Nyxoah to Participate in the Oppenheimer 35th Annual Healthcare MedTech & Services Conference
March 03 2025 - 3:30PM
UK Regulatory
Nyxoah to Participate in the Oppenheimer 35th Annual Healthcare
MedTech & Services Conference
Nyxoah to
Participate in the
Oppenheimer
35th
Annual Healthcare
MedTech & Services
Conference
Mont-Saint-Guibert, Belgium – March 3,
2025, 10:30pm CET / 4:30pm ET – Nyxoah SA (Euronext
Brussels/Nasdaq: NYXH) (“Nyxoah” or the “Company”), that
develops breakthrough treatment alternatives for Obstructive Sleep
Apnea (OSA) through neuromodulation, today announced that the
Company will participate in the Oppenheimer 35th Annual
Healthcare MedTech & Services Conference, which takes place
March 17 – 20, 2025.
Olivier Taelman, Nyxoah’s Chief Executive
Officer, will deliver a corporate presentation on Monday, March 17,
2025, at 8:40am ET. A webcast of the presentation will be available
in the Events section of Nyxoah’s Investor Relations website. The
Company will be available for 1x1 meetings with institutional
investors.
Nyxoah’s Investor Presentation can be accessed
on the Shareholder Information section of the Company’s
Investor Relations page.
About
Nyxoah
Nyxoah is reinventing sleep for the billion people that suffer from
obstructive sleep apnea (OSA). We are a medical technology company
that develops breakthrough treatment alternatives for OSA through
neuromodulation. Our first innovation is Genio®, a battery-free
hypoglossal neuromodulation device that is inserted through a
single incision under the chin and controlled by a wearable.
Through our commitment to innovation and clinical evidence, we have
shown best-in-class outcomes for reducing OSA burden.
Following the successful completion of the BLAST
OSA study, the Genio® system received its European CE Mark in 2019.
Nyxoah completed two successful IPOs: on Euronext Brussels in
September 2020 and NASDAQ in July 2021. Following the positive
outcomes of the BETTER SLEEP study, Nyxoah received CE mark
approval for the expansion of its therapeutic indications to
Complete Concentric Collapse (CCC) patients, currently
contraindicated in competitors’ therapy. Additionally, the Company
announced positive outcomes from the DREAM IDE pivotal study for
FDA and U.S. commercialization approval.
For more information, please visit
http://www.nyxoah.com/.
Caution – CE marked since 2019. Investigational
device in the United States. Limited by U.S. federal law to
investigational use in the United States.
Contacts:
Nyxoah
John Landry, CFO
IR@nyxoah.com
- ENGLISH_Oppenheimer Press Release_FINAL


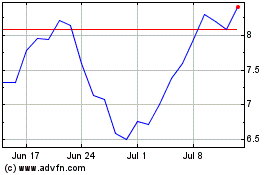
Nyxoah (EU:NYXH)
Historical Stock Chart
From Feb 2025 to Mar 2025
Nyxoah (EU:NYXH)
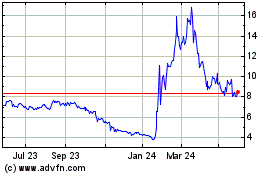
Historical Stock Chart
From Mar 2024 to Mar 2025