Orthofix Announces FDA 510(k) Clearance for the Rodeo Telescopic Nail
May 09 2024 - 6:00AM
Business Wire
Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and
orthopedics company, today announced that it has received U.S. Food
and Drug Administration (FDA) 510(k) clearance to market its Rodeo™
Telescopic Nail.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20240509384510/en/
The Rodeo Telescopic Nail by Orthofix is
an innovative device to surgically treat deformities or fractures
in patients suffering from osteogenesis imperfecta. (Photo:
Business Wire)
The Rodeo Telescopic Nail is an innovative device indicated to
surgically treat deformities or fractures in patients suffering
from osteogenesis imperfecta (OI). The nail implant serves to
stabilize the patient’s limb while also elongating (or telescoping)
to accommodate the natural growth of pediatric patients.
The Rodeo system is designed to address many of the
biomechanical and procedural challenges associated with current OI
telescopic rod systems. Specifically, its patented design provides
the strength and reliable bone fixation required when implanting in
the inherently fragile bone associated with OI patients. The
implant procedure is streamlined and the system’s instrumentation
and sterile pack configurations aid in optimizing efficiency in the
operating room and eliminate the need to sterilize trays leading up
to the surgery, thereby reducing procedural costs, O.R. time, and
risks of contamination.
“The launch of the Rodeo Telescopic Nail represents Orthofix’s
continued commitment to address the underserved pediatric market
with specialized solutions tailored to the specific needs and
unique conditions, such as OI, of this patient population,” said
Kim Elting, President of Global Orthopedics. “The Rodeo system has
been very well received in Europe, and we are pleased to be able to
announce this limited U.S. market release during National OI
Awareness Week and join in educating others about this genetic bone
disorder that is present at birth.”
Often referred to as brittle bone disease, OI affects both males
and females equally throughout the world with a prevalence
estimated to be one in 10,000 births. A child born with OI may have
bones that break easily or form abnormally. In more severe cases,
it can involve hundreds of fractures that occur without apparent
cause.
Orthofix will be exhibiting at the EPOSNA Annual Meeting (Booth
#24) May 8-11 in National Harbor, Maryland, and featuring the Rodeo
Telescopic Nail in an educational symposium entitled “Osteogenesis
Imperfecta Disease: Shared Experiences on Different Techniques and
the Latest Solutions.”
The Rodeo Telescopic Nail will be available soon at select
institutions in the U.S.; it has been in clinical use in a limited
number of European countries since 2022 with more than 170
surgeries performed to date.
For more information about Orthofix’s full portfolio of
pediatric solutions, please visit OrthofixKids.com.
About Orthofix
Orthofix is a leading global spine and orthopedics company with
a comprehensive portfolio of biologics, innovative spinal hardware,
bone growth therapies, specialized orthopedic solutions, and a
leading surgical navigation system. Its products are distributed in
more than 60 countries worldwide.
The Company is headquartered in Lewisville, Texas, where it
conducts general business, product development, medical education
and manufacturing, and has primary offices in Carlsbad, CA, with a
focus on spine and biologics product innovation and surgeon
education, and Verona, Italy, with an emphasis on product
innovation, production, and medical education for orthopedics. The
combined Company’s global R&D, commercial and manufacturing
footprint also includes facilities and offices in Irvine, CA,
Toronto, Canada, Sunnyvale, CA, Maidenhead, UK, Munich, Germany,
Paris, France and São Paulo, Brazil.
Forward-Looking Statements
This news release may include forward-looking statements within
the meaning of Section 21E of the Securities Exchange Act of 1934,
as amended, and Section 27A of the Securities Act of 1933, as
amended. In some cases, you can identify forward-looking statements
by terminology such as “may,” “will,” “should,” “expects,” “plans,”
“anticipates,” “believes,” “estimates,” “projects,” “intends,”
“predicts,” “potential,” “continue” or other comparable
terminology. Orthofix cautions you that statements included in this
news release that are not a description of historical facts are
forward-looking statements that are based on the Company’s current
expectations and assumptions. Each forward-looking statement
contained in this news release is subject to risks and
uncertainties that could cause actual results to differ materially
from those expressed or implied by such statement. Applicable risks
and uncertainties include, among others: the ability of newly
launched products to perform as designed and intended and to meet
the needs of surgeons and patients, including as a result of the
lack of robust clinical validation; and the risks identified under
the heading “Risk Factors” in Orthofix Medical Inc.’s Annual Report
on Form 10-K for the fiscal year ended December 31, 2023, which was
filed with the Securities and Exchange Commission (SEC) on March 5,
2024. The Company’s public filings with the Securities and Exchange
Commission are available at www.sec.gov. You are cautioned not to
place undue reliance on forward-looking statements, which speak
only as of the date when made. Orthofix does not intend to revise
or update any forward-looking statement set forth in this news
release to reflect events or circumstances arising after the date
hereof, except as may be required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240509384510/en/
Media Relations Denise Landry DeniseLandry@orthofix.com
214.937.2529 Investor Relations Louisa Smith, Gilmartin
Group IR@orthofix.com
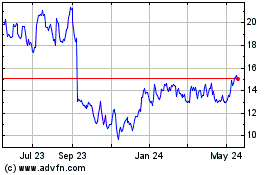
Orthofix Medical (NASDAQ:OFIX)
Historical Stock Chart
From Nov 2024 to Dec 2024
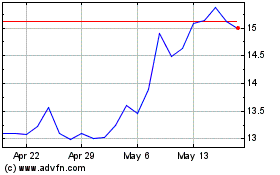
Orthofix Medical (NASDAQ:OFIX)
Historical Stock Chart
From Dec 2023 to Dec 2024