Revance Shares Rally Premarket on FDA Expanded OK for Daxxify
August 14 2023 - 8:09AM
Dow Jones News
By Colin Kellaher
Shares of Revance Therapeutics rose more than 10% in premarket
trading Monday after the U.S. Food and Drug Administration approved
the expanded use of its Daxxify antiwrinkle treatment in its first
therapeutic indication.
The Nashville biotechnology company on Monday said the FDA
approved Daxxify for the treatment of adults with cervical
dystonia, a painful condition in which the neck muscles contract
involuntarily.
Revance last year won FDA approval of Daxxify for the temporary
improvement of frown lines in adults, launching a competitor to
AbbVie's Botox antiwrinkle injection.
Revance said the approval of Daxxify for cervical dystonia gives
the company entry into the $2.5 billion U.S. therapeutic
neuromodulator market.
Revance shares, which closed Friday at $18.91, were recently up
11% to $21 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
August 14, 2023 08:54 ET (12:54 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
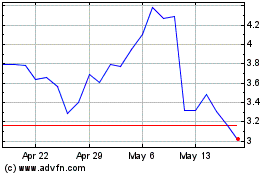
Revance Therapeutics (NASDAQ:RVNC)
Historical Stock Chart
From Apr 2024 to May 2024
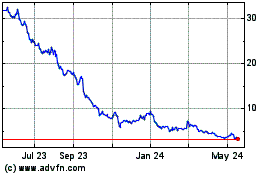
Revance Therapeutics (NASDAQ:RVNC)
Historical Stock Chart
From May 2023 to May 2024