Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2024
☐ TRANSITION REPORT UNDER SECTION 13 OR
15 (d) OF THE EXCHANGE ACT
For the transition period from _________ to _________
Commission File Number: 000-12641
DALRADA FINANCIAL CORPORATION
(Name of Small Business Issuer in its charter)
| Wyoming |
38-3713274 |
| (state or other jurisdiction of incorporation or organization) |
(I.R.S. Employer ID. No.) |
600 La Terraza Blvd., Escondido, California 92025
(Address of principal executive offices)
858-283-1253
Issuer’s telephone number
Securities registered pursuant to Section 12(b) of
the Act: None
Securities registered pursuant to Section 12(g) of
the Act: None
| Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
| Common Stock, $0.005 par value per share |
|
DFCO |
|
None |
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required
to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1)
has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days. Yes ☐ No ☒
Indicate by check mark whether the registrant has
submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of
this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☐
No ☒
Indicate by check mark whether the registrant is a
large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See
the definitions of “large accelerated filer”, “accelerated filer” “smaller reporting company,” and
“emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ |
|
Accelerated filer ☐ |
| Non-accelerated filer ☒ |
Smaller reporting company ☒ |
| Emerging growth company ☐ |
|
If an emerging growth company, indicate by check mark
if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has
filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting
under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its
audit report. Yes ☐ No ☒
If securities are registered pursuant to Section 12(b)
of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of
an error to previously issued financial statements. Indicate by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act). Yes ☐ No ☐
Indicate by check mark whether any of those error
corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s
executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a
shell company (as defined in Rule 12b-2 of the Act) Yes ☐ No ☒
State the aggregate market value of the voting and
non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average
bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal
quarter: $17,158,525.
As of January 8, 2025, the registrant’s outstanding stock consisted
of 120,157,113 common shares.
DALRADA FINANCIAL CORPORATION
Table of Contents
PART I
Item 1. Description of Business
Company Overview
Moving the world forward takes bold resolve that turns
ideas into actions and builds real-time solutions that positively impact people and the planet. Dalrada Financial Corporation (“Dalrada”
or the “Company”) accelerates positive change for current and future generations by harnessing true potential and developing
products and services that become transformative innovations.
Dalrada was incorporated in September 1982 under the
laws of the State of California. It was reincorporated in May 1983 under the laws of the State of Delaware and reincorporated again on
May 5, 2020, under the laws of the state of Wyoming. Dalrada Financial Corporation trades under the symbol, OTC: DFCO.
Dalrada has five business divisions: Genefic,
Dalrada Climate Technology, Dalrada Precision Manufacturing, Dalrada Technologies and Dalrada Corporate. Within
each of these divisions, the Company drives transformative innovation while creating solutions that are sustainable, accessible, and affordable.
Dalrada’s global solutions directly address climate change, gaps in the health care industry, and technology needs that facilitate
a new era of human behavior and interaction and ensure a bright future for the world around us.
Genefic
Genefic delivers advanced health care solutions with
dedicated products, services, and systems. From virus and disease screening capabilities to pharmaceutical goods and holistic wellness
clinics, When the world needs advanced health care, Genefic delivers with ingenuity, accessibility, and affordability. This specialized
division is committed to developing key health products, lifesaving medications and building comprehensive systems to increase capability,
strive to keep people healthy with the goals of improving their quality of life and increasing their longevity– on a global level.
Genefic Specialty Pharmacy
(“Genefic Pharmacy”)- Genefic Pharmacy (formerly Genefic Specialty Pharmacy Rx Solutions) is an Alabama-based pharmacy
with more than 30 years of experience in the retail medical and pharmaceutical industries. Genefic Pharmacy specializes in providing expert
care and managing disease states through comprehensive prescription management, education, nursing, and total health solutions. Genefic
Pharmacy maintains pharmacy licenses in all 50 States as well as Washington D.C.
Genefic Infusion Rx- Genefic
Infusion Rx is a Louisiana-based infusion pharmacy which handles all aspects of fluid and medication infusion, via intravenous or subcutaneous
application. Genefic Infusion Rx serves as an essential with healthcare systems, enhancing the infusion process through efficient authorization
and prescription management. Its state-of-the-art compounding facility is led by one of only eight pharmacists in Louisiana with a sterile
compounding board certification, ensuring top-tier precision and quality in medication preparation.
Boost Diagnostics- Boost
Diagnostics (formerly Empower Genomics and Genefic Diagnostics) is Dalrada’s wholly owned diagnostic laboratory subsidiary which
processes molecular diagnostic and antibody tests to support the diagnosis of COVID-19 and the detection of immune response to the virus.
Boost Diagnostics has built up and maintained the testing capacity to handle surges in COVID-19 testing demands. Boost Diagnostics also
offers genetic testing capabilities including Pharmacogenomics, Nutraceutical, Nutrition/Diet DNA and Exercise/Fitness DNA tests.
Pala Diagnostics (“Pala”)-
Pala was a joint venture diagnostic laboratory entity which processed both molecular diagnostic and antibody tests to support the diagnosis
of COVID-19 and the detection of immune response to the virus. Pala was no longer an operational entity as of June 30, 2023.
Dalrada Career Institute (“DCI”)
(aka International Health Group (“IHG”)) - IHG provides highly trained nursing and medical assistants for hospitals and
home health facilities since 2006. IHG Medical Assistant programs include Certified Nursing Assistant (“CNA") and Home Health
Aide (“HHA”) training and the fast-track 22-Day CNA Certification Program at its state-approved testing facility. DCI started
its first RN, nursing class in February of 2024 and this first class will be completed in February 2025. It is the intent of DCI to double
their class size when it begins its second class in 2025.
Dalrada Climate Technology (formerly Dalrada
Energy Services)
Dalrada Climate Technology (“DCT”) is
a segment which incapsulates energy services and state-of-the-art technology within the climate sustainability space. DCT employs next-generation
technology and services which enhances clean energy efforts while reducing the world’s carbon footprint. As a premier industrial
heat pump manufacturer, Dalrada delivers innovation and efficiency, building solutions that reduce energy consumption and minimize carbon
footprints, increase operational efficiencies, meet environmental, social, and governance (ESG) goals, and lower energy costs for clients.
Dalrada Technology Limited
(“DTL”) - DTL is a holding company for all United Kingdom and European based Dalrada Climate Technology entities.
Likido Ltd. (“Likido”)
- Likido is an international engineering company developing advanced solutions for the harvesting and recycling of energy. Using its novel,
heat pump systems (patent pending), Likido is working to revolutionize the renewable energy sector with the provision of innovative modular
process technologies to maximize the capture and reuse of thermal energy for integrated heating and cooling applications. With uses across
industrial, commercial and residential sectors, Likido provides cost savings and minimized carbon emissions across global supply chains.
Likido's technologies enable the effective recovery and recycling of process energy, mitigating against climate change and expected enhancement
of quality of life through the provision of low-carbon heating and cooling systems. Likido’s products currently include the
DCT One Heat Pumps (formerly Likido®ONE) and DCT Cryo Chiller. Likido also offers heat pump solutions specifically designed for residential
purposes.
During the prior year, the U.S.
Government selected DCT One Series high-performance, low-carbon heat pump for real-world testing in a prestigious clean energy program.
The implementation of the DCT One Series testing is still in process. The expected positive results should not only increase market acceleration
and adoption within the federal government acceptance of groundbreaking eco-friendly technology but should also accelerate adoption within
the commercial building industry.
Dalrada Technology Spain L.T.
(“DTS”)- DTS was established as a Spanish subsidiary of DTL for the expansion of the manufacturing and sale of the DCT
One Series and DCT Cryo Chiller throughout Europe.
Dalrada Energy Services (“DES”)-
DES provides end-to-end comprehensive energy service solutions in a robust commercial capacity. DES helps organizations meet ESG goals
and standards while mitigating negative environmental impacts.
Bothof Brothers Construction
(“Bothof”)- Bothof is a licensed general contractor which provides a wide range of development, construction and design
capabilities and expertise throughout the United States. Through Bothof’s extensive experience in construction and contracting,
the DES division can provide a myriad of additional services to its private and public works customers.
Dalrada Home Corporation (“Dalrada
Home”)- Dalrada Home Corporation was established in February of 2024. Dalrada Home’s cutting-edge sustainability solutions
are designed specifically for residential purposes. Our home heat pumps help us lead the way in providing innovative climate technology
products and services to residential customers.
Dalrada Precision Manufacturing
Dalrada Precision Manufacturing creates total manufacturing
solutions that start with the design and development of high-quality machine parts and components, and end with an efficient global supply
chain. This specialized business division can meet today’s high demands and solves industry challenges. Dalrada Precision Manufacturing
is confident that it redefines the critical quality of the world’s top components and responds with in-house research, design, engineering,
and distribution through a highly reliable global supply chain and improved time-to-market capabilities.
Dalrada Precision Parts (“Precision”)
- Precision extends the client its engineering and operations team by helping devise unique manufacturing solutions tailored to their
products. Dalrada Precision can enter at any stage of the product lifecycle from concept and design to mass production and logistics.
Deposition Technologies (“DepTec”)
- DepTec designs, develops, manufactures, and services chemical vapor and physical vapor deposition systems for the microchip and semiconductor
industries.
DepTec has built an impressive
catalogue of precision OEM parts for PVD (Physical vapor deposition) systems and the Company’s refurbished systems which allows
clients the option of purchasing the same model of system they’ve been using for decades –but with significant upgrades and
improved efficiencies. Older systems can now operate more reliably with additional control and monitoring plus longer lifespans. DepTec
also has its own PVD and CVD (Chemical Vapor Deposition) systems, EVOS-PVD and EVOS -CVD, which deposits metals and non-metals for microchips
used in almost every standard and specialized microdevices made today and in the future. These systems can produce a superior film layer
utilized in rugged high-stress environment designs.
Ignite I.T. (“Ignite”)
- Ignite is a manufacturer and seller of eco-friendly deep cleaners, parts washers and degreasers that are specially formulated to lift
hydrocarbon-based dirt and grease from virtually all surfaces with minimal effort. Ignite products are non-flammable, non-corrosive, non-toxic,
butyl-free, water-based, and leave a light citrus scent. Ignite is developed for all surfaces suitable for water and meets or exceed the
most stringent industry-testing specifications.
Dalrada Technologies
Dalrada Technologies has worked with some of the world’s
most recognizable companies, providing digital engineering for cutting-edge software systems and offering a host of robust digital services.
This business division connects the world with integrated technology and innovative solutions, delivering advanced capabilities and error-free
results. Dalrada Technologies creates digital products with expert computer information technology and software engineering services for
a variety of technical industries and clients in both B2B and B2C environments.
Prakat (“Prakat”)
- Prakat is an ISO 9001-certified company that provides end-to-end technology services across various industries, improving the value
chain. The Company specializes in test engineering, accessibility engineering, product engineering, application modernization, billing
and revenue management, CRM, and block chain. Prakat provides global customers with software and technology solutions specializing in
Test Engineering, Accessibility Engineering, Product Engineering and Application Modernization.
Dalrada Corporate
Dalrada Corporate covers the activities which support
the entire suite of Dalrada subsidiaries. Dalrada Corporate includes the areas of administration, finance, human resources, legal advice,
information technology, and marketing. It also contains executive management and shareholder-related services.
Research and Development
We spent $0 and $120,000 on research and development
activities during the years ended June 30, 2024, and 2023, respectively. We anticipate that we will incur additional expenses on research
and development over the next 12 months. Our planned expenditures on our operations or a business combination are summarized under the
section of this Annual Report on form 10-K entitled “Management’s Discussion and Analysis of Financial Position and Results
of Operations”.
Item 1A. Risk Factors
Not applicable to smaller reporting companies.
Item 1B. Unresolved Staff Comments
Not applicable to smaller reporting companies.
Item 1C. Cybersecurity
Risk management and strategy
The Information Technology “IT” environment
of the Company is critical in efforts to effectively perform day-to-day processes and expand on current opportunities and investments.
Our security policies and processes are based on best practices of the industry and are regularly reviewed by our management to ensure
the current technological capabilities are maintained. We review System and Organization Controls 1 (SOC 1 Type II) certifications where
relevant from third-party partners and service providers as needed.
Governance
The Board of Directors works directly with, and
is in frequent communication with, management and our third-party IT providers to protect the Company’s information systems from
cybersecurity threats. To date, there have not been any cybersecurity threats that have materially affected the Company.
Item 2. Description of Property
We currently lease 110,838 square feet of office,
medical, pharmacy and warehouse space in California, Alabama, Texas, Louisiana, Scotland, and India, with leases that expire through
2028.
| |
|
|
|
Square |
|
|
|
| |
|
|
|
Footage |
|
Lease |
|
| Location |
|
Type |
|
(approximate) |
|
Expiration |
|
| Escondido, California |
|
Corporate Headquarters |
|
|
49,530 |
|
|
6/30/2027 |
|
| San Diego, California |
|
Office |
|
|
8,228 |
|
|
3/14/2028 |
|
| Escondido, California |
|
Office |
|
|
2,992 |
|
|
6/30/2027 |
|
| Chula Vista, California |
|
Office, Medical Suite |
|
|
3,200 |
|
|
11/12/2024 |
|
| San Diego, California |
|
Office, Medical Suite |
|
|
9,016 |
|
|
8/31/2028 |
|
| Bengaluru, India |
|
Office |
|
|
3,300 |
|
|
4/1/2026 |
|
| Coronado, California |
|
Office, Medical Suite |
|
|
462 |
|
|
12/31/2024 |
|
| Florence, Alabama |
|
Pharmacy |
|
|
1,443 |
|
|
5/31/2025 |
|
| Livingston, Scotland |
|
Office, Warehouse |
|
|
4,500 |
|
|
8/27/2025 |
|
| Escondido, California |
|
Office |
|
|
167 |
|
|
12/31/2024 |
|
| Livingston, Scotland |
|
Office, Warehouse |
|
|
19,000 |
|
|
11/2/2027 |
|
| Bergondo, Spain |
|
Office, Warehouse |
|
|
9,000 |
|
|
5/31/2028 |
|
| Metairie, Louisiana |
|
Office, Medical Suite |
|
|
6,468 |
|
|
9/30/2025 |
|
Item 3. Legal Proceedings
Genefic Products (“Dalrada Health”), a
subsidiary of Dalrada Financial Corporation, formed a joint venture with Vivera Pharmaceuticals, Inc. (“Vivera”), whereby
Vivera is the minority member. As the managing member of the joint venture, Genefic Products, in December 2021, filed suit against Vivera
and Paul Edalat, Vivera’s Chairman and CEO, for misappropriation of funds on behalf of the joint venture in the amount of $2,104,509.
In addition to filing a cross-complaint against Genefic Products, Vivera filed a separate complaint against Dalrada Financial Corporation,
Empower Genomics (a subsidiary of Dalrada Financial Corporation), Dalrada Financial Corporation’s officers, and other unrelated
parties. The proceedings are being held at the Superior Court of the State of California, for the County of Orange – Central Justice
Center.
In September 2023, Kroger Specialty Pharmacy LLC (“Kroger”)
filed lawsuits/preliminary injunctions against Genefic Specialty Pharmacy and two of its employees who were former employees of Kroger.
The lawsuits were filed in Tennessee and Alabama, respectively. The basis for the injunction arose from a non-compete clause in the contract
between the two employees and a company which was later acquired by Kroger. In April 2024, the Court in the Tennessee case granted the
preliminary injunction on the Tennessee employee, which is due to expire in April 2025. The case against the Tennessee employee is under
appeal. No injunction has yet been issued against the Alabama employee.
In September 2023, Asset Group, Inc. (“Asset”)
filed a breach of contract with Dalrada Health Products (“DHP”) in the Superior Court of San Diego. The case arises out of
a Purchase Order wherein Asset agreed to pay DHP the sum of $3,240,000 for the purchase of 1,800,000 IRIS Ear Loop Face Masks during the
COVID-19 pandemic. Asset filed a complaint alleging DHP did not have authority to sell the masks. However, DHP have provided their counsel
with proof of authority and are preparing a Cross-Complaint for Asset’s material breach of the contract. This matter is currently
set for trial January 31, 2025.
In March 2024, MDIQ filed a breach of contract with
Dalrada Financial Corporation (“DFCO”) in Collin County Texas Superior Court. MDIQ was hired to process insurance claims for
COVID-19 testing performed by Empower Genomics. MDIQ failed to perform yet filed a civil collection case against DFCO for failure to pay
the invoices. DFCO is now in the process of counter suing for approximately $2,000,000 of unpaid claims that we would have benefitted
from had MDIQ performed according to the contract.
A former consultant, Simon Gray, and distribution
representative, DePrey Company, acted in concert with supplier Zhongshan Mide Hardware Products Co., Ltd. (“Mide”) to steal
Fastenal Company purchase orders and effectively try to cut Dalrada Manufacturing out of its contractual relationship. DFCO has filed
a lawsuit against DePrey Company and Simon Gray in July for a breach of contract and intentional interference with contractual relationships
in the California Southern District Court.
In June 2024, Dalrada Financial Corporation (“DFCO”)
filed a case in the California Southern District Court alleging, among other causes of action, fraud, breach of contract, unjust enrichment
following DFCO purchasing Likido company from Stuart Cox and his failure to disclose pertinent financial liabilities he had incurred prior
to the sale of the company to DFCO. Mr. Cox resides in the Philippines and service of the summons and complaint is pending.
Item 4. Mine Safety Disclosures
Not applicable to our Company.
PART II
Item 5. Market for Common Equity, Related Stockholder Matters and Small
Business Issuer Purchases of Equity Securities
Market Information
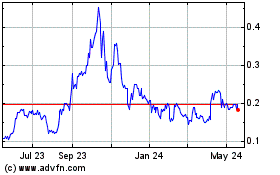
Our shares of common stock are quoted on the OTC Markets
Group’s Pink® Open Market under the symbol DFCO. Set forth below are high and low bid prices for our common stock
for each quarterly period in the two most recent fiscal years. Such quotations reflect inter-dealer prices, without retail mark-up, markdown
or commissions and may not necessarily represent actual transactions in the common stock.
| Period |
|
High |
|
|
Low |
|
| Fiscal 2024 |
|
|
|
|
|
|
|
|
| First Quarter ended September 30, 2023 |
|
$ |
0.2375 |
|
|
$ |
0.2100 |
|
| Second Quarter ended December 31, 2023 |
|
$ |
0.4600 |
|
|
$ |
0.1850 |
|
| Third Quarter ended March 31, 2024 |
|
$ |
0.2070 |
|
|
$ |
0.1450 |
|
| Fourth Quarter ended June 30, 2024 |
|
$ |
0.2325 |
|
|
$ |
0.1535 |
|
| |
|
|
|
|
|
|
|
|
| Fiscal 2023 |
|
|
|
|
|
|
|
|
| First Quarter ended September 30, 2022 |
|
$ |
0.3700 |
|
|
$ |
0.0900 |
|
| Second Quarter ended December 31, 2022 |
|
$ |
0.1700 |
|
|
$ |
0.0700 |
|
| Third Quarter ended March 31, 2023 |
|
$ |
0.0200 |
|
|
$ |
0.0800 |
|
| Fourth Quarter ended June 30, 2023 |
|
$ |
0.1900 |
|
|
$ |
0.0600 |
|
Number of Holders
As of January 8, 2025, there were 120,157,113 issued
and outstanding shares of common stock held by a total of 605 shareholders of record.
Dividends
No cash dividends were paid on our shares of common
stock during the fiscal years ended June 30, 2024, and 2023. We have not paid any cash dividends since our inception and do not foresee
declaring any dividends on our common stock in the foreseeable future.
Recent Sales of Unregistered Securities
In July 2023, the Company issued 500,000 shares of
common stock in connection with a fee for a third-party loan in the amount of 1,200,000. The company ascribed $60,000 to those shares
recorded as a debt discount. The Company issued these shares of common stock pursuant to the exemption from registration provided by Section
4(a)(2) of the Act in that such issuance did not constitute a public offering.
In July 2023, the Company issued 109,637 shares of
common stock pursuant to the Stock Purchase Agreement with Prakat Solutions Inc. for $14,413. This issuance was a follow on with certain
legacy stockholders of Prakat to the 2020 purchase by the Company of 72% of Prakat. The Company issued these shares of common stock pursuant
to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public offering.
In September and December 2023, April and May 2024,
the Company issued a total of 500,000 shares of common stock related to earn-out payments in the acquisition of Genefic Specialty Pharmacy.
The company ascribed $106,250 to those shares recorded at the value of the shares upon issuance. The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public
offering.
In October 2023 the company issued 500,000 shares
of common stock pursuant to a loan agreement for $173,000. The Company issued these shares of common stock pursuant to the exemption from
registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public offering.
In December 2023 and April 2024, the Company issued
a total of 1,000,002 shares of common stock related to the acquisition of DepTec (SSCe) for $200,947. The Company issued these shares
of common stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute
a public offering.
In February 2024, the Company issued 4,666,665 shares
of common stock related to a Company conducted private placement for aggregate proceeds of $604,001, or $0.13 per share. The Company used
the proceeds for operating capital. The Company issued these shares of common stock pursuant to the exemption from registration abiding
by Rule 506 under Regulation D.
In February 2024, the Company issued 1,200,000 shares
of common stock pursuant to consulting agreements resulting in $241,200 in consultancy fees. The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public
offering.
Preferred Stock:
On March 29, 2024, the Company converted $13,318,943
of related party debt principal and interest into 15,951 shares (effective price of $835 per share) of Series I Convertible Preferred
Stock (“Series I Stock”). The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock
(equivalent to converting the related dollars into common shares at $0.167 per share). The Company issued these shares of common stock
pursuant to the exemption from registration provided by Section 3(a)(9) of the Act in that such issuance did not constitute a public offering.
Pursuant to the acquisition agreement dated April
6, 2022 between the Company and Silicon Services Consortium Ltd. (“SSCe”), the sellers of SSCe were to be issued 3,000,000
shares of its common stock evenly every quarter for 24 months with the initial distribution to take place on the effective date (the “Share
Consideration”). If at the end of the 24-month stock distribution period, beginning on the effective date of April 7, 2022 (the
“Distribution Period”), the value of common stock consideration does not equate to 4,000,000 GBP (the “Target Amount”)
in value, then the Company shall issue additional stock equal to the shortfall between the value of the Share Consideration and the Target
Amount (the “Valuation Shortfall”). At the end of the Distribution Period, the sellers of SSCe were to be issued an additional
$4,440,000 in stock as a result of the Valuation Shortfall. The Company share price at the end of the Distribution Period was $0.20, creating
an additional 22,200,000 shares of common stock due to the sellers of SSCe. Pursuant to board resolution dated May 22, 2024, Valuation
Shortfall shares were issued into 4,440 shares of Series I Convertible Preferred Stock (“Series I Stock”) as opposed to common
stock. The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock (equivalent to converting the related
dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights. The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public
offering.
On June 30, 2024, the Company converted $3,924,499
of related party debt principal and interest into 4,700 shares (effective price of $835 per share) of Series I Convertible Preferred Stock
(“Series I Stock”). The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock (equivalent
to converting the related dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights. The Company
issued these shares of common stock pursuant to the exemption from registration provided by Section 3(a)(9) of the Act in that such issuance
did not constitute a public offering.
Other Stockholder Matters
None.
Item 6. Selected Financial Data
Not applicable to smaller reporting companies.
Item 7. Management's Discussion and Analysis of Financial Condition
and Results of Operations
You should read the following discussion and analysis
in conjunction with our financial statements, including the notes thereto, included in this Report. Some of the information contained
in this Report may contain forward-looking statements within the meaning of Section 27A of the Securities Exchange Act of 1933, as amended
(the “Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). This information
may involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance, or achievements
to be materially different from future results, performance or achievements expressed or implied by any forward-looking statements. Forward-looking
statements which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by the use
of the words “may,” “will,” “should,” “expect,” “anticipate,” “estimate,”
“believe,” “intend” or “project” or the negative of these words or other variations on these words
or comparable terminology. These forward-looking statements are based on assumptions that may be incorrect, and there can be no assurance
that the projections included in these forward-looking statements will come to pass. Our actual results could differ materially from those
expressed or implied by the forward-looking statements as a result of various factors. We undertake no obligation to update publicly any
forward-looking statements for any reason, even if new information becomes available or other events occur in the future.
Our Independent Registered Public Accounting Firm’s
report contains a statement that our net loss and limited working capital raise substantial doubt about our ability to continue as a going
concern. Our independent registered public accountants have stated in their report (included in Item 8 of the Financial Statements) that
our significant operating losses and working capital deficit raise substantial doubt about our ability to continue as a going concern.
We incurred a net loss of $23,250,181 and 20,627,896, respectively, for the years ended June 30, 2024 and 2023. Although the Company continues
to rely on equity and debt investors to finance its losses, it is implementing plans to achieve cost savings and other strategic objectives
to address Company profitability. In addition to raising debt and equity financing, the Company continues to focus on growing the subsidiaries
anticipated to be most profitable while reducing investments in areas that are not expected to have long-term benefits. The Company will
continue to pursue synergistic opportunities to enhance its business portfolio.
Acquisitions
During the year ended June 30, 2024, the Company acquired
a business to complement the Genefic segment.
Refer to “Note 4. Business Combinations and
Asset Acquisition” to the Condensed Consolidated Financial Statements for discussion regarding the Company’s acquisitions.
RESULTS OF OPERATIONS
The following table sets forth the results of our
operations for the years ended June 30, 2024, and 2023:
| | |
Year Ended June 30, 2024 | |
| | |
Genefic | | |
Dalrada Climate Technology | | |
Dalrada Precision Manufacturing | | |
Dalrada Technologies | | |
Corporate | | |
Consolidated | |
| Revenues | |
$ | 17,684,765 | | |
$ | 3,674,697 | | |
$ | 2,447,148 | | |
$ | 1,373,136 | | |
$ | – | | |
$ | 25,179,746 | |
| Income (Loss) from Operations | |
| (1,825,270 | ) | |
| (5,956,460 | ) | |
| (1,349,321 | ) | |
| (235,104 | ) | |
| (11,409,690 | ) | |
| (20,775,845 | ) |
| | |
Year Ended June 30, 2023 | |
| | |
Genefic | | |
Dalrada Energy | | |
Dalrada Precision Manufacturing | | |
Dalrada Technologies | | |
Corporate | | |
Consolidated | |
| Revenues | |
$ | 15,740,919 | | |
$ | 7,075,414 | | |
$ | 4,873,225 | | |
$ | 2,049,411 | | |
$ | – | | |
$ | 29,738,969 | |
| Income (Loss) from Operations | |
| (5,783,441 | ) | |
| (1,065,221 | ) | |
| (2,461,219 | ) | |
| 10,634 | | |
| (11,660,710 | ) | |
| (20,959,957 | ) |
Dalrada Financial Corporation manages five primary
segments: 1) Genefic (formerly Dalrada Health); 2) Dalrada Climate Technology; 3) Dalrada Precision Manufacturing; 4) Dalrada Technologies;
and 5) Dalrada Corporate. The business segment data (see “Note 13. Segment Reporting”) should be read in conjunction with
this discussion.
Revenues and Cost of Revenues
Genefic
Total Revenues for Genefic increased to $17,684,765,
or 12.3% from last year’s revenue of $15,740,919.
Genefic Specialty Pharmacy (formerly ‘Watson’)
revenue increased $13,049,089, or 453.8% compared to $2,875,326 in the prior year. The increase was a result of obtaining additional accreditations
including the Healthcare Merchant Accreditation from the National Association of Boards of Pharmacy (NABP) where it can be listed on the
official Accredited Merchants’ list along with larger pharmacy retailers CVS, Walgreens, and Walmart, among others. With Healthcare
merchant Accreditation, Genefic Specialty Pharmacy has proven its standards and practices to be in line with the requirements set by large
online advertising platforms such as Google and Bing. Genefic Specialty Pharmacy also obtained the Specialty Pharmacy Accreditation and
Mail Service Pharmacy Accreditation from the Utilization Review Accreditation Commission (URAC). NABP’s Specialty Pharmacy Accreditation
signifies to patients, payers, and providers that the pharmacy organization is recognized for providing an advanced level of pharmacy
services and disease management for patients taking medications that meet special handling, storage and distribution requirements. NABP’s
Digital Pharmacy Accreditation signifies to patients, payers, and providers that the pharmacy organization is recognized for its commitment
to the highest quality health care and safe pharmacy practices over the internet. The specific Digital Pharmacy Accreditation was created
to recognize safe and legitimate pharmacies with an internet presence that stands out against the ever-growing list of rogue pharmacy
websites. These accreditations allowed Genefic Specialty Pharmacy to ramp up a sales team in conjunction with the ability to fill specialty
medications. Lastly, Genefic Specialty Pharmacy was granted a number of hemophilia contracts throughout the year. The cost of revenue
was $10,612,296.
Pala Diagnostics (“Pala”) and Empower
Genomics (“Empower”) generated $27,910 of the total revenue for Genefic through its complexity CLIA diagnostic laboratories
compared with $10,338,768 in the prior year. The decrease in revenue was a result of the closure of the CLIA diagnostic laboratories,
which focused primarily on COVID-19 testing services with validated PCR and Rapid antigen testing.
DCI generated $1,338,960, or 7.6% of the total revenue
for Genefic. DCI’s revenue increased by $247,026 from the prior year, or 22.6%. The increase in revenue was a result of obtaining
Licensed Vocational Nursing (“LVN”) accreditation along with a rising number of students entering and graduating from DCI’s
Certified Nursing Assistant (“CNA”), Medical Assistant and Home Health Aid (“HHA”) Certification programs.
Dalrada Precision Manufacturing
Total Revenues for Dalrada Precision Manufacturing
decreased to $2,447,148, or 49.8% from last year’s revenue of $4,873,225.
Dalrada Precision Parts generated $1,130,905, or 46.2%
of the total revenue for Dalrada Precision Manufacturing. Revenue for Dalrada Precision Parts decreased by $1,550,001, or 57.8% from the
prior year. The decrease in revenue was due to the loss of its primary customer in precision parts manufacturing. The cost of revenue
was $427,364, or 37.8% of revenue.
DepTec generated $1,242,642, or 50.8% of the total
revenue for Dalrada Precision Manufacturing. Revenue for DepTec decreased by $49,661, or 3.8% from the prior year. DepTec records its
revenue using a cost-based input method, by which we use actual costs incurred relative to the total estimated contract costs to determine,
as a percentage, progress toward contract completion. The cost of revenues was $1,279,752, or 103.0% of revenue.
Ignite’s cleaners, parts washers and degreaser
products generated $73,601, or 3.0% of total revenue for Dalrada Precision Manufacturing. Revenue for Ignite decreased by $231,657, or
75.9% from the prior year. The decrease in revenue was due to ramping down operations of the company. The cost of revenue was $77,536,
or 105.3% of revenue, and includes inventory adjustments.
Dalrada Climate Technology (Formerly Dalrada Energy
Services)
Total Revenues for Dalrada Climate Technology decreased
to $3,674,697, or 48.1% from last year’s revenue of $7,075,414.
Dalrada Energy Services generated $567,930, or 15.5%
of the total revenue for the Dalrada Climate Technology segment. Revenue for Dalrada Energy Services decreased by $3,943,603, or 87.4%
from the prior year. The decrease in revenue was a result of nearing completion of the Averett University project.
Bothof Brothers Construction (“Bothof”) generated $2,796,199,
or 76.1% of the total revenue for the Dalrada Climate Technology segment. Bothof revenue increased by $232,318, or 9.1% from last year.
Bothof generated revenue in its construction and contracting services throughout the United States. Bothof Brothers’ customers include
both residential and commercial projects in the private and public sectors. During the year, $1,697,485 of revenue was generated through
related parties. The cost of revenue was $3,176,886, or 113.6% of revenue
Dalrada Technologies
Total Revenue for Dalrada Technologies” sole
subsidiary, Prakat, decreased to $1,373,136, or 33.0% from the prior year’s revenue of $2,049,411. The decrease in revenue was a
result of several contracts ending their terms during the year.
Operating Expenses
Total Operating Expenses decreased to $26,584,681,
or 11.4%, compared to last year’s expenses of $30,019,876.
Corporate
Total Corporate expenses decreased to $11,377,290,
or 2.4%, compared to last year’s expenses of $11,660,710.
The Corporate segment’s Selling, general and
administrative (“SG&A”) expenses consist of the following:
| |
· |
Employee compensation and benefits decreased by $522,450, or 12.2% from the prior year and is a result of a reduction in corporate employees. |
| |
|
|
| |
· |
Legal and professional fees increased by $396,486, or 39.2% from the prior year and is a result of an increase in corporate litigation as well as audit related costs. |
| |
|
|
| |
· |
Sales and marketing costs increased by $27,326, or 36.9% from the prior year due to increased costs associated with third party investor relations, paid media, and content creation expenses. |
| |
|
|
| |
· |
Other general and administrative costs for general corporate expenses, including information technology, rent, travel, and insurance decreased by $256,222, or 13.5% from the prior year and is a result of decreased in travel expenses, computer software expenses, and management fees. |
Interest Expense decreased by $1,354,290 or 53.0%
from the prior year as a result of increases in related party debt as well as PPP loans and convertible debt issued in prior years. See
“Note 7. Notes Payable” to our audited condensed consolidated financial statements included in this Annual Report on Form
10-K for more information regarding our outstanding debt.
Stock-based compensation includes expenses related
to equity awards issued to employees and non-employee directors. Stock-based compensation increased by $221,440, or 5.5% from the prior
year. See “Note 12. Stock-Based Compensation” to our audited condensed consolidated financial statements included in this
Annual Report on Form 10-K for more information regarding our stock-based compensation.
Genefic
Total Genefic expenses decreased to $7,266,439, or 36.6%, compared to last
year’s expenses of $11,468,627.
The Genefic segment’s Selling, general and administrative
(“SG&A”) expenses consist of the following:
| |
· |
Employee compensation and benefits increased by $1,255,151, or 52.4% from the prior year and a result of growth of the pharmacy business. |
| |
|
|
| |
· |
Legal and Professional Fees decreased by $1,764,960, or 71.8% from the prior year and primarily a result of a reduction in professional fees associated with COVID-19 testing services with validated PCR and Rapid antigen testing. |
| |
|
|
| |
· |
Sales and marketing costs decreased by $16,314, or 34.8% from the prior year due to reduced costs associated with third party advertising, paid media, and content creation expenses. |
| |
|
|
| |
· |
Other general and administrative costs decreased by $3,242,509, or 52.9% from the prior year and is a result of bad debt expense recorded in the prior fiscal year for Boost, Pala, and Genefic Wellness in the amounts of $1,648,562, $1,296,825, and $1,100,675, respectively. |
Dalrada Precision Manufacturing
Total Dalrada Precision Manufacturing expenses decreased
to $2,011,817, or 51.4%, compared to last year’s expenses of $4,136,885.
The Dalrada Precision Manufacturing Segment’s
Selling, general and administrative (“SG&A”) expenses consist of the following:
| |
· |
Employee compensation and benefits decreased by $1,035,630 or 70.4% from the prior year as a reduced activity in the segment. |
| |
|
|
| |
· |
Legal and Professional Fees decreased by $1,019,258, or 89.2% from the prior year. The decrease in legal fees was primarily a result of the settlement Likido Ltd.’s lawsuit with MAPtech Packaging, Inc. Pursuant to the settlement, the Company shall pay the sum of $558,252 in damages, legal costs, and reimbursement for arbitration fees and expenses paid on account by MAPtech recognized in the prior year. |
| |
|
|
| |
· |
Sales and marketing costs decreased by $2,246, or 13.3% from the prior year due to reduced costs associated with third party advertising, paid media, and content creation expenses. |
| |
|
|
| |
· |
Other general and administrative costs decreased by $67,934, or 4.5% from the prior year and is a result of a decrease in travel, trade shows and other overhead expenses required for the expansion in the Precision Parts and Ignite businesses. |
Dalrada Climate Technology
Total Dalrada Climate Technology expenses increased
to $5,204,881, or 164.2%, compared to last year’s expenses of $1,969,829.
The Dalrada Climate Technology Segment’s Selling,
general and administrative (“SG&A”) expenses consist of the following:
| |
· |
Employee compensation and benefits increased to $2,878,201, compared to $484,719 in the prior year as the energy segment continued to grow during the year. The employee resources were focused on the development of the current projects and building a future pipeline. |
| |
|
|
| |
· |
Legal and Professional Fees decreased by $292,415, or 51.0% from the prior year and is a result of the growth of the energy segment throughout the prior year. Professional fees included services for management fees and other services specific to the energy industry. |
| |
|
|
| |
· |
Sales and marketing costs increased to $19,988, a 58.6% increase from the prior year. The Company’s internal marketing generates most of the sales and marketing services which is included in the Corporate segment employee compensation and benefits expenses. |
| |
|
|
| |
· |
Other general and administrative costs increased to $2,026,225, a 125.2% increase from the prior year and is a result of management fees, travel and other general overhead costs associated with Dalrada Climate Technology’s energy upgrade business and Bothof Brothers Contracting. |
Dalrada Technologies
Total Dalrada Technologies expenses decreased to $724,254,
or 7.6%, compared to last year’s expenses of $783,825.
The Dalrada Technologies segment’s Selling,
general and administrative (“SG&A”) expenses consist of the following:
| |
· |
Employee compensation and benefits decreased by $51,363, or 15.1% from the prior year and is a result of employee wage fluctuations. |
| |
|
|
| |
· |
Legal and Professional Fees increased by $51,158, or 37.2% from the prior year and is a result of increased third-party engineering fees related to its projects. |
| |
|
|
| |
· |
Sales and marketing costs increased by $15,844, or 1,039.0% from the prior year and is a result of increased third-party advertising and marketing costs. |
| |
|
|
| |
· |
Other general and administrative costs decreased by $75,210, or 24.7% and is a result of decreases in information technology, rent, travel, and insurance costs. |
Other (Expense) Income
Other (Expense) Income increased by $2,806,572 or
844.8% from a $332,236 in Other Income in the prior year to a $2,474,336 Other Expense in the current year. The change in Other Expense
was a result of $2,090,978 of “Gain on expiration of accrued payroll taxes” due to quarterly tax liabilities that expiring
during fiscal 2023, $500,000 related to the sale of the Dalrada Energy Services intellectual property, and a $585,411 change in the fair
value of contingent liability all incurred in the prior year. Other expenses incurred for the fiscal year ended June 30, 2024 consisted
of interest expense of $1,213,441 and a change in fair value of the contingent liability of $511,892.
Net Income (Loss)
Net loss for the year ended June 30, 2024, was $23,250,181
compared to a Net loss of $20,627,721 during the year ended June 30, 2023.
Liquidity and Capital Resources
As of June 30, 2024, the Company had current assets
of $13,145,412 and current liabilities of $13,844,784 compared with current assets of $9,817,045 and current liabilities of $10,019,465
at June 30, 2023. The continuation of the Company as a going concern is dependent upon successful financing through equity and/or debt
investors and growing the subsidiaries anticipated to be profitable while reducing investments in areas that are not expected to have
long-term benefits.
The Company anticipates an increase in sales of Likido’s
Likido®ONE heat pump through its current and future customer base. Furthermore, the United States General Services Administration
(GSA) and the Department of Energy (DOE) have chosen the Company’s Likido®ONE heat pump to help reduce greenhouse emissions
from commercial buildings through high performance, low-carbon solutions set forth by the Green Proving Ground (GPG) program.
Cash Flows
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Net cash used in operating activities | |
$ | (7,925,238 | ) | |
$ | (4,612,798 | ) |
| Net cash used in investing activities | |
| (552,658 | ) | |
| (1,063,427 | ) |
| Net cash provided by financing activities | |
| 8,117,078 | | |
| 5,717,144 | |
| Net change in cash during the period, before effects of foreign currency | |
$ | (360,818 | ) | |
$ | 40,919 | |
Cash flow from Operating Activities
During the year ended June 30, 2024, the Company used
$7,925,238 of cash for operating activities compared to $4,612,798 used during the year ended June 30, 2023. The increase in the use of
cash for operating activities was primarily due to an overall increase in funding from related parties.
Cash flow from Investing Activities
During the year ended June 30, 2024, the Company used
$552,658 of cash for investing activities compared to $1,063,427 used during the year ended June 30, 2023. The decrease in the use of
cash for investing activities was due to the purchase of property plant and equipment in the prior year.
Cash flow from Financing Activities
During the year ended June 30, 2024, the Company received
$8,117,078 of cash for financing activities compared to $5,717,144 received during the year ended June 30, 2023. The increase in financing
activities was primarily due to the draw down of various note payables to fund the company.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have
or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, Revenues or expenses,
results of operations, liquidity, capital expenditures or capital resources.
Subsequent Events
On July 10, 2024, the Company entered into a promissory
note with 1800 Diagonal Lending, LLC for $87,975. The promissory note includes a one-time interest charge of 14%, which was applied on
the issuance date, and matures on May 15, 2025. There are 4 monthly payments of $10,029 and one payment of $60,175 for a total payback
of $100,291.
On July 18, 2024, the Company executed a cash advance
agreement with Cali Flower Capital Inc. with a total advance of $200,00 and payback of $299,800.
On July 25, 2024, the Company executed a revenue purchase
agreement with 24 Capital with a total advance of $125,000 and payback of $187,375.
On July 29, 2024, the Company executed a revenue purchase
agreement with Tycoon Capital Group with a total advance of $125,000 and payback of $187,375.
On August 12, 2024, the Company entered into an Exclusive
Master Distribution Agreement (the “Agreement”) with Applied Technologies of NY, Inc. (“ATI”). The Agreement establishes
the sales goals of 50 commercial heat pumps and 25 residential heat pumps in the first 18 months followed by a total of 600 heat pumps
(combined commercial and residential heat pumps) in the following 12-month period.
On August 15, 2024, the Company signed a lease for
5,650 square feet of manufacturing and office space in Portland, Oregon related to the deposition technology business. The base monthly
lease cost is $5,254 per month and expires on April 30, 2027.
On August 19, 2024, the Company acquired Grand Entrances
for the consideration of $100 in cash, including its current liabilities and assuming its lease, which includes a monthly lease cost of
$10,291 and expires on April 11, 2030.
On August 23, 2024, the Company executed a revenue
purchase agreement with Quick Funding with a total advance of $170,000 and payback of $254,150.
On September 20, 2024, the Company executed a revenue
purchase agreement with QFS Capital, LLC with a total advance of up to $1,573,781 and payback of $2,359,097.
On October 11, 2024, Vince Monteparte resigned as
a member of the Company’s Board of Directors.
On October 12, 2024, Assurance Dimensions resigned
as the Company’s auditor.
On October 15, 2024, Heather McMahon resigned as member
of the Company’s Board of Directors.
On October 18, 2024, the Company engaged CM3 Advisory
as its new auditor for the fiscal year ended June 30, 2024.
On October 23, 2024, the Company nominated Roger Campos
as a member of the Company’s Board of Directors.
Critical Accounting Policies
Our condensed consolidated financial statements and
accompanying notes have been prepared in accordance with accounting principles generally accepted in the United States of America and
applied on a consistent basis. The preparation of financial statements in conformity with accounting principles generally accepted in
the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities,
the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses
during the reporting periods.
We regularly evaluate the accounting policies and
estimates that we use to prepare our condensed consolidated financial statements. A complete summary of these policies is included in
Note 2. Summary of Significant Accounting Policies of the notes to our condensed consolidated financial statements. In general, management's
estimates are based on historical experience, on information from third party professionals, and on various other assumptions that are
believed to be reasonable under the facts and circumstances. Actual results could differ from those estimates made by management.
Accrued Payroll Taxes
The total balance for Federal Accrued Payroll Taxes
is accumulated on a quarterly basis beginning on their respective quarterly filing dates. Accrued Interest is compounded daily at an Effective
Annual Interest Rate of approximately seven percent. The individual quarterly sub-totals have a calculated expiration date of ten years
according to the Internal Revenue Service (“IRS”) statute of limitations. This timeline can be extended because of bankruptcy
or other legal action that is filed by the Company (Code 520 per IRS Federal Account Transcripts). Code 520 effectively stops the clock
for the Statute of limitations until the bankruptcy or other legal action has been removed (Code 521 per IRS Federal Account Transcripts).
In addition to the number of days between Code 520 and 521, every Code 520 automatically extends the IRS Statute of limitations by 30
days. As the quarterly sub-totals surpass their respective “Calculated Expiration Date” the Company removes the liability
from the Condensed Consolidated Balance Sheets and an equivalent amount is recognized as “Gain on expiration of accrued payroll
taxes” on the Condensed Consolidated Statements of Operations and Comprehensive Loss. The amount owing may be subject to additional
late filing fees and penalties that are not quantifiable as at the date of these condensed consolidated financial statements.
Revenue Recognition
The Company recognizes and accounts for revenue in
accordance with Accounting Standards Codification (“ASC”) 606 as a principal on the sale of goods and services. Pursuant to
ASC 606, revenue is measured based on a consideration specified in a contract with a customer, and excludes any sales incentives and amounts
collected on behalf of third parties. The Company recognizes revenue when it satisfies its performance obligation by transferring control
over a product or service to a customer.
Use of Estimates
Our condensed consolidated financial statements and
accompanying notes have been prepared in accordance with accounting principles generally accepted in the United States of America and
requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of revenues and expenses
during the reporting period. The Company regularly evaluates estimates and assumptions related to the revenue, valuation of inventory,
valuation of acquired assets and liabilities, variables used in the computation of share-based compensation, litigation, and evaluation
of goodwill and intangible assets for impairment.
The Company bases its estimates and assumptions on
current facts, historical experience, and various other factors that it believes to be reasonable under the circumstances, the results
of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses
that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from
the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results
of operations will be affected.
Stock-Based Compensation
The Company records stock-based compensation in accordance
with ASC 718, Compensation – Stock Compensation, using the fair value method. All transactions in which goods or services
are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received
or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the
cost of the services received as consideration are measured and recognized based on the fair value using quoted market prices of the equity
instruments issued.
Business
Combination
ASC 805, Business
Combinations (“ASC 805”), applies the acquisition method of accounting for business combinations to all acquisitions where
the acquirer gains a controlling interest, regardless of whether consideration was exchanged. ASC 805 establishes principles and requirements
for how the acquirer: a) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed,
and any non-controlling interest in the acquiree; b) recognizes and measures the goodwill acquired in the business combination or a gain
from a bargain purchase; and c) determines what information to disclose to enable users of the financial statements to evaluate the nature
and financial effects of the business combination. Accounting for acquisitions requires the Company to recognize, separately
from goodwill, the assets acquired, and the liabilities assumed at their acquisition-date fair values. Goodwill as of the acquisition
date is measured as the excess of consideration transferred and the net of the acquisition-date fair values of the assets acquired and
the liabilities assumed. While the Company uses its best estimates and assumptions to accurately value assets acquired and liabilities
assumed at the acquisition date, the estimates are inherently uncertain and subject to refinement. As a result, during the measurement
period, which may be up to one year from the acquisition date, the Company may record adjustments to the assets acquired and liabilities
assumed with the corresponding offset to goodwill. Upon the conclusion of the measurement period or final determination of the values
of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded with a corresponding gain or
loss being recognized in the Condensed Consolidated Statements of Operations and Comprehensive Loss.
Goodwill and Intangible Assets
The Company accounts for goodwill and intangible assets
in accordance with ASC 350, Intangibles – Goodwill and Other (“ASC 350”). ASC 350 requires that goodwill and other intangibles
with indefinite lives should be tested for impairment annually or on an interim basis if events or circumstances indicate that the fair
value of an asset has decreased below its carrying value.
Goodwill is tested for impairment at the reporting
unit level (operating segment or one level below an operating segment) on an annual basis (June 30 for the Company) and between annual
tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying
value. The Company considers its market capitalization and the carrying value of its assets and liabilities, including goodwill, when
performing its goodwill impairment test. When conducting its annual goodwill impairment assessment, the Company initially performs a qualitative
evaluation of whether it is more likely than not that goodwill is impaired. If it is determined by a qualitative evaluation that it is
more likely than not that goodwill is impaired, the Company then applies a two-step impairment test. The two-step impairment test first
compares the fair value of the Company's reporting unit to its carrying or book value. If the fair value of the reporting unit exceeds
its carrying value, goodwill is not impaired and the Company is not required to perform further testing. If the carrying value of the
reporting unit exceeds its fair value, the Company determines the implied fair value of the reporting unit's goodwill and if the carrying
value of the reporting unit's goodwill exceeds its implied fair value, then an impairment loss equal to the difference is recorded in
the Condensed Consolidated Statements of Operations and Comprehensive Loss. The Company recorded an impairment of goodwill in the amount
of $0 and $433,556 during the years ended June 30, 2024 and 2023, respectively.
An intangible asset is an identifiable non-monetary
asset without physical substance. Such an asset is identifiable when it is separable, or when it arises from contractual or other legal
rights. Separable assets can be sold, transferred, licensed, etc. Examples of intangible assets include computer software, licenses, trademarks,
patents, films and copyrights. The Company’s intangible assets are finite lived assets and are amortized on a straight-line basis
over the estimated useful lives of the assets.
Purchase Price Allocation
Upon the completion of a business combination, the
consideration transferred as well as the assets and liabilities acquired must be recorded at their acquisition date fair values. Upon
identification of the acquirer and determination of the acquisition date, business combinations are accounted for through the preparation
of a Purchase Price Allocation (PPA). We take into consideration the five steps when completing a PPA:
Step 1: Determine the fair value of consideration
paid;
Step 2: Revalue all existing assets and liabilities
(excluding intangible assets and goodwill which are addressed in step 3 to 5 below) to their acquisition date fair values;
Step 3: Identify the intangible assets acquired;
Step 4: Determine the fair value of identifiable intangible
assets acquired; and,
Step 5: Allocate the remaining consideration to goodwill
and assess the reasonableness of the overall conclusion
Related Party Transactions
Related party transactions are conducted with parties
with which the Company has a close association, such as majority owned subsidiaries, its executive, managers, and their families. The
types of transactions that can be conducted between related parties are many, such as sales, asset transfers, leases, lending arrangements,
guarantees, allocations of common costs, and the filing of consolidated tax returns. The Company discloses any transaction that would
impact the decision making of the users of its condensed consolidated financial statements. This involves the following disclosures:
| |
· |
General. The Company discloses all material related party transactions, including the nature of the relationship, the nature of the transactions, the dollar amounts of the transactions, the amounts due to or from related parties. |
| |
· |
Receivables. The Company separately discloses any receivables from officers, employees, or affiliated entities. |
Recently Issued Accounting Pronouncements
In November 2023, the Financial Accounting Standards
Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2023-07, Improvements to Reportable Segment Disclosures
(Topic 280). This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses
that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a
segment's profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an
explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding
how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal
years beginning after December 15, 2024. Adoption of the ASU should be applied retrospectively to all prior periods presented in the financial
statements. Early adoption is also permitted. This ASU will likely result in us including the additional required disclosures when adopted.
We are currently evaluating the provisions of this ASU and expect to adopt them for the year ending June 30, 2025.
Contractual Obligations
We are a smaller reporting company as defined by Rule
12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
Item 7A. Quantitative and Qualitative Disclosures
about Market Risk
Not applicable to smaller reporting companies.
Item 8. Financial Statements
DALRADA FINANCIAL CORPORATION
Consolidated Financial Statements
For the Years Ended June 30, 2024 and 2023
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
[EXPLANATORY NOTE]
The financial statements contained in this Annual Report on Form 10-K meet
the Alternative Reporting Standards of the OTC Markets Group Inc. and are believed by management to fairly present the financial statements
of the Company as at June 30, 2024 and for the 12 months then ended. However, they are deficient as an annual report filed under Section
13 of the Securities Exchange Act of 1934 because such financial statements do not contain a report of the Company’s PCAOB registered
independent public accounting firm. As of the date of this filing, that firm had not completed its audit procedures in respect of the
Company’s financial statements for the fiscal year ended June 30, 2024; hence, no report was included in this filing. The Company
plan to remedy this deficiency through the filing of an amended Annual Report on Form 10-K as promptly as possible, which amendment will
include an audit report and a footnote to the Company’s financial statements that will explain in tabular form any variances between
this filing and the amended filing.
DALRADA FINANCIAL CORPORATION
Condensed Consolidated Balance Sheets
| | |
| | |
| |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
| |
| Assets | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 501,927 | | |
$ | 812,806 | |
| Accounts receivable, net | |
| 7,403,933 | | |
| 4,453,104 | |
| Accounts receivable, net - related parties | |
| 1,236,484 | | |
| 752,348 | |
| Other receivables | |
| 680,598 | | |
| 376,604 | |
| Inventories | |
| 2,647,652 | | |
| 2,078,692 | |
| Prepaid expenses and other current assets | |
| 674,818 | | |
| 1,343,491 | |
| Total current assets | |
| 13,145,412 | | |
| 9,817,045 | |
| Noncurrent receivables | |
| 20,742 | | |
| 41,722 | |
| Noncurrent receivables - related parties | |
| 1,136,508 | | |
| 1,173,893 | |
| Property and equipment, net | |
| 1,452,282 | | |
| 1,476,082 | |
| Goodwill | |
| 4,175,758 | | |
| 3,803,147 | |
| Intangible assets, net | |
| 3,547,266 | | |
| 3,858,086 | |
| Right-of-use asset, net | |
| 2,437,034 | | |
| 2,771,854 | |
| Right-of-use asset, net - related party | |
| 1,689,806 | | |
| 2,227,286 | |
| Total assets | |
$ | 27,604,808 | | |
$ | 25,169,115 | |
| | |
| | | |
| | |
| Liabilities and Stockholders' Equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 6,382,691 | | |
$ | 5,178,897 | |
| Accrued liabilities | |
| 1,023,286 | | |
| 1,084,008 | |
| Accounts payable and accrued liabilities – related parties | |
| 136,976 | | |
| 547,949 | |
| Deferred revenue | |
| 452,411 | | |
| 1,337,259 | |
| Notes payable, current portion | |
| 4,468,760 | | |
| 439,562 | |
| Notes payable, current portion – related parties | |
| – | | |
| 251,605 | |
| Lease liability, current portion | |
| 832,142 | | |
| 660,394 | |
| Lease liability, current portion - related party | |
| 548,518 | | |
| 519,791 | |
| Total current liabilities | |
| 13,844,784 | | |
| 10,019,465 | |
| Noncurrent payables | |
| – | | |
| 48,888 | |
| Notes payable, net of current portion | |
| 2,332,003 | | |
| 1,011,395 | |
| Notes payable, net of current portion – related parties | |
| 53,957 | | |
| 1,648,478 | |
| Contingent consideration | |
| 47,343 | | |
| 4,285,389 | |
| Lease liability, net of current portion | |
| 1,694,804 | | |
| 2,160,834 | |
| Lease liability, net of current portion - related party | |
| 1,193,312 | | |
| 1,741,830 | |
| Total liabilities | |
| 19,166,203 | | |
| 20,916,279 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 13) | |
| – | | |
| – | |
| | |
| | | |
| | |
| Stockholders' equity: | |
| | | |
| | |
| Preferred stock, $0.01 par value, 100,000 shares authorized: | |
| – | | |
| – | |
| Series I preferred stock, $0.01 par value, 51,059 and 35,108 shares authorized, issued and outstanding as of June 30, 2024 and June 30, 2023, respectively | |
| 351 | | |
| 351 | |
| Series H preferred stock, $0.01 par value, 15,002 shares authorized, issued and outstanding as of June 30, 2024 and June 30, 2023, respectively | |
| 150 | | |
| 150 | |
| Series G preferred stock, $0.01 par value, 10,002 shares authorized, issued and outstanding as of both June 30, 2024 and June 30, 2023, respectively | |
| 100 | | |
| 100 | |
| Series F preferred stock, $0.01 par value, 5,000 shares authorized, issued and outstanding as of both June 30, 2024 and June 30, 2023, respectively | |
| 50 | | |
| 50 | |
| Common stock, $0.005 par value, 500,000,000 shares authorized, 97,175,443 and 88,699,139 shares issued and outstanding at June 30, 2024 and June 30, 2023, respectively | |
| 485,877 | | |
| 443,478 | |
| Common stock to be issued | |
| – | | |
| 192,925 | |
| Preferred stock to be issued | |
| 21,839,776 | | |
| – | |
| Additional paid-in capital | |
| 150,948,583 | | |
| 145,251,822 | |
| Accumulated deficit | |
| (164,741,349 | ) | |
| (141,729,009 | ) |
| Accumulated other comprehensive loss | |
| (909 | ) | |
| (50,848 | ) |
| Total Dalrada Financial Corp's stockholders' equity | |
| 8,532,629 | | |
| 4,109,019 | |
| Noncontrolling interests | |
| (94,024 | ) | |
| 143,817 | |
| Total stockholders' equity | |
| 8,438,605 | | |
| 4,252,836 | |
| Total liabilities and stockholders' equity | |
$ | 27,604,808 | | |
$ | 25,169,115 | |
(The accompanying notes are an integral part of these
condensed consolidated financial statements)
DALRADA FINANCIAL CORPORATION
Condensed Consolidated Statements of Operations and
Comprehensive Loss
| | |
| | | |
| | |
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Revenues | |
$ | 23,461,914 | | |
$ | 27,456,223 | |
| Revenues - related party | |
| 1,717,832 | | |
| 2,282,746 | |
| Total revenues | |
| 25,179,746 | | |
| 29,738,969 | |
| Cost of revenues | |
| 19,370,910 | | |
| 20,679,050 | |
| Gross profit | |
| 5,808,836 | | |
| 9,059,919 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| Selling, general and administrative | |
| 26,584,681 | | |
| 29,466,320 | |
| Research and development | |
| – | | |
| 120,000 | |
| Loss on impairment of goodwill | |
| – | | |
| 433,556 | |
| Total operating expenses | |
| 26,584,681 | | |
| 30,019,876 | |
| Loss from operations | |
| (20,775,845 | ) | |
| (20,959,957 | ) |
| | |
| | | |
| | |
| Other (expense) income: | |
| | | |
| | |
| Interest expense | |
| (1,213,441 | ) | |
| (2,552,918 | ) |
| Interest income | |
| 85,659 | | |
| 79,758 | |
| Other (expense) income | |
| (1,340,147 | ) | |
| 722,620 | |
| Gain on expiration of accrued tax liability | |
| – | | |
| 2,090,978 | |
| (Loss) gain on foreign exchange | |
| (6,407 | ) | |
| (8,202 | ) |
| Total other (expense) income,
net | |
| (2,474,336 | ) | |
| 332,236 | |
| Loss before taxes | |
| (23,250,181 | ) | |
| (20,627,721 | ) |
| Income taxes | |
| – | | |
| – | |
| Net loss | |
| (23,250,181 | ) | |
| (20,627,721 | ) |
| | |
| | | |
| | |
| Other comprehensive loss | |
| | | |
| | |
| Foreign currency translation | |
| 49,939 | | |
| (175 | ) |
| Comprehensive loss | |
$ | (23,200,242 | ) | |
$ | (20,627,896 | ) |
| | |
| | | |
| | |
| Net loss attributable to noncontrolling interests | |
| (237,841 | ) | |
| (335,202 | ) |
| Net loss attributable to Dalrada Financial Corporation
stockholders | |
$ | (23,012,340 | ) | |
$ | (20,292,519 | ) |
| | |
| | | |
| | |
| Net loss per common share to Dalrada stockholders - basic | |
$ | (0.25 | ) | |
$ | (0.24 | ) |
| Net loss per common share to Dalrada stockholders - diluted | |
$ | (0.25 | ) | |
$ | (0.24 | ) |
| | |
| | | |
| | |
| Weighted average common shares outstanding — basic | |
| 92,135,080 | | |
| 83,761,903 | |
| Weighted average common shares outstanding — diluted | |
| 92,135,080 | | |
| 83,761,903 | |
(The accompanying notes are an integral part of these
condensed consolidated financial statements)
DALRADA FINANCIAL CORPORATION
Condensed Consolidated Statements of Changes in Stockholders’
Equity (Deficit)
| | |
| |
| | | |
| |
| | | |
| |
| | | |
| |
| | | |
| |
| | |
| | |
Preferred Stock | | |
| |
| |
| | |
Series I | | |
Series H | | |
Series G | | |
Series F | | |
Common Stock | |
| | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | |
| Balance at June 30, 2023 | |
35,108 | |
$ | 351 | | |
15,022 | |
$ | 150 | | |
10,002 | |
$ | 100 | | |
5,000 | |
$ | 50 | | |
88,699,139 | |
$ | 443,478 | |
| Common stock issued pursuant to acquisitions | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
1,609,639 | |
| 8,048 | |
| Common stock issued pursuant to debt agreement | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
1,000,000 | |
| 5,000 | |
| Conversion of related party notes into preferred stock | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Warrants issued pursuant to acquisitions | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Common stock issued pursuant to consulting agreement | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
1,200,000 | |
| 6,000 | |
| Common stock issued pursuant to private placement | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
4,666,665 | |
| 23,351 | |
| Stock-based compensation | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Net loss | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Foreign currency translation | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Balance at June 30, 2024 | |
35,108 | |
$ | 351 | | |
15,022 | |
$ | 150 | | |
10,002 | |
$ | 100 | | |
5,000 | |
$ | 50 | | |
97,175,443 | |
$ | 485,877 | |
| | |
Preferred Stock | | |
| |
| |
| | |
Series I | | |
Series H | | |
Series G | | |
Series F | | |
Common Stock | |
| | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | | |
Shares | |
Amount | |
| Balance at June 30, 2022 | |
– | |
$ | – | | |
– | |
$ | – | | |
10,002 | |
$ | 100.00 | | |
5,000 | |
$ | 50 | | |
72,174,620 | |
$ | 360,855 | |
| | |
| |
| | | |
| |
| | | |
| |
| | | |
| |
| | | |
| |
| | |
| Common stock issued for conversion of convertibles notes, accrued interest
and premium | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
10,974,520 | |
| 54,873 | |
| Common stock issued pursuant to acquisitions | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
3,049,999 | |
| 15,250 | |
| Common stock issued pursuant to consultant agreement | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
2,000,000 | |
| 10,000 | |
| Conversion of related party notes into preferred stock | |
35,108 | |
| 351 | | |
15,022 | |
| 150 | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Warrants issued pursuant to acquisitions | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Stock-based compensation | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
500,000 | |
| 2,500 | |
| Net income (loss) | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Foreign currency translation | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | | |
– | |
| – | |
| Balance at June 30, 2023 | |
35,108 | |
$ | 351 | | |
15,022 | |
$ | 150 | | |
10,002 | |
$ | 100 | | |
5,000 | |
$ | 50 | | |
88,699,139 | |
$ | 443,478 | |
(Continued)
(The accompanying notes are an integral part of these
condensed consolidated financial statements)
DALRADA FINANCIAL CORPORATION
Condensed Consolidated Statements of Changes in Stockholders’
Equity (Deficit)
(continued)
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Common Stock to be | | |
Preferred Stock to be | | |
Additional Paid-in | | |
Accumulated | | |
Accumulated Other Comprehensive Income | | |
Total
Dalrada
Financial
Corp's Stockholders' | | |
Noncontrolling | | |
Total Stockholders' Equity | |
| | |
Issued | | |
Issued | | |
Capital | | |
Deficit | | |
(Loss) | | |
Deficit | | |
Interests | | |
(Deficit) | |
| Balance at June 30, 2023 | |
192,925 | | |
– | | |
$ | 145,251,822 | | |
$ | (141,729,009 | ) | |
$ | (50,848 | ) | |
$ | 4,109,019 | | |
$ | 143,817 | | |
$ | 4,252,836 | |
| Common stock issued pursuant to acquisitions | |
(192,925 | ) | |
4,596,334 | | |
| 386,093 | | |
| – | | |
| – | | |
| 4,797,550 | | |
| – | | |
| 4,797,550 | |
| Common stock issued pursuant to debt agreement | |
– | | |
– | | |
| 228,000 | | |
| – | | |
| – | | |
| 233,000 | | |
| – | | |
| 233,000 | |
| Conversion of related party notes into preferred stock | |
– | | |
17,243,442 | | |
| – | | |
| – | | |
| – | | |
| 17,243,442 | | |
| – | | |
| 17,243,442 | |
| Warrants issued pursuant to acquisitions | |
– | | |
– | | |
| 22,722 | | |
| – | | |
| – | | |
| 22,722 | | |
| – | | |
| 22,722 | |
| Common stock issued pursuant to consulting agreement | |
– | | |
– | | |
| 235,200 | | |
| – | | |
| – | | |
| 241,200 | | |
| – | | |
| 241,200 | |
| Common stock issued pursuant to private placement | |
– | | |
– | | |
| 580,650 | | |
| – | | |
| – | | |
| 604,001 | | |
| – | | |
| 604,001 | |
| Stock-based compensation | |
– | | |
– | | |
| 4,244,096 | | |
| – | | |
| – | | |
| 4,244,096 | | |
| – | | |
| 4,244,096 | |
| Net loss | |
– | | |
– | | |
| – | | |
| (23,012,340 | ) | |
| – | | |
| (23,012,340 | ) | |
| (237,841 | ) | |
| (23,250,181 | ) |
| Foreign currency translation | |
– | | |
– | | |
| – | | |
| – | | |
| 49,939 | | |
| 49,939 | | |
| – | | |
| 49,939 | |
| Balance at June 30, 2024 | |
– | | |
21,839,776 | | |
$ | 150,948,583 | | |
$ | (164,741,349 | ) | |
$ | (909 | ) | |
$ | 8,532,629 | | |
$ | (94,024 | ) | |
$ | 8,438,605 | |
| | |
Common Stock to be | | |
Preferred Stock to be | | |
Additional Paid-in | | |
Accumulated | | |
Accumulated Other Comprehensive Income | | |
Total
Dalrada
Financial
Corp's Stockholders' | | |
Noncontrolling | | |
Total Stockholders' Equity | |
| | |
Issued | | |
Issued | | |
Capital | | |
Deficit | | |
(Loss) | | |
Deficit | | |
Interests | | |
(Deficit) | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at June 30, 2022 | |
1,066,925 | | |
– | | |
$ | 104,627,032 | | |
$ | (121,436,490 | ) | |
$ | (50,673 | ) | |
$ | (15,432,201 | ) | |
$ | 479,019 | | |
$ | (14,953,182 | ) |
| | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common stock issued for conversion of convertibles notes, accrued interest
and premium | |
– | | |
– | | |
| 1,392,615 | | |
| – | | |
| – | | |
| 1,447,488 | | |
| – | | |
| 1,447,488 | |
| Common stock issued pursuant to acquisitions | |
(699,000 | ) | |
– | | |
| 998,225 | | |
| – | | |
| – | | |
| 314,475 | | |
| – | | |
| 314,475 | |
| Common stock issued pursuant to consultant agreement | |
– | | |
– | | |
| 174,000 | | |
| – | | |
| – | | |
| 184,000 | | |
| – | | |
| 184,000 | |
| Conversion of related party notes into preferred stock | |
– | | |
– | | |
| 33,859,043 | | |
| – | | |
| – | | |
| 33,859,544 | | |
| – | | |
| 33,859,544 | |
| Warrants issued pursuant to acquisitions | |
– | | |
– | | |
| 5,751 | | |
| – | | |
| – | | |
| 5,751 | | |
| – | | |
| 5,751 | |
| Stock-based compensation | |
(175,000 | ) | |
– | | |
| 4,195,156 | | |
| – | | |
| – | | |
| 4,022,656 | | |
| – | | |
| 4,022,656 | |
| Net income (loss) | |
– | | |
– | | |
| – | | |
| (20,292,519 | ) | |
| – | | |
| (20,292,519 | ) | |
| (335,202 | ) | |
| (20,627,721 | ) |
| Foreign currency translation | |
– | | |
– | | |
| – | | |
| – | | |
| (175 | ) | |
| (175 | ) | |
| – | | |
| (175 | ) |
| Balance at June 30, 2023 | |
192,925 | | |
– | | |
$ | 145,251,822 | | |
$ | (141,729,009 | ) | |
$ | (50,848 | ) | |
$ | 4,109,019 | | |
$ | 143,817 | | |
$ | 4,252,836 | |
(The accompanying notes are an integral part of these
condensed consolidated financial statements)
DALRADA FINANCIAL CORPORATION
Condensed Consolidated Statements of Cash Flows
| | |
| | | |
| | |
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (23,250,181 | ) | |
$ | (20,627,721 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 885,965 | | |
| 707,572 | |
| Stock compensation | |
| 4,244,096 | | |
| 4,022,656 | |
| Stock consideration issued to vendor | |
| 241,200 | | |
| 184,000 | |
| Amortization of debt discount | |
| – | | |
| 1,224,472 | |
| Convertible debt premium satisfied with common stock | |
| – | | |
| 200,000 | |
| Change in fair value of contingent consideration | |
| 752,429 | | |
| (270,936 | ) |
| Provision for credit losses | |
| 2,175,397 | | |
| 4,783,357 | |
| Loss on impairment of goodwill | |
| – | | |
| 433,556 | |
| Gain on expiration of accrued tax liability | |
| – | | |
| (2,090,978 | ) |
| Changes in operating assets and
liabilities, net of amounts acquired or assumed in connection with acquisition: | |
| | | |
| | |
| Accounts receivable | |
| (5,610,362 | ) | |
| (3,897,875 | ) |
| Other receivables | |
| (303,994 | ) | |
| (60,660 | ) |
| Inventories | |
| (568,960 | ) | |
| (454,071 | ) |
| Prepaid expenses and other current assets | |
| 726,900 | | |
| (722,147 | ) |
| Noncurrent receivables | |
| 58,365 | | |
| 35,883 | |
| Accounts payable | |
| 1,203,794 | | |
| 2,822,813 | |
| Noncurrent payables | |
| (48,888 | ) | |
| (71,646 | ) |
| Accounts payable and accrued liabilities - related
parties | |
| 12,254,613 | | |
| 7,936,820 | |
| Accrued liabilities | |
| 199,236 | | |
| 640,529 | |
| Accrued payroll taxes, penalties and interest | |
| – | | |
| 35,242 | |
| Deferred revenue | |
| (884,848 | ) | |
| 556,336 | |
| Net cash used in operating activities | |
| (7,925,238 | ) | |
| (4,612,798 | ) |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchase of property and equipment | |
| (434,845 | ) | |
| (693,201 | ) |
| Purchase of intangibles | |
| (117,813 | ) | |
| (470,680 | ) |
| Acquisition of business, net of
cash | |
| – | | |
| 100,454 | |
| Net cash used in investing activities | |
| (552,658 | ) | |
| (1,063,427 | ) |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from related party notes payable | |
| 2,923,418 | | |
| 6,757,688 | |
| Repayments of related party notes payable | |
| (428,924 | ) | |
| 350,028 | |
| Repayments of convertible note payable | |
| – | | |
| (1,680,000 | ) |
| Proceeds from notes payable | |
| 6,262,536 | | |
| – | |
| Repayments of notes payable | |
| (1,243,953 | ) | |
| – | |
| Net proceeds (repayments) from notes payable | |
| – | | |
| 289,428 | |
| Proceeds from private placement | |
| 604,001 | | |
| – | |
| Net cash provided by financing
activities | |
| 8,117,078 | | |
| 5,717,144 | |
| Net change in cash and cash equivalents | |
| (360,818 | ) | |
| 40,919 | |
| Effect of exchange rate changes on cash | |
| 49,939 | | |
| (175 | ) |
| Cash and cash equivalents at beginning of period | |
| 812,806 | | |
| 772,062 | |
| Cash and cash equivalents at end of period | |
$ | 501,927 | | |
$ | 812,806 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for income taxes | |
$ | – | | |
$ | 139,941 | |
| Cash paid for interest | |
$ | 621,735 | | |
$ | 53,760 | |
| | |
| | | |
| | |
| Supplemental disclosure of non-cash investing and financing
activities: | |
| | | |
| | |
| Conversion of related party notes
and interest into preferred stock | |
$ | 14,878,016 | | |
$ | 33,859,544 | |
| Conversion of accounts payable-related
parties to note payable-related parties | |
$ | 2,365,426 | | |
$ | 8,676,605 | |
| Common stock issued pursuant to
business combination | |
$ | 70,936 | | |
$ | 314,475 | |
| Conversion of convertible note
payable, accrued interest and premium into common stock | |
$ | – | | |
$ | 1,447,488 | |
| Warrants issued pursuant to acquisitions | |
$ | 22,722 | | |
$ | – | |
| Net assets acquired upon acquisition | |
$ | 371,298 | | |
$ | – | |
| (Decrease) Increase in right-of-use
asset and liability | |
$ | (58,227 | ) | |
$ | 2,227,830 | |
(The accompanying notes are an integral part of these
condensed consolidated financial statements)
DALRADA FINANCIAL CORPORATION
Notes to the Condensed Consolidated Financial Statements
Years ended June 30, 2024, and 2023
| 1. |
Organization and Nature of Operations |
Unless otherwise stated or the context requires
otherwise, references herein to the “Company,” “Dalrada,” “we,” “us,” and “our”
mean Dalrada Financial Corporation and its direct and indirect subsidiaries, and controlled and managed entities.
Dalrada Financial Corporation, (“Dalrada”),
was incorporated in September 1982 under the laws of the State of California. It was reincorporated in May 1983 under the laws of the
State of Delaware and reincorporated again on May 5, 2020, under the laws of the state of Wyoming. Dalrada Financial Corporation trades
under the symbol, OTC: DFCO.
Dalrada has five primary business divisions:
Genefic, Dalrada Climate Technology, Dalrada Precision Manufacturing, Dalrada Technologies and Dalrada
Corporate. Within each of these divisions, the Company drives transformative innovation while creating solutions that are sustainable,
accessible, and affordable. Dalrada’s global solutions directly address climate change, gaps in the health care industry, and technology
needs that facilitate a new era of human behavior and interaction and ensure a bright future for the world around us.
Genefic
Genefic delivers advanced health care solutions
with dedicated products, services, and systems. From virus and disease screening capabilities to pharmaceutical goods and holistic wellness
clinics, When the world needs advanced health care, Genefic delivers with ingenuity, accessibility, and affordability. This specialized
division is committed to developing key health products, lifesaving medications and building comprehensive systems to increase capability,
strive to keep people healthy with the goals of improving their quality of life and increasing their longevity– on a global level.
Genefic Specialty
Pharmacy (“Genefic Pharmacy”)- Genefic Pharmacy (formerly Genefic Specialty Pharmacy Rx Solutions) is an Alabama-based
pharmacy with more than 30 years of experience in the retail medical and pharmaceutical industries. Genefic Pharmacy specializes in providing
expert care and managing disease states through comprehensive prescription management, education, nursing, and total health solutions.
Genefic Pharmacy maintains pharmacy licenses in all 50 States as well as Washington D.C.
Genefic Infusion
Rx- Genefic Infusion Rx is a Louisiana-based infusion pharmacy which handles all aspects of fluid and medication infusion, via intravenous
or subcutaneous application. Genefic Infusion Rx serves as an essential with healthcare systems, enhancing the infusion process through
efficient authorization and prescription management. Its state-of-the-art compounding facility is led by one of only eight pharmacists
in Louisiana with a sterile compounding board certification, ensuring top-tier precision and quality in medication preparation.
Boost Diagnostics-
Boost Diagnostics (formerly Empower Genomics and Genefic Diagnostics) is Dalrada’s wholly owned diagnostic laboratory subsidiary
which processes molecular diagnostic and antibody tests to support the diagnosis of COVID-19 and the detection of immune response to the
virus. Boost Diagnostics has built up and maintained the testing capacity to handle surges in COVID-19 testing demands. Boost Diagnostics
also offers genetic testing capabilities including Pharmacogenomics, Nutraceutical, Nutrition/Diet DNA and Exercise/Fitness DNA tests.
Pala Diagnostics
(“Pala”)- Pala was a joint venture diagnostic laboratory entity which processed both molecular diagnostic and antibody
tests to support the diagnosis of COVID-19 and the detection of immune response to the virus. Pala was no longer an operational entity
as of June 30, 2023.
Dalrada Career Institute
(“DCI”) (aka International Health Group (“IHG”)) - IHG provides highly trained nursing and medical assistants
for hospitals and home health facilities since 2006. IHG Medical Assistant programs include Certified Nursing Assistant (“CNA")
and Home Health Aide (“HHA”) training and the fast-track 22-Day CNA Certification Program at its state-approved testing facility.
DCI started its first RN, nursing class in February of 2024 and this first class will be completed in December 2024. It is the intent
of DCI to double their class size when they begin their second class in 2025.
Dalrada Climate Technology
(formerly Dalrada Energy Services)
Dalrada Climate Technology (“DCT”)
is a segment which incapsulates energy services and state-of-the-art technology within the climate sustainability space. DCT employs next-generation
technology and services which enhances clean energy efforts while reducing the world’s carbon footprint. As a premier industrial
heat pump manufacturer, Dalrada delivers innovation and efficiency, building solutions that reduce energy consumption and minimize carbon
footprints, increase operational efficiencies, meet environmental, social, and governance (ESG) goals, and lower energy costs for clients.
Dalrada Technology
Limited (“DTL”)- DTL is a holding company for all United Kingdom and European based Dalrada Climate Technology entities.
Likido Ltd. (“Likido”)-
Likido is an international engineering company developing advanced solutions for the harvesting and recycling of energy. Using its novel,
heat pump systems (patent pending), Likido is working to revolutionize the renewable energy sector with the provision of innovative modular
process technologies to maximize the capture and reuse of thermal energy for integrated heating and cooling applications. With uses across
industrial, commercial and residential sectors, Likido provides cost savings and minimized carbon emissions across global supply chains.
Likido's technologies enable the effective recovery and recycling of process energy, mitigating against climate change and expected enhancement
of quality of life through the provision of low-carbon heating and cooling systems. Likido’s products currently include the
DCT One Heat Pumps (formerly Likido®ONE) and DCT Cryo Chiller. Likido also offers heat pump solutions specifically designed for residential
purposes.
During the prior year,
the U.S. Government selected DCT One Series high-performance, low-carbon heat pump for real-world testing in a prestigious clean energy
program. The implementation of the DCT One Series testing is still in process. The expected positive results should not only increase
market acceleration and adoption within the federal government acceptance of groundbreaking eco-friendly technology but should also accelerate
adoption within the commercial building industry.
Dalrada Technology
Spain L.T. (“DTS”)- DTS was established as a Spanish subsidiary of DTL for the expansion of the manufacturing and sale
of the DCT One Series and DCT Cryo Chiller throughout Europe.
Dalrada Energy Services
(“DES”)- DES provides end-to-end comprehensive energy service solutions in a robust commercial capacity. DES helps organizations
meet ESG goals and standards while mitigating negative environmental impacts.
Bothof Brothers Construction
(“Bothof”)- Bothof is a licensed general contractor which provides a wide range of development, construction and design
capabilities and expertise throughout the United States. Through Bothof’s extensive experience in construction and contracting,
the DES division can provide a myriad of additional services to its private and public works customers.
Dalrada Home Corporation
(“Dalrada Home”)- Dalrada Home Corporation was established in February of 2024. Dalrada Home’s cutting-edge sustainability
solutions are designed specifically for residential purposes. Our home heat pumps help us lead the way in providing innovative climate
technology products and services to residential customers.
Dalrada Precision Manufacturing
Dalrada Precision Manufacturing creates
total manufacturing solutions that start with the design and development of high-quality machine parts and components, and end with an
efficient global supply chain. This specialized business division can meet today’s high demands and solves industry challenges.
Dalrada Precision Manufacturing is confident that it redefines the critical quality of the world’s top components and responds with
in-house research, design, engineering, and distribution through a highly reliable global supply chain and improved time-to-market capabilities.
Dalrada Precision
Parts (“Precision”) - Precision extends the client its engineering and operations team by helping devise unique manufacturing
solutions tailored to their products. Dalrada Precision can enter at any stage of the product lifecycle from concept and design to mass
production and logistics.
Deposition Technologies
(“DepTec”) - DepTec designs, develops, manufactures, and services chemical vapor and physical vapor deposition systems
for the microchip and semiconductor industries.
DepTec has built an impressive catalogue
of precision OEM parts for PVD (Physical vapor deposition) systems and the Company’s refurbished systems which allows clients the
option of purchasing the same model of system they’ve been using for decades –but with significant upgrades and improved efficiencies.
Older systems can now operate more reliably with additional control and monitoring plus longer lifespans. DepTec also has its own PVD
and CVD (Chemical Vapor Deposition) systems, EVOS-PVD and EVOS -CVD, which deposits metals and non-metals for microchips used in almost
every standard and specialized microdevices made today and in the future. These systems can produce a superior film layer utilized in
rugged high-stress environment designs.
Ignite I.T. (“Ignite”)
- Ignite is a manufacturer and seller of eco-friendly deep cleaners, parts washers and degreasers that are specially formulated to lift
hydrocarbon-based dirt and grease from virtually all surfaces with minimal effort. Ignite products are non-flammable, non-corrosive, non-toxic,
butyl-free, water-based, and leave a light citrus scent. Ignite is developed for all surfaces suitable for water and meets or exceed the
most stringent industry-testing specifications.
Dalrada Technologies
Dalrada Technologies has worked with some
of the world’s most recognizable companies, providing digital engineering for cutting-edge software systems and offering a host
of robust digital services. This business division connects the world with integrated technology and innovative solutions, delivering
advanced capabilities and error-free results. Dalrada Technologies creates digital products with expert computer information technology
and software engineering services for a variety of technical industries and clients in both B2B and B2C environments.
Prakat (“Prakat”)-
Prakat is an ISO 9001-certified company that provides end-to-end technology services across various industries, improving the value chain.
The Company specializes in test engineering, accessibility engineering, product engineering, application modernization, billing and revenue
management, CRM, and block chain. Prakat provides global customers with software and technology solutions specializing in Test Engineering,
Accessibility Engineering, Product Engineering and Application Modernization.
Dalrada Corporate
Dalrada Corporate covers the activities
which support the entire suite of Dalrada subsidiaries. Dalrada Corporate includes the areas of administration, finance, human resources,
legal advice, information technology, and marketing. It also contains executive management and shareholder-related services.
Going Concern
These condensed consolidated financial statements
have been prepared on a going concern basis, which implies that the Company will continue to realize its assets and discharge its liabilities
in the normal course of business. As of June 30, 2024, and 2023, the Company had a positive working capital deficit of $377,228 and $202,420,
respectively. The Company incurred negative cash flows from operations for the years ended June 30, 2024, and 2023, and raises substantial
doubt about the Company’s ability to continue as a going concern. The continuation of the Company as a going concern is dependent
upon the successful financing through equity and/or debt investors and growing the subsidiaries anticipated to be profitable while reducing
investments in areas that are not expected to have long-term benefits. The Company expects to fund any short-term operational deficits
primarily through collection of outstanding accounts receivable from medical insurance providers, Medicare, pharmaceutical sales, the
sale of DCT commercial and residential heat pumps as well as loans from related parties.
| 2. |
Summary of Significant Accounting Policies |
| |
(a) |
Basis of Presentation |
These condensed consolidated financial
statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America
(“US GAAP”) and are expressed in U.S. dollars. The Company’s fiscal year end is June 30.
| |
(b) |
Principles of Consolidation |
The condensed consolidated financial statements
include the accounts of the Company and its subsidiaries, as well as the accounts of any entities over which the Company has a controlling
financial interest in accordance with Accounting Standards Codification (“ASC”) 810 Consolidation. All transactions and balances
between these entities have been eliminated upon consolidation.
The condensed consolidated financial statements
include the accounts of Dalrada Financial Corporation, Solas Corp., Boost Genomics Inc. (formerly Empower Genomics, Inc.), Dalrada Career
Institute (formerly International Health Group, Inc.), Pala Diagnostics, LLC, Pacific Stem Cells, LLC, Genefic Specialty Rx (formerly
Genefic Specialty Pharmacy Rx Solutions, Inc.),Genefic Infusion Rx., Shark Innovative Technologies Corp., Dalrada Precision Corp., Dalrada
Energy Services, Inc., Dalrada Home Corp., Likido Corp., Dalrada Technology Spain S.L., Ignite I.T., Bothof Brothers Construction Inc.,
Prakat Solutions, Inc., Prakat Solutions Private Limited, Likido Ltd., Deposition Technologies Ltd. and Dalrada Technology Ltd., controlled
by the Company through its direct or indirect ownership of a majority voting interest. Additionally, the condensed consolidated financial
statements include the accounts of variable interest entities (“VIEs”) in which the Company has a variable interest and for
which the Company is the “primary beneficiary” as it has both: (1) the power to direct the activities of the VIE that most
significantly impact the VIE’s economic performance and (2) the obligation to absorb losses of the VIE that potentially could be
significant to the VIE or the right to receive benefits from the VIE that potentially could be significant to the VIE. All significant
intercompany accounts and transactions are eliminated in consolidation.
Income attributable to the minority interest
in the Company’s majority owned and controlled consolidated subsidiaries is recorded as net income attributable to noncontrolling
interests in the Condensed Consolidated Statements of Operations and Comprehensive Loss and the noncontrolling interest is reflected as
a separate component of the statement of stockholders’ equity, condensed consolidated balance sheet, and statement of cash flows.
The preparation of these condensed consolidated
financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts
of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements
and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions
related to the revenue, valuation of inventory, valuation of acquired assets and liabilities, variables used in the computation of share-based
compensation, litigation, and evaluation of goodwill and intangible assets for impairment.
The Company bases its estimates and assumptions
on current facts, historical experience, and various other factors that it believes to be reasonable under the circumstances, the results
of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses
that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from
the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results
of operations will be affected.
| |
(d) |
Cash and Cash Equivalents |
Cash and cash equivalents include cash
deposits in financial institutions, and the Company considers all highly liquid instruments with a maturity of three months or less at
the time of issuance to be cash equivalents.
| |
(e) |
Concentrations of Credit Risk |
Financial instruments that potentially
subject the Company to concentrations of credit risk consist principally of cash, accounts receivable, and cash equivalents. The Company
generally maintains balances in various operating accounts at financial institutions that management believes to be of high credit quality,
in amounts that may exceed federally insured limits. The Company has not experienced any losses related to its cash and cash equivalents
and does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
When estimating its allowance for credit
losses related to revenues from COVID-19 Testing, the Company differentiates its receivables based on the following customer types: healthcare
insurers, government payers, and cash payers. Additionally, the Company applies assumptions and judgments for assessing collectability
and determining net revenues and accounts receivable from its customers. Management considers various historical collection factors for
assessing collectability and determining net revenues and accounts receivable from our customers which include the period that the receivables
have been outstanding, history of payment amounts, status of collections due, and applicable statutes of limitations.
During the year ended June 30, 2024 and
2023, healthcare insurers and government payers accounted for over 61% and 42% of total revenues, respectively. Also, healthcare insurers
and government payers amounted to total revenues of $17,543,688 and $12,546,849 for the years ended June 30, 2024 and 2023, respectively.
The accounts receivable related to both healthcare insurers and government payers is $6,613,175 and $1,499,415 as of June 30, 2024 and
2023, respectively.
As of June 30, 2024 and 2023, $0 and $829,239
is owed by customers from the sale of Likido units, respectively.
| |
(f) |
Fair Value Measurements |
Pursuant to ASC 820, Fair Value Measurements
and Disclosures, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring
fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used
to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of
input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure
fair value:
Level 1 - applies to assets or liabilities
for which there are quoted prices in active markets for identical assets or liabilities.
Level 2 - applies to assets or liabilities
for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets
or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent
transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally
from, or corroborated by, observable market data.
Level 3 - applies to assets or liabilities
for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the
assets or liabilities.
The Company’s financial instruments
consist principally of cash, accounts receivable, accounts payable and accrued liabilities, notes payable, and amounts due to related
parties. Pursuant to ASC 820, the fair value of cash is determined based on “Level 1” inputs, which consist of quoted prices
in active markets for identical assets. The recorded values of all other financial instruments approximate their current fair values because
of their short-term nature and respective maturity dates or durations.
The fair value of the contingent consideration
obligations was based on a probability weighted approach derived from the estimates of earn-out criteria and the probability assessment
with respect to the likelihood of achieving those criteria. The measurement was based on significant inputs that were not observable
in the market, therefore, the Company classified this liability as Level 3 in the following tables:
| Schedule of fair value measurements | |
| | | |
| | | |
| | | |
| | |
| | |
Fair Value Measurements
as of June 30, 2024 Using: | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | | |
| | | |
| | | |
| | |
| Contingent consideration | |
| – | | |
| – | | |
$ | 47,343 | | |
$ | 47,343 | |
| | |
$ | – | | |
$ | – | | |
$ | 47,343 | | |
$ | 47,343 | |
| | |
Fair Value Measurements
as of June 30, 2023 Using: | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities: | |
| | | |
| | | |
| | | |
| | |
| Contingent consideration | |
| – | | |
| – | | |
$ | 4,285,389 | | |
$ | 4,285,389 | |
| | |
$ | – | | |
$ | – | | |
$ | 4,285,389 | | |
$ | 4,285,389 | |
The Company records a contingent consideration
liability relating to stock price guarantees included in its acquisition and consulting agreements. The estimated fair value of the contingent
consideration is recorded using a significant observable measure and is therefore classified as a Level 3 financial instrument.
The fair value of the contingent consideration
liability related to the Company’s business combinations is valued based on a forward contract and the guaranteed equity value at
settlement as defined in the acquisition agreement (see “Note 4. Business Combinations and Asset Acquisition). The fair value of
the contingent consideration is then calculated based on the guaranteed equity value at settlement as defined in the acquisition agreement.
(See “Note 14. Commitments and Contingencies”).
Changes in contingent consideration liability
during the year ended June 30, 2024, and 2023, are as follows:
| Schedule of contingent consideration liability | |
| | |
| | |
Contingent | |
| | |
Consideration | |
| | |
Liability | |
| Balance as of June 30, 2023 | |
$ | 4,285,389 | |
| Change in fair value | |
| 310,945 | |
| Transfer of contingent liability to current liability | |
| (4,596,334 | ) |
| Recognition of contract contingencies | |
| 47,343 | |
| Balance as of June 30, 2024 | |
$ | 47,343 | |
| | |
Contingent | |
| | |
Consideration | |
| | |
Liability | |
| Balance as of June 30, 2022 | |
$ | 4,870,800 | |
| Change in fair value | |
| (585,411 | ) |
| Balance as of June 30, 2023 | |
$ | 4,285,389 | |
| |
(g) |
Convertible Instruments |
The Company evaluates and accounts for
conversion options embedded in convertible instruments in accordance with ASC Topic 815, Derivatives and Hedging Activities (“ASC
815”).
Applicable U.S. GAAP requires companies
to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial instruments according
to certain criteria. The criteria includes circumstances in which (a) the economic characteristics and risks of the embedded derivative
instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument
that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable
generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with
the same terms as the embedded derivative instrument would be considered a derivative instrument.
The Company accounts for convertible instruments
(when the Company has determined that the embedded conversion options should not be bifurcated from their host instruments) as follows.
The Company records, when necessary, deemed dividends for the intrinsic value of conversion options embedded in shares based upon the
differences between the fair value of the underlying common stock at the commitment date of the transaction and the effective conversion
price embedded in the shares.
Accounts receivables are derived from products
and services delivered to customers and are stated at their net realizable value. Each month, the Company reviews its receivables on a
customer-by-customer basis and evaluates whether an allowance for expected credit losses is necessary based on any known or perceived
collection issues. Any balances that are eventually deemed uncollectible are written off against the allowance after all means of collection
have been exhausted and the potential for recovery is considered remote. As of June 30, 2024, and 2023, the Company had an allowance for
expected credit losses of $860,200 and $2,430,615, respectively.
Genefic Pharmacy, Genefic Infusion, Boost,
and Pala have a standardized approach to estimate the amount of consideration that we expect to be entitled to for its pharmaceutical
revenue, and COVID-19 testing including the impact of contractual allowances (including payer denials), and patient price concessions.
The Company principally estimates the allowance for credit losses by pool based on historical collection experience, the current credit
worthiness of the customers, current economic conditions, expectations of future economic conditions and the period of time that the receivables
have been outstanding. Adjustments to our estimated contractual allowances and implicit patient price concessions are recorded in the
current period as changes in estimates.
Inventory is recorded at the lower of cost
or net realizable value on a first-in first-out (“FIFO”) basis. As of June 30, 2024 and 2023, inventory is comprised of raw
materials purchased from suppliers, work-in-progress, and finished goods produced or purchased for resale. The Company establishes inventory
reserves for estimated obsolete or unsaleable inventory equal to the difference between the cost of inventory and the estimated realizable
value based upon assumptions about future market conditions. No reserve was established for as of June 30, 2024 and 2023, respectively.
| |
(j) |
Property and Equipment |
Property and equipment are stated at cost
less accumulated depreciation and amortization. Depreciation and amortization expense is recognized using the straight-line method over
the estimated useful life of each asset, as follows:
| Schedule of property and equipment estimated useful life |
|
| |
Estimated Useful Life |
| Computer and office equipment |
3 - 5 years |
| Machinery and equipment |
5 years |
| Leasehold improvements |
Shorter of lease term or useful life |
Estimated useful lives are periodically
assessed to determine if changes are appropriate. Maintenance and repairs are charged to expense as incurred. When assets are retired
or otherwise disposed of, the cost of these assets and related accumulated depreciation or amortization are eliminated from the Condensed
Consolidated Balance Sheet and any resulting gains or losses are included in the Condensed Consolidated Statement of Operations and Comprehensive
Loss in the period of disposal.
| |
(k) |
Business Combinations and Asset Acquisitions |
The Company accounts for acquisitions in
which it obtains control of one or more businesses as a business combination. The purchase price of the acquired businesses is allocated
to the tangible and intangible assets acquired and liabilities assumed based on their estimated fair values at the acquisition date. The
excess of the purchase price over those fair values is recognized as goodwill. During the measurement period, which may be up to one year
from the acquisition date, the Company may record adjustments, in the period in which they are determined, to the assets acquired and
liabilities assumed with the corresponding offset to goodwill. If the assets acquired are not a business, the Company accounts for the
transaction or other event as an asset acquisition. Under both methods, the Company recognizes the identifiable assets acquired, the liabilities
assumed, and any noncontrolling interest in the acquired entity. In addition, for transactions that are business combinations, the Company
evaluates the existence of goodwill or a gain from a bargain purchase.
| |
(l) |
Contingent Consideration |
A Company acquisition includes contingent
consideration as part of the purchase price. The fair value of the contingent consideration is estimated as of the acquisition date based
on the present value of the contingent payments to be made using a weighted probability of possible payments. The unobservable inputs
used in the determination of the fair value of the contingent consideration include managements assumptions about the likelihood of payment
based on the established benchmarks and discount rates based on internal rate of return analysis. The fair value measurement includes
inputs that are Level 3 measurement as discussed in Note 2. Summary of Significant Accounting Policies of our condensed consolidated financial
statements included in this Annual Report on Form 10-K. Should actual results increase or decrease as compared to the assumption used
in our analysis, the fair value of the contingent consideration obligations will increase or decrease, up to the contracted limit, as
applicable. Changes in the fair value of the contingent earn-out consideration could cause a material impact and volatility in our operating
results. The contingent consideration decreased by $4,238,046 to a balance of $47,343 during the year ended June 30, 2024, and the balance
was subsequently transferred to a current liability. The outstanding balance of as of June 30, 2024 is in relation to the recognition
of contract related contingencies.
| |
(m) |
Impairment of Long-Lived Assets |
The
Company reviews its long-lived assets (property and equipment and amortizable intangible assets) for impairment whenever events or circumstances
indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected cash flows, undiscounted, is less than
the carrying amount of the asset, an impairment loss is recognized as the amount by which the carrying amount of the asset exceeds its
fair value.
Goodwill is tested annually at June 30
for impairment and upon the occurrence of certain events or substantive changes in circumstances.
The annual goodwill impairment test allows
for the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting
unit is less than its carrying amount. An entity may choose to perform the qualitative assessment on none, some or all of its reporting
units or an entity may bypass the qualitative assessment for any reporting unit and proceed directly to step one of the quantitative impairment
tests. If it is determined, on the basis of qualitative factors, that the fair value of a reporting unit is, more likely than not, less
than its carrying value, the quantitative impairment test is required. The quantitative impairment test calculates any goodwill impairment
as the difference between the carrying amount of a reporting unit and its fair value, but not to exceed the carrying amount of goodwill.
As of June 30, 2024 and 2023, there were quantitative factors that indicated goodwill was impaired in the amounts of $0 and $433,556,
respectively.
An intangible asset is an identifiable
non-monetary asset without physical substance. Such an asset is identifiable when it is separable, or when it arises from contractual
or other legal rights. Separable assets can be sold, transferred, licensed, etc. Examples of intangible assets include computer software,
licenses, trademarks, patents, films, and copyrights. The Company’s intangible assets are finite lived assets and are amortized
on a straight-line basis over the estimated useful lives of the assets.
The Company determines revenue recognition
in accordance with ASU 2014-09, Revenue from Contracts with Customers, and its related amendments (collectively known as “ASC
606”) through the following steps:
| |
- |
Identification of a contract with a customer; |
| |
- |
Identification of the performance obligations in the contract; |
| |
- |
Determination of the transaction price; |
| |
- |
Allocation of the transaction price to the performance obligations in the contract; and |
| |
- |
Recognition of revenue when or as the performance obligations are satisfied. |
Revenue is recognized when control of the
promised goods or services is transferred to customers, in an amount that reflects the consideration the Company expects to be entitled
to in exchange for those goods or services. As a practical expedient, the Company does not adjust the transaction price for the effects
of a significant financing component if, at contract inception, the period between customer payment and the transfer of goods or services
is expected to be one year or less.
The Company’s revenue is derived
from the sales of its products, which represents net sales recorded in the Company’s Condensed Consolidated Statements of Operations
and Comprehensive Loss. Product sales are recognized when performance obligations under the terms of the contract with the customer are
satisfied. Typically, this would occur upon transfer of control, including passage of title to the customer and transfer of risk of loss
related to those goods. The Company measures revenue as the amount of consideration to which it expects to be entitled in exchange for
transferring goods (transaction price). The Company records reductions to revenue for estimated customer returns, allowances, markdowns,
and discounts. The Company bases its estimates on historical rates of customer returns and allowances as well as the specific identification
of outstanding returns, markdowns and allowances that have not yet been received by the Company. The actual amount of customer returns
and allowances is inherently uncertain and may differ from the Company’s estimates. If the Company determines that actual or expected
returns or allowances are significantly higher or lower than the reserves it established, it will record a reduction or increase, as appropriate,
to net sales in the period in which it makes such a determination. Reserves for returns and markdowns are included within accrued expenses
and other liabilities in the Company’s Condensed Consolidated Balance Sheets. Allowance and discounts are recorded in accounts receivable,
net and the value of inventory associated with reserves for sales returns are included within prepaid expenses and other current assets
in the Condensed Consolidated Balance Sheets.
The Company estimates warranty claims reserves
based on historical results and research and determined that a warranty reserve was not necessary as of June 30, 2024, or 2023.
Net revenues from specialty pharmacy accounted
for over 61% of the Company’s total net revenues for the year ended June 30, 2024. Sales are recognized at an amount that reflects
the consideration to which the Company expects to be entitled in exchange for transferring control of goods or services to the customer.
The Company recognizes revenue, net of taxes and expected returns, at the time it sells merchandise, provides services or dispenses prescription
drugs to the customer. The Company estimates revenue based on expected reimbursements from third-party payors (e.g., pharmacy benefit
managers, insurance companies and governmental agencies) for dispensing prescription drugs. The estimates are based on all available information
including historical experience and are updated to actual reimbursement amounts.
The Company recognizes revenue when control
of the prescription drugs is transferred to customers, in an amount that reflects the consideration the Company expects to be entitled
to receive in exchange for those prescription drugs. The Company has established the following revenue recognition policies for the Pharmacy
Services segment:
Revenues generated from prescription drugs
sold by mail service dispensing pharmacies are recognized when the prescription drug is delivered to the client plan member. At the time
of delivery, the Company has performed substantially all of its performance obligations under its client contracts and does not experience
a significant level of returns or reshipments.
The Company’s retail drugstores recognize
revenue at the time the customer takes possession of the merchandise. For pharmacy sales, each prescription claim is its own arrangement
with the customer and is a performance obligation, separate and distinct from other prescription claims under other retail network arrangements.
Revenues are adjusted for refunds owed to third party payers resulting from pricing guarantees and performance against defined value-based
service and performance metrics. The inputs to these estimates are not subject to a high degree of subjectivity or volatility.
Boost, which provides clinical testing
services and other services, satisfies its performance obligations and recognizes revenues primarily upon completion of the testing process
(when results are reported) or when services have been rendered. Pala does not invoice the patients themselves for testing but relies
on healthcare insurers and government payers for reimbursement for COVID-19 testing. Boost has a standardized approach to estimate the
amount of consideration that we expect to be entitled to, including the impact of contractual allowances (including payer denials), and
patient price concessions. We regularly assess the state of our billing operations in order to identify issues which may impact the collectability
of receivables or revenue estimates. We believe that the collectability of our receivables is directly linked to the quality of our billing
processes, most notably those related to obtaining the correct information in order to bill effectively for the services we provide. As
such, we strive to implement “best practices” and work with our third-party billing company to reduce the number of requisitions
that we receive from healthcare providers with missing or incorrect billing information. We believe that our collection and revenue estimation
processes, along with our close monitoring of our billing operations, help to reduce the risk associated with material adjustments to
reserve estimates. However, changes to our estimate of the impact of contractual allowances (including payer denials) and patient price
concessions could have a material impact on our results of operations and financial condition in the period that the estimates are adjusted.
Adjustments to our estimated contractual allowances and implicit patient price concessions are recorded in the current period as changes
in estimates. Although we have limited track record, further adjustments to the allowances, based on actual receipts, may be recorded
upon settlement.
DES recognizes revenue on energy savings
contracts where it provides design, engineering and equipment upgrades to obtain energy savings through Environmental, Social, and Governance
(“ESG”) targets. DES recognizes revenue through two performance obligations: 1) the Energy Savings Report (point in time);
and 2) functional IP license (point in time with a significant financing component and royalty and variable consideration constraint).
Up to and upon completion of an energy savings project, DES calculates the monthly energy savings based on prior and current energy consumption
totals. Upon completion of a project, the customer pays monthly fixed payments which represents a financing component. DES recognized
monthly interest income and “royalty” revenue when the constraint from the energy savings percentage is known. DES records
revenue as it provides additional management, consulting, and other services as they are incurred.
DES records a sales-type where the Company
is the lessor. The Company records its investment in the plant and equipment, used to upgrade a customer’s real property, leased
to franchisees on a net basis, which is comprised of the present value of fixed lease payments not yet received over the course of the
energy savings agreements. The current and noncurrent portions of our net investment in sales-type leases are included in “Accounts
Receivable, net – related parties” and “Noncurrent receivables – related parties” respectively in the Condensed
Consolidated Balance Sheets. Unearned income is recognized as interest income over the lease term. Sales-type leases result in the recognition
of gain or loss at the commencement of the lease, which is recorded to “Revenues – related party” in the Condensed Consolidated
Statements of Operations and Comprehensive Loss.
DepTec and Bothof recognize revenues using
a cost-based input method, by which we use actual costs incurred relative to the total estimated contract costs to determine, as a percentage,
progress toward contract completion. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses
are determined.
The Company also earns service revenue
from its other subsidiaries, including information technology and consulting services via Prakat, educational programs, and courses from
Dalrada Career Institute, energy services and solutions from Dalrada Technology Spain and Likido, and custom parts manufacturing for Dalrada
Precision Parts. For Prakat, Dalrada Precision Parts, Dalrada Technology Spain and Likido, revenues are recognized when performance obligations
have been satisfied and the services are complete. This is generally at a point of time upon written completion and client acceptance
of the project or product, which represents transfer of control to the customer. For IHG, revenues are recognized over the course of a
semester while services are performed.
Disaggregation of Revenue
The following table presents the Company's
revenue disaggregated by revenue source:
| Schedule of disaggregated revenue | |
| | | |
| | |
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Product sales - third parties | |
$ | 18,384,320 | | |
$ | 7,324,522 | |
| Product sales - related party | |
| 140 | | |
| 77,308 | |
| Service revenue - third parties | |
| 5,077,594 | | |
| 20,131,701 | |
| Service revenue - related party | |
| 1,717,692 | | |
| 2,205,438 | |
| Total revenue | |
$ | 25,179,746 | | |
$ | 29,738,969 | |
Accounts Receivable and Deferred Revenue
The following table provides information
about receivables and contract liabilities from contracts with customers:
| Schedule of receivables and contract liabilities | |
| | | |
| | |
| |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| Accounts receivable, net | |
$ | 7,403,933 | | |
$ | 4,453,104 | |
| Accounts receivable, net - related parties | |
| 1,236,484 | | |
| 752,348 | |
| Noncurrent receivables | |
| 20,742 | | |
| 41,722 | |
| Noncurrent receivables - related parties | |
| 1,136,508 | | |
| 1,173,893 | |
| Deferred revenue | |
| 452,411 | | |
| 1,337,259 | |
The Company invoices customers based upon
contractual billing schedules, and accounts receivable are recorded when the right to consideration becomes unconditional. Contract liabilities
represent a set-up fee prepayment received from a customer in advance of performance obligations met. For the fiscal years ended June
30, 2024 and 2023, $993,962 and $242,015 of beginning deferred revenue was recognized as revenue, respectively.
Cost of revenue consists primarily of
inventory sold and related freight for product sales and direct labor for information technology and consulting services. The following
table is a breakdown of cost of revenue:
| Schedule of cost of revenue | |
| | | |
| | |
| | |
Year Ended | |
| | |
June 30 | |
| | |
2024 | | |
2023 | |
| Product sales | |
$ | 12,628,731 | | |
$ | 5,546,015 | |
| Service revenue | |
| 6,742,179 | | |
| 15,133,035 | |
| Total cost of revenue | |
$ | 19,370,910 | | |
$ | 20,679,050 | |
Advertising costs are expensed as incurred.
During the fiscal years ended June 30, 2024, and 2023, advertising expenses were $185,261 and $292,473, respectively.
| |
(q) |
Stock-based Compensation |
The Company records stock-based compensation
in accordance with ASC 718, Compensation – Stock Compensation using the fair value method. All transactions in which goods
or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration
received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees
and the cost of the services received as consideration are measured and recognized based on the quoted market price of the equity instruments
issued. During the years ended June 30, 2024, and 2023, stock-based compensation expenses were $4,244,096 and $4,022,656, respectively.
| |
(r) |
Foreign Currency Translation |
The functional currency of the Company
is the United States dollar. The functional currency of the Likido, DepTec, and Dalrada Technology subsidiaries is the Great British Pound.
The functional currency of Prakat is the Indian Rupee. The functional currency of Dalrada Technology Spain is the Euro. The financial
statements of the Company’s subsidiaries were translated to United States dollars in accordance with ASC 830, Foreign Currency
Translation Matters, using period-end rates of exchange for assets and liabilities, and average rates of exchange for the year for
revenues and expenses. Gains and losses arising on foreign currency denominated transactions are included in the Other Comprehensive Loss
section of the Condensed Consolidated Statements of Operations and Comprehensive Loss.
ASC
220, Comprehensive Income, establishes standards for the reporting and display of comprehensive loss and its components in the
condensed consolidated financial statements. During the years ended June 30, 2024, and 2023,
the Company’s only component of comprehensive loss was foreign currency translation adjustments.
| |
(t) |
Non-controlling Interests |
Non-controlling interests are classified
as a separate component of equity in the Company's Condensed Consolidated Balance Sheets and Statements of Changes in Stockholders’
Equity. Net loss attributable to non-controlling interests are reflected separately from condensed consolidated net loss in the Condensed
Consolidated Statements of Comprehensive Loss and Statements of Changes in Stockholders’ Equity (Deficit). Any change in ownership
of a subsidiary while the controlling financial interest is retained is accounted for as an equity transaction between the controlling
and non-controlling interests. In addition, when a subsidiary is deconsolidated, any retained non-controlling equity investment in the
former subsidiary will be initially measured at fair value and the difference between the carrying value and fair value of the retained
interest will be recorded as a gain or loss.
As of June 30, 2024, and 2023, non-controlling
interests pertained to the Company’s Prakat and Pala subsidiaries in the amount of 25.4%, and 51.0%, respectively.
| |
(u) |
Basic and Diluted Net Loss per Share |
The Company computes net income (loss)
per share in accordance with ASC 260, Earnings per Share. ASC 260 requires presentation of both basic and diluted earnings per
share (“EPS”) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common
shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect
to all dilutive potential common shares outstanding during the periods using the treasury stock method and convertible preferred stock
using the if-converted method. In computing diluted EPS, the average stock price for the periods is used in determining the number of
shares assumed to be purchased from the exercise of warrants.
There were no adjustments to the numerator
during the years ended June 30, 2024 and 2023.
The Company accounts for income taxes using
the asset and liability method in accordance with ASC 740, Accounting for Income Taxes. The asset and liability method provides
that deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the
financial reporting and tax bases of assets and liabilities, and for operating loss and tax credit carryforwards. Deferred tax assets
and liabilities are measured using the currently enacted tax rates and laws that will be in effect when the differences are expected to
reverse. The Company records a valuation allowance to reduce deferred tax assets to the amount that is believed more likely than not to
be realized. The Company had a full valuation allowance at June 30, 2024 and 2023, respectively.
| |
(w) |
Recent Accounting Pronouncements |
In November 2023, the Financial Accounting
Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2023-07, Improvements to Reportable Segment
Disclosures (Topic 280). This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable
segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported
measure of a segment's profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the
CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance
and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods
within fiscal years beginning after December 15, 2024. Adoption of the ASU should be applied retrospectively to all prior periods presented
in the financial statements. Early adoption is also permitted. This ASU will likely result in us including the additional required disclosures
when adopted. We are currently evaluating the provisions of this ASU and expect to adopt them for the year ending June 30, 2025.
| 3. |
Investment in Pala Diagnostics |
In August 2021, Dalrada entered a joint
venture (“JV”) with Vivera Pharmaceuticals, Inc (“Vivera”) for a 51% ownership and controlling interest to operate
a CLIA-certified diagnostics lab focused on SARS-CoV-2 testing. The JV has been treated as a business combination.
The Company determined that Pala is a Variable
Interest Entity (“VIE”); we believe that the Company has the power to direct the activities that most significantly impact
the economic performance of Pala, and accordingly, Dalrada is considered the primary beneficiary of the VIE. The Company has consolidated
the activities of the VIE.
Pursuant to the partnership agreement, Dalrada
contributed equity in the amount of $500,000 for operating capital and Vivera contributed property and equipment at a fair value of $111,185.
This amount was recorded to non-controlling interest equity balance in the Company’s Condensed Consolidated Balance Sheets.
Pursuant to the JV agreement, Dalrada
issued 250,000
shares of common stock to Vivera in October 2021. The fair value of $58,560
was recorded to goodwill as of June 30, 2022. This goodwill balance was written down to $0
as of June 30, 2023.
In December 2021, Dalrada Health filed suit
against Vivera and Paul Edalat, Vivera’s Chairman and CEO, for misappropriation of funds on behalf of the joint venture in the amount
of $2,104,509, accounted for as an unauthorized distribution. In addition to filing a cross-complaint against Dalrada Health Products,
Vivera filed a separate complaint against Dalrada Financial Corporation, Empower Genomics, Dalrada Financial Corporation’s officers,
and other unrelated parties. See Note 14. Commitments and Contingencies for legal proceedings.
| 4. |
Business Combinations and Asset Acquisition |
Fiscal 2024 Transactions
IV Services, LLC dba Genefic Infusion
Rx
On May 13, 2024, the Company acquired 100%
of IV Services, LLC dba Genefic Infusion Rx (“IVS”) through a Membership Interest Purchase Agreement. IVS is a Louisiana based
pharmacy that holds pharmacy licenses in Louisiana and Mississippi and specializes in home infusion services. Pursuant to the terms of
the transaction, the Company acquired all assets, tangible and intangible, which relate to, or are used or held for use in connection
with the business for cash, closing date inventory, closing date rent payment, closing date cash on hand, and total liabilities of $156,926.
The Company acquired IVS to expand the physical
footprint of Genefic Specialty Rx as well as its ability to ship a larger volume of prescriptions and to enter the infusion pharmacy business.
The IVS transaction was accounted for as
a business combination in accordance with Accounting Standards Codification (“ASC”) Topic 805, Business Combinations (“ASC
805”). The Company has determined preliminary fair values of the assets acquired and liabilities assumed. These values are subject
to change as we perform additional reviews of our assumptions utilized.
The Company has made a preliminary allocation
of the purchase price regarding the acquisition related to the assets acquired and liabilities assumed as of the purchase date. The following
table summarizes the purchase price allocation as of May 13, 2024:
| Schedule of purchase price allocation | |
| |
| | |
Preliminary
Purchase Price Allocation | |
| Cash and cash equivalents | |
$ | 2,991 | |
| Inventory | |
| 149,216 | |
| Prepaid expenses | |
| 2,390 | |
| Deposits | |
| 10,700 | |
| Fixed assets, net | |
| 166,526 | |
| IP-technology-license | |
| 99,000 | |
| Non-competes | |
| 17,500 | |
| Goodwill | |
| 371,298 | |
| Accounts payable | |
| (97,494 | ) |
| Accrued compensation – PTO | |
| (12,933 | ) |
| Loan payable | |
| (46,500 | ) |
| Purchase price consideration | |
$ | 662,694 | |
Fiscal 2023 Transactions
Bothof Brothers Construction Inc.
On October 17, 2022, the Company acquired
100% of the common stock of Bothof Brothers Construction Inc. (“Bothof”). The Company assumed the net liabilities of the Bothof
in exchange for the employment services of the selling shareholder. All considerations in the transaction required the continued employment
of the selling shareholder and thus is not consideration transferred under ASC 805.
The Company entered into a 36-month employment
agreement with the selling shareholder for $30,000 monthly and additionally issued 3,000,000 cashless warrants, at a strike price of $0.15
per share, to equal $450,000, which vest quarterly over a period of 24 months (the “Warrant Consideration”).
If at the end of the 24-month warrant distribution
period, beginning on the effective date of October 17, 2022 (the “Distribution Period”), the value of cashless warrants does
not equate to $6,000,000 (the “Target Amount”) in value, then the Company shall issue additional cashless warrants equal to
the shortfall between the value of the Warrants Consideration and the Target Amount (the “Valuation Shortfall”).
The value of the Warrant
Consideration to the selling shareholder is $3,482,550. The Company records the value as stock-based compensation on a straight-line basis
over the vesting period of 24-months.
The Warrant Consideration is contingent
on the selling shareholder’s continued employment with the Company; therefore, it is treated as stock-based compensation expense
and recognized ratably over a 24-month period.
The Company acquired Bothof to facilitate
the work of and expand the Dalrada Energy Services segment. Bothof’s selling shareholder holds certain licenses, construction/engineering
design expertise and management skills which will leverage synergies with Dalrada Energy Services.
The Bothof transaction was accounted for
as a business combination in accordance with ASC 805. The Company has determined preliminary fair values of the assets acquired and liabilities
assumed.
The Company has made a preliminary allocation
of the purchase price regarding the acquisition related to the assets acquired and liabilities assumed as of the purchase date. The following
table summarizes the purchase price allocation as of October 17, 2022:
| Schedule of purchase price allocation | |
| |
| | |
Preliminary
Purchase Price Allocation | |
| Cash and cash equivalents | |
$ | 70,979 | |
| Other receivables | |
| 27,289 | |
| Right of use asset, net | |
| 18,618 | |
| Property and equipment, net | |
| 17,179 | |
| Trade name | |
| 6,776 | |
| Accounts payable | |
| (24,165 | ) |
| Accrued liabilities | |
| (18,807 | ) |
| Deferred revenue | |
| (60,000 | ) |
| Lease liability | |
| (18,618 | ) |
| Notes payable, current portion | |
| (19,251 | ) |
| Purchase price consideration | |
$ | – | |
Trade name is amortized on a straight-line
basis over one month. The fair value estimate of the trade name for the purchase price allocation was based on an analysis of the present
value of future cash flows and relief from royalty method.
Dalrada Technology LTD EU (“DTL”)
On March 1, 2023, the Company acquired 100%
of the common stock of DTL in an asset acquisition. In consideration for the asset acquisition, the Company issued 1,000,000 cashless
warrants, at a strike price of $0.10 per share, which shall vest quarterly over 36 months.
The value of the Warrant Consideration to
the selling shareholder is $68,975. The value was calculated using the Black-Scholes model. The Company recorded a liability for the warrants
at the acquisition date as the warrants are not contingent on employment of the sellers.
The Company acquired DTL as a holding company
for its European operations, including Likido Ltd. and DepTec. DTL will also be utilized to pursue certain European grants and other governmental
funding opportunities. The two sellers of DTL are related parties to the Chairman and CEO of the Company.
The DTL transaction was accounted for as
an asset acquisition in accordance with ASC 805. The Company has determined preliminary fair values of the assets acquired and liabilities
assumed.
The Company has made a preliminary allocation
of the purchase price regarding the asset acquisition related to the assets acquired and liabilities assumed as of the purchase date.
The following table summarizes the purchase price allocation as of March 1, 2023:
| Schedule of purchase price allocation | |
| |
| | |
Preliminary
Purchase Price Allocation | |
| Cash and cash equivalents | |
$ | 9,108 | |
| Deposits | |
| 13,536 | |
| Prepaids | |
| 24,666 | |
| Furniture and Fixtures | |
| 64,533 | |
| Trade name | |
| 206,336 | |
| Loan Payable | |
| (249,204 | ) |
| Purchase price consideration | |
$ | 68,975 | |
Trade name is amortized on a straight-line basis over
two years.
| 5. |
Selected Balance Sheet Elements |
Inventories
Inventories consisted of the following
as of June 30, 2024, and 2023:
| Schedule of inventories | |
| | | |
| | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| Raw materials | |
$ | 910,679 | | |
$ | 658,175 | |
| Work-in-progress | |
| 1,129,348 | | |
| 708,007 | |
| Finished goods | |
| 607,625 | | |
| 712,510 | |
| Inventories | |
$ | 2,647,652 | | |
$ | 2,078,692 | |
Property and Equipment, Net
Property and equipment, net consisted of
the following as of June 30, 2024, and 2023:
| Schedule of property and equipment | |
| | | |
| | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| Machinery and equipment | |
$ | 1,769,470 | | |
$ | 1,448,556 | |
| Leasehold improvements | |
| 416,854 | | |
| 208,689 | |
| Computer and office equipment | |
| 458,689 | | |
| 426,162 | |
| Construction in progress | |
| – | | |
| 249,613 | |
| Property and equipment, gross | |
| 2,645,013 | | |
| 2,333,020 | |
| Less: Accumulated depreciation | |
| (1,192,731 | ) | |
| (856,938 | ) |
| Property and equipment, net | |
$ | 1,452,282 | | |
$ | 1,476,082 | |
Depreciation expense of $473,183 and $360,544
for the years ended June 30, 2024, and 2023, respectively, were included in selling, general and administrative expenses in the Condensed
Consolidated Statements of Operations and Comprehensive Loss.
Goodwill
Goodwill consisted of the following by
entity as of June 30, 2024, and 2023:
| Schedule of goodwill | |
| | | |
| | | |
| | |
| | |
Precision | | |
Genefic | | |
Total | |
| Balance: June 30, 2023 | |
$ | 3,106,090 | | |
$ | 697,057 | | |
$ | 3,803,147 | |
| Additions | |
| – | | |
| 372,611 | | |
| 372,611 | |
| Balance: June 30, 2024 | |
$ | 3,106,090 | | |
$ | 1,069,668 | | |
$ | 4,175,758 | |
| | |
| | | |
| | | |
| | |
| | |
Precision | | |
Genefic | | |
Total | |
| Balance: June 30, 2022 | |
$ | 3,122,811 | | |
$ | 1,130,613 | | |
$ | 4,253,424 | |
| Additions | |
| – | | |
| – | | |
| – | |
| Adjustments to purchase price allocation | |
| (16,721 | ) | |
| – | | |
| (16,721 | ) |
| Less: loss on impairment | |
| – | | |
| (433,556 | ) | |
| (433,556 | ) |
| Balance: June 30, 2023 | |
$ | 3,106,090 | | |
$ | 697,057 | | |
$ | 3,803,147 | |
Intangible Assets, Net
Intangible assets, net consisted of the
following as of June 30, 2024, and June 30, 2023:
| Schedule of intangible assets, net | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | |
| | |
| | |
| | |
Developed | | |
| |
| | |
| | |
| | |
| | |
| | |
technology, | | |
| |
| | |
Curriculum | | |
| | |
Customer | | |
| | |
software, | | |
| |
| | |
development | | |
Licenses | | |
relationships | | |
Trademarks | | |
and other | | |
Totals | |
| Balance: June 30, 2023 | |
$ | 693,385 | | |
$ | 1,064,000 | | |
$ | 1,244,480 | | |
$ | 535,547 | | |
$ | 813,479 | | |
$ | 4,350,891 | |
| Additions | |
| – | | |
| – | | |
| – | | |
| – | | |
| 116,500 | | |
| 116,500 | |
| Balance: June 30, 2024 | |
| 693,385 | | |
| 1,064,000 | | |
| 1,244,480 | | |
| 535,547 | | |
| 929,979 | | |
| 4,467,391 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Less: Accumulated amortization | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance: June 30, 2023 | |
| (172,230 | ) | |
| (55,378 | ) | |
| (153,770 | ) | |
| (54,595 | ) | |
| (56,832 | ) | |
| (492,805 | ) |
| Additions | |
| (69,339 | ) | |
| (51,118 | ) | |
| (124,448 | ) | |
| (116,324 | ) | |
| (66,091 | ) | |
| (427,320 | ) |
| Balance: June 30, 2024 | |
| (241,569 | ) | |
| (106,496 | ) | |
| (278,218 | ) | |
| (170,919 | ) | |
| (122,923 | ) | |
| (920,125 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net book value: June 30, 2024 | |
$ | 451,816 | | |
$ | 957,504 | | |
$ | 966,262 | | |
$ | 364,628 | | |
$ | 807,056 | | |
$ | 3,547,266 | |
| | |
| | |
| | |
| | |
| | |
Developed | | |
| |
| | |
| | |
| | |
| | |
| | |
technology, | | |
| |
| | |
Curriculum | | |
| | |
Customer | | |
| | |
software, | | |
| |
| | |
development | | |
Licenses | | |
relationships | | |
Trademarks | | |
and other | | |
Totals | |
| Balance: June 30, 2022 | |
$ | 693,385 | | |
$ | 1,064,000 | | |
$ | 1,230,159 | | |
$ | 348,100 | | |
$ | 335,021 | | |
$ | 3,670,665 | |
| Additions | |
| – | | |
| – | | |
| – | | |
| 186,047 | | |
| 477,458 | | |
| 663,505 | |
| Adjustments to purchase price allocation | |
| – | | |
| – | | |
| 14,321 | | |
| 1,400 | | |
| 1,000 | | |
| 16,721 | |
| Balance: June 30, 2023 | |
| 693,385 | | |
| 1,064,000 | | |
| 1,244,480 | | |
| 535,547 | | |
| 813,479 | | |
| 4,350,891 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Less: Accumulated amortization | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance: June 30, 2022 | |
| (102,891 | ) | |
| (4,260 | ) | |
| (30,754 | ) | |
| (380 | ) | |
| (7,492 | ) | |
| (145,777 | ) |
| Additions | |
| (69,339 | ) | |
| (51,118 | ) | |
| (123,016 | ) | |
| (54,215 | ) | |
| (49,340 | ) | |
| (347,028 | ) |
| Balance: June 30, 2023 | |
| (172,230 | ) | |
| (55,378 | ) | |
| (153,770 | ) | |
| (54,595 | ) | |
| (56,832 | ) | |
| (492,805 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net book value: June 30, 2023 | |
$ | 521,155 | | |
$ | 1,008,622 | | |
$ | 1,090,710 | | |
$ | 480,952 | | |
$ | 756,647 | | |
$ | 3,858,086 | |
Amortization expense of $426,220 and $347,028
for the years ended June 30, 2024, and 2023, respectively, were included in selling, general and administrative expenses in the statements
of operations and comprehensive loss. The Company’s intangible assets are subject to amortization and are amortized over the straight-line
methods over their estimated period of benefit.
Future amortization expense is as follows:
| Schedule of future amortization expense | |
| | |
| Year Ending June 30, | |
| |
| 2025 | |
$ | 416,132 | |
| 2026 | |
| 338,891 | |
| 2027 | |
| 338,891 | |
| 2028 | |
| 338,891 | |
| 2029 | |
| 332,358 | |
| Thereafter | |
| 1,782,102 | |
| Total | |
$ | 3,547,266 | |
As of June 30, 2024, and 2023, the Company
had $0 and $0, respectively, of accrued payroll taxes, penalties and interest relating to calendar years 2004 - 2007. The total balance
for accrued payroll taxes accumulated on a quarterly basis beginning on their respective quarterly filing dates. Accrued interest was
compounded daily at an estimated effective interest rate of 7.33%. The quarterly sub-totals that made up the balance had a calculated
expiration date of 10 years according to the Internal Revenue Service statute of limitations. As the tax periods surpassed their estimated
expiration date, the Company removed the liability from the Condensed Consolidated Balance Sheets, and an equivalent amount was recognized
as “Gain on expiration of accrued tax liability” within other income on the Condensed Consolidated Statements of Operations
and Comprehensive Loss. For the years ended June 30, 2024, and 2023, the Company recognized $0 and $35,242, respectively, of penalties
and interest within interest expense on the Condensed Consolidated Statements of Operations and Comprehensive Loss. For the years ended
June 30, 2024, and 2023, the Company recognized $0 and $2,090,978, respectively, within “Gain on expiration of accrued tax liability”
related to quarterly tax liabilities that expired during the respective fiscal years.
Notes Payable – Related Parties
The following is a summary of notes payable
– related parties as of June 30, 2024, and 2023:
| Schedule of notes payable –
related parties | |
| | | |
| | |
| | |
June 30, 2024 | |
| | |
Outstanding | | |
Accrued | |
| | |
Principal | | |
Interest | |
| Related entity 1 | |
$ | – | | |
$ | – | |
| Related entity 2 | |
| 52,887 | | |
| – | |
| Related entity 3 | |
| – | | |
| – | |
| Related entity 4 | |
| 1,070 | | |
| – | |
| Related entity 5 | |
| – | | |
| – | |
| Related entity 6 | |
| – | | |
| – | |
| | |
$ | 53,957 | | |
$ | – | |
| | |
June 30, 2023 | |
| | |
Outstanding | | |
Accrued | |
| | |
Principal | | |
Interest | |
| Related entity 1 | |
$ | 1,380,672 | | |
$ | 3,038 | |
| Related entity 2 | |
| 126,864 | | |
| – | |
| Related entity 3 | |
| 105,000 | | |
| – | |
| Related entity 4 | |
| 50,074 | | |
| – | |
| Related entity 5 | |
| – | | |
| – | |
| Related entity 6 | |
| 237,473 | | |
| 11,144 | |
| | |
$ | 1,900,083 | | |
$ | 14,182 | |
The following is a summary of current and
noncurrent notes payable – related parties as of June 30, 2024, and 2023:
| Schedule of current and
noncurrent notes payable – related parties | |
| | | |
| | | |
| | |
| | |
June 30, 2024 | |
| | |
Current | | |
Long-Term | | |
| |
| | |
Portion | | |
Portion | | |
Total | |
| Related entity 1 | |
$ | – | | |
$ | – | | |
$ | – | |
| Related entity 2 | |
| – | | |
| 52,887 | | |
| 52,887 | |
| Related entity 3 | |
| – | | |
| – | | |
| – | |
| Related entity 4 | |
| – | | |
| 1,070 | | |
| 1,070 | |
| Related entity 5 | |
| – | | |
| – | | |
| – | |
| Related entity 6 | |
| – | | |
| – | | |
| – | |
| | |
$ | – | | |
$ | 53,957 | | |
$ | 53,957 | |
| | |
June 30, 2023 | |
| | |
Current | | |
Long-Term | | |
| |
| | |
Portion | | |
Portion | | |
Total | |
| Related entity 1 | |
$ | – | | |
$ | 1,380,672 | | |
$ | 1,380,672 | |
| Related entity 2 | |
| – | | |
| 126,864 | | |
| 126,864 | |
| Related entity 3 | |
| – | | |
| 105,000 | | |
| 105,000 | |
| Related entity 4 | |
| 14,132 | | |
| 35,942 | | |
| 50,074 | |
| Related entity 5 | |
| – | | |
| – | | |
| – | |
| Related entity 6 | |
| 237,473 | | |
| – | | |
| 237,473 | |
| | |
$ | 251,605 | | |
$ | 1,648,478 | | |
$ | 1,900,083 | |
All notes dated December 31, 2022, and prior
are unsecured, bear interest at 3% per annum, and are due 360 days from the date of issuance, ranging from June 25, 2020, to December
30, 2022. All notes dated after December 31, 2022, are unsecured, bear interest at 8% per annum, and are due 1095 days from the date of
issuance. Each related party has significant influence or common ownership with the Company’s Chief Executive Officer. Several of
these notes are in default. The Company has not received any notices of default or demands for payment. All notes are unsecured and those
which are past-due are due on demand. As of June 30, 2024 and 2023, total accrued interest for Notes Payable-Related Parties was $0 and
$14,182, respectively. The Company recorded interest expense from Notes Payable-Related Party for fiscal years ending June 30, 2024, and
2023, of $244,750 and $814,240, respectively.
There were various related party debt convertible
notes that occurred during 2024 and 2023 (see “Note 8. Convertible Note Payable – Related Parties” for more information).
The following are the expected future payments
as of June 30, 2024:
| Schedule of expected payments - related parties | |
| |
| Fiscal Year Ended June 30, | |
| |
| 2025 | |
$ | – | |
| 2026 | |
| 53,957 | |
| 2027 | |
| – | |
| 2028 | |
| – | |
| 2029 | |
| – | |
| Total | |
$ | 53,957 | |
Notes Payable
Notes payable includes the following:
| Schedule of note payable | |
| | |
| |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| Current portion | |
$ | 4,468,760 | | |
$ | 439,562 | |
| Noncurrent portion | |
| 2,332,003 | | |
| 1,011,395 | |
| Total | |
$ | 6,800,763 | | |
$ | 1,450,957 | |
The Company’s Economic Injury Disaster
Loan (“EIDL”) dated May 10, 2020, include a 3.75% interest rate for up to 30 years; the payments are deferred for the first
two years (during which interest will accrue), and payments of principal and interest are made over the remaining 28 years. The EIDL loan
has no penalty for prepayment. The EIDL loan attaches collateral which includes the following property that EIDL borrower owns or shall
acquire or create immediately upon the acquisition or creation thereof: all tangible and intangible personal property, including, but
not limited to: (a) inventory, (b) equipment, (c) instruments, including promissory notes (d) chattel paper, including tangible chattel
paper and electronic chattel paper, (e) documents, (f) letter of credit rights, (g) accounts, including health-care insurance receivables
and credit card receivables, (h) deposit accounts, (i) commercial tort claims, (j) general intangibles, including payment intangibles
and software and (k) as-extracted collateral as such terms may from time to time be defined in the Uniform Commercial Code. The security
interest the EIDL borrower grants includes all accessions, attachments, accessories, parts, supplies and replacements for the collateral,
all products, proceeds and collections thereof and all records and data relating thereto. The balance of IHG’s EIDL is $150,000
and $147,807 for the years ended June 30, 2024, and 2023, respectively. The EIDL loan is technically in default as a result of a change
in ownership without the Small Business Administration (“SBA”) prior written consent. The Company has contacted the SBA regarding
the transfer of ownership and has not yet finalized the transfer of ownership.
The Company’s COVID-19 Government
Loan includes a 2.5% interest rate for up to six years; the payments are deferred for the first year (during which interest will accrue).
The balance of COVID-19 Government Loan is $24,206 and $36,938 for the years ended June 30, 2024, and 2023, respectively.
The Company has a loan totaling $95,060
and $320,709 as of June 30, 2024, and 2023, respectively, which includes an interest rate of 5% with a maturity date of April 29, 2025.
The loan is collateralized by personal property and includes monthly payments in the amount of $2,656, with a balloon payment at the maturity
date in the amount of $336,898. The Company renewed a loan on June 26, 2023, for $176,836, which includes an interest rate equal to the
Wall Street Journal Prime Rate, or 8.25% as of June 30, 2023, and a maturity date of June 26, 2024. The loan is collateralized by the
accounts receivable of the Company and includes four payments of $46,838.
On July 25, 2023, the Company entered into
an agreement with OnPoint LTB, LLC, for a credit line and funding of up to $2,000,000. The terms of the credit line include a 24-month
term loan, with interest only for 6 months, then amortizing over 18 months down to 50%, with the remaining 50% of the balance due at the
end of term. Interest is fixed at 20% per annum, with an origination fee of $20,000 which is added to the loan balance. The Company borrowed
the first installment of $1,200,000 at the time of closing and the remaining $800,000 was borrowed on October 4, 2023. As part of the
loan origination fee, the Company issued 500,000 shares of its common stock. The transaction includes a debt discount of $189,971 which
is amortized using an effective interest method over a 24-month period. The net balance of the loan is $1,561,395 as of June 30, 2024.
On January 4, 2024, the Company executed
a revenue purchase agreement with NewCo Capital Group LLC for $350,000, which includes a 17% purchase percentage and a total purchased
amount of $507,500 at the end of the term. The agreement includes a $10,500 underwriting fee and a $10,500 origination fee.
On January 22, February 22, a loan and security
agreement was executed with Nautilus Parent Holding, LLC whereby the Company can borrow 80% of the estimated accounts receivable at 2%
interest per month for up to a maximum draw down of $750,000. On April 18, 2024, the Company board of directors approved to increase the
maximum draw down to $8,000,000. As of June 30, 2024, the total drawdown was $2,900,000. The agreement includes a $5,000 expense deposit.
On April 8, 2024, the Company entered into
a promissory note with 1800 Diagonal Lending, LLC for $172,500. The promissory note includes a one-time interest charge of 14%, which
was applied on the issuance date, and matures on February 15, 2025. There are 10 monthly payments in the amount of $19,665 for a total
payback of $196,650. In the event of default, 1800 Diagonal Lending, LLC shall have the right to convert all or part of the outstanding
and unpaid amount of the note into common shares equal to 61% multiplied by the lowest trading price of the Company common stock during
the 10 trading days prior to the conversion date.
On May 2, 2024, the Company executed a revenue
purchase agreement with Credit Line Capital Group for $600,000, which includes a 14% purchase percentage and a total purchased amount
of $786,000 at the end of the term. The agreement includes a $6,000 underwriting fee and a $6,000 origination fee.
On May 16, 2024, the Company entered into
a promissory note with 1800 Diagonal Lending, LLC for $122,475. The promissory note includes a one-time interest charge of 14%, which
was applied on the issuance date, and matures on March 30, 2025. There are 10 monthly payments in the amount of $13,962 for a total payback
of $139,621. In the event of default, 1800 Diagonal Lending, LLC shall have the right to convert all or part of the outstanding and unpaid
amount of the note into common shares equal to 61% multiplied by the lowest trading price of the Company common stock during the 10 trading
days prior to the conversion date.
On May 16, 2024, the Company entered into
a term loan with Agile Capital Funding, LLC for $525,000 and includes an administrative fee in the amount of $23,625. There are 32 weekly
payments in the amount of $22,641 for a total payback of $724,500.
On June 25, 2024, the Company executed a
revenue purchase agreement with Cucumber Capital LLC for $325,000, which includes a 9% purchase percentage and a total purchased amount
of $487,175 at the end of the term. The agreement includes a $19,500 origination fee.
The following are the expected future payments
as of June 30, 2024:
| Schedule of expected future payments | |
| |
| Fiscal Year Ending June 30, | |
| |
| 2025 | |
$ | 4,468,760 | |
| 2026 | |
| 2,167,053 | |
| 2027 | |
| 19,902 | |
| 2028 | |
| 23,068 | |
| 2029 | |
| 17,222 | |
| Thereafter | |
| 104,759 | |
| Total | |
$ | 6,800,763 | |
| 8. |
Convertible Note Payable – Related Parties |
On February 1, 2022, $6,532,206 of related
party debt principal and interest related to promissory notes issued by the Company, with the option for conversion, was converted into
10,002 shares of Series G Convertible Preferred Stock (“Series G Stock”). The Series G Stock shall convert at one share of
Series G Stock to 2,177 shares of common stock (equivalent to converting the related dollars into common shares at $0.30 per share).
On April 4, 2023, $4,544,224 of related
party debt principal and interest related to promissory notes issued by the Company, with the option for conversion, was converted into
15,002 shares of Series H Convertible Preferred Stock (“Series H Stock”). The Series H Stock shall convert at one share of
Series H Stock to 3,029 shares of common stock (equivalent to converting the related dollars into common shares at $0.10 per share).
On June 23, 2023, $29,315,320 of related
party debt principal and interest related to promissory notes issued by the Company, with the option for conversion, was converted into
35,108 shares of Series I Convertible Preferred Stock (“Series I Stock”). The Series I Stock shall convert at one share of
Series I Stock to 5,000 shares of common stock (equivalent to converting the related dollars into common shares at $0.167 per share).
On March 29, 2024, $13,318,783 of related
party debt principal and interest related to promissory notes issued by the Company, with the option for conversion, was converted into
15,951 shares of Series I Convertible Preferred Stock (“Series I Stock”). The Series I Stock shall convert at one share of
Series I Stock to 5,000 shares of common stock (equivalent to converting the related dollars into common shares at $0.167 per share).
Pursuant to the acquisition agreement dated
April 6, 2022, between the Company and Silicon Services Consortium Ltd. (“SSCe”), the sellers of SSCe were to be issued 3,000,000
shares of its common stock evenly every quarter for 24 months with the initial distribution to take place on the effective date (the “Share
Consideration”). If at the end of the 24-month stock distribution period, beginning on the effective date of April 7, 2022 (the
“Distribution Period”), the value of common stock consideration does not equate to 4,000,000 GBP (the “Target Amount”)
in value, then the Company shall issue additional stock equal to the shortfall between the value of the Share Consideration and the Target
Amount (the “Valuation Shortfall”). At the end of the Distribution Period, the sellers of SSCe were to be issued an additional
$4,440,000 in stock as a result of the Valuation Shortfall. The Company share price at the end of the Distribution Period was $0.20, creating
an additional 22,200,000 shares of common stock due to the sellers of SSCe. Pursuant to board resolution dated May 22, 2024, Valuation
Shortfall shares were issued into 4,440 shares of Series I Convertible Preferred Stock (“Series I Stock”) as opposed to common
stock. The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock (equivalent to converting the related
dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights.
On June 30, 2024, the Company converted
$3,924,499 of related party debt principal and interest into 4,700 shares of Series I Convertible Preferred Stock (“Series I Stock”).
The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock (equivalent to converting the related
dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights.
| 9. |
Related Party Transactions |
Fiscal 2024 Transactions
During the year ended June 30, 2024, the
Company received cash funding or expenses paid on behalf of the Company from related parties totaling $2,923,418. The expenses paid on
their behalf primarily relate to operational expenditure and payroll. In most cases, promissory notes were created on a quarterly basis
totaling the amounts referenced above. The remaining amounts are included within accounts payable – related parties for which the
related parties expect repayment. The above-referenced expenses relate to three corporations that the Company has classified as related
parties. These corporations are all owned and/or operated by an individual who has a familial relationship with the Company’s CEO.
As of June 30, 2024, amounts included within
accounts payable and accrued liabilities – related parties for related party expenses were $136,976.
Fiscal 2023 Transactions
During the year ended June 30, 2023, the
Company received cash funding or expenses paid on its behalf from related parties totaling $5,439,249. The expenses paid on their behalf
primarily relate to operational expenditure and payroll. In most cases, promissory notes were created on a quarterly basis totaling the
amounts referenced above. The remaining amounts are included within accounts payable – related parties on the Company’s Condensed
Consolidated Balance Sheet for which the related parties expect repayment. The above-referenced expenses relate to three corporations
that the Company has classified as related parties. These corporations are all owned and/or operated by an individual who has a familial
relationship with the Company’s CEO.
During the year ended June 30, 2023, the
Company incurred expenses for services provided by related parties totaling $5,312,020. Services provided to the Company include management
services, payroll processing services, rent and chartered flight services. The corporations are either owned and/or operated by a relative
of the Company’s CEO, is a corporation in which the Company’s CEO can exercise control or is an individual who has a familial
relationship with the Company’s CEO.
During the year ended June 30, 2023, the
Company’s Bothof Brothers subsidiary recognized revenue for construction services totaling $2,134,470 from corporations owned and/or
operated by a related party who has a familial relationship with the Company’s CEO.
During the year ended June 30, 2023, the
Company’s DES subsidiary recognized monthly interest income and royalty revenue totaling $45,968 from corporations owned and/or
operated by a related party who has a familial relationship with the Company’s CEO.
During the year ended June 30, 2023, the
Company incurred $1,669,788 in services performed by non-employee board members.
As of June 30, 2023, amounts included within
accounts payable and accrued liabilities – related parties on the Company’s Condensed Consolidated Balance Sheet for related
party expenses were $500,288.
The following is a summary of revenues
recorded by the Company’s to related parties with common ownership:
| Schedule of revenues | |
| | | |
| | |
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Dalrada Health | |
$ | – | | |
$ | 76,912 | |
| Dalrada Energy Services | |
| 5,207 | | |
| 45,968 | |
| Ignite | |
| 140 | | |
| 396 | |
| Prakat | |
| 15,000 | | |
| 25,000 | |
| Bothof Brothers | |
| 1,697,485 | | |
| 2,134,470 | |
| | |
$ | 1,717,832 | | |
$ | 2,282,746 | |
See Notes 7, 8, 9, 10, and 14 for additional
related party transactions.
On March 29, 2024, the Company converted
$13,318,943 of related party debt principal and interest into 15,951 shares (effective price of $835 per share) of Series I Convertible
Preferred Stock (“Series I Stock”). The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common
stock (equivalent to converting the related dollars into common shares at $0.167 per share). The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 3(a)(9) of the Act in that such issuance did not constitute a public
offering.
Pursuant to the acquisition agreement dated
April 6, 2022 between the Company and Silicon Services Consortium Ltd. (“SSCe”), the sellers of SSCe were to be issued 3,000,000
shares of its common stock evenly every quarter for 24 months with the initial distribution to take place on the effective date (the “Share
Consideration”). If at the end of the 24-month stock distribution period, beginning on the effective date of April 7, 2022 (the
“Distribution Period”), the value of common stock consideration does not equate to 4,000,000 GBP (the “Target Amount”)
in value, then the Company shall issue additional stock equal to the shortfall between the value of the Share Consideration and the Target
Amount (the “Valuation Shortfall”). At the end of the Distribution Period, the sellers of SSCe were to be issued an additional
$4,440,000 in stock as a result of the Valuation Shortfall. The Company share price at the end of the Distribution Period was $0.20, creating
an additional 22,200,000 shares of common stock due to the sellers of SSCe. Pursuant to board resolution dated May 22, 2024, Valuation
Shortfall shares were issued into 4,440 shares of Series I Convertible Preferred Stock (“Series I Stock”) as opposed to common
stock. The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common stock (equivalent to converting the related
dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights. The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public
offering.
On June 30, 2024, the Company converted
$3,924,499 of related party debt principal and interest into 4,700 shares (effective price of $835 per share) of Series I Convertible
Preferred Stock (“Series I Stock”). The Series I Stock shall convert at one share of Series I Stock to 5,000 shares of common
stock (equivalent to converting the related dollars into common shares at $0.167 per share). The Series I Stock does not have voting rights.
The Company issued these shares of common stock pursuant to the exemption from registration provided by Section 3(a)(9) of the Act in
that such issuance did not constitute a public offering.
Common Stock Transactions – Fiscal 2024
In July 2023, the Company issued 500,000
shares of common stock in connection with a fee for a third-party loan in the amount of 1,200,000. The company ascribed $60,000 to those
shares recorded as a debt discount. The Company issued these shares of common stock pursuant to the exemption from registration provided
by Section 4(a)(2) of the Act in that such issuance did not constitute a public offering.
In July 2023, the Company issued 109,637
shares of common stock pursuant to the Stock Purchase Agreement with Prakat Solutions Inc. for $14,413. This issuance was a follow on
with certain legacy stockholders of Prakat to the 2020 purchase by the Company of 72% of Prakat. The Company issued these shares of common
stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public
offering.
In September and December 2023, April and
May 2024, the Company issued a total of 500,000 shares of common stock related to earn-out payments in the acquisition of Genefic Specialty
Pharmacy. The company ascribed $106,250 to those shares recorded at the value of the shares upon issuance. The Company issued these shares
of common stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute
a public offering.
In October 2023 the company issued 500,000
shares of common stock pursuant to a loan agreement for $173,000. The Company issued these shares of common stock pursuant to the exemption
from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute a public offering.
In December 2023 and April 2024, the Company
issued a total of 1,000,002 shares of common stock related to the acquisition of DepTec (SSCe) for $200,947. The Company issued these
shares of common stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not
constitute a public offering.
In February 2024, the Company issued 4,666,665
shares of common stock related to a Company conducted private placement for aggregate proceeds of $604,001, or $0.13 per share. The Company
used the proceeds for operating capital. The Company issued these shares of common stock pursuant to the exemption from registration abiding
by Rule 506 under Regulation D.
In February 2024, the Company issued 1,200,000
shares of common stock pursuant to consulting agreements resulting in $241,200 in consultancy fees. The Company issued these shares of
common stock pursuant to the exemption from registration provided by Section 4(a)(2) of the Act in that such issuance did not constitute
a public offering.
| 12. |
Stock-Based Compensation |
Dalrada Financial Corporation 2020 Stock
Compensation Plan
On July 9, 2020, the Board authorized the
Dalrada Financial Corporation 2020 Stock Compensation Plan (the “Plan”) to be used to compensate the company’s board
of directors. The Plan allocates the issuance of up to 3,500,000 shares. On February 25, 2021, the Company amended the Plan to issue up
to 4,500,000 shares and issued an aggregate of 4,500,000 common shares, or 500,000 shares to each board member. Condensed Consolidated
Statements of Operations and Comprehensive Loss. On November 10, 2021, the Company cancelled 6,500,000 shares
issued under the Plan to the Board of Directors and issued 6,500,000 cashless warrants. A total of 4,500,000 cashless warrants were
to vest immediately, and the remaining 2,000,000 cashless warrants were to vest over a 12-month period. All cashless warrants carry a
$0.45 exercise price and a ten-year term. The Company recorded stock-based compensation of $730,000 related to the 6,500,000 shares. The
issuance of the warrants was treated as a modification and, as a result of the value of the stock-based compensation of the shares cancelled
being greater than the stock-based compensation related to the cashless warrants issued, no additional stock-based compensation expense
was recorded for the year ended June 30, 2022.
On
November 30, 2021, the Company issued 2,275,000 cashless warrants to employees and consultants for services performed. A total of 825,000
cashless warrants were vested immediately and the remaining 1,450,000 cashless warrants vests over a 36-month period. The cashless warrants
include an exercise price of $0.45 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless
warrants granted was $0.73 per share, or $1,651,093 which was calculated using the Black-Scholes model.
On
February 16, 2022, the Company issued 2,250,000 cashless warrants to new members of the Board of Directors. The cashless warrants vest
over a 12-month period and hold an exercise price of $0.45 per share. The cashless warrants expire in ten years after issuance. The fair
value of the cashless warrants granted was $0.59 per share, or $1,338,644, which was calculated using the Black-Scholes model.
On August 11, 2022, the Company issued 2,200,000
cashless warrants to new members of the Board of Directors and Advisors. A total of 1,500,000 cashless warrants vest over a 12-month period
and hold an exercise price of $0.45 per share. 450,000 cashless warrants vest over a 12-month period and hold an exercise price of $0.41
per share, and the remaining 250,000 cashless warrants vest over a 12-month period beginning April 8, 2023, and hold an exercise price
of $0.45 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was $0.18
per share, or $397,890, which was calculated using the Black-Scholes model.
On October 7, 2022, the Company issued 3,000,000
cashless warrants to the selling shareholder of Bothof in connection with the acquisition of Bothof. The warrants vest over a 24-month
period and hold an exercise price of $0.15 per share. The cashless warrants expire in ten years after issuance. The fair value of the
cashless warrants granted was $1.26 per share, or $5,101,223, which was calculated using the Fair Value method. The cashless warrants
are contingent on the selling shareholder’s continued employment with the Company; therefore, it is treated as stock-based compensation
expense and recognized ratably over a 24-month period.
On March 1, 2023, the Company issued 1,000,000
cashless warrants to the selling shareholders of Dalrada Technology Ltd with the acquisition of Dalrada Technology Ltd. The warrants vest
over a 36-month period and hold an exercise price of $0.10 per share. The cashless warrants expire in ten years after issuance. The fair
value of the cashless warrants granted was $0.07 per share, or $68,975, which was calculated using the Fair Value method.
On April 14, 2023, the Company authorized
and issued 26,638,500 cashless warrants to various officers, employees, and consultants of the Company. A total of 5,575,000 cashless
warrants vest over a 36-month period and hold an exercise price of $0.45 per share. A total of 3,600,000 cashless warrants vest over a
24-month period and hold an exercise price of $0.45 per share. A total of 5,000,000 cashless warrants vest over a 36-month period and
hold an exercise price of $0.33 per share. A total of 1,300,000 cashless warrants vest over a 12-month period and hold an exercise price
of $0.20 per share. A total of 500,000 cashless warrants vest over a 12-month period and hold an exercise price of $0.12 per share. A
total of 250,000 cashless warrants vest over a 12-month period and hold an exercise price of $0.45 per share. A total of 20,000 cashless
warrants vest over a 12-month period and hold an exercise price of $0.09 per share. A total of 6,200,000 cashless warrants vest over a
36-month period and hold an exercise price of $0.16 per share. A total of 2,250,000 cashless warrants vest over a 36-month period and
hold an exercise price of $0.25 per share. A total of 1,143,500 cashless warrants vest over a 36-month period and hold an exercise price
of $0.08 per share. The remaining 800,000 cashless warrants vest over a 24-month period and hold an exercise price of $0.14 per share.
The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was $0.08 per share, or $2,143,402,
which was calculated using the Black-Scholes model.
On May 25, 2023, the Company authorized
and issued 587,634 cashless warrants to various employees of the Company. A total of 537,634 cashless warrants vest over a 36-month period
and hold an exercise price of $0.45 per share, and the remaining 50,000 cashless warrants vest over a 36-month period and hold an exercise
price of $0.08 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was
$0.10 per share, or $47,408, which was calculated using the Black-Scholes model.
On September 6, 2023, the Company authorized
and issued 15,861,000 cashless warrants to various officers, employees, and consultants of the Company. A total of 6,000,000 cashless
warrants vest over a 24-month period and hold an exercise price of $0.10 per share. A total of 4,200,000 cashless warrants vest over a
36-month period and hold an exercise price of $0.12 per share. A total of 5,161,000 cashless warrants vest over a 36-month period and
hold an exercise price of $0.17 per share. A total of 500,000 cashless warrants vest over a 36-month period and hold an exercise price
of $0.12 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was $0.08
per share, or $2,064,699, which was calculated using the Black-Scholes model.
On December 14, 2023, the Company authorized
and issued 250,000 cashless warrants to various employees of the Company. All 250,000 cashless warrants vest over a 36-month period and
hold an exercise price of $0.17 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants
granted was $0.17 per share, or $42,056, which was calculated using the Black-Scholes model.
On January 30, 2024, the Company authorized
and issued 5,455,000
cashless warrants to various employees and consultants of the company. A total of 3,250,000
cashless warrants vest over a 36-month
period and hold an exercise price of $0.20
per share. A total of 1,875,000
cashless warrants vest over a 36-month
period and hold an exercise price of $0.12
per share. A total of 80,000
cashless warrants vest over a 30-month
period and hold an exercise price of $0.20
per share. A total of 250,000
cashless warrants vest over a 12-month
period and hold an exercise price of $0.20
per share.
| Schedule of warrants outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Weighted |
|
| |
|
Common |
|
|
Weighted |
|
|
Average |
|
| |
|
Stock |
|
|
Average |
|
|
Remaining |
|
| |
|
Warrants |
|
|
Exercise Price |
|
|
Contractual Life |
|
| Outstanding - June 30, 2022 |
|
|
12,025,000 |
|
|
$ |
– |
|
|
|
8.82 |
|
| Granted |
|
|
33,426,134 |
|
|
$ |
0.29 |
|
|
|
|
|
| Exercised |
|
|
– |
|
|
|
– |
|
|
|
|
|
| Forfeited |
|
|
– |
|
|
|
– |
|
|
|
|
|
| Outstanding - June 30, 2023 |
|
|
45,451,134 |
|
|
$ |
0.33 |
|
|
|
8.83 |
|
| Granted |
|
|
21,566,000 |
|
|
$ |
0.14 |
|
|
|
|
|
| Exercised |
|
|
– |
|
|
|
– |
|
|
|
|
|
| Forfeited |
|
|
(12,840,673) |
|
|
|
– |
|
|
|
|
|
| Outstanding - June 30, 2024 |
|
|
54,176,461 |
|
|
$ |
0.26 |
|
|
|
8.28 |
|
| Exercisable at June 30, 2024 |
|
|
35,986,922 |
|
|
$ |
0.31 |
|
|
|
7.90 |
|
| Schedule of assumptions | |
| | |
| |
| | |
Years Ended June 30, | |
| | |
2024 | | |
2023 | |
| Risk-free interest rate | |
| 4.00% | | |
| 3.61% | |
| Expected volatility (1) | |
| 109.7% | | |
| 158.3% | |
| Expected dividend yield | |
| 0.00% | | |
| 0.00% | |
| Expected life (years) | |
| 5.26 | | |
| 5.27 | |
The
intrinsic value of outstanding warrants was $0 as of June 30, 2024, and 2023.
During
the year ended June 30, 2024, and 2023, stock-based compensation expenses were $4,244,097 and $4,022,656, respectively. Total unrecognized
compensation cost of non-vested options was $2,924,625 and $4,985,051 on June 30, 2024, and 2023, respectively, which will be recognized
over the next two and half fiscal years.
The Company manages its business and makes
its decisions within its operating segments. The Company classifies its operations into five segments: Genefic, Climate Technology,
Manufacturing, Technology, and Corporate. The Company evaluates the performance of its segments primarily based on revenues and operating
income (loss).
Segment information for the years ended
June 30, 2024, and 2023 is as follows:
| Schedule of segment information | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Year Ended June 30, 2024 | |
| | |
Genefic | | |
Dalrada Climate Technology | | |
Dalrada Precision Manufacturing | | |
Dalrada Technologies | | |
Corporate | | |
Consolidated | |
| Revenues | |
$ | 17,684,765 | | |
$ | 3,674,697 | | |
$ | 2,447,148 | | |
$ | 1,373,136 | | |
$ | – | | |
$ | 25,179,746 | |
| Income (Loss) from Operations | |
| (1,825,270 | ) | |
| (5,956,460 | ) | |
| (1,349,321 | ) | |
| (235,104 | ) | |
| (11,409,690 | ) | |
| (20,775,845 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Year Ended June 30, 2023 | |
| | |
Genefic | | |
Dalrada Energy | | |
Dalrada Precision Manufacturing | | |
Dalrada Technologies | | |
Corporate | | |
Consolidated | |
| Revenues | |
$ | 15,740,919 | | |
$ | 7,075,414 | | |
$ | 4,873,225 | | |
$ | 2,049,411 | | |
$ | – | | |
$ | 29,738,969 | |
| Income (Loss) from Operations | |
| (5,783,441 | ) | |
| (1,065,221 | ) | |
| (2,461,219 | ) | |
| 10,634 | | |
| (11,660,710 | ) | |
| (20,959,957 | ) |
Geographic Information
The following table presents revenue by
country:
| Schedule of revenue by country | |
| | | |
| | |
| | |
Year Ended, | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| United States | |
$ | 22,532,068 | | |
$ | 26,208,432 | |
| Scotland | |
| 1,242,642 | | |
| 1,887,061 | |
| Spain | |
| 310,568 | | |
| – | |
| India | |
| 1,094,468 | | |
| 1,643,476 | |
| | |
$ | 25,179,746 | | |
$ | 29,738,969 | |
The following table presents inventories by country:
| Schedule of inventories by country | |
| | | |
| | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| United States | |
$ | 544,334 | | |
$ | 584,330 | |
| Scotland | |
| 2,103,318 | | |
| 1,494,362 | |
| | |
$ | 2,647,652 | | |
$ | 2,078,692 | |
The following table presents property and equipment, net, by
country:
| Schedule of property and equipment by country | |
| | | |
| | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | |
| United States | |
$ | 1,277,282 | | |
$ | 1,284,834 | |
| Scotland | |
| 121,180 | | |
| 182,657 | |
| Spain | |
| 48,121 | | |
| – | |
| India | |
| 5,699 | | |
| 8,591 | |
| | |
$ | 1,452,282 | | |
$ | 1,476,082 | |
| 14. |
Commitments and Contingencies |
Lease Commitments
The Company determines if an arrangement
is a lease at inception. This determination generally depends on whether the arrangement conveys to the Company the right to control the
use of an explicitly or implicitly identified fixed asset for a period of time in exchange for consideration. Control of an underlying
asset is conveyed to the Company if the Company obtains the rights to direct the use of and to obtain substantially all the economic benefits
from using the underlying asset. The Company has lease agreements which include lease and non-lease components, which the Company has
elected to account for as a single lease component for all classes of underlying assets. Lease expense for variable lease components is
recognized when the obligation is probable.
Operating lease right of use (“ROU”)
assets and lease liabilities are recognized at commencement date based on the present value of lease payments over the lease term. Operating
lease payments are recognized as lease expense on a straight-line basis over the lease term. The Company primarily leases buildings (real
estate) which are classified as operating leases. ASC 842 requires a lessee to discount its unpaid lease payments using the interest rate
implicit in the lease or, if that rate cannot be readily determined, its incremental borrowing rate. As an implicit interest rate is not
readily determinable in the Company's leases, the incremental borrowing rate is used based on the information available at commencement
date in determining the present value of lease payments.
The lease term for all the Company's leases
includes the non-cancellable period of the lease plus any additional periods covered by either a Company option to extend (or not to terminate)
the lease that the Company is reasonably certain to exercise, or an option to extend (or not to terminate) the lease controlled by the
lessor. Options for lease renewals have been excluded from the lease term (and lease liability) for the majority of the Company's leases
as the reasonably certain threshold is not met.
Lease payments included in the measurement
of the lease liability are comprised of fixed payments, variable payments that depend on index or rate, and amounts probable to be payable
under the exercise of the Company option to purchase the underlying asset if reasonably certain.
Variable lease payments not dependent on
a rate or index associated with the Company's leases are recognized when the event, activity, or circumstance in the lease agreement on
which those payments are assessed as probable. Variable lease payments are presented as operating expenses in the Company's Condensed
Consolidated Statement of Operations and Comprehensive Loss in the same line item as expense arising from fixed lease payments. As of
and during the year ended June 30, 2021, management determined that there were no variable lease costs.
Right of Use Asset
In July 2022, the Company entered into a
five-year lease agreement to lease a commercial building in Escondido, California. The building is owned by a related party. The Company
recognized a right of use asset and lease liability of $2,405,540 and used an effective borrowing rate of 3.0% within the calculation.
Imputed interest is $192,521. The lease agreements mature in June 2027.
In April 2023, the Company’s Prakat
subsidiary entered into a lease agreement to lease office space through March 2026. The Company recognized a right of use asset and liability
of $99,060 and used an effective borrowing rate of 8% within the calculation.
In May 2021, the Company’s PSC subsidiary
entered into a three-year and 6-month lease agreement to lease a medical office space in Poway, California. The Company recognized a right
of use asset and lease liability of $277,856 and used an effective borrowing rate of 3.0% within the calculation.
In January 2022, the Company’s IHG
subsidiary entered into a five-year and 5-month lease agreement to lease a medical office space in Chula Vista, California. The Company
recognized a right of use asset and lease liability of $287,345 and used an effective borrowing rate of 3.0% within the calculation.
In May 2022, the Company’s IHG subsidiary
entered into a six-year and 3-month lease agreement to lease an office space in San Diego, California. The Company recognized a right
of use asset and lease liability of $919,722 and used an effective borrowing rate of 4.0% within the calculation.
In August 2020, the Company’s DepTec
subsidiary entered into a five-year lease agreement to lease office space. The Company recognized a right of use asset and lease liability
of $146,622 and used an effective borrowing rate of 3.0%
In May 2021, the Company’s Genefic
Specialty Pharmacy subsidiary entered into a three-year lease agreement to lease a building in Florence, Alabama. The Company recognized
a right of use asset and lease liability of $90,827 and used an effective borrowing rate of 3.0%
In July 2022, the Company’s Empower
subsidiary entered into a five-year lease agreement to lease a commercial space in Escondido, California. The building is owned by a related
party. The Company recognized a right-of-use asset and lease liability of $322,756 and used an effective borrowing rate of 3.0% within
the calculation. Imputed interest is $25,838. The lease agreement matures in June 2027.
In October 2022, the Company’s Pala
Diagnostics entered into a one-year lease agreement to lease a research and development laboratory space in San Diego, California. The
Company recognized a right-of-use asset and lease liability of $37,239 And used an effective borrowing rate of 8% within the calculation.
Imputed interest is $1,761. The lease agreement matures in October 2023.
In October 2022, the Company acquired Bothof
Brothers which had an existing lease to a commercial building in Escondido, California. The building is owned by a related party. Upon
acquisition, the company recognized a right-of-use asset and lease liability of $33,454 and used an effective borrowing rate of 3.0% within
the calculation. Imputed interest is $2,174. The lease agreement matured in December 2024.
In January 2023, the Company’s Solas
subsidiary entered into a one-year lease agreement to lease an office and medical suite in Coronado, California. The company recognized
a right-of-use asset and lease liability of $47,211 and used an effective borrowing rate of 8%.
In March 2023, the Company acquired Dalrada
Technology Ltd. which had an existing lease to a commercial building in Livingston, Scotland. Upon acquisition, the company recognized
a right-of-use asset and lease liability of $540,615 and used an effective borrowing rate of 8.0% within the calculation. Imputed interest
is $125,761. The lease agreement matures in October 2027.
In March 2023, Genefic entered into a five-year
lease agreement to lease a commercial building in San Diego, California. The Company recognized a right-of-use asset and lease liability
of $844,242 and used an effective borrowing rate of 8.0% within the calculation. Imputed interest is $185,976. The lease agreement matures
in March 2028.
In March 2023, Dalrada Technology Spain
S.L. entered into a five-year lease agreement to lease a commercial building in Bergondo, Spain. The Company recognized a right-of-use
asset and lease liability of $125,780 and used an effective borrowing rate of 8.0% within the calculation. Imputed interest is $28,129.
The lease agreement matures in May 2028.
In July, 2023, Bothof Brothers entered into
a 3-year lease agreement to lease a warehouse in Escondido, California. The Company recognized a right-of-use asset and lease liability
of $342,211 and used an effective borrowing rate of 8.0% within the calculation. Imputed interest is $39,366. The lease agreement matures
in February 2026.
In October 2022, the Company acquired Genefic
Infusion which had an existing lease to a building in Metairie, Louisiana. Upon acquisition, the company recognized a right-of-use asset
and lease liability of $109,421 and $122,541, respectively, and used an effective borrowing rate of 8.0% within the calculation. Imputed
interest is $8,838. The lease agreement matures in September 2025.
The following are the expected maturities
of lease liabilities for operating leases as of June 30, 2024, including the total amount of imputed interested related:
| Schedule of minimum lease payments | |
| | |
| Fiscal Year Ended June 30, | |
| |
| 2025 | |
$ | 1,564,119 | |
| 2026 | |
| 1,375,296 | |
| 2027 | |
| 1,232,901 | |
| 2028 | |
| 430,722 | |
| 2029 | |
| 32,627 | |
| Thereafter | |
| – | |
| Total lease payments | |
| 4,635,665 | |
| Less: imputed interest | |
| (366,889 | ) |
| Present value of lease liability | |
$ | 4,268,776 | |
Other information related to operating
leases for the years ended June 30, 2024, and 2023, respectively, were as follows:
| Schedule of lease information | |
| | | |
| | |
| | |
June 30, 2024 | | |
June 30, 2023 | |
| Weighted average remaining lease term - years | |
| 3.1 | | |
| 4.0 | |
| Weighted average discount rate | |
| 6.87% | | |
| 6.75% | |
We file income tax returns in the United
States federal jurisdiction and in various state, local, and foreign jurisdictions. In the normal course of business, we are subject to
examination by taxing authorities. The Company is currently on extension and has yet to file their income tax return for the year ended
June 30, 2024.
As of June 30, 2024 and 2023, the Company
had federal and state net operating loss carry forwards of $14,945,830, and $37,237,922, respectively, that may be offset against future
taxable income which will begin to expire in 2038 through 2041.
The reconciliation of income tax expense
computed at the U.S. federal statutory rate to the income tax provision for the years ended June 30, 2024, and 2023 is as follows:
| Schedule of reconciliation of income taxes | |
| | |
| |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Current: | |
| | | |
| | |
| Federal | |
$ | – | | |
$ | – | |
| State | |
| – | | |
| – | |
| Foreign | |
| – | | |
| – | |
| Total current income tax expense | |
| 5,600 | | |
| – | |
| Deferred: | |
| | | |
| | |
| Federal | |
| (4,065,132 | ) | |
| (3,267,961 | ) |
| State | |
| (1,143,034 | ) | |
| (907,392 | ) |
| Total deferred income tax expense | |
| (5,208,166 | ) | |
| (4,175,893 | ) |
| | |
| | | |
| | |
| Valuation allowance | |
| 5,208,166 | | |
| 4,175,893 | |
| Total provision for income taxes | |
$ | – | | |
$ | – | |
The
provision for income tax for the year ended June 30, 2024, is included in selling, general and administrative expenses.
Deferred
income taxes reflect the net tax effects of: (a) temporary differences between the carrying amounts of assets and liabilities for financial
reporting purposes and the amounts used for income tax purposes; and (b) operating loss and tax credit carryforwards. We record net deferred
tax assets to the extent we believe these assets will more likely than not be realized. In making such a determination, we consider all
available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable
income, tax planning strategies and recent financial operations. Significant components of deferred tax assets as of June 30, 2024, and
2023 were as follows:
| Schedule of deferred taxes | |
| | |
| |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| Depreciation & Amortization | |
$ | 237,475 | | |
$ | 93,044 | |
| Reserves and Accruals | |
| 111,487 | | |
| 780,876 | |
| Research & Development Credits | |
| – | | |
| – | |
| Net Operating Loss Carryforwards | |
| 14,596,868 | | |
| 9,185,104 | |
| Gross Deferred Tax Assets | |
| 14,945,830 | | |
| 10,059,024 | |
| | |
| | | |
| | |
| Valuation Allowance | |
| (14,945,830 | ) | |
| (10,059,024 | ) |
| | |
| | | |
| | |
| Net Deferred Tax Assets | |
$ | – | | |
$ | – | |
Reconciliation
of the statutory federal income tax to the Company's effective tax:
| Schedule of reconciliation of the statutory federal income tax | |
| | |
| |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| Tax at Federal Statutory Rate | |
| 21.0% | | |
| 21.0% | |
| State, Net of Federal Benefit | |
| 4.9% | | |
| 4.3% | |
| Foreign Tax | |
| 0.0% | | |
| 0.0% | |
| Tax Exempt Interest Income | |
| 0.0% | | |
| (0.06)% | |
| Gain on Expiration of Accrued Tax Liability | |
| 0.0% | | |
| 0.0% | |
| Stock Based Compensation | |
| (3.8)% | | |
| (4.1)% | |
| Nondeductible Interest | |
| 0.0% | | |
| (20.1)% | |
| Change in Valuation Allowance | |
| (22.5)% | | |
| (20.6)% | |
| | |
| | | |
| | |
| Provision for Taxes | |
| (1.0)% | | |
| (0.4)% | |
The
difference in the effective rate and the statutory rate is due to permanent differences, primarily deductibility of penalties and interest
on accrued payroll tax liabilities and the gains related to the expiration of the statute of limitations for accrued payroll tax liabilities.
On July 10, 2024, the Company entered into
a promissory note with 1800 Diagonal Lending, LLC for $87,975. The promissory note includes a one-time interest charge of 14%, which was
applied on the issuance date, and matures on May 15, 2025. There are 4 monthly payments of $10,029 and one payment of $60,175 for a total
payback of $100,291.
On July 18, 2024, the Company executed a
cash advance agreement with Cali Flower Capital Inc. with a total advance of $200,00 and payback of $299,800.
On July 25, 2024, the Company executed a
revenue purchase agreement with 24 Capital with a total advance of $125,000 and payback of $187,375.
On July 29, 2024, the Company executed a
revenue purchase agreement with Tycoon Capital Group with a total advance of $125,000 and payback of $187,375.
On August 12, 2024, the Company entered
into an Exclusive Master Distribution Agreement (the “Agreement”) with Applied Technologies of NY, Inc. (“ATI”).
The Agreement establishes the sales goals of 50 commercial heat pumps and 25 residential heat pumps in the first 18 months followed by
a total of 600 heat pumps (combined commercial and residential heat pumps) in the following 12-month period.
On August 15, 2024, the Company signed a
lease for 5,650 square feet of manufacturing and office space in Portland, Oregon related to the deposition technology business. The base
monthly lease cost is $5,254 per month and expires on April 30, 2027.
On August 19, 2024, the Company acquired
Grand Entrances for the consideration of $100 in cash, including its current liabilities and assuming its lease, which includes a monthly
lease cost of $10,291 and expires on April 11, 2030.
On August 23, 2024, the Company executed
a revenue purchase agreement with Quick Funding with a total advance of $170,000 and payback of $254,150.
On September 20, 2024, the Company executed
a revenue purchase agreement with QFS Capital, LLC with a total advance of up to $1,573,781 and payback of $2,359,097.
On October 11, 2024, Vince Monteparte resigned
as a member of the Company’s Board of Directors.
On October 12, 2024, Assurance Dimensions
resigned as the Company’s auditor.
On October 15, 2024, Heather McMahon resigned
as member of the Company’s Board of Directors.
On October 18, 2024, the Company engaged
CM3 Advisory as its new auditor for the fiscal year ended June 30, 2024.
On October 23, 2024, the Company nominated
Roger Campos as a member of the Company’s Board of Directors.
Item 9. Changes in and Disagreements with Accountants on Accounting
and Financial Disclosure
On February 14, 2024, Macias Gini & O’Connell,
LLP (“MGO”), the auditor for Dalrada for the year ended June 30, 2023, ceased performing any audit or review services for
the Company. Dalrada and MGO did not have any disagreements as of the date services ceased. On March 7, 2024, Dalrada engaged Assurance
Dimensions for audit and review services for the year ended June 30, 2024. On October 12, 2024, Assurance Dimensions ceased performing
any audit or review services for the Company. Dalrada and Assurance Dimensions did not have any disagreements as of the date services
ceased. On October 18, 2024, Dalrada engaged CM3 Advisory for audit and review services for the year ended June 30, 2024.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls
and Procedures
Our disclosure controls and procedures are designed
to ensure that information we are required to disclose in reports that we file or submit under the Securities Exchange Act of 1934, as
amended (the “Exchange Act”) is recorded, processed, summarized, and reported within the time periods specified in SEC rules
and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief
Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. As required by Rule 13a-15(b) and Rule 15d-15(b)
under the Exchange Act, our management, including our Chief Executive Officer and Chief Financial Officer, evaluated, as of December 31,
2022, the effectiveness of our disclosure controls and procedures as defined in Exchange Act Rule 13a-15(c) and Rule 15d-15(e).
Management’s Annual Report
on Internal Control over Financial Reporting
Our management is responsible for establishing and
maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal
control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United
States.
Because of its inherent limitations, internal control
over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide
only reasonable assurance of achieving their control objectives.
The Company’s management, with the participation
of the Company’s Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure
controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended
(the “Exchange Act”)) as of the end of the period covered by this report. Based on such an evaluation, the Company’s
Chief Executive Officer and Chief Financial Officer, have concluded that certain internal controls over financial reporting were not effective
as of June 30, 2024 due to the material weaknesses described in further detail below.
Previously Reported Material Weaknesses in Internal
Control over Financial Reporting
As previously disclosed in our Annual Report on Form
10-K for the year ended June 30, 2023, we identified material weaknesses in the Company’s control environment.
To address our material weaknesses, we developed a
remediation plan which included the implementation and enhancement of various policies, procedures, and controls. In executing our remediation
plan, during Fiscal 2024, the Company hired accounting experts to address technical accounting transactions, purchase price allocations
and other accounting activity. We also implemented a strict travel and reimbursement policy and enhanced our Board of Directors and Audit
Committee for better oversight of our financial reporting processes.
Material Weaknesses in Internal Control over Financial
Reporting
A material weakness is a deficiency, or combination
of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement
of the annual or interim consolidated financial statements will not be prevented or detected on a timely basis. As of June 30, 2024, we
continue to have material weaknesses in the Company’s control environment. Regarding the overall financial reporting structure of
the Company, there is not currently a comprehensive and formalized financial reporting policies and procedures manual in place. The following
sets forth internal control weakness identified:
| |
- |
preparing adequate and complete schedules across the various consolidated entities including roll forwards, revenue recognition, and allowance estimates |
Management’s remediation plan will focus on
standardizing our reconciliation process including roll forwards and revenue recognition procedures. We look to enhance the continued
improvement of our policies, procedures, and internal controls over our control environment and risk assessment.
No Attestation Report by Independent
Registered Accountant
The effectiveness of our internal control over financial
reporting as of June 30, 2024, has not been audited by our independent registered public accounting firm by virtue of our exemption from
such requirement as a smaller reporting company.
Changes in Internal Control Over Financial Reporting
There have been no changes in internal control over
financial reporting except as noted above.
Item 9B. Other Information.
During the year ended June 30, 2024, no
director or officer adopted or terminated any Rule 10b5-1 trading arrangement or non-Rule 10b5-1 trading arrangement, as each term
is defined in Item 408(a) of Regulation S-K.
PART III
Item 10. Directors, Executive Officers, Promoters and Control
Persons; Compliance with Section 16(a) of the Exchange Act
Directors, Executive Officers, and Key Employees
The following table sets forth certain information regarding our directors,
executive officers and key employees as of June 30, 2024 and as of the date of the filing of this report:
| Name and Address |
|
Age |
|
Position(s) Held |
| |
|
|
|
|
| Brian Bonar |
|
76 |
|
CEO and Director |
| Kyle McCollum |
|
41 |
|
CFO |
| Pauline Gourdie |
|
51 |
|
Director |
| Brian Kendrick |
|
60 |
|
Director |
| Anthony Zolezzi |
|
69 |
|
Director |
| Amy Scannell |
|
33 |
|
Director |
| Roger Campos 1 |
|
78 |
|
Director |
| Heather McMahon 2 |
|
51 |
|
Director |
| Vincent Monteparte 3 |
|
59 |
|
Director |
1 Mr. Campos commenced
service as a director as of October 23, 2024.
2 Ms. McMahon resigned
as a director as of October 15, 2024.
3 Mr. Monteparte resigned
as a director as of October 11, 2024.
Background of Directors and Executive Officers
Brian Bonar, CEO and Director has over 16 years
with Dalrada. Prior to joining Dalrada, Mr. Bonar worked for more than eighteen years with IBM in the US, Asia, and Europe. He has formerly
worked at QMS, and Rastek Corporation, The Solvis Group, and was on the Board of Directors of Allegiant Professional and Alliance National
Insurance Company. Mr. Bonar is also the founder of Bezier Systems.
Mr. Bonar is involved with various private entities
and has been recognized by the “Cambridge Who’s Who” on several occasions as the executive of the year. In 2012, he
was recognized as the CEO of the fastest growing public company in Orange County. He is the Chairman of Trucept, Inc. as well as President
and Chief Executive Officer of various privately held corporations. He is also on the board of American Marine LLC and founded American
Management Services (AMS) Outsourcing, a PEO-focused company.
Mr. Bonar holds the honorary title, Lord Bonar of
Wilcrick, Cardiff, Wales United Kingdom. He received a BSC in Mechanical Engineering from Strathclyde University, Glasgow Scotland and
an MBA and PHD in the field of International Business Development Studies from the Stafford University, England UK.
Kyle McCollum, has served as our Chief Financial
Officer since May 2021. Prior to that, Mr. McCollum was with Better Choice Company, Inc. (NYSE: BTTR) for three years where Mr. McCollum
acted as Corporate Secretary and SVP of Corporate Finance.
In 2018, Mr. McCollum helped form Bona Vida, Inc.,
a pet CBD company, where he served as Chief Financial Officer. In May 2019, Bona Vida merged into Better Choice Company, Inc., a New York
Stock Exchange-listed pet health and wellness company (NYSE: BTTR), where Mr. McCollum acted as Corporate Secretary and SVP of Corporate
Finance. With Better Choice Company, he assisted in the merger of TruPet, LLC, as well as the acquisition of Halo Purely for Pets. Mr.
McCollum also guided Better Choice Company in the filing of several of its 10-Q’s and its 2019 audited 10-K.
From March 2014 to July 2018, Mr. McCollum was Chief
Financial Officer of Das & Co., a New York-based family office. At Das & Co., he managed all accounting, tax, audit, portfolio
valuation, asset management, financial/investment reporting, and operations for Landmark Banyan Real Estate Fund, a $200 million India
Real Estate Fund comprised of over 10 developments. Mr. McCollum was also Chief Operating Officer of all Das & Co.’s holding
and operating subsidiaries, including Apex Resources, its mining company in Tanzania, and LDC Developers, its real estate development
company.
Mr. McCollum also served as Director of Finance at
29th Street Capital, a California-based real estate investment firm with a publicly stated asset value base of US $500 million. Prior
to 29th Street Capital, Mr. McCollum was Director of Finance and Corporate Compliance at Fletcher Robbe International LLP Attorneys at
Law, an international transactional and securities-based law firm with offices in Century City, New York, Hong Kong and Beijing. While
at Fletcher Robbe, Mr. McCollum focused on complex finance transactions, mergers & acquisitions, securities and guided several foreign
listed public companies on international cross-listing to U.S. public exchanges. Mr. McCollum earned a Bachelor of Science and Master
of Accountancy degree from the University of Montana and holds a Certified Public Accounting license in Montana.
Pauline Gourdie, Director - Ms. Gourdie is
currently the owner/operator of CSL Staffing (“CSL”), which she established in 2016. CSL is a boutique general staffing service,
providing staffing solutions for businesses in the San Diego and greater Southern California areas. For seven years prior to that, Ms.
Gourdie was the President/Owner of Gourdie Consulting Corp which provided business consulting services across Americas & Europe. Ms.
Gourdie possesses over 20 years of experience managing individuals and teams and was instrumental in the implementation of fulfilment
and manufacturing centers for IBM and Lenovo in the United States, United Kingdom, Eastern Europe, and China.
Ms. Gourdie holds a Bachelor of Science degree in
Industrial and Labor Relations from Cornell University and brings to Dalrada an extensive knowledge of supply chain management, customer
account and relationship management, and recruitment and development. Ms. Gourdie was appointed to the Dalrada board on July 29, 2019
and does not receive compensation in her role as a director.
Brian Kendrick, Director – Mr. Kendrick
has been the Managing Director of Allegro Jet Capital Management, LLC since 2014. Mr. Kendrick has over 30 years of business experience
starting with a short stint with Burroughs as a computer programmer. Mr. Kendrick developed one of the industry's first systems for tracking
owners of aircraft throughout the world and managed all aspects from the inspection and purchase of aircraft to delivery. Mr. Kendrick
was appointed to the Dalrada board on July 29, 2019.
Anthony Zolezzi, Director – Mr. Zolezzi
has been the CEO of Diomics Inc. since August 2019 and is on the Board of Directors of TwinLab and Wild Oats Organic Marketplace. Previously,
in 2018, Mr. Zolezzi was named the CEO of Twinlab Consolidated Holdings, Inc., and appointed to Twinlab’s Board of Directors in
May 2018. Mr. Zolezzi, for the last six years, is also an operating partner at Pegasus Capital Advisors, a private asset management company
focused on the wellness sectors. As a serial entrepreneur, Mr. Zolezzi has dedicated his career to the well-being of both people and the
planet, co-founding businesses that provide potential solutions to both health concerns and key environmental issues as well as focusing
on ways that biotech breakthroughs can enhance consumer health and wellness. Zolezzi has authored and co-authored six books.
Mr. Zolezzi is a graduate of Loyola Marymount University,
earned an MBA at San Diego State University and completed the Executive Development Program at the Kellogg School of Management at Northwestern
University. Mr. Zolezzi is a former board member of Vitamin Angels, a non-profit focused on providing nutritional support in impoverished
countries. He also co-founded and is a former board member of the Organic Center for Education and Promotion, and a former member of the
Organic Alliance Board of Directors. Mr. Zolezzi also serves as an advisory board member with the Menus of Change program, a joint venture
between The Culinary Institute of America and Harvard T.H.Chan School of Public Health, and the Keurig Corporation. Mr. Zolezzi was appointed
to the Dalrada board in February 2021.
Amy Scannell is currently General Counsel of
Tipp Investments, LLC, in Escondido California since June 2019. Tipp Investments, LLC is a company with broad investment interests including
commercial real estate, agriculture, and aviation. Scannell was admitted to the California bar in 2019 and the Massachusetts bar in 2015.
Prior to Tipp Investments, Scannell worked as an associate attorney for Baskin & Associates, LLC, a family law litigation firm in
Boston, Massachusetts. Scannell received her Juris Doctorate from Suffolk University Law School in Boston Massachusetts and Bachelor of
Arts from Westfield State University in Westfield, Massachusetts.
Roger Campos is the Founder and Chairman/CEO
of the Minority Business RoundTable (MBRT), established in 2002 as the first national organization for CEOs of the nation’s Asian
American, Hispanic American, African American, and Native American-owned businesses. MBRT provides a national forum for CEOs of large
and small, minority, veteran, HUBZone and women-owned businesses to address public policy issues; and serves as a unique resource on business
issues including federal procurement, contracting, access to capital and coaching CEO’s on how to do business with large corporations,
federal and state governments. Appointed by Maryland Governor Larry Hogan, during his first term, Mr. Campos served in the Cabinet as
Maryland’s first Business Ombudsman responsible for investigating and resolving complaints, issues or problems between businesses,
economic development organizations, communities, and federal, state, and local agencies; and overseeing and administering Maryland’s
first customer service standards. During the Governor’s second term, Mr. Campos was appointed as Assistant Secretary for Project
C.O.R.E (Creating Opportunities for Renewal & Enterprise) and small business development, an economic and revitalization initiative
in Baltimore City to expand business and community growth, provide new green space, affordable housing, mixed- use development, encourage
investment through attractive financing, generate jobs and strengthen the partnership between Baltimore and the State of Maryland. Prior
to founding MBRT, Mr. Campos was Vice President of Government Relations for the Hispanic Association of Colleges and Universities where
he served as chief executive managing Washington, D.C. operations including overseeing the nationally recognized internship program that
has educated more than 12,000 interns from over 500 colleges and universities. He was also Co-Founder of U.S. Hispanic Youth Entrepreneurship
& Education, a nonprofit that provided students with leadership skills and college stipends. He has a distinguished public service
career having served four years in the White House, Executive Office of the President, Office of Management and Budget setting up Presidential
Commissions, Councils and reorganizations of federal programs; Special Assistant to the Secretary of Energy; Served as Special Consultant
to the Administrator, Small Business Administration where he drafted the standard operating procedures for the U.S. federal government’s
8(a) minority business program; and served in the Office of the General Counsel, U.S. Department of Agriculture. He has also served on
several Cabinet National Advisory Boards and is widely recognized as a national business leader on small business, supplier diversity,
access to contracts and capital with federal, state, and local governments. Mr. Campos holds a Juris Doctorate degree from California
Western School of Law in San Diego, CA and a Bachelor of Arts degree in social sciences from the University of California at Santa Barbara.
Heather McMahon is one of the nation’s
top experts on counterintelligence, cybersecurity, supply chain risk management, and critical technology protection for the defense and
intelligence communities. She is currently a Professor of Practice at the at the University of Maryland’s Applied Research Laboratory
for Intelligence and Security (ARLIS). At ARLIS, she leads research that integrates social and behavioral sciences, computing, and artificial
intelligence to advance applied research and development of capabilities in areas of critical need for the Department of Defense and the
intelligence community (IC). Heather formerly served as the ARLIS Deputy Executive Direct
Previously, Heather served in the White House as Senior
Director to the President’s Intelligence Advisory Board (PIAB) where she advised the Executive Office of the President and National
Security Council on national security, counterintelligence, risk management, industrial security, and insider threat matters. In 2023,
she was recognized with the Presidential Rank Order Award for her service there.
Prior to the White House, Heather served as a senior
defense intelligence executive for the Undersecretary of Defense for Intelligence (USDI) and the Department of the Army’s Intelligence
Staff where she devised, implemented, and led programs to protect DoD technology, information, personnel, facilities, and critical infrastructure
from internal and external threats. Heather also served as a business development executive at a cybersecurity company.
Heather is a seasoned U.S. Army combat veteran and
highly skilled human intelligence and counterintelligence officer with nearly three decades of global operational experience. This includes
extensive tours in Afghanistan, Iraq, Bosnia, Europe, and Asia, serving the Army at every echelon between platoon and corps as well as
in the IC’s strategic enterprise. For her exemplary service, she received the Legion of Merit, Bronze Star Medal, Meritorious Service
Medal (3), DoD Counterintelligence Award for Best Counterintelligence Operations Team (2006; Iraq), and Defense Medal for Exceptional
Civilian Service (2021), among other awards.
Today, Heather applies her experience and expertise
to corporations on how to manage risk and defend against state-sponsored cyberattacks, insider threats, and intellectual property theft.
She currently serves as a member of the Grant Committee for the Gula Tech Foundation, a civic effort focused on closing the cyber skills
gap in America. She was also a national security fellow at the Foundation for Defense of Democracies and serves as a volunteer Senior
Advisor to Maker Mask, a nonprofit technology-based initiative to support effective community responses to the COVID-19 crisis.
Heather is a graduate of the United States Military
Academy at West Point along with numerous advanced Intelligence Community and military schools including the Army’s Jumpmaster School
and Airborne School. As a writer, she has appeared in the Washington Post and the US Army War College War Room (co-author with Michael
Schellhammer).
Vincent Monteparte is Principal and Venture
Partner in the venture capital and investment banking industry, having managed transactions and investments ranging from $40M to $500M.
His leadership transformed uniquely positioned mid-market organizations in the enterprise software sector to upwards of $2 billion in
enterprise valuations. A diverse background in technology, aerospace, transportation, logistics, real estate and healthcare has led Mr.
Monteparte to roles as Venture Partner at Sway Ventures, a US-based venture capital firm investing
in early to mid-stage technology companies and Principal at Global Capital Markets, an investment banking firm focusing on mid-market
transactions for technology, manufacturing, distribution, service and retail companies.
Mr. Monteparte
began his career as an entrepreneur and founded various companies, most recently Miro Technologies. At Miro, he led the development of
a SaaS solution to modernize maintenance, repair, overhaul, and supply chain operations for complex assets. The business was sold to Boeing
for 14x return on invested capital with an IRR of 48%. Additionally, Mr. Monteparte has since held various senior level executive roles
leading teams and positioning multinational corporations to growth.
Mr. Monteparte holds a series 63 and 79 license and
Board Advisory positions for BlueSky eLearn, a leading learning management software platform and Measurabl, a global ESG SaaS Software
company. He received a B.A. in Aeronautical Engineering from Embry-Riddle Aeronautical University and an MBA from the Pepperdine University
Graziadio School of Business, where he earned the Most Distinguished Alumni Award. In his spare time, Mr. Monteparte co-chairs the Business
& Entrepreneurship Committee at Pepperdine, where he acts as a mentor to young entrepreneurs and executives in career and portfolios.
Term of Office of Directors
Our directors are appointed for a one-year term to
hold office until the next annual general meeting of our stockholders or until removed from office in accordance with our bylaws. Our
officers are appointed by our Board of Directors and hold office until the officer dies or resigns or the Board elects a successor or
removes the officer.
Key Employees
David Pickett, is currently Executive Vice
President of Sales and Marketing. Mr. Pickett’s professional background includes 25 years’ experience in executive relationship
development and business growth with several companies including Celestica Inc. and Axxion Inc. Mr. Pickett has worked with the largest
OEM and Fortune 500 companies in the world. Mr. Pickett’s vast knowledge base of engineering and manufacturing operational and supply
chain requirements has proven to be a strategic asset for accelerating the growth of Dalrada Precision’s global manufacturing and
clean energy initiatives through its portfolio company Likido.
Family Relationships
Pauline Gourdie is the daughter of Brian Bonar.
Audit Committee Financial Expert
No determination has been made as to whether any member
of the audit committee qualified as an audit committee financial expert as defined in Item 401 of Regulation S-K.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act
of 1934, as amended, requires our directors and executive officers, and persons who beneficially own more than 10% of a registered class
of our equity securities, to file reports of beneficial ownership and changes in the beneficial ownership of our securities with the SEC
of Forms 3 (Initial Statement of Beneficial Ownership), 4 (Statement of Changes of Beneficial Ownership of Securities) and 5 (Annual Statement
of Beneficial Ownership of Securities). Directors, executive officers and beneficial owners of more than 10% of our Common Stock are required
by SEC regulations to furnish us with copies of all Section 16(a) forms that they file. The required filings will be made.
Code of Ethics
We have adopted an informal Code of Ethics that applies
to our officers and directors, which we feel is sufficient at this time.
Item 11. Executive and Director Compensation
SUMMARY COMPENSATION TABLE
| Name and Principal | |
| | |
| | |
| | |
Stock | | |
Option | | |
All Other | | |
| |
| Position | |
Year | | |
Salary | | |
Bonus | | |
Awards | | |
Awards | | |
Compensation | | |
Total | |
| Brian Bonar, CEO | |
2024 | | |
| 432,300 | | |
| – | | |
| 158,928 | | |
| – | | |
| – | | |
| 591,228 | |
| Kyle McCollum, CFO | |
2024 | | |
| 375,000 | | |
| 100,000 | | |
| – | | |
| – | | |
| – | | |
| 475,000 | |
| Pauline Gourdie | |
2024 | | |
| – | | |
| – | | |
| 158,928 | | |
| – | | |
| – | | |
| 158,928 | |
| Brian Kendrick | |
2024 | | |
| – | | |
| – | | |
| 158,928 | | |
| – | | |
| – | | |
| 158,928 | |
| Anthony Zolezzi | |
2024 | | |
| 43,824 | | |
| – | | |
| 158,928 | | |
| – | | |
| – | | |
| 202,752 | |
| Amy Scannell | |
2024 | | |
| – | | |
| | | |
| 158,928 | | |
| – | | |
| – | | |
| 158,928 | |
| Vincent Monteparte | |
2024 | | |
| – | | |
| – | | |
| 158,928 | | |
| – | | |
| – | | |
| 158,928 | |
| Heather McMahon | |
2024 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Total | |
| | |
| 851,124 | | |
| 100,000 | | |
| 953,568 | | |
| – | | |
| – | | |
| 1,904,692 | |
| Name and Principal | |
| | |
| | |
| | |
Stock | | |
Option | | |
All Other | | |
| |
| Position | |
Year | | |
Salary | | |
Bonus | | |
Awards | | |
Awards | | |
Compensation | | |
Total | |
| Brian Bonar, CEO | |
2023 | | |
| 393,000 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 393,000 | |
| Brian McGoff, COO | |
2023 | | |
| 350,719 | | |
| – | | |
| 479,623 | | |
| – | | |
| – | | |
| 830,342 | |
| Kyle McCollum, CFO | |
2023 | | |
| 375,062 | | |
| 75,000 | | |
| 248,916 | | |
| – | | |
| – | | |
| 698,978 | |
| Pauline Gourdie | |
2023 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Brian Kendrick | |
2023 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Anthony Zolezzi | |
2023 | | |
| 23,415 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 23,415 | |
| Amy Scannell | |
2023 | | |
| – | | |
| | | |
| – | | |
| – | | |
| – | | |
| – | |
| Vincent Monteparte | |
2023 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Anuradha Biswas | |
2023 | | |
| – | | |
| – | | |
| 90,345 | | |
| – | | |
| – | | |
| 90,345 | |
| Total | |
| | |
| 1,142,196 | | |
| 75,000 | | |
| 818,844 | | |
| – | | |
| – | | |
| 2,036,080 | |
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
TABLE
| |
| |
OPTION AWARDS | | |
| |
STOCK AWARDS | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
| | |
Equity | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
Equity | | |
Incentive | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
Incentive | | |
Plan | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
Plan | | |
Awards: | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
Awards: | | |
Market | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
Number | | |
or Payout | |
| |
| |
| | |
| | |
| | |
| |
| | |
| | |
of | | |
Value of | |
| |
| |
| | |
| | |
| | |
| |
| | |
Market | | |
Unearned | | |
Unearned | |
| |
| |
Number of | | |
Number of | | |
| | |
| |
Number | | |
Value of | | |
Shares, | | |
Shares, | |
| |
| |
Securities | | |
Securities | | |
| | |
| |
of Shares | | |
Shares or | | |
Units or | | |
Units or | |
| |
| |
Underlying | | |
Underlying | | |
| | |
| |
or Units | | |
Units | | |
Other | | |
Other | |
| |
| |
Unexercised | | |
Unexercised | | |
| | |
| |
of Stock | | |
of Stock | | |
Rights | | |
Rights | |
| |
| |
Options and | | |
Options and | | |
Option | | |
| |
That Have | | |
That Have | | |
That | | |
That | |
| |
| |
Warrants | | |
Warrants | | |
Exercise | | |
Option | |
Not | | |
Not | | |
Have Not | | |
Have Not | |
| Name and Principal |
| |
(#) | | |
(#) | | |
Price | | |
Expiration | |
Vested | | |
Vested | | |
Vested | | |
Vested | |
| Position(s) |
| |
(Exercisable) | | |
(Unexercisable) | | |
($) | | |
Date | |
(#) | | |
($) | | |
(#) | | |
($) | |
| Brian Bonar, CEO |
| |
500,000 | | |
– | | |
0.45 | | |
7/7/2030 | |
– | | |
– | | |
– | | |
– | |
| Brian Bonar, CEO |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
| Kyle McCollum, CFO |
| |
1,000,000 | | |
– | | |
0.47 | | |
5/10/2031 | |
– | | |
– | | |
– | | |
– | |
| Kyle McCollum, CFO |
| |
500,000 | | |
– | | |
0.45 | | |
7/7/2030 | |
– | | |
– | | |
– | | |
– | |
| Kyle McCollum, CFO |
| |
1,413,699 | | |
1,586,301 | | |
0.16 | | |
1/28/2033 | |
– | | |
– | | |
– | | |
– | |
| Pauline Gourdie |
| |
500,000 | | |
– | | |
0.45 | | |
7/7/2030 | |
– | | |
– | | |
– | | |
– | |
| Pauline Gourdie |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
| Brian Kendrick |
| |
500,000 | | |
– | | |
0.45 | | |
7/7/2030 | |
– | | |
– | | |
– | | |
– | |
| Brian Kendrick |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
| Anthony Zolezzi |
| |
500,000 | | |
– | | |
0.45 | | |
7/7/2030 | |
– | | |
– | | |
– | | |
– | |
| Anthony Zolezzi |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
| Amy Scannell |
| |
500,000 | | |
– | | |
0.45 | | |
2/14/2032 | |
– | | |
– | | |
– | | |
– | |
| Amy Scannell |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
| Vincent Monteparte |
| |
500,000 | | |
– | | |
0.45 | | |
2/14/2032 | |
– | | |
– | | |
– | | |
– | |
| Vincent Monteparte |
| |
515,068 | | |
484,932 | | |
0.10 | | |
6/19/2033 | |
– | | |
– | | |
– | | |
– | |
Director Compensation
On July 9, 2020, the Board authorized the Dalrada
Financial Corporation 2020 stock compensation plan to be used to compensate the company board of directors. The plan allocates the issuance
of up to 3,500,000 shares. On February 25, 2021, the Company amended the plan to issue up to 4,500,000 shares and issued an aggregate
of 4,500,000 common shares, or 500,000 shares to each board member (9). 3,500,000 shares of common stock were granted on July 9, 2020,
at $0.08 per share and 1,000,000 shares of common stock were granted on February 25, 2021, at $0.45 per share, for a total fair value
of $730,000, which is included in the Condensed Consolidated Statements of Operations and Comprehensive Loss.
On August 16, 2021, the Company approved Amendment
No.1 of the 2020 Stock Compensation Plan used to compensate the Company board of directors. The Amended plan allocates the issuance of
up to 6,500,000 shares which includes an additional 2,000,000 shares valued at $0.28 per shares or $560,000.
On September 30, 2021, the Board of Directors through
email correspondence, approved Amendment No. 3 of the 2020 Stock Compensation Plan used to compensate the Company board of directors.
The Board Directors agree to the following changes;
| |
1) |
Cancellation and return to treasury of all the commons shares issued under the previous 2020 Stock Compensation Plan and amendments totaling 6,500,000 shares of common stock. |
| |
|
|
| |
2) |
Ability to issue Cashless Warrants for up to 10,000,000 shares of the Company’s Common Stock, at $.005 par value per share (“Common Stock”) at 500,000 shares per board member, key employees, or consultants with an exercise price at $0.45 per common share. |
| |
|
|
| |
3) |
The vesting period for the Warrants shall be as follows: |
| |
a) |
Warrants issued to replace the 4,500,000 shares issued pursuant to the July 9, 2020, grant date will be fully vested; |
| |
|
|
| |
b) |
Warrants issued to replace the 2,000,000 shares issued pursuant to the July 19, 2021, grant date and any later grant dates will vest over a one-year period. |
On February 16, 2022, the Company issued 2,250,000
cashless warrants to new members of the Board of Directors. The cashless warrants vest over a 12-month period and hold an exercise price
of $0.45 per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was $0.59
per share, or $1,338,644, which was calculated using the Black-Scholes model.
On June 20, 2023, the Company issued 1,000,000 cashless
warrants to new members of the Board of Directors. The cashless warrants vest over a 24-month period and hold an exercise price of $0.10
per share. The cashless warrants expire in ten years after issuance. The fair value of the cashless warrants granted was $0.18 per share,
or $953,565 which was calculated using the Black-Scholes model.
Employment Agreements
On July 1, 2019, the Company entered into an employment
agreement with the Chief Executive Officer of the Company. Pursuant to the agreement, the Company will compensate the Chief Executive
Officer a base salary of $393,000 per annum, annual increases of 10% and a quarterly bonus based on whether the Company achieves a net
profit. He will be issued common stock of the Company sufficient to provide a 10% ownership position only upon a reverse split, which
shares are to be maintained for a period of two years. In addition to all other benefits and compensation, he shall be eligible for a
quarterly bonus of $47,000 based on if the Company achieves a net profit for that quarter. The Chief Executive Officers base salary has
been deferred and accrued by the Company.
Report on Repricing of Options
None.
Item 12. Security Ownership of Certain Beneficial Owners and Management
and Related Stockholder Matters
The following table provides certain information regarding
the ownership of our common stock, as of June 30, 2024, and as of the date of the filing of this Annual Report on Form 10-K by:
| |
· |
each of our executive officers; |
| |
|
|
| |
· |
each director; |
| |
|
|
| |
· |
each person known to us to own more than 5% of our outstanding common stock; and |
| |
|
|
| |
· |
all our executive officers and directors act as a group. |
As of January 8, 2025, we had a total of 120,157,113
shares of common stock issued and outstanding held by a total of 605 shareholders of record. Except as indicated in footnotes to this
table, the persons named in this table have sole voting and investment power with respect to all shares of common stock indicated below.
| Name and Address of Beneficial Owner | |
Title of Class | |
Amount and Nature of Beneficial Ownership (1) (#) | | |
Percent of Class (2) (%) | |
| Brian Bonar, Chief Executive Officer and Director3 | |
Common Shares | |
| 25,810,046 | | |
| 20.99 | |
| Kyle McCollum, Chief Financial Officer4 | |
Common Shares | |
| 3,445,205 | | |
| 3.42 | |
| Pauline Gourdie, Director5 | |
Common Shares | |
| 1,280,822 | | |
| 1.30 | |
| Brian Kendrick, Director5 | |
Common Shares | |
| 1,280,822 | | |
| 1.30 | |
| Anthony Zolezzi, Director5 | |
Common Shares | |
| 1,280,822 | | |
| 1.30 | |
| Amy Scannell, Director5 | |
Common Shares | |
| 1,280,822 | | |
| 1.30 | |
| Roger Campos, Director | |
Common Shares | |
| 0 | | |
| 0 | |
| All officers and Directors as a group (seven persons) | |
Common Shares | |
| 34,378,539 | | |
| 27.44 | |
| 1 | Unless otherwise noted, each person or group identified possesses sole
voting and investment power with respect to such shares. |
| 2 | Applicable percentage of ownership is based on 97,175443 shares of Common
Stock outstanding as of January 10, 2025, plus, for each stockholder, all shares that such stockholder could acquire within 60 days of
January 10, 2025, upon the exercise of existing warrants or conversion of existing convertible securities. |
| 3 | Includes 6,258,198 common shares; 18,271,026 common shares that can
be purchased within 60 days of January 10, 2025, upon the conversion of 4,537 Series I Preferred Shares, 247 Series H Preferred Shares,
and 1,529 Series I Preferred Shares; and 1,280,822 shares that can be purchased within 60 days of January 10, 2025, upon the exercise
of 1,280,822 warrants. |
| 4 | Includes 3,445,205 shares that can be purchased within 60 days of January
10, 2025, upon the exercise of 3,445,205 warrants. |
| 5 | Includes 1,280,222 shares that can be purchased within 60 days of January
10, 2025, upon the exercise of 1,280,222 warrants. |
The address for each of the persons listed in the chart is 600 La Terraza
Blvd., Escondido, California 92025.
Item 13. Certain Relationships, Related Transactions and Director Independence
Notes Payable – Related Parties
| | |
June 30, 2024 | |
| | |
Outstanding | | |
Accrued | |
| | |
Principal | | |
Interest | |
| Related entity 1 | |
$ | – | | |
$ | – | |
| Related entity 2 | |
| 52,887 | | |
| – | |
| Related entity 3 | |
| – | | |
| – | |
| Related entity 4 | |
| 1,070 | | |
| – | |
| Related entity 5 | |
| – | | |
| – | |
| Related entity 6 | |
| – | | |
| – | |
| | |
$ | 53,957 | | |
$ | – | |
| | |
June 30, 2023 | |
| | |
Outstanding | | |
Accrued | |
| | |
Principal | | |
Interest | |
| Related entity 1 | |
$ | 1,380,672 | | |
$ | 3,038 | |
| Related entity 2 | |
| 126,864 | | |
| – | |
| Related entity 3 | |
| 105,000 | | |
| – | |
| Related entity 4 | |
| 50,074 | | |
| – | |
| Related entity 5 | |
| – | | |
| – | |
| Related entity 6 | |
| 237,473 | | |
| 11,144 | |
| | |
$ | 1,900,083 | | |
$ | 14,182 | |
All notes dated December 31, 2022, and prior are unsecured,
bear interest at 3% per annum, and are due 360 days from the date of issuance, ranging from June 25, 2020, to December 30, 2022. All notes
dated after December 31, 2022, are unsecured, bear interest at 8% per annum, and are due 1095 days from the date of issuance.
Convertible Note Payable – Related Parties
On June 30, 2019, the Company issued a convertible
note for $1,875,000 to the Chief Executive Officer of the Company for compensation. Under the terms of the note, the amount due is unsecured,
bears interest at 3% per annum, and was due 360 days from the date of issuance. On June 30, 2019, the Company issued a note agreement
which included a conversion feature of the outstanding balance at $0.034 per share. As the conversion price was equal to the fair value
of the common shares on the date of the agreement, there was no beneficial conversion feature. As of June 30, 2024, the principal balance
was $0 and the accrued interest was $0.
Related Party Transactions
On July 1, 2019, the Company formalized an employment
agreement with its Chief Executive Officer, which entitles him to compensation of three hundred and ninety-three thousand dollars ($393,000)
per year. Annual increases will be up to 10% based on performance criteria to be determined at a later date. He will be issued common
stock of the Company sufficient to provide a 10% ownership position post reverse split, which shares be maintained for a period of two
years. In addition to all other benefits and compensation, he shall be eligible for a quarterly bonus of $47,000 based on if the Company
achieves a net profit for that quarter. As of June 30, 2024, and 2023, the Company had $0 and $0, accrued within accounts payable and
accrued liabilities – related parties, respectively.
The following is a summary of revenues recorded by
the Company’s to related parties with common ownership:
| | |
Year Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Dalrada Health | |
$ | – | | |
$ | 76,912 | |
| Dalrada Energy Services | |
| 5,207 | | |
| 45,968 | |
| Ignite | |
| 140 | | |
| 396 | |
| Prakat | |
| 15,000 | | |
| 25,000 | |
| Bothof Brothers | |
| 1,697,485 | | |
| 2,134,470 | |
| | |
$ | 1,717,832 | | |
$ | 2,282,746 | |
Director Independence
The OTC Markets Group Inc. does not have a requirement
that a majority of our Board of Directors be independent. However, with respect to the definition of independence utilized by NASDAQ,
our officers, other than Mr. Bonar, would be deemed to be independent.
Our Audit Committee is comprised of our officers and
directors. NASDAQ requires at least three members on the Audit Committee, each of whom must be independent. NASDAQ also requires that,
if the Chief Executive Officer’s compensation is determined by its Compensation Committee, the Compensation Committee must be comprised
solely of independent directors. The Company currently does not meet either of these requirements.
The NASDAQ rules have both objective tests and a subjective
test for determining who is an “independent director.” The objective tests state, for example, that a director is not considered
independent if he or she is an employee of the Company or is a partner, executive officer or controlling stockholder of an entity to which
the Company made, or from which the Company received, payments in the current or any of the past three fiscal years that exceed the greater
of $200,000 or 5% of the recipient’s consolidated gross revenue for that year or a family member serves in the current fiscal year
or has served at any time during the last three fiscal years as an executive officer of the Company. The subjective test states that an
independent director must be a person who lacks a relationship that, in the opinion of the Board, would interfere with the exercise of
independent judgment in carrying out the responsibilities of a director.
Item 14. Principal Accountant Fees and Services
The Company incurred $236,424 in audit feeds for the
year ended June 30, 2023. Fees for the year ended June 30, 2024 are to be provided upon issuance of the audited Form 10-K.
"Audit Fees" consisted of fees billed for
services rendered for the audit of the Company’s annual financial statements and “Audit-Related Fees are for review of the
financial statements included in the Company’s quarterly reports on Form 10-Q.
Item 15. Exhibits
The financial statement schedules are omitted because
they are inapplicable, or the requested information is shown in our financial statements or related notes thereto.
Exhibits
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange
Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
Dalrada Financial Corporation |
| |
|
| |
By: /s/ Brian Bonar |
| Date: January 13, 2025 |
Brian Bonar |
| |
Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange
Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates
indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Brian Bonar |
|
Principal Executive Officer and Director |
|
January 13, 2025 |
| Brian Bonar |
|
|
|
|
| |
|
|
|
|
| /s/ Kyle McCollum |
|
Principal Financial and Accounting Officer |
|
January 13, 2025 |
| Kyle McCollum |
|
|
|
|
| |
|
|
|
|
| /s/ Pauline Gourdie |
|
Director |
|
January 13, 2025 |
| Pauline Gourdie |
|
|
|
|
| |
|
|
|
|
| /s/ Brian Kendrick |
|
Director |
|
January 13, 2025 |
| Brian Kendrick |
|
|
|
|
| |
|
|
|
|
| /s/ Anthony Zolezzi |
|
Director |
|
January 13, 2025 |
| Anthony Zolezzi |
|
|
|
|
| |
|
|
|
|
| /s/ Amy Scannell |
|
Director |
|
January 13, 2025 |
| Amy Scannell |
|
|
|
|
| |
|
|
|
|
| /s/ Roger Campos |
|
Director |
|
January 13, 2025 |
| Roger Campos |
|
|
|
|
EXHIBIT 31.1
CERTIFICATION PURSUANT TO
18 USC, ss 1350, AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES OXLEY ACT OF 2002
I, Brian Bonar, certify that:
| |
1. |
I have reviewed this Annual Report on Form 10-K of Dalrada Financial Corporation; |
| |
|
|
| |
2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| |
|
|
| |
3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| |
|
|
| |
4. |
I am responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal controls over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| |
a. |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedure to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| |
|
|
| |
b. |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| |
|
|
| |
c. |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based upon such evaluation; and |
| |
|
|
| |
d. |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| |
5. |
I have disclosed, based on my most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| |
a. |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| |
|
|
| |
b. |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Dated: January 13, 2025 |
|
| |
|
| /s/ Brian Bonar |
|
Brian Bonar
President, Chief Financial Officer, and Director
(Principal Executive Officer, Principal Financial Officer
and Principal Accounting Officer) |
|
EXHIBIT 32.1
CERTIFICATION PURSUANT
TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY
ACT OF 2002
I, Brian Bonar, hereby certify,
pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| (1) |
the Annual Report on Form 10-K of Dalrada Financial Corporation for the year ended June 30, 2024 (the “Report”) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Dalrada Financial Corporation |
| Dated: January 13, 2025 |
|
|
| |
|
|
| |
|
|
| |
|
/s/ Brian Bonar |
| |
|
Brian Bonar |
| |
|
President, Chief Financial Officer, and Director |
| |
|
(Principal Executive Officer, Principal Financial Officer, and Principal Accounting Officer) |
Dalrada Financial (CE) (USOTC:DFCO)
Historical Stock Chart
From Feb 2025 to Mar 2025
Dalrada Financial (CE) (USOTC:DFCO)
Historical Stock Chart
From Mar 2024 to Mar 2025