Virpax Confirms Positive Results with US Army with Probudur™ for Combat Care Study
February 13 2025 - 3:01PM
Business Wire
Virpax Pharmaceuticals, Inc. (Nasdaq: VRPX) (“Virpax” or the
“Company”), a company specializing in developing non-addictive
products for pain management, post-traumatic stress disorder,
central nervous system (CNS) disorders and anti-viral barrier
indications, today announced the completion of the full study that
followed the initial Probudur™ pilot study performed by the U.S.
Army Institute of Surgical Research (USAISR) under an existing
Cooperative Research and Development Agreement (CRADA).
The USAISR is the U.S. Department of Defense’s premier research
organization for developing solutions for trauma and critical care
challenges in combat casualties. This study was designed to
determine if Probudur reduces pain behaviors in a rat model of
incisional pain. The surgical procedures and assessments were
identical to those in the pilot study. The study compared Probudur
with free bupivacaine and EXPAREL®.
Various concentrations of Probudur were injected into the tissue
around the incision site as well as a saline solution for the
control group. The doses of Probudur showed reduction in
incision-induced pain behaviors. “These positive results are
consistent with what we at Virpax have previously observed and we
are encouraged by these findings,” commented Jatinder Dhaliwal,
Chief Executive Officer of Virpax.
Probudur is being developed to achieve the overall goal of safe
and effective pain control during the perioperative period and to
significantly reduce or eliminate the need for opioids after
surgery in approved indications. Probudur is a local anesthetic
that binds to the sodium channels, preventing pain signals from
reaching the brain. In preclinical studies, Probudur has shown long
duration pain control for at least 96 hours, with a rat incisional
model demonstrating analgesia for up to five days and in vitro
studies demonstrating a slow release of bupivacaine that lasted for
up to six (6) days.
About Virpax Pharmaceuticals, Inc. Virpax is developing
branded, non-addictive pain management products candidates using
its proprietary technologies to optimize and target drug delivery.
Virpax is initially seeking the U.S. Food and Drug Administration
(FDA) approval for two prescription drug candidates that employ two
different patented drug delivery platforms. Probudur™ is a single
injection liposomal bupivacaine formulation being developed to
manage post-operative pain and Envelta™ is an intranasal molecular
envelope enkephalin formulation being developed to manage acute and
chronic pain, including pain associated with cancer. Virpax is also
using its intranasal Molecular Envelope Technology (MET) to develop
one other prescription product candidate, NobrXiol™, which is being
developed for the nasal delivery of a pharmaceutical-grade
cannabidiol (CBD) for the management of rare pediatric epilepsy.
Virpax has competitive cooperative research and development
agreements (CRADAs) for two of its prescription drug candidates,
one with the National Institutes of Health (NIH) and one with the
Department of Defense (DOD). Virpax is also seeking partners for
two nonprescription product candidates: AnQlar, which is being
developed to inhibit viral replication caused by influenza or
SARS-CoV-2, and Epoladerm™, which is a topical diclofenac spray
film formulation being developed to manage pain associated with
osteoarthritis. For more information, please visit
https://www.virpaxpharma.com.and follow us on Twitter, LinkedIn and
YouTube.
Forward-Looking Statements
This press release contains certain forward-looking statements
as that term is defined in the Private Securities Litigation Reform
Act of 1995, as amended, including those described below. These
forward-looking statements are based on current expectations,
estimates, forecasts and projections about the industry and markets
in which we operate and management’s current beliefs and
assumptions.
These statements may be identified by the use of forward-looking
expressions, including, but not limited to, “expect,” “anticipate,”
“intend,” “plan,” “believe,” “estimate,” “potential,” “predict,”
“project,” “should,” “would” and similar expressions and the
negatives of those terms. These statements relate to future events
and involve known and unknown risks, uncertainties, and other
factors, including the additional capital which will be necessary
to complete studies and clinical trials that the Company plans to
initiate and other factors listed under “Risk Factors” in the
Company’s Annual Report on Form 10-K and Quarterly Reports on Form
10-Q that the Company has filed with the U.S. Securities and
Exchange Commission. Prospective investors are cautioned not to
place undue reliance on such forward-looking statements, which
speak only as of the date of this press release. The Company
undertakes no obligation to publicly update any forward-looking
statement, whether as a result of new information, future events or
otherwise.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20250213717392/en/
Investor: info@virpaxpharma.com
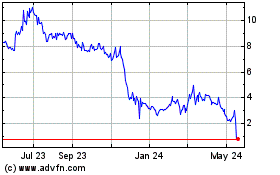
Virpax Pharmaceuticals (NASDAQ:VRPX)
Historical Stock Chart
From Jan 2025 to Feb 2025
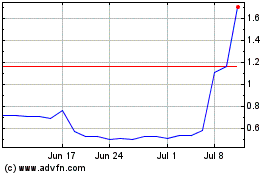
Virpax Pharmaceuticals (NASDAQ:VRPX)
Historical Stock Chart
From Feb 2024 to Feb 2025