| PROSPECTUS SUPPLEMENT |
Filed pursuant to Rule 424(b)(5) |
| (To Prospectus dated February 6, 2024) |
File No. 333-276481 |

CHINA PHARMA
HOLDINGS, INC.
Up to $600,000 of Shares
of Common Stock
China Pharma Holdings, Inc. (the “Company”, “we”,
“our”, or “us”) have entered into a securities purchase agreement (the “SPA”) with an institutional
investor (the “Investor”), relating to the sale of shares of common stock of the Company, par value $0.001 per share (the
“Common Stock”) over a commitment period from December 12, 2024, the effective date of the SPA through December 31, 2024.
In accordance with the terms of the SPA, we may offer and sell shares of our Common Stock, from time to time, with an aggregate offering
price of up to $600,000 covered by this prospectus supplement (“Pro Supp”) to the Investor.
Shares of our Common Stock
covered by this Pro Supp may be sold by any method deemed to be an “at the market offering” as defined in Rule 415(a)(4) under
the Securities Act of 1933, as amended (the “Securities Act”). The Investor may acquire our Common Stock through one or more
closings at a per share price equal to the lower of (i) the closing price the day prior to notice date or (ii) the five (5) day average
closing price as reported by Bloomberg or on the NYSE American Market’s website (the “Purchase Price”) upon our receipt of purchase notices. The Purchase Price shall not be less than $0.15 per share.
The transaction under the
SPA will allow us to raise capital by selling shares of our Common Stock in open market transactions; however, it is at the Investor’s
discretion. There is no assurance that all or any of our shares of the Common Stock covered by this Pro Supp will be issued.
We are currently subject to
General Instruction I.B.6 of Form S-3, which limits the amounts of securities that we may sell under the registration statement of which
the Pro Supp and the accompanying prospectus form a part. The aggregate market value of our outstanding Common Stock held by non-affiliates
pursuant to General Instruction I.B.6 of Form S-3, or public float, is $2,588,182.33, which is based on 10,039,497 shares of our outstanding
Common Stock held by non-affiliates as of the date of this Pro Supp, at a price of $0.26 per share, which was the last reported sale price
of our Common Stock on Nasdaq on November 4, 2024. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell shares
pursuant to this Pro Supp with a value of more than one-third of the aggregate market value of our Common Stock held by non-affiliates
in any 12-month period, which is $862,727.44. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during
the prior 12 calendar month period.
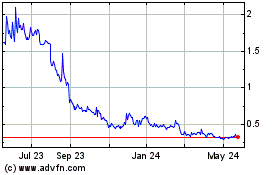
Our Common Stock trades on the NYSE American under the symbol “CPHI”.
The last reported sale price of our Common Stock on the NYSE American on December 12, 2024 was $0.186 per share.
The date of this prospectus supplement is December 12, 2024.
TABLE
OF CONTENTS
ABOUT THIS PROSPECTUS
SUPPLEMENT
This Pro Supp is a supplement
to the accompanying prospectus that is also a part of this document. This Pro Supp and the accompanying prospectus, dated February 6,
2024, are part of a registration statement on Form S-3 (File No. 333-276481, the “Registration Statement”) that we filed with
the Securities and Exchange Commission, or the SEC, utilizing a “shelf” registration process. Under this shelf registration
process, we may offer and sell from time to time in one or more offerings the securities described in the accompanying prospectus.
This document is in two parts.
The first part is this Pro Supp, which describes the securities we are offering and the terms of the offering and also adds to and updates
information contained in the accompanying prospectus and the documents incorporated by reference into the accompanying prospectus. The
second part is the accompanying prospectus, which provides more general information, some of which may not apply to the securities offered
by this Pro Supp. Generally, when we refer to this “prospectus,” we are referring to both documents combined. To the extent
there is a conflict between the information contained in this Pro Supp, on the one hand, and the information contained in the accompanying
prospectus or any document incorporated by reference therein, on the other hand, you should rely on the information in this Pro Supp.
We urge you to carefully read this Pro Supp and the accompanying prospectus, together with the information incorporated herein and therein
by reference as described under the heading “Where You Can Find Additional Information,” before buying any of the securities
being offered.
You should not consider any
information in this Pro Supp or the accompanying prospectus to be investment, legal or tax advice. You should consult your own counsel,
accountants and other advisors for legal, tax, business, financial and related advice regarding the purchase of any of the securities
offered by this Pro Supp. You should assume that the information in this Pro Supp and the accompanying prospectus is accurate only as
of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of
the document incorporated by reference, regardless of the time of delivery of this Pro Supp and the accompanying prospectus or any sale
of a security.
This Pro Supp contains summaries
of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete
information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to
herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this Pro
Supp is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
FORWARD-LOOKING STATEMENTS
This Pro Supp, the accompanying
prospectus and the documents that we have filed with the SEC that are incorporated by reference in this Pro Supp contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and
Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and may involve material risks, assumptions
and uncertainties. Forward-looking statements typically are identified by the use of terms such as “may,” “will,”
“should,” “believe,” “might,” “expect,” “anticipate,” “intend,”
“plan,” “estimate,” and similar words, although some forward-looking statements are expressed differently.
Any forward looking statements
contained in this Pro Supp, the accompanying prospectus and the documents that we have filed with the SEC that are incorporated by reference
in this Pro Supp are only estimates or predictions of future events based on information currently available to our management and management’s
current beliefs about the potential outcome of future events. Whether these future events will occur as management anticipates, whether
we will achieve our business objectives, and whether our revenues, operating results, or financial condition will improve in future periods
are subject to numerous risks. There are a number of important factors that could cause actual results to differ materially from the results
anticipated by these forward-looking statements. These important factors include those that we discuss under the heading “Risk Factors”
and in other sections of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (the “Form 10-K”), as
well as in our other reports filed from time to time with the SEC that are incorporated by reference into this Pro Supp and the accompanying
prospectus. You should read these factors and the other cautionary statements made in this Pro Supp, the accompanying prospectus and in
the documents we incorporate by reference into this Pro Supp and the accompanying prospectus as being applicable to all related forward-looking
statements wherever they appear in this Pro Supp or the documents we incorporate by reference into this Pro Supp and the accompanying
prospectus. If one or more of these factors materialize, or if any underlying assumptions prove incorrect, our actual results, performance
or achievements may vary materially from any future results, performance or achievements expressed or implied by these forward-looking
statements. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future
events or otherwise, except as required by law.
PROSPECTUS SUPPLEMENT SUMMARY
This summary is not complete
and does not contain all of the information that you should consider before investing in the shares of Common Stock offered by this Pro
Supp. You should read this summary together with the entire Pro Supp and the accompanying prospectus, including our risk factors (as provided
for herein and incorporated by reference), financial statements, the notes to those financial statements and the other documents that
are incorporated by reference in this Pro Supp, before making an investment decision. You should carefully read the information described
under the heading “Where You Can Find More Information.” We have not authorized anyone to provide you with information different
from that contained in this Pro Supp. The information contained in this Pro Supp is accurate only as of the date of this Pro Supp, regardless
of the time of delivery of this Pro Supp or of any sale of our securities.
Unless the context otherwise
requires, references to “we”, “us”, “the Company” or “our Company” refer to China Pharma
Holdings, Inc. and its wholly owned subsidiaries. “China” and the “PRC” refer to the People’s Republic of
China.
Organization and Nature of Operations
The Company, a Nevada corporation,
owns 100% of Onny Investment Limited (“Onny”), a British Virgin Islands corporation, which owns 100% of Hainan Helpson Medical
& Biotechnology Co., Ltd (“Helpson”), a company organized under the laws of the People’s Republic of China (the
“PRC”).
Onny acquired 100% of the
ownership in Helpson on May 25, 2005, by entering into an Equity Transfer Agreement with Helpson’s three former shareholders. The
transaction was approved by the Commercial Bureau of Hainan Province on June 12, 2005 and Helpson received the Certificate of Approval
for Establishment of Enterprises with Foreign Investment in the PRC on the same day. Helpson received its business license evidencing
its WFOE (Wholly Foreign Owned Enterprise) status on June 21, 2005.
Our
corporate organizational chart is set forth below.

The Company has acquired and
continues to acquire well-accepted medical formulas to add to its diverse portfolio of Western and Chinese medicines.
We
are not a Chinese operating company but a Nevada holding company. All of our operations are conducted in the PRC through Hainan Helpson
Medical & Biotechnology Co., Ltd (“Helpson”), our wholly owned subsidiary incorporated under the laws of the People’s
Republic of China (the “PRC”), where the manufacturing facilities are located. Helpson is principally engaged in the development,
manufacture and marketing of pharmaceutical products for human use in connection with a variety of high-incidence and high-mortality diseases
and medical conditions prevalent in the PRC. It manufactures pharmaceutical products in the form of dry powder injectables, liquid injectables,
tablets, capsules, and cephalosporin oral solutions. The majority of its pharmaceutical products are sold on a prescription basis and
all of them have been approved for at least one or more therapeutic indications by the National Medical Products Administration (the “NMPA”,
formerly China Food and Drug Administration, or CFDA) based upon demonstrated safety and efficacy.
China’s
consistency evaluation of generic drugs continues to proceed in 2024. Helpson has always taken the task of promoting the consistency evaluation
as a top priority, and worked on them actively. However, for each drug’s consistency evaluation, due to the continuous dynamic changes
of the detailed consistency evaluation policies, market trends, expected investments, and expected returns of investment (“ROI”),
the whole industry, including Helpson, has been making slow progresses in terms of the consistency evaluation. One of the flagship products,
Candesartan tablets, a hypertension product, has passed generic-drug-consistency-evaluation in early August 2023.
Helpson
has taken a more cautious and flexible attitude towards initiating and progressing any project for existing products’ consistency
evaluation to cope with the changing macro environment of drug sales in China. In 2018, relevant Chinese authorities decided to implement
trial Centralized Procurement (“CP”) activities in 11 selected pilot cities (including 4 municipalities and 7 other cities),
since then, nine rounds of CP activities have been carried out as of November 13, 2024, which significantly reduced the price of the drugs
that won the bids. In addition, the consistency evaluation has been adopted as one of the qualification standards for participating in
the CP activities. As a result, Helpson needs to balance between the market access brought by CP, the investment of financial resources
and time to obtain the qualification of CP, and the sharp decline in the price of drugs included in CP before making decisions regarding
CP for any products.
In
addition, Helpson continues to explore the field of comprehensive healthcare. Comprehensive healthcare is a general concept proposed by
the Chinese government according to the development of the times, social needs and changes in disease spectrum. According to the Outline
of “Healthy China 2030” issued by Chinese government in October 2016, the total size of China’s health service industry
is expected to reach RMB 16 trillion (approximately $2.5 trillion) by 2030. This industry focuses on people’s daily life, aging
and diseases, pays attention to all kinds of risk factors and misunderstandings affecting health, calls for self-health management, and
advocates the comprehensive care throughout the entire process of life. It covers all kinds of health-related information, products, and
services, as well as actions taken by various organizations to meet the health needs. In response to this trend, Helpson launched Noni
enzyme, a natural, Xeronine-rich antioxidant food supplement at the end of 2018. It also launched wash-free sanitizers and masks, in 2020,
to address the market needs caused by COVID-19 in China. As Chinese government officially terminated its zero-case policy, now the responsibility
to protect people from the impact of COVID-19 falls more to the citizens themselves, and masks and sanitizers have been popular since
COVID-19. Helpson has sufficient production capacity for medical masks, surgical masks, KN95 masks, and N95 masks, which also meets the
personal needs for protection against other respiratory infectious disease. Helpson’s N95 medical protective mask has received registration
certificate at the end of 2022 and has been on the market in the mainland China nationwide.
Helpson
will continue to optimize its product structure and actively respond to the current health needs of human beings.
Market Trends
As
a generic drug company, Helpson is presented with a huge domestic market. Helpson believes that through further upgrades and better conformity
with Chinese consistency evaluations, which are based on European and American production standards, it will be able to export the products
to overseas markets. In China’s market, Helpson believes that in the future, cost management and control ability will gradually
become important factors in determining the competitiveness of generic pharmaceutical enterprises. Although price control leads to a decline
in the profitability, enterprises who win the CP have a good chance of achieving bulk pricing to increase their market share and support
their continuous innovation and transformation. On a separate note, growing and advancing consumer demand in China drives the increase
of discretionary consumption. With the improvement of residents’ quality of life, the healthcare demand is also changing. Helpson
believes that there is a large number of unmet demands in comprehensive healthcare and internet healthcare sectors.
In
addition, the Office of the State Council issued “Pilot Plan for Marketing Authorization Holders” on May 24, 2016,
allowing eligible drug research and development institutions and scientific researchers to become Marketing Authorization Holders (“MAH”)
by obtaining drug marketing authorization and drug approval numbers from the State Council. This policy uses a management model of separating
drug marketing authorization and drug production licenses, thereby allowing MAHs to produce pharmaceuticals themselves or to consign production
to other pharmaceutical manufacturers. This policy not only transitions the production practices to meet the European and United States
standards by separating drug approval and production qualifications, thereby changing the existing model of bundling drug approval codes
to pharmaceutical manufacturers in China, but also serves as a supplement to the ongoing consistency evaluations policy.
In
general, demand for pharmaceutical products continues its steady growth in China. Helpson believes the ongoing generic drug consistency
evaluations and reform of China’s drug production registration and review policies will have major effects on the future development
of our industry and may change its business patterns. Helpson will continue to actively adapt to the national policy guidance and further
evaluate market conditions for the existing products then adjust accordingly, and compete in the market in order to optimize the development
strategy.
RISK FACTORS
An investment in our shares
of Common Stock involves a high degree of risk. Before making any investment decision, you should carefully consider the risk factors
set forth in this Pro Supp, the accompanying prospectus and the information incorporated by reference herein and therein, including under
the caption “Risk Factors” in our most recent annual report on Form 10-K, as well as in any applicable Pro Supp, as updated
by our subsequent filings under the Exchange Act.
These risks could materially
affect our business, results of operation or financial condition and affect the value of our securities. Additional risks and uncertainties
that are not yet identified may also materially harm our business, operating results and financial condition and could result in a complete
loss of your investment. You could lose all or part of your investment. For more information, see “Where You Can Find More Information.”
+
The Chinese government may intervene with
or influence our business at any time. That may negatively influence our operation, our ability to continue listing on U.S. exchange and
the value of our shares may significantly decline or be worthless, which would materially affect the interest of our stockholders.
The Chinese central or local
governments may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures
and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future,
including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional
or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular
regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
As such, our business segments
may be subject to various government and regulatory interference in the provinces in which they operate. The Company could be subject
to regulation by various political and regulatory entities, including various local and municipal agencies and government sub-divisions.
The Company may incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure
to comply. The Chinese government may intervene with or influence our operations at any time with little advance notice, which could result
in a material change in our operations and in the value of our shares.
Future sales or
the potential for future sales of our securities may cause the trading price of our Common Stock to decline and could impair our ability
to raise capital through subsequent equity offerings.
Sales
of a substantial number of our Common Stock or other securities in the public markets, or the perception that these sales may occur, could
cause the market price of our Common Stock or other securities to decline and could materially impair our ability to raise capital through
the sale of additional securities.
We plan to sell
shares of our Common Stock in “at the market offerings” and the Common Stock the investor buys will likely pay different prices.
The
Common Stock the Investor to purchase at different times through this offering will likely have different prices and may experience different
outcomes in their investment results. Investors may experience a decline in the value of their shares of our Common Stock. The trading
price of our Common Stock has been volatile and subject to wide fluctuations. Many factors could have an impact on the market price of
our Common Stock, including the factors described above and in the accompanying prospectus and those incorporated by reference herein
and therein.
We cannot predict
the actual number of shares, if any, of our Common Stock that we will sell under the SPA, or the gross proceeds resulting from those sales.
The
number of shares of our Common Stock to be sold to the Investor through the SPA will fluctuate based on a number of factors, including
the market price of our Common Stock during the sales period, and the demand for our Common Stock during the sales period. Because the
price per share of each share sold will fluctuate during the sales period, it is not possible to predict the number of shares that will
be sold or the gross proceeds we will raise in connection with those sales.
In
addition, the Purchase Price in this offering is subject to repricing adjustments as contemplated under the SPA as follows. In the event
the Company’s delivery of the shares is not confirmed by 1:00 pm E.T. on the trading day the purchase notice is submitted, the Investor
has the right to adjust the purchase price to match the at-the-market price on the date of the delivery of the purchase notice, which
is only permitted if the market price on the delivery day is lower than the previously established price. Further, the Investor, has the
right, in its sole discretion, to return to the Company any or all the shares issued under the SPA within one business day following the
initial receipt of such shares and prior to the payment of the purchase price to the Company if, based on price discovery or market conditions,
the Investor determines that the issuance of such shares is unfavorable.
USE OF PROCEEDS
We may issue and sell shares
of Common Stock having aggregate sales proceeds of up to $600,000 from time to time. The amount of proceeds from this offering will depend
upon the number of shares of our Common Stock sold and the market price at which they are sold. Because there is no minimum offering amount
required as a condition of this offering, the actual total public offering amount, and proceeds to us, if any, are not determinable
at this time. There can be no assurance that we will be able to sell any shares under the SPA.
We currently intend to use
the net proceeds from this offering for general working capital purposes.
PLAN OF DISTRIBUTION
Pursuant to the SPA, we may issue and sell from time to time shares
during the commitment period from December 12, 2024, the effective date of the SPA through December 31, 2024, of our Common Stock having
an aggregate gross sales price of up to $600,000 to the Investor, subject to certain limitations, pursuant to this Pro Supp and the accompanying
prospectus. The SPA will be filed with the SEC and is incorporated by reference into this Pro Supp. This is a brief summary of the anticipated
material terms of the SPA and does not purport to be a complete statement of its terms and conditions.
Under the terms of the SPA,
in no event will the Company issue or sell to the Investor dollar amount of shares of Common Stock that would (i) exceed the number or
dollar amount of shares of Common Stock registered and available on the registration statement of which this Pro Supp forms a part, (ii)
exceed the number of authorized but unissued shares of Common Stock, (iii) exceed the number or dollar amount of shares of Common Stock
permitted to be sold under Form S-3 (including General Instruction I.B.6 thereof, if applicable), or (iv) exceed the number or dollar
amount of Common Stock for this Pro Supp which forms a part of the registration statemen.
The Investor may acquire our
Common Stock through one or more closings upon our receipt of purchase notices. The number of our Common Stock will be determined based
on the at-the-market price, but in no event shall the per share price be lower than $0.15, which is to be stated in the purchase notice
subject to repricing adjustments as contemplated under the SPA as follows. In the event the Company’s delivery of the shares is
not confirmed by 1:00 pm E.T. on the trading day the purchase notice is submitted, the Investor has the right to adjust the purchase price
to match the at-the-market price on the date of the delivery of the purchase notice, which is only permitted if the market price on the
delivery day is lower than the previously established price. Further, the Investor, has the right, in its sole discretion, to return to
the Company any or all the shares issued under the SPA within one business day following the initial receipt of such shares and prior
to the payment of the purchase price to the Company if, based on price discovery or market conditions, the Investor determines that the
issuance of such shares is unfavorable.
Additionally, we also agreed to provide “most favored nation”
treatment (the “MFN”) to the Investor should the Company enter into any financing, transaction, settlement, or similar agreement
with more favorable terms within thirty (30) days after the effective date of this SPA. In the event any of the forgoing events occurs
during the term above referenced, such more favorable terms shall be retroactively applied to all closed purchases and any future purchases
under the SPA (the “MFN Adjustments”) as if the more favorable terms had been in effect prior to each closing. MFN Adjustments
may include but are not limited to, at-the-market price discounts, the inclusion of warrants, or anti-dilution/true-up provisions. The
difference in value from the MFN adjustments shall be issued to the Investor as a convertible note.
Sales of our Common Stock by the Company as contemplated in this Pro
Supp shall be delivered according to the DWAC (Deposit/Withdrawal at Custodian) instructions specified in the purchase notice.
The actual proceeds to us
will vary depending on the number of shares sold and the prices of such sales. Because there is no minimum offering amount required as
a condition to close this offering, the actual total offering amount, and proceeds to us, if any, are not determinable at
this time.
The offering of our Common
Stock pursuant to the SPA will terminate on the earlier of (i) the date on which the Investor has purchased our Common Stock in a value
equal to $600,000, (ii) the date on which the Registration Statement is no longer effective, or (iii) December 31, 2024, unless extended
or terminated earlier in accordance with the terms of the SPA.
Our Common Stock is traded
on the NYSE American under the symbol “CPHI”.
The foregoing does not purport
to be a complete statement of the terms and conditions of the SPA. A copy of the SPA is included as an exhibit to our Current Report on
Form 8-K that will be filed with the SEC and incorporated by reference into the registration statement of which this Pro Supp and the
accompanying base prospectus form a part.
LEGAL MATTERS
The validity of the issuance
of the securities offered hereby will be passed upon for us by FLANGAS LAW GROUP. Any underwriters will also be advised about the validity
of the securities and other legal matters by their own counsel, which will be named in the Pro Supp.
EXPERTS
The consolidated balance sheets
of China Pharma Holdings, Inc. and the subsidiaries as of December 31, 2023 and 2022, the related consolidated statements of operations
and comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2023, and
the related notes (collectively referred to as the “financial statements”), incorporated in this prospectus by reference to
our Annual Report on Form 10-K for the year ended December 31, 2023, have been so incorporated in reliance upon the report of BF Borgers
CPA, P.C., an independent registered public accounting firm and the Company’s previous auditor, given on the authority of such firm
as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of
a registration statement that we have filed with the SEC. Certain information in the registration statement has been omitted from this
prospectus in accordance with the rules of the SEC. We are subject to the information requirements of the Securities Exchange Act
of 1934, as amended, or the Exchange Act, and, in accordance therewith, file annual, quarterly and current reports, proxy statements
and other information with the SEC. Such annual, quarterly and current reports, proxy statements and other information are available at
the website of the SEC at https://www.sec.gov.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The following documents filed
by us with the Securities and Exchange Commission are incorporated by reference in this prospectus:
| |
● |
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed on April 1, 2024; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2024, filed on November 13, 2024; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2024, filed on August 14, 2024; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2024, filed on May 15, 2024; |
| |
● |
Definitive Proxy Statement on Schedule 14A, filed with the SEC on November 12, 2024; |
| |
● |
The description of China Pharma’s securities contained in Exhibit 4.2 of the Company’s Annual Report on Form 10-K filed on March 30, 2022; and |
| |
● |
All of our filings made with the Securities and Exchange Commission pursuant to the Exchange Act after the date of the initial registration statement of which this prospectus forms a part and prior to the effectiveness of the registration statement of which this prospectus forms a part. |
All documents subsequently
filed with the Securities and Exchange Commission by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the
date of this prospectus and prior to the filing of a post-effective amendment which indicates that all securities offered herein have
been sold or which deregisters all securities then remaining unsold, shall be deemed to be incorporated by reference herein and to be
part of this prospectus from the respective dates of filing of such documents. Any statement contained herein or in a document incorporated
or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes hereof or of the related Pro
Supp to the extent that a statement contained herein or in any other subsequently filed document which is also incorporated or deemed
to be incorporated herein modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except
as so modified or superseded, to constitute a part of this prospectus.
We will provide to each
person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated
by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings by written or oral request,
excluding the exhibits to such filings which we have not specifically incorporated by reference in such filings, at no cost, by writing
us at China Pharma Holdings, Inc., 2nd Floor, No. 17, Jinpan Road, Haikou, Hainan Province, China, or calling us at 86-10-898-66811730.
This prospectus is part of
a registration statement we filed with the Securities and Exchange Commission. You should only rely on the information or representations
contained in this prospectus and any accompanying Pro Supp. We have not authorized anyone to provide information other than that provided
in this prospectus and any accompanying Pro Supp. We are not making an offer of these securities in any state where the offer is not permitted.
You should not assume that the information in this prospectus or any accompanying Pro Supp is accurate as of any date other than the date
on the front of the document.

CHINA PHARMA HOLDINGS,
INC.
Up to $600,000 of Shares
of Common Stock
PROSPECTUS SUPPLEMENT
December 12, 2024
Prospectus

CHINA PHARMA HOLDINGS, INC.
$50,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
China Pharma Holdings, Inc. is a Nevada incorporated
holding company (“China Pharma”, the “Company”, “we”, “our” or “us”) with
all of its operations conducted in China through its subsidiaries. Through this prospectus, we may offer and sell, from time to time in
one or more offerings, any combination of common stock, preferred stock, debt securities (which may be convertible into or exchangeable
for common stock), warrants, rights or units that include any of these securities up to an aggregate offering price not exceeding $50,000,000.
When we decide to sell a particular class or series of securities, we will provide specific terms of the offered securities in a prospectus
supplement. The prospectus supplement may also add, update or change information contained in or incorporated by reference into this prospectus.
You should read both this prospectus and the accompanying prospectus supplement together with the additional information described under
the heading “Where You Can Find More Information” in this prospectus.
We may offer and sell these securities directly
to investors, through agents designated from time to time or to or through underwriters or dealers, including on a continuous or delayed
basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution”
in this prospectus. If any agents or underwriters are involved in the sale of any securities with respect to which this prospectus is
being delivered, the names of such agents or underwriters and any applicable fees, commissions, discounts or over-allotment options will
be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such
sale will also be set forth in a prospectus supplement.
Our common stock is traded on the NYSE American
(formerly known as AMEX) under the symbol “CPHI”. The closing price for our common stock on NYSE American on February 5,
2024 was $0.09 per share. Each prospectus supplement will contain information, where applicable, as to any listing on the NYSE American
or any other securities exchange of the securities covered by the prospectus supplement.
INVESTING IN OUR SECURITIES INVOLVES SUBSTANTIAL
RISKS. CHINA PHARMA IS NOT A CHINESE OPERATING COMPANY, BUT A HOLDING COMPANY INCORPORATED IN NEVADA. AS A HOLDING COMPANY WITH NO MATERIAL
OPERATIONS OF ITS OWN, THE COMPANY CONDUCTS A SUBSTANTIAL MAJORITY OF ITS OPERATIONS THROUGH THE OPERATING ENTITY ESTABLISHED IN THE PEOPLE’S
REPUBLIC OF CHINA (THE “PRC”), PRIMARILY THE COMPANY’S WHOLLY OWNED PRC SUBSIDIARY.
ADDITIONALLY, HELPSON, CHINA PHARMA’S
PRC SUBSIDIARY, IS SUBJECT TO CERTAIN LEGAL AND OPERATIONAL RISKS ASSOCIATED WITH ITS OPERATIONS IN CHINA. PRC LAWS AND REGULATIONS GOVERNING
HELPSON’S CURRENT BUSINESS OPERATIONS ARE SOMETIMES VAGUE AND UNCERTAIN, AND THEREFORE, THESE RISKS MAY RESULT IN A MATERIAL CHANGE
IN HELPSON’S OPERATIONS, SIGNIFICANT DEPRECIATION OF THE VALUE OF OUR COMMON STOCK, OR A COMPLETE HINDRANCE OF THE COMPANY’S
ABILITY TO OFFER OR CONTINUE TO OFFER ITS SECURITIES TO INVESTORS. RECENTLY, THE PRC GOVERNMENT INITIATED A SERIES OF REGULATORY ACTIONS
AND STATEMENTS TO REGULATE BUSINESS OPERATIONS IN CHINA WITH LITTLE ADVANCE NOTICE, INCLUDING CRACKING DOWN ON ILLEGAL ACTIVITIES IN THE
SECURITIES MARKET, ADOPTING NEW MEASURES TO EXTEND THE SCOPE OF CYBERSECURITY REVIEWS, AND EXPANDING THE EFFORTS IN ANTI-MONOPOLY ENFORCEMENT.
SINCE THESE STATEMENTS AND REGULATORY ACTIONS ARE NEW, IT IS HIGHLY UNCERTAIN HOW SOON LEGISLATIVE OR ADMINISTRATIVE REGULATION MAKING
BODIES WILL RESPOND AND WHAT EXISTING OR NEW LAWS OR REGULATIONS OR DETAILED IMPLEMENTATIONS AND INTERPRETATIONS WILL BE MODIFIED OR PROMULGATED,
IF ANY, AND THE POTENTIAL IMPACT OF SUCH MODIFIED OR NEW LAWS AND REGULATIONS WILL HAVE ON THE COMPANY’S DAILY BUSINESS OPERATION,
THE ABILITY TO ACCEPT FOREIGN INVESTMENTS AND LIST ON AN U.S. OR OTHER FOREIGN EXCHANGE.
ON FEBRUARY 17, 2023, THE CHINA SECURITIES
REGULATORY COMMISSION (THE “CSRC”), PROMULGATED THE TRIAL ADMINISTRATIVE MEASURES OF OVERSEAS SECURITIES OFFERING AND LISTING
BY DOMESTIC COMPANIES (THE “TRIAL MEASURES”), WHICH BECAME EFFECTIVE ON MARCH 31, 2023. ON THE SAME DATE, THE CSRC CIRCULATED
SUPPORTING GUIDANCE RULES NO.1 THROUGH NO.5, NOTES ON THE TRIAL MEASURES, NOTICE ON ADMINISTRATION ARRANGEMENTS FOR THE FILING OF OVERSEAS
LISTINGS BY DOMESTIC ENTERPRISES AND RELEVEANT CSRC ANSWERS TO REPORT OR QUESTIONS (COLLECTIVELY, THE “GUIDANCE RULES AND NOTICE”),
ON CSRC’S OFFICIAL WEBSITE. THE TRIAL MEAURES, TOGETHER WITH THE GUIDANCE RULES AND NOTICE REITERATE THE BASIC PRINCIPLES OF DRAFT
ADMINSTRATIVE PROVISIONS AND DRAFT FILING MEASURES AND IMPOSE SUBSTANTIALLY THE SAME REQUIREMENTS FOR THE OVERSEAS SECURITIES OFFERING
AND LISTING BY DOMESTIC ENTERPRISES, AND CLARIFIED AND EMPHASIZED SEVERAL ASPECTS. BECAUSE WE ARE ALREADY PUBLICLY LISTED IN THE U.S.,
THE TRIAL MEASURES AND THE GUIDANCE RULES AND NOTICE DO NOT IMPOSE OBVIOUS ADDITIONAL REGULATORY BURDEN ON US BEYOND THE OBLIGATION TO
REPORT TO THE CSRC AND COMPLY WITH THE FILING REQUIREMENTS ON ANY FUTURE OFFERINGS OF OUR SECURITIES, OR MATERIAL EVENTS SUCH AS A CHANGE
OF CONTROL OR DELISTING. AS THE TRIAL MEASURES AND THE GUIDANCE RULES AND NOTICE ARE NEWLY ISSUED, THERE REMAINS UNCERTAINTY AS TO HOW
THEY WILL BE INTERPRETED OR IMPLEMENTED. THEREFORE, WE ARE SUBJECT TO SUCH FILING REQUIREMENTS UNDER THE TRIAL MEASURES UPON FUTURE SUBSEQUENT
OFFERINGS, AND MAY BE SUBJECT TO ADDITIONAL FILING REQUIREMENTS AND, IF THERE ARE ANY CHANGES TO THE TRIAL MEASURES, AT THAT TIME WE
MAY NOT BE ABLE TO GET CLEARANCE FROM THE CSRC IN A TIMELY FASHION.
ON MAY 20, 2020, THE U.S. SENATE PASSED THE
HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT REQUIRING FOREIGN COMPANIES TO CERTIFY THEY ARE NOT OWNED OR CONTROLLED BY A FOREIGN GOVERNMENT
IF THE PCAOB IS UNABLE TO AUDIT SPECIFIED REPORTS BECAUSE THE COMPANY USES A FOREIGN AUDITOR NOT SUBJECT TO PCAOB INSPECTION. IF THE PCAOB
IS UNABLE TO INSPECT THE COMPANY’S AUDITORS FOR THREE CONSECUTIVE YEARS, THE ISSUER’S SECURITIES ARE PROHIBITED FROM TRADING
ON A U.S. STOCK EXCHANGE. ON DECEMBER 2, 2020, THE U.S. HOUSE OF REPRESENTATIVES APPROVED THE HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT.
ON DECEMBER 18, 2020, THE HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT WAS SIGNED INTO LAW. PURSUANT TO THE HOLDING FOREIGN COMPANIES ACCOUNTABLE
ACT, THE PCAOB ISSUED A DETERMINATION REPORT ON DECEMBER 16, 2021 WHICH FOUND THAT THE PCAOB IS UNABLE TO INSPECT OR INVESTIGATE COMPLETELY
REGISTERED PUBLIC ACCOUNTING FIRMS HEADQUARTERED IN: (1) MAINLAND CHINA OF THE PRC BECAUSE OF A POSITION TAKEN BY ONE OR MORE AUTHORITIES
IN MAINLAND CHINA; AND (2) HONG KONG, A SPECIAL ADMINISTRATIVE REGION AND DEPENDENCY OF THE PRC, BECAUSE OF A POSITION TAKEN BY ONE OR
MORE AUTHORITIES IN HONG KONG. ON AUGUST 26, 2022, THE PCAOB ANNOUNCED AND SIGNED A STATEMENT OF PROTOCOL (THE “PROTOCOL”)
WITH THE CHINA SECURITIES REGULATORY COMMISSION AND THE MINISTRY OF FINANCE OF THE PEOPLE’S REPUBLIC OF CHINA. THE PROTOCOL PROVIDES
THE PCAOB WITH: (1) SOLE DISCRETION TO SELECT THE FIRMS, AUDIT ENGAGEMENTS AND POTENTIAL VIOLATIONS IT INSPECTS AND INVESTIGATES, WITHOUT
ANY INVOLVEMENT OF CHINESE AUTHORITIES; (2) PROCEDURES FOR PCAOB INSPECTORS AND INVESTIGATORS TO VIEW COMPLETE AUDIT WORK PAPERS WITH
ALL INFORMATION INCLUDED AND FOR THE PCAOB TO RETAIN INFORMATION AS NEEDED; (3) DIRECT ACCESS TO INTERVIEW AND TAKE TESTIMONY FROM ALL
PERSONNEL ASSOCIATED WITH THE AUDITS THE PCAOB INSPECTS OR INVESTIGATES. OUR AUDITOR IS HEADQUARTERED IN SINGAPORE, SINGAPORE AND WILL
BE INSPECTED BY THE PCAOB ON A REGULAR BASIS.
OUR AUDITOR IS NOT SUBJECT TO THE DETERMINATION.
OUR AUDITOR IS SUBJECT TO LAWS IN THE UNITED STATES PURSUANT TO WHICH THE PCAOB CONDUCTS REGULAR INSPECTIONS TO ASSESS OUR AUDITOR’S
COMPLIANCE WITH THE APPLICABLE PROFESSIONAL STANDARDS. ON JUNE 22, 2021, THE U.S. SENATE PASSED THE ACCELERATING HOLDING FOREIGN COMPANIES
ACCOUNTABLE ACT (“AHFCAA”) WHICH, PROPOSED TO REDUCE THE NUMBER OF CONSECUTIVE NON-INSPECTION YEARS REQUIRED FOR TRIGGERING
THE PROHIBITIONS UNDER THE HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT FROM THREE YEARS TO TWO. ON DECEMBER 29, 2022, THE CONSOLIDATED APPROPRIATIONS
ACT, 2023 (THE “CAA”) WAS SIGNED INTO LAW, WHICH OFFICIALLY REDUCED THE NUMBER OF CONSECUTIVE NON-INSPECTION YEARS REQUIRED
FOR TRIGGERING THE PROHIBITIONS UNDER THE HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT FROM THREE YEARS TO TWO, THUS, REDUCING THE TIME BEFORE
AN APPLICABLE ISSUER’S SECURITIES MAY BE PROHIBITED FROM TRADING OR DELISTED. CURRENTLY, OUR AUDITOR IS SUBJECT TO INSPECTION BY
PCAOB. HOWEVER, IF AHFCAA WERE ENACTED INTO LAW, IT MAY POSE MORE RISKS OF POTENTIAL DELISTING AS WELL AS DEPRESS THE PRICE OF COMPANY’S
COMMON STOCK. ON DECEMBER 15, 2022, THE PCAOB ISSUED A NEW DETERMINATION REPORT WHICH CONCLUDED THAT IT WAS ABLE TO INSPECT AND INVESTIGATE
COMPLETELY PCAOB-REGISTERED ACCOUNTING FIRMS HEADQUARTERED IN MAINLAND CHINA AND HONG KONG IN 2022, AND THE PCAOB VACATED THE DECEMBER
16, 2021 DETERMINATION REPORT. SHOULD THE PCAOB AGAIN ENCOUNTER IMPEDIMENTS TO INSPECTIONS AND INVESTIGATIONS IN MAINLAND CHINA OR HONG
KONG AS A RESULT OF POSITIONS TAKEN BY ANY AUTHORITY IN EITHER JURISDICTION, INCLUDING BY THE CSRC OR THE MOF, THE PCAOB WILL MAKE DETERMINATIONS
UNDER THE HFCAA AS AND WHEN APPROPRIATE. HOWEVER, WHETHER THE PCAOB WILL CONTINUE TO CONDUCT INSPECTIONS AND INVESTIGATIONS COMPLETELY
TO ITS SATISFACTION OF PCAOB-REGISTERED PUBLIC ACCOUNTING FIRMS HEADQUARTERED IN MAINLAND CHINA AND HONG KONG IS SUBJECT TO UNCERTAINTY
AND DEPENDS ON A NUMBER OF FACTORS OUT OF CHINA PHARMA’S, AND CHINA PHARMA’S AUDITOR’S, CONTROL, INCLUDING POSITIONS
TAKEN BY AUTHORITIES OF THE PRC. THE PCAOB IS EXPECTED TO CONTINUE TO DEMAND COMPLETE ACCESS TO INSPECTIONS AND INVESTIGATIONS AGAINST
ACCOUNTING FIRMS HEADQUARTERED IN MAINLAND CHINA AND HONG KONG IN THE FUTURE AND STATES THAT IT HAS ALREADY MADE PLANS TO RESUME REGULAR
INSPECTIONS IN EARLY 2023 AND BEYOND. THE PCAOB IS REQUIRED UNDER THE HOLDING FOREIGN COMPANIES ACCOUNTABLE ACT TO MAKE ITS DETERMINATION
ON AN ANNUAL BASIS WITH REGARDS TO ITS ABILITY TO INSPECT AND INVESTIGATE COMPLETELY ACCOUNTING FIRMS BASED IN THE MAINLAND CHINA AND
HONG KONG. SHOULD THE PCAOB AGAIN ENCOUNTER IMPEDIMENTS TO INSPECTIONS AND INVESTIGATIONS IN MAINLAND CHINA OR HONG KONG AS A RESULT OF
POSITIONS TAKEN BY ANY FOREIGN AUTHORITY INCLUDING BUT IS NOT LIMITED TO MAINLAND CHINA OR HONG KONG JURISDICTION, THE PCAOB WILL ACT
EXPEDITIOUSLY TO CONSIDER WHETHER IT SHOULD ISSUE A NEW DETERMINATION.
THE CHINESE REGULATORY AUTHORITIES COULD DISALLOW
THE COMPANY’S STRUCTURE, WHICH COULD RESULT IN A MATERIAL CHANGE IN THE COMPANY’S OPERATIONS AND THE VALUE OF THE COMPANY’S
SECURITIES COULD DECLINE OR BECOME WORTHLESS. FOR A DESCRIPTION OF THE COMPANY’S CORPORATE STRUCTURE, SEE “PROSPECTUS SUMMARY”
STARTING PAGE 1. SEE ALSO “RISK FACTORS - RISKS RELATED TO DOING BUSINESS IN CHINA” INCORPORATED BY REFERENCE INTO THIS PROSPECTUS.
SEE THE SECTION TITLED “RISK FACTORS”
BEGINNING ON PAGE 4 OF THIS PROSPECTUS, AND THE RISK FACTORS IN ANY ACCOMPANYING PROSPECTUS SUPPLEMENT TO READ ABOUT FACTORS YOU SHOULD
CONSIDER BEFORE BUYING SHARES OF CHINA PHARMA’S COMMON STOCK.
OUR SUBSIDIARIES HAVE NEVER ISSUED ANY DIVIDENDS
OR DISTRIBUTIONS TO US, OR TO ANY INVESTORS AS OF THE DATE OF THIS PROSPECTUS. HELPSON, OUR PRC SUBSIDIARY, GENERATES AND RETAINS CASH
GENERATED FROM OPERATING ACTIVITIES AND RE-INVEST IT IN DAILY BUSINESS. IN THE FUTURE, CASH PROCEEDS RAISED FROM OVERSEAS FINANCING ACTIVITIES
AND THE EXERCISE OF WARRANTS BY WARRANT HOLDERS, MAY BE TRANSFERRED BY US TO HELPSON VIA CAPITAL CONTRIBUTION AND SHAREHOLDER LOANS, AS
THE CASE MAY BE.
THE MAJORITY OF OUR INCOME IS RECEIVED IN RENMINBI
(“RMB”) AND RESTRICTIONS IN FOREIGN CURRENCIES MAY LIMIT THE COMPANY’S ABILITY TO PAY DIVIDENDS OR OTHER PAYMENTS, OR
OTHERWISE SATISFY THE COMPANY’S FOREIGN CURRENCY DENOMINATED OBLIGATIONS, IF ANY. UNDER EXISTING PRC FOREIGN EXCHANGE REGULATIONS,
PAYMENTS OF CURRENT ACCOUNT ITEMS, INCLUDING PROFIT DISTRIBUTIONS, INTEREST PAYMENTS AND EXPENDITURES FROM TRADE-RELATED TRANSACTIONS,
CAN BE MADE IN FOREIGN CURRENCIES WITHOUT PRIOR APPROVAL FROM THE STATE ADMINISTRATION OF THE FOREIGN EXCHANGE (“SAFE”) IN
THE PRC AS LONG AS CERTAIN PROCEDURAL REQUIREMENTS ARE MET. APPROVAL FROM APPROPRIATE GOVERNMENT AUTHORITIES IS REQUIRED IF RMB IS CONVERTED
INTO FOREIGN CURRENCY AND REMITTED OUT OF THE PRC TO PAY CAPITAL EXPENSES SUCH AS THE REPAYMENT OF LOANS DENOMINATED IN FOREIGN CURRENCIES.
THE PRC GOVERNMENT MAY, AT ITS DISCRETION, IMPOSE RESTRICTIONS ON ACCESS TO FOREIGN CURRENCIES FOR CURRENT ACCOUNT TRANSACTIONS AND IF
THIS OCCURS IN THE FUTURE, WE MAY NOT BE ABLE TO PAY DIVIDENDS IN FOREIGN CURRENCIES TO OUR STOCKHOLDERS.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION
NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES, OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS.
ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is February 6,
2024
Table
of Contents
You should rely only on the information contained
or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with information
different from that contained or incorporated by reference into this prospectus. If any person does provide you with information that
differs from what is contained or incorporated by reference in this prospectus, you should not rely on it. No dealer, salesperson or other
person is authorized to give any information or to represent anything not contained in this prospectus. You should assume that the information
contained in this prospectus or any prospectus supplement is accurate only as of the date on the front of the document and that any information
contained in any document we have incorporated by reference is accurate only as of the date of the document incorporated by reference,
regardless of the time of delivery of this prospectus or any prospectus supplement or any sale of a security. These documents are not
an offer to sell or a solicitation of an offer to buy these securities in any circumstances under which the offer or solicitation is unlawful.
ABOUT THIS PROSPECTUS
This prospectus is part of
a Registration Statement on Form S-3 that we filed with the Securities and Exchange Commission utilizing a “shelf” registration
process. Under this shelf process, we may sell common stock, preferred stock, debt securities (which may be convertible into or exchangeable
for common stock), warrants, rights or units from time to time in one or more offerings at indeterminate prices, up to an aggregate offering
price for all such securities of $50,000,000. This prospectus provides you with a general description of the securities we may offer.
Each time we sell any securities under this prospectus, we will provide a prospectus supplement that will contain specific information
about the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus. You
should read both this prospectus and any prospectus supplement together with additional information described below under the heading
“Where You Can Find More Information.”
You should rely only on the
information contained in or incorporated by reference in this prospectus, any accompanying prospectus supplement or in any related free
writing prospectus filed by us with the SEC. China Pharma has not authorized anyone to provide you with different information. This prospectus
and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other
than the securities described in the accompanying prospectus supplement or an offer to sell or the solicitation of an offer to buy such
securities in any circumstances in which such offer or solicitation is unlawful. Persons who come into possession of this prospectus in
jurisdictions outside the United States are required to inform themselves about, and to observe, any restrictions as to the offering and
the distribution of this prospectus applicable to those jurisdictions. You should assume that the information appearing in this prospectus,
any prospectus supplement, the documents incorporated by reference and any related free writing prospectus is accurate only as of their
respective dates, and our business, financial condition, results of operations and prospects may have changed since such date. Other than
as required under the federal securities laws, we undertake no obligation to publicly update or revise such information, whether as a
result of new information, future events or any other reason.
This prospectus and any accompanying
prospectus supplement or other offering materials do not contain all of the information included in the registration statement as permitted
by the rules and regulations of the SEC. For further information, we refer you to the registration statement on Form S-3, including its
exhibits. We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
and, therefore, file reports and other information with the SEC. Statements contained in this prospectus and any accompanying prospectus
supplement, or other offering materials about the provisions or contents of any agreement or other document are only summaries. If SEC
rules require that any agreement or document be filed as an exhibit to the registration statement, you should refer to that agreement
or document for its complete contents.
You should not assume that
the information in this prospectus, any prospectus supplement or any other offering materials is accurate as of any date other than the
date on the front of each document. Our business, financial condition, results of operations and prospects may have changed since then.
Unless otherwise stated in
this prospectus, references to:
| |
● |
“China” or the “PRC” refers to the People’s Republic of China, excluding, for the purposes of this prospectus only, Hong Kong, Macau and Taiwan; |
| |
|
|
| |
● |
“Helpson” refers to Hainan Helpson Medical & Biotechnology Co., Ltd. |
| |
|
|
| |
● |
“Onny” refers to Onny Investment Ltd. |
| |
|
|
| |
● |
“RMB” and “Renminbi” refer to the legal currency of China; and |
| |
|
|
| |
● |
“US$,” “U.S. dollars,” “$,” and “dollars” refer to the legal currency of the United States. |
We use RMB as the functional
currency and U.S. dollars as reporting currency in our financial statements and in this prospectus. Monetary assets and liabilities denominated
in Renminbi are translated into U.S. dollars at the rates of exchange as of the balance sheet date, equity accounts are translated at
historical exchange rates, and revenues, expenses, gains and losses are translated using the average rate for the period. In other parts
of this prospectus, any Renminbi denominated amounts are accompanied by translations. We make no representation that the Renminbi or U.S.
dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or Renminbi, as the case may be,
at any particular rate or at all. The PRC government restricts or prohibits the conversion of Renminbi into foreign currency and foreign
currency into Renminbi for certain types of transactions.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in
this Form S-3 that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act, and
Section 21E of the Exchange Act. These include statements about the Company’s expectations, beliefs, intentions or strategies for
the future, which are indicated by words or phrases such as “anticipate”, “expect”, “intend”, “plan”,
“will”, “the Company believes”, “management believes” and similar words or phrases. The forward-looking
statements are based on the Company’s current expectations and are subject to certain risks, uncertainties and assumptions. The
Company’s actual results could differ materially from results anticipated in these forward-looking statements. All forward-looking
statements included in this document are based on information available to the Company on the date hereof, and the Company assumes no
obligation to update any such forward-looking statements.
These forward-looking statements
are neither promises nor guarantees of future performance, due to a variety of risks and uncertainties and other factors more fully discussed
in the “Risk Factors” section in this prospectus, the section of any accompanying prospectus supplement entitled “Risk
Factors” and the risk factors and cautionary statements described in other documents that China Pharma files from time to time with
the SEC. Given these uncertainties, readers should not place undue reliance on China Pharma’s forward-looking statements. These
forward-looking statements speak only as of the date on which the statements were made and are not guarantees of future performance. Except
as may be required by applicable law, China Pharma does not undertake to update any forward-looking statements after the date of this
prospectus or the respective dates of documents incorporated by reference herein or therein that include forward-looking statements.
Except as required by law,
China Pharma assumes no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements to
reflect events or developments occurring after the date of this prospectus, even if new information becomes available in the future.
PROSPECTUS
SUMMARY
Organization and Nature of Operations
China Pharma, a Nevada corporation,
owns 100% of Onny, a British Virgin Islands corporation, which owns 100% of Helpson, a company organized under the laws of the People’s
Republic of China (the “PRC”).
Onny acquired 100% of the
ownership in Helpson on May 25, 2005, by entering into an Equity Transfer Agreement with Helpson’s three former shareholders. The
transaction was approved by the Commercial Bureau of Hainan Province on June 12, 2005 and Helpson received the Certificate of Approval
for Establishment of Enterprises with Foreign Investment in the PRC on the same day. Helpson received its business license evidencing
its WFOE (Wholly Foreign Owned Enterprise) status on June 21, 2005.
Our corporate organizational
chart is set forth below:

Helpson has acquired and continues
to acquire well-accepted medical formulas to add to its diverse portfolio of Western and Chinese medicines.
Business Overview & Recent Developments
Helpson is principally engaged in the development, manufacture and
marketing of pharmaceutical products for human use in connection with a variety of high-incidence and high-mortality diseases and medical
conditions prevalent in the PRC. As a Nevada holding company without any operations, all of China Pharma’s operations are conducted
in the PRC, where the manufacturing facilities are located, through Helpson, China Pharma’s indirectly wholly owned PRC subsidiary.
Helpson manufactures pharmaceutical products in the form of dry powder injectables, liquid injectables, tablets, capsules, and cephalosporin
oral solutions. The majority of our pharmaceutical products are sold on a prescription basis and all have been approved for at least one
or more therapeutic indications by the National Medical Products Administration (the “NMPA”, formerly China Food and Drug
Administration, CFDA) based upon demonstrated safety and efficacy.
As
of the date of this prospectus, Helpson manufactured 19 pharmaceutical products for a wide variety of diseases and medical indications,
each of which may be classified into one of three general categories:
| |
● |
Basic generic drugs, which are common drugs in the PRC for which there is a very large market demand; |
| |
● |
First-to-market generic drugs, which are generic western drugs that are new to the PRC marketplace; or |
| |
● |
Modern Traditional Chinese Medicines, which are generally comprised of non-synthetic, plant-based medicinal compounds that have been widely used in the PRC for thousands of years. Helpson applies modern production techniques to produce pharmaceutical products in different formulations, such as tablets, capsules or powders. |
In
selecting generic drugs to develop and manufacture, Helpson considers several factors, including the number of other manufacturers currently
producing this particular drug, the size of the market for that drug, the proposed or required method of distribution, the existing and
expected pricing for that particular drug in the marketplace, the costs of manufacturing that drug, and the costs of acquiring or developing
the formula for that drug. Helpson believes that generic drugs that it has selected to manufacture have large addressable markets and
higher profit margins relative to other generic drugs manufactured and distributed in the PRC.
In
addition, Helpson manufactured comprehensive healthcare products and protective products in two production facilities it owns and operates
in Haikou, Hainan Province, PRC. One has a construction area of 663.94 square meters, the other factory has two buildings with production
area of 20,282.42 square meters and 6,593.20 square meters.
China’s consistency
evaluation of generic drugs continued to proceed in 2023. Helpson has always taken the task of promoting the consistency evaluation as
a top priority, and worked on them actively. However, for each drug’s consistency evaluation, due to the continuous dynamic changes
of the detailed consistency evaluation policies, market trends, expected investments, and expected returns of investment (“ROI”),
all companies in this industry have been adjusting to the policies under consistency evaluation. One of the flagship products, Candesartan
tablets, a hypertension product, passed generic-drug-consistency-evaluation in early August 2023.
Helpson has taken a more cautious
and flexible attitude towards the initiating and progressing any project for an existing product’s consistency evaluation and to
cope with the changing macro environment of drug sales in China. Since initiated in 2018, when relevant Chinese authorities decided to
implement trial Centralized Procurement (“CP”) activities in 11 selected pilot cities (including four municipalities and seven
other cities), nine rounds of CP activities have been carried out as of November 6, 2023, which significantly reduced the price of
the drugs that won the bids. In addition, the consistency evaluation has been adopted as one of the qualification standards for participating
in the GPO activities. As a result, Helpson must balance at least the investment of financial resources and time to obtain the qualification
of CP, and the sharp decline in the price of drugs included in CP, before making decisions for any products.
In addition, Helpson continues
to explore the field of comprehensive healthcare. Comprehensive healthcare is a general concept proposed by the Chinese government according
to the development of the times, social needs and changes in disease spectrum. According to the Outline of “Healthy China 2030”
issued by Chinese government in October 2016, the total size of China’s health service industry is expected to reach RMB 16 trillion
(approximately $2.5 trillion) by 2030. This industry focuses on people’s daily life, aging and diseases, pays attention to all kinds
of risk factors and misunderstandings affecting health, calls for self-health management, and advocates the comprehensive care throughout
the entire process of life. It covers all kinds of health-related information, products, and services, as well as actions taken by various
organizations to meet the health needs. In response to this trend, Helpson launched Noni enzyme, a natural, Xeronine-rich antioxidant
food supplement at the end of 2018. Helpson also launched wash-free sanitizers and masks, in 2020, to address the market needs caused
by COVID-19 in China. As Chinese government officially terminated its zero-case policy, now the responsibility to protect people from
the impact of COVID-19 falls more to the citizens themselves, and masks and sanitizers have been more and more popular due to increasing
demand. Helpson has sufficient production capacity for medical masks, surgical masks, KN95 masks, and N95 masks, which meets the personal
needs for protection against the epidemic outbreak. Thanks to the green channel provided by Hainan Medical Products Administration, Helpson
received the Registration Certificate of N95 medical protective mask at the fastest speed by the end of 2022, when the infection of COVID-19
had surged in China.
Helpson plans to continue
to optimize its product structure and actively respond to the current health needs of human beings.
Market Trends
As a generic drug company,
Helpson is presented with a huge domestic market. We believe that through further upgrades and consistency evaluations, which are based
on European and American production standards, Helpson will be able to export the products to overseas markets. In China’s market,
we believe that in the future, cost management and control ability will gradually become an important factor in determining the competitiveness
of generic pharmaceutical enterprises. Although price control leads to a decline in the profitability, the CP’s winning enterprise
has a good chance of achieving price-for-volume in order to increase its market share and support its continuous innovation transformation.
Additionally, rising and advancing consumer demand in China drives increases in discretionary consumption, and with the improvement of
residents’ quality of life, the healthcare demand is also changing. We believe that there are a large number of unmet demands in comprehensive
healthcare and Internet healthcare sectors.
In addition, the Office of
the State Council issued “Pilot Plan for Marketing Authorization Holders” on May 24, 2016, allowing eligible drug research
and development institutions and scientific researchers to become Marketing Authorization Holders (“MAH”) by obtaining drug
marketing authorization and drug approval numbers from the State Council. This policy uses a management model of separating drug marketing
authorization and drug production licenses, thereby allowing MAHs to produce pharmaceuticals themselves or to consign production to other
pharmaceutical manufacturers. This policy not only transitions our production practices to meet the European and United States standards
by separating drug approval and production qualifications, thereby changing the existing model of bundling drug approval numbers to pharmaceutical
manufacturers in China, but also serves as a supplement to the ongoing consistency evaluations policy.
In general, demand for pharmaceutical
products is still experiencing steady growth in China. We believe the ongoing generic drug consistency evaluations and reform of China’s
drug production registration and review policies will have major effects on the future development of our industry and may change its
business patterns. Helpson will continue to actively adapt to the national policy guidance and further evaluate market conditions for
its existing products, and competition in the market in order to optimize its development strategy.
Intercompany activities between the holding company and our subsidiaries
As of the date of this
prospectus, none of our subsidiaries have distributed any dividends to China Pharma, nor has China Pharma distributed any dividends to
its investors. China Pharma currently has no intention to distribute earnings to its stockholders and investors. The tables below present
cash flow transfer between China Pharma and Helpson, through China Pharma’s wholly owned subsidiary Onny for the years ended December
31, 2022 and 2021. China Pharma’s management believes that there are no tax consequences for cash flow transfers between China
Pharma and Helpson through Onny.
| For the year ended December 31, 2022 |
| No. |
|
Transfer from |
|
Transfer to |
|
Approximate value ($) |
|
|
Note |
| 1 |
|
China Pharma (via Onny) |
|
Helpson |
|
|
1,300,000 |
|
|
For Helpson’s operations |
| For the year ended December 31, 2021 |
| No. |
|
Transfer from |
|
Transfer to |
|
Approximate value ($) |
|
|
Note |
| 1 |
|
China Pharma (via Onny) |
|
Helpson |
|
|
3,000,000 |
|
|
For Helpson’s operations |
| 2 |
|
Helpson (via Onny) |
|
China Pharma |
|
|
320,000 |
|
|
For the payment of the agent service fees of China Pharma |
Executive Offices
Our principal executive offices
are located at 2nd Floor, No. 17, Jinpan Road, Haikou, Hainan Province, China. Our telephone number at that address is +86-898-66811730.
RISK FACTORS
Investing in our common stock
involves risk. You should carefully consider the specific risks discussed or incorporated by reference into the applicable prospectus
supplement, together with all the other information contained in the prospectus supplement or incorporated by reference into this prospectus
and the applicable prospectus supplement. You should also consider the risks, uncertainties and assumptions discussed under the caption
“Risk Factors” included in our Annual Report on Form 10-K for the year ended December 31, 2022, and in subsequent filings,
which are incorporated by reference into this prospectus. These risk factors may be amended, supplemented or superseded from time to time
by other reports we file with the SEC in the future or by a prospectus supplement relating to a particular offering of securities. These
risks and uncertainties are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us,
or that we currently view as immaterial, may also impair our business. If any of the risks or uncertainties described in our SEC filings
or any prospectus supplement or any additional risks and uncertainties actually occur, our business, financial condition and results of
operations could be materially and adversely affected. In that case, the trading price of our securities could decline and you might lose
all or part of your investment.
Risks Related to Doing Business in China
There
are Legal and Operational Risks Associated with Having the Majority of the Company’s Operations in the PRC.
The
PRC legal system is based on written statutes. The laws, regulations and legal requirements of China are relatively new and change often,
and their interpretation and enforcement depend to a large extent on relevant government policy and involve significant uncertainties
that could limit the reliability of the legal protections available to us.
The
PRC government has broad discretion in dealing with violations of laws and regulations, including levying fines, revoking business and
other licenses and requiring actions necessary for compliance. We cannot predict the effect of the interpretation of existing or new PRC
laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation
of any current or future PRC laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required
to restructure our operations or cease to provide certain services.
In
addition, the enforcement of laws and regulations in China can change quickly with little advance notice. In 2021, the PRC government
initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including
cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting
new measures to extend the scope of cybersecurity reviews, and expanding the efforts in anti-monopoly enforcement. Since these statements
and regulatory actions are new, it is highly uncertain how soon legislative or administrative regulation making bodies will respond and
what existing or new laws or regulations or detailed implementations and interpretations will be modified or promulgated, if any, and
the potential impact such modified or new laws and regulations will have on our daily business operation, the ability to accept foreign
investments and list on an U.S. or other foreign exchange. Any action by the Chinese government to exert more oversight and control over
foreign investment in China-based companies could result in a material change in our operation, cause the value of our shares to significantly
decline or become worthless, and significantly limit, or completely hinder our ability to offer or continue to offer our shares to investors
and cause the value of such securities to significantly decline or be worthless.
We
cannot predict the effects of future developments in government policy or the PRC legal system in general. We may be required in the future
to procure additional permits, authorizations and approvals for our existing and future operations, which may not be obtainable in a timely
fashion or at all, or may involve substantial costs and unforeseen risks.
Certain PRC regulations may make it more difficult
for us to pursue growth through acquisitions.
Anti-Monopoly
Law of the People’s Republic of China promulgated by the Standing Committee of the National People’s Congress, which became
effective in 2008 and amended in 2022 (“Anti-Monopoly Law”), established additional procedures and requirements that could
make merger and acquisition activities by foreign investors more time-consuming and complex. Such regulation requires, among other things,
that State Administration for Market Regulation (“SAMR”) be notified in advance of any change-of-control transaction in which
a foreign investor acquires control of a PRC domestic enterprise or a foreign company with substantial PRC operations, if certain thresholds
under the Provisions of the State Council on the Standard for Declaration of Concentration of Business Operators, issued by the State
Council in 2008 and amended in 2018, are triggered. Moreover, the Anti-Monopoly Law requires that transactions which involve the national
security, the examination on the national security shall also be conducted according to the relevant provisions of the State. In addition,
PRC Measures for the Security Review of Foreign Investment which became effective in January 2021 require acquisitions by foreign investors
of PRC companies engaged in military-related or certain other industries that are crucial to national security be subject to security
review before consummation of any such acquisition. We may pursue potential strategic acquisitions that are complementary to our business
and operations.
Complying
with the requirements of these regulations to complete such transactions could be time-consuming, and any required approval processes,
including obtaining approval or clearance from the MOFCOM, may delay or inhibit our ability to complete such transactions, which could
affect our ability to expand our business or maintain our market share.
The Chinese government
may intervene or influence our business or operations at any time. Any such intervention or influence may negatively affect our operation
or interfere with our continued listing on a U.S. exchange, and could cause the value of our shares to significantly decline or be worthless,
which would materially affect the interest of our stockholders.
The Chinese central or
local governments may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures
and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, Chinese government actions in
the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy
or regional or local variations in the implementation of economic policies, could have a significant adverse effect on economic conditions
in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
As such, our business
segments may be subject to various government and regulatory interference in the provinces in which they operate. We could be subject
to regulation by various political and regulatory entities, including various local and municipal agencies and government sub-divisions.
We may incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to
comply. The Chinese government may intervene or influence our operations at any time with little advance notice, which could result in
a material change in our operations and in the value of our shares.
Adverse regulatory developments
in China may subject us to additional regulatory review, and additional disclosure requirements and regulatory scrutiny to be adopted
by the SEC in response to risks related to recent regulatory developments in China may impose additional compliance requirements for companies
like us with significant China-based operations, all of which could increase our compliance costs, subject us to additional disclosure
requirements. In addition, uncertainties with respect to the PRC legal system could adversely affect us.
We
conduct all of our business through Helpson, our PRC subsidiary, in China. The operations in China are governed by PRC laws and regulations.
Helpson is generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws and regulations
applicable to wholly foreign-owned enterprises. The PRC legal system is based on statutes. Prior court decisions may be cited for reference
but have limited precedential value.
The
recent regulatory developments in China, in particular with respect to restrictions on China-based companies raising capital offshore,
may lead to additional regulatory review in China over our financing and capital raising activities in the United States. In addition,
we may be subject to industry-wide regulations that may be adopted by the relevant PRC authorities, which may have the effect of limiting
our service offerings, restricting the scope of our operations in China, or causing the suspension or termination of our business operations
in China entirely, all of which will materially and adversely affect our business, financial condition and results of operations. We may
have to adjust, modify, or completely change our business operations in response to adverse regulatory changes or policy developments,
and we cannot assure you that any remedial action adopted by us can be completed in a timely, cost-efficient, or liability-free manner
or at all.
On February 17, 2023, the CSRC promulgated the Trial Administrative
Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Measures”), which took effect on March
31, 2023. On the same date, the CSRC circulated Supporting Guidance Rules No. 1 through No. 5, Notes on the Trial Measures, Notice on
Administration Arrangements for the Filing of Overseas Listings by Domestic Enterprises and relevant CSRC Answers to Reporter Questions
(collectively, the “Guidance Rules and Notice”), on CSRC’s official website. The Trial Measures, together with the Guidance
Rules and Notice, reiterate the basic principles for the overseas securities offering and listing by domestic enterprises, and clarified
and emphasized several aspects, which include but are not limited to: (1) criteria to determine whether an issuer will be required to
go through the filing procedures under the Trial Measures; (2) exemptions from immediate filing requirements for issuers including those
that have already been listed in foreign securities markets, including U.S. markets, prior to the effective date of the Trial Measures,
but these issuers shall still be subject to filing procedures if they conduct refinancing or are involved in other circumstances that
require filing with the CSRC; (3) a negative list of types of issuers banned from listing or offering overseas, such as issuers whose
affiliates have been recently convicted of bribery and corruption; (4) issuers’ compliance with web security, data security, and
other national security laws and regulations; (5) issuers’ filing and reporting obligations, such as obligation after offering or
listing overseas to file with the CSRC after it completes subsequent offerings and to report to the CSRC material events including change
of control or voluntary or forced delisting of the issuer; and (6) the CSRC’s authority to fine both issuers and their relevant
shareholders for failure to comply with the Trial Measures, including failure to comply with filing obligations or committing fraud and
misrepresentation. Specifically, pursuant to the Trial Measures, our future securities offerings in the NYSE American where we have previously
offered and listed shall also be filed with the CSRC within 3 working days after the offering is completed. The Trial Measures provide
the CSRC with power to warn, fine, and issue injunctions against both PRC domestic companies, their controlling shareholders, and their
advisors in listing or offering securities (collectively, the “Subject Entities”), as well as individuals directly responsible
for these Subject Entities (the “Subject Individuals”). For failure to comply with the Trial Measures Negative List or the
Trial Measures Filing Obligations, or materially disclose false or misleading statements in the filing and reporting required by the Trial
Measures: (1) PRC domestic companies, and their controlling shareholders if the controlling shareholders induced the PRC domestic companies’
failure to comply, severally, may face warnings, injunctions to comply, and fines between RMB1 million and RMB10 million (approximately
$145,647 and $1,456,473); the Subject Individuals in these entities may severally, face warnings and fines between RMB0.5 million and
RMB5 million (approximately $72,824 and $728,237). (2) Advisors in listing or offering securities that failed to dutifully advise the
PRC domestic companies and their controlling shareholders in complying with the Trial Measures and caused such failures to comply can
face warnings and fines between RMB0.5 million and RMB5 million (approximately $72,824 and $728,237); the Subject Individuals in these
advisor entities may, severally, face warnings and fines between RMB0.2 million and RMB2 million (approximately $29,129 and $291,295).
As the Trial Measures are newly issued, there remain uncertainties regarding its interpretation and implementation. Therefore, we cannot
assure you that we will be able to complete the filings for our future offerings and fully comply with the relevant new rules on a timely
basis, if at all. In addition, we cannot guarantee that we will not be subject to tightened regulatory review and we could be exposed
to government interference in China.
Recent greater oversight by the Cyberspace
Administration of China (the “CAC”) over data security, particularly for companies seeking to list on a foreign exchange,
could adversely impact our business and our offering.
On December 28, 2021,
the CAC and other relevant PRC governmental authorities jointly promulgated the Cybersecurity Review Measures, which took effect on February 15,
2022. The Cybersecurity Review Measures provide that, in addition to critical information infrastructure operators (“CIIOs”)
that intend to purchase Internet products and services, net platform operators engaging in data processing activities that affect or
may affect national security must be subject to cybersecurity review by the Cybersecurity Review Office of the PRC. According to the
Cybersecurity Review Measures, a cybersecurity review assesses potential national security risks that may be brought about by any procurement,
data processing, or overseas listing. The Cybersecurity Review Measures require that an online platform operator which possesses the
personal information of at least one million users must apply for a cybersecurity review by the CAC if it intends to be listed in foreign
countries.
On November 14, 2021,
the CAC promulgated the draft Regulations on the Administration of Cyber Data Security for public comment, pursuant to which data processors
conducting certain activities must apply for cybersecurity review. The draft regulations also require that data processors processing
important data or going public overseas shall conduct an annual data security self-assessment or entrust a data security service institution
to do so, and submit the data security assessment report of the previous year to the local branch of the CAC before January 31 each year.
Further, the draft regulations would require internet platform operators to establish platform rules, privacy policies and algorithm strategies
related to data, and solicit public comments on their official websites and personal information protection related sections for no less
than 30 working days when they formulate platform rules or privacy policies or makes any amendments that may have a significant impact
on users’ rights and interests. In addition, platform rules and privacy policies formulated by operators of large internet platforms
with more than 100 million daily active users, or amendments to such rules or policies by operators of large internet platforms with more
than 100 million daily active users that may have significant impacts on users’ rights and interests shall be evaluated by a third-party
organization designated by the CAC and reported to local branch of the CAC for approval. The CAC has solicited comments on this draft
until December 13, 2021, but there is no definite timetable as to when the draft regulations will be enacted. As such, substantial uncertainties
exist with respect to the enactment timetable, final content, interpretation and implementation of such regulations.
As of the date of this prospectus,
we, and Helpson, our PRC subsidiary, (i) are not required to obtain permissions from the CSRC, CAC or any other government authorities
on Helpson’s operations, and (ii) have not received or been denied such permissions by any PRC government authorities. If the Security
Administration Draft is enacted as proposed, we believe that the operations of Helpson and our listing will not be affected and that we
will not be subject to cybersecurity review by the CAC, given that Helpson possess personal data of fewer than one million individual
clients and do not collect data that affects or may affect national security in their business operations as of the date of this prospectus
and do not anticipate that they will be collecting over one million users’ personal information or data that affects or may affect
national security in the near future. There remains uncertainty, however, as to how the Cybersecurity Review Measures and the Security
Administration Draft will be interpreted or implemented and whether the PRC regulatory agencies, including the CAC, may adopt new laws,
regulations, rules, or detailed implementation and interpretation related to the Cybersecurity Review Measures and the Security Administration
Draft. If any such new laws, regulations, rules, or implementation and interpretation come into effect, we will take all reasonable measures
and actions to comply and to minimize the adverse effect of such laws on us. We cannot guarantee, however, that we will not be subject
to cybersecurity review and network data security review in the future. During such reviews, we may be required to suspend our operation
or experience other disruptions to operations. Cybersecurity review and network data security review could also result in negative publicity
with respect to our Company and diversion of our managerial and financial resources, which could materially and adversely affect our business,
financial conditions, and results of operations.
As of the date of this prospectus,
we have not received any notice from any authorities identifying Helpson as CIIOs. However, given the uncertainties surrounding the interpretation
and implementation of the Cyber Security Law, Data Security Law and relevant regulations, we cannot rule out the possibility that we,
or certain of our customers or suppliers may be deemed as a CIIO, or an operator processing “important data.” First, if we
are deemed as a CIIO, our purchase of network products or services, if deemed to be affecting or may affect national security, will need
to be subject to cybersecurity review, before we can enter into agreements with relevant customers or suppliers, and before the conclusion
of such procedure, these customers will not be allowed to use our products or services, and we are not allowed to purchase products or
services from our suppliers. There can be no assurance that we would be able to complete the applicable cybersecurity review procedures
in a timely manner, or at all, if we are required to follow such procedures. Any failure or delay in the completion of the cybersecurity
review procedures may prevent us from using certain network products and services, and may result in fines of up to ten times the purchase
price of such network products and services being imposed upon us, if we are deemed a CIIO using network products or services without
having completed the required cybersecurity review procedures. If the reviewing authority is of the view that the use of such network
products or services by us, or by certain of our customers or suppliers, involves risk of disruption, is vulnerable to external attacks,
or may negatively affect, compromise, or weaken the protection of national security, we may not be able to provide such products or services
to relevant customers, or purchase products or services from relevant suppliers. This could have a material adverse effect on our results
of operations and business prospects. Second, the notion of “important data” is not clearly defined by the Cyber Security
Law or the Data Security Law. In order to comply with the statutory requirements, we will need to determine whether we possess important
data, monitor the important data catalogs that are expected to be published by local governments and departments, perform risk assessments
and ensure we are complying with reporting obligations to applicable regulators. We may also be required to disclose to regulators business-sensitive
or network security-sensitive details regarding our processing of important data, and may need to pass the government security review
or obtain government approval in order to share important data with offshore recipients, which can include foreign licensors, or share
data stored in China with judicial and law enforcement authorities outside of China. If judicial and law enforcement authorities outside
China require us to provide data stored in China, and we are not able to pass any required government security review or obtain any required
government approval to do so, we may not be able to meet the foreign authorities’ requirements. The potential conflicts in legal
obligations could have adverse impact on our operations in and outside of China.
We May be Required to
Obtain Additional Permissions and Approvals for Business Operations in the PRC.
As of the date of this prospectus,
Helpson has obtained all the required permissions and approvals from PRC authorities that are required to operate its business. Helpson
has never failed to receive or maintain any permissions or approvals, nor have any applications been rejected. However, the PRC regulatory
authorities may in the future promulgate laws, regulations, or implementing rules that require Helpson to obtain additional permissions
or approvals to operate business. If that occurs, we cannot assure you that we will receive such additional permissions and approvals
on time. If we do not receive or maintain the approval, or incorrectly conclude that such approval is not required, or applicable laws,
regulations, or interpretations change such that we are required to obtain approval in the future, we may be subject to an investigation
by competent regulators, fines or penalties, and these risks could result in a material adverse change in our operations and the value
of our common stock, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, or cause
such securities to significantly decline in value or become worthless.
Risks Related to Our Business
We rely on distributors
for all of our revenues and failure to maintain relationships with and collect payment from our distributors or to otherwise expand our
distribution network would materially and adversely affect our business.
We sell our products exclusively
to pharmaceutical distributors in the PRC and rely on distributors for all of our revenues. We have business relationships with over
1,000 distributors in the PRC. For the year ended December 31, 2022, no customer accounted for more than 10.0% of sales, and three customers
accounted for 52.9%, 11.4%, and 10.4% of accounts receivable. In line with industry practices in the PRC, we enter into written sales
agreements with our distributors. However, such sales agreements are not in substance equivalent to a typical distribution agreement
in the United States. Each sales agreement resembles a sales order, and specifies one or several purchases of one or more products without
any continuing obligation to purchase any additional products. There are no written contracts between us and any of its distributors
requiring the distributors to pay us our account receivable upon their receipt of funds from its customers, or state-owned hospitals.
Pharmaceutical distributors typically process the payment of the account receivable to the companies upon their receipt of payment from
their customers, i.e., the state-owned hospitals, as a matter of implied consensus. In the event the length of collection term deviates
from the past pattern of any particular customer, we will adjust its credit term.
Any potential default
in repaying the accounts receivable without recourse by us may materially and negatively affect our profitability and business. In the
event certain distributors choose not to continue their relationship with us after completing their existing sales agreements, they can
do so without breaching any contract or agreement, and our financial results could be adversely affected if we cannot quickly find substantially
similar distributors under such circumstances. In addition, some of our distributors may sell products that compete with our products.
We compete for desired distributors with other pharmaceutical manufacturers, many of which may have higher visibility, greater name recognition,
greater financial resources, and broader product selection than we do. Consequently, maintaining relationships with existing distributors
and replacing distributors may be difficult and time-consuming. Any disruption of our distribution network, including our failure to
renew our existing distribution agreements with our desired distributors, could negatively affect our ability to effectively sell our
products and would materially and adversely affect our business, financial condition and results of operations.
Risks Related to Our Securities and the Offering
Future sales or other
dilution of our equity could depress the market price of our common stock.
Sales of our common stock,
the common stock underlying our preferred stock, warrants, rights or convertible debt securities, or any combination of the foregoing,
in the public market, or the perception that such sales could occur, could negatively impact the price of our common stock.
In addition, the issuance
of additional shares of our common stock, securities convertible into or exercisable for our common stock, other equity-linked securities,
including preferred stock, warrants or rights or any combination of these securities pursuant to this prospectus will dilute the ownership
interest of our common shareholders and could depress the market price of our common stock and impair our ability to raise capital through
the sale of additional equity securities.
We may need to seek additional
capital. If this additional financing is obtained through the issuance of equity securities, debt securities convertible into equity or
options, warrants or rights to acquire equity securities, our existing shareholders could experience significant dilution upon the issuance,
conversion or exercise of such securities.
Our management will
have broad discretion over the use of the proceeds we receive from the sale our securities pursuant to this prospectus and might not apply
the proceeds in ways that increase the value of your investment.
Our management will have broad
discretion to use the net proceeds from any offerings under this prospectus, and you will be relying on the judgment of our management
regarding the application of these proceeds. Except as described in any prospectus supplement or in any related free writing prospectus
that we may authorize to be provided to you, the net proceeds received by us from our sale of the securities described in this prospectus
will be added to our general funds and will be used for general corporate purposes. Our management might not apply the net proceeds from
offerings of our securities in ways that increase the value of your investment and might not be able to yield a significant return, if
any, on any investment of such net proceeds. You may not have the opportunity to influence our decisions on how to use such proceeds.
USE OF PROCEEDS
Except as otherwise provided
in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities covered by this prospectus
for general corporate purposes, which may include working capital, capital expenditures, research and development expenditures, acquisitions
of new technologies or businesses and investments. Additional information on the use of net proceeds from an offering of securities covered
by this prospectus may be set forth in the prospectus supplement relating to the specific offering.
THE SECURITIES WE MAY OFFER
The descriptions of the securities
contained in this prospectus, together with any applicable prospectus supplement, summarize all the material terms and provisions of the
various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to a particular offering
the specific terms of the securities offered by that prospectus supplement. If we indicate in the applicable prospectus supplement, the
terms of the securities may differ from the terms we have summarized below. We will also include in the prospectus supplement information,
where applicable, about material United States federal income tax considerations relating to the securities, and the securities exchange,
if any, on which the securities will be listed.
We may sell from time to time,
in one or more offerings:
| |
● |
shares of our common stock; |
| |
● |
shares of our preferred stock; |
| |
● |
debt securities (which may be convertible into or exchangeable for common stock); |
| |
● |
warrants to purchase our common stock; |
| |
● |
rights to purchase our common stock; and/or |
| |
● |
units consisting of the forgoing. |
This prospectus may not be
used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
DESCRIPTION OF CAPITAL STOCK
General
The following description
of our capital stock together with the additional information we include in any applicable prospectus supplement, summarizes the material
terms and provisions of the capital stock that we may offer under this prospectus but is not complete. For the complete terms of our capital
stock, please refer to our articles of incorporation and our bylaws, as amended from time to time. While the terms we have summarized
below will apply generally to any future capital stock that we may offer, we will describe the specific terms of any series of these securities
in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any capital stock we
offer under that prospectus supplement may differ from the terms we describe below.
Our authorized capital stock
consists of five hundred million (500,000,000) shares of common stock, $0.001 par value per share and five million (5,000,000) shares
of preferred stock, $0.001 par value per share. The authorized and unissued shares of capital stock are available for issuance without
further action by our shareholders, unless such action is required by applicable law or the rules of any stock exchange on which our securities
may be listed. Unless approval of our shareholders is so required, our board of directors will not seek shareholder approval for the issuance
and sale of our capital stock.
Common Stock
As of February 5, 2024,
there were 56,981,244 shares of our common stock outstanding. The holders of our common stock are entitled to such dividends as our board
of directors may declare from legally available funds. The holders of our common stock are entitled to one vote per share on any matter
to be voted upon by shareholders. Our articles of incorporation or our bylaws, as amended from time to time, do not provide for cumulative
voting. No holder of our common stock has any preemptive right to subscribe for any shares of capital stock issued in the future under
the Nevada Revised Statutes, our articles of incorporation or our bylaws, as amended from time to time. Our common stock has no preemptive
or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock.
All shares of common stock
offered hereby will, when issued, be fully paid and non-assessable, including shares of common stock issued upon exercise of common stock
warrants or common stock purchase rights, if any.
Our common stock is quoted on the NYSE American
under the symbol “CPHI”. The transfer agent and registrar for our common stock is Equiniti Trust Company (f/k/a Corporate
Stock Transfer), 1110 Centre Pointe Curve, Suite 101, Mendota Heights, MN 55120.
Preferred Stock
As of February 5, 2024,
no shares of preferred stock had been issued or were outstanding.
Our board of directors has
the authority to issue up to 5,000,000 shares of preferred stock in one or more series and to determine the rights and preferences of
the shares of any such series without stockholder approval. Our board of directors has the authority to fix the designation and powers,
rights and preferences and the qualifications, limitations or restrictions with respect to each class or series of such class without
further vote or action by the stockholders, unless action is required by applicable law or the rules of any stock exchange on which our
securities may be listed. The ability of our board of directors to issue preferred stock without stockholder approval could have the effect
of delaying, deferring or preventing a change of control of us or the removal of existing management. Further, our board of directors
may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights
of the holders of our common stock. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of
our common stock.
We will file as an exhibit
to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the
SEC, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance
of that series of preferred stock. This description will include, but not be limited to, the following:
| |
● |
the title and stated value; |
| |
● |
the number of shares we are offering; |
| |
● |
the liquidation preference per share; |
| |
● |
the dividend rate, period and payment date and method of calculation for dividends; |
| |
● |
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| |
● |
the provisions for a sinking fund, if any; |
| |
● |
the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| |
● |
whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price, or how it will be calculated, and the conversion period; |
| |
● |
whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price, or how it will be calculated, and the exchange period; |
| |
● |
voting rights, if any, of the preferred stock; |
| |
● |
preemptive rights, if any; |
| |
● |
restrictions on transfer, sale or other assignment, if any; |
| |
● |
a discussion of any material United States federal income tax considerations applicable to the preferred stock; |
| |
● |
the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
| |
● |
any limitations on the issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and |
| |
● |
any other specific terms, preferences, rights or limitations of, or restrictions on, the preferred stock. |
DESCRIPTION OF DEBT SECURITIES
We may issue debt securities,
in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. When we offer to sell debt
securities, we will describe the specific terms of any debt securities offered from time to time in a supplement to this prospectus, which
may supplement or change the terms outlined below. Senior debt securities will be issued under one or more senior indentures, dated as
of a date prior to such issuance, between us and a trustee to be named in a prospectus supplement, as amended or supplemented from time
to time. Any subordinated debt securities will be issued under one or more subordinated indentures, dated as of a date prior to such issuance,
between us and a trustee to be named in a prospectus supplement, as amended or supplemented from time to time. The indentures will be
subject to and governed by the Trust Indenture Act of 1939, as amended.
Before we issue any debt securities,
the form of indentures will be filed with the SEC and incorporated by reference as an exhibit to the registration statement of which this
prospectus is a part or as an exhibit to a current report on Form 8-K. For the complete terms of the debt securities, you should refer
to the applicable prospectus supplement and the form of indentures for those particular debt securities. We encourage you to read the
applicable prospectus supplement and the form of indenture for those particular debt securities before you purchase any of our debt securities.
We will describe in the applicable
prospectus supplement the terms of the series of debt securities being offered, including:
| |
● |
whether or not such debt securities are guaranteed; |
| |
● |
the principal amount being offered, and if a series, the total amount authorized and the total amount outstanding; |
| |
● |
any limit on the amount that may be issued; |
| |
● |
whether or not we will issue the series of debt securities in global form, the terms and who the depositary will be; |
| |
● |
the annual interest rate, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
| |
● |
whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| |
● |
the terms of the subordination of any series of subordinated debt; |
| |
● |
the place where payments will be payable; |
| |
● |
restrictions on transfer, sale or other assignment, if any; |
| |
● |
our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| |
● |
the date, if any, after which, and the price at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
| |
● |
the date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| |
● |
any restrictions of our ability and/or the ability of our subsidiaries to: |
| |
● |
incur additional indebtedness; |
| |
● |
issue additional securities; |
| |
● |
pay dividends and make distributions in respect of our capital stock and the capital stock of our subsidiaries; |
| |
● |
place restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer assets; |
| |
● |
make investments or other restricted payments; |
| |
● |
sell or otherwise dispose of assets; |
| |
● |
enter into sale-leaseback transactions; |
| |
● |
engage in transactions with stockholders and affiliates; |
| |
● |
issue or sell stock of our subsidiaries; or |
| |
● |
effect a consolidation or merger; |
| |
● |
whether the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based, asset-based or other financial ratios; |
| |
● |
a discussion of any material United States federal income tax considerations applicable to the debt securities; |
| |
● |
information describing any book-entry features; |
| |
● |
provisions for a sinking fund purchase or other analogous fund, if any; |
| |
● |
the denominations in which we will issue the series of debt securities; |
| |
● |
the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; and |
| |
● |
any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, including any additional events of default or covenants provided with respect to the debt securities, and any terms that may be required by us or advisable under applicable laws or regulations. |
DESCRIPTION OF WARRANTS
The following description,
together with the additional information we may include in any applicable prospectus supplement, summarizes the material terms and provisions
of the warrants that we may offer under this prospectus and any related warrant agreement and warrant certificate. While the terms summarized
below will apply generally to any warrants that we may offer, we will describe the specific terms of any series of warrants in more detail
in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants offered under that prospectus
supplement may differ from the terms described below. Specific warrant agreements will contain additional important terms and provisions
and will be incorporated by reference as an exhibit to the registration statement which includes this prospectus.
General
We may issue warrants for
the purchase of common stock in one or more series. We may issue warrants independently or together with common stock, and the warrants
may be attached to or separate from common stock. We may issue the warrants under warrant agreements to be entered into between us and
a bank or trust company, as warrant agent, all as described in the prospectus supplement. If we issue the warrants under warrant agreements,
the warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency
or trust for or with any holders or beneficial owners of warrants.
While the terms summarized
below will apply generally to any warrants that we may offer, we will describe the particular terms of any series of warrants in more
detail in the applicable prospectus supplement. The terms of any warrants offered under a prospectus supplement may differ from the terms
described below:
| |
● |
the offering price and aggregate number of warrants offered; |
| |
● |
if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security; |
| |
● |
if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| |
● |
in the case of warrants to purchase common stock, the number or amount of shares of common stock purchasable upon the exercise of one warrant and the price at which and currency in which these shares may be purchased upon such exercise; |
| |
● |
the manner of exercise of the warrants, including any cashless exercise rights; |
| |
● |
the warrant agreement under which the warrants will be issued; |
| |
● |
the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; |
| |
● |
anti-dilution provisions of the warrants, if any; |
| |
● |
the terms of any rights to redeem or call the warrants; |
| |
● |
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| |
● |
the dates on which the right to exercise the warrants will commence and expire or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will be exercisable; |
| |
● |
the manner in which the warrant agreement and warrants may be modified; |
| |
● |
the identities of the warrant agent and any calculation or other agent for the warrants; |
| |
● |
federal income tax consequences of holding or exercising the warrants; |
| |
● |
the terms of the securities issuable upon exercise of the warrants; |
| |
● |
any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed or quoted; and |
| |
● |
any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants,
holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| |
● |
in the case of warrants to purchase common stock the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
Exercise of Warrants
Each warrant will entitle
the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in
the applicable prospectus supplement. Holders of the warrants may exercise the warrants at any time up to the close of business on the
expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised
warrants will become void.
Holders of the warrants may
exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information,
and paying the required exercise price by the methods provided in the applicable prospectus supplement. We will set forth on the reverse
side of the warrant certificate, and in the applicable prospectus supplement, the information that the holder of the warrant will be required
to deliver to the warrant agent or any other office indicated in the prospectus supplement.
Upon receipt of the required
payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other
office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If
fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for
the remaining amount of warrants.
Enforceability of Rights By Holders of Warrants
Any warrant agent will act
solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with
any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent
will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty
or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without
the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action the holder’s right
to exercise, and receive the securities purchasable upon exercise of, its warrants in accordance with their terms.
Warrant Agreement Will Not Be Qualified Under Trust Indenture Act
No warrant agreement will
be qualified as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore,
holders of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Governing Law
Unless we provide otherwise
in the applicable prospectus supplement, each warrant agreement and any warrants issued under the warrant agreements will be governed
by New York law.
DESCRIPTION OF RIGHTS
We may issue rights to purchase
our common stock or preferred stock, in one or more series. Rights may be issued independently or together with any other offered security
and may or may not be transferable by the person purchasing or receiving the subscription rights. In connection with any rights offering
to our stockholders, we may enter into a standby underwriting arrangement with one or more underwriters pursuant to which such underwriters
will purchase any offered securities remaining unsubscribed after such rights offering. In connection with a rights offering to our stockholders,
we will distribute certificates evidencing the rights and a prospectus supplement to our stockholders on the record date that we set for
receiving rights in such rights offering. The applicable prospectus supplement or free writing prospectus will describe the following
terms of rights in respect of which this prospectus is being delivered:
| |
● |
the title of such rights; |
| |
● |
the securities for which such rights are exercisable; |
| |
● |
the exercise price for such rights; |
| |
● |
the date of determining the security holders entitled to the rights distribution; |
| |
● |
the number of such rights issued to each security holder; |
| |
● |
the extent to which such rights are transferable; |
| |
● |
if applicable, a discussion of the material United States federal income tax considerations applicable to the issuance or exercise of such rights; |
| |
● |
the date on which the right to exercise such rights shall commence, and the date on which such rights shall expire (subject to any extension); |
| |
● |
the conditions to completion of the rights offering; |
| |
● |
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the rights; |
| |
● |
the extent to which such rights include an over-subscription privilege with respect to unsubscribed securities; |
| |
● |
if applicable, the material terms of any standby underwriting or other purchase arrangement that we may enter into in connection with the rights offering; and |
| |
● |
any other terms of such rights, including terms, procedures and limitations relating to the exchange and exercise of such rights. |
Each right will entitle the
holder thereof the right to purchase for cash such amount of shares of common stock or preferred stock, or any combination thereof, at
such exercise price as shall in each case be set forth in, or be determinable as set forth in, the prospectus supplement relating to the
rights offered thereby. Rights may be exercised at any time up to the close of business on the expiration date for such rights set forth
in the prospectus supplement. After the close of business on the expiration date, all unexercised rights will become void. Rights may
be exercised as set forth in the prospectus supplement relating to the rights offered thereby. Upon receipt of payment and the proper
completion and due execution of the rights certificate at the office of the rights agent, if any, or any other office indicated in the
prospectus supplement, we will forward, as soon as practicable, the shares of common stock and/or preferred stock purchasable upon such
exercise. We may determine to offer any unsubscribed offered securities directly to persons other than stockholders, to or through agents,
underwriters or dealers or through a combination of such methods, including pursuant to standby underwriting arrangements, as set forth
in the applicable prospectus supplement.
DESCRIPTION OF UNITS
We may issue units comprised
of one or more of the other securities described in this prospectus in any combination. Each unit will be issued so that the holder of
the unit is also the holder, with the rights and obligations of a holder, of each security included in the unit. The unit agreement under
which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or
at any time before a specified date or upon the occurrence of a specified event or occurrence.
The applicable prospectus
supplement will describe:
| |
● |
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| |
● |
any unit agreement under which the units will be issued; |
| |
● |
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| |
● |
whether the units will be issued in fully registered or global form. |
PLAN OF DISTRIBUTION
We may sell the securities
being offered pursuant to this prospectus to or through underwriters, through dealers, through agents, or directly to one or more purchasers
or through a combination of these methods. The applicable prospectus supplement will describe the terms of the offering of the securities,
including:
| |
● |
the name or names of any underwriters, if any, and if required, any dealers or agents; |
| |
● |
the purchase price of the securities and the proceeds we will receive from the sale; |
| |
● |
any underwriting discounts and other items constituting underwriters’ compensation; |
| |
● |
any discounts or concessions allowed or reallowed or paid to dealers; and |
| |
● |
any securities exchange or market on which the securities may be listed or traded. |
We may distribute the securities from time to
time in one or more transactions at:
| |
● |
a fixed price or prices, which may be changed; |
| |
● |
market prices prevailing at the time of sale; |
| |
● |
prices related to such prevailing market prices; or |
Sales Through Underwriters or Dealers
Only underwriters named in
the prospectus supplement are underwriters of the securities offered by the prospectus supplement.
If underwriters are used in
an offering, we will execute an underwriting agreement with such underwriters and will specify the name of each underwriter and the terms
of the transaction (including any underwriting discounts and other terms constituting compensation of the underwriters and any dealers)
in a prospectus supplement. The securities may be offered to the public either through underwriting syndicates represented by managing
underwriters or directly by one or more investment banking firms or others, as designated. If an underwriting syndicate is used, the managing
underwriter(s) will be specified on the cover of the prospectus supplement. If underwriters are used in the sale, the offered securities
will be acquired by the underwriters for their own accounts and may be resold from time to time in one or more transactions, including
negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Any public offering price
and any discounts or concessions allowed or reallowed or paid to dealers may be changed from time to time. Unless otherwise set forth
in the prospectus supplement, the obligations of the underwriters to purchase the offered securities will be subject to conditions precedent
and the underwriters will be obligated to purchase all of the offered securities, if any are purchased.
We may grant to the underwriters
options to purchase additional securities to cover over-allotments, if any, at the public offering price, with additional underwriting
commissions or discounts, as may be set forth in a related prospectus supplement. The terms of any over-allotment option will be set forth
in the prospectus supplement for those securities.
If we use a dealer in the
sale of the securities being offered pursuant to this prospectus or any prospectus supplement, we will sell the securities to the dealer,
as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of
resale. The names of the dealers and the terms of the transaction will be specified in a prospectus supplement.
Direct Sales and Sales Through Agents
We may sell the securities
directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and
we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise,
any agent will act on a best-efforts basis for the period of its appointment.
We may sell the securities
directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect
to any sale of those securities. The terms of any such sales will be described in the prospectus supplement.
Delayed Delivery Contracts
We may authorize agents or
underwriters to solicit offers by institutional investors to purchase securities from us at the public offering price set forth in the
prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We
will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus
supplement.
General Information
In connection with the sale
of the securities, underwriters, dealers or agents may receive compensation from us or from purchasers of the securities for whom they
act as agents, in the form of discounts, concessions or commissions. Underwriters may sell the securities to or through dealers, and those
dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers
for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of the securities, and any institutional
investors or others that purchase securities directly for the purpose of resale or distribution, may be deemed to be underwriters, and
any discounts or commissions received by them from us and any profit on the resale of the common stock by them may be deemed to be underwriting
discounts and commissions under the Securities Act.
We may provide agents, underwriters
and other purchasers with indemnification against particular civil liabilities, including liabilities under the Securities Act, or contribution
with respect to payments that the agents, underwriters or other purchasers may make with respect to such liabilities. Agents and underwriters
may engage in transactions with, or perform services for, us in the ordinary course of business.
To facilitate the public offering
of a series of securities, persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise affect
the market price of the securities. This may include over-allotments or short sales of the securities, which involves the sale by persons
participating in the offering of more securities than have been sold to them by us. In those circumstances, such persons would cover such
over-allotments or short positions by purchasing in the open market or by exercising the over-allotment option granted to those persons.
In addition, those persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market
or by imposing penalty bids, whereby selling concessions allowed to underwriters or dealers participating in any such offering may be
reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may
be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market.
Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as to the direction or magnitude
of any effect that the transactions described above, if implemented, may have on the price of our securities.
Unless otherwise specified
in the applicable prospectus supplement, any common stock sold pursuant to a prospectus supplement will be eligible for listing on the
NYSE American, subject to official notice of issuance. Any underwriters to whom securities are sold by us for public offering and sale
may make a market in the securities, but such underwriters will not be obligated to do so and may discontinue any market making at any
time without notice.
LEGAL MATTERS
The validity of the issuance
of the securities offered hereby will be passed upon for us by FLANGAS LAW GROUP. Any underwriters will also be advised about the validity
of the securities and other legal matters by their own counsel, which will be named in the prospectus supplement.
EXPERTS
The consolidated balance sheets of China Pharma Holdings, Inc. and
the subsidiaries as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, stockholders’
equity, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to
as the “financial statements”), incorporated in this prospectus by reference to our Annual Report on Form 10-K for the year
ended December 31, 2022, have been so incorporated in reliance upon the report of BF Borgers CPA, P.C., an independent registered public
accounting firm, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of
a registration statement that we have filed with the SEC. Certain information in the registration statement has been omitted from this
prospectus in accordance with the rules of the SEC. We are subject to the information requirements of the Securities Exchange Act
of 1934, as amended, or the Exchange Act, and, in accordance therewith, file annual, quarterly and current reports, proxy statements
and other information with the SEC. Such annual, quarterly and current reports, proxy statements and other information are available at
the website of the SEC at https://www.sec.gov.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The following documents filed
by us with the Securities and Exchange Commission are incorporated by reference in this prospectus:
| |
● |
Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 30, 2023; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2023, filed on November 13, 2023; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2023, filed on August 14, 2023; |
| |
● |
Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023, filed on May 12, 2023; |
| |
● |
Definitive Proxy Statement on Schedule 14A, filed with the SEC on November 7, 2023; |
| |
● |
The description of China Pharma’s securities contained in Exhibit 4.2 of the Company’s Annual Report on Form 10-K filed on March 30, 2022; and |
| |
● |
All of our filings made with the Securities and Exchange Commission pursuant to the Exchange Act after the date of the initial registration statement of which this prospectus forms a part and prior to the effectiveness of the registration statement of which this prospectus forms a part. |
All documents subsequently
filed with the Securities and Exchange Commission by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the
date of this prospectus and prior to the filing of a post-effective amendment which indicates that all securities offered herein have
been sold or which deregisters all securities then remaining unsold, shall be deemed to be incorporated by reference herein and to be
part of this prospectus from the respective dates of filing of such documents. Any statement contained herein or in a document incorporated
or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes hereof or of the related prospectus
supplement to the extent that a statement contained herein or in any other subsequently filed document which is also incorporated or deemed
to be incorporated herein modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except
as so modified or superseded, to constitute a part of this prospectus.
We will provide to each
person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated
by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings by written or oral request,
excluding the exhibits to such filings which we have not specifically incorporated by reference in such filings, at no cost, by writing
us at China Pharma Holdings, Inc., 2nd Floor, No. 17, Jinpan Road, Haikou, Hainan Province, China, or calling us at 86-10-898-66811730.
This prospectus is part of
a registration statement we filed with the Securities and Exchange Commission. You should only rely on the information or representations
contained in this prospectus and any accompanying prospectus supplement. We have not authorized anyone to provide information other than
that provided in this prospectus and any accompanying prospectus supplement. We are not making an offer of these securities in any state
where the offer is not permitted. You should not assume that the information in this prospectus or any accompanying prospectus supplement
is accurate as of any date other than the date on the front of the document.
PROSPECTUS

CHINA PHARMA HOLDINGS, INC.
$50,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
The date of this prospectus is February 6,
2024.
China Pharma (AMEX:CPHI)
Historical Stock Chart
From Jan 2025 to Feb 2025
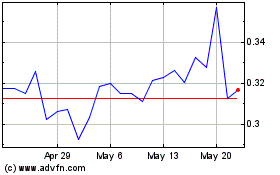
China Pharma (AMEX:CPHI)
Historical Stock Chart
From Feb 2024 to Feb 2025