Ocular Therapeutix™ Reports Inducement Grant Under Nasdaq Listing Rule 5635(c)(4)
March 07 2025 - 3:05PM

Ocular Therapeutix, Inc. (NASDAQ: OCUL, “Ocular”), a
biopharmaceutical company committed to redefining the retina
experience, today announced that it has granted inducement awards
to one newly hired employee. The awards were made as inducements
material to the individual’s acceptance of employment with Ocular
under Ocular’s 2019 Inducement Stock Incentive Plan in accordance
with Nasdaq Listing Rule 5635(c)(4).
The inducement equity awards were granted effective
as of March 3, 2025 and consist of (i) a non-statutory stock option
to purchase up to 48,000 shares of Ocular’s common stock at a per
share exercise price equal to the closing price of Ocular’s common
stock on The Nasdaq Global Market on the effective date of grant,
and (ii) a restricted stock unit award representing the right to
receive 15,500 shares of Ocular’s common stock. The stock option
has a ten-year term and is scheduled to vest over four years, with
25% of the original number of shares vesting on March 3, 2026 and
the remainder vesting in equal monthly installments over the three
years thereafter, subject to the individual’s continued service to
Ocular through the applicable vesting dates. The restricted stock
unit award is scheduled to vest over three years, in equal annual
installments, with the first annual installment vesting on March 3,
2026, and subject to the recipient’s continued service to Ocular
through the applicable vesting dates.
The inducement equity awards are subject to the
terms and conditions of the award agreements covering the grants
and Ocular’s 2019 Inducement Stock Incentive Plan.
About Ocular Therapeutix, Inc.Ocular
Therapeutix, Inc. is a biopharmaceutical company committed to
redefining the retina experience. AXPAXLI™ (axitinib intravitreal
hydrogel, also known as OTX-TKI), Ocular’s product candidate for
retinal disease, is based on its ELUTYX™ proprietary bioresorbable
hydrogel-based formulation technology. AXPAXLI is currently in
Phase 3 clinical trials for wet age-related macular degeneration
(wet AMD).
Ocular’s pipeline also leverages the ELUTYX
technology in its commercial product DEXTENZA®, an FDA-approved
corticosteroid for the treatment of ocular inflammation and pain
following ophthalmic surgery and ocular itching associated with
allergic conjunctivitis, and in its product candidate PAXTRAVA™
(travoprost intracameral hydrogel or OTX-TIC), which is currently
in a Phase 2 clinical trial for the treatment of open-angle
glaucoma or ocular hypertension.
Follow the Company on its website, LinkedIn, or
X.
The Ocular Therapeutix logo and DEXTENZA® are
registered trademarks of Ocular Therapeutix, Inc. AXPAXLI™,
PAXTRAVA™, ELUTYX™, and Ocular Therapeutix™ are trademarks of
Ocular Therapeutix, Inc.
Investors & MediaOcular
Therapeutix, Inc.Bill SlatteryVice President, Investor
Relationsbslattery@ocutx.com
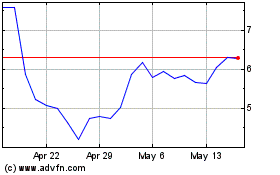
Ocular Therapeutix (NASDAQ:OCUL)
Historical Stock Chart
From Feb 2025 to Mar 2025
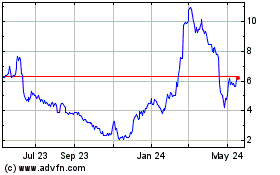
Ocular Therapeutix (NASDAQ:OCUL)
Historical Stock Chart
From Mar 2024 to Mar 2025