0001690585false00016905852024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 8, 2024 |
DIANTHUS THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-38541 |
81-0724163 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
7 Times Square 43rd Floor |
|
New York, New York |
|
10036 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 929 999-4055 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 Par Value |
|
DNTH |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
Dianthus Therapeutics, Inc.’s (the “Company”) audited financial statements for the fiscal year ended December 31, 2023 are not yet available. Beginning on January 8, 2024, the Company plans to disclose in a presentation to investors (the "Presentation") that it expects to report cash, cash equivalents, and short-term investments of approximately $173 million as of December 31, 2023. A copy of the Presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The estimated cash, cash equivalents and short-term investments amount is preliminary and unaudited, represents management’s estimate as of the date of this report, is subject to completion of the Company’s financial closing procedures for the fourth quarter and fiscal year ended December 31, 2023, and does not present all necessary information for a complete understanding of the Company’s financial condition as of December 31, 2023, or the Company’s results of operations for the year ended December 31, 2023. The actual financial results may differ materially from the preliminary estimated financial information.
The information in this Item 2.02, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 7.01 Regulation FD Disclosure.
Spokespersons of the Company plan to present the information in the Presentation at various meetings beginning on January 8, 2024, including investor and analyst meetings. Marino Garcia, the Company's President and Chief Executive Officer, will also present the information in the Presentation at the 42nd Annual J.P. Morgan Healthcare Conference on January 11, 2024.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Cautionary Note Regarding Forward-Looking Statements. The Presentation contains forward-looking statements that involve certain risks and uncertainties that could cause actual results to differ materially from those expressed or implied by these statements. Please refer to the cautionary notes in the Presentation regarding these forward-looking statements.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
DIANTHUS THERAPEUTICS, INC. |
|
|
|
|
Date: |
January 8, 2024 |
By: |
/s/ Adam M. Veness, Esq. |
|
|
|
Adam M. Veness, Esq.
SVP, General Counsel and Secretary |
Corporate Presentation January 2024 Exhibit 99.1

FORWARD-LOOKING STATEMENTS Certain statements in this presentation (“Presentation”), other than purely historical information, may constitute “forward-looking statements” within the meaning of the federal securities laws, including for purposes of the safe harbor provisions under the United Stated Private Securities Litigation Reform Act of 1995, concerning Dianthus Therapeutics, Inc. (the “Company”). These forward-looking statements include statements regarding the Company’s future plans and prospects, including statements regarding the expectations or plans for discovery, preclinical studies, clinical trials and research and development programs, in particular with respect to DNTH103, and any developments or results in connection therewith, including the target product profile of DNTH103; the anticipated timing of the results from those studies and trials; expectations regarding the use of proceeds and the time period over which the Company’s capital resources will be sufficient to fund its anticipated operations; and expectations regarding the market and potential opportunities for complement therapies, in particular with respect to DNTH103. The words “opportunity,” “potential,” “milestones,” “runway,” “will,” “anticipate,” “achieve,” “near-term,” “catalysts,” “pursue,” “pipeline,” “believe,” continue,” “could,” “estimate,” “expect,” “ intend,” “may,” “might,” “plan,” “possible,” “predict,” “project,” “ should,” “ strive,” “would,” “aim,” “target,” “commit,” and similar expressions (including the negatives of these terms or variations of them) generally identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Actual results could differ materially from those included in the forward-looking statements due to various factors, risks and uncertainties, including, but not limited to, that preclinical testing of DNTH103 and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that the development of DNTH103 or the Company's compounds may take longer and/or cost more than planned, that the Company may be unable to successfully complete the clinical development of the Company’s compounds, that the Company may be delayed in initiating, enrolling or completing any clinical trials, and that the Company's compounds may not receive regulatory approval or become commercially successful products. These and other risks and uncertainties are identified under the heading "Risk Factors" included in Exhibit 99.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 12, 2023 (as amended on September 21, 2023), and other filings that the Company has made and may make with the SEC in the future. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. Dianthus undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

Lead program, DNTH103, is a potent investigational monoclonal antibody that targets the classical complement pathway by selectively inhibiting active C1s protein Top-line Ph. 1 data confirm a ~60-day half-life, potent classical pathway inhibition, and a potentially differentiated safety profile Cash runway expected to fund operations into Q2’26 DNTH103 intended to be the first subcutaneous, self-administered injection dosed as infrequently as once-every-two-weeks to treat generalized Myasthenia Gravis On track to initiate multiple Ph. 2 trials in neuromuscular indications starting with generalized Myasthenia Gravis in Q1’24 with top-line results in 2H’25; Ph. 2 trials in CIDP and MMN to be initiated in ’24 Founded in 2019 to develop next-generation complement therapies to treat severe autoimmune diseases Advancing next-generation complement therapies to improve the lives of autoimmune disease patients Riliprubart, an active C1s inhibitor, recently showed clinical PoC in a Ph. 2 open-label trial in CIDP, demonstrating PoC in humans for active C1s-inhibition in autoimmune neuromuscular diseases
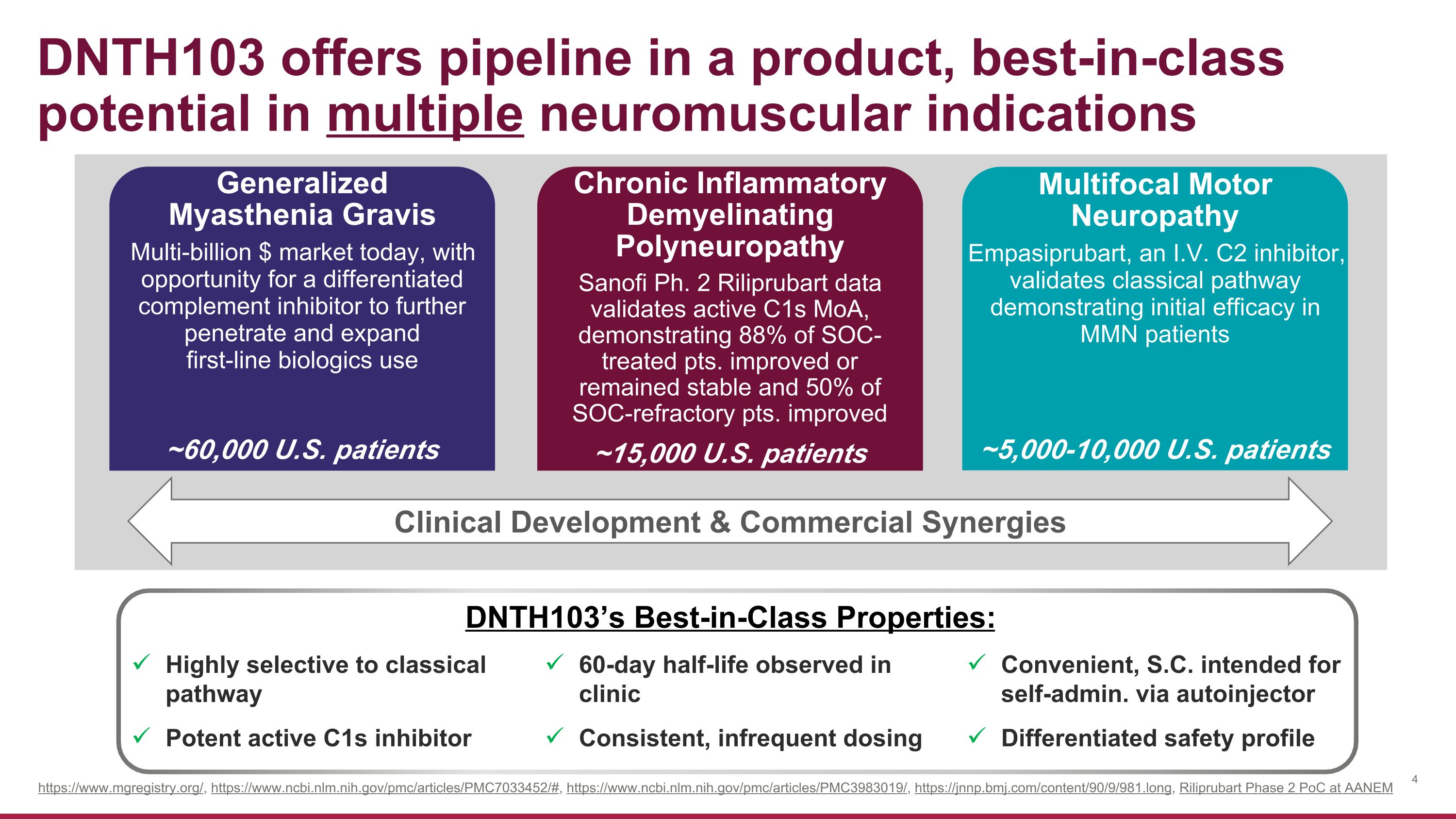
DNTH103 offers pipeline in a product, best-in-class potential in multiple neuromuscular indications Generalized �Myasthenia Gravis Multi-billion $ market today, with opportunity for a differentiated complement inhibitor to further penetrate and expand first-line biologics use ~60,000 U.S. patients Chronic Inflammatory Demyelinating Polyneuropathy Sanofi Ph. 2 Riliprubart data validates active C1s MoA, demonstrating 88% of SOC-treated pts. improved or remained stable and 50% of SOC-refractory pts. improved ~15,000 U.S. patients Multifocal Motor Neuropathy Empasiprubart, an I.V. C2 inhibitor, validates classical pathway demonstrating initial efficacy in MMN patients ~5,000-10,000 U.S. patients https://www.mgregistry.org/, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/#, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983019/, https://jnnp.bmj.com/content/90/9/981.long, Riliprubart Phase 2 PoC at AANEM Highly selective to classical pathway Potent active C1s inhibitor 60-day half-life observed in clinic Consistent, infrequent dosing Convenient, S.C. intended for self-admin. via autoinjector Differentiated safety profile DNTH103’s Best-in-Class Properties: Clinical Development & Commercial Synergies
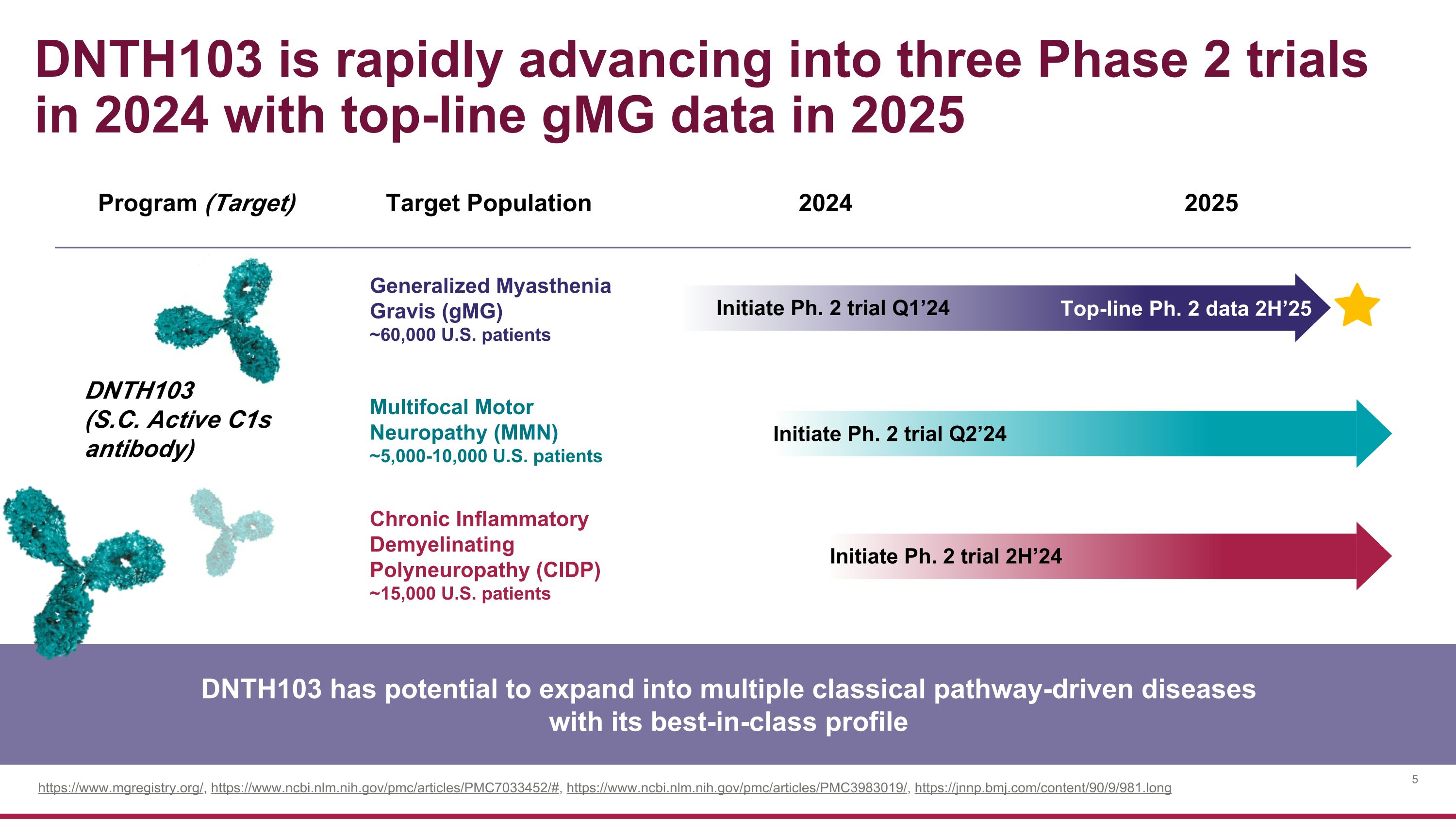
Program (Target) Target Population 2024 2025 DNTH103 (S.C. Active C1s antibody) Generalized Myasthenia �Gravis (gMG) ~60,000 U.S. patients Multifocal Motor �Neuropathy (MMN) ~5,000-10,000 U.S. patients Chronic Inflammatory Demyelinating �Polyneuropathy (CIDP) ~15,000 U.S. patients DNTH103 is rapidly advancing into three Phase 2 trials in 2024 with top-line gMG data in 2025 Initiate Ph. 2 trial Q1’24 Top-line Ph. 2 data 2H’25 Initiate Ph. 2 trial Q2’24 Initiate Ph. 2 trial 2H’24 DNTH103 has potential to expand into multiple classical pathway-driven diseases with its best-in-class profile https://www.mgregistry.org/, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/#, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983019/, https://jnnp.bmj.com/content/90/9/981.long

DNTH103 Opportunity in Myasthenia Gravis
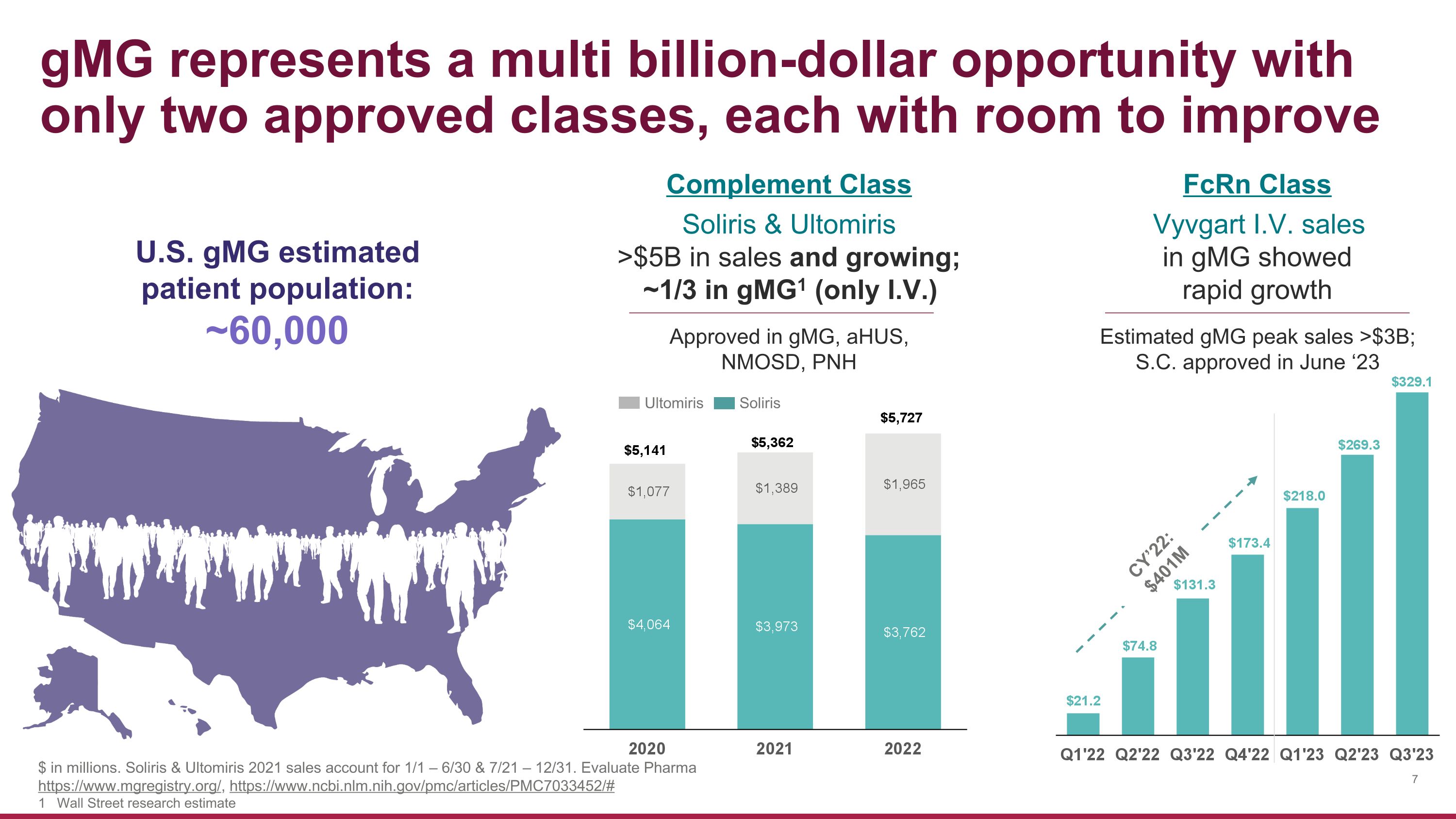
gMG represents a multi billion-dollar opportunity with�only two approved classes, each with room to improve Approved in gMG, aHUS, NMOSD, PNH Soliris Ultomiris Soliris & Ultomiris >$5B in sales and growing; ~1/3 in gMG1 (only I.V.) U.S. gMG estimated patient population: ~60,000 Vyvgart I.V. sales in gMG showed rapid growth Estimated gMG peak sales >$3B; S.C. approved in June ‘23 CY’22: $401M Complement Class FcRn Class $ in millions. Soliris & Ultomiris 2021 sales account for 1/1 – 6/30 & 7/21 – 12/31. Evaluate Pharma https://www.mgregistry.org/, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7033452/# 1 Wall Street research estimate
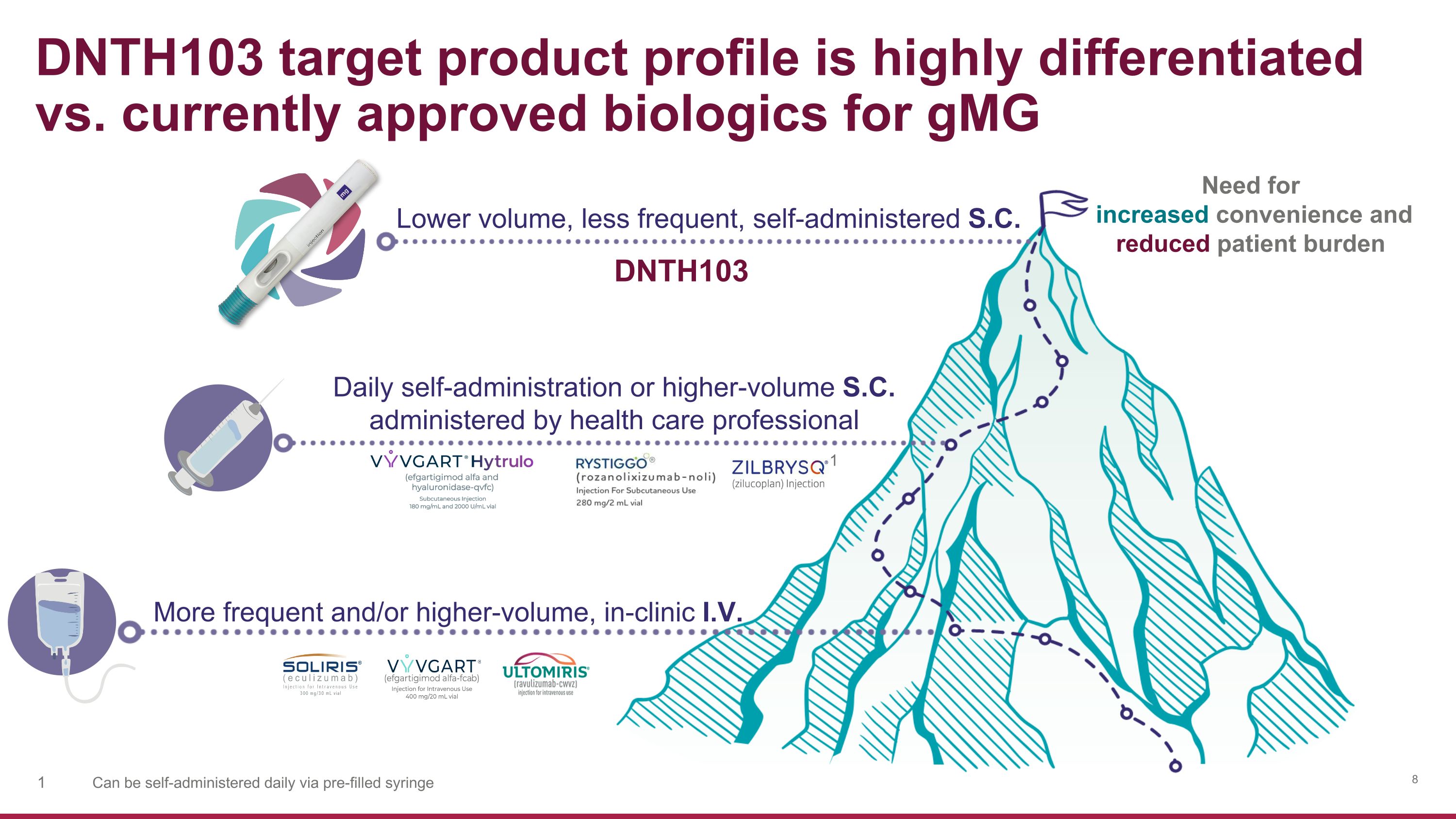
DNTH103 Lower volume, less frequent, self-administered S.C. More frequent and/or higher-volume, in-clinic I.V. Need for increased convenience and reduced patient burden DNTH103 target product profile is highly differentiated vs. currently approved biologics for gMG 1 Can be self-administered daily via pre-filled syringe Daily self-administration or higher-volume S.C. administered by health care professional
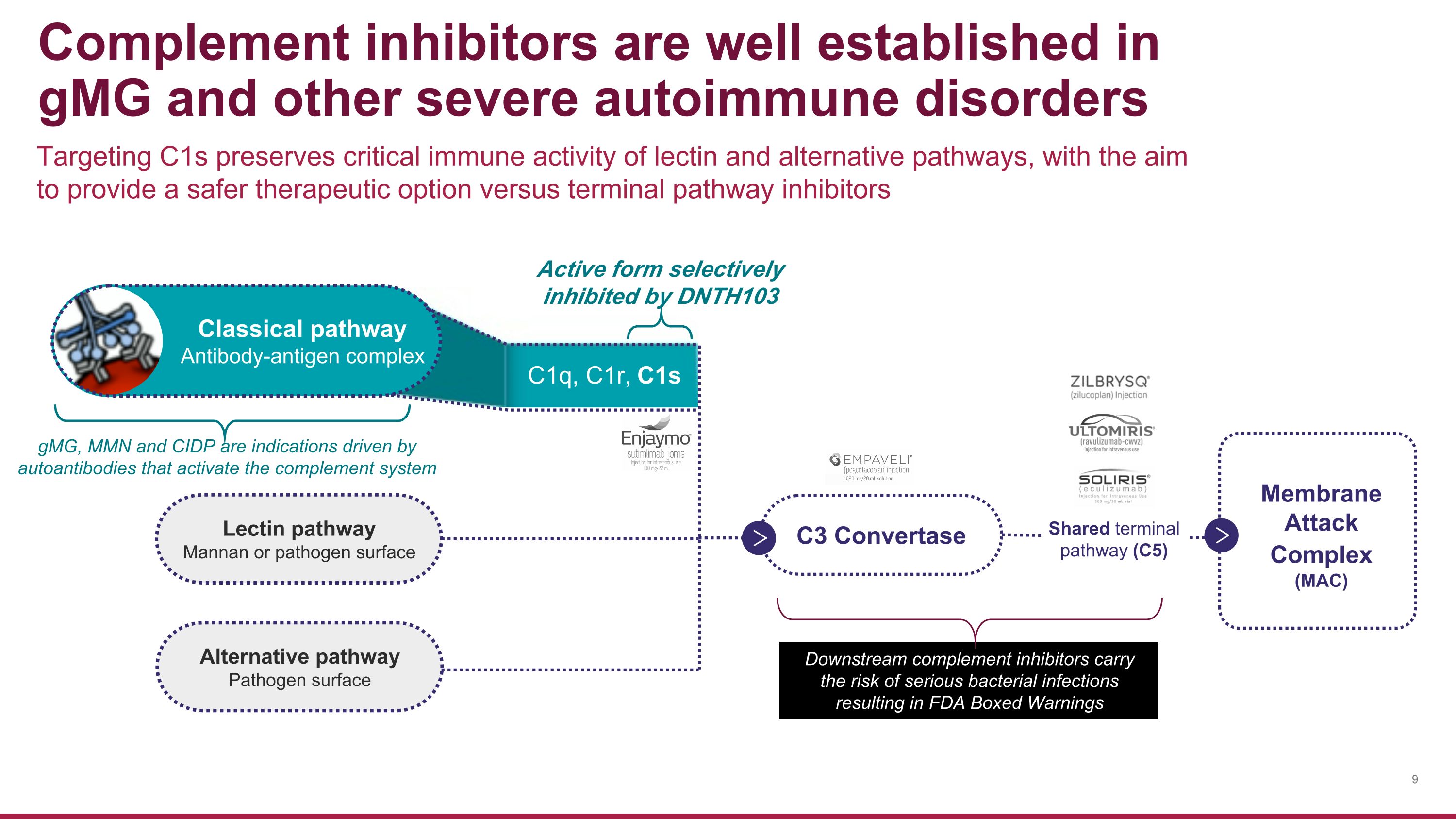
Membrane �Attack Complex �(MAC) Targeting C1s preserves critical immune activity of lectin and alternative pathways, with the aim to provide a safer therapeutic option versus terminal pathway inhibitors Complement inhibitors are well established in gMG and other severe autoimmune disorders Downstream complement inhibitors carry the risk of serious bacterial infections resulting in FDA Boxed Warnings Lectin pathway Mannan or pathogen surface Alternative pathway Pathogen surface C3 Convertase Shared terminal pathway (C5) Classical pathway Antibody-antigen complex Active form selectively inhibited by DNTH103 C1q, C1r, C1s gMG, MMN and CIDP are indications driven by autoantibodies that activate the complement system
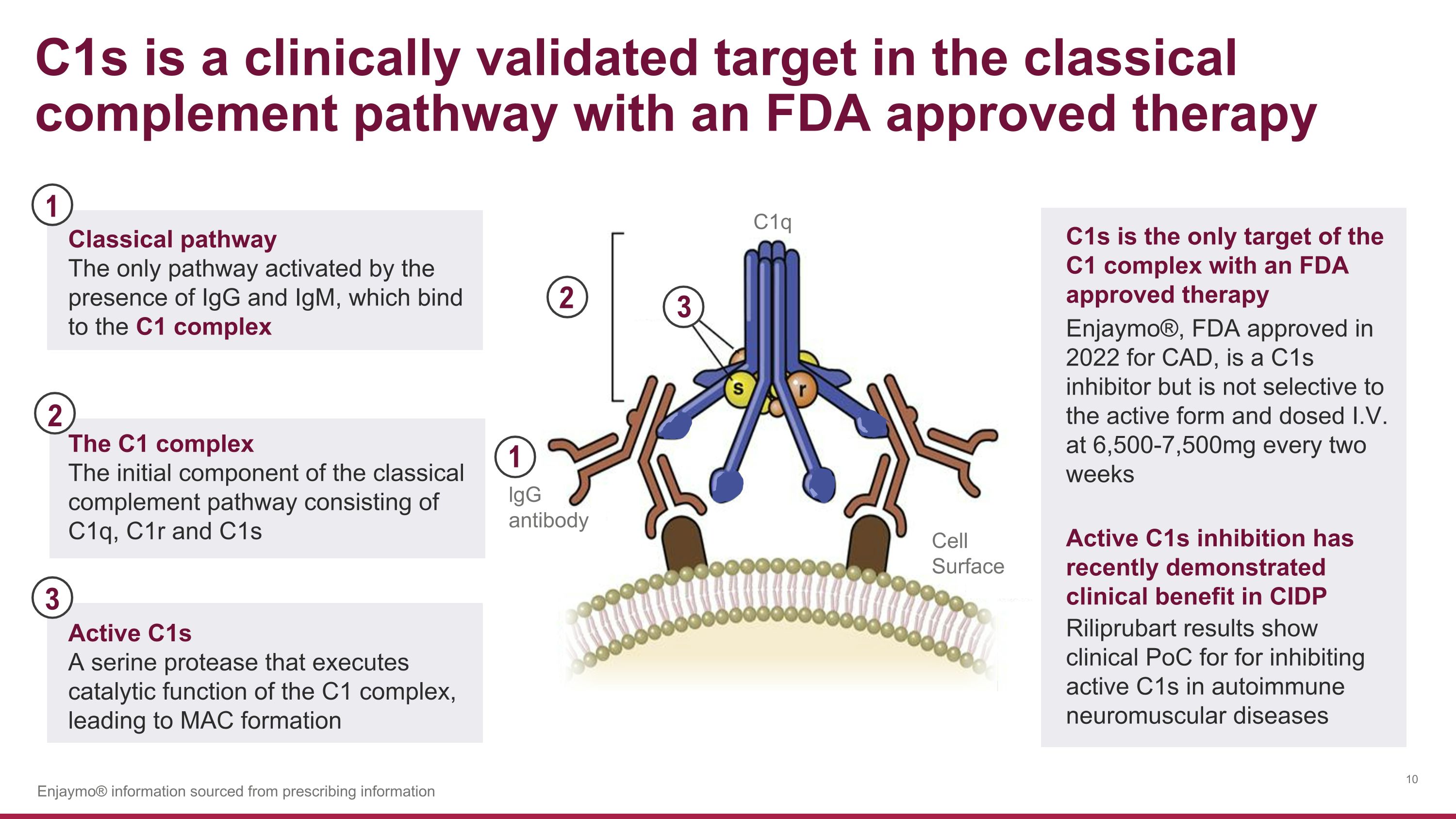
C1q lgG�antibody Cell�Surface C1s is a clinically validated target in the classical complement pathway with an FDA approved therapy The C1 complex The initial component of the classical complement pathway consisting of C1q, C1r and C1s 3 2 2 3 Active C1s A serine protease that executes catalytic function of the C1 complex, leading to MAC formation C1s is the only target of the C1 complex with an FDA approved therapy Enjaymo®, FDA approved in 2022 for CAD, is a C1s inhibitor but is not selective to the active form and dosed I.V. at 6,500-7,500mg every two weeks Enjaymo® information sourced from prescribing information 1 Classical pathway The only pathway activated by the presence of IgG and IgM, which bind to the C1 complex 1 Active C1s inhibition has recently demonstrated clinical benefit in CIDP Riliprubart results show clinical PoC for for inhibiting active C1s in autoimmune neuromuscular diseases
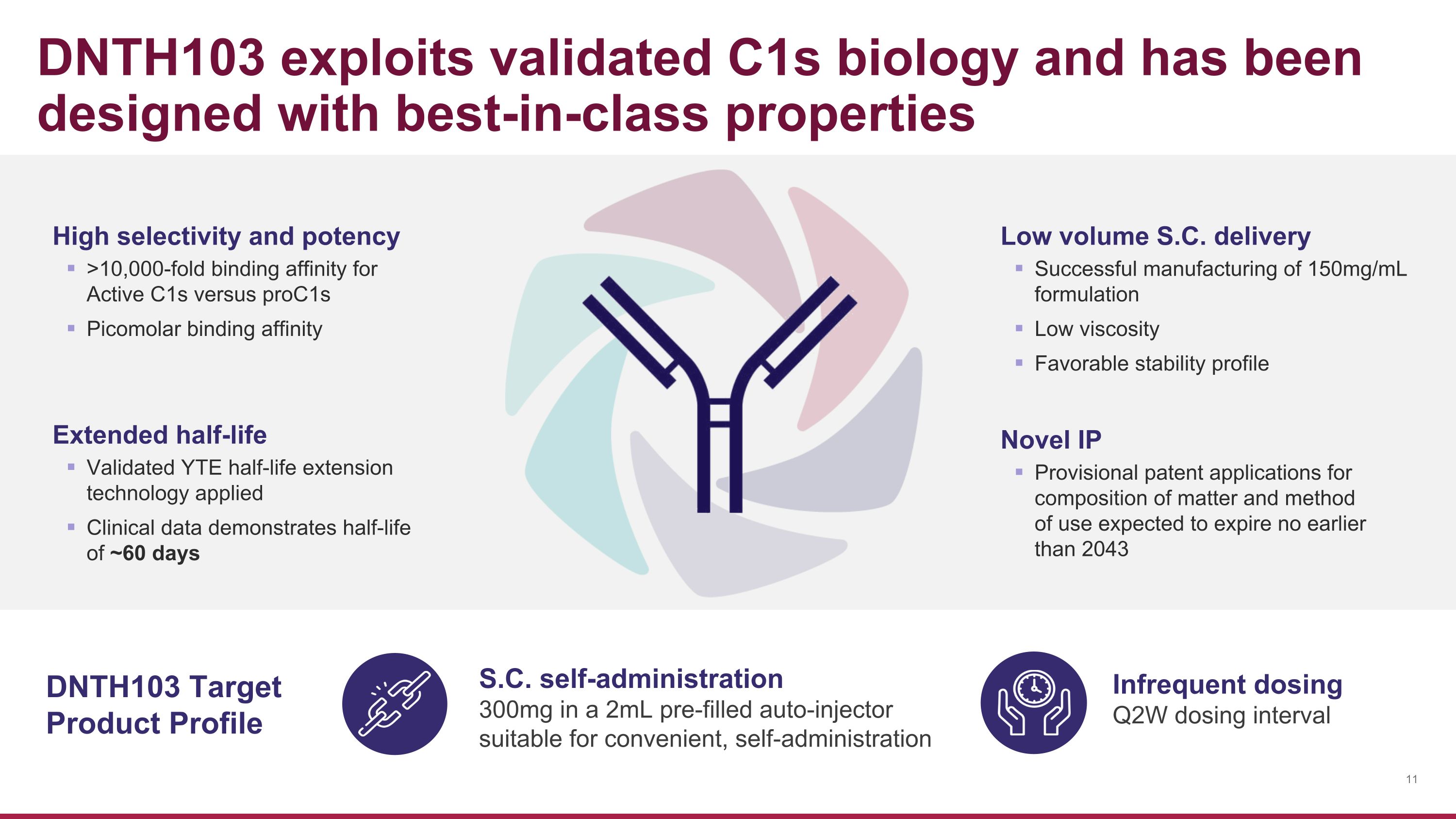
DNTH103 exploits validated C1s biology and has been designed with best-in-class properties Extended half-life Validated YTE half-life extension technology applied Clinical data demonstrates half-life of ~60 days Novel IP Provisional patent applications for composition of matter and method of use expected to expire no earlier than 2043 Low volume S.C. delivery Successful manufacturing of 150mg/mL formulation Low viscosity Favorable stability profile DNTH103 Target Product Profile S.C. self-administration 300mg in a 2mL pre-filled auto-injector suitable for convenient, self-administration Infrequent dosing Q2W dosing interval High selectivity and potency >10,000-fold binding affinity for Active C1s versus proC1s Picomolar binding affinity

DNTH103 Clinical Development
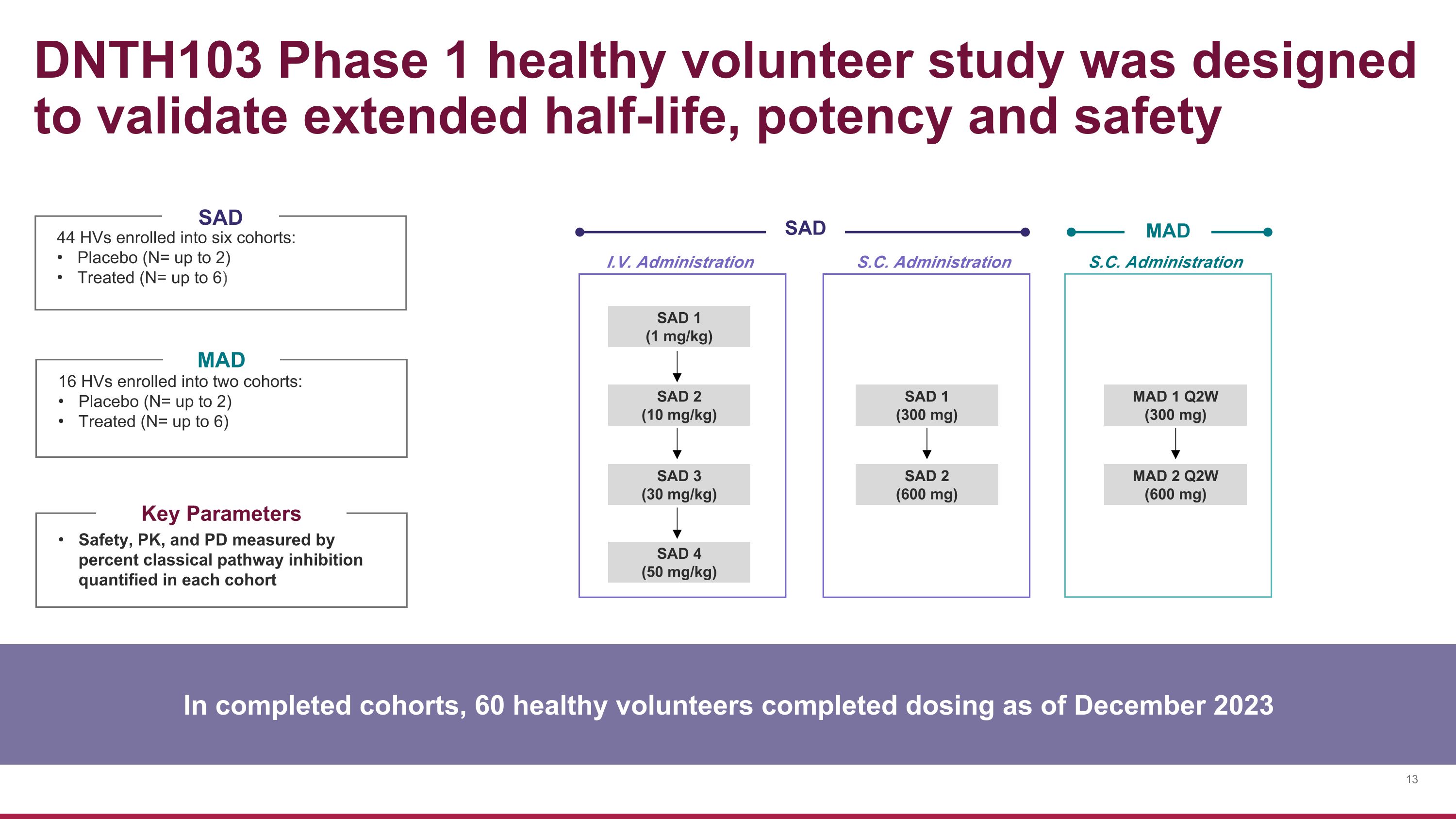
SAD 1 (1 mg/kg) SAD 3 (30 mg/kg) SAD 2 (10 mg/kg) MAD 1 Q2W (300 mg) MAD 2 Q2W (600 mg) SAD 1 (300 mg) SAD 2 (600 mg) I.V. Administration S.C. Administration S.C. Administration SAD MAD DNTH103 Phase 1 healthy volunteer study was designed to validate extended half-life, potency and safety 44 HVs enrolled into six cohorts: Placebo (N= up to 2) Treated (N= up to 6) 16 HVs enrolled into two cohorts: Placebo (N= up to 2) Treated (N= up to 6) SAD MAD Safety, PK, and PD measured by percent classical pathway inhibition quantified in each cohort Key Parameters In completed cohorts, 60 healthy volunteers completed dosing as of December 2023 SAD 4 (50 mg/kg)
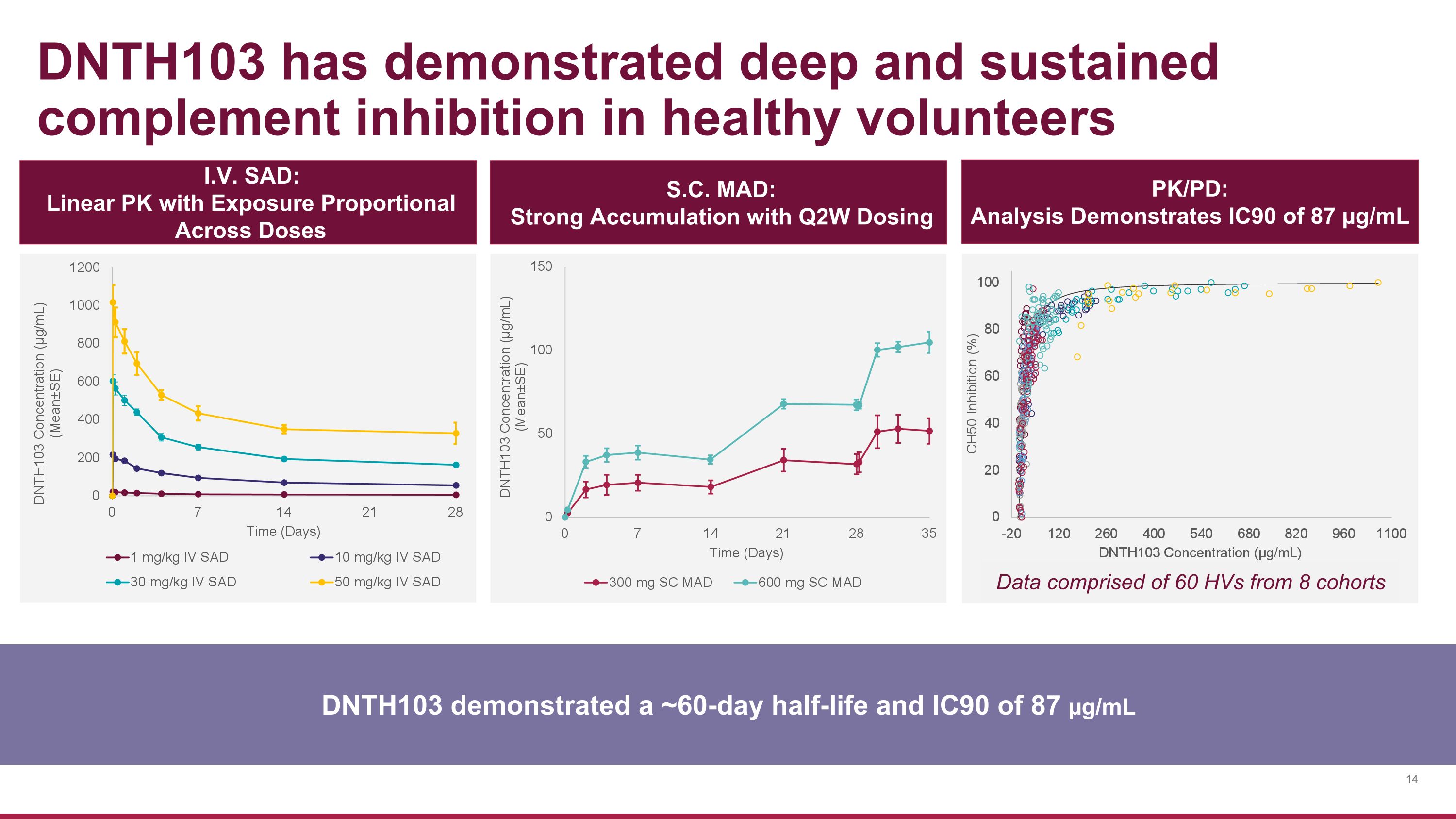
DNTH103 has demonstrated deep and sustained complement inhibition in healthy volunteers DNTH103 demonstrated a ~60-day half-life and IC90 of 87 µg/mL I.V. SAD: Linear PK with Exposure Proportional Across Doses S.C. MAD: Strong Accumulation with Q2W Dosing PK/PD: Analysis Demonstrates IC90 of 87 µg/mL Data comprised of 60 HVs from 8 cohorts
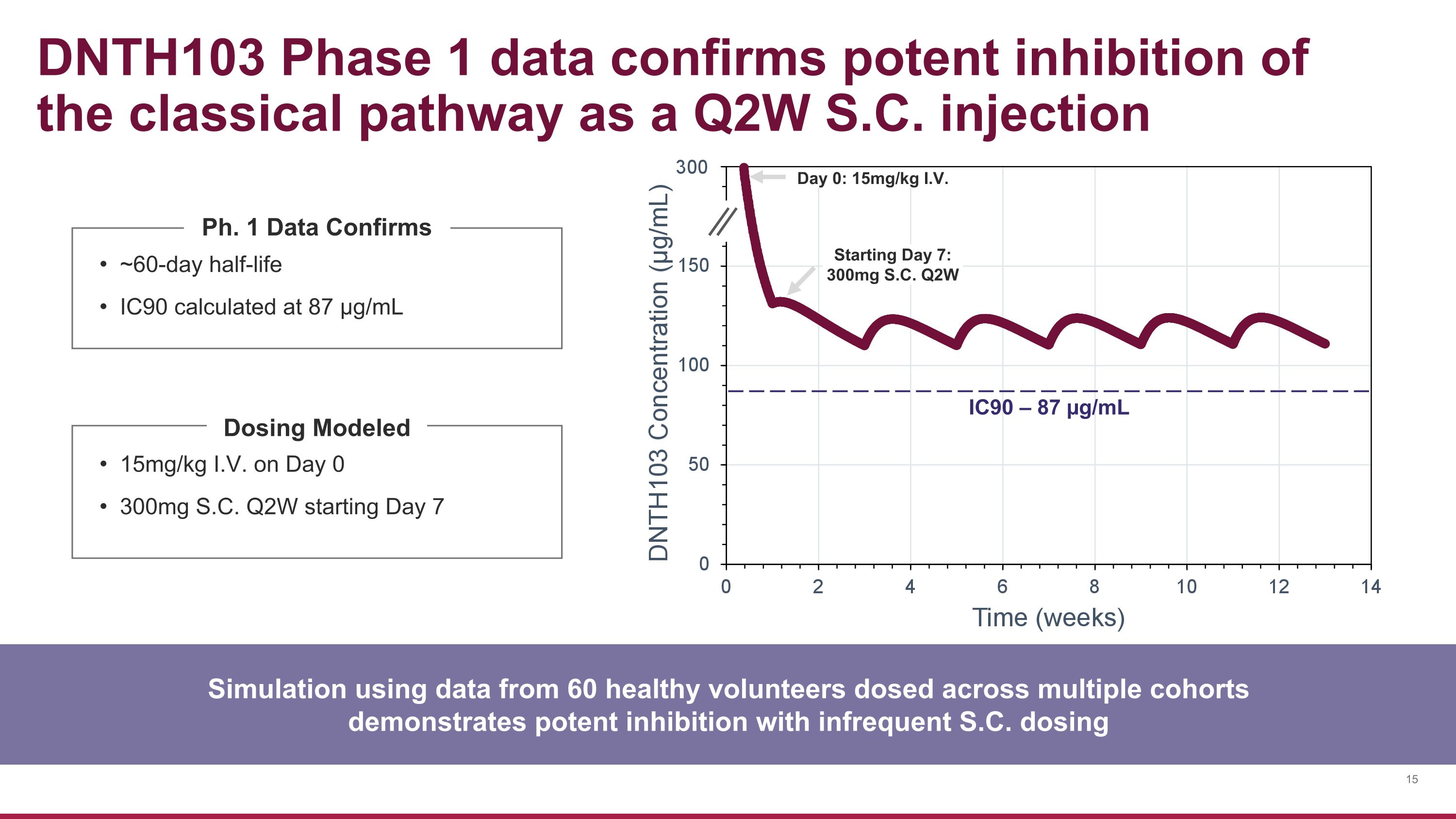
15mg/kg I.V. on Day 0 300mg S.C. Q2W starting Day 7 DNTH103 Phase 1 data confirms potent inhibition of the classical pathway as a Q2W S.C. injection ~60-day half-life IC90 calculated at 87 µg/mL Ph. 1 Data Confirms Simulation using data from 60 healthy volunteers dosed across multiple cohorts demonstrates potent inhibition with infrequent S.C. dosing IC90 – 87 µg/mL Day 0: 15mg/kg I.V. Starting Day 7: 300mg S.C. Q2W Dosing Modeled
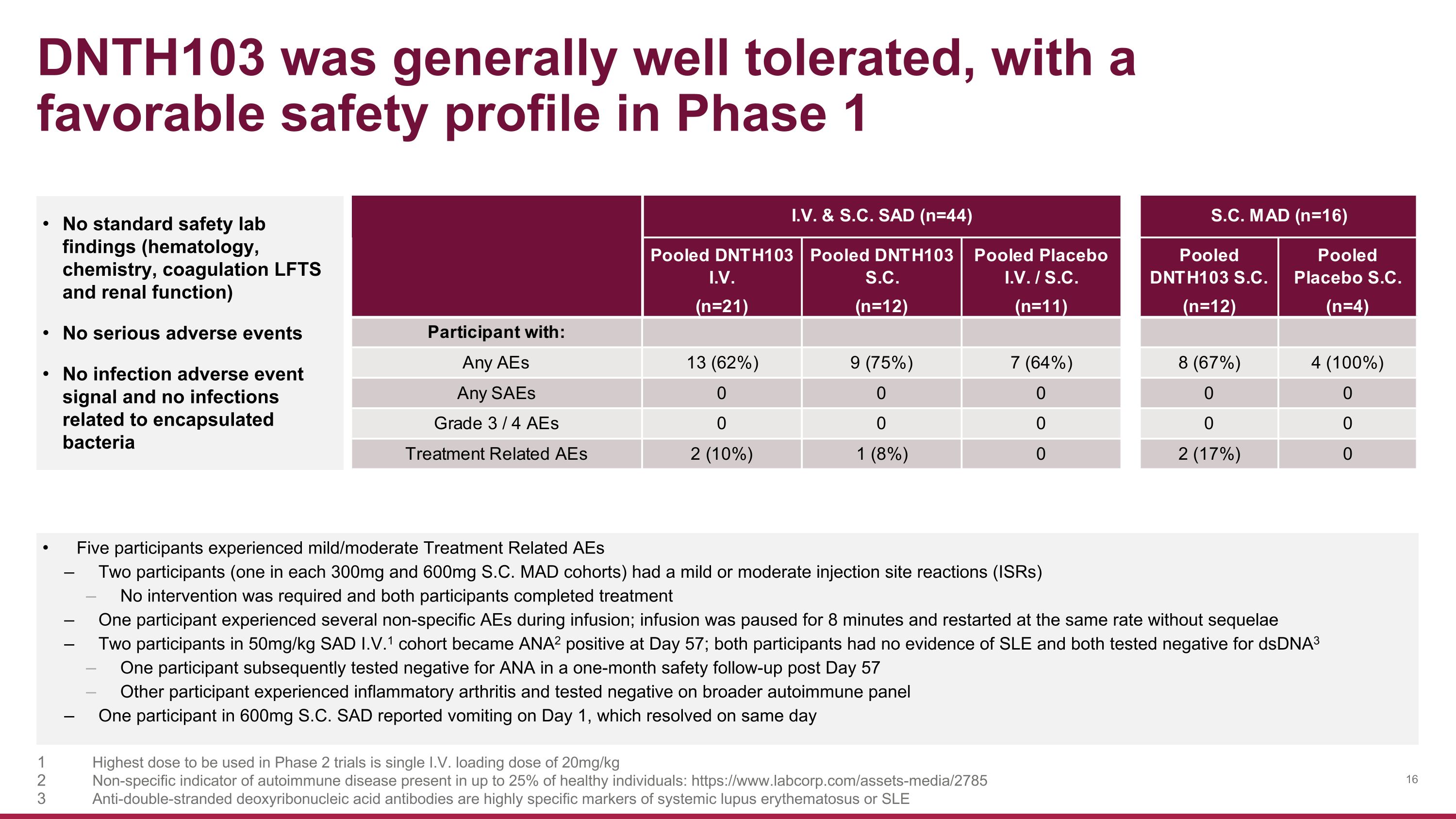
DNTH103 was generally well tolerated, with a favorable safety profile in Phase 1 No standard safety lab findings (hematology, chemistry, coagulation LFTS and renal function) No serious adverse events No infection adverse event signal and no infections related to encapsulated bacteria Five participants experienced mild/moderate Treatment Related AEs Two participants (one in each 300mg and 600mg S.C. MAD cohorts) had a mild or moderate injection site reactions (ISRs) No intervention was required and both participants completed treatment One participant experienced several non-specific AEs during infusion; infusion was paused for 8 minutes and restarted at the same rate without sequelae Two participants in 50mg/kg SAD I.V.1 cohort became ANA2 positive at Day 57; both participants had no evidence of SLE and both tested negative for dsDNA3 One participant subsequently tested negative for ANA in a one-month safety follow-up post Day 57 Other participant experienced inflammatory arthritis and tested negative on broader autoimmune panel One participant in 600mg S.C. SAD reported vomiting on Day 1, which resolved on same day Highest dose to be used in Phase 2 trials is single I.V. loading dose of 20mg/kg Non-specific indicator of autoimmune disease present in up to 25% of healthy individuals: https://www.labcorp.com/assets-media/2785 Anti-double-stranded deoxyribonucleic acid antibodies are highly specific markers of systemic lupus erythematosus or SLE
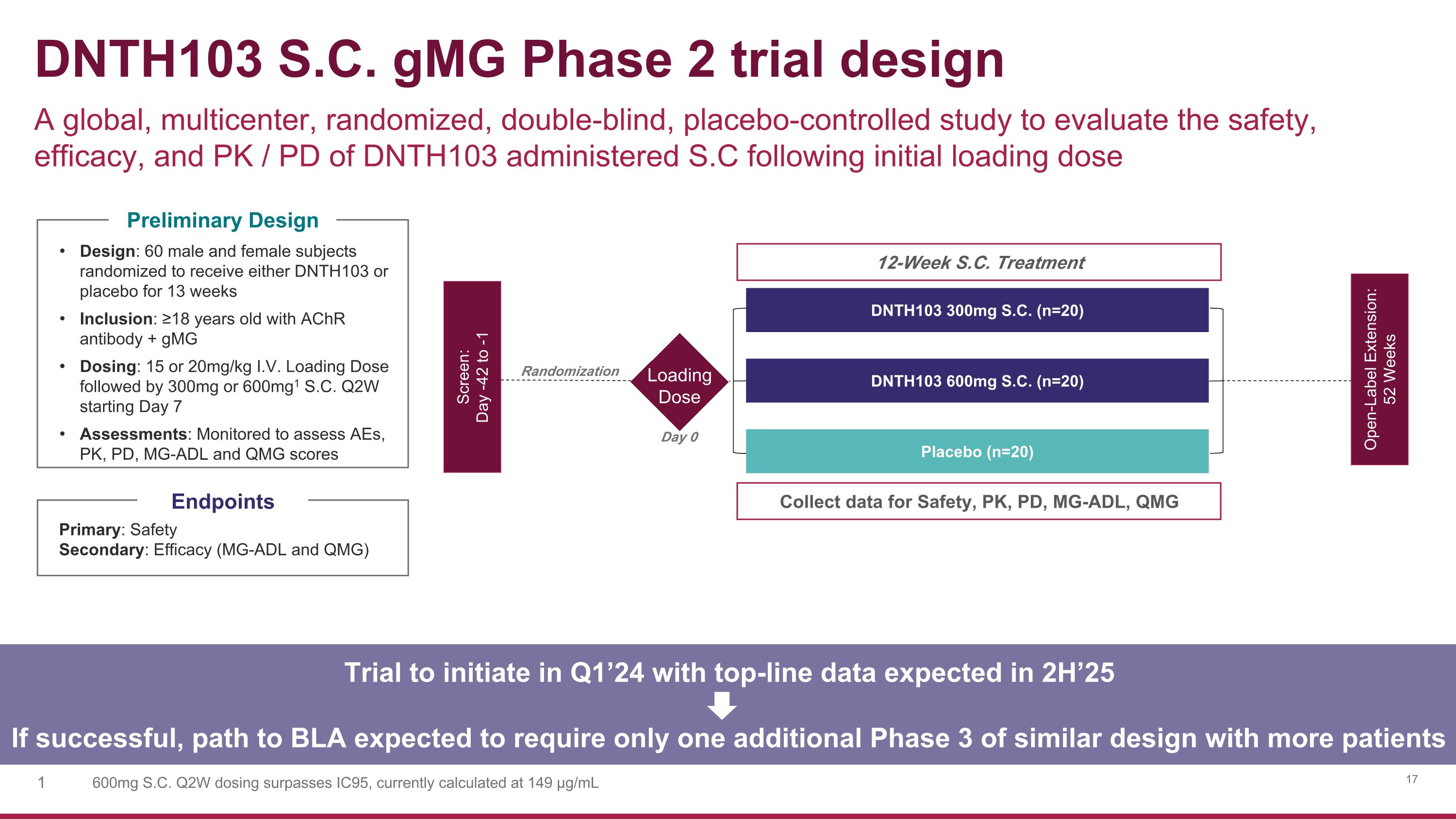
DNTH103 S.C. gMG Phase 2 trial design A global, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, efficacy, and PK / PD of DNTH103 administered S.C following initial loading dose 12-Week S.C. Treatment Screen: Day -42 to -1 Open-Label Extension: 52 Weeks DNTH103 300mg S.C. (n=20) DNTH103 600mg S.C. (n=20) Collect data for Safety, PK, PD, MG-ADL, QMG Randomization Primary: Safety Secondary: Efficacy (MG-ADL and QMG) Design: 60 male and female subjects randomized to receive either DNTH103 or placebo for 13 weeks Inclusion: ≥18 years old with AChR antibody + gMG Dosing: 15 or 20mg/kg I.V. Loading Dose followed by 300mg or 600mg1 S.C. Q2W starting Day 7 Assessments: Monitored to assess AEs, PK, PD, MG-ADL and QMG scores Endpoints Preliminary Design Placebo (n=20) Trial to initiate in Q1’24 with top-line data expected in 2H’25 If successful, path to BLA expected to require only one additional Phase 3 of similar design with more patients Loading Dose Day 0 600mg S.C. Q2W dosing surpasses IC95, currently calculated at 149 µg/mL
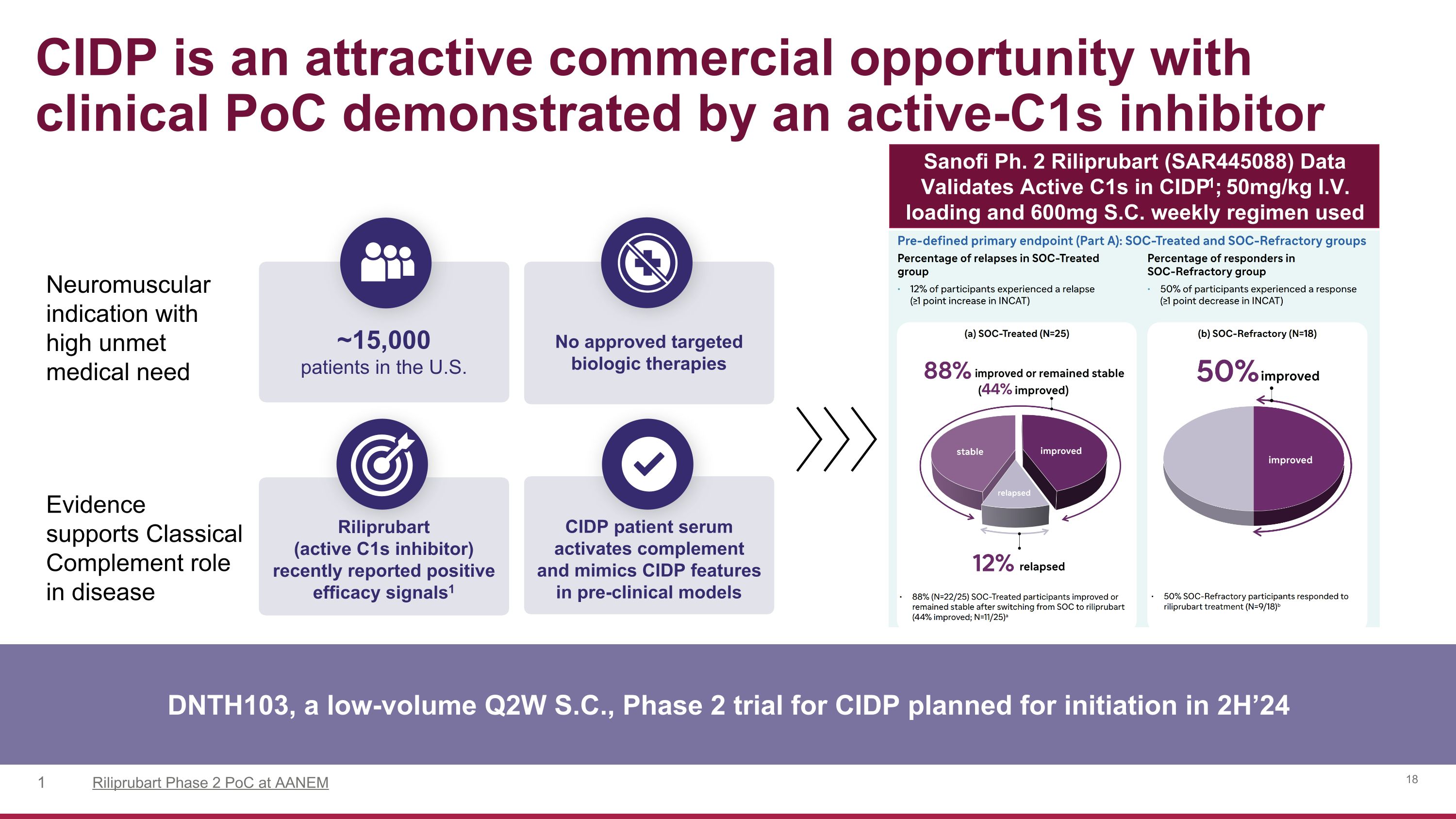
Neuromuscular indication with high unmet medical need Evidence �supports Classical Complement role in disease CIDP is an attractive commercial opportunity with clinical PoC demonstrated by an active-C1s inhibitor DNTH103, a low-volume Q2W S.C., Phase 2 trial for CIDP planned for initiation in 2H’24 ~15,000�patients in the U.S. No approved targeted biologic therapies Riliprubart (active C1s inhibitor) recently reported positive efficacy signals1 CIDP patient serum activates complement and mimics CIDP features in pre-clinical models Riliprubart Phase 2 PoC at AANEM Sanofi Ph. 2 Riliprubart (SAR445088) Data Validates Active C1s in CIDP1; 50mg/kg I.V. loading and 600mg S.C. weekly regimen used
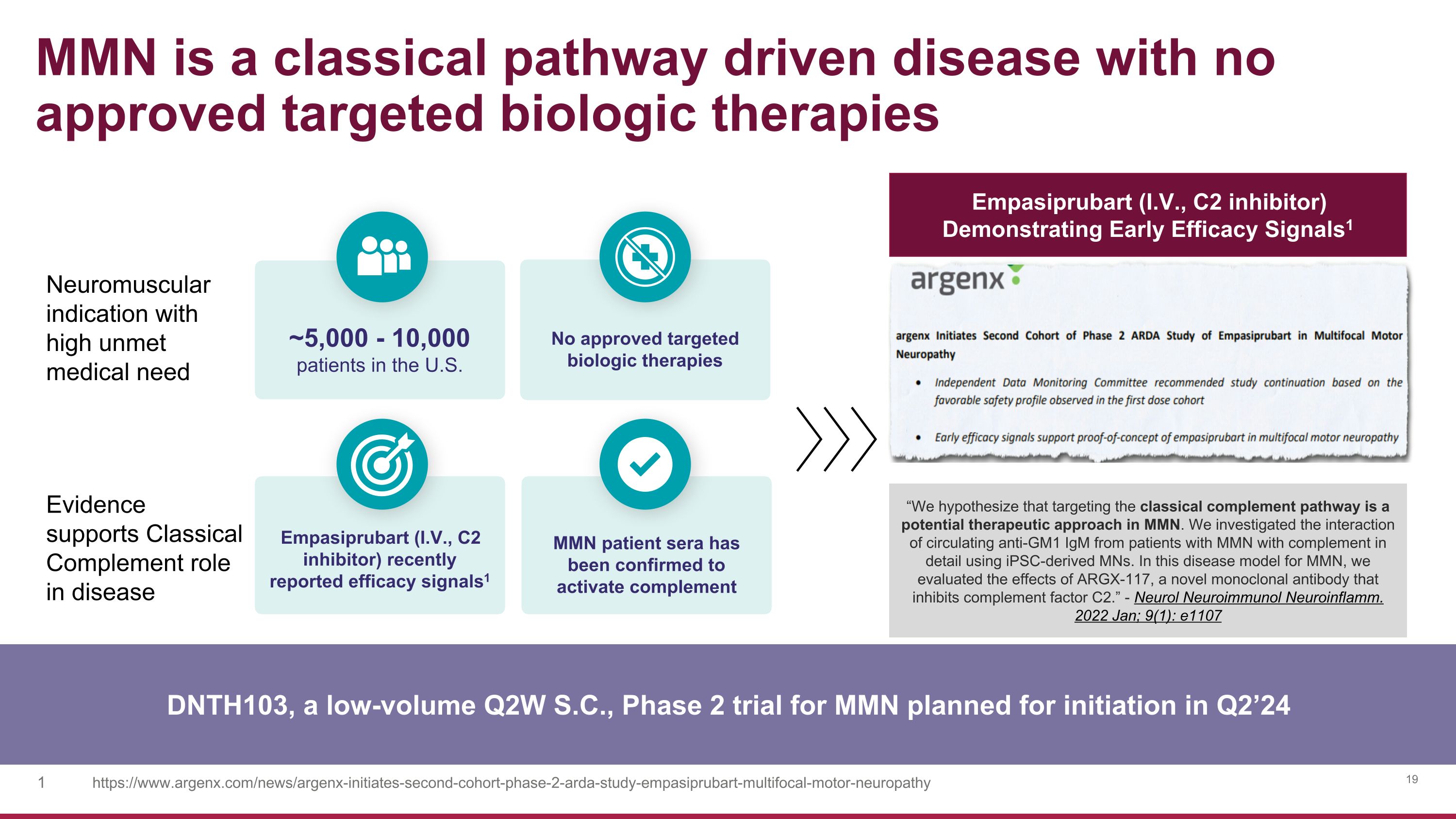
Neuromuscular indication with high unmet medical need Evidence �supports Classical Complement role in disease MMN is a classical pathway driven disease with no approved targeted biologic therapies DNTH103, a low-volume Q2W S.C., Phase 2 trial for MMN planned for initiation in Q2’24 No approved targeted biologic therapies Empasiprubart (I.V., C2 inhibitor) recently reported efficacy signals1 MMN patient sera has been confirmed to activate complement ~5,000 - 10,000�patients in the U.S. https://www.argenx.com/news/argenx-initiates-second-cohort-phase-2-arda-study-empasiprubart-multifocal-motor-neuropathy Empasiprubart (I.V., C2 inhibitor) Demonstrating Early Efficacy Signals1 “We hypothesize that targeting the classical complement pathway is a potential therapeutic approach in MMN. We investigated the interaction of circulating anti-GM1 IgM from patients with MMN with complement in detail using iPSC-derived MNs. In this disease model for MMN, we evaluated the effects of ARGX-117, a novel monoclonal antibody that inhibits complement factor C2.” - Neurol Neuroimmunol Neuroinflamm. 2022 Jan; 9(1): e1107

Corporate
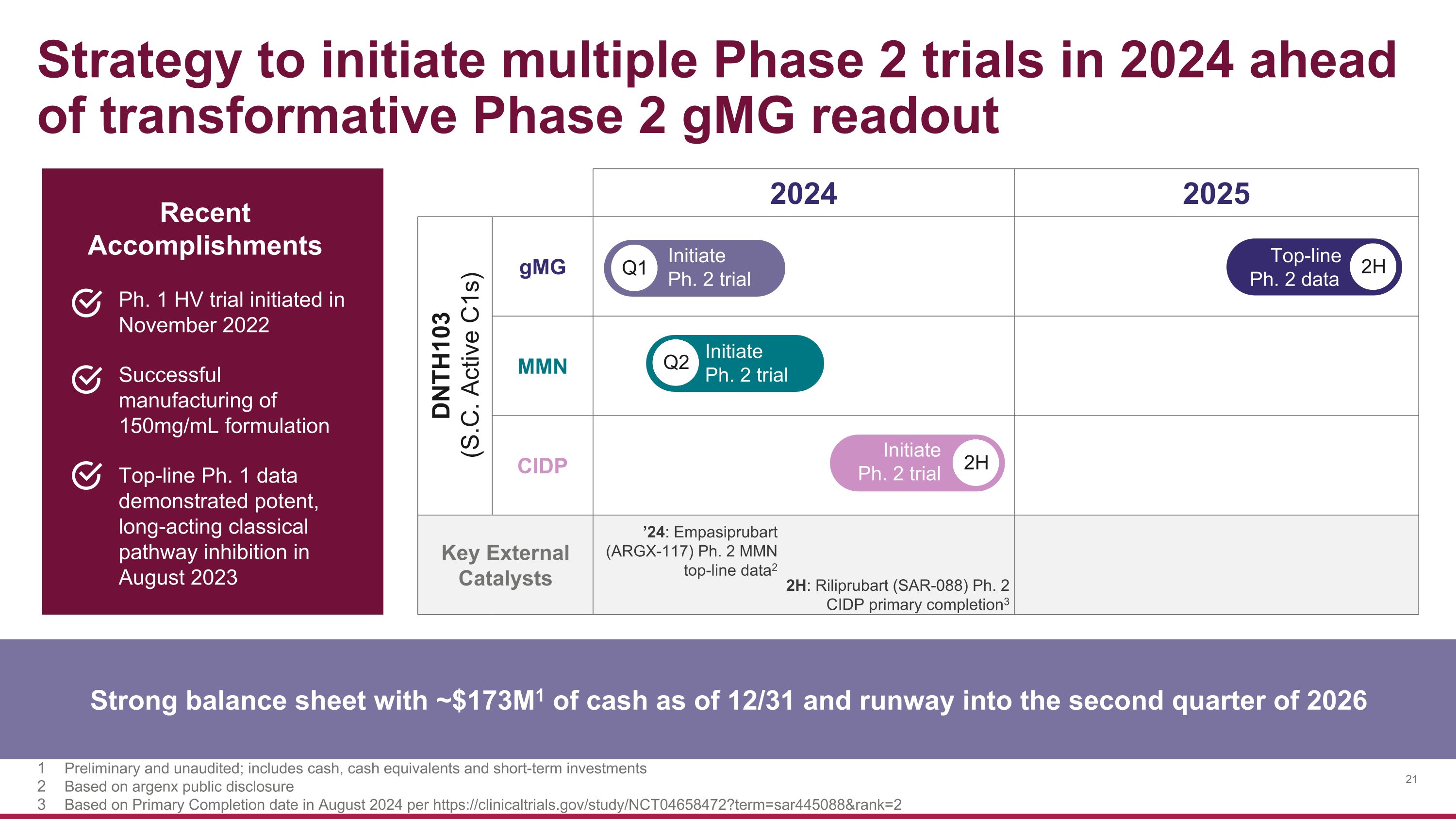
Strategy to initiate multiple Phase 2 trials in 2024 ahead of transformative Phase 2 gMG readout 2024 2025 DNTH103 (S.C. Active C1s) gMG MMN CIDP Key External Catalysts Key External Catalysts Strong balance sheet with ~$173M1 of cash as of 12/31 and runway into the second quarter of 2026 Q1 Initiate �Ph. 2 trial 2H Top-line �Ph. 2 data Q2 Initiate �Ph. 2 trial 2H Initiate �Ph. 2 trial Ph. 1 HV trial initiated in November 2022 Successful manufacturing of 150mg/mL formulation Top-line Ph. 1 data demonstrated potent, long-acting classical pathway inhibition in August 2023 Recent Accomplishments Preliminary and unaudited; includes cash, cash equivalents and short-term investments Based on argenx public disclosure Based on Primary Completion date in August 2024 per https://clinicaltrials.gov/study/NCT04658472?term=sar445088&rank=2 ’24: Empasiprubart (ARGX-117) Ph. 2 MMN top-line data2 2H: Riliprubart (SAR-088) Ph. 2 CIDP primary completion3

Accomplished team of biotech industry veterans and scientists committed to bringing innovation to market Select Auto-Immune Drugs Developed by Dianthus Team Select Experience Includes: Marino Garcia President & CEO Simrat Randhawa, M.D. Chief Medical Officer Ryan Savitz Chief Financial Officer Adam Veness, Esq. General Counsel Rivka Gluck, R.N. Head of Clinical Development Operations Kristina Maximenko Chief People Officer Jennifer Davis Ruff Head of Investor Relations & Corporate Affairs Jeffrey Stavenhagen, Ph.D. Chief Scientific Officer Sankalp Gokhale, M.D. Head of Clinical Development, Neurology Debra Segal Head of Regulatory Affairs BOARD OF DIRECTORS SENIOR MANAGEMENT Lonnie Moulder Chairman of the Board, Dianthus Tomas Kiselak Managing Member, Fairmount Alison Lawton Board Member, ProQR and X4, Prior Chair of Board, Magenta Anne McGeorge Board Member, The Oncology Institute, Board Member, Be the Match Lei Meng Senior Therapeutics Analyst, Avidity Partners Paula Soteropoulos Venture Partner, 5AM Ventures Jonathan Violin, Ph.D. Venture Partner, Fairmount, Co-founder of Dianthus, Board Member, Astria Therapeutics, and former President/CEO of Viridian Therapeutics Marino Garcia President & CEO, Dianthus Jud Taylor Head of Technical Operations Robert McGarr, Ph.D. Head of Program & Alliance Management

Appendix
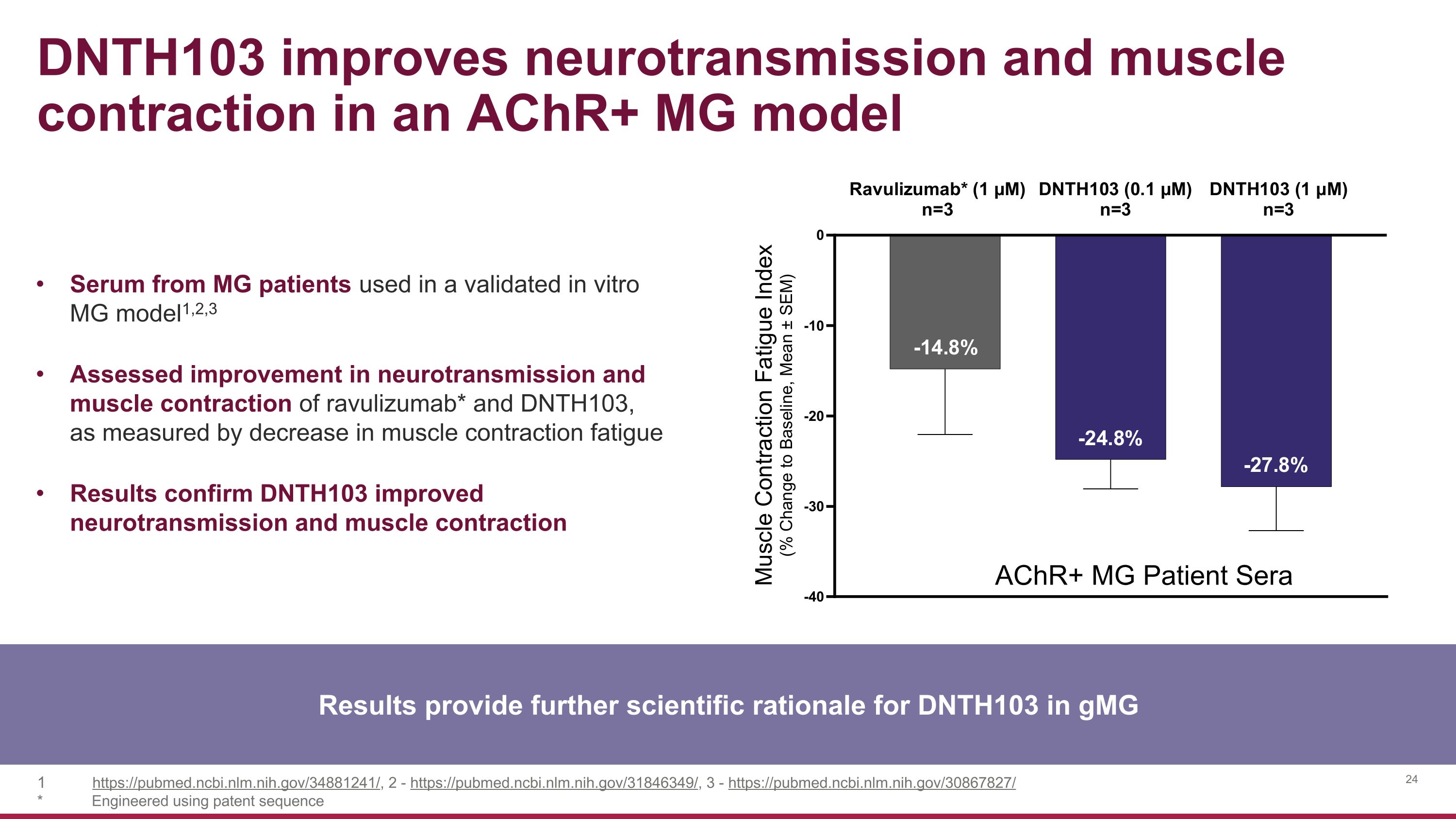
DNTH103 improves neurotransmission and muscle contraction in an AChR+ MG model Results provide further scientific rationale for DNTH103 in gMG Serum from MG patients used in a validated in vitro MG model1,2,3 Assessed improvement in neurotransmission and muscle contraction of ravulizumab* and DNTH103, as measured by decrease in muscle contraction fatigue Results confirm DNTH103 improved neurotransmission and muscle contraction https://pubmed.ncbi.nlm.nih.gov/34881241/, 2 - https://pubmed.ncbi.nlm.nih.gov/31846349/, 3 - https://pubmed.ncbi.nlm.nih.gov/30867827/ * Engineered using patent sequence AChR+ MG Patient Sera
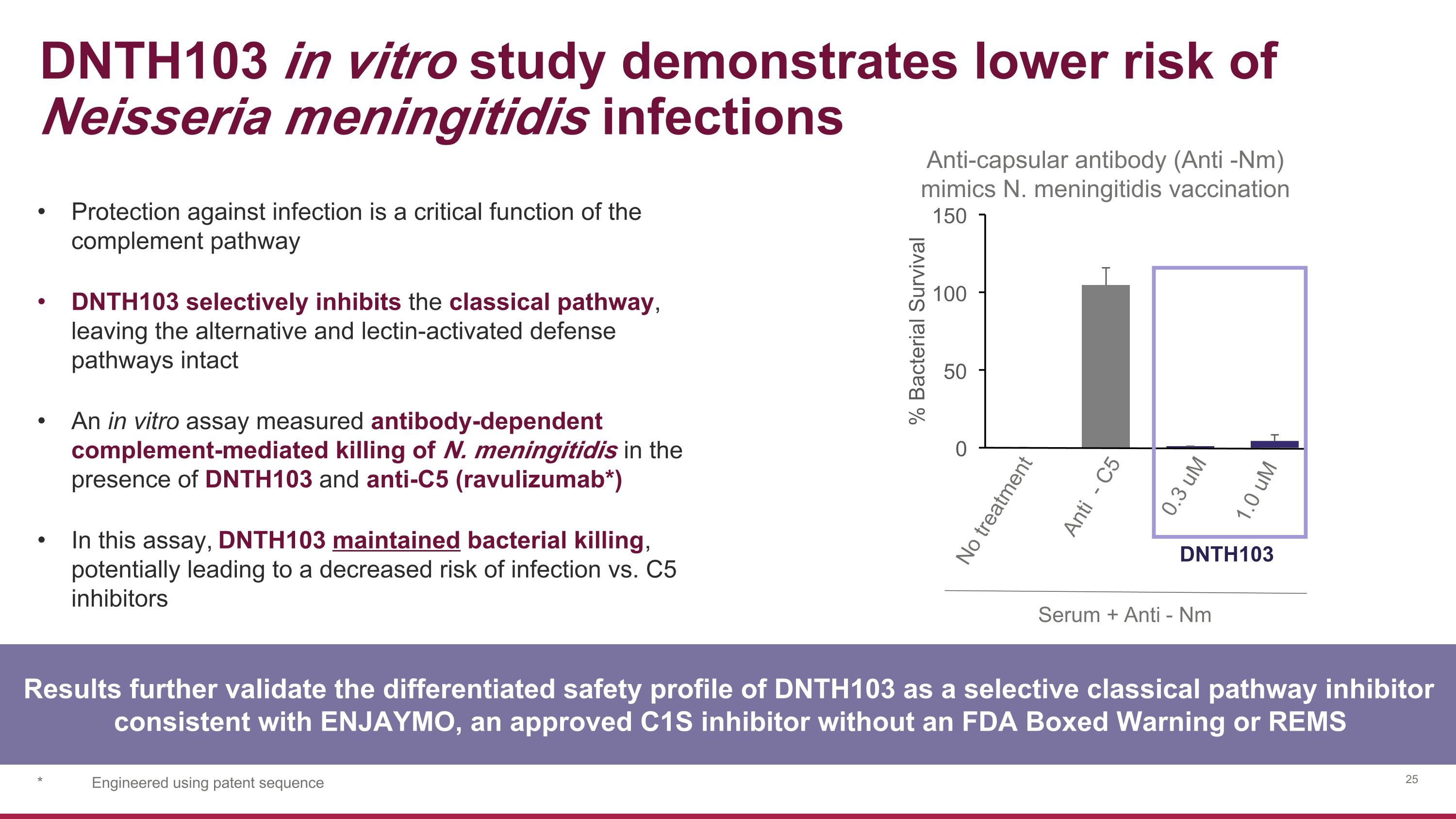
DNTH103 in vitro study demonstrates lower risk of Neisseria meningitidis infections Anti-capsular antibody (Anti -Nm) mimics N. meningitidis vaccination 0 50 100 150 % Bacterial Survival Serum +Anti - Nm 0.3 uM 1.0 uM DNTH103 Anti - C5 No treatment Results further validate the differentiated safety profile of DNTH103 as a selective classical pathway inhibitor consistent with ENJAYMO, an approved C1S inhibitor without an FDA Boxed Warning or REMS Protection against infection is a critical function of the complement pathway DNTH103 selectively inhibits the classical pathway, leaving the alternative and lectin-activated defense pathways intact An in vitro assay measured antibody-dependent complement-mediated killing of N. meningitidis in the presence of DNTH103 and anti-C5 (ravulizumab*) In this assay, DNTH103 maintained bacterial killing, potentially leading to a decreased risk of infection vs. C5 inhibitors * Engineered using patent sequence
v3.23.4
Document and Entity Information
|
Jan. 08, 2024 |
| Document And Entity Information [Line Items] |
|
| Document Type |
8-K
|
| Document Period End Date |
Jan. 08, 2024
|
| Entity Registrant Name |
DIANTHUS THERAPEUTICS, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-38541
|
| Entity Tax Identification Number |
81-0724163
|
| Entity Address, Address Line One |
7 Times Square
|
| Entity Address, Address Line Two |
43rd Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10036
|
| City Area Code |
929
|
| Local Phone Number |
999-4055
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 Par Value
|
| Trading Symbol |
DNTH
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| Entity Central Index Key |
0001690585
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Magenta Therapeutics (NASDAQ:MGTA)
Historical Stock Chart
From May 2024 to Jun 2024
Magenta Therapeutics (NASDAQ:MGTA)
Historical Stock Chart
From Jun 2023 to Jun 2024