Lumos Pharma to Participate in the Oppenheimer 2023 Montauk Life Sciences Summit
July 12 2023 - 7:00AM

Lumos Pharma, Inc. (NASDAQ:LUMO), a biopharmaceutical company
advancing an oral therapeutic candidate for Pediatric Growth
Hormone Deficiency (PGHD) through Phase 2 clinical trials,
announced that the Company’s President & Chief Scientific
Officer, John McKew, PhD, will participate in the Oppenheimer 2023
Life Sciences Summit to be held July 18th – 20th in Montauk, NY,
during which discussions with investors will take place.
Please contact your Oppenheimer sales
representative to attend the Montauk Life Sciences Summit or Lumos
Pharma Investor Relations with questions about our clinical
programs and corporate strategy.
About Lumos Pharma
Lumos Pharma, Inc. is a clinical stage
biopharmaceutical company focused on the development and
commercialization of therapeutics for rare diseases. Lumos
Pharma was founded and is led by a management team with
longstanding experience in rare disease drug development. Lumos
Pharma’s lead therapeutic candidate is LUM-201, an oral growth
hormone stimulating small molecule, currently being evaluated in a
Phase 2 clinical trial, the OraGrowtH210 Trial, a PK/PD trial, the
OraGrowtH212 Trial, and a switch trial, the OraGrowtH213 Trial for
the treatment of Pediatric Growth Hormone Deficiency (PGHD). If
approved by the FDA, LUM-201 would provide an orally administered
alternative to recombinant growth hormone injections that PGHD
subjects otherwise endure for many years of treatment. LUM-201 has
received Orphan Drug Designation in both the US and EU. For more
information, please visit https://lumos-pharma.com/.
Investor & Media Contact:
Lisa MillerLumos Pharma Investor
Relations512-792-5454ir@lumos-pharma.com
Source: Lumos Pharma, Inc.
Lumos Pharma (NASDAQ:LUMO)
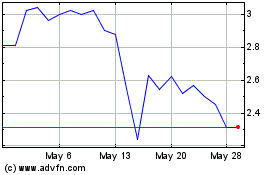
Historical Stock Chart
From Apr 2024 to May 2024
Lumos Pharma (NASDAQ:LUMO)
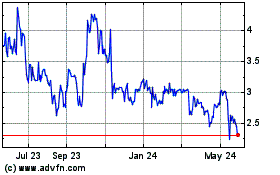
Historical Stock Chart
From May 2023 to May 2024