0001497253falseFYhttp://fasb.org/us-gaap/2023#RoyaltyMemberhttp://fasb.org/us-gaap/2023#RoyaltyMemberP1Y2024-030001497253us-gaap:FairValueInputsLevel1Member2023-04-012024-03-310001497253us-gaap:RetainedEarningsMember2022-03-310001497253srt:MaximumMemberonvo:CommonWarrantsMemberus-gaap:IPOMemberus-gaap:SubsequentEventMember2024-05-080001497253us-gaap:RestrictedStockUnitsRSUMember2023-04-012024-03-310001497253srt:MaximumMemberonvo:ProspectusTo2021ShelfMemberonvo:AtTheMarketFacilityMember2021-01-290001497253onvo:ProspectusToTwoThousandAndTwentyFourShelfMemberonvo:AtTheMarketFacilityMember2023-04-012024-03-310001497253onvo:ClemsonUniversityMember2022-04-012023-03-310001497253us-gaap:OperatingSegmentsMemberonvo:ResearchDevelopmentSegmentMember2023-04-012024-03-310001497253onvo:DaysOfFdaApprovalOfFirstLicensedProductMemberonvo:SalkInstituteForBiologicalStudiesMemberus-gaap:LicensingAgreementsMember2023-04-012024-03-310001497253onvo:ClemsonUniversityMember2016-03-310001497253onvo:TwoThousandAndTwentyFourShelfMemberonvo:AtTheMarketFacilityMember2023-04-012024-03-310001497253onvo:ViscientBiosciencesMember2022-04-012023-03-310001497253onvo:ProspectusToTwoThousandAndTwentyFourShelfMemberonvo:AtTheMarketFacilityMember2024-03-310001497253onvo:CommonWarrantsMemberus-gaap:SubsequentEventMember2024-05-082024-05-080001497253onvo:EmployeeStockPurchasePlanMemberus-gaap:EmployeeStockOptionMember2024-03-310001497253onvo:TwoThousandTwentyThreeEmployeeStockPurchasePlanMember2024-03-310001497253us-gaap:AdditionalPaidInCapitalMember2023-03-310001497253us-gaap:AdditionalPaidInCapitalMember2024-03-310001497253us-gaap:SubsequentEventMember2024-05-082024-05-080001497253onvo:MetacrineIncMemberonvo:MetacrinesFxrProgramMember2024-03-3100014972532023-09-300001497253us-gaap:CommonStockMember2023-03-310001497253onvo:EquityIncentivePlanTwoThousandTwelveMember2018-07-310001497253us-gaap:OperatingSegmentsMember2024-03-310001497253us-gaap:RestrictedStockUnitsRSUMemberonvo:EquityIncentivePlanTwoThousandTwelveMember2024-03-310001497253us-gaap:CommonStockMember2023-04-012024-03-310001497253us-gaap:OperatingSegmentsMember2023-03-310001497253us-gaap:TreasuryStockCommonMember2023-03-310001497253us-gaap:OperatingSegmentsMemberus-gaap:RoyaltyMemberonvo:ResearchDevelopmentSegmentMember2023-04-012024-03-3100014972532023-04-012024-03-310001497253us-gaap:CommonStockMember2022-03-310001497253us-gaap:OperatingSegmentsMember2022-04-012023-03-310001497253us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012023-03-310001497253srt:MaximumMemberonvo:ProspectusToTwoThousandAndTwentyFourShelfMemberonvo:AtTheMarketFacilityMember2024-01-260001497253us-gaap:EmployeeStockOptionMemberonvo:EquityIncentivePlanTwoThousandTwelveMember2024-03-310001497253onvo:ATMOfferingsMemberus-gaap:SubsequentEventMember2024-04-012024-05-300001497253onvo:SublicenseAgreementsMemberonvo:ClemsonUniversityMember2023-04-012024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMemberus-gaap:CommonStockMember2022-10-120001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMembersrt:MaximumMemberus-gaap:CommonStockMember2022-10-120001497253onvo:SalkInstituteForBiologicalStudiesMemberonvo:DaysOfFirstCommercialSaleOfALicensedProductInTerritoryMemberus-gaap:LicensingAgreementsMember2023-04-012024-03-310001497253srt:MaximumMember2024-03-310001497253onvo:ExecutiveChairmanMemberus-gaap:SellingGeneralAndAdministrativeExpensesMember2023-08-282023-08-280001497253onvo:ProspectusTo2021And2024ATMProspectusShelfMember2023-04-012024-03-310001497253onvo:CommonWarrantsMemberus-gaap:IPOMemberus-gaap:SubsequentEventMember2024-05-082024-05-0800014972532023-03-310001497253us-gaap:RetainedEarningsMember2023-03-310001497253onvo:ClemsonUniversityMemberus-gaap:LicensingAgreementsMember2011-05-012011-05-310001497253onvo:TwoThousandAndTwentyFourShelfMember2024-01-260001497253onvo:TwoThousandAndTwentyTwoInducementEquityPlanMember2022-10-120001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMember2023-04-012024-03-310001497253us-gaap:AdditionalPaidInCapitalMember2022-03-310001497253onvo:CommonWarrantsMemberus-gaap:SubsequentEventMember2024-05-080001497253onvo:PreFundedWarrantMemberus-gaap:SubsequentEventMember2024-05-080001497253us-gaap:SubsequentEventMember2024-05-290001497253us-gaap:RetainedEarningsMember2023-04-012024-03-310001497253us-gaap:GeneralAndAdministrativeExpenseMember2023-04-012024-03-310001497253us-gaap:DomesticCountryMember2024-03-310001497253onvo:ClemsonUniversityMember2023-04-012024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMemberus-gaap:RestrictedStockUnitsRSUMember2024-03-310001497253onvo:SalkInstituteForBiologicalStudiesMemberonvo:DaysOfTheDosingOfTheFirstPatientInAPhaseIiiClinicalTrialMemberus-gaap:LicensingAgreementsMember2023-04-012024-03-310001497253us-gaap:CommonStockMember2024-03-310001497253us-gaap:RestrictedStockUnitsRSUMember2024-03-310001497253onvo:SalkInstituteForBiologicalStudiesMemberus-gaap:LicensingAgreementsMember2023-04-012024-03-310001497253onvo:UniversityOfMissouriMember2009-03-012009-03-310001497253us-gaap:TreasuryStockCommonMember2022-04-012023-03-3100014972532022-04-012023-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMember2022-10-120001497253onvo:ESPPSharesMember2022-04-012023-03-310001497253onvo:BICOGroupABMember2022-02-222022-02-220001497253onvo:EquityIncentivePlanTwoThousandTwelveMember2012-01-3100014972532022-03-310001497253us-gaap:IPOMemberus-gaap:SubsequentEventMember2024-05-082024-05-080001497253us-gaap:TreasuryStockCommonMember2024-03-310001497253us-gaap:TreasuryStockCommonMember2022-03-310001497253us-gaap:EmployeeStockOptionMember2023-04-012024-03-310001497253us-gaap:LicensingAgreementsMember2023-04-012024-03-310001497253onvo:TwoThousandAndTwentyFourShelfMember2024-03-310001497253us-gaap:LicensingAgreementsMember2024-03-310001497253onvo:InducementAwardAgreementsMember2022-02-280001497253onvo:IncentiveAwardPlanMemberus-gaap:EmployeeStockOptionMember2024-03-310001497253onvo:MetacrinesFxrProgramMember2023-04-012024-03-310001497253onvo:MetacrineIncMemberonvo:MetacrinesFxrProgramMember2022-04-012023-03-310001497253us-gaap:OperatingSegmentsMemberus-gaap:RoyaltyMemberonvo:ResearchDevelopmentSegmentMember2022-04-012023-03-310001497253onvo:TwoThousandAndTwentyOneShelfMemberonvo:AtTheMarketFacilityMember2023-04-012024-03-310001497253us-gaap:OperatingSegmentsMemberus-gaap:ProductMemberonvo:MosaicSegmentMember2023-04-012024-03-310001497253us-gaap:OperatingSegmentsMemberonvo:MosaicSegmentMember2023-03-310001497253onvo:SanDiegoPermanentLeaseMember2020-11-232020-11-2300014972532024-05-250001497253onvo:TwoThousandEighteenSalesAgreementMember2023-04-012024-03-310001497253srt:MaximumMember2023-04-012024-03-310001497253us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001497253onvo:AtTheMarketFacilityMember2024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMember2024-03-310001497253onvo:TwoThousandTwentyThreeEmployeeStockPurchasePlanMember2023-10-310001497253us-gaap:OperatingSegmentsMemberonvo:MosaicSegmentMember2023-04-012024-03-310001497253us-gaap:LicensingAgreementsMember2022-04-012023-03-310001497253onvo:EquityIncentivePlanTwoThousandTwelveMember2022-10-120001497253us-gaap:OperatingSegmentsMember2023-04-012024-03-310001497253srt:MinimumMember2024-03-310001497253us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-310001497253us-gaap:GeneralAndAdministrativeExpenseMember2022-04-012023-03-310001497253onvo:BICOGroupABMember2023-04-012024-03-310001497253onvo:TwoThousandAndTwentyOneInducementEquityPlanMember2021-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMember2022-07-250001497253us-gaap:RetainedEarningsMember2024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwelveMember2024-03-310001497253onvo:ProspectusTo2021ShelfMemberonvo:AtTheMarketFacilityMember2023-04-012024-03-310001497253us-gaap:ResearchAndDevelopmentExpenseMember2023-04-012024-03-310001497253us-gaap:OperatingSegmentsMemberus-gaap:ProductMemberonvo:MosaicSegmentMember2022-04-012023-03-310001497253onvo:ExecutiveChairmanMember2023-08-282023-08-280001497253us-gaap:StateAndLocalJurisdictionMember2024-03-310001497253onvo:UniversityOfMissouriMember2022-12-052022-12-050001497253us-gaap:DomesticCountryMembersrt:MaximumMember2023-04-012024-03-310001497253onvo:SanDiegoPermanentLeaseMember2020-11-230001497253us-gaap:OperatingSegmentsMemberonvo:ResearchDevelopmentSegmentMember2023-03-310001497253onvo:TwoThousandAndTwentyOneInducementEquityPlanMemberus-gaap:EmployeeStockOptionMember2024-03-310001497253us-gaap:RestrictedStockUnitsRSUMember2023-03-310001497253us-gaap:OperatingSegmentsMemberonvo:MosaicSegmentMember2024-03-310001497253us-gaap:LicensingAgreementsMember2023-03-310001497253onvo:AtTheMarketFacilityMember2023-04-012024-03-310001497253us-gaap:RetainedEarningsMember2022-04-012023-03-310001497253us-gaap:ResearchAndDevelopmentExpenseMember2022-04-012023-03-310001497253onvo:MosaicSegmentMember2024-03-310001497253onvo:SanDiegoPermanentLeaseMember2021-11-172021-11-170001497253us-gaap:IPOMemberus-gaap:SubsequentEventMemberonvo:JonestradingInstitutionalServicesLlcMember2024-05-082024-05-080001497253us-gaap:AdditionalPaidInCapitalMember2022-04-012023-03-3100014972532024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwelveMember2022-07-252022-10-120001497253onvo:EmployeeStockPurchasePlanMember2024-03-310001497253onvo:ViscientBiosciencesMember2023-04-012024-03-310001497253us-gaap:TreasuryStockCommonMember2023-04-012024-03-310001497253us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012024-03-310001497253onvo:ESPPSharesMember2023-04-012024-03-310001497253us-gaap:IPOMember2024-03-310001497253us-gaap:OperatingSegmentsMemberonvo:ResearchDevelopmentSegmentMember2024-03-3100014972532021-12-170001497253onvo:BICOGroupABMember2022-04-012023-03-310001497253us-gaap:FairValueInputsLevel1Member2024-03-3100014972532024-01-012024-03-310001497253onvo:EquityIncentivePlanTwoThousandTwentyTwoMemberus-gaap:EmployeeStockOptionMember2024-03-310001497253us-gaap:IPOMemberus-gaap:SubsequentEventMember2024-05-132024-05-130001497253us-gaap:CommonStockMember2022-04-012023-03-310001497253onvo:TwoThousandTwentyThreeEmployeeStockPurchasePlanMember2023-04-012024-03-310001497253us-gaap:FairValueInputsLevel1Member2023-03-310001497253us-gaap:OperatingSegmentsMemberonvo:ResearchDevelopmentSegmentMember2022-04-012023-03-310001497253us-gaap:OperatingSegmentsMemberonvo:MosaicSegmentMember2022-04-012023-03-310001497253us-gaap:AdditionalPaidInCapitalMember2023-04-012024-03-3100014972532023-08-182023-08-180001497253us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-31xbrli:pureutr:sqftxbrli:sharesonvo:Participantonvo:Compoundsonvo:Employeesiso4217:USD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended March 31, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Transition Period from to
Commission File No. 001-35996
ORGANOVO HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
|
27-1488943 |
(State or other jurisdiction of incorporation or organization) |
|
(IRS Employer Identification No.) |
|
|
|
11555 Sorrento Valley Rd, Suite 100 San Diego, CA |
|
92121 |
(Address of principal executive offices) |
|
(Zip code) |
Registrant’s telephone number, including area code: 858-224-1000
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol |
Name of each exchange on which registered |
Common Stock, par value $0.001 per share |
ONVO |
The Nasdaq Capital Market |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “accelerated filer”, “large accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
|
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
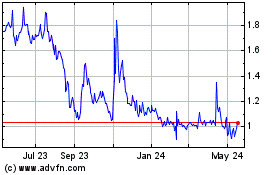
The aggregate market value of the voting and non-voting common equity held by non-affiliates based on the closing stock price as reported on the Nasdaq Capital Market on September 30, 2023, the last trading day of the registrant’s second fiscal quarter, was $10,340,301. For purposes of this computation only, shares of common stock held by each executive officer, director, and 10% or greater stockholders have been excluded in that such persons may be deemed affiliates.
The number of outstanding shares of the registrant’s common stock, as of May 25, 2024 was 14,371,826.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required for Part III of this report is incorporated herein by reference to the definitive proxy statement for the 2024 annual meeting of the registrant’s stockholders, expected to be filed within 120 days of the end of the registrant’s fiscal year.
|
|
|
|
|
|
Auditor Firm Id: |
89 |
Auditor Name: |
Rosenberg Rich Baker Berman, P.A. |
Auditor Location: |
Somerset, NJ |
Organovo Holdings, Inc.
Annual Report on Form 10-K
For the Year Ended March 31, 2024
Table of Contents
Important Information Regarding Forward-Looking Statements
Portions of this Annual Report on Form 10-K (including information incorporated by reference) (“Annual Report”) include “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995, based on our current beliefs, expectations and projections regarding any strategic transaction process; the ability to advance our research and development activities and pursue development of any of our pipeline products; our technology; our product and service development opportunities and timelines; our business strategies; customer acceptance and the market potential of our technology; products and services; our future capital requirements; our future financial performance; and other matters. This includes, in particular, Item 1. “Business” and Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Annual Report, as well as other portions of this Annual Report. The words “believe,” “expect,” “anticipate,” “project,” “could,” “would,” and similar expressions, among others, generally identify “forward-looking statements,” which speak only as of the date the statements were made. The matters discussed in these forward-looking statements are subject to risks, uncertainties and other factors that could cause our actual results to differ materially from those projected, anticipated or implied in the forward-looking statements. As a result, you should not place undue reliance on any forward-looking statements. The most significant of these risks, uncertainties and other factors are described in Item 1A. “Risk Factors” of this Annual Report. Except to the limited extent required by applicable law, we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
PART I
Item 1. Business.
Overview
Organovo Holdings, Inc. (“Organovo,” “we,” “us,” “our,” the “Company” and “our Company”) is a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in our established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC.
Our current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). We plan to start a Phase 2a clinical trial in UC in the calendar year 2024. We released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis ("MASH") in April 2024 that are supportive of ongoing development, and we believe FXR314 has a commercial opportunity in MASH, most likely in combination therapy. We are exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and our IBD disease models.
Our second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. We use our proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function, and disease. We believe these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, we are initially focusing on the intestine and have ongoing 3D tissue development efforts in human tissue models of UC and CD. We use these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. We intend to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Our current understanding of intestinal tissue models and IBD disease models leads us to believe that we can create models that provide greater insight into the biology of these diseases than are generally currently available. We are creating high fidelity disease models, leveraging our prior work including the work found in our peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Our advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures.
Using these disease models, we intend to identify and validate novel therapeutic targets. After finding therapeutic drug targets, we intend to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials.
We expect to broaden our work into additional therapeutic areas over time and are currently exploring specific tissues for development. In our work to identify the areas of interest, we evaluate areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, we are building upon both our external and in house scientific expertise, which will be essential to our drug development effort.
Recent Developments
Mosaic Cell Sciences Division
In February 2024, we formed our Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain primary human cells we utilize in our research and development efforts. We believe Mosaic can help us optimize our supply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials we use are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. We intend for Mosaic to provide us with qualified human cells for use in our clinical research and development programs. In addition to supplying us with primary human cells, we intend for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which we expect to offset costs and over time become a profit center that offsets overall research and development ("R&D") spending by Organovo.
Best Efforts Public Offering
On May 8, 2024, we priced a best efforts public offering (the “Offering”) of: (i) 1,562,500 shares of our common stock and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock at a combined public offering price of $0.80 per share and accompanying Common Warrant to purchase one share of common stock and (ii) pre-funded warrants (“Pre-Funded Warrants”) to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock at a combined public offering price of $0.799 per Pre-Funded Warrant and accompanying Common Warrant to purchase one share of common stock. In connection with the Offering, we entered into Securities Purchase Agreements with the purchasers of the securities in the Offering on May 8, 2024.
The per share exercise price for the Pre-Funded Warrants is $0.001, subject to adjustment as provided therein. The Pre-Funded Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire when exercised in full. The holders may exercise the Pre-Funded Warrants by means of a “cashless exercise.”
The per share exercise price for the Common Warrants is $0.80, subject to adjustment as provided therein. The Common Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire on the date that is five years following the original issuance date. If a registration statement covering the issuance of the shares of common stock issuable upon exercise of the Common Warrants is not available for the issuance, then the holders may exercise the Common Warrants by means of a “cashless exercise.”
In connection with the Offering, we paid JonesTrading Institutional Services LLC, which acted as the placement agent in connection with the Offering, a cash fee of 5.0% of the aggregate gross proceeds raised in the Offering.
The closing of the Offering occurred on May 13, 2024. We received net proceeds of approximately $4.7 million from the Offering, after deducting the estimated offering expenses payable by us, including the Placement Agent fees.
Our Platform Technology
Our 3D human tissue platform is multifaceted. We approach each tissue agnostic to specific technologies, and intend to apply the best 3D technology to a given disease. We are developing novel disease models using high throughput systems, bioprinted and flow/stretch capable 3D systems as appropriate. Our proprietary NovoGen Bioprinters® and related technologies for preparing bio-inks and bioprinting multicellular tissues with complex architecture are grounded in over a decade of peer-reviewed scientific publications, deriving originally from research led by Dr. Gabor Forgacs, one of our founders and a former George H. Vineyard Professor of Biological Physics at the University of Missouri-Columbia (“MU”). We have a broad portfolio of intellectual property rights covering the principles, enabling instrumentation, applications, tissue constructs and methods of cell-based printing, including exclusive licenses to certain patented and patent pending technologies from MU and Clemson University. We own or exclusively license more than 160 patents and pending applications worldwide covering specific tissue designs, uses, and methods of manufacture.
The NovoGen Bioprinter® Platform
Our NovoGen Bioprinters® are automated devices that enable the fabrication of 3D living tissues comprised of mammalian cells. A custom graphic user interface (“GUI”) facilitates the 3D design and execution of scripts that direct precision movement of multiple dispensing heads to deposit defined cellular building blocks called bio-ink. Bio-ink can be formulated as a 100% cellular composition or as a mixture of cells and other matter (hydrogels, particles). Our NovoGen Bioprinters® can also dispense pure hydrogel formulations, provided the physical properties of the hydrogel are compatible with the dispensing parameters. Most typically, hydrogels are deployed to create void spaces within specific locations in a 3D tissue or to aid in the deposition of specific cell types. We are able to employ a wide variety of proprietary cell- and hydrogel-based bio-inks in the fabrication of tissues. Our NovoGen Bioprinters® also serve as important components of our tissue prototyping and manufacturing platform, as they are able to rapidly and precisely fabricate intricate small-scale tissue models for in vitro use as well as larger-scale tissues suitable for in vivo use.
Generation of bio-ink comprising human cells is the first step in our standard bioprinting. A wide variety of cells and cell-laden hydrogels can be formulated into bio-ink and bioprinted tissues, including cell lines, primary cells, and stem/progenitor cells. The majority of tissue designs employ two or more distinct varieties of bio-ink, usually comprised of cells that represent distinct compartments within a target tissue. For example, a 3D liver tissue might consist of two to three distinct bio-inks that are each made from a single cell type, a combination of cell types, and/or a combination of primary cells and one or more bio-inert hydrogels that serve as physical supports for the bioprinted tissue during its maturation period, or to transiently occupy negative spaces in a tissue design.
Research Collaborations
We continue to collaborate with several academic institutions by providing them with access to our NovoGen Bioprinters® for research purposes, including: Yale School of Medicine, Knight Cancer Institute at Oregon Health & Science University, and the
University of Virginia. We believe that the use of our bioprinting platform by major research institutions may help to advance the capabilities of the platform and generate new applications for bioprinted tissues. In prior instances, an academic institution or other third party provided funding to support the academic collaborator’s access to our technology platform. This funding was typically reflected as collaboration revenues in our financial statements. Our academic research collaborations typically involve both parties contributing resources directly to projects. We are not currently generating any revenues from these collaborations.
Intellectual Property
We rely on a combination of patents, trademarks, trade secrets, confidential know-how, copyrights, and a variety of contractual mechanisms such as confidentiality, material transfer, licenses, research collaboration, limited technology access, and invention assignment agreements, to protect our intellectual property. Our intellectual property portfolio for our core technology was initially built through licenses from MU and the Medical University of South Carolina. We subsequently expanded our intellectual property portfolio by filing our own patent and trademark applications worldwide and negotiating additional licenses and purchases.
On an ongoing basis we review and analyze our full intellectual property portfolio to align it with our current business needs, strategies and objectives. Based on that ongoing review, selected patents and patent applications in various countries are or will be abandoned or allowed to lapse. The numbers provided herein are reflective of those changes.
We solely own or hold exclusive licenses to 34 issued U.S. patents and more than 45 issued international patents in foreign jurisdictions including Australia, Canada, China, Denmark, France, Great Britain, Germany, Ireland, Japan, South Korea, Sweden, the Netherlands and Switzerland. We solely or jointly own or hold exclusive licenses to 17 pending U.S. patent applications and more than 5 pending international applications in foreign jurisdictions including Australia, Canada, China, the European Patent Office, Japan and South Korea. These patent families relate to our bioprinting technology and our engineered tissue products and services, including our various uses in areas of tissue creation, in vitro testing, utilization in drug discovery, and in vivo therapeutics.
In connection with the recent acquisition of the FXR program from Metacrine, we acquired the related patent portfolio by way of assignment. This includes filings on the lead candidate, FXR314, and selected filings on the prior candidate (no longer in development), FXR125. With respect to this FXR portfolio, we solely own 7 issued patents and 15 international patents in jurisdictions, including Australia, China, Eurasia, India, Israel, Mexico, Japan and South Africa. We solely own 8 pending U.S. patent applications and more than 50 pending international applications in foreign jurisdictions, including Argentina, Australia, Brazil, Chile, Canada, Eurasia, Europe, Israel, India, Japan, South Korea, Mexico, Philippines, Singapore, South Africa, Hong Kong and Taiwan. These patent families relate to FXR125 and FXR314, including generic coverage, species coverage, methods of use, formulations and polymorph crystals.
In-Licensed Intellectual Property
In 2009 and 2010, we obtained world-wide exclusive licenses to intellectual property owned by MU and the Medical University of South Carolina, which now includes 7 issued U.S. patents, 2 pending U.S. applications and 16 issued international patents. Dr. Gabor Forgacs, one of our founders and a former George H. Vineyard Professor of Biophysics at MU, was one of the co-inventors of all of these works (collectively, the “Forgacs Intellectual Property”). The Forgacs Intellectual Property provides us with intellectual property rights relating to cellular aggregates, the use of cellular aggregates to create engineered tissues, and the use of cellular aggregates to create engineered tissue with no scaffold present. The intellectual property rights derived from the Forgacs Intellectual Property also enables us to utilize our NovoGen Bioprinter® to create engineered tissues.
In 2011, we obtained an exclusive license to a U.S. patent (U.S. Patent No. 7,051,654) owned by the Clemson University Research Foundation that provides us with intellectual property rights relating to methods of using ink-jet printer technology to dispense cells and relating to the creation of matrices of bioprinted cells on gel materials.
In connection with the acquisition of the FXR program from Metacrine in 2023, we were assigned and assumed a license agreement with the Salk Institute for Biological Studies requiring milestone and royalty payments based on the development and commercialization of FXR314.
The patent rights we obtained through these exclusive licenses are not only foundational within the field of 3D bioprinting and FXR agonist therapies but provide us with favorable priority dates. We are required to make ongoing royalty payments under these exclusive licenses based on net sales of products and services that rely on the intellectual property we in-licensed. For additional information regarding our royalty obligations see “Note 6. Collaborative Research, Development, and License Agreements” in the Notes to the Consolidated Financial Statements included in this Annual Report.
Company Owned Intellectual Property
In addition to the intellectual property we have in-licensed, we have historically innovated and grown our intellectual property portfolio.
With respect to our bioprinting platform, we have 11 issued U.S. patents and 13 issued foreign patents directed to our NovoGen Bioprinter® and methods of bioprinting: U.S. Patent Nos. 8,931,880; 9,149,952; 9,227,339; 9,315,043; 9,499,779; 9,855,369; 10,174,276, 10,967,560, 11,577,450, 11,577,451 and 11,413,805 ; Australia Patent Nos. 2015202836, and 2014296246; Canada Patent No. 2,812,766; China Patent Nos. ZL201180050831.4 and ZL201480054148.1; European Patent Nos. 2838985, 2629975, and 3028042; Japan Patent Nos. 6333231, 6566426 and 6842918, and Russian Patent No. 2560393. These issued patents and pending patent applications carry remaining patent terms ranging from over 20 years to just over 7 years. We have additional U.S. continuation applications pending in these families as well foreign counterpart applications in multiple countries.
Our ExVive™ Human Liver Tissue is protected by U.S. Patent Nos. 9,222,932, 9,442,105, 10,400,219 and 11,127,774; Australia Patent Nos. 2014236780 and 2017200691; and Canada Patent No. 2,903,844. Our ExVive™ Human Kidney Tissue is protected by U.S. Patent Nos. 9,481,868, 10,094,821 and 10,962,526; Australian Patent No. 2015328173, Canadian Patent No. 2,962,778, European Patent No. 3204488 and Japan Patent No. 7021177. These issued patents and pending patent applications carry remaining patent terms ranging from over 11 years to just over 9 years. We have additional U.S. patent applications pending in these families, as well as foreign counterpart applications in multiple countries. We currently have pending numerous patent applications in the U.S. and globally that are directed to additional features on bioprinters, additional tissue types, their methods of fabrication, and specific applications.
Our U.S. Patent Nos. 9,855,369 and 9,149,952, which relate to our bioprinter technology, were the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”), one of our competitors. Likewise, U.S. Patent Nos. 9,149,952, 9,855,369, 8,931,880, 9,227,339, 9,315,043 and 10,967,560 (all assigned to Organovo, Inc.) and U.S. Patent Nos. 7,051,654, 8,241,905, 8,852,932 and 9,752,116 (assigned to Clemson University and the University of Missouri, respectively) were implicated in a declaratory judgment complaint filed against Organovo, Inc., our wholly owned subsidiary, by BICO Group AB and certain of its subsidiaries in the United States District Court for the District of Delaware. All of these matters have since been settled in a favorable manner for the Company. Specifically, on February 23, 2022, we announced an agreement of a non-exclusive license for BICO Group AB and its affiliate companies to Organovo’s foundational patent portfolio in 3D bioprinting. For more information regarding these proceedings, see the section titled Part I, Item 3 of this Annual Report on Form 10-K.
With respect to our FXR agonist program covering FXR314 and FXR125, we have 7 issued U.S. patents and 15 issued foreign patents directed to composition of matter protection (generic and specific) for FXR314 and FXR125, as well claims directed to methods of treatment of GI diseases, formulations of FXR314 and polymorphs of the FXR314 molecule including United States Patent Nos.11,214,538, 10,703,712, 10,927,082, 10,961,198, 11,236,071 and 11,084,817, granted Australian Patent Nos. 2016323992 and 2018236275, Chinese Patent Nos. 201680066917 and 269065, Eurasian Patent Nos. 040003 and 040704, Israeli Patent Nos. 258011, 296068 and 296065, Indian Patent No. 380510, Japanese Patent Nos. 6905530 and 717709, Mexican Patent Nos. 386,752 and 397265 and South African Patent No. 2018/01750. In addition, we have 8 pending U.S. patent applications and over 50 pending foreign patent applications, including U.S. Patent Application Nos. 18/156,069, 18/174,393, 17/349,757, 17/906,580, 17/906,582 and 17/906,585 and over 50 pending international patent applications in a number of countries including, Australia, Brazil, Canada, Chile, China, the Eurasian Patent Office, the European Patent Office, Israel, India, Japan, South Korea, Mexico, Singapore, Philippines and Hong Kong. These issued patents and pending patent applications carry remaining patent terms ranging from over 18 years to just over 15 years.
Employees and Human Capital
As of May 1, 2024, we had 20 employees, of which 12 are full-time. We have also retained some of our former employees as consultants, in addition to a number of expert consultants in specific scientific and operational areas. Our employees are not represented by labor unions or covered under any collective bargaining agreements. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and additional employees. The principal purposes of our equity incentive plans are to attract, retain and motivate selected employees, consultants, and directors through the granting of equity-based compensation awards.
Corporate Information
We are operating the business of our subsidiaries, including Organovo, Inc., our wholly-owned subsidiary, which we acquired in February 2012. Organovo, Inc. was incorporated in Delaware in April 2007. Our common stock has traded on The Nasdaq Stock Market LLC under the symbol “ONVO” since August 8, 2016 and our common stock currently trades on the Nasdaq Capital Market. Prior to that time, it traded on the NYSE MKT under the symbol “ONVO” and prior to that was quoted on the OTC Market.
Our principal executive offices are located at 11555 Sorrento Valley Rd, Suite 100, San Diego CA 92121 and our phone number is (858) 224-1000. Our Internet website can be found at http://www.organovo.com. The content of our website is not intended to be incorporated by reference into this Annual Report or in any other report or document that we file.
Available Information
Our investor relations website is located at http://ir.organovo.com. We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Reports filed with the Securities and Exchange Commission (the “SEC”) pursuant to the Exchange Act, including annual and quarterly reports, and other reports we file, are available free of charge, through our website. The content of our website is not intended to be incorporated by reference into this Annual Report or in any other report or document that we file. We make them available on our website as soon as reasonably possible after we file them with the SEC. The reports we file with the SEC are also available on the SEC’s website (http://www.sec.gov).
Item 1A. Risk Factors.
Investment in our common stock involves a substantial degree of risk and should be regarded as speculative. As a result, the purchase of our common stock should be considered only by persons who can reasonably afford to lose their entire investment. Before you elect to purchase our common stock, you should carefully consider the risk and uncertainties described below in addition to the other information incorporated herein by reference. Additional risks and uncertainties of which we are unaware or which we currently believe are immaterial could also materially adversely affect our business, financial condition or results of operations. If any of the risks or uncertainties discussed in this Annual Report occur, our business, prospects, liquidity, financial condition and results of operations could be materially and adversely affected, in which case the trading price of our common stock could decline, and you could lose all or part of your investment.
Risk Factor Summary
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below and should be carefully considered, together with other information in this Annual Report on Form 10-K and our other filings with the Securities and Exchange Commission before making investment decisions regarding our common stock.
•We will incur substantial additional operating losses over the next several years as our research and development activities increase.
•Using our platform technology to develop human tissues and disease models for drug discovery and development is new and unproven.
•As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our development activities.
•We may require substantial additional funding. Raising additional capital would cause dilution to our existing stockholders and may restrict our operations or require us to relinquish rights to our technologies or to a product candidate.
•Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes, and results of earlier studies and trials may not be predictive of future results.
•The near and long-term viability of our drug discovery and development efforts will depend on our ability to successfully establish strategic relationships.
•Current and future legislation may increase the difficulty and cost of commercializing our drug candidates and may affect the prices we may obtain if our drug candidates are approved for commercialization.
•Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern. Separately, our independent registered public accounting firm has included in its opinion for the year ended March 31, 2024 an explanatory paragraph expressing substantial doubt in our ability to continue as a going concern, which may hinder our ability to obtain future financing.
•Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations.
•We have a history of operating losses and expect to incur significant additional operating losses.
•There is no assurance that an active market in our common stock will continue at present levels or increase in the future.
•The price of our common stock may continue to be volatile, which could lead to losses by investors and costly securities litigation.
•Patents covering our products could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
•We may be involved in lawsuits or other proceedings to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Risks Related to our Business
We are a clinical stage biotechnology company focusing on clinical drug development of the farnesoid X receptor (“FXR”) agonist FXR314, which involves a substantial degree of uncertainty, and on 3D bioprinting technology to develop human tissues and disease models for drug discovery and development, which is an unproven business strategy that may never achieve profitability.
We are a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease ("IBD"), including ulcerative colitis ("UC"), based on demonstration of clinical promise in three-dimensional ("3D") human tissues as well as strong preclinical data. Our current clinical focus is in advancing FXR314 in IBD, including UC and Crohn's disease. Our secondary focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. Our success will depend upon our ability to advance the development of FXR314, our ability to determine the appropriate clinical focus for FXR314, our ability to identify additional drug candidates to pursue and the viability of our platform technology and any disease models we develop. Our success will also depend on our ability to select an appropriate development strategy for FXR314 and any other drug candidates we may identify, including internal development or partnering or licensing arrangements with pharmaceutical companies. We may not be able to partner or license our drug candidates. We may never achieve profitability, or even if we achieve profitability, we may not be able to maintain or increase our profitability.
We will incur substantial additional operating losses over the next several years as our research and development activities increase.
We will incur substantial additional operating losses over the next several years as our research and development activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things:
•successfully developing human tissues and disease models for drug discovery and development that enable us to identify drug candidates;
•successfully outsourcing certain portions of our development efforts;
•entering into partnering or licensing arrangements with pharmaceutical companies to further develop and conduct clinical trials for any drug candidates we identify;
•obtaining any necessary regulatory approval for any drug candidates we identify; and
•raising sufficient funds to finance our activities and long-term business plan.
We might not succeed at any of these undertakings. If we are unsuccessful at one or more of these undertakings, our business, prospects, and results of operations will be materially adversely affected.
Using our platform technology to develop human tissues and disease models for drug discovery and development is new and unproven.
Utilizing our 3D bioprinting platform technology to develop human tissues and disease models for drug discovery and development will involve new and unproven technologies, disease models and approaches, each of which is subject to the risk associated with new and evolving technologies. To date, we have not identified or developed any drug candidates utilizing our new business model. Our future success will depend on our ability to utilize our 3D bioprinting platform to develop human tissues and disease models that will enable us to identify and develop viable drug candidates. We may experience unforeseen technical complications, unrecognized defects and limitations in our technology or our ability to develop disease models or identify viable drug candidates. These complications could materially delay or substantially increase the anticipated costs and time to identify and develop viable drug candidates, which would have a material adverse effect on our business and financial condition and our ability to continue operations.
We will face intense competition in our drug discovery efforts.
The biotechnology and pharmaceutical industry is subject to intense competition and rapid and significant technological change. There are many potential competitors for the disease indications we may pursue, including major drug companies, specialized biotechnology firms, academic institutions, government agencies and private and public research institutions. Many of these competitors have significantly greater financial and technical resources, experience and expertise in the following areas than we have, including:
•research and technology development;
•development of or access to disease models;
•identification and development of drug candidates;
•regulatory processes and approvals; and
•identifying and entering into agreements with potential collaborators.
Principal competitive factors in our industry include: the quality, scientific and technical support, management and the execution of drug development and regulatory approval strategies; skill and experience of employees, including the ability to recruit and retain skilled, experienced employees; intellectual property portfolio; range of capabilities, including drug identification, development and regulatory approval; and the availability of substantial capital resources to fund these activities.
In order to effectively compete, we may need to make substantial investments in our research and technology development, drug candidate identification and development, testing and regulatory approval and licensing and business development activities. There is no assurance that we will be successful in discovering effective drug candidates using our 3D bioprinted tissues or disease models. Our technologies and drug development plans also may be rendered obsolete or noncompetitive as a result of drugs, intellectual property, technologies, products and services introduced by competitors. Any of these risks may prevent us from building a successful drug discovery business or entering into a strategic partnership or collaboration related to, any drug candidates we identify on favorable terms, or at all.
As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our development activities.
As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our 3D tissue development activities. We purchase human cells from selected third-party suppliers based on quality assurance, cost effectiveness, and regulatory requirements. We need to continue to identify additional sources of qualified human cells and there can be no guarantee that we will be able to access the quantity and quality of raw materials needed at a cost-effective price. Any failure to obtain a reliable supply of sufficient human cells or a supply at cost effective prices would harm our business and our results of operations and could cause us to be unable to support our drug development efforts.
We may not be successful in establishing our Mosaic Cell Sciences division (“Mosaic”) as a profitable commercial business.
We formed Mosaic to serve as a key source of certain of the primary human cells we utilize in our research and development efforts. In addition to supplying human cells for our business requirements, we believe there is an opportunity for Mosaic to operate as a commercial business by selling human cells to other pharmaceutical, biotech and research organizations. We intend for Mosaic to begin selling its human cell offerings to end users both directly and through distribution partners during fiscal 2025. Operating and developing Mosaic’s business is subject to a number of risks and uncertainties, including:
•failing to source a sufficient supply of high-quality human organs or cells;
•failing to achieve market acceptance for its human cell offerings;
•failing to demonstrate the quality and reliability of its human cell offerings; • failing to be both cost effective and competitive with the products offered by third parties;
•failing to obtain any necessary regulatory approvals;
•failing to be able to produce its human cell offerings on a large enough scale;
•failing to establish and maintain distribution relationships with reliable third parties;
•failing to hire and retain qualified personnel; and
•infringing the proprietary rights of third parties or failing to protect our own intellectual property.
If any of these or any other risks and uncertainties occur, our efforts to establish Mosaic as a commercial business may be unsuccessful, which would harm our business and results of operations.
Our business will be adversely impacted if we are unable to successfully attract, hire and integrate key additional employees or contractors.
Our future success depends in part on our ability to successfully attract and then retain key additional executive officers and other key employees and contractors to support our drug discovery plans. Recruiting and retaining qualified scientific and clinical personnel is critical to our success. Competition to hire qualified personnel in our industry is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. If we are unable to attract and retain high quality personnel, our ability to pursue our drug discovery business will be limited, and our business, prospects, financial condition, and results of operations may be adversely affected.
We may require substantial additional funding. Raising additional capital would cause dilution to our existing stockholders and may restrict our operations or require us to relinquish rights to our technologies or to a product candidate.
We currently do not have any committed external source of funds and do not expect to generate any meaningful revenue in the foreseeable future. If our board of directors decides that we should pursue further research and development activities than already proposed, we will require substantial additional funding to operate our proposed business, including expanding our facilities and hiring additional qualified personnel, and we would expect to finance these cash needs through a combination of equity offerings, debt financings, government or other third-party funding and licensing or collaboration arrangements.
To the extent that we raise additional capital through the sale of equity or convertible debt, the ownership interests of our stockholders will be diluted. In addition, the terms of any equity or convertible debt we agree to issue may include liquidation or other preferences that adversely affect the rights of our stockholders. Convertible debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, and declaring dividends, and may impose limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Moreover, we have the ability to sell up to $0.8 million of additional shares of our common stock to the public through an “at the market offering” pursuant to a Sales Agreement that we entered into with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC on March 16, 2018 (the “Sales Agreement”). Any shares of common stock issued in the “at the market offering” (“ATM offering”) will result in dilution to our existing stockholders.
We currently have an effective shelf registration statement on Form S-3 filed with the Securities and Exchange Commission (the “SEC”), which we may use to offer from time to time any combination of debt securities, common and preferred stock and warrants. On March 16, 2018, we entered into the Sales Agreement pursuant to which we have the ability to sell shares of our common stock to the public through an ATM offering. As of March 31, 2024, we have issued and sold pursuant to the Sales Agreement an aggregate of 5,371,418 shares of our common stock for gross proceeds of approximately $46.9 million. However, in the event that the aggregate market value of our common stock held by non-affiliates (“public float”) is less than $75.0 million, the amount we can raise through primary public offerings of securities, including sales under the Sales Agreement, in any twelve-month period using shelf registration statements is limited to an aggregate of one-third of our public float. As of May 15, 2024, our public float was less than $75.0 million, and therefore we are limited to an aggregate of one-third of our public float in the amount we could raise through primary public offerings of securities in any twelve-month period using shelf registration statements, or $2,474,091. Although we would still maintain the ability to raise funds through other means, such as through the filing of a registration statement on Form S-1 or in private placements, the rules and regulations of the SEC or any other regulatory agencies may restrict our ability to conduct certain types of financing activities, or may affect the timing of and amounts we can raise by undertaking such activities.
Further, additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations. Raising additional funding through debt or equity financing is likely to be difficult or unavailable altogether given the early stage of our technology and any drug candidates we identify. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline further and existing stockholders may not agree with our financing plans or the terms of such financings.
Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes, and results of earlier studies and trials may not be predictive of future results.
Before obtaining marketing approval from regulatory authorities for the sale of any drug candidates we identify, any such drug candidates must undergo extensive clinical trials to demonstrate the safety and efficacy of the drug candidates in humans. Human clinical testing is expensive and can take many years to complete, and we cannot be certain that any clinical trials will be conducted as planned or completed on schedule, if at all. We may elect to complete this testing, or some portion thereof, internally or enter into a partnering or development agreement with a pharmaceutical company to complete these trials. Our inability, or the inability of any third party with whom we enter into a partnering or development agreement, to successfully complete preclinical and clinical development could result in additional costs to us and negatively impact our ability to generate revenues or receive development or milestone payments. Our future success is dependent on our ability, or the ability of any pharmaceutical company with whom we enter into a partnering or development agreement, to successfully develop, obtain regulatory approval for, and then successfully commercialize any drug candidates we identify.
Any drug candidates we identify will require additional clinical development, management of clinical, preclinical and manufacturing activities, regulatory approval in applicable jurisdictions, achieving and maintaining commercial-scale supply, building of a commercial organization, substantial investment and significant marketing efforts. We are not permitted to market or promote any of our drug candidates before we receive regulatory approval from the U.S. Food and Drug Administration (“FDA”) or comparable foreign regulatory authorities, and we may never receive such regulatory approval for any of our drug candidates.
We, or any third party with whom we enter into a partnering or development agreement, may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to earn development or milestone payments or for any drug candidates to obtain regulatory approval, including:
•delays in or failure to reach agreement on acceptable terms with prospective contract research organizations (“CROs”) and clinical sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
•failure to obtain sufficient enrollment in clinical trials or participants may fail to complete clinical trials;
•clinical trials of our drug candidates that may produce negative or inconclusive results, and as a result we, or any pharmaceutical company with who we enter into a partnering or development agreement, may decide, or regulators may require, additional clinical trials;
•suspension or termination of clinical research, either by us, any third party with whom we enter into a partnering or development agreement, regulators or institutional review boards, for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
•additional or unanticipated clinical trials required by regulators or institutional review boards to obtain approval or any drug candidates may be subject to additional post-marketing testing requirements to maintain regulatory approval;
•regulators may revise the requirements for approving any drug candidates, or such requirements may not be as anticipated;
•the cost of clinical trials for any drug candidates may be greater than anticipated;
•the supply or quality of any drug candidates or other materials necessary to conduct clinical trials of our drug candidates may be insufficient or inadequate or may be delayed; and
•regulatory authorities may suspend or withdraw their approval of a product or impose restrictions on its distribution.
If we, or any third party with whom we enter into a partnering or development agreement, experience delays in the completion of, or termination of, any clinical trial of any drug candidates that we develop, or are unable to achieve clinical endpoints due to unforeseen events, the commercial prospects of our drug candidates will be harmed, and our ability to develop milestones, development fees or product revenues from any of these drug candidates will be delayed.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates that we identify for specific indications among many potential options. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Currently, we are focused on developing FXR314 in IBD, including UC, based on demonstration of clinical promise in 3D human tissues as well as strong preclinical data. Our resource allocation decisions may cause us to fail to capitalize on viable commercial medicines or profitable market opportunities. Our
projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on estimates. If any of our estimates are inaccurate, the market opportunities for any of our product candidates could be significantly diminished and have an adverse material impact on our business. Additionally, the potentially addressable patient population for our product candidates may be limited, or may not be amenable to treatment with our product candidates. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing, or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Any such event could have a material adverse effect on our business, financial condition, results of operations and prospects.
We will rely upon third-party contractors and service providers for the execution of critical aspects of any future development programs. Failure of these collaborators to provide services of a suitable quality and within acceptable timeframes may cause the delay or failure of any future development programs.
We plan to outsource certain functions, tests and services to CROs, medical institutions and collaborators as well as outsource manufacturing to collaborators and/or contract manufacturers, and we will rely on third parties for quality assurance, clinical monitoring, clinical data management and regulatory expertise. We may elect, in the future, to engage a CRO to run all aspects of a clinical trial on our behalf. There is no assurance that such individuals or organizations will be able to provide the functions, tests, biologic supply or services as agreed upon or in a quality fashion and we could suffer significant delays in the development of our drug candidates or development programs.
In some cases, there may be only one or few providers of such services, including clinical data management or manufacturing services. In addition, the cost of such services could be significantly increased over time. We may rely on third parties and collaborators to enroll qualified patients and conduct, supervise and monitor our clinical trials. Our reliance on these third parties and collaborators for clinical development activities reduces our control over these activities. Our reliance on these parties, however, does not relieve us of our regulatory responsibilities, including ensuring that our clinical trials are conducted in accordance with Good Clinical Practice (“GCP”) regulations and the investigational plan and protocols contained in the regulatory agency applications. In addition, these third parties may not complete activities on schedule or may not manufacture under Current Good Manufacturing Practice (“cGMP”) conditions. Preclinical or clinical studies may not be performed or completed in accordance with Good Laboratory Practices (“GLP”) regulatory requirements or our trial design. If these third parties or collaborators do not successfully carry out their contractual duties or meet expected deadlines, obtaining regulatory approval for manufacturing and commercialization of our drug candidates may be delayed or prevented. We may rely substantially on third-party data managers for our clinical trial data. There is no assurance that these third parties will not make errors in the design, management or retention of our data or data systems. There is no assurance these third parties will pass FDA or regulatory audits, which could delay or prohibit regulatory approval.
In addition, we will exercise limited control over our third-party partners and vendors, which makes us vulnerable to any errors, interruptions or delays in their operations. If these third parties experience any service disruptions, financial distress or other business disruption, or difficulties meeting our requirements or standards, it could make it difficult for us to operate some aspects of our business.
The near and long-term viability of our drug discovery and development efforts will depend on our ability to successfully establish strategic relationships.
The near and long-term viability of our drug discovery and development efforts depend in part on our ability to successfully establish new strategic partnering, collaboration and licensing arrangements with biotechnology companies, pharmaceutical companies, universities, hospitals, insurance companies and or government agencies. Establishing strategic relationships is difficult and time-consuming. Potential partners and collaborators may not enter into relationships with us based upon their assessment of our technology or drug candidates or our financial, regulatory or intellectual property position. If we fail to establish a sufficient number of strategic relationships on acceptable terms, we may not be able to develop and obtain regulatory approval for our drug candidates or generate sufficient revenue to fund further research and development efforts. Even if we establish new strategic relationships, these relationships may never result in the successful development or regulatory approval for any drug candidates we identify for a number of reasons both within and outside of our control.
Investors’ expectations of our performance relating to environmental, social and governance factors may impose additional costs and expose us to new risks.
There is an increasing focus from certain investors, employees, regulators and other stakeholders concerning corporate responsibility, specifically related to environmental, social and governance (“ESG”) factors. Some investors and investor advocacy groups may use
these factors to guide investment strategies and, in some cases, investors may choose not to invest in our company if they believe our policies relating to corporate responsibility are inadequate. Third-party providers of corporate responsibility ratings and reports on companies have increased to meet growing investor demand for measurement of corporate responsibility performance, and a variety of organizations currently measure the performance of companies on such ESG topics, and the results of these assessments are widely publicized. Investors, particularly institutional investors, use these ratings to benchmark companies against their peers and if we are perceived as lagging with respect to ESG initiatives, certain investors may engage with us to improve ESG disclosures or performance and may also make voting decisions, or take other actions, to hold us and our board of directors accountable. In addition, the criteria by which our corporate responsibility practices are assessed may change, which could result in greater expectations of us and cause us to undertake costly initiatives to satisfy such new criteria. If we elect not to or are unable to satisfy such new criteria, investors may conclude that our policies with respect to corporate responsibility are inadequate. We may face reputational damage in the event that our corporate responsibility procedures or standards do not meet the standards set by various constituencies.
We may face reputational damage in the event our corporate responsibility initiatives or objectives do not meet the standards set by our investors, stockholders, lawmakers, listing exchanges or other constituencies, or if we are unable to achieve an acceptable ESG or sustainability rating from third-party rating services. A low ESG or sustainability rating by a third-party rating service could also result in the exclusion of our common stock from consideration by certain investors who may elect to invest with our competition instead. Ongoing focus on corporate responsibility matters by investors and other parties as described above may impose additional costs or expose us to new risks. Any failure or perceived failure by us in this regard could have a material adverse effect on our reputation and on our business, share price, financial condition, or results of operations, including the sustainability of our business over time.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
Our business, financial condition and share price could be adversely affected by general conditions in the global economy and in the global financial markets. As widely reported, in the past several years, global credit and financial markets have experienced volatility and disruptions, and especially in 2020, 2021 and 2022 due to the impacts of the COVID-19 pandemic, and, more recently, the ongoing conflict between Ukraine and Russia and the global impact of restrictions and sanctions imposed on Russia, including, for example, severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. Moreover, the global impacts of the Israel-Hamas war are still unknown. There can be no assurances that further deterioration in credit and financial markets and confidence in economic conditions will not occur. For example, U.S. debt ceiling and budget deficit concerns have increased the possibility of additional credit-rating downgrades and economic slowdowns, or a recession in the United States. Although U.S. lawmakers passed legislation to raise the federal debt ceiling on multiple occasions, including a suspension of the federal debt ceiling in June 2023, ratings agencies have lowered or threatened to lower the long-term sovereign credit rating on the United States. The impact of this or any further downgrades to the U.S. government’s sovereign credit rating or its perceived creditworthiness could adversely affect the U.S. and global financial markets and economic conditions. Absent further quantitative easing by the Federal Reserve, these developments could cause interest rates and borrowing costs to rise, which may negatively impact our results of operations or financial condition.
Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and share price and could require us to delay or abandon clinical development plans. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
The impact of the Russian invasion of Ukraine and the Israel-Hamas war on the global economy, energy supplies and raw materials is uncertain, but may prove to negatively impact our business and operations.
The short and long-term implications of Russia’s invasion of Ukraine and the Israel-Hamas war are difficult to predict at this time. We continue to monitor any adverse impact that the outbreak of war in Ukraine and the subsequent institution of sanctions against Russia by the United States and several European and Asian countries, and the Israel-Hamas war may have on the global economy in general, on our business and operations and on the businesses and operations of our suppliers and other third parties with which we conduct business. For example, a prolonged conflict in Ukraine or Israel may result in increased inflation, escalating energy prices and constrained availability, and thus increasing costs, of raw materials. We will continue to monitor this fluid situation and develop contingency plans as necessary to address any disruptions to our business operations as they develop. To the extent the wars in Ukraine or Israel may adversely affect our business as discussed above, they may also have the effect of heightening many of the other risks described herein. Such risks include, but are not limited to, adverse effects on macroeconomic conditions, including inflation; disruptions to our technology infrastructure, including through cyberattack, ransom attack, or cyber-intrusion; adverse changes in
international trade policies and relations; disruptions in global supply chains; and constraints, volatility, or disruption in the capital markets, any of which could negatively affect our business and financial condition.
Risks Related to Government Regulation
In the past, we have used hazardous chemicals, biological materials and infectious agents in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our product manufacturing, research and development, and testing activities have involved the controlled use of hazardous materials, including chemicals, biological materials and infectious disease agents. We cannot eliminate the risks of accidental contamination or the accidental spread or discharge of these materials, or any resulting injury from such an event. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our insurance coverage and our total assets. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. We were also subject to various laws and regulations relating to safe working conditions, laboratory and manufacturing practices, and the experimental use of animals. Our operations may have required that environmental permits and approvals be issued by applicable government agencies. If we failed to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance.
If we fail to obtain and sustain an adequate level of reimbursement for our potential products by third-party payors, potential future sales would be materially adversely affected.
There will be no viable commercial market for our drug candidates, if approved, without reimbursement from third-party payors. Reimbursement policies may be affected by future healthcare reform measures. We cannot be certain that reimbursement will be available for our current drug candidates or any other drug candidate we may develop. Additionally, even if there is a viable commercial market, if the level of reimbursement is below our expectations, our anticipated revenue and gross margins will be adversely affected.
Third-party payors, such as government or private healthcare insurers, carefully review and increasingly question and challenge the coverage of and the prices charged for drugs. Reimbursement rates from private health insurance companies vary depending on the Company, the insurance plan and other factors. Reimbursement rates may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. There is a current trend in the U.S. healthcare industry toward cost containment.
Large public and private payors, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and reimbursement levels for, particular treatments. Such third-party payors, including Medicare, may question the coverage of, and challenge the prices charged for, medical products and services, and many third-party payors limit coverage of or reimbursement for newly approved healthcare products. In particular, third-party payors may limit the covered indications. Cost-control initiatives could decrease the price we might establish for products, which could result in product revenues being lower than anticipated. We believe our drugs will be priced significantly higher than existing generic drugs and consistent with current branded drugs. If we are unable to show a significant benefit relative to existing generic drugs, Medicare, Medicaid and private payors may not be willing to provide reimbursement for our drugs, which would significantly reduce the likelihood of our products gaining market acceptance.
We expect that private insurers will consider the efficacy, cost-effectiveness, safety and tolerability of our potential products in determining whether to approve reimbursement for such products and at what level. Obtaining these approvals can be a time consuming and expensive process. Our business, financial condition and results of operations would be materially adversely affected if we do not receive approval for reimbursement of our potential products from private insurers on a timely or satisfactory basis. Limitations on coverage could also be imposed at the local Medicare carrier level or by fiscal intermediaries. Medicare Part D, which provides a pharmacy benefit to Medicare patients as discussed below, does not require participating prescription drug plans to cover all drugs within a class of products. Our business, financial condition and results of operations could be materially adversely affected if Part D prescription drug plans were to limit access to, or deny or limit reimbursement of, our drug candidates or other potential products.
Reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis. In many countries, the product cannot be commercially launched until reimbursement is approved. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. The negotiation process in some countries can exceed 12 months. To obtain reimbursement or pricing
approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our products to other available therapies.
If the prices for our potential products are reduced or if governmental and other third-party payors do not provide adequate coverage and reimbursement of our drugs, our future revenue, cash flows and prospects for profitability will suffer.
Current and future legislation may increase the difficulty and cost of commercializing our drug candidates and may affect the prices we may obtain if our drug candidates are approved for commercialization.
In the U.S. and some foreign jurisdictions, there have been a number of adopted and proposed legislative and regulatory changes regarding the healthcare system that could prevent or delay regulatory approval of our drug candidates, restrict or regulate post-marketing activities and affect our ability to profitably sell any of our drug candidates for which we obtain regulatory approval.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“MMA”) changed the way Medicare covers and pays for pharmaceutical products. Cost reduction initiatives and other provisions of this legislation could limit the coverage and reimbursement rate that we receive for any of our approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
In addition, on August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which, among other things, includes policies that are designed to have a direct impact on drug prices and reduce drug spending by the federal government, which shall take effect in 2023. Under the Inflation Reduction Act of 2022, Congress authorized Medicare beginning in 2026 to negotiate lower prices for certain costly single-source drug and biologic products that do not have competing generics or biosimilars. This provision is limited in terms of the number of pharmaceuticals whose prices can be negotiated in any given year and it only applies to drug products that have been approved for at least 9 years and biologics that have been licensed for 13 years. Drugs and biologics that have been approved for a single rare disease or condition are categorically excluded from price negotiation. Further, the new legislation provides that if pharmaceutical companies raise prices in Medicare faster than the rate of inflation, they must pay rebates back to the government for the difference. The new law also caps Medicare out-of-pocket drug costs at an estimated $4,000 a year in 2024 and, thereafter beginning in 2025, at $2,000 a year.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively the “PPACA”), was enacted. The PPACA was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The PPACA increased manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate amount for both branded and generic drugs and revised the definition of “average manufacturer price”, which may also increase the amount of Medicaid drug rebates manufacturers are required to pay to states. The legislation also expanded Medicaid drug rebates and created an alternative rebate formula for certain new formulations of certain existing products that is intended to increase the rebates due on those drugs. The Centers for Medicare & Medicaid Services (“CMS”), which administers the Medicaid Drug Rebate Program, also has proposed to expand Medicaid rebates to the utilization that occurs in the territories of the U.S., such as Puerto Rico and the Virgin Islands. Further, beginning in 2011, the PPACA imposed a significant annual fee on companies that manufacture or import branded prescription drug products and required manufacturers to provide a 50% discount off the negotiated price of prescriptions filled by beneficiaries in the Medicare Part D coverage gap, referred to as the “donut hole.” Legislative and regulatory proposals have been introduced at both the state and federal level to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
There have been public announcements by members of the U.S. Congress, regarding plans to repeal and replace the PPACA and Medicare. For example, on December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act of 2017, which, among other things, eliminated the individual mandate requiring most Americans (other than those who qualify for a hardship exemption) to carry a minimum level of health coverage, effective January 1, 2019. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, or the Texas District Court Judge, ruled that the individual mandate is a critical and inseverable feature of the PPACA, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act of 2017, the remaining provisions of the PPACA are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the Fifth Circuit upheld the District Court’s ruling with respect to the individual mandate but remanded the case to the District Court to consider whether other parts of the law can remain in effect. While the Texas District Court Judge has stated that the ruling will have no immediate effect, it is unclear how this decision, subsequent appeals, and other efforts to repeal and replace the PPACA will impact the law and our business. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our drug candidates, if any, may be. In addition, increased scrutiny by
the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing approval testing and other requirements.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under government payor programs, and review the relationship between pricing and manufacturer patient programs. The U.S. Department of Health and Human Services has started soliciting feedback on some of these measures and, at the same time, is implementing others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. While any proposed measures will require authorization through additional legislation to become effective, Congress has indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our drug candidates, if approved for commercialization.
In Europe, the United Kingdom formally withdrew from the European Union on January 31, 2020, and entered into a transition period that ended on December 31, 2020. A significant portion of the regulatory framework in the United Kingdom is derived from the regulations of the European Union. We cannot predict what consequences the recent withdrawal of the United Kingdom from the European Union will have on the regulatory frameworks of the United Kingdom or the European Union, or on our future operations, if any, in these jurisdictions, and the United Kingdom is in the process of negotiating trade deals with other countries. Additionally, the United Kingdom’s withdrawal from the European Union may increase the possibility that other countries may decide to leave the European Union again.
Actions that we have taken to restructure our business to align our cost structure with our strategic priorities may not have the anticipated effects.
In August 2023, we announced a plan to reduce our workforce by six employees, which represented approximately 24% of our employees as of August 18, 2023. The decision to reduce our workforce was made in order to focus spending on our clinical program for FXR314, reduce ongoing operating expenses not related to clinical expenses, and extend our cash runway. As a result of the reduction in force, we incurred approximately $0.4 million of cash expenditures in connection with the reduction in force, which relate to severance pay, in the year ended March 31, 2024. We may incur additional expenses not currently contemplated due to events associated with the reduction in force; for example, the reduction in force may have a future impact on other areas of our liabilities and obligations, which could result in losses in future periods. Moreover, we may not realize, in full or in part, the anticipated benefits and savings from this restructuring due to unforeseen difficulties, delays or unexpected costs. If we are unable to realize the expected operational efficiencies and cost savings from the restructuring, our operating results and financial condition would be adversely affected. In addition, we may need to undertake additional workforce reductions or restructuring activities in the future.
Risks Related to Our Capital Requirements, Finances and Operations
Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern. Separately, our independent registered public accounting firm has included in its opinion for the year ended March 31, 2024 an explanatory paragraph expressing substantial doubt in our ability to continue as a going concern, which may hinder our ability to obtain future financing.
Our financial statements as of March 31, 2024 have been prepared under the assumption that we will continue as a going concern for the next twelve months. Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern. Separately, our independent registered public accounting firm included in its opinion for the year ended March 31, 2024 an explanatory paragraph referring to our recurring losses from operations and expressing substantial doubt in our ability to continue as a going concern without additional capital becoming available. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, obtain government grants, reduce expenditures, and generate significant revenue. Our financial statements as of March 31, 2024 do not include any adjustments that might result from the outcome of this uncertainty. The reaction of investors to the inclusion of a going concern statement by management and our auditors, and our potential inability to continue as a going concern, in future years could materially adversely affect our share price and our ability to raise new capital or enter into strategic alliances.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations.
There can be no assurance that we will be able to raise sufficient additional capital on acceptable terms or at all. Raising additional funding through debt or equity financing is likely to be difficult or unavailable altogether given the early stage of our therapeutic candidates. If such additional financing is not available on satisfactory terms, or is not available in sufficient amounts, we may be required to delay, limit or eliminate the development of business opportunities and our ability to achieve our business objectives, our competitiveness, and our business, financial condition and results of operations will be materially adversely affected. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing stockholders, increased fixed payment obligations and the existence of securities with rights that may be senior to those of our common stock. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline further and existing stockholders may not agree with our financing plans or the terms of such financings. In addition, if we seek funds through arrangements with collaborative partners, these arrangements may require us to relinquish rights to our technology or potential future product candidates or otherwise agree to terms unfavorable to us. Although we raised net proceeds of approximately $4.7 million in May 2024 in connection with the sale and issuance of (i) 1,562,500 shares of our common stock, and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock and (ii) pre-funded warrants to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock in a best efforts public offering, we will need to raise additional financing to continue our products’ development for the foreseeable future, and will continue to need to do so until we become profitable.
We have a history of operating losses and expect to incur significant additional operating losses.
We have generated operating losses each year since we began operations, including $15.1 million and $17.7 million for the years ended March 31, 2024 and 2023, respectively. As of March 31, 2024, we had an accumulated deficit of $339.7 million. We expect to incur substantial additional operating losses over the next several years as our research and development activities increase.
The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things:
•successfully developing human tissues and disease models for drug discovery and development that enable us to identify drug candidates;
•successfully outsourcing certain portions of our development efforts;
•entering into collaboration or licensing arrangements with pharmaceutical companies to further develop and conduct clinical trials for any drug candidates we identify;
•obtaining any necessary regulatory approvals for any drug candidates we identify; and
•raising sufficient funds to finance our activities and long-term business plan.
We might not succeed at any of these undertakings. If we are unsuccessful at one or more of these undertakings, our business, prospects, and results of operations will be materially adversely affected. We may never generate significant revenue, and even if we do generate significant revenue, we may never achieve profitability.
Our quarterly operating results may vary, which could negatively affect the market price of our common stock.
Our results of operations in any quarter may vary from quarter to quarter and are influenced by such factors as expenses related to:
•evaluating and implementing strategic alternatives, technology licensing opportunities, potential collaborations, and other strategic transactions;
•research and development expenditures, including commencement of preclinical studies and clinical trials;
•the timing of the hiring of new employees, which may require payments of signing, retention or similar bonuses; and
•changes in costs related to the general global economy.
We believe that operating results for any particular quarter are not necessarily a meaningful indication of future results. Nonetheless, fluctuations in our quarterly operating results could negatively affect the market price of our common stock.
We may identify material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements of our financial statements.
Our management team is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
We cannot assure you that we will not have material weaknesses or significant deficiencies in our internal control over financial reporting. If we identify any material weaknesses or significant deficiencies that may exist, the accuracy and timing of our financial reporting may be adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, and our stock price may decline materially as a result.
Future strategic investments could negatively affect our business, financial condition and results of operations if we fail to achieve the desired returns on our investment.
Our ability to benefit from future external strategic investments depends on our ability to successfully conduct due diligence, evaluate prospective opportunities, and buy the equity of our target investments at acceptable market prices. Our failure in any of these tasks could result in unforeseen loses associated with the strategic investments.
We may also discover deficiencies in internal controls, data adequacy and integrity, product quality, regulatory compliance, product liabilities or other undisclosed liabilities that we did not uncover prior to our investment, which could result in us becoming subject asset impairments, including potential loss of our investment capital. In addition, if we do not achieve the anticipated benefits of an external investment as rapidly as expected, or at all, investors or analysts may downgrade our stock.
We also expect to continue to carry out strategic investments that we believe are necessary to expand our business. There are no assurances that such initiatives will yield favorable results for us. Accordingly, if these initiatives are not successful, our business, financial condition and results of operations could be adversely affected. If these risks materialize, our stock price could be materially adversely affected. Any difficulties in such investments could have a material adverse effect on our business, financial condition and results of operations.
Our business could be adversely impacted if we are unable to retain our executive officers and other key personnel.
Our future success will depend to a significant degree upon the continued contributions of our key personnel, especially our executive officers. We do not currently have long-term employment agreements with our executive officers or our other key personnel, and there is no guarantee that our executive officers or key personnel will remain employed with us. Moreover, we have not obtained key man life insurance that would provide us with proceeds in the event of the death, disability or incapacity of any of our executive officers or other key personnel. Further, the process of attracting and retaining suitable replacements for any executive officers and other key personnel we lose in the future would result in transition costs and would divert the attention of other members of our senior management from our existing operations. Additionally, such a loss could be negatively perceived in the capital markets. Finally, our Executive Chairman also provides services to Viscient Biosciences, Inc. (“Viscient”). Executives that provide services to us and Viscient do not dedicate all of their time to us, as disclosed in our filings, and we may therefore compete with Viscient for the time commitments of our Executive Chairman from time to time.
We may be subject to security breaches or other cybersecurity incidents that could compromise our information and expose us to liability.
We routinely collect and store sensitive data (such as intellectual property, proprietary business information and personally identifiable information) for ourselves, our employees and our suppliers and customers. We make significant efforts to maintain the security and integrity of our computer systems and networks and to protect this information. However, like other companies in our industry, our networks and infrastructure may be vulnerable to cyber-attacks or intrusions, including by computer hackers, foreign governments, foreign companies or competitors, or may be breached by employee error, malfeasance or other disruption. Any such breach could result in unauthorized access to (or disclosure of) sensitive, proprietary or confidential information of ours, our employees or our suppliers or customers, and/or loss or damage to our data. Any such unauthorized access, disclosure, or loss of information could cause competitive harm, result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and/or cause reputational harm.
Compliance with global privacy and data security requirements could result in additional costs and liabilities to us or inhibit our ability to collect and process data globally, and the failure to comply with such requirements could subject us to significant fines and penalties, which may have a material adverse effect on our business, financial condition and results of operations.
The regulatory framework for the collection, use, safeguarding, sharing, transfer, and other processing of information worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. Globally, virtually every jurisdiction in which we operate has established its own data security and privacy frameworks with which we must comply. For example, the collection, use, disclosure, transfer, or other processing of personal data regarding individuals in the European Union, including personal health data, is subject to the EU General Data Protection Regulation (the “GDPR”), which took effect across all member states of the European Economic Area (the “EEA”) in May 2018. The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process personal data, including requirements relating to processing health and other sensitive data, obtaining consent of the individuals to whom the personal data relates, providing information to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, providing notification of data breaches, and taking certain measures when engaging third-party processors. The GDPR increases our obligations with respect to clinical trials conducted in the EEA by expanding the definition of personal data to include coded data and requiring changes to informed consent practices and more detailed notices for clinical trial subjects and investigators. In addition, the GDPR imposes strict rules on the transfer of personal data to countries outside the European Union, including the United States, and, as a result, increases the scrutiny that clinical trial sites located in the EEA should apply to transfers of personal data from such sites to countries that are considered to lack an adequate level of data protection, such as the United States. The GDPR also permits data protection authorities to require destruction of improperly gathered or used personal information and/or impose substantial fines for violations of the GDPR, which can be up to four percent of global revenues or 20 million Euros, whichever is greater, and it also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. In addition, the GDPR provides that European Union member states may make their own further laws and regulations limiting the processing of personal data, including genetic, biometric or health data.
Further, Brexit has led to, and could continue to lead to legislative and regulatory changes, which may increase our compliance costs. As of January 1, 2021 and the expiry of transitional arrangements agreed to between the United Kingdom and the European Union, data processing in the United Kingdom is governed by a United Kingdom version of the GDPR (combining the GDPR and the Data Protection Act 2018), exposing us to two parallel regimes, each of which authorizes similar fines and other potentially divergent enforcement actions for certain violations. On June 28, 2021, the European Commission adopted an Adequacy Decision for the United Kingdom, allowing for the relatively free exchange of personal information between the European Union and the United Kingdom, however, the European Commission may suspend the Adequacy Decision if it considers that the United Kingdom no longer provides for an adequate level of data protection. The UK has announced plans to reform the country’s data protection legal framework in its Data Reform Bill, which may introduce significant changes from the GDPR, which may lead to additional compliance costs. Other jurisdictions outside the European Union are similarly introducing or enhancing privacy and data security laws, rules and regulations.
Similar actions are either in place or under way in the United States. There are a broad variety of data protection laws that are applicable to our activities, and a wide range of enforcement agencies at both the state and federal levels that can review companies for privacy and data security concerns based on general consumer protection laws. The Federal Trade Commission and state Attorneys General all are aggressive in reviewing privacy and data security protections for consumers. New laws also are being considered at both the state and federal levels and several states have passed comprehensive privacy laws. For example, the California Consumer Privacy Act — which went into effect on January 1, 2020 — is creating similar risks and obligations as those created by the GDPR, though the California Consumer Privacy Act does exempt certain information collected as part of a clinical trial subject to the Federal Policy for the Protection of Human Subjects (the Common Rule). As of January 1, 2023, the California Consumer Privacy Act (as amended and expanded by the California Privacy Rights Act) is in full effect, with enforcement by California’s dedicated privacy enforcement agency expected to start later in 2023. While California was first among the states in adopting comprehensive data privacy legislation similar to the GDPR, many other states are following suit. Similar laws passed in Virginia, Colorado, Connecticut, and Utah took effect in 2023. Additionally, Delaware, Indiana, Iowa, Montana, Oregon, Tennessee and Texas have adopted
privacy laws, which take effect from July 1, 2024 through 2026. Many other states are considering similar legislation. Additionally, a broad range of legislative measures also have been introduced at the federal level. Accordingly, failure to comply with federal and state laws (both those currently in effect and future legislation) regarding privacy and security of personal information could expose us to fines and penalties under such laws. There also is the threat of consumer class actions related to these laws and the overall protection of personal data. This is particularly true with respect to data security incidents, and sensitive personal information, including health and biometric data. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our reputation and business.
Given the breadth and depth of changes in data protection obligations, preparing for and complying with these requirements is rigorous and time intensive and requires significant resources and a review of our technologies, systems and practices, as well as those
of any third-party collaborators, service providers, contractors or consultants that process or transfer personal data collected in the European Union. The GDPR, new state privacy laws and other changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, such as healthcare data or other personal information from our clinical trials, could require us to change our business practices and put in place additional compliance mechanisms, may interrupt or delay our development, regulatory and commercialization activities and increase our cost of doing business, and could lead to government enforcement actions, private litigation and significant fines and penalties against us and could have a material adverse effect on our business, financial condition and results of operations.
We and our partners may be subject to stringent privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security, and changes in such laws, regulations, policies or how they are interpreted or changes in contractual obligations could adversely affect our business.
There are numerous U.S. federal and state data privacy and protection laws and regulations that apply to the collection, transmission, processing, storage and use of personally-identifying information, which among other things, impose certain requirements relating to the privacy, security and transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve in jurisdictions worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Failure to comply with any of these laws and regulations could result in enforcement action against us, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
If we are unable to properly protect the privacy and security of health-related information or other sensitive or confidential information in our possession, we could be found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face significant administrative, civil and criminal penalties. Enforcement activity can also result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents.
We may experience conflicts of interest with Viscient Biosciences, Inc. with respect to business opportunities and other matters.
Keith Murphy, our Executive Chairman, is the Chief Executive Officer, Chairman and principal stockholder of Viscient, a private company that he founded in 2017 that is focused on drug discovery and development utilizing 3D tissue technology and multi-omics (genomics, transcriptomics, metabolomics). Jeffrey N. Miner, our former Chief Scientific Officer, is a co-founder, the Chief Scientific Officer and a significant stockholder of Viscient. In addition, Adam Stern, Douglas Jay Cohen and David Gobel (through the Methuselah Foundation and the Methuselah Fund), members of our Board, have invested funds through a convertible promissory note in Viscient, but do not serve as an employee, officer or director of Viscient. Additional members of our Research and Development organization also work at Viscient, and we expect that additional employees or consultants of ours will also be employees of or consultants to Viscient. We use certain Viscient-owned facilities and equipment and allow Viscient to use certain of our facilities and equipment. During fiscal 2024, we provided services to Viscient, and we expect to continue to provide services to Viscient and enter into additional agreements with Viscient in the future.
In addition, we license, as well as cross-license, certain intellectual property to and from Viscient and expect to continue to do so in the future. In particular, pursuant to an Asset Purchase and Non-Exclusive Patent License Agreement with Viscient, dated November 6, 2019, as amended, we have provided a paid up, worldwide, irrevocable, perpetual, non-exclusive license to Viscient under certain of our patents and know-how to (a) make, have made, use, sell, offer to sell, import and otherwise exploit the inventions and subject matter covered by certain patents regarding certain bioprinter devices and bioprinting methods, engineered liver tissues, engineered renal tissues, engineered intestinal tissue and engineered tissue for in vitro research use, (b) to use and internally repair the bioprinters, and (c) to make additional bioprinters for internal use only in connection with drug discovery and development research, target identification and validation, compound screening, preclinical safety, absorption, distribution, metabolism, excretion and toxicology (ADMET) studies, and in vitro research to complement clinical development of a therapeutic compound. Although we have entered, and expect to enter, into agreements and arrangements that we believe appropriately govern the ownership of intellectual property created by joint employees or consultants of Viscient and/or using our or Viscient’s facilities or equipment, it is possible that we may disagree with Viscient as to the ownership of intellectual property created by shared employees or consultants, or using shared equipment or facilities.
On December 28, 2020, we entered into an intercompany agreement with Viscient and Organovo, Inc., our wholly-owned subsidiary (the “Intercompany Agreement”). Pursuant to the Intercompany Agreement, we agreed to provide Viscient certain services related to 3D bioprinting technology, which includes, but is not limited to, histology services, cell isolation, and proliferation of cells, and Viscient agreed to provide us certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, Viscient and we each agreed to share certain facilities and equipment and, subject to further agreement, to each
make certain employees available for specified projects to the other party at prices to be determined in good faith by the parties. Under the Intercompany Agreement, each party will retain its own prior intellectual property and will obtain new intellectual property rights within their respectively defined fields of use.
Due to the interrelated nature of Viscient with us, conflicts of interest may arise with respect to transactions involving business dealings between us and Viscient, potential acquisitions of businesses or products, the development and ownership of technologies and products, the sale of products, markets and other matters in which our best interests and the best interests of our stockholders may conflict with the best interests of the stockholders of Viscient. In addition, we and Viscient may disagree regarding the interpretation of certain terms of the arrangements we previously entered into with Viscient or may enter into in the future. We cannot guarantee that any conflict of interest will be resolved in our favor, or that, with respect to our transactions with Viscient, we will negotiate terms that are as favorable to us as if such transactions were with another third-party. In addition, executives that provide services to us and Viscient may not dedicate all of their time to us and we may therefore compete with Viscient for the time commitments of our executive officers from time to time.
Risks Related to Our Common Stock and Liquidity Risks
We could fail to maintain the listing of our common stock on the Nasdaq Capital Market, which could seriously harm the liquidity of our stock and our ability to raise capital or complete a strategic transaction.
The Nasdaq Stock Market LLC (“Nasdaq”) has established continued listing requirements, including a requirement to maintain a minimum closing bid price of at least $1 per share. If a company trades for 30 consecutive business days below such minimum closing bid price, it will receive a deficiency notice from Nasdaq. Assuming it is in compliance with the other continued listing requirements, Nasdaq would provide such company a period of 180 calendar days in which to regain compliance by maintaining a closing bid price at least $1 per share for a minimum of ten consecutive business days. There can be no assurance that we will continue to maintain compliance with the minimum bid price requirement or other listing requirements necessary for us to maintain the listing of our common stock on the Nasdaq Capital Market.
A delisting from the Nasdaq Capital Market and commencement of trading on the Over-the-Counter Bulletin Board would likely result in a reduction in some or all of the following, each of which could have a material adverse effect on stockholders:
•the liquidity of our common stock;
•the market price of our common stock (and the accompanying valuation of our Company);
•our ability to obtain financing or complete a strategic transaction;
•the number of institutional and other investors that will consider investing in shares of our common stock;
•the number of market markers or broker-dealers for our common stock; and
•the availability of information concerning the trading prices and volume of shares of our common stock.
There is no assurance that an active market in our common stock will continue at present levels or increase in the future.
Our common stock is currently traded on the Nasdaq Capital Market, but there is no assurance that an active market in our common stock will continue at present levels or increase in the future. As a result, an investor may find it difficult to dispose of our common stock on the timeline and at the volumes they desire. This factor limits the liquidity of our common stock and may have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
The price of our common stock may continue to be volatile, which could lead to losses by investors and costly securities litigation.
The trading price of our common stock is likely to be highly volatile and could fluctuate in response to factors such as:
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
•our ability to execute on our new strategic plan;
•reduced government funding for research and development activities;
•actual or anticipated variations in our operating results;
•adoption of new accounting standards affecting our industry;
•additions or departures of key personnel;
•sales of our common stock or other securities in the open market;
•degree of coverage of securities analysts and reports and recommendations issued by securities analysts regarding our business;
•volume fluctuations in the trading of our common stock; and
•other events or factors, many of which are beyond our control.
The stock market is subject to significant price and volume fluctuations. The trading price of our common stock is, and is likely to continue to be, volatile. For example, during the fiscal year ended March 31, 2024, our closing stock price ranged from $0.90 to $2.24 per share. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been initiated against such a company. Litigation initiated against us, whether or not successful, could result in substantial costs and diversion of our management’s attention and resources, which could harm our business and financial condition.
Investors may experience dilution of their ownership interests because of the future issuance of additional shares of our capital stock.
We are authorized to issue 200,000,000 shares of common stock and 25,000,000 shares of preferred stock. As of March 31, 2024, there were an aggregate of 10,077,726 shares of our common stock issued and outstanding and available for issuance on a fully diluted basis and no shares of preferred stock outstanding. That total for our common stock includes 2,462,899 shares of our common stock that may be issued upon the vesting of restricted stock units, the exercise of outstanding stock options, or is available for issuance under our equity incentive plans, and 45,000 shares of common stock that may be issued through our 2023 Employee Stock Purchase Plan (“ESPP”).
In the future, we may issue additional authorized but previously unissued equity securities to raise funds to support our continued operations and to implement our business plan. We may also issue additional shares of our capital stock or other securities that are convertible into or exercisable for our capital stock in connection with hiring or retaining employees, future acquisitions, or for other business purposes. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders may result. In addition, the future issuance of any such additional shares of capital stock may create downward pressure on the trading price of our common stock. There can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the price at which shares of our common stock is currently traded on the Nasdaq Capital Market. Moreover, depending on market conditions, we cannot be sure that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or to our stockholders.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our common stock to date and it is not anticipated that any dividends will be paid to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of our business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. As an investor, you should take note of the fact that a lack of a dividend can further affect the market value of our stock and could significantly affect the value of any investment.
Anti-takeover provisions in our organizational documents and Delaware law may discourage or prevent a change of control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
Our Certificate of Incorporation, as amended (“Certificate of Incorporation”), and Amended and Restated Bylaws, as amended (“Bylaws”) contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions:
•authorize the issuance of preferred stock which can be created and issued by our board of directors without prior stockholder approval, with rights senior to those of the common stock;
•provide for a classified board of directors, with each director serving a staggered three-year term;
•provide that each director may be removed by the stockholders only for cause;
•prohibit our stockholders from filling board vacancies, calling special stockholder meetings, or taking action by written consent; and
•require advance written notice of stockholder proposals and director nominations.
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These and other provisions in our
Certificate of Incorporation, Bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including delaying or impeding a merger, tender offer, or proxy contest involving our company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
Risks Related to Our Intellectual Property
If we are not able to adequately protect our proprietary rights, our business could be harmed.
Our success will depend to a significant extent on our ability to obtain patents and maintain adequate protection for our technologies, intellectual property and products and service offerings in the United States and other countries. If we do not protect our intellectual property adequately, competitors may be able to use our technologies and gain a competitive advantage.
To protect our products and technologies, we, and our collaborators and licensors, must prosecute and maintain existing patents, obtain new patents and pursue other intellectual property protection. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies. Moreover, the patent positions of many biotechnology and pharmaceutical companies are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, we cannot guarantee that:
•any patent applications filed by us will issue as patents;
•third parties will not challenge our proprietary rights, and if challenged that a court or an administrative board of a patent office will hold that our patents are valid and enforceable;
•third parties will not independently develop similar or alternative technologies or duplicate any of our technologies by inventing around our claims;
•any patents issued to us will cover our technology and products as ultimately developed;
•we will develop additional proprietary technologies that are patentable;
•the patents of others will not have an adverse effect on our business; or
•as issued patents expire, we will not lose some competitive advantage.
As previously disclosed, we have recommenced certain historical operations and are now focusing our future efforts on developing highly customized 3D human tissues as living, dynamic models for healthy and diseased human biology for drug development. Previously, we focused our efforts on developing our in vivo liver tissues to treat end-stage liver disease and a select group of life-threatening, orphan diseases, for which there were limited treatment options other than organ transplant. We also explored the development of other potential pipeline in vivo tissue constructs. As we focus our business on developing highly customized 3D human tissues, we may sell, discontinue, adjust or abandon certain patents and patent applications relating to our historical operations. There can be no assurance that we will be successful at such efforts or sell or otherwise monetize such assets on acceptable terms, if at all. There is also no guarantee that our remaining patents will be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies.
We may not be able to protect our intellectual property rights throughout the world.
Certain foreign jurisdictions have an absolute requirement of novelty that renders any public disclosure of an invention immediately fatal to patentability in such jurisdictions. Therefore, there is a risk that we may not be able to protect some of our intellectual property in the United States or abroad due to disclosures, which we may not be aware of, by our collaborators or licensors. Some foreign jurisdictions prohibit certain types of patent claims, such as “method-of-treatment/use-type” claims; thus, the scope of protection available to us in such jurisdictions is limited.
Moreover, filing, prosecuting and defending patents on all of our potential products and technologies throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not sought or obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our future products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to
enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Patents covering our products could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. We may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office (the “USPTO”), or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review (“IPR”), or interference proceedings or other similar proceedings challenging our patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we may have to participate in interference proceedings declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge our priority of invention or other features of patentability with respect to our patents and patent applications. Such challenges may result in loss of patent rights, in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology or products. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
For example, our U.S. Patent Nos. 9,855,369 and 9,149,952, which relate to our bioprinter technology, were the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”), one of our competitors. Likewise, U.S. Patent Nos. 9,149,952, 9,855,369, 8,931,880, 9,227,339, 9,315,043 and 10,967,560 (all assigned to Organovo, Inc.) and U.S. Patent Nos. 7,051,654, 8,241,905, 8,852,932 and 9,752,116 (assigned to Clemson University and the University of Missouri, respectively) were implicated in a declaratory judgment complaint filed against Organovo, Inc., our wholly owned subsidiary, by BICO Group AB and certain of its subsidiaries in the United States District Court for the District of Delaware. All of these matters were eventually settled in February 2022.
Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition or results of operations. We may become involved in lawsuits to protect or enforce our inventions, patents or other intellectual property or the patents of our licensors, which could be expensive and time consuming.
In addition, if we initiate legal proceedings against a third party to enforce a patent covering our products, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may also raise claims challenging the validity or enforceability of our patents before administrative bodies in the United States or abroad, even outside the context of litigation, including through re-examination, post-grant review, IPR, interference proceedings, derivation proceedings and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of or amendment to our patents in such a way that they no longer cover our products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our products. Such a loss of patent protection would have a material adverse effect on our business, financial condition, and results of operations.
We may be involved in lawsuits or other proceedings to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our collaborators or licensors or our licensors may breach or otherwise prematurely terminate the provisions of our license agreements with them. To counter infringement or unauthorized use, we may be required to file infringement claims or lawsuits, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our collaborators or licensors is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our other patent applications at risk of not issuing. Additionally, our licensors
may continue to retain certain rights to use technologies licensed by us for research purposes. Patent disputes can take years to resolve, can be very costly and can result in loss of rights, injunctions or substantial penalties. Moreover, patent disputes and related proceedings can distract management’s attention and interfere with running our business.
Furthermore, because of the potential for substantial discovery in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments which could harm our business.
As more companies file patents relating to bioprinters and bioprinted tissues, it is possible that patent claims relating to bioprinters or bioprinted human tissue may be asserted against us. In addition, the drug candidates we pursue may also be pursued by other companies, and it is possible that patent claims relating to such drug candidates may also be asserted against us. Any patent claims asserted against us could harm our business. Moreover, we may face claims from non-practicing entities, which have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation and diversion of resources, cause product shipment or delays or require us to enter into royalty or license agreements. These licenses may not be available on acceptable terms, or at all. Even if we are successful in defending such claims, infringement and other intellectual property litigation can be expensive and time-consuming to litigate and divert management’s attention from our core business. Any of these events could harm our business significantly.
Our current and future research, development and commercialization activities also must satisfy the obligations under our license agreements. Any disputes arising under our license agreements could be costly and distract our management from the conduct of our business. Moreover, premature termination of a license agreement could have an adverse impact on our business.
In addition to infringement claims against us, if third parties have prepared and filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the United States Patent and Trademark Office (“PTO”) to determine the priority of invention and opposition proceedings outside of the United States. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party.
Third parties may also attempt to initiate reexamination, post grant review or inter partes review of our patents or those of our collaborators or licensors in the PTO. We may also become involved in similar opposition proceedings in the European Patent Office or similar offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology.
Changes in U.S. patent law or the patent law of other countries or jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is costly, time-consuming and inherently uncertain. For example, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act included a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and that may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application is typically entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO, and may become involved in post-grant proceedings, including opposition, derivation, reexamination, inter partes review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope or enforceability of, or invalidate, our patent rights, which could adversely affect our competitive position.
In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we might obtain in the future.
Similarly, changes in patent law and regulations in other countries or jurisdictions or changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our ability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future. For example, the complexity and uncertainty of European patent laws have also increased in recent years. In Europe, in June 2023, a new unitary patent system was introduced, which will significantly impact European patents, including those granted before the introduction of the system. Under the unitary patent system, after a European patent is granted, the patent proprietor can request unitary effect, thereby getting a European patent with
unitary Effect, or a Unitary Patent. Each Unitary Patent is subject to the jurisdiction of the Unitary Patent Court, or the UPC. As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty of any litigation. Patents granted before the implementation of the UPC will have the option of opting out of the jurisdiction of the UPC and remaining as national patents in the UPC countries. Patents that remain under the jurisdiction of the UPC may be potentially vulnerable to a single UPC-based revocation challenge that, if successful, could invalidate the patent in all countries who are signatories to the UPC. We cannot predict with certainty the long-term effects of the new unitary patent system.
We depend on license agreements with University of Missouri, Clemson University and the Salk Institute for Biological Studies for rights to use certain patents, pending applications, and know how. Failure to comply with or maintain obligations under these agreements and any related or other termination of these agreements could materially harm our business and prevent us from developing or commercializing new product candidates.
We are party to license agreements with University of Missouri, Clemson University and the Salk Institute for Biological Studies under which we were granted exclusive rights to patents and patent applications that are important to our business and to our ability to develop and commercialize our 3D tissue products fabricated using our NovoGen Bioprinters and our FXR314 agonist in gastrointestinal disease. Our rights to use these patents and patent applications and employ the inventions claimed in these licensed patents are subject to the continuation of and our compliance with the terms of our license agreements. If we were to breach the terms of these license agreements and the agreements were terminated as a result, our ability to continue to develop and commercialize our NovoGen Bioprinters, 3D tissue products and the FXR314 agonist and to operate our business could be adversely impacted.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary and licensed technology and processes, we rely in part on confidentiality agreements with our corporate partners, employees, consultants, manufacturers, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of our confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We employ or engage individuals who were previously employed at other biopharmaceutical companies. Although we have no knowledge of any such claims against us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and even if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees. To date, none of our employees have been subject to such claims.
General Risk Factors
Compliance with the reporting requirements of federal securities laws can be expensive.
We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including the compliance obligations of the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley Act”). The costs of complying with the reporting requirements of the federal securities laws, including preparing and filing annual and quarterly reports and other information with the Securities and Exchange Commission (the “SEC”) and furnishing audited reports to stockholders, can be substantial.
If we fail to comply with the rules of Section 404 of the Sarbanes-Oxley Act related to accounting controls and procedures, or, if we discover material weaknesses and deficiencies in our internal control and accounting procedures, we may be subject to sanctions by regulatory authorities and our stock price could decline.
Section 404 of the Sarbanes-Oxley Act (“Section 404”) requires that we evaluate and determine the effectiveness of our internal control over financial reporting. We believe our system and process evaluation and testing comply with the management certification requirements of Section 404. We cannot be certain, however, that we will be able to satisfy the requirements in Section 404 in all future periods. If we are not able to continue to meet the requirements of Section 404 in a timely manner or with adequate compliance, we may be subject to sanctions or investigation by regulatory authorities, such as the SEC or Nasdaq. Any such action could adversely affect our financial results or investors’ confidence in us and could cause our stock price to fall. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we identify deficiencies in our internal controls that are deemed to be material weaknesses, we may be required to incur significant additional financial and management resources to achieve compliance.
None.
Item 1C. Cybersecurity
We believe cybersecurity is critical to advancing our technological advancements. As a clinical stage biotechnology company, we face a multitude of cybersecurity threats that include attacks common in most industries, such as ransomware and denial-of service. Our customers, suppliers, subcontractors, and business partners face similar cybersecurity threats, and a cybersecurity incident impacting us or any of these entities could materially adversely affect our operations, performance, and results of operations. These cybersecurity threats and related risks make it imperative that we expend resources on cybersecurity.
Assessing, identifying, and managing cybersecurity related risks are factored into our overall business approach. We have implemented a governance structure and processes to assess, identify, manage, and report cybersecurity risks and have developed our own practices and framework, which we believe enhance our ability to identify and manage cybersecurity risks. Our Audit Committee oversees management’s processes for identifying and mitigating risks, including cybersecurity risks, to help align our risk exposure with our strategic objectives. The IT Risk Committee and our cybersecurity consultant, which has extensive information technology and program management experience, regularly brief the Audit Committee on our cybersecurity and information security posture and the Audit Committee is apprised of any cybersecurity incidents deemed to have a moderate or higher business impact, even if immaterial to us. The Audit Committee retains oversight of cybersecurity because of its importance. In the event of any incident, we intend to follow our detailed incident response playbook, which outlines the steps to be followed from incident detection to mitigation, recovery, and notification, including notifying functional areas (e.g., legal), as well as senior leadership and the Audit Committee, as appropriate.
As a clinical stage biotechnology company, we must also comply with extensive regulations, including requirements imposed by the U.S. Food and Drug Administration related to adequately safeguarding patient information. We work with our cybersecurity consultant on assessing cybersecurity risk and on policies and practices aimed at mitigating these risks. We believe we are positioned to meet SEC disclosure requirements. Third parties also play a role in our cybersecurity. We engage third-party services to conduct evaluations of our security controls, whether through penetration testing, independent audits, or consulting on best practices to address new challenges.
We rely heavily on our supply chain to deliver our products and services, and a cybersecurity incident at a supplier, subcontractor or business partner could materially adversely impact us. We will require that our subcontractors report cybersecurity incidents to us so that we can assess the impact of the incident on us.
Notwithstanding the extensive approach we take to cybersecurity, we may not be successful in preventing or mitigating a cybersecurity incident that could have a material adverse effect on us. See “Risk Factors” for a discussion of cybersecurity risks, including, without limitation, the risk factor under the heading “We may be subject to security breaches or other cybersecurity incidents that could compromise our information and expose us to liability”. Except as set forth therein, risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, have not materially affected and are not reasonably likely to materially affect our company, including our business strategy, results of operations, or financial condition.
Item 2. Properties.
In November 2020, we entered into a sixty-two month lease agreement for our long term permanent premises, consisting of approximately 8,051 square feet of lab and office space. In November 2021, we amended the permanent lease agreement to add an additional 2,892 square of office space in the same building. In December 2021, we took occupancy of the aforementioned lab and office space, located at 11555 Sorrento Valley Road, San Diego, CA 92121. See “Note 8. Leases” of the Notes to the Consolidated Financial Statements contained within this Annual Report for a further discussion of properties.
Item 3. Legal Proceedings.
In addition to commitments and obligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business.
The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its financial statements. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not meaningful indicators of its potential liability.
We are not involved in any material legal proceedings or legal matters at this time. See “Note 9. Commitments and Contingencies” of the Notes to the Consolidated Financial Statements contained within this Annual Report for a further discussion of potential commitments and contingencies related to legal proceedings.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information for Common Stock
Our common stock is traded on the Nasdaq Capital Market under the symbol “ONVO.”
Holders of Record
As of May 25, 2024, we had 14,371,826 outstanding shares of common stock and approximately 82 holders of record of our common stock. The number of beneficial owners is substantially greater than the number of record holders because a large portion of our common stock is held of record through brokerage firms in “street name.”
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain all future earnings, if any, for use in our business and do not anticipate paying any cash dividends on our common stock in the foreseeable future.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Performance Graph
This performance graph is furnished and shall not be deemed “filed” with the SEC or subject to Section 18 of the Exchange Act, nor shall it be deemed incorporated by reference in any of our filings under the Securities Act of 1933, as amended.
The graph set forth below compares the cumulative total stockholder return data on our common stock with the cumulative return data of (i) the Nasdaq Stock Market Composite Index, and (ii) the Nasdaq Biotechnology Index over the five-year period ending March 31, 2024. This graph assumes the investment of $100 on March 31, 2019 in our common stock and each of the comparative indices and assumes the reinvestment of dividends. No cash dividends have been declared or paid on our common stock.
The comparisons in the graph and related information is not intended to forecast or be indicative of possible future performance of our common stock, and we do not make or endorse any predictions as to future stockholder returns.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Organovo Holdings, Inc.,
the Nasdaq Composite Index, and the Nasdaq Biotechnology Index

* $100 invested on March 31, 2019 in stock or index, including reinvestment of dividends.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following management’s discussion and analysis of financial condition and results of operations should be read in conjunction with our historical consolidated financial statements and the related notes. This management’s discussion and analysis contains forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. These forward-looking statements are subject to risks and uncertainties that could cause our actual results or events to differ materially from those expressed or implied by the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below and those discussed in section Item 1A. “Risk Factors” in this Annual Report. Except as required by applicable law we do not undertake any obligation to update our forward-looking statements to reflect events or circumstances occurring after the date of this Annual Report.
Overview
We are a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in our established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC.
Our current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). We plan to start a Phase 2a clinical trial in UC in the calendar year 2024. We released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis ("MASH") in April 2024 that are supportive of ongoing development, and we believe FXR314 has a commercial opportunity in MASH, most likely in combination therapy. We are exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and our IBD disease models.
Our second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. We use our proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. We believe these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, we are initially focusing on the intestine and have ongoing 3D tissue development efforts in human tissue models of UC and CD. We use these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. We intend to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Our current understanding of intestinal tissue models and IBD disease models leads us to believe that we can create models that provide greater insight into the biology of these diseases than are generally currently available. We are creating high fidelity disease models, leveraging our prior work including the work found in our peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Our advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures.
Using these disease models, we intend to identify and validate novel therapeutic targets. After finding therapeutic drug targets, we intend to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials.
We expect to broaden our work into additional therapeutic areas over time and are currently exploring specific tissues for development. In our work to identify the areas of interest, we evaluate areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, we are building upon both our external and in house scientific expertise, which will be essential to our drug development effort.
Recent Developments
Mosaic Cell Sciences Division
In February 2024, we formed our Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain of the primary human cells we utilize in our research and development efforts. We believe Mosaic can help us optimize our supply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials we use are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. We intend for Mosaic to provide us
with qualified human cells for use in our clinical research and development programs. In addition to supplying us with primary human cells, we intend for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which we expect to offset costs and over time become a profit center that offsets overall R&D spending by Organovo.
Best Efforts Public Offering
On May 8, 2024, we priced a best efforts public offering (the “Offering”) of: (i) 1,562,500 shares of our common stock and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock at a combined public offering price of $0.80 per share and accompanying Common Warrant to purchase one share of common stock and (ii) pre-funded warrants (“Pre-Funded Warrants”) to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock at a combined public offering price of $0.799 per Pre-Funded Warrant and accompanying Common Warrant to purchase one share of common stock. In connection with the Offering, we entered into Securities Purchase Agreements with the purchasers of the securities in the Offering on May 8, 2024.
The per share exercise price for the Pre-Funded Warrants is $0.001, subject to adjustment as provided therein. The Pre-Funded Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire when exercised in full. The holders may exercise the Pre-Funded Warrants by means of a “cashless exercise.”
The per share exercise price for the Common Warrants is $0.80, subject to adjustment as provided therein. The Common Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire on the date that is five years following the original issuance date. If a registration statement covering the issuance of the shares of common stock issuable upon exercise of the Common Warrants is not available for the issuance, then the holders may exercise the Common Warrants by means of a “cashless exercise.”
In connection with the Offering, we paid JonesTrading Institutional Services LLC, which acted as the placement agent in connection with the Offering, a cash fee of 5.0% of the aggregate gross proceeds raised in the Offering.
The closing of the Offering occurred on May 13, 2024. We received net proceeds of approximately $4.7 million from the Offering, after deducting the estimated offering expenses payable by us, including the Placement Agent fees.
Critical Accounting Policies, Estimates, and Judgments
Our financial statements are prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). Any reference in this annual report to applicable guidance is meant to refer to the authoritative accounting principles generally accepted in the United States as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We continually evaluate our estimates and judgments used in preparing our financial statements and related disclosures, none of which are considered critical. All estimates affect reported amounts of assets, liabilities, revenues and expenses, as well as disclosures of contingent assets and liabilities. These estimates and judgments are also based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known.
Our significant accounting policies are set forth in “Note 1. Description of Business and Summary of Significant Accounting Policies” in the Notes to Consolidated Financial Statements contained within this Annual Report. Of those policies, we believe that the policies discussed below may involve a higher degree of judgment and may be more critical to an accurate reflection of our financial condition and results of operations. Accounting policies regarding stock-based compensation are considered critical, as they require significant assumptions. If there is a difference between the assumptions used in determining our stock-based compensation expense and the actual factors that become known over time, specifically with respect to anticipated forfeitures, we may change the input factors used in determining stock-based compensation costs for future grants. These changes, if any, may materially impact our results of operations in the period such changes are made.
Stock-based compensation
For purposes of calculating stock-based compensation, we estimate the fair value of stock options and shares acquirable under our 2022 Equity Incentive Plan (“2022 Plan”), Amended and Restated 2012 Equity Incentive Plan (the “2012 Plan”), our 2016 Employee Stock Purchase Plan (the “2016 ESPP”), our 2023 Employee Stock Purchase Plan (the “2023 ESPP”), or our 2021 Inducement Equity Plan (the “Inducement Plan”) using a Black-Scholes option-pricing model. The determination of the fair value of share-based payment awards utilizing the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, expected life, risk-free interest rate and expected dividends. Expected volatility is based on the Company-specific historical volatility
rate. For certain options granted with vesting criteria contingent on market conditions, we engage with valuation specialists to calculate fair value and requisite service periods using Monte Carlo simulations. For certain options granted with vesting criteria contingent on pre-defined Company performance criteria, we periodically assess and adjust the expense based on the probability of achievement of such performance criteria. For shares acquirable under our 2016 ESPP and 2023 ESPP, we use our Company-specific volatility rate. The expected life of the stock options is based on historical and other economic data trended into the future. The risk-free interest rate assumption is based on observed interest rates appropriate for the expected terms of our stock options. The dividend yield assumption is based on our history and expectation of no dividend payouts. If factors change and we employ different assumptions, our stock-based compensation expense may differ significantly from what we have recorded in the past.
For purposes of calculating stock-based compensation, we estimate the fair value of restricted stock units (“RSUs”) with pre-defined performance criteria, based on the closing stock price on the date of grant. No exercise price or other monetary payment is required for receipt of the shares issued in settlement of the respective award; instead, consideration is furnished in the form of the participant’s service to us.
If there is a difference between the assumptions used in determining our stock-based compensation expense and the actual factors that become known over time, we may change the input factors used in determining stock-based compensation costs for future grants. These changes, if any, may materially impact our results of operations in the period such changes are made.
Revenue
We assess whether our license agreements are considered a contract with a customer under ASC Topic 606, Revenue from Contracts with Customers (“Topic 606”) or an arrangement with a collaborator subject to guidance under ASC Topic 808, Collaborative Arrangements (“Topic 808”). These agreements can include one or more of the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales. At contract inception, we assess the goods or services agreed upon within each contract and assess whether each good or service is distinct and determine those that are performance obligations. We then recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
For the year ended March 31, 2024, the performance obligations assessed were sales-based royalties on a quarterly basis. We evaluate the performance obligation to determine if it can be satisfied at a point in time or over time. For agreements that include sales-based royalties, we estimate and recognize revenue in the period the underlying sales occur. Key factors considered in the estimate include sales of products that include the underlying licensed intellectual property (“IP”) and the location of customers related to the jurisdictions of the licensed IP. In addition, variable consideration must be evaluated to determine if it is constrained and, therefore, excluded from the transaction price. Differences in the allocation of the transaction price between delivered and undelivered performance obligations can impact the timing of revenue recognition but do not change the total revenue recognized under any agreement.
Results of Operations
Comparison of the Years Ended March 31, 2024 and 2023
The following table summarizes our results of operations for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31, |
|
|
Increase (decrease) |
|
|
2024 |
|
|
2023 |
|
|
$ |
|
|
% |
|
Revenues |
$ |
109 |
|
|
$ |
370 |
|
|
$ |
(261 |
) |
|
|
(71 |
%) |
Research and development |
$ |
5,498 |
|
|
$ |
8,885 |
|
|
$ |
(3,387 |
) |
|
|
(38 |
%) |
Selling, general and administrative |
$ |
9,697 |
|
|
$ |
9,216 |
|
|
$ |
481 |
|
|
|
5 |
% |
Other income |
$ |
417 |
|
|
$ |
474 |
|
|
$ |
(57 |
) |
|
|
(12 |
%) |
Revenues
We had $0.1 million of royalty revenue for the year ended March 31, 2024, compared to $0.4 million of royalty revenue for the year ended March 31, 2023. Royalty revenue for each of the years ended March 31, 2024 and 2023, was related to the sales-based royalty revenue earned from the aforementioned licensing of IP. The decrease in royalty revenue year over year relates to a decrease in sales of royalty-bearing products by the licensee.
Research and Development Expenses
The following table summarizes our research and development expenses for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31, |
|
|
Increase (decrease) |
|
|
2024 |
|
|
2023 |
|
|
$ |
|
|
% |
|
Research and development |
$ |
5,133 |
|
|
$ |
8,247 |
|
|
$ |
(3,114 |
) |
|
|
(38 |
%) |
Non-cash stock-based compensation |
|
138 |
|
|
|
473 |
|
|
|
(335 |
) |
|
|
(71 |
%) |
Depreciation and amortization |
|
227 |
|
|
|
165 |
|
|
|
62 |
|
|
|
38 |
% |
Total research and development expenses |
$ |
5,498 |
|
|
$ |
8,885 |
|
|
$ |
(3,387 |
) |
|
|
(38 |
%) |
Total research and development expenses decreased by $3.4 million, or 38%, from approximately $8.9 million for the year ended March 31, 2023 to approximately $5.5 million for the year ended March 31, 2024. Our full-time research and development staff increased from an average of fifteen employees for the year ended March 31, 2023 to an average of sixteen employees for the year ended March 31, 2024. The decrease in total research and development activities consisted of a $4.1 million decrease in lab and research expenses, which was offset by a $0.4 million increase in consulting costs, a $0.2 million increase in personnel related costs, and a $0.1 million increase in facilities costs and depreciation. Of the $4.1 million decrease in lab and research expenses, $4.0 million related to acquired in-process research and development (“IPR&D”) of Metacrine's FXR program, related research data, and IP in the year ended March 31, 2023.
Selling, General and Administrative Expenses
The following table summarizes our selling, general and administrative expenses for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31, |
|
|
Increase (decrease) |
|
|
2024 |
|
|
2023 |
|
|
$ |
|
|
% |
|
Selling, general and administrative |
$ |
8,274 |
|
|
$ |
7,184 |
|
|
$ |
1,090 |
|
|
|
15 |
% |
Non-cash stock-based compensation |
|
1,370 |
|
|
|
1,904 |
|
|
|
(534 |
) |
|
|
(28 |
%) |
Depreciation and amortization |
|
53 |
|
|
|
128 |
|
|
|
(75 |
) |
|
|
(59 |
%) |
Total selling, general and administrative
expenses |
$ |
9,697 |
|
|
$ |
9,216 |
|
|
$ |
481 |
|
|
|
5 |
% |
Total selling, general and administrative expenses increased approximately $0.5 million, or 5%, from $9.2 million for the year ended March 31, 2023 to approximately $9.7 million for the year ended March 31, 2024. The increase year over year relates to increases in legal and other corporate expenses, including a $0.7 million increase in investor relations expenses and a $0.2 million increase in legal costs, offset by a $0.4 million decrease in personnel related costs. Our full-time selling, general, and administrative employees decreased from an average of five employees for the year ended March 31, 2023 to an average of four employees for the year ended March 31, 2024.
Other Income (Expense)
Other income was $0.4 million and $0.5 million for the years ended March 31, 2024 and 2023, respectively. Other income primarily consisted of interest income in both years.
Financial Condition, Liquidity and Capital Resources
Going forward, we intend to focus on clinical drug development of FXR314, the lead compound in our established FXR program. Our current clinical focus is in advancing FXR314 in IBD, including UC and CD. We plan to start a Phase 2 clinical trial in UC in the calendar year 2024. We released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis (“MASH”) in April 2024 that is supportive of ongoing development, and we believe FXR314 has a commercial opportunity in MASH, most likely in combination therapy. Additionally, we plan to leverage our proprietary technology platform to develop therapeutic drugs, focusing on IBD, including CD and UC, with a goal of broadening our work into additional therapeutic areas over time.
The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of March 31, 2024, we had cash and cash equivalents of approximately $2.9 million and an accumulated deficit of $339.7 million. As of March 31, 2023, we had cash and cash equivalents of $15.3 million and an accumulated deficit of $325.0 million. We had negative cash flows from operations of $14.7 million and $12.4 million for the years ended March 31, 2024 and 2023, respectively.
As of March 31, 2024, we had total current assets of approximately $3.9 million and current liabilities of approximately $1.9 million, resulting in working capital of $2.0 million. At March 31, 2023, we had total current assets of approximately $17.0 million and current liabilities of approximately $3.7 million, resulting in working capital of $13.3 million.
The following table sets forth a summary of the primary sources and uses of cash for the years ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended March 31, |
|
|
2024 |
|
|
2023 |
|
Net cash (used in) provided by: |
|
|
|
|
|
Operating activities |
$ |
(14,653 |
) |
|
$ |
(12,408 |
) |
Investing activities |
|
816 |
|
|
|
(966 |
) |
Financing activities |
|
1,437 |
|
|
|
— |
|
Net decrease in cash, cash equivalents, and restricted cash |
$ |
(12,400 |
) |
|
$ |
(13,374 |
) |
Operating activities
Net cash used in operating activities was approximately $14.7 million and $12.4 million for the years ended March 31, 2024 and 2023, respectively. The $2.3 million increase in operating cash usage, for the year ended March 31, 2024, was attributable primarily to the $2.0 million cash payment in fiscal 2024 for acquired IPR&D, in addition to an increase in our research and development activities.
Investing activities
Net cash provided by investing activities was $0.8 million for the year ended March 31, 2024. Net cash provided by investing activities for the year ended March 31, 2024 consisted of the liquidation of equity securities of $0.7 million and $0.1 million of investment income. Net cash used in investing activities for the year ended March 31, 2023 consisted of $0.7 million of purchases of equity securities, net of sales, and $0.4 million of fixed asset purchases, which was slightly offset by $0.1 million of investment income.
Financing activities
Net cash provided by financing activities was $1.4 million for the year ended March 31, 2024. Financing activities consisted of the sale of common stock through at-the-market (“ATM”) share offerings. There were no financing activities for the year ended March 31, 2023. Refer to “Operations funding requirements” below for further information regarding financing activities.
Operations funding requirements
Through March 31, 2024, we have financed our operations primarily through the sale of common stock through public and ATM offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research-based services, grants, and collaborative research agreements, and from the sale of convertible notes.
Our ongoing cash requirements include research and development expenses, compensation for personnel, consulting fees, legal and accounting support, insurance premiums, facilities, maintenance of our intellectual property portfolio, license and collaboration agreements, listing on the Nasdaq Capital Market, and other miscellaneous fees to support our operations. We expect our total operating expense for the fiscal year ending March 31, 2025 to be approximately $23.5 million. Based on our current operating plan and available cash resources, we will need substantial additional funding to support future operating activities. We have concluded that the prevailing conditions and ongoing liquidity risks faced by us raise substantial doubt about our ability to continue as a going concern for at least one year following the date these financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern.
We previously had an effective shelf registration statement on Form S-3 (File No. 333-252224), declared effective by the SEC on January 29, 2021 (the “2021 Shelf”), which registered $150.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing, that expired on January 29, 2024. On January 26, 2024, we filed a new shelf registration statement on Form S-3 (File No. 333-276722) to register $150.0 million of common stock, preferred stock, debt securities, warrants and units, or
any combination of the foregoing (the “2024 Shelf”). The 2024 Shelf was declared effective by the SEC on February 8, 2024 and replaced the 2021 Shelf at that time.
On March 16, 2018, we entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, we filed a prospectus supplement to the 2021 Shelf (the “2021 ATM Prospectus Supplement”), pursuant to which we could offer and sell, from time to time through the Agents, shares of our common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold were issued pursuant to our 2021 Shelf until it was replaced by the 2024 Shelf.
On January 26, 2024, we filed a prospectus to the 2024 Shelf (the “2024 ATM Prospectus”), pursuant to which we may offer and sell, from time to time, through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $2,605,728. Any shares offered and sold in these ATM transactions are issued pursuant to the 2024 Shelf.
During the year ended March 31, 2024, we sold 1,172,342 shares of common stock in ATM offerings, of which 1,135,940 shares were sold pursuant to the 2021 Shelf, and 36,402 shares were sold pursuant to the 2024 Shelf. As of March 31, 2024, we have sold an aggregate of 2,753,204 shares of common stock in ATM offerings under the 2021 ATM Prospectus Supplement and 2024 ATM Prospectus, for gross proceeds of approximately $23.2 million. As of March 31, 2024, there was approximately $100.0 million available in future offerings under the 2024 Shelf, and approximately $2.6 million available for future offerings through our ATM program under the 2024 ATM Prospectus.
In May 2024, we closed a best efforts public offering conducted off a Registration Statement on Form S-1 (File No. 333-278668) and issued and sold (i) 1,562,500 shares of our common stock, and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock and (ii) pre-funded warrants (“Pre-Funded Warrants”) to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock for estimated net proceeds of $4.7 million.
As of May 29, 2024, we had cash and cash equivalents of approximately $7.0 million.
Having insufficient funds may require us to relinquish rights to our technology on less favorable terms than we would otherwise choose. Failure to obtain adequate financing could adversely affect our operations. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business. We cannot be sure that additional financing will be available if and when needed, or that, if available, we can obtain financing on terms favorable to our stockholders. Any failure to obtain financing when required will have a material adverse effect on our business, operating results, and financial condition.
Effect of Inflation and Changes in Prices
Management does not believe that inflation and changes in price will have a material effect on our operations.
Recent Accounting Pronouncements
For information regarding recently adopted and issued accounting pronouncements, see “Note 14. Recent Accounting Pronouncements” in the Notes to the Consolidated Financial Statements contained in this Annual Report.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We invest our excess cash in short term, high quality interest bearing securities including US government and US government agency securities and high-grade corporate commercial paper. The primary objective of our investment activities is to preserve our capital for the purpose of funding our operations. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash, cash equivalents, and short-term investments in a variety of securities, including money market funds. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because the majority of our investments are comprised of cash and cash equivalents. We currently do not hedge interest rate exposure. Due to the nature of our short-term investments, we believe that we are not subject to any material market risk exposure. We have limited foreign currency risk exposure as our business operates primarily in U.S. dollars. We do not have significant foreign currency nor any other derivative financial instruments.
Item 8. Consolidated Financial Statements.
Organovo Holdings, Inc.
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of:
Organovo Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Organovo Holdings, Inc. (“Company”) as of March 31, 2023, and the related consolidated statements of operations and other comprehensive loss, stockholders’ equity, and cash flows for the year ended March 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2023, and the results of its operations and its cash flows for the year ended March 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring losses and negative cash flows from operations and is dependent on additional financing to fund operations. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there were no critical audit matters.
/s/ Mayer Hoffman McCann P.C.
We served as the Company's auditor from 2011 to 2023.
San Diego, California
July 13, 2023
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of:
Organovo Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Organovo Holdings, Inc. (the “Company”) as of March 31, 2024, and the related statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the year ended March 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2024, and the results of its operations and its cash flows for the year ended March 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring losses and negative cash flows from operations and is dependent on additional financing to fund operations. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Rosenberg Rich Baker Berman P.A.
We have served as the Company’s auditor since 2023.
Somerset, New Jersey
May 31, 2024
ORGANOVO HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands except for share and per share data)
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
Assets |
|
|
|
|
|
|
Current Assets |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
2,901 |
|
|
$ |
15,301 |
|
Accounts receivable |
|
|
33 |
|
|
|
152 |
|
Inventory |
|
|
297 |
|
|
|
— |
|
Investment in equity securities |
|
|
— |
|
|
|
706 |
|
Prepaid expenses and other current assets |
|
|
705 |
|
|
|
889 |
|
Total current assets |
|
|
3,936 |
|
|
|
17,048 |
|
Fixed assets, net |
|
|
669 |
|
|
|
902 |
|
Restricted cash |
|
|
143 |
|
|
|
143 |
|
Operating lease right-of-use assets |
|
|
1,299 |
|
|
|
1,705 |
|
Prepaid expenses and other assets, net |
|
|
302 |
|
|
|
515 |
|
Total assets |
|
$ |
6,349 |
|
|
$ |
20,313 |
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
Accounts payable |
|
$ |
627 |
|
|
$ |
331 |
|
Accrued expenses |
|
|
727 |
|
|
|
2,848 |
|
Operating lease liability, current portion |
|
|
506 |
|
|
|
492 |
|
Total current liabilities |
|
|
1,860 |
|
|
|
3,671 |
|
Operating lease liability, net of current portion |
|
|
888 |
|
|
|
1,313 |
|
Total liabilities |
|
|
2,748 |
|
|
|
4,984 |
|
Commitments and Contingencies (Notes 8 and 9) |
|
|
|
|
|
|
Stockholders' Equity |
|
|
|
|
|
|
Common stock, $0.001 par value; 200,000,000 shares authorized,
10,077,726 and 8,716,906 shares issued and outstanding at
March 31, 2024 and 2023, respectively |
|
|
10 |
|
|
|
9 |
|
Additional paid-in capital |
|
|
343,261 |
|
|
|
340,317 |
|
Accumulated deficit |
|
|
(339,669 |
) |
|
|
(324,998 |
) |
Accumulated other comprehensive income |
|
|
— |
|
|
|
2 |
|
Treasury stock, 46 shares at cost |
|
|
(1 |
) |
|
|
(1 |
) |
Total stockholders' equity |
|
|
3,601 |
|
|
|
15,329 |
|
Total Liabilities and Stockholders' Equity |
|
$ |
6,349 |
|
|
$ |
20,313 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE LOSS
(in thousands except for share and per share data)
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Revenues |
|
|
|
|
|
|
Royalty revenue |
|
$ |
109 |
|
|
$ |
370 |
|
Total Revenues |
|
|
109 |
|
|
|
370 |
|
Research and development expenses |
|
|
5,498 |
|
|
|
8,885 |
|
Selling, general, and administrative expenses |
|
|
9,697 |
|
|
|
9,216 |
|
Total costs and expenses |
|
|
15,195 |
|
|
|
18,101 |
|
Loss from Operations |
|
|
(15,086 |
) |
|
|
(17,731 |
) |
Other Income (Expense) |
|
|
|
|
|
|
Loss on fixed asset disposals |
|
|
— |
|
|
|
(9 |
) |
Gain on investment in equity securities |
|
|
12 |
|
|
|
29 |
|
Interest income |
|
|
405 |
|
|
|
454 |
|
Total Other Income |
|
|
417 |
|
|
|
474 |
|
Income Tax Expense |
|
|
(2 |
) |
|
|
(2 |
) |
Net Loss |
|
$ |
(14,671 |
) |
|
$ |
(17,259 |
) |
Other comprehensive income: |
|
|
|
|
|
|
Unrealized gain (loss) on available-for-sale debt securities |
|
|
(2 |
) |
|
|
2 |
|
Comprehensive loss |
|
$ |
(14,673 |
) |
|
$ |
(17,257 |
) |
Net loss per common share—basic and diluted |
|
$ |
(1.60 |
) |
|
$ |
(1.98 |
) |
Weighted average shares used in computing net loss per common share—basic
and diluted |
|
|
9,144,922 |
|
|
|
8,713,032 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
|
|
|
Treasury Stock |
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
|
Amount |
|
|
Additional Paid-in Capital |
|
|
Shares |
|
Amount |
|
|
Accumulated Deficit |
|
|
Accumulated Other Comprehensive Income |
|
Total |
|
Balance at March 31, 2022 |
|
|
8,711 |
|
|
$ |
9 |
|
|
$ |
337,940 |
|
|
|
— |
|
$ |
(1 |
) |
|
$ |
(307,739 |
) |
|
|
— |
|
$ |
30,209 |
|
Issuance of common stock under employee and
director stock option, RSU and purchase plans |
|
|
6 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
2,377 |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
2,377 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
(17,259 |
) |
|
|
— |
|
|
(17,259 |
) |
Unrealized gain on available-for-sale debt securities, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
2 |
|
|
2 |
|
Balance at March 31, 2023 |
|
|
8,717 |
|
|
$ |
9 |
|
|
$ |
340,317 |
|
|
|
— |
|
$ |
(1 |
) |
|
$ |
(324,998 |
) |
|
$ |
2 |
|
$ |
15,329 |
|
Issuance of common stock under employee and
director stock option, RSU and purchase plans |
|
|
122 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
Stock-based compensation expense |
|
|
66 |
|
|
|
— |
|
|
|
1,508 |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
1,508 |
|
Issuance of common stock from public offering,
net |
|
|
1,173 |
|
|
|
1 |
|
|
|
1,436 |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
1,437 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
(14,671 |
) |
|
|
— |
|
|
(14,671 |
) |
Unrealized loss on available-for-sale debt securities, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
(2 |
) |
Balance at March 31, 2024 |
|
|
10,078 |
|
|
$ |
10 |
|
|
$ |
343,261 |
|
|
|
— |
|
$ |
(1 |
) |
|
$ |
(339,669 |
) |
|
$ |
— |
|
$ |
3,601 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
Cash Flows From Operating Activities |
|
|
|
|
|
|
Net loss |
|
$ |
(14,671 |
) |
|
$ |
(17,259 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Gain on investment in equity securities |
|
|
(12 |
) |
|
|
(29 |
) |
Loss on disposal of fixed assets |
|
|
— |
|
|
|
9 |
|
Accretion on investments |
|
|
(142 |
) |
|
|
(105 |
) |
Depreciation and amortization |
|
|
280 |
|
|
|
293 |
|
Stock-based compensation |
|
|
1,508 |
|
|
|
2,377 |
|
Increase (decrease) in cash resulting from changes in: |
|
|
|
|
|
|
Accounts receivable |
|
|
119 |
|
|
|
(152 |
) |
Inventory |
|
|
(297 |
) |
|
|
— |
|
Prepaid expenses and other assets |
|
|
392 |
|
|
|
177 |
|
Accounts payable |
|
|
296 |
|
|
|
(148 |
) |
Accrued expenses |
|
|
(2,121 |
) |
|
|
2,359 |
|
Operating right-of-use asset and lease liability, net |
|
|
(5 |
) |
|
|
70 |
|
Net cash used in operating activities |
|
|
(14,653 |
) |
|
|
(12,408 |
) |
Cash Flows From Investing Activities |
|
|
|
|
|
|
Purchases of fixed assets |
|
|
(42 |
) |
|
|
(396 |
) |
Purchases of investments |
|
|
(10,860 |
) |
|
|
(9,893 |
) |
Maturities of investments |
|
|
11,000 |
|
|
|
10,000 |
|
Purchases of equity securities |
|
|
— |
|
|
|
(1,061 |
) |
Proceeds from sales of equity securities |
|
|
— |
|
|
|
384 |
|
Liquidation of equity securities |
|
|
718 |
|
|
|
— |
|
Net cash provided by (used in) investing activities |
|
|
816 |
|
|
|
(966 |
) |
Cash Flows From Financing Activities |
|
|
|
|
|
|
Proceeds from issuance of common stock, net |
|
|
1,437 |
|
|
|
— |
|
Net cash provided by financing activities |
|
|
1,437 |
|
|
|
— |
|
Net Decrease in Cash, Cash Equivalents, and Restricted Cash |
|
|
(12,400 |
) |
|
|
(13,374 |
) |
Cash, cash equivalents, and restricted cash at beginning of period |
|
|
15,444 |
|
|
|
28,818 |
|
Cash, cash equivalents, and restricted cash at end of period |
|
$ |
3,044 |
|
|
$ |
15,444 |
|
Reconciliation of cash, cash equivalents, and restricted cash to the
consolidated balance sheets |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
2,901 |
|
|
$ |
15,301 |
|
Restricted cash |
|
|
143 |
|
|
|
143 |
|
Total cash, cash equivalents and restricted cash |
|
$ |
3,044 |
|
|
$ |
15,444 |
|
Supplemental Disclosure of Cash Flow Information: |
|
|
|
|
|
|
Income taxes paid |
|
$ |
2 |
|
|
$ |
2 |
|
Purchases of fixed assets in accounts payable |
|
$ |
— |
|
|
$ |
64 |
|
The accompanying notes are an integral part of these consolidated financial statements.
Organovo Holdings, Inc.
Notes to Consolidated Financial Statements
Note 1. Description of Business and Summary of Significant Accounting Policies
Nature of operations and basis of presentation
Organovo Holdings, Inc. (“Organovo Holdings,” “Organovo,” and the “Company”) is a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in the Company's established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC.
The Company's current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024. The Company released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis (“MASH”) in April 2024 that is supportive of ongoing development, and the Company believes FXR314 has a commercial opportunity in MASH, most likely in combination therapy. The Company is exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and the Company's IBD disease models.
The Company's second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. The Company believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
The Company's current understanding of intestinal tissue models and IBD disease models leads the Company to believe that it can create models that provide greater insight into the biology of these diseases than are generally currently available. The Company is creating high fidelity disease models, leveraging its prior work including the work found in its peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) The Company's advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures.
Using these disease models, the Company intends to identify and validate novel therapeutic targets. After finding therapeutic drug targets, the Company intends to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials.
The Company expects to broaden its work into additional therapeutic areas over time and is currently exploring specific tissues for development. In its work to identify the areas of interest, the Company evaluates areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, the Company is building upon both its external and in house scientific expertise, which will be essential to its drug development effort.
In February 2024, the Company formed its Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain primary human cells that the Company utilizes in its research and development efforts. The Company believes Mosaic can help optimize its supply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials the Company uses are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. The Company intends for Mosaic to provide it with qualified human cells for use in its clinical research and development programs. In addition to supplying the Company with primary human cells, the Company intends for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which the Company expects to offset costs and over time become a profit center that offsets overall research and development ("R&D") spending by the Company.
Liquidity and Going Concern
The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of March 31, 2024, the Company had cash and cash equivalents of approximately $2.9 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $339.7 million. The restricted cash was pledged as collateral for a letter of credit that the Company is required to maintain as a security deposit under the terms of the lease agreements for its facilities. The Company also had negative cash flows from operations of approximately $14.7 million during the year ended March 31, 2024.
Through March 31, 2024, the Company has financed its operations primarily through the sale of common stock through public and at-the-market (“ATM”) offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research-based services, grants, and collaborative research agreements, and from the sale of convertible notes. During the year ended March 31, 2024, the Company issued 1,172,342 shares of its common stock through its ATM facility.
Based on the Company's current operating plan and available cash resources, including the total net proceeds of $4.7 million from the best efforts public offering that closed in May 2024, the Company will need substantial additional funding to support future operating activities. The Company has concluded that the prevailing conditions and ongoing liquidity risks faced by the Company raise substantial doubt about its ability to continue as a going concern for at least one year following the date these consolidated financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern. As the Company continues its operations and is focusing its efforts on drug discovery and development, the Company will need to raise additional capital to implement this business plan. The Company cannot predict with certainty the exact amount or timing for any future capital raises. The Company will seek to raise additional capital through debt or equity financings, or through some other financing arrangement. However, the Company cannot be sure that additional financing will be available if and when needed, or that, if available, it can obtain financing on terms favorable to its stockholders. Any failure to obtain financing when required will have a material adverse effect on the Company’s business, operating results, and financial condition.
Use of estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions.
Investments
Investments consist of investments in debt securities and investments in equity securities.
Investments in debt securities consist of investments in U.S. Treasury bills. As of March 31, 2024 and 2023, all investments that have original maturities of three months or less are classified as cash equivalents on the Consolidated Balance Sheets. As of March 31, 2024, all investments are classified as available-for-sale, as the sale of such investments may be required prior to maturity to implement management strategies. Available-for-sale debt securities are recorded at fair value. Any unrealized gains and losses are included in accumulated other comprehensive income as a component of stockholders' equity until realized. As U.S. Treasury bills are risk-free, any declines in fair value are considered temporary.
Investments in equity securities consist of investments in the common stock of entities traded in active markets. The Company does not have the ability to exercise significant influence over any entities. Therefore, initial investments are recorded at cost, and are remeasured at fair value as of the balance sheet date. Any gains or losses resulting from the change in fair value are recorded in net income. The investments in equity securities are classified as current assets.
Fair value measurement
Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
•Level 1 — Quoted prices in active markets for identical assets or liabilities.
•Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Financial instruments
For certain of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other assets, accounts payable, accrued expenses, the carrying amounts are generally considered to be representative of their respective fair values because of the short-term nature of those instruments.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents.
Restricted cash
As of each of March 31, 2024 and 2023, the Company had approximately $0.1 million of restricted cash, deposited with a financial institution. The entire amount was held in certificates of deposit to support a letter of credit agreement related to the Company’s facility leases entered into in November 2020 and amended in November 2021.
Inventory
Inventories are stated at the lower of cost or net realizable value. Inventory as of March 31, 2024 consisted of approximately $0.3 million in finished goods, which is related to the Mosaic Cell Sciences division. There were no finished goods inventory as of March 31, 2023. Please refer to "Note 16. Business Segment Information" for further information.
Fixed assets and depreciation
Fixed assets are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the remaining lease term. The estimated useful lives of the fixed assets range between one and seven years.
Impairment of long-lived assets
In accordance with authoritative guidance, the Company reviews its long-lived assets, including fixed assets and other assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred as of March 31, 2024 and 2023.
Research and development
Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Research and development costs are expensed as incurred.
Acquired in-process research and development
The Company has acquired drug candidates in development. The costs to acquire a drug candidate are immediately expensed as acquired in-process research and development, provided that the drug candidate has no alternative future use. Acquired in-process research and development expenses are included in total research and development expenses on the Consolidated Statements of Operations and Other Comprehensive Loss.
FXR Program
In March 2023, the Company acquired Metacrine's FXR program for $4.0 million. The FXR program was determined to have no alternative future use, and therefore was considered acquired in-process research and development and fully expensed. In the year
ended March 31, 2023, the Company paid a $2.0 million upfront payment, and the remaining $2.0 million was paid in the year ended March 31, 2024, upon the final transfer of the drug compounds, related data, and IP.
Segment Reporting
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance. As of March 31, 2024, the Company identified two operating segments. Please refer to "Note 16. Business Segment Information" for further information.
Income taxes
Deferred income taxes are recognized for the tax consequences in future years for differences between the tax basis of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the combination of the tax payable for the year and the change during the year in deferred tax assets and liabilities. The Company’s policy regarding uncertainty in income taxes is pursuant to Accounting Standard Codification (“ASC”) Topic 740-10. Interest and penalties that would be assessed in relation to the settlement value of unrecognized tax benefits is recognized as a component of income tax expense.
Revenue recognition
The Company has generated revenues from payments received from licensing intellectual property.
The Company has entered into a license agreement with a company that includes the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales, if any. At the initiation of the agreement, the Company has analyzed whether it results in a contract with a customer under Topic 606.
The Company has considered a variety of factors in determining the appropriate estimates and assumptions under these arrangements, such as whether the Company is a principal vs. agent, whether the elements are distinct performance obligations, whether there are determinable stand-alone prices, and whether any licenses are functional or symbolic. The Company has evaluated each performance obligation to determine if it can be satisfied and recognized as revenue at a point in time or over time. Typically, non-refundable upfront fees have been considered fixed, while sales-based royalty payments have been identified as variable consideration which must be evaluated to determine if it has been constrained and, therefore, excluded from the transaction price. Please refer to “Note 6. Collaborative Research, Development, and License Agreements” for further information.
Stock-based compensation
The Company accounts for stock-based compensation in accordance with the ASC Topic 718, Compensation — Stock Compensation, which establishes accounting for equity instruments exchanged for employee and non-employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair value of the award (determined using either the Black-Scholes or Monte Carlo option-pricing models, depending on the complexity of the equity grant), and is recognized as an expense, under the straight-line method, over the employee or non-employee's requisite service period (generally the vesting period of the equity grant). The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses its Company-specific historical volatility rate. The risk-free interest rate assumption is based on U.S. Treasury rates. The weighted average expected life of options is estimated using the average of the contractual term and the weighted average vesting term of the options. Option forfeitures are treated as a reduction of stock-based compensation expense and accounted for as they occur.
Comprehensive income (loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the consolidated financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at comprehensive income (loss).
Net loss per share
Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. The weighted-average number of shares used to compute diluted loss per share excludes any assumed exercise of stock options and warrants, shares reserved for purchase under the Company’s 2023 Employee Stock Purchase Plan (“2023 ESPP”), the assumed release of restriction of restricted stock units (“RSUs”), and shares subject to repurchase as the effect would be anti-dilutive. No dilutive effect was calculated for the years ended March 31, 2024 and 2023 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive.
Common stock equivalents excluded from computing diluted net loss per share were approximately 0.8 million shares and 1.6 million shares for the years ended March 31, 2024 and 2023, respectively.
Note 2. Investments and fair value measurement
Investments in debt securities
As of March 31, 2024, the Company held $1.0 million of investments in debt securities (which are included in the $2.9 million of cash and cash equivalents). For the year ended March 31, 2024, there was $0.1 million of interest income related to the investments in debt securities. There were less than $0.1 million of unrealized gains recorded on investments in debt securities for the year ended March 31, 2024. As the investments in debt securities consist of U.S. Treasury bills from active markets, the fair value is measured using level 1 inputs.
The following table summarizes the Company's investments in debt securities that are measured at fair value as of March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortized costs basis |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair value |
|
As of March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
997 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
998 |
|
As of March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
4,943 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
4,945 |
|
Investments in equity securities
For the year ended March 31, 2024, $0.7 million of equity securities were liquidated and there was less than a $0.1 million gain on the investment in equity securities. As of March 31, 2024, the fair value of investment in equity securities was zero, as a result of the liquidation of the shares by the underlying company on June 26, 2023. As the investments in equity securities consist of common stock from active markets, the fair value is measured using level 1 inputs.
The following table presents the activity for investments in equity securities measured at fair value for the year ended March 31, 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Investment in Equity Securities
(in thousands) |
|
Balance at March 31, 2023 |
|
$ |
706 |
|
Sales |
|
|
(718 |
) |
Gain on investment in equity securities |
|
|
12 |
|
Balance at March 31, 2024 |
|
$ |
— |
|
Note 3. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Prepaid insurance |
|
$ |
445 |
|
|
$ |
507 |
|
Prepaid expenses |
|
|
256 |
|
|
|
313 |
|
Other current assets |
|
|
4 |
|
|
|
69 |
|
|
|
$ |
705 |
|
|
$ |
889 |
|
Note 4. Fixed Assets
Fixed assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Laboratory equipment |
|
$ |
1,617 |
|
|
$ |
1,575 |
|
Furniture and fixtures |
|
|
66 |
|
|
|
66 |
|
Computer software and equipment |
|
|
244 |
|
|
|
537 |
|
Fixed Assets, gross |
|
|
1,927 |
|
|
|
2,178 |
|
Less accumulated depreciation |
|
|
(1,258 |
) |
|
|
(1,276 |
) |
Fixed Assets, net |
|
$ |
669 |
|
|
$ |
902 |
|
As of March 31, 2024 and 2023, all of the Company’s fixed assets were active and in use. Depreciation expense for the years ended March 31, 2024 and 2023 was approximately $0.3 million and $0.2 million, respectively.
Note 5. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Accrued compensation |
|
$ |
536 |
|
|
$ |
609 |
|
Accrued legal and professional fees |
|
|
93 |
|
|
|
193 |
|
Acquired in-process research and development |
|
|
— |
|
|
|
2,000 |
|
Other accrued expenses |
|
|
98 |
|
|
|
46 |
|
|
|
$ |
727 |
|
|
$ |
2,848 |
|
Note 6. Collaborative Research, Development, and License Agreements
License Agreements
BICO Group AB
From June 2021 to February 2022, certain patents owned or sublicensed by the Company became the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”). The Company and BICO Group AB were also engaged in litigation regarding patent infringement during the same time period. On February 22, 2022, the Company and BICO Group AB signed a settlement and patent license agreement (“License Agreement”) to close all matters noted above. In addition to closing all legal matters and patent disputes noted above, as part of the agreement, the Company agreed to grant a non-exclusive license to BICO
Group AB to use the Company’s aforementioned patents for its business operations of manufacturing and selling bioprinters as well as bioinks. The Company concluded that the nature of the license granted represents functional intellectual property.
As part of the License Agreement, BICO Group AB agreed to pay the Company a one time, nonrefundable upfront fee of $1,500,000. Based on ASC Topic 606, the Company concluded that the performance obligation related to this upfront fee consisted of the Company filing stipulations of dismissal of all legal matters noted above, as well as the Company granting the non-exclusive license of the aforementioned patents within five days of receiving the upfront payment. The conditions of the performance obligation were satisfied, and therefore the Company recognized revenue of $1,500,000 on February 22, 2022, the executed date of the License Agreement.
Additionally, as part of the License Agreement, BICO Group AB agreed to pay the Company ongoing sales-based royalties (based on percentages of BICO Group AB’s net sales) for the use of the granted license. The sales-based royalties became effective beginning on February 22, 2022, the effective date of the License Agreement, and continue until the expiration of the last surviving licensed patent. As the sales-based royalties are required to be paid 45 days after the end of every quarter, there is variable consideration that must be estimated to determine royalty revenue within a given reporting period. Once actual revenue earned is determined in the following fiscal quarter, an adjustment is made from the previously estimated amount. For the years ended March 31, 2024 and 2023, the Company recorded $109,000 and $370,000, respectively, of royalty revenue based on sales-based royalties from the License Agreement.
Also as part of the License Agreement, certain patents involved in the agreement are sublicensed by the Company from the University of Missouri and Clemson University. See below for further information.
University of Missouri
In March 2009, the Company entered into a license agreement with the Curators of the University of Missouri to in-license certain technology and intellectual property relating to self-assembling cell aggregates and to intermediate cellular units. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay the University of Missouri royalties ranging from 1% to 3% of net sales of covered tissue products, and of the fair market value of covered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales achieved by the Company each year.
On December 5, 2022, the Company amended the license agreement with the University of Missouri, whereby the Company agreed to pay a single, upfront payment of $50,000 to the University of Missouri in exchange for the aforementioned licensed intellectual property to be fully paid up by the Company. As a result, the Company will continue to have rights to the licensed intellectual property until its expiration in June 2028, but will no longer owe minimum annual royalty payments, royalty payments based on net sales, or any other payments (other than patent annuities and any prosecution costs) in the future.
Clemson University
In May 2011, the Company entered into a license agreement with Clemson University Research Foundation ("CURF") to in-license certain technology and intellectual property relating to ink-jet printing of viable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company was required to pay the university royalties ranging from 1.5% to 3% of net sales of covered tissue products and the fair market value of covered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales reached each year. The license agreement terminates upon expiration of the patents licensed, which are expected to expire in May 2024, and is subject to certain conditions as defined in the license agreement. Minimum annual royalty payments of $40,000 per year were due beginning in calendar 2016. The royalty payments of $40,000 were made for the years ended March 31, 2024 and 2023. The annual minimum royalty is creditable against royalties owed during the same calendar year.
In addition to the annual royalties noted above, CURF is owed 40% of all payments including but not limited to, upfront payments, license fees, issue fees, maintenance fees, and milestone payments received from third parties, including sublicensees, in consideration for sublicensing rights to licensed products. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. As of March 31, 2024, the Company’s legal expenses exceeded royalties owed from the upfront payment and sales-based royalties related to the license agreement. Therefore, no royalty expense to CURF was recorded for the year ended March 31, 2024. No royalty expense related to sales-based royalties has been recorded to date
Capitalized License Fees
Capitalized license fees consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
License fees |
|
$ |
109 |
|
|
$ |
114 |
|
Less accumulated amortization |
|
|
(101 |
) |
|
|
(101 |
) |
License fees, net |
|
$ |
8 |
|
|
$ |
13 |
|
The above license fees, net of accumulated amortization, are included in prepaid expenses and other assets in the accompanying consolidated balance sheets and are being amortized over the life of the related patents. Amortization expense of licenses was approximately $5,000 and $82,000 for the years ended March 31, 2024 and 2023, respectively. At March 31, 2024, the weighted average remaining amortization period for all licenses was approximately 3 years. The annual amortization expense of licenses for the next five years is estimated to be approximately $3,000 per year.
The Salk Institute for Biological Studies
In March 2023, the Company acquired the FXR Agonist program from Metacrine. All patent rights related to this program have been assigned to the Company in connection with the acquisition. In addition, the Company assumed and was assigned a license agreement with the Salk Institute for Biological Studies (hereafter “Salk”) that provides certain payments to Salk upon the successful development and commercialization of the lead compound, FXR314. The Company is required to pay Salk royalties ranging from 1% to 1.125% of net sales of therapeutics based on FXR314. In addition, the Company is required to make certain milestone payments based on the successful initiation and/or completion of certain development milestones, including $500,000 within 45 days of the dosing of the first patient in a phase III clinical trial, $1,000,000 within 45 days of FDA approval of the first Licensed Product and $1,500,000 within 45 days of the first commercial sale of a Licensed Product in the Territory. There are also reduced milestone payments application to a second or third licensed product, if any. Should the Company sublicense a licensed product to a third party, then it must pay a 3.5% of sublicensing revenue attributable to such a sublicense.
Note 7. Stockholders’ Equity
Preferred stock
The Company is authorized to issue 25,000,000 shares of preferred stock. There are no shares of preferred stock currently outstanding, and the Company has no present plans to issue shares of preferred stock.
Common stock
In January 2012, the Company's Board of Directors ("Board") approved the 2012 Amended and Restated Equity Incentive Plan ("2012 Plan"). The 2012 Plan initially authorized the issuance of up to 327,699 shares of common stock for awards of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards, and the number of shares issuable pursuant thereto was increased several times to an aggregate of 2,327,699 shares.
In March 2021, the Board approved the 2021 Inducement Equity Incentive Plan ("Inducement Plan"). The Inducement Plan authorized the issuance of up to 750,000 shares of common stock for awards of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards. In February 2022, 50,000 incentive stock options were issued under the Inducement Plan.
On October 12, 2022, the Company's stockholders and the Board approved the 2022 Equity Incentive Plan ("2022 Plan"), and it became effective on that date. The 2022 Plan replaced the 2012 Plan on the effective date. Upon the effective date, the Company ceased granting awards under the 2012 Plan and any shares remaining available for future issuance under the 2012 Plan were cancelled and are no longer available for future issuance. The 2012 Plan continues to govern awards previously granted under it. At the time the Board approved the 2022 Plan, an aggregate of 1,363,000 shares of the Company’s common stock was initially reserved for issuance under the 2022 Plan. The Company committed to reducing the new 2022 Plan share reserve by the number of shares that were granted under the 2012 Plan and the Inducement Plan between July 25, 2022 and October 12, 2022. From July 25, 2022 to October 12, 2022, the Company issued 126,262 shares of its common stock under the 2012 Plan. As a result, the number of shares reserved for future issuance under the 2022 Plan is 1,236,738 shares of common stock. The Company also committed to reducing the
aggregate number of shares of its common stock issuable pursuant to the Inducement Plan from 750,000 shares to 51,000 shares (which includes 50,000 shares of its common stock issuable pursuant to an outstanding option to purchase common stock with an exercise price of $2.75 per share, leaving only 1,000 shares available for future issuance under the Inducement Plan) and the share reserve was reduced effective October 12, 2022.
The Company previously had an effective shelf registration statement on Form S-3 (File No. 333-252224), declared effective by the SEC on January 29, 2021 (the “2021 Shelf”), which registered $150.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing, that expired on January 29, 2024. On January 26, 2024, the Company filed a new shelf registration statement on Form S-3 (File No. 333-276722) to register $150.0 million of the Company's common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the "2024 Shelf"). The 2024 Shelf was declared effective by the SEC on February 8, 2024 and replaced the 2021 Shelf at that time.
On March 16, 2018, the Company entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, the Company filed a prospectus supplement to the 2021 Shelf (the “2021 ATM Prospectus Supplement”), pursuant to which the Company may offer and sell, from time to time through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold were issued pursuant to the 2021 Shelf until it was replaced by the 2024 Shelf.
On January 26, 2024, the Company filed a prospectus to the 2024 Shelf (the "2024 ATM Prospectus"), pursuant to which the Company may offer and sell, from time to time through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $2,605,728. Any shares offered and sold in these ATM transactions will be issued pursuant to the 2024 Shelf.
During the year ended March 31, 2024, the Company issued 1,172,342 shares of common stock in ATM offerings, of which 1,135,940 shares were sold pursuant to the 2021 Shelf, and 36,402 shares were sold pursuant to the 2024 Shelf. As of March 31, 2024, the Company has sold an aggregate of 2,753,204 shares of common stock in ATM offerings under the 2021 ATM Prospectus Supplement and 2024 ATM Prospectus, with gross proceeds of approximately $23.2 million. As of March 31, 2024, there was approximately $100.0 million available for future offerings under the 2024 Shelf, and approximately $2.6 million available for future offerings through the Company’s ATM program under the 2024 ATM Prospectus.
Restricted stock units
The following table summarizes the Company’s RSUs activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
|
|
Weighted
Average Price |
|
Unvested at March 31, 2023 |
|
|
127,717 |
|
|
$ |
2.22 |
|
Granted |
|
|
117,642 |
|
|
$ |
1.39 |
|
Vested |
|
|
(122,717 |
) |
|
$ |
1.90 |
|
Cancelled / forfeited |
|
|
— |
|
|
$ |
— |
|
Unvested at March 31, 2024 |
|
|
122,642 |
|
|
$ |
1.75 |
|
Stock options
During the year ended March 31, 2024, under the 2022 Plan, 184,158 stock options were granted at various exercise prices.
On August 28, 2023, the Company's Executive Chairman voluntarily forfeited 462,500 outstanding stock options, of which 312,918 were unvested and therefore cancelled, and 149,582 were vested and therefore treated as expired. The forfeited stock option awards were not replaced by other awards or other compensation and there is no plan to replace the forfeited awards. Therefore, all previous unrecognized compensation expense associated with the forfeited awards, approximately $519,000, was recognized as a selling, general, and administrative expense on the date of forfeiture.
The following table summarizes stock option activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options
Outstanding |
|
|
Weighted-
Average
Exercise Price |
|
|
Aggregate
Intrinsic
Value |
|
Outstanding at March 31, 2023 |
|
|
1,451,217 |
|
|
$ |
6.49 |
|
|
$ |
38,327 |
|
Options granted |
|
|
184,158 |
|
|
$ |
1.67 |
|
|
$ |
— |
|
Options canceled |
|
|
(937,368 |
) |
|
$ |
7.20 |
|
|
$ |
— |
|
Options exercised |
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
Outstanding at March 31, 2024 |
|
|
698,007 |
|
|
$ |
4.26 |
|
|
$ |
— |
|
Vested and Exercisable at March 31, 2024 |
|
|
401,536 |
|
|
$ |
5.61 |
|
|
$ |
— |
|
The weighted-average remaining contractual term of stock options exercisable and outstanding at March 31, 2024 was approximately 7.38 years.
During the years ended March 31, 2024 and 2023, the Company issued zero shares of common stock upon exercise of stock options.
Employee Stock Purchase Plan
In June 2016, the Board, and subsequently in August 2016, the Company's stockholders approved, the 2016 ESPP. The Company reserved 75,000 shares of common stock for issuance thereunder. As of October 31, 2023, the 2016 ESPP was replaced by the 2023 ESPP (as defined below).
In July 2023, the Board adopted, and subsequently on October 31, 2023, the Company's stockholders approved, the 2023 Employee Stock Purchase Plan (the “2023 ESPP”). The 2023 ESPP became effective on October 31, 2023 and replaced the 2016 ESPP on that date. The Company reserved 45,000 shares of common stock for issuance thereunder. The 2023 ESPP permits employees to purchase common stock through payroll deductions, limited to 15 percent of each employee’s compensation up to $25,000 per employee per year or 500 shares per employee per six-month purchase period. Shares under the 2023 ESPP are purchased at 85 percent of the fair market value at the lower of (i) the closing price on the first trading day of the six-month purchase period or (ii) the closing price on the last trading day of the six-month purchase period. The initial offering under the 2023 ESPP commenced on March 1, 2024.
Common stock reserved for future issuance
Common stock reserved for future issuance consisted of the following at March 31, 2024:
|
|
|
|
|
Common stock issuable pursuant to options outstanding and reserved under the 2012 Plan |
|
|
431,416 |
|
Common stock reserved under the 2012 Plan |
|
|
— |
|
Common stock issuable pursuant to options outstanding and reserved under the 2022 Plan |
|
|
216,591 |
|
Common stock reserved under the 2022 Plan |
|
|
1,641,250 |
|
Common stock reserved under the ESPP |
|
|
45,000 |
|
Common stock reserved under the 2021 Inducement Equity Plan |
|
|
1,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2012 Plan |
|
|
5,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2022 Plan |
|
|
117,642 |
|
Common stock issuable pursuant to options outstanding and reserved under the Inducement Plan |
|
|
50,000 |
|
Total at March 31, 2024 |
|
|
2,507,899 |
|
Stock-based compensation expense and valuation information
Stock-based awards include stock options and RSUs under the Company's 2022 Plan, 2012 Plan, inducement awards, performance-based RSUs under an Incentive Award Performance-Based Restricted Stock Unit Agreement, the Inducement Plan, and rights to purchase stock under the 2023 ESPP.
Stock-based compensation expense for all stock-based awards consists of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Research and development |
|
$ |
138 |
|
|
$ |
473 |
|
General and administrative |
|
|
1,370 |
|
|
|
1,904 |
|
Total |
|
$ |
1,508 |
|
|
$ |
2,377 |
|
The total unrecognized compensation cost related to unvested stock option grants as of March 31, 2024 was approximately $485,000 and the weighted average period over which these grants are expected to vest is 2.17 years.
The total unrecognized stock-based compensation cost related to unvested RSUs as of March 31, 2024 was approximately $151,000 which will be recognized over a weighted average period of 0.73 years.
As of March 31, 2024, there was one participant enrolled into the 2023 ESPP for the current purchase period, beginning March 1, 2024.
The fair value of stock options was estimated at the grant date using the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
99.04 |
% |
|
|
95.53 |
% |
Risk-free interest rate |
|
|
4.11 |
% |
|
|
3.32 |
% |
Expected life of options |
|
6 years |
|
|
6 years |
|
Weighted average grant date fair value |
|
$ |
1.34 |
|
|
$ |
1.83 |
|
The fair value of each RSU is recognized as stock-based compensation expense over the vesting term of the award. The fair value is based on the closing stock price on the date of the grant.
The Company uses the Black-Scholes valuation model to calculate the fair value of shares issued pursuant to the 2016 ESPP and the 2023 ESPP. Stock-based compensation expense is recognized over the purchase period using the straight-line method. The fair value of ESPP shares was estimated at the purchase period commencement date using the following assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
95.20 |
% |
|
|
86.58 |
% |
Risk-free interest rate |
|
|
5.27 |
% |
|
|
3.34 |
% |
Expected term |
|
6 months |
|
|
6 months |
|
Grant date fair value |
|
$ |
0.39 |
|
|
$ |
0.82 |
|
The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses the Company-specific historical volatility rate as the indicator of expected volatility. The risk-free interest rate assumption was based on U.S. Treasury rates. The expected life is the 6-month purchase period.
Note 8. Leases
After the initial adoption of ASC Topic 842, on an on-going basis, the Company evaluates all contracts upon inception and determines whether the contract contains a lease by assessing whether there is an identified asset and whether the contract conveys the right to control the use of identified asset in exchange for consideration over a period of time. If a lease is identified, the Company will apply the guidance from ASC Topic 842 to properly account for the lease.
Operating Leases
On November 23, 2020, the Company entered into a lease agreement, pursuant to which the Company permanently leased approximately 8,051 square feet of office space (the “Permanent Lease”) in San Diego once certain tenant improvements were completed by the landlord and the premises were ready for occupancy. Additionally, on November 17, 2021, the Permanent Lease was amended to add an additional 2,892 square feet of office space in the same building. The Permanent Lease commenced on December
17, 2021 and is intended to serve as the Company’s permanent premises for approximately sixty-two months. Monthly rental payments are approximately $40,800 with 3% annual escalators.
The Company determined that the Permanent Lease is considered an operating lease under ASC Topic 842, and therefore upon the lease commencement date of December 17, 2021, recognized lease liabilities and corresponding right-of-use assets of $2.3 million. The Company records operating lease expense on a straight-line basis over the life of the lease (referred to as “operating lease expense”). Variable lease expenses associated with the Company’s leases, such as payments for additional monthly fees to cover the Company’s share of certain facility expenses (common area maintenance) are expensed as incurred.
The table below summarizes the Company’s lease liabilities and corresponding right-of-use assets as of March 31, 2024 (in thousands):
|
|
|
|
|
|
|
March 31, 2024 |
|
ASSETS |
|
|
|
Operating lease right-of-use assets |
|
$ |
1,299 |
|
Total lease right-of-use assets |
|
$ |
1,299 |
|
|
|
|
|
LIABILITIES |
|
|
|
Current |
|
|
|
Operating lease liability |
|
$ |
506 |
|
Noncurrent |
|
|
|
Operating lease liability, net of current portion |
|
$ |
888 |
|
Total lease liabilities |
|
$ |
1,394 |
|
|
|
|
|
Weighted average remaining lease term: |
|
2.83 |
|
Weighted average discount rate: |
|
|
6 |
% |
Variable lease expense was approximately $153,000 and $146,000 for the years ended March 31, 2024 and 2023, respectively. Operating lease expense was approximately $503,000 and $499,000 for the years ended March 31, 2024 and 2023, respectively.
Cash outflows associated with the Company’s operating lease for the years ended March 31, 2024 and 2023 were $509,000 and $430,000, respectively.
Future lease payments relating to the Company’s operating lease liabilities as of March, 31, 2024 are as follows (in thousands):
|
|
|
|
|
Fiscal year ending March 31, 2025 |
|
|
523 |
|
Fiscal year ending March 31, 2026 |
|
|
538 |
|
Fiscal year ending March 31, 2027 |
|
|
460 |
|
Total future lease payments |
|
|
1,521 |
|
Less: Imputed Interest |
|
|
(127 |
) |
Total lease obligations |
|
|
1,394 |
|
Less: Current obligations |
|
|
(506 |
) |
Noncurrent lease obligations |
|
$ |
888 |
|
Note 9. Commitments and Contingencies
Legal matters
In addition to commitments and obligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business.
The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its consolidated financial statements. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In
addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not meaningful indicators of its potential liability.
The Company regularly reviews contingencies to determine the adequacy of its accruals and related disclosures. During the period presented, the Company has not recorded any accrual for loss contingencies associated with any claims or legal proceedings; determined that an unfavorable outcome is probable or reasonably possible; or determined that the amount or range of any possible loss is reasonably estimable. However, the outcome of legal proceedings and claims brought against the Company is subject to significant uncertainty. Therefore, although management considers the likelihood of such an outcome to be remote, if one or more legal matters were resolved against the Company in a reporting period, the Company’s consolidated financial statements for that reporting period could be materially adversely affected.
Note 10. Income Taxes
A reconciliation of the statutory federal rate and the effective rate, for operations, is as follows for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
|
|
March 31,
2023 |
|
|
|
Tax computed at federal statutory rate |
$ |
(3,081 |
) |
|
21 |
% |
|
$ |
(3,624 |
) |
21% |
|
State income tax, net of federal benefit |
|
(110 |
) |
|
0.7 |
% |
|
|
(44 |
) |
0.2% |
|
Stock-based compensation |
|
721 |
|
|
-4.9 |
% |
|
|
167 |
|
-1% |
|
Research credits |
|
— |
|
|
0 |
% |
|
|
60 |
|
-0.4% |
|
Change in tax rate |
|
(62 |
) |
|
0.4 |
% |
|
|
157 |
|
-0.9% |
|
Removal of net operating losses and research development credits |
|
1,910 |
|
|
-13 |
% |
|
|
1,410 |
|
-8.2% |
|
Uncertain tax positions |
|
111 |
|
|
-0.8 |
% |
|
|
— |
|
|
0 |
% |
Other |
|
(16 |
) |
|
0.2 |
% |
|
|
1 |
|
0% |
|
Valuation allowance |
|
527 |
|
|
-3.6 |
% |
|
|
1,873 |
|
-10.7% |
|
Provision (benefit) for income taxes |
$ |
— |
|
0.0% |
|
|
$ |
— |
|
0.0% |
|
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows as of March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Deferred tax assets: |
|
|
|
|
|
Amortization |
$ |
593 |
|
|
$ |
598 |
|
Section 174 R&D capitalization |
|
1,793 |
|
|
|
855 |
|
Accrued expenses and reserves |
|
105 |
|
|
|
116 |
|
Operating lease liability |
|
307 |
|
|
|
384 |
|
Stock-based compensation |
|
315 |
|
|
|
755 |
|
Inventory |
|
259 |
|
|
|
251 |
|
Other, net |
|
4 |
|
|
|
3 |
|
Total deferred tax assets |
|
3,376 |
|
|
|
2,962 |
|
Valuation allowance |
|
(2,983 |
) |
|
|
(2,458 |
) |
Net deferred tax assets |
$ |
393 |
|
|
$ |
504 |
|
Deferred tax liabilities: |
|
|
|
|
|
Operating lease right-of-use assets |
|
(286 |
) |
|
|
(363 |
) |
Depreciation |
|
(107 |
) |
|
|
(135 |
) |
Investment in equity securities |
|
— |
|
|
|
(6 |
) |
Total deferred tax liabilities |
$ |
(393 |
) |
|
$ |
(504 |
) |
|
$ |
— |
|
|
$ |
— |
|
A full valuation allowance has been established to offset the deferred tax assets as management cannot conclude that realization of such assets is more likely than not. Under the Internal Revenue Code (“IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research tax credit carryforwards to offset taxable income may be limited based on cumulative changes in ownership. The Company has not completed an analysis to determine whether any such limitations have been triggered as of March 31, 2024. Until this analysis is completed, the Company has removed the deferred tax assets related to net operating losses from its
deferred tax asset schedule. Further, until a study is completed and any limitation known, approximately $1.7 million and $1.6 million for the years ended March 31, 2024 and 2023, respectively, would be considered as an uncertain tax position if netted against the deferred tax asset. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. Any carryforwards that will expire prior to utilization as a result of such limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. The valuation allowance increased by approximately $525,000 and approximately $1,875,000 for the years ended March 31, 2024 and 2023, respectively.
The Company had federal and state net operating loss carryforwards of approximately $219.4 million and $42.8 million, respectively, as of March 31, 2024. Federal net operating loss carryforwards of approximately $75.8 million will carryforward indefinitely and be available to offset up to 80% of future taxable income each year subject to revisions made by the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The remaining federal net operating losses will begin to expire in 2028, unless previously utilized. The state net operating loss carryforwards (“NOLs”) will begin to expire in 2028, unless previously utilized.
The Company had federal and state research tax credit carryforwards of approximately $5.0 million and $4.5 million at March 31, 2024, respectively. The federal research tax credit carryforwards begin expiring in 2028. The state research tax credit carryforwards do not expire.
The Company did not record any accruals for income tax accounting uncertainties for the year ended March 31, 2024.
The Company did not accrue either interest or penalties from inception through March 31, 2024.
The Company does not expect its unrecognized tax benefits to significantly increase or decrease within the next 12 months.
The Company is subject to tax in the United States and in California. As of March 31, 2024, the Company’s tax years from inception are subject to examination by the tax authorities due to the generation of net operating losses. The Company is not currently under examination by any jurisdiction.
Note 11. Concentrations
Credit risk and significant customers
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions located within the United States. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. Balances may exceed federally insured limits. The Company is also potentially subject to concentrations of credit risk in its revenues and accounts receivable. However, the Company only receives royalty revenue from one licensee and has not historically experienced any accounts receivable write-downs.
Note 12. Related Parties
From time to time, the Company enters into agreements with one or more related parties in the ordinary course of its business. These agreements are ratified by the Board or a committee thereof pursuant to its related party transaction policy.
Viscient Biosciences (“Viscient”) is an entity for which Keith Murphy, the Company’s Executive Chairman, serves as the Chief Executive Officer and President. Dr. Jeffrey Miner, the Company’s former Chief Scientific Officer, is also the Chief Scientific Officer of Viscient, and Thomas Jurgensen, the Company’s former General Counsel, previously served as outside legal counsel to Viscient through his law firm, Optima Law Group, APC.
On December 28, 2020, the Company entered into an intercompany agreement (the “Intercompany Agreement”) with Viscient and Organovo, Inc., the Company’s wholly-owned subsidiary, which included an asset purchase agreement for certain lab equipment. Pursuant to the Intercompany Agreement, the Company agreed to provide Viscient certain services related to 3D bioprinting technology, which includes, but is not limited to, histology services, cell isolation, and proliferation of cells and Viscient agreed to provide the Company certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, the Company and Viscient each agreed to share certain facilities and equipment and, subject to further agreement, to each make certain employees available for specified projects for the other party at prices to be determined in good faith by the parties. The Company evaluated the accounting for the Intercompany Agreement and concluded that any services provided by Viscient to the Company will be expensed as incurred, and any compensation for services provided by the Company to Viscient will be considered a reduction of personnel related expenses. Any services provided to Viscient do not fall under Topic 606 as the Intercompany Agreement is not a contract with a customer. For the years ended March 31, 2024 and 2023, the Company incurred no
consulting expenses from Viscient. Additionally, for the years ended March 31, 2024 and 2023, the Company provided approximately $14,000 and $59,000 of histology services to Viscient, respectively.
Note 13. Defined Contribution Plan
The Company has a defined contribution 401(k) plan covering substantially all employees. During the year ended March 31, 2015, the 401(k) plan was amended (the “Amended Plan”) to include an employer matching provision. Under the terms of the Amended Plan, the Company will make matching contributions on up to the first 6% of compensation contributed by its employees. Amounts expensed under the Company’s 401(k) plan for the years ended March 31, 2024 and 2023 were approximately $61,000 and $10,000, respectively.
Note 14. Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies. Unless otherwise stated, the Company believes that the impact of the recently issued accounting pronouncements that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption.
In December 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The update requires a public business entity to disclose, on an annual basis, a tabular rate reconciliation using both percentages and currency amounts, broken out into specified categories with certain reconciling items further broken out by nature and jurisdiction to the extent those items exceed a specified threshold. In addition, all entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received. Adoption of the ASU allows for either the prospective or retrospective application of the amendment and is effective for annual periods beginning after December 15, 2024, with early adoption permitted. The Company has not yet completed its assessment of the impact of ASU 2023-09 on the Company’s Consolidated Financial Statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment's profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company is currently evaluating the impact of this guidance.
Note 15. Restructuring
On August 18, 2023, the Company announced to its employees a plan to reduce the Company’s workforce, effective August 25, 2023, by approximately six employees, which represented approximately 24% of its employees as of August 18, 2023. The Company refocused operations on FXR314, its clinical drug candidate. This decision to reduce the Company’s workforce was made in order to focus spending on the Company’s clinical program for FXR314, reduce ongoing operating expenses not related to clinical expenses, and extend the Company’s cash runway. The Company incurred approximately $0.4 million of cash expenditures for the year ended March 31, 2024, in connection with the reduction in force, which related to severance pay. As of March 31, 2024, all restructuring costs were paid in full. The Company anticipates annual cost savings of approximately $1.5 million resulting from the reduction in force.
Note 16. Business Segment Information
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance. The Company's operating segments were identified in fiscal 2024 and did not impact prior periods. The Company's operating segments are as follows:
Research & Development
The research and development (“R&D”) segment consists of the Company's drug development efforts. The Company's current clinical focus is in advancing FXR314 in IBD, including UC and CD. The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024. The Company released Phase 2 data for FXR314 for the treatment of MASH in April 2024 that are supportive of
ongoing development, and believes FXR314 has a commercial opportunity in MASH, most likely in combination therapy. The Company is exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and its IBD disease models.
The Company's second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. The Company believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Mosaic Cell Sciences
The Mosaic Cell Sciences segment, which began operations in February 2024, consists of our Mosaic Cell Sciences division (“Mosaic”) which will serve as a key source of certain of the primary human cells that the Company utilizes in its research and development efforts. The Company believes Mosaic can help optimize its supply chain, reduce operating expenses related to cell sourcing and procurement, and ensure that the cellular raw materials it uses are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. The Company intends for Mosaic to provide the Company with qualified human cells for use in its clinical research and development programs. In addition to supplying the Company with primary human cells, the Company intends for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which the Company expects to offset costs and over time become a profit center that offsets overall R&D spending by the Company.
Business Segment Information
The Company's R&D segment generates royalty revenue related to its IP. The R&D segment's identifiable assets are its fixed assets. The Company expects that the Mosaic segment will generate future product revenue as part of its core operations, and its identifiable assets are its fixed assets and inventory. Other Company assets are comprised of all other assets that are not distinctly identifiable to a segment, which are presented on the Company's Consolidated Balance Sheets as of March 31, 2024.
R&D segment revenues consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Royalty revenue |
$ |
109 |
|
|
$ |
370 |
|
Mosaic |
|
|
|
|
|
Product revenue |
|
— |
|
|
|
— |
|
Total segment revenue |
$ |
109 |
|
|
$ |
370 |
|
Total company revenue |
$ |
109 |
|
|
$ |
370 |
|
Operating segment expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
$ |
4,404 |
|
|
$ |
8,885 |
|
Mosaic |
|
1,094 |
|
|
|
— |
|
Total segment operating expenses |
$ |
5,498 |
|
|
$ |
8,885 |
|
Selling, general, and administrative expenses |
|
9,697 |
|
|
|
9,216 |
|
Total company operating expenses |
$ |
15,195 |
|
|
$ |
18,101 |
|
Operating segment assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Fixed assets, net |
$ |
423 |
|
|
$ |
902 |
|
Mosaic |
|
|
|
|
|
Fixed assets, net |
|
183 |
|
|
|
— |
|
Inventory |
|
297 |
|
|
|
— |
|
Total segment assets |
$ |
903 |
|
|
$ |
902 |
|
Other company assets |
|
5,446 |
|
|
|
19,411 |
|
Total company assets |
$ |
6,349 |
|
|
$ |
20,313 |
|
Note 17. Subsequent Events
On May 8, 2024, the Company priced a best efforts public offering (the “Offering”) of: (i) 1,562,500 shares of its common stock and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock at a combined public offering price of $0.80 per share and accompanying Common Warrant to purchase one share of common stock and (ii) pre-funded warrants (“Pre-Funded Warrants”) to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock at a combined public offering price of $0.799 per Pre-Funded Warrant and accompanying Common Warrant to purchase one share of common stock. In connection with the Offering, the Company entered into Securities Purchase Agreements with the purchasers of the securities in the Offering on May 8, 2024.
The per share exercise price for the Pre-Funded Warrants is $0.001, subject to adjustment as provided therein. The Pre-Funded Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire when exercised in full. The holders may exercise the Pre-Funded Warrants by means of a “cashless exercise.”
The per share exercise price for the Common Warrants is $0.80, subject to adjustment as provided therein. The Common Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire on the date that is five years following the original issuance date. If a registration statement covering the issuance of the shares of common stock issuable upon exercise of the Common Warrants is not available for the issuance, then the holders may exercise the Common Warrants by means of a “cashless exercise.”
In connection with the Offering, the Company paid JonesTrading Institutional Services LLC, which acted as the placement agent in connection with the Offering, a cash fee of 5.0% of the aggregate gross proceeds raised in the Offering.
The closing of the Offering occurred on May 13, 2024. The Company received net proceeds of approximately $4.7 million from the Offering, after deducting the estimated offering expenses payable by the Company, including the Placement Agent fees.
Additionally, between April 1, 2024 and the date of the filing of this Annual Report on Form 10-K, the Company issued 1,389,002 shares of common stock in ATM offerings under the 2024 ATM Prospectus for net proceeds of approximately $1.8 million.
As of May 29, 2024, the Company's cash and cash equivalents balance was approximately $7.0 million.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed pursuant to the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our executive chairman and our principal financial and accounting officer, as appropriate, to allow timely decisions regarding required disclosure.
Under the supervision of our Executive Chairman and our Chief Financial Officer, and with the participation of all members of management, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act. Based on this evaluation, our executive chairman and our principal financial officer concluded that our disclosure controls and procedures were designed and operating effectively as of the end of the period covered by this Annual Report.
Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our management’s annual report on internal control over financial reporting is set forth below.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our system of internal control over financial reporting is designed to provide reasonable assurance to our management and the Board regarding the preparation and fair presentation of our consolidated financial statements for external purposes in accordance with generally accepted accounting principles.
Our management, under the supervision of our Executive Chairman and our Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of March 31, 2024. In making this assessment, we used the framework included in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the criteria set forth in Internal Control — Integrated Framework (2013), our management concluded that our internal control over financial reporting was effective as of March 31, 2024.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act) that occurred during the fourth quarter of the fiscal year ended March 31, 2024, to which this report relates that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Executive Chairman and our Chief Financial Officer, do not expect that our disclosure controls or our internal control over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Item 9B. Other Information.
During the fiscal quarter ended March 31, 2024, none of our directors or officers (as defined in Section 16 of the Securities Exchange Act of 1934, as amended) adopted or terminated any contract, instruction or written plan for the purchase or sale of our securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) or any “non-Rule 10b5-1 trading arrangement,” as defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information relating to our directors, executive officers and corporate governance, including our Code of Business Conduct, will be included in the proxy statement for the 2024 annual meeting of the Company’s stockholders, expected to be filed within 120 days of the end of our most recently completed fiscal year, which is incorporated herein by reference. The full text of our Code of Business Conduct, which is the code of ethics that applies to all of our officers, directors and employees, can be found in the “Investors” section of our website accessible to the public at www.organovo.com.
Item 11. Executive Compensation.
Information relating to executive compensation will be included in the proxy statement for the 2024 annual meeting of the Company’s stockholders, expected to be filed within 120 days of the end of our most recently completed fiscal year, which is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table summarizes information about the Company’s equity compensation plans by type as of March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(C) |
|
|
(A) |
|
|
|
|
Number of |
|
|
Number of |
|
|
|
|
securities available |
|
|
securities to be |
|
(B) |
|
|
for future issuance |
|
|
issued upon |
|
Weighted-average |
|
|
under Equity |
|
|
exercise/vesting |
|
exercise price |
|
|
Compensation Plans |
|
|
of outstanding |
|
of outstanding |
|
|
(excluding securities |
|
|
options, warrants, |
|
options, warrants, |
|
|
reflected in |
Plan category |
|
units and rights |
|
units and rights |
|
|
column (A)) |
Equity compensation plans approved by security holders (1) |
|
770,649 (2) |
|
$ |
4.38 |
|
|
1,686,250 (3) |
Equity compensation plans not approved by security holders (4) |
|
50,000 (5) |
|
$ |
2.75 |
|
|
1,000 (6) |
(1)Includes the 2012 Plan, the 2022 Plan, and the 2023 ESPP.
(2)Includes stock options to purchase 648,007 shares of common stock with a per share weighted-average exercise price of $6.62. Also includes 122,642 restricted stock units with no exercise price.
(3)Includes 45,000 shares of common stock available for purchase under the 2023 ESPP as of March 31, 2024.
(4)Includes the Inducement Plan.
(5)Includes 50,000 stock options with a per share exercise price of $2.75 granted pursuant to the Inducement Plan.
(6)Includes 1,000 shares of common stock reserved for issuance pursuant to the Inducement Plan.
Information relating to the beneficial ownership of our common stock will be included in the proxy statement for the 2024 annual meeting of the Company’s stockholders, expected to be filed within 120 days of the end of our most recently completed fiscal year, which is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information relating to certain relationships and related transactions and director independence will be included in the proxy statement for the 2024 annual meeting of the Company’s stockholders, expected to be filed within 120 days of the end of our most recently completed fiscal year, which is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
Information relating to principal accountant fees and services will be included in the proxy statement for the 2024 annual meeting of the Company’s stockholders, expected to be filed within 120 days of the end of our most recently completed fiscal year, which is incorporated herein by reference.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)The following documents have been filed as part of this Annual Report:
1.Consolidated Financial Statements: The information required by this item is included in Item 8 of Part II of this annual report.
2.Financial Statement Schedules: Financial statement schedules required under the related instructions are not applicable for the years ended March 31, 2024 and 2023 and have therefore been omitted.
3.Exhibits: The exhibits listed in the Exhibit Index attached to this report are filed or incorporated by reference as part of this annual report.
(b)The exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this Annual Report.
EXHIBIT INDEX
|
|
|
Exhibit No. |
|
Description |
|
|
|
|
|
|
3.1 |
|
Certificate of Incorporation of Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 3, 2012). |
|
|
|
3.2 |
|
Certificate of Amendment of Certificate of Incorporation of Organovo Holdings, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on July 27, 2018). |
|
|
|
3.3 |
|
Certificate of Second Amendment of Certificate of Incorporation of Organovo Holdings, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K as filed with the SEC on August 17, 2020). |
|
|
|
3.4 |
|
Amended and Restated Bylaws of Organovo Holdings, Inc. (incorporated by reference from Exhibit 3.4 to the Company's Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
4.1 |
|
Form of Common Warrant (incorporated by reference from Exhibit 4.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 13, 2024). |
|
|
|
4.2 |
|
Form of Pre-Funded Warrant (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 13, 2024). |
|
|
|
4.3* |
|
Description of Securities. |
|
|
|
10.1+ |
|
Organovo Holdings, Inc. Amended and Restated 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on October 6, 2021). |
|
|
|
10.2+ |
|
Form of Stock Option Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.16 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012). |
|
|
|
10.3+ |
|
Form of Non-Employee Director Stock Option Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.35 to the Company’s Annual Report on Form 10-K, as filed with the SEC on June 9, 2015). |
|
|
|
10.4+ |
|
Form of Executive Stock Option Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.36 to the Company’s Annual Report on Form 10-K, as filed with the SEC on June 9, 2015). |
|
|
|
10.5+ |
|
Form of Indemnification Agreement (incorporated by reference from Exhibit 10.17 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012). |
|
|
|
10.6† |
|
License Agreement dated as of March 24, 2009, by and between Organovo, Inc. and the Curators of the University of Missouri (incorporated by reference from Exhibit 10.23 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.7† |
|
License Agreement dated as of March 12, 2010 by and between the Company and the Curators of the University of Missouri (incorporated by reference from Exhibit 10.24 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.8† |
|
License Agreement dated as of May 2, 2011, by and between the Company and Clemson University Research Foundation (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.9+ |
|
Organovo Holdings, Inc. Severance and Change in Control Plan (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 9, 2015). |
|
|
|
10.10+ |
|
Amendment to the Organovo Holdings, Inc. Severance and Change in Control Plan, dated May 19, 2020 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 20, 2020). |
|
|
|
10.11+ |
|
Form of Organovo Holdings, Inc. Severance and Change in Control Plan Participation Agreement (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 9, 2015). |
|
|
|
Exhibit No. |
|
Description |
|
|
|
10.12+ |
|
Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement (Retention Form) under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.13+ |
|
Form of Employee Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.14+ |
|
Form of Non-Employee Director Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.15+ |
|
Consulting Agreement, dated September 15, 2020, by and between Organovo and Multi Dimensional Bio Insight LLC. (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.16+ |
|
Consulting Agreement, dated August 25, 2020, by and between Organovo and Danforth Advisors (incorporated by reference from Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.17+ |
|
Amendment No. 5 dated October 4, 2021 to Consulting Agreement dated August 25, 2021 by and between Company and Danforth Advisors LLC (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K, as filed with the SEC on October 6, 2021). |
|
|
|
10.18 |
|
Lease Agreement dated November 23, 2020, between Organovo Holdings, Inc. and San Diego Inspire 1, LLC (Permanent Lease Agreement 176640186.8) (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on November 25, 2020). |
|
|
|
10.19 |
|
Amended and Restated Lease Agreement dated November 23, 2020, between Organovo, Inc., as Tenant, and San Diego Inspire 2, LLC, as Landlord, as amended by First Amendment to Amended & Restated Lease, dated November 17, 2021, between San Diego Inspire 2, LLC, as Landlord, and Organovo, Inc., as Tenant (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on November 19, 2021). |
|
|
|
10.20 |
|
Intercompany Agreement, dated December 28, 2020, by and among Organovo Holdings, Inc., Organovo, Inc. and Viscient Biosciences, Inc. (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on December 31, 2020). |
|
|
|
10.21 |
|
Sales Agreement, dated March 16, 2018, by and among Organovo Holdings, Inc., H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC. (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2018). |
|
|
|
10.22+ |
|
Organovo Holdings, Inc. 2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 10, 2021). |
|
|
|
10.23+ |
|
Form of Stock Option Agreement under the Organovo Holdings, Inc. 2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 4.2 to the Company’s Registration Statement on Form S-8 (File No. 333-254714), as filed with the SEC on March 25, 2021). |
|
|
|
10.24+ |
|
Form of Restricted Stock Unit Agreement under the Organovo Holdings, Inc. 2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 4.3 to the Company’s Registration Statement on Form S-8 (File No. 333-254714), as filed with the SEC on March 25, 2021). |
|
|
|
10.25 |
|
Settlement and Patent License Agreement, dated February 22, 2022, by and between Organovo Holdings, Inc. and BICO Group AB (incorporated by reference to Exhibit 10.34 to the Company’s Annual Report on Form 10-K, as filed with the SEC on June 10, 2022). |
|
|
|
10.26+ |
|
Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on October 14, 2022) |
|
|
|
10.27+ |
|
Form of Global Stock Option Award Agreement under the Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 4.2 to the Company's Registration Statement on Form S-8 (No. 333-268001), filed with the SEC on October 25, 2022). |
|
|
|
10.28+ |
|
Form of Global Restricted Stock Unit Award Agreement under the Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 4.3 to the Company's Registration Statement on Form S-8 (No. 333-268001), filed with the SEC on October 25, 2022). |
|
|
|
|
|
|
Exhibit No. |
|
Description |
10.29 |
|
Purchase Agreement, dated March 10, 2023, by and between Organovo Holdings, Inc. and Metacrine, Inc. (incorporated by reference from Exhibit 10.38 to the Company’s Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
10.30 |
|
Separation Agreement and General Release, effective as of September 15, 2023, between Organovo Holdings, Inc. and Tom Jurgensen (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on September 12, 2023). |
|
|
|
10.31 |
|
Separation Agreement and General Release, effective as of September 27, 2023, between Organovo Holdings, Inc. and Jeffrey Miner (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on September 22, 2023). |
|
|
|
10.32+ |
|
Organovo Holdings, Inc. 2023 Employee Stock Purchase Plan (incorporated by reference from Exhibit 10.1 to the Company's Current Report on Form 8-K, as filed with the SEC on November 3, 2023). |
|
|
|
10.33 |
|
Placement Agency Agreement, dated May 8, 2024, between the Company and JonesTrading Institutional Services LLC (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 13, 2024). |
|
|
|
10.34^ |
|
Form of Securities Purchase Agreement, dated May 8, 2024 (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 13, 2024). |
|
|
|
21.1* |
|
Subsidiaries of Organovo Holdings, Inc. |
|
|
|
23.1* |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
23.2* |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
24.1* |
|
Power of Attorney (included on signature page hereto). |
|
|
|
31.1* |
|
Certification of Chief Executive Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. |
|
|
|
31.2* |
|
Certification of Chief Financial Officer a Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. |
|
|
|
32.1* |
|
Certifications Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and to 18 U.S.C. Section 1350. |
|
|
|
97* |
|
Organovo Holdings, Inc. Clawback Policy. |
|
|
|
101.INS* |
|
Inline XBRL Instance Document |
|
|
|
101.SCH* |
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
+ Designates management contracts and compensation plans.
† This Exhibit has been filed separately with the Secretary of the Securities and Exchange Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
# Certain identified information has been omitted pursuant to Item 601(b)(10) of Regulation S-K because such information is both (i) not material and (ii) would likely cause competitive harm to the Registrant if publicly disclosed. The Registrant hereby undertakes to furnish supplemental copies of the unredacted exhibit upon request by the Securities and Exchange Commission.
^ Non-material schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company hereby undertakes to furnish supplemental copies of any of the omitted schedules and exhibits upon request by the Securities and Exchange Commission.
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
ORGANOVO HOLDINGS, INC. |
|
|
|
|
By: |
/s/ Keith Murphy |
|
|
Keith Murphy |
|
|
Executive Chairman |
|
|
|
|
Date: |
May 31, 2024 |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Keith Murphy and Thomas Hess, and each of them individually, as the undersigned’s true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for the undersigned and in the undersigned’s name, place, and stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their respective substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
|
|
|
|
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Keith Murphy |
|
Executive Chairman |
|
May 31, 2024 |
Keith Murphy |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Thomas Hess |
|
Chief Financial Officer |
|
May 31, 2024 |
Thomas Hess |
|
(Principal Financial and Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Adam Stern |
|
Director |
|
May 31, 2024 |
Adam Stern |
|
|
|
|
|
|
|
|
|
/s/ Douglas Cohen |
|
Director |
|
May 31, 2024 |
Douglas Cohen |
|
|
|
|
|
|
|
|
|
/s/ David Gobel |
|
Director |
|
May 31, 2024 |
David Gobel |
|
|
|
|
|
|
|
|
|
/s/ Vaidehi Joshi |
|
Director |
|
May 31, 2024 |
Vaidehi Joshi |
|
|
|
|
|
|
|
|
|
/s/ Alison Milhous |
|
Director |
|
May 31, 2024 |
Alison Milhous |
|
|
|
|
|
|
|
|
|
DESCRIPTION OF ORGANOVO HOLDINGS, INC.’S SECURITIES
REGISTERED PURSUANT TO SECTION 12 OF THE
SECURITIES EXCHANGE ACT OF 1934
The following description of the common stock, par value $0.001 per share, of Organovo Holdings, Inc. (“us,” “our,” “we,” or the “Company”), which is the only security of the Company registered under Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), summarizes certain information regarding the common stock in our certificate of incorporation, as amended, our by-laws, as amended, and applicable provisions of Delaware general corporate law (the “DGCL”), and is qualified by reference to our certificate of incorporation, our certificate of amendment of certificate of incorporation, our second certificate of amendment of certificate of incorporation, and our amended and restated bylaws, which are incorporated by reference as Exhibit 3.1, 3.2, 3.3, and 3.4, respectively, to the Annual Report on Form 10-K for the fiscal year ending March 31, 2024.
Our authorized capital stock consists of 200,000,000 shares of common stock, par value $0.001 per share and 25,000,000 shares of preferred stock, par value $0.001 per share.
General
As of March 31, 2024, our certificate of incorporation, as amended (the “Certificate of Incorporation”), authorizes us to issue up to (i) 200,000,000 shares of common stock, par value $0.001 per share, and (ii) 25,000,000 shares of preferred stock, par value $0.001 per share.
On August 18, 2020, we effected a 1-for-20 reverse stock split of our outstanding common stock. As a result of the reverse stock split, every twenty (20) shares of our pre-reverse split common stock were combined and reclassified into one (1) share of common stock. The reverse stock split had no effect on the number of authorized shares of common or preferred stock, or on the stated par value per share of our common stock.
The following is a summary of the material provisions of the common stock and preferred stock provided for in our Certificate of Incorporation and amended and restated bylaws, as amended (the “Bylaws”). For additional detail about our capital stock, please refer to our Certificate of Incorporation and Bylaws.
Common Stock
Our common stock is listed on the Nasdaq Capital Market under the symbol “ONVO”.
Voting: Holders of our common stock are entitled to one vote for each share on all matters submitted to a stockholder vote, except matters that relate only to a series of our preferred stock.
The holders of common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of common stock that are present in person or represented by proxy. Except as otherwise provided by law, amendments to the Certificate of Incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of common stock. The Certificate of Incorporation does not provide for cumulative voting in the election of directors. The common stock holders will be entitled to such cash dividends as may be declared from time to time by our board of directors from funds available. Upon our liquidation, dissolution or winding up, the common stock holders will be entitled to receive pro rata all assets available for distribution to such holders.
Dividends: Subject to limitations under Delaware law and preferences that may apply to any then-outstanding shares of preferred stock, holders of common stock are entitled to share ratably in dividends, if any, as may be declared from time to time by our board of directors in its discretion from funds legally available therefor.
Dividends, if any, will be contingent upon our revenues and earnings, if any, and capital requirements and financial conditions. The payment of dividends, if any, will be within the discretion of our board of directors. We presently intend to retain all earnings, if any, and accordingly our board of directors does not anticipate declaring any dividends prior to a business combination.
Liquidation: In the event of a liquidation, dissolution or winding up, the holders of common stock are entitled to share pro rata all assets remaining after payment in full of all liabilities and after providing for each class of stock, if any, having preference over the common stock, subject to the liquidation preference of any then outstanding shares of preferred stock.
Miscellaneous: Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our Certificate of Incorporation, our board of directors is authorized to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock. There are no restrictions presently on the repurchase or redemption of any shares of our preferred stock.
The issuance of preferred stock will affect, and may adversely affect, the rights of holders of common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred stock. The effects of issuing preferred stock could include one or more of the following:
•restricting dividends on the common stock;
•diluting the voting power of the common stock;
•impairing the liquidation rights of the common stock; or
•delaying or preventing changes in control or management of our company.
We have no present plans to issue any shares of preferred stock nor are any shares of our preferred stock presently outstanding.
Warrants
As of May 31, 2024, the Company had outstanding warrants to purchase an aggregate of 10,183,500 shares of common stock as follows :
•warrants to purchase an aggregate of 3,621,000 shares of common stock with an exercise price of $0.001 per share, all of which are current exercisable (subject to certain beneficial ownership requirements); and
•warrants to purchase an aggregate of 6,562,500 shares of common stock with an exercise price of $0.80 per share, all of which are current exercisable (subject to certain beneficial ownership requirements) and expire on May 13, 2029.
All of the outstanding warrants contain provisions for the adjustment of the exercise price in the event of stock dividends, stock splits or similar transactions. In addition, certain of the warrants contain a “cashless exercise” feature that allows the holders thereof to exercise the warrants without a cash payment to the Company under certain circumstances. Certain of the warrants also contain provisions that provide certain rights to warrant holders in the event of a fundamental transaction, including a merger or consolidation with or into another entity, such as:
•the right to receive the same amount and kind of consideration paid to the holders of Common Stock in the fundamental transaction;
•the right to require the Company or a successor entity to purchase the unexercised portion of certain warrants at the warrant’s respective fair value using the Black Scholes option pricing formula; or
•the right to require the Company or a successor entity to redeem the unexercised portion of certain warrants for the same consideration paid to holders of Common Stock in the fundamental transaction at the warrant’s respective fair value using the Black Scholes option pricing formula.
Effect of Certain Provisions of our Certificate of Incorporation and Bylaws
Provisions of our Certificate of Incorporation and our Bylaws could have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, may have the effect of discouraging takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
Classified Board. Our Certificate of Incorporation and our Bylaws provide that our board of directors is divided into three classes, consisting of two Class I directors, two Class II directors and two Class III directors. The directors designated as Class I directors have a term expiring at our annual meeting of stockholders in 2024. The directors designated as Class II directors have a term expiring at our annual meeting of stockholders in 2025, and the directors designated as a Class III directors have a term expiring at our annual meeting of stockholders in 2026. Directors for each class will be elected at the annual meeting of stockholders held in the year in which the term for that class expires and thereafter will serve for a term of three years. At any meeting of stockholders for the election of directors at which a quorum is present, the election will be determined by a plurality of the votes cast by the stockholders entitled to vote at the election. Under the classified board provisions, it will take at least two elections of directors for any individual or group to gain control of our board. Accordingly, these provisions could discourage a third party from initiating a proxy contest, making a tender offer or otherwise attempting to gain control of us.
Undesignated preferred stock. The authority of our board of directors to issue preferred stock could potentially be used to discourage attempts by third parties to obtain control of our company through a merger, tender offer, proxy contest, or otherwise by making it more difficult or more costly to obtain control of our company. Our board of directors may issue preferred stock with voting rights or conversion rights that, if exercised, could adversely affect the voting power of the holders of common stock.
Advanced Notice Requirement. Stockholder nominations of individuals for election to our board of directors and stockholder proposals of other matters to be brought before an annual meeting of our stockholders must comply with the advance notice procedures set forth in our Bylaws. Generally, to be timely, such notice must be received at our principal executive offices no later than the date specified in our proxy statement released to stockholders in connection with the preceding year’s annual meeting of stockholders, which date shall be not earlier than the 75th day, nor later than the close of business on the 45th day, prior to the one-year anniversary of the date on which we first mailed our proxy materials or a notice of availability of proxy materials (whichever is earlier) for the preceding year’s annual meeting and the stockholder must have complied in all respects with the requirements of Section 14 of the 1934 Act, including, without limitation, the requirements of Rule 14a-19 under the 1934 Act.
Special Meeting Requirements. Our Bylaws provide that special meetings of our stockholders may only be called at the request of a majority of the authorized number of members of the board of directors, chairperson of the board of directors, chief executive officer, president or secretary. Only such business shall be considered at a special meeting as shall have been stated in the notice for such meeting.
No Stockholder Action by Written Consent Except with Prior Board Approval: Our Certificate of Incorporation and Bylaws provide that no action shall be taken by our stockholders except at an annual or special meeting of the stockholders called in accordance with the Bylaws, and no action shall be taken by our stockholders
by written consent, except if the action to be effected by written consent and the taking of such action by written action is approved in advance by resolution of the board of directors.
No Cumulative Voting. Our Certificate of Incorporation does not include a provision for cumulative voting for directors.
Removal of Directors. Our Certificate of Incorporation and Bylaws provide that the holders of our voting stock may only remove our directors for cause.
Authorized but Unissued Shares. Our authorized but unissued shares of common stock and preferred stock will be available for future issuance without stockholder approval. We may use additional shares for a variety of purposes, including future public offerings to raise additional capital, to fund acquisitions and as employee compensation. The existence of authorized but unissued shares of common stock and preferred stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Size of Board and Vacancies. Our Bylaws provide that the number of directors on our board of directors is fixed exclusively by our board of directors. Vacancies and newly created directorships resulting from any increase in our authorized number of directors will be filled by a majority of our board of directors then in office, although less than a quorum, or by a sole remaining director.
Indemnification. Our Certificate of Incorporation and our Bylaws provide that we will indemnify our officers and directors against losses as they incur in investigations and legal proceedings resulting from their services to us, which may include service in connection with takeover defense measures.
Delaware Anti-Takeover Statute
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware regulating corporate takeovers. In general, Section 203 generally prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years following the date on which the person became an interested stockholder unless:
•prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
•upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
•at or subsequent to the date of the transaction, the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder.
In general, Section 203 defines business combination to include the following:
•any merger or consolidation involving the corporation and the interested stockholder;
•any sale, lease, exchange, mortgage, transfer, pledge or other disposition of 10% or more of either the assets or outstanding stock of the corporation involving the interested stockholder;
•subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
•any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or
•the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation.
In general, Section 203 defines interested stockholder as an entity or person who, together with affiliates and associates, beneficially owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The provisions of Delaware law and our Certificate of Incorporation and our Bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in our management. It is possible that these provisions may make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Exhibit 21.1
Subsidiaries of Organovo Holdings, Inc.
I.Organovo, Inc., a Delaware corporation
II.Opal Merger Sub, Inc., a Delaware corporation
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in Registration Statement Nos. 333-275845, 333-268001, 333-260910, 333-254714, 333-226839, 333-213345, 333-209395, 333-192248 and 333-181324 on Form S-8 and Registration Statement No. 333-276722 on Form S-3, of our report dated July 13, 2023, with respect to the consolidated financial statements of Organovo Holdings, Inc. as of March 31, 2023 and for the year then ended (which report includes an explanatory paragraph regarding the existence of substantial doubt about the Company's ability to continue as a going concern), included in this annual report on Form 10-K for the year ended March 31, 2024.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
May 31, 2024
Exhibit 23.2
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in Registration Statements on Form S-8 (No. 333-275845), (No. 333-268001), (No. 333-254714), (No. 333-226839), (No. 333-213345), (No. 333-209395), (No. 333-192248), and (No. 333-181324), and Form S-3 (No. 333-276722) of our report dated May 31, 2024, relating to the consolidated financial statements of Organovo Holdings, Inc. as of March 31, 2024 and for the year then ended, included in this Annual Report on Form 10-K for the year ended March 31, 2024.
/s/ Rosenberg Rich Baker Berman P.A.
Somerset, New Jersey
May 31, 2024
Exhibit 31.1
CERTIFICATION PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Keith Murphy, certify that:
1. I have reviewed this annual report on Form 10-K of Organovo Holdings, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
Dated: May 31, 2024 |
|
/s/ Keith Murphy |
|
|
Keith Murphy |
|
|
Executive Chairman (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Thomas Hess, certify that:
1. I have reviewed this annual report on Form 10-K of Organovo Holdings, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
Dated: May 31, 2024 |
|
/s/ Thomas Hess |
|
|
Thomas Hess |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report on Form 10-K of Organovo Holdings, Inc. (the “Company”) for the year ended March 31, 2024, as filed with the Securities and Exchange Commission (the “Report”), Keith Murphy and Thomas Hess, do hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
•The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
•The information in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: May 31, 2024
|
/s/ Keith Murphy |
Keith Murphy |
Executive Chairman (Principal Executive Officer) |
|
/s/ Thomas Hess |
Thomas Hess |
Chief Financial Officer (Principal Financial Officer) |
A signed original of this written statement required by Section 906 has been provided to Organovo Holdings, Inc. and will be retained by Organovo Holdings, Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission, and is not to be incorporated by reference into any filing of Organovo Holdings, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
Exhibit 97
Organovo Holdings, Inc.
Clawback Policy
The Board of Directors (the “Board”) of Organovo Holdings, Inc. (the “Company”) believes that it is in the best interests of the Company and its stockholders to adopt this Clawback Policy (this “Policy”), which provides for the recovery of certain incentive compensation in the event of an Accounting Restatement (as defined below). This Policy is designed to comply with, and shall be interpreted to be consistent with, Section 10D of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10D-1 promulgated under the Exchange Act (“Rule 10D-1”) and Nasdaq Listing Rule 5608 (the “Listing Standards”).
Unless otherwise determined by the Board, the Compensation Committee of the Board (or another committee of the Board) shall administer this Policy (the Board or such committee charged with administration of this Policy, the “Administrator”). Unless otherwise determined by the Board, the Compensation Committee of the Board (the “Compensation Committee”) shall be the Administrator. The Administrator is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate or advisable for the administration of this Policy. Any determinations made by the Administrator shall be final and binding on all affected individuals and need not be uniform with respect to each individual covered by this Policy. In the administration of this Policy, the Administrator is authorized and directed to consult with the full Board or such other committees of the Board, as may be necessary or appropriate as to matters within the scope of such other committee’s responsibility and authority. Subject to any limitation at applicable law, the Administrator may authorize and empower any officer or employee of the Company to take any and all actions necessary or appropriate to carry out the purpose and intent of this Policy (other than with respect to any recovery under this Policy involving such officer or employee).
As used in this Policy, the following definitions shall apply:
•“Accounting Restatement” means an accounting restatement of the Company’s financial statements due to the Company’s material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
•“Administrator” has the meaning set forth in Section 1 hereof.
•“Applicable Period” means the three completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement, as well as any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years (except that a transition period that comprises a period of at least nine months shall count as a completed fiscal year). The “date on which the Company is required to prepare an Accounting Restatement” is the earlier to occur of (a) the date the Board, a committee of the Board or the officer or officers of the Company authorized to take such action if Board action is not required, concludes or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement or (b) the date a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement, in each case regardless of if or when the restated financial statements are filed.
•“Code” means the U.S. Internal Revenue Code of 1986, as amended. Any reference to a section of the Code or regulation thereunder includes such section or regulation, any valid regulation or other
official guidance promulgated under such section, and any comparable any future legislation or regulation amending, supplementing, or superseding such section or regulation.
•“Compensation Committee” has the meaning set forth in Section 1 hereof.
•“Covered Executives” means the Company’s current and former executive officers, as determined by the Administrator in accordance with the definition of executive officer set forth in Rule 10D-1 and the Listing Standards; provided that executive officers for purposes of this Policy shall include at a minimum executive officers identified pursuant to 17 C.F.R. 229.401(b).
•“Effective Date” has the meaning set forth in Section 9 hereof.
•“Erroneously Awarded Compensation” has the meaning set forth in Section 5 hereof.
•A “Financial Reporting Measure” is any measure that is determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measure that is derived wholly or in part from such measure. Financial Reporting Measures include but are not limited to the following (and any measures derived from the following): Company stock price; total shareholder return (“TSR”); revenues; net income; operating income; profitability of one or more reportable segments; financial ratios (e.g., accounts receivable turnover and inventory turnover rates); earnings before interest, taxes, depreciation and amortization; funds from operations and adjusted funds from operations; liquidity measures (e.g., working capital, operating cash flow); return measures (e.g., return on invested capital, return on assets); earnings measures (e.g., earnings per share); sales per square foot or same store sales, where sales is subject to an Accounting Restatement; revenue per user, or average revenue per user, where revenue is subject to an Accounting Restatement; cost per employee, where cost is subject to an Accounting Restatement; any of such financial reporting measures relative to a peer group, where the Company’s financial reporting measure is subject to an Accounting Restatement; and tax basis income. A Financial Reporting Measure need not be presented within the Company’s financial statements or included in a filing with the Securities and Exchange Commission.
•“Incentive-Based Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure. Incentive-Based Compensation is “received” for purposes of this Policy in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-Based Compensation award is attained, even if the payment or grant of such Incentive-Based Compensation occurs after the end of that period.
•“Nasdaq” has the meaning set forth in Section 5 hereof.
3.Covered Executives; Incentive-Based Compensation
This Policy applies to Incentive-Based Compensation received by a Covered Executive (a) after beginning services as a Covered Executive; (b) if that person served as a Covered Executive at any time during the performance period for such Incentive-Based Compensation; and (c) while the Company had a listed class of securities on a national securities exchange.
4.Required Recoupment of Erroneously Awarded Compensation in the Event of an Accounting Restatement
In the event the Company is required to prepare an Accounting Restatement, the Company shall promptly demand in writing and recoup the amount of any Erroneously Awarded Compensation received by any Covered Executive, as calculated pursuant to Section 5 hereof, during the Applicable Period. Recovery under this Policy with respect to a Covered Executive shall not require the finding of any misconduct by such Covered Executive or such Covered Executive being found responsible for the accounting error leading to an Accounting Restatement. If a Covered Executive fails to repay Erroneously Awarded Compensation that is owed to the Company under this Policy, the Company shall take all appropriate action to recover such Erroneously Awarded
Compensation from the Covered Executive, and the Covered Executive shall be required to reimburse the Company for all expenses (including legal expenses) incurred by the Company in recovering such Erroneously Awarded Compensation.
5.Erroneously Awarded Compensation: Amount Subject to Recovery
The amount of “Erroneously Awarded Compensation” subject to recovery under this Policy, as determined by the Administrator, is the amount of Incentive-Based Compensation received by the Covered Executive that exceeds the amount of Incentive-Based Compensation that otherwise would have been received by the Covered Executive had it been determined based on the restated amounts.
Erroneously Awarded Compensation shall be computed by the Administrator without regard to any taxes paid by the Covered Executive in respect of the Erroneously Awarded Compensation.
For Incentive-Based Compensation based on stock price or TSR, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculations directly from the information in the Accounting Restatement: (a) the Administrator shall determine the amount of Erroneously Awarded Compensation based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or TSR upon which the Incentive-Based Compensation was received; and (b) the Company shall maintain documentation of the determination of that reasonable estimate and provide such documentation to The Nasdaq Stock Market LLC (“Nasdaq”).
The Administrator shall determine, in its sole discretion, the timing and method for promptly recouping Erroneously Awarded Compensation hereunder, which may include without limitation (a) seeking reimbursement of all or part of any cash or equity-based award, (b) cancelling prior cash or equity-based awards, whether vested or unvested or paid or unpaid, (c) cancelling or offsetting against any planned future cash or equity-based awards, (d) forfeiture of deferred compensation, subject to compliance with Section 409A of the Code and the regulations promulgated thereunder and (e) any other method authorized by applicable law or contract. Subject to compliance with any applicable law, the Administrator may effect recovery under this Policy from any amount otherwise payable to the Covered Executive, including amounts payable to such individual under any otherwise applicable Company plan or program, including base salary, bonuses or commissions and compensation previously deferred by the Covered Executive.
The Company is authorized and directed pursuant to this Policy to recoup Erroneously Awarded Compensation in compliance with this Policy unless the Compensation Committee has determined that recovery would be impracticable solely for the following limited reasons, and subject to the following procedural and disclosure requirements:
•The direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered. Before concluding that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on expense of enforcement, the Administrator must make a reasonable attempt to recover such erroneously awarded compensation, document such reasonable attempt(s) to recover and provide that documentation to Nasdaq;
•Recovery would violate home country law of the issuer where that law was adopted prior to November 28, 2022. Before concluding that it would be impracticable to recover any amount of Erroneously Awarded Compensation based on violation of home country law of the issuer, the Administrator must satisfy the applicable opinion and disclosure requirements of Rule 10D-1 and the Listing Standards; or
•Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder.
7.No Indemnification of Covered Executives
Notwithstanding the terms of any indemnification or insurance policy or any contractual arrangement with any Covered Executive that may be interpreted to the contrary, the Company shall not indemnify any Covered
Executives against (a) the loss of any Erroneously Awarded Compensation, including any payment or reimbursement for the cost of third-party insurance purchased by any Covered Executives to fund potential clawback obligations under this Policy, or (b) any claims relating to the Company’s enforcement of its rights under this Policy.
8.Administrator Indemnification
Any members of the Administrator, and any other members of the Board who assist in the administration of this Policy, shall not be personally liable for any action, determination or interpretation made with respect to this Policy and shall be fully indemnified by the Company to the fullest extent under applicable law and Company policy with respect to any such action, determination or interpretation. The foregoing sentence shall not limit any other rights to indemnification of the members of the Board under applicable law or Company policy.
9.Effective Date; Retroactive Application
This Policy shall be effective as of October 2, 2023 (the “Effective Date”). The terms of this Policy shall apply to any Incentive-Based Compensation that is received by Covered Executives on or after the Effective Date, even if such Incentive-Based Compensation was approved, awarded, granted or paid to Covered Executives prior to the Effective Date. Without limiting the generality of Section 6 hereof, and subject to applicable law, the Administrator may effect recovery under this Policy from any amount of compensation approved, awarded, granted, payable or paid to the Covered Executive prior to, on or after the Effective Date.
10.Amendment; Termination
The Board or the Compensation Committee may amend, modify, supplement, rescind or replace all or any portion of this Policy at any time and from time to time in its discretion, and shall amend this Policy as it deems necessary to comply with applicable law or any rules or standards adopted by a national securities exchange on which the Company’s securities are listed. Notwithstanding anything in this Section 10 to the contrary, no amendment or other modification of this Policy shall be effective if such amendment or other modification would (after taking into account any actions taken by the Company contemporaneously with such amendment or other modification) cause the Company to violate any federal securities laws, Securities and Exchange Commission rule or the rules of any national securities exchange or national securities association on which the Company’s securities are listed.
11.Other Recoupment Rights; Company Claims
The Board intends that this Policy shall be applied to the fullest extent of the law. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company under applicable law or pursuant to the terms of any similar policy in any employment agreement, equity award agreement or similar agreement and any other legal remedies available to the Company. This Policy is also in addition to (and not in lieu of) any right of repayment, forfeiture or right of offset against any employees that is required pursuant to any statutory repayment requirement (regardless of whether implemented at any time prior to or following the adoption or amendment of this Policy), including Section 304 of the Sarbanes-Oxley Act of 2002. Any amounts paid to the Company pursuant to Section 304 of the Sarbanes-Oxley Act of 2002 shall be considered in determining any amounts recovered under this Policy. The Compensation Committee may require that any employment agreement, equity award agreement or any other agreement entered into on or after the Effective Date shall, as a condition to the grant of any benefit thereunder, require a Covered Executive to agree to abide by the terms of this Policy. The application and enforcement of this Policy does not preclude the Company from taking any other action to enforce a Covered Executive’s obligations to the Company, including termination of employment or institution of legal proceedings.
Nothing contained in this Policy, and no recoupment or recovery as contemplated by this Policy, shall limit any claims, damages or other legal remedies the Company or any of its affiliates may have against a Covered Executive (including reimbursement of legal fees incurred by or on behalf of the Company or any of its affiliates) arising out of or resulting from any actions or omissions by the Covered Executive.
This Policy shall be binding and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives.
13.Exhibit Filing Requirement
A copy of this Policy and any amendments thereto shall be posted on the Company’s website and filed as an exhibit to the Company’s annual report on Form 10-K.
This Policy and all rights and obligations hereunder are governed by and construed in accordance with the internal laws of the State of [Delaware], excluding any choice of law rules or principles that may direct the application of the laws of another jurisdiction. All actions arising out of or relating to this Policy shall be heard and determined exclusively in the Court of Chancery of the State of Delaware or, if such court declines to exercise jurisdiction or if subject matter jurisdiction over the matter that is the subject of any such legal action or proceeding is vested exclusively in the U.S. federal courts, the U.S. District Court for the District of Delaware.
If any provision of this Policy or the application of such provision to any Covered Executive shall be adjudicated to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provisions of this Policy, and the invalid, illegal or unenforceable provisions shall be deemed amended to the minimum extent necessary to render any such provision (or the application of such provision) valid, legal or enforceable.
Clawback Policy Acknowledgment
I, the undersigned, agree and acknowledge that I am fully bound by, and subject to, all of the terms and conditions of the Organovo Holdings, Inc.’s Clawback Policy (as may be amended, restated, supplemented or otherwise modified from time to time, the “Policy”). In the event of any inconsistency between the Policy and the terms of any employment agreement to which I am a party, or the terms of any compensation plan, program or agreement under which any compensation has been granted, awarded, earned or paid, the terms of the Policy shall govern. The Policy will apply both during and after the undersigned’s employment with the Company. In the event it is determined by the Administrator that any amounts granted, awarded, earned or paid to me must be forfeited or reimbursed to the Company, I will promptly take any action necessary to effectuate such forfeiture and/or reimbursement. Any capitalized terms used in this Acknowledgment without definition shall have the meaning set forth in the Policy.
|
|
By: ___________________________________
[Name]
[Title]
|
_____________________________________
Date
|
v3.24.1.1.u2
Document and Entity Information - USD ($)
|
12 Months Ended |
|
|
Mar. 31, 2024 |
May 25, 2024 |
Sep. 30, 2023 |
| Cover [Abstract] |
|
|
|
| Document Type |
10-K
|
|
|
| Amendment Flag |
false
|
|
|
| Document Period End Date |
Mar. 31, 2024
|
|
|
| Document Fiscal Year Focus |
2024
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| Trading Symbol |
ONVO
|
|
|
| Entity Registrant Name |
ORGANOVO HOLDINGS, INC.
|
|
|
| Entity Central Index Key |
0001497253
|
|
|
| Current Fiscal Year End Date |
--03-31
|
|
|
| Entity Well-known Seasoned Issuer |
No
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
| Entity Small Business |
true
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Common Stock, Shares Outstanding |
|
14,371,826
|
|
| Entity Public Float |
|
|
$ 10,340,301
|
| Entity File Number |
001-35996
|
|
|
| Entity Tax Identification Number |
27-1488943
|
|
|
| Entity Address, Address Line One |
11555 Sorrento Valley Rd
|
|
|
| Entity Address, Address Line Two |
Suite 100
|
|
|
| Entity Address, City or Town |
San Diego
|
|
|
| Entity Address, State or Province |
CA
|
|
|
| Entity Address, Postal Zip Code |
92121
|
|
|
| City Area Code |
858
|
|
|
| Local Phone Number |
224-1000
|
|
|
| Document Annual Report |
true
|
|
|
| Document Transition Report |
false
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
|
| Security Exchange Name |
NASDAQ
|
|
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
|
|
| Auditor Name |
Rosenberg Rich Baker Berman, P.A.
|
|
|
| Auditor Firm ID |
89
|
|
|
| Auditor Location |
Somerset, NJ
|
|
|
| Documents Incorporated by Reference |
DOCUMENTS INCORPORATED BY REFERENCE Certain information required for Part III of this report is incorporated herein by reference to the definitive proxy statement for the 2024 annual meeting of the registrant’s stockholders, expected to be filed within 120 days of the end of the registrant’s fiscal year.
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDocuments incorporated by reference. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-23
| Name: |
dei_DocumentsIncorporatedByReferenceTextBlock |
| Namespace Prefix: |
dei_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Consolidated Balance Sheets - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Current Assets |
|
|
| Cash and cash equivalents |
$ 2,901
|
$ 15,301
|
| Accounts receivable |
33
|
152
|
| Inventory |
297
|
0
|
| Investment in equity securities |
0
|
706
|
| Prepaid expenses and other current assets |
705
|
889
|
| Total current assets |
3,936
|
17,048
|
| Fixed assets, net |
669
|
902
|
| Restricted cash |
143
|
143
|
| Operating lease right-of-use assets |
1,299
|
1,705
|
| Prepaid expenses and other assets, net |
302
|
515
|
| Total assets |
6,349
|
20,313
|
| Current Liabilities |
|
|
| Accounts payable |
627
|
331
|
| Accrued expenses |
727
|
2,848
|
| Operating lease liability, current portion |
506
|
492
|
| Total current liabilities |
1,860
|
3,671
|
| Operating lease liability, net of current portion |
888
|
1,313
|
| Total liabilities |
2,748
|
4,984
|
| Commitments and Contingencies (Notes 8 and 9) |
|
|
| Stockholders' Equity |
|
|
| Common stock, $0.001 par value; 200,000,000 shares authorized, 10,077,726 and 8,716,906 shares issued and outstanding at March 31, 2024 and 2023, respectively |
10
|
9
|
| Additional paid-in capital |
343,261
|
340,317
|
| Accumulated deficit |
(339,669)
|
(324,998)
|
| Accumulated other comprehensive income |
0
|
2
|
| Treasury stock, 46 shares at cost |
(1)
|
(1)
|
| Total stockholders' equity |
3,601
|
15,329
|
| Total Liabilities and Stockholders' Equity |
$ 6,349
|
$ 20,313
|
| X |
- DefinitionEquity securities current.
| Name: |
onvo_EquitySecuritiesCurrent |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed after one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount allocated to previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481520/505-30-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TreasuryStockCommonValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Mar. 31, 2024 |
Mar. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Common stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares authorized |
200,000,000
|
200,000,000
|
| Common stock, shares issued |
10,077,726
|
8,716,906
|
| Common stock, shares outstanding |
10,077,726
|
8,716,906
|
| Treasury stock, shares |
46
|
46
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of previously issued common shares repurchased by the issuing entity and held in treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockCommonShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.1.1.u2
Consolidated Statements of Operations and Other Comprehensive Loss - USD ($)
$ in Thousands |
12 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Revenues |
|
|
| Total Revenues |
$ 109
|
$ 370
|
| Revenue From Contract With Customer Product And Service [Extensible List] |
Royalty [Member]
|
Royalty [Member]
|
| Research and development expenses |
$ 5,498
|
$ 8,885
|
| Selling, general, and administrative expenses |
9,697
|
9,216
|
| Total costs and expenses |
15,195
|
18,101
|
| Loss from Operations |
(15,086)
|
(17,731)
|
| Other Income (Expense) |
|
|
| Loss on fixed asset disposals |
0
|
(9)
|
| Gain on investment in equity securities |
12
|
29
|
| Interest income |
405
|
454
|
| Total Other Income |
417
|
474
|
| Income Tax Expense |
(2)
|
(2)
|
| Net Loss |
(14,671)
|
(17,259)
|
| Other comprehensive income: |
|
|
| Unrealized gain (loss) on available-for-sale debt securities |
(2)
|
2
|
| Comprehensive Loss |
$ (14,673)
|
$ (17,257)
|
| Net loss per common share basic |
$ (1.6)
|
$ (1.98)
|
| Net loss per common share diluted |
$ (1.6)
|
$ (1.98)
|
| Weighted average shares used in computing net loss per common share basic |
9,144,922
|
8,713,032
|
| Weighted average shares used in computing net loss per common share diluted |
9,144,922
|
8,713,032
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionTotal costs of sales and operating expenses for the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DisaggregationOfRevenueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized and realized gain (loss) on investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 321
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479536/321-10-50-4
| Name: |
us-gaap_EquitySecuritiesFvNiGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, excluding oil and gas property and timber property. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-3
| Name: |
us-gaap_GainLossOnDispositionOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income on nonoperating securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPeriodIncreaseDecreaseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after tax and before adjustment, of unrealized holding gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Excludes unrealized gain (loss) on investment in debt security measured at amortized cost (held-to-maturity) from transfer to available-for-sale. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-9
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
| Name: |
us-gaap_OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates product and service for revenue from satisfaction of performance obligation by transferring promised product and service to customer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479777/606-10-55-91
| Name: |
us-gaap_RevenueFromContractWithCustomerProductAndServiceExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Consolidated Statements of Stockholders' Equity - USD ($)
shares in Thousands, $ in Thousands |
Total |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Treasury Stock [Member] |
Accumulated Deficit [Member] |
Accumulated Other Comprehensive Income [Member] |
| Beginning balance at Mar. 31, 2022 |
$ 30,209
|
$ 9
|
$ 337,940
|
$ (1)
|
$ (307,739)
|
$ 0
|
| Beginning balance, Shares at Mar. 31, 2022 |
|
8,711
|
|
0
|
|
|
| Issuance of common stock under employee and director stock option, RSU and purchase plans |
0
|
$ 0
|
0
|
$ 0
|
0
|
0
|
| Issuance of common stock under employee and director stock option, RSU and purchase plans, Shares |
|
6
|
|
0
|
|
|
| Stock-based compensation expense |
2,377
|
$ 0
|
2,377
|
$ 0
|
0
|
0
|
| Stock-based compensation expense, Shares |
|
0
|
|
|
|
|
| Net loss |
(17,259)
|
$ 0
|
0
|
0
|
(17,259)
|
0
|
| Unrealized loss on available-for-sale debt securities, net of tax |
2
|
$ 0
|
0
|
0
|
0
|
2
|
| Unrealized loss on available-for-sale debt securities, net of tax, Shares |
|
0
|
|
|
|
|
| Ending balance at Mar. 31, 2023 |
15,329
|
$ 9
|
340,317
|
$ (1)
|
(324,998)
|
2
|
| Ending balance, Shares at Mar. 31, 2023 |
|
8,717
|
|
0
|
|
|
| Issuance of common stock under employee and director stock option, RSU and purchase plans |
0
|
$ 0
|
0
|
$ 0
|
0
|
0
|
| Issuance of common stock under employee and director stock option, RSU and purchase plans, Shares |
|
122
|
|
0
|
|
|
| Stock-based compensation expense |
1,508
|
$ 0
|
1,508
|
$ 0
|
0
|
0
|
| Stock-based compensation expense, Shares |
|
66
|
|
0
|
|
|
| Issuance of common stock from public offering, net |
1,437
|
$ 1
|
1,436
|
$ 0
|
0
|
0
|
| Issuance of common stock from public offering, Shares |
|
1,173
|
|
0
|
|
|
| Net loss |
(14,671)
|
$ 0
|
0
|
$ 0
|
(14,671)
|
0
|
| Unrealized loss on available-for-sale debt securities, net of tax |
(2)
|
$ 0
|
0
|
0
|
0
|
(2)
|
| Unrealized loss on available-for-sale debt securities, net of tax, Shares |
|
0
|
|
|
|
|
| Ending balance at Mar. 31, 2024 |
$ 3,601
|
$ 10
|
$ 343,261
|
$ (1)
|
$ (339,669)
|
$ 0
|
| Ending balance, Shares at Mar. 31, 2024 |
|
10,078
|
|
0
|
|
|
| X |
- DefinitionAdjustments To Additional Paid In Capital Share based Compensation Requisite Service Period Recognition Share
| Name: |
onvo_AdjustmentsToAdditionalPaidInCapitalShareBasedCompensationRequisiteServicePeriodRecognitionShare |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period shares public offering.
| Name: |
onvo_StockIssuedDuringPeriodSharesPublicOffering |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period shares under employee and director stock option RSU and purchase plans.
| Name: |
onvo_StockIssuedDuringPeriodSharesUnderEmployeeAndDirectorStockOptionRSUAndPurchasePlans |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value public offering.
| Name: |
onvo_StockIssuedDuringPeriodValuePublicOffering |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value under employee and director stock option RSU and purchase plans.
| Name: |
onvo_StockIssuedDuringPeriodValueUnderEmployeeAndDirectorStockOptionRSUAndPurchasePlans |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionUnrealized gain (loss) on shares available-for-sale securities
| Name: |
onvo_UnrealizedGainLossOnSharesAvailableForSaleSecurities |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Consolidated Statements of Cash Flows - USD ($)
$ in Thousands |
12 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Cash Flows From Operating Activities |
|
|
| Net loss |
$ (14,671)
|
$ (17,259)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Gain on investment in equity securities |
(12)
|
(29)
|
| Loss on disposal of fixed assets |
0
|
9
|
| Accretion on investments |
(142)
|
(105)
|
| Depreciation and amortization |
280
|
293
|
| Stock-based compensation |
1,508
|
2,377
|
| Increase (decrease) in cash resulting from changes in: |
|
|
| Accounts receivable |
119
|
(152)
|
| Inventory |
(297)
|
0
|
| Prepaid expenses and other assets |
392
|
177
|
| Accounts payable |
296
|
(148)
|
| Accrued expenses |
(2,121)
|
2,359
|
| Operating right-of-use asset and lease liability, net |
(5)
|
70
|
| Net cash used in operating activities |
(14,653)
|
(12,408)
|
| Cash Flows From Investing Activities |
|
|
| Purchases of fixed assets |
(42)
|
(396)
|
| Purchases of investments |
(10,860)
|
(9,893)
|
| Maturities of investments |
11,000
|
10,000
|
| Purchases of equity securities |
0
|
(1,061)
|
| Proceeds from sales of equity securities |
0
|
384
|
| Liquidation of equity securities |
718
|
0
|
| Net cash provided by (used in) investing activities |
816
|
(966)
|
| Cash Flows From Financing Activities |
|
|
| Proceeds from issuance of common stock, net |
1,437
|
0
|
| Net cash provided by financing activities |
1,437
|
0
|
| Net Decrease in Cash, Cash Equivalents, and Restricted Cash |
(12,400)
|
(13,374)
|
| Cash, cash equivalents, and restricted cash at beginning of period |
15,444
|
28,818
|
| Cash, cash equivalents, and restricted cash at end of period |
3,044
|
15,444
|
| Reconciliation of cash, cash equivalents, and restricted cash to the consolidated balance sheets |
|
|
| Cash and cash equivalents |
2,901
|
15,301
|
| Restricted cash |
143
|
143
|
| Cash, cash equivalents, and restricted cash at end of period |
3,044
|
15,444
|
| Supplemental Disclosure of Cash Flow Information: |
|
|
| Income taxes paid |
2
|
2
|
| Purchases of fixed assets in accounts payable |
$ 0
|
$ 64
|
| X |
- DefinitionAccretion on Investments.
| Name: |
onvo_AccretionOnInvestments |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease decrease in operating lease right-of-use assets and liabilities, net.
| Name: |
onvo_IncreaseDecreaseInOperatingLeaseRightOfUseAssetsAndLiabilitiesNet |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLiquidation of equity securities.
| Name: |
onvo_LiquidationOfEquitySecurities |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPayments to acquire investment.
| Name: |
onvo_PaymentsToAcquireInvestment |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from maturities prepayments and calls of investments.
| Name: |
onvo_ProceedsFromMaturitiesPrepaymentsAndCallsOfInvestments |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized and realized gain (loss) on investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 321
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479536/321-10-50-4
| Name: |
us-gaap_EquitySecuritiesFvNiGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, including oil and gas property and timber property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnSaleOfPropertyPlantEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_PaymentsToAcquireEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_ProceedsFromSaleOfEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Description of Business and Summary of Significant Accounting Policies
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Description of Business and Summary of Significant Accounting Policies |
Note 1. Description of Business and Summary of Significant Accounting Policies Nature of operations and basis of presentation Organovo Holdings, Inc. (“Organovo Holdings,” “Organovo,” and the “Company”) is a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in the Company's established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC. The Company's current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024. The Company released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis (“MASH”) in April 2024 that is supportive of ongoing development, and the Company believes FXR314 has a commercial opportunity in MASH, most likely in combination therapy. The Company is exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and the Company's IBD disease models. The Company's second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. The Company believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas. As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development. The Company's current understanding of intestinal tissue models and IBD disease models leads the Company to believe that it can create models that provide greater insight into the biology of these diseases than are generally currently available. The Company is creating high fidelity disease models, leveraging its prior work including the work found in its peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) The Company's advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures. Using these disease models, the Company intends to identify and validate novel therapeutic targets. After finding therapeutic drug targets, the Company intends to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials. The Company expects to broaden its work into additional therapeutic areas over time and is currently exploring specific tissues for development. In its work to identify the areas of interest, the Company evaluates areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, the Company is building upon both its external and in house scientific expertise, which will be essential to its drug development effort. In February 2024, the Company formed its Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain primary human cells that the Company utilizes in its research and development efforts. The Company believes Mosaic can help optimize its supply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials the Company uses are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. The Company intends for Mosaic to provide it with qualified human cells for use in its clinical research and development programs. In addition to supplying the Company with primary human cells, the Company intends for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which the Company expects to offset costs and over time become a profit center that offsets overall research and development ("R&D") spending by the Company. Liquidity and Going Concern The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of March 31, 2024, the Company had cash and cash equivalents of approximately $2.9 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $339.7 million. The restricted cash was pledged as collateral for a letter of credit that the Company is required to maintain as a security deposit under the terms of the lease agreements for its facilities. The Company also had negative cash flows from operations of approximately $14.7 million during the year ended March 31, 2024. Through March 31, 2024, the Company has financed its operations primarily through the sale of common stock through public and at-the-market (“ATM”) offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research-based services, grants, and collaborative research agreements, and from the sale of convertible notes. During the year ended March 31, 2024, the Company issued 1,172,342 shares of its common stock through its ATM facility. Based on the Company's current operating plan and available cash resources, including the total net proceeds of $4.7 million from the best efforts public offering that closed in May 2024, the Company will need substantial additional funding to support future operating activities. The Company has concluded that the prevailing conditions and ongoing liquidity risks faced by the Company raise substantial doubt about its ability to continue as a going concern for at least one year following the date these consolidated financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern. As the Company continues its operations and is focusing its efforts on drug discovery and development, the Company will need to raise additional capital to implement this business plan. The Company cannot predict with certainty the exact amount or timing for any future capital raises. The Company will seek to raise additional capital through debt or equity financings, or through some other financing arrangement. However, the Company cannot be sure that additional financing will be available if and when needed, or that, if available, it can obtain financing on terms favorable to its stockholders. Any failure to obtain financing when required will have a material adverse effect on the Company’s business, operating results, and financial condition. Use of estimates The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions. Investments Investments consist of investments in debt securities and investments in equity securities. Investments in debt securities consist of investments in U.S. Treasury bills. As of March 31, 2024 and 2023, all investments that have original maturities of three months or less are classified as cash equivalents on the Consolidated Balance Sheets. As of March 31, 2024, all investments are classified as available-for-sale, as the sale of such investments may be required prior to maturity to implement management strategies. Available-for-sale debt securities are recorded at fair value. Any unrealized gains and losses are included in accumulated other comprehensive income as a component of stockholders' equity until realized. As U.S. Treasury bills are risk-free, any declines in fair value are considered temporary. Investments in equity securities consist of investments in the common stock of entities traded in active markets. The Company does not have the ability to exercise significant influence over any entities. Therefore, initial investments are recorded at cost, and are remeasured at fair value as of the balance sheet date. Any gains or losses resulting from the change in fair value are recorded in net income. The investments in equity securities are classified as current assets. Fair value measurement Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value: •Level 1 — Quoted prices in active markets for identical assets or liabilities. •Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. •Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Financial instruments For certain of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other assets, accounts payable, accrued expenses, the carrying amounts are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. Cash and cash equivalents The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. Restricted cash As of each of March 31, 2024 and 2023, the Company had approximately $0.1 million of restricted cash, deposited with a financial institution. The entire amount was held in certificates of deposit to support a letter of credit agreement related to the Company’s facility leases entered into in November 2020 and amended in November 2021. Inventory Inventories are stated at the lower of cost or net realizable value. Inventory as of March 31, 2024 consisted of approximately $0.3 million in finished goods, which is related to the Mosaic Cell Sciences division. There were no finished goods inventory as of March 31, 2023. Please refer to "Note 16. Business Segment Information" for further information. Fixed assets and depreciation Fixed assets are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the remaining lease term. The estimated useful lives of the fixed assets range between one and seven years. Impairment of long-lived assets In accordance with authoritative guidance, the Company reviews its long-lived assets, including fixed assets and other assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred as of March 31, 2024 and 2023. Research and development Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Research and development costs are expensed as incurred. Acquired in-process research and development The Company has acquired drug candidates in development. The costs to acquire a drug candidate are immediately expensed as acquired in-process research and development, provided that the drug candidate has no alternative future use. Acquired in-process research and development expenses are included in total research and development expenses on the Consolidated Statements of Operations and Other Comprehensive Loss. FXR Program In March 2023, the Company acquired Metacrine's FXR program for $4.0 million. The FXR program was determined to have no alternative future use, and therefore was considered acquired in-process research and development and fully expensed. In the year ended March 31, 2023, the Company paid a $2.0 million upfront payment, and the remaining $2.0 million was paid in the year ended March 31, 2024, upon the final transfer of the drug compounds, related data, and IP. Segment Reporting Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance. As of March 31, 2024, the Company identified two operating segments. Please refer to "Note 16. Business Segment Information" for further information. Income taxes Deferred income taxes are recognized for the tax consequences in future years for differences between the tax basis of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the combination of the tax payable for the year and the change during the year in deferred tax assets and liabilities. The Company’s policy regarding uncertainty in income taxes is pursuant to Accounting Standard Codification (“ASC”) Topic 740-10. Interest and penalties that would be assessed in relation to the settlement value of unrecognized tax benefits is recognized as a component of income tax expense. Revenue recognition The Company has generated revenues from payments received from licensing intellectual property. The Company has entered into a license agreement with a company that includes the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales, if any. At the initiation of the agreement, the Company has analyzed whether it results in a contract with a customer under Topic 606. The Company has considered a variety of factors in determining the appropriate estimates and assumptions under these arrangements, such as whether the Company is a principal vs. agent, whether the elements are distinct performance obligations, whether there are determinable stand-alone prices, and whether any licenses are functional or symbolic. The Company has evaluated each performance obligation to determine if it can be satisfied and recognized as revenue at a point in time or over time. Typically, non-refundable upfront fees have been considered fixed, while sales-based royalty payments have been identified as variable consideration which must be evaluated to determine if it has been constrained and, therefore, excluded from the transaction price. Please refer to “Note 6. Collaborative Research, Development, and License Agreements” for further information. Stock-based compensation The Company accounts for stock-based compensation in accordance with the ASC Topic 718, Compensation — Stock Compensation, which establishes accounting for equity instruments exchanged for employee and non-employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair value of the award (determined using either the Black-Scholes or Monte Carlo option-pricing models, depending on the complexity of the equity grant), and is recognized as an expense, under the straight-line method, over the employee or non-employee's requisite service period (generally the vesting period of the equity grant). The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses its Company-specific historical volatility rate. The risk-free interest rate assumption is based on U.S. Treasury rates. The weighted average expected life of options is estimated using the average of the contractual term and the weighted average vesting term of the options. Option forfeitures are treated as a reduction of stock-based compensation expense and accounted for as they occur. Comprehensive income (loss) Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the consolidated financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at comprehensive income (loss). Net loss per share Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. The weighted-average number of shares used to compute diluted loss per share excludes any assumed exercise of stock options and warrants, shares reserved for purchase under the Company’s 2023 Employee Stock Purchase Plan (“2023 ESPP”), the assumed release of restriction of restricted stock units (“RSUs”), and shares subject to repurchase as the effect would be anti-dilutive. No dilutive effect was calculated for the years ended March 31, 2024 and 2023 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive. Common stock equivalents excluded from computing diluted net loss per share were approximately 0.8 million shares and 1.6 million shares for the years ended March 31, 2024 and 2023, respectively. |
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the general note to the financial statements for the reporting entity which may include, descriptions of the basis of presentation, business description, significant accounting policies, consolidations, reclassifications, new pronouncements not yet adopted and changes in accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 250
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//250/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationBasisOfPresentationBusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Investments and Fair Value Measurement
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Investments, All Other Investments [Abstract] |
|
| Investments and Fair Value Measurement |
Note 2. Investments and fair value measurement Investments in debt securities As of March 31, 2024, the Company held $1.0 million of investments in debt securities (which are included in the $2.9 million of cash and cash equivalents). For the year ended March 31, 2024, there was $0.1 million of interest income related to the investments in debt securities. There were less than $0.1 million of unrealized gains recorded on investments in debt securities for the year ended March 31, 2024. As the investments in debt securities consist of U.S. Treasury bills from active markets, the fair value is measured using level 1 inputs. The following table summarizes the Company's investments in debt securities that are measured at fair value as of March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortized costs basis |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair value |
|
As of March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
997 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
998 |
|
As of March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
4,943 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
4,945 |
|
Investments in equity securities For the year ended March 31, 2024, $0.7 million of equity securities were liquidated and there was less than a $0.1 million gain on the investment in equity securities. As of March 31, 2024, the fair value of investment in equity securities was zero, as a result of the liquidation of the shares by the underlying company on June 26, 2023. As the investments in equity securities consist of common stock from active markets, the fair value is measured using level 1 inputs. The following table presents the activity for investments in equity securities measured at fair value for the year ended March 31, 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Investment in Equity Securities
(in thousands) |
|
Balance at March 31, 2023 |
|
$ |
706 |
|
Sales |
|
|
(718 |
) |
Gain on investment in equity securities |
|
|
12 |
|
Balance at March 31, 2024 |
|
$ |
— |
|
|
| X |
- DefinitionThe entire disclosure for investment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//320/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//321/tableOfContent
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Topic 325
-Publisher FASB
-URI https://asc.fasb.org//325/tableOfContent
| Name: |
us-gaap_InvestmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsAllOtherInvestmentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionPrepaid expenses and other current assets.
| Name: |
onvo_PrepaidExpensesAndOtherCurrentAssetsTextBlock |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Fixed Assets
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Property, Plant and Equipment [Abstract] |
|
| Fixed Assets |
Note 4. Fixed Assets Fixed assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Laboratory equipment |
|
$ |
1,617 |
|
|
$ |
1,575 |
|
Furniture and fixtures |
|
|
66 |
|
|
|
66 |
|
Computer software and equipment |
|
|
244 |
|
|
|
537 |
|
Fixed Assets, gross |
|
|
1,927 |
|
|
|
2,178 |
|
Less accumulated depreciation |
|
|
(1,258 |
) |
|
|
(1,276 |
) |
Fixed Assets, net |
|
$ |
669 |
|
|
$ |
902 |
|
As of March 31, 2024 and 2023, all of the Company’s fixed assets were active and in use. Depreciation expense for the years ended March 31, 2024 and 2023 was approximately $0.3 million and $0.2 million, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Accrued Expenses
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Accrued Expenses |
Note 5. Accrued Expenses Accrued expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Accrued compensation |
|
$ |
536 |
|
|
$ |
609 |
|
Accrued legal and professional fees |
|
|
93 |
|
|
|
193 |
|
Acquired in-process research and development |
|
|
— |
|
|
|
2,000 |
|
Other accrued expenses |
|
|
98 |
|
|
|
46 |
|
|
|
$ |
727 |
|
|
$ |
2,848 |
|
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a),20,24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Collaborative Research, Development, and License Agreements
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Collaborative Research, Development, and License Agreements |
Note 6. Collaborative Research, Development, and License Agreements License Agreements BICO Group AB From June 2021 to February 2022, certain patents owned or sublicensed by the Company became the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”). The Company and BICO Group AB were also engaged in litigation regarding patent infringement during the same time period. On February 22, 2022, the Company and BICO Group AB signed a settlement and patent license agreement (“License Agreement”) to close all matters noted above. In addition to closing all legal matters and patent disputes noted above, as part of the agreement, the Company agreed to grant a non-exclusive license to BICO Group AB to use the Company’s aforementioned patents for its business operations of manufacturing and selling bioprinters as well as bioinks. The Company concluded that the nature of the license granted represents functional intellectual property. As part of the License Agreement, BICO Group AB agreed to pay the Company a one time, nonrefundable upfront fee of $1,500,000. Based on ASC Topic 606, the Company concluded that the performance obligation related to this upfront fee consisted of the Company filing stipulations of dismissal of all legal matters noted above, as well as the Company granting the non-exclusive license of the aforementioned patents within five days of receiving the upfront payment. The conditions of the performance obligation were satisfied, and therefore the Company recognized revenue of $1,500,000 on February 22, 2022, the executed date of the License Agreement. Additionally, as part of the License Agreement, BICO Group AB agreed to pay the Company ongoing sales-based royalties (based on percentages of BICO Group AB’s net sales) for the use of the granted license. The sales-based royalties became effective beginning on February 22, 2022, the effective date of the License Agreement, and continue until the expiration of the last surviving licensed patent. As the sales-based royalties are required to be paid 45 days after the end of every quarter, there is variable consideration that must be estimated to determine royalty revenue within a given reporting period. Once actual revenue earned is determined in the following fiscal quarter, an adjustment is made from the previously estimated amount. For the years ended March 31, 2024 and 2023, the Company recorded $109,000 and $370,000, respectively, of royalty revenue based on sales-based royalties from the License Agreement. Also as part of the License Agreement, certain patents involved in the agreement are sublicensed by the Company from the University of Missouri and Clemson University. See below for further information. University of Missouri In March 2009, the Company entered into a license agreement with the Curators of the University of Missouri to in-license certain technology and intellectual property relating to self-assembling cell aggregates and to intermediate cellular units. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay the University of Missouri royalties ranging from 1% to 3% of net sales of covered tissue products, and of the fair market value of covered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales achieved by the Company each year. On December 5, 2022, the Company amended the license agreement with the University of Missouri, whereby the Company agreed to pay a single, upfront payment of $50,000 to the University of Missouri in exchange for the aforementioned licensed intellectual property to be fully paid up by the Company. As a result, the Company will continue to have rights to the licensed intellectual property until its expiration in June 2028, but will no longer owe minimum annual royalty payments, royalty payments based on net sales, or any other payments (other than patent annuities and any prosecution costs) in the future. Clemson University In May 2011, the Company entered into a license agreement with Clemson University Research Foundation ("CURF") to in-license certain technology and intellectual property relating to ink-jet printing of viable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company was required to pay the university royalties ranging from 1.5% to 3% of net sales of covered tissue products and the fair market value of covered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales reached each year. The license agreement terminates upon expiration of the patents licensed, which are expected to expire in May 2024, and is subject to certain conditions as defined in the license agreement. Minimum annual royalty payments of $40,000 per year were due beginning in calendar 2016. The royalty payments of $40,000 were made for the years ended March 31, 2024 and 2023. The annual minimum royalty is creditable against royalties owed during the same calendar year. In addition to the annual royalties noted above, CURF is owed 40% of all payments including but not limited to, upfront payments, license fees, issue fees, maintenance fees, and milestone payments received from third parties, including sublicensees, in consideration for sublicensing rights to licensed products. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. As of March 31, 2024, the Company’s legal expenses exceeded royalties owed from the upfront payment and sales-based royalties related to the license agreement. Therefore, no royalty expense to CURF was recorded for the year ended March 31, 2024. No royalty expense related to sales-based royalties has been recorded to date Capitalized License Fees Capitalized license fees consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
License fees |
|
$ |
109 |
|
|
$ |
114 |
|
Less accumulated amortization |
|
|
(101 |
) |
|
|
(101 |
) |
License fees, net |
|
$ |
8 |
|
|
$ |
13 |
|
The above license fees, net of accumulated amortization, are included in prepaid expenses and other assets in the accompanying consolidated balance sheets and are being amortized over the life of the related patents. Amortization expense of licenses was approximately $5,000 and $82,000 for the years ended March 31, 2024 and 2023, respectively. At March 31, 2024, the weighted average remaining amortization period for all licenses was approximately 3 years. The annual amortization expense of licenses for the next five years is estimated to be approximately $3,000 per year. The Salk Institute for Biological Studies In March 2023, the Company acquired the FXR Agonist program from Metacrine. All patent rights related to this program have been assigned to the Company in connection with the acquisition. In addition, the Company assumed and was assigned a license agreement with the Salk Institute for Biological Studies (hereafter “Salk”) that provides certain payments to Salk upon the successful development and commercialization of the lead compound, FXR314. The Company is required to pay Salk royalties ranging from 1% to 1.125% of net sales of therapeutics based on FXR314. In addition, the Company is required to make certain milestone payments based on the successful initiation and/or completion of certain development milestones, including $500,000 within 45 days of the dosing of the first patient in a phase III clinical trial, $1,000,000 within 45 days of FDA approval of the first Licensed Product and $1,500,000 within 45 days of the first commercial sale of a Licensed Product in the Territory. There are also reduced milestone payments application to a second or third licensed product, if any. Should the Company sublicense a licensed product to a third party, then it must pay a 3.5% of sublicensing revenue attributable to such a sublicense.
|
| X |
- DefinitionThe entire disclosure for collaborative arrangements in which the entity is a participant, including a) information about the nature and purpose of such arrangements; b) its rights and obligations thereunder; c) the accounting policy for collaborative arrangements; and d) the income statement classification and amounts attributable to transactions arising from the collaborative arrangement between participants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//808/tableOfContent
| Name: |
us-gaap_CollaborativeArrangementDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders' Equity
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Equity [Abstract] |
|
| Stockholders' Equity |
Note 7. Stockholders’ Equity Preferred stock The Company is authorized to issue 25,000,000 shares of preferred stock. There are no shares of preferred stock currently outstanding, and the Company has no present plans to issue shares of preferred stock. Common stock In January 2012, the Company's Board of Directors ("Board") approved the 2012 Amended and Restated Equity Incentive Plan ("2012 Plan"). The 2012 Plan initially authorized the issuance of up to 327,699 shares of common stock for awards of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards, and the number of shares issuable pursuant thereto was increased several times to an aggregate of 2,327,699 shares. In March 2021, the Board approved the 2021 Inducement Equity Incentive Plan ("Inducement Plan"). The Inducement Plan authorized the issuance of up to 750,000 shares of common stock for awards of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards. In February 2022, 50,000 incentive stock options were issued under the Inducement Plan. On October 12, 2022, the Company's stockholders and the Board approved the 2022 Equity Incentive Plan ("2022 Plan"), and it became effective on that date. The 2022 Plan replaced the 2012 Plan on the effective date. Upon the effective date, the Company ceased granting awards under the 2012 Plan and any shares remaining available for future issuance under the 2012 Plan were cancelled and are no longer available for future issuance. The 2012 Plan continues to govern awards previously granted under it. At the time the Board approved the 2022 Plan, an aggregate of 1,363,000 shares of the Company’s common stock was initially reserved for issuance under the 2022 Plan. The Company committed to reducing the new 2022 Plan share reserve by the number of shares that were granted under the 2012 Plan and the Inducement Plan between July 25, 2022 and October 12, 2022. From July 25, 2022 to October 12, 2022, the Company issued 126,262 shares of its common stock under the 2012 Plan. As a result, the number of shares reserved for future issuance under the 2022 Plan is 1,236,738 shares of common stock. The Company also committed to reducing the aggregate number of shares of its common stock issuable pursuant to the Inducement Plan from 750,000 shares to 51,000 shares (which includes 50,000 shares of its common stock issuable pursuant to an outstanding option to purchase common stock with an exercise price of $2.75 per share, leaving only 1,000 shares available for future issuance under the Inducement Plan) and the share reserve was reduced effective October 12, 2022. The Company previously had an effective shelf registration statement on Form S-3 (File No. 333-252224), declared effective by the SEC on January 29, 2021 (the “2021 Shelf”), which registered $150.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing, that expired on January 29, 2024. On January 26, 2024, the Company filed a new shelf registration statement on Form S-3 (File No. 333-276722) to register $150.0 million of the Company's common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the "2024 Shelf"). The 2024 Shelf was declared effective by the SEC on February 8, 2024 and replaced the 2021 Shelf at that time. On March 16, 2018, the Company entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, the Company filed a prospectus supplement to the 2021 Shelf (the “2021 ATM Prospectus Supplement”), pursuant to which the Company may offer and sell, from time to time through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold were issued pursuant to the 2021 Shelf until it was replaced by the 2024 Shelf. On January 26, 2024, the Company filed a prospectus to the 2024 Shelf (the "2024 ATM Prospectus"), pursuant to which the Company may offer and sell, from time to time through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $2,605,728. Any shares offered and sold in these ATM transactions will be issued pursuant to the 2024 Shelf. During the year ended March 31, 2024, the Company issued 1,172,342 shares of common stock in ATM offerings, of which 1,135,940 shares were sold pursuant to the 2021 Shelf, and 36,402 shares were sold pursuant to the 2024 Shelf. As of March 31, 2024, the Company has sold an aggregate of 2,753,204 shares of common stock in ATM offerings under the 2021 ATM Prospectus Supplement and 2024 ATM Prospectus, with gross proceeds of approximately $23.2 million. As of March 31, 2024, there was approximately $100.0 million available for future offerings under the 2024 Shelf, and approximately $2.6 million available for future offerings through the Company’s ATM program under the 2024 ATM Prospectus. Restricted stock units The following table summarizes the Company’s RSUs activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
|
|
Weighted
Average Price |
|
Unvested at March 31, 2023 |
|
|
127,717 |
|
|
$ |
2.22 |
|
Granted |
|
|
117,642 |
|
|
$ |
1.39 |
|
Vested |
|
|
(122,717 |
) |
|
$ |
1.90 |
|
Cancelled / forfeited |
|
|
— |
|
|
$ |
— |
|
Unvested at March 31, 2024 |
|
|
122,642 |
|
|
$ |
1.75 |
|
Stock options During the year ended March 31, 2024, under the 2022 Plan, 184,158 stock options were granted at various exercise prices. On August 28, 2023, the Company's Executive Chairman voluntarily forfeited 462,500 outstanding stock options, of which 312,918 were unvested and therefore cancelled, and 149,582 were vested and therefore treated as expired. The forfeited stock option awards were not replaced by other awards or other compensation and there is no plan to replace the forfeited awards. Therefore, all previous unrecognized compensation expense associated with the forfeited awards, approximately $519,000, was recognized as a selling, general, and administrative expense on the date of forfeiture. The following table summarizes stock option activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options
Outstanding |
|
|
Weighted-
Average
Exercise Price |
|
|
Aggregate
Intrinsic
Value |
|
Outstanding at March 31, 2023 |
|
|
1,451,217 |
|
|
$ |
6.49 |
|
|
$ |
38,327 |
|
Options granted |
|
|
184,158 |
|
|
$ |
1.67 |
|
|
$ |
— |
|
Options canceled |
|
|
(937,368 |
) |
|
$ |
7.20 |
|
|
$ |
— |
|
Options exercised |
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
Outstanding at March 31, 2024 |
|
|
698,007 |
|
|
$ |
4.26 |
|
|
$ |
— |
|
Vested and Exercisable at March 31, 2024 |
|
|
401,536 |
|
|
$ |
5.61 |
|
|
$ |
— |
|
The weighted-average remaining contractual term of stock options exercisable and outstanding at March 31, 2024 was approximately 7.38 years. During the years ended March 31, 2024 and 2023, the Company issued zero shares of common stock upon exercise of stock options. Employee Stock Purchase Plan In June 2016, the Board, and subsequently in August 2016, the Company's stockholders approved, the 2016 ESPP. The Company reserved 75,000 shares of common stock for issuance thereunder. As of October 31, 2023, the 2016 ESPP was replaced by the 2023 ESPP (as defined below). In July 2023, the Board adopted, and subsequently on October 31, 2023, the Company's stockholders approved, the 2023 Employee Stock Purchase Plan (the “2023 ESPP”). The 2023 ESPP became effective on October 31, 2023 and replaced the 2016 ESPP on that date. The Company reserved 45,000 shares of common stock for issuance thereunder. The 2023 ESPP permits employees to purchase common stock through payroll deductions, limited to 15 percent of each employee’s compensation up to $25,000 per employee per year or 500 shares per employee per six-month purchase period. Shares under the 2023 ESPP are purchased at 85 percent of the fair market value at the lower of (i) the closing price on the first trading day of the six-month purchase period or (ii) the closing price on the last trading day of the six-month purchase period. The initial offering under the 2023 ESPP commenced on March 1, 2024. Common stock reserved for future issuance Common stock reserved for future issuance consisted of the following at March 31, 2024:
|
|
|
|
|
Common stock issuable pursuant to options outstanding and reserved under the 2012 Plan |
|
|
431,416 |
|
Common stock reserved under the 2012 Plan |
|
|
— |
|
Common stock issuable pursuant to options outstanding and reserved under the 2022 Plan |
|
|
216,591 |
|
Common stock reserved under the 2022 Plan |
|
|
1,641,250 |
|
Common stock reserved under the ESPP |
|
|
45,000 |
|
Common stock reserved under the 2021 Inducement Equity Plan |
|
|
1,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2012 Plan |
|
|
5,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2022 Plan |
|
|
117,642 |
|
Common stock issuable pursuant to options outstanding and reserved under the Inducement Plan |
|
|
50,000 |
|
Total at March 31, 2024 |
|
|
2,507,899 |
|
Stock-based compensation expense and valuation information Stock-based awards include stock options and RSUs under the Company's 2022 Plan, 2012 Plan, inducement awards, performance-based RSUs under an Incentive Award Performance-Based Restricted Stock Unit Agreement, the Inducement Plan, and rights to purchase stock under the 2023 ESPP. Stock-based compensation expense for all stock-based awards consists of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Research and development |
|
$ |
138 |
|
|
$ |
473 |
|
General and administrative |
|
|
1,370 |
|
|
|
1,904 |
|
Total |
|
$ |
1,508 |
|
|
$ |
2,377 |
|
The total unrecognized compensation cost related to unvested stock option grants as of March 31, 2024 was approximately $485,000 and the weighted average period over which these grants are expected to vest is 2.17 years. The total unrecognized stock-based compensation cost related to unvested RSUs as of March 31, 2024 was approximately $151,000 which will be recognized over a weighted average period of 0.73 years. As of March 31, 2024, there was one participant enrolled into the 2023 ESPP for the current purchase period, beginning March 1, 2024. The fair value of stock options was estimated at the grant date using the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
99.04 |
% |
|
|
95.53 |
% |
Risk-free interest rate |
|
|
4.11 |
% |
|
|
3.32 |
% |
Expected life of options |
|
6 years |
|
|
6 years |
|
Weighted average grant date fair value |
|
$ |
1.34 |
|
|
$ |
1.83 |
|
The fair value of each RSU is recognized as stock-based compensation expense over the vesting term of the award. The fair value is based on the closing stock price on the date of the grant. The Company uses the Black-Scholes valuation model to calculate the fair value of shares issued pursuant to the 2016 ESPP and the 2023 ESPP. Stock-based compensation expense is recognized over the purchase period using the straight-line method. The fair value of ESPP shares was estimated at the purchase period commencement date using the following assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
95.20 |
% |
|
|
86.58 |
% |
Risk-free interest rate |
|
|
5.27 |
% |
|
|
3.34 |
% |
Expected term |
|
6 months |
|
|
6 months |
|
Grant date fair value |
|
$ |
0.39 |
|
|
$ |
0.82 |
|
The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses the Company-specific historical volatility rate as the indicator of expected volatility. The risk-free interest rate assumption was based on U.S. Treasury rates. The expected life is the 6-month purchase period.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for shareholders' equity and share-based payment arrangement. Includes, but is not limited to, disclosure of policy and terms of share-based payment arrangement, deferred compensation arrangement, and employee stock purchase plan (ESPP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
| Name: |
us-gaap_ShareholdersEquityAndShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Leases
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Leases [Abstract] |
|
| Leases |
Note 8. Leases After the initial adoption of ASC Topic 842, on an on-going basis, the Company evaluates all contracts upon inception and determines whether the contract contains a lease by assessing whether there is an identified asset and whether the contract conveys the right to control the use of identified asset in exchange for consideration over a period of time. If a lease is identified, the Company will apply the guidance from ASC Topic 842 to properly account for the lease. Operating Leases On November 23, 2020, the Company entered into a lease agreement, pursuant to which the Company permanently leased approximately 8,051 square feet of office space (the “Permanent Lease”) in San Diego once certain tenant improvements were completed by the landlord and the premises were ready for occupancy. Additionally, on November 17, 2021, the Permanent Lease was amended to add an additional 2,892 square feet of office space in the same building. The Permanent Lease commenced on December 17, 2021 and is intended to serve as the Company’s permanent premises for approximately sixty-two months. Monthly rental payments are approximately $40,800 with 3% annual escalators. The Company determined that the Permanent Lease is considered an operating lease under ASC Topic 842, and therefore upon the lease commencement date of December 17, 2021, recognized lease liabilities and corresponding right-of-use assets of $2.3 million. The Company records operating lease expense on a straight-line basis over the life of the lease (referred to as “operating lease expense”). Variable lease expenses associated with the Company’s leases, such as payments for additional monthly fees to cover the Company’s share of certain facility expenses (common area maintenance) are expensed as incurred. The table below summarizes the Company’s lease liabilities and corresponding right-of-use assets as of March 31, 2024 (in thousands):
|
|
|
|
|
|
|
March 31, 2024 |
|
ASSETS |
|
|
|
Operating lease right-of-use assets |
|
$ |
1,299 |
|
Total lease right-of-use assets |
|
$ |
1,299 |
|
|
|
|
|
LIABILITIES |
|
|
|
Current |
|
|
|
Operating lease liability |
|
$ |
506 |
|
Noncurrent |
|
|
|
Operating lease liability, net of current portion |
|
$ |
888 |
|
Total lease liabilities |
|
$ |
1,394 |
|
|
|
|
|
Weighted average remaining lease term: |
|
2.83 |
|
Weighted average discount rate: |
|
|
6 |
% |
Variable lease expense was approximately $153,000 and $146,000 for the years ended March 31, 2024 and 2023, respectively. Operating lease expense was approximately $503,000 and $499,000 for the years ended March 31, 2024 and 2023, respectively. Cash outflows associated with the Company’s operating lease for the years ended March 31, 2024 and 2023 were $509,000 and $430,000, respectively. Future lease payments relating to the Company’s operating lease liabilities as of March, 31, 2024 are as follows (in thousands):
|
|
|
|
|
Fiscal year ending March 31, 2025 |
|
|
523 |
|
Fiscal year ending March 31, 2026 |
|
|
538 |
|
Fiscal year ending March 31, 2027 |
|
|
460 |
|
Total future lease payments |
|
|
1,521 |
|
Less: Imputed Interest |
|
|
(127 |
) |
Total lease obligations |
|
|
1,394 |
|
Less: Current obligations |
|
|
(506 |
) |
Noncurrent lease obligations |
|
$ |
888 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Commitments and Contingencies
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Note 9. Commitments and Contingencies Legal matters In addition to commitments and obligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business. The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its consolidated financial statements. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not meaningful indicators of its potential liability. The Company regularly reviews contingencies to determine the adequacy of its accruals and related disclosures. During the period presented, the Company has not recorded any accrual for loss contingencies associated with any claims or legal proceedings; determined that an unfavorable outcome is probable or reasonably possible; or determined that the amount or range of any possible loss is reasonably estimable. However, the outcome of legal proceedings and claims brought against the Company is subject to significant uncertainty. Therefore, although management considers the likelihood of such an outcome to be remote, if one or more legal matters were resolved against the Company in a reporting period, the Company’s consolidated financial statements for that reporting period could be materially adversely affected.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant arrangements with third parties, which includes operating lease arrangements and arrangements in which the entity has agreed to expend funds to procure goods or services, or has agreed to commit resources to supply goods or services, and operating lease arrangements. Descriptions may include identification of the specific goods and services, period of time covered, minimum quantities and amounts, and cancellation rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Income Taxes
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Note 10. Income Taxes A reconciliation of the statutory federal rate and the effective rate, for operations, is as follows for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
|
|
March 31,
2023 |
|
|
|
Tax computed at federal statutory rate |
$ |
(3,081 |
) |
|
21 |
% |
|
$ |
(3,624 |
) |
21% |
|
State income tax, net of federal benefit |
|
(110 |
) |
|
0.7 |
% |
|
|
(44 |
) |
0.2% |
|
Stock-based compensation |
|
721 |
|
|
-4.9 |
% |
|
|
167 |
|
-1% |
|
Research credits |
|
— |
|
|
0 |
% |
|
|
60 |
|
-0.4% |
|
Change in tax rate |
|
(62 |
) |
|
0.4 |
% |
|
|
157 |
|
-0.9% |
|
Removal of net operating losses and research development credits |
|
1,910 |
|
|
-13 |
% |
|
|
1,410 |
|
-8.2% |
|
Uncertain tax positions |
|
111 |
|
|
-0.8 |
% |
|
|
— |
|
|
0 |
% |
Other |
|
(16 |
) |
|
0.2 |
% |
|
|
1 |
|
0% |
|
Valuation allowance |
|
527 |
|
|
-3.6 |
% |
|
|
1,873 |
|
-10.7% |
|
Provision (benefit) for income taxes |
$ |
— |
|
0.0% |
|
|
$ |
— |
|
0.0% |
|
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows as of March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Deferred tax assets: |
|
|
|
|
|
Amortization |
$ |
593 |
|
|
$ |
598 |
|
Section 174 R&D capitalization |
|
1,793 |
|
|
|
855 |
|
Accrued expenses and reserves |
|
105 |
|
|
|
116 |
|
Operating lease liability |
|
307 |
|
|
|
384 |
|
Stock-based compensation |
|
315 |
|
|
|
755 |
|
Inventory |
|
259 |
|
|
|
251 |
|
Other, net |
|
4 |
|
|
|
3 |
|
Total deferred tax assets |
|
3,376 |
|
|
|
2,962 |
|
Valuation allowance |
|
(2,983 |
) |
|
|
(2,458 |
) |
Net deferred tax assets |
$ |
393 |
|
|
$ |
504 |
|
Deferred tax liabilities: |
|
|
|
|
|
Operating lease right-of-use assets |
|
(286 |
) |
|
|
(363 |
) |
Depreciation |
|
(107 |
) |
|
|
(135 |
) |
Investment in equity securities |
|
— |
|
|
|
(6 |
) |
Total deferred tax liabilities |
$ |
(393 |
) |
|
$ |
(504 |
) |
|
$ |
— |
|
|
$ |
— |
|
A full valuation allowance has been established to offset the deferred tax assets as management cannot conclude that realization of such assets is more likely than not. Under the Internal Revenue Code (“IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research tax credit carryforwards to offset taxable income may be limited based on cumulative changes in ownership. The Company has not completed an analysis to determine whether any such limitations have been triggered as of March 31, 2024. Until this analysis is completed, the Company has removed the deferred tax assets related to net operating losses from its deferred tax asset schedule. Further, until a study is completed and any limitation known, approximately $1.7 million and $1.6 million for the years ended March 31, 2024 and 2023, respectively, would be considered as an uncertain tax position if netted against the deferred tax asset. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. Any carryforwards that will expire prior to utilization as a result of such limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. The valuation allowance increased by approximately $525,000 and approximately $1,875,000 for the years ended March 31, 2024 and 2023, respectively. The Company had federal and state net operating loss carryforwards of approximately $219.4 million and $42.8 million, respectively, as of March 31, 2024. Federal net operating loss carryforwards of approximately $75.8 million will carryforward indefinitely and be available to offset up to 80% of future taxable income each year subject to revisions made by the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The remaining federal net operating losses will begin to expire in 2028, unless previously utilized. The state net operating loss carryforwards (“NOLs”) will begin to expire in 2028, unless previously utilized. The Company had federal and state research tax credit carryforwards of approximately $5.0 million and $4.5 million at March 31, 2024, respectively. The federal research tax credit carryforwards begin expiring in 2028. The state research tax credit carryforwards do not expire. The Company did not record any accruals for income tax accounting uncertainties for the year ended March 31, 2024. The Company did not accrue either interest or penalties from inception through March 31, 2024. The Company does not expect its unrecognized tax benefits to significantly increase or decrease within the next 12 months. The Company is subject to tax in the United States and in California. As of March 31, 2024, the Company’s tax years from inception are subject to examination by the tax authorities due to the generation of net operating losses. The Company is not currently under examination by any jurisdiction.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Concentrations
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Risks and Uncertainties [Abstract] |
|
| Concentrations |
Note 11. Concentrations Credit risk and significant customers Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions located within the United States. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. Balances may exceed federally insured limits. The Company is also potentially subject to concentrations of credit risk in its revenues and accounts receivable. However, the Company only receives royalty revenue from one licensee and has not historically experienced any accounts receivable write-downs.
|
| X |
- DefinitionThe entire disclosure for any concentrations existing at the date of the financial statements that make an entity vulnerable to a reasonably possible, near-term, severe impact. This disclosure informs financial statement users about the general nature of the risk associated with the concentration, and may indicate the percentage of concentration risk as of the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
| Name: |
us-gaap_ConcentrationRiskDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RisksAndUncertaintiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Related Parties
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Related Party Transactions [Abstract] |
|
| Related Parties |
Note 12. Related Parties From time to time, the Company enters into agreements with one or more related parties in the ordinary course of its business. These agreements are ratified by the Board or a committee thereof pursuant to its related party transaction policy. Viscient Biosciences (“Viscient”) is an entity for which Keith Murphy, the Company’s Executive Chairman, serves as the Chief Executive Officer and President. Dr. Jeffrey Miner, the Company’s former Chief Scientific Officer, is also the Chief Scientific Officer of Viscient, and Thomas Jurgensen, the Company’s former General Counsel, previously served as outside legal counsel to Viscient through his law firm, Optima Law Group, APC. On December 28, 2020, the Company entered into an intercompany agreement (the “Intercompany Agreement”) with Viscient and Organovo, Inc., the Company’s wholly-owned subsidiary, which included an asset purchase agreement for certain lab equipment. Pursuant to the Intercompany Agreement, the Company agreed to provide Viscient certain services related to 3D bioprinting technology, which includes, but is not limited to, histology services, cell isolation, and proliferation of cells and Viscient agreed to provide the Company certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, the Company and Viscient each agreed to share certain facilities and equipment and, subject to further agreement, to each make certain employees available for specified projects for the other party at prices to be determined in good faith by the parties. The Company evaluated the accounting for the Intercompany Agreement and concluded that any services provided by Viscient to the Company will be expensed as incurred, and any compensation for services provided by the Company to Viscient will be considered a reduction of personnel related expenses. Any services provided to Viscient do not fall under Topic 606 as the Intercompany Agreement is not a contract with a customer. For the years ended March 31, 2024 and 2023, the Company incurred no consulting expenses from Viscient. Additionally, for the years ended March 31, 2024 and 2023, the Company provided approximately $14,000 and $59,000 of histology services to Viscient, respectively.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Defined Contribution Plan
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Retirement Benefits [Abstract] |
|
| Defined Contribution Plan |
Note 13. Defined Contribution Plan The Company has a defined contribution 401(k) plan covering substantially all employees. During the year ended March 31, 2015, the 401(k) plan was amended (the “Amended Plan”) to include an employer matching provision. Under the terms of the Amended Plan, the Company will make matching contributions on up to the first 6% of compensation contributed by its employees. Amounts expensed under the Company’s 401(k) plan for the years ended March 31, 2024 and 2023 were approximately $61,000 and $10,000, respectively.
|
| X |
- DefinitionThe entire disclosure for an entity's employee compensation and benefit plans, including, but not limited to, postemployment and postretirement benefit plans, defined benefit pension plans, defined contribution plans, non-qualified and supplemental benefit plans, deferred compensation, share-based compensation, life insurance, severance, health care, unemployment and other benefit plans. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 710
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//710/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 712
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//712/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 715
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//715/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
| Name: |
us-gaap_CompensationAndEmployeeBenefitPlansTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CompensationAndRetirementDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Recent Accounting Pronouncements
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Changes and Error Corrections [Abstract] |
|
| Recent Accounting Pronouncements |
Note 14. Recent Accounting Pronouncements From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies. Unless otherwise stated, the Company believes that the impact of the recently issued accounting pronouncements that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption. In December 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The update requires a public business entity to disclose, on an annual basis, a tabular rate reconciliation using both percentages and currency amounts, broken out into specified categories with certain reconciling items further broken out by nature and jurisdiction to the extent those items exceed a specified threshold. In addition, all entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received. Adoption of the ASU allows for either the prospective or retrospective application of the amendment and is effective for annual periods beginning after December 15, 2024, with early adoption permitted. The Company has not yet completed its assessment of the impact of ASU 2023-09 on the Company’s Consolidated Financial Statements. In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment's profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company is currently evaluating the impact of this guidance. |
| X |
- References
+ Details
| Name: |
us-gaap_AccountingChangesAndErrorCorrectionsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for change in accounting principle. Includes, but is not limited to, nature, reason, and method of adopting amendment to accounting standards or other change in accounting principle. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 11.M.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480530/250-10-S99-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (f)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480530/250-10-S99-6
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 250
-Publisher FASB
-URI https://asc.fasb.org//250/tableOfContent
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(1)
-SubTopic 20
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(2)
-SubTopic 20
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
| Name: |
us-gaap_NewAccountingPronouncementsAndChangesInAccountingPrinciplesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Restructuring
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Restructuring and Related Activities [Abstract] |
|
| Restructuring |
Note 15. Restructuring On August 18, 2023, the Company announced to its employees a plan to reduce the Company’s workforce, effective August 25, 2023, by approximately six employees, which represented approximately 24% of its employees as of August 18, 2023. The Company refocused operations on FXR314, its clinical drug candidate. This decision to reduce the Company’s workforce was made in order to focus spending on the Company’s clinical program for FXR314, reduce ongoing operating expenses not related to clinical expenses, and extend the Company’s cash runway. The Company incurred approximately $0.4 million of cash expenditures for the year ended March 31, 2024, in connection with the reduction in force, which related to severance pay. As of March 31, 2024, all restructuring costs were paid in full. The Company anticipates annual cost savings of approximately $1.5 million resulting from the reduction in force.
|
| X |
- DefinitionThe entire disclosure for restructuring and related activities. Description of restructuring activities such as exit and disposal activities, include facts and circumstances leading to the plan, the expected plan completion date, the major types of costs associated with the plan activities, total expected costs, the accrual balance at the end of the period, and the periods over which the remaining accrual will be settled. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//420/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringAndRelatedActivitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Business Segment Information
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Segment Reporting [Abstract] |
|
| Business Segment Information |
Note 16. Business Segment Information Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance. The Company's operating segments were identified in fiscal 2024 and did not impact prior periods. The Company's operating segments are as follows: Research & Development The research and development (“R&D”) segment consists of the Company's drug development efforts. The Company's current clinical focus is in advancing FXR314 in IBD, including UC and CD. The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024. The Company released Phase 2 data for FXR314 for the treatment of MASH in April 2024 that are supportive of ongoing development, and believes FXR314 has a commercial opportunity in MASH, most likely in combination therapy. The Company is exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and its IBD disease models. The Company's second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. The Company believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas. As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development. Mosaic Cell Sciences The Mosaic Cell Sciences segment, which began operations in February 2024, consists of our Mosaic Cell Sciences division (“Mosaic”) which will serve as a key source of certain of the primary human cells that the Company utilizes in its research and development efforts. The Company believes Mosaic can help optimize its supply chain, reduce operating expenses related to cell sourcing and procurement, and ensure that the cellular raw materials it uses are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. The Company intends for Mosaic to provide the Company with qualified human cells for use in its clinical research and development programs. In addition to supplying the Company with primary human cells, the Company intends for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which the Company expects to offset costs and over time become a profit center that offsets overall R&D spending by the Company. Business Segment Information The Company's R&D segment generates royalty revenue related to its IP. The R&D segment's identifiable assets are its fixed assets. The Company expects that the Mosaic segment will generate future product revenue as part of its core operations, and its identifiable assets are its fixed assets and inventory. Other Company assets are comprised of all other assets that are not distinctly identifiable to a segment, which are presented on the Company's Consolidated Balance Sheets as of March 31, 2024. R&D segment revenues consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Royalty revenue |
$ |
109 |
|
|
$ |
370 |
|
Mosaic |
|
|
|
|
|
Product revenue |
|
— |
|
|
|
— |
|
Total segment revenue |
$ |
109 |
|
|
$ |
370 |
|
Total company revenue |
$ |
109 |
|
|
$ |
370 |
|
Operating segment expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
$ |
4,404 |
|
|
$ |
8,885 |
|
Mosaic |
|
1,094 |
|
|
|
— |
|
Total segment operating expenses |
$ |
5,498 |
|
|
$ |
8,885 |
|
Selling, general, and administrative expenses |
|
9,697 |
|
|
|
9,216 |
|
Total company operating expenses |
$ |
15,195 |
|
|
$ |
18,101 |
|
Operating segment assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Fixed assets, net |
$ |
423 |
|
|
$ |
902 |
|
Mosaic |
|
|
|
|
|
Fixed assets, net |
|
183 |
|
|
|
— |
|
Inventory |
|
297 |
|
|
|
— |
|
Total segment assets |
$ |
903 |
|
|
$ |
902 |
|
Other company assets |
|
5,446 |
|
|
|
19,411 |
|
Total company assets |
$ |
6,349 |
|
|
$ |
20,313 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_SegmentReportingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//280/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-26
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 34
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-34
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_SegmentReportingDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Subsequent Events
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Note 17. Subsequent Events On May 8, 2024, the Company priced a best efforts public offering (the “Offering”) of: (i) 1,562,500 shares of its common stock and accompanying common warrants (“Common Warrants”) to purchase up to 1,562,500 shares of common stock at a combined public offering price of $0.80 per share and accompanying Common Warrant to purchase one share of common stock and (ii) pre-funded warrants (“Pre-Funded Warrants”) to purchase 5,000,000 shares of common stock and accompanying Common Warrants to purchase up to 5,000,000 shares of common stock at a combined public offering price of $0.799 per Pre-Funded Warrant and accompanying Common Warrant to purchase one share of common stock. In connection with the Offering, the Company entered into Securities Purchase Agreements with the purchasers of the securities in the Offering on May 8, 2024. The per share exercise price for the Pre-Funded Warrants is $0.001, subject to adjustment as provided therein. The Pre-Funded Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire when exercised in full. The holders may exercise the Pre-Funded Warrants by means of a “cashless exercise.” The per share exercise price for the Common Warrants is $0.80, subject to adjustment as provided therein. The Common Warrants were immediately exercisable, subject to certain beneficial ownership limitations, and will expire on the date that is five years following the original issuance date. If a registration statement covering the issuance of the shares of common stock issuable upon exercise of the Common Warrants is not available for the issuance, then the holders may exercise the Common Warrants by means of a “cashless exercise.” In connection with the Offering, the Company paid JonesTrading Institutional Services LLC, which acted as the placement agent in connection with the Offering, a cash fee of 5.0% of the aggregate gross proceeds raised in the Offering. The closing of the Offering occurred on May 13, 2024. The Company received net proceeds of approximately $4.7 million from the Offering, after deducting the estimated offering expenses payable by the Company, including the Placement Agent fees. Additionally, between April 1, 2024 and the date of the filing of this Annual Report on Form 10-K, the Company issued 1,389,002 shares of common stock in ATM offerings under the 2024 ATM Prospectus for net proceeds of approximately $1.8 million. As of May 29, 2024, the Company's cash and cash equivalents balance was approximately $7.0 million.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Description of Business and Summary of Significant Accounting Policies (Policies)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Nature of operations and basis of presentation |
Nature of operations and basis of presentation Organovo Holdings, Inc. (“Organovo Holdings,” “Organovo,” and the “Company”) is a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in the Company's established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC. The Company's current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024. The Company released Phase 2 data for FXR314 for the treatment of metabolic function-associated steatohepatitis (“MASH”) in April 2024 that is supportive of ongoing development, and the Company believes FXR314 has a commercial opportunity in MASH, most likely in combination therapy. The Company is exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and the Company's IBD disease models. The Company's second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. The Company believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas. As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development. The Company's current understanding of intestinal tissue models and IBD disease models leads the Company to believe that it can create models that provide greater insight into the biology of these diseases than are generally currently available. The Company is creating high fidelity disease models, leveraging its prior work including the work found in its peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) The Company's advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures. Using these disease models, the Company intends to identify and validate novel therapeutic targets. After finding therapeutic drug targets, the Company intends to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials. The Company expects to broaden its work into additional therapeutic areas over time and is currently exploring specific tissues for development. In its work to identify the areas of interest, the Company evaluates areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, the Company is building upon both its external and in house scientific expertise, which will be essential to its drug development effort. In February 2024, the Company formed its Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain primary human cells that the Company utilizes in its research and development efforts. The Company believes Mosaic can help optimize its supply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials the Company uses are of the highest quality and are derived from tissues that are ethically sourced in full compliance with state and federal guidelines. The Company intends for Mosaic to provide it with qualified human cells for use in its clinical research and development programs. In addition to supplying the Company with primary human cells, the Company intends for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which the Company expects to offset costs and over time become a profit center that offsets overall research and development ("R&D") spending by the Company.
|
| Liquidity and Going Concern |
Liquidity and Going Concern The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of March 31, 2024, the Company had cash and cash equivalents of approximately $2.9 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $339.7 million. The restricted cash was pledged as collateral for a letter of credit that the Company is required to maintain as a security deposit under the terms of the lease agreements for its facilities. The Company also had negative cash flows from operations of approximately $14.7 million during the year ended March 31, 2024. Through March 31, 2024, the Company has financed its operations primarily through the sale of common stock through public and at-the-market (“ATM”) offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research-based services, grants, and collaborative research agreements, and from the sale of convertible notes. During the year ended March 31, 2024, the Company issued 1,172,342 shares of its common stock through its ATM facility. Based on the Company's current operating plan and available cash resources, including the total net proceeds of $4.7 million from the best efforts public offering that closed in May 2024, the Company will need substantial additional funding to support future operating activities. The Company has concluded that the prevailing conditions and ongoing liquidity risks faced by the Company raise substantial doubt about its ability to continue as a going concern for at least one year following the date these consolidated financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern. As the Company continues its operations and is focusing its efforts on drug discovery and development, the Company will need to raise additional capital to implement this business plan. The Company cannot predict with certainty the exact amount or timing for any future capital raises. The Company will seek to raise additional capital through debt or equity financings, or through some other financing arrangement. However, the Company cannot be sure that additional financing will be available if and when needed, or that, if available, it can obtain financing on terms favorable to its stockholders. Any failure to obtain financing when required will have a material adverse effect on the Company’s business, operating results, and financial condition.
|
| Use of estimates |
Use of estimates The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions.
|
| Investments |
Investments Investments consist of investments in debt securities and investments in equity securities. Investments in debt securities consist of investments in U.S. Treasury bills. As of March 31, 2024 and 2023, all investments that have original maturities of three months or less are classified as cash equivalents on the Consolidated Balance Sheets. As of March 31, 2024, all investments are classified as available-for-sale, as the sale of such investments may be required prior to maturity to implement management strategies. Available-for-sale debt securities are recorded at fair value. Any unrealized gains and losses are included in accumulated other comprehensive income as a component of stockholders' equity until realized. As U.S. Treasury bills are risk-free, any declines in fair value are considered temporary. Investments in equity securities consist of investments in the common stock of entities traded in active markets. The Company does not have the ability to exercise significant influence over any entities. Therefore, initial investments are recorded at cost, and are remeasured at fair value as of the balance sheet date. Any gains or losses resulting from the change in fair value are recorded in net income. The investments in equity securities are classified as current assets.
|
| Fair value measurement |
Fair value measurement Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value: •Level 1 — Quoted prices in active markets for identical assets or liabilities. •Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. •Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
| Financial instruments |
Financial instruments For certain of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other assets, accounts payable, accrued expenses, the carrying amounts are generally considered to be representative of their respective fair values because of the short-term nature of those instruments.
|
| Cash and cash equivalents |
Cash and cash equivalents The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents.
|
| Restricted cash |
Restricted cash As of each of March 31, 2024 and 2023, the Company had approximately $0.1 million of restricted cash, deposited with a financial institution. The entire amount was held in certificates of deposit to support a letter of credit agreement related to the Company’s facility leases entered into in November 2020 and amended in November 2021.
|
| Inventory |
Inventory Inventories are stated at the lower of cost or net realizable value. Inventory as of March 31, 2024 consisted of approximately $0.3 million in finished goods, which is related to the Mosaic Cell Sciences division. There were no finished goods inventory as of March 31, 2023. Please refer to "Note 16. Business Segment Information" for further information.
|
| Fixed assets and depreciation |
Fixed assets and depreciation Fixed assets are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the remaining lease term. The estimated useful lives of the fixed assets range between one and seven years.
|
| Impairment of long-lived assets |
Impairment of long-lived assets In accordance with authoritative guidance, the Company reviews its long-lived assets, including fixed assets and other assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred as of March 31, 2024 and 2023.
|
| Research and development |
Research and development Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Research and development costs are expensed as incurred.
|
| Acquired in-process research and development |
Acquired in-process research and development The Company has acquired drug candidates in development. The costs to acquire a drug candidate are immediately expensed as acquired in-process research and development, provided that the drug candidate has no alternative future use. Acquired in-process research and development expenses are included in total research and development expenses on the Consolidated Statements of Operations and Other Comprehensive Loss. FXR Program In March 2023, the Company acquired Metacrine's FXR program for $4.0 million. The FXR program was determined to have no alternative future use, and therefore was considered acquired in-process research and development and fully expensed. In the year ended March 31, 2023, the Company paid a $2.0 million upfront payment, and the remaining $2.0 million was paid in the year ended March 31, 2024, upon the final transfer of the drug compounds, related data, and IP.
|
| Income taxes |
Income taxes Deferred income taxes are recognized for the tax consequences in future years for differences between the tax basis of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the combination of the tax payable for the year and the change during the year in deferred tax assets and liabilities. The Company’s policy regarding uncertainty in income taxes is pursuant to Accounting Standard Codification (“ASC”) Topic 740-10. Interest and penalties that would be assessed in relation to the settlement value of unrecognized tax benefits is recognized as a component of income tax expense.
|
| Revenue recognition |
Revenue recognition The Company has generated revenues from payments received from licensing intellectual property. The Company has entered into a license agreement with a company that includes the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales, if any. At the initiation of the agreement, the Company has analyzed whether it results in a contract with a customer under Topic 606. The Company has considered a variety of factors in determining the appropriate estimates and assumptions under these arrangements, such as whether the Company is a principal vs. agent, whether the elements are distinct performance obligations, whether there are determinable stand-alone prices, and whether any licenses are functional or symbolic. The Company has evaluated each performance obligation to determine if it can be satisfied and recognized as revenue at a point in time or over time. Typically, non-refundable upfront fees have been considered fixed, while sales-based royalty payments have been identified as variable consideration which must be evaluated to determine if it has been constrained and, therefore, excluded from the transaction price. Please refer to “Note 6. Collaborative Research, Development, and License Agreements” for further information.
|
| Stock-based compensation |
Stock-based compensation The Company accounts for stock-based compensation in accordance with the ASC Topic 718, Compensation — Stock Compensation, which establishes accounting for equity instruments exchanged for employee and non-employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair value of the award (determined using either the Black-Scholes or Monte Carlo option-pricing models, depending on the complexity of the equity grant), and is recognized as an expense, under the straight-line method, over the employee or non-employee's requisite service period (generally the vesting period of the equity grant). The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses its Company-specific historical volatility rate. The risk-free interest rate assumption is based on U.S. Treasury rates. The weighted average expected life of options is estimated using the average of the contractual term and the weighted average vesting term of the options. Option forfeitures are treated as a reduction of stock-based compensation expense and accounted for as they occur.
|
| Comprehensive income (loss) |
Comprehensive income (loss) Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the consolidated financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at comprehensive income (loss).
|
| Net loss per share |
Net loss per share Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. The weighted-average number of shares used to compute diluted loss per share excludes any assumed exercise of stock options and warrants, shares reserved for purchase under the Company’s 2023 Employee Stock Purchase Plan (“2023 ESPP”), the assumed release of restriction of restricted stock units (“RSUs”), and shares subject to repurchase as the effect would be anti-dilutive. No dilutive effect was calculated for the years ended March 31, 2024 and 2023 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive. Common stock equivalents excluded from computing diluted net loss per share were approximately 0.8 million shares and 1.6 million shares for the years ended March 31, 2024 and 2023, respectively.
|
| X |
- DefinitionLiquidity and going concern.
| Name: |
onvo_LiquidityAndGoingConcernPolicyTextBlock |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEntity's cash and cash equivalents accounting policy with respect to restricted balances. Restrictions may include legally restricted deposits held as compensating balances against short-term borrowing arrangements, contracts entered into with others, or company statements of intention with regard to particular deposits; however, time deposits and short-term certificates of deposit are not generally included in legally restricted deposits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsRestrictedCashAndCashEquivalentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents with respect to unrestricted balances. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsUnrestrictedCashAndCashEquivalentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs assigned to identifiable tangible and intangible assets of an acquired entity to be used in the research and development activities of the combined enterprise. An entity also may disclose the appraisal method or significant assumptions used to value acquired research and development assets.
| Name: |
us-gaap_InProcessResearchAndDevelopmentPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue from contract with customer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-18
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-20
Reference 9: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org//606/tableOfContent
| Name: |
us-gaap_RevenueFromContractWithCustomerPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.C.Q3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.1.Q5)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.3.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.2.Q6)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Recent Accounting Pronouncements (Policies)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Recent Accounting Pronouncements |
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies. Unless otherwise stated, the Company believes that the impact of the recently issued accounting pronouncements that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption. In December 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The update requires a public business entity to disclose, on an annual basis, a tabular rate reconciliation using both percentages and currency amounts, broken out into specified categories with certain reconciling items further broken out by nature and jurisdiction to the extent those items exceed a specified threshold. In addition, all entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received. Adoption of the ASU allows for either the prospective or retrospective application of the amendment and is effective for annual periods beginning after December 15, 2024, with early adoption permitted. The Company has not yet completed its assessment of the impact of ASU 2023-09 on the Company’s Consolidated Financial Statements. In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment's profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company is currently evaluating the impact of this guidance.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Investments and Fair Value Measurement (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Investments, All Other Investments [Abstract] |
|
| Summary of Investments in Debt Securities that are Measured at Fair Value |
The following table summarizes the Company's investments in debt securities that are measured at fair value as of March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortized costs basis |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair value |
|
As of March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
997 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
998 |
|
As of March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
Investments in debt securities |
|
$ |
4,943 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
4,945 |
|
|
| Schedule of Activity for Investments in Equity Securities Measured at Fair Value |
The following table presents the activity for investments in equity securities measured at fair value for the year ended March 31, 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Investment in Equity Securities
(in thousands) |
|
Balance at March 31, 2023 |
|
$ |
706 |
|
Sales |
|
|
(718 |
) |
Gain on investment in equity securities |
|
|
12 |
|
Balance at March 31, 2024 |
|
$ |
— |
|
|
| X |
- DefinitionSchedule of investments in equity securities measured at fair value.
| Name: |
onvo_ScheduleOfInvestmentsInEquitySecuritiesMeasuredAtFairValueTableTextBlock |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-9
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsAllOtherInvestmentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionTabular disclosure of the amounts paid in advance for capitalized costs that will be expensed with the passage of time or the occurrence of a triggering event, and will be charged against earnings within one year or the normal operating cycle, if longer; the aggregate carrying amount of current assets, not separately presented elsewhere in the balance sheet; and other deferred costs.
| Name: |
us-gaap_DeferredCostsCapitalizedPrepaidAndOtherAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Fixed Assets (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Property, Plant and Equipment [Abstract] |
|
| Schedule of Fixed Assets |
Fixed assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Laboratory equipment |
|
$ |
1,617 |
|
|
$ |
1,575 |
|
Furniture and fixtures |
|
|
66 |
|
|
|
66 |
|
Computer software and equipment |
|
|
244 |
|
|
|
537 |
|
Fixed Assets, gross |
|
|
1,927 |
|
|
|
2,178 |
|
Less accumulated depreciation |
|
|
(1,258 |
) |
|
|
(1,276 |
) |
Fixed Assets, net |
|
$ |
669 |
|
|
$ |
902 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Accrued Expenses (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Summary of Accrued Expenses |
Accrued expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Accrued compensation |
|
$ |
536 |
|
|
$ |
609 |
|
Accrued legal and professional fees |
|
|
93 |
|
|
|
193 |
|
Acquired in-process research and development |
|
|
— |
|
|
|
2,000 |
|
Other accrued expenses |
|
|
98 |
|
|
|
46 |
|
|
|
$ |
727 |
|
|
$ |
2,848 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionSchedule of capitalized license fees.
| Name: |
onvo_ScheduleOfCapitalizedLicenseFeesTableTextBlock |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders' Equity (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Class Of Stock [Line Items] |
|
| Summary of Stock Option Activity |
The following table summarizes stock option activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options
Outstanding |
|
|
Weighted-
Average
Exercise Price |
|
|
Aggregate
Intrinsic
Value |
|
Outstanding at March 31, 2023 |
|
|
1,451,217 |
|
|
$ |
6.49 |
|
|
$ |
38,327 |
|
Options granted |
|
|
184,158 |
|
|
$ |
1.67 |
|
|
$ |
— |
|
Options canceled |
|
|
(937,368 |
) |
|
$ |
7.20 |
|
|
$ |
— |
|
Options exercised |
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
Outstanding at March 31, 2024 |
|
|
698,007 |
|
|
$ |
4.26 |
|
|
$ |
— |
|
Vested and Exercisable at March 31, 2024 |
|
|
401,536 |
|
|
$ |
5.61 |
|
|
$ |
— |
|
|
| Common Stock Reserved for Future Issuance |
Common stock reserved for future issuance consisted of the following at March 31, 2024:
|
|
|
|
|
Common stock issuable pursuant to options outstanding and reserved under the 2012 Plan |
|
|
431,416 |
|
Common stock reserved under the 2012 Plan |
|
|
— |
|
Common stock issuable pursuant to options outstanding and reserved under the 2022 Plan |
|
|
216,591 |
|
Common stock reserved under the 2022 Plan |
|
|
1,641,250 |
|
Common stock reserved under the ESPP |
|
|
45,000 |
|
Common stock reserved under the 2021 Inducement Equity Plan |
|
|
1,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2012 Plan |
|
|
5,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2022 Plan |
|
|
117,642 |
|
Common stock issuable pursuant to options outstanding and reserved under the Inducement Plan |
|
|
50,000 |
|
Total at March 31, 2024 |
|
|
2,507,899 |
|
|
| Schedule of Stock-based Compensation Expense |
Stock-based compensation expense for all stock-based awards consists of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Research and development |
|
$ |
138 |
|
|
$ |
473 |
|
General and administrative |
|
|
1,370 |
|
|
|
1,904 |
|
Total |
|
$ |
1,508 |
|
|
$ |
2,377 |
|
|
| Fair Value of Employee Stock Options |
The fair value of stock options was estimated at the grant date using the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
99.04 |
% |
|
|
95.53 |
% |
Risk-free interest rate |
|
|
4.11 |
% |
|
|
3.32 |
% |
Expected life of options |
|
6 years |
|
|
6 years |
|
Weighted average grant date fair value |
|
$ |
1.34 |
|
|
$ |
1.83 |
|
|
| Restricted stock units (RSUs) [Member] |
|
| Class Of Stock [Line Items] |
|
| Summary of Company's RSUs Activity |
The following table summarizes the Company’s RSUs activity for the year ended March 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
|
|
Weighted
Average Price |
|
Unvested at March 31, 2023 |
|
|
127,717 |
|
|
$ |
2.22 |
|
Granted |
|
|
117,642 |
|
|
$ |
1.39 |
|
Vested |
|
|
(122,717 |
) |
|
$ |
1.90 |
|
Cancelled / forfeited |
|
|
— |
|
|
$ |
— |
|
Unvested at March 31, 2024 |
|
|
122,642 |
|
|
$ |
1.75 |
|
|
| ESPP Shares [Member] |
|
| Class Of Stock [Line Items] |
|
| Fair Value of Employee Stock Options |
The fair value of ESPP shares was estimated at the purchase period commencement date using the following assumptions:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
March 31, 2024 |
|
|
Year Ended
March 31, 2023 |
|
Dividend yield |
|
|
— |
|
|
|
— |
|
Volatility |
|
|
95.20 |
% |
|
|
86.58 |
% |
Risk-free interest rate |
|
|
5.27 |
% |
|
|
3.34 |
% |
Expected term |
|
6 months |
|
|
6 months |
|
Grant date fair value |
|
$ |
0.39 |
|
|
$ |
0.82 |
|
|
| X |
- DefinitionCommon stock capital shares reserved for future issuance.
| Name: |
onvo_CommonStockCapitalSharesReservedForFutureIssuanceTableTextBlock |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of the number and weighted-average grant date fair value for restricted stock and restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock and restricted stock units that were granted, vested, or forfeited during the year.
| Name: |
us-gaap_ScheduleOfSharebasedCompensationRestrictedStockAndRestrictedStockUnitsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_EmployeeStockOwnershipPlanESOPDisclosuresByPlanAxis=onvo_ESPPSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Leases (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Leases [Abstract] |
|
| Schedule of Lease Liabilities and Corresponding Right-of-use Assets |
The table below summarizes the Company’s lease liabilities and corresponding right-of-use assets as of March 31, 2024 (in thousands):
|
|
|
|
|
|
|
March 31, 2024 |
|
ASSETS |
|
|
|
Operating lease right-of-use assets |
|
$ |
1,299 |
|
Total lease right-of-use assets |
|
$ |
1,299 |
|
|
|
|
|
LIABILITIES |
|
|
|
Current |
|
|
|
Operating lease liability |
|
$ |
506 |
|
Noncurrent |
|
|
|
Operating lease liability, net of current portion |
|
$ |
888 |
|
Total lease liabilities |
|
$ |
1,394 |
|
|
|
|
|
Weighted average remaining lease term: |
|
2.83 |
|
Weighted average discount rate: |
|
|
6 |
% |
|
| Schedule of Future Lease Payments |
Future lease payments relating to the Company’s operating lease liabilities as of March, 31, 2024 are as follows (in thousands):
|
|
|
|
|
Fiscal year ending March 31, 2025 |
|
|
523 |
|
Fiscal year ending March 31, 2026 |
|
|
538 |
|
Fiscal year ending March 31, 2027 |
|
|
460 |
|
Total future lease payments |
|
|
1,521 |
|
Less: Imputed Interest |
|
|
(127 |
) |
Total lease obligations |
|
|
1,394 |
|
Less: Current obligations |
|
|
(506 |
) |
Noncurrent lease obligations |
|
$ |
888 |
|
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Income Taxes (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Summary of Reconciliation of Statutory Federal Rate and Effective Rate for Operations |
A reconciliation of the statutory federal rate and the effective rate, for operations, is as follows for the years ended March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
|
|
March 31,
2023 |
|
|
|
Tax computed at federal statutory rate |
$ |
(3,081 |
) |
|
21 |
% |
|
$ |
(3,624 |
) |
21% |
|
State income tax, net of federal benefit |
|
(110 |
) |
|
0.7 |
% |
|
|
(44 |
) |
0.2% |
|
Stock-based compensation |
|
721 |
|
|
-4.9 |
% |
|
|
167 |
|
-1% |
|
Research credits |
|
— |
|
|
0 |
% |
|
|
60 |
|
-0.4% |
|
Change in tax rate |
|
(62 |
) |
|
0.4 |
% |
|
|
157 |
|
-0.9% |
|
Removal of net operating losses and research development credits |
|
1,910 |
|
|
-13 |
% |
|
|
1,410 |
|
-8.2% |
|
Uncertain tax positions |
|
111 |
|
|
-0.8 |
% |
|
|
— |
|
|
0 |
% |
Other |
|
(16 |
) |
|
0.2 |
% |
|
|
1 |
|
0% |
|
Valuation allowance |
|
527 |
|
|
-3.6 |
% |
|
|
1,873 |
|
-10.7% |
|
Provision (benefit) for income taxes |
$ |
— |
|
0.0% |
|
|
$ |
— |
|
0.0% |
|
|
| Summary of Net Deferred Tax Assets |
Significant components of the Company’s net deferred tax assets are as follows as of March 31, 2024 and 2023 (in thousands, except percentages):
|
|
|
|
|
|
|
|
|
March 31,
2024 |
|
|
March 31,
2023 |
|
Deferred tax assets: |
|
|
|
|
|
Amortization |
$ |
593 |
|
|
$ |
598 |
|
Section 174 R&D capitalization |
|
1,793 |
|
|
|
855 |
|
Accrued expenses and reserves |
|
105 |
|
|
|
116 |
|
Operating lease liability |
|
307 |
|
|
|
384 |
|
Stock-based compensation |
|
315 |
|
|
|
755 |
|
Inventory |
|
259 |
|
|
|
251 |
|
Other, net |
|
4 |
|
|
|
3 |
|
Total deferred tax assets |
|
3,376 |
|
|
|
2,962 |
|
Valuation allowance |
|
(2,983 |
) |
|
|
(2,458 |
) |
Net deferred tax assets |
$ |
393 |
|
|
$ |
504 |
|
Deferred tax liabilities: |
|
|
|
|
|
Operating lease right-of-use assets |
|
(286 |
) |
|
|
(363 |
) |
Depreciation |
|
(107 |
) |
|
|
(135 |
) |
Investment in equity securities |
|
— |
|
|
|
(6 |
) |
Total deferred tax liabilities |
$ |
(393 |
) |
|
$ |
(504 |
) |
|
$ |
— |
|
|
$ |
— |
|
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Business Segment Information (Tables)
|
12 Months Ended |
Mar. 31, 2024 |
|---|
| Segment Reporting [Abstract] |
|
| Summary of R&D Segment Revenues |
R&D segment revenues consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Royalty revenue |
$ |
109 |
|
|
$ |
370 |
|
Mosaic |
|
|
|
|
|
Product revenue |
|
— |
|
|
|
— |
|
Total segment revenue |
$ |
109 |
|
|
$ |
370 |
|
Total company revenue |
$ |
109 |
|
|
$ |
370 |
|
|
| Summary of Operating Segment Expenses |
Operating segment expenses consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
$ |
4,404 |
|
|
$ |
8,885 |
|
Mosaic |
|
1,094 |
|
|
|
— |
|
Total segment operating expenses |
$ |
5,498 |
|
|
$ |
8,885 |
|
Selling, general, and administrative expenses |
|
9,697 |
|
|
|
9,216 |
|
Total company operating expenses |
$ |
15,195 |
|
|
$ |
18,101 |
|
|
| Summary of Operating Segment Assets |
Operating segment assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
R&D |
|
|
|
|
|
Fixed assets, net |
$ |
423 |
|
|
$ |
902 |
|
Mosaic |
|
|
|
|
|
Fixed assets, net |
|
183 |
|
|
|
— |
|
Inventory |
|
297 |
|
|
|
— |
|
Total segment assets |
$ |
903 |
|
|
$ |
902 |
|
Other company assets |
|
5,446 |
|
|
|
19,411 |
|
Total company assets |
$ |
6,349 |
|
|
$ |
20,313 |
|
|
| X |
- DefinitionTabular disclosure of all significant reconciling items in the reconciliation of total assets from reportable segments to the entity's consolidated assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ReconciliationOfAssetsFromSegmentToConsolidatedTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation of profit (loss) from reportable segments to the consolidated income (loss) before income tax expense (benefit) and discontinued operations. Includes, but is not limited to, reconciliation after income tax if income tax is allocated to the reportable segment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of all significant reconciling items in the reconciliation of total revenues from reportable segments to the entity's consolidated revenues. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ReconciliationOfRevenueFromSegmentsToConsolidatedTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_SegmentReportingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Description of Business and Summary of Significant Accounting Policies - Additional Information (Details)
|
|
12 Months Ended |
|
|
May 13, 2024
USD ($)
|
Mar. 31, 2024
USD ($)
Compounds
shares
|
Mar. 31, 2023
USD ($)
shares
|
May 29, 2024
USD ($)
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Cash and cash equivalents |
|
$ 2,901,000
|
$ 15,301,000
|
|
| Restricted cash |
|
143,000
|
143,000
|
|
| Accumulated deficit |
|
(339,669,000)
|
(324,998,000)
|
|
| Cash flow from operations |
|
$ 14,653,000
|
$ 12,408,000
|
|
| Issuance of common stock | shares |
|
10,077,726
|
8,716,906
|
|
| Proceeds from sale of common stock shares |
|
$ 1,437,000
|
$ 0
|
|
| Restricted cash |
|
$ 100,000
|
100,000
|
|
| Inventory finished goods |
|
|
0
|
|
| Estimated useful life of the fixed assets |
|
The estimated useful lives of the fixed assets range between one and seven years.
|
|
|
| Impairment of long-lived assets |
|
$ 0
|
0
|
|
| Acquired in-process research and development accrued expenses |
|
0
|
2,000,000
|
|
| Dilutive effect |
|
$ 0
|
$ 0
|
|
| Common stock equivalents excluded from computing diluted net loss per share | shares |
|
800,000
|
1,600,000
|
|
| Subsequent Event [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Cash and cash equivalents |
|
|
|
$ 7,000,000
|
| Maximum [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Maturity of highly liquid investment |
|
3 months
|
|
|
| Useful life of fixed assets, range |
|
7 years
|
|
|
| Minimum [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Useful life of fixed assets, range |
|
1 year
|
|
|
| Mosaic Cell Sciences [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Inventory finished goods |
|
$ 0.3
|
|
|
| At-The-Market Facility [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Issuance of common stock | shares |
|
1,172,342
|
|
|
| Public Offering [Member] | Subsequent Event [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Net proceeds from issuance of offerings |
$ 4,700,000
|
|
|
|
| Metacrine's FXR Program [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Number of clinically tested compounds | Compounds |
|
2
|
|
|
| Number of discovery or preclinical compounds | Compounds |
|
2,000
|
|
|
| Metacrine, Inc. [Member] | Metacrine's FXR Program [Member] |
|
|
|
|
| Description Of Business And Summary Of Significant Accounting Policies [Line Items] |
|
|
|
|
| Asset Acquisition, Price of Acquisition, Expected |
|
|
$ 4,000,000
|
|
| Upfront payment paid |
|
|
$ 2,000,000
|
|
| Acquired in-process research and development accrued expenses |
|
$ 2,000,000
|
|
|
| X |
- DefinitionAcquired in-process research and development accrued expenses
| Name: |
onvo_AcquiredInProcessResearchAndDevelopmentAccruedExpenses |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCash and cash equivalents maturity period.
| Name: |
onvo_CashAndCashEquivalentsMaturityPeriod |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of business and summary of significant accounting policies.
| Name: |
onvo_DescriptionOfBusinessAndSummaryOfSignificantAccountingPoliciesLineItems |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of clinically tested compounds.
| Name: |
onvo_NumberOfClinicallyTestedCompounds |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of discovery or preclinical compounds.
| Name: |
onvo_NumberOfDiscoveryOrPreclinicalCompounds |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionProperty plant and equipment estimated useful lives description.
| Name: |
onvo_PropertyPlantAndEquipmentEstimatedUsefulLivesDescription |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPurchase price of expected asset acquisition prior to consideration being transferred. Excludes business acquisition. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 805
-SubTopic 50
-Name Accounting Standards Codification
-Section 15
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480123/805-50-15-3
| Name: |
us-gaap_AssetAcquisitionPriceOfAcquisitionExpected |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) to net income used for calculating diluted earnings per share (EPS), resulting from the assumed exercise stock options, restrictive stock units (RSUs), convertible preferred stock of an employee stock ownership plan (ESOP), and other dilutive convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_DilutiveSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of write-downs for impairments recognized during the period for long lived assets held for use (including those held for disposal by means other than sale). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-4
| Name: |
us-gaap_ImpairmentOfLongLivedAssetsHeldForUse |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of completed merchandise or goods expected to be sold within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryFinishedGoods |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
| Name: |
us-gaap_RestrictedCashAndCashEquivalentsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_MosaicSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=onvo_AtTheMarketFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Investments and Fair Value Measurement - Additional Information (Details) - USD ($)
|
12 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Investments [Line Items] |
|
|
| Investments in debt securities |
$ 1,000,000
|
|
| Cash and cash equivalents |
2,901,000
|
$ 15,301,000
|
| Interest income on investments in debt securities |
100,000
|
|
| Liquidation of equity securities |
700,000
|
|
| (Loss) gain on investment in equity securities |
12,000
|
29,000
|
| Purchase of equity securities |
0
|
1,061,000
|
| Sale of equity securities |
0
|
$ 384,000
|
| Fair value of investment in equity securities |
0
|
|
| Maximum [Member] |
|
|
| Investments [Line Items] |
|
|
| Unrealized gains on investments in debt securities |
100,000
|
|
| (Loss) gain on investment in equity securities |
$ 100,000
|
|
| X |
- DefinitionInterest income on investments in debt securities.
| Name: |
onvo_InterestIncomeOnInvestmentsInDebtSecurities |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLiquidation of equity securities fvni.
| Name: |
onvo_LiquidationOfEquitySecuritiesFvni |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), investment in debt security measured at amortized cost (held-to-maturity) and investment in debt security measured at fair value with change in fair value recognized in net income (trading). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(d)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
| Name: |
us-gaap_DebtSecuritiesUnrealizedGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482736/825-10-45-1A
| Name: |
us-gaap_EquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrealized and realized gain (loss) on investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 321
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479536/321-10-50-4
| Name: |
us-gaap_EquitySecuritiesFvNiGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all investments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(h))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
| Name: |
us-gaap_Investments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_PaymentsToAcquireEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_ProceedsFromSaleOfEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionAmount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
| Name: |
us-gaap_ScheduleOfAvailableForSaleSecuritiesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionSchedule of investments in equity securities measured at fair value.
| Name: |
onvo_ScheduleOfInvestmentsInEquitySecuritiesMeasuredAtFairValueLineItems |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482736/825-10-45-1A
| Name: |
us-gaap_EquitySecuritiesFVNINoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrealized and realized gain (loss) on investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 321
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479536/321-10-50-4
| Name: |
us-gaap_EquitySecuritiesFvNiGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_PaymentsToAcquireEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as investing activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 10
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479567/321-10-45-1
| Name: |
us-gaap_ProceedsFromSaleOfEquitySecuritiesFvNi |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for insurance that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInsurance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Fixed Assets - Schedule of Fixed Assets (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Property, Plant and Equipment [Abstract] |
|
|
| Laboratory equipment |
$ 1,617
|
$ 1,575
|
| Furniture and fixtures |
66
|
66
|
| Computer software and equipment |
244
|
537
|
| Fixed Assets, gross |
1,927
|
2,178
|
| Less accumulated depreciation |
(1,258)
|
(1,276)
|
| Fixed Assets, net |
$ 669
|
$ 902
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated amortization of capitalized costs for computer software, including but not limited to, acquired and internally developed computer software. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_CapitalizedComputerSoftwareGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation of equipment commonly used in offices and stores that have no permanent connection to the structure of a building or utilities. Examples include, but are not limited to, desks, chairs, tables, and bookcases. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_FurnitureAndFixturesGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation of tangible personal property used to produce goods and services, including, but is not limited to, tools, dies and molds, computer and office equipment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_MachineryAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionAcquired in-process research and development accrued expenses
| Name: |
onvo_AcquiredInProcessResearchAndDevelopmentAccruedExpenses |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Collaborative Research, Development, and License Agreements - Additional Information (Details) - USD ($)
|
|
|
1 Months Ended |
12 Months Ended |
|
Dec. 05, 2022 |
Feb. 22, 2022 |
May 31, 2011 |
Mar. 31, 2009 |
Mar. 31, 2024 |
Mar. 31, 2023 |
Mar. 31, 2016 |
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Total Revenues |
|
|
|
|
$ 109,000
|
$ 370,000
|
|
| Licensing Agreements [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Amortization expense of licenses |
|
|
|
|
$ 5,000
|
82,000
|
|
| Weighted average remaining amortization period for all licenses |
|
|
|
|
3 years
|
|
|
| Annual amortization expense of licenses year 1 |
|
|
|
|
$ 3,000
|
|
|
| Annual amortization expense of licenses year 2 |
|
|
|
|
3,000
|
|
|
| Annual amortization expense of licenses year 3 |
|
|
|
|
3,000
|
|
|
| Annual amortization expense of licenses year 4 |
|
|
|
|
3,000
|
|
|
| Annual amortization expense of licenses year 5 |
|
|
|
|
3,000
|
|
|
| BICO Group AB [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Nonrefundable upfront fee paid |
|
$ 1,500,000
|
|
|
|
|
|
| Total Revenues |
|
$ 1,500,000
|
|
|
109,000
|
370,000
|
|
| University of Missouri [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Royalty fees percentage minimum |
|
|
|
1.00%
|
|
|
|
| Royalty fees percentage maximum |
|
|
|
3.00%
|
|
|
|
| Upfront payment |
$ 50,000,000
|
|
|
|
|
|
|
| Clemson University [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Minimum annual royalty paid |
|
|
|
|
40,000
|
$ 40,000
|
|
| Upfront payment |
|
|
|
|
$ 0
|
|
|
| Minimum annual royalty payment due |
|
|
|
|
|
|
$ 40,000
|
| Clemson University [Member] | Sublicense Agreements [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Percentage of royalty revenue from sublicensee |
|
|
|
|
40.00%
|
|
|
| Clemson University [Member] | Licensing Agreements [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Royalty fees percentage minimum |
|
|
1.50%
|
|
|
|
|
| Royalty fees percentage maximum |
|
|
3.00%
|
|
|
|
|
| Expected expiration year of license agreement |
|
|
2024-05
|
|
|
|
|
| Salk Institute for Biological Studies [Member] | Licensing Agreements [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Royalty fees percentage minimum |
|
|
|
|
1.00%
|
|
|
| Royalty fees percentage maximum |
|
|
|
|
1.125%
|
|
|
| Percentage of sublicensing revenue |
|
|
|
|
3.50%
|
|
|
| Salk Institute for Biological Studies [Member] | Licensing Agreements [Member] | 45 days of Dosing of First Patient in a Phase III Clinical Trial [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Milestone payments |
|
|
|
|
$ 500,000
|
|
|
| Salk Institute for Biological Studies [Member] | Licensing Agreements [Member] | 45 days of FDA Approval of First Licensed Product [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Milestone payments |
|
|
|
|
1,000,000
|
|
|
| Salk Institute for Biological Studies [Member] | Licensing Agreements [Member] | 45 Days of First Commercial Sale of Licensed Product in Territory [Member] |
|
|
|
|
|
|
|
| Collaborative Arrangements And Noncollaborative Arrangement Transactions [Line Items] |
|
|
|
|
|
|
|
| Milestone payments |
|
|
|
|
$ 1,500,000
|
|
|
| X |
- DefinitionLicense agreement expiration month.
| Name: |
onvo_LicenseAgreementExpirationMonth |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:gYearMonthItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionMinimum annual royalty payment.
| Name: |
onvo_MinimumAnnualRoyaltyPayment |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNonrefundable upfront fee paid.
| Name: |
onvo_NonrefundableUpfrontFeePaid |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of royalty revenue from sublicensee.
| Name: |
onvo_PercentageOfRoyaltyRevenueFromSublicensee |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of sublicensing revenue.
| Name: |
onvo_PercentageOfSublicensingRevenue |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRoyalty fees percentage maximum.
| Name: |
onvo_RoyaltyFeesPercentageMaximum |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRoyalty fees percentage minimum.
| Name: |
onvo_RoyaltyFeesPercentageMinimum |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRoyalty payments under license agreement due year two.
| Name: |
onvo_RoyaltyPaymentsUnderLicenseAgreementDueYearTwo |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average period before the next renewal or extension (both explicit and implicit) for intangible assets that have been renewed or extended, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-SubTopic 30
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetWeightedAveragePeriodBeforeNextRenewalOrExtension |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the next rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextRollingTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the fifth rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the fourth rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the third rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the second rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_LicensingAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=onvo_SublicenseAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=onvo_DaysOfTheDosingOfTheFirstPatientInAPhaseIiiClinicalTrialMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=onvo_DaysOfFdaApprovalOfFirstLicensedProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=onvo_DaysOfFirstCommercialSaleOfALicensedProductInTerritoryMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 928
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483147/928-340-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
| Name: |
us-gaap_FiniteLivedIntangibleAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_LicensingAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.1.1.u2
Stockholders' Equity - Common Stock - Additional Information (Details) - USD ($)
|
3 Months Ended |
12 Months Ended |
|
|
|
|
|
|
|
Oct. 12, 2022 |
Mar. 31, 2024 |
Mar. 31, 2023 |
Jan. 26, 2024 |
Jul. 25, 2022 |
Feb. 28, 2022 |
Mar. 31, 2021 |
Jan. 29, 2021 |
Jul. 31, 2018 |
Jan. 31, 2012 |
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
0
|
0
|
|
|
|
|
|
|
|
| IPO [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Securities authorized for offer and sale, amount |
|
$ 150,000,000
|
|
|
|
|
|
|
|
|
| At-The-Market Facility [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
1,172,342
|
|
|
|
|
|
|
|
|
| 2018 Sales Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Equity sales agreement expiration date |
|
Jan. 29, 2024
|
|
|
|
|
|
|
|
|
| 2021 ATM Prospectus Supplement [Member] | At-The-Market Facility [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
2,753,204
|
|
|
|
|
|
|
|
|
| 2021 ATM Prospectus Supplement [Member] | At-The-Market Facility [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common stock value reserved for future issuance |
|
|
|
|
|
|
|
$ 50,000,000
|
|
|
| 2021 Shelf [Member] | At-The-Market Facility [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
1,135,940
|
|
|
|
|
|
|
|
|
| 2024 Shelf [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Securities authorized for offer and sale, amount |
|
|
|
$ 150,000,000
|
|
|
|
|
|
|
| Common stock value reserved for future issuance |
|
$ 100,000,000
|
|
|
|
|
|
|
|
|
| 2024 Shelf [Member] | At-The-Market Facility [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
36,402
|
|
|
|
|
|
|
|
|
| 2024 ATM Prospectus [Member] | At-The-Market Facility [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common stock value reserved for future issuance |
|
$ 2,600,000
|
|
|
|
|
|
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
2,753,204
|
|
|
|
|
|
|
|
|
| 2024 ATM Prospectus [Member] | At-The-Market Facility [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common stock value reserved for future issuance |
|
|
|
$ 2,605,728
|
|
|
|
|
|
|
| 2021 and 2024 ATM Prospectus [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Value of shares sold under equity distribution agreement |
|
$ 23,200,000
|
|
|
|
|
|
|
|
|
| Equity Incentive Plan 2012 [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
1,236,738
|
|
|
|
|
|
|
|
|
327,699
|
| Shares available for issuance |
|
|
|
|
|
|
|
|
2,327,699
|
|
| Common stock shares issued |
126,262
|
|
|
|
|
|
|
|
|
|
| Inducement Award Agreements [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
|
|
|
|
|
50,000
|
|
|
|
|
| 2021 Inducement Equity Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
|
|
|
|
|
|
750,000
|
|
|
|
| Equity Incentive Plan 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
1,363,000
|
|
|
|
750,000
|
|
|
|
|
|
| Excercise price per share |
$ 2.75
|
|
|
|
|
|
|
|
|
|
| Equity Incentive Plan 2022 [Member] | Common Stock [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
50,000
|
|
|
|
|
|
|
|
|
|
| Equity Incentive Plan 2022 [Member] | Common Stock [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
51,000
|
|
|
|
|
|
|
|
|
|
| 2022 Inducement Equity Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common shares authorized to be issued |
1,000
|
|
|
|
|
|
|
|
|
|
| X |
- DefinitionCombination of securities authorized for offer and sale.
| Name: |
onvo_CombinationOfSecuritiesAuthorizedForOfferAndSale |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCommon stock capital value reserved for future issuance.
| Name: |
onvo_CommonStockCapitalValueReservedForFutureIssuance |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionEquity sales agreement expiration date.
| Name: |
onvo_EquitySalesAgreementExpirationDate |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesIssuedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=onvo_AtTheMarketFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_TwoThousandEighteenSalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_ProspectusTo2021ShelfMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_TwoThousandAndTwentyOneShelfMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_TwoThousandAndTwentyFourShelfMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_ProspectusToTwoThousandAndTwentyFourShelfMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OtherCommitmentsAxis=onvo_ProspectusTo2021And2024ATMProspectusShelfMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EquityIncentivePlanTwoThousandTwelveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_InducementAwardAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_TwoThousandAndTwentyOneInducementEquityPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EquityIncentivePlanTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_TwoThousandAndTwentyTwoInducementEquityPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Stockholders' Equity - Summary of Company's RSUs Activity (Details) - Restricted stock units (RSUs) [Member]
|
12 Months Ended |
|
Mar. 31, 2024
$ / shares
shares
|
|---|
| Class Of Stock [Line Items] |
|
| Beginning balance, Unvested, Number of Shares | shares |
127,717
|
| Granted, Number of Shares | shares |
117,642
|
| Vested, Number of Shares | shares |
(122,717)
|
| Cancelled / forfeited, Number of Shares | shares |
0
|
| Ending balance, Unvested, Number of Shares | shares |
122,642
|
| Beginning balance, Unvested, Weighted Average Price | $ / shares |
$ 2.22
|
| Granted, Weighted Average Price | $ / shares |
1.39
|
| Vested, Weighted Average Price | $ / shares |
1.9
|
| Cancelled / forfeited, Weighted Average Price | $ / shares |
0
|
| Ending balance, Unvested, Weighted Average Price | $ / shares |
$ 1.75
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares under non-option equity instrument agreements that were either cancelled or expired. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(3)-(4)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsForfeituresAndExpirations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Stockholders' Equity - Stock Options and Employee Stock Purchase Plan - Additional Information (Details) - USD ($)
|
|
12 Months Ended |
|
Aug. 28, 2023 |
Mar. 31, 2024 |
Mar. 31, 2023 |
Oct. 31, 2023 |
| Class Of Stock [Line Items] |
|
|
|
|
| Stock-based compensation |
|
$ 1,508,000
|
$ 2,377,000
|
|
| Revenue recognized |
|
$ 109,000
|
$ 370,000
|
|
| Weighted-average remaining contractual term of stock options exercisable |
|
7 years 4 months 17 days
|
|
|
| Weighted-average remaining contractual term of stock options outstanding |
|
7 years 4 months 17 days
|
|
|
| Issuance of common stock from stock options exercises, net, Shares |
|
0
|
0
|
|
| Number of common stock shares approved under ESPP |
|
2,507,899
|
|
|
| Expected life of options |
|
6 years
|
6 years
|
|
| Executive Chairman [Member] |
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
| Voluntarily forfeited outstanding stock options |
462,500
|
|
|
|
| Unvested, cancelled shares |
312,918
|
|
|
|
| Expired, Options Outstanding |
149,582
|
|
|
|
| Executive Chairman [Member] | Selling, General and Administrative Expenses [Member] |
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
| Stock-based compensation |
$ 519,000
|
|
|
|
| Equity Incentive Plan 2022 [Member] |
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
| Stock options granted |
|
184,158
|
|
|
| Number of common stock shares approved under ESPP |
|
1,641,250
|
|
|
| 2016 Employee Stock Purchase Plan [Member] |
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
| Number of common stock shares approved under ESPP |
|
75,000
|
|
|
| 2023 Employee Stock Purchase Plan [Member] |
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
| Number of common stock shares approved under ESPP |
|
|
|
45,000
|
| Employee subscription rate |
|
15.00%
|
|
|
| Compensation amount per employee |
|
$ 25,000,000
|
|
|
| Number of shares per employee |
|
500
|
|
|
| Fair market value at discount |
|
85.00%
|
|
|
| Purchase period |
|
6 months
|
|
|
| Initial offering period |
|
2024-03
|
|
|
| Description of plan |
|
Shares under the 2023 ESPP are purchased at 85 percent of the fair market value at the lower of (i) the closing price on the first trading day of the six-month purchase period or (ii) the closing price on the last trading day of the six-month purchase period.
|
|
|
| X |
- DefinitionShare based compensation arrangement by share based payment award initial offering period.
| Name: |
onvo_ShareBasedCompensationArrangementByShareBasedPaymentAwardInitialOfferingPeriod |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:gYearMonthItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare based compensation arrangement by share based payment award maximum amount per employee.
| Name: |
onvo_ShareBasedCompensationArrangementByShareBasedPaymentAwardMaximumAmountPerEmployee |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionShare based compensation arrangement by share based payment award purchase period.
| Name: |
onvo_ShareBasedCompensationArrangementByShareBasedPaymentAwardPurchasePeriod |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of terms of share-based payment arrangement. Includes, but is not limited to, type of award or grantee and reason for issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate from fair value on purchase date that participants pay for shares. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPricePurchaseDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe highest percentage of annual salary that an employee is permitted to utilize with respect to the plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardMaximumEmployeeSubscriptionRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe highest quantity of shares an employee can purchase under the plan per period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardMaximumNumberOfSharesPerEmployee |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-vested options forfeited.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsNonvestedOptionsForfeitedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=onvo_ExecutiveChairmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SellingGeneralAndAdministrativeExpensesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EquityIncentivePlanTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_TwoThousandTwentyThreeEmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Stockholders' Equity - Summary of Stock Option Activity (Details)
|
12 Months Ended |
|
Mar. 31, 2024
USD ($)
$ / shares
shares
|
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Options Outstanding, Beginning balance | shares |
1,451,217
|
| Granted, Options Outstanding | shares |
184,158
|
| Canceled, Options Outstanding | shares |
(937,368)
|
| Exercised, Options Outstanding | shares |
0
|
| Options Outstanding, Ending balance | shares |
698,007
|
| Vested and Exercisable, Options Outstanding | shares |
401,536
|
| Weighted-Average Exercise Price, Options Beginning balance | $ / shares |
$ 6.49
|
| Options granted, Weighted-Average Exercise Price | $ / shares |
1.67
|
| Options canceled, Weighted-Average Exercise Price | $ / shares |
7.2
|
| Options exercised, Weighted-Average Exercise Price | $ / shares |
0
|
| Weighted-Average Exercise Price, Options Ending balance | $ / shares |
4.26
|
| Vested and Exercisable, Weighted-Average Exercise Price | $ / shares |
$ 5.61
|
| Aggregate Intrinsic Value, Options Beginning balance | $ |
$ 38,327
|
| Options Exercised, Aggregate Intrinsic Value | $ |
0
|
| Aggregate Intrinsic Value, Options Ending balance | $ |
0
|
| Vested and Exercisable, Aggregate Intrinsic Value | $ |
$ 0
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFor presentations that combine terminations, the number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan or that expired. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price of options that were either forfeited or expired. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders' Equity - Common Stock Reserved for Future Issuance (Details)
|
Mar. 31, 2024
shares
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
2,507,899
|
| Equity Incentive Plan 2012 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
0
|
| 2016 Employee Stock Purchase Plan [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
75,000
|
| Equity Incentive Plan 2022 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
1,641,250
|
| Stock options [Member] | Equity Incentive Plan 2012 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
431,416
|
| Stock options [Member] | 2016 Employee Stock Purchase Plan [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
45,000
|
| Stock options [Member] | Equity Incentive Plan 2022 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
216,591
|
| Stock options [Member] | 2021 Inducement Equity Plan [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
1,000
|
| Stock options [Member] | Incentive Award Plan [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
50,000
|
| Restricted stock units (RSUs) [Member] | Equity Incentive Plan 2012 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
5,000
|
| Restricted stock units (RSUs) [Member] | Equity Incentive Plan 2022 [Member] |
|
| Share Based Compensation Arrangement By Share Based Payment Award [Line Items] |
|
| Common stock reserved for future issuance |
117,642
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EquityIncentivePlanTwoThousandTwelveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_EquityIncentivePlanTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_TwoThousandAndTwentyOneInducementEquityPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_IncentiveAwardPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionNumber of participants enrolled in employee stock purchase plan.
| Name: |
onvo_NumberOfParticipantsEnrolledInEmployeeStockPurchasePlan |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost to be recognized for nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedShareBasedAwardsOtherThanOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=onvo_TwoThousandTwentyThreeEmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_EmployeeStockOwnershipPlanESOPDisclosuresByPlanAxis=onvo_ESPPSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Leases - Additional Information (Details)
|
|
|
12 Months Ended |
|
|
Nov. 17, 2021
ft²
|
Nov. 23, 2020
USD ($)
ft²
|
Mar. 31, 2024
USD ($)
|
Mar. 31, 2023
USD ($)
|
Dec. 17, 2021
USD ($)
|
| Operating Lease [Line Items] |
|
|
|
|
|
| Operating lease, liability |
|
|
$ 1,394,000
|
|
$ 2,300,000
|
| Operating lease right-of-use assets |
|
|
1,299,000
|
$ 1,705,000
|
$ 2,300,000
|
| Rent expense |
|
|
503,000
|
499,000
|
|
| Variable lease expense |
|
|
153,000
|
146,000
|
|
| Operating cash flows from operating leases |
|
|
$ 509,000
|
$ 430,000
|
|
| San Diego Permanent Lease [Member] |
|
|
|
|
|
| Operating Lease [Line Items] |
|
|
|
|
|
| Lab and Office space under lease agreement | ft² |
2,892
|
8,051
|
|
|
|
| Lease term |
|
62 months
|
|
|
|
| Monthly rental payments |
|
$ 40,800
|
|
|
|
| Base rent escalators |
|
3.00%
|
|
|
|
| X |
- DefinitionLab and office space area under lease agreement.
| Name: |
onvo_LabAndOfficeSpaceAreaUnderLeaseAgreement |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:areaItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOperating leases monthly rent expense.
| Name: |
onvo_OperatingLeasesMonthlyRentExpense |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate used by lessee to determine present value of operating lease payments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseDiscountRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of variable lease cost, excluded from lease liability, recognized when obligation for payment is incurred for finance and operating leases. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_VariableLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=onvo_SanDiegoPermanentLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Leases - Schedule of Lease Liabilities and Corresponding Right-of-use Assets (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Mar. 31, 2023 |
Dec. 17, 2021 |
| Assets |
|
|
|
| Operating lease right-of-use assets |
$ 1,299
|
|
|
| Total lease right-of-use assets |
1,299
|
$ 1,705
|
$ 2,300
|
| Current Liabilities |
|
|
|
| Operating lease liability, current portion |
506
|
492
|
|
| Noncurrent |
|
|
|
| Operating lease liability, net of current portion |
888
|
$ 1,313
|
|
| Total lease liabilities |
$ 1,394
|
|
$ 2,300
|
| Weighted average remaining lease term: |
2 years 9 months 29 days
|
|
|
| Weighted average discount rate: |
6.00%
|
|
|
| X |
- DefinitionOperating lease right of use asset non current.
| Name: |
onvo_OperatingLeaseRightOfUseAssetNonCurrent |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.1.1.u2
Leases - Schedule of Future Lease Payments (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Mar. 31, 2023 |
Dec. 17, 2021 |
| Leases [Abstract] |
|
|
|
| Fiscal year ending March 31, 2025 |
$ 523
|
|
|
| Fiscal year ending March 31, 2026 |
538
|
|
|
| Fiscal year ending March 31, 2027 |
460
|
|
|
| Total future lease payments |
1,521
|
|
|
| Less: Imputed Interest |
(127)
|
|
|
| Total lease liabilities |
1,394
|
|
$ 2,300
|
| Less: Current obligations |
(506)
|
$ (492)
|
|
| Noncurrent lease obligations |
$ 888
|
$ 1,313
|
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.1.1.u2
v3.24.1.1.u2
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset or (liability) attributable to deductible temporary differences from property, plant, and equipment.
| Name: |
onvo_DeferredTaxAssetLiabilityPropertyPlantAndEquipment |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDeferred tax assets operating lease liability.
| Name: |
onvo_DeferredTaxAssetsOperatingLeaseLiability |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDeferred tax assets research and development capitalization.
| Name: |
onvo_DeferredTaxAssetsResearchAndDevelopmentCapitalization |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDeferred tax liabilities investment in equity securities.
| Name: |
onvo_DeferredTaxLiabilitiesInvestmentInEquitySecurities |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionDeferred tax liability operating lease right-of-use assets.
| Name: |
onvo_DeferredTaxLiabilitiesOperatingLeaseRightOfUseAssets |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from intangible assets including goodwill.
| Name: |
us-gaap_DeferredTaxAssetsGoodwillAndIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from inventory. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxAssetsNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from reserves and accruals. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccruals |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible temporary differences from reserves and accruals, classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccrualsOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxLiabilitiesNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Income Taxes - Additional Information (Details) - USD ($)
|
12 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Operating Loss Carryforwards [Line Items] |
|
|
| Unrecognized tax benefits |
$ 0
|
|
| Uncertain tax position netted against deferred tax asset |
1,700,000
|
$ 1,600,000
|
| Increase (decrease) in valuation allowance |
525,000
|
$ 1,875,000
|
| Federal net operating loss carryforwards |
219,400,000
|
|
| State net operating loss carryforwards |
$ 42,800,000
|
|
| Federal net operating loss carryforwards (“NOLs”) expiring year |
2028
|
|
| State net operating loss carryforwards (“NOLs”) expiring year |
2028
|
|
| Federal research tax credit carryforwards expiration period |
2028
|
|
| Accrued expense regarding interest or penalties |
$ 0
|
|
| Federal [Member] |
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
| Operating loss carry forwards |
75,800,000
|
|
| Research tax credit carryforwards |
$ 5,000,000.0
|
|
| Federal [Member] | Maximum [Member] |
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
| Percentage of future taxable income to be offset |
80.00%
|
|
| State [Member] |
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
| Research tax credit carryforwards |
$ 4,500,000
|
|
| X |
- DefinitionFederal net operating loss carry forwards expiration year start.
| Name: |
onvo_FederalNetOperatingLossCarryForwardsExpirationYearStart |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFederal research tax credit carry forwards expiration year start.
| Name: |
onvo_FederalResearchTaxCreditCarryForwardsExpirationYearStart |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of future taxable income to be offset.
| Name: |
onvo_PercentageOfFutureTaxableIncomeToBeOffset |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionState net operating loss carry forwards expiration year start.
| Name: |
onvo_StateNetOperatingLossCarryForwardsExpirationYearStart |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible domestic operating loss carryforwards. Excludes state and local operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwardsDomestic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible state and local operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwardsStateAndLocal |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible research tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwardsResearch |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount recognized for uncertainty in income taxes classified as noncurrent. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilityForUncertainTaxPositionsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OperatingLossCarryforwardsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-10B
| Name: |
us-gaap_UnrecognizedTaxBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
v3.24.1.1.u2
| X |
- References
+ Details
| Name: |
us-gaap_CompensationAndRetirementDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of administration expense of defined benefit plan which decreases plan assets. Excludes plan administration expense paid by employer.
| Name: |
us-gaap_DefinedBenefitPlanAdministrationExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum percentage of employee gross pay the employee may contribute to a defined contribution plan.
| Name: |
us-gaap_DefinedContributionPlanMaximumAnnualContributionsPerEmployeePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionRestructuring annual cost savings.
| Name: |
onvo_RestructuringAnnualCostSavings |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
| Name: |
us-gaap_RestructuringCostAndReserveLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses for special or contractual termination benefits provided to current employees involuntarily terminated under a benefit arrangement associated exit or disposal activities pursuant to an authorized plan. Excludes expenses related to one-time termination benefits, a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_SeveranceCosts1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SegmentReportingRevenueReconcilingItemLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_OperatingSegmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_ResearchDevelopmentSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_RoyaltyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_MosaicSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Business Segment Information - Summary of Operating Segment Expenses (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Segment Reporting, Reconciling Item for Operating Profit (Loss) from Segment to Consolidated [Line Items] |
|
|
| Total segment operating expenses |
$ 5,498
|
$ 8,885
|
| Selling, general, and administrative expenses |
9,697
|
9,216
|
| Total costs and expenses |
15,195
|
18,101
|
| Operating Segments [Member] |
|
|
| Segment Reporting, Reconciling Item for Operating Profit (Loss) from Segment to Consolidated [Line Items] |
|
|
| Total segment operating expenses |
5,498
|
8,885
|
| Selling, general, and administrative expenses |
9,697
|
9,216
|
| Total costs and expenses |
15,195
|
18,101
|
| Operating Segments [Member] | R&D [Member] |
|
|
| Segment Reporting, Reconciling Item for Operating Profit (Loss) from Segment to Consolidated [Line Items] |
|
|
| Total segment operating expenses |
4,404
|
8,885
|
| Operating Segments [Member] | Mosaic [Member] |
|
|
| Segment Reporting, Reconciling Item for Operating Profit (Loss) from Segment to Consolidated [Line Items] |
|
|
| Total segment operating expenses |
$ 1,094
|
$ 0
|
| X |
- DefinitionTotal costs of sales and operating expenses for the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SegmentReportingReconcilingItemForOperatingProfitLossFromSegmentToConsolidatedLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_OperatingSegmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_ResearchDevelopmentSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_MosaicSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Business Segment Information - Summary of Operating Segment Assets (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Segment Reporting, Asset Reconciling Item [Line Items] |
|
|
| Fixed assets, net |
$ 669
|
$ 902
|
| Inventory |
297
|
0
|
| Total assets |
6,349
|
20,313
|
| Operating Segments [Member] |
|
|
| Segment Reporting, Asset Reconciling Item [Line Items] |
|
|
| Total segment assets |
903
|
902
|
| Other company assets |
5,446
|
19,411
|
| Total assets |
6,349
|
20,313
|
| Operating Segments [Member] | R&D [Member] |
|
|
| Segment Reporting, Asset Reconciling Item [Line Items] |
|
|
| Fixed assets, net |
423
|
902
|
| Operating Segments [Member] | Mosaic [Member] |
|
|
| Segment Reporting, Asset Reconciling Item [Line Items] |
|
|
| Fixed assets, net |
183
|
0
|
| Inventory |
$ 297
|
$ 0
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SegmentReportingAssetReconcilingItemLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidationItemsAxis=us-gaap_OperatingSegmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_ResearchDevelopmentSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=onvo_MosaicSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Subsequent Events - Additional Information (Details) - USD ($)
$ / shares in Units, $ in Thousands |
|
|
2 Months Ended |
12 Months Ended |
|
May 13, 2024 |
May 08, 2024 |
May 30, 2024 |
Mar. 31, 2024 |
Mar. 31, 2023 |
May 29, 2024 |
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Issuance of common stock |
|
|
|
0
|
0
|
|
| Cash and cash equivalents |
|
|
|
$ 2,901
|
$ 15,301
|
|
| Subsequent Event [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Subsequent event, date |
|
May 08, 2024
|
|
|
|
|
| Cash and cash equivalents |
|
|
|
|
|
$ 7,000
|
| Subsequent Event [Member] | Common Warrants [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Stock offering price per share |
|
$ 0.8
|
|
|
|
|
| Issuance of warrants |
|
1,562,500
|
|
|
|
|
| Warrants exercise price per share |
|
$ 0.8
|
|
|
|
|
| Warrant expiration period |
|
5 years
|
|
|
|
|
| Subsequent Event [Member] | Pre-Funded Warrants [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Stock offering price per share |
|
$ 0.799
|
|
|
|
|
| Warrants exercise price per share |
|
$ 0.001
|
|
|
|
|
| Subsequent Event [Member] | Public Offering [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Issuance of common stock |
|
1,562,500
|
|
|
|
|
| Initial offering date |
|
May 8, 2024
|
|
|
|
|
| Net proceeds from issuance of offerings |
$ 4,700
|
|
|
|
|
|
| Subsequent Event [Member] | Public Offering [Member] | Common Warrants [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Issuance of common stock |
|
5,000,000
|
|
|
|
|
| Subsequent Event [Member] | ATM Offerings [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Issuance of common stock |
|
|
1,389,002
|
|
|
|
| Net proceeds from issuance of offerings |
|
|
$ 1,800
|
|
|
|
| Maximum [Member] | Subsequent Event [Member] | Public Offering [Member] | Common Warrants [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Issuance of warrants |
|
5,000,000
|
|
|
|
|
| JonesTrading Institutional Services LLC [Member] | Subsequent Event [Member] | Public Offering [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Cash fee percentage of aggregate gross proceeds raised in offering |
|
5.00%
|
|
|
|
|
| X |
- DefinitionCash fee percentage of aggregate gross proceeds raised in offering.
| Name: |
onvo_CashFeePercentageOfAggregateGrossProceedsRaisedInOffering |
| Namespace Prefix: |
onvo_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWarrant expiration period.
| Name: |
onvo_WarrantExpirationPeriod |
| Namespace Prefix: |
onvo_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA description of the time period when the company made its initial offering of a class of securities.
| Name: |
us-gaap_InitialOfferingPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDate the event occurred after the balance sheet date but before financial statements are issues or available to be issued, in YYYY-MM-DD format. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 855
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=onvo_CommonWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=onvo_PreFundedWarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=onvo_ATMOfferingsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=onvo_JonestradingInstitutionalServicesLlcMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Organovo (NASDAQ:ONVO)
Historical Stock Chart
From Dec 2024 to Jan 2025
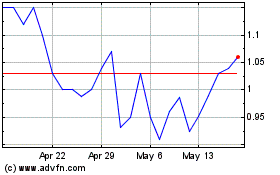
Organovo (NASDAQ:ONVO)
Historical Stock Chart
From Jan 2024 to Jan 2025