false
0001817640
0001817640
2024-09-24
2024-09-24
0001817640
BRZH:CommonStockParValue0.0001PerShareMember
2024-09-24
2024-09-24
0001817640
BRZH:RightsExchangeableIntoOnetwentiethOfOneShareOfCommonStockMember
2024-09-24
2024-09-24
0001817640
BRZH:WarrantsEachWholeWarrantExercisableForOneShareOfCommonStockAtExercisePriceOf11.50PerWholeShareMember
2024-09-24
2024-09-24
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or Section 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
September 24, 2024
BREEZE HOLDINGS ACQUISITION CORP.
(Exact name of registrant as specified in its charter)
| Delaware |
|
001-39718 |
|
85-1849315 |
(State or other jurisdiction of
incorporation or organization) |
|
(Commission File Number) |
|
(IRS Employer
Identification Number) |
955 W.
John Carpenter Freeway, Suite 100-929
Irving,
TX 75039
(Address of principal executive offices)
(619) 500-7747
(Registrant’s telephone number, including
area code)
Not Applicable
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation to the registrant under any of the following provisions:
| ☒ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on
which registered |
| Common Stock, par value $0.0001 per share |
|
BRZH |
|
N/A |
| Rights exchangeable into one-twentieth of one share of common stock |
|
BRZHR |
|
N/A |
| Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 per whole share |
|
BRZHW
|
|
N/A |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☒
If an emerging growth company, indicate by
check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 1.01. Entry into a Material Definitive Agreement.
Merger Agreement
On
September 24, 2024, Breeze Holdings Acquisition Corp., a Delaware corporation (“Breeze”), entered into a Merger Agreement
and Plan of Reorganization (the “Merger Agreement”), by and among (i) Breeze, (ii) a Cayman Islands exempted company and wholly-owned
subsidiary of Parent expected to be named “YD Bio Limited,” which is in the process of being formed, and once formed will
enter into a joinder to the Merger Agreement (“Pubco”), (iii) Breeze Merger Sub, Inc., a Delaware corporation and which
will be a direct, wholly-owned subsidiary of Pubco (“Parent Merger Sub”), (iv) a Cayman Islands exempted company which will
be a wholly-owned subsidiary of Pubco, expected to be named “BH Biopharma Merger Sub Limited,” and once formed, will enter
into a joinder to the Merger Agreement (“Company Merger Sub,” with Company Merger Sub and Parent Merger Sub together referred
to herein as the “Merger Subs”), and (v) YD Biopharma Limited, a Cayman Islands exempted company (“YD Biopharma”).
Capitalized terms used herein and not defined shall have the meaning attributed to them in the Merger Agreement.
The
Merger Agreement and the transactions contemplated thereby were approved by the boards of directors of each of Breeze and YD Biopharma.
The Business Combination
Pursuant
to and in accordance with the terms set forth in the Merger Agreement, (a) Parent Merger Sub will merge with and into Breeze, with
Breeze continuing as the surviving entity (the “Parent Merger”), as a result of which, (i) Breeze will become a wholly-owned
subsidiary of Pubco, and (ii) each issued and outstanding security of Breeze immediately prior to the effective time of the Parent
Merger (the “Parent Merger Effective Time”) (other than shares of Breeze common stock that have been redeemed or are owned
by Breeze or any of its direct or indirect subsidiaries as treasury shares and any Dissenting Parent Shares (as defined in the Merger
Agreement)) shall no longer be outstanding and shall automatically be cancelled in exchange for the issuance to the holder thereof of
a substantially equivalent security of Pubco (other than the Parent Rights, which shall be automatically converted into ordinary shares
of Pubco), and, (b) immediately following the consummation of the Parent Merger but on the same day, Company Merger Sub will merge
with and into YD Biopharma, with YD Biopharma continuing as the surviving entity (the “Company Merger” and, together with
the Parent Merger, the “Mergers”), as a result of which, (i) YD Biopharma will become a wholly-owned subsidiary of Pubco,
and (ii) each issued and outstanding security of YD Biopharma immediately prior to the effective time of the Company Merger (the
“Company Merger Effective Time”) (other than any Cancelled Shares or Dissenting Shares) shall no longer be outstanding and
shall automatically be cancelled in exchange for the issuance to the holder thereof of a substantially equivalent security of Pubco. The
Mergers and the other transactions contemplated by the Merger Agreement are hereinafter referred to as the “Business Combination.”
The
Business Combination is expected to close in February 2025, subject to customary closing conditions, the receipt of certain governmental
approvals and the required approval by the stockholders of Breeze and YD Biopharma.
Business Combination
Consideration
Pursuant to and in accordance
with the terms set forth in the Merger Agreement, at the Parent Merger Effective Time, (a) each share of Breeze common stock, par value
$0.0001 per share (“Breeze Common Stock”) outstanding immediately prior to the Parent Merger Effective Time that has not been
redeemed, is not owned by Breeze or any of its direct or indirect subsidiaries as treasury shares and is not a Dissenting Parent Share
will automatically convert into one ordinary share of Pubco (each, a “Pubco Ordinary Share”), (b) each Breeze Warrant shall
automatically convert into one warrant to purchase a Pubco Ordinary Share (each, a “Pubco Warrant”) on substantially the same
terms and conditions; and (c) each Breeze Right will be automatically converted into the number of Pubco Ordinary Shares that would have
been received by the holder of such Breeze Right if it had been converted upon the consummation of a business combination in accordance
with Breeze’s organizational documents.
The aggregate consideration
to be received by the equity holders of YD Biopharma is based on a pre-transaction equity value of $647,304,110. In accordance with
the terms and subject to the conditions of the Merger Agreement, at the Company Merger Effective Time, each issued and outstanding
ordinary share of YD Biopharma shall be cancelled and converted into a number of Pubco Ordinary Shares based on that Exchange Ratio described
below. The Exchange Ratio will be equal to (i) $647,304,110, divided by (ii) the number of fully-diluted shares of YD Biopharma
Common Stock outstanding as of the Closing, further divided by (iii) an assumed value of Pubco Ordinary Shares of $10.00 per share.
Governance
The
parties have agreed to take actions such that, effective immediately after the Closing of the Business Combination, Pubco’s board
of directors shall consist of seven directors, consisting of two Breeze designees (at least one of whom shall be an “independent
director”), four YD Biopharma designees (at least three of whom shall be “independent directors”). Additionally, certain
current YD Biopharma management personnel will become officers of Pubco. To qualify as an “independent director” under the
Merger Agreement, a designee shall qualify as “independent” under the rules of the Nasdaq Stock Market.
Representations
and Warranties; Covenants
The Merger Agreement contains
representations, warranties and covenants of each of the parties thereto that are customary for transactions of this type, including,
among others, covenants providing for (i) certain limitations on the operation of the parties’ respective businesses prior to consummation
of the Business Combination, (ii) the parties’ efforts to satisfy conditions to consummation of the Business Combination, including
by obtaining any necessary approvals from governmental agencies (including U.S. federal antitrust authorities and under the Hart-Scott-Rodino
Antitrust Improvements Act of 1976, as amended (the “HSR Act”)), (iii) prohibitions on the parties soliciting alternative
transactions, (iv) Pubco preparing and filing a registration statement on Form F-4 with the Securities and Exchange Commission (the “SEC”)
and taking certain other actions to obtain the requisite approval of Breeze’s stockholders to vote in favor of certain matters,
including the adoption of the Merger Agreement and approval of the Business Combination, at a special meeting to be called for the approval
of such matters, and (v) the protection of, and access to, confidential information of the parties.
The representations, warranties
and covenants in the Merger Agreement were made solely for the benefit of the parties to the Merger Agreement and are subject to limitations
agreed upon by the contracting parties, including being qualified by confidential disclosures made the parties to the Merger Agreement
which are not filed publicly and which are subject to a contractual standard of materiality different from that generally applicable to
stockholders and were used for the purpose of allocating risk among the parties rather than establishing matters as facts. Breeze does
not believe that these schedules contain information that is material to an investment decision.
In
addition, Pubco has agreed to adopt an equity incentive plan, as described in the Merger Agreement.
Conditions to the
Closing
The
obligations of Breeze, Pubco, Parent Merger Sub and Company Merger Sub (the “Breeze Parties”) and YD Biopharma to consummate
the Business Combination are subject to certain closing conditions, including, but not limited to, (i) the approval of Breeze’s
stockholders, (ii) the approval of YD Biopharma’s stockholders, and (iii) Pubco’s Form F-4 registration statement
becoming effective.
In addition, the obligations
of the Breeze Parties to consummate the Business Combination are also subject to the fulfillment (or waiver) of other closing conditions,
including, but not limited to, (i) the representations and warranties of YD Biopharma being true and correct to the standards applicable
to such representations and warranties and each of the covenants of YD Biopharma having been performed or complied with in all material
respects, (ii) delivery of certain ancillary agreements required to be executed and delivered in connection with the Business Combination,
and (iii) no Material Adverse Effect having occurred.
The obligation of YD Biopharma
to consummate the Business Combination is also subject to the fulfillment (or waiver) of other closing conditions, including, but not
limited to, (i) the representations and warranties of the Breeze Parties being true and correct to the standards applicable to such
representations and warranties and each of the covenants of the Breeze Parties having been performed or complied with in all material
respects, and (ii) the shares of Pubco Common Stock issuable in connection with the Business Combination being listed on the Nasdaq
Stock Market.
Termination
The Merger Agreement may be
terminated under certain customary and limited circumstances prior to the Closing of the Business Combination, including, but not limited
to, (i) by mutual written consent of Breeze and YD Biopharma, (ii) by Breeze, on the one hand, or YD Biopharma, on the other
hand, if there is any breach of the representations, warranties, covenant or agreement of the other party as set forth in the Merger Agreement,
in each case, such that certain conditions to closing cannot be satisfied and the breach or breaches of such representations or warranties
or the failure to perform such covenant or agreement, as applicable, are not cured or cannot be cured within certain specified time periods,
(iii) by either Breeze or YD Biopharma if the Business Combination is not consummated by April 30, 2025 (which date may be extended
by mutual agreement of the parties to the Merger Agreement), (iv) by either Breeze or YD Biopharma if a meeting of Breeze’s
stockholders is held to vote on proposals relating to the Business Combination and the stockholders do not approve the proposals, and
(v) by Breeze if the YD Biopharma stockholders do not approve the Merger Agreement.
Under
certain circumstances as described further in the Merger Agreement, if the Merger Agreement is validly terminated by Breeze, YD Biopharma
will pay Breeze a fee equal to the trust extension payments made by on behalf of Breeze to the trust in connection with the Business Combination
of up to $150,000.
PIPE Investment
The
Merger Agreement contemplates that Breeze, Pubco and YD Biopharma shall use their commercially reasonable efforts to enter into and consummate
a subscription with investors related to a private placement of shares in the Company, Breeze and/or Pubco (the “PIPE Investment”).
Sponsor Support Agreement
Concurrently with the execution
of the Merger Agreement, Breeze, Pubco, YD Biopharma and the Parent Initial Stockholders (as defined in the Merger Agreement) entered
into an Sponsor Support Agreement (the “Sponsor Support Agreement”), pursuant to which, among other things, the Parent Initial
Stockholders: (a) agreed to vote all of their shares of Breeze Common Stock in favor of the Parent Proposals, including the adoption
of the Merger Agreement and the approval of the Transactions; (b) agreed to vote against any other matter, action, agreement, transaction
or proposal that would reasonably be expected to result in (i) a breach of any of the Breeze Parties’ representations, warranties,
covenants, agreements or obligations under the Merger Agreement or (ii) any of the mutual or YD Biopharma conditions to the Closing
in the Merger Agreement not being satisfied; (c) (i) waived, subject to and conditioned upon the Closing and to the fullest
extent permitted by applicable law and the Breeze organizational documents, and (ii) agreed not to assert or perfect, any rights
to adjustment or other anti-dilution protections to which such Breeze Initial Stockholder may be entitled in connection with the
Mergers or the other Transactions; (d) agreed to take, or cause to be taken, all actions and to do, or cause to be done, all things
reasonably necessary under applicable laws to consummate the Mergers and the other Transactions on the terms and subject to the conditions
set forth in the Merger Agreement prior to any valid termination of the Merger Agreement; (e) agreed not to transfer or pledge any
of their shares of Breeze Common Stock, or enter into any arrangement with respect thereto, after the execution of the Merger Agreement
and prior to the Closing Date, subject to certain customary conditions and exceptions; and (f) waived their rights to redeem any
of their shares of Breeze Common Stock in connection with the approval of the Parent Proposals.
The foregoing description
of the Sponsor Support Agreement is subject to and qualified in its entirety by reference to the full text of the Sponsor Support Agreement,
a copy of which is attached hereto as Exhibit 10.1, and the terms of which are incorporated herein by reference. Any capitalized terms
used in this section entitled “Sponsor Support Agreement” and not otherwise defined herein shall have the meanings
assigned to them in the Sponsor Support Agreement.
YD Biopharma Support
Agreement
Concurrently with the execution
of the Merger Agreement, Breeze, Pubco, YD Biopharma, and certain shareholders of YD Biopharma representing the requisite votes necessary
to approve the Merger Agreement (the “YD Biopharma Equity Holders”) entered into Shareholder Support Agreements (the “Shareholder
Support Agreement”), pursuant to which the YD Biopharma Equity Holders: (a) agreed to vote in favor of the adoption of the Merger
Agreement and approve the Mergers and the other Transactions to which YD Biopharma is a party; (b) agreed to waive any appraisal
or similar rights they may have pursuant to Cayman law with respect to the Mergers and the other Transactions; (d) agreed to vote
against any other matter, action, agreement, transaction or proposal that would reasonably be expected to result in (i) a breach
of any of YD Biopharma’s representations, warranties, covenants, agreements or obligations under the Merger Agreement or (ii) any
of the mutual or the Breeze Parties’ conditions to the Closing in the Merger Agreement not being satisfied; and (e) agreed
not to sell, assign, transfer or pledge any of their YD Biopharma ordinary shares (or enter into any arrangement with respect thereto)
after the execution of the Merger Agreement and prior to the Closing Date, subject to certain customary conditions and exceptions.
The
foregoing description of the Shareholder Support Agreement is subject to and qualified in its entirety by reference to the full text of
the Shareholder Support Agreement, a copy of which is attached hereto as Exhibit 10.2, and the terms of which are incorporated herein
by reference. Any capitalized terms used in this section entitled “Shareholder Support Agreement” and not otherwise
defined herein shall have the meanings assigned to them in the Shareholder Support Agreement.
Lock-Up Agreement
Concurrently with the execution
of the Merger Agreement, Breeze, Pubco, YD Biopharma, the Parent Initial Stockholders and certain YD Biopharma Equity Holders entered
into a Lock-Up Agreement (the “Lock-Up Agreement”), pursuant to which the Parent Initial Stockholders and such YD Biopharma
Equity Holders agreed, among other things, to refrain from selling or transferring their shares of Pubco Common Stock for a period of
eight (8) months following the Closing, subject to early release (a) of 10% of their shares of Pubco Common Stock if the daily
volume weighted average closing sale price of Pubco Common Stock quoted on the Nasdaq for any 20 trading days within any 30 consecutive trading
day period beginning on the four-month anniversary of the Closing exceeds $12.50 per share, (b) of an additional 10% of their shares
of Pubco Common Stock if the daily volume weighted average closing sale price of Pubco Common Stock quoted on the Nasdaq for any 20 trading
days within any 30 consecutive trading day period beginning on the four-month anniversary of the Closing exceeds $15.00 per share;
(c) of all of their shares of Pubco Common Stock upon the occurrence of a Subsequent Transaction; and (d) upon the determination
of the Pubco board of directors (including a majority of the independent directors) following the six month anniversary of the Closing
Date.
The
foregoing description of the Lock-Up Agreement is subject to and qualified in its entirety by reference to the full text of the Lock-Up
Agreement, a copy of which is attached hereto as Exhibit 10.3, and the terms of which are incorporated herein by reference. Any capitalized
terms used in this section entitled “Lock-Up Agreement” and not otherwise defined herein shall have the meanings assigned
to them in the Lock-Up Agreement.
Registration Rights Agreement
In accordance with the Merger
Agreement, within thirty (30) days after the execution of the Merger Agreement, Breeze, the Parent Initial Stockholders, Pubco, and certain
YD Biopharma Equity Holders are expected to enter into a registration rights agreement (the “Registration Rights Agreement”),
pursuant to which Pubco will, among other things, be obligated to file a registration statement to register the resale of certain securities
of Pubco held by the Parent Initial Stockholders and such YD Biopharma Equity Holders. The Registration Rights Agreement will also provide
the Parent Initial Stockholders and such YD Biopharma Equity Holders with “piggy-back” registration rights, subject to certain
requirements and customary conditions.
Item 7.01. Regulation FD Disclosure.
On
September 25, 2024, Breeze and YD Biopharma issued a joint press release announcing their entry into the Merger Agreement. The press release
is attached hereto as Exhibit 99.1 and incorporated by reference herein.
Furnished
as Exhibit 99.2 hereto and incorporated into this Item 7.01 by reference is the investor presentation that Breeze and YD Biopharma have
prepared for use in connection with the announcement of the Business Combination.
The
foregoing (including Exhibits 99.1 and 99.2) is being furnished pursuant to Item 7.01 of Form 8-K and will not be deemed to be filed for
purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise be subject to
the liabilities of that section, nor will it be deemed to be incorporated by reference in any filing under the Securities Act or the Exchange
Act.
Additional Information
and Where to Find It
This
Current Report relates to a proposed business combination transaction among Breeze, Pubco, and YD Biopharma pursuant to which Breeze and
YD Biopharma would become wholly-owned subsidiaries of Pubco. In connection with the proposed transaction, Pubco intends to file with
the SEC a registration statement/proxy statement on Form F-4 that will that also will constitute a proxy statement of Breeze with respect
to the Breeze Common Stock to be issued in the proposed transaction (the “proxy statement/prospectus”). This Current Report
is not a substitute for the Proxy Statement/Prospectus. The definitive Proxy Statement/Prospectus (if and when available) will be delivered
to Breeze’s and YD Biopharma’s stockholders. Pubco and/or Breeze may also file other relevant documents regarding the proposed
transaction with the SEC. BEFORE MAKING ANY VOTING OR INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS OF BREEZE AND YD BIOPHARMA AND
OTHER INTERESTED PARTIES ARE URGED TO READ THE REGISTRATION STATEMENT, PROXY STATEMENT/PROSPECTUS AND ALL OTHER RELEVANT DOCUMENTS THAT
ARE FILED OR WILL BE FILED WITH THE SEC IN CONNECTION WITH THE PROPOSED TRANSACTION, INCLUDING ANY AMENDMENTS OR SUPPLEMENTS TO THESE
DOCUMENTS, CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT BREEZE, YD
BIOPHARMA, THE PROPOSED TRANSACTION AND RELATED MATTERS.
Investors
and security holders may obtain free copies of the proxy statement/prospectus (if and when available) and other documents that are filed
or will be filed with the SEC by Breeze through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the
SEC by Breeze will be available free of charge at Breeze Holdings Acquisition Corp., 955 W. John Carpenter Fwy., Suite 100-929, Irving,
TX 75039, attention: J. Douglas Ramsey, Ph.D.
Participants in the Solicitation
Breeze,
YD Biopharma and certain of their respective directors and executive officers may be deemed to be participants in the solicitation of
proxies from the stockholders of Breeze and YD Biopharma in respect of the proposed transaction. Information about Breeze’s directors
and executive officers and their ownership of Breeze common stock is set forth in Breeze’s filings with the SEC, including its Annual
Report on Form 10-K/A for the year ended December 31, 2023 filed with the SEC on April 25, 2024 (as amended, the “Annual Report”).
To the extent that holdings of Breeze’s securities have changed since the amounts included in the Annual Report, such changes have
been or will be reflected on Statements of Change in Ownership on Form 4 filed with the SEC. Other information regarding the participants
in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained
in the Proxy Statement/Prospectus and other relevant materials to be filed with the SEC in respect of the proposed transaction when they
become available. You may obtain free copies of these documents as described in the preceding paragraph.
Cautionary Note Regarding Forward-Looking Statements
This
Current Report contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of
1995, including, among other things, statements regarding the anticipated benefits and impact of the proposed transaction on the combined
company’s business and future financial and operating results, the anticipated timing of closing of the proposed transaction, the
anticipated growth of the industries and markets in which YD Biopharma competes, the success and customer acceptance of YD Biopharma’s
product and service offerings and other aspects of YD Biopharma’s operations, plans, objectives, opportunities, expectations or
operating results, the expected ownership structure of the combined company and the likelihood and ability of the parties to successfully
consummate the proposed transaction. Words such as “may,” “should,” “will,” “believe,”
“expect,” “anticipate,” “intend,” “estimated,” “target,” “project,”
and similar phrases or words of similar meaning that denote future expectations or intent regarding the combined company’s financial
results, operations and other matters are intended to identify forward-looking statements. You should not rely upon forward-looking statements
as predictions of future events. Such forward-looking statements are based upon the current beliefs and expectations of management and
are inherently subject to significant business, economic and competitive risks, uncertainties and other factors, both known and unknown,
which are difficult to predict and generally beyond our control and that may cause actual results and the timing of future events to differ
materially from the results and timing of future events anticipated by the forward-looking statements in this Current Report, including
but not limited to: (i) the ability of the parties to complete the proposed transaction within the time frame anticipated or at all, which
may adversely impact the price of Breeze’s securities; (ii) the failure to realize the anticipated benefits of the proposed transaction
or those benefits taking longer than anticipated to be realized; (iii) the risk that the proposed transaction may not be completed by
Breeze’s business combination deadline; (iv) the failure to satisfy the conditions to the consummation of the proposed transaction,
including the adoption of the definitive merger agreement by the stockholders of Breeze or YD Biopharma, the receipt of any required governmental
or regulatory approvals or the failure to meet the Nasdaq listing standards in connection with the closing of the proposed transaction;
(v) the occurrence of any event, change or other circumstance that could give rise to the termination of the definitive merger agreement;
(vi) the effect of the announcement or pendency of the proposed transaction on YD Biopharma’s business relationships, performance
and business generally; (vii) risks that the proposed transaction disrupts current plans and operations of YD Biopharma and any potential
difficulties in YD Biopharma employee retention as a result of the proposed transaction; (viii) the outcome of any legal proceedings that
may be instituted against YD Biopharma or Breeze related to the definitive merger agreement or the proposed transaction or any product
liability or regulatory lawsuits or proceedings relating to YD Biopharma’s products or services; (ix) the ability to maintain the
listing of Pubco’s securities on the Nasdaq Capital Market after the closing; (x) potential volatility in the price of Breeze’s
securities due to a variety of factors, including changes in the competitive and highly regulated industries in which YD Biopharma operates,
variations in performance across competitors, changes in laws and regulations affecting YD Biopharma’s business, and changes in
the combined company’s capital structure; (xi) the ability to implement business plans, identify and realize additional opportunities
and achieve forecasts and other expectations after the completion of the proposed transaction; (xii) the risk of downturns and the possibility
of rapid change in the highly competitive industries in which YD Biopharma operates or the markets that YD Biopharma targets; (xiii) the
inability of YD Biopharma and its current and future collaborators to successfully develop and commercialize YD Biopharma’s products
and services in the expected time frame or at all; (xiv) the risk that the combined company may never achieve or sustain profitability
or may need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; and (xv)
the costs of the proposed transaction. The forward-looking statements contained in this Current Report are also subject to additional
risks, uncertainties and factors, including those described in Breeze’s most recent Annual Report on Form 10-K and Quarterly Reports
on Form 10-Q and other documents filed or to be filed with the SEC by Pubco and/or Breeze from time to time. You are cautioned not to
place undue reliance on forward-looking statements as a predictor of future performance as projected financial information and other information
are based on estimates and assumptions that are inherently subject to various significant risks, uncertainties and other factors, many
of which are beyond our control. The forward-looking statements included in this Current Report are made only as of the date hereof, and
we disclaim any intention or obligation to update any forward-looking statements as a result of developments occurring after the date
hereof.
No Offer or Solicitation
This
Current Report is not intended to and shall not constitute an offer to sell or the solicitation of an offer to sell or to buy any securities
or a solicitation of any vote or approval and is not a substitute for the proxy statement/prospectus or any other document that Breeze
may file with the SEC or send to Breeze’s, Pubco’s or YD Biopharma’s stockholders in connection with the proposed transaction.
No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| † |
Certain of the exhibits and schedules to this exhibit have been omitted in accordance with Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of all omitted exhibits and schedules to the SEC upon its request. |
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
BREEZE HOLDINGS ACQUISITION CORP. |
| |
|
|
| Date: September 25, 2024 |
By: |
/s/ J. Douglas Ramsey |
| |
Name: |
J. Douglas Ramsey, Ph.D. |
| |
Title: |
Chief Executive Officer and
Chief Financial Officer |
Exhibit 2.1
Execution Version
MERGER AGREEMENT AND PLAN OF REORGANIZATION
BY AND AMONG
BREEZE HOLDINGS ACQUISITION CORP.,
BREEZE MERGER SUB, INC.,
AND
YD BIOPHARMA LIMITED
DATED AS OF SEPTEMBER 24, 2024
Table of Contents
| |
|
|
Page |
| |
|
|
|
| ARTICLE I DEFINITIONS |
3 |
| |
Section 1.01 |
Certain Definitions |
3 |
| |
Section 1.02 |
Further Definitions |
15 |
| |
Section 1.03 |
Construction |
17 |
| |
|
|
|
| ARTICLE II AGREEMENT AND PLAN OF MERGER |
18 |
| |
Section 2.01 |
The Mergers |
18 |
| |
Section 2.02 |
Effective Time; Closing |
18 |
| |
Section 2.03 |
Effect of the Mergers |
19 |
| |
Section 2.04 |
Governing Documents |
19 |
| |
Section 2.05 |
Directors and Officers |
20 |
| |
Section 2.06 |
Closing Deliverables |
20 |
| |
|
|
|
| ARTICLE III CONVERSION and exchange OF SECURITIES |
21 |
| |
Section 3.01 |
Conversion of Company Securities |
21 |
| |
Section 3.02 |
Effect of Parent Merger on Issued and Outstanding Securities of Parent |
22 |
| |
Section 3.03 |
Effect of Parent Merger on Issued and Outstanding Securities of Parent Merger Sub and Pubco |
23 |
| |
Section 3.04 |
Exchange of Company Securities |
23 |
| |
Section 3.05 |
Stock Transfer Books |
25 |
| |
Section 3.06 |
Payment of Expenses |
25 |
| |
Section 3.07 |
Dissenters’ Rights |
26 |
| |
|
|
|
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
27 |
| |
Section 4.01 |
Organization and Qualification; Subsidiaries |
27 |
| |
Section 4.02 |
Organizational Documents |
28 |
| |
Section 4.03 |
Capitalization |
28 |
| |
Section 4.04 |
Authority Relative to This Agreement |
29 |
| |
Section 4.05 |
No Conflict; Required Filings and Consents |
30 |
| |
Section 4.06 |
Permits; Compliance |
30 |
| |
Section 4.07 |
Financial Statements |
31 |
| |
Section 4.08 |
Absence of Certain Changes or Events |
33 |
| |
Section 4.09 |
Absence of Litigation |
33 |
| |
Section 4.10 |
Employee Benefit Plans |
34 |
| |
Section 4.11 |
Labor and Employment Matters |
36 |
| |
Section 4.12 |
Real Property; Title to Assets |
36 |
| |
Section 4.13 |
Intellectual Property |
37 |
| |
Section 4.14 |
Taxes |
41 |
| |
Section 4.15 |
Environmental Matters |
43 |
| |
Section 4.16 |
Material Contracts |
44 |
| |
Section 4.17 |
Insurance |
46 |
| |
Section 4.18 |
Vote Required |
47 |
| |
Section 4.19 |
Certain Business Practices |
47 |
| |
Section 4.20 |
Interested Party Transactions |
47 |
| |
Section 4.21 |
Brokers |
48 |
| |
Section 4.22 |
FDA |
48 |
| |
Section 4.23 |
Exclusivity of Representations and Warranties |
49 |
| |
|
|
|
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF Parent Parties |
49 |
| |
Section 5.01 |
Corporate Organization |
49 |
| |
Section 5.02 |
Governing Documents |
49 |
| |
Section 5.03 |
Capitalization |
50 |
| |
Section 5.04 |
Authority Relative to this Agreement |
50 |
| |
Section 5.05 |
No Conflict; Required Filings and Consents |
51 |
| |
Section 5.06 |
Compliance |
52 |
| |
Section 5.07 |
SEC Filings; Financial Statements; Sarbanes-Oxley |
52 |
| |
Section 5.08 |
Absence of Certain Changes or Events |
54 |
| |
Section 5.09 |
Absence of Litigation |
54 |
| |
Section 5.10 |
Board Approval; Vote Required |
54 |
| |
Section 5.11 |
No Prior Operations of Pubco and the Merger Subs |
55 |
| |
Section 5.12 |
Brokers |
55 |
| |
Section 5.13 |
Parent Trust Fund |
55 |
| |
Section 5.14 |
Employees |
56 |
| |
Section 5.15 |
Taxes |
56 |
| |
Section 5.16 |
Registration and Listing |
58 |
| |
Section 5.17 |
Prior Business Operations |
58 |
| |
Section 5.18 |
Parent Material Contracts |
58 |
| |
Section 5.19 |
Proxy Statement and Registration Statement |
59 |
| |
Section 5.20 |
Investment Company Act |
59 |
| |
Section 5.21 |
Transactions with Affiliates |
59 |
| |
Section 5.22 |
Legacy Parent Transaction Expenses |
59 |
| |
Section 5.23 |
The Parent Parties’ Investigation and Reliance |
60 |
| |
|
|
|
| ARTICLE VI CONDUCT OF BUSINESS PENDING THE Company MERGER |
60 |
| |
Section 6.01 |
Conduct of Business by the Company Pending the Company Merger |
60 |
| |
Section 6.02 |
Conduct of Business by the Parent Parties Pending the Mergers |
64 |
| |
Section 6.03 |
Claims Against Trust Account |
66 |
| ARTICLE VII ADDITIONAL AGREEMENTS |
67 |
| |
Section 7.01 |
Proxy Statement; Registration Statement |
67 |
| |
Section 7.02 |
Parent Stockholders’ Meeting; Pubco and Merger Subs Stockholder’s Approval |
69 |
| |
Section 7.03 |
Requisite Approval |
69 |
| |
Section 7.04 |
Access to Information; Confidentiality |
70 |
| |
Section 7.05 |
Non-Solicitation |
71 |
| |
Section 7.06 |
Exclusivity |
72 |
| |
Section 7.07 |
Employee Benefits Matters |
73 |
| |
Section 7.08 |
Directors’ and Officers’ Indemnification |
74 |
| |
Section 7.09 |
Notification of Certain Matters |
75 |
| |
Section 7.10 |
Further Action; Reasonable Best Efforts |
75 |
| |
Section 7.11 |
Public Announcements |
76 |
| |
Section 7.12 |
Tax Matters |
76 |
| |
Section 7.13 |
Stock Exchange Listing |
76 |
| |
Section 7.14 |
Antitrust |
77 |
| |
Section 7.15 |
Trust Account |
78 |
| |
Section 7.16 |
Directors |
79 |
| |
Section 7.17 |
Equity Incentive Plan |
79 |
| |
Section 7.18 |
Related Party Agreements |
79 |
| |
Section 7.19 |
Assignment of Legacy Parent Transaction Expenses |
79 |
| |
Section 7.20 |
PIPE Investment |
79 |
| |
|
|
|
| ARTICLE VIII CONDITIONS TO THE MERGERs |
80 |
| |
Section 8.01 |
Conditions to the Obligations of Each Party |
80 |
| |
Section 8.02 |
Conditions to the Obligations of the Parent Parties |
81 |
| |
Section 8.03 |
Conditions to the Obligations of the Company |
82 |
| |
|
|
|
| ARTICLE IX TERMINATION, AMENDMENT AND WAIVER |
83 |
| |
Section 9.01 |
Termination |
83 |
| |
Section 9.02 |
Effect of Termination |
85 |
| |
Section 9.03 |
Amendment |
85 |
| |
Section 9.04 |
Waiver |
85 |
| |
|
|
|
| ARTICLE X GENERAL PROVISIONS |
86 |
| |
Section 10.01 |
Notices |
86 |
| |
Section 10.02 |
Nonsurvival of Representations, Warranties and Covenants |
87 |
| |
Section 10.03 |
Severability |
87 |
| |
Section 10.04 |
Entire Agreement; Assignment |
87 |
| |
Section 10.05 |
Parties in Interest |
88 |
| |
Section 10.06 |
Governing Law |
88 |
| |
Section 10.07 |
Waiver of Jury Trial |
88 |
| |
Section 10.08 |
Headings |
88 |
| |
Section 10.09 |
Counterparts; Electronic Delivery |
89 |
| |
Section 10.10 |
Specific Performance |
89 |
| |
Section 10.11 |
No Recourse |
89 |
| |
Section 10.12 |
Conflicts and Privilege |
90 |
| |
|
|
|
| Schedule A |
Key Employees |
|
| Schedule B |
Transaction Expenses Example |
|
MERGER AGREEMENT AND PLAN OF REORGANIZATION
This MERGER AGREEMENT AND
PLAN OF REORGANIZATION (this “Agreement”), dated as of September 24, 2024 (the “Effective Date”),
is made by and among Breeze Holdings Acquisition Corp., a Delaware corporation (“Parent”), a Cayman Islands
exempted company and a wholly-owned subsidiary of Parent, expected to be named “YD Bio Limited,” which is in the process of
being formed by Parent, and once formed, Parent shall cause it to enter into a joinder to this Agreement (“Pubco”),
Breeze Merger Sub, Inc., a Delaware corporation which will be a direct, wholly owned Subsidiary of Pubco (“Parent Merger Sub”),
a Cayman Islands exempted company that will be a wholly-owned subsidiary of Pubco, expected to be named “BH Biopharma Merger Sub
Limited,” which is in the process of being formed by Parent, and once formed, Parent shall cause it to enter into a joinder to this
Agreement (“Company Merger Sub,” Company Merger Sub and Parent Merger Sub are together referred to herein as
the “Merger Subs”), and YD Biopharma Limited, a Cayman Islands exempted company (the “Company”).
Certain terms used herein are defined in ARTICLE I.
RECITALS
WHEREAS, upon the terms and
subject to the conditions of this Agreement, the parties hereto desire and intend to effect a business combination transaction pursuant
to which (a) Parent Merger Sub will merge with and into Parent, with Parent continuing as the surviving corporation (the “Parent
Merger”), and (b) immediately following the consummation of the Parent Merger but on the same day, Company Merger Sub
will merge with and into the Company (the “Company Merger,” the Company Merger and the Parent Merger are together
referred to herein as the “Mergers”), with the Company continuing as the surviving corporation;
WHEREAS, as a result of the
Mergers, Parent and the Company will become wholly owned Subsidiaries of Pubco, and Pubco will become a publicly traded company listed
on Nasdaq;
WHEREAS, the Board of Directors
of the Company (the “Company Board”) has unanimously (a) determined that this Agreement, the Ancillary
Agreements to which the Company is a party, the Company Merger and the other Transactions to which the Company is a party are fair to,
and in the best interests of, the Company and its stockholders, and declared their advisability, (b) approved this Agreement, the
Ancillary Agreements to which the Company is a party, the Company Merger and the other Transactions to which the Company is a party, and
(c) recommended the adoption of this Agreement and the approval of the Company Merger and the other Transactions to which the Company
is a party by the stockholders of the Company;
WHEREAS, the Board of
Directors of Parent (the “Parent Board”) has (a) determined that (i) this Agreement, the
Ancillary Agreements to which Parent is a party, the Mergers and the other Transactions are fair to, and in the best interests of,
Parent and its stockholders, and declared their advisability and (ii) the fair market value of the Company is equal to at least
eighty percent (80%) of the balance of the Trust Fund, (b) approved this Agreement, the Ancillary Agreements to which Parent is
a party, the Mergers and the other Transactions to which Parent is a party, and (c) adopted a resolution recommending that the
stockholders of Parent vote in favor of all Parent Proposals, including, without limitation, adoption of this Agreement and approval
of the Transactions, and directing that this Agreement and the Mergers and the other Transactions to which Parent is a party be
submitted for consideration by the stockholders of Parent at the Parent Stockholders’ Meeting;
WHEREAS, the Board of Directors
of Parent Merger Sub (the “Parent Merger Sub Board”) has (a) determined that this Agreement, the Ancillary
Agreements to which Parent Merger Sub is a party, the Parent Merger and the other Transactions to which Parent Merger Sub is a party are
fair to, and in the best interests of, Parent Merger Sub and Pubco as its sole stockholder, and declared their advisability, (b) adopted
this Agreement and approved the Parent Merger and the other Transactions to which Parent Merger Sub is a party, and (c) recommended the
adoption of this Agreement and the approval of the Parent Merger and the other Transactions to which Parent Merger Sub is a party by Pubco
as the sole stockholder of Parent Merger Sub and directed that this Agreement, the Parent Merger and the other Transactions to which Parent
Merger Sub is a party be submitted for consideration by Pubco as the sole stockholder of Parent Merger Sub;
WHEREAS, immediately following
the execution of this Agreement (and in any event within twenty-four (24) hours herefrom), Pubco will submit this Agreement and the Transactions
to Parent for adoption and approval as the sole stockholder of Pubco, and Parent will so adopt this Agreement and approve the Transactions
in such capacity by irrevocable written consent;
WHEREAS, immediately following
the execution of this Agreement (and in any event within twenty-four (24) hours herefrom), Parent Merger Sub will submit this Agreement
and the Transactions to Pubco for adoption and approval as the sole stockholder of Parent Merger Sub, and Pubco will so adopt this Agreement
and approve the Transactions in such capacity by irrevocable written consent;
WHEREAS, immediately following
the execution of this Agreement (and in any event within twenty-four (24) hours herefrom), Company Merger Sub will submit this Agreement
and the Transactions to Pubco for adoption and approval as the sole stockholder of Company Merger Sub, and Pubco will so adopt this Agreement
and approve the Transactions in such capacity by irrevocable written consent;
WHEREAS, as promptly as practicable
following the execution of this Agreement (and in any event within thirty (30) days thereafter), Pubco, Parent, the Company and the Specified
Stockholders shall enter into a Stockholder Support Agreement (the “Stockholder Support Agreement”), providing
that, among other things, the Specified Stockholders will provide their written consent to (a) adopt this Agreement and approve the Company
Merger and the other Transactions to which the Company is a party, and (b) waive any appraisal or similar rights they may have pursuant
to the Companies Act of the Cayman Islands (Revised), as amended (the “Cayman Act”) with respect to the Company
Merger and the other Transactions;
WHEREAS, as promptly as
practicable following the execution of this Agreement (and in any event within thirty (30) days thereafter), Pubco, Parent, Breeze
Sponsor, LLC, a Delaware limited liability company (the “Sponsor”), each of the directors and officers of
Parent (together with the Sponsor, the “Parent Initial Stockholders”) and the Specified Stockholders shall
enter into a Registration Rights Agreement (the “Registration Rights Agreement”), providing that, among
other things, Pubco will grant to the Parent Initial Stockholders and the Specified Stockholders certain demand and piggyback
registration rights with respect to Pubco Ordinary Shares (or any securities convertible into or exercisable for Pubco Ordinary
Shares) to be held by such Persons immediately following the Closing;
WHEREAS, as promptly as practicable
following the execution of this Agreement (and in any event within thirty (30) days thereafter), Pubco, Parent, the Parent Initial Stockholders,
the Company and the Specified Stockholders shall enter into a Lock-Up Agreement (the “Lock-Up Agreement”) providing
that, among other things, certain Pubco Ordinary Shares held by the Parent Initial Stockholders and the Specified Stockholders will be
subject to the limitations on disposition as set forth therein;
WHEREAS, concurrently with
the execution and delivery of this Agreement, Pubco, Parent, the Company and the Parent Initial Stockholders entered into a Sponsor Support
Agreement (the “Sponsor Support Agreement”), pursuant to which, among other things, (a) the Parent Initial
Stockholders agreed to (i) vote all of their shares of Parent Common Stock in favor of the Parent Proposals, including the adoption
of this Agreement and approval of the Transactions, and if necessary and applicable, any Extension Proposal, and (ii) abstain from
exercising any Redemption Rights in connection with the Parent Merger or the other Transactions, and (b) the Sponsor has agreed to
assume and pay all of the Legacy Parent Transaction Expenses in full and indemnify Parent and its Subsidiaries (including, following the
Effective Time, the Company) from any and all liabilities related thereto;
WHEREAS, each individual listed
on Schedule A (each, a “Key Employee”) has entered into an employment agreement with the Company
(collectively, the “Employment Agreements”), which Employment Agreements shall continue in effect at and which
shall be assigned to, and assumed by, Pubco at the Closing; and
WHEREAS, for U.S. federal
and applicable state income Tax purposes, the parties hereto intend that, (a) taken together, the Mergers and any PIPE Investment will
qualify as a transaction under Section 351 of the Code and the Treasury Regulations promulgated thereunder, (b) the Company Merger will
qualify as a “reorganization” within the meaning of Section 368(a) of the Code and the Treasury Regulations promulgated thereunder
and (c) that this Agreement be, and hereby is adopted as, a “plan of reorganization” (within the meaning of Section 368(a)
of the Code and Treasury Regulations Sections 1.368-2(g) and 1.368-3) to which each of Pubco, Company Merger Sub, and the Company are
parties under Section 368(b) of the Code (the “Intended Tax Treatment”).
NOW, THEREFORE, in consideration
of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, the parties hereto
hereby agree as follows:
ARTICLE
I
DEFINITIONS
Section 1.01
Certain Definitions. For purposes of this Agreement:
“Action”
means any litigation, suit, claim, action, proceeding, audit, or investigation by or before any Governmental Authority.
“Affiliate”
of a specified Person means a Person who, directly or indirectly through one or more intermediaries, controls, is controlled by, or is
under common control with, such specified Person.
“Aggregate Company
Merger Consideration” means the aggregate Per Share Company Merger Consideration payable pursuant to this Agreement to the
Participating Securityholders.
“Ancillary Agreements”
means the Stockholder Support Agreement, the Sponsor Support Agreement, the Registration Rights Agreement, the Lock-Up Agreement, the
Employment Agreements and all other agreements, certificates and instruments executed and delivered by Parent, Pubco, the Merger Subs
or the Company in connection with the Transactions and specifically contemplated by this Agreement.
“Anti-Corruption
Laws” means, as applicable (i) the U.S. Foreign Corrupt Practices Act of 1977, as amended, (ii) the UK Bribery
Act 2010, (iii) anti-bribery legislation promulgated by the European Union and implemented by its member states, (iv) legislation
implementing the OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transactions, and (v) similar
legislation applicable to the Company or any Company Subsidiary from time to time.
“Business Data”
means all business information and data, including Personal Information (whether of employees, contractors, consultants, customers, consumers,
or other Persons and whether in electronic or any other form or medium) that is accessed, collected, used, stored, shared, distributed,
transferred, disclosed, destroyed, disposed of or otherwise processed by any of the Business Systems or otherwise in the course of the
conduct of the business of the Company or any Company Subsidiaries.
“Business Day”
means any day on which the principal offices of the SEC in Washington, D.C. are open to accept filings, or, in the case of determining
a date when any payment is due, any day on which banks are not required or authorized to close in New York, NY; provided that banks
shall not be deemed to be required or authorized to be closed due to a “shelter in place”, “non-essential
employee” or similar closure of physical branch locations at the direction of any Governmental Authority if such banks’
electronic funds transfer systems (including for wire transfers) are open for use by customers on such day.
“Business Systems”
means all Software, firmware, middleware, equipment, workstations, routers, hubs, computer hardware (whether general or special purpose),
electronic data processors, databases, communications, telecommunications, networks, interfaces, platforms, servers, peripherals, and
computer systems, including any outsourced systems and processes, and any Software and systems provided via the cloud or “as
a service,” that are owned or used in the conduct of the business of the Company or any Company Subsidiaries.
“Capital Stock”
means the Company Ordinary Shares.
“Cayman Registrar”
means the Registrar of Companies of the Cayman Islands.
“Code”
means the Internal Revenue Code of 1986, as amended.
“Company Convertible
Securities” means, collectively, all options, warrants or rights to subscribe for or purchase any ordinary shares of the
Company or securities convertible into or exchangeable for, or otherwise confer on the holder any right to acquire any ordinary shares
of the Company.
“Company Equity
Value” means $647,304,110.
“Company IP”
means, collectively, all Company Owned IP and Company Licensed IP.
“Company Licensed
IP” means all Intellectual Property rights owned or purported to be owned by a third party that are licensed to the Company
or any Company Subsidiary or that the Company or any Company Subsidiary otherwise has a right to use.
“Company
Material Adverse Effect” means any event, circumstance, change or effect that, individually or in the aggregate with
any one or more other events, circumstances, changes and effects, (i) is or would reasonably be expected to be materially
adverse to the business, financial condition, assets and liabilities or results of operations of the Company and the Company
Subsidiaries taken as a whole or (ii) would prevent, materially delay or materially impede the performance by the Company of
its obligations under this Agreement or the consummation of the Merger or any of the other Transactions; provided, however,
that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of
whether, there has been or will be a Company Material Adverse Effect: (a) any change or proposed change in or change in the
interpretation of any Law or GAAP; (b) events or conditions generally affecting the industries or geographic areas in which the
Company and the Company Subsidiaries operate; (c) any downturn in general economic conditions, including changes in the credit,
debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market
index or commodity or any disruption of such markets); (d) any geopolitical conditions, outbreak of hostilities, acts of war,
sabotage, civil unrest, cyberterrorism, terrorism, military actions, earthquakes, volcanic activity, hurricanes, tsunamis,
tornadoes, floods, mudslides, wild fires or other natural disasters, weather conditions, epidemics, pandemics or other outbreaks of
illness or public health events and other force majeure events (including any escalation or general worsening of any of the
foregoing); (e) any actions taken or not taken by the Company or the Company Subsidiaries as required by this Agreement or any
Ancillary Agreement; (f) any event, circumstance, change or effect attributable to the announcement or execution, pendency,
negotiation or consummation of the Merger or any of the other Transactions (including the impact thereof on relationships with
customers, suppliers, employees or Governmental Authorities); (g) any failure to meet any projections, forecasts, guidance,
estimates, milestones, budgets or financial or operating predictions of revenue, earnings, cash flow or cash position
(provided that this clause (g) shall not prevent a determination that any event, circumstance, change or effect
which is the underlying cause of such failure has resulted in a Company Material Adverse Effect to the extent not excluded by
another exception herein); or (h) any actions taken, or failures to take action, or such other changes or events, in each case,
which Parent has requested or to which it has consented, except in the cases of clauses (a) through (d), to the
extent that the Company and the Company Subsidiaries, taken as a whole, are disproportionately and adversely affected thereby as
compared with other participants in the industries in which the Company and the Company Subsidiaries operate.
“Company Memorandum
and Articles” means the Company’s memorandum and articles of association filed with the Cayman Registrar on March
14, 2024 and as they may be amended and/or restated from time to time.
“Company Merger
Sub Ordinary Shares” means the ordinary shares of Company Merger Sub designated as ordinary shares in the Company Merger
Sub memorandum and articles of association filed with the Cayman Registrar.
“Company Merger
Sub Organizational Documents” means the memorandum and articles of association filed with the Cayman Registrar of Company
Merger Sub, as amended, modified or supplemented from time to time.
“Company Ordinary
Shares” means ordinary shares, par value $0.10 per share, of the Company.
“Company Organizational
Documents” means the Company Memorandum and Articles as amended, modified or supplemented from time to time.
“Company Owned
IP” means all Intellectual Property rights owned or purported to be owned by the Company or any of the Company Subsidiaries.
“Company Reference
Share Value” means a dollar amount equal to (i) the sum of the Company Equity Value, divided by (ii) the number of
Fully Diluted Company Shares.
“Company Securities”
means the Company Ordinary Shares and the Company Convertible Securities.
“Confidential
Information” means any information, knowledge or data concerning the businesses and affairs of the Company, the Company
Subsidiaries, or any Suppliers or customers of the Company or any Company Subsidiaries or Parent or its subsidiaries (as applicable) that
is not already generally available to the public, including any Intellectual Property rights.
“Consent Solicitation
Statement” means the consent solicitation statement included as part of the Registration Statement with respect to the solicitation
by the Company of the Company Stockholder Approval.
“Contracts”
means any legally binding contracts, agreements, subcontracts, instruments, conditional sales contracts, indentures, notes, bonds, loans,
credit agreements, licenses, sublicenses, mortgages, deeds of trust, powers of attorney, guaranties, leases and subleases and all amendments,
modifications, supplements, schedules, annexes and exhibits thereto.
“control”
(including the terms “controlled by” and “under common control with”) means the possession,
directly or indirectly, or as trustee or executor, of the power to direct or cause the direction of the management and policies of a Person,
whether through the ownership of voting securities, as trustee or executor, by contract or otherwise.
“Disabling Devices”
means Software, viruses, time bombs, logic bombs, trojan horses, trap doors, back doors, spyware, malware, worms, other computer instructions,
intentional devices, techniques, other technology, disabling codes, instructions, or other similar code or software routines or components
that are designed to threaten, infect, assault, vandalize, defraud, disrupt, damage, disable, delete, maliciously encumber, hack into,
incapacitate, perform unauthorized modifications, infiltrate or slow or shut down a computer system or data, software, system, network,
other device, or any component of such computer system, including any such device affecting system security or compromising or disclosing
user data in an unauthorized manner, other than those incorporated by the Company or the applicable third party intentionally to protect
Company Owned IP or Business Systems from misuse.
“Employee Benefit
Plan” means each “employee benefit plan,” as defined in Section 3(3) of ERISA (whether
or not subject to ERISA), any nonqualified deferred compensation plan subject to Section 409A of the Code, and each other retirement,
health, welfare, cafeteria, bonus, commission, stock option, stock purchase, restricted stock, other equity or equity-based compensation,
performance award, incentive, deferred compensation, retiree medical or life insurance, death or disability benefit, supplemental retirement,
severance, retention, change in control, employment, consulting, fringe benefit, sick pay, vacation, and similar plan, program, policy,
practice, agreement, or arrangement, whether written or unwritten.
“Environmental
Laws” means any United States federal, state or local or non-United States Laws relating to: (i) releases or threatened
releases of, or exposure of any Person to, Hazardous Substances or materials containing Hazardous Substances; (ii) the manufacture,
handling, transport, use, treatment, storage or disposal of Hazardous Substances or materials containing Hazardous Substances; or (iii) pollution
or protection of the environment, natural resources or human health and safety.
“ERISA”
means the Employee Retirement Income Security Act of 1974.
“Ex-Im Laws”
means all applicable Laws relating to export, re-export, transfer, and import controls, including the U.S. Export Administration
Regulations, the customs and import Laws administered by U.S. Customs and Border Protection, and the EU Dual Use Regulation.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended.
“Exchange Ratio”
means the following ratio: the quotient obtained by dividing (i) the Company Reference Share Value by (ii) the Pubco Per Share
Value.
“Fully Diluted
Company Shares” means, as of the Company Merger Effective Time, the sum of: (i) the number of Company Ordinary Shares
outstanding immediately prior to the Effective Time; and (ii) the number of Company Ordinary Shares issuable in respect of all issued
and outstanding Company Convertible Securities.
“Fraud”
means actual and intentional common law fraud committed by a party to the Agreement with respect to the making of the representations
and warranties by such party set forth in ARTICLE IV or ARTICLE V as applicable. Under no circumstances shall “Fraud”
include any equitable fraud, constructive fraud, negligent misrepresentation, unfair dealings, or any other fraud or torts based on recklessness
or negligence.
“Hazardous Substance(s)”
means: (i) any substances, wastes, or materials defined, identified or regulated as hazardous or toxic or as a pollutant or a contaminant
under any Environmental Law; (ii) petroleum and petroleum products, including crude oil and any fractions thereof; (iii) natural
gas, synthetic gas, and any mixtures thereof; (iv) polychlorinated biphenyls, per- and polyfluoroalkyl substances, asbestos and radon;
and (v) any other substance, material or waste regulated by, or for which standards of care may be imposed under any Environmental
Law.
“HIPAA”
means the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations, including as amended by the Health
Information Technology for Economic and Clinical Health Act provisions of the American Recovery and Reinvestment Act of 2009, Pub. Law
No. 111-5 and its implementing regulations.
“HSR Act”
means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended.
“Incentive Sponsor
Shares” means the aggregate shares of Parent Common Stock held by the Sponsor.
“Intellectual
Property” means (i) issued patents and pending patent applications (including provisional and non-provisional
applications), design patents, certificates of invention and patent disclosures, together with all reissues, continuations,
continuations-in-part, divisionals, renewals, substitutions, revisions, extensions (including supplementary protection certificates)
or reexaminations thereof, as well as any other applications worldwide claiming priority to any of the foregoing
(“Patents”), (ii) trademarks and service marks, trade dress, logos, trade names, corporate names, brands,
slogans, and other source identifiers together with all translations, adaptations, derivations, combinations and other variants of
the foregoing, and all applications, registrations, and renewals in connection therewith, together with all of the goodwill
associated with the foregoing, (iii) copyrights, and other works of authorship (whether or not copyrightable), and moral rights, and
registrations and applications for registration, renewals and extensions thereof, (iv) trade secrets, know-how (including ideas,
formulas, compositions, inventions (whether or not patentable or reduced to practice)), customer and supplier lists, improvements,
protocols, processes, methods and techniques, research and development information, industry analyses, algorithms, architectures,
layouts, drawings, specifications, designs, plans, methodologies, proposals, industrial models, technical data, financial and
accounting and all other data, databases, database rights, including rights to use any Personal Information, pricing and cost
information, business and marketing plans and proposals, and customer and supplier lists (including lists of prospects) and related
information (“Trade Secrets”), (v) rights in Software, Internet domain names and social media accounts,
(vi) rights of publicity and all other intellectual property or proprietary rights of any kind or description, (vii) copies and
tangible embodiments of any of the foregoing, in whatever form or medium, including all Software, and (viii) all legal rights
arising from items (i) through (vi), including the right to prosecute, enforce and perfect such interests and rights to sue, oppose,
cancel, interfere, enjoin and collect damages based upon such interests, including such rights based on past infringement, if any,
in connection with any of the foregoing.
“IRS”
means the Internal Revenue Service of the United States.
“Knowledge”
or “to the Knowledge” of a Person means in the case of the Company, the actual knowledge of the individuals
listed on Section 1.01(A) of the Company Disclosure Schedule after reasonable inquiry (and for all purposes of Section 4.13
hereof, “reasonable inquiry” shall not require Company to have conducted patent clearance or similar freedom
to operate searches, or other Intellectual Property searches), and in the case of Parent, the actual knowledge of the individuals listed
on Section 1.01(A) of the Parent Disclosure Schedule after reasonable inquiry.
“Leased Real Property”
means the real property leased by the Company or Company Subsidiaries as tenant, together with, to the extent leased by the Company or
Company Subsidiaries, all buildings and other structures, facilities or improvements located thereon and all easements, licenses, rights
and appurtenances of the Company or Company Subsidiaries relating to the foregoing.
“Legacy Parent
Transaction Expenses” means all liabilities reflected on Parent’s financial statements as of and for the period ended
June 30, 2024.
“Lien”
means any lien, security interest, mortgage, deed of trust, defect of title, easement, right of way, pledge, adverse claim or other encumbrance
of any kind that secures the payment or performance of an obligation (other than those created under applicable securities Laws).
“Off-the-Shelf
Software” means any commercially available, off-the-shelf Software that is licensed other than through a written agreement
executed by the licensee (such as via clickwrap, browsewrap, or shrinkwrap licenses) or that has license or user-fees less than $50,000
per year.
“Open Source Software”
means any Software in source code form that is licensed pursuant to (i) any license that is a license now or in the future approved
by the open source initiative and listed at http://www.opensource.org/licenses, which licenses include all versions of the GNU
General Public License (GPL), the GNU Lesser General Public License (LGPL), the GNU Affero GPL, the MIT license, the Eclipse Public License,
the Common Public License, the CDDL, the Mozilla Public License (MPL), the Artistic License, the Netscape Public License, the Sun Community
Source License (SCSL), and the Sun Industry Standards License (SISL), (ii) any license to Software that is considered “free”
or “open source software” by the open source foundation or the free software foundation, (iii) the Server Side
Public License, or (iv) any Reciprocal License.
“Parent Bylaws”
means the Bylaws of Parent, adopted as of June 11, 2020.
“Parent Certificate
of Incorporation” means the Amended and Restated Certificate of Incorporation of Parent, dated as of June 11, 2020.
“Parent Common
Stock” means the common stock of the Parent, par value of $0.0001 per share, designated as Common Stock in the Parent Certificate
of Incorporation.
“Parent Material
Adverse Effect” means any event, circumstance, change or effect that, individually or in the aggregate with any one or more
other events, circumstances, changes and effects, (i) is or would reasonably be expected to be materially adverse to the business,
financial condition, assets and liabilities or results of operations of the Parent Parties; or (ii) would prevent, materially delay
or materially impede the performance by any Parent Party of its respective obligations under this Agreement or the consummation of the
Mergers or any of the other Transactions; provided, however, that none of the following shall be deemed to constitute, alone
or in combination, or be taken into account in the determination of whether, there has been or will be a Parent Material Adverse Effect:
(a) any change or proposed change in or change in the interpretation of any Law or GAAP; (b) events or conditions generally
affecting the industries or geographic areas in which Parent operates; (c) any downturn in general economic conditions, including
changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any
security or market index or commodity or any disruption of such markets); (d) any geopolitical conditions, outbreak of hostilities,
acts of war, sabotage, civil unrest, cyberterrorism, terrorism, military actions, earthquakes, volcanic activity, hurricanes, tsunamis,
tornadoes, floods, mudslides, wild fires or other natural disasters, weather conditions, epidemics, pandemics or other outbreaks of illness
or public health events and other force majeure events (including any escalation or general worsening of any of the foregoing); (e) any
actions taken or not taken by any Parent Party as required by this Agreement or any Ancillary Agreement; (f) any event, circumstance
change or effect attributable to the announcement or execution, pendency, negotiation or consummation of the Mergers or any of the other
Transactions or (g) any actions taken, or failures to take action, or such other changes or events, in each case, which the Company
has requested or to which it has consented, except in the cases of clauses (a) through (d), to the extent that any
Parent Party is disproportionately and adversely affected thereby as compared with other participants in the industry in which such Parent
Party operates.
“Parent Merger
Sub Common Stock” means the common stock of Parent Merger Sub, par value of $0.001 per share, designated as Common Stock
in the Parent Merger Sub certificate of incorporation.
“Parent Merger
Sub Organizational Documents” means the certificate of incorporation and bylaws of Parent Merger Sub, as amended, modified
or supplemented from time to time.
“Parent Organizational
Documents” means the Parent Certificate of Incorporation and the Parent Bylaws, in each case, as amended, modified or supplemented
from time to time.
“Parent Parties”
means Parent, Pubco and the Merger Subs.
“Parent Preferred
Stock” means the preferred stock of the Parent, par value of $0.0001 per share, designated as Preferred Stock in the Parent
Certificate of Incorporation.
“Parent Right”
means a right to acquire 1/20th of a share of Parent Common Stock as set forth in the Amended and Restated Rights Agreement, dated January
26, 2022, between Parent and the Trustee.
“Parent Units”
means the units issued in the IPO or the overallotment consisting of one (1) share of Parent Common Stock, one (1) Parent Right, and one
(1) Parent Warrant.
“Parent Stockholder
Approval” means the approval of the Parent Proposals by an affirmative vote of the holders of the requisite number of shares
of Parent Common Stock (as determined in accordance with applicable Law and the Parent Governing Documents) at a Parent Stockholders’
Meeting duly called by the Parent Board and held for such purpose.
“Parent Warrants”
means the warrants to purchase Parent Common Stock that are outstanding immediately prior to the Closing.
“Participating
Securityholders” means, as of immediately prior to the Closing, each holder of Company Ordinary Shares.
“PCAOB”
means the United States Public Company Accounting Oversight Board and any division or subdivision thereof.
“PCI DSS”
means the Payment Card Industry Data Security Standard, issued by the Payment Card Industry Security Standards Council.
“Permitted Financing
Securities” means any equity securities or debt securities of the Company (or any securities convertible into or exercisable
for equity securities of the Company) issued in any Permitted Financing, notes that are convertible into shares of Capital Stock and warrants
exercisable for shares of Capital Stock.
“Permitted Liens”
means (i) such imperfections of title, easements, encumbrances, Liens or restrictions that do not materially impair or interfere
with the current use of the Company’s or any Company Subsidiary’s assets that are subject thereto, (ii) materialmen’s,
mechanics’, carriers’, workmen’s, warehousemen’s, repairmen’s, landlord’s and other similar Liens
arising in the ordinary course of business, or deposits to obtain the release of such Liens, (iii) Liens for Taxes not yet due and
delinquent or, if delinquent, being contested in good faith and for which appropriate reserves have been made, (iv) zoning, entitlement,
conservation restriction and other land use and environmental regulations promulgated by Governmental Authorities that are not violated
in any material respect by the Company’s or any Company Subsidiary’s current use of the assets that are subject thereto, (v) revocable,
non-exclusive licenses (or sublicenses) of Company Owned IP granted in the ordinary course of business, (vi) non-monetary Liens,
encumbrances and restrictions on real property (including easements, covenants, rights of way and similar restrictions of record) that
do not materially interfere with the present uses of such real property, (vii) Liens identified in the Annual Financial Statements,
and (viii) Liens on leases, subleases, easements, licenses, rights of use, rights to access and rights of way arising from the provisions
of such agreements or benefiting or created by any superior estate, right or interest.
“Person”
means an individual, corporation, partnership, limited partnership, limited liability company, syndicate, person (including, without limitation,
a “Person” as defined in Section 13(d)(3) of the Exchange Act), trust, association or entity or government,
political subdivision, agency or instrumentality of a government.
“Personal Information”
means “personal information,” “personal data,” “personally
identifiable information” or equivalent terms as defined by applicable Privacy/Data Security Laws.
“Pubco Bylaws”
means the Bylaws of Pubco, adopted as of February 9, 2024.
“Pubco Certificate
of Incorporation” means the Certificate of Incorporation of Pubco, dated as of February 6, 2024.
“Pubco Memorandum
and Articles” means the Memorandum and Articles of Pubco as filed with the Cayman Registrar, as the same may be amended
and/or restated from time to time.
“Pubco Ordinary
Shares” means the ordinary shares of Pubco designated in the Pubco Memorandum and Articles.
“Pubco Organizational
Documents” means the Pubco Reincorporation and the Pubco Memorandum and Articles, as amended, modified or supplemented from
time to time.
“Pubco Per Share
Value” means $10.00.
“Privacy/Data
Security Laws” means all Laws governing the creation, receipt, collection, use, storage, maintenance, protection, processing,
sharing, security, disclosure, or transfer (collectively, “Processing”) of Personal Information, such as, to
the extent applicable, the following Laws and their implementing regulations: the Fair Credit Reporting Act, the Federal Trade Commission
Act, the CAN-SPAM Act, the Telephone Consumer Protection Act, the Telemarketing and Consumer Fraud and Abuse Prevention Act, Children’s
Online Privacy Protection Act, California Consumer Privacy Act, the General Data Protection Regulation (GDPR), the Data Protection Law
Enforcement Directive, HIPAA, state data security Laws, state data breach notification Laws, applicable Laws relating to the transfer
of Personal Information, PCI DSS, and any applicable Laws concerning requirements for website and mobile application privacy policies
and practices, call or electronic monitoring or recording or any outbound communications (including outbound calling and text messaging,
telemarketing, and e-mail marketing).
“Products”
means any products or services under development, developed, manufactured, performed, out-licensed, sold, distributed or otherwise made
available by or on behalf of the Company or any Company Subsidiary, including those from which the Company or any Company Subsidiary has
derived previously, is currently deriving or is scheduled or intends to derive, revenue from the sale or provision thereof, including
the products and product candidates set out on Section 1.01(B) of the Company Disclosure Schedule.
“Reciprocal License”
means a license of an item of Software that requires or that conditions any rights granted in such license upon (i) the disclosure,
distribution or licensing of any other Software (other than such item of Software as provided by a third party in its unmodified form),
(ii) a requirement that any disclosure, distribution or licensing of any other Software (other than such item of Software in its
unmodified form) be at no charge, (iii) a requirement that any other licensee of the Software be permitted to access the source code
of, modify, make derivative works of, or reverse-engineer any such other Software, (iv) a requirement that such other Software be
redistributable by other licensees, or (v) the grant of any patent rights (other than patent rights in such item of Software), including
non-assertion or patent license obligations (other than patent obligations relating to the use of such item of Software).
“Redemption Date”
means the deadline for exercising Redemption Rights in connection with the Merger.
“Redemption Rights”
means the redemption rights provided for in Section 9.2 of Article IX of the Parent Certificate of Incorporation.
“Registered Intellectual
Property” means all Intellectual Property that is the subject of an application, registration, issue, or grant, including
any issued or granted patents, registered trademarks, registered copyrights, domain names, social media accounts, and applications therefor.
“Release”
means any spill, discharge, leach, leak, emission, escape, injection, dumping, pouring, emptying, disposal or other release of any materials,
wastes or substances into the environment, whether or not notification or reporting to any governmental authority was or is required,
including any Release which is subject to Environmental Laws.
“Required Parent
Stockholder Approval” means the approval of those Parent Proposals identified in clauses (A)-(C) of Section 7.01(a)
by an affirmative vote of the holders of at least a majority of the outstanding Parent Common Stock entitled to vote (as determined in
accordance with applicable Law and the Parent Organizational Documents) at a Parent Stockholders’ Meeting duly called by the Parent
Board and held for such purpose.
“Requisite Approval”
means the affirmative vote of the holders of at least a majority of the shares of outstanding Company Ordinary Shares.
“Sanctioned Person”
means at any time any Person (i) listed on any Sanctions-related list of designated or blocked Persons, (ii) the government
of, resident in, or organized under the laws of a country or territory that is the subject of comprehensive restrictive Sanctions from
time to time (which includes, as of the date of this Agreement, Cuba, Iran, North Korea, Syria, and the Crimea region), or (iii) majority-owned
or controlled by any of the foregoing.
“Sanctions”
means those applicable, economic and financial sanctions Laws, regulations, embargoes, and restrictive measures administered or enforced
by (i) the United States (including without limitation the U.S. Treasury Department’s Office of Foreign Assets Control),
(ii) the European Union and enforced by its member states, (iii) the United Nations, (iv) His Majesty’s Treasury,
or (v) any other similar governmental authority with jurisdiction over the Company or any Company Subsidiary from time to time.
“Securities Act”
means the Securities Act of 1933, as amended.
“Software”
means all computer software (in object code or source code format), data and databases, and related documentation and materials.
“Specified Stockholders”
means the Persons or entities listed on Section 1.01(C) of the Company Disclosure Schedule.
“Stockholder”
means a holder of stock or shares, as appropriate.
“Subsidiary”
or “Subsidiaries” of the Company, the Parent Surviving Subsidiary, the Company Surviving Subsidiary, Parent,
Pubco or any other Person means an Affiliate controlled by such Person, directly or indirectly, through one or more intermediaries.
“Supplier”
means any Person that supplies inventory or other materials or personal property, components, or other goods or services (including, design,
development and manufacturing services) that comprise or are utilized in, including in connection with the design, development, manufacture
or sale of, the Products of the Company or any Company Subsidiary.
“Tax”
or “Taxes” means any and all taxes (including any duties, levies or other similar governmental fees, assessments
or charges of any kind in the nature of taxes), including, but not limited to, income, estimated, business, occupation, corporate, capital,
gross receipts, transfer, stamp, registration, employment, payroll, social security (or similar), unemployment, withholding, occupancy,
license, severance, capital, production, ad valorem, excise, windfall profits, customs, duties, environmental, premium, real property
gains, real property, personal property, sales, use, turnover, value added and franchise taxes, in each case imposed by any Governmental
Authority, whether disputed or not, together with all interest, penalties, and additions to tax imposed with respect to such amounts thereto.
“Tax Return”
means any return, declaration, report, claim for refund or information return or statement relating to Taxes, including any schedule or
attachment thereto and any amendment thereof, in each case filed or required to be filed with a Governmental Authority.
“Trading Day”
means any day on which shares of Pubco Common Stock is actually traded on the principal securities exchange or securities market on which
shares of Pubco Common Stock are then traded.
“Transaction Documents”
means this Agreement, including all schedules and exhibits hereto, and the Ancillary Agreements.
“Transactions”
means the transactions contemplated by the Transaction Documents.
“Treasury Regulations”
means the regulations promulgated under the Code.
“Virtual Data
Room” means the virtual data room established by the Company or its Representatives, hosted by iDeals, with access made
available to Parent and its Representatives.
“Willful Breach”
means a party’s material breach of any of its representations or warranties as set forth in this Agreement or any other Transaction
Document, or such party’s material breach of any of its covenants set forth in this Agreement or any other Transaction Document,
which material breach, in each case, constitutes, or is a consequence of, a purposeful act or failure to act by such party with the knowledge
that the taking of such act or failure to take such act would, or would reasonably be expected to, cause a material breach of this Agreement
or such Transaction Document.
Section 1.02
Further Definitions. The following terms have the meaning set forth in the Sections set forth
below:
| Defined Term |
Location of Definition |
| Acquisition Proposal |
Section 7.05(b) |
| AFS |
Section 10.12 |
| Agreement |
Preamble |
| Antitrust Laws |
Section 7.14(a) |
| Annual Financial Statements |
Section 4.07(a) |
| Blue Sky Laws |
Section 4.05(b) |
| Board |
Recitals |
| Break-Up Fee |
Section 9.02(b) |
| Business Combination |
Section 6.03 |
| Business Combination Proposal |
Section 7.06 |
| Cancelled Shares |
Section 3.01(b)(ii) |
| Cayman Act |
Recitals |
| Certificates |
Section 3.04(a) |
| Certificates of Merger |
Section 2.02(a) |
| Closing |
Section 2.02(b) |
| Closing Date |
Section 2.02(b) |
| Company |
Preamble |
| Company Board |
Recitals |
| Company Board Recommendation |
Section 7.03 |
| Company Certificate of Merger |
Section 2.02(a) |
| Company Disclosure Schedule |
ARTICLE IV |
| Company Merger Effective Time |
Section 2.02(a) |
| Company Merger |
Recitals |
| Company Merger Payment Schedule |
Section 3.04(i) |
| Company Merger Sub |
Preamble |
| Company Merger Sub Board |
Recitals |
| Company Officer’s Certificate |
Section 8.02(c) |
| Company Permits |
Section 4.06(a) |
| Company Service Provider |
Section 6.01(b)(vii) |
| Company Stockholder Approval |
Section 1.01 |
| Company Subsidiary |
Section 4.01(a) |
| Company Surviving Subsidiary |
Section 2.01(b) |
| Continuing Employees |
Section 7.07(a) |
| Data Security Requirements |
Section 4.13(l) |
| DGCL |
Recitals |
| Dissenting Parent Shares |
Section 3.07(b) |
| Dissenting Company Shares |
Section 3.07(a) |
| Employment Agreement |
Recitals |
| Environmental Permits |
Section 1.01 |
| ERISA Affiliate |
Section 4.10(c) |
| Exchange Agent |
Section 3.04(a) |
| Exchange Agent Agreement |
Section 3.04(a) |
| Exchange Fund |
Section 3.04(b)(i) |
| Extension Proposal |
Section 2.06(b) |
| Extension Proxy Statement |
Section 3.06(b) |
| Defined Term |
Location of Definition |
| FDA |
Section 4.22 |
| FDCA |
Section 4.22 |
| GAAP |
Section 4.07(a) |
| Governmental Authority |
Section 4.05(b) |
| Intended Tax Treatment |
Recitals |
| Interim Financial Statements |
Section 4.07(b) |
| Interim Financial Statements Date |
Section 4.07(b) |
| Interim Period |
Section 6.01(a) |
| IPO |
Section 6.03 |
| Key Employee |
Recitals |
| Law |
Section 4.05(a) |
| Lease |
Section 4.12(b) |
| Lease Documents |
Section 4.12(b) |
| Lock-Up Agreement |
Recitals |
| Material Contracts |
Section 4.16(a) |
| Maximum Annual Premium |
Section 7.08(b) |
| Mergers |
Recitals |
| Merger Subs |
Preamble |
| Non-Disclosure Agreement |
Section 7.04(b) |
| Nonparty Affiliate |
Section 10.11 |
| Ordinary Commercial Agreement |
Section 4.14(b) |
| Parent |
Preamble |
| Parent Board |
Recitals |
| Parent Board Recommendation |
Section 7.02(a) |
| Parent Certificate of Merger |
Section 2.02(a) |
| Parent Closing Liability Max |
Section 8.03(e) |
| Parent Disclosure Schedule |
ARTICLE V |
| Parent Initial Stockholders |
Recitals |
| Pharmaceutical Product |
Section 4.22 |
| PIPE Investment |
Section 7.21 |
| Pubco LTIP |
Section 7.01(a) |
| Parent Merger |
Recitals |
| Parent Merger Effective Time |
Section 2.02(a) |
| Parent Merger Sub |
Preamble |
| Parent Merger Sub Board |
Recitals |
| Parent Proposals |
Section 7.01(a) |
| Parent Related Party |
Section 5.21 |
| Parent Related Party Transactions |
Section 5.21 |
| Parent SEC Reports |
Section 5.07(a) |
| Parent Stockholders’ Meeting |
Section 7.01(a) |
| Per Share Company Merger Consideration |
Section 3.01(b)(i) |
| Permitted Financings |
Section 6.01(b)(ii) |
| Plans |
Section 4.10(a) |
| Privileged Communications |
Section 10.12 |
| Pro Rata Share |
Section 3.04(i) |
| Defined Term |
Location of Definition |
| Prospectus |
Section 6.03 |
| Proxy Statement |
Section 7.01(a) |
| Pubco |
Preamble |
| Pubco Assumed Company Warrant |
Section 3.01(b)(v) |
| Pubco Assumed Parent Warrant |
Section 3.02(b) |
| Pubco Board |
Recitals |
| Pubco Restricted Stock |
Section 3.01(b)(i) |
| Public Stockholders |
Section 6.03 |
| Registration Rights Agreement |
Recitals |
| Registration Statement |
Section 7.01(a) |
| Related Party |
Section 7.18 |
| Released Claims |
Section 6.03 |
| Remedies Exceptions |
Section 4.04 |
| Representatives |
Section 7.04(a) |
| SEC |
Section 5.07(a) |
| SPAC Surviving Subsidiary |
Section 2.01(a) |
| Sponsor |
Recitals |
| Sponsor Support Agreement |
Recitals |
| Stockholder Support Agreement |
Recitals |
| Surviving Provisions |
Section 9.02(a) |
| Tax Claim |
Section 4.14(a) |
| Terminating Company Breach |
Section 9.01(h) |
| Terminating Parent Breach |
Section 9.01(i) |
| Trust Account |
Section 5.13 |
| Trust Agreement |
Section 5.13 |
| Trust Fund |
Section 5.13 |
| Trustee |
Section 5.13 |
| Waiving Parties |
Section 10.12 |
| Written Consent |
Section 7.03 |
| YD Group |
Section 10.12 |
Section 1.03
Construction.
(a)
Unless the context of this Agreement otherwise requires, (i) words of any gender include each other gender, (ii) words
using the singular or plural number also include the plural or singular number, respectively, (iii) the definitions contained in
this agreement are applicable to the other grammatical forms of such terms, (iv) the terms “hereof,”
“herein,” “hereby,” “hereto” and derivative
or similar words refer to this entire Agreement, (v) the terms “Article,” “Section,”
“Schedule” and “Exhibit” refer to the specified Article, Section, Schedule or Exhibit of
or to this Agreement, (vi) the word “including” means “including without limitation,”
(vii) the word “or” shall be disjunctive but not exclusive, (viii) references to agreements and other documents
shall be deemed to include all subsequent amendments and other modifications thereto and references to any Law shall include all rules
and regulations promulgated thereunder, and (ix) references to any Law shall be construed as including all statutory, legal, and regulatory
provisions consolidating, amending or replacing such Law.
(b)
The language used in this Agreement shall be deemed to be the language chosen by the parties to express their mutual intent and
no rule of strict construction shall be applied against any party.
(c)
Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified,
and when counting days, the date of commencement will not be included as a full day for purposes of computing any applicable time periods
(except as otherwise may be required under any applicable Law). If any action is to be taken or given on or by a particular calendar day,
and such calendar day is not a Business Day, then such action may be deferred until the next Business Day.
(d)
All accounting terms used herein and not expressly defined herein shall have the meanings given to them under GAAP.
ARTICLE
II
AGREEMENT
AND PLAN OF MERGER
Section 2.01
The Mergers.
(a)
Upon the terms and subject to the conditions set forth herein, and in accordance with the DGCL, at the Parent Merger Effective
Time, Parent Merger Sub shall be merged with and into Parent. As a result of the Parent Merger, (a) the separate corporate existence of
Parent Merger Sub shall cease, (b) Parent shall continue as the surviving corporation of the Parent Merger, and (c) Parent shall become
a wholly owned subsidiary of Pubco. Parent as the surviving company in the Parent Merger is hereinafter sometimes referred to as “SPAC
Surviving Subsidiary” (and references to Parent for periods after the Parent Merger Effective Time shall include SPAC Surviving
Subsidiary).
(b)
Upon the terms and subject to the conditions set forth herein, and in accordance with the Cayman Act, at the Company Merger Effective
Time, Company Merger Sub shall be merged with and into the Company. As a result of the Merger, (a) the separate corporate existence of
Company Merger Sub shall cease, (b) the Company shall continue as the surviving corporation of the Company Merger, and (c) the Company
shall become a wholly owned subsidiary of Pubco. The Company as the surviving corporation in the Company Merger is hereinafter sometimes
referred to as “Company Surviving Subsidiary” (and references to the Company for periods after the Company Merger
Effective Time shall include Company Surviving Subsidiary). Notwithstanding the Company Merger, for purposes of this Agreement, the Company
will not be included within the meaning of the term Parent Parties for periods prior to the Company Merger Effective Time.
Section 2.02
Effective Time; Closing.
(a) As promptly as practicable,
but in no event later than three (3) Business Days, after the satisfaction or, if permissible, waiver of the conditions set forth
in ARTICLE VIII (other than those conditions that by their nature are to be satisfied at the Closing, it being understood that
the occurrence of the Closing shall remain subject to the satisfaction or, if permissible, waiver of such conditions at the Closing),
the parties hereto shall cause (i) the Parent Merger to be consummated by filing a certificate of merger with the Secretary of State
of the State of Delaware, in such form as is required by, and executed in accordance with, the relevant provisions of the DGCL (the “Parent
Certificate of Merger”), in each case, in form as mutually agreed by the parties (the date and time of the filing of such
Parent Certificate of Merger (or such later time as may be agreed by each of the parties hereto and specified in such Parent Certificate
of Merger) being the “Parent Merger Effective Time”), and (ii) immediately following the consummation of the
Parent Merger but on the same day, the Company Merger to be consummated by filing a plan of merger and such other document(s) required
by the Cayman Act (the “Company Certificate of Merger,” the Company Certificate of Merger together with the
Parent Certificates of Merger are herein referred to as the “Certificates of Merger”) with the Cayman Registrar,
in such form as is required by, and executed in accordance with, the relevant provisions of the Cayman Act, and mutually agreed by the
parties (the date and time of the filing of such Company Certificate of Merger (or such later time as may be agreed by each of the parties
hereto and specified in such Company Certificate of Merger) being the “Company Merger Effective Time”).
(b)
Immediately prior to such filing of the Certificates of Merger in accordance with Section 2.02(a), the closing (the “Closing”)
shall be held by electronic exchange of deliverables and release of signatures, for the purpose of confirming the satisfaction or waiver,
as the case may be, of the conditions set forth in ARTICLE VIII. The date on which the Closing shall occur is referred to herein
as the “Closing Date.”
Section 2.03
Effect of the Mergers.
(a)
At the Parent Merger Effective Time, the effect of the Parent Merger shall be as provided herein and in the applicable provisions
of the DGCL. Without limiting the generality of the foregoing, and subject thereto, at the Parent Merger Effective Time, all the property,
rights, privileges, immunities, powers, franchises, licenses and authority of Parent and Parent Merger Sub shall vest in the SPAC Surviving
Subsidiary, and all debts, liabilities, obligations, restrictions, disabilities and duties of Parent and Parent Merger Sub shall become
the debts, liabilities, obligations, restrictions, disabilities and duties of the SPAC Surviving Subsidiary.
(b)
At the Company Merger Effective Time, the effect of the Company Merger shall be as provided herein and in the applicable provisions
of the Cayman Act. Without limiting the generality of the foregoing, and subject thereto, at the Company Merger Effective Time, all the
property, rights, privileges, immunities, powers, franchises, licenses and authority of the Company and Company Merger Sub shall vest
in the Company Surviving Subsidiary, and all debts, liabilities, obligations, restrictions, disabilities and duties of the Company and
Company Merger Sub shall become the debts, liabilities, obligations, restrictions, disabilities and duties of the Company Surviving Subsidiary.
Section 2.04
Governing Documents.
(a) At the Parent Merger
Effective Time, the Parent Certificate of Incorporation, as in effect immediately prior to the Parent Merger Effective Time, shall
be amended and restated substantially in the form of the certificate of incorporation of Parent Merger Sub as in effect immediately
prior to the Parent Merger Effective Time, and duly filed with the Secretary of State of the State of Delaware, and, as so amended
and restated, shall be the certificate of incorporation of the Parent Surviving Subsidiary until
thereafter amended in accordance with the terms thereof and the DGCL.
(b)
At the Company Merger Effective Time, the Company Memorandum and Articles, as in effect immediately prior to the Company Merger
Effective Time, shall be amended and restated as mutually agreed by the parties hereto and duly filed with the Cayman Registrar, and,
as so amended and restated, shall be the memorandum and articles of association of the Company Surviving Subsidiary until thereafter amended
in accordance with the Cayman Act and such Company Memorandum and Articles (subject to Section 7.08).
(c)
At the Closing, Pubco shall amend and restate, effective as of the Parent Merger Effective Time, the Pubco Memorandum and Articles
as mutually agreed by the parties hereto and duly file such amended and restated Pubco Memorandum and Articles with the Cayman Registrar.
Section 2.05
Directors and Officers.
(a)
The parties will take all requisite actions such that the initial directors of the Company Surviving Subsidiary and the initial
officers of the Company Surviving Subsidiary at and as of immediately after the Company Merger Effective Time shall be the individuals
indicated on Section 2.05(a) of the Company Disclosure Schedule, each to hold office in accordance with the provisions of the Cayman
Act and the memorandum and articles of association of the Company Surviving Subsidiary and until their respective successors are, in the
case of the initial directors, duly elected or appointed and qualified and, in the case of the initial officers, duly appointed. At the
Parent Merger Effective Time, the individuals indicated on Section 2.05(a) of the Company Disclosure Schedule shall be the directors
and officers of the SPAC Surviving Subsidiary, each to hold office in accordance with the provisions of the Cayman Act and the memorandum
and articles of association of the SPAC Surviving Subsidiary.
(b)
At or prior to the Company Merger Effective Time, the parties shall take all necessary action, including causing the directors
of Pubco to resign, so that effective as of the Closing, Pubco’s board of directors will consist of seven (7) individuals, with
such number of independent directors as is necessary to satisfy applicable Nasdaq rules. The Company shall have the right to designate
five (5) members of Pubco’s board of directors and Parent shall have the right to designate two (2) members of Pubco’s board
of directors, with one of Parent’s designees to serve as an independent member of Pubco’s audit committee. For purposes of
this Section 2.05(b), to qualify as an “independent director,” a Person shall qualify as “independent”
under the rules of the Nasdaq Capital Market.
Section 2.06
Closing Deliverables.
(a)
At the Closing, the Company will deliver or cause to be delivered to Parent:
(i)
a copy of the Registration Rights Agreement duly executed the Specified Stockholders;
and
(ii)
a copy of the Lock-Up Agreement duly executed by the Specified Stockholders.
(b)
At the Closing, Parent will deliver or cause to be delivered to the Company:
(i) a copy of the
Registration Rights Agreement duly executed by duly authorized representatives of Parent and the Parent Initial Stockholders;
and
(ii) a copy of the Lock-Up
Agreement duly executed by duly authorized representatives of the Parent Initial Stockholders.
ARTICLE
III
CONVERSION
and exchange OF SECURITIES
Section 3.01
Conversion of Company Securities.
(a)
At the Company Merger Effective Time, by virtue of the Company Merger and without any action on the part of Pubco, Parent, the
Company Merger Sub, the Company or the holders of any of the following securities:
(i) each Company Ordinary
Share (excluding any Cancelled Shares or Dissenting Shares) that is issued and outstanding immediately prior to the Company Merger
Effective Time shall be cancelled and converted into the number of Pubco Ordinary Shares equal to the Exchange Ratio (rounded to the
nearest whole number) (which consideration shall hereinafter be referred to as the “Per Share Company Merger
Consideration”). Each Company Ordinary Share converted into the right to receive the Per Share Company Merger
Consideration pursuant to this Section 3.01(b)(i) will no longer be outstanding, will automatically be cancelled and retired
and will cease to exist, and each holder of (A) any Certificate formerly representing any such Company Ordinary Shares or
(B) any book-entry account which immediately prior to the Company Merger Effective Time represented Company Ordinary Shares
will, subject to applicable Law in the case of Dissenting Shares, cease to have any rights with respect thereto, except the right to
receive the Per Share Company Merger Consideration for each such Company Ordinary Share in accordance with this Section
3.01(b)(i);
(ii) each share of Capital
Stock owned by Pubco, Parent or the Merger Subs or held in the treasury of the Company, or owned by any of their respective direct
or indirect wholly-owned Subsidiaries immediately prior to the Company Merger Effective Time (collectively, the
“Cancelled Shares”), shall be canceled without any conversion thereof and no payment or distribution shall
be made with respect thereto;
(iii) each Company Merger
Sub Ordinary Share issued and outstanding immediately prior to the Company Merger Effective Time shall be converted into and
exchanged for one validly issued, fully paid and nonassessable ordinary share of the Company Surviving Subsidiary;
Section 3.02 Effect of
Parent Merger on Issued and Outstanding Securities of Parent. At the Parent Merger Effective Time,
by virtue of the SPAC Merger and without any action on the part of Pubco, Parent, the Parent Merger Sub or the holders of any of the
following securities:
(a)
Parent Common Stock. Each share Parent Common Stock issued and outstanding immediately prior to the Parent Merger Effective
Time that is not redeemed pursuant to the Redemption Rights (other than Dissenting Parent Shares and those shares described in Section
3.02(d) below) shall be automatically cancelled and extinguished and converted into the right to receive one (1) share of Pubco Common
Stock, following which, all shares of Parent Common Stock shall cease to be outstanding and shall automatically be canceled pursuant to
the terms of this Agreement and shall cease to exist. The holders of outstanding shares of Parent Common Stock immediately prior to the
Parent Merger Effective Time shall cease to have any rights with respect to such shares except as provided herein or required under applicable
Law. Each certificate previously evidencing shares of Parent Common Stock (other than Dissenting Parent Shares) shall be exchanged for
a certificate (if requested) representing the same number of Parent Common Stock upon the surrender of such certificate. Each certificate
formerly representing Parent Common Stock (other than Dissenting Parent Shares) shall thereafter represent the same number of Pubco Common
Stock.
(b)
Parent Warrants. Each issued and outstanding Parent Warrant shall automatically, without any action on the part of the holder
thereof, be converted into a warrant to purchase shares of Pubco Common Stock (each, a “Pubco Assumed Parent Warrant”)
determined in accordance with the terms of such Parent Warrants. At the Parent Merger Effective Time, the Parent Warrants shall cease
to be outstanding and shall automatically be canceled and retired and shall cease to exist. Each of the Pubco Assumed Parent Warrants
shall have, and be subject to, substantially the same terms and conditions set forth in the Parent Warrants, except that in each case
they shall represent the right to acquire shares of Pubco Common Stock in lieu of shares Parent Common Stock, in each case, determined
in accordance with the terms of such Parent Warrant. At or prior to the Parent Merger Effective Time, Pubco shall take all corporate action
necessary to reserve for future issuance, and shall maintain such reservation for so long as any of the Pubco Assumed Parent Warrants
remain outstanding, a sufficient number of shares of Pubco Common Stock for delivery upon the exercise of such Pubco Assumed Parent Warrants.
(c)
Parent Rights. Each issued and outstanding Parent Right shall be automatically converted into the number of shares of Pubco
Common Stock that would have been received by the holder thereof if the Parent Right had been converted in accordance with the Parent
Organizational Documents into shares of Parent Common Stock. At the Parent Merger Effective Time, the Parent Rights shall cease to be
outstanding and shall automatically be canceled and retired and shall cease to exist. The holders of certificates previously evidencing
Parent Rights outstanding immediately prior to the Parent Merger Effective Time shall cease to have any rights with respect to such Parent
Rights, except as provided herein or by applicable Law. Each certificate formerly representing Parent Rights shall thereafter represent
only the right to receive shares of Pubco Common Stock as set forth herein.
(d)
Treasury Shares. If there are any shares of Parent Common Stock that are owned by Parent as treasury shares or by any direct
or indirect Subsidiary of Parent, such shares shall be canceled and extinguished without any conversion thereof or consideration therefor.
Section 3.03 Effect of
Parent Merger on Issued and Outstanding Securities of Parent Merger Sub and Pubco. At the Parent Merger
Effective Time:
(a)
each share of Parent Merger Sub Common Stock issued and outstanding immediately prior to the Parent Merger Effective Time shall
be converted into and exchanged for one validly issued, fully paid and nonassessable share of common stock, par value $0.0001 per share,
of the SPAC Surviving Subsidiary; and
(b)
by virtue of the Parent Merger and without any action on the part of any party hereto or any action on the part of the holders
of securities of any party hereto, all of the securities of Pubco issued and outstanding immediately prior to the Parent Merger Effective
Time shall be canceled and extinguished without any conversion thereof or consideration therefor.
Section 3.04
Exchange of Company Securities.
(a)
Exchange Agent. Pubco shall appoint an exchange agent reasonably
acceptable to the Company (the “Exchange Agent”) (it being understood and agreed, for the avoidance of doubt,
that Continental Stock Transfer & Trust Company (or any of its Affiliates) shall be deemed to be acceptable to the Company) and enter
into a paying and exchange agent agreement, in form and substance reasonably acceptable to Pubco and the Company (the “Exchange
Agent Agreement”) for the purpose of exchanging certificates representing the Capital Stock (collectively, the “Certificates”),
if any, and each share of Capital Stock held in book-entry form on the stock transfer books of the Company immediately prior to the Company
Merger Effective Time, in either case, for the portion of the Aggregate Company Merger Consideration issuable in respect of such Capital
Stock pursuant to Section 3.01(b) on the terms and subject to the other conditions set forth in this Agreement. The Company shall
reasonably cooperate with Pubco and the Exchange Agent in connection with the appointment of the Exchange Agent, the entry into the Exchange
Agent Agreement (including, if necessary or advisable, as determined in good faith by Pubco, by also entering into the Exchange Agent
Agreement in form and substance reasonably acceptable to the Company) and the performance of the covenants and agreements in this Section
3.04(a) (including the provision of any information, or the entry into any agreements or documentation, necessary or advisable, as
determined in good faith by Pubco, or otherwise required by the Exchange Agent Agreement for the Exchange Agent to fulfill its duties
as the Exchange Agent in connection with the transactions contemplated hereby).
(b)
Exchange Procedures.
(i) On the Closing Date,
Pubco shall deposit, or shall cause to be deposited, with the Exchange Agent, for the benefit of the Participating Securityholders,
for exchange and issuance in accordance with this ARTICLE III, the number of Pubco Ordinary Shares sufficient to deliver the
Aggregate Company Merger Consideration issuable pursuant to this Agreement (such Pubco Ordinary Shares being hereinafter referred to
as the “Exchange Fund”).
(ii) Pubco shall cause the
Exchange Agent, pursuant to irrevocable instructions, to issue such Aggregate Company Merger Consideration out of the Exchange Fund
in accordance with the Company Merger Payment Schedule and the other applicable provisions
contained in this Agreement. The Exchange Fund shall not be used for any other purpose.
(iii)
Upon the delivery of the Company Merger Payment Schedule to the Exchange Agent in accordance with Section 3.04(i), Pubco,
Parent and the Company shall take reasonable steps to cause the applicable Aggregate Company Merger Consideration to be issued to the
applicable Participating Securityholder in book-entry form as soon as reasonably practicable following the Closing Date; provided
that the applicable Aggregate Company Merger Consideration shall not be issued with respect to shares of Capital Stock represented by
a Certificate until the applicable holder of such Capital Stock has surrendered such Certificate to the Exchange Agent.
(iv)
If any Aggregate Company Merger Consideration is to be issued to a Person other than the holder of Capital Stock in whose name
the surrendered Certificate or the transferred shares of Capital Stock in book-entry form is registered, it shall be a condition to the
issuance of the applicable Aggregate Company Merger Consideration that, in addition to any other requirements set forth in the Exchange
Agent Agreement, (A) either such Certificate shall be properly endorsed or shall otherwise be in proper form for transfer or such share
of Capital Stock in book-entry form shall be properly transferred and (B) the Person requesting such consideration pay to the Exchange
Agent any transfer or similar Taxes required as a result of such consideration being issued to a Person other than the registered holder
of such Certificate or share of Capital Stock in book-entry form or establish to the satisfaction of the Exchange Agent that such transfer
or similar Taxes have been paid or are not payable.
(c)
No Further Rights in Capital Stock. The Aggregate Company
Merger Consideration issuable upon conversion of the Capital Stock in accordance with the terms hereof shall be deemed to have been issued
in full satisfaction of all rights pertaining to such Capital Stock.
(d)
Adjustments to Aggregate Company Merger Consideration. The
Aggregate Company Merger Consideration shall be equitably adjusted to reflect the effect of any stock split, reverse stock split, stock
dividend (including any dividend or distribution of securities convertible into shares of Pubco Common Stock), reorganization, recapitalization,
reclassification, combination, merger, sale or exchange of shares or other like change with respect to shares of Pubco Common Stock occurring
on or after the date hereof and prior to the Company Merger Effective Time.
(e)
Termination of Exchange Fund. Any portion of the Exchange
Fund that remains undistributed to the Participating Securityholders for one (1) year after the Company Merger Effective Time shall be
delivered to Pubco, upon demand, and any Participating Securityholder who has not theretofore complied with this Section 3.04 shall
thereafter look only to Parent for such holder’s Per Share Company Merger Consideration. Any portion of the Exchange Fund remaining
unclaimed by Participating Securityholders as of a date which is immediately prior to such time as such amounts would otherwise escheat
to or become property of any Governmental Authority shall, to the extent permitted by applicable Law, become the property of Pubco free
and clear of any claims or interest of any Person previously entitled thereto.
(f) No
Liability. None of the Exchange Agent, Pubco, Parent or the Company Surviving Subsidiary shall be liable to any
Participating Securityholder for any Pubco Common Stock (or dividends or distributions with respect thereto) or cash delivered to a
public official pursuant to any abandoned property, escheat or similar Law.
(g) Withholding
Rights. Notwithstanding anything in this Agreement to the contrary, each of Pubco, Parent, the Company Merger Sub, the
Company, the Company Surviving Subsidiary and the Exchange Agent shall be entitled to deduct and withhold from amounts (including
shares, options or other property) otherwise payable, issuable or transferable pursuant to this Agreement such amounts as it is
required to deduct and withhold with respect to such payment, issuance or transfer under the Code or any provision of state, local
or non-U.S. Tax Law. To the extent that amounts are so deducted or withheld and timely paid to the applicable Governmental Authority
in accordance with applicable Law, such deducted or withheld amounts shall be treated for all purposes of this Agreement as having
been paid, issued or transferred to the Participating Securityholder (or intended recipients) in respect of which such deduction and
withholding was made. The parties hereto shall cooperate in good faith to eliminate or reduce any such deduction or withholding.
(h) Fractional
Shares. No certificates or scrip or shares representing fractional Pubco Ordinary Shares shall be issued upon the
exchange of Capital Stock and such fractional share interests will not entitle the owner thereof to vote or to have any rights of a
stockholder of Pubco or a holder of shares of Pubco Common Stock. In lieu of any fractional share of Pubco Common Stock to which any
holder of Capital Stock would otherwise be entitled, the Exchange Agent shall round up or down to the nearest whole share of Pubco
Common Stock, as applicable, with a fraction of 0.5 rounded up. No cash settlements shall be made with respect to fractional shares
eliminated by rounding.
(i) Company
Merger Payment Schedule. At least two (2) Business Days prior to the Closing Date, the Company shall deliver to Pubco,
Parent and the Exchange Agent a schedule (the “Company Merger Payment Schedule”) showing the percentage
allocation of the aggregate Per Share Company Merger Consideration to each Participating Securityholder at the Closing (such
Participating Securityholder’s “Pro Rata Share”) and the corresponding number of shares of Pubco
Common Stock to be issued to such Participating Securityholder pursuant to Section 3.01.
Section 3.05
Stock Transfer Books. At the Company Merger Effective Time, the stock transfer books of the
Company shall be closed and there shall be no further registration of transfers of Company Ordinary Shares thereafter on the records of
the Company. From and after the Company Merger Effective Time, the holders of the Capital Stock outstanding immediately prior to the Company
Merger Effective Time shall cease to have any rights with respect to such Capital Stock, except as otherwise provided in this Agreement
or by Law.
Section 3.06
Payment of Expenses.
(a) The Company and Parent
shall each pay one-half (1/2) of all costs relating to the Transactions, including (A) any EDGAR agent typesetting fees incurred in connection
with the preparation and filing with the SEC of the Proxy Statement and the Registration Statement,
(B) any fees relating to SEC or other regulatory filing fees (including those incurred in connection with the Proxy Statement, the Registration
Statement and the Notification and Report Forms filed under the HSR Act and any other applicable Antitrust Law), and (C) all transfer
taxes associated with the issuance of Pubco’s securities at Closing; provided, that each party shall bear the fees
and expenses of its advisors incurred in connection with the Transactions.
(b)
No sooner than five (5) nor later than two (2) Business Days prior to the Closing Date, Parent shall provide to the Company
a written report setting forth a list of all of the Legacy Parent Transaction Expenses. On or prior to the Closing Date, Parent shall
cause the Legacy Parent Transaction Expenses to be forgiven, assigned to Sponsor without recourse to Parent, or to be satisfied in full
with no further recourse to Parent.
Section 3.07
Dissenters’ Rights.
(a) Notwithstanding any
provision of this Agreement to the contrary and to the extent available under the Cayman Act, Company Ordinary Shares that are
outstanding immediately prior to the Company Merger Effective Time and that are held by shareholders of the Company who have validly
exercised in writing their dissenters’ rights for such Company Ordinary Shares in accordance with Section 238 of the Cayman
Action, and have otherwise complied in all respects with the provisions of the Cayman Act relevant to the exercise and perfection of
dissenters’ rights (collectively, the “Dissenting Company Shares”) shall not be converted into, and
such stockholders shall have no right to receive, the applicable Aggregate Company Merger Consideration unless and until such
stockholder withdraws or otherwise loses such dissenters’ rights (through failure to perfect such dissenters’ rights or
otherwise) under the Cayman Act. From and after the Company Merger Effective Time, (A) the Dissenting Company Shares shall no longer
be outstanding and shall automatically be cancelled and extinguished by virtue of the Company Merger and shall cease to exist and
(B) the holders of Dissenting Company Shares shall be entitled only to such rights as may be granted to them under Section 238 of
the Cayman Act and shall not be entitled to exercise any of the voting rights or other rights of a shareholder of the Company
Surviving Subsidiary or any of its Affiliates (including Pubco); provided, however, that if any holder of Dissenting
Company Shares effectively withdraws or loses such dissenters’ rights (through failure to perfect such dissenters’
rights or otherwise) under the Cayman Act, then the Company Ordinary Shares held by such Dissenting Company Shareholder (1) shall no
longer be deemed to be Dissenting Company Shares and (2) shall be treated as if they had been converted automatically at the Company
Merger Effective Time into the right to receive the applicable portion of the Company Merger Consideration pursuant to Section
3.01(a)(i) in accordance with the terms and conditions of this Agreement. Each holder of Dissenting Company Shares who becomes
entitled to payment for his, her or its Dissenting Company Shares pursuant to the Cayman Act shall receive payment thereof from
Company in accordance with the Cayman Act. The Company shall give Parent (prior to the Closing) or the Sponsor (after the Closing)
prompt notice of any written demands for dissenters’ rights in respect of any Company Ordinary Share, attempted withdrawals of
such demands and any other material developments related to any such demands and provide copies of all documents, instruments or
other communications received by Company, any of its Subsidiaries or any of their respective Representatives related thereto and
shall otherwise keep Parent (prior to the Closing) or the Sponsor (after the Closing) reasonably apprised as to the status and
developments related to such matters, and Parent (prior to the Closing) or the Sponsor (after the Closing) shall have the opportunity
to participate in all negotiations and proceedings with respect to all such demands. The Company shall not, except with the prior
written consent (not to be unreasonably withheld, conditioned or delayed) of Parent (prior to the Closing) or the Sponsor (after the
Closing), make any payment or deliver any consideration (including Pubco Ordinary Shares) with respect to, settle or offer or agree
to settle any such demands.
(b)
Notwithstanding any provision of this Agreement to the contrary and to the extent available under the DGCL, shares of Parent Common
Stock that are outstanding immediately prior to the Parent Merger Effective Time and that are held by stockholders of Parent who shall
have neither voted in favor of the Parent Merger nor consented thereto in writing and who shall have demanded properly in writing appraisal
for such shares of Parent Common Stock in accordance with the DGCL and otherwise complied with all of the provisions of the DGCL relevant
to the exercise and perfection of dissenters’ rights (“Dissenting Parent Shares”) shall not be converted
into, and such stockholders shall have no right to receive, shares of Pubco Common Stock in accordance with the terms of this ARTICLE
III unless and until such stockholder fails to perfect or withdraws or otherwise loses his, her or its right to appraisal and payment
under the DGCL. Shares held by any stockholder of Parent who fails to perfect or who effectively withdraws or otherwise loses his, her
or its dissenters’ rights to appraisal of such shares of Parent Common Stock under the DGCL, shall thereupon be deemed to have been
converted into, and to have become exchangeable for, as of the Effective Time, shares of Pubco Common Stock, without any interest thereon,
in accordance with the terms of this ARTICLE III.
ARTICLE
IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except as set forth in the
Company’s disclosure schedule delivered by the Company to Parent and the Company Merger Sub on the date of this Agreement (the “Company
Disclosure Schedule”), (each of which qualifies (a) the correspondingly numbered representation, warranty or covenant specified
therein and (b) such other representations, warranties or covenants where its relevance as an exception to (or disclosure for purposes
of) such other representation, warranty or covenant is reasonably apparent on its face or cross-referenced), the Company hereby represents
and warrants to the Parent Parties as follows:
Section 4.01
Organization and Qualification; Subsidiaries.
(a)
The Company and each Subsidiary of the Company (each a “Company Subsidiary”) is a corporation, company
or other organization duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation or
organization and has the requisite corporate or other organizational power and authority and all necessary governmental approvals to own,
lease and operate its properties and to carry on its business as it is now being conducted. The Company and each Company Subsidiary is
duly qualified or licensed as a foreign corporation or other organization to do business, and is in good standing, in each jurisdiction
where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing
necessary, except for such failures to be so qualified or licensed and in good standing that would not, individually or in the aggregate,
be expected to have a Company Material Adverse Effect.
(b)
A true and complete list of all the Company Subsidiaries and each other entity in which the Company or any Company Subsidiary
owns any equity or similar interest, together with the jurisdiction of incorporation of each Company Subsidiary or such other entity and
the percentage of the equity interest of each Company Subsidiary or such other entity that is owned by the Company and each other Company
Subsidiary, in each case, as of the date of this Agreement, is set forth in Section 4.01(b) of the Company Disclosure Schedule.
As of the date of this Agreement, the Company does not directly or indirectly own any equity or similar interest in, or any interest convertible
into or exchangeable or exercisable for any equity or similar interest in, any other corporation, partnership, joint venture or business
association or other entity other than any entity set forth on Section 4.01(b) of the Company Disclosure Schedule.
Section 4.02
Organizational Documents. The Company has prior to the date of this Agreement made available
to Parent in the Virtual Data Room a complete and correct copy of the Company Memorandum and Articles and the memorandum and articles
of association, certificate of incorporation and the bylaws or equivalent organizational documents, each as amended, restated or otherwise
modified as of the date of this Agreement, of each Company Subsidiary. Such memoranda and articles of association, certificates of incorporation,
bylaws or equivalent organizational documents are in full force and effect. Neither the Company nor any Company Subsidiary is in violation
of any of the provisions of its memorandum and articles of association, certificate of incorporation, bylaws or equivalent organizational
documents.
Section 4.03
Capitalization.
(a)
The authorized capital stock of the Company consists of 5,000,000 Company Ordinary Shares. As of the date of this Agreement, (A)
1,051,997 Company Ordinary Shares are issued and outstanding and (B) 0 Company Ordinary Shares are subject to outstanding Company
Convertible Securities.
(b)
There are no options, warrants, preemptive rights, calls, convertible securities, conversion rights or other rights, agreements,
arrangements or commitments of any character relating to the issued or unissued share capital of the Company or any Company Subsidiary
or obligating the Company or any Company Subsidiary to issue or sell any shares of, or other equity or voting interests in, or any securities
convertible into or exchangeable or exercisable for shares or other equity or other voting interests in, the Company or any Company Subsidiary.
As of the date hereof, neither the Company nor any Company Subsidiary is a party to, or otherwise bound by, and neither the Company nor
any Company Subsidiary has granted, any outstanding equity appreciation rights, participations, phantom equity, restricted stock, restricted
stock units, performance shares, contingent value rights or similar securities or rights that are derivative of, or provide economic benefits
based, directly or indirectly, on the value or price of, any shares, or other securities or ownership interests in, the Company or any
Company Subsidiary. There are no voting trusts, voting agreements, proxies, stockholder agreements or other agreements to which the Company
or any Company Subsidiary is a party, or to the Company’s knowledge, among any holder of Capital Stock or any other equity interests
or other securities of the Company or any Company Subsidiary to which the Company or any Company Subsidiary is not a party, with respect
to the voting or transfer of the Capital Stock or any of the equity interests or other securities of the Company or any of the Company
Subsidiaries. Except for the Company Subsidiaries, the Company does not own any equity interests in any Person.
(c)
There are no outstanding contractual obligations of the Company or any Company Subsidiary to repurchase, redeem or otherwise acquire
any shares of the Company or any capital stock of any Company Subsidiary or to provide funds to or make any investment (in the form of
a loan, capital contribution or otherwise) in any Person other than a Company Subsidiary.
(d)
All outstanding Capital Stock and all outstanding shares of capital stock or other equity securities (as applicable) of each Company
Subsidiary have been issued and granted in compliance with (A) all applicable securities Laws and other applicable Laws and (B) all
preemptive rights and other requirements set forth in applicable Contracts to which the Company or any Company Subsidiary is a party and
the organizational documents of the Company and the Company Subsidiaries, as applicable.
(e)
Each outstanding share of capital stock of each Company Subsidiary is duly authorized, validly issued, fully paid and nonassessable,
and each such share is owned 100% by the Company or another Company Subsidiary free and clear of all Liens, options, rights of first refusal
and limitations on the Company’s or any Company Subsidiary’s voting rights, other than transfer restrictions under applicable
securities Laws and their respective organizational documents.
(f)
Except for the Capital Stock held by the stockholders of the Company, no shares or other equity or voting interest of the Company,
or options, warrants or other rights to acquire any such shares or other equity or voting interest, of the Company is authorized or issued
and outstanding.
(g)
Except as set forth on Section 4.02(h) of the Company Disclosure Schedule, all outstanding Capital Stock and all outstanding
shares of capital stock or other equity securities (as applicable) of each Company Subsidiary have been issued and granted (i) in compliance
in all material respects with applicable securities Laws and other applicable Laws and (ii) in compliance with any preemptive rights and
other similar requirements set forth in applicable Contracts to which the Company or any Company Subsidiary is a party.
Section 4.04 Authority
Relative to This Agreement. The Company has all necessary corporate power and authority to execute
and deliver this Agreement and each Ancillary Agreement to which it is a party, to perform its obligations hereunder and thereunder
and, subject to receiving the Company Stockholder Approval, to consummate the Transactions. The execution and delivery of this
Agreement by the Company and the consummation by the Company of the Transactions have been, and each Ancillary Agreement to which
the Company is a party will be, duly and validly authorized by all necessary corporate action, and no other corporate proceedings on
the part of the Company are necessary to authorize this Agreement and each Ancillary Agreement to which it is a party or to
consummate the Transactions (other than, with respect to the Merger, the Company Stockholder Approval, which the Written Consent
shall satisfy, and the filing and recordation of appropriate merger documents as required by the Cayman Act). This Agreement has
been duly and validly executed and delivered by the Company and, assuming the due authorization, execution and delivery by the
Parent Parties, constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with
its terms, except as limited by applicable bankruptcy, insolvency, reorganization, moratorium and other Laws of general
application affecting enforcement of creditors’ rights generally, by general equitable principles (the “Remedies
Exceptions”). The Company Board has unanimously approved this Agreement and the Transactions. To the knowledge of the
Company, except as provided under the Cayman Act, no state takeover Law is applicable to the Company Merger or the other
Transactions.
Section 4.05
No Conflict; Required Filings and Consents.
(a)
The execution and delivery of this Agreement by the Company does not, and subject to receipt of the filing and recordation of appropriate
merger documents as required by the Cayman Act and of the consents, approvals, authorizations or permits, filings and notifications, expiration
or termination of waiting periods after filings and other actions set forth on Section 4.05(a) of the Company Disclosure Schedule,
including the Written Consent, being made, obtained or given, the performance of this Agreement by the Company will not (i) conflict
with or violate the Company Memorandum and Articles or any equivalent organizational documents of the Company or any Company Subsidiary,
(ii) conflict with or violate any United States or non-United States statute, law, ordinance, regulation, rule, code, executive order,
injunction, judgment, decree or other order (“Law”) applicable to the Company or any Company Subsidiary or by
which any property or asset of the Company or any Company Subsidiary is bound or affected, or (iii) result in any breach of or constitute
a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any right of termination,
amendment, acceleration or cancellation of, or result in the creation of a Lien (other than any Permitted Lien) on any material property
or asset of the Company or any Company Subsidiary pursuant to, any Material Contract, except, with respect to clauses (ii) and (iii),
for any such conflicts, violations, breaches, defaults or other occurrences which would not have or reasonably be expected to have a Company
Material Adverse Effect.
(b)
The execution and delivery of this Agreement by the Company does not, and the performance of this Agreement by the Company will
not, require any consent, approval, authorization or permit of, or filing with or notification to, or expiration or termination of any
waiting period by, any United States federal, state, county or local or non-United States government, governmental, regulatory or administrative
authority, agency, instrumentality or commission or any court, tribunal, or judicial or arbitral body (a “Governmental Authority”),
except (i) for applicable requirements, if any, of the Exchange Act, the Securities Act, state securities or “blue sky”
laws (“Blue Sky Laws”) and state takeover Laws, the pre-merger notification requirements of the HSR Act, and
filing with and recordation of appropriate merger documents as required by the Cayman Act, and (ii) where the failure to obtain such
consents, approvals, authorizations or permits, or to make such filings or notifications, would not have or would not reasonably be expected
to have a Company Material Adverse Effect.
Section 4.06
Permits; Compliance.
(a) As of the date of
this Agreement, each of the Company and the Company Subsidiaries is in possession of all material franchises, grants,
authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, registrations, approvals and orders of
any Governmental Authority, necessary for each of the Company or the Company Subsidiaries to own, lease and operate its properties
or to carry on its business as it is now being conducted (the “Company
Permits”), except where the failure to have such Company Permits would not have or would not reasonably be expected to
have a Company Material Adverse Effect. As of the date of this Agreement, no suspension or cancellation of any of the Company
Permits is pending or, to the knowledge of the Company, threatened in writing. As of the date of this Agreement, neither the Company
nor any Company Subsidiary is in conflict with, or in default, breach or violation of, (a) any Law applicable to the Company or any
Company Subsidiary or by which any property or asset of the Company or any Company Subsidiary is bound or affected, or (b) any
Material Contract or Company Permit, except, in each case, for any such conflicts, defaults, breaches or violations that would not
have or would not reasonably be expected to have a Company Material Adverse Effect.
(b)
The business of the Company and its Subsidiaries, and each Company Product that is or has been developed or tested by or on behalf
of the Company or the Subsidiaries, is in compliance in all material respects with all applicable Laws. This Section 4.06(b) shall
not apply to Tax matters.
Section 4.07
Financial Statements.
(a)
The Company has prior to the date of this Agreement made available to Parent in the Virtual Data Room true and complete copies
of the unaudited consolidated balance sheet of the Company and the Company Subsidiaries as of December 31, 2023 and the unaudited
consolidated balance sheet of the Company and the Company Subsidiaries as of December 31, 2022, and the related consolidated statements
of operations and cash flows of the Company and the Company Subsidiaries for each of the years then ended (collectively, the “Annual
Financial Statements”), which are attached as Section 4.07(a) of the Company Disclosure Schedule. Each of the Annual
Financial Statements (including the notes thereto) (i) was prepared in all material respects in accordance with United States generally
accepted accounting principles in effect as of the date of this Agreement (“GAAP”) applied on a consistent basis
throughout the periods indicated (except as may be indicated in the notes thereto) and (ii) fairly presents, in all material respects,
the financial position, results of operations and cash flows of the Company and the Company Subsidiaries as of and at the date thereof
and for the period indicated therein, except (A) as otherwise noted therein or (B) for any changes made in connection with the preparation
of financial statements of the Company audited in accordance with the auditing standards of the PCAOB.
(b)
The Company has prior to the date of this Agreement made available to Parent in the Virtual Data Room true and complete copies
of the unaudited consolidated balance sheet of the Company and the Company Subsidiaries as of June 30, 2024 (the “Interim
Financial Statements Date”), and the related unaudited consolidated statements of operations and cash flows of the Company
and the Company Subsidiaries for the six-month period then ended (collectively, the “Interim Financial Statements”),
which are attached as Section 4.07(b) of the Company Disclosure Schedule. The Interim Financial Statements (i) were prepared in
accordance with GAAP applied on a consistent basis throughout the periods indicated (except for the omission of footnotes and subject
to year-end adjustments) and (ii) fairly present, in all material respects, the financial position, results of operations and cash flows
of the Company and the Company Subsidiaries as of and at the date thereof and for the period indicated therein, except as otherwise noted
therein and subject to normal and recurring year-end adjustments.
(c)
Except as and to the extent set forth on the Annual Financial Statements or the Interim Financial Statements, neither the Company
nor any Company Subsidiary has any liability or obligation of a nature (whether accrued, absolute, contingent or otherwise) required to
be reflected on a balance sheet prepared in accordance with GAAP, except for: (i) liabilities that were incurred in the ordinary
course of business or in connection with the consummation of the Transactions since the Interim Financial Statements Date, (ii) obligations
for future performance under any Contract to which the Company or any Company Subsidiary is a party or (iii) such other liabilities
and obligations which are not, individually or in the aggregate, expected to result in a Company Material Adverse Effect.
(d)
In the two (2) years prior to the date of this Agreement, (i) neither the Company nor any Company Subsidiary nor, to the Company’s
knowledge, any director, officer, employee, auditor, accountant or Representative of the Company or any Company Subsidiary, has received
or otherwise had or obtained knowledge of any complaint, allegation, assertion or claim, whether written or, to the knowledge of the Company,
oral, regarding the accounting or auditing practices, procedures, methodologies or methods of the Company or any Company Subsidiary or
their respective internal accounting controls, including any such complaint, allegation, assertion or claim that the Company or any Company
Subsidiary has engaged in questionable accounting or auditing practices and (ii) there have been no internal investigations regarding
accounting or revenue recognition discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial
officer or general counsel of the Company, the Company Board or any committee thereof.
(e)
To the knowledge of the Company, no employee of the Company or any Company Subsidiary has provided or is providing information
to any law enforcement agency regarding the commission or possible commission of any crime or the violation or possible violation of any
applicable Law. None of the Company, any Company Subsidiary or, to the knowledge of the Company, any officer, employee, contractor, subcontractor
or agent of the Company or any Company Subsidiary has discharged, demoted, suspended, threatened, harassed or in any other manner discriminated
against an employee of the Company or any Company Subsidiary in the terms and conditions of employment because of any act of such employee
described in 18 U.S.C. sec. 1514A(a).
(f)
All accounts receivable of the Company and the Company Subsidiaries reflected on the Interim Financial Statements or arising thereafter
have arisen from bona fide transactions in the ordinary course of business consistent with past practices and in accordance with
GAAP and are collectible, subject to bad debts reserved in the Interim Financial Statements. To the knowledge of the Company, such accounts
receivables are not subject to valid defenses, setoffs or counterclaims, other than routine credits granted for errors in ordering, shipping,
pricing, discounts, rebates, returns in the ordinary course of business and other similar matters. The Company’s reserve for contractual
allowances and doubtful accounts is adequate in all material respects and has been calculated in a manner consistent with past practices.
Since December 31, 2023, neither the Company nor any of the Company Subsidiaries has modified or changed in any material respect
its sales practices or methods including, without limitation, such practices or methods in accordance with which the Company or any of
the Company Subsidiaries sell goods, fill orders or record sales.
(g)
All accounts payable of the Company and the Company Subsidiaries reflected on the Interim Financial Statements or arising thereafter
are the result of bona fide transactions in the ordinary course of business and have been paid or are not yet due or payable. Since
December 31, 2023 through the date of this Agreement, the Company and the Company Subsidiaries have not altered in any material respects
their practices for the payment of such accounts payable, including the timing of such payment.
(h)
The Company has established and maintains a system of internal accounting controls designed to provide reasonable assurance that
(i) all transactions are executed in accordance with management’s specific authorization; (ii) the preparation of the Company’s
financial statements for external purposes are in conformity with GAAP and maintain asset accountability; (iii) access to assets is only
permitted in accordance with management’s specific authorization and (iv) the Company’s records accurately reflect the transaction
and disposition of assets, in all material respects.
(i)
Neither the Company (including any employee thereof) nor the Company’s independent auditors has identified or been made aware
of (i) any significant deficiency or material weakness in the system of internal accounting controls utilized by the Company as of the
date of this Agreement,, (ii) any fraud, whether or not material, that involves the Company’s management or other employees who
have a role in the preparation of financial statements or the internal accounting controls utilized by the Company or (iii) any claim
or allegation regarding any of the foregoing.
Section 4.08
Absence of Certain Changes or Events. Since December 31, 2023 through and until the date of
this Agreement, except as otherwise reflected in the Annual Financial Statements or the Interim Financial Statements, or as expressly
contemplated by this Agreement, (a) the Company and the Company Subsidiaries have conducted their respective businesses in all material
respects in the ordinary course and in a manner consistent with past practice, other than due to any actions taken due to a “shelter
in place,” “non-essential employee” or similar direction of any Governmental Authority, (b) neither
the Company or any of the Company Subsidiaries have sold, assigned, transferred, permitted to lapse, abandoned, or otherwise disposed
of any right, title or interest in or to any of their respective material assets (including Company Owned IP) other than revocable non-exclusive
licenses or sublicenses of Company Owned IP granted in the ordinary course of business in which grants of rights to use such Company Owned
IP are incidental to performance under the agreement, (c) there has not been a Company Material Adverse Effect, and (d) none
of the Company or any Company Subsidiary has taken any action that, if taken after the date of this Agreement, would constitute a material
breach of any of the covenants set forth in Section 6.01(b), excluding the covenants set forth in Sections 6.01(b)(i), (vii),
(viii), (ix) or (xiii).
Section 4.09
Absence of Litigation. There is no material Action pending or, to the knowledge of the Company,
threatened against the Company or any Company Subsidiary, or any property or asset of the Company or any Company Subsidiary. Neither the
Company nor any Company Subsidiary nor any property or asset of the Company or any Company Subsidiary is subject to any continuing order
of, consent decree, settlement agreement or other similar written agreement with, or, to the knowledge of the Company, continuing investigation
by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority.
This Section 4.09 shall not apply to Tax matters.
Section 4.10 Employee Benefit
Plans.
(a)
Section 4.10(a) of the Company Disclosure Schedule lists, as of the date of this Agreement, all Employee Benefit Plans that
are maintained, contributed to, required to be contributed to, or sponsored by the Company or any Company Subsidiary for the benefit of
any current or former employee, officer, director or consultant, or under which the Company or any Company Subsidiary has or could incur
any liability (contingent or otherwise) (collectively, whether or not material, the “Plans”).
(b)
With respect to each Plan, the Company has made available to Parent, if applicable, as of the date of this Agreement, (i) a
true and complete copy of the current plan document and all amendments thereto (or a written summary if not reduced to writing) and each
trust or other funding arrangement, (ii) copies of the most recent summary plan description and any summaries of material modifications,
(iii) a copy of the 2020 filed IRS Form 5500 annual report and accompanying schedules (or, if not yet filed, the most recent
draft thereof), (iv) copies of the most recently received IRS determination, opinion or advisory letter, and (v) any non-routine
correspondence to or from any Governmental Authority with respect to any Plan in the three (3) years prior to the date of this Agreement.
Neither the Company nor any Company Subsidiary has any express commitment to modify, change or terminate any Plan, other than with respect
to a modification, change or termination required by ERISA or the Code, or other applicable Law.
(c)
None of the Plans is or was in the two (2) years prior to the date of this Agreement, nor does the Company, any Company Subsidiary
or any ERISA Affiliate have or reasonably expect to have any liability or obligation (contingent or otherwise) under, (i) a multiemployer
plan (within the meaning of Section 3(37) or 4001(a)(3) of ERISA), (ii) a single employer pension plan (within the meaning of
Section 4001(a)(15) of ERISA) subject to Section 412 of the Code or Title IV of ERISA, (iii) a multiple employer plan
subject to Section 413(c) of the Code, (iv) a multiple employer welfare arrangement under ERISA, or (v) a “voluntary employees’
beneficiary association” within the meaning of Section 509(c)(9) of the Code. For purposes of this Agreement, “ERISA
Affiliate” means any entity that together with the Company or any Company Subsidiary would be deemed a “single
employer” for purposes of Section 4001(b)(1) of ERISA or Sections 414 of the Code.
(d)
As of the date of this Agreement and except as disclosed to the Parent in writing, neither the Company nor any Company Subsidiary
is or will be obligated, whether under any Plan or otherwise, to pay separation, severance, termination or similar benefits to any Person
as a result of any Transaction (whether alone or in connection with another event), nor will any such Transaction (whether alone or in
connection with another event) (i) accelerate the time of payment or vesting, (ii) increase the amount or cause the funding of, of any
benefit or other compensation due to any individual, (iii) result in the triggering or imposition of any restrictions or limitations on
the rights of the Company or any other Person to amend or terminate any Employee Benefit Plan; (iv) entitle the recipient of any payment
or benefit to receive a “gross up” payment for any income or other taxes that might be owed with respect to such payment or
benefit; or (v) result in the payment of any amount that would, individually or in combination with any other such payment, constitute
an “excess parachute payment,” as defined in 280G(b)(1) of the Code.
(e)
Except as disclosed to the Parent in writing, none of the Plans provides, nor does the Company or any Company Subsidiary have
or reasonably expect to have any obligation to provide, medical or other welfare benefits to any current or former employee, officer,
director or consultant of the Company or any Company Subsidiary after termination of employment or service except as may be required under
Section 4980B of the Code and Part 6 of Title I of ERISA and the regulations thereunder (at the sole cost of such current
or former employee, officer, director or consultant).
(f)
Each Plan is and has been for the six (6) years prior to the date of this Agreement in compliance, in all material respects, in
accordance with its terms and the requirements of all applicable Laws including, without limitation, ERISA and the Code. The Company and
each Company Subsidiary have performed, in all material respects, all obligations required to be performed by them under, are not in any
material respect in default under or in violation, and have no knowledge, of any default or violation in any material respect by any party
to, any Plan. No Action is pending or, to the knowledge of the Company, threatened with respect to any Plan (other than claims for benefits
in the ordinary course) and, to the knowledge of the Company, no fact or event exists that could reasonably be expected to give rise to
any such Action.
(g)
Each Plan that is intended to be qualified under Section 401(a) of the Code has (i) timely received a favorable determination
letter from the IRS covering all of the provisions applicable to the Plan for which determination letters are currently available that
the Plan is so qualified and each trust established in connection with such Plan is exempt from U.S. federal income Tax under Section 501(a)
of the Code or (ii) is entitled to rely on a favorable opinion or advisory letter from the IRS, and to the knowledge of Company,
no fact or event has occurred since the date of such determination or opinion letter or letters from the IRS that could reasonably be
expected to adversely affect the qualified status of any such Plan or the exempt status of any such trust.
(h)
There has not been any non-exempt prohibited transaction (within the meaning of Section 406 of ERISA or Section 4975
of the Code) nor any reportable events (within the meaning of Section 4043 of ERISA) with respect to any Plan that could reasonably
be expected to result in material liability to the Company or any of the Company Subsidiaries. There have been no acts or omissions by
the Company, any Company Subsidiary or any ERISA Affiliate that have given or could reasonably be expected to give rise to any material
fines, penalties, Taxes or related charges under Sections 502 or 4071 of ERISA or Section 511 or Chapter 43 of the Code
for which the Company, any Company Subsidiary or any ERISA Affiliate may be liable.
(i)
All contributions, premiums or payments required to be made with respect to any Plan have been made to the extent due on or before
their respective due dates or properly accrued on the consolidated financial statements of the Company and the Company Subsidiaries, except
as would not result in material liability to the Company and the Company Subsidiaries.
(j) Each
Plan that constitutes a nonqualified deferred compensation plan subject to Section 409A of the Code is in documentary
compliance with, and has been administered and operated in compliance with, the provisions of Section 409A of the Code and the
Treasury Regulations promulgated thereunder, and no additional Tax under Section 409A(a)(1)(B) of
the Code has been or could reasonably be expected to be incurred by a participant in any such Plan.
Section 4.11
Labor and Employment Matters.
(a)
Section 4.11(a) of the Company Disclosure Schedules sets forth, as of the date of this Agreement, a true, correct and complete
list of all employees of the Company and any Company Subsidiary, including any employee who is on a leave of absence of any nature, authorized
or unauthorized, and sets forth, as of the date of this Agreement, for each such individual the following, on a no name basis: (i) title
or position (including whether full or part time); (ii) hire date and service commencement date (if different); (iii) current
annualized base salary or (if paid on an hourly basis) hourly rate of pay; and (iv) commission, bonus or other incentive based compensation.
As of the date hereof, all compensation, including wages, commissions and bonuses, due and payable to all employees of the Company and
any Company Subsidiary for services performed on or prior to the date hereof have been paid in full (or accrued in full in the Company’s
financial statements).
(b)
There are no material Actions pending or, to the knowledge of the Company, threatened against the Company or any Company Subsidiary
by any of their respective current or former employees; (ii) neither the Company nor any Company Subsidiary is, nor has either the
Company or any Company Subsidiary been in the two (2) years prior to the date of this Agreement, a party to, bound by, or negotiating
any collective bargaining agreement or other contract with a union, works council or labor organization applicable to Persons employed
by the Company or any Company Subsidiary, nor, to the knowledge of the Company, are there any activities or proceedings of any labor union
to organize any such employees; (iii) there are no unfair labor practice complaints pending against the Company or any Company Subsidiary
before the National Labor Relations Board; and (iv) there has never been, nor, to the knowledge of the Company, has there been any
threat of, any strike, slowdown, work stoppage, lockout, concerted refusal to work overtime or other similar labor disruption or dispute
affecting, or, to the knowledge of the Company, threat thereof, by or with respect to any employees of the Company or any Company Subsidiary.
(c)
The Company and the Company Subsidiaries are and have been in the two (2) years prior to the date of this Agreement in compliance
in all material respects with all applicable Laws relating to the employment, employment practices, employment discrimination, terms and
conditions of employment, mass layoffs and plant closings (including the Worker Adjustment and Retraining Notification Act of 1988, as
amended, or any similar state or local Laws), immigration, meal and rest breaks, pay equity, workers’ compensation, family and medical
leave, and occupational safety and health requirements, payment of wages, hours of work, and collective bargaining as required by the
appropriate Governmental Authority and are not liable for any material arrears of wages, penalties or other sums for failure to comply
with any of the foregoing.
Section 4.12
Real Property; Title to Assets.
(a)
The Company does not own any real property.
(b)
Section 4.12(b) of the Company Disclosure Schedule lists, as of the date of this Agreement, the street address of each
parcel of Leased Real Property, and sets forth, as of the date of this Agreement, a list of each lease, sublease, license or occupancy
agreement pursuant to which the Company or any Company Subsidiary leases, subleases, licenses or occupies any real property (each, a “Lease”),
with the name of the lessor or any other party thereto, and the date of the Lease in connection therewith and each material amendment
to any of the foregoing (collectively, the “Lease Documents”). True, correct and complete copies of all Lease
Documents have prior to the date of this Agreement been made available to Parent in the Virtual Data Room. Except as otherwise set forth
in Section 4.12(b) of the Company Disclosure Schedule as of the date of this Agreement, (i) there are no leases, subleases,
sublicenses, concessions or other contracts granting to any Person other than the Company or Company Subsidiaries the right to use or
occupy any Leased Real Property, and (ii) all such Leases are in full force and effect, are valid and enforceable in accordance with
their respective terms, subject to the Remedies Exceptions, and there is not, under any of such Leases, any existing default or event
of default (or event which, with notice or lapse of time, or both, would constitute a default) by the Company or any Company Subsidiary
or, to the Company’s knowledge, by the other party to such Leases, except as would not, individually or in the aggregate, be material
to the Company and the Company Subsidiaries, taken as a whole. As of the date of this Agreement, neither the Company, nor any Company
Subsidiary, has subleased, sublicensed or otherwise granted to any Person any right to use, occupy or possess any portion of the Leased
Real Property.
(c)
Other than any actions taken due to a “shelter in place,” “non-essential employee” or similar direction
of any Governmental Authority, there are no contractual or legal restrictions that preclude or restrict the ability of the Company or
any Company Subsidiary to use any Leased Real Property by such party for the purposes for which it is currently being used, except as
would not, individually or in the aggregate, be material to the Company and the Company Subsidiaries, taken as a whole. There are no latent
defects or adverse physical conditions affecting the Leased Real Property, and improvements thereon, other than those that would not have
a Company Material Adverse Effect.
(d)
Each of the Company and the Company Subsidiaries has legal and valid title to, or, in the case of Leased Real Property and assets,
valid leasehold or subleasehold interests in, all of its properties and assets, tangible and intangible, real, personal and mixed, used
or held for use in its business, free and clear of all Liens other than Permitted Liens, except as would not, individually or in the aggregate,
be material to the Company and the Company Subsidiaries, taken as a whole.
Section 4.13
Intellectual Property.
(a) Section 4.13(a)
of the Company Disclosure Schedule, as updated, contains a true, correct and complete list, as of the date of this Agreement, of all
of the following that are (as applicable) owned or purported to be owned, used or held for use by the Company or the Company Subsidiaries: (i)
Registered Intellectual Property constituting Company Owned IP (showing in each, as applicable, the filing date, date of issuance, expiration
date and registration or application number, and registrar), (ii) all material, unregistered trademarks and brand names constituting
Company Owned IP, (iii) domain names and social media accounts used or held for use by the Company in the conduct of the business
and (iv) all material Contracts to use any Company Licensed IP (other than (x) Contracts
for Off-the-Shelf Software, (y) commercially available service agreements to Business Systems (other than Software), and (z) any
Intellectual Property licenses ancillary to the purchase or use of services, equipment, reagents or other materials incorporated into
the Products. The Company shall be permitted to provide an updated Section 4.13(a) of the Company Disclosure Schedule within
fifteen (15) Business Days after the date hereof.
(b)
Except as set forth in Section 4.13(b) of the Company Disclosure Schedule, the Company and the Company Subsidiaries own,
have valid and enforceable licenses for, or otherwise have adequate rights to use, all Intellectual Property and technology that are or
would reasonably be expected to be material to their business as currently conducted (including upon the commercialization of products
or services described in the Registration Statement, the Company Disclosure Schedule or the Prospectus as under development) or to the
development, manufacture, operation and sale of any products and services sold by the Company or any Company Subsidiary, and the consummation
of the Transactions will not conflict with, alter or impair any such rights. No Company IP, or, to the Company’s Knowledge, Company
Licensed IP, has been adjudged by a court of competent jurisdiction invalid or unenforceable in whole or in part. The Company IP constitutes
all Intellectual Property rights necessary for, or to the knowledge of the Company, otherwise used in, the operation of the business of
the Company and the Company Subsidiaries as currently conducted and is sufficient for the conduct of such business as currently conducted,
and the consummation of the transactions contemplated hereby will not conflict with, alter or impair any such rights.
(c)
Other than as set forth in Section 4.13(c) of the Company Disclosure Schedule, the Company or one of the Company Subsidiaries
(i) exclusively owns (beneficially and, with respect to Registered Intellectual Property, as record owner), free and clear of all Liens
(other than Permitted Liens), all right, title and interest in and to the Company Owned IP and, (ii) has the right to use, pursuant
to a valid and enforceable Contract, all Company Licensed IP. All Company Owned IP is subsisting and, to the knowledge of the Company,
valid and enforceable. No loss or expiration of any of the Company Owned IP is threatened in writing, or, to the Company’s knowledge,
pending, and, to the Company’s knowledge, no loss or expiration of exclusively in-licensed Company IP is threatened in writing or
pending. To the Company’s knowledge, the Company and the Company Subsidiaries have complied in all material respects with the terms
of each agreement pursuant to which Intellectual Property has been licensed to the Company or one of the Company Subsidiaries, and all
such agreements are in full force and effect.
(d)
The Company and each of its applicable Company Subsidiaries have taken and take reasonable actions to maintain, protect and enforce
the secrecy, confidentiality and value of its Trade Secrets and other material Confidential Information, including requiring all Persons
having access thereto to execute written non-disclosure agreements. Neither the Company nor any Company Subsidiary has disclosed any Trade
Secrets or other Confidential Information to any other Person other than pursuant to a written confidentiality agreement under which such
other Person agrees to maintain the confidentiality of and protect such Confidential Information. To the Company’s knowledge, no
Trade Secrets of the Company have been disclosed by the Company in a manner that has resulted or is likely to result in the loss of trade
secret or other rights in and to such information.
(e)
Other than as set forth in Section 4.13(e) of the Company Disclosure Schedules, (i) during the three (3) years prior to
the date of this Agreement there have been no claims filed or claims threatened in writing, against the Company or any Company Subsidiary,
by any Person (A) contesting the validity, use, ownership, enforceability, scope, patentability or registrability of any of the Company
IP, or (B) alleging any infringement or misappropriation of, or other violation of, any valid Intellectual Property rights of other
Persons (including any unsolicited written demands or written offers to license any Intellectual Property rights from any other Person);
(ii) the operation of the business of the Company and the Company Subsidiaries (including the Products) as currently conducted does
not infringe, misappropriate or violate, any Intellectual Property rights of other Persons; (iii) to the Company’s knowledge
and except as disclosed to the Parent in writing, no other Person has infringed, misappropriated or violated any of the Company IP, and
no such action, suit, proceeding or claim alleging such infringement, misappropriation or violation of Company IP has been filed or threatened
in writing by the Company or its Subsidiaries against any other Person; (iv) to the Company’s knowledge, there would be no threatened
action, suit, proceeding or claim by others that the Company or one of the Company Subsidiaries would, upon the commercialization of any
product or service described in the Registration Statement, the Company Disclosure Schedules or the Prospectus, infringe, misappropriate
or otherwise violate, any patent, trademark, tradename, service name, copyright, trade secret or other Intellectual Property or proprietary
right of another; and (v) neither the Company nor any of the Company Subsidiaries has received written notice of any of the foregoing
or received any formal written opinion of counsel regarding the foregoing, and the Company is unaware of any facts which could form a
reasonable basis for any such action, suit, proceeding or claim.
(f) To the
Company’s knowledge, there is no prior art or other information that may render any Patent within the Company Owned IP invalid
or unenforceable or that may render any Patent application within such Intellectual Property unpatentable that has not been
disclosed to the U.S. Patent and Trademark Office or any foreign equivalent thereto. To the Company’s knowledge, there are no
material defects in any of the Company Owned IP. The product candidates described in the Company Disclosure Schedules as under
development or commercialization by the Company or any Company Subsidiary fall within the scope of the claims of one or more Patent
or pending Patent application owned by, or exclusively licensed to, the Company or any Company Subsidiary.
(g)
Other than as set forth in Section 4.13(g) of the Company Disclosure Schedule, no funding, facilities or personnel of any
Governmental Authority were used, directly or indirectly, to develop or create, in whole or in part, any Company Owned IP.
(h) Other than as set
forth in Section 4.13(h) of the Company Disclosure Schedule, all Persons who have contributed, developed or conceived any
Company Owned IP have executed valid and enforceable written agreements with the Company or one of the Company Subsidiaries,
pursuant to which such Persons assigned or have an obligation to assign to the Company or the applicable Company Subsidiary all of
their entire right, title, and interest in and to any Intellectual Property created, conceived or otherwise developed by such Person
in the course of or related to his, her or its relationship with the Company or the applicable Company Subsidiary, without further
ongoing consideration or any restrictions or obligations whatsoever, including on the use or other disposition or ownership of such
Intellectual Property; or, with respect to Intellectual Property rights that cannot be assigned
(e.g., “moral rights” in certain jurisdictions), such Person has unconditionally and irrevocably waived the enforcement
thereof, and no such Person has excluded works or inventions from such assignment. To the Company’s knowledge, no
current or former employee, director or officer of the Company or one of the Company Subsidiaries or any consultant who has
contributed, developed or conceived of any Company Owned IP is or has been in violation of any term of any employment or consulting
contract, patent disclosure agreement, invention assignment agreement, non-competition agreement, non-solicitation agreement,
nondisclosure agreement or any restrictive covenant to or with a former employer where the basis of such violation relates to such
employee’s employment or other engagement with the Company or Company Subsidiary.
(i) No Related Party,
nor any current or former partner, director, stockholder, officer or employee of the Company or Company Subsidiaries or of any
Related Party will, after giving effect to the transactions contemplated hereby, own, license or retain any rights in any of the
Intellectual Property owned, used or held for use (including for defensive purposes) by the Company in the conduct of the business
as currently conducted.
(j) Section
4.13(j) of the Company Disclosure Schedule sets forth a list, as of the date of this Agreement, of all Open Source Software that
has been used in connection with any Products.
(k)
The Company and the Company Subsidiaries owns, leases, licenses, or otherwise has the legal right to use all Business Systems,
and such Business Systems are sufficient in all material respects for the current needs of the business of the Company or any of the Company
Subsidiaries as currently conducted by the Company or the Company Subsidiaries. The Company and each of the Company Subsidiaries maintain
commercially reasonable disaster recovery, business continuity and risk assessment plans, procedures and facilities. To the Company’s
knowledge, in the three (3) years prior to the date of this Agreement, there has not been any failure with respect to any of the Business
Systems that are material to the conduct of the Company’s and the Company Subsidiaries’ business that has not been remedied
or replaced in all material respects.
(l) The Company and each
of the Company Subsidiaries currently and during the three (3) years prior to the date of this Agreement have complied in all
material respects with (i) all Privacy/Data Security Laws applicable to the Company or a Company Subsidiary, (ii) any
applicable privacy or other policies of the Company or a Company Subsidiary, respectively, published on a Company website or
otherwise made publicly available by the Company or a Company Subsidiary concerning the collection, dissemination, storage, use or
other Processing of Personal Information or Business Data, (iii) industry standards to which the Company or any Company
Subsidiary is bound to adhere, and (iv) all Contracts that the Company or any Company Subsidiary has entered into or is
otherwise bound with respect to privacy or data security (collectively, the “Data Security Requirements”).
The Company and the Company Subsidiaries have each implemented data security safeguards that are designed to protect the security
and integrity of the Business Systems and any Personal Information and that are otherwise consistent with the Data Security
Requirements. The Company’s and the Company Subsidiaries’ employees and contractors receive commercially reasonable
training on information security issues. Section 4.13(l) of the Company Disclosure Schedule identifies any Contracts under
which Business Data or Personal Information of the Company or the Company Subsidiaries is hosted
or processed on the systems or networks of third parties, including cloud computing arrangements. To the Company’s knowledge
there is no Disabling Device in any of the Business Systems constituting Company Owned IP or Product components. For the two (2)
years prior to the date of this Agreement, neither the Company nor any of the Company Subsidiaries has (i) experienced any data
security breaches, unauthorized access or use of any of the Business Systems, or unauthorized acquisition, destruction, damage,
disclosure, loss, corruption, alteration, or use of any Personal Information or Business Data; or (ii) been subject to or
received written notice of any audits, proceedings or investigations by any Governmental Authority or any customer, or received any
material claims or complaints regarding the collection, dissemination, storage or use of Personal Information, or the violation of
any applicable Data Security Requirements. All processing, storing and transmitting of payment card data by or for the
Company and the Company Subsidiaries is compliant with PCI DSS.
(m) The Company or one
of the Company Subsidiaries (i) owns the Business Data constituting Company Owned IP free and clear of any restrictions other
than those imposed by applicable Privacy/Data Security Laws, or (ii) has the right, as applicable, to use, exploit, publish,
reproduce, distribute, license, sell, and create derivative works of and otherwise Process the other Business Data, in whole or in
part, in the manner in which the Company and the Company Subsidiaries receive and use such Business Data prior to the Closing Date.
The Company and the Company Subsidiaries are not subject to any material legal obligations, including based on the Transactions
contemplated hereunder, that would prohibit Merger Sub, or Parent from receiving, using or otherwise Processing Personal Information
after the Closing Date, in a similar manner in which the Company and the Company Subsidiaries receive, use and otherwise Process
such Personal Information immediately prior to the Closing Date or result in material liabilities in connection with Data Security
Requirements.
Section 4.14
Taxes.
(a)
The Company and each Company Subsidiary: (i) have duly and timely filed all material Tax Returns that they are required to
have filed as of the date hereof (taking into account any extension of time within which to file) and all such filed Tax Returns are complete
and accurate in all material respects; (ii) have paid all Taxes that are shown as due on such filed Tax Returns and any other material
Taxes that they are required to have paid as of the date hereof to avoid penalties or charges for late payment; (iii) with respect
to all material Tax Returns filed by them, have not waived any statute of limitations with respect to Taxes or agreed to any extension
of time with respect to a Tax assessment or deficiency (other than pursuant to customary extensions of the due date for filing a Tax Return
obtained in the ordinary course of business); and (iv) do not have any material deficiency, assessment, claim, audit, examination,
investigation, litigation or other proceeding in respect of Taxes (each, a “Tax Claim”) pending or asserted,
proposed or threatened in writing for a Tax period for which the statute of limitations for a Tax assessment remains open, other than
any Tax Claims that have since been resolved. The unpaid Taxes of the Company and the Company Subsidiaries as of the Interim Financial
Statements Date did not materially exceed the reserves for Taxes (other than any reserves for deferred Taxes established to reflect timing
differences between book and taxable income) of the Company and the Company Subsidiaries set forth in the Interim Financial Statements.
(b)
Neither the Company nor any Company Subsidiary is a party to, is bound by or has an obligation under any Tax sharing agreement,
Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement, in each case other than an agreement, contract
or arrangement the primary purpose of which does not relate to Taxes (each, an “Ordinary Commercial Agreement”).
(c)
Neither the Company nor any Company Subsidiary will be required to include any material item of income in, or exclude any material
item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) adjustment
under Section 481(c) of the Code (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) by reason
of any change in method of accounting made prior to the Closing; (ii) “closing agreement” as described
in Section 7121 of the Code (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) executed prior
to the Closing; (iii) installment sale or open transaction disposition made prior to the Closing; (iv) intercompany transaction
or any excess loss account described in the Treasury Regulations promulgated under Section 1502 of the Code (or any corresponding
or similar provision of state, local or non-U.S. income Tax Law) entered into or created, respectively, prior to the Closing; or (v) prepaid
amount received or deferred revenue booked prior to the Closing.
(d)
The Company and each Company Subsidiary have withheld and paid to the appropriate Governmental Authority all material Taxes required
to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor,
stockholder or other third party and, to the Company’s knowledge, have complied in all material respects with all applicable Laws
relating to the reporting and withholding of Taxes.
(e)
Neither the Company nor any Company Subsidiary has been a member of an affiliated group filing a consolidated, combined or unitary
income Tax Return (other than a group of which the Company or a Company Subsidiary was the common parent).
(f) Neither the Company
nor any Company Subsidiary has any material liability for the Taxes of any Person (other than the Company and the Company
Subsidiaries) under Treasury Regulation Section 1.1502-6 (or any corresponding or similar provision of state, local or non-U.S.
income Tax Law), as a transferee or successor, or, except pursuant to an Ordinary Commercial Agreement, by contract.
(g)
Neither the Company nor any Company Subsidiary has any request for a material “closing agreement” as
described in Section 7121 of the Code (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) or private
letter ruling from any Governmental Authority.
(h)
The Company has prior to the date of this Agreement made available to Parent complete and accurate copies of the U.S. federal
income Tax Returns filed by the Company for the Tax year ended December 31, 2020, and each of the four prior Tax years prior to such Tax
year.
(i) Neither the Company nor
any Company Subsidiary has in any year for which the applicable statute of limitations remains open distributed stock of another Person,
or has had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part
by Sections 355 or 361 of the Code.
(j) Neither the Company
nor any Company Subsidiary has engaged in or entered into a “listed transaction” within the meaning of
Treasury Regulation Section 1.6011-4(b)(2).
(k)
Neither the IRS nor any other Governmental Authority has asserted in writing against the Company or any Company Subsidiary any
deficiency or claim for any material Taxes or interest thereon or penalties in connection therewith.
(l) There are no Liens
for Taxes (other than Permitted Liens) upon any assets of the Company or any Company Subsidiary.
(m) Neither the Company
nor any Company Subsidiary has been a “United States real property holding corporation” within the meaning of
Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(n)
Neither the Company nor any Company Subsidiary has received any written notice from a non-U.S. Governmental Authority that
it has a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business
in a country other than the country in which it is organized.
(o)
Neither the Company nor any Company Subsidiary has received any written claim from a Governmental Authority in a jurisdiction in
which the Company or such Company Subsidiary does not file Tax Returns stating that the Company or such Company Subsidiary is or may be
subject to Tax in such jurisdiction.
(p)
For U.S. federal income Tax purposes, the Company is, and
has been since its formation, classified as a corporation.
(q)
The Company has not taken or agreed to take any action, and does not intend to or plan to take any action, or has any knowledge
of any fact or circumstance that could reasonably be expected to prevent the Mergers from qualifying for the Intended Tax Treatment.
Section 4.15 Environmental
Matters. Each of the Company and each Company Subsidiary is not materially violating, and for the
five (5) years prior to the date of this Agreement has not materially violated, any applicable Environmental Laws; (b) to the
knowledge of the Company, none of the properties currently or formerly owned, leased or operated by the Company or any Company
Subsidiary (including, without limitation, soils and surface and ground waters) are contaminated with, and no Company or Company
Subsidiary has Released, any Hazardous Substance which requires reporting, investigation, remediation, monitoring or other response
action by the Company or any Company Subsidiary pursuant to applicable Environmental Laws; (c) none of the Company or any of the
Company Subsidiaries is, in any material respect, actually or allegedly liable, or to the Company’s knowledge, potentially
liable, pursuant to applicable Environmental Laws for any off-site contamination by Hazardous Substances; (d) each of the
Company and each Company Subsidiary has all material permits, licenses and other authorizations required of the Company under
applicable Environmental Law (“Environmental Permits”), and the Company and each Company Subsidiary is,
and has since January 1, 2018 been, in compliance in all material respects with such Environmental Permits; and (e) neither the
Company nor any Company Subsidiary is the subject of any pending or, or to the Company’s knowledge, threatened Action, nor has
the Company or any Company Subsidiary received any written notice, alleging any material violation of or, or material liability
under, Environmental Laws.
Section 4.16
Material Contracts.
(a)
Section 4.16(a) of the Company Disclosure Schedule lists, as of the date of this Agreement, the following types of Contracts
to which the Company or any Company Subsidiary is a party, excluding for this purpose, any purchase orders submitted by customers (such
Contracts as are required to be set forth in Section 4.16(a) of the Company Disclosure Schedule, along with any Plan listed on
Section 4.10(a) of the Company Disclosure Schedule, being the “Material Contracts”):
(i) all Contracts with
consideration payable to the Company or any of the Company Subsidiaries of more than $150,000, in the aggregate, over any 12-month
period;
(ii) each Contract
requiring payment by or to the Company after the date of this Agreement in excess of $150,000 pursuant to its express terms relating
to (A) any agreement involving provision of services or products with respect to any pre-clinical development activities of the
Company or (B) any alliance, joint venture, cooperation, development or other agreement currently in force under which the Company
has continuing obligations to develop any product, technology or service, or any agreement pursuant to which the Company has
continuing obligations to develop any Intellectual Property rights that will not be owned, in whole or in part, by the Company;
(iii) all Contracts
pursuant to which the Company or any Company Subsidiary has engaged any third party to manage the business of the Company or any
Company Subsidiary (excluding contracts for employment), to the extent material to the business of the Company or any Company
Subsidiary;
(iv) all Contracts with
any Governmental Authority to which the Company or any Company Subsidiary is a party or which otherwise govern the use of any
Company Owned IP, other than any Company Permits;
(v)
all Contracts evidencing indebtedness for borrowed money in an amount greater than $150,000, and any pledge agreements, security
agreements or other collateral agreements in which the Company or any Company Subsidiary granted to any Person a Lien on any of the property
or assets of the Company or any Company Subsidiary, and all agreements or instruments guaranteeing the debts or other obligations of any
Person;
(vi) all Contracts
pursuant to which the Company or a Company Subsidiary has continuing obligations or interests involving (A) “milestone”
or other similar contingent payments, including upon the achievement of regulatory or commercial
milestones which would result in a payment in excess of $150,000 or (B) payment of royalties or other amounts calculated based upon
any revenues or income of the Company, in each case that cannot be terminated by the Company without penalty, or without more than
sixty (60) days’ notice without material payment or penalty;
(vii) all Contracts
establishing any partnership, joint venture, strategic alliance or other collaboration or similar arrangement between the Company or
any Company Subsidiary, on the one hand, and any third party, on the other hand (including with respect to the Products);
(viii)
any Contract relating to the acquisition or disposition of any business or asset (whether by merger, sale of stock, sale of assets
or otherwise) under which the Company or any of its Affiliates has or will have obligations with respect to an “earn out,”
contingent purchase price or similar contingent payment obligation;
(ix) all Contracts that
limit, or purport to limit, the ability of the Company or any Company Subsidiary to compete in any line of business or with any
Person or entity or in any geographic area or during any period of time excluding customary confidentiality clauses;
(x)
all Contracts that result in any Person or entity holding a power of attorney from the Company or any Company Subsidiary that materially
relates to the Company, any Company Subsidiary or materially impacts their respective business;
(xi) all Leases, and all
leases or master leases of personal property, reasonably likely to result in annual payments of $150,000 or more in a 12-month
period;
(xii) all Contracts
involving use of or granting licenses to the Company or any of the Company Subsidiaries with respect to any Company Licensed IP that
are material to the business of the Company;
(xiii)
all Contracts which involve the license or grant of rights to Company Owned IP by the Company or the Company Subsidiaries, other
than (A) collaboration agreements entered into on the form of such agreement made available in the Virtual Data Room or (B) and license
agreements granted in the ordinary course of business to customers in connection with Products or to suppliers or service providers in
the ordinary course of business solely for the purpose of enabling such suppliers or service providers to provide services for the benefit
of the Company or the Company Subsidiaries;
(xiv) all Contracts under
which the Company has agreed to purchase goods or services from a vendor, Supplier or other Person on a preferred supplier or
“most favored supplier” basis or which otherwise establishes any exclusive sale or distribution obligation
with respect to any Product or geographic area;
(xv) all Contracts for the
development of Company Owned IP for the benefit of the Company that are material to the Company, other than employment, consulting and
collaboration agreements entered into on the form of such agreement made available in the Virtual Data Room, without material
modification;
(xvi) all Contracts under
which any broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection
with the Transactions, or which has a fee tail still in effect, based upon arrangements made by or on behalf of the Company or any
Company Subsidiary;
(xvii) all
Contracts that provide for the settlement of any material Action that contains any ongoing material obligation on the Company or the
Company Subsidiaries; and
(xviii) all
Contracts between the Company and any holders of more than 2% of the Company’s Capital Stock (assuming the full conversion or
exercise of all Company Securities held by such Person) that relate to such stockholder’s ownership of Company Securities.
(b)
Except as has not been, and would not reasonably be expected to be, individually or in the aggregate, material to the Company and
the Company Subsidiaries, taken as a whole, as of the date of this Agreement (i) each Material Contract is a legal, valid and binding
obligation of the Company or the Company Subsidiaries and, to the knowledge of the Company, the other parties thereto, and neither the
Company nor any Company Subsidiary is in material breach or violation of, or material default under, any Material Contract nor has any
Material Contract been canceled by the other party; (ii) to the Company’s knowledge, no other party is in material breach or
violation of, or material default under, any Material Contract; and (iii) the Company and the Company Subsidiaries have not received
any written, or to the knowledge of the Company, oral claim of any material default under any such Material Contract. As of the date of
this Agreement, the Company has furnished or made available to Parent in the Virtual Data Room true and complete copies, in all respects,
of all Material Contracts, including amendments thereto that are material in nature.
Section 4.17
Insurance.
(a)
Section 4.17(a) of the Company Disclosure Schedule sets forth, with respect to each material insurance policy under which
the Company or any Company Subsidiary is an insured, a named insured or otherwise the principal beneficiary of coverage as of the date
of this Agreement (i) the names of the insurer, the principal insured and each named insured, (ii) the policy number, (iii) the
period, scope and amount of coverage and (iv) the premium most recently charged.
(b) With respect to each
such insurance policy, except as would not be expected to result in a Company Material Adverse Effect: (i) the policy is legal,
valid, binding and enforceable in accordance with its terms (subject to the Remedies Exceptions) and, except for policies that have
expired under their terms in the ordinary course, is in full force and effect; (ii) neither the Company nor any Company
Subsidiary is in material breach or default (including any such breach or default with respect to the payment of premiums or the
giving of notice), and no event has occurred which, with notice or the lapse of time, would constitute such a material breach or
default, or permit termination or modification, under the policy; and (iii) to the knowledge
of the Company, no insurer on the policy has been declared insolvent or placed in receivership, conservatorship or
liquidation.
Section 4.18
Vote Required. The Requisite Approval (the “Company Stockholder Approval”)
is the only vote of the holders of any class or series of capital stock or other securities of the Company necessary to adopt this Agreement
and approve the Transactions. The Written Consent, if executed and delivered, would qualify as the Company Stockholder Approval and no
additional approval or vote from any holders of any class or series of capital stock of the Company would then be necessary to adopt this
Agreement and approve the Transactions.
Section 4.19
Certain Business Practices.
(a)
For the three (3) years prior to the date of this Agreement, none of the Company, any Company Subsidiary, any of their respective
directors, officers, or employees or, to the Company’s knowledge, agents, while acting on behalf of the Company or any Company Subsidiary,
has: (i) used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to political activity;
(ii) made any unlawful payment to foreign or domestic government officials or employees or to foreign or domestic political parties
or campaigns or violated any provision of any applicable Anti-Corruption Law; or (iii) to the extent not covered by subclause (i)
and (ii), made any payment in the nature of criminal bribery.
(b)
For the three (3) years prior to the date of this Agreement, none of the Company, any Company Subsidiary, any of their respective
directors, officers, or employees or, to the Company’s knowledge, agents (i) is or has been a Sanctioned Person; (ii) has
transacted business with or for the benefit of any Sanctioned Person or has otherwise violated applicable Sanctions, while acting on behalf
of the Company or any Company Subsidiary; or (iii) has violated any Ex-Im Laws while acting on behalf of the Company or any Company
Subsidiary.
(c)
There are no, and for the three (3) years prior to the date of this Agreement, there have not been any, material internal investigations,
external investigations to which the Company has knowledge of, audits, actions or proceedings pending, or any voluntary or involuntary
disclosures made to a Governmental Authority, with respect to any apparent or suspected violation by the Company, any Company Subsidiary,
or any of their respective officers, directors, employees, or agents with respect to any Anti-Corruption Laws, Sanctions, or Ex-Im Laws.
Section 4.20 Interested
Party Transactions. Except for employment relationships and the payment of compensation, benefits
and expense reimbursements and advances in the ordinary course of business, no director, officer or other Affiliate of the Company
or any Company Subsidiary, or any immediate family of any of the foregoing, to the Company’s knowledge, has or has had,
directly or indirectly as of the date of this Agreement: (a) an economic interest in any Person that has furnished or sold, or
furnishes or sells, services or Products that the Company or any Company Subsidiary furnishes or sells, or proposes to furnish or
sell; (b) an economic interest in any Person that purchases from or sells or furnishes to, the Company or any Company
Subsidiary, any goods or services; (c) a beneficial interest in any Contract disclosed in Section 4.16(a) of the Company
Disclosure Schedule; or (d) any Contract with the Company or any Company Subsidiary, other than customary indemnity
arrangements; provided, however, that ownership of no more than five percent (5%) of the outstanding voting
stock of a publicly traded corporation shall not be deemed an “economic interest in any Person” for purposes of this Section
4.20. The Company and the Company Subsidiaries have not, for the two (2) years prior to the date of this Agreement,
(i) extended or maintained credit, arranged for the extension of credit or renewed an extension of credit in the form of a
personal loan to or for any director or executive officer (or equivalent thereof) of the Company, or (ii) materially modified
any term of any such extension or maintenance of credit.
Section 4.21
Brokers. Except as set forth on Section 4.21 of the Company Disclosure Schedule, no
broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions
based upon arrangements made by or on behalf of the Company or any Company Subsidiary.
Section 4.22
FDA. As to each product subject to the jurisdiction of the U.S. Food and Drug Administration
(“FDA”) under the Federal Food, Drug and Cosmetic Act, as amended, and the regulations thereunder (“FDCA”)
that is manufactured, packaged, labeled, tested, distributed, sold, and/or marketed by the Company or any Company Subsidiary (each such
product, a “Pharmaceutical Product”), such Pharmaceutical Product is being manufactured, packaged, labeled,
tested, distributed, sold and/or marketed by the Company in compliance with all applicable requirements under FDCA and similar laws, rules
and regulations relating to registration, investigational use, premarket clearance, licensure, or application approval, good manufacturing
practices, good laboratory practices, good clinical practices, product listing, quotas, labeling, advertising, record keeping and filing
of reports, except where the failure to be in compliance would not have a Material Adverse Effect. There is no pending, completed or,
to the Company's knowledge, threatened, action (including any lawsuit, arbitration, or legal or administrative or regulatory proceeding,
charge, complaint, or investigation) against the Company or any Company Subsidiary, and none of the Company or any Company Subsidiary
has received any notice, warning letter or other communication from the FDA or any other governmental entity, which (i) contests the premarket
clearance, licensure, registration, or approval of, the uses of, the distribution of, the manufacturing or packaging of, the testing of,
the sale of, or the labeling and promotion of any Pharmaceutical Product, (ii) withdraws its approval of, requests the recall, suspension,
or seizure of, or withdraws or orders the withdrawal of advertising or sales promotional materials relating to, any Pharmaceutical Product,
(iii) imposes a clinical hold on any clinical investigation by the Company or any of its Subsidiaries, (iv) enjoins production at any
facility of the Company or any Company Subsidiary, (v) enters or proposes to enter into a consent decree of permanent injunction with
the Company or any Company Subsidiary, or (vi) otherwise alleges any violation of any laws, rules or regulations by the Company or any
Company Subsidiary, and which, either individually or in the aggregate, would have a Material Adverse Effect. The properties, business
and operations of the Company have been and are being conducted in all material respects in accordance with all applicable laws, rules
and regulations of the FDA. The Company has not been informed by the FDA that the FDA will prohibit the marketing, sale, license or use
in the United States of any product proposed to be developed, produced or marketed by the Company nor has the FDA expressed any concern
as to approving or clearing for marketing any product being developed or proposed to be developed by the Company.
Section 4.23 Exclusivity
of Representations and Warranties. Except as otherwise expressly provided in this ARTICLE
IV (as modified by the Company Disclosure Schedule) or in the Company Officer’s Certificate, the Company hereby
expressly disclaims and negates any other express or implied representation or warranty whatsoever (whether at Law or in equity)
with respect to the Company, its Affiliates, and any matter relating to any of them, including their affairs, the condition, value
or quality of the assets, liabilities, financial condition or results of operations, or with respect to the accuracy or completeness
of any other information made available to the Parent Parties, their respective Affiliates or any of their respective
Representatives by, or on behalf of, the Company, and any such representations or warranties are expressly disclaimed. Without
limiting the generality of the foregoing, except as expressly set forth in this Agreement (as modified by the Company Disclosure
Schedule) or in the Company Officer’s Certificate, neither the Company nor any other Person on behalf of the Company has made
or makes, any representation or warranty, whether express or implied, with respect to any projections, forecasts, estimates or
budgets made available to the Parent Parties, their respective Affiliates or any of their respective Representatives of future
revenues, future results of operations (or any component thereof), future cash flows or future financial condition (or any component
thereof) of the Company (including the reasonableness of the assumptions underlying any of the foregoing), whether or not included
in any management presentation or in any other information made available to the Parent Parties, their respective Affiliates or any
of their respective Representatives or any other Person, and any such representations or warranties are expressly disclaimed.
ARTICLE
V
REPRESENTATIONS
AND WARRANTIES OF Parent Parties
Except as set forth in Parent’s
disclosure schedule delivered by Parent to the Company on the date of this Agreement (the “Parent Disclosure Schedule”)
and in Parent SEC Reports (to the extent the qualifying nature of such disclosure is readily apparent from the content of such Parent
SEC Reports, but excluding disclosures referred to in “Forward-Looking Statements,” “Risk Factors” and any other
disclosures therein to the extent they are of a predictive or cautionary nature or related to forward-looking statements), the Parent
Parties hereby represent and warrant to the Company as follows:
Section 5.01
Corporate Organization.
(a)
Each Parent Party is a company duly organized, validly existing and in good standing under the Laws of the jurisdiction of its
incorporation or organization and has the requisite corporate or limited liability power and authority and all necessary governmental
approvals to own, lease and operate its properties and to carry on its business as it is now being conducted, except where the failure
to have such power, authority and governmental approvals would not result in a Parent Material Adverse Effect.
(b)
Pubco is the only Subsidiary of Parent and the Merger Subs are the only Subsidiaries of Pubco. Except for Pubco and the Merger
Subs, Parent does not directly or indirectly own any equity or similar interest in, or any interest convertible into or exchangeable or
exercisable for any equity or similar interest in, any corporation, partnership, joint venture, business association or other Person.
Section 5.02 Governing
Documents. Each of the Parent Parties has heretofore furnished to the Company complete and correct
copies of the Parent Organizational Documents, Pubco Organizational Documents, the Parent Merger Sub Organizational Documents and the
Company Merger Sub Organizational Documents. The Parent Organizational Documents, Pubco Organizational Documents, the Parent Merger Sub
Organizational Documents and the Company Merger Sub Organizational Documents are in full force and effect. No Parent Party is in violation
of any of the provisions of its respective organizational documents.
Section 5.03
Capitalization.
(a)
The authorized capital stock of Parent consists of (i) 100,000,000 shares of Parent Common Stock, par value $0.0001 per share,
and (ii) 1,000,000 shares of Parent Preferred Stock, par value $0.0001 per share. As of the date of this Agreement, (A) 7,907,013 shares
of Parent Common Stock are issued and outstanding (which includes 893,712 shares subject to Redemption Rights), (B) no shares of Parent
Preferred Stock are issued and outstanding, (C) no shares of Parent Common Stock are held in the treasury of Parent, (D) 11,500,000 redeemable
warrants to purchase Parent Common Stock and 5,425,000 private placement warrants to purchase Parent Common Stock are issued and outstanding
and (E) 11,500,000 Parent Rights are issued and outstanding. Each Parent Warrant is exercisable for the number of shares of Parent Common
Stock stated in each Parent Warrant at an exercise price of $11.50 per share.
(b)
All outstanding shares of Parent Common Stock, Parent Warrants and Parent Rights (i) are duly authorized, validly issued, fully
paid and nonassessable, (ii) are not subject to any preemptive rights, (iii) have been issued and granted in compliance with all applicable
securities Laws and other applicable Laws and (iv) were issued free and clear of all Liens other than transfer restrictions under applicable
securities Laws and the Parent Organizational Documents.
(c)
Other than the Parent Warrants, there are no options, warrants, preemptive rights, calls, convertible securities, conversion rights
or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued capital stock of Parent or
obligating Parent to issue or sell any shares of capital stock of, or other equity interests in, Parent. Parent is not a party to, or
otherwise bound by, and has not granted, any equity appreciation rights, participations, phantom equity or similar rights. There are no
voting trusts, voting agreements, proxies, shareholder agreements or other agreements with respect to the voting or transfer of Parent
Common Stock or any of the equity interests or other securities of Parent. Except for Pubco and the Merger Subs, Parent does not own any
equity interests in any Person.
(d)
Other than Redemption Rights, there are no outstanding contractual obligations of Parent to repurchase, redeem or otherwise acquire
any Parent Common Stock or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any
Persons.
Section 5.04 Authority
Relative to this Agreement. Each Parent Party has all necessary corporate power and authority to
execute and deliver this Agreement and each Ancillary Agreement to which they are a party, to perform its obligations hereunder and
thereunder and to consummate the Transactions, in each case subject to obtainment of the Parent Stockholder Approval or the
approval of Pubco as the sole stockholder of each of the Merger Subs, as applicable. The execution and delivery of this Agreement by
each Parent Party and the consummation by each Parent Party of the Transactions have been, and each Ancillary Agreement to which
they are a party will be, duly and validly authorized by all necessary corporate or limited liability company action, as applicable,
and no other corporate or limited liability company proceedings on the part of any Parent Party is necessary to authorize this
Agreement and each Ancillary Agreement to which it is a party or to consummate the Transactions (other than (a) with respect to
the Mergers, (i) the Parent Stockholder Approval, the approval by Parent, as the sole stockholder of Pubco Merger Sub, and the
approval of Pubco as the sole stockholder of each of the Merger Subs, and (ii) the filing and recordation of appropriate merger
documents as required by the DGCL and the Cayman Act, as applicable, and (b) with respect to the issuance of Pubco Ordinary
Shares and the amendment and restatement of the Pubco Memorandum and Articles pursuant to this Agreement, the Parent Stockholder
Approval). This Agreement has been duly and validly executed and delivered by each of the Parent Parties and, assuming due
authorization, execution and delivery by the Company, constitutes a legal, valid and binding obligation of each Parent Party
enforceable against it, in accordance with its terms subject to the Remedies Exceptions.
Section 5.05
No Conflict; Required Filings and Consents.
(a)
The execution and delivery of this Agreement by each Parent Party does not, and the performance of this Agreement by each Parent
Party will not, (i) conflict with or violate such Parent Party’s organizational documents, (ii) assuming that all consents,
approvals, authorizations, expiration or termination of waiting periods and other actions described in Section 5.05(b) have
been obtained and all filings and obligations described in Section 5.05(b) have been made, conflict with or violate any Law applicable
to such Parent Party or by which any of its property or assets is bound or affected, or (iii) result in any breach of, or constitute
a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination,
amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of such Parent Party pursuant
to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which
such Parent Party is a party or by which such Parent Party or any of its property or assets is bound or affected, except, with respect
to clauses (ii) and (iii), for any such conflicts, violations, breaches, defaults or other occurrences which would not have or reasonably
be expected to have a Parent Material Adverse Effect.
(b)
The execution and delivery of this Agreement by each Parent Party does not, and the performance of this Agreement by each Parent
Party will not, require any consent, approval, authorization or permit of, or filing with or notification to, or expiration or termination
of any waiting period by, any Governmental Authority, except (i) for applicable requirements, if any, of the Exchange Act, the Securities
Act, Blue Sky Laws and state takeover Laws, the pre-merger notification requirements of the HSR Act, and filing and recordation of appropriate
merger documents as required by the DGCL and Cayman Act, as applicable, and (ii) where the failure to obtain such consents, approvals,
authorizations or permits, or to make such filings or notifications, would not, individually or in the aggregate, prevent or materially
delay consummation of any of the Transactions or otherwise prevent any Parent Party from performing its material obligations under this
Agreement.
Section 5.06 Compliance.
No Parent Party is or has been in conflict with, or in default, breach or violation of, (a) any Law applicable to it, by which any
property or asset of such Parent Party is bound or affected, or (b) any note, bond, mortgage, indenture, contract, agreement, lease,
license, permit, franchise or other instrument or obligation to which such Parent Party is a party or by which such Parent Party or any
property or asset of Parent Party is bound, except, in each case, for any such conflicts, defaults, breaches or violations that would
not have or reasonably be expected to have a Parent Material Adverse Effect. Each Parent Party is in possession of all material franchises,
grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental
Authority necessary for it to own, lease and operate its properties or to carry on its business as it is now being conducted. This Section
5.06 shall not apply to Tax matters.
Section 5.07
SEC Filings; Financial Statements; Sarbanes-Oxley.
(a)
Except as set forth on Section 5.7(a) of the Parent Disclosure Schedule, Parent has filed all forms, reports, schedules,
statements and other documents, including any exhibits thereto, required to be filed by it with the Securities and Exchange Commission
(the “SEC”) since November 23, 2020, together with any amendments, restatements or supplements thereto (collectively,
the “Parent SEC Reports”). Parent has heretofore furnished to the Company true and correct copies of all amendments
and modifications that have not been filed by Parent with the SEC to all agreements, documents and other instruments that previously had
been filed by Parent with the SEC and are currently in effect. As of their respective dates, the Parent SEC Reports (i) complied with
the applicable requirements of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act, and the rules and regulations promulgated
thereunder, and (ii) did not, at the time they were filed, or, if amended, as of the date of such amendment, contain any untrue statement
of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements made therein,
in the light of the circumstances under which they were made, not misleading. Each director and executive officer of Parent has filed
with the SEC on a timely basis all documents required with respect to Parent by Section 16(a) of the Exchange Act and the rules and regulations
thereunder.
(b)
Except as set forth on Section 5.7(b) of the Parent Disclosure Schedule, each of the financial statements (including, in
each case, any notes thereto) contained in the Parent SEC Reports was prepared in accordance with GAAP (applied on a consistent basis)
and Regulation S-X and Regulation S-K, as applicable, throughout the periods indicated (except as may be indicated in the notes thereto
or, in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC) and each fairly presents, in all material respects,
the financial position, results of operations, changes in stockholders equity and cash flows of Parent as at the respective dates thereof
and for the respective periods indicated therein, (subject, in the case of unaudited statements, to normal and recurring year-end adjustments).
Parent has no off-balance sheet arrangements that are not disclosed in the Parent SEC Reports. No financial statements other than those
of Parent are required by GAAP to be included in the consolidated financial statements of Parent.
(c) Except as and to the
extent set forth in the Parent SEC Reports, Parent has no liability or obligation of a nature (whether accrued, absolute, contingent
or otherwise) required to be reflected on a balance sheet prepared in accordance with GAAP,
except for liabilities and obligations arising in the ordinary course of Parent’s business.
(d)
Parent is in compliance with the applicable listing and corporate governance rules and regulations of Nasdaq Capital Market.
(e)
Parent has established and maintains disclosure controls and procedures (as defined in Rule 13a-15 under the Exchange Act). Such
disclosure controls and procedures are designed to ensure that material information relating to Parent and other material information
required to be disclosed by Parent in the reports and other documents that it files or furnishes under the Exchange Act is recorded, processed,
summarized and reported within the time periods specified in the rules and forms of the SEC, and that all such material information is
accumulated and communicated to Parent’s principal executive officer and its principal financial officer as appropriate to allow
timely decisions regarding required disclosure and to make the certifications required pursuant to Sections 302 and 906 of the Sarbanes-Oxley
Act. Such disclosure controls and procedures are effective in timely alerting Parent’s principal executive officer and principal
financial officer to material information required to be included in Parent’s periodic reports required under the Exchange Act.
(f) Parent maintains
systems of internal control over financial reporting that are sufficient to provide reasonable assurance regarding the reliability
of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including policies
and procedures sufficient to provide reasonable assurance: (i) that Parent maintains records that in reasonable detail accurately
and fairly reflect, in all material respects, its transactions and dispositions of assets; (ii) that transactions are recorded as
necessary to permit the preparation of financial statements in conformity with GAAP; (iii) that receipts and expenditures are being
made only in accordance with authorizations of management and its board of directors; and (iv) regarding prevention or timely
detection of unauthorized acquisition, use or disposition of its assets that could have a material effect on its financial
statements. Parent has delivered to the Company a true and complete copy of any disclosure (or, if unwritten, a summary thereof) by
any representative of Parent to Parent’s independent auditors relating to any material weaknesses in internal controls and any
significant deficiencies in the design or operation of internal controls that would adversely affect the ability of Parent to
record, process, summarize and report financial data. Parent has no knowledge of any fraud or whistle-blower allegations, whether or
not material, that involves management or other employees or consultants who have or had a significant role in the internal control
over financial reporting of Parent. Since December 31, 2019, there have been no material changes in Parent internal control over
financial reporting.
(g)
There are no outstanding loans or other extensions of credit made by Parent to any executive officer (as defined in Rule 3b-7 under
the Exchange Act) or director of Parent. Parent has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
(h) Neither Parent
(including any employee thereof) nor Parent’s independent auditors has identified or been made aware of (i) any significant
deficiency or material weakness in the system of internal accounting controls utilized by Parent, (ii) any fraud, whether or not
material, that involves Parent’s management or other employees who have a role in the preparation
of financial statements or the internal accounting controls utilized by Parent or (iii) any claim or allegation regarding any of the
foregoing.
(i) As of the date
hereof, there are no outstanding SEC comments from the SEC with respect to the Parent SEC Reports. To the knowledge of Parent, none
of the Parent SEC Reports filed on or prior to the date hereof is subject to ongoing SEC review or investigation as of the date
hereof.
Section 5.08
Absence of Certain Changes or Events. Since December 31, 2021, except as expressly contemplated
by this Agreement, (a) Parent has conducted its business in all material respects in the ordinary course and in a manner consistent
with past practice, other than due to any actions taken due to a “shelter in place,” “non-essential
employee” or similar direction of any Governmental Authority, (b) there has not been any Parent Material Adverse Effect,
and (c) Parent has not taken any action that, if taken after the date of this Agreement, would constitute a material breach of any of
the covenants set forth in Section 6.02(b).
Section 5.09
Absence of Litigation. There is no Action pending or, to the knowledge of Parent, threatened
against Parent, or any property or asset of Parent, before any Governmental Authority. Neither Parent nor any material property or asset
of Parent is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to
the knowledge of Parent, continuing investigation by, any Governmental Authority, or any order, writ, judgment, injunction, decree, determination
or award of any Governmental Authority. This Section 5.09 shall not apply to Tax matters.
Section 5.10
Board Approval; Vote Required.
(a)
The Parent Board, by resolutions duly adopted by unanimous vote of the members of the Parent Board at a meeting duly called and
held and not subsequently rescinded or modified in any way, has duly (i) determined that this Agreement, the Ancillary Agreements
to which Parent is a party, the Mergers and the other Transactions are fair to, and in the best interests of, Parent and its stockholders,
and declared their advisability, (ii) approved (A) this Agreement, the Ancillary Agreements, the Mergers and the other Transactions
to which Parent is a party, (B) the payment of the Aggregate Company Merger Consideration to the Participating Securityholders pursuant
to this Agreement, (C) the issuance of Pubco Ordinary Shares in connection with the Mergers, (D) the amendment and restatement of the
Pubco Memorandum and Articles, and (E) the Pubco LTIP, and (iii) adopted a resolution recommending that the stockholders of Parent
vote in favor of all Parent Proposals, including, without limitation, adoption of this Agreement and approval of the Mergers and the other
Transactions to which Parent is a party, and directing that this Agreement, the Mergers and the other Transactions to which Parent is
a party be submitted for consideration by the stockholders of Parent at the Parent Stockholders’ Meeting.
(b)
The only vote of the holders of any class or series of capital stock of Parent necessary to approve the Transactions is the affirmative
vote of the holders of a majority of the outstanding shares of Parent Common Stock.
(c)
Each of the Pubco Board, the Parent Merger Sub Board and the Company Merger Sub Board, by resolutions duly adopted by unanimous
written consent and not subsequently rescinded or modified in any way, has duly (i) determined that this Agreement, the Ancillary
Agreements to which such Parent Party is a party, the Parent Merger and/or the Company Merger, as applicable, and the other Transactions
to which such Parent Party is a party are fair to and in the best interests of such Parent Party and its sole stockholder, and declared
their advisability, (ii) adopted this Agreement and approved the Parent Merger and/or the Company Merger, as applicable, and the
other Transactions to which such Parent Party is a party, and (iii) recommended that Parent as the sole stockholder of Pubco, and
Pubco as the sole stockholder of each of the Merger Subs, as applicable, adopt this Agreement and approve the Parent Merger and/or the
Company Merger, as applicable, and the other Transactions to which such Parent Party is a party and directed that this Agreement, the
Parent Merger and/or the Company Merger, as applicable, and the other Transactions to which such Parent Party is a party be submitted
for consideration by Parent as the sole stockholder of Pubco or by Pubco as the sole stockholder of each of the Merger Subs, as applicable.
(d)
The only vote of the holders of any class or series of capital stock of the Merger Subs that is necessary to approve this Agreement,
the Mergers and the other Transactions is the affirmative vote of Pubco as the sole stockholder of the Merger Subs. The only vote of the
holders of any class or series of capital stock of Pubco that is necessary to approve this Agreement, the Mergers and the other Transactions
is the affirmative vote of Parent as the sole stockholder of Pubco.
Section 5.11
No Prior Operations of Pubco and the Merger Subs. Each of Pubco, Parent Merger Sub and Company
Merger Sub was formed solely for the purpose of engaging in the Transactions and has not engaged in any business activities or conducted
any operations or incurred any obligation or liability, other than as contemplated by this Agreement.
Section 5.12
Brokers. Except as set forth on Section 5.12 of the Parent Disclosure Schedule, no
broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions
based upon arrangements made by or on behalf of any Parent Party.
Section 5.13 Parent
Trust Fund. As of the date of this Agreement, Parent has no less than $17,700,000 held in the
trust fund established by Parent for the benefit of its public stockholders (the “Trust Fund”) maintained
in a trust account (the “Trust Account”). The monies of such Trust Account are invested in cash and held
in trust by Continental Stock Transfer & Trust Company (the “Trustee”) pursuant to the Investment
Management Trust Agreement, dated as of November 23, 2020, between Parent and the Trustee (the “Trust
Agreement”). Except as set forth in Section 5.13 of the Parent Disclosure Schedule or in connection with the
Extension Proposal, the Trust Agreement has not been amended or modified and is valid and in full force and effect and is
enforceable in accordance with its terms, subject to the Remedies Exceptions. Parent has complied in all material respects with the
terms of the Trust Agreement and is not in breach thereof or default thereunder and there does not exist under the Trust Agreement
any event which, with the giving of notice or the lapse of time, would constitute such a breach or default by Parent or, to the
knowledge of Parent, the Trustee. There are no separate contracts, agreements, side letters or other understandings (whether written
or unwritten, express or implied): (i) between Parent and the Trustee that would cause the description of the Trust
Agreement in the Parent SEC Reports to be inaccurate in any material respect; or (ii) to the knowledge of Parent, that would
entitle any Person (other than stockholders of Parent who shall have elected to exercise their Redemption Rights pursuant to the
Parent Organizational Documents) to any portion of the proceeds in the Trust Account. Prior to the Closing, none of the funds held
in the Trust Account have been released except: (A) to pay income and franchise Taxes from any interest income earned in the
Trust Account; and (B) upon the exercise of Redemption Rights in accordance with the provisions of the Parent Organizational
Documents. As of the date hereof, there are no Actions pending or, to the knowledge of Parent, threatened in writing with respect to
the Trust Account. As of the date hereof, assuming the accuracy of the representations and warranties of the Company herein and the
compliance by the Company with its obligations hereunder, Parent has no reason to believe that any of the conditions to the use of
funds in the Trust Account will not be satisfied or funds available in the Trust Account will not be available to Parent at the
Effective Time.
Section 5.14
Employees. Other than any officers of Parent as described in the Parent SEC Reports, Parent
has never employed any employees. Other than consultants and advisors retained in the ordinary course of business (including in connection
with the Transactions) or as described in the Parent SEC Reports, Parent has never retained any contractors. Other than reimbursement
of any out-of-pocket expenses incurred by Parent’s officers and directors in connection with activities on Parent’s behalf
in an aggregate amount not in excess of the amount of cash held by Parent outside of the Trust Account, Parent has no unsatisfied material
liability with respect to any employee, officer or director. Parent has never and does not currently maintain, sponsor, contribute to
or have any direct liability under any employee benefit plan (as defined in Section 3(3) of the Employee Retirement Income Security Act
of 1974, as amended), nonqualified deferred compensation plan subject to Section 409A of the Code, bonus, stock option, stock purchase,
restricted stock, incentive, deferred compensation, retiree medical or life insurance, supplemental retirement, severance, change in control,
fringe benefit, sick pay and vacation plans or arrangements or other employee benefit plans, programs or arrangements. Except as set forth
in Section 5.14 of the Parent Disclosure Schedule, neither the execution and delivery of this Agreement nor the other Ancillary
Agreements nor the consummation of the Transactions will (i) result in any payment becoming due to any director, officer or employee
of Parent, (ii) result in the acceleration of the time of payment or vesting of any such benefits, or (iii) give rise to any “excess
parachute payment” within the meaning of Section 280G of the Code. There is no contract, agreement, plan or arrangement to which
Parent is a party which requires payment by any party of a Tax gross-up or Tax reimbursement payment to any Person.
Section 5.15
Taxes.
(a) Parent (i) has duly
and timely filed (taking into account any extension of time within which to file) all material Tax Returns required to be filed by
it as of the date hereof and all such filed Tax Returns are complete and accurate in all material respects; (ii) has timely paid all
Taxes that are shown as due on such filed Tax Returns and any other material Taxes that Parent is otherwise obligated to pay, except
with respect to current Taxes that are not yet due and payable or are otherwise being contested in good faith; (iii) with respect to
all material Tax Returns filed by or with respect to it, has not waived any statute of limitations with respect to Taxes or agreed
to any extension of time with respect to a Tax assessment or deficiency which assessment or deficiency has not yet been resolved;
and (iv) does not have any deficiency, audit, examination, investigation or other proceeding in
respect of a material amount of Taxes or material Tax matters pending or threatened in writing, for a Tax period which the statute
of limitations for assessments remains open.
(b)
Parent is not party to, bound by or has an obligation under any Tax sharing agreement, Tax indemnification agreement, Tax allocation
agreement or similar contract or arrangement (including any agreement, contract or arrangement providing for the sharing or ceding of
credits or losses) or has a potential liability or obligation to any Person as a result of or pursuant to any such agreement, contract,
arrangement or commitment other than an agreement, contract, arrangement or commitment the primary purpose of which does not relate to
Taxes.
(c)
To the knowledge of Parent, as of the date hereof, Parent is not required to include any material item of income in, or exclude
any material item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result
of any: (i) change in method of accounting for a taxable period ending on or prior to the Closing Date under Section 481(c) of the Code
(or any corresponding or similar provision of state, local or foreign income Tax law); (ii) “closing agreement” as described
in Section 7121 of the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or prior
to the Closing Date; or (iii) installment sale made on or prior to the Closing Date; (iv) prepaid amount received or deferred revenue
accrued on or prior to the Closing Date; (v) intercompany transaction or excess loss account described in Treasury Regulations under Section
1502 of the Code (or any corresponding or similar provision of state, local or non-United States income Tax law) in existence on or prior
to the Closing Date; (vi) any use of an improper method of accounting use for any tax period or portion thereof ending or ended on or
prior to the Closing Date; or (vii) income arising or accruing prior to the Closing and includable after the Closing under Subchapter
K, Section 951, 951A or 956 of the Code.
(d)
Parent has withheld and paid to the appropriate Tax authority all material Taxes required to have been withheld and paid in connection
with amounts paid or owing to any current or former employee, independent contractor, creditor, shareholder or other third party and has
complied in all material respects with all applicable laws, rules and regulations relating to the payment and withholding of Taxes, including
all reporting and record keeping requirements related thereto.
(e)
Parent has not been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or foreign
income Tax Return.
(f) Parent does not have
any material liability for the Taxes of any Person under Treasury Regulation Section 1.1502-6 (or any similar provision of state,
local or foreign law), as a transferee or successor, by contract, or otherwise.
(g)
Parent does not have any request for a material ruling in respect of Taxes pending between Parent, on the one hand, and any Tax
authority, on the other hand.
(h)
Parent has made available to the Company true, correct and complete copies of the U.S. federal income Tax Returns filed by Parent
for the 2020 tax year.
(i) Parent has not since
incorporation distributed stock of another Person, or had its stock distributed by another Person, in a transaction that was
purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(j) Parent has not
engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2).
(k)
There are no Tax liens upon any assets of Parent except for Permitted Liens.
(l) Parent (A) is not
and has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the
applicable period specified in Code Section 897(c)(2) or (B) has not received written notice from a jurisdiction where it does not
file Tax Returns that it is subject to Tax in that jurisdiction. Parent has not made an election under Section 965(h) of the
Code.
(m) Parent has not taken
or agreed to take any action and does not intend to or plan to take any action, or has any knowledge of any fact or circumstance
that could reasonably be expected to prevent the Mergers from qualifying for the Intended Tax Treatment.
(n)
Notwithstanding anything in this Agreement to the contrary, the representations and warranties set forth in this Section 5.15
shall constitute the only representations and warranties by the Parent with respect to Taxes.
Section 5.16
Registration and Listing. The issued and outstanding shares of Parent Common Stock are registered
pursuant to Section 12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “BREZ”.
The issued and outstanding Parent Rights are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on the
Nasdaq Capital Market under the symbol “BREZR”. The issued and outstanding Parent Warrants are registered pursuant to Section
12(b) of the Exchange Act and are listed for trading on the Nasdaq Capital Market under the symbol “BREZW”. As of the date
of this Agreement, there is no Action pending or, to the knowledge of Parent, threatened in writing against Parent by the Nasdaq Capital
Market or the SEC with respect to any intention by such entity to deregister the shares of Parent Common Stock, Parent Warrants or Parent
Rights or terminate the listing of Parent on the Nasdaq Capital Market. None of Parent or any of its Affiliates has taken any action in
an attempt to terminate the registration of the shares of Parent Common Stock, the Parent Warrants or the Parent Rights under the Exchange
Act.
Section 5.17
Prior Business Operations. Parent has limited its activities in all material respects to those
activities (a) contemplated in the prospectus of Parent, dated as of November 23, 2020, or (b) otherwise necessary to consummate the Transactions.
Section 5.18 Parent
Material Contracts. The SPAC SEC Reports include true and complete copies of each “material
contract” (as such term is defined in Regulation S-K of the SEC) to which Parent is party (the “Parent Material
Contracts”). Each Parent Material Contract is in full force and effect and, to the knowledge of Parent, is valid and
binding upon and enforceable against each of the parties thereto (subject to the Remedies Exception), except insofar as
enforceability may be limited by the Remedies Exceptions. True and complete copies of all Parent Material Contracts have been
made available to the Company.
Section 5.19
Proxy Statement and Registration Statement. None of the information relating to Parent or
Pubco supplied by such Parent Party in writing for inclusion in the Proxy Statement will, as of the date the Registration Statement is
made effective, as of the date the Proxy Statement (or any amendment or supplement thereto) is first mailed to the Parent Stockholders,
at the time of the Parent Stockholders’ Meeting, or at the Parent Merger Effective Time or Company Merger Effective Time, contain
any misstatement of a material fact or omission of any material fact necessary to make the statements therein, in light of the circumstances
under which they are made, not misleading; provided, however, that neither Parent nor Pubco makes any representation with
respect to any forward-looking statements supplied by or on behalf of such Parent Party for inclusion in, or relating to information to
be included in the Proxy Statement or Registration Statement.
Section 5.20
Investment Company Act. Parent is not an “investment company” or a Person directly
or indirectly “controlled” by or acting on behalf of an “investment company”, or required to register as an “investment
company”, in each case within the meaning of the Investment Company Act of 1940.
Section 5.21
Transactions with Affiliates. Section 5.21 of the Parent Disclosure Schedule sets forth
all Contracts between (a) Parent, on the one hand, and (b) any officer, director, employee, partner, member, manager director or indirect
equityholder (including the Sponsor) or Affiliate of either Parent or the Sponsor, on the other hand (each Person identified in this part
(b), a “Parent Related Party”). Except as set forth in Section 5.21 of the Parent Disclosure Schedule,
no Parent Related Party (i) owns any interest in any material asset used in the business of Parent, (ii) possesses, directly or indirectly,
any material financial interest in, or is a director or executive officer of, any Person which is a material client, supplier, customer,
lessor or lessee of Parent, or (iii) owes any material amount to, or is owed any material amount by, Parent. All Contracts, arrangements,
understandings, interests and other matters that are required to be disclosed pursuant to this Section 5.21 are referred to herein
as “Parent Related Party Transactions.”
Section 5.22 Legacy
Parent Transaction Expenses. The Legacy Parent Transaction Expenses set forth on Section
1.01 of the Parent Disclosure Schedule include in all material respects all costs, fees and expenses incurred by the Parent
Parties in connection with (a) any proposed Business Combination of Parent other than the Transactions, including any fees and
expenses of legal counsel to the Parent Parties and of any other agents, advisors, consultants, experts, financial advisors and
other service providers engaged by or on behalf of the Parent Parties in connection with any such transactions, (b) the
preparation and filing with the SEC of any proxy statement prior to the date hereof for the purpose of amending the Parent
Organizational Documents and the Trust Agreement to extend the time period for Parent to consummate a Business Combination,
including the value of any additional securities or economic inducements offered to stockholders of Parent in connection therewith
and the costs, fees and expenses of any legal counsel or any other service providers engaged in connection therewith, (c) any
amounts due to the underwriters of Parent’s IPO in connection with any proposed Business Combination of Parent other than the
Transactions and which are not duplicative with such amounts due in connection with the Transactions, or (d) entering into any
agreements with any stockholders of Parent to incentivize them to either unwind or facilitate the unwinding of their
respective exercise of applicable Redemption Rights in connection with any proposed Business Combination of Parent other than the
Transactions or any proxy statement prior to the date hereof for the purpose of amending the Parent Organizational Documents and the
Trust Agreement to extend the time period for Parent to consummate a Business Combination.
Section 5.23
The Parent Parties’ Investigation and Reliance. Each Parent Party is a sophisticated
purchaser and has made its own independent investigation, review and analysis regarding the Company and any Company Subsidiary and the
Transactions, which investigation, review and analysis were conducted by the Parent Parties, together with expert advisors, including
legal counsel, that they have engaged for such purpose. The Parent Parties and their Representatives have been provided with full and
complete access to the Representatives, properties, offices, plants and other facilities, books and records of the Company and any Company
Subsidiary and other information that they have requested in connection with their investigation of the Company and the Company Subsidiaries
and the Transactions. No Parent Party is relying on any statement, representation or warranty, oral or written, express or implied, made
by the Company or any Company Subsidiary or any of their respective Representatives, except as expressly set forth in ARTICLE IV
(as modified by the Company Disclosure Schedule) or the Company Officer’s Certificate. Neither the Company nor any of its respective
stockholders, Affiliates or Representatives shall have any liability to any Parent Party or any of their respective stockholders, Affiliates
or Representatives resulting from the use of any information, documents or materials made available to the Parent Parties, or any of their
Representatives, whether orally or in writing, in any confidential information memoranda, “data rooms,” management presentations,
due diligence discussions or in any other form in expectation of the Transactions. Neither the Company nor any of its stockholders, Affiliates
or Representatives is making, directly or indirectly, any representation or warranty with respect to any estimates, projections or forecasts
involving the Company or any Company Subsidiary.
ARTICLE
VI
CONDUCT OF BUSINESS PENDING THE Company MERGER
Section 6.01
Conduct of Business by the Company Pending the Company Merger.
(a)
The Company agrees that, between the Effective Date and the Closing or the earlier termination of this Agreement (the “Interim
Period”), except as (1) expressly contemplated by any other provision of this Agreement or any Ancillary Agreement,
(2) as set forth in Section 6.01 of the Company Disclosure Schedule or (3) as required by applicable Law (including as
may be requested or compelled by any Governmental Authority), unless Parent shall otherwise consent in writing (which consent shall not
be unreasonably conditioned, withheld or delayed):
(i) the Company shall use
its reasonable best efforts to, and shall cause each Company Subsidiary to use its reasonable best efforts to, conduct its business
in the ordinary course of business; and
(ii) the Company shall use
its reasonable best efforts to preserve substantially intact the business organization of the Company and the Company Subsidiaries,
to keep available the services of the current officers and Key Employees of the Company and the Company Subsidiaries and to preserve
the current relationships of the Company and the Company Subsidiaries with customers, Suppliers and other Persons with which the
Company or any Company Subsidiary has significant business relations.
(b)
By way of amplification and not limitation, except as (1) expressly contemplated by any other provision of this Agreement
or any Ancillary Agreement, (2) as set forth in Section 6.01 of the Company Disclosure Schedule or (3) as required by
applicable Law (including as may be requested or compelled by any Governmental Authority), the Company shall not, and shall cause each
Company Subsidiary not to, during the Interim Period, directly or indirectly, do any of the following without the prior written consent
of Parent (which consent shall not be unreasonably conditioned, withheld or delayed):
(i) amend or otherwise
change the Company Memorandum and Articles or equivalent organizational documents;
(ii) issue, sell, pledge,
dispose of, grant or encumber or subject to any Lien, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of,
or otherwise amend any terms of, (A) any shares of any class of capital stock of the Company or any Company Subsidiary, or any
options, warrants, restricted stock units, convertible securities or other rights of any kind to acquire any shares of such capital
stock, or any other ownership interest (including, without limitation, any phantom interest), of the Company or any Company
Subsidiary, provided that none of (1) the consummation of one or more private placement transactions by the Company of any
equity securities (or securities convertible into or exercisable for equity securities) of the Company prior to the Company Merger
Effective Time which raise no more than $100,000,000 in the aggregate, and (2) the consummation of any initial sale of any shares of
capital stock of the Company in an underwritten public offering registered under the Securities Act or any direct listing of any
shares of capital stock of the Company on a securities exchange or securities market (collectively, the “Permitted
Financings”), or the issuance of any Permitted Financing Securities in connection therewith, shall require the consent
of Parent; or (B) any material assets of the Company or any Company Subsidiary, other than sales of assets in the ordinary
course of business;
(iii) adopt a plan of, or
otherwise enter into or effect a, complete or partial liquidation, dissolution, restructuring, recapitalization or other
reorganization of the Company or the Company Subsidiaries (other than the Company Merger or in connection with any Permitted
Financing), acquire any equity interest or other interest in any other entity other than a Company Subsidiary or enter into a joint
venture, partnership, business association or other similar arrangement with any other entity;
(iv) declare, set aside,
make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, excluding any dividend payable in the
form of shares of Capital Stock;
(v)
reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of its capital
stock, other than redemptions of equity securities from former employees upon the terms set forth in the underlying agreements governing
such equity securities;
(vi) (A) acquire
(including, without limitation, by merger, consolidation, or acquisition of stock or substantially all of the assets or any other
business combination) any corporation, partnership, other business organization or any division thereof, in each case, other than a
Company Subsidiary; or (B) incur any indebtedness for borrowed money or issue any debt securities or assume, guarantee or
endorse, or otherwise become responsible for, the obligations of any Person, or make any loans or advances, or grant any security
interest in any of its assets, in excess of $5,000,000 in the aggregate, other than in connection with a Permitted Financing;
(vii) (A) except as
provided for through the Employment Agreements, grant any increase in the compensation or incentives payable or to become payable to
any current or former director, officer, employee (including any Key Employee) or service provider of the Company or any Company
Subsidiary that has a base salary or compensation in excess of $150,000 (each, a “Company Service
Provider”), (B) except through or in connection with the Employment Agreements, enter into any new, or terminate or
amend any existing, employment, retention, bonus, change in control, or termination agreement with any Company Service Provider, (C)
except as provided for through the Employment Agreements, accelerate or commit to accelerate the funding, payment, or vesting of any
compensation or benefits to any Company Service Provider, or (D) establish or become obligated under any collective bargaining
agreement or other contract or agreement with a labor union, trade union, works council, or other representative of employees; provided, however,
that notwithstanding anything herein to the contrary, the Company may (1) provide increases in salary, wages, bonuses or benefits to
employees as required under the terms of any Plan in existence as of the date of this Agreement and reflected on Section
4.10(a) of the Company Disclosure Schedule or, for employees (other than Key Employees), in the ordinary course of business
consistent with past practice, (2) change the title of its employees (other than Key Employees) in the ordinary course of business,
and (3) make annual or quarterly bonus or commission payments in the ordinary course of business consistent with past practice
and in accordance with the bonus or commission plans existing on the date of this Agreement;
(viii)
other than as required by Law or pursuant to the terms of a Plan entered into prior to the date of this Agreement and reflected
on Section 4.10(a) of the Company Disclosure Schedule or as provided for through the Employment Agreements, grant any severance
or termination pay to (A) any Key Employee or any director or officer of the Company or of any Company Subsidiary, or (B) other than in
the ordinary course of business consistent with past practice, any other current employee of the Company or of any Company Subsidiary;
(ix) adopt, amend or
terminate any material Plan or any Employee Benefit Plan that would be a Plan if in effect as of the date hereof except (A) as may
be required by applicable Law, (B) as is required in order to consummate the Transactions or (C) in connection with health and
welfare plan renewals in the ordinary course of business consistent with past practice (provided that such renewals do not
materially increase the cost to the Company or any Company Subsidiary of providing such benefits);
(x)
waive the restrictive covenant obligations of any employee of the Company or any Company Subsidiary;
(xi) materially
amend or change any of the Company’s or any Company Subsidiary’s accounting policies or procedures, other than reasonable
and usual amendments in the ordinary course of business or as may be required by a change in GAAP;
(xii) make, change or
revoke any material Tax election, amend any income or other material Tax Return, settle or compromise any material income Tax
liability, adopt or change any accounting method in respect of material Taxes, consent to any extension or waiver of the statute of
limitations applicable to any claim or assessment in respect of material Taxes, execute any material “closing agreement”
as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) or
enter into any Tax sharing or similar agreement in respect of material Taxes (other than an Ordinary Commercial Agreement);
(xiii)
materially amend, or modify or consent to the termination (excluding any expiration in accordance with its terms) of any Material
Contract or amend, waive, modify or consent to the termination (excluding any expiration in accordance with its terms) of the Company’s
or any Company Subsidiary’s material rights thereunder, in each case in a manner that is adverse to the Company or any Company Subsidiary,
taken as a whole, except in the ordinary course of business;
(xiv) (A) exclusively
license, sell, transfer, assign or otherwise dispose of, divest or spin-off, any material Company IP or other material Intellectual
Property used or held for use in the business of the Company and the Company Subsidiaries, (B) abandon, relinquish, permit to lapse
or to be abandoned, invalidated, dedicated to the public, or disclaimed, or fail to perform or make any applicable filings,
recordings or other similar actions or filings, or fail to pay all required fees and Taxes required to maintain and protect its
interest in, any material Company IP, or (C) disclose or otherwise make available to any Person who is not subject to a written
agreement to maintain the confidentiality of such trade secrets any material Trade Secret included in the Company IP;
(xv) waive, release,
assign, settle or compromise any Action, other than waivers, releases, assignments, settlements or compromises that are solely
monetary in nature and do not exceed $250,000 individually or $1,000,000 in the aggregate; or
(xvi) enter into any
formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
Nothing herein shall
require the Company to obtain consent from Parent to do any of the foregoing if obtaining such consent might reasonably be expected
to violate applicable Law, and nothing contained in this Section 6.01 shall give to Parent, directly or indirectly, the right
to control or direct the ordinary course of business operations of the Company or any of the Company Subsidiaries prior to the
Closing Date. During the Interim Period, each of Parent and the Company shall exercise, consistent with the terms and conditions
hereof, complete control and supervision of its respective operations.
Section 6.02
Conduct of Business by the Parent Parties Pending the Mergers.
(a)
Except as expressly contemplated by any other provision of this Agreement or any Ancillary Agreement, and except as set forth on
Section 6.02 of the Parent Disclosure Schedule and as required by applicable Law (including as may be requested or compelled by
any Governmental Authority), Parent agrees that during the Interim Period, unless the Company shall otherwise consent in writing (which
consent shall not be unreasonably withheld, delayed or conditioned), the businesses of the Parent Parties shall be conducted in the ordinary
course of business and in a manner consistent with past practice.
(b)
By way of amplification and not limitation, except as expressly contemplated by any other provision of this Agreement or any Ancillary
Agreement, as set forth on Section 6.02 of the Parent Disclosure Schedule or as required by applicable Law (including as may
be requested or compelled by any Governmental Authority), no Parent Party shall, during the Interim Period, directly or indirectly, do
any of the following without the prior written consent of the Company, which consent shall not be unreasonably withheld, delayed or conditioned:
(i) amend or otherwise
change such Parent Party’s organizational documents or form any Subsidiary of Parent other than Pubco and the Merger Subs;
(ii) declare, set aside,
make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any of its capital
stock, other than redemptions from the Trust Fund that are required pursuant to the Parent Organizational Documents;
(iii) reclassify, combine,
split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any of the Parent Common Stock, Parent Rights
or Parent Warrants except for redemptions from the Trust Fund that are required pursuant to the Parent Organizational Documents;
(iv) issue, sell, pledge,
dispose of, grant or encumber, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any
class of capital stock or other securities of any Parent Party, or any options, warrants, convertible securities or other rights of
any kind to acquire any shares of such capital stock, or any other ownership interest (including, without limitation, any phantom
interest), of any Parent Party;
(v)
acquire (including, without limitation, by merger, consolidation, or acquisition of stock or assets or any other business combination)
any corporation, partnership, other business organization or enter into any strategic joint ventures, partnerships or alliances with any
other Person;
(vi) incur any
indebtedness for borrowed money or guarantee any such indebtedness of another Person or Persons, issue or sell any debt securities
or options, warrants, calls or other rights to acquire any debt securities of Parent, as applicable, enter into any “keep
well” or other agreement to maintain any financial statement condition or enter into any arrangement having the
economic effect of any of the foregoing;
(vii) make any change in
any method of financial accounting or financial accounting principles, policies, procedures or practices, except as required by a
concurrent amendment in GAAP or applicable Law made subsequent to the date hereof, as agreed to by its independent accountants;
(viii) make, change or
revoke any material Tax election, amend any income or other material Tax Return, settle or compromise any material income Tax
liability, adopt or change any accounting method in respect of material Taxes, consent to any extension or waiver of the statute of
limitations applicable to any claim or assessment in respect of material Taxes, execute any material “closing agreement”
as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) or
enter into any Tax sharing or similar agreement in respect of material Taxes (other than an Ordinary Commercial Agreement);
(ix) liquidate, dissolve,
reorganize or otherwise wind up the business and operations of any Parent Party;
(x)
amend, waive, modify or consent to the termination of the Trust Agreement or any other agreement related to the Trust Account;
(xi) (A) enter into,
materially amend, or modify or consent to the termination (excluding any expiration in accordance with its terms) of any Contracts
to which a Parent Party is party (including engagement letters with financial advisors) in a manner that would materially and
adversely affect Parent or any of its Subsidiaries after the Closing or would impose material liabilities on any Parent or any of
its Subsidiaries after the Closing, or (B) enter into any Contract that would entitle any third party to any bonuses, payments or
other fees upon or conditioned upon the consummation of the Closing, other than any services providers engaged by Parent prior to
the Closing for printing, mailing and solicitation services with respect to the Proxy Statement or the Registration Statement;
or
(xii) enter into, renew,
modify or revise any Parent Related Party Transaction (or any Contract or agreement that if entered into prior to the execution and
delivery of this Agreement would be a Parent Related Party Transaction); or
(xiii)
enter into any formal or informal agreement or otherwise make a binding commitment to do any of the foregoing.
Nothing in this Section
6.02 shall give to the Company, directly or indirectly, the right to control or direct the ordinary course of business operations
of Parent prior to the Closing Date. Prior to the Closing Date, each of Parent and the Company shall exercise, consistent with the terms
and conditions hereof, complete control and supervision of its respective operations, as required by Law.
Section 6.03 Claims
Against Trust Account. Reference is made to the final prospectus of Parent, dated as of November
23, 2020 and filed with the SEC (Registration No. 333-249677) on November 24, 2020 (the
“Prospectus”). The Company hereby represents and warrants that it understands that Parent has established
the Trust Account containing the proceeds of its initial public offering (the “IPO”) and the overallotment
shares acquired by its underwriters and from certain private placements occurring simultaneously with the IPO (including interest
accrued from time to time thereon) for the benefit of Parent’s public stockholders (including overallotment shares acquired by
Parent’s underwriters, the “Public Stockholders”), and that, except as otherwise described in the
Prospectus, Parent may disburse monies from the Trust Account only: (a) to the Public Stockholders in the event they elect to redeem
their Parent Common Stock in connection with the consummation of Parent’s initial business combination (as such term is used
in the Prospectus) (the “Business Combination”) or in connection with an extension of its deadline to
consummate a Business Combination, (b) to the Public Stockholders if Parent fails to consummate a Business Combination within 12
months after the closing of the IPO, subject to extension by amendment to Parent’s organizational documents, (c) with respect
to any interest earned on the amounts held in the Trust Account, amounts as necessary to pay any Taxes and up to $100,000 in
dissolution expenses, or (d) to Parent after or concurrently with the consummation of a Business Combination. For and in
consideration of Parent entering into this Agreement, and for other good and valuable consideration, the receipt and sufficiency of
which is hereby acknowledged, the Company hereby agrees on behalf of itself and its Affiliates that, notwithstanding anything to the
contrary in this Agreement, neither the Company nor any of its Affiliates do now or shall at any time hereafter have any right,
title, interest or claim of any kind in or to any monies in the Trust Account or distributions therefrom, or make any claim against
the Trust Account (including any distributions therefrom), regardless of whether such claim arises as a result of, in connection
with or relating in any way to, this Agreement or any proposed or actual business relationship between Parent or its
Representatives, on the one hand, and the Company or its Representatives, on the other hand, or any other matter, and regardless of
whether such claim arises based on contract, tort, equity or any other theory of legal liability (any and all such claims are
collectively referred to hereafter as the “Released Claims”). The Company on behalf of itself and its
Affiliates hereby irrevocably waives any Released Claims that the Company or any of its Affiliates may have against the Trust
Account (including any distributions therefrom) now or in the future as a result of, or arising out of, any negotiations, contracts
or agreements with Parent or its Representatives and will not seek recourse against the Trust Account (including any distributions
therefrom) for any reason whatsoever (including for an alleged breach of this Agreement or any other agreement with Parent or its
Affiliates). The Company agrees and acknowledges that such irrevocable waiver is material to this Agreement and specifically relied
upon by Parent and its Affiliates to induce Parent to enter into this Agreement, and the Company further intends and understands
such waiver to be valid, binding and enforceable against the Company and each of its Affiliates under applicable Law. To the extent
the Company or any of its Affiliates commences any action or proceeding based upon, in connection with, relating to or arising out
of any matter relating to Parent or its Representatives, which proceeding seeks, in whole or in part, monetary relief against Parent
or its Representatives, the Company hereby acknowledges and agrees that the Company’s and its Affiliates’ sole remedy
shall be against funds held outside of the Trust Account and that such claim shall not permit the Company or its Affiliates (or any
Person claiming on any of their behalf or in lieu of any of them) to have any claim against the Trust Account (including any
distributions therefrom) or any amounts contained therein. In the event (a) the Company or any of its Affiliates commences any
action or proceeding based upon, in connection with, relating to or arising out of any matter relating to Parent or its
Representatives, which proceeding seeks, in whole or in part, relief against the Trust Account (including any distributions
therefrom) or the Public Stockholders of Parent, whether in the form of money damages or injunctive relief, and (b) Parent and its
Representatives, as applicable, prevails in such action or proceeding, Parent or its Representatives, as applicable, shall be
entitled to recover from the Company and its Affiliates the associated legal fees and costs in connection with any such action.
Notwithstanding anything in this Agreement to the contrary, the provisions of this paragraph shall survive indefinitely with respect
to the obligations set forth in this Agreement.
ARTICLE
VII
ADDITIONAL AGREEMENTS
Section 7.01
Proxy Statement; Registration Statement.
(a)
As soon as reasonably practicable following the date of this Agreement, (i) Parent (with the assistance and cooperation of
the Company as reasonably requested by Parent) shall prepare and Pubco shall file with the SEC a proxy statement (as amended or supplemented,
the “Proxy Statement”) to be sent to the stockholders of Parent, in which Parent shall solicit proxies from
Parent’s stockholders to vote at the special meeting of Parent’s stockholders called for the purpose of voting on the following
matters (the “Parent Stockholders’ Meeting”) in favor of (A) the adoption of this Agreement and approval
of the Mergers, (B) the issuance of Pubco Ordinary Shares as contemplated by this Agreement, (C) the approval and adoption of
the Amended and Restated Pubco Memorandum and Articles, (D) the approval and adoption of an equity incentive plan, in form and substance
reasonably acceptable to Parent and the Company that provides for grant of awards to employees and other service providers of the Company
Surviving Subsidiary and its Subsidiaries in the form of options, restricted stock, restricted stock units or other equity-based awards
based on Pubco Ordinary Shares with a total pool of awards of Pubco Ordinary Shares not exceeding, together with the number of shares
of Parent Common Stock that would be issuable immediately after the Company Merger Effective Time and the Parent Merger Effective Time
upon the vesting of all Converted RSUs or Converted Options, 10% of the number of Pubco Ordinary Shares outstanding as of immediately
following the Closing (the “Pubco LTIP”), which Pubco LTIP shall have an annual “evergreen”
increase of not more than 3% of Pubco Ordinary Shares outstanding as of the day prior to such increase, and (E) any approval of other
proposals the parties deem necessary to effectuate the Mergers and the other Transactions (collectively, the “Parent Proposals”),
and (ii) Parent shall prepare and Pubco shall file with the SEC a registration statement on Form F-4 (together with all amendments
thereto, the “Registration Statement”), which Registration Statement shall include the Proxy Statement in connection
with the registration under the Securities Act of Pubco Ordinary Shares and the Pubco Assumed Parent Warrants to be issued to Participating
Securityholders and/or holders of Parent securities, as applicable, pursuant to this Agreement.
(b) Pubco and Parent
shall (w) cause the Proxy Statement and Registration Statement when filed with the SEC to comply in all material respects with
all legal requirements applicable thereto, (x) respond as promptly as reasonably practicable to and resolve all comments
received from the SEC concerning the Proxy Statement or the Registration Statement, (y) cause the Registration Statement to be
declared effective under the Securities Act as promptly as practicable and (z) keep the Registration Statement effective as
long as is necessary to consummate the Transactions. As promptly as practicable after the
Registration Statement becomes effective, Parent shall mail (or cause to be mailed) the Proxy Statement to its stockholders. Each of
Parent, Pubco and the Company shall promptly furnish all information concerning it as may reasonably be requested by the other party
in connection with such actions and the preparation of the Registration Statement and the Proxy Statement.
(c)
No filing of, or amendment or supplement to the Proxy Statement or the Registration Statement will be made by Pubco or Parent without
the approval of the Company (such approval not to be unreasonably withheld, conditioned or delayed). Parent and Pubco, on the one hand,
and the Company, on the other hand, each will advise the other, promptly after they receive notice thereof, of the time when the Registration
Statement has become effective or any supplement or amendment thereto has been filed, of the issuance of any stop order, of the suspension
of the qualification of the Parent Common Stock or the Pubco Assumed Parent Warrants to be issued or issuable to Participating Securityholders
and/or holders of Parent securities, as applicable, in connection with this Agreement for offering or sale in any jurisdiction, or of
any request by the SEC for amendment of the Proxy Statement or the Registration Statement or comments thereon and responses thereto or
requests by the SEC for additional information. Each of Pubco, Parent and the Company shall cooperate and mutually agree upon (such agreement
not to be unreasonably withheld, conditioned or delayed), any response to comments of the SEC with respect to the Proxy Statement or the
Registration Statement and any amendment to the Proxy Statement or the Registration Statement filed in response thereto.
(d)
Each of Parent and Pubco represents that the information supplied by it for inclusion in the Registration Statement and the Proxy
Statement shall not contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or
necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading at (i) the
time the Registration Statement is declared effective, (ii) the time the Proxy Statement (or any amendment thereof or supplement
thereto) is first mailed to the stockholders of Parent, (iii) the time of the Parent Stockholders’ Meeting, (iv) the Company
Merger Effective Time, and (v) the Parent Merger Effective Time. If, at any time prior to the Company Merger Effective Time, any event
or circumstance relating to any of the Parent Parties, or their respective officers or directors, should be discovered by Parent or Pubco
which should be set forth in an amendment or a supplement to the Registration Statement or the Proxy Statement, Parent shall promptly
inform the Company. All documents that each of Parent and Pubco is responsible for filing with the SEC in connection with the Mergers
or the other Transactions will comply as to form and substance in all material respects with the applicable requirements of the Securities
Act and the Exchange Act.
(e) The Company
represents that the information supplied by the Company for inclusion in the Registration Statement and the Proxy Statement shall
not contain any untrue statement of a material fact or fail to state any material fact required to be stated therein or necessary in
order to make the statements therein, in light of the circumstances under which they were made, not misleading, at (i) the time
the Registration Statement is declared effective, (ii) the time the Proxy Statement (or any amendment thereof or supplement
thereto) is first mailed to the stockholders of Parent, (iii) the time of the Parent Stockholders’ Meeting, (iv) the
Parent Merger Effective Time, and (iv) the Company Merger Effective Time. If, at any time prior to the Company Merger Effective
Time, any event or circumstance relating to the Company or any Company Subsidiary, or their
respective officers or directors, should be discovered by the Company which should be set forth in an amendment or a supplement to
the Registration Statement or the Proxy Statement, the Company shall promptly inform Parent.
(f)
As promptly as practicable after the initial filing of the Registration Statement, the Company (with the assistance and cooperation
of Parent as reasonably requested by the Company) shall prepare an information statement relating to the action to be taken
by the stockholders of the Company pursuant to the Written Consent. As promptly as practicable after the date on which the Registration
Statement becomes effective, the Company shall deliver the Consent Solicitation Statement and the prospectus contained in the Registration
Statement to its stockholders.
Section 7.02
Parent Stockholders’ Meeting; Pubco and Merger Subs Stockholder’s Approval.
(a)
Parent shall call and hold the Parent Stockholders’ Meeting as promptly as practicable after the date on which the Registration
Statement becomes effective (but in any event no later than 30 days after the date on which the Proxy Statement is mailed to stockholders
of Parent) for the purpose of voting solely upon the Parent Proposals; provided that, with the prior consultation of the Company,
Parent may postpone or adjourn the Parent Stockholders’ Meeting on one or more occasions for up to thirty (30) days in the
aggregate upon the good faith determination by the Parent Board that such postponement or adjournment is necessary to solicit additional
proxies to obtain approval of the Parent Proposals or otherwise take actions consistent with Parent’s obligations pursuant to Section
7.10 of this Agreement. Parent shall use its reasonable best efforts to obtain the approval of the Parent Proposals at the Parent
Stockholders’ Meeting, including by soliciting from its stockholders proxies as promptly as possible in favor of the Parent Proposals.
The Parent Board shall recommend to its stockholders that they approve the Parent Proposals (the “Parent Board Recommendation”)
and shall include such recommendation in the Proxy Statement. The Parent Board shall not (and no committee or subgroup thereof shall)
(i) change, withdraw, withhold, qualify or modify the Parent Board Recommendation, (ii) publicly propose to change, withdraw, withhold,
qualify or modify the Parent Board Recommendation or (iii) fail to include the Parent Board Recommendation in the Proxy Statement.
(b)
Promptly following the execution of this Agreement (and in any event within twenty-four (24) hours herefrom), (i) Parent shall
adopt this Agreement and approve the Mergers and the other Transactions in its capacity as the sole stockholder of Pubco, and (ii) Pubco
shall adopt this Agreement and approve the Mergers and the other Transactions in its capacity as the sole stockholders of each of the
Merger Subs.
Section 7.03 Requisite
Approval. Upon the terms set forth in this Agreement, (a) the Company shall (i) obtain the
irrevocable written consent, in form and substance reasonably acceptable to Parent, of holders of Capital Stock constituting the
Requisite Approval in favor of the adoption of this Agreement and the approval of the Company Merger and the other Transactions (the
“Written Consent”), as soon as reasonably practicable after the Registration Statement becomes effective,
and in any event within five (5) Business Days after the Registration Statement becomes effective, and (b) the Company Board shall
recommend to its stockholders that they adopt this Agreement and approve the Company Merger and the other Transactions to
which the Company is a party (the “Company Board Recommendation”). The
Company Board shall not (and no committee or subgroup thereof shall) (i) change, withdraw, withhold, qualify or modify, or publicly
propose to change, withdraw, withhold, qualify or modify, the Company Board Recommendation, (ii) approve, recommend or declare
advisable, or propose publicly to approve, recommend or declare advisable, any Acquisition Proposal or (iii) fail to include the
Company Board Recommendation in the Consent Solicitation Statement.
Section 7.04
Access to Information; Confidentiality.
(a)
During the Interim Period, the Company and Parent shall (and shall cause their respective Subsidiaries to): (i) provide to
the other party (and the other party’s officers, directors, employees, accountants, consultants, legal counsel, agents and other
representatives, collectively, “Representatives”) reasonable access at reasonable times upon prior notice to
the officers, employees, agents, properties, offices and other facilities of such party and its subsidiaries and to the books and records
thereof; and (ii) furnish promptly to the other party such information concerning the business, properties, contracts, assets, liabilities,
personnel and other aspects of such party and its subsidiaries as the other party or its Representatives may reasonably request, including
in connection with any Tax disclosure in any statement, filing, notice or application relating to the Intended Tax Treatment or any Tax
opinion requested or required to be filed pursuant to Section 7.12(c). Notwithstanding the foregoing, neither the Company nor Parent
shall be required to provide access to or disclose information where the access or disclosure would jeopardize the protection of attorney-client
privilege or contravene applicable Law (it being agreed that the parties shall use their reasonable best efforts to cause such information
to be provided in a manner that would not result in such jeopardy or contravention).
(b)
All information obtained by the parties pursuant to this Section 7.04 shall be kept confidential in accordance with the
Nondisclosure Agreement, dated as of August 9, 2024 (the “Non-Disclosure Agreement”), between Parent and the
Company.
(c)
Notwithstanding anything in this Agreement to the contrary, each party hereto (and its respective Representatives) may consult
any Tax advisor as is reasonably necessary regarding the Tax treatment and Tax structure of the Transactions and may disclose to such
Tax advisor as reasonably necessary such treatment and structure of the Transactions and all materials (including any Tax analysis) that
are provided relating to such treatment or structure, in each case in accordance with the Non-Disclosure Agreement.
Section 7.05
Non-Solicitation.
(a) During the Interim Period,
the Company shall not, shall cause its Subsidiaries not to and shall use its reasonable best efforts to cause its and their respective
Representatives not to, directly or indirectly, (i) initiate, solicit, propose or knowingly induce the making, submission or announcement
of, or knowingly encourage, facilitate or assist, any inquiries or requests for information with respect to, or the making of, any inquiry
regarding, or any proposal or offer that constitutes, or could reasonably be expected to result in or lead to, any Acquisition Proposal,
(ii) engage in, continue or otherwise participate in any negotiations or discussions concerning, or provide access to its properties,
business, assets, books, records or any confidential information or data to, any Person relating
to any proposal, offer, inquiry or request for information that constitutes, or could reasonably be expected to result in or lead to,
any Acquisition Proposal, (iii) approve, endorse or recommend, or propose publicly to approve, endorse or recommend, any Acquisition
Proposal, (iv) execute or enter into, any letter of intent, memorandum of understanding, agreement in principle, confidentiality agreement,
merger agreement, acquisition agreement, exchange agreement, joint venture agreement, partnership agreement, option agreement or other
similar agreement for or relating to any Acquisition Proposal or (v) resolve or agree to do, or do, any of the foregoing. The Company
also agrees that, immediately following the execution of this Agreement, it shall, and shall cause each of its Subsidiaries and its and
their Representatives to, cease any solicitations, discussions or negotiations with any Person (other than the parties hereto and their
respective Representatives) conducted heretofore in connection with an Acquisition Proposal or any inquiry or request for information
that could reasonably be expected to lead to, or result in, an Acquisition Proposal. The Company also agrees that within five (5) Business
Days of the execution of this Agreement, the Company shall request each Person (other than the parties hereto and their respective Representatives)
that has prior to the date hereof executed a confidentiality agreement in connection with its consideration of an Acquisition Proposal
(and with whom the Company has had contact in the twelve (12) months prior to the date of this Agreement regarding an Acquisition Proposal)
to return or destroy all confidential information furnished to such Person by or on behalf of it or any of its Subsidiaries prior to
the date hereof in accordance with the terms of the confidentiality agreement executed with such Person and terminate access to any physical
or electronic data room maintained by or on behalf of the Company or any of its Subsidiaries. If a party or any of its Subsidiaries or
any of its or their respective Representatives receives any inquiry or proposal with respect to an Acquisition Proposal at any time prior
to the Closing, then such party shall promptly (and in no event later than two (2) Business Days after such party becomes aware of such
inquiry or proposal) notify such Person in writing of the terms of this Section 7.05. Without limiting the foregoing, it
is understood that any violation of the restrictions contained in this Section 7.05 by any of the Company Subsidiaries, or any
of the Company’s or its Subsidiaries’ respective Representatives acting on the Company’s or one of its Subsidiaries’
behalf, shall be deemed to be a breach of this Section 7.05 by the Company.
(b) For purposes of this
Agreement, “Acquisition Proposal” means any proposal or offer from any Person or “group” (as defined
in the Exchange Act) (other than the Parent Parties, or their respective Affiliates) relating to, in a single transaction or series of
related transactions, (i) any direct or indirect acquisition or purchase of a business that constitutes fifty percent (50%) or more of
the net revenues, net income or assets of the Company and its Subsidiaries, taken as a whole, (ii) any direct or indirect acquisition
of fifty percent (50%) or more of the consolidated assets of the Company and its Subsidiaries, taken as a whole (based on the fair market
value thereof, as determined in good faith by the Company Board), including through the acquisition of one or more Subsidiaries of the
Company owning such assets, (iii) acquisition of beneficial ownership, or the right to acquire beneficial ownership, of fifty percent
(50%) or more of the total voting power of the equity securities of the Company, any tender offer or exchange offer that if consummated
would result in any Person beneficially owning fifty percent (50%) or more of the total voting power of the equity securities of the
Company, or any merger, reorganization, consolidation, share exchange, business combination, recapitalization, liquidation, dissolution
or similar transaction involving the Company (or any Subsidiary of the Company whose business constitutes fifty percent (50%) or more
of the net revenues, net income or assets of the Company and its Subsidiaries, taken as a whole)
or (iv) any issuance or sale or other disposition (including by way of merger, reorganization, division, consolidation, share exchange,
business combination, recapitalization or other similar transaction) of fifty percent (50%) or more of the total voting power of the
equity securities of the Company; provided that, for the avoidance of doubt, no Permitted Financing shall constitute an
Acquisition Proposal.
Section 7.06
Exclusivity. During the Interim Period, Parent shall not, shall cause its Subsidiaries not
to and shall use its reasonable best efforts to cause its and their respective Representatives not to, directly or indirectly, (i) initiate,
solicit, propose or knowingly induce the making, submission or announcement of, or knowingly encourage, facilitate or assist, any inquiries
or requests for information with respect to, or the making of, any inquiry regarding, or any proposal or offer that constitutes, or could
reasonably be expected to result in or lead to, any Business Combination other than the Transactions (a “Business Combination
Proposal”), (ii) engage in, continue or otherwise participate in any negotiations or discussions concerning, or provide
access to its properties, business, assets, books, records or any confidential information or data to, any Person relating to any proposal,
offer, inquiry or request for information that constitutes, or could reasonably be expected to result in or lead to, any Business Combination
Proposal, (iii) approve, endorse or recommend, or propose publicly to approve, endorse or recommend, any Business Combination Proposal,
(iv) execute or enter into, any letter of intent, memorandum of understanding, agreement in principle, confidentiality agreement, merger
agreement, acquisition agreement, exchange agreement, joint venture agreement, partnership agreement, option agreement or other similar
agreement for or relating to any Business Combination Proposal or (v) propose, resolve or agree to do, or do, any of the foregoing.
Parent also agrees that, immediately following the execution of this Agreement, it and the Sponsor shall, and shall cause each of their
respective Subsidiaries and its and their Representatives to, cease any solicitations, discussions or negotiations with any Person (other
than the parties hereto and their respective Representatives) conducted heretofore in connection with a Business Combination Proposal
or any inquiry or request for information that could reasonably be expected to lead to, or result in, a Business Combination Proposal.
Parent also agrees that within five (5) Business Days of the execution of this Agreement, Parent shall request each Person (other than
the parties hereto and their respective Representatives) that has prior to the date hereof executed a confidentiality agreement in connection
with its consideration of a Business Combination Proposal (and with whom Parent has had contact in the twelve (12) months prior to the
date of this Agreement regarding a Business Combination Proposal) to return or destroy all confidential information furnished to such
Person by or on behalf of it or any of its Subsidiaries prior to the date hereof in accordance with the terms of the confidentiality agreement
executed with such Person and terminate access to any physical or electronic data room maintained by or on behalf of Parent or any of
its Subsidiaries. If a party or any of its Subsidiaries or any of its or their respective Representatives receives any inquiry or proposal
with respect to a Business Combination Proposal at any time prior to the Closing, then such party shall promptly (and in no event later
than two (2) Business Days after such party becomes aware of such inquiry or proposal) notify such Person in writing of the terms of this
Section 7.06. Without limiting the foregoing, it is understood that any violation of the restrictions contained in this Section
7.06 by any of Parent’s Subsidiaries, or any of Parent’s or its Subsidiaries’ respective Representatives acting
on Parent’s or one of its Subsidiaries’ behalf, shall be deemed to be a breach of this Section 7.06 by Parent.
Section 7.07 Employee Benefits
Matters.
(a)
Pubco shall, or shall cause the Company Surviving Subsidiary and each of its Subsidiaries, as applicable, to provide the employees
of the Company and the Company Subsidiaries who remain employed immediately after the Effective Time (the “Continuing Employees”)
credit for purposes of eligibility to participate, vesting and determining the level of benefits, as applicable, under any Employee Benefit
Plan established or maintained by the Company Surviving Subsidiary or any of its Subsidiaries (excluding any retiree health plans or programs
or defined benefit retirement plans or programs) for service accrued or deemed accrued prior to the Company Merger Effective Time with
the Company or any Company Subsidiary; provided, however, that such crediting of service shall not operate to duplicate
any benefit or the funding of any such benefit. In addition, Parent shall use reasonable best efforts to (i) cause to be waived any
eligibility waiting periods, any evidence of insurability requirements and the application of any pre-existing condition limitations under
each of the Employee Benefit Plans established or maintained by the Company Surviving Subsidiary or any of its Subsidiaries that cover
the Continuing Employees or their dependents, and (ii) cause any eligible expenses incurred by any Continuing Employee and his or
her covered dependents, during the portion of the plan year in which the Closing occurs, under those health and welfare benefit plans
in which such Continuing Employee currently participates to be taken into account under those health and welfare benefit plans in which
such Continuing Employee participates subsequent to the Closing Date for purposes of satisfying all deductible, coinsurance, and maximum
out-of-pocket requirements applicable to such Continuing Employee and his or her covered dependents for the applicable plan year. Following
the Closing, the Company Surviving Subsidiary will honor all accrued but unused vacation and other paid time off of the Continuing Employees
that existed immediately prior to the Closing with respect to the calendar year in which the Closing occurs. The Company shall provide
Pubco or its designee with all information reasonably requested and necessary to allow Pubco or its designee to comply with such obligations.
(b)
The Company shall cause all notices to be timely provided to each optionee under the Company Equity Incentive Plan as required
by the Company Equity Incentive Plan in connection with the Transactions.
(c)
The provisions of this Section 7.07 are solely for the benefit of the parties to the Agreement, and nothing contained in
this Agreement, express or implied, shall confer upon any Continuing Employee or legal representative or beneficiary or dependent thereof,
or any other Person, any rights or remedies of any nature or kind whatsoever under or by reason of this Agreement, whether as a third-party
beneficiary or otherwise, including, without limitation, any right to employment or continued employment for any specified period, or
level of compensation or benefits. Nothing contained in this Agreement, express or implied, shall constitute an amendment or modification
of any Employee Benefit Plan or other employee benefit arrangement or shall require any of the Company, Pubco, Parent, the Parent Surviving
Subsidiary, the Company Surviving Subsidiary or any of its subsidiaries to continue any Plan or other employee benefit arrangements, or
prevent their amendment, modification or termination.
Section 7.08 Directors’
and Officers’ Indemnification.
(a)
The certificate of incorporation of the Company Surviving Subsidiary and Pubco shall each contain provisions no less favorable
with respect to indemnification, advancement or expense reimbursement than are set forth in the Company Memorandum and Articles, which
provisions shall not be amended, repealed or otherwise modified for a period of six (6) years from the Company Merger Effective Time in
any manner that would affect adversely the rights thereunder of individuals who, at or prior to the Company Merger Effective Time, were
directors, officers, employees, fiduciaries or agents of the Company, unless such modification shall be required by applicable Law. From
and after the Company Merger Effective Time, Pubco agrees that it shall indemnify and hold harmless each present and former director and
officer of the Company against any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, damages
or liabilities incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative
or investigative, arising out of or pertaining to matters existing or occurring at or prior to the Company Merger Effective Time whether
asserted or claimed prior to, at or after the Company Merger Effective Time, to the fullest extent that the Company would have been permitted
under applicable Law and the Company Memorandum and Articles in effect on the date of this Agreement to indemnify such Person (including
the advancing of expenses as incurred to the fullest extent permitted under applicable Law). Pubco further agrees that with respect to
the provisions of the bylaws and certificate of incorporation or limited liability company agreement, as applicable, of the Company Subsidiaries
relating to indemnification, advancement or expense reimbursement, such provisions shall not be amended, repealed or otherwise modified
for a period of six (6) years from the Company Merger Effective Time in any manner that would affect adversely the rights thereunder of
individuals who, at or prior to the Company Merger Effective Time, were directors, officers, employees, fiduciaries or agents of such
Company Subsidiary, unless such modification shall be required by applicable Law.
(b)
For a period of six (6) years from the Company Merger Effective Time, Pubco shall maintain in effect directors’ and officers’
liability insurance covering those Persons who are currently covered by the Company’s directors’ and officers’ liability
insurance policy (true, correct and complete copies of which have been heretofore made available to Pubco or its agents or Representatives
in the Virtual Data Room) on terms not less favorable than the terms of such current insurance coverage, except that in no event shall
Pubco be required to pay an annual premium for such insurance in excess of 250% of the aggregate annual premium payable by the Company
for such insurance policy for the year ended December 31, 2023 (the “Maximum Annual Premium”); provided,
however, that (i) Pubco may cause coverage to be extended under the current directors’ and officers’ liability insurance
by obtaining a six (6)-year “tail” policy containing terms not less favorable than the terms of such current
insurance coverage with respect to claims existing or occurring at or prior to the Company Merger Effective Time, and (ii) if any claim
is asserted or made within such six (6)-year period, any insurance required to be maintained under this Section 7.08(b) shall be
continued in respect of such claim until the final disposition thereof.
(c) On the Closing Date,
to the extent not already entered into, Pubco shall enter into customary indemnification agreements reasonably satisfactory to each
of the Company and Parent with the post-Closing directors and officers of Pubco, which
indemnification agreements shall continue to be effective following the Closing.
Section 7.09
Notification of Certain Matters. The Company shall give prompt notice in writing to Parent,
and Parent shall give prompt notice in writing to the Company, of any event which a party becomes aware of between the date of this Agreement
and the Closing (or the earlier termination of this Agreement in accordance with ARTICLE IX), the occurrence, or non-occurrence
of which causes or would reasonably be expected to cause any of the conditions set forth in ARTICLE VIII to fail.
Section 7.10
Further Action; Reasonable Best Efforts.
(a)
Upon the terms and subject to the conditions of this Agreement, each of the parties hereto shall use its reasonable best efforts
to take, or cause to be taken, appropriate action, and to do, or cause to be done, such things as are necessary, proper or advisable under
applicable Laws or otherwise, and each shall cooperate with the other, to consummate and make effective the Transactions, including using
its reasonable best efforts to make all filings with, respond to questions from, obtain all permits, consents, approvals, authorizations,
qualifications and orders of, and the expiration or termination of waiting periods by, Governmental Authorities and parties to Contracts
with the Company and the Company Subsidiaries as set forth in Section 4.05 necessary for the consummation of the Transactions and
to fulfill the conditions to the Mergers. If at any time after the Parent Merger Effective Time or Company Merger Effective Time further
action is necessary or desirable to the parties to carry out the purposes of this Agreement, the proper officers and directors of each
party shall use their reasonable best efforts to take all such action.
(b)
During the Interim Period, each of the parties shall keep each other apprised of the status of matters relating to the Transactions,
including promptly notifying the other parties of any communication it or any of its Affiliates receives from any Governmental Authority
relating to the matters that are the subject of this Agreement and permitting the other parties to review in advance, and to the extent
practicable consult about, any proposed communication by such party to any Governmental Authority in connection with the Transactions.
During the Interim Period, no party to this Agreement shall agree to participate in any meeting, video or telephone conference, or other
communications with any Governmental Authority in respect of any filings, investigation or other inquiry unless it consults with the other
parties in advance and, to the extent permitted by such Governmental Authority, gives the other parties the opportunity to attend and
participate at such meeting, conference or other communications. Subject to the terms of the Non-Disclosure Agreement, during the Interim
Period, the parties will coordinate and cooperate fully with each other in exchanging such information and providing such assistance as
the other parties may reasonably request in connection with the foregoing. Subject to the terms of the Non-Disclosure Agreement, the parties
will provide each other with copies of all material correspondence, filings or communications, including any documents, information and
data contained therewith, between them or any of their Representatives, on the one hand, and any Governmental Authority, on the other
hand, with respect to this Agreement and the Transactions contemplated hereby during the Interim Period. No party shall take or cause
to be taken any action before any Governmental Authority that is inconsistent with or intended to delay its action on requests for a consent
or the consummation of the Transactions.
(c)
During the Interim Period, each of Parent and Pubco will provide such information and such other assistance as is reasonably requested
by the Company in connection with the Permitted Financings.
(d)
During the Interim Period, Parent shall use reasonable best efforts to cause holders of Parent Common Stock not to exercise or
otherwise waive their Redemption Rights, including by entry into binding non-redemption agreements. Parent shall not enter into any Contracts
between Parent or any of its Affiliates and any holder of Parent Common Stock or any of its Affiliates relating to any such waiver of
Redemption Rights without the prior written consent of the Company; provided that the Sponsor shall be expressly permitted to transfer,
assign or convey shares of Parent Common Stock beneficially owned by the Sponsor in connection with such Contracts to secure waivers of
the Redemption Rights; provided, further, that any shares of Parent Common Stock transferred, assigned or conveyed in connection
with securing such waivers of Redemption Rights shall remain obligated under the terms of the Sponsor Support Agreement.
Section 7.11
Public Announcements. The initial press release relating to this Agreement shall be a joint
press release, the text of which has been agreed to by each of Parent and the Company. Thereafter, between the date of this Agreement
and the Closing Date (or the earlier termination of this Agreement in accordance with ARTICLE IX) unless otherwise prohibited by
applicable Law or the requirements of the Nasdaq Capital Market, each of Parent and Pubco, on the one hand, and the Company, on the other
hand, shall use its reasonable best efforts to consult with each other before issuing any press release or otherwise making any public
statements with respect to this Agreement, the Mergers or any of the other Transactions, and shall not issue any such press release or
make any such public statement without the prior written consent of the other party. Furthermore, nothing contained in this Section
7.11 shall prevent Parent or the Company or its respective Affiliates from furnishing customary or other reasonable information concerning
the Transactions to their investors and prospective investors that is substantively consistent with public statements previously consented
to by the other party in accordance with this Section 7.11.
Section 7.12
Tax Matters.
(a)
None of the parties hereto shall (and each shall cause its Affiliates not to) take or cause to be taken (or fail to take or cause
to be taken) any action, which action (or failure to act), whether before or after the Effective Time, would reasonably be expected to
prevent or impede the Mergers from qualifying for the Intended Tax Treatment.
(b) For U.S. federal and
applicable state income Tax purposes, the parties hereto intend that (i) taken together, the Mergers and any PIPE Investment shall
together qualify as a transaction described in Section 351(a) of the Code, (ii) the Company Merger shall qualify as a
“reorganization” within the meaning of Section 368(a) of the Code and the Treasury Regulations promulgated thereunder
and (iii) that this Agreement be, and hereby is adopted as, a “plan of reorganization” (within the meaning of Section
368(a) of the Code and Treasury Regulations Sections 1.368-2(g) and 1.368-3) to which each of Pubco, Company Merger Sub, and the
Company are parties under Section 368(b) of the Code. The parties hereto shall prepare and file all Tax Returns and otherwise report
the Mergers consistent with the Intended Tax Treatment (including attaching a statement described in Treasury Regulations Sections
1.368-3(a) and 1.368-3(b) to applicable Tax Returns), unless otherwise required pursuant to a
“determination” within the meaning of Section 1313(a) of the Code.
Each party hereto shall promptly notify the other party in writing of any challenge to the Intended Tax Treatment by any
Governmental Authority. The parties hereto shall use commercially reasonable efforts to cooperate in connection with
fulfilling Tax reporting requirements under Treasury Regulations Sections 1.351-3, 1.368-3(a) and 1.368-3(b), as applicable.
(c)
Each party hereto shall promptly notify the other party in writing if, before the Closing, such party knows or has reason to believe
that the Mergers may not qualify for the Intended Tax Treatment (and whether the terms of this Agreement could be reasonably amended in
order to facilitate the Mergers qualifying for the Intended Tax Treatment). In the event either (i) Parent or the Company seeks a Tax
opinion from its respective Tax advisor regarding the Intended Tax Treatment or (ii) the SEC requests or requires such Tax opinion, each
party hereto shall use reasonable efforts to execute and deliver customary Tax representation letters as the applicable Tax advisor may
reasonably request in form and substance reasonably satisfactory to such Tax advisor. In the event the SEC requests or requires a Tax
opinion with respect to the Mergers, Parent shall use reasonable best efforts to cause its legal counsel to deliver such Tax opinion,
subject to customary assumptions and limitations, to Parent, and the Company shall use reasonable best efforts to cause a nationally recognized
accounting firm to deliver such Tax opinion, subject to customary assumptions and limitations, to the Company.
Section 7.13
Stock Exchange Listing. Pubco will cause the Pubco Ordinary Shares and the Pubco Assumed Parent
Warrants issued in connection with the Transactions to be approved for listing on the Nasdaq Capital Market at the Closing. During the
period from the date hereof until the Closing, Parent shall keep the Parent Common Stock, Parent Rights and Parent Warrants listed for
trading on the Nasdaq Capital Market.
Section 7.14
Antitrust.
(a)
To the extent required under any Laws that are designed to prohibit, restrict or regulate actions having the purpose or effect
of monopolization or restraint of trade, including the HSR Act (“Antitrust Laws”), each party hereto agrees
to promptly make any required filing or application under Antitrust Laws, as applicable, and no later than ten (10) Business Days after
the date of this Agreement, the Company and Parent each shall file (or cause to be filed) with the Antitrust Division of the U.S. Department
of Justice and the U.S. Federal Trade Commission a Notification and Report Form as required by the HSR Act. The parties hereto agree to
supply as promptly as reasonably practicable any additional information and documentary material that may reasonably be requested pursuant
to Antitrust Laws and to take all other actions necessary, proper or advisable to cause the expiration or termination of the applicable
waiting periods or obtain required approvals, as applicable under Antitrust Laws as soon as practicable.
(b) During the Interim Period,
Parent and the Company each shall, in connection with its efforts to obtain all requisite approvals and expiration or termination of
waiting periods for the Transactions under any Antitrust Law, use its reasonable best efforts to: (i) cooperate in all respects
with each other party or its Affiliates in connection with any filing or submission and in connection with any investigation or other
inquiry, including any proceeding initiated by a private Person; (ii) keep the other reasonably informed of any communication received
by such party from, or given by such party to, any Governmental Authority and of any communication
received or given in connection with any proceeding by a private Person, in each case regarding any of the Transactions, and promptly
furnish the other with copies of all such written communications; (iii) permit the other to review in advance any written communication
to be given by it to, and consult with each other in advance of any meeting or conference with, any Governmental Authority or, in connection
with any proceeding by a private Person, with any other Person, and to the extent permitted by such Governmental Authority or other Person,
give the other party the opportunity to attend and participate in such meetings and conferences; (iv) in the event a party is prohibited
from participating in or attending any meetings or conferences, keep such party promptly and reasonably apprised with respect thereto;
and (v) cooperate in the filing of any memoranda, white papers, filings, correspondence or other written communications explaining or
defending the Transactions, articulating any regulatory or competitive argument, or responding to requests or objections made by any
Governmental Authority; provided that materials required to be provided pursuant to this Section 7.14(b) may be
limited to outside counsel and may be redacted (x) to remove references to the valuation of the Company, and (y) as necessary
to comply with contractual arrangements.
(c)
No party hereto shall take any action that could reasonably be expected to adversely affect or materially delay the approval of
any Governmental Authority, or the expiration or termination of any waiting period of any required filings or applications under Antitrust
Laws, including by agreeing to merge with or acquire any other Person or acquire a substantial portion of the assets of or equity in any
other Person. The parties hereto further covenant and agree, with respect to a threatened or pending preliminary or permanent injunction
or other order, decree or ruling or statute, rule, regulation or executive order that would adversely affect the ability of the parties
to consummate the Transactions, to use reasonable best efforts to prevent or lift the entry, enactment or promulgation thereof, as the
case may be.
Section 7.15 Trust
Account. As of the Parent Merger Effective Time, the obligations of Parent to dissolve or
liquidate within a specified time period as contained in the Parent Certificate of Incorporation will be terminated and Parent shall
have no obligation whatsoever to dissolve and liquidate the assets of Parent by reason of the consummation of the Mergers or
otherwise, and, except to the extent they elect to redeem their shares of Parent Common Stock in connection with the Mergers
pursuant to the Parent Organizational Documents, no stockholder of Parent shall be entitled to receive any amount from the Trust
Account; provided that the foregoing shall not modify or restrict the obligations of Parent to consummate the redemption of
any shares of Parent Common Stock pursuant to a valid exercise of Redemption Rights prior to the Parent Merger Effective Time in
accordance with the Parent Organizational Documents. At least forty-eight (48) hours prior to the Parent Merger Effective Time,
Parent shall provide notice to the Trustee in accordance with the Trust Agreement and shall deliver any other documents, opinions or
notices required to be delivered to the Trustee pursuant to the Trust Agreement and cause the Trustee prior to the Parent Merger
Effective Time to, and the Trustee shall thereupon be obligated to, transfer all funds held in the Trust Account to Parent (other
than funds required to be paid from the Trust Account to stockholders of the Parent that elected to redeem their shares of Parent
Common Stock in connection with the Mergers pursuant to the Parent Organizational Documents pursuant to the Trust Agreement) (to be
held as available cash on the balance sheet of Parent, and to be used to pay (a) as and when due all amounts payable to the
stockholders of Parent holding shares of Parent Common Stock in the event they elect to redeem their Parent Common Stock pursuant to
the Parent Organizational Documents, (b) any Outstanding Transaction Expenses payable by Parent on the Closing Date pursuant
to Section 3.06 or (c) for working capital and other general corporate purposes of the business
following the Closing) and thereafter shall cause the Trust Account and the Trust Agreement to terminate.
Section 7.16
Directors. Parent and Pubco shall take all necessary action to cause the Pubco Board as of
and immediately after the Company Merger Effective Time to be comprised of the individuals designated by Parent and the Company pursuant
to Section 2.05.
Section 7.17
Equity Incentive Plan. Prior to the Closing and effective as of the Closing, Parent shall,
and shall cause Pubco to, adopt the Pubco LTIP.
Section 7.18
Related Party Agreements. Prior to the Closing, (a) the Company shall have terminated, or
caused to be terminated, all Contracts set forth in Section 4.20 of the Company Disclosure Schedule and any other Contracts between
the Company and any of its directors, officers or holder of more than ten percent (10%) of the Capital Stock (assuming the full conversion
or exercise of all Company Securities held by such Person), or any immediate family member of any of the foregoing (whether directly or
indirectly through an Affiliate of such Person) (a “Related Party”) or that would otherwise be required to be
disclosed pursuant to Item 404 of Regulation S-K without any liability to the Company, other than (i) ordinary course agreements relating
to director and employee compensation and benefits and (ii) the Contracts set forth on Section 7.18 of the Company Disclosure Schedule,
and (b) Parent shall have terminated, or caused to be terminated, all Parent Related Party Transactions, other than the Contracts set
forth on Section 7.18 of the Parent Disclosure Schedule.
Section 7.19
Assignment of Legacy Parent Transaction Expenses. Prior to the close of business on the Business
Day immediately preceding the Closing Date, Parent shall (a) obtain the consent, in form and substance reasonably acceptable to the Company,
of each payee of Legacy Transaction Expenses set forth on Section 1.01 of the Parent Disclosure Schedule, and each payee of any
other transaction expenses reasonably determined by Parent and the Company to have been omitted from Section 1.01 of the Parent
Disclosure Schedule but which would otherwise constitute Legacy Parent Transaction Expenses, to the assignment of such Legacy Parent Transaction
Expenses to the Sponsor, and that such payee will not seek any recourse from Pubco or any of its Subsidiaries (including, following the
Company Merger Effective Time, the Company) with respect to such Legacy Parent Transaction Expenses, and (b) assign to the Sponsor all
of the Legacy Parent Transaction Expenses set forth on Section 1.01 of the Parent Disclosure Schedule and any other transaction
expenses reasonably determined by Parent and the Company to have been omitted from Section 1.01 of the Parent Disclosure Schedule
but which would otherwise constitute Legacy Parent Transaction Expenses.
Section 7.20 PIPE
Investment. Each of the Company, the Parent and Pubco agree that each shall use their commercially reasonable efforts to enter
into and consummate subscription agreements with investors relating to a private placement of shares (including, for the avoidance
of doubt, preferred equity) in the Company, the Parent and/or Pubco, convertible loan agreements with the Company, Parent and/or
Pubco, and/or enter into backstop arrangements with potential investors provided always that the terms of any such private placement
or backstop arrangement must be mutually agreeable to, and approved in advance in writing by each of the Company, Parent and Pubco
(a “PIPE Investment”). Each of the Company, Parent and Pubco shall use, and shall cause their respective
representatives to use, their respective commercially reasonable efforts to cause such PIPE Investment to occur, and having the
senior management of the Company, Parent and/or Pubco participate in any investor meetings and roadshows with respect to a PIPE
Investment as reasonably requested; provided, that any such PIPE Investment must not adversely impact the Intended Tax Treatment.
Each of the Company, Parent and Pubco agree that Parent or Pubco may pursue a PIPE Investment on terms that are aligned with those
attached as Schedule 7.20; provided, however, that, notwithstanding Schedule 7.20 or anything to the contrary in this Agreement, the
terms and conditions of any PIPE Investment must still be agreed upon, in writing, by the Company, Parent and Pubco prior to the
offering of such PIPE Investment.
ARTICLE
VIII
CONDITIONS TO THE MERGERs
Section 8.01
Conditions to the Obligations of Each Party. The obligations of the Company and the Parent
Parties to consummate the Transactions, including the Mergers, are subject to the satisfaction or waiver (where permissible) at or prior
to the Closing of the following conditions:
(a)
Company Stockholder Approval. The Company Stockholder Approval
shall have been obtained.
(b)
Parent Stockholders’ Approval. The
Required Parent Stockholder Approval shall have been obtained in accordance with the Proxy Statement, the DGCL, the Parent Organizational
Documents and the rules and regulations of the Nasdaq Capital Market.
(c)
No Order. No Governmental Authority shall have enacted,
issued, promulgated, enforced or entered any Law, rule, regulation, judgment, decree, executive order or award which is then in effect
and has the effect of making the Transactions, including the Mergers, illegal or otherwise prohibiting consummation of the Transactions,
including the Mergers.
(d)
Antitrust Approvals and Waiting Periods. All required filings
under the HSR Act shall have been completed and any applicable waiting period (and any extension thereof) applicable to the consummation
of the Transactions under the HSR Act shall have expired or been terminated.
(e)
Registration Statement. The Registration Statement shall
have been declared effective under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall
be in effect, and no proceedings for purposes of suspending the effectiveness of the Registration Statement shall have been initiated
or be threatened by the SEC.
(f)
Parent Net Tangible Assets. Parent shall have at least $5,000,001
of net tangible assets following the exercise of Redemption Rights in accordance with the Parent Organizational Documents.
Section 8.02 Conditions
to the Obligations of the Parent Parties. The obligations of the Parent Parties to consummate the
Transactions, including the Mergers, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing
of the following additional conditions:
(a)
Representations and Warranties. The representations and
warranties of the Company contained in (i) Section 4.01 (Organization and Qualification; Subsidiaries), Section 4.04
(Authority Relative to This Agreement), Section 4.08 (Absence of Certain Changes or Events) and Section 4.21
(Brokers) shall each be true and correct in all material respects as of the date hereof and as of the Closing Date as though made
on and as of such date (without giving effect to any limitation as to “materiality” or “Company
Material Adverse Effect” or any similar limitation set forth therein), except to the extent of any changes that reflect
actions permitted in accordance with Section 6.01 of this Agreement and except to the extent that any such representation and warranty
expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date,
(ii) Section 4.03 (Capitalization) shall be true and correct in all respects except for de minimis inaccuracies
as of the date hereof and as of the Closing Date as though made on and as of such date (except to the extent of any changes that reflect
actions constituting Permitted Financings and any other actions permitted in accordance with Section 6.01 of this Agreement and
except to the extent that any such representation or warranty expressly is made as of an earlier date, in which case such representation
and warranty shall be true and correct as of such specified date) and (iii) all other representations and warranties of the Company set
forth in ARTICLE IV shall be true and correct (without giving any effect to any limitation as to “materiality”
or “Company Material Adverse Effect” or any similar limitation set forth therein) in all respects as of the
date hereof and as of the Closing Date, as though made on and as of such date, except (A) to the extent that any such representation
and warranty expressly speaks as of an earlier date, in which case such representation and warranty shall be true and correct as of such
earlier date, and (B) where the failure of such representations and warranties to be true and correct (whether as of the Closing
Date or such earlier date) does not result in a Company Material Adverse Effect.
(b)
Agreements and Covenants. The Company shall have performed
or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by
it on or prior to the Closing.
(c)
Officer’s Certificate. The Company shall have delivered
to Parent a certificate (the “Company Officer’s Certificate”), dated as of the Closing Date, signed by
an officer of the Company, certifying as to the satisfaction of the conditions specified in Section 8.02(a), Section 8.02(b)
and Section 8.02(f).
(d)
Material Adverse Effect. Since the date hereof, there shall
not have occurred any Company Material Adverse Effect that is continuing on the Closing Date.
(e)
Lock-Up Agreements. The Specified Stockholders shall have delivered, or have caused to be delivered, to Parent duly executed
copies of the Lock-Up Agreements.
(f) PIPE Investment. The PIPE Investment shall have closed.
(g)
Exclusive License Agreement. The Company shall have delivered to Parent a duly executed copy of an exclusive licensing
agreement with EG Biomed Co., Ltd. granting the Company exclusive rights to EG Biomed Co., Ltd.’s patents, technologies and detection
products, and EG-Breast Cancer Detection-P1-Blood Monitoring Tool for the United States, European and Asian markets.
Section 8.03
Conditions to the Obligations of the Company. The obligations of the Company to consummate
the Transactions, including the Company Merger, are subject to the satisfaction or waiver (where permissible) at or prior to Closing of
the following additional conditions:
(a)
Representations and Warranties. The representations and
warranties of the Parent Parties contained in (i) Section 5.01 (Corporation Organization), Section 5.04 (Authority Relative
to this Agreement), Section 5.08 (Absence of Certain Changes or Events) and Section 5.12 (Brokers) shall
each be true and correct in all material respects as of the date hereof, as of the Effective Date (with respect to representations and
warranties of Pubco and Parent Merger Sub), and as of the Closing Date as though made on and as of such date (without giving effect to
any limitation as to “materiality” or “Parent Material Adverse Effect” or any similar
limitation set forth therein), except to the extent that any changes that reflect actions permitted in accordance with Section 6.02
of this Agreement and except to the extent that any such representation and warranty expressly speaks as of an earlier date, in which
case such representation and warranty shall be true and correct as of such earlier date, (ii) Section 5.03 (Capitalization)
shall be true and correct in all respects except for de minimis inaccuracies as of the date hereof and as of the Closing Date as
though made on and as of such date (except to the extent of any changes that reflect actions permitted in accordance with Section 6.02
of this Agreement and except to the extent that any such representation or warranty expressly is made as of an earlier date, in which
case such representation and warranty shall be true and correct as of such specified date) and (iii) other representations and warranties
of the Parent Parties contained in this Agreement shall be true and correct (without giving any effect to any limitation as to “materiality”
or “Parent Material Adverse Effect” or any similar limitation set forth therein) in all respects as of the date
hereof, as of the Effective Date (with respect to representations and warranties of Pubco and Parent Merger Sub), and as of the Closing
Date, as though made on and as of such date, except (A) to the extent that any such representation and warranty expressly speaks
as of an earlier date, in which case such representation and warranty shall be true and correct as of such earlier date, and (B) where
the failure of such representations and warranties to be true and correct (whether as of the Closing Date or such earlier date) does not
result in a Parent Material Adverse Effect.
(b)
Agreements and Covenants. Each of the Parent Parties shall
have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied
with by it on or prior to the Closing.
(c)
Officer’s Certificate. Parent shall have delivered
to the Company a certificate, dated as of the Closing Date, signed by an officer of Parent, certifying as to the satisfaction of the conditions
specified in Section 8.03(a), Section 8.03(b) and Section 8.03(d).
(d)
D&O Resignations. Except as otherwise agreed by Parent and the Company in writing, all directors and officers of Parent
shall have resigned or otherwise been removed effective as of the Closing.
(e)
Parent Liabilities. At or prior to Closing, Parent shall have paid or otherwise satisfied in full all costs relating to
the Transactions payable by Parent pursuant to Section 3.06(a). To the extent Parent’s total liabilities at the time
of Closing exceed $50,000 (the “Parent Closing Liability Max”), Sponsor shall have agreed to assume, pay or
otherwise satisfy in full any amounts owed by Parent in excess of the Parent Closing Liability Max. All advances made by Sponsor to Parent
prior to Closing shall have been forgiven, assumed by Sponsor in writing without recourse to Parent or otherwise satisfied by Sponsor
and Parent. Parent shall have paid all amounts owed to Parent’s service providers and advisors, including any and all success fees
at or before Closing.
(f)
Material Adverse Effect. Since the date hereof, there shall not have occurred any Parent Material Adverse Effect
that is continuing on the Closing Date.
(g)
Stock Exchange Listing. The Pubco Ordinary Shares shall be listed on the Nasdaq Capital Market as of the Closing
Date.
(h)
Pubco Board. All directors and officers of Pubco that have not been designated to serve as directors and officers of Pubco
as of and immediately following the Company Merger Effective Time pursuant to Section 2.05(b) shall have resigned or been removed
by Pubco prior to the Closing.
(i)
Closing Price of Parent Common Stock. The closing price of a share of Parent Common Stock on the OTCQX on the trading date
immediately preceding the Closing Date shall not be less than $8.00 per share.
ARTICLE
IX
TERMINATION, AMENDMENT AND WAIVER
Section 9.01
Termination. This Agreement may be terminated and the Mergers and the other Transactions may
be abandoned at any time prior to the Closing, notwithstanding any requisite approval and adoption of this Agreement and the Transactions
by the stockholders of the Company or Parent, as follows:
(a)
by mutual written consent of Parent and the Company;
(b)
by written notice from either Parent or the Company to the other on or after April 30, 2025 (such date, as may be so extended by
mutual agreement of the parties);
(c) by written notice
from either Parent or the Company to the other if any Governmental Authority in the United States shall have enacted, issued,
promulgated, enforced or entered any injunction, order, decree or ruling (whether temporary, preliminary or permanent) which has
become final and nonappealable and has the effect of making consummation of the Transactions,
including the Mergers, illegal or otherwise preventing or prohibiting consummation of the Transactions;
(d)
by written notice from either Parent or the Company to the other if the Parent Stockholders’ Meeting has been held (including
any adjournment or postponement thereof permitted by Section 7.02(a)), has concluded, the Parent stockholders have duly voted and
the Required Parent Stockholder Approval has not been obtained;
(e)
by the Company if Parent shall have failed to deliver the consent of Parent, as the sole stockholder of Pubco, and the consents
of Pubco, as the sole stockholder of each of the Merger Subs, to the adoption of this Agreement and the approval of the Transactions within
twenty-four (24) hours after the execution of this Agreement;
(f)
by written notice from Parent to the Company if the Stockholder Support Agreements have not been delivered by a number of Company
stockholders sufficient to deliver the Company Stockholder Approval within thirty (30) days of the execution and delivery of this Agreement;
provided, however, that if the Stockholder Support Agreements signed by such number of holders have been delivered, Parent
may not terminate this Agreement pursuant to this Section 9.01(f);
(g)
by written notice from Parent to the Company if the Company shall have failed to obtain the Company Stockholder Approval within
five (5) Business Days after the Registration Statement becomes effective; provided, however, that if the Written Consent
evidencing the Requisite Approval has been obtained, Parent may not terminate this Agreement pursuant to this Section 9.01(g);
(h)
by written notice from Parent to the Company upon a breach of any representation, warranty, covenant or agreement on the part of
the Company set forth in this Agreement, or if any representation or warranty of the Company shall have become untrue, in either case
such that the conditions set forth in Sections Section 8.02(a) and 8.02(b) would not be satisfied (“Terminating
Company Breach”); provided that Parent has not waived such Terminating Company Breach and the Parent Parties are
not then in material breach of their representations, warranties, covenants or agreements in this Agreement; provided, further,
that if such Terminating Company Breach is curable by the Company, Parent may not terminate this Agreement under this Section 9.01(h)
for so long as the Company continues to exercise its reasonable efforts to cure such breach, unless such breach is not cured within thirty
(30) days after notice of such breach is provided by Parent to the Company; or
(i) by
written notice from the Company to Parent upon a breach of any representation, warranty, covenant or agreement on the part of the
Parent Parties set forth in this Agreement, or if any representation or warranty of a Parent Party shall have become untrue, in
either case such that the conditions set forth in Sections Section 8.03(a) and 8.03(b) would not be satisfied
(“Terminating Parent Breach”); provided that the Company has not waived such Terminating Parent
Breach and the Company is not then in material breach of its representations, warranties, covenants or agreements in this Agreement; provided, further,
that if such Terminating Parent Breach is curable by the Parent Parties, the Company may not terminate this Agreement under this Section
9.01(i) for so long as the Parent Parties continue to exercise their reasonable efforts to
cure such breach, unless such breach is not cured within thirty (30) days after notice of such breach is provided by the
Company to Parent.
Section 9.02
Effect of Termination.
(a)
In the event of the termination of this Agreement pursuant to Section 9.01, this Agreement shall forthwith become void
and have no effect, without any liability on the part of any party hereto or its respective Affiliates, officers, directors, employees
or stockholders, other than liability of any party hereto for any Willful Breach by such party occurring prior to such termination subject
to Section 6.03. The provisions of Section 6.03, Section 7.04(b) and Article X (collectively,
the “Surviving Provisions”) and the Non-Disclosure Agreement, and any other Section or Article of this
Agreement referenced in the Surviving Provisions, which are required to survive in order to give appropriate effect to the Surviving Provisions,
shall in each case survive any termination of this Agreement.
(b)
If this Agreement is validly terminated by Parent (i) pursuant to and in accordance with Section 9.01(g) or Section 9.01(h)
(provided that such breach by the Company is a Willful Breach), the Company shall pay to Parent (by wire transfer of immediately
available funds), within two (2) Business Days after such termination, a fee in an amount equal to the actual documented monthly extension
payments made by or on behalf of the Company to the Trust Account on or after the Effective Date (provided that Parent shall provide
in good faith the amount of such payments no later than one (1) Business Day following such termination), which such amount shall constitute
liquidated damages under this Agreement and which amount shall not exceed $150,000 (the “Break-Up Fee”).
(c)
The Parent Parties agree that in the event this Agreement is terminated by Parent pursuant to Section 9.01(g) or Section
9.01(h) and the Break-Up Fee is paid to Parent pursuant to Section 9.02(b), (i) the payment of such Break-Up Fee shall be the
sole and exclusive remedy (whether at law, in equity, in contract, in tort or otherwise) of the Parent Parties and their respective equityholders
and Affiliates against the Company or any of its directors, officers and other Affiliates for, and (ii) in no event will the Parent Parties
or any of their respective equityholders or Affiliates be entitled to recover any other money damages or any other remedy based on a claim
in law or equity with respect to, (A) any loss suffered as a result of the failure of the Mergers to be consummated, (B) the termination
of this Agreement, (C) any liabilities or obligations arising under this Agreement or (D) any claims or Actions arising out of or relating
to any breach, termination or failure of or under this Agreement, and upon payment to Parent of the Break-Up Fee in accordance with Section
9.02(b), neither the Company nor any of its directors, officers or other Affiliates shall have any further liability or obligation
to the Parent Parties or any of their equityholders or Affiliates relating to or arising out of this Agreement or the Transactions.
Section 9.03
Amendment. This Agreement may be amended in writing by the parties hereto at any time prior
to the Closing. This Agreement may not be amended except by an instrument in writing signed by each of the parties hereto.
Section 9.04 Waiver.
At any time prior to the Closing, (a) Parent may (i) extend the time for the performance of any obligation or other act of
the Company, (ii) waive any inaccuracy in the representations and warranties of the Company contained herein or in any document
delivered by the Company pursuant hereto and (iii) waive compliance with any agreement of the Company or any condition
to its own obligations contained herein and (b) the Company may (i) extend the time for the performance of any obligation
or other act of any Parent Party, (ii) waive any inaccuracy in the representations and warranties of any Parent Party contained
herein or in any document delivered by any Parent Party pursuant hereto and (iii) waive compliance with any agreement of any
Parent Party or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in
an instrument in writing signed by the party or parties to be bound thereby. No failure or delay by any party in exercising any
right, power or privilege hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude any
other or further exercise thereof or the exercise of any other right, power or privilege.
ARTICLE
X
GENERAL PROVISIONS
Section 10.01 Notices.
All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be
deemed to have been duly given upon receipt) by delivery in person, by email or by registered or certified mail (postage prepaid,
return receipt requested) to the respective parties at the following addresses (or at such other address for a party as shall be
specified in a notice given in accordance with this Section 10.01):
if to the Parent Parties:
Breeze Holdings Acquisition Corp.
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039
Attention: J. Douglas Ramsey, Ph.D.
Email: doug@breezeacquisition.com
with a copy to:
Woolery & Co.
1 Pier 76
408 12th Ave
New York, NY 10018
Attention: Jim Woolery; Mathew J. Saur
Email: james@wooleryco.com; matt@ekpyrosis.co
if to the Company:
YD Biopharma Limited
12th Floor, No. 101, Section 2
Nanjing East Road, Zhongshan District
Taipei City, Taiwan 54186159
Attn: Dr. Ethan Shen
E-mail: ethanshen@eg-bio.com
with a copy to:
ArentFox Schiff LLP
1717 K Street NW
Suite 700
Washington, DC 20006
Attention: Ralph V. De Martino
Email: ralph.demartino@afslaw.com
Section 10.02 Nonsurvival
of Representations, Warranties and Covenants. None of the representations, warranties, covenants,
obligations or other agreements in this Agreement or in any certificate, statement or instrument delivered pursuant to this
Agreement, including any rights arising out of any breach of such representations, warranties, covenants, obligations, agreements
and other provisions, shall survive the Closing and all such representations, warranties, covenants, obligations or other agreements
shall terminate and expire upon the occurrence of the Closing (and there shall be no liability after the Closing in respect
thereof), except for (a) those covenants and agreements contained herein that by their terms expressly apply in whole or in
part after the Closing and then only with respect to any breaches occurring after the Closing and (b) this ARTICLE X and
any corresponding definitions set forth in ARTICLE I.
Section 10.03 Severability.
If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of law, or public
policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the
economic or legal substance of the Transactions is not affected in any manner materially adverse to any party. Upon such
determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall
negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a
mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the fullest extent
possible.
Section 10.04 Entire
Agreement; Assignment. This Agreement and the Ancillary Agreements constitute the entire agreement
among the parties with respect to the subject matter hereof and supersede all prior agreements and undertakings, both written and
oral, among the parties, or any of them, with respect to the subject matter hereof, except for the Non-Disclosure Agreement or as
set forth in Section 7.04(b). This Agreement shall not be assigned (whether pursuant to a merger, by operation of law
or otherwise) by any party without the prior express written consent of the other parties hereto.
Section 10.05 Parties
in Interest. This Agreement shall be binding upon and inure solely to the benefit of each party
hereto, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other Person any right, benefit
or remedy of any nature whatsoever under or by reason of this Agreement, other than Section 7.08 (which is intended to be for
the benefit of the Persons covered thereby and may be enforced by such Persons).
Section 10.06 Governing
Law. This Agreement shall be governed by, and construed in accordance with, the Laws of the State
of Delaware applicable to contracts executed in and to be performed in that State. All legal actions and proceedings arising out of
or relating to this Agreement shall be heard and determined exclusively in any Delaware Chancery Court; provided that, if
jurisdiction is not then available in the Delaware Chancery Court, then any such legal Action may be brought in any federal court
located in the State of Delaware or any other Delaware state court. The parties hereto hereby (a) irrevocably submit to the
exclusive jurisdiction of the aforesaid courts for themselves and with respect to their respective properties for the purpose of any
Action arising out of or relating to this Agreement brought by any party hereto, and (b) agree not to commence any Action
relating thereto except in the courts described above in Delaware, other than Actions in any court of competent jurisdiction to
enforce any judgment, decree or award rendered by any such court in Delaware as described herein. Each of the parties further agrees
that notice as provided herein shall constitute sufficient service of process and the parties further waive any argument that such
service is insufficient. Each of the parties hereby irrevocably and unconditionally waives, and agrees not to assert, by way of
motion or as a defense, counterclaim or otherwise, in any Action arising out of or relating to this Agreement or the transactions
contemplated hereby, (a) any claim that it is not personally subject to the jurisdiction of the courts in Delaware as described
herein for any reason, (b) that it or its property is exempt or immune from jurisdiction of any such court or from any legal
process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of
judgment, execution of judgment or otherwise) and (c) that (i) the Action in any such court is brought in an inconvenient
forum, (ii) the venue of such Action is improper or (iii) this Agreement, or the subject matter hereof, may not be
enforced in or by such courts.
Section 10.07 Waiver
of Jury Trial. Each of the parties hereto hereby waives to the fullest extent permitted by
applicable Law any right it may have to a trial by jury with respect to any litigation directly or indirectly arising out of, under
or in connection with this Agreement or the Transactions. Each of the parties hereto (a) certifies that no representative,
agent or attorney of any other party has represented, expressly or otherwise, that such other party would not, in the event of
litigation, seek to enforce that foregoing waiver and (b) acknowledges that it and the other hereto have been induced to enter
into this Agreement and the Transactions, as applicable, by, among other things, the mutual waivers and certifications in this Section
10.07.
Section 10.08 Headings.
The descriptive headings contained in this Agreement are included for convenience of reference only and shall not affect in any way
the meaning or interpretation of this Agreement.
Section 10.09 Counterparts;
Electronic Delivery. This Agreement and each other Transaction Document may be executed and
delivered (including by facsimile or portable document format (pdf) transmission) in one or more counterparts, and by the
different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which
taken together shall constitute one and the same agreement. Delivery by email to counsel for the other parties of a counterpart
executed by a party shall be deemed to meet the requirements of the previous sentence.
Section 10.10
Specific Performance. The parties agree that irreparable damage would occur if any provision
of this Agreement were not performed in accordance with the terms hereof, and, accordingly, that the parties shall be entitled to an injunction
or injunctions to prevent breaches of this Agreement or to enforce specifically the performance of the terms and provisions hereof (including
the parties’ obligation to consummate the Mergers) in the Court of Chancery of the State of Delaware or, if that court does not
have jurisdiction, any court of the United States located in the State of Delaware without proof of actual damages or otherwise, in addition
to any other remedy to which they are entitled at law or in equity as expressly permitted in this Agreement. Each of the parties hereby
further waives (a) any defense in any action for specific performance that a remedy at law would be adequate and (b) any requirement
under any Law to post security or a bond as a prerequisite to obtaining equitable relief.
Section 10.11 No
Recourse. Except in the case of Fraud, all actions, claims, obligations, liabilities or causes of
actions (whether in contract or in tort, in law or in equity, or granted by statute whether by or through attempted piercing of the
corporate, limited partnership or limited liability company veil) that may be based upon, in respect of, arise under, out or by
reason of, be connected with, or relate in any manner to: (a) this Agreement, (b) the negotiation, execution or
performance of this Agreement (including any representation or warranty made in, in connection with, or as an inducement to, this
Agreement), (c) any breach of this Agreement and (d) any failure of the Mergers to be consummated, may be made only
against (and, without prejudice to the rights of any express third party beneficiary to whom rights under this Agreement inure
pursuant to Section 10.11), are those solely of the Persons that are expressly identified as parties to this Agreement and
not against any Nonparty Affiliate (as defined below). Except in the case of Fraud, no other Person, including any director,
officer, employee, incorporator, member, partner, manager, stockholder, optionholder, Affiliate, agent, attorney or representative
of, or any financial advisor or lender to, any party to this Agreement, or any director, officer, employee, incorporator, member,
partner, manager, stockholder, Affiliate, agent, attorney or representative of, or any financial advisor or lender to (each of the
foregoing, a “Nonparty Affiliate”) any of the foregoing shall have any liabilities (whether in contract or in
tort, in law or in equity, or granted by statute whether by or through attempted piercing of the corporate, limited partnership or
limited liability company veil) for any claims, causes of action, obligations or liabilities arising under, out of, in connection
with or related in any manner to the items in the immediately preceding clauses (a) through (d) and each party, on behalf of
itself and its Affiliates, hereby irrevocably releases and forever discharges each of the Nonparty Affiliate from any such liability
or obligation.
Section 10.12 Conflicts
and Privilege. Each of Pubco, Parent and the Company hereby agrees on behalf of its directors,
members, partners, officers, employees and Affiliates and each of their respective successors and assigns (including, after the
Closing, the Parent Surviving Subsidiary and the Company Surviving Subsidiary) (all such Parties the “Waiving
Parties”), that ArentFox Schiff LLP (“AFS”) may represent the shareholders or holders
of other equity interests of the Company or any of their respective directors, members, partners, officers, employees or Affiliates
(other than the Company Surviving Subsidiary (collectively, the “YD Group”)), in each case solely in
connection with any Action or obligation arising out of or relating to this Agreement, any other Transaction Documents or the
transactions contemplated hereby or thereby, notwithstanding its prior representation of the Company and its Subsidiaries or other
Waiving Parties, and each of Pubco, Parent and the Company, on behalf of itself and the Waiving Parties hereby consents thereto and
irrevocably waives (and will not assert) any conflict of interest, breach of duty or any other objection arising from or relating to
AFS’s prior representation of the Company, its Subsidiaries or of Waiving Parties. Each of Pubco, Parent and the Company, for
itself and the Waiving Parties, hereby further irrevocably acknowledges and agrees that all privileged communications, written or
oral, between the Company and its Subsidiaries or any member of the Waiving Parties and AFS, made in connection with the
negotiation, preparation, execution, delivery and performance under, or any dispute or Action arising out of or relating to, this
Agreement, any other Transaction Documents or the transactions contemplated hereby or thereby, or any matter relating to any of the
foregoing, are privileged communications that do not pass to the Company Surviving Subsidiary notwithstanding the Company Merger,
and instead survive, remain with and are controlled by the YD Group (the “Privileged Communications”),
without any waiver thereof. Parent, Pubco and the Company, together with any of their respective Affiliates, Subsidiaries,
successors or assigns, agree that no Person may use or rely on any of the Privileged Communications, whether located in the records
or email server of the Company Surviving Subsidiary and its Subsidiaries, in any Action against or involving any of the Parties
after the Closing, and Parent, Pubco and the Company agree not to assert that any privilege has been waived as to the Privileged
Communications, by virtue of the Mergers.
[Signature Page Follows]
IN WITNESS WHEREOF, Parent,
Pubco, Parent Merger Sub, Company Merger Sub and the Company have caused this Agreement to be executed as of the date first written above
by their respective officers thereunto duly authorized.
| |
BREEZE HOLDINGS ACQUISITION CORP.
|
| |
|
|
|
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
|
Name: |
J. Douglas Ramsey, Ph.D. |
| |
|
Title: |
CEO & CFO |
| |
|
|
|
BREEZE MERGER SUB, INC. |
| |
|
|
|
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
|
Name: |
J. Douglas Ramsey, Ph.D. |
| |
|
Title: |
President |
| |
|
|
|
YD BIOPHARMA LIMITED |
| |
|
|
|
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
|
Name: |
Dr. Hsieh Tsung Shen |
| |
|
Title: |
CEO |
[Signature Page to Merger Agreement and Plan of
Reorganization]
Exhibit 10.1
Execution Version
SPONSOR SUPPORT AGREEMENT
This SPONSOR SUPPORT AGREEMENT
(this “Agreement”), dated as of September 24, 2024, is made by and among Breeze Holdings Acquisition Corp.,
a Delaware corporation (“Parent”), YD Biopharma Limited, a Cayman Islands exempted company (the “Company”),
Breeze Sponsor, LLC, a Delaware limited liability company (the “Sponsor”), and the undersigned stockholders of Parent
(the “Parent Stockholders” and together with the Sponsor, the “Parent Initial Stockholders”).
Parent, the Company and each of the Parent Initial Stockholders are sometimes referred to herein individually as a “Party”
and collectively as the “Parties”. Capitalized terms used but not otherwise defined herein shall have the meanings
ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
A. On
September 24, 2024, Parent, the Company, a Cayman Islands exempted company and a wholly-owned subsidiary of Parent, expected to be named
“YD Bio Limited,” which is in the process of being formed by the Parent, and once formed, the Parent shall cause it to enter
into a joinder to this Agreement (“Pubco”), Breeze Merger Sub, Inc., a Delaware corporation that will be a direct,
wholly-owned Subsidiary of Pubco (“Parent Merger Sub”), and a Cayman Islands exempted company and that will
be a wholly-owned subsidiary of Pubco, expected to be named “BH Biopharma Merger Sub Limited,” which is in the process of
being formed by the Parent, and once formed, the Parent shall cause it to enter into a joinder to this Agreement (“Company
Merger Sub”), entered into that certain Merger Agreement and Plan of Reorganization (the “Merger Agreement”),
pursuant to which, among other matters, (a) Parent Merger Sub will merge with and into Parent, with Parent continuing as the surviving
company (the “Parent Merger”), and with the security holders of Parent receiving substantially equivalent securities
of Pubco and (b) Company Merger Sub will merge with and into the Company, with the Company continuing as the surviving company (the “Company
Merger”; Company Merger and the Parent Merger are together referred to herein as the “Mergers”),
and with the shareholders of the Company receiving Pubco Ordinary Shares, and as a result of the Mergers, Parent and the Company will
become wholly-owned subsidiaries of Pubco, and Pubco will become a publicly traded company listed on Nasdaq, all upon the terms and subject
to the conditions set forth in the Merger Agreement and in accordance with the provisions of applicable law.
B. Each
of the Parent Initial Stockholders is the record and beneficial owner of the number of issued and outstanding shares of Parent set forth
on Schedule A hereto (the “Shares”).
C. In
order to induce Parent and the Company to enter into the Merger Agreement and the Specified Stockholders to enter into the Stockholder
Support Agreement, the Parties desire to enter into this Agreement on the terms and conditions set forth herein.
AGREEMENT
NOW, THEREFORE, in consideration
of the premises and the mutual promises contained herein, and for other good and valuable consideration, the receipt and sufficiency of
which are hereby acknowledged, the Parties, each intending to be legally bound, hereby agree as follows:
1. Agreement
to Vote; No Redemption. Each of the Parent Initial Stockholders hereby irrevocably and unconditionally agrees (a) to vote, at
any meeting of the stockholders of Parent or in any action by written consent of the stockholders of Parent, all of such Parent Initial
Stockholder’s Shares (together with any other shares of Parent that such Parent Initial Stockholder acquires record or beneficial
ownership of or the power to vote after the date hereof, collectively, the “Subject Parent Stock”) (i) in favor of
the Parent Proposals, including without limitation the adoption of the Merger Agreement and approval of the Transactions, and if necessary
and applicable, the Extension Proposal, and (ii) against, and withhold consent with respect to, any other matter, action, agreement, transaction
or proposal that would reasonably be expected to result in (A) a breach of any of the Parent Parties’ representations, warranties,
covenants, agreements or obligations under the Merger Agreement or (B) any of the conditions to the Closing set forth in Sections
8.01 or 8.03 of the Merger Agreement not being satisfied, (b) if a meeting of the stockholders of Parent is held in respect of the matters
set forth in clause (a), to appear at such meeting, in person or by proxy, or otherwise cause all of its Subject Parent Stock to be counted
as present thereat for purposes of establishing a quorum, and (c) not to redeem, elect to redeem or tender or submit any of its Subject
Parent Stock for redemption in connection with such Parent stockholder approval, the Mergers or the other Transactions, or if necessary
and applicable, the Extension Proposal. Prior to any valid termination of the Merger Agreement, each of the Parent Initial Stockholders
shall take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary under applicable Laws to
consummate the Mergers and the other Transactions on the terms and subject to the conditions set forth therein. The obligations of the
Parent Initial Stockholders specified in this Section 1 shall apply whether or not the Mergers, any of the other Transactions or
any action described above, or if necessary and applicable, the Extension Proposal, is recommend by the Parent Board. Each of the Parent
Initial Stockholders acknowledges receipt and review of a copy of the Merger Agreement.
2. Waiver
of Anti-dilution Protection. Each of the Parent Initial Stockholders hereby irrevocably (a) waives, subject to, and conditioned upon,
the occurrence of the Closing, to the fullest extent permitted by applicable Law and the Parent Organizational Documents, and (b) agrees
not to assert or perfect, any rights to adjustment or other anti-dilution protections to which such Parent Initial Stockholder may be
entitled in connection with the Mergers or the other Transactions, or if necessary and applicable, the Extension Proposal.
3. Transfer
of Shares.
(a) Each
of the Parent Initial Stockholders hereby agrees that it shall not, directly or indirectly, (i) sell, assign, transfer (including
by operation of law), place a lien on, pledge, hypothecate, grant an option to purchase, distribute, dispose of or otherwise encumber
any of its Subject Parent Stock or otherwise enter into any contract, option or other arrangement or undertaking to do any of the foregoing
(each, a “Transfer”), (ii) deposit any of its Subject Parent Stock into a voting trust or enter into a
voting agreement or arrangement or grant any proxy or power of attorney with respect to any of its Subject Parent Stock that conflicts
with any of the covenants or agreements set forth in this Agreement or (iii) take any action that would have the effect of preventing
or materially delaying the performance of its obligations hereunder; provided, however, that the foregoing shall not apply
to any Transfer (A) to an Affiliate of such Parent Initial Stockholder, (B) to another Parent Initial Stockholder that is a Party
and bound by the terms and obligations hereof or (C) made in connection with the Mergers or the other Transactions; provided,
that any transferee of any Transfer of the type set forth in clause (A) must enter into a joinder agreement agreeing to become a Party.
(b) In
furtherance of the foregoing, Parent hereby agrees to (i) place a revocable stop order on all Subject Parent Stock subject to Section 3(a),
including those which may be covered by a registration statement, and (ii) notify Parent’s transfer agent in writing of such
stop order and the restrictions on such Subject Parent Stock under Section 3(a) and direct Parent’s transfer agent not
to process any attempts by any Parent Initial Stockholder to Transfer any Subject Parent Stock except in compliance with Section 3(a)
and which shall first be approved by Parent; for the avoidance of doubt, the obligations of Parent under this Section 3(b)
shall be deemed to be satisfied by the existence of any similar stop order and restrictions currently existing on the Subject Parent Stock.
4. Reserved.
5. Other
Covenants and Agreements.
(a) Each
of the Parent Initial Stockholders hereby agrees to be bound by and subject to (i) Section 7.04(b) (Confidentiality) and Section
7.11 (Public Announcements) of the Merger Agreement to the same extent as such provisions apply to the parties to the Merger Agreement
and (ii) Section 7.06 (Exclusivity) and Section 7.02 (Parent Stockholders’ Meeting; Pubco and Merger Subs Stockholder’s
Approval) of the Merger Agreement to the same extent as such provisions apply to Parent, in each case, mutatis mutandis and as
if such Parent Initial Stockholder was a party thereto.
(b) The
Sponsor hereby agrees in accordance with Section 7.19 of the Merger Agreement to assume and pay all of the Legacy Parent Transaction Expenses
in full and will indemnify Pubco, Parent, the Company and their respective Subsidiaries from any and all liabilities relating to the Legacy
Parent Transaction Expenses. Notwithstanding anything herein to the contrary, the Sponsor agrees that it shall not Transfer any of its
Shares or distribute any of its assets unless and until such time as it has assumed and paid in full all Legacy Parent Transaction Expenses
in accordance with this Section 5(b).
(c) Each
of the Parent Initial Stockholders acknowledges and agrees that the Company is entering into the Merger Agreement and the Specified Stockholders
are entering into the A&R Stockholder Support Agreement in reliance upon the Parent Initial Stockholders entering into this Agreement
and agreeing to be bound by, and perform, or otherwise comply with, as applicable, the agreements, covenants and obligations contained
in this Agreement, and but for the Parent Initial Stockholders entering into this Agreement and agreeing to be bound by, and perform,
or otherwise comply with, as applicable, the agreements, covenants and obligations contained in this Agreement, the Company would not
have entered into the Merger Agreement or agreed to consummate the Transactions and the Specified Stockholders would not have entered
into the Stockholder Support Agreement or agreed to consummate the transactions contemplated thereby.
6. Representations
and Warranties. Each of the Parent Initial Stockholders, severally and not jointly, represents and warrants to Parent and the Company
as follows:
(a) If
such Parent Initial Stockholder is a natural person, he or she is legally competent to execute and deliver this Agreement. If such Parent
Initial Stockholder is not a natural person, it is duly organized, validly existing and in good standing under the Laws of the jurisdiction
of its incorporation or organization.
(b) The
execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby are within such Parent
Initial Stockholder’s power and have been duly authorized by all necessary actions on the part of such Parent Initial Stockholder.
(c) The
execution and delivery of this Agreement by such Parent Initial Stockholder does not, and the performance by such Parent Initial Stockholder
of his, her or its obligations hereunder will not, (i) conflict with or violate any Law applicable to such Parent Initial Stockholder,
(ii) result in the creation of any Lien on any of its Subject Parent Stock (other than under this Agreement, the Merger Agreement or the
Ancillary Agreements), (iii) if applicable, conflict with or result in a breach or violation of or constitute a default under its
organizational documents, or (iv) require any consent, authorization or approval of, declaration, filing or registration with, or
notice to, any Person, in each case that has not been given or made as of the date hereof.
(d) There
are no Actions pending against such Parent Initial Stockholder or, to the knowledge of such Parent Initial Stockholder, threatened against
such Parent Initial Stockholder, before (or, in the case of threatened Actions, that would be before) any arbitrator or any Governmental
Authority, which in any manner challenges or seeks to prevent, enjoin or materially delay the performance by such Parent Initial Stockholder
of its obligations under this Agreement.
(e) This
Agreement has been duly executed and delivered by such Parent Initial Stockholder and, assuming due authorization, execution and delivery
by the other Parties, this Agreement constitutes a legally valid and binding obligation of such Parent Initial Stockholder, enforceable
against him, her or it in accordance with the terms hereof (except as enforceability may be limited by applicable bankruptcy Laws, other
applicable, similar Laws affecting creditors’ rights and general principles of equity affecting the availability of specific performance
and other equitable remedies),
(f) Such
Parent Initial Stockholder has not entered into, and shall not enter into, any agreement that would restrict, limit or interfere with
the performance of his, her or its obligations hereunder.
(g) Such
Parent Initial Stockholder is the exclusive record and beneficial owner of, and has good and valid title to, all of the Shares set forth
opposite such Parent Initial Stockholder’s name on Schedule A hereto, and there exist no Liens, pledge, proxy, security interest,
option, right of first refusal, adverse claim of ownership or any other limitations or restrictions (including, without limitation, any
restriction on the right to vote, sell or otherwise dispose of such Shares), other than pursuant to (i) this Agreement, (ii) the
Parent Organizational Documents, (iii) the Merger Agreement or the Ancillary Agreements, (iv) the Letter Agreement, dated as of November
23, 2020, by and between Parent and the Sponsor, (v) the Securities Escrow Agreement, dated as of November 23, 2020, by and among Parent,
certain stockholders of Parent and Continental Stock Transfer & Trust Company, and (vi) any applicable securities Laws, and as of
the date of this Agreement, such Parent Initial Stockholder has the sole power (as currently in effect) to vote, and the right, power
and authority to sell, transfer and deliver, such Shares, and such Parent Initial Stockholder does not own, directly or indirectly, any
other Shares.
7. Termination.
This Agreement shall automatically terminate, without any notice or other action by any Party, upon the earliest of (a) the termination
of the Merger Agreement in accordance with its terms and (b) the mutual written agreement of all of the Parties. Upon termination of this
Agreement as provided in the immediately preceding sentence, none of the Parties shall have any further obligations or liabilities under,
or with respect to, this Agreement. Notwithstanding the foregoing or anything to the contrary in this Agreement, (i) the termination of
this Agreement pursuant to Section 7(a) shall not affect any liability on the part of any Party for a Willful Breach
of any covenant or agreement set forth in this Agreement prior to such termination or Fraud, (ii) Sections 2, 6 and
12 shall each survive any termination of this Agreement pursuant to Section 7(a), and (iii) Sections 7
through 16 shall survive any termination of this Agreement. For purposes of this Section 7, “Fraud”
means an act or omission by a Party, and requires: (i) a false or incorrect representation or warranty expressly set forth in this
Agreement, (ii) with actual knowledge (as opposed to constructive, imputed or implied knowledge) by the Party making such representation
or warranty that such representation or warranty expressly set forth in this Agreement is false or incorrect, (iii) an intention
to deceive another Party to induce him, her or it to enter into this Agreement, (iv) another Party, in justifiable or reasonable
reliance upon such false or incorrect representation or warranty expressly set forth in this Agreement, causing such Party to enter into
this Agreement, and (v) causing such Party to suffer damage by reason of such reliance.
8. No
Recourse. Except for claims pursuant to the Merger Agreement or any other Ancillary Agreement by any party thereto against any other
party thereto, each Party agrees that (a) this Agreement may only be enforced against, and any action for breach of this Agreement may
only be made against, the Parties, and no claims of any nature whatsoever (whether in tort, contract or otherwise) arising under or relating
to this Agreement, the negotiation hereof or its subject matter, or the transactions contemplated hereby shall be asserted against any
Affiliate of the Company or any Affiliate of Parent (other than the Parent Initial Stockholders, on the terms and subject to the conditions
set forth herein), and (b) none of the Affiliates of the Company or the Affiliates of Parent (other than the Parent Initial Stockholders,
on the terms and subject to the conditions set forth herein) shall have any liability arising out of or relating to this Agreement, the
negotiation hereof or its subject matter, or the transactions contemplated hereby, including with respect to any claim (whether in tort,
contract or otherwise) for breach of this Agreement or in respect of any written or oral representations made or alleged to be made in
connection herewith, as expressly provided herein, or for any actual or alleged inaccuracies, misstatements or omissions with respect
to any information or materials of any kind furnished in connection with this Agreement, the negotiation hereof or the transactions contemplated
hereby.
9. Notices.
All notices and other communications among the Parties shall be in writing and shall be deemed to have been duly given (a) when delivered
in person, (b) when delivered after posting in the United States mail having been sent registered or certified mail return receipt
requested, postage prepaid, (c) when delivered by FedEx or other nationally recognized overnight delivery service or (d) when
e-mailed during normal business hours (and otherwise as of the immediately following Business Day), addressed as follows:
Breeze Holdings Acquisition Corp.
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039
Attention: J. Douglas Ramsey, Ph.D.
Email: doug@breezeacquisition.com
with a copy to:
Woolery & Co.
1 Pier 76
408 12th Ave
New York, NY 10018
Attention: Jim Woolery; Mathew J. Saur
Email: james@wooleryco.com; matt@ekpyrosis.co
| |
(b) |
If
to the Company, to: |
YD Biopharma Limited
12th Floor, No. 101, Section 2
Nanjing East Road, Zhongshan District
Taipei City, Taiwan 54186159
Attn: Dr. Ethan Shen
E-mail: ethanshen@eg-bio.com
with a copy to:
ArentFox Schiff LLP
1717 K Street NW
Suite 700
Washington, DC 20006
Attention: Ralph V. De Martino
Email: ralph.demartino@afslaw.com
| |
(c) |
If
to the Sponsor, to: |
Breeze Sponsor, LLC
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039
Attention: J. Douglas Ramsey, Ph.D.
Email: doug@breezeacquisition.com
with a copy to:
Woolery & Co.
1 Pier 76
408 12th Ave
New York, NY 10018
Attention: Jim Woolery; Mathew J. Saur
Email: james@wooleryco.com; mathew@wooleryco.com
If to a Parent Initial Stockholder (other than
the Sponsor), to the address or email address set forth for such Parent Initial Stockholder on his, her or its signature page hereof,
or to such other address or addresses as the Parties may from time to time designate in writing.
10. Entire
Agreement. This Agreement and the Merger Agreement constitute the entire agreement of the Parties with respect to the subject matter
of this Agreement and supersede all prior agreements and undertakings, both written and oral, among the Parties with respect to the subject
matter of this Agreement, except as otherwise expressly provided in this Agreement.
11. Amendments
and Waivers; Assignment. Any provision of this Agreement may be amended or waived if, and only if, such amendment or waiver is in
writing and signed by the Parties and the Parties to be bound thereby, respectively. Notwithstanding the foregoing, no failure or delay
by any Party in exercising any right hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude
any other or further exercise of any other right hereunder. Neither this Agreement nor any of the rights, interests or obligations hereunder
shall be assignable by any Party without the prior written consent of the other Parties.
12. Fees
and Expenses. Except as otherwise expressly set forth herein or in the Merger Agreement, all fees and expenses incurred in connection
with this Agreement and the transactions contemplated hereby, including the fees and disbursements of counsel, financial advisors and
accountants, shall be paid by the Party incurring such fees or expenses.
13. Fiduciary
Duties. Notwithstanding anything in this Agreement to the contrary, (a) none of the Parent Initial Stockholders makes any agreement
or understanding herein in any capacity other than in its capacity as a record holder and beneficial owner of the Subject Parent Stock
and (b) nothing herein will be construed to limit or affect any action or inaction by any representative of such Parent Initial Stockholder
in its capacity as a member of the Parent Board or other similar governing body of any of its Affiliates or as an officer, employee or
fiduciary of Parent or any of its Affiliates, in each case, acting in such person’s capacity as a director, officer, employee or
fiduciary of Parent or such Affiliate.
14. Remedies.
Except as otherwise expressly provided herein, any and all remedies provided herein will be deemed cumulative with and not exclusive of
any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude
the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be
an adequate remedy, would occur in the event that a Party does not perform its respective obligations under the provisions of this Agreement
in accordance with their specific terms or otherwise breaches such provisions. It is accordingly agreed that each Party shall be entitled
to an injunction or injunctions, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce
specifically the terms and provisions of this Agreement, in each case, without posting a bond or undertaking and without proof of damages
and this being in addition to any other remedy to which they are entitled at law or in equity. Each Party agrees that it will not oppose
the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this
Agreement on the basis that the other Parties have an adequate remedy at law or an award of specific performance is not an appropriate
remedy for any reason at law or equity.
15. No
Third Party Beneficiaries. This Agreement shall be for the sole benefit of the Parties and their respective successors and permitted
assigns and is not intended, nor shall be construed, to give any Person, other than the Parties and their respective successors and permitted
assigns, any legal or equitable right, benefit or remedy of any nature whatsoever by reason this Agreement. Nothing in this Agreement,
expressed or implied, is intended to or shall constitute the Parties acting as partners or participants in a joint venture.
16. Incorporation
by Reference. Sections 1.03 (Construction)), 10.03 (Severability), 10.06 (Governing Law), 10.07 (Waiver of Jury Trial),
10.08 (Headings) and 10.09 (Counterparts; Electronic Delivery) of the Merger Agreement are incorporated herein by reference
and shall apply to this Agreement mutatis mutandis.
[Signature page follows]
IN WITNESS WHEREOF, each of
the Parties has executed and delivered this Sponsor Support Agreement as of the date first above written.
| |
BREEZE HOLDINGS ACQUISITION CORP. |
| |
|
| |
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
|
Name: |
J. Douglas Ramsey, Ph.D. |
| |
|
Title: |
CEO & CFO |
| |
BREEZE SPONSOR, LLC |
| |
|
| |
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
|
Name: |
J. Douglas Ramsey, Ph.D. |
| |
|
Title: |
Manager |
| |
YD BIOPHARMA LIMITED |
| |
|
| |
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
|
Name: |
Dr. Hsieh Tsung Shen |
| |
|
Title: |
CEO |
[Signature
Page to Sponsor Support Agreement]
IN WITNESS WHEREOF,
each of the Parties has executed and delivered this Sponsor Support Agreement as of the date first above written.
| |
PARENT STOCKHOLDERS: |
| |
|
| |
I-BANKERS SECURITIES, INC. |
| |
|
|
| |
By: |
/s/ Matthew J. McCloskey |
| |
Name: |
Matthew J. McCloskey |
| |
Title: |
President |
| |
Email: |
|
| |
Address: |
|
| |
/s/ Albert McLelland |
| |
Albert McLelland |
| |
Email: |
| |
Address: |
| |
/s/ Daniel L. Hunt |
| |
Daniel L. Hunt |
| |
Email: |
| |
Address: |
| |
/s/ Robert Lee Thomas |
| |
Robert Lee Thomas |
| |
Email: |
| |
Address: |
| |
/s/ Bill Stark |
| |
Bill Stark |
| |
Email: |
| |
Address: |
[Signature
Page to Sponsor Support Agreement]
SCHEDULE A
PARENT SHARES
| Name |
|
Number of Shares of Common Stock |
| Breeze Sponsor, LLC |
|
2,475,000 |
| I-Bankers Securities, Inc. |
|
300,000 |
| Albert McLelland |
|
25,000 |
| Daniel L. Hunt |
|
25,000 |
| Robert Lee Thomas |
|
25,000 |
| Bill Stark |
|
25,000 |
Exhibit 10.2
Execution Version
SHAREHOLDER SUPPORT AGREEMENT
This SHAREHOLDER SUPPORT AGREEMENT (this
“Agreement”), dated as of September 24, 2024, is made by and among Breeze Holdings Acquisition Corp., a Delaware
corporation (“Parent”), YD Biopharma Limited, a Cayman Islands exempted company (the “Company”),
and the undersigned shareholders of the Company (each, a “Shareholder” and collectively, the “Shareholders”).
Parent, the Company and each of the Shareholders are sometimes referred to herein individually as a “Party”
and collectively as the “Parties”. Capitalized terms used but not otherwise defined herein shall have the meanings
ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
A. On
September 24, 2024, Parent, the Company, a Cayman Islands exempted company and a wholly-owned subsidiary of Parent, expected to be named
“YD Bio Holdings Limited,” which is in the process of being formed by the Parent, and once formed, the Parent shall cause
it to enter into a joinder to this Agreement (“Pubco”), Breeze Merger Sub, Inc., a Delaware corporation that
will be a direct, wholly-owned Subsidiary of Pubco (“Parent Merger Sub”), and a Cayman Islands exempted company
that will be a wholly-owned subsidiary of Pubco, expected to be named “BH Biopharma Merger Sub Limited,” which is in the process
of being formed by the Parent, and once formed, the Parent shall cause it to enter into a joinder to this Agreement (“Company
Merger Sub”), entered into that certain Merger Agreement and Plan of Reorganization (the “Merger Agreement”),
pursuant to which, among other matters, (a) Parent Merger Sub will merge with and into Parent, with Parent continuing as the surviving
company (the “Parent Merger”), and with the security holders of Parent receiving substantially equivalent securities
of Pubco and (b) Company Merger Sub will merge with and into the Company, with the Company continuing as the surviving company (the “Company
Merger”; Company Merger and the Parent Merger are together referred to herein as the “Mergers”),
and with the shareholders of the Company receiving Pubco Ordinary Shares, and as a result of the Mergers, Parent and the Company will
become wholly-owned subsidiaries of Pubco, and Pubco will become a publicly traded company listed on Nasdaq, all upon the terms and subject
to the conditions set forth in the Merger Agreement and in accordance with the provisions of applicable law
B. Each
of the Shareholders is the record and beneficial owner of the number and type of issued and outstanding ordinary shares of the Company
set forth on Schedule A hereto (the “Shares”).
C. In
order to induce Parent and the Company to enter into the Merger Agreement and the Parent Initial Shareholders to enter into the Sponsor
Support Agreement, the Parties desire to enter into this Agreement on the terms and conditions set forth herein.
AGREEMENT
NOW, THEREFORE, in consideration
of the premises and the mutual promises contained herein, and for other good and valuable consideration, the receipt and sufficiency of
which are hereby acknowledged, the Parties, each intending to be legally bound, hereby agree as follows:
1. Company
Shareholder Consent and Related Matters. As promptly as reasonably practicable (and in any event within five (5) Business Days) following
the time at which the Registration Statement becomes effective under the Securities Act, each of the Shareholders shall (a) duly execute
and deliver to the Company, Pubco and Parent the Written Consent, which Written Consent shall constitute the Requisite Approval, pursuant
to which it shall irrevocably and unconditionally (i) adopt the Merger Agreement and approve the Company Merger and the other Transactions
to which the Company is a party, (ii) approve, in accordance with the terms and subject to the conditions of the Company Organizational
Documents, the Company Preferred Conversion to take effect immediately prior to the Closing and (iii) waive any appraisal or similar rights
they may have pursuant to Cayman Law with respect to the Company Merger and the other Transactions, and (b) vote (or cause to be
voted) all of such Shareholder’s Shares (together with any shares of the Company that such Shareholder acquires record or beneficial
ownership of or the power to vote after the date hereof, collectively, the “Subject Company Stock”) against, and withhold
consent with respect to, any other matter, action, agreement, transaction or proposal that would reasonably be expected to result in (i) a
breach of any of the Company’s representations, warranties, covenants, agreements or obligations under the Merger Agreement or (ii) any
of the conditions to the Closing set forth in Sections 8.01 or 8.02 of the Merger Agreement not being satisfied; provided, that
in the case of clauses (a) and (b), the Merger Agreement shall not have been amended or modified without such Shareholder’s consent
(A) to decrease the Aggregate Company Merger Consideration payable under the Merger Agreement or (B) to change the form of the Aggregate
Company Merger Consideration, in each case in a manner adverse to such Shareholder. Each of the Shareholders acknowledges receipt and
review of a copy of the Merger Agreement.
2. Transfer
of Shares. Each Shareholder hereby agrees that it shall not, directly or indirectly, (a) sell, assign, transfer (including by
operation of law), place a lien on, pledge, hypothecate, grant an option to purchase, distribute, dispose of or otherwise encumber any
of its Subject Company Stock or otherwise enter into any contract, option or other arrangement or undertaking to do any of the foregoing
(each, a “Transfer”), (b) deposit any of its Subject Company Stock into a voting trust or enter into a voting
agreement or arrangement or grant any proxy or power of attorney with respect to any of its Subject Company Stock that conflicts with
any of the covenants or agreements set forth in this Agreement or (c) take any action that would have the effect of preventing or
materially delaying the performance of its obligations hereunder; provided, however, that the foregoing shall not apply
to any Transfer (i) to an Affiliate of such Shareholder, (ii) to another Shareholder that is a Party and bound by the terms
and obligations hereof or (iii) made in connection with the Company Merger or the other Transactions; provided, that any transferee
of any Transfer of the type set forth in clause (i) must enter into a joinder agreement agreeing to become a Party.
3. Other
Covenants and Agreements.
(a) [Reserved.]
(b) Each
of the Shareholders hereby agrees to be bound by and subject to (i) Section 7.04(b) (Confidentiality) and Section 7.11 (Public Announcements)
of the Merger Agreement to the same extent as such provisions apply to the parties to the Merger Agreement and (ii) Section 6.03 (Claims
Against the Trust Account), Section 7.03 (Requisite Approval) and Section 7.05 (Non-Solicitation) of the Merger Agreement to the same
extent as such provisions apply to the Company, in each case, mutatis mutandis and as if such Shareholder was a party thereto.
Notwithstanding anything in this Agreement to the contrary, (x) no Shareholder shall be responsible for the actions of the Company
or the Company Board (or any committee thereof) or any officers, directors (in their capacity as such), employees or professional advisors
of any of the foregoing (the “Company Related Parties”), including with respect to any of the matters contemplated
by this Section 3(b), (y) no Shareholder is making any representations or warranties with respect to the actions of any of
the Company Related Parties, and (z) any breach by the Company of its obligations under the Merger Agreement shall not be considered
a breach of this Section 3(b) (it being understood for the avoidance of doubt that each Shareholder shall remain responsible for
any breach by it of this Section 3(b)).
(c) Each
of the Shareholders acknowledges and agrees that Pubco, Parent, Parent Merger Sub and Company Merger Sub are entering into the Merger
Agreement and the Parent Initial Shareholders are entering into the A&R Sponsor Support Agreement in reliance upon the Shareholders
entering into this Agreement and agreeing to be bound by, and perform, or otherwise comply with, as applicable, the agreements, covenants
and obligations contained in this Agreement, and but for the Shareholders entering into this Agreement and agreeing to be bound by, and
perform, or otherwise comply with, as applicable, the agreements, covenants and obligations contained in this Agreement, Parent and Parent
Merger Sub would not have entered into the Merger Agreement or agreed to consummate the Transactions and the Parent Initial Shareholders
would not have entered into the A&R Sponsor Support Agreement or agreed to consummate the transactions contemplated thereby.
4. Shareholder
Representations and Warranties. Each of the Shareholders, severally and not jointly, represents and warrants to Parent and the Company
as follows:
(a) If
such Shareholder is a natural person, he or she is legally competent to execute and deliver this Agreement. If such Shareholder is not
a natural person, it is duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation
or organization.
(b) The
execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby are within such Shareholder’s
power and have been duly authorized by all necessary actions on the part of such Shareholder.
(c) The
execution and delivery of this Agreement by such Shareholder does not, and the performance by such Shareholder of his, her or its obligations
hereunder will not, (i) conflict with or violate any Law applicable to such Shareholder, (ii) result in the creation of any
Lien on any of its Subject Company Stock (other than under this Agreement, the Merger Agreement or the Ancillary Agreements), (iii) if
applicable, conflict with or result in a breach or violation of or constitute a default under its organizational documents, or (iv) require
any consent, authorization or approval of, declaration, filing or registration with, or notice to, any Person, in each case that has not
been given or made as of the date hereof.
(d) There
are no Actions pending against such Shareholder or, to the knowledge of such Shareholder, threatened against such Shareholder, before
(or, in the case of threatened Actions, that would be before) any arbitrator or any Governmental Authority, which in any manner challenges
or seeks to prevent, enjoin or materially delay the performance by such Shareholder of its obligations under this Agreement.
(e) This
Agreement has been duly executed and delivered by such Shareholder and, assuming due authorization, execution and delivery by the other
Parties, this Agreement constitutes a legally valid and binding obligation of such Shareholder, enforceable against him, her or it in
accordance with the terms hereof (except as enforceability may be limited by applicable bankruptcy Laws, other applicable, similar Laws
affecting creditors’ rights and general principles of equity affecting the availability of specific performance and other equitable
remedies).
(f) Such
Shareholder has not entered into, and shall not enter into, any agreement that would restrict, limit or interfere with the performance
of his, her or its obligations hereunder.
(g) Such
Shareholder is the exclusive record and beneficial owner of, and has good and valid title to, all of the Shares set forth opposite such
Shareholder’s name on Schedule A hereto, and there exist no Liens, pledge, proxy, security interest, option, right of first
refusal, adverse claim of ownership or any other limitations or restrictions (including, without limitation, any restriction on the right
to vote, sell or otherwise dispose of such Shares), other than pursuant to (i) this Agreement, (ii) the Company Organizational
Documents, (iii) the Merger Agreement or the Ancillary Agreements and (iv) any applicable securities Laws, and as of the date of this
Agreement, such Shareholder has the sole power (as currently in effect) to vote, and the right, power and authority to sell, transfer
and deliver, such Shares, and such Shareholder does not own, directly or indirectly, any other Shares.
5. Termination.
This Agreement shall automatically terminate, without any notice or other action by any Party, upon the earliest of (a) the Company
Merger Effective Time, (b) the termination of the Merger Agreement in accordance with its terms and (c) the mutual written agreement
of all of the Parties. Upon termination of this Agreement as provided in the immediately preceding sentence, none of the Parties shall
have any further obligations or liabilities under, or with respect to, this Agreement. Notwithstanding the foregoing or anything to the
contrary in this Agreement, (i) the termination of this Agreement pursuant to Section 5(b) shall not affect any liability
on the part of any Party for a Willful Breach of any covenant or agreement set forth in this Agreement prior to such termination or Fraud,
(ii) Sections 5 and 10 shall each survive any termination of this Agreement pursuant to Section 5(a), and (iii) Sections
5 through 14 shall survive any termination of this Agreement. For purposes of this Section 5, “Fraud”
means an act or omission by a Party, and requires: (A) a false or incorrect representation or warranty expressly set forth in this
Agreement, (B) with actual knowledge (as opposed to constructive, imputed or implied knowledge) by the Party making such representation
or warranty that such representation or warranty expressly set forth in this Agreement is false or incorrect, (C) an intention to
deceive another Party to induce him, her or it to enter into this Agreement, (D) another Party, in justifiable or reasonable reliance
upon such false or incorrect representation or warranty expressly set forth in this Agreement, causing such Party to enter into this Agreement,
and (E) causing such Party to suffer damage by reason of such reliance.
6. No
Recourse. Except for claims pursuant to the Merger Agreement or any other Ancillary Agreement by any party thereto against any other
party thereto, each Party agrees that (a) this Agreement may only be enforced against, and any action for breach of this Agreement may
only be made against, the Parties, and no claims of any nature whatsoever (whether in tort, contract or otherwise) arising under or relating
to this Agreement, the negotiation hereof or its subject matter, or the transactions contemplated hereby shall be asserted against any
Affiliate of the Company (other than the Shareholders, on the terms and subject to the conditions set forth herein) or any Affiliate of
Parent, and (b) none of the Affiliates of the Company (other than the Shareholders, on the terms and subject to the conditions set forth
herein) or the Affiliates of Parent shall have any liability arising out of or relating to this Agreement, the negotiation hereof or its
subject matter, or the transactions contemplated hereby, including with respect to any claim (whether in tort, contract or otherwise)
for breach of this Agreement or in respect of any written or oral representations made or alleged to be made in connection herewith, as
expressly provided herein, or for any actual or alleged inaccuracies, misstatements or omissions with respect to any information or materials
of any kind furnished in connection with this Agreement, the negotiation hereof or the transactions contemplated hereby.
7. Notices.
All notices and other communications among the Parties shall be in writing and shall be deemed to have been duly given (a) when delivered
in person, (b) when delivered after posting in the United States mail having been sent registered or certified mail return receipt
requested, postage prepaid, (c) when delivered by FedEx or other nationally recognized overnight delivery service or (d) when
e-mailed during normal business hours (and otherwise as of the immediately following Business Day), addressed as follows:
Breeze Holdings Acquisition Corp.
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039
Attention: J. Douglas Ramsey, Ph.D.
Email: doug@breezeacquisition.com
with a copy to:
Woolery & Co.
1 Pier 76
408 12th Ave
New York, NY 10018
Attention: Jim Woolery; Mathew J. Saur
Email: james@wooleryco.com; matt@ekpyrosis.co
| (b) | If to the Company, to: |
YD Biopharma Limited
12th Floor, No. 101, Section 2
Nanjing East Road, Zhongshan District
Taipei City, Taiwan 54186159
Attn: Dr. Ethan Shen
E-mail: ethanshen@eg-bio.com
with a copy to:
ArentFox Schiff LLP
1717 K Street NW
Suite 700
Washington, DC 20006
Attention: Ralph V. De Martino
Email: ralph.demartino@afslaw.com
(c) If
to a Shareholder, to the address or email address set forth for such Shareholder on his, her or its signature page hereof, or to such
other address or addresses as the Parties may from time to time designate in writing.
8. Entire
Agreement. This Agreement and the Merger Agreement constitute the entire agreement of the Parties with respect to the subject matter
of this Agreement and supersede all prior agreements and undertakings, both written and oral, among the Parties with respect to the subject
matter of this Agreement, except as otherwise expressly provided in this Agreement.
9. Amendments
and Waivers; Assignment. Any provision of this Agreement may be amended or waived if, and only if, such amendment or waiver is in
writing and signed by the Parties and the Parties to be bound thereby, respectively. Notwithstanding the foregoing, no failure or delay
by any Party in exercising any right hereunder shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude
any other or further exercise of any other right hereunder. Neither this Agreement nor any of the rights, interests or obligations hereunder
shall be assignable by any Party without the prior written consent of the other Parties.
10. Fees
and Expenses. Except as otherwise expressly set forth in the Merger Agreement, all fees and expenses incurred in connection with this
Agreement and the transactions contemplated hereby, including the fees and disbursements of counsel, financial advisors and accountants,
shall be paid by the Party incurring such fees or expenses.
11. Fiduciary
Duties. Notwithstanding anything in this Agreement to the contrary, (a) none of the Shareholders makes any agreement or understanding
herein in any capacity other than in its capacity as a record holder and beneficial owner of the Subject Company Stock and (b) nothing
herein will be construed to limit or affect any action or inaction by any representative of such Shareholder in its capacity as a member
of the Company Board or other similar governing body of any of its Affiliates or as an officer, employee or fiduciary of the Company or
any of its Affiliates, in each case, acting in such person’s capacity as a director, officer, employee or fiduciary of the Company
or such Affiliate.
12. Remedies.
Except as otherwise expressly provided herein, any and all remedies provided herein will be deemed cumulative with and not exclusive of
any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude
the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be
an adequate remedy, would occur in the event that a Party does not perform its respective obligations under the provisions of this Agreement
in accordance with their specific terms or otherwise breaches such provisions. It is accordingly agreed that each Party shall be entitled
to an injunction or injunctions, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce
specifically the terms and provisions of this Agreement, in each case, without posting a bond or undertaking and without proof of damages
and this being in addition to any other remedy to which they are entitled at law or in equity. Each Party agrees that it will not oppose
the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this
Agreement on the basis that the other Parties have an adequate remedy at law or an award of specific performance is not an appropriate
remedy for any reason at law or equity.
13. No
Third Party Beneficiaries. This Agreement shall be for the sole benefit of the Parties and their respective successors and permitted
assigns and is not intended, nor shall be construed, to give any Person, other than the Parties and their respective successors and permitted
assigns, any legal or equitable right, benefit or remedy of any nature whatsoever by reason this Agreement. Nothing in this Agreement,
expressed or implied, is intended to or shall constitute the Parties acting as partners or participants in a joint venture.
14. Incorporation
by Reference. Sections 1.03 (Construction), 10.03 (Severability), 10.06 (Governing Law), 10.07 (Waiver of Jury Trial), 10.08 (Headings)
and 10.09 (Counterparts; Electronic Delivery) of the Merger Agreement are incorporated herein by reference and shall apply to this
Agreement mutatis mutandis.
[Signature page follows]
IN WITNESS WHEREOF, each of
the Parties has executed and delivered this Shareholder Support Agreement as of the date first above written.
| |
PARENT: |
| |
|
| |
BREEZE HOLDINGS ACQUISITION CORP. |
| |
|
| |
By: |
/s/ J. Douglas Ramsey, Ph.D.
|
| |
|
Name: |
J. Douglas Ramsey, Ph.D. |
| |
|
Title: |
CEO & CFO |
[Signature Page to Shareholder Support Agreement]
IN WITNESS WHEREOF, each of
the Parties has executed and delivered this Shareholder Support Agreement as of the date first above written.
| |
COMPANY: |
| |
|
| |
YD
Biopharma limited |
| |
|
| |
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
|
Name: |
Dr. Hsieh Tsung Shen |
| |
|
Title: |
CEO |
[Signature Page to Shareholder Support Agreement]
IN WITNESS WHEREOF, each of
the Parties has executed and delivered this Shareholder Support Agreement as of the date first above written.
| |
SHAREHOLDER: |
| |
|
| |
YD
Biopharma Holding limited |
| |
|
| |
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
|
Name: |
Dr. Hsieh Tsung Shen |
| |
|
Title: |
CEO |
[Signature Page to Shareholder Support Agreement]
SCHEDULE A
COMPANY SHARES
| Name | |
Ordinary Shares | | |
% | |
| YD Biopharma Holding Limited | |
| 883,179 | | |
| 83.95 | % |
Exhibit 10.3
Execution Version
LOCK-UP AGREEMENT
THIS LOCK-UP AGREEMENT (this
“Agreement”) is made and entered into as of September 24, 2024, by and among YD Biopharma Limited, a Cayman
Islands exempted company (the “Company”), the undersigned shareholders of the Company (collectively, the “Company
Shareholders”), Breeze Holdings Acquisition Corp., a Delaware corporation (“Parent”), Breeze Sponsor,
LLC, a Delaware limited liability company (the “Sponsor”), and the undersigned stockholders of Parent (the “Parent
Stockholders” and together with the Sponsor, the “Parent Initial Stockholders”). The Company Shareholders
and the Parent Initial Stockholders are sometimes referred to herein individually as a “Stockholder” and collectively
as the “Stockholders.” The Company, Parent, and the Stockholders are sometimes referred to herein individually
as a “Party” and collectively as the “Parties”. Capitalized terms used but not otherwise
defined herein shall have the meanings ascribed to such terms in the Merger Agreement (as defined below).
A. Each
of the Parent Initial Stockholders holds the number of shares of Parent common stock (“Parent Common Stock”)
set forth opposite such Parent Initial Stockholder’s name on Exhibit A hereto.
B. On
September 24, 2024, Parent, the Company, a Cayman Islands exempted company and a wholly-owned subsidiary of Parent, expected to be named
“YD Bio Limited,” which is in the process of being formed by the Parent, and once formed, the Parent shall cause it to enter
into a joinder to this Agreement (“Pubco”), Breeze Merger Sub, Inc., a Delaware corporation that will be a direct,
wholly-owned Subsidiary of Pubco (“Parent Merger Sub”), and a Cayman Islands exempted company that will be a
wholly-owned subsidiary of Pubco, expected to be named “BH Biopharma Merger Sub Limited,” which is in the process of being
formed by the Parent, and once formed, the Parent shall cause it to enter into a joinder to this Agreement (“Company Merger
Sub”), entered into that certain Merger Agreement and Plan of Reorganization (the “Merger Agreement”),
pursuant to which, among other matters, (a) Parent Merger Sub will merge with and into Parent, with Parent continuing as the surviving
company (the “Parent Merger”), and with the security holders of Parent receiving substantially equivalent securities
of Pubco and (b) Company Merger Sub will merge with and into the Company, with the Company continuing as the surviving company (the “Company
Merger”; Company Merger and the Parent Merger are together referred to herein as the “Mergers”),
and with the shareholders of the Company receiving Pubco Ordinary Shares, and as a result of the Mergers, Parent and the Company will
become wholly-owned subsidiaries of Pubco, and Pubco will become a publicly traded company listed on Nasdaq, all upon the terms and subject
to the conditions set forth in the Merger Agreement and in accordance with the provisions of applicable law.
C. The
Merger Agreement contemplates that the Parties will enter into this Agreement, pursuant to which the ordinary shares of Pubco (“Pubco
Ordinary Shares”), held by the Stockholders immediately following the Parent Merger Effective Time and the Company Merger
Effective Time, as applicable (together with any securities paid as dividends or distributions with respect to such securities or into
which such securities are exchanged or converted), shall become subject to the limitations on disposition set forth herein.
D. The
Parties desire to enter into this Agreement on the terms and conditions set forth herein.
NOW, THEREFORE, in
consideration of the premises set forth above, which are incorporated in this Agreement as if fully set forth below, and the mutual promises
set forth herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending
to be legally bound hereby, the Parties hereby agree as follows:
1. For
purposes of this Agreement:
(a) the
term “Early Release Period” means the period beginning on the date that is the day after the four-month anniversary
of the Closing Date and ending on the date that is the eight-month anniversary of the Closing Date;
(b) the
term “Lock-up Period” means the period beginning on the Closing Date and ending on the date that is eight (8) months
after the Closing Date; provided, that the Lock-Up Period may be shortened in accordance with the terms of Section 2(a) or Section
2(b);
(c) the
term “Lock-up Shares” means any Pubco Ordinary Shares held by the Stockholders immediately following each of the Parent
Merger Effective Time and the Company Merger Effective Time, as applicable, or acquired thereafter;
(d) the
term “Permitted Transferee” means any Person to whom a Stockholder is permitted to transfer Lock-up Shares prior to
the expiration of the Lock-up Period pursuant to Section 2(c);
(e) the
term “Stock Price Level” means a daily volume weighted average closing sale price of Pubco Ordinary Shares quoted on
the Nasdaq Capital Market (or such other principal securities exchange or securities market on which the Pubco Ordinary Shares are then
traded) for any twenty (20) Trading Days within any thirty (30) consecutive Trading Day period; and
(f) the
term “Transfer” means any (i) voluntary or involuntary transfer, sale of, offer to sell, contract or agreement to sell,
hypothecation, pledge, grant of any option to purchase or otherwise dispose of (whether by operation of law or otherwise) or agreement
to dispose of or establishment or increase of a put equivalent position or liquidation with respect to or decrease of a call equivalent
position within the meaning of Section 16 of the Exchange Act, and the rules and regulations promulgated thereunder, with respect to,
any security, (ii) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences
of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (iii)
public announcement of any intention to effect any transaction specified in clause (i) or (ii).
2. Lock-Up
Provisions.
(a) Each
of the Stockholders hereby agrees that it shall not, and shall cause its Permitted Transferees not to, Transfer any Lock-Up Shares during
the Lock-Up Period (the “Transfer Restriction”), except as permitted in accordance with the following:
| (i) | during the Early Release Period, the Transfer Restriction shall expire with respect to ten percent (10%)
of the Lock-Up Shares held by each Stockholder (the “First Tranche”) if a Stock Price Level equal to or greater than
$12.50 is achieved; |
| (ii) | during the Early Release Period and after or concurrently with the satisfaction of the conditions precedent
for the early expiration of the Transfer Restriction with respect to the First Tranche under Section 2(a)(i), the Transfer Restriction
shall expire with respect to an additional ten percent (10%) of the Lock-Up Shares (the “Second Tranche”), if a Stock
Price Level equal to or greater than $15.00 is achieved; and |
| (iii) | on the date on which Pubco completes a Subsequent Transaction, the Transfer Restriction will expire with
respect to all Lock-Up Shares. |
(b) Notwithstanding
anything to the contrary in this Agreement, each of the Parties hereby acknowledges and agrees that, at any time during the Lock-Up Period
and no earlier than six (6) months after the Closing Date, the board of directors of Pubco (the “Board”) may, in its
sole discretion, resolve to terminate, in whole or in part, the Transfer Restriction; provided, that any termination of the Transfer
Restriction in part shall apply pro rata to the Stockholders; and, provided, further, that the approval of such resolution
of the Board shall (i) be given at a meeting of the Board called for the purpose of considering and voting upon such resolution and (ii)
include at least a majority of the “independent” (as such term is defined in Rule 5605(a)(2) of the Nasdaq Capital Market
Listing Requirements) members of the Board.
(c) Notwithstanding
the provisions set forth in Section 2(a), each of the Stockholders and its respective Permitted Transferees may Transfer, in whole
or in part, its Lock-up Shares during the Lock-up Period (i) to any Affiliate(s) of such Stockholder, (ii) in the case of an individual
Stockholder, to a member of such individual’s immediate family (including such Stockholder’s spouse or ancestors, descendants
or siblings (in each case, whether by blood, marriage or adoption)) or to a trust, the beneficiary of which is such Stockholder or a member
of such Stockholder’s immediate family, or (iii), in the case of an individual Stockholder, by virtue of laws of descent and distribution
upon the death of such Stockholder.
(d) The
per share prices of the Pubco Ordinary Shares referenced in this Agreement shall be equitably adjusted on account of any changes in the
equity securities of Pubco by way of stock split, stock dividend, combination or reclassification, or through any merger, consolidation,
reorganization, recapitalization or business combination, or by any other means.
(e) If
any Transfer is made or attempted contrary to the provisions of this Agreement, such Transfer shall be null and void ab initio,
and Pubco shall refuse to recognize any such Transfer and any such transferee of Lock-Up Shares as one of its equity holders for any purpose.
In order to enforce this Section 2(e), Pubco may impose stop-transfer instructions with respect to the Lock-Up Shares in accordance
with the terms of this Agreement until the end of Lock-Up Period.
(f) During
the Lock-Up Period, each certificate (if any are issued) evidencing any Lock-Up Shares shall be stamped or otherwise imprinted with a
legend in substantially the following form, in addition to any other applicable legends:
“THE SECURITIES REPRESENTED BY THIS
CERTIFICATE ARE SUBJECT TO RESTRICTIONS ON TRANSFER AS SET FORTH IN THAT CERTAIN LOCK-UP AGREEMENT, DATED AS OF SEPTEMBER 24, 2024, BY
AND AMONG THE ISSUER OF SUCH SECURITIES (THE “ISSUER”) THE ISSUER’S SECURITY HOLDER NAMED IN THIS CERTIFICATE
AND THE OTHER PARTIES THERETO. A COPY OF SUCH LOCK-UP AGREEMENT WILL BE FURNISHED WITHOUT CHARGE BY THE ISSUER TO THE HOLDER HEREOF UPON
WRITTEN REQUEST.”
(g) For
the avoidance of any doubt, each of the Stockholders shall retain all of his, her or its rights as a stockholder of Pubco with respect
to his, her or its Lock-Up Shares during the Lock-Up Period, including the right to vote any Lock-Up Shares.
3. Miscellaneous.
(a) Effective
Date. This Agreement shall become effective at the Parent Merger Effective Time.
(b) Termination
of the Merger Agreement. Notwithstanding anything to the contrary contained herein, in the event that the Merger Agreement is terminated
in accordance with its terms prior to the Parent Merger Effective Time, this Agreement and all rights and obligations of the Parties hereunder
shall automatically terminate and be of no further force or effect.
(c) Binding
Effect; Assignment. This Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of the Parties
hereto and their respective permitted successors and assigns. Except as otherwise provided in this Agreement, this Agreement and all obligations
of the Parties are personal to the Parties and may not be transferred or delegated by the Parties at any time.
(d) Third
Parties. Nothing contained in this Agreement or in any instrument or document executed by any Party in connection with the transactions
contemplated hereby shall create any rights in, or be deemed to have been executed for the benefit of, any person or entity that is not
a Party hereto or thereto.
(e) Governing
Law; Jurisdiction. This Agreement shall be governed by and construed in accordance with the Laws of the State of Delaware, without
giving effect to any choice of Law or conflict of Law provision or rule (whether of the State of Delaware or any other jurisdiction) that
would cause the application of the law of any jurisdiction other than the State of Delaware. Each Party (a) irrevocably consents to the
service of the summons and complaint and any other process in any Action relating to the transactions contemplated by this Agreement,
for and on behalf of itself or any of its properties or assets, in accordance with this Section 3(e) or in such other manner as
may be permitted by applicable Law and that such process may be served in the manner of giving notices in Section 3(h) and that
nothing in this Section 3(e) shall affect the right of any Party to serve legal process in any other manner permitted by applicable
Law, (b) irrevocably and unconditionally consents and submits itself and its properties and assets in any Action or proceeding to the
exclusive general jurisdiction of the Court of Chancery of the State of Delaware (the “Chancery Court”) (or, only if
the Chancery Court declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware)
in the event any dispute or controversy arises out of this Agreement or the transactions contemplated hereby, or for recognition and enforcement
of any Order in respect thereof, (c) agrees that it will not attempt to deny or defeat such personal jurisdiction by motion or other request
for leave from any such court, (d) waives any objection that it may now or hereafter have to the venue of any such Action in any such
court or that such Action was brought in an inconvenient court and agrees not to plead or claim the same, and (e) agrees that it will
not bring any such Action in any court other than the aforesaid courts. Each Party agrees that a final Order in any Action in such courts
as provided above shall be conclusive and may be enforced in other jurisdictions by suit on the Order or in any other manner provided
by applicable Law.
(f) WAIVER
OF JURY TRIAL. EACH OF THE PARTIES HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT MAY HAVE TO A TRIAL
BY JURY WITH RESPECT TO ANY ACTION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR THE TRANSACTIONS
CONTEMPLATED HEREBY. EACH PARTY HEREBY (i) CERTIFIES THAT NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE,
THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF ANY ACTION, SEEK TO ENFORCE THE FOREGOING WAIVER AND (ii) ACKNOWLEDGES THAT IT AND THE
OTHER PARTIES HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION
3(f).
(g) Interpretation.
The titles and subtitles used in this Agreement are for convenience only and are not to be considered in construing or interpreting this
Agreement. In this Agreement, unless the context otherwise requires: (i) any pronoun used in this Agreement shall include the corresponding
masculine, feminine or neuter forms, and the singular form of nouns, pronouns and verbs shall include the plural and vice versa; (ii)
“including” (and with correlative meaning “include”) means “including” without limiting the generality
of any description preceding or succeeding such term and shall be deemed in each case to be followed by the words “without limitation”;
(iii) the words “herein,” “hereto,” and “hereby” and other words of similar import in this Agreement
shall be deemed in each case to refer to this Agreement as a whole and not to any particular section or other subdivision of this Agreement;
and (iv) the term “or” means “and/or”. The Parties have participated jointly in the negotiation and drafting of
this Agreement. Consequently, in the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed
as if drafted jointly by the Parties, and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of
the authorship of any provision of this Agreement.
(h) Notices.
All notices, consents, waivers and other communications hereunder shall be in writing and shall be deemed to have been duly given when
delivered (i) in person, (ii) by e-mail (having obtained electronic delivery confirmation thereof), (iii) one (1) Business Day after being
sent, if sent by reputable, nationally recognized overnight courier service or (iv) three (3) Business Days after being mailed, if sent
by registered or certified mail, pre-paid and return receipt requested; provided, however, that notice given pursuant to clauses (iii)
and (iv) above shall not be effective unless a duplicate copy of such notice is also given in person or by e-mail (having obtained electronic
delivery confirmation thereof), in each case to the addresses specified on the signature pages hereto (or at such other addresses for
a Party as shall be specified by like notice).
(i) Amendments
and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally
or in a particular instance, and either retroactively or prospectively) only with the written consent of the Parties or the Party agreeing
to be bound thereby, respectively. No failure or delay by a Party in exercising any right hereunder shall operate as a waiver thereof.
No waivers of or exceptions to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed to be
or construed as a further or continuing waiver of any such term, condition, or provision.
(j) Severability.
In case any provision in this Agreement shall be held invalid, illegal or unenforceable in a jurisdiction, such provision shall be modified
or deleted, as to the jurisdiction involved, only to the extent necessary to render the same valid, legal and enforceable, and the validity,
legality and enforceability of the remaining provisions hereof shall not in any way be affected or impaired thereby nor shall the validity,
legality or enforceability of such provision be affected thereby in any other jurisdiction. Upon such determination that any term or other
provision is invalid, illegal or incapable of being enforced, the Parties will substitute for any invalid, illegal or unenforceable provision
a suitable and equitable provision that carries out, so far as may be valid, legal and enforceable, the intent and purpose of such invalid,
illegal or unenforceable provision.
(k) Specific
Performance. Each of the Parties acknowledges that its obligations under this Agreement are unique and recognizes and affirms that
in the event of a breach of this Agreement by such Party, money damages will be inadequate and the other Parties will have no adequate
remedy at law, and agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed
by the other Parties in accordance with their specific terms or were otherwise breached. Accordingly, each of the Parties shall be entitled
to an injunction or restraining order to prevent breaches of this Agreement by the other Parties and to enforce specifically the terms
and provisions hereof, without the requirement to post any bond or other security or to prove that money damages would be inadequate,
this being in addition to any other right or remedy to which such Parties may be entitled under this Agreement, at law or in equity.
(l) Entire
Agreement. This Agreement constitutes the full and entire understanding and agreement among the Parties with respect to the subject
matter hereof and supersedes any other written or oral agreement relating to the subject matter hereof existing between the Parties; provided,
that, for the avoidance of doubt, the foregoing shall not affect the rights and obligations of the Parties under the Merger Agreement
or any other Ancillary Agreement. Notwithstanding the foregoing, nothing in this Agreement shall limit any of the rights or remedies or
obligations of the Parties under any other agreement between any of the Parties, or any certificate or instrument executed by any of the
Parties.
(m) Further
Assurances. From time to time, at another Party’s reasonable request and without further consideration (but at the requesting
Party’s reasonable cost and expense), each Party shall execute and deliver such additional documents and take all such further action
as may be reasonably necessary to consummate the transactions contemplated by this Agreement.
(n) Counterparts;
Facsimile. This Agreement may also be executed and delivered by facsimile signature or by email in portable document or other
electronic format in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one
and the same instrument.
[Remainder of Page Intentionally Left Blank;
Signature Pages Follow]
IN WITNESS WHEREOF,
the Parties have executed this Agreement as of the date first written above.
| |
Parent: |
| |
|
| |
BREEZE HOLDINGS ACQUISITION CORP. |
| |
|
| |
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
Name: |
J. Douglas Ramsey, Ph.D. |
| |
Title: |
CEO & CFO |
| |
Email: |
doug@breezeacquisition.com |
| |
Address: |
Breeze Holdings Acquisition Corp.
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039 |
| |
Attention: |
J. Douglas Ramsey, Ph.D. |
| |
SPONSOR: |
| |
|
| |
BREEZE SPONSOR, LLC |
| |
|
| |
By: |
/s/ J. Douglas Ramsey, Ph.D. |
| |
Name: |
J. Douglas Ramsey, Ph.D. |
| |
Title: |
Manager |
| |
Email: |
doug@breezeacquisition.com |
| |
Address: |
Breeze Holdings Acquisition Corp.
955 W. John Carpenter Fwy., Suite 100-929
Irving, TX 75039 |
| |
Attention: |
J. Douglas Ramsey, Ph.D. |
[Signature Page to Lock-Up Agreement]
IN WITNESS WHEREOF,
the Parties have executed this Agreement as of the date first written above.
| |
PARENT STOCKHOLDERS: |
| |
|
| |
I-BANKERS SECURITIES, INC. |
| |
|
| |
By: |
/s/ Matthew J. McCloskey |
| |
Name: |
Matthew J. McCloskey |
| |
Title: |
CEO |
| |
Email: |
|
| |
Address: |
|
| |
/s/ Albert McLelland |
| |
Albert McLelland |
| |
Email: |
| |
Address: |
| |
/s/ Daniel L. Hunt |
| |
Daniel L. Hunt |
| |
Email: |
| |
Address: |
| |
/s/ Robert Lee Thomas |
| |
Robert Lee Thomas |
| |
Email: |
| |
Address: |
| |
/s/ Bill Stark |
| |
Bill Stark |
| |
Email: |
| |
Address: |
[Signature Page to Lock-Up Agreement]
IN WITNESS WHEREOF,
the Parties have executed this Agreement as of the date first written above.
| |
COMPANY: |
| |
|
| |
YD Biopharma Limited |
| |
|
| |
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
Name: |
Dr. Hsieh Tsung Shen |
| |
Title: |
CEO |
| |
Email: |
|
| |
Address: |
YD Biopharma Limited
12th Floor, No. 101, Section 2
Nanjing East Road, Zhongshan District
Taipei City, Taiwan 54186159 |
[Signature Page to Lock-Up Agreement]
IN WITNESS WHEREOF,
the Parties have executed this Agreement as of the date first written above.
| |
COMPANY SHAREHOLDERS: |
| |
|
| |
YD Biopharma Holding Limited |
| |
|
| |
By: |
/s/ Dr. Hsieh Tsung Shen |
| |
Name: |
Dr. Hsieh Tsung Shen |
| |
Title: |
CEO |
| |
|
|
| |
Email: |
|
| |
|
|
| |
Address: |
|
[Signature Page to Lock-Up Agreement]
Exhibit 99.1
Breeze Holdings Acquisition Corp. Announces
Definitive Agreement to Merge with YD Biopharma Limited
YD Biopharma is a Clinical-Stage Biopharmaceutical
Company Focusing on Cancer Prevention Diagnostics and Seeking to Transform the Treatment of a Wide Spectrum of Diseases
Pro Forma for the Transaction, Combined Company
is Expected to Have an Estimated Enterprise Value of Nearly $700 Million
The Proposed Merger is Expected to Close by
Early 2025; After Closing, the Combined Company is Expected to be Listed on Nasdaq Capital Market
YD Biopharma has Recently Obtained Patents,
Technology, and U.S. Authorization for Core Methylation Detection of Pancreatic Cancer, Along with Entering into an Agreement to Acquire
Licenses for Breast Cancer Detection Upon the Closing of the Merger
Irving, Texas, September 25, 2024 –
Breeze Holdings Acquisition Corp. (OTCQX: BRZH, BRZHR, BRZHW) (“Breeze” or the “Company”), a publicly traded special
purpose acquisition company, has entered into a definitive agreement to merge with YD Biopharma Limited (“YD Biopharma”),
a clinical-stage biopharmaceutical company focusing on cancer prevention medical diagnostics and the development of exosome-based therapeutics
with the potential to transform the treatment of a wide spectrum of diseases with high unmet medical need. Following the closing, the
combined company is expected to be listed on the Nasdaq Capital Market.
Using Technology to Detect Health Problems
Early On
YD Biopharma specializes in the biopharmaceutical
business and serves as a supplier of drugs and medical materials for clinical trials. In 2015, YD Biopharma was appointed as a clinical
testing drug supplier by Novartis and has since expanded its offerings to include development and supply of ancillary products post-launch.
YD Biopharma’s mission is to create a cancer-free world through advancements in biotechnology.
More recently, YD Biopharma obtained patent and
technology authorization from 3D Global Biotech Inc. (“3D Biotech”) to pioneer the application of corneal mesenchymal stem
cells and their exosomes for treating eye diseases. YD Biopharma has introduced new advanced drugs and treatments for conditions such
as dry eye disease, glaucoma, and corneal repair. YD Biopharma aims to optimize the treatment market for eye diseases by distribution
through pharmacies, optometrists, and other channels.
Earlier this year, YD Biopharma obtained patents,
technology and U.S. market authorization from EG Biomed Taiwan for core methylation detection of pancreatic cancer with high sensitivity,
specificity and accuracy. This partnership has led to the establishment of an independent laboratory in the U.S. dedicated to pancreatic
cancer early detection and monitoring technology that marks a significant expansion of YD Biopharma’s research and development capabilities
to collaborate with hospitals, insurance companies and pharmaceutical companies to reach new patients.
YD Biopharma has also recently negotiated related
authorizations for breast cancer detection to further expand the Company’s product offerings. YD Biopharma is in the process of
acquiring licenses from EG BioMed Taiwan for advanced breast cancer detection technology in the U.S., E.U., and Asia-Pacific that has
high sensitivity, specificity and accuracy. The acquisition of the licenses for EG Biomed’s breast cancer detection technology in
the U.S., E.U., and Asia-Pacific is expected to be consummated simultaneously with the closing of the merger with Breeze.
Management Commentary
Dr. Ethan Shen, the Founder, Chairman and CEO
of YD Biopharma, has an extensive background in the pharmaceutical industry having worked at a well-known global pharmaceutical company.
Inspired by his father’s struggle with cancer and subsequent passing, Dr. Shen is dedicated to eradicating cancer and helping people
to avoid chronic and painful treatments through early detection.
Dr. Shen stated the following regarding the proposed
transaction, “I’m pleased to announce the next phase of our strategy as we embark on a public listing in the U.S. through
the proposed business combination with Breeze. Since our founding in 2013, we’ve made significant strides in expanding our capabilities
through organic innovation, licensing agreements, and notable strategic partnerships. We have a strategic roadmap in place for accelerated
growth and a compelling story to tell in the U.S. market as we aim to deliver health problem detection at an earlier stage than ever before
through minimal intervention.”
J. Douglas Ramsey, Ph.D., Chairman and CEO of
Breeze, commented, “From day one, it has been our mission at Breeze to find a company with innovative and disruptive technology
that has the potential to deliver significant growth to our shareholders. We are highly optimistic about the proposed business combination
with YD Biopharma, a company that we believe is a true outlier in the biotech industry with strong growth potential in a variety of healthcare
markets. We are working closely with their team to expeditiously close the transaction by early 2025 and move forward with YD Biopharma
as a publicly traded company in the U.S.”
YD Biopharma Key Investment Highlights
| ● | Proven Capabilities Across a Broad Spectrum of Solutions:
YD Biopharma has an extensive suite of solutions ranging from ophthalmology cellular drug development to pancreatic and breast cancer
diagnostics to nutritional product sales. |
| ● | Notable Strategic Partnerships, Offering Validation and
Growth Potential: YD Biopharma is a clinical testing drug supplier for Novartis, a top five global pharmaceutical company, as well
as having licensing partnerships with EG BioMed for pancreatic cancer detection and 3D Global Biotech to develop treatment for eye disorders. |
| ● | Proprietary Technology Supported by Licensing Agreements
and IP Portfolio: Multi-decade, exclusive licensing agreements and owned, patented technology provides YD Biopharma with significant
competitive first-mover advantage in each of its clinical markets. |
| ● | Large and Underserved Markets for Each Solution Showcase
Untapped Growth Potential: Multi-billion-dollar global market sizes and high single digit CAGRs over the next decade provide significant
growth potential for YD Biopharma’s solutions. |
| ● | Strong Leadership Team with Deep Expertise in Biotech
and Finance: YD Biopharma has a founder-led management team with experience in new drug development, medical-grade health product
development, pharmacy channel development, and financial management and accounting. |
Transaction Overview
Under the terms of the business combination agreement,
Breeze and YD Biopharma will each merge into wholly-owned subsidiaries of a newly formed Cayman holding company expected to be named “YD
Biopharma Holdings Limited” and is anticipated to be listed on the Nasdaq Capital Market.
Assuming no redemptions, the combined company
will have an estimated post-transaction enterprise value of $694 million, consisting of an estimated equity value of $715 million, $21.0
million in cash and no debt. Cash proceeds raised will consist of Breeze’s $10.1 million cash in trust (before redemptions and payment
of any transaction expenses) and $15 million in anticipated new capital.
YD Biopharma intends to use the proceeds from
the transaction to expand production and continue development, approval and launch of new technologies.
The transaction has been unanimously approved
by the boards of directors of both YD Biopharma and Breeze. It is expected to close by early 2025, subject to regulatory and stockholder
approvals, and other customary closing conditions. Additional information may be found in the Current Report on Form 8-K that was filed
by Breeze Holdings today with the U.S. Securities and Exchange Commission.
Upon completion of the transaction, YD Biopharma
will continue to be led by Founder, Chairman, and CEO Dr. Ethan Shen. Wu Cheng-fend will serve as Chief Medical Officer, and May Tsai
will serve as Chief Business Officer.
Advisors
ArentFox Schiff LLP and Ogier are acting as legal
advisors to YD Biopharma. I-Bankers Securities, Inc. is acting as financial advisor to Breeze Holdings. Woolery & Co. PLLC is acting
as legal advisor to Breeze Holdings.
About YD Biopharma
YD Biopharma Limited is a clinical-stage biopharmaceutical
company focusing on cancer prevention medical diagnostics and the development of exosome-based therapeutics with the potential to transform
the treatment of a wide spectrum of diseases with high unmet medical need. Through continuous effort and innovation, the Company has also
become a recognized supplier of clinical trial drugs and has begun developing and supplying post-market auxiliary products.
For more information, please visit www.yd-biopharma.com.
About Breeze Holdings Acquisition Corp.
Breeze Holdings is a blank check company organized
for the purpose of effecting a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization, or other similar
business combinations with one or more businesses or entities.
Additional Information and Where to Find It
This press release relates to a proposed business
combination transaction involving Breeze Holdings and YD Biopharma. In connection with the proposed transaction, a newly-formed Cayman
exempted company expected to be named “YD Biopharma Holdings Limited” (“YD Holdings”) intends to file with the
U.S. Securities and Exchange Commission (the “SEC”) a registration statement on Form F-4 that will include a proxy statement
of Breeze and that also will constitute a prospectus of YD Holdings with respect to the ordinary shares of YD Holdings to be issued in
the proposed transaction (the “Proxy Statement/Prospectus”). This document is not a substitute for the Proxy Statement/Prospectus.
The definitive Proxy Statement/Prospectus (if and when available) will be delivered to Breeze Holdings’ and YD Biopharma’s
stockholders. Breeze Holdings may also file other relevant documents regarding the proposed transaction with the SEC. BEFORE MAKING ANY
VOTING OR INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS OF BREEZE HOLDINGS AND YD BIOPHARMA AND OTHER INTERESTED PARTIES ARE URGED
TO READ THE REGISTRATION STATEMENT, PROXY STATEMENT/PROSPECTUS AND ALL OTHER RELEVANT DOCUMENTS THAT ARE FILED OR WILL BE FILED WITH THE
SEC IN CONNECTION WITH THE PROPOSED TRANSACTION, INCLUDING ANY AMENDMENTS OR SUPPLEMENTS TO THESE DOCUMENTS, CAREFULLY AND IN THEIR ENTIRETY
WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT BREEZE HOLDINGS, YD HOLDINGS, YD BIOPHARMA, THE PROPOSED
TRANSACTION AND RELATED MATTERS.
Investors and security holders of Breeze Holdings
and YD Biopharma may obtain free copies of the Registration Statement and Proxy Statement/Prospectus (if and when available) and other
documents that are filed or will be filed with the SEC by Breeze Holdings through the website maintained by the SEC at www.sec.gov. Copies
of the documents filed with the SEC by Breeze Holdings will be available free of charge at Breeze Holdings Acquisition Corp., 955 W. John
Carpenter Fwy., Suite 100-929, Irving, TX 75039, attention: J. Douglas Ramsey.
Participants in the Solicitation
Breeze Holdings, YD Biopharma and certain of their
respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Breeze
Holdings and YD Biopharma in respect of the proposed transaction. Information about Breeze Holdings’ directors and executive officers
and their ownership of Breeze Holdings common stock is set forth in Breeze Holdings’ filings with the SEC, including its Annual
Report on Form 10-K/A for the year ended December 31, 2023 filed with the SEC on April 25, 2024 (the “Annual Report”). To
the extent that holdings of Breeze Holdings’ securities have changed since the amounts included in the Annual Report, such changes
have been or will be reflected on Statements of Change in Ownership of Form 4 filed with the SEC. Other information regarding the participants
in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained
in the Proxy Statement/Prospectus and other relevant materials to be filed with the SEC in respect of the proposed transaction when they
become available. You may obtain free copies of these documents as described in the preceding paragraph.
Cautionary Note Regarding Forward-Looking Statements
This press release contains “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, among other things, statements
regarding the anticipated benefits and impact of the proposed transaction on the combined company’s business and future financial
and operating results, the anticipated timing of closing of the proposed transaction, the anticipated growth of the industries and markets
in which YD Biopharma competes, the success and customer acceptance of YD Biopharma’s product and service offerings and other aspects
of YD Biopharma’s operations, plans, objectives, opportunities, expectations or operating results, the expected ownership structure
of the combined company and the likelihood and ability of the parties to successfully consummate the proposed transaction. Words such
as “may,” “should,” “will,” “believe,” “expect,” “anticipate,”
“intend,” “estimated,” “target,” “project,” and similar phrases or words of similar meaning
that denote future expectations or intent regarding the combined company’s financial results, operations and other matters are intended
to identify forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Such forward-looking
statements are based upon the current beliefs and expectations of management and are inherently subject to significant business, economic
and competitive risks, uncertainties and other factors, both known and unknown, which are difficult to predict and generally beyond our
control and that may cause actual results and the timing of future events to differ materially from the results and timing of future events
anticipated by the forward-looking statements in this press release, including but not limited to: (i) the ability of the parties to complete
the proposed transaction within the time frame anticipated or at all, which may adversely impact the price of Breeze Holdings’ securities;
(ii) the failure to realize the anticipated benefits of the proposed transaction or those benefits taking longer than anticipated to be
realized; (iii) the risk that the proposed transaction may not be completed by Breeze Holdings’ business combination deadline and
the potential failure to obtain further extensions of the business combination deadline if sought by Breeze Holdings; (iv) the failure
to satisfy the conditions to the consummation of the proposed transaction, including the adoption of the definitive merger agreement by
the stockholders of Breeze Holdings or YD Biopharma, the receipt of any required governmental or regulatory approvals or the failure to
meet the Nasdaq listing standards in connection with the closing of the proposed transaction; (v) the lack of a third party valuation
in determining whether or not to pursue the proposed transaction; (vi) the occurrence of any event, change or other circumstance that
could give rise to the termination of the definitive merger agreement; (vii) the impact of the COVID-19 pandemic or related governmental
or regulatory orders ; (viii) the effect of the announcement or pendency of the proposed transaction on YD Biopharma’s business
relationships, performance and business generally; (ix) risks that the proposed transaction disrupts current plans and operations of YD
Biopharma and any potential difficulties in YD Biopharma employee retention as a result of the proposed transaction; (x) the outcome of
any legal proceedings that may be instituted against YD Biopharma or Breeze Holdings related to the definitive merger agreement or the
proposed transaction or any product liability or regulatory lawsuits or proceedings relating to YD Biopharma’s products or services;
(xi) the ability to maintain the listing of YD Holdings’ securities on the Nasdaq Capital Market after the closing of the proposed
transaction; (xii) potential volatility in the price of Breeze Holdings’ securities due to a variety of factors, including changes
in the competitive and highly regulated industries in which YD Biopharma operates, variations in performance across competitors, changes
in laws and regulations affecting YD Biopharma’s business, and changes in the combined company’s capital structure; (xiii)
the ability to implement business plans, identify and realize additional opportunities and achieve forecasts and other expectations after
the completion of the proposed transaction; (xiv) the risk of downturns and the possibility of rapid change in the highly competitive
industries in which YD Biopharma operates or the markets that YD Biopharma targets; (xv) the inability of YD Biopharma and its current
and future collaborators to successfully develop and commercialize YD Biopharma’s products and services in the expected time frame
or at all; (xvi) the risk that the combined company may never achieve or sustain profitability or may need to raise additional capital
to execute its business plan, which may not be available on acceptable terms or at all; and (xvii) the costs of the proposed transaction.
The forward-looking statements contained in this press release are also subject to additional risks, uncertainties and factors, including
those described in Breeze Holdings’ most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q and other documents
filed or to be filed with the SEC by Breeze Holdings from time to time. You are cautioned not to place undue reliance on forward-looking
statements as a predictor of future performance as projected financial information and other information are based on estimates and assumptions
that are inherently subject to various significant risks, uncertainties and other factors, many of which are beyond our control. The forward-looking
statements included in this press release are made only as of the date hereof, and we disclaim any intention or obligation to update any
forward-looking statements as a result of developments occurring after the date hereof. Forecasts and estimates regarding YD Biopharma’s
industry and end markets are based on sources we believe to be reliable, however there can be no assurance these forecasts and estimates
will prove accurate in whole or in part. Annualized, pro forma, projected and estimated numbers are used for illustrative purposes only,
are not forecasts and may not reflect actual results.
No Offer or Solicitation
This press release is for informational purposes
only and is not intended to and shall not constitute an offer to sell or the solicitation of an offer to sell or to buy any securities
or a solicitation of any proxy, consent, vote or approval with respect to any securities in respect of the proposed transaction and is
not a substitute for the Proxy Statement/Prospectus or any other document that Breeze Holdings may file with the SEC or send to Breeze
Holdings’ or YD Biopharma’s stockholders in connection with the proposed transaction. No offer, sale, issuance or transfer
of securities shall be made in any jurisdiction in which such offer, sale, issuance or transfer would be unlawful prior to registration
or qualification under the securities laws of any such jurisdiction.
Contacts:
YD Biopharma Limited
Bob Chiu
bobc95@udn-pharm.com
Breeze Holdings Acquisition Corp.
Investor Relations
Cody Slach and Cody Cree
Gateway Group
(949) 574-3860
BREZ@gateway-grp.com
4
Exhibit 99.2

Investor Presentation YD BIOPHARMA LIMITED September 2024

©2024, YD BIOPHARMA ., LTD. Disclaimer YD BIOPHARMA This investor presentation (this “ Presentation ”) has been prepared for use in connection with a potential business combination (the “ Business Combination ”) between Breeze Holdings Acquisition Corp . (“ SPAC ”) and YD Biopharma Limited (” YD Biopharma ” or “ YD BIO ” or “ Target ” and together with SPAC, the “ Parties ”) . This Presentation is for information purposes only and may not be reproduced or redistributed, in whole or in part, without the prior written consent of the Parties . This Presentation does not and, if hereafter supplemented, will not be all inclusive or contain all of the information that may be required to evaluate the Business Combination . The Parties reserve the right to update or supplement the information provided in this Presentation but disclaim any obligation to do so . You should not definitively rely upon it or use it to form the definitive basis for any decision, contract, commitment, or action whatsoever, with respect to the Business Combination or otherwise . You should consult your own legal, regulatory, tax, business, financial and accounting advisors to the extent you deem necessary, and you must make your own investment decision and perform your own independent investigation and analysis of the Parties and the Business Combination . To the fullest extent permitted by law, in no circumstances will either Party or any of their respective affiliates, officers, directors, employees, representatives, advisors or agents be responsible or liable for any direct, indirect or consequential loss or loss of profit arising from the use of this Presentation, its contents, its omissions, reliance on the information contained within it or on opinions communicated in relation thereto or otherwise arising in connection therewith . The information in this Presentation is highly confidential . This Presentation shall remain the property of the Parties, and the distribution or disclosure of this Presentation by you to any other person is unauthorized except to your affiliates, officers, directors, employees, representatives, advisors or agents who have a need to receive this Presentation in connection with evaluating the Business Combination and who have been advised of, and have agreed to abide by, the confidentiality obligations described herein . Any unauthorized disclosure, forwarding, reproduction or photocopying, or alteration of the contents, of this Presentation or any portion thereof is prohibited . You shall keep this Presentation and its contents confidential, shall not use this Presentation or its contents for any purpose other than as expressly authorized by the Parties and shall be required to return or destroy all copies of this Presentation or portions thereof in your possession promptly following a request by the Parties . By accepting delivery of this Presentation, you are deemed to agree to the foregoing confidentiality requirements . Any unauthorized distribution or reproduction of any part of this Presentation may result in a violation of the United States Securities Act of 1933 , as amended (the “ Act ”) . Neither Party nor any of their respective affiliates, officers, directors, employees, representatives, advisors or agents makes any representation or warranty, express or implied, in relation to the accuracy or completeness of the information contained in this Presentation or any oral information provided in connection herewith, or any data it generates, or that any transaction has been or may be effected on the terms or in the manner stated or implied by this Presentation, or as to the achievement or reasonableness of future projections, management targets, estimates, prospects or returns, if any, and the Parties accept no responsibility, obligation or liability (whether direct or indirect, in contract, tort or otherwise) in relation to any of such information . The Parties and their respective affiliates, officers, directors, employees, representatives, advisors, and agents expressly disclaim any and all liability which may be based on this Presentation and any errors therein or omissions therefrom . This Presentation and the information contained herein do not constitute : (a) (i) a solicitation of a proxy, consent or authorization with respect to any securities in respect of the Business Combination or (ii) an offer to sell or exchange or the solicitation of an offer to buy or exchange any security, commodity or instrument or related derivative, nor shall there be any sale of any securities, commodities or instruments or related derivatives in any jurisdiction in which such offer, solicitation, sale or exchange would be unlawful prior to the registration or qualification under the securities laws of any such jurisdiction or (b) an offer or commitment to lend, syndicate or arrange a financing, underwrite or purchase or act as an agent or advisor or in any other capacity with respect to any transaction, or commit capital, or to participate in any trading strategies . You should not construe the contents of this Presentation as legal, regulatory, tax, accounting or investment advice or a recommendation . We recommend that you seek independent third party legal, regulatory, business, financial, accounting and tax advice regarding the contents of this Presentation . This Presentation does not constitute and should not be considered as any form of financial or fairness opinion or recommendation by either Party or any of their respective affiliates, officers, directors, employees, representatives, advisors or agents . This Presentation is not a research report . By accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision . This Presentation is for distribution only to persons reasonably believed to be sufficiently expert to understand the risks involved in the Business Combination . You should determine the economic risks and merits, as well as the legal, regulatory, business, financial, accounting and tax characterizations and consequences of the Business Combination and independently determine that you are able to assume the risks associated with the foregoing . By accepting delivery of this Presentation, you acknowledge that you have been advised that (a) neither Party is providing you any legal, regulatory, business, financial, accounting or tax advice, (b) you understand that there will be legal, regulatory, business, financial, accounting and tax risks associated with the Business Combination, (c) you should receive legal, regulatory, business, financial, accounting and tax advice from advisors with appropriate expertise to assess relevant risks and (d) you should apprise senior management in your organization as to the legal, regulatory, business, financial, accounting and tax advice (and, if applicable, risks) associated with the Business Combination and these disclaimers as to these matters . You confirm that you are not relying upon the information contained herein to make any decision . NEITHER THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (“SEC”) NOR ANY STATE OR TERRITORIAL SECURITIES COMMISSION OR ANY OTHER REGULATORY AUTHORITY HAS APPROVED OR DISAPPROVED OF THIS PRESENTATION OR DETERMINED THAT IT IS TRUTHFUL OR COMPLETE . ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE . 2

©2024, YD BIOPHARMA ., LTD. Disclaimer YD BIOPHARMA Forward - Looking Statements This Presentation contains certain “forward - looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995 , Section 27 A of the Act and Section 21 E of the Securities Exchange Act of 1934 , as amended . All statements other than statements of historical fact contained in this Presentation, including, but not limited to, statements as to the transactions contemplated by the Business Combination and related agreements, the benefits or timing of the Business Combination, the effects of regulations, projected future results of operations and financial position, revenue and other metrics, planned products and services, business strategy and plans, objectives of management for future operations, market size and growth opportunities, competitive position and technological and market trends, are forward - looking statements . Some of these forward - looking statements can be identified by the use of forward - looking words, including, but not limited to, “may,” “should,” “expect,” “intend,” “will,” “estimate,” “anticipate,” “believe,” “predict,” “plan,” “targets,” “projects,” “could,” “would,” “continue,” “forecast,” “strategy,” and “opportunity” or the negatives of these terms or variations of them or similar expressions . All forward - looking statements are subject to risks, uncertainties, and other factors (including those which are beyond the control of either Party) which could cause actual results to differ materially from those expressed or implied by such forward - looking statements . All forward - looking statements are based upon estimates, forecasts and assumptions that, while considered reasonable by the Parties, are inherently uncertain, and many factors may cause the actual results to differ materially from current expectations, which include, but are not limited to : (a) the occurrence of any event, change or other circumstances that could give rise to the termination of the definitive agreements executed by the Parties with respect to the Business Combination ; (b) the outcome of any legal proceedings that may be instituted against either Party or the combined company, or any of their respective directors or officers, following the announcement of the Business Combination and the transactions contemplated thereby ; (c) the inability to complete the Business Combination due to the failure to obtain approval of the stockholders of either Party, or to satisfy other conditions to closing of the Business Combination ; (d) changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining applicable regulatory approvals for the Business Combination ; (e) the ability to meet or maintain the Nasdaq Stock Market’s listing standards prior to or following the consummation of the Business Combination ; (f) the risk that the announcement or consummation of the Business Combination disrupts current plans and operations of either Party ; (g) the inability to recognize the anticipated benefits of the Business Combination ; (h) the ability of the combined company to successfully increase market penetration into its target markets, to execute on its business strategy or to compete effectively ; (i) the addressable markets that the Parties intend to target do not grow as expected ; (j) the loss of any key executives ; (k) the loss of any relationships with key suppliers or customers (as may be applicable) or the inability to attract and retain customers ; (l) the inability to protect patents and other intellectual property ; (n) costs related to the Business Combination ; (o) changes in applicable laws or regulations ; (p) the possibility that either Party or the combined company may be adversely affected by other economic, business and/or competitive factors ; (q) the Parties’ estimates of growth and projected financial results and meeting or satisfying the underlying assumptions with respect thereto ; (r) the risk that the Business Combination may not be completed in a timely manner or at all, which may adversely affect the price of either Party’s securities ; (s) the risk that the transaction may not be completed by SPAC’s business combination deadline (as may be extended pursuant to SPAC’s governing documents) ; (t) the impact of the novel coronavirus disease pandemic, including any mutations or variants thereof, and its effect on business and financial conditions ; and (u) other risks and uncertainties set forth in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward - Looking Statements” in SPAC’s Form S - 1 (File No . 333 - 249677 ), annual report on Form 10 - K/A for the year ended December 31 , 2023 , quarterly report on Form 10 - Q for the period ended June 30 , 2024 and, when available, the registration statement on Form F - 4 to be filed with the SEC in connection with the Business Combination, which will include a document that serves as a preliminary prospectus and proxy statement of SPAC, referred to as a proxy statement/prospectus, and other documents filed or to be filed from time to time with the SEC in connection with the Business Combination . The foregoing list is not exhaustive, and new risks may emerge from time to time . These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward - looking statements . Nothing in this Presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . Neither Party, nor any of their respective affiliates, gives any assurance that the combined company will achieve its currently expected results . Neither Party nor the combined company undertakes any duty to update or revise these forward - looking statements, except as otherwise required by law . Use of Projections This Presentation may contain financial forecasts of the Parties and the combined company which are based on currently available information and estimates . Neither Party nor its independent auditors, nor the independent registered public accounting firm of either Party, has audited, reviewed, compiled or performed any procedures with respect to the projections for the purpose of their inclusion in this Presentation, and accordingly, none of the foregoing has expressed an opinion or provided any other form of assurance with respect thereto for the purpose of this Presentation . These projections are forward - looking statements and should not be relied upon as being necessarily indicative of future results . The projected financial information contained in this Presentation constitutes forward - looking information, and neither Party nor the combined company undertakes any duty to update or revise the projected financial information . The assumptions and estimates underlying such projected financial information are inherently uncertain and are subject to a wide variety of significant business, economic, competitive, and other risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information . See the section titled “Forward - Looking Statements” above . Accordingly, there can be no assurance that the prospective results are indicative of future performance or that actual results will not differ materially from the results presented in the prospective financial information contained in this Presentation . Actual results may differ materially from the results contemplated by the projected financial information contained in this Presentation . The inclusion of such information in this Presentation should not be regarded as a representation by any person that the results reflected in such projections will be achieved . 3

©2024, YD BIOPHARMA ., LTD. Disclaimer YD BIOPHARMA Financial Information Certain financial information and data contained in this Presentation is unaudited and may not conform to Regulation S - X . Such information and data may not be included in, may be adjusted in or may be presented differently in the registration statement to be filed with the SEC in connection with the Business Combination and the preliminary proxy statement/prospectus contained therein . Trademarks Each Party owns or has rights to various trademarks, service marks, copyrights, trade names and products that they use in connection with the operation of its business . This Presentation may also contain trademarks, service marks, copyrights, trade names or products of other companies, which are the property of their respective owners . The use or display of third parties’ trademarks, service marks, copyrights, trade names or products in this Presentation is not intended to and does not imply a relationship with either Party, or an endorsement or sponsorship by or of either Party . Solely for convenience, the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM, © or ® symbols, but such references are not intended to indicate, in any way, that each Party will not assert, to the fullest extent under applicable law, its rights or the rights of the applicable owners, if any, to these trademarks, service marks, copyrights, trade names and products . Industry and Market Data This Presentation contains and relies on certain information obtained from third - party sources and the internal sources . This information involves many assumptions and limitations ; therefore, there can be no guarantee as to the accuracy or reliability of such assumptions, and you are cautioned not to give undue weight to this information . Further, no representation or warranty, express or implied, is made by either Party as to the reasonableness of the assumptions made within or the accuracy or completeness of any projections or any other information contained herein, and to the fullest extent permitted by law, the Parties hereby disclaim any responsibility or liability for any direct, indirect or consequential loss or loss of profit arising from the use of such information . Any data on past performance or modelling contained herein are not an indication as to future performance . Neither Party nor any of their respective affiliates, officers, directors, employees, representatives or advisors has independently verified the accuracy or completeness of any such third - party information . Similarly, other third - party survey data and research reports commissioned by the Parties, while believed to be reliable, are based on limited sample sizes and have not been independently verified by either Party . In addition, projections, assumptions, estimates, goals, targets, plans and trends of the future performance of the industry in which Target operates, and its future performance, are necessarily subject to uncertainty and risk due to a variety of factors, including those described above . These and other factors could cause results to differ materially from those expressed in the estimates made by independent parties and by the Parties . Additional Information and Where to Find It The Parties intend to file a definitive proxy statement/prospectus of SPAC with the SEC in connection with the Business Combination (the “ Definitive Proxy Statement/Prospectus ”) . The Definitive Proxy Statement/Prospectus will be sent to all stockholders of SPAC and Target as of a record date to be established . The Parties will also file other documents regarding the Business Combination with the SEC . Before making any voting or other investment decision, investors and security holders of SPAC and Target are urged to read the Definitive Proxy Statement/Prospectus and all other relevant documents filed or that will be filed with the SEC in connection with the Business Combination as they become available because they will contain important information about the Parties and the Business Combination . Investors and security holders will be able to obtain free copies of the Definitive Proxy Statement/Prospectus and all other relevant documents filed or that will be filed with the SEC by the Parties in connection with the Business Combination through the website maintained by the SEC at www . sec . gov . Participants in the Solicitation The Parties and certain other parties to the definitive agreement executed in connection with the Business Combination, and certain of their respective directors, executive officers and other members of management and employees, may, under SEC rules, be deemed to be participants in the solicitation of proxies from SPAC’s stockholders in connection with the Business Combination . A list of the names of such persons and information regarding their interests in the Business Combination will be contained in the Definitive Proxy Statement/Prospectus when available . You may obtain free copies of these documents free of charge by directing a written request to SPAC at doug@breezeacquisition . com . This Presentation does not contain all the information that should be considered in connection with the Business Combination . It is not intended to form any basis of any investment decision or any decision in respect to the Business Combination . 4

©2024, YD BIOPHARMA ., LTD. YD BIO Merger w/ Breeze Holdings Acquisition Corp. YD BIOPHARMA Key Management • SPAC Overview : Breeze Holdings Acquisition Corp . (OTCQX : BRZH) is a publicly - listed special purpose acquisition company with approximately $ 10 M in cash . • Valuation : Company pro forma equity value of approximately $ 700 m . • Structure : Breeze and YD BIO will each merge into wholly - owned subsidiaries of a newly formed Cayman holding company expected to be named “YD Bio Limited . ” Post - merger YD Bio Limited is expected to be listed on Nasdaq . • Ownership : Existing YD BIO shareholders : 90 . 6 % , PIPE shareholders : 2 . 6 % , SPAC public shares : 2 . 1 % , SPAC insider shares : 4 . 7 % . • Timing : The transaction is expected to close 1 Q 25 , subject to customary closing conditions and any required regulatory approvals . • Management and Board Composition : Upon completion of the transaction, YD BIO will continue to be led by Dr . Ethan Shen . The Board will consist of YD BIO’s CEO, five ( 5 ) directors designated by YD BIO and two ( 2 ) directors designated by Breeze Holdings . Breeze and YD Biopharma Partnership Combines Public Company Experience and Sector Expertise Transaction Details Experience Name And Position ~25 Years Doug Ramsey, Ph.D. Chairman & CEO ~35 Years Russ Griffin Director & President ~23 Years Dr. Ethan Shen Chairman & CEO ~17 Years Wu Cheng - feng Chief Medical Officer ~35 Years May Tsai Chief Business Officer ~18 Years Jean Chen Accounting Manager Breeze Holdings YD Biopharma 5
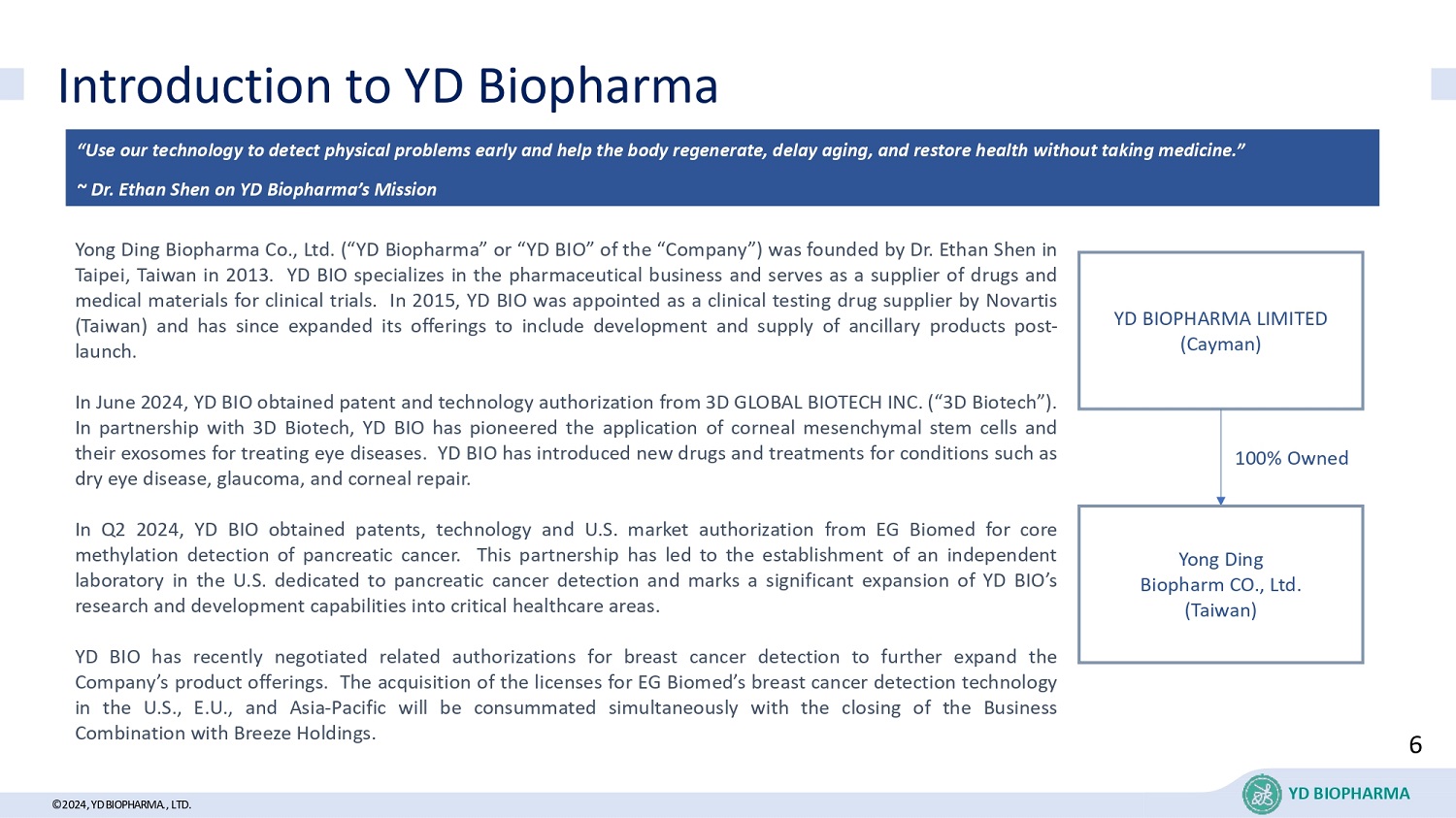
©2024, YD BIOPHARMA ., LTD. Introduction to YD Biopharma YD BIOPHARMA Yong Ding Biopharma Co . , Ltd . (“YD Biopharma” or “YD BIO” of the “Company”) was founded by Dr . Ethan Shen in Taipei, Taiwan in 2013 . YD BIO specializes in the pharmaceutical business and serves as a supplier of drugs and medical materials for clinical trials . In 2015 , YD BIO was appointed as a clinical testing drug supplier by Novartis (Taiwan) and has since expanded its offerings to include development and supply of ancillary products post - launch . In June 2024 , YD BIO obtained patent and technology authorization from 3 D GLOBAL BIOTECH INC . (“ 3 D Biotech”) . In partnership with 3 D Biotech, YD BIO has pioneered the application of corneal mesenchymal stem cells and their exosomes for treating eye diseases . YD BIO has introduced new drugs and treatments for conditions such as dry eye disease, glaucoma, and corneal repair . In Q 2 2024 , YD BIO obtained patents, technology and U . S . market authorization from EG Biomed for core methylation detection of pancreatic cancer . This partnership has led to the establishment of an independent laboratory in the U . S . dedicated to pancreatic cancer detection and marks a significant expansion of YD BIO’s research and development capabilities into critical healthcare areas . YD BIO has recently negotiated related authorizations for breast cancer detection to further expand the Company’s product offerings . The acquisition of the licenses for EG Biomed’s breast cancer detection technology in the U . S . , E . U . , and Asia - Pacific will be consummated simultaneously with the closing of the Business Combination with Breeze Holdings . “ Use our technology to detect physical problems early and help the body regenerate, delay aging, and restore health without ta kin g medicine. ” ~ Dr. Ethan Shen on YD Biopharma’s Mission YD BIOPHARMA LIMITED (Cayman) Yong Ding Biopharm CO., Ltd. (Taiwan) 100% Owned 6
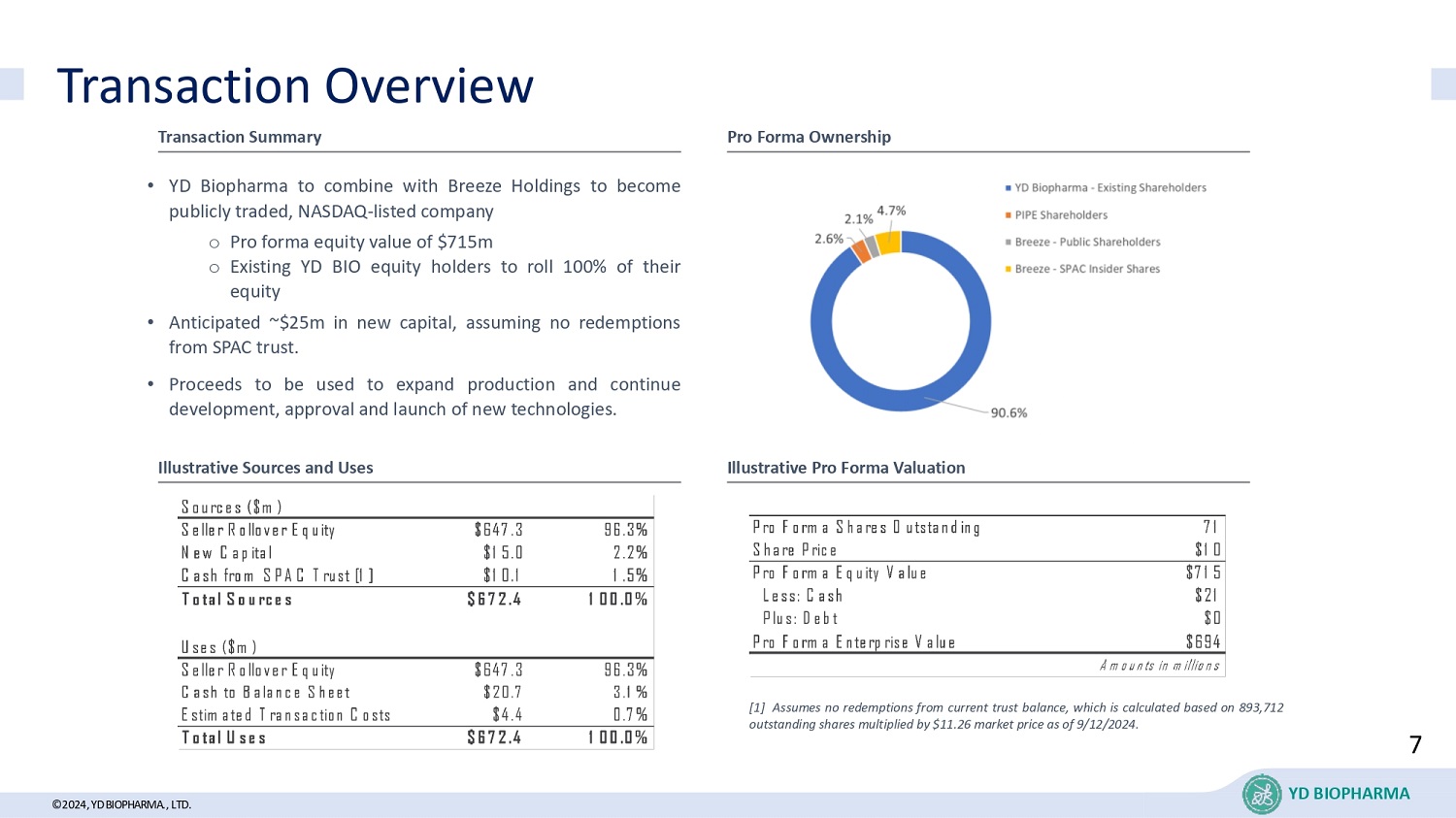
©2024, YD BIOPHARMA ., LTD. Transaction Overview YD BIOPHARMA Transaction Summary Pro Forma Ownership Illustrative Sources and Uses Illustrative Pro Forma Valuation • YD Biopharma to combine with Breeze Holdings to become publicly traded, NASDAQ - listed company o Pro forma equity value of $ 715 m o Existing YD BIO equity holders to roll 100 % of their equity • Anticipated ~ $ 25 m in new capital, assuming no redemptions from SPAC trust . • Proceeds to be used to expand production and continue development, approval and launch of new technologies . 95.4% Pro Forma Shares Outstanding 71 Share Price $10 Pro Forma Equity Value $715 Less: Cash $21 Plus: Debt $0 Pro Forma Enterprise Value $694 Amounts in millions [ 1 ] Assumes no redemptions from current trust balance, which is calculated based on 893 , 712 outstanding shares multiplied by $ 11 . 26 market price as of 9 / 12 / 2024 . Sources ($m) Seller Rollover Equity $647.3 96.3% New Capital $15.0 2.2% Cash from SPAC Trust [1] $10.1 1.5% Total Sources $672.4 100.0% Uses ($m) Seller Rollover Equity $647.3 96.3% Cash to Balance Sheet $20.7 3.1% Estimated Transaction Costs $4.4 0.7% Total Uses $672.4 100.0% 7
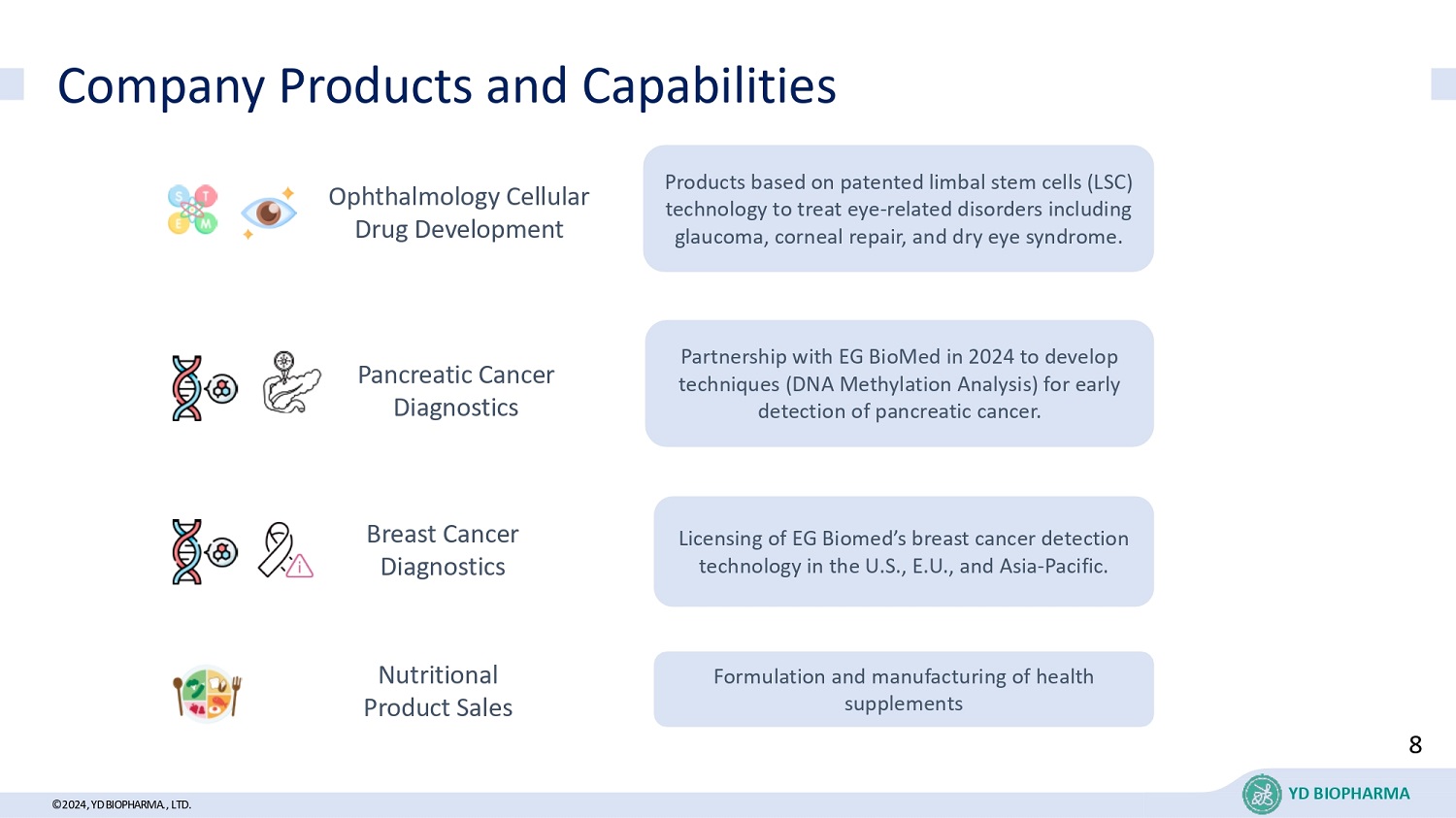
©2024, YD BIOPHARMA ., LTD. Company Products and Capabilities YD BIOPHARMA 8 Formulation and manufacturing of health supplements Products based on patented limbal stem cells (LSC) technology to treat eye - related disorders including glaucoma, corneal repair, and dry eye syndrome. Partnership with EG BioMed in 2024 to develop techniques (DNA Methylation Analysis) for early detection of pancreatic cancer. Licensing of EG Biomed’s breast cancer detection technology in the U.S., E.U., and Asia - Pacific. Pancreatic Cancer Diagnostics Breast Cancer Diagnostics Ophthalmology Cellular Drug Development Nutritional Product Sales
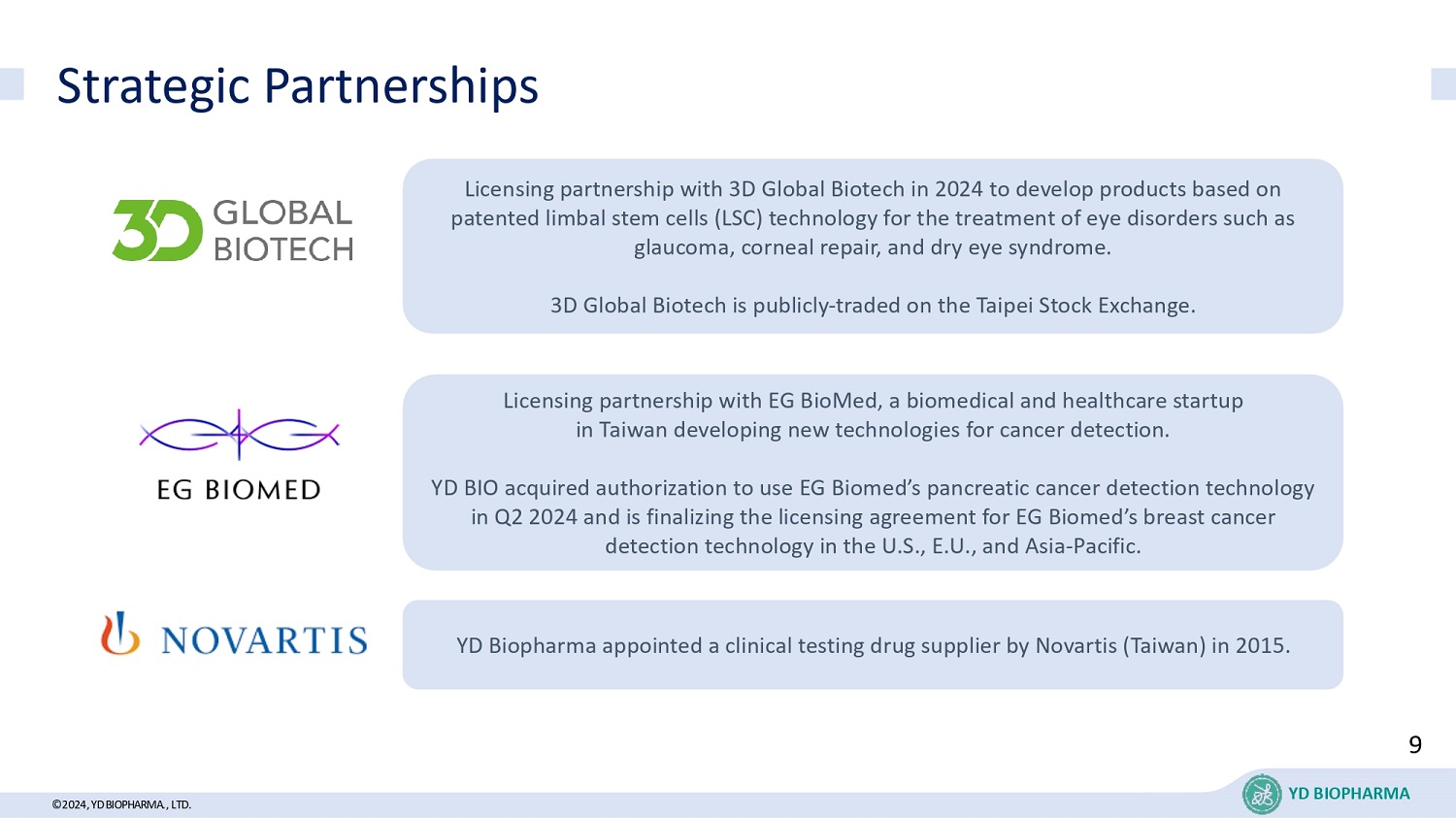
©2024, YD BIOPHARMA ., LTD. Strategic Partnerships YD BIOPHARMA YD Biopharma appointed a clinical testing drug supplier by Novartis (Taiwan) in 2015. Licensing partnership with 3D Global Biotech in 2024 to develop products based on patented limbal stem cells (LSC) technology for the treatment of eye disorders such as glaucoma, corneal repair, and dry eye syndrome. 3D Global Biotech is publicly - traded on the Taipei Stock Exchange. Licensing partnership with EG BioMed, a biomedical and healthcare startup in Taiwan developing new technologies for cancer detection. YD BIO acquired authorization to use EG Biomed’s pancreatic cancer detection technology in Q2 2024 and is finalizing the licensing agreement for EG Biomed’s breast cancer detection technology in the U.S., E.U., and Asia - Pacific. 9
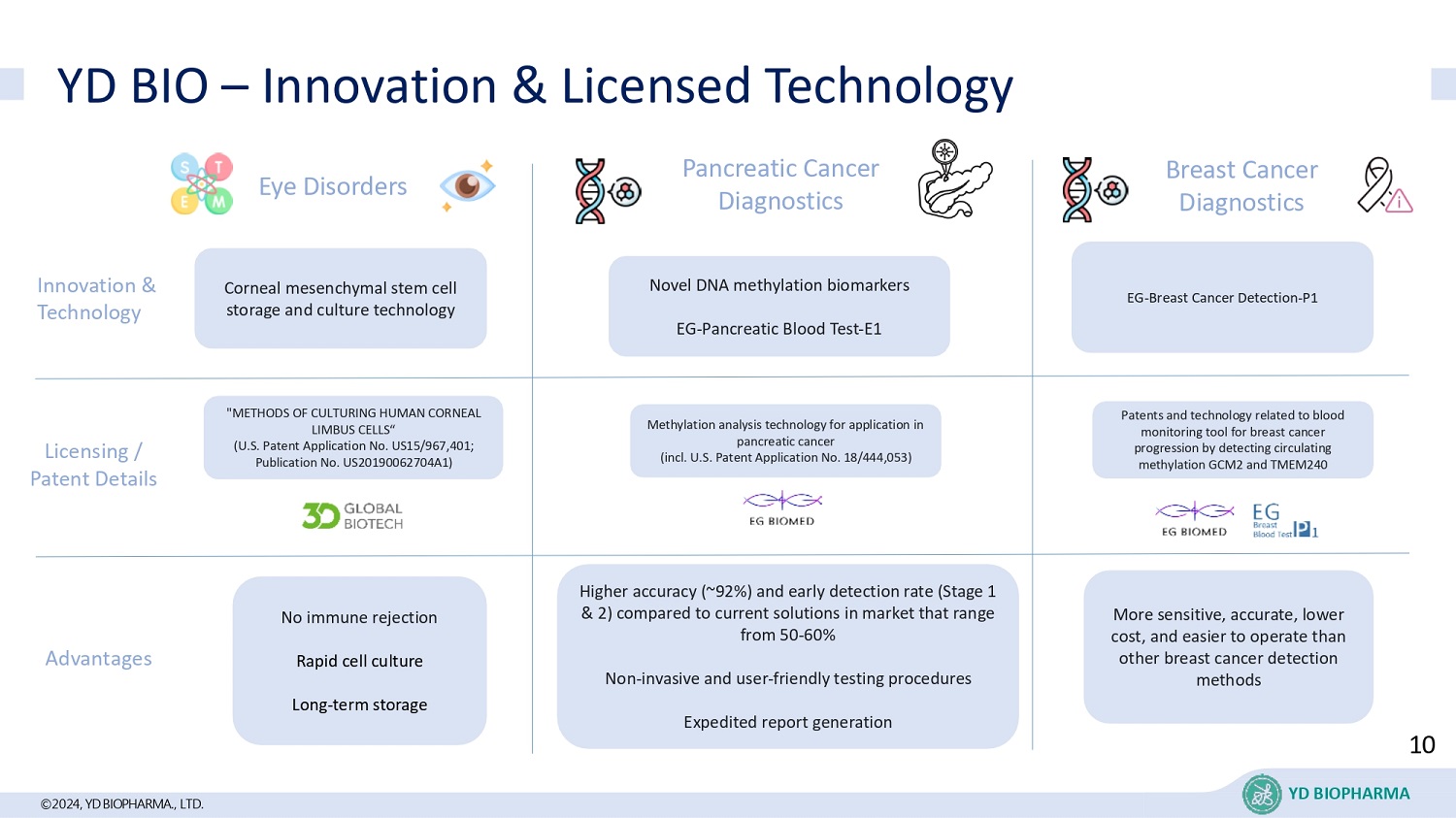
©2024, YD BIOPHARMA ., LTD. YD BIO – Innovation & Licensed Technology Eye Disorders No immune rejection Rapid cell culture Long - term storage Pancreatic Cancer Diagnostics Higher accuracy (~92%) and early detection rate (Stage 1 & 2) compared to current solutions in market that range from 50 - 60% Non - invasive and user - friendly testing procedures Expedited report generation Breast Cancer Diagnostics More sensitive, accurate, lower cost, and easier to operate than other breast cancer detection methods Licensing / Patent Details Advantages Novel DNA methylation biomarkers EG - Pancreatic Blood Test - E1 Corneal mesenchymal stem cell storage and culture technology EG - Breast Cancer Detection - P1 Innovation & Technology "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS“ (U.S. Patent Application No. US15/967,401; Publication No. US20190062704A1) Patents and technology related to blood monitoring tool for breast cancer progression by detecting circulating methylation GCM2 and TMEM240 Methylation analysis technology for application in pancreatic cancer (incl. U.S. Patent Application No. 18/444,053) 10 YD BIOPHARMA
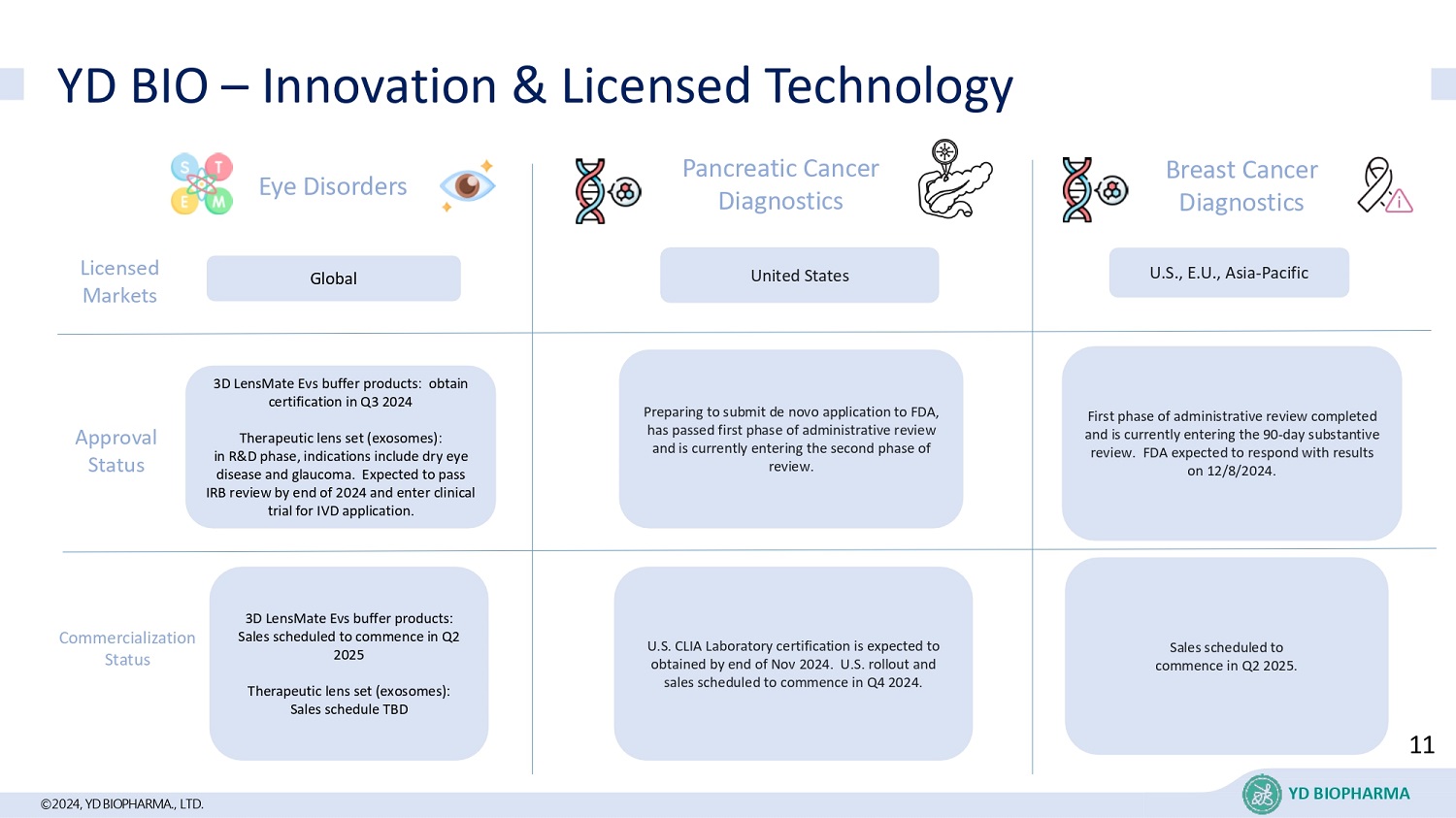
©2024, YD BIOPHARMA ., LTD. YD BIO – Innovation & Licensed Technology Global Pancreatic Cancer Diagnostics United States Breast Cancer Diagnostics U.S., E.U., Asia - Pacific L icensed Markets Approval Status 3D LensMate Evs buffer products: obtain certification in Q3 2024 Therapeutic lens set (exosomes): in R&D phase, indications include dry eye disease and glaucoma. Expected to pass IRB review by end of 2024 and enter clinical trial for IVD application. First phase of administrative review completed and is currently entering the 90 - day substantive review. FDA expected to respond with results on 12/8/2024. Preparing to submit de novo application to FDA, has passed first phase of administrative review and is currently entering the second phase of review. Commercialization Status U.S. CLIA Laboratory certification is expected to obtained by end of Nov 2024. U.S. rollout and sales scheduled to commence in Q4 2024. Sales scheduled to commence in Q2 2025. 3D LensMate Evs buffer products: Sales scheduled to commence in Q2 2025 Therapeutic lens set (exosomes): Sales schedule TBD Eye Disorders 11 YD BIOPHARMA
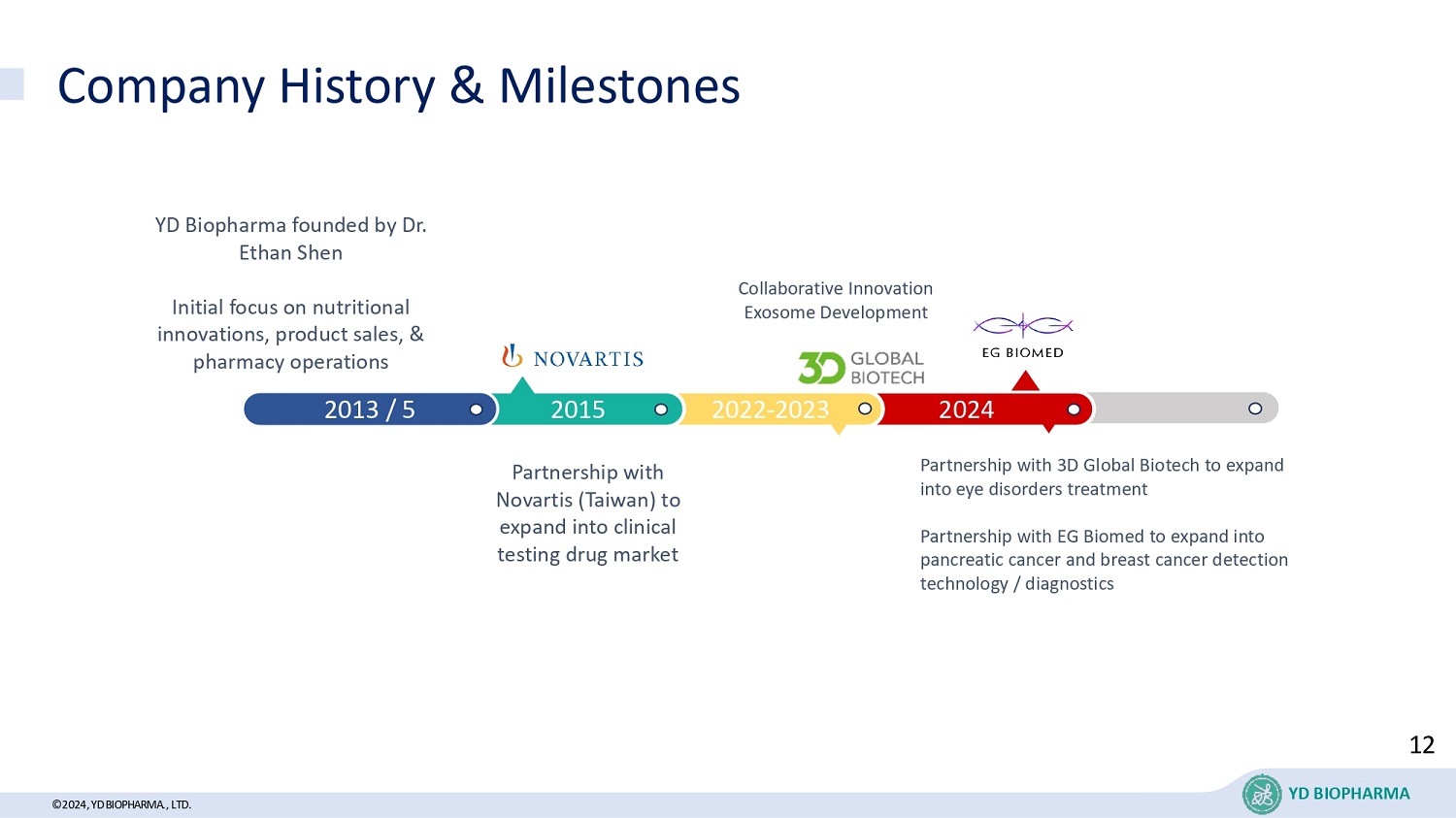
©2024, YD BIOPHARMA ., LTD. Company History & Milestones YD BIOPHARMA YD Biopharma founded by Dr. Ethan Shen Initial focus on nutritional innovations, product sales, & pharmacy operations Partnership with Novartis (Taiwan) to expand into clinical testing drug market Collaborative Innovation Exosome Development Partnership with 3D Global Biotech to expand into eye disorders treatment Partnership with EG Biomed to expand into pancreatic cancer and breast cancer detection technology / diagnostics 2024 2022 - 2023 2015 2013 / 5 12
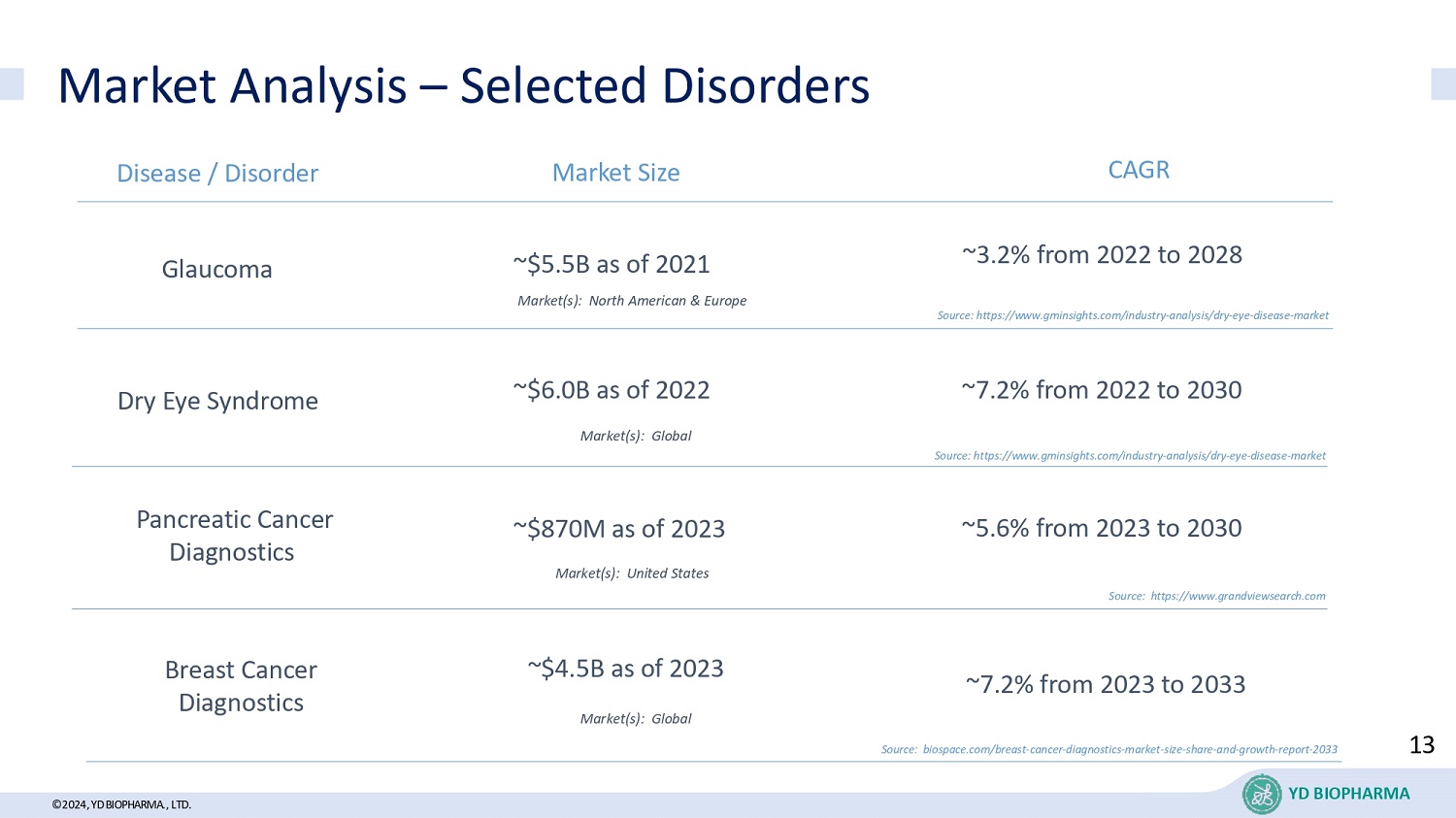
©2024, YD BIOPHARMA ., LTD. Market Analysis – Selected Disorders YD BIOPHARMA Pancreatic Cancer Diagnostics Glaucoma Dry Eye Syndrome Disease / Disorder Market Size ~$870M as of 2023 CAGR ~5.6% from 2023 to 2030 ~$5.5B as of 2021 ~3.2% from 2022 to 2028 ~$6.0B as of 2022 Source: https://www.grandviewsearch.com ~7.2% from 2022 to 2030 Source: https://www.gminsights.com/industry - analysis/dry - eye - disease - market Breast Cancer Diagnostics ~$4.5B as of 2023 ~7.2% from 2023 to 2033 Source: biospace.com/breast - cancer - diagnostics - market - size - share - and - growth - report - 2033 Market(s): Global Market(s): North American & Europe Source: https://www.gminsights.com/industry - analysis/dry - eye - disease - market Market(s): Global Market(s): United States 13
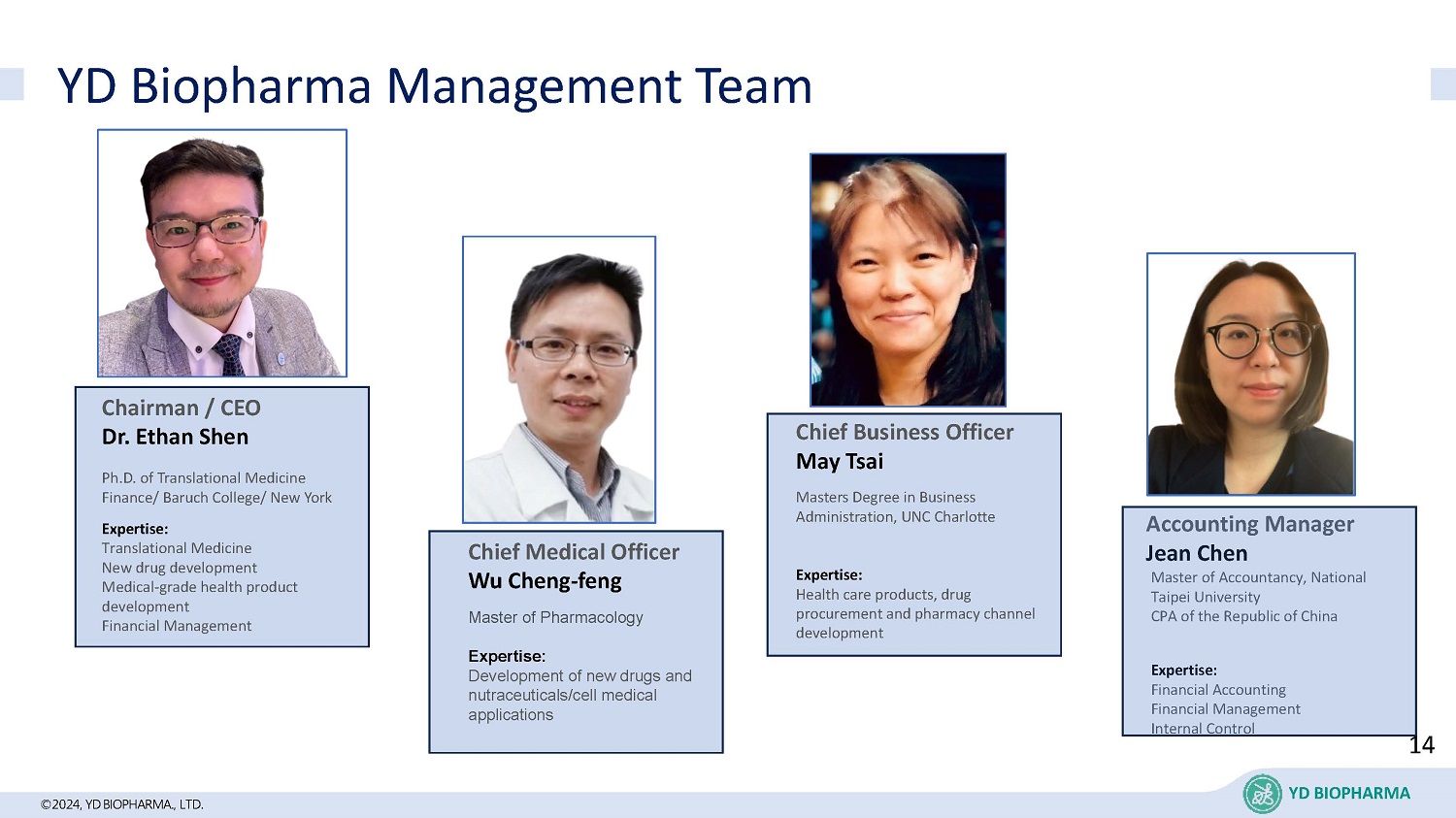
©2024, YD BIOPHARMA ., LTD. YD Biopharma Management Team Ph.D. of Translational Medicine Finance/ Baruch College/ New York Expertise: Translational Medicine New drug development Medical - grade health product development Financial Management Chairman / CEO Dr. Ethan Shen C hief Medical Officer Wu Cheng - feng Master of Pharmacology Expertise: Development of new drugs and nutraceuticals/cell medical applications C hief Business Officer May Tsai Masters Degree in Business Administration, UNC Charlotte Expertise: H ealth care products, drug procurement and pharmacy channel development Master of Accountancy, National Taipei University CPA of the Republic of China Expertise: Financial Accounting Financial Management Internal Control Accounting Manager Jean Chen 14 YD BIOPHARMA

Doug Ramsey, Ph.D. Chairman, CEO, CFO and Director ▪ Finance professor at SMU, Baylor and Cal Poly Pomona ▪ BS in Finance from Cal Poly Pomona, MBA from the University of Chicago Booth School of Business and an MA and Ph.D. in Business and Financial Economics from Claremont Graduate University ▪ Public - company CFO experience ▪ National Association of Corporate Directors (NACD) – Directorship Certified ©2024, YD BIOPHARMA ., LTD. Breeze Holdings Acquisition Corp. Management Team – Selected Highlights Russ Griffin President and Director ▪ BS in Petroleum Engineering Technology from Nicholls State University ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international ▪ National Association of Corporate Directors (NACD) – Directorship Certified Charles Ross Chief Operating Officer ▪ BS in Engineering from UT Austin ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international Aaron Ortega Executive VP of Business Development ▪ BA from Duke University, and an MBA from Southern Methodist University’s Cox School of Business ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international SPAC Board Members: Albert McLelland, Robert Thomas, Bill Stark and Gen. James Williams. Various Roles & Credentials ▪ Director of the Chairman’s Asian Cross - Border Transactions Initiative for PwC ▪ Adjunct Professor at SMU Caruth Institute for Entrepreneurship in the Cox School of Business ▪ Extensive international operating, capital markets and corporate governance experience including multiple director roles on b eha lf of the Carlyle Group and other prominent financial services firms 15 YD BIOPHARMA
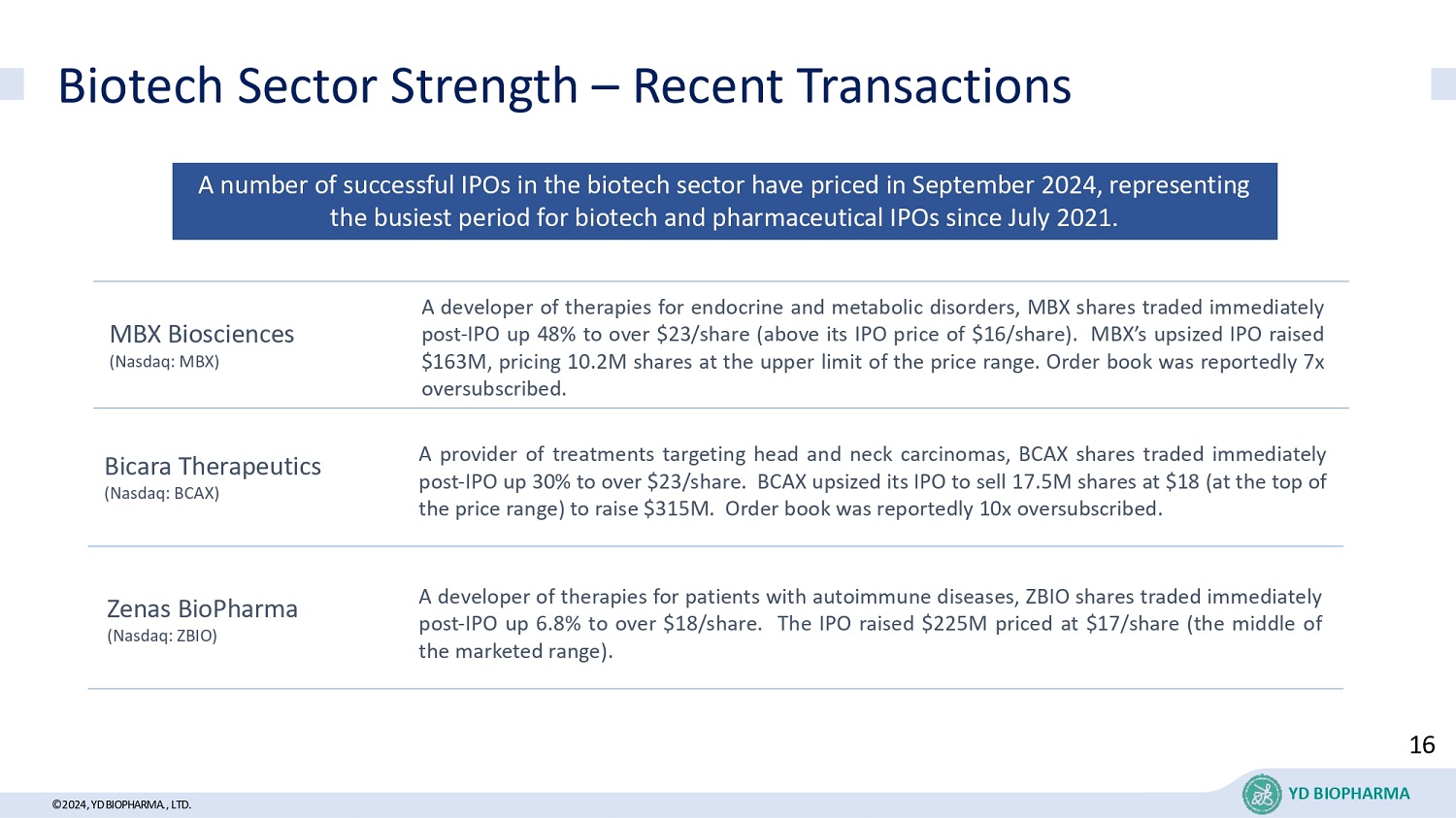
©2024, YD BIOPHARMA ., LTD. Biotech Sector Strength – Recent Transactions YD BIOPHARMA MBX Biosciences (Nasdaq: MBX) Bicara Therapeutics (Nasdaq: BCAX) Zenas BioPharma (Nasdaq: ZBIO) A developer of therapies for endocrine and metabolic disorders, MBX shares traded immediately post - IPO up 48 % to over $ 23 /share (above its IPO price of $ 16 /share) . MBX’s upsized IPO raised $ 163 M, pricing 10 . 2 M shares at the upper limit of the price range . Order book was reportedly 7 x oversubscribed . A provider of treatments targeting head and neck carcinomas, BCAX shares traded immediately post - IPO up 30 % to over $ 23 /share . BCAX upsized its IPO to sell 17 . 5 M shares at $ 18 (at the top of the price range) to raise $ 315 M . Order book was reportedly 10 x oversubscribed . A number of successful IPOs in the biotech sector have priced in September 2024, representing the busiest period for biotech and pharmaceutical IPOs since July 2021. A developer of therapies for patients with autoimmune diseases, ZBIO shares traded immediately post - IPO up 6 . 8 % to over $ 18 /share . The IPO raised $ 225 M priced at $ 17 /share (the middle of the marketed range) . 16

APPENDIX

We will strive to surpass our competitors within two years Galleri EGPT - E1 Test Grail EG BIOMED Company NGS platform qPCR platform platform High Low Testing Cost Analysis takes a long time and reporting is slow Short analysis time and quick report generation Test of Time High construction costs Unable to quickly deploy globally Low construction cost Rapid global layout Global layout Through low detection cost, fast speed and low construction cost, we hope to surpass our competitors in 2 years . 18 YD BIOPHARMA
©2024, YD BIOPHARMA ., LTD. Publications Related to Licensed Technology 19 YD BIOPHARMA
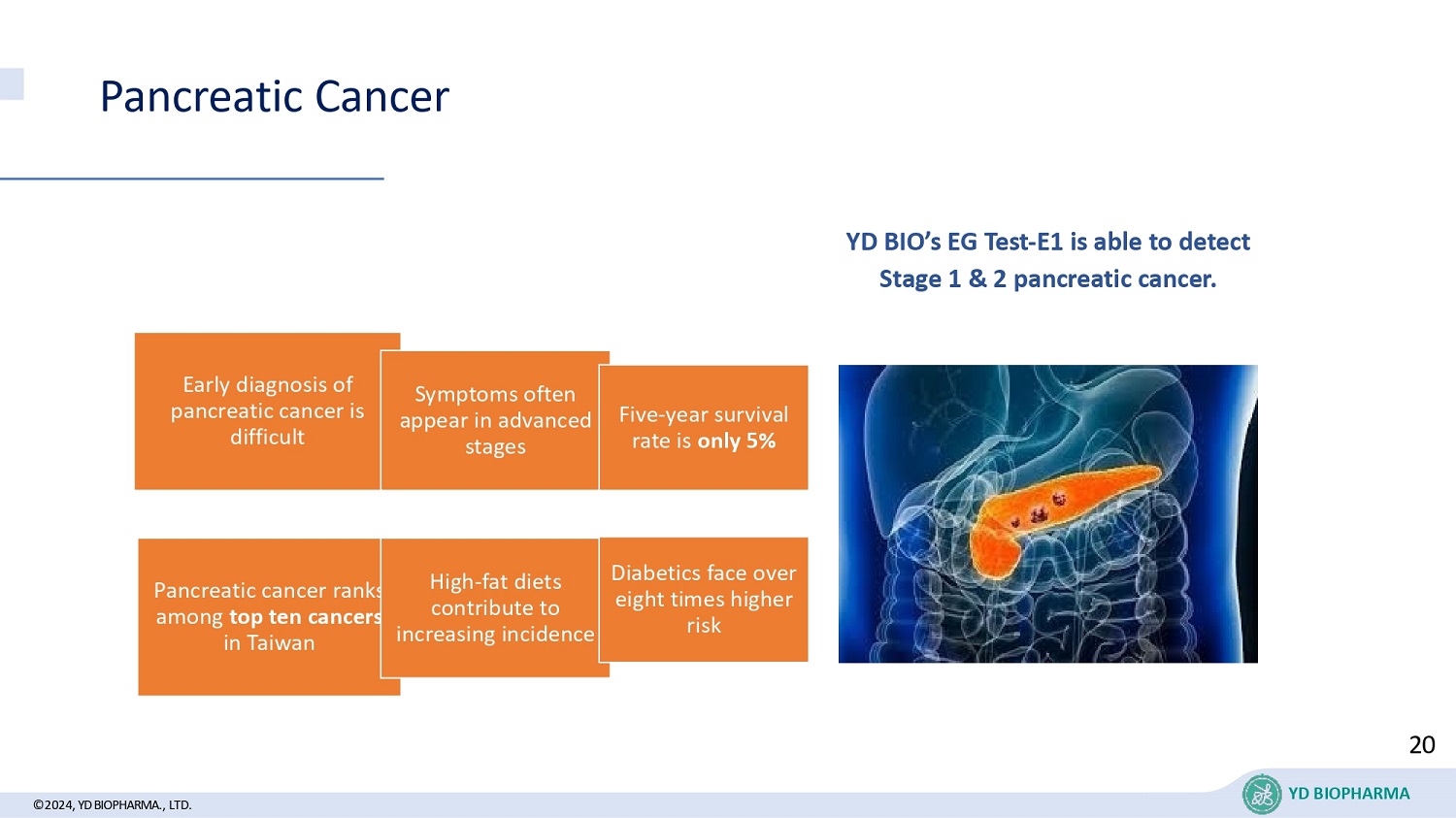
Pancreatic cancer ranks among top ten cancers in Taiwan Pancreatic Cancer ©2024, YD BIOPHARMA ., LTD. Early diagnosis of pancreatic cancer is difficult Symptoms often appear in advanced stages Five - year survival rate is only 5% High - fat diets contribute to increasing incidence Diabetics face over eight times higher risk YD BIO’s EG Test - E1 is able to detect Stage 1 & 2 pancreatic cancer. 20 YD BIOPHARMA
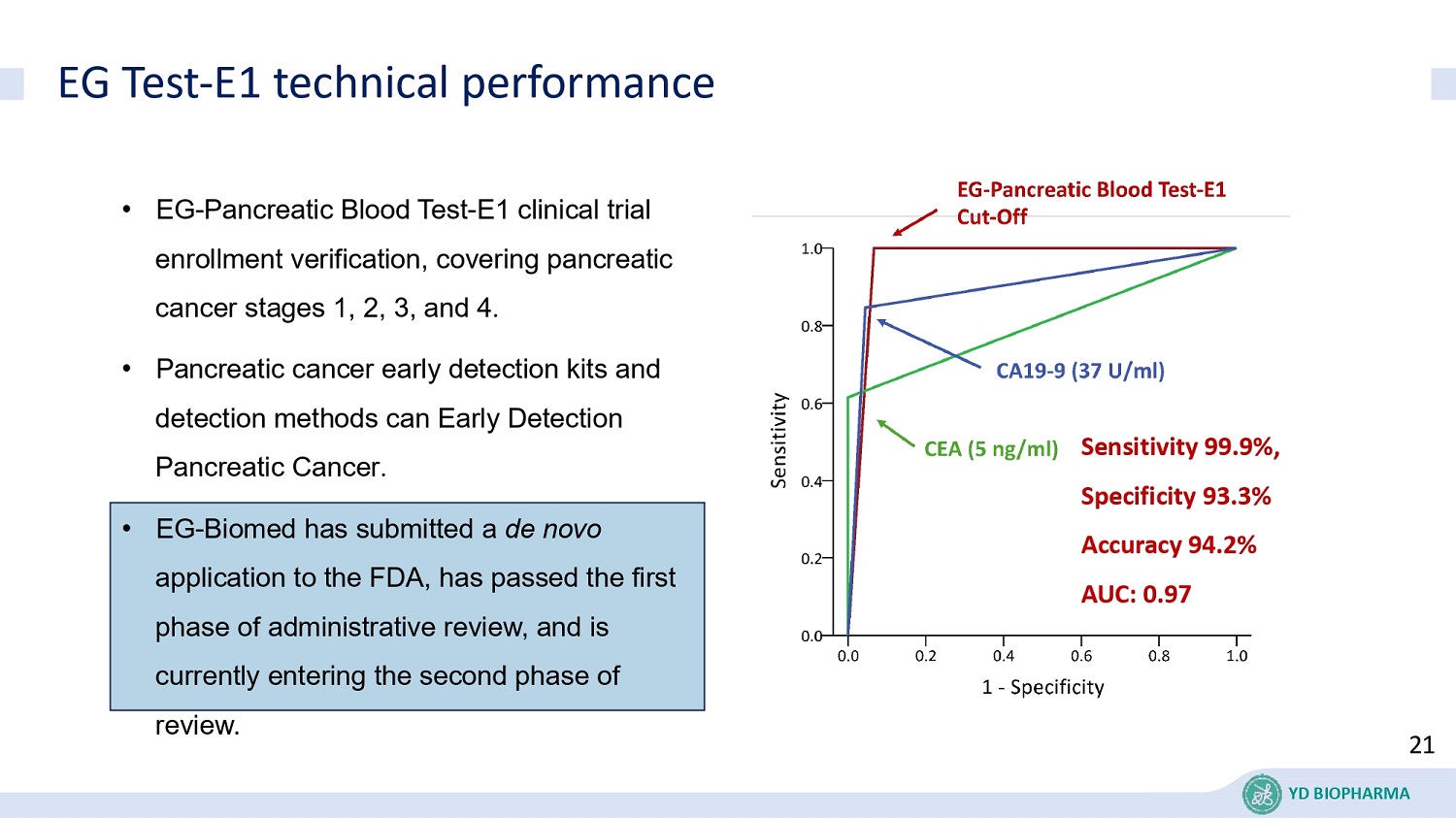
EG Test - E1 technical performance • EG - Pancreatic Blood Test - E1 clinical trial enrollment verification, covering pancreatic cancer stages 1, 2, 3, and 4 . • Pancreatic cancer early detection kits and detection methods can Early Detection Pancreatic Cancer . • EG - Biomed has submitted a de novo application to the FDA, has passed the first phase of administrative review, and is currently entering the second phase of review. Sensitivity 99.9%, Specificity 93.3% Accuracy 94.2% AUC: 0.97 21 YD BIOPHARMA

Breast cancer diagnosis requires invasive and anesthetic breast biopsy procedures, which can be stressful for patients. Current blood tests for breast cancer monitoring lack sufficient sensitivity . The Clinical Need for Precise, Non - Invasive Blood Cancer Detection Breast Cancer 22 ©2024, YD BIOPHARMA ., LTD. YD BIOPHARMA
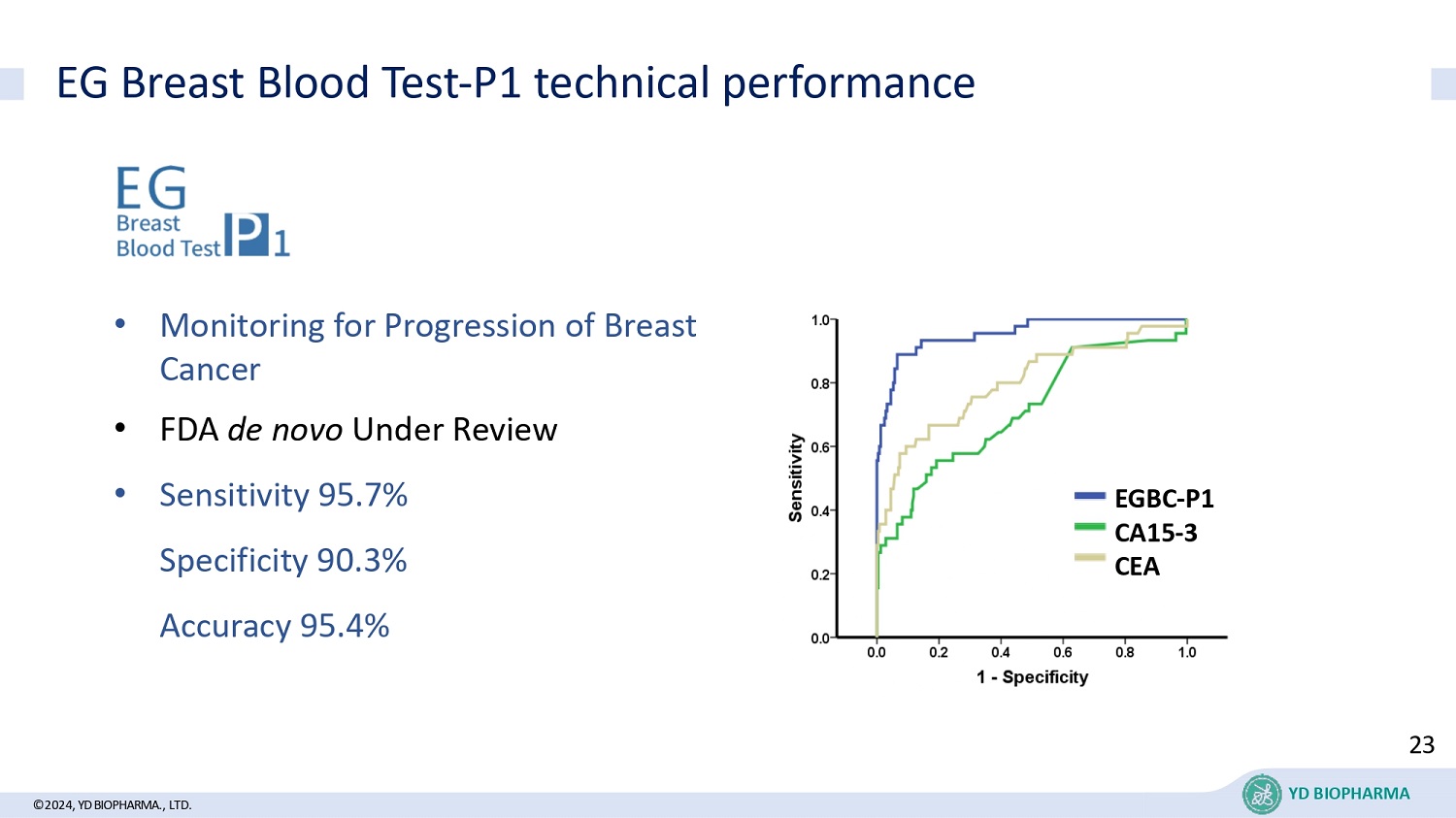
EG Breast Blood Test - P1 technical performance • Monitoring for Progression of Breast Cancer • FDA de novo Under Review • Sensitivity 95.7% Specificity 90.3% Accuracy 95.4% EGBC - P1 CA15 - 3 CEA 23 ©2024, YD BIOPHARMA ., LTD. YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement from 3D GLOBAL BIOTECH INC. • Licensed Patent and Know - How: "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS" (U.S. Patent Application No. US15/967,401; Publication No. US20190062704A1), and owns the relevant know - how and technical data. • Scope of License: 3D GLOBAL 's Patent and Know - How, as well as its know - how in the technology of cell culture process, technology of cell bank construction , exosome purification and authentication technology, and exosome production , which are related to and/or derived from "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS" . YD Biopharma obtains a global, exclusive license from 3D GLOBAL to use the licensed subject to develop, manufacture, offer for sale, sell, use, or import for the above purpose the Product. • Method of License: Global, exclusive license. and agrees YD Biopharma can sublicense to a third party. » Authorization tim e: From the effective date of this Agreement until the expiration of twenty (20) years after all the Product have/has been put on the market. » License Fees and Royalties from selling the Product: Total Licensing fee is USD 5,000,000, Signature Deposit is USD 1,000,0000, Other USD4,000,000 authorization fees will be paid upon milestone completion. and a royalty of 10% of the " Gross Sales" of the Product on a quarterly basis. » Sub - licenses Fee: 20% of the sub - license fees 24 YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement (Pancreatic cancer Detection) from EG BioMed • Licensed Patent and Know - How: methylation analysis technology for application in pancreatic cancer (including U.S. Patent Application No. 18/444,053) and the relevant know - how, and agrees to have the aforesaid patent(s) and relevant know - how • Scope of License: use of the Licensed Patent and Know - How in the Licensed Territory (United States) for manufacturing, offering for sale, selling, using, or importing the Product for the aforementioned purposes. High - fat diets contribute to increasing incidence • Method of License: E xclusive license in U.S. » Authorization tim e : 10 years and will be automatically renewed for another five years or until the expiration of the Licensed Patent » License Fees and Royalties from selling the Product: NT$60,000,000 (tax excluded) and a royalty of 7% of the " Gross Sales" of the Product on a quarterly basis. 25 YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement (Breast cancer Detection) from EG BioMed * • Licensed Patent and Know - How: methylation analysis technology for application in breast cancer (including U.S. Patent Application No. 17/053,688, Europe 3790984, China Patent No. 201980031343.5,Taiwan Patent No. I721414 and other Patent in Asian countries) and the relevant know - how, and agrees to have the aforesaid patent(s) and relevant know - how • Scope of License: use of the Licensed Patent and Know - How in the Licensed Territory(American, European and Asian) for manufacturing, offering for sale, selling, using, or importing the Product for the aforementioned purposes. High - fat diets contribute to increasing incidence • Method of License: E xclusive license • Authorization tim e : 20 years and will be automatically renewed for another five years or until the expiration of the Licensed Patent » License Fees and Royalties from selling the Product: A royalty of 20% of the " Gross Sales" of the Product on a quarterly basis. *YD Biopharma and EG BioMed have reached an agreement in principle on the above terms and are currently working on definitive documentation. 26 YD BIOPHARMA

YD BIOPHARMA LIMITED THANK YOU
v3.24.3
Cover
|
Sep. 24, 2024 |
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 24, 2024
|
| Entity File Number |
001-39718
|
| Entity Registrant Name |
BREEZE HOLDINGS ACQUISITION CORP.
|
| Entity Central Index Key |
0001817640
|
| Entity Tax Identification Number |
85-1849315
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
955 W.
John Carpenter Freeway
|
| Entity Address, Address Line Two |
Suite 100-929
|
| Entity Address, City or Town |
Irving
|
| Entity Address, State or Province |
TX
|
| Entity Address, Postal Zip Code |
75039
|
| City Area Code |
619
|
| Local Phone Number |
500-7747
|
| Written Communications |
true
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Common Stock, par value $0.0001 per share |
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
BRZH
|
| Rights exchangeable into one-twentieth of one share of common stock |
|
| Title of 12(b) Security |
Rights exchangeable into one-twentieth of one share of common stock
|
| Trading Symbol |
BRZHR
|
| Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 per whole share |
|
| Title of 12(b) Security |
Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 per whole share
|
| Trading Symbol |
BRZHW
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=BRZH_CommonStockParValue0.0001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=BRZH_RightsExchangeableIntoOnetwentiethOfOneShareOfCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=BRZH_WarrantsEachWholeWarrantExercisableForOneShareOfCommonStockAtExercisePriceOf11.50PerWholeShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Breeze Holdings Acquisit... (QX) (USOTC:BRZHW)
Historical Stock Chart
From Dec 2024 to Jan 2025

Breeze Holdings Acquisit... (QX) (USOTC:BRZHW)
Historical Stock Chart
From Jan 2024 to Jan 2025
