GammaCan Announces Appointment of Richard Spritz, M.D. to Scientific Advisory Board; Leading Geneticist and Pediatric Specialis
September 05 2006 - 6:30AM
Business Wire
GammaCan International (OTCBB: GCAN) today announced the
appointment of Richard Spritz, M.D. to the Company's Scientific
Advisory Board. Bringing over thirty years of medical and
scientific acumen to GammaCan, and specifically to the Company's
mission of bringing VitiGam to the clinic, Dr. Spritz is a member
of the Medical Advisory Board of Vitiligo Support International and
a Council Member of PanAmerican Pigment Cell Society. "We are
extremely pleased that Richard has joined our Scientific Advisory
Board and are honored by his participation. Richard's work on the
identification and cloning of genes involved in the pathogenesis
vitiligo is groundbreaking and undoubtedly will lead to new
insights into this and other immune-mediated disorders. We both
recognized the connection between our respective research efforts,
and the entire Team at GammaCan is exited to work with Richard as
we develop VitiGam to treat stage III and IV melanoma," stated
Patrick Schnegelsberg, CEO. Dr. Spritz is presently Director Human
Medical Genetics Program and Professor of Pediatrics, Biochemistry
and Molecular Genetics at the University of Colorado Health Science
Center. Prior to his tenure at the University of Colorado, Dr.
Spritz served as Assistant and Associate Professor of Medical
Genetics and Pediatrics at the University of Wisconsin. Among his
numerous accomplishments, Dr. Spritz sits on the Medical Advisory
Board of Vitiligo Support International, is a member of The
Council, PanAmerican Pigment Cell Society, Chaired a number of NIH
Committees, and has for many years served on National Research
Advisory Committees for the March of Dimes Birth Defects
Foundation. He has been published over 170 peer-reviewed papers and
receives ongoing research support from the NIH, specifically in the
genetics of human pigmentation and autoimmune disorders. Richard
Spritz holds an M.D. from Pennsylvania State University and was a
Fellow in Human Genetics at the Yale University School of Medicine.
"I am delighted to be associated with GammaCan, and look forward to
contributing to the company's efforts, to develop VitiGam. I
believe this has the potential to be an important therapeutic to
help patients suffering from advanced melanoma," said Spritz. About
VitiGam: VitiGam is GammaCan's second generation intravenous
IgG-based product and a first-in-class anti-cancer immunotherapy.
GammaCan plans on having VitiGam to enter phase I/II testing under
a US IND in the near future after it recently held a pre-IND
meeting with the FDA. VitiGam is being designed to target
metastatic melanoma patients with Stage III and IV melanoma.
VitiGam is an IgG product that is different from standard IgGs: It
is manufactured from the plasma of donors with vitiligo, a benign
autoimmune skin condition affecting up to 2% of the general
population. GammaCan scientists have shown that this "enriched"
vitiligo IgG (VitiGam) contains potent anti-melanoma activity in
both in vitro and mouse xenograft melanoma models. Thus, GammaCan
expects VitiGam to provide (1) anti-melanoma activity directed
specifically against malignant melanoma cells and (2) non-specific
anti-cancer activity - as is the case with IgG in general. About
GammaCan: GammaCan is a biopharmaceutical company focused on
clinical-phase development and commercialization. GammaCan's
initial therapy under development is a first-in-class anti-cancer
immunotherapy aimed at preventing metastasis (the spread of cancer
to other parts of the body) of a variety of cancers. GammaCan's
first generation product (GCAN101) is currently completing phase II
- clinical trials. For more information about GammaCan visit
www.GammaCan.com or call the company's headquarters in Kiryat Ono,
Israel at +972 (03) 738-2616 or toll free 1-866-308-0396 (from
North America). Safe Harbor Statement: Statements in this document
that are not purely historical are forward-looking statements.
Forward-looking statements in this release include statements
regarding the commercialization of an anti-cancer immunotherapy and
the Company developing the boosting of cancer patients' immune
systems with IVIg into an effective treatment. Actual outcomes and
the Company's actual results could differ materially from those in
such forward-looking statements. Factors that could cause actual
results to differ materially include risks and uncertainties such
as the inability to finance the planned development of the
technology, unforeseen technical difficulties in developing the
technology, the inability to obtain regulatory approval for human
use, competitors' therapies proving more effective, cheaper or
otherwise preferable for consumers, inability to market the product
we produce, among other factors, all of which could among other
things, delay or prevent product release or cause our company to
fail. For further risk factors see the risk factors associated with
other early state medical research and development companies filed
with the SEC on Edgar.
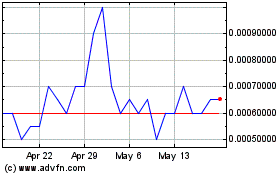
Greater Cannabis (PK) (USOTC:GCAN)
Historical Stock Chart
From Jun 2024 to Jul 2024
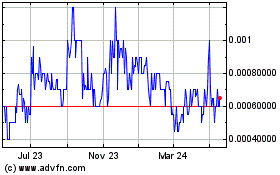
Greater Cannabis (PK) (USOTC:GCAN)
Historical Stock Chart
From Jul 2023 to Jul 2024