false
0000921114
0000921114
2024-12-19
2024-12-19
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
December 19, 2024
ARMATA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in
its charter)
| Washington |
|
001-37544 |
|
91-1549568 |
(State or other jurisdiction of
incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
| 5005
McConnell Avenue, Los Angeles,
California |
|
90066 |
| (Address of principal executive offices) |
|
(Zip Code) |
(310) 655-2928
(Registrant’s
telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging Growth Company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Securities registered pursuant to Section 12(b) of
the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common Stock |
|
ARMP |
|
NYSE American |
| Item 7.01 |
Regulation FD Disclosure. |
On December 19,
2024, Armata Pharmaceuticals, Inc. (the “Company”) issued a press release announcing results from its Phase
2 Tailwind study of inhaled AP-PA02 in non-cystic fibrosis bronchiectasis subjects with chronic pulmonary Pseudomonas aeruginosa
infection. The full text of the press release issued in connection with this announcement is furnished as Exhibit 99.1 to this Current
Report on Form 8-K.
The Company posted
an updated corporate presentation dated December 19, 2024 (the “Corporate Presentation”) to its website at https://investor.armatapharma.com/events-and-presentations.
The Corporate Presentation, which is furnished as Exhibit 99.2 hereto, replaces the corporate presentation dated November 15,
2024 previously posted to the Company’s website. The Company plans to use its website to disseminate future updates to the Corporate
Presentation and may not file or furnish a Current Report on Form 8-K alerting investors if the Corporate Presentation is updated.
The information
contained in the Corporate Presentation is summary information that is intended to be considered in the context of the Company’s
Securities and Exchange Commission (“SEC”) filings and other public announcements that the Company may make, by press
release or otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained
in this report, except as may be required by the federal securities laws, although it may do so from time to time as its management believes
is warranted. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through
other public disclosure. In addition, the Company disclaims any inferences regarding the materiality of such information that may arise
as a result of it furnishing such information under Item 7.01 of this Current Report on Form 8-K.
The information
in this Item 7.01 and the attached Exhibits 99.1 and 99.2 is being furnished and shall not be deemed “filed” for the purposes
of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section. The information
in this Item 7.01 and the attached Exhibits 99.1 and 99.2 shall not be incorporated by reference into any registration statement or other
document pursuant to the Securities Act of 1933, as amended.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| Date: December 19, 2024 |
Armata Pharmaceuticals, Inc. |
| |
|
| |
By: |
/s/ David D. House |
| |
Name: |
David D. House |
| |
Title: |
Senior Vice President, Finance and Principal Financial officer |
Exhibit 99.1

Armata Pharmaceuticals Announces Encouraging
Results from the Phase 2 Tailwind Study of Inhaled AP-PA02 in Non-Cystic Fibrosis Bronchiectasis Subjects with Chronic Pulmonary
Pseudomonas aeruginosa Infection
Results demonstrate that inhaled AP-PA02 provides
a durable reduction of Pseudomonas aeruginosa in the lung, with a favorable safety and tolerability profile
LOS
ANGELES, Calif., December 19, 2024 -- Armata Pharmaceuticals, Inc. (NYSE American: ARMP) (“Armata” or the
“Company”), a biotechnology company focused on the development of high-purity pathogen-specific bacteriophage therapeutics
for chronic pulmonary diseases and antibiotic-resistant bacterial pathogens, today announced encouraging topline results from its Phase
2 (“Tailwind”) trial evaluating AP-PA02, a novel, inhaled multi-phage therapeutic for the treatment of chronic pulmonary
Pseudomonas aeruginosa (“P.a.” or “P. aeruginosa”) infections in non-cystic fibrosis bronchiectasis
(“NCFB”) patients. This is the second successful clinical trial AP-PA02, Armata’s lead pulmonary candidate, which was
first evaluated in cystic fibrosis patients in the Phase 1b/2a SWARM-P.a. trial, completed in 2023.
The
Tailwind study (NCT05616221) was a multicenter, randomized, double-blind, placebo-controlled trial that
evaluated the safety, pharmacokinetics and efficacy of inhaled AP-PA02. The Tailwind study was conducted in two cohorts
running in parallel: subjects in one cohort (cohort A) received inhaled AP-PA02 as monotherapy, while subjects in another
cohort (cohort B) received inhaled AP-PA02 in combination with inhaled anti-pseudomonal antibiotic treatment. Subjects in both
cohorts were dosed at home by nebulization with study drug administered every 12 hours for 10 days and were followed for
approximately four weeks after receiving their last dose of study drug.
The primary efficacy endpoint was the reduction
in P.a. colony forming units (“CFUs”) in the lung sputum at one week following completion of dosing (day 17) compared
to baseline. Per the statistical analysis plan, efficacy analysis of each independent cohort showed no significant difference between
subjects treated with AP-PA02 and placebo due to small numbers of subjects in each cohort. Notably, a post-hoc intent-to-treat analysis
(n=33 active and n=15 placebo; all subjects from both cohorts) demonstrated a statistically significant reduction of P.a. CFUs
in the lung at day 17 (AP-PA02 vs. placebo; P=0.05). The reduction in P.a. CFUs persisted two weeks following completion of dosing
with AP-PA02 when compared with placebo at day 24 (AP-PA02 vs. placebo; P=0.015). Additionally, paired analysis of P.a. CFU density
at baseline compared to day 10 (P=0.03), day 11 (P=0.01), day 17 (P=0.003) and day 24 (P=0.018) was significant in the AP-PA02-treated
cohort. We believe the data suggest that AP-PA02 alone is as effective as the combination therapy of phage and antibiotics in reducing
P.a. CFUs in the lung. Additionally, approximately one-third of subjects treated with phage monotherapy exhibited at least a 2-log
CFU reduction in P.a. compared to no reduction in placebo treated subjects. The study data indicate the potential for phage therapy
to reduce reliance on chronic antibiotic use. Armata's latest corporate presentation with topline results from the Phase 2 Tailwind
study can be found here.
Safety data indicate that inhaled AP-PA02 was
well-tolerated with treatment-emergent adverse events mild and self-limiting. There was one possibly related serious adverse event that
was linked to an acute pulmonary event requiring hospitalization that was responsive to antibiotics. We believe the safety and tolerability
of AP-PA02 exhibited a promising profile for treating chronically infected NCFB patients.

“The positive results from our Tailwind
study further demonstrate the potential of our high-purity phage cocktail as a new monotherapy treatment alternative for chronic
pulmonary disease caused by P. aeruginosa infection, including drug-resistant bacteria,” stated Dr. Deborah Birx, Chief
Executive Officer of Armata. “From the beginning, our mission at Armata has been to evaluate phage-based therapeutics in randomized
controlled clinical trials that evaluate safety and efficacy to support potential regulatory approval.”
About Armata Pharmaceuticals, Inc.
Armata is a clinical-stage biotechnology company
focused on the development of pathogen-specific bacteriophage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat
bacterial infections using its proprietary bacteriophage-based technology. Armata is developing and advancing a broad pipeline of natural
and synthetic phage candidates, including clinical candidates for Pseudomonas aeruginosa, Staphylococcus aureus, and other
pathogens. Armata is committed to advancing phage therapy with drug development expertise that spans bench to clinic, including in-house
phage-specific cGMP manufacturing to support full commercialization.
Forward Looking Statements
This communication contains “forward-looking”
statements as defined by the Private Securities Litigation Reform Act of 1995. These statements relate to future events, results or to
Armata’s future financial performance and involve known and unknown risks, uncertainties and other factors which may cause Armata’s
actual results, performance or events to be materially different from any future results, performance or events expressed or implied by
the forward-looking statements. In some cases, you can identify these statements by terms such as “anticipate,” “believe,”
“could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,”
“predict,” “project,” “should,” “will,” “would” or the negative of those terms,
and similar expressions. These forward-looking statements reflect management’s beliefs and views with respect to future events and
are based on estimates and assumptions as of the date of this communication and are subject to risks and uncertainties including risks
related to Armata’s development of bacteriophage-based therapies; ability to staff and maintain its production facilities under
fully compliant current Good Manufacturing Practices; ability to meet anticipated milestones in the development and testing of the relevant
product; ability to be a leader in the development of phage-based therapeutics; ability to achieve its vision, including improvements
through engineering and success of clinical trials; ability to successfully complete preclinical and clinical development of, and obtain
regulatory approval of its product candidates and commercialize any approved products on its expected timeframes or at all; and Armata’s
estimates regarding anticipated operating losses, capital requirements and needs for additional funds. Additional risks and uncertainties
relating to Armata and its business can be found under the caption “Risk Factors” and elsewhere in Armata’s filings
and reports with the SEC, including in Armata’s Annual Report on Form 10-K, filed with the SEC on March 21, 2024, and
in its subsequent filings with the SEC.
Armata expressly disclaims any obligation or undertaking
to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Armata’s
expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.

Media Contacts:
At Armata:
Pierre Kyme
Armata Pharmaceuticals, Inc.
ir@armatapharma.com
310-665-2928 x234
Investor Relations:
Joyce Allaire
LifeSci Advisors, LLC
jallaire@lifesciadvisors.com
212-915-2569
Exhibit 99.2
Corporate Presentation December 19, 2024 NYSE American: ARMP

2 This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions . If the risks or uncertainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward - looking statements . All statements other than statements of historical fact could be deemed forward - looking, including, but not limited to : our estimates regarding anticipated operating losses, capital requirements and needs for additional funds ; our ability to raise additional capital when needed and to continue as a going concern ; our ability to manufacture, or otherwise secure the manufacture of, sufficient amounts of our product candidates for our preclinical studies and clinical trials ; our clinical development plans, including planned clinical trials ; our research and development plans, including our clinical development plans ; our ability to select combinations of phages to formulate our product candidates ; our development of bacteriophage - based therapies ; the potential use of bacteriophages to treat bacterial infections ; the potential future of antibiotic resistance ; our ability for bacteriophage therapies to disrupt and destroy biofilms and restore sensitivity to antibiotics ; our planned development strategy, presenting data to regulatory agencies and defining planned clinical studies ; the expected timing of additional clinical trials, including Phase 1 b/Phase 2 or registrational clinical trials ; our ability to manufacture and secure sufficient quantities of our product candidates for clinical trials ; the drug product candidates to be supplied by us for clinical trials ; the potential for bacteriophage technology being uniquely positioned to address the global threat of antibiotic resistance ; the safety and efficacy of our product candidates ; our anticipated regulatory pathways for our product candidates ; the activities to be performed by specific parties in connection with clinical trials ; our ability to successfully complete preclinical and clinical development of, and obtain regulatory approval of our product candidates and commercialize any approved products on our expected timeframes or at all ; our pursuit of additional indications ; the content and timing of submissions to and decisions made by the U . S . Food and Drug Administration (the “FDA”) and other regulatory agencies ; our ability to leverage the experience of our management team and to attract and retain management and keep management and other key personnel ; the capacities and performance of our suppliers, manufacturers, contract research organizations (“CROs”) and other third parties over whom we have limited control ; our ability to staff and maintain our production facilities under fully compliant current Good Manufacturing Practices ; the actions of our competitors and success of competing drugs or other therapies that are or may become available ; our expectations with respect to future growth and investments in our infrastructure, and our ability to effectively manage any such growth ; the size and potential growth of the markets for any of our product candidates, and our ability to capture share in or impact the size of those markets ; the benefits of our product candidates ; potential market growth and market and industry trends ; maintaining collaborations with third parties including our partnership with the Cystic Fibrosis Foundation and the U . S . Department of Defense (the “DoD”) ; potential future collaborations with third parties and the potential markets and market opportunities for product candidates ; our ability to achieve our vision, including improvements through engineering and success of clinical trials ; our ability to meet anticipated milestones for 2024 ; our ability to be a leader in the development of phage - based therapeutics ; the expected use of proceeds from the $ 21 . 6 million DoD grant ; the effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage to comply with applicable regulatory requirements ; the accuracy of our estimates regarding future expenses, revenues, capital requirements and need for additional financing ; our expectations regarding future planned expenditures ; our ability to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes - Oxley Act ; our ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of any of our products and product candidates ; our ability to protect our intellectual property, including pending and issued patents ; our ability to operate our business without infringing the intellectual property rights of others ; our ability to advance our clinical development programs, which could be impacted by the COVID - 19 pandemic ; the expected impact of the COVID - 19 pandemic on our operations and any statements of assumptions underlying any of the items mentioned ; and statements of belief and any statement of assumptions underlying any of the items mentioned . These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance . Actual results could differ materially from our current expectations as a result of these risks and uncertainties, which include, without limitation, risks related to the ability of our lead clinical candidates, AP - PA 02 and AP - SA 02 (including any modifications thereto) to be more effective than previous candidates ; our ability to enhance AP - PA 02 to treat both CF and NCFB patients ; our ability to develop products as expected ; our expected market opportunity for our products ; our ability to sufficiently fund our operations as expected, including obtaining additional funding as needed, and to refinance, repay or restructure its debt ; and whether Armata will incur unforeseen expenses or liabilities . You should not rely upon forward - looking statements as predictions of future events . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur . Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statements to actual results or to changes in our expectations except as required by law . We refer you to the documents that we file from time to time with the Securities and Exchange Commission, including our most recently filed Annual Report on Form 10 - K, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K . These documents, including the sections therein entitled “Risk Factors,” identify important factors that could cause the actual results to differ materially from those contained in forward - looking statements . In addition, this presentation also contains estimates, projections and other information concerning our industry, our business, and the markets for our product candidates, as well as data regarding market research, estimates and forecasts prepared by our management . Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information . These statements are based upon information available to us as of the date of this presentation, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information . Forward Looking Statements

3 Armata Highlights A leading developer of high - purity, pathogen - specific phage therapeutics: • Potential alternative to antibiotics – effective while protecting normal human microbiome • Activity independent of antibiotic resistance, providing critical alternative in setting of increasing MDR worldwide • De - risked modality: worldwide usage (pre - antibiotic era), decades of therapeutic use data ex - US (post - antibiotic era) Three phase 2 clinical trials across two programs: • AP - PA02 : Completed two Phase 2 studies across two chronic Pseudomonas aeruginosa respiratory indications (CF and NCFB) • Positive top - line results from Phase 2 trial (“Tail wind ”) of inhaled AP - PA02 in patients with NCFB announced December 2024 • Positive top - line results from Phase 1b/2a trial (“SWARM - Pa ”) of inhaled AP - PA02 in patients with CF announced March 2023 • AP - SA02: Phase 1b/2a fully enrolled (4Q24) for Staphylococcus aureus bacteremia (“diSArm”); readout anticipated in 1Q25 Clinical strategy with “parallel pathways” optimizing for both rapid regulatory approval and large commercial opportunity • Commercial models project peak year sales exceeding $2B for AP - PA02 and AP - SA02 across 4 lead indications • Products will benefit from durability and pricing advantages of biologics in U.S. market Agile phage product development platform to efficiently develop programs for new or expanded clinical indications Industry leading phage - specific drug manufacturing platform provides competitive advantage and partnerships • In - house cGMP excellence which creates competitive advantage for internal pipeline with optimized purity allowing for higher dose escalation and longer treatments • State of the art fill and finish line with significant proprietary process knowledge • Potential for additional revenue source through large - molecule third party manufacturing contracts Seasoned leadership team brings track record and differentiated relationships with partners • Demonstrated operational excellence and delivery across multiple functional areas • Successful track record in capital raises, M&A, and exits • Deep industry and government relationships have led to non - dilutive financing and potential for future support (e.g., CF Foundation, U.S. Department of Defense) Harnessing significant advantages of phage - based anti - infectives Diversified pipeline allows multiple shots on goal with compelling market opportunities World - class manufacturing facilities and development capabilities Strong leadership team and key partnerships
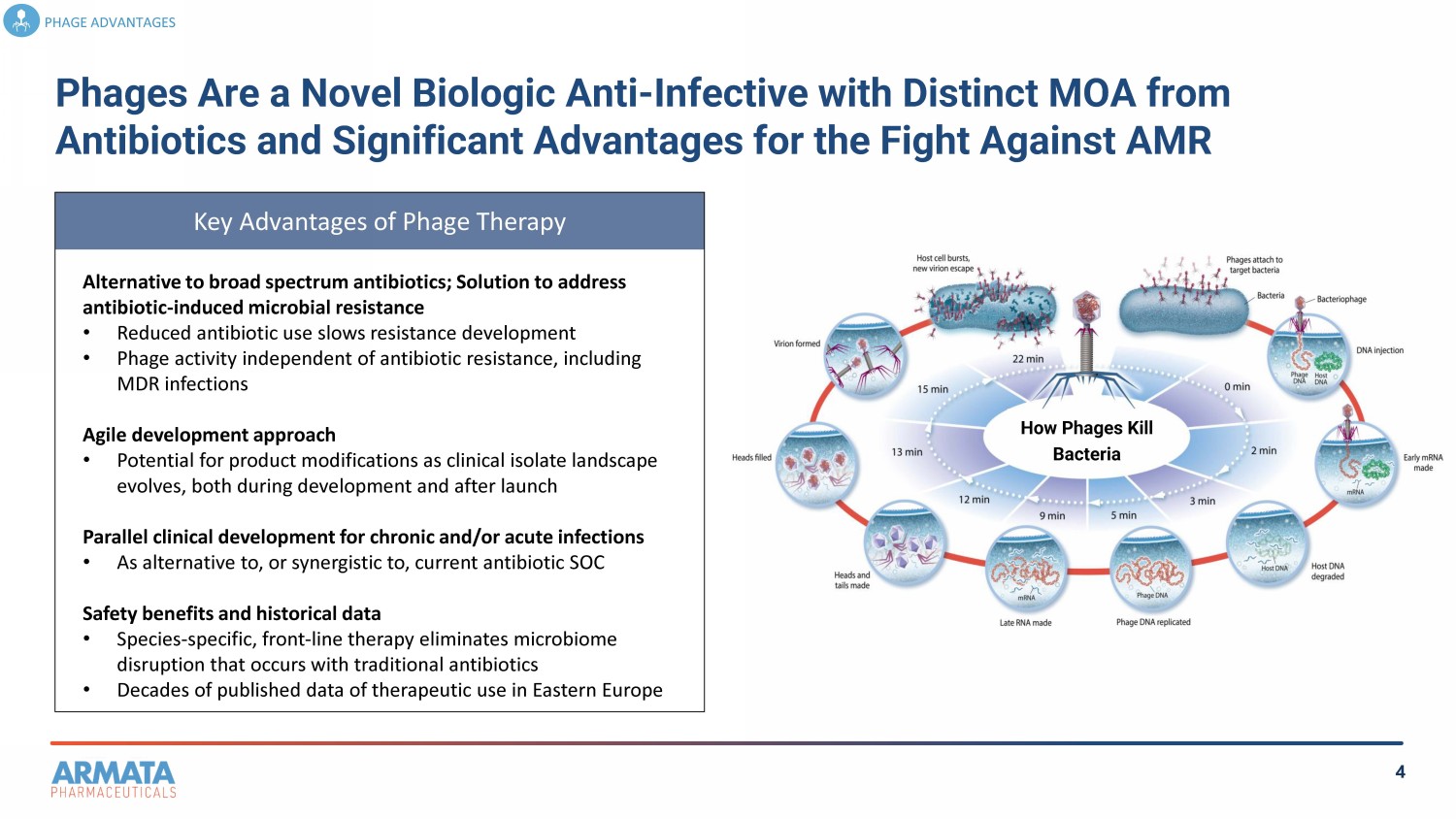
4 How Phages Kill Bacteria Key Advantages of Phage Therapy Phages Are a Novel Biologic Anti - Infective with Distinct MOA from Antibiotics and Significant Advantages for the Fight Against AMR PHAGE ADVANTAGES Alternative to broad spectrum antibiotics; Solution to address antibiotic - induced microbial resistance • Reduced antibiotic use slows resistance development • Phage activity independent of antibiotic resistance, including MDR infections Agile development approach • Potential for product modifications as clinical isolate landscape evolves, both during development and after launch Parallel clinical development for chronic and/or acute infections • As alternative to, or synergistic to, current antibiotic SOC Safety benefits and historical data • Species - specific, front - line therapy eliminates microbiome disruption that occurs with traditional antibiotics • Decades of published data of therapeutic use in Eastern Europe
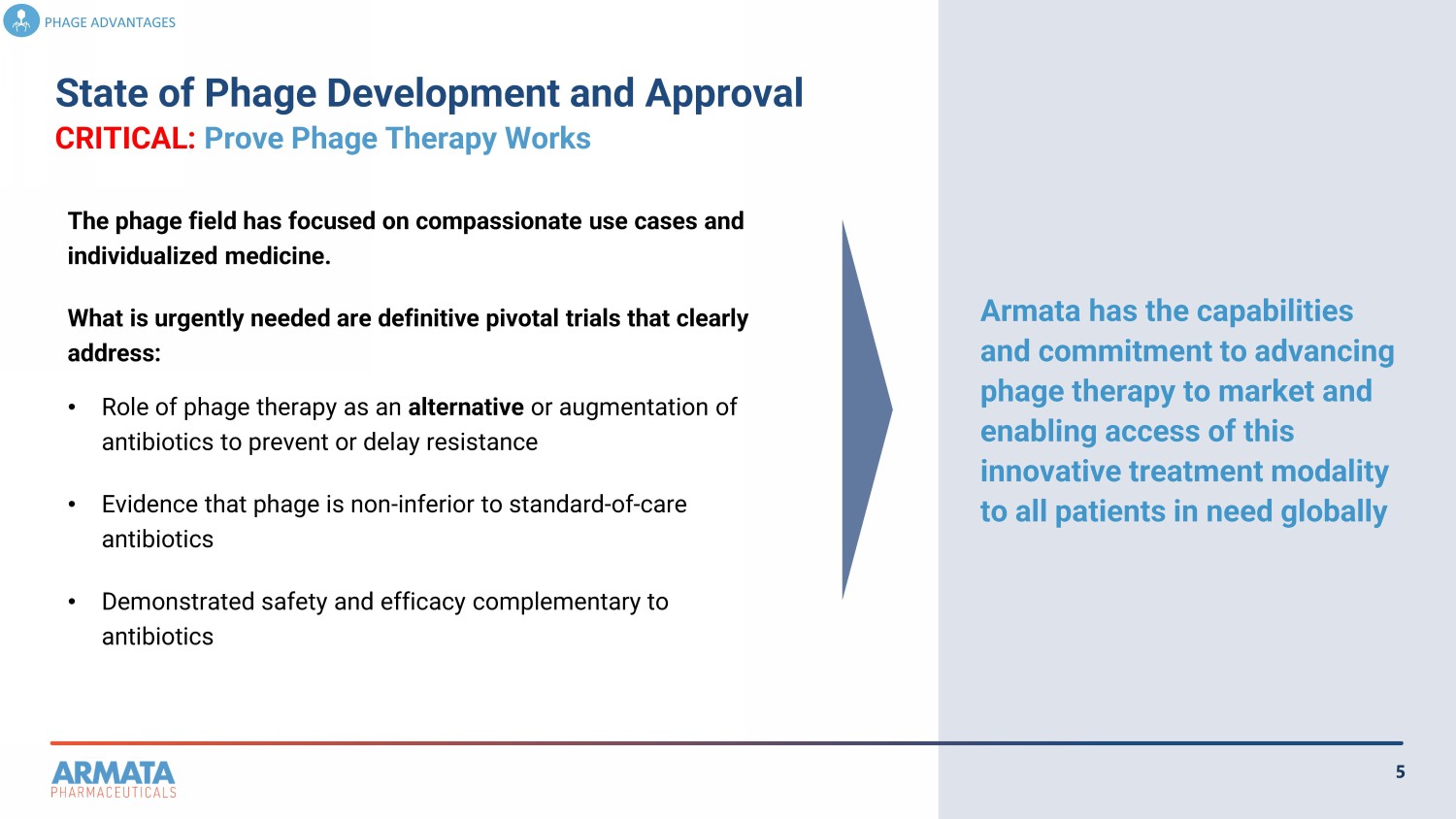
5 State of Phage Development and Approval PHAGE ADVANTAGES The phage field has focused on compassionate use cases and individualized medicine. What is urgently needed are definitive pivotal trials that clearly address: • Role of phage therapy as an alternative or augmentation of antibiotics to prevent or delay resistance • Evidence that phage is non - inferior to standard - of - care antibiotics • Demonstrated safety and efficacy complementary to antibiotics Armata has the capabilities and commitment to advancing phage therapy to market and enabling access of this innovative treatment modality to all patients in need globally 5 State of Phage Development and Approval CRITICAL: Prove Phage Therapy Works
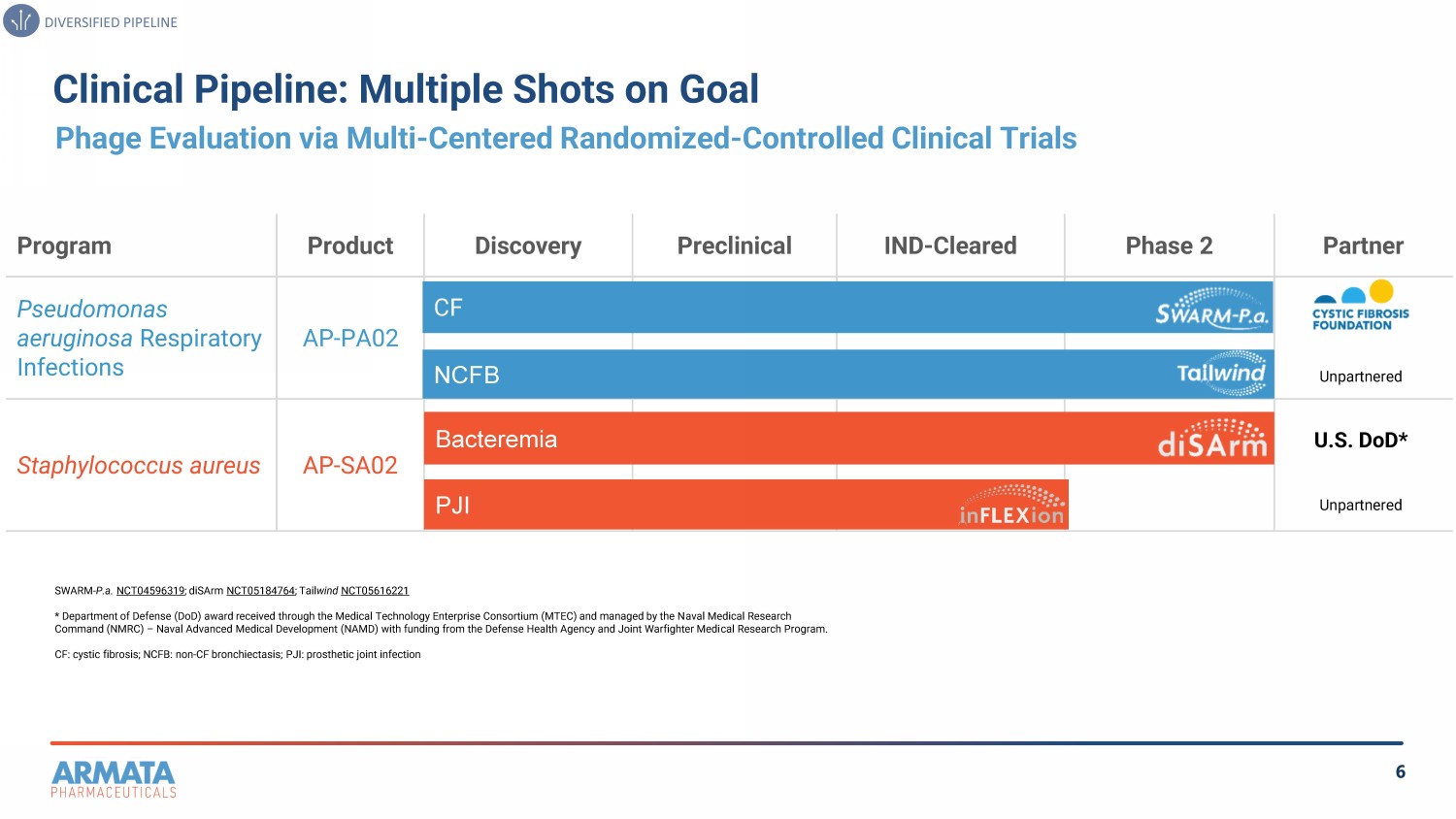
6 Clinical Pipeline: Multiple Shots on Goal Phage Evaluation via Multi - Centered Randomized - Controlled Clinical Trials SWARM - P.a. NCT04596319 ; diSArm NCT05184764 ; Tail wind NCT05616221 * Department of Defense (DoD) award received through the Medical Technology Enterprise Consortium (MTEC) and managed by the N ava l Medical Research Command (NMRC) – Naval Advanced Medical Development (NAMD) with funding from the Defense Health Agency and Joint Warfighter Medi cal Research Program. CF: cystic fibrosis; NCFB: non - CF bronchiectasis; PJI: prosthetic joint infection DIVERSIFIED PIPELINE Partner Phase 2 IND - Cleared Preclinical Discovery Product Program AP - PA02 Pseudomonas aeruginosa Respiratory Infections AP - SA02 Staphylococcus aureus CF NCFB Bacteremia PJI Unpartnered U.S. DoD* Unpartnered
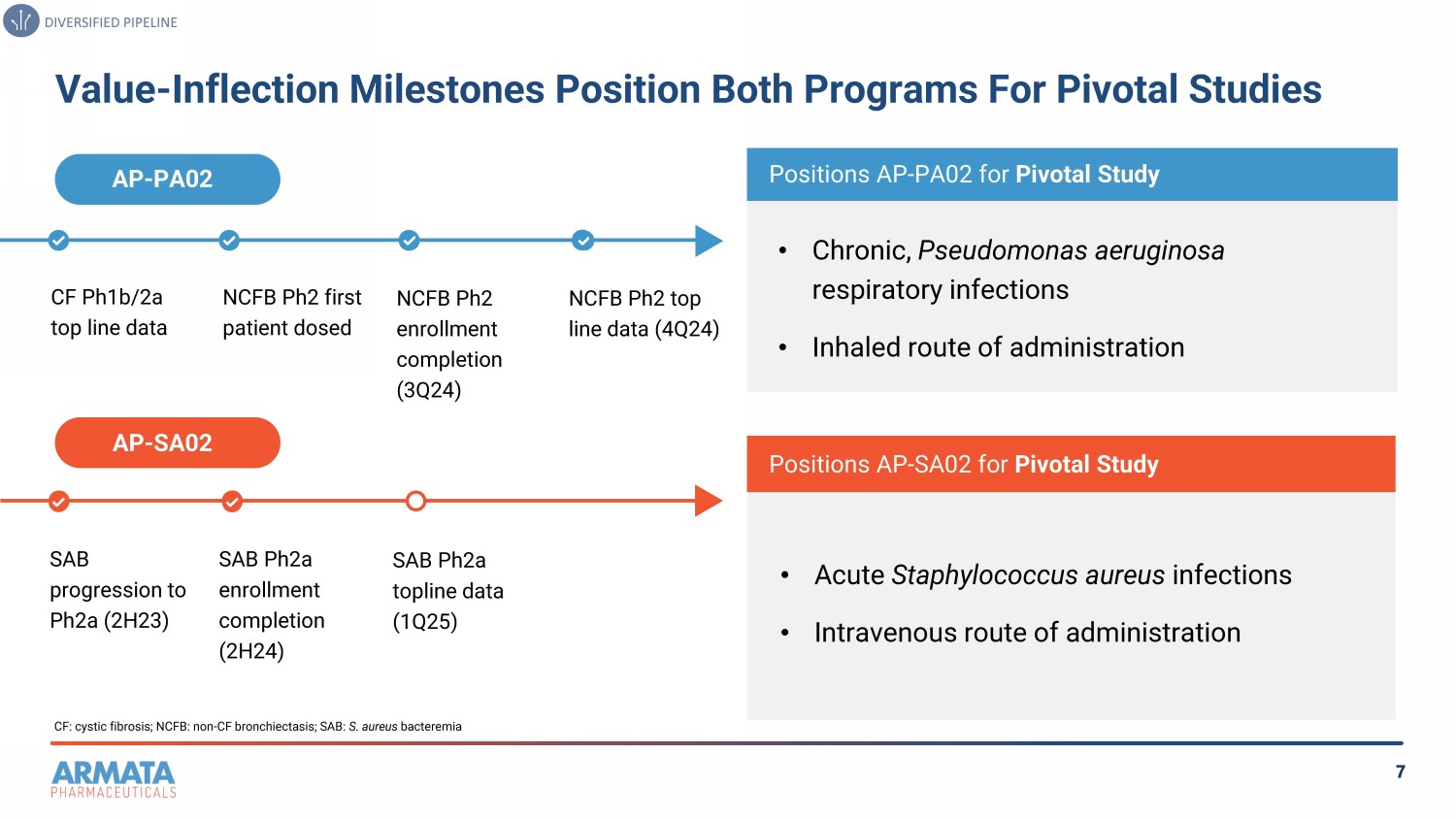
7 Value - Inflection Milestones Position Both Programs For Pivotal Studies CF: cystic fibrosis; NCFB: non - CF bronchiectasis; SAB: S. aureus bacteremia AP - PA02 CF Ph1b/2a top line data NCFB Ph2 first patient dosed NCFB Ph2 enrollment completion (3Q24) NCFB Ph2 top line data ( 4Q 24) AP - SA02 Positions AP - PA02 for Pivotal Study • Chronic, Pseudomonas aeruginosa respiratory infections • Inhaled route of administration Positions AP - SA02 for Pivotal Study • Acute Staphylococcus aureus infections • Intravenous route of administration SAB progression to Ph2a (2H23) SAB Ph2a enrollment completion (2H24) SAB Ph2a topline data (1Q25) DIVERSIFIED PIPELINE
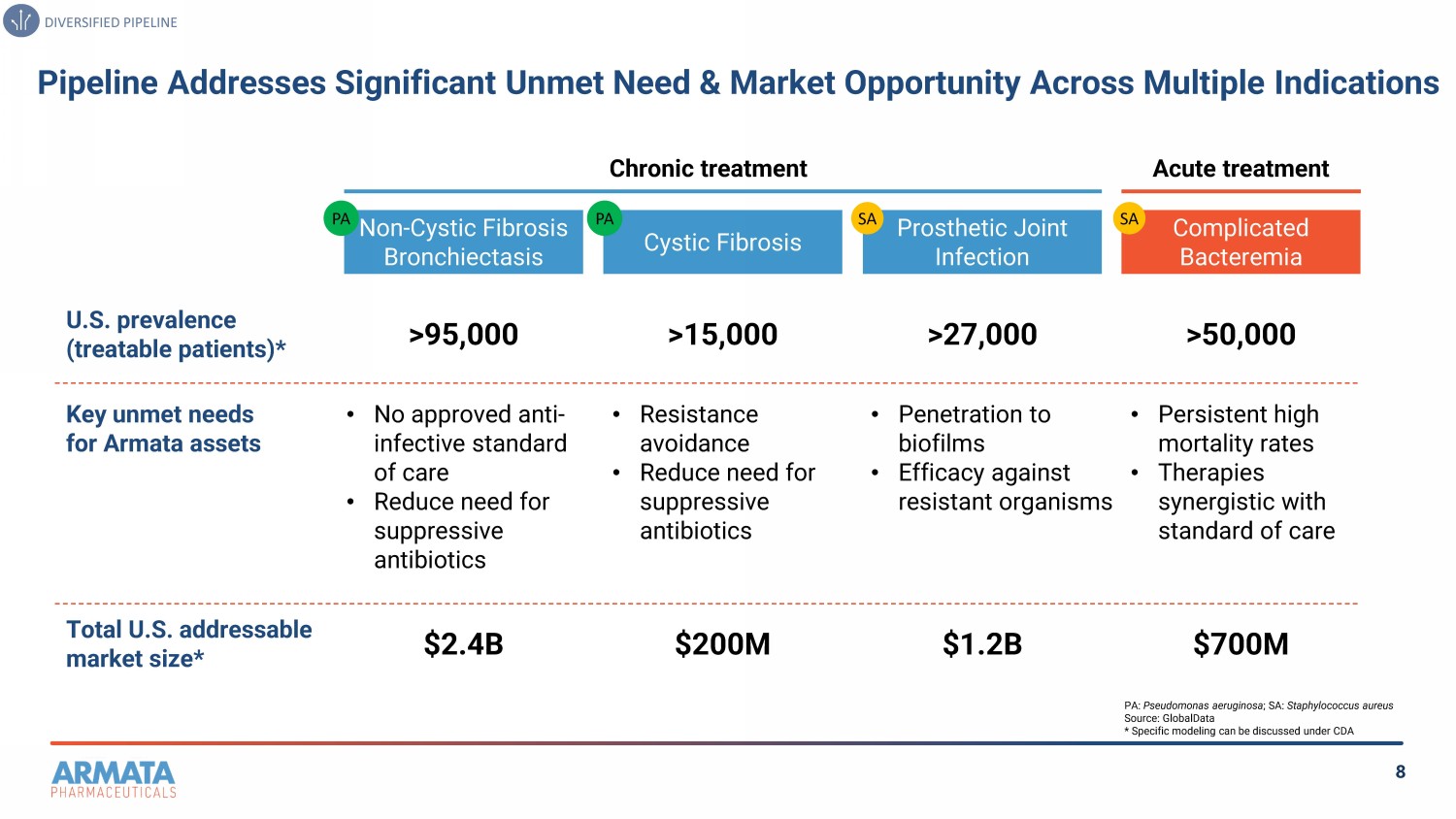
8 Pipeline Addresses Significant Unmet Need & Market Opportunity Across Multiple Indications DIVERSIFIED PIPELINE Non-Cystic Fibrosis Bronchiectasis U.S. prevalence (treatable patients)* Key unmet needs for Armata assets Cystic Fibrosis >95,000 >15,000 Total U.S. addressable market size* • Resistance avoidance • Reduce need for suppressive antibiotics • No approved anti - infective standard of care • Reduce need for suppressive antibiotics $2.4B $200M Complicated Bacteremia >50,000 • Persistent high mortality rates • Therapies synergistic with standard of care $700M Prosthetic Joint Infection >27,000 • Penetration to biofilms • Efficacy against resistant organisms $1.2B Chronic treatment Acute treatment PA: Pseudomonas aeruginosa ; SA: Staphylococcus aureus Source: GlobalData * Specific modeling can be discussed under CDA PA PA SA SA
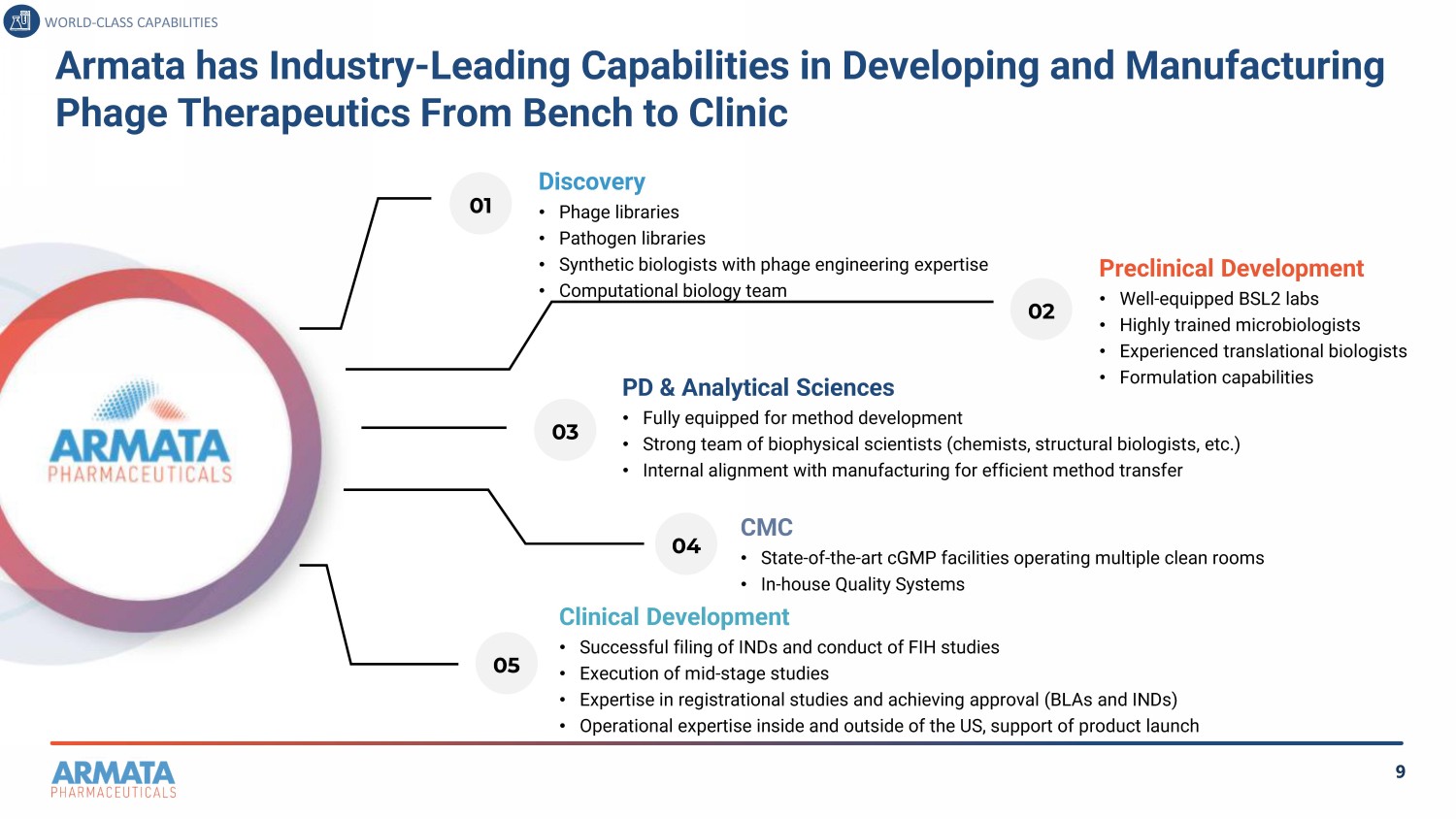
9 Armata has Industry - Leading Capabilities in Developing and Manufacturing Phage Therapeutics From Bench to Clinic Discovery • Phage libraries • Pathogen libraries • Synthetic biologists with phage engineering expertise • Computational biology team Preclinical Development • Well - equipped BSL2 labs • Highly trained microbiologists • Experienced translational biologists • Formulation capabilities PD & Analytical Sciences • Fully equipped for method development • Strong team of biophysical scientists (chemists, structural biologists, etc.) • Internal alignment with manufacturing for efficient method transfer CMC • State - of - the - art cGMP facilities operating multiple clean rooms • In - house Quality Systems Clinical Development • Successful filing of INDs and conduct of FIH studies • Execution of mid - stage studies • Expertise in registrational studies and achieving approval (BLAs and INDs) • Operational expertise inside and outside of the US, support of product launch 01 03 02 04 05 WORLD - CLASS CAPABILITIES
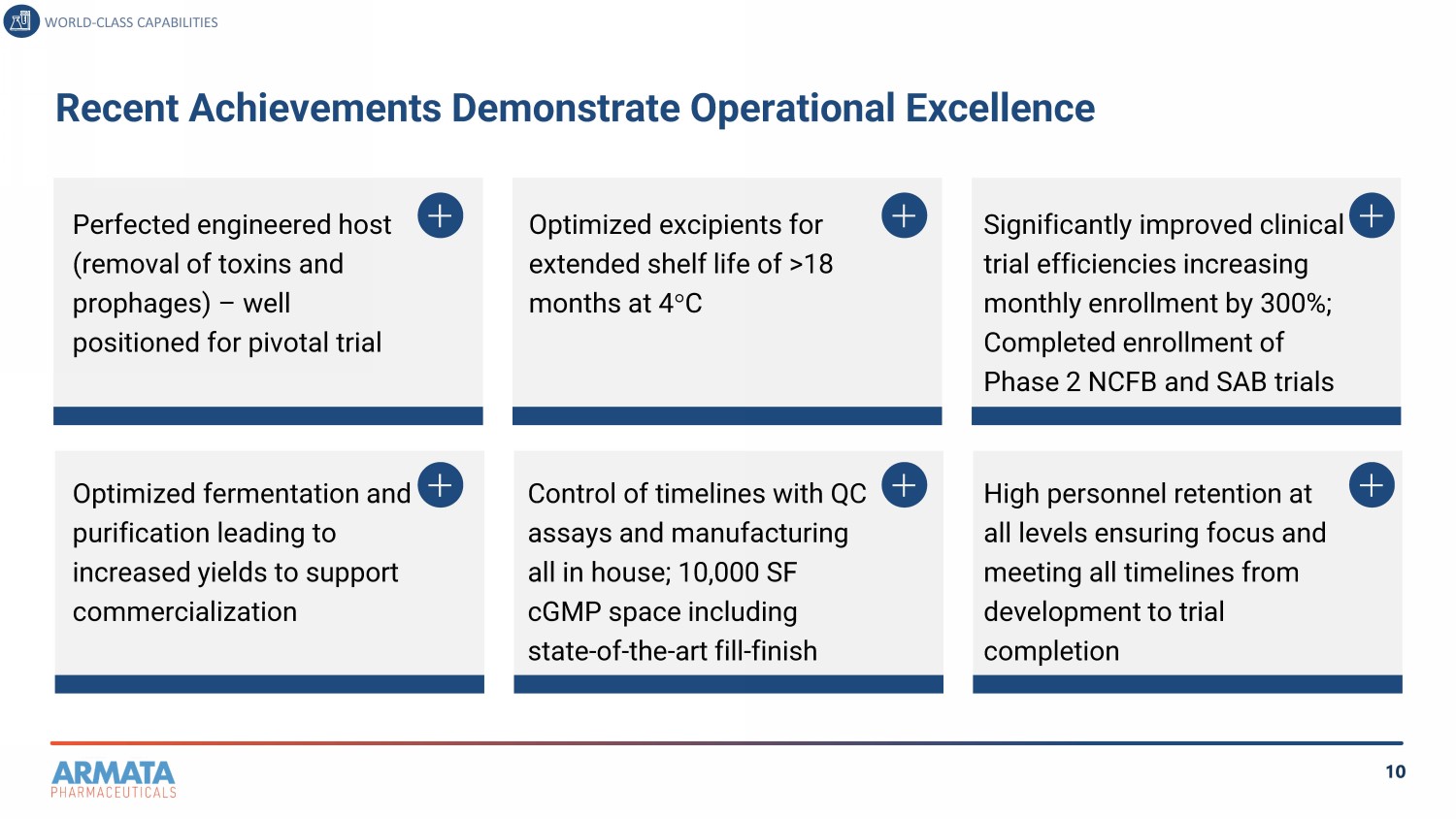
10 Recent Achievements Demonstrate Operational Excellence WORLD - CLASS CAPABILITIES Perfected engineered host (removal of toxins and prophages) – well positioned for pivotal trial Optimized fermentation and purification leading to increased yields to support commercialization Optimized excipients for extended shelf life of >18 months at 4 C Control of timelines with QC assays and manufacturing all in house; 10,000 SF cGMP space including state - of - the - art fill - finish Significantly improved clinical trial efficiencies increasing monthly enrollment by 300%; Completed enrollment of Phase 2 NCFB and SAB trials High personnel retention at all levels ensuring focus and meeting all timelines from development to trial completion

11 Manufacturing Infrastructure Creates Competitive Advantage and Alternate Revenue Streams State of the art cGMP manufacturing facility • 10,000 square foot purpose - built facility • Essential infrastructure for phage production - Two independent production lines with dedicated upstream and downstream cleanrooms - Ability to manufacture multiple products in parallel - Additional independent Flex Suite with potential to act as a third production line - High - throughput semi - automated aseptic filling line - Versatile configurations for final product form (liquid, powder, vials, syringes) - System turn - around time for different drug product: within 24 hours Scalability provides capacity for contract manufacturing as well as in - house programs • Additional revenue stream from contracting additional space - CMC capabilities and infrastructure adaptable to other advanced biologics - Profitable manufacturing agreement(s) anticipated in 2024/2025 • In - house capabilities derisk late - stage trials and allow for efficient commercial scale production with fewer supply chain disruption threats WORLD - CLASS CAPABILITIES
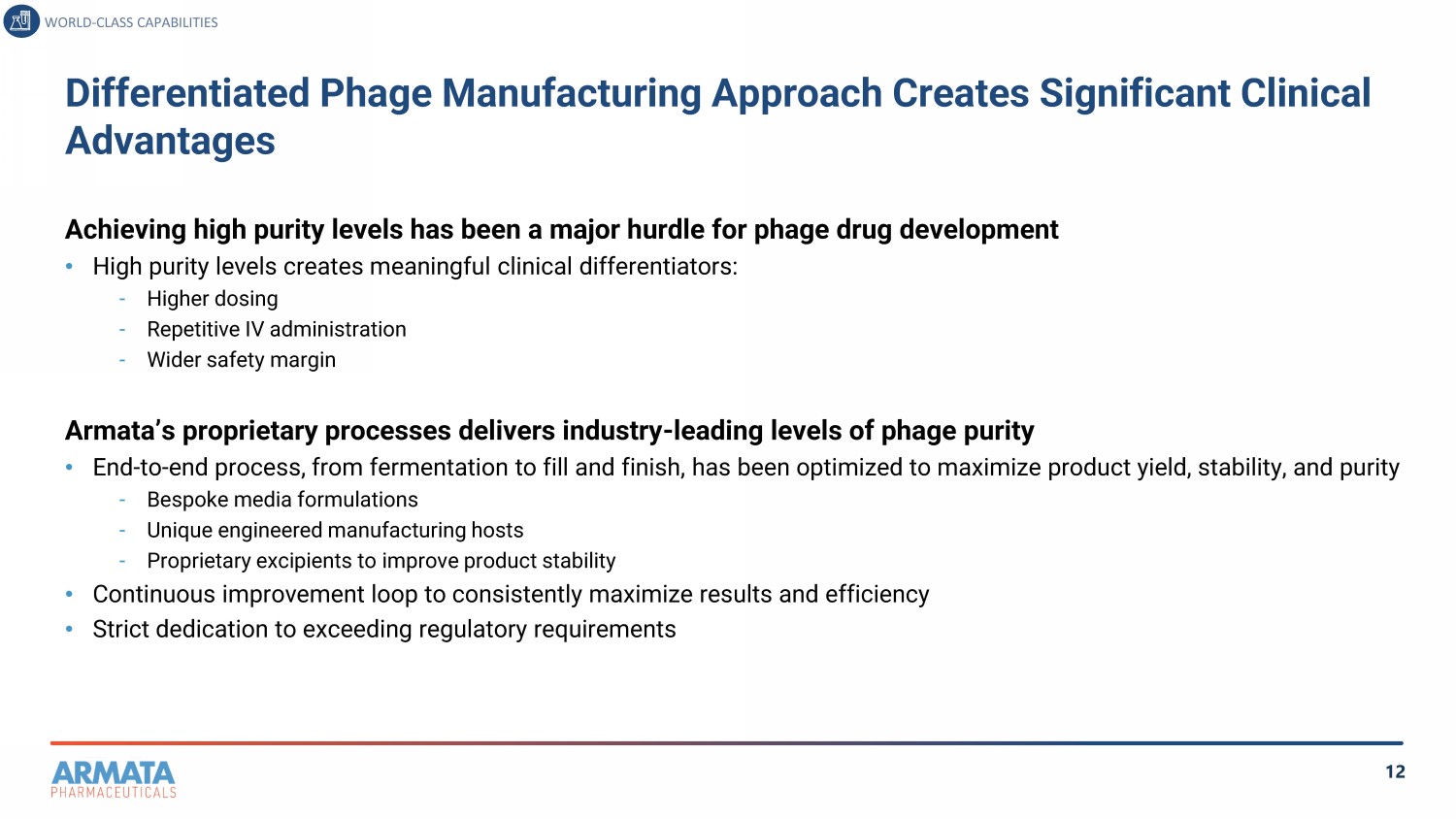
12 Differentiated Phage Manufacturing Approach Creates Significant Clinical Advantages WORLD - CLASS CAPABILITIES Achieving high purity levels has been a major hurdle for phage drug development • High purity levels creates meaningful clinical differentiators: - Higher dosing - Repetitive IV administration - Wider safety margin Armata’s proprietary processes delivers industry - leading levels of phage purity • End - to - end process, from fermentation to fill and finish, has been optimized to maximize product yield, stability, and purity - Bespoke media formulations - Unique engineered manufacturing hosts - Proprietary excipients to improve product stability • Continuous improvement loop to consistently maximize results and efficiency • Strict dedication to exceeding regulatory requirements

13 Leadership and Board of Directors Diverse Public Company Drug Development Expertise Deborah Birx, MD CEO Arwa Shurrab Regulatory Strategy and Operations Management Board of Directors Jules Haimovitz Robin Kramer, Chair Todd Peterson, PhD Deborah Birx, MD Odysseas Kostas, MD Joseph Patti, PhD Sarah Schlesinger, MD Peter Hubbard, MBA VP, Operations Suparna Mishra Sarkar, PhD VP, Quality David House SVP, Finance LEADERSHIP AND PARTNERS Pierre Kyme, PhD CBO
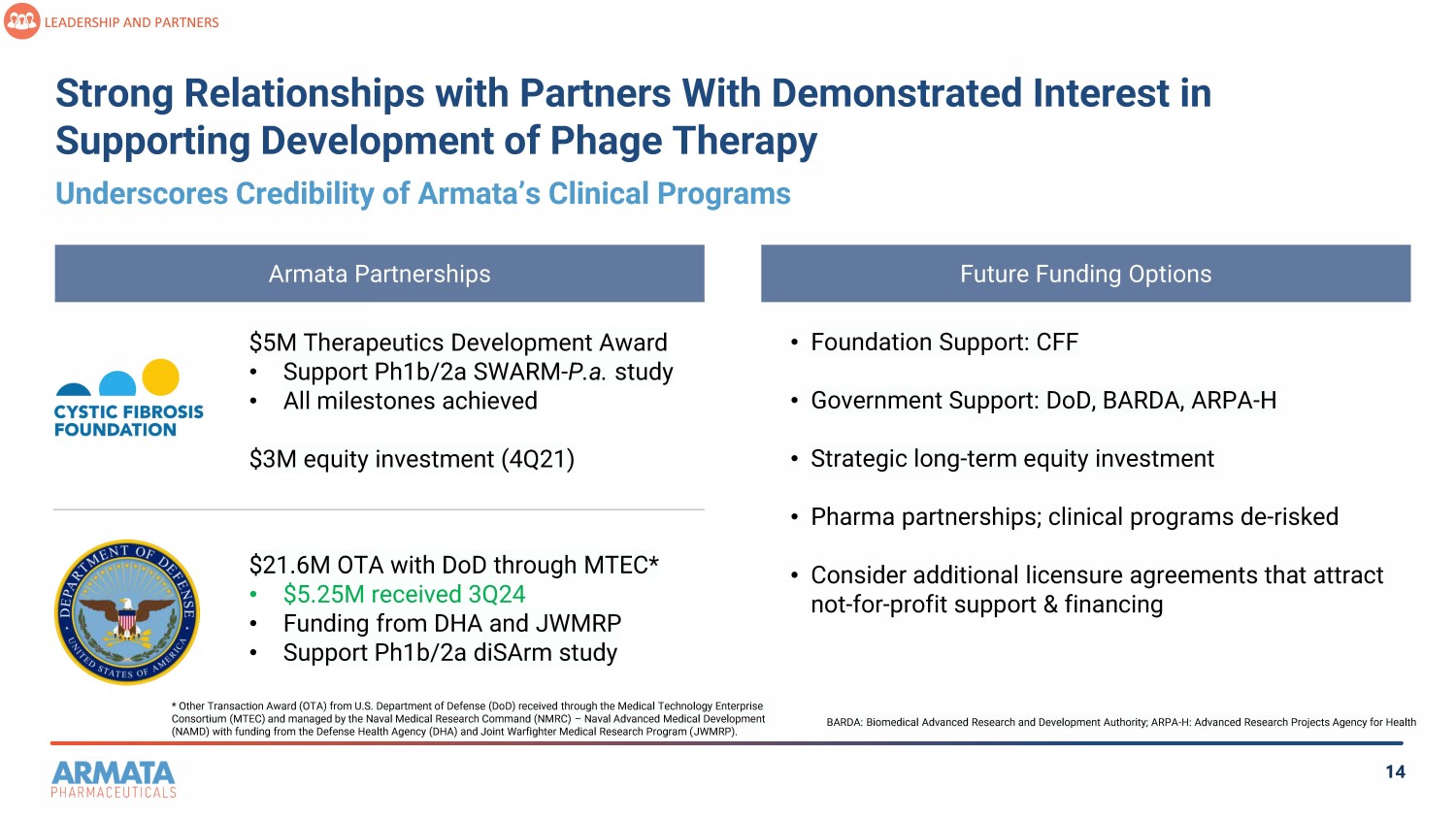
14 Strong Relationships with Partners With Demonstrated Interest in Supporting Development of Phage Therapy LEADERSHIP AND PARTNERS Armata Partnerships $5M Therapeutics Development Award • Support Ph1b/2a SWARM - P.a. study • All milestones achieved $3M equity investment (4Q21) $21.6M OTA with DoD through MTEC* • $5.25M received 3Q24 • Funding from DHA and JWMRP • Support Ph1b/2a diSArm study Future Funding Options • Foundation Support: CFF • Government Support: DoD, BARDA, ARPA - H • Strategic long - term equity investment • Pharma partnerships; clinical programs de - risked • Consider additional licensure agreements that attract not - for - profit support & financing Underscores Credibility of Armata’s Clinical Programs * Other Transaction Award (OTA) from U.S. Department of Defense (DoD) received through the Medical Technology Enterprise Consortium (MTEC) and managed by the Naval Medical Research Command (NMRC) – Naval Advanced Medical Development (NAMD) with funding from the Defense Health Agency (DHA) and Joint Warfighter Medical Research Program ( JWMRP). BARDA: Biomedical Advanced Research and Development Authority; ARPA - H: Advanced Research Projects Agency for Health
Cystic Fibrosis (CF) Non - CF Bronchiectasis (NCFB) Pseudomonas aeruginosa Program (AP - PA02)
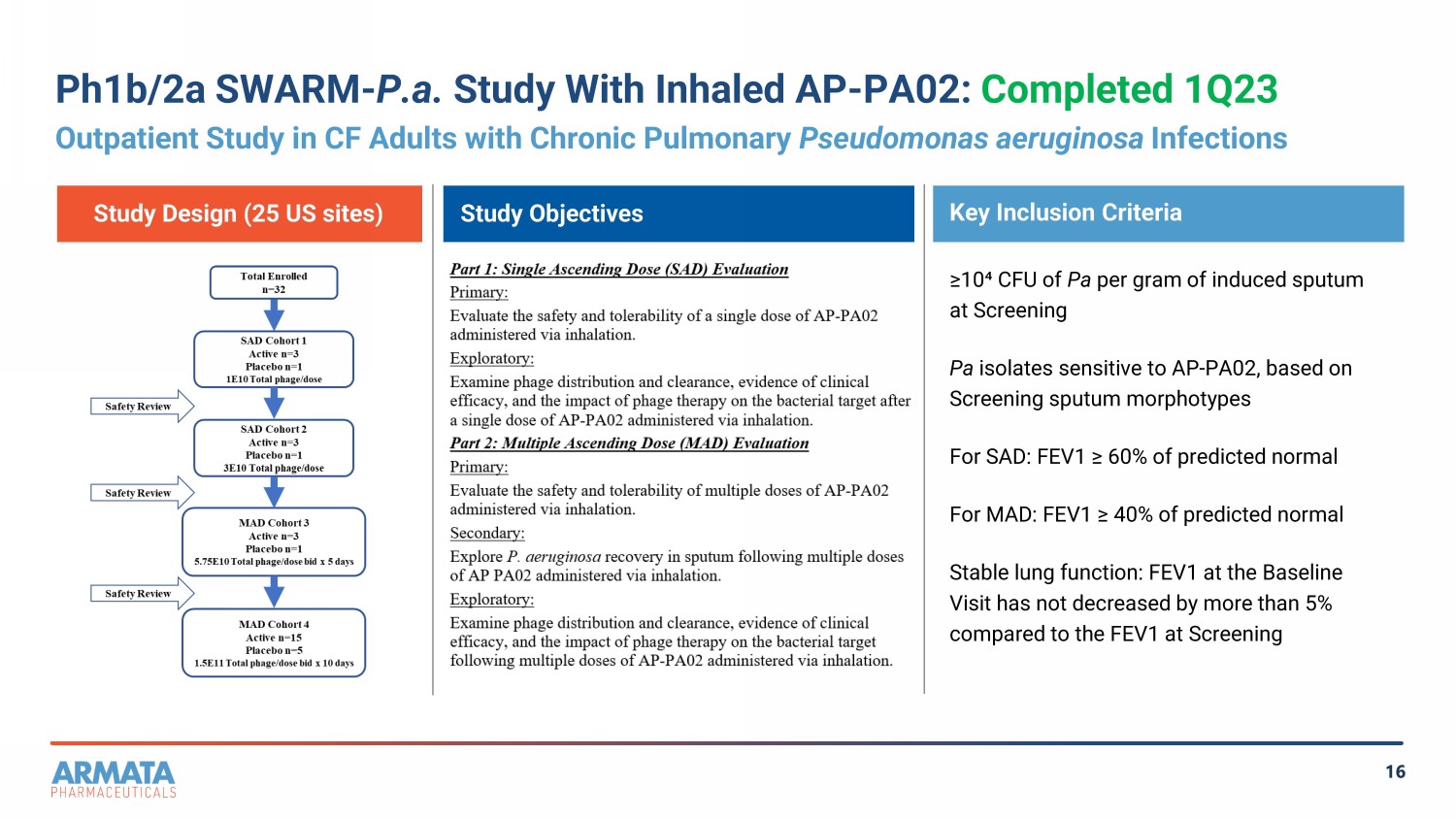
16 Ph1b/2a SWARM - P.a. Study With Inhaled AP - PA02: Completed 1Q23 Outpatient Study in CF Adults with Chronic Pulmonary Pseudomonas aeruginosa Infections Study Objectives Key Inclusion Criteria Study Design (25 US sites) ≥10⁴ CFU of Pa per gram of induced sputum at Screening Pa isolates sensitive to AP - PA02, based on Screening sputum morphotypes For SAD: FEV1 ≥ 60% of predicted normal For MAD: FEV1 ≥ 40% of predicted normal Stable lung function: FEV1 at the Baseline Visit has not decreased by more than 5% compared to the FEV1 at Screening
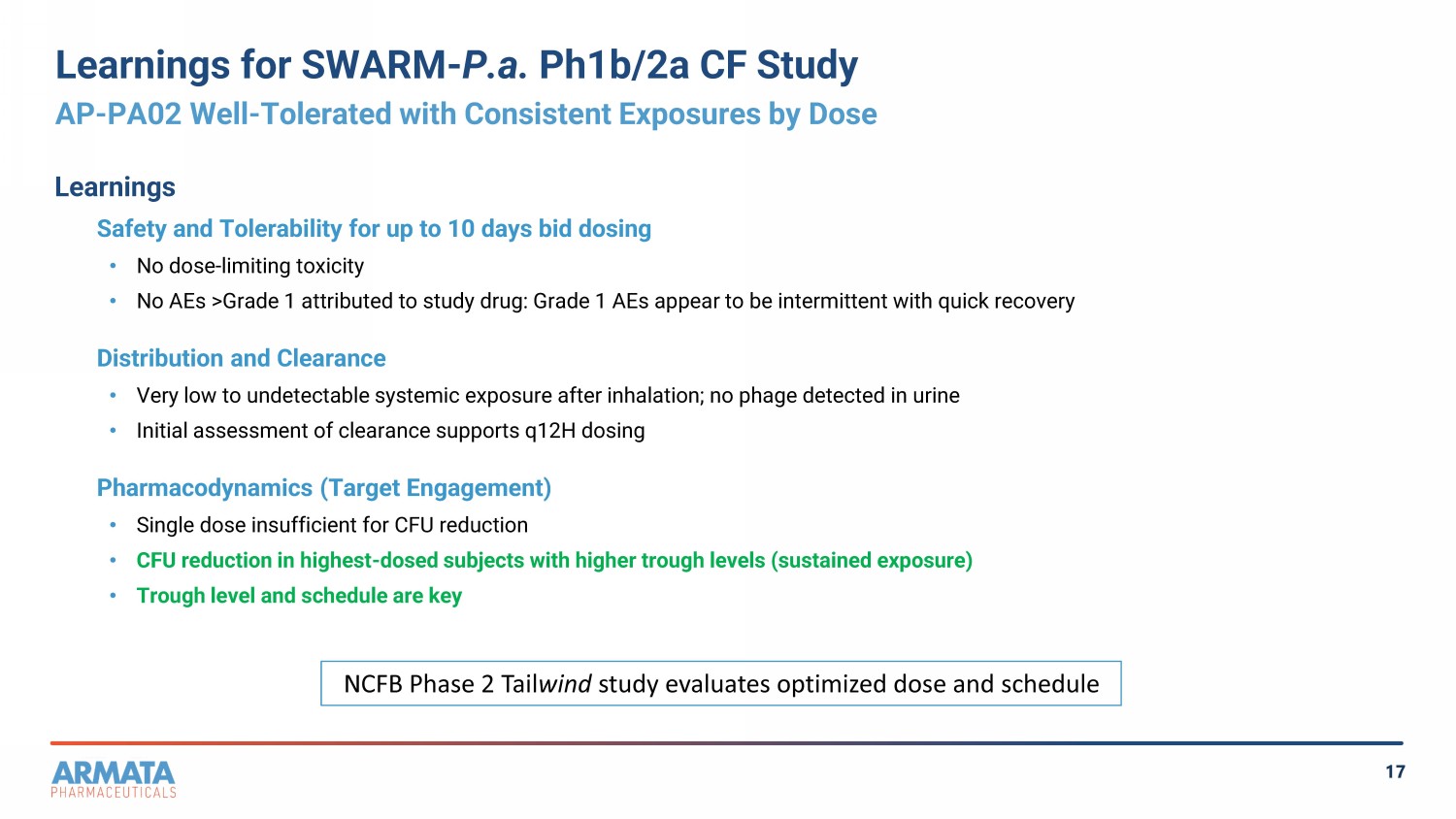
17 Learnings for SWARM - P.a. Ph1b/2a CF Study AP - PA02 Well - Tolerated with Consistent Exposures by Dose Learnings Safety and Tolerability for up to 10 days bid dosing • No dose - limiting toxicity • No AEs >Grade 1 attributed to study drug: Grade 1 AEs appear to be intermittent with quick recovery Distribution and Clearance • Very low to undetectable systemic exposure after inhalation; no phage detected in urine • Initial assessment of clearance supports q12H dosing Pharmacodynamics (Target Engagement) • Single dose insufficient for CFU reduction • CFU reduction in highest - dosed subjects with higher trough levels (sustained exposure) • Trough level and schedule are key NCFB Phase 2 Tail wind study evaluates optimized dose and schedule

* Data are preliminary and remains subject to further review and quality control; findings are subject to change pending complete data that will be presented in the future NCFB Phase 2 Trial Top Line Data*
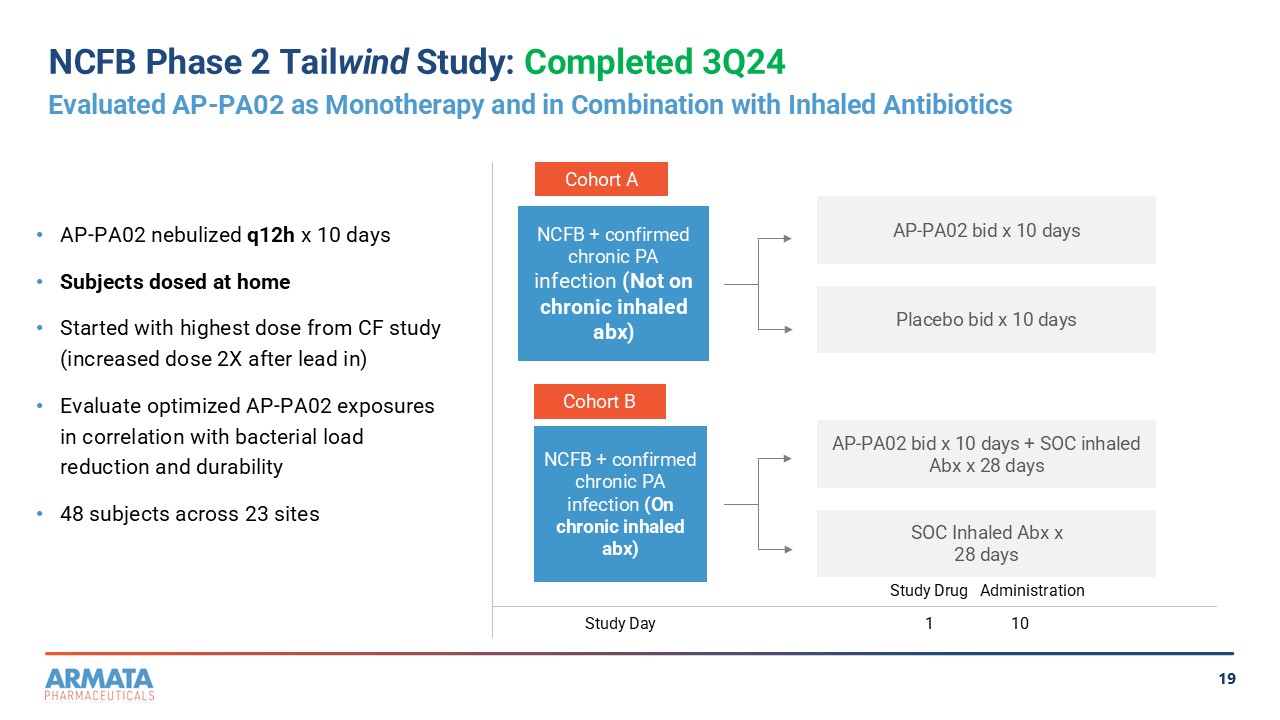
19 NCFB Phase 2 Tail wind Study: Completed 3Q23 Evaluated AP - PA02 as Monotherapy and in Combination with Inhaled Antibiotics • AP - PA02 nebulized q12h x 10 days • Subjects dosed at home • Started with highest dose from CF study (increased dose 2X after lead in) • Evaluate optimized AP - PA02 exposures in correlation with bacterial load reduction and durability • 48 subjects across 23 sites Cohort A Cohort B NCFB + confirmed chronic PA infection (Not on chronic inhaled abx) NCFB + confirmed chronic PA infection (On chronic inhaled abx) AP - PA02 bid x 10 days Placebo bid x 10 days AP - PA02 bid x 10 days + SOC inhaled Abx x 28 days SOC Inhaled Abx x 28 days Study Day Study Drug Administration 1 10
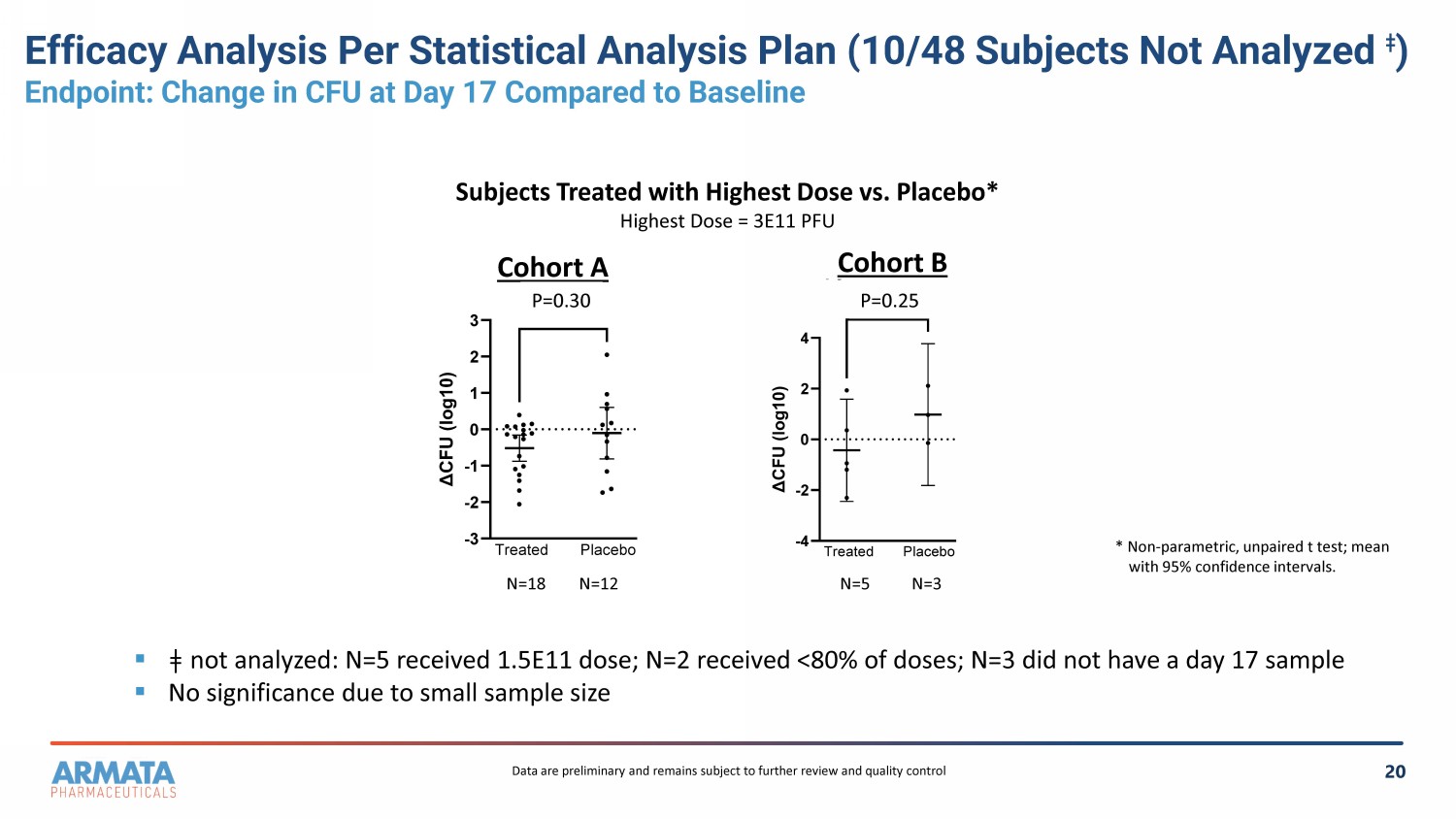
20 Efficacy Analysis Per Statistical Analysis Plan (10/48 Subjects Not Analyzed ǂ ) Endpoint: Change in CFU at Day 17 Compared to Baseline -4 -2 0 2 4 Copy of B T v P 3.0 Δ C F U ( l o g 1 0 ) 0.2500 Treated Placebo -3 -2 -1 0 1 2 3 Copy of A T v P 3.0 Δ C F U ( l o g 1 0 ) 0.3048 Treated Placebo Cohort A Cohort B Subjects Treated with Highest Dose vs. Placebo* Highest Dose = 3E11 PFU N=18 N=12 N=5 N=3 ▪ ǂ n ot analyzed: N=5 received 1.5E11 dose; N=2 received <80% of doses; N=3 did not have a day 17 sample ▪ No significance due to small sample size P=0.30 P=0.25 * Non - parametric, unpaired t test; mean with 95% confidence intervals. Data are preliminary and remains subject to further review and quality control
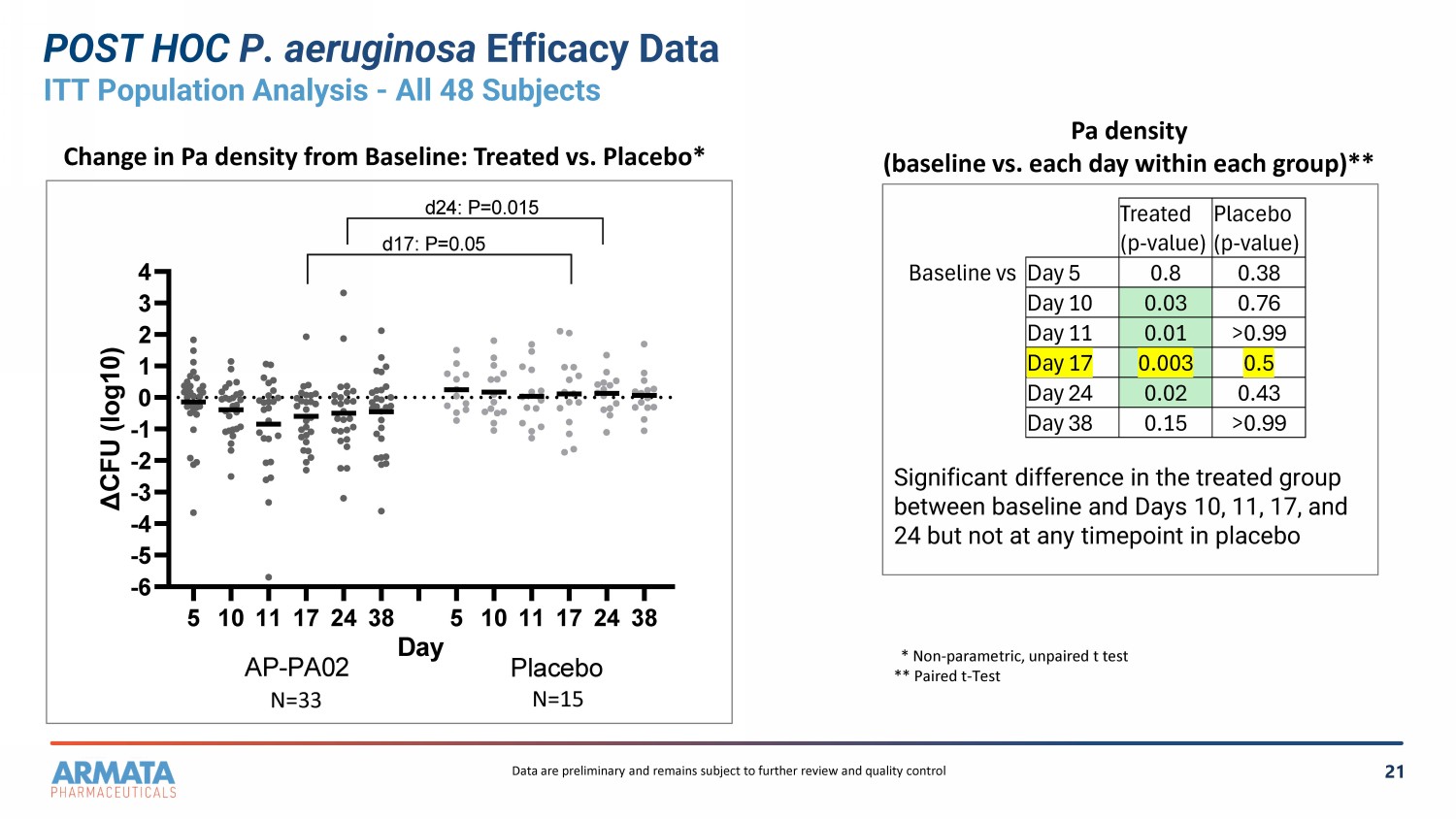
21 POST HOC P. aeruginosa Efficacy Data ITT Population Analysis - All 48 Subjects * Non - parametric, unpaired t test ** Paired t - Test Significant difference in the treated group between baseline and Days 10, 11, 17, and 24 but not at any timepoint in placebo Change in Pa density from Baseline: Treated vs. Placebo* Pa density (baseline vs. each day within each group)** Placebo (p - value) Treated (p - value) 0.38 0.8 Day 5 Baseline vs 0.76 0.03 Day 10 >0.99 0.01 Day 11 0.5 0.003 Day 17 0.43 0.02 Day 24 >0.99 0.15 Day 38 5 1011172438 5 1011172438 -6 -5 -4 -3 -2 -1 0 1 2 3 4 AP-PA02 (A +B) vs Placebo (A+ B) Day Δ C F U ( l o g 1 0 ) AP-PA02 Placebo d17: P=0.05 d24: P=0.015 N=33 N=15 Data are preliminary and remains subject to further review and quality control
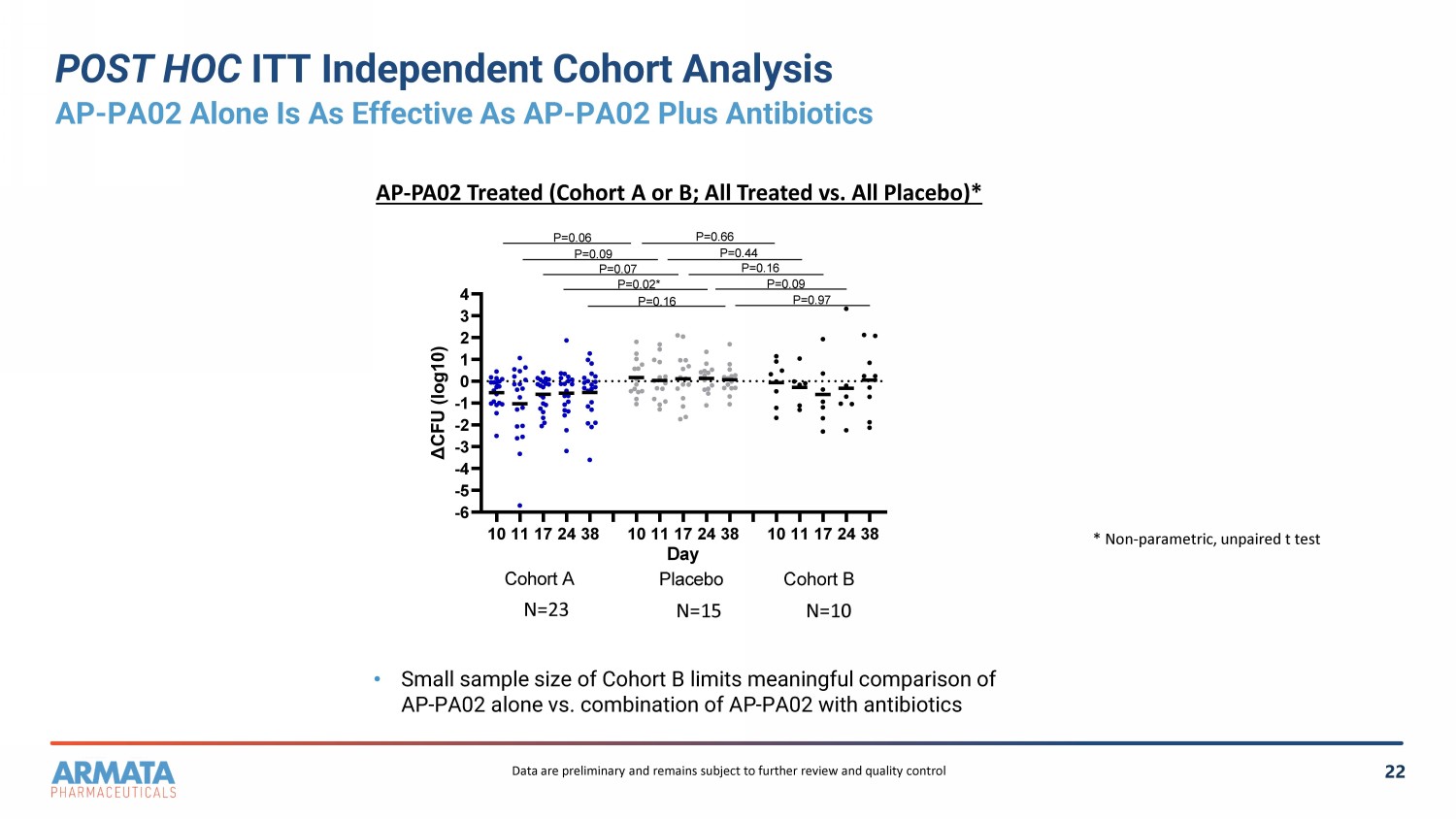
22 POST HOC ITT Independent Cohort Analysis AP - PA02 Alone Is As Effective As AP - PA02 Plus Antibiotics • Small sample size of Cohort B limits meaningful comparison of AP - PA02 alone vs. combination of AP - PA02 with antibiotics N=23 N=15 N=10 * Non - parametric, unpaired t test AP - PA02 Treated (Cohort A or B ; A ll Treated vs . All P lacebo )* 1011172438 1011172438 1011172438 -6 -5 -4 -3 -2 -1 0 1 2 3 4 Day Δ C F U ( l o g 1 0 ) Cohort A Placebo Cohort B P=0.97 P=0.09 P=0.16 P=0.16 P=0.07 P=0.02* P=0.09 P=0.06 P=0.44 P=0.66 Data are preliminary and remains subject to further review and quality control
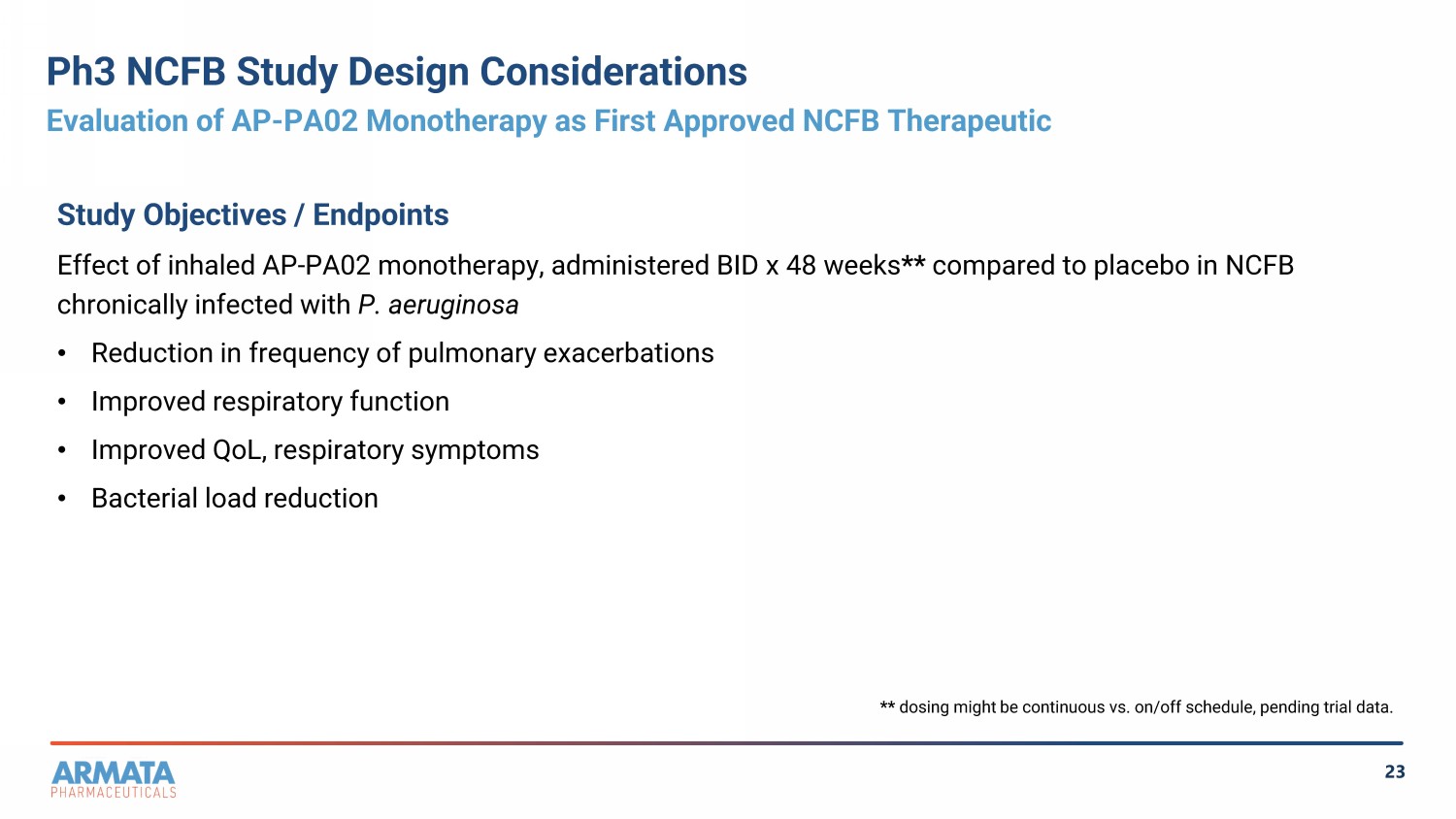
23 Ph3 NCFB Study Design Considerations Evaluation of AP - PA02 Monotherapy as First Approved NCFB Therapeutic Study Objectives / Endpoints Effect of inhaled AP - PA02 monotherapy, administered BID x 48 weeks ** compared to placebo in NCFB chronically infected with P. aeruginosa • Reduction in frequency of pulmonary exacerbations • Improved respiratory function • Improved QoL, respiratory symptoms • Bacterial load reduction ** dosing might be continuous vs. on/off schedule, pending trial data.
Complicated Bacteremia Prosthetic Joint Infection Staphylococcus aureus Program (AP - SA02)
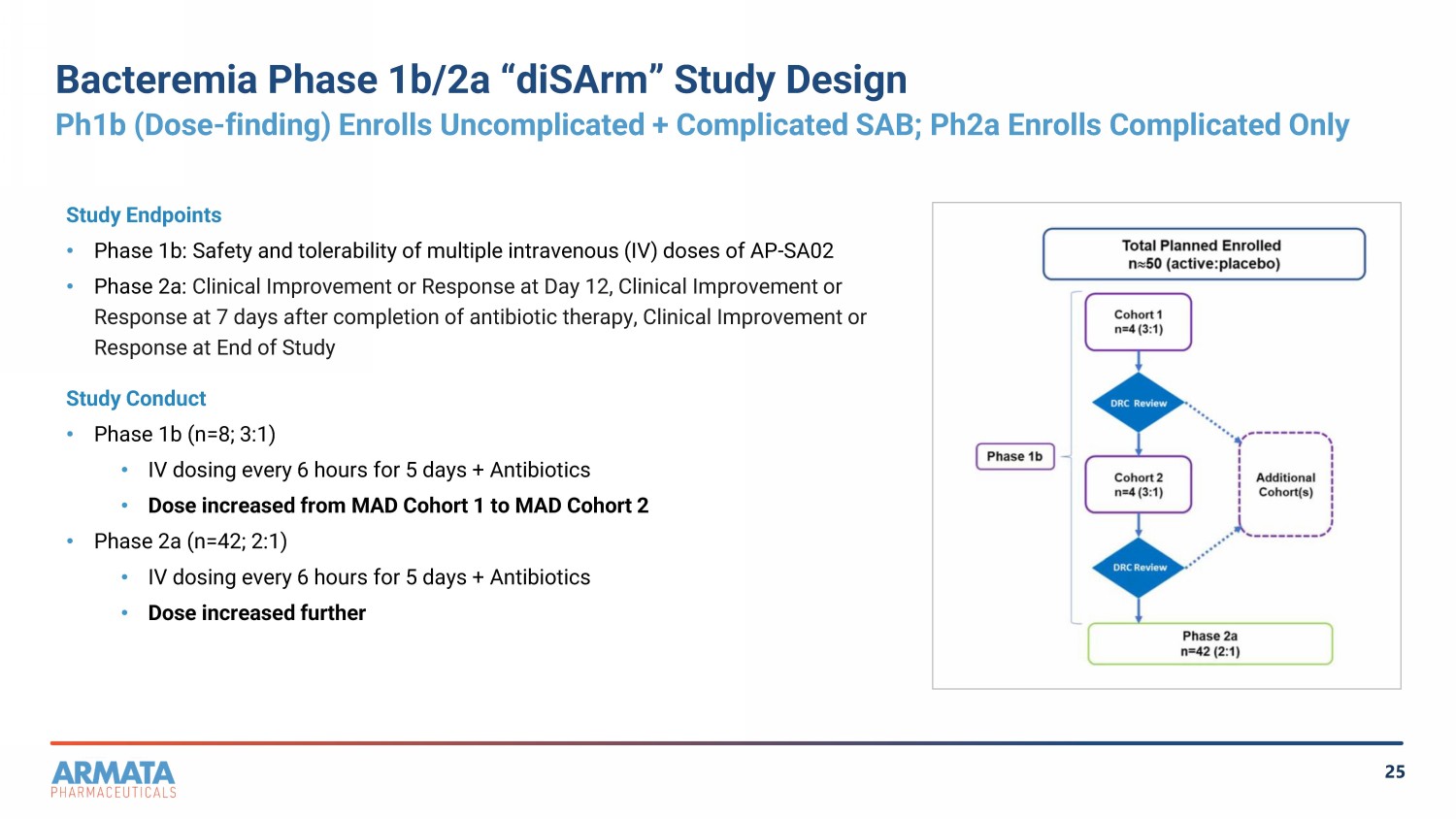
25 Bacteremia Phase 1b/2a “diSArm” Study Design Ph1b (Dose - finding) Enrolls Uncomplicated + Complicated SAB; Ph2a Enrolls Complicated Only Study Endpoints • Phase 1b: Safety and tolerability of multiple intravenous (IV) doses of AP - SA02 • Phase 2a: Clinical Improvement or Response at Day 12, Clinical Improvement or Response at 7 days after completion of antibiotic therapy, Clinical Improvement or Response at End of Study Study Conduct • Phase 1b (n=8; 3:1) • IV dosing every 6 hours for 5 days + Antibiotics • Dose increased from MAD Cohort 1 to MAD Cohort 2 • Phase 2a (n=42; 2:1) • IV dosing every 6 hours for 5 days + Antibiotics • Dose increased further
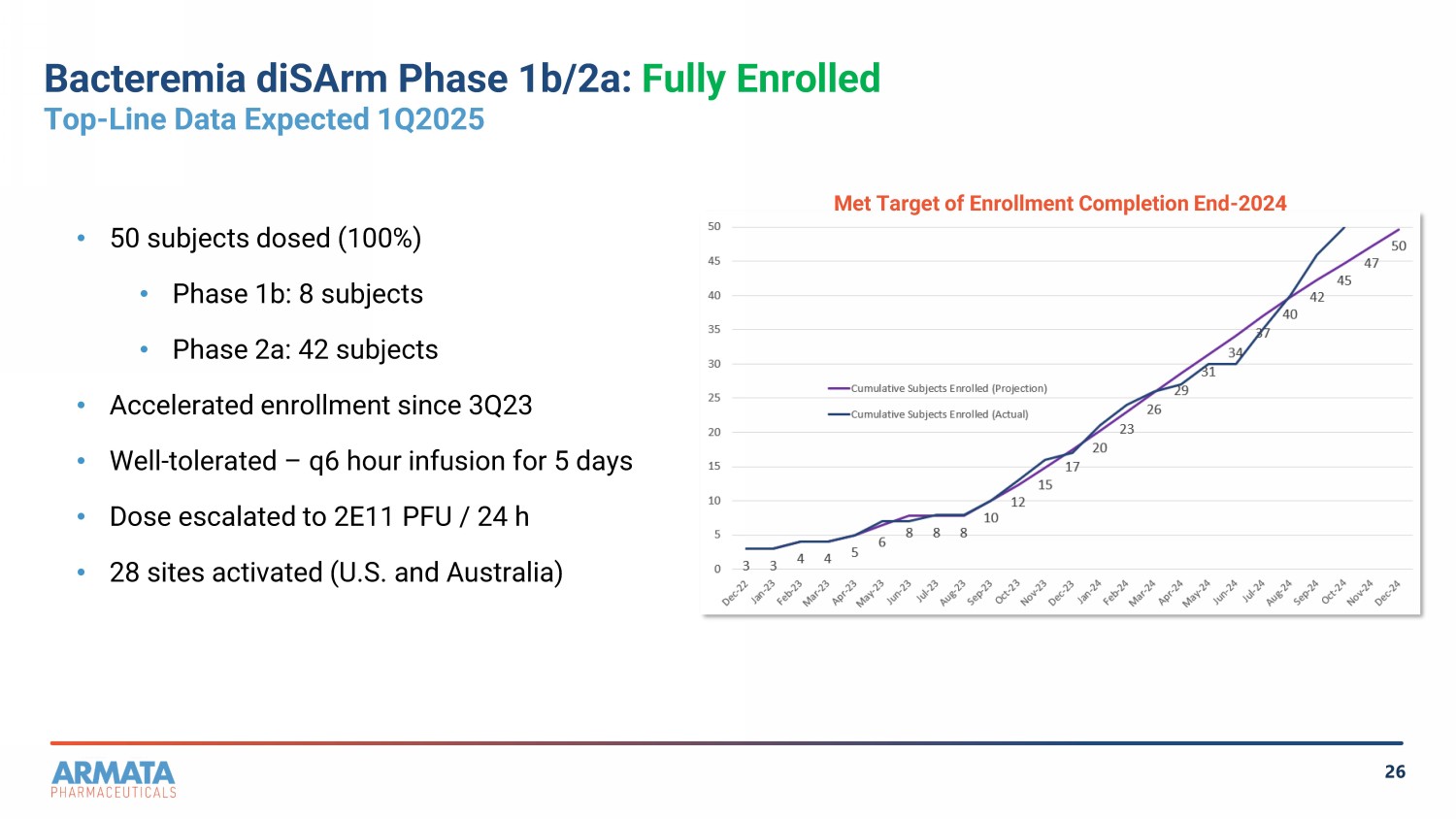
26 Bacteremia diSArm Phase 1b/2a: Fully Enrolled Top - Line Data Expected 1Q2025 • 50 subjects dosed (100%) • Phase 1b: 8 subjects • Phase 2a : 42 subjects • Accelerated enrollment since 3Q23 • Well - tolerated – q6 hour infusion for 5 days • Dose escalated to 2 E11 PFU / 24 h • 28 sites activated (U.S. and Australia) Met Target of Enrollment Completion End - 2024
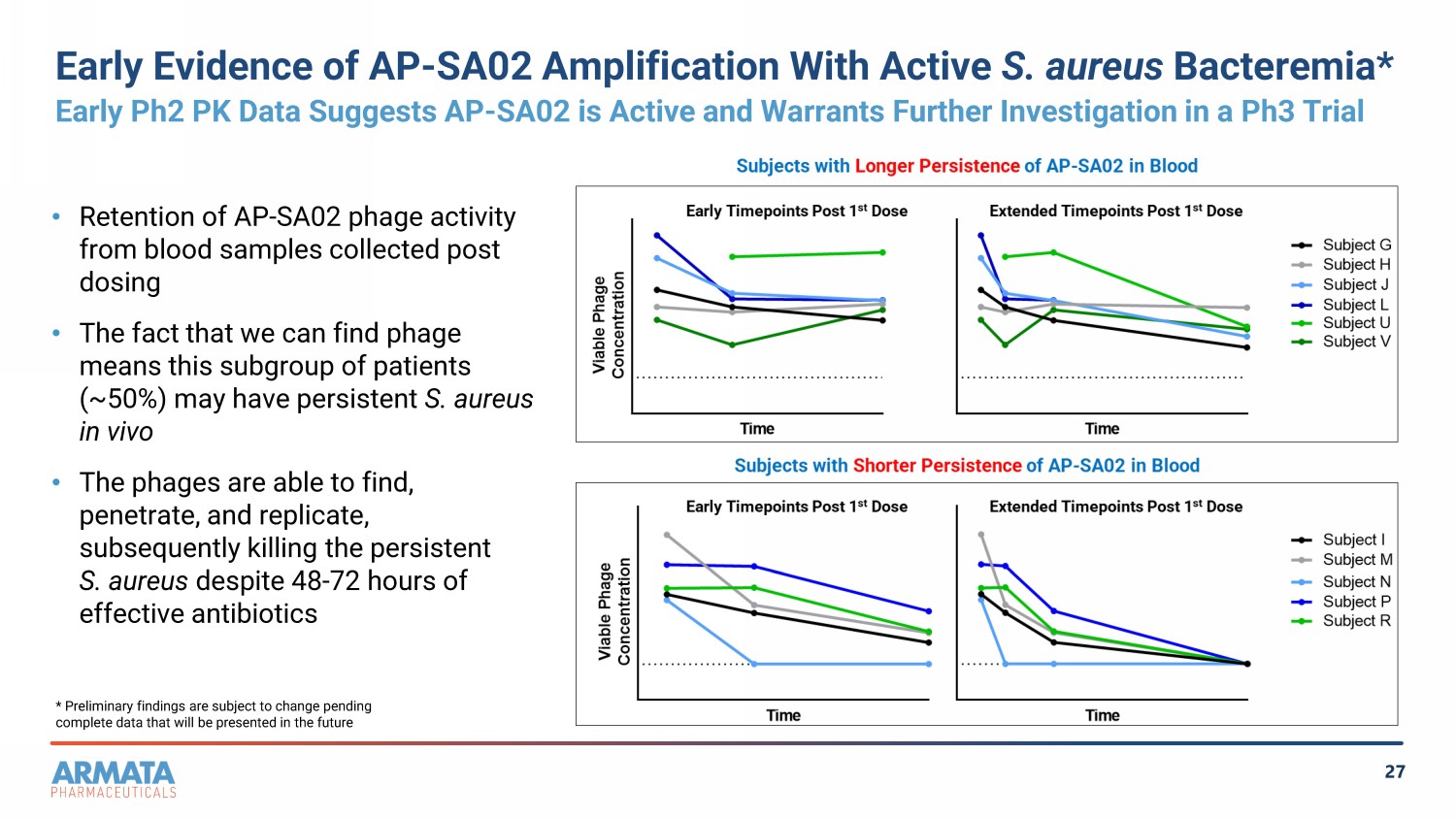
27 Early Evidence of AP - SA02 Amplification With Active S. aureus Bacteremia* Early Ph2 PK Data Suggests AP - SA02 is Active and Warrants Further Investigation in a Ph3 Trial • Retention of AP - SA02 phage activity from blood samples collected post dosing • The fact that we can find phage means this subgroup of patients (~50%) may have persistent S. aureus in vivo • The phages are able to find, penetrate, and replicate, subsequently killing the persistent S. aureus despite 48 - 72 hours of effective antibiotics * Preliminary findings are subject to change pending complete data that will be presented in the future
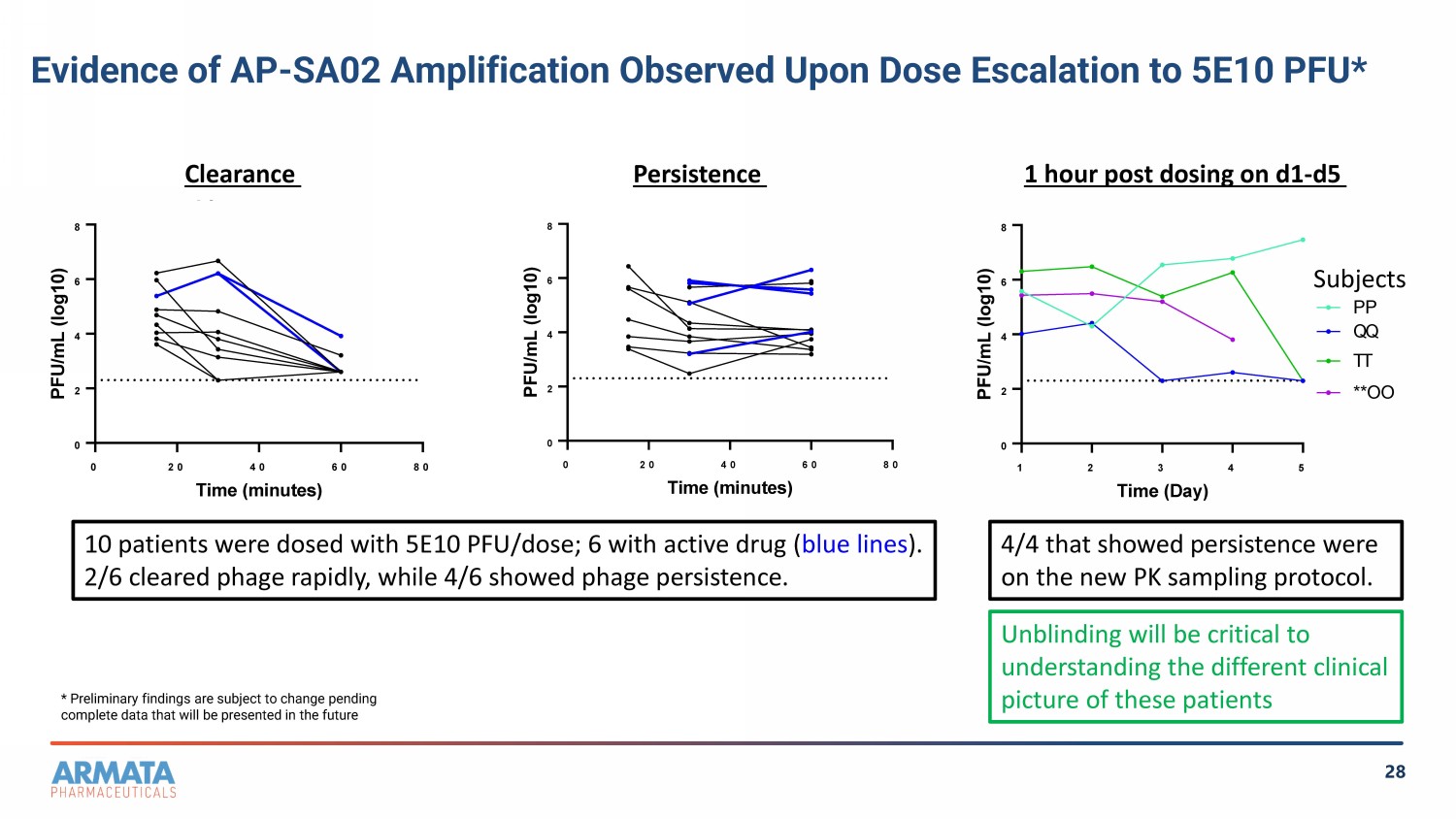
28 0 20 40 60 80 0 2 4 6 8 Copy of persis Time (minutes) P F U / m L ( l o g 1 0 ) Subject G Subject H Subject J Subject U Subject V Subject L Subject P Subject CC Subject S Subject X Subject PP (5E10) Subject TT(5E10) Subject QQ (5e10) Subject OO (5e10) 0 20 40 60 80 0 2 4 6 8 Copy of clearance Time (minutes) P F U / m L ( l o g 1 0 ) Subject I Subject N Subject R Subject M Subject P Subject X Subject Z Subject BB Subject MM (5E10) Subject LL (5E10) Subject EE Persistence Clearance Evidence of AP - SA02 Amplification Observed Upon Dose Escalation to 5E10 PFU* 1 hour post dosing on d1 - d5 Subjects 10 patients were dosed with 5E10 PFU/dose; 6 with active drug ( blue lines ). 2/6 cleared phage rapidly, while 4/6 showed phage persistence. * Preliminary findings are subject to change pending complete data that will be presented in the future 4/4 that showed persistence were on the new PK sampling protocol. Unblinding will be critical to understanding the different clinical picture of these patients
29 Ph3 SAB Study Design Considerations Non - Inferiority Antibiotic Sparing/Reducing Study for Uncomplicated and Complicated SAB Study Objectives/Endpoints • Primary Objective − Demonstrate non - inferiority of AP - SA02 to antibiotics in uncomplicated SAB − Demonstrate non - inferiority of 2 wks of AP - SA02 plus 2 - 3 wks of antibiotics to 4 - 6 wks of antibiotics in complicated SAB • Primary Endpoint: Overall success in mITT population • Patient alive at end of study • No new metastatic foci or complications • Resolution or improvement of clinical signs/symptoms • Two negative blood cultures for S. aureus
Corporate Summary

31 Funding and Capitalization Cash Position • $17.1 million unrestricted cash and cash equivalents at end 3Q24* Financing History • Q1 2020: $25M private placement of common stock and warrants with Innoviva, Inc. • Q1 2021: $20M private placement of common stock and warrants with Innoviva Strategic Opportunities LLC (a subsidiary of Innov iva Inc.) • Q3 2021: $7M private placement of common stock with two investors – Cystic Fibrosis Foundation ($3M) and Innoviva Strategic Opportunities LLC ($4M) • Q1 2022: $45M private placement of common stock and warrants with Innoviva Strategic Opportunities • January 2023: $30M secured convertible credit and security agreement with Innoviva Strategic Opportunities − Mandatory conversion into Armata common stock upon completion of a qualified equity financing − Maturity date: extended to January 10, 2026 • July 2023: $25M secured credit agreement with Innoviva Strategic Opportunities − Maturity date: extended to January 10, 2026 – Collateralized by all assets of Armata and its subsidiaries • March 2024: $35M secured credit agreement with Innoviva Strategic Opportunities – Maturity date: June 4, 2025 – Collateralized by all assets of Armata and its subsidiaries Capitalization • 36.2 million common shares outstanding at end 3Q24* *unaudited and subject to change
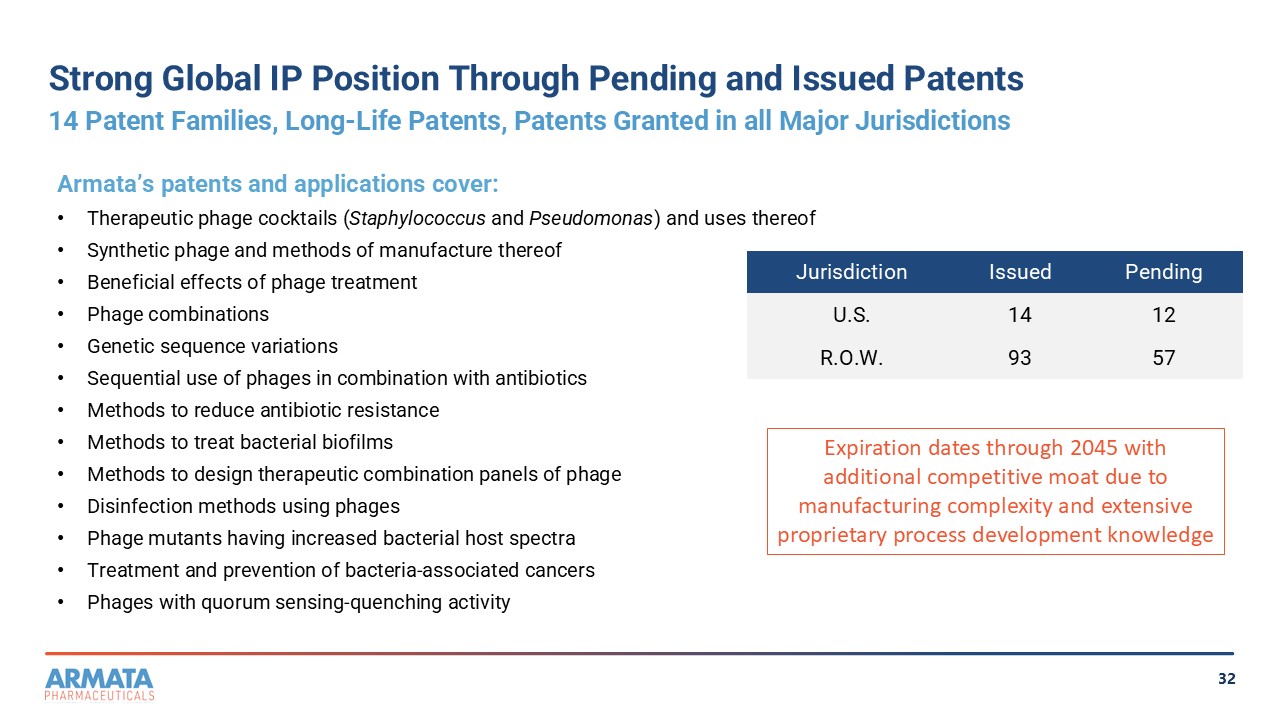
32 Strong Global IP Position Through Pending and Issued Patents 14 Patent Families, Long - Life Patents, Patents Granted in all Major Jurisdictions Expiration dates through 2045 with additional competitive moat due to manufacturing complexity and extensive proprietary process development knowledge Pending Issued Jurisdiction 12 14 U.S. 58 92 R.O.W. Armata’s patents and applications cover: • Therapeutic phage cocktails ( Staphylococcus and Pseudomonas ) and uses thereof • Synthetic phage and methods of manufacture thereof • Beneficial effects of phage treatment • Phage combinations • Genetic sequence variations • Sequential use of phages in combination with antibiotics • Methods to reduce antibiotic resistance • Methods to treat bacterial biofilms • Methods to design therapeutic combination panels of phage • Disinfection methods using phages • Phage mutants having increased bacterial host spectra • Treatment and prevention of bacteria - associated cancers • Phages with quorum sensing - quenching activity

33 Grateful to the patients, the research team and site investigators that make this work possible, and to our funders We are privileged to work across the United States and Australia in nearly 75 sites for the three Phase 2 trials We are effective due to top - notch employees that work at Armata everyday to ensure optimized engineered host/phage combinations to reliably produce highly purified phages at high titers meeting quality testing requirements and demonstrating biologic relevance We are grateful to the patient volunteers that understand the need for carefully controlled clinical trials to demonstrate the potential of new investigational drugs

www.armtapharma.com
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Armata Pharmaceuticals (AMEX:ARMP)
Historical Stock Chart
From Jan 2025 to Feb 2025
Armata Pharmaceuticals (AMEX:ARMP)
Historical Stock Chart
From Feb 2024 to Feb 2025