false000181381400018138142023-11-022023-11-02
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 2, 2023
MIND MEDICINE (MINDMED) INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
British Columbia, Canada |
001-40360 |
98-1582438 |
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
One World Trade Center, Suite 8500 New York, New York |
|
10007 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (212) 220-6633
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Shares |
MNMD |
The Nasdaq Stock Market LLC |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On November 2, 2023, Mind Medicine (MindMed) Inc. (the “Company”) issued a press release (the "Press Release:) announcing its financial results for its fiscal quarter ended September 30, 2023 as well as information regarding a conference call to discuss these financial results and the Company’s recent corporate highlights. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in this Item 2.02 of this Current Report (including Exhibit 99.1) is being furnished and shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
Item 7.01 Regulation FD Disclosure
The Company presented the slides attached as Exhibit 99.2 at its earnings call on November 2, 2023. A copy of the slides is posted on the Company’s website and is furnished as Exhibit 99.2 and incorporated herein by reference.
The information contained in this Item 7.01 of this Current Report (including Exhibit 99.2) is being furnished and shall not be deemed filed for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
MIND MEDICINE (MINDMED) INC. |
|
|
|
|
Date: November 2, 2023 |
|
By: |
/s/ Robert Barrow |
|
|
Name: |
Robert Barrow |
|
|
Title: |
Chief Executive Officer |

Exhibit 99.1
MindMed Reports Third Quarter 2023 Financial Results and Business Highlights
– Topline readout of MM-120 in GAD (Phase 2b) expected in Q4 2023 –
– Topline readout of MM-120 in ADHD (Phase 2a proof-of-concept) anticipated by the end of Q1 2024 –
– MM-402 in ASD on track for Phase 1 clinical trial initiation in Q4 2023 –
– Cash and cash equivalents of $117.7 million at September 30, 2023 –
– Company to host conference call today at 4:30 PM ET –
NEW YORK, November 2, 2023 -- Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today reported its financial results for the quarter ended September 30, 2023.
“During the third quarter we continued to focus on execution ahead of several key data readouts in the coming months. This includes, in particular, topline results from our Phase 2b study of MM-120 in generalized anxiety disorder (GAD) which are anticipated by the end of this year. Additionally, we anticipate the initiation of our Phase 1 study of MM-402 by the end of this year and reporting topline data from our proof-of-concept study of MM-120 in ADHD by the end of Q1 2024.” said Robert Barrow, Chief Executive Officer and Director of MindMed. “The continued growth in prevalence and impact of GAD, ASD and other brain health disorders highlights the importance and timeliness of our innovative programs. Our team remains singularly focused on delivering novel treatments for brain health disorders to the millions of patients in need and we are eager to share these important milestones in the months ahead.”
Recent Highlights and Anticipated Upcoming Milestones:
Phase 2b study evaluating MM-120 for generalized anxiety disorder (GAD) in adults.
•Study MMED008 is a multi-center, parallel, randomized, double-blind, placebo-controlled, dose-optimization study. The trial enrolled 198 participants who were randomized to receive a single oral administration of MM-120 (25 µg, 50 µg, 100 µg or 200 µg) or placebo.
•The primary objective of the study is to determine the dose-response relationship of four doses of MM-120 versus placebo as measured by the change in Hamilton Anxiety Rating Scale (HAM-A) from Baseline to Week 4.
•Dosing is complete with topline results for the primary endpoint (Week 4) expected to be announced in Q4 2023.
•We anticipate the announcement of 12-week safety and efficacy results by the end of Q1 2024 and the presentation of the full data from the study at a scientific meeting in 2024.
Proof-of-Concept study evaluating repeated low-dose administration of MM-120 for attention-deficit/hyperactivity disorder (ADHD) in adults.
•Study MMED007 is a multi-center, randomized, double-blind, placebo-controlled study. The trial enrolled 53 participants who were randomized to receive twice-weekly oral doses of MM-120 20 μg or placebo for 6 weeks.
•Topline results expected by the end of Q1 2024.
•The primary endpoint of this study is the mean change from baseline at Week 6 in ADHD symptoms, as assessed by the Adult ADHD Investigator Rating Scale (AISRS).
Advancing development of MM-402 (R(-)-MDMA) for autism spectrum disorder (ASD) into first clinical trial in Q4 2023.
•The Company plans to initiate its first clinical trial of MM-402 in Q4 2023. This Phase 1 study is intended to characterize the tolerability, pharmacokinetics and pharmacodynamics of MM-402, and to evaluate early signals of efficacy to support the Company’s approach in targeting core symptoms of ASD in adults.
•University Hospital Basel (UHB) in Switzerland, the Company’s collaborator, is currently enrolling participants in a Phase 1 investigator-initiated trial of R(-)-MDMA, S(+)-MDMA and R/S-MDMA in healthy adult volunteers. This trial is designed to assess the tolerability, pharmacokinetics and acute subjective, physiological and endocrine effects of the three molecules. The Company anticipates topline results to be presented in the first half of 2024.
•In October 2023, the Company presented results from a MM-402 nonclinical study in a model of ASD, titled “MM-402 demonstrates better efficacy than S(+)-3,4-MDMA or (±)-3,4-MDMA in Fmr1 knockout mice, an animal model of autism spectrum disorder” at the 36th Annual European College of Neuropsychopharmacology (ECNP) Congress.
Third Quarter 2023 Financial Results
Cash and Cash Equivalents Balance. As of September 30, 2023, MindMed had cash and cash equivalents totaling $117.7 million compared to $142.1 million as of December 31, 2022. The Company believes its available cash and cash equivalents as well as its committed credit facility are expected to fund operations into 2026, if certain milestones are achieved that unlock additional capital.
Net Cash Used in Operating Activities. For the nine months ended September 30, 2023, net cash used in operating activities was $43.8 million, compared to $37.3 million in the nine months ended September 30, 2022.
Research and Development (R&D). R&D expenses were $13.2 million for the quarter ended September 30, 2023, compared to $7.8 million for the quarter ended September 30, 2022, an increase of $5.4 million. The increase was primarily due to increases of $6.4 million in expenses related to clinical research and product development for the MM-120 GAD study, and $0.4 million in internal personnel
costs as a result of increasing research and development capacities, partially offset by a decrease of $0.4 million in expenses related to our MM-402 program, a decrease of $0.2 million related to our paused MM-110 program, a decrease of $0.5 million in preclinical activities, and a decrease of $0.2 million in connection with various external R&D collaborations.
General and Administrative (G&A). G&A expenses were $8.4 million for the quarter ended September 30, 2023, compared to $9.2 million for the quarter ended September 30, 2022, a decrease of $0.8 million. The decrease was primarily related to issuance costs related to the Company's 2022 USD Financing Warrants that were issued as part of the Company's public equity offering which closed on September 30, 2022.
Net Loss. Net loss for the quarter ended September 30, 2023, was $17.9 million, compared to $16.5 million for the same period in 2022.
Conference Call and Webcast Reminder
MindMed management will host a conference call at 4:30 PM EST today to provide a corporate update and review the Company’s third quarter 2023 financial results. Individuals may participate in the live call via telephone by dialing (855) 327-6837 (domestic) or (631) 891-4304 (international). The webcast can be accessed live here on the Financials page in the Investors section of the MindMed website, https://mindmed.co/. The webcast will be archived on the Company’s website for at least 30 days after the conference call.
About MindMed
MindMed is a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders. Our mission is to be the global leader in the development and delivery of treatments that unlock new opportunities to improve patient outcomes. We are developing a pipeline of innovative product candidates, with and without acute perceptual effects, targeting neurotransmitter pathways that play key roles in brain health disorders.
MindMed trades on NASDAQ under the symbol MNMD and on the Canadian NEO Exchange under the symbol MMED.
Forward-Looking Statements
Certain statements in this news release related to the Company constitute “forward-looking information” within the meaning of applicable securities laws and are prospective in nature. Forward-looking information is not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “will”, “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe”, “potential” or “continue”, or the negative thereof or similar variations. Forward-looking information in this news release includes, but is not limited to, statements regarding the progress of trials and studies; results and timing of and reporting of topline data from clinical trials; the potential benefits of the Company’s product candidates, and the Company’s cash runway and committed credit facility funding its operations into 2026 if certain milestones are achieved that unlock additional capital. There are numerous risks and
uncertainties that could cause actual results and the Company’s plans and objectives to differ materially from those expressed in the forward-looking information, including history of negative cash flows; limited operating history; incurrence of future losses; availability of additional capital; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to herein and the risks described in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2023 under headings such as “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and other filings and furnishings made by the Company with the securities regulatory authorities in all provinces and territories of Canada which are available under the Company’s profile on SEDAR at www.sedar.com and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Except as required by law, the Company undertakes no duty or obligation to update any forward-looking statements contained in this release as a result of new information, future events, changes in expectations or otherwise.
For Media & Investor Inquiries, please contact:
Maxim Jacobs, CFA
Vice President, Investor Relations and Corporate Communications
Mind Medicine (MindMed) Inc.
ir@mindmed.co
media@mindmed.co
Mind Medicine (MindMed) Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited; in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
Ended September 30, |
|
|
Nine Months
Ended September 30, |
|
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
13,203 |
|
|
$ |
7,772 |
|
|
$ |
40,578 |
|
|
$ |
27,339 |
|
General and administrative |
|
|
8,413 |
|
|
|
9,211 |
|
|
|
31,083 |
|
|
|
25,092 |
|
Total operating expenses |
|
|
21,616 |
|
|
|
16,983 |
|
|
|
71,661 |
|
|
|
52,431 |
|
Loss from operations |
|
|
(21,616 |
) |
|
|
(16,983 |
) |
|
|
(71,661 |
) |
|
|
(52,431 |
) |
Other income/(expense): |
|
|
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
|
1,163 |
|
|
|
360 |
|
|
|
3,759 |
|
|
|
443 |
|
Foreign exchange (loss)/gain, net |
|
|
(439 |
) |
|
|
138 |
|
|
|
(244 |
) |
|
|
94 |
|
Change in fair value of 2022 USD Financing Warrants |
|
|
3,020 |
|
|
|
— |
|
|
|
(3,671 |
) |
|
|
— |
|
Other (expense)/income |
|
|
(51 |
) |
|
|
— |
|
|
|
(51 |
) |
|
|
1 |
|
Total other income/(expense), net |
|
|
3,693 |
|
|
|
498 |
|
|
|
(207 |
) |
|
|
538 |
|
Net loss |
|
|
(17,923 |
) |
|
|
(16,485 |
) |
|
|
(71,868 |
) |
|
|
(51,893 |
) |
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
Gain/(loss) on foreign currency translation |
|
|
415 |
|
|
|
(107 |
) |
|
|
150 |
|
|
|
(303 |
) |
Comprehensive loss |
|
$ |
(17,508 |
) |
|
$ |
(16,592 |
) |
|
$ |
(71,718 |
) |
|
$ |
(52,196 |
) |
Net loss per common share, basic and diluted |
|
$ |
(0.45 |
) |
|
$ |
(0.56 |
) |
|
$ |
(1.85 |
) |
|
$ |
(1.82 |
) |
Weighted-average common shares, basic and diluted |
|
|
39,720,007 |
|
|
|
29,296,333 |
|
|
|
38,798,374 |
|
|
|
28,566,161 |
|
Mind Medicine (MindMed) Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023
(unaudited) |
|
|
December 31, 2022 |
|
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
117,699 |
|
|
$ |
142,142 |
|
Prepaid and other current assets |
|
|
2,387 |
|
|
|
3,913 |
|
Total current assets |
|
|
120,086 |
|
|
|
146,055 |
|
Goodwill |
|
|
19,918 |
|
|
|
19,918 |
|
Intangible assets, net |
|
|
1,317 |
|
|
|
3,689 |
|
Other non-current assets |
|
|
229 |
|
|
|
331 |
|
Total assets |
|
$ |
141,550 |
|
|
$ |
169,993 |
|
|
|
|
|
|
|
|
Liabilities and Shareholders’ Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
7,686 |
|
|
$ |
2,111 |
|
Accrued expenses |
|
|
9,957 |
|
|
|
5,877 |
|
2022 USD Financing Warrants |
|
|
13,511 |
|
|
|
9,904 |
|
Total current liabilities |
|
|
31,154 |
|
|
|
17,892 |
|
Credit facility, long-term |
|
|
14,068 |
|
|
|
— |
|
Other liabilities, long-term |
|
|
349 |
|
|
|
1,184 |
|
Total liabilities |
|
|
45,571 |
|
|
|
19,076 |
|
|
|
|
|
|
|
|
Shareholders' Equity: |
|
|
|
|
|
|
Common shares, no par value, unlimited authorized as of September 30, 2023 and December 31, 2022; 40,094,708 and 37,979,136 issued and outstanding as of September 30, 2023 and December 31, 2022, respectively |
|
|
— |
|
|
|
— |
|
Additional paid-in capital |
|
|
361,538 |
|
|
|
344,758 |
|
Accumulated other comprehensive income |
|
|
777 |
|
|
|
627 |
|
Accumulated deficit |
|
|
(266,336 |
) |
|
|
(194,468 |
) |
Total shareholders' equity |
|
|
95,979 |
|
|
|
150,917 |
|
Total liabilities and shareholders' equity |
|
$ |
141,550 |
|
|
$ |
169,993 |
|
Exhibit 99.2

Logo Third Quarter 2023 Financial Results and Business Update November 2, 2023

Disclaimer This presentation (the “Presentation”) has been preparedby Mind Medicine(MindMed) Inc. (“MindMed” or the “Company”) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omissions from, this Presentation. This Presentation does not constitute an offering of, or a solicitation of an offer to purchase, securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the propertyof the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the Securities and Exchange Commission (the "SEC") or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracyor adequacy of this Presentation. Any representation to the contraryis a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains,and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. PrivateSecurities Litigation Reform Act of 1995 and other applicable securities laws. Forward-looking statementscan often, but not always,be identified by words such as “plans”,“expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases,or statements that certain actions,events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and similar references to future periods.Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertainingto: the development and commercialization of any medicine or treatment, or the efficacyof either of the foregoing, the success and timing of our development activities; the success and timing of our planned clinical trials; our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals; the likelihood of obtaining patents or the efficacy of such patents once granted and the potential for the markets that MindMed is anticipating to access. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business,future plans and strategies, projections, anticipated eventsand trends, the economyand other future conditions as of the date of this Presentation. While MindMed considersthese assumptions to be reasonable, the assumptions are inherently subjectto significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of MindMed’s control, and actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated medicines and treatments being developed; MindMed’s history of negative cash flows; MindMed’s limited operating history; incurrence of future losses; compliance with laws and regulations; difficultyassociated with researchand development; risks associated with clinical trialsor studies; heightened regulatory scrutiny; early stage product development; clinical trialrisks; regulatory approvalprocesses; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to throughout the “Risk Factors” sections of MindMed’s most recently filed Annual Report on Form 10-K filed with the SEC, the Company’s Quarterly Report on Form 10-Q for the fiscal quarterended September 30, 2023, and in other filings we make in the futurewith the SEC and the securities regulatoryauthorities in all provinces and territories of Canada, available under the Company’s profileon SEDAR at www.sedar.com. Any forward-looking statement made by MindMed in this Presentation is based only on information currently available to the Companyand speaks only as of the date on whichit is made. MindMed undertakesno obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whetheras a result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. The Company works with a non-hallucinogenic synthetic derivative of the psychedelic substance ibogaine, known as zolunicant which is a synthetic organic molecule designed around a common coronaridine chemical backbone. Zolunicant is not a Schedule I substance in the United States and the Company does not foresee it becoming a Schedule I substance due to its non-hallucinogenic properties. While the Company is focusedon programs using psychedelic or hallucinogenic compoundsand non-hallucinogenic derivatives of these compounds, the Company does not have any direct or indirectinvolvement with the illegal selling,production or distribution of any substances in the jurisdictions in which it operates.The Company is a neuro-pharmaceutical drug development companyand does not deal with psychedelic or hallucinogenic substances except within laboratory and clinical trial settingsconducted within approved regulatory frameworks. The Company’s products will not be commercialized prior to applicable regulatory approval, which will only be grantedif clinical evidenceof safety and efficacy for the intendeduses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referredto in this Presentation or ascertained the underlying economicassumptions relied upon by such sources. References in this Presentation to research reportsor to articles and publications should be not construedas depicting the complete findingsof the entire referenced report or article. MindMeddoes not make any representation as to the accuracy of such information. Q3 2023 Earnings Presentation November 2023 2 logo

MindMed Third Quarter Financial Results and Business Update Call Participants Robert Barrow Chief Executive Officer and Board Director Schond Greenway, MBA Chief Financial Officer Daniel Karlin, MD, MA Chief Medical Officer Francois Lilienthal, MD, MBA Chief Commercial Officer Q3 2023 Earnings Presentation November 2023 3 logo
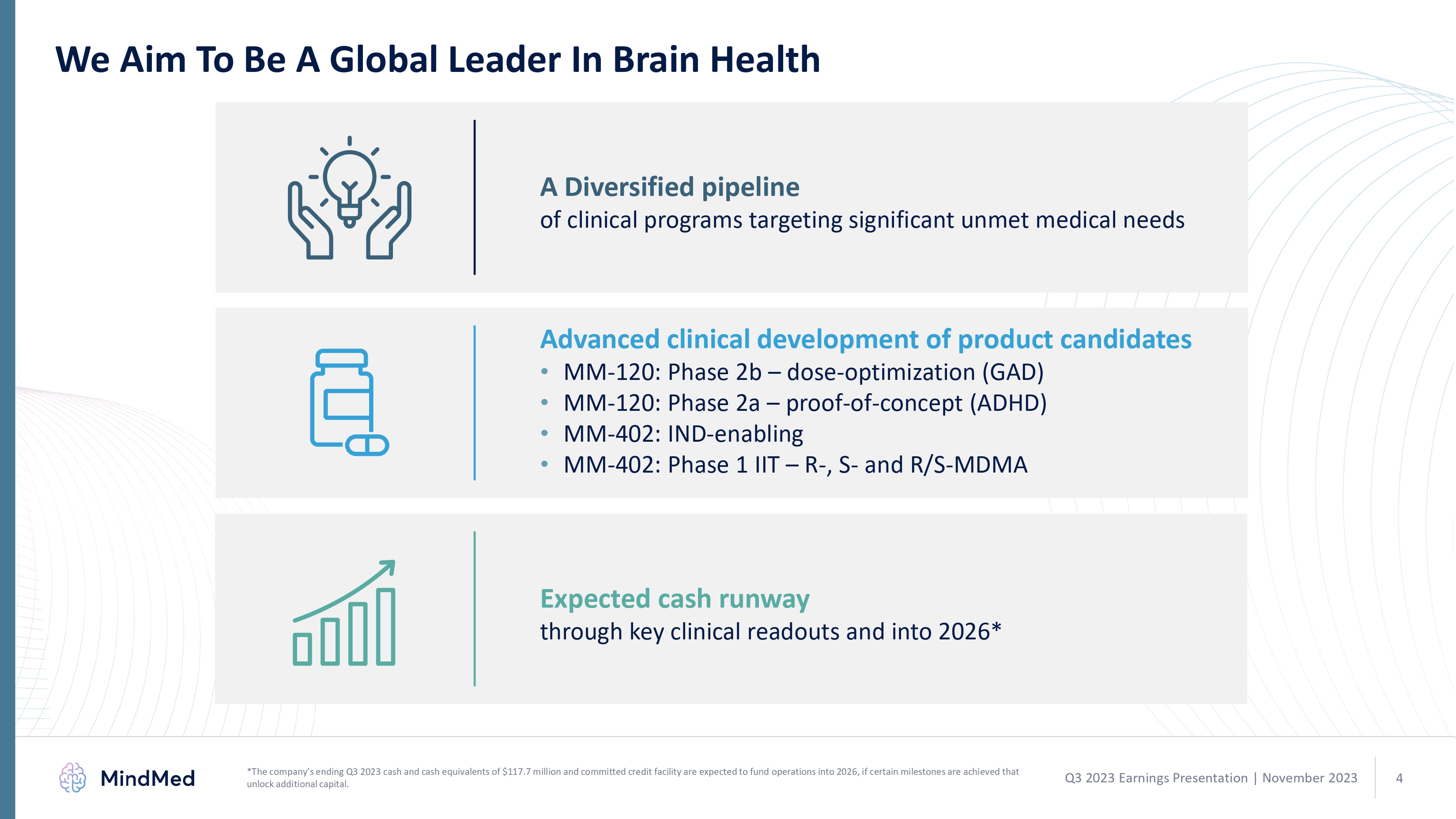
We Aim To Be A Global Leader In Brain Health A Diversified pipeline of clinical programs targeting significant unmet medical needs Advanced clinical development of product candidates MM-120: Phase 2b – dose-optimization (GAD) MM-120: Phase 2a – proof-of-concept (ADHD) MM-402: IND-enabling MM-402: Phase 1 IIT – R-, S- and R/S-MDMA Expected cash runway through key clinical readouts and into 2026* graphic *The company’s ending Q3 2023 cash and cash equivalents of $117.7 million and committed credit facility are expected to fund operations into 2026, if certain milestones are achieved that unlock additional capital. Q3 2023 Earnings Presentation November 2023 4 logo

Urgent Need for Better Treatments for Brain Health Disorders 10% 4.4% $461B 1-year prevalence of anxiety disorders in the US1estimated prevalence of ADHD among US adults2 economic cost of ASD in the US predicted by 20253 GAD ADHD ASD 1 in 4 U.S. Adults has a Diagnosable Mental Health Disorder1 1. Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023. 2. Kessler RC, Adler L, Barkley R, et al. 2005; Am J Psychiatry; 163(4). 3. Leigh JP and Du J 2015; J. Autism Dev. Disord.; 45(12). Q3 2023 Earnings Presentation November 2023 5 Q3 2023 Earnings Presentation November 2023 5 logo
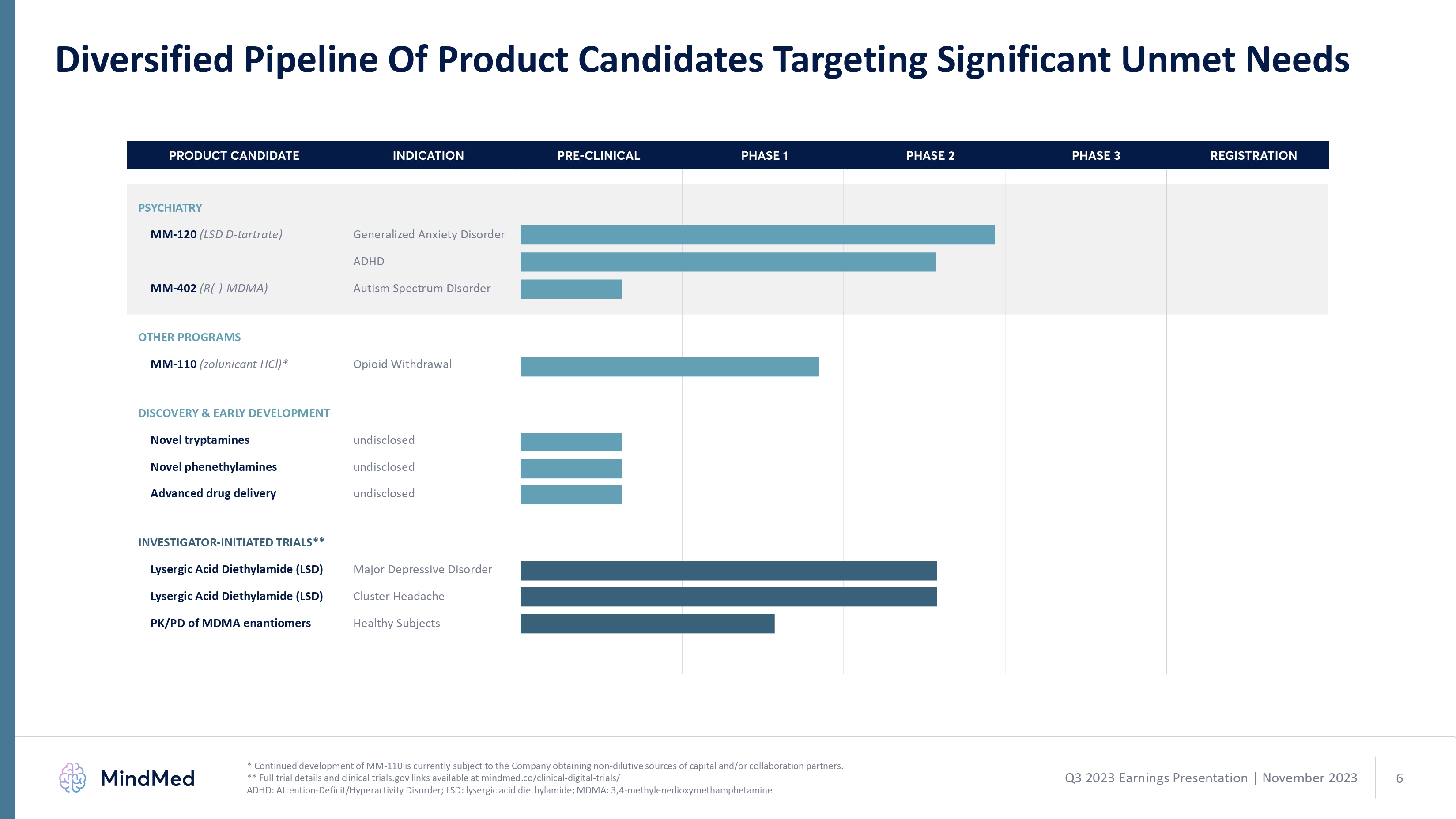
Diversified Pipeline Of Product Candidates Targeting Significant Unmet Needs PSYCHIATRY MM-120 (LSD D-tartrate) Generalized Anxiety Disorder ADHD MM-402 (R(-)-MDMA) Autism Spectrum Disorder OTHER PROGRAMS MM-110 (zolunicant HCl)* Opioid Withdrawal DISCOVERY & EARLY DEVELOPMENT Novel tryptamines undisclosed Novel phenethylamines undisclosed Advanced drug delivery undisclosed INVESTIGATOR-INITIATED TRIALS** Lysergic Acid Diethylamide (LSD) Lysergic Acid Diethylamide (LSD) Major Depressive Disorder Cluster Headache * Continued development of MM-110 is currently subject to the Company obtaining non-dilutive sources of capital and/or collaboration partners. ** Full trial details and clinical trials.gov links available at mindmed.co/clinical-digital-trials/ ADHD: Attention-Deficit/Hyperactivity Disorder; LSD: lysergic acid diethylamide; MDMA: 3,4-methylenedioxymethamphetamine PRODUCT CANDIDATE INDICATION PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 REGISTRATION Bargraph Q3 2023 Earnings Presentation November 2023 6 logo
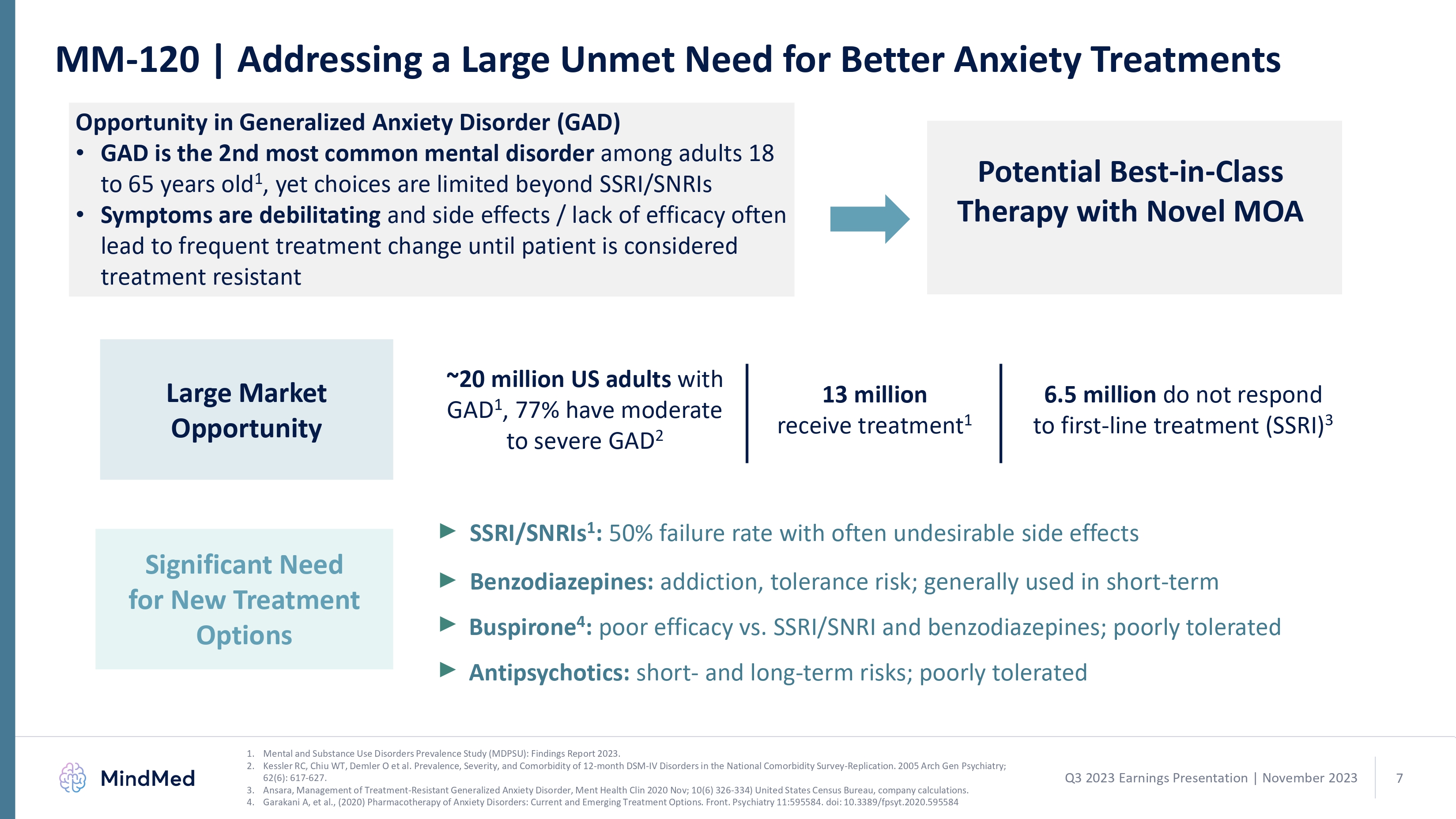
MM-120 | Addressing a Large Unmet Need for Better Anxiety Treatments Opportunity in Generalized Anxiety Disorder (GAD)GAD is the 2nd most common mental disorder among adults 18 to 65 years old1, yet choices are limited beyond SSRI/SNRIs Symptoms are debilitating and side effects / lack of efficacy often lead to frequent treatment change until patient is considered treatment resistant Potential Best-in-Class Therapy with Novel MOA ~20 million US adults with GAD1, 77% have moderate to severe GAD2 13 million
receive treatment1 6.5 million do not respond to first-line treatment (SSRI)3 Large Market Opportunity Significant Need for New Treatment Options SSRI/SNRIs1: 50% failure rate with often undesirable side effects Benzodiazepines: addiction, tolerance risk; generally used in short-term Buspirone4: poor efficacy vs. SSRI/SNRI and benzodiazepines; poorly tolerated Antipsychotics: short- and long-term risks; poorly tolerated 1. Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023. 2. Kessler RC, Chiu WT, Demler O et al. Prevalence, Severity, and Comorbidity of 12-month DSM-IV Disorders in the National Comorbidity Survey-Replication. 2005 Arch Gen Psychiatry; 62(6): 617-627. 3. Ansara, Management of Treatment-Resistant Generalized Anxiety Disorder, Ment Health Clin 2020 Nov; 10(6) 326-334) United States Census Bureau, company calculations. Garakani A, et al., (2020) Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 11:595584. doi: 10.3389/fpsyt.2020.595584 Q3 2023 Earnings Presentation November 2023 7 logo
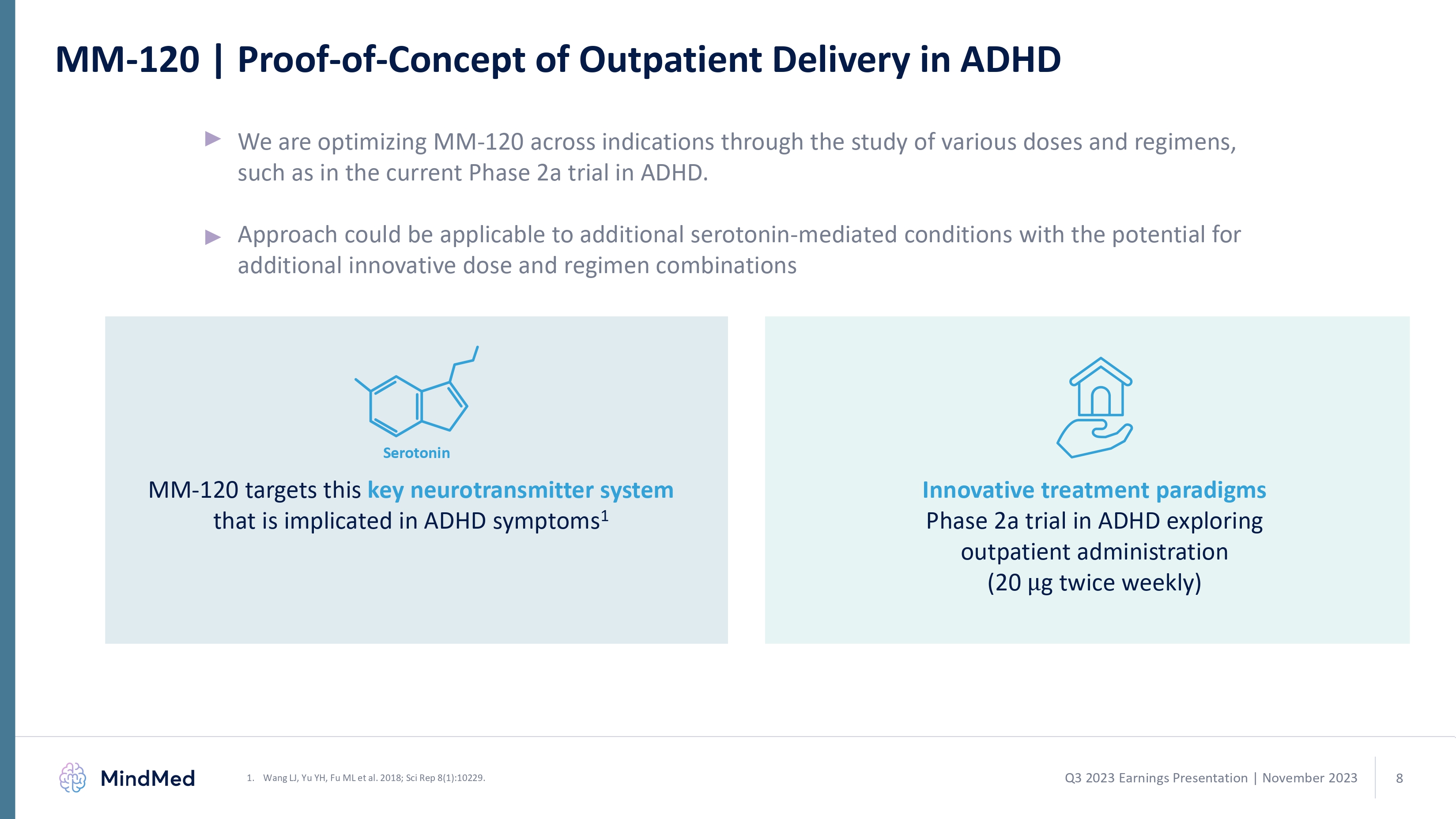
MM-120 | Proof-of-Concept of Outpatient Delivery in ADHD We are optimizing MM-120 across indications through the study of various doses and regimens, such as in the current Phase 2a trial in ADHD. Approach could be applicable to additional serotonin-mediated conditions with the potential for additional innovative dose and regimen combinations MM-120 targets this key neurotransmitter system that is implicated in ADHD symptoms1Innovative treatment paradigms Phase 2a trial in ADHD exploring outpatient administration (20g twice weekly) 1. Wang LJ, Yu YH, Fu ML et al. 2018; Sci Rep 8(1):10229. Serotonin Q3 2023 Earnings Presentation November 2023 8 logo
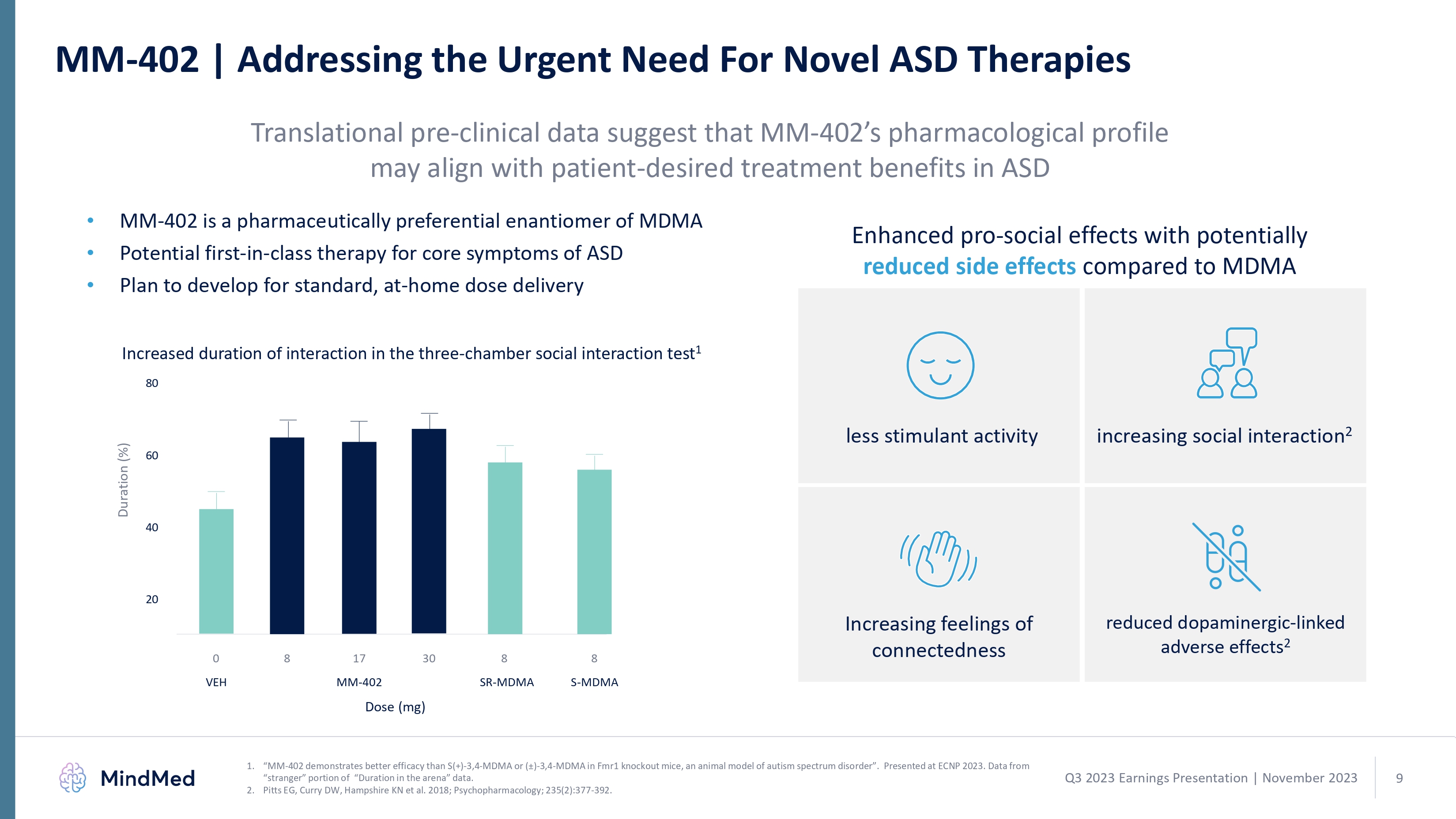
MM-402 | Addressing the Urgent Need For Novel ASD Therapies MM-402 | Addressing the Urgent Need For Novel ASD Therapies Translational pre-clinical data suggest that MM-402’s pharmacological profile may align with patient-desired treatment benefits in ASD MM-402 is a pharmaceutically preferential enantiomer of MDMA Potential first-in-class therapy for core symptoms of ASD Plan to develop for standard, at-home dose delivery Enhanced pro-social effects with potentially reduced side effects compared to MDMA creased duration of interaction in the three-chamber social interaction test 1 less stimulant activity increasing social interaction 2 80 60 40 20 0 8 17 30 8 8 VEH MM-402 SR- S-MDMA MM-402 demonstrates better efficacy than S(+)-3,4-MDMA or (±)-3,4-MDMA in Fmr1 knockout mice, an animal model of autism spectrum disorder”. Presented at ECNP 2023. Data from “stranger” portion of “Duration in the arena” data. Pitts EG, Curry DW, Hampshire KN et al. 2018; Psychopharmacology; 235(2):377-392. 1 2 Q3 2023 Earnings Presentation | November 2023 mindmed 9 logo

MM-120 LSD D-tartrate for Generalized Anxiety Disorder (GAD) Q3 2023 Earnings Presentation | November 2023 mindmed 10 logo
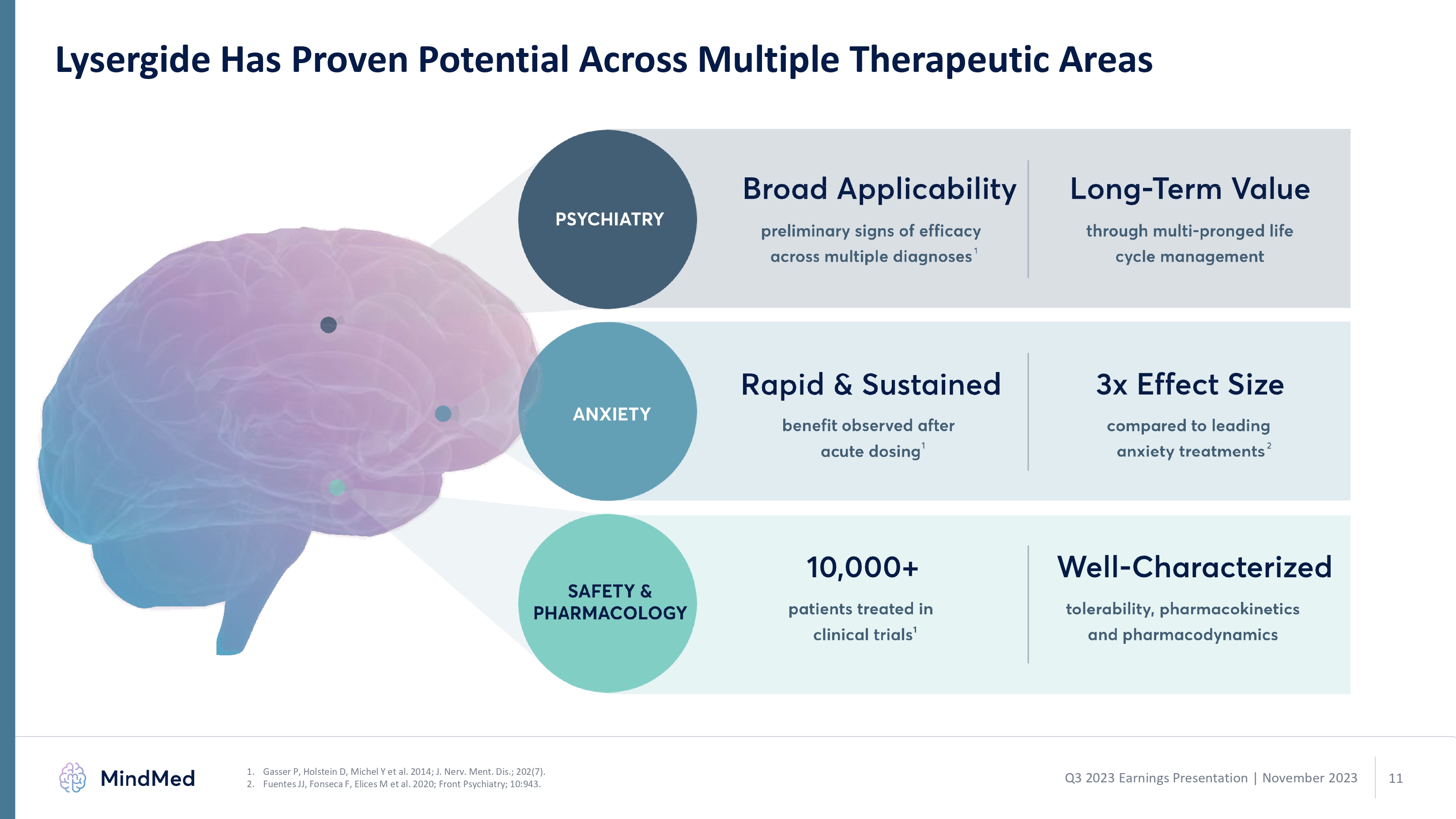
Lysergide Has Proven Potential Across Multiple Therapeutic Areas Gasser P, Holstein D, Michel Y et al. 2014; J. Nerv. Ment. Dis.; 202(7). Fuentes JJ, Fonseca F, Elices M et al. 2020; Front Psychiatry; 10:943.1 2 Q3 2023 Earnings Presentation | November 2023 psychiatry broad applicability preliminary sings of efficacy across multiple diagnoses1 raid & sustained benefit observed after acute dosing 10,000+ patients treated in clinical trials 1 long term value through multi ponged life cycle management 3x effect sixe compared to leading anxiety treatments 2 well characterized tolerability pharmacokinetics and pharmacodynamics. 11 logo
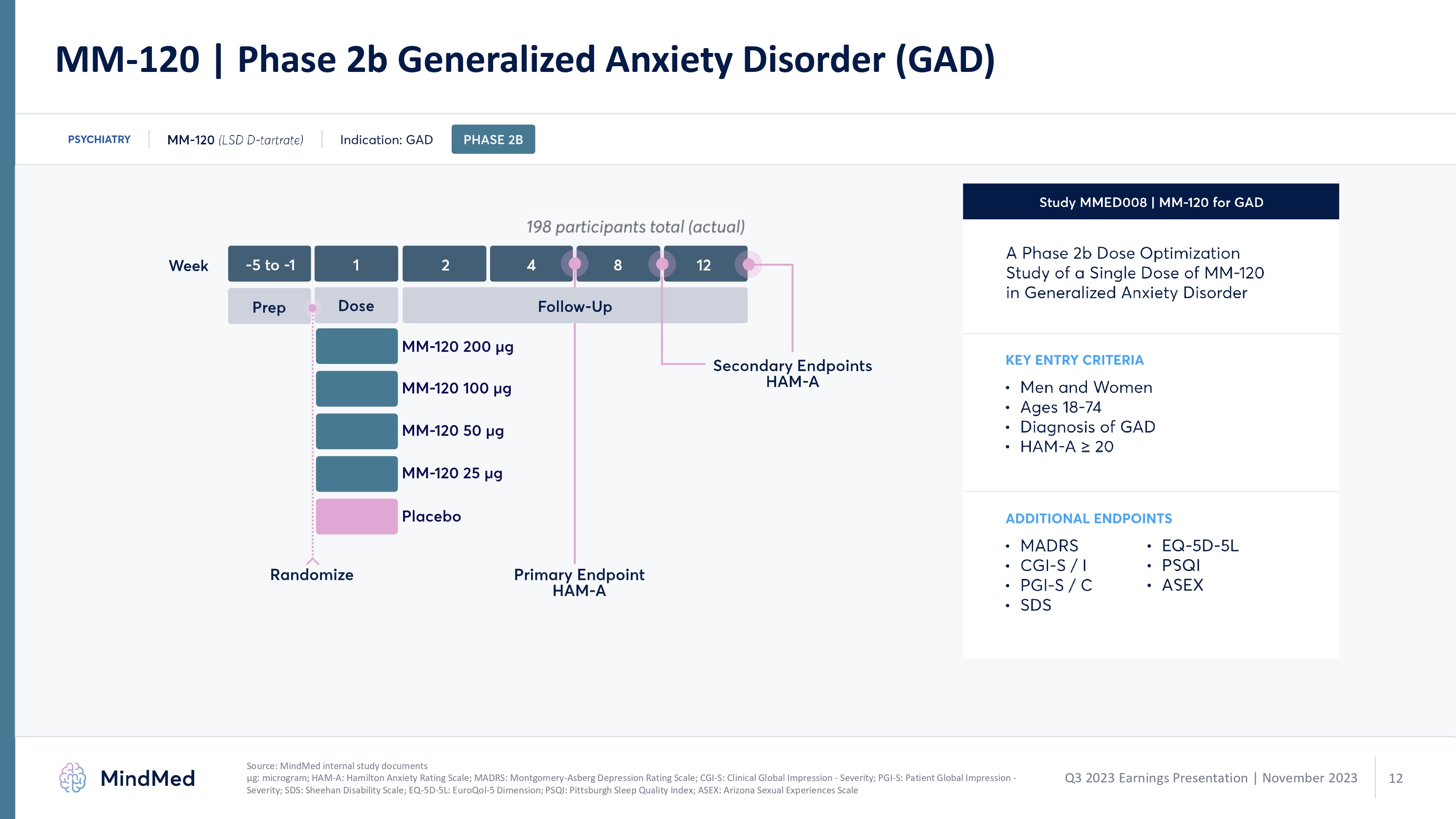
MM-120 | Phase 2b Generalized Anxiety Disorder (GAD) psychiatry mm 120 (lds d-tartarte indication gad phase2b 198 participants total actual week 5 to 1 1 2 4 8 12 prep dose follow up mm120 200 ug mm 120 100 ug mm 120 50 ug placebo primary endpoint ham a randomize study mmed008 mm 120 for gad a phase 2b dose optimization study of a single dose of mm 120 in generalized anxiety disorder key entry criteria men and women ages 18-74 diagnosis of gad ham a > 20 additional endpoints madrs cgi s/ I pgi s/ c sds eq 5d 5l Source: MindMed internal study documents μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impression - Severity; PGI-S Patient Global logoImpression - Severity; SDS: Sheehan Disability Scale; EQ-5D-5L: EuroQol-5 Dimension; PSQI Pittsburgh Sleep Quality Index; ASEX: Arizona Sexual Experiences Scale Q3 2023 Earnings Presentation | November 2023 mindmed 12 logo

MM-120 | Trial Design Milestones for Psychedelic Drug Class FDA guidance and Phase 2b dose-finding study align with MindMed’s framework for designing well-controlled, scientifically rigorous trials to assess safety and efficacy in the psychedelic drug class Phase 2b design aligns well with FDA guidance No concurrent psychotherapy – “Psychotherapeutic interventions have the potential to increase expectancy and performance biases 1 Placebo-controlled – “allows for better contextualization of safety findings 1 Dose-ranging – “The dose-response relationship for most psychedelic drugs is poorly understood. Sponsors should take appropriate steps to characterize the dose-response relationship 1 . FDA. “Psychedelic Drugs: Considerations for Clinical Investigations 1 FDA issues first draft guidance on clinical trials with psychedelic drugs Agency provides clarity on regulatory expectations and R&D considerations Guidance will “help researchers design studies that will yield interpretable results that will be capable of supporting future drug Q3 2023 Earnings Presentation | November 2023 mindmed 13 logo

MM-120 | Phase 2b Generalized Anxiety Disorder (GAD) - Primary Analysis Multiple Comparison Procedure Modelling (MCP-Mod) Statistical methodology for dose-response developed by Novartis in 2004 1 Involves establishing a dose-response signal using multiple comparison procedures and then estimating the dose-response curve and target doses of interest using modelling techniques Qualification opinions from both FDA and EMA FDA: “MCP-Mod method is found more effective than pairwise comparison due toits ability to utilize all available data 2 EMA: “The MCP-Mod approach is efficient in the sense that it uses the available data Novartis. “The MCP-Mod methodology – A statistical methodology for dose-response 1 FDA, Office of Clinical Pharmacology, Division of Pharmacometrics 2 EMA, Committee for Medicinal Products for Human Use 3 Comparisons better than the commonly applied pairwise 0 50 100 150 200 Candidate curve 1 Candidate curve Candidate curve Candidate curve Candidate curve Candidate curve 2 3 4 5 6 dose 14 logo

MM-120 | Illustrative Analysis of Pharmacologic Treatments for GAD1 Reported Mean Effect Size by Existing Therapeutic Drug Class1,2 Buspirone SSRIs Benzodiazapines 0.17 0.36* 0.38* 0 0.1 0.2 0.3 0.4 Effect size (ES) presents the adjusted to the mean difference in treatment response between placebo and active treatment ES useful to compare overall treatment effects across trials Results from published review of effect sizes for double-blind, placebo-controlled trials of GAD treatments primarily using HAM-A as the main outcome measure SSRIs and benzodiazepines, the major therapeutic classes of drugs approved for GAD, have mean effect sizes that range between 0.36 to 0.38 MindMed 1. Examination of baseline group assignment for all of the studies (20 studies utilizing the HAM-A (Hamilton Anxiety Scale) and 1 study using the PARS (Pediatric Anxiety Scale) for the primary outcome measurement. 2. Source: RB Hidalgo, J Psychopharmacol. 2007 Nov;21(8):864-72. 3. ES is calculated by dividing the difference between the mean values of the two groups by the standard deviation value. Q3 2023 Earnings Presentation | November 2023 15 logo

Potential to Leverage Existing Monitored Delivery Infrastructure Spravato® (esketamine) for the treatment of Major Depressive Disorder (MDD) Monitored Delivery Paradigm Established for Spravato 8 intranasal 2-hr treatments over a 4-week period (16 hours)1 with 4 additional 2-hr treatments over 4 weeks (8 hours)1; translating into at least 24 hours in treatment sessions over the first 8 weeks of treatment alone1 Once a week or every 2 weeks thereafter on an individualized basis1 Attractive Commercial Opportunity Over 3,000 treatment centers nationwide2 Certified clinicians and physicians Acceptance by major insurers (United, Cigna, Blue Cross/Blue Shield, etc.)2 Reported 9M sales of $483m, up 89% compared to the first nine months of 20223 Geographic Distribution of Spravato Treatment Centers2 Reported Spravato Sales3 Sales (Millions, $USD) 600 500 400 300 200 100 0 89% Growth 9M 2022 9M 2023 US International MindMed 1. Spravato FDA Prescribing Information 2. Johnson & Johnson; Spravato website. Compiled by company 3. Company Report Johnson & Johnson; July 20, 2023 Financial Results for 9 months ending September 30, 2023 and September 30, 2022 Q3 2023 Earnings Presentation | November 2023 16 logo

Financial Results MindMed Q3 2023 Earnings Presentation | November 2023 17 logo
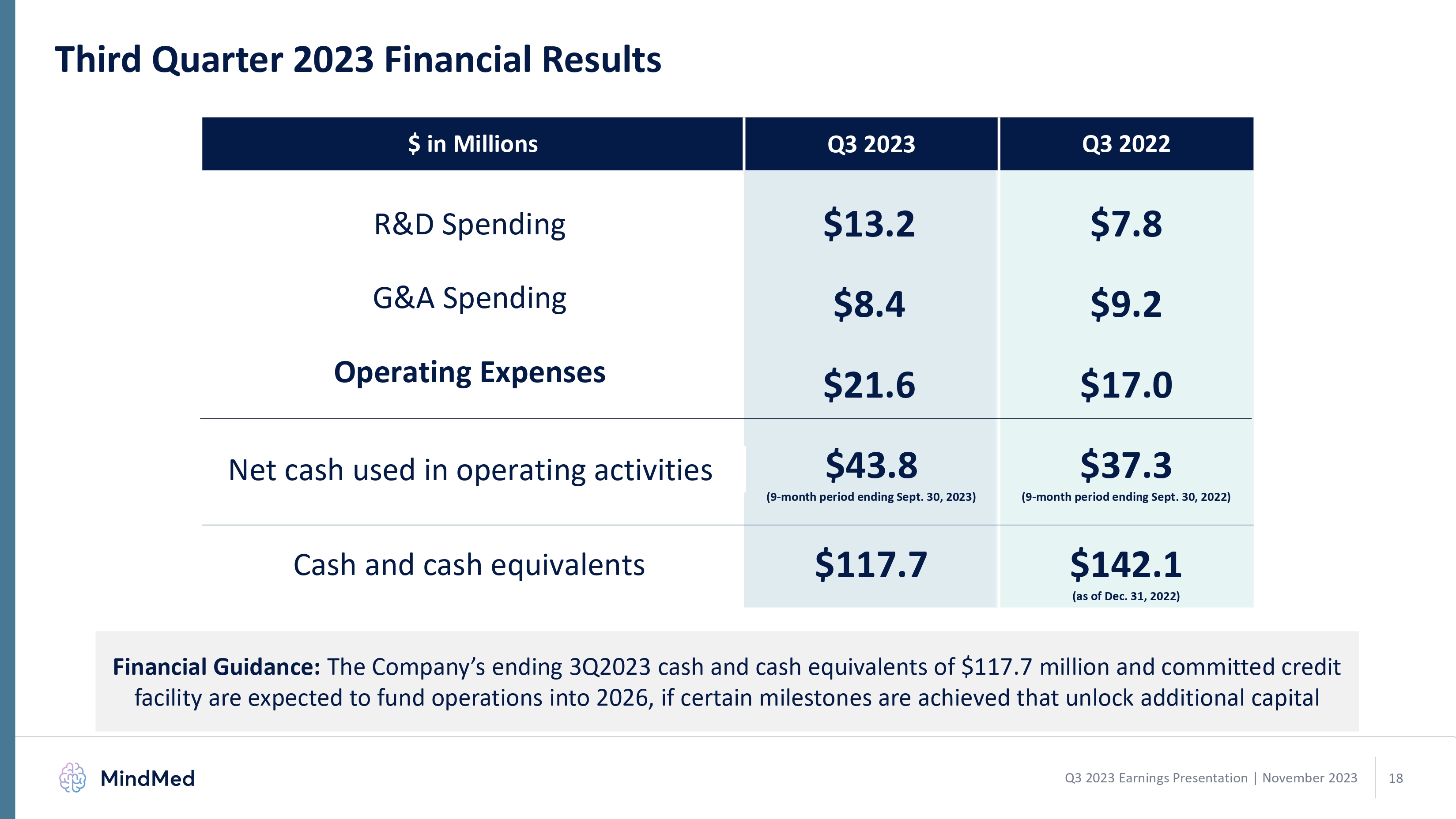
Third Quarter 2023 Financial Results $ in Millions Q3 2023 Q3 2022 R&D Spending $13.2 $7.8 G&A Spending $8.4 $9.2 Operating Expenses $21.6 $17.0 Net cash used in operating activities $43.8 (9-month period ending Sept. 30, 2023) $37.3 (9-month period ending Sept. 30, 2022) Cash and cash equivalents $117.7 $142.1 (as of Dec. 31, 2022) Financial Guidance: The Company’s ending 3Q2023 cash and cash equivalents of $117.7 million and committed credit facility are expected to fund operations into 2026, if certain milestones are achieved that unlock additional capital MindMed Q3 2023 Earnings Presentation | November 2023 18 logo

Anticipated Near-Term Milestones Q4 2023 Q1 2024 Q2 2024 Q3 2024 Q4 2024 MM-120 GAD Phase 2b 4-wk Topline MM-120 GAD Phase 2b 12-wk Topline MM-120 GAD Full data presentation at scientific meeting MM-120 ADHD Phase 2a Topline MM-402 Phase 1 initiation MM-402/R-MDMA Phase 1 IIT (UHB-sponsored) Topline MindMed Q3 2023 Earnings Presentation | November 2023 19 logo

MindMed Q&A MindMed Q3 2023 Earnings Presentation | November 2023 20 logo
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Mind Medicine MindMed (NASDAQ:MNMD)
Historical Stock Chart
From Apr 2024 to May 2024
Mind Medicine MindMed (NASDAQ:MNMD)
Historical Stock Chart
From May 2023 to May 2024