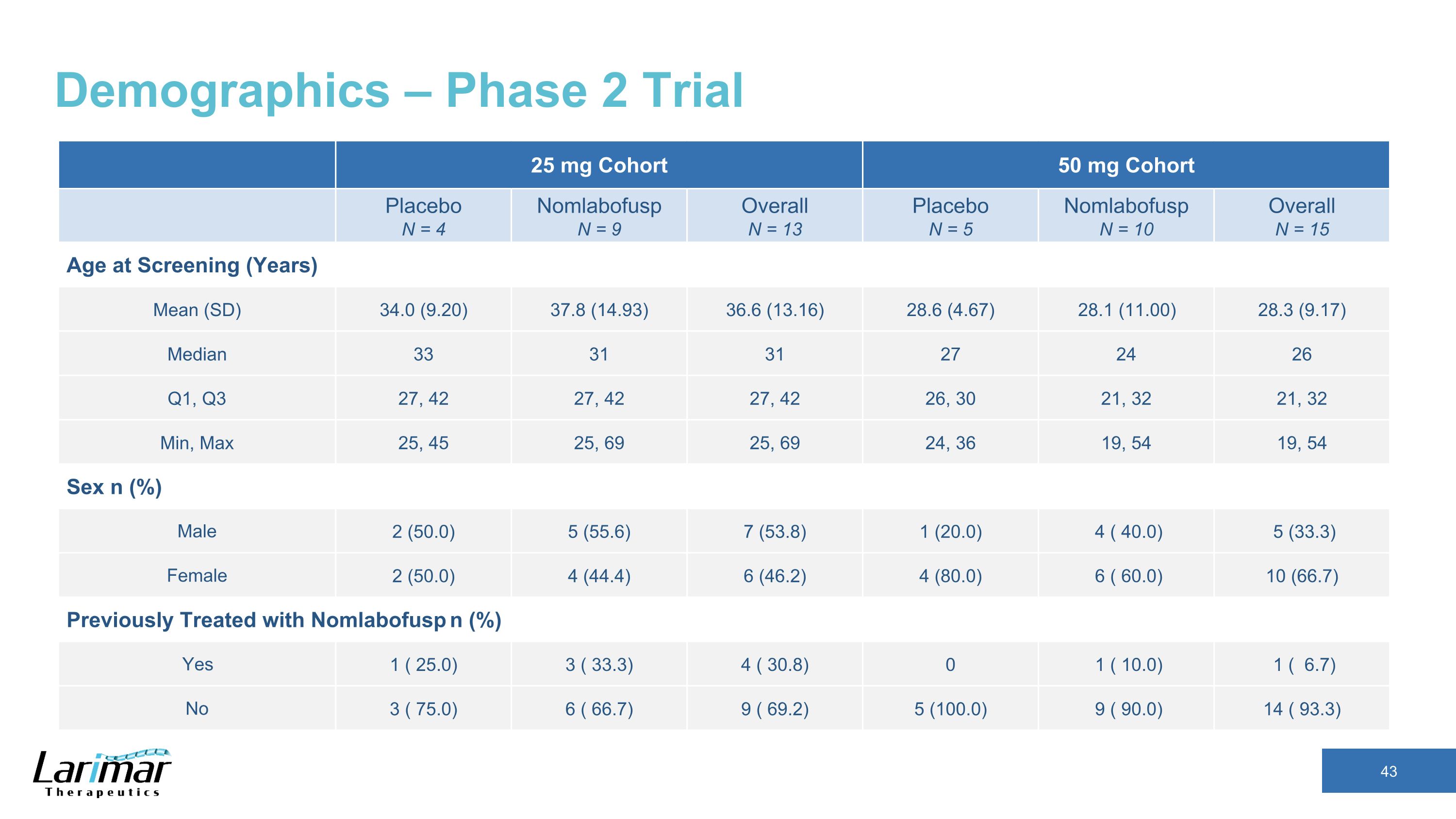
Demographics – Phase 2 Trial 25 mg Cohort 50 mg Cohort Placebo N = 4 Nomlabofusp N = 9 Overall N = 13 Placebo N = 5 Nomlabofusp N = 10 Overall N = 15 Age at Screening (Years) Mean (SD) 34.0 (9.20) 37.8 (14.93) 36.6 (13.16) 28.6 (4.67) 28.1 (11.00) 28.3 (9.17) Median 33 31 31 27 24 26 Q1, Q3 27, 42 27, 42 27, 42 26, 30 21, 32 21, 32 Min, Max 25, 45 25, 69 25, 69 24, 36 19, 54 19, 54 Sex n (%) Male 2 (50.0) 5 (55.6) 7 (53.8) 1 (20.0) 4 ( 40.0) 5 (33.3) Female 2 (50.0) 4 (44.4) 6 (46.2) 4 (80.0) 6 ( 60.0) 10 (66.7) Previously Treated with Nomlabofusp n (%) Yes 1 ( 25.0) 3 ( 33.3) 4 ( 30.8) 0 1 ( 10.0) 1 ( 6.7) No 3 ( 75.0) 6 ( 66.7) 9 ( 69.2) 5 (100.0) 9 ( 90.0) 14 ( 93.3)

Disease Characteristics – Phase 2 Study 25 mg Cohort 50 mg Cohort Placebo N = 4 Nomlabofusp N = 9 Overall N = 13 Placebo N = 5 Nomlabofusp N = 10 Overall N = 15 Age at Symptom Onset (Years) Mean (SD) 14.5 (4.93) 13.0 (10.47) 13.5 (8.77) 15.2 (7.26) 13.7 (8.37) 14.2 (7.78) Median 14.5 10 11 14 12.5 14 Q1, Q3 11, 19 8, 13 9, 15 11, 16 7, 18 7, 18 Min, Max 9, 20 5, 38 5, 38 8, 27 5, 30 5, 30 Age at Diagnosis (Years) Mean (SD) 17.5 (5.57) 18.6 (11.20) 18.2 (9.58) 18.6 (6.80) 16.6 (8.03) 17.3 (7.46) Median 16.5 16 16 19 13.5 14 Q1, Q3 14, 22 14, 20 14, 20 13, 20 10, 21 12, 21 Min, Max 12, 25 5, 42 5, 42 12, 29 9, 30 9, 30 Time Since Diagnosis (Years) Mean (SD) 16.1 (5.97) 18.5 (11.52) 17.8 (9.94) 9.5 (3.72) 11.9 (7.05) 11.1 (6.10) Median 13.42 14.32 13.5 11 11.26 11 Q1, Q3 12.9, 19.3 12.8, 21.6 12.8, 21.6 5.8, 11.3 7.4, 15.3 5.8, 15.2 Min, Max 12.5, 25.0 5.4, 45.0 5.4, 45.0 5.6, 14.0 2.3, 25.1 2.3, 25.1

Non-Interventional Study Data
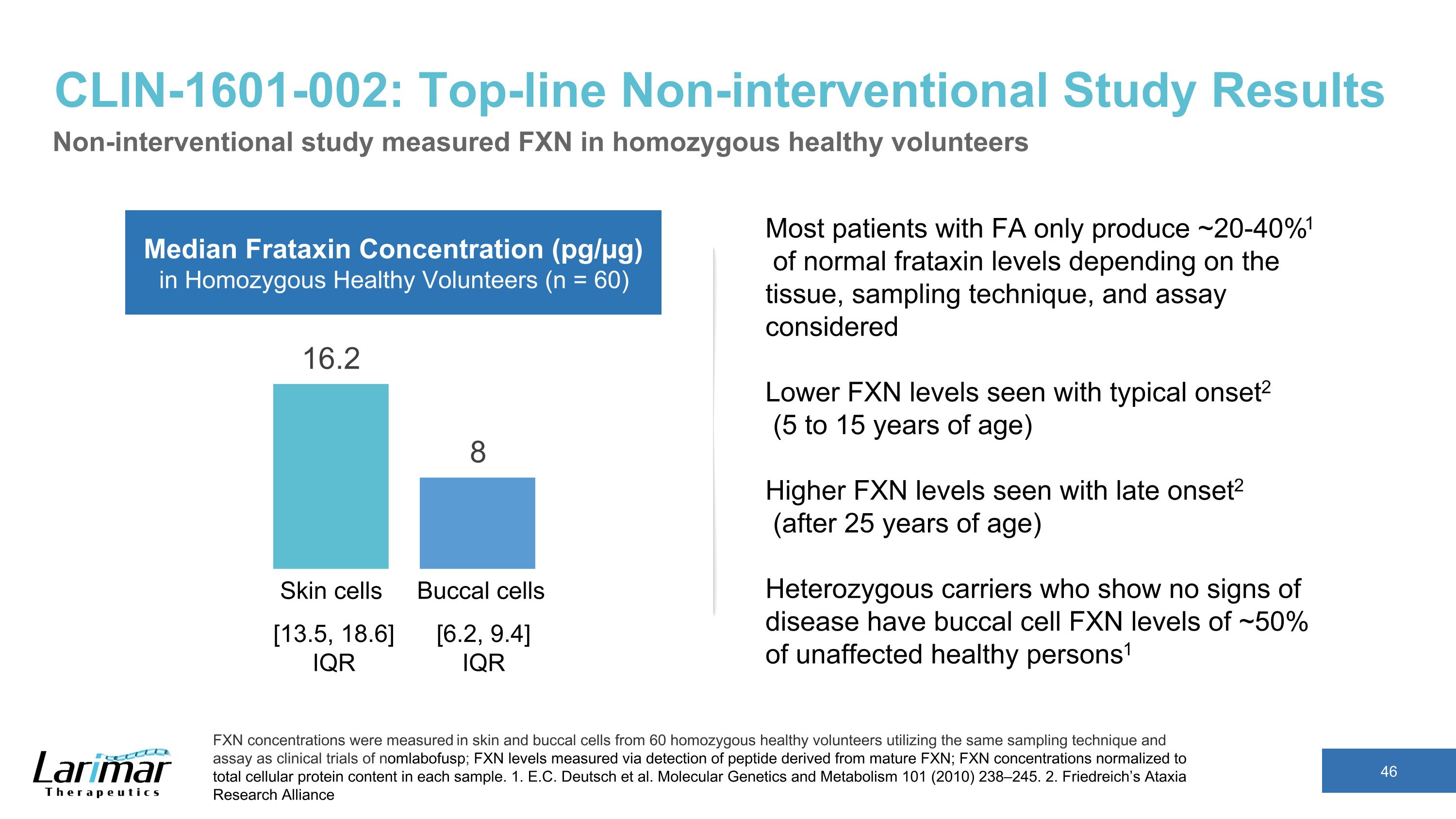
CLIN-1601-002: Top-line Non-interventional Study Results Non-interventional study measured FXN in homozygous healthy volunteers FXN concentrations were measured in skin and buccal cells from 60 homozygous healthy volunteers utilizing the same sampling technique and assay as clinical trials of nomlabofusp; FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations normalized to total cellular protein content in each sample. 1. E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. 2. Friedreich’s Ataxia Research Alliance Skin cells Buccal cells Median Frataxin Concentration (pg/µg) in Homozygous Healthy Volunteers (n = 60) Most patients with FA only produce ~20-40%1 of normal frataxin levels depending on the tissue, sampling technique, and assay considered Lower FXN levels seen with typical onset2 (5 to 15 years of age) Higher FXN levels seen with late onset2 (after 25 years of age) Heterozygous carriers who show no signs of disease have buccal cell FXN levels of ~50% of unaffected healthy persons1 [13.5, 18.6] IQR [6.2, 9.4] IQR
v3.24.1.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From Dec 2024 to Jan 2025
Larimar Therapeutics (NASDAQ:LRMR)
Historical Stock Chart
From Jan 2024 to Jan 2025