false 0001876588 0001876588 2024-10-30 2024-10-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 30, 2024
ZimVie Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-41242 |
|
87-2007795 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
| 4555 Riverside Drive |
|
|
| Palm Beach Gardens, FL |
|
|
|
33410 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (800) 342-5454
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.01 per share |
|
ZIMV |
|
The Nasdaq Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On October 30, 2024, ZimVie Inc. (the “Company”) issued a press release reporting its financial results for the quarter ended September 30, 2024. The press release is attached hereto as Exhibit 99.1 and the information set forth therein is incorporated herein by reference and constitutes a part of this report.
| Item 7.01 |
Regulation FD Disclosure. |
On October 30, 2024, the Company also made available a presentation that contains supplemental financial information, including additional full-year 2024 financial guidance. A copy of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K and the information set forth therein is incorporated herein by reference.
The information contained in Item 2.02 and Item 7.01 of this report, including Exhibit 99.1 and Exhibit 99.2 hereto, is being furnished and shall not be deemed to be “filed” with the Securities and Exchange Commission for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section and is not incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
ZimVie Inc. |
|
|
|
|
| Date: October 30, 2024 |
|
|
|
By: |
|
/s/ Heather Kidwell |
|
|
|
|
Name: |
|
Heather Kidwell |
|
|
|
|
Title: |
|
Senior Vice President, Chief Legal, Compliance and Human
Resources Officer and Corporate Secretary |
Exhibit 99.1

ZimVie Reports Third Quarter 2024 Financial Results
| |
• |
|
Third Party Net Sales from Continuing Operations of $103.2 million |
| |
• |
|
Net Loss from Continuing Operations of $(3.0) million; Net Loss margin of (3.0%) |
| |
• |
|
Adjusted EBITDA[1] from Continuing Operations of
$13.1 million; Adjusted EBITDA[1] margin of 12.7% |
| |
• |
|
GAAP diluted EPS from Continuing Operations of $(0.11) and adjusted diluted EPS of $0.12
|
PALM BEACH GARDENS, Florida, October 30, 2024 (GLOBE NEWSWIRE) – ZimVie Inc. (Nasdaq: ZIMV), a global life sciences
leader in the dental market, today reported financial results for the quarter ended September 30, 2024. Management will host a corresponding conference call today, October 30, 2024, at 4:30 p.m. Eastern Time.
“In the third quarter we saw an improvement to revenue growth in our largest market of North America, achieved manufacturing efficiencies, and saw
increased adoption of our digital offerings,” said Vafa Jamali, President and Chief Executive Officer. “In addition to our continued focus on driving operational improvement, we made incremental investments in our sales force, R&D
initiatives and training programs designed to ensure ZimVie is well positioned for expansion into the future.”
Third Quarter 2024 Financial
Results: Continuing Operations
Third party net sales for the third quarter of 2024 were $103.2 million, a decrease of 2.0% on a reported basis
and 2.2% in constant currency[1], versus the third quarter of 2023.
Net loss for the third quarter
of 2024 was $(3.0) million, an improvement of $7.2 million versus a net loss of $(10.2) million in the third quarter of 2023. Net loss margin for the third quarter of 2024 was 3.0% of third party net sales, an improvement of 670 basis points
versus a net loss margin of 9.7% in the third quarter of 2023.
Adjusted net income[1] for the third quarter of 2024 was $3.3 million, an increase of $2.1 million versus the third quarter of 2023.
Basic and diluted EPS were $(0.11) and adjusted diluted EPS[1] was $0.12 for the third
quarter of 2024. Weighted average shares outstanding for both basic and adjusted diluted EPS was 27.6 million.
Adjusted EBITDA[1] for the third quarter of 2024 was $13.1 million, or 12.7% of third party net sales, an increase of $0.9 million or 110 basis points versus the third quarter of 2023.
Updated Full Year 2024 Continuing Operations Financial Guidance:
|
|
|
|
|
| Projected Year Ending December 31, 2024 |
|
Guidance |
|
| Net Sales |
|
$ |
450M to $455M |
|
| Adjusted EBITDA[2] |
|
$ |
60M to $62M |
|
| Adjusted EPS[2] |
|
$ |
0.57 to $0.62 |
|
| [1] |
This is a non-GAAP financial measure. Refer to “Note on Non-GAAP Financial Measures” and the reconciliations in this release for further information. |
| [2] |
This is a non-GAAP financial measure for which a reconciliation to
the most directly comparable GAAP financial measure is not available without unreasonable efforts. Refer to “Forward-Looking Non-GAAP Financial Measures” in this release, which identifies the
information that is unavailable without unreasonable efforts and provides additional information. It is probable that this forward-looking non-GAAP financial measure may be materially different from the
corresponding GAAP financial measure. |
Conference Call
ZimVie will host a conference call today, October 30, 2024, at 4:30 p.m. ET to discuss its third quarter 2024 financial results. To access the call,
please register online at https://investor.zimvie.com/events-presentations/event-calendar. A live and archived audio webcast will also be available on this site.
About ZimVie
ZimVie is a global life sciences leader in
the dental market that develops, manufactures, and delivers a comprehensive portfolio of products and solutions designed to support dental tooth replacement and restoration procedures. From its headquarters in Palm Beach Gardens, Florida, and
additional facilities around the globe, ZimVie works to improve smiles, function, and confidence in daily life by offering comprehensive tooth replacement solutions, including trusted dental implants, biomaterials, and digital workflow solutions. As
a worldwide leader in this space, ZimVie is committed to advancing clinical science and technology foundational to restoring daily life. For more information about ZimVie, please visit us at www.ZimVie.com. Follow @ZimVie on Twitter, Facebook,
LinkedIn, or Instagram.
Note on Non-GAAP Financial Measures
This press release includes non-GAAP financial measures that differ from financial measures calculated in accordance
with U.S. generally accepted accounting principles (“GAAP”). These non-GAAP financial measures may not be comparable to similar measures reported by other companies and should be considered in
addition to, and not as a substitute for, or superior to, other measures prepared in accordance with GAAP.
Adjusted EBITDA is a non-GAAP financial measure provided in this release for certain periods and is calculated by excluding certain items from net income (loss) from Continuing Operations on a GAAP basis, as detailed in the
reconciliations presented later in this press release. Adjusted EBITDA margin is Adjusted EBITDA divided by third party net sales from Continuing Operations for the applicable period.
Sales change information in this release is presented on a GAAP (reported) basis and on a constant currency basis. Constant currency percentage changes
exclude the effects of foreign currency exchange rates. They are calculated by translating current and prior-period sales from Continuing Operations at the same predetermined exchange rate. The translated results are then used to determine
year-over-year percentage increases or decreases.
Net income (loss) and diluted earnings (loss) per share in this release are presented on a GAAP
(reported) basis and on an adjusted basis. Adjusted net income (loss) and adjusted diluted earnings (loss) per share exclude the effects of certain items, which are detailed in the reconciliations of these
non-GAAP financial measures to the most directly comparable GAAP financial measures presented later in this press release.
Reconciliations of these non-GAAP measures to the most directly comparable GAAP financial measures are included in
this press release.
Management uses non-GAAP financial measures internally to evaluate the performance of the
business. Additionally, management believes these non-GAAP measures provide meaningful incremental information to investors to consider when evaluating the performance of the company. Management believes these
measures offer the ability to make period-to-period comparisons that are not impacted by certain items that can cause dramatic changes in reported income, but that do
not impact the fundamentals of our operations. The non-GAAP measures enable the evaluation of operating results and trend analysis by allowing a reader to better identify operating trends that may otherwise be
masked or distorted by these types of items that are excluded from the non-GAAP measures.
Forward-Looking Non-GAAP Financial Measures
This press release also includes certain forward-looking
non-GAAP financial measures for the year ending December 31, 2024. We calculate forward-looking non-GAAP financial measures based on internal forecasts that omit
certain amounts that would be included in GAAP financial measures. We have not provided quantitative reconciliations of these forward-looking non-GAAP financial measures to the most directly comparable
forward-looking GAAP financial measures because the excluded items are not available on a prospective basis without unreasonable efforts. For example, the timing of certain transactions is difficult to predict because management’s plans may
change. In addition, the company believes such reconciliations would imply a degree of precision and certainty that could be confusing to investors. It is probable that these forward-looking non-GAAP financial
measures may be materially different from the corresponding GAAP financial measures.
2
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of federal securities laws, including, among others, any statements about our
expectations, plans, intentions, strategies, or prospects. We generally use the words “may,” “will,” “expects,” “believes,” “anticipates,” “plans,” “estimates,”
“projects,” “assumes,” “guides,” “targets,” “forecasts,” “sees,” “seeks,” “should,” “could,” “would,” “predicts,” “potential,”
“strategy,” “future,” “opportunity,” “work toward,” “intends,” “guidance,” “confidence,” “positioned,” “design,” “strive,” “continue,”
“track,” “look forward to,” “optimistic” and similar expressions to identify forward-looking statements. All statements other than statements of historical or current fact are or may be deemed to be forward-looking
statements. Such statements are based upon the current beliefs, expectations, and assumptions of management and are subject to significant risks, uncertainties, and changes in circumstances that could cause actual outcomes and results to differ
materially from the forward-looking statements. These risks, uncertainties and changes in circumstances include, but are not limited to: dependence on new product development, technological advances and innovation; shifts in the product category or
regional sales mix of our products and services; supply and prices of raw materials and products; pricing pressures from competitors, customers, dental practices and insurance providers; changes in customer demand for our products and services
caused by demographic changes or other factors; challenges relating to changes in and compliance with governmental laws and regulations affecting our U.S. and international businesses, including regulations of the U.S. Food and Drug Administration
and foreign government regulators, such as more stringent requirements for regulatory clearance of products; competition; the impact of healthcare reform measures; reductions in reimbursement levels by third-party payors; cost containment efforts
sponsored by government agencies, legislative bodies, the private sector and healthcare group purchasing organizations, including the volume-based procurement process in China; control of costs and expenses; dependence on a limited number of
suppliers for key raw materials and outsourced activities; the ability to obtain and maintain adequate intellectual property protection; breaches or failures of our information technology systems or products, including by cyberattack, unauthorized
access or theft; the ability to retain the independent agents and distributors who market our products; our ability to attract, retain and develop the highly skilled employees we need to support our business; the effect of mergers and acquisitions
on our relationships with customers, suppliers and lenders and on our operating results and businesses generally; the ability to form and implement alliances; changes in tax obligations arising from tax reform measures, including European Union
rules on state aid, or examinations by tax authorities; product liability, intellectual property and commercial litigation losses; changes in general industry and market conditions, including domestic and international growth rates; changes in
general domestic and international economic conditions, including inflation and interest rate and currency exchange rate fluctuations; the effects of global pandemics and other adverse public health developments on the global economy, our business
and operations and the business and operations of our suppliers and customers, including the deferral of elective procedures and our ability to collect accounts receivable; and the impact of the ongoing financial and political uncertainty on
countries in the Euro zone on the ability to collect accounts receivable in affected countries. You are cautioned not to rely on these forward-looking statements, since there can be no assurance that these forward-looking statements will prove to be
accurate. Forward-looking statements speak only as of the date they are made, and we expressly disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or
otherwise.
Media Contact Information:
ZimVie
Grace Flowers • Grace.Flowers@ZimVie.com
(561) 319-6130
Investor Contact Information:
Gilmartin Group LLC
Marissa Bych • Marissa@gilmartinir.com
3
ZIMVIE INC.
CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS (UNAUDITED)
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Three Months
Ended September 30, |
|
|
For the Nine Months
Ended September 30, |
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Net Sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Third party, net |
|
$ |
103,222 |
|
|
$ |
105,311 |
|
|
$ |
338,228 |
|
|
$ |
344,131 |
|
| Related party, net |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
236 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Net Sales |
|
|
103,222 |
|
|
|
105,311 |
|
|
|
338,228 |
|
|
|
344,367 |
|
| Cost of products sold, excluding intangible asset amortization |
|
|
(35,820 |
) |
|
|
(36,907 |
) |
|
|
(123,596 |
) |
|
|
(124,246 |
) |
| Related party cost of products sold, excluding intangible asset amortization |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(231 |
) |
| Intangible asset amortization |
|
|
(6,037 |
) |
|
|
(6,778 |
) |
|
|
(18,059 |
) |
|
|
(20,378 |
) |
| Research and development |
|
|
(6,926 |
) |
|
|
(5,677 |
) |
|
|
(20,285 |
) |
|
|
(19,365 |
) |
| Selling, general and administrative |
|
|
(57,313 |
) |
|
|
(56,505 |
) |
|
|
(180,024 |
) |
|
|
(186,054 |
) |
| Restructuring and other cost reduction initiatives |
|
|
(687 |
) |
|
|
(1,391 |
) |
|
|
(3,664 |
) |
|
|
(3,929 |
) |
| Acquisition, integration, divestiture and related |
|
|
(1,276 |
) |
|
|
(1,936 |
) |
|
|
(6,934 |
) |
|
|
(4,647 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses |
|
|
(108,059 |
) |
|
|
(109,194 |
) |
|
|
(352,562 |
) |
|
|
(358,850 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Loss |
|
|
(4,837 |
) |
|
|
(3,883 |
) |
|
|
(14,334 |
) |
|
|
(14,483 |
) |
| Other income (expense), net |
|
|
3,462 |
|
|
|
(990 |
) |
|
|
6,161 |
|
|
|
(1,189 |
) |
| Interest income |
|
|
2,466 |
|
|
|
569 |
|
|
|
4,938 |
|
|
|
1,929 |
|
| Interest expense |
|
|
(4,827 |
) |
|
|
(5,553 |
) |
|
|
(14,766 |
) |
|
|
(17,187 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from continuing operations before income taxes |
|
|
(3,736 |
) |
|
|
(9,857 |
) |
|
|
(18,001 |
) |
|
|
(30,930 |
) |
| Benefit (provision) for income taxes from continuing operations |
|
|
688 |
|
|
|
(325 |
) |
|
|
(6,161 |
) |
|
|
(1,555 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss from Continuing Operations of ZimVie Inc. |
|
|
(3,048 |
) |
|
|
(10,182 |
) |
|
|
(24,162 |
) |
|
|
(32,485 |
) |
| Earnings (loss) from discontinued operations, net of tax |
|
|
764 |
|
|
|
5,093 |
|
|
|
10,103 |
|
|
|
(25,945 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss of ZimVie Inc. |
|
$ |
(2,284 |
) |
|
$ |
(5,089 |
) |
|
$ |
(14,059 |
) |
|
$ |
(58,430 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic (Loss) Earnings Per Common Share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Continuing operations |
|
$ |
(0.11 |
) |
|
$ |
(0.38 |
) |
|
$ |
(0.88 |
) |
|
$ |
(1.23 |
) |
| Discontinued operations |
|
|
0.03 |
|
|
|
0.19 |
|
|
|
0.37 |
|
|
|
(0.98 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss |
|
$ |
(0.08 |
) |
|
$ |
(0.19 |
) |
|
$ |
(0.51 |
) |
|
$ |
(2.21 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Diluted (Loss) Earnings Per Common Share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Continuing operations |
|
$ |
(0.11 |
) |
|
$ |
(0.38 |
) |
|
$ |
(0.88 |
) |
|
$ |
(1.23 |
) |
| Discontinued operations |
|
|
0.03 |
|
|
|
0.19 |
|
|
|
0.37 |
|
|
|
(0.98 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss |
|
$ |
(0.08 |
) |
|
$ |
(0.19 |
) |
|
$ |
(0.51 |
) |
|
$ |
(2.21 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4
ZIMVIE INC.
CONDENSED CONSOLIDATED BALANCE SHEETS (UNAUDITED)
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
| |
|
As of |
|
| |
|
September 30, 2024 |
|
|
December 31, 2023 |
|
| ASSETS |
|
|
|
|
|
|
|
|
| Current Assets: |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
66,808 |
|
|
$ |
71,511 |
|
| Accounts receivable, less allowance for credit losses of $2,392
and $3,222 respectively |
|
|
69,581 |
|
|
|
65,168 |
|
| Inventories |
|
|
77,087 |
|
|
|
79,600 |
|
| Prepaid expenses and other current assets |
|
|
24,162 |
|
|
|
23,825 |
|
| Current assets of discontinued operations |
|
|
28,036 |
|
|
|
242,773 |
|
|
|
|
|
|
|
|
|
|
| Total Current Assets |
|
|
265,674 |
|
|
|
482,877 |
|
| Property, plant and equipment, net of accumulated depreciation
of $131,717 and $126,624, respectively |
|
|
49,614 |
|
|
|
54,167 |
|
| Goodwill |
|
|
262,767 |
|
|
|
262,111 |
|
| Intangible assets, net |
|
|
98,251 |
|
|
|
114,354 |
|
| Note receivable |
|
|
63,072 |
|
|
|
— |
|
| Other assets |
|
|
31,271 |
|
|
|
26,747 |
|
| Noncurrent assets of discontinued operations |
|
|
12,299 |
|
|
|
265,089 |
|
|
|
|
|
|
|
|
|
|
| Total Assets |
|
$ |
782,948 |
|
|
$ |
1,205,345 |
|
|
|
|
|
|
|
|
|
|
| LIABILITIES AND EQUITY |
|
|
|
|
|
|
|
|
| Current Liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
27,403 |
|
|
$ |
27,785 |
|
| Income taxes payable |
|
|
2,440 |
|
|
|
2,863 |
|
| Other current liabilities |
|
|
58,363 |
|
|
|
67,108 |
|
| Current liabilities of discontinued operations |
|
|
48,432 |
|
|
|
75,858 |
|
|
|
|
|
|
|
|
|
|
| Total Current Liabilities |
|
|
136,638 |
|
|
|
173,614 |
|
| Deferred income taxes |
|
|
276 |
|
|
|
265 |
|
| Lease liability |
|
|
9,477 |
|
|
|
9,080 |
|
| Other long-term liabilities |
|
|
9,269 |
|
|
|
9,055 |
|
| Non-current portion of debt |
|
|
220,281 |
|
|
|
508,797 |
|
| Noncurrent liabilities of discontinued operations |
|
|
369 |
|
|
|
95,041 |
|
|
|
|
|
|
|
|
|
|
| Total Liabilities |
|
|
376,310 |
|
|
|
795,852 |
|
|
|
|
|
|
|
|
|
|
| Commitments and Contingencies |
|
|
|
|
|
|
|
|
| Stockholders’ Equity: |
|
|
|
|
|
|
|
|
| Common stock, $0.01 par value, 150,000 shares authorized Shares, issued and outstanding, of 27,587
and 27,076, respectively |
|
|
276 |
|
|
|
271 |
|
| Preferred stock, $0.01 par value, 15,000 shares authorized, 0 shares issued and
outstanding |
|
|
— |
|
|
|
— |
|
| Additional paid in capital |
|
|
933,735 |
|
|
|
922,996 |
|
| Accumulated deficit |
|
|
(454,873 |
) |
|
|
(440,814 |
) |
| Accumulated other comprehensive loss |
|
|
(72,500 |
) |
|
|
(72,960 |
) |
|
|
|
|
|
|
|
|
|
| Total Stockholders’ Equity |
|
|
406,638 |
|
|
|
409,493 |
|
|
|
|
|
|
|
|
|
|
| Total Liabilities and Stockholders’ Equity |
|
$ |
782,948 |
|
|
$ |
1,205,345 |
|
|
|
|
|
|
|
|
|
|
5
ZIMVIE INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
(in thousands)
|
|
|
|
|
|
|
|
|
| |
|
For the Nine Months Ended September 30, |
|
| |
|
2024 |
|
|
2023 |
|
| Cash flows (used in) provided by operating activities: |
|
|
|
|
|
|
|
|
| Net loss of ZimVie Inc. |
|
$ |
(14,059 |
) |
|
$ |
(58,430 |
) |
| Adjustments to reconcile net loss to net cash (used in) provided by operating
activities: |
|
|
|
|
|
| Depreciation and amortization |
|
|
25,697 |
|
|
|
95,088 |
|
| Share-based compensation |
|
|
12,473 |
|
|
|
16,129 |
|
| Deferred income tax provision |
|
|
(4,201 |
) |
|
|
(11,967 |
) |
| Loss on disposal of fixed assets |
|
|
418 |
|
|
|
2,411 |
|
| Other non-cash items |
|
|
2,818 |
|
|
|
2,762 |
|
| Gain on sale of spine disposal group |
|
|
(22,427 |
) |
|
|
— |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Income taxes |
|
|
2,548 |
|
|
|
(34,061 |
) |
| Accounts receivable |
|
|
(5,742 |
) |
|
|
13,019 |
|
| Related party receivables |
|
|
— |
|
|
|
8,483 |
|
| Inventories |
|
|
7,139 |
|
|
|
18,246 |
|
| Prepaid expenses and other current assets |
|
|
(2,447 |
) |
|
|
4,187 |
|
| Accounts payable and accrued liabilities |
|
|
(6,314 |
) |
|
|
(18,216 |
) |
| Related party payable |
|
|
— |
|
|
|
(13,177 |
) |
| Other assets and liabilities |
|
|
(3,179 |
) |
|
|
(8,780 |
) |
|
|
|
|
|
|
|
|
|
| Net cash (used in) provided by operating activities |
|
|
(7,276 |
) |
|
|
15,694 |
|
|
|
|
|
|
|
|
|
|
| Cash flows provided by (used in) investing activities: |
|
|
|
|
|
|
|
|
| Additions to instruments |
|
|
(1,316 |
) |
|
|
(4,341 |
) |
| Additions to other property, plant and equipment |
|
|
(2,677 |
) |
|
|
(5,340 |
) |
| Proceeds from sale of spine disposal group, net of cash disposed |
|
|
291,123 |
|
|
|
— |
|
| Other investing activities |
|
|
(1,961 |
) |
|
|
(2,762 |
) |
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) investing activities |
|
|
285,169 |
|
|
|
(12,443 |
) |
|
|
|
|
|
|
|
|
|
| Cash flows used in financing activities: |
|
|
|
|
|
|
|
|
| Proceeds from debt |
|
|
— |
|
|
|
4,760 |
|
| Payments on debt |
|
|
(290,000 |
) |
|
|
(22,291 |
) |
| Business combination contingent consideration payments |
|
|
(3,712 |
) |
|
|
— |
|
| Payments related to tax withholding for share-based compensation |
|
|
(1,729 |
) |
|
|
(419 |
) |
| Proceeds from stock plan activity |
|
|
— |
|
|
|
1,167 |
|
|
|
|
|
|
|
|
|
|
| Net cash used in financing activities |
|
|
(295,441 |
) |
|
|
(16,783 |
) |
|
|
|
|
|
|
|
|
|
| Effect of exchange rates on cash and cash equivalents |
|
|
(2,124 |
) |
|
|
(620 |
) |
|
|
|
|
|
|
|
|
|
| Decrease in cash and cash equivalents |
|
|
(19,672 |
) |
|
|
(14,152 |
) |
| Cash and cash equivalents, beginning of year |
|
|
87,768 |
|
|
|
89,601 |
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents, end of period |
|
$ |
68,096 |
|
|
$ |
75,449 |
|
|
|
|
|
|
|
|
|
|
| Presentation includes cash of both continuing and discontinued operations |
|
6
RECONCILIATION OF CONSTANT CURRENCY NET SALES
Continuing Operations ($ in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Three Months
Ended September 30, |
|
|
|
|
|
|
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
Change (%) |
|
|
Foreign
Exchange
Impact |
|
|
Constant
Currency %
Change |
|
| United States |
|
$ |
65,350 |
|
|
$ |
65,003 |
|
|
|
0.5 |
% |
|
|
0.0 |
% |
|
|
0.5 |
% |
| International |
|
|
37,872 |
|
|
|
40,308 |
|
|
|
(6.0 |
%) |
|
|
0.6 |
% |
|
|
(6.6 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Dental Third Party Sales |
|
|
103,222 |
|
|
|
105,311 |
|
|
|
(2.0 |
%) |
|
|
0.2 |
% |
|
|
(2.2 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Net Sales |
|
|
— |
|
|
|
— |
|
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Dental Net Sales |
|
$ |
103,222 |
|
|
$ |
105,311 |
|
|
|
(2.0 |
%) |
|
|
0.2 |
% |
|
|
(2.2 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Nine Months
Ended September 30, |
|
|
|
|
|
|
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
Change (%) |
|
|
Foreign
Exchange
Impact |
|
|
Constant
Currency %
Change |
|
| United States |
|
$ |
202,414 |
|
|
$ |
204,173 |
|
|
|
(0.9 |
%) |
|
|
0.0 |
% |
|
|
(0.9 |
%) |
| International |
|
|
135,814 |
|
|
|
139,958 |
|
|
|
(3.0 |
%) |
|
|
(1.0 |
%) |
|
|
(2.0 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Dental Third Party Sales |
|
|
338,228 |
|
|
|
344,131 |
|
|
|
(1.7 |
%) |
|
|
(0.4 |
%) |
|
|
(1.3 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Net Sales |
|
|
— |
|
|
|
236 |
|
|
|
(100.0 |
%) |
|
|
0.0 |
% |
|
|
(100.0 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Dental Net Sales |
|
$ |
338,228 |
|
|
$ |
344,367 |
|
|
|
(1.8 |
%) |
|
|
(0.4 |
%) |
|
|
(1.4 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7
RECONCILIATION OF ADJUSTED NET INCOME AND DILUTED EPS
Continuing Operations (in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Three Months Ended September 30, 2024 |
|
| |
|
Net Sales |
|
|
Cost of
products sold,
excluding
intangible
asset
amortization |
|
|
Operating
expenses,
excluding
cost of
products
sold |
|
|
Operating
(Loss)
Income |
|
|
Net
(Loss)
Income |
|
|
Diluted EPS |
|
| Reported |
|
$ |
103,222 |
|
|
$ |
(35,820 |
) |
|
$ |
(72,239 |
) |
|
$ |
(4,837 |
) |
|
$ |
(3,048 |
) |
|
$ |
(0.11 |
) |
| Restructuring and other cost reduction initiatives
[1] |
|
|
— |
|
|
|
— |
|
|
|
687 |
|
|
|
687 |
|
|
|
687 |
|
|
|
0.02 |
|
| Acquisition, integration, divestiture and related
[2] |
|
|
— |
|
|
|
— |
|
|
|
1,276 |
|
|
|
1,276 |
|
|
|
1,276 |
|
|
|
0.05 |
|
| European union medical device regulation
[3] |
|
|
— |
|
|
|
— |
|
|
|
406 |
|
|
|
406 |
|
|
|
406 |
|
|
|
0.01 |
|
| Other charges [4] |
|
|
— |
|
|
|
287 |
|
|
|
— |
|
|
|
287 |
|
|
|
287 |
|
|
|
0.01 |
|
| Intangible asset amortization |
|
|
— |
|
|
|
— |
|
|
|
6,037 |
|
|
|
6,037 |
|
|
|
6,037 |
|
|
|
0.22 |
|
| Share-based compensation modification
[5] |
|
|
— |
|
|
|
— |
|
|
|
(521 |
) |
|
|
(521 |
) |
|
|
(521 |
) |
|
|
(0.02 |
) |
| Tax effect of above adjustments & other
[6] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,841 |
) |
|
|
(0.06 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted |
|
$ |
103,222 |
|
|
$ |
(35,533 |
) |
|
$ |
(64,354 |
) |
|
$ |
3,335 |
|
|
$ |
3,283 |
|
|
$ |
0.12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Three Months Ended September 30, 2023 |
|
| |
|
Net Sales |
|
|
Cost of
products sold,
excluding
intangible
asset
amortization |
|
|
Operating
expenses,
excluding
cost of
products
sold |
|
|
Operating
(Loss)
Income |
|
|
Net
(Loss)
Income |
|
|
Diluted EPS |
|
| Reported |
|
$ |
105,311 |
|
|
$ |
(36,907 |
) |
|
$ |
(72,287 |
) |
|
$ |
(3,883 |
) |
|
$ |
(10,182 |
) |
|
$ |
(0.38 |
) |
| Restructuring and other cost reduction initiatives
[1] |
|
|
— |
|
|
|
— |
|
|
|
1,391 |
|
|
|
1,391 |
|
|
|
1,391 |
|
|
|
0.05 |
|
| Acquisition, integration, divestiture and related
[2] |
|
|
— |
|
|
|
— |
|
|
|
1,936 |
|
|
|
1,936 |
|
|
|
1,936 |
|
|
|
0.07 |
|
| European union medical device regulation
[3] |
|
|
— |
|
|
|
— |
|
|
|
295 |
|
|
|
295 |
|
|
|
295 |
|
|
|
0.01 |
|
| Intangible asset amortization |
|
|
— |
|
|
|
— |
|
|
|
6,778 |
|
|
|
6,778 |
|
|
|
6,778 |
|
|
|
0.26 |
|
| Other charges [4] |
|
|
— |
|
|
|
293 |
|
|
|
— |
|
|
|
293 |
|
|
|
293 |
|
|
|
0.01 |
|
| Spin-related share-based compensation expense
[7] |
|
|
— |
|
|
|
— |
|
|
|
800 |
|
|
|
800 |
|
|
|
800 |
|
|
|
0.03 |
|
| Tax effect of above adjustments & other
[6] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(100 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted |
|
$ |
105,311 |
|
|
$ |
(36,614 |
) |
|
$ |
(61,087 |
) |
|
$ |
7,610 |
|
|
$ |
1,211 |
|
|
$ |
0.05 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| [1] |
Restructuring activities to better position the organization and the expenses incurred were primarily related
to severance and professional fees. |
| [2] |
Acquisition, integration, divestiture and related expenses for the three months ended September 30, 2024
include professional services fees ($1.9 million) and stranded costs ($0.4 million) related to sale of the spine segment, partially offset by a fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million).
Acquisition, integration, divestiture and related expenses for the three months ended September 30, 2023 include professional services fees ($1.6 million) and rebranding costs ($0.3 million) related to the separation from our former parent.
|
| [3] |
Expenses incurred for initial compliance with the European Union (“EU”) Medical Device Regulation
(“MDR”) for previously-approved products. |
| [4] |
Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. |
| [5] |
Net impact to share-based compensation expense of converting outstanding restricted stock units
(“RSUs”) with performance-based metrics based on the consolidated results of the spine and dental segments into time-based RSUs following the sale of the spine segment. |
8
| [6] |
Reflects the tax effect of the adjustments from reported to adjusted, as well as an adjustment for
management’s expectation of ZimVie’s statutory tax rate based on current tax law and adjusted pre-tax income. |
| [7] |
Spin-related share-based compensation expense from grants provided due to the successful separation from Zimmer
Biomet. |
9
RECONCILIATION OF ADJUSTED EBITDA:
Continuing Operations ($ in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
For the Three Months
Ended September 30, |
|
|
For the Nine Months
Ended September 30, |
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Net Sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Third Party Sales |
|
$ |
103,222 |
|
|
$ |
105,311 |
|
|
$ |
338,228 |
|
|
$ |
344,131 |
|
| Related Party Sales |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
236 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Net Sales |
|
$ |
103,222 |
|
|
$ |
105,311 |
|
|
$ |
338,228 |
|
|
$ |
344,367 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss |
|
$ |
(3,048) |
|
|
$ |
(10,182 |
) |
|
$ |
(24,162 |
) |
|
$ |
(32,485 |
) |
| Interest expense, net |
|
|
2,361 |
|
|
|
4,984 |
|
|
|
9,828 |
|
|
|
15,258 |
|
| Income tax (benefit) provision |
|
|
(688 |
) |
|
|
325 |
|
|
|
6,161 |
|
|
|
1,555 |
|
| Depreciation and amortization |
|
|
8,490 |
|
|
|
8,415 |
|
|
|
25,383 |
|
|
|
26,057 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EBITDA |
|
|
7,115 |
|
|
|
3,542 |
|
|
|
17,210 |
|
|
|
10,385 |
|
| Share-based compensation |
|
|
3,323 |
|
|
|
4,741 |
|
|
|
11,761 |
|
|
|
14,159 |
|
| Restructuring and other cost reduction initiatives
[1] |
|
|
687 |
|
|
|
1,391 |
|
|
|
3,664 |
|
|
|
3,929 |
|
| Acquisition, integration, divestiture and related
[2] |
|
|
1,276 |
|
|
|
1,936 |
|
|
|
6,934 |
|
|
|
4,647 |
|
| Related party gain |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5 |
) |
| European Union medical device regulation
[3] |
|
|
406 |
|
|
|
295 |
|
|
|
1,118 |
|
|
|
2,227 |
|
| Other charges [4] |
|
|
287 |
|
|
|
293 |
|
|
|
860 |
|
|
|
864 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted EBITDA |
|
$ |
13,094 |
|
|
$ |
12,198 |
|
|
$ |
41,547 |
|
|
$ |
36,206 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Loss Margin [5] |
|
|
-3.0 |
% |
|
|
-9.7 |
% |
|
|
-7.1 |
% |
|
|
-9.4 |
% |
| Adjusted EBITDA Margin [6] |
|
|
12.7 |
% |
|
|
11.6 |
% |
|
|
12.3 |
% |
|
|
10.5 |
% |
| [1] |
Restructuring activities to better position our organization for future success based on the current business
environment and sale of the spine business. |
| [2] |
Acquisition, integration, divestiture and related expenses for the three and nine months ended
September 30, 2024 include professional services fees ($1.9 million and $5.8 million, respectively) and stranded costs ($0.4 million and $0.9 million, respectively) related to sale of the spine segment, partially offset by
fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million and $0, respectively). Acquisition, integration, divestiture and related expenses for the three and nine months ended September 30,
2023 include professional services fees ($1.6 million and $3.2 million, respectively), rebranding costs related to the separation from our former parent ($0.3 million and $0.5 million, respectively) and technology costs ($0 and
$0.7 million, respectively) incurred to prepare for and complete the separation from our former parent. |
| [3] |
Expenses incurred for initial compliance with the EU MDR for previously-approved products.
|
| [4] |
Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. |
| [5] |
Net Loss Margin is calculated as Net Loss divided by third party net sales for the applicable period.
|
| [6] |
Adjusted EBITDA Margin is Adjusted EBITDA divided by third party net sales for the applicable period.
|
10
RECONCILIATION OF COST OF PRODUCTS SOLD (excluding intangible asset amortization), R&D and SG&A:
Continuing Operations ($ in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months
Ended September 30, |
|
|
Percentage of Third
Party Net Sales |
|
|
Nine Months
Ended September 30, |
|
|
Percentage of Third
Party Net Sales |
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Cost of products sold, excluding intangible asset amortization |
|
$ |
(35,820 |
) |
|
$ |
(36,907 |
) |
|
|
(34.7 |
%) |
|
|
(35.0 |
%) |
|
$ |
(123,596 |
) |
|
$ |
(124,246 |
) |
|
|
(36.5 |
%) |
|
|
(36.1 |
%) |
| Other charges [1] |
|
|
287 |
|
|
|
293 |
|
|
|
0.3 |
% |
|
|
0.2 |
% |
|
|
860 |
|
|
|
864 |
|
|
|
0.2 |
% |
|
|
0.2 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted cost of products sold, excluding intangible asset amortization |
|
$ |
(35,533 |
) |
|
$ |
(36,614 |
) |
|
|
(34.4 |
%) |
|
|
(34.8 |
%) |
|
$ |
(122,736 |
) |
|
$ |
(123,382 |
) |
|
|
(36.3 |
%) |
|
|
(35.9 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Research and development |
|
$ |
(6,926 |
) |
|
$ |
(5,677 |
) |
|
|
(6.7 |
%) |
|
|
(5.4 |
%) |
|
$ |
(20,285 |
) |
|
$ |
(19,365 |
) |
|
|
(6.0 |
%) |
|
|
(5.6 |
%) |
| European union medical device regulation
[2] |
|
|
406 |
|
|
|
295 |
|
|
|
0.3 |
% |
|
|
0.3 |
% |
|
|
1,118 |
|
|
|
2,227 |
|
|
|
0.3 |
% |
|
|
0.6 |
% |
| Share-based compensation modification
[3] |
|
|
(45 |
) |
|
|
— |
|
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
(45 |
) |
|
|
— |
|
|
|
0.0 |
% |
|
|
0.0 |
% |
| Spin-related share-based compensation expense
[4] |
|
|
— |
|
|
|
80 |
|
|
|
0.0 |
% |
|
|
0.1 |
% |
|
|
— |
|
|
|
240 |
|
|
|
0.0 |
% |
|
|
0.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted research and development |
|
$ |
(6,565 |
) |
|
$ |
(5,302 |
) |
|
|
(6.4 |
%) |
|
|
(5.0 |
%) |
|
$ |
(19,212 |
) |
|
$ |
(16,898 |
) |
|
|
(5.7 |
%) |
|
|
(4.9 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Selling, general and administrative |
|
$ |
(57,313 |
) |
|
$ |
(56,505 |
) |
|
|
(55.5 |
%) |
|
|
(53.7 |
%) |
|
$ |
(180,024 |
) |
|
$ |
(186,054 |
) |
|
|
(53.2 |
%) |
|
|
(54.1 |
%) |
| Share-based compensation modification
[3] |
|
|
(476 |
) |
|
|
— |
|
|
|
(0.5 |
%) |
|
|
0.0 |
% |
|
|
(476 |
) |
|
|
— |
|
|
|
(0.2 |
%) |
|
|
0.0 |
% |
| Spin-related share-based compensation expense
[4] |
|
|
— |
|
|
|
720 |
|
|
|
0.0 |
% |
|
|
0.7 |
% |
|
|
— |
|
|
|
2,160 |
|
|
|
0.0 |
% |
|
|
0.7 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Adjusted selling, general and administrative |
|
$ |
(57,789 |
) |
|
$ |
(55,785 |
) |
|
|
(56.0 |
%) |
|
|
(53.0 |
%) |
|
$ |
(180,500 |
) |
|
$ |
(183,894 |
) |
|
|
(53.4 |
%) |
|
|
(53.4 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| [1] |
Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. |
| [2] |
Expenses incurred for initial compliance with the EU MDR for previously-approved products.
|
| [3] |
Net impact to share-based compensation expense of converting outstanding RSUs with performance-based metrics
based on the consolidated results of the spine and dental segments to time-based RSUs following the sale of the spine segment. |
| [4] |
Spin-related share-based compensation expense from grants provided due to the successful separation from Zimmer
Biomet. |
11

A Global Dental Leader October 2024
Exhibit 99.2

Forward-Looking Statements and
Non-GAAP Measures Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995 This presentation contains forward-looking statements within the meaning of federal securities laws, including, among others, any statements
about our expectations, plans, intentions, strategies, or prospects. We generally use the words “may,” “will,” “expects,” “believes,” “anticipates,” “plans,”
“estimates,” “projects,” “assumes,” “guides,” “targets,” “forecasts,” “sees,” “seeks,” “should,” “could,” “would,”
“predicts,” “potential,” “strategy,” “future,” “opportunity,” “work toward,” “intends,” “guidance,” “confidence,” “positioned,”
“design,” “strive,” “continue,” “track,” “look forward to,” “optimistic” and similar expressions to identify forward-looking statements. All statements other than statements of
historical or current fact are or may be deemed to be forward-looking statements. Such statements are based upon the current beliefs, expectations, and assumptions of management and are subject to significant risks, uncertainties, and changes in
circumstances that could cause actual outcomes and results to differ materially from the forward-looking statements. These risks, uncertainties and changes in circumstances include, but are not limited to: dependence on new product development,
technological advances and innovation; shifts in the product category or regional sales mix of our products and services; supply and prices of raw materials and products; pricing pressures from competitors, customers, dental practices and insurance
providers; changes in customer demand for our products and services caused by demographic changes or other factors; challenges relating to changes in and compliance with governmental laws and regulations affecting our U.S. and international
businesses, including regulations of the U.S. Food and Drug Administration and foreign government regulators, such as more stringent requirements for regulatory clearance of products; competition; the impact of healthcare reform measures; reductions
in reimbursement levels by third-party payors; cost containment efforts sponsored by government agencies, legislative bodies, the private sector and healthcare group purchasing organizations, including the volume-based procurement process in China;
control of costs and expenses; dependence on a limited number of suppliers for key raw materials and outsourced activities; the ability to obtain and maintain adequate intellectual property protection; breaches or failures of our information
technology systems or products, including by cyberattack, unauthorized access or theft; the ability to retain the independent agents and distributors who market our products; our ability to attract, retain and develop the highly skilled employees we
need to support our business; the effect of mergers and acquisitions on our relationships with customers, suppliers and lenders and on our operating results and businesses generally; the ability to form and implement alliances; changes in tax
obligations arising from tax reform measures, including European Union rules on state aid, or examinations by tax authorities; product liability, intellectual property and commercial litigation losses; changes in general industry and market
conditions, including domestic and international growth rates; changes in general domestic and international economic conditions, including inflation and interest rate and currency exchange rate fluctuations; the effects of global pandemics and
other adverse public health developments on the global economy, our business and operations and the business and operations of our suppliers and customers, including the deferral of elective procedures and our ability to collect accounts receivable;
and the impact of the ongoing financial and political uncertainty on countries in the Euro zone on the ability to collect accounts receivable in affected countries. You are cautioned not to rely on these forward-looking statements, since there can
be no assurance that these forward-looking statements will prove to be accurate. Forward-looking statements speak only as of the date they are made, and we expressly disclaim any intention or obligation to update or revise any forward-looking
statements, whether as a result of new information, future events, or otherwise. Non-GAAP Financial Measures This presentation contains financial measures which have not been calculated in accordance with United States generally
accepted accounting principles (“GAAP”), because they are a basis upon which our management assesses our performance. Although we believe these measures may be useful for investors for the same reason, these financial measures should not
be considered as an alternative to GAAP financial measures as a measure of our financial condition, performance or liquidity. In addition, these financial measures may not be comparable to similar measures used by other companies. In the Appendix to
this presentation, we provide further descriptions of these non-GAAP measures and reconciliations of these non-GAAP measures to the most directly comparable GAAP measures.
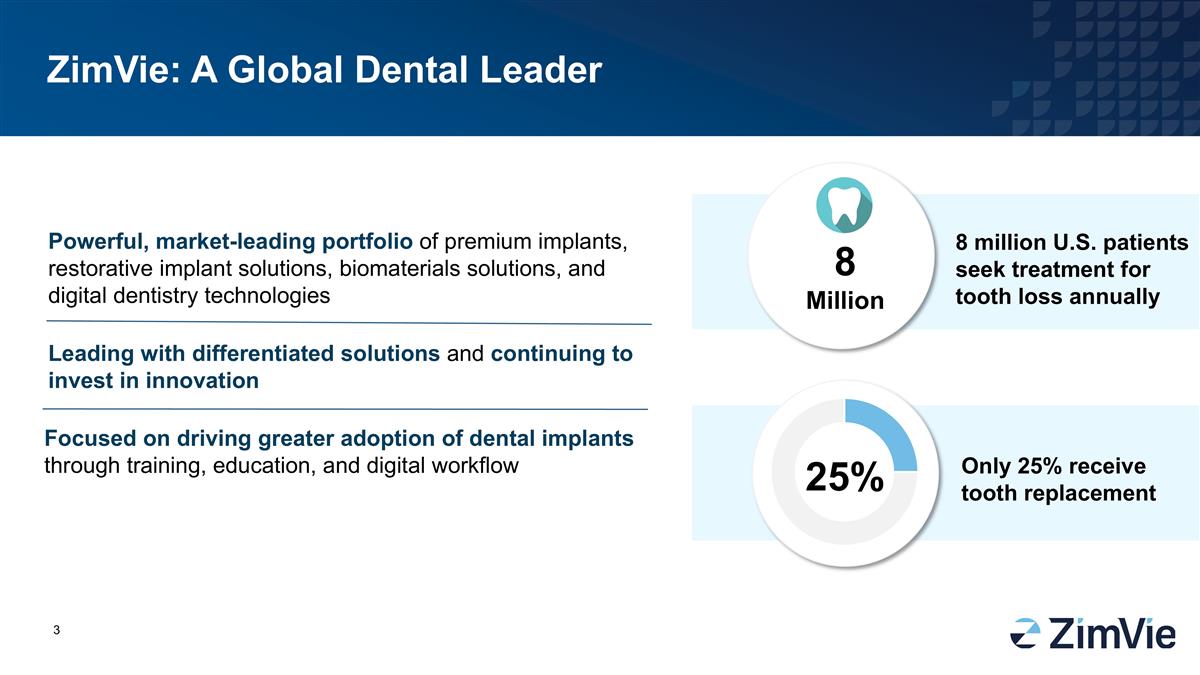
ZimVie: A Global Dental Leader
Powerful, market-leading portfolio of premium implants, restorative implant solutions, biomaterials solutions, and digital dentistry technologies 8 Million 8 million U.S. patients seek treatment for tooth loss annually 25% Only 25% receive tooth
replacement Focused on driving greater adoption of dental implants through training, education, and digital workflow Leading with differentiated solutions and continuing to invest in innovation
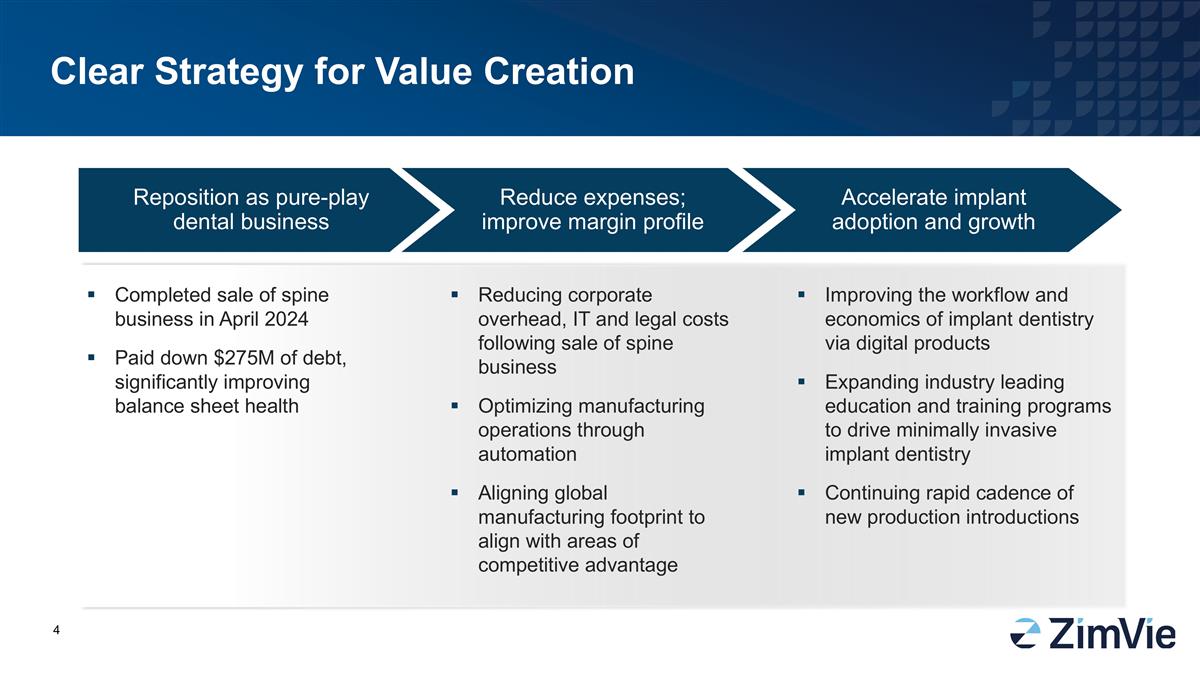
Clear Strategy for Value Creation
Completed sale of spine business in April 2024 Paid down $275M of debt, significantly improving balance sheet health Reducing corporate overhead, IT and legal costs following sale of spine business Optimizing manufacturing operations through
automation Aligning global manufacturing footprint to align with areas of competitive advantage Improving the workflow and economics of implant dentistry via digital products Expanding industry leading education and training programs to drive
minimally invasive implant dentistry Continuing rapid cadence of new production introductions Reposition as pure-play dental business Reduce expenses; improve margin profile Accelerate implant adoption and growth
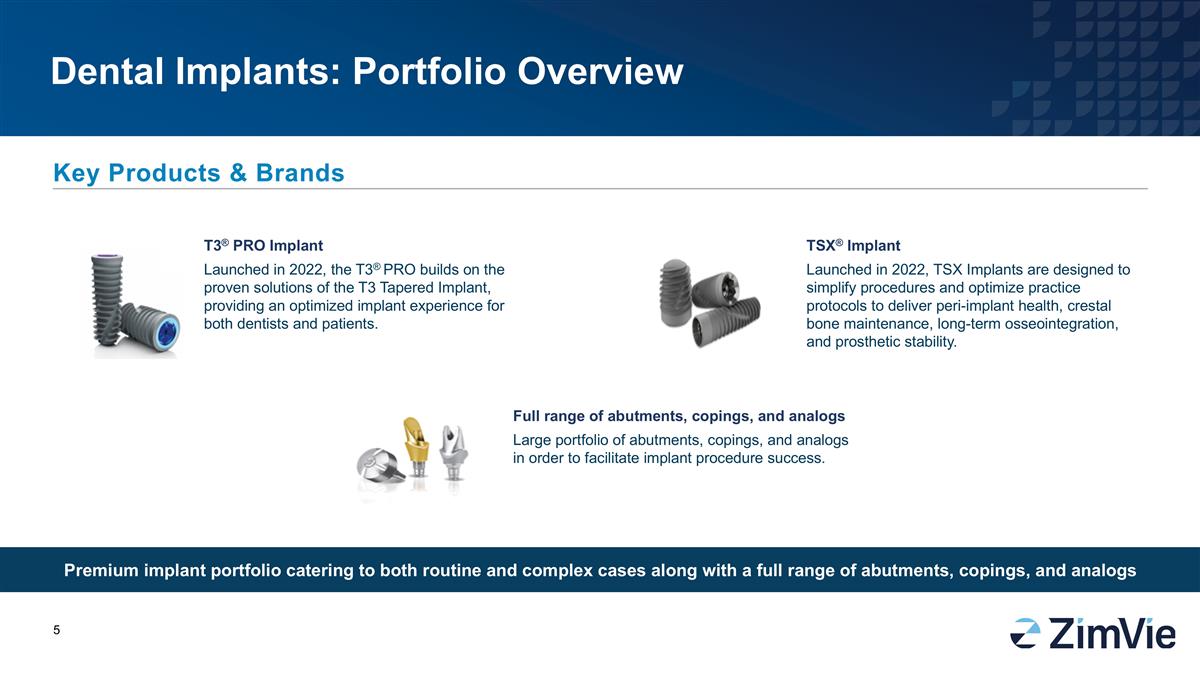
Dental Implants: Portfolio Overview
Premium implant portfolio catering to both routine and complex cases along with a full range of abutments, copings, and analogs Key Products & Brands TSX® Implant Launched in 2022, TSX Implants are designed to simplify procedures and
optimize practice protocols to deliver peri-implant health, crestal bone maintenance, long-term osseointegration, and prosthetic stability. T3® PRO Implant Launched in 2022, the T3® PRO builds on the proven solutions of the T3 Tapered
Implant, providing an optimized implant experience for both dentists and patients. Full range of abutments, copings, and analogs Large portfolio of abutments, copings, and analogs in order to facilitate implant procedure success.

Biomaterials: Portfolio Overview
Biomaterial solutions that are used for soft tissue and bone rehabilitation, helping build sufficient bone necessary for dental implant surgery Key Products & Brands Barrier Membranes By providing a reliable barrier during the critical phases of
wound healing, these membranes help maintain bone growth material. Puros® Allograft Products Products used in implant procedures to provide a foundation for the implant and create a desirable aesthetic outcome. Puros® Allograft Bone Block
Human-donor sourced bone graft material that allows patients with damaged or inadequate bone quality to be provided with a stable surface for implant application. Xenograft and Synthetic Bone Grafts Synthetic bone material that can be used to create
a suitable surface of implantation.
Digital Dentistry: Portfolio Overview
End-to-end solutions ranging from intraoral scanning technology to open architecture CAD/CAM systems, guided surgery solutions, and patient-specific restorations Key Products & Brands GenTek™ System End-to-end prosthetic offerings designed
to support CAD/CAM restorations. Implant Concierge Virtual treatment planning through Implant Concierge™ provides outsourced treatment planning services and guided surgery solutions, taking significant workflow out of the dental office.
RealGUIDE® Software Software suite that offers precise planning, designing, and predictable placement of dental implants and restorations, helping users manage procedural risk more effectively and plan complex cases in a fraction of the time.
BellaTek® System Patient-specific abutments, bars, implant bridges, and hybrid restorations designed to match each patient’s tooth anatomy.

Revitalizing the Portfolio with Recent
Launches T3® PRO Implant Encode® Emergence Healing Abutment TSX® Implant Azure™ Multi-Platform Solutions Portfolio RegenerOss® Cortico–Cancellous Particulate RegenerOss® Bone Graft Plug Biotivity™ A/C
Plus Membrane RealGUIDE® 5.4 Software CAD/CAM Workflow Systems MEDIT Intraoral Scanners Dental Implants Biomaterials Digital Dentistry Biotivity™ Hyaluronic Acid

Virtual Treatment Planning Custom
Surgical Guide Kits Delivering digital workflow enhancements to save clinician time and improve patient satisfaction AI facilitated reconstruction procedures require 3 fewer hours of human labor* ZimVie Encode Emergence workflow reduces chair time
and saves one restorative impression appointment Seeing rapid adoption of guided surgery software End-to-End Solutions Save Time and Improve the Clinician and Patient Experience *Internal data
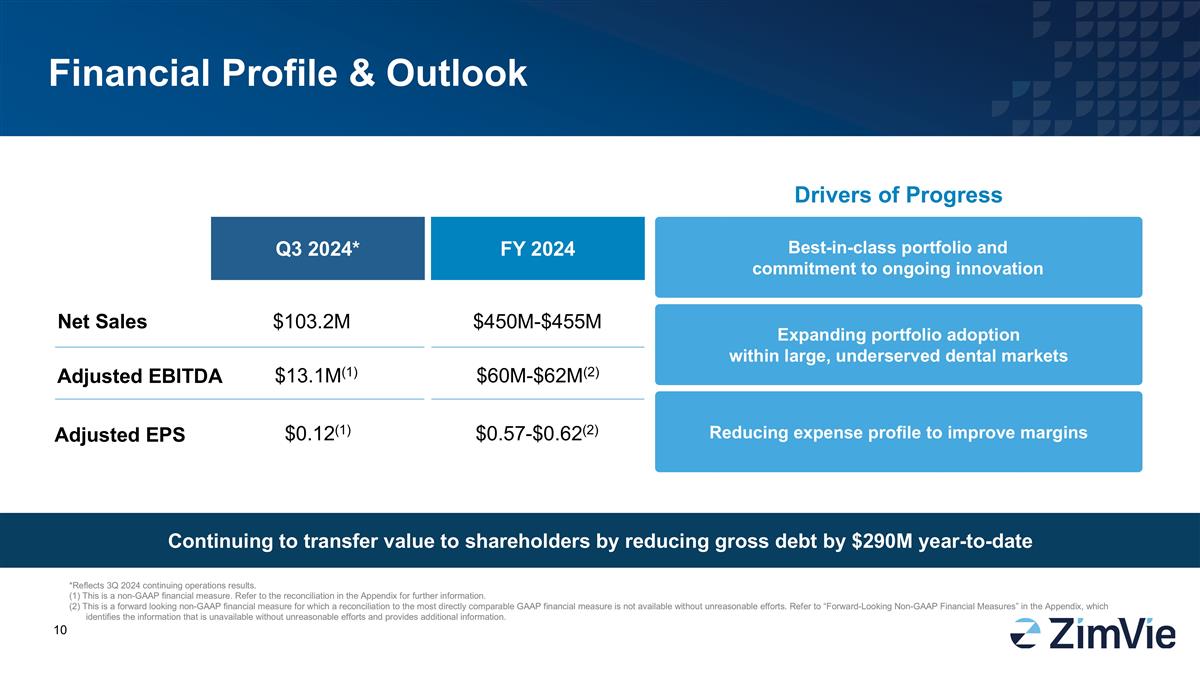
Financial Profile & Outlook Net
Sales Adjusted EBITDA Q3 2024* FY 2024 $103.2M $13.1M(1) $450M-$455M $60M-$62M(2) Drivers of Progress Expanding portfolio adoption within large, underserved dental markets Reducing expense profile to improve margins Best-in-class portfolio and
commitment to ongoing innovation Adjusted EPS $0.12(1) $0.57-$0.62(2) Continuing to transfer value to shareholders by reducing gross debt by $290M year-to-date *Reflects 3Q 2024 continuing operations results. (1) This is a non-GAAP financial
measure. Refer to the reconciliation in the Appendix for further information. (2) This is a forward looking non-GAAP financial measure for which a reconciliation to the most directly comparable GAAP financial measure is not available without
unreasonable efforts. Refer to “Forward-Looking Non-GAAP Financial Measures” in the Appendix, which identifies the information that is unavailable without unreasonable efforts and provides additional information.

Committed to Executing Strategic
Transformation Commercialize new product introductions across major geographies Drive digital workflow adoption to expand implant adoption Address and reduce stranded costs Optimize manufacturing & supply chain capabilities Position the business
for sustainable growth Innovate digital workflow to drive efficiency and outcomes for the most complex implant procedures Expand geographically with direct representation Continue to invest in training and education to drive adoption Transformed to
pure-play dental business Paid down $275M of debt Launched RealGUIDE® software update Introduced GenTek Restorative Components (U.S.) Recent Accomplishments Current Priorities Market Expansion Opportunities

Appendix

Note on Non-GAAP Financial Measures
This presentation includes non-GAAP financial measures that differ from financial measures calculated in accordance with U.S. generally accepted accounting principles (“GAAP”). These non-GAAP financial measures may not be comparable to
similar measures reported by other companies and should be considered in addition to, and not as a substitute for, or superior to, other measures prepared in accordance with GAAP. Adjusted EBITDA is a non-GAAP financial measure provided in this
presentation for certain periods and is calculated by excluding certain items from net loss from Continuing Operations on a GAAP basis, as detailed in the reconciliations presented later in this presentation. Adjusted EBITDA margin is Adjusted
EBITDA divided by third party net sales from Continuing Operations for the applicable period. Adjusted diluted earnings (loss) per share is a non-GAAP financial measure provided in this presentation for certain periods and is calculated by excluding
the effects of certain items from diluted earnings (loss) per share on a GAAP basis, as detailed in the reconciliations presented later in this presentation. Reconciliations of these non-GAAP measures to the most directly comparable GAAP
financial measures are included in this presentation. Management uses non-GAAP financial measures internally to evaluate the performance of the business. Additionally, management believes these non-GAAP measures provide meaningful incremental
information to investors to consider when evaluating the performance of the company. Management believes these measures offer the ability to make period-to-period comparisons that are not impacted by certain items that can cause dramatic changes in
reported income but that do not impact the fundamentals of our operations. The non-GAAP measures enable the evaluation of operating results and trend analysis by allowing a reader to better identify operating trends that may otherwise be masked or
distorted by these types of items that are excluded from the non-GAAP measures. Forward-Looking Non-GAAP Financial Measures This presentation also includes certain forward-looking non-GAAP financial measures for the year ending December 31, 2024. We
calculate forward-looking non-GAAP financial measures based on internal forecasts that omit certain amounts that would be included in GAAP financial measures. We have not provided quantitative reconciliations of these forward-looking non-GAAP
financial measures to the most directly comparable forward-looking GAAP financial measures because the excluded items are not available on a prospective basis without unreasonable efforts. For example, the timing of certain transactions is difficult
to predict because management’s plans may change. In addition, the company believes such reconciliations would imply a degree of precision and certainty that could be confusing to investors. It is probable that these forward-looking non-GAAP
financial measures may be materially different from the corresponding GAAP financial measures.
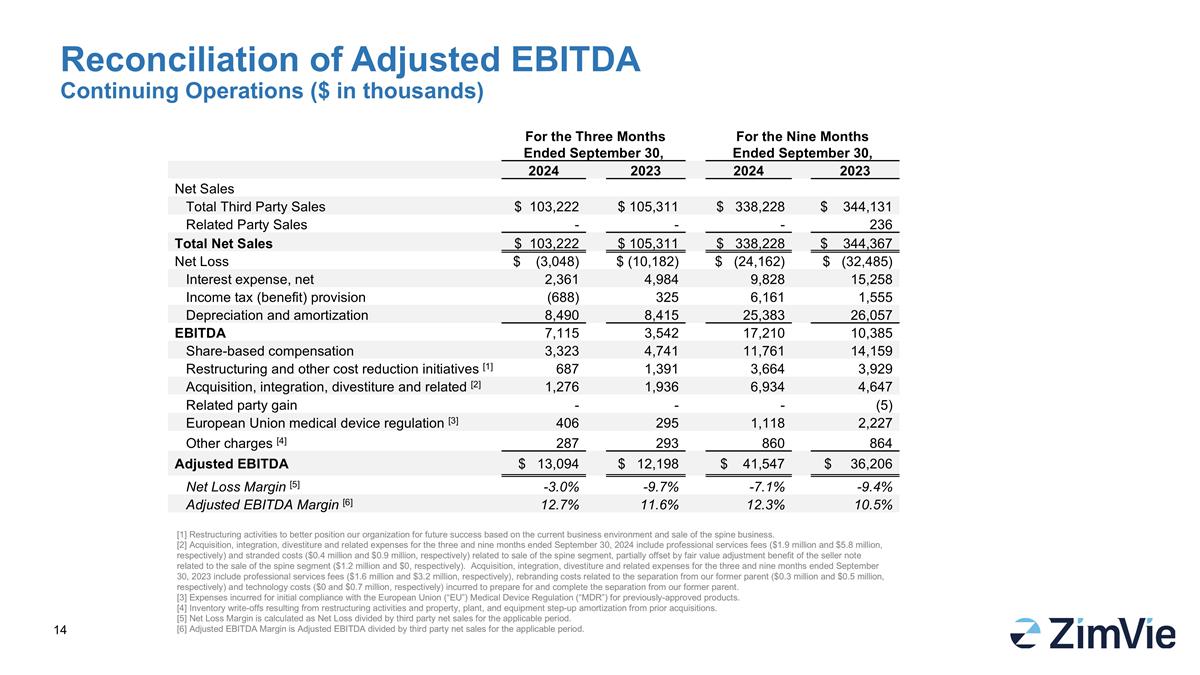
Reconciliation of Adjusted EBITDA
Continuing Operations ($ in thousands) [1] Restructuring activities to better position our organization for future success based on the current business environment and sale of the spine business. [2] Acquisition, integration, divestiture and
related expenses for the three and nine months ended September 30, 2024 include professional services fees ($1.9 million and $5.8 million, respectively) and stranded costs ($0.4 million and $0.9 million, respectively) related to sale of the spine
segment, partially offset by fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million and $0, respectively). Acquisition, integration, divestiture and related expenses for the three and nine months
ended September 30, 2023 include professional services fees ($1.6 million and $3.2 million, respectively), rebranding costs related to the separation from our former parent ($0.3 million and $0.5 million, respectively) and technology costs ($0 and
$0.7 million, respectively) incurred to prepare for and complete the separation from our former parent. [3] Expenses incurred for initial compliance with the European Union (“EU”) Medical Device Regulation (“MDR”) for
previously-approved products. [4] Inventory write-offs resulting from restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. [5] Net Loss Margin is calculated as Net Loss divided by third party net
sales for the applicable period. [6] Adjusted EBITDA Margin is Adjusted EBITDA divided by third party net sales for the applicable period. For the Three Months Ended September 30, For the Nine Months Ended September 30, 2024 2023
2024 2023 Net Sales Total Third Party Sales $ 103,222 $ 105,311 $ 338,228 $ 344,131 Related Party Sales - - - 236 Total Net Sales $ 103,222 $ 105,311 $ 338,228 $ 344,367
Net Loss $ (3,048) $ (10,182) $ (24,162) $ (32,485) Interest expense, net 2,361 4,984 9,828 15,258 Income tax (benefit) provision (688) 325 6,161 1,555 Depreciation and amortization 8,490 8,415 25,383
26,057 EBITDA 7,115 3,542 17,210 10,385 Share-based compensation 3,323 4,741 11,761 14,159 Restructuring and other cost reduction initiatives [1] 687 1,391 3,664 3,929 Acquisition, integration,
divestiture and related [2] 1,276 1,936 6,934 4,647 Related party gain - - - (5) European Union medical device regulation [3] 406 295 1,118 2,227 Other charges [4] 287 293 860 864 Adjusted
EBITDA $ 13,094 $ 12,198 $ 41,547 $ 36,206 Net Loss Margin [5] -3.0% -9.7% -7.1% -9.4% Adjusted EBITDA Margin [6] 12.7% 11.6% 12.3% 10.5%
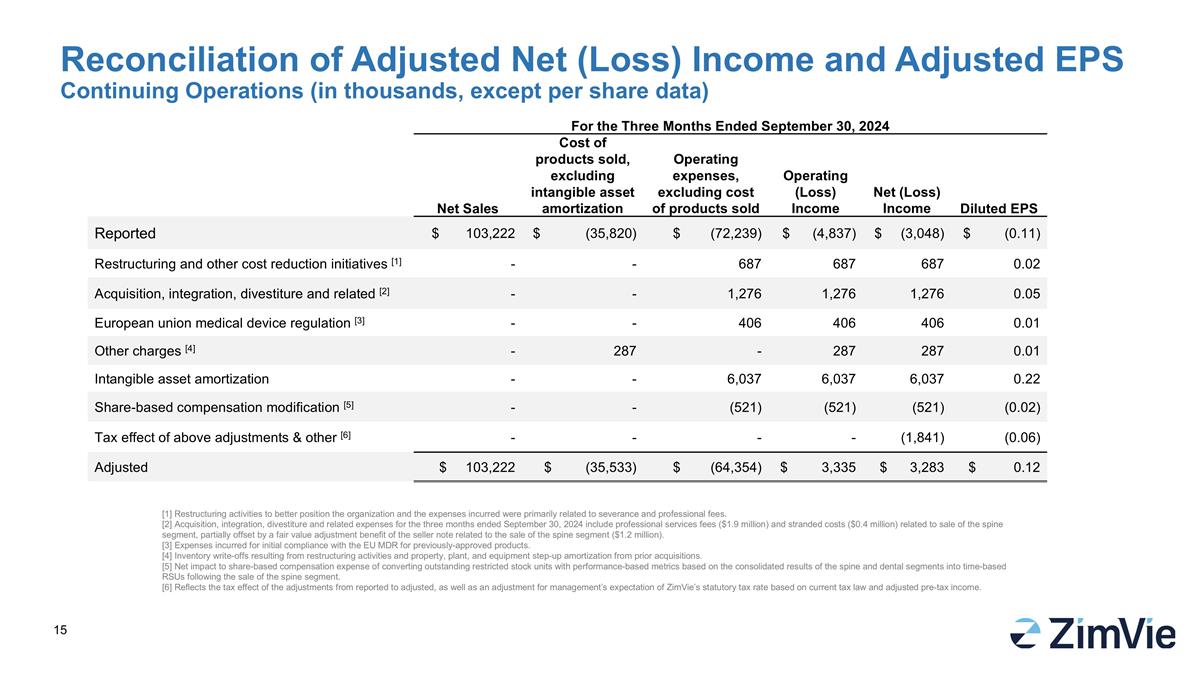
Reconciliation of Adjusted Net
(Loss) Income and Adjusted EPS Continuing Operations (in thousands, except per share data) [1] Restructuring activities to better position the organization and the expenses incurred were primarily related to severance and professional fees. [2]
Acquisition, integration, divestiture and related expenses for the three months ended September 30, 2024 include professional services fees ($1.9 million) and stranded costs ($0.4 million) related to sale of the spine segment, partially offset by a
fair value adjustment benefit of the seller note related to the sale of the spine segment ($1.2 million). [3] Expenses incurred for initial compliance with the EU MDR for previously-approved products. [4] Inventory write-offs resulting from
restructuring activities and property, plant, and equipment step-up amortization from prior acquisitions. [5] Net impact to share-based compensation expense of converting outstanding restricted stock units with performance-based metrics based on the
consolidated results of the spine and dental segments into time-based RSUs following the sale of the spine segment. [6] Reflects the tax effect of the adjustments from reported to adjusted, as well as an adjustment for management’s expectation
of ZimVie’s statutory tax rate based on current tax law and adjusted pre-tax income. For the Three Months Ended September 30, 2024 Net Sales Cost of products sold, excluding intangible asset amortization Operating expenses, excluding cost of
products sold Operating (Loss) Income Net (Loss) Income Diluted EPS Reported $ 103,222 $ (35,820) $ (72,239) $ (4,837) $ (3,048) $ (0.11) Restructuring and other cost reduction initiatives [1] - - 687 687 687 0.02 Acquisition, integration,
divestiture and related [2] - - 1,276 1,276 1,276 0.05 European union medical device regulation [3] - - 406 406 406 0.01 Other charges [4] - 287 - 287 287 0.01 Intangible asset amortization - - 6,037 6,037 6,037 0.22 Share-based compensation
modification [5] - - (521) (521) (521) (0.02) Tax effect of above adjustments & other [6] - - - - (1,841) (0.06) Adjusted $ 103,222 $ (35,533) $ (64,354) $ 3,335 $ 3,283 $ 0.12
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
ZimVie (NASDAQ:ZIMV)
Historical Stock Chart
From Mar 2025 to Apr 2025
ZimVie (NASDAQ:ZIMV)
Historical Stock Chart
From Apr 2024 to Apr 2025