false
0001742927
0001742927
2024-12-16
2024-12-16
0001742927
rvph:CommonStockParValue00001PerShareCustomMember
2024-12-16
2024-12-16
0001742927
rvph:WarrantsToPurchaseOneShareOfCommonStockCustomMember
2024-12-16
2024-12-16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 16, 2024
|
REVIVA PHARMACEUTICALS HOLDINGS, INC.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
|
001-38634
|
|
85-4306526
|
|
(State or other jurisdiction
of incorporation)
|
|
(Commission File Number)
|
|
(IRS Employer
Identification No.)
|
|
10080 N Wolfe Road, Suite SW3-200, Cupertino, CA
|
|
95014
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
Registrant’s telephone number, including area code: (408) 501-8881
|
Not Applicable
|
|
(Former name or former address, if changed since last report.)
|
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Trading Symbol(s)
|
|
Name of each exchange on which
registered
|
|
Common Stock, par value $0.0001 per share
|
|
RVPH
|
|
Nasdaq Capital Market
|
|
Warrants to purchase one share of Common Stock
|
|
RVPHW
|
|
Nasdaq Capital Market
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
Item 7.01.
|
Regulation FD Disclosure.
|
On December 16, 2024, Reviva Pharmaceuticals Holdings, Inc. (the “Company”) issued a press release announcing positive preliminary topline data for the open-label extension (the “OLE”) portion of the Company’s ongoing Phase 3 RECOVER trial evaluating the long-term safety and tolerability of brilaroxazine in patients with schizophrenia. A copy of the press release is attached hereto as Exhibit 99.1.
The information in this Current Report on Form 8-K under Item 7.01, including the information contained in Exhibit 99.1, is being furnished to the Securities and Exchange Commission, and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by a specific reference in such filing.
On December 16, 2024, the Company announced positive preliminary topline data from its OLE evaluating the long-term safety and tolerability of brilaroxazine in patients with schizophrenia. Administration of brilaroxazine once daily led to robust broad-spectrum efficacy that was sustained over 1 year. Brilaroxazine was generally well tolerated with no single side effect >5% and favorable compliance, with a discontinuation rate of 35% in the OLE part of this study. All three doses of brilaroxazine (15 mg, 30 mg and 50 mg) tested were efficacious and generally well-tolerated.
Key safety, efficacy and compliance findings for pooled analysis of brilaroxazine at 15, 30, and 50 mg include:
| |
●
|
A total number of 435 patients were enrolled in the OLE across three dose groups: 139 in brilaroxazine 15 mg, 155 in brilaroxazine 30mg and 141 in brilaroxazine 50mg
|
| |
●
|
156 (35.86%) rollover participants from the double-blind portion of the Phase 3 trial, while 279 (64.13%) de novo participants enrolled in the OLE
|
| |
●
|
Preliminary efficacy results are presented for 113 patients who completed 52 weeks (1 year) of treatment; preliminary safety results are presented for all 435 patients who enrolled in the OLE, including patients that are still participating in the trial
|
Brilaroxazine across doses improved major symptom domains of schizophrenia after 1-year of treatment:
| |
●
|
Dose dependent efficacy at the 15, 30, and 50 mg doses was observed, with decreases in PANSS total scores of -15.2, -18.6 and -20.8 points, respectively, from baseline to end-of-treatment at 52 -week (1 -year)
|
| |
●
|
Pooled data of brilaroxazine at the 15, 30, and 50 mg doses (N = 113) demonstrated clinically meaningful and sustained long-term (1-year) efficacy for schizophrenia with a significant decrease in PANSS total scores, PANSS positive symptoms, and PANSS negative symptoms compared to baseline
|
| |
o
|
PANSS Total scores: 18.6-point decrease (71.6 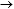 53), p ≤ 0.0001 |
| |
o
|
PANSS Positive Symptoms: 5.2-point decrease (17.7 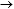 12.5), p ≤ 0.0001 |
| |
o
|
PANSS Negative Symptoms: 4.5-point decrease (19.5 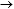 15.0), p ≤ 0.0001 |
| |
●
|
Brilaroxazine demonstrated strong sustained efficacy from acute through maintenance treatment over 1 -year with a decrease in PANSS Total score in rollover patients from the double-blind portion of the trial
|
| |
o
|
≥30-point decrease of PANSS total in 86.76% of patients
|
| |
o
|
≥40-point decrease of PANSS total in 64.70% of patients
|
| |
o
|
≥50-point decrease of PANSS total in 33.82% of patients
|
Long-term clinical safety, tolerability and adherence findings of brilaroxazine administered for up to one year support a well-tolerated safety profile:
| |
●
|
15.2% of participants reported at least one treatment-related adverse event (TRAE), which were mostly mild (12.2%) or moderate (3%) in severity and transient in nature
|
| |
●
|
Most common TRAEs ≥1% were weight increase (3.2%), insomnia (1.8%) and somnolence (1.6%)
|
| |
●
|
Brilaroxazine was not associated with any clinically meaningful changes in movement disorder scales over 1 -year treatment
|
| |
●
|
No drug-related serious adverse events (SAEs) observed or major safety concerns reported for brilaroxazine after up to 1 -year of treatment; 3 serious adverse events were reported and none were related to brilaroxazine treatment
|
| |
●
|
Treatment discontinuation rate of 35% reported in this OLE, primarily due to withdrawal of consent (22%), participant lost to follow up (7%), and treatment-related adverse events (1.6%)
|
Collectively, the findings from the OLE (52-week/1-year) portion of the Phase 3 RECOVER study further strengthen the safety, efficacy and treatment adherence findings from the double-blind (4-week) portion of RECOVER.
The OLE portion of the RECOVER Study is being conducted globally at multiple centers to assess the safety, and efficacy of brilaroxazine at flexible doses of 15, 30 or 50 mg, administered once daily for 52- weeks (1 -year) in patients with stable schizophrenia. The OLE included both rollover participants from the double-blind portion of RECOVER study and de novo participants with stable schizophrenia. Long-term safety data from a minimum of 100 patients who have completed 1-year of treatment is a requirement for brilaroxazine’s NDA submission to the FDA.
|
Item 9.01.
|
Financial Statements and Exhibits.
|
(d) The following exhibit is furnished with this report:
|
Exhibit
No.
|
|
Description
|
|
99.1
|
|
|
|
104
|
|
Cover Page Interactive Data File (embedded within the Inline XBRL document)
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
REVIVA PHARMACEUTICALS HOLDINGS, INC.
|
| |
|
|
|
Dated: December 16, 2024
|
By:
|
/s/ Narayan Prabhu
|
| |
Name:
Title:
|
Narayan Prabhu
Chief Financial Officer
|
Exhibit 99.1
Reviva Announces Positive Preliminary Topline Data for the Long-Term Open Label Extension Portion of the Phase 3 RECOVER Study Evaluating Brilaroxazine in Schizophrenia
– Once daily brilaroxazine demonstrated favorable long-term safety and robust broad-spectrum efficacy sustained over 1-year –
– Generally well-tolerated with low rates of adverse events and discontinuation –
– Full data set from open-label extension (OLE) of RECOVER expected in Q1 2025 –
Cupertino, Calif., December 16, 2024 – Reviva Pharmaceuticals Holdings, Inc. (NASDAQ: RVPH) (“Reviva” or the “Company”), a late-stage pharmaceutical company developing therapies that seek to address unmet medical needs in the areas of central nervous system (CNS), inflammatory and cardiometabolic diseases, today announced positive preliminary topline data for the open-label extension (OLE) portion of the Company’s ongoing Phase 3 RECOVER study evaluating the long-term safety and tolerability of brilaroxazine in patients with schizophrenia. Administration of brilaroxazine once daily led to robust broad-spectrum efficacy that was sustained over 1 year. Brilaroxazine was generally well tolerated with no single side effect >5% and favorable compliance, with a discontinuation rate of 35% in the OLE part of this study. All three doses of brilaroxazine (15 mg, 30 mg and 50 mg) tested were efficacious and generally well-tolerated.
“We believe these topline preliminary long-term data build on the strong clinical evidence demonstrating that brilaroxazine can improve all major symptom domains of schizophrenia, and now importantly, show sustained efficacy over time. Moreover, the generally well-tolerated safety profile and high compliance rate following one year of treatment highlight the potential once daily brilaroxazine holds to address major barriers to successful long-term treatment in schizophrenia,” said Laxminarayan Bhat, Ph.D., Founder, President, and CEO of Reviva. “We look forward to reporting the full data set from the OLE portion of the RECOVER study, which will include long-term safety, tolerability and efficacy data, as well as vocal and blood biomarker data as additional independent measures of efficacy, expected in the first quarter of 2025.”
Dr. Scott Bartley, MD, Chief Medical Officer and Principal Investigator for Pillar Clinical Research and Investigator in the RECOVER trials added, “No current therapy addresses all needs of patients with schizophrenia. The broad-spectrum improvements in all major symptom domains, including negative symptoms, along with the low treatment discontinuation rates with long-term use of brilaroxazine are encouraging and support the potential of brilaroxazine to reduce the current burden on people affected by this debilitating and life-long mental illness.”
Key safety, efficacy and compliance findings for pooled analysis of brilaroxazine at 15, 30, and 50 mg include:
| |
●
|
A total number of 435 patients were enrolled in the OLE across three dose groups: 139 in brilaroxazine 15 mg, 155 in brilaroxazine 30mg and 141 in brilaroxazine 50mg
|
| |
●
|
156 (35.86%) rollover participants from the double-blind portion of the Phase 3 trial, while 279 (64.13%) de novo participants enrolled in the OLE
|
| |
●
|
Preliminary efficacy results are presented for 113 patients who completed 52 weeks (1 year) of treatment; preliminary safety results are presented for all 435 patients who enrolled in the OLE, including patients that are still participating in the trial.
|
Brilaroxazine across doses improved major symptom domains of schizophrenia after 1-year of treatment:
| |
■
|
Dose dependent efficacy at the 15, 30, and 50 mg doses was observed, with decreases in PANSS total scores of -15.2, -18.6 and -20.8 points, respectively, from baseline to end-of-treatment at 52 weeks (1 year)
|
| |
■
|
Pooled data of brilaroxazine at the 15, 30, and 50 mg doses (N = 113) demonstrated clinically meaningful and sustained long-term (1-year) efficacy for schizophrenia with a significant decrease in PANSS total scores, PANSS positive symptoms, and PANSS negative symptoms compared to baseline
|
| |
o
|
PANSS Total scores: 18.6-point decrease (71.6 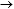 53), p ≤ 0.0001 53), p ≤ 0.0001
|
| |
o
|
PANSS Positive Symptoms: 5.2-point decrease (17.7 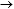 12.5), p ≤ 0.0001 12.5), p ≤ 0.0001
|
| |
o
|
PANSS Negative Symptoms: 4.5-point decrease (19.5 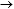 15.0), p ≤ 0.0001 15.0), p ≤ 0.0001
|
| |
■
|
Brilaroxazine demonstrated strong sustained efficacy from acute through maintenance treatment over 1 year with a decrease in PANSS Total score in rollover patients from the double-blind portion of the trial
|
| |
o
|
≥30-point decrease of PANSS total in 86.76% of patients
|
| |
o
|
≥40-point decrease of PANSS total in 64.70% of patients
|
| |
o
|
≥50-point decrease of PANSS total in 33.82% of patients
|
Long-term clinical safety, tolerability and adherence findings of brilaroxazine administered for up to one year support a well-tolerated safety profile:
| |
■
|
15.2% of participants reported at least one treatment-related adverse event (TRAE), which were mostly mild (12.2%) or moderate (3%) in severity and transient in nature
|
| |
■
|
Most common TRAEs ≥1% were weight increase (3.2%), insomnia (1.8%) and somnolence (1.6%)
|
| |
■
|
Brilaroxazine was not associated with any clinically meaningful changes in movement disorder scales over 1 year treatment
|
| |
■
|
No drug-related serious adverse events (SAEs) observed or major safety concerns reported for brilaroxazine after up to 1 year of treatment; 3 serious adverse events were reported and none were related to brilaroxazine treatment
|
| |
■
|
Treatment discontinuation rate of 35% reported in this OLE, primarily due to withdrawal of consent (22%), participant lost to follow up (7%), and treatment-related adverse events (1.6%)
|
Collectively, the findings from the OLE (52-week/1-year) portion of the Phase 3 RECOVER study further strengthen the safety, efficacy and treatment adherence findings from the double-blind (4-week) portion of RECOVER.
The OLE portion of the RECOVER Study is being conducted globally at multiple centers to assess the safety, and efficacy of brilaroxazine at flexible doses of 15, 30 or 50 mg, administered once daily for 52 weeks (1 year) in patients with stable schizophrenia. The OLE included both rollover participants from the double-blind portion of RECOVER study and de novo participants with stable schizophrenia. Long-term safety data from a minimum of 100 patients who have completed 1 year of treatment is a requirement for brilaroxazine’s NDA submission to the FDA.
About Brilaroxazine
Brilaroxazine is an in-house discovered new chemical entity with potent affinity and selectivity against key serotonin and dopamine receptors implicated in the pathophysiology of several conditions including schizophrenia, psoriasis and interstitial lung diseases like pulmonary hypertension, pulmonary arterial hypertension (PAH) and idiopathic pulmonary fibrosis (IPF).
Positive topline data from the global Phase 3 RECOVER trial in schizophrenia demonstrated the trial successfully met all primary and secondary endpoints with statistically significant and clinically meaningful reductions across all major symptom domains, including reduction in key proinflammatory cytokines implicated in the pathophysiology of schizophrenia and comorbid inflammatory conditions at week 4 with 50 mg of brilaroxazine vs. placebo, with a generally well-tolerated side effect profile comparable to placebo and discontinuation rates lower than placebo. Positive data from a clinical drug-drug interaction (DDI) study investigating the potential effect of the CYP3A4 enzyme on brilaroxazine in healthy subjects supports no clinically significant interaction when combined with CYP3A4 inhibitors. Reviva believes that a full battery of regulatory compliant toxicology and safety pharmacology studies has been completed for brilaroxazine. Reviva intends to develop brilaroxazine for other neuropsychiatric indications, including bipolar disorder, major depressive disorder (MDD) and attention-deficit/hyperactivity disorder (ADHD).
Additionally, brilaroxazine has shown promising nonclinical activity for inflammatory diseases, such as psoriasis, pulmonary arterial hypertension (PAH) and idiopathic pulmonary fibrosis (IPF), with mitigation of fibrosis and inflammation in translational animal models. Brilaroxazine has already received Orphan Drug Designation by the U.S. FDA for the treatment of PAH and IPF conditions. To learn more about the clinical and preclinical data available for brilaroxazine, please visit revivapharma.com/publications.
About Reviva
Reviva is a late-stage biopharmaceutical company that discovers, develops, and seeks to commercialize next-generation therapeutics for diseases representing unmet medical needs and burdens to society, patients, and their families. Reviva’s current pipeline focuses on the central nervous system (CNS), inflammatory and cardiometabolic diseases. Reviva’s pipeline currently includes two drug candidates, brilaroxazine (RP5063) and RP1208. Both are new chemical entities discovered in-house. Reviva has been granted composition of matter patents for both brilaroxazine and RP1208 in the United States, Europe, and several other countries.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act, as amended, including those relating to the Company’s 1-year open label extension (OLE) trial evaluating the long-term safety and tolerability for brilaroxazine in schizophrenia, the Company’s planned registrational Phase 3 RECOVER-2 trial, the Company’s expectations regarding the anticipated clinical profile of its product candidates, including statements regarding anticipated efficacy or safety profile, and those relating to the Company’s expectations, intentions or beliefs regarding matters including product development, clinical and regulatory timelines and expenses, planned or additional studies, planned or intended regulatory submissions, the timing of availability of additional data or initiation of additional trials, market opportunity, ability to raise sufficient funding, competitive position, possible or assumed future results of operations, business strategies, potential opportunities for development including partnerships, growth or expansion opportunities and other statements that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions.
These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential, “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2023, and the Company’s other filings from time to time with the Securities and Exchange Commission. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
Corporate Contact:
Reviva Pharmaceuticals Holdings, Inc.
Laxminarayan Bhat, PhD
www.revivapharma.com
Investor Relations Contact:
LifeSci Advisors, LLC
Bruce Mackle
bmackle@lifesciadvisors.com
v3.24.4
Document And Entity Information
|
Dec. 16, 2024 |
| Document Information [Line Items] |
|
| Entity, Registrant Name |
REVIVA PHARMACEUTICALS HOLDINGS, INC.
|
| Document, Type |
8-K
|
| Document, Period End Date |
Dec. 16, 2024
|
| Entity, Incorporation, State or Country Code |
DE
|
| Entity, File Number |
001-38634
|
| Entity, Tax Identification Number |
85-4306526
|
| Entity, Address, Address Line One |
10080 N Wolfe Road
|
| Entity, Address, Address Line Two |
Suite SW3-200
|
| Entity, Address, City or Town |
Cupertino
|
| Entity, Address, State or Province |
CA
|
| Entity, Address, Postal Zip Code |
95014
|
| City Area Code |
408
|
| Local Phone Number |
501-8881
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity, Emerging Growth Company |
false
|
| Amendment Flag |
false
|
| Entity, Central Index Key |
0001742927
|
| CommonStockParValue00001PerShare Custom [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
RVPH
|
| Security Exchange Name |
NASDAQ
|
| WarrantsToPurchaseOneShareOfCommonStock Custom [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants to purchase one share of Common Stock
|
| Trading Symbol |
RVPHW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=rvph_CommonStockParValue00001PerShareCustomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=rvph_WarrantsToPurchaseOneShareOfCommonStockCustomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Reviva Pharmaceuticals (NASDAQ:RVPHW)
Historical Stock Chart
From Dec 2024 to Jan 2025

Reviva Pharmaceuticals (NASDAQ:RVPHW)
Historical Stock Chart
From Jan 2024 to Jan 2025
