Shield Therapeutics PLC Abstract to be presented at Healthcare Conference
October 20 2023 - 5:45AM
RNS Non-Regulatory
TIDMSTX
Shield Therapeutics PLC
20 October 2023
Shield Therapeutics plc
("Shield" or the "Company" or the "Group")
Shield to Present Abstract at 26th Annual Women's Healthcare
Conference 2023 on Accrufer(R)
London, UK - 20 October 2023: Shield Therapeutics plc (LSE:
STX), a commercial stage pharmaceutical company that delivers
Accrufer(R)/Feraccru(R) (ferric maltol), an innovative and
differentiated specialty pharmaceutical product, to address a
significant unmet need for patients suffering from iron deficiency
(with or without anemia) reports that an abstract has been accepted
for presentation at the National Association of Nurse Practitioners
in Women's Health 26th Annual Women's Healthcare Conference taking
place October 29 - November 1 in San Diego, California. Dr Michael
Cody M.D. will present during Monday, October 30(th) 11:45 am -
1:45 pm and Tuesday, October 31st, 11:30 am - 12:30 pm.
Poster title: Iron absorption is maintained over time following
repeated twice-daily ferric maltol dosing: Post hoc analysis of a
multicenter, open-label, randomized, phase 1 clinical study.
Poster Presentation Dates and times: Monday, October 30th; 11:45
am - 1:45 pm; Tuesday, October 31st, 11:30 am - 12:30 pm
NPWH is the leading membership organization for Women's Health
Nurse Practitioners and all NPs who perform women's and
gender-related healthcare. Through continuing education, advocacy,
professional development, and research, we support our members as
they strive to continuously improve access to and quality of
healthcare for women.
Find out more about the 26th Annual conference here National
Association of Nurse Practitioners in Women's Health
(npwh.org).
For further information please contact:
Shield Therapeutics plc www.shieldtherapeutics.com
Greg Madison, CEO +44 (0) 191 511 8500
Nominated Adviser and Joint
Broker
Peel Hunt LLP
James Steel/Patrick Birkholm +44 (0)20 7418 8900
Joint Broker
Cavendish Capital Markets Ltd
Geoff Nash/ George Dollemore/Nigel
Birks +44 (0)20 7220 0563
Financial PR & IR Advisor
Walbrook PR
Paul McManus/ Alice Woodings +44 (0)20 7933 8780 or shield@walbrookpr.com
Investor Contact (US Advisor)
LifeSci Advisors, LLC
John Mullaly +1 617 429 3548 or jmullaly@lifesciadvisors.com
About Iron Deficiency and Accrufer(R)/Feraccru(R)
Clinically low iron levels (aka iron deficiency, ID) can cause
serious health problems for adults of all ages, across multiple
therapeutic areas. Together, ID and ID with anemia (IDA) affect
about 20 million people in the US and represent a $2.3B market
opportunity. As the first and only FDA approved oral iron to treat
ID/IDA, Accrufer(R) has the potential to meet an important unmet
medical need for both physicians and patients.
Accrufer(R)/Feraccru(R) (ferric maltol) is a novel, stable,
non-salt-based oral therapy for adults with ID/IDA.
Accrufer(R)/Feraccru(R) has a novel mechanism of absorption
compared to other oral iron therapies and has been shown to be an
efficacious and well-tolerated therapy in a range of clinical
trials. More information about Accrufer(R)/ Feraccru(R) , including
the product label, can be found at: www.accrufer.com and
www.feraccru.com .
About Shield Therapeutics plc
Shield is a commercial stage specialty pharmaceutical company
that delivers Accrufer(R)/Feraccru(R) (ferric maltol), an
innovative and differentiated pharmaceutical product, to address a
significant unmet need for patients suffering from iron deficiency,
with or without anemia. The Company launched Accrufer(R) in the
U.S. with an exclusive, multi-year collaboration agreement with
Viatris. Outside of the U.S., the Company licensed the rights to
four specialty pharmaceutical companies. Feraccru(R) is
commercialized in the UK and European Union by Norgine B.V.
(Norgine), which also has marketing rights in Australia and New
Zealand. Shield also has an exclusive license agreement with
Beijing Aosaikang Pharmaceutical Co., Ltd., for the development and
commercialization of Accrufer(R)/ Feraccru(R) in China, Hong Kong,
Macau and Taiwan, with Korea Pharma Co., Ltd. for the Republic of
Korea (Korea Pharma), and with KYE Pharmaceuticals Inc. for
Canada.
Accrufer(R)/Feraccru(R) has patent coverage until the
mid-2030s.
Accrufer(R)/Feraccru(R) are registered trademarks of Shield
Therapeutics.
This information is provided by Reach, the non-regulatory press
release distribution service of RNS, part of the London Stock
Exchange. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
NRAPPGCWUUPWGUU
(END) Dow Jones Newswires
October 20, 2023 06:45 ET (10:45 GMT)
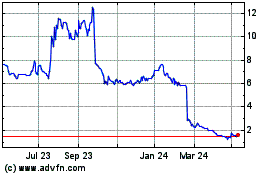
Shield Therapeutics (LSE:STX)
Historical Stock Chart
From Oct 2024 to Nov 2024
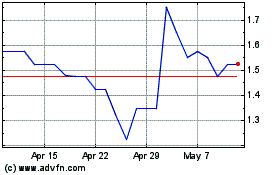
Shield Therapeutics (LSE:STX)
Historical Stock Chart
From Nov 2023 to Nov 2024