false 0001786205 0001786205 2023-12-11 2023-12-11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 11, 2023
Arcellx, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-41259 |
|
47-2855917 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 25 West Watkins Mill Road |
|
|
| Suite A |
|
|
| Gaithersburg, Maryland |
|
20878 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 240 327-0603
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.001 par value per share |
|
ACLX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On December 11, 2023, Arcellx, Inc. (the “Company”) hosted a webcast investor relations event at the 65th American Society of Hematology Annual Meeting in San Diego, CA, highlighting results from the Company’s CART-ddBCMA Phase 1 expansion clinical trial in patients with relapsed or refractory multiple myeloma. During the webcast, the Company presented the slides attached as Exhibit 99.1 to this Current Report on Form 8-K, which are incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
Arcellx, Inc. |
|
|
|
|
| Date: December 12, 2023 |
|
|
|
By: |
|
/s/ Rami Elghandour |
|
|
|
|
|
|
Rami Elghandour Chief Executive Officer |

Exhibit 99.1 th Investor Relations Event at the 65 ASH Annual Meeting
December 11, 2023

Forward-Looking Statements This presentation contains forward-looking
statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on our management’s beliefs and assumptions and on information currently
available to our management. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial condition, results of operations, business strategy, operations and prospects,
the potential of and expectations regarding our product candidates and programs, including our ability to launch and scale, and the plans and objectives of management, as well as statements regarding industry trends, are forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “can,” “contemplate,” “continue,” “could,” “design,”
“estimate,” “expect,” “imagine,” “intend,” “likely,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,”
“predict,” “project,” “should,” “target,” “will” or “would,” or the negative of these terms or other similar expressions or other comparable terminology are intended to identify
forward-looking statements, although not all forward-looking statements contain these identifying words. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we
believe may affect our financial condition, results of operations, business strategy and financial needs, and these statements represent our views as of the date of this presentation. We may not actually achieve the plans, intentions or expectations
disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or
quantified. Information regarding certain risks, uncertainties and assumptions may be found in our filings with the Securities and Exchange Commission. New risk factors emerge from time to time and it is not possible for our management team to
predict all risk factors or assess the impact of all factors on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking
statements. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any
date subsequent to the date of this presentation. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration or any
other regulatory authority. The presentation also includes select interim and preliminary results from an ongoing clinical trial as of specific data cutoff dates. Such results should be viewed with caution as final results may differ as additional
data becomes available. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. No representation is made as to the safety or
effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation also contains estimates and other statistical data made by independent parties or publicly available information, as well as
other information based on our internal sources. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the accuracy or completeness of the
data contained in these industry publications and other publicly available information. Accordingly, we makes no representations as to the accuracy or completeness of that data. Cross-trial comparisons are not based on head-to-head studies and no
direct comparisons can be made. Cross-trial data interpretation should be considered with caution as it is limited by differences in study population, design and other factors. 2 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Agenda Topic Presenter Time Opening Remarks Rami Elghandour 10 Minutes
Chairman and Chief Executive Officer, Arcellx Phase I Study of anito-cel: A CART- Matthew J. Frigault, M.D., M.S. 20 Minutes Therapy Utilizing a Novel Synthetic iMMagine-1 and ACLX-001 Clinical Study Investigator, Binding Domain for the Treatment of
Clinical Director of the Cellular Therapy Service at Mass Subjects with Relapsed or Refractory General Cancer Center and Assistant Professor at Harvard Multiple Myeloma Medical School Panel Discussion and Q&A Panelists: 30 Minutes Matthew J.
Frigault, M.D., M.S. Krina K. Patel, M.D., M.Sc. iMMagine-1 Clinical Study Investigator, Associate Professor, Department of Lymphoma/Myeloma, Division of Cancer Medicine at the University of Texas MD Anderson Cancer Center Moderator: Chris Heery,
M.D. Chief Medical Officer, Arcellx Q&A 30 Minutes 3 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Arcellx is a Different Kind of Cell Therapy Company Potential
best-in-class therapy partnered with Kite, the global leader in cell therapy. Scalable manufacturing and commercial footprint to support leadership in a $12B+ Multiple Myeloma cell therapy market. Sufficient capital to fund operations into
2027.

Arcellx is well positioned for launch and scale D-Domain, A
Differentiated Potential Best-in-Class Positioned for Scale and Technology Platform Multiple Myeloma Franchise Success in CAR-T Space ‣ High Expression: Novel synthetic binder ‣ Potential best-in-class profile for ‣ Well
capitalized: Strong cash enables high CAR positive drug product CAR-T in MM: data from phase 1 position of $483M as of 9/30 with a low overall cell dose and high ($768M pro-forma post Kite trial demonstrates deep and surface expression durable
responses with expansion) expected to fund manageable safety profile operations into 2027 ‣ Manufacturability: Small size and high ‣ Large market with high unmet stability may allow for consistent, ‣ Favorable collaboration need:
Global Myeloma Market $12B+ scalable manufacturability structure: Co/co structure nd th across 2 to 5 line increases operating leverage, with CMC commercial readiness ‣ Versatility: Single infusion CAR-T and ‣ Partnered with Kite/Gilead:
Proven costs covered by Kite/Gilead dosable/controllable CAR-T platforms CMC capability and largest through collaboration provide novel approaches in commercial footprint with 17,000+ challenging hematologic and solid patients treated and 300+ ATC
tumor cancer settings‣ D-Domain IP: Growing pipeline is presence in 25+ countries wholly owned outside of CART- ddBCMA and ACLX-001 5 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Anitocabtagene autoleucel (anito-cel/CART-ddBCMA) 1 Autologous
BCMA-directed CAR T-cell therapy using a novel, D-Domain binder Bi-Valent 2 TCR 4 Camelid VHh (50kDa) (25kDa) 3 scFv (25kDa) D-Domain (8kDa) 41BB 41BB 41BB CD3zCD3z CD3z CD3z CD3z 1 Rotte, et al. Immuno-Oncology Insights
2022; 3(1), 13–24 2 Chan, KF. et al. 2018.,Nat Commun 9:1026-1026 3 Bjerragaard-Anderson, K., et al 2018. Sci. Rep., 8:10836-10836. 6 4 https://commons.wikimedia.org/wiki/File:1I3V_(Lama_VHH_domain_unligated).png#file Arcellx | 2 C0 o2 r3 p o
ArS a H t e IR P E rv ee sn et ntation
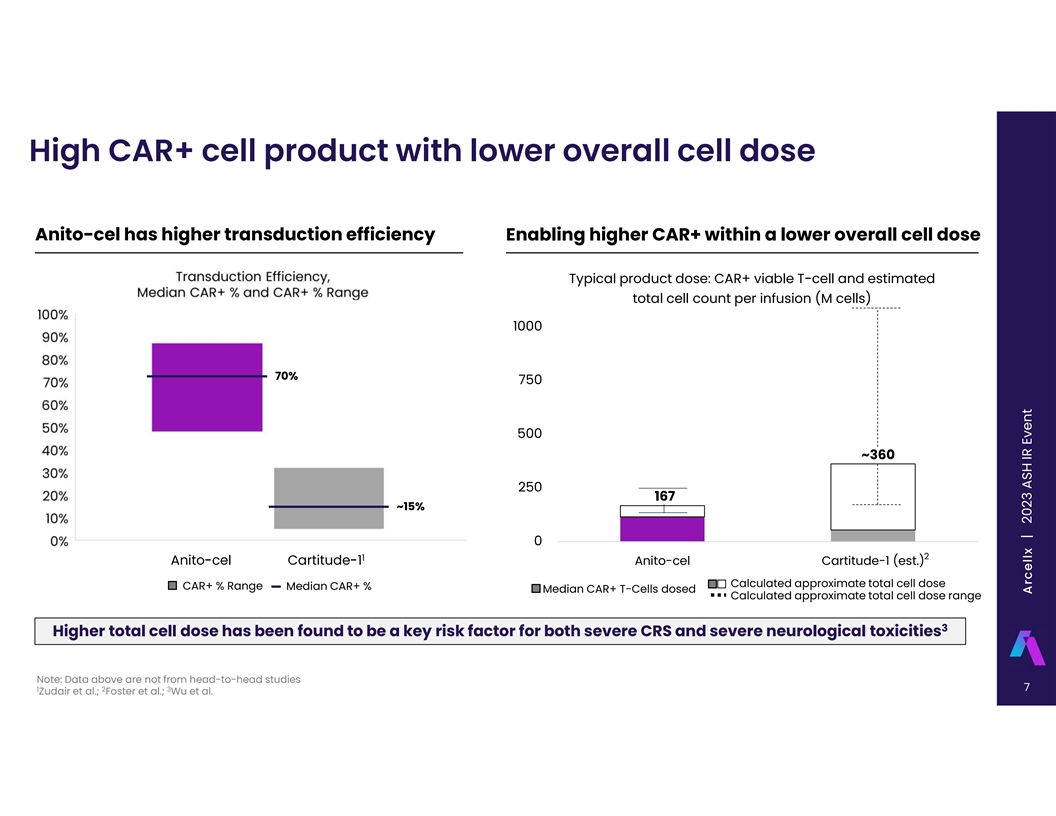
High CAR+ cell product with lower overall cell dose Anito-cel has higher
transduction efficiency Enabling higher CAR+ within a lower overall cell dose Typical product dose: CAR+ viable T-cell and estimated total cell count per infusion (M cells) 1000 70% 750 500 ~360 250 167 ~15% 0 2 1 Anito-cel Cartitude-1 Anito-cel
Cartitude-1 (est.) Calculated approximate total cell dose CAR+ % Range Median CAR+ % Median CAR+ T-Cells dosed Calculated approximate total cell dose range 3 Higher total cell dose has been found to be a key risk factor for both severe CRS and
severe neurological toxicities Note: Data above are not from head-to-head studies 7 1 2 3 Zudair et al.; Foster et al.; Wu et al. Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Phase 1 Clinical Profile Supports Potential Best-in-Class Candidate 100%
ORR Median PFS No Delayed not reached Neurotoxicities 76% CR/sCR at median follow-up of 26.5 mos. Including no Parkinsonian Symptoms No grade ≥3 CRS and 1 case of Grade 3 CR/sCR rate maintained across In the overall population studied, the
high-risk subgroups, including EMD, estimated median PFS has not been ICANS at RP2D. All events resolved without high-risk cytogenetics, age ≥65 reached at 24 months sequelae with routine management 32 patients at DL1 have had at least the
12-month follow-up visit and are evaluable for safety 0% Grade ≥3 CRS in DL1 and 3% Grade ≥3 ICANS in DL1 No tissue-targeted toxicities, no Guillain-Barré syndrome, no cranial nerve palsies observed as of latest data cut-off Phase 2
Pivotal Study Currently Enrolling 8 Phase 1 Study: October 15, 2023, data cut-off; ASH Annual Meeting, December 2023, abstract #1023 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Anito-cel Phase 1 in a higher risk patient population Extramedullary
Disease B2M ≥ 5.5 (ISS stage 3) High-Risk Cytogenetics** 50% 30% 40% 25% 40% 30% 20% 30% 15% 20% 28% 36% 35% 20% 39% 29% 34% 10% 18% 24% 16% 10% 14% 10% 20% 5% 13% 0% 0% 0% A An ni it to o- -C ce ell Cartitude-1 Legend-2 KarMMa* A An niit to
o- -C ce el l Cartitude-1 Legend-2 KarMMa A An niit to o- -C ce ell Cartitude-1 Legend-2 KarMMa Bone marrow plasma cells ≥ 60% Age group ≥ 65 Penta Refractory 30% 60% 80% 25% 50% 60% 20% 40% 15% 30% 40% 53% 68% 24% 22% 10% 20% 36% 35%
20% 42% 5% 10% 26% NA NA NA NA 0% 0% 0% Anito-cel Anito-cel Anito-cel Anito-Cel Cartitude-1 Legend-2 KarMMa Anito-Cel Cartitude-1 Legend-2 KarMMa Anito-Cel Cartitude-1 Legend-2 KarMMa *KarMMa EMD figure includes bone-based lesions; **Defined as the
presence of Del 17p, t(14;16), t(4;14); for Anito-cel, high risk cytogenetics including +1q gain is n=26 (68%); Data above are not from head-to-head studies. 9 4 6 7 KarMMa: Munshi et al.; Legend-2: Zhao et al.; Cartitude-1: Martin et al. (2023)
Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Multiple Myeloma is a Significant Market Opportunity Growing
opportunity for CAR-T 3rd most solutions as more effective common therapies move to blood cancer earlier line patients Incurable Impacting Multiple disease with 100,000 patients life expectancy annually Myeloma of just over 5 years Limited Therapies
Total addressable comprise ~$20B global market (2L+) of $12B+ market today in CAR-T 10 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

A Rich Development Pipeline with Growth in Mind Clinical and
Preclinical Pipeline Discovery/ Phase 2 / Indication Platform Phase 1 Current Status / Anticipated Milestone Preclinical Pivotal iMMagine-1 pivotal/ anito-cel Present preliminary data 2H’24 ddCAR Multiple Earlier line Ph 3 Confirmatory RCT /
anito-cel Initiation planned for 2024 Myeloma ACLX-001: BCMA ARC-SparX Kite exercised option ACLX-002: CD123 Phase 1 enrolling ARC-SparX AML/MDS ACLX-003 SCLC ARC-SparX Solid Tumors ddCAR HCC 11 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn
et ntation

Arcellx Reimagining Cell Therapy + Novel Synthetic Positive Interim
Phase Partnered with Global Platform Built for Binding Domain 1 Clinical Results Leader in Cell Therapy Potential Success Single-infusion ddCAR 100% ORR; 76% CR/sCR Combining potential ACLX-001 Phase 1 clinical Strong investor base platform and deep
durability in best in class program trial in MM initiated in Exceptional team CART-ddBCMA with Kite’s established 2Q22 and multiple myeloma commercial and Wholly owned IP ACLX-002 Phase 1 clinical Dosable, controllable Phase 1 study with mPFS
manufacturing expertise trial in AML/MDS Well capitalized ARC-SparX platform not reached at 26.5 mo. initiated in 4Q22 median follow-up Pivotal study enrolling 12 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Agenda Topic Presenter Time Opening Remarks Rami Elghandour 10 Minutes
Chairman and Chief Executive Officer, Arcellx Phase I Study of anito-cel: A CART- Matthew J. Frigault, M.D., M.S. 20 Minutes Therapy Utilizing a Novel Synthetic iMMagine-1 and ACLX-001 Clinical Study Investigator, Binding Domain for the Treatment of
Clinical Director of the Cellular Therapy Service at Mass Subjects with Relapsed or Refractory General Cancer Center and Assistant Professor at Harvard Multiple Myeloma Medical School Panel Discussion and Q&A Panelists: 30 Minutes Matthew J.
Frigault, M.D., M.S. Krina K. Patel, M.D., M.Sc. iMMagine-1 Clinical Study Investigator, Associate Professor, Department of Lymphoma/Myeloma, Division of Cancer Medicine at the University of Texas MD Anderson Cancer Center Moderator: Chris Heery,
M.D. Chief Medical Officer, Arcellx Q&A 30 Minutes 13 Arcellx | 2 C0 o2 r3 p o ArS a H t e IR P E rv ee sn et ntation

Abstract: 1023 Phase 1 Study Of Anito-cel For The Treatment Of Patients
With Relapsed And/Or Refractory Multiple Myeloma: Results From At Least 1-year Follow-up In All Patients 1 2 3 4 5* Matthew Frigault, MD, MS , Jacalyn Rosenblatt, MD , Binod Dhakal, MBBS , Noopur Raje, MD , Daniella Cook, BS , 6 7* 8* 9* 9* Mahmoud
R. Gaballa, MD , Estelle Emmanuel-Alejandro , Danielle Nissen , Kamalika Banerjee , Anand Rotte, PhD , 9 10 11 12 Christopher R. Heery, MD , David Avigan, MD , Andrzej Jakubowiak, MD, PhD and Michael R. Bishop, MD

Anitocabtagene autoleucel (anito-cel/CART-ddBCMA) 1 Autologous
BCMA-directed CAR T-cell therapy using a novel, D-Domain binder D-Domain Attributes: 1,2 Non-Antibody Derived Synthetic Protein Small D-Domain construct facilitates high transduction Expression efficiency, CAR positivity, and CAR density on the
T-cell 2-4 surface Rapid D-Domain folding, lack of disulfide bonds, and a Stability hydrophobic core enables stability at and beyond 5,6 physiologic conditions Due to small size and compact structure, D-Domain CARs have a scFv Bivalent camelid VHh
D-Domain 6 Structure low risk of tonic signaling and (~25 kDa) (~25 kDa) (~8 kDa) potentially more efficient Multiple Myeloma cell killing 1 2 3 4 Rotte, et al. Immuno-Oncology Insights 2022; 3(1), 13–24; Frigault, et al. Blood Adv. 2023;
7(5):768-777; Cante-Barrett, et al. BMC Res. Notes 2016; 9:13; Buonato, et al. Mol. Cancer Ther. 2022; 15 5 6 21(7):1171-1183; Zhu, et al. Proc. Nat. Acad. Sci. 2003; 100(26): 15486-15491; Qin, et al. Mol. Ther. 2019; 27(7): 1262-1274. A Arrc c ee
llx ll x | 2023 ASH IR Event

Anito-cel Phase 1: Background and Methods Phase 1 first-in-human trial
is in patients with relapsed and/or refractory myeloma • Prior IMiD, PI, and CD38-targeted therapy Anito-cel • Received ≥3 prior lines of therapies or triple refractory 2 Dose Levels evaluated, 6 patients in each dose escalation
cohort 6 • DL1 = 100 + 20% x 10 CAR+ cells BCMA Binding Domain 6 • DL2 = 300 + 20% x 10 CAR+ cells Expansion cohort is enrolled at DL1 Phase 2 pivotal study (NCT05396885) is enrolling patients 16 A Arrc c ee llx ll x | 2023 ASH IR
Event

Anito-cel Phase 1: Patient Disposition Enrolled and Leukapheresed n=40
Successful Manufacture of Anito-cel n=40 Discontinued: Infection, n=1 Lymphodepletion n=39 Discontinued: Hypoxia/Heart Failure, n=1 Total Dosed n=38 6 6 DL1, 100x10 CAR+ cells DL2, 300x10 CAR+ cells n=32 n=6 Safety and Efficacy evaluable in all
dosed patients n=38 Dose escalation Dose escalation Expansion cohort n=6 n=6 n=26 Median administered dose at DL1, 115 million cells (range, 112-120 million cells) 17 A Arrc c ee llx ll x | 2023 ASH IR Event

Anito-cel Phase 1: A higher risk patient population 4 6 7 KarMMa
Legend-2 Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS
stage III, EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High
risk cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA
35 (36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 18 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

Greater proportion of patients with ISS Stage III 4 6 7 KarMMa Legend-2
Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS stage III,
EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High risk
cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA 35
(36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 19 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

Greater proportion of patients with EMD 4 6 7 KarMMa Legend-2
Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS stage III,
EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High risk
cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA 35
(36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 20 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

Greater proportion of patients with High-Risk Cytogenetics 4 6 7 KarMMa
Legend-2 Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS
stage III, EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High
risk cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA
35 (36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 21 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

Greater proportion of patients > 65 4 6 7 KarMMa Legend-2
Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS stage III,
EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High risk
cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA 35
(36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 22 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

Greater proportion of patients that are Penta Refractory 4 6 7 KarMMa
Legend-2 Cartitude-1 Anito-cel ph1 ‣ Greater percentage of patients N=128 N=74 N=97 N=38 with poor prognostic features: Anito-cel Phase 1 has higher rates BMPC > 60%, # (%) NA NA 21 (22%) 9 (24%) of patients with high tumor burden, ISS
stage III, EMD, and B2M > 5.5 (ISS stage 3), # (%) 21 (16%) 21 (28%) 14 (14%) 7 (18%) high-risk cytogenetics, which are all poor prognostic features for 50 (39%) EMD, # (%) 15 (20%) 13 (13%) 13 (34%) {incl. bone-based lesions} cell therapy High
risk cytogenetics, # (%)* 45 (35%) 15 (36%) 23 (24%) 11 (29%) ‣ Greater percentage of patients ECOG 0 57 (45%) 30 (41%) 39 (40%) 12 (32%) that are difficult to treat: Anito- cel Phase 1 has older patients Age group > 65, # (%) 45 (35%) NA
35 (36%) 20 (53%) (age ≥ 65), higher disease burden (BMPC ≥ 60%) and fewer ECOG 0 Triple refractory, # (%) 108 (84%) NA 85 (88%) 38 (100%) patients Penta refractory, # (%) 33 (26%) NA 41 (42%) 26 (68%) ‣ Greater percentage of
refractory Previous ASCT 120 (94%) 18 (24%) 87 (90%) 29 (76%) patients: Anito-cel Phase 1 Bridging Therapy, # (%) 112 (88%) NA 73 (75%) 26 (68%) enrolled all triple-refractory patients and had more penta- Median prior therapies 6 [3-16] 3 [1-9] 6.0
[3-18] 4 [3-16] refractory disease patients, unresponsive to other therapies *Defined as the presence of Del 17p, t(14;16), t(4;14); Anito-cel high-risk cytogenetics including +1q gain is n = 26 (68%); Cross-trial data interpretation should be
considered with caution as it is limited by differences in study population, study design, and other factors 23 4 6 7 Munshi et al.; Zhao et al.; Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

High CR/sCR rate of 76%, maintained across high-risk groups 100% 8% 8%
8% 9% 10% 90% 8% 8% 5% 9% 16% 80% 70% 60% PR VGPR 50% 85% sCR/CR 85% 83% 82% 40% 76% 30% 20% 10% 0% All Subjects High Risk* EMD High Risk Cytogenetics Age ≥65 (N=38) (N=24) (N=13) (N=11) (N=20) Note: Data cut-off October 15, 2023; * High Risk
defined as a patient with EMD, ISS Stage III (B2M ≥5.5), or BMPC ≥60% 24 A Arrc c ee llx ll x | 2023 ASH IR Event
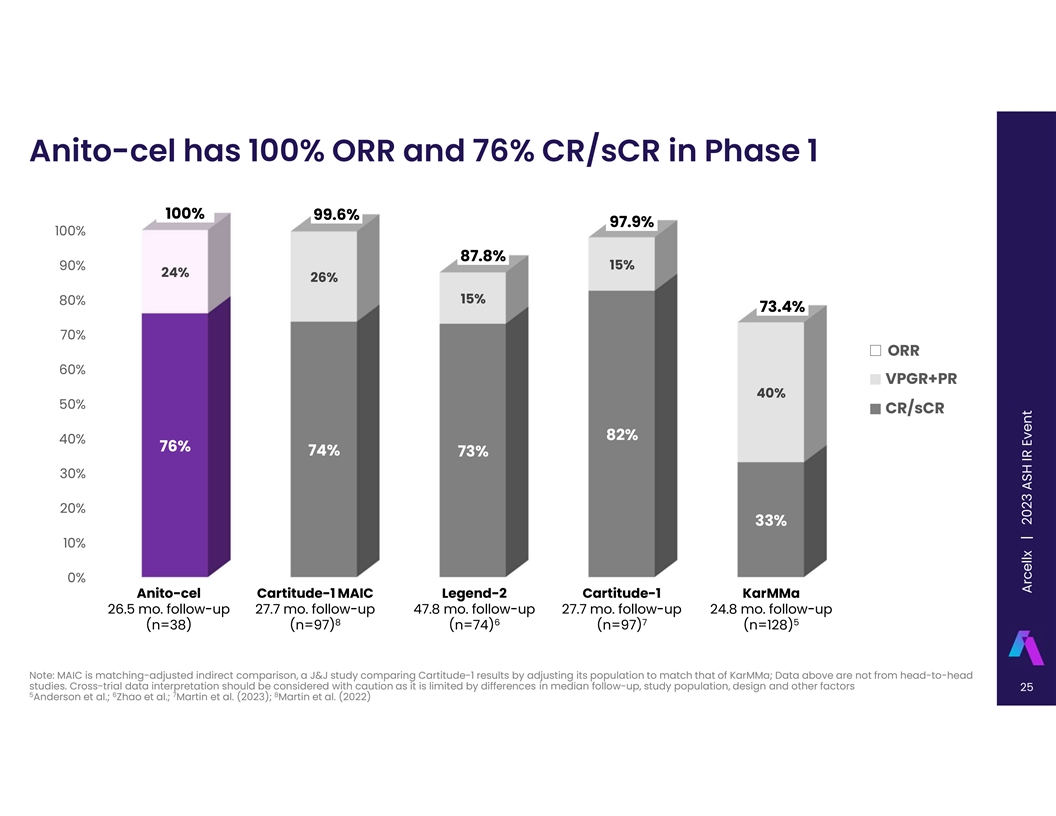
Anito-cel has 100% ORR and 76% CR/sCR in Phase 1 100% 99.6% 97.9% 100%
87.8% 15% 90% 24% 26% 15% 80% 73.4% 70% ORR 60% VPGR+PR 40% 50% CR/sCR 82% 40% 76% 74% 73% 30% 20% 33% 10% 0% Anito-cel Cartitude-1 MAIC Legend-2 Cartitude-1 KarMMa 26.5 mo. follow-up 27.7 mo. follow-up 47.8 mo. follow-up 27.7 mo. follow-up 24.8 mo.
follow-up 8 6 7 5 (n=38) (n=97) (n=74) (n=97) (n=128) Note: MAIC is matching-adjusted indirect comparison, a J&J study comparing Cartitude-1 results by adjusting its population to match that of KarMMa; Data above are not from head-to-head
studies. Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors 25 5 6 7 8 Anderson et al.; Zhao et al.; Martin et al. (2023); Martin et al.
(2022) A Arrc c ee llx ll x | 2023 ASH IR Event

mPFS not reached at 26.5 mo median follow-up (all patients) Time PFS
Estimate 95% Confidence (months) (%) Interval (%) 6 92.1 77.5, 97.4 12 75.9 58.7, 86.6 All Patients (n = 38) 18 63.7 45.7, 77.2 24 56.0 37.3, 71.1 § Median PFS not reached for all patients (n=38) § Median PFS not reached for CR/sCR
patients (n=29, 76%) All patients § 89% (n=25/28) of evaluable* patients MRD negative at All patients -5 minimum of 10 sensitivity Note: Data cut-off October 15, 2023; * Evaluable patients had identifiable malignant clone in the baseline bone
marrow aspirate 26 A Arrc c ee llx ll x | 2023 ASH IR Event

mPFS not reached at 26.5 mo median follow-up (all patients) Anito-Cel
Phase 1 median follow-up 26.5 mos Cartitude-1 8 median follow-up 27.7 mos Cartitude-1 MAIC 8 median follow-up 27.7 mos KarMMA 8 median follow-up 15.4 mos Note: MAIC is matching-adjusted indirect comparison, a J&J study comparing Cartitude-1
results by adjusting its population to match that of KarMMa; Data above are not from head-to-head studies. Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population,
design and other factors 27 8 Martin et al. (2022) A Arrc c ee llx ll x | 2023 ASH IR Event

Anito-cel mPFS not reached in EMD and Non-EMD patients PFS Time 95%
Confidence Estimate (months) Interval (%) (%) 6 92.3 56.6, 98.9 12 67.1 34.2, 86.2 With EMD (n = 13) 18 67.1 34.2, 86.2 24 57.5 25.7, 79.9 § Median PFS not reached for patients with EMD (n=13) § Median PFS not reached for Non-EMD patients
(n=25) Note: Data cut-off October 15, 2023 28 A Arrc c ee llx ll x | 2023 ASH IR Event

Durability tracking to >24 mo. mPFS in high-risk populations
Kaplan-Meier High Risk Extramedullary High Risk Overall ≥ 65 years PFS Estimates Features* disease Cytogenetics Patients n 38 24 13 11 20 (%) (100%) (63.2%) (34.2%) (28.9%) (52.6%) 6-month PFS % 92.1% 91.7% 92.3% 81.8% 95.0% (95% CI) (77.5%,
97.4%) (70.6%, 97.8%) (56.6%, 98.9%) (44.7%, 95.1%) (69.5%, 99.3%) 12-month PFS % 75.9% 74.2% 67.1% 71.6% 85.0% (95% CI) (58.7%, 86.6%) (51.3%, 87.5%) (34.2%, 86.2%) (35.0%, 89.9%) (60.4%, 94.9%) 18-month PFS % 63.7% 64.6% 67.1% 71.6% 74.3% (95% CI)
(45.7%, 77.2%) (41.3%, 80.6%) (34.2%, 86.2%) (35.0%, 89.9%) (48.7%, 88.4%) 24-month PFS % 56.0% 58.7% 57.5% 71.6% 61.3% (95% CI) (37.3%, 71.1%) (35.1%, 76.3%) (25.7%, 79.9%) (35.0%, 89.9%) (34.9%, 79.7%) In all risk subgroups, including High Risk,
the est. median PFS has not been reached at 24 months Note: Data cut-off October 15, 2023; * High Risk defined as a patient with EMD, ISS Stage III (B2M ≥5.5), or BMPC ≥60% 29 A Arrc c ee llx ll x | 2023 ASH IR Event

Durability highlights potential best-in-class profile mPFS 0 6 12 18 24
30 36 42 Anito-cel Not Reached 27-month estimated PFS: 51% 26.5-mo. (at 26.5 mo. median follow-up) median follow-up Cartitude-1 Not Reached 27.7-mo. 27-month estimated PFS: 55% 7 (at 27.7 mo. median follow-up) median follow-up Cartitude-1 MAIC
27.7-mo. 22.8 - 25.2 months* 8 median follow-up Cartitude-4 and † 24.3 months Cartitude-1 MAIC 10 2-4 prior LoT RWE CAR-T ‡ 18.6-mo. <18.6 months 9 median follow-up Legend-2 47.8-mo. 18.0 months 6 median follow-up KarMMa 24.8-mo. 8.6
months 5 median follow-up † *All variable adjusted comparison using FDA-approved doses cohort and base case adjusted comparison using “all doses” cohort shown; Cartitude-4 and Cartitude-1 MAIC had both trials ‡ used in
matching adjusted indirect comparison; 77 of 134 patients had a progression event at 18.6 months of median follow-up; MAIC is matching-adjusted indirect comparison, a J&J study comparing Cartitude-1 results by adjusting its population to match
that of KarMMa; RWE refers to real world evidence for Carvykti and Abecma. Data above are not from head-to-head 30 studies. Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study
population, design and other factors; LoT is Lines of Therapy 5 6 7 8 9 10 Anderson et al.; Zhao et al.; Martin et al. (2023); Martin et al. (2022); Pan et al.; Bar et al. All Patients A Arrc c ee llx ll x | 2023 ASH IR Event
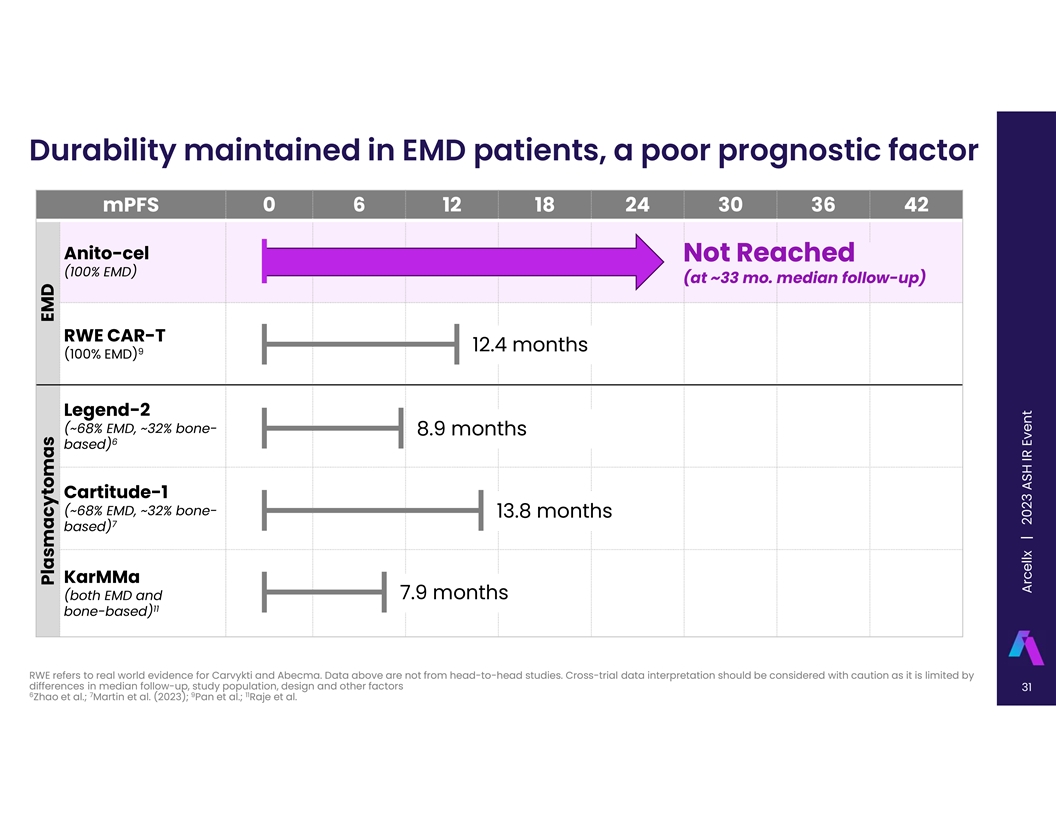
Durability maintained in EMD patients, a poor prognostic factor mPFS 0
6 12 18 24 30 36 42 Anito-cel Not Reached (100% EMD) (at ~33 mo. median follow-up) RWE CAR-T 12.4 months 9 (100% EMD) Legend-2 (~68% EMD, ~32% bone- 8.9 months 6 based) Cartitude-1 (~68% EMD, ~32% bone- 13.8 months 7 based) KarMMa (both EMD and 7.9
months 11 bone-based) RWE refers to real world evidence for Carvykti and Abecma. Data above are not from head-to-head studies. Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up,
study population, design and other factors 31 6 7 9 11 Zhao et al.; Martin et al. (2023); Pan et al.; Raje et al. EMD Plasmacytomas A Arrc c ee llx ll x | 2023 ASH IR Event

The typical patient in the Anito-Cel Phase 1 had a high-risk feature,
where approved CAR-Ts have had poor outcomes mPFS 0 6 12 18 24 30 Anito-cel Ph 1 High-Risk Features* 24-mo est. PFS: 59% (n=24/38, 63%) Cartitude-1 Plasmacytoma 13.8 months 7 (~68% EMD; ~32% bone-based lesions) 63% Cartitude-1 ISS Stage 3 15.0
months 7 (B2M ≥ 5.5 mg/L) of Anito-cel Ph1 pts Cartitude-1 BMPC > 60% 24.1 months 7 (High tumor burden) Anito-cel Ph 1 High-Risk Cytogenetics 24-mo est. PFS: 72% (n=11/38, 29%) 29% Cartitude-1 High-Risk of Anito-cel Cytogenetics 21.1 months
Ph1 pts 7 (Del 17p, t(14;16), t(4;14)) * High Risk defined as a patient with EMD, ISS Stage III (B2M ≥5.5), or BMPC ≥60%; Data above are not from head-to-head studies. Cross-trial data interpretation should be considered with caution as
it is limited by differences in median follow-up, study population, design and other factors 32 7 Martin et al. (2023) A Arrc c ee llx ll x | 2023 ASH IR Event

At 2-yrs follow-up, Anito-cel has favorable safety profile • No
delayed neurotoxicities, no Parkinsonian-like syndromes • No cranial nerve palsies, no Guillain-Barré syndrome, in the entire population through follow-up • One Grade 5 AE post study treatment (unrelated cardiac arrest due to
non-study drug overdose) CAR-T-associated AEs 100 million 300 million Grade 3/4 AEs (non-CRS/ICANS) ≥5% after cell infusion (N=38) Per ASTCT criteria (N=32) (N=6) Hematologic Grade 1/2 Grade 3 Grade 1/2 Grade 3 Cytokine Release Syndrome (CRS)
Neutrophil count dec. 31 (81.6%) 30 (94%) 0 5 (83%) 1 (17%) Anemia 22 (57.9%) Thrombocytopenia 16 (42.1%) Median onset (min-max) 2 days (1-12 days) 2 day (1-2 days) Lymphocyte count decreased 15 (39.5%) White blood cell count decreased 7 (18.4%)
Median duration (min-max) 6 days (1-10 days) 5 days (3-9 days) Febrile Neutropenia 5 (13.2%) Grade 1/2 Grade 3 Grade 1/2 Grade 3 Non-hematologic Neurotoxicity (ICANs) 5 (16%) 1 (3%) 0 1 (17%) Hypertension 3 (7.9%) AST increased 2 (5.3%) Median onset
(min-max) 4.5 days (3-6 days) 7 days Cellulitis 2 (5.3%) Hypokalemia 2 (5.3%) Median duration (min-max) 3.5 days (1-9 days) 17 days Hyponatraemia 2 (5.3%) Hypophosphatemia 2 (5.3%) Toxicity Management Lung Infection 2 (5.3%) Tocilizumab 27 (84%) 5
(83%) Pain in extremity 2 (5.3%) Dexamethasone 20 (63%) 2 (33%) Sepsis 2 (5.3%) 33 A Arrc c ee llx ll x | 2023 ASH IR Event

iMMagine-1 Phase 2 Pivotal Trial Currently Enrolling A multicenter,
open-label study of CART-ddBCMA in patients with r/r MM Primary Endpoint Overall Response Rate (ORR) per IMWG criteria by Independent Review Committee (IRC) ‣ The primary analysis is planned when all subjects have a minimum of 13 months follow
up after infusion of CART-ddBCMA Key Secondary Endpoint Stringent complete response (sCR) or complete response (CR) rate per IMWG criteria ORR per IMWG by IRC in patients with 3 prior lines • At least 3 prior lines of therapy, including PI,
ImiD, and anti-CD38 antibody, and refractory to last line • Eligibility Criteria Measurable disease • ECOG 0-1 • N=~110 Enrollment and Dose • Dose = 115 (+/-10) million CAR+ cells 34 A Arrc c ee llx ll x | 2023 ASH IR
Event

Conclusions § Anito-cel utilizes a novel, synthetic, compact and
stable D-Domain binder o D-Domain facilitates high CAR surface expression, low risk of tonic signaling o Recommended Phase 2 Dose selected as 115±10 million CAR+ T cells § CR/sCR rate 76%; 100% ORR per IMWG o CR/sCR rate >80% in all
evaluated sub-groups including high-risk (EMD, high-risk cytogenetics, age ≥65) -5 o 89% of MRD evaluable patients (n=25/28) were MRD negative at 10 or lower § Median PFS, DOR, and OS not reached at 2 years of follow-up (median 26.5
months) o CAR-T-ddBCMA continues to demonstrate deep and durable efficacy, including in high-risk patient sub-groups § At 2 years of follow-up (median 26.5 months), manageable safety profile o No grade ≥3 CRS and 1 case of Grade 3 ICANS
at RP2D. All events resolved without sequelae with routine management o No delayed neurotoxicity, no cranial nerve palsy, no Parkinsonian symptoms, no Guillain-Barré syndrome Pivotal phase 2, iMMagine-1 trial (NCT05396885) is now enrolling in
co-development with Kite 35 A Arrc c ee llx ll x | 2023 ASH IR Event

Arcellx Panel Discussion Christopher Heery, Matthew J. Frigault, Krina
K. Patel, M.D. M.D., M.S. M.D., M.Sc. Chief Medical Officer, Arcellx iMMagine-1 and ACLX-001 Clinical iMMagine-1 Clinical Study Study Investigator, Clinical Investigator, Associate Director of the Cellular Therapy Professor, Department of Service at
Mass General Cancer Lymphoma/Myeloma, Division Center and Assistant Professor at of Cancer Medicine at the Harvard Medical School University of Texas MD Anderson Cancer Center 36 Arcellx Corporate Presentation | September 2023

Q&A 37 A Arrc c ee llx ll x | 2023 ASH IR Event

References 1. Zudair et al., “Translational learnings from
Cilta-cel, a BCMA-targeted CAR-T Cell Therapy”, CAR-TCR (2022) 2. Foster et al. “Cross-study safety analysis of risk factors in CAR T cell clinical trials: An FDA database pilot project”, Molecular Therapy Oncolytics, Vol 27: Dec
2022. https://doi.org/10.1016/j.omto.2022.10.006 3. Wu LS, Su Y, Li C, et al (2022) Population‐based cellular kinetic characterization of Ciltacabtagene Autoleucel in subjects with relapsed or refractory multiple myeloma. Clinical and
Translational Science 15:3000–3011. doi: 10.1111/cts.13421 4. Munshi NC, Anderson LD, Shah N, et al (2021) Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine 384:705–716. doi:
10.1056/nejmoa2024850 5. Anderson LD, Shah N, Jagannath S, et al (2021) OAB-027: Idecabtagene vicleucel (IDE-Cel, BB2121), a BCMA-directed car T-cell therapy, for the treatment of patients with relapsed and refractory multiple myeloma (RRMM):
Updated results from Karmma. Clinical Lymphoma Myeloma and Leukemia. doi: 10.1016/s2152-2650(21)02101-7 6. Zhao W-H, Wang B-Y, Chen L-J, et al (2022) Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: A phase 1, single-arm,
open-label, Multicenter Study in China (Legend-2). Journal of Hematology & Oncology. doi: 10.1186/s13045-022-01301-8 7. Martin T, Usmani SZ, Berdeja JG, et al (2023) Ciltacabtagene Autoleucel, an anti–B-cell maturation antigen chimeric
antigen receptor T- cell therapy, for relapsed/refractory multiple myeloma: Cartitude-1 2-year follow-up. Journal of Clinical Oncology 41:1265–1274. doi: 10.1200/jco.22.00842 8. Martin T, Usmani SZ, Schecter JM, et al (2022) Updated results
from a matching-adjusted indirect comparison of efficacy outcomes for Ciltacabtagene Autoleucel in cartitude-1 versus Idecabtagene Vicleucel in Karmma for the treatment of patients with relapsed or refractory multiple myeloma. Current Medical
Research and Opinion 39:81–89. doi: 10.1080/03007995.2022.2139052 9. Pan, et al. Abstract 1006: Outcomes after CAR T Cells in Multiple Myeloma Patients with Extramedullary and Paramedullary disease (ASH 2023) 10. Bar, et al. “Comparative
Efficacy of Ciltacabtagene Autoleucel Versus Idecabtagene Vicleucel in the Treatment of Patients With Relapsed or Refractory Multiple Myeloma Previously Treated With 2–4 Prior Lines of Therapy Using a Matching-Adjusted Indirect
Comparison”; American Society of Hematology (2023) 11. Raje NS, Siegel DS, Jagannath S, et al (2020) Idecabtagene Vicleucel (IDE-Cel, BB2121) in relapsed and refractory multiple myeloma: Analyses of high-risk subgroups in the KARMMA study.
Blood 136:37–38. doi: 10.1182/blood-2020-134319 38 A Arrc c ee llx ll x | 2023 ASH IR Event
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Arcellx (NASDAQ:ACLX)
Historical Stock Chart
From Apr 2024 to May 2024
Arcellx (NASDAQ:ACLX)
Historical Stock Chart
From May 2023 to May 2024