Aptar’s N-Sorb Nitrosamine Mitigation Solution Accepted to US FDA’s Emerging Technology Program
September 23 2024 - 4:00PM
Business Wire
N-Sorb leverages company’s proven 3-Phase
Activ-Polymer™ platform technology to address recent concerns
related to N-nitrosamine impurities in pharmaceuticals
AptarGroup, Inc. (NYSE: ATR), a global leader in drug and
consumer product dosing, dispensing and protection technologies,
announced that its N-Sorb nitrosamine mitigation solution has been
accepted into the U.S. Food & Drug Administration’s (FDA)
Emerging Technology Program (ETP), which helps promote the adoption
of innovative approaches to pharmaceutical product design and
manufacturing.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240923964121/en/
Aptar’s N-Sorb Nitrosamine Mitigation
Solution. (Photo: Business Wire)
N-Sorb leverages Aptar CSP Technologies’ proven 3-Phase
Activ-Polymer™ platform technology to address the pressing issue of
N-nitrosamine impurities in pharmaceuticals. These impurities,
classified as probable human carcinogens, have raised significant
regulatory concerns and prompted numerous drug product recalls.
Nitrosamines can form during drug product storage or transport,
posing risks to patient health.
N-Sorb technology can be deployed in multiple formats by
integrating active material science into polymers. For example, the
technology can be incorporated into a blister format that
integrates a piece of N-Sorb Activ-Film™ material into each
individual Activ-Blister™ cavity. This same platform is currently
trusted by global brands to protect sensitive Active Pharmaceutical
Ingredients (APIs) from degradation due to moisture and oxygen
exposure. Alternatively, the technology can be seamlessly
integrated into a container closure system. N-Sorb’s intelligent
design allows it to react with nitrosamine precursors in the
packaging headspace to inhibit nitrosamine formation and scavenge
nitrosamine impurities post-formation.
By delivering this Generally Recognized as Safe (GRAS) material
directly within the packaging, N-Sorb can eliminate the need for
pharmaceutical developers to reformulate their drug products, which
could support compliance with US FDA and EU EMA regulations
regarding safe nitrosamine levels. The active packaging
intervention represents a paradigm shift in managing impurities and
degradation, which could significantly enhance overall mitigation
strategies and aligns with the latest FDA guidance (updated Sept.
4, 2024) that recognizes packaging changes as a potential
mitigation strategy. By addressing nitrosamine concerns with active
packaging, N-Sorb technology can help accelerate drug product
development and help alleviate the burden of drug shortages due to
recalls.
“The FDA’s Emerging Technology Program is highly selective,
reserved for the most promising pharma and healthcare sector
solutions,” said Badre Hammond, VP Global Commercial Operations and
GM for Aptar CSP Technologies. “Our ability to mitigate nitrosamine
formation with active material science introduces a critical
quality control element, designed to ensure patient safety. We are
eager to collaborate with the FDA’s Emerging Technology team to
empower pharma brands with this innovative offering.”
As part of the program, industry representatives meet with the
FDA’s Emerging Technology Team members to discuss, identify and
resolve potential technical and regulatory issues related to the
development and implementation of novel technologies prior to
regulatory submission. This multi-stakeholder effort aligns with
the FDA’s mission to facilitate modernization in the pharmaceutical
industry, reducing time and cost requirements for introducing novel
solutions.
About Aptar
Aptar is a global leader in drug and consumer product dosing,
dispensing and protection technologies. Aptar serves a number of
attractive end markets including pharmaceutical, beauty, food,
beverage, personal care and home care. Aptar CSP Technologies
leverages its active material science expertise to transform ideas
into market opportunities, accelerate and de-risk the product
development process, and provide complete solutions that improve
consumers’ and patients’ lives. The company offers a complete set
of services from concept ideation, to design and engineering, to
product development, global production, quality control, and
regulatory support that results in expedited speed-to-market. For
more information, please visit www.csptechnologies.com and
www.aptar.com.
This press release contains forward-looking statements.
Forward-looking statements generally can be identified by the fact
that they do not relate strictly to historical or current facts and
by use of words such as “expects,” “anticipates,” “believes,”
“estimates,” “future,” “potential,” “continues” and other similar
expressions or future or conditional verbs such as “will,”
“should,” “would” and “could” are intended to identify such
forward-looking statements. Forward-looking statements are made
pursuant to the safe harbor provisions of Section 27A of the
Securities Act of 1933 and Section 21E of the Securities Exchange
Act of 1934 and are based on our beliefs as well as assumptions
made by and information currently available to us. Accordingly, our
actual results or other events may differ materially from those
expressed or implied in such forward-looking statements due to
known or unknown risks and uncertainties that exist in our
operations and business environment including, but not limited to:
the successful integration of acquisitions; the regulatory
environment; and competition, including technological advances. For
additional information on these and other risks and uncertainties,
please see our filings with the Securities and Exchange Commission,
including the discussion under “Risk Factors” and “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” in our Form 10-K and Forms 10-Q. We undertake no
obligation to update publicly any forward-looking statements,
whether as a result of new information, future events or otherwise,
except as otherwise required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240923964121/en/
Aptar Investor Relations
Contact: Mary Skafidas mary.skafidas@aptar.com +1 347
351 6407
Aptar Media Contact Katie
Reardon katie.reardon@aptar.com +1 815 479 5671
Aptar CSP Technologies Media
Contact: Tricia Dozier tricia.dozier@aptar.com
315-521-4555
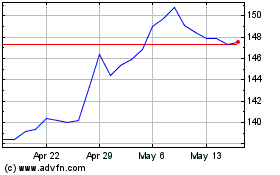
AptarGroup (NYSE:ATR)
Historical Stock Chart
From Nov 2024 to Dec 2024
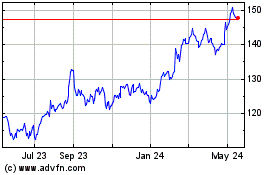
AptarGroup (NYSE:ATR)
Historical Stock Chart
From Dec 2023 to Dec 2024