Antibe Announces Unfavorable Decision in Arbitration With Nuance Pharma
March 04 2024 - 6:00AM
Business Wire
Antibe Therapeutics Inc. (TSX: ATE, OTCQX: ATBPF), a
clinical-stage biotechnology company leveraging its hydrogen
sulfide platform to target pain and inflammation, today announced
that the arbitrator has found in favor of Nuance Pharma in the
dispute regarding the license agreement for the commercialization
of otenaproxesul in the Greater China region. The confidential
ruling from the Singapore International Arbitration Centre rescinds
the license agreement and requires Antibe to refund the US$20
million upfront payment and pay interest and costs of approximately
US$4 million. The decision is not subject to appeal. Antibe views
this unexpected result as highly unusual based on best practices
for licensing deals in the biotech industry.
“We strongly disagree with this decision,” commented Dan
Legault, Antibe’s CEO. “Nonetheless, we acknowledge the fact that
we agreed to binding arbitration in a foreign jurisdiction. Antibe
respects the confidentiality and final nature of the arbitration
proceedings and will accept the decision in good faith. We continue
to believe that the comprehensive data package shared with Nuance
for the previous chronic pain formulation fully reflected the
drug’s safety and efficacy characteristics. Although this ruling
presents a significant short-term challenge, we remain committed to
developing otenaproxesul -- particularly due to the strength of
recent clinical results for acute pain that have significantly
de-risked its final trials.”
In light of the arbitral ruling, the Company is evaluating its
development and milestone plans for the balance of 2024, which may
include adjusting timelines as circumstances require. The strong
data from the recent PK/PD study highlight otenaproxesul’s
potential and the priority remains conducting the Phase II trial as
soon as possible. More information will be shared as the Company
determines the best path forward for all stakeholders.
About Antibe Therapeutics Inc.
Antibe is a clinical-stage biotechnology company leveraging its
proprietary hydrogen sulfide platform to develop next-generation
therapies to target pain and inflammation arising from a wide range
of medical conditions. The Company’s current pipeline includes
assets that seek to overcome the gastrointestinal ulcers and
bleeding associated with nonsteroidal anti-inflammatory drugs
(“NSAIDs”). Antibe’s lead drug, otenaproxesul, is in clinical
development as a safer alternative to opioids and today’s NSAIDs
for acute pain. Antibe’s second pipeline drug, ATB-352, is being
developed for a specialized pain indication. The Company’s next
target is inflammatory bowel disease (“IBD”), a condition long in
need of safer, more effective therapies. Learn more at
antibethera.com.
Forward Looking Statements
This news release includes certain forward-looking statements
under applicable securities laws, which may include, but are not
limited to, statements concerning the payment of amounts due to
Nuance under the arbitral award, the anticipated scope, timing,
duration and completion of certain of the Company’s pre-clinical
and clinical trial programs and studies and the anticipated timing
for seeking market approval for certain of the Company’s drugs and
therapies for certain additional indications. Any statements
contained herein that are not statements of historical facts may be
deemed to be forward-looking, including those identified by the
expressions “will”, “anticipate”, “believe”, “plan”, “estimate”,
“expect”, “intend”, “propose” and similar wording. Forward-looking
statements involve known and unknown risks and uncertainties that
could cause actual results, performance, or achievements to differ
materially from those expressed or implied in this news release.
Factors that could cause actual results to differ materially from
those anticipated in this news release include, but are not limited
to, the Company’s inability to timely execute on its business
strategy and timely and successfully complete its clinical trials
and studies, the Company’s inability to obtain the necessary
regulatory approvals related to its activities, risks associated
with drug development generally and those risk factors set forth in
the Company’s public filings made in Canada and available on
sedarplus.com. The Company assumes no obligation to update the
forward-looking statements or to update the reasons why actual
results could differ from those reflected in the forward-looking
statements except as required by applicable law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240304390845/en/
Antibe Therapeutics Inc. Christina Cameron VP Investor Relations
+1 416-577-1443 christina@antibethera.com
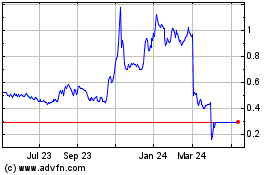
Antibe Therapeutics (TSX:ATE)
Historical Stock Chart
From Nov 2024 to Dec 2024
Antibe Therapeutics (TSX:ATE)
Historical Stock Chart
From Dec 2023 to Dec 2024