Crescita Expands Medical Aesthetic Portfolio with MicronJetTM600 The World’s Smallest Intradermal Delivery Device
July 17 2024 - 7:00AM
Business Wire
Crescita Therapeutics Inc. (TSX: CTX and OTC US:
CRRTF) (“Crescita” or the “Company”), a growth-oriented,
innovation-driven Canadian commercial dermatology company, today
announced that it has signed an exclusive distribution agreement
(the “Agreement”) with NanoPass Technologies Ltd. (“NanoPass”), a
pioneer in the development and commercialization of an advanced
intradermal delivery device, to launch and distribute
MicronJetTM600 (“MicronJet”) in the Canadian medical aesthetics
market.
MicronJet is an innovative intradermal injection device,
leveraging the proven MEMS technology, that offers a highly
effective, consistent and virtually pain-free delivery of aesthetic
products and therapeutic substances. With three 0.6mm, silicon
crystal-made delivery pyramids, MicronJet can be attached to
standard syringes and will provide aesthetic clinicians with the
least invasive and most precise intradermal delivery on the market
today, allowing administration to delicate and sensitive areas such
as around the eyes, neck and décolleté area, as well as to the full
face, for optimal patient outcomes.
“MicronJet is a great addition to our medical aesthetic
portfolio, enhancing our offerings alongside NCTF®135 HA, Art
Filler® and Pliaglis®. This novel device provides precision in
penetration depth, no bruising and comfortable injection, all of
which are welcomed by medical practitioners and patients alike,”
stated Serge Verreault, President and CEO of Crescita. “Bringing
innovative products to Canadian practitioners and patients aligns
with our strategic vision to establish ourselves as a leading
player in our market,” added Mr. Verreault.
Crescita will be responsible for obtaining regulatory approval
for MicronJet from Health Canada and plans to launch the product
promptly thereafter, which is currently anticipated to be in the
first half of 2025. MicronJet has been accepted by patients
worldwide and is supported by over 70 clinical studies and multiple
peer reviewed publications. MicronJet is CE marked, U.S. FDA
cleared, ISO 13485 certified and is currently approved in Australia
and several other jurisdictions in Europe, Asia and the Middle
East.
Daniel Levitats, CEO of NanoPass, added, “We're thrilled to
collaborate with Crescita to bring advanced delivery solutions to
the Canadian market and support more clinicians in expanding their
practice, delivering improved patient outcomes, and increasing
treatment satisfaction. This collaboration marks an exciting step
in NanoPass’s commercial growth goals towards becoming the new
intradermal delivery standard.”
About Crescita Therapeutics Inc. Crescita (TSX: CTX and
OTC US: CRRTF) is a growth-oriented, innovation-driven Canadian
commercial dermatology company with in-house R&D and
manufacturing capabilities. The Company offers a portfolio of
high-quality, science-based non-prescription skincare products and
a commercial stage prescription product. We also own multiple
proprietary transdermal delivery platforms that support the
development of patented formulations to facilitate the delivery of
active ingredients into or through the skin. For more information
visit, www.crescitatherapeutics.com.
About NanoPass NanoPass is a leading innovator in
intradermal delivery technology, specializing in developing
cutting-edge therapeutic and medical aesthetic delivery solution
platforms. NanoPass is redefining the injection therapy standard of
care, supporting superior clinical results and virtually pain-free
procedures through continues clinical innovation. MicronJetTM600 is
FDA cleared, CE marked and has regulatory approval in Brazil, South
Korea, China and more. It is supported by extensive clinical data
and approved for the delivery of any substance or drug under the
surface of the skin that is approved for this delivery route. For
more information, visit www.nanopass.com.
Forward-looking Information All information in this press
release, other than statements of current and historical fact,
represents forward-looking information within the meaning of
applicable securities laws and is qualified by this cautionary
note. Forward-looking information can be identified by words such
as: “believe”, “expect”, “become”, “future”, “contingent”, “will”,
“may” and similar references to future periods. Forward-looking
information in this news release includes, but is not limited to,
statements with respect to obtaining regulatory approval for
MicronJet, its anticipated launch date, as well as its acceptance
in the Canadian market.
Forward-looking information is neither historical fact nor an
assurance of future performance. Instead, it is based only on
current beliefs, expectations, and assumptions regarding the future
of the Company’s business, future plans and strategies,
projections, anticipated events and trends, the economy and other
future conditions.
Because forward-looking information relates to the future, it is
subject to inherent uncertainties, risks and changes in
circumstances that are difficult to predict and many of which are
outside of the Company’s control.
Actual results may differ materially from those indicated in
forward-looking information. Therefore, you should not unduly rely
on any forward-looking information. Important factors and risks
that could cause actual results to differ materially from those
indicated in forward-looking information include, among others:
- economic and market conditions, including factors impacting
global supply chains such as pandemics and geopolitical conflicts
and tensions;
- the impact of inflation and changing interest rates;
- the Company’s ability to execute its growth strategies;
- reliance on third parties for marketing, distribution and
commercialization, and clinical trials;
- the impact of variations in the values of the Canadian dollar
in relation to the U.S. dollar and Euro;
- increasing competition in the industries in which the Company
operates;
- the Company’s ability to retain members of its management team
and key personnel;
- the degree or lack of market acceptance of MicronJet;
- manufacturing and supply risks;
- the Company’s ability to meet its contractual obligations;
- the impact of litigation involving the Company and/or
MicronJet;
- the impact of changes in the relationship with NanoPass
Technologies Ltd.;
- the failure to obtain regulatory approval for the Product from
Health Canada;
- the failure to launch MicronJet in the Canadian medical
aesthetics market; and
- the failure to further develop the Canadian medical aesthetics
market.
As a result of the foregoing and other factors, no assurance can
be given that future results, levels of activity or achievements
indicated in any forward-looking information will actually be
achieved. Any forward-looking information in this press release is
based only on information currently available to management and
speaks only as of the date on which it is provided. Except as
required by applicable securities laws, Crescita undertakes no
obligation to publicly update any forward-looking information,
whether written or oral, that may be provided from time to time,
whether as a result of new information, future developments or
otherwise.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240717950170/en/
FOR MORE INFORMATION: Linda Kisa, CPA, CA Vice-President,
Reporting and Corporate Affairs Email:
lkisa@crescitatx.com
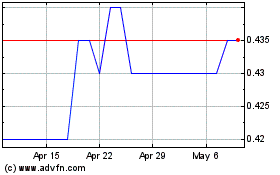
Crescita Therapeutics (TSX:CTX)
Historical Stock Chart
From Jan 2025 to Feb 2025
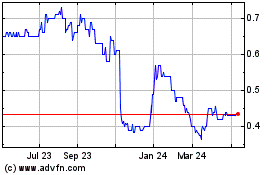
Crescita Therapeutics (TSX:CTX)
Historical Stock Chart
From Feb 2024 to Feb 2025