Bayer's Menopause Drug Elinzanetant Met Primary Endpoints in Phase 3 Studies
January 08 2024 - 2:05AM
Dow Jones News
By Pierre Bertrand
Bayer said that its menopause drug Elinzanetant met the primary
endpoints of its Oasis 1 and 2 Phase 3 studies.
When compared with placebo, the drug showed a statistically
significant reduction in the frequency and the severity of moderate
to severe vasomotor symptoms, also known as hot flashes.
It also demonstrated statistically significant improvements in
sleep disturbances and menopause-related quality of life, the
company said.
The Oasis 1 and 2 studies assessed the efficacy and safety of
Elinzanetant when administered orally once daily. The result of the
Oasis 3 Phase 3 study is expected in the coming months, Bayer
said.
The company added that it plans to submit the data from the
Oasis studies to health authorities for marketing
authorizations.
Write to Pierre Bertrand at pierre.bertrand@wsj.com
(END) Dow Jones Newswires
January 08, 2024 02:50 ET (07:50 GMT)
Copyright (c) 2024 Dow Jones & Company, Inc.
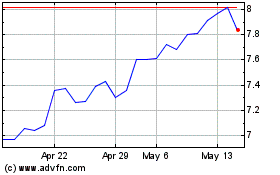
Bayer Aktiengesellschaft (PK) (USOTC:BAYRY)
Historical Stock Chart
From Jan 2025 to Feb 2025
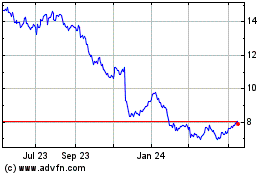
Bayer Aktiengesellschaft (PK) (USOTC:BAYRY)
Historical Stock Chart
From Feb 2024 to Feb 2025
Real-Time news about Bayer Aktiengesellschaft (PK) (OTCMarkets): 0 recent articles
More Bayer Aktiengesellschaft (PK) News Articles