Stryker launches Gamma4 Hip Fracture Nailing System in Europe
March 26 2024 - 2:10AM
Business Wire
New system streamlines workflows and enhances usability
Stryker (NYSE:SYK), a global leader in medical technologies,
today announced the release of its Gamma4 Hip Fracture Nailing
System in most of the European markets. The newest Gamma system
will provide surgeons with the next generation of Stryker’s
intramedullary nailing system. The Gamma4 system is designed to
treat hip and femur fractures and streamline procedural workflows
for surgeons. The system received the CE certification in Europe in
September 2023.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240326868923/en/
The Gamma4 System is indicated for the
treatment of fractures in the intracapsular, trochanteric,
subtrochanteric and shaft regions of the femur (including
osteoporotic and osteopenic bone). (Photo: Business Wire)
“The Gamma4 system represents the latest chapter of the Gamma
legacy, and we are excited to expand availability to our European
customers after a successful launch in the U.S.,” said Markus Ochs,
vice president and general manager of Stryker’s European Trauma
& Extremities division. “This latest system demonstrates our
commitment to working together with our customers to understand
their needs. With evolved features to the trusted Gamma system, we
believe the system will help our customers rebuild patient
lives.”
Used in over 25,000 cases in North America and Japan, the Gamma4
System is indicated for the treatment of fractures in the
intracapsular, trochanteric, subtrochanteric and shaft regions of
the femur (including osteoporotic and osteopenic bone). Key
features include:
- A redefined nail design. Designed using our SOMA
database of over 34,000 3D bone models generated from CT scans1,
Gamma4 has a chamfered distal tip, a 5mm shorter proximal body
compared to Gamma3, and a length-dependent radius of curvature
(RoC) for long nails2, enhancing anatomical fit and usability.
- Improved workflow. The Precision Pin™ offers more
resistance to pull out, and the potential for skiving is reduced by
66% compared to a standard ∅3.2 K-wire.3
- Integrated instrument platform. Gamma4 and T2Alpha nails
now all work on the same instrument platform, standardizing
surgical procedures.
“The Gamma system has evolved through streamlined processes,
standardization and meticulous attention to detail, all crafted to
elevate operative techniques and enhance usability,” said Dr.
Gilbert Taglang, chairman of the OTCF Education Committee. “With an
unwavering focus on surgeon experience and feedback, our goal was
to not only enhance ease of use but also prioritize the well-being
of the patient. The new Gamma4 system redefines hip fracture care
with sleek instrumentation, advanced nail design and improved
geometry collectively, ensuring a lasting impact on orthopaedic
practices for years to come.”
About Stryker
Stryker is a global leader in medical technologies and, together
with its customers, is driven to make healthcare better. The
company offers innovative products and services in MedSurg,
Neurotechnology, Orthopaedics and Spine that help improve patient
and healthcare outcomes. Alongside its customers around the world,
Stryker impacts more than 150 million patients annually. More
information is available at www.stryker.com.
A surgeon must always rely on his or her own professional
clinical judgment when deciding whether to use a particular product
when treating a particular patient. Stryker does not dispense
medical advice and recommends that surgeons be trained in the use
of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth
of Stryker’s product offerings. A surgeon must always refer to the
package insert, product label and/or instructions for use before
using any of Stryker’s products. Products may not be available in
all markets because product availability is subject to the
regulatory and/or medical practices in individual markets. Please
contact your sales representative if you have questions about the
availability of products in your area.
Content ID: G4-AD-13, 02-2024
References
- Internal report D0000124129, Rev AD
- Internal Report № D0000089997, Rev AA
- Stryker: Internal test report D0000099352, Rev AE
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240326868923/en/
Media Contact Andrea Sampson President/CEO, Sampson
Public Relations Group asampson@sampsonprgroup.com 562.304.0301
Stryker (NYSE:SYK)
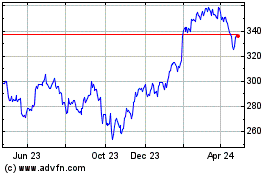
Historical Stock Chart
From Jan 2025 to Feb 2025
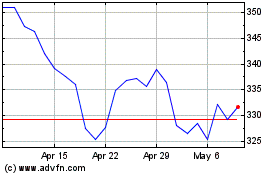
Stryker (NYSE:SYK)
Historical Stock Chart
From Feb 2024 to Feb 2025