UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1−K
ANNUAL REPORT PURSUANT TO REGULATION A
OF THE SECURITIES ACT OF 1933
For the fiscal year ended December 31, 2023
20/20 GeneSystems, Inc.
(Exact name of issuer as specified in its charter)
| Delaware |
|
57-2272107 |
| (State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
15810 Gaither Road, Suite 235, Gaithersburg,
MD 20877
(Full mailing address of principal executive offices)
(240) 453-6339
(Issuer’s telephone number, including area
code)
Series B Preferred Stock and Series C Preferred
Stock
Title of each class of securities issued pursuant
to Regulation A
TABLE OF CONTENTS
Use of Terms
Except as otherwise indicated by the context and
for the purposes of this report only, references in this report to “we,” “us,” “our” or “our
company” refer to 20/20 GeneSystems, Inc., a Delaware corporation.
Special Note Regarding Forward Looking Statements
This report contains forward-looking statements
that are based on our management’s beliefs and assumptions and on information currently available to us. All statements other than
statements of historical facts are forward-looking statements. The forward-looking statements are contained principally in, but not limited
to, Item 1 “Business” and Item 2 “Management’s Discussion and Analysis of Financial Condition and Results
of Operations.” These statements relate to future events or to our future financial performance and involve known and unknown
risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially
different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Forward-looking statements include, but are not limited to, statements about:
| ● | our goals and strategies; |
| ● | our future business development, financial condition and results of operations; |
| ● | expected changes in our revenue, costs or expenditures; |
| ● | growth of and competition trends in our industry; |
| ● | our expectations regarding demand for, and market acceptance of, our products; |
| ● | our expectations regarding our relationships with investors, institutional funding partners and other
parties we collaborate with; |
| ● | fluctuations in general economic and business conditions in the market in which we operate; and |
| ● | relevant government policies and regulations relating to our industry. |
In some cases, you can identify forward-looking
statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,”
“plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,”
“potential,” “project” or “continue” or the negative of these terms or other comparable terminology.
These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and
unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results.
Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the
heading Item 1 “Business─Risk Factors” and elsewhere in this report. If one or more of these risks or uncertainties
occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected
by the forward-looking statements. No forward-looking statement is a guarantee of future performance.
The forward-looking statements made in this report
relate only to events or information as of the date on which the statements are made in this report. We do not intend to update or otherwise
revise the forward-looking statements in this report, whether as a result of new information, future events or otherwise.
ITEM 1. BUSINESS
Overview
We are a commercial stage diagnostics company
with the core mission of developing and commercializing clinical laboratory tests for early disease detection and prevention and associated
software that is powered by machine learning and real-world data to improve diagnostic accuracy and clinical utility.
Our lead tests currently focus on early cancer
detection. Of the ten deadliest cancers in the U.S., only three—breast, colon, and prostate—have widely adopted screening
modalities. This is despite growing evidence that early detection saves or extends lives for cancers of the lung, liver, pancreas, esophagus,
and ovaries which are not yet the subject of widespread asymptomatic screening. To address this deficiency, we are offering what
we believe to be one of the first multi-cancer early detection, or MCED, blood tests to enter the American market. Known as OneTest, we
believe our test may be the first and only MCED test to enter the U.S. market based on the levels of tumor antigens rather than circulating
tumor DNA, or ctDNA. Tumor antigen measurement is a widely deployed technology (see “Carcinoembryonic Antigen, Carbohydrate Antigen
19-9, Cancer Antigen 125, Prostate-Specific Antigen and Other Cancer Markers: A Primer on Commonly Used Cancer Markers” World Journal
of Oncology (2023) 14(1):4-14; “Clinically Meaningful Use of Blood Tumor Markers in Oncology” (2016) BioMed Research International,
2016:9795269, doi:10.1155/2016/9795269). Throughout East Asia, these biomarkers are used for screening as part of yearly health checkups.
In the U.S. and other Western nations, tumor antigens are widely used to monitor therapy responses or disease recurrence in persons being
treated for cancer. Furthermore, each of the biomarkers detected in the OneTest panel uses an existing in vitro diagnostic test
platform that has been cleared or approved by the U.S. Food and Drug Administration, or the FDA, for at least one disease indication and
is automated, easy to use, and widely available. This proteomic approach permits significantly lower costs and easier access as compared
to DNA-based testing with little if any demonstrable loss in test accuracy, especially for early-stage detection of the major cancers
for which there is no widespread screening.
MCEDs gained significant attention in 2022 as
the White House included MCEDs as a core component of its “Cancer Moonshot” program and bi-partisan legislation has been introduced
in Congress to make it easier for these types of screening tests to achieve reimbursement by government payers (See H.R.2407 - Nancy Gardner
Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act reintroduced in the 118th Congress (2023-2024)). Additionally H.R.
5212 the “Firefighter Investments to Recognize Exposure to Cancer Act,” or the FIRE Act, was introduced in August 2023 to
allocate $700 million in grants to American firefighters to receive MCED tests. Several states, including Maryland and New Jersey, already
provide MCED funds for their firefighters (firefighters have proven higher incidents and death rates for several cancers and is a major
segment of our customer base).
This focus on MCEDs has been further bolstered
by the activities of high-profile companies offering or developing ctDNA based tests following technological advances in next-generation
DNA sequencing and machine learning techniques. While ctDNA-based tests are newer and are seeing growing use by scientists, clinicians,
and self-insured employers, they are significantly more expensive, are lacking in the level and number of analytical and clinical validation
studies to support them and generally have not performed any better than protein-based technologies in terms of sensitivities for early-stage
cancers in asymptomatic populations. Additionally, ctDNA tests require larger quantities of blood that require venipuncture whereas proteomic-based
MCEDs work well with capillary blood that can be easily collected in retail locations or at home without a phlebotomist.
As discussed below, we believe that there are
considerable advantages of our unique, patented technical approach to the development of MCED tests via the application of sophisticated
machine learning algorithms to analyze tumor antigen data collected from large cohorts of asymptomatic real-world populations. Our use
of this technical approach has been demonstrated to substantially improve the accuracy of using tumor antigen-based tests for screening
and risk assessment (see “Improving Multi-Tumor Biomarker Health Check-up Tests with Machine Learning Algorithms” Cancers
2020 Jun 1;12(6):1442). We have directly demonstrated this advantage in real-world population studies including 27,938 individuals
performed in collaboration with researchers in East Asia, where tumor antigens are currently used to test millions of individuals without
the added value of our AI-enhanced methods (see “Cancers Screening in an Asymptomatic Population by Using Multiple Tumour Markers.”
PLoS One. 2016;11(6) and “Improving Multi-Tumor Biomarker Health Check-up Tests with Machine Learning Algorithms” Cancers
2020 Jun 1;12(6):1442). These studies/publications indicate clear and significant improvements in area under the curve (AUC), sensitivity,
and specificity for overall cancers as well as individual cancers.
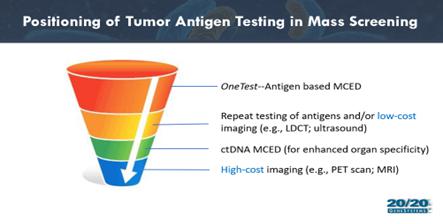
We have positioned OneTest as a “top of
funnel” first screening test rather than as a diagnostic test for cancer. Whereas a diagnostic test is typically used to make a
determination of the presence or absence of disease, a screening test is used to identify individuals at elevated risk for disease and
funnel them into further work-up, ultimately including definitive diagnostic tests. By way of example, suspicious results on a “top
of funnel” first screening test might be used to indicate a second screening test which could be a molecular (ctDNA sequencing)
or imaging modality which in turn might lead to biopsy as the definitive diagnostic. This approach very much differentiates OneTest from
competing tests, including other MCED tests whether based on ctDNA, protein biomarkers or other modalities. Most of these other screening
tests are placed further down in the funnel and lead directly to more expensive and more invasive definitive diagnostic tests. As such,
these competing modalities focus more on achieving the highest levels of specificity in order to reduce the number of false positive results
that could lead directly to an expensive and invasive test. Because OneTest is positioned at the “top of the funnel” meaning
that immediate follow-up tests are less expensive and generally not invasive (beyond a second blood draw), the performance characteristics
of OneTest are more focused on sensitivity, the detection of true positives, while accepting a lower specificity, as false positives will
be removed further down the funnel.
On March 22, 2024, we executed an option to
exclusively license certain intellectual properties developed and owned by The University of Texas M.D. Anderson Cancer Center, or
MD Anderson, and on April 26, 2024, we entered into a collaborative research agreement with MD Anderson, both of which will become
effective if we raise at least $23 million within six months (see also Item 2 “Management’s Discussion and Analysis
of Financial Condition and Results of Operations—Recent Developments”). The research relationship with and
intellectual properties from this institution are expected to help lead to improvements to our MCED, including new biomarkers,
algorithms, and evidence of clinical utility. Additionally, we believe it will help us bring to market a blood test
specifically for the early detection of lung cancer in smokers and former smokers. That test was developed by a team at MD
Anderson with over $60 million in funding from federal and state agencies as well as various philanthropies. Validated using
blood specimens from diverse, blinded cohorts comprising thousands of pre-symptomatic individuals, the lung cancer test analyzes
several of the same tumor antigens that are part of OneTest, along with a novel biomarker (ProSurfactant B) discovered by members of
that team. This test will also be used primarily as a “top of funnel” to screen individuals with a history of tobacco
use to improve both the compliance and effectiveness of low-dose CT, or LDCT, scans which are now part of U.S. screening
guidelines.
To increase our menu of innovative tests faster
and at a lower cost and risk than through internal development, in 2021 we established our Clinical Laboratory Innovation Accelerator,
or CLIAx, which permits diagnostics start-up companies from around the world to launch their laboratory developed tests in our CLIA (Clinical
Laboratory Improvement Amendments) licensed laboratory using shared equipment and laboratory personnel. To date, we have enrolled one
company in our CLIAx, Minomic International, or Minomic, and helped it validate and launch its blood test to help determine whether prostate
specific antigen, or PSA, levels should be followed up with a biopsy. Our CLIAx, which we believe to be the first such shared CLIA laboratory
facility in the U.S., reduces the costs and expense for start-up companies to launch their novel tests in the American market while providing
us with sales and marketing rights to additional products. In 2022, it earned an “Honorable Mention” in Fast Company
magazine’s list of “World Changing Ideas.”
In response to the novel coronavirus pandemic
that began in early 2020, we expanded our business and offered several COVID-19 testing solutions, both rapid kits and laboratory-based
tests. In the third quarter of 2020, in response to substantial and urgent demand for expanded viral testing in Maryland, we also began
to provide COVID-19 viral testing using polymerase chain reaction (PCR) analytical equipment in our clinical laboratory. This pandemic-associated
testing resulted in several years of profitability and forged business alliances that are being leveraged to support our core business.
However, following the expiration of the public health emergency in May 2023, all testing from both the State of Maryland and the Montgomery
County Health Department has ceased, and we do not anticipate additional COVID-19 testing absent a new variant resulting in a significant
increase in cases.
Our legacy business also includes a pioneering
field test kit for screening suspicious powders for bioterror agents known as BioCheck, which hundreds of first responder organizations
use regularly. Our BioCheck kits for screening suspicious powders remain profitable, but with limited growth potential.
Key Products
As of the date of this report, we sell three products:
(i) OneTest, rebranded to OneTest Standard, which was first introduced in 2019, (ii) OneTest Premium, which was launched in October 2023
(on a combined basis, we now process and report an average of 800+ OneTests per month), and (iii) BioCheck, which was first introduced
in 2001, for which we make and sell approximately 800 kits per month. Our other products are either in development or in a pre-commercial
mode.
For the years ended December 31, 2023 and 2022,
our COVID-19 testing business represented approximately 18% and 95% of our total revenues, respectively, with sales of OneTest accounting
for approximately 65% and 3% of our revenues, respectively, and sales of BioCheck accounting for approximately 13% and 1% of our revenues,
respectively. The remaining revenues were generated from our CLIAx, which accounted for approximately 4% and 1% of our revenues for the
years ended December 31, 2023 and 2022, respectively.
OneTest for Cancer—A Multi-Cancer Early Detection Blood
Test
The survival rate for the deadliest cancers is
closely linked to the stage at the time of diagnosis. With lung cancer, for example, some studies show a five-year survival rate approaching
90% for screen-detected Stage 1 cancers (see Henschke, et al. “Survival of patient with Stage 1 Lung Cancer Detected on CT Screening,”
N. Engl. J. Med. 355 (2006)). That survival plummets to under 5% for cancers first diagnosed in Stage 4. For these reasons in certain
regions of the world, especially East Asia, an aggressive cancer screening posture is commonplace. Tens of millions of individuals in
Japan, Korea, China, and Taiwan undertake 3-5 hour “health checks” each year that usually include blood tests for an array
of cancers. Typically, these blood tests measure the levels of between three to eight tumor antigens, which are proteins secreted by tumors
that can be detected using antibodies. Large-scale observational studies by our collaborators in Taiwan using data from cancer registries
demonstrate that these tests are useful for detecting even early-stage cancers (see Y.-H. We et al., “Cancer screening through a
multi-analyte serum biomarker panel during health check-up examinations: Results from a 12-year experience,” Clinica Chemica
Acta 450 (2015)). However, using our patented methodology, this screening approach can be rendered significantly more accurate using
machine learning algorithms that integrate the outcomes of tens of thousands of tested individuals together with clinical factors (e.g.,
age, gender, smoking history, etc.) with the biomarker levels (see “Improving Multi-Tumor Biomarker Health Check-up Tests with Machine
Learning Algorithms,” Cancers, 2020 Jun 1;12(6):1442).
OneTest is our MCED test and algorithm to screen
for multiple cancer types from a single blood sample. OneTest is powered by our patented machine-learning algorithms developed in the
manner described above. Studies by MD Anderson have found very little variability in the levels of these biomarkers across ethnicities
and geographies. The algorithm combines the levels of protein biomarkers such as carcinoembryonic antigen, or CEA, alpha-fetoprotein,
or AFP, PSA, and others, with patient information (e.g., age, gender, smoking history, etc.). We report the values of the biomarkers along
with a proprietary score indicating the likelihood of being diagnosed with cancer within a year of the test date (a sample lab report
is shown below).
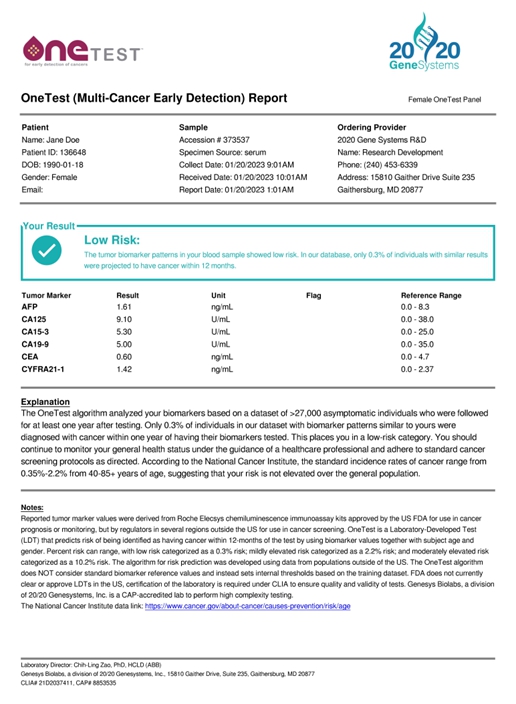
The goal is to encourage those with the highest
likelihood of having cancer to obtain follow-up imaging (ultrasound, CT, MRI, etc.) with the objective of finding early tumors that can
be surgically removed or otherwise successfully treated before becoming fatal. Among the cancers for which OneTest screens, accuracies
are strongest for those of the lung, liver, pancreas, and prostate (see “Improving Multi-Tumor Biomarker Health Check-up Tests with
Machine Learning Algorithms” Cancers 2020 Jun 1;12(6):1442). The foundation of this product is the measurement of a panel
to tumor antigens—CEA, cancer antigen 125, Cyfra, AFP, cancer antigen 19.9, cancer antigen 15.3, and PSA.
In Asia, several hundred million individuals receive
yearly blood tests for many of the tumor markers that are part of OneTest. These tests are typically private pay (i.e., not covered by
health insurance) averaging about $100 per test, depending on the number of biomarkers measured. Our list price is currently $189 with
discounts for volume and special offers. According to 2020 US Census Data, there are about 115 million Americans between the ages of 45-75,
the optimal ages for cancer screening. Thus, we estimate that OneTest addresses a market of over $15 billion annually in the U.S. alone
based on our current list price. We believe that for cancer screening to be impactful it must be affordable and accessible. Our technical
approach will help advance that goal.
In the U.S., our CLIA-licensed clinical laboratory
utilizes immunoassay detection kits and analyzers from Roche Diagnostics. For overseas customers, our algorithms have been optimized to
accommodate data from the following kits and analyzers: Roche Diagnostics, Abbott Diagnostics, Siemens Healthcare and Beckman Coulter.
The OneTest Machine Learning Algorithm—A Unique and Patented
Technical Approach
OneTest is built around the installed base of
existing FDA-approved tumor marker detection kits which run on automated instruments available from companies like Roche Diagnostics,
Abbott Diagnostics, Siemens Diagnostics, and others. In the U.S., approval for most of these kits, except PSA, is for monitoring of disease
recurrence, not screening. While we are using these approved kits in an off-label manner, this practice is permitted under the laboratory-developed
test CLIA framework. One advantage to using these kits is that the analytical performance of these kits has been fully vetted by regulatory
authorities ensuring the accuracy of individual marker value results. Furthermore, these tests and instruments are used in thousands of
clinical testing labs worldwide, thereby permitting us to obtain data from around the world. Throughout East Asia in particular millions
of individuals have their tumor antigen levels tested each year at physical examination or health checkup centers. In many cases these
tumor markers are tested using the same kits and instrumentation that we use in our CLIA laboratory. This has permitted us to develop
machine learning algorithms based on historical outcome data from cancer registries that would otherwise require long and expensive prospective
clinical trials if novel biomarkers are incorporated. One further advantage is that these markers are known and are meaningful to clinicians
and specifically to oncologists. While their use in an MCED test is novel and proprietary, the individual marker values are always listed
as a part of the OneTest standard report, and these values can help healthcare professionals to better guide follow-up testing and year-over-year
monitoring.
Evidence of our approach was first published in
a respected oncology journal in May 2020 co-authored by several of our scientists (“Improving Multi-Tumor Biomarker Health Check-up
Tests with Machine Learning Algorithms,” Cancers 2020, 12, 1442). Incorporation of changes to the levels of these
biomarkers over time (a/k/a/ biomarker “trends” or “velocity”) has also been shown in numerous studies to improve
diagnostic accuracy and usefulness. An updated machine learning algorithm that we developed was published in March 2022 in “Long
short-term memory model – A deep learning approach for medical data with irregularity in cancer predication with tumor markers”
Computers in Biology and Medicine 144 (2022) 105362. This research was also presented as a poster at the 2021 Annual Meeting of
the American Association for Clinical Chemistry where it won the First-Place award for industry submissions.
In July 2023, a report “A panel of seven
protein tumor markers for effective and affordable multi-cancer early detection by artificial intelligence: a large-scale and multicenter
case–control study,” appeared in The Lancet, eClinical Medicine, Vol. 61. This study uses essentially the same biomarker
panel (AFP, CA125, CA15-3, CA19-9, CA72-4, CEA and CYFRA 21-1) and algorithm approach (machine learning/AI) as our OneTest and provides
further validation and confirmation of our accuracy levels from independent cohorts from China and the U.S. The test developed from the
study has a reported sensitivity of 51.7% at a specificity of 92.9% with an overall accuracy of 84.3%. In comparison, OneTest at the moderate
cutoff yields a sensitivity of 22.8% at a specificity of 98.4% (accuracy 97.8%) and at the mild cutoff a sensitivity of 73.2% at a specificity
of 80.9% (accuracy 80.8%).
In short, our unique technical approach involves
the following three elements: (i) obtain “real-world” data from tens of thousands of apparently healthy individuals (i.e.
no apparent signs of symptoms of cancer when tested) who are screened for cancer using blood tests that are routine in certain parts of
the world (e.g. East Asia), (ii) use this data to build machine learning algorithms that improve the accuracy of those tests by integrating
cancer outcomes and clinical factors (age, gender, etc.), and (iii) introduce those tests and algorithms worldwide, even in parts of the
world where this testing approach is less common (e.g. North America), while examining variability across patient populations.
Artificial intelligence (AI) and machine learning
are expected to transform healthcare by helping physicians diagnose and treat patients with greater accuracy and precision. As we continue
to collect reliable outcome data (i.e., whether cancer was diagnosed) from individuals tested with the OneTest biomarkers (either from
our customers or from research collaborators), our ability to leverage the latest and most powerful forms of machine learning will increase.
On April 4, 2023, U.S. Patent No. 11,621,080 titled
“Methods and Machine Learning Systems for Predicting the Likelihood or Risk of Having Cancer” was issued to us. Additionally,
in January 2024 we received a Notice of Allowance from the U.S. Patent and Trademark office for a second patent covering OneTest. Similar
notices of patentability have also been received in early 2024 from patent offices in Japan and China. Our inventors were among the first
to apply machine learning and AI to prospective outcome data from thousands of persons tested with protein tumor markers to predict a
newly tested individual’s likelihood of having cancer. We expect to continue to build out a formidable patent estate in this arena.
We are aware of several large companies that have expressed interest in MCEDs with technical approaches covered by our patents which may
create opportunities for licensing revenues.
MCED Research, Development and Product Improvements
In October 2023, we introduced a “premium”
version of OneTest, at a higher price point, together with the “basic” version that we are now providing. To that end, in
August 2022, we executed a technology license and access agreement with Korean-based BioInfra Life Science, Inc., or BioInfra. BioInfra
commercializes an MCED in Korea primarily based on the levels of tumor antigens, such as CEA, CA-125, etc. However, their panel also includes
several inflammatory markers such as C-reactive protein, Transthyretin, Beta-2-Microglobulin, etc. that BioInfra has demonstrated to result
in improved accuracy. This data is reported in the peer-reviewed journal article “Diagnostic value of combining tumor and inflammatory
biomarkers in detecting common cancers in Korea,” Clinica Chimica Acta 516 (2021) 169–178.
BioInfra developed its I-Finder/OneTest Premium
using case-control cohorts. Resulting data from these training/validation cohorts are reported in the table below:
| Cancer | |
Stage | |
% Sensitivity @98% Specificity | | |
Cancer | |
Stage | |
% Sensitivity @98% Specificity | |
| Lung | |
Overall | |
| 51 | | |
Prostate | |
Overall | |
| 75.5 | |
| | |
Stage I | |
| 33.3 | | |
| |
Stage I | |
| 100 | |
| | |
Stage II | |
| 61.1 | | |
| |
Stage II | |
| 58.3 | |
| | |
Stage Ill | |
| 52.9 | | |
| |
Stage III | |
| 88.9 | |
| | |
Stage IV | |
| 90.5 | | |
| |
| |
| | |
| Liver | |
Overall | |
| 88.6 | | |
Ovary | |
Overall | |
| 73.7 | |
| | |
Stage I | |
| 85.7 | | |
| |
Stage I | |
| 25 | |
| | |
Stage II | |
| 90.9 | | |
| |
Stage II | |
| 100 | |
| | |
Stage Ill | |
| 100 | | |
| |
Stage III | |
| 100 | |
| | |
Stage IV | |
| 100 | | |
| |
Stage IV | |
| 80 | |
| Colorectal | |
Overall | |
| 72.1 | | |
Gastric | |
Overall | |
| 33.3 | |
| | |
Stage I | |
| 64.3 | | |
| |
Stage I | |
| 27.3 | |
| | |
Stage II | |
| 80 | | |
| |
Stage II | |
| 50 | |
| | |
Stage Ill | |
| 75.9 | | |
| |
Stage III | |
| 80 | |
| | |
Stage IV | |
| 100 | | |
Breast | |
Overall | |
| 18.8 | |
| Pancreas | |
Overall | |
| 92.7 | | |
| |
Stage I | |
| 15.4 | |
| | |
Stage I | |
| 85.7 | | |
| |
Stage II | |
| 15.4 | |
| | |
Stage II | |
| 95.7 | | |
| |
Stage III | |
| 57.1 | |
| | |
Stage Ill | |
| 100 | | |
| |
| |
| | |
| | |
Stage IV | |
| 85.7 | | |
| |
| |
| | |
In the first quarter of 2023, BioInfra conducted
a real-world analysis of their test performance based on data from Korean governmental cancer registries. This study is currently being
prepared for peer-reviewed publication. It looked at results of the BioInfra test as reported in the health records of individual clients
who purchased the test over several years (n=42,364) and correlated these results to health outcomes (cancer diagnoses) in the ensuing
12 months. The test performance was excellent compared to testing individual biomarkers alone, without our algorithms. BioInfra in their
peer-reviewed publication, “Diagnostic value of combining tumor and inflammatory biomarkers in detecting common cancers in Korea”
(2021) Clinica Chimica Acta, 516, 169-178, directly compared the AUC of the ROC curves for the MCED to that of single tumor markers
(CEA for colon cancer, Cyfra 21.1 or CEA for lung cancer, PSA for prostate cancer). Note that a higher AUC indicates better performance
and that the best possible AUC is 1.0.
| Cancer | |
MCED AUC | | |
Single Marker AUC | |
| Colon | |
| 0.9603 | | |
| 0.7183 | |
| Liver | |
| 0.9685 | | |
| 0.7943 | |
| Lung | |
| 0.9424 | | |
| 0.7609 | |
| Prostate | |
| 0.9848 | | |
| 0.9635 | |
Based on the data available to date, the premium
version is expected to have improved sensitivity and better organ specificity to help identify the tumor of origin. The following table
summarizes the data available to date.
| Cancer | |
Sensitivity | | |
Specificity | |
| Liver | |
| 47.1 | % | |
| 98.7 | % |
| Lung | |
| 45.5 | % | |
| 94.9 | % |
| Pancreatic | |
| 42.9 | % | |
| 99.2 | % |
| Prostate | |
| 42.2 | % | |
| 98.3 | % |
| Colorectal | |
| 34.0 | % | |
| 97.8 | % |
| Ovarian | |
| 29.7 | % | |
| 97.5 | % |
| Breast | |
| 20.2 | % | |
| 96.5 | % |
| Stomach | |
| 8.6 | % | |
| 98.4 | % |
Typically, data generated from a pre-diagnostic
cohort (i.e. specimens collected before a diagnosis) such as that shown above is less compelling data from newly diagnosed patients. It
should also be noted that reducing the specificity to around 85% would substantially boost the sensitivity in a manner that would avoid
missing many cancers while not a consequential number of false positives.
Under the terms of our agreement with BioInfra,
we have the exclusive right to commercialize BioInfra’s test panel and algorithm in the United States, having paid the requisite
up-front license fee of $300,000 and commenced bridging studies to validate those algorithms on a Western population. In addition, we
have agreed to pay per-test royalty fees in the range of $12-$25 per test for sales of our products using BioInfra’s technology.
Our agreement with BioInfra is for a term of three (3) years and may be extended for an additional three (3) years if certain minimum
royalties are met or if we conduct, or arrange for another party to conduct, a prospective clinical trial in the U.S. BioInfra may terminate
the agreement upon thirty (30) days written notice.
Another promising source for improvements to our
MCED may be our potential collaboration with MD Anderson discussed above. Of the $23 million that we plan to raise within six months,
$4 million would be allocated to collaborative research funding. The biomarkers, methodology, and intellectual property associated with
the lung cancer test panel that they developed and validated over ten years overlaps with that of our MCED, and the MD Anderson team has
access to one million blood specimens from individuals collected before any cancer diagnoses. If the agreement becomes effective upon
the payment of fees before the deadline, we believe these unique resources, coupled with the scientific and clinical acumen of MD Anderson’s
team, may yield several important and novel biomarkers and algorithms in the months and years to come that may function to improve the
performance of the biomarkers measured in our current OneTest.
In 2024, our scientific and laboratory personnel
successfully demonstrated the equivalency in the performance of OneTest using capillary blood with that of venous blood. The requirement
of engaging with a phlebotomist adds cost and burden to many of our consumers, especially those who purchase OneTest online. Since our
test requires only a fraction of the blood typically collected through venipuncture, we have shown that the test can function comparatively
with capillary blood collected from fingerstick or the upper arm. Fortunately, several new devices are entering the market to improve
capillary collection. Obviating the need for a phlebotomist should permit our test to be more easily accessed at pharmacy counters and
even at home thereby increasing uptake and adoption.
In terms of establishing clinical utility (i.e.,
demonstrating a mortality benefit), the U.S. National Cancer Institute, or NCI, is planning to sponsor randomized clinical trials of various
MCEDs as part of the White House “Cancer Moonshot” program. We have taken steps to have our test evaluated by NCI for possible
inclusion in those trials which will likely commence in 2025 and span at least seven years.
Blood Test for the Early Detection of Lung Cancer
Lung cancer is the third most common cancer and
the leading cause of cancer deaths among both men and women, according to the American Cancer Society. The intellectual properties developed
and owned by MD Anderson which we have an option to license include a lung cancer blood test developed by one of the world’s leading
experts in early cancer detection. The MD Anderson team, led by Sam Hanash, MD, Ph.D., has received over $60 million in funding from federal
and state governments as well as philanthropies in support of developing this test.
Validated using blood specimens from diverse,
blinded cohorts comprising thousands of pre-symptomatic individuals, the blood test analyzes several of the same tumor antigens that are
part of OneTest (CEA, CA-125, Cyfra) along with a novel biomarker (ProSurfactant B) discovered by members of that team. The main purpose
of the test is to screen individuals with a history of tobacco use for their increased lung cancer risk.
Large scale clinical trials have proven that screening
of those with a history of tobacco use using LDCT scans can reduce the death rate from lung cancer by 20% (see “Reduced lung-cancer
mortality with low-dose computed tomographic screening” N Engl J Med 2011;365:395-409). Unfortunately, despite heavy promotion
by the American Lung Association and others, according to the National Cancer Institute, fewer than 6% of Americans who meet the current
guidelines for yearly scans (based on smoking history) comply with these recommended screening guidelines and get a yearly scan, according
to the National Cancer Institute. The MD Anderson team believes that a blood test used to direct those with the highest risk to LDCT
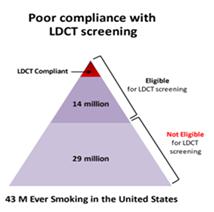
will substantially boost compliance and result in over 5,000 more lives saved per year over current screening paradigms. As illustrated
in the figure below left, of the 43 million Americans with a smoking history, only 14 million are eligible to get yearly LDCTs (based
on age and 20-pack year smoking history) but only about 6% of these individuals (about 840,000 people) actually do so. Under a new screening
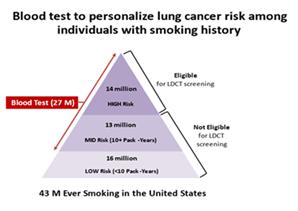
paradigm advocated by MD Anderson (right figure), the blood test would be provided to those with at least a 10-pack year smoking history
(27 million Americans) with compliance approaching 40% (the current compliance rate for PSA testing among American men). Those with a
positive blood test would be encouraged by their physician to follow-up with a CT scan, thereby saving more lives.
 |
|
 |
The lung cancer test will be positioned both before
and after LDCT screening. The pre-CT applications include screening of smokers and former smokers while post-CT the test will be used
to help resolve ambiguous pulmonary nodules. The later will likely require a distribution agreement with a channel partner that employs
a dedicated sales team calling on pulmonary medicine specialists as well as participation in trade shows such as the American Thoracic
Society annual meeting.
The far larger lung cancer screening market will
rely on many of the same sales and marketing strategies employed with OneTest, including large, self-insured employers and direct-to-consumer
advertising. Prior to that, we are targeting large, self-insured employers in occupations like transportation, construction and manufacturing
with large numbers of tobacco users in their workforce.
The MD Anderson developed test will expand the
pool of those eligible to receive LDCT from 20 pack year smokers (i.e., those who smoked an average of a pack a day for 20 years) to 10
pack-year smokers. Eventually it may be utilized by never-smokers for which incidents of lung cancer have been on the rise.
We estimate that the market for the lung cancer
test alone to be over $600 million by the year 2030. That projection is based on the following assumptions:
| ● | According to data from the U.S. Department of Health & Human Services, Substance Abuse and Mental
Health Services Administration, about 27 million Americans have smoked an average of one pack of cigarettes per day for 10 or more years
(before becoming smoke free for 15 years) and would benefit from the MD Anderson developed blood test on a yearly basis. |
| ● | Based on an estimated annual uptake of PSA blood tests of 40%, we estimate that 20% of those 27 million
eligible Americans will undertake yearly blood testing for lung cancer by 2030, or 5.4 million tests per year. |
| ● | At an average selling price of $170 per test per year, this creates an over $640 million annual revenue
opportunity over the next 7 years. |
Currently, no marketed tests are known to be addressing
this market in a meaningful way. Accordingly, we have an opportunity for a first-mover advantage.
Other Lab Tests for Early Disease Detection
We intend to introduce other lab tests to aid
in the early detection or prevention of chronic diseases such as cardiovascular and neurological disorders. Our clinical lab can now run
most of the routine tests (those ordered as part of a yearly check-up) and we are able to attract and acquire innovative tests through
our CLIAx facility. Our strategy is to upsell additional wellness and screening tests to our OneTest customers, since only 20% of the
quantity of blood we receive is required for the MCED tumor markers alone. The residual blood can be used to screen for other diseases.
Self-insured employers and occupational health
practices provide an especially attractive opportunity in this regard as they typically conduct thousands of blood draws per year. Since
the amount of blood collected and shipped to our lab is more than five times the amount needed to run OneTest, the residual blood is more
than ample to run dozens of other routine analytes such as lipid profiles, vitamins, glucose, metabolic panels, etc. Providing ancillary
testing to our MCED and lung cancer test customers saves them time and helps us improve the economics of our operations. In short, we
obtain more revenue for each dollar spent on marketing, selling, and shipping.
To make us an attractive choice for routine cardiovascular
testing, we have developed a machine-learning algorithm to predict the risk of cardiac arrest based on cholesterol values and other common
cardiac markers. This “OneTestforCardio” is built with data from over 50,000 patient records.
We are also laying the groundwork for “OneTest
for Longevity” that will measure biomarkers associated with healthy aging, especially markers of inflammation. Evidence suggests
that many of these biomarker levels fall within weeks of implementing health diet and exercise programs.
The aforementioned CLIAx is expected to serve
as a magnet for new test developers from around the world interested in launching their tests in the American market. This will help us
increase our test menu faster and with less expense than organic research and development.
COVID-19 Tests
In the third quarter of 2020, in response to a
substantial and urgent demand for expanded pandemic-related testing in Maryland, we began to provide COVID-19 viral testing using polymerase
chain reaction (PCR) analytical equipment in our clinical laboratory. Initially, most of our customers were nursing/assisted living facilities.
In the first quarter of 2021, we began receiving and testing specimens under contract with and collected by the Montgomery Department
of Health and Human Services. In August of 2021, we were one of five CLIA-certified laboratories to be awarded a contract with the Maryland
Department of Health to perform coronavirus screening at K-12 schools. Since then, we have collected and run PCR lab tests on over 138,000
specimens from over 80 public and private schools throughout the State of Maryland. However, following the expiration of the public health
emergency in May 2023, all testing from both the State of Maryland and the Montgomery County Health Department has ceased, and we do not
anticipate additional COVID-19 testing absent a new variant resulting in a significant increase in cases.
Profits from COVID-19 testing were deployed to
grow our core cancer diagnostics business. Additionally, some of the commercial partnerships we entered for COVID-19 testing are being
extended for non-pandemic-related testing.
Field Tests for Screening Suspicious Powders
We have a longstanding business that makes and
sells a proprietary test kit for screening suspicious powders called BioCheck. These kits are widely used by fire departments and other
emergency responders to quickly screen unknown suspicious powders for compounds such as ricin, anthrax, and other bioweapon agents and
to identify false alarms in minutes at the site of a suspected bioterror threat. The powder screening kit works by quickly identifying
the presence or absence of protein, a biomolecule found in all living materials. It therefore provides a rapid screen for the possible
presence of multiple bioterrorism agents while ruling out most of the ordinary substances that citizens have frequently feared to be possible
bio-agents of terror. Such ordinary substances include, for example, talc, ceiling tile dust, powdered sugar, etc., none of which are
expected to contain detectable levels of protein.
Lab Facility
We operate a high-complexity CLIA-licensed clinical
laboratory facility where our lab tests are performed at our Gaithersburg facility. This clinical lab became accredited by the College
of American Pathologists, or CAP, in 2022. Our CLIA lab is currently equipped with immunodiagnostic, clinic chemistry, and molecular (PCR)
analyzers, extractors, and liquid-handling robots. CAP and CLIA regulations establish standards for proficiency testing, facility administration,
general laboratory systems, preanalytic, analytic, and postanalytic systems, personnel qualifications and responsibilities, quality control,
quality assessment, and specific cytology provisions for labs performing moderate to high complexity tests. Our laboratory is inspected
biennially as part of its ongoing certification under the CLIA.
In connection with our lease agreement for a new,
larger facility in Gaithersburg Maryland, we have established what we believe to be the country’s first accelerator facility specifically
for diagnostics innovators worldwide seeking to launch novel diagnostic tests in a CLIA laboratory. Our CLIAx is expected to help drive
growth for us over the next few years. We signed up our first CLIAx tenant in August 2022, Minomic, which we helped to launch a novel
blood test and algorithm to help predict prostate cancer following an abnormal PSA test.
In July 2021, we entered a lab services and marketing
agreement with Minomic under which its testing technology and reagents were transferred to our CLIA lab, installed, and validated under
CLIA regulations. Under the agreement, Minomic maintains its ownership of all intellectual property. Minomic compensates us on a “cost
plus” basis (i.e., our fully burdened costs for labor, materials, space and testing analyzers plus a 10% profit). Furthermore, we
have the right, but not the obligation, to help market their test with a 25% commission. We have not yet opted to promote the Minomic
test since it does not target our typical consumer base. However, we believe this framework will be apt for other lab tests that address
the early detection, disease prevention and wellness market. The agreement with Minomic is for a term of three years and may be terminated
by either party upon 30 days’ written notice if there has been a material breach of the agreement that has not been cured with 60
days of notice of such breach. Either party may also terminate the agreement in the event of insolvency, bankruptcy, assignment for the
benefit of creditors of the other party or an admission of the party’s inability to pay its debts as they become due.
We plan to seek co-marketing rights to all tests
run out of our CLIAx. Our CLIAx received an Honorable Mention by Fast Company magazine as part of their 2022 “World Changing Ideas”
competition.
Supply Chain
For OneTest, we rely on a supply chain through
Roche Diagnostics IVD kits for Cobas E411, with all reagents used also available on other immunoassay platforms offered by major companies
such as Abbott, Beckman, Siemens, and ThermoFisher, except for one reagent, CYFRA. CYFRA is only available in the United States on our
current Roche equipment; however, we can also source this assay on a Luminex system.
In addition to our OneTest, we also rely on a
supply chain for general chemistry markers. Currently, these markers are run on Abbott Alinity C, but they are available through all major
manufacturers, including Roche.
We have established reagent contracts with Roche
and Abbott that guarantee pricing for all immunoassay and chemistry markers currently used in our diagnostic test panels. These contracts
ensure that we can continue to provide our customers with high-quality diagnostic tests at predictable pricing. Additionally, these contracts
provide us with supply chain stability and allow us to manage cost fluctuations associated with reagent pricing.
We depend on our suppliers and contract manufacturers
to provide us and our customers with materials in a timely manner that meets our and their quality, quantity, and cost requirements. We
have initiated a second source qualification process for most of these critical components, but we may not be successful in securing second
sourcing for all of them on a timely basis. Moreover, while we are confident that other suppliers could meet our quality, quantity and
cost requirements, the time required to transition to a new supplier could have negative impact on our ability to perform these tests
until an alternative supplier could be validated. Our supply chain for OneTest is critical to our ability to deliver high-quality diagnostic
tests to our customers.
Overall, we remain committed to building strong
relationships with our suppliers and contract manufacturers to ensure that our supply chain for all our diagnostic tests is reliable,
resilient, and able to meet the needs of our customers. We continuously monitor and improve our supply chain processes to minimize the
risk of disruptions and ensure that we can provide high-quality diagnostic tests to our customers when they need them.
Please see “—Risk Factors—Risks
Related to Our Business and Industry” for a description of the risks related to our supplier relationships.
Sales and Marketing Strategy
To date, our largest market segments for our MCED
are (i) self-insured employers, especially those whose workers are believed to have higher incidences of cancer than normal (e.g., firefighters),
(ii) medical providers specializing in wellness and disease prevention and (iii) consumers who purchase on-line. Based on our sales in
the first quarter of 2024, we estimate that the percentage of our sales from each of these three market segments for this year will be
approximately 42%, 4% and 54% respectively.
We believe that the most reliable near-term market
for our cancer tests in the U.S. is occupational health, and more specifically, organizations that employ or care for individuals with
perceived high risk for cancers. One such occupation is firefighters. Studies by several research groups, including the National Institutes
of Occupational Safety & Health, have proven that firefighters have increased incidence and mortality for several types of cancers,
including those of the digestive, respiratory, and urinary tracts. Importantly, for many of these high-incidence cancers (e.g. lung cancer
and mesothelioma), the biomarkers that we measure have been shown to be elevated in numerous published studies (see “Exposure–response
relationships for select cancer and non-cancer health outcomes in a cohort of US firefighters from San Francisco, Chicago and Philadelphia
(1950–2009)”, Occup Environ Med 2015;72:699–706.) Thus, OneTest Standard has become a popular tool for cancer
screening of current and former firefighters.
Penetration of this large occupational health
market will require significant business-to-business sales and marketing campaigns as well as consumer-initiated test campaigns that must
be coupled with convenient access to phlebotomy services and telemedicine practitioners to provide guidance on the test and its results.
Retail (walk-in) clinics such as urgent care centers and pharmacy chains present the best opportunities to grow the consumer-initiated
test market for OneTest Standard and OneTest Premium.
We currently have engagements in place with over
1,000 retail clinics located throughout the U.S., mostly urgent care centers, to conduct blood draws for OneTest products and include
over 200 locations of AnyLabTestNow. These clinics, coupled with a dedicated telemedicine service, have made it practical for us to initiate
a consumer-initiated test campaign. In the future we expect to offer capillary collection options at retail venues and at home.
The lung cancer test, in particular, presents
compelling opportunities outside of the U.S. as it includes an important biomarker (ProsurfactantB) previously unavailable. At this time,
we are exploring opportunities for the lung cancer test with our strategic shareholder Ping An, which currently provides diagnostic testing
services to over 200 million Chinese individuals through its Ping An Good Doctor program.
Competition
Because of the substantial unmet medical need
worldwide, many companies (and associated academic entities) are actively seeking to develop and commercialize tests of various types
to detect cancers early, when it can be treated most effectively. Current approaches include in-vivo radiographic imaging as well
as in-vitro tests using diverse bodily tissues and fluids including blood (serum or whole blood), urine, saliva, stool, sputum,
and exhaled breath.
In the U.S., we know of no MCED blood tests that
large numbers of Americans routinely utilize. Furthermore, there do not appear to currently be any companies in the U.S. that have adopted
our approach of testing a panel of tumor antigens together with a machine learning algorithm. However, there is significant and growing
competition in the MCED space with most tests using next-generation sequencing to analyze ctDNA. Most notably, Grail Inc., which was acquired
by Illumina for $8 billion in 2020, introduced its Galleri test in the second quarter of 2021 at a price of $949. Additionally, Thrive,
Inc. was acquired by Exact Sciences for $2 billion, but they have not publicly announced when they plan to launch their test CancerGuard
MCED. These tests may present both competitive threats but also opportunities for OneTest. The fact that our test measures well known
biomarkers creates several important competitive advantages. Our lower cost OneTest Standard with a list price of under $200 could be
followed up with more expensive ctDNA tests and/or imaging for those individuals with high biomarkers levels or a high algorithm score.
In East Asia, where such biomarker tests are commonly
offered as part of annual health check-ups, we are unaware of any widely used algorithms of the type we have developed, namely an algorithm
built with real-world data from a large screening population with known cancer outcomes. However, there are many emerging companies seeking
to use “liquid biopsy” and “next-gen sequencing” for pan-cancer testing. Furthermore, many companies are actively
utilizing AI and machine learning to improve health outcomes, and at least some of those companies are likely seeking to use these techniques
to improve cancer screening blood tests.
Competitive Strengths
We believe the following competitive strengths
should enable us to compete effectively in and capitalize on the growing demand for novel screening, prevention, and wellness testing,
especially in the fast-growing MCED market.
| ● | Our MCED test and anticipated lung cancer tests and algorithms are supported by data from pre-symptomatic
patient populations and therefore should translate well into real-world screening populations. The reported diagnostic
accuracy of our tests — typically quantified as a function of clinical sensitivity and specificity — are
generally comparable to those reported by our competitors in various publications (it is important to note that this comparison is on
the basis of reported data, and no head-to-head studies have been performed). However, most of our competitors derive their accuracy numbers
mainly from retrospective studies of blood specimens from newly diagnosed individuals (“case-control” studies). Accuracy reports
from retrospective studies tend to be artificially higher than what occurs when the test is administered for real-world screening purposes
when blood is collected before presentation of signs or symptoms of cancer. Most competing products were developed in a laboratory setting
involving blood samples from individuals after they were presented with symptoms of cancer when it has often advanced to a later stage.
We believe that the accuracies of tests developed using this “case/control” model may consistently fail to hold up in real-world
screening practice. A recent report, Jamshidi, et al., “Evaluation of cell-free DNA approaches for multi-cancer early detection”
(2022) Cancer Cell 40, 1537–1549, concluded that a whole genome methylation pattern approach yielded the highest sensitivities
for cancer when specificity was held at 98%. The overall sensitivity was reported as 34%. This was a case-control study and interestingly
when broken down by stage the sensitivities drop significantly to only 20% for stage II and 10% for stage I cancers. The corresponding
authors on this study were from Grail Inc., which was acquired by Illumina for $8 billion in 2020. In September of 2022, Grail reported
interim results of its Pathfinder real-world (prospective) clinical trial of its Galleri MCED test which is based on cfDNA methylation
patterns. In this interim analysis, they report a sensitivity of 29% at a 99% specificity. OneTest, which was developed in a real-world
cohort of over 27,000 asymptomatic individuals, achieves an overall sensitivity of 23.2% at 98% specificity; however, given that it is
positioned as a “top of funnel,” a significantly greater overall sensitivity (79%) can be achieved if the specificity is allowed
to drop to 80%. OneTest Premium achieved a sensitivity of 30.4% at 97.7% specificity in a Korean real-world study of over 42,000 subjects. |
| ● | Our tests are designed to be compatible with widely installed lab systems. Our tests are
designed to be compatible with standard instrument systems manufactured and distributed by companies such as Roche Diagnostics, Abbott
Diagnostics, and Siemens Healthcare. We believe that this dramatically lowers the barriers to adoption by hundreds of clinical diagnostics
laboratories worldwide. Furthermore, it helps to pave the way for new sources of “big data” from individuals tested worldwide
using standardized test kits and instruments. |
| ● | Our tests are far more affordable than DNA based liquid biopsies. We project that the average
selling price of the basic version of our MCED (blood test plus algorithm) at scale to range from $125 to $189 (with bulk discounts provided
to companies). In contrast, the list price for Grail’s Galleri test which is listed as $949. It simply costs far less to run tumor
antigen tests on automated platforms than next-generation sequencing. |
| ● | Our tests require small quantities of blood making them adaptable to capillary collection at pharmacies
and in homes. At the start of 2023, we demonstrated with a small pilot program that the biomarkers in our MCED can likely be analyzed
with capillary collected blood in a manner generally comparable to venous collection by a phlebotomist. Several novel devices have been
developed to improve the volume of capillary blood collected from the finger or upper arm while reducing the pain and anxiety associated
with traditional large needle blood draws. In April 2023, one such device, the TAP II manufactured by YourBio Health received a 510K clearance
from the FDA It is unlikely that ctDNA can be measured with those small quantities of blood. This gives us a competitive advantage by
permitting our tests to be offered at pharmacies where phlebotomists are generally unavailable. |
| ● | Our test reports include the absolute levels of the biomarkers and relative changes from prior reports.
Numerous reports in the scientific and medical literature conclude that tumor antigens such as CEA, CA-125, AFP, and PSA tend to rise
in the months before diagnosis of lung, pancreas, ovarian, liver, and prostate cancers respectively. Thus, an important feature of our
test report—which differs from that of our competitors with ctDNA based tests—is to include the levels of each biomarker tested
and the change from prior test reports. We have found this to be desired data for both individuals getting our tests and their physicians.
In contrast, Grail’s Galleri test report simply indicates whether the patient is positive or negative for various cancers. |
Potential Limitations of our Approach
As stated, there are compelling advantages to
protein-based screening instead of ctDNA sequencing. While this approach may yield greater sensitivity, especially for earlier stage cancers,
it will likely result in less specificity, as it is harder to localize high biomarker levels or risk scores to particular organs. Therefore,
we believe that OneTest and the lung cancer test we expect to introduce are best positioned as “top of funnel” first screens
that can be followed up with imaging tests and/or ctDNA based blood tests.
Growth Strategies and Path to Profitability
We will strive to increase shareholder value by
pursuing the following growth strategies:
| ● | Facilitate access to our tests at retail clinics, pharmacy counters, and at-home. COVID-19
testing caused a paradigm shift in the way Americans seek access to testing. Previously, most testing was done at doctor’s offices
and at specialty patient service centers maintained by the large national lab chains. During the pandemic, testing was conducted at retail
establishments and at home. OneTest Standard and OneTest Premium currently require a venipuncture blood collection. For those consumers
without easy access to a phlebotomist, we currently make available over 1,000 locations throughout the U.S. where they can have their
specimen collected. About 400 of these venues are urgent care facilities and the balance locations of the company Any Lab Test Now. We
also have a telemedicine provider available to authorize the test and be available to consult with the patient in the event of a high-risk
score. Going forward, we plan to validate both OneTest products using a novel capillary collection device so that we can substantially
expand the number of testing locations to venues that do not employ phlebotomists, especially to pharmacies nationwide. The OneTest capillary
panel, which utilizes capillary collection instead of venous draws, has already undergone rigorous analytic validation. Currently, we
are in the final stages of completing the necessary paperwork under CLIA/CAP regulations, and we expect to have everything finalized by
the end of June 2024. In summary, we are right on schedule to launch the OneTest capillary panel in the seven selected Giant Food stores
as planned. |
| ● | Strategic partnerships and cooperative advertising. To facilitate scale while mitigating
expenses, we have initiated an ambitious plan of marketing alliances and partnerships with an array of other companies, large and small,
including suppliers, other clinical labs, and organizations that offer wellness and screening tests. In many cases we seek to introduce
the cooperative advertising model where marketing expenses are shared pro-rata based on revenue allotments. |
| ● | Targeting of employers, especially in high-risk occupations. Certain professions, such as
firefighters, have proven higher incidence and mortality rates for multiple cancer types and are therefore actively looking for new, affordable
early detection solutions. We have found these communities to be accessible and early adopters for OneTest. |
| ● | Expanding our test menu. We are now offering our MCED consumers different versions of that
test (standard and premium). We soon expect to offer optional biomarker add-ons that address other routine disease conditions. The volume
of venous blood collected can easily facilitate multiple tests. We are finishing OneTest Standard capillary validation, and expect to
launch commercially soon. We are conducting trials for OneTest Premium markers, and within 1- 3 months, we plan to finish capillary validation
for OneTest Premium. Our tests and algorithms measure the levels of biomarkers that can be assayed using kits and instruments widely available
in thousands of clinical laboratories worldwide. The proprietary algorithms will be separate from the testing service so there is virtually
no limit on scalability, both in volume and geography. Because the specimens can be tested in a local lab, costly shipping can be avoided
so specimens do not need to be sent out using expensive overnight shipping services. In the future, we expect our tests to become available
at pharmacy chains and walk-in clinics that have on-site blood sample collection capabilities and trained healthcare practitioners to
educate consumers. To date, we have made our algorithms available over the cloud to a commercial partner in Taiwan. |
| ● | Consumer initiated testing. We have had considerable success to date with consumer initiated
testing by leveraging digital marketing platforms such as Facebook, Google, and LinkedIn. This approach has proven cost-effective, especially
when repeat (yearly) testing is factored in. In many cases, individuals refer us to their employers or medical providers which multiplies
the revenues derived from these advertisements. Based on our first quarter 2024 sales, we anticipate that approximately 33% of our sales
will come directly from consumers who purchase on-line (as opposed to the other two segments of self-insured employers and medical providers).
While consumers often initiate the test purchase process, in all cases we require a medical provider to order the test and be available
to consult the patient in the event of an abnormal test result. Usually, a telemedicine provider we have engaged provides these services. |
Facilities
On March 18, 2021, we entered into a lease agreement
for a new office and laboratory space totaling 5,511 square feet in Gaithersburg, Maryland. The term of the lease commenced on December
8, 2021 and expires 88 months thereafter. The initial monthly rent is $14,315 with annual increases to $17,308 for the final year of the
lease. We will also pay our 7.75% pro rata portion of the property taxes, operating expenses and insurance costs and are also responsible
for paying for the utilities used on the premises.
We believe that all our properties have been adequately
maintained, are generally in good condition, and are suitable and adequate for our business.
Intellectual Property
The following table summarizes our patent portfolio.
All of these patents and patent applications are owned by us.
| Description | |
Serial No./Patent No. | |
Jurisdiction | |
Projected Expiry |
| Algorithms and AI for the Early Detection of Lung and other Cancers |
| 1 | |
Algorithm for assessing the likelihood a patient has lung cancer | |
US 9,753,043; US 10,156,575; 11,733,249 | |
US and CA | |
2032 |
| 2 | |
Methods for aiding in distinguishing between benign and malignant pulmonary nodules | |
WO 2017/173428 | |
US and CN | |
2037 |
| 3 | |
Algorithm for assessing the likelihood a patient has cancer | |
US 11,621,080 | |
US and CN | |
2035-37 |
| 4 | |
Cancer Classifier Models | |
PCT/US19/40075 | |
US, CN and JP | |
2039 |
| 5 | |
Methods and algorithms for identifying a patient for follow-up cancer diagnostic testing | |
WO 2021/247577 | |
US | |
2041 |
| 6 | |
Pan cancer universal algorithm | |
WO 2022/015700 | |
US and CN | |
2041 |
| 7 | |
Use of multiple tumor markers in a machine learning model for cancer detection | |
US 2018/0173847 | |
US and TW | |
2036 |
| Other - Biocheck |
| 8 | |
Methods for processing dry powder for protein analysis and detection of bacterial spores | |
10,774,358 | |
US | |
2036 |
No assurance is made that any pending patent applications
within the portfolio will result in a granted patent.
To protect our intellectual property, we rely
on a combination of laws and regulations, as well as contractual restrictions. We rely on Federal patent laws to protect our intellectual
property, including our patented technology. We also rely on the protection of laws regarding unregistered copyrights for certain content
we create and trade secret laws to protect our proprietary technology and know-how. To further protect our intellectual property, we enter
into confidentiality agreements with our employees, executive officers and directors.
Employees
As of December 31, 2023, we had a total of 21
employees of which 12 were full-time and 9 were part-time.
We believe that we maintain a satisfactory working
relationship with our employees, and we have not experienced any significant labor disputes or any difficulty in recruiting staff for
our operations. None of our employees are represented by a labor union.
Legal Proceedings
From time to time, we may become involved in various
lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties
and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of
any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating
results.
Government Regulation
The healthcare industry, and thus our business,
is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws and regulations have not been definitively
interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In
addition, these laws and their interpretations are subject to change.
Both United States federal and state governmental
agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement
efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other
things, the marketing, labeling, promotion, manufacturing, and export of diagnostic healthcare products. The federal government also has
increased funding in recent years to fight healthcare fraud, and various agencies, such as the United States Department of Justice, the
Office of Inspector General of the Department of Health and Human Services, and state Medicaid fraud control units, are coordinating their
enforcement efforts.
FDA and CLIA
Based on widespread industry practice, we believe
that our products do not require pre-market approval from the FDA. In the U.S., our current products are Laboratory Developed Tests, or
LDTs, regulated under the CLIA and the Maryland Department of Health. If in the future we elect to license or distribute software as a
service those products would likely be deemed to be Clinical Decisions Support Software, or CDSS. As explained below, products in both
of those categories do not require FDA pre-market approval but could become subject to the FDA’s policy of “enforcement discretion.”
Laboratory Developed Tests. LDTs
are tests run in the laboratory of the company that developed them. With very rare exceptions, LDTs are not regulated by the FDA but rather
under a different regulatory regime called CLIA (Clinical Laboratory Improvement Amendments), state law and regulations, and organizations
such as CAP. Our laboratory is fully certified and compliant with CLIA as a “High Complexity Lab.” Furthermore, as of 2023
our lab has been accredited by CAP.
Under current law there is no requirement for
CLIA regulated LDTs to obtain approval or clearance from the FDA prior to being marketed (outside the context of tests used in response
to a declared pandemic emergency under which the FDA has been given special statutory authorities). In November 2016, the FDA issued a
formal statement clarifying that LDTs can be marketed without pre-market approval, but that the agency maintains “enforcement discretion”
to require their approval for those LDTs that are marketed in a way that is unsafe or could mislead or cause harm to patients. Since November
2016, such enforcement discretion has been exercised very rarely, and when it has been exercised, the tests were not ordered by independent
medical professionals. To reduce the likelihood that our tests will face enforcement discretion by the FDA, we request that our tests
be ordered by a physician who is independent of our company and that the physician aid the patient/consumer in interpreting the test results.
On September 29, 2023, the FDA issued a proposed
regulation under which they would begin to regulate LDTs starting in late 2027. The proposed rule, which will likely be finalized
in April 2024, is expected to be challenged in court and may also be overridden by legislation in Congress. However, if the rules
survive, they could significantly increase the cost and burden and affect our ability to market or improve existing LDTs and/or introduce
new lab tests.
A bill was introduced in the 117th
Congress which ended in December 2022 called “the VALID Act” that would for the first-time mandate FDA pre-market approval
of LDTs. That legislation generated significant opposition from stakeholders and failed to pass. In March 2023, the VALID Act bill was
re-introduced in the 118th Congress. The likelihood of passage of this bill cannot be predicted at this time. In the event
of passage, the VALID Act includes a “Grandfather clause” permitting tests on the market before passage of the law to remain
so without FDA approval.
CDSS. On December 13, 2016, the
21st Century Cures Act was signed into law. Among the many provisions of the Cures Act was the exclusion of certain medical decision support
software from the FDA’s jurisdiction. On December 8, 2017, the FDA issued its first set of Draft Guidance to implement those provisions
of the Cures Act relating to CDSS. Based on our reading of this Draft Guidance, we believe that there may be aspects of our current or
planned OneTest software package that would be exempt from pre-market approval. If we elect to proceed with an independent software product
in the U.S. (as we will likely do overseas), outside laboratories could run the OneTest biomarker panels (all of the detection instruments
and kits are FDA approved).
Operating under the assumption that seeking FDA
approval for our products is optional, but that approval could improve the adoption rates and permit greater scale, we may seek FDA approval
when test volume exceeds the capacity of our CLIA laboratory. In so doing, we will present to the FDA real-world evidence, data from tens
of thousands of individuals tested with our products in the U.S. and overseas. On August 31, 2017, the FDA issued Guidance on the “Use
of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices.” The Guidance provides that “in some cases,
a ‘traditional’ clinical trial may be impractical or excessively challenging to conduct” and that use of real-world
data “may in some cases provide similar information with comparable or even superior characteristics to information collected and
analyzed through a traditional clinical trial.”
Federal and State Fraud and Abuse Laws
We are subject to federal fraud and abuse laws
such as the federal Anti-Kickback Statute, or AKS, the federal prohibition against physician self-referral, commonly known as the Stark
Law, the Eliminating Kickbacks in Recovery Act, or EKRA, and the federal False Claims Act, or the FCA. We are also subject to similar
state and foreign fraud and abuse laws.
The AKS prohibits knowingly and willfully offering,
paying, soliciting, or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in return for or to induce
such person to refer an individual, or to purchase, lease, order, arrange for, or recommend purchasing, leasing or ordering, any item
or service that may be reimbursable, in whole or in part, under a federal healthcare program, such as Medicare or Medicaid. There are
a number of statutory exceptions and regulatory safe harbors to the AKS that provide protection from AKS liability to arrangements that
fully satisfy the applicable requirements.
EKRA prohibits knowingly and willfully soliciting,
receiving, offering or paying remuneration, directly or indirectly, in return for the referral of a patient to, or in exchange for an
individual using the services of certain entities, including laboratories, if the services are covered by a health care benefit program.
The term “health care benefit program” is broadly defined such that EKRA extends to referrals reimbursed by both governmental
and commercial third-party payers. EKRA includes a number of statutory exceptions that provide protection from EKRA liability if the applicable
requirements are met.
The Stark Law generally prohibits, among other
things, clinical laboratories and other so-called “designated health services” entities from billing Medicare for any designated
health services when the physician ordering the service, or any member of such physician’s immediate family, has a financial relationship,
such as a direct or indirect investment interest in or compensation arrangement with the billing entity, unless the arrangement meets
an exception to the prohibition. The Stark Law also prohibits physicians from making such referrals to a designated health services entity.
There are also similar state laws that apply where Medicaid and/or commercial payers are billed.
The FCA imposes penalties against individuals
or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment to the government that are false
or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent
claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal
government. This statute also permits a private individual acting as a “qui tam” whistleblower to bring actions on behalf
of the federal government alleging violations of the FCA and to share in any monetary recovery. FCA liability is potentially significant
in the healthcare industry because the statute provides for treble damages and mandatory penalties of $13,508 to $27,018 per false claim
or statement for penalties assessed after January 30, 2023, with respect to violations occurring after November 2, 2015.
Other federal statutes pertaining to healthcare
fraud and abuse include the civil monetary penalties statute, which prohibits, among other things, the offer or payment of remuneration
to a Medicaid or Medicare beneficiary that the offeror or payer knows or should know is likely to influence the beneficiary to order or
receive a reimbursable item or service from a particular provider, practitioner, or supplier, and contracting with an individual or entity
that the person knows or should know is excluded from participation in a federal health care program. In addition, federal criminal statutes
created by the Health Insurance Portability and Accountability Act of 1996, or HIPAA, prohibit, among other things, knowingly and willfully
executing or attempting to execute a scheme to defraud any healthcare benefit program or obtain by means of false or fraudulent pretenses,
representations or promises any money or property owned by or under the control of any healthcare benefit program in connection with the
delivery of or payment for healthcare benefits, items or services.
In addition to these federal laws, there are often
similar state anti-kickback and false claims laws that typically apply to arrangements involving reimbursement by a state-funded Medicaid
or other health care program. Often, these laws closely follow the language of their federal law counterparts, although they do not always
have the same exceptions or safe harbors. In some states, these anti-kickback laws apply with respect to all payers, including commercial
payers.
A number of states have enacted laws that require
pharmaceutical and medical device companies to monitor and report payments, gifts and other remuneration made to physicians and other
healthcare providers, and, in some states, marketing expenditures. In addition, some state statutes impose outright bans on certain manufacturer
gifts to physicians or other health care professionals. Some of these laws, referred to as “aggregate spend” or “gift”
laws, carry substantial fines if they are violated.
Efforts to ensure that our business arrangements
with third parties will comply with applicable healthcare laws and regulations will involve substantial costs and extensive annual trainings
for all of our employees and contractors. If our operations are found to be in violation of any of these laws or any other governmental
regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment,
exclusion from participation in government-funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages,
reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate
integrity agreement or other agreement to resolve allegations of non-compliance with the law, and the curtailment or restructuring of
our operations, any of which could adversely affect our ability to operate our business and our results of operations. If any of the physicians
or other healthcare providers or entities with whom we do business is found to be not in compliance with applicable laws, they may be
subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
Anti-Corruption
The Foreign Corrupt Practices Act of 1977, or
the FCPA, and similar international bribery laws make it unlawful for persons or entities to make payments to foreign government officials
to assist in obtaining and maintaining business. Specifically, the anti-bribery provisions of the FCPA prohibit any offer, payment, promise
to pay, or authorizing the payment of money or anything of value to any person, while knowing that all or a portion of such money or thing
of value will be offered, given or promised, directly or indirectly, to a foreign official to do or omit to do an act in violation of
his or her duty, or to secure any improper advantage in order to assist in obtaining or retaining business for or with, or directing business,
to any person. In addition to the anti-bribery provisions of the FCPA, the statute also contains accounting requirements designed to operate
in tandem with the anti-bribery provisions. Covered companies are required to make and keep books and records that accurately and fairly
reflect the transactions of the company and devise and maintain an adequate system of internal accounting controls. With our international
operations through our third-party partnerships, we could incur significant fines and penalties, as well as criminal liability, if we
fail to comply with either the anti-bribery or accounting requirements of the FCPA, or similar international bribery laws. Even an unsuccessful
challenge of our compliance with these laws could cause us to incur adverse publicity and significant legal and related costs.
Privacy and Data Protection Laws
Numerous federal and state laws and regulations,
including HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, govern the collection,
dissemination, security, use and confidentiality of protected health information, or PHI, and personal information. In the course of performing
our business we obtain personal information, including PHI. Laws and regulations relating to privacy, data protection, and consumer protection
are evolving and, in some cases, particularly with regard to newer laws, may be subject to potentially differing interpretations. Under
HIPAA and HITECH, the Department of Health & Human Services, or the HHS, issues regulations
that establish uniform standards governing the conduct of certain electronic healthcare transactions and requirements for protecting the
privacy and security of PHI, used or disclosed by covered entities, or CEs, and their authorized business associates, or BAs. Because
we electronically transmit health care information, and we also provide certain services to CEs and receive PHI from them, we are at times
either a CE or a BA, as defined by HIPAA. Our subcontractors that create, receive, maintain, transmit or otherwise process PHI on our
behalf are HIPAA BAs and must also comply with HIPAA, as applicable.
HIPAA and HITECH include the privacy and security
rules, breach notification requirements and electronic transaction standards. The privacy rule governs the use and disclosure of PHI,
generally prohibits the use or disclosure of PHI except as permitted under the rule, and mandates certain safeguards to protect the privacy
of PHI. The privacy rule also sets forth individual rights, such as the right to access or amend certain records containing such individual’s
PHI, or to request restrictions on the use or disclosure of such individual’s PHI. The security rule requires CEs and BAs to safeguard
the confidentiality, integrity, and availability of electronically transmitted or stored PHI (also referred to as ePHI) by implementing
administrative, physical and technical safeguards. Under HIPAA’s breach notification rule, a CE must notify individuals, the Secretary
of HHS, and in some circumstances, the media of certain breaches of unsecured PHI or ePHI, and similar breach notification provisions
apply to certain BAs under HITECH.
Penalties for failure to comply with a requirement
of HIPAA and HITECH vary depending on the number and nature of the violations and any history of prior violations, but can be significant
and include civil monetary or criminal penalties. HIPAA is enforced by the HHS, Office for Civil Rights, and HIPAA also authorizes state
attorneys general to file suit on behalf of their residents for violations. Courts are able to award damages, costs and attorneys’
fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to file
suit in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such
as those for negligence or recklessness in improper use, access to or disclosure of PHI. In addition, HIPAA mandates that the Secretary
of HHS conduct periodic compliance audits of HIPAA CEs, such as us, and their BAs for compliance with the HIPAA privacy and security standards
and breach notification rules. It also tasks HHS with establishing a methodology whereby harmed individuals who were the victims of breaches
of unsecured PHI may receive a percentage of the civil monetary penalty paid by the violator.
In addition, we may be subject to state privacy,
cybersecurity, and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and
other personal information. California, for example, has enacted the Confidentiality of Medical Information Act, which, in addition to
HIPAA and HITECH, sets forth standards with which all California health care providers must abide. Colorado has enacted the Colorado Privacy
Act, and Virginia has enacted the Consumer Data Protection Act, both of which also have standards that must be complied with that supplement
Federal data protection requirements. State laws may be more stringent, broader in scope or offer greater individual rights with respect
to PHI than HIPAA, and state laws may differ from each other in regards to personal information treatment, which may complicate compliance
efforts. For instance, the California Consumer Privacy Act, or CCPA, became effective on January 1, 2020 and was amended by the passage
of the California Privacy Rights Act, or CPRA, in November of 2020, which amendments came into force on January 1, 2023. The CCPA, among
other things, gives California residents expanded rights to access and delete their personal information, opt out of certain personal
information sharing and receive detailed information about how their personal information is used by requiring covered companies to provide
new disclosures to California consumers (as that term is broadly defined) and provide such consumers new ways to opt-out of certain sales
of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches
that is expected to increase data breach litigation. The CCPA has been amended from time to time, and it remains unclear what, if any,
further modifications will be made to this legislation or how it will be interpreted. Although there are certain exemptions for PHI and
clinical trial data, the CCPA’s implementation standards and enforcement practices are likely to remain uncertain for the foreseeable
future and the CCPA may increase our compliance costs and potential liability. Additionally, the CPRA imposes additional data protection
obligations on companies doing business in California, including additional consumer rights processes and opt outs for certain uses of
sensitive data. It also creates a new California data protection agency – the California Privacy Protection Agency – specifically
tasked to enforce the law, which would likely result in increased regulatory scrutiny of California businesses in the areas of data protection
and security. Similar laws have been proposed in other states and at the federal level, and if passed, such laws may have potentially
conflicting requirements that could continue to make compliance challenging and costly.
Additionally, the FTC and state attorneys general
enforce consumer protection laws that prohibit unfair and deceptive acts and practices, including Section 5 of the FTC Act, which creates
standards for the collection, use, dissemination and security of health-related and other personal information. Claims of unfair or deceptive
trade practices regarding privacy and security can lead to significant liabilities and consequences, including regulatory investigations,
penalties, fines and orders as well as civil claims, which could impact our data practices and operations or cause reputational damage.
We may also be subject to laws and regulations
in foreign countries covering data privacy and other protection of health and employee information that may add additional compliance
burden and complexity. For example, in the European Economic Area, the collection and use
of personal data is governed by the European Union’s General Data Protection Regulation, or the GDPR. In the United Kingdom, the
GDPR has been adopted in substantially the same form, however the UK may potentially make revisions in the coming years. The GDPR, together
with national legislation, regulations and guidelines of the European Union member states and the United Kingdom governing the processing
of personal data, impose strict obligations and restrictions on the ability to collect, analyze, store, transfer and otherwise process
personal data. European and United Kingdom data protection authorities may interpret the GDPR and national laws differently and impose
additional requirements, which adds to the complexity of processing personal data in or from the European
Economic Area or United Kingdom. Guidance on implementation and compliance practices is often updated or otherwise revised. The
GDPR applies extra-territorially under certain circumstances and imposes stringent requirements on controllers and processors of personal
data, including, for example, requirements to ensure a legal bases to process personal information, provide robust disclosures to individuals,
facilitate data subject rights, provide data security breach notifications within 72 hours after discovering a breach in certain circumstances,
limit retention of personal information and apply enhanced protections to health data and other categories of sensitive personal information.
The GDPR also has requirements around international transfers of personal data. Requirements around transfers to the United States and
other jurisdictions have increased since a July 2020 decision by the Court of Justice of the European Union invalidated the Privacy Shield
as a basis to transfer personal data from Europe to the United States, and added requirements for reliance on Standard Contractual Clauses.
Regulatory guidance on requirements for international transfers, and other GDPR compliance matters, continues to evolve. For example,
the European Commission in December 2022 announced that it was beginning the process of drafting a new adequacy decision that would ease
regulatory barriers for data transfers to the United States. However, it is widely expected that the new adequacy decision will itself
face scrutiny from the Court of Justice, underscoring that GDPR compliance is an ongoing endeavor. Failure to comply with the requirements
of the GDPR may result in fines of up to €20 million or up to 4% of the total worldwide annual turnover of our preceding fiscal year,
whichever is higher, and other administrative penalties. To comply with the GDPR and other applicable international data protection laws
and regulations, we may be required to put in place additional mechanisms ensuring compliance, which may result in other substantial expenditures.
Cybersecurity
Our business relies on secure and continuous processing
of information and the availability of our IT networks and IT resources, as well as critical IT vendors that support our technology, research
and other data processing operations. While we take steps to protect our systems and data, security incidents, data breaches, computer
malware and computer hacking attacks have become more prevalent across industries, including the life sciences sector, and may occur on
our systems or those of our third-party service providers. Unauthorized persons may in the future be able to exploit weaknesses in the
security systems of our (or our third-party service providers) IT networks and gain access to PHI and other personal information, sensitive
trade secrets, or other proprietary information. Any wrongful use or disclosure of PHI, other personal information, trade secrets or other
proprietary information by us or our third-party service providers could subject us to regulatory fines or penalties, third-party claims
or otherwise could adversely affect our business and results of operations. Although HIPAA and the regulations promulgated thereunder
do not provide for a private right of action, failures to adequately protect PHI or our IT systems could be viewed as violations of the
HIPAA security rule or violations of other applicable information security laws, regulations, contractual obligations or industry standards,
and could further result in costly data breach notification obligations that negatively impact our reputation.
Moreover, data security incidents or data breaches,
as well as attacks on our IT systems, could result in operational disruptions or data loss or corruption that could adversely impact our
business and operations, resulting in substantial investment of resources to investigate, recover and remediate and subject us to heightened
regulatory scrutiny.
International Regulations
Many countries in which we may offer any of our
diagnostic tests in the future have anti-kickback regulations prohibiting providers from offering, paying, soliciting or receiving remuneration,
directly or indirectly, in order to induce business that is reimbursable under any national health care program. In situations involving
physicians employed by state-funded institutions or national healthcare agencies, violation of the local anti-kickback law may also constitute
a violation of the FCPA.
The FCPA prohibits any United States individual,
business entity or employee of a United States business entity to offer or provide, directly or through a third party, including any potential
distributors we may rely on in certain markets, anything of value to a foreign government official with corrupt intent to influence an
award or continuation of business or to gain an unfair advantage, whether or not such conduct violates local laws. In addition, it is
illegal for a company that reports to the U.S. Securities and Exchange Commission, or the SEC, to have false or inaccurate books or records
or to fail to maintain a system of internal accounting controls. We will also be required to maintain accurate information and control
over sales and distributors’ activities that may fall within the purview of the FCPA, its books and records provisions and its anti-bribery
provisions.
The standard of intent and knowledge in anti-bribery
cases is minimal. Intent and knowledge are usually inferred from that fact that bribery took place. The accounting provisions do not require
intent. Violations of the FCPA’s anti-bribery provisions for corporations and other business entities are subject to a fine of up
to $2 million and officers, directors, stockholders, employees, and agents are subject to a fine of up to $100,000 and imprisonment for
up to five years. Other countries, including the United Kingdom and other OECD Anti-Bribery Convention members, have similar anti-corruption
regulations, such as the United Kingdom Anti-Bribery Act.
When marketing our diagnostic tests outside of
the United States, we may be subject to foreign regulatory requirements governing human clinical testing, prohibitions on the import of
tissue necessary for us to perform our diagnostic tests or restrictions on the export of tissue imposed by countries outside of the United
States or the import of tissue into the United States, and marketing approval. These requirements vary by jurisdiction, differ from those
in the United States and may in some cases require us to perform additional pre-clinical or clinical testing. In many countries outside
of the United States, coverage, pricing and reimbursement approvals are also required.
Market access, sales and marketing of medical
devices in non-U.S. countries are subject to foreign regulatory requirements that vary widely from country to country. For example, in
the European Economic Area, a medical device must meet the Medical Devices Directive’s/In
Vitro Medical Devices Directive’s, or MDD/IVDD, Essential Requirements or, applicable on May 26, 2021, the Medical Devices Regulation’s,
or MDR, or applicable on May 26, 2022, In Vitro Medical Devices Regulation’s, or IVDR, General Safety and Performance Requirements
which apply to it, taking into account its intended purpose as defined by the data supplied by the manufacturer on the label, in the instructions
for use or in promotional or sales materials or statements and as specified by the manufacturer in the clinical evaluation. Before placing
a medical device on the European Economic Area market, the manufacturer must draw up a declaration
of conformity, certifying that the device complies with the MDD/IVDD/MDR/IVDR, and must then affix the CE mark. For medium and high-risk
devices as well as low risk devices that are placed on the market in sterile condition, have a measuring function, or are reusable surgical
instruments, the manufacturer must obtain a CE certificate from a notified body. The notified body typically audits and examines the device’s
technical documentation, including the clinical evaluation, and the quality system for the manufacture, design and final inspection of
the relevant device before issuing a CE certificate. Following the issuance of this CE certificate, manufacturers may draw up the declaration
of conformity and affix the CE mark to the devices covered by this CE certificate.
Manufacturers of medical devices must document
in a clinical evaluation report, or CER, the evaluation of the clinical data related to the
device. The CER is part of the device’s technical file. The evaluation shall document that the applicable Essential Requirements/General
Safety and Performance Requirements are met and document the evaluation of the undesirable side-effects and the acceptability of the benefit-risk
ratio. The CER must be updated based on information from the post-market surveillance and vigilance activities related to the device.
The CER shall consist, inter alia, of analyzed clinical data collected from a clinical investigation of the device, or the results
of other studies on substantially equivalent devices. Reliance on “substantially equivalent” devices is very restrictive and
requires, inter alia, that the manufacturer has full access to the technical documentation of the equivalent device on an ongoing
basis and, if the “equivalent device” is not its own, that the manufacture has in place a contract with the manufacturer of
the “equivalent device.”
Environmental, Health and Safety Regulations
We are subject to various federal, state, local,
and foreign environmental, health and safety laws and regulations and permitting and licensing requirements. Such laws include those governing
laboratory practices, the generation, storage, use, manufacture, handling, transportation, treatment, remediation, release and disposal
of, and exposure to, hazardous materials and wastes and worker health and safety. Our operations involve the generation, use, storage
and disposal of hazardous materials, and the risk of injury, contamination or non-compliance with environmental, health and safety laws
and regulations or permitting or licensing requirements cannot be eliminated. Compliance with environmental laws and regulations has not
had a material effect on our capital expenditures, earning or competitive position.
Corporate History
We were incorporated in the State of Delaware
on August 7, 2000 under the name 20/20 BioSystems, Inc. On September 19, 2000, our name was changed to 20/20 Gene Systems, Inc. and on
June 27, 2021, our name was changed to 20/20 GeneSystems, Inc. We do not have any subsidiaries.
Risk Factors
Investing in our securities involves a significant
degree of risk. In evaluating our company and an investment in our securities, careful consideration should be given to the following
risk factors, in addition to the other information included in this report. Each of these risk factors could materially adversely affect
our business, operating results, or financial condition, as well as adversely affect the value of an investment in our securities. The
following is a summary of the most significant factors. We are still subject to all the same risks that all companies in our industry,
and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and
technological developments (such as cyber-security). Additionally, early-stage companies are inherently riskier than more developed companies.
You should consider general risks as well as specific risks when deciding whether to invest.
Risks Related to Our Business and Industry
Prior to the establishment of our COVID-19
testing business, we incurred losses, and expect to continue to generate losses now that COVID-19 testing has ceased.
While we achieved profitability in 2021 and 2022,
such profitability was mainly a result of COVID-19 testing, which ceased in the second quarter of 2023. Prior to 2021, we incurred losses
since inception. We have financed our operations through the sale of our securities, product revenues and government research grants and
contracts. There is no assurance that we will be able to obtain adequate financing that we may need, or that any such financing that may
become available will be on terms that are favorable to us and our stockholders. Ultimately, our ability to generate sufficient operating
revenue to earn a profit depends upon our success in developing and marketing or licensing our diagnostic tests and technology. Any failure
to do so could result in the possible closure of our business or force us to seek additional capital through loans or additional sales
of our equity securities to continue business operations, which could dilute the value of any securities you hold, or could result in
the loss of your entire investment.
Now that the pandemic emergency has ended,
our success will depend heavily on our cancer screening tests.
Now that the pandemic emergency has ended, the
bulk of our revenues depends almost entirely on the commercial success of our cancer tests unless we can also develop or acquire new tests
to other diseases or chronic conditions. The commercial success and our ability to generate revenues will depend on a variety of factors,
including the following:
| ● | patient acceptance of and demand for our tests; |
| ● | acceptance in the medical community; |
| ● | successful sales, marketing, and educational programs, including successful direct-to-patient marketing
such as online advertising; |
| ● | the amount and nature of competition from other multi- cancer screening products and procedures; |
| ● | the ease of use of our ordering process for physicians; |
| ● | maintaining and defending patent protection of our intellectual property; and |
| ● | our ability to establish and maintain adequate commercial manufacturing, distribution, sales and CLIA
laboratory testing capabilities. |
If we are unable to develop and maintain substantial
sales of our tests or if we are significantly delayed or limited in doing so, our business prospects, financial condition and results
of operation will be adversely affected.
We will need to attract additional capital
to scale our business but have no assurance that we can do so successfully.
We will be incurring significant sales and marketing
costs as we commercialize our diagnostic test products. We will need to raise additional capital to pay operating expenses until we are
able to generate sufficient revenues from diagnostic test sales, royalties, and license fees, and we will need to sell additional equity
or debt securities to meet those capital needs. Our ability to raise additional equity or debt capital will depend not only on progress
made marketing and selling our diagnostic tests, but also will depend on access to capital and conditions in the capital markets. There
is no assurance that we will be able to raise capital at times and in amounts needed to finance the development and commercialization
of our diagnostic tests, maintenance of our CLIA certified diagnostic laboratory, and general operations. Even if capital is available,
it may not be available on terms that we or our stockholders would consider favorable. Furthermore, sales of additional equity securities
could result in the dilution of the interests of our stockholders.
We will spend a substantial amount of our
capital on test validation, biomarker and data acquisitions, data analytics and algorithm development, but our products might not succeed
in gaining widespread market acceptance.
We have developed and will continually refine
new biomarker test panels and associated algorithms. The main focus of these products is on early detection of cancer. Our technologies
may not prove to be sufficiently efficacious or medically useful to gain widespread adoption or market share. The diagnostics tests and
software that we have introduced to the market to date have not yet generated significant revenues. Without diagnostic test sales or licensing
fee revenues, we will not be able to operate at a profit, and we will not be able to cover our operating expenses without raising additional
capital.
Medical organizations, physicians and employers
may be reluctant to try a new diagnostic test due to the high degree of risk associated with the application of new technologies and diagnostic
tests in the field of human medicine, especially if the new test differs from the current standard of care for detecting cancer in patients.
Competing tests for the screening or initial diagnosis of cancer are being developed by more established and significantly better-financed
diagnostics or biotech companies, and academic laboratories.
There also is a risk that our competitors may
succeed in developing more accurate or more cost-effective diagnostic tests that could render our diagnostic tests and technologies obsolete
or noncompetitive. Even if our tests are technically superior, we may not be able to differentiate our products sufficiently from our
competition.
The success of our diagnostic tests depends
on the degree of market acceptance by physicians, patients, government agencies and others who influence medical decision making.
The value of our diagnostic products is thus far
proven mainly with real world evidence, rather than traditional clinical trials; and there is no assurance that real world evidence will
gain wide acceptance by the medical establishment or regulators in the countries in which we conduct business. Also, there is no assurance
that data derived from East Asia will be accepted in Western nations and generating data from Western populations could be time consuming
and expensive. The value of machine learning and artificial intelligence in our algorithms is novel, not entirely proven, and might not
be widely embraced by the medical establishment or regulators in the countries in which we conduct business.
Our diagnostics tests may not gain market acceptance
by physicians and others in the medical community. The degree of market acceptance of our tests will depend on a number of factors, including:
| ● | demonstrated sensitivity and specificity for detecting cancers; |
| ● | the availability and attractiveness of alternative screening methods; |
| ● | the willingness of physicians to recommend or prescribe our tests; |
| ● | the ease of use of our ordering process for physicians; and |
| ● | evidence that our tests confer a mortality benefit rather than merely shifting the stage of cancer at
time of diagnosis. |
If our diagnostics tests do not achieve an adequate
level of acceptance, we may not generate the substantial revenues we need to generate to remain profitable.
We are expecting patient self-pay to constitute
a significant portion of our revenues for the foreseeable future and this revenue growth is contingent upon individuals’ willingness
to pay out of pocket for our diagnostic tests.
We expect that a substantial portion of the patients
for whom we will perform diagnostic tests will have Medicare as their primary medical insurance. Medicare coverage is not expected for
several years. Patients who are not covered by Medicare will generally rely on health insurance provided by private health insurance companies.
If we are considered a “non-contracted provider” by a third-party payer, that payer may not reimburse patients for diagnostic
tests performed by us or doctors within the payer’s network of covered physicians may not use our services to perform diagnostic
tests for their patients. As a result, we may need to enter into contracts with health insurance companies or other private payers to
provide diagnostic tests to their insured patients at specified rates of reimbursement which may be lower than the rates we might otherwise
collect.
Until our diagnostic tests are covered by Medicare
or private insurance, we expect that self-pay will constitute a significant portion of our revenues for the foreseeable future. This revenue
growth will be contingent on individuals’ willingness to pay out of pocket for our diagnostic tests.
We face substantial competition.
The development and commercialization of diagnostics
tests, especially MCEDs, is highly competitive and subject to rapid technological advances. We face competition with respect to our
current products and any product candidates we may seek to develop or commercialize in the future. Our competitors may develop comparable
tests that are safer, more effective, more convenient or less costly than any products that we may develop or market or may obtain marketing
approval for their products from the FDA, or equivalent foreign regulatory bodies more rapidly than we may obtain approval for our product
candidates. Our competitors may devote greater resources to market or sell their tests, research and development capabilities, adapt more
quickly to new technologies, scientific advances or patient preferences and needs, initiate or withstand substantial price competition more
successfully, or more effectively negotiate third-party licensing and collaborative arrangements. As a result, physicians and other key
healthcare decision makers may choose other products over our products, switch from our products to new products or choose to use our
products only in limited circumstances, which could adversely affect our business, financial condition, and results of operations.
If our diagnostics tests do not perform
as expected, are misused or misinterpreted, or the reliability of the technology is questioned, we could experience delayed or reduced
market acceptance of the tests, increased costs and damage to our reputation. False positives or false negatives could cause harm to patients
and could result in action taken against our company.
Our success depends on the market’s confidence
that we can provide a reliable, high-quality diagnostic tests. We believe that customers are likely to be particularly sensitive to product
defects and errors. Our reputation and the public image of our diagnostic tests may be impaired if they fail to perform as expected or
are perceived as difficult to use. Despite clinical verification studies, quality control and quality assurance testing, defects or errors
could occur with tests.
In the future, if our diagnostic tests experience
a material defect or error, this could result in loss or delay of revenues, delayed market acceptance, damaged reputation, diversion of
development resources, legal claims, increased insurance costs or increased service and warranty costs, any of which could harm our business.
Such defects or errors could also prompt us to amend certain warning labels or narrow the scope of the use of our diagnostic tests, either
of which could hinder our success in the market. Even after any underlying concerns or problems are resolved, any widespread concerns
regarding our technology or any manufacturing defects or performance errors in the test could result in lost revenue, delayed market acceptance,
damaged reputation, increased service and warranty costs and claims against us.
Our inability to manage growth could harm
our business.
We have added, and expect to continue to add,
additional personnel in the areas of sales and marketing, laboratory operations, billing and collections, quality assurance and compliance.
As we build our commercialization efforts and expand research and development activities, the scope and complexity of our operations is
increasing significantly. As a result of our growth, our operating expenses and capital requirements have also increased, and we expect
that they will continue to increase, significantly. Our ability to manage our growth effectively requires us to forecast expenses accurately,
and to properly forecast and expand operational and testing facilities, if necessary, to expend funds to improve our operational, financial
and management controls, reporting systems and procedures. As we move forward in commercializing our tests, we will also need to effectively
manage our growing manufacturing, laboratory operations and sales and marketing needs. If we are unable to manage our anticipated growth
effectively, our business could be harmed.
We currently manufacture our tests predominantly
in one facility and perform our testing in one laboratory facility. As demand for our tests grow, we may lack adequate facility space
and capabilities to meet increased processing requirements. Moreover, if these or any future facilities or our equipment were damaged
or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business
could be materially harmed.
We currently perform testing in a single laboratory
facility in Gaithersburg, Maryland. Our headquarters and manufacturing facilities are also located in Maryland. As we expand sales and
increase the number of tests processed by our laboratory facility, we may need to expand or modify our existing laboratory facility or
acquire new laboratory facilities to increase our processing capacity. Any failure to do so on terms acceptable to us, if at all, may
significantly delay our processing times and capabilities, which may adversely affect our business, financial condition, and results of
operation.
If these, or any future facilities, were to be
damaged, destroyed or otherwise unable to operate, whether due to fire, floods, storms, tornadoes, other inclement weather events or natural
disasters, employee malfeasance, terrorist acts, power outages, or otherwise, our business could be severely disrupted. If our laboratory
is disrupted, we may not be able to perform testing or generate test reports as promptly as patients and healthcare providers require
or expect, or possibly not at all. If we are unable to perform testing or generate test reports within a timeframe that meets patient
and healthcare provider expectations, our business, financial results, and reputation could be materially harmed.
We currently maintain insurance against damage
to our property and equipment and against business interruption and research and development restoration expenses, subject to deductibles
and other limitations. If we have underestimated our insurance needs with respect to an interruption, or if an interruption is not subject
to coverage under our insurance policies, we may not be able to cover our losses.
There are a limited number of manufacturers
of molecular diagnostic equipment and related chemical reagents necessary for the provision of our diagnostic tests.
The test panels and algorithms that we have developed
and will continue to develop rely on certain analytic equipment. There are only a few manufacturers of the equipment we will need and
the chemical reagents that are required for use with a particular manufacturer’s equipment will be available only from that equipment
manufacturer. If the manufacturer of the equipment we acquire discontinues operation or if we and other testing laboratories experience
supply or quality issues with their equipment or reagents, it may become necessary for us to adjust our products for different analytic
equipment, which would require additional experiments to ensure reproducibility of our test results using the new equipment. As a result,
we may be unable to provide our diagnostic products for a period of time.
Our suppliers may experience development
or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We may encounter unforeseen situations in the
manufacturing of our diagnostic tests that could result in delays or shortfalls in production. Suppliers may also face similar delays
or shortfalls. In addition, suppliers’ production processes may have to change to accommodate any significant future expansion of
manufacturing capacity, which may increase suppliers’ manufacturing costs, delay production of diagnostic tests, reduce our product
gross margin and adversely impact our business. If we are unable to keep up with demand for tests by successfully securing supply and
shipping our diagnostic tests in a timely manner, our revenue could be impaired, market acceptance for the tests could be adversely affected
and our customers might instead purchase our competitors’ diagnostic tests.
To achieve widespread use of our diagnostic
test and commercial scale, individual consumers will need convenient access to blood draw services, but we cannot guarantee that these
service providers will be willing to perform them.
Currently, our cancer tests require venous blood
collected by a licensed phlebotomist. While our business customers, such as employers, typically have little difficulty finding phlebotomists,
this can be a challenge for many of our individual consumers. To address this need, we have about 1,000 retail establishments that can
draw blood for our test customers. These establishments perform these services based on contracts we have with the companies Any Lab Test
Now and My One Medical Source. If those contracts were to terminate or expire or if they are unable to maintain their franchisees or networks
of clinics willing to draw blood, this could limit our ability to serve our customers and grow.
We have limited sales and marketing resources
and few distribution resources for the commercialization of any diagnostic tests that we have developed.
We currently have limited sales and marketing
resources. If we are successful in developing marketable diagnostic tests, we will need to build our own marketing and sales capability,
which would require the investment of significant financial and management resources to recruit, train, and manage a sales force.
The sizes of the markets for our diagnostic
tests and services and any future diagnostic tests and services may be smaller than we estimate and may decline.
Our estimates of the annual total addressable
market for our diagnostic tests and services are based on a number of internal and third-party estimates and assumptions, including, without
limitation, the assumed prices at which we can sell our diagnostic tests and services in the market. While we believe our assumptions
and the data underlying our estimates are reasonable, these assumptions and estimates may not be correct and the conditions supporting
our assumptions or estimates may change at any time, thereby reducing the predictive accuracy of these underlying factors. As a result,
our estimates of the annual total addressable market for our diagnostic tests and services in different market segments may prove to be
incorrect. If the actual number of patients who would benefit from our diagnostic tests, the price at which we can sell them or the annual
total addressable market for them is smaller than we have estimated, it may impair our sales growth and negatively affect our business,
financial condition and results of operations.
If we fail to enter into and maintain successful
strategic alliances for diagnostic tests that we elect to co-develop, co-market, or out-license, we may have to reduce or delay our diagnostic
test development or increase our expenditures.
To facilitate the development, manufacture, and
commercialization of our diagnostic tests we may enter into strategic alliances with hospitals and biomedical research institutes, biotechnology
and diagnostics companies, clinical testing reference laboratories, and marketing firms in many of the countries in which we do business.
We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms,
if at all. If we fail to create and maintain suitable alliances, we may have to limit the size or scope of, or delay, one or more of our
product development or research programs, or we may have to increase our expenditures and may need to obtain additional funding, which
may be unavailable or available only on unfavorable terms.
In some countries we may license marketing rights
to diagnostics or clinical laboratory companies or to a joint venture company formed with those companies. Under such arrangements we
might receive only a royalty on sales of the diagnostic tests developed or an equity interest in a joint venture company that develops
the diagnostic test. As a result, our revenues from the sale of those diagnostic tests may be substantially less than the amount of revenues
and gross profits that we might receive if we were to market and run the diagnostic tests ourselves.
We may become dependent on possible future
collaborations to develop and commercialize many of our diagnostic test candidates and to provide the manufacturing, regulatory compliance,
sales, marketing and distribution capabilities required for the success of our business.
We may enter into various kinds of collaborative
research and development, manufacturing, and diagnostic test marketing agreements to develop and commercialize our diagnostic tests. There
is a risk that we could become dependent upon one or more collaborative arrangements. A collaborative arrangement, upon which we might
depend might be terminated by our collaboration partner or they might determine not to actively pursue the co-development of our diagnostic
tests. A collaboration partner also may not be precluded from independently pursuing competing diagnostic tests or technologies.
The success of our business is substantially
dependent upon the efforts of our senior management team and our ability to attract additional personnel.
Our success depends largely on the skills, experience,
and performance of key members of our senior management team who are critical to directing and managing our growth and development in
the future. Our success is substantially dependent upon our senior management’s ability to lead our company, implement successful
corporate strategies and initiatives, develop key relationships, including relationships with collaborators and business partners, and
successfully commercialize products and services. While our management team has significant experience developing diagnostic products,
we have considerably less experience in commercializing these products or services. The efforts of our management team will be critical
to us as we develop our technologies and seek to commercialize our tests and other products and services.
Our success also depends in large part on our
ability to attract and retain managerial personnel. Competition for desirable personnel is intense, and there can be no assurance that
we will be able to attract and retain the necessary staff. The failure to maintain management or to attract sales personnel could materially
adversely affect our business, financial condition, and results of operations.
Certain jurisdictions in which we may do
business may not provide the same level of legal protections and enforcement of contract and intellectual property rights to which investors
are accustomed in the United States.
We may conduct business in China and other foreign
jurisdictions. In order to do business in these countries, we will be required to comply with the laws of those countries, including restrictions
on exporting currency, requirements for local partners, tax laws and other legal requirements. Doing business in such foreign jurisdictions
also entails political risk over which we have no control and for which we are unable to obtain insurance on acceptable terms. These countries
also have different judicial systems, which may not provide the same level of legal protections and enforcement of contract and intellectual
property rights to which investors are accustomed in the United States. We can provide no assurance that the applicable laws of such foreign
jurisdictions will not be changed in ways unfavorable to us, or that applicable laws will be adequately enforced in order to provide the
same levels of protection accorded to us in the United States.
Risks Related to Intellectual Property
If we are unable to obtain and enforce patents
and to protect our trade secrets, others could use our technology to compete with us, which could create undue competition and pricing
pressures. There is no certainty that our pending or future patent applications will result in the issuance of patents or that our issued
patents will be deemed enforceable.
The success of our business depends significantly
on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a
patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing
research, development, and commercialization of diagnostic tests that rely on that technology, unless we are able to obtain a license
to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at
an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a diagnostic test
with which our diagnostic test would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to
alternative technologies, which could prove costly and could cause delays in diagnostic test development, or we could be forced to discontinue
the development or marketing of any diagnostic tests that were developed using the technology covered by the patent.
We have issued patents and patent applications
pending worldwide that are owned by or exclusively licensed to us. We and our collaborators expect to continue to file and prosecute patent
applications covering the products and technology that we commercialize. However, there is no assurance that any of our licensed patent
applications, or any patent applications that we have filed or that we may file in the future in the United States or abroad, will result
in the issuance of patents.
Our success will depend in part on our ability
to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining
and enforcing patents, our competitors could use our technology and create diagnostic tests that compete with our diagnostic tests, without
paying license fees or royalties to us.
The relatively recent Supreme Court decisions
in Mayo Collaborative Services v. Prometheus Laboratories, Inc. and Alice Corp. v. CLS Bank Int'l may adversely impact our
ability to obtain strong patent protection for some or all of our diagnostic tests and associated algorithms.
The preparation, filing, and prosecution
of patent applications can be costly and time consuming.
The preparation and filing of patent applications,
and the maintenance of patents that are issued, may require substantial time and money. A patent interference proceeding may be instituted
with the United States Patent and Trademark Office, or USPTO, when more than one-person files a patent application covering the same technology,
or if someone wishes to challenge the validity of an issued patent. Furthermore, our limited financial resources may not permit us to
pursue patent protection of all of our technology and diagnostic tests throughout the world, even where we have legally binding patent
protection and trade secret rights. Even if we are able to obtain issued patents covering our technology or diagnostic tests, we may have
to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and diagnostic tests
from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret
rights.
Our patents may not protect our diagnostic
tests from competition.
We might not be able to obtain any patents beyond
those that have been issued by the USPTO, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful
patent protection. There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability
of any patent issued to us.
If we fail to meet our obligations under
various license, license option, and technology transfer agreements, we may lose our rights to key technologies or data sources on which
our business depends.
Our business will depend on several critical technologies
and data sources that have licenses from various domestic and overseas companies and research centers. Importantly, if we fail to meet
our obligations under our technology access agreement with BioInfra, this would adversely impact our ability to introduce an enhanced
or premium version of our MCED test. These and other license agreements typically impose obligations on us, including payment obligations
and obligations to pursue development and commercialization of diagnostic tests under the licensed patents and technology. If licensors
believe that we have failed to meet our obligations under a license agreement, they could seek to limit or terminate our license rights,
which could lead to costly and time-consuming dispute resolution and, potentially, a loss of the licensed rights. During the period of
any such litigation our ability to carry out the development and commercialization of potential diagnostic tests, and our ability to raise
any capital that we might then need, could be significantly and negatively affected. If our license rights were restricted or ultimately
lost, we would not be able to continue to use the licensed patents and technology in our business.
Risks Related to Healthcare Government
Regulation, Reimbursement, Product Safety and Effectiveness
We have relied and expect to continue to
rely on third parties to conduct studies of our diagnostics tests that will be required to meet our obligations under CLIA, CAP and/or
other regulatory authorities and those third parties may not perform satisfactorily.
We rely on third parties, such as academic, medical
and commercial entities, to conduct studies for our diagnostics tests. These include MD Anderson, the Chang Gung Memorial Hospital in
Taiwan and BioInfra. Our reliance on these third parties will reduce our control over these activities. These third-party contractors
may not complete activities on schedule or conduct studies in accordance with regulatory requirements or our study design. We cannot control
whether they devote sufficient time, skill, and resources to our studies. Our reliance on third parties that we do not control will not
relieve us of any applicable requirement to prepare, and ensure compliance with, various procedures required under good scientific and
clinical practices. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected
deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their
failure to adhere to our clinical protocols or regulatory requirements under the CLIA or CAP, or for other reasons, our studies may be
extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for additional diagnostic tests.
We must successfully maintain and/or upgrade
our information technology systems, and our failure to do so could have a material adverse effect on our business, financial condition
or results of operations.
We rely on various information technology systems
to manage our operations. Recently, we have implemented, and we continue to implement, modifications and upgrades to such systems and
acquired new systems with new functionality. These types of activities subject us to inherent costs and risks associated with replacing
and changing these systems, including impairment of our ability to fulfill customer orders, potential disruption of our internal control
structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel
to implement and operate the new systems, demands on management time and other risks and costs of delays or difficulties in transitioning
to or integrating new systems into our current systems. These implementations, modifications and upgrades may not result in productivity
improvements at a level that outweighs the costs of implementation, or at all. In addition, the difficulties with implementing new technology
systems may cause disruptions in our business operations and have a material adverse effect on our business, financial condition or results
of operations.
Our business and operations could suffer
in the event of system failures.
Despite the implementation of security measures,
our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized
access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations.
For example, the loss of data for our diagnostic test candidates could result in delays in our regulatory filings and development efforts
and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our
data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our diagnostic
test candidates could be delayed.
International operations could subject us
to risks and expenses that could adversely impact the business and results of operations.
To date, we have not undertaken substantial commercial
activities outside the United States. We have evaluated commercialization in Asian countries. If we seek to expand internationally, or
launch other products or services internationally, in the future, those efforts would expose us to risks from the failure to comply with
foreign laws and regulations that differ from those under which we operate in the U.S., as well as U.S. rules and regulations that govern
foreign activities such as the FCPA. In addition, we could be adversely affected by other risks associated with operating in foreign countries.
Economic uncertainty in some of the geographic regions in which we might operate, including developing regions, could result in the disruption
of commerce and negatively impact cash flows from our operations in those areas.
These and other factors may have a material adverse
effect on any international operations we may seek to undertake and, consequently, on our financial condition and results of operations.
Our business is subject to various complex
laws and regulations. We could be subject to significant fines and penalties if we or our partners fail to comply with these laws and
regulations.
As a provider of clinical diagnostic products
and services, we and our partners are subject to extensive and frequently changing federal, state, and local laws and regulations governing
various aspects of our business. In particular, the clinical laboratory industry is subject to significant governmental certification
and licensing regulations, as well as federal and state laws regarding:
| ● | test ordering and billing practices; |
| ● | marketing, sales and pricing practices; |
| ● | the Eliminating Kickbacks in Recovery Act of 2018; |
| ● | health information privacy and security, including HIPAA, as amended by HITECH, and comparable state laws; |
| ● | anti-markup legislation; and |
We are also required to comply with FDA regulations,
including with respect to our labeling and promotion activities. In addition, advertising of our tests is subject to regulation by the
Federal Trade Commission, or the FTC. Violation of any FDA requirement could result in enforcement actions, such as seizures, injunctions,
civil penalties and criminal prosecutions, and violation of any FTC requirement could result in injunctions and other associated remedies,
all of which could have a material adverse effect on our business. Most states also have similar regulatory and enforcement authority
for devices. Additionally, most foreign countries have authorities comparable to the FDA and processes for obtaining marketing approvals.
Obtaining and maintaining these approvals, and complying with all laws and regulations, may subject us to similar risks and delays as
those we could experience under FDA and FTC regulation. We incur various costs in complying and overseeing compliance with these laws
and regulations.
Healthcare policy has been a subject of extensive
discussion in the executive and legislative branches of the federal and many state governments and healthcare laws and regulations are
subject to change. Development of the existing commercialization strategy for our tests have been based on existing healthcare policies.
We cannot predict what additional changes, if any, will be proposed or adopted or the effect that such proposals or adoption may have
on our business, financial condition and results of operations.
If we or our partners, including independent sales
representatives, fail to comply with these laws and regulations, we could incur significant fines and penalties and our reputation and
prospects could suffer. Additionally, our partners could be forced to cease offering our products and services in certain jurisdictions,
which could materially disrupt our business.
We could be unexpectedly required to obtain
pre-market regulatory approval of our diagnostic test products in the U.S. or overseas.
Our diagnostic test products are classified as
LTDs, which, in general, are not currently regulated by the FDA. However, FDA policies and practices could be interpreted or evolve to
deem our products under their jurisdiction and in need of approval as a condition to continued marketing in the U.S. This may also be
the case for corresponding foreign regulatory authorities. On September 29, 2023, FDA issued a proposed regulation under which they would
begin to regulate LDTs starting in late 2027. The proposed rule, which will likely be finalized in April 2024, is expected to be
challenged in court and may also be overridden by legislation in Congress. However, if the rules survive, they could significantly
increase the cost and burden and affect our ability to market or improve existing LDTs and/or introduce new lab tests.
If we unexpectedly are required to obtain regulatory
approval of our diagnostic test products, it may take two years or more to conduct the clinical studies and trials necessary to obtain
pre-market approval from the FDA. Even if our clinical trials are completed as planned, we cannot be certain that the results will support
our test claims or that the FDA will agree with our conclusions regarding our test results. Success in early clinical trials does not
ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior
clinical trials and studies. If we are required to conduct pre-market clinical trials, delays in the commencement or completion of clinical
testing could significantly increase our test development costs and delay commercialization. Many of the factors that may cause or lead
to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or
approval. The clinical trial process may fail to demonstrate that our tests are effective for the proposed indicated uses, which could
cause us to abandon a test candidate and may delay development of other tests.
We are required to comply with federal and
state laws governing the privacy of health information, and any failure to comply with these laws could result in material criminal and
civil penalties.
HIPAA sets forth security regulations that establish
administrative, physical, and technical standards for maintaining the confidentiality, integrity and availability of protected health
information in electronic form. We also may be required to comply with state laws that are more stringent than HIPAA or that provide individuals
with greater rights with respect to the privacy or security of, and access to, their health care records. HITECH established certain health
information security breach notification obligations that require covered entities to notify each individual whose protected health information
is breached.
We may incur significant compliance costs related
to HIPAA and HITECH privacy regulations and varying state privacy regulations and varying state privacy and security laws. Given the complexity
of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are
subject to changing and potentially conflicting interpretation, our ability to comply with the HIPAA, HITECH and state privacy requirements
is uncertain and the costs of compliance are significant. The costs of complying with any changes to the HIPAA, HITECH and state privacy
restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant
monetary penalties as well as reputational damage.
We are subject to federal and state healthcare
fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
We are subject to healthcare fraud and abuse regulation
and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations
include the following:
| ● | The Eliminating Kickbacks in Recovery Act of 2018; |
| ● | The federal Anti-Kickback Statute; |
| ● | The federal physician self-referral prohibition, commonly known as the Stark Law; |
| ● | The federal false claims and civil monetary penalties laws; |
| ● | The federal Physician Payment Sunshine Act requirements under the Affordable Care Act; and |
| ● | State law equivalents of each of the federal laws enumerated above. |
Any action brought against us for violation of
these laws or regulations, even if we are in compliance and successfully defend against it, could cause us to incur significant legal
expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation
of any of these laws and regulations, we may be subject to applicable penalties associated with the violation, including, among others,
administrative, civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, Medicaid programs, including
the California Medical Assistance Program (Medi-Cal—the California version of the Medicaid program) or other state or federal health
care programs. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our
operations.
If we become subject to claims relating
to the receipt and handling of bio-hazardous materials (including infected blood), we could incur significant cost and liability.
Our quality control quality assurance process
might involve the receipt and handling of whole blood, serum, or plasma from one or more individuals. We are subject to Federal, state
and local regulations governing the use, manufacture, storage, handling and disposal of biological materials and waste products. We may
incur significant costs complying with both existing and future environmental laws and regulations. In particular, we are subject to regulation
by the Maryland Department of Health, the CLIA, Occupational Safety and Health Administration, or OSHA, and the Environmental Protection
Agency, or EPA, and to regulation under the Toxic Substances Control Act and the Resource Conservation and Recovery Act in the United
States. OSHA or the EPA may adopt additional regulations in the future that may affect our research and development programs. The risk
of accidental contamination or injury from hazardous materials cannot be eliminated completely. In the event of an accident, we could
be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of our workers’
compensation insurance. We may not be able to maintain insurance on acceptable terms, if at all.
In the event that one or more lawsuits are
filed against us, we could be subject to reputational risk.
Our diagnostic tests are intended for use only
as screening devices, which trigger more in-depth diagnostic procedures. If our tests failed and the patient sued us, we could incur reputational
damage if doctors or patients were dissuaded from using our tests. Repeated lawsuits could also precipitate regulatory scrutiny that could
negatively impact our ability to sell our products.
Risks Related to Ownership of Our Common Stock
There is no public market for our common
stock. You cannot be certain that an active trading market or a specific share price will be established, and you may not be able to resell
your securities at or above the purchase price.
There is currently no public market for our common
stock. We may apply for the listing of our common stock on a national exchange (i.e., NYSE or NASDAQ) or for the quotation of our common
stock on the OTCQB or OTCQX markets maintained by OTC Markets Group Inc. However, an active trading market may not develop even
if we are successful in arranging for our common stock to be listed or quoted. We also cannot assure you that the market price of our
common stock will not fluctuate or decline significantly, including a decline below the offering price, in the future.
The market price of our common stock may
fluctuate, and you could lose all or part of your investment.
Our financial performance, our industry’s
overall performance, changing consumer preferences, technologies and government regulatory action, tax laws and market conditions in general
could have a significant impact on the future market price of our common stock. Some of the other factors that could negatively affect
our share price or result in fluctuations in our share price include:
| ● | actual or anticipated variations in our periodic operating results; |
| ● | increases in market interest rates that lead purchasers of our common stock to demand a higher yield; |
| ● | changes in earnings estimates; |
| ● | changes in market valuations of similar companies; |
| ● | actions or announcements by our competitors; |
| ● | adverse market reaction to any increased indebtedness we may incur in the future; |
| ● | additions or departures of key personnel; |
| ● | actions by stockholders; |
| ● | speculation in the press or investment community; and |
| ● | our intentions and ability to list our common stock on a national securities exchange and our subsequent
ability to maintain such listing. |
Future issuances of our common stock or
securities convertible into our common stock could cause the market price of our common stock to decline and would result in the dilution
of your shareholding.
Future issuances of our common stock or securities
convertible into our common stock could cause the market price of our common stock to decline. We cannot predict the effect, if any, of
future issuances of our common stock or securities convertible into our common stock on the price of our common stock. In all events,
future issuances of our common stock would result in the dilution of your shareholding. In addition, the perception that new issuances
of our common stock, or other securities convertible into our common stock, could occur, could adversely affect the market price of our
common stock.
Future issuances of debt securities, which
would rank senior to our capital stock upon our bankruptcy or liquidation, and future issuances of preferred stock may adversely affect
the level of return you may be able to achieve from an investment in our securities.
In the future, we may attempt to increase our
capital resources by offering debt securities. Upon bankruptcy or liquidation, holders of our debt securities, and lenders with respect
to other borrowings we may make, would receive distributions of our available assets prior to any distributions being made to holders
of our capital stock. Moreover, if we issue additional preferred stock, the holders of such preferred stock could be entitled to preferences
over existing holders of common stock and preferred stock in respect of the payment of dividends and the payment of liquidating distributions.
Because our decision to issue debt or preferred securities in any future offering, or borrow money from lenders, will depend in part on
market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any such future
offerings or borrowings. You must bear the risk that any future offerings we conduct or borrowings we make may adversely affect the level
of return you may be able to achieve from an investment in our securities.
We have never paid cash dividends on our
common stock and we do not intend to pay dividends for the foreseeable future.
We have paid no cash dividends on our common stock
to date, and we do not anticipate paying cash dividends in the near term. For the foreseeable future, we intend to retain any earnings
to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our stock. Accordingly,
investors must be prepared to rely on sales of their common stock after price appreciation to earn an investment return, which may never
occur. Investors seeking cash dividends should not purchase our common stock. Any determination to pay dividends in the future will be
made at the discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions,
restrictions imposed by applicable law and other factors our board deems relevant.
Anti-takeover provisions in our charter
documents and under Delaware law could make an acquisition of our company more difficult, and limit attempts by our stockholders to replace
or remove our current management.
Provisions in our certificate of incorporation
and bylaws may have the effect of delaying or preventing a change of control of our company or changes in our management. Our authorized
but unissued shares of common stock are available for our board of directors to issue without stockholder approval. We may use these additional
shares for a variety of corporate purposes, including raising additional capital, corporate acquisitions and employee stock plans. The
existence of our authorized but unissued shares of common stock could render it more difficult or discourage an attempt to obtain control
of our company by means of a proxy context, tender offer, merger or other transaction since our board of directors can issue large amounts
of capital stock as part of a defense to a take-over challenge. In addition, we have authorized in our certificate of incorporation 20,000,000
shares of preferred stock. Our board acting alone and without approval of our stockholders can designate and issue one or more series
of preferred stock containing super-voting provisions, enhanced economic rights, rights to elect directors, or other dilutive features,
that could be utilized as part of a defense to a take-over challenge.
In addition, various provisions of our bylaws
may also have an anti-takeover effect. These provisions may delay, defer or prevent a tender offer or takeover attempt of our company
that a stockholder might consider in his or her best interest, including attempts that might result in a premium over the market price
for the shares held by our stockholders. Our bylaws contain limitations as to who may call special meetings as well as require advance
notice of stockholder matters to be brought at a meeting. Additionally, our bylaws also provide that no director may be removed by less
than a majority of the issued and outstanding shares entitled to vote on the removal. Our bylaws also permit the board of directors to
establish the number of directors and fill any vacancies and newly created directorships. These provisions will prevent a stockholder
from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with
its own nominees.
Our bylaws also establish an advance notice procedure
for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election
to the board of directors. Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the notice
of meeting or brought before the meeting by or at the direction of the board of directors or by a stockholder who was a stockholder of
record on the record date for the meeting, who is entitled to vote at the meeting and who has given us timely written notice, in proper
form, of the stockholder’s intention to bring that business before the meeting. Although our bylaws do not give the board of directors
the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special
or annual meeting, our bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures
are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors
or otherwise attempting to obtain control of our company.
Moreover, Section 203 of the General Corporation
Law of the State of Delaware may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions
on mergers, business combinations and other transactions between us and holders of 15% or more of our common stock.
These provisions may frustrate or prevent any
attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members
of our board of directors, which is responsible for appointing the members of our management.
We are subject to ongoing public reporting
requirements that are less rigorous than rules for more mature public companies, and our stockholders receive less information.
We are required to publicly report on an ongoing
basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are
more relaxed than for public companies reporting under the Securities Exchange Act of 1934, as amended, or the Exchange Act. The differences
include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports.
Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within
90 calendar days after the end of the first six months of the issuer’s fiscal year.
We may elect to become a public reporting company
under the Exchange Act. If we elect to do so, we will be required to publicly report on an ongoing basis as an emerging growth company,
as defined in Jumpstart Our Business Startups Act, or the JOBS Act, under the reporting rules set forth under the Exchange Act. For so
long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are
applicable to other Exchange Act reporting companies that are not emerging growth companies, including but not limited to:
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley
Act; |
| ● | being permitted to comply with reduced disclosure obligations regarding executive compensation in our
periodic reports and proxy statements; and |
| ● | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and stockholder
approval of any golden parachute payments not previously approved. |
In addition, Section 107 of the JOBS Act also
provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities
Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an emerging growth
company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have
elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable
to those of companies that comply with such new or revised accounting standards.
We would expect to take advantage of these reporting
exemptions until we are no longer an emerging growth company. We would remain an emerging growth company for up to five years, or until
the earliest of (i) the last day of the first fiscal year in which our total annual gross revenues exceed $1 billion, (ii) the date that
we become a large accelerated filer as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common
shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter
or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
If we decide to apply for the quotation of our
Common Stock on the OTCQB or OTCQX market, we will be subject to the OTC Market’s Reporting
Standards, which can be satisfied in a number of ways, including by remaining in compliance with (i) SEC reporting requirements, if we
elect to become a public reporting company under the Exchange Act, or (ii) Regulation A reporting requirements, if we elect not to become
a reporting company under the Exchange Act.
In either case, we will be subject to ongoing
public reporting requirements that are less rigorous than Exchange Act rules for companies that are not emerging growth companies, and
our stockholders could receive less information than they might expect to receive from more mature public companies.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a commercial-stage diagnostics company
with the core mission of developing and commercializing clinical laboratory tests for early disease detection and prevention and associated
software that is powered by machine learning and real-world data to improve diagnostic accuracy and clinical utility.
Our lead tests currently focus on early cancer
detection. Of the ten deadliest cancers in the U.S., only three—breast, colon, and prostate—have widely adopted screening
modalities. This is despite growing evidence that early detection saves or extends lives for cancers of the lung, liver, pancreas, esophagus,
and ovaries which are not yet the subject of widespread asymptomatic screening. To address this deficiency, we are offering what
we believe to be one of the MCED blood tests to enter the American market. Known as OneTest, we believe our test may be the first and
only MCED test to enter the U.S. market based on the levels of tumor antigens rather than ctDNA. Tumor antigen measurement is a widely
deployed technology (see “Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, Cancer Antigen 125, Prostate-Specific Antigen and
Other Cancer Markers: A Primer on Commonly Used Cancer Markers” World Journal of Oncology (2023) 14(1):4-14; “Clinically Meaningful
Use of Blood Tumor Markers in Oncology” (2016) BioMed Research International, 2016:9795269, doi:10.1155/2016/9795269). Throughout
East Asia, these biomarkers are used for screening as part of yearly health checkups. In the U.S. and other Western nations, tumor antigens
are widely used to monitor therapy responses or disease recurrence in persons being treated for cancer. Furthermore, each of the biomarkers
detected in the OneTest panel uses an existing in vitro diagnostic test platform that has been cleared or approved by the FDA for
at least one disease indication and is automated, easy to use, and widely available. This proteomic approach permits significantly lower
costs and easier access as compared to DNA-based testing with little if any demonstrable loss in test accuracy, especially for early-stage
detection of the major cancers for which there is no widespread screening.
We are also planning to bring to market a blood
test specifically for the early detection of lung cancer in smokers and former smokers. That test was developed by a team at MD Anderson
with over $60 million in funding from federal and state agencies as well as various philanthropies. Validated using blood specimens from
diverse, blinded cohorts comprising thousands of pre-symptomatic individuals, the lung cancer test analyzes several of the same tumor
antigens that are part of OneTest, along with a novel biomarker (ProSurfactant B) discovered by members of that team. The test will be
used primarily to screen individuals with a history of tobacco use to improve both the compliance and effectiveness of LDCT scans which
are now part of U.S. screening guidelines.
To increase our menu of innovative tests faster
and at a lower cost and risk than through internal development, in 2021 we established our CLIAx, which permits diagnostics start-up companies
from around the world to launch their laboratory-developed tests in our CLIA-licensed laboratory using shared equipment and laboratory
personnel. To date, we have enrolled one company in our CLIAx, Minomic, and helped it validate and launch its blood test to help determine
whether specific antigen or PSA, levels should be followed up with a biopsy. Our CLIAx, which we believe to be the first such shared CLIA
laboratory facility in the U.S., reduces the costs and expenses for start-up companies to launch their novel tests in the American market
while providing us with sales and marketing rights to additional products. In 2022, it earned an “Honorable Mention” in Fast
Company magazine’s list of “World Changing Ideas.”
In response to the novel coronavirus pandemic
that began in early 2020, we expanded our business and offered several COVID-19 testing solutions, both rapid kits and laboratory-based
tests. In the third quarter of 2020, in response to substantial and urgent demand for expanded viral testing in Maryland, we also began
to provide COVID-19 viral testing using polymerase chain reaction (PCR) analytical equipment in our clinical laboratory. This pandemic-associated
testing resulted in several years of profitability and forged business alliances that are being leveraged to support our core business.
However, following the expiration of the public health emergency in May 2023, all testing from both the State of Maryland and the Montgomery
County Health Department has ceased, and we do not anticipate additional COVID-19 testing absent a new variant resulting in a significant
increase in cases.
Our legacy business also includes a pioneering
field test kit for screening suspicious powders for bioterror agents known as BioCheck, which hundreds of first responder organizations
use regularly. Our BioCheck kits for screening suspicious powders remain profitable, but with limited growth potential.
Recent Developments
Option Agreement
On March 22, 2024, we entered into an option agreement
with The Board of Regents, or the Board, of The University of Texas System, an agency of the State of Texas, on behalf of MD Anderson,
pursuant to which MD Anderson has granted us an exclusive six-month option to enter into a royalty-bearing, exclusive license to certain
patent rights and technology, which option may be exercised by us upon (i) our completion of an equity financing with proceeds of at least
$23 million based on a pre-money valuation of at least $70 million (which such financing may not be obtained through a crowdfunding or
Regulation A offering) and (ii) payment of a fee in the amount of $4,457,069.15. Upon exercise of the option, we will enter into a patent
and technology license agreement with the Board and MD Anderson, in the form attached to the option agreement, pursuant to which MD Anderson
will grant the license to us in exchange for certain royalties, fees and shares of common stock set forth in the patent and technology license agreement.
Convertible Bonds Subscription Agreement
On March 20, 2024, we entered into a convertible
bonds subscription agreement with Cornerstone Investment Inc., or the Investor, pursuant to which we agreed to issue a convertible bond
in the principal amount of $23 million to the Investor (or its designee) for a purchase price of $23 million. The issuance of the convertible
bond is subject to customary closing conditions, as well as execution of the option agreement described above and a collaborative research
agreement with MD Anderson. The convertible bond will have a term of five (5) years and will not bear interest; provided that (i) if any
portion of the convertible bond has not been converted prior to the maturity date or the date on which an event of default (as defined
in the convertible bond) occurs, as applicable, and (ii) the Investor desires to receive a cash payment with respect to such unconverted
portion on the maturity date or the date on which an event of default occurs, as applicable, we shall be required to pay the Investor,
in addition any other amounts required under the convertible bond, interest accrued on the aggregate principal sum of the convertible
bond at a rate equal to 6% per annum from the date on which the convertible bond is issued up to the maturity date or the date on which
an event of default occurs, as applicable. In addition, if the convertible bond is still outstanding after the maturity date or the date
on which an event of default occurs, as applicable, then interest shall accrue beginning on the day after the maturity date on the outstanding
principal balance and the default amount at a rate equal to 12% per annum. We may not prepay the convertible bond prior to the maturity
date.
The convertible bond will be convertible at any
time at the option of the holder into shares of our common stock, or, subject to stockholder approval, a new series of our preferred stock
to be designated as series E preferred stock with the terms and conditions set forth in Annex A to the convertible bond. In addition,
the convertible bond shall automatically be converted into series E preferred stock upon the earlier to occur of (i) an initial public
offering of our common stock and concurrent listing on a national securities exchange, including without limitation the New York Stock
Exchange, NYSE American or the Nasdaq Stock Market (any tier), (ii) a direct listing of our common stock on a national securities exchange,
including without limitation the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier) or (iii) upon such stockholder
approval. The number of shares to be issued upon conversion shall be equal (i) the outstanding principal amount of the convertible bond
and all accrued and unpaid default interest, if any, divided by (ii) the conversion price then in effect. The initial conversion price
will be $5.34 per share, subject to customary adjustments for stock dividends, stock splits, stock combinations, reclassifications, mergers,
consolidations, sales of all assets, or similar events. In addition, subject to certain exceptions, if we issue any equity securities
with an implied price per share of less than the conversion price then in effect, then the conversion price shall be adjusted, concurrently
with such equity issuance, to the implied price per share received by us for such equity issuance. Finally, the conversion price will
be subject to adjustment in the event that we complete an initial public offering or a direct listing of our common stock on a national
securities exchange that does not meet the requirements of a Q-IPO or a Direct Listing (each as defined below).
The convertible bond will be unsecured and will
contain customary covenants and events of default for a loan of this type. We also agreed that the Investor will be entitled to nominate
at least one (1) director to our board of directors as long as the convertible bond is outstanding. Subject to certain exceptions, we
also agreed that the Investor will have the right to participate in any subsequent financing transactions involving the issuance of common
stock or securities convertible into or exercisable or exchangeable for common stock for cash consideration in an amount required to maintain
the Investor’s fully diluted ownership in our company. We also agreed to use our best efforts to (i) close a firm commitment underwritten
public offering and concurrent listing on a national securities exchange, including without limitation the New York Stock Exchange, NYSE
American or the Nasdaq Stock Market (any tier), with a per share offering price of at least $5.34 plus interest accrued on $5.34 at a
rate equal to 6% per annum from the issuance date of the convertible bond up to the date of listing, or the Target Price (which we refer
to as a Q-IPO), or (ii) complete a direct listing of our common stock on a national securities exchange, including without limitation
the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), with the reference price of at least the Target Price
(which we refer to as a Direct Listing), within three (3) years following the issuance date of the convertible bond, which period may
be extended by one (1) year by mutual agreement between us and the Investor.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by
the following factors:
| ● | our ability to access additional capital and the size and timing of subsequent financings; |
| ● | the costs of acquiring additional data, technology, and/or intellectual property to successfully reach
our goals and to remain competitive; |
| ● | personnel and facilities costs in any region in which we seek to introduce and market our products; |
| ● | the costs of sales, marketing, and customer acquisition; |
| ● | the average price per test paid by consumers; |
| ● | the number of tests ordered per quarter; |
| ● | the costs of third-party laboratories to run our tests; |
| ● | the willingness of healthcare providers (including telemedicine providers) to prescribe and encourage
our tests and the fees charged by them to do so; |
| ● | the costs of compliance with any unforeseen regulatory obstacles or governmental mandates in any states
or countries in which we seek to operate; |
| ● | the costs of any additional clinical studies which are deemed necessary for us to remain viable and competitive
in any region of the world; |
| ● | the extent and duration of demand for COVID-19 viral and serology testing; and |
| ● | our ability to identify additional tests and revenue sources to make up for the drop in COVID-19 testing. |
Results of Operations
The following table sets forth key components
of our results of operations during the years ended December 31, 2023 and 2022, both in dollars and as a percentage of our revenues.
| |
|
December 31, 2023 |
|
|
December 31, 2022 |
|
| |
|
Amount |
|
|
% of Revenues |
|
|
Amount |
|
|
% of Revenues |
|
| Revenues |
|
$ |
1,424,304 |
|
|
|
100.00 |
% |
|
$ |
11,059,145 |
|
|
|
100.00 |
% |
| Cost of revenues |
|
|
1,315,166 |
|
|
|
92.34 |
% |
|
|
5,937,398 |
|
|
|
53.69 |
% |
| Gross profit |
|
|
109,138 |
|
|
|
7.66 |
% |
|
|
5,121,747 |
|
|
|
46.31 |
% |
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sales, general and administrative |
|
|
5,061,450 |
|
|
|
355.36 |
% |
|
|
3,322,835 |
|
|
|
30.05 |
% |
| Research and development |
|
|
1,409,150 |
|
|
|
98.94 |
% |
|
|
120,043 |
|
|
|
1.09 |
% |
| Loss on impairment of fixed assets |
|
|
209,073 |
|
|
|
14.68 |
% |
|
|
- |
|
|
|
- |
|
| Total operating expenses |
|
|
6,679,673 |
|
|
|
468.98 |
% |
|
|
3,442,878 |
|
|
|
31.13 |
% |
| Operating income (loss) |
|
|
(6,570,535 |
) |
|
|
(461.32 |
)% |
|
|
1,678,869 |
|
|
|
15.18 |
% |
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
|
(27,915 |
) |
|
|
(1.96 |
)% |
|
|
(15,685 |
) |
|
|
(0.14 |
)% |
| Interest income |
|
|
209,150 |
|
|
|
14.68 |
% |
|
|
68,421 |
|
|
|
0.62 |
% |
| Gain on sale of asset |
|
|
- |
|
|
|
- |
|
|
|
2,371 |
|
|
|
0.02 |
% |
| Other expense |
|
|
(2,009 |
) |
|
|
(0.14 |
)% |
|
|
- |
|
|
|
- |
|
| Other income |
|
|
- |
|
|
|
- |
|
|
|
452,899 |
|
|
|
4.10 |
% |
| Total other (income) expense |
|
|
179,226 |
|
|
|
12.58 |
% |
|
|
508,006 |
|
|
|
4.59 |
% |
| Net income (loss) |
|
$ |
(6,391,309 |
) |
|
|
(448.73 |
)% |
|
$ |
2,186,875 |
|
|
|
19.77 |
% |
Revenues. We generated revenues
from sales of COVID-19 tests, OneTest, BioCheck and from our CLIAx during the years ended December 31, 2023 and 2022. Our total revenues
decreased by $9,634,841, or 87.12%, to $1,424,304 for the year ended December 31, 2023 from $11,059,145 for the year ended December 31,
2022. Such decrease was due to a significant decrease in revenues from sales of our COVID-19 tests and a decrease in revenues from our
CLIAx, offset by increases in revenues from sales of OneTest and BioCheck. The following table summarizes our revenues by product:
| | |
December 31, 2023 | | |
December 31, 2022 | |
| | |
Amount | | |
% of Revenues | | |
Amount | | |
% of Revenues | |
| COVID-19 PCR Tests | |
$ | 250,145 | | |
| 17.56 | % | |
$ | 10,393,256 | | |
| 93.98 | % |
| COVID-19 Antibody/Antigen Tests | |
| 2,375 | | |
| 0.17 | % | |
| 97,452 | | |
| 0.88 | % |
| OneTest | |
| 921,502 | | |
| 64.70 | % | |
| 323,414 | | |
| 2.92 | % |
| BioCheck | |
| 187,926 | | |
| 13.19 | % | |
| 154,660 | | |
| 1.40 | % |
| CLIAx | |
| 62,356 | | |
| 4.38 | % | |
| 90,363 | | |
| 0.82 | |
| Total revenues | |
$ | 1,424,304 | | |
| | | |
$ | 11,059,145 | | |
| | |
Revenues from our COVID-19 tests are derived from
two classes of tests: (i) rapid point-of-care tests (antibody and antigen) that we distributed after validating and (ii) lab-based PCR
testing of nasal swabs sent to our CLIA lab from area nursing homes, numerous county school systems in the State of Maryland and the Montgomery
County Health Department. Revenues from our COVID-19 tests decreased by $10,238,188, or 97.59%, to $252,520 for the year ended December
31, 2023 from $10,490,708 for the year ended December 31, 2022. Such decrease was due to the significant decrease in demand for COVID-19
testing as the pandemic has subsided. As of the date of this report, all testing has ceased at both the State of Maryland and Montgomery
County Health Departments, and we do not anticipate additional COVID-19 testing absent a new variant resulting in a significant increase
in cases.
Revenues from sales of OneTest increased by $598,088,
or 184.93%, to $921,502 for the year ended December 31, 2023 from $323,414 for the year ended December 31, 2022. Such an increase was
the result of adding additional sales leadership and personnel over the last year and increased digital advertising during the past twelve
months.
Revenues from sales of BioCheck increased by $33,266,
or 21.51%, to $187,926 for the year ended December 31, 2023 from $154,660 for the year ended December 31, 2022. Such an increase was due
to our efforts to re-engage past customers to order the product again.
Revenues from our CLIAx decreased by $28,007,
or 30.99%, to $62,356 for the year ended December 31, 2023 from $90,363 for the year ended December 31, 2022. Such decrease was due to
the shift from tech transfer activities to ongoing laboratory activities for the processing of tests. The revenue for 2022 was predominantly
for tech transfer of their lab developed test which yielded slightly higher revenue than for 2023, which was for ongoing laboratory activities
both of which were billed to them monthly. The agreement with the CLIAx customer includes future revenue sharing and co-marketing of their
test into the US market if we are involved in the selling of these tests.
Cost of revenues. Our cost of revenues
includes materials, labor, and laboratory expenses. Our cost of revenues decreased by $4,622,232, or 77.85%, to $1,315,166 for the year
ended December 31, 2023 from $5,937,398 for the year ended December 31, 2022. As a percentage of revenues, cost of revenues was 92.34%
and 53.69% for the years ended December 31, 2023 and 2022, respectively. This significant decrease was due to the significant decrease
in COVID-19 test revenue as detailed in the table below. The cost to provide COVID-19 testing in 2023 exceeded the revenue generated and
as a result we assessed the viability of generating revenue on COVID-19-related equipment and remaining inventory as described further
under loss on impairment of fixed assets below.
| | |
December 31, 2023 | | |
December 31, 2022 | |
| | |
Revenues | | |
Cost of Revenues | | |
Gross Profit | | |
Gross Margin | | |
Revenues | | |
Cost of Revenues | | |
Gross Profit | | |
Gross Margin | |
| COVID-19 Tests | |
$ | 252,520 | | |
$ | 260,556 | | |
$ | (8,036 | ) | |
| (3.18 | )% | |
$ | 10,490,708 | | |
$ | 5,508,534 | | |
$ | 4,982,174 | | |
| 47.49 | % |
| OneTest | |
| 921,502 | | |
| 939,924 | | |
| (18,422 | ) | |
| (2.00 | )% | |
| 323,414 | | |
| 333,354 | | |
| (9,940 | ) | |
| (3.07 | )% |
| BioCheck | |
| 187,926 | | |
| 100,335 | | |
| 87,591 | | |
| 46.61 | % | |
| 154,660 | | |
| 61,321 | | |
| 93,339 | | |
| 60.35 | % |
| CLIAx | |
| 62,356 | | |
| 14,351 | | |
| 48,005 | | |
| 76.99 | % | |
| 90,363 | | |
| 34,189 | | |
| 56,174 | | |
| 62.16 | % |
| | |
$ | 1,424,304 | | |
$ | 1,315,166 | | |
$ | 109,138 | | |
| 7.66 | % | |
$ | 11,059,145 | | |
$ | 5,937,398 | | |
$ | 5,121,747 | | |
| 46.31 | % |
Gross profit and gross margin. Our
gross profit decreased by $5,012,609, or 97.87%, to $109,138 for the year ended December 31, 2023 from $5,121,747 for the year ended December
31, 2022. Gross profit as a percentage of revenues (gross margin) was 7.66% and 46.31% for the years ended December 31, 2023 and 2022,
respectively. From the table above, it is evident that the costs to provide COVID-19 testing exceeded the revenue earned during 2023.
Fixed lab costs are allocated on a percent of revenue by product type basis thus putting downward pressure on all gross margins as COVID-19
revenue declined.
Sales, general and administrative expenses.
Our sales, general and administrative expenses include sales, marketing, office leases, overhead, executive compensation, legal, regulatory,
government relations, and similar expenses. Our sales, general and administrative expenses increased by $1,738,615, or 52.32%, to $5,061,450
for the year ended December 31, 2023 from $3,322,835 for the year ended December 31, 2022. As a percentage of revenues, sales, general
and administrative expenses were 355.36% and 30.05% for the years ended December 31, 2023 and 2022, respectively. Such increase was primarily
due to the recognition of $892,780 in stock compensation expense recorded upon the granting of stock options in 2023 as compared to $176,082
in 2022. Other attributors to the increase include sales and marketing costs for hiring of additional salespeople and advertising activities
in excess of the prior year by $341,219 and $397,859, respectively, as well as professional fees for accounting, legal, regulatory and
business development activities in excess of 2022 by $169,242 related to increased regulatory filings and negotiation of license agreements
for technology to enhance our product offerings.
Loss on impairment of fixed assets.
In the year ended December 31, 2023, we performed an impairment analysis of laboratory equipment utilized in COVID-19 testing due
to the significant material decrease in revenue and cash flow related to the COVID-19 testing and recorded an impairment charge of $209,073.
It was determined after discussion with lab personnel that certain PCR laboratory equipment could be repurposed for potential future products
and would be retained for research and development. The net book value of this equipment that remains in fixed assets equals $122,056
and will continue to be depreciated to research and development costs. As of December 31, 2023, we had no PCR testing inventory since
the supplies were expensed to research and development.
Research and development expenses.
Our research and development expenses include clinical data acquisitions, laboratory validation and bridging studies, data analysis algorithms,
and non-capitalizable machine learning software development. It also includes laboratory test validation and technical consultation. Our
research and development expenses increased by $1,289,107, or 1,073.87%, to $1,409,150 for the year ended December 31, 2023 from $120,043
for the year ended December 31, 2022. As a percentage of revenues, research and development expenses were 98.94% and 1.09% for the years
ended December 31, 2023 and 2022, respectively. Approximately 53%, or $745,522, of the expenses in 2023 were due to a focus on the LDT
validation of OneTest Premium (BioInfra I-Finder) technology with the remaining $663,628 distributed equally across the tech transfer
of LungSPOT (lung cancer test), cardiovascular disease algorithms development and a capillary blood collection method study.
Total other income (expense). We
had total other income, net, of $179,226 for the year ended December 31, 2023, as compared to other income, net, of $508,006 for the year
ended December 31, 2022. Total other income, net, for the year ended December 31, 2023 consisted of $209,150 of interest income offset
by $27,915 of interest expense and other expenses of $2,009, while total other income, net, for the year ended December 31, 2022 consisted
of other income of $452,899 related to the reversal of contingent liabilities and other estimated accruals no longer deemed a liability,
interest income of $68,421 and a gain on sale of asset of $2,371 related to equipment sales, offset by interest expense of $15,685.
Net income (loss). As a result of
the cumulative effect of the factors described above, we generated a net loss of $6,391,309 for the year ended December 31, 2023, as compared
to a net income of $2,186,875 for the year ended December 31, 2022, a decrease of $8,578,184, or 392.26%.
Liquidity and Capital Resources
As of December 31, 2023, we had cash and cash
equivalents of $4,089,461. Historically, our sources of cash have included private placements of equity securities and cash generated
from revenues.
Management has prepared estimates of operations
believes that sufficient funds will be generated from operations to fund our operations and to service our debt obligations for at least
the next twelve months. We may, however, in the future require additional cash resources due to changing business conditions, implementation
of our strategy to expand our business, or investments or acquisitions we may decide to pursue. If our own financial resources are insufficient
to satisfy our capital requirements, we may seek to sell additional equity or debt securities or obtain additional credit facilities.
The sale of additional equity securities could result in dilution to our stockholders. The incurrence of indebtedness would result in
increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations.
Financing may not be available in amounts or on terms acceptable to us, if at all. Any failure by us to raise additional funds on terms
favorable to us, or at all, could limit our ability to expand our business operations and could harm our overall business prospects.
Summary of Cash Flows
The following table provides detailed information
about our net cash flow for the period indicated:
| | |
Years Ended December 31, | |
| | |
2023 | | |
2022 | |
| Net cash provided by (used in) operating activities | |
$ | (4,479,971 | ) | |
$ | 5,803,059 | |
| Net cash used in investing activities | |
| (43,764 | ) | |
| (462,552 | ) |
| Net cash provided by (used in) financing activities | |
| (194,379 | ) | |
| 112,599 | |
| Net increase (decrease) in cash and cash equivalents | |
| (4,718,114 | ) | |
| 5,453,106 | |
| Cash and cash equivalents at beginning of period | |
| 8,807,575 | | |
| 3,354,469 | |
| Cash and cash equivalent at end of period | |
$ | 4,089,461 | | |
$ | 8,807,575 | |
Net cash used in operating activities was $4,479,971
for the year ended December 31, 2023, as compared to net cash provided by operating activities of $5,803,059 for the year ended December
31, 2022. Cash used in operating activities for the year ended December 31, 2023 was mainly attributed to the net loss of $6,391,309 and
the addition of non-cash adjustments that positively impact operating cashflows which includes $1,303,952 of stock-based compensation
and $209,073 of impairment of fixed assets. The remaining net decrease was primarily attributed to net positive cash from accounts receivable
of $696,090 offset by a reduction to accounts payable and accrued expenses of $521,113. Cash provided by operating activities for the
year ended December 31, 2022 was mainly attributed to our net income of $2,186,875 and the addition of non-cash adjustments that positively
impact operating cashflows which includes $290,218 of depreciation and amortization expense and $176,082 of stock-based compensation.
The remaining net increase was attributed to the following net positive changes in the asset accounts: net positive cash from accounts
receivable of $3,484,807 and net decrease in inventory and prepaid expenses of $159,584, and the net negative impact to operating cashflows
from reductions to accounts payable and accrued expenses of $720,299, offset from a net cash increase from deferred revenue of $163,524.
Net cash used in investing activities was $43,764
for the year ended December 31, 2023, as compared to $462,552 for the year ended December 31, 2022. The net cash used in investing activities
for the year ended December 31, 2023 consisted of the acquisition of technology under a license agreement of $34,381 and purchases of
capital equipment of $9,383, while the net cash used in investing activities for the year ended December 31, 2022 consisted of purchases
of capital equipment of $261,793 and acquisition of technology under a license agreement and related validation costs of $206,509, offset
by proceeds from sale of equipment of $5,750.
Net cash used in financing activities was $194,379
for the year ended December 31, 2023, as compared to net cash provided by financing activities was $112,599 for the year ended December
31, 2022. The net cash used in financing activities for the year ended December 31, 2023 consisted of deferred offering costs of $148,387
and principal payments on financing lease payments of $46,575, offset by net of proceeds from the exercise of warrants of $583, while
the net cash provided by financing activities for the year ended December 31, 2022 consisted the proceeds from notes payable of $183,166,
net of debt discount costs of $11,715 and proceeds from the exercise of warrants of $12, offset by principal payments on financing lease
liabilities of $58,864.
Convertible Note Offering
On August 15, 2022, we launched an equity crowdfunding
offering under Section 4(a)(6) of the Securities Act and Regulation Crowdfunding promulgated thereunder, pursuant to which we offered
convertible promissory notes. As of December 31, 2023, we issued convertible promissory notes in the aggregate principal amount of $213,010.
The notes bear interest at rates ranging from 6% to 11.10% and are due and payable twenty-four (24) months after the date of issuance.
The notes are unsecured, contain customary events of default and are convertible into common stock upon certain events. As of December
31, 2023, the outstanding balance of these notes is $229,164 consisting of principal of $213,010, net of unamortized debt issuance cost
of $4,980 and an accrued interest balance of $21,134.
Contractual Obligations
Our principal commitments consist mostly of obligations
under the convertible notes described above and the operating leases described under Item 1 “Business—Facilities.”
Other than indicated above, at December 31, 2023, we did not have other long-term debt obligations, capital (finance) lease obligations,
operating lease obligations, purchase obligations or other long-term liabilities reflected on our balance sheet.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that
have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues
or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
The preparation of financial statements in conformity
with United States generally accepted accounting principles requires our management to make assumptions, estimates and judgments that
affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified
certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important
for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important
to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex
judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in
subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and
because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments.
We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of
our financial statements:
Revenue Recognition. In accordance
with Accounting Standards Codification, or ASC, Topic 606, Revenue from Contracts with Customers, we recognize revenue when the
customer obtains control of promised goods or services, in an amount that reflects the consideration which we expect to receive in exchange
for those goods and services. To determine revenue recognition for arrangements that we deem are within the scope of ASC Topic 606, we
perform the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in
the contract; (iii) calculate transfer price; (iv) allocate the transaction price to the performance obligation in the contract;
and (v) recognize revenue when (or as) the entity satisfies a performance obligation. Performance obligations for four different
types of services are discussed below:
| ● | OneTest ‒ Revenues from the sale of OneTest are recognized when returned serum specimens are analyzed
in our CLIA laboratory and the results are reported to the customer. The specific transaction price is provided to the customer at the
time of purchase either through the on-line portal or via a sales quote for commercial clients, which may be discounted from list price
based on volume of tests ordered. Periodically, discounts are provided to individuals when purchased through our online portal. No estimates
or adjustments are made to the transaction price for returns or refunds, since these events rarely occur. There are three customer groups:
(i) individuals who purchase tests through our online portal; (ii) commercial clients that pay upfront for test kits and (iii) professional
health organizations that purchase collection kits and are all billed upon completion of testing and when results are reported to the
customer. Contracts with customers do not contain significant financing components based on the typical period between performance of
services and collection of consideration. There are very little requests for returns or refunds. |
| ● | BioCheck ‒ Revenues for kits are recognized when kits are shipped to the customer. The specific
transaction price is provided to the customer at the time of purchase, which may be discounted from list price based on the volume of
tests ordered. No estimates or adjustments are made to the transaction price for returns or refunds, since these events rarely occur.
Customers’ payment terms are due upon receipt and are not provided significant financing components based on the typical period
between shipment of the product and collection of consideration. There are no requests for returns or refunds. |
| o | Point-of-Care (POC) Test Kits ‒ Revenues for COVID-19 distributed test kits for use at the POC (i.e.,
rapid antigen and antibody tests) are recognized when test kits are shipped to the customer based on negotiated prices per individual
contracts. Customers’ payment terms are due upon receipt of the invoice and are not provided significant financing components based
on the typical period between shipment of the product and collection of consideration. There are no requests for returns or refunds. |
| o | COVID-19 Lab Tests (PCR) ‒ Revenues from the sale of COVID-19 viral (PCR) tests are recognized when
returned nasal swabs are analyzed in our CLIA laboratory and the results are reported to the customer. |
| § | For direct billing to customers, revenue is recorded based on the agreed contracted amount for each test
completed. Customers’ payment terms are net 30 days and are not provided significant financing components based on the typical period
between completed tests and collection of consideration. |
| § | For insurance, we estimate the amount of consideration we expect to be entitled to receive from customer
groups in exchange for providing services using the portfolio approach practical expedient. The use of the expedient is not expected to
differ materially from applying the guidance to an individual contract. These estimates are based on utilizing the expected value method
and include the impact of contractual allowances (including payer denials). The portfolios determined using the portfolio approach consist
of the following groups of customers which are similar since they are all insurance providers with similar reimbursement practices: healthcare
insurers and government payers (Medicare and Medicaid programs). The process for estimating revenues and the ultimate collection of accounts
receivable involves significant judgment and estimation. We follow a standard process, which considers historical denial and collection
experience and other factors (including the period of time that the receivables have been outstanding), to estimate contractual allowances
and recording adjustments in the current period as changes in estimates. Further adjustments to the allowances, based on actual receipts,
may be recorded upon settlement. We rely on a third part billing company to process all claims to be paid by insurance providers. As a
result, the average days to receive payment on these types of claims exceeds ninety days in some cases. As of December 31, 2023, we were
owed $2,078 from insurance companies. These claims are no longer billable directly to the customer and if not reimbursed by the insurance
providers, the balance will be written off against the allowance for doubtful accounts. |
| ● | CLIAx – Contractually, we can earn revenue in two ways: (i) by providing laboratory services and
(ii) through co-marketing activities of the CLIAx clients laboratory developed tests. Revenue for laboratory services is recognized monthly
based on agreed laboratory activities for space, equipment use and contracted personnel. The revenue that can be earned through co-marketing
activities would be recognized if we sell any of the customer’s products. As of December 31, 2023, the CLIAx customer is working
through its marketing plan and we have not yet performed any co-marketing activities and as a result have not sold any CLIAx products
or recognized any related revenue. |
Impairment of Long-Lived Assets.
The long-lived assets held and used by us are reviewed for impairment no less frequently than annually or whenever events or changes in
circumstances indicate that the carrying amount of an asset may not be recoverable. In the event that facts and circumstances indicate
that the cost of any long-lived assets may be impaired, an evaluation of recoverability is performed. The impairment losses for the year
ended December 31, 2023 and 2022 were $209,073 and $0 for certain fixed assets, respectively. There can be no assurance, however, that
market conditions will not change or demand for our products and services will continue, which could result in impairment of long-lived
assets in the future.
Preferred Stock.
ASC 480, Distinguishing Liabilities from Equity, includes standards for how an issuer of equity (including equity shares issued
by consolidated entities) classifies and measures on its balance sheet certain financial instruments with characteristics of both liabilities
and equity. Management is required to determine the presentation for the preferred stock as a result of the redemption and conversion
provisions, among other provisions in the agreement. Specifically, management is required to determine whether the embedded conversion
feature in the preferred stock is clearly and closely related to the host instrument, and whether the bifurcation of the conversion feature
is required and whether the conversion feature should be accounted for as a derivative instrument. If the host instrument and conversion
feature are determined to be clearly and closely related (both more akin to equity), derivative liability accounting under ASC 815, Derivatives
and Hedging, is not required. Management determined that the host contract of the preferred stock is more akin to equity, and accordingly,
derivative liability accounting is not required by us. Costs incurred directly for the issuance of the preferred stock are recorded as
a reduction of gross proceeds received by us.
Shipping and Handling. Amounts billed
to a customer for shipping and handling are reported as revenues. Costs related to shipments to the Company are classified as cost of
sales and totaled $134,824 and $258,837 for the years ended December 31, 2023 and 2022, respectively.
Research and Development. We incur
research and development costs during the process of researching and developing our laboratory tests, algorithms, information technologies,
and other intellectual properties. Our research and development costs consist primarily of data acquisition and personnel costs of scientists
and laboratory technicians. We expense these costs as incurred until the resulting product has been completed, tested, validated, and
made ready for commercial use.
Stock-Based Compensation. We account
for stock awards issued under ASC 718, Compensation – Stock Compensation. Under ASC 718, stock-based compensation cost is
measured at the grant date, based on the estimated fair value of the award. Stock-based compensation is recognized as an expense over
the employee’s requisite vesting period and over the non-employee’s period of providing goods or services. The fair value
of each stock option or warrant award is estimated on the date of grant using the Black-Scholes option valuation model. Restricted shares
are measured based on the fair market value of the underlying stock on the grant date.
Recently Issued Accounting Pronouncements
Management does not believe any other recently
issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying consolidated
financial statements.
ITEM 3. DIRECTORS AND OFFICERS
Directors and Executive Officers
The following table sets forth the name and position
of each of our current executive officers, directors, and significant employees.
| Name | |
Position | |
Age | | |
Term of Office | | |
Approximate hours per week for part-time employees | |
| Jonathan Cohen | |
Chief Executive Officer, President and Director | |
| 61 | | |
| From August 2000 | | |
| N/A | |
| Anne Shiflett | |
Acting Chief Financial Officer | |
| 58 | | |
| From April 2023 | | |
| 30 | |
| Jiming Zhou, Ph.D. | |
Chief Operating Officer | |
| 57 | | |
| From August 2020 | | |
| N/A | |
| Ron Baker | |
Chief Business Officer | |
| 73 | | |
| From October 2019 | | |
| N/A | |
| Michael Lebowitz, Ph.D. | |
Chief Scientific Officer | |
| 56 | | |
| From January 2020 | | |
| 24 | |
| John G. Compton, Ph.D. | |
Chairman of the Board | |
| 75 | | |
| From July 2016 | | |
| N/A | |
| Richard M. Cohen | |
Director | |
| 73 | | |
| From July 2016 | | |
| N/A | |
| Wei Lu | |
Director | |
| 40 | | |
| From June 2023 | | |
| N/A | |
| Prasanth Reddy | |
Director | |
| 49 | | |
| From November 2023 | | |
| N/A | |
| John W. Rollins | |
Director | |
| 79 | | |
| From November 2017 | | |
| N/A | |
| Michael A. Ross, M.D. | |
Director | |
| 74 | | |
| From July 2016 | | |
| N/A | |
Jonathan Cohen. Mr. Cohen is the
founder of our company and has served as Chief Executive Officer, President, and a director since its inception. He is a co-inventor of
two of our most successful products, OneTest and BioCheck, and has led the commercial launch and sales of both. He has also spearheaded
license, research, technology transfer, investment, and sales and marketing agreements with Fortune 500 companies such as Eastman Kodak,
Abbott Diagnostics, Johnson & Johnson, IBM, and Ping An, the largest health insurance company in China. Mr. Cohen has also been a
leading advocate in Annapolis, MD and on Capitol Hill on behalf of small and emerging biotechnology and diagnostics companies. Before
founding our company, Mr. Cohen was patent and general counsel for two publicly traded companies, Ventana Medical Systems Inc. (acquired
by Roche diagnostics in 2008), from 1999 to 2000, and Oncor Inc., from 1997 to 1999. Mr. Cohen is a registered patent attorney with more
than 25 years of experience in biotechnology patents and licensing matters. Mr. Cohen has a Master of Science Degree in Biotechnology
from Johns Hopkins University and a law degree from the American University. We believe that Mr.
Cohen is qualified to serve on our board of directors due to his experience in our industry and knowledge of our company.
Anne Shiflett. Ms. Shiflett has
served as our Acting Chief Financial Officer since April 1, 2023 and previously served as our Director of Finance from February 21, 2022
to March 31, 2023. Ms. Shiflett has over 30 years of managerial, financial and accounting experience, including expertise in leading the
start-up and rapid growth of new and emerging companies in information technology, real estate brokerage and life sciences. She has been
in the life sciences industry for the past eighteen years, most recently as chief business officer of Gypsy Basin Genomics from December
2020 to January 2022 and vice president, finance and administration at Catalent Pharma Solutions, Cell and Gene Therapy Business Unit
(formerly known as Paragon Bioservices, Inc.) from August 18, 2014 to September 1, 2020. Ms. Shiflett has been involved in raising over
$100 million in various forms of financing to include preferred stock, bridge financing, bank financing and venture back debt and in facilitating
the sale of Paragon Bioservices, Inc. to Catalent Pharma Solutions for $1.2 billion dollars in May 2019. Ms. Shiflett received a BBA in
Accounting and an MBA in Business Management from Loyola College of Maryland.
Jiming
Zhou, PhD. Dr. Zhou has served as our Chief Operating Officer since August 2020. He is an expert in healthcare and biotech industries,
with over 20 years of experience in both academia and industry. Dr. Zhou began his academic career as an associate professor at Sichuan
University in China, where he received his PhD of Biology. Afterward, he moved to the United States to conduct research at the University
of Iowa, where he spent 7 years publishing over 30 peer-reviewed research papers and receiving numerous grants and patents. In 2005, Dr.
Zhou transitioned into industrial R&D, where he led a joint pharmaceutical project that reached significant milestones totaling $330
million. He then went on to manage multiple clinical labs and co-founded companies, collaborating with prominent healthcare institutes
both in the US and China. Prior to joining us in July 2019, Dr. Zhou held various leadership roles, including serving as president and
co-founder of Baltimore-based biotech firm Firefox Pharmaceuticals, LLC from April 2017 to July 2019, partner and co-founder of Virginia-based
Fairfax Medical Consulting International, LLC from October 2013, and managing director of Diagnostic Operation and Strategic Alliance
of the Genetics and IVF Institute, an international company based in Virginia, from September 2009 to September 2013. Dr. Zhou’s
extensive experience in the biotech industry, along with his research expertise, make him a valuable member of our team. He continues
to play a crucial role in our success and growth.
Ron
Baker. Mr. Baker has served as our Chief Business Officer since October 2019 and previously served as our Director of Sales from
October 2019 to January 2023. Prior to joining us, he held executive management positions in clinical research, operations, technical,
sales, marketing and business development with international, national and start-up companies, all related to specialized oncology laboratory
services, including as executive director of U.S. sales for SGS Life Sciences (Belgium) from December 2006 to March 2018. He previously
worked with Roche Diagnostics and Roche Clinical Labs, International Clinical Labs and Molecular Oncology (start-up sold to Dianon). Mr.
Baker earned his BS in Biology from Loyola University.
Michael Lebowitz, Ph.D. Dr. Lebowitz
has served as our Chief Scientific Officer since January 2020 and was previously our Director of
Research & Development from 2009-2012. Dr. Lebowitz has more than 30 years of research experience, including 22 years in our industry
and more than 18 years in research management. He has been directly involved in the
commercial launch of six LDTs for the early detection of cancer and
the establishment of two CLIA-certified labs. He has also spearheaded the R&D supporting an anti-cancer vaccine from discovery through
phase I clinical development. He is concurrently chief scientific officer of Athanor Biosciences, Inc., a cancer therapeutics company
he cofounded in 2020. Prior to his current positions, he was senior director and vice president of research at Sensei Biotherapeutics
from 2014-2019. Dr. Lebowitz holds a Ph.D. from the Johns Hopkins University School of Medicine in biochemistry, cellular, and
molecular biology where he subsequently completed a three-year fellowship in immunology in the department of pathology, division of immunopathology.
He is currently an adjunct faculty at both Johns Hopkins University and University of Maryland,
Baltimore County teaching in their respective Biotechnology programs.
John G. Compton, Ph.D. Dr. Compton
has served as Chairman of the Board since July 2016. He has over 30 years of experience in the development and application of molecular
biological techniques to answer questions about genetics and epidermal differentiation and has authored more than 80 publications in the
field. Dr. Compton served as vice-president of BioReference Laboratories from 2007 to 2013. Previously, Dr. Compton was founder, and served
as scientific director and co-president of GeneDx Inc, from 2000 to 2006, the assets of which were acquired by BioReference Laboratories
(now part of Opko) in September 2006. Dr. Compton also serves as Mayor of the Town of Washington Grove, MD (2000-2008, 2018-present),
on the Board of Directors of Quertle Inc. and chairs the Boards of the non-profit BlackRock Center for the Arts and the Pinkney Center
for Science and Technology at Montgomery College Germantown Campus. Dr. Compton holds B.S. degrees in Physics and Biology from the Massachusetts
Institute of Technology, received his Ph.D. from the University of California, Berkeley, in Biophysics, and was a Staff Scientist at the
NIAMS, National Institutes of Health, Bethesda, from 1991-2000. In 2003, he was awarded the Entrepreneur of the Year award by the Technology
Council of Maryland. We believe that Dr. Compton is qualified to serve on our board of directors
due to his extensive experience in our industry.
Richard M. Cohen. Mr. Cohen has
served as a member of our board of directors since July 2016. He is an experienced CEO/CFO at public and private companies. His professional
experience includes biotech, financial services and diversified media and he maintains excellent contacts with capital financing sources
on and off Wall Street. He has been the president of Richard M Cohen Consultants since 1995, a company providing financial consulting
services to both public and private companies. From March 2012 to July 2015, he was the founder and managing partner of Chord
Advisors, a firm providing outsourced CFO services to both public and private companies. He was the chief executive officer and chief
financial officer of CorMedix Inc., a publicly traded medical device/biotechnology company with an intrapericardial therapy product targeted
to markets in the U.S. and Europe, from 2010 to 2013. He has served on the board of directors and audit committees of Ondas Holdings Inc.
(2018 to present), Helix BioMedix, Inc. (2006 to present), CorMedix Inc. (2010 to 2013), and Rodman & Renshaw (2008 to 2012). Mr.
Cohen’s academic credentials include an MBA from Stanford University and B.S. with honors from Wharton School, University of Pennsylvania.
We believe that Mr. Cohen is qualified to serve on our board of directors due to his extensive management
and board experience.
Wei Lu. Ms. Lu has served as a member
of our board of directors since June 2023. Ms. Lu has over 10 years of experience in private equity investment and post investment management.
She has served as the Vice President of Ping An Ventures since January 2019, where she is mainly responsible for post investment management
of medical investments, including biotechnology, medical devices, medical services, etc. Ms. Wei Lu holds Master’s degree in Finance
from Chongqing University.
Prasanth Reddy. Dr. Reddy has served
as a member of our board of directors since November 2023. He is triple board-certified in internal medicine, medical oncology,
and hematology, and practiced medicine and served in leadership positions for more than 14 years in various clinical settings including
academia, private practice, managed care, and life sciences. Dr. Reddy was most recently senior vice president, global enterprise oncology
head of Labcorp from January 2021 to July 2023. Previously he served as vice president of medical affairs at Foundation Medicine from
February 2018 to December 2020. He currently serves in the Air Force Reserve as a Lt Colonel. Dr. Reddy earned a bachelor’s degree
in microbiology and psychology from Kansas State University, and a medical degree from the University of Kansas Medical Center, where
he also completed his internal medicine residency and clinical hematology and oncology fellowship. Dr. Reddy has a master’s degree
in public health and is an alumnus of Harvard Business School. Additionally, he is a fellow of the American College of Physicians and
is a Certified Physician Executive. We believe that Dr. Reddy is qualified to serve on our board of directors due to his extensive
experience in our industry.
John W. Rollins. Mr. Rollins has
served as a member of our board of directors since November 2017. He has served on multiple boards and chairs the board of directors of
the MedStar Southern Maryland Hospital Center (2014 to present). From 2001 to 2010, he taught Entrepreneurship at the George Washington
University School of Business and founded the GW New Venture Competition and served as its Director from 2007 to 2014. In 2003, Mr. Rollins
founded StreamCenter, Inc., a firm that pioneered online education using video streaming, and served as chair of the board of directors
from 2003 to 2008, and chief executive officer from 2008 to 2010. Prior to 2001, he founded and served for three decades as the chief
executive officer and chairman of AZTECH Software Corporation, the nation’s first specialized provider of information technology
services to non-profit organizations. Mr. Rollins’s board experience has included serving as Trustee of the National Park Trust
(Vice Chair and Treasurer) (1990 to present), Director of the MedStar Georgetown University Hospital (Vice Chair) (2002 to 2013), the
Washington Hospital Center (Vice Chair and Treasurer) (1977 to 2002), and the U.S. Association for Small Business & Entrepreneurship
(2004 to 2006). Mr. Rollins earned his A.B. in Mathematics from Dartmouth and his M.B.A. in Finance from the Stanford University Graduate
School of Business. We believe that Mr. Rollins is qualified to serve on our board of directors
due to his extensive board experience.
Michael A. Ross, M.D. Dr. Ross has
served as a member of our board of directors since July 2016. He has served as the chairman and chief executive
officer of Euclid Systems Corporation since 2015, where he led the growth of this ophthalmic medical device company from $3.1 million
to over $20 million in five years. The bulk of Euclid’s sales are in China and East Asia where Dr. Ross visits 4-5 times per year.
Prior to joining Euclid, he was chief executive officer of E-P Therapeutics from 2010 to 2012, and was a medical and scientific advisor
to StemCyte, Inc. 2009 to 2010. He is Board-certified in Obstetrics and Gynecology and is a founding member of an OB-GYN-Infertility
practice in Northern Virginia from 1980 to 2007. Dr. Ross has been a Clinical Professor of Obstetrics and Gynecology, George
Washington University Medical Center since 1979, and has served on the boards of directors of several biotech and medical device companies. He
has a B.S. in Chemistry and Biology from Dickinson College and an M.D. from George Washington University. We
believe that Dr. Ross is qualified to serve on our board of directors due to his extensive experience in our industry.
Our directors currently have terms which will
end at our next annual meeting of the stockholders or until their successors are elected and qualify, subject to their prior death, resignation
or removal. Officers serve at the discretion of the board of directors.
Wei Lu
was elected by the holders of our series A-1 preferred stock and Mr. Rollins was elected by the holders of all series of our preferred
stock. Except for the rights of such holders to elect a director, which will expire upon conversion of such shares, there are no agreements
or understandings for any of our executive officers or directors to resign at the request of another person and no officer or director
is acting on behalf of nor will any of them act at the direction of any other person.
Family Relationships
There are no family relationships between any
director, executive officer, person nominated or chosen to become a director or executive officer or any significant employee.
Legal Proceedings
To the best of our knowledge, none of our directors
or executive officers has, during the past five years:
| ● | been convicted in a criminal proceeding (excluding traffic violations and other minor offences); or |
| ● | had any petition under the federal bankruptcy laws or any state insolvency law was filed by or against,
or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership
in which he was general partner at or within two years before the time of such filing, or any corporation or business association of which
he was an executive officer at or within two years before the time of such filing. |
Corporate Governance
Our board of directors currently has three standing
committees, an audit committee, a compensation committee and nominating and corporate governance committee, which perform various duties
on behalf of and report to the board of directors. From time to time, the board of directors may establish other committees.
The Board’s Role in Risk Oversight
The board of directors oversees that the assets
of our company are properly safeguarded, that the appropriate financial and other controls are maintained, and that our business is conducted
wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the board’s
oversight of the various risks facing our company. In this regard, our board seeks to understand and oversee critical business risks.
Our board does not view risk in isolation. Risks are considered in virtually every business decision and as part of our business strategy.
Our board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is
essential for our company to be competitive on a global basis and to achieve its objectives.
While the board oversees risk management, company
management is charged with managing risk. Management communicates routinely with the board and individual directors on the significant
risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management.
Our board administers its risk oversight function
as a whole by making risk oversight a matter of collective consideration; however, much of the work is delegated to committees, which
meet regularly and report back to the full board. We have established a standing audit committee, compensation committee and nominating
and corporate governance committee of our board of directors. The audit committee oversees risks related to our financial statements,
the financial reporting process, and accounting and legal matters, the compensation committee evaluates the risks and rewards associated
with our compensation philosophy and programs, and the nominating and corporate governance committee evaluates risks associated with management
decisions and strategic direction.
Audit Committee
Richard M. Cohen, John G. Compton and Michael
A. Ross currently serve on our audit committee, with Mr. Cohen serving as chairman. The audit committee oversees our accounting and financial
reporting processes and the audits of the financial statements of our company.
The audit committee is responsible for, among
other things: (i) retaining and overseeing our independent accountants; (ii) assisting the board in its oversight of the integrity of
our financial statements, the qualifications, independence and performance of our independent auditors and our compliance with legal and
regulatory requirements; (iii) reviewing and approving the plan and scope of the internal and external audit; (iv) pre-approving any audit
and non-audit services provided by our independent auditors; (v) approving the fees to be paid to our independent auditors; (vi) reviewing
with our chief executive officer and chief financial officer and independent auditors the adequacy and effectiveness of our internal controls;
(vii) reviewing hedging transactions; and (viii) reviewing and approving related party transactions; and (ix) reviewing and assessing
annually the audit committee’s performance and the adequacy of its charter.
Compensation Committee
Richard M. Cohen, John G. Compton and John W.
Rollins currently serve on our compensation committee, with Mr. Rollins serving as chairman. The compensation committee assists the board
in reviewing and approving the compensation structure, including all forms of compensation relating to our directors and executive officers.
The compensation committee is responsible for,
among other things: (i) reviewing and approving the remuneration of our executive officers; (ii) determining the compensation of our independent
directors; (iii) making recommendations to the board regarding equity-based and incentive compensation plans, policies and programs; and
(iv) reviewing and assessing annually the compensation committee’s performance and the adequacy of its charter.
Nominating and Corporate Governance Committee
Richard M. Cohen, John G. Compton and Michael
A. Ross currently serve on our nominating and corporate governance committee, with Mr. Ross serving as the chair. The nominating and corporate
governance committee assists the board of directors in selecting individuals qualified to become our directors and in determining the
composition of the board and its committees.
The nominating and corporate governance committee
is responsible for, among other things: (i) recommending the number of directors to comprise our board; (ii) identifying and evaluating
individuals qualified to become members of the board; (iii) recommending to the board the director nominees for each annual stockholders’
meeting; (iv) recommending to the board the candidates for filling vacancies that may occur between annual stockholders’ meetings;
(v) reviewing independent director compensation and board processes, self-evaluations and policies; (vi) overseeing compliance with our
code of ethics; and (vii) monitoring developments in the law and practice of corporate governance.
The nominating and corporate governance committee’s
methods for identifying candidates for election to our board of directors (other than those proposed by our stockholders, as discussed
below) will include the solicitation of ideas for possible candidates from a number of sources - members of our board of directors, our
executives, individuals personally known to the members of our board of directors, and other research. The nominating and corporate governance
committee may also, from time-to-time, retain one or more third-party search firms to identify suitable candidates.
In making director recommendations, the nominating
and corporate governance committee may consider some or all of the following factors: (i) the candidate’s judgment, skill, experience
with other organizations of comparable purpose, complexity and size, and subject to similar legal restrictions and oversight; (ii) the
interplay of the candidate’s experience with the experience of other board members; (iii) the extent to which the candidate would
be a desirable addition to the board and any committee thereof; (iv) whether or not the person has any relationships that might impair
his or her independence; and (v) the candidate’s ability to contribute to the effective management of our company, taking into account
the needs of our company and such factors as the individual’s experience, perspective, skills and knowledge of the industry in which
we operate.
A stockholder may nominate one or more persons
for election as a director at an annual meeting of stockholders if the stockholder complies with the notice and information provisions
contained in our bylaws. Such notice must be in writing to our company not less than 90 days and not more than 120 days prior
to the anniversary date of the preceding year’s annual meeting of stockholders or as otherwise required by requirements of the Exchange
Act. In addition, stockholders furnishing such notice must be a holder of record on both (i) the date of delivering such notice and
(ii) the record date for the determination of stockholders entitled to vote at such meeting.
Compensation of Directors and Executive Officers
Summary Compensation Table
The following table sets forth information concerning
all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the
noted periods. No other executive officers received total annual salary and bonus compensation in excess of $100,000.
| Name and Principal Position | |
Year | | |
Salary ($) | | |
Bonus ($) | | |
Option Awards ($)(1) | | |
All Other Compensation ($) | | |
Total ($) | |
| Jonathan Cohen, | |
| 2023 | | |
| 250,000 | | |
| 200,000 | | |
| 481,120 | | |
| 45,364 | | |
| 976,484 | |
| Chief Executive Officer(2) | |
| 2022 | | |
| 250,000 | | |
| 150,000 | | |
| - | | |
| 44,936 | | |
| 444,936 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Jiming Zhou, | |
| 2023 | | |
| 215,000 | | |
| 160,000 | | |
| 436,480 | | |
| - | | |
| 811,480 | |
| Chief Operating Officer | |
| 2022 | | |
| 200,004 | | |
| 180,726 | | |
| - | | |
| - | | |
| 380,730 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Anne Shiflett, Acting | |
| 2023 | | |
| 112,500 | | |
| - | | |
| 130,000 | | |
| 61,431 | | |
| 303,931 | |
| Chief Financial Officer | |
| 2022 | | |
| - | | |
| - | | |
| - | | |
| 120,750 | | |
| 120,750 | |
| (1) | The amount is equal to the aggregate grant-date fair value with
respect to the awards, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. |
| (2) | Other compensation represents fringe benefits for insurance and
employer 401(k) matches. |
Employment Agreements
On May 6, 2019, we entered into an employment
agreement with Jonathan Cohen, our founder, Chief Executive Officer and President, with an initial term commencing as of January 1, 2019
and ending on December 31, 2019, which automatically renews for additional one (1) year periods unless either party provides written notice
at least sixty (60) days prior to the expiration of the initial term or any renewal period. Pursuant to the employment agreement, Mr.
Cohen is entitled to an annual base salary of $250,000. Mr. Cohen will also be entitled to a cash bonus for 2019 of up to 30% of the base
salary at the discretion of the compensation committee and based on certain criteria set forth in the employment agreement, which shall
be paid within 60 days after year end. Following sharp increases in revenues resulting from COVID-19 testing, the cash bonus cap was increased
to 60% and 80% of base salary for 2021 and 2022. Mr. Cohen is also permitted during the term, if and to the extent eligible, to participate
in all employee benefit plans, policies and practices maintained by or on behalf of our company commensurate with Mr. Cohen’s position.
Either party may terminate the employment agreement at any time without cause (as defined in the employment agreement) upon sixty (60)
days’ written notice. In addition, we may terminate the employment agreement immediately for cause. If we terminate the employment
agreement without cause, all compensation payable to Mr. Cohen under the employment agreement shall cease as of the date of termination,
and we shall pay to Mr. Cohen the following sums: (i) the base salary on the termination date for twelve (12) months (the applicable period
being referred to as the severance period), payable in equal installments in accordance with our normal payroll procedures beginning with
the termination date; (ii) benefits under group health and life insurance plans in which Mr. Cohen participated prior to termination through
the severance period; (iii) all previously earned, accrued, and unpaid benefits from us and our employee benefit plans, including any
such benefits under our pension, disability, and life insurance plans, policies, and programs; and (iv) bonus, if any, at the discretion
of the compensation committee; provided that if, prior to the date on which our foregoing obligations cease, Mr. Cohen violates certain
covenants set forth in the employment agreement, then we shall have no obligation to make any of the payments that remain payable by us
under clauses (i), (ii) and (iv) above on or after the date of such violation. The payment of severance may be conditioned by us on the
delivery by Mr. Cohen of a release of any and all claims that he may have against our company. In addition, if the employment agreement
is terminated by us for cause, then Mr. Cohen is only entitled to receive the amounts specified in clause (iii), and if the employment
agreement is terminated by Mr. Cohen or due to his death or disability, then Mr. Cohen (or his estate or representative as applicable)
shall receive only the amounts specified in clauses (iii) and (iv). In the event that the term expires and is not renewed by us, then
Mr. Cohen shall receive the amounts specified in clauses (i), (ii), (iii) and (iv), provided however, that this shall not apply if we
enter into a new employment agreement with Mr. Cohen. Finally, in the event that the employment agreement is terminated by us within one
year following a change of control (as defined in the employment agreement), then Mr. Cohen shall receive, in addition to the amount of
any accrued and unpaid salary then due Mr. Cohen, the amounts specified in clauses (i), (ii), (iii) and (iv). Mr. Cohen’s employment
agreement contains restrictive covenants prohibiting him from owning or operating a business that competes with our company or soliciting
our customers or employees for one year following the termination of his employment.
As of April 1, 2023, we have agreed to pay Jiming
Zhou, our Chief Operating Officer, an annual salary of $220,000 and he is also eligible for (i) a bonus equal to 4% of our gross profit
and (ii) a bonus equal to 20% of our revenues derived from China and Taiwan, each as determined by our independent registered public accounting
firm in accordance with GAAP. He is also eligible for discretionary bonuses, as determined by our board of directors, for all investments
or business endeavors in China and Taiwan, for all new products launched in 2023 and based on efficiency, execution, speed, and regulatory
compliance of all clinical laboratory operations. Mr. Zhou is also permitted, if and to the extent eligible, to participate in all employee
benefit plans, policies and practices maintained by or on behalf of our company commensurate with his position.
As of April 1, 2023, we have agreed to pay Anne
Shiflett, our Acting Chief Financial Officer, an annual salary of $150,000 and she is also eligible for a bonus tied to financial raises
prorated based on amount raised and for discretionary bonuses, as determined by our board of directors. Ms. Shiflett is also permitted,
if and to the extent eligible, to participate in all employee benefit plans, policies, and practices maintained by or on behalf of our
company commensurate with her position.
Retirement Benefits
We have not maintained, and do not currently maintain,
a defined benefit pension plan or nonqualified deferred compensation plan. We currently make available a retirement plan intended to provide
benefits under Section 401(k) of the Internal Revenue Code of 1986, as amended, or the Code, pursuant to which employees, including
the executive officers named above, can make voluntary pre-tax contributions. We match 3.5% of the first 6% of employee contributions.
Potential Payments Upon Termination or Change in Control
As described under “—Employment
Agreements” above, Mr. Cohen is entitled to severance under certain circumstances described above.
Outstanding Equity Awards at Fiscal Year-End
The following table includes certain information
with respect to the value of all unexercised options and unvested shares of restricted stock previously awarded to the executive officers
named above at the fiscal year ended December 31, 2023.
| | |
Option Awards |
| Name | |
Number of Securities Underlying Unexercised Options (#) Exercisable | | |
Number of Securities Underlying Unexercised Options (#) Unexercisable | | |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | | |
Option Exercise Price ($) | | |
Option Expiration Date |
| Jonathan Cohen | |
| 287,779 | | |
| 100,221 | | |
| - | | |
$ | 1.74 | | |
01/01/2033 |
| Jiming Zhou | |
| 256,667 | | |
| 95,333 | | |
| - | | |
$ | 1.74 | | |
01/01/2033 |
| Anne Shiflett | |
| 0 | | |
| 100,000 | | |
| - | | |
$ | 1.74 | | |
01/01/2033 |
Director Compensation
The table below sets forth the compensation paid
to our independent directors during the fiscal year ended December 31, 2023.
| Name | |
Fees Earned or Paid in Cash
($) | | |
Option Awards
($)(1) | | |
Total
($) | |
| John G. Compton | |
| 20,000 | | |
| 39,300 | | |
| 59,300 | |
| Richard M. Cohen | |
| 17,500 | | |
| 39,300 | | |
| 56,800 | |
| Ming Li(2) | |
| - | | |
| - | | |
| - | |
| Wei Lu(2) | |
| - | | |
| - | | |
| - | |
| Prasanth Reddy(3) | |
| 2,500 | | |
| - | | |
| 2,500 | |
| John W. Rollins | |
| 17,500 | | |
| 39,300 | | |
| 56,800 | |
| Michael A. Ross | |
| 15,000 | | |
| 39,300 | | |
| 54,300 | |
| (1) | The amount is equal to the aggregate grant-date fair value with respect to the awards, computed in accordance
with Financial Accounting Standards Board Accounting Standards Codification Topic 718. |
| (2) | In June 2023, Ming Li resigned from the board and was replaced by Wei Lu. |
| (3) | Prasanth Reddy was appointed to the board in November 2023. |
Effective as of January 1, 2022, our independent
directors, except for Ming Li and Wei Lu, who represent an investor, are paid a cash fee of $15,000 per year, payable quarterly, with
the chairman receiving an additional $5,000 per year and the committee chairs receiving an additional $2,500 per year.
On January 1, 2023, each independent director,
except for Ming Li, was granted an option for the purchase of 30,000 shares of common stock that vests monthly over one year, each at
an exercise price of $1.74 per share.
Stock Incentive Plan
On January 26, 2022, our board of directors adopted
the 20/20 GeneSystems, Inc. 2022 Stock Incentive Plan, or the Plan, which was approved by stockholders on June 15, 2022. Awards that may
be granted include incentive stock options as described in section 422(b) of the Code, non-qualified stock options (i.e., options that
are not incentive stock options) and awards of restricted stock. These awards offer our employees, consultants, advisors and outside directors
the possibility of future value, depending on the long-term price appreciation of our common stock and the award holder’s continuing
service with our company or one or more of its subsidiaries.
All of the permissible types of awards under the
Plan are described in more detail as follows:
Purposes of Plan: The purpose
of the Plan is to offer selected employees, consultants, advisors and outside directors the opportunity to acquire equity in our company.
Administration of the Plan: Administration
of the Plan is entrusted to the compensation committee of the board of directors. Among other things, the committee has the authority
to select persons who will receive awards, determine the types of awards and the number of shares to be covered by awards, and to establish
the terms, conditions, restrictions and other provisions of awards.
Eligible Recipients: Persons
eligible to receive awards under the Plan will be those employees, consultants, advisors and outside directors of our company and its
subsidiaries who are selected by the compensation committee.
Shares Available Under the Plan: The
maximum number of shares of common stock that may be delivered to participants under the Plan is 3,000,000, subject to adjustment for
certain corporate changes affecting the shares, such as stock splits. Shares subject to an award under the Plan for which the award is
canceled, forfeited or expires again become available for grants under the Plan. Shares subject to an award that is settled in cash will
not again be made available for grants under the Plan.
Stock Options:
General. Subject to the provisions
of the Plan, the compensation committee has the authority to determine all grants of stock options. That determination will include: (i)
the number of shares subject to any option; (ii) the exercise price per share; (iii) the expiration date of the option; (iv) the manner,
time and date of permitted exercise; (v) other restrictions, if any, on the option or the shares underlying the option; and (vi) any other
terms and conditions as the compensation committee may determine.
Option Price. The exercise price for stock
options will be determined at the time of grant. Normally, the exercise price will not be less than the fair market value on the date
of grant, as determined in good faith by the compensation committee. As a matter of tax law, the exercise price for any incentive stock
option awarded may not be less than the fair market value of the shares on the date of grant. However, incentive stock option grants to
any person owning more than 10% of our voting stock must have an exercise price of not less than 110% of the fair market value on the
grant date.
Exercise of Options. An option may
be exercised only in accordance with the terms and conditions for the option agreement as established by the compensation committee at
the time of the grant. The option must be exercised by notice to us, accompanied by payment of the exercise price. Payments may be made
in cash or, at the option of the compensation committee, by actual or constructive delivery of shares of common stock to the holder of
the option based upon the fair market value of the shares on the date of exercise.
Expiration or Termination. Options,
if not previously exercised, will expire on the expiration date established by the compensation committee at the time of grant; provided
that such term cannot exceed ten years and that such term of an incentive stock option granted to a holder of more than 10% of our voting
stock cannot exceed five years. Options will terminate before their expiration date if the holder’s service with us terminates before
the expiration date. The option may remain exercisable for specified periods after certain terminations of service, including terminations
as a result of death, disability or retirement, with the precise period during which the option may be exercised to be established by
the compensation committee and reflected in the grant evidencing the award.
Stock Awards: Stock awards
can also be granted under the Plan. A stock award is a grant of shares of common stock. These awards will be subject to such conditions,
restrictions and contingencies as the compensation committee shall determine at the date of grant. Those may include requirements for
continuous service and/or the achievement of specified performance goals.
Other Material Provisions: Awards
will be evidenced by a written agreement, in such form as may be approved by the compensation committee. In the event of various changes
to the capitalization of our company, such as stock splits, stock dividends and similar re-capitalizations, an appropriate adjustment
will be made by the compensation committee to the number of shares covered by outstanding awards or to the exercise price of such awards.
The compensation committee is also permitted to include in the written agreement provisions that provide for certain changes in the award
in the event of a change of control of our company, including acceleration of vesting. Except as otherwise determined by the compensation
committee at the date of grant, awards will not be transferable, other than by will or the laws of descent and distribution. Prior to
any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any employee withholding tax requirements.
The board also has the authority, at any time, to discontinue the granting of awards. The board also has the authority to alter or amend
the Plan or any outstanding award or may terminate the Plan as to further grants, provided that no amendment will, without the approval
of our stockholders, increase the number of shares available under the Plan or change the persons eligible for awards under the Plan.
No amendment that would adversely affect any outstanding award made under the Plan can be made without the consent of the holder of such
award.
Except as set forth above, we do not have any
ongoing plan or arrangement for the compensation of directors and executive officers.
ITEM 4. SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table sets forth information regarding
beneficial ownership of our voting stock as of April 25, 2024 (i) by each of our executive officers and directors who beneficially owns
more than 10% of any class of our voting securities; (ii) by all of our executive officers and directors as a group; and (iii) by each
person who is known by us to beneficially own more than 10% of any class our voting securities. Since none of the foregoing own any of
our series A-1 preferred stock, series B preferred stock or series C preferred stock, we have excluded columns for these shares from the
table below. Unless otherwise specified, the address of each of the persons set forth below is in care of our company at 15810 Gaither
Road, Suite 235, Gaithersburg, MD 20877.
| | |
Amount Acquirable(1) | | |
| | |
Percent of | | |
Percent of | | |
Percent of | |
| Name and Address of Beneficial Owner | |
Total
Common
Stock | | |
Series A
Preferred
Stock | | |
Series A-2
Preferred
Stock | | |
Percent of
Common
Stock(2) | | |
Series A
Preferred
Stock(3) | | |
Series A-2
Preferred
Stock(4) | | |
Total
Voting
Stock(5) | |
| Jonathan Cohen(6) | |
| 1,694,595 | | |
| 0 | | |
| 0 | | |
| 33.22 | % | |
| * | | |
| * | | |
| 17.44 | % |
| All directors and officers as a group(7) | |
| 3,054,005 | | |
| 13,029 | | |
| 21,535 | | |
| 60.34 | % | |
| 1.54 | % | |
| 4.87 | % | |
| 31.92 | % |
| (1) | Beneficial Ownership is determined in accordance with the rules
of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has
direct ownership of and sole voting power and investment power with respect to the shares. For each beneficial owner above, any securities
acquirable within 60 days have been included in the denominator in accordance with SEC Rule 13d-3(d)(1). |
| (2) | Based on 4,773,128 shares of our common stock outstanding as
of April 25, 2024. |
| (3) | Based on 846,368 shares of our Series A Preferred Stock outstanding
as of April 25, 2024. |
| (4) | Based on 442,402 shares of our Series A-2 Preferred Stock outstanding
as of April 25, 2024. |
| (5) | percentage of total voting stock represents total ownership
with respect to all shares of our common stock, series A preferred stock, series A-1 preferred stock, series A-2 preferred stock, series
B preferred stock and series C preferred stock, as a single class and on an as-converted to common stock basis. As of April 25, 2024,
there were 651,465 shares of series A-1 preferred stock, 1,471,487 shares of series B preferred stock and 1,204,040 shares of series
C preferred stock issued and outstanding. Shares of series A preferred stock, series A-1 preferred stock, series A-2 preferred stock,
series B preferred stock and series C preferred stock are convertible into shares of common stock on the basis of 1 share of common stock
for each share of such preferred stock (subject to adjustment). Holders of series A preferred stock, series A-1 preferred stock, series
A-2 preferred stock, series B preferred stock and series C preferred stock vote with the holders of common stock on all matters on an
as-converted to common stock basis. |
| (6) | Includes 1,366,400 shares of common stock and options for the
purchase of 328,195 shares of common stock exercisable within 60 days. |
| (7) | Includes 1,384,177 shares of common stock, options for the purchase
of 1,667,162 shares of common stock and warrants for the purchase of 2,666 shares of common stock exercisable within 60 days. |
ITEM 5. INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
Since the beginning of our 2022 fiscal year, we
have not entered into any transactions, nor is there any currently proposed transaction, in which we were or are to be a participant and
the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last
two completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation
described under Item 3 “Directors and Officers—Compensation of Directors and Executive Officers” above).
ITEM 6. OTHER INFORMATION
We have no information to disclose that was required
to be in a report on Form 1-U during the last six months of the fiscal year ended December 31, 2023, but was not reported.
ITEM 7. FINANCIAL STATEMENTS
INDEX TO FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the Board of Directors and Stockholders
20/20 GeneSystems, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets
of 20/20 GeneSystems, Inc. (the “Company”) as of December 31, 2023 and 2022, and the related statement of operations, stockholders’
equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”).
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December
31, 2023 and 2022, and the results of their operations and their cash flows for each of the years then ended, in conformity with accounting
principles generally accepted in the United States of America.
Basis for Opinion
The financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ dbbmckennon
San Diego, California
April 26, 2024
We have been the Company’s auditor since 2018.
20/20 GENESYSTEMS, INC.
BALANCE SHEETS
DECEMBER 31, 2023 AND 2022
| | |
2023 | | |
2022 | |
| Assets | |
| | |
| |
| Current assets: | |
| | |
| |
| Cash and cash equivalents | |
$ | 4,089,461 | | |
$ | 8,807,575 | |
| Accounts receivable, net | |
| 68,834 | | |
| 764,924 | |
| Inventory | |
| 60,668 | | |
| 87,074 | |
| Prepaid expenses | |
| 81,469 | | |
| 72,270 | |
| Total current assets | |
| 4,300,432 | | |
| 9,731,843 | |
| License agreement, net | |
| 316,143 | | |
| 340,929 | |
| Property and equipment, net | |
| 244,203 | | |
| 580,911 | |
| Intangible assets, net | |
| 210,386 | | |
| 179,403 | |
| Right of use assets | |
| 933,394 | | |
| 1,088,783 | |
| Due from affiliated entities | |
| - | | |
| 2,699 | |
| Deferred offering costs | |
| 148,387 | | |
| - | |
| Other assets | |
| 214,883 | | |
| 290,453 | |
| Total assets | |
$ | 6,367,828 | | |
$ | 12,215,021 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 360,279 | | |
$ | 464,282 | |
| Accrued liabilities | |
| 232,685 | | |
| 649,795 | |
| Deferred revenue | |
| 254,871 | | |
| 316,222 | |
| Financing lease liabilities – current | |
| - | | |
| 46,575 | |
| Lease liability – current | |
| 163,788 | | |
| 153,297 | |
| Total current liabilities | |
| 1,011,623 | | |
| 1,630,171 | |
| | |
| | | |
| | |
| Long-term liabilities: | |
| | | |
| | |
| Convertible note payable, net of unamortized debt discount | |
| 229,164 | | |
| 207,246 | |
| Lease liability – long term | |
| 839,549 | | |
| 1,003,338 | |
| Total long-term liabilities | |
| 1,068,713 | | |
| 1,210,584 | |
| | |
| | | |
| | |
| Total liabilities | |
| 2,080,336 | | |
| 2,840,755 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 8) | |
| - | | |
| - | |
| | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.01 par value; 20,000,000 authorized; 15,384,238 and 5,384,238 undesignated as of December 31, 2023 and 2022, respectively | |
| - | | |
| - | |
| Series C preferred stock, $0.01 par value; 3,340,909 authorized; 1,204,040 shares issued and outstanding as of December 31, 2023 and 2022; liquidation preference of $5,297,776 | |
| 12,043 | | |
| 12,043 | |
| Series B preferred stock, $0.01 par value; 3,569,405 authorized; 1,471,487 shares issued and outstanding as of December 31, 2023 and 2022; liquidation preference of $5,194,349 | |
| 14,715 | | |
| 14,715 | |
| Series A-2 preferred stock, $0.01 par value; 800,000 authorized; 442,402 shares issued and outstanding as of December 31, 2023 and 2022; liquidation preference of $1,442,231 | |
| 4,424 | | |
| 4,424 | |
| Series A-1 preferred stock, $0.01 par value; 978,000 authorized; 651,465 shares issued and outstanding as of December 31, 2023 and 2022; liquidation preference of $1,999,998 | |
| 6,515 | | |
| 6,515 | |
| Series A preferred stock, $0.01 par value; 1,303,000 authorized; 846,368 shares issued and outstanding as of December 31, 2023 and 2022; liquidation preference of $2,598,350 | |
| 8,464 | | |
| 8,464 | |
| Common stock, $0.01 par value; 50,000,000 authorized; 4,773,128 and 4,764,811 shares issued and outstanding as of December 31, 2023 and 2022, respectively | |
| 47,731 | | |
| 47,648 | |
| Additional paid-in capital | |
| 28,150,331 | | |
| 26,845,879 | |
| Accumulated deficit | |
| (23,956,731 | ) | |
| (17,565,422 | ) |
| Total stockholders’ equity | |
| 4,287,492 | | |
| 9,374,266 | |
| Total liabilities and stockholders’ equity | |
$ | 6,367,828 | | |
$ | 12,215,021 | |
See accompanying notes to the financial statements
20/20 GENESYSTEMS, INC.
STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 1,424,304 | | |
$ | 11,059,145 | |
| | |
| | | |
| | |
| Cost of revenues (including stock-based compensation $82,941 and $0, respectively) | |
| 1,315,166 | | |
| 5,937,398 | |
| | |
| | | |
| | |
| Gross profit | |
| 109,138 | | |
| 5,121,747 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| Sales, general and administrative (including stock-based compensation $892,332 and $176,081, respectively) | |
| 5,061,450 | | |
| 3,322,835 | |
| Research and development (including stock-based compensation $328,679 and $0, respectively) | |
| 1,409,150 | | |
| 120,043 | |
| Loss on impairment of fixed assets | |
| 209,073 | | |
| - | |
| Total operating expenses | |
| 6,679,673 | | |
| 3,442,878 | |
| | |
| | | |
| | |
| Operating income (loss) | |
| (6,570,535 | ) | |
| 1,678,869 | |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Interest expense | |
| (27,915 | ) | |
| (15,685 | ) |
| Interest income | |
| 209,150 | | |
| 68,421 | |
| Gain on sale of asset | |
| - | | |
| 2,371 | |
| Other expense | |
| (2,009 | ) | |
| - | |
| Other income | |
| - | | |
| 452,899 | |
| Total other income (expense) | |
| 179,226 | | |
| 508,006 | |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net income (loss) | |
$ | (6,391,309 | ) | |
$ | 2,186,875 | |
| | |
| | | |
| | |
| Basic net income (loss) per common share | |
$ | (1.34 | ) | |
$ | 0.46 | |
| Diluted net income (loss) per common share | |
$ | (1.34 | ) | |
$ | 0.23 | |
| Weighted-average common shares outstanding, basic | |
| 4,768,799 | | |
| 4,763,561 | |
| Weighted-average common shares outstanding, diluted | |
| 4,768,799 | | |
| 9,487,385 | |
See accompanying notes to the financial statements
20/20 GENESYSTEMS, INC.
STATEMENTS OF STOCKHOLDERS’
EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | |
Series
C
Preferred Stock | | |
Series
B
Preferred Stock | | |
Series
A-2
Preferred Stock | | |
Series
A-1
Preferred Stock | | |
Series
A
Preferred Stock | | |
Common
Stock | | |
Additional
Paid-in | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| Balance,
December 31, 2021 | |
| 1,205,069 | | |
$ | 12,051 | | |
| 1,471,487 | | |
$ | 14,715 | | |
| 442,402 | | |
$ | 4,424 | | |
| 651,465 | | |
$ | 6,515 | | |
| 846,368 | | |
$ | 8,464 | | |
| 4,762,572 | | |
$ | 47,626 | | |
$ | 26,548,299 | | |
$ | (19,752,297 | ) | |
$ | 6,889,797 | |
| Stock
based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 297,582 | | |
| - | | |
| 297,582 | |
| Exercise
of warrants | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,210 | | |
| 12 | | |
| - | | |
| - | | |
| 12 | |
| Conversion
of preferred stock | |
| (1,029 | ) | |
| (8 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,029 | | |
| 10 | | |
| (2 | ) | |
| - | | |
| - | |
| Net
income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,186,875 | | |
| 2,186,875 | |
| Balance,
December 31, 2022 | |
| 1,204,040 | | |
$ | 12,043 | | |
| 1,471,487 | | |
$ | 14,715 | | |
| 442,402 | | |
$ | 4,424 | | |
| 651,465 | | |
$ | 6,515 | | |
| 846,368 | | |
$ | 8,464 | | |
| 4,764,811 | | |
$ | 47,648 | | |
$ | 26,845,879 | | |
$ | (17,565,422 | ) | |
$ | 9,374,266 | |
| Stock
based compensation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,303,952 | | |
| - | | |
| 1,303,952 | |
| Exercise
of warrants | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 8,317 | | |
| 83 | | |
| 500 | | |
| - | | |
| 583 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,391,309 | ) | |
| (6,391,309 | ) |
| Balance,
December 31, 2023 | |
| 1,204,040 | | |
$ | 12,043 | | |
| 1,471,487 | | |
$ | 14,715 | | |
| 442,402 | | |
$ | 4,424 | | |
| 651,465 | | |
$ | 6,515 | | |
| 846,368 | | |
$ | 8,464 | | |
| 4,773,128 | | |
$ | 47,731 | | |
$ | 28,150,331 | | |
$ | (23,956,731 | ) | |
$ | 4,287,492 | |
See accompanying notes to the financial statements
20/20 GENESYSTEMS, INC.
STATEMENTS OF CASH
FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | |
2023 | | |
2022 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | |
| | |
| |
| Net income (loss) | |
$ | (6,391,309 | ) | |
$ | 2,186,875 | |
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 140,416 | | |
| 194,053 | |
| Stock based compensation | |
| 1,303,952 | | |
| 176,082 | |
| Amortization of license fees | |
| 24,786 | | |
| 26,784 | |
| Amortization of ROU assets, net of liabilities | |
| 2,091 | | |
| 67,852 | |
| Amortization of debt discount | |
| 5,208 | | |
| 1,529 | |
| Gain on sale of asset | |
| - | | |
| 3,379 | |
| Impairment of intangibles | |
| - | | |
| 24,091 | |
| Loss on impairment of fixed assets | |
| 209,073 | | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 696,090 | | |
| 1,937,689 | |
| Other receivables | |
| - | | |
| 1,547,118 | |
| Inventory | |
| 26,406 | | |
| 159,584 | |
| Prepaid expenses and other assets | |
| 66,371 | | |
| 34,798 | |
| Accounts payable | |
| (104,003 | ) | |
| (248,487 | ) |
| Related party payable | |
| 2,699 | | |
| - | |
| Interest payable | |
| 16,710 | | |
| - | |
| Accrued liabilities | |
| (417,110 | ) | |
| (471,812 | ) |
| Deferred revenue | |
| (61,351 | ) | |
| 163,524 | |
| Net cash (used in) provided by operating activities | |
| (4,479,971 | ) | |
| 5,803,059 | |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | |
| | | |
| | |
| Purchase of property and equipment | |
| (9,383 | ) | |
| (261,793 | ) |
| Proceeds from the sales of equipment | |
| - | | |
| 5,750 | |
| Acquisition of license agreement and patent cost | |
| (34,381 | ) | |
| (206,509 | ) |
| Net cash used in investing activities | |
| (43,764 | ) | |
| (462,552 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | |
| | | |
| | |
| Proceeds from convertible notes payable | |
| - | | |
| 183,166 | |
| Convertible notes payable financing costs | |
| - | | |
| (11,715 | ) |
| Deferred offering costs | |
| (148,387 | ) | |
| - | |
| Principal payments on financing lease liabilities | |
| (46,575 | ) | |
| (58,864 | ) |
| Proceeds from exercise of warrant | |
| 583 | | |
| 12 | |
| Net cash provided by (used in) financing activities | |
| (194,379 | ) | |
| 112,599 | |
| | |
| | | |
| | |
| Increase (decrease) in cash and cash equivalents | |
| (4,718,114 | ) | |
| 5,453,106 | |
| Cash and cash equivalents, beginning of year | |
| 8,807,575 | | |
| 3,354,469 | |
| Cash and cash equivalents, end of year | |
$ | 4,089,461 | | |
$ | 8,807,575 | |
| | |
| | | |
| | |
| Supplemental disclosures of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Cash paid for income taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash disclosures of cash flow information: | |
| | | |
| | |
| Conversion of Series C Preferred Stock to Common Stock | |
$ | - | | |
$ | 8 | |
| Escrow of convertible notes payable principal | |
$ | - | | |
$ | 29,843 | |
| Accrued liability reclassed to equity | |
$ | - | | |
| 121,500 | |
| Operating lease, ROU assets and liabilities | |
$ | - | | |
$ | 103,276 | |
See accompanying notes to the financial statements
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
NOTE 1
– BUSINESS AND NATURE OF OPERATIONS
20/20 GeneSystems, Inc. (the “Company”),
founded in May 2000, is a commercial stage diagnostics company with the core mission of developing and commercializing clinical laboratory
tests for early disease detection and prevention and associated software that is powered by machine learning and real-world data to improve
diagnostic accuracy and clinical utility.
For early cancer detection, the Company uses machine
learning and real-world data analytics approaches to substantially improve the accuracy of tumor biomarkers that are currently tested
in millions of individuals around the world. The Company’s cancer product, known as OneTest, is a multi-cancer test for screening
at least five types of cancer from one blood sample.
In response to the novel coronavirus pandemic
that began in early 2020, the Company expanded its business and acquired and commercialized several COVID-19 serology (antibody) and viral
(RT-PCR) tests, both rapid kits and laboratory-based tests.
The Company’s legacy business includes a
patented field test kit for screening suspicious powders for bioterror agents that is used regularly by hundreds of first responder organizations
worldwide, known as BioCheck.
To increase its menu of innovative tests faster
and at a lower cost and risk than through internal development, in 2021, the Company established its Clinical Laboratory Innovation Accelerator
(“CLIAx”), which permits diagnostics start-up companies from around the world to launch their laboratory developed tests in
the Company’s CLIA (Clinical Laboratory Improvement Amendments) licensed laboratory using shared equipment and laboratory personnel.
Management Plans
The Company had incurred operating losses since
its inception up to December 31, 2020; however, the Company experienced profitability from January 1, 2021 through December 31, 2022 from
revenue generated from COVID-19 testing. With the winding down and lifting of the government funding of COVID-19 testing reimbursement,
the Company incurred operating losses for 2023. Historically during the years of losses, the Company has relied on debt and equity financing
for working capital. The Company expects to fund its operations through cash on hand, increased revenue from operations, planned
reductions in spending, and the remaining capital raised through its planned Regulation CF offering and institutional financing in the
form of a convertible bond.
Based on
the Company’s plans, management believes the doubt regarding the Company’s ability to continue as a going concern has been
alleviated.
NOTE 2
– SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The preparation of financial statements in conformity
with United States generally accepted accounting principles (“U.S. GAAP”) requires management to make certain estimates and
assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements, and the reported amount
of revenues and expenses during the reporting periods. Actual results could materially differ from these estimates. It is reasonably possible
that changes in estimates will occur in the near term. The use of estimates include revenue recognition, impairment of long-lived assets
and stock-based compensation.
Business Segments
The Company has determined that its current business
and operations consist of one reporting segment.
Reclassifications
The Company has reclassified, combined or separately
disclosed certain amounts in the prior years’ financial statements and accompanying footnotes to conform with the current year’s
presentation. On the Balance Sheet, prior period presentation of $206,509 of “License Agreements, net” is now contained within
“Other Assets”.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Fair Value of Financial Instruments
Fair value is defined as the exchange price that
would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset
or liability in an orderly transaction between market participants as of the measurement date. Applicable accounting guidance provides
an established hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of
unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants
would use in valuing the asset or liability and are developed based on market data obtained from sources independent of the Company. Unobservable
inputs are inputs that reflect the Company’s assumptions about the factors that market participants would use in valuing the asset
or liability. There are three levels of inputs that may be used to measure fair value:
Level 1 – Observable
inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 – Include other
inputs that are directly or indirectly observable in the marketplace.
Level 3 – Unobservable
inputs which are supported by little or no market activity.
The fair value hierarchy
also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
Fair value estimates
discussed herein are based upon certain market assumptions and pertinent information available to management as of December 31, 2023 and
2022. The respective carrying value of certain on-balance-sheet financial instruments approximated their fair values. These financial
instruments include cash, accounts payable and accrued liabilities. Fair values for these items were assumed to approximate carrying values
because of their short-term nature or they are payable on demand.
Cash and Cash Equivalents
The Company considers
time deposits, certificates of deposit, and certain investments with an original maturity of three months or less to be cash equivalents.
Accounts Receivable
Accounts receivable
represent amounts due from commercial customers. On December 31, 2023, 2022 and 2021, customer accounts receivable totaled $68,834,
$764,924 and $4,215,465, respectively. Receivables through a third-party provider for insurance claims of $0 and $547,438 are included in this
balance at December 31, 2023 and 2022, respectively. The payment of consideration related to these third-party receivables is
subject only to the passage of time. Management reviews open accounts monthly and takes appropriate steps for collection. When
needed, an allowance for doubtful accounts is recorded to reflect management’s determination of the amount deemed
uncollectable. An allowance for doubtful accounts of $29,346 and $62,460 is included in accounts receivable at December 31, 2023 and
2022, respectively.
Inventories
Inventories are stated at the lower of cost or
market using the first-in, first out (FIFO) method. Inventories consisted entirely of finished goods as of December 31, 2023 and 2022.
Internal Use Software
The Company incurs software
development costs to develop software programs to be used solely to meet its internal needs and cloud-based applications used to deliver
its services. In accordance with Accounting Standards Codification (“ASC”) 350-40, Internal-Use Software, the Company
capitalizes development costs related to these software applications once the preliminary project stage is complete and it is probable
that the project will be completed, the software will be used to perform the function intended, and the value will be recoverable. Reengineering
costs, minor modifications and enhancements that do not significantly improve the overall functionality of the software are expensed as
incurred.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Property and Equipment
Property and equipment
are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful life
of three (3) to seven (7) years. Significant renewals and betterments are capitalized while maintenance and repairs are charged to expense
as incurred. Leasehold improvements are amortized on the straight-line basis over the lesser of their estimated useful lives or the term
of the related lease, whichever is shorter. Gains or losses on dispositions of assets are reflected in other income or expense.
Intangible Assets – Patents
The Company capitalizes
patent filing fees, and it expenses legal fees, in connection with internally developed pending patents. The Company also will capitalize
patent defense costs to the extent these costs enhance the economic value of an existing patent. The Company evaluates the capitalized
costs annually to determine if any amounts should be written down. Patent costs begin amortizing upon approval by the corresponding government
and are generally amortized over the expected period to be benefitted, not to exceed the patent lives, which may be as long as 20 years.
Intangible Assets
- License Agreements
In accordance with ASC
730-10-25-2.c, Topic 350-30 paragraph 805-50-30-2, license fees incurred through license agreements for technology supporting specific
products to be sold are either expensed or recognized as intangible assets. The Company recognizes intangible assets when the following
criteria are met: (1) the asset is identifiable, (2) the Company has control over the asset, (3) the cost of the asset can be measured
reliably, and (4) it is probable that economic benefits will flow to the Company. In accordance with Topic 350-30 paragraph 805-50-30-2,
the costs that are capitalized are measured by the cash paid to the licensor for the licensing of their technology in accordance with
the license agreement. Any costs incurred during the validation of the technology are expensed once incurred. The license fees are amortized
either beginning when the technology is validated internally and is ready to be included within the Company’s product offerings
over the period covered by the agreement which might include extensions or based on other terms specific to the agreement.
Impairment of Long-Lived Assets
The long-lived assets
held and used by the Company are reviewed for impairment no less frequently than annually or whenever events or changes in circumstances
indicate that the carrying amount of an asset may not be recoverable. In the event that facts and circumstances indicate that the cost
of any long-lived assets may be impaired, an evaluation of recoverability is performed. The impairment losses for the years ended December
31, 2023 and 2022 were $209,073 and $24,091 for certain equipment and patent costs, respectively. There can be no assurance, however,
that market conditions will not change or demand for the Company’s products and services will continue, which could result in impairment
of long-lived assets in the future.
Offering Costs
The Company complies
with the requirements of ASC 340 with regards to offering costs. Prior to the completion of an offering, offering costs will be capitalized
as deferred offering costs on the balance sheet. The deferred offering costs will be charged to stockholders’ equity upon the completion
of an offering or to expense if the offering is not completed. The total deferred offering costs at December 31, 2023 and 2022 was $148,387
and $0, respectively.
Preferred Stock
ASC 480, Distinguishing
Liabilities from Equity, includes standards for how an issuer of equity (including equity shares issued by consolidated entities)
classifies and measures on its balance sheet certain financial instruments with characteristics of both liabilities and equity.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Management is required
to determine the presentation for the Preferred Stock as a result of the redemption and conversion provisions, among other provisions
in the agreement. Specifically, management is required to determine whether the embedded conversion feature in the Preferred Stock is
clearly and closely related to the host instrument, and whether the bifurcation of the conversion feature is required and whether the
conversion feature should be accounted for as a derivative instrument. If the host instrument and conversion feature are determined to
be clearly and closely related (both more akin to equity), derivative liability accounting under ASC 815, Derivatives and Hedging,
is not required. Management determined that the host contract of the Preferred Stock is more akin to equity, and accordingly, derivative
liability accounting is not required by the Company.
Costs incurred directly
for the issuance of the Preferred Stock are recorded as a reduction of gross proceeds received by the Company.
Basic and Diluted
Loss Per Share
The Company follows Financial
Accounting Standards Board (“FASB”) ASC 260, Earnings per Share, to account for earnings per share. Basic earnings
per share calculations are determined by dividing net loss by the weighted average number of shares of common stock outstanding during
the year. Diluted earnings per share calculations are determined by dividing net income by the weighted average number of common shares
and dilutive common share equivalents outstanding. Dilutive common share equivalents include the dilutive effect of in-the-money share
equivalents, which are calculated, based on the average share price for each period using the treasury stock method. Under the treasury
stock method, the exercise price of an award, if any, the amount of compensation cost, if any, for future service that the Company has
not yet recognized, and the estimated tax benefits that would be recorded in paid-in capital, if any, when an award is settled are assumed
to be used to repurchase shares in the current period. During periods when common stock equivalents, if any, are anti-dilutive they are
not considered in the computation.
The following is a summary
of outstanding securities which have been included in the calculation of diluted net income per share and reconciliation of net income
to net income available to common stockholders for the years ended December 31, 2023 and 2022.
| | |
2023 | | |
2022 | |
| Weighted average common shares outstanding used in calculating basic earnings per share | |
| 4,768,799 | | |
| 4,763,561 | |
| Warrants to purchase Common Stock | |
| 47,093 | | |
| 54,751 | |
| Options to purchase Common Stock | |
| 2,394,415 | | |
| 53,311 | |
| Convertible notes | |
| 47,302 | | |
| - | |
| Series C Preferred Stock | |
| 1,204,040 | | |
| 1,204,040 | |
| Series B Preferred Stock | |
| 1,471,487 | | |
| 1,471,487 | |
| Series A-2 Preferred Stock | |
| 442,402 | | |
| 442,402 | |
| Series A-1 Preferred Stock | |
| 651,465 | | |
| 651,465 | |
| Series A Preferred Stock | |
| 846,368 | | |
| 846,368 | |
| Dilutive effect excluded from earnings per share | |
| (7,104,572 | ) | |
| - | |
| Weighted average common shares outstanding used in calculating diluted earnings per share | |
| 4,768,799 | | |
| 9,487,385 | |
The Company excluded
all Preferred Stock, warrants and options from the computation of diluted net loss per share the year ended December 31, 2023.
The Company excluded
163,196 options and 15,069 warrants from the computation of diluted net income per share for the year ended December 31, 2022 as their
exercise prices were in excess of the most recent valuation of the Company’s common stock during that period. There are no material
reconciling items to net income to diluted net income for common shareholders.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Revenue Recognition
In accordance with ASC
Topic 606, Revenue from Contracts with Customers, the Company recognizes revenue when the customer obtains control of promised
goods or services, in an amount that reflects the consideration which it expects to receive in exchange for those goods and services.
To determine revenue recognition for arrangements that the Company deems are within the scope of ASC Topic 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract;
(iii) calculate transfer price; (iv) allocate the transaction price to the performance obligation in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation.
Disaggregated Revenue ‒
The Company disaggregates revenue from contracts with customers by contract type, as it believes it best depicts how the nature, amount,
timing and uncertainty of revenue and cash flows are affected by economic factors.
The Company’s revenue by contract type is
as follows:
| | |
For the Years Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| BioCheck | |
$ | 187,926 | | |
$ | 154,660 | |
| OneTest | |
| 921,502 | | |
| 323,414 | |
| COVID-19 PCR Tests | |
| 250,145 | | |
| 10,393,256 | |
| COVID-19 Antibody/Antigen Tests | |
| 2,375 | | |
| 97,452 | |
| CLIAx | |
| 62,356 | | |
| 90,363 | |
| Total revenues | |
$ | 1,424,304 | | |
$ | 11,059,145 | |
Performance Obligations ‒ Performance
obligations for four different types of services are discussed below:
| ● | OneTest ‒ Revenues from the sale of OneTest are recognized when returned serum specimens are analyzed
in the Company’s CLIA laboratory and the results are reported to the customer. The specific transaction price is provided to the
customer at the time of purchase either through the on-line portal or via a sales quote for commercial clients, which may be discounted
from list price based on volume of tests ordered. Periodically, discounts are provided to individuals when purchased through the Company’s
online portal. No estimates or adjustments are made to the transaction price for returns or refunds, since these events rarely occur.
There are three customer groups: (i) individuals who purchase tests through the Company’s online portal; (ii) commercial clients
that pay upfront for test kits and (iii) professional health organizations that purchase collection kits and are all billed upon completion
of testing and when results are reported to the customer. Contracts with customers do not contain significant financing components based
on the typical period between performance of services and collection of consideration. There are very little requests for returns or refunds. |
| ● | BioCheck ‒ Revenues for kits are recognized when kits are shipped to the customer. The specific
transaction price is provided to the customer at the time of purchase, which may be discounted from list price based on the volume of
tests ordered. No estimates or adjustments are made to the transaction price for returns or refunds, since these events rarely occur.
Customers’ payment terms are due upon receipt and are not provided significant financing components based on the typical period
between shipment of the product and collection of consideration. There are no requests for returns or refunds. |
| o | Point-of-Care (POC) Test Kits ‒ Revenues for COVID-19 distributed test kits for use at the POC (i.e.,
rapid antigen and antibody tests) are recognized when test kits are shipped to the customer based on negotiated prices per individual
contracts. Customers’ payment terms are due upon receipt of the invoice and are not provided significant financing components based
on the typical period between shipment of the product and collection of consideration. There are no requests for returns or refunds. |
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
| o | COVID-19 Lab Tests (PCR) ‒ Revenues from the sale of COVID-19 viral (PCR) tests are recognized when
returned nasal swabs are analyzed in the Company’s CLIA laboratory and the results are reported to the customer. |
| § | For direct billing to customers, revenue is recorded based on the agreed contracted amount for each test
completed. Customers’ payment terms are net 30 days and are not provided significant financing components based on the typical period
between completed tests and collection of consideration. |
| § | For insurance, the Company estimates the amount of consideration it expects to be entitled to receive
from customer groups in exchange for providing services using the portfolio approach practical expedient. The use of the expedient is
not expected to differ materially from applying the guidance to an individual contract. These estimates are based on utilizing the expected
value method and include the impact of contractual allowances (including payer denials). The portfolios determined using the portfolio
approach consist of the following groups of customers which are similar since they are all insurance providers with similar reimbursement
practices: healthcare insurers and government payers (Medicare and Medicaid programs). The process for estimating revenues and the ultimate
collection of accounts receivable involves significant judgment and estimation. The Company follows a standard process, which considers
historical denial and collection experience and other factors (including the period of time that the receivables have been outstanding),
to estimate contractual allowances and recording adjustments in the current period as changes in estimates. Further adjustments to the
allowances, based on actual receipts, may be recorded upon settlement. The Company relies on a third part billing company to process all
claims to be paid by insurance providers. As a result, the average days to receive payment on these types of claims exceeds ninety days
in some cases. As of December 31, 2023, the Company was owed $2,078 from insurance companies. These claims are no longer billable directly
to the customer and if not reimbursed by the insurance providers, the balance will be written off against the allowance for doubtful accounts. |
| ● | CLIAx – Contractually, the Company can earn revenue in two ways: (i) by providing laboratory services
and (ii) through co-marketing activities of the CLIAx clients laboratory developed tests. Revenue for laboratory services is recognized
monthly based on agreed laboratory activities for space, equipment use and contracted personnel. The revenue that can be earned through
co-marketing activities would be recognized if the Company sells any of the customer’s products. As of December 31, 2023, the CLIAx
customer is working through its marketing plan and the Company has not yet performed any co-marketing activities and as a result has not
sold any CLIAx products or recognized any related revenue. |
Deferred revenue represents contract
liabilities that are recorded when cash payments are received or are due in advance of the Company’s satisfaction of
performance obligations. The deferred revenue for the years ended December 31, 2023, 2022 and 2021 were $254,871, $316,222
and $152,698, respectively, and are related to OneTest.
Seasonality
The Company’s significant
growth in COVID-19 viral testing solutions is affected by the pattern of seasonality subject to the unpredictable demand for
viral testing in Maryland. With the significant decline in incidences and requirement for testing, the Company has anticipated the material
decrease in revenue and cash flow related to the COVID-19 testing.
Shipping and Handling
Amounts billed to a customer
for shipping and handling are reported as revenues. Costs related to shipments to the Company are classified as cost of sales and totaled
$134,824 and $258,837 for the years ended December 31, 2023 and 2022, respectively.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Research and Development
The Company incurs research
and development costs during the process of researching and developing the Company’s laboratory tests, algorithms, information technologies,
and other intellectual properties. The Company’s research and development costs consist primarily of data acquisition and personnel
costs of scientists and laboratory technicians. The Company expenses these costs as incurred until the resulting product has been completed,
tested, validated, and made ready for commercial use.
Advertising
The Company expenses
advertising costs as incurred. Advertising expenses were $780,127 and $358,337 for the years ended December 31, 2023 and 2022, respectively.
Stock-Based Compensation
The Company accounts
for stock awards issued under ASC 718, Compensation – Stock Compensation. Under ASC 718, stock-based compensation cost is
measured at the grant date, based on the estimated fair value of the award. Stock-based compensation is recognized as expense over the
employee’s requisite vesting period and over the nonemployee’s period of providing goods or services. The fair value of each
stock option or warrant award is estimated on the date of grant using the Black-Scholes option valuation model. Restricted shares are
measured based on the fair market value of the underlying stock on the grant date.
Income Taxes
The Company applies ASC
740, Income Taxes. Deferred income taxes are recognized for the tax consequences in future years of differences between the tax
bases of assets and liabilities and their financial statement reported amounts at each period end, based on enacted tax laws and statutory
tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established,
when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax
expense for the period, if any and the change during the period in deferred tax assets and liabilities. As
of December 31, 2023, and 2022, the Company has a valuation allowance on the net deferred assets due to the continued likelihood that
realization of any future benefit from deductible temporary differences and net operating loss carryforwards cannot be sufficiently assumed.
ASC 740 also provides
criteria for the recognition, measurement, presentation, and disclosure of uncertain tax positions. A tax benefit from an uncertain position
is recognized only if it is “more likely than not” that the position is sustainable upon examination by the relevant taxing
authority based on its technical merit. Interest and penalties, if any, are accrued as a component of operating expenses when assessed.
Concentrations
The Company maintains
its cash at various financial institutions located in the United States of America which it believes to be credit worthy. Balances are
insured by the Federal Deposit Insurance Corporation up to $250,000. At times, the Company maintains balances in excess of the federally
insured limits. The Company has not experienced any losses with respect to its cash balances.
As of December 31, 2023,
approximately 51% of total accounts receivable were due from two sources. As of December 31, 2022, approximately 51% of total accounts
receivable were due from one source. During the year ended December 31, 2023, approximately 37% of total revenues were received from one
source. During the year ended December 31, 2022, approximately 94% of total revenues were received from two sources. With the decline
in COVID-19 incidences and the US Government no longer funding this testing, the Company’s customers no longer require COVID-19
testing services’ and the Company’s revenue in this area is now zero.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Risks and Uncertainties
In response to the novel
coronavirus pandemic that began in early 2020, the Company expanded its business and offered several COVID-19 testing solutions, both
rapid kits and laboratory-based tests. The revenues in these areas have ceased entirely due to the end of the pandemic and emergency funding
by the US Government.
Recent Accounting Pronouncements
Management
does not believe that any recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material
impact on the Company’s financial statement presentation or disclosures.
NOTE 3
– PROPERTY AND EQUIPMENT
Property and equipment
consisted of the following:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| Office equipment | |
$ | 147,259 | | |
$ | 160,669 | |
| Furniture and fixtures | |
| 57,691 | | |
| 54,112 | |
| Laboratory equipment | |
| 463,719 | | |
| 896,636 | |
| Vehicles | |
| 40,555 | | |
| 40,555 | |
| Leasehold improvements | |
| 12,221 | | |
| 12,221 | |
| Total property and equipment | |
| 721,445 | | |
| 1,164,193 | |
| Less accumulated depreciation | |
| (477,242 | ) | |
| (583,282 | ) |
| | |
$ | 244,203 | | |
$ | 580,911 | |
In the year ended
December 31, 2023, the Company performed an impairment analysis of laboratory equipment utilized in COVID-19 testing due to the significant
material decrease in revenue and cash flow related to the COVID-19 testing and recorded an impairment charge of $209,073. It was
determined after discussion with lab personnel that certain PCR laboratory equipment could be repurposed for potential future products
and would be retained for research and development. The net book value of this equipment that remains in fixed assets equals $122,056
and will continue to be depreciated to research and development costs.
Depreciation expense
was $137,018 and $183,662 for the years ended December 31, 2023 and 2022, respectively.
NOTE 4
– INTANGIBLE ASSETS
Intangible assets consisted
of the following:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| Issued patents (amortized) | |
$ | 31,840 | | |
$ | 31,840 | |
| Unissued patents (unamortized) | |
| 207,150 | | |
| 177,423 | |
| Software development costs | |
| 4,654 | | |
| 45,575 | |
| Total | |
| 243,644 | | |
| 254,838 | |
| Less accumulated amortization | |
| (33,258 | ) | |
| (75,435 | ) |
| | |
$ | 210,386 | | |
$ | 179,403 | |
Amortization expense
for intangible assets was $3,398 and $10,391 for the years ended December 31, 2023 and 2022, respectively. Unissued patents represent
the legal fees incurred to file and prosecute patents prior to issuance. The unissued patents are for active pending patents only. Any
accumulated legal fees associated with abandoned unissued patents are expensed in the period they are abandoned.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
NOTE 5
– FINANCING LEASES
In January 2021, the Company leased certain equipment
under separate non-cancelable equipment loan and security agreements. The agreements mature in December 2023. The agreements
require various monthly payments of principal and interest through maturity and are secured by the assets under lease. As of December
31, 2022, $173,915 of financing lease equipment and $47,507 of accumulated depreciation are included in property and equipment on the
balance sheets. The weighted average interest rate was 6.2% at December 31, 2022. The lease was paid off in 2023.
NOTE 6
– OPERATING LEASES
On March 18, 2021, the Company entered into a
lease agreement with Shady Grove Development Park IX L.L.L.P. for a new office and laboratory space totaling 5,511 square feet in Gaithersburg,
Maryland. The term of the lease commenced on December 1, 2021 and shall expire 88 months thereafter. The initial monthly rent is $10,676
with annual increases to $17,308 for the final year of the lease. The Company will also pay its 7.75% pro rata portion of the property
taxes, operating expenses and insurance costs and is also responsible to pay for the utilities used on the premises.
On September 29, 2022, the Company entered into
a lease agreement with Abbott Laboratories, Inc. for laboratory equipment (analyzer). The term of the lease commenced on December 1, 2022
and shall expire 84 months thereafter. The monthly rental payments are $1,488 throughout the term of the lease. The Company also has a
commitment to purchase $86 thousand of consumables annually during the term of the lease.
Supplemental balance sheet information related
to this lease is as follows:
| | |
December 31,
2023 | |
| Operating lease right-of-use lease asset | |
$ | 1,242,936 | |
| Accumulated amortization | |
| (309,542 | ) |
| Net balance | |
$ | 933,394 | |
| | |
| | |
| Lease liability, current portion | |
| 163,788 | |
| Lease liability, long term | |
| 839,549 | |
| Total operating lease liabilities | |
$ | 1,003,337 | |
| | |
| | |
| Weighted Average Remaining Lease Term – operating leases | |
| 63 months | |
| | |
| | |
| Weighted Average Discount Rate – operating leases | |
| 3.8 | % |
Future minimum lease payments under this operating
lease as of December 31, 2023, were as follows:
| 2024 | |
$ | 199,629 | |
| 2025 | |
| 204,632 | |
| 2026 | |
| 209,767 | |
| 2027 | |
| 215,036 | |
| 2028 | |
| 220,460 | |
| Thereafter | |
| 68,293 | |
| Total lease payments | |
| 1,117,817 | |
| Less imputed interest | |
| (114,480 | ) |
| Maturities of lease liabilities | |
$ | 1,003,337 | |
In August 2011, the Company entered into a lease
commencing in December 2011 which expired in November 2016. Under the lease agreement, the Company was to pay an annual rent of $134,975,
plus additional operating expenses. Upon expiration, this lease had continued on a month-to-month basis until March 2022. Total rent expense,
including additional operating expenses related to this property, was $0 and $22,720 for the years ended December 31, 2023 and 2022, respectively.
In early 2022, the Company vacated the property and entered into an agreement to settle any remaining obligations due.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Lease expense for the year ended December 31,
2023 was comprised of the following:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| Operating lease expense | |
$ | 196,851 | | |
$ | 177,041 | |
NOTE 7
– CONVERTIBLE NOTE PAYABLE
On August 15, 2022, the Company launched an equity
crowdfunding offering under Section 4(a)(6) of the Securities Act of 1933, as amended (the “Securities Act”), and Regulation
Crowdfunding promulgated thereunder, pursuant to which the Company offered convertible promissory notes. As of December 31, 2023, the
Company issued convertible promissory notes in the aggregate principal amount of $213,010. The notes bear interest at rates ranging from
6% to 11.10%, cannot be prepaid without a majority investor vote and are due and payable twenty-four (24) months after the date of issuance.
The notes are unsecured, contain customary events of default and are convertible into Common Stock as follows:
| ● | In the event that the Company issues and sells Common Stock or Preferred Stock to investors in a transaction
or series of transactions resulting in gross proceeds of at least $100,000, excluding debt or the issuance of Common Stock or Preferred
Stock in asset purchase or strategic merger or acquisition (a “Qualified Financing”), then the entire unpaid principal amount
and all accrued, but unpaid interest shall convert into Common Stock at conversion price equal to the lesser of (i) 90% of the per share
price paid by such investors or (ii) the price equal to the quotient of $58,400,000 divided by the aggregate number of outstanding shares
of Common Stock as of immediately prior to the initial closing of the Qualified Financing (assuming full conversion or exercise of all
convertible and exercisable securities then outstanding other than these notes); |
| ● | In the event the Company completes an equity financing in which it sells Common Stock or Preferred Stock
in a transaction that does not constitute a Qualified Financing, then the note holder has the option to treat such equity financing as
a Qualified Financing on the same terms set forth above; |
| ● | Upon the earlier to occur of (i) the closing of the sale of Common Stock to the public at a price of at
least $8.15 per share (subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar
recapitalization with respect to the Common Stock) in a public offering pursuant to an effective registration statement or offering statement
(Regulation A) under the Securities Act resulting in at least $5,000,000 of gross proceeds, (ii) the date on which the Company’s
Common Stock is listed on a national stock exchange, including without limitation, NYSE American or the Nasdaq Capital Market, or (iii)
the date and time, or the occurrence of an event, specified by vote or written consent of the holders of at least a majority in principal
amount of the then outstanding notes, then the entire unpaid principal amount and all accrued, but unpaid interest shall convert into
Common Stock at conversion price equal to the quotient of $58,400,000 divided by the aggregate number of outstanding shares of Common
Stock as of immediately prior to the consummation of the event described above; and |
| ● | The entire outstanding principal balance and all unpaid accrued interest shall automatically be converted
into Common Stock at a conversion price equal to the quotient of $58,400,000 divided by the aggregate number of outstanding shares of
Common Stock as of immediately prior to the conversion (assuming full conversion or exercise of all convertible and exercisable securities
then outstanding other than these notes) as soon a reasonably practicable following the maturity date. |
As of December 31, 2023 and 2022, the outstanding
balance of these notes is $229,164 and $207,246, respectively, consisting of principal of $213,010, net of unamortized debt issuance cost
of $4,980 and $10,187, respectively, and an accrued interest balance of $21,134 and $4,423, respectively.
Interest expense on the notes totaled $16,711
and $4,423 for the year ended December 31, 2023 and 2022, respectively, and the Company recorded amortization of debt discount
in the amount of $5,207 and $1,529 during the year ended December 31, 2023 and 2022, respectively.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
NOTE 8
– COMMITMENTS AND CONTINGENCIES
Royalties and License Agreements
License agreements:
| | |
December 31,
2023 | | |
December 31,
2022 | |
| International license agreement | |
| 450,008 | | |
| 450,008 | |
| Total license agreements | |
| 450,008 | | |
| 450,008 | |
| Less accumulated amortization | |
| (133,865 | ) | |
| (109,079 | ) |
| | |
$ | 316,143 | | |
$ | 340,929 | |
The Company is amortizing the license agreement
over the term amounting to an amortization expense of $24,786 and $26,784 for the years ended December 31, 2023 and 2022, respectively.
In November 2017, the Company executed a
license agreement with a foreign entity to obtain and secure an exclusive license to certain technology, intellectual property, and data
relating to the Company’s OneTest in exchange for $150,000 of certain up-front fees and $300,008 in Common Stock and ongoing royalty
fees. In accordance with ASC 720-10-25-2.c, Topic 350-30-25-1, the Company recognized the $150,000 in up-front fees paid and the $300,000
in Common Stock as an Intangible Asset – License fee since the technology is deemed to provide a future benefit in its use to the
Company by way of its sales of OneTest. The Company entered an exclusive license to the technology until the last patent included in the
specified technology expires, or 20 years. The Company has amortized the license agreement over the term amounting to an accumulative
amortization of $133,865 and $109,079 as of December 31, 2023 and 2022, respectively.
In August 2022, the Company entered into a three-year
agreement to obtain and secure an exclusive license to certain multi-cancer diagnostic testing technology that incorporates additional
biomarkers not currently part of the Company’s OneTest. This product once validated will be marketed as OneTest Premium. In addition
to OneTest Premium, the license agreement provides access to other technology for tests that assess various chronic diseases such as immune
function, cardiovascular function and diabetic propensity that utilizes measurement of additional biomarkers. As of December 31, 2022,
in accordance with ASC 720-10-25-2.c, Topic 350-30-25-1, the Company recognized the $150,000 in up-front fees paid along with an additional
$56,509 in equipment validation materials in other asset and was recognized as research and development costs upon completion of the validation
study as of December 31, 2023. The initial up-front license fee of $150,000 will be amortized through the recognition of royalty fees
incurred on each sale and is included within the other assets on the accompanying balance sheet. Upon validation, the Company will recognize
future per-test royalty fees in the range of $12-$25 per test.
On January 6, 2023, the Company entered into an
option agreement to license certain proprietary technology from a leading cancer research institute for their in vitro diagnostics in
the field of lung cancer blood-based predisposition evaluation tool. The initial six-month option costs the Company $70,000 and a portion
of the patent fees. The agreement provides the potential for an exclusive license to the technology upon achievement of certain financing
and partnership goals that need to be accomplished by early July 2023. The option agreement provides for up to three one-month extensions
if needed upon mutual consent and additional option fees of $10,000 for each month extended. In accordance with ASC 720-10-25-2.c, Topic
350-30-25-1, the Company recognized the $70,000 in up-front option fee paid as an Intangible Asset – License fee since the technology
once licensed will be deemed to provide a future benefit in its use to the Company by way of potential sales of LungSpot-lung cancer test.
The initial up-front license fee of $70,000 will be amortized over the life of the final license agreement once finalized or expensed
if a final agreement is not consummated. The agreement was not extended on July 6, 2023 and as a result, the $70,000 upfront fee was expensed
to research and development costs as there was not alterative use for the license fee paid.
NOTE 9
– STOCKHOLDERS’ EQUITY
On July 18, 2023, the Company filed a Certificate
of Amendment of Second Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware, pursuant
to which the authorized Common Stock was increased from 25,000,000 shares to 50,000,000 shares and the authorized Preferred Stock was
increased from 10,000,000 shares to 20,000,000 shares.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Preferred Stock
The Company has authorized the issuance of 10,000,000
shares of Preferred Stock with par value of $0.01, of which 1,303,000 have been designated as Series A Preferred Stock, 978,000 have been
designated as Series A-1 Preferred Stock, 800,000 shares have been designated as Series A-2 Preferred Stock, 3,569,405 shares have been
designated as Series B Preferred Stock and 3,340,909 shares have been designated as Series C Preferred Stock (collectively, the “Designated
Preferred Stock”). Below is a summary of the terms of the Designated Preferred Stock.
Ranking. With respect to dividend
rights and rights on liquidation, winding up and dissolution, shares of Designated Preferred Stock rank pari passu to each other
and senior to all shares of Common Stock.
Voting Rights. Shares of Designated
Preferred Stock vote together with the holders of Common Stock on an as-converted basis on all matters for which the holders of Common
Stock vote at an annual or special meeting of stockholders or act by written consent, except as required by law. For so long as shares
of Designated Preferred Stock are outstanding, the holders of such shares vote together, as a separate class, to elect one director to
the Company’s board, and for so long as shares of Series A-1 Preferred Stock are outstanding, the holders of Series A-1 Preferred
Stock vote together, as a separate class, to elect one director to the Company’s board.
Conversion Rights. Each share of
Designated Preferred Stock is convertible at any time at the option of the holder at the then current conversion rate. The conversion
rate for the Designated Preferred Stock is currently one share of Common Stock for each share of Designated Preferred Stock, calculated
by dividing the liquidation preference of such share by the conversion price then in effect. In addition, all outstanding shares of Designated
Preferred Stock, plus accrued but unpaid dividends thereon, shall automatically be converted into shares of Common Stock, at the then
effective conversion rate, upon the earlier to occur of (a) the closing of the sale of shares of Common Stock to the public at a price
of at least $8.15 per share (subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar
recapitalization with respect to the Common Stock), in a public offering pursuant to an effective registration statement or offering statement
under the Securities Act of 1933, as amended (the “Securities Act”), resulting in at least $5,000,000 of gross proceeds to
the Company, (b) the date on which the shares of Common Stock are listed on a national stock exchange, including without limitation the
New York Stock Exchange or the Nasdaq Stock Market, or (c) the date and time, or the occurrence of an event, specified by vote or written
consent of the holders of at least 67% of the then outstanding shares of Designated Preferred Stock, voting together on an as-converted
to Common Stock basis (which vote or consent shall include the holders of at least 67% of the shares of Series A-1 Preferred Stock outstanding
voting as a separate class).
Liquidation Rights. In the event
of any voluntary or involuntary liquidation, dissolution or winding up of the Company or a deemed liquidation event, each holder of Designated
Preferred Stock then outstanding shall be entitled to be paid out of the cash and other assets of the Company available for distribution
to its stockholders, prior and in preference to all shares of Common Stock, an amount in cash equal to the aggregate liquidation preference
of all shares held by such holder. The shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series
B Preferred Stock and Series C Preferred Stock have a liquidation preference of $3.07, $3.07, $3.26, $3.53 and $4.40, respectively (subject
to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar recapitalization) plus any accrued
and unpaid dividends. If upon any liquidation or deemed liquidation event the remaining assets available for distribution are insufficient
to pay the holders of Designated Preferred Stock the full preferential amount to which they are entitled, the holders of Designated Preferred
Stock shall share ratably in any distribution of the remaining assets and funds in proportion to the respective full preferential amounts
which would otherwise be payable, and the Company shall not make or agree to make any payments to the holders of Common Stock. A “deemed
liquidation event” means, unless otherwise determined by the holders of at least a majority of the Designated Preferred Stock then
outstanding (voting together as a single class on an as-converted basis), (a) a sale of all or substantially all of the Company’s
assets to a non-affiliate of the Company, (b) a merger, acquisition, change of control, consolidation or other transactions or series
of transactions in which stockholders prior to such transaction or series of transactions do not retain a majority of the voting power
of the surviving entity immediately following such transaction or series of transactions, or (c) the grant of an exclusive license to
all or substantially all of the Company’s technology or intellectual property rights except where such exclusive license is made
to one or more wholly-owned subsidiaries of the Company.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Dividends. The Designated Preferred
Stock will not be entitled to dividends or distributions unless and until the board declares a dividend or distribution in cash or other
property to holders of outstanding shares of Common Stock, in which event, the aggregate amount of such each distribution shall be distributed
as follows: (a) first, seventy percent (70%) of the distribution amount to the holders of shares of Designated Preferred Stock, on a pro
rata basis, until such time as such holders have received an aggregate amount in distributions or other payments in respect of such holder’s
shares that is equal to the number of shares owned by such holders multiplied by the liquidation preference stated above, and (b) second,
thirty percent (30%) of the distribution amount to the holders of shares of Common Stock, on a pro rata basis. Notwithstanding the foregoing,
at such time as the holders of Designated Preferred Stock and Common Stock have received the amounts described above, the holders of the
Designated Preferred Stock shall receive Distributions pari passu with the holders of the Common Stock on an as-converted basis,
using the then-current conversion rate of such shares of Designated Preferred Stock.
Preemptive Rights. Until the Company’s
initial public offering of Common Stock occurs and unless otherwise waived by the prior express written consent of the holders of the
majority of the voting power of all then outstanding Designated Preferred Stock, voting together on an as-converted to Common Stock basis,
in the event that the Company proposes to issue any Common Stock or shares convertible or exercisable for Common Stock, except for excluded
issuances, the Company must first offer those additional equity securities to holders of Designated Preferred Stock for a period of no
less than thirty (30) days prior to selling or issuing any such additional equity securities to any person, in accordance with the procedures
set forth in the Company’s certificate of incorporation, as amended. For purposes hereof, “excluded securities” means
the issuance of shares of Common Stock or securities convertible into shares of Common Stock (a) granted pursuant to or issued upon the
exercise of stock options granted under an equity incentive plan to employees, officers, directors, consultants or strategic partners,
(b) granted to employees, officers, directors, consultants or strategic partners for services, including in connection with an incentive
plan, or other fair value received or committed, (c) in consideration for a transaction approved by the board which does not result in
the issuance for cash of more than five percent (5%) of the outstanding shares of Common Stock, (d) in connection with an acquisition
transaction approved by the board, (e) to vendors, commercial partners, financial institutions or lessors in connection with commercial
credit transactions, equipment financings or similar transaction approved by the board (provided that such securities do not exceed 10%
of the consideration in such transaction), (f) pursuant to conversion or exchange rights included in securities previously issued by the
Company or (g) in connection with a stock split, stock division, reclassification, stock dividend or other recapitalization.
Redemption. Shares of each series
of Designated Preferred Stock are not redeemable without the prior express written consent of the holders of the majority of the voting
power of all then outstanding shares of such applicable series of Designated Preferred Stock, voting as a separate class.
Protective Rights. So long as at
least twenty-five percent (25%) of the Designated Preferred Stock collectively remains outstanding, in addition to any other vote or consent
of stockholders required by law, the vote or consent of the holders of at least a majority of all shares of Designated Preferred Stock
then outstanding and entitled to vote thereon, voting together and on an as-converted to Common Stock basis, given in person or by proxy,
either in writing without a meeting or by vote at any meeting called for the purpose, including the consent of the holders of Series A-1
Preferred Stock, shall be necessary for effecting or validating, either directly or indirectly by amendment, merger, consolidation or
otherwise:
| (a) | the authorization, creation and/or issuance of any equity
security, other than shares of Common Stock or options to purchase Common Stock issued to investors, employees, managers, officers or
directors of, or consultants or advisors to, the Company or any of its subsidiaries pursuant to a plan, agreement or arrangement approved
by the board; |
| (b) | the amendment, alteration or repeal of any provision of the
certificate of incorporation or bylaws or otherwise alter or change any right, preference or privilege of any Designated Preferred Stock
in a manner adverse to the holders thereof; |
| (c) | any increase or decrease in the size of the board; |
| (d) | the purchase, redemption, or acquisition of any shares other
than from a selling holder pursuant to the provisions of the certificate of incorporation or any other restriction provisions applicable
to any shares in agreements approved by the board or in the operating agreement of any limited liability company utilized for the purpose
of facilitating investment in the Company; |
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
| (e) | the liquidation or dissolution of the Company or the sale,
lease, pledge, mortgage, or other disposal of all or substantially all of its assets; |
| (f) | any election to engage in any business that deviates in any
material respect from the Company’s business as contemplated under any operating plan approved by the board; |
| (g) | the waiver of any adjustment to the conversion price applicable
to the Designated Preferred Stock; or |
| (h) | any declaration or payment of any cash dividend or other
cash distribution to any holders of capital stock. |
Series A Preferred Stock
As of December 31, 2023 and 2022, there were 846,368
shares of Series A Preferred Stock issued and outstanding. No shares of Series A Preferred Stock were issued during the years ended December
31, 2023 and 2022.
Series A-1 Preferred Stock
As of December 31, 2023 and 2022, there were 651,465
shares of Series A-1 Preferred Stock issued and outstanding. No shares of Series A-1 Preferred Stock were issued during the years ended
December 31, 2023 and 2022.
Series A-2 Preferred Stock
As of December 31, 2023 and 2022, there were 442,402
shares of Series A-2 Preferred Stock issued and outstanding. No shares of Series A-2 Preferred Stock were issued during the years ended
December 31, 2023 and 2022.
Series B Preferred Stock
As of December 31, 2023 and 2022, there were 1,471,487
shares of Series B Preferred Stock issued and outstanding. No shares of Series B Preferred Stock were issued during the years ended December
31, 2023 and 2022.
Series C Preferred Stock
As of December 31, 2023 and 2022, there were 1,204,040
shares of Series C Preferred Stock issued and outstanding.
On January 8, 2020, the Company launched an offering
under Regulation A of Section 3(6) of the Securities Act for Tier 2 offerings, pursuant to which the Company offered up to 3,340,909
shares of Series C Preferred Stock at an offering price of $4.40 per share for gross proceeds of up to $14,700,000 on a “best efforts”
basis. This offering was terminated on June 15, 2021.
During the year ended December 31, 2021, the Company
issued 369,750 shares of Series C Preferred Stock for gross proceeds of $1,510,076 and net proceeds of $1,246,088. The Company also issued
7,357 shares of Series C Preferred Stock to the placement agent as partial compensation for its services. Additionally, 30,365 shares
of Series C Preferred Stock were converted into 30,365 shares of Common Stock.
In March 2022, an aggregate of 1,029 shares of
series C preferred stock were converted into 1,029 shares of common stock.
Common Stock
As of December 31, 2023 and 2022, there were 4,773,128
and 4,764,811 shares of Common Stock and outstanding, respectively.
During the year ended December 31, 2023, the Company
issued 8,317 shares of Common Stock upon the exercise of warrants for proceeds of $583.
During the year ended December 31, 2022, the Company
issued 1,210 shares of Common Stock upon the exercise of warrants for proceeds of $12.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
During the year ended December 31, 2022, the Company
issued 1,029 shares of Common Stock upon the conversion of 1,029 shares of Series C Preferred Stock.
Stock Options
On January 26, 2022, the board of directors adopted
the 20/20 GeneSystems, Inc. 2022 Stock Incentive Plan (the “2022 Plan”), which was approved by stockholders on June 15, 2022.
Awards that may be granted include incentive stock options as described in section 422(b) of Internal Revenue Code of 1986, as amended,
non-qualified stock options (i.e., options that are not incentive stock options) and awards of restricted stock. Up to 3,000,000 shares
of Common Stock may be issued under the 2022 Plan.
On February 1, 2022, the Company granted non-qualified
stock options under the 2022 Plan for the purchase of 300,668 shares of Common Stock at an exercise price of $1.0643 per share, which
represented the fair market value of the Company’s Common Stock on date of grant, to certain directors of the Company. An aggregate
of 150,332 shares vested in full on the date of grant and an aggregate of 150,336 shares vest monthly over one year. Management determines
the value of options granted using the calculated value method and the Black-Scholes option pricing model for a total fair market value
of $183,708.
On January 1, 2023, the Company issued non-qualified
stock options for the purchase of an aggregate of 1,485,000 shares of Common Stock at an exercise price of $1.74 per share, which represented
the fair market value of the Company’s Common Stock on date of grant, under the 2022 Plan, which 1,155,000 options issued to certain
employees and officers vest 50% upon the date of grant and the remainder vest over 24 months, 210,000 options issued to certain employees
and officers vest 25% on the first anniversary of the date of grant and monthly thereafter for remaining 36 months, and 120,000 options
to certain directors that vest over a term of one year. Management determines the value of options granted using the calculated value
method and the Black-Scholes option pricing model. The fair value of the stock options issued in 2023 was determined using the Black Scholes
option pricing model with the following assumptions: dividend yield: 0%; volatility: 79.7% to 92.7%; risk free rate: 3.99% to 4.73%; estimated
term of five and ½ to seven years for a total fair market value of $1,862,400.
On April 1, 2023, the Company issued a non-qualified
stock option for the purchase of 50,000 shares at an exercise price of $1.74 per share to an officer under the 2022 Plan which vests 25%
on the first anniversary of the date of grant and monthly thereafter for remaining 36 months. Management determines the value of
options granted using the calculated value method and the Black-Scholes option pricing model. The fair value of the stock options issued
in 2023 was determined using the Black Scholes option pricing model with the following assumptions: dividend yield: 0%; volatility: 82.8%;
risk free rate 3.6%; estimated term seven years for a total fair market value of $66,000.
With the assistance of third parties, the Company
determined the fair market value of its Common Stock underlying the stock options comparing a market approach through analysis of comparable
public companies and a venture funding approach taking into account the senior terms of the Company’s Preferred Stock as compared
to the Common Stock to arrive at a fair market value per share estimate for a common share. Once calculated, the Company applied a discount
of 27% to account for the lack of marketability.
The risk-free interest rate assumption for options
granted is based upon observed interest rates on the United States government securities appropriate for the expected term of the Company’s
employee stock options.
The expected term of employee stock options is
calculated using the simplified method because it has insufficient history upon which to base an assumption about the terms,
which takes into consideration the contractual life and vesting terms of the options.
The Company determined the expected volatility
assumption for options granted using the historical volatility of comparable public companies’ common stock. The Company will continue
to monitor peer companies and other relevant factors used to measure expected volatility for future stock option grants, until such time
that the Company’s Common Stock has enough market history to use historical volatility.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
The dividend yield assumption for options granted
is based on the Company’s history and expectation of dividend payouts. The Company has never declared or paid any cash dividends
on its Common Stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.
The Company recognizes stock option forfeitures
as they occur as there is insufficient historical data to accurately determine future forfeitures rates.
During the years ended December 31, 2023 and 2022,
the Company recorded stock-based compensation of $1,303,952 and $297,582, respectively, which is an expense of $82,941 and $0 in cost
of revenues, $892,332 and $297,582 in the sales, general and administrative expenses, $328,679 and $0 in research and development, respectively.
As of December 31, 2023, there was approximately $569,675 of total unrecognized share-based compensation related to unvested stock options,
which the Company expects to recognize over approximately three years.
A summary of the incentive stock option activity
is as follows:
| | |
Total Options | | |
Weighted Average Exercise Price Per Share | | |
Total Weighted Average Remaining Contractual Life | |
| Options outstanding, December 31, 2021 | |
| 153,362 | | |
$ | 4.50 | | |
| 1.0 | |
| Granted | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Expired | |
| (132,000 | ) | |
| 4.50 | | |
| - | |
| Options outstanding, December 31, 2022 | |
| 21,362 | | |
$ | 4.50 | | |
| 0.83 | |
| Granted | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Expired | |
| (21,362 | ) | |
| 4.50 | | |
| - | |
| Options outstanding, December 31, 2023 | |
| - | | |
$ | - | | |
| - | |
| | |
| | | |
| | | |
| | |
| Options exercisable, December 31, 2023 | |
| - | | |
$ | - | | |
| - | |
There is no remaining unvested expense related
to these stock options.
A summary of the Company’s non-qualified
stock option activity is as follows:
| | |
Total Options | | |
Weighted Average Exercise Price Per Share | | |
Total Weighted Average Remaining Contractual Life | |
| Options outstanding, December 31, 2021 | |
| 626,747 | | |
$ | 1.09 | | |
| 8.07 | |
| Granted | |
| 300,668 | | |
| 1.06 | | |
| 10.0 | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Forfeited | |
| - | | |
| - | | |
| - | |
| Expired | |
| - | | |
| - | | |
| - | |
| Options outstanding, December 31, 2022 | |
| 927,415 | | |
$ | 1.08 | | |
| 7.72 | |
| Granted | |
| 1,535,000 | | |
| 1.74 | | |
| 10.0 | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Forfeited | |
| (68,000 | ) | |
| 1.66 | | |
| - | |
| Expired | |
| - | | |
| - | | |
| - | |
| Options outstanding, December 31, 2023 | |
| 2,394,415 | | |
$ | 1.48 | | |
| 8.14 | |
| | |
| | | |
| | | |
| | |
| Options exercisable, December 31, 2023 | |
| 1,891,915 | | |
$ | 1.41 | | |
| 7.90 | |
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
The Company recognizes compensation expense for
stock option awards on a straight-line basis over the applicable service period of the award. The service period is generally the vesting
period. The following assumptions were used to calculate share-based compensation expense for years ended December 31, 2023 and 2022:
| | |
2023 | | |
2022 | |
| Exercise price | |
| $1.735 | | |
| $1.0643 | |
| Share price | |
| $1.740 | | |
| $1.0643 | |
| Volatility | |
| 79.7% -92.7% | | |
| 68.5% | |
| Risk-free interest rate | |
| 3.6% - 4.73% | | |
| 1.63% | |
| Dividend yield | |
| 0.0% | | |
| 0.0% | |
| Expected term | |
| 5.5 to 7.0 years | | |
| 5.0 years | |
Warrants
On April 19, 2022, the Company issued a five-year
warrant for the purchase of 91 shares of Common Stock at an exercise price of $4.40 (subject to standard adjustments) to a consultant
for a value of $28 as partial compensation for services rendered and recorded in general and administrative costs.
A summary of the Company’s warrant activity
is as follows:
| | |
Warrants | | |
Weighted Average Exercise Price Per Share | | |
Total Weighted Average Remaining Contractual Life | |
| Warrants outstanding, December 31, 2021 | |
| 103,637 | | |
$ | .71 | | |
| 2.70 | |
| Granted | |
| 91 | | |
| 4.40 | | |
| 5.00 | |
| Exercised | |
| (1,210 | ) | |
| 0.01 | | |
| - | |
| Forfeited/Expired | |
| (30,025 | ) | |
| 0.01 | | |
| - | |
| Warrants outstanding, December 31, 2022 | |
| 72,493 | | |
$ | 1.02 | | |
| 2.63 | |
| Granted | |
| - | | |
| - | | |
| - | |
| Exercised | |
| (8,317 | ) | |
| 0.01 | | |
| - | |
| Forfeited/Expired | |
| (17,083 | ) | |
| 0.01 | | |
| - | |
| Warrants outstanding, December 31, 2023 | |
| 47,093 | | |
$ | 1.56 | | |
| 2.70 | |
| | |
| | | |
| | | |
| | |
| Warrants exercisable, December 31, 2023 | |
| 47,093 | | |
$ | 1.56 | | |
| 2.70 | |
NOTE 10
– RELATED PARTY TRANSACTIONS
The Company utilizes the services of the brother
of the Chief Executive Officer, who is trained as a computer engineer and has over seven years’ experience with clinical lab operations,
to oversee the Company’s laboratory information systems and patient/physician portals. During the years ended December 31, 2023
and 2022, the Company paid $101,978 and $58,078, respectively, to this related party.
The Chief Executive Officer founded an organization
in January 2021 to create an alliance of clinical labs, entrepreneurs, scientists, healthcare providers, and concerned citizens who oppose
Congressional legislation to require FDA pre-approval for new laboratory tests, known as the VALID Act. The Company contributed $31,050
and $75,000 in 2023 and 2022 to this organization, respectively.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
NOTE 11
– INCOME TAXES
The following table presents the current and deferred
income tax provision for federal and state income taxes for the years ended December 31, 2023 and 2022:
| | |
| 2023 | | |
| 2022 | |
| Current provision for income taxes | |
$ | - | | |
$ | - | |
| Deferred income tax benefit | |
| - | | |
| - | |
| Total provision for income taxes | |
$ | - | | |
$ | - | |
The provision for federal income taxes differs
from that computed by applying federal and state statutory rates to the loss before federal income tax provision, as indicated in the
following analysis:
| | |
2023 | | |
2022 | |
| Expected federal tax (expense) benefit | |
$ | 1,342,200 | | |
$ | (459,200 | ) |
| Expected state tax (expense) benefit | |
| 527,300 | | |
| (180,400 | ) |
| Nondeductible expenses and other | |
| (416,700 | ) | |
| (126,900 | ) |
| (Increase) decrease in valuation allowance | |
| (1,452,800 | ) | |
| 766,500 | |
| Total provision for income taxes | |
$ | - | | |
$ | - | |
The major components of the deferred taxes are
as follows at December 31, 2023 and 2022:
| | |
2023 | | |
2022 | |
| Account receivable, net | |
$ | 7,400 | | |
$ | 18,300 | |
| Accumulated depreciation | |
| (1,500 | ) | |
| (1,500 | ) |
| Intangible assets, net | |
| (69,300 | ) | |
| (78,000 | ) |
| Accrued expenses | |
| 62,100 | | |
| 39,400 | |
| Net operating loss | |
| 6,077,100 | | |
| 4,644,800 | |
| Deferred tax asset valuation allowance | |
| (6,075,800 | ) | |
| (4,623,000 | ) |
| | |
$ | - | | |
$ | - | |
The Company files income tax returns for U.S.
federal income tax purposes and in Maryland, Virginia, and Pennsylvania. Based on federal tax returns filed or to be filed through December
31, 2023, the Company had available approximately $20.8 million in U.S. tax net operating loss carryforwards which assesses the utilization
of a Company’s net operating loss carryforwards resulting from retaining continuity of its business operations and changes within
its ownership structure. Net operating loss carryforwards expire in 20 years for federal income tax reporting purposes. For Federal income
tax purposes, the net operating losses begin to expire in 2020, however, carryforward losses for years beginning in 2018 have no expiration.
State net operating loss carryforwards through December 31, 2023 are approximately $20.7 million and have begun to expire in 2020. There
is a full valuation allowance as of December 31, 2023 and 2022 which may be reversed in future periods at a point when the Company can
make the determination that recoverability will be probable. The valuation allowance for deferred tax assets increased and decreased by
approximately $1,452,800 and $766,500 during the years ended December 31, 2023 and 2022, respectively.
The United States Federal and applicable state
returns from 2018 forward are still subject to tax examination by the United States Internal Revenue Service; however, the Company does
not currently have any ongoing tax examinations.
NOTE 12
– SUBSEQUENT EVENTS
The Company has evaluated subsequent events that
occurred after December 31, 2022 through April 26, 2024, the issuance date of these financial statements. Except as set forth below, there
have been no events or transactions during this time which would have a material effect on these financial statements.
20/20 GENESYSTEMS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2023 AND 2022
Option Agreement
On March 22, 2024, the Company entered into an
option agreement with The Board of Regents (the “Board”) of The University of Texas System, an agency of the State of Texas,
on behalf of by The University of Texas M.D. Anderson Cancer Center (“MD Anderson”), pursuant to which MD Anderson has granted
the Company an exclusive six-month option to enter into a royalty-bearing, exclusive license to certain patent rights and technology,
which option may be exercised by the Company upon (i) the Company’s completion of an equity financing with proceeds of at least
$23 million based on a pre-money valuation of at least $70 million (which such financing may not be obtained through a crowdfunding or
Regulation A offering) and (ii) payment of a fee in the amount of $4,457,069.15. Upon exercise of the option, the Company will enter into
a patent and technology license agreement with the Board and MD Anderson, in the form attached to the option agreement, pursuant to which
MD Anderson will grant the license to the Company in exchange for certain royalties, fees and shares of Common Stock set forth in the patent and technology
license agreement.
Convertible Bonds Subscription Agreement
On March 20, 2024, the Company entered into a
convertible bonds subscription agreement with Cornerstone Investment Inc. (the “Investor”), pursuant to which the Company
agreed to issue a convertible bond in the principal amount of $23 million to the Investor (or its designee) for a purchase price of $23
million. The issuance of the convertible bond is subject to customary closing conditions, as well as execution of the option agreement
described above and a collaborative research agreement with MD Anderson. The convertible bond will have a term of five (5) years and will
not bear interest; provided that (i) if any portion of the convertible bond has not been converted prior to the maturity date or the date
on which an event of default (as defined in the convertible bond) occurs, as applicable, and (ii) the Investor desires to receive a cash
payment with respect to such unconverted portion on the maturity date or the date on which an event of default occurs, as applicable,
the Company shall be required to pay the Investor, in addition any other amounts required under the convertible bond, interest accrued
on the aggregate principal sum of the convertible bond at a rate equal to 6% per annum from the date on which the convertible bond is
issued up to the maturity date or the date on which an event of default occurs, as applicable. In addition, if the convertible bond is
still outstanding after the maturity date or the date on which an event of default occurs, as applicable, then interest shall accrue beginning
on the day after the maturity date on the outstanding principal balance and the default amount at a rate equal to 12% per annum. The Company
may not prepay the convertible bond prior to the maturity date.
The convertible bond will be convertible at any
time at the option of the holder into shares of Common Stock, or, subject to stockholder approval, a new series of Preferred Stock to
be designated as Series E Preferred Stock with the terms and conditions set forth in Annex A to the convertible bond. In addition, the
convertible bond shall automatically be converted into Series E Preferred Stock upon the earlier to occur of (i) an initial public offering
of the Company’s Common Stock and concurrent listing on a national securities exchange, including without limitation the New York
Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), (ii) a direct listing of the Company’s Common Stock on a national
securities exchange, including without limitation the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier) or
(iii) upon such stockholder approval. The number of shares to be issued upon conversion shall be equal (i) the outstanding principal amount
of the convertible bond and all accrued and unpaid default interest, if any, divided by (ii) the conversion price then in effect. The
initial conversion price will be $5.34 per share, subject to customary adjustments for stock dividends, stock splits, stock combinations,
reclassifications, mergers, consolidations, sales of all assets, or similar events. In addition, subject to certain exceptions, if the
Company issues any equity securities with an implied price per share of less than the conversion price then in effect, then the conversion
price shall be adjusted, concurrently with such equity issuance, to the implied price per share received by the Company for such equity
issuance. Finally, the conversion price will be subject to adjustment in the event that the Company completes an initial public offering
or a direct listing of its Common Stock on a national securities exchange that does not meet the requirements of a Q-IPO or a Direct Listing
(each as defined below).
The convertible bond will be unsecured and will
contain customary covenants and events of default for a loan of this type. The Company also agreed that the Investor will be entitled
to nominate at least one (1) director to the board of directors as long as the convertible bond is outstanding. Subject to certain exceptions,
the Company also agreed that the Investor will have the right to participate in any subsequent financing transactions involving the issuance
of Common Stock or securities convertible into or exercisable or exchangeable for Common Stock for cash consideration in an amount required
to maintain the Investor’s fully diluted ownership in the Company. The Company also agreed to use its best efforts to (i) close
a firm commitment underwritten public offering and concurrent listing on a national securities exchange, including without limitation
the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), with a per share offering price of at least $5.34 plus
interest accrued on $5.34 at a rate equal to 6% per annum from the issuance date of the convertible bond up to the date of listing (the
“Target Price” and such offering, a “Q-IPO”), or (ii) complete a direct listing of Common Stock on a national
securities exchange, including without limitation the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), with
the reference price of at least the Target Price (a “Direct Listing”), within three (3) years following the issuance date
of the convertible bond, which period may be extended by one (1) year by mutual agreement between the Company and the Investor.
ITEM 8. EXHIBITS
| Exhibit No. |
|
Description |
| 2.1 |
|
Second Amended and Restated Articles of Incorporation of 20/20 GeneSystems, Inc. (incorporated by reference to Exhibit 2.1 to the Semiannual Report on Form 1-SA filed on November 15, 2018) |
| 2.2 |
|
Certificate of Amendment to Second Amended and Restated Articles of Incorporation of 20/20 GeneSystems, Inc. (incorporated by reference to Exhibit 2.2 to the Semiannual Report on Form 1-SA filed on September 8, 2023) |
| 2.3 |
|
Certificate of Designation of Series C Preferred Stock (incorporated by reference to Exhibit 2.2 to the Annual Report on Form 1-K filed on July 6, 2020) |
| 2.4 |
|
Amended and Restated Bylaws of 20/20 GeneSystems, Inc. (incorporated by reference to Exhibit 2.3 to the Annual Report on Form 1-K filed on July 6, 2020) |
| 3.1 |
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 3.1 to the Offering Statement on Form 1-A/A filed on December 31, 2019) |
| 6.1* |
|
Convertible Bonds Subscription Agreement, dated March 20, 2024, between 20/20 GeneSystems, Inc. and Cornerstone Investment Inc. |
| 6.2+* |
|
Option Agreement, dated March 22, 2024, between 20/20 GeneSystems, Inc. and The Board of Regents of The University of Texas System, on behalf of The University of Texas M. D. Anderson Cancer Center |
| 6.3+* |
|
Collaborative Research Agreement, dated April 26, 2024 between 20/20 GeneSystems, Inc. and The University of Texas M. D. Anderson Cancer Center |
| 6.4* |
|
Technology License and Access Agreement, dated August 10, 2022, between BioInfra Life Science, Inc. and 20/20 GeneSystems, Inc. |
| 6.5* |
|
Lab Services and Marketing Agreement, dated August 5, 2021, between Minomic International and 20/20 GeneSystems, Inc. |
| 6.6 |
|
Lease Agreement, dated March 18, 2021, between Shady Grove Development Park IX L.L.L.P. and 20/20 GeneSystems, Inc. (incorporated by reference to Exhibit 6.11 to the Annual Report on Form 1-K filed on April 30, 2021) |
| 6.7 |
|
Employment Agreement, dated May 5, 2019, between 20/20 GeneSystems, Inc. and Jonathan Cohen (incorporated by reference to Exhibit 6.8 to the Offering Statement on Form 1-A filed on August 12, 2019) |
| 6.8 |
|
20/20 GeneSystems, Inc. Stock Incentive Plan (incorporated by reference to Exhibit 6.3 to the Annual Report on Form 1-K filed on May 26, 2022) |
| 6.9 |
|
Form of Stock Option Agreement (incorporated by reference to Exhibit 6.4 to the Annual Report on Form 1-K filed on May 26, 2022) |
| 6.10 |
|
Form of Restricted Stock Award Agreement (incorporated by reference to Exhibit 6.5 to the Annual Report on Form 1-K filed on May 26, 2022) |
| + | Certain confidential information contained these exhibits has
been omitted in accordance with Item 17 of Form 1-A because we customarily and actually treat that information as private or confidential
and the omitted information is not material. |
SIGNATURES
Pursuant to the requirements
of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: April 29, 2024 |
20/20 GENESYSTEMS, INC. |
| |
|
| |
/s/ Jonathan Cohen |
| |
Name: Jonathan Cohen |
| |
Title: Chief Executive Officer |
| |
(Principal Executive Officer) |
| |
|
| |
/s/ Anne Shiflett |
| |
Name: Anne Shiflett |
| |
Title: Acting Chief Financial Officer |
| |
(Principal Financial and Accounting Officer) |
Pursuant to the requirements
of Regulation A, this report has been signed below by the following persons on behalf of the issuer and in the capacities and on the dates
indicated.
| SIGNATURE |
|
TITLE |
|
DATE |
| |
|
|
|
|
| /s/ Jonathan Cohen |
|
Chief Executive Officer, President and Director |
|
April 29, 2024 |
| Jonathan Cohen |
|
(principal executive officer) |
|
|
| |
|
|
|
|
| /s/ Anne Shiflett |
|
Acting Chief Financial Officer |
|
April 29, 2024 |
| Anne Shiflett |
|
(principal financial and accounting officer) |
|
|
| |
|
|
|
|
| /s/ John G. Compton |
|
Chairman of the Board |
|
April 29, 2024 |
| John G. Compton |
|
|
|
|
| |
|
|
|
|
| /s/ Richard M. Cohen |
|
Director |
|
April 29, 2024 |
| Richard M. Cohen |
|
|
|
|
| |
|
|
|
|
| /s/ Wei Lu |
|
Director |
|
April 29, 2024 |
| Wei Lu |
|
|
|
|
| |
|
|
|
|
| /s/ Prasanth Reddy |
|
Director |
|
April 29, 2024 |
| Prasanth Reddy |
|
|
|
|
| |
|
|
|
|
| /s/ John W. Rollins |
|
Director |
|
April 29, 2024 |
| John W. Rollins |
|
|
|
|
| |
|
|
|
|
| /s/ Michael A. Ross |
|
Director |
|
April 29, 2024 |
| Michael A. Ross |
|
|
|
|
54
Exhibit 6.1
CONVERTIBLE BONDS SUBSCRIPTION AGREEMENT
This Convertible Bonds Subscription
Agreement, dated as of March 20, 2024 (this “Agreement”), is entered into by and between 20/20 GeneSystems, Inc., a
Delaware corporation having its main office at 15810 Gaither Dr., Suite 235, Gaithersburg, Maryland, U.S.A. (the “Company”),
and Cornerstone Investment Inc., a Korean corporation having its registered office at 21F S-Tower, 82 Saemunan-ro, Jongno-gu, Seoul, Korea
(the “Investor”).
RECITALS
A. On the terms and subject
to the conditions set forth herein, the Company desires to issue and sell to the Investor, and the Investor desires to subscribe for,
convertible bonds with aggregate principal amount of US$23,000,000.
B. Capitalized terms not otherwise
defined herein shall have the meaning set forth in the form of Bond (defined below) attached hereto as Exhibit A.
AGREEMENT
NOW THEREFORE, in consideration
of the foregoing, and the representations, warranties, and conditions set forth below, the parties hereto, intending to be legally bound,
hereby agree as follows:
1. The
Bonds.
(a) Issuance
of Bonds. Subject to all of the terms and conditions hereof, the Company agrees to issue and sell to the Investor, and the Investor
agrees to subscribe for, convertible bonds in the form of Exhibit A hereto (each, a “Bond” and, collectively,
the “Bonds”) in the aggregate principal amount of US$23,000,000.
(b) Delivery.
The sale and purchase of the Bonds shall take place at a closing (the “Closing”) to be held at such place and time
as the Company and the Investor may determine; provided that the Investor will use commercially reasonable efforts to close within six
(6) weeks from the date that the condition set forth in Section 4(f) is satisfied, but in no event later than eight (8) weeks following
the satisfaction of the condition set forth in Section 4(f) (the date of the Closing, the “Closing Date”). At
the Closing, the Company will deliver to the Investor the Bonds against receipt by the Company of the corresponding subscription price
(the “Subscription Price”). Each of the Bonds will be registered in the Investor’s name in the Company’s
records.
(c) Use
of Proceeds. The Company shall use the proceeds of the transactions contemplated hereby for working capital and general corporate
purposes. The Company will make such disclosures regarding its expenditures as are required in reports filed with the Securities and Exchange
Commission (the “SEC”); provided that as soon as reasonably practical upon receiving the Investor’s request,
the Company shall provide to the Investor such information regarding its expenditures as reasonably requested by the Investor.
(d) Payments.
The Company will make all cash payments due under the Bonds in immediately available funds by 1:00 p.m. Eastern time on the date such
payment is due at the address for such purpose specified below the Investor’s name on Schedule I hereto, or at such other
address, or in such other manner, as the Investor or other registered holder of a Bond may from time to time direct in writing.
2. Representations
and Warranties of the Company. Except as set forth on the disclosure schedule, attached as Schedule II (the “Disclosure
Schedule”), the Company represents and warrants to the Investor that the following statements are true and correct as of the
date hereof and as of the Closing Date, except to the extent such representations and warranties are specifically made as of a particular
date (in which case such representations and warranties will be true and correct as of such date):
(a) Due
Incorporation, Qualification, etc. The Company (i) is a corporation duly organized, validly existing and in good standing under the
laws of the State of Delaware; (ii) has the power and authority to own, lease and operate its properties and carry on its business as
now conducted; and (iii) is duly qualified, licensed to do business and in good standing as a foreign corporation in each jurisdiction
where the failure to be so qualified or licensed could reasonably be expected to have a material adverse effect on the Company.
(b) Authority.
Except for Stockholder Approval, if required, the execution, delivery and performance by the Company of each Transaction Document to be
executed by the Company and the consummation of the transactions contemplated thereby (i) are within the power of the Company and (ii)
have been duly authorized by all necessary actions on the part of the Company.
(c) Enforceability.
Each Transaction Document executed, or to be executed, by the Company has been, or will be prior to the Closing, duly executed and delivered
by the Company and constitutes, or will constitute duly executed and delivered by the Company, a legal, valid and binding obligation of
the Company, enforceable against the Company in accordance with its terms, except as limited by bankruptcy, insolvency or other laws of
general application relating to or affecting the enforcement of creditors’ rights generally and general principles of equity.
(d) Non-Contravention.
Subject to Stockholder Approval, if required, the execution and delivery by the Company of the Transaction Documents executed by the Company
and the performance and consummation of the transactions contemplated thereby do not and will not (i) violate the Company’s Certificate
of Incorporation or Bylaws (as amended, the “Charter Documents”) or any judgment, order, writ, decree, statute, rule
or regulation applicable to the Company; (ii) violate any provision of, or result in the breach or the acceleration of, or entitle any
other Person to accelerate (whether after the giving of notice or lapse of time or both), any mortgage, indenture, agreement, instrument
or contract to which the Company is a party or by which it is bound; or (iii) result in the creation or imposition of any Lien upon any
property, asset or revenue of the Company or the suspension, revocation, impairment, forfeiture, or nonrenewal of any material permit,
license, authorization or approval applicable to the Company, its business or operations, or any of its assets or properties.
(e) Capitalization.
Section 2(e) of the Disclosure Schedule sets forth an accurate and complete list as of the date hereof of the authorized capital stock
of the Company, and the outstanding shares of capital stock of the Company. The outstanding shares of capital stock or equity interests
of the Company are validly issued, fully paid and non-assessable. Except as set forth on Section 2(e) of the Disclosure Schedule, there
is neither (i) any outstanding options, warrants, purchase rights, subscription rights, conversion rights, exchange rights or other contracts
or commitments that could require the Company to either issue any of the capital stock or other equity interests of the Company or acquire
any existing shares of the capital stock or other equity interests from other stockholders of the Company, other than statutory pre-emptive
rights under applicable laws; nor (ii) any securities or rights convertible into, or exchangeable with, shares of any capital stock of
the Company.
(f) Subsidiaries.
The Company has no subsidiaries.
(g) Approvals.
No consent, approval, order or authorization of, or registration, declaration or filing with, any governmental authority or other Person
is required in connection with the execution and delivery of the Transaction Documents executed by the Company and the performance and
consummation of the transactions contemplated thereby, other than (i) the filing of Form D with the SEC and such filings as are required
to be made under applicable State securities laws and (ii) Stockholder Approval, if required.
(h) No
Violation or Default. The Company is not, and has not been, in violation of or in default with respect to (i) its Charter Documents,
(ii) any material judgment, order, writ, decree, statute, rule or regulation applicable to the Company; or (iii) any material mortgage,
indenture, agreement, instrument or contract to which the Company is a party or by which it is bound (nor is there any waiver in effect
which, if not in effect, would result in such a violation or default). The Company is not, and has not been, in material violation of
any federal or state statute, rule or regulation applicable to the Company. The Company has not received any written notice from any governmental
authority regarding any actual, alleged, or potential violation of, or failure to comply with, any term or requirement of any applicable
law. Each contract and other financial arrangement and relationship entered into by the Company with customers, vendors, suppliers, employees,
and contractors is, and has been, in compliance in all material respects with all applicable laws.
(i) No
“Bad Actor” Disqualification. The Company has exercised reasonable care, in accordance with SEC rules and guidance, to
determine whether any Covered Person (as defined below) is subject to any of the “bad actor” disqualifications described in
Rule 506(d)(1)(i) through (viii) under the Securities Act (“Disqualification Events”). To the Knowledge of the Company,
no Covered Person is subject to a Disqualification Event, except for a Disqualification Event covered by Rule 506(d)(2) or (d)(3) under
the Securities Act. The Company has complied, to the extent applicable, with any disclosure obligations under Rule 506(e) under the Securities
Act. “Covered Persons” are those persons specified in Rule 506(d)(1) under the Securities Act, including the Company;
any predecessor or affiliate of the Company; any director, executive officer, other officer participating in the offering, general partner
or managing member of the Company; any beneficial owner of 20% or more of the Company’s outstanding voting equity securities, calculated
on the basis of voting power; any promoter (as defined in Rule 405 under the Securities Act) connected with the Company in any capacity
at the time of the sale of the Bonds; and any person that has been or will be paid (directly or indirectly) remuneration for solicitation
of purchasers in connection with the sale of the Bonds (a “Solicitor”), any general partner or managing member of any
Solicitor, and any director, executive officer or other officer participating in the offering of any Solicitor or general partner or managing
member of any Solicitor.
(j) Litigation.
There is no claim, action, suit, proceeding, arbitration, complaint, charge or investigation pending, or to the Knowledge of the Company,
currently threatened (i) against the Company or any officer, director or Key Employee of the Company; (ii) that questions the validity
of the Transaction Documents or the right of the Company to enter into them, or to consummate the transactions contemplated by the Transaction
Documents; or (iii) to the Knowledge of the Company, that would reasonably be expected to have, either individually or in the aggregate,
a material adverse effect on the Company. Neither the Company nor, to the Knowledge of the Company, any of its officers, directors or
Key Employees is a party or is named as subject to the provisions of any order, writ, injunction, judgment or decree of any court or government
agency or instrumentality (in the case of officers, directors or Key Employees, such as would affect the Company). There is no action,
suit, proceeding or investigation by the Company pending or which the Company intends to initiate. The foregoing includes, without limitation,
actions, suits, proceedings or investigations pending or threatened in writing (or any basis therefor to the Knowledge of the Company)
involving the prior employment of any of the Company’s employees, their services provided in connection with the Company’s
business, any information or techniques allegedly proprietary to any of their former employers or their obligations under any agreements
with prior employers.
(k) Intellectual
Property. The Company owns or possesses sufficient legal rights to all Company’s intellectual property without any known conflict
with, or infringement of, the rights of others, including prior employees or consultants, or academic institutions with which any of them
may be affiliated now or may have been affiliated in the past. To the Knowledge of the Company, no product or service marketed or sold
(or proposed to be marketed or sold) by the Company violates or will violate any license or infringes or will infringe any intellectual
property rights of any Person. Other than with respect to commercially available software products under standard end-user object code
license agreements, there are no outstanding options, licenses, agreements, claims, encumbrances or shared ownership interests of any
kind relating to the Company’s intellectual property, nor is the Company bound by or a party to any options, licenses or agreements
of any kind with respect to the patents, trademarks, service marks, trade names, copyrights, trade secrets, licenses, information, proprietary
rights and processes of any other person. The Company has not received any communications alleging that the Company has violated, or by
conducting its business, would violate any of the patents, trademarks, service marks, tradenames, copyrights, trade secrets, mask works
or other proprietary rights or processes of any Person. The Company has obtained and possesses valid licenses to use all of the software
programs present on the computers and other software-enabled electronic devices that it owns or leases or that it has otherwise provided
to its employees for their use in connection with the Company’s business. To the Knowledge of the Company, it will not be necessary
to use any inventions of any of its employees or consultants (or persons it currently intends to hire) made prior to their employment
by the Company, including prior employees or consultants, or academic or medical institutions with which any of them may be affiliated
now or may have been affiliated in the past. Each employee and consultant has assigned to the Company all intellectual property rights
he or she owns that are related to the Company’s business as now conducted and as presently proposed to be conducted and all intellectual
property rights that he, she or it solely or jointly conceived, reduced to practice, developed or made during the period of his, her or
its employment or consulting relationship with the Company that (i) related, at the time of conception, reduction to practice, development,
or making of such intellectual property right, to the Company’s business as then conducted or as then proposed to be conducted,
(ii) were developed on any amount of the Company’s time or with the use of any of the Company’s equipment, supplies, facilities
or information or (iii) resulted from the performance of services for the Company. The Company has not embedded any open source, copyleft
or community source code in any of its products generally available or in development, including but not limited to any libraries or code
licensed under any general public license, lesser general public license or similar license arrangement. For purposes of this Section 2(k),
the Company shall be deemed to have knowledge of a patent right if the Company has actual knowledge of the patent right or would be found
to be on notice of such patent right as determined by reference to United States patent laws. No government funding, facilities of a university,
college, other educational institution or research center, or funding from third parties was used in the development of any of the Company’s
intellectual property. No person who was involved in, or who contributed to, the creation or development of any of the Company’s
intellectual property, has performed services for the government, university, college, or other educational institution or research center
in a manner that would affect Company’s rights in the Company’s intellectual property.
(l) Financial
Statements. The Company has delivered to the Investor its audited financial statements as of and for the fiscal year ended December
31, 2022 and 2021, respectively, and unaudited financial data for the 6-month period ended June 30, 2023 (collectively, the “Financial
Statements”). The Financial Statements have been prepared in accordance with United States generally accepted accounting principles
(the “Accounting Principles”) applied on a consistent basis throughout the periods indicated. The Financial Statements
fairly present in all material respects the financial condition and operating results of the Company as of the dates, and for the periods,
indicated therein, subject in the case of the unaudited Financial Statements to normal year-end audit adjustments. Except as set forth
in the Financial Statements, the Company has no material liabilities or obligations, contingent or otherwise, other than (i) liabilities
incurred in the ordinary course of business; (ii) obligations under contracts and commitments incurred in the ordinary course of
business; and (iii) liabilities and obligations of a type or nature not required under the Accounting Principles to be reflected in the
Financial Statements, which, in all such cases, individually and in the aggregate would not have a material adverse effect. The Company
maintains and will continue to maintain a standard system of accounting established and administered in accordance with the Accounting
Principles.
(m)
Absence of Certain Changes; No Material Adverse Change. Since June 30, 2023, (i) the Company has conducted its business only in
the ordinary course and (ii) there has been no material adverse effect in the financial condition, assets, liabilities, business, operations,
customers or prospects of the Company or in the value or condition of its properties and assets. To the Knowledge of the Company, there
is no fact or contingency which could reasonably be expected to result in a material adverse effect in the financial condition, assets,
liabilities, business, operations, customers or prospects of the Company or in the value or condition of its properties and assets.
(n) Conversion
Securities. The shares of the Company issuable upon conversion of the Bonds (the “Conversion Securities”) shall
be duly and validly issued, non-assessable and fully paid, and shall be free of any preemptive or similar rights or any Lien. Subject
to the accuracy of the representations and warranties of the Investor in this Agreement, the offer and issuance by the Company of the
Bonds and the Conversion Securities is exempt from registration under the Securities Act.
(o) Title
to Properties; Liens. The Company has (i) good and marketable title to all its properties and assets, both real and personal, and
(ii) good title to all its leasehold interests, in each case not being subject to any Lien, other than Liens relating to current taxes
not yet due and payable. With respect to the properties and assets leased by the Company it is in compliance with such leases, which are
in force and effect. All properties of the Company (i) have been maintained in accordance with normal industry practice, are in good operating
condition and repair (subject to normal wear and tear) and are suitable for the purposes for which they are presently used; and (ii) when
taken as a whole, constitute all properties and assets necessary to permit the Company to carry on its business as conducted by the Company.
(p) Permits.
The Company has all franchises, permits, licenses and any similar governmental approval necessary for the conduct of its business as currently
conducted (the “Licenses”), and the Company will be able to obtain and maintain, without undue burden or expense, all
such Licenses for the conduct of its business as proposed. The Licenses are in full force and effect. The Company is not in default under
any of its Licenses, nor has it received any notice relating to the suspension, revocation or modification of any of its Licenses.
(q) Labor
Relations. The Company is in compliance with all laws respecting employment and employment practices, terms and conditions of employment
and wages and hours in all material respects, and is not, and has not, engaged in any unfair labor practice. There is no unfair labor
practice claim or complaint, or workers’ compensation claim, against the Company pending or, to the Knowledge of the Company, threatened
before any governmental authority. There is no labor strike, work stoppage, production or work slowdown or other labor dispute involving
the Company or any of its employees pending or, to the Knowledge of the Company, threatened against the Company and, to the Knowledge
of the Company, there is no labor union or other employee organizing effort pending or threatened against the Company and there has not
been any such labor strike, work stoppage, production or work slowdown or other labor dispute. To the Knowledge of the Company, no Key
Employee intends to terminate employment with the Company or is otherwise likely to become unavailable to continue as a Key Employee,
nor does the Company have a present intention to terminate the employment of any of the foregoing.
(r) Tax.
The Company has filed all U.S. federal, and material state and foreign tax returns required to be filed, has paid all taxes required to
be paid in respect of any and all periods, has established an adequate accrual or reserve for the payment of all taxes payable in respect
of the periods subsequent to the periods covered by the most recent applicable tax returns, has made all necessary estimated tax payments,
and has no liability for taxes in excess of the amount so paid or accruals or reserves so established. The Company is not delinquent in
the payment of any tax and is not delinquent in the filing of any material tax return, and no deficiencies for any tax have been threatened,
claimed, proposed or assessed against the Company. The Company is not currently under any action, audit, investigation, examination, litigation
or any other proceedings, pending or threatened, nor has the Company been audited, by any relevant tax authority or any other governmental
authority, with respect to any tax or tax return other than any such item that is being contested in good faith. The Company has withheld
and paid to the proper governmental authority all taxes required to have been withheld in connection with amounts paid or owing to any
employee, independent contractor, creditor, shareholder or other third party. The Company has not waived any statute of limitations in
respect of taxes or agreed to any extension of time with respect to a tax assessment or deficiency.
(s) Related
Party Transactions. All transactions between the Company, on the one hand, and any of its and its affiliates’ respective directors,
executive officers, employees, stockholders or affiliates, or any director or executive officer of any entity in which any director or
executive officer of the Company is a manager or directly or indirectly holds equity securities or its respective assets, on the other
hand (collectively, “Related Party Transactions”) were entered into on an arm’s-length basis and, to the extent
not expired or terminated, are in full force and effect and enforceable in accordance with their terms. Except as set forth in Section
2(s) of the Disclosure Schedule, there is no Related Party Transaction.
(t) Data
Privacy. In connection with its collection, storage, transfer (including, without limitation, any transfer across national borders)
and/or use of any personally identifiable information from any individuals, including, without limitation, any customers, prospective
customers, employees and/or other third parties (collectively, “Personal Information”), the Company is and has been
in compliance with all applicable laws in all relevant jurisdictions, the Company’s privacy policies and the requirements of any
contract or codes of conduct to which the Company is a party. The Company has commercially reasonable physical, technical, organizational
and administrative security measures and policies in place to protect all Personal Information collected by it or on its behalf from and
against unauthorized access, use and/or disclosure. The Company is and has been in compliance in all material respects with all laws relating
to data loss, theft and breach of security notification obligations.
3. Representations
and Warranties of Investor. The Investor represents and warrants to the Company that the following statements are true and correct
as of the date hereof and as of the Closing Date:
(a) Binding
Obligation. The Investor has full legal capacity, power and authority to execute and deliver this Agreement and to perform its obligations
hereunder. This Agreement and the Transaction Documents constitute valid and binding obligations of the Investor, enforceable in accordance
with their terms, except as limited by bankruptcy, insolvency or other laws of general application relating to or affecting the enforcement
of creditors’ rights generally and general principles of equity.
(b) Securities
Law Compliance. The Investor has been advised that the Bonds and the underlying securities have not been registered under the Securities
Act, or any state securities laws and, therefore, cannot be resold unless they are registered under the Securities Act and applicable
state securities laws or unless an exemption from such registration requirements is available. The Investor is purchasing the Bonds hereunder
for its own account for investment, not as a nominee or agent, and not with a view to, or for resale in connection with, the distribution
thereof, and the Investor has no present intention of selling, granting any participation in, or otherwise distributing the same. Further,
the representations in Section 3(b)(i) and/or Section 3(b)(ii), as applicable, are true and correct:
(i) U.S. Investor Investment
Representations. The Investor is an accredited investor as such term is defined in Rule 501 of Regulation D under the Securities
Act. The Investor has furnished or made available any and all information requested by the Company or otherwise necessary to satisfy any
applicable verification requirements as to accredited investor status.
(ii) Non-U.S. Investor
Investment Representations. The Investor is not a “U.S. Person,” as such term is defined in Regulation S promulgated
under the Securities Act. No offer or sale of the Bonds was made to the Investor in the United States. The Investor is not acquiring
the Bonds for the account or on behalf of any U.S. Person. The Investor has not made any prearrangement to transfer the Bonds or underlying
securities to a U.S. Person or to return the Bonds or underlying securities to the United States securities markets (which includes short
sales in the United States within the applicable “distribution compliance period,” as defined in Regulation S (hereinafter
referred to as the “restricted period”)) and is not purchasing the Bonds as part of any plan or scheme to evade the
registration requirements of the Securities Act. All offers and sales of the Bonds or the underlying securities by the Investor in the
United States or to U.S. Persons or otherwise whether prior to the expiration or after the expiration of the applicable restricted period
shall be made only pursuant to a registration of the Bonds and/or the underlying securities under the Securities Act or an exemption from
registration, and in compliance with Regulation S. The Investor is not a “distributor,” as defined in Regulation S.
The Investor acknowledges that the Investor may only be able to resell the Bonds and the underlying securities pursuant to the provisions
of Regulation S and otherwise pursuant to the Securities Act, and that it may not be possible for the Investor to liquidate its investment
in the Bonds or the underlying securities. The Investor is prepared, therefore, to hold its Bonds and/or the underlying securities indefinitely.
(c) Foreign
Investor. If the Investor is not a U.S. Person, the Investor hereby represents that, except for the foreign direct investment
report to be filed in Korea prior to the Closing and other required Korean filings that will be made by the Investor when due following
the Closing, it has satisfied itself as to the full observance of the laws of its jurisdiction in connection with any invitation to subscribe
for the Bonds or any use of this Agreement, including (i) the legal requirements within its jurisdiction for the purchase of the
Bonds, (ii) any foreign exchange restrictions applicable to such purchase, (iii) any governmental or other consents that may
need to be obtained, and (iv) the income tax and other tax consequences, if any, that may be relevant to the purchase, holding, redemption,
sale, or transfer of the Bonds. The Investor’s subscription and payment for the Bonds does not violate any applicable securities
or other laws of the Investor’s jurisdiction.
(d) Access
to Information. The Investor acknowledges that the Company has given the Investor access to the corporate records and accounts of
the Company and to all information in its possession relating to the Company, has made its officers and representatives available for
interview by the Investor, and has furnished the Investor with all documents and other information reasonably required for the Investor
to make an informed decision with respect to the purchase of the Bonds.
4. Conditions
to Closing of the Investor. The Investor’s obligations at the Closing are subject to the fulfillment, on or prior to the
Closing Date, of all of the following conditions, any of which may be waived in whole or in part by the Investor:
(a) Representations
and Warranties. The representations and warranties made by the Company in Section 2 hereof shall have been true and correct
when made, and shall be true and correct on the Closing Date, except to the extent such representations and warranties are specifically
made as of a particular date in which case such representations and warranties will be true and correct as of such date.
(b) Governmental
Approvals and Filings. Except for any notices required or permitted to be filed after the Closing Date with certain federal and state
securities commissions, the Company shall have obtained all governmental approvals required in connection with the lawful sale and issuance
of the Bonds.
(c) Legal
Requirements. At the Closing, the sale and issuance by the Company, and the subscription by the Investor, of the Bonds shall be legally
permitted by all laws and regulations to which the Investor or the Company is subject.
(d) Proceedings
and Documents. All corporate and other proceedings in connection with the transactions contemplated at the Closing and all documents
and instruments incident to such transactions shall be reasonably satisfactory in substance and form to the Investor.
(e) Transaction
Documents. The Company shall have duly executed and delivered to the Investor the Transaction Documents.
(f) MD
Anderson Cancer Center Agreement. The Company shall have duly executed and delivered to the Investor an executed copy of (i) the option
agreement by and between the Company and the Board of Regents of the University of Texas System (the “Regents”) (on
behalf of the University of Texas M. D. Anderson Cancer Center (the “MDACC”)), substantially in the form attached hereto
as Exhibit B, and (ii) the collaborative research agreement by and between the Company and the Regents (on behalf of the MDACC),
substantially in the form attached hereto as Exhibit C (collectively, the “MDACC Agreements”).
5. Conditions
to Obligations of the Company. The Company’s obligation to issue and sell the Bonds at the Closing is subject to the fulfillment,
on or prior to the Closing Date, of the following conditions, any of which may be waived in whole or in part by the Company:
(a) Representations
and Warranties. The representations and warranties made by the Investor in Section 3 hereof shall be true and correct when
made, and shall be true and correct on the Closing Date.
(b) Governmental
Approvals and Filings. The Investor shall have obtained all governmental approvals required in connection with the lawful subscription
for the Bonds.
(c) Legal
Requirements. At the Closing, the sale and issuance by the Company, and the subscription by the Investor, of the Bonds shall be legally
permitted by all laws and regulations to which the Investor or the Company is subject.
(d) Subscription
Price. The Investor shall have delivered to the Company the Subscription Price in respect of the Bonds.
6. Miscellaneous.
(a) Waivers
and Amendments. Any provision of this Agreement, and the Bonds may be amended, waived or modified only upon the written consent of
the Company and the Investor.
(b) Fees
and Expenses. Each party shall be entirely responsible for its own respective legal and administrative costs in connection with the
issuance and sale of the Bonds.
(c) Governing
Law; Dispute Resolution. This Agreement shall be governed by the internal law of the State of Delaware, without regard to conflict
of law principles that would result in the application of any law other than the law of the State of Delaware. All disputes, controversies
or claims between the parties hereto arising out of or in connection with this Agreement (including its existence, validity or termination)
that cannot be amicably resolved shall be finally resolved and settled by arbitration administered by the London Court of International
Arbitration in accordance with its arbitration rules, which arbitration rules are deemed to be incorporated by reference into this Section
6(c). The arbitration tribunal shall be composed of one (1) arbitrator. The arbitration will take place in London, England, and shall
be conducted in the English language. The arbitration award shall be final and binding on the parties, and judgment on the award rendered
by the arbitrator may be entered in any court having jurisdiction thereof. Each party hereto irrevocably submits to the non-exclusive
jurisdiction of the federal and state courts located in the City of New York, NY or the courts located in Seoul, Korea for such purpose,
and each party hereby waives any objection such person may have based on improper venue or inconvenient forum in connection with any such
action or proceeding in any such court.
(d) Survival.
The representations, warranties, covenants and agreements made herein shall survive the execution and delivery of this Agreement.
(e) Successors
and Assigns. Subject to the restrictions on transfer described in Section 6(f) below, the rights and obligations of the Company
and the Investor shall be binding upon and benefit the successors, assigns, heirs, administrators and transferees of the parties. Notwithstanding
anything to the contrary contained herein, the Investor may assign or transfer this Agreement in its entirety to a fund advised or managed
by the Investor prior to the Closing with a prior written notice to the Company, and upon such assignment or transfer, the original Investor
shall be released from its obligations hereunder and the fund shall for all purposes be considered the “Investor” party to
this Agreement and shall have all the rights and obligations of the Investor under this Agreement to the same extent as if it were an
original party hereto.
(f) Registration,
Transfer and Replacement of the Bonds. The Bonds issuable under this Agreement shall be bonds registered under the Investor’s
name. The Company will keep at its principal executive office books for the registration and registration of transfer of the Bonds. Prior
to presentation of any Bond for registration of transfer, the Company shall treat the Person in whose name such Bond is registered as
the owner and holder of such Bond for all purposes whatsoever, whether or not such Bond shall be overdue, and the Company shall not be
affected by notice to the contrary. Subject to any restrictions on or conditions to transfer set forth in any Bond, the holder of any
Bond, at its option, may in person or by duly authorized attorney surrender the same for exchange at the Company’s chief executive
office, and promptly thereafter and at the Company’s expense, except as provided below, receive in exchange therefor one or more
new Bond(s), each in the principal requested by such holder, dated the date to which interest shall have been paid on the Bond so surrendered
or, if no interest shall have yet been so paid, dated the date of the Bond so surrendered and registered in the name of such Person or
Persons as shall have been designated in writing by such holder or its attorney for the same principal amount as the then unpaid principal
amount of the Bond so surrendered. Upon receipt by the Company of evidence reasonably satisfactory to it of the ownership of and the loss,
theft, destruction or mutilation of any Bond and (i) in the case of loss, theft or destruction, of indemnity reasonably satisfactory to
it; or (ii) in the case of mutilation, upon surrender thereof, the Company, at its expense, will execute and deliver in lieu thereof a
new Bond executed in the same manner as the Bond being replaced, in the same principal amount as the unpaid principal amount of such Bond
and dated the date to which interest shall have been paid on such Bond or, if no interest shall have yet been so paid, dated the date
of such Bond.
(g) Voting
of Securities. Unless otherwise prohibited by any regulatory authorities or applicable law, including those of the Republic of Korea,
the Investor shall not vote against any person nominated by the Company’s board of directors or the nominating committee, in either
case, in the Investor’s capacity as a holder of the Conversion Stock issued upon conversion of the Bond or common stock of the Company
issued upon conversion of the Conversion Stock for a period of three (3) years from the Original Issuance Date (as defined in Annex A
to the Bond); provided, however, that such three year period shall commence on the original issuance date of the Bond if the Company is
not listed on a national securities exchange within twelve (12) months of the original issuance date of the Bond.
(h) Market
Standoff. In connection with any underwritten public offering by the Company of its equity securities pursuant to an effective registration
statement filed under the Securities Act, including the Company’s initial public offering, the Investor shall not directly or indirectly
sell, make any short sale of, loan, hypothecate, pledge, offer, grant or sell any option or other contract for the purchase of, purchase
any option or other contract for the sale of, or otherwise dispose of or transfer, or agree to engage in any of the foregoing transactions
with respect to, the Bond and the securities underlying the Bond without the prior written consent of the Company or its managing underwriter.
Such restriction shall be in effect for such period of time following the date of the final prospectus for the offering as may be requested
by the Company or such underwriter. In no event, however, shall such period exceed one hundred eighty (180) days plus such additional
period as may reasonably be requested by the Company or such underwriter and as agreed by the Investor (which agreement shall not be unreasonably
withheld or delayed) to accommodate regulatory restrictions on (i) the publication or other distribution of research reports or (ii) analyst
recommendations and opinions.
(i) Entire
Agreement. This Agreement together with the other Transaction Documents constitute and contain the entire agreement among the Company
and Investor and supersede any and all prior agreements, negotiations, correspondence, understandings and communications among the parties,
whether written or oral, respecting the subject matter hereof.
(j) Notices.
All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given
upon the earlier of (i) personal delivery to the party to be notified, (ii) when sent, if sent by electronic mail during normal business
hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (iii) five (5)
days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (iv) one (1) business day after
deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification
of receipt. All communications shall be sent to the respective parties at their address as set forth below or to such e-mail address or
address as subsequently modified by written notice given in accordance with this Section 6(j).
If to the Investor:
Cornerstone Investment Inc.
E-mail:
Attention:
If to the Company:
20/20 GeneSystems, Inc.
15810 Gaither Dr., Suite 235
Gaithersburg, MD 20877
E-mail:
Attention: Jonathan Cohen, Chief Executive Officer
The Investor consents to
the delivery of any stockholder notice pursuant to General Corporation Law of the State of Delaware (as amended or superseded from time
to time, “DGCL”), by electronic transmission pursuant to Section 232 of the DGCL (or any successor thereto) at the
e-mail address set forth in this Section 6(j) or updated from time to time by notice to the Company. To the extent that any notice
given by means of electronic transmission is returned or undeliverable for any reason, the foregoing consent shall be deemed to have been
revoked until a new or corrected e-mail address has been provided, and such attempted electronic notice shall be ineffective and deemed
to not have been given. The Investor agrees to promptly notify the Company of any change in its e-mail address, and that failure to do
so shall not affect the foregoing.
(k) Severability
of this Agreement. If any provision of this Agreement shall be judicially determined to be invalid, illegal or unenforceable, the
validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
(l) Counterparts.
This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall
constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any electronic signature complying
with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered
shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(Signature Page Follows)
The parties have caused this
Agreement to be duly executed and delivered by their proper and duly authorized officers as of the date and year first written above.
| COMPANY: 20/20 GENESYSTEMS, INC. |
|
| a Delaware corporation |
|
| |
|
| By: |
/s/ Jonathan Cohen |
|
| Name: |
Jonathan Cohen |
|
| Title: |
President and CEO |
|
| INVESTOR: CORNERSTONE INVESTMENT INC. |
|
| a Korean corporation |
|
| |
|
| By: |
/s/ Chang Kyu Choi |
|
| Name: |
Chang Kyu Choi |
|
| Title: |
President and CEO |
|
SCHEDULE I
SCHEDULE OF INVESTOR
| Name and Address | |
Principal Amount | |
| Cornerstone Investment Inc. | |
US$ | 23,000,000 | |
Exhibit A
FORM OF BOND
NEITHER THIS SECURITY NOR THE SECURITIES INTO
WHICH THIS SECURITY IS CONVERTIBLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY
STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT, AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT
TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT
TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL
OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
20/20 GENESYSTEMS, INC.
CONVERTIBLE BOND
| US$23,000,000 |
| ______________,2024 |
FOR VALUE RECEIVED, 20/20 GeneSystems, Inc., a
Delaware corporation (the “Company”), promises to pay to __________, or its registered assigns (the “Investor”),
in lawful money of the United States of America the principal sum of $23,000,000, or such lesser amount as shall equal the outstanding
principal amount hereof, together with Default Interest (as defined below), if any. All unpaid principal, together with any then unpaid
and accrued the Default Interest and other amounts payable hereunder (including, without limitation, such amounts payable under Section
1(a)), shall be due and payable upon demand by the Investor after the earlier of (i) ___________, 2029 or (ii) upon an Event of Default
(as defined below) (the “Maturity Date”). This Bond is the “Bond” issued pursuant to the Subscription Agreement.
The following is a statement of the rights of
the Investor and the conditions to which this Bond is subject, and to which the Investor, by the acceptance of this Bond, agrees:
1. Interest;
Payments.
(a) Interest.
Prior to the Maturity Date or the date on which an Event of Default occurs, as applicable, no interest shall be accrued on this Bond;
provided that if (i) any portion of this Bond has not been converted to the Conversion Stock (as defined below) prior to the Maturity
Date or the date on which an Event of Default occurs, as applicable, and (ii) the Investor desires to receive a cash payment with respect
to such unconverted portion on the Maturity Date or the date on which an Event of Default occurs, as applicable, the Company shall be
required to pay the Investor, in addition to the amounts required under the applicable sections of this Bond, interest accrued on the
aggregate principal sum of this Bond at a rate equal to 6% per annum from the date on which this Bond is issued (the “Issuance
Date”) up to the Maturity Date or the date on which an Event of Default occurs, as applicable, computed on the basis of the
actual number of days elapsed and a year of 365 days (the “YTM Amount”). If this Bond is still outstanding after the
Maturity Date or the date on which an Event of Default occurs, as applicable, then interest shall accrue beginning on the day after the
Maturity Date on the outstanding principal balance and the YTM Amount at a rate equal to 12% per annum, computed on the basis of the actual
number of days elapsed and a year of 365 days (the “Default Interest”).
(b) No
Voluntary Prepayment. The Company may not prepay this Bond in whole or in part prior to the Maturity Date.
2. Events
of Default. The occurrence of any of the following shall constitute an “Event of Default” under this Bond and
the other Transaction Documents:
(a) Failure
to Pay. The Company fails to pay (i) when due any principal payment on the due date hereunder or (ii) any Default Interest payment
or other payment required under the terms of this Bond or any other Transaction Document on the date due and such payment shall not have
been made within five (5) business days of the Company’s receipt of written notice to the Company of such failure to pay;
(b) Breaches
of Covenants. The Company intentionally fails to observe or perform any other covenant, obligation, condition or agreement contained
in this Bond or the other Transaction Documents (other than those specified in Section 2(a)) and such failure shall continue for
sixty (60) days after the Company’s receipt of written notice to the Company of such failure;
(c) Representations
and Warranties. Any representation or warranty made by the Company in the Transaction Documents is intentionally false or incorrect
in any material respect when made (for the avoidance of doubt, the Company does not warrant that it will achieve any results derived from
projections provided by the Company);
(d) Voluntary
Bankruptcy or Insolvency Proceedings. The Company (i) applies for or consent to the appointment of a receiver, trustee, liquidator
or custodian of itself or of all or a substantial part of its property, (ii) admits in writing its inability to pay its debts generally
as they mature, (iii) makes a general assignment for the benefit of its or any of its creditors, (iv) is dissolved or liquidated, (v)
commences a voluntary case or other proceeding seeking liquidation, reorganization or other relief with respect to itself or its debts
under any bankruptcy, insolvency or other similar law now or hereafter in effect or consent to any such relief or to the appointment of
or taking possession of its property by any official in an involuntary case or other proceeding commenced against it, or (vi) takes any
action for the purpose of effecting any of the foregoing;
(e) Involuntary
Bankruptcy or Insolvency Proceedings. Proceedings for the appointment of a receiver, trustee, liquidator or custodian of the Company,
or of all or a substantial part of the property thereof, or an involuntary case or other proceedings seeking liquidation, reorganization
or other relief with respect to the Company or any of its subsidiaries, if any, or the debts thereof under any bankruptcy, insolvency
or other similar law now or hereafter in effect is commenced and an order for relief entered or such proceeding is not dismissed or discharged
within forty-five (45) days of commencement; or
(f) Judgments.
A final judgment or order for the payment of money in an amount in excess of $500,000 is rendered against the Company and the same remains
undischarged for a period of ninety (90) days during which execution is not effectively stayed, or any judgment, writ, assessment, warrant
of attachment, or execution or similar process is issued or levied against a substantial part of the property of the Company or any of
its subsidiaries, if any, and such judgment, writ, or similar process is not released, stayed, vacated or otherwise dismissed within ninety
(90) days after issue or levy.
3. Rights
of Investor.
(a) Rights
of Investor upon Default. Upon the occurrence or existence of any Event of Default, the Investor shall receive the rights under Section
3(b), for the avoidance of doubt, as if the Maturity Date had already occurred.
(b) Rights
of Investor upon Maturity Date. On or after the Maturity Date, the Investor may, by written notice to the Company, declare all outstanding
Obligations payable by the Company hereunder to be immediately due and payable without presentment, demand, protest or any other notice
of any kind, all of which are hereby expressly waived.
4. Covenants.
(a) Negative
Pledge. For so long as this Bond is outstanding, the Company shall not take or permit any of its directors, officers, committee members,
committees, employees, agents or delegates to take (whether in a single transaction or a series of related transactions, whether directly
or indirectly, and whether or not by amendment, merger, consolidation, scheme or arrangement, amalgamation, or otherwise) any of the following
without a prior written approval by the Investor, which shall not be unreasonably withheld, conditioned or delayed:
(i) any amendment of the organizational
documents of the Company or its subsidiaries, if any, that would adversely affect the Investor;
(ii) any liquidation, dissolution,
reconstitution, restructuring, winding-up, rehabilitation or bankruptcy of the Company or its subsidiaries, if any;
(iii) any issuance, delivery,
sale, redemption, repurchase or other acquisition or disposition of any equity interests of the Company or any of its subsidiaries, if
any (including any stock options); provided that the foregoing shall not apply to Exempt Issuances or to repurchases of Common Stock or
other equity interests of departing officers, directors, employees or consultants of the Company pursuant to agreements providing for
the repurchase of such Common Stock or equity interests at either the original purchase price thereof or the then-current fair market
value thereof to the extent that such agreements have been disclosed to the Investor if executed prior to the issuance of this Bond, or
in the case of such agreements executed after the issuance of this Bond, to the extent that such agreement have been disclosed to, and
approved in advance in writing by, the Investor;
(iv) any entry, acquisition,
investment in or disposition of any Person, asset or business (by way of merger, share acquisition or disposition, acquisition or disposition
of assets or similar business combination) involving aggregate consideration in excess of $1,500,000 individually and $7,500,000 in the
aggregate, other than a Change of Control;
(v) any capital expenditures
in excess of $1,500,000 individually and $7,500,000 in the aggregate;
(vi) any distribution of profits
or assets, or any bonus or other payment of any nature, in favor of the existing shareholders of the Company or any its subsidiaries in
their capacity as shareholders, if any;
(vii) any creation, incurrence
of, or increase in, any indebtedness in excess of $1,500,000 individually and $7,500,000 in the aggregate that is not expressly subordinated
to the Company’s obligations hereunder, other than to banks, equipment lessors or other financial institutions or other lenders,
or to real property lessors, pursuant to a debt financing, equipment leasing or real property leasing transaction, approved by a majority
of the directors of the Company;
(viii) any sale, assignment,
transfer, licensing or disposition of any material intellectual property rights owned by the Company or its subsidiaries, in each case
outside the ordinary course of business, if any, or failure to make any filing, pay any fee or take any other action necessary to prosecute
and maintain in full force and effect any material registered intellectual property;
(ix) any transactions with
(including any loan to) the Company’s directors, officers, committee members, employees, or affiliated companies or the affiliates
of any of the foregoing, except for those transactions disclosed in Section 2(s) of the Disclosure Schedule to the Subscription Agreement
or that are approved by the majority of disinterested directors of the Company;
(x) any settlement or commencement
of a proceeding by the Company and/or its subsidiaries, if any, which could reasonably be expected to have a material adverse effect on
the Company and/or subsidiaries, if any;
(xi) the termination of Key
Employees; or
(xii) any entry into any contract
to do any of the foregoing.
(b) Board
of Directors. For so long as this Bond is outstanding, the Investor shall be entitled to nominate at least one (1) director (the “Investor
Director”) of the Company. The Company shall, and shall cause its stockholders to, take all necessary actions to cause the election
of the designees of the Investor to the Company’s board of directors as the Investor Director. In the event that a vacancy is created
at any time by the death, disability, retirement, resignation, removal (with or without cause) or expiration of the term of office of
the Investor Director, the Investor shall have the exclusive right to fill the vacancy created thereby with a new designee of the Investor,
and the Company shall, and shall cause its stockholders to, take all necessary actions to procure the election of such designee as a registered
director as soon as possible. The Company shall take all actions necessary to ensure that the director indemnity and directors’
insurance to the reasonable satisfaction of the Investor shall remain in place for so long as any Investor Director remains on the Company’s
board of directors. Notwithstanding the foregoing, any Investor Director nominated by the Investor shall not be subject to any Disqualification
Events (as defined in the Subscription Agreement).
(c) Best
Efforts to Close Q-IPO or Direct Listing. The Company shall use its best efforts to (i) close a Q-IPO or (ii) complete a Direct Listing
within three (3) years following the date of issuance of this Bond, which period may be extended by one (1) year by mutual agreement between
the Company and the Investor.
5. Conversion.
(a) Conversion
Price. Subject to the adjustments effected under Section 5(b), this Bond may be converted in accordance with Section 5(c)
and Section 5(d) below at a conversion price equal to US$5.34 (the “Conversion Price”). The total number of
shares to be issued upon conversion shall equal (i) the outstanding principal amount of this Bond and all accrued and unpaid Default Interest
on this Bond, if any, divided by (ii) the Conversion Price then in effect.
(b)
Adjustments.
(i) Adjustment for Corporate
Actions. If the Company shall at any time or from time to time after the issuance of this Bond (w) pay a stock dividend or otherwise
make a distribution or distributions on shares of its equity or equity equivalent securities, (x) effect a subdivision of the outstanding
equity or equity equivalent securities into a larger number of equity or equity equivalent securities (including by way of a stock split),
(y) combine (including by way of reverse stock split) outstanding equity or equity equivalent securities into a smaller number of equity
or equity equivalent securities, or (z) issue by reclassification of equity or equity equivalent securities any equity or equity equivalent
securities of the Company, then the Conversion Price then in effect immediately before such action shall be multiplied by a fraction,
of which the numerator shall be the number of shares of equity or equity equivalent securities (excluding treasury shares, if any) outstanding
before such event and of which the denominator shall be the number of shares of equity or equity equivalent securities outstanding after
such event. Any adjustment made pursuant to this Section 5(b)(i) shall become effective immediately after the record date for the
determination of equity holders entitled to receive such dividend or distribution and shall become effective immediately after the effective
date in the case of a subdivision, combination or reclassification.
(ii) Subsequent Equity
Sales. Except for the issuance of securities pursuant to clauses (a), (b), (c) and (i) of the definition of Exempt Issuances below
and other issuances of equity or equity equivalent securities disclosed to, and approved in writing not to adjust the Conversion Price
then in effect in advance by, the Investor, in the event the Company issues any equity or equity equivalent securities with an implied
price per share of less than the Conversion Price (such issuance, the “Equity Issuance”), the Conversion Price shall
be adjusted, concurrently with the Equity Issuance, to the implied price per share received by the Company for such Equity Issuance. Notwithstanding
the foregoing, the Conversion Price as adjusted pursuant to this Section 5(b)(ii) shall not be less than the par value of the Common
Stock, subject to adjustments for stock splits and stock dividends on the Common Stock.
(iii) Effect of Merger,
Etc. In the case of any consolidation with, or merger of the Company into, any other Person or any merger or consolidation of another
Person into the Company (other than a merger in which the Company is the continuing corporation or a consolidation or merger which does
not result in any reclassification, conversion, exchange or cancellation of outstanding equity or equity equivalent securities), or any
sale, transfer or lease of all, or substantially all, of the properties or assets of the Company, the Person resulting from such consolidation
or merger, or which acquires or leases such properties or assets of the Company, as the case may be, shall execute and deliver to the
Investor a new Bond and procure that the Investor will have the right thereafter to convert this Bond into the kind and amount of shares
and other securities, cash and property receivable upon such consolidation, merger, sale, transfer or lease by a holder of the number
of shares of Conversion Stock into which this Bond might have been converted immediately prior to such consolidation, merger, sale, transfer
or lease.
(iv) IPO Adjustment.
If the Company completes an initial public offering or a direct listing of its Common Stock on a national securities exchange that does
not meet the requirements of a Q-IPO or a Direct Listing, as applicable, the Conversion Price shall be adjusted, effective as of immediately
prior to such an initial public offering or a direct listing, as applicable, as follows:
CP = A – B
For purposes of the foregoing formula,
the following definitions shall apply:
“CP” shall mean the Conversion
Price to be in effect immediately after the completion of initial public offering or direct listing, as applicable;
“A” shall mean the actual
per share offering price to be offered at the initial public offering or the actual per share reference price at the direct listing, as
applicable; and
“B” shall mean the interest
accrued on CP at a rate equal to 6% per annum from the Issuance Date and the date of listing, computed on the basis of the actual number
of days elapsed and a year of 365 days.
Notwithstanding the foregoing,
the Conversion Price as adjusted pursuant to this Section 5(b)(iv) shall not be less than the par value of the Common Stock, subject
to adjustments for stock splits and stock dividends on the Common Stock.
(v) Notice of Adjustments.
Whenever the Conversion Price is adjusted pursuant to any provision of this Section 5(b), the Company shall promptly mail to the
Investor a notice setting forth the Conversion Price after such adjustment and setting forth in reasonable detail the facts requiring
such adjustment.
(c) Optional
Conversion. At any time while this Bond is outstanding, at the option of the Investor in such Investor’s sole discretion, this
Bond may be converted, in whole or in part, into Common Stock (or preferred stock of the Company with the terms and conditions set forth
in Annex A; provided; however, that if the Investor desires to convert to preferred stock, it shall request that the Company obtain
Stockholder Approval and the Company will use commercially reasonable efforts to do so) (such stock issued upon conversion of this Bond,
the “Conversion Stock”). To exercise its conversion right, the Investor shall deliver a written notice to the Company,
specifying the principal amount of this Bond as well as the accrued and unpaid Default Interest, if any, to be converted, together with
the original Bond to be converted (or a notice to the effect that the original Bond has been lost, stolen or destroyed and an agreement
acceptable to the Company whereby the Investor agrees to indemnify the Company from any loss incurred by it in connection with this Bond).
If this Bond is surrendered for partial conversion, the Company shall execute and deliver to the Investor a new Bond in an aggregate principal
amount equal to the unconverted portion of the surrendered Bond, without payment of any service charge borne by the Investor. The Company
shall, as soon as practicable thereafter, issue and deliver to the Investor a certificate or certificates (or a notice of issuance of
uncertificated shares, if applicable) for the number of shares to which the Investor shall be entitled upon such conversion. Any conversion
of this Bond pursuant to this Section 5(c) shall be deemed to have been made on the date when the Investor’s notice referred
to the previous provisions and the original Bond to be converted (or a notice to the effect that the original Bond has been lost, stolen
or destroyed and an agreement acceptable to the Company whereby the Investor agrees to indemnify the Company from any loss incurred by
it in connection with this Bond) are delivered to the Company and on and after such date the Persons entitled to receive the shares issuable
upon such conversion shall be treated for all purposes as the record holder of such shares.
(d) Automatic
Conversion. This Bond shall automatically be converted into preferred stock of the Company with the terms and conditions set forth
in Annex A upon the earlier to occur of (i) an initial public offering of the Common Stock and concurrent listing on a national
securities exchange, including without limitation the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), (ii)
a direct listing of the Common Stock on a national securities exchange, including without limitation the New York Stock Exchange, NYSE
American or the Nasdaq Stock Market (any tier) or (iii) upon Stockholder Approval (the “Conversion Event”). Written
notice shall be delivered to the Investor at the address last shown on the records of the Company for the Investor or given by the Investor
to the Company for the purpose of notice by at least ten (10) business days prior to the anticipated closing date of the Conversion Event,
notifying the Investor of the conversion to be effected, specifying the anticipated Conversion Price, the principal amount of this Bond
to be converted, together with all accrued and unpaid Default Interest, if any, the date on which such conversion is expected to occur
and calling upon the Investor to surrender to the Company, in the manner and at the place designated, this Bond. The Investor agrees to
deliver the original of this Bond (or a notice to the effect that the original Bond has been lost, stolen or destroyed and an agreement
acceptable to the Company whereby the holder agrees to indemnify the Company from any loss incurred by it in connection with this Bond)
at the closing of the Conversion Event for cancellation; provided, however, that upon the closing of the Conversion Event, this Bond shall
be deemed converted and of no further force and effect, whether or not it is delivered for cancellation as set forth in this sentence.
The Company shall, as soon as practicable thereafter, issue and deliver to the Investor a certificate or certificates (or a notice of
issuance of uncertificated shares, if applicable) for the number of shares to which the Investor shall be entitled upon such conversion.
Any conversion of this Bond pursuant to this Section 5(d) shall be deemed to have been made immediately prior to the closing of
the Conversion Event but immediately after the adjustment of the Conversion Price pursuant to Section 5(b)(iv), if applicable,
and on and after such date the Persons entitled to receive the shares shall issuable upon such conversion be treated for all purposes
as the record holder of such shares.
(e) Fractional
Shares; Effect of Conversion. No fractional shares shall be issued upon conversion of this Bond. Any fractional shares which would
otherwise be issuable upon conversion of this Bond will be rounded up to the next whole share. Upon conversion of this Bond in full, the
Company shall be forever released from all its obligations and liabilities under this Bond and this Bond shall be deemed of no further
force or effect, whether or not the original of this Bond has been delivered to the Company for cancellation.
6. Participation
in Future Financing.
(a) Participation
Right. From the date hereof until the date that this Bond is no longer outstanding, upon any issuance by the Company of Common Stock
or securities convertible into or exercisable or exchangeable for Common Stock (“Common Stock Equivalents”) for cash
consideration pursuant to clause (e) of the definition of Exempt Issuances below (a “Subsequent Financing”), the Investor
shall have the right to participate in the Subsequent Financing in the manner set forth in Section 6(b) on the same terms, conditions
and price provided for in the Subsequent Financing.
(b) Mechanics
of Participation Right.
(i) The Company shall deliver
to the Investor a written notice of the Company’s intention to effect a Subsequent Financing (a “Subsequent Financing Notice”)
at least thirty (30) business days prior to the date of the expected consummation of the Subsequent Financing, which notice shall describe
in reasonable detail the proposed terms of such Subsequent Financing, the amount of proceeds intended to be raised thereunder and the
Person or Persons through or with whom such Subsequent Financing is proposed to be effected (the “Subsequent Investor(s)”)
and shall include a term sheet and transaction documents relating thereto as an attachment. If the Investor desires to participate in
such Subsequent Financing, it must provide written notice (the “Participation Notice”) to the Company within one hundred
and twenty (120) days following the date on which the Subsequent Financing Notice is delivered to the Investor (the “Notice Termination
Time”) which notice specifies the Investor’s willingness to participate in the Subsequent Financing, directly and/or through
its designated affiliate, as well as the number of Common Stock or Common Stock Equivalents, as applicable, to be subscribed by the Investor
and/or its designated affiliate.
(ii) In order to protect the
Investor’s right to maintain its shareholding ratio on a fully-diluted basis, (x) if the Investor is to participate in the Subsequent
Financing simultaneously with the Subsequent Investor(s), the Investor may subscribe for, or cause its designated affiliate to subscribe
for, up to such number of Common Stock or Common Stock Equivalents (whichever is to be issued at the Subsequent Financing) calculated
by multiplying (A) the aggregate number of Common Stock or Common Stock Equivalents, as applicable, available for subscription at the
Subsequent Financing and (B) the Investor’s Pro Rata Percentage (defined below); and (y) if the Investor is to participate in the
Subsequent Financing after the consummation of the Subsequent Financing by the Subsequent Investor(s), the Investor may subscribe for,
or cause its designated affiliate to subscribe for, up to such number of Common Stock or Common Stock Equivalents (whichever to be issued
at the Subsequent Financing) calculated as follows:

For purposes of the foregoing formula,
the following definitions shall apply:
“ISF” shall mean the number
of Common Stock or Common Stock Equivalents (whichever is to be issued at the Subsequent Financing) that the Investor may subscribe for
or cause its designated affiliate to subscribe for.
“SF” shall mean the aggregate
number of Common Stock or Common Stock Equivalents, as applicable, issued to the Subsequent Investor(s) at the Subsequent Financing.
“A” shall mean the number
of shares of Common Stock outstanding on a fully-diluted basis that is not held by the Investor immediately prior to the consummation
of the Subsequent Financing.
“B” shall mean the sum of
(A) the number of shares of Common Stock held by the Investor on a fully-diluted basis immediately prior to the consummation of the Subsequent
Financing and (B) the number of shares of Common Stock or Common Stock Equivalents that the Investor is eligible to subscribe for, or
cause its designated affiliate to subscribe for, in connection with any prior Subsequent Financing as of the date of consummation of the
Subsequent Financing.
(iii) Assuming that the Investor
delivers the Participation Notice within the Notice Termination Time, (x) if the Investor delivers the Participation Notice within twenty
(20) business days following the delivery of the Subsequent Financing Notice (the “Simultaneous Participation Deadline”),
the Company shall effect the Subsequent Financing to the Investor and the Subsequent Investor(s) simultaneously in accordance with the
Participation Notice; and (y) if the Investor delivers the Participation Notice after the Simultaneous Participation Deadline, the Company
may consummate the Subsequent Financing to the Subsequent Investor(s) on the terms set forth in the Subsequent Financing Notice and shall
allow the Investor to exercise its participation right set forth in Section 6(b)(ii)(y).
(iv) For the avoidance of
doubt, the Investor and/or its designated affiliate, at its sole and absolute discretion, may, in the Subsequent Financing, subscribe
for any number of Common Stock or Common Stock Equivalents, as applicable, equal to or less than such number set forth in Section 6(b)(ii)(x)
or 6(b)(ii)(y), as applicable.
(v) If the Company receives
no such notice from the Investor as of the Notice Termination Time, the Investor shall be deemed to have notified the Company that it
does not elect to participate in such Subsequent Financing.
7. Definitions.
As used in this Bond, the following capitalized terms have the following meanings:
“Change of Control”
shall mean (i) any “person” or “group” (within the meaning of Section 13(d) and 14(d) of the Securities Exchange
Act of 1934, as amended) becoming the “beneficial owner” (as defined in Rule 13d-3 under the Securities Exchange Act of 1934,
as amended), directly or indirectly, of more than 50% of the outstanding voting securities of the Company having the right to vote for
the election of members of the board of directors of the Company, (ii) any reorganization, merger or consolidation of the Company, other
than a transaction or series of related transactions in which the holders of the voting securities of the Company outstanding immediately
prior to such transaction or series of related transactions retain, immediately after such transaction or series of related transactions,
at least a majority of the total voting power represented by the outstanding voting securities of the Company or such other surviving
or resulting entity, (iii) a sale, lease or other disposition of all or substantially all of the assets of the Company or (iv) an exclusive,
irrevocable licensing of all or substantially all of the Company’s intellectual property to a third party.
“Common Stock”
shall mean the common stock, par value $0.01, of the Company.
“Direct Listing”
shall mean a direct listing of the Common Stock on a national securities exchange, including without limitation the New York Stock Exchange,
NYSE American or the Nasdaq Stock Market (any tier), with the reference price of at least $5.34 plus interest accrued on $5.34
at a rate equal to 6% per annum from the Issuance Date up to the date of listing, computed on the basis of the actual number of days elapsed
and a year of 365 days (as adjusted for stock splits, stock dividends and the like) (the “Target Price”).
“Exempt Issuances”
shall mean the issuance of (a) shares of Common Stock or options to purchase shares of Common Stock issued to employees, officers, directors
or consultants of the Company pursuant to any stock or option plan duly adopted for such purpose by a majority of the directors of the
Company or a majority of a committee of established for such purpose; (b) securities upon the conversion of this Bond; (c) shares of Common
Stock issued pursuant to Common Stock Equivalents issued and outstanding on the date of this Bond or commitments to issue shares of Common
Stock or Common Stock Equivalents, which commitments are in existence on the date of this Bond, or amended after the date hereof if such
amendment is approved in advance by the Investor (which approval shall not be unreasonably withheld, conditioned or delayed) and the majority
of the directors of the Company; (d) Common Stock or Common Stock Equivalents issued pursuant to acquisitions or strategic transactions,
unless the Investor’s approval is required pursuant to Section 4; (e) subject to the Investor’s participation right
contained in Section 6, Common Stock or Common Stock Equivalents issued in financing transactions, the primary purpose of which
is to raise capital; (f) Common Stock or Common Stock Equivalents issued to banks, equipment lessors or other financial institutions or
other lenders, or to real property lessors, pursuant to a debt financing, equipment leasing or real property leasing transaction, approved
by a majority of the directors of the Company; (g) Common Stock or Common Stock Equivalents issued in connection with the provision of
goods pursuant to transactions approved by a majority of the non-employee disinterested directors of the Company; (h) Common Stock or
Common Stock Equivalents issued in connection with sponsored research, collaboration, technology license, development, marketing, investor
relations or other similar agreements or strategic partnerships approved a majority of the directors of the Company; and (i) the License
Equity (as defined in the MDACC Agreements) issued to the Board of Regents of the University of Texas System (as described in Exhibit
II of the option agreement included in the MDACC Agreements); provided, however, if the sum of the number of shares of Common Stock and
Common Stock Equivalents issued under subclauses (d), (f), (g) and/or (h) above exceeds 10% of the outstanding Common Stock on a fully
diluted basis as of the Issuance Date, any amount of such shares of Common Stock or Common Stock Equivalents in excess of such amount
shall not constitute an Exempt Issuance.
“Key Employee”
shall mean any executive-level employee (including division director and C-level positions) as well as any employee or consultant who
either alone or in concert with others develops, invents, programs or designs any intellectual property owned or used by the Company in
the conduct of the Company’s business as now conducted and as presently proposed to be conducted.
“Knowledge of the
Company” shall mean the actual knowledge of any of the Company’s officers, directors or Key Employees and such knowledge
as such individuals should have had in their capacity as the Company’s officers, directors or Key Employees, as applicable, after
reasonable due inquiry and investigation.
“Lien”
shall mean, with respect to any property, any security interest, mortgage, pledge, lien, claim, charge or other encumbrance.
“MDACC Agreements”
shall have the meaning assigned to such term in the Subscription Agreement.
“Obligations”
shall mean and include all loans, advances, debts, liabilities and obligations, howsoever arising, owed by the Company to the Investor
of every kind and description, now existing or hereafter arising under or pursuant to the terms of this Bond and the other Transaction
Documents, including, all interest, fees, charges, expenses, attorneys’ fees and costs and accountants’ fees and costs chargeable
to and payable by the Company hereunder and thereunder, in each case, whether direct or indirect, absolute or contingent, due or to become
due, and whether or not arising after the commencement of a proceeding under Title 11 of the United States Code (11 U. S. C. Section 101
et seq.), as amended from time to time (including post-petition interest) and whether or not allowed or allowable as a claim in
any such proceeding.
“Person”
shall mean and include an individual, a partnership, a corporation (including a business trust), a joint stock company, a limited liability
company, an unincorporated association, a joint venture or other entity or a governmental authority.
“Pro Rata Percentage”
shall mean the fraction the numerator of which is the number of shares of Common Stock held by the Investor on a fully-diluted basis and
the denominator of which is the number of shares of Common Stock outstanding on a fully-diluted basis.
“Q-IPO”
shall mean a firm commitment underwritten public offering and concurrent listing on a national securities exchange, including without
limitation the New York Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), with a per share offering price of at least
the Target Price.
“Securities Act”
shall mean the Securities Act of 1933, as amended.
“Stockholder Approval”
shall mean the approval of the issuance of preferred stock upon conversion of this Bond (but not the issuance of the Bond itself, which
does not require approval of stockholders) by a majority of the Company’s outstanding series A preferred stock, series A-1 preferred
stock, series A-2 preferred stock, series B preferred stock, series C preferred stock, and series D preferred stock, which majority must
include the holder of the Company’s series A-1 preferred stock; provided, however, that such approval shall not be required from
and after the time that the Company completes a Q-IPO or Direct Listing.
“Subscription Agreement”
shall mean the Convertible Bonds Subscription Agreement, dated as of the date hereof (as amended, modified or supplemented), by and between
the Company and the Investor.
“Transaction Documents”
shall mean this Bond and the Subscription Agreement.
8. Miscellaneous.
(a) Change
of Control. If there is a Change of Control prior to the full conversion of this Bond for any reason, then at the election of the
Investor in the Investor’s sole discretion, this Bond may be redeemed, prior to and in preference to any equity securities, at a
price equal to the sum of the principal amount of this Bond plus an interest accrued on the unconverted portion of this Bond at a rate
equal to 28% per annum from the Issuance Date up to the date on which the Change of Control is consummated, computed on the basis of the
actual number of days elapsed and a year of 365 days. The Company shall deliver to the Investor written notice of any Change of Control
immediately upon becoming aware of such Change of Control but in any event at least sixty (60) days prior to consummation of any Change
of Control.
(b) Successors
and Assigns. Subject to the restrictions on transfer described below, the rights and obligations of the Company and the Investor shall
be binding upon and benefit the successors, assigns, heirs, administrators and transferees of the parties.
(c) Transfers.
With respect to any offer, sale or other disposition of this Bond, the Investor will give written notice to the Company prior thereto,
describing briefly the manner thereof, together with a written opinion of the Investor’s counsel, or other evidence if reasonably
satisfactory to the Company, to the effect that such offer, sale or other distribution may be effected without registration or qualification
(under any federal or state law then in effect). Upon receiving such written notice and reasonably satisfactory opinion, if so requested,
or other evidence, the Company, as promptly as practicable, shall notify the Investor that the Investor may sell or otherwise dispose
of this Bond, all in accordance with the terms of the notice delivered to the Company. If a determination has been made pursuant to this
Section 8(c) that the opinion of counsel for the Investor, or other evidence, is not reasonably satisfactory to the Company, the
Company shall so notify the Investor promptly after such determination has been made. Each Bond thus transferred shall bear a legend as
to the applicable restrictions on transferability in order to ensure compliance with the Securities Act, unless in the opinion of counsel
for the Company such legend is not required in order to ensure compliance with the Securities Act. Transfers of this Bond shall be registered
upon registration books maintained for such purpose by or on behalf of the Company as provided in the Subscription Agreement. Prior to
presentation of this Bond for registration of transfer, the Company shall treat the registered holder hereof as the owner and holder of
this Bond for the purpose of receiving all payments hereon and for all other purposes whatsoever, whether or not this Bond shall be overdue
and the Company shall not be affected by notice to the contrary.
(d) Waiver
and Amendment. Any provision of this Bond may be amended, waived or modified upon the written consent of the Company and the Investor.
(e) Notices.
All notices and other communications to be given or delivered pursuant to this Bond shall be delivered in the manner provided in the Subscription
Agreement. In the event of: (i) any taking by the Company of a record of the holders of any class of securities of the Company for the
purpose of determining the holders thereof who are entitled to receive any dividend or other distribution or any right to subscribe for,
purchase or otherwise acquire any shares of stock of any class or any other securities or property, or to receive any other right; (ii)
any capital reorganization of the Company, any reclassification or recapitalization of the capital stock of the Company or any transfer
of all or substantially all of the assets of the Company to any other Person or any consolidation or merger involving the Company; or
(iii) any voluntary or involuntary dissolution, liquidation or winding-up of the Company, the Company will mail to the Investor at least
ten (10) days prior to the earliest date specified therein, a notice specifying (A) the date on which any such record is to be taken for
the purpose of such dividend, distribution or right and the amount and character of such dividend, distribution or right; or (B) the date
on which any such reorganization, reclassification, recapitalization, transfer, consolidation, merger, dissolution, liquidation or winding-up
is expected to become effective and the record date for determining stockholders entitled to vote thereon.
(f) Payment.
Unless converted into the Company’s equity interests pursuant to the terms hereof, payment shall be made in lawful tender of
the United States.
(g) Usury.
In the event any interest is paid on this Bond which is deemed to be in excess of the then legal maximum rate, then that portion of
the interest payment representing an amount in excess of the then legal maximum rate shall be deemed a payment of principal and applied
against the principal of this Bond.
(h) Governing
Law; Dispute Resolution. All questions concerning the construction, validity, enforcement and interpretation of this Bond shall be
determined in accordance with the provisions of the Subscription Agreement.
(i) Tax
Withholding. Notwithstanding any other provision to the contrary, the Company shall be entitled to deduct and withhold from any amounts
payable or otherwise deliverable with respect to this Bond such amounts as may be required to be deducted or withheld therefrom under
any provision of applicable law, and to be provided any necessary tax forms and information, including Internal Revenue Service Form W-9
or appropriate version of IRS Form W-8, as applicable, from each beneficial owner of this Bond. To the extent such amounts are so deducted
or withheld and paid over to the appropriate taxing authority, such amounts shall be treated for all purposes as having been paid to the
person to whom such amounts otherwise would have been paid.
(Signature Page Follows)
The Company has caused this Bond to be issued
as of the date first written above.
| 20/20 GENESYSTEMS, INC. |
|
| a Delaware corporation |
|
| |
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
ANNEX A
TERMS AND CONDITIONS OF PREFERRED SHARES
| 1. | Designation; Number of Shares; Stated Value. There is hereby created out of the authorized and
unissued shares of Preferred Stock of the Company a series of Preferred Stock designated as the “Series E Preferred Stock”
(the “Series E Preferred Stock”). The number of shares constituting such series shall be __________. Such number of
shares may from time to time be increased or decreased (but not below the number of shares then outstanding) by the Board in accordance
with the Certificate of Incorporation and applicable law. Each share of Series E Preferred Stock shall have a stated value equal to the
lower of $5.34 per share or the conversion price (as defined in, and as may be adjusted pursuant to, that certain convertible bond (the
“Bond”) issued by the Company to the initial holder of the Series E Preferred Stock on ___________, 2024) (the “Bond
Conversion Price”), subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other
similar recapitalization with respect to the Series E Preferred Stock (the “Stated Value”). Each share of Series E
Preferred Stock shall be identical in all respects to every other share of Series E Preferred Stock. |
| 2. | Ranking. With respect to payment of dividends and distribution of assets upon liquidation, dissolution
or winding up of the Company, whether voluntary or involuntary, all shares of Series E Preferred Stock shall rank (i) pari passu
with all Parity Securities; (ii) senior to all Junior Securities; and (iii) junior to all Senior Securities, if any. As used herein, “Parity
Securities” means any class of securities hereafter authorized that is specifically designated as ranking pari passu
with the Series E Preferred Stock, “Junior Securities” means the Series A Preferred Stock, Series A-1 Preferred Stock,
Series A-2 Preferred Stock, Series B Preferred Stock, Series C Preferred Stock, Series D Preferred Stock and Common Stock of the Company
and any other class of securities hereafter authorized that is specifically designated as junior to the Series E Preferred Stock, and
“Senior Securities” means any class of securities hereafter authorized that is specifically designated as senior to
the Series E Preferred Stock. |
| 3. | Dividends. From and after the date of the issuance of any share of Series E Preferred Stock (the
“Original Issuance Date”), dividends at the rate per annum of 1.0% of the Stated Value shall accrue on such share of
Series E Preferred Stock. Dividends shall accrue from day to day, whether or not declared, and shall be cumulative. The holders of Series
E Preferred Stock shall not be entitled to participate in any dividend or other distribution made on the Junior Securities unless and
until the Series E Preferred Stock is converted in accordance with the terms hereof and then only in connection with dividends or other
distributions having a record date that occurs from or after such conversion. However, in no event shall the Company declare any dividend
on any Junior Security if such dividend would impair the ability of the Company to pay any dividends due on the Series E Preferred Stock. |
| (a) | Liquidation Preference. In the event of any voluntary or involuntary liquidation, dissolution or
winding up of the Company (a “Liquidation Event”) or a Deemed Liquidation Event (as defined below), each holder of
shares of Series E Preferred Stock then outstanding shall be entitled to be paid out of the cash and other assets of the Company available
for distribution to its stockholders, before any payment shall be made to the holders of Junior Securities, but pari passu with
the holders of Parity Securities, by reason of their ownership thereof, an amount per share equal to the greater of (i) one (1) times
the Stated Value, plus any accrued and unpaid dividends thereon or (ii) such amount per share of Series E Preferred Stock as would have
been payable had all shares of Series E Preferred Stock been converted into Common Stock immediately prior to such Liquidation Event or
Deemed Liquidation Event, plus an interest accrued on the greater of (i) or (ii), as applicable, at a rate equal to 4% per annum from
the Original Issuance Date (or with respect to any accrued and unpaid dividends, from the date on which such dividends are accrued) up
to the date of the Liquidation Event or Deemed Liquidation Event, computed on the basis of the actual number of days elapsed and a year
of 365 days. |
| (b) | Insufficient Assets. If upon any Liquidation Event or Deemed Liquidation Event the remaining assets
of the Company available for distribution to its stockholders shall be insufficient to pay the holders of the shares of Series E Preferred
Stock the full preferential amount to which they are entitled under Section 4(a) and the holders of Parity Securities, if any, the full
preferential amount to which they are entitled under the terms of the relevant instrument governing such Parity Securities, (i) the holders
of the shares of Series E Preferred Stock and any such Parity Securities shall share ratably in any distribution of the remaining assets
and funds of the Company in proportion to the respective full preferential amounts which would otherwise be payable in respect thereof
upon such Liquidation Event or Deemed Liquidation Event if all amounts payable on or with respect to such shares and Parity Securities
were paid in full, and (ii) the Company shall not make or agree to make any payments to the holders of Junior Securities. |
| (c) | Deemed Liquidation Events. A “Deemed Liquidation Event” means (i) a sale, lease
or transfer of all or substantially all of the Company’s assets to a non-affiliate of the Company; (ii) a merger, acquisition, change
of control, consolidation or other transactions or series of transactions in which the Company’s stockholders prior to such transaction
or series of transactions do not retain a majority of the voting power of the surviving entity immediately following such transaction
or series of transactions; or (iii) the grant of an (by territory, field of use or market) exclusive license to all or substantially all
of the Company’s technology or intellectual property rights (determined on a consolidated basis with all of the Company’s
direct and indirect subsidiaries) except where such exclusive license is made to one or more wholly-owned subsidiaries of the Company. |
| (a) | Voting Generally. On any matter presented to the stockholders of the Company for their action or
consideration at any meeting of stockholders of the Company (or by written consent of stockholders in lieu of meeting), each holder of
Series E Preferred Stock shall be entitled to cast the number of votes equal to the number of whole shares of Common Stock into which
the shares of Series E Preferred Stock held by such holder are convertible as of the record date for determining stockholders entitled
to vote on such matter. Except as provided by law or by the other provisions of this Certificate of Designation, the holders of Series
E Preferred Stock shall vote together with the holders of shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred
Stock, Series B Preferred Stock, Series C Preferred Stock, Series D Preferred Stock and Common Stock as a single class. |
| (b) | Board Composition. For so long as shares of Series E Preferred Stock remain outstanding, the holders
of Series E Preferred Stock shall together, as a separate class, have the right to elect: (i) if the number of directors on the Board
is equal to or fewer than seven (7), two (2) directors reasonably acceptable to the Board or the nominating committee of the Board (or
one (1) director if the holders of Series E Preferred Stock, as a holder of the Bond, have elected one (1) director) to the Board and
(ii) if the number of directors on the Board is eight (8) or more, three (3) directors reasonably acceptable to the Board or the nominating
committee of the Board (or two (2) directors if the holders of Series E Preferred Stock, as a holder of the Bond, have elected one (1)
director) to the Board (the “Designated Directors”); provided, however, (x) the holders of Series E Preferred
Stock shall be entitled to elect three (3) directors reasonably acceptable to the Board or the nominating committee of the Board (or two
(2) directors if the holders of Series E Preferred Stock, as a holder of the Bond, have elected one (1) director) to the Board regardless
of the number of directors on the Board if the aggregate amount invested by the holders of Series E Preferred Stock in the Company in
the form of convertible bonds, Common Stock or Common Stock Equivalents (as defined below) is equal to or more than $50,000,000 and (y)
in no event shall the number of directors on the Board be greater than ten (10). The nominee shall be deemed reasonably acceptable to
the Board or the nominating committee of the Board other than where the Board or the nominating committee determines that (A) the nominee
is a bad actor as defined in Rule 506(d) of the Securities Act of 1933, as amended, or is otherwise legally prohibited from serving on
the Board, (B) the nominee does not have business, finance or other relevant experience and educational background, (C) the nominee is
not independent under NYSE American Company Guide Rule 803, or (D) the nominee does not have sufficient financial literacy to review and
understand the financial statements of the Company, in which case the Board or the nominating committee, as applicable, shall communicate
its grounds for rejecting such nominee in writing to the holders of Series E Preferred Stock. If the Board or the nominating committee
of the Board rejects any director elected by the holders of Series E Preferred Stock and the holders of Series E Preferred Stock disagree
with such decision of the Board or the nominating committee of the Board, the holders of Series E Preferred Stock shall have a right to
(x) call a meeting of stockholders with regard to such director nominee and (y) require that the Board or the nominating committee of
the Board accept any director nominee approved by a majority of the stockholders. In the event that a vacancy is created at any time by
the death, disability, retirement, resignation, removal (with or without cause) or expiration of the term of office of a Designated Director,
the holders of Series E Preferred Stock shall have the exclusive right to fill the vacancy created thereby with a new Designated Director.
With respect to the remaining directors constituting the Board, the holders of Series E Preferred Stock shall not vote against any person
nominated by the Board or the nominating committee of the Board between the Original Issuance Date and the third anniversary of the Original
Issuance Date; provided, however, that such three (3) year period shall commence on the original issuance date of the Bond
if the Company is not listed on a national securities exchange within twelve (12) months of the original issuance date of the Bond; provided
further that the foregoing obligation of the holders of Series E Preferred Stock shall not apply if the holders of Series E Preferred
Stock, in their reasonable discretion, determine that the person nominated by the Board or the nominating committee of the Board falls
under any of the following: (A) the nominee is a bad actor as defined in Rule 506(d) of the Securities Act of 1933, as amended, or is
otherwise legally prohibited from serving on the Board, (B) the nominee does not have business, finance or other relevant experience and
educational background, (C) the nominee is not independent under NYSE American Company Guide Rule 803, or (D) the nominee does not have
sufficient financial literacy to review and understand the financial statements of the Company. |
| (c) | Preferred Stock Protective Provisions. For so long as shares of Series E Preferred Stock remain
outstanding, in addition to any other vote or consent of stockholders required by law or this Certificate of Designation, the vote or
consent of the holders of a majority of the shares of Series E Preferred Stock then outstanding (the “Requisite Holders”)
shall be necessary for effecting or validating, either directly or indirectly, by amendment, merger, consolidation or otherwise: (i) any
amendment, alteration or repeal of any provision of this Certificate of Designation or of the Certificate of Incorporation or Bylaws of
the Company if such amendment, alteration or repeal will adversely affect the rights of the holders of the Series E Preferred Stock in
a manner different from any other series of preferred stock or (ii) the liquidation or dissolution of the Company. |
| (a) | Redemption Rights. If, but only if, the Company materially breaches any provision of this Certificate
of Designation and is unable to cure the same within thirty (30) days of receiving written notice from a holder of such breach (a “Default”),
the holders of the Series E Preferred Stock shall have redemption rights to request the Company to redeem all or part of the Series E
Preferred Stock in accordance with applicable laws at a redemption price per share equal to the sum of the Stated Value plus accrued,
but unpaid, dividends thereon, plus interest accrued on the Stated Value and such dividends at a rate equal to 4% per annum from the Original
Issuance Date (or with respect to any accrued and unpaid dividends, from the date on which such dividends are accrued) up to the date
of redemption, computed on the basis of the actual number of days elapsed and a year of 365 days (the “Redemption Price”).
Upon delivery of the redemption notice following a Default, the Company shall redeem the Series E Preferred Stock as requested within
thirty (30) days. If the Company fails to redeem the Series E Preferred Stock as requested following a Default, the redemption period
shall be extended until the redemption is fully completed. The exercise of redemption rights shall not affect the claim on any unpaid
dividends up until the date of the request for redemption. |
| (b) | Default Interest. If the Company fails to redeem following a Default in accordance with this Section
6, the Company shall pay the holders of the Series E Preferred Stock a default interest at an annual compounding interest rate equal to
the lower of (i) 12% of the Redemption Price from the following day of the date on which redemption should have been made or (ii) the
legal maximum rate. |
| (a) | Optional Conversion. Each Share of Series E Preferred Stock shall be convertible at any time and
from time to time at the option of the holder into such number of fully paid and non-assessable shares of Common Stock as is determined
by dividing the Stated Value, plus the value of the accrued, but unpaid, dividends thereon, by the Conversion Price in effect on the conversion
date. The “Conversion Price” means the lower of $5.34 per share or the Bond Conversion Price, subject to adjustment
as provided below. |
| (b) | Mechanics of Optional Conversion. Holders shall effect conversions
by providing the Company with a notice of conversion (a “Notice of Conversion”).
Each Notice of Conversion shall specify the number of shares of Series E Preferred Stock to be converted, the number of shares
of Series E Preferred Stock owned prior to the conversion at issue, the number of shares of Series E Preferred Stock owned subsequent
to the conversion at issue, the number of shares of Common Stock to be issued upon conversion and the date on which such conversion is
to be effected, which date may not be prior to the date the applicable holder delivers by email or facsimile such Notice of Conversion
to the Company (such date, the “Optional Conversion Date”). If no Optional Conversion Date is specified in a Notice
of Conversion, the Optional Conversion Date shall be the date that such Notice of Conversion to the Company is deemed delivered hereunder.
No ink-original Notice of Conversion shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization)
of any Notice of Conversion form be required. The calculations and entries set forth in the Notice of Conversion shall control in the
absence of manifest or mathematical error. To effect conversions of shares of Series E Preferred Stock, a holder shall not be required
to surrender the certificate(s) representing the shares of Series E Preferred Stock to the Company unless all of the shares of Series
E Preferred Stock represented thereby are so converted, in which case such holder shall deliver the certificate representing such shares
of Series E Preferred Stock promptly following the Optional Conversion Date at issue. The Company shall, as soon as practicable after
the Optional Conversion Date, issue and deliver to such holder of Series E Preferred Stock, or to his, her or its nominees, a certificate
or certificates, or a notification of book entry, for the number of full shares of Common Stock issuable upon such conversion in accordance
with the provisions hereof and a certificate or certificates, or a notification of book entry, for the number (if any) of the shares of
Series E Preferred Stock represented by the surrendered certificate that were not converted into Common Stock. |
| (c) | Mandatory Conversion. If all Conditions (as defined below) have been satisfied, then the Series
E Preferred Stock shall automatically, and without any action on the part of the Company or the holder, convert into such number of fully
paid and non-assessable shares of Common Stock as is determined by dividing the Stated Value, plus the value of the accrued, but unpaid,
dividends thereon, by the Conversion Price in effect on the conversion date. Notwithstanding the foregoing, if no Condition has been satisfied,
the Company may nevertheless issue a written notice of mandatory conversion to the holder on or after the third (3rd) anniversary
of the date of original issuance of the Series E Preferred Stock as long as at least three (3) months has elapsed after the first Listing
Event (as defined below); provided that the Conversion Price shall automatically be adjusted to 80% of the value weighted average price
of the Company’s Common Stock for the trailing three (3) months. The date on which mandatory conversion is effected under this Section
7(c) shall be referred to as the “Mandatory Conversion Time.” For purposes of this provision, “Conditions”
means: (i) the volume weighted average price of the Common Stock for the trailing three (3) months is at least 1.5 times the initial Conversion
Price and (ii) (A) if the total investment amount invested by the initial holder of the Series E Preferred Stock is at least $23,000,000
but less than $50,000,000, the Company has achieved revenues for the trailing twelve-month period of at least $25,000,000 or (B) if the
total investment amount invested by the initial holder of the Series E Preferred Stock is at least $50,000,000, the Company has achieved
revenues for the trailing twelve-month period of at least $45,000,000. |
| (d) | Mechanics of Mandatory Conversion. All holders of record of shares of Series E Preferred Stock
shall be sent written notice of the mandatory conversion at least ten (10) business days prior to the Mandatory Conversion Time. Upon
receipt of such notice, each holder of shares in certificated form shall surrender his, her or its certificate or certificates for all
such shares, if any (or, if such holder alleges that such certificate has been lost, stolen or destroyed, a lost certificate affidavit
and agreement reasonably acceptable to the Company to indemnify the Company against any claim that may be made against the Company on
account of the alleged loss, theft or destruction of such certificate), to the Company at the place designated in such notice. If so required
by the Company, any certificates surrendered for conversion shall be endorsed or accompanied by written instrument or instruments of transfer,
in form satisfactory to the Company, duly executed by the registered holder or by his, her or its attorney duly authorized in writing.
All rights with respect to the shares converted pursuant to this Section 7(d), including the rights, if any, to receive notices and vote
(other than as a holder of Common Stock), will terminate at the Mandatory Conversion Time (notwithstanding the failure of the holder or
holders thereof to surrender any certificates at or prior to such time), except only the rights of the holders thereof, upon surrender
of any certificate or certificates of such holders (or lost certificate affidavit and agreement) therefor, to receive the shares of Common
Stock. As soon as practicable after the Mandatory Conversion Time and, if applicable, the surrender of any certificate or certificates
(or lost certificate affidavit and agreement) for the shares, the Company shall issue and deliver to such holder of Series E Preferred
Stock, or to his, her or its nominees, a certificate or certificates, or a notification of book entry, for the number of full shares of
Common Stock issuable upon such conversion in accordance with the provisions hereof. |
| (e) | Fractional Shares. No fractional shares of Common Stock or scrip shall be issued upon conversion
of the Series E Preferred Stock. Any fractional shares which would otherwise be issuable upon conversion of the Series E Preferred Stock
will be rounded up to the next whole share. |
| (a) | Adjustment for Corporate Actions. If the Company shall at any time or from time to time after the
issuance of the Series E Preferred Stock (i) pay a stock dividend or otherwise make a distribution or distributions on shares of its Common
Stock or any other equity or equity equivalent securities payable in shares of Common Stock, (ii) effect a subdivision of the outstanding
Common Stock into a larger number of shares (including by way of a stock split), (iii) combine (including by way of reverse stock split)
outstanding shares of Common Stock into a smaller number of shares, or (iv) issue by reclassification of shares of the Common Stock any
shares of capital stock of the Company, then the Conversion Price then in effect immediately before such action shall be multiplied by
a fraction, of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding before
such event and of which the denominator shall be the number of shares of Common Stock outstanding after such event. Any adjustment made
pursuant to this Section 8(a) shall become effective immediately after the record date for the determination of stockholders entitled
to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision,
combination or reclassification. |
| (b) | Subsequent Equity Sales. In the event the Company issues any Common Stock at a price per share,
or any securities convertible or exchangeable into, or exercisable for, Common Stock (“Common Stock Equivalents”),
with an implied price per share, of less than the Conversion Price (such issuance, the “Issuance”), the Conversion
Price shall be adjusted, concurrently with the Issuance, to the implied price per share received by the Company for such Issuance; provided
that the Conversion Price as adjusted pursuant to this Section 8(b) shall not be less than the par value of the Common Stock, subject
to adjustments for stock splits and stock dividends on the Common Stock. Notwithstanding the foregoing, this Section 8(b) shall not apply
to an Exempt Issuance and other issuances of Common Stock or Common Stock Equivalents disclosed to, and approved in writing not to adjust
the Conversion Price then in effect in advance by, each holder of shares of Series E Preferred Stock then outstanding. For purposes hereof,
“Exempt Issuance” shall mean the issuance of (a) shares of Common Stock or options to purchase shares of Common Stock
issued to employees, officers, directors or consultants of the Company pursuant to any stock or option plan duly adopted for such purpose
by a majority of the directors of the Company or a majority of a committee of established for such purpose; (b) shares of Common Stock
issued pursuant to Common Stock Equivalents issued and outstanding on the Original Issuance Date or commitments to issue shares of Common
Stock or Common Stock Equivalents, which commitments are in existence on the Original Issuance Date, or amended after the Original Issuance
Date if such amendment is approved by a majority of the directors of the Company, including a majority of the Designated Directors; and
(c) the License Equity, as defined in the MDACC Agreements (as defined in the Bond), issued to the Board of Regents of the University
of Texas System (as described in Exhibit II of the option agreement included in the MDACC Agreements). |
| (c) | Adjustment Following IPO or Direct Listing. If the Series E Preferred Stock has not been converted
or redeemed and the volume-weighted average price per share of the Common Stock between the closing date of (i) an initial public offering
of the Company’s Common Stock and concurrent listing on a national securities exchange, including without limitation the New York
Stock Exchange, NYSE American or the Nasdaq Stock Market (any tier), or (ii) a direct listing of the Company’s common stock on any
such national securities exchange (each, a “Listing Event”), and the six-month anniversary of the closing date of the
Listing Event (the “Listing Price”) is lower than (i) the Conversion Price then in effect plus (ii) the interest calculated
on the Conversion Price then in effect by applying an annual compounding interest rate of 6% for the foregoing six-month period (the “Target
Price”), the Conversion Price shall be adjusted to the Listing Price; provided that the Conversion Price shall not be adjusted
to become lower than 70% of the Target Price. Notwithstanding the foregoing, the Conversion Price as adjusted pursuant to this Section
8(c) shall not be less than the par value of the Common Stock, subject to adjustments for stock splits and stock dividends on the Common
Stock. |
| (d) | Effect of Merger, Etc. In the case of any consolidation with, or merger of the Company into, any
other person or any merger or consolidation of another person into the Company (other than a merger in which the Company is the continuing
corporation or a consolidation or merger which does not result in any reclassification, conversion, exchange or cancellation of outstanding
Common Stock), or any sale, transfer or lease of all, or substantially all, of the properties or assets of the Company, the person resulting
from such consolidation or merger, or which acquires or leases such properties or assets of the Company, as the case may be, shall execute
and deliver to the holders of Series E Preferred Stock a new certificate representing the same security that the holder of the Series
E Preferred Stock would have received if such holder converted to Common Stock immediately prior to such consolidation, merger, sale,
transfer or lease. |
| (e) | Calculations. All calculations under this Section 8 shall be made to the nearest cent or the nearest
1/100th of a share, as the case may be. For purposes of this Section 8, the number of shares of Common Stock deemed to be issued and outstanding
as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding. |
| (f) | Notice of Adjustments. Whenever the Conversion Price is adjusted pursuant to any provision of this
Section 8, the Company shall promptly mail to each holder a notice setting forth the Conversion Price after such adjustment and setting
forth in reasonable detail the facts requiring such adjustment. |
| (g) | Notice to Allow Conversion by Holders. If (i) the Company shall
declare a dividend (or any other distribution in whatever form) or redemption on the Common Stock, (ii) the Company shall authorize the
granting to all holders of the Common Stock of rights or warrants to subscribe for or purchase any shares of
capital stock of any class or of any rights, (iii) the approval of stockholders shall be required in connection with any reclassification
of the Common Stock, any consolidation or merger to which the Company is a party, any sale or
transfer of all or substantially all of the assets of the Company, or any compulsory share exchange whereby the Common Stock is converted
into other securities, cash or property or (iv) the Company shall authorize a Liquidation Event or Deemed Liquidation Event, then, in
each case, the Company shall cause to be delivered to each holder of Series E Preferred Stock at its last address as it shall appear upon
the stock books of the Company, at least ten (10) calendar days prior to the applicable record or effective date hereinafter specified,
a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants,
or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions,
redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer
or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of
record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification,
consolidation, merger, sale, transfer or share exchange, provided that the failure to deliver such notice or any defect therein or in
the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. The holder shall remain
entitled to convert its Series E Preferred Stock (or any part hereof) during the 10-day period commencing on the date of such notice through
the effective date of the event triggering such notice except as may otherwise be expressly set forth herein. |
| 9. | Participation in Future Financing. |
| (a) | Participation Right. From the date hereof until the date that the Series E Preferred Stock is no
longer outstanding, upon any issuance by the Company of Common Stock or Common Stock Equivalents for cash consideration in a financing
transaction, the primary purpose of which is to raise capital (a “Subsequent Financing”), the holder of the Series
E Preferred Stock shall have the right to participate in the Subsequent Financing in the manner set forth in Section 9(b) on the same
terms, conditions and price provided for in the Subsequent Financing. |
| (b) | Mechanics of Participation Right. |
| (i) | The Company shall deliver to the holder of the Series E Preferred Stock a written notice of the Company’s
intention to effect a Subsequent Financing (a “Subsequent Financing Notice”) at least thirty (30) business days prior
to the date of the expected consummation of the Subsequent Financing, which notice shall describe in reasonable detail the proposed terms
of such Subsequent Financing, the amount of proceeds intended to be raised thereunder and the person or persons through or with whom such
Subsequent Financing is proposed to be effected (the “Subsequent Investor(s)”) and shall include a term sheet and transaction
documents relating thereto as an attachment. If the holder desires to participate in such Subsequent Financing, it must provide written
notice (the “Participation Notice”) to the Company within one hundred and twenty (120) days following the date on which
the Subsequent Financing Notice is delivered to the holder (the “Notice Termination Time”) which notice specifies the
holder’s willingness to participate in the Subsequent Financing, directly and/or through its designated affiliate, as well as the
number of Common Stock or Common Stock Equivalents, as applicable, to be subscribed by the holder and/or its designated affiliate. |
| (ii) | In order to protect the holder’s right to maintain its shareholding ratio on a fully-diluted basis,
(x) if the holder is to participate in the Subsequent Financing simultaneously with the Subsequent Investor(s), the holder may subscribe
for, or cause its designated affiliate to subscribe for, up to such number of Common Stock or Common Stock Equivalents (whichever is to
be issued at the Subsequent Financing) calculated by multiplying (A) the aggregate number of Common Stock or Common Stock Equivalents,
as applicable, available for subscription at the Subsequent Financing and (B) the holder’s Pro Rata Percentage (as defined below);
and (y) if the holder is to participate in the Subsequent Financing after the consummation of the Subsequent Financing by the Subsequent
Investor(s), the holder may subscribe for, or cause its designated affiliate to subscribe for, up to such number of Common Stock or Common
Stock Equivalents (whichever is to be issued at the Subsequent Financing) calculated as follows: |
For purposes of the foregoing formula,
the following definitions shall apply:
“HSF” shall mean the number
of Common Stock or Common Stock Equivalents (whichever is to be issued at the Subsequent Financing) that the holder may subscribe for
or cause its designated affiliate to subscribe for.
“SF” shall mean the aggregate
number of Common Stock or Common Stock Equivalents, as applicable, issued to the Subsequent Investor(s) at the Subsequent Financing.
“A” shall mean the number
of shares of Common Stock outstanding on a fully-diluted basis that is not held by the holder immediately prior to the consummation of
the Subsequent Financing.
“B” shall mean the sum
of (A) the number of shares of Common Stock held by the holder on a fully-diluted basis immediately prior to the consummation of the Subsequent
Financing and (B) the number of shares of Common Stock or Common Stock Equivalents that the holder is eligible to subscribe for, or cause
its designated affiliate to subscribe for, in connection with any prior Subsequent Financing as of the date of consummation of the Subsequent
Financing.
| (iii) | Assuming that the holder delivers the Participation Notice within the Notice Termination Time, (x) if
the holder delivers the Participation Notice within twenty (20) business days following the delivery of the Subsequent Financing Notice
(the “Simultaneous Participation Deadline”), the Company shall effect the Subsequent Financing to the holder and the
Subsequent Investor(s) simultaneously in accordance with the Participation Notice; and (y) if the holder delivers the Participation Notice
after the Simultaneous Participation Deadline, the Company may consummate the Subsequent Financing to the Subsequent Investor(s) on the
terms set forth in the Subsequent Financing and shall allow the holder to exercise its participation right set forth in Section 9(b)(ii)(y). |
| (iv) | For the avoidance of doubt, the holder and/or its designated affiliate, at its sole and absolute discretion,
may, in the Subsequent Financing, subscribe for any number of Common Stock or Common Stock Equivalents, as applicable, equal to or less
than such number set forth in Section 9(b)(ii)(x) or 9(b)(ii)(y), as applicable. |
| (v) | If the Company receives no such notice from the holder as of the Notice Termination Time, the holder shall
be deemed to have notified the Company that it does not elect to participate in such Subsequent Financing. |
(c) For
the purpose of this Section 9, “Pro Rata Percentage” shall mean the fraction the numerator of which is the number of
shares of Common Stock held by the holder on a fully-diluted basis and the denominator of which is the number of shares of Common Stock
outstanding on a fully-diluted basis.
| (a) | Notices. Any and all notices or other communications or deliveries
to be provided by the holders hereunder including, without limitation, any Notice of Conversion, shall be in writing and delivered personally,
by email, or sent by a nationally recognized overnight courier service, addressed to the Company at its principal office, email address
investors@2020gene.com or such other address or email address as the Company may specify for
such purposes by notice to the holders of Series E Preferred Stock delivered in accordance with this Section 10(a). Any and all notices
or other communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by email,
or sent by a nationally recognized overnight courier service addressed to each holder of Series E Preferred Stock at the address or email
address of such holder appearing on the books of the Company, or if no such address or email address appears on the books of the Company,
at the principal place of business of such holder. Any notice or other communication or deliveries hereunder shall be deemed given and
effective on the earliest of (i) the date of transmission, if such notice or communication is delivered via email at prior to 5:30 p.m.
(New York City time) on any business day, (ii) the next business day after the date of transmission, if such notice or communication is
delivered via email on a day that is not a business day or later than 5:30 p.m. (New York City time) on a business day, (iii) the second
business day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual receipt
by the party to whom such notice is required to be given. |
| (b) | Lost or Mutilated Stock Certificate. If a holder’s Series
E Preferred Stock certificate shall be mutilated, lost, stolen or destroyed, the Company shall execute and deliver, in exchange and substitution
for and upon cancellation of a mutilated certificate, or in lieu of or in substitution for a lost, stolen or destroyed certificate, a
new certificate for the shares of Series E Preferred Stock so mutilated, lost, stolen or destroyed, but only upon receipt of evidence
of such loss, theft or destruction of such certificate, and of the ownership hereof reasonably
satisfactory to the Company. |
| (c) | Governing Law. All questions concerning
the construction, validity, enforcement and interpretation of this Certificate of Designation shall be governed by and construed and enforced
in accordance with the internal laws of the State of Delaware, without regard to the principles of conflict of laws thereof. |
| (d) | Amendments; Waiver. This Certificate of Designation may be amended
or any provision of this Certificate of Designation may be waived with the affirmative vote of the Requisite Holders. Any waiver by the
Company or a holder of Series E Preferred Stock of a breach of any provision of this Certificate
of Designation shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other
provision of this Certificate of Designation or a waiver by any other holders, except that a waiver by the Requisite Holders will constitute
a waiver of all holders. The failure of the Company or a holder of Series E Preferred Stock to insist upon strict adherence to any term
of this Certificate of Designation on one or more occasions shall not be considered a waiver or deprive that party (or any other holder)
of the right thereafter to insist upon strict adherence to that term or any other term of this Certificate of Designation on any other
occasion. Any waiver by the Company or a holder of Series E Preferred Stock must be in writing. Any action that is required or permitted
to be taken by the Requisite Holders may be taken at any meeting called by any holder or holders of Series E Preferred Stock or by a written
consent or action by such holders in lieu of any such meeting. With respect to any meeting of holders, unless otherwise specified in this
Certificate of Designation, the procedures for calling and holding a meeting of the holders of Common Stock of the Company shall be applicable
with respect to a meeting of the holders of Series E Preferred Stock, mutatis mutandis. The Company shall promptly provide to any
holder a list of the other holders upon the request of any holder, and otherwise provide such cooperation and assistance to any holder
for the purposes of calling and holding a meeting of the holders as from time to time reasonably requested by a holder. |
| (e) | Severability. If any provision of this Certificate of Designation
is invalid, illegal or unenforceable, the balance of this Certificate of Designation shall remain in effect, and if any provision is inapplicable
to any person or circumstance, it shall nevertheless remain applicable to all other persons and circumstances.
If it shall be found that any interest or other amount deemed interest due hereunder violates the applicable law governing usury, the
applicable rate of interest due hereunder shall automatically be lowered to equal the maximum rate of interest permitted under applicable
law. |
| (f) | Headings. The headings contained herein
are for convenience only, do not constitute a part of this Certificate of Designation and shall not be deemed to limit or affect any of
the provisions hereof. |
| (g) | Status of Converted or Redeemed Series E Preferred Stock. If
any shares of Series E Preferred Stock shall be converted, redeemed or reacquired by the Company, such shares shall resume the status
of authorized but unissued shares of preferred stock and shall no longer be designated as Series
E Preferred Stock. |
ANNEX A-12
Exhibit 6.2
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
Option
Agreement
This Option Agreement (“Agreement”)
is made as of the date of the last authorized signature below (the “Effective Date”), by and between The Board of Regents
(“Board”) of The University of Texas System (“System”), an agency of the State of Texas,
whose address is 210 West 7th Street, Austin, Texas 78701, on behalf of The University of Texas M. D. Anderson Cancer Center (“MD
Anderson”), a member institution of System, and 20/20 GeneSystems Inc., having a principal place of business located at 15810
Gaither Drive, Suite 235, Gaithersburg MD 20877 (“Company”).
Recitals
A. Board
owns, on behalf of MD Anderson, Licensed Subject Matter (defined below).
B. Company
desires a period of time in which to evaluate Licensed Subject Matter, potential products arising therefrom and markets therefor.
C. Company
desires to secure a license to practice the Licensed Subject Matter as set in the License Agreement (defined below), effective upon exercise
of the Option, including, without limitation, obtaining Investor Financing and payment of the Option Fee (each as defined below), as set
forth herein.
NOW, THEREFORE, in consideration of the premises
and mutual covenants contained herein, and intending to be legally bound hereby, the parties hereto agree as follows:
I. Definitions
| 1.1 | Agreement has the meaning set forth in the preamble above. |
| 1.2 | Board has the meaning set forth in the preamble above. |
| 1.3 | Company Pre-Money Valuation shall have the meaning set forth in Section 2.2. |
| 1.4 | Effective Date has the meaning set forth in the preamble above. |
| 1.5 | Investor
Financing shall have the meaning set forth in Section 2.2. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.6 | Company has the meaning set forth in the preamble above. |
| 1.7 | MD Anderson has the meaning set forth in the preamble above. |
| 1.8 | Option has the meaning set forth in Section 2.1. |
| 1.9 | Option Fee has the meaning set forth in Section 2.2. |
| 1.10 | Option Period means the period beginning on the Effective Date and ending six (6) months thereafter. |
| 1.11 | Patent Rights means the Board’s rights in: |
| (a) | the patents and patent applications listed in the License Agreement as shown in Exhibit A; and |
| (b) | all patent applications that claim priority to any of the provisional applications listed in License Agreement
as shown in Exhibit A; and |
| (c) | all divisionals, continuations and continuations-in-part of the non-provisional patent applications identified
in (a) and (b), above provided that the claims of such continuations-in-part are entitled to claim priority to at least one of the patent
applications identified in (a) or (b), above; and |
| (d) | all reissues, reexaminations, extensions, and PCT International Applications, foreign counterparts of
any of the patents or patent applications identified in (a), (b) or (c), above; and |
| (e) | any patents that issue with respect to any of the patent applications listed in (a), (b), (c) or (d),
above. |
| 1.12 | System has the meaning set forth in the preamble above. |
| 1.13 | Technology Rights means Board’s rights within the Licensed Field in the information in Exhibit
VII of the License Agreement of Exhibit A, and other technical information, know-how, processes, procedures, compositions, devices, methods,
formulas, protocols, techniques, designs, drawings, Laboratory Analytics, laboratory test protocols or procedures or data created at MD
Anderson before the Effective Date by the inventor(s) listed in Exhibit I to the License Agreement attached as Exhibit A while employed
at MD Anderson, to the extent the foregoing are not covered by Patent Rights, but are (a) described in the invention disclosure reports
listed on Exhibit I; or (b) facilitate the practice, development, manufacture, use, and/or selling of invention(s) claimed in the patents
and/or patent applications listed in the definition of Patent Rights, whether outstanding, expired or abandoned, and with respect to this
clause (b) have no obligations or encumbrances in favor of or benefitting any third party and are not otherwise subject to contractual
or legal restrictions that would preclude the licensure to Company as contemplated by the License Agreement; provided, however,
in the event of the existence of such obligation and/or encumbrance, MD Anderson will use its best efforts to remove such obligation and/or
encumbrance or obtain rights and/or permissions necessary for Licensee to exercise the rights granted hereunder, at MD Anderson’s
expense, further provided that if such obligation and/or encumbrance is removed such rights, permissions, licenses, and interests automatically
shall be deemed as part of the Technology Rights for the full Term of the Option and the License stated in Exhibit A, and further provided
that if MD Anderson is unable to remove such a third party obligation and/or encumbrance, or lacks the power to make the full scope of
the grants set forth herein, despite having applied its best efforts, then the Parties agree to negotiate in good faith to modify the
financial terms of Agreement to account for the inability of MD Anderson to confer the full scope of the grants in this Agreement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.14 | [***] License means [***]. |
II. Option
| 2.1 | MD Anderson hereby grants to the Company an exclusive option (“Option”) to enter into a royalty-bearing,
exclusive license to Patent Rights and Technology Rights during the Option Period. Subject to the rights, if any, of the United States
government pursuant to 35 U.S.C. §200, et seq., during the Option Period, MD Anderson shall not offer a license to any third party
which is inconsistent with the Option granted herein. |
| 2.2 | During the Option Period, Company may exercise the Option by providing MD Anderson with written notice
thereof after satisfying the following conditions precedent, each of which must be met (unless waived by all parties signatory hereto)
prior to expiry of the Option Period: |
| (a) | Company must have completed a transaction or series of transactions pursuant to which the Company completes
an equity financing for aggregate proceeds of $23,000,000 USD and such issuance shall (i) be based on a pre-money valuation of Company
of $70,000,000 USD (the “Company Pre-Money Valuation”) and (ii) not be completed in reliance on Regulation Crowdfunding
(as defined in the Jumpstart Our Business Startups Act (JOBS Act)) or Regulation A (as defined in Section 3(g) of the Securities Act of
1933, as amended) (the transactions described in subsections (i) and (ii) above, the “Investor Financing”); and |
| (b) | MD Anderson’s receipt from Company of documentation evidencing fulfillment, to MD Anderson’s
reasonable satisfaction, of the Investor Financing contemplated by 2.2(a) above; and |
| (c) | MD Anderson’s receipt of payment by Company of an option fee in the amount of $4,457,069.15 (“Option
Fee”) within five (5) days of MD Anderson’s written confirmation of its satisfaction with the documents providing under
Section 2.2(b). Such amount shall be nonrefundable and shall be noncreditable against any future license consideration. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
Licensee shall deliver
to MD Anderson (i) copies of all agreements entered into in the Investor Financing (including all stockholder agreements, purchase agreements,
charters, and ancillary documentation); (ii) the term sheet for the Investor Financing, if any; and (iii) a document evidencing fulfillment
of the Investor Financing referenced in Section 2.2(b) in the form of Exhibit III. Delivery of the items set forth in subsection (i).
| 2.3. | Upon Company’s exercise of the Option, Board and Company agree to execute the License Agreement
in the form attached as Exhibit A hereto. If Company has not executed the License Agreement by the end of the Option Period or has not
timely paid the Option Fee, this Agreement shall be deemed terminated and Board and MD Anderson shall be free to enter into an exclusive
or non-exclusive license to Patent Rights, Technology Rights and/or Board’s Rights under the [***] License with any other entity. |
III. General
| 3.1. | The Company and MD Anderson agree that any information disclosed by either party to the other pursuant
to this Agreement shall be maintained in strict confidence, and each will use all reasonable diligence to prevent disclosure except to
necessary employees, and to affiliates and consultants who agree to be bound by this confidentiality provision and for which the receiving
party agrees to be fully responsible for such parties’ compliance. The Company’s and MD Anderson’s obligations under
this confidentiality clause shall remain in effect for the Option Period and a period of three (3) years thereafter. The Company and MD
Anderson shall not have any obligation of confidentiality with respect to information that: |
| (a) | is in the public domain by use and/or publication; or |
| (b) | is developed independently of information received from the disclosing party; or |
| (c) | was already in the recipient’s possession prior to receipt from disclosing party; or |
| (d) | is properly obtained by recipient from a third party with a valid legal right to disclose such information
and such third party is not under a confidentiality obligation to the disclosing party; or |
| (e) | is required by law or regulation to be disclosed, provided that the recipient shall notify the disclosing
party as soon as practicable of such requirement or regulation to enable the disclosing party to seek a protective order or similar remedy. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 3.2. | MD ANDERSON MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED,
INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR VALIDITY OF PATENT RIGHTS CLAIMS, ISSUED
OR PENDING, WITH RESPECT TO THE PATENT RIGHTS AND/OR TECHNOLOGY RIGHTS. MD ANDERSON MAKES NO REPRESENTATION THAT ANY INVENTIONS CONTAINED
IN PATENT RIGHTS AND/OR TECHNOLOGY RIGHTS DO NOT INFRINGE ANY OTHER PATENTS HELD BY OTHERS. The Company has not been induced in any way
by MD Anderson to enter into this Agreement. The Company has conducted its own due diligence with respect to this Agreement and is not
relying on any representations or warranties by MD Anderson. |
| 3.3. | This Agreement is not assignable and any attempt to do so shall be null and void. |
| 3.4. | All notices required to be given under this Option shall be in writing and shall be deemed to have been
sufficiently given for all purposes thereof when mailed by certified first class or overnight mail and shall be evidenced by the signature
of a representative of the party receiving the notice at the point of receipt or by the time-stamped receipt of the overnight carrier. |
All notices and any correspondence respecting
this Agreement shall be addressed and sent out as follows:
To MD Anderson:
The University of Texas M. D. Anderson
Cancer Center
Office of Technology Commercialization,
To the Company:
Chief Executive Officer
15810 Gaither Drive,
Suite 235
Gaithersburg, MD 20877
| 3.5. | Upon fifteen (15) days written notice and to the extent that Company has not fully exercised the Option
as set forth in Section 2.2, Board and/or MD Anderson may terminate this Agreement at any time prior to Company’s full exercise
of the Option. The obligations of Section 3.6 shall survive termination. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 3.6. | Neither party shall use the name of the other party in any form of promotion or in connection with the
sale of products, processes, devices or designs without the prior written approval of such party. |
| 3.7. | This Agreement contains the entire agreement of the parties hereto with respect to the matters covered
hereby and all prior negotiations and agreements with respect hereto are of no force and effect. No subsequent modification hereof shall
be made except in a writing executed by MD Anderson and the Company. |
| 3.8. | This Agreement and all claims arising out of or relating thereto will be governed, construed and enforced
in accordance with the laws of the United States of America and of the State of Texas, without regard to its conflict of law provisions.
The Texas State Courts of Harris County, Texas (or, if there is exclusive federal jurisdiction, the United States District Court for the
Southern District of Texas) shall have exclusive jurisdiction and venue over any dispute arising out of this Agreement, and Licensee consents
to the jurisdiction and venue of such courts and hereby explicitly waives the rights to any other venue to which it might be entitled
by cause of action, domicile or otherwise. |
| 3.9. | This Agreement may be executed in one (1) or more counterparts, by original, facsimile or PDF signature,
each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Signatures to this
Agreement transmitted by facsimile, by email in “portable document format” (“.pdf”), or by any other electronic
means intended to preserve the original graphic and pictorial appearance of this Agreement shall have the same effect as physical delivery
of the paper document bearing original signature. In the event signatures are exchanged by facsimile and/or in “.pdf” format,
each party shall, if requested, thereafter promptly provide an original signature page to the other party. |
| 3.10. | MD Anderson, as an agency of the State of Texas and a member institution of The University of Texas System,
is subject to the constitution and laws of the State of Texas and, under the constitution and laws of the State of Texas, possesses certain
rights and privileges, is subject to certain limitations and restrictions, and only has such authority as is granted under the constitution
and laws of the State of Texas. Moreover, notwithstanding the generality or specificity of any provision of this Agreement, the provisions
of this Agreement as they pertain to MD Anderson are enforceable only to the extent authorized by the constitution and laws of the State
of Texas. No party to this Agreement will be required to perform or commit any act or omission that would violate any applicable law,
including the constitution and laws of the State of Texas. Nothing in this Agreement shall be deemed as a waiver by Board, System or MD
Anderson of its sovereign immunity. |
[Signatures on Following
Page]
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
IN WITNESS WHEREOF, the parties have executed
this Agreement effective on the date set forth in the first paragraph hereof.
BOARD OF REGENTS OF THE
UNIVERSITY OF TEXAS System, on behalf of
THE UNIVERSITY OF TEXAS M. D. ANDERSON CANCER CENTER
Date: 03/20/2024
Approved as to Content:
THE UNIVERSITY OF TEXAS
M. D. ANDERSON CANCER CENTER
| By: |
/s/ Ferran Prat |
|
| |
Ferran Prat, J.D., Ph.D. |
|
| |
Vice President, Research Admin &
Industry Relations |
|
Date: 03/19/2024
COMPANY
| Printed Name: |
Jonathan Cohen |
|
| Title: |
President & CEO |
|
Date: 03/22/2024
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
EXHIBIT A
Patent and Technology License Agreement
This Patent and Technology License Agreement (“Agreement”)
is made as of the date of the last authorized signature below (the “Effective Date”), by and between The Board of Regents
(“Board”) of The University of Texas System (“System”), an agency of the State of Texas,
whose address is 210 West 7th Street, Austin, Texas 78701, on behalf of The University of Texas M. D. Anderson Cancer Center (“MD
Anderson”), a member institution of System, and 20/20 GeneSystems Inc., a Delaware corporation, having a principal place of
business located at 15810 Gaither Drive, Suite 235, Gaithersburg MD 20877 (“Licensee”).
Recitals
| A. | Board owns Licensed Subject Matter (defined below). |
| B. | Board, through MD Anderson, has determined that development and commercialization of the Licensed Subject
Matter is in the public’s best interest and is consistent with Board’s educational and research missions and goals. |
| C. | Board desires to have the Licensed Subject Matter developed and commercialized for the benefit of Licensee,
the inventors, Board, System, MD Anderson, and the public. |
| D. | Licensee desires to secure a license to practice the Licensed Subject Matter as set forth herein, effective
upon meeting certain financial obligations and exercise of the Option as set forth herein. |
| E. | Licensee and Board desire to enter into the Sponsored Research Agreement dated __________ and effective
upon the effective date thereof, to pursue research in the area of discovery, development, and validation of multi-cancer early detection
(MCED) laboratory developed tests and in vitro diagnostic (IVD) assays. |
NOW, THEREFORE, in consideration of the mutual
covenants and promises herein contained, the parties agree as follows:
As used in this Agreement, the following terms
have the meanings indicated:
| 1.1 | 510K Clearance means (a) an order, in the form of a letter, from FDA in response to a Pre-Market
Notification submission to the FDA in accordance with 21 CFR Part 807, Subpart E, or any future revisions or substitutes thereof, in order
to obtain clearance to market a medical device in the United States as substantially equivalent to a legally marketed device consistent
with section 513(i)(1)(A) FD&C Act); or (b) an equivalent foreign filing, approval, clearance, or permission in any jurisdiction other
than the United States. For clarification and for purposes of this Agreement, 510K includes, without limitation, the submission of any
application for, or the making of a declaration for, or the use of, a CE Mark for commercialization of a Class I device in the European
Union. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.2 | Affiliate means any business entity more than fifty percent (50%) owned, directly or indirectly,
by Licensee, any business entity which, directly or indirectly, owns more than fifty percent (50%) of Licensee, or any business entity
that is more than fifty percent (50%) owned, directly or indirectly, by a business entity that owns more than fifty percent (50%) of Licensee. |
| 1.3 | Agreement has the meaning set forth in the preamble above. |
| 1.4 | Anticipated Costs has the meaning set forth in Section 4.5. |
| 1.5 | Board has the meaning set forth in the preamble above. |
| 1.6 | Breakthrough Device Designation means the designation provided by the FDA through the FDA’s
Breakthrough Device Program. |
| 1.7 | BPCIA means the U.S. Biologics Price Competition and Innovation Act of 2009, as amended from time
to time. |
| 1.8 | Calendar Quarter means the respective periods of three (3) consecutive calendar months ending on
March 31, June 30, September 30 and December 31; provided, however, that (a) the first Calendar Quarter of the Term (defined below) shall
extend from the Effective Date to the first to occur of March 31, June 30, September 30 or December 31; and (b) the last Calendar Quarter
of the Term shall end on the effective date of the expiration or termination of this Agreement. |
| 1.9 | Committees shall have the meaning set forth in Section 3.6(a). |
| 1.10 | Combination Product means any Licensed Product that Licensee desires to sell, for a single price,
integrated or in combination with one or more other functional components (such as biomarker panels or algorithms) for which no royalty
would be due hereunder if sold separately. |
| 1.11 | Development Milestone shall have the meaning set forth in Section 3.6(b)(iii). |
| 1.12 | Effective Date has the meaning set forth in the preamble above. |
| 1.13 | FDA means the United States Food and Drug Administration or its successor. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.14 | First Commercial Sale shall mean, with respect to any Licensed Product, the first sale to a third
party for which revenue has been recognized by Licensee or a Sublicensee, for use or consumption of such Licensed Product in any country
in the Licensed Territory; excluding, however, any transfer or disposition for compassionate or similar use for which no value
is received by Licensee or any Sublicensee. For clarity, First Commercial Sale shall be determined on a License Product-by-Product and
country-by-country basis and shall only occur if such sale is included in Net Sales. |
| 1.15 | Fully-Diluted Capitalization shall mean, the aggregate number of issued and outstanding equity
securities of Licensee, plus the amount of any reserves for issuances of equity compensation, and assuming full conversion and exercise
of all options, warrants or other convertible and exercisable securities then outstanding. |
| 1.16 | Graphic Logos means graphic logos or symbols as set forth on Exhibit IV of this Agreement relating
to the MD Anderson Licensed Marks. |
| 1.17 | Gross Up has the meaning set forth in Section 3.9. |
| 1.18 | Joint SRA Technology Rights means SRA Technology Rights created or developed jointly by, or on
behalf of, Licensee and by, or on behalf of, MD Anderson. |
| 1.19 | Laboratory Analytics means algorithms (i.e. a finite set of well-defined rules for the solution
of a problem in a finite number of steps), including without limitation, algorithms that mathematically analyze, compute, or interpret
the levels or values of biomarkers, with or without clinical factors, at one time or over time, generated by a clinical laboratory. |
| 1.20 | Laboratory Developed Test means an in vitro diagnostic product (IVDs) that is a Licensed
Product and is intended for clinical use and is designed, manufactured, and used within a single clinical laboratory certified under the
Clinical Laboratory Improvement Amendments of 1988 (42 USC 263a) and the associated regulations (42 CFR 493) (collectively, “CLIA”)
and meets the regulatory requirements under CLIA to perform high complexity testing or equivalent designation by a Regulatory Authority
of a jurisdiction other than the United States. |
| 1.21 | Laboratory Test Protocols means all
steps or protocols used in a research laboratory or clinical laboratory setting, and for
the avoidance of doubt includes Laboratory Analytics. |
| 1.22 | License Equity shall have the meaning set forth in Section 3.6(b). |
| 1.23 | Licensed Field means in vitro (related to fluids outside of a patient’s body) diagnostic
in the field of blood-based predisposition evaluation, screening, detection, diagnosis, prognosis, relapse monitoring, monitoring of response
to therapy, and development of companion diagnostics for therapies; excludes all in vivo (related to fluids within a patient’s
body) diagnostics and therapeutic applications. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.24 | Licensed Product(s) means (A) any product or component that: (i) comprises, uses, or is made using
subject matter covered by one or more Valid Claims; (ii) enables a user, acting either alone, independently with other users or in concert
with other users, to perform or practice one or more Valid Claims; or (iii) incorporates, uses, or is made using subject matter covered
by Technology Rights or SRA Technology Rights; and/or (B) any method or process that, when performed by one or more people and/or entities
acting either alone, independently with others or in concert with others, (i) practices one or more Valid Claims or (ii) incorporates
or uses subject matter comprising Technology Rights or SRA Technology Rights that at any time qualifies as MD Anderson’s “Confidential
Information” consistent with Section 9.1 of this Agreement; |
| 1.25 | Licensed Subject Matter means (a) Patent Rights, (b) Technology Rights, (c) inventions and/or discoveries
covered by Patent Rights and/or Technology Rights, and/or MD Anderson’s rights under the [***] License (d) SRA Patent Rights and
(e) SRA Technology Rights. |
| 1.26 | Licensed Territory means worldwide. |
| 1.27 | Licensee has the meaning set forth in the preamble above. |
| 1.28 | Licensee Board shall have the meaning set forth in Section 3.6(a). |
| 1.29 | Licensee’s OneTest means that test as sold by Licensee, as such test exists as of the Effective
Date and as described in Exhibit VI, that detects multiple cancers and/or establishes the risk of developing cancers other than lung and
pancreatic cancer. |
| 1.30 | [***]/ Pre-CT means use of a Licensed Subject Matter to determine if an individual has the same
or higher risk for lung cancer as a person who is eligible under US national guidelines for LDCT-based annual lung cancer screening or
a use that is substantially the same. |
| 1.31 | [***]/ Post-CT means use of a Licensed Subject Matter to help determine if a lung biopsy is medically
advisable in a person who has an indeterminate nodule in their lungs as shown by chest x-ray, or CT imaging of the chest, irrespective
of whether that nodule was found in a screening program or incidentally, or a use that is substantially the same. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.32 | Major Insurer means a healthcare insurer that is either (a) one of the top four private insurers
in the US, as determined by size of membership, at the time of coverage of the applicable Licensed Product, (b) US Centers of Medicare
and Medicaid Services (CMS), and (c) US Veteran’s Administration (VA). |
| 1.33 | MD Anderson has the meaning set forth in the preamble above. |
| 1.34 | MD Anderson Licensed Marks means (a) [***] and [***] and the foreign language translations thereof;
(the foregoing, whether prior to or after the trademark registrations thereof), and any Graphic Logos. |
| 1.35 | MDA Observer shall have the meaning set forth in Section 3.6(a). |
| 1.36 | MCED
means an in vitro diagnostic test performing one or more of the functions of the
Licensed Field for at least two cancers. |
| 1.37 | Net Sales means the gross revenues
received by Licensee or its Sublicensees from a Sale less sales discounts actually granted,
sales taxes actually paid, use taxes actually paid, import taxes actually paid, export duties
actually paid, transportation shipping and insurance costs related to transportation shipping
actually paid or allowed, the actual amount of any write-offs for bad debt directly relating
to Sales, expenses related to research and product development including clinical trials,
regulatory compliance, and improvements; costs related to product recalls or replacement
of defective products, and amounts actually allowed or credited due to returns (not exceeding
the original billing or invoice amount), all as recorded by Licensee or its Sublicensees
in their official books and records in accordance with applicable generally accepted accounting
practices or International Financial Reporting Standards and consistent with their financial
statements and/or, if applicable, regulatory filings with the United States Securities and
Exchange Commission. Net Sales excludes any revenue from Licensed Products donated for charitable
purposes, including but not limited to donations to non-profit organizations, charitable
institutions or for humanitarian aid. Net Sales does not include surcharges or pass-through
revenues collected for disbursement, and actually dispersed, to third parties such as physicians
who provide a medical authorization or prescriptions for the Licensed Product, blood collection
devices, payments to third parties for blood collections, or payments for marketing or cooperative
advertising, as long as said surcharges and pass-through revenues are reasonable, arms-length,
and for fair market value in each case to the extent reasonably necessary for the sale of
a Licensed Product. |
| 1.38 | Other Party(ies) means any party, other than Licensee, to a Semi-Exclusive License. |
| 1.39 | Other Party Consideration means all consideration received by MD Anderson and resulting directly
from an Other Party’s exercise of rights to Licensed Subject Matter derived from [***] in the United States from any Semi-Exclusive
License granted to such Other Party; provided, however, (a) to the extent Other Party Consideration includes equity, MD Anderson
shall be obligated to share such consideration only after liquidation, which to liquidate shall be at MD Andersons sole discretion and
(b) Other Party Consideration shall not include (i) amounts due to any third party resulting from the rights granted hereunder, including
without limitation, amounts due under the [***] License and/or to any funding source, and (ii) funds paid by an Other Party for future
research to be performed by MD Anderson at reasonably and customary market standard rates if (A) the respective Semi-Exclusive License
expressly states that such funds are for research to be performed by MD Anderson after the actual date of signatory execution of the Semi-Exclusive
License, and (B) MD Anderson does in fact perform such research after execution of, and in accordance with, the Semi-Exclusive Agreement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.40 | Patent Expenses means out-of-pocket expenses incurred by MD Anderson in preparing (including conducting
prior art searches, if any), filing, prosecuting (including any type of post-grant or post-issuance proceedings), defending, enforcing
and maintaining patent applications and patents under Licensed Subject Matter. |
| 1.41 | Patent Rights means the Board’s rights in: |
| (a) | the patents and patent applications listed in the Exhibit I to this Agreement; and |
| (b) | all patent applications that claim priority to any of the provisional applications listed in Exhibit I;
and |
| (c) | all divisionals, continuations and continuations-in-part of the patent applications identified in (a)
and (b), above provided that the claims of such continuations-in-part are entitled to claim priority to at least one of the patent applications
identified in (a) or (b), above; and |
| (d) | all reissues, reexaminations, extensions, PCT International Applications and foreign counterparts of any
of the patents or patent applications identified in (a), (b) or (c), above; and |
| (e) | any patents that issue with respect to any of the patent applications listed in (a), (b), (c) or (d),
above. |
| 1.42 | PMA
Approval means the premarket approval obtained from a premarket approval application
submitted to the FDA in accordance with 21 CFR Part 814, or any future revisions or substitutes
thereof, in order to obtain approval to market a medical device in the United States, or
an equivalent submission, filing, clearance, approval, or permission in any national jurisdiction
other than the United States. |
| 1.43 | Refunded
Amount has the meaning set forth in Section 3.9. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.44 | Regulatory Approval means the approval needed in a particular jurisdiction by a Regulatory Authority
to market or Sell a Licensed Product or Licensed Service in that jurisdiction. |
| 1.45 | Regulatory Authority means the governmental authority responsible for granting any necessary licenses
or approvals for the marketing, Sale or use of a Licensed Product or Licensed Service in a particular jurisdiction, including without
limitation, the United States Food and Drug Administration (FDA), European Medicines Agency or Koseisho (i.e. the Japanese Ministry of
Health and Welfare). |
| 1.46 | Royalty-Free Practitioner means MD Anderson and the following individuals: [***] (“Practitioner
Inventors”), and any partner or associate who practices medicine with one or more of the Practitioner Inventors, but with respect
to such partner or associate, only for such time as he/she is engaged in a bona fide medical practice with one or more of the Practitioner
Inventors. |
| 1.47 | Sale, Sell, or Sold means the
transfer or disposition of a Licensed Product for value; provided, however, that a transfer
or disposition of a Licensed Product for value shall not be included in Sales if (a) the
transfer is to Licensee or a Sublicensee for resale and not for end use by Licensee or a
Sublicensee or (b) the transfer is to a Royalty Free Practitioner. |
| 1.48 | Semi-Exclusive means, with respect the grant of Section 2.1(c), that Board retains the right to
enter into a Semi-Exclusive License to an Other Party; provided, however, at no time shall there be more than two (2) active Semi-Exclusive
Licenses granting rights to an Other Party. |
| 1.49 | Semi-Exclusive License a license granting rights consistent with those granted to Licensee in 2.1(c). |
| 1.50 | Sponsored Research Agreement or SRA means the Collaborative Research Agreement between MD
Anderson and Company dated _______. |
| 1.51 | Sponsored
Research Inventions means SRA Patent Rights and SRA Technology Rights. |
| 1.52 | SRA Patent Rights means the Board’s rights in: |
| (a) | the patents and patent applications covering an Invention (as defined in the SRA) that is the subject
of an Invention Notice and elected by Licensee pursuant to Section 7.3 of the SRA; and |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (b) | all patent applications that claim priority to any of patent or patent application of (a); and |
| (c) | all divisionals, continuations, continuations-in-part of the patent applications identified in (a) and
(b), above provided that the claims of such continuations-in-part are entitled to claim priority to at least one of the patent applications
identified in (a) or (b), above; and |
| (d) | all reissues, reexaminations, extensions, PCT International Applications, and foreign counterparts of
any of the patents or patent applications identified in (a), (b) or (c), above; and |
| (e) | any patents that issue with respect to any of the patent applications listed in (a), (b), (c) or (d),
above. |
| 1.53 | SRA
Technology Rights means any technical information, know-how, processes, procedures, compositions,
devices, methods, formulas, protocols, techniques, Laboratory Analytics, Laboratory Test
Protocols or procedures, designs, drawings or data created by or on behalf of MD Anderson
in during and in the course of performance of the activities of the Sponsored Research Agreement. |
| 1.54 | Sublicense Agreement means any agreement or arrangement pursuant to which Licensee (or a Sublicensee)
grants to any third party any of the license rights granted to the Licensee under this Agreement. An assignment of all rights and obligations
under this Agreement shall not be deemed to be a Sublicense Agreement. |
| 1.55 | Sublicensee means any entity to whom an express sublicense has been granted under the Licensed
Subject Matter. For clarity, a third party wholesaler or distributor who has no significant responsibility for marketing and promotion
of a Licensed Product within its distribution territory or field (i.e., the third party simply functions as a reseller), and who does
not pay any consideration to Licensee for such wholesale or distributor rights, shall not be deemed a Sublicensee, and the resale by such
a wholesaler or distributor shall not be treated as royalty bearing Sales by a Sublicensee provided that a royalty is being paid by Licensee
for the initial transfer to the wholesaler or distributor as required under this Agreement. |
| 1.56 | Substantial Infringer has the meaning set forth in Section 5.1. |
| 1.57 | System has the meaning set forth in the preamble above. |
| 1.58 | Technology Rights means Board’s rights within the Licensed Field in the information in Exhibit
VII of the License Agreement of Exhibit A, and other technical information, know-how, processes, procedures, compositions, devices, methods,
formulas, protocols, techniques, designs, drawings, Laboratory Analytics, laboratory test protocols or procedures or data created at MD
Anderson before the Effective Date by the inventor(s) listed in Exhibit I to the License Agreement attached as Exhibit A while employed
at MD Anderson, to the extent the foregoing are not covered by Patent Rights, but are (a) described in the invention disclosure reports
listed on Exhibit I; or (b) facilitate the practice, development, manufacture, use, and/or selling of invention(s) claimed in the patents
and/or patent applications listed in the definition of Patent Rights, whether outstanding, expired or abandoned, and with respect to this
clause (b) have no obligations or encumbrances in favor of or benefitting any third party and are not otherwise subject to contractual
or legal restrictions that would preclude the licensure to Company as contemplated by the License Agreement; provided, however,
in the event of the existence of such obligation and/or encumbrance, MD Anderson will use its best efforts to remove such obligation and/or
encumbrance or obtain rights and/or permissions necessary for Licensee to exercise the rights granted hereunder, at MD Anderson’s
expense, further provided that if such obligation and/or encumbrance is removed such rights, permissions, licenses, and interests automatically
shall be deemed as part of the Technology Rights for the full Term of the Option and the License stated in Exhibit A, and further provided
that if MD Anderson is unable to remove such a third party obligation and/or encumbrance, or lacks the power to make the full scope of
the grants set forth herein, despite having applied its best efforts, then the Parties agree to negotiate in good faith to modify the
financial terms of Agreement to account for the inability of MD Anderson to confer the full scope of the grants in this Agreement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 1.59 | Term shall have the meaning set forth in Section 11.1. |
| 1.60 | [***] License means [***]. |
| 1.61 | [***] Antibodies means [***]. |
| 1.62 | [***] Field means [***]. |
| 1.63 | [***] Products shall have the same meaning as “Products”, as defined in the [***] License. |
| 1.64 | U.S. Government has the meaning set forth in Section 12.1. |
| 1.65 | USPSTF means the United States Preventive Services Task Force. |
| 1.66 | Valid Claim means (a) a claim of an
issued and unexpired patent included within the Licensed Subject Matter unless the claim
has been held unenforceable or invalid by the final, un-reversed, and un-appealable decision
of a court or other governmental body of competent jurisdiction, has been irretrievably abandoned
or disclaimed, or has otherwise been finally admitted or finally determined by the relevant
governmental authority to be invalid, un-patentable or unenforceable, whether through reissue,
reexamination, disclaimer or otherwise, or (b) for [***] ([***]) years after its priority
date, a claim of a pending patent application identified on Exhibit I as of the Effective
Date to the extent the claim continues to be prosecuted in good faith or (c) for [***] ([***])
years after its priority date, a claim of a pending patent application other than those identified
on Exhibit I as of the Effective Date within Licensed Subject Matter to the extent the claim
continues to be prosecuted in good faith. |
If a claim in a patent application within
Licensed Subject Matter has been pending more than the periods set forth above, and a patent subsequently issues containing such a claim
that is substantially identical to the invention as claimed in the published patent application, then such claim shall, as of the date
published, be considered a Valid Claim (“Valid Claim Reinstatement”).
II. License
& Commercialization
| 2.1 | Board, through MD Anderson, hereby grants to Licensee: |
| (a) | a royalty-bearing, exclusive, sublicensable (as set forth in Section 2.3) license under Patent Rights,
in the Licensed Field, to manufacture, have manufactured, develop, have developed, use, import, export, offer to sell and/or sell Laboratory
Developed Tests (“LDTs”) in such jurisdictions in the Licensed Territory that permit the commercialization of LDTs; and |
| (b) | a royalty-bearing, exclusive, sublicensable (as set forth in Section 2.3) license under Technology Rights,
in the Licensed Field, to manufacture, have manufactured, develop, have developed, use, import, export, offer to sell and/or sell, Laboratory
Developed Tests (“LDTs”) in such jurisdictions in the Licensed Territory that permit the commercialization of LDTs; and |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (c) | a royalty-bearing, Semi-Exclusive, non-sublicensable license under Patent Rights, Technology Rights, in
the Licensed Field, to manufacture, have manufactured, develop, have developed, use, import, export, offer to sell and/or sell Licensed
Products that are 1) in vitro diagnostic (IVD) assays other than Laboratory Developed Tests and 2) manufactured and distributed
under either a 510K Clearance or PMA Approval within the Licensed Territory; and |
| (d) | a royalty-bearing, Semi-Exclusive, sublicensable (as set forth in Section 2.3) sublicense under MD Anderson’s
rights pursuant to the [***] License, and subject to Licensee’s compliance with all applicable terms and conditions therein, in
the [***] Field, to manufacture distribute and Sell [***] Antibodies and [***] Products within the Licensed Territory; and |
| (e) | a royalty-bearing, exclusive, sublicensable (as set forth in Section 2.3) license under Sponsored Research
Inventions, in the Licensed Field, to manufacture, have manufactured, develop, have developed, use, import, export, offer to sell and/or
sell Licensed Products within the Licensed Territory; and |
| (f) | subject to the restrictions set forth in Section 6.2, the right to use the following MD Anderson names
and marks to brand the Licensed Products within the Licensed Field within the Licensed Territory. |
| 2.2 | The grants of Section 2.1 are subject to Sections 12.2 and 12.3, the payment by Licensee to MD Anderson
of all consideration as provided herein, the timely payment of at least $[***] USD under the Sponsored Research Agreement not later than
the [***] ([***]) anniversary thereof, Schedule E of the [***] License, and are further subject to the following rights retained by Board
and MD Anderson to: |
| (a) | Publish the general scientific findings from research related to Licensed Subject Matter, subject to the
terms of Article IX–Confidential Information and Publication; and |
| (b) | Use Licensed Subject Matter for patient care at MD Anderson to the extent Licensee does not have a commercially
available Licensed Product that is suitable, in MD Anderson’s reasonable discretion, for such patient care, research, teaching and
other academically-related purposes; and |
| (c) | Transfer tangible embodiments of the Licensed Subject Matter to academic or research institutions for
non-commercial research use; and |
| (d) | In addition and with respect to each of the grants of Section 2.1(a) (in countries or jurisdictions that
do not provide for Laboratory Developed Tests) and 2.1(c), Board retains the right to enter into Semi-Exclusive Licenses with Other Parties;
provided, however, at no time shall there be more than two (2) active Semi-Exclusive Licenses. |
The parties hereby acknowledge and agree
that any exercise by Licensee (or a Sublicensee) of Board’s rights outside the scope of any of the Licensed Territory or the Licensed
Field or the [***] Field or the exercise of rights exceeding the scope of Licensed Products, shall be deemed a material breach of this
Agreement. For clarity, this Agreement will not terminate solely due to termination of the Sponsored Research Agreement prior to the payment
of the third $[***] USD payment thereunder.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 2.3 | Licensee has the right to grant Sublicense Agreements under the Licensed Subject Matter consistent with
the terms of this Agreement, as part of good faith, arms-length transactions, subject to the following: |
| (a) | A Sublicense Agreement shall not exceed the scope and rights granted to Licensee hereunder. Sublicensee
must agree in writing to be bound by terms and conditions consistent with this Agreement and shall agree that Board and MD Anderson are
third party beneficiaries of the Sublicense Agreement. In the event of termination of this Agreement, continued sublicense rights shall
be governed by Section 2.4. Licensee may grant a Sublicensee the right to grant further sub-Sublicense Agreements consistent with this
Agreement, in which case such sub-Sublicense Agreements shall be treated as “Sublicense Agreements” and such sub-Sublicensees
shall be treated as “Sublicensees” for purposes of this Agreement. |
| (b) | Licensee shall deliver to MD Anderson a complete and accurate copy of each Sublicense Agreement granted
by Licensee or Sublicensee, and any modification or termination thereof, within thirty (30) calendar days following the applicable execution,
modification, or termination of such Sublicense Agreement. If the Sublicense Agreement is not in English, Licensee shall provide MD Anderson
an accurate English translation in addition to a copy of the original agreement. |
| (c) | Notwithstanding any such Sublicense Agreement, Licensee will remain primarily liable to Board and MD Anderson
for all of the Licensee’s duties and obligations contained in this Agreement, including without limitation the payment of all amounts
due to MD Anderson whether or not paid to Licensee by a Sublicensee. Any act or omission of a Sublicensee that would be a breach of this
Agreement if performed by Licensee will be deemed to be a breach by Licensee. Each Sublicense Agreement shall contain a right of termination
by Licensee in the event that the Sublicensee breaches the payment or reporting obligations affecting Board and/or MD Anderson or any
other terms and conditions of the Sublicense Agreement that would constitute a breach of this Agreement if such acts were performed by
Licensee. |
| 2.4 | All rights and licenses of each Sublicensee shall terminate upon termination of this Agreement; provided,
however, that MD Anderson agrees to negotiate in good faith with each existing Sublicensee that (a) is in good standing under the
respective Sublicense Agreement as of the date of termination of this Agreement, and (b) provides written notice to MD Anderson within
thirty (30) calendar days after termination of this Agreement stating that such Sublicensee desires to enter into negotiations for an
agreement with MD Anderson granting rights under Licensed Subject Matter. MD Anderson shall negotiate in good faith in accordance with
this Section 2.4 but shall have no obligation to enter into an agreement with any Sublicensee. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (a) | it shall exercise its best efforts to develop, make available, and
promote, within [***] of the Effective Date, a Laboratory Developed Test, that performs the functions contemplated by the Licensed Field related to lung cancer
that consists solely of a biomarker combination selected from the embodiments set forth in Exhibit V and shall be Sold as a supplement,
enhancement or confirmatory test of Licensee’s OneTest or Licensee’s MCED for individuals at high risk of lung cancer due
to smoking history if neither (b) nor (c) of this Section 2.5 are met at the time of such Sale; and |
| (b) | any Laboratory Developed Test Sold by Licensee or a Sublicensee during the first [***] from the
Effective Date, that is specifically intended to identify lung cancer in individuals at high risk due to smoking history shall, with
respect to any functions contemplated by the Licensed Field related to lung cancer, consist solely of a biomarker combination
selected from the embodiments set forth in Exhibit V. |
| 2.6 | Within thirty (30) calendar days following each anniversary of the Effective Date, Licensee will deliver
to MD Anderson a written progress report as to Licensee’s (and any Sublicensee’s) efforts and accomplishments during the preceding
year in diligently commercializing Licensed Subject Matter in the Licensed Territory and Licensee’s (and Sublicensees’) commercialization
plans for the upcoming year. |
| 2.7 | Promptly after execution hereof by both Parties and request by Licensee, MD Anderson shall transfer items
1, 2 and 4 of Exhibit VII to Licensee. Transfer of materials described in items 3 and 5 of Exhibit VII shall be transferred pursuant to
the Sponsored Research Agreement. |
III. Consideration,
Payments and Sales Reports
| 3.1 | In consideration of rights granted by Board to Licensee under this Agreement, Licensee agrees to pay MD
Anderson each of the following: |
| (a) | All Patent Expenses incurred by or for MD Anderson before or after the Effective Date for so long as this
Agreement remains in effect as follows: |
| (i) | $[***] in Patent Expenses incurred prior to the Effective Date, due and payable within five (5) days of
the Effective Date and receipt of MD Anderson’s invoice therefor; and |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (ii) | all Patent Expenses incurred prior to the Effective Date in excess of the amounts set forth in Section
3.1(a)(i), due and payable within thirty (30) days of Licensee’s receipt of MD Anderson’s invoice therefor; and |
| (iii) | after the Effective Date and for each Calendar Quarter thereafter Licensee’s pro-rata share of all
other Patent Expense such pro-rata share determined by the total number of licensees of Licensed Subject Matter in the Licensed Field
on a country-by-country basis at the time such expenses are incurred, due and payable by Licensee within thirty (30) calendar days of
MD Anderson’s invoice therefor. |
For clarity, each of Licensee’s
and the any other co-licensee’s share with respect to a country will be calculated as an equal fraction of the total Patent Expenses,
where the denominator is the total number of licensees in such country and the numerator is one. To aid Licensee’s financial planning,
MD Anderson will promptly inform Licensee after MD Anderson grants any Other Party rights consistent with those granted in Section 2.1(c).
| (b) | A running royalty on Net Sales, as follows: |
| (i) | Subject to Section 3.1(b)(iii), for Licensed Products Sold prior to and on the [***] anniversary of [***] and covered by a Valid Claim in the country or jurisdiction of Sale, [***] percent ([***]%) of Net Sales and for
Licensed Products Sold during such period that are not covered by a Valid Claim in the country or jurisdiction of Sale, [***] percent
([***]%) of Net Sales; and |
| (ii) | Subject to Section 3.1(b)(iii), for Licensed Products Sold after the [***] anniversary of [***] and covered by a Valid Claim in the country or jurisdiction of Sale, [***] percent ([***]%) of Net Sales and for Licensed Products
Sold during such period that are not covered by a Valid Claim in the country or jurisdiction of Sale, [***] percent ([***]%) of
Net Sales; |
| (iii) | For Licensed Products that comprise, use, or are made using [***] and that are covered by a Valid Claim in the country
or jurisdiction of Sale, [***] percent ([***]%) of Net Sales and for such Licensed Products Sold during such period that are not
covered by a Valid Claim in the country or jurisdiction of Sale, [***] percent ([***]%) of Net Sales; and |
| (c) | Valid Claim Reinstatement. Adjustments due to Valid Claim Reinstatement as described in
Section 1.65 as follows: (i) MD Anderson shall provide written notice to Licensee of a Valid Claim Reinstatement, and (ii) Licensee
shall retroactively recalculate any amounts owed under this Article III as though the Valid Claim had not lapsed or ceased to
exist. Within [***] of notice of such Valid Claim Reinstatement, Licensee shall provide to MD Anderson an accounting of, and payment
for, the adjustments of the amounts owed as a result of such Valid Claim Reinstatement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 3.2 | Notwithstanding any other provision in this Agreement, if a Licensed Product is covered by more than one
patent or Valid Claim within the scope of this Agreement, or by both Technology Rights and SRA Technology Rights, the royalties payable
for such Licensed Product shall not be cumulative. Instead, the highest single royalty rate applicable among the Valid Claims or among
the Technology Rights and SRA Technology Rights covering the Licensed Product shall be applied, to the exclusion of royalty rates for
other patents or claims. |
| 3.3 | Licensee acknowledges and agrees that the subject matter covered by Technology Rights and/or SRA Technology
Rights not included in a patent application or patent under Licensed Subject Matter is valuable, provides Licensee with a competitive
advantage and head start in the further research, development, and commercialization of Licensed Products, and will inherently be used
in products and services useful in the Licensed Field that are developed by Licensee or its Sublicensees after the Effective Date. The
reduced royalty payments for Licensed Products not covered by a Valid Claim are appropriate in light of the foregoing. |
| 3.4 | Royalty Stacking. If, during the Term, Licensee, or a Sublicensees is required to obtain a royalty-bearing
license under patent or technology rights owned by a third party to Sell any Licensed Product in the Licensed Field in one or more jurisdictions,
Licensee may, provided that Licensee is not otherwise reducing the royalties that would otherwise be payable (e.g., reducing royalties
for one or more Combination Products), deduct from any royalty for such Sale due under this Agreement on the Net Sales of such Licensed
Products in the Field in that jurisdiction [***] percent ([***]%) of royalties actually paid by Licensee to such third party during a
Calendar Quarter to the extent such payments and license related to such Licensed Product for a given Calendar Quarter; provided, however,
that in no event shall the royalty payments payable to MD Anderson for a given Licensed Product for a given Calendar Quarter be reduced
pursuant to this Section 3.4 to less than [***] percent ([***]%) of the amounts otherwise payable under Section 3.1(b) with respect to
such Licensed Product for such Calendar Quarter. |
| 3.5 | Licensee will not sell any Licensed Product as a Combination Product until the Parties have agreed in
writing regarding the fair contribution of each of the other functional components and that of the Licensed Product without the other
functional components to the overall value of such Combination Product. Licensee will propose an allocation of fair contributions in a
draft writing and MD Anderson will make a first response within three (3) weeks of receipt of the draft writing. If necessary, the Parties
shall negotiate in good faith to agree upon a final allocation for a period not longer that thirty (30) days after MD Anderson’s
first receipt of the draft writing. In the event the Parties cannot reach such final allocation within the foregoing time period, the
fair allocation shall be referred to the Parties’ respective Senior Officers designated below (“Senior Officers”),
for resolution through good faith negotiations within thirty (30) days. Each Party shall ensure that its Senior Officer has all necessary
and appropriate authority to fully resolve the Dispute on behalf of such Party. Such Senior Officers are as follows: |
For MD Anderson: SrVP, Rsch Admin &
Ind Rltns (or successor office), and
For Licensee: Chief Scientific Officer
(or his/her designee)
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
To facilitate reaching an agreement
on the writing and notwithstanding the foregoing, in such writing, the royalty payments payable to MD Anderson for a Combination Product
that shows improved detection of one or more tumor types shall range from [***] percent ([***]%) to [***] percent ([***]%) based on the
number of tumor types with improved detection with respect to Licensee’s OneTest resulting from Licensed Products using the following
guidance: (a) up to [***] tumor types, [***] percent ([***]%) royalty; (b) [***] to [***] tumor types, [***] percent ([***]%) royalty;
(c) more than [***] tumor types, [***] percent ([***]%) royalty. In no event shall the royalty payments payable to MD Anderson for a Combination
Product for which royalties are earned under Section 3.1(b)(iii) be reduced to less than [***] percent ([***]%).
| 3.6 | In addition to the license consideration set forth in Section 3.1, as consideration for the grant of licenses
made herein: |
| (a) | Licensee shall grant to MD Anderson one (1) observer seat (the “MDA Observer”) who
is reasonably acceptable to the Licensee Board and who shall be entitled to, in accordance with reasonable board policies, (i) attend
all meetings of the board of directors of Licensee (the “Licensee Board”), all committees of the Licensee Board (“Committees”),
and all governing bodies of any subsidiaries of Licensee, (ii) participate in discussions of matters brought to the Licensee Board, Committees,
or subsidiary governing bodies, as applicable, and (iii) receive, concurrently with delivery to the Licensee Board, Committees, governing
bodies , as applicable, copies of all notices, minutes, consents, and other materials provided to the Licensee Board, Committees, or governing
bodies, as applicable; provided, however, that (x) such MDA Observer shall agree to hold in confidence and trust and to act in a fiduciary
manner with respect to all information so provided, and (y) the Licensee reserves the right to withhold any information and to exclude
such MDA Observer from any meeting or portion thereof if access to such information or attendance at such meeting could adversely affect
the attorney-client privilege between the Licensee and its counsel. Licensee hereby covenants and agrees that any employee of MD Anderson
that is appointed by MD Anderson as the MDA Observer shall be an MDA Observer reasonably acceptable to the Licensee Board in compliance
with this subsection. |
| (b) | Licensee shall issue to Board (or its designee) a number of Common Stock shares of the Licensee specified
below, for no additional consideration (for purposes of the MD Anderson Intellectual Property Policy, such shares are considered “License
Equity” as defined therein), according to the following schedule and subject to the following terms and conditions: |
| (i) | Licensee shall issue to the Board (or its designee) a number of shares of License Equity equivalent to
[***] percent ([***]%) of Licensee’s Fully Diluted Capitalization as of the date that the Investor Financing is completed (the “Target
Ownership Percentage”). The License Equity shall be issued to the Board (or its designee) in two tranches. The first tranche, representing
half of the shares necessary to meet the Target Ownership Percentage, as of the date that the Investor Financing is completed, shall be
issued to the Board (or its designee) within five (5) days of MD Anderson’s written confirmation of its satisfaction with the documents
provided under Section 2.2(b) of the Option Agreement. The second tranche, representing the remaining shares necessary to meeting the
Target Ownership Percentage as of the date of the Investor Financing is completed, shall be issued to the Board (or its designee) upon
the first Anniversary of the Effective Date of the Sponsored Research Agreement. For clarity and notwithstanding the foregoing, the second
tranche will be paid by Licensee to Board unless the payment pursuant to Section 3.1 of the Sponsored Research Agreement of the Payment
Event due within twelve (12) months of the effective date is not made because (A) MD Anderson has materially breached the SRA or (B) the
SRA has been terminated pursuant to Section 10.1(b) thereof. |
| (ii) | The transactions set forth in Section 3.6(b)(i) shall be subject to Exhibit II to this Agreement. |
| (iii) | Upon achievement by Licensee or a Sublicensee of the Development Milestones set forth in the table below,
the Board, or its designee, shall receive the corresponding Equity Award: |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| Development Milestone |
Equity Award |
| [***] / Pre-CT: earn a Grade B from the USPSTF |
A number of shares of License Equity equivalent to [***] percent ([***]%) of the Licensee’s Fully Diluted Capitalization as of the Effective Date of this Agreement. |
| [***] / Post-CT: earn a CMS Coverage Determination |
A number of shares of License Equity equivalent to [***] percent ([***]%) of the Licensee’s Fully Diluted Capitalization as of the Effective Date of this Agreement. |
| MCED: Selected for National Cancer Institute (NCI) “Vanguard” multi-year Randomized Clinical Trial |
A number of shares of License Equity equivalent to [***] percent ([***]%) of the Licensee’s Fully Diluted Capitalization as of the Effective Date of this Agreement. |
| MCED: earn a CMS Coverage Determination with a product that incorporates or uses Licensed Subject Matter |
A number of shares of License Equity equivalent to [***] percent ([***]%) of the Licensee’s Fully Diluted Capitalization as of the Effective Date of this Agreement. |
| MCED: prove, to the satisfaction of at least one major payer, a mortality benefit obtained for at least 3 cancers |
A number of shares of License Equity equivalent to [***] percent ([***]%) of the Licensee’s Fully Diluted Capitalization as of the Effective Date of this Agreement. |
| 3.7 | Unless otherwise provided, all such payments are payable within thirty (30) calendar days after each Calendar
Quarter during the Term, at which time Licensee will also deliver to MD Anderson a true and accurate report, giving such particulars of
the business conducted by Licensee and any Sublicensees during the respective Calendar Quarter as necessary for MD Anderson to account
for Licensee’s payments hereunder. This report will include pertinent data, including, but not limited to each of the following: |
| (a) | The accounting methodologies used to account for and calculate the items included in the report and any
differences in such accounting methodologies used by Licensee since the previous report. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (b) | A list of Licensed Products produced for the respective Calendar Quarter categorized by the respective
technology under Licensed Subject Matter. |
| (c) | The total quantities of Licensed Products produced categorized by the respective technology under Licensed
Subject Matter. |
| (d) | The total Sales categorized by the respective technology under Licensed Subject Matter. |
| (e) | The calculation of Net Sales, segregated on a Licensed Product-by-Licensed Product and a country-by-country
basis, by the respective technology under Licensed Subject Matter. The calculation of Net Sales shall itemize the permitted deductions
from the gross consideration from a Sale used to arrive at the resulting Net Sales, on a Licensed Product-by-Licensed Product and country-by-country
basis. |
| (f) | The royalties so computed and due MD Anderson by the respective technology under Licensed Subject Matter. |
| (g) | All consideration received from each Sublicensee or assignee or other payments due MD Anderson. |
| (h) | All other amounts due MD Anderson herein. |
Simultaneously with the delivery of
each such report, Licensee agrees to pay MD Anderson the amount due, if any, for the period of such report. These reports are required
even if no payments are due.
| 3.8 | Audit and Recordkeeping: Subject to applicable law, |
| a) | During the term of this Agreement and for one (1) year thereafter, Licensee agrees to keep complete and
accurate records of its, and its Sublicensees’, Sales and Net Sales in sufficient detail to enable the royalties and other payments due
hereunder to be determined. Licensee agrees to permit MD Anderson or its representatives, at MD Anderson’s expense, to periodically examine
Licensee’s books, ledgers, and records during regular business hours for the purpose of and to the extent necessary to verify any
report required under this Agreement [***] ([***]) years prior to the audit. If any amounts due MD Anderson are determined to have been
underpaid in an amount equal to or greater than [***] percent ([***]%) of the total amount due during the period so examined, then Licensee
will pay the reasonable cost of the examination. The findings of any such audit shall be treated as Confidential Information pursuant
to the terms of this Agreement. Licensee shall pay accrued interest at [***] percent ([***]%) simple annual interest, or the highest rate
allowed by applicable law, whichever is lower, on any and all late payments under this Agreement (regardless of whether the deficiency
is identified by audit or otherwise), with such interest commencing on the date after the due date of each such late payment. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| b) | During the term of this Agreement and for one (1) year thereafter, MD Anderson agrees to keep complete
and accurate records of its Other Party revenues in sufficient detail to enable the royalties and other payments due hereunder to be determined.
MD Anderson agrees to permit Licensee’s representatives, at Licensee’s expense, to periodically examine MD Anderson’s books,
ledgers, and records during regular business hours for the purpose of and to the extent necessary to verify any report required under
this Agreement [***] ([***]) years prior to the audit. If any amounts due Licensee are determined to have been underpaid in an amount
equal to or greater than [***] percent ([***]%) of the total amount due during the period so examined, then Licensee will be entitled
to take a credit for the reasonable cost of the examination to be applied against any amount due under the License Agreement. The findings
of any such audit shall be treated as Confidential Information pursuant to the terms of this Agreement. Licensee’s credit shall
accrue interest at [***] percent ([***]%) simple annual interest, or the highest rate allowed by applicable law, whichever is lower, on
any and all late payments under this Agreement (regardless of whether the deficiency is identified by audit or otherwise), with such interest
commencing on the date after the due date of each such late payment. |
| 3.9 | All amounts payable hereunder by Licensee shall be made in United States Dollar denominated funds, free
and clear and without any deduction, set-off, or reduction for or on account of any tax, levy, impost, duty, charge, fee or withholding
of any nature now or hereafter imposed by any governmental, fiscal or other authority. If Licensee is compelled by law to make any such
deduction, set-off, or reduction, it shall pay MD Anderson such additional amounts as are necessary to ensure receipt by MD Anderson of
the full amounts payable hereunder that MD Anderson would have received but for the deduction, set-off or reduction (“Gross Up”).
In the event that Licensee pays a Gross Up to MD Anderson, MD Anderson shall reasonably cooperate with Licensee to execute forms required
by a governmental, fiscal, or other authority to claim such Gross Up was entitled to a tax, levy, impost, duty, charge, fee or other withholding
exemption. In the event MD Anderson receives a payment from a governmental, fiscal or other authority for the Gross Up (“Refunded
Amount”), it shall provide notice to Licensee of the Refunded Amount and Licensee shall be entitled to deduct the Refunded Amount
from the amounts due MD Anderson for the next reporting and payment period as set forth in Section 3.7. |
| 3.10 | All payments to MD Anderson under this Agreement shall be by checks made payable to The University of
Texas M. D. Anderson Cancer Center, and sent by United States mail to, or by wire transfer to: |
REFERENCE: include title and Effective
Date of Agreement and type of payment (e.g., license upfront fee, milestone payment, royalty, maintenance fee, etc.) and list applicable
patent/application identified by MD Anderson reference number and patent number or application serial number.
| 3.11 | No payments due or royalty rates owed to MD Anderson under this Agreement will be reduced as the result
of co-ownership of Licensed Subject Matter by Board and another person or entity, including, but not limited to, Licensee. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 3.12 | If a payment to MD Anderson requires delivery of an invoice, then MD Anderson’s delay in providing
an invoice shall not excuse or waive any payment obligation of Licensee, but the deadline for Licensee’s payment shall be extended
by the period of such delay. An invoice shall be deemed to be delivered to Licensee if transmitted to Licensee’s address in Section
13.2. Any failure by Licensee to update its billing address shall not excuse timely payment. |
| 3.13 | In consideration for Licensee’s agreement to forego an exclusive grant under Sections 2.1(c) and
(d) in countries or jurisdictions that do not provide for Laboratory Developed Tests such that Board’s has right to enter into Semi-Exclusive
Licenses as set forth in this Agreement, MD Anderson shall pay Licensee the following percentages of Other Party Consideration received
by MDACC to the extent Licensee continues to make Sales of a Licensed Product that solely contains the biomarkers listed in Exhibit V
that is covered by a Valid Claim and for which such Other Party Consideration is received: |
| (a) | if Licensee is the first to receive Breakthrough Device Designation from the FDA on such Licensed Product
that is subject to a Semi-Exclusive License, [***] percent ([***]%); or |
| (b) | if Licensee is the first to receive national coverage in the United States, as evidenced by payer including
such Licensed Product in its reimbursement coverage, from at least one Major Insurer in the United States on a Licensed Product that is
subject to a Semi-Exclusive License, [***]percent ([***]%); with respect to this Section 3.13(b) License will be deemed to have obtained
national coverage if Licensee obtains an NCD (National Coverage Decision) or an LCD (Local Coverage Decision) from a MAC (Medicare Administrative
Contractor) and receives reimbursement for commercial testing from a patient inside the area covered by the MAC and also receives reimbursement
from at least one patient in at least [***] ([***]) states outside the MAC region in which the LCD was granted or an successor designation
if reimbursement rules change after the Effective Date; or |
| (c) | if Licensee is the first to receive assignment of grade B from the USPSTF in the US on a Licensed Product
that is subject to a Semi-Exclusive License, as evidenced by (i) USPSTF posting final recommendation statement and (ii) evidence summary
on its website and publication in peer reviewed journal, [***]percent ([***]%) of all Other Party Consideration; or |
| (d) | if Licensee is the first to receive either 510(k) Clearance or PMA Approval from the FDA on a Licensed
Product that is subject to a Semi-Exclusive License, [***] percent ([***]%) of all Other Party Consideration. |
MD Anderson shall make written reports
of the amounts due to Licensee under this Section 3.13 within [***] ([***]) days after each calendar quarter and substantially concomitantly
make the payments due to Licensee to Licensee in accordance with Section 3.14.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
With respect to the percentages set
forth in this Section 3.13(a)–(d), only the highest applicable highest percentage applicable applies. For example, if Licensee achieves
Breakthrough Device Designation under Section 3.13(a) and later national coverage from one Major Insurer in the United States consistent
with Section 3.13b), the MD Anderson would be obligated to pay Licensee [***] percent ([***]%) of Other Party Consideration until payment
under Section 3.13(b) is met and at such time would be then obligated to pay Licensee [***]percent ([***]%) of Other Party Consideration
received after the date Section 3.13(b) is met. In no event shall MD Anderson be obligated to pay Licensee under more than one of Sections
3.13(a)-(d).
For clarity, Licensee must be the first,
relative to any Other Party, to achieve the applicable event set forth in Section 3.13 (a)-(d) in order to trigger any payment thereunder.
For example, if one or both of the Other Parties achieves national coverage by a Major Insurer as described in Section 3.13(b), even if
Licensee thereafter achieves such national coverage, MD Anderson is not obligated to pay Licensee the percentage under Section 3.13(b).
| 3.14 | All payments to Licensee under this Agreement shall be by ACH to: |
| 3.15 | If a payment to Licensee requires delivery of an invoice, then Licensee’s delay in providing an
invoice shall not excuse or waive any payment obligation of Licensee, but the deadline for MD Anderson’s payment shall be extended
by the period of such delay. An invoice shall be deemed to be delivered to MD Anderson if transmitted to MD Anderson’s address in
Section 13.2. Any failure by MD Anderson to update its billing address shall not excuse timely payment. |
IV. Patents,
Inventions, Material Transfer
| 4.4 | If Licensee is more than thirty (30) calendar days in arrears on any payment or obligation due under this
Agreement, Board, MD Anderson, and the counsel prosecuting licensed patents and patent applications shall have no obligation to confer
or otherwise communicate with, or provide any information to, Licensee under this Article IV of this Agreement unless and until Licensee
is no longer in arrears on all payments and obligations under this Agreement. |
| 4.5 | Notwithstanding Section 4.1, prior to instructing patent counsel
to respond or take action with respect to a patent or patent application directed to Licensed Subject Matter and/or prior to incurring
costs with respect thereto, MD Anderson shall have the right, at its election and in its sole discretion, to require upfront, advance
payment from Licensee of the anticipated Patent Expenses for any patent or patent application directed to Licensed Subject Matter (“Anticipated
Costs”) as follows: MD Anderson shall provide a reasonable estimate of the Anticipated Costs to Licensee and shall specify
a due date for the advance payment of such Anticipated Costs. With respect to such due date, MD Anderson may require Licensee to
make such advance payment for Anticipated Costs at any time up to [***] prior to the date MD Anderson has chosen for the
legal work to be completed, provided that such due date for payment is at least fifteen (15) calendar days after the estimate is provided
to Licensee. Unless otherwise agreed in writing, such estimate may be sent by email to patents@2020gene.com or in accordance with
the Notice provisions in Section 13.2. In the event the payment for Anticipated Costs actually made by Licensee to MD Anderson
exceeds the actual costs, any unused balance will be credited towards future patent expenses, or, upon written request, returned to Licensee.
In the event the actual costs incurred by MD Anderson exceed the estimate of Anticipated Costs, MD Anderson shall invoice Licensee for
the excess costs. Within thirty (30) calendar days of receiving an invoice from MD Anderson for such costs incurred in excess of the
reasonable estimate of Anticipated Costs, Licensee shall reimburse MD Anderson for such excess amount. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
V. Infringement
by Third Parties
| 5.1 | Licensee, at its expense, shall have the first right, but not the obligation, to enforce any patent exclusively
licensed hereunder against infringement by third parties in the Licensed Field within the Licensed Territory. A potential infringer shall
be deemed a “Substantial Infringer”: if gross sales revenue from allegedly infringing commercial activities exceeds [***]
([***]) times Licensee’s bona fide estimate of the cost of enforcement beginning with investigation costs, continuing through
discovery, trial, and all reasonably forecast appeals and remands. Licensee shall be responsible for payment of all fees and expenses
associated with such enforcement incurred by Licensee and incurred by MD Anderson in providing cooperation in such enforcement. Any recovery
for actual damages or punitive or enhanced damages in excess of Licensee’s documented, third-party expenses in enforcing the Licensed
Subject Matter and amounts actually reimbursed by Licensee to MD Anderson under this Section 5.1 shall be shared by Licensee with MD Anderson
as follows: [***]% of such recovery shall be provided to MD Anderson and [***]% shall be retained by Licensee. Licensee must notify MD
Anderson in writing of any potential Substantial Infringer in the Licensed Field within the Licensed Territory within thirty (30) calendar
days of knowledge thereof. If Licensee does not actively seek to file suit against a Substantial Infringer Licensee knows about in the
Licensed Field within the Licensed Territory within [***] ([***]) months of knowledge thereof, then Board or MD Anderson may, at its sole
discretion, enforce any patent licensed hereunder on behalf of itself and Licensee, with MD Anderson retaining all recoveries from such
enforcement, and/or reduce the licenses that cover the infringing activity to co-exclusives. If Licensee knows of a potential infringer
who is not a Substantial Infringer and Licensee does not file suit or use other measures to deter infringement, then Licensee shall confer
with MD Anderson’s Office of Technology Commercialization to reach consensus on how to deal with the non-Substantial Potential Infringer
and if Licensee does not follow a course of action acceptable to MD Anderson, then MD Anderson will give Licensee notice that Licensee
must take an action or series of actions to protect the patent exclusivity, and if Licensee does not then report affirmative actions (which
may include filing suit) or does not use other reasonable measures to enforce the rights granted hereunder against such potential infringer
within [***] ([***]) months after receipt of MD Anderson’s notice, then Board or MD Anderson may, at its sole discretion, enforce
any patent licensed hereunder on behalf of itself and Licensee, with MD Anderson retaining all recoveries from such enforcement, and/or
reduce the licenses that cover the infringing activity to co-exclusive. |
| 5.2 | If it is necessary to name Board or MD Anderson as a party in such action, then Licensee must first obtain
Board’s and MD Anderson’s prior written permission, which permission shall not be unreasonably withheld, provided that Board and
MD Anderson shall have reasonable prior input on choice of counsel on any matter where such counsel represents Board or MD Anderson, and
Licensee and such counsel agree to follow all required procedures of the Texas Attorney General regarding retention of outside counsel
for state entities. |
| 5.3 | MD Anderson and/or Board may require Licensee to provide substantial, competent evidence and analysis
of the alleged third party infringement for MD Anderson and/or Board to consider before deciding whether to grant permission to name Board
or MD Anderson as a party in an infringement action. If MD Anderson and/or Board declines to grant permission to name Board or MD Anderson,
then Licensee’s obligations to enforce the patents shall be deemed to be discharged and satisfied. Such evidence shall be treated
as Confidential Information pursuant to the terms of this Agreement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 5.4 | Notwithstanding anything to the contrary herein, nothing in this Agreement shall obligate The University
of Texas System, The Board of Regents of The University of Texas System, MD Anderson, or any other agency of The State of Texas to join,
or permit the use of its name or otherwise participate, as a litigant in any litigation or adversarial judicial proceeding. |
| 5.3 | Licensee shall consult and cooperate in good faith with MD Anderson regarding strategy with respect to
certifications, notices and patent enforcement procedures under the BPCIA, including without limitation the listing of any Licensed Subject
Matter in any such procedure or engaging in any patent resolution provisions of the BPCIA. MD Anderson shall have final decision-making
authority, in its sole discretion, with respect to actions under the BPCIA. |
VI. Patent
Marking
| 6.1 | Licensee agrees that all packaging containing individual Licensed Product(s), documentation therefor,
and, when possible, actual Licensed Product(s) sold by Licensee and/or Sublicensees will be appropriately marked with the number of any
applicable patent(s) licensed hereunder in accordance with each country’s patent laws, including Title 35, United States Code, to the
extent such marking is necessary or required to fully preserve Patent Rights and/or SRA Patent Rights in each such country or the right
to recover damages for infringement thereof. |
| 6.2 | Licensee may use the MD Anderson Licensed Marks to brand Licensed Products; provided, however,
[***] shall be used only on those Licensed Products wherein functions contemplated by the Licensed Field related to lung cancer consist
solely of a biomarker combinations selected from the embodiments set forth in Exhibit V, and in advertising, promotional or sales materials
in various medium for Licensed Products including without limitation the parts of the website of Licensee describing the Licensed Products.
Any such use will be subject to and will comply with the terms and conditions of Schedule 6.2 attached hereto and incorporated herein,
and Section 6.3 hereof. To the extent that MD Anderson elects at its sole discretion to produce Graphic Logos, MD Anderson will incorporate
such Graphic Logos as MD Anderson Licensed Marks hereunder and will provide an updated Exhibit IV that shows the Graphic Logos, which
Licensee may use in accordance with the terms and conditions applicable to the MD Anderson Licensed Marks. As between the Parties MD Anderson
will solely own and retain all right, title, and interest to MD Anderson Licensed Marks and all goodwill from any use of the MD Anderson
Licensed Marks will inure to the benefit of MD Anderson. The Parties acknowledge and agree that the MD Anderson Licensed Marks may be
the subject of one (1) or more pending trademark registrations, and to the extent that any applicable governmental agency requires any
change or modification to the MD Anderson Licensed Marks as a condition of granting applicable trademark protection, MD Anderson reserves
the right to reasonably modify the specific MD Anderson Licensed Marks accordingly, and Licensee’s right to use such MD Anderson Licensed
Marks as set forth herein will be subject to any such modification. |
For the avoidance of doubt, MD Anderson
will have the right to review and approve any use of MD Anderson Licensed Marks as permitted by this Agreement. Additionally, Licensee
will not use any Graphic Logo until MD Anderson has approved, in writing, of its incorporation as an MD Anderson Licensed Mark and its
inclusion on Exhibit IV of this Agreement. Specific use of any Graphic Logos by Licensee must also be approved by MD Anderson in writing.
Any additional use of the MD Anderson name except as an element of the MD Anderson Licensed Marks will require the prior written approval
of MD Anderson, and will comply with the terms and conditions of Schedule 6.2 attached hereto and incorporated herein, and incorporated
herein, and Section 6.3 hereof. Additionally, except as required by applicable law or for regulatory purposes or otherwise provided herein,
the parties will not use the names (including any trademark or other identifier) of the other parties or the other parties’ employee or
staff member for any other purpose without the written approval of the other parties.
| 6.3 | Licensee will not state or imply in any publication, advertisement, or other medium
that any product or service bearing Licensee’s names or trademarks and/or manufactured, sold or distributed by Licensee has been tested,
approved, or endorsed by MD Anderson. |
| 6.4 | Licensee will not use MD Anderson Licensed Marks on any product in a manner that
is inconsistent with Section 6.2. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
VII. Indemnification
and Insurance
| 7.1 | LICENSEE AGREES TO HOLD HARMLESS, INDEMNIFY, AND, TO THE EXTENT AUTHORIZED BY THE TEXAS CONSTITUTION AND
THE LAWS OF THE STATE OF TEXAS AND SUBJECT TO THE STATUTORY DUTIES OF THE TEXAS ATTORNEY GENERAL, DEFEND BOARD, SYSTEM, MD ANDERSON, THEIR
REGENTS, OFFICERS, EMPLOYEES, STUDENTS AND AGENTS (the “UT INDEMNITEES”) FROM AND AGAINST ANY CLAIMS, DEMANDS, OR CAUSES OF
ACTION WHATSOEVER, COSTS OF SUIT AND REASONABLE ATTORNEY’S FEES (INCLUDING WITHOUT LIMITATION, THOSE COSTS ARISING ON ACCOUNT OF
ANY INJURY OR DEATH OF PERSONS OR DAMAGE TO PROPERTY) CAUSED BY, OR ARISING OUT OF, OR RESULTING FROM, THE EXERCISE OR PRACTICE BY LICENSEE,
ITS OFFICERS, ITS SUBLICENSEES OR THEIR OFFICERS, EMPLOYEES, AGENTS, TRANSFEREES, OR REPRESENTATIVES, OF THE RIGHTS GRANTED HEREUNDER. |
| 7.2 | IN NO EVENT SHALL BOARD, SYSTEM OR MD ANDERSON BE LIABLE FOR ANY INDIRECT, SPECIAL, CONSEQUENTIAL OR PUNITIVE
DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS OR EXPECTED SAVINGS OR OTHER ECONOMIC LOSSES, OR FOR INJURY TO PERSONS
OR PROPERTY) ARISING OUT OF, OR IN CONNECTION WITH, THIS AGREEMENT OR ITS SUBJECT MATTER, REGARDLESS OF WHETHER BOARD, SYSTEM OR MD ANDERSON
KNOWS OR SHOULD KNOW OF THE POSSIBILITY OF SUCH DAMAGES. |
| 7.3 | SCOPE OF INDEMNIFICATION OF UT INDEMNITEES. LICENSEE’S OBLIGATIONS TO HOLD HARMLESS, INDEMNIFY,
AND DEFEND THE UT INDEMNITEES IN SECTION 7.1 AND THE LIMITATION OF LIABILITY IN SECTION 7.2 SHALL INCLUDE, BUT ARE NOT LIMITED TO, ANY
CLAIM ALLEGING STRICT STATUTORY LIABILITY, PRODUCT DEFECT LIABILITY, OR ANY UT INDEMNITEE’S OWN NEGLIGENCE (WHETHER SOLE OR CONCURRENT)
THAT ARISES OUT OF, RELATES TO, IS CAUSED IN WHOLE OR IN PART BY, OR RESULTS FROM THE USE OR SALE OF ANY TANGIBLE MATERIALS (INCLUDING
BIOLOGICAL MATERIALS) PROVIDED TO LICENSEE BY BOARD OR MD ANDERSON UNDER OR IN CONNECTION WITH THIS AGREEMENT OR PRODUCTS USED OR SOLD
BY LICENSEE OR ANY AFFILIATE OR SUBLICENSEE. |
| 7.4 | Licensee shall procure and maintain insurance as follows: |
| (a) | Beginning at the time when any Licensed Subject Matter or any Licensed Product is being distributed or
sold (including for the purpose of obtaining regulatory approvals) by Licensee, an Affiliate, or by a Sublicensee, Licensee shall, at
its sole cost and expense, procure and maintain commercial general liability insurance in amounts not less than $[***] per incident and
$[***] annual aggregate, and Licensee shall use reasonable efforts to have the Board, System, MD Anderson, their Regents, officers, employees,
students and agents named as additional insureds. Such commercial general liability insurance shall provide: (i) product liability coverage;
(ii) broad form contractual liability coverage for Licensee’s indemnification under this Agreement; and (iii) coverage for litigation
costs. The minimum amounts of insurance coverage required herein shall not be construed to create a limit of Licensee’s liability with
respect to its indemnification under this Agreement. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (b) | Licensee shall provide MD Anderson with written evidence of such insurance within thirty (30) calendar
days of its procurement. Additionally, Licensee shall provide MD Anderson with written notice of at least fifteen (15) calendar days prior
to the cancellation, non-renewal or material change in such insurance. |
| (c) | Licensee shall maintain such commercial general liability insurance beyond the expiration or termination
of this Agreement during: (i) the period that any Licensed Subject Matter developed pursuant to this Agreement is being commercially distributed
or sold by Licensee, an Affiliate or by a Sublicensee or agent of Licensee; and (ii) the five (5) year period immediately after such period. |
7.5 To
the extent authorized by the Constitution and the laws of the State of Texas, Licensee’s obligations under this Section 7 shall
not apply to the extent that such claim arises from or results from any of Indemnitee’s negligence or willful misconduct.
VIII.
Use of Name
| 8.1 | Except to the extent expressly permitted by the Sponsored Research Agreement, Licensee will not use the
name of (or the name of any employee of), or make reference to, MD Anderson, System or Board in any advertising, promotional or sales
literature, on its Web site, or for the purpose of raising capital without the advance written consent of Board secured through: |
The University of Texas
M. D. Anderson Cancer Center
| 8.2 | Notwithstanding Section 8.1, Licensee may use the name of (or name of employee of) MD Anderson, System
or Board in routine business correspondence, or as needed in appropriate regulatory submissions without written consent. |
IX.
Confidential Information and Publication
| 9.1 | MD Anderson and Licensee each agree that all information contained in documents marked “confidential”
and, if not so marked, information confidential nature would be reasonably recognized by the recipient party from the subject matter or
subject type of the information disclosed (“Confidential Information”) and forwarded to one by the other (i) are to be received
in strict confidence, (ii) are to be used only for the purposes of this Agreement, and (iii) will not be disclosed by the recipient party,
its agents or employees without the prior written consent of the disclosing party, except to the extent that the recipient party can establish
by competent written proof that such information: |
| (a) | was in the public domain at the time of disclosure by the disclosing party to the recipient party; |
| (b) | became part of the public domain, after disclosure by the disclosing party to the recipient party, through
no act or omission of the recipient party, its employees, agents, successors or assigns; |
| (c) | was lawfully disclosed to the recipient party by a third party that was under no obligation of confidentiality
with respect to such information; |
| (d) | was already known by the recipient party at the time of disclosure by the disclosing party to the recipient
party; or |
| (e) | was independently developed by the recipient party without use of the disclosing party’s Confidential
Information. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 9.2 | Each party’s obligation of confidence hereunder will be fulfilled by using at least the same degree
of care with the disclosing party’s confidential information as it uses to protect its own confidential information, but always at least
a reasonable degree of care. This obligation will exist while this Agreement is in force and for a period of [***] ([***]) years thereafter. |
| 9.3 | MD Anderson reserves the right to publish or share with third parties the general scientific findings
from research related to Licensed Subject Matter, but only to the extent that said findings omit Licensee’s Confidential Information.
MD Anderson will submit the manuscript of any proposed publication to Licensee at least [***] ([***]) calendar days before publication,
and Licensee shall have the right to review and comment upon the publication in order to protect Licensee’s Confidential Information.
Upon Licensee’s request, publication may be delayed up to [***] ([***]) additional calendar days to enable Licensee to secure adequate
intellectual property protection and/or omit Licensee’s Confidential Information that would otherwise be affected by the publication. |
Notwithstanding the foregoing, nothing
in this Agreement shall require MD Anderson or Board to refrain from publishing any information if doing so would (a) cause MD Anderson
or Board to violate any export control laws or laws that provide tax-exempt status for any bonds issued by MD Anderson or The University
of Texas System, or (b) result in the inapplicability to MD Anderson or The University of Texas System of the fundamental research exclusion
or exemption from U.S. export control laws for such information.
| 9.4 | In the event that the recipient party is required to disclose the disclosing party’s Confidential
Information under operation of applicable law, regulation, or order of a court or governmental administrative body having competent jurisdiction,
the recipient party shall, to the extent practicable, provide the disclosing party reasonable notice of such potential disclosure so that
that the disclosing party may seek a protective order or other appropriate protection or legal relief to prevent or limit such disclosure.
If, in the absence of, or pursuant to the terms of, such protection or legal relief, the recipient party is nonetheless required by applicable
law, regulation, or order of a court or governmental administrative body having competent jurisdiction to disclose any portion of the
disclosing party’s Confidential Information, the required disclosure shall be permitted under this Agreement but shall be limited
to only that portion of the disclosing party’s Confidential Information for which disclosure is so required. |
X. Assignment
| 10.1 | (a) Except in connection with the sale of all or substantially all of Licensee’s assets to a third party
or in connection with any merger, acquisition, consolidation, or business combination, neither this Agreement nor any rights, interests,
duties or obligations of Licensee under this Agreement may be assigned (either directly or indirectly) by Licensee without the prior written
consent of MD Anderson, which will not be unreasonably withheld. A merger or other transaction in which the equity holders of Licensee
prior to such event hold less than a majority of the equity of the surviving or acquiring entity shall be considered an assignment of
this Agreement. |
| 10.2 | For any permitted assignment by Licensee to be effective, the assignee must assume in writing (a copy
of which writing will be provided to MD Anderson) all of Licensee’s interests, rights, duties, and obligations under the Agreement and
agree to comply with all terms and conditions of the Agreement as if the assignee were the original party (i.e., the Licensee) to the
Agreement. |
| 10.3 | Any attempted assignment in violation of the foregoing shall be null and void. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
XI. Term
and Termination
| 11.1 | Subject to Sections 11.2, 11.3 and 11.4, the term of this Agreement is from the Effective Date until the
last to occur of: (a) the expiration of all patents issued under Patent Rights and SRA Patents (if any) and the cancellation, withdrawal,
or express abandonment of all patent applications under Patents Rights and SRA Patents (if any), or (b) the date that is the [***] ([***])
anniversary of the Effective Date. |
| 11.2 | In addition to any other rights under this Agreement, at any time after [***] from the Effective
Date, Board or MD Anderson has the right to terminate the license granted in Section 2.1 in any country or jurisdiction within the
Licensed Territory if Licensee, within [***] after receiving written notice from MD Anderson of the intended
termination, fails to provide reasonably satisfactory written evidence to MD Anderson that Licensee or its Sublicensee(s) has
commercialized or is actively and effectively attempting to commercialize a Licensed Product in such jurisdiction(s). The following
definitions apply to Section 11.2: (a) “commercialized” means having Sales in such jurisdiction; and
(b) “actively and effectively attempting to commercialize” means having an effective, ongoing and active research,
development, manufacturing, marketing or sales program as appropriate, directed toward obtaining regulatory approval, and/or
production and/or Sales in any jurisdiction, and providing reasonably acceptable plans to MD Anderson, to commercialize licensed
inventions in the jurisdiction(s) in which MD Anderson intends to terminate. |
| 11.3 | Subject to any rights herein which survive termination, this Agreement will earlier terminate in its entirety: |
| (a) | automatically, if Licensee becomes bankrupt or insolvent and/or if the business of Licensee shall be placed
in the hands of a receiver, assignee, or trustee, whether by voluntary act of Licensee or otherwise; or |
| (b) | upon [***] ([***]) calendar days written notice from MD Anderson, if Licensee breaches or defaults on
the payment or report of obligations of Article III, or use of name obligations of Article VIII unless, before the end of such [***] ([***])
calendar day notice period, Licensee has cured the default or breach to MD Anderson’s reasonable satisfaction, and so notifies MD
Anderson, stating the manner of the cure; provided, however, with respect to any such breach that is not capable of being cured,
Licensee shall be deemed to have cured such breach if it takes reasonable action to ameliorate or mitigate such breach, which action may
include ceasing any further use that resulted in such breach or issuing a corrective press release, in any case as may be appropriate
under the circumstances; or |
| (c) | immediately, if after written notice from MD Anderson of a default, if Licensee fails to pay the past
Patent Expenses within [***] ([***]) calendar days; |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (d) | upon [***] ([***]) calendar days written notice from either Party if the other Party materially breaches
or defaults on any other obligation under this Agreement, unless, before the end of such [***] ([***]) calendar day notice period, the
recipient of such notice has cured the default or breach to notifying Party’s reasonable satisfaction and so notifies the notifying
Party, stating the manner of the cure; or |
| (e) | at any time by mutual written agreement between Licensee and MD Anderson upon [***] ([***]) calendar days
written notice to all parties and subject to any terms herein which survive termination; or |
| (f) | if Section 13.8 is invoked; or |
| (f) | immediately, upon written notice from MD Anderson, if Licensee has defaulted or been late on its payment
obligations pursuant to the terms of this Agreement on any [***] ([***]) occasions in a [***] period; provided, however,
any payment obligation that is more than [***] past due shall not serve as the basis for notice and termination under this
Section11.3(g) nor shall late payments and underpayments discovered by audit shall form the basis for termination of this Agreement to
the extent that Licensee makes a corrective payment to MD Anderson of its own; or |
| (g) | immediately, upon written notice from Licensee, for any reason or no reason; or |
| (h) | immediately, upon written notice from MD Anderson, if MD Anderson and Licensee have not, despite good
faith negotiations, executed the Sponsored Research Agreement within [***] ([***]) calendar days, unless extended by mutual agreement
of the Parties, of the date that this Agreement is fully executed by both Parties. |
11.4 Upon
termination of this Agreement:
| (a) | nothing herein will be construed to release either party of any obligation maturing prior to the effective
date of the termination; |
| (b) | Licensee covenants and agrees to remain bound by the provisions of Articles VII (Indemnification and Insurance),
VIII (Use of Board and MD Anderson’s Name) and IX (Confidential Information and Publication) of this Agreement; |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| (c) | Subject to Licensee’s payment of any earned royalty or any other amounts due pursuant to Article
III of this Agreement, Licensee may, for a period of one year after the effective date of the termination, sell all Licensed Products
and parts therefor that it has on hand at the date of termination; |
| (d) | Subject to Section 11.4(c), Licensee agrees to cease and desist any use and all Sale of the Patent Rights,
SRA Patent Rights and Technology Rights and upon termination of this Agreement; provided, however, Licensee may. following termination,
continue to use any SRA Technology following termination provided that Licensee pays MD Anderson an amount of [***] Dollars ($[***]) within
[***] of the effective date of termination. Licensee shall be under no obligation to cease and desist from the exercise
of its rights in Joint SRA Technology or the Sale of Products that incorporate such Joint SRA Technology nor shall there be any payment
obligations in association therewith. |
| (e) | Consistent with Section 13(a) of the [***] License, should MDA’s license with [***] terminate, then
MDA will use its best efforts to enable Licensee to obtain a license from [***] such that Licensee is not prejudiced, financially or otherwise,
by such termination of the license agreement between MDA and [***]. |
XII. Warranty:
Superior-Rights
| 12.1 | Except for the rights, if any, of the government of the United States of America (“U.S. Government”)
as set forth below, Board represents and warrants its belief that (a) it is the owner of the right, title, and interest in and to Licensed
Subject Matter, (b) it has the right to grant licenses thereunder, and (c) it has not knowingly granted licenses thereunder to any other
entity that would restrict rights granted hereunder except as stated herein. |
| 12.2 | Licensee understands that the Licensed Subject Matter may have been developed under a funding agreement
with the U.S. Government and, if so, that the U.S. Government may have certain rights relative thereto. This Agreement is explicitly made
subject to the U.S. Government’s rights under any such agreement and any applicable law or regulation. To the extent that there is a conflict
between any such agreement, applicable law or regulation and this Agreement, the terms of such U.S. Government agreement, applicable law
or regulation shall prevail. Licensee agrees that Licensed Products used or Sold in the United States will be manufactured substantially
in the United States, unless a written waiver is obtained in advance from the U.S. Government. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 12.3 | LICENSEE UNDERSTANDS AND AGREES THAT BOARD AND MD ANDERSON, BY THIS AGREEMENT, MAKE NO REPRESENTATION
AS TO THE OPERABILITY OR FITNESS FOR ANY USE, SAFETY, EFFICACY, APPROVABILITY BY REGULATORY AUTHORITIES, TIME AND COST OF DEVELOPMENT,
PATENTABILITY, AND/OR BREADTH OF THE LICENSED SUBJECT MATTER. BOARD AND MD ANDERSON, BY THIS AGREEMENT, ALSO MAKE NO REPRESENTATION AS
TO WHETHER ANY PATENT INCLUDED WITHIN LICENSED SUBJECT MATTER IS VALID OR AS TO WHETHER THERE ARE ANY PATENTS NOW HELD, OR WHICH WILL
BE HELD, BY OTHERS OR BY BOARD OR MD ANDERSON IN THE LICENSED FIELD, NOR DO BOARD AND MD ANDERSON MAKE ANY REPRESENTATION THAT THE INVENTIONS
CONTAINED IN LICENSED SUBJECT MATTER DO NOT INFRINGE ANY OTHER PATENTS NOW HELD OR THAT WILL BE HELD BY OTHERS OR BY BOARD. |
| 12.4 | Licensee, by execution hereof, acknowledges, covenants and agrees that Licensee has not been induced in
any way by Board, System, MD Anderson or employees thereof to enter into this Agreement, and further warrants and represents that (a) Licensee
is entering into this Agreement voluntarily; (b) Licensee has conducted sufficient due diligence with respect to all items and issues
pertaining to this Agreement; and (c) Licensee has adequate knowledge and expertise, or has used knowledgeable and expert consultants,
to adequately conduct such due diligence, and agrees to accept all risks inherent herein. |
| 12.5 | Board represents and warrants that, to the actual knowledge as of the Effective Date of MD Anderson’s
personnel in its Office of Technology Commercialization and/or Strategic Industry Venture familiar who are with this transaction, there
are no known obligations, encumbrances, or contractual or legal restrictions in favor of or benefitting any third party that would preclude
the licensure of Board’s rights as contemplated by this Agreement. |
XIII. General
| 13.1 | This Agreement, together with a Sponsored Research Agreement being entered simultaneously therewith, and
any exhibits and/or fully executed amendments hereto, constitutes the entire and only agreement between the parties for Licensed Subject
Matter and all other prior negotiations, representations, agreements and understandings related to the subject matter of this Agreement
are superseded hereby. Neither party has relied on any such prior communication in entering into this Agreement. No agreements altering
or supplementing the terms hereof will be made except by a written document signed by both parties. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 13.2 | Any notice required by this Agreement shall be in writing and shall be deemed to have been sufficiently
given for all purposes thereof when sent by first class mail or reputable international courier (e.g., Federal Express or UPS) and shall
be evidenced by the postmark at the point of mailing or by the dated delivery receipt of the courier. All notices and any correspondence
respecting this Agreement shall be transmitted as follows: |
To MD Anderson, if by mail:
The University of Texas M. D. Anderson
Cancer Center
Strategic Industry Ventures/Office
of Technology Commercialization
To MD Anderson, if by courier:
The University of Texas M. D. Anderson
Cancer Center
Strategic Industry Ventures/Office
of Technology Commercialization
Contact phone number
for use by courier:
To Licensee by mail
or courier:
20/20 GeneSystems
Inc
15810 Gaither Drive,
Suite 235
Gaithersburg MD
20877
Attn: Jonathan Cohen
or other addresses as may be given from
time to time under the terms of this notice provision.
Communications regarding
patent prosecution may be transmitted by electronic mail. For such communications to MD Anderson sent via electronic mail, the electronic
mail shall be addressed or copied to
| 13.3 | Licensee must comply with all applicable federal, state and local laws and regulations in connection with
its activities pursuant to this Agreement or Licensed Subject Matter, including U.S. Export Administration Regulations, as well as end-user,
end-use, and destination restrictions applied by the United States. Licensee acknowledges that the Licensed Subject Matter may be subject
to U.S. export control jurisdiction. |
| 13.4 | This Agreement and all claims arising out of or relating thereto will be governed, construed and enforced
in accordance with the laws of the United States of America and of the State of Texas, without regard to its conflict of law provisions.
The Texas State Courts of Harris County, Texas (or, if there is exclusive federal jurisdiction, the United States District Court for the
Southern District of Texas) shall have exclusive jurisdiction and venue over any dispute arising out of this Agreement, and Licensee consents
to the jurisdiction and venue of such courts and hereby explicitly waives the rights to any other venue to which it might be entitled
by cause of action, domicile or otherwise. |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| 13.5 | Failure of Licensee, Board or MD Anderson to enforce a right under this Agreement will not act as a waiver
of right or the ability to later assert that right relative to the particular situation involved. |
| 13.6 | Headings included herein are for convenience only and will not be used to construe this Agreement. The
Parties acknowledge and agree that both Parties substantially participated in negotiating the provisions of this Agreement; therefore,
both Parties agree that any ambiguity in this Agreement shall not be construed more favorably toward one Party than the other Party, regardless
of which Party primarily drafted this Agreement. |
| 13.7 | If any provision of this Agreement is for any reason found to be invalid or unenforceable, such provision
shall be interpreted to fulfill its intended purpose to the maximum extent permitted by applicable law and all other provisions of this
Agreement nevertheless will remain enforceable. |
| 13.8 | In the event that Licensee (or its Affiliate or Sublicensee) brings an action, or participates as an adverse
party in any action, before any court, agency or tribunal seeking to invalidate or otherwise challenge the enforceability of or Board’s
ownership of any patent included in the Licensed Subject Matter, then MD Anderson may immediately terminate this Agreement upon written
notice to Licensee and with no opportunity for Licensee to cure. Any dispute regarding the validity, enforceability or ownership of any
patent included in the Licensed Subject Matter shall be litigated in the courts located in Houston, Texas, and Licensee agrees not to
challenge personal jurisdiction in that forum. To the extent that Licensee (or its Affiliate or Sublicensee) unsuccessfully challenges,
or participates as an adverse party in an action that unsuccessfully challenges, the validity or enforceability of any patent included
in the Licensed Subject Matter, Licensee agrees to reimburse MD Anderson and Board for all costs and fees (including attorney’s
fees) paid by MD Anderson and Board in defending against such challenge. Licensee understands and agrees that, in the event Licensee (or
its Affiliate or Sublicensee) successfully challenges the validity or enforceability of any patent included in the Licensed Subject Matter,
all payments or other consideration made or otherwise provided by Licensee to MD Anderson prior to a final, non-appealable adjudication
of invalidity and/or unenforceability shall be non-refundable. The obligations of this Section shall survive the expiration or termination
of this Agreement. |
| 13.9 | This Agreement may be executed in one (1) or more counterparts, by original, facsimile or PDF signature,
each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Signatures to
this Agreement transmitted by facsimile, by email in “portable document format” (“.pdf”), or by any other electronic
means intended to preserve the original graphic and pictorial appearance of this Agreement shall have the same effect as physical delivery
of the paper document bearing original signature. In the event signatures are exchanged by facsimile and/or in “.pdf”
format, each party shall, if requested, thereafter promptly provide an original signature page to the other party. |
| 13.10 | MD Anderson, as an agency of the State of Texas and a member institution of The University of Texas System,
is subject to the constitution and laws of the State of Texas and, under the constitution and laws of the State of Texas, possesses certain
rights and privileges, is subject to certain limitations and restrictions, and only has such authority as is granted under the constitution
and laws of the State of Texas. Moreover, notwithstanding the generality or specificity of any provision of this Agreement, the provisions
of this Agreement as they pertain to MD Anderson are enforceable only to the extent authorized by the constitution and laws of the State
of Texas. No party to this Agreement will be required to perform or commit any act or omission that would violate any applicable law,
including the constitution and laws of the State of Texas. Nothing in this Agreement shall be deemed as a waiver by Board, System or MD
Anderson of its sovereign immunity. |
[Signatures Appear on Following
Page]
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
IN WITNESS WHEREOF, the parties hereto
have caused their duly authorized representatives to execute this Agreement.
|
BOARD OF REGENTS OF THE
UNIVERSITY OF TEXAS System, on behalf of
THE UNIVERSITY OF TEXAS M. D. ANDERSON
CANCER CENTER
By ______________________________
Printed Name: ____________________
Title: ___________________________
Date: _______________ |
|
Approved as to Content:
By __________________________________
Ferran Prat, J.D., Ph.D.
Senior Vice President
Research Administration & Industry Relations
M. D. Anderson Cancer Center
Date: ________________ |
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
20/20 GENESYSTEMS INC.
By ________________________________
Printed Name: ______________________
Title: _____________________________
Date:_______________
40
Exhibit 6.3
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
COLLABORATIVE RESEARCH AGREEMENT
This Collaborative Research
Agreement (“Agreement”), effective as of Effective Date as defined in the License Agreement referenced in Recital C
(the “Effective Date”) is made by and between The University of Texas M. D. Anderson Cancer Center, (“MD Anderson”),
a member institution of The University of Texas System (“System”), with a place of business at 1515 Holcombe Blvd.,
Houston, Texas, 77030, and 20/20 GeneSystems Inc., a corporation with a place of business at 15810 Gaither Drive, Suite 235, Gaithersburg,
MD 20877 (“Sponsor”). MD Anderson and Sponsor hereinafter may be referred to each as a “Party” and
collectively as the “Parties.”
RECITALS
| A. | Sponsor has developed, together with various third-party research collaborators, a multi-cancer early
detection (“MCED”) blood test which it currently commercializes under the trademark “OneTest.” |
| B. | MD Anderson and Sponsor (collectively “the Parties”) are interested in pursuing [***]
that are Licensed Subject Matter as defined by the License Agreement (Recital C). |
| C. | As part of and contemporaneous with (i) that certain Option Agreement, entered by the parties on March
22, 2024 (the “Option Agreement”) (ii) including that Patent and Technology License Agreement contemplated between The Board
of Regents of The University of Texas System, on behalf of MD Anderson, and Sponsor, (the “License Agreement”), and (iii)
an investment from a Korean based private equity firm known as Cornerstone, MD Anderson agrees to conduct and Sponsor desires to collaborate
with MD Anderson and is willing to sponsor MD Anderson’s research study/ies or workscopes (each, a “Study”),
as described in the scope of work found under Exhibit A, attached hereto which may be updated or amended from time-to-time by the mutual
agreement of the Parties. |
| D. | Sponsor and MD Anderson are entering into this Agreement to set forth the rights and obligations of the
Parties with respect to the Study. |
NOW THEREFORE, in consideration
of the mutual covenants and promises herein contained, MD Anderson and Sponsor agree as follows:
1. TERM
This Agreement shall be effective
as of the Effective Date, and shall continue in effect for a period of [***] ([***]) years following the Effective Date (“Term”)
unless such Term is extended by mutual written agreement of the Parties, or the Agreement is earlier terminated in accordance with Section
11 of this Agreement.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
2. STUDY CONDUCT, MATERIALTRANSFER, AND TECHNOLOGY
TRANSFER
2.1 MD
Anderson will use its own facilities and its best efforts to conduct the Study under the direction of [***], (the “Principal
Investigator”) in accordance with Exhibit A and applicable laws and regulations. In the event of any conflict between Exhibit
A and this Agreement, this Agreement shall control. MD Anderson shall provide all necessary personnel, equipment, supplies, facilities
and resources to perform MD Anderson’s obligations in the Study according to the Collaborative Research Plan attached hereto as
Exhibit A, its obligations therein undertaken according to a timeframe and schedule mutually agreed to by the Parties, and shall be fully
responsible for the activities of any MD Anderson personnel to whom Study activities are delegated (collectively, “MD Anderson
Study Participants”). Sponsor shall provide all necessary personnel, equipment, supplies, facilities and resources to perform
Sponsor’s obligations in the Study according to Exhibit A, and shall be fully responsible for the activities of any employee, contractor
or agent of Sponsor who participates in the Study (collectively, “Sponsor Study Participants”, and collectively with
MD Anderson Study Participants, “Project Participants”).
2.2 Project
Participants. Each Party will ensure that before commencing any work on the Study, all Project Participants are covered by written
obligation or written policies that include (a) obligations of confidentiality and non-use with respect to Confidential Information that
are consistent with the terms of this Agreement; and (b) a requirement to assign to such Party any and all rights that such Project Participants
might otherwise have in the results of their work without any obligation of the other Party to pay any royalties or other consideration
to such Project Participants.
2.3 Sponsor
understands and acknowledges that MD Anderson’s primary mission is the development and dissemination of scientific knowledge, and
that MD Anderson makes no representations, warranties, or guarantees with respect to any specific results of the Study.
2.4 Sponsor
understands and acknowledges that MD Anderson may be involved in similar research through other researchers on behalf of itself and others.
Except as expressly set forth herein, nothing in this Agreement will limit or prohibit MD Anderson or any of its personnel, including
the Principal Investigator, from conducting any research or from performing research for or with any entity or person, including any other
outside sponsors. Sponsor acknowledges that this provision is intended to preserve the academic freedom and integrity of MD Anderson and
its faculty and to ensure that MD Anderson and its faculty are not regarded as captive researchers for Sponsor.
2.5 Material
Transfer. Under the scope of work described in Exhibit A, MD Anderson will provide to Sponsor certain materials, information, and/or
data for the conduct of the Study as may be further described in Exhibit A (the “MD Anderson Materials”), and Sponsor
will provide to MD Anderson certain materials, information and/or data for the conduct of the Study as may be further described in Exhibit
A (the “Sponsor Study Materials”). The Parties agree that all research conducted in connection with the Study will
be conducted in compliance with all laws, governmental regulations including without limitation current U.S. National Institutes of Health
guidelines, the Health Information Portability and Accountability Act (HIPAA) of 1996 and any applicable regulations or guidelines pertaining
to research with human specimens and human clinical and patient data.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
Sponsor will use the MD Anderson
Materials solely for the Study and shall not sell, distribute, transfer or otherwise provide any MD Anderson Materials to any third party
without MD Anderson’s prior written consent, other than its Sponsor Study Participants who require access to the MD Anderson Materials
to carry out the Study, who are under Sponsor’s supervision, and who are informed of the proprietary nature of the MD Anderson Materials.
Sponsor will take reasonable precautions at least as stringent as those observed to protect its own proprietary material of a similar
nature to ensure that all confidentiality and non-use obligations under this Agreement are observed. MD Anderson will supply to Sponsor,
[***], such quantities of the MD Anderson Materials as are specified in Exhibit A. Sponsor will be responsible for the actions or inactions,
as the case may be, of Sponsor Study Participants who require access to the MD Anderson Materials to carry out the Study. Sponsor acknowledges
and agrees that the MD Anderson Materials are and will at all times remain the exclusive property of MD Anderson. Sponsor acknowledges
and agrees that the MD Anderson Materials may be hazardous in nature. Sponsor will use, store, and handle all MD Anderson Materials in
accordance with any instruction provided by MD Anderson and with all applicable laws relating thereto.
MD Anderson will use the Sponsor
Materials solely for the Study and shall not sell, distribute, transfer or otherwise provide any Sponsor Materials to any third party,
or use them for the benefit of any third party, without Sponsor’s prior written consent, other than its MD Anderson Study Participants
who require access to the Sponsor Materials to carry out the Study, who are under Principal Investigator’s supervision, and who
are informed of the proprietary nature of the Sponsor Materials. MD Anderson will not reverse engineer or modify the Sponsor Materials,
except as required to conduct the Study. MD Anderson will take reasonable precautions at least as stringent as those observed to protect
its own proprietary material of a similar nature to ensure that all confidentiality and non-use obligations under this Agreement are observed.
Sponsor will supply to MD Anderson, free of charge, such quantities of the Sponsor Materials as are specified in Exhibit A. MD Anderson
will be responsible for the actions or inactions, as the case may be, of the Principal Investigator and other MD Anderson Study Participants
who require access to the Sponsor Materials to carry out the Study. MD Anderson acknowledges and agrees that the Sponsor Materials are
and will at all times remain the exclusive property of Sponsor. MD Anderson acknowledges and agrees that the Sponsor Materials may be
hazardous in nature. MD Anderson will use, store, and handle all Sponsor Materials in accordance with any instruction provided by Sponsor
and with all applicable laws relating thereto.
3. STUDY FINANCIAL PAYMENTS
3.1 MD
Anderson shall be responsible for its research expenses incurred in the conduct of the Study. In support for the expenditures and reasonable
overhead of MD Anderson in conducting the Study in accordance with the Collaborative Research Plan attached hereto as Exhibit A, Sponsor
agrees to pay MD Anderson the following in the amount and on the schedule (each, a “Payment Event”) as set forth below and
in the Collaborative Research Plan Budget described in Exhibit B attached hereto (up to a maximum aggregate amount of $[***]). MD Anderson
shall use the funding from Sponsor solely for the Study and not for any other purpose:
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
| Payment Event |
Payment Amount (US$) |
| No. 1: Within [***] ([***]) days of the Effective Date of this Agreement |
[***] Dollars ($[***]) |
| No. 2: Within [***] ([***]) business days of full execution of the License Agreement by both Parties of the Option Agreement |
[***] Dollars ($[***]) |
| No. 3: Within [***] ([***]) months of payment of Payment Event No. 2 |
[***] Dollars ($[***]) |
| No. 4: Within [***] ([***]) months of payment of Payment Event No. 2 |
[***] Dollars ($[***]) |
| No. 5: Within [***] ([***]) months of payment of Payment Event No. 2 |
[***] Dollars ($[***]) |
The foregoing notwithstanding, and for the avoidance
of doubt, if as contemplated in the Option Agreement Sponsor fails to raise a total of at least $[***] within [***] ([***]) months of
the effective date of the Option Agreement, it shall have no obligation to pay MD Anderson the $[***] that would otherwise be due for
Payment Event No. 2 or the $[***] that would otherwise be due for Payment Event No. 3 and MD Anderson may terminate the Agreement pursuant
to Section 10.2, and furthermore, if Sponsor fails to raise at least $[***] within [***] ([***]) months of the Effective Date, it shall
have no obligation to pay MD Anderson the $[***] that would otherwise be due for Payment Events Nos. 4 and 5. Furthermore, in the event
of a material breach by MD Anderson that is not cured within [***] days, Sponsor shall have no obligation to make further payments thereafter.
In the event of any conflict between Exhibit B and this Agreement, this Agreement shall control.
3.2 Upon
receipt by MD Anderson of payment of the Payment Amount set forth for Payment Event No.1, MD Anderson will solely conduct activities under
Aim 5 of the Collaborative Research Plan described in Exhibit A. Following execution of the License Agreement and upon receipt by MD Anderson
of payment of the Payment Amount set forth for Payment Event No. 2, MD Anderson will expand its undertakings in the conduct of the Study
as described in the Collaborative Research Plan included in Exhibit A according to a timeframe and schedule mutually agreed to by the
Parties.
4. DATA
4.1
Subject to Section 7.3 below, the Parties shall [***] generated
in the conduct of the Study (“Data”). Except as expressly provided for pursuant to the License Agreement, neither Party
shall, directly or indirectly, commercialize, exploit, encumber, transfer or license, assign or dispose of, to third party, any right,
title or interest to Data without the prior written consent of the other Party. MD Anderson shall always have the right to use Data for
its internal research and academic purposes, as well as publication purposes in accordance with Section 5.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
4.2 Each
Party shall provide the other Party with its Data (both in raw and aggregate form) in a Study report pursuant to a timetable mutually
agreed to by the Parties. All Data shall be de-identified, and where indicated by the workscope certain Data will be coded with a Study
ID number. Sponsor shall have the right to use Data [***] subject to Section 4.1 above, provided that Sponsor shall maintain such Data
in confidence until the earlier of: (a) publication or public disclosure of such Data by MD Anderson and/or Principal Investigator; or
(b) [***] ([***]) months following receipt of the Study report upon completion of the Study. In addition to other rights of Sponsor, Sponsor
shall have the right to use the raw Data (x) in its own internal research, (y) to collaborate with academic researchers outside of MD
Anderson and Sponsor’s organization, and (z) [***].
4.3 The
Parties shall use Data solely for the purposes of the Collaboration and hold Data in confidence subject to each Party’s right to
publish in accordance with Section 5 below, the License Agreement, and MD Anderson’s right to use Data for internal research and
academic purposes. Data and relevant materials will be stored on a shared cloud data storage system; electronic lab book software and/or
other electronic communications and data storage systems/platforms will be mutually agreed upon by the Parties. The Parties will maintain
regular communications regarding Data resulting from research activities under the Collaboration and to evaluate the work performed in
relation to the goals of this Agreement and the applicable Collaborative Research Plan.
5. PUBLICATION AND PUBLICITY
5.1 MD
Anderson and Principal Investigator shall have the right to publish or present Data in scientific journals and/or at scientific meetings
at MD Anderson’s and/or the Principal Investigator’s sole discretion, and to submit Data to a public data registry. MD Anderson
and Principal Investigator shall provide Sponsor with a copy of a proposed publication or presentation for review and comment [***] ([***])
days prior to submission to any publishing source or at least ([***]) days prior to presentation at a scientific meeting or conference.
MD Anderson and Principal Investigator shall consider in good faith Sponsor’s comments and suggestions on manuscripts to
be published and materials to be used for presentation, provided however, MD Anderson and Principal Investigator shall have the final
authority to determine the scope and content of any presentation and/or publication of Data provided that MD Anderson and Principal Investigator
comply with any request from Sponsor to (i) delete Sponsor’s Confidential Information in any such manuscript or material, and (ii)
delay any submission for publication or presentation for a period of up to an additional [***] ([***]) days for the purpose of preparing
and filing appropriate patent applications. Notwithstanding the foregoing, nothing in this Agreement shall require MD Anderson to refrain
from publishing any information if doing so would (a) cause MD Anderson to violate any export control laws or laws that provide tax-exempt
status for any bonds issued by MD Anderson or The University of Texas System, or (b) result in the inapplicability to MD Anderson or The
University of Texas System of the fundamental research exclusion or exemption from U.S. export control laws for such information.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
5.2 Except
as required by law or to acknowledge the contributions of the other Party in an academic or scientific publication, no Party shall use
the name, symbol, trademark, trade name or logo of the other Party (or any abbreviation or adaptation thereof) in any publication, press
release, promotional material or other form of publicity without the prior written consent of the other Party in each instance. Unless
otherwise agreed to by MD Anderson in writing, Sponsor will not state or imply that MD Anderson endorses any of Sponsor’s products
or services, nor use MD Anderson’s logo (or variation or adaptation thereof) under any circumstances. All materials using the name,
trademarks, service marks, or symbols of MD Anderson or The University of Texas for any purpose, including, but not limited to, the use
in advertising, marketing, and sales promotion materials or any other materials or mediums (such as the internet, domain names, or URL
addresses), must be submitted to MD Anderson’s Public Relations team for prior written approval at the following email address:,
or to such other person or contact as indicated by MD Anderson in writing.
6. CONFIDENTIAL INFORMATION
6.1 In
conjunction with the Study, the Parties may wish to disclose certain of their respective confidential and/or proprietary information (“Confidential
Information”) to each other. Each Party will use Confidential Information of the other Party solely for the purpose of conducting
the Study, and shall use reasonable efforts to prevent the disclosure of such other Party’s Confidential Information to third parties
during the Term and for a period of [***] ([***]) years after expiration or termination of this Agreement, provided that the receiving
Party’s obligation of confidentiality and nonuse hereunder shall not apply to information that: (a) is already in the receiving Party’s
possession at the time of disclosure; (b) is or later becomes part of the public domain through no fault of the receiving Party; (c) is
received from a third party having no obligations of confidentiality or nonuse to the disclosing Party; (d) independently developed by
the receiving Party; (e) is required by law or regulation to be disclosed; (f) is published in accordance with Section 5 of this Agreement;
(g) is necessary to disclose in order to file a patent application or enforce a patent related to this Agreement; or (h) is communicated
to MD Anderson’s scientific and/or institutional review committees under confidentiality obligations equivalent to those herein.
6.2 In
the event that information is required to be disclosed pursuant to Section 6.1(e), the Party required to make disclosure shall notify
the other Party to allow the other Party to assert whatever exclusions or exemptions may be available to such Party under applicable law
or regulation.
6.3 In
the event that Sponsor shall come into contact with any “Protected Health Information” (as such term is defined under
HIPAA) of MD Anderson or any information that could be used to identify any of MD Anderson’s patients or research subjects, Sponsor
shall maintain any such Protected Health Information or other information confidential in accordance with laws and regulations as applicable
to MD Anderson, including without limitation HIPAA, and shall not use or disclose any such Protected Health Information or other information
in any manner that would constitute a violation of any applicable law or regulation if such use or disclosure was made by MD Anderson.
MD Anderson represents and warrants that it shall comply with all applicable data privacy and protection laws and regulations in the collection,
use, and handling of personal data under this Agreement.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
7. INTELLECTUAL PROPERTY
7.1 Sponsor
and MD Anderson understand and agree that the performance of the Study may require use of information and/or materials developed: (a)
before the Effective Date; or (b) independent of this Agreement, and that may be protected by patents or other proprietary rights owned
by or licensed to either Party (“Background Intellectual Property”). Nothing in this Agreement will be deemed or construed
to convey or transfer to either Party any rights or license with respect to the Background Intellectual Property of the other Party except
insofar as contemplated by this Agreement.
7.2 “Invention”
shall mean any invention or discovery, whether or not patentable, conceived during the conduct of the Study and arising from the performance
of the Study including the legal rights relating to inventions, patent applications, patents, trade secrets, algorithms, mathematical
formulae, and any other legally protectable information. Inventorship will be determined in accordance with United States patent law,
and ownership of Inventions shall follow inventorship. “Joint Intellectual Property” means any Invention invented by at least
one employee or agent of MD Anderson in combination with at least one employee or agent of Sponsor. “MD Anderson Intellectual Property”
means any Invention invented solely by employees or agents of MD Anderson. MD Anderson will have sole ownership of such MD Anderson Intellectual
Property. MD Anderson Intellectual Property further includes any rights MD Anderson may have in Joint Intellectual Property. “Sponsor
Intellectual Property” means any Invention invented solely by employees or agents of Sponsor. Sponsor will have sole ownership of
such Sponsor Intellectual Property. Sponsor Intellectual Property further includes any rights Sponsor may have in Joint Intellectual Property.
Each Party shall promptly after conception of any Invention, notify the other Party of such Invention, providing with such notification
sufficient information or such other Party to evaluate the Invention (“Invention Notice”). The Parties agree to cooperate
in good faith regarding decisions regarding filing of patent applications related to any such Invention. Such Invention Notices will be
considered Confidential Information of both Parties.
7.3 Notwithstanding
anything to the contrary herein, all Data and Inventions invented by MD Anderson prior to execution of the License Agreement shall be
owned solely by MD Anderson. Following execution of the License Agreement and receipt by MD Anderson of payment of the Payment Amount
set forth for Payment Event No. 2 according to Section 3.1 above, ownership of all arising Data and Inventions will be determined according
to Sections 4.1 and 7.2 herein, respectively.
7.4 Sponsor
shall have a period of [***] ([***]) days after receiving MD Anderson’s receipt of such Invention Notice, to evaluate the Invention
(“Evaluation Period”). Subject to Section 7.5, if Sponsor notifies MD Anderson in writing during Evaluation Period of its
desire to license such MD Anderson Intellectual Property or MD Anderson’s interest in any Joint Intellectual Property, such Invention
shall be a Sponsored Research Invention (as that term is defined in the License Agreement) under the License Agreement.
7.5 [***].
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
8. INDEMNIFICATION
8.1 Sponsor
agrees to indemnify, hold harmless, and subject to the statutory duties of the Texas State Attorney General defend MD Anderson, System,
their Regents, officers, agents and employees (“MD Anderson Indemnitees”) from any liability, loss or damage they may
suffer as a result of claims, demands, costs or judgments against them arising out of Sponsor’s rights and obligations under this
Agreement, including but not limited to Sponsor’s use of Data; provided, however, that Sponsor shall not be obligated to hold harmless
any MD Anderson Indemnitee from claims arising out of the negligence or willful malfeasance of any MD Anderson Indemnitee.
8.2 To the extent authorized by the constitution
and laws of the State of Texas, MD Anderson agrees to indemnify and hold harmless Sponsor, its officers, agents and employees (“Sponsor
Indemnitees”) from any liability, loss or damage they may suffer as a result of claims, demands, costs or judgments against
them arising out of MD Anderson’s negligence in conducting the Study, provided, however, that MD Anderson shall not be obligated
to hold harmless any Sponsor Indemnitee from claims arising out of the negligence or willful malfeasance of any Sponsor Indemnitee.
8.3 Both
Parties agree that upon receipt of a notice of claim or action arising out of the Study, the Party receiving such notice will notify the
other Party promptly.
9. INDEPENDENT CONTRACTOR
For the purposes of this Agreement
and the Study, the Parties shall be, and shall be deemed to be, independent contractors and not agents or employees of the other Party.
Neither Party shall have authority to make any statements, representations or commitments of any kind, or to take any action that shall
be binding on the other Party, except as may be expressly provided for herein or authorized in writing.
10. TERMINATION
10.1 This
Agreement may be terminated: (a) immediately by the written agreement of both Parties; or (b) immediately by either Party if at any time
Principal Investigator becomes unable to conduct the Study, and the Parties cannot agree upon a mutually acceptable successor to the Principal
Investigator.
10.2 MD
Anderson shall have the right to terminate this Agreement immediately if, within [***] after the effective date of the
Option Agreement: (a) the License Agreement is not signed; or (b) the Payment Amount set forth for Payment Event No. 2 in Section 3.1
above is not received in full by MD Anderson. [***].
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
10.3 [***].
10.4 In
the event that either Party shall be in default of its material obligations under this Agreement and shall fail to remedy such default
within [***] ([***]) days after receipt of written notice thereof, this Agreement shall terminate upon expiration of the [***] ([***])
day period.
10.5 Termination
or cancellation of this Agreement shall not affect the rights and obligations of the Parties accrued prior to termination. Upon termination:
(a) Sponsor shall pay MD Anderson for all reasonable expenses incurred or committed to be expended as of the effective termination date,
including salaries for appointees for the remainder of their appointment as applicable; and (b) each Party shall return to the other Party
or destroy any Confidential Information of such other Party remaining in the Party’s possession, provided that such Party may retain
one (1) copy of such Confidential Information for purposes of compliance with this Agreement and with applicable laws and regulations.
10.6 Any
provisions of this Agreement that by their nature extend beyond expiration or termination of the Agreement shall survive such termination.
11. MISCELLANEOUS PROVISIONS
11.1 Except
in connection with the sale of all or substantially all of Sponsor’s assets to a third party or in connection with any merger, acquisition,
consolidation, or business combination, neither this Agreement nor any rights, interests, duties or obligations of Sponsor under this
Agreement may be assigned (either directly or indirectly) by Sponsor without the prior written consent of MD Anderson, which will not
be unreasonably withheld. A merger or other transaction in which the equity holders of Sponsor prior to such event hold less than a majority
of the equity of the surviving or acquiring entity shall be considered an assignment of this Agreement.
11.2 This
Agreement constitutes the entire and only agreement between the Parties relating to each Study, and all prior negotiations, representations,
agreements, and understandings are superseded hereby. No agreements altering or supplementing the terms hereof may be made except by means
of a written document signed by the duly authorized representatives of the Parties.
11.3 Principal
Investigator and Sponsor may be parties to a consulting agreement or other outside agreement to which MD Anderson is not a party. Sponsor
acknowledges and agrees that MD Anderson has no involvement with or responsibility for these consulting or outside agreements. MD Anderson
acknowledges and represents that MD Anderson has not entered into other agreements that conflict with or result in inconsistent obligations
with the terms of this Agreement.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
11.4 Any
notice required by this Agreement shall be given by prepaid, first class, certified mail, return receipt requested, addressed in the case
of MD Anderson to:
The University of Texas
M. D. Anderson Cancer Center
With a copy to:
The University of
Texas System
M. D. Anderson Cancer
Center
or in the case of Sponsor
to:
20/20 GeneSystems, Inc.
15810 Gaither Drive, Suite
235
Gaithersburg, MD 20877
ATTN: President & CEO
With a copy to:
20/20 GeneSystems, Inc.
15810 Gaither Drive, Suite
235
Gaithersburg, MD 20877
ATTN: Chief Scientific
Officer
or at such other addresses as
may be given from time to time in accordance with the terms of this notice provision.
11.5 This
Agreement shall be governed by, construed, and enforced in accordance with the laws of the State of Texas.
11.6 MD
Anderson is an agency of the State of Texas and under the Constitution and laws of the State of Texas possesses certain rights and privileges
and only such authority as is granted to it under the Constitution and laws of the State of Texas. Notwithstanding any provision hereof,
nothing herein is intended to be, nor will it be construed to be, a waiver of the sovereign immunity of the State of Texas or a prospective
waiver or restriction of any of the rights, remedies, claims, and privileges of the State of Texas. Moreover, notwithstanding the generality
or specificity of any provision hereof, the provisions of this agreement as they pertain to MD Anderson are enforceable only to the extent
authorized by the Constitution and laws of the State of Texas.
11.7 Neither
MD Anderson nor Sponsor will be required to perform any act or to refrain from any act or be bound to any act that would violate any state
or federal law applicable to it. In this regard, this Agreement is subject to, and MD Anderson and Sponsor agree to comply with, all applicable
local, state, federal, national and international laws, statutes, rules and regulations. Any provision of any law, statute, rule or regulation
that invalidates any provision of this Agreement, that is inconsistent with any provision of this Agreement, or that would cause one or
any of the Parties hereto to be in violation of law will be deemed to have superseded the terms of this Agreement. MD Anderson and Sponsor,
however, will use all reasonable efforts to accommodate the terms and intent of this Agreement to the greatest extent possible consistent
with the requirements of the law and negotiate in good faith toward amendment of this Agreement in such respect. If the Parties cannot
reach agreement on an appropriate amendment, then this Agreement may be immediately terminated by either Party.
11.8 No
Party shall be liable to any other for any delay or non-performance of its obligations under this Agreement arising from any Force Majeure
Event. “Force Majeure Event” means any act or event, in whole or in part, whether foreseen or unforeseen, that is beyond the
reasonable control of a Party, but excludes economic hardship or insufficiency of funds.
| CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS INFORMATION THAT THE ISSUER CUSTOMARILY AND ACTUALLY TREATS AS PRIVATE OR CONFIDENTIAL AND THE OMITTED INFORMATION IS NOT MATERIAL. |
IN WITNESS WHEREOF, the Parties
have caused this Agreement to be executed by their duly authorized representatives.
| 20/20 GENESYSTEMS, INC. |
|
THE UNIVERSITY OF TEXAS |
| |
|
|
M. D. ANDERSON CANCER CENTER |
| |
|
|
|
|
| By |
/s/ Jonathan Cohen |
|
By |
/s/ Amy M Moritz |
| Name: |
Jonathan Cohen |
|
Name: |
Amy M Moritz |
| Title: |
President & CEO |
|
Title: |
Associate Director, ORA |
| |
|
|
|
|
| Date: 04/26/2024 |
|
Date: 04/25/2024 |
| |
|
|
|
|
| |
|
|
READ AND UNDERSTOOD BY: |
| |
|
|
|
|
| |
|
|
/s/ Samir Hanash |
| |
|
|
Name: |
Samir Hanash |
| |
|
|
Principal Investigator |
11
Exhibit 6.4










Exhibit 6.5

















20 20 GeneSystems (GM) (USOTC:TWTG)
Historical Stock Chart
From Apr 2024 to May 2024

20 20 GeneSystems (GM) (USOTC:TWTG)
Historical Stock Chart
From May 2023 to May 2024
