TIDMGSK
RNS Number : 7647G
GlaxoSmithKline PLC
28 July 2021
Issued: Wednesday, 28 July 2021, London U.K.
GSK delivers strong Q2 sales of GBP8.1 billion, +6% AER, +15% CER
Total EPS 27.9p -39% AER, -28% CER; Adjusted EPS 28.1p +46% AER +71%
CER
Highlights
Sales growth driven by strong commercial execution and favourable
prior year comparison
-- Pharmaceuticals GBP4.2 billion +3% AER, +12% CER with growth in
New and Specialty products (+25% CER) including Respiratory +36%
CER, Immuno-Inflammation +46% CER, Oncology +69% CER, total HIV
+14% CER
-- Vaccines GBP1.6 billion +39% AER, +49% CER reflecting strong growth
in Meningitis +46% CER, Established Vaccines +28% CER, Shingrix
+1% CER with improved performance notably in the US and GBP258
million pandemic adjuvant sales. Continue to expect strong growth
from Shingrix in H2
-- Consumer Healthcare GBP2.3 billion -4% AER, +3% CER (+7% CER excluding
divestments/brands under review)
Effective cost control supports delivery of adjusted earnings per
share growth
-- Total Group operating margin 20.7%. Total EPS 27.9p -39% AER, -28%
CER
-- Adjusted Group operating margin 26.7%. Adjusted EPS 28.1p +46%
AER, +71% CER (H1 -10% AER, +2% CER). This included a contribution
to growth from COVID-19 solutions of approximately +20% AER, +21%
CER in Q2 (+7% AER, +7% CER in H1)
-- Q2 net cash flow from operations GBP1.3 billion. Free cash flow
GBP316 million
Continued R&D delivery and strengthening of pipeline
-- FDA rolling review of cabotegravir for prevention of HIV (PrEP)
completed
-- Positive phase III headline results for daprodustat, potential
transformative medicine for anaemia due to chronic kidney disease
-- 3 new strategic collaborations announced, iTeos, Alector* and Halozyme
strengthen pipeline in next generation immuno-oncology, immuno-neurology
and HIV
-- Emergency use authorisations for sotrovimab; Phase III started
for Sanofi-GSK adjuvanted COVID-19 vaccine and EMA rolling review
initiated
Investor Update in June outlined new outlooks for growth and plans
to maximise shareholder value
-- GSK expects to deliver step-change in sales, operating profit growth
and performance from 2022, driven by high quality Vaccines and
Specialty Medicines portfolio and late-stage pipeline
-- Proposed demerger to create new world-leading Consumer Healthcare
company confirmed for mid-2022
Confident in delivering 2021 EPS guidance and reconfirm 2022 outlook
-- 2021 Adjusted EPS to decline by mid-to-high single-digit percentage
at CER
-- 2022 meaningful improvements expected in revenues and margins
-- 2021 guidance and 2022 outlook exclude any contribution from COVID-19
solutions
Dividend of 19p/share declared for Q2 2021. Continue to expect 80p/share
for 2021
Emma Walmsley, Chief Executive Officer, GSK said: "GSK delivered
an excellent performance in Q2. We expect this positive momentum
to continue through the second half of the year driving us towards
the better end of our earnings guidance range for 2021, and meaningful
performance improvement in 2022. We continue to strengthen our pipeline
and are advancing well towards separation. Our clear priority is
to focus on execution, unlocking the value of Consumer Healthcare
and delivering the step-change in growth and performance we now see
for GSK."
The Total results are presented in summary on page 2 and under 'Financial
performance' on pages 12 and 27 and Adjusted results reconciliations
are presented on pages 23 , 24, 38 and 39. Adjusted results are a
non-IFRS measure that may be considered in addition to, but not as
a substitute for, or superior to, information presented in accordance
with IFRS. Adjusted results are defined on page 10 and GBP% or AER%
growth, CER% growth, free cash flow and other non-IFRS measures are
defined on page 67, COVID-19 solutions are also defined on page 67.
GSK provides guidance on an Adjusted results basis only, for the
reasons set out on page 11. All expectations, guidance and targets
regarding future performance and dividend payments should be read
together with 'Outlook, assumptions and cautionary statements' on
pages 68 and 69. * Subject to HSR clearance.
Q2 2021 results
Q2 2021 Growth H1 2021 Growth
------------ ------------
GBPm GBP% CER% GBPm GBP% CER%
-------- ----- ----- -------- ----- -----
Turnover 8,092 6 15 15,510 (7) (1)
Total operating profit 1,675 (41) (30) 3,368 (31) (21)
Total earnings per
share 27.9p (39) (28) 49.4p (36) (27)
Adjusted operating
profit 2,158 23 43 4,039 (9) 3
Adjusted earnings per
share 28.1p 46 71 51.0p (10) 2
Net cash from operating
activities 1,292 (53) 1,623 (56)
Free cash flow 316 (84) 313 (87)
2021 guidance
We reconfirm our guidance range for 2021 for a decline of mid to
high-single digit percent Adjusted EPS at CER, excluding any contribution
from COVID-19 solutions.
In 2021, as planned we will continue to increase investment in our
pipeline, build on our top-line momentum for key growth drivers and
largely complete readiness for separation. Assuming healthcare systems
and consumer trends approach normality in the second half of the
year, we continue to expect Pharmaceutical revenue to grow flat to
low-single digits at CER and Consumer Healthcare revenue to grow
low to mid-single digits at CER (excluding brands divested/under
review) with above market growth. For our Vaccines business, as noted
at the time of announcing full-year 2020 results, we anticipated
disruption during the first half of the year, given governments'
prioritisation of COVID-19 vaccination programmes and ongoing measures
to contain the pandemic. This was expected to impact adult and adolescent
immunisations, including Shingrix, notably in the US and this is
reflected in our first-half year 2021 Vaccines performance. We are
encouraged by the rate at which COVID-19 vaccinations are being deployed
in many countries, particularly the US and UK, which provides support
for healthcare systems returning to normal, though we are seeing
global differentiation in the pace of deployment in other major markets.
There remains, however, uncertainty as to the impact of COVID-19,
the speed of deployment of mass immunisation programmes and easing
of pandemic conditions. In the second half of the year we continue
to expect strong recovery and contribution to growth but, with Shingrix
sales recovering more slowly in ex-US markets, we now expect Vaccines
revenue for 2021 to be broadly flat. We remain confident in the underlying
demand for our Vaccine products.
Our strong Q2 2021 performance gives us confidence that, providing
we continue to see improving demand for adult vaccinations through
the balance of 2021, as well as healthcare systems and consumer trends
approaching normality, we are likely to deliver full-year Adjusted
EPS towards the better end of our guidance range which is for a decline
of mid-to-high single-digit percentage at CER excluding any contribution
from COVID-19 solutions.
2021 COVID-19 solutions expectations
In H1 2021, we had COVID-19 solution sales of GBP276 million including
GBP260 million of pandemic vaccines of which GBP258 million were
pandemic adjuvant sales and GBP16 million of the treatment sotrovimab.
The contribution to H1 Adjusted EPS was approximately 7%. For the
full year, we expect that the COVID-19 solutions will contribute
approximately between 4% to 6% of Adjusted EPS growth. The outcome
within that range is dependent upon the success of sotrovimab contracting
for 2021, and of pandemic adjuvant contracting for 2022 and the resulting
potential charges within COGS as we continue to manufacture for this
potential.
All expectations, guidance and targets regarding future performance
and dividend payments should be read together with 'Outlook, assumptions
and cautionary statements' on pages 68 and 69. If exchange rates
were to hold at the closing rates on 30 June 2021 ($1.39/GBP1, EUR1.17/GBP1
and Yen 153/GBP1) for the rest of 2021, the estimated negative impact
on 2021 Sterling turnover growth would be 5% and if exchange gains
or losses were recognised at the same level as in 2020, the estimated
negative impact on 2021 Sterling Adjusted EPS growth would be around
10%.
Results presentation
A webcast of the quarterly results presentation hosted by Emma Walmsley,
GSK CEO, will be held at 2pm BST on 28 July 2021. Presentation materials
will be published on www.gsk.com prior to the webcast and a transcript
of the webcast will be published subsequently.
Information available on GSK's website does not form part of, and
is not incorporated by reference into, this Results Announcement.
Operating performance - Q2 2021
Turnover Q2 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Pharmaceuticals 4,229 3 12
Vaccines 1,571 39 49
Consumer Healthcare 2,292 (4) 3
------ ------- -------
Group turnover 8,092 6 15
------ ------- -------
Group turnover was GBP8,092 million in the quarter, up 6% AER, 15%
CER.
Pharmaceuticals turnover in the quarter was GBP4,229 million, up
3% AER, 12% CER. The quarter results show significant growth over
the same quarter last year, driven by strong growth in New and Specialty
products, favourable US RAR adjustments and a prior year comparator
that was impacted by destocking of COVID-19 related first quarter
additional demand.
Vaccines turnover grew 39% AER, 49% CER to GBP1,571 million, primarily
driven by pandemic adjuvant sales, higher demand for DTPa-containing
vaccines in the US and higher demand for Bexsero in the US and in
Europe. Vaccines turnover excluding pandemic vaccines grew 16% AER,
24% CER to GBP1,311 million.
Consumer Healthcare turnover declined 4% AER, but increased 3% CER
to GBP2,292 million. Sales excluding brands divested/under review
declined 1% AER but increased 7% CER supported by a favourable comparative
in Q2 2020 as a result of destocking including the reversal of the
benefit of the accelerated purchases in the first quarter in 2020
across all categories as a result of the COVID-19 pandemic.
Operating profit
Total operating profit was GBP1,675 million in Q2 2021 compared
with GBP2,850 million in Q2 2020. The total operating margin was
20.7%. This decrease in Total operating profit primarily reflected
the net profit on disposal of the Horlicks and other Consumer brands
of GBP2,304 million in the prior period partly offset by the related
loss on sale of the shares in Hindustan Unilever of GBP476 million.
Adjusted operating profit was GBP2,158 million, 23% higher than
Q2 2020 at AER, 43% higher at CER on a turnover increase of 15%
CER. The Adjusted operating margin of 26.7% was 3.7 percentage points
higher at AER, and 5.6 percentage points higher on a CER basis than
in Q2 2020. The increase in Adjusted operating profit primarily
reflected leverage from a favourable comparison to destocking in
Q2 2020 in Pharmaceuticals and Consumer Healthcare, GBP258 million
of pandemic adjuvant sales, increased demand for Meningitis and
DTPa-containing Vaccines, a favourable prior period RAR adjustment
in Pharmaceuticals, continued tight control of ongoing costs and
benefits from continued restructuring. This was partly offset by
increased investment behind launches and increased investment in
R&D.
Earnings per share
Total EPS was 27.9p, compared with 45.5p in Q2 2020. This primarily
reflected an unfavourable comparison to net profit on disposal in
Q2 2020 of the Horlicks and other Consumer brands partly offset
by the related loss on sale of the shares in Hindustan Unilever.
In Q2 2021 a credit of GBP325 million to Taxation was recorded resulting
from the revaluation of deferred tax assets following enactment
of the proposed change of UK corporation tax rate from 19% to 25%
(effective 2023).
Adjusted EPS was 28.1p compared with 19.2p in Q2 2020, up 46% AER,
71% CER, on a 43% CER increase in Adjusted operating profit reflecting
sales increases in Pharmaceuticals and Consumer Healthcare including
a benefit from destocking in Q2 2020 and increased Vaccines sales
including pandemic adjuvant sales, lower interest costs, a lower
effective tax rate and a lower non-controlling interest allocation
of Consumer Healthcare and ViiV profits. The contribution to growth
from COVID-19 solutions was approximately 20% AER, 21% CER.
Cash flow
The net cash inflow from operating activities for the quarter was
GBP1,292 million (Q2 2020: GBP2,760 million). Free cash inflow was
GBP316 million for the quarter (Q2 2020: GBP1,949 million inflow).
The decrease primarily reflected an adverse comparison to the significant
reduction in trade receivables in Q2 2020 as a result of collections
following strong sales in Q1 2020, adverse timing of returns and
rebates and taxes compared to Q2 2020, increased purchases of intangible
assets and reduced proceeds from disposal of intangible assets as
the Consumer Brands Disposal programme is now complete. This was
partly offset by increased operating profit, a lower seasonal increase
in inventory and lower dividends to non-controlling interests.
Operating performance - H1 2021
Turnover H1 2021
-------------------------
Growth Growth
GBPm GBP% CER%
------- ------- -------
Pharmaceuticals 8,111 (5) 2
Vaccines 2,795 (5) -
Consumer Healthcare 4,604 (12) (7)
------- ------- -------
Group turnover 15,510 (7) (1)
Group turnover was GBP15,510 million in the six months, down 7%
AER, 1% CER.
Pharmaceuticals turnover in the six months was GBP8,111 million,
down 5% AER but up 2% CER, with growth from Respiratory and HIV
partly offset by declines in Established Pharmaceuticals. In the
first half of last year, the additional COVID-19 related demand
experienced towards the end of the first quarter was broadly reversed
in the second quarter. In the current year, the market environment
continues to be impacted by COVID-19, impacting our Established
Pharmaceuticals products in International and Europe regions.
Vaccines turnover declined 5% AER, but was flat at CER to GBP2,795
million, primarily driven by pandemic adjuvant sales, offset by
the adverse impact of the COVID-19 pandemic on Shingrix. Vaccines
turnover excluding pandemic vaccines declined 14% AER, 9% CER .
Consumer Healthcare turnover for the six months declined 12% AER,
7% CER to GBP4,604 million largely driven by the divestment programme
which completed in Q1 2021 as well as the H1 2020 comparative including
a particularly strong first quarter given accelerated purchasing
due to the COVID-19 pandemic.
Operating profit
Total operating profit was GBP3,368 million in H1 2021 compared
with GBP4,864 million in H1 2020. The total operating margin was
21.7%. The decrease in total operating profit primarily reflected
an unfavourable comparison to the net profit on disposal in Q2 2020
of the Horlicks and other Consumer brands and resultant sale of
shares in Hindustan Unilever.
Adjusted operating profit was GBP4,039 million, 9% lower than H1
2020 at AER, but 3% higher at CER on a turnover decline of 1% CER.
The Adjusted operating margin of 26.0% was 0.4 percentage points
lower at AER, but 1.1 percentage points higher on a CER basis than
in H1 2020. The increase in Adjusted operating profit primarily
reflected a benefit from incremental pandemic adjuvant sales, sales
growth in Pharmaceuticals and tight control of ongoing costs including
reduced promotional and variable spending across all three businesses
as a result of the COVID-19 lockdowns, favourable legal settlements
compared to increased legal costs in 2020 and benefits from continued
restructuring across the Group. This was partly offset by an adverse
mix in Vaccines as well as higher supply chain costs and under-recoveries,
divestments in Consumer Healthcare and increased investment in R&D
across Vaccines and Pharmaceuticals.
Earnings per share
Total EPS was 49.4p, compared with 77.0p in H1 2020. This primarily
reflected an unfavourable comparison to the net profit on disposal
in Q2 2020 of the Horlicks and other Consumer brands partly offset
by the related loss on sale of the shares in Hindustan Unilever.
These factors were partly offset by lower major restructuring costs
and lower re-measurement charges on the contingent consideration
liabilities. In Q2 2021 a credit of GBP325 million to Taxation was
recorded resulting from the revaluation of deferred tax assets following
enactment of the proposed change of the UK corporation tax rate
from 19% to 25% (effective 2023).
Adjusted EPS was 51.0p compared with 56.9p in H1 2020, down 10%
AER but up 2% CER, on a 3% CER increase in Adjusted operating profit
reflecting incremental pandemic adjuvant sales, sales increases
in Pharmaceuticals, tight cost control and favourable legal settlements,
lower interest costs, a lower non-controlling interest allocation
of Consumer Healthcare and ViiV profits, partly offset by lower
non-pandemic sales in Vaccines, primarily Shingrix and a higher
effective tax rate. The contribution to growth from COVID-19 solutions
was approximately 7% AER, 7% CER.
Cash flow
The net cash inflow from operating activities for the six months
was GBP1,623 million (H1 2020: GBP3,725 million). Free cash inflow
was GBP313 million for the six months (H1 2020: GBP2,480 million
inflow). The decrease primarily reflected adverse exchange impacts,
reduction in trade receivables in H1 2020 as a result of collections
following strong sales in Q1 2020, adverse timing of returns and
rebates and taxes compared to Q2 2020 and increased inventory, increased
purchases of intangible assets and reduced proceeds from disposal
of intangible assets as the Consumer Brands Disposal programme is
now complete. This was partly offset by increased operating profit
and lower dividends to non-controlling interests.
R&D pipeline
We focus on the science of the immune system, human genetics and
advanced technologies to develop Vaccines and Specialty Medicines
in four core therapeutic areas - Infectious Diseases, HIV, Oncology
and Immunology/Respiratory. We also remain open to opportunities
outside these core therapy areas where there are scale opportunities
consistent with the science of the immune system and human genetic
validation.
As disclosed at the Investor Update on 23 June 2021, the company
has a robust late-stage R&D pipeline with many assets having the
potential to be first-in-class or best-in-class, as well as offering
significant strategic lifecycle opportunities . The late-stage pipeline
is expected to help deliver the sales ambition set by the company
for 2021-2026 and beyond.
O ur R&D pipeline currently comprises 63 Vaccines and Specialty Medicines.
Pipeline news flow highlights since Q1 2021 Results are listed below
in chronological order.
Infectious diseases
Shingrix
-- Received FDA approval for the prevention of HZ (herpes zoster)
in adults who are immunodeficient or immunosuppressed due to disease
or therapy.
Klebsiella candidate vaccine
-- Started a Phase I study of the tetravalent bioconjugate candidate
vaccine Kleb4V that includes O-antigen combined with our proprietary
adjuvant system.
MenABCWY candidate vaccine
-- Started a Phase I study of the Meningococcal ABCWY 2(nd) generation
candidate vaccine to assess immunogenicity and safety in healthy
adolescents and adults aged 15 to 25 years who had previously been
vaccinated with the MenACWY vaccine.
Priorix
-- Delivered a regulatory submission to the FDA for immunisation against
measles, mumps and rubella.
Respiratory Syncytial Virus (RSV) candidate vaccines
-- Positive phase I/II data for RSV maternal candidate vaccine (recombinant
PreF protein) presented at the European Society for Paediatric
Infectious Diseases annual meeting.
-- Development of phase II RSV paediatric candidate vaccine (viral
vector) discontinued following assessment that target efficacy
profile was unlikely to be met.
HIV
Cabenuva (cabotegravir/rilpivirine)
-- Presented data from the CUSTOMIZE study showing new long-acting
HIV regimen Cabenuva can be successfully implemented in a broad
range of US healthcare practices, even during COVID-19.
Dovato (dolutegravir/lamivudine)
-- Presented data from SALSA the second Dovato switch study confirming
non-inferior efficacy and no virologic failure versus a broad range
of regimens of at least 3 drugs.
GSK3810109 (VRC-N6LS; broadly neutralising antibody)
-- Started a Phase II study with a broadly neutralising antibody for
the treatment of HIV.
Cabotegravir (long-acting integrase inhibitor)
-- Completed a rolling submission of a new drug application with the
FDA for long-acting cabotegravir for the prevention of HIV.
ENHANZE(R) drug delivery technology
-- ViiV Healthcare and Halozyme entered a global collaboration and
license agreement to enable the development of "ultra-long-acting"
medicines for HIV.
Oncology
GSK4428859 (EOS-448; TIGIT antagonist)
-- Announced a co-development and co-commercialisation collaboration
with iTeos Therapeutics for EOS-448, an anti-TIGIT monoclonal antibody.
Zejula (niraparib; PARP inhibitor)
-- The MOONSTONE study evaluating the efficacy and safety of niraparib
plus dostarlimab in the treatment of platinum resistant ovarian
cancer without a known BRCAm has been stopped as a pre-planned
analysis suggested the data would not meet the high bar set for
this single arm ORR study.
feladilimab (GSK3359609; ICOS agonist)
-- The ENTRÉE-Lung sub study 1 investigating feladilimab plus
docetaxel versus docetaxel alone did not meet its primary endpoint
at the interim analysis.
Immunology/Respiratory
AL001 and AL101 (progranulin-elevating monoclonal antibodies )
-- Announced a global collaboration in immuno-neurology with Alector*
for two clinical stage first-in-class monoclonal antibodies for
neurodegenerative diseases.
* Subject to HSR clearance
Benlysta (belimumab)
-- Announced European Commission approval of Benlysta in combination
with background immunosuppressive therapies for the treatment of
adult patients with active lupus nephritis in Europe.
Nucala (mepolizumab)
-- EMA accepted a filing submission for the 40mg pre-filled syringe
to provide an appropriate paediatric presentation for at home administration.
Opportunity driven
daprodustat ( oral hypoxia-inducible factor prolyl hydroxylase inhibitor)
-- Announced positive headline results from five Phase III studies
of daprodustat for patients with anaemia due to chronic kidney
disease.
COVID-19
Vaccines collaborations
-- EMA initiated a rolling review of Sanofi and GSK's COVID-19 adjuvanted
protein recombinant vaccine candidate, Vidprevtyn.
-- Sanofi and GSK started a global Phase III clinical efficacy study
of their vaccine candidate. Pending positive Phase III outcomes
and regulatory reviews, the vaccine could be approved in Q4 2021.
-- Medicago and GSK announced positive interim Phase II results for
their adjuvanted COVID-19 vaccine candidate, demonstrating that
neutralising antibody responses were ten-times higher than in people
recovering from COVID-19.
-- Sanofi and GSK announced Phase II results for their vaccine candidate,
achieving strong rates of neutralising antibody responses, in line
with those measured in people who have recovered from COVID-19
across all adult age groups.
Sotrovimab (GSK4182136; dual-action SARS-CoV-2 monoclonal antibody)
-- Vir and GSK announced the first patient was dosed in the Phase
III COMET-TAIL study investigating intramuscular sotrovimab for
patients with mild/moderate COVID-19.
-- Vir and GSK announced full results from the Phase III COMET-ICE
trial demonstrating a 79% reduction in hospitalisation for more
than 24 hours or death due to any cause with early treatment with
sotrovimab in adult patients with mild-to-moderate COVID-19 who
are at high risk of progression to severe disease.
-- Received Emergency Use Authorisation from the FDA for the treatment
of mild-to-moderate COVID-19 in high-risk adults and paediatric
patients.
-- Received a positive scientific opinion from the EMA's Committee
for Human Medicinal Products (CHMP)
for the early treatment of COVID-19. The CHMP opinion under Article
5(3) can now be considered by the national authorities in EU member
states when taking evidence-based decisions on the early use of
the medicine prior to marketing authorisation.
Contents Page
Total and Adjusted results 10
Financial performance - Q2 2021 12
Financial performance - H1 2021 27
Cash generation 43
Returns to shareholders 44
Income statements 46
Statement of comprehensive income - three months ended 30 June
2021 47
Statement of comprehensive income - six months ended 30 June
2021 48
Pharmaceuticals turnover - three months ended 30 June 2021 49
Pharmaceuticals turnover - six months ended 30 June 2021 50
Vaccines turnover - three months ended 30 June 2021 51
Vaccines turnover - six months ended 30 June 2021 52
Balance sheet 53
Statement of changes in equity 54
Cash flow statement - six months ended 30 June 2021 55
Segment information 56
Legal matters 58
Additional information 59
Reconciliation of cash flow to movements in net debt 65
Net debt analysis 65
Free cash flow reconciliation 65
Principal risks and uncertainties 66
Reporting definitions 67
Outlook, assumptions and cautionary statements 68
Directors' responsibility statement 70
Independent review report 71
Contacts
GSK - one of the world's leading research-based pharmaceutical and
healthcare companies - is committed to improving the quality of human
life by enabling people to do more, feel better and live longer.
For further information please visit www.gsk.com .
GSK enquiries:
Media enquiries: Tim Foley +44 (0) 20 8047 (London)
5502
Simon Moore +44 (0) 20 8047 (London)
5502
Madeleine Breckon +44 (0) 20 8047 (London)
5502
Kristen Neese +1 215 751 3335 (Philadelphia)
Kathleen Quinn +1 202 603 5003 (Washington)
Analyst/Investor enquiries: James Dodwell +44 (0) 20 8047 (London)
2406
Sonya Ghobrial +44 (0) 7392 784784 (Consumer)
Mick Readey +44 (0) 7990 339653 (London)
Jeff McLaughlin +1 215 751 7002 (Philadelphia)
Frannie DeFranco +1 215 751 4855 (Philadelphia)
Registered in England & Wales:
No. 3888792
Registered Office:
980 Great West Road
Brentford, Middlesex
TW8 9GS
Total and Adjusted results
Total reported results represent the Group's overall performance.
GSK also uses a number of adjusted, non-IFRS, measures to report
the performance of its business. Adjusted results and other non-IFRS
measures may be considered in addition to, but not as a substitute
for or superior to, information presented in accordance with IFRS.
Adjusted results are defined below and other non-IFRS measures are
defined on page 67.
GSK believes that Adjusted results, when considered together with
Total results, provide investors, analysts and other stakeholders
with helpful complementary information to understand better the
financial performance and position of the Group from period to period,
and allow the Group's performance to be more easily compared against
the majority of its peer companies. These measures are also used
by management for planning and reporting purposes. They may not
be directly comparable with similarly described measures used by
other companies.
GSK encourages investors and analysts not to rely on any single
financial measure but to review GSK's quarterly results announcements,
including the financial statements and notes, in their entirety.
GSK is committed to continuously improving its financial reporting,
in line with evolving regulatory requirements and best practice.
In line with this practice, GSK expects to continue to review and
refine its reporting framework.
Adjusted results exclude the following items from Total results,
together with the tax effects of all of these items:
-- amortisation of intangible assets (excluding computer software)
-- impairment of intangible assets (excluding computer software) and
goodwill
-- Major restructuring costs, which include impairments of tangible
assets and computer software, (under specific Board approved programmes
that are structural, of a significant scale and where the costs
of individual or related projects exceed GBP25 million), including
integration costs following material acquisitions
-- transaction-related accounting or other adjustments related to
significant acquisitions
-- proceeds and costs of disposal of associates, products and businesses;
significant legal charges (net of insurance recoveries) and expenses
on the settlement of litigation and government investigations;
other operating income other than royalty income, and other items
including the one-off impact of the revaluation of deferred tax
assets and liabilities following enactment of the increase in the
headline rate of UK corporation tax from 19% to 25% (effective
2023)
-- separation costs
Costs for all other ordinary course smaller scale restructuring
and legal charges and expenses are retained within both Total and
Adjusted results.
As Adjusted results include the benefits of Major restructuring
programmes but exclude significant costs (such as significant legal,
major restructuring and transaction items) they should not be regarded
as a complete picture of the Group's financial performance, which
is presented in Total results. The exclusion of other Adjusting
items may result in Adjusted earnings being materially higher or
lower than Total earnings. In particular, when significant impairments,
restructuring charges and legal costs are excluded, Adjusted earnings
will be higher than Total earnings.
GSK has undertaken a number of Major restructuring programmes in
response to significant changes in the Group's trading environment
or overall strategy, or following material acquisitions. Costs,
both cash and non-cash, of these programmes are provided for as
individual elements are approved and meet the accounting recognition
criteria. As a result, charges may be incurred over a number of
years following the initiation of a Major restructuring programme.
Significant legal charges and expenses are those arising from the
settlement of litigation or government investigations that are not
in the normal course and materially larger than more regularly occurring
individual matters. They also include certain major legacy matters.
The enactment of the increase in the headline rate of UK Corporation
tax from 19% to 25% (effective 2023) resulted in a credit of GBP325
million in Q2 2021. Due to the magnitude, GSK has reported this
credit as an Adjusting item in Q2 2021 and H1 2021 so that it does
not obscure the key trends in the Group's performance for the period.
Reconciliations between Total and Adjusted results, providing further
information on the key Adjusting items, are set out on pages 23,
24, 38 and 39.
GSK provides earnings guidance to the investor community on the
basis of Adjusted results. This is in line with peer companies and
expectations of the investor community, supporting easier comparison
of the Group's performance with its peers. GSK is not able to give
guidance for Total results as it cannot reliably forecast certain
material elements of the Total results, particularly the future
fair value movements on contingent consideration and put options
that can and have given rise to significant adjustments driven by
external factors such as currency and other movements in capital
markets.
ViiV Healthcare
ViiV Healthcare is a subsidiary of the Group and 100% of its operating
results (turnover, operating profit, profit after tax) are included
within the Group income statement.
Earnings are allocated to the three shareholders of ViiV Healthcare
on the basis of their respective equity shareholdings (GSK 78.3%,
Pfizer 11.7% and Shionogi 10%) and their entitlement to preferential
dividends, which are determined by the performance of certain products
that each shareholder contributed. As the relative performance of
these products changes over time, the proportion of the overall earnings
allocated to each shareholder also changes. In particular, the increasing
proportion of sales of dolutegravir-containing products has a favourable
impact on the proportion of the preferential dividends that is allocated
to GSK. Adjusting items are allocated to shareholders based on their
equity interests. GSK was entitled to approximately 86% of the Total
earnings and 83% of the Adjusted earnings of ViiV Healthcare for
2020.
As consideration for the acquisition of Shionogi's interest in the
former Shionogi-ViiV Healthcare joint venture in 2012, Shionogi received
the 10% equity stake in ViiV Healthcare and ViiV Healthcare also
agreed to pay additional future cash consideration to Shionogi, contingent
on the future sales performance of the products being developed by
that joint venture, principally dolutegravir. Under IFRS 3 'Business
combinations', GSK was required to provide for the estimated fair
value of this contingent consideration at the time of acquisition
and is required to update the liability to the latest estimate of
fair value at each subsequent period end. The liability for the contingent
consideration recognised in the balance sheet at the date of acquisition
was GBP659 million. Subsequent re-measurements are reflected within
other operating income/(expense) and within Adjusting items in the
income statement in each period. At 30 June 2021, the liability,
which is discounted at 8.0%, stood at GBP5,199 million, on a post-tax
basis.
Cash payments to settle the contingent consideration are made to
Shionogi by ViiV Healthcare each quarter, based on the actual sales
performance of the relevant products in the previous quarter. These
payments reduce the balance sheet liability and hence are not recorded
in the income statement. The cash payments made to Shionogi by ViiV
Healthcare in H1 2021 were GBP419 million.
Because the liability is required to be recorded at the fair value
of estimated future payments, there is a significant timing difference
between the charges that are recorded in the Total income statement
to reflect movements in the fair value of the liability and the actual
cash payments made to settle the liability.
Further explanation of the acquisition-related arrangements with
ViiV Healthcare are set out on pages 52 and 53 of the Annual Report
2020.
Financial performance - Q2 2021
Total results
The Total results for the Group are set out below.
Q2 2021 Q2 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Turnover 8,092 7,624 6 15
Cost of sales (2,554) (2,449) 4 9
-------- -------- ------- -------
Gross profit 5,538 5,175 7 18
Selling, general and administration (2,642) (2,709) (2) 3
Research and development (1,222) (1,301) (6) -
Royalty income 77 75 3 -
Other operating income/(expense) (76) 1,610
-------- -------- ------- -------
Operating profit 1,675 2,850 (41) (30)
Finance income 7 1
Finance expense (192) (229)
Loss on disposal of interest
in associates (36) -
Share of after tax profits
of
associates and joint ventures 16 19
-------- -------- ------- -------
Profit before taxation 1,470 2,641 (44) (32)
Taxation 68 (201)
Tax rate % (4.6)% 7.6%
-------- -------- ------- -------
Profit after taxation 1,538 2,440 (37) (26)
-------- -------- ------- -------
Profit attributable to non-controlling
interests 143 177
Profit attributable to shareholders 1,395 2,263
-------- -------- ------- -------
1,538 2,440 (37) (26)
-------- -------- ------- -------
Earnings per share 27.9p 45.5p (39) (28)
-------- -------- ------- -------
Adjusted results
The Adjusted results for the Group are set out below. Reconciliations
between Total results and Adjusted results for Q2 2021 and Q2 2020
are set out on pages 23 and 24.
Q2 2021
--------------------------------------
% of Growth Growth
GBPm turnover GBP% CER%
-------- ---------- ------- -------
Turnover 8,092 100 6 15
Cost of sales (2,348) (29.0) 4 9
Selling, general and administration (2,498) (30.9) (1) 5
Research and development (1,165) (14.4) (1) 6
Royalty income 77 1.0 3 -
-------- ---------- ------- -------
Adjusted operating profit 2,158 26.7 23 43
-------- ---------- ------- -------
Adjusted profit before tax 1,989 29 50
Adjusted profit after tax 1,623 32 54
Adjusted profit attributable
to shareholders 1,407 47 72
-------- ------- -------
Adjusted earnings per share 28.1p 46 71
-------- ------- -------
Operating profit by business Q2 2021
% of Growth Growth
GBPm turnover GBP% CER%
------ ---------- ------- -------
Pharmaceuticals 2,094 49.5 11 25
Pharmaceuticals R&D* (853) (6) 1
------ ---------- ------- -------
Total Pharmaceuticals 1,241 29.3 27 46
Vaccines 514 32.7 94 >100
Consumer Healthcare 498 21.7 (4) 5
------ ---------- ------- -------
2,253 27.8 28 46
Corporate & other unallocated
costs (95)
Adjusted operating profit 2,158 26.7 23 43
------ ---------- ------- -------
* Operating profit of Pharmaceuticals R&D segment, which is the responsibility
of the Chief Scientific Officer and President, R&D. It excludes
ViiV Healthcare R&D expenditure, which is reported within the Pharmaceuticals
segment.
Turnover
Pharmaceuticals turnover
Q2 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Respiratory 717 25 36
HIV 1,235 4 14
Immuno-inflammation 233 32 46
Oncology 119 55 69
------ ------- -------
New and Specialty 2,304 14 25
Established Pharmaceuticals 1,925 (8) -
------
4,229 3 12
------
US 1,986 10 23
Europe 963 3 6
International 1,280 (7) 3
------ ------- -------
4,229 3 12
------ ------- -------
Pharmaceuticals turnover in the quarter was GBP4,229 million, up
3% AER, 12% CER.
In the quarter results show significant growth over the same quarter
last year, driven by strong growth in New and Specialty products,
favourable US RAR adjustments and a prior year comparator that was
impacted by destocking in the quarter.
Q2 2020 was weakened as it was the first full quarter of COVID-19
pandemic impact and included some destocking of COVID-19 related
additional demand in the first quarter. The impact of this prior
year destocking accounted for approximately 3 percentage points of
growth at CER in the current quarter. The prior period RAR adjustment,
including the impact of lower than expected Medicaid usage on a number
of products, also accounted for approximately 4 percentage points
of growth at CER in the quarter.
New and Specialty sales of GBP2,304 million grew 14% AER, 25% CER,
with ongoing growth from Respiratory, Oncology, Immuno-Inflammation
and HIV.
Respiratory sales were up 25% AER, 36% CER, to GBP717 million, on
growth of Trelegy and Nucala, and our Immuno-Inflammation and Oncology
portfolios continue to show double digit growth. HIV sales grew 4%
AER, 14% CER, to GBP1,235 million, including the favourable impact
on growth of prior year destocking, with growth of Dovato exceeding
the decline in Triumeq. Sales of Established Pharmaceuticals declined
8% AER but were flat at CER, to GBP1,925 million.
In the US, sales grew 10% AER, 23% CER. Continued growth of Nucala,
Trelegy, Benlysta and Dovato was supplemented by growth in Established
Products. This reflected strong demand for Established Respiratory
products in the COVID-19 environment and certain supply challenges
faced by generic competitor products, plus the benefit of a favourable
prior period RAR adjustment in the quarter.
In Europe, sales grew 3% AER, 6% CER, including the favourable impact
on growth of prior year destocking, with double digit growth of Trelegy,
Nucala, Anoro, Benlysta and Zejula. HIV two-drug regimens growth
exceeded the declines in Tivicay and Triumeq. The Established Pharmaceuticals
portfolio declined 8% AER, 6% CER, impacted by generic competition
including Seretide, Duodart and Volibris.
International declined 7% AER but grew 3% CER. Growth from the Respiratory
portfolio and HIV including the impact of tender phasing was offset
by declines in Established Pharmaceuticals which continue to be impacted
by COVID-19 suppressed antibiotics markets and increased generic
competition in Japan on Xyzal and Avolve.
Respiratory
Total Respiratory sales were up 25% AER, 36% CER, with growth from
Trelegy, Nucala and Anoro. International Respiratory sales grew 41%
AER, 51% CER including Nucala, up 22% AER, 35% CER and Trelegy up
more than 100% AER and CER including the impact of Trelegy Asthma
launched in Japan in Q4 2020. In Europe, Respiratory grew 23% AER,
25% CER with double digit growth of Anoro, Trelegy and Nucala. In
the US, Respiratory grew 22% AER, 37% CER, driven by Trelegy and
Nucala and the impact of a prior period RAR adjustment.
Sales of Nucala were GBP292 million in the quarter and grew 21% AER,
32% CER, with consistent, strong growth across all three regions.
US sales were up 21% AER, 35% CER to GBP182 million and International
sales of GBP45 million grew 22% AER, 35% CER. Europe sales of GBP65
million grew 20% AER, 22% CER.
Trelegy sales were up 50% AER, 64% CER to GBP291 million driven by
growth in all regions. In the US, sales benefited from the new asthma
indication approved and launched in Q3 2020, with sales up 46% AER,
64% CER. In Europe, sales grew 36% AER, 36% CER and in International,
where Trelegy asthma was approved in Japan in Q4 2020, sales grew
more than 100% AER and CER to GBP38 million.
HIV
HIV sales were GBP1,235 million with growth of 4% AER, 14% CER in
the quarter. Q2 2021 growth benefited from customer destocking in
the Q2 2020 comparator, following customer stock building in Q1 2020
due to COVID-19 mainly in the US and Europe. Together with the timing
of Tivicay tenders in International, these two factors accounted
for 5 and 6 percentage points of CER growth respectively. The mature
portfolio drove 1 percentage point of CER decline. Triumeq sales
were GBP466 million, down 20% AER, 13% CER and Tivicay sales were
GBP407 million, up 9% AER, 21% CER.
New HIV products Juluca, Dovato, Rukobia and Cabenuva delivered sales
of GBP330 million representing 27% of the total HIV portfolio. Sales
of the two drug regimens Juluca and Dovato were GBP132 million and
GBP184 million respectively with combined growth of 75% AER, 90%
CER. Rukobia sales were GBP10 million. Cabenuva, the first long acting
injectable, recorded quarterly sales of GBP4 million.
In the US, total sales were GBP716 million with decline of 3% AER,
but growth of 8% CER. New HIV products delivered sales of GBP216
million, including: Dovato GBP102 million growth of more than 100%
AER and more than 100% CER, Juluca GBP101 million growth of 12% AER,
24% CER, Rukobia GBP10 million and Cabenuva GBP3 million. Combined
Tivicay and Triumeq sales were GBP488 million declining 17% AER,
7% CER. In Europe, total sales were GBP292 million with growth of
8% AER, 11% CER. New HIV products delivered sales of GBP97 million,
including: Dovato GBP69 million growth of more than 100% AER and
more than 100% CER and Juluca GBP27 million growth of 29% AER, 38%
CER. Combined Tivicay and Triumeq sales were GBP184 million declining
17% AER, 14% CER.
Oncology
Sales of Zejula, the PARP inhibitor treatment for ovarian cancer
were GBP98 million in the quarter, up 27% AER, 38% CER. Sales included
GBP54 million in the US and GBP41 million in Europe.
Blenrep for the treatment of patients with relapsed or refractory
multiple myeloma was approved and launched in the US and Europe in
Q3 2020 and reported sales of GBP21 million in the quarter.
Immuno-inflammation
Sales of Benlysta in the quarter were up 21% AER, 34% CER to GBP214
million, including the impact of lupus nephritis launches in US and
Japan in H2 2020. In the quarter, the first sales of sotrovimab,
the monoclonal antibody treatment for COVID-19 patients at risk of
hospitalisation totalled GBP16 million.
Established Pharmaceuticals
Sales of Established Pharmaceuticals in the quarter were GBP1,925
million, down 8% AER, but flat at CER.
Established Respiratory products declined 2% AER but grew 6% CER
to GBP1,089 million. The ongoing impact of generic competition to
Xyzal in Japan, and to Advair/Seretide in all regions was more than
offset by the favourable impact on growth from destocking last year
and the benefit of a prior period RAR adjustment in the quarter.
The remainder of the Established Pharmaceuticals portfolio declined
by 14% AER, 7% CER to GBP836 million driven by generic impacts including
Volibris in Europe and Avolve/Avodart in Japan.
Vaccines turnover
Q2 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Meningitis 225 35 46
Influenza 33 >100 >100
Shingles 295 (9) 1
Established Vaccines 758 21 28
------
1,311 16 24
Pandemic Vaccines 260 - -
Total Vaccines 1,571 39 49
------ ------- -------
US 796 78 97
Europe 297 3 6
International 478 20 26
------ ------- -------
1,571 39 49
------ ------- -------
Vaccines turnover grew 39% AER, 49% CER to GBP1,571 million, primarily
driven by pandemic adjuvant sales, higher demand for DTPa-containing
vaccines in the US and higher demand for Bexsero in the US and in
Europe.
Vaccines turnover excluding pandemic vaccines grew 16% AER, 24% CER
to GBP1,311 million.
Vaccines performance in Q2 2021 continued to be affected by lower
demand for routine adult vaccination due to COVID-19 vaccination
programme deployment mainly in the US and Europe. Demand for paediatric
and adolescent vaccination improved as lifting of stay-at-home directives
resulted in increased visits to healthcare practitioners and points
of vaccination.
Higher demand in the US for paediatric and adolescent vaccination
represented a recovery trend including partial catch up of prior
period missed vaccinations and CDC purchasing patterns.
In Q2 2020, sales declined 29% CER due to the significant impact
of the COVID-19 pandemic across all regions.
Meningitis
Meningitis sales grew 35% AER, 46% CER to GBP225 million. Bexsero
sales grew 53% AER, 63% CER to GBP165 million reflecting higher demand
and increased market share in the US, together with strong performance
in Europe and International.
Menveo sales were up 55% AER, 71% CER to GBP59 million, primarily
driven by higher demand in the US, partly offset by unfavorable phasing
and lower demand in International.
Influenza
Fluarix/FluLaval sales grew by more than 100% AER and CER, to GBP33
million driven by strong southern hemisphere demand in International.
Shingles
Shingrix d eclined by 9% AER but grew 1% CER to GBP295 million.
Growth from launches in the UK and in China was mostly offset by
a decline in Germany where COVID-19 related restrictions and prioritisation
of COVID-19 mass vaccination limited Shingrix uptake. In the US,
increased market demand in the current quarter was offset by favourable
prior period returns and rebates movements and channel stocking in
the comparator quarter.
Established Vaccines
Hepatitis vaccines sales were up 28% AER, 38% CER to GBP110 million,
largely driven by favourable US wholesaler purchasing patterns and
higher demand in paediatric vaccines, partly offset by higher competitive
pressure in the US.
Sales of DTPa-containing vaccines (Infanrix, Pediarix and Boostrix)
grew by 45% AER, 55% CER. Infanrix/Pediarix sales rose 14% AER, 24%
CER to GBP136 million, reflecting increased channel stock replenishment
on wholesaler purchasing patterns and demand in the US , partly offset
by a change in recommendation for the dosing schedule in Germany
and supply constraints in Europe. Boostrix sales grew 92% AER and
more than 100% CER to GBP146 million largely driven by higher demand
in the US and in International.
Rotarix sales were up 3% AER, 9% CER to GBP132 million, reflecting
increased channel stocking on wholesaler purchasing patterns in the
US.
Synflorix sales declined by 6% AER, 2% CER to GBP97 million, primarily
due to lower tender demand in Emerging Markets and Europe.
MMRV vaccines sales were flat at AER, but grew 6% CER to GBP54 million,
largely driven by favourable phasing in International.
Pandemic Vaccines
Pandemic vaccines sales of GBP260 million included GBP258 million
of pandemic adjuvant sales, which represented delivery of around
two-thirds of the contracted volumes with the US and Canadian governments.
Consumer Healthcare turnover
Q2 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Oral health 663 4 12
Pain relief 563 6 13
Vitamins, minerals and supplements 359 (11) (6)
Respiratory health 210 (2) 6
Digestive health and other 464 (5) 3
------ ------- -------
2,259 (1) 7
Brands divested/under review 33 (72) (70)
------ ------- -------
2,292 (4) 3
------ ------- -------
US 734 (11) (1)
Europe 608 1 3
International 950 (1) 6
------ ------- -------
2,292 (4) 3
------ ------- -------
Consumer Healthcare sales declined 4% AER, but increased 3% CER to
GBP2,292 million in the second quarter with growth in the quarter
more than offsetting dilution from the divestment programme which
completed at the end of Q1 2021.
Sales excluding brands divested/under review declined 1% AER but
increased 7% CER supported by a favourable comparative in Q2 2020
as a result of destocking. In the second quarter last year, sales
at CER excluding brands divested/under review were flat at pro-forma
and included the reversal of the benefit of the accelerated purchases
in the first quarter in 2020 across all categories as a result of
the COVID-19 pandemic. Additionally, Q2 2020 benefited by approximately
2 percentage points from increased retailer stocking ahead of a systems
cutover in North America which reversed in Q3 2020.
On a 2 year CAGR sales excluding brands divested/under review increased
3%. Consistent with disclosure in the Q1 2021 press release given
the significant volatility with the Q1 2020 and Q2 2020 comparatives
as a result of the COVID-19 pandemic the 2 year category CAGRs are
shared below. These are shared for the first half of this year only,
as this is more indicative of underlying trends than looking at the
quarters in isolation.
International sales excluding brands divested/under review grew high
single digit CER with strong performance in the emerging markets
such as China, Latin America, and India.
Oral health
Oral health sales increased 4% AER, 12% CER to GBP663 million. In
Q2 2020 Oral health sales declined low single digit. Sensodyne delivered
growth in the mid-teens compared with low single digit growth in
Q2 2020, reflecting underlying brand strength and continued innovation
particularly in the US, Middle East and Africa and India. Gum health
and Denture care reported mid-teens growth and high single digit
growth respectively in the second quarter. Denture care sales, whilst
up in the quarter helped by a favourable comparative to Q2 2020,
continued to be negatively impacted by social distancing due to the
pandemic. On a 2 year CAGR growth was 5%, consistent with the 2 year
CAGR for the first quarter and similar to trends in the second half
of 2020.
Pain relief
Pain relief sales increased 6% AER, 13% CER to GBP563 million. In
Q2 2020 Pain relief sales declined low single digit on a pro-forma
basis. Advil and Panadol increased double digit helped by favourable
comparatives due to destocking in Q2 2020. Voltaren increased low
double digit helped by the successful Rx to OTC switch in May last
year. The 2 year CAGR for the category grew 5% similar to the 2 year
CAGR in the prior quarter.
Vitamins, minerals and supplements
Vitamins, minerals and supplements sales declined 11% AER, 6% CER
to GBP359 million. In Q2 2020 Vitamins, minerals and supplements
sales increased high-teens per cent on a pro-forma basis. Centrum
grew low single digit although this was more than offset by a double
digit decline in Emergen-C which faced particularly challenging comparatives
(sales almost doubled in Q2 2020) and a mid-single digit decline
in Caltrate. On a 2 year CAGR the category grew 6%.
Respiratory health
Respiratory health sales declined 2% AER but increased 6% CER to
GBP210 million. In Q2 2020 Respiratory health sales declined low
double digit on a pro-forma basis. Theraflu and Robitussin declined
significantly. Otrivin and Flonase were up double digit although
helped by easier comparatives in Q2 2020 when sales declined in the
high teens. The category 2 year CAGR declined 3%. Seasonal cold,
flu and nasal brands continued to be adversely impacted by the weaker
cold and flu season as a result of the pandemic and social distancing,
and its 2 year CAGR declined high single digit. In contrast the allergy
2 year CAGR was up high single digit.
Digestive health and other
Digestive health and other brands sales declined 5% AER but increased
3% CER at GBP464 million. In Q2 2020 Digestive health and other brands
had decreased low-single digits on a pro-forma basis. Digestive health
products saw double digit growth, with Smokers health products up
mid single digit and Skin health products up high teens although
brands such as Chapstick remained muted given reduced impulse purchase.
The 2 year category CAGR was flat.
Operating performance
Cost of sales
Total cost of sales as a percentage of turnover was 31.6%, 0.6 percentage
points lower at AER and 1.8 percentage points lower in CER terms
compared with Q2 2020. This included a similar level of write downs
in manufacturing sites as in Q2 2020.
Excluding these and other Adjusting items, Adjusted cost of sales
as a percentage of turnover was 29.0%, 0.5 percentage points lower
at AER and 1.6 percentage points lower at CER compared with Q2 2020.
This reflected price benefits in Pharmaceuticals, including the benefit
from a prior period RAR adjustment, reduced supply chain costs and
a favourable mix in Vaccines, including pandemic adjuvants.
Selling, general and administration
Total SG&A costs as a percentage of turnover were 32.6%, 2.9 percentage
points lower at AER and 3.6 percentage points lower at CER compared
with Q2 2020.
Excluding Adjusting items, Adjusted SG&A costs as a percentage of
turnover were 30.9%, 2.3 percentage points lower at AER than in Q2
2020 and 3.0 percentage points lower on a CER basis. Adjusted SG&A
costs declined 1% AER but increased 5% CER which reflected increased
investment for launches in Pharmaceuticals and Vaccines compared
to reduced spend in Q2 2020 as a result of the COVID-19 lockdowns
partly offset by continued tight control of ongoing costs and the
continuing benefit of restructuring in Pharmaceuticals, Consumer
Healthcare and support functions.
Research and development
Total R&D expenditure was GBP1,222 million (15.1% of turnover), down
6% AER, but flat at CER, including a decrease in impairments. Adjusted
R&D expenditure was GBP1,165 million (14.4% of turnover), 1% lower
at AER, 6% higher at CER than in Q2 2020.
Pharmaceuticals R&D expenditure was GBP883 million, down 4% AER,
up 3% CER, primarily driven by increases in the Specialty portfolio
excluding Oncology, offset by a net reduction compared to Q2 2020
in Oncology. Efficiency savings continue from the implementation
of the One Development programme for Pharmaceuticals and Vaccines
as part of the Separation preparation restructuring programme and
variable spending as a result of COVID-19 lockdowns.
In the Specialty portfolio excluding Oncology there has been an increase
in investment, specifically related to our two key COVID-19 treatment
programmes (sotrovimab and otilimab) and also in bepirovirsen, the
HBV antisense oligonucleotide programme, depemokimab, the anti-IL5
for asthma and otilimab for rheumatoid arthritis. There has also
been an increase in spend for the early stage non-Oncology research
portfolio. In Oncology there is increased investment from the progression
of Zejula, Jemperli and NY-ESO, offset by a reduction in spend on
Blenrep following successful approval in Q3 2020 and feladilimab
following the decision to terminate two studies in April.
R&D expenditure in Vaccines was GBP224 million, up 28% AER, 34% CER,
reflecting increased investment in meningitis and RSV, partly offset
by efficiency savings from the implementation of the One Development
programme. R&D expenditure in Consumer Healthcare was GBP58 million.
Royalty income
Royalty income was GBP77 million (Q2 2020: GBP75 million), up 3%
AER, but flat at CER.
Other operating income/(expense)
Net other operating expense of GBP76 million (Q2 2020: GBP1,610 million
income) primarily reflected accounting charges of GBP101 million
(Q2 2020: GBP368 million) arising from the re-measurement of the
contingent consideration liabilities related to the acquisitions
of the former Shionogi-ViiV Healthcare joint venture and the former
Novartis Vaccines business and the liabilities for the Pfizer put
option and Pfizer and Shionogi preferential dividends in ViiV Healthcare.
This included a re-measurement charge of GBP125 million (Q2 2020:
GBP343 million) for the contingent consideration liability due to
Shionogi, primarily as a result of the unwinding of the discount
for GBP92 million and a charge for GBP33 million from adjustments
to sales forecasts partly offset by updated exchange rate assumptions.
This was partly offset by fair value gains on investments and a number
of asset disposals. Q2 2020 included the net profit on disposal of
the Horlicks and other Consumer brands of GBP2,304 million partly
offset by the loss on sale of the shares in Hindustan Unilever of
GBP476 million.
Operating profit
Total operating profit was GBP1,675 million in Q2 2021 compared with
GBP2,850 million in Q2 2020. This primarily reflected an unfavourable
comparison to the net profit on disposal of the Horlicks and other
Consumer brands of GBP2,304 million partly offset by the related
loss on sale of the shares in Hindustan Unilever of GBP476 million.
Excluding these and other Adjusting items, Adjusted operating profit
was GBP2,158 million, 23% higher than Q2 2020 at AER, 43% higher
at CER on a turnover increase of 15% CER. The Adjusted operating
margin of 26.7% was 3.7 percentage points higher at AER, and 5.6
percentage points higher on a CER basis than in Q2 2020.
The increase in Adjusted operating profit primarily reflected leverage
from a favourable comparison to destocking in Q2 2020 in Pharmaceuticals
and Consumer Healthcare, GBP258 million of pandemic adjuvant sales,
increased demand for Meningitis and DTPa-containing Vaccines and
a favourable prior period RAR adjustment in Pharmaceuticals, continued
tight control of ongoing costs and benefits from continued restructuring
across the business. This was partly offset by increased investment
behind launches and increased investment in R&D.
Contingent consideration cash payments which are made to Shionogi
and other companies reduce the balance sheet liability and hence
are not recorded in the income statement. Total contingent consideration
cash payments in Q2 2021 amounted to GBP205 million (Q2 2020: GBP240
million). This included cash payments made to Shionogi of GBP203
million (Q2 2020: GBP232 million).
Adjusted operating profit by business
Pharmaceuticals operating profit was GBP1,241 million, up 27% AER,
and 46% CER on a turnover increase of 12% CER. The operating margin
of 29.3% was 5.6 percentage points higher at AER than in Q2 2020
and 7.2 percentage points higher on a CER basis. This primarily reflected
positive leverage from a favourable comparison to destocking in Q2
2020 and a favourable prior period RAR adjustment, as well as continued
tight control of ongoing costs and benefits from continued restructuring.
This was partly offset by increased investment in R&D.
Vaccines operating profit was GBP514 million, up 94% AER, >100% CER
on a turnover increase of 49% CER. The operating margin of 32.7%
was 9.3 percentage points higher at AER than in Q2 2020 and 11.4
percentage points higher on a CER basis. This was primarily driven
by positive operating leverage from sales growth including pandemic
adjuvant sales mix. This was partly offset by higher R&D spend to
support key strategic priorities along with increased SG&A investment
to support business growth.
Consumer Healthcare operating profit was GBP498 million, down 4%
AER but up 5% CER on a turnover increase of 3% CER. The operating
margin of 21.7% was 0.1 percentage point lower at AER but 0.5 percentage
points higher on a CER basis than in Q2 2020. The margin increase
at CER reflected incremental synergy benefits from the Pfizer Joint
Venture integration, price increases and leverage from volume growth
partially offset by the impact of divestments (1.1 percentage points),
increased advertising and promotion investment to resume to pre-COVID-19
levels, increased commodity costs and investment into manufacturing
sites.
Net finance costs
Total net finance costs were GBP185 million compared with GBP228
million in Q2 2020. Adjusted net finance costs were GBP185 million
compared with GBP227 million in Q2 2020. The decrease primarily reflects
reduced interest expense from lower debt levels, favourable movements
in foreign exchange rates and reduced swap interest expense on foreign
currency hedges.
Share of after tax profits of associates and joint ventures
The share of after tax profits of associates and joint ventures was
GBP16 million (Q2 2020: GBP19 million).
Loss on disposal of interest in associates
In the quarter the net loss on disposal of interest in associates
was GBP36 million, primarily driven by a loss on disposal of the
interest in Innoviva Inc.
Taxation
The credit of GBP68 million represented an effective tax rate on
Total results of (4.6)% (Q2 2020: 7.6%) and reflected the different
tax effects of the various Adjusting items including a credit of
GBP325 million in Q2 2021 resulting from the revaluation of deferred
tax assets following enactment of the proposed change of UK corporation
tax rate from 19% to 25% (effective 1 April 2023). Q2 2020 reflected
the disposal of the Horlicks and other Consumer brands and the subsequent
disposal of shares received in Hindustan Unilever. Tax on Adjusted
profit amounted to GBP366 million and represented an effective Adjusted
tax rate of 18.4% (Q2 2020: 20.5%).
Issues related to taxation are described in Note 14, 'Taxation' in
the Annual Report 2020. The Group continues to believe it has made
adequate provision for the liabilities likely to arise from periods
which are open and not yet agreed by tax authorities. The ultimate
liability for such matters may vary from the amounts provided and
is dependent upon the outcome of agreements with relevant tax authorities.
Non-controlling interests
The allocation of Total earnings to non-controlling interests amounted
to GBP143 million (Q2 2020: GBP177 million). The reduction was primarily
due to a reduced allocation of Consumer Healthcare Joint Venture
profits of GBP76 million (Q2 2020: GBP137 million), partly offset
by an increased allocation of ViiV Healthcare profits of GBP60 million
(Q2 2020: GBP24 million), including reduced credits for re-measurement
of contingent consideration liabilities.
The allocation of Adjusted earnings to non-controlling interests
amounted to GBP216 million (Q2 2020: GBP267 million). The reduction
in allocation primarily reflected a reduced allocation of Consumer
Healthcare Joint Venture profits of GBP108 million (Q2 2020: GBP138
million) and a reduced allocation of ViiV Healthcare profits of GBP101
million (Q2 2020: GBP113 million), as well as lower net profits in
some of the Group's other entities with non-controlling interests.
Earnings per share
Total EPS was 27.9p, compared with 45.5p in Q2 2020. This primarily
reflected an unfavourable comparison to the net profit on disposal
in Q2 2020 of the Horlicks and other Consumer brands partly offset
by the related loss on sale of the shares in Hindustan Unilever,
as well as by a credit of GBP325 million to Taxation in Q2 2021 resulting
from the revaluation of deferred tax assets following enactment of
the proposed change of UK corporation tax rate from 19% to 25% (effective
1 April 2023).
Adjusted EPS was 28.1p compared with 19.2p in Q2 2020, up 46% AER
and 71% CER, on a 43% CER increase in Adjusted operating profit reflecting
sales increases in Pharmaceuticals and Consumer Healthcare including
a benefit from destocking in Q2 2020 and increased Vaccines sales
including pandemic adjuvant sales, lower interest costs, a lower
effective tax rate and a lower non-controlling interest allocation
of Consumer Healthcare and ViiV profits. The contribution to growth
from COVID-19 solutions was approximately 20% AER, 21% CER.
Currency impact on Q2 2021 results
The results for Q2 2021 are based on average exchange rates, principally
GBP1/$1.40, GBP1/EUR1.16 and GBP1/Yen 152. Comparative exchange rates
are given on page 59. The period-end exchange rates were GBP1/$1.39,
GBP1/EUR1.17 and GBP1/Yen 153.
In the quarter, turnover increased 6% AER, 15% CER. Total EPS was
27.9p compared with 45.5p in Q2 2020. Adjusted EPS was 28.1p compared
with 19.2p in Q2 2020, up 46% AER and 71% CER. The adverse currency
impact primarily reflected the strengthening in Sterling, particularly
against the US as well as Yen. Exchange gains or losses on the settlement
of intercompany transactions had a 1% impact on the negative currency
impact of 25 percentage points on Adjusted EPS.
Adjusting items
The reconciliations between Total results and Adjusted results for
Q2 2021 and Q2 2020 are set out below.
Three months ended 30 June 2021
Divestments,
significant
legal
Intangible Intangible Major and
Total amort- impair- restruct- Transaction- other Separation Adjusted
results isation ment uring related items costs results
GBPm GBPm GBPm GBPm GBPm GBPm GBPm GBPm
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Turnover 8,092 8,092
Cost of sales (2,554) 171 28 7 (2,348)
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Gross profit 5,538 171 28 7 5,744
Selling, general
and
administration (2,642) 83 (13) 74 (2,498)
Research and
development (1,222) 25 6 26 (1,165)
Royalty income 77 77
Other operating
income/(expense) (76) 1 123 (48) -
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Operating profit 1,675 196 6 138 130 (61) 74 2,158
Net finance costs (185) (185)
Loss on disposal
of interest in
associates (36) 36 -
Share of after
tax profits
of associates
and joint
ventures 16 16
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit before
taxation 1,470 196 6 138 130 (25) 74 1,989
Taxation 68 (38) (1) (29) (33) (319) (14) (366)
Tax rate % (4.6)% 18.4%
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit after
taxation 1,538 158 5 109 97 (344) 60 1,623
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit
attributable
to
non-controlling
interests 143 73 216
Profit
attributable
to
shareholders 1,395 158 5 109 24 (344) 60 1,407
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Earnings per
share 27.9p 3.2p 0.1p 2.1p 0.5p (6.9)p 1.2p 28.1p
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Weighted average
number of shares
(millions) 5,004 5,004
------------ ------------
Three months ended 30 June 2020
Divestments,
significant
legal
Intangible Intangible Major and
Total amort- impair- restruct- Transaction- other Separation Adjusted
results isation ment uring related items costs results
GBPm GBPm GBPm GBPm GBPm GBPm GBPm GBPm
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Turnover 7,624 7,624
Cost of sales (2,449) 180 (2) 12 10 (2,249)
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Gross profit 5,175 180 (2) 12 10 5,375
Selling, general
and
administration (2,709) 3 182 (20) (4) 18 (2,530)
Research and
development (1,301) 17 116 (2) (1) (1,171)
Royalty income 75 75
Other operating
income/(expense) 1,610 1 359 (1,970) -
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Operating profit 2,850 197 117 193 349 (1,975) 18 1,749
Net finance costs (228) 1 (227)
Share of after
tax profits
of associates
and joint
ventures 19 19
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit before
taxation 2,641 197 117 193 349 (1,974) 18 1,541
Taxation (201) (34) (22) (47) (56) 47 (3) (316)
Tax rate % 7.6% 20.5%
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit after
taxation 2,440 163 95 146 293 (1,927) 15 1,225
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit
attributable
to
non-controlling
interests 177 90 267
Profit
attributable
to
shareholders 2,263 163 95 146 203 (1,927) 15 958
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Earnings per
share 45.5p 3.2p 1.9p 2.9p 4.1p (38.7)p 0.3p 19.2p
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Weighted average
number of shares
(millions) 4,977 4,977
------------ ------------
Major restructuring and integration
Within the Pharmaceuticals sector, the highly regulated manufacturing
operations and supply chains and long lifecycle of the business mean
that restructuring programmes, particularly those that involve the
rationalisation or closure of manufacturing or R&D sites are likely
to take several years to complete.
Total Major restructuring charges incurred in Q2 2021 were GBP138
million (Q2 2020: GBP193 million), analysed as follows:
Q2 2021 Q2 2020
------------------------- -------------------------
Cash Non-cash Total Cash Non-cash Total
GBPm GBPm GBPm GBPm GBPm GBPm
------ --------- ------ ------ --------- ------
2018 major restructuring
programme (incl. Tesaro) 3 - 3 30 15 45
Consumer Healthcare
Joint
Venture integration
programme 35 (2) 33 82 15 97
Separation Preparation
restructuring programme 104 (10) 94 42 3 45
Combined restructuring
and
integration programme 4 4 8 (3) 9 6
146 (8) 138 151 42 193
------ --------- ------ ------ --------- ------
Cash charges of GBP104 million under the Separation Preparation programme
primarily arose from restructuring of some administrative functions
as well as commercial pharmaceuticals and R&D functions. Non-cash
credits of GBP10 million primarily reflected a write back on disposal
of a site.
Cash charges of GBP35 million on the Consumer Healthcare Joint Venture
programme primarily related to severance and integration costs.
Total cash payments made in Q2 2021 were GBP197 million (Q2 2020:
GBP163 million), GBP114 million (Q2 2020: GBP20 million) relating
to the Separation Preparation restructuring programme, a further
GBP48 million (Q2 2020: GBP65 million) relating to the Consumer Healthcare
Joint Venture integration programme, GBP21 million (Q2 2020: GBP47
million) under the 2018 major restructuring programme including the
settlement of certain charges accrued in previous quarters and GBP14
million (Q2 2020: GBP31 million) for the existing Combined restructuring
and integration programme.
The analysis of Major restructuring charges by business was as follows:
Q2 2021 Q2 2020
GBPm GBPm
-------- --------
Pharmaceuticals 54 44
Vaccines 4 (14)
Consumer Healthcare 36 105
-------- --------
94 135
Corporate & central functions 44 58
-------- --------
Total Major restructuring costs 138 193
-------- --------
The analysis of Major restructuring charges by Income statement line
was as follows:
Q2 2021 Q2 2020
GBPm GBPm
-------- --------
Cost of sales 28 12
Selling, general and administration 83 182
Research and development 26 (2)
Other operating income 1 1
Total Major restructuring costs 138 193
-------- --------
The benefit in the quarter from restructuring programmes was GBP0.1
billion, with contributions from the Consumer Healthcare Joint Venture
integration and the Separation Preparation restructuring programme.
Transaction-related adjustments
Transaction-related adjustments resulted in a net charge of GBP130
million (Q2 2020: GBP349 million). This included a net GBP101 million
accounting charge for the re-measurement of the contingent consideration
liabilities related to the acquisitions of the former Shionogi-ViiV
Healthcare joint venture and the former Novartis Vaccines business
and the liabilities for the Pfizer put option and Pfizer and Shionogi
preferential dividends in ViiV Healthcare.
Q2 2021 Q2 2020
Charge/(credit) GBPm GBPm
-------- --------
Contingent consideration on former Shionogi-ViiV
Healthcare joint venture
(including Shionogi preferential dividends) 125 343
ViiV Healthcare put options and Pfizer preferential
dividends (37) 10
Contingent consideration on former Novartis Vaccines
business 13 15
Other adjustments 29 (19)
-------- --------
Total transaction-related charges 130 349
-------- --------
The GBP125 million charge relating to the contingent consideration
for the former Shionogi-ViiV Healthcare joint venture represented
an increase in the valuation of the contingent consideration due
to Shionogi, primarily as a result of the unwind of the discount
for GBP92 million and a charge of GBP33 million primarily from adjustments
to sales forecasts partly offset by updated exchange rate assumptions.
The GBP37 million credit relating to the ViiV Healthcare put option
and Pfizer preferential dividends represented a reduction in the
valuation of the put option primarily as a result of lower cash following
preference dividend payments.
The ViiV Healthcare contingent consideration liability is fair valued
under IFRS. The potential impact of the COVID-19 pandemic remains
uncertain and at 30 June 2021, it has been assumed that there will
be no significant impact on the long-term value of the liability.
This position remains under review and the amount of the liability
will be updated in future quarters as further information on the
impact of the pandemic becomes available. An explanation of the accounting
for the non-controlling interests in ViiV Healthcare is set out on
page 11.
Divestments, significant legal charges and other items
Divestments and other items also included fair value gains on investments
and a number of asset disposals and certain other Adjusting items,
including the impact of the proposed change of UK Corporation tax
rate as discussed on page 11. In the quarter, the net loss on disposal
of interests in associates was GBP36 million, primarily driven by
a loss on disposal of the interest in Innoviva Inc. There was a credit
of GBP11 million (Q2 2020: GBP1 million charge) for significant legal
matters arising in the quarter. Significant legal cash payments were
GBP1 million (Q2 2020: GBP1 million).
Separation costs
From Q2 2020, the Group started to report additional costs to prepare
for Consumer Healthcare separation. Separation costs incurred in
the quarter were GBP74 million (Q2 2020: GBP18 million).
Financial performance - H1 2021
Total results
The Total results for the Group are set out below.
H1 2021 H1 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Turnover 15,510 16,714 (7) (1)
Cost of sales (5,034) (5,648) (11) (8)
-------- -------- ------- -------
Gross profit 10,476 11,066 (5) 2
Selling, general and administration (5,069) (5,625) (10) (6)
Research and development (2,340) (2,488) (6) (1)
Royalty income 168 142 18 18
Other operating income/(expense) 133 1,769
-------- -------- ------- -------
Operating profit 3,368 4,864 (31) (21)
Finance income 17 42
Finance expense (393) (458)
Loss on disposal of interest
in associates (36) -
Share of after tax profits
of
associates and joint ventures 32 28
-------- -------- ------- -------
Profit before taxation 2,988 4,476 (33) (23)
Taxation (190) (357)
Tax rate % 6.4% 8.0%
-------- -------- ------- -------
Profit after taxation 2,798 4,119 (32) (23)
-------- -------- ------- -------
Profit attributable to non-controlling
interests 330 291
Profit attributable to shareholders 2,468 3,828
-------- -------- ------- -------
2,798 4,119 (32) (23)
-------- -------- ------- -------
Earnings per share 49.4p 77.0p (36) (27)
-------- -------- ------- -------
Adjusted results
The Adjusted results for the Group are set out below. Reconciliations
between Total results and Adjusted results for H1 2021 and H1 2020
are set out on pages 38 and 39.
H1 2021
--------------------------------------
% of Growth Growth
GBPm turnover GBP% CER%
-------- ---------- ------- -------
Turnover 15,510 100 (7) (1)
Cost of sales (4,584) (29.6) (6) (3)
Selling, general and administration (4,813) (31.0) (9) (6)
Research and development (2,242) (14.5) (1) 5
Royalty income 168 1.1 18 18
-------- ---------- ------- -------
Adjusted operating profit 4,039 26.0 (9) 3
-------- ---------- ------- -------
Adjusted profit before tax 3,696 (8) 4
Adjusted profit after tax 3,012 (11) 1
Adjusted profit attributable
to shareholders 2,550 (10) 3
-------- ------- -------
Adjusted earnings per share 51.0p (10) 2
-------- ------- -------
Operating profit by business H1 2021
--------------------------------------
% of Growth Growth
GBPm turnover GBP% CER%
-------- ---------- ------- -------
Pharmaceuticals 4,004 49.4 3 12
Pharmaceuticals R&D* (1,644) (6) -
-------- ---------- ------- -------
Total Pharmaceuticals 2,360 29.1 9 22
Vaccines 820 29.3 (27) (17)
Consumer Healthcare 1,033 22.4 (20) (13)
-------- ---------- ------- -------
4,213 27.2 (8) 3
Corporate & other unallocated
costs (174)
Adjusted operating profit 4,039 26.0 (9) 3
-------- ---------- ------- -------
* Operating profit of Pharmaceuticals R&D segment, which is the responsibility
of the Chief Scientific Officer and President, R&D. It excludes
ViiV Healthcare R&D expenditure, which is reported within the Pharmaceuticals
segment.
Turnover
Pharmaceuticals turnover
H1 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Respiratory 1,336 22 31
HIV 2,266 (5) 1
Immuno-inflammation 413 26 37
Oncology 229 45 53
------ ------- -------
New and Specialty 4,244 7 14
Established Pharmaceuticals 3,867 (15) (9)
------
8,111 (5) 2
------
US 3,699 4 14
Europe 1,913 (8) (7)
International 2,499 (13) (6)
------ ------- -------
8,111 (5) 2
------ ------- -------
Pharmaceuticals turnover in the six months was GBP8,111 million,
down 5% AER but up 2% CER with Sterling strengthening over the period.
HIV sales were down 5% AER but up 1% CER, to GBP2,266 million, with
growth in Juluca and Dovato partly offset by Tivicay and Triumeq.
Respiratory sales were up 22% AER, 31% CER, to GBP1,336 million,
on growth of Trelegy and Nucala. Sales of Established Pharmaceuticals
declined 15% AER, 9% CER to GBP3,867 million.
In the first half of last year, the additional COVID-19 related demand
experienced towards the end of the first quarter was broadly reversed
in the second quarter. In the current year, the market environment
continues to be impacted by COVID-19, impacting our Established Pharmaceuticals
products in International and Europe regions.
In the US, sales grew 4% AER, 14% CER with continued growth of New
and Specialty products supplemented by growth in Established Products.
This reflected strong demand for Established Respiratory products
in the COVID-19 environment and certain supply challenges faced by
generic competitor products, plus the benefit of a favourable prior
period RAR adjustment.
In Europe, sales declined 8% AER and 7% CER, with growth of Trelegy,
Nucala, Benlysta and Oncology products offset by declines in the
Established Pharmaceuticals portfolio. This portfolio was impacted
by generic competition including Seretide, Duodart and Volibris,
lower antibiotic demand, and a one-off UK Relenza contract last year.
Growth in HIV two-drug regimens was offset by declines in Tivicay
and Triumeq.
International declined 13% AER, 6% CER. Growth from the Respiratory
and HIV portfolios was offset by declines in Established Pharmaceuticals
which continued to be impacted by COVID-19 suppressed antibiotics
markets and increased generic competition in Japan on Xyzal and Avolve.
Respiratory
Total Respiratory sales were up 22% AER, 31% CER, with strong growth
from all Regions. International Respiratory sales grew 37% AER, 45%
CER including Nucala up 24% AER, 34% CER, and Trelegy up 94% AER
and more than 100% CER including the impact of Trelegy Asthma launched
in Japan in Q4 2020. In Europe, Respiratory grew 12% AER, 12% CER
with double digit growth of Trelegy and high single digit growth
of Nucala. In the US, Respiratory grew 23% AER, 35% CER, driven by
Trelegy and Nucala and the impact of a prior period RAR adjustment.
Sales of Nucala were GBP546 million in the six months and grew 21%
AER, 29% CER, with consistent, strong growth across all three regions.
US sales were up 25% AER, 37% CER to GBP332 million and International
sales of GBP87 million grew 24% AER, 34% CER. Europe sales of GBP127
million grew 9% AER, 9% CER.
Trelegy sales were up 39% AER, 49% CER to GBP539 million driven by
growth in all regions. In the US, sales benefited from the new asthma
indication approved and launched in Q3 2020, with sales up 38% AER,
51% CER. In Europe, sales grew 21% AER, 21% CER and in International,
where Trelegy asthma was approved in Japan in Q4 2020, sales grew
94% AER and more than 100% CER to GBP68 million.
HIV
HIV sales were GBP2,266 million with decline of 5% AER but growth
of 1% CER in the six months. Triumeq sales were GBP902 million, down
21% AER, 16% CER and Tivicay sales were GBP708 million, down 10%
AER, 3% CER. In the first six months, growth was reduced by customer
stock building due to COVID-19 in 2020, mainly in the US and Europe,
accounting for 1 percentage point of CER growth. The mature portfolio
drove 1 percentage point of CER decline.
New HIV products Juluca, Dovato, Rukobia and Cabenuva delivered sales
of GBP592 million representing 26% of the total HIV portfolio. Sales
of the two drug regimens Juluca and Dovato were GBP244 million and
GBP325 million, respectively, with combined growth of 55% AER, 65%
CER. Rukobia sales were GBP17 million. Cabenuva, the first long acting
injectable, recorded half year sales of GBP6 million.
In the US, total sales were GBP1,313 million with decline of 9% AER
and 1% CER. New HIV products delivered sales of GBP382 million, including:
Dovato GBP176 million growth of 87% AER and more than 100% CER, Juluca
GBP184 million flat at AER, but growth of 9% CER, Rukobia GBP17 million
and Cabenuva GBP5 million. Combined Tivicay and Triumeq sales were
GBP907 million declining 20% AER, 13% CER. In Europe, total sales
were GBP579 million with decline of 2% AER, 2% CER. New HIV products
delivered sales of GBP181 million, including: Dovato GBP127 million
growth of more than 100% AER and more than 100% CER and Juluca GBP53
million growth of 18% AER, 20% CER. Combined Tivicay and Triumeq
sales were GBP380 million declining 21% AER, 21% CER.
Oncology
Sales of Zejula, the PARP inhibitor treatment for ovarian cancer
were GBP186 million, up 18% AER, 24% CER. Sales included GBP105 million
in the US and GBP77 million in Europe.
Blenrep for the treatment of patients with relapsed or refractory
multiple myeloma was approved and launched in the US and Europe in
Q3 2020. Launches in Europe included four markets in the first half
of the year, and sales globally totalled GBP42 million.
Immuno-inflammation
Sales of Benlysta were up 20% AER, 30% CER to GBP392 million, including
the impact of lupus nephritis launches in US and Japan in H2 2020.
The first sales of sotrovimab, the monoclonal antibody treatment
for COVID-19 patients at risk of hospitalisation, totalled GBP16
million.
Established Pharmaceuticals
Sales of Established Pharmaceuticals in the six months were GBP3,867
million, down 15% AER, 9% CER.
Established Respiratory products declined 9% AER 3% CER to GBP2,216
million. This reflects the ongoing impact of generic competition
to Xyzal in Japan, and to Advair/Seretide globally. The decline was
partially offset by the favourable impact on growth of favourable
prior period RAR adjustments.
The remainder of the Established Pharmaceuticals portfolio declined
by 21% AER, 16% CER to GBP1,651 million on lower demand for antibiotics
during the COVID-19 pandemic period, the impact of government mandated
changes increasing use of generics in markets including France, Japan
and China and a strong comparator, including the European Relenza
contract.
Vaccines turnover
H1 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Meningitis 415 6 12
Influenza 51 42 53
Shingles 622 (36) (31)
Established Vaccines 1,447 (6) (2)
------
2,535 (14) (9)
Pandemic Vaccines 260 - -
------ ------- -------
2,795 (5) -
------ ------- -------
US 1,301 (11) (3)
Europe 604 (5) (5)
International 890 6 10
------ ------- -------
2,795 (5) -
------ ------- -------
Vaccines turnover declined 5% AER, but was flat at CER to GBP2,795
million, primarily driven by pandemic adjuvant sales, offset by the
adverse impact of the COVID-19 pandemic on Shingrix.
Vaccines turnover excluding pandemic vaccines declined 14% AER, 9%
CER to GBP2,535 million.
Vaccines performance in the first half of 2021 was affected by lower
demand for routine adult vaccination due to COVID-19 vaccination
programme deployment mainly in the US and Europe, resulting in lower
Shingrix sales. Demand for paediatric and adolescent vaccination
improved, particularly in the second quarter, as lifting of stay-at-home
directives resulted in increased visits to healthcare practitioners
and points of vaccination.
Higher demand in the US for paediatric and adolescent vaccination
represents a recovery trend related to improved COVID-19 pandemic
conditions.
In the comparator period, vaccines sales declined by 6% CER primarily
driven by the adverse impact of the COVID-19 pandemic in the second
quarter.
Meningitis
Meningitis sales grew 6% AER, 12% CER to GBP415 million. Bexsero
sales grew 10% AER, 15% CER to GBP299 million, reflecting higher
demand together with increased market share in the US and strong
performance in Europe.
Menveo sales were up 26% AER, 36% CER to GBP98 million, primarily
driven by higher demand and increased market share in the US.
Influenza
Fluarix/FluLaval sales grew 42% AER, 53% CER, to GBP51 million driven
by higher southern hemisphere demand in International.
Shingles
Shingrix declined by 36% AER, 31% CER to GBP622 million, primarily
driven by lower demand in the US due to prioritised focus on COVID-19
mass vaccination of older adults together with US channel stocking
and favourable prior period returns and rebates movements in the
comparator period. Growth from launches in the UK and in China was
mostly offset by a decline in Germany and in Canada where COVID-19
related restrictions and prioritisation of COVID-19 mass vaccination
limited Shingrix uptake.
Established Vaccines
Hepatitis vaccines were down 31% AER, 27% CER to GBP205 million,
adversely impacted in the US and Europe by lower demand due to COVID-19
pandemic conditions, travel restrictions in Europe and competitive
pressure in the US market.
Sales of DTPa-containing vaccines (Infanrix, Pediarix and Boostrix)
grew by 5% AER, 11% CER. Infanrix/Pediarix sales declined 9% AER,
3% CER to GBP272 million, reflecting supply constraints and lower
tender demand in Europe together with the change in recommendation
for the dosing schedule in Germany, partly offset by increased channel
stock replenishment on wholesaler purchasing patterns and demand
in the US. Boostrix sales grew 28% AER, 34% CER to GBP240 million,
largely driven by higher demand in International together with favourable
US wholesaler purchasing patterns and higher demand.
Rotarix sales were down 12% AER, 8% CER to GBP246 million, reflecting
unfavourable phasing in Emerging Markets.
Synflorix sales declined by 12% AER, 10% CER to GBP199 million,
primarily due to lower tender demand in Emerging Markets and Europe.
MMRV vaccines sales grew 5% AER, 9% CER to GBP117 million, largely
driven by improved supply in International.
Pandemic Vaccines
Pandemic vaccines sales of GBP260 million included GBP258 million
of pandemic adjuvant sales, which represented delivery of around
two-thirds of the contracted volumes with the US and Canadian governments.
Consumer Healthcare turnover
H1 2021
------------------------
Growth Growth
GBPm GBP% CER%
------ ------- -------
Oral health 1,358 (1) 5
Pain relief 1,109 (3) 2
Vitamins, minerals and supplements 708 (8) (4)
Respiratory health 453 (31) (26)
Digestive health and other 892 (5) 2
------ ------- -------
4,520 (7) (2)
Brands divested/under review 84 (78) (77)
------ ------- -------
4,604 (12) (7)
------ ------- -------
US 1,447 (20) (12)
Europe 1,219 (10) (9)
International 1,938 (8) (2)
------ ------- -------
4,604 (12) (7)
------ ------- -------
Consumer Healthcare sales for the six months sales declined 12% AER,
7% CER to GBP4,604 million largely driven by the divestment programme
which completed in Q1 2021 as well as the H1 2020 comparative including
a particularly strong first quarter given accelerated purchasing
due to the COVID-19 pandemic.
Sales excluding brands divested/under review declined 2% CER, which
compares to the same six months last year when sales increased 7%
CER excluding brands divested/under review on a pro-forma basis.
In addition to benefitting from the particularly strong first quarter
last year, H1 2020 was helped by 1 percentage point from the increased
retailer stocking ahead of a systems cutover in North America, which
subsequently reversed in the third quarter of 2020. Consistent with
the Q2 2021 results, the first half 2 year CAGRs are shared below,
to give more perspective on underlying performance trends adjusting
for the volatility in the first two quarters last year as a result
of the pandemic. The unprecedented negative impact of a historically
weak seasonal cold flu and nasal products continued to adversely
impact sales growth.
International sales excluding brands divested/under review grew high
single digit CER in emerging markets, with double digit growth in
China, Latin America, India and high single digit growth in Middle
East and Africa.
Oral health
Oral health sales decreased 1% AER and increased 5% CER to GBP1,358
million. In H1 2020 Oral health sales increased mid single digit.
Sensodyne delivered high single digit growth reflecting underlying
brand strength, continued innovation and good consumer up take in
traditional retail and ecommerce channels particularly in India and
in the US. Gum health delivered double digit growth, whilst Denture
care was flat. On a 2 year CAGR growth for the category was up 5%.
Pain relief
Pain relief sales decreased 3% AER and increased 2% CER to GBP1,109
million. In H1 2020, Pain relief sales increased mid single digit
on a pro-forma basis. Advil declined in the high teens (compared
to double digit growth in H1 2020) which was more than offset mainly
by growth in the mid-teens for Voltaren and to a lesser extent by
Panadol which was up low single digit. Voltaren growth was helped
by the successful Rx to OTC switch in the US last year. On a 2 year
CAGR growth for the category was up 4%.
Vitamins, minerals and supplements
Vitamins, minerals and supplements sales declined 8% AER, 4% CER
to GBP708 million. In H1 2020 Vitamins, minerals and supplements
sales increased high-teens per cent on a pro-forma basis. Caltrate
delivered double digit growth and Centrum grew low single digit (compared
with growth in the high teens in H1 2020) although this was more
than offset by double digit decline in Emergen-C which faced a particularly
challenging comparator in H1 2020 when volumes almost doubled. On
a 2 year CAGR the growth for the category was up 7%.
Respiratory health
Respiratory health sales declined 31% AER, 26% CER to GBP453 million.
In H1 2020, Respiratory health sales increased low double digit on
a pro-forma basis benefiting from accelerated purchasing in the first
quarter particularly on cold and flu products. Theraflu and Robitussin
declined double digit given the continued impact of a significantly
lower cold and flu season due to the COVID-19 pandemic and social
distancing and a strong H1 2020 comparator. Otrivin declined double
digit and Flonase was flat. On a 2 year CAGR the category was down
10%, with seasonal cold, flu and nasal down mid-teens and allergy
up low single digit.
Digestive health and other
Digestive health and other brands sales decreased 5% AER and increased
2% CER at GBP892 million. In H1 2020, Digestive health and other
brands declined low-single digits on a pro-forma basis. Growth in
Digestive health products offset a decline Smokers' health products,
with Skin health products up low single digit. On a 2 year CAGR the
growth for the category was flat.
Operating performance
Cost of sales
Total cost of sales as a percentage of turnover was 32.5%, 1.3 percentage
points lower at AER and 2.4 percentage points lower in CER terms
compared with H1 2020. This primarily reflected lower write downs
in a number of manufacturing sites and the unwind in Q1 2020 of the
fair market value uplift on inventory arising on completion of the
Consumer Healthcare Joint Venture with Pfizer.
Excluding these and other Adjusting items, Adjusted cost of sales
as a percentage of turnover was 29.6%, 0.5 percentage points higher
at AER but 0.4 percentage points lower at CER compared with H1 2020.
This reflected price benefits in Pharmaceuticals, including the benefit
from a prior period RAR adjustment, as well as reduced supply chain
costs partly offset by an adverse mix in Vaccines, primarily due
to the reduction in Shingrix sales in the US partly offset by beneficial
pandemic adjuvant mix, as well as higher supply chain costs and under-recoveries
resulting from lower demand.
Selling, general and administration
Total SG&A costs as a percentage of turnover were 32.7%, 1.0 percentage
points lower at AER and 1.6 percentage points lower at CER compared
with H1 2020.
Excluding Adjusting items, Adjusted SG&A costs as a percentage of
turnover were 31.0%, 0.8 percentage points lower at AER than in H1
2020 and 1.3 percentage points lower on a CER basis. Adjusted SG&A
costs declined 9% AER, 6% CER which reflected the tight control of
ongoing costs and reduced variable spending across all three businesses
as a result of the COVID-19 lockdowns, and the continuing benefit
of restructuring in Pharmaceuticals, Consumer Healthcare and support
functions. Over a third of this decline also reflected a favourable
legal settlement in H1 2021 compared to increased legal costs in
2020.
Research and development
Total R&D expenditure was GBP2,340 million (15.1% of turnover), down
6% AER, 1% CER, including a decrease in major restructuring charges
and impairments. Adjusted R&D expenditure was GBP2,242 million (14.5%
of turnover), 1% lower at AER, 5% higher at CER than in H1 2020.
Pharmaceuticals R&D expenditure was GBP1,717 million, down 3% AER,
up 3% CER, primarily driven by the increased investment in our Specialty
portfolio offset by a net reduction in Oncology. Efficiency savings
continued from the implementation of the One Development programme
for Pharmaceuticals and Vaccines as part of the Separation Preparation
restructuring programme and variable spending as a result of COVID-19
lockdowns.
The growth of the Specialty portfolio excluding Oncology investment
was driven primarily by our two COVID-19 treatment programmes (sotrovimab
and otilimab) as well as the progression of a number of other key
programmes, including bepirovirsen, the HBV antisense oligonucleotide
programme, depemokimab, the anti-IL5 for asthma and otilimab for
rheumatoid arthritis. In Oncology there has been increased investment
in Zejula and Jemperli, offset by a reduction in spend on Blenrep
following successful approval in Q3 2020 and feladilimab following
the decision to terminate two studies in April.
R&D expenditure in Vaccines was GBP412 million, up 24% AER, 26% CER,
reflecting increased investment in clinical programmes for meningitis
ABCWY and RSV, partly offset by efficiency savings from the implementation
of the One Development programme and variable spending as a result
of COVID-19 lockdowns. R&D expenditure in Consumer Healthcare was
GBP113 million.
Royalty income
Royalty income was GBP168 million (H1 2020: GBP142 million), up 18%
AER, 18% CER, primarily driven by higher sales of Gardasil.
Other operating income/(expense)
Net other operating income of GBP133 million (H1 2020: GBP1,769 million
income) primarily reflected a number of asset disposals including
the disposal of royalty rights on cabozantinib, disposal of a number
of Consumer brands and fair value uplifts on investments partly offset
by accounting charges of GBP208 million (H1 2020: GBP841 million)
arising from the re-measurement of the contingent consideration liabilities
related to the acquisitions of the former Shionogi-ViiV Healthcare
joint venture and the former Novartis Vaccines business and the liabilities
for the Pfizer put option and Pfizer and Shionogi preferential dividends
in ViiV Healthcare. This included a re-measurement charge of GBP259
million (H1 2020: GBP778 million) for the contingent consideration
liability due to Shionogi, primarily as a result of the unwinding
of the discount for GBP185 million and a charge for GBP74 million
from adjustments to sales forecasts partly offset by updated exchange
rate assumptions. H1 2020 included the net profit on disposal of
the Horlicks and other Consumer Healthcare brands of GBP2,815 million,
partly offset by the related loss on sale of the shares in Hindustan
Unilever of GBP476 million.
Operating profit
Total operating profit was GBP3,368 million in H1 2021 compared with
GBP4,864 million in H1 2020. This primarily reflected an unfavourable
comparison to the net profit on disposal in Q2 2020 of the Horlicks
and other Consumer brands and resultant sale of shares in Hindustan
Unilever. This was partly offset by lower major restructuring costs,
lower re-measurement charges on the contingent consideration liabilities
and the unwind in 2020 of the fair market value uplift on inventory
arising on completion of the Consumer Healthcare Joint Venture with
Pfizer.
Excluding these and other Adjusting items, Adjusted operating profit
was GBP4,039 million, 9% lower than H1 2020 at AER, but 3% higher
at CER on a turnover decline of 1% CER. The Adjusted operating margin
of 26.0% was 0.4 percentage points lower at AER, but 1.1 percentage
points higher on a CER basis than in H1 2020.
The increase in Adjusted operating profit primarily reflected the
benefit from incremental pandemic adjuvant sales, sales growth in
Pharmaceuticals and tight control of ongoing costs including reduced
promotional and variable spending across all three businesses as
a result of the COVID-19 lockdowns, favourable legal settlements
compared to increased legal costs in 2020 and benefits from continued
restructuring across the business. This was partly offset by lower
non-pandemic sales in Vaccines, primarily Shingrix, an adverse mix
in Vaccines as well as higher supply chain costs and under-recoveries,
divestments in Consumer Healthcare and increased investment in R&D
across Vaccines and Pharmaceuticals.
Contingent consideration cash payments which are made to Shionogi
and other companies reduce the balance sheet liability and hence
are not recorded in the income statement. Total contingent consideration
cash payments in H1 2021 amounted to GBP426 million (H1 2020: GBP455
million). This included cash payments made to Shionogi of GBP419
million (H1 2020: GBP445 million).
Adjusted operating profit by business
Pharmaceuticals operating profit was GBP2,360 million, up 9% AER,
22% CER on a turnover increase of 2% CER. The operating margin of
29.1% was 3.7 percentage points higher at AER than in H1 2020 and
5.0 percentage points higher on a CER basis. This primarily reflected
price benefits in Pharmaceuticals, including the benefit from a prior
period RAR adjustment, as well as reduced supply chain costs, the
tight control of ongoing costs, reduced variable spending as a result
of the COVID-19 lockdowns, a favourable legal settlement in 2021
compared to increased legal costs in 2020 and the continuing benefit
of restructuring. This was partly offset by increased investment
in R&D.
Vaccines operating profit was GBP820 million, down 27% AER, 17% CER
on flat turnover at CER. The operating margin of 29.3% was 8.9 percentage
points lower at AER than in H1 2020 and 6.7 percentage points lower
on a CER basis. This was primarily driven by higher supply chain
costs resulting from lower demand and adverse mix due to Shingrix
sales in the US, along with higher R&D spend to support key strategic
priorities. This was partly offset by pandemic adjuvant beneficial
mix and higher royalty income.
Consumer Healthcare operating profit was GBP1,033 million, down 20%
AER, 13% CER on a turnover decrease of 7% CER. The operating margin
of 22.4% was 2.1 percentage points lower at AER and 1.4 percentage
points lower on a CER basis than in H1 2020. This primarily reflected
the impact of divestments (1.7 percentage points), increased advertising
and promotion investment to resume pre-COVID-19 levels and increased
commodity costs and investment into manufacturing sites, partially
offset by synergy benefits from the Pfizer Joint Venture integration,
price increases and tight cost control.
Net finance costs
Total net finance costs were GBP376 million compared with GBP416
million in H1 2020. Adjusted net finance costs were GBP375 million
compared with GBP414 million in H1 2020. The decrease is primarily
as a result of reduced interest expense from lower debt levels and
favourable movements in foreign exchange rates, partly offset by
an adverse comparison to a fair value gain on interest rate swaps
in the 2020 comparator and lower interest income on overseas cash
post-closing of the divestment of Horlicks and other Consumer Healthcare
nutrition products in India and a number of other countries.
Share of after tax profits of associates and joint ventures
The share of after tax losses of associates and joint ventures was
GBP32 million (H1 2020: GBP28 million profits).
Loss on disposal of interest in associates
The net loss on disposal of interests in associates was GBP36 million,
primarily driven by a loss of disposal of our interest in the associate
Innoviva Inc.
Taxation
The charge of GBP190 million represented an effective tax rate on
Total results of 6.4% (H1 2020: 8.0%) and reflected the different
tax effects of the various Adjusting items, including a credit of
GBP325 million in Q2 2021 resulting from the revaluation of deferred
tax assets following enactment of the proposed change of UK corporation
tax rate from 19% to 25% (effective 1 April 2023). H1 2020 reflected
the disposal of the Horlicks and other Consumer brands and the subsequent
disposal of shares received in Hindustan Unilever. Tax on Adjusted
profit amounted to GBP684 million and represented an effective Adjusted
tax rate of 18.5% (H1 2020: 16.3%).
Issues related to taxation are described in Note 14, 'Taxation' in
the Annual Report 2020. The Group continues to believe it has made
adequate provision for the liabilities likely to arise from periods
which are open and not yet agreed by tax authorities. The ultimate
liability for such matters may vary from the amounts provided and
is dependent upon the outcome of agreements with relevant tax authorities.
Non-controlling interests
The allocation of Total earnings to non-controlling interests amounted
to GBP330 million (H1 2020: GBP291 million). The increase was primarily
due to an increased allocation of ViiV Healthcare profits of GBP136
million (H1 2020: GBP64 million), including reduced credits for re-measurement
of contingent consideration liabilities, partly offset by a reduced
allocation of Consumer Healthcare Joint Venture profits of GBP163
million (H1 2020: GBP196 million).
The allocation of Adjusted earnings to non-controlling interests
amounted to GBP462 million (H1 2020: GBP549 million). The reduction
in allocation primarily reflected a reduced allocation of Consumer
Healthcare Joint Venture profits of GBP222 million (H1 2020: GBP277
million) and a reduced allocation of ViiV Healthcare profits of GBP209
million (H1 2020: GBP241 million).
Earnings per share
Total EPS was 49.4p, compared with 77.0p in H1 2020. This primarily
reflected an unfavourable comparison to the net profit on disposal
in Q2 2020 of the Horlicks and other Consumer brands partly offset
by the related loss on sale of the shares in Hindustan Unilever,
as well as by a credit of GBP325 million to Taxation in Q2 2021 resulting
from the revaluation of deferred tax assets following enactment of
the proposed change of UK corporation tax rate from 19% to 25% (effective
1 April 2023), lower major restructuring costs and lower re-measurement
charges on the contingent consideration liabilities.
Adjusted EPS was 51.0p compared with 56.9p in H1 2020, down 10% AER
but up 2% CER, on a 3% CER increase in Adjusted operating profit
reflecting incremental pandemic adjuvant sales, sales increases in
Pharmaceuticals, tight cost control and favourable legal settlements,
lower interest costs and a lower non-controlling interest allocation
of Consumer Healthcare and ViiV profits, partly offset by lower non-pandemic
sales in Vaccines, primarily Shingrix and a higher effective tax
rate. The contribution to growth from COVID-19 solutions was approximately
7% AER, 7% CER.
Currency impact on H1 2021 results
The results for H1 2021 are based on average exchange rates, principally
GBP1/$1.39, GBP1/EUR1.15 and GBP1/Yen 149. Comparative exchange rates
are given on page 59. The period-end exchange rates were GBP1/$1.39,
GBP1/EUR1.17 and GBP1/Yen 153.
In the six months, turnover decreased 7% AER, 1% CER. Total EPS was
49.4p compared with 77.0p in H1 2020. Adjusted EPS was 51.0p compared
with 56.9p in H1 2020, down 10% AER but up 2% CER. The adverse currency
impact primarily reflected the strengthening in Sterling, particularly
against the US Dollar as well as the Japanese Yen. Exchange gains
or losses on the settlement of intercompany transactions had a negligible
impact on the negative currency impact of twelve percentage points
on Adjusted EPS.
Adjusting items
The reconciliations between Total results and Adjusted results for
H1 2021 and H1 2020 are set out below.
Six months ended 30 June 2021
Divestments,
significant
legal
Intangible Intangible Major and
Total amort- impair- restruct- Transaction- other Separation Adjusted
results isation ment uring related items costs results
GBPm GBPm GBPm GBPm GBPm GBPm GBPm GBPm
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Turnover 15,510 15,510
Cost of sales (5,034) 346 1 62 14 27 (4,584)
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Gross profit 10,476 346 1 62 14 27 10,926
Selling, general
and
administration (5,069) 158 (11) 109 (4,813)
Research and
development (2,340) 51 19 28 (2,242)
Royalty income 168 168
Other operating
income/(expense) 133 232 (365) -
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Operating profit 3,368 397 20 248 246 (349) 109 4,039
Net finance costs (376) 1 (375)
Loss on disposal
of interest in
associates (36) 36 -
Share of after
tax profits
of associates
and joint
ventures 32 32
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit before
taxation 2,988 397 20 249 246 (313) 109 3,696
Taxation (190) (77) (4) (53) (64) (275) (21) (684)
Tax rate % 6.4% 18.5%
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit after
taxation 2,798 320 16 196 182 (588) 88 3,012
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit
attributable
to
non-controlling
interests 330 132 462
Profit
attributable
to
shareholders 2,468 320 16 196 50 (588) 88 2,550
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Earnings per
share 49.4p 6.4p 0.3p 3.9p 1.0p (11.7)p 1.7p 51.0p
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Weighted average
number of shares
(millions) 4,999 4,999
------------ ------------
Six months ended 30 June 2020
Divestments,
significant
legal
Intangible Intangible Major and
Total amort- impair- restruct- Transaction- other Separation Adjusted
results isation ment uring related items costs results
GBPm GBPm GBPm GBPm GBPm GBPm GBPm GBPm
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Turnover 16,714 16,714
Cost of sales (5,648) 351 27 305 106 (4,859)
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Gross profit 11,066 351 27 305 106 11,855
Selling, general
and
administration (5,625) 17 288 (20) 6 18 (5,316)
Research and
development (2,488) 34 116 82 (1) (2,257)
Royalty income 142 142
Other operating
income/(expense) 1,769 1 832 (2,602) -
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Operating profit 4,864 385 160 676 918 (2,597) 18 4,424
Net finance costs (416) 1 1 (414)
Share of after
tax profits of
associates and
joint ventures 28 28
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit before
taxation 4,476 385 160 677 918 (2,596) 18 4,038
Taxation (357) (73) (28) (152) (114) 69 (3) (658)
Tax rate % 8.0% 16.3%
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit after
taxation 4,119 312 132 525 804 (2,527) 15 3,380
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Profit
attributable
to
non-controlling
interests 291 258 549
Profit
attributable
to
shareholders 3,828 312 132 525 546 (2,527) 15 2,831
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Earnings per
share 77.0p 6.3p 2.6p 10.5p 11.0p (50.8)p 0.3p 56.9p
------------ ------------ ------------ ------------ ------------ ------------ ------------ ------------
Weighted average
number of shares
(millions) 4,971 4,971
------------ ------------
Major restructuring and integration
Within the Pharmaceuticals sector, the highly regulated manufacturing
operations and supply chains and long lifecycle of the business mean
that restructuring programmes, particularly those that involve the
rationalisation or closure of manufacturing or R&D sites are likely
to take several years to complete.
Total Major restructuring charges incurred in H1 2021 were GBP248
million (H1 2020: GBP676 million), analysed as follows:
H1 2021 H1 2020
------------------------- -------------------------
Cash Non-cash Total Cash Non-cash Total
GBPm GBPm GBPm GBPm GBPm GBPm
------ --------- ------ ------ --------- ------
2018 major restructuring
programme (incl. Tesaro) 10 3 13 56 170 226
Consumer Healthcare
Joint
Venture integration
programme 75 2 77 139 17 156
Separation Preparation
restructuring programme 183 (1) 182 279 3 282
Combined restructuring
and
integration programme 4 (28) (24) - 12 12
272 (24) 248 474 202 676
------ --------- ------ ------ --------- ------
Cash charges of GBP183 million under the Separation Preparation programme
primarily arose from restructuring of some administrative and central
manufacturing functions as well as commercial pharmaceuticals and
R&D functions.
Cash charges of GBP75 million on the Consumer Healthcare Joint Venture
programme primarily related to severance and integration costs. The
non-cash credit in the Combined restructuring and integration programme
primarily reflected a write back on disposal of a site.
Total cash payments made in H1 2021 were GBP408 million (H1 2020:
GBP331 million), GBP214 million (H1 2020: GBP31 million) relating
to the Separation Preparation restructuring programme, a further
GBP108 million (H1 2020: GBP135 million) relating to the Consumer
Healthcare Joint Venture integration programme, GBP54 million (H1
2020: GBP100 million) under the 2018 major restructuring programme
including the settlement of certain charges accrued in previous quarters
and GBP32 million (H1 2020: GBP65 million) for the existing Combined
restructuring and integration programme.
H1 2021 H1 2020
GBPm GBPm
-------- --------
Pharmaceuticals 91 216
Vaccines (40) 196
Consumer Healthcare 85 179
-------- --------
136 591
Corporate & central functions 112 85
-------- --------
Total Major restructuring costs 248 676
-------- --------
The analysis of Major restructuring charges by Income statement line
was as follows:
H1 2021 H1 2020
GBPm GBPm
-------- --------
Cost of sales 62 305
Selling, general and administration 158 288
Research and development 28 82
Other operating income - 1
Total Major restructuring costs 248 676
-------- --------
The benefit in the six months from restructuring programmes was GBP0.3
billion, the Consumer Healthcare Joint Venture integration was GBP0.1
billion, the benefit from the Separation Preparation restructuring
programme was GBP0.1 billion and the benefit from the 2018 Restructuring
programme was GBP0.1 billion.
The 2018 major restructuring programme, including Tesaro, is now
expected to cost GBP1.6 billion to the end of 2021, with cash costs
of GBP0.7 billion and non-cash costs of GBP0.9 billion, and is expected
to deliver annual savings of around GBP0.45 billion by the end of
2021 (at 2019 rates). These savings are intended to be fully re-invested
to help fund targeted increases in R&D and commercial support of
new products.
The completion of the Consumer Healthcare Joint Venture with Pfizer
is expected to realise substantial cost synergies, generating total
annual cost savings of GBP0.5 billion by 2022 for expected cash costs
of GBP0.7 billion and non-cash charges expected to be GBP0.1 billion,
plus additional capital expenditure of GBP0.2 billion. Up to 25%
of the cost savings are intended to be reinvested in the business
to support innovation and other growth opportunities.
The Group initiated in Q1 2020 a two-year Separation Preparation
programme to prepare for the separation of GSK into two companies:
New GSK, a biopharma company with an R&D approach focused on science
related to the immune system, the use of genetics and new technologies,
and a new leader in Consumer Healthcare. The programme aims to
-- Drive a common approach to R&D with improved capital allocation
-- Align and improve the capabilities and efficiency of global support
functions to support New GSK
-- Further optimise the supply chain and product portfolio, including
the divestment of non-core assets. A strategic review of prescription
dermatology is underway
-- Prepare Consumer Healthcare to operate as a standalone company
The programme now expects to deliver GBP0.8 billion of annual savings
by 2022 and GBP1.0 billion by 2023, with total costs estimated at
GBP2.4 billion, of which GBP1.6 billion is expected to be cash costs.
The proceeds of delivered and anticipated divestments are largely
expected to cover the cash costs of the programme.
Transaction-related adjustments
Transaction-related adjustments resulted in a net charge of GBP246
million (H1 2020: GBP918 million). This included a net GBP208 million
accounting charge for the re-measurement of the contingent consideration
liabilities related to the acquisitions of the former Shionogi-ViiV
Healthcare joint venture and the former Novartis Vaccines business
and the liabilities for the Pfizer put option and Pfizer and Shionogi
preferential dividends in ViiV Healthcare.
H1 2021 H1 2020
Charge/(credit) GBPm GBPm
-------- --------
Contingent consideration on former Shionogi-ViiV
Healthcare joint venture
(including Shionogi preferential dividends) 259 778
ViiV Healthcare put options and Pfizer preferential
dividends (90) 59
Contingent consideration on former Novartis Vaccines
business 39 4
Release of fair value uplift on acquired Pfizer
inventory - 91
Other adjustments 38 (14)
-------- --------
Total transaction-related charges 246 918
-------- --------
The GBP259 million charge relating to the contingent consideration
for the former Shionogi-ViiV Healthcare joint venture represented
an increase in the valuation of the contingent consideration due
to Shionogi, primarily as a result of the unwind of the discount
for GBP185 million and a charge of GBP74 million primarily from adjustments
to sales forecasts partly offset by updated exchange rate assumptions.
The GBP90 million credit relating to the ViiV Healthcare put option
and Pfizer preferential dividends represented a reduction in the
valuation of the put option as a result of lower cash following preference
dividend payments and updated exchange rate assumptions.
The ViiV Healthcare contingent consideration liability is fair valued
under IFRS. The potential impact of the COVID-19 pandemic remains
uncertain and at 30 June 2021, it has been assumed that there will
be no significant impact on the long-term value of the liability.
This position remains under review and the amount of the liability
will be updated in future quarters as further information on the
impact of the pandemic becomes available. An explanation of the accounting
for the non-controlling interests in ViiV Healthcare is set out on
page 11.
Divestments, significant legal charges and other items
Divestments and other items also included gains from a number of
asset disposals, including the disposal of royalty rights on cabozantinib
and disposal of a number of Consumer brands, fair value gains on
investments and certain other Adjusting items. The Consumer Brands
disposal programme is complete and has delivered net proceeds of
GBP1.1 billion. In Q2 2021 the net loss on disposal of interests
in associates was GBP36 million, primarily driven by a loss on disposal
of the interest in the associate Innoviva Inc. A credit of GBP12
million (H1 2020: GBP6 million charge) was recorded for significant
legal matters arising in the quarter. Significant legal cash payments
were GBP2 million (H1 2020: GBP6 million).
Separation costs
From Q2 2020, the Group started to report additional costs to prepare
for Consumer Healthcare separation. Separation costs incurred in
H1 2021 were GBP109 million (H1 2020: GBP18 million). Separation
costs incurred to date were GBP177 million. Total separation costs
are estimated to be GBP600-700 million, excluding transaction costs.
Cash generation
Cash flow
Q2 2021 H1 2021 H1 2020
-------- -------- --------
Net cash inflow from operating activities
(GBPm) 1,292 1,623 3,725
Free cash inflow* (GBPm) 316 313 2,480
Free cash flow growth (%) (84)% (87)% >100%
Free cash flow conversion* (%) 23% 13% 65%
Net debt** (GBPm) 21,921 21,921 23,435
-------- -------- --------
* Free cash flow and free cash flow conversion are defined on page
67 .
** Net debt is analysed on page 65.
Q2 2021
The net cash inflow from operating activities for the quarter was
GBP1,292 million (Q2 2020: GBP2,760 million). The decrease primarily
reflected an adverse comparison to the significant reduction in trade
receivables in Q2 2020 as a result of collections following strong
sales in Q1 2020, adverse timing of returns and rebates and taxes
compared to Q2 2020, partly offset by increased operating profit
net of adverse exchange and a lower seasonal increase in inventory.
Total cash payments to Shionogi in relation to the ViiV Healthcare
contingent consideration liability in the quarter were GBP203 million
(Q2 2020: GBP232 million), of which GBP177 million was recognised
in cash flows from operating activities and GBP26 million was recognised
in contingent consideration paid within investing cash flows. These
payments are deductible for tax purposes.
Free cash inflow was GBP316 million for the quarter (Q2 2020: GBP1,949
million inflow). The decrease primarily reflected an adverse comparison
to the significant reduction in trade receivables in Q2 2020 as a
result of collections following strong sales in Q1 2020, adverse
timing of returns and rebates and taxes compared to Q2 2020, increased
purchases of intangible assets and reduced proceeds from intangible
assets as the Consumer Brands Disposal programme is now complete.
This was partly offset by increased operating profit, a lower seasonal
increase in inventory and lower dividends to non-controlling interests.
H1 2021
The net cash inflow from operating activities for the six months
was GBP1,623 million (H1 2020: GBP3,725 million). The decrease primarily
reflected adverse exchange impacts, reduction in trade receivables
in H1 2020 as a result of collections following strong sales in Q1
2020, adverse timing of returns and rebates and taxes compared to
Q2 2020 and increased inventory, partly offset by increased operating
profit.
Total cash payments to Shionogi in relation to the ViiV Healthcare
contingent consideration liability in the six months were GBP419
million (H1 2020: GBP445 million), of which GBP366 million was recognised
in cash flows from operating activities and GBP53 million was recognised
in contingent consideration paid within investing cash flows. These
payments are deductible for tax purposes.
Free cash inflow was GBP313 million for the six months (H1 2020:
GBP2,480 million inflow). The decrease primarily reflected adverse
exchange impacts, reduction in trade receivables in H1 2020 as a
result of collections following strong sales in Q1 2020, adverse
timing of returns and rebates and taxes compared to Q2 2020 increased
inventory, increased purchases of intangible assets and reduced proceeds
from intangible assets as the Consumer Brands Disposal programme
is now complete. This was partly offset by increased operating profit
and lower dividends to non-controlling interests.
Net debt
At 30 June 2021, net debt was GBP21.9 billion, compared with GBP20.8
billion at 31 December 2020, comprising gross debt of GBP25.5 billion
and cash and liquid investments of GBP3.6 billion. Net debt increased
due to the dividends paid to shareholders of GBP2.1 billion and additional
investments of GBP0.1 billion, partly offset by GBP0.3 billion free
cash flow, GBP0.4 billion proceeds from investments, including GBP0.3
billion proceeds from the Innoviva disposal and GBP0.4 billion of
net favourable exchange impacts from the translation of non-Sterling
denominated debt and exchange on other financing items.
At 30 June 2021, GSK had short-term borrowings (including overdrafts
and lease liabilities) repayable within 12 months of GBP5.0 billion
with loans of GBP2.6 billion repayable in the subsequent year.
Returns to shareholders
Quarterly dividends
The Board has declared a second interim dividend for 2021 of 19 pence
per share (Q2 2020: 19 pence per share).
GSK recognises the importance of dividends to shareholders and aims
to distribute regular dividend payments that will be determined primarily
with reference to the free cash flow generated by the business after
funding the investment necessary to support the Group's future growth.
The Board currently intends to maintain the dividend for 2021 at
the current level of 80p per share, subject to any material change
in the external environment or performance expectations.
At the New GSK Investor Update on 23 June GSK set out that from 2022
a progressive dividend policy will be implemented, guided by a 40
to 60 percent pay-out ratio through the investment cycle. This is
a key part of the capital allocation framework. For 2022, for the
first half of the year, GSK expects to declare a 27p dividend for
the current group. GSK is on track to separate into two companies
early in the second half of 2022. GSK expects the aggregate dividend,
across the two new businesses to be 28p per share for the second
half. In aggregate this would represent on a full year 2022 basis
the equivalent of a Group dividend of 55p per share, representing
a 31% decrease from the 80p/share dividend expected for 2021. This
expected, aggregate 55p per share dividend for full year 2022 is
comprised of 44p representing New GSK's policy, and an expected 11p
from the Consumer Healthcare business. Dividend policy for the new
Consumer Healthcare company will be set by its Board of Directors.
In 2023, the first full year of standalone operations for New GSK,
GSK expects to declare a full year dividend of 45p per share.
Payment of dividends
The equivalent interim dividend receivable by ADR holders will be
calculated based on the exchange rate on 5 October 2021. An annual
fee of $0.03 per ADS (or $0.0075 per ADS per quarter) is charged
by the Depositary.
The ex-dividend date will be 19 August 2021, with a record date of
20 August 2021 and a payment date of 7 October 2021.
Paid/ Pence per
payable share GBPm
------------- ---------- -----
2021
First interim 8 July 2021 19 951
7 October
Second interim 2021 19 951
----------
2020
First interim 9 July 2020 19 946
8 October
Second interim 2020 19 946
14 January
Third interim 2021 19 946
Fourth interim 8 April 2021 23 1,151
--- ------
80 3,989
--- ------
Weighted average number of shares
Q2 2021 Q2 2020
millions millions
---------- ----------
Weighted average number of shares
- basic 5,004 4,977
Dilutive effect of share options
and share awards 56 46
---------- ----------
Weighted average number of shares
- diluted 5,060 5,023
---------- ----------
Weighted average number of shares
H1 2021 H1 2020
millions millions
---------- ----------
Weighted average number of shares
- basic 4,999 4,971
Dilutive effect of share options
and share awards 43 46
---------- ----------
Weighted average number of shares
- diluted 5,042 5,017
---------- ----------
At 30 June 2021, 5,005 million shares (30 June 2020: 4,977 million)
were in free issue (excluding Treasury shares and shares held by
the ESOP Trusts). GSK made no share repurchases during the period.
The company issued 0.3 million shares under employee share schemes
in the quarter for proceeds of GBP4 million (Q2 2020: GBP3 million).
At 30 June 2021, the ESOP Trust held 26.8 million GSK shares against
the future exercise of share options and share awards. The carrying
value of GBP93 million has been deducted from other reserves. The
market value of these shares was GBP386 million.
At 30 June 2021, the company held 355.2 million Treasury shares at
a cost of GBP4,969 million, which has been deducted from retained
earnings.
Financial information
Income statements
Q2 2021 Q2 2020 H1 2021 H1 2020
GBPm GBPm GBPm GBPm
-------- -------- -------- --------
TURNOVER 8,092 7,624 15,510 16,714
Cost of sales (2,554) (2,449) (5,034) (5,648)
-------- -------- -------- --------
Gross profit 5,538 5,175 10,476 11,066
Selling, general and administration (2,642) (2,709) (5,069) (5,625)
Research and development (1,222) (1,301) (2,340) (2,488)
Royalty income 77 75 168 142
Other operating income/(expense) (76) 1,610 133 1,769
-------- -------- -------- --------
OPERATING PROFIT 1,675 2,850 3,368 4,864
Finance income 7 1 17 42
Finance expense (192) (229) (393) (458)
Loss on disposal of interests
in associates (36) - (36) -
Share of after tax profits
of
associates and joint ventures 16 19 32 28
-------- -------- -------- --------
PROFIT BEFORE TAXATION 1,470 2,641 2,988 4,476
Taxation 68 (201) (190) (357)
Tax rate % (4.6)% 7.6% 6.4% 8.0%
-------- -------- -------- --------
PROFIT AFTER TAXATION 1,538 2,440 2,798 4,119
-------- -------- -------- --------
Profit attributable to non-controlling
interests 143 177 330 291
Profit attributable to shareholders 1,395 2,263 2,468 3,828
-------- -------- -------- --------
1,538 2,440 2,798 4,119
-------- -------- -------- --------
EARNINGS PER SHARE 27.9p 45.5p 49.4p 77.0p
-------- -------- -------- --------
Diluted earnings per share 27.6 p 45.0p 48.9 p 76.3p
-------- -------- -------- --------
Statement of comprehensive income
Q2 2021 Q2 2020
GBPm GBPm
-------- --------
Profit for the period 1,538 2,440
Items that may be reclassified subsequently to income
statement:
Exchange movements on overseas net assets and net
investment hedges 60 182
Reclassification of exchange movements on liquidation
or disposal of overseas
subsidiaries and associates (10) 36
Fair value movements on cash flow hedges 9 (5)
Reclassification of cash flow hedges to income statement 2 51
Deferred tax on fair value movements on cash flow
hedges (3) (3)
58 261
-------- --------
Items that will not be reclassified to income statement:
Exchange movements on overseas net assets of non-controlling
interests (3) 42
Fair value movements on equity investments (78) 224
Tax on fair value movements on equity investments (16) (24)
Re-measurement gains/(losses) on defined benefit
plans 258 (1,445)
Tax on re-measurement gains/(losses) on defined
benefit plans (40) 279
-------- --------
121 (924)
-------- --------
Other comprehensive income/(expense) for the period 179 (663)
-------- --------
Total comprehensive income for the period 1,717 1,777
-------- --------
Total comprehensive income for the period attributable
to:
Shareholders 1,577 1,558
Non-controlling interests 140 219
-------- --------
1,717 1,777
-------- --------
Statement of comprehensive income
H1 2021 H1 2020
GBPm GBPm
-------- --------
Profit for the period 2,798 4,119
Items that may be reclassified subsequently to income
statement:
Exchange movements on overseas net assets and net
investment hedges (207) 360
Reclassification of exchange movements on liquidation
or disposal of overseas
subsidiaries and associates (10) 36
Fair value movements on cash flow hedges (2) (23)
Reclassification of cash flow hedges to income statement 16 52
Deferred tax on fair value movements on cash flow
hedges (3) (3)
(206) 422
-------- --------
Items that will not be reclassified to income statement:
Exchange movements on overseas net assets of non-controlling
interests (37) 95
Fair value movements on equity investments 158 185
Tax on fair value movements on equity investments 38 (14)
Re-measurement gains/(losses) on defined benefit
plans 281 (445)
Tax on re-measurement gains/(losses) on defined
benefit plans (52) 92
-------- --------
388 (87)
-------- --------
Other comprehensive income for the period 182 335
-------- --------
Total comprehensive income for the period 2,980 4,454
-------- --------
Total comprehensive income for the period attributable
to:
Shareholders 2,687 4,068
Non-controlling interests 293 386
-------- --------
2,980 4,454
-------- --------
Pharmaceuticals turnover - three months ended 30 June 2021
Total US Europe International
------------------------------------- ------------------------------------- ------------------------------------- -------------------------------------
Growth Growth Growth Growth
----------------------- ----------------------- ----------------------- -----------------------
GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER%
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Respiratory 717 25 36 463 22 37 150 23 25 104 41 51
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Anoro Ellipta 134 (4) 4 77 (13) (5) 36 12 19 21 11 16
Trelegy Ellipta 291 50 64 204 46 64 49 36 36 38 >100 >100
Nucala 292 21 32 182 21 35 65 20 22 45 22 35
HIV 1,235 4 14 716 (3) 8 292 8 11 227 30 43
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Dolutegravir
products 1,189 4 14 691 (5) 6 280 8 12 218 40 54
Tivicay 407 9 21 196 (6) 5 72 (17) (15) 139 78 >100
Triumeq 466 (20) (13) 292 (23) (14) 112 (16) (14) 62 (16) (11)
Juluca 132 17 28 101 12 24 27 29 38 4 >100 >100
Dovato 184 >100 >100 102 >100 >100 69 >100 >100 13 >100 >100
Rukobia 10 - - 10 - - - - - - - -
Cabenuva 4 - - 3 - - 1 - - - - -
Other 32 (30) (31) 12 (22) (18) 11 - (18) 9 (53) (48)
Immuno-
inflammation 233 32 46 179 17 30 17 42 42 37 >100 >100
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Benlysta 214 21 34 179 17 30 17 42 42 18 50 83
Sotrovimab 16 - - - - - - - - 16 - -
Oncology 119 55 69 68 45 62 49 58 61 2 >100 >100
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Zejula 98 27 38 54 15 30 41 37 40 3 >100 >100
Blenrep 21 - - 14 - - 8 - - (1) - -
Jemperli 1 - - - - - 1 - - - - -
New and Specialty
Pharmaceuticals 2,304 14 25 1,426 8 21 508 17 19 370 42 58
Established
Pharmaceuticals 1,925 (8) - 560 16 29 455 (8) (6) 910 (18) (10)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Established
Respiratory 1,089 (2) 6 478 27 42 250 (9) (7) 361 (22) (15)
Arnuity Ellipta 10 25 50 9 50 67 - - - 1 (50) -
Avamys/Veramyst 63 2 10 - - - 20 5 5 43 - 12
Flixotide/Flovent 105 (10) (1) 68 26 39 15 (12) (12) 22 (52) (43)
Incruse Ellipta 53 (10) (2) 29 (12) (3) 19 - - 5 (29) -
Relvar/Breo
Ellipta 312 29 40 153 84 >100 84 8 10 75 (7) 2
Seretide/Advair 347 (18) (11) 132 (8) 3 79 (30) (27) 136 (18) (12)
Ventolin 167 16 27 89 53 74 25 4 13 53 (15) (11)
Other Respiratory 32 (48) (46) (2) >(100) >(100) 8 33 17 26 (53) (53)
Dermatology 102 7 16 - - - 35 17 20 67 5 16
Augmentin 91 (9) (1) - - - 29 38 38 62 (22) (11)
Avodart 85 (37) (32) - - - 30 (23) (21) 55 (41) (35)
Imigran/Imitrex 26 (4) 4 7 (30) (20) 12 - - 7 40 60
Lamictal 116 (14) (6) 55 (17) (6) 28 - - 33 (20) (10)
Seroxat/Paxil 30 (17) (8) - - - 8 - - 22 (21) (11)
Valtrex 23 (8) - 2 (33) - 9 29 29 12 (20) (13)
Other 363 (14) (7) 18 (25) (29) 54 (28) (23) 291 (10) (2)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Pharmaceuticals 4,229 3 12 1,986 10 23 963 3 6 1,280 (7) 3
-------- -------- -------- -------- ---------- -------- -------- --------- -------- -------- --------- --------
Pharmaceuticals turnover - six months ended 30 June 2021
Total US Europe International
------------------------------------- ------------------------------------- ------------------------------------- -------------------------------------
Growth Growth Growth Growth
----------------------- ----------------------- ----------------------- -----------------------
GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER%
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Respiratory 1,336 22 31 849 23 35 293 12 12 194 37 45
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Anoro Ellipta 251 (2) 4 140 (7) 1 72 6 6 39 5 11
Trelegy Ellipta 539 39 49 377 38 51 94 21 21 68 94 >100
Nucala 546 21 29 332 25 37 127 9 9 87 24 34
HIV 2,266 (5) 1 1,313 (9) (1) 579 (2) (2) 374 5 13
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Dolutegravir
products 2,179 (5) 1 1,267 (11) (2) 560 (1) - 352 10 19
Tivicay 708 (10) (3) 359 (15) (7) 147 (24) (23) 202 19 32
Triumeq 902 (21) (16) 548 (23) (16) 233 (20) (19) 121 (15) (11)
Juluca 244 5 12 184 - 9 53 18 20 7 75 75
Dovato 325 >100 >100 176 87 >100 127 >100 >100 22 >100 >100
Rukobia 17 - - 17 - - - - - - - -
Cabenuva 6 - - 5 - - 1 - - - - -
Other 64 (30) (28) 24 (18) (13) 18 (31) (38) 22 (39) (34)
Immuno-
inflammation 413 26 37 324 16 27 33 27 27 56 >100 >100
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Benlysta 392 20 30 324 16 27 33 27 27 35 52 70
Sotrovimab 16 - - - - - - - - 16 - -
Oncology 229 45 53 133 40 53 92 44 44 4 >100 >100
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Zejula 186 18 24 105 11 21 77 22 22 4 >100 >100
Blenrep 42 - - 28 - - 15 - - (1) - -
Jemperli 1 - - - - - 1 - - - - -
New and Specialty
Pharmaceuticals 4,244 7 14 2,619 4 14 997 6 6 628 21 30
Established
Pharmaceuticals 3,867 (15) (9) 1,080 3 12 916 (19) (19) 1,871 (20) (14)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Established
Respiratory 2,216 (9) (3) 920 11 21 508 (16) (15) 788 (21) (15)
Arnuity Ellipta 16 (6) 6 13 - 8 - - - 3 (25) -
Avamys/Veramyst 166 (3) 4 - - - 36 (5) (5) 130 (2) 6
Flixotide/Flovent 222 (7) - 138 33 44 31 (31) (31) 53 (42) (36)
Incruse Ellipta 105 (9) (4) 56 (11) (3) 37 (5) (5) 12 (14) (7)
Relvar/Breo
Ellipta 580 10 17 265 34 46 166 1 1 149 (9) (2)
Seretide/Advair 698 (14) (10) 249 - 9 174 (28) (27) 275 (16) (11)
Ventolin 356 (10) (4) 201 (2) 7 50 (19) (18) 105 (19) (15)
Other Respiratory 73 (50) (47) (2) >(100) >100 14 - - 61 (54) (53)
Dermatology 202 (2) 4 - - - 69 1 1 133 (3) 6
Augmentin 182 (32) (27) - - - 52 (33) (33) 130 (32) (25)
Avodart 168 (39) (36) 1 (67) (67) 60 (32) (31) 107 (42) (38)
Imigran/Imitrex 51 (16) (13) 15 (40) (36) 24 (4) (4) 12 9 18
Lamictal 232 (15) (9) 110 (19) (11) 56 (7) (7) 66 (14) (8)
Seroxat/Paxil 63 (12) (7) - - - 16 (11) (11) 47 (13) (6)
Valtrex 45 (15) (9) 5 (29) (14) 17 6 6 23 (23) (17)
Other 708 (20) (15) 29 (38) (38) 114 (35) (34) 565 (15) (9)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Pharmaceuticals 8,111 (5) 2 3,699 4 14 1,913 (8) (7) 2,499 (13) (6)
-------- -------- -------- -------- ---------- -------- -------- --------- -------- -------- --------- --------
Vaccines turnover - three months ended 30 June 2021
Total US Europe International
------------------------------------- ------------------------------------- ------------------------------------- -------------------------------------
Growth Growth Growth Growth
----------------------- ----------------------- ----------------------- -----------------------
GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER%
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Meningitis 225 35 46 109 >100 >100 96 25 27 20 (55) (43)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Bexsero 165 53 63 60 >100 >100 89 25 28 16 60 90
Menveo 59 55 71 49 >100 >100 5 - - 5 (64) (57)
Other 1 (95) (90) - - - 2 100 100 (1) >(100) (100)
Influenza 33 >100 >100 - - - - - - 33 >100 >100
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Fluarix,
FluLaval 33 >100 >100 - - - - - - 33 >100 >100
Shingles 295 (9) 1 237 (12) - 44 - 2 14 27 18
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Shingrix 295 (9) 1 237 (12) - 44 - 2 14 27 18
Established
Vaccines 758 21 28 239 78 99 157 (6) (4) 362 11 16
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Infanrix,
Pediarix 136 14 24 78 90 >100 27 (36) (33) 31 (14) (8)
Boostrix 146 92 >100 66 94 >100 35 30 33 45 >100 >100
Hepatitis 110 28 38 64 56 76 25 - 4 21 5 5
Rotarix 132 3 9 26 53 76 27 (4) - 79 (5) (2)
Synflorix 97 (6) (2) - - - 9 (10) (10) 88 (5) (1)
Priorix,
Priorix
Tetra,
Varilrix 54 - 6 - - - 24 (11) (7) 30 11 19
Cervarix 36 6 9 - - - 7 40 40 29 - 3
Other 47 68 79 5 >100 >100 3 - (33) 39 63 87
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Vaccines
excluding
pandemic
vaccines 1,311 16 24 585 31 46 297 3 6 429 8 14
Pandemic
vaccines 260 - - 211 - - - - - 49 - -
Pandemic
adjuvant 258 - - 211 - - - - - 47 - -
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Total
Vaccines 1,571 39 49 796 78 97 297 3 6 478 20 26
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Vaccines turnover - six months ended 30 June 2021
Total US Europe International
------------------------------------- ------------------------------------- ------------------------------------- -------------------------------------
Growth Growth Growth Growth
----------------------- ----------------------- ----------------------- -----------------------
GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER% GBPm GBP% CER%
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Meningitis 415 6 12 164 30 41 186 8 9 65 (31) (22)
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Bexsero 299 10 15 91 12 22 174 12 13 34 (6) 11
Menveo 98 26 36 73 62 76 9 (36) (36) 16 (16) (5)
Other 18 (57) (57) - - - 3 - - 15 (62) (62)
Influenza 51 42 53 - - - - - - 51 50 62
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Fluarix,
FluLaval 51 42 53 - - - - - - 51 50 62
Shingles 622 (36) (31) 506 (42) (36) 75 17 17 41 8 5
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Shingrix 622 (36) (31) 506 (42) (36) 75 17 17 41 8 5
Established
Vaccines 1,447 (6) (2) 420 (10) (1) 343 (14) (14) 684 1 4
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Infanrix,
Pediarix 272 (9) (3) 142 10 20 67 (30) (30) 63 (15) (9)
Boostrix 240 28 34 109 18 30 71 15 15 60 76 76
Hepatitis 205 (31) (27) 115 (32) (25) 49 (39) (38) 41 (18) (16)
Rotarix 246 (12) (8) 48 (17) (9) 57 (3) (2) 141 (13) (10)
Synflorix 199 (12) (10) - - - 21 (28) (28) 178 (10) (8)
Priorix,
Priorix
Tetra,
Varilrix 117 5 9 - - - 56 - 2 61 11 16
Cervarix 81 76 78 - - - 15 67 67 66 78 81
Other 87 (5) (3) 6 (65) (65) 7 (22) (44) 74 12 18
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Vaccines
excluding
pandemic
vaccines 2,535 (14) (9) 1,090 (25) (18) 604 (5) (5) 841 - 4
Pandemic
vaccines 260 - - 211 - - - - - 49 - -
Pandemic
adjuvant 258 - - 211 - - - - - 47 - -
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Total
Vaccines 2,795 (5) - 1,301 (11) (3) 604 (5) (5) 890 6 10
-------- -------- -------- -------- -------- -------- -------- -------- -------- -------- -------- --------
Balance sheet
31 December
30 June 2021 30 June 2020 2020
GBPm GBPm GBPm
------------- ------------- ------------
ASSETS
Non-current assets
Property, plant and equipment 9,831 10,490 10,176
Right of use assets 758 941 830
Goodwill 10,435 10,998 10,597
Other intangible assets 29,333 31,263 29,824
Investments in associates and
joint ventures 76 390 364
Other investments 3,159 2,174 3,060
Deferred tax assets 4,711 4,455 4,287
Derivative financial instruments 6 5 5
Other non-current assets 1,136 946 1,041
------------- ------------- ------------
Total non-current assets 59,445 61,662 60,184
------------- ------------- ------------
Current assets
Inventories 6,330 6,396 5,996
Current tax recoverable 605 328 671
Trade and other receivables 6,902 7,168 6,952
Derivative financial instruments 104 421 152
Liquid investments 59 87 78
Cash and cash equivalents 3,503 8,166 6,292
Assets held for sale 63 412 106
------------- ------------- ------------
Total current assets 17,566 22,978 20,247
------------- ------------- ------------
TOTAL ASSETS 77,011 84,640 80,431
------------- ------------- ------------
LIABILITIES
Current liabilities
Short-term borrowings (5,041) (5,964) (3,725)
Contingent consideration liabilities (743) (804) (765)
Trade and other payables (14,267) (15,450) (15,840)
Derivative financial instruments (91) (245) (221)
Current tax payable (411) (685) (545)
Short-term provisions (834) (776) (1,052)
------------- ------------- ------------
Total current liabilities (21,387) (23,924) (22,148)
------------- ------------- ------------
Non-current liabilities
Long-term borrowings (20,442) (25,726) (23,425)
Corporation tax payable (176) (195) (176)
Deferred tax liabilities (3,587) (3,967) (3,600)
Pensions and other post-employment
benefits (3,357) (3,999) (3,650)
Other provisions (653) (814) (707)
Derivative financial instruments (5) (24) (10)
Contingent consideration liabilities (5,017) (5,026) (5,104)
Other non-current liabilities (809) (828) (803)
------------- ------------- ------------
Total non-current liabilities (34,046) (40,579) (37,475)
------------- ------------- ------------
TOTAL LIABILITIES (55,433) (64,503) (59,623)
------------- ------------- ------------
NET ASSETS 21,578 20,137 20,808
------------- ------------- ------------
EQUITY
Share capital 1,347 1,346 1,346
Share premium account 3,299 3,278 3,281
Retained earnings 7,379 6,622 6,755
Other reserves 3,352 2,347 3,205
------------- ------------- ------------
Shareholders' equity 15,377 13,593 14,587
Non-controlling interests 6,201 6,544 6,221
------------- ------------- ------------
TOTAL EQUITY 21,578 20,137 20,808
------------- ------------- ------------
Statement of changes in equity
Share- Non-
Share Share Retained Other holders' controlling Total
capital premium earnings reserves equity interests equity
GBPm GBPm GBPm GBPm GBPm GBPm GBPm
------------ ------------ ------------ ------------ ------------ ------------ ------------
At 1 January 2021 1,346 3,281 6,755 3,205 14,587 6,221 20,808
Profit for the
period 2,468 2,468 330 2,798
Other
comprehensive
income/(expense)
for the period 14 205 219 (37) 182
------------ ------------ ------------ ------------ ------------
Total
comprehensive
income
for the period 2,482 205 2,687 293 2,980
------------ ------------ ------------ ------------ ------------
Distributions to
non-controlling
interests (320) (320)
Contributions from
non-controlling
interests 7 7
Dividends to
shareholders (2,097) (2,097) (2,097)
Shares issued 1 18 19 19
Realised after tax
profits
on disposal of
equity
investments 145 (145) - -
Share of
associates and
joint ventures
realised profits
on disposal
of equity
investments 9 (9) - -
Write-down on
shares held
by ESOP Trusts (96) 96 - -
Share-based
incentive plans 181 181 181
------------ ------------ ------------ ------------ ------------ ------------ ------------
At 30 June 2021 1,347 3,299 7,379 3,352 15,377 6,201 21,578
------------ ------------ ------------ ------------ ------------ ------------ ------------
At 1 January 2020 1,346 3,174 4,530 2,355 11,405 6,952 18,357
Profit for the
period 3,828 3,828 291 4,119
Other
comprehensive
income
for the period 41 199 240 95 335
------------ ------------ ------------ ------------ ------------
Total
comprehensive
income
for
the period 3,869 199 4,068 386 4,454
------------ ------------ ------------ ------------ ------------
Distributions to
non-controlling
interests (652) (652)
Contributions from
non-controlling
interests 3 3
Changes to
non-controlling
interests (145) (145)
Dividends to
shareholders (2,085) (2,085) (2,085)
Shares issued - 26 26 26
Realised after tax
profits
on disposal of
equity
investments 36 (36) - -
Shares acquired by
ESOP
Trusts 78 361 (439) - -
Write-down on
shares held
by ESOP Trusts (268) 268 - -
Share-based
incentive plans 179 179 179
------------ ------------ ------------ ------------ ------------ ------------ ------------
At 30 June 2020 1,346 3,278 6,622 2,347 13,593 6,544 20,137
------------ ------------ ------------ ------------ ------------ ------------ ------------
Cash flow statement - six months ended 30 June 2021
H1 2021 H1 2020
GBPm GBPm
-------- --------
Profit after tax 2,798 4,119
Tax on profits 190 357
Share of after tax profits of associates and
joint ventures (32) (28)
Loss on disposal of interests in associates 36 -
Net finance expense 376 416
Depreciation, amortisation and other adjusting
items 1,025 (971)
Increase in working capital (1,072) (476)
Contingent consideration paid (371) (393)
(Decrease)/increase in other net liabilities
(excluding contingent
consideration paid) (627) 1,251
-------- --------
Cash generated from operations 2,323 4,275
Taxation paid (700) (550)
-------- --------
Net cash inflow from operating activities 1,623 3,725
-------- --------
Cash flow from investing activities
Purchase of property, plant and equipment (440) (420)
Proceeds from sale of property, plant and equipment 102 12
Purchase of intangible assets (575) (326)
Proceeds from sale of intangible assets 384 636
Purchase of equity investments (122) (208)
Proceeds from sale of equity investments 171 2,871
Purchase of businesses, net of cash acquired 1 (6)
Contingent consideration paid (55) (62)
Disposal of businesses (19) 237
Investment in associates and joint ventures (1) (1)
Proceeds from disposal of associates and joint
ventures 277 -
Interest received 17 26
Decrease in liquid investments 18 -
Dividends from associates and joint ventures 9 14
-------- --------
Net cash (outflow)/inflow from investing activities (233) 2,773
-------- --------
Cash flow from financing activities
Issue of share capital 19 26
Increase in long-term loans - 2,354
Repayment of short-term loans (352) (3,018)
Repayment of lease liabilities (108) (111)
Interest paid (439) (476)
Dividends paid to shareholders (2,097) (2,085)
Distributions to non-controlling interests (320) (652)
Contributions from non-controlling interests 7 3
Other financing items (131) 278
-------- --------
Net cash outflow from financing activities (3,421) (3,681)
-------- --------
(Decrease)/increase in cash and bank overdrafts
in the period (2,031) 2,817
-------- --------
Cash and bank overdrafts at beginning of the
period 5,261 4,831
Exchange adjustments (34) 28
(Decrease)/increase in cash and bank overdrafts (2,031) 2,817
-------- --------
Cash and bank overdrafts at end of the period 3,196 7,676
-------- --------
Cash and bank overdrafts at end of the period
comprise:
Cash and cash equivalents 3,503 8,166
Cash and cash equivalents reported in assets
held for sale - 2
-------- --------
3,503 8,168
Overdrafts (307) (492)
-------- --------
3,196 7,676
-------- --------
Segment information
Operating segments are reported based on the financial information
provided to the Chief Executive Officer and the responsibilities
of the Corporate Executive Team (CET). GSK reports results under
four segments: Pharmaceuticals; Pharmaceuticals R&D; Vaccines and
Consumer Healthcare, and individual members of the CET are responsible
for each segment.
The Pharmaceuticals R&D segment is the responsibility of the Chief
Scientific Officer and President, R&D and is reported as a separate
segment. The operating profit of this segment excludes the ViiV Healthcare
operating profit (including R&D expenditure) that is reported within
the Pharmaceuticals segment.
The Group's management reporting process allocates intra-Group profit
on a product sale to the market in which that sale is recorded, and
the profit analyses below have been presented on that basis.
Corporate and other unallocated turnover and costs include the results
of certain Consumer Healthcare products which are being held for
sale in a number of markets in order to meet anti-trust approval
requirements, together with the costs of corporate functions.
Turnover by segment
Q2 2021 Q2 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Pharmaceuticals 4,229 4,102 3 12
Vaccines 1,571 1,133 39 49
Consumer Healthcare 2,292 2,389 (4) 3
-------- -------- ------- -------
Total turnover 8,092 7,624 6 15
-------- -------- ------- -------
Operating profit by segment
Q2 2021 Q2 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Pharmaceuticals 2,094 1,886 11 25
Pharmaceuticals R&D (853) (910) (6) 1
-------- -------- ------- -------
Pharmaceuticals including R&D 1,241 976 27 46
Vaccines 514 265 94 >100
Consumer Healthcare 498 521 (4) 5
-------- -------- ------- -------
Segment profit 2,253 1,762 28 46
Corporate and other unallocated
costs (95) (13)
-------- -------- ------- -------
Adjusted operating profit 2,158 1,749 23 43
Adjusting items (483) 1,101
-------- -------- ------- -------
Total operating profit 1,675 2,850 (41) (30)
Finance income 7 1
Finance costs (192) (229)
Loss on disposal of interests
in associates (36) -
Share of after tax profits of
associates
and joint ventures 16 19
-------- -------- ------- -------
Profit before taxation 1,470 2,641 (44) (32)
-------- -------- ------- -------
Turnover by segment
H1 2021 H1 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Pharmaceuticals 8,111 8,498 (5) 2
Vaccines 2,795 2,938 (5) -
Consumer Healthcare 4,604 5,251 (12) (7)
-------- -------- ------- -------
15,510 16,687 (7) (1)
Corporate and other unallocated
turnover - 27
-------- -------- ------- -------
Total turnover 15,510 16,714 (7) (1)
-------- -------- ------- -------
Operating profit by segment
H1 2021 H1 2020 Growth Growth
GBPm GBPm GBP% CER%
-------- -------- ------- -------
Pharmaceuticals 4,004 3,904 3 12
Pharmaceuticals R&D (1,644) (1,745) (6) -
-------- -------- ------- -------
Pharmaceuticals including R&D 2,360 2,159 9 22
Vaccines 820 1,123 (27) (17)
Consumer Healthcare 1,033 1,287 (20) (13)
-------- -------- ------- -------
Segment profit 4,213 4,569 (8) 3
Corporate and other unallocated
costs (174) (145)
-------- -------- ------- -------
Adjusted operating profit 4,039 4,424 (9) 3
Adjusting items (671) 440
-------- -------- ------- -------
Total operating profit 3,368 4,864 (31) (21)
Finance income 17 42
Finance costs (393) (458)
Loss on disposal of interests
in associates (36) -
Share of after tax profits of
associates
and joint ventures 32 28
-------- -------- ------- -------
Profit before taxation 2,988 4,476 (33) (23)
-------- -------- ------- -------
Legal matters
The Group is involved in significant legal and administrative proceedings,
principally product liability, intellectual property, tax, anti-trust,
consumer fraud and governmental investigations, which are more fully
described in the 'Legal Proceedings' note in the Annual Report 2020.
At 30 June 2021, the Group's aggregate provision for legal and other
disputes (not including tax matters described on page 22) was GBP0.2
billion (31 December 2020: GBP0.3 billion).
The Group may become involved in significant legal proceedings in
respect of which it is not possible to make a reliable estimate of
the expected financial effect, if any, that could result from ultimate
resolution of the proceedings. In these cases, the Group would provide
appropriate disclosures about such cases, but no provision would
be made.
The ultimate liability for legal claims may vary from the amounts
provided and is dependent upon the outcome of litigation proceedings,
investigations and possible settlement negotiations. The Group's
position could change over time, and, therefore, there can be no
assurance that any losses that result from the outcome of any legal
proceedings will not exceed by a material amount the amount of the
provisions reported in the Group's financial accounts.
Significant developments since the date of the Annual Report 2020
are as follows:
On 1 June 2021, the Court overseeing the Zofran Multidistrict Litigation
(MDL) in the District of Massachusetts granted GSK's motion for summary
judgment on federal preemption grounds. The District Court granted
judgment for GSK in all cases pending in the MDL and closed the MDL
proceeding. On 1 July 2021, Plaintiffs filed a notice to appeal the
preemption decision with respect to all cases in the MDL to the United
States Court of Appeals for the First Circuit.
Additional information
Accounting policies and basis of preparation
This unaudited Results Announcement contains condensed financial
information for the three and six months ended 30 June 2021, is prepared
in accordance with the Disclosure Guidance and Transparency Rules
(DTR) of the Financial Conduct Authority and United Kingdom adopted
IAS34 'Interim financial reporting' and should be read in conjunction
with the Annual Report 2020, which was prepared in accordance with
United Kingdom adopted International Financial Reporting Standards.
This Results Announcement has been prepared applying consistent accounting
policies to those applied by the Group in the Annual Report 2020.
The Group has not identified any changes to its key sources of accounting
judgements or estimations of uncertainty compared with those disclosed
in the Annual Report 2020.
This Results Announcement does not constitute statutory accounts
of the Group within the meaning of sections 434(3) and 435(3) of
the Companies Act 2006. The full Group accounts for 2020 were published
in the Annual Report 2020, which has been delivered to the Registrar
of Companies and on which the report of the independent auditor was
unqualified and did not contain a statement under section 498 of
the Companies Act 2006.
COVID-19 pandemic
The potential impact of the COVID-19 pandemic on GSK's trading performance
and all our principal risks has been assessed with mitigation plans
put in place. In the first half of 2021, as anticipated, the pandemic
impacted Group performance primarily in demand for Vaccines as a
result of governments' prioritisation of COVID-19 vaccination programmes
and of ongoing containment measures impacting customers' ability
and willingness to access vaccination services across all regions.
We remain confident in the underlying demand for our Vaccine products
and are encouraged by the rate at which COVID-19 vaccinations are
being deployed in many countries, particularly the US and UK, which
provides support for healthcare systems returning to normal, though
the pace varies in other key markets. We continue to monitor the
situation closely, as this continues to be a very dynamic and uncertain
situation, with the ultimate severity, duration and impact unknown
at this point including potential impacts on trading results, clinical
trials, supply continuity and our employees. The situation could
change at any time and there can be no assurance that the COVID-19
pandemic will not have a material adverse impact on the future results
of the Group.
Exchange rates
GSK operates in many countries, and earns revenues and incurs costs
in many currencies. The results of the Group, as reported in Sterling,
are affected by movements in exchange rates between Sterling and
other currencies. Average exchange rates, as modified by specific
transaction rates for large transactions, prevailing during the period,
are used to translate the results and cash flows of overseas subsidiaries,
associates and joint ventures into Sterling. Period-end rates are
used to translate the net assets of those entities. The currencies
which most influenced these translations and the relevant exchange
rates were:
Q2 2021 Q2 2020 H1 2021 H1 2020 2020
-------- -------- -------- -------- -----
Average rates:
US$/GBP 1.40 1.25 1.39 1.27 1.29
Euro/GBP 1.16 1.13 1.15 1.15 1.13
Yen/GBP 152 134 149 137 137
Period-end rates:
US$/GBP 1.39 1.23 1.39 1.23 1.36
Euro/GBP 1.17 1.10 1.17 1.10 1.11
Yen/GBP 153 132 153 132 141
During Q2 2021 average Sterling exchange rates were stronger against
the US Dollar, the Yen and the Euro compared with the same period
in 2020. During the six months ended 30 June 2021, average Sterling
exchange rates were stronger against the US Dollar and the Yen but
flat against the Euro compared with the same period in 2020. Period-end
Sterling exchange rates were stronger against the US Dollar, the
Euro and the Yen compared with the 2020 period-end rates.
Net assets
The book value of net assets increased by GBP770 million from GBP20,808
million at 31 December 2020 to GBP21,578 million at 30 June 2021.
This primarily reflected the Total profit for the period, the re-measurement
gains on the defined benefit plans and the increase in the fair value
of equity investments exceeding the adverse exchange movements and
the dividends paid during the period.
The carrying value of investments in associates and joint ventures
at 30 June 2021 was GBP76 million (31 December 2020: GBP364 million),
with a market value of GBP76 million (31 December 2020: GBP364 million).
During Q2 2021, the Group sold all of its shares in Innoviva Inc
back to Innoviva for GBP277 million.
At 30 June 2021, the net deficit on the Group's pension plans was
GBP1,882 million compared with GBP2,104 million at 31 December 2020.
The decrease in the net deficit primarily arose from an increase
in the rates used to discount UK pension liabilities from 1.4% to
1.9%, and US pension liabilities from 2.3% to 2.7%, partly offset
by an increase in the UK inflation rate from 2.8% to 3.1% and lower
UK assets.
The estimated present value of the potential redemption amount of
the Pfizer put option related to ViiV Healthcare, recorded in Other
payables in Current liabilities, was GBP870 million (31 December
2020: GBP960 million).
Contingent consideration amounted to GBP5,760 million at 30 June
2021 (31 December 2020: GBP5,869 million), of which GBP5,199 million
(31 December 2020: GBP5,359 million) represented the estimated present
value of amounts payable to Shionogi relating to ViiV Healthcare
and GBP504 million (31 December 2020: GBP477 million) represented
the estimated present value of contingent consideration payable to
Novartis related to the Vaccines acquisition.
Of the contingent consideration payable (on a post-tax basis) to
Shionogi at 30 June 2021, GBP717 million (31 December 2020: GBP745
million) is expected to be paid within one year.
Movements in contingent consideration are as follows :
ViiV Healthcare Group
H1 2021 GBPm GBPm
---------------- ------
Contingent consideration at beginning of the
period 5,359 5,869
Re-measurement through income statement 259 317
Cash payments: operating cash flows (366) (371)
Cash payments: investing activities (53) (55)
Contingent consideration at end of the period 5,199 5,760
---------------- ------
ViiV Healthcare Group
H1 2020 GBPm GBPm
---------------- ------
Contingent consideration at beginning of the
period 5,103 5,479
Re-measurement through income statement 778 806
Cash payments: operating cash flows (388) (393)
Cash payments: investing activities (57) (62)
Contingent consideration at end of the period 5,436 5,830
---------------- ------
Contingent liabilities and commitments
There were contingent liabilities at 30 June 2021 in respect of guarantees
and indemnities entered into as part of the ordinary course of the
Group's business. No material losses are expected to arise from such
contingent liabilities. Provision is made for the outcome of legal
and tax disputes where it is both probable that the Group will suffer
an outflow of funds and it is possible to make a reliable estimate
of that outflow. Descriptions of the significant legal disputes to
which the Group is a party are set out on page 58.
During the quarter on 14 June 2021 GSK signed an agreement with iTeos
Therapeutics. On 2 July 2021 GSK signed an agreement with Alector.
These agreements result in total contractual commitments of approximately
GBP956 million for upfront payments as at 30 June 2021 closing Dollar
spot rate. The agreement with Alector is conditional on review by
the appropriate regulatory agencies under the Hart-Scott-Rodino Act.
Financial instruments fair value disclosures
The following tables categorise the Group's financial assets and
liabilities held at fair value by the valuation methodology applied
in determining their fair value. Where possible, quoted prices in
active markets are used (Level 1). Where such prices are not available,
the asset or liability is classified as Level 2, provided all significant
inputs to the valuation model used are based on observable market
data. If one or more of the significant inputs to the valuation model
is not based on observable market data, the instrument is classified
as Level 3. Other investments classified as Level 3 in the tables
below comprise equity investments in unlisted entities with which
the Group has entered into research collaborations and also investments
in emerging life science companies.
Level Level Level
1 2 3 Total
At 30 June 2021 GBPm GBPm GBPm GBPm
------ ------ ------ ------
Financial assets at fair value
Financial assets at fair value
through other
comprehensive income (FVTOCI):
Other investments designated at
FVTOCI 2,796 - 183 2,979
Trade and other receivables - 1,653 - 1,653
Financial assets mandatorily at
fair value through
profit or loss (FVTPL):
Other investments - - 180 180
Other non-current assets - - 29 29
Trade and other receivables - 60 - 60
Held for trading derivatives that
are not in a
designated and effective hedging
relationship - 49 5 54
Cash and cash equivalents 915 - - 915
Derivatives designated and effective
as hedging
instruments - 56 - 56
3,711 1,818 397 5,926
------ ------ ------ ------
Financial liabilities at fair value
Financial liabilities mandatorily
at fair value through
profit or loss (FVTPL):
Contingent consideration liabilities - - (5,760) (5,760)
Held for trading derivatives that
are not in a
designated and effective hedging
relationship - (78) (5) (83)
Derivatives designated and effective
as hedging
instruments. - (13) - (13)
- (91) (5,765) (5,856)
---- ----- -------- --------
Level Level Level
1 2 3 Total
At 31 December 2020 GBPm GBPm GBPm GBPm
------ ------ ------ ------
Financial assets at fair value
Financial assets at fair value
through other
comprehensive income (FVTOCI):
Other investments designated at
FVTOCI 2,281 - 658 2,939
Trade and other receivables - 1,942 - 1,942
Financial assets mandatorily measured
at fair value
through profit or loss (FVTPL):
Other investments - - 121 121
Other non-current assets - - 30 30
Trade and other receivables - 46 - 46
Held for trading derivatives that
are not in a
designated and effective hedging
relationship - 63 5 68
Cash and cash equivalents 3,292 - - 3,292
Derivatives designated and effective
as hedging
instruments - 89 - 89
5,573 2,140 814 8,527
------ ------ ------ ------
Level Level Level
1 2 3 Total
At 31 December 2020 GBPm GBPm GBPm GBPm
------- ------ -------- --------
Financial liabilities at fair value
Financial liabilities mandatorily
at fair value through
profit or loss (FVTPL):
Contingent consideration liabilities - - (5,869) (5,869)
Held for trading derivatives that
are not in a
designated and effective hedging
relationship - (191) (9) (200)
Derivatives designated and effective
as hedging
instruments - (31) - (31)
- (222) (5,878) (6,100)
-------- ------ -------- --------
Movements in the six months to 30 June 2021 and the six months to
30 June 2020 for financial instruments measured using Level 3 valuation
methods are presented below:
Financial Financial
assets liabilities
GBPm GBPm
---------- -------------
At 1 January 2021 814 (5,878)
Gains/(losses) recognised in the income statement 47 (313)
Gains recognised in other comprehensive income 90 -
Additions 51 -
Disposals (10) -
Transfer from Level 3 (595) -
Payments in the period - 426
At 30 June 2021 397 (5,765)
---------- -------------
At 1 January 2020 757 (5,479)
Gains/(losses) recognised in the income statement 6 (806)
Gains recognised in other comprehensive income 151 -
Additions 52 (24)
Disposals (10) -
Payments in the period - 455
At 30 June 2020 956 (5,854)
----- --------
Net losses of GBP267 million (H1 2020: net losses of GBP800 million)
reported in other operating income and net gains of GBP90 million
(H1 2020: net gains of GBP151 million) reported in other comprehensive
income were attributable to Level 3 financial instruments held at
the end of the period. Net gains of GBP90 million (H1 2020: GBP151
million) attributable to Level 3 financial instruments reported in
Other comprehensive income as Fair value movements on equity investments
were all attributable to financial instruments held at the end of
the period, of which net gains of GBP99 million (H1 2020: nil) arose
prior to transfer from Level 3 on equity investments which transferred
to a Level 1 valuation methodology as a result of listing on a recognised
stock exchange during the period. Net gains and losses include the
impact of exchange movements.
Financial liabilities measured using Level 3 valuation methods at
30 June included GBP5,199 million (H1 2020: GBP5,436 million) of
contingent consideration for the acquisition in 2012 of the former
Shionogi-ViiV Healthcare joint venture and GBP504 million (H1 2020:
GBP349 million) of contingent consideration for the acquisition of
the Novartis Vaccines business in 2015. Contingent consideration
is expected to be paid over a number of years and will vary in line
with the future performance of specified products, the achievement
of certain milestone targets and movements in certain foreign currencies.
The financial liabilities are measured at the present value of expected
future cash flows, the most significant inputs to the valuation models
being future sales forecasts, the discount rate and the Sterling/US
Dollar exchange.
The table below shows, on an indicative basis, the income statement
and balance sheet sensitivity to reasonably possible changes in key
inputs to the valuation of the largest contingent consideration liabilities.
Shionogi- Novartis
ViiV Healthcare Vaccines
Increase/(decrease) in financial liability GBPm GBPm
----------------- ----------
10% increase in sales forecasts 524 83
10% decrease in sales forecasts (520) (83)
1% (100 basis points) increase in discount rate (201) (41)
1% (100 basis points) decrease in discount rate 217 48
10 cent appreciation of US Dollar 296 2
10 cent depreciation of US Dollar (253) (2)
10 cent appreciation of Euro 112 21
10 cent depreciation of Euro (94) (18)
The Group transfers financial instruments between different levels
in the fair value hierarchy when, as a result of an event or change
in circumstances, the valuation methodology applied in determining
their fair values alters in such a way that it meets the definition
of a different level. There were no transfers between the Level 1
and Level 2 fair value measurement categories. Transfers from Level
3 relate to equity investments in companies which were listed on
stock exchanges during the period.
The following methods and assumptions were used to measure the fair
value of the significant financial instruments carried at fair value
on the balance sheet:
-- Other investments - equity investments traded in an active market
determined by reference to the relevant stock exchange quoted bid
price; other equity investments determined by reference to the
current market value of similar instruments, recent financing rounds
or the discounted cash flows of the underlying net assets
-- Trade receivables carried at fair value - based on invoiced amount
-- Interest rate swaps, foreign exchange forward contracts, swaps
and options - based on the present value of contractual cash flows
or option valuation models using market-sourced data (exchange
rates or interest rates) at the balance sheet date
-- Cash and cash equivalents carried at fair value - based on net
asset value of the funds
-- Contingent consideration for business acquisitions and divestments
- based on present values of expected future cash flows
There are no material differences between the carrying value of the
Group's other financial assets and liabilities and their estimated
fair values, with the exception of bonds, for which the carrying
values and fair values are set out in the table below:
30 June 2021 31 December 2020
-------------------- --------------------
Carrying Fair Carrying Fair
value value value value
GBPm GBPm GBPm GBPm
--------- --------- --------- ---------
Bonds in a designated hedging relationship (6,801) (7,202) (7,681) (8,171)
Other bonds (17,034) (20,718) (17,205) (21,966)
--------- --------- --------- ---------
(23,835) (27,920) (24,886) (30,137)
--------- --------- --------- ---------
The following methods and assumptions are used to estimate the fair
values of financial assets and liabilities which are not measured
at fair value on the balance sheet:
-- Company owned life insurance policies - based on cash surrender
value
-- Receivables and payables, including put options, carried at amortised
cost - approximates to the carrying amount
-- Liquid investments - approximates to the carrying amount
-- Cash and cash equivalents carried at amortised cost - approximates
to the carrying amount
-- Short-term loans, overdrafts and commercial paper - approximates
to the carrying amount because of the short maturity of these instruments
-- Long-term loans - based on quoted market prices (a Level 1 fair
value measurement) in the case of European and US Medium Term Notes
and other fixed rate borrowings; approximates to the carrying amount
in the case of other fixed rate borrowings and floating rate bank
loans
Put option
Other payables in Current liabilities includes the present value
of the expected redemption amount of the Pfizer put option over its
non-controlling interest in ViiV Healthcare of GBP870 million. This
reflects a number of assumptions around future sales and profit forecasts,
multiples and forecast exchange rates. The forecast exchange rates
used are consistent with market rates at 30 June 2021.
The table below shows on an indicative basis the income statement
and balance sheet sensitivity to reasonably possible changes in the
key inputs to the measurement of this liability.
ViiV
Healthcare
put option
Increase/(decrease) in financial liability GBPm
------------
10% increase in sales forecasts 91
10% decrease in sales forecasts (91)
1% (100 basis points) increase in discount rate (30)
1% (100 basis points) decrease in discount rate 32
------------
Reconciliation of cash flow to movements in net debt
H1 2021 H1 2020
GBPm GBPm
--------- ---------
Net debt at beginning of the period (20,780) (25,215)
(Decrease)/increase in cash and bank overdrafts (2,031) 2,817
Decrease in liquid investments (18) -
Net decrease in short-term loans 352 3,018
Increase in long-term loans - (2,354)
Repayment of lease liabilities 108 111
Exchange adjustments 525 (1,769)
Other non-cash movements (77) (43)
--------- ---------
(Increase)/decrease in net debt (1,141) 1,780
--------- ---------
Net debt at end of the period (21,921) (23,435)
--------- ---------
Net debt analysis
30 June 30 June 31 December
2021 2020 2020
GBPm GBPm GBPm
--------- --------- ------------
Liquid investments 59 87 78
Cash and cash equivalents 3,503 8,166 6,292
Cash and cash equivalents reported
in assets held for sale - 2 -
Short-term borrowings (5,041) (5,964) (3,725)
Long-term borrowings (20,442) (25,726) (23,425)
---------
Net debt at end of the period (21,921) (23,435) (20,780)
--------- --------- ------------
Free cash flow reconciliation
Q2 2021 H1 2021 H1 2020
GBPm GBPm GBPm
-------- -------- --------
Net cash inflow from operating activities 1,292 1,623 3,725
Purchase of property, plant and
equipment (239) (440) (420)
Proceeds from sale of property,
plant and equipment 65 102 12
Purchase of intangible assets (422) (575) (326)
Proceeds from disposals of intangible
assets 56 384 636
Net finance costs (335) (422) (450)
Dividends from joint ventures and
associates 9 9 14
Contingent consideration paid (reported
in investing
activities) (26) (55) (62)
Distributions to non-controlling
interests (84) (320) (652)
Contributions from non-controlling
interests - 7 3
Free cash inflow 316 313 2,480
-------- -------- --------
Principal risks and uncertainties
The principal risks and uncertainties affecting the Group are those
described under the headings below. These are detailed in the 'Principal
risks and uncertainties' section of the Annual Report 2020. See 'Additional
information' on page 59 for risks and uncertainties relating to the
COVID-19 pandemic.
Patient safety Failure to appropriately collect, review, follow up,
or report human safety information, including adverse
events from all potential sources, and to act on any
relevant findings in a timely manner.
Product quality Failure by GSK, its contractors or suppliers to ensure
appropriate controls and governance of quality in
product development; compliance with good manufacturing
practice or good distribution practice regulations
in commercial or clinical trials manufacture and distribution
activities; compliance with the terms of GSK product
licences and supporting regulatory activities.
Financial controls Failure to comply with current tax laws or incurring
and reporting significant losses due to treasury activities; failure
to report accurate financial information in compliance
with accounting standards and applicable legislation.
Anti-bribery The risk comprises five sub-risk areas: bribery of
and corruption public officials by GSK; bribery of commercial and
other non-public entities by GSK; bribery by third
parties acting on behalf of GSK; GSK employees receiving
and/or requesting bribes and/or other undue personal
benefit; other corruption-non-compliance with laws
and regulations related to money laundering or facilitation
of tax evasion by third parties/clients/partners.
Commercial practices Failure to engage in commercial activities that are
consistent with the letter and spirit of the law,
industry regulations or the Group's requirements relating
to sales and promotion of our medicines and vaccines;
appropriate interactions with healthcare professionals/organisations
and patients; legitimate and transparent transfers
of value; and pricing and competition (or antitrust)
regulations in commercial practices, including trade
channel activities and tendering business.
Non-promotional Failure to engage in non-promotional activities that
engagement are consistent with external regulations, internal
policies and GSK values regarding scientific engagement
with healthcare professionals and patients, including
communications relating to our medicines or associated
disease areas, appropriate conduct of interactions
and legitimacy and transparency of those interactions.
Privacy Failure to collect, secure, use and destroy personal
information in accordance with data privacy laws can
lead to harm to individuals (e.g. financial, stress,
prejudice) and GSK (e.g. fines, operational, financial
and reputational).
Research practices Failure to adequately conduct ethical and sound pre-clinical
and clinical research. In addition, failure to engage
in scientific activities that are consistent with
the letter and spirit of the law, industry, or the
Group's requirements. It comprises the following sub-risks:
non-clinical and laboratory research; human subject
research; data integrity; care, welfare and treatment
of animals; human biological samples management; data
disclosure; regulatory filings and engagement; and
patents.
Environment, Failure in management of: execution of hazardous activities;
health & safety GSK's physical assets and infrastructure; handling
and processing of hazardous chemicals and biological
agents; control of releases of substances harmful
to the environment in both the short and long term,
leading to incidents which could disrupt our R&D and
supply activities, harm employees, harm the communities
and harm the local environments in which we operate.
Environmental Failure in management of: physical climate and environmental
sustainability risks, current and future regulatory requirements
for environmental policies and taxes, delivery and
performance of management environmental objectives,
leading to reduced supply chain resilience, product
life cycle management issues, loss of trust/reputation
with employees, investors, customers, regulators and
other stakeholders, increased costs, loss of sales
or market access and negative impacts on the environment.
Information security The risk that unauthorised disclosure, theft, unavailability
or corruption of GSK's information or key information
systems may lead to harm to our patients, workforce
and customers, disruption to our business and/or loss
of commercial or strategic advantage, damage to our
reputation or regulatory sanction.
Supply continuity Failure to deliver a continuous supply of compliant
finished product; inability to respond effectively
to a crisis incident in a timely manner to recover
and sustain critical operations.
Transformation Failure to deliver the plan for successful transformation
and separation of GSK into two competitive standalone
companies; New GSK, a biopharma company and new Consumer
Healthcare.
Reporting definitions
Total and Adjusted results
Total reported results represent the Group's overall performance.
GSK also uses a number of adjusted, non-IFRS, measures to report
the performance of its business. Adjusted results and other non-IFRS
measures may be considered in addition to, but not as a substitute
for or superior to, information presented in accordance with IFRS.
Adjusted results are defined on page 10 and other non-IFRS measures
are defined below.
Free cash flow
Free cash flow is defined as the net cash inflow/outflow from operating
activities less capital expenditure on property, plant and equipment
and intangible assets, contingent consideration payments, net finance
costs, and dividends paid to non-controlling interests plus proceeds
from the sale of property, plant and equipment and intangible assets,
and dividends received from joint ventures and associates. It is
used by management for planning and reporting purposes and in discussions
with and presentations to investment analysts and rating agencies.
Free cash flow growth is calculated on a reported basis. A reconciliation
of net cash inflow from operations to free cash flow is set out on
page 65.
Free cash flow conversion
Free cash flow conversion is free cash flow as a percentage of earnings.
Working capital
Working capital represents inventory and trade receivables less trade
payables.
CER and AER growth
In order to illustrate underlying performance, it is the Group's
practice to discuss its results in terms of constant exchange rate
(CER) growth. This represents growth calculated as if the exchange
rates used to determine the results of overseas companies in Sterling
had remained unchanged from those used in the comparative period.
CER% represents growth at constant exchange rates. GBP% or AER% represents
growth at actual exchange rates.
Pro-forma growth
The acquisition of the Pfizer consumer healthcare business completed
on 31 July 2019 and so GSK's reported results for H1 2020 included
six months of results of the former Pfizer consumer healthcare business
from 1 January 2020.
The Group has presented in this Results Announcement pro-forma growth
rates at CER in H1 2020 for sales excluding brands divested/under
review for Consumer Healthcare and sales for certain categories of
consumer healthcare products taking account of this transaction.
Pro-forma growth rates for the half year are calculated comparing
reported results for H1 2020, calculated applying the exchange rates
used in the comparative period, with the results for H1 2019 adjusted
to include the equivalent six months of results of the former Pfizer
consumer healthcare business during H1 2019, as consolidated (in
US$) and included in Pfizer's US GAAP results.
2 year Compound Annual Growth Rate
CAGR is defined as the compound annual growth rate and shows the
annualised average rate of pro-forma revenue growth between two given
years, assuming growth takes place at an exponentially compounded
rate. For Consumer Healthcare, the 2 year revenue CAGR has been shared
showing the annualised average rate of pro-forma revenue growth between
2019 and 2021.
COVID-19 solutions
COVID-19 solutions include the sales of pandemic adjuvant and other
COVID-19 solutions including vaccine manufacturing and sotrovimab
and the associated costs but does not include reinvestment in R&D.
This categorisation is used by management and we believe is helpful
to investors through providing clarity on the results of the Group
by showing the contribution to growth from COVID-19 solutions .
Brand names and partner acknowledgements
Brand names appearing in italics throughout this document are trademarks
of GSK or associated companies or used under licence by the Group.
Outlook, assumptions and cautionary statements
2021 guidance
Our guidance range for 2021 is a decline of mid to high-single digit
percent adjusted EPS at CER and excludes any contribution from COVID-19
related solutions.
2021-2026 sales and adjusted operating profit growth outlooks, 2026
cash generated from operations outlook, 2031 sales ambition and 2021-2023
dividend expectations
In June 2021, GSK announced that it expected New GSK to deliver sales
growth and adjusted operating profit growth of more than 5% and more
than 10%, respectively, CAGR at constant exchange rates over the
five year period 2021-2026 (with 2021 as the base year). These financial
outlooks exclude any contribution from COVID-19 related revenues.
New GSK expects to improve adjusted operating margin from the mid-20s%
in 2021 to over 30% by 2026 and cash generated from operations is
expected to exceed GBP10 billion by 2026. By 2031, New GSK aims to
deliver sales of more than GBP33 billion (at constant exchange rates).
Assumptions related to 2021 guidance, 2021-2026 outlooks, 2031 sales
ambition and 2021-2023 dividend expectations
In outlining the guidance for 2021 and future five-year 2021-26 outlook,
2031 ambition and dividend expectations, the Group has made certain
assumptions about the healthcare sector (including regarding possible
governmental, legislative and regulatory reform), the different markets
and competitive landscape in which it operates and the delivery of
revenues and financial benefits from its current portfolio, its development
pipeline of drugs and vaccines, its restructuring programmes and
its plans for the separation of Consumer Healthcare.
2021 guidance
The Group has made planning assumptions for 2021 that healthcare
systems and consumer trends will approach normality in the second
half of the year, and we expect turnover to be flat to low single
digit growth for the Pharmaceuticals and Vaccines businesses and
low to mid-single digit growth for Consumer Healthcare excluding
brands divested/under review. These planning assumptions as well
as earnings guidance and dividend expectations assume no material
interruptions to supply of the Group's products, no material mergers,
acquisitions or disposals, no material litigation or investigation
costs for the Company (save for those that are already recognised
or for which provisions have been made), no share repurchases by
the Company, and no change in the Group's shareholdings in ViiV Healthcare.
The assumptions also assume no material changes in the healthcare
environment. The 2021 guidance factors in all divestments and product
exits announced to date, including product divestments planned in
connection with the formation of the Consumer Healthcare Joint Venture
with Pfizer, and the non-core divestments planned to fund the cash
costs of the Separation Preparation restructuring programme.
The Group's guidance assumes successful delivery of the Group's integration
and restructuring plans. It also assumes that the integration and
investment programmes following the creation of the Consumer Healthcare
Joint Venture with Pfizer are delivered successfully. Material costs
for investment in new product launches and R&D have been factored
into the expectations given. Given the potential development options
in the Group's pipeline, the outlook may be affected by additional
data-driven R&D investment decisions. The guidance is given on a
constant currency basis.
New GSK's revenue, operating profit, operating margin and cash flow
outlooks, revenue ambition and dividend expectations
GSK expects and assumes the next several years to be challenging
for the healthcare industry with continued uncertainty related to
the impact of the COVID-19 pandemic on adult vaccinations and continued
pressure on pricing of pharmaceuticals. GSK also expects volume demand
for its products to increase, particularly for Shingrix in the US,
as healthcare systems are expected to return to normal following
disruption from governments' prioritisation of COVID-19 vaccination
programmes and ongoing measures to contain the pandemic, and for
Shingrix in China.
The assumptions for New GSK's revenue, operating profit, operating
margin and cash flow outlooks, revenue ambition and dividend expectations
assume successful delivery of the ongoing and planned integration
and restructuring plans and the planned demerger of Consumer Healthcare;
the delivery of revenues and financial benefits from its current
and development pipeline portfolio of drugs and vaccines (which have
been assessed for this purpose on a risk-adjusted basis, as described
further below); regulatory approvals of the pipeline portfolio of
drugs and vaccines that underlie these expectations (which have also
been assessed for this purpose on a risk-adjusted basis, as described
further below); no material interruptions to supply of the Group's
products; no material mergers, acquisitions or disposals or other
material business development transactions; no material litigation
or investigation costs for the company (save for those that are already
recognised or for which provisions have been made); no share repurchases
by the company; and no change in the shareholdings in ViiV Healthcare.
GSK assumes no premature loss of exclusivity for key products over
the period.
The assumptions for New GSK's revenue, operating profit, operating
margin and cash flow outlooks, revenue ambition and dividend expectations
also factor in all divestments and product exits announced to date
as well as material costs for investment in new product launches
and R&D. Pipeline risk-adjusted sales are based on the latest internal
estimate of the probability of technical and regulatory success for
each asset in development.
Notwithstanding these outlooks and expectations, there is still uncertainty
as to whether our assumptions, targets, outlooks expectations and
ambitions will be achieved, including based on the other assumptions
outlined above.
All outlook and ambition statements are given on a constant currency
basis and use 2021 forecast exchange rates as a base, assuming a
continuation of Q1 2021 closing rates (GBP1/$1.38, GBP1/EUR1.17,
GBP1/Yen 152). 2021-2026 outlook refers to the 5 years to 2026 with
2021 as the base year.
Assumptions and cautionary statement regarding forward-looking statements
The Group's management believes that the assumptions outlined above
are reasonable, and that the guidance, outlooks, ambitions and expectations
described in this report are achievable based on those assumptions.
However, given the forward-looking nature of these guidance, outlooks,
ambitions and expectations, they are subject to greater uncertainty,
including potential material impacts if the above assumptions are
not realised, and other material impacts related to foreign exchange
fluctuations, macro-economic activity, the impact of outbreaks, epidemics
or pandemics, such as the COVID-19 pandemic and ongoing challenges
and uncertainties posed by the COVID-19 pandemic for businesses and
governments around the world, changes in legislation, regulation,
government actions or intellectual property protection, product development
and approvals, actions by our competitors, and other risks inherent
to the industries in which we operate.
This document contains statements that are, or may be deemed to be,
"forward-looking statements". Forward-looking statements give the
Group's current expectations or forecasts of future events. An investor
can identify these statements by the fact that they do not relate
strictly to historical or current facts. They use words such as 'anticipate',
'estimate', 'expect', 'intend', 'will', 'project', 'plan', 'believe',
'target', 'aim', 'ambition' and other words and terms of similar
meaning in connection with any discussion of future operating or
financial performance. In particular, these include statements relating
to future actions, prospective products or product approvals, future
performance or results of current and anticipated products, sales
efforts, expenses, the outcome of contingencies such as legal proceedings,
dividend payments and financial results. Other than in accordance
with its legal or regulatory obligations (including under the Market
Abuse Regulation, the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no
obligation to update any forward-looking statements, whether as a
result of new information, future events or otherwise. The reader
should, however, consult any additional disclosures that the Group
may make in any documents which it publishes and/or files with the
SEC. All readers, wherever located, should take note of these disclosures.
Accordingly, no assurance can be given that any particular expectation
will be met and investors are cautioned not to place undue reliance
on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks
and uncertainties, many of which relate to factors that are beyond
the Group's control or precise estimate. The Group cautions investors
that a number of important factors, including those in this document,
could cause actual results to differ materially from those expressed
or implied in any forward-looking statement. Such factors include,
but are not limited to, those discussed under Item 3.D 'Risk Factors'
in the Group's Annual Report on Form 20-F for 2020 and any impacts
of the COVID-19 pandemic. Any forward looking statements made by
or on behalf of the Group speak only as of the date they are made
and are based upon the knowledge and information available to the
Directors on the date of this report.
Directors' responsibility statement
The Board of Directors approved this Half-yearly Financial Report
on 28 July 2021.
The Directors confirm that to the best of their knowledge the unaudited
condensed financial information has been prepared in accordance with
IAS 34 as contained in UK-adopted International Financial Reporting
Standards (IFRS) and that the interim management report includes
a fair review of the information required by DTR 4.2.7 and DTR 4.2.8.
After making enquiries, the Directors considered it appropriate to
adopt the going concern basis in preparing this Half-yearly Financial
Report.
The Directors of GlaxoSmithKline plc are as follows:
Sir Jonathan Symonds Non-Executive Chairman, Nominations & Corporate
Governance Committee Chair
Dame Emma Walmsley Chief Executive Officer (Executive Director)
Iain Mackay Chief Financial Officer (Executive Director)
Dr Hal Barron Chief Scientific Officer and President, R&D (Executive
Director)
Vindi Banga Senior Independent Non-Executive Director
Charles Bancroft Independent Non-Executive Director, Audit & Risk
Committee Chair
Dr Anne Beal Independent Non-Executive Director
Dr Vivienne Cox Independent Non-Executive Director, Workforce
Engagement Director
Lynn Elsenhans Independent Non-Executive Director, Corporate
Responsibility Committee Chair
Dr Laurie Glimcher Independent Non-Executive Director
Dr Jesse Goodman Independent Non-Executive Director, Science Committee
Chair
Urs Rohner Independent Non-Executive Director, Remuneration
Committee Chair
By order of the Board
Emma Walmsley Iain Mackay
Chief Executive Officer Chief Financial Officer
28 July 2021
Independent review report to GlaxoSmithKline plc
We have been engaged by GlaxoSmithKline plc ("the Company") to review
the condensed financial information in the Results Announcement of
the Company for the three and six months ended 30 June 2021.
What we have reviewed
The condensed financial information comprises:
-- the income statements and statements of comprehensive income for
the three and six month periods ended 30 June 2021 on pages 46
to 48;
-- the balance sheet as at 30 June 2021 on page 53;
-- the statement of changes in equity for the six month period then
ended on page 54;
-- the cash flow statement for the six month period then ended on
page 55; and
-- the accounting policies and basis of preparation and the explanatory
notes to the condensed financial statements on pages 49 to 52
and 56 to 64 .
We have read the other information contained in the Results Announcement,
including the non-IFRS measures contained on pages 49 to 52 and 56
to 64, and considered whether it contains any apparent misstatements
or material inconsistencies with the information in the condensed
set of financial statements.
This report is made solely to the Company in accordance with International
Standard on Review Engagements (UK and Ireland) 2410 "Review of Interim
Financial Information Performed by the Independent Auditor of the
Entity" issued by the Financial Reporting Council. Our work has been
undertaken so that we might state to the Company those matters we
are required to state to it in an independent review report and for
no other purpose. To the fullest extent permitted by law, we do not
accept or assume responsibility to anyone other than the Company,
for our review work, for this report, or for the conclusions we have
formed.
Directors' responsibilities
The Results Announcement of the Company, including the condensed
financial statements, is the responsibility of, and has been approved
by, the directors. The directors are responsible for preparing the
Results Announcement of the Company in accordance with the Disclosure
Guidance and Transparency Rules of the United Kingdom's Financial
Conduct Authority.
As disclosed in note 1, the annual financial statements of the Company
are prepared in accordance with United Kingdom adopted International
Financial Reporting Standards. The condensed financial statements
included in this Results Announcement have been prepared in accordance
with United Kingdom adopted International Accounting Standard 34,
"Interim Financial Reporting".
Our responsibility
Our responsibility is to express to the Company a conclusion on the
condensed financial statements in the Results Announcement based
on our review.
Scope of review
We conducted our review in accordance with International Standard
on Review Engagements (UK and Ireland) 2410 "Review of Interim Financial
Information Performed by the Independent Auditor of the Entity" issued
by the Financial Reporting Council for use in the United Kingdom.
A review of interim financial information consists of making inquiries,
primarily of persons responsible for financial and accounting matters,
and applying analytical and other review procedures. A review is
substantially less in scope than an audit conducted in accordance
with International Standards on Auditing (UK) and consequently does
not enable us to obtain assurance that we would become aware of all
significant matters that might be identified in an audit. Accordingly,
we do not express an audit opinion.
Conclusion
Based on our review, nothing has come to our attention that causes
us to believe that the condensed financial statements in the Results
Announcement for the three and six months ended 30 June 2021 are
not prepared, in all material respects, in accordance with United
Kingdom adopted International Accounting Standard 34 and the Disclosure
Guidance and Transparency Rules of the United Kingdom's Financial
Conduct Authority.
Deloitte LLP
Statutory Auditor
London, United Kingdom
28 July 2021
This information is provided by RNS, the news service of the
London Stock Exchange. RNS is approved by the Financial Conduct
Authority to act as a Primary Information Provider in the United
Kingdom. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
IR PPURWMUPGUBR
(END) Dow Jones Newswires
July 28, 2021 07:00 ET (11:00 GMT)
Gsk (LSE:GSK)
Historical Stock Chart
From Jan 2025 to Feb 2025
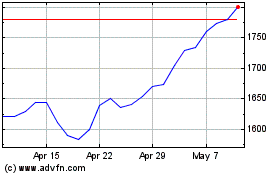
Gsk (LSE:GSK)
Historical Stock Chart
From Feb 2024 to Feb 2025