Form 6-K - Report of foreign issuer [Rules 13a-16 and 15d-16]
February 06 2025 - 5:02AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of February, 2025
Commission File No. 001-39621
OPTHEA LIMITED
(Translation of registrant’s name into English)
Level 4
650 Chapel Street
South Yarra, Victoria, 3141
Australia
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
|
|
OPTHEA LIMITED |
|
(Registrant) |
|
|
|
|
By: |
/s/ Frederic Guerard |
|
Name: |
Frederic Guerard |
|
Title: |
Chief Executive Officer |
Date: 02/06/2025
Exhibit 99.1

ASX, Nasdaq and Media Release
February 6, 2025
Opthea Wet AMD Data Featured at Macula Society Meeting
Melbourne, Australia, and Princeton, NJ, US, February 6, 2025 -- Opthea Limited (ASX/NASDAQ:OPT, “Opthea”, the “Company”), a clinical-stage biopharmaceutical company developing novel therapies to treat highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD), today announced that sozinibercept will be highlighted during an oral presentation at the Macula Society 48th Annual Meeting being held February 12-15, 2025 in Charlotte Harbor, Florida. The Macula Society is a forum for new research in retinal vascular and macular diseases.
Details are as follows:
Session: Neovascular AMD I: Trials
Timing: Friday, February 14, 2025, 8:00 to 8:05 AM ET
Presentation: Sozinibercept Combination Therapy for Neovascular Age-Related Macular Degeneration: Subgroup Analysis of a Phase 2b Study to Assess the Angiographic Predictors of Response
Presenter: David S. Boyer, MD
Program: https://www.xcdsystem.com/maculasociety/program/5G2onFx/index.cfm?pgid=397&RunRemoveSessionFilter=1
David S. Boyer, MD will present data from the sozinibercept Phase 2b wet AMD trial demonstrating that superior vision and anatomic outcomes were observed with sozinibercept 2 mg combination therapy, compared with standard-of-care ranibizumab alone, with baseline angiographic lesion characteristics predictive of response.
About Opthea
Opthea (ASX/NASDAQ:OPT) is a biopharmaceutical company developing novel therapies to address the unmet needs in the treatment of highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME).
Opthea’s lead product candidate, sozinibercept, is being evaluated in two fully enrolled pivotal Phase 3 clinical trials (COAST, NCT04757636, and ShORe, NCT04757610) for use in combination with standard-of-care anti-VEGF-A therapies to improve overall efficacy and deliver superior vision gains compared to standard-of-care anti-VEGF-A agents alone.
To learn more, visit our website at www.opthea.com and follow us on X and LinkedIn.
Authorized for release to ASX by Frederic Guerard, PharmD, CEO
|
|
Investor Inquiries |
Join our email database to receive program updates: |
PJ Kelleher LifeSci Advisors LLC Email: pkelleher@lifesciadvisors.com Phone: 617-430 7579 Media Inquiries Silvana Guerci-Lena NorthStream Global Partners Email:silvana@nsgpllc.com |
Tel: +61 (0) 3 9826 0399 Email: info@opthea.com Web: www.opthea.com Source: Opthea Limited |
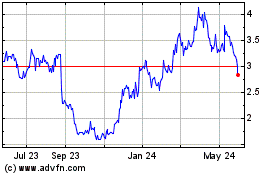
Opthea (NASDAQ:OPT)
Historical Stock Chart
From Jan 2025 to Feb 2025
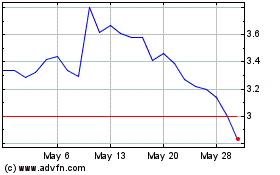
Opthea (NASDAQ:OPT)
Historical Stock Chart
From Feb 2024 to Feb 2025