
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Sera Prognostics Update September 25, 2023

Disclaimer ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The company has no obligation to provide any updates to these forward-looking statements, even if its expectations change, whether as a result of new information, future events or otherwise, except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Further information on potential factors, risks and uncertainties that could affect operating and financial results is included in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, including in each case under the heading RISK FACTORS, and in the company’s other filings with the SEC. The information in this presentation should be considered in conjunction with a review of the company’s filings with the SEC including the information in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, under the heading MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.

Sera Prognostics aims to be a global leader in high-value women’s health diagnostics, delivering pivotal pregnancy information to improve the health of women and newborns, and to simultaneously improve the economics of healthcare Our Vision ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Who We Are – The Pregnancy Company® ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. • Proteomics and bioinformatics platform company focusing on creating valuable pregnancy information to improve the well-being of mothers and babies • Long-term goal to comprehensively characterize pregnancy and build pregnancy information tools/ apps/services that benefit patients, doctors, insurers, researchers and other pregnancy stakeholders • We are working to extend our strong clinical and scientific data in our journey to build expansive data and insights into pregnancy • We have assembled a team of dedicated individuals with demonstrated expertise and knowledge to accomplish our vision
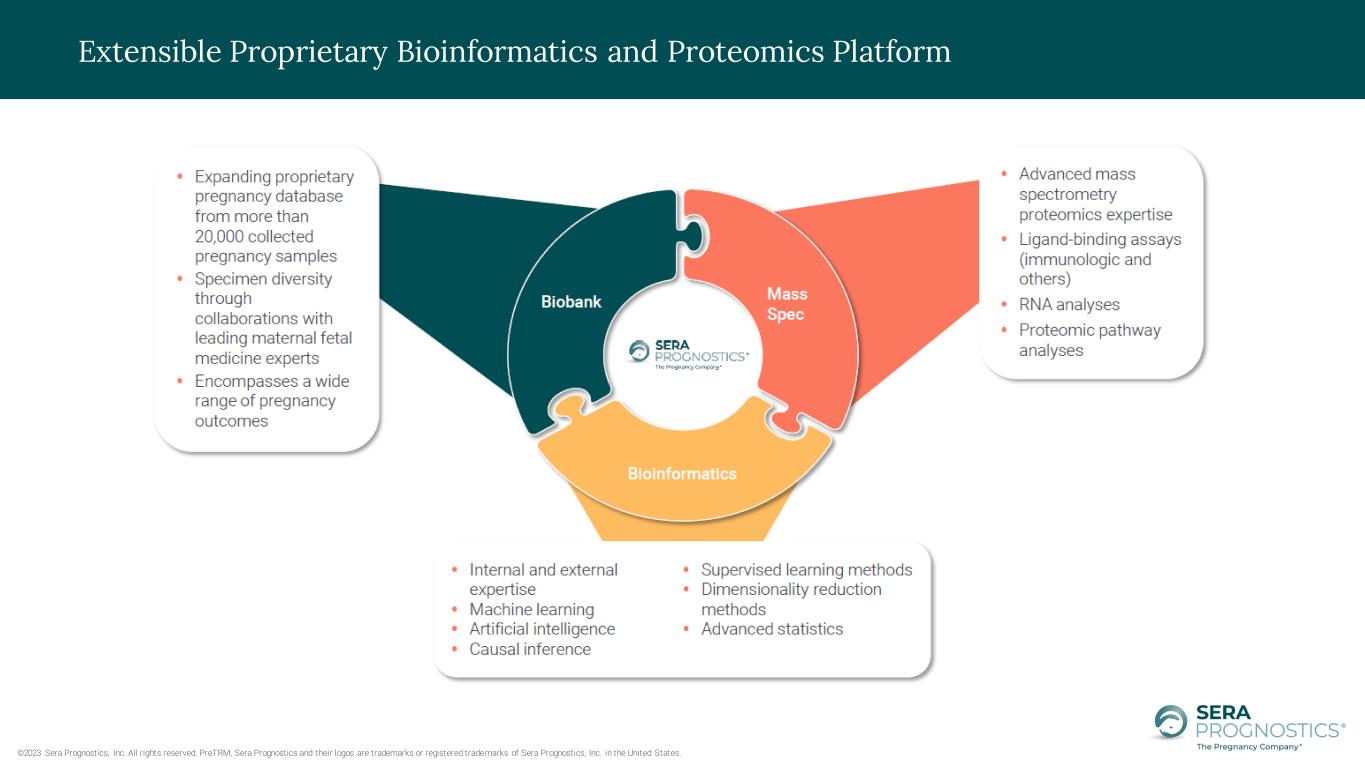
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Extensible Proprietary Bioinformatics and Proteomics Platform
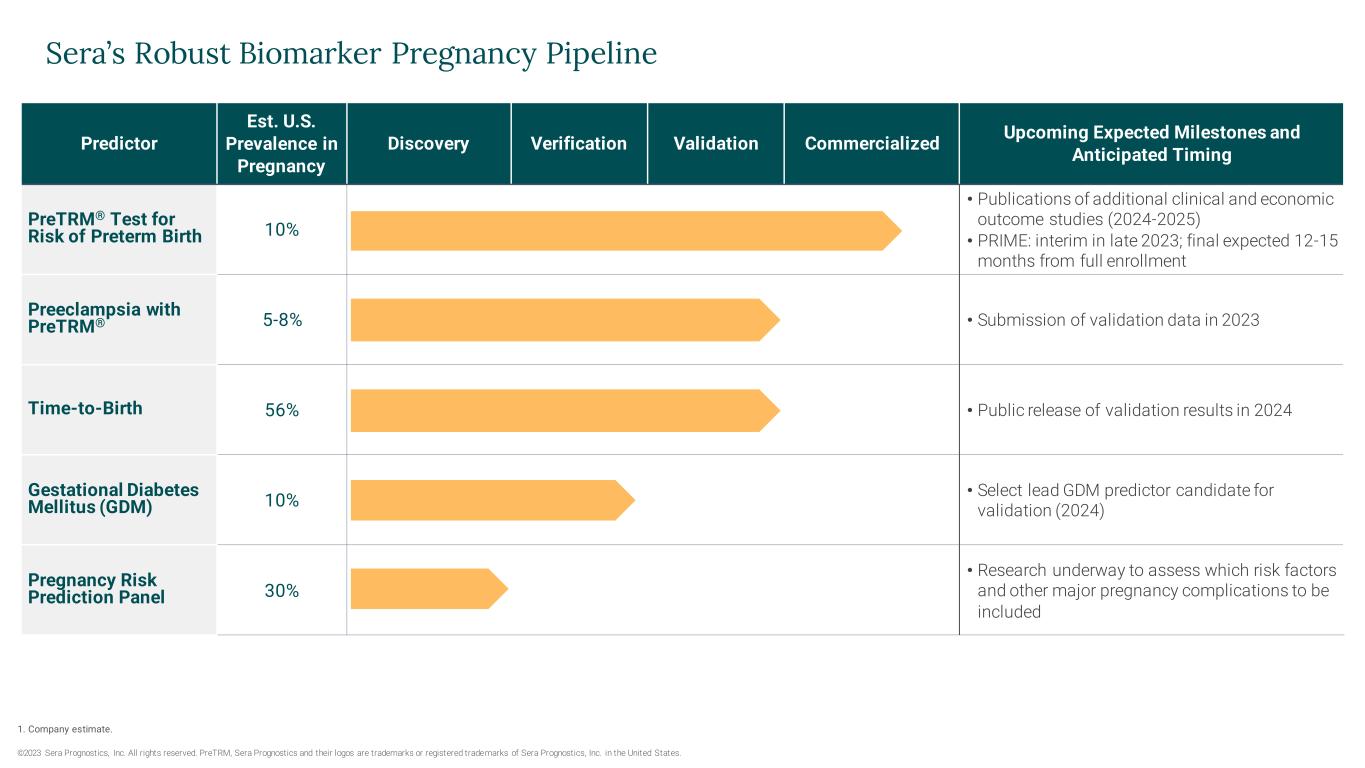
Predictor Est. U.S. Prevalence in Pregnancy Discovery Verification Validation Commercialized Upcoming Expected Milestones and Anticipated Timing PreTRM® Test for Risk of Preterm Birth 10% • Publications of additional clinical and economic outcome studies (2024-2025) • PRIME: interim in late 2023; final expected 12-15 months from full enrollment Preeclampsia with PreTRM® 5-8% • Submission of validation data in 2023 Time-to-Birth 56% • Public release of validation results in 2024 Gestational Diabetes Mellitus (GDM) 10% • Select lead GDM predictor candidate for validation (2024) Pregnancy Risk Prediction Panel 30% • Research underway to assess which risk factors and other major pregnancy complications to be included Sera’s Robust Biomarker Pregnancy Pipeline ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. 1. Company estimate.
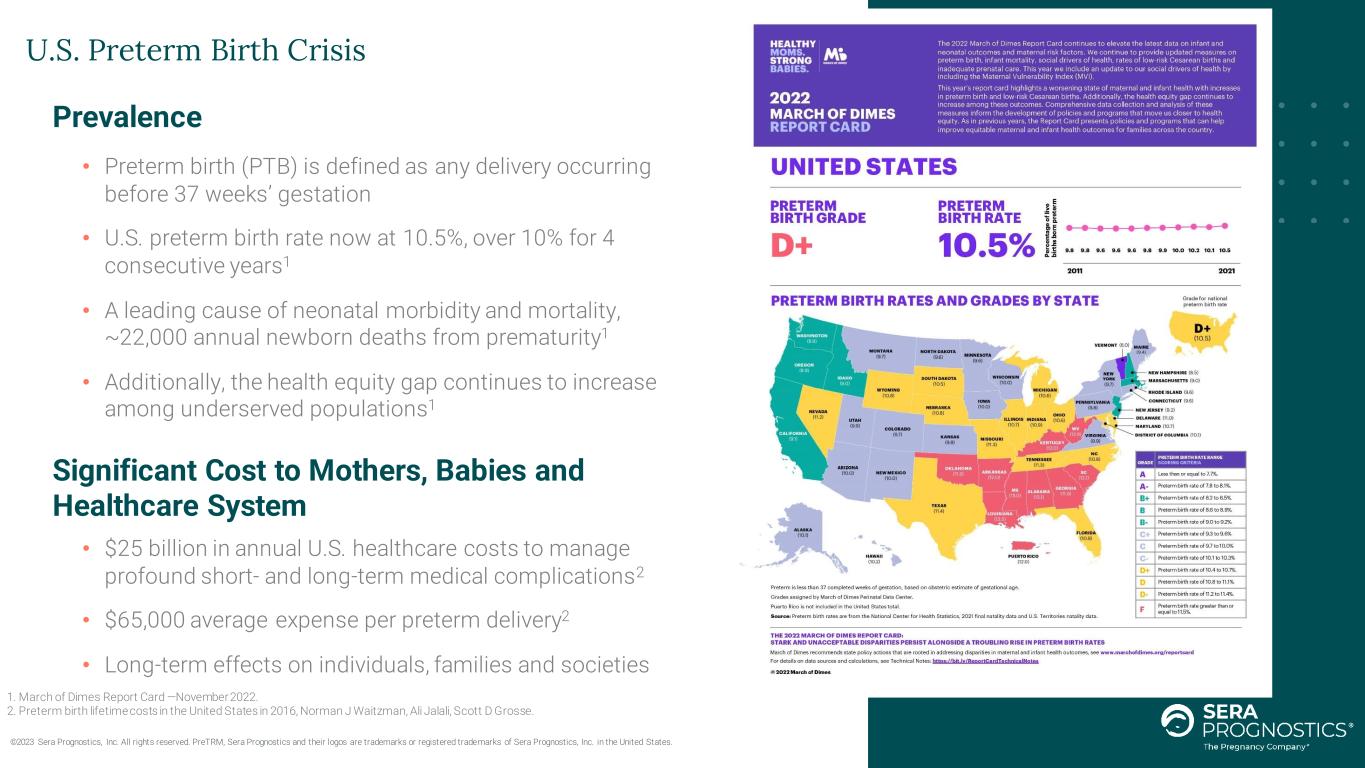
U.S. Preterm Birth Crisis ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Prevalence • Preterm birth (PTB) is defined as any delivery occurring before 37 weeks’ gestation • U.S. preterm birth rate now at 10.5%, over 10% for 4 consecutive years1 • A leading cause of neonatal morbidity and mortality, ~22,000 annual newborn deaths from prematurity1 • Additionally, the health equity gap continues to increase among underserved populations1 Significant Cost to Mothers, Babies and Healthcare System • $25 billion in annual U.S. healthcare costs to manage profound short- and long-term medical complications2 • $65,000 average expense per preterm delivery2 • Long-term effects on individuals, families and societies 1. March of Dimes Report Card —November 2022. 2. Preterm birth lifetime costs in the United States in 2016, Norman J Waitzman, Ali Jalali, Scott D Grosse.
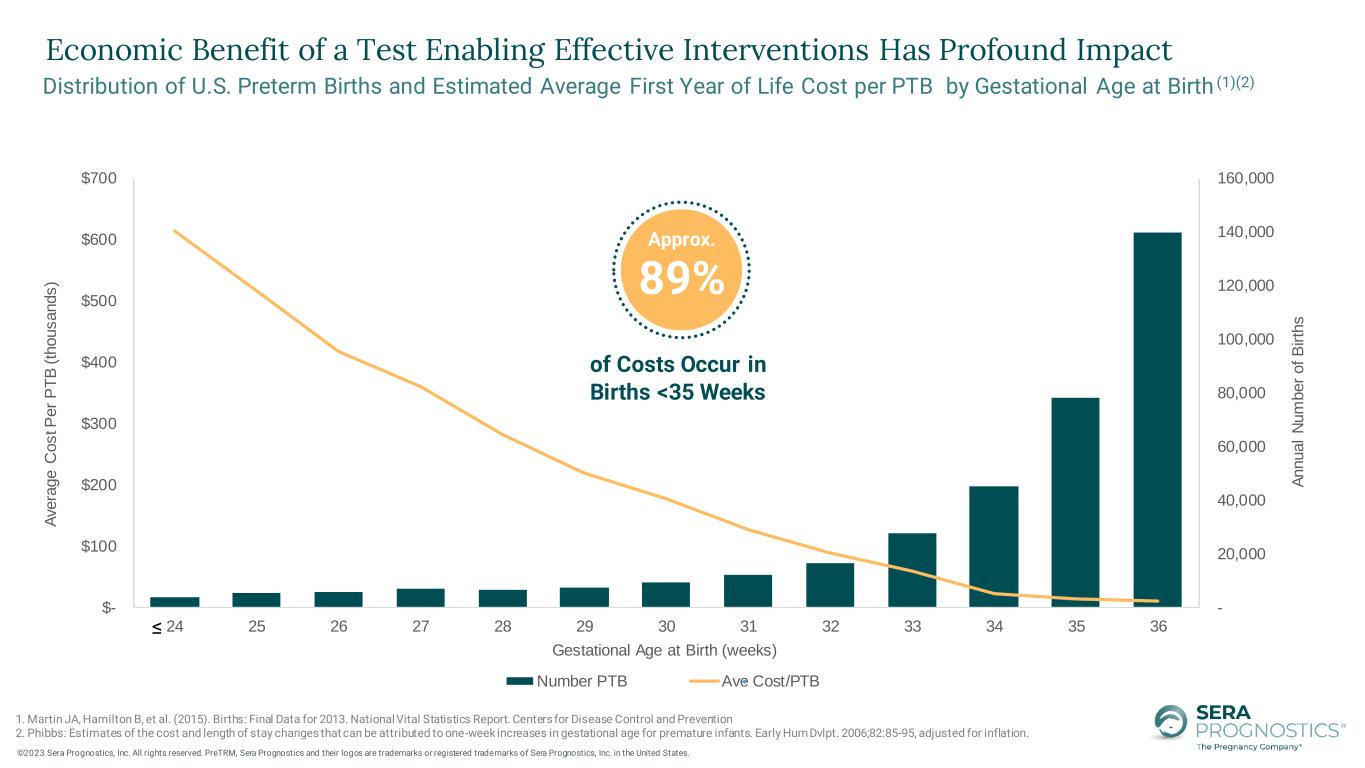
- 20,000 40,000 60,000 80,000 100,000 120,000 140,000 160,000 $- $100 $200 $300 $400 $500 $600 $700 24 25 26 27 28 29 30 31 32 33 34 35 36 A n n u a l N u m b e r o f B ir th s A ve ra g e C o s t P e r P T B ( th o u s a n d s ) Gestational Age at Birth (weeks) Number PTB Ave Cost/PTB of Costs Occur in Births <35 Weeks Approx. 89% 1. Martin JA, Hamilton B, et al. (2015). Births: Final Data for 2013. National Vital Statistics Report. Centers for Disease Control and Prevention 2. Phibbs: Estimates of the cost and length of stay changes that can be attributed to one-week increases in gestational age for premature infants. Early Hum Dvlpt. 2006;82:85-95, adjusted for inflation. Distribution of U.S. Preterm Births and Estimated Average First Year of Life Cost per PTB by Gestational Age at Birth (1)(2) ≤ . ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Economic Benefit of a Test Enabling Effective Interventions Has Profound Impact

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PAPR Study • Groundbreaking prediction validation study • 5,501 patients, 11 U.S. sites • Validated proprietary biomarker signature highly predictive of preterm birth risk • Results demonstrate strong prediction of preterm birth occurring before 37 weeks, and even stronger prediction before 35 weeks TREETOP Study • 5,011 patients, 18 U.S. sites • Broadly validates biomarker prediction of: – Any preterm delivery <32 weeks’ gestation – Adverse neonatal outcomes – Neonatal hospital length of stay • Identifies pregnancies at highest risk for severe complications PAPR & TREETOP Studies: Validating Biomarker Signature for Preterm Birth Risk
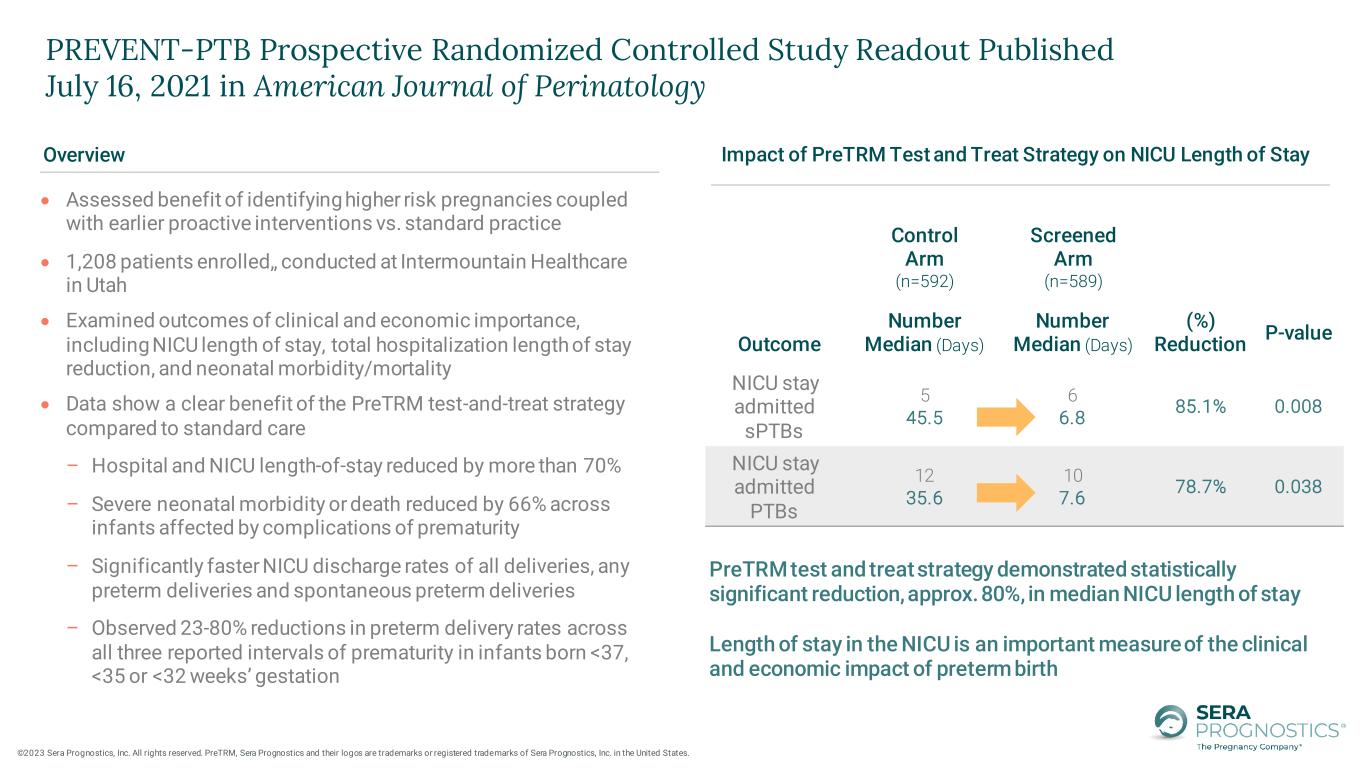
• Assessed benefit of identifying higher risk pregnancies coupled with earlier proactive interventions vs. standard practice • 1,208 patients enrolled,, conducted at Intermountain Healthcare in Utah • Examined outcomes of clinical and economic importance, including NICU length of stay, total hospitalization length of stay reduction, and neonatal morbidity/mortality • Data show a clear benefit of the PreTRM test-and-treat strategy compared to standard care – Hospital and NICU length-of-stay reduced by more than 70% – Severe neonatal morbidity or death reduced by 66% across infants affected by complications of prematurity – Significantly faster NICU discharge rates of all deliveries, any preterm deliveries and spontaneous preterm deliveries – Observed 23-80% reductions in preterm delivery rates across all three reported intervals of prematurity in infants born <37, <35 or <32 weeks’ gestation PreTRM test and treat strategy demonstrated statistically significant reduction, approx. 80%, in median NICU length of stay Length of stay in the NICU is an important measure of the clinical and economic impact of preterm birth Control Arm (n=592) Screened Arm (n=589) Outcome Number Median (Days) Number Median (Days) (%) Reduction P-value NICU stay admitted sPTBs 5 45.5 6 6.8 85.1% 0.008 NICU stay admitted PTBs 12 35.6 10 7.6 78.7% 0.038 Impact of PreTRM Test and Treat Strategy on NICU Length of StayOverview PREVENT-PTB Prospective Randomized Controlled Study Readout Published July 16, 2021 in American Journal of Perinatology ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

• Collaboration with Elevance based on health economic analysis from HealthCore • Multicenter prospective randomized controlled study within Elevance network • Up to 6,500 participants in approx. 15 U.S. sites • Evaluates benefit of PreTRM identification of higher risk pregnancies coupled with proactive interventions • Primary outcomes are hospital length of stay and neonatal morbidity / mortality • Builds data that can be a template for future clinical use of PreTRM PRIME Study: Prematurity Risk Assessment Combined with Clinical Interventions for Improved Neonatal OutcoMEs Elevance Health (formerly Anthem) and Sera Collaborate to Improve Maternal and Neonatal Health

Anthem Claims Data • Analysis of >40,000 mothers and babies within commercially insured Elevance membership – Evaluated screening with PreTRM along with proactive interventions given to PreTRM-higher risk patients vs. standard care – Demonstrated robust clinical and economic impacts of the PreTRM test-and-treat strategy Results(1) • 20% reduction in PTB • $1,608 gross savings, excluding a $745 PreTRM list price per pregnant woman (amortized over all pregnancies including non-tested) • 10% reduction in neonatal intensive care admissions • 7% reduction in overall hospital length-of-stay • 33% reduction in births <32 weeks • The test-and-treat strategy is dominant with respect to cost savings across all conservative probabilistic sensitivity analyses and scenarios * Grabner M, et al. Cost-effectiveness of a proteomic pest for preterm birth prediction; ClinicoEconomics and Outcomes Research 2021:13 809–820 Published Elevance (formerly Anthem) Cost-Effectiveness Model* ©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Near-term Execution Accelerate PreTRM Adoption and Revenue

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. 1 Reengaging institutions with the data • PREVENT Sub-analysis • AVERT PRETERM TRIAL • PRIME • Vietnam Study • RWEs Piloting a care coordination offering Launching real-world evidence studies illustrating the value of PreTRM Distinct Levers to Near-Term Revenue

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Adding outcome evidence to our data story
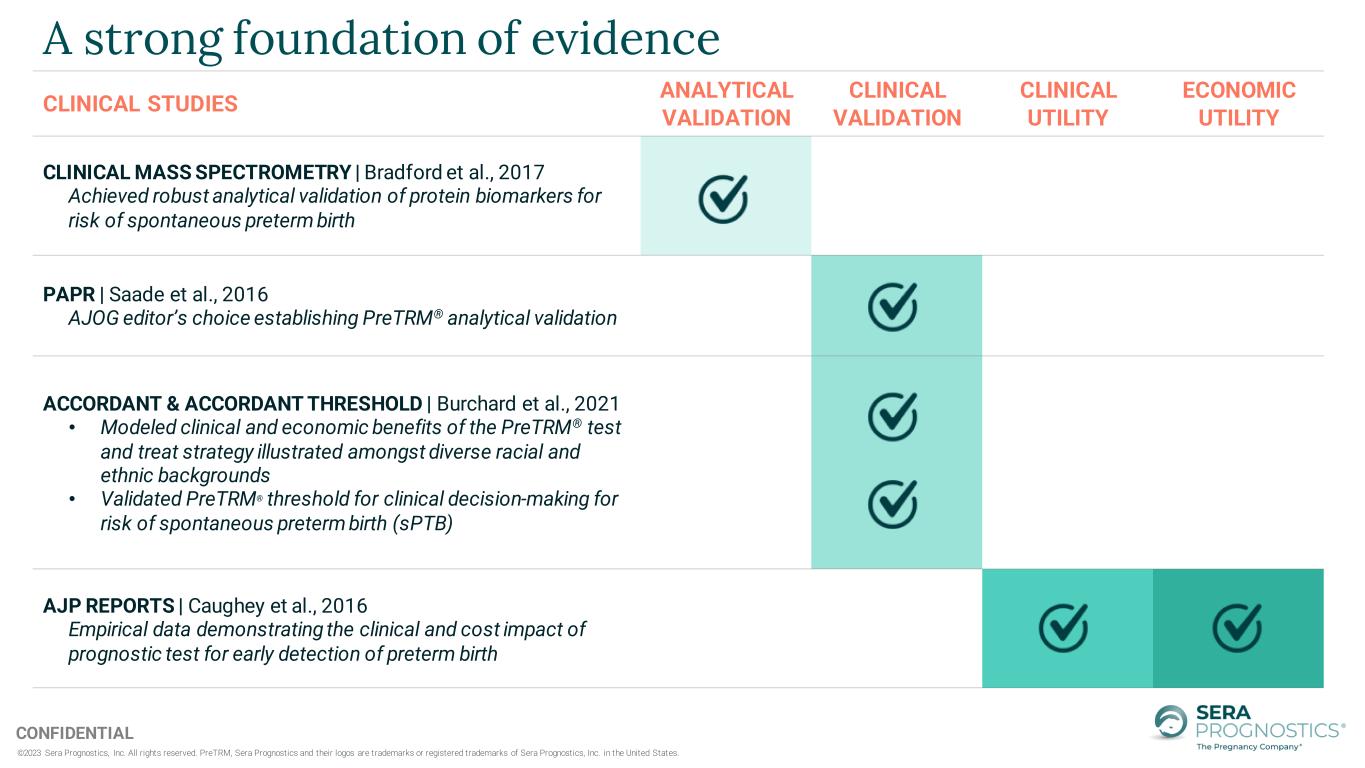
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL A strong foundation of evidence CLINICAL STUDIES ANALYTICAL VALIDATION CLINICAL VALIDATION CLINICAL UTILITY ECONOMIC UTILITY CLINICAL MASS SPECTROMETRY | Bradford et al., 2017 Achieved robust analytical validation of protein biomarkers for risk of spontaneous preterm birth PAPR | Saade et al., 2016 AJOG editor’s choice establishing PreTRM® analytical validation ACCORDANT & ACCORDANT THRESHOLD | Burchard et al., 2021 • Modeled clinical and economic benefits of the PreTRM® test and treat strategy illustrated amongst diverse racial and ethnic backgrounds • Validated PreTRM® threshold for clinical decision-making for risk of spontaneous preterm birth (sPTB) AJP REPORTS | Caughey et al., 2016 Empirical data demonstrating the clinical and cost impact of prognostic test for early detection of preterm birth
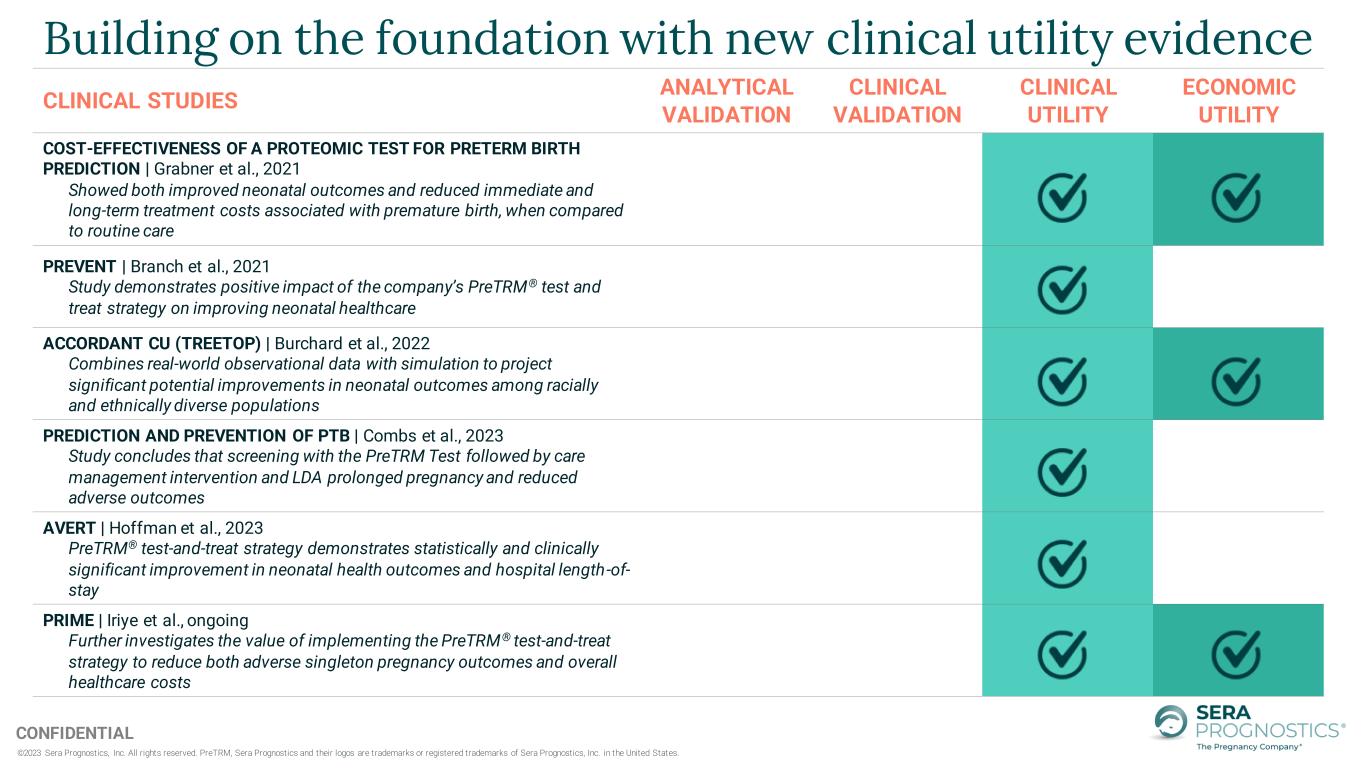
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Building on the foundation with new clinical utility evidence CLINICAL STUDIES ANALYTICAL VALIDATION CLINICAL VALIDATION CLINICAL UTILITY ECONOMIC UTILITY COST-EFFECTIVENESS OF A PROTEOMIC TEST FOR PRETERM BIRTH PREDICTION | Grabner et al., 2021 Showed both improved neonatal outcomes and reduced immediate and long-term treatment costs associated with premature birth, when compared to routine care PREVENT | Branch et al., 2021 Study demonstrates positive impact of the company’s PreTRM® test and treat strategy on improving neonatal healthcare ACCORDANT CU (TREETOP) | Burchard et al., 2022 Combines real-world observational data with simulation to project significant potential improvements in neonatal outcomes among racially and ethnically diverse populations PREDICTION AND PREVENTION OF PTB | Combs et al., 2023 Study concludes that screening with the PreTRM Test followed by care management intervention and LDA prolonged pregnancy and reduced adverse outcomes AVERT | Hoffman et al., 2023 PreTRM® test-and-treat strategy demonstrates statistically and clinically significant improvement in neonatal health outcomes and hospital length-of- stay PRIME | Iriye et al., ongoing Further investigates the value of implementing the PreTRM® test-and-treat strategy to reduce both adverse singleton pregnancy outcomes and overall healthcare costs

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. AVERT PRETERM TRIAL
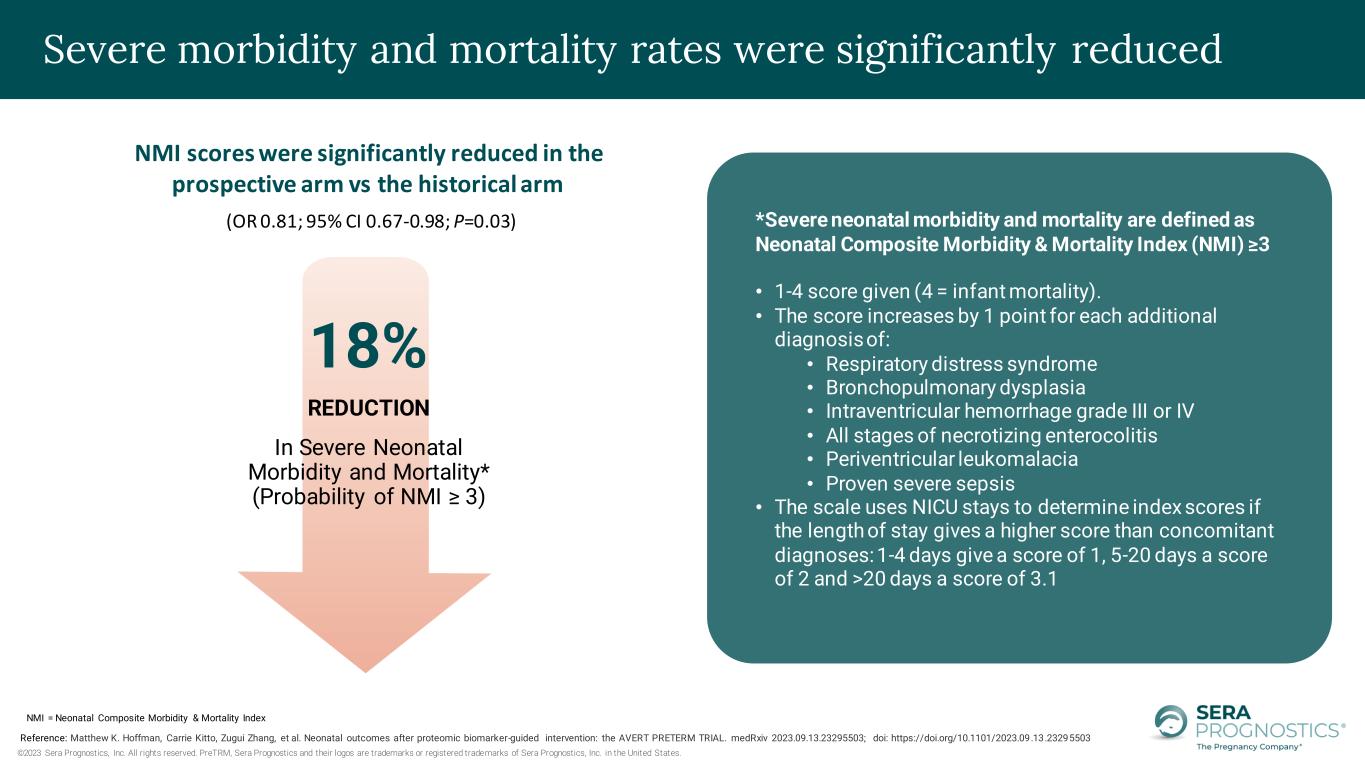
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Severe morbidity and mortality rates were significantly reduced *Severe neonatal morbidity and mortality are defined as Neonatal Composite Morbidity & Mortality Index (NMI) ≥3 • 1-4 score given (4 = infant mortality). • The score increases by 1 point for each additional diagnosis of: • Respiratory distress syndrome • Bronchopulmonary dysplasia • Intraventricular hemorrhage grade III or IV • All stages of necrotizing enterocolitis • Periventricular leukomalacia • Proven severe sepsis • The scale uses NICU stays to determine index scores if the length of stay gives a higher score than concomitant diagnoses: 1-4 days give a score of 1, 5-20 days a score of 2 and >20 days a score of 3.1 NMI scores were significantly reduced in the prospective arm vs the historical arm 18% REDUCTION In Severe Neonatal Morbidity and Mortality* (Probability of NMI ≥ 3) (OR 0.81; 95% CI 0.67-0.98; P=0.03) Reference: Matthew K. Hoffman, Carrie Kitto, Zugui Zhang, et al. Neonatal outcomes after proteomic biomarker-guided intervention: the AVERT PRETERM TRIAL. medRxiv 2023.09.13.23295503; doi: https://doi.org/10.1101/2023.09.13.23295503 NMI = Neonatal Composite Morbidity & Mortality Index

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Gestational age at birth was increased for those at risk of earliest delivery Control Arm (Standard of Care) N=155 Mean Gestational Age at Birth (weeks) Screened Arm (PreTRM Test + Care Management) N=15 Increased Gestational Age at Birth on Average by 2.48 Weeks Gestational Age at Birth <32 Weeks Reference: Matthew K. Hoffman, Carrie Kitto, Zugui Zhang, et al. Neonatal outcomes after proteomic biomarker-guided intervention: the AVERT PRETERM TRIAL. medRxiv 2023.09.13.23295503; doi: https://doi.org/10.1101/2023.09.13.23295503
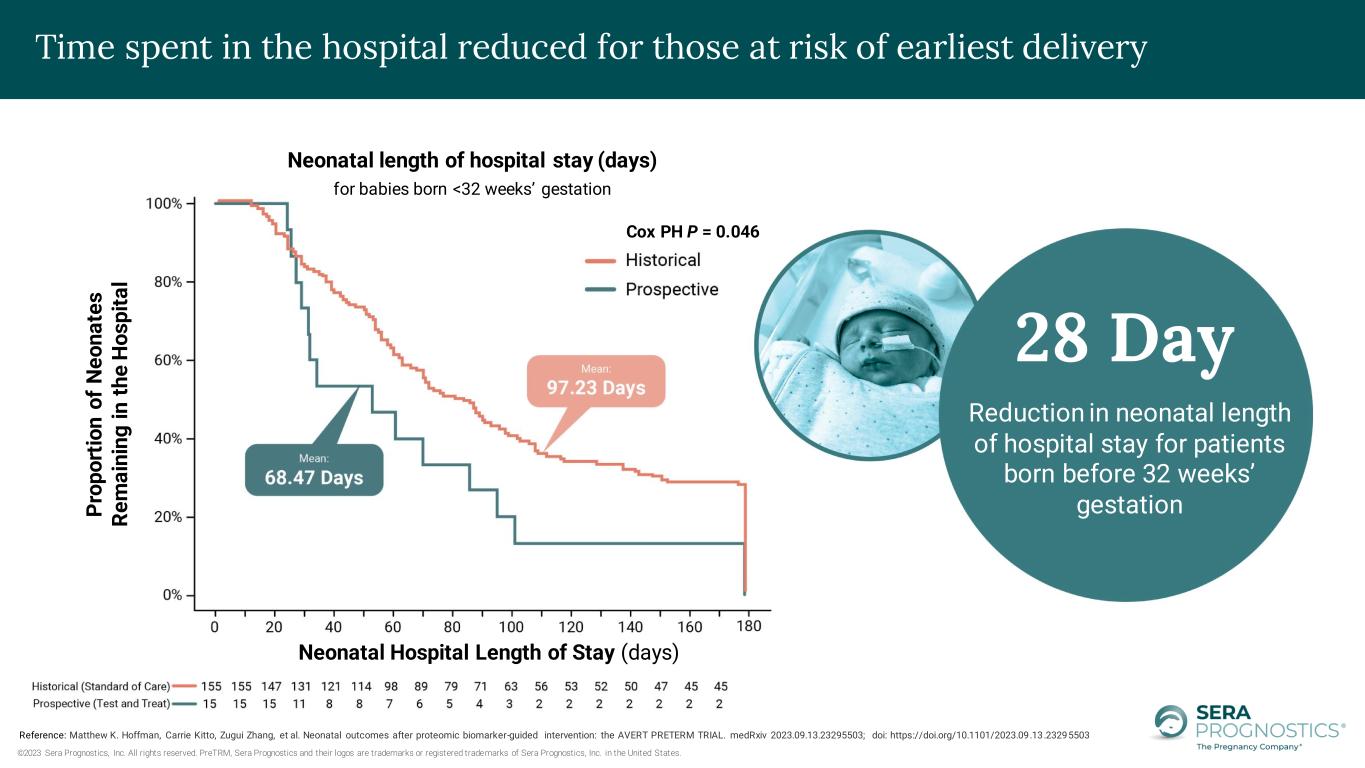
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. P ro p o rt io n o f N e o n a te s R e m a in in g i n t h e H o sp it a l Neonatal Hospital Length of Stay (days) Neonatal length of hospital stay (days) for babies born <32 weeks’ gestation 28 Day Reduction in neonatal length of hospital stay for patients born before 32 weeks’ gestation Reference: Matthew K. Hoffman, Carrie Kitto, Zugui Zhang, et al. Neonatal outcomes after proteomic biomarker-guided intervention: the AVERT PRETERM TRIAL. medRxiv 2023.09.13.23295503; doi: https://doi.org/10.1101/2023.09.13.23295503 Time spent in the hospital reduced for those at risk of earliest delivery Cox PH P = 0.046
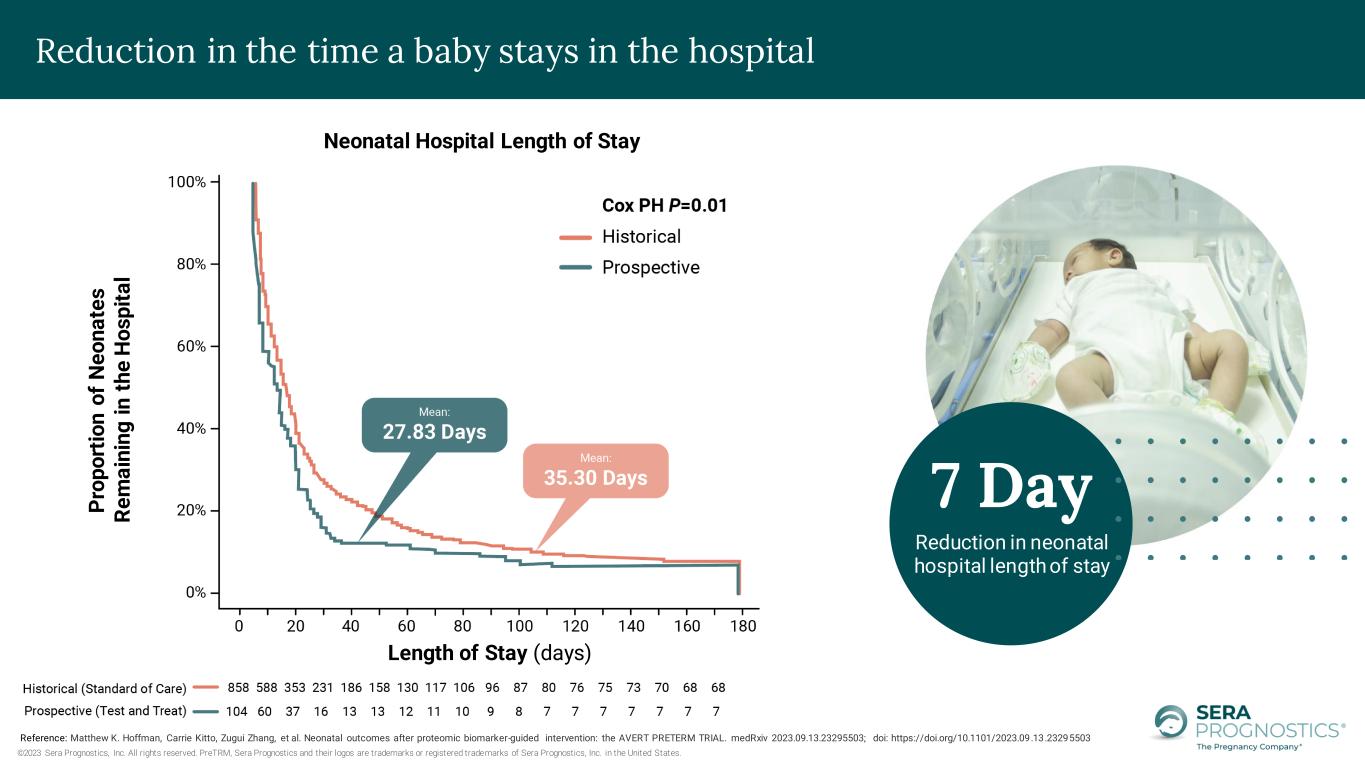
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Neonatal Hospital Length of Stay P ro p o rt io n o f N e o n a te s R e m a in in g i n t h e H o s p it a l Length of Stay (days) Reference: Matthew K. Hoffman, Carrie Kitto, Zugui Zhang, et al. Neonatal outcomes after proteomic biomarker-guided intervention: the AVERT PRETERM TRIAL. medRxiv 2023.09.13.23295503; doi: https://doi.org/10.1101/2023.09.13.23295503 7 Day Reduction in neonatal hospital length of stay Reduction in the time a baby stays in the hospital

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PREVENT-PTB Study – Sub-analysis
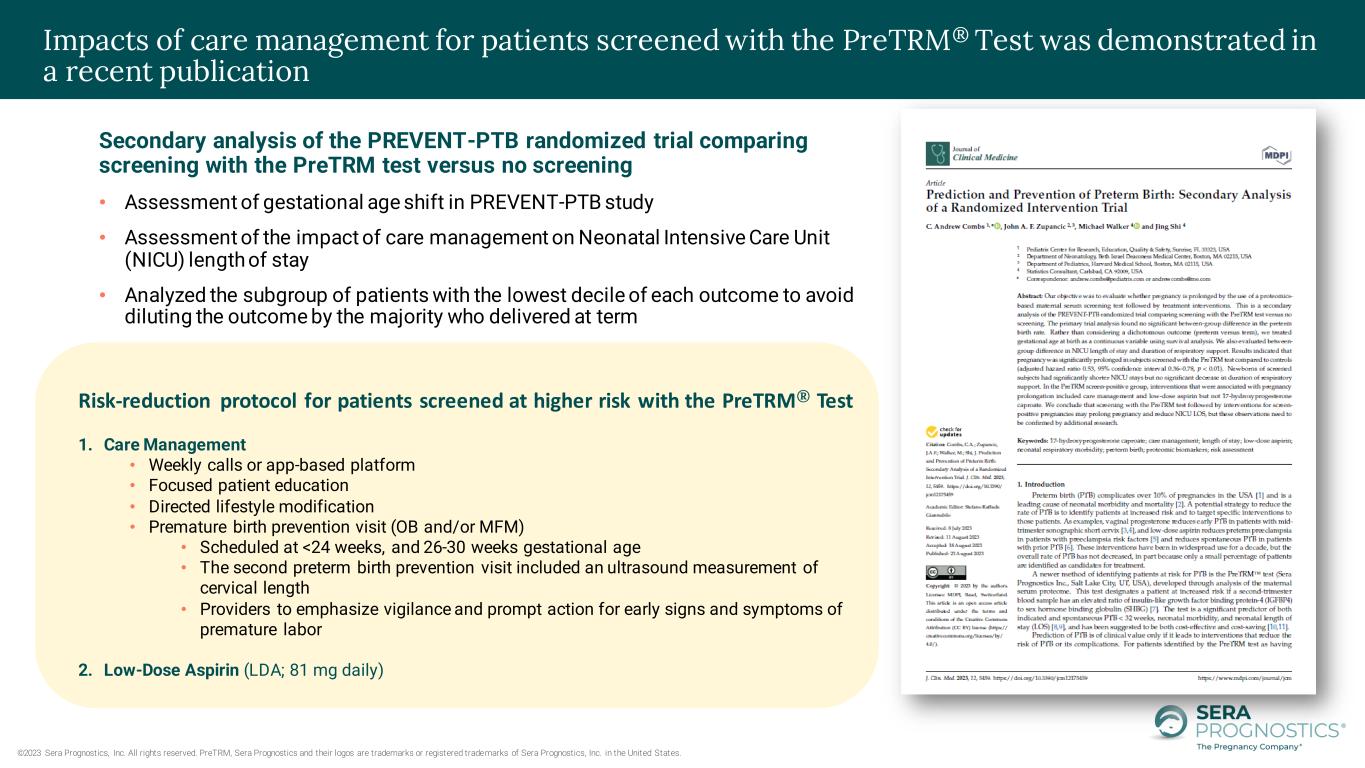
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Impacts of care management for patients screened with the PreTRM® Test was demonstrated in a recent publication Secondary analysis of the PREVENT-PTB randomized trial comparing screening with the PreTRM test versus no screening • Assessment of gestational age shift in PREVENT-PTB study • Assessment of the impact of care management on Neonatal Intensive Care Unit (NICU) length of stay • Analyzed the subgroup of patients with the lowest decile of each outcome to avoid diluting the outcome by the majority who delivered at term Risk-reduction protocol for patients screened at higher risk with the PreTRM® Test 1. Care Management • Weekly calls or app-based platform • Focused patient education • Directed lifestyle modification • Premature birth prevention visit (OB and/or MFM) • Scheduled at <24 weeks, and 26-30 weeks gestational age • The second preterm birth prevention visit included an ultrasound measurement of cervical length • Providers to emphasize vigilance and prompt action for early signs and symptoms of premature labor 2. Low-Dose Aspirin (LDA; 81 mg daily)
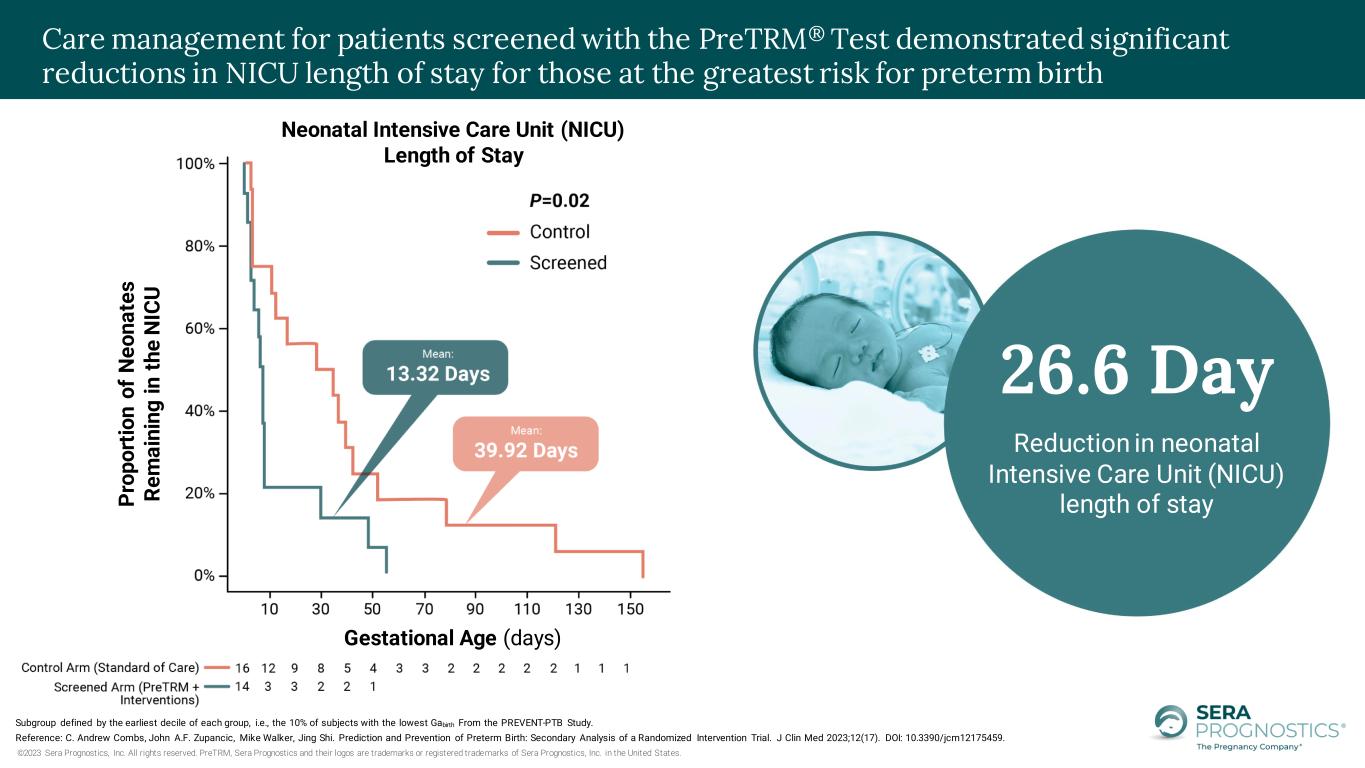
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Subgroup defined by the earliest decile of each group, i.e., the 10% of subjects with the lowest Gabirth From the PREVENT-PTB Study. Reference: C. Andrew Combs, John A.F. Zupancic, Mike Walker, Jing Shi. Prediction and Prevention of Preterm Birth: Secondary Analysis of a Randomized Intervention Trial. J Clin Med 2023;12(17). DOI: 10.3390/jcm12175459. Care management for patients screened with the PreTRM® Test demonstrated significant reductions in NICU length of stay for those at the greatest risk for preterm birth P ro p o rt io n o f N e o n a te s R e m a in in g i n t h e N IC U Gestational Age (days) Neonatal Intensive Care Unit (NICU) Length of Stay 26.6 Day Reduction in neonatal Intensive Care Unit (NICU) length of stay
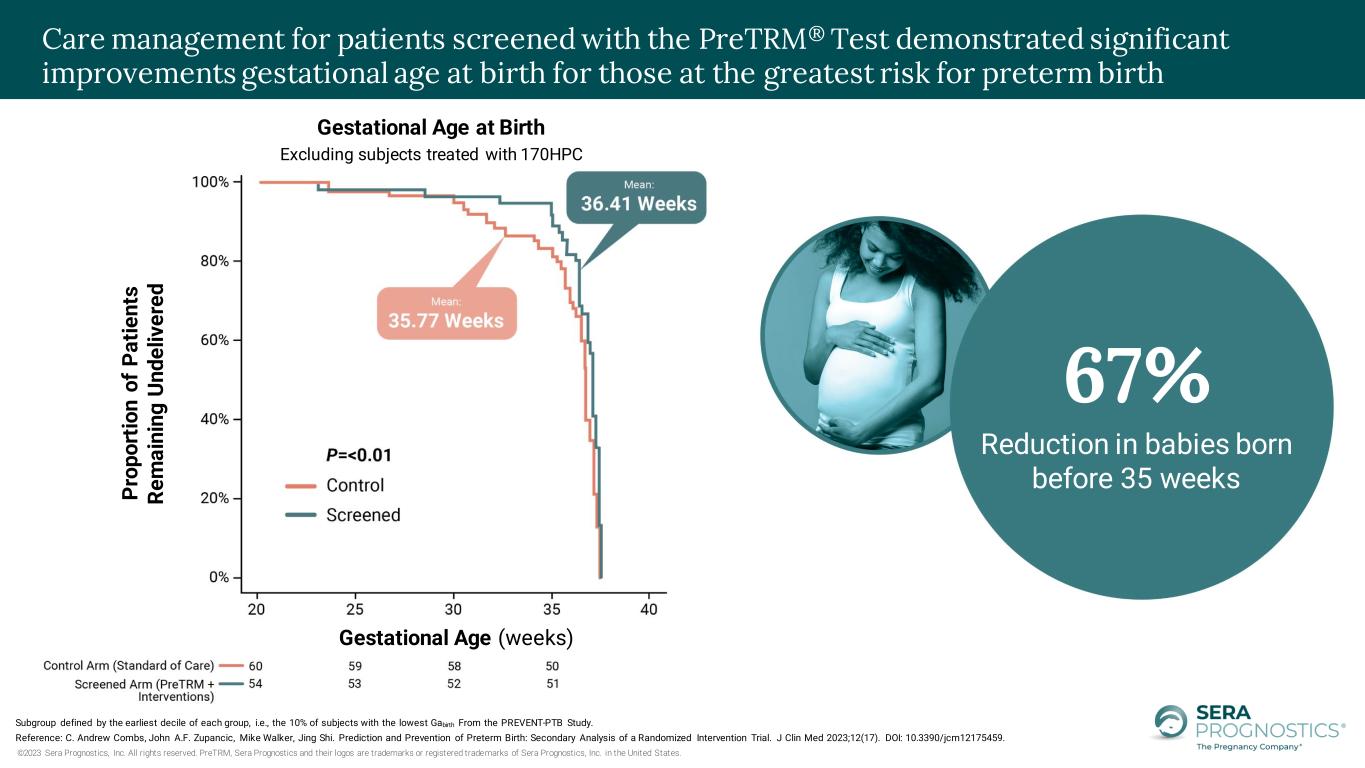
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Lorem ipsum P ro p o rt io n o f P a ti e n ts R e m a in in g U n d e li v e re d Gestational Age (weeks) Gestational Age at Birth Excluding subjects treated with 170HPC Subgroup defined by the earliest decile of each group, i.e., the 10% of subjects with the lowest Gabirth From the PREVENT-PTB Study. Reference: C. Andrew Combs, John A.F. Zupancic, Mike Walker, Jing Shi. Prediction and Prevention of Preterm Birth: Secondary Analysis of a Randomized Intervention Trial. J Clin Med 2023;12(17). DOI: 10.3390/jcm12175459. Care management for patients screened with the PreTRM® Test demonstrated significant improvements gestational age at birth for those at the greatest risk for preterm birth 67% Reduction in babies born before 35 weeks

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Care Coordination Pilot

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Piloting offering to support higher risk patients by coordinating with physician's care • Addresses a hurdle to test adoption by increasing the comfort level of physicians • May help establish care guidelines as soon as possible Care Coordination

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Real-world Evidence Development
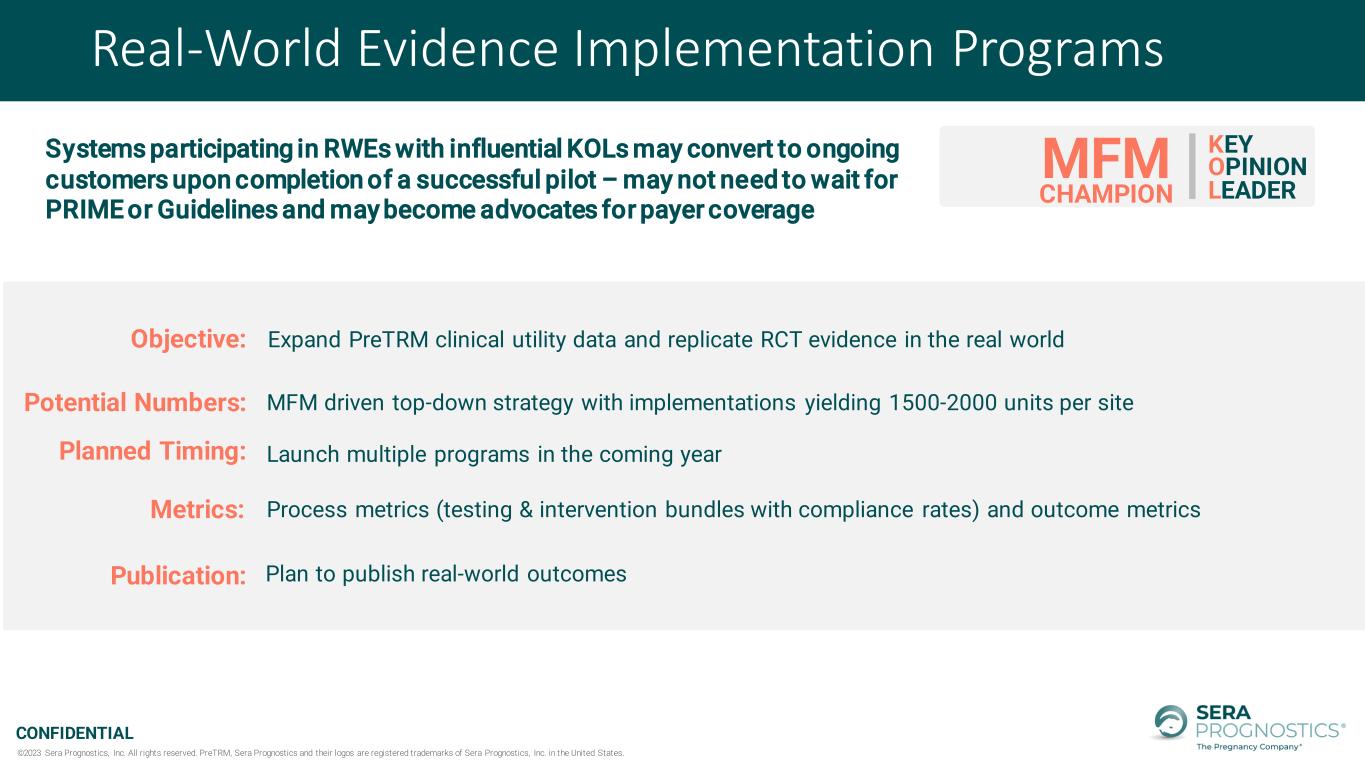
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Real-World Evidence Implementation Programs KEY OPINION LEADER MFM CHAMPION Objective: Expand PreTRM clinical utility data and replicate RCT evidence in the real world Launch multiple programs in the coming yearPlanned Timing: Publication: Plan to publish real-world outcomes Potential Numbers: MFM driven top-down strategy with implementations yielding 1500-2000 units per site Process metrics (testing & intervention bundles with compliance rates) and outcome metricsMetrics: Systems participating in RWEs with influential KOLs may convert to ongoing customers upon completion of a successful pilot – may not need to wait for PRIME or Guidelines and may become advocates for payer coverage

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Medium-term Vision Building a multi-product platform

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Product Portfolio: Time To Birth (TTB) Intended Use To provide a more accurate estimate of when a mother’s baby will be delivered for the purposes of planning maternity leave, travel, visitors, etc. This is not a clinical test and should not be used to plan interventions. Only reports results for term pregnancies Value Proposition For a significant population of Moms-to-be, TTB can offer planning, preparations, and expectations filled with hope and anticipation rather than anxiety and uncertainty by avoiding unwelcome surprises Market Assessment Two independent market surveys of consumers show a strong demand for the TTB test Validation Status Verification and validation done on two cohorts (PAPR, TREETOP) Performance More accurate than the traditionally estimated due date. Commercial Model Consumer pay, direct-to-consumer and business-to-business arrangements

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Product Portfolio: Pregnancy Risk Prediction Panel Intended Use To provide a woman and her physician a risk assessment for multiple pregnancy complications assessing both High Risk (rule-in) and Low Risk (rule-out) results in a single test Value Proposition For OB/GYNs managing high patient volume and focused on preventive care; For Moms seeking to understand the individual risks to their pregnancy journey; For Systems seeking to triage pregnancies to the appropriate level of care Market Assessment Market survey of consumers show a strong demand for predictors of pregnancy complications. Validation Status Clinical validation in 2024 Performance Prototypes have shown very high positive and negative predictive value Commercial Model Physician ordered, payer billed

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Long-term Vision A Bigger Picture
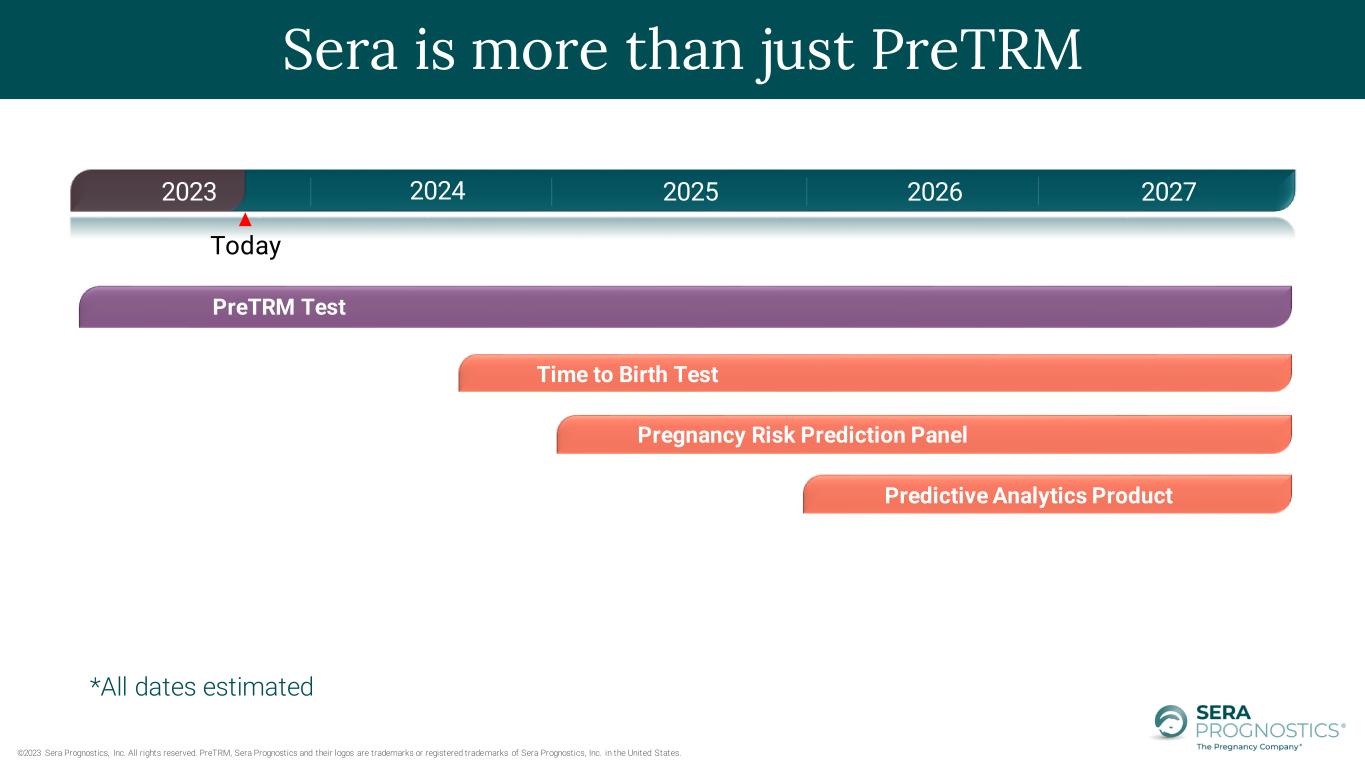
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Today 2023 2024 2025 2026 PreTRM Test Time to Birth Test Pregnancy Risk Prediction Panel Predictive Analytics Product Sera is more than just PreTRM 2027 *All dates estimated

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are registered trademarks of Sera Prognostics, Inc. in the United States. As many as 30% of pregnancies are affected by various complications (i.e., a high-risk pregnancy), including: preterm birth, preeclampsia, fetal growth restriction, stillbirth, hypertension of pregnancy, gestational diabetes, and others The economic consequences of preterm birth alone are estimated to be approximately $25 billion annually We see a compelling opportunity to partner on the use of proteomics and predictive analytics using our high-value data set across nearly 20,000 pregnancies Data As An Asset 30% $25B 20k We have the capability to integrate our pregnancy complication datasets with clinical and demographic data within our proprietary pregnancy assays
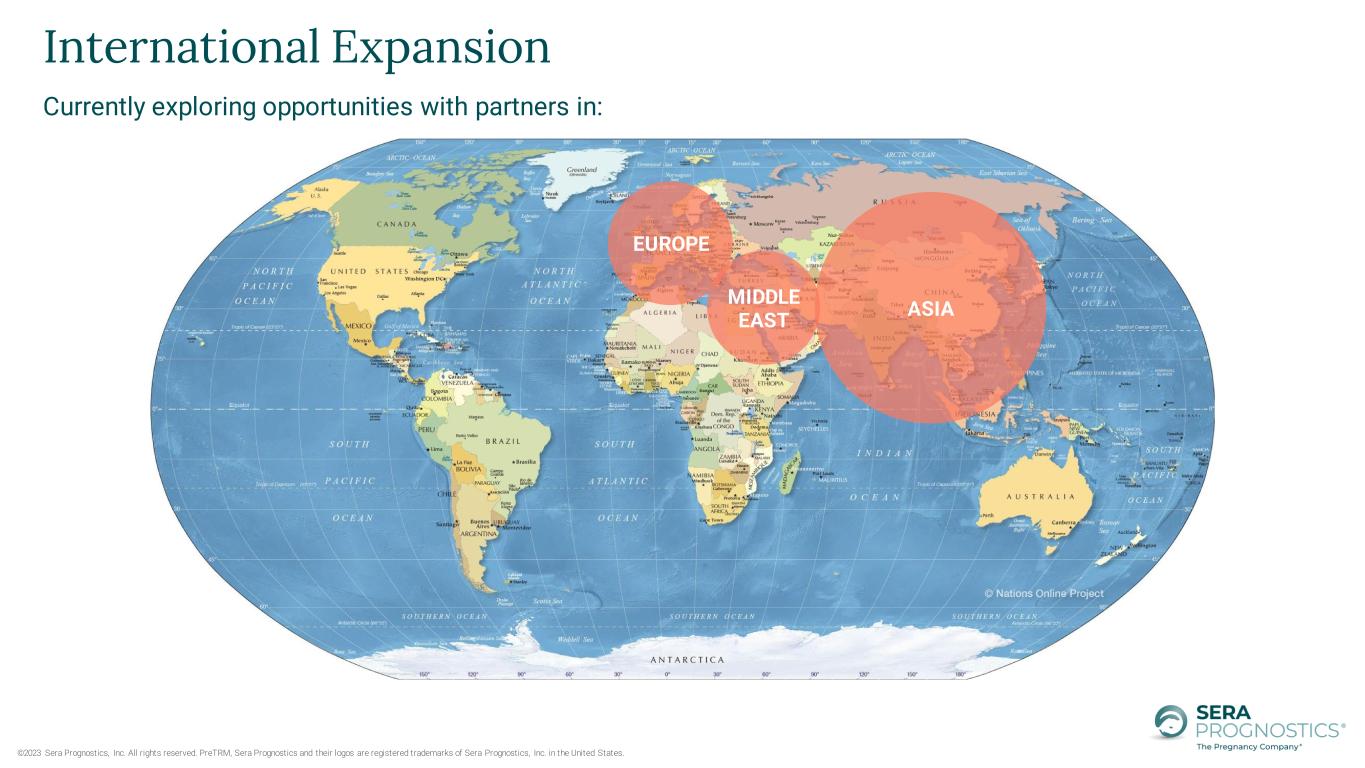
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are registered trademarks of Sera Prognostics, Inc. in the United States. Currently exploring opportunities with partners in: International Expansion EUROPE ASIA MIDDLE EAST

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Key Upcoming Events
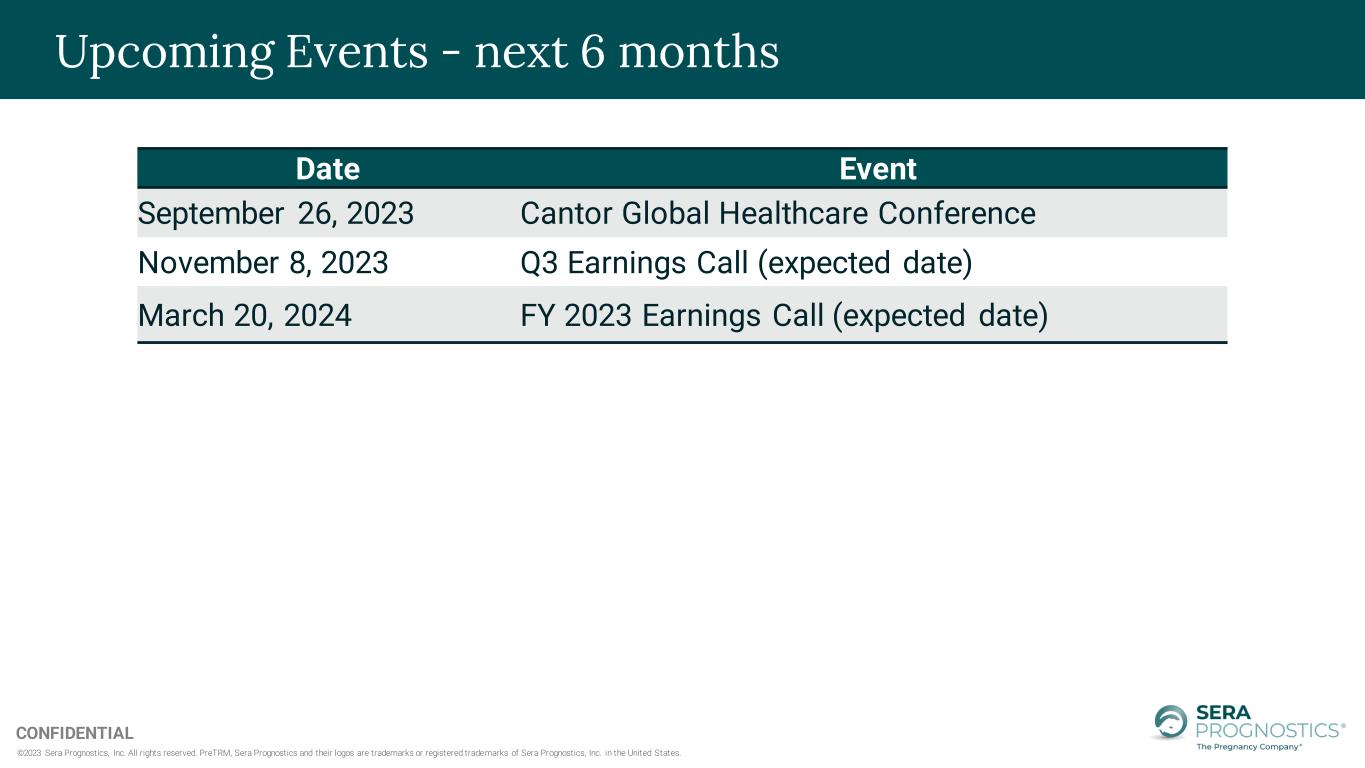
©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. CONFIDENTIAL Upcoming Events - next 6 months Date Event September 26, 2023 Cantor Global Healthcare Conference November 8, 2023 Q3 Earnings Call (expected date) March 20, 2024 FY 2023 Earnings Call (expected date)

©2023 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. 1 Questions?