false
0001500198
0001500198
2024-10-25
2024-10-25
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of Earliest Event Reported):
October 25, 2024
NeuroOne Medical Technologies Corporation
(Exact name of registrant as specified in its charter)
| Delaware |
|
001-40439 |
|
27-0863354 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
7599 Anagram Dr., Eden Prairie, MN 55344
(Address of principal executive offices and zip
code)
952-426-1383
(Registrant’s telephone number, including
area code)
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
|
NMTC |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging Growth Company ☐
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement.
On October 25, 2024 (the “Effective Date”),
NeuroOne Medical Technologies Corporation (“NeuroOne”) entered into an amended and restated exclusive development and distribution
agreement (the “A&R Distribution Agreement”) with Zimmer, Inc. (“Zimmer”), pursuant to which NeuroOne granted
Zimmer the exclusive right and license to distribute NeuroOne’s OneRF Ablation System.
Pursuant to the A&R Distribution Agreement,
Zimmer will make an upfront payment of $3.0 million to NeuroOne within 10 business days of the Effective Date of the A&R Distribution
Agreement. Additionally, NeuroOne will be eligible for an additional $1.0 million payment from Zimmer upon achievement of certain specified
net sales milestones.
The A&R Distribution Agreement will expire
on September 30, 2034, unless terminated earlier pursuant to its terms. Either party may terminate the A&R Distribution Agreement
(x) with written notice for the other party’s material breach following a cure period or (y) if the other party becomes subject
to certain insolvency proceedings. In addition, Zimmer may terminate the A&R Distribution Agreement for any reason with 90 days’
written notice, and NeuroOne may terminate the A&R Distribution Agreement if Zimmer acquires or directly or indirectly owns a controlling
interest in certain competitors of NeuroOne.
Each of Zimmer and NeuroOne has agreed to indemnify
the other party against certain losses and expenses relating to the development or commercialization of a product by the indemnifying
party, the negligence or willful misconduct of the indemnifying party or its directors, officers, employees or agents or a breach of the
indemnifying party’s representations, warranties or covenants.
The foregoing description of the A&R Distribution
Agreement is not complete and is qualified in its entirety by reference to the A&R Distribution Agreement, which is attached hereto
as Exhibit 10.1 and incorporated herein by reference.
Item 8.01 Other Events.
Attached as Exhibit 99.1 is a copy of the press
release issued by NeuroOne on October 31, 2024 announcing the execution of the A&R Distribution Agreement.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| # | Certain schedules and exhibits
have been omitted pursuant to Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the
SEC upon request. Certain portions of the A&R Distribution Agreement that are not material and would be competitively harmful if
publicly disclosed have been redacted pursuant to Item 601(b)(10)(iv) of Regulation S-K. A copy of the unredacted A&R Distribution
Agreement will be furnished to the SEC upon request. |
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| |
NEUROONE MEDICAL TECHNOLOGIES CORPORATION |
| Dated: October 31, 2024 |
|
|
| |
By: |
/s/ David Rosa |
| |
|
David Rosa |
| |
|
Chief Executive Officer |
Exhibit 10.1
Certain
identified information has been excluded from the exhibit because it is both (i) not material and (ii) is of the type that the Company
treats as private or confidential. Double asterisks denote omissions.
amended
and restated EXCLUSIVE DEVELOPMENT
AND
DISTRIBUTION AGREEMENT
This
Amended and Restated Exclusive Development and Distribution Agreement (this “Agreement”) is entered into as of October
25, 2024 (the “Effective Date”), by and between Zimmer, Inc., a Delaware corporation (“Zimmer”),
and NeuroOne Medical Technologies Corporation, a Delaware corporation (the “Company”). Zimmer and the Company are
referred to individually as a “Party” and together as the “Parties”. Capitalized terms used herein,
to the extent not otherwise defined, have the meanings specified in Exhibit A.
BACKGROUND
A.
Zimmer and its Affiliates develop, manufacture and sell medical products.
B.
The Company developed technology, including Strip/Grid Products, relating to the use of electrodes for neurosurgical applications, including
the diagnosis of epilepsy.
C.
The Parties entered into an Exclusive Development and Distribution Agreement dated July 20, 2020, related to the SEEG and Strip/Grid
Product Systems, which was subsequently amended pursuant to the terms and conditions of a letter agreement dated January 6, 2021, a Second
Amendment to Exclusive Development and Distribution Agreement dated June 28, 2022, and a Third Amendment to Exclusive Development and
Distribution Agreement dated August 2, 2022 (collectively, the “EDDA”).
D.
The Parties desire to amend and restate the terms of the EDDA in its entirety pursuant to Section 11.3 of the EDDA to include certain
distribution rights for the OneRF™ Product System on the terms and conditions set forth in this Agreement.
E.
Pursuant to the terms of the EDDA, the Parties collaborated in the development of SEEG Products and Electrode Cable Assembly Products
for neurosurgical diagnostic applications, including the diagnosis of epilepsy.
F.
The Parties desire for Zimmer and its Affiliates to become the exclusive distributor of certain products of the Company, including the
Strip/Grid Products and the SEEG Products that are developed as a result of the Parties’ efforts under the EDDA, as well as the
OneRF™ Product System in accordance with the terms and conditions of this Agreement.
AGREEMENT
In
consideration of the mutual covenants set forth in this Agreement, and intending to be legally bound, the Parties hereby agree as follows:
ARTICLE
I
SEEG AND STRIP/GRID Product SYSTEMS DEVELOPMENT
1.1.
Project Scope. The Parties will collaborate in carrying out a project (the “Project”) to Develop the following
Products for use in neurosurgical applications:
(a)
Strip/Grid Products;
(b)
SEEG Products; and
(c)
Electrode Cable Assembly Products.
For
purposes of this Agreement, Sections 1.1(a) – (c) collectively shall be defined as the “SEEG and Strip/Grid Product Systems”).
1.2.
Development Plan. The initial development plan for the Project is set forth in the documents attached as Exhibit B and
the documents referenced therein (collectively, as amended as provided herein, the “Development Plan”). At any time
within ninety (90) days after the Original Effective Date, Zimmer shall have the right, but not the obligation, to cause the initial
Development Plan to be amended to include an Approved Design Modification by delivering written notice to the Company (a “Design
Modification Notice”). Upon receipt of a Design Modification Notice, the Parties shall mutually determine, in good faith, the
amendments to be made to the Development Plan. Following agreement on the required amendments, the Parties shall continue to implement
the Development Plan, as so amended. The initial Development Plan may be further amended from time to time by the JDC as contemplated
by Section 1.8.
1.3.
Development Responsibility; Approval Rights; Project Managers. Except as otherwise expressly stated in this Agreement or the Development
Plan, the Company shall be responsible for performing all development activities contemplated by the Development Plan. Following the
Original Effective Date, the Company shall not enter into any consulting agreement or other services agreement with, or pay any compensation
to, any health care professional for any purpose related to the SEEG and Strip/Grid Product Systems without the prior written consent
of Zimmer (which shall not be unreasonably withheld). Zimmer shall be responsible for providing reasonable support, assistance and consultation
to the Company with respect to the Project and shall have the right to review and approve any and all aspects of the design of the SEEG
Products and associated instrumentation and Electrode Cable Assembly Products pertaining to the critical features identified for each
such Product on Exhibit C. The Company hereby designates [**] as its project manager for its responsibilities in connection with
the Project and Zimmer hereby designates [**] as its project manager for its responsibilities in connection with the Project. Either
Party may designate in writing to the other Party an alternative project manager at its sole discretion.
1.4.
Efforts; Costs. Each Party will use Commercially Reasonable Efforts to perform and complete in a timely fashion its responsibilities
in connection with the Project. Except as otherwise expressly stated in this Agreement or the Development Plan, each Party shall be responsible
for such costs and expenses incurred by such Party in connection with the Project. For the avoidance of doubt, Zimmer shall be responsible
for all costs and expenses related to the Commercialization of the Products in the Territory and the Company shall be responsible for
all costs and expenses related to the Development of the SEEG and Strip/Grid Product Systems and the submission and prosecution of all
regulatory filings required for Regulatory Approvals for the Products in the Territory; provided, however, Zimmer and the Company acknowledge
and agree that the Products for all countries in the Territory outside of United States will be based upon the Products for which Regulatory
Approval has been received by the Company in the United States and to the extent Zimmer or any Regulatory Authority outside of the United
States requires additional changes to the Products or additional actions or requirements beyond the submission and prosecution of regulatory
applications for such Products in such country, then the Company and Zimmer shall negotiate in good faith and agree to the allocation
between the Parties of the costs and expenses related such additional changes to the Products or such additional actions or requirements.
Zimmer acknowledges and agrees that all Regulatory Approvals for the Products in the Territory will be owned by the Company.
1.5.
Records. The Company will maintain records of its activities under the Development Plan in sufficient detail and in a good scientific
manner appropriate for patent and regulatory purposes, including (a) the Design History Files, laboratory notebooks and invention disclosures
in reasonable detail to enable the preparation and submission of patent applications covering all patentable subject matter, and (b)
data and documentation (including instructions for use, user manuals and risk analysis) sufficient in form and substance to enable the
Regulatory Filings contemplated by Section 7.2. The records maintained by the Company for the Products shall include all information
required to be included therein by Zimmer’s quality systems.
1.6.
Audit Rights. Zimmer will have the right, solely with respect to the Project and the Products, at Zimmer’s sole cost and
expense, upon fifteen (15) days’ prior notice to the Company and during regular business hours, to inspect and audit the Company’s
records, facilities and operations for the purpose of verifying that the Company is using Commercially Reasonable Efforts to develop
the SEEG and Strip/Grid Product Systems. Zimmer shall not be permitted to exercise the audit rights set forth in this Section 1.6
more than twice during any calendar year of the Term (as defined below).
1.7.
Exclusivity. The Company and Zimmer shall be (a) exclusive development and commercial partners with respect to the development
and Commercialization of the SEEG and Strip/Grid Product Systems and related components to the SEEG and Strip/Grid Product Systems during
the SEEG and Strip/Grid Product Term and (b) exclusive commercial partner with respect to Commercialization of the OneRF™ Product
System and related components to the OneRF™ Product System during the RF Term, and during such respective time periods the Company
and Zimmer shall not (i) engage in or perform any development activities with respect to the SEEG and Strip/Grid Product Systems or OneRF™
Product System and related components to such systems, with or for the benefit of any other Person, or (ii) manufacture, market, sell
or distribute the SEEG and Strip/Grid Product Systems or OneRF™ Product System and related components to such systems, to any other
person or entity except as provided under this Agreement or the MS Agreement; provided, however, that nothing in this Section
1.7 shall prevent the Company or Zimmer from (x) utilizing third-party contract engineering, manufacturing or other development resources
in connection with the internal development of the SEEG and Strip/Grid Product Systems or the OneRF™ Product System or such related
components or (y) acquiring and owning any entity engaged in the business of developing, manufacturing, marketing, selling or distributing
electrodes or other products or technology that is similar to or competitive with the SEEG and Strip/Grid Product Systems or the OneRF™
Product System; provided, however, if Zimmer acquires or directly or indirectly owns a controlling interest in any entity engaged in
the business of developing, manufacturing, marketing, selling or distributing electrodes for the diagnosis or RF treatment any indication
within the Field (a “Competitive Triggering Event”), the Company shall have the right to terminate this Agreement,
in the Company’s sole discretion pursuant to the terms and conditions of Section 10.3(d) of this Agreement following such
Competitive Triggering Event provided that the Company pays to Zimmer the Competitive Triggering Event Termination Fee.
1.8.
Joint Development Committee.
(a)
The Project Participants will coordinate their development activities associated with the SEEG and Strip/Grid Product Systems through
a joint development committee (the “JDC”) comprising two representatives of each Project Participant. The initial
representatives of the Company will be [**] and [**] and the initial representatives of Zimmer will be [**] and[**]. The JDC will be
co-chaired by one representative of each Project Participant selected by such Project Participant. The Project Participants will consult
in good faith regarding any changes to the individuals serving on the JDC. Subject to reasonable advance notice, a Project Participant
may invite other members of its organization to attend a particular meeting, but any such additional attendee shall not have any of the
rights or responsibilities of a formally appointed representative.
(b)
Authority. The JDC shall have the authority to approve modifications to the Development Plan, the Regulatory Filing strategy,
the Specifications and the Other Product Specifications; otherwise, the JDC shall be a consultative, rather than decision-making, body.
For the avoidance of doubt, the JDC shall have no authority to amend any of the terms or conditions of this Agreement or the MS Agreement.
All approvals of the JDC shall require unanimous consent; provided that, if the JDC is unable to reach unanimous agreement on a matter
within its authority, then the Company’s CEO and a designated representative to be identified by Zimmer from Zimmer will negotiate
in good faith to decide the matter; provided, however the Parties acknowledge and agree in the event of any modifications to the Development
Plan, the Regulatory Filing strategy, the Specifications or the Other Product Specifications, the Parties shall revise the deadlines
set forth in Section 6.1(d) to reflect such modifications.
(c)
Meetings. The JDC will meet in accordance with a schedule established by agreement of the Project Participants, but no less frequently
than quarterly, from the Effective Date until the Date of the First Commercial Sale of the last Product to achieve commercial sales,
and as needed thereafter. Meetings may be held remotely if so agreed. Each Project Participant will use Commercially Reasonable Efforts
to cause its representatives to attend the JDC meetings and will be responsible for the expenses incurred by its own representatives
in participating in the JDC. The co-chairs will be responsible for distributing an agenda for each meeting at least three days in advance
of the meeting. Each Project Participant will have the right to request the co-chairs to include any matter or issue within the purview
of the JDC, which requests will be accommodated by the co-chairs to the extent feasible. The co-chairs will alternate responsibility
for generating and circulating minutes of each meeting (which will include a summary of any actions agreed at the meeting) to each appointed
representative for review and comment. Any corrections or comments must be submitted to the co-chairs within ten Business Days after
the draft minutes have been circulated.
(d)
Duration. The JDC will operate until the Date of the First Commercial Sale of the last Product to achieve a First Commercial Sale,
and as needed thereafter.
ARTICLE
II
INTELLECTUAL PROPERTY
2.1.
Development License Grant. In consideration of the potential benefits to the Company by reason of the Project, during the Term,
the Company hereby grants to Zimmer a non-exclusive, royalty-free license under the IP that is owned by the Company in the Territory
and within the Field, under the control of the Company or to which the Company otherwise has access and that is necessary for or otherwise
useful to the Project (the “Company Background IP”), without the right to grant sublicenses (except to its Affiliates),
for the sole purpose of conducting the Project within the Field. Such license and any such sublicenses shall survive completion of the
Project and shall continue for the remaining Term of this Agreement and for so long as Zimmer continues to sell or distribute any Products
under this Agreement. The Company shall own and retain all right, title and interest in and to the Company Background IP.
2.2.
Program IP.
(a)
Ownership. The rights of ownership of Program IP shall be determined in accordance with United States patent and other intellectual
property laws (regardless of where the Program IP was invented, conceived, discovered, created, made, developed, reduced to practice
or otherwise perfected or exists). Notwithstanding the foregoing, as between Zimmer and the Company, all right, title and interest in
and to Program IP shall be determined as follows:
(i)
Zimmer shall own all right, title and interest in all Program IP that is invented, conceived, discovered, created, made, developed, reduced
to practice or otherwise perfected solely by one or more employees, agents or consultants of Zimmer, its Affiliates or subcontractors
(“Zimmer Program IP”);
(ii)
the Company shall own all right, title and interest in all Program IP that is invented, conceived, discovered, created, made, developed,
reduced to practice or otherwise perfected solely by one or more employees, agents or consultants of the Company, its Affiliates or subcontractors
(“Company Program IP”).
(iii)
Zimmer and the Company shall jointly own all right, title and interest in all Program IP that is invented, conceived, discovered, created,
made, developed, reduced to practice or otherwise perfected by one or more employees, agents or consultants of Zimmer, its Affiliates
or subcontractors together with one or more employees, agents or consultants of the Company, its Affiliates or subcontractors (“Joint
Program IP”).
(b)
Assignment and Assistance. Each of Zimmer and the Company hereby, on behalf of themselves and each of their respective Affiliates,
employees and contractors, assigns to one another ownership of rights, title and interest in and to such Program IP to effect the ownership
allocation set forth in Section 2.2(a). In furtherance of the foregoing, each such Party shall, upon request by the other, promptly
undertake and perform (and/or cause its Affiliates and its and their respective contractors, employees and/or agents to promptly undertake
and perform) such further actions as are reasonably necessary for Zimmer and the Company (as between them) to each perfect its title
in any such Program IP, including by causing the execution of any assignments or other legal documentation, and/or providing the other
Party or its patent counsel with reasonable access to any contractors, employees or agents who may be inventors of such Program IP.
(c)
Rights of Use in Joint Program IP. For clarity, Zimmer and the Company shall co-own any and all Joint Program IP with the ability
to use and sublicense such Joint Program IP for any and all purposes, in perpetuity, without the need to account to the other (subject,
in all cases, to any other applicable terms of this Agreement).
2.3.
Program Patent Rights.
(a)
Prosecution. The Company will use Commercially Reasonable Efforts to Prosecute Program Patent Rights claiming Company Program
IP of commercial interest or importance in the Designated Jurisdictions and shall be responsible for all costs and expenses associated
therewith. The Company will use Commercially Reasonable Efforts to Prosecute Program Patent Rights claiming Joint Program IP of commercial
interest or importance in the Designated Jurisdictions, in consultation with Zimmer, and the Company and Zimmer shall equally split the
external costs associated therewith. At the request and expense of the Company, Zimmer shall provide reasonable assistance in connection
with the Prosecution of Program Patent Rights claiming Joint Program IP. The Parties shall actively consult with each other in connection
with the Prosecution of such Program Patent Rights and shall have the right to participate in all strategic decisions and to review and
comment on all filings. The Company shall keep Zimmer informed of all material developments relating to such Prosecution. Notwithstanding
the foregoing, in the event the Company is unwilling or unable to Prosecute Program Patent Rights as set forth in this Section 2.3(a),
the Company shall give Zimmer full control of all such Prosecution efforts and shall assign any such Program Patent Rights to Zimmer,
after which (i) Zimmer shall bear all costs and expenses associated with the Prosecution of such Program Patent Rights; (ii) the Company
shall cooperate with Zimmer to complete, execute, and provide to Zimmer any and all documents necessary to facilitate such control by
Zimmer, including documents sufficient to appoint and convey powers of attorney to Prosecution counsel of Zimmer’s choosing in
each of the foregoing jurisdictions and, if necessary, to withdraw any Prosecution counsel previously appointed by the Company; (iii)
Zimmer shall have no obligation to consult with the Company or otherwise keep the Company informed in connection with the Prosecution
of such Program Patent Rights; and (iv) absent a request from Zimmer, the Company shall have no right to participate in any strategic
decision or to review or comment on any filings with respect to such Prosecution.
(b)
Zimmer Program IP. For the avoidance of doubt, Zimmer shall have the sole and exclusive right, in its discretion, to Prosecute
Program Patent Rights contained within the Zimmer Program IP and the Company shall have no rights in connection therewith.
2.4.
Zimmer Distribution Licenses.
(a)
The Company hereby grants to Zimmer and its Affiliates the exclusive right and license under the Company IP or any other intellectual
property owned or licensed to Company to use, promote, market, sell, offer for sale, distribute, import/export and otherwise Commercialize
and to have others do any of the foregoing on their behalf the Products in the Territory for all uses and applications in the Field (the
rights to the SEEG and Strip/Grid Product Systems the “SEEG and Strip/Grid Distribution License”, the rights to the
OneRF™ Product System the “RF Distribution License”, and collectively the “Zimmer Distribution License”).
During the Term, the Company shall grant Zimmer and its Affiliates access to Company’s Intellectual Property to the extent necessary
to facilitate their ability to exploit the Zimmer Distribution License.
(b)
During the SEEG and Strip/Grid Product Term with respect to the SEEG and Strip/Grid Product Systems, and during the RF Term with respect
to the OneRF™ Product System, each Zimmer Distribution License shall be exclusive, even as to the Company itself. So long as a
Zimmer Distribution License is exclusive, the Company will not, directly or indirectly, promote, market, sell, distribute or otherwise
Commercialize the Products in the Territory for any use in the Field, either on its own behalf or through any Affiliate or Third Party
without Zimmer’s prior written consent.
(c)
Zimmer shall have the right to grant sublicenses under the Zimmer Distribution Licenses, including the right to authorize its Affiliates
and Distributors to participate (each a “Subdistributor”), subject to the terms of this Agreement, in the use, promotion,
marketing, sale, distribution and Commercialization of the Products in accordance with this Agreement and all Applicable Laws anywhere
in the Territory for any use in the Field. In the event that any Subdistributor takes any action that would constitute a breach of the
terms of this Agreement or violates any laws relating to the sale, marketing, promotion or distribution of the Product in any country
within the Territory, then Zimmer shall use its Commercially Reasonable Efforts to cause such Subdistributor to remedy such breach or
violation.
(d)
The Company shall not grant distribution rights with respect to the SEEG and Strip/Grid Product Systems or the OneRF™ Product System
in the Field to any Third Party during the Strip/Grid Product Term or the RF Term respectively, or make any commitment or enter into
any contract, license or agreement that would materially adversely impact or render ineffective Zimmer’s rights under this Section
2.4(d).
(e)
With respect to the Territory for the OneRF™ Product System, Zimmer shall be granted the exclusive right to negotiate until December
31, 2024 the inclusion of any additional countries within the definition of “Territory” as set forth in this Agreement.
Accordingly, the Company agrees that it will not engage with, negotiate or otherwise discuss distribution rights with any third party
for the OneRF™ Product System in any country or territory until January 1, 2025.
2.5.
Third Party Intellectual Property. Except as specifically provided for in this Agreement, in a written agreement between the Company
and a Third Party existing on the date hereof, or as may be specifically agreed between the Company and Zimmer during the Term of the
Project, the Company shall not use any third-party intellectual property rights in undertaking its development efforts.
2.6.
Enforcement of Rights. If either Party learns of any infringement or violation by a Third Party of any Program IP, it shall notify
the other Party as soon as practicable. Thereafter, the Parties shall consult on a course of action with respect to such infringement
or violation. Unless otherwise agreed to by the Parties in the course of their consultations, the Company shall have the first right
to bring and control any claim, action or proceeding against such Third Party for such infringement or violation of the Program IP (an
“Enforcement Action”) by counsel of its own choice. If the Company fails to initiate any such Enforcement Action within
the earlier of 90 days following notice of the basis therefor or 30 days before the expiration of any applicable time limit for bringing
such Enforcement Action, then Zimmer shall have the right to bring and control any such Enforcement action by counsel of its own choice.
If Zimmer lacks standing to bring such Enforcement Action, then the Company shall be obligated to bring such Enforcement Action if so
requested by Zimmer. In all events, the non-controlling Party shall have the right to be represented in any such Enforcement Action by
counsel of its own choice. If the Party bringing any such Enforcement Action is unable to initiate or Prosecute it solely in its own
name, the other Party shall join in voluntarily and shall execute all documents necessary to enable the initiating Party to Prosecute
and maintain such Enforcement Action. In connection therewith, the Parties shall cooperate fully and provide each other with any information
or assistance reasonably requested. Each Party shall keep the other informed of developments, including the status of settlement negotiations.
Neither Party shall settle such Enforcement Action without the prior written consent of the other Party, which consent shall not be unreasonably
withheld, conditioned or delayed. Each Party shall bear its own costs and expenses in connection with the Enforcement Action, but shall
be entitled to full reimbursement thereof out of any recovery or monetary award realized in connection therewith. After such reimbursement,
the balance of any recovery or monetary award shall be allocated between the Parties in proportion to the relative economic losses suffered
by each, as determined by the Parties in good faith.
2.7.
Defense of Infringement Claims. If any Third Party asserts a Claim against a Party (or any of its Affiliates or Distributors)
alleging that any Product, the use or practice of the Program IP or any activity pursuant to this Agreement infringes, misappropriates
or violates the Intellectual Property Rights of any Third Party (any such Claim being referred to as an “Infringement Claim”),
the Party first having notice of the Infringement Claim shall promptly notify the other Party thereof in writing specifying the facts,
to the extent known, in reasonable detail. The Company shall indemnify Zimmer with respect to Infringement Claims pursuant to Section
8.4.
2.8.
Use of Zimmer Trademarks. If directed by Zimmer in writing, the Company shall place onto the Products shipped to Zimmer (or the
packaging for such Products) the designated Zimmer Trademarks and part numbers. In the event that any Products so labeled are not delivered
to Zimmer, whether due to scrap, rejection, cancellation of orders or otherwise, the Company shall promptly remove and destroy or, at
the request of Zimmer, return to Zimmer, any and all labels, name plates, or other Trademarks placed on the Products. The Company shall
not use the “Zimmer Biomet”, “Zimmer” or “Biomet” names or any Zimmer Trademarks except as provided
in this Agreement and in accordance with Zimmer’s policies regarding the use of such names or Trademarks, and any use of the names
“Zimmer Biomet “, “Zimmer” or “Biomet” by the Company in labeling, advertisements and other similar
usages shall be subject to the prior written approval of Zimmer. Upon termination of this Agreement or upon the request of Zimmer, the
Company will discontinue the use of all Zimmer Trademarks, including the names “Zimmer Biomet”, “Zimmer” and
“Biomet”, and, thereafter, will not use any of the Zimmer names or Trademarks in any manner except as may be expressly permitted
by other agreements between the Company and Zimmer.
2.9.
Promotion Limitation. The Company covenants that it shall not use the “Zimmer Biomet”, “Zimmer” or “Biomet”
names or any Zimmer Trademarks, in any advertising or promotion by the Company (whether by including reference to Zimmer in any list
of customers, advertising that its services and products are used by Zimmer, denying or confirming the foregoing or for any other purposes)
without advance written permission from Zimmer. Notwithstanding the above, the Company can use the “Zimmer Biomet”, “Zimmer”
or “Biomet” names or any Zimmer Trademarks, for: (a) press announcements or other communications consistent with Zimmer’s
own public announcements or regulatory filings or this Agreement; (b) press announcements or other communications consistent with medical
publications and/or abstracts related to the Product; and (c) as required by law or regulation.
2.10.
Trademark License. Zimmer and its Affiliates and Distributors will have the right to use the Company’s Trademarks associated
with the Products as required by applicable Regulatory Laws or as otherwise necessary in connection with Zimmer’s marketing and
distribution of the Products. Zimmer and its Affiliates and Distributors shall comply with the reasonable quality control instructions
of the Company as to the form and manner in which such Trademarks are used. Other than as expressly provided in this Agreement, no Party
shall acquire or have any right to use the name or Trademarks of the other Party without its prior written consent.
2.11.
Rights upon Company Insolvency. Each of the Zimmer Distribution License and the licenses granted under Section 2.1 herein
shall be deemed to be, for purposes of Section 365(n) of the United States Bankruptcy Code (and any similar laws of other countries),
a license of rights to “intellectual property” as defined therein. Zimmer and its Affiliates, as licensees of such rights,
shall have the rights and elections with respect thereto as specified in the United States Bankruptcy Code (and any similar laws of other
countries). If a bankruptcy or similar proceeding is commenced by or against the Company and the Company (or a trustee or other Person
acting on its behalf) thereafter rejects this Agreement or fails to perform all of its obligations hereunder, then Zimmer and its Affiliates
shall be entitled to retain their rights under this Agreement (and under any agreement supplementary hereto) as such rights existed prior
to commencement of such proceeding.
2.12.
No Reverse Engineering. The Parties agree not to reverse engineer any Products, except to the extent permitted by this Agreement
or to the extent enforcement of the foregoing is prohibited by Applicable Law.
2.13.
Development of Related Instruments. Zimmer shall have the sole right (at Zimmer’s sole cost and expense) to design, Develop
and Commercialize Related Instruments itself or through third-party developers or vendors, which may include the Company. No Intellectual
Property created, discovered or developed directly related to the design, Development or Commercialization of the Related Instruments
shall be Program IP for purposes of this Agreement and all Intellectual Property Rights with respect thereto shall belong to Zimmer.
To the extent requested by Zimmer, the Company shall include one or more of the Related Instruments in its Regulatory Filings and Regulatory
Approvals, and Zimmer shall provide to the Company all information reasonably requested by the Company for the purpose of making such
Regulatory Filings or obtaining such Regulatory Approvals and with respect to such Related Instruments that are included by the Company
in any such Regulatory Filings and Regulatory Approvals, Zimmer hereby grants to the Company a non-exclusive license to the Intellectual
Property Rights related to the Related Instruments during the Term and for a period of nine (9) months following the expiration or termination
of this Agreement.
ARTICLE
III
DATA PRIVACY AND SECURITY
3.1.
Company Data Privacy and Security Program. The Company shall establish and maintain a data privacy and security program that complies
with all Applicable Laws regarding data privacy and security and is consistent with industry standards for privacy, confidentiality and
data security in industries involving the collection, storage and processing of Protected Data. Zimmer, together with its outside counsel
and an independent, third-party auditor, shall have the right to review and assess the Company’s data privacy and security program
to assess its compliance with Applicable Laws. Zimmer shall not be permitted to exercise the audit rights set forth in this Section
3.1 more than twice during any calendar year of the Term.
ARTICLE
IV
DISTRIBUTION
4.1.
Marketing and Sales Activities. Zimmer will make the Products available to its sales force and will use Commercially Reasonable
Efforts to promote, market and sell the Products. Notwithstanding the foregoing, Zimmer will have sole discretion to establish the pricing
and other terms and conditions of sale of the Products to its customers.
4.2.
Branding. The Parties acknowledge that Zimmer may establish separate brand(s) and Trademark(s) for use and association exclusively
with the Products to be marketed and sold by Zimmer pursuant to this Agreement. Zimmer shall own the associated Trademark(s). The Company
shall be responsible for the development, quality and regulatory efforts required in conjunction with labeling changes for the Products
required under Applicable Law as a result of branding decisions. The Company shall cooperate as requested by Zimmer to obtain approval
of such brand names as may be required by Regulatory Laws and shall package the Products consistent with the branding specifications
for such brand(s). Until any new Zimmer brand(s) are established and at any time thereafter, the Parties acknowledge that Zimmer and
its Affiliates and Distributors may distribute and sell Products using the brand(s) associated with such Products. Zimmer shall reimburse
the Company for all reasonable costs incurred by the Company in connection with any branding or labeling changes for the OneRF™
Product System.
4.3.
Training, Marketing and Education Support.
(a)
Company Training for Zimmer. Throughout the Term, the Company will provide, for no additional consideration, (i) periodic Product
sales training sessions for sales personnel of Zimmer, its Affiliates and Distributors, at times and locations as shall be mutually agreed,
(ii) a system for real-time advice regarding the Products for Zimmer’s sales personnel, (iii) reasonable customer and field support
(including a system to respond effectively to Product Complaints and warranty claims), and (iv) reasonable assistance in developing training
and sales/marketing literature as needed to facilitate the marketing, sale and distribution of the Products.
(b)
Training Compliance. All Company training sessions for Zimmer personnel and all Product informational materials created or used
by the Company with respect thereto shall comply with all Zimmer corporate policies, practices and procedures related to advertising,
promotional and informational materials. In addition, all surgeon education and training events and programs conducted by either party
online or in person shall comply with all Zimmer compliance policies, practices and procedures related to arrangements with healthcare
professionals.
4.4.
Right to Product Improvements. Zimmer shall have the distribution right in the Field and Territory to any and all Product Improvements
developed or produced by the Company (i) during the SEEG and Strip/Grid Term, for the SEEG and Strip/Grid System, and (ii) during the
RF Term, for the OneRF™ Product System. The Company shall promptly notify Zimmer of the completion of each Product Improvement
and shall collaborate with Zimmer to integrate such Product Improvement into the Products.
4.5.
[**].
4.6.
Territory Expansion. During the Term, the Parties will negotiate in good faith regarding including additional countries
to be included in the definition of Territory. In the event that Zimmer seeks to directly or indirectly (e.g., through a distributor)
market and sell the Products in a country not identified in the Territory, it shall notify Company of its desire to include the country
within the Territory and provide information reasonably requested by Company. Company, exercising commercially reasonable judgment,
shall respond to Zimmer within [**] days of Zimmer’s request and, if agreed, the Parties shall amend this Agreement to include
the additional country(ies) within the definition of Territory. Further, to the extent Company seeks to directly or indirectly
through a third party market or sell Products in a country not identified in the definition of Territory, it shall inform Zimmer of such
opportunity and provide Zimmer with information as reasonably requested regarding such opportunity. Upon receipt of such notice,
Zimmer shall have the right to propose an alternative sales and/or distribution channel to Company within [**] days of such notice from
Company. Company, exercising commercially reasonable judgment, shall consider such proposal and, if mutually agreed to by the Parties,
amend the Agreement to include the additional country(ies) with the definition of Territory.
ARTICLE
V
MANUFACTURING AND SUPPLY
5.1.
Manufacturing and Supply Agreement. On or prior to the date of the LMR Order for the Strip/Grid Products, the Parties shall enter
into a Manufacturing and Supply Agreement in substantially the form attached hereto as Exhibit D (the “MS Agreement”).
The Parties agree to amend the MS Agreement on or prior to November 15, 2024 to include the OneRF Product System to be manufactured and
supplied by the Company under the MS Agreement. Following the execution or amendment thereof, the Parties shall comply at all times with
the requirements set forth in the MS Agreement (as so amended, if applicable). In the event of conflict between the provisions of this
Agreement and the MS Agreement (as so amended, if applicable), this Agreement shall control.
5.2.
Manufacturing Permits and Approvals. At all times during the Term of this Agreement, the Company shall obtain and maintain in
full force and effect (at the Company’s expense) all permits, certifications, approvals and licenses required by Applicable Laws
(including, as applicable, ISO certification, FDA registration, and all other U.S. or international permits and approvals required),
and shall make all filings with Governmental Authorities, that are necessary and/or legally required to conduct any business involving
the development, manufacture, sale, distribution or promotion of the Products and otherwise for the performance of the Company’s
duties and obligations under this Agreement. The Company shall notify Zimmer as soon as practicable after receiving notice of any claim
or action by the FDA or other Governmental Authority with respect to its permits, certifications or licenses that could adversely affect
the Company’s performance of its obligations under this Agreement.
5.3.
Quality Agreement. On the date of execution of the MS Agreement, the Company and Zimmer shall enter into a supplier quality agreement
similar to the form Quality Agreement attached hereto as Exhibit E (the “Quality Agreement”). Following the
execution thereof, the Parties shall comply at all times with the quality requirements set forth in the Quality Agreement. In the event
of conflict between the provisions of this Agreement and the Quality Agreement, this Agreement shall control. The Parties agree to amend
the Quality Agreement on or prior to November 15, 2024 to include the OneRF Product System.
ARTICLE
VI
FEES AND TRANSFER PRICING
6.1.
Exclusivity Fees and Milestone Payment.
(a)
In consideration for the exclusive distribution rights granted to Zimmer under the EDDA, Zimmer shall make an initial payment to the
Company in the amount of $2,000,000 (the “Initial Exclusivity Fee”) in immediately available funds on the tenth (10th)
Business Day after the Original Effective Date. The Company agrees to use the proceeds from the Initial Exclusivity Fee first, to pay
expenses associated with the Project and thereafter for general working capital purposes.
(b)
In consideration for the exclusive RF Distribution License granted to Zimmer hereunder, Zimmer agrees to pay the Company the RF Exclusivity
Fee in immediately available funds on the tenth (10th) Business Day after the Effective Date.
(c)
The Company shall be paid a milestone fee of one million dollars ($1,000,000) (the “OneRF™ Product System Sales Milestone
Payment”) if Net Sales of the RF Generator and Temperature Accessory Kit (collectively the “RF Milestone Products”)
only from the OneRF™ Product System meets or exceeds [**].
(d)
Except where Zimmer timely delivers a Design Modification Notice pursuant to Section 1.2, if one or more of the events set forth
in the table below occurs on or before the deadline indicated for such event and the Product Availability Date for the SEEG Products
occurs on or before [**], then the Company shall receive the additional amount indicated for such event as part of the SEEG Exclusivity
Maintenance Fee.
| Event | |
| Deadline | | |
| Interim
Fee Bonus | |
| (i)
[**] | |
| [**] | | |
| [**] | |
| (ii)
[**] | |
| [**] | | |
| [**] | |
Notwithstanding
the foregoing, if Zimmer timely delivers a Design Modification Notice to the Company pursuant to Section 1.2, and one or more
of the events set forth in the table below occurs on or before the deadline indicated for such event and the Product Availability Date
for the SEEG Products occurs on or before [**], then the Company shall receive the additional amount indicated for such event as part
of the SEEG Exclusivity Maintenance Fee:
| Event | |
| Deadline | | |
| Interim
Fee Bonus | |
| (iii)
[**] | |
| [**] | | |
| [**] | |
| (iv)
[**] | |
| [**] | | |
| [**] | |
Each
additional amount described in clauses (i) through (iv) of this Section 6.1(d) is referred to in this Agreement as an “Interim
Fee Bonus”. For purposes of this Agreement, each of the foregoing events shall have occurred only if the Company has demonstrated
the achievement of the event to Zimmer’s reasonable satisfaction. Notwithstanding the foregoing, the events in Sections 6.1(d)(ii),
(iii) and (iv) shall not be deemed to be met if FDA Approval for the SEEG Products is not received prior to the applicable
deadline.
(e)
Reserved.
(f)
Commencing on [**] and continuing until [**], Zimmer shall submit to the Company a written quarterly report including (i) its computation
of the aggregate Net Sales of the RF Milestone Products and (ii) a list of aggregate sales during the month by RF Milestone Products
for each quarter of the milestone earnout period set forth in Section 6(c). The quarterly report shall be provided to Company within
[**] days of the end of each quarter as set forth herein (e.g., the 2024 Q4 report shall be due on or before [**] to Company). For clarity,
the last quarterly report shall contain the sales information referenced in this Section 6(f) and shall be provided to Company on or
before [**].
(g)
The Initial Exclusivity Fee, the SEEG Exclusivity Maintenance Fee (including any Interim Fee Bonus(es)), the RF Exclusivity Fee, and
the OneRF™ Product System Sales Milestone Payment, once paid, are non-refundable.
For
clarity, the Parties agree that as of the Effective Date, Zimmer has paid Company the consideration set forth in Sections 6.1(a) and
6.1(d) and no additional consideration is due and owing to Company pursuant to such provisions.
6.2.
Transfer Pricing; Initial Orders and Minimum Purchase Requirements .
(a)
Reserved.
(b)
Transfer Price for the Products. With respect to the transfer pricing for the first three (3) years after the Effective Date,
the pricing for the products shall be as set forth below and on Exhibit F hereto:
RF
Product Pricing
[**]
Non
RF Product Pricing
[**]
The
transfer price for calendar year 2027 et seq. for all the products listed above will be mutually agreed to by the Parties on or
before [**] of each calendar year for the subsequent year negotiating in good faith. In the event the Parties are unable to reach agreement,
the transfer prices listed above will be set at a [**] increase from the prior year.
Within
ten (10) business days after the Effective Date, Zimmer will provide Company with purchase orders for RF Products. A number of RF Products
equal to at least the Minimum Purchase Requirements for 2024 will be delivered in 2024, and if the purchase orders exceed the Minimum
Purchase Requirements for 2024, the remainder may be delivered in 2025.
(c)
Minimum Purchase Requirements for RF Products. Zimmer agrees to the Minimum Purchase Requirements for calendar years 2024, 2025 and 2026
below:
| Product | |
2024 | | |
2025 | | |
2026 | |
| SEEG-RF Electrode | |
| [**] | 1 | |
| [**] | | |
| [**] | |
| Temperature Accessory Kit | |
| [**] | | |
| [**] | | |
| [**] | |
| RF Generator | |
| [**] | | |
| [**] | | |
| | |
(The
SEEG-RF Electrode, Temperature Accessory Kit and RF Generator are collectively referred to as “Minimum Purchase Requirements”).
The
Minimum Purchase Requirements for SEEG-RF Electrode and Temperature Accessory Kit for calendar year 2027 et seq. will be mutually
agreed to by the Parties on or before [**] of each calendar year for the subsequent year. In the event the Parties are unable to reach
agreement, the Minimum Purchase Requirement for SEEG-RF Electrode and Temperature Accessory Kit will be set at a [**] increase from the
prior year. For clarity, there shall be no minimum purchase requirements for the RF Generator (after 2025) or any other product not listed
in the table above.
In
the event that Zimmer fails to meet the Minimum Purchase Requirements during any one (1) year period, Zimmer shall have the option, in
its sole discretion, to take one of the following actions, within [**] days of the end of the calendar year in which the annual Minimum
Purchase Requirement was not met (“Cure Period”):
(i)
Place a purchase order for the difference in the amount of Minimum Purchase Requirements purchased (or subject to outstanding purchase
order for that year) and the Minimum Purchase Requirements for each product segment. By way of illustration if in [**]; or
(ii)
Make a payment to the Company in an amount equal to the product of the respective Transfer Price multiplied by the difference between
(1) the annual Minimum Purchase Requirement for the product(s) in the table set forth above, minus (2) the number of units of
such products purchased by Zimmer (including outstanding purchase orders for that year,) in the applicable year.
If
Zimmer fails to meet its Minimum Purchase Requirements in any year and does not take one of the actions set forth above within [**] days
after the end of each calendar year (i.e., by [**] of the respective year), the Company shall have the right to issue a written notice
of termination of this Agreement which shall take effect [**] days upon receipt of such written notice. During the [**] period beginning
on the date that the Company provides written notice of such termination, notwithstanding anything herein to the contrary, the Company
shall have the right to enter into discussions and negotiations with third parties regarding the potential licensing of the Products.
| 1 | This
quantity excludes non-RF SEEG electrode quantities that have already shipped or are in process
for 2024 as of the Effective Date. |
(d)
Initial Orders for SEEG and Strip/Grid Product System. Not later than thirty (30) days following the execution of the MS Agreement
(in the case of Strip/Grid Products) and no later than thirty (30) days following the Acceptance of all Deliverables for SEEG Products
under the Development Plan, Zimmer, or its Affiliates or Distributors, will place an initial order or orders under the MS Agreement to
purchase a sufficient number of Strip/Grid Products or SEEG Products, as applicable, to conduct a Limited Market Release of such Products
(an “LMR Order”). Thereafter, Zimmer will have the right with respect to each Product to place an initial stocking
order (an “Initial Stocking Order”) for such Product in a quantity determined by Zimmer in its sole discretion to
be sufficient to support the commercial launch of such Product.
6.3.
Taxes. Zimmer shall pay all sales, use and transfer taxes and other charges arising out of the purchase and sale of the Products,
including any VAT and personal property taxes and all inspection fees and duties, applicable to the sale and transport of the Products
by Zimmer or any Subdistributors in the Territory which are applicable thereto. The Company shall not be responsible for any business,
occupation, withholding or similar tax, or any taxes of any kind, relating to the purchase and sale of the Products.
6.4.
Customer Support. Zimmer shall be solely responsible for providing, and shall provide the customers in the Territory with, all
training with respect to the Products that is necessary to support the normal use of the Products by customers. Zimmer shall, at its
cost, cause all of its sales force personnel to become sufficiently knowledgeable and competent with respect to the Products, to allow
Zimmer to meet its customer support obligations under this Section 6.4. The Company will provide required training on the
Products at the Company’s expense pursuant to Section 4.3 above, although Zimmer is responsible for travel and lodging and
other expenses related to attending such required training.
6.5.
OUS Forecast. On or before [**], Zimmer shall provide Company with a nonbinding OUS forecast for the Products for a period of
at least one (1) year.
ARTICLE
VII
COMPLIANCE AND QUALITY CONTROL
7.1.
Compliance with Laws.
(a)
Each Party shall comply in all material respects with all Applicable Laws that pertain to its activities under this Agreement and, except
as otherwise provided for herein, will bear the entire cost and expense of such compliance.
(b)
To the extent the Company retains Health Care Professionals to assist with Development of the Products, those Health Care Professionals
will be engaged (i) through a written consulting agreement that requires the Health Care Professional to abide by the laws and regulations
enumerated herein under the definition of Applicable Laws, (ii) following completion of needs assessment and due diligence processes,
and (iii) compensated hourly at a fair market value rate. All related compensation must be accurately recorded and reported under the
Physician Payments Sunshine Act and other Applicable Laws.
(c)
The Company will ensure that its employees receive anti-bribery and anti-corruption compliance training.
7.2.
Regulatory Approvals.
(a)
Company Responsibilities. The Company will make all Regulatory Filings and obtain and maintain all required Regulatory Approvals
(including all required FDA Approvals) for the Products in the Designated Jurisdictions. The Company will have primary responsibility
for all communications, submissions and interactions with Regulatory Authorities for the purpose of obtaining and maintaining Regulatory
Approvals; provided, however, that solely with respect to Products in the Field and in the Territory, Zimmer will have
the right to (i) review and provide input into each, including the preparation of each Regulatory Filing and the proposed indications
and instructions for use (or equivalent labeling) included in each Regulatory Filing, (ii) require the Company to include one or more
Related Instruments in each Regulatory Filing, and (iii) participate generally in the regulatory process at its own expense. To facilitate
such participation, solely with respect to Products in the Field and in the Territory, the Company will (x) notify Zimmer of all planned
Regulatory Filings and regulatory actions, communications and meetings sufficiently in advance to allow Zimmer a reasonable opportunity
to review, comment and/or participate, as applicable; and (y) provide Zimmer copies of all relevant communications, correspondence and
documents received from Regulatory Authorities promptly after receipt, including, where applicable, copies of the following items and
equivalent information for non-U.S. jurisdictions: FDA clearance letters, 510(k) submissions, Declarations of Conformity, ISO 13485 certification,
redacted Essential Requirements verification checklists, and any licenses or evidence of registration of Products in its possession and
control as applicable for sale in the Territory. The Company will be responsible for all costs and expenses relating to obtaining and
maintaining Regulatory Approvals for the Products (including, if requested by Zimmer, any Related Instruments included in the applicable
Regulatory Filing) in the Designated Jurisdictions. The Company hereby grants to Zimmer the fully paid-up right and license to use any
and all Regulatory Approvals related to the Products in the Territory owned by or licensed to the Company and existing as of the date
of this Agreement or obtained during the Term.
(b)
Material Communications. The Company will keep Zimmer informed on a regular and timely basis of all material communications and
filings with, to or from all Regulatory Authorities relating to the Products or the Regulatory Approvals solely with respect to Products
in the Field and in the Territory.
(c)
Other. Zimmer will have primary responsibility for all communications, submissions and interactions with the FDA and other applicable
Regulatory Authorities for the purpose of obtaining and maintaining regulatory approvals for any devices or products associated with
the Products, including, as applicable, any mobile applications designed by Zimmer using information from the Products, any Related Instruments
that are not included in the Company’s Regulatory Filings, or, to the extent relevant, other medical devices incorporating the
Products. The Company shall provide all information and support necessary to enable Zimmer to obtain and maintain such approvals.
7.3.
Regulatory Proceedings. The Company shall be responsible to Regulatory Authorities throughout the Territory as the manufacturer
of the Products. If either Party receives notice of an actual or threatened inspection, investigation, inquiry, import or export ban,
product seizure, enforcement proceeding or similar action by a Regulatory Authority with respect to a Product or a Party’s activities
in connection with a Product, it will notify the other Party within two Business Days after its receipt of notice of the action and will
promptly deliver to the other Party, within a further one Business Day, copies of all relevant documents received from the Regulatory
Authority. The Parties shall cooperate in response to the action, including providing information and documentation as requested by the
Regulatory Authority. If the action primarily concerns Zimmer’s activities, then Zimmer will have primary responsibility to respond
to the Regulatory Authority; otherwise, the Company will have primary responsibility to respond. In either case, upon request of the
responding Party, the other Party shall cooperate in good faith and provide consulting advice and assistance with the response.
7.4.
Product Labels. All Products will be labeled to reflect the Company as the manufacturer and Zimmer as the distributor. The Company
will work with Zimmer to ensure Product Part numbers avoid conflicts for Zimmer. Zimmer shall have the right to review and approve all
labeling for the Products (including package inserts/IFUs, product brochures, surgical techniques and web-site materials), and the Company
agrees to make modifications to such labeling for the Products as reasonably requested by Zimmer. If applicable, the Company will have
sole responsibility for obtaining all necessary Product labels and for negotiating the language of the Product labels and package insert/IFU
with the applicable Regulatory Authorities in the Territory; however, the Company will not propose or agree to specific content without
Zimmer’s prior approval.
7.5.
Debarment. The Company certifies that it has not and will not use in any capacity in connection with the performance of its obligations
under this Agreement, the services of any individual or entity debarred, excluded, suspended or disqualified by the Office of Inspector
General of the Department of Health and Human Services or otherwise deemed ineligible to participate in any federal or state healthcare
program.
7.6.
Federal EEO and Affirmative Action Obligations. In as much as Zimmer is a federal contractor, federal law requires that Zimmer
notify the Company of its equal employment opportunity and affirmative action obligations. This Section 7.6 applies only to the
performance of this Agreement in the United States of America. When applicable, the Company shall comply with the EO Clause in Section
202 of Executive Order 11246, as amended, which is incorporated herein by specific reference. When applicable, the Company shall abide
by the requirements of 41 C.F.R. § 60-300.5(a). This regulation prohibits discrimination against qualified protected veterans, and
requires affirmative action by covered prime contractors and subcontractors to employ and advance in employment qualified protected veterans.
When applicable, the Company shall abide by the requirements of 41 C.F.R. § 60-741.5(a). This regulation prohibits discrimination
against qualified individuals on the basis of disability, and requires affirmative action by covered prime contractors and subcontractors
to employ and advance in employment qualified individuals with disabilities.
7.7.
Reporting. Each Party shall maintain a system of collecting and recording Product Complaints and reportable events under Applicable
Laws relating to the Products in accordance with its standard procedures and policies in effect from time to time and shall provide to
the other Party reports of such complaints and events within two Business Days after receipt. Each Party shall immediately notify the
other of any material information such Party learns concerning the accuracy, performance, safety or efficacy of the Products, regardless
of whether formal reporting to any Regulatory Authority is required. The Company shall be responsible for investigating all Product Complaints
and reportable events and reporting to Zimmer, Regulatory Authorities and customers, as appropriate; provided, however, that Zimmer will
provide reasonable assistance and cooperation in all communications with customers. The Company will be responsible for all post-market
surveillance required by a Regulatory Authority or Applicable Laws and for submitting to applicable Regulatory Authorities all required
reports and other materials, including annual reports, distribution reports, product performance reports, medical device reports, safety
reports and similar reports.
7.8.
Field Actions. If either Party in good faith determines that a Field Action involving a Product or its labeling is warranted (whether
or not required by a Regulatory Authority), such Party will immediately notify the other Party in writing and will advise the other Party
of the reasons underlying its determination that a Field Action is warranted. The Parties will consult with each other as to any action
to be taken in regard to such Field Action. If, after consultation, either Party in good faith believes that a Field Action should be
undertaken with respect to a Product or its labeling, the Parties will cooperate in carrying out the same. The Company will be responsible
for all of Zimmer’s reasonable out of pocket costs and expenses, including the cost of the Products and the replacement cost of
the Products, in the event of Field Action with respect to any Product unless such Field Action was due to an intentional act or omission
of Zimmer. The Company will be responsible for any required reporting to Regulatory Authorities with respect to any Field Action involving
a Product or its labeling.
ARTICLE
VIII
REPRESENTATIONS AND WARRANTIES; INDEMNIFICATION.
8.1.
Mutual Representations and Warranties. Each Party hereby represents and warrants to the other that:
(a)
it is a corporation duly organized, validly existing and, if relevant in its jurisdiction of organization, in good standing under the
laws of its jurisdiction of organization and has all requisite power and authority to enter into this Agreement and to perform its obligations
hereunder;
(b)
the execution, delivery and performance by it of this Agreement and the consummation by it of the transactions contemplated hereby have
been duly authorized and approved by all necessary corporate or equivalent action on its part;
(c)
this Agreement has been duly executed and delivered by it and constitutes its legal, valid and binding obligation, enforceable against
it in accordance with its terms;
(d)
the execution, delivery and performance by it of this Agreement and the consummation by it of the transactions contemplated hereby do
not and will not: (i) violate any Applicable Laws; (ii) conflict with, or result in the breach of any provision of its organizational
documents; (iii) result in the creation of any lien or encumbrance of any nature upon any property being transferred by it pursuant to
this Agreement; or (iv) violate, conflict with, result in the breach or termination of, or constitute a default under (or event which,
with notice, lapse of time or both, would constitute a default under) any permit, contract or agreement to which it is a party or by
which any of its properties or businesses are bound;
(e)
no authorization, consent or approval of, or notice to or filing with, any Person is required for the execution, delivery and performance
by it of this Agreement (excluding approvals of Regulatory Authorities as contemplated herein); and
(f)
neither it nor any of its Affiliates is debarred under the FFDCA or on the U.S. Department of Health and Human Service’s List of
Excluded Individuals/Entities or the U.S. General Services Administration’s Lists of Parties Excluded from Federal Procurement
and Non-Procurement Programs.
(g)
as of the Effective Date, the obligations of both Parties set forth in Section 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.8, 6.1(a) and 6.1(d) have
been completed in full and no ongoing obligations in those sections remain.
8.2.
Company Representations and Warranties. The Company hereby represents and warrants to, and covenants with, Zimmer as follows:
(a)
neither it nor any of its Affiliates has employed (or used any contractor or consultant that employs) any Person who is debarred under
the FFDCA or under investigation by the FDA or other governmental authority for debarment thereunder; and
(b)
no health care professional holds any equity interest in the Company or any of its Affiliates, other than in compliance with Applicable
Laws;
(c)
the Company’s operation of its business has complied (and will continue to comply), and the Company is currently in compliance,
in each case in all material respects, with all Applicable Laws, and no event or state of facts has occurred or exists that (with or
without notice or lapse of time or both) would constitute or result in a material violation by the Company of, or a material failure
on the part of the Company to comply with, any Applicable Law;
(d)
neither the Company nor any Person acting on its behalf has or will, directly or indirectly, (i) used or agree to use any funds for unlawful
contributions, gifts, entertainment or expenses relating to political or government activity; (ii) made or agree to make, or attempt
to make or agree to make, any unlawful payment to any Government Official or violated any provision of any Anti-Corruption Law; (iii)
take(en) any action that would constitute a violation of any Anti-Corruption Law; (iv) made or agreed to make, or attempted to make or
agree to make, any other unlawful payment; or (v) have knowledge of a third party violating any provision of any Anti-Corruption Law
on behalf of the Company;
(e)
neither the Company nor any Person acting on its behalf has taken or will take any action in furtherance of making an offer, payment,
gift, promise to pay, or authorization of the giving of anything of value, to any Government Official (or to any Person while knowing
or having reason to know that all or some portion of the consideration remitted to that Person will be offered, given, or promised to
a Government Official) for the purpose of (i) influencing any act, decision, or failure to act by a Government Official in his or her
official capacity; (ii) inducing such Government Official to use his or her influence to affect any act or decision of a Governmental
Entity; (iii) obtaining, retaining or directing any business; or (iv) pursuing the issuance of any consent or any other commercial or
regulatory benefits in connection with the establishment or operation of the Company;
(f)
the Company has obtained FDA Approval for the Strip/Grid Products and such approval is in full force and effect on the date hereof; and
(g)
there are no circumstances that may affect the accuracy of the foregoing representations and warranties, including FDA or similar investigations
of, or debarment proceedings against, Company or any Person performing services or rendering assistance relating thereto and Company
will promptly notify Zimmer if it becomes aware of any such circumstances during the Term. Further Zimmer will have the right, solely
with respect to the Project and the Products, at Zimmer’s sole cost and expense, upon reasonable prior notice to the Company and
during regular business hours, to inspect and audit the Company’s records, facilities and operations for the purpose of verifying
its compliance with the Applicable Law and provisions in this Paragraph.
8.3.
Intellectual Property Matters. The Company hereby represents and warrants to, and covenants with, Zimmer as follows:
(a)
Ownership. Each of the following statements are true and correct as of the Effective Date and shall remain true and correct for
the duration of the Term: (i) the Company has sole and exclusive ownership of, or the unqualified right to use the Company IP; (ii) the
Company has not granted to any Person other than Zimmer a license, covenant not to sue or similar right with respect to any component
of the Company IP in the Field in the Territory that would be inconsistent with or conflict with the grant of the exclusive distribution
rights and license under this Agreement; (iii) the Company IP is free of any lien, encumbrance, security interest, mortgage or claim
of any nature; (iv) except for payments due under the WARF License, which shall be the sole responsibility of the Company, the Company
has no obligation to make any royalty, license or other payment in respect of the use or practice of the Company IP, or in respect of
the development, design, use, operation, manufacture, distribution or Commercialization of the Products; and (v) all Intellectual Property
Rights required for the development, design, use, operation, manufacture, distribution and Commercialization of the Products in accordance
with the Specifications for the Field in the Territory are included in the Company IP.
(b)
Right to Make Grant of License. The Company has the right to make any grants of Company IP to Zimmer that it makes or is required
to make under this Agreement. Without limitation of the foregoing, each of the employees, agents, consultants, contractors and others
who may contribute to or participate in the invention, discovery, creation or development of any Company IP is under a valid and enforceable
legal obligation to assign to the Company, all right, title and interest in such Company IP. The Company shall not grant to any Person
other than Zimmer a license, covenant not to sue or similar right with respect to any component of the Company IP that would be inconsistent
with or conflict with Zimmer’s exercise of its rights under any license granted to Zimmer hereunder in any manner.
(c)
Protection. The Company has administered and maintained all Patent Rights, Trademarks, Copyrights, Industrial Designs and other
components of the Company IP, and has made and will continue to make all filings with Governmental Agencies, whether U.S. or foreign,
arising from or associated with any Company IP or related Intellectual Property Rights to protect the Intellectual Property of the Products
in accordance with commercially reasonable intellectual property portfolio filing and maintenance practices. The Company is current in
payment of all patent maintenance fees and similar costs related to such portfolio maintenance. The Company has taken responsible and
prudent steps to safeguard and maintain the secrecy and confidentiality of all Proprietary Information included in the Company Background
IP.
(d)
Non-Infringement. The use and practice of the Company IP for the development, design, use, operation, manufacture, distribution
and Commercialization of the Products in accordance with the Specifications for the Field in the Territory do not infringe, violate or
misappropriate the Intellectual Property Rights of any Person.
(e)
Claims. No Person has asserted a Claim against the Company with respect to any of the Company IP, which Claim (i) challenges the
ownership, validity or enforceability of any of the Company IP, (ii) alleges that the Company’s use or practice of the Company
IP infringes, misappropriates or violates the Intellectual Property Rights of any Person, or (iii) seeks to enjoin or restrain the Company’s
use or practice of the Company IP in any manner that would interfere with the transactions contemplated by this Agreement. The Company
has no knowledge that any Person intends to assert such a Claim or that any Person has a valid basis to do so.
(f)
Infringement by Others. Except as disclosed by the Company to Zimmer prior to the Effective Date, The Company has no reason to
believe that any Person has infringed, violated or misappropriated any granted patents in the Company IP as it relates to the Product
in the Field.
8.4.
Indemnification.
(a)
Scope.
(i)
Each Party shall indemnify and hold harmless the other Party (and its Affiliates and their respective shareholders, officers, directors,
employees and agents) from any liability, damage, loss, action, cause of action, tax, cost or expense (whether or not arising out of
a Third Party legal claim or action) (including reasonable attorneys’, consultants’ and experts’ fees and expenses
and all amounts paid in investigation, defense and settlement of any of the foregoing) (collectively, “Losses”) arising
out of, in connection with or relating to (x) any breach or inaccuracy of any representation or warranty made by such indemnifying Party
in this Agreement; (y) any noncompliance with or breach or nonperformance of any agreement, covenant or obligation to be performed by
such indemnifying Party pursuant to this Agreement; or (z) any violation of Applicable Law or negligence or willful misconduct in connection
with this Agreement by the indemnifying Party or any of its Affiliates or their respective representatives, employees or agents. If the
Parties have indemnification obligations to one another in connection with a single Third Party legal claim or action, they shall contribute
to the aggregate damages and costs in proportion to their relative responsibilities therefor based upon all relevant equitable considerations.
(ii)
In addition to its obligations under Section 8.4(a)(i), the Company shall indemnify and hold harmless Zimmer (and its Affiliates
and their respective shareholders, officers, directors, employees and agents) from any Losses arising out of, in connection with or relating
to (x) any Infringement Claim and (y) any claim of a failure to pay royalties or other amounts due to Third Parties under license agreements
between the Company and such Third Parties, including any such claims made under or in connection with the WARF License.
(b)
Notice. The indemnified Party shall promptly notify the indemnifying Party in writing and in reasonable detail of each indemnity
claim, but any delay or deficiency of such notice shall not excuse the indemnifying Party’s indemnification obligations except
to the extent that its legal position is materially prejudiced due to the delay or deficiency.
(c)
Defense. The indemnifying Party shall have the right to assume and control the defense and settlement of any Third Party legal
claim or action if the predominant claims therein are covered by its indemnity, unless the Parties have a conflict of interest with respect
to such legal claim or action or the claimant is seeking relief that, if awarded, could have an adverse effect on the indemnified Party’s
ongoing business (other than an award of money damages). If the indemnifying Party assumes the defense, it shall employ counsel reasonably
acceptable to the indemnified Party and shall defend the claim or action with diligence. In all events, the Party not controlling the
defense shall reasonably cooperate with the controlling Party and shall be permitted to participate in the defense at its own expense.
(d)
Settlement. Neither Party shall settle a Third Party legal claim or action covered by indemnification under this Section 8.4
without the other Party’s prior written consent, which shall not be unreasonably withheld, conditioned or delayed, except that
the Party controlling the defense shall have the right to do so if (i) it will pay in full all monetary elements of the settlement, (ii)
the settlement does not include non-monetary elements, findings or admissions that would be detrimental to the other Party’s ongoing
business, and (iii) the settlement includes a full release in favor of the other Party from all Losses arising out of, in connection
with or relating to such legal claim or action.
ARTICLE
IX
CONFIDENTIALITY AND PUBLICITY
9.1.
Scope. In the course of their activities pursuant to this Agreement, the Parties anticipate that they may disclose Confidential
Information to one another and that either Party may, from time to time, be a disclosing Party or a recipient of Confidential Information.
The Parties wish to protect such Confidential Information in accordance with this ARTICLE IX. The provisions of this ARTICLE
IX will apply to disclosures furnished to or received by a Party and its agents and representatives (which may include agents and
representatives of its Affiliates). Each Party will (a) advise its employees, agents and representatives of the requirements of this
ARTICLE IX and ensure that its employees, agents and representatives are bound by confidentiality obligations substantially consistent
with those set forth in this ARTICLE IX; and (b) be responsible to ensure their compliance with such provisions and be responsible
for any breach of such provisions. The provisions of this ARTICLE IX will supersede and replace any prior agreements between the
Parties relating to Confidential Information covered hereby. In addition to any other remedies available in law or equity, the disclosing
Party will be entitled to temporary and permanent injunctive relief in the event of a breach by the recipient under this ARTICLE IX.
9.2.
Definition of Confidential Information. For purposes hereof, “Confidential Information” with respect to a disclosing
Party means all Proprietary Information, in any form or media, concerning the disclosing Party or its Affiliates that the disclosing
Party or its Affiliates furnishes to the recipient, whether furnished before or after the date hereof, and all notes, analyses, compilations,
studies and other materials, whether prepared by the recipient or others, that contain or reflect such Proprietary Information and Zimmer’s
Confidential Information includes all Program IP and all data, deliverables and other information developed by either Party in connection
with this Agreement; provided, however, that Confidential Information does not include information that (a) is or hereafter becomes generally
available to the public other than as a result of a disclosure by the recipient in violation of this Agreement, (b) was already known
to the recipient prior to receipt from the disclosing Party as evidenced by prior written documents in its possession not subject to
an existing confidentiality obligation to the disclosing Party, (c) is disclosed to the recipient on a non-confidential basis by a Person
who is not in default of any confidentiality obligation to the disclosing Party, or (d) is independently developed by or on behalf of
the recipient without reliance on or use of information received hereunder. The contents of this Agreement will be deemed to be Confidential
Information of each Party.
9.3.
Confidentiality Obligations. The recipient of Confidential Information will (a) use such Confidential Information solely and exclusively
in connection with the exercise of its rights and the discharge of its obligations under this Agreement, and (b) not disclose such Confidential
Information without the prior written consent of the disclosing Party to any Person other than those of its agents and representatives
who need to know such Confidential Information for such permitted use and who are bound by appropriate written obligations of confidentiality
with respect thereto. Notwithstanding the foregoing, the recipient of Confidential Information may disclose it to the extent necessary
to comply with Applicable Laws or with an order issued by a court or regulatory body with competent jurisdiction; provided that, in connection
with such disclosure, the recipient will (A) provide, if allowable, reasonable advance notice of such disclosure to the disclosing Party;
(B) limit the disclosure to the information that is legally required to be disclosed, and (C) use Commercially Reasonable Efforts to
obtain confidential treatment or an appropriate protective order, to the extent available, with respect to such Confidential Information.
The obligations under this Section 9.3 will remain in effect from the Effective Date through the fifth anniversary of the termination
or expiration of this Agreement. Notwithstanding the foregoing, the obligations to protect Confidential Information identified by the
disclosing Party as a trade secret shall extend until such information becomes publicly available. In addition to the foregoing, each
Party may disclose Confidential Information belonging to the other Party as expressly permitted by this Agreement or if and to the extent
such disclosure is reasonably necessary in the following instances:
(a)
filing, prosecuting, or maintaining Patents as permitted by this Agreement;
(b)
regulatory filings for the Products that such Party has a license or right to Develop or Commercialize hereunder in a given country or
Territory;
(c)
prosecuting or defending litigation as permitted by this Agreement;
(d)
complying with applicable court orders or governmental regulations, including regulations promulgated by securities exchanges and the
SEC, provided that any Party making such disclosure shall promptly notify such other Party of such order or regulation upon the receipt
thereof, and provide reasonable assistance to such other Party in seeking confidential treatment of such Confidential Information;
(e)
disclosure to its and its Affiliates’ employees, consultants, contractors, and agents, to its licensees and sublicensees, in each
case on a need-to-know basis in connection with the Development or Commercialization of the Products in accordance with the terms of
this Agreement, in each case under written obligations of confidentiality and non-use at least as stringent as those herein; and
(f)
disclosure to actual and bona fide potential investors, acquirors, licensees, and other financial or commercial partners solely for the
purpose of evaluating or carrying out an actual or potential investment, acquisition, or collaboration, in each case under written obligations
of confidentiality and non-use at least as stringent (except with respect to duration, which may be shorter as long as not less than
two (2) years) as those herein, provided that if this Agreement is being disclosed the disclosing Party redacts the financial terms and
other provisions of this Agreement that are not reasonably required to be disclosed in connection with such potential investment, acquisition,
or collaboration, which redaction shall be prepared in consultation with the other Party.
Notwithstanding
the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to
Section 9.3(c) or 9.3(d), it will, except where impracticable, give reasonable advance notice to the other Party of such
disclosure and use the same diligent efforts to secure confidential treatment of such Confidential Information as such Party would use
to protect its own confidential information, but in no event less than reasonable efforts. In any event, the Parties agree to take all
reasonable action to avoid disclosure of Confidential Information hereunder. Any information disclosed pursuant to Section 9.3(c)
or 9.3(d) shall remain Confidential Information and subject to the restrictions set forth in this Agreement, including the
foregoing provisions of this Article IX.
9.4.
Limitations on Obligations. Except as otherwise required by Applicable Law, upon the termination or expiration of this Agreement,
if requested by the disclosing Party, the recipient of Confidential Information:
(a)
will promptly return or destroy, at the recipient’s election, all Confidential Information of the disclosing Party, including all
notes or other work product prepared by the recipient based upon or incorporating Confidential Information of the disclosing Party;
(b)
will not retain any copies, extracts or other reproductions, in whole or in part, of such Confidential Information, notes or other work
product; provided, however, that the recipient will be permitted to retain (but not use) (i) one file copy of all Confidential Information
on a confidential basis to evidence the scope of and to enforce the Party’s obligation of confidentiality under this ARTICLE
IX; and (ii) all back up electronic media maintained in the ordinary course of business for archival purposes; and
(c)
will certify in writing to the disclosing Party that the recipient has complied with this Section 9.4.
9.5.
Publicity. It is understood that each Party may desire or be required to issue press releases relating to this Agreement or activities
hereunder. The Parties agree to consult with each other reasonably and in good faith with respect to the text and timing of such press
releases prior to the issuance thereof, to the extent practicable, and not to issue any such release without the other Party’s
consent, provided that a Party may not unreasonably withhold, condition, or delay consent to such releases by more than four (4)
Business Days, and that either Party may issue such press releases or make such disclosures as it determines, based on advice of counsel,
is required to comply with Applicable Laws or with the rules and regulations promulgated by the SEC or any exchange on which such Party’s
securities are listed. Each Party shall provide the other Party with advance notice of, and an opportunity to review, legally required
disclosures to the extent practicable. The Parties will consult with each other on the provisions of this Agreement to be redacted in
any filings required by Applicable Laws; provided that each Party shall have the right to make any such filing as it reasonably
determines is required under Applicable Laws. In addition, either Party shall be free to disclose, without the other Party’s prior
written consent, the existence of this Agreement, the identity of the other Party and those terms of the Agreement which have already
been publicly disclosed in accordance with this Section 9.5, each of the Parties may make internal announcements to their respective
employees regarding the transactions contemplated by this Agreement, subject to the other Party’s review and approval of the announcing
Party’s announcement, which approval shall not be unreasonably withheld. Nothing herein shall prohibit or prevent either Party
or any of its Affiliates from disclosing any information of a nature that would typically be provided by companies to their investors
or prospective investors.
ARTICLE
X
TERM AND TERMINATION
10.1.
Term. The term of this Agreement (the “Term”) will begin on the Effective Date and will remain in effect until
October 31, 2034. Upon the expiration of the Term, this Agreement may be renewed upon the mutual written agreement of the Parties. This
Agreement may be terminated before expiration of the Term only by agreement of the Parties or in accordance with Section 10.3.
10.2.
Term of Exclusivity.
(a)
The license rights granted to Zimmer under this Agreement shall be exclusive (i) from the Original Effective Date until September 30,
2032 for the SEEG Products and Strip/Grid Products (the “SEEG and Strip/Grid Product Term”); and (ii) from the Effective
Date until October 31, 2034 for the OneRF™ Product System (the “RF Term”).
10.3.
Termination. This Agreement may be terminated before the end of the Term or any renewal term as follows:
(a)
If either Party believes the other Party is in material breach of this Agreement, it may give written notice of such breach to the other
Party, which notice shall specify the breach in reasonable detail. If the alleged breach is not remedied within sixty (60) days after
such written notice, the non-breaching Party may terminate this Agreement immediately upon delivery to the other Party of a written notice
of termination. The termination right provided herein will not constitute an exclusive remedy for the material breach.
(b)
Either Party may terminate this Agreement by delivering written notice of its decision to do so if the other Party is dissolved under
applicable corporate law or becomes subject to an Insolvency Event.
(c)
Zimmer may terminate this Agreement for any reason or no reason by delivering written notice to the Company specifying the effective
date of such termination, which notice must be provided at least ninety (90) days prior to such effective date.
(d)
The Company may terminate this Agreement immediately upon delivery of written notice to Zimmer upon the occurrence of a Competitive Triggering
Event and the payment by the Company to Zimmer of the Competitive Triggering Event Termination Fee; provided that any such notice
must be delivered and the Competitive Triggering Event Termination Fee must be paid by the Company no later than six (6) months following
the occurrence of the Competitive Triggering Event. Upon the termination of this Agreement pursuant to this Section 10.3(d), Zimmer
and its Affiliates and Subdistributors shall have the right, for a period of nine (9) months after the termination of this Agreement
(the “Transition Period”), to continue to place orders (including bulk orders) and purchase Products under the MS
Agreement for sale to their respective then-existing customers and to sell off all inventory of the Products held or purchased for sale
by Zimmer or its Affiliates or Subdistributors in the Territory during the Transition Period and wind down the accounts for the Territory.
The Company shall continue to manufacture Products and sell Products to Zimmer pursuant to the MS Agreement during the Transition Period.
At the end of the Transition Period, the Company shall repurchase any non-expired Products previously purchased and not resold by Zimmer
and its Affiliates and Subdistributors at a price equal to [**].
(e)
The Company may terminate this Agreement pursuant to the provisions set forth in Section 6.2(c).
10.4.
Survival. The following provisions will survive termination or expiration of this Agreement: (a) Section 2.2, relating
to Program IP, Section 2.12, and the first two sentences of Section 2.13; (b) ARTICLE VIII, relating to representations
and warranties and indemnification; (c) ARTICLE IX, relating to confidentiality and publicity; (d) ARTICLE X, relating
to termination and post-termination rights and obligations; and (e) any provisions required for the interpretation or enforcement of
any of the foregoing.
10.5.
Accrued Obligations. Termination or expiration of this Agreement will not relieve any Party of any obligation that is expressly
indicated to survive termination and will be without prejudice to any rights that have accrued to the benefit of any Party prior to such
termination.
ARTICLE
XI
MISCELLANEOUS
11.1.
Interpretive Conventions. Whenever the words “include,” “includes” or “including” are used
in this Agreement, they will be understood to be followed by the words “without limitation.” Pronouns, including “he,”
“she” and “it,” when used in reference to any Person, will be deemed applicable to entities or individuals, male
or female, as appropriate in any given case. Standard variations on defined terms (such as the plural form of a term defined in the singular
form, and the past tense of a term defined in the present tense) will be deemed to have meanings that correlate to the meanings of the
defined terms. Article, Section and other headings contained in this Agreement are for reference purposes only and are not intended to
describe, interpret, define or limit the scope, extent or intent of any provision of this Agreement. When a reference is made in this
Agreement to a Recital, an Article, a Section, a Schedule, an Attachment or an Exhibit, such reference is to a Recital, Article or Section
of, or a Schedule, Attachment or Exhibit to, this Agreement, unless otherwise indicated. All references to “dollars” or “$”
will be deemed to be references to the lawful currency of the United States.
11.2.
Compliance; Conflicts. Each Party and its Affiliates and their respective employees and agents will comply in all material respects
with all Applicable Laws that pertain to its activities under this Agreement and, except as otherwise provided herein, will bear the
entire cost and expense of such compliance. The Parties will not, directly or indirectly, take any action (including the grant of any
right or the undertaking of any obligation) that is in conflict with any provision of this Agreement.
11.3.
Entire Agreement; Amendments. This Agreement together with the MS Agreement constitutes the entire agreement among the Parties
concerning the subject matter hereof and supersedes all previous negotiations, agreements and commitments with respect thereto, including,
without limitation, the EDDA and that certain Term Sheet dated September 24, 2024 entered into by and between the Parties. This Agreement
will not be amended or modified in any manner except by a written instrument signed by duly authorized officers or representatives of
each of the Parties.
11.4.
Governing Law. Any claim or controversy relating in any way to this Agreement will be governed by and interpreted exclusively
in accordance with the laws of the State of Indiana, without regard to the conflicts of law principles thereof.
11.5.
Dispute Resolution. Any claim, dispute, or controversy as to the breach, enforcement, interpretation or validity of this Agreement
(each, a “Dispute”) will be referred to the Chief Executive Officer of the Company and the President (or equivalent)
of the applicable Zimmer division for Zimmer for attempted resolution prior to the institution of litigation. In the event such executives
are unable to resolve such Dispute within 30 days of such Dispute being referred to them, then either Party may commence an action in
the Chosen Courts seeking resolution of the Dispute.
11.6.
Jurisdiction; Court Proceedings; Waiver of Jury Trial.
(a)
Any Claim, litigation, proceeding hearing or other action (collectively, “Proceeding”) against any Party arising out
of, in connection with or relating to this Agreement shall be brought solely in any federal court sitting in the Northern District of
Indiana (the “Chosen Courts”) and each of the Parties submits to the exclusive jurisdiction of the Chosen Courts for
the purpose of any such Proceeding; provided that a final judgment in any such Proceeding may be enforced in other jurisdictions by suit
on the judgment or in any other manner provided by Applicable Law. Each Party irrevocably and unconditionally agrees not to assert any
(i) objection that it may ever have to the laying of venue of any such Proceeding in any Chosen Court, (ii) Claim that any such Proceeding
brought in any Chosen Court has been brought in an inconvenient forum and (iii) Claim that any Chosen Court does not have personal jurisdiction
over such Party with respect to such Proceeding.
(b)
Each Party agrees that service of process in any Proceeding may be made by mailing a copy thereof by registered or certified mail or
by overnight courier service, postage prepaid, to it at its address specified herein. Nothing in this Agreement will affect the right
of any Party to serve process in any other manner permitted by Applicable Law.
(c)
EACH OF THE PARTIES TO THIS AGREEMENT HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY ACTION
(I) ARISING UNDER THIS AGREEMENT OR (II) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES, OR ANY OF
THE TRANSACTIONS RELATED HERETO, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER AT LAW OR IN EQUITY, BASED IN CONTRACT
OR IN TORT OR OTHERWISE. EACH PARTY HEREBY FURTHER AGREES AND CONSENTS THAT ANY SUCH ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A
JURY AND THAT THE PARTIES MAY FILE A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES TO THE WAIVER
OF THEIR RIGHT TO TRIAL BY JURY.
11.7.
Partial Illegality. If any provision of this Agreement or the application thereof to either Party or any circumstances will be
declared void, illegal or unenforceable, the remainder of this Agreement will be valid and enforceable to the extent permitted by Applicable
Laws. In such event, the Parties will use their best efforts to replace the invalid or unenforceable provision by a provision that, to
the extent permitted by the Applicable Laws, achieves the purposes intended under the invalid or unenforceable provision. Any deviation
by either Party from the terms and provisions of this Agreement in order to comply with Applicable Laws will not be considered a breach
of this Agreement.
11.8.
Waiver of Compliance. No provision of this Agreement will be waived by any act, omission or knowledge of a Party or its agents
or employees, except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving
Party, which waiver will be effective only with respect to the specific obligation and instance described therein.
11.9.
Notices. All notices and other communications in connection with this Agreement shall be in writing and shall be sent to the respective
Parties at the following addresses, or to such other addresses as may be designated by the Parties in writing from time to time in accordance
with this Section 11.9, by (i) registered or certified mail, postage prepaid, (ii) express courier service, service fee prepaid,
or (iii) electronic mail:
To
the Company:
NeuroOne
Medical Technologies Corporation
7599
Anagram Drive
Eden
Prairie, MN 55344
Attn:
Dave Rosa
Email:
[**]
with
a copy (which shall not constitute notice) to:
Honigman
LLP
650
Trade Centre Way, Suite 200
Kalamazoo,
MI 49002
Attention:
Phillip D. Torrence
Email:
[**]
To
Zimmer:
Zimmer,
Inc.
c/o
General Manager
Zimmer
Biomet CMF and Thoracic LLC
1520
Tradeport Drive
Jacksonville,
FL 32218
Email:
[**]
with
a copy (which shall not constitute notice) to:
Zimmer,
Inc.
345
East Main Street
Warsaw,
Indiana 46580
Attn:
General Counsel
Email:
[**]
All
notices shall be deemed given and received (i) if delivered by hand, immediately, (ii) if sent by mail, five Business Days after posting,
or (iii) if delivered by email or express courier service, the next Business Day in the jurisdiction of the recipient.
11.10.
Counterparts; Electronic or Facsimile Transmission. This Agreement may be executed in counterparts, each of which will be deemed
to be an original and all of which together will be deemed to be one and the same instrument. This Agreement may be delivered by one
or both Parties by facsimile or electronic transmission with the same effect as if delivered personally.
11.11.
Further Assurances. From time to time, as and when requested by either Party, the other Party will execute and deliver, or cause
to be executed and delivered, all such documents and instruments and will take, or cause to be taken, all such further actions as such
other Party may reasonably deem necessary or desirable to carry out the intentions of the Parties embodied in this Agreement.
11.12.
Jointly Prepared. This Agreement has been prepared jointly and will not be strictly construed against either Party.
11.13.
Assignment. Except as expressly provided hereunder, neither this Agreement nor any rights or obligations hereunder may be assigned
or otherwise transferred by either Party without the prior written consent of the other Party (which consent shall not be unreasonably
withheld); provided, however, that either Party may assign or otherwise transfer this Agreement and its rights and obligations hereunder
without the other Party’s consent:
(a)
in connection with the transfer or sale of all or substantially all of the business or assets of such Party relating to the Products
to a Third Party, whether by merger, consolidation, divesture, restructure, sale of stock, sale of assets, or otherwise; or
(b)
to an Affiliate, provided that the assigning Party shall remain liable and responsible to the non-assigning Party hereto for the performance
and observance of all such duties and obligations by such Affiliate, and provided further that if the entity to which this Agreement
is assigned ceases to be an Affiliate of the assigning Party, the Agreement shall be automatically assigned back to the assigning Party
or its successor.
The
rights and obligations of the Parties under this Agreement shall be binding upon and inure to the benefit of the successors and permitted
assigns of the Parties specified above, and the name of a Party appearing herein will be deemed to include the name of such Party’s
successors and permitted assigns to the extent necessary to carry out the intent of this Section 11.13. Any assignment not in
accordance with this Section 11.13 shall be null and void.
11.14.
Relationship of Parties. Each Party to this Agreement is an independent contractor. Employees and agents of one Party are not
employees or agents of the other Party, will not hold themselves out as such, and will not have any authority or power to bind the other
Party to any contract or other obligation.
11.15.
Third-Party Beneficiaries. Nothing in this Agreement, whether express or implied, is intended to confer any rights or remedies
under or by reason of this Agreement on any Persons other than the Parties hereto and their respective successors, assigns and Affiliates.
11.16.
Force Majeure. Neither Party shall be deemed to breach this Agreement to the extent that such Party is prevented from or delayed
in performing its obligations hereunder due to any causes that are, and that would notwithstanding the exercise of reasonable diligence
be expected to be, beyond such Party’s reasonable control (such causes, “Force Majeure Events”), but only to
the extent that (a) the effects of such Force Majeure Events upon the functions, activities and efforts of the affected Party to
perform its obligations hereunder is not greater or more than its effects on such similar or related functions, activities or efforts
of such Party or its vendors performed in connection with the Party’s own business and (b) the affected Party uses its Commercially
Reasonably Efforts to mitigate the effects of the Force Majeure Events, including without limitation, by working around any constraints
on such Party’s ability to perform its obligations hereunder that arise as a result of such Force Majeure Event (“Work-Around
Efforts”). Upon any Force Majeure Event, the affected Party shall give notice of such event as soon as reasonably practicable
to the other Party stating the extent and duration of the impact that such event will have on its performance of its obligations hereunder
and the cause thereof, and the affected Party shall resume the performance of its obligations as soon as reasonably practicable. Neither
Party shall be liable for the nonperformance or delay in performance of its obligations under this Agreement when such failure is due
to a Force Majeure Event, provided that the affected Party implements any feasible Work-Around Efforts.
The
following Exhibits form an integral part of the Agreement:
| Exhibit A |
Definitions |
| Exhibit B |
Development Plan |
| Exhibit C |
Critical Features |
| Exhibit D |
Form of Supply and Manufacturing Agreement |
| Exhibit E |
Form of Quality Agreement |
| Exhibit F |
Transfer Price |
| Exhibit G |
OneRF™ Product System Diagram |
[remainder
of page intentionally blank; signature page follows]
The
Parties have executed this Agreement as of the Effective Date to evidence their agreement to the terms and provisions set forth herein.
| |
Zimmer,
Inc. |
| |
|
| |
By: |
/s/
Chad F. Phipps |
| |
|
Chad F. Phipps, Senior Vice
President |
| |
|
| |
NeuroOne
Medical Technologies Corporation |
| |
|
| |
By: |
/s/
David Rosa |
| |
|
David Rosa, President and Chief
Executive Officer |
[Signature
page to Exclusive Development and Distribution Agreement]
EXHIBIT
A
DEFINITIONS
“Acceptance”
means, with respect to Deliverables, confirmation by Zimmer, acting reasonably, that such Deliverables satisfy the applicable Acceptance
Criteria.
“Acceptance
Criteria”, with respect to any Deliverables, has the meaning set forth in the Development Plan for such Deliverables.
“Affiliate”
means, with respect to any Person, any other Person that directly or indirectly controls, is controlled by or is under common control
with such Person. A Person shall be deemed to control another Person if such Person (i) possesses the power to direct or cause the direction
of the management, business and policies of such Person, whether by contract or otherwise, and/or (ii) owns fifty percent (50%) or more
of the voting securities of such Person.
“Agreement”
has the meaning set forth in the opening paragraph.
“Anti-Corruption
Law” means the U.S. Foreign Corrupt Practices Act of 1977, as amended, and all anti-corruption or anti-bribery Applicable Laws
of any jurisdiction where any Company directly or indirectly designs, develops, manufactures, markets, distributes or sells products
or that is otherwise applicable to the business of the Company.
“Applicable
Law(s)” means all applicable laws, ordinances, rules and regulations of any kind whatsoever of any governmental (including
international, foreign, federal, state and local) or Regulatory Authority, including HIPAA and all other data privacy and security laws
and laws governing the use, disclosure and protection of information and the U.S. Foreign Corrupt Practices Act of 1977; the U.S. Travel
Act, 18 U.S.C. § 1952; the Anti-Kickback Statute, 42 U.S.C. § 1320a-7b(b); the Physician Self-Referral Law, 42 U.S.C. §
1395nn; the U.K. Bribery Act 2010; any applicable Law enacted in connection with, or arising under, the OECD Convention on Combating
Bribery of Foreign Public Officials in International Business Transactions; or any other applicable Law or regulation of any foreign
or domestic jurisdiction relating to bribery or corruption.
“Approved
Design Modification” means [**].
“[**]”.
“Business
Day” means any day other than a Saturday, a Sunday or a day on which banks in New York City are authorized or obligated by
law or executive order to remain closed.
“Chosen
Courts” has the meaning set forth in Section 11.6(a).
“Change
in Control Transaction” means: (a) the sale of all or substantially all of the assets of the Company to an unrelated person
or entity; (b) a merger, reorganization, consolidation or similar transaction pursuant to which the holders of the Company’s outstanding
voting power immediately prior to such transaction do not own a majority of the outstanding voting power of the resulting or successor
entity (or its ultimate parent, if applicable) immediately upon completion of such transaction; or (c) the sale of all of the stock of
the Company to an unrelated person or entity.
“Claim”
means any and all manner of claims, actions, suits, damages, demands and liabilities whatsoever in law or equity, whether known or unknown,
liquidated or unliquidated, fixed, contingent, direct or indirect.
“Commercialize”
or “Commercialization” means the conduct of all activities undertaken before and after Regulatory Approval relating
to the promotion, sales, marketing, medical support, and distribution (including importing, exporting, transporting, customs clearance,
warehousing, invoicing, handling, and delivering Products to customers) of Products in the Field, including sales force efforts, detailing,
advertising, market research, medical education and information services, marketing, sales force training, and sales (including receiving,
accepting and filling Product orders) and distribution.
“Commercially
Reasonable Efforts” means, with respect to a Party and its obligations under this Agreement, those commercially reasonable
efforts and resources consistent with the usual practices of a similarly situated company for the development and commercialization of
a product originating from its own research and development department without a royalty obligation to others, which is at a similar
stage of research, development, or commercialization, taking into account that product’s profile of efficacy and safety; proprietary
position, including patent exclusivity; regulatory status, including anticipated or approved labeling and anticipated or approved post-approval
requirements; present and future market and commercial potential, including competitive market conditions (but not taking into account
any payment owed to the other Party under this Agreement), and all other relevant factors, including technical, legal, scientific and/or
medical factors.
“Company”
has the meaning set forth in the opening paragraph.
“Company
Background IP” has the meaning set forth in Section 2.1.
“Company
IP” means, collectively, the Company Background IP, the Company Program IP and all rights of the Company in the Joint Program
IP (which, for the avoidance of doubt, does
not
include any rights of Zimmer in the Joint Program IP).
“Company
Program IP” has the meaning set forth in Section 2.2(a)(ii).
“Competitive
Triggering Event” has the meaning set forth in Section 1.7.
“Competitive
Triggering Event Termination Fee” means [**].
“Confidential
Information” has the meaning set forth in Section 9.2.
“Copyrights”
means all rights in original works of authorship fixed in any tangible medium of expression under the copyright laws of the United States
or any other country (and including all rights accruing by virtue of bilateral or international copyright treaties and conventions),
including, but not limited to, all renewals, extensions, reversions or restorations of copyrights now or hereafter provided for by law
and all rights to make applications for copyright registrations and recordations, regardless of the medium of fixation or means of expression.
“Cure
Period” has the meaning set forth in Section 6.2(c).
“Debarred/Excluded”
means any Person becoming debarred or suspended under 21 U.S.C. §335(a) or (b), the subject of a conviction described in Section
306 of the FFDCA, excluded, or having previously been excluded, from a federal or governmental health care program, debarred from federal
contracting, convicted of or pled nolo contendere to any felony, or to any federal or state legal violation (including misdemeanors)
relating to prescription drug products or fraud, the subject to Office of Foreign Assets Control (“OFAC”) sanctions
or on the OFAC list of specially designated nationals, or the subject of any similar sanction of any Governmental Authority.
“Deliverables”
means, with respect to a Product, the Deliverables for such Product set forth in the Development Plan.
“Design
History File” means, with respect to any Product, each of the following (i) design control process and revision, (ii) design
and development plan, (iii) user needs / intended uses / design inputs, DCTM (inputs), (iv) design review I meeting minutes and attachments,
(v) design outputs, DCTM (outputs), (vi) design review II meeting minutes and attachments, (vii) design verification protocols / reports,
(viii) design validation protocols / reports, (ix) design verification / validation, DCTM (complete), (x) design review III meeting minutes
and attachments, (xi) additional design review minutes and attachments (if applicable), (xii) final responses to design review observations,
(xiii) risk management file, (xiv) usability engineering file, (xv) design transfer activities (specification review and checklist),
(xvi) design transfer checklist, (xvii) design closure checklist and (xviii) the information required to be created and/or maintained
under the Company’s SOP-01004, as amended from time to time.
“Design
Modification Notice” has the meaning set forth in Section 1.2.
“Designated
Jurisdictions” means (a) the United States and (b) any jurisdictions other than the United States where Zimmer determines,
after consultation with the Company, to market and sell one or more Products.
“Development”
or “Develop” means any act or process for the purposes of (i) creating or causing something to be created; or (ii)
making something more advanced. For clarity, Development does not include marketing or sales of a commercial product.
“Development
Plan” has the meaning set forth in Section 1.2.
“Dispute”
has the meaning set forth in Section 11.5.
“Distributor”
means a Third Party that markets, distributes, promotes and/or sells a Party’s products under a contractual arrangement with the
Party.
“Effective
Date” has the meaning set forth in the opening paragraph.
“Electrode
Cable Assembly Products” means an assembly consisting of EEG cables and a ZIF connector box, which serves to connect cortical
electrodes or SEEG electrodes to an EEG recording headbox.
“Enforcement
Action” has the meaning set forth in Section 2.6.
“FDA”
means the United States Food and Drug Administration and any successor agency.
“FDA
Approval” means, with respect to any Product, clearance by the FDA of a 510(k) application for such Product.
“FFDCA”
means the United States Federal Food, Drug, and Cosmetic Act, 21 U.S.C. 301, et. seq., as amended, and the rules, regulations, guidance,
guidelines and requirements promulgated or issued thereunder.
“Field”
means (i) sEEG brain surgeries for monitoring, recording or stimulation of brain tissue at the subsurface level of the brain for less
than 30 days use and/or (ii) sEEG RF ablation surgeries limited in the brain (specifically excluding ablation of nervous tissue that
connects to the brain from other areas of the body).
“Field
Action” means any action by a Party that meets the criteria of “recall,” “correction,” or “removal”
or similar field or customer action as defined by applicable Regulatory Law.
“First
Commercial Sale” means the first commercial sale by Zimmer or its Affiliates on an arms’ length basis to an end user
of a Product (other than any RF Product) in any country following receipt of Regulatory Approval to do so. For avoidance of doubt, sales
for purposes of test marketing (including a Limited Market Release), sampling, or promotion shall not constitute a First Commercial Sale.
“Foot
Pedal” means a foot pedal for the RF Generator provided as an optional accessory to enable the physician to apply ablation
energy while freeing up their hands. The foot pedal is a re-usable, non-patient contacting accessory, that is not part of the sterile
field.
“Force
Majeure Events” has the meaning set forth in Section 11.16.
“Generator
Cart” means a dedicated RF Generator cart that may be easily moved into the correct position within the operating environment
and still be easily accessible. The cart also provides additional storage capacity for accessory devices as needed. The cart
is a re-usable, nonpatient contacting accessory, that is not part of the sterile field.
“Generator
Interface Cable (GIC)” means an adapter that acts between the RF Generator and the RFCB for both temperature monitoring and
RF energy delivery. The GIC is a re-usable, non-patient contacting accessory, that is not part of the sterile field.
“Governmental
Authority” means any country in which any of the Products is manufactured, marketed, sold, tested, investigated or otherwise
regulated, and all states or other political subdivisions thereof and supranational bodies applicable thereto, including the European
Union, and all agencies, commissions, officials, courts or other instrumentalities of the foregoing.
“Governmental
Entity” means any:
(i)
U.S. federal, state, local or municipal government or non-U.S. government or subdivision thereof;
(ii)
governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, instrumentality
or entity and any court or other tribunal);
(iii)
multi-national organization or body; or
(iv)
body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory or taxing authority
or power.
“Government
Official” means any (i) officer, employee or agent of any Governmental Entity; (ii) any official, employee or agent of a public
international organization; (iii) any Person acting in an official capacity on behalf of such government, instrumentality or public international
organization; (iv) any political party official or candidate for political office; (v) a member of a royal family; or (vi) any officer
or employee of a government-owned or controlled enterprise, company or organization. In many instances, Health Care Professionals who
work at or are otherwise affiliated with public hospitals or universities may be considered Government Officials.
“Health
Care Professional” means an individual, entity, or employee of such entity, within the continuum of care of patients, which
may purchase, lease, recommend, use, prescribe, or arrange for the purchase or lease of medical device products and services.
“HIPAA”
means the Health Insurance Portability and Accountability Act of 1996, as amended, and the rules and regulations implementing the same.
“Industrial
Designs” means all features of shape, configuration, pattern, ornament and the like that are or can be registered as designs
or industrial designs under Applicable Laws and all applications, registrations and renewals in connection therewith.
“Infringement
Claim” has the meaning set forth in Section 2.7.
“Initial
Exclusivity Fee” has the meaning set forth in Section 6.1(a).
“Initial
Stocking Order” has the meaning set forth in Section 6.2(d).
“Insolvency
Event” means that the Party has (i) commenced a voluntary proceeding under any insolvency law, or (ii) had an involuntary proceeding
commenced against it under any insolvency law which has continued undismissed or unstayed for 60 consecutive days, or (iii) had a receiver,
trustee or similar official appointed for it or for any substantial part of its property, or (iv) made an assignment for the benefit
of creditors, or (v) had an order for relief entered with respect to it by a court of competent jurisdiction under any insolvency law.
For purposes hereof, the term “insolvency law” means any applicable bankruptcy, insolvency or other similar law now or hereafter
in effect.
“Intellectual
Property” means Patent Rights, Copyrights, Trademarks, Industrial Designs, and Proprietary Information.
“Intellectual
Property Rights” means all forms of legal rights and protections that may be obtained under Applicable Laws for Intellectual
Property.
“Interim
Fee Bonus” has the meaning set forth in Section 6.1(d).
“IP”
means Patent Rights and Know-How.
“JDC”
has the meaning set forth in Section 1.8.
“Joint
Program IP” has the meaning set forth in Section 2.2(a)(iii).
“Know-How”
means information and materials (including but not limited to ideas, discoveries, improvements, inventions, know-how, trade secrets,
processes, methods, protocols, formulas, data and designs), whether patentable or not, that are in the possession or under the control
of a Party and are not generally known or publicly available.
“Limited
Market Release” means, with respect to a Product, the sale or transfer of such Product by Zimmer or its Affiliates or Distributors
to one or more Third Parties or end-users to conduct the initial clinical cases. For context, this occurs in a limited number of centers
and patients over a limited time (estimated twenty-five (25) patients over three (3) months.
“LMR
Order” has the meaning set forth in Section 6.2(d).
“Losses”
has the meaning set forth in Section 8.4(a).
“Medical
Device Tax” means an excise tax on the sale of certain medical devices by the manufacturer or importer of the device imposed
by Section 4191 of the Internal Revenue Code of 1986, as amended.
“Minimum
Purchase Requirements” has the meaning set forth in Section 6.2(c).
“Modified
Connector” means the connector that connects the electrode tail to the cable box for SEEG Products as modified pursuant to
an Approved Design Modification.
“MS
Agreement” has the meaning set forth in Section 5.1.
“Net
Sales” means the cumulative gross invoice price at which the RF Milestone Products are sold to end users (e.g., hospitals or
physicians) during the measurement period by Zimmer, less (i) trade, cash, quantity and other discounts and coupons; (ii) returns,
rebates, chargebacks and other allowances; (iii) retroactive price reductions that are actually allowed or granted; and (iv) taxes or
fees levied on, determined or imposed with respect to such sales by Governmental Authority, including import taxes, export taxes, excise
taxes, sales taxes, value-added taxes, consumption taxes, duties and other taxes. For the avoidance of doubt, the calculations of Net
Sales will not be reduced by sales commissions paid to sales representatives. All components of Net Sales shall be determined from the
books and records of Zimmer in accordance with GAAP using its then-current standard procedures and methodology for applying GAAP and
for converting foreign currencies to United States dollars.
“OneRF™
Product System” shall be defined as a system comprised of SEEG-RF Electrodes, TA, Spacer Tubes, RF Connector Box, RF Generator,
GIC, Foot Pedal, Universal Cable Assembly, Generator Cart, bin, and cart handle (bin and cart handle as referenced in Section 6.2(b)).
The OneRF Product System provides a therapeutic capability in addition to the diagnostic capabilities of the existing Evo® sEEG electrodes
as of the Effective Date. See Diagram attached as Exhibit G.
“OneRF™
Product System Sales Milestone Payment” has the meaning set forth in Section 6.1(c).
“Original
Effective Date” means July 20, 2020.
“Other
Product Specifications” means, with respect to any Product (other than any RF Product), the specifications and requirements
for the Development of such Product as set forth in Exhibit B or as otherwise determined by the JDC by amendment of the Development
Plan in accordance with Section 1.8.
“OUS”
means the countries set forth in the Territory outside the United States of America.
“Party”
or “Parties” has the meaning set forth in the opening paragraph.
“Patent
Rights” means patents and patent applications, including (i) certificates of invention and applications therefor; (ii) divisionals,
continuations, continuations-in-part, reissues, renewals, substitutions, registrations, re-examinations, post-grant reviews, revalidations,
extensions, supplementary protection certificates, and the like of any of the foregoing; and (iii) foreign equivalents of any of the
foregoing.
“Person”
means a natural person, a corporation, a partnership, a trust, a joint venture, a limited liability company, any Governmental Authority,
or any other entity or organization.
“Proceeding”
has the meaning set forth in Section 11.6(a).
“Product
Availability Date” means, with respect to a Product (other than any RF Product), the first date on which all of the following
have occurred: (a) FDA Approval of the Product has been received, (b) the MS Agreement has been entered into or amended to provide for
the manufacturing and supply of the Product by the Company; (c) Zimmer has placed the LMR Order for the Product under the MS Agreement,
and (d) the units of the Product included in the LMR Order have been received and accepted by Zimmer following inspection as contemplated
by the MS Agreement.
“Product
Complaint” means any written, electronic or oral communication that alleges deficiencies related to the identity, quality,
durability, reliability, safety, efficacy or performance of any Product, including actual or suspected product tampering, contamination,
mislabeling or misformulation.
“Product
Improvement” means any changes, optimizations or improvements to any Product or any of its components, whether or not patentable
or subject to a claim of copyright; provided, however, Product Improvements do not include any therapeutics devices or products used
for treatment.
“Products”
means Strip/Grid Products, SEEG Products, Electrode Cable Assembly Products and RF Products, and “Product” means any
of them.
“Product
Transaction” has the meaning set forth in Section 4.5.
“Program
Copyrights” means all Copyrights authored by or on behalf of a Party or its Affiliates or subcontractors in the course of the
Project within the Field.
“Program
IP” means all Program Copyrights, Program Know-How and Program Patent Rights.
“Program
Know-How” means all Know-How invented, conceived, discovered, created, made, developed, reduced to practice or otherwise perfected
in the course of the Project within the Field.
“Program
Patent Rights” means all Patent Rights that claim or disclose Program Know-How within the Field.
“Project”
has the meaning set forth in Section 1.1.
“Project
Participant” means each of Zimmer and the Company.
“Proprietary
Information” means a Party’s trade secrets, know how, business plans, manufacturing processes, source code, clinical
strategies, product specifications, scientific data, market analyses, formulae, designs, training manuals and other non-public information
(whether business, financial, commercial, scientific, clinical, regulatory or otherwise) that the Party treats as proprietary and uses
commercially reasonable efforts to protect.
“Prosecute”
means, in relation to any Patent Rights, (i) to prepare and file patent applications (including re-examinations or re-issues thereof)
and to represent applicant(s) or assignee(s) before relevant patent offices or other relevant authorities during examination, re-examination
and re-issue thereof in appeal processes and interferences or any equivalent proceedings, (ii) to secure the grant of any Patent Rights
arising from such patent applications, (iii) to maintain in force any issued Patent Right (including through payment of any relevant
maintenance fees), and (iv) to make all decisions with regard to any of the foregoing activities. “Prosecution” has a corresponding
meaning.
“Protected
Data” means information protected by Applicable Laws, including but not limited to all data related to patients, all protected
health information, as defined by HIPAA, personal data as defined by the General Data Protection Regulation, and any other personal and
identifiable data that is produced, analyzed, collected or stored using or within Products, regardless of the form or location of the
storage, provided that such Protected Data was made available in accordance with Applicable Laws for use by the Company and/or Zimmer.
“Quality
Agreement” has the meaning set forth in Section 5.3.
“Regulatory
Approval” means, with respect to any country or region, any approval, product and establishment license, registration or authorization
of any Regulatory Authority required for the manufacture, use, storage, importation, exportation, transport, distribution or sale of
the Product in such country or region.
“Regulatory
Authority” or “Regulatory Authorities” means, with respect to any country or jurisdiction, any Governmental
Authority, entity or agency involved in granting regulatory approval for the manufacture, marketing, sale, reimbursement and/or pricing
of the Product, or in administering Regulatory Laws in that country or jurisdiction, including the FDA in the United States.
“Regulatory
Filings” means all applications, dossiers, notifications, requests and other documents that may be filed with a Regulatory
Authority seeking approval to engage in development, manufacturing or Commercialization activities with respect to the Product or seeking
any regulatory designation or status with respect to the Product, as well as all supplements and amendments to the foregoing.
“Regulatory
Laws” means all Applicable Laws governing the import, export, testing, investigation, manufacture, marketing or sale of a product,
or establishing recordkeeping or reporting obligations for product complaints or adverse events, or relating to product recalls or similar
regulatory matters.
“Related
Instruments” means a ruler, a stylet, drill bits, a driver and a coagulator to be used with the SEEG Products.
“Representatives”
will be deemed to include each Person that is or during the term of this Agreement becomes (i) an Affiliate of such Party or (ii) an
officer, director, member, manager, executive partner, employee, partner, advisor (including without limitation accountants, attorneys,
financial advisors, and consultants), agent or other representative of such Party or of such Party’s affiliates, and the term “Person,”
will be broadly interpreted to include any individual and any corporation, partnership, entity, group, tribunal or Governmental Authority.
“RF”
means radio frequency.
“RF
Connector Box (RFCB)” means a connector box that enables the SEEG-RF Electrodes and the TA to be connected to the RF Generator.
“RF
Distribution License” has the meaning set forth in Section 2.4(a).
“RF
Exclusivity Fee” shall be three million dollars ($3,000,000 (USD)).
“RF
Generator” means a radiofrequency generator which enables the capability to transmit energy to the SEEG-RF Electrodes for the
creation of RF ablation lesions in nervous tissue, used in the Field.
“RF
Milestone Products” has the meaning set forth in Section 6.1(c).
“RF
Products” means the OneRF™ Product System.
“ROFN
Notice” has the meaning set forth in Section 4.5.
“ROFN
Period” has the meaning set forth in Section 4.5.
"SEEG
and Strip/Grid Distribution License" has the meaning set forth in Section 2.4(a).
“SEEG
and Strip/Grid Product Systems” has the meaning set forth in Section 1.1.
“SEEG
and Strip/Grid Product Term” has the meaning set forth in Section 10.2.
“SEEG
Exclusivity Maintenance Fee” means (a) if the Product Availability Date for the SEEG Products occurs on or before [**], then
$[**], plus the amount of any Interim Fee Bonuses earned pursuant to Section 6.1(b), including any such Interim Fee Bonus
earned after [**] pursuant to Section 6.1(d)(iv) following the delivery of a Design Modification Notice; (b) if the Product Availability
Date for the SEEG Products occurs after [**], but on or before [**], then $[**], plus if Zimmer timely issues a Design Modification
Notice, any Interim Fee Bonus earned pursuant to Section 6.1(d)(iv); (c) if the Product Availability Date for the SEEG Products
occurs after [**], but on or before [**], then $[**]; and (d) if the Product Availability Date for the SEEG Products occurs after [**],
then $[**].
“SEEG
Products” means depth electrodes of any size or configuration, consisting of implants placed into the brain for monitoring
or mapping the subsurface levels of the brain for the surgical diagnosis of neurological conditions, including epilepsy, together with
anchor bolts, caps and associated instrumentation; provided, however, SEEG Products do not include any therapeutics devices or products
used for treatment.
“SEEG-RF
Electrodes” means depth electrodes which include both diagnostic and RF treatment capabilities used in the Field.
“Spacer
Tubes” means tubes that are used to position the TA temperature sensor behind the contact intended to be used for ablation
and consists of an assortment of tubes to align the TA with the appropriate location on the SEEG-RF Electrodes being used for ablation.
“Specifications”
means, collectively, (i) the Company’s design and functionality specifications relating to the Products in the Company’s
sales literature and other Product documentation made available to Zimmer, and (ii) any specifications for manufacturing, testing, storing,
packaging, shipping or labeling the Products set forth in any approved application for Regulatory Approval and any supplements and amendments
thereto.
“Strip/Grid
Products” means cortical probe electrodes (strips and grids) of any size or configuration, consisting of implants placed onto
the surface of the brain for monitoring or mapping surface levels of the brain for the surgical diagnosis of neurological conditions,
including epilepsy; provided, however, Strip/Grid Products do not include any therapeutics devices or products used for treatment.
“Subdistributor”
has the meaning set forth in Section 2.4(c).
“Taxes”
means any and all federal, state and local taxes, including the Medical Device Tax, excise or gross receipts taxes, personal property
taxes, customs, duties or levies, and any foreign taxes.
“Temperature
Accessory (TA)” means an accessory that is inserted into the lumen of the SEEG-RF Electrodes and connected to the RFCB which
enables temperature monitoring within the SEEG-RF Electrodes inside the brain at the lesion site.
“Term”
has the meaning set forth in Section 10.1.
“Territory”
means (a) the entire world (except China) with respect to the SEEG Products and Strip/Grid Products and (b) United States of America,
Canada, Ireland, France, Belgium, Switzerland, Germany, Poland, Italy, Israel, Australia, Japan, Taiwan, Thailand, Korea, India, United
Kingdom and Hong Kong with respect to the OneRF™ Product System.
“Third
Party” means Persons other than the Parties or Affiliates thereof.
“Trademarks”
means all trademarks, service marks, trade dress, logos, labels, domain names, websites and trade names, together with all translations,
adaptations, derivations and combinations thereof (including all goodwill associated therewith), and all applications, registrations
and renewals in connection therewith.
“Transition
Period” has the meaning set forth in Section 10.3(d).
“WARF
License” means that certain Amended and Restated Exclusive Start-Up Company License Agreement, dated January 21, 2020 entered
into by and between the Company and Wisconsin Alumni Research Foundation, as amended, restated, supplemented or otherwise modified from
time to time.
“Work-Around
Efforts” has the meaning set forth in Section 11.16.
“Zimmer”
has the meaning set forth in the opening paragraph.
“Zimmer
Distribution License(s)” has the meaning set forth in Section 2.4(a).
“Zimmer
Program IP” has the meaning set forth in Section 2.2(a)(i).
Exhibit 99.1

NeuroOne®
Announces Expansion of Existing Distribution Agreement with Zimmer Biomet for Commercialization of OneRF™ Ablation System
License Provides
Exclusive Commercialization Rights for US Distribution and Certain OUS Markets
Agreement expected
to boost NeuroOne sales revenue and profitability
EDEN PRAIRIE, Minn. –
October 31, 2024 (GLOBE NEWSWIRE) – NeuroOne Medical Technologies Corporation (Nasdaq: NMTC) (“NeuroOne” or the “Company”),
a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders,
today announced the execution of an amendment to its existing distribution agreement with Zimmer Biomet that will provide Zimmer Biomet
with certain exclusive rights to distribute NeuroOne’s OneRF™ Ablation System for use in the brain.
NeuroOne will receive
an upfront payment of $3 million with the potential to earn an additional milestone payment if certain performance criteria is achieved.
NeuroOne expects that the agreement will generate meaningful revenue and drive improved profitability for the Company.
The OneRF Ablation System
is the only FDA cleared radiofrequency ablation system in the United States for both diagnostic and therapeutic use. It has been used
in a number of ablation cases since its limited launch in April. Cases were reported as being successful using the same device to identify
the brain tissue triggering seizure activity and ablate the targeted tissue to reduce or eliminate brain-related seizure activity. In
addition, the technology has the potential to reduce hospital stays, number of surgeries and adverse events while offering temperature
control to enhance patient safety. The devices are initially placed in the operating room. To date, all the ablations have been performed
at the patient’s bedside saving additional operating costs while allowing the patient to be diagnosed and treated in one hospitalization
instead of multiple visits.
“The expanded agreement
with Zimmer Biomet to include distribution of our OneRF Ablation System is a significant catalyst for the Company,” says Dave Rosa,
president and CEO of NeuroOne. “We are confident that the partnership will allow NeuroOne to leverage Zimmer Biomet’s leadership
position in robotic technology and extensive distribution channel both in the United States and abroad. As the world’s first FDA
cleared system for both diagnostic and therapeutic procedures, our ablation system provides clear advantages over existing competitive
electrode technologies. We look forward to continuing to expand the indications for use in the future.”
Brian Hatcher, President,
SET and CMFT at Zimmer Biomet said, “We are excited to expand the relationship with NeuroOne to include the OneRF Ablation System,
which builds on our existing agreement to distribute NeuroOne’s Cortical and sEEG diagnostic electrode technology, and we look forward
to launching this product in the near future.”
About NeuroOne
NeuroOne Medical Technologies
Corporation is a developmental stage company committed to providing minimally invasive and hi-definition solutions for EEG recording,
brain stimulation and ablation solutions for patients suffering from epilepsy, Parkinson’s disease, dystonia, essential tremors,
chronic pain due to failed back surgeries and other related neurological disorders that may improve patient outcomes and reduce procedural
costs. The Company may also pursue applications for other areas such as depression, mood disorders, pain, incontinence, high blood pressure,
and artificial intelligence. For more information, visit www.nmtc1.com.
Forward Looking Statements
This press release may
include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the
Securities Exchange Act of 1934, as amended. Except for statements of historical fact, any information contained in this press release
may be a forward–looking statement that reflects NeuroOne’s current views about future events and are subject to known and
unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be
materially different from the information expressed or implied by these forward-looking statements. In some cases, you can identify forward–looking
statements by the words or phrases “may,” “might,” “will,” “could,” “would,”
“should,” “expect,” “intend,” “plan,” “objective,” “anticipate,”
“believe,” “estimate,” “predict,” “project,” “potential,” “target,”
“seek,” “contemplate,” “continue, “focused on,” “committed to” and “ongoing,”
or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward–looking
statements may include statements regarding the potential for the expanded distribution agreement to increase revenues and profitability,
the potential to receive any milestone payment, the potential for future FDA submissions for additional ablation applications, business
strategy, market size, potential growth opportunities, and future operations. Although NeuroOne believes that we have a reasonable basis
for each forward-looking statement, we caution you that these statements are based on a combination of facts and factors currently known
by us and our expectations of the future, about which we cannot be certain. Our actual future results may be materially different from
what we expect due to factors largely outside our control, including risks that our partnerships may not facilitate the commercialization
or market acceptance of our technology; whether due to supply chain disruptions, labor shortages, risks that our technology will not perform
as expected based on results of our pre-clinical and clinical trials; risks related to uncertainties associated with the Company’s
capital requirements to achieve its business objectives and ability to raise additional funds: the risk that we may not be able to secure
or retain coverage or adequate reimbursement for our technology; uncertainties inherent in the development process of our technology;
risks related to changes in regulatory requirements or decisions of regulatory authorities; that we may not have accurately estimated
the size and growth potential of the markets for our technology; risks related to our ability to protect our intellectual property rights;
and other risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in our filings
with the Securities and Exchange Commission. These forward–looking statements speak only as of the date of this press release and
NeuroOne undertakes no obligation to revise or update any forward–looking statements for any reason, even if new information becomes
available in the future.
“Caution: Federal law restricts this
device to sale by or on the order of a physician”
Contact:
800-631-4030
ir@nmtc1.com
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
NeuroOne Medical Technol... (NASDAQ:NMTC)
Historical Stock Chart
From Nov 2024 to Dec 2024
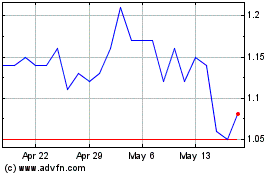
NeuroOne Medical Technol... (NASDAQ:NMTC)
Historical Stock Chart
From Dec 2023 to Dec 2024