false 0001623526 0001623526 2023-07-25 2023-07-25
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 25, 2023
Stoke Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-38938 |
|
47-1144582 |
| (State or other jurisdiction of incorporation or organization) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 45 Wiggins Ave Bedford, Massachusetts |
|
01730 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (781) 430-8200
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
STOK |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On July 25, 2023, Stoke Therapeutics, Inc. (the “Company”) announced new safety and efficacy data from patients treated with STK-001 in its ongoing Phase 1/2a MONARCH and ADMIRAL studies and its SWALLOWTAIL open-label extension (OLE) study in children and adolescents with Dravet syndrome.
A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K. A copy of the presentation that the Company will present on July 25, 2023 that further describes the data from the ongoing Phase 1/2a MONARCH and ADMIRAL studies and the SWALLOWTAIL open-label extension (OLE) study is attached as Exhibit 99.2 to this Current Report on Form 8-K.
The information furnished under this Item 7.01, including Exhibit 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
Cautionary Note Regarding Forward-Looking Statements
This report contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to the ability of STK-001 to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in non-seizure comorbidities at the indicated dosing levels or at all, and the timing and expected progress of clinical trials, data readouts and presentations. Statements including words such as “plan,” “will,” “continue,” “expect,” or “ongoing” and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties related to: the Company’s ability to advance, obtain regulatory approval of and ultimately commercialize its product candidates; the timing and results of preclinical and clinical trials; the risk that positive results in a clinical trial may not be replicated in subsequent trials or successes in early stage clinical trials may not be predictive of results in later stage trials and preliminary interim data readouts of ongoing trials may show results that change when such trials are completed; the Company’s ability to fund development activities and achieve development goals; the Company’s ability to protect its intellectual property; and other risks and uncertainties described under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, its quarterly reports on Form 10-Q, and the other documents the Company files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this report, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
STOKE THERAPEUTICS, INC. |
|
|
|
|
| Date: July 25, 2023 |
|
|
|
By: |
|
/s/ Stephen J. Tulipano |
|
|
|
|
|
|
Stephen J. Tulipano |
|
|
|
|
|
|
Chief Financial Officer |
Exhibit 99.1
Stoke Therapeutics Announces Positive New Safety & Efficacy Data from Patients Treated with
STK-001 in the Phase 1/2a Studies (MONARCH & ADMIRAL) and the SWALLOWTAIL Open-Label Extension (OLE) Study in Children and Adolescents with Dravet Syndrome
– Totality of data from these ongoing studies suggest clinical benefit for patients ages 2 to 18 years old, including reductions in
seizures and improvements in cognition and behavior that support the potential for disease modification –
– Phase 1/2a
ADMIRAL Study Data STK-001 (70mg): Patients treated with 2 or 3 initial doses experienced substantial and sustained reductions in convulsive seizure frequency; Median reductions at 3 months after last dose
(n=6) of 80% and 89% (n=3) at 6 months after last dose, compared to baseline –
– OLE Study Data STK-001 (30mg, 45mg): Sustained reductions in convulsive seizure frequency and improvements in cognition and behavior–
– MONARCH & ADMIRAL Study Safety Data: STK-001 has been generally well-tolerated among
74 patients treated with single and multiple doses of 10mg to 70mg –
– Additional data anticipated in Q1 2024 following
completion of Phase 1/2a studies –
– Management will host a webinar and conference call for analysts and investors at
8:00 a.m. Eastern Time today –
BEDFORD, Mass., July 25, 2023 – Stoke Therapeutics, Inc. (Nasdaq: STOK), a biotechnology
company dedicated to addressing the underlying cause of severe diseases by upregulating protein expression with RNA-based medicines, today announced positive new safety and efficacy data from patients
treated with STK-001 in the two ongoing Phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension (OLE) study in children and adolescents with Dravet syndrome. These new data suggest
clinical benefit for patients 2 to 18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior
support the potential for disease modification in a highly refractory patient population.
“Together these data support the potential for STK-001 to address the underlying cause of Dravet syndrome by treating both seizures and the cognitive and behavioral issues that make this disease so complex and devastating. Our ongoing studies are providing a
better understanding of a dose and dosing regimen that may generate substantial and sustained benefits for patients, while continuing to be generally well tolerated,” said Edward M. Kaye, M.D., Chief Executive Officer of Stoke Therapeutics.
“We are on track to complete the Phase 1/2a studies by year-end and look forward to sharing these data, and data from the open-label extension studies, in the first quarter of 2024.”
“The patients in these studies were already taking the best available
anti-seizure medicines, making the additional observed reductions in seizures quite meaningful. One of the most exciting things we are seeing is the early sign that, for the first time, we may have a therapy
that can address the syndrome, in addition to the seizures,” said Joseph Sullivan, M.D., Professor of Neurology and Pediatrics and Director of the Pediatric Epilepsy Center of Excellence at the University of California San
Francisco, and a prominent researcher into Dravet Syndrome. “What we know from the natural history data is that the profound deficits in cognitive functioning among patients with Dravet syndrome do not tend to improve on their own, which makes
the improvements indicated in multiple assessments of cognition and behavior compelling.”
About the Phase 1/2a Studies
MONARCH and ADMIRAL are multi-center, Phase 1/2a studies of children and adolescents who have an established diagnosis of Dravet syndrome. The primary
objectives for MONARCH in the United States and ADMIRAL in the United Kingdom are to assess the safety and tolerability of STK-001, as well as to determine the pharmacokinetics in plasma and exposure in
cerebrospinal fluid. A secondary objective is to assess the efficacy of STK-001 as an adjunctive antiepileptic treatment with respect to the percentage change from baseline in convulsive seizure frequency.
Key Efficacy Findings from a Combined Analysis of Phase 1/2a Studies MONARCH & ADMIRAL
The combined efficacy analysis reported today was based on clinically evaluable data from 45 patients who were treated with multiple doses (30mg, 45mg, 70mg)
in either of these two ongoing studies. The greatest reduction in convulsive seizure frequency has been observed among the small number (n=11) of patients treated with two or three doses of 70mg in the ADMIRAL study. The analysis of the 70mg
multiple dose cohort from ADMIRAL study consists primarily of patients treated with three doses of STK-001 (n=5). The Company anticipates that the remaining ADMIRAL study data will consist primarily of
patients treated with two doses of 70mg (n=6). (See Key Safety Findings below.)
Based on these new data and an increasing understanding of the STK-001 mechanism of action and time necessary to produce a clinical effect, the Company performed multiple analyses, including a “through” analysis that incorporates data from a period of time during and
after dosing and an “at” analysis that captured data at a specific timepoint after dosing was completed. The results of both analyses are reported below. The Company believes that the “at” analysis more accurately captured the
effect of STK-001 and will be the most relevant for use in future studies, and the overall development program for STK-001.
Reductions in Convulsive Seizure Frequency Were Observed Across Dose Cohorts*
|
|
|
|
|
|
|
| Median % Reduction from Baseline
in Convulsive Seizure Frequency |
|
30mg MAD
(3 doses, n=18) |
|
45mg MAD
(3 doses, n=16) |
|
70mg MAD**
(3 doses, n=5) (2 doses,
n=6) |
| At 3 Months After Last Dose |
|
27% (n=16) |
|
19% (n=14) |
|
80% (n=6†) |
|
|
|
|
| At 6 Months After Last Dose |
|
4% (n=13) |
|
45% (n=8) |
|
89% (n=3†) |
|
|
|
|
| Day 29 Through 3 Months After Last Dose |
|
28% (n=17) |
|
18% (n=16) |
|
42% (n=8†) |
|
|
|
|
| Day 29 Through 6 Months After Last Dose |
|
24% (n=16) |
|
26% (n=14) |
|
42% (n=6†) |
| * |
Patient numbers were primarily variable due to the fact that patients with ≥50% of the data points
in each time period were included in the applicable “through” cohort (bottom two data rows), even if the patient had not yet reached the last timepoint in the time period. |
| ** |
ADMIRAL patients only. The MONARCH study is evaluating single doses of 70mg and data from this cohort are not
yet available. |
| † |
5/6 patients (at 3 months), 3/3 patients (at 6 months), 5/8 patients (day 29 through 3 months) and 5/6 patients
(day 29 through 6 months) after last dose were treated with 3 doses of 70mg |
Key Efficacy Findings From the SWALLOWTAIL Open-Label
Extension Study
Following treatment in the Phase 1/2a MONARCH study, patients who meet study entry criteria are eligible to continue treatment with STK-001 in SWALLOWTAIL. An analysis of a subset of these patients was performed to assess the potential impact of ongoing treatment with STK-001. This analysis was based only
on the group of patients who received a cumulative total dose of at least 30mg of STK-001 in MONARCH and then continued treatment in SWALLOWTAIL with 30mg or 45mg doses every four months. Twenty-six patients met these criteria when they began treatment in SWALLOWTAIL.
Data from this analysis provide
evidence of the potential for disease modification with ongoing treatment with STK-001. Durable reductions in convulsive seizure frequency were observed throughout the course of treatment. Data from a mixed
model repeated measures (MMRM) analysis indicated substantial improvements from baseline through 12 months in multiple assessments of cognition and behavior, including:
| |
• |
|
Expressive and receptive communication as measured by the Vineland Adaptive Behavior Scale (VABS-III) |
| |
• |
|
Gross motor skills as measured by VABS-III |
| |
• |
|
Executive function as measured by the Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P) |
| |
• |
|
Global Impression of Change scores as reported by caregivers and by clinicians |
Data from the Company’s BUTTERFLY natural history study showed little to no change in these assessments among patients treated with currently available anti-seizure medicines.
Key Safety Findings from an Analysis of the Phase 1/2a MONARCH and ADMIRAL Studies:
The safety analysis for the Phase 1/2a studies reported today was based on data from 74 patients who were treated with single or multiple doses of STK-001 (10mg, 20mg, 30mg, 45mg, 70mg) and followed for up to six months after their last dose.
| |
• |
|
STK-001 was generally well-tolerated among 74 patients treated with
single and multiple doses of 10mg to 70mg in the Phase 1/2a studies and there were no discontinuations related to study drug. |
| |
• |
|
32% (24/74) of patients experienced a treatment-emergent adverse event (TEAE) that was related to study drug. The
most common TEAEs related to study drug were CSF protein elevations, vomiting, and irritability. |
| |
• |
|
20% (15/74) of patients had a treatment-emergent serious adverse event (TESAE). The TESAEs experienced by 14 of
the 15 were not considered related to study drug. |
| |
• |
|
One patient who received multiple doses of 70mg STK-001 in the ADMIRAL
study experienced Suspected Unexpected Serious Adverse Reactions (SUSARs) that were attributed by the investigator to STK-001. The patient went on to complete the study. |
| |
• |
|
Subsequently, the study protocol for ADMIRAL was amended to allow investigators to decide whether to administer
two or three doses of STK-001 (70mg) in the ADMIRAL study before patients would be eligible to enroll in the LONGWING OLE. |
Safety findings from patients who continued treatment in SWALLOWTAIL OLE (n=44) were consistent with the findings from MONARCH and ADMIRAL with the exception
of a greater incidence of CSF protein elevation. In SWALLOWTAIL, 64% (28/44) of patients had at least 1 CSF protein value >50 mg/dL. No clinical manifestations have been observed in these patients, although one patient discontinued treatment in
SWALLOWTAIL due to elevated CSF protein.
Key PK and CSF Exposure Findings:
| |
• |
|
A dose-dependent increase in study drug exposure was observed in plasma. The plasma PK profile was consistent
across MONARCH and ADMIRAL patients who were treated at the same dose level. |
| |
• |
|
STK-001 drug levels increased in CSF following 3 doses of 30mg and 45mg,
suggesting STK-001 accumulation in CNS tissues. CSF exposure was measurable up to six months following multiple intrathecal doses of STK-001, indicating sustained
exposure of STK-001 in the brain. CSF exposure data from the 70mg cohort will be included in the end of study analysis. |
Clinical Progress Updates and Next Steps
| |
• |
|
These data are planned for presentation at the 35th International Epilepsy Congress September 2-6, 2023 in Dublin, Ireland, and also at the American Epilepsy Society (AES) December 1-5, 2023 in Orlando, Fla. |
| |
• |
|
The Company anticipates additional data, including the end of study data from MONARCH (including patients treated
with a single dose of 70mg) and ADMIRAL, as well as additional data from the SWALLOWTAIL and LONGWING OLEs, in the first quarter of 2024. |
| |
• |
|
The Company plans to share an update on Phase 3 planning in the first half of 2024, pending the results from the
completed Phase 1/2a studies and ongoing OLEs. |
Stoke Webinar and Conference Call for Analysts and Investors
Stoke will host a webinar and conference call for analysts and investors at 8:00 a.m. Eastern Time on Tuesday, July 25, 2023, to present positive new data
from the two ongoing Phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study in children and adolescents with Dravet syndrome. The webinar will be broadcast live on the Investors & News section of
Stoke’s website at https://investor.stoketherapeutics.com/. An archived replay of the webinar will be available for at least 90 days following the event. Participants who want to join the call and ask a question may register here
to receive the dial-in numbers and unique PIN to seamlessly access the call. Otherwise please access the listen-only webinar by clicking here.
About Dravet Syndrome
Dravet syndrome is a severe and
progressive genetic epilepsy characterized by frequent, prolonged and refractory seizures, beginning within the first year of life. Dravet syndrome is difficult to treat and has a poor long-term prognosis. Complications of the disease often
contribute to a poor quality of life for patients and their caregivers. The effects of the disease go beyond seizures and often include intellectual disability, developmental delays, movement and balance issues, language and speech disturbances,
growth defects, sleep abnormalities, disruptions of the autonomic nervous system and mood disorders. The disease is classified as developmental and epileptic encephalopathy due to the developmental delays and cognitive impairment associated with the
disease. Compared with the general epilepsy population, people living with Dravet syndrome have a higher risk of sudden unexpected death in epilepsy, or SUDEP. There are no approved disease-modifying therapies for people living with Dravet syndrome.
One out of 16,000 babies are born with Dravet syndrome, which is not concentrated in a particular geographic area or ethnic group.
About STK-001
STK-001 is an investigational new medicine for the treatment of
Dravet syndrome currently being evaluated in ongoing clinical trials. Stoke believes that STK-001, a proprietary antisense oligonucleotide (ASO), has the potential to be the first disease-modifying therapy to
address the genetic cause of Dravet syndrome. STK-001 is designed to upregulate NaV1.1 protein expression by leveraging the non-mutant (wild-type) copy of the
SCN1A gene to restore physiological NaV1.1 levels, thereby reducing both occurrence of seizures and significant non-seizure comorbidities. STK-001 has been
granted orphan drug designation by the FDA and the EMA, and rare pediatric disease designation by the FDA as a potential new treatment for Dravet syndrome.
About the Phase 1/2a MONARCH Study (United States)
The
MONARCH study is a Phase 1/2a open-label study of children and adolescents ages 2 to 18 who have an established diagnosis of Dravet syndrome and have evidence of a genetic mutation in the SCN1A gene. The primary objectives for the study are
to assess the safety and tolerability of STK-001, as well as to determine the pharmacokinetics in plasma and exposure in cerebrospinal fluid. A secondary objective is to assess the efficacy as an adjunctive
antiepileptic treatment with respect to the percentage change from baseline in convulsive seizure frequency. Stoke also intends to measure non-seizure aspects of the disease, such as quality of life, as
secondary endpoints. Additional information about the MONARCH study can be found at https://www.monarchstudy.com/.
Patients who participated in the MONARCH study and meet study entry criteria are eligible to continue
treatment in SWALLOWTAIL, an open-label extension (OLE) study designed to evaluate the long-term safety and tolerability of repeat doses of STK-001. We expect that SWALLOWTAIL will also provide valuable
information on the preliminary effects of STK-001 on seizures along with non-seizure aspects of the disease, such as quality of life and cognition. Enrollment and dosing
in SWALLOWTAIL are ongoing.
About the Phase 1/2a ADMIRAL Study (United Kingdom)
The ADMIRAL study is a Phase 1/2a open-label study of children and adolescents ages 2 to <18 who have an established diagnosis of Dravet syndrome and have
evidence of a genetic mutation in the SCN1A gene. The primary objectives for the study are to assess the safety and tolerability of multiple doses of STK-001, as well as to determine the
pharmacokinetics in plasma and exposure in cerebrospinal fluid. A secondary objective is to assess the effect of multiple doses of STK-001 as an adjunctive antiepileptic treatment with respect to the
percentage change from baseline in convulsive seizure frequency. Stoke also intends to measure non-seizure aspects of the disease, such as overall clinical status and quality of life, as secondary endpoints.
Patients who participated in the ADMIRAL study and meet study entry criteria are eligible to continue treatment in LONGWING, an open-label extension
(OLE) study designed to evaluate the long-term safety and tolerability of repeat doses of STK-001. We expect that LONGWING will also provide valuable information on the preliminary effects of STK-001 on seizures along with non-seizure aspects of the disease, such as quality of life and cognition.
Enrollment and dosing in LONGWING are ongoing.
About TANGO
TANGO (Targeted Augmentation of Nuclear Gene Output) is Stoke’s proprietary research platform. Stoke’s initial application for this
technology are diseases in which one copy of a gene functions normally and the other is mutated, also called haploinsufficiencies. In these cases, the mutated gene does not produce its share of protein, resulting in disease. Using the TANGO approach
and a deep understanding of RNA science, Stoke researchers design antisense oligonucleotides (ASOs) that bind to pre-mRNA and help the functional (or wild-type) genes produce more protein. TANGO aims to
restore missing proteins by increasing – or stoking – protein output from healthy genes, thus compensating for the mutant copy of the gene.
About Stoke Therapeutics
Stoke Therapeutics (Nasdaq:
STOK), is a biotechnology company dedicated to addressing the underlying cause of severe diseases by upregulating protein expression with RNA-based medicines. Using Stoke’s proprietary TANGO (Targeted
Augmentation of Nuclear Gene Output) approach, Stoke is developing antisense oligonucleotides (ASOs) to selectively restore protein levels. Stoke’s first compound, STK-001, is in clinical testing for the
treatment of Dravet syndrome, a severe and progressive genetic epilepsy. Dravet syndrome is one of many
diseases caused by a haploinsufficiency, in which a loss of ~50% of normal protein levels leads to disease. Stoke is pursuing the development of STK-002
for the treatment of autosomal dominant optic atrophy (ADOA), the most common inherited optic nerve disorder. Stoke’s initial focus is haploinsufficiencies and diseases of the central nervous system and the eye, although proof of concept has
been demonstrated in other organs, tissues, and systems, supporting its belief in the broad potential for its proprietary approach. Stoke is headquartered in Bedford, Massachusetts with offices in Cambridge, Massachusetts. For more information,
visit https://www.stoketherapeutics.com/ or follow Stoke on Twitter at @StokeTx.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform
Act of 1995, including, but not limited to the ability of STK-001 to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in
non-seizure comorbidities at the indicated dosing levels or at all, and the timing and expected progress of clinical trials, data readouts and presentations. Statements including words such as
“plan,” “will,” “continue,” “expect,” or “ongoing” and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as well as
assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties related
to: the Company’s ability to advance, obtain regulatory approval of and ultimately commercialize its product candidates; the timing and results of preclinical and clinical trials; the risk that positive results in a clinical trial may not be
replicated in subsequent trials or successes in early stage clinical trials may not be predictive of results in later stage trials and preliminary interim data readouts of ongoing trials may show results that change when such trials are completed;
the Company’s ability to fund development activities and achieve development goals; the Company’s ability to protect its intellectual property; and other risks and uncertainties described under the heading “Risk Factors” in the
Company’s Annual Report on Form 10-K for the year ended December 31, 2022, its quarterly reports on Form 10-Q, and the other documents the Company files from time
to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or
circumstances after the date hereof.
Stoke Media & Investor Contacts:
Dawn Kalmar
Chief Communications Officer
dkalmar@stoketherapeutics.com
781-303-8302
Eric Rojas
Vice President, Investor Relations
IR@stoketherapeutics.com
617-312-2754

Analysis of STK-001 for the Treatment
of Dravet Syndrome Stoke Therapeutics July 25, 2023 Exhibit 99.2

Agenda Introduction Eric Rojas, Head
of Investor Relations Introductory Remarks Edward M. Kaye, M.D., Chief Executive Officer Phase 1/2a Interim Analysis Barry Ticho, M.D., Ph.D., Chief Medical Officer Kimberly Parkerson, M.D., Ph.D., Head of Neurology Clinical Development
Closing Remarks Edward M. Kaye, M.D., Chief Executive Officer Q&A (to include additional Stoke leadership) Shamim Ruff, Chief Regulatory Officer

Forward Looking Statements This
presentation has been prepared by Stoke Therapeutics, Inc. (“Stoke” or “we”) for information purposes only and for no other purpose. Nothing contain in this presentation is, or should be construed as, a recommendation,
promise or representation by the presenter or Stoke or any officer, director, employee, agent or advisor of Stoke. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. This presentation contains
forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to the ability of STK-001 to treat the underlying causes of Dravet syndrome
and reduce seizures or show improvements in non-seizure comorbidities at the indicated dosing levels or at all, and the timing and expected progress of clinical trials, data readouts and presentations. Statements including words such as
“plan,” “will,” “continue,” “expect,” or “ongoing” and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as
well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties
related to: our ability to advance, obtain regulatory approval of and ultimately commercialize its product candidates; the timing and results of preclinical and clinical trials; the risk that positive results in a clinical trial may not be
replicated in subsequent trials or successes in early stage clinical trials may not be predictive of results in later stage trials and preliminary interim data readouts of ongoing trials may show results that change when such trials are completed;
our ability to fund development activities and achieve development goals; our ability to protect its intellectual property; and other risks and uncertainties described under the heading “Risk Factors” in our Annual Report on Form 10-K
for the year ended December 31, 2022, our quarterly reports on Form 10-Q, and the other documents we file from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press
release, and Stoke undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. By attending or receiving this presentation you acknowledge that you are cautioned not to place
undue reliance on these forward-looking statements, which speak only as of the date such statements are made; you will be solely responsible for your own assessment of the market and our market position; and that you will conduct your own analysis
and be solely responsible for forming your own view of the potential future performance of Stoke.

Introductory Remarks Edward M. Kaye,
M.D. Chief Executive Officer
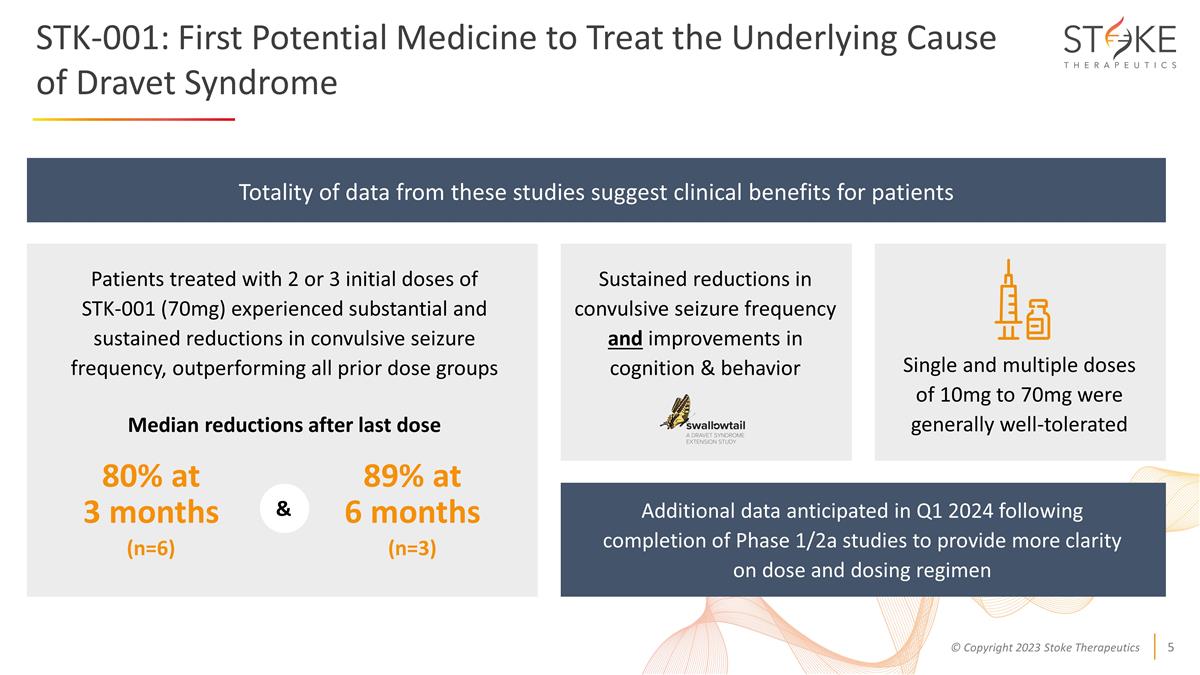
STK-001: First Potential Medicine to
Treat the Underlying Cause of Dravet Syndrome Patients treated with 2 or 3 initial doses of STK-001 (70mg) experienced substantial and sustained reductions in convulsive seizure frequency, outperforming all prior dose groups Sustained reductions in
convulsive seizure frequency and improvements in cognition & behavior Totality of data from these studies suggest clinical benefits for patients Median reductions after last dose & Single and multiple doses of 10mg to 70mg were generally
well-tolerated Additional data anticipated in Q1 2024 following completion of Phase 1/2a studies to provide more clarity on dose and dosing regimen 80% at 3 months (n=6) 89% at 6 months (n=3)

~35,000 Seizures are not adequately
controlled in 90% of people with Dravet syndrome 85% of cases caused by a HAPLOINSUFFICIENCY of the SCN1A gene people affected in the U.S., Canada, Japan, Germany, France and the UK of children and adolescents with Dravet syndrome die before
adulthood, due to SUDEP1, prolonged seizures, seizure-related accidents or infections 20% Up to Dravet syndrome is not concentrated in a particular geographic area or ethnic group 50% NaV1.1 protein expression 1 out of 16,000 babies are born with
Dravet syndrome RESULTING in Today: No Disease-Modifying Medicines for Dravet Syndrome 1 Sudden Unexpected Death in Epilepsy Sources: 2018 Health Advances Report; Djémié et al., Molecular Genetics & Genomic Medicine, 2016; Lagae et
al., Developmental Medicine & Child Neurology, 2017; Nabbout et al., Orphanet Journal of Rare Diseases, 2013

Our Goal: Syndrome Management, Not
Just Seizures No medicines available for Syndrome management STK-001 The only potential disease-modifying approach currently in the clinic for Dravet syndrome Available medicines used to control seizures: Acetazolamide Benzodiazepines Brivaracetam
Cannabidiol Carbamazepine Clobazam Ethosuximide Felbamate Fenfluramine Lamotrigine Levetiracetam Mesuximide Oxcarbazepine Phenytoin Rufinamide Stiripentol Topiramate Valproate products Zonisamide Multiple medicines available for Seizure management
Despite these treatments, seizures are not adequately controlled in 90% of patients with Dravet syndrome
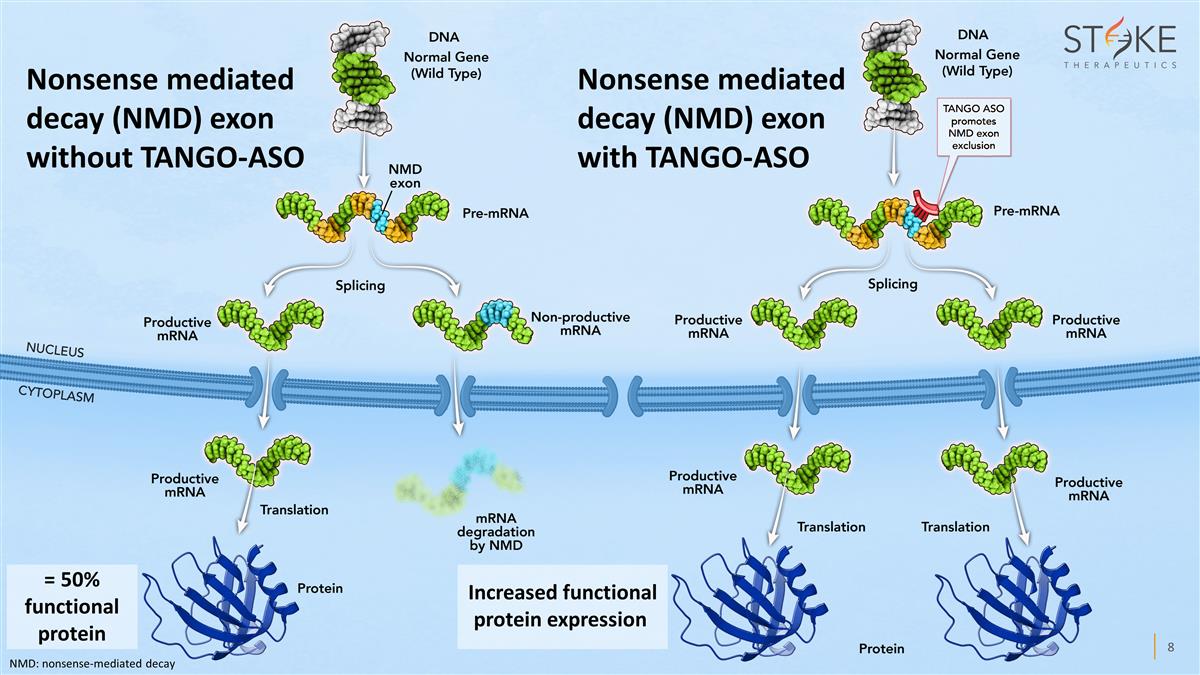
Nonsense mediated decay (NMD) exon
without TANGO-ASO Increased functional protein expression Nonsense mediated decay (NMD) exon with TANGO-ASO = 50% functional protein NMD: nonsense-mediated decay
Analysis of Phase 1/2a MONARCH and
ADMIRAL Studies of STK-001 Barry Ticho, M.D., Ph.D. Chief Medical Officer

Phase 1/2a Study Designs: MONARCH
(US) and ADMIRAL (UK) *Study protocol for ADMIRAL was amended to allow investigators to decide whether to administer two or three doses of STK-001 (70mg) in the ADMIRAL study before patients would be eligible to enroll in the LONGWING OLE. * SAD MAD
MAD
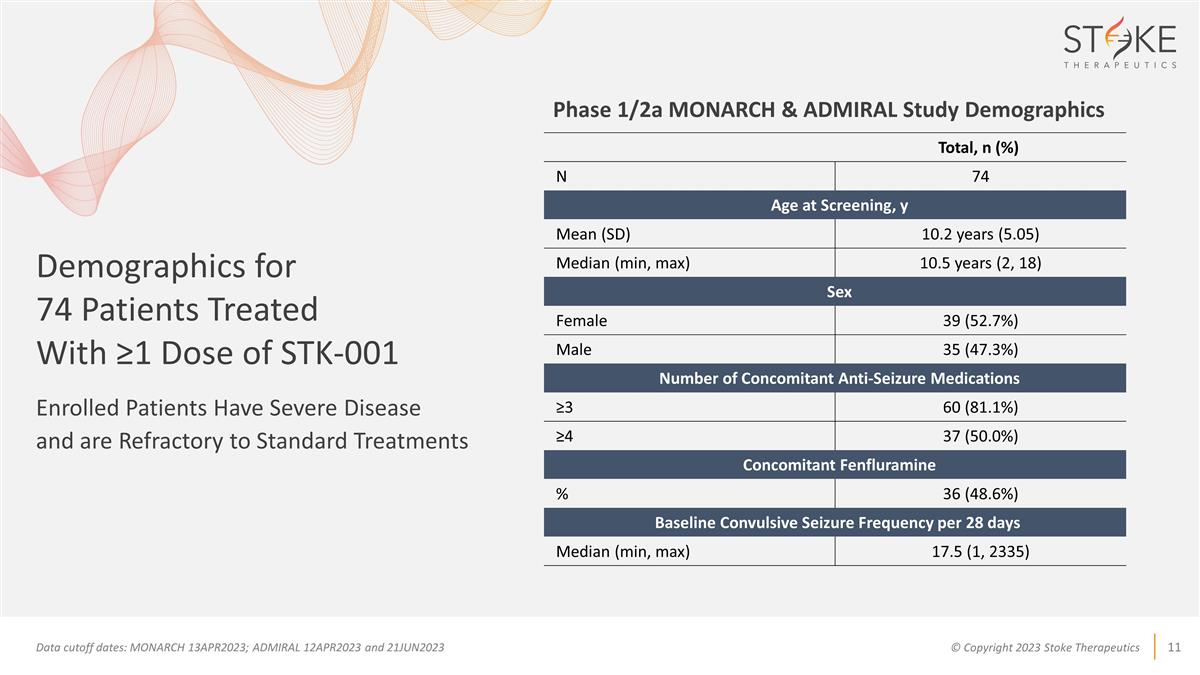
Demographics for 74 Patients
Treated With ≥1 Dose of STK-001 Enrolled Patients Have Severe Disease and are Refractory to Standard Treatments Data cutoff dates: MONARCH 13APR2023; ADMIRAL 12APR2023 and 21JUN2023 Total, n (%) N 74 Age at
Screening, y Mean (SD) 10.2 years (5.05) Median (min, max) 10.5 years (2, 18) Sex Female 39 (52.7%) Male 35 (47.3%) Number of Concomitant Anti-Seizure Medications ≥3 60 (81.1%) ≥4 37
(50.0%) Concomitant Fenfluramine % 36 (48.6%) Baseline Convulsive Seizure Frequency per 28 days Median (min, max) 17.5 (1, 2335) Phase 1/2a MONARCH & ADMIRAL Study Demographics
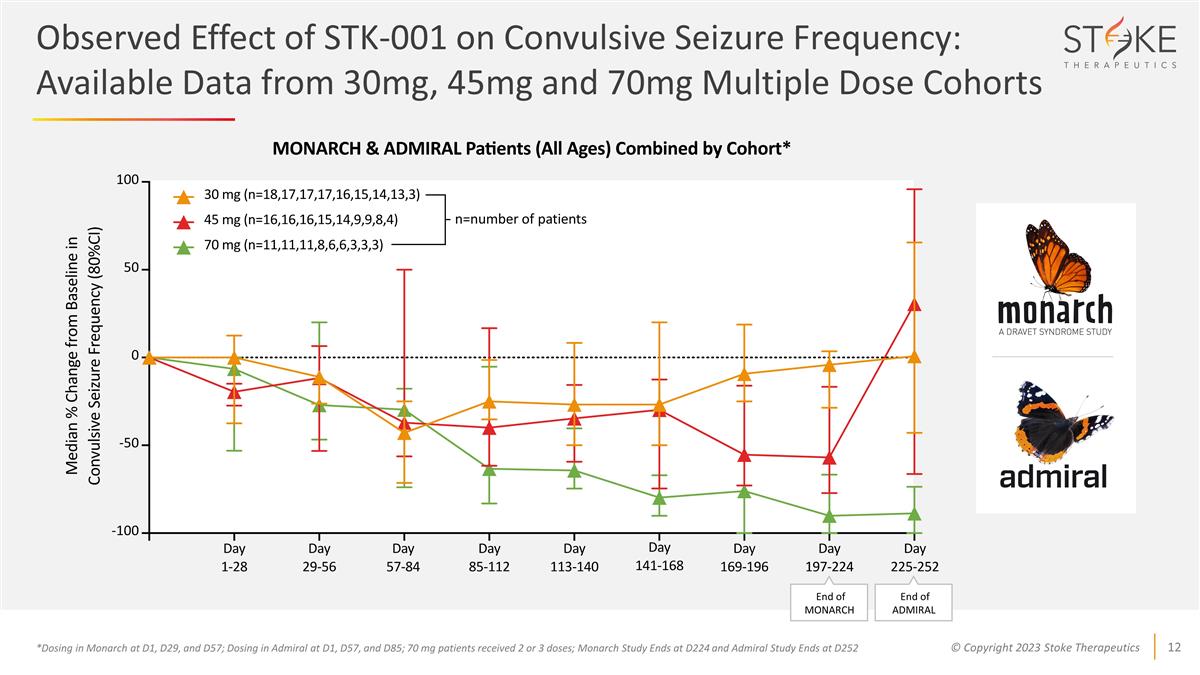
Observed Effect of STK-001 on
Convulsive Seizure Frequency: Available Data from 30mg, 45mg and 70mg Multiple Dose Cohorts *Dosing in Monarch at D1, D29, and D57; Dosing in Admiral at D1, D57, and D85; 70 mg patients received 2 or 3 doses; Monarch Study Ends at D224 and Admiral
Study Ends at D252 n=number of patients End of MONARCH End of ADMIRAL
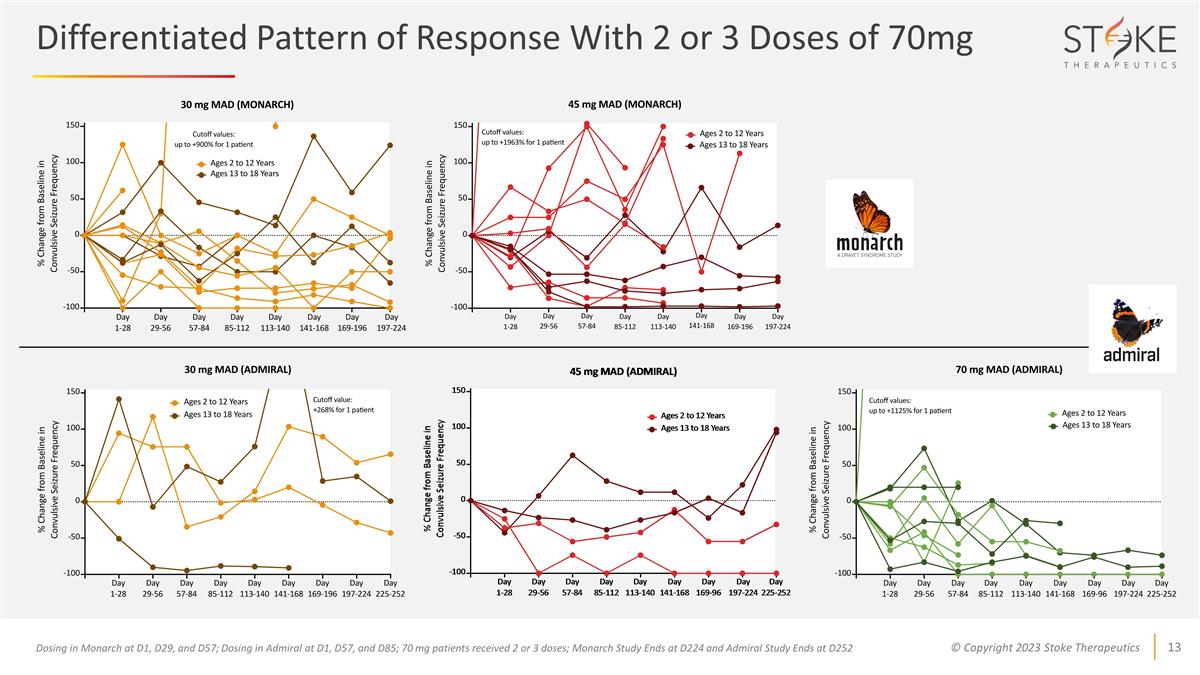
Differentiated Pattern of Response
With 2 or 3 Doses of 70mg Dosing in Monarch at D1, D29, and D57; Dosing in Admiral at D1, D57, and D85; 70 mg patients received 2 or 3 doses; Monarch Study Ends at D224 and Admiral Study Ends at D252
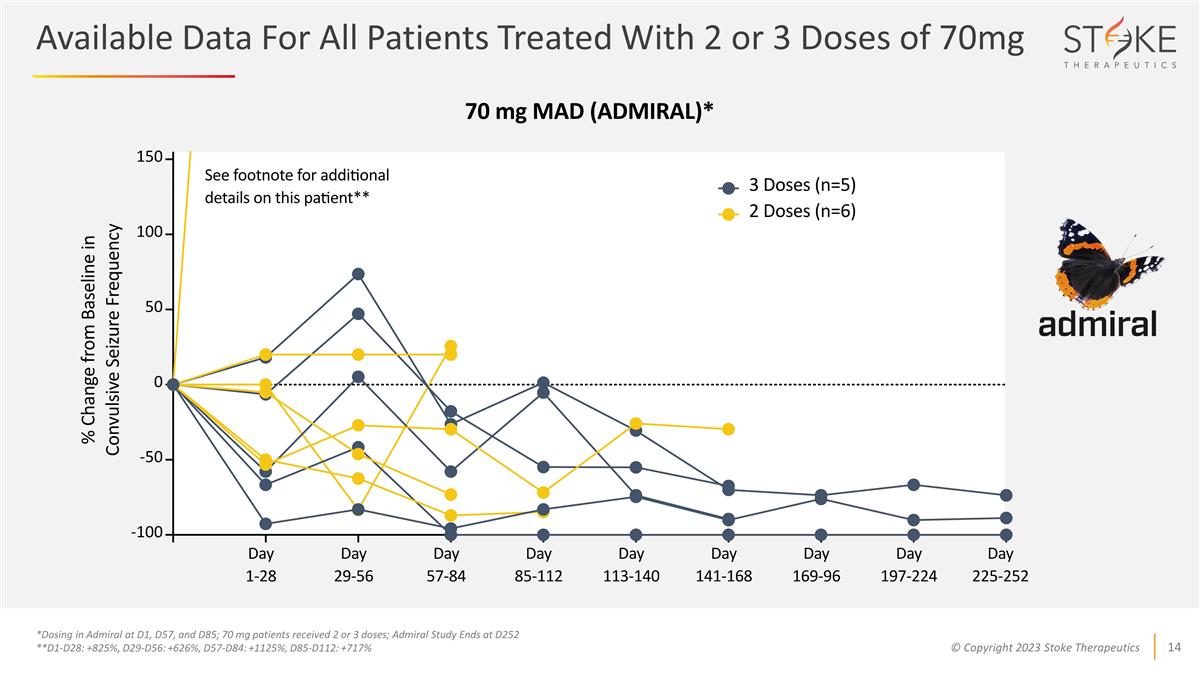
Available Data For All Patients
Treated With 2 or 3 Doses of 70mg *Dosing in Admiral at D1, D57, and D85; 70 mg patients received 2 or 3 doses; Admiral Study Ends at D252 **D1-D28: +825%, D29-D56: +626%, D57-D84: +1125%, D85-D112: +717%
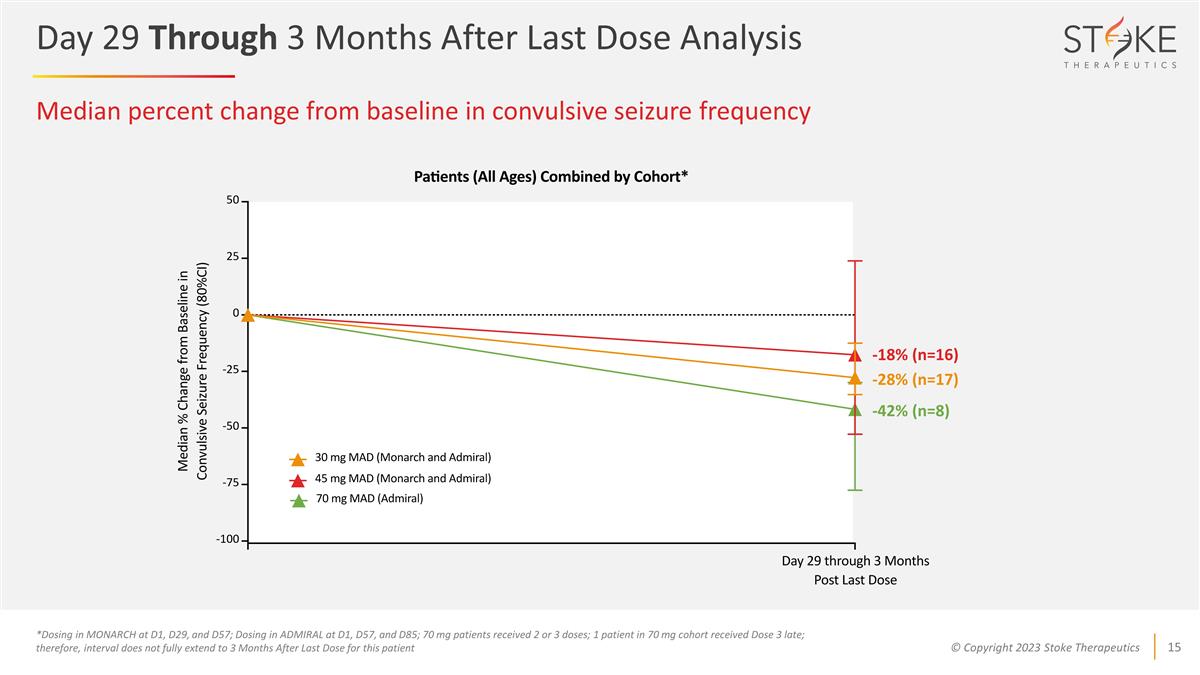
Median percent change from baseline
in convulsive seizure frequency *Dosing in MONARCH at D1, D29, and D57; Dosing in ADMIRAL at D1, D57, and D85; 70 mg patients received 2 or 3 doses; 1 patient in 70 mg cohort received Dose 3 late; therefore, interval does not fully extend to 3
Months After Last Dose for this patient -42% (n=8) -18% (n=16) -28% (n=17) Day 29 Through 3 Months After Last Dose Analysis
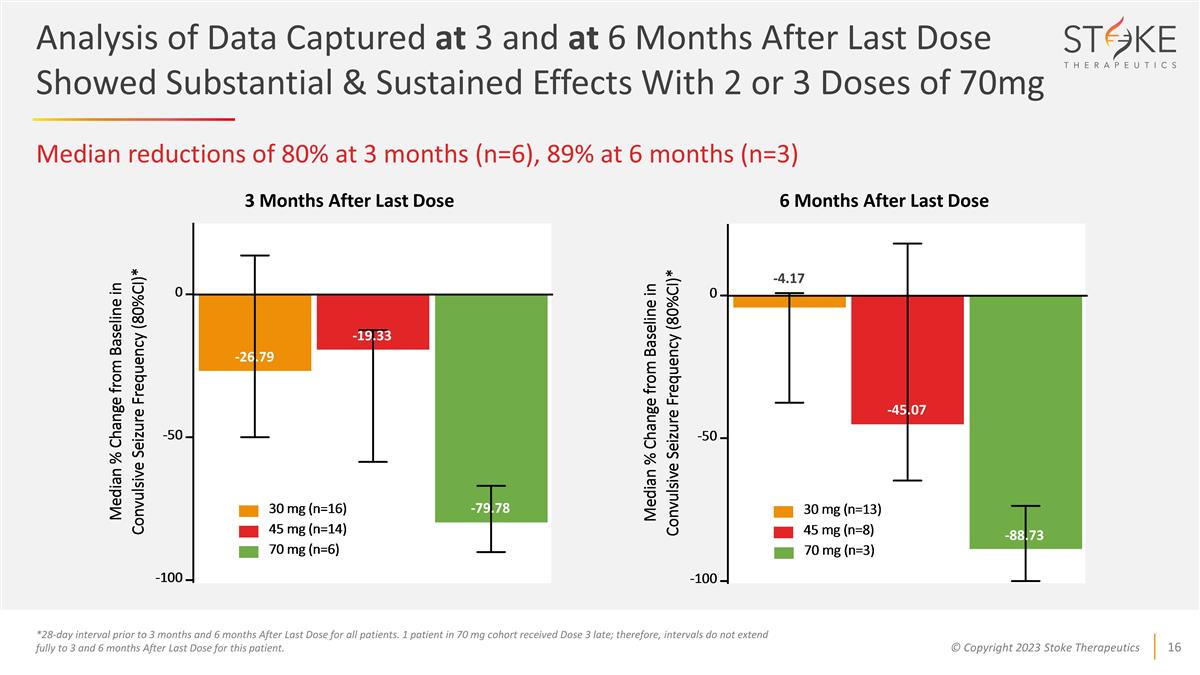
Analysis of Data Captured at 3 and
at 6 Months After Last Dose Showed Substantial & Sustained Effects With 2 or 3 Doses of 70mg Median reductions of 80% at 3 months (n=6), 89% at 6 months (n=3) *28-day interval prior to 3 months and 6 months After Last Dose for all patients. 1
patient in 70 mg cohort received Dose 3 late; therefore, intervals do not extend fully to 3 and 6 months After Last Dose for this patient. 3 Months After Last Dose -45.07 -4.17 -88.73 -79.78 -19.33 -26.79 6 Months After Last Dose
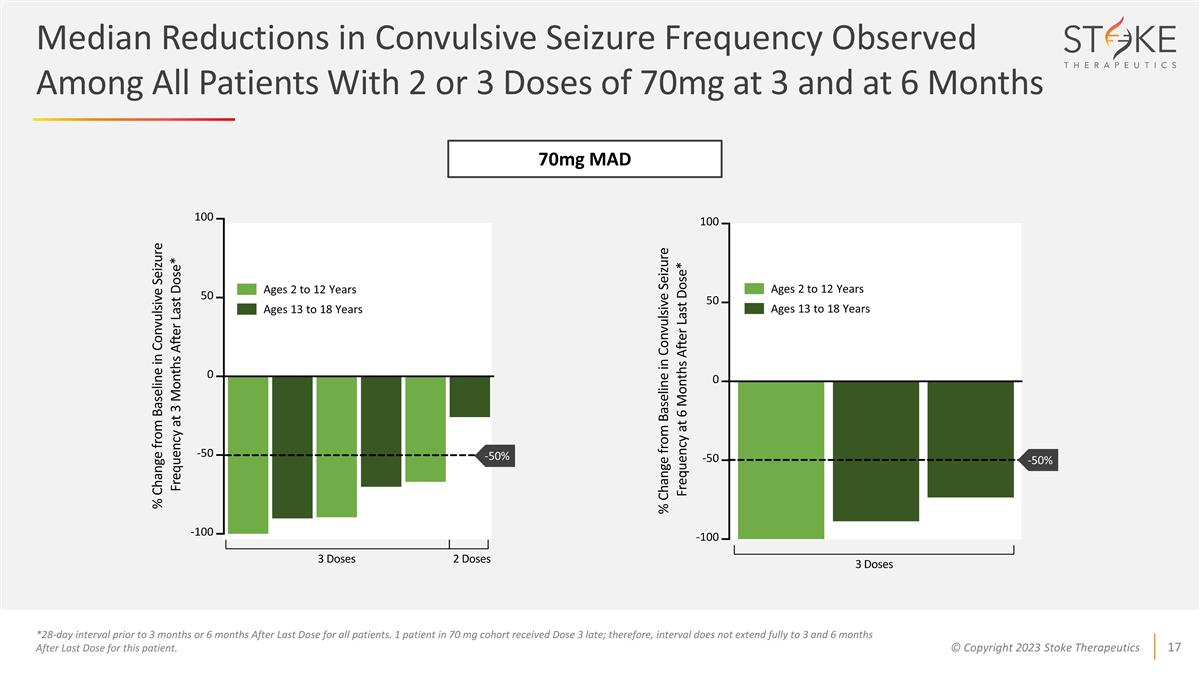
*28-day interval prior to 3 months
or 6 months After Last Dose for all patients. 1 patient in 70 mg cohort received Dose 3 late; therefore, interval does not extend fully to 3 and 6 months After Last Dose for this patient. 70mg MAD Ages 2 to 12 Years Ages 13 to 18 Years Ages 2 to 12
Years Ages 13 to 18 Years Median Reductions in Convulsive Seizure Frequency Observed Among All Patients With 2 or 3 Doses of 70mg at 3 and at 6 Months -50% -50%

Analyses of SWALLOWTAIL OLE
Kimberly Parkerson, M.D., Ph.D. Head of Neurology Clinical Development

An open-label extension study
designed to evaluate the long-term safety and tolerability of repeat doses of STK-001
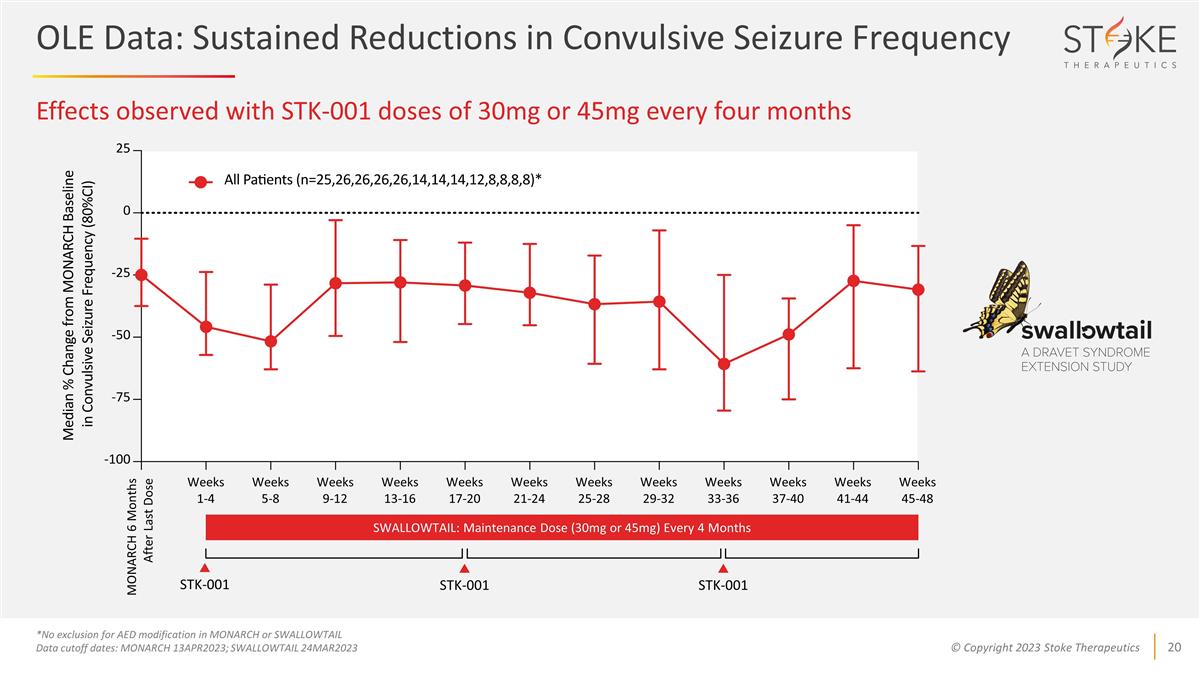
Effects observed with STK-001 doses
of 30mg or 45mg every four months *No exclusion for AED modification in MONARCH or SWALLOWTAIL Data cutoff dates: MONARCH 13APR2023; SWALLOWTAIL 24MAR2023 Weeks 1-4 Weeks 5-8 Weeks 9-12 Weeks 13-16 Weeks 17-20 Weeks 21-24 Weeks 25-28 Weeks 29-32
Weeks 33-36 Weeks 37-40 Weeks 41-44 Weeks 45-48 STK-001 STK-001 STK-001 MONARCH 6 Months After Last Dose SWALLOWTAIL: Maintenance Dose (30mg or 45mg) Every 4 Months OLE Data: Sustained Reductions in Convulsive Seizure Frequency

Dravet Syndrome is More Than
“Just Seizures” Source: Voice of the Patient Report Published by the Dravet Syndrome Foundation, May 2022 Intellectual Disability & Developmental Delays “Over time, we have seen slow, steady decline in all areas, from speech,
to mobility, endurance, loss of energy, tolerance for stimulation, stamina, etc.” Movement & Balance “We're disappointed when [our son’s] physical activity is limited and the short walk or visit that we plan with his
grandmothers must now be changed to a longer wheelchair ride.” Language & Speech Disturbances “At age 19, [our son] stopped talking, seemingly losing his capacity for speech overnight. Most days he is silent, and though he can
understand simple conversation he is largely unable to express himself.” Sleep Abnormalities “Every single night, he has seizures in his sleep. In addition to all of the other comorbidities of DS, he's robbed of the basic human necessity
of getting a good night's sleep. This impacts our entire family, as it is hard to function on so little sleep day after day."

Vineland Adaptive Behavior Scale
(VABS-III) An all-around measure of how a person performs in daily situations I would love to see him regain some speech so he can communicate and be less frustrated. Communication Daily living skills Socialization Motor skills – Barbara S.,
Mom of son with Dravet syndrome “ ” Source: Courtesy of The Dravet Syndrome Foundation
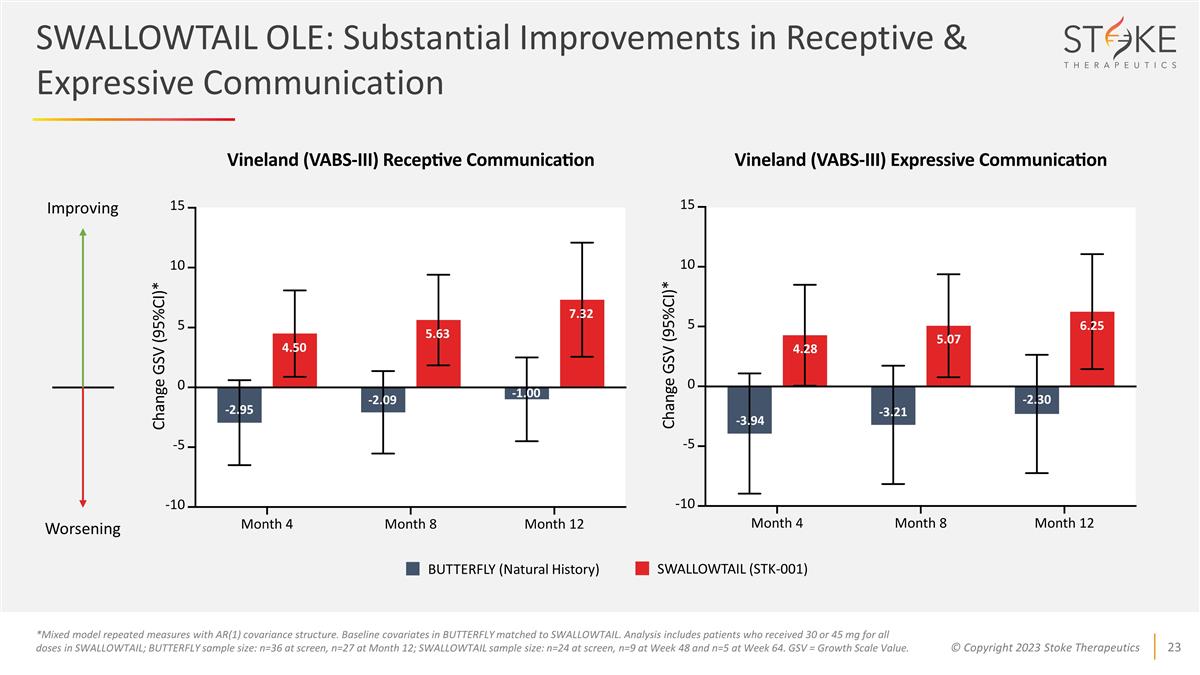
-2.95 -2.09 4.50 5.63 7.32 -1.00
BUTTERFLY (Natural History) SWALLOWTAIL (STK-001) *Mixed model repeated measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes patients who received 30 or 45 mg for all doses in
SWALLOWTAIL; BUTTERFLY sample size: n=36 at screen, n=27 at Month 12; SWALLOWTAIL sample size: n=24 at screen, n=9 at Week 48 and n=5 at Week 64. GSV = Growth Scale Value. Improving Worsening -3.94 4.28 5.07 6.25 -3.21 -2.30 SWALLOWTAIL OLE:
Substantial Improvements in Receptive & Expressive Communication
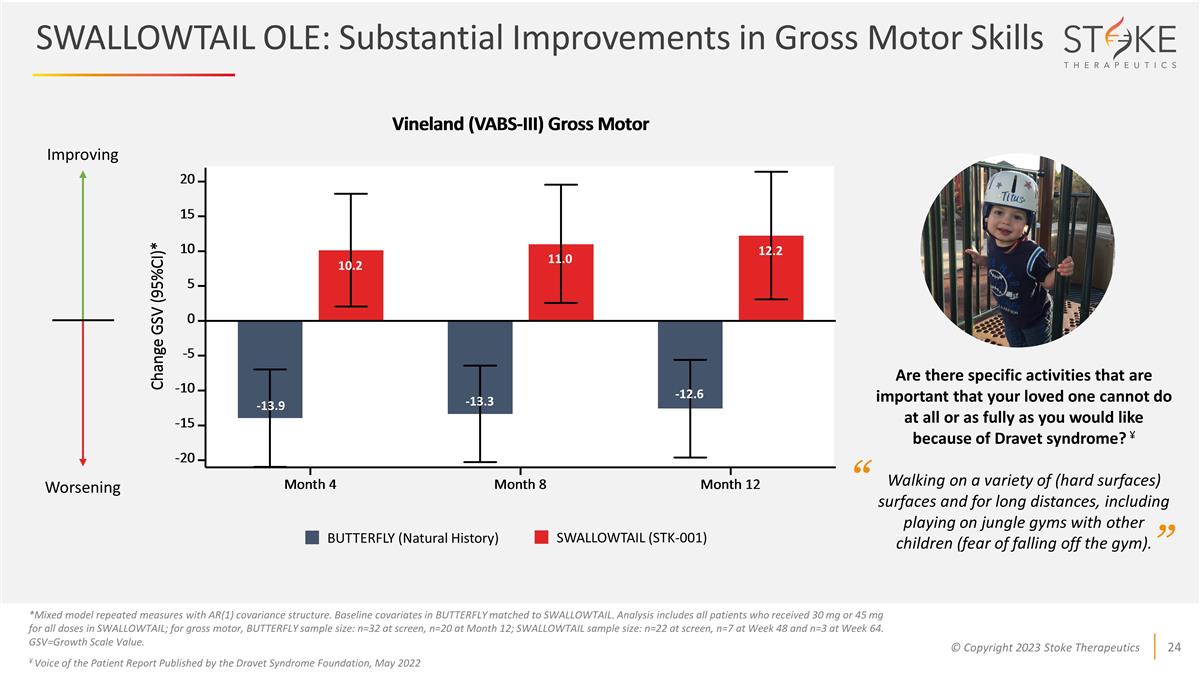
SWALLOWTAIL OLE: Substantial
Improvements in Gross Motor Skills *Mixed model repeated measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes all patients who received 30 mg or 45 mg for all doses in SWALLOWTAIL; for
gross motor, BUTTERFLY sample size: n=32 at screen, n=20 at Month 12; SWALLOWTAIL sample size: n=22 at screen, n=7 at Week 48 and n=3 at Week 64. GSV=Growth Scale Value. ¥ Voice of the Patient Report Published by the Dravet Syndrome Foundation,
May 2022 -13.9 10.2 11.0 12.2 -13.3 -12.6 Improving Worsening BUTTERFLY (Natural History) SWALLOWTAIL (STK-001) Are there specific activities that are important that your loved one cannot do at all or as fully as you would like because of Dravet
syndrome? ¥ Walking on a variety of (hard surfaces) surfaces and for long distances, including playing on jungle gyms with other children (fear of falling off the gym). “ ”
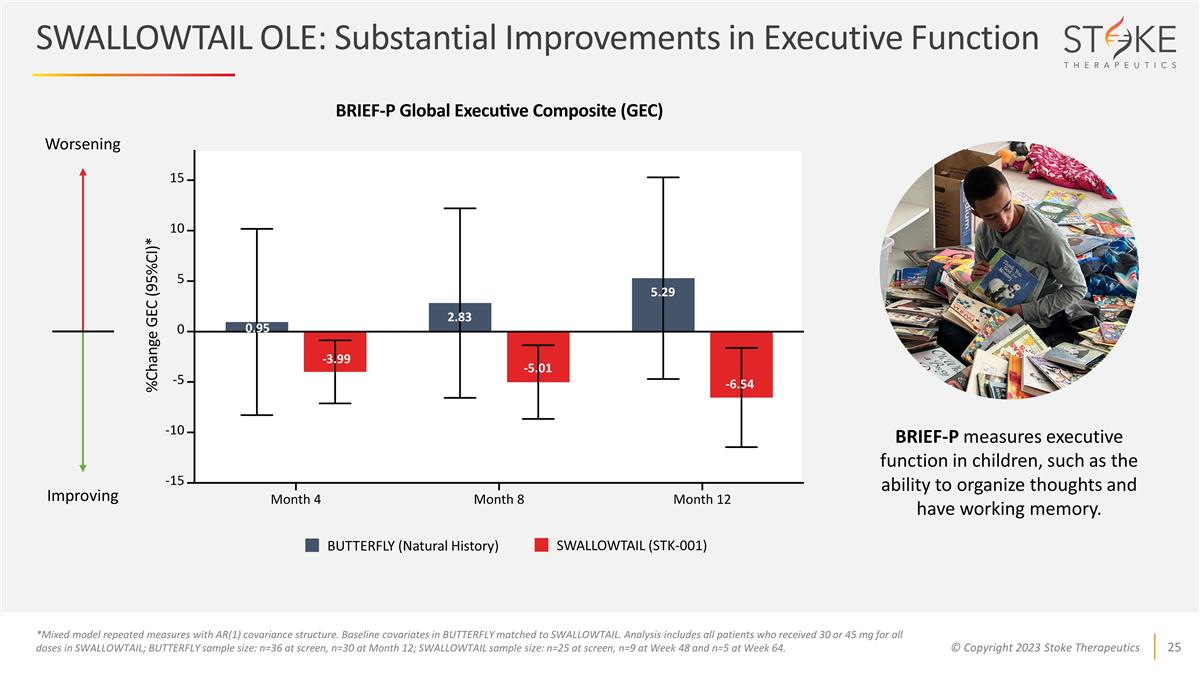
SWALLOWTAIL OLE: Substantial
Improvements in Executive Function 0.95 -3.99 -5.01 5.29 2.83 -6.54 Worsening Improving BUTTERFLY (Natural History) SWALLOWTAIL (STK-001) BRIEF-P measures executive function in children, such as the ability to organize thoughts and have working
memory. *Mixed model repeated measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes all patients who received 30 or 45 mg for all doses in SWALLOWTAIL; BUTTERFLY sample size: n=36 at
screen, n=30 at Month 12; SWALLOWTAIL sample size: n=25 at screen, n=9 at Week 48 and n=5 at Week 64.

No change Very much worse Very much
improved BUTTERFLY (Natural History) SWALLOWTAIL (STK-001) SWALLOWTAIL OLE: Substantial Improvements in Overall Condition Clinician and Caregiver Global Impression of Change scales measure a patient’s overall condition *Mixed model repeated
measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes all patients who received 30 or 45 mg for all doses in SWALLOWTAIL. For CGI-C, BUTTERFLY sample size: n=32 at Month 3, n=29 at Month
12; and for CaGI-C, BUTTERFLY sample size: n=27 at Month 3, n=24 at Month 12. For both CGI-C and CaGI-C, SWALLOWTAIL sample size: n=25 at Week 16, n=9 at Week 48 and n=5 at Week 64. CGI and CaGI in BUTTERFLY were adapted for cognition.
CGI-C=Clinical Global Impression of Change and CaGI-C=Caregiver Global Impression of Change. 4.40 2.80 4.32 2.68 4.24 2.50 4.48 2.23 4.23 1.95 3.98 1.53

Single & Multiple Doses up to
70mg Were Generally Well Tolerated MONARCH and ADMIRAL (n=74) 32% (24/74) of patients experienced a treatment-emergent adverse event (TEAE) related to study drug Most common TEAEs related to study drug were CSF protein elevations, vomiting,
and irritability 20% (15/74) had a treatment-emergent serious adverse event (TESAE). The TESAEs experienced by 14 of 15 patients were not considered related to study drug. 1 patient experienced Suspected Unexpected Serious Adverse Reactions (SUSARs)
that the investigator attributed to STK-001. The patient completed the study. An amendment to the ADMIRAL study protocol allowed investigators to decide whether to administer 2 or 3 doses of STK-001 (70mg) SWALLOWTAIL (n=44) A greater incidence of
CSF protein elevations was observed compared to MONARCH & ADMIRAL CSF protein values >50 mg/dL have been observed after dosing without any associated clinical manifestations 35% (26/74) of patients in MONARCH & ADMIRAL 64% (28/44) of
patients in SWALLOWTAIL: 1 patient discontinued study treatment; 1 patient missed 1 dose

Key Takeaways Data to date support
STK-001 as the first potential disease-modifying therapy for Dravet syndrome Efficacy Reductions in seizures and improvements in assessments of cognition and behavior suggest clinical benefit for patients ages 2 to 18 years old Median reductions of
80% at 3 months (n=6) and 89% at 6 months (n=3) after last dose with 2 or 3 initial doses of 70mg Safety STK-001 has been generally well-tolerated Most common TEAEs related to study drug were CSF protein elevations, vomiting, and irritability Next
Steps Data to be presented at the International Epilepsy Congress (September) and American Epilepsy Society (December) Additional data anticipated in Q1 2024 End of study for MONARCH & ADMIRAL Additional SWALLOWTAIL & LONGWING OLE data

Closing Remarks Edward M. Kaye,
M.D. Chief Executive Officer

Next Steps and Path to Phase 3
Dosing complete in 70mg SAD in the US and 70mg MAD in the UK Additional data in Q1 2024 : End of study data from ADMIRAL and MONARCH More OLE data from SWALLOWTAIL (30mg, 45mg) and LONGWING (45mg) Engage with regulatory agencies to agree on Phase 3
design, including dose and dosing regimen Continue to engage with key stakeholders on plans and study design Determine cognitive and behavioral endpoints for Phase 3 study An update on Phase 3 planning anticipated in the first half of 2024
Q&A

v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Stoke Therapeutics (NASDAQ:STOK)
Historical Stock Chart
From Oct 2024 to Oct 2024

Stoke Therapeutics (NASDAQ:STOK)
Historical Stock Chart
From Oct 2023 to Oct 2024
