Stratasys Expands Market Opportunities for Medical Device Manufacturers to Leverage 3D Printing to Enhance Innovation and Improve Patient Outcomes
February 04 2025 - 7:15AM
Business Wire
Stratasys Direct’s New ISO 13485 Certification
Meets Stringent Regulatory Requirements Essential for Medical
Device Manufacturers
Stratasys Ltd. (NASDAQ: SSYS) today announced that its Stratasys
Direct manufacturing facility in Tuscon, Arizona has achieved ISO
13485 certification, a globally recognized standard for quality
management systems in medical device manufacturing. Stratasys plans
to extend this certification to its other Stratasys Direct
manufacturing facilities in Texas and Minnesota.
By achieving ISO 13485 certification, Stratasys Direct is
formally recognized for its safety, precision, and reliability of
its 3D-printed components. This certification addresses critical
regulatory requirements, removing barriers to adoption and enabling
medical device manufacturers to scale production reliably while
accelerating innovation, reducing costs, and improving patient
outcomes.
Stratasys Direct 3D prints multiple components for the medical
industry making complex geometries and patient-specific components
that traditional methods cannot achieve.
"ISO 13485 certification is a game-changer for medical
manufacturing," said Gurvinder Kahlon, General Manager and Vice
President, Stratasys Direct. "This achievement reflects our
commitment to delivering solutions that not only meet rigorous
regulatory standards but also empower the medical industry to
innovate faster and deliver better patient care."
Stratasys Direct has been a trusted partner to the medical field
for years, delivering custom 3D-printed models and components. The
ISO 13485 certification builds on that legacy, positioning 3D
printing as an essential tool for the medical device industry. By
adopting 3D printing, medical manufacturers can reduce development
times, create highly customized components, and innovate faster
than with traditional manufacturing methods. Stratasys Direct’s ISO
13485 certification provides a clear pathway to regulatory
compliance, ensuring consistent quality and expanding market access
for these advanced manufacturing solutions.
Stratasys Direct will showcase its capabilities and commitment
to the medical industry at MD&M West, taking place February
4-6, 2025, in Anaheim, California.
About Stratasys
Stratasys is leading the global shift to additive manufacturing
with innovative 3D printing solutions for industries such as
aerospace, automotive, consumer products, and healthcare. Through
smart and connected 3D printers, polymer materials, a software
ecosystem, and parts on demand, Stratasys solutions deliver
competitive advantages at every stage in the product value chain.
The world’s leading organizations turn to Stratasys to transform
product design, bring agility to manufacturing and supply chains,
and improve patient care.
To learn more about Stratasys, visit www.stratasys.com, the
Stratasys blog, X/Twitter, LinkedIn, or Facebook. To learn more
about Stratasys Direct’s 3D printing services, visit
https://www.stratasys.com/en/stratasysdirect/. Stratasys reserves
the right to utilize any of the foregoing social media platforms,
including Stratasys’ websites, to share material, non-public
information pursuant to the SEC’s Regulation FD. To the extent
necessary and mandated by applicable law, Stratasys will also
include such information in its public disclosure filings.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20250204095120/en/
Media and Investor Contacts:
Stratasys Corporate, North America & EMEA Chris Reese
chris.reese@stratasys.com +1 651 357 0877
Stratasys Corporate, Israel & EMEA Erik Snider
Erik.Snider@stratasys.com +972 74 745 6053
Investor Relations Yonah Lloyd Yonah.Lloyd@stratasys.com +972 74
745 4919
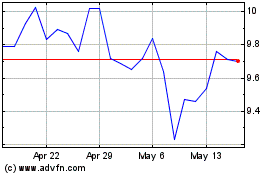
Stratasys (NASDAQ:SSYS)
Historical Stock Chart
From Jan 2025 to Feb 2025
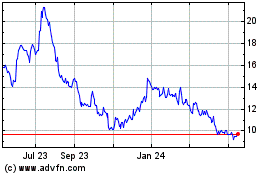
Stratasys (NASDAQ:SSYS)
Historical Stock Chart
From Feb 2024 to Feb 2025