false
0001533040
0001533040
2024-01-01
2024-09-30
0001533040
2024-09-30
0001533040
2023-12-31
0001533040
2024-07-01
2024-09-30
0001533040
2023-07-01
2023-09-30
0001533040
2023-01-01
2023-09-30
0001533040
PHIO:PreferredStockSeriesDMember
2023-12-31
0001533040
us-gaap:CommonStockMember
2023-12-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-12-31
0001533040
us-gaap:RetainedEarningsMember
2023-12-31
0001533040
PHIO:PreferredStockSeriesDMember
2024-03-31
0001533040
us-gaap:CommonStockMember
2024-03-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-03-31
0001533040
us-gaap:RetainedEarningsMember
2024-03-31
0001533040
2024-03-31
0001533040
PHIO:PreferredStockSeriesDMember
2024-06-30
0001533040
us-gaap:CommonStockMember
2024-06-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-06-30
0001533040
us-gaap:RetainedEarningsMember
2024-06-30
0001533040
2024-06-30
0001533040
PHIO:PreferredStockSeriesDMember
2022-12-31
0001533040
us-gaap:CommonStockMember
2022-12-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001533040
us-gaap:RetainedEarningsMember
2022-12-31
0001533040
2022-12-31
0001533040
PHIO:PreferredStockSeriesDMember
2023-03-31
0001533040
us-gaap:CommonStockMember
2023-03-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001533040
us-gaap:RetainedEarningsMember
2023-03-31
0001533040
2023-03-31
0001533040
PHIO:PreferredStockSeriesDMember
2023-06-30
0001533040
us-gaap:CommonStockMember
2023-06-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001533040
us-gaap:RetainedEarningsMember
2023-06-30
0001533040
2023-06-30
0001533040
PHIO:PreferredStockSeriesDMember
2024-01-01
2024-03-31
0001533040
us-gaap:CommonStockMember
2024-01-01
2024-03-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-01-01
2024-03-31
0001533040
us-gaap:RetainedEarningsMember
2024-01-01
2024-03-31
0001533040
2024-01-01
2024-03-31
0001533040
PHIO:PreferredStockSeriesDMember
2024-04-01
2024-06-30
0001533040
us-gaap:CommonStockMember
2024-04-01
2024-06-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-04-01
2024-06-30
0001533040
us-gaap:RetainedEarningsMember
2024-04-01
2024-06-30
0001533040
2024-04-01
2024-06-30
0001533040
PHIO:PreferredStockSeriesDMember
2024-07-01
2024-09-30
0001533040
us-gaap:CommonStockMember
2024-07-01
2024-09-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-07-01
2024-09-30
0001533040
us-gaap:RetainedEarningsMember
2024-07-01
2024-09-30
0001533040
PHIO:PreferredStockSeriesDMember
2023-01-01
2023-03-31
0001533040
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-03-31
0001533040
us-gaap:RetainedEarningsMember
2023-01-01
2023-03-31
0001533040
2023-01-01
2023-03-31
0001533040
PHIO:PreferredStockSeriesDMember
2023-04-01
2023-06-30
0001533040
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001533040
us-gaap:RetainedEarningsMember
2023-04-01
2023-06-30
0001533040
2023-04-01
2023-06-30
0001533040
PHIO:PreferredStockSeriesDMember
2023-07-01
2023-09-30
0001533040
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001533040
us-gaap:RetainedEarningsMember
2023-07-01
2023-09-30
0001533040
PHIO:PreferredStockSeriesDMember
2024-09-30
0001533040
us-gaap:CommonStockMember
2024-09-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2024-09-30
0001533040
us-gaap:RetainedEarningsMember
2024-09-30
0001533040
PHIO:PreferredStockSeriesDMember
2023-09-30
0001533040
us-gaap:CommonStockMember
2023-09-30
0001533040
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001533040
us-gaap:RetainedEarningsMember
2023-09-30
0001533040
2023-09-30
0001533040
2024-07-04
2024-07-05
0001533040
PHIO:ClinicalCoDevelopmentAgreementMember
PHIO:AgonOxMember
2024-09-30
0001533040
PHIO:ClinicalCoDevelopmentAgreementMember
PHIO:AgonOxMember
2024-07-01
2024-09-30
0001533040
PHIO:ClinicalCoDevelopmentAgreementMember
PHIO:AgonOxMember
2024-01-01
2024-09-30
0001533040
PHIO:ClinicalCoDevelopmentAgreementMember
PHIO:AgonOxMember
2023-07-01
2023-09-30
0001533040
PHIO:ClinicalCoDevelopmentAgreementMember
PHIO:AgonOxMember
2023-01-01
2023-09-30
0001533040
us-gaap:CashAndCashEquivalentsMember
2024-09-30
0001533040
us-gaap:CashAndCashEquivalentsMember
us-gaap:FairValueInputsLevel1Member
2024-09-30
0001533040
us-gaap:CashAndCashEquivalentsMember
us-gaap:FairValueInputsLevel2Member
2024-09-30
0001533040
us-gaap:CashAndCashEquivalentsMember
us-gaap:FairValueInputsLevel3Member
2024-09-30
0001533040
us-gaap:FairValueInputsLevel1Member
2024-09-30
0001533040
us-gaap:FairValueInputsLevel2Member
2024-09-30
0001533040
us-gaap:FairValueInputsLevel3Member
2024-09-30
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
2024-03-01
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
2024-02-29
2024-03-01
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
2024-01-01
2024-09-30
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
PHIO:LaboratoryFacilityMember
2024-07-01
2024-09-30
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
PHIO:LaboratoryFacilityMember
2024-01-01
2024-09-30
0001533040
us-gaap:PropertySubjectToOperatingLeaseMember
2024-09-30
0001533040
PHIO:May2024FinancingMember
2024-05-16
0001533040
PHIO:May2024FinancingMember
2024-05-15
2024-05-16
0001533040
PHIO:July2024FinancingMember
2024-07-11
0001533040
PHIO:July2024FinancingMember
PHIO:SeriesCWarrantsMember
2024-07-11
0001533040
PHIO:July2024FinancingMember
PHIO:SeriesDWarrantsMember
2024-07-11
0001533040
PHIO:July2024FinancingMember
2024-07-10
2024-07-11
0001533040
PHIO:EquityClassifiedWarrantsMember
2024-07-01
2024-07-31
0001533040
PHIO:PlacementAgentWarrantsMember
2024-07-01
2024-07-31
0001533040
PHIO:July2024AbeyanceSharesMember
PHIO:July2024InducementLetterAgreementMember
2024-07-31
0001533040
PHIO:July2024AbeyanceSharesMember
PHIO:July2024InducementLetterAgreementMember
2024-07-01
2024-09-30
0001533040
PHIO:April2023FinancingMember
2023-04-19
2023-04-20
0001533040
PHIO:April2023FinancingMember
2023-04-20
0001533040
PHIO:April2023FinancingMember
PHIO:SeriesAWarrantsMember
2023-04-20
0001533040
PHIO:April2023FinancingMember
PHIO:SeriesBWarrantsMember
2023-04-20
0001533040
PHIO:April2023FinancingMember
PHIO:PlacementAgentWarrantsMember
2023-04-20
0001533040
PHIO:April2023FinancingMember
PHIO:WarrantAmendmentAgreementsMember
PHIO:PreviouslyIssuedWarrantsMember
2023-04-19
2023-04-20
0001533040
PHIO:April2023FinancingMember
PHIO:WarrantAmendmentAgreementsMember
PHIO:PreviouslyIssuedWarrantsMember
2023-04-20
0001533040
PHIO:June2023FinancingMember
PHIO:RegisteredSharesMember
2023-06-01
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:UnregisteredSharesMember
2023-06-01
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:UnregisteredSharesMember
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:UnregisteredPreFundedWarrantsMember
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:SeriesAWarrantsMember
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:SeriesBWarrantsMember
2023-06-02
0001533040
PHIO:June2023FinancingMember
PHIO:PlacementAgentWarrantsMember
2023-06-02
0001533040
PHIO:June2023FinancingMember
2023-06-01
2023-06-02
0001533040
PHIO:June2023PrefundedWarrantsMember
2023-07-01
2023-09-30
0001533040
PHIO:July2024AbeyanceSharesMember
PHIO:July2024InducementLetterAgreementMember
2024-01-01
2024-09-30
0001533040
PHIO:June2023PrefundedWarrantsMember
2023-01-01
2023-09-30
0001533040
us-gaap:WarrantMember
2023-12-31
0001533040
us-gaap:WarrantMember
2024-01-01
2024-09-30
0001533040
us-gaap:WarrantMember
2024-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
PHIO:EmployeesMember
2024-01-01
2024-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2023-07-01
2023-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2023-01-01
2023-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2024-07-01
2024-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2024-01-01
2024-09-30
0001533040
us-gaap:EmployeeStockOptionMember
PHIO:EmployeesMember
2024-01-01
2024-09-30
0001533040
us-gaap:EmployeeStockOptionMember
PHIO:NonEmployeeMembersMember
2024-01-01
2024-09-30
0001533040
us-gaap:EmployeeStockOptionMember
2024-01-01
2024-09-30
0001533040
us-gaap:EmployeeStockOptionMember
2023-07-01
2023-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2023-12-31
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2024-09-30
0001533040
us-gaap:EmployeeStockOptionMember
2023-12-31
0001533040
us-gaap:EmployeeStockOptionMember
2024-09-30
0001533040
us-gaap:ResearchAndDevelopmentExpenseMember
2024-07-01
2024-09-30
0001533040
us-gaap:ResearchAndDevelopmentExpenseMember
2023-07-01
2023-09-30
0001533040
us-gaap:ResearchAndDevelopmentExpenseMember
2024-01-01
2024-09-30
0001533040
us-gaap:ResearchAndDevelopmentExpenseMember
2023-01-01
2023-09-30
0001533040
us-gaap:GeneralAndAdministrativeExpenseMember
2024-07-01
2024-09-30
0001533040
us-gaap:GeneralAndAdministrativeExpenseMember
2023-07-01
2023-09-30
0001533040
us-gaap:GeneralAndAdministrativeExpenseMember
2024-01-01
2024-09-30
0001533040
us-gaap:GeneralAndAdministrativeExpenseMember
2023-01-01
2023-09-30
0001533040
PHIO:StockOptionsMember
2024-01-01
2024-09-30
0001533040
PHIO:StockOptionsMember
2023-01-01
2023-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2024-01-01
2024-09-30
0001533040
us-gaap:RestrictedStockUnitsRSUMember
2023-01-01
2023-09-30
0001533040
us-gaap:WarrantMember
2024-01-01
2024-09-30
0001533040
us-gaap:WarrantMember
2023-01-01
2023-09-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
utr:sqft
Table of Contents
As filed with the Securities and Exchange Commission
on January 21, 2025
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
PHIO PHARMACEUTICALS CORP.
(Exact name of Registrant as specified in its
charter)
|
Delaware
(State or other jurisdiction of
incorporation or organization) |
|
2834
(Primary Standard Industrial
Classification Code Number) |
|
45-3215903
(I.R.S. Employer
Identification Number) |
11 Apex Drive, Suite 300A, PMB 2006
Marlborough, Massachusetts 01752
(508) 767-3861
(Address, including zip code, and telephone
number, including area code, of Registrant’s principal executive offices)
Robert J. Bitterman
President & CEO
Phio Pharmaceuticals Corp.
11 Apex Drive, Suite 300A, PMB 2006
Marlborough, Massachusetts 01752
(508) 767-3861
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
Copies to:
Steven J. Abrams, Esq.
Hogan Lovells US LLP
1735 Market Street, Suite 2300
Philadelphia, Pennsylvania 19103
(267) 675-4600
Approximate date of commencement
of proposed sale to the public:
From time to time after the
effective date of this Registration Statement.
If any of the securities being registered on this
Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.
☒
If this Form is filed to register additional securities
for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
|
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
|
☒ |
| |
|
Emerging growth company |
|
☐ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration
statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which
specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission,
acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus
is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange
Commission is effective. This preliminary prospectus is not an offer to sell these securities, and it is not soliciting an offer to buy
these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated January 21, 2025
Preliminary Prospectus

Up to 5,930,016 Shares of Common Stock
Pursuant to this prospectus, the selling stockholders
identified herein (the “Selling Stockholders”) are offering on a resale basis an aggregate of up to 5,930,016 shares
of common stock of Phio Pharmaceuticals Corp. (the “Company,” “we,” “us” or “our”),
par value $0.0001 per share (the “Common Stock) consisting of (a) up to 437,192 shares of Common Stock that are issuable
upon exercise of warrants with a five year term (the “Series E Warrants”) purchased pursuant to a securities purchase
agreement by and among the Company and certain of the Selling Stockholders, dated December 19, 2024, (b) up to 240,000 shares of Common
Stock that are issuable upon exercise of warrants with a five year term (the “Series F Warrants”) purchased pursuant
to a securities purchase agreement by and among the Company and certain of the Selling Stockholders, dated December 23, 2024, (c) up to
2,127,340 shares of Common Stock that are issuable upon exercise of warrants with a twenty-four month term (the “Series G Warrants”)
purchased pursuant to a securities purchase agreement by and among the Company and certain of the Selling Stockholders, dated January
13, 2025, (d) up to 1,666,670 shares of Common Stock that are issuable upon exercise of warrants with a twenty-four month term (the “Series
H Warrants”) purchased pursuant to a securities purchase agreement by and among the Company and certain of the Selling Stockholders,
dated January 14, 2025, (e) up to 1,220,000 shares of Common Stock that are issuable upon exercise of warrants with a twenty-four month
term (the “Series I Warrants”) purchased pursuant to a securities purchase agreement by and among the Company and certain
of the Selling Stockholders, dated January 16, 2025 and (f) up to 238,814 shares of Common Stock that are issuable upon the exercise of
certain warrants (together with the Series E Warrants, the Series F Warrants, the Series G Warrants, the Series H Warrants and the Series
I Warrants, the “Warrants”) issued to our placement agent pursuant to an engagement letter in connection with the offerings
described herein.
We will not receive any of the proceeds from the
sale by the Selling Stockholders of the Common Stock covered by this prospectus. Upon any exercise of the Warrants by payment of cash,
however, we will receive the exercise price of the Warrants, which, if exercised in cash with respect to the 5,930,016 shares of Common
Stock offered hereby, would result in gross proceeds to us of approximately $17.5 million. However, we cannot predict when and in what
amounts or if the Warrants will be exercised by payments of cash and it is possible that the Warrants may expire and never be exercised,
in which case we would not receive any cash proceeds.
The Selling Stockholders may sell or otherwise
dispose of the Common Stock covered by this prospectus in a number of different ways and at varying prices. We provide more information
about how the Selling Stockholders may sell or otherwise dispose of the Common Stock covered by this prospectus in the section entitled
“Plan of Distribution” on page 50. Discounts, concessions, commissions and similar selling
expenses attributable to the sale of Common Stock covered by this prospectus will be borne by the Selling Stockholders. We will pay all
expenses (other than discounts, concessions, commissions and similar selling expenses) relating to the registration of the Common Stock
with the Securities and Exchange Commission (the “SEC”).
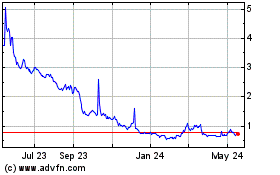
Our Common Stock is listed on The Nasdaq Capital
Market under the symbol “PHIO.” On January 17, 2025, the last reported sale price of our Common Stock on The Nasdaq Capital
Market was $2.77 per share.
Investing in our securities involves a high
degree of risk. Before making any investment in these securities, you should consider carefully the risks and uncertainties described
in the section entitled “Risk Factors” beginning on page 10 of this prospectus.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
The date of this prospectus is ,
2025
TABLE OF CONTENTS
Page
ABOUT THIS PROSPECTUS
This prospectus relates to the resale by the Selling
Stockholders identified in this prospectus under the caption “Selling Stockholders,” from time to time, of up to an aggregate
of 5,930,016 shares of Common Stock. We are not selling any shares of Common Stock under this prospectus, and we will not receive any
proceeds from the sale of shares of Common Stock offered hereby by the Selling Stockholders, although we may receive cash from the exercise
of the Warrants.
You should rely only on the information provided
in this prospectus. We have not authorized anyone to provide you with any other information and we take no responsibility for, and can
provide no assurances as to the reliability of, any other information that others may give you. The information contained in this prospectus
speaks only as of the date set forth on the cover page and may not reflect subsequent changes in our business, financial condition, results
of operations and prospects.
We are not, and the Selling Stockholders are not,
making offers to sell these securities in any jurisdiction in which an offer or solicitation is not authorized or permitted or in which
the person making such offer or solicitation is not qualified to do so or to any person to whom it is unlawful to make such an offer or
solicitation. You should read this prospectus in its entirety before making an investment decision. You should also read and consider
the information in the documents to which we have referred you in the section entitled “Where You Can Find More Information”
below, including the registration statement and the other reports we file with the SEC.
In this prospectus, unless otherwise noted, (1)
the term “Phio” refers to Phio Pharmaceuticals Corp. and our subsidiary, MirImmune, LLC and (2) the terms “Company,”
“we,” “us” and “our” refer to the ongoing business operations of Phio and MirImmune, LLC, whether
conducted through Phio or MirImmune, LLC.
CAUTIONARY NOTE
REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements
within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such
as “intends,” “believes,” “anticipates,” “indicates,” “plans,” “expects,”
“suggests,” “may,” “would,” “should,” “potential,” “designed to,”
“will,” “ongoing,” “estimate,” “forecast,” “target,” “predict,”
“could,” and similar references, although not all forward-looking statements contain these words. Forward-looking statements
are neither historical facts nor assurances of future performance. These statements are based only on our current beliefs, expectations
and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy
and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks
and changes in circumstances that are difficult to predict and many of which are outside of our control. Risks that could cause actual
results to vary from expected results expressed in our forward-looking statements include, but are not limited to:
| |
· |
we are dependent on the success of our INTASYL™ technology, and our product candidates based on this technology, which is unproven and may never lead to approved and marketable products; |
| |
· |
our product candidates are in an early stage of development and we may fail, experience significant delays, never advance in clinical development or not be successful in our efforts to identify or discover additional product candidates, which may materially and adversely impact our business; |
| |
· |
if we experience delays or difficulties in identifying and enrolling subjects in clinical trials, it may lead to delays in generating clinical data and the receipt of necessary regulatory approvals; |
| |
· |
topline data may not accurately reflect or may materially differ from the complete results of a clinical trial; |
| |
· |
we rely upon third parties for the manufacture of the clinical supply for our product candidates; |
| |
· |
our business and operations would suffer in the event of computer system failures, cyberattacks or a deficiency in our cybersecurity; |
| |
· |
we are dependent on the patents we own and the technologies we license, and if we fail to maintain our patents or lose the right to license such technologies, our ability to develop new products would be harmed; |
| |
· |
we will require substantial additional funds to complete our research and development activities; |
| |
· |
future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our stockholders or may otherwise adversely affect our business; |
| |
· |
we may not be able to maintain compliance with the continued listing requirements of The Nasdaq Capital Market; and |
| |
· |
the price of our common stock has been and may continue to be volatile. |
The risks set forth above are not exhaustive and
additional factors, including those identified in this prospectus under the heading “Risk Factors,” for reasons described
elsewhere in this prospectus and in other filings Phio Pharmaceuticals Corp. periodically makes with the Securities and Exchange Commission,
including the other risks identified in Item 1A. Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2023 and
our subsequent Quarterly Reports on Form 10-Q, could adversely affect our business and financial performance. Therefore, you should not
rely unduly on any of these forward-looking statements. Forward-looking statements contained in this prospectus speak as of the date hereof
and Phio Pharmaceuticals Corp. does not undertake to update any of these forward-looking statements to reflect a change in its views or
events or circumstances that occur after the date of this report, except as required by law.
Prospectus Summary
The following summary
highlights certain information contained elsewhere in this prospectus. This summary provides an overview of selected information and
does not contain all of the information you should consider in making your investment decision. Therefore, you should read the
entire prospectus carefully before investing in our securities. Investors should carefully consider the information set forth under
“Risk Factors” beginning on page 10 of this prospectus and the financial statements
and other information in this prospectus.
Overview
Phio (“Phio”,
“we”, “our”, or the “Company”) is a clinical stage biotechnology company whose
proprietary INTASYL® small interfering RNA gene silencing technology is designed to make immune cells more effective in killing tumor
cells. We are developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that reduce the body’s
ability to fight cancer, without the need for specialized formulations or drug delivery systems. We are committed to discovering and developing
innovative cancer treatments for patients by creating new pathways toward a cancer-free future.
INTASYL Technology
Overall, RNA is involved in
the synthesis, regulation and expression of proteins. RNA takes the instructions from DNA and turns those instructions into proteins within
the body’s cells. RNA interference, or RNAi, is a biological process that inhibits the expression of genes or the production of
proteins. Diseases are often related to the incorrect protein being made, excessive amounts of a specific protein being made, or the correct
protein being made, but at the wrong location or time. RNAi offers a novel approach to drug development because RNAi compounds can be
designed to silence any one of the thousands of human genes, many of which are considered “undruggable” by traditional therapeutics.
Our development efforts are
based on our proprietary INTASYL small interfering RNA technology. It is a patented technology from which specific patented compounds
are developed. INTASYL compounds are comprised of a unique sequence of chemically modified nucleotides (modified small interfering RNA,
or siRNAs) that target a broad range of cell types and tissues. The compounds are designed to effectively silence genes that tumors use
to evade the immune system.
Since the initial discovery
of RNAi, drug delivery has been the primary challenge in developing RNAi-based therapeutics. Other siRNA technologies require cell targeting
chemical conjugates which limit delivery to specific cell types. INTASYL is based on proprietary chemistry that is designed to maximize
the activity and adaptability of the compound and is unique in that it can be delivered to any cell type or tissue without the need to
modify the chemistry. This is designed to eliminate the need for formulations or delivery systems (for example, nanoparticles or electroporation).
This provides efficient, spontaneous, cellular uptake with potent, long-lasting intracellular activity.
We believe that our INTASYL technology provides
the following benefits including, but not limited to:
| |
· |
Ability to target a broad range of cell types and tissues; |
| |
· |
Ability to target both intracellular and extracellular protein targets; |
| |
· |
Efficient uptake by target cells, avoiding the need for assisted delivery; |
| |
· |
Sustained, or long-term, effect in vivo; |
| |
· |
Ability to target multiple genes in one drug product; |
| |
· |
Favorable clinical safety profile with local administration; and |
| |
· |
Readily manufactured under current good manufacturing practices. |
PH-762
PH-762 is an INTASYL compound
designed to reduce the expression of cell death protein 1 (“PD-1”). PD-1 is a protein that inhibits T cells’
ability to kill cancer cells and is a clinically validated target in immunotherapy. Decreasing the expression of PD-1 can thereby increase
the capacity of T cells, which protect the body from cancer cells and infections, to kill cancer cells.
Our preclinical studies have
demonstrated that direct-to-tumor application of PH-762 resulted in potent anti-tumoral effects and have shown that direct-to-tumor treatment
with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Importantly, direct-to-tumor administration
of PH-762 resulted in activity against distant untreated tumors, indicative of a systemic anti-tumor response. We believe these data further
support the potential for PH-762 to provide a strong local immune response without the dose immune-related adverse effects seen with systemic
antibody therapy.
PH-762 is currently being
evaluated in a U.S. multi-center Phase 1b dose-escalating clinical trial through the intratumoral injection of PH-762 for the treatment
of patients with cutaneous squamous cell carcinoma, melanoma and Merkel cell carcinoma. The trial (NCT 06014086) is designed to evaluate
the safety and tolerability of neoadjuvant use of intratumorally injected PH-762, assess the tumor response, and determine the dose or
dose range for continued study of PH-762 and is expected to enroll up to 30 patients. In November 2023, we announced the dosing of the
first patient under a previously cleared Investigational New Drug (“IND”) application by the U.S. Food and Drug Administration,
and the trial is currently open for the continued enrollment of patients. In May and December 2024, respectively, a Safety Monitoring
Committee (SMC) reviewed data from the first and second dose cohorts treated with PH-762 and in both instances recommended the escalation
to the next dose concentration. A total of seven (7) patients with cutaneous
carcinomas have been enrolled in dose cohorts 1 and 2. The second cohort enrolled a total of 4 patients who were diagnosed with cutaneous
squamous cell carcinoma. At Day 36 (tumor excision), while patients in the first cohort had stable disease, a complete response (100%
tumor clearance) was reported for 2 patients with cutaneous squamous cell carcinoma. Partial response (90% tumor clearance) was reported
for 1 patient with cutaneous squamous cell carcinoma and 1 patient had stable disease, having not progressed.
Intratumoral
injection of PH-762 has been well tolerated in all patients enrolled in the trial to date. There were no dose-limiting toxicities or clinically
relevant treatment-emergent adverse effects in the patients receiving intratumoral PH-762. The trial is open for the continued
enrollment of patients and expects to complete enrollment of patients in the third quarter of 2025.
Due to INTASYL’s ease
of administration, we have shown that our compounds can easily be incorporated into current adoptive cell therapy (ACT) manufacturing
processes. In ACT, immune cells such as T cells, Natural killer cells or dendritic cells are taken from a patient or donor's blood or
tumor tissue, grown in large numbers ex vivo, and then given back to the patient to help the immune system fight cancer. By treating T
cells with our INTASYL compounds while they are being grown outside the body, we believe our INTASYL compounds can improve these immune
cells to make them more effective in killing cancer. Preclinical data generated in collaboration with AgonOx, Inc. (“AgonOx”),
a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer, demonstrated
that treating AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP TIL”) with PH-762 increased
their tumor killing activity by two-fold.
In February 2021, we
entered into a clinical co-development collaboration agreement (the “Clinical Co-Development Agreement”) with AgonOx
to develop a T cell-based therapy using PH-762 and AgonOx’s DP TIL. Under the Clinical Co-Development Agreement, we had agreed to
reimburse AgonOx up to $4 million in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients with advanced
melanoma and other advanced solid tumors. We were also eligible to receive certain future development milestones and low single-digit
sales-based royalty payments from AgonOx’s licensing of its DP TIL technology.
In May 2024, we terminated
the Clinical Co-Development Agreement with AgonOx, which such termination was effective immediately. Effective as of the date of termination,
the Clinical Co-Development Agreement and our continuing obligations and those of AgonOx thereunder were terminated in their entirety.
We are no longer required to provide financial support for the development of costs incurred under the Clinical Co-Development Agreement,
and we are no longer entitled to future development milestones or royalty payments from AgonOx’s licensing of its DP TIL technology.
We will pay to AgonOx all monetary obligations that accrued prior to the termination of the Clinical Co-Development Agreement. Remaining
payments to be made to AgonOx as of Sept 30, 2024 total $35,000, which primarily relate to accrued obligations for patient fees and other
miscellaneous costs as of the date of termination. Pursuant to the terms of the Clinical Co-Development Agreement, each of the Company
and AgonOx, shall be responsible for its own costs and expenses incurred in connection with the wind-down of the Phase 1 clinical trial.
Prior to the termination of
the Clinical Co-Development Agreement with AgonOx, PH-762 treated DP TIL were being evaluated in a Phase 1 clinical trial in the United
States with up to 18 patients with advanced melanoma and other advanced solid tumors by AgonOx. The primary trial objectives were to evaluate
the safety and to study the potential for enhanced therapeutic benefit from the administration of PH-762 treated DP TIL. AgonOx had enrolled
three patients. The first two patients were treated with DP TIL only and the third patient was treated with a combination of DP TIL and
PH-762.
The Offerings
December 19, 2024 Concurrent Registered Direct
Offering and Private Placement
On December 19, 2024, we entered
into a securities purchase agreement (the “December 19, 2024 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “December 19, 2024 Registered Direct Offering”)
and concurrent private placement (the “December 19, 2024 Private Placement” and, together with the December 19, 2024
Registered Direct Offering, the “December 19, 2024 Offerings”). The December 19, 2024 Offerings closed on December
20, 2024.
We offered and sold in
the December 19, 2024 Registered Direct Offering 437,192 shares of Common Stock at a purchase price of $2.635 per share. In the December
19, 2024 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 437,192 shares of common
stock (the “Series E Warrants”). Under the terms of the December 19, 2024 Securities Purchase Agreement, for each share
of common stock issued in the December 19, 2024 Registered Direct Offering, an accompanying Series E Warrant was issued to the purchaser
thereof. Each Series E Warrant is exercisable for one share of common stock (a “December 19, 2024 Warrant Share”) at
an exercise price of $2.51 per share and will expire on December 20, 2029. The Series E Warrants were offered and sold at a purchase price
of $0.125 per Series E Warrant, which purchase price is included in the offering price per share of common stock issued in the December
19, 2024 Registered Direct Offering.
December 23, 2024 Concurrent Registered Direct
Offering and Private Placement
On December 23, 2024, we entered
into a securities purchase agreement (the “December 23, 2024 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “December 23, 2024 Registered Direct Offering”)
and concurrent private placement (the “December 23, 2024 Private Placement” and, together with the December 23, 2024
Registered Direct Offering, the “December 23, 2024 Offerings”). The December 23, 2024 Offerings closed on December
24, 2024.
We offered and sold in
the December 23, 2024 Registered Direct Offering 240,000 shares of common stock at a purchase price of $2.00 per share. In the December
23, 2024 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 240,000 shares of common
stock (the “Series F Warrants”). Under the terms of the December 23, 2024 Securities Purchase Agreement, for each
share of common stock issued in the December 23, 2024 Registered Direct Offering, an accompanying Series F Warrant was issued to the
purchaser thereof. Each Series F Warrant is exercisable for one share of common stock (a “December 23, 2024 Warrant Share”)
at an exercise price of $2.00 per share and will expire on December 24, 2029. The Series F Warrants were offered and sold at a purchase
price of $0.125 per Series F Warrant, which purchase price is included in the offering price per share of common stock issued in the
December 19, 2024 Registered Direct Offering.
January 13, 2025 Concurrent Registered Direct
Offering and Private Placement
On January 13, 2025, we entered
into a securities purchase agreement (the “January 13, 2025 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “January 13, 2025 Registered Direct Offering”)
and concurrent private placement (the “January 13, 2025 Private Placement” and, together with the January 13, 2025
Registered Direct Offering, the “January 13, 2025 Offerings”). The January 13, 2025 Offerings closed on January 14,
2025.
We offered and sold in the
January 13, 2025 Registered Direct Offering 1,063,670 shares of common stock at a purchase price of $3.00 per share. In the January 13,
2025 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 2,127,340 shares of common
stock (the “Series G Warrants”). Under the terms of the January 13, 2025 Securities Purchase Agreement, for each share
of common stock issued in the January 13, 2025 Registered Direct Offering, two accompanying Series G Warrants were issued to the purchaser
thereof. Each Series G Warrant is exercisable for one share of common stock (a “January 13, 2025 Warrant Share”) at
an exercise price of $3.00 per share and will expire on January 14, 2027. The Series G Warrants were offered and sold at a purchase price
of $0.125 per Series G Warrant, which purchase price is included in the offering price per share of common stock issued in the January
13, 2025 Registered Direct Offering.
January 14, 2025 Concurrent Registered Direct
Offering and Private Placement
On January 14, 2025, we
entered into a securities purchase agreement (the “January 14, 2025 Securities Purchase Agreement”) with certain of
the Selling Stockholders in connection with a registered direct public offering (the “January 14, 2025 Registered Direct Offering”)
and concurrent private placement (the “January 14, 2025 Private Placement”). The January 14, 2025 Registered Direct
Offering and the January 14, 2025 Private Placement (together, the “January 14, 2025 Offerings”) closed on January
15, 2025.
We offered and sold in
the January 14, 2025 Registered Direct Offering 833,335 shares of common stock at a purchase price of $3.00 per share. In the January
14, 2025 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 1,666,670
shares of common stock (the “Series H Warrants”). Under the terms of the January 14, 2025 Securities Purchase
Agreement, for each share of common stock issued in the January 14, 2025 Registered Direct Offering, two accompanying Series
H Warrants were issued to the purchaser thereof. Each Series H Warrant is exercisable for one share of common stock (a “January
14, 2025 Warrant Share”) at an exercise price of $3.00 per share and will expire on January 15, 2027. The Series
H Warrants were offered and sold at a purchase price of $0.125 per Series H Warrant, which purchase price is included in the offering
price per share of common stock issued in the January 14, 2025 Registered Direct Offering.
January 16, 2025 Concurrent Registered Direct
Offering and Private Placement
On January 16, 2025, we
entered into a securities purchase agreement (the “January 16, 2025 Securities Purchase Agreement” and, together with
the December 19, 2024 Securities Purchase Agreement, the December 23, 2024 Securities Purchase Agreement, the January 13, 2025 Securities
Purchase Agreement and the January 14, 2025 Securities Purchase Agreement, the “Purchase Agreements”) with certain
of the Selling Stockholders in connection with a registered direct public offering (the “January 16, 2025 Registered Direct Offering”
and, together with the December 19, 2024 Registered Direct Offering, the December 23, 2024 Registered Direct Offering, the January 13,
2025 Registered Direct Offering and the January 14, 2025 Registered Direct Offering, the “Registered Direct Offerings”)
and concurrent private placement (the “January 16, 2025 Private Placement” and, together with the December 19, 2024
Private Placement, the December 23, 2024 Private Placement, the January 13, 2025 Private Placement and the January 14, 2025 Private Placement,
the “Private Placements”). The January 16, 2025 Registered Direct Offering and the January 16, 2025 Private Placement
(together, the “January 16, 2025 Offerings”) closed on January 17, 2025.
We offered and sold in
the January 16, 2025 Registered Direct Offering 610,000 shares of common stock at a purchase price of $3.00 per share. In the January
16, 2025 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 1,220,000
shares of common stock (the “Series I Warrants” and, together with the Series E Warrants, the Series F Warrants, the
Series G Warrants and the Series H Warrants, the “Warrants”). Under the terms of the January 16, 2025 Securities
Purchase Agreement, for each share of common stock issued in the January 16, 2025 Registered Direct Offering, two accompanying
Series I Warrants were issued to the purchaser thereof. Each Series I Warrant is exercisable for one share of common stock (a “January
16, 2025 Warrant Share” and, together with the December 19, 2024 Warrant Shares, the December 23, 2024 Warrant Shares,
the January 13, 2025 Warrant Shares and the January 14, 2025 Warrant Shares, the “Warrant Shares”) at an exercise
price of $3.00 per share and will expire on January 19, 2027. The Series I Warrants were offered and sold at a purchase price of
$0.125 per Series I Warrant, which purchase price is included in the offering price per share of common stock issued in the January
16, 2025 Registered Direct Offering.
Pursuant to an engagement
letter, dated as of June 27, 2024, as amended, between us and H.C. Wainwright & Co., LLC (the “Placement Agent”),
we agreed to pay the Placement Agent a total cash fee equal to 7.5% of the gross proceeds received in the Registered Direct Offerings
and the Private Placements. We also agreed to pay the Placement Agent in connection with the Registered Direct Offerings and the Private
Placements a management fee equal to 1.0% of the gross proceeds raised in the Registered Direct Offerings and the Private Placements.
Further, we agreed to pay the Placement Agent (i) in connection with each of the December 19, 2024 Offerings, the January 13, 2025 Offerings
and the January 14, 2025 Offerings, $25,000 for non-accountable expenses and $15,950 for clearing fees, (ii) in connection with the January
16, 2025 Offerings, $20,000 for non-accountable expenses and $10,000 for clearing fees and (iii) in connection with the December 23,
2024 Offerings, $10,000 for non-accountable expenses and $10,000 for clearing fees.
In addition, in connection
with the Registered Direct Offerings and the Private Placements, we agreed to issue to the Placement Agent, or its designees, warrants
to purchase up to an aggregate of 238,814 shares of common stock (the “Placement Agent Warrants”), which represent
7.5% of the aggregate number of shares of common stock sold in the Registered Direct Offerings. The Placement Agent Warrants have substantially
the same terms as the Warrants, except that (i) 32,789 of the Placement Agent Warrants have an exercise price equal to $3.2938, or 125%
of the offering price per share of common stock sold in the December 19, 2024 Registered Direct Offering, and are exercisable until December
19, 2029, (ii) 18,000 of the Placement Agent Warrants have an exercise price equal to $2.50, or 125% of the offering price per share
of common stock sold in the December 23, 2024 Registered Direct Offering, and are exercisable until December 23, 2029, (iii) 79,775 of
the Placement Agent Warrants have an exercise price equal to $3.75, or 125% of the offering price per share of common stock sold in the
January 13, 2025 Registered Direct Offering, and are exercisable until January 14, 2027, (iv) 62,500 of the Placement Agent Warrants
have an exercise price equal to $3.75, or 125% of the offering price per share of common stock sold in the January 14, 2025 Registered
Direct Offering, and are exercisable until January 15, 2027 and (v) 45,750 of the Placement Agent Warrants have an exercise price equal
to $3.75, or 125% of the offering price per share of common stock sold in the January 16, 2025 Registered Direct Offering, and are exercisable
until January 19, 2027.
The net proceeds to us from
the Registered Direct Offerings and the Private Placements are approximately $7.95 million, after deducting fees and expenses.
Corporate Information
The Company was incorporated
in the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, we changed our name to Phio Pharmaceuticals
Corp., to reflect our transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology therapeutics.
Our principal mailing address is 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, Massachusetts 01752, and our telephone number is (508)
767-3861. Our website address is http://www.phiopharma.com. Our website and the information contained on that site, or connected
to that site, is not part of or incorporated by reference into this prospectus.
Effective July 5, 2024, we
completed a 1-for-9 reverse stock split of our outstanding Common Stock, including reclassifying an amount equal to the reduction
in par value to additional paid-in capital. The reverse stock split did not reduce the number of authorized shares of our Common Stock
or preferred stock. All share and per share amounts have been adjusted to give effect to the reverse stock split.
THE OFFERING
The Selling Stockholders identified
in this prospectus are offering on a resale basis up to 5,930,016 shares of Common Stock issuable upon exercise of the Warrants, as more
fully described above.
| Common Stock to be offered by the Selling Stockholders |
|
Up to 5,930,016 shares of Common Stock |
| |
|
|
| Common Stock outstanding prior to this offering |
|
958,219 shares of Common Stock as of September 30, 2024 |
| |
|
|
| Common Stock to be outstanding after this offering |
|
6,888,2351 shares of Common Stock |
| |
|
|
| Use of proceeds: |
|
We will not receive any proceeds from the sale of the shares of Common Stock by the Selling Stockholders, except for the Warrant exercise price paid for the Common Stock offered hereby and issuable upon the exercise of the Warrants. See “Use of Proceeds” on page 21 of this prospectus. |
| |
|
|
| Risk factors: |
|
You should read the “Risk Factors” section beginning on page 10 of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our securities. |
| |
|
|
| Nasdaq Capital Market symbol: |
|
Our Common Stock is listed on The Nasdaq Capital Market under the symbol “PHIO.” We do not intend to apply for listing of the Warrants on any securities exchange or nationally recognized trading system. |
1 The number of shares of Common Stock
to be outstanding after this offering is based on 958,219 shares of Common Stock outstanding as of September 30, 2024. Unless specifically
stated otherwise, the information in this prospectus is as of September 30, 2024 and excludes:
| |
|
|
| |
· |
1,133 shares of Common Stock issuable upon the exercise of stock options outstanding as of September 30, 2024, having a weighted average exercise price of $3,371.79 per share; |
| |
|
|
| |
· |
71,000 shares of Common Stock issuable upon the vesting of restricted stock units outstanding as of September 30, 2024; |
| |
|
|
| |
· |
1,294,886 shares of Common Stock issuable upon the exercise of warrants outstanding as of September 30, 2024, having a weighted average exercise price of $21.62 per share; |
| |
|
|
| |
· |
360 shares of Common Stock reserved for future issuance under our 2020 Long-Term Incentive Plan as of September 30, 2024; |
| |
|
|
| |
· |
437,192 shares of Common Stock issued in the December 19, 2024 Private Placement; |
| |
|
|
| |
· |
437,192 shares of Common Stock issuable upon the exercise of the Series E Warrants issued in the December 19, 2024 Private Placement at an exercise price of $2.51 per share; |
| |
|
|
| |
· |
32,789 shares of Common Stock issuable upon the exercise of placement agent warrants issued as compensation to the placement agent (or its designee) in connection with the December 19, 2024 Offerings at an exercise price of $3.2938 per share; |
| |
|
|
| |
· |
240,000 shares of Common Stock issued in the December 23, 2024 Private Placement; |
| |
|
|
| |
· |
240,000 shares of Common Stock issuable upon the exercise of the Series F Warrants issued in the December 23, 2024 Private Placement at an exercise price of $2.00 per share; |
| |
|
|
| |
· |
18,000 shares of Common Stock issuable upon the exercise of placement agent warrants issued as compensation to the placement agent (or its designee) in connection with the December 23, 2024 Offerings at an exercise price of $2.50 per share; |
| |
|
|
| |
· |
1,063,670 shares of Common Stock issued in the January 13, 2025 Private Placement; |
| |
|
|
| |
· |
2,127,340 shares of Common Stock issuable upon the exercise of the Series G Warrants issued in the
January 13, 2025 Private Placement at an exercise price of $3.00 per share; |
| |
|
|
| |
· |
79,775 shares of Common Stock issuable upon the exercise of placement agent warrants issued as compensation
to the placement agent (or its designee) in connection with the January 13, 2025 Offerings at an exercise price of $3.75 per share; |
| |
|
|
| |
· |
833,335 shares of Common Stock issued in the January 14, 2025 Private Placement; |
| |
|
|
| |
· |
1,666,670 shares of Common Stock issuable upon the exercise of the Series H Warrants issued in the
January 14, 2025 Private Placement at an exercise price of $3.00 per share; |
| |
|
|
| |
· |
62,500 shares of Common Stock issuable upon the exercise of placement agent warrants issued as compensation
to the placement agent (or its designee) in connection with the January 14, 2025 Offerings at an exercise price of $3.75 per share; |
| |
|
|
| |
· |
610,000 shares of Common Stock issued in the January 16, 2025 Private Placement; |
| |
|
|
| |
· |
1,220,000 shares of Common Stock issuable upon the exercise of the Series I Warrants issued in the
January 16, 2025 Private Placement at an exercise price of $3.00 per share; and |
| |
|
|
| |
· |
45,750 shares of Common Stock issuable upon the exercise of placement agent warrants issued as compensation
to the placement agent (or its designee) in connection with the January 16, 2025 Offerings at an exercise price of $3.75 per share. |
RISK FACTORS
Investing in our securities
involves a high degree of risk. Before investing in our securities, you should carefully consider the following risk factors as well
as other information we include in this prospectus. The risks are not the only ones we face, but those that we consider to be material.
There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse
effects on our future results. Our business, financial condition, results of operations and future growth prospects could be materially
and adversely affected by any of these risks. In these circumstances, the market price of our Common Stock could decline, and you may
lose all or part of your investment.
Risks Relating to Our Business and Industry
We are dependent on
the success of our INTASYL technology, and our product candidates based on this technology, which is unproven and may never lead to approved
and marketable products.
Our efforts have been focused
on the development of product candidates based on our INTASYL technology. We have invested, and we expect to continue to invest, significant
financial resources and efforts developing our product candidates. Our ability to eventually generate revenue is highly dependent on
the successful development, regulatory approval and commercialization of our INTASYL product candidates by us or by collaborative partners,
which may not occur for the foreseeable future, if ever, and is highly uncertain and depends on a number of factors, many of which are
beyond our control. Therefore, it is difficult to accurately predict challenges we may face with our product candidates as they move
through the discovery, preclinical and clinical development stages. We will spend large amounts of money developing our INTASYL technology
and may never succeed in obtaining regulatory approval. In addition, our research methodology may be unsuccessful in identifying product
candidates and results from preclinical studies and clinical trials may not predict the results that will be obtained in later phase
trials of our product candidates or our product candidates may interact with patients in unforeseen or harmful ways that may make it
impractical or impossible to manufacture, receive regulatory approval or commercialize. If we are not successful in bringing an INTASYL
product candidate to market, it could negatively impact our business and financial condition and we may not be able to identify and successfully
implement an alternative product development strategy.
Our product candidates
are in an early stage of development and we may fail, experience significant delays, never advance clinical development or not be successful
in our efforts to identify or discover additional product candidates, which may materially and adversely impact our business.
Our success depends heavily
on the successful development of our product candidates, which may never occur. Our product candidates, which are in early stages of
development, could be delayed, not advance into the clinic, or unexpectedly fail at any stage of development. Our ability to identify,
develop and commercialize product candidates is dependent on extensive preclinical and other non-clinical tests in order to support an
IND in the United States, or the equivalent with regulatory authorities in other jurisdictions, if applicable. These research programs
to identify new product candidates require substantial financial and human resources, are difficult to design and can take many years
to complete.
We cannot be certain of the
outcome of our research studies and clinical trials and the results from these studies and clinical trials may not predict the results
that will be obtained in later stages of development and we may focus our efforts and resources on product candidates that may prove
to be unsuccessful. There is no assurance that we will be able to successfully develop our product candidates, and we may forego opportunities
with certain product candidates or for indications that later prove to have greater commercial potential. If we are not able to successfully
develop our product candidates, we may be forced to abandon or delay our development efforts, which may materially and adversely affect
our business, financial condition, and results of operations.
Further, the FDA may not
accept the results of our preclinical studies or clinical trials and may require us to complete additional studies or impose stricter
approval conditions than we expect, which could impact the value of a particular program, the approvability or commercialization of the
particular product candidate or product and our Company in general. Because of these factors, it is difficult to predict the time and
cost of the development of our product candidates. Any delay or failure in obtaining required approvals may prevent us from completing
our preclinical studies or clinical trials and could have a material adverse effect on our ability to initiate or commercialize drug
or biologic candidate on a timely basis, or at all. Additionally, preclinical studies and clinical trials are lengthy and expensive and
if our cash resources become limited we may not be able to commence, continue or complete such preclinical studies or clinical trials.
If we experience delays
or difficulties in identifying and enrolling patients in clinical trials, it may lead to delays in generating clinical data and the receipt
of necessary regulatory approvals.
Clinical trials of a new
drug or biologic candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease
or condition the drug or biologic candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment
are affected by many factors, and delays in patient enrollment can result in increased costs and longer development times, which could
materially and adversely impact our business and financial condition. We may experience slower than expected patient enrollment in our
current or future clinical trials. In addition, clinical trials for drug or biologic candidates that treat the same indications as our
product candidates may result in patients who would otherwise be eligible for our clinical trials instead enrolling in clinical trials
for other drug or biologic candidates.
Topline data may not
accurately reflect or may materially differ from the complete results of a clinical trial.
From time to time, we may
publicly disclose topline or interim data from our clinical trials based on a preliminary analysis of then-available data, of which the
results, related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular
trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received
or had the opportunity to fully and carefully evaluate all data. Preliminary observations made in early stages of clinical trials are
not necessarily indicative of results that will be obtained when full data sets are analyzed or in subsequent clinical trials. As a result,
topline data may differ from future results from the same studies or different conclusions may qualify such results once additional data
has been received and evaluated. Topline or interim data also remain subject to audit and verification procedures that may result in
the final data being materially different from the preliminary data that we publicly disclose and should be viewed with caution until
the complete data is available. If the topline data we report differs from future analysis of results, or if others, including regulatory
authorities, disagree with the conclusions reached, our business, financial condition, and results of operations could be materially
and adversely affected.
We rely upon third-parties
to conduct our clinical trials and other studies for our product candidates, and if they do not successfully fulfill their obligations,
the development of our product candidates may be materially impacted.
We rely upon third-party
CROs, medical institutions, collaborators, clinical investigators, consultants and other third-parties to support and conduct our clinical
trials and we rely on these third-party CROs for the execution of certain of our preclinical studies and expect to continue to do so.
Because we rely on these third-parties, we cannot necessarily control the timing, quality of work or amount of resources that our contract
partners will devote to these activities. We, our collaborators, and our CROs are responsible for ensuring that our clinical trials are
conducted in accordance with applicable regulations and protocols. If we, our collaborators, or our CROs fail to comply with these applicable
regulations, the FDA may not accept these data and may require us to complete additional preclinical studies and clinical trials, which
could result in significant additional costs and delays to us.
As we only control certain
aspects of their activities, we cannot guarantee that these partners will fulfill their obligations to us under these arrangements. If
these third-parties do not successfully carry out their responsibilities, as well as within a timely fashion, our clinical trials and
preclinical studies may be delayed, unsuccessful or otherwise adversely affected. If we have to enter into alternative arrangements it
may delay or adversely affect the development of our product candidates and our business operations. This could be difficult, costly
or impossible, and our preclinical studies or clinical trials may need to be extended, delayed, terminated or repeated, and we may not
be able to obtain regulatory approval in a timely fashion, or at all, for the applicable drug or biologic candidate, or to commercialize
such drug or biologic candidate being tested in such studies or trials.
A number of different
factors could prevent us from advancing into clinical development, obtaining regulatory approval, and ultimately commercializing our
product candidates on a timely basis, or at all.
Before obtaining regulatory
approval for the sale of any drug or biologic candidate, we must conduct extensive preclinical tests and successful clinical trials to
demonstrate the safety and efficacy of our product candidates in humans. Before human clinical trials may commence, we must submit to
the FDA an IND. An IND involves the completion of preclinical studies and the submission of the results, together with proposed clinical
protocols, manufacturing information, analytical data and other data in the IND submission. The FDA may require us to complete additional
preclinical studies or disagree with our clinical trial study design. Also, animal models may not exist for some of the disease areas
we choose to develop our product candidates for. As a result, our clinical trials may be delayed or we may be required to incur more
expense than we anticipated.
Clinical trials require the
review and oversight of IRBs, which approve and continually review clinical investigations and protect the rights and welfare of patients.
Before our clinical trials can begin, we must also submit to the FDA a clinical protocol accompanied by the approval of the IRB at the
institution(s) participating in the clinical trial. An inability or delay in obtaining IRB approval could prevent or delay the initiation
and completion of our clinical trials, and the FDA may decide not to consider any data or information derived from a clinical investigation
not subject to initial and continuing IRB review and approval.
Preclinical studies and clinical
trials are lengthy and expensive, and their outcome is highly uncertain. Historical failure rates are high due to a number of factors,
such as safety and efficacy of drug or biologic candidates. We, our collaborators, the FDA, or an IRB may suspend clinical trials of
a drug or biologic candidate at any time for various reasons, including if we or they believe the patients participating in such trials
are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a drug or biologic candidate on patients
in a clinical trial could result in the FDA suspending or terminating the clinical trial and refusing to approve a particular drug or
biologic candidate for any or all indications of use.
An additional number of factors could affect the
timing, cost or outcome of our drug development efforts, including the following:
| |
· |
Delays in filing or acceptance of INDs, NDAs, or BLA for our product candidates; |
| |
|
|
| |
· |
Difficulty in securing centers to conduct clinical trials; |
| |
|
|
| |
· |
Conditions imposed on us by the FDA regarding the scope or design of our clinical trials; |
| |
|
|
| |
· |
Problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies; |
| |
|
|
| |
· |
Difficulty in enrolling patients in conformity with required protocols or projected timelines; |
| |
|
|
| |
· |
Third-party contractors failing to comply with regulatory requirements or to meet their contractual obligations to us in a timely
manner; |
| |
|
|
| |
· |
Our drug or biologic candidates having unexpected and different chemical and pharmacological properties in humans than in laboratory
testing and interacting with human biological systems in unforeseen, ineffective or harmful ways; |
| |
|
|
| |
· |
The need to suspend or terminate clinical trials, for example, if the participants are being exposed to unacceptable health risks; |
| |
|
|
| |
· |
Insufficient or inadequate supply or quality of our product candidates or other necessary materials necessary to conduct our
clinical trials; |
| |
|
|
| |
· |
Effects of our product candidates not having the desired effects or including undesirable side effects or the product candidates
having other unexpected characteristics; |
| |
|
|
| |
· |
The cost of our clinical trials being greater than we anticipate; |
| |
|
|
| |
· |
Negative or inconclusive results from our clinical trials or the clinical trials of others for similar product candidates or
inability to generate statistically significant data confirming the efficacy of the product being tested; |
| |
|
|
| |
· |
Changes in the FDA’s requirements or expectations for testing during the course of that testing; |
| |
|
|
| |
· |
Reallocation of our limited financial and other resources to other clinical programs; and |
| |
|
|
| |
· |
Adverse results obtained by other companies developing similar drugs. |
A failure of any preclinical
study or clinical trial can occur at any stage of testing. Any delay or failure in obtaining required approvals may prevent us from completing
our preclinical studies or clinical trials and could have a material adverse effect on our ability to initiate or commercialize any drug
or biologic candidate on a timely basis, or at all. Additionally, preclinical studies and clinical trials are lengthy and expensive and
if our cash resources become limited we may not be able to commence, continue or complete our clinical trials, which could have a material
impact on our business, financial condition, and results of operations.
We are subject to significant
competition and may not be able to compete successfully.
The biotechnology and pharmaceutical
industries are intensely competitive, contain a high degree of risk and there are many other companies actively engaged in the discovery,
development and commercialization of products that may compete with our product candidates. Many of our competitors have substantially
greater experience and greater research and development capabilities, staffing, financial, manufacturing, marketing, technical and other
resources than us, and we may not be able to successfully compete with them. These companies include large and small pharmaceutical and
biotechnology companies, academic institutions, government agencies and other private and public research organizations.
In addition, even if we are
successful in developing our product candidates, in order to compete successfully we may need to be first to market or to demonstrate
that our products are superior to therapies based on different technologies. Some of our competitors may develop and commercialize products
that are introduced to market earlier than our product candidates or on a more cost-effective basis. A number of our competitors have
already commenced clinical testing of product candidates and may be more advanced than we are in the process of developing such product
candidates. If we are not first to market or are unable to demonstrate superiority, on a cost-effective basis or otherwise, any products
for which we are able to obtain approval may not be successful.
We also face competition
acquiring technologies complementary to our INTASYL technology. Further, we may face competition with respect to product efficacy and
safety, ease of use and adaptability to modes of administration, acceptance by physicians, timing and scope of regulatory approvals,
reimbursement coverage, price and patent position, including dominant patent positions of others. If we are not able to successfully
obtain regulatory approval or commercialize our product candidates, we may not be able to establish market share and generate revenues
from our technology.
If we fail to attract,
hire and retain qualified personnel, we may not be able to design, develop, market or sell our products or successfully manage our business.
We have a small core management
team and are particularly dependent on them. Accordingly, our business prospects are dependent on the principal members of our executive
team, the loss of whose services could make it difficult for us to manage our business successfully and achieve our business objectives.
While we have entered into an employment agreement with our Chief Executive Officer, he could leave at any time, in addition to our other
employees, who are all “at will” employees. Our ability to identify, attract, retain and integrate additional qualified key
personnel is also critical to our success. Competition for skilled research, product development, regulatory and technical personnel
is intense, and we may not be able to recruit and retain the personnel we need. The loss of the services of any key personnel, or our
inability to hire new personnel with the requisite skills, could restrict our ability to develop our product candidates.
We are subject to potential
liabilities from clinical testing and future product liability claims.
The use of our product candidates
in clinical trials and, if any of our product candidates receive regulatory approval, the sale of our product candidates for commercial
use expose us to the risk of product liability claims. Product liability claims may be brought against us by patients, healthcare providers,
consumers or others who come into contact with our product candidates or approved products. We have, and will seek to obtain, clinical
trial insurance for current and any future clinical trials that we conduct, as well as liability insurance for any products that we market.
However, there is no assurance that we will be able to obtain insurance in the amounts we seek, or at all. We anticipate that licensees
who develop our products will carry liability insurance covering the clinical testing of our product candidates and the marketing of
those product candidates, if approved. There is no assurance, however, that any insurance maintained by us or our licensees will prove
adequate in the event of a claim against us. If we cannot successfully defend against product liability claims, we could incur substantial
liabilities. Even if claims asserted against us are unsuccessful, they may divert management’s attention from our operations and
we may have to incur substantial costs to defend such claims. Any of these outcomes could materially impact our business and financial
condition.
We rely upon third
parties for the manufacture of the clinical supply for our product candidates.
We rely on third-party suppliers
and manufacturers to provide us with the materials and services to manufacture our product candidates for certain preclinical studies
and for our clinical trials, and we expect that we will continue to rely on third-party manufacturers for the supply of our product candidates
in the future. We have limited in-house manufacturing capabilities and resources, and we do not own or lease manufacturing facilities
or have our own supply source for the required materials to manufacture our compounds. Further, we have limited cGMP manufacturing capabilities
and limited experience scaling up of clinical supply as our internal capabilities are limited to small-scale production of research material.
Accordingly, we are dependent upon third-party suppliers and contract manufacturers to obtain supplies and manufacture our product candidates
and we will need to either develop, contract for, or otherwise arrange for the necessary manufacturers for these supplies.
There are a limited number
of manufacturers that make oligonucleotides and we currently contract with multiple manufacturers for the supply of our product candidates
to reduce the risk of supply interruption or availability. However, there is no assurance that our supply of our product candidates will
not be limited, interrupted, of satisfactory quality or be available at acceptable prices. For example, constraints on the supply chain
and availability of resources have resulted in delays and shortages at manufacturing facilities. While we have engaged with multiple
manufacturers for the supply of our product candidates in order to mitigate the impact of the loss or delay of any one manufacturer,
there can be no assurance that our efforts will be successful. If for any reason we are unable to obtain the clinical supply of our product
candidates from our current manufacturers, we would have to seek to contract with another major manufacturer. If we or any of these manufacturers
are unable or unwilling to increase its manufacturing capacity or if we are unable to establish alternative arrangements on a timely
basis or on acceptable terms, the development and commercialization of such an approved product may be delayed or there may be a shortage
in supply. Any inability to manufacture our product candidates or future approved drugs in sufficient quantities when needed would seriously
harm our business.
Approval of any of our product
candidates will not occur unless the manufacturing facilities are in compliance with the FDA’s cGMP regulations in order to ensure
that drug products are safe and that they consistently meet applicable requirements and specifications. These requirements are enforced
by the FDA through periodic inspections of the manufacturing facilities and can result in enforcement action, such as warning letters,
fines and suspension of production if they are found to not be in compliance with the regulations. If our suppliers or manufacturers
do not comply with the FDA regulations for our product candidates, we may experience delays in timing or supply, be forced to manufacture
our product candidates ourselves or seek to contract with another supplier or manufacturer. If we are required to switch suppliers or
manufacturers, we will be required to verify that the new supplier or manufacturer maintains facilities and processes in line with cGMP
regulations, which may result in delays, additional expenses, and may have a material adverse effect on our ability to complete the development
of our product candidates.
Unstable market and
economic conditions, including elevated and sustained inflation, may have serious adverse consequences on our business, financial condition
and stock price.
As has been widely reported,
we are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted
by domestic and global monetary and fiscal policy, geopolitical instability, ongoing military conflicts, and high domestic and global
inflation. The U.S. Federal Reserve and other central banks may be unable to contain inflation through more restrictive monetary policy
and inflation may increase or continue for a prolonged period of time. Inflationary factors, such as increases in the cost of clinical
supplies, interest rates, overhead costs and transportation costs may adversely affect our operating results. We continue to monitor
these events and the potential impact on our business. Although we do not believe that inflation has had a material impact on our financial
position or results of operations to date, we may be adversely affected in the future due to domestic and global monetary and fiscal
policy, supply chain constraints, consequences associated with the coronavirus pandemic and the ongoing military conflicts, and such
factors may lead to increases in the cost of manufacturing our product candidates and delays in initiating studies. In addition, global
credit and financial markets have experienced extreme volatility and disruptions in the past several years and the foregoing factors
have led to and may continue to cause diminished liquidity and credit availability, declines in consumer confidence, declines in economic
growth, uncertainty about economic stability and increased inflation.
There can be no assurance
that deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy
may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market
conditions. If the current equity and credit markets deteriorate, or do not improve, it may make any necessary debt or equity financings
more difficult, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could
have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon
clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners
may not survive these difficult economic times, which could directly affect our ability to attain our operating goals.
Our business and operations
would suffer in the event of computer system failures, cyberattacks or a deficiency in our cybersecurity.
Despite the implementation
of security measures, our internal computer systems and those of our third-party contractors and collaborators are vulnerable to damage
from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures, cyberattacks
or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside
our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer
hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted
attacks and intrusions from around the world have increased. Such an event could cause interruption of our operations. As part of our
business, we and our third-party contractors and collaborators maintain large amounts of confidential information, including non-public
personal information on patients and our employees. Breaches in security could result in the loss or misuse of this information, which
could, in turn, result in potential regulatory actions or litigation, including material claims for damages, interruption to our operations,
damage to our reputation or otherwise have a material adverse effect on our business, financial condition and operating results. We expect
to have appropriate information security policies and systems in place in order to prevent unauthorized use or disclosure of confidential
information, including non-public personal information, but there can be no assurance that such use or disclosure will not occur.
Risks Relating to Our Intellectual Property
We may be involved
in litigation to protect our patents and intellectual property rights and our ability to protect our patents and intellectual property
rights is uncertain and may subject us to potential liabilities.
We have filed patent applications,
have pending patents that we have licensed and those that we own and expect to continue to file patent applications. We may also need
to license patents and patent applications from research sponsored by us with third-parties. There is no assurance that these applications
will result in any issued patents or that those patents would withstand possible legal challenges or protect our technologies from competition.
The patent granting authorities have upheld stringent standards for the RNAi patents that have been prosecuted so far and, consequently,
pending patents that we have licensed and those that we own may continue to experience long and difficult prosecution challenges and
may ultimately issue with much narrower claims than those in the pending applications.
In addition, others may challenge
the patents or patent applications that we currently license or may license in the future or that we own and, as a result, these patents
could be narrowed, invalidated or rendered unenforceable, which would negatively affect our ability to exclude others from using the
technologies described in these patents. There is no assurance that these patents or other pending applications or issued patents we
license or that we own will withstand possible legal challenges. Moreover, the laws of some foreign countries may not protect our proprietary
rights to the same extent as do the laws of the United States. Our efforts to enforce and maintain our intellectual property rights may
not be successful and may result in substantial costs and diversion of management and key employee’s time. If we are unable to
defend our licensed or owned intellectual property, it may have a materially and adverse impact on our business, results of operations
and financial condition.
Third-parties may claim
that we infringe their patents, which may result in substantial liabilities and prevent us from pursuing the development of our product
candidates.
Because the field we operate
in is constantly changing and patent applications are still being processed by government patent offices around the world, there is a
great deal of uncertainty about which patents will issue, when, to whom and with what claims. Although we are not aware of any blocking
patents or other proprietary rights, it is likely that there will be significant litigation and other proceedings, such as interference
and opposition proceedings in various patent offices, relating to patent rights in the field we operate. Further, many patents in the
fields we are pursuing have already been exclusively licensed to third-parties, including our competitors. It is possible that we may
become a party to such proceedings.
If a claim should be brought
against us and we are found to infringe the rights of others, we may be required to pay substantial damages, be forced to stop the development
of product candidates affected by the claim, and/or establish licenses or similar arrangements. Furthermore, any such licenses may not
be available when needed, on commercially reasonable terms or at all. Whether an infringement claim is successful or not, the cost of
these proceedings may be significant and divert the attention of management and other key employees. As a result, we cannot be certain
that our patents or those we license will not be challenged by others, which could have a material adverse effect on our business, results
of operations and financial condition.
We are dependent on
the patents we own and the technologies we license, and if we fail to maintain our patents or lose the right to license such technologies,
our ability to develop new products would be harmed.
Our success depends upon
our ability to obtain and maintain intellectual property protection for our product candidates. Any patents issued to us or our licensors
may not provide us with any competitive advantages, and there is no assurance that the patents of others will not have an adverse effect
on our ability to do business or to continue to develop our product candidates freely. Pending patents that we have licensed and those
that we own may continue to experience long and difficult prosecution challenges and may ultimately issue with much narrower claims than
those in the pending applications. Because of the extensive time required for development, testing, and regulatory review of a potential
product, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in
force for only a short period following commercialization, thus reducing any advantage provided by the patent. Further, even if our rights
are valid, enforceable and broad in scope, competitors may develop products based on technology that is not covered by our licenses or
patents or patent applications that we own. If we are unable to derive value from our licensed or owned intellectual property, it may
have a materially and adverse impact on our business, results of operations and financial condition.
Third parties may hold or
seek to obtain additional patents that could make it more difficult or impossible for us to develop products based on our technologies
without obtaining a license to such patents, which licenses may not be available on attractive terms, or at all. If there is any dispute
or issue of non-performance between us and the respective licensing partner regarding the rights or obligations under the license agreements,
the ability to develop and commercialize the affected product candidate may be adversely affected. Moreover, if any of our existing licenses
are terminated, the development of the product candidates contemplated by the licenses could be delayed or terminated and we may not
be able to negotiate additional licenses on acceptable terms, if at all, which would have a material adverse effect on our business.
To the extent that we are required and are able to obtain multiple licenses from third parties to develop or commercialize a product
candidate, the aggregate licensing fees and milestones and royalty payments made to these parties may materially reduce our economic
returns or even cause us to abandon development or commercialization of a product candidate.
Risks Relating to Our Financial Condition
We will require substantial
additional funds to complete our research and development activities.
We have used substantial
funds to develop our product candidates and will need to raise additional substantial funds to continue our drug development efforts
and support our operations. Our future capital requirements and the period for which our existing resources are able to support our operations
may vary significantly from what we expect. We anticipate that we will need to raise substantial amounts of money to fund a variety of
future activities integral to the development of our business, which may include but is not limited to the following:
| |
· |
To conduct research and development to successfully develop our product candidates; |
| |
|
|
| |
· |
To obtain regulatory approval for our product candidates; |
| |
|
|
| |
· |
To file and prosecute patent applications and to defend and assess patents to protect our technologies; |
| |
|
|
| |
· |
To retain qualified employees, particularly in light of intense competition for qualified personnel; |
| |
|
|
| |
· |
To manufacture products ourselves or through third parties; |
| |
|
|
| |
· |
To market our products, either through building our own sales and distribution capabilities or relying
on third parties; and |
| |
|
|
| |
· |
To acquire new technologies, licenses or products. |
We are dependent on obtaining
funding from third parties, such as proceeds from the issuance of debt, sale of equity or strategic opportunities, in order to maintain
our operations. We cannot assure you that additional financing will be available to us on acceptable terms, or at all. If we cannot,
or are limited in the ability to, issue equity, incur debt or enter into strategic collaborations, we may be unable to fund the discovery
and development of our product candidates or improve our technology. If we fail to obtain additional funding when needed, we may ultimately
be unable to continue to develop and potentially commercialize our product candidates, and we may be forced to scale back or terminate
our operations or seek to merge with or be acquired by another company.
We have a history of
net losses, and we expect to continue to incur net losses for the foreseeable future and may not achieve or maintain profitability.
We have generated significant
losses to date, have not generated any product revenue and may not generate product revenue in the foreseeable future, or ever. We expect
to incur significant operating losses as we advance our product candidates through drug development and the regulatory process. Our ability
to achieve profitability, if ever, will depend on, among other things, us or our collaborators, obtaining regulatory approvals and successfully
commercializing our drug or biologic candidates. Even if we are able to successfully commercialize our drug or biologic candidates, we
may not be able to achieve or sustain profitability, which could have a material adverse effect on our business, financial condition
and results of operations.
Future financing may
be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect
on our stockholders or may otherwise adversely affect our business.
If we raise funds through
the issuance of debt or equity, any debt securities or preferred stock issued will have rights, preferences and privileges senior to
those of holders of our common stock in the event of a liquidation. In such event, there is a possibility that once all senior claims
are settled, there may be no assets remaining to pay out to the holders of common stock. The terms of debt securities may also impose
restrictions on our operations, which may include limiting our ability to incur additional indebtedness, to pay dividends on or repurchase
our capital stock, or to make certain acquisitions or investments. In addition, we may be subject to covenants requiring us to satisfy
certain financial tests and ratios, and our ability to satisfy such covenants may be affected by events outside of our control. If we
raise funds through the issuance of additional equity, whether through private placements or public offerings, such an issuance would
dilute current stockholders’ ownership in us, perhaps substantially. The issuance of a significant amount of shares of common stock
could cause the market price of our common stock to decline or become highly volatile.
We expect to continue
to incur significant research and development expenses, which may make it difficult for us to attain profitability, and may lead to uncertainty
as to our ability to continue as a going concern.
We expend substantial funds
to develop our technologies, and additional substantial funds will be required for further research and development, including preclinical
testing and clinical trials of any product candidates, and to manufacture and market any products that are approved for commercial sale.
Because the successful development of our products is uncertain, we are unable to precisely estimate the actual funds we will require
to develop and potentially commercialize them. In addition, we may not be able to generate enough revenue, even if we are able to commercialize
any of our product candidates, to become profitable.
Changes in our operating
plans, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, increased expenses,
potential acquisitions or other events will all affect our ability to continue as a going concern. We have limited cash resources, have
reported recurring losses from operations since inception, negative operating cashflows and have not yet received product revenues. These
factors raise substantial doubt regarding our ability to continue as a going concern, and the Company’s current cash resources
may not provide sufficient capital to fund operations for at least the next 12 months from the date of the release of the consolidated
financial statements included elsewhere in this Annual Report. The continuation of the Company as a going concern depends upon our ability
to raise additional capital through equity offerings, debt offerings and/or strategic opportunities to fund our operations. There can
be no assurance that we will be successful in accomplishing these plans in order to continue as a going concern. Any such inability to
continue as a going concern may result in our common stockholders losing their entire investment. There is no guarantee that we will
become profitable or secure additional financing.
Our ability to utilize
net operating loss carryforwards and other tax benefits may be limited.
We have historically incurred
net losses and may never achieve or sustain profitability. Under the Internal Revenue Code of 1986, as amended (the “Code”),
a corporation is generally allowed a deduction for net operating losses carried forward from a prior taxable year. Under that provision,
we can carryforward our net operating losses to offset our future taxable income, if any, until such net operating losses are used or
expire. Net operating losses incurred in tax years beginning after December 31, 2017 may be carried forward indefinitely, but are limited
to offset up to 80% of future taxable income. Certain of our net operating loss carryforwards predating December 31, 2017 could expire
unused before offsetting potential future income tax liabilities.
Additionally, an ownership
change, as defined by Section 382 and 383 of the Code, results from transactions increasing the ownership of certain stockholders or
public groups in the stock of a corporation by more than 50% over a three-year period. Pursuant to Section 382 and 383 of the code, if
the Company has experienced a change of control at any time since inception, utilization of the Company’s net operating loss or
tax credit carryforwards then in existence would be subject to an annual limitation. Any limitation may result in expiration of a portion
of the net operating loss or tax credit carryforwards before utilization.
We have completed multiple
assessments of the available net operating loss and tax credit carryforwards under Sections 382 and 383 of the Code through the year
ended December 31, 2023 and determined that we underwent multiple ownership changes during the period from inception to 2023. As a result,
our net operating losses and tax credit carryforwards are subject to substantial annual limitations under Sections 382 and 383 of the
Code due to these ownership changes. The Company has adjusted its net operating loss and tax credit carryforwards to address the impact
of the ownership changes. We assess the need to conduct an ownership change analysis to determine whether any changes occurred in ownership
that would limit net operating loss or tax credit carryforwards on an annual basis. We may experience ownership changes in the future
as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If an ownership change occurs and
our ability to use our net operating loss and tax credit carryforwards is materially limited, it could harm our future operating results
by effectively increasing our future tax obligations.
Risks Relating to Our Securities
The price of our common
stock has been and may continue to be volatile.
Our stock price has historically
fluctuated widely and is likely to continue to be volatile. Because we are at an early stage of development and in the absence of product
revenue as a measure of operating performance, we anticipate that the market price for our common stock may be influenced by, but not
limited to, such factors as:
| |
· |
Announcements regarding the initiation or completion, and the results of preclinical studies and
clinical trials of our product candidates; |
| |
|
|
| |
· |
Announcements regarding clinical trial results or development announcements concerning our competitors
product candidates; |
| |
|
|
| |
· |
Regulatory or legal developments in the United States; |
| |
|
|
| |
· |
The recruitment or departure of key personnel; |
| |
|
|
| |
· |
The issuance of competitive patents or disallowance or loss of our patent rights; |
| |
|
|
| |
· |
Our ability to raise additional capital and the terms on which additional capital is raised; |
| |
|
|
| |
· |
To acquire new technologies, licenses or products; and |
| |
|
|
| |
· |
General economic, industry and market conditions. |
The stock markets, in general,
and the markets for drug delivery and pharmaceutical company stocks, in particular, have experienced extreme volatility, that has often
been unrelated to the operating performance of these particular companies. These broad market fluctuations may adversely affect the trading
price of our common stock and could result in the loss of all or part of your investment. In addition, the limited trading volume of
our stock may contribute to its volatility. Moreover, if we are unable to trade above $1.00 for a certain period of time, or fulfill
the other continued listing standards, The Nasdaq Stock Market (“Nasdaq”) may delist our common stock. Delisting our
common stock from Nasdaq would adversely affect our trading volume and would likely negatively impact our trading price.
We may not be able
to maintain compliance with the continued listing requirements of The Nasdaq Capital Market.
Nasdaq Listing Rule 5550(b)(1)
requires companies listed on the Nasdaq Capital Market to maintain stockholders’ equity of at least $2.5 million for continued
listing. As of September 30, 2024, our stockholders’ equity was $4.9 million and there can be no assurance that we will be able
to maintain or increase our stockholders’ equity in the future. If our stockholders’ equity falls below $2.5 million, as
a result of operating losses or for other reasons, or if we are unable to demonstrate to Nasdaq’s satisfaction that we subsequently
regained compliance with this requirement, Nasdaq will notify us of such non-compliance. If we receive such notice from Nasdaq, in accordance
with the Nasdaq Listing Rules, we will have 45 calendar days from the date of the notification to submit a plan to regain compliance
with Nasdaq Listing Rule 5550(b)(1). If our compliance plan is accepted, we may be granted up to 180 calendar days from the date of the
initial notification to evidence compliance. If our compliance plan is not accepted or we are otherwise unable to evidence compliance
within Nasdaq’s allotted timeframe, Nasdaq may take steps to delist our common stock.
Such a delisting would have
an adverse effect on the market liquidity of our securities, decrease the market price of our securities, result in the potential loss
of confidence by investors, suppliers, customers and employees and fewer business development opportunities, and adversely affect our
ability to obtain financing for the continuation of our operations. We are actively monitoring our stockholders’ equity and will
consider any and all options available to us to maintain compliance with Nasdaq Listing Rule 5550(b)(1).
Our Board of Directors
has the authority to issue shares of “blank check” preferred stock and the terms of the preferred stock may reduce the value
of our common stock.
We are authorized to issue
up to 10,000,000 shares of preferred stock in one or more series. Our Board of Directors (the “Board”) may determine
the terms of future preferred stock offerings without further action by our stockholders. The issuance of our preferred stock could affect
the rights of existing stockholders or reduce the value of our outstanding preferred stock or common stock. In particular, rights granted
to holders of certain series of preferred stock may include voting rights, preferences as to dividends and liquidation, conversion and
redemption rights and restrictions on our ability to merge with or sell our assets to a third party.
We may acquire other
businesses or form joint ventures that may be unsuccessful and could dilute your ownership interest in the Company.
As part of our business strategy,
we may pursue future acquisitions of other complementary businesses and technology licensing arrangements. We also may pursue strategic
alliances. We have limited experience with respect to acquiring other companies and with respect to the formation of collaborations,
strategic alliances and joint ventures. We may not be able to integrate such acquisitions successfully into our existing business, and
we could assume unknown or contingent liabilities. We also could experience adverse effects on our reported results of operations from
acquisition related charges, amortization of acquired technology and other intangibles and impairment charges relating to write-offs
of goodwill and other intangible assets from time to time following the acquisition. Integration of an acquired company requires management
resources that otherwise would be available for ongoing development of our existing business. We may not realize the anticipated benefits
of any acquisition, technology license or strategic alliance. There is no assurance that we will be successful in developing such assets,
and a failure to successfully develop such assets could diminish our prospects.
To finance future acquisitions,
we may choose to issue shares of our common stock or preferred stock as consideration, which would dilute current stockholders’
ownership interest in us. Alternatively, it may be necessary for us to raise additional funds through public or private financings. Additional
funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders.
Any future acquisitions by us also could result in large and immediate write-offs, the incurrence of contingent liabilities or amortization
of expenses related to acquired intangible assets, any of which could harm our operating results.
Provisions of our certificate
of incorporation and bylaws and Delaware law might discourage, delay or prevent a change of control of the Company or changes in our
management and, as a result, depress the trading price of our common stock.
Our certificate of incorporation
and bylaws contain provisions that could discourage, delay or prevent a change of control of the Company or changes in our management
that the stockholders of the Company may deem advantageous. These provisions:
| |
· |
Authorize the issuance of “blank check” preferred stock that our Board could issue to
increase the number of outstanding shares and to discourage a takeover attempt; |
| |
|
|
| |
· |
Prohibit stockholder action by written consent, which requires all stockholder actions to be taken
at a meeting of our stockholders; |
| |
|
|
| |
· |
Provide that the Board is expressly authorized to adopt, alter or repeal our bylaws; and |
| |
|
|
| |
· |
Establish advance notice requirements for nominations for election to our Board or for proposing
matters that can be acted upon by stockholders at stockholder meetings. |
Although we believe these
provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our Board,
they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or
prevent any attempts by our stockholders to replace or remove our current management team by making it more difficult for stockholders
to replace members of our Board, which is responsible for appointing the members of our management.
Moreover, because we are
incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a
person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after
the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination
is approved in a prescribed manner.
Use of Proceeds
We will not receive any of
the proceeds from the sale of the Common Stock by the Selling Stockholders. The shares offered hereby are issuable upon the exercise
of the Warrants. Upon exercise of the Warrants for cash, we will receive, subject to fees to the Placement Agent, the applicable cash
exercise price paid by the holders of the Warrants of approximately $17.5 million (assuming the full exercise of the Warrants).
We intend to use any proceeds
received by us from the cash exercise of the Warrants for working capital and other general corporate purposes. We may also use a portion
of any proceeds received by us from the cash exercise of the Warrants to acquire or invest in complementary businesses, products and
technologies or to fund the development of any such complementary businesses, products or technologies. We currently have no plans for
any such acquisitions.
DIVIDEND POLICY
We have never paid any cash
dividends and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We expect to retain future earnings,
if any, for use in our development activities and the operation of our business. The payment of any future dividends will be subject
to the discretion of our Board of Directors and will depend, among other things, upon our results of operations, financial condition,
cash requirements, prospects and other factors that our Board of Directors may deem relevant.
DETERMINATION
OF OFFERING PRICE
The prices at which the shares
of Common Stock covered by this prospectus may actually be sold will be determined by the prevailing public market price for shares of
our Common Stock or by negotiations between the Selling Stockholders and buyers of our Common Stock in private transactions or as otherwise
described in “Plan of Distribution.”
MARKET INFORMATION
Our common stock is listed
on The Nasdaq Capital Market under the symbol “PHIO.”
HOLDERS
As of December 31, 2024,
there were approximately 14 holders of record of our common stock. Because many of our shares are held by brokers and other institutions
on behalf of stockholders, we are unable to estimate the total number of individual stockholders represented by these holders of record.
BuSINESS
Overview
Phio Pharmaceuticals Corp.
(“Phio,” “we,” “our” or the “Company”) is a clinical stage
biotechnology company whose proprietary INTASYL® small interfering RNA technology is designed to make immune cells more effective
in killing tumor cells. We are developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that
reduce the body’s ability to fight cancer, without the need for specialized formulations or drug delivery systems. We are committed
to discovering and developing innovative cancer treatments for patients by creating new pathways toward a cancer-free future.
In 2023, we implemented a
cost rationalization program driven by our transition from discovery research to product development. This resulted in a decision not
to renew the lease for office and laboratory space in Marlborough, Massachusetts, which expired on March 31, 2024. As of April 1, 2024,
we have continued operations primarily as a remote business with a laboratory facility in Worcester, Massachusetts. Additionally, we
rationalized discovery research personnel resulting in an overall headcount reduction by greater than 50%. Expense reductions have been
redirected to funding the Phase 1b clinical trial with PH-762 directed toward skin cancer.
INTASYL Technology
Overall, RNA is involved
in the synthesis, regulation and expression of proteins. RNA takes the instructions from DNA and turns those instructions into proteins
within the body’s cells. RNA interference, or RNAi, is a biological process that inhibits the expression of genes or the production
of proteins. Diseases are often related to the incorrect protein being made, excessive amounts of a specific protein being made, or the
correct protein being made, but at the wrong location or time. RNAi offers a novel approach to drug development because RNAi compounds
can be designed to silence any one of the thousands of human genes, many of which are considered “undruggable” by traditional
therapeutics.
Our development efforts are
based on our proprietary INTASYL small interfering RNA technology. It is a patented technology from which specific patented compounds
are developed. INTASYL compounds are comprised of a unique sequence of chemically modified nucleotides (modified small interfering RNA,
or siRNAs) that target a broad range of cell types and tissues. The compounds are designed to effectively silence genes that tumors use
to evade the immune system.
Since the initial discovery
of RNAi, drug delivery has been the primary challenge in developing RNAi-based therapeutics. Other siRNA technologies require cell targeting
chemical conjugates which limit delivery to specific cell types. INTASYL is based on proprietary chemistry that is designed to maximize
the activity and adaptability of the compound and is unique in that it can be delivered to any cell type or tissue without the need to
modify the chemistry. This is designed to eliminate the need for formulations or delivery systems (for example, nanoparticles or electroporation).
This provides efficient, spontaneous, cellular uptake with potent, long-lasting intracellular activity.
We believe that our INTASYL
technology provides the following benefits including, but not limited to:
| |
· |
Ability to target a broad range of cell types and tissues; |
| |
· |
Ability to target both intracellular and extracellular protein targets; |
| |
· |
Efficient uptake by target cells, avoiding the need for assisted delivery; |
| |
· |
Sustained, or long-term, effect in vivo; |
| |
· |
Ability to target multiple genes in one drug product; |
| |
· |
Favorable clinical safety profile with local administration; and |
| |
· |
Readily manufactured under current good manufacturing practices. |
Our Pipeline
INTASYL compounds are designed
to precisely target specific proteins that reduce the body’s ability to fight cancer, without the need for specialized formulations
or drug delivery systems, and are designed to make immune cells more effective in killing tumor cells. Our efforts are focused on developing
immuno-oncology therapeutics using our INTASYL technology. We have demonstrated preclinical activity against multiple gene targets including
PD-1, BRD4, CTLA-4, TIGIT and CTGF and have demonstrated preclinical efficacy in both direct-to-tumor injection and adoptive cell therapy
(“ACT”) applications with our INTASYL compounds.
The following table summarizes
our product pipeline. Below we provide important information and context regarding each compound.
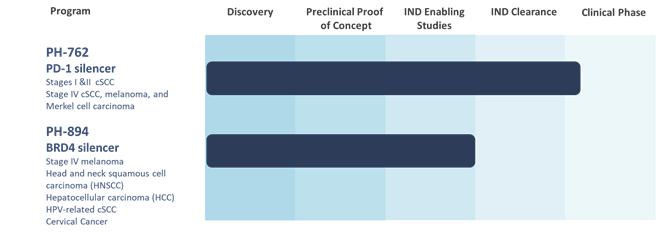
PH-762
PH-762 is an INTASYL compound
designed to reduce the expression of cell death protein 1 (“PD-1”). PD-1 is a protein that inhibits T cells’
ability to kill cancer cells and is a clinically validated target in immunotherapy. Decreasing the expression of PD-1 can thereby increase
the capacity of T cells, which protect the body from cancer cells and infections, to kill cancer cells.
Our preclinical studies have
demonstrated that direct-to-tumor application of PH-762 resulted in potent anti-tumoral effects and have shown that direct-to-tumor treatment
with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Importantly, direct-to-tumor
administration of PH-762 resulted in activity against distant untreated tumors, indicative of a systemic anti-tumor response. We believe
these data further support the potential for PH-762 to provide a strong local immune response without the dose immune-related adverse
effects seen with systemic antibody therapy.
PH-762 is currently being
evaluated in a U.S. multi-center Phase 1b dose-escalating clinical trial through the intratumoral injection of PH-762 for the treatment
of patients with cutaneous squamous cell carcinoma, melanoma and Merkel cell carcinoma. The trial (NCT 06014086) is designed to evaluate
the safety and tolerability of neoadjuvant use of intratumorally injected PH-762, assess the tumor response, and determine the dose or
dose range for continued study of PH-762 and is expected to enroll up to 30 patients. In November 2023, we announced the dosing of the
first patient under a previously cleared Investigational New Drug (“IND”) application by the U.S. Food and Drug Administration.
In May and December 2024, respectively, a Safety Monitoring Committee (SMC) reviewed data from the first and second dose cohorts treated
with PH-762, and in both instances recommended the escalation to the next dose concentration. A total of seven (7) patients with cutaneous
carcinomas have been enrolled in Cohorts 1 and 2. The second cohort enrolled a total of 4 patients who were diagnosed with
cutaneous squamous cell carcinoma. At Day 36 (tumor excision), while patients in the first cohort had stable disease, a complete response
(100% tumor clearance) was reported for 2 patients with cutaneous squamous cell carcinoma. Partial response (90% tumor clearance)
was reported for 1 patient with cutaneous squamous cell carcinoma and 1 patient had stable disease, having not progressed. Intratumoral
injection of PH-762 has been well tolerated in all patients enrolled in the trial to date. There were no dose-limiting adverse events
or clinically relevant treatment-emergent adverse effects in the patients receiving intratumoral PH-762. The trial is open for the continued
enrollment of patients and expects to complete enrollment of patients in the third quarter of 2025.
Due to INTASYL’s ease
of administration, we have shown that our compounds can easily be incorporated into current ACT manufacturing processes. In ACT, immune
cells such as T cells, Natural killer cells or dendritic cells are taken from a patient or donor's own blood or tumor tissue, grown in
large numbers ex vivo, and then given back to the patient to help the immune system fight cancer. By treating T cells with our INTASYL
compounds while they are being grown outside the body, we believe our INTASYL compounds can improve these immune cells to make them more
effective in killing cancer. Preclinical data generated in collaboration with AgonOx, Inc. (“AgonOx”), a private company
developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer, demonstrated that treating
AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP TIL”) with PH-762 increased their
tumor killing activity by two-fold.
In February 2021, we
entered into a clinical co-development collaboration agreement (the “Clinical Co-Development Agreement”) with AgonOx
to develop a T cell-based therapy using PH-762 and AgonOx’s DP TIL. Under the Clinical Co-Development Agreement, we had agreed
to reimburse AgonOx up to $4 million in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients with
advanced melanoma and other advanced solid tumors. We were also eligible to receive certain future development milestones and low single-digit
sales-based royalty payments from AgonOx’s licensing of its DP TIL technology.
In May 2024, we terminated
the Clinical Co-Development Agreement with AgonOx, which such termination was effective immediately. Effective as of the date of termination,
the Clinical Co-Development Agreement and our continuing obligations and those of AgonOx thereunder were terminated in their entirety.
We are no longer required to provide financial support for the development of costs incurred under the Clinical Co-Development Agreement,
and we are no longer entitled to future development milestones or royalty payments from AgonOx’s licensing of its DP TIL technology.
We will pay to AgonOx all monetary obligations that accrued prior to the termination of the Clinical Co-Development Agreement. Remaining
payments to be made to AgonOx as of September 30, 2024 total $35,000, which primarily relate to accrued obligations for patient fees
and other miscellaneous costs as of the date of termination. Pursuant to the terms of the Clinical Co-Development Agreement, each of
the Company and AgonOx, shall be responsible for its own costs and expenses incurred in connection with the wind-down of the Phase 1
clinical trial.
Prior to the termination
of the Clinical Co-Development Agreement with AgonOx, PH-762 treated DP TIL were being evaluated in a Phase 1 clinical trial in the U.S.
with up to 18 patients with advanced melanoma and other advanced solid tumors by AgonOx. The primary trial objectives were to evaluate
the safety and to study the potential for enhanced therapeutic benefit from the administration of PH-762 treated DP TIL. AgonOx had enrolled
three patients. The first two patients were treated with DP TIL only and a third patient was treated with a combination of DP TIL and
PH-762.
PH-894
PH-894 is an INTASYL compound
that is designed to silence BRD4, a protein that controls gene expression in both T cells and tumor cells, thereby effecting the immune
system as well as the tumor. Intracellular and/or commonly considered “undruggable” targets, such as BRD4, represent a challenge
for small molecule and antibody therapies. Therefore, what sets this compound apart is its dual mechanism: PH-894 suppression of BRD4
in T cells results in T cell activation, and suppression of BRD4 in tumor cells results in tumors becoming more sensitive to being killed
by T cells.
Preclinical studies conducted
have demonstrated that PH-894 resulted in a strong, concentration dependent and durable silencing of BRD4 in T cells and in various cancer
cells. Similar to PH-762, preclinical studies have also shown that direct-to-tumor application of PH-894 resulted in potent and statistically
significant anti-tumoral effects against distant untreated tumors, indicative of a systemic anti-tumor response. These preclinical data
indicate that PH-894 can reprogram T cells and other cells in the tumor microenvironment to provide enhanced immunotherapeutic activity.
We have completed the investigational new drug application (“IND”)-enabling studies and are in the process of finalizing
the study reports required for an IND submission with PH-894. As a result of the reprioritization to advance our clinical trial with
PH-762 in the U.S., we have elected to defer the IND submission for PH-894.
Synergies With Other Therapies
Preclinical studies with
our INTASYL compounds in combination with antibodies resulted in enhanced potency in vivo. The combination of INTASYL with antibodies
may also increase the number of addressable drug targets. Unlike other antibody combination approaches, INTASYL can target multiple protein
drug targets in a specific therapeutic dose, thereby enhancing potency while maintaining a favorable tolerability and safety profile.
We have demonstrated preclinical
efficacy with INTASYL in ACT applications. In preclinical studies, INTASYL was shown to enhance the activity of ACT therapies, including
with tumor infiltrating lymphocytes and natural killer cells. As demonstrated in these preclinical studies, INTASYL is easily incorporated
into current ACT manufacturing processes.
Intellectual Property
INTASYL compounds have a
single-stranded phosphorothioate region, a short duplex region, and contain a variety of nuclease-stabilizing and lipophilic chemical
modifications that we believe combine the beneficial properties of both conventional RNAi and antisense technologies. We protect our
proprietary information by means of United States and foreign patents, trademarks and copyrights. In addition, we rely upon trade secret
protection and contractual arrangements to protect certain of our proprietary information and products. We have pending patent applications
that relate to potential drug targets, compounds we are developing to modulate those targets, methods of making or using those compounds,
and proprietary elements of our drug discovery technology.
We have also obtained rights
to various patents and patent applications under licenses with third parties, which require us to pay royalties, milestone payments,
or both.
The degree of patent protection
for biotechnology products and processes, including ours, remains uncertain, both in the U.S. and in other important markets, because
the scope of protection depends on decisions of patent offices, courts and lawmakers in these countries. There is no certainty that our
existing patents or others, if obtained, will afford us substantial protection or commercial benefit. Similarly, there is no assurance
that our pending patent applications or patent applications licensed from third parties will ultimately be granted as patents or that
those patents that have been issued or are issued in the future will stand if they are challenged in court. We assess our license agreements
on an ongoing basis and may from time to time terminate licenses to technology that we do not intend to employ in our technology platforms,
or in our product discovery or development activities.
Patents and Patent Applications
We are actively seeking protection
for our intellectual property and are prosecuting a number of patents and pending patent applications covering our compounds and technologies.
A combined summary of these patents and patent applications is set forth below in the following table:
| | |
Pending
Applications | |
Issued
Patents |
| United States | |
10 | |
32 |
| Canada | |
2 | |
3 |
| Europe | |
6 | |
25 |
| Japan | |
5 | |
10 |
| Other Markets | |
1 | |
6 |
Our portfolio includes 76
issued patents, 68 of which cover our INTASYL technology. There are 19 patent families broadly covering both the composition and methods
of use of our self-delivering INTASYL technology and uses of our INTASYL compounds targeting immune checkpoint, cellular differentiation
and metabolism targets for ex vivo cell-based cancer immunotherapies. The INTASYL technology patents are scheduled to expire between
2029 and 2038.
Furthermore, there are 27
patent applications, encompassing what we believe to be important new RNAi compounds and their use as therapeutics, chemical modifications
of RNAi compounds that improve the compounds’ suitability for therapeutic uses (including delivery) and compounds directed to specific
targets (i.e., that address specific disease states). The patents that may issue from these pending patent applications will,
if issued, be set to expire between 2029 and 2044, not including any patent term extensions that may be afforded under the Federal Food,
Drug, and Cosmetic Act (“FFDCA”) (and the equivalent provisions in foreign jurisdictions) for any delays incurred
during the regulatory approval process relating to human drug products (or processes for making or using human drug products).
Key Intellectual Property License Agreements
As we develop our own proprietary
compounds, we continue to evaluate our in-licensed portfolio as well as the field for new technologies that could be in-licensed to further
enhance our intellectual property portfolio and unique intellectual property position.
In September 2011, the Company
entered into an agreement with Advanced RNA Technologies, LLC (“Advirna”), pursuant to which Advirna assigned to us
its existing patent and technology rights related to the INTASYL technology in exchange for an annual maintenance fee, a one-time milestone
payment upon the future issuance of the first patent with valid claims covering the assigned patent and technology rights and the issuance
of shares of common stock of the Company equal to 5% of the Company’s fully-diluted shares outstanding at the time of issuance.
In 2012, we issued shares of common stock of the Company to Advirna equal to 5% of our fully-diluted shares outstanding at the time of
issuance and paid $350,000 to Advirna upon the issuance of the first patent in 2014. Additionally, we also pay to Advirna an annual maintenance
fee of $100,000 and are required to pay low single-digit royalties on any licensing revenue received by us with respect to future licensing
of the assigned Advirna patent and technology rights. To date, any royalties owed to Advirna under the Advirna agreement have been minimal.
Our rights under the Advirna
agreement will expire upon the later of: (i) the expiration of the last-to-expire of the “patent rights” (as defined therein)
included in the Advirna agreement or (ii) the abandonment of the last-to-be abandoned of such patents, unless earlier terminated in accordance
with the provisions of the Advirna agreement. Further, the Company also granted back to Advirna a license under the assigned patent and
technology rights for fields of use outside human therapeutics.
Manufacturing and Supply
We do not have any manufacturing
capability and therefore we currently rely on and intend to continue to rely on contract manufacturing organizations to produce our product
candidates in accordance with regulatory requirements.
We currently rely on and
contract with third parties for the manufacture of drug substances and drug products for use in our preclinical studies and clinical
trials in accordance with regulatory requirements. We expect that we will continue to rely on and contract with third parties to manufacture
our product candidates in the future.
Competition
The biotechnology and pharmaceutical
industries, including the immuno-oncology field, are a constantly evolving landscape with rapidly advancing technologies and significant
competition. There are a number of competitors in the immuno-oncology field including large and small pharmaceutical and biotechnology
companies, academic institutions, government agencies and other private and public research organizations. Many of these companies are
larger than us and have greater financial resources and human capital to develop competing products.
Government Regulation
Review and Approval of Drugs in the United
States
The United States and many
other countries extensively regulate the preclinical and clinical testing, manufacturing, labeling, storage, record-keeping, advertising,
promotion, export, marketing and distribution of drugs and biologic products. The U.S. Food and Drug Administration (“FDA”)
regulates pharmaceutical and biologic products under the FFDCA, the Public Health Service Act and other federal statutes and regulations.
To obtain approval of our
future product candidates from the FDA, we must, among other requirements, submit data supporting safety and efficacy for the intended
indication as well as detailed information on the manufacture and composition of the product candidate. In most cases, this will require
extensive laboratory tests, preclinical studies and clinical trials. The collection of these data, as well as the preparation of applications
for review by the FDA involve significant time and expense. The FDA also may require post-marketing testing to monitor the safety and
efficacy of approved products or place conditions on any approvals that could restrict the therapeutic claims and commercial applications
of these products. Regulatory authorities may withdraw product approvals if we fail to comply with regulatory standards or if we encounter
problems at any time following initial marketing of our products.
The first stage of the FDA
approval process for a new biologic or drug involves completion of preclinical studies and the submission of the results of these studies
to the FDA. These data, together with proposed clinical protocols, manufacturing information, analytical data and other information submitted
to the FDA through an IND, must become effective before human clinical trials may commence. Preclinical studies generally involve evaluation
of product characteristics and animal studies to assess the efficacy and safety of the product candidate. Many of these studies must
be conducted in accordance with the FDA’s current Good Laboratory Practices, the Animal Welfare Act, and other applicable regulations.
After the IND becomes effective,
a company may commence human clinical trials. These are typically conducted in three sequential phases, but the phases may overlap. Phase
1 trials consist of testing the product candidate in a small number of patients or healthy volunteers, primarily for safety at one or
more doses. Phase 2 trials, in addition to safety, evaluate the efficacy of the product candidate in a patient population somewhat larger
than Phase 1 trials. Phase 3 trials typically involve additional testing for safety and clinical efficacy in an expanded population at
multiple test sites. A company must submit to the FDA a clinical protocol, accompanied by the approval of the Institutional Review Board
(“IRB”) at the institutions participating in the trials, prior to commencement of each clinical trial.
To obtain FDA marketing authorization,
a company must submit to the FDA the results of the preclinical and clinical testing, together with, among other things, detailed information
on the manufacture and composition of the product candidate, in the form of a new drug application (“NDA”), or, in
the case of a biologic, a biologics license application (“BLA”).
The amount of time taken
by the FDA to approve a NDA or BLA will depend upon a number of factors, including whether the product candidate has received priority
review, the quality of the submission and studies presented, the potential contribution that the compound will make in improving the
treatment of the disease in question and agency resources.
The FDA maintains several
programs to facilitate and expedite the development and review of applications that are intended for the treatment of a serious or life-threatening
disease or condition that meet certain other criteria, including Fast Track Designation, Breakthrough Designation, Priority Review, and
the Accelerated Approval pathway.
We anticipate that our products
will be manufactured by our strategic partners, licensees or other third parties. Before approving an NDA or BLA, the FDA will inspect
the facilities at which the product is manufactured and will not approve the product unless the manufacturing facilities are in compliance
with the FDA’s current good manufacturing practice regulations (“cGMP”), which are regulations that govern the
manufacture, holding and distribution of a product. Manufacturers of biologics also must comply with the FDA’s general biological
product standards. Our manufacturers also will be subject to regulation under the Occupational Safety and Health Act, the Nuclear Energy
and Radiation Control Act, the Toxic Substance Control Act and the Resource Conservation and Recovery Act and other applicable environmental
statutes. Following approval, the FDA and certain state agencies periodically inspects drug and biologic manufacturing facilities to
ensure continued compliance with the cGMP. Our manufacturers will have to continue to comply with those requirements. Failure to comply
with these requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing or recall
or seizure of product. Adverse patient experiences with the product must be reported to the FDA and could result in the imposition of
marketing restrictions through labeling changes or market removal. Product approvals may be withdrawn if compliance with regulatory requirements
is not maintained or if problems concerning safety or efficacy of the product occur following approval.
The labeling, advertising,
promotion, marketing and distribution of a drug or biologic product also must be in compliance with FDA and Federal Trade Commission
requirements which include, among others, standards and regulations for off-label promotion, industry sponsored scientific and educational
activities, promotional activities involving the internet, and direct-to-consumer advertising. We also will be subject to state and local
requirements governing the manufacturing and distribution of pharmaceutical products. In addition, we will be subject to a variety of
federal, state and local regulations relating to the use, handling, storage and disposal of hazardous materials, including chemicals
and radioactive and biological materials. In addition, we will be subject to various laws and regulations governing laboratory practices
and the experimental use of animals. In each of these areas, failure to comply with the applicable requirements could result in administrative
or judicial enforcement action, which could include refusal to permit clinical trials, refusal to approve an application, withdrawal
of an approval, issuance of a warning letter, product recall, product seizure, suspension of production or distribution, fines, refusals
of government contracts, and restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could
have a material adverse effect on us.
Environmental Compliance
Our research and development
activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various
radioactive compounds. We are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal
of these materials and specific waste products. We are also subject to numerous environmental, health and workplace safety laws and regulations,
including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of bio-hazardous materials. The cost
of compliance with these laws and regulations could be significant and may adversely affect capital expenditures to the extent we are
required to procure expensive capital equipment to meet regulatory requirements. However, to date, compliance with such environmental
laws and regulations has not had a material impact on our capital expenditures.
Human Capital Management
We currently have five full-time
employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement, nor have we experienced
any work stoppages.
We continually evaluate our
business needs and weigh the use of in-house expertise and capacity with outsourced expertise and capacity. We currently outsource substantially
all preclinical and clinical trial work to third party contract research organizations and drug manufacturing contractors.
Our ability to identify,
attract, retain and integrate additional qualified key personnel is also critical to our success and the competition for skilled research,
product development, regulatory and technical personnel is intense. To attract qualified applicants, we offer a total rewards package
consisting of base salary and cash target bonus, a comprehensive benefit package and equity compensation for every employee. Bonus opportunity
and equity compensation increase as a percentage of total compensation based on level of responsibility. Actual bonus payouts are based
on performance.
A majority of Phio’s
employees have obtained advanced degrees in their professions and we support our employees’ further development with individualized
development plans, mentoring, coaching, group training, conference attendance and financial support including tuition reimbursement.
Corporate Information
Effective July 5, 2024, the
Company completed a 1-for-9 reverse stock split of the Company’s outstanding common stock, including reclassifying an amount equal
to the reduction in par value to additional paid-in capital. The reverse stock split did not reduce the number of authorized shares of
the Company’s common or preferred stock. All share and per share amounts have been adjusted to give effect to the reverse stock
split.
We were incorporated in the
state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, we changed our name to Phio Pharmaceuticals Corp.,
to reflect our transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology therapeutics.
Our executive offices are located at 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, MA 01752, and our telephone number is (508) 767-3861.
The Company’s website
address is http://www.phiopharma.com. We make available on our website, free of charge, copies of our annual reports on Form 10-K,
our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) as soon as reasonably
practicable after these reports are filed electronically with, or otherwise furnished to, the Securities and Exchange Commission (the
“SEC”). We also make available on our website the charters of our audit, compensation, nominating and governance committees,
as well as our corporate code of ethics and conduct.
The SEC maintains an internet
site that contains reports, proxy and information statements, and other information regarding Phio and other issuers that file electronically
with the SEC. The SEC’s website address is http://www.sec.gov. The contents of this website, and our website, are not incorporated
by reference into this report and should not be considered to be part of this report.
Management’s
discussion and analysis
The following discussion
and analysis of our financial condition and results of operations should be read together with our consolidated financial statements
and the related notes included in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties
because they are based on current expectations and relate to future events and our future financial performance. Our actual results may
differ materially from those anticipated in these forward-looking statements because of many important factors, including those set forth
under “Risk Factors” and elsewhere in this prospectus.
Overview and Recent Developments
Phio is a clinical stage
biotechnology company whose proprietary INTASYL® small interfering RNA gene silencing technology is designed to make immune cells
more effective in killing tumor cells. We are developing therapeutics that are designed to leverage INTASYL to precisely target specific
proteins that reduce the body’s ability to fight cancer, without the need for specialized formulations or drug delivery systems.
Cost Rationalization
In
2023, we implemented a cost rationalization program driven by our transition from a research
company to a product development company. This transition resulted in a decision not to renew
the lease for our corporate headquarters and primary research facility in Marlborough, Massachusetts,
which expired on March 31, 2024. As of April 1, 2024, we have continued operations primarily
as a remote business with a laboratory facility in Worcester, Massachusetts. Additionally,
we rationalized research personnel and reduced our headcount by approximately 36%. These
expense reductions have been redirected to funding the Phase 1b clinical trial with PH-762
directed toward skin cancer.
PH-762
PH-762 is an INTASYL compound
designed to reduce the expression of cell death protein 1 (“PD-1”). PD-1 is a protein that inhibits T cells’
ability to kill cancer cells and is a clinically validated target in immunotherapy. Decreasing the expression of PD-1 can thereby increase
the capacity of T cells, which protect the body from cancer cells and infections, to kill cancer cells.
Our preclinical studies have
demonstrated that direct-to-tumor application of PH-762 resulted in potent anti-tumoral effects and have shown that direct-to-tumor treatment
with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Importantly, direct-to-tumor
administration of PH-762 resulted in activity against distant untreated tumors, indicative of a systemic anti-tumor response. We believe
these data further support the potential for PH-762 to provide a strong local immune response without the dose immune-related adverse
effects seen with systemic antibody therapy.
PH-762 is currently being
evaluated in a U.S. multi-center Phase 1b dose-escalating clinical trial through the intratumoral injection of PH-762 for the treatment
of patients with cutaneous squamous cell carcinoma, melanoma and Merkel cell carcinoma. The trial is designed to evaluate the safety
and tolerability of neoadjuvant use of intratumorally injected PH-762, assess the tumor response, and determine the dose or dose range
for continued study of PH-762 and is expected to enroll up to 30 patients. In November 2023, we announced the dosing of the first patient
under a previously cleared Investigational New Drug (“IND”) application by the U.S. Food and Drug Administration.
In May 2024, a safety monitoring committee reviewed data from the first dose cohort treated and recommended the escalation to the next
dose concentration. Five (5) patients with cutaneous carcinomas have enrolled in Cohorts 1 and 2. Intratumoral injection of PH-762 has
been well tolerated in all patients enrolled in the trial to date. There were no related adverse events, no serious adverse events, and
no dose limiting toxicities or dose adjustments. The trial is open for the continued enrollment of patients and expects to complete enrollment
of patients in the third quarter of 2025.
AgonOx Collaboration
Due
to INTASYL’s ease of administration, we have shown that our compounds can easily be
incorporated into current adoptive cell therapy (“ACT”) manufacturing
processes. In ACT, T cells are usually taken from a patient own blood or tumor tissue, grown
in large numbers in a laboratory, and then given back to the patient to help the immune system
fight cancer. By treating T cells with our INTASYL compounds while they are being grown in
a laboratory, we believe our INTASYL compounds can improve these immune cells to make them
more effective in killing cancer. Preclinical data generated in collaboration with AgonOx,
Inc. (“AgonOx”), a private company developing a pipeline of novel immunotherapy
drugs targeting key regulators of the immune response to cancer, demonstrated that treating
AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP
TIL”) with PH-762 increased their tumor killing activity by two-fold.
In
February 2021, we entered into a clinical co-development collaboration agreement (the “Clinical
Co-Development Agreement”) with AgonOx to develop a T cell-based therapy using
PH-762 and AgonOx’s DP TIL. Under the Clinical Co-Development Agreement, we and AgonOx
were working to develop a T cell-based therapy using our lead product candidate, PH-762,
and AgonOx’s DP TIL Technology. We had agreed to reimburse AgonOx up to $4 million
in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients
with advanced melanoma and other advanced solid tumors. We were also eligible to receive
certain future development milestones and low single-digit sales-based royalty payments from
AgonOx’s licensing of its DP TIL technology.
In May 2024, we terminated
the Clinical Co-Development Agreement with AgonOx, which such termination was effective immediately. Effective as of the date of termination,
the Clinical Co-Development Agreement and our continuing obligations and those of AgonOx thereunder were terminated in their entirety.
We are no longer required to provide financial support for the development of costs incurred under the Clinical Co-Development Agreement,
and we are no longer entitled to future development milestones or royalty payments from AgonOx’s licensing of its DP TIL technology.
We will pay to AgonOx all payment obligations that accrued prior to the termination of the Clinical Co-Development Agreement. Remaining
payments to be made to AgonOx as of September 30, 2024 total $35,000, which primarily relate to accrued obligations for patient fees
and other miscellaneous costs as of the date of termination. Pursuant to the terms of the Clinical Co-Development Agreement, we and AgonOx
are coordinating the orderly wind-down of the Phase 1 clinical trial. Each of us and AgonOx shall be responsible for its own costs and
expenses incurred in connection with the wind-down of the Phase 1 clinical trial.
Prior to the termination
of the Clinical Co-Development Agreement with AgonOx, PH-762 treated DP TIL were being evaluated in a Phase 1 clinical trial in the United
States with up to 18 patients with advanced melanoma and other advanced solid tumors by AgonOx. The primary trial objectives were to
evaluate the safety and to study the potential for enhanced therapeutic benefit from the administration of PH-762 treated DP TIL. AgonOx
had enrolled three patients. The first two patients were treated with DP TIL only and the third patient was treated with a combination
of DP TIL and PH-762.
On December 19, 2024, we
announced that the Safety Monitoring Committee (SMC) recommended dose escalation in our Phase 1b clinical trial designed to evaluate
the safety and tolerability of PH-762 in the treatment of Stages 1, 2, and 4 cutaneous squamous cell carcinoma, Stage 4 melanoma and
Stage 4 Merkel cell carcinoma.
On January 13, 2025, we announced
that in this Phase 1b clinical trial with PH-762, dosed intratumorally, the second cohort had enrolled 4 patients who were diagnosed with
cutaneous squamous cell carcinoma. At Day 36 (tumor excision), the first two patients who completed treatment showed a complete response
(100% tumor clearance) and a partial response (90% clearance), respectively. Pathology data assessing efficacy data on the remaining 2
patients is forthcoming.
The intratumoral injections
have been well tolerated in our Phase 1b clinical trial. There have been no dose-limiting toxicities, or serious adverse events in participants
receiving intratumoral PH-762 in our Phase 1b clinical trial.
Recent Offerings
In December 2024 and January 2025,
we conducted a number of concurrent registered direct offerings and private placements with certain of the Selling Stockholders. The net
proceeds to us from the such registered direct offerings and private placements are approximately $7.95 million, after deducting fees
and expenses, and the details of such offerings are as follows:
December 19, 2024 Concurrent Registered Direct
Offering and Private Placement
On December 19, 2024, we entered
into a securities purchase agreement (the “December 19, 2024 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “December 19, 2024 Registered Direct Offering”)
and concurrent private placement (the “December 19, 2024 Private Placement” and, together with the December 19, 2024
Registered Direct Offering, the “December 19, 2024 Offerings”). The December 19, 2024 Offerings closed on December
20, 2024.
We offered and sold in the
December 19, 2024 Registered Direct Offering 437,192 shares of Common Stock at a purchase price of $2.635 per share. In the December 19,
2024 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 437,192 shares of common stock
(the “Series E Warrants”). Under the terms of the December 19, 2024 Securities Purchase Agreement, for each share of
common stock issued in the December 19, 2024 Registered Direct Offering, an accompanying Series E Warrant was issued to the purchaser
thereof. Each Series E Warrant is exercisable for one share of common stock (a “December 19, 2024 Warrant Share”) at
an exercise price of $2.51 per share and will expire on December 20, 2029. The Series E Warrants were offered and sold at a purchase price
of $0.125 per Series E Warrant, which purchase price is included in the offering price per share of common stock issued in the December
19, 2024 Registered Direct Offering.
December 23, 2024 Concurrent Registered Direct
Offering and Private Placement
On December 23, 2024, we entered
into a securities purchase agreement (the “December 23, 2024 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “December 23, 2024 Registered Direct Offering”)
and concurrent private placement (the “December 23, 2024 Private Placement” and, together with the December 23, 2024
Registered Direct Offering, the “December 23, 2024 Offerings”). The December 23, 2024 Offerings closed on December
24, 2024.
We offered and sold in the December
23, 2024 Registered Direct Offering 240,000 shares of common stock at a purchase price of $2.00 per share. In the December 23, 2024 Private
Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 240,000 shares of common stock (the “Series
F Warrants”). Under the terms of the December 23, 2024 Securities Purchase Agreement, for each share of common stock issued
in the December 23, 2024 Registered Direct Offering, an accompanying Series F Warrant was issued to the purchaser thereof. Each Series
F Warrant is exercisable for one share of common stock (a “December 23, 2024 Warrant Share”) at an exercise price of
$2.00 per share and will expire on December 24, 2029. The Series F Warrants were offered and sold at a purchase price of $0.125 per Series
F Warrant, which purchase price is included in the offering price per share of common stock issued in the December 19, 2024 Registered
Direct Offering.
January 13, 2025 Concurrent Registered Direct Offering
and Private Placement
On January 13, 2025, we entered
into a securities purchase agreement (the “January 13, 2025 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “January 13, 2025 Registered Direct Offering”)
and concurrent private placement (the “January 13, 2025 Private Placement” and, together with the January 13, 2025
Registered Direct Offering, the “January 13, 2025 Offerings”). The January 13, 2025 Offerings closed on January 14,
2025.
We offered and sold in the January
13, 2025 Registered Direct Offering 1,063,670 shares of common stock at a purchase price of $3.00 per share. In the January 13, 2025 Private
Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 2,127,340 shares of common stock (the “Series
G Warrants”). Under the terms of the January 13, 2025 Securities Purchase Agreement, for each share of common stock issued in
the January 13, 2025 Registered Direct Offering, two accompanying Series G Warrants were issued to the purchaser thereof. Each Series
G Warrant is exercisable for one share of common stock (a “January 13, 2025 Warrant Share”) at an exercise price of
$3.00 per share and will expire on January 14, 2027. The Series G Warrants were offered and sold at a purchase price of $0.125 per Series
G Warrant, which purchase price is included in the offering price per share of common stock issued in the January 13, 2025 Registered
Direct Offering.
January 14, 2025 Concurrent Registered Direct Offering
and Private Placement
On January 14, 2025, we entered
into a securities purchase agreement (the “January 14, 2025 Securities Purchase Agreement”) with certain of the Selling
Stockholders in connection with a registered direct public offering (the “January 14, 2025 Registered Direct Offering”)
and concurrent private placement (the “January 14, 2025 Private Placement”). The January 14, 2025 Registered Direct
Offering and the January 14, 2025 Private Placement (together, the “January 14, 2025 Offerings”) closed on January
15, 2025.
We offered and sold in the January
14, 2025 Registered Direct Offering 833,335 shares of common stock at a purchase price of $3.00 per share. In the January 14,
2025 Private Placement, we also issued to such Selling Stockholders unregistered warrants to purchase up to 1,666,670 shares
of common stock (the “Series H Warrants”). Under the terms of the January 14, 2025 Securities Purchase Agreement,
for each share of common stock issued in the January 14, 2025 Registered Direct Offering, two accompanying Series H Warrants
were issued to the purchaser thereof. Each Series H Warrant is exercisable for one share of common stock (a “January 14, 2025 Warrant
Share”) at an exercise price of $3.00 per share and will expire on January 15, 2027. The Series H Warrants were offered
and sold at a purchase price of $0.125 per Series H Warrant, which purchase price is included in the offering price per share of common
stock issued in the January 14, 2025 Registered Direct Offering.
January 16, 2025 Concurrent Registered Direct Offering
and Private Placement
On January 16, 2025, we
entered into a securities purchase agreement (the “January 16, 2025 Securities Purchase Agreement” and, together with
the December 19, 2024 Securities Purchase Agreement, the December 23, 2024 Securities Purchase Agreement, the January 13, 2025 Securities
Purchase Agreement and the January 14, 2025 Securities Purchase Agreement, the “Purchase Agreements”) with certain
of the Selling Stockholders in connection with a registered direct public offering (the “January 16, 2025 Registered Direct
Offering” and, together with the December 19, 2024 Registered Direct Offering, the December 23, 2024 Registered Direct Offering,
the January 13, 2025 Registered Direct Offering and the January 14, 2025 Registered Direct Offering, the “Registered Direct
Offerings”) and concurrent private placement (the “January 16, 2025 Private Placement” and, together with
the December 19, 2024 Private Placement, the December 23, 2024 Private Placement, the January 13, 2025 Private Placement and the January
14, 2025 Private Placement, the “Private Placements”). The January 16, 2025 Registered Direct Offering and the January
16, 2025 Private Placement (together, the “January 16, 2025 Offerings”) closed on January 17, 2025.
We offered and
sold in the January 16, 2025 Registered Direct Offering 610,000 shares of common stock at a purchase price of $3.00 per
share. In the January 16, 2025 Private Placement, we also issued to such Selling Stockholders unregistered warrants
to purchase up to 1,220,000 shares of common stock (the “Series I Warrants” and, together with the Series E Warrants,
the Series F Warrants, the Series G Warrants and the Series H Warrants, the “Warrants”). Under the terms of the January
16, 2025 Securities Purchase Agreement, for each share of common stock issued in the January 16, 2025 Registered Direct
Offering, two accompanying Series I Warrants were issued to the purchaser thereof. Each Series I Warrant is exercisable for one share
of common stock (a “January 16, 2025 Warrant Share” and, together with the December 19, 2024 Warrant Shares,
the December 23, 2024 Warrant Shares, the January 13, 2025 Warrant Shares and the January 14, 2025 Warrant Shares, the “Warrant
Shares”) at an exercise price of $3.00 per share and will expire on January 19, 2027. The Series I Warrants were offered
and sold at a purchase price of $0.125 per Series I Warrant, which purchase price is included in the offering price per share of common
stock issued in the January 16, 2025 Registered Direct Offering.
Pursuant to an engagement letter,
dated as of June 27, 2024, as amended, between us and H.C. Wainwright & Co., LLC (the “Placement Agent”), we agreed
to pay the Placement Agent a total cash fee equal to 7.5% of the gross proceeds received in the Registered Direct Offerings and the Private
Placements. We also agreed to pay the Placement Agent in connection with the Registered Direct Offerings and the Private Placements a
management fee equal to 1.0% of the gross proceeds raised in the Registered Direct Offerings and the Private Placements. Further, we agreed
to pay the Placement Agent (i) in connection with each of the December 19, 2024 Offerings, the January 13, 2025 Offerings and the January
14, 2025 Offerings, $25,000 for non-accountable expenses and $15,950 for clearing fees, (ii) in connection with the January 16, 2025 Offerings,
$20,000 for non-accountable expenses and $10,000 for clearing fees and (iii) in connection with the December 23, 2024 Offerings, $10,000
for non-accountable expenses and $10,000 for clearing fees.
In addition, in connection with
the Registered Direct Offerings and the Private Placements, we agreed to issue to the Placement Agent, or its designees, warrants to purchase
up to an aggregate of 238,814 shares of common stock (the “Placement Agent Warrants”), which represent 7.5% of the
aggregate number of shares of common stock sold in the Registered Direct Offerings. The Placement Agent Warrants have substantially the
same terms as the Warrants, except that (i) 32,789 of the Placement Agent Warrants have an exercise price equal to $3.2938, or 125% of
the offering price per share of common stock sold in the December 19, 2024 Registered Direct Offering, and are exercisable until December
19, 2029, (ii) 18,000 of the Placement Agent Warrants have an exercise price equal to $2.50, or 125% of the offering price per share of
common stock sold in the December 23, 2024 Registered Direct Offering, and are exercisable until December 23, 2029, (iii) 79,775 of the
Placement Agent Warrants have an exercise price equal to $3.75, or 125% of the offering price per share of common stock sold in the January
13, 2025 Registered Direct Offering, and are exercisable until January 14, 2027, (iv) 62,500 of the Placement Agent Warrants have an exercise
price equal to $3.75, or 125% of the offering price per share of common stock sold in the January 14, 2025 Registered Direct Offering,
and are exercisable until January 15, 2027 and (v) 45,750 of the Placement Agent Warrants have an exercise price equal to $3.75, or 125%
of the offering price per share of common stock sold in the January 16, 2025 Registered Direct Offering, and are exercisable until January
19, 2027.
Results of Operations for the Three and Nine Months Ended September
30, 2024 and 2023
The
following data summarizes the results of our operations for the periods indicated, in thousands:
| | |
Three
Months Ended September
30, | | |
| | |
Nine
Months Ended September
30, | | |
| |
| Description | |
2024 | | |
2023 | | |
Dollar Change | | |
2024 | | |
2023 | | |
Dollar
Change | |
| Operating expenses | |
$ | 1,590 | | |
$ | 2,776 | | |
$ | (1,186 | ) | |
$ | 5,713 | | |
$ | 8,925 | | |
$ | (3,212 | ) |
| Operating loss | |
$ | (1,590 | ) | |
$ | (2,776 | ) | |
$ | 1,186 | | |
$ | (5,713 | ) | |
$ | (8,925 | ) | |
$ | 3,212 | |
| Net loss | |
$ | (1,524 | ) | |
$ | (2,780 | ) | |
$ | 1,256 | | |
$ | (5,524 | ) | |
$ | (8,931 | ) | |
$ | 3,407 | |
Comparison of the Three and Nine Months Ended September 30, 2024
and 2023
Operating Expenses
The following table summarizes our total operating
expenses, for the periods indicated, in thousands:
| | |
Three Months Ended
September 30, | | |
| | |
Nine
Months Ended September
30, | | |
| |
| Description | |
2024 | | |
2023 | | |
Dollar Change | | |
2024 | | |
2023 | | |
Dollar Change | |
| Research and development | |
$ | 644 | | |
$ | 1,808 | | |
$ | (1,164 | ) | |
$ | 2,658 | | |
$ | 5,325 | | |
$ | (2,667 | ) |
| General and administrative | |
| 946 | | |
| 968 | | |
| (22 | ) | |
| 3,055 | | |
| 3,600 | | |
| (545 | ) |
| Total operating expenses | |
$ | 1,590 | | |
$ | 2,776 | | |
$ | (1,186 | ) | |
$ | 5,713 | | |
$ | 8,925 | | |
$ | (3,212 | ) |
Research and Development Expenses
Research and development
expenses relate to compensation and benefits for research and development personnel, facility-related expenses, supplies, external services,
costs to acquire technology licenses, research activities under our research collaboration agreement, expenses associated with preclinical
and clinical development activities and other operating costs. Our research and development programs are focused on the development of
immuno-oncology therapeutics based on our INTASYL therapeutic platform. Since we commenced operations, research and development expenses
have been a significant portion of our total operating expenses and are expected to constitute the majority of our spending for the foreseeable
future.
Research and development
expenses for the three months ended September 30, 2024 decreased 64% as compared with the three months ended September 30, 2023. The
decrease in research and development expenses was primarily driven by our cost rationalization measures in transitioning from a research
company to a product development company resulting in a decrease of $286,000 in salary-related costs, including stock-based compensation
expense, and $85,000 due to the wind-down of preclinical studies, and $835,000 primarily related to our former Clinical Co-Development
Agreement from the prior year period.
Research and development
expenses for the nine months ended September 30, 2024 decreased 50% as compared with the nine months ended September 30, 2023. The decrease
in research and development expenses was primarily driven by our cost rationalization measures in transitioning from a research company
to a product development company resulting in a decrease of $880,000 of expense due to the wind-down of preclinical studies, $1,108,000
in salary-related costs, including stock-based compensation expense, and $204,000 in lab supplies associated with the reduction in headcount,
in addition to decreases in clinical consulting fees of $350,000 incurred in connection with our IND filing for PH-762 in the prior period,
decreases in clinical trial-related fees for our former PH-762 trials in ACT and European clinical trial, and a decrease of $208,000
in manufacturing fees for PH-762.
We anticipate our research
and development expenses will remain relatively consistent for the remainder of 2024.
General and Administrative Expenses
General and administrative
expenses relate to compensation and benefits for general and administrative personnel, facility-related expenses, professional fees for
legal and patent-related activities, audit, tax and consulting services, as well as other general corporate expenses.
General and administrative
expenses for the three months ended September 30, 2024 decreased 2% as compared with the three months ended September 30, 2023. The decrease
in general and administrative expenses was primarily due to a decrease in salary-related expenses.
General and administrative
expenses for the nine months ended September 30, 2024 decreased 15% as compared with the nine months ended September 30, 2023. The decrease
in general and administrative expenses was primarily due to decreases in salary-related expenses due to reductions in headcount of $196,000,
in professional fees for a total of $229,000 primarily related to legal and patent expenses, and in our D&O insurance premium of
$74,000 as compared to the prior year period.
We anticipate our general and
administrative expenses will remain relatively consistent for the remainder of 2024.
Results of Operations for the Years Ended December 31, 2023 and
2022
The following data summarizes the results of our
operations for the years ended December 31, 2023 and 2022, in thousands:
| | |
Years Ended
December 31, | | |
Dollar | |
| | |
2023 | | |
2022 | | |
Change | |
| Operating expenses | |
$ | 10,824 | | |
$ | 11,462 | | |
$ | (638 | ) |
| Operating loss | |
$ | (10,824 | ) | |
$ | (11,462 | ) | |
$ | 638 | |
| Net loss | |
$ | (10,826 | ) | |
$ | (11,480 | ) | |
$ | 654 | |
Comparison of the Years Ended December 31, 2023 and 2022
Operating Expenses
The following table summarizes our total operating
expenses, for the periods indicated, in thousands:
| | |
Years Ended
December 31, | | |
Dollar | |
| | |
2023 | | |
2022 | | |
Change | |
| Research and development | |
$ | 6,332 | | |
$ | 7,012 | | |
$ | (680 | ) |
| General and administrative | |
| 4,366 | | |
| 4,450 | | |
| (84 | ) |
| Impairment of property and equipment | |
| 126 | | |
| – | | |
| 126 | |
| Total operating expenses | |
$ | 10,824 | | |
$ | 11,462 | | |
$ | (638 | ) |
Research and Development Expenses
Research and development
expenses for the year ended December 31, 2023 decreased 10% as compared to the year ended December 31, 2022. The decrease was primarily
due to a decrease in costs related to the completion of our IND-enabling preclinical studies for PH-894 of approximately $1,979,000 and
reduced lab supplies of approximately $298,000 as a result of a decrease in lab personnel and a shift in focus on clinical development
and, partially offset by an increase in clinical-related costs of approximately $1,580,000 for the two U.S. PH-762 Phase 1 clinical trials
as compared to the prior year period.
General and Administrative
Expenses
General and administrative
expenses for the year ended December 31, 2023 decreased 2% as compared to the year ended December 31, 2022. The decrease was primarily
due to decreases in personnel-related expenses of approximately $230,000 due to departmental organizational changes and one-time executive
search-related fees of approximately $78,000 for the Company’s President & CEO and reduced D&O insurance premiums of $92,000,
partially offset by increased legal fees of approximately $302,000.
Impairment of Property
and Equipment
Loss on impairment of property
and equipment for the year ended December 31, 2023 increased 100% as compared to the year ended December 31, 2022. The impairment charge
to our long-lived assets was associated with our non-renewal of our office lease to operate as a remote business. The carrying value
of these assets totaling $126,000 was deemed no longer recoverable and an impairment charge of $126,000 was recorded to adjust those
assets to their fair value.
Liquidity and Capital Resources
Historically, our primary
source of funding has been through the sale of our securities. In the future, we will be dependent on obtaining funding from third parties,
such as proceeds from the issuance of debt, sale of equity or strategic opportunities, in order to maintain our operations. We have reported
recurring losses from operations since inception and expect that we will continue to have negative cash flows from our operations for
the foreseeable future. At September 30, 2024, we had cash of $5,390,000 as compared with $8,490,000 at December 31, 2023.
On May 16, 2024, we entered
into a purchase agreement (the “Purchase Agreement”) with Triton Funds LP (“Triton”), pursuant
to which we agreed to sell, and Triton agreed to purchase, upon our request in one or more transactions, up to 95,833 shares of our common
stock at a purchase of $6.48 per share (the “Purchase Price”), for aggregate gross proceeds of up to $621,000. On
July 3, 2024, we terminated the Purchase Agreement with Triton effective immediately. No shares of common stock were sold by us pursuant
to the Purchase Agreement prior to termination.
In July 2024, we entered
into inducement letter agreements (the “July 2024 Inducement Letter Agreements”) with certain holders of certain of
our existing warrants to purchase up to an aggregate of 545,286 shares of the Company’s common stock. The existing warrants were
originally issued in February 2020 through December 2023, having exercise prices between $324.00 and $9.72 per share. Pursuant to the
July 2024 Inducement Letter Agreements, these warrants were exercised for cash at a reduced exercise of $5.45 per share in consideration
of our agreement to issue new unregistered five and one-half year term Series C warrants to purchase up to 583,098 shares of common stock
at an exercise price of $5.45 and new unregistered eighteen month term Series D warrants to purchase up to 507,474 shares of common stock
at an exercise price of $5.45, both issued and sold at a price of $0.125 per warrant share (the “July 2024 Financing”).
The net proceeds to us from the July 2024 Financing were approximately $2,646,000, after deducting placement agent fees and offering
expenses.
We have limited cash resources,
have reported recurring losses from operations since inception, have negative operating cash flows and have not yet received product
revenues. These factors raise substantial doubt regarding our ability to continue as a going concern, and our current cash resources
may not provide sufficient capital to fund operations for at least the next 12 months from the date of the release of the condensed consolidated
financial statements included elsewhere in this Quarterly Report. Our continuation as a going concern depends upon our ability to raise
additional capital through equity offerings, debt offerings and/or strategic opportunities to fund our operations. There can be no assurance
that we will be successful in accomplishing any of these plans in order to continue as a going concern. The condensed consolidated financial
statements included elsewhere in this Quarterly Report do not include any adjustments to the recoverability and classification of recorded
asset amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
The following table summarizes
our cash flows for the periods indicated, in thousands:
| | |
Nine Months Ended
September 30, | |
| | |
2024 | | |
2023 | |
| Net cash used in operating activities | |
$ | (5,741 | ) | |
$ | (8,379 | ) |
| Net cash used in investing activities | |
| – | | |
| (5 | ) |
| Net cash provided by financing activities | |
| 2,641 | | |
| 5,010 | |
| Net decrease in cash, cash equivalents and restricted cash | |
$ | (3,100 | ) | |
$ | (3,374 | ) |
Net Cash Flow from Operating Activities
Net cash used in operating
activities was $5,741,000 for the nine months ended September 30, 2024 as compared with $8,379,000 for the nine months ended September
30, 2023. The decrease was primarily due to a decrease in net loss of $3,407,000, a decrease in non-cash related items of $298,000 primarily
related to reduced stock-based compensation expense, and a decrease in the changes in operating assets and liabilities of $472,000 primarily
as a result of liabilities owed for the completion of preclinical studies in the prior year period.
Net Cash Flow from Investing Activities
There were no investing activities
for the nine months ended September 30, 2024 as compared with $5,000 for the nine months ended September 30, 2023. The decrease in net
cash used in investing activities was primarily due to laboratory and computer equipment purchases for our facility during the prior
year period.
Net Cash Flow from Financing Activities
Net cash provided by financing
activities was $2,641,000 for the nine months ended September 30, 2024 as compared with $5,010,000 for the nine months ended September
30, 2023. The decrease was primarily due to the net proceeds from the completion of our financing activities during the prior year period
as compared with the current year period.
The following table summarizes
our cash flows for the years ended December 31, 2023 and 2022, in thousands:
| | |
Years Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Net cash used in operating activities | |
$ | (10,749 | ) | |
$ | (12,129 | ) |
| Net cash used in investing activities | |
| (5 | ) | |
| (121 | ) |
| Net cash provided by (used in) financing activities | |
| 7,413 | | |
| (26 | ) |
| Net decrease in cash and restricted cash | |
$ | (3,341 | ) | |
$ | (12,276 | ) |
Net Cash Flow from Operating Activities
Net cash used in operating
activities for the year ended December 31, 2023 decreased 11% as compared to the year ended December 31, 2022, primarily due to decreased
cash outflows from changes in operating assets and liabilities of $720,000 as a result of liabilities owed for the payments related to
the IND-enabling studies with PH-894 and clinical supply manufacturing of PH-762 and PH-894 in the prior year period and a decrease in
net loss of $654,000.
Net Cash Flow from Investing Activities
Net cash used in investing
activities for the year ended December 31, 2023 decreased 96% as compared to the year ended December 31, 2022, primarily due to changes
in laboratory and computer equipment purchases for our facility over the comparative period.
Net Cash Flow from Financing Activities
Net cash provided by financing
activities for the year ended December 31, 2023 increased as compared to the net cash used in financing activities for the year ended
December 31, 2022, primarily due to the Company’s capital raise activities over the comparative period (see Note 9 to our consolidated
annual consolidated financial statements included elsewhere in this Annual Report).
Critical Accounting Policies and Estimates
The discussion and analysis
of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in
accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of these consolidated
financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and
expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and base our estimates
on historical experience and various other assumptions that are believed to be reasonable under the circumstances. Actual results may
differ from these estimates under different assumptions or conditions and could have a material impact on our reported results. While
our significant accounting policies are more fully described in Note 1 to our consolidated financial statements included elsewhere in
this Annual Report, we believe the following addresses our accounting policies to be the most critical in understanding the judgments
and estimates we use in preparing our consolidated financial statements.
Research and Development Expenses
Research and development
expenses are charged to expense as incurred. Payments made by us in advance for research and development services not yet provided and/or
for materials not yet received are recorded as prepaid expenses and expensed when the service has been performed or when the goods have
been received. Accrued liabilities are recorded with respect to services provided and/or materials that we have received for which vendors
have not yet billed us. The financial terms of these contracts are subject to negotiation, vary from provider to provider and may result
in uneven payment flows. There may be instances in which payments made to our vendors exceed the level of services provided and result
in a prepayment of the expense. In other instances, payment depends on factors such as the successful completion of milestones.
We are required to estimate
our accrued research and development expenses, of which a significant portion relate to third party providers we have contracted with
to perform various research activities on our behalf for the continued development of our product candidates. This process includes reviewing
open contracts and purchase orders, estimating the service performed and the associated cost incurred for research and development services
not yet billed or otherwise notified of actual cost. Accrued liabilities for the services provided by contract research organizations
are recorded during the period incurred based on such estimates and assumptions as expected cost, passage of time, the level of effort
to be expended in each period, the achievement of milestones and other information available to us. Estimates of our research and development
accruals are assessed on a quarterly basis based on an evaluation of the progress to completion of specific tasks using information and
data provided to us by our vendors and facts and circumstances known to us at that time, and adjusted accordingly.
Actual results may differ
from these estimates and could have a material impact on our reported results. Our historical accrual estimates have not been materially
different from our actual costs. Due to the nature of estimates, we cannot provide assurance that we will not make changes to our estimates
in the future as we become aware of additional information about the conduct of our research activities.
Collaborative Arrangements
We follow the provisions
of the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”)
Topic 808, “Collaborative Arrangements,” (“Topic 808”) when collaboration agreements involve joint
operating activities in which both parties are active participants and that are also both exposed to significant risks and rewards. We
also consider the guidance in the FASB ASC Topic 606, “Revenue from Contracts with Customers,” (“Topic 606”)
in determining the appropriate treatment for activities between us and our collaborative partners that are more reflective of a vendor-customer
relationship and therefore, within the scope of Topic 606, as well as other accounting literature. Under Topic 808, we determine an appropriate
recognition method, either by analogy to appropriate accounting literature or by applying a reasonable accounting policy election. Generally,
the classification of transactions under the collaborative arrangement is determined based on the nature and contractual terms of the
arrangement along with the nature of the operations of the participants. We recognize our share of costs arising from research and development
activities performed by collaborators in the period our collaborators incur such expense. Reimbursements that are the result of a collaborative
relationship instead of a customer relationship, such as co-development activities, are evaluated on a quarterly basis and recorded as
an offset to research and development expense incurred. Payments in excess of our collaboration expense will be recorded as revenue.
Derivative Financial Instruments
During the normal course
of business we may issue warrants as part of a debt or equity financing. Warrants and other derivative financial instruments are accounted
for either as equity or as an asset or liability, depending on the characteristics of each derivative financial instrument. Financial
instruments that do not meet the definition of a derivative are classified as equity and measured at fair value and recorded as additional
paid in capital in stockholders’ equity at the date of issuance. No further adjustments to their valuation are made. Financial
instruments that meet the definition of a derivative are classified as an asset or liability are measured at fair value on the issuance
date and are revalued on each subsequent balance sheet date. The changes in the fair value are recognized as current period income or
loss.
Contractual Obligations
Details
of our obligations under the Clinical Co-Development Agreement with our former collaboration
partner AgonOx can be found in Note 2 of the condensed consolidated financial statements.
Outside of the above, there have been no material changes to the contractual obligations
as disclosed in our 2023 Form 10-K.
Future Funding Requirements
At September 30, 2024, we
had cash and cash equivalents of $5,390,000 and received estimated net proceeds of $2,646,000 from our July 2024 Financing. At September
30, 2024, we expect that our cash and cash equivalents will enable us to fund our current operating plan into Q2 2025. Due to the difficulty
and uncertainty associated with the design and implementation of preclinical studies and clinical trials, we will continue to assess our
cash and cash equivalents and future funding requirements. However, there is no assurance that additional funding will be achieved and
that we will succeed in our future operations. We expect to continue to incur substantial additional operating losses for at least the
next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval,
the eventual commercialization of our product candidates. If we obtain marketing approval for any of our product candidates, we will incur
significant sales, marketing and manufacturing expenses. We also expect to continue to incur significant costs to comply with corporate
governance, internal controls and similar requirements associated with operating as a public reporting company.
Actual cash requirements
could differ from management’s projections due to many factors including additional investments in research and development programs,
clinical trial expenses for PH-762, competing technological and market developments, general and administrative expenses, and the costs
of any strategic acquisitions and/or development of complementary business opportunities.
We expect to seek additional
funding to sustain our future operations and while we have successfully raised capital in the past, the ability to raise capital in future
periods is not assured. We do not know if additional capital will be available when needed or on terms favorable to us or our stockholders.
Collaboration, licensing or other agreements may not be available on favorable terms, or at all. If we seek to sell our equity securities,
we do not know whether and to what extent we will be able to do so, or on what terms. If available, additional equity financing may be
dilutive to stockholders, debt financing may involve restrictive covenants or other unfavorable terms and dilute our existing stockholders’
equity, and funding through collaboration, licensing or other commercial agreements may be on unfavorable terms, including requiring
us to relinquish rights to certain of our technologies or products. If adequate financing is not available if and when needed, we may
delay, reduce the scope of, or eliminate research or development programs, if any, postpone or cancel the pursuit of product candidates,
or otherwise significantly curtail our operations to reduce our cash requirements and extend our capital.
Management
Set forth below are the present
directors and executive officers of the Company as of December 31, 2024. There are no arrangements or understandings between any of the
directors, officers and other persons pursuant to which such person was selected as a director or an officer.
| Name |
|
Age |
|
Position(s) with
the Company |
|
|
| Robert J. Bitterman |
|
73 |
|
President, Chief Executive Officer and Chairman of the Board of Directors |
|
|
| Robert M. Infarinato |
|
79 |
|
Vice President, Chief Financial Officer |
|
|
| Patricia A. Bradford |
|
74 |
|
Director |
|
|
| Robert L. Ferrara |
|
73 |
|
Lead Independent Director |
|
|
| Jonathan E. Freeman, Ph.D. |
|
57 |
|
Director |
|
|
| Curtis A. Lockshin, Ph.D. |
|
64 |
|
Director |
|
|
Biographies
Set forth below are brief accounts of the business experience of each
director and executive officer of the Company.
Robert J. Bitterman has served as a member
and the Chairman of the Board since 2012 and as our President and Chief Executive Officer since February 2023. Mr. Bitterman served as
the Interim Executive Chairman of the Company from September 2022 to February 2023 until his appointment as President and Chief Executive
Officer. Mr. Bitterman served as the President and Chief Executive Officer of Cutanea Life Sciences, Inc., a private company he founded
in 2005 that focused on developing innovative technologies to treat diseases and disorders of the skin and subcutaneous tissue, until
its acquisition by Biofrontera, Inc., USA in March 2019. Since leaving Cutanea, Mr. Bitterman was retired until commencing the Interim
Executive Chairman role with the Company in September 2022. Prior to his role at Cutanea Life Sciences, Inc., Mr. Bitterman also held
the position of President and Chief Executive Officer of Isolagen, Inc., President and General Manager of Dermik Laboratories and various
positions of increasing responsibility in financial and commercial capacities within Aventis S.A. Mr. Bitterman holds an A.B. degree
in Economics from The College of the Holy Cross and a Master of Business Administration degree from Boston University. He also holds
a Doctor of Humane Letters (Honoris Causa) from the New York College of Podiatric Medicine. Mr. Bitterman’s executive leadership
experience and his experience in the pharmaceutical industry qualifies him to serve as a member of the Board.
Robert M. Infarinato has served as our
Vice President, Chief Financial Officer since August 1, 2024. Mr. Infarinato, an attorney and certified public accountant, has over 40
years of leadership roles, including in the pharmaceutical and biotech industries. From 2000 to 2024, Mr. Infarinato served as a self-employed
business consultant doing business as International Business Consulting. From 2010 to 2019, Mr. Infarinato served in various positions
within Cutanea Life Sciences, Inc., a privately held dermatology company, including chief compliance officer, as well as a member of
the board of directors and the audit committee thereof. From 1996 to 2013, Mr. Infarinato served as a member of the board of trustees
for Abington Health Systems, serving as chairman from 2010 to 2013. Following Abington Health Systems’ merger with Jefferson Health
System, Mr. Infarinato served on the finance committee until September 2021 and the Abington Jefferson Compliance Committee until 2023.
From January 2022 to January 2023, Mr. Infarinato served as a tax specialist for Steinberg, Shebairo LLP CPAs. Mr. Infarinato obtained
his Juris Doctor degree from Fordham University School of Law, and his B.S. from the Syracuse University Whitman School of Management.
Patricia A. Bradford has served as a member
of the Board since 2022. Ms. Bradford served as Senior Vice President Global Human Resources at Unisys Corporation, a global information
technology solutions company, where her total service at Unisys spanned from 1982 until her retirement in 2013. In her role at Unisys,
Ms. Bradford strategically led all global human resource programs and initiatives, including talent management, at multiple levels of
the organization. Ms. Bradford’s roles at Unisys progressively included all areas of human resources, including an overseas assignment
at the Unisys European headquarters where she provided human resources leadership to the region. Prior to Unisys, Ms. Bradford was employed
by Deloitte, an audit, consulting, tax, and advisory services firm, from 1978 to 1982. Since 2014, Ms. Bradford has maintained a consulting
practice focused on individual coaching for senior executives and high potential employees recommended by management. Ms. Bradford received
a B.S. degree with an emphasis on accounting and statistics from Walsh College and is a Certified Public Accountant. Ms. Bradford’s
executive leadership experience, global business perspective, and human capital management and financial backgrounds qualifies her to
serve as a member of the Board.
Robert L. Ferrara has served as a member
of the Board since 2019 and currently serves as our Lead Independent Director. He most recently served as the Chief Financial Officer
of Cutanea Life Sciences, Inc., a private company focused on developing innovative technologies to treat diseases and disorders of the
skin and subcutaneous tissue, from January 2012 to his retirement in June 2019. Prior to Cutanea, Mr. Ferrara served as the Chief Financial
Officer of Storeroom Solutions Inc., a venture capital financed, technology enhanced, integrated supply chain solutions company, from
2004 to 2011, and NER Data Products, Inc., an IT service management company, from 2000 to 2003, as well as holding other senior level
financial positions in national and international public companies in the greater Philadelphia area. Mr. Ferrara received a B.S. in Accounting
from Lehigh University and is a Certified Public Accountant. Mr. Ferrara’s financial expertise and his extensive experience in
both publicly traded and venture capital backed companies in a variety of industries, including the life sciences, qualifies him to serve
as a member of the Board.
Jonathan E. Freeman, Ph.D. has served
as a member of the Board since 2017. Dr. Freeman currently serves as the Chief Operating Officer of Anthos Therapeutics Inc., a clinical-stage
biopharmaceutical company developing therapies for cardiovascular patients, a position he has held since July 2021. Anthos Therapeutics
Inc. was launched by Novartis and Blackstone Life Sciences, a private investment firm, where Dr. Freeman has also served as a Senior
Advisor since July 2018. From 2017 to June 2018, Dr. Freeman held the position of Chief Business Officer of Vedanta Biosciences, a clinical-stage
company developing therapies for immune-mediated diseases. Prior to his role with Vedanta Biosciences, Dr. Freeman was the Senior Vice
President of Strategy and Portfolio Management and Head of Business Development and Licensing at Merck KGaA, a leading science and technology
company, from 2008 to 2016. Dr. Freeman received a Ph.D. in Molecular Pharmacology and Drug Metabolism from the Imperial Cancer Research
Fund (now CRUK), an M.A. and First Class Honours in Biochemistry from Cambridge University and a MBA with a finance major from Webster
University, St. Louis. Dr. Freeman’s executive leadership experience and his background in immunology qualifies him to serve as
a member of the Board.
Curtis A. Lockshin, Ph.D. has served as
a member of the Board since 2013. Dr. Lockshin served as the Chief Scientific Officer of Xenetic Biosciences, Inc., a biopharmaceutical
company focused on the development of novel oncology therapeutics, from January 2017 to May 2024. Prior to this appointment, Dr. Lockshin
served as Xenetic Biosciences, Inc.’s Vice President of Research and Operations from March 2014 to January 2017. From July 2016
to December 2016, Dr. Lockshin served as Chief Technical Officer of VBI Vaccines, Inc., a company developing vaccines in infectious disease
and immuno-oncology. VBI Vaccines, Inc. merged with SciVac Therapeutics, Inc. and its subsidiary SciVac, Ltd., a commercial-stage biologics
and vaccine company, in July 2016 where Dr. Lockshin had served as its Chief Executive Officer and director since September 2014. Since
2004, Dr. Lockshin has served as a Director of the Ruth K. Broad Biomedical Research Foundation, a Duke University Support Corporation.
Since May 2013, Dr. Lockshin has also served as President and Chief Executive Officer of Guardum Pharmaceuticals, LLC, a private pharmaceutical
company. Dr. Lockshin holds a PhD in Biological Chemistry and a BS in Life Sciences, both from the Massachusetts Institute of Technology.
Our Nominating Committee believes that Dr. Lockshin’s scientific background, his significant industry knowledge and management
experience qualifies him to serve as a member of the Board.
Director Independence
We believe that the Company benefits from having
a strong and independent Board. For a director to be considered independent, the Board must determine that the director does not have
any direct or indirect material relationship with the Company that would affect his or her exercise of independent judgment. On an annual
basis, the Board reviews the independence of all directors under the applicable Commission rules and Nasdaq listing standards. The Board
also considers each director’s affiliations with the Company and members of management, as well as significant holdings of Company
securities. This review considers all known relevant facts and circumstances in making an independence determination. Based on this review,
the Board has made an affirmative determination that all directors are independent, other than our President and Chief Executive Officer
and Chairman of the Board, Mr. Bitterman.
In addition, Nasdaq listing standards require
that, subject to specified exceptions, each member of our Audit, Compensation, Governance and Nominating Committees of the Board be independent
and that members of our Audit Committee of the Board also satisfy independence criteria set forth in Rule 10A-3 under the Securities
and Exchange Act of 1934, as amended (the “Exchange Act”). The Board has determined that all members of the Audit
Committee, Compensation Committee, Governance Committee, and Nominating Committee are independent under the applicable Nasdaq listing
standards and the Exchange Act.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and
Ethics that applies to all employees, officers and directors. Our Code of Business Conduct and Ethics, as well as other corporate governance
materials, is located on our website at www.phiopharma.com. Waivers of our Code of Business Conduct and Ethics may only be granted
by the Board. We intend to disclose on our website any amendments to, or waivers from, the Code of Business Conduct and Ethics that are
required to be disclosed pursuant to the disclosure requirements of Item 5.05 of Form 8-K within four business days following the date
of such amendment or waiver.
Related Party Transactions
The Board, with the assistance of the Audit Committee,
reviews and approves all transactions with directors, officers and holders of more than 5% of our voting securities and their affiliates.
Prior to the Board’s consideration of a transaction with such a related party, the material facts as to the related party’s
relationship or interest in the transaction must be disclosed to the Board, and the transaction will not be considered approved by the
Board unless a majority of the directors who are not interested in the transaction (if applicable) approve the transaction. Furthermore,
when stockholders are entitled to vote on a transaction with a related party, the material facts of the related party’s relationship
or interest in the transaction must be disclosed to the stockholders, who must approve the transaction in good faith.
During the past two years, there has not been,
nor is there currently proposed, any transaction or series of related transactions to which we were or will be a party in which the amount
involved exceeded or will exceed the lesser of (i) $120,000 and (ii) one percent of the average of Company's total assets at year-end
for the last two completed fiscal years and in which the other parties included or will include any of our directors, executive officers,
holders of 5% or more of our voting securities, or any member of the immediate family of any of the foregoing persons, other than compensation
arrangements with our directors and executive officers.
DIRECTOR AND EXECUTIVE
OFFICER COMPENSATION
Director Compensation
We compensate our non-employee directors for
their service as a member of the Board. Each non-employee director is entitled to receive an annual cash retainer of $35,000. The chairs
of the Board and Audit Committee are entitled to receive an additional annual cash retainer of $15,000 and the chairs of the Compensation
Committee, the Governance Committee and the Nominating Committee are entitled to receive an additional cash retainer of $7,500. In addition,
the Lead Independent Director, if any, is entitled to receive an additional annual cash retainer of $12,500. Each non-employee director
is also entitled to receive an annual grant of restricted stock units (“RSUs”) as determined by the board, which vest
in full on the one-year anniversary of the respective date of grant.
The Compensation Committee and the Board reassess
the appropriate levels of cash and equity compensation for non-employee directors on an annual basis.
Non-employee directors are also reimbursed for
their travel and reasonable out-of-pocket expenses incurred in connection with attending Board and committee meetings and in attending
continuing education seminars, to the extent that attendance is required by the Board or the committee(s) on which that director serves.
The following table shows the compensation to our
non-employee directors in fiscal year 2024. We compensate our non-employee directors for their service as a member of the Board. Compensation
paid to Robert J. Bitterman, our President, Chief Executive Officer and Chairman of the Board, is set forth in the Summary Compensation
Table due to Mr. Bitterman’s status as one of our named executive officers (“NEOs”).
| Name | |
Fees Earned or
Paid in Cash ($) | | |
Stock
Awards ($)(1) | | |
Total ($) | |
| Patricia A. Bradford | |
| 50,000 | | |
| 22,320 | | |
| 72,320 | |
| Robert L. Ferrara | |
| 62,500 | | |
| 27,900 | | |
| 90,400 | |
| Jonathan E. Freeman, Ph.D. | |
| 35,000 | | |
| 2,790 | | |
| 37,790 | |
| Curtis A. Lockshin, Ph.D. | |
| 42,500 | | |
| 5,580 | | |
| 48,080 | |
As of December 31, 2024, the aggregate number
of shares underlying stock options and RSUs by our non-employee directors is as follows: Patricia A. Bradford 8,000 RSUs, Robert L. Ferrara
10,000 RSUs, Jonathan E. Freeman, Ph.D. 1,000 RSUs, and Curtis A. Lockshin, Ph.D. 2,000 RSUs. Mr. Bitterman’s outstanding equity
awards are also included in the Outstanding Equity Awards at Fiscal Year-End table due to his status as a NEO during the fiscal year
ended December 31, 2024.
Executive Compensation
Compensation of Named Executive Officers
Our NEOs with respect to the fiscal year that
ended on December 31, 2024 are Robert J. Bitterman, who serves as our President and Chief Executive Officer, and Robert M. Infarinato,
who serves as our Vice President and Chief Financial Officer. Mr. Bitterman and Mr. Infarinato are referred to as our “named executive
officers” or NEOs.
Summary Compensation Table
The following table provides information regarding the compensation
earned by our NEOs:
| Name and principal position | |
Year | |
Salary
($) | | |
Stock
awards
($)(1) | | |
Non-equity
incentive plan
compensation
($)(2) | | |
All other
compensation
($)(3) | | |
Total
($) | |
| Robert J. Bitterman (4) | |
2024 | |
| 342,615 | | |
| 43,245 | | |
| – | | |
| 1,159 | | |
| 387,019 | |
| (President and Chief Executive Officer) | |
2023 | |
| 380,000 | | |
| 57,640 | | |
| – | | |
| 252 | | |
| 437,892 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| | |
| Robert M. Infarinato (5) | |
2024 | |
| 75,462 | | |
| 29,330 | | |
| – | | |
| 414 | | |
| 105,206 | |
| (Vice President and Chief Financial Officer) | |
| |
| | | |
| | | |
| | | |
| | | |
| | |
| (1) |
The amounts shown, consisting entirely of RSUs, reflect
the grant date fair value of RSUs computed in accordance with the FASB ASC Topic 718, “Compensation — Stock Compensation”
for the indicated year. |
| (2) |
Reflects the annual cash bonus earned for performance for each respective
year under our Incentive Bonus Program. Mr. Bitterman did not receive an annual cash bonus in 2024 or 2023. |
| (3) |
Represents amounts for the dollar value of life insurance premiums paid. |
| (4) |
Mr. Bitterman has served as a member of the Board since 2012 and served
as our Interim Executive Chairman from September 2022 to February 2023 and was appointed as our President and Chief Executive Officer
in February 2023. Upon his appointment to Interim Executive Chairman, Mr. Bitterman ceased receiving compensation in connection with
his position as a director of the Board, including as Chairman of the Board. |
| (5) |
Mr. Infarinato was appointed Vice President and Chief Financial Officer
effective as of August 1, 2024. |
Outstanding Equity Awards at Fiscal Year-End
The following table shows information regarding outstanding equity
awards as of December 31, 2024 for our NEOs:
| | |
| |
Option Awards | | |
Stock Awards | |
| Name | |
Grant Date | |
Number
of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | |
Number
of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | | |
Option
Exercise
Price ($) | | |
Option
Expiration
Date | | |
Number
of
Shares or
Units of
Stock That
Have Not
Vested (#) | | |
Market
Value of Shares or
Units of
Stock That
Have Not
Vested ($) | | |
Equity
Incentive
Plan
Awards:
Number of
Unearned
Shares or
Units of
Stock That
Have Not
Vested (#) | | |
Equity
Incentive
Plan
Awards:
Market Value of
Unearned
Shares or
Units of
Stock That
Have Not
Vested
($)(1) | |
Robert J. Bitterman
(2) | |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
6/1/2015 | |
| 1 | | |
| – | | |
| 225,720.00 | | |
| 6/1/2025 | | |
| – | | |
| – | | |
| – | | |
| – | |
| | |
2/10/2016 | |
| 1 | | |
| – | | |
| 169,884.00 | | |
| 2/10/2026 | | |
| – | | |
| – | | |
| – | | |
| – | |
| | |
2/1/2017 | |
| 1 | | |
| – | | |
| 37,362.60 | | |
| 2/1/2027 | | |
| – | | |
| – | | |
| – | | |
| – | |
| | |
9/11/2024 | |
| | | |
| | | |
| | | |
| | | |
| 15,500 | | |
| 43,245 | | |
| – | | |
| – | |
| Robert M. Infarinato(3) | |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
8/13/2024 | |
| – | | |
| – | | |
| – | | |
| – | | |
| 8,000 | | |
| 20,960 | | |
| – | | |
| – | |
| | |
9/11/2024 | |
| – | | |
| – | | |
| – | | |
| – | | |
| 3,000 | | |
| 8,370 | | |
| – | | |
| – | |
| (1) |
Value is based on the closing price of $1.80 of the Common
Stock on December 31, 2024. |
| (2) |
The equity awards granted to Mr. Bitterman on June 2, 2014, June 1,
2015, February 10, 2016, and February 1, 2017 vested in one installment on the first anniversary of the grant date. The equity award
granted to Mr. Bitterman on September 11, 2024 will vest in full on the first anniversary of the grant date. |
| (3) |
The equity awards granted to Mr. Infarinato on August 13, 2024 and September
11, 2024 will each vest in full on the first anniversary of the respective grant date. |
Base Salary
When reviewing and approving our executive compensation
arrangements, including the base salaries paid to our executive officers, the Compensation Committee considers a number of factors, including,
but not limited to: the performance of the executive officer to our overall performance, the performance of the executive officer against
our corporate objectives, the executive officer’s skills, experience and qualifications in such executive officer’s role,
review of compensation surveys of base salaries paid by comparable organizations and market compensation data. These factors provide
the framework for decisions regarding the base salary compensation for each executive officer. No single factor is determinative in setting
base salary levels, nor was the impact of any factor on the determination of pay levels quantifiable.
Incentive Compensation
Annual Incentive Bonus
Annual bonuses are based on the achievement of
corporate goals typically comprised of a mix of clinical development, discovery, financial, business development, and investor relations
related performance objectives. The corporate goals are approved by the Board on an annual basis at the start of each year. Annual bonuses
for all employees, including executive officers, take into account the achievement of specified business objectives and individual performance
objectives, with the exception of our President and Chief Executive Officer, whose annual bonus is determined solely by the achievement
of business objectives. The Compensation Committee reviews our achievements against these corporate goals and their assessment of the
goals and recommendations regarding funding is presented to our full Board for approval. The Compensation Committee maintains full discretion
in determining overall performance under the annual bonus and may adjust bonus payouts based on factors it deems relevant. Our NEOs received
no annual incentive bonus payments in 2024 or 2023.
Equity Incentive
We maintain our 2020 Long Term Incentive Plan
(“2020 Plan”) pursuant to which we currently grant RSU awards to eligible participants. Grants of RSUs under this
plan to our NEOs are disclosed in the Summary Compensation Table and Outstanding Equity Awards at Fiscal Year-End table above.
Employment and Change of Control Agreements
The following provides descriptions of the employment
agreements that are currently in effect for our NEOs:
Robert J. Bitterman
Mr. Bitterman was appointed President and Chief Executive
Officer and entered into an employment agreement, dated February 20, 2023, pursuant to which he was entitled to an initial annual base
salary of $440,000 and is eligible to receive an annual bonus of up to 40% of his annual base salary, based on the achievement of certain
performance goals established annually by the Board. In connection with his appointment, we granted Mr. Bitterman RSUs settleable for
11,000 shares of Common Stock under the 2020 Plan. The RSUs vest in two equal annual installments, commencing on the first anniversary
of the date of grant, subject to Mr. Bitterman’s continuous service with us through each such vesting date. Effective as of October
16, 2023, Mr. Bitterman voluntarily reduced his annual base salary by $100,000 to $340,000.
If Mr. Bitterman’s employment is terminated
by us due to death or disability, we shall pay to Mr. Bitterman or to his estate, as applicable, any earned, but unpaid, base salary
and any amounts owed to Mr. Bitterman for reimbursement of expenses properly incurred which are reimbursable, in each case as earned
or incurred, as applicable through the date of termination (the “Accrued Benefits”), as well as pay any accrued but
unpaid bonus then due to Mr. Bitterman and all equity awards that have been granted will immediately vest on a pro-rata basis. If Mr.
Bitterman’s employment is terminated by the Board for cause or by Mr. Bitterman without good reason, we shall pay to Mr. Bitterman
the Accrued Benefits through the date of termination. If Mr. Bitterman’s employment is terminated by Mr. Bitterman for good reason
or by us other than as a result of death or disability and other than for cause, then we shall pay to Mr. Bitterman the Accrued Benefits
through the date of termination, continue to pay Mr. Bitterman his base salary for three months from the date of separation, pay any
accrued but unpaid bonus and if, and only if, such termination occurs within one year of a change in control all equity awards that have
been granted but are not exercisable at the time of such termination shall immediately become exercisable in full.
Mr. Bitterman is eligible to participate in the
2020 Plan and other benefits available to our executive officers.
Robert M. Infarinato
Mr. Infarinato was appointed Vice President and
Chief Financial Officer and entered into an offer letter and employment agreement, effective August 1, 2024, pursuant to which he is
entitled to an initial annual base salary of $180,000 and is eligible to receive an annual bonus of up to 30% of his annual base salary,
based on the achievement of certain performance goals established annually by the Board. In connection with his appointment, we granted
Mr. Infarinato RSUs settleable for 8,000 shares of Common Stock under the 2020 Plan. The RSUs vest in equal annual installments over
three years, commencing on the first anniversary of the date of grant, subject to Mr. Infarinato’s continuous service with us through
each such vesting date.
Mr. Infarinato’s employment is at-will,
which means that we or Mr. Infarinato may terminate his employment with us at any time, with or without notice or cause.
Mr. Infarinato is eligible to participate in
the 2020 Plan and other benefits available to our executive officers.
Equity Incentive
We maintain our 2020 Long Term Incentive Plan
(“2020 Plan”) pursuant to which we currently grant RSU awards to eligible participants. Grants of RSUs under this
plan to our NEOs are disclosed in the Summary Compensation Table and Outstanding Equity Awards at Fiscal Year-End table above.
The Compensation Committee last granted a stock option
in October 2023. We have no program, practice or plan to grant stock options in coordination with the release of material nonpublic information.
We also have not timed the release of material nonpublic information for the purpose of affecting the value of stock options or other
compensation, and we have no plan to do so. These considerations are not applicable to RSUs or other types of equity awards that do not
include an exercise price related to the market price of our stock on the date of grant.
SECURITY OWNERSHIP
OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Based
on information available to us and filings with the SEC, the following table sets forth certain
information regarding the beneficial ownership (as defined by Rule 13d-3 under the Exchange
Act) of our outstanding Common Stock for (i) each of our directors, (ii) each of our NEOs,
(iii) all of our directors and executive officers as a group and (iv) persons known to us
to beneficially own more than 5% of our outstanding Common Stock. The following information
is presented as of January 17, 2025 or such other date as may be reflected below.
Beneficial ownership and percentage ownership are
determined in accordance with the rules of the SEC and include voting or investment power with respect to shares of stock. This information
does not necessarily indicate beneficial ownership for any other purpose. Under these rules, shares of Common Stock not outstanding but
deemed beneficially owned by virtue of the right of a person to acquire them as of January 17, 2025, or within 60 days of January 17,
2025, are deemed outstanding for the purpose of computing the percentage ownership of each person, but are not deemed outstanding for
the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated and subject to applicable
community property laws, to our knowledge, each stockholder named in the following table possesses sole voting and investment power over
their shares of Common Stock, except for those jointly owned with that person’s spouse. Unless otherwise indicated below, the address
of each person listed on the table is c/o Phio Pharmaceuticals Corp., 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, MA 01752.
| |
|
Shares Beneficially Owned |
|
| Name and Address of Beneficial Owner |
|
Number (1) |
|
|
Percent of
Class (2) |
|
| Greater than 5% Holder |
|
|
|
|
|
|
|
|
| Intracoastal Capital LLC(3) |
|
|
472,907 |
|
|
|
9.99% |
|
| Directors and Named Executive Officers: |
|
|
|
|
|
|
|
|
| Robert J. Bitterman |
|
|
1,499 |
|
|
|
* |
|
| Robert M. Infarinato |
|
|
– |
|
|
|
* |
|
| Patricia A. Bradford |
|
|
352 |
|
|
|
* |
|
| Robert Ferrara |
|
|
445 |
|
|
|
* |
|
| Jonathan E. Freeman, Ph.D. |
|
|
356 |
|
|
|
* |
|
| Curtis A. Lockshin, Ph.D. |
|
|
356 |
|
|
|
* |
|
| All current directors and executive officers as a group (six persons) |
|
|
3,008 |
|
|
|
* |
|
| * |
Indicates less than 1%. |
| |
|
| (1) |
Represents shares of Common Stock held as of January 17, 2025 plus shares of Common Stock that may
be acquired upon the exercise of options and warrants within 60 days of January 17, 2025. |
| |
|
| (2) |
Based on 4,776,848 shares of Common Stock that were issued and outstanding as of January 17, 2025.
Shares not outstanding but deemed beneficially owned by virtue of the right of a person to acquire them as of January 17, 2025, or
within 60 days of January 17, 2025, are treated as outstanding only when determining the ownership and voting power for each person
(or all directors and executive officers as a group). |
| |
|
| (3) |
Based on information provided by Intracoastal Capital LLC (“Intracoastal”).
Each of Intracoastal, Mitchell P. Kopin (“Mr. Kopin”) and Daniel B. Asher (“Mr. Asher”) be deemed
to have beneficial ownership of 472,907 shares of Common Stock issuable upon the exercise of certain warrants held by Intracoastal. Certain
of the warrants held by Intracoastal contain a blocker provision under which the holder thereof does not have the right to exercise its
warrants to the extent (but only to the extent) that such exercise would result in beneficial ownership by the holder thereof, together
with the holder’s affiliates, and any other persons acting as a group together with the holder or any of the holder’s affiliates,
of more than 9.99% of the Company’s Common Stock. Based upon information provided by Intracoastal, Intracoastal and Messrs. Kopin
and Asher would be deemed to have beneficial ownership of 1,045,576 shares of Common Stock in the absence of such blocker provisions.
The principal business office of Mr. Kopin and Intracoastal is 245 Palm Trail, Delray Beach, Florida 33483. The principal business office
of Mr. Asher is 111 W. Jackson Boulevard, Suite 2000, Chicago, Illinois 60604. |
| |
|
SELLING STOCKHOLDERS
This
prospectus covers the possible resale by the Selling Stockholder identified in the table
below of 5,930,016 shares of Common Stock issuable upon the exercise of the Warrants. The
Selling Stockholders acquired the Warrants pursuant to the Purchase Agreements or the Engagement
Letter, as applicable, and we are filing the registration statement of which this prospectus
is a part pursuant to the provisions of the Purchase Agreements and the Engagement Letter.
The
table below is based on information supplied to us by the Selling Stockholders, and lists
the Selling Stockholders and other information regarding the beneficial ownership of the
shares of Common Stock by each of the Selling Stockholders. The second column lists the number
of shares of Common Stock beneficially owned by each Selling Stockholder, based on its ownership
of the Warrants, as of January 17, 2025, assuming exercise of the Warrants held by the Selling
Stockholders on that date, without regard to any limitations on exercises.
The third column lists the shares
of Common Stock being offered under this prospectus by the Selling Stockholders. The fourth column assumes the sale of all of the shares
offered by the Selling Stockholders pursuant to this prospectus. The percentage of beneficial ownership after this offering is based
on 4,776,848 shares of Common Stock outstanding as of January 17, 2025.
Under the terms of the Warrants,
a Selling Stockholder may not exercise the Warrants to the extent such exercise would cause such Selling Stockholder, together with its
affiliates and attribution parties, to beneficially own a number of shares of Common Stock which would exceed 4.99% or 9.99%, as applicable,
of our then outstanding Common Stock following such exercise, excluding for purposes of such determination shares of Common Stock issuable
upon exercise of such Warrants which have not been exercised. The number of shares in the second and fourth columns do not reflect this
limitation. The Selling Stockholder may sell all, some or none of their shares in this offering. See “Plan of
Distribution” below for further information.
Except for (a) the ownership
of the Warrants and other equity sold by us to the Selling Stockholders in past offerings and (b) with respect to the Warrants issued
as compensation to the Placement Agent, or its designees, who has acted as our Placement Agent in a number of past offerings, the Selling
Stockholders have not had any material relationship with us within the past three years.
| |
|
|
|
|
|
|
|
Shares Beneficially Owned
After this Offering |
|
| Selling Stockholders |
|
Number of Shares Beneficially
Owned Before this
Offering(1) |
|
|
Number of Shares to be Sold in
this Offering |
|
|
Number of
Shares |
|
|
Percentage of Total Outstanding Common Stock(1) |
|
| Anson Investments Master Fund LP(2) |
|
|
1,307,494 |
|
|
|
1,307,494 |
|
|
|
– |
|
|
|
* |
|
| Anson East Master Fund LP(3) |
|
|
321,030 |
|
|
|
321,030 |
|
|
|
– |
|
|
|
* |
|
| Orca Capital AG(4) |
|
|
1,628,524 |
|
|
|
1,628,524 |
|
|
|
– |
|
|
|
* |
|
| CVI Investments, Inc.(5) |
|
|
1,531,939 |
|
|
|
1,529,856 |
|
|
|
2,083 |
|
|
|
* |
|
| Intracoastal Capital, LLC(6) |
|
|
1,045,576 |
|
|
|
904,298 |
|
|
|
141,278 |
|
|
|
2.87% |
|
| Michael Vasinkevich(7) |
|
|
201,646 |
|
|
|
153,139 |
|
|
|
48,507 |
|
|
|
1.01% |
|
| Noam Rubinstein(7) |
|
|
99,429 |
|
|
|
75,226 |
|
|
|
60,815 |
|
|
|
1.26% |
|
| Craig Schwabe(7) |
|
|
10,614 |
|
|
|
8,060 |
|
|
|
6,516 |
|
|
|
* |
|
| Charles Worthman(7) |
|
|
3,160 |
|
|
|
2,389 |
|
|
|
1,931 |
|
|
|
* |
|
* Represents beneficial ownership of less than one percent.
(1) The ability to exercise the Warrants held
by the Selling Stockholders is subject to a beneficial ownership limitation that, at the time of initial issuance of the Warrants, was
capped at either 4.99% or 9.99% beneficial ownership of our issued and outstanding Common Stock (post-exercise). These beneficial ownership
limitations may be adjusted up or down, subject to providing advanced notice to us. Beneficial ownership as reflected in the selling
stockholder table reflects the total number of shares potentially issuable underlying the Warrants and does not give effect to these
beneficial ownership limitations. Accordingly, actual beneficial ownership, as calculated in accordance with Section 13(d) and Rule 13d-3
thereunder may be lower than as reflected in the table.
(2) Anson Advisors Inc. and Anson Funds Management
LP, the Co-Investment Advisers of Anson Investments Master Fund LP (“Anson”), hold voting and dispositive power over
the Common Stock held by Anson. Tony Moore is the managing member of Anson Management GP LLC, which is the general partner of Anson Funds
Management LP. Moez Kassam and Amin Nathoo are directors of Anson Advisors Inc. Mr. Moore, Mr. Kassam and Mr. Nathoo each disclaim beneficial
ownership of these shares of Common Stock except to the extent of their pecuniary interest therein. The principal business address of
Anson is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands.
(3) Anson Advisors Inc. and Anson Funds Management
LP, the Co-Investment Advisers of Anson East Master Fund LP (“Anson East”), hold voting and dispositive power over
the Common Shares held by Anson East. Tony Moore is the managing member of Anson Management GP LLC, which is the general partner of Anson
Funds Management LP. Moez Kassam and Amin Nathoo are directors of Anson Advisors Inc. Mr. Moore, Mr. Kassam and Mr. Nathoo each disclaim
beneficial ownership of these Common Shares except to the extent of their pecuniary interest therein. The principal business address
of Anson East is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands.
(4) Roman Grodon, Thomas Koenig, and Beate Ruhle-Burkhardt
have shared voting control and investment discretion over the securities reported herein that are held by Orca Capital AG (“Orca
Capital”). As a result, each of Roman Grodon, Thomas Koenig, and Beate Ruhle-Burkhardt may be deemed to have beneficial ownership
(as determined under Section 13(d) of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”)) of
the securities reported herein that are held by Orca Capital. The principal business address of Orca Capital is Sperl-Ring 2, 85276 Hettenshausen,
Germany.
(5) Heights Capital Management, Inc., the authorized
agent of CVI Investments, Inc. (“CVI”), has discretionary authority to vote and dispose of the shares held by CVI
and may be deemed to be the beneficial owner of these shares. Martin Kobinger, in his capacity as President of Heights Capital Management,
Inc., may also be deemed to have investment discretion and voting power over the shares held by CVI. Mr. Kobinger disclaims any such
beneficial ownership of the shares. CVI is affiliated with one or more FINRA members, none of whom are currently expected to participate
in the sale pursuant to this registration statement of the securities reported herein. The principal business address of CVI is c/o Heights
Capital Management, Inc., 101 California Street, Suite 3250, San Francisco, CA 94111.
(6) Mitchell P. Kopin (“Mr. Kopin”)
and Daniel B. Asher (“Mr. Asher”), each of whom are managers of Intracoastal Capital, LLC (“Intracoastal”),
have shared voting control and investment discretion over the securities reported herein that are held by Intracoastal. As a result,
each of Mr. Kopin and Mr. Asher may be deemed to have beneficial ownership (as determined under Section 13(d) of the Exchange Act) of
the securities reported herein that are held by Intracoastal. The principal business address of Intracoastal is 245 Palm Trail, Delray
Beach, FL 33483.
(7) Each of these Selling Stockholders is affiliated
with Wainwright, a registered broker dealer with a registered address of H.C. Wainwright & Co., LLC, 430 Park Ave, 3rd Floor, New
York, NY 10022, and has sole voting and dispositive power over the securities held. The number of shares beneficially owned prior to
this offering consist of shares of common stock issuable upon exercise of placement agent warrants, which were received as compensation.
The Selling Stockholder acquired the placement agent warrants in the ordinary course of business and, at the time the placement agent
warrants were acquired, the Selling Stockholder had no agreement or understanding, directly or indirectly, with any person to distribute
such securities.
PLAN OF DISTRIBUTION
Each Selling Stockholder
of the securities and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any or all of its securities
covered hereby on the principal trading market or any other stock exchange, market or trading facility on which the securities are traded
or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following
methods when selling securities:
| |
· |
ordinary brokerage transactions and transactions in which the broker dealer solicits purchasers; |
| |
· |
block trades in which the broker-dealer will attempt to sell the securities as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| |
· |
purchases by a broker-dealer as principal and resale by the broker dealer for its account; |
| |
· |
an exchange distribution in accordance with the rules of the applicable exchange; |
| |
· |
privately negotiated transactions; |
| |
· |
settlement of short sales; |
| |
· |
in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified
number of such securities at a stipulated price per security; |
| |
· |
through the writing or settlement of options or other hedging transactions, whether through an options
exchange or otherwise; |
| |
· |
a combination of any such methods of sale; or |
| |
· |
any other method permitted pursuant to applicable law. |
The Selling Stockholders
may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities
Act”), if available, rather than under this prospectus.
Broker-dealers engaged by
the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts
from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts
to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of
a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown
in compliance with FINRA Rule 2121.
In connection with the sale
of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial
institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling
Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities
to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with
broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer
or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution
may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders
and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the
meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents
and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the
Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding, directly
or indirectly, with any person to distribute the securities.
We are required to pay certain
fees and expenses incurred incident to the registration of the securities.
We have agreed to keep this
prospectus effective until all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any
other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under
applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have
been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available
and is complied with.
Under applicable rules and
regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in
market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to
the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Common
Stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and
have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
DESCRIPTION OF
SECURITIES TO BE REGISTERED
The following summary description of our capital
stock is based on the provisions of our amended and restated certificate of incorporation and amended and restated bylaws and the applicable
provisions of the Delaware General Corporation Law (“DGCL”). This information is qualified entirely by reference to
the applicable provisions of our amended and restated certificate of incorporation, amended and restated bylaws and the DGCL. For information
on how to obtain copies of our amended and restated certificate of incorporation and amended and restated bylaws, which are exhibits
to the registration statement of which this prospectus forms a part, see the section titled “Where You Can Find
More Information” in this prospectus.
General
Our authorized capital stock
consists of 100,000,000 shares of Common Stock, par value $0.0001 per share and 10,000,000 shares of preferred stock, par value $0.0001
per share.
Common Stock
Holders of our Common Stock
are entitled to one vote per share for the election of members of our Board of Directors and on all other matters that require stockholder
approval. Holders of our Common Stock may not cumulate votes for the election of directors. Subject to any preferential rights of any
outstanding preferred stock, in the event of our liquidation, dissolution or winding up, holders of our Common Stock are entitled to
share ratably in the assets remaining after payment of liabilities and the liquidation preferences of any outstanding preferred stock.
Holders of Common Stock have the right to receive dividends when, as and if, declared by the Board of Directors. Our Common Stock does
not carry any preemptive rights enabling a holder to subscribe for, or receive shares of, any class of our Common Stock or any other
securities convertible into shares of any class of our Common Stock. There are no redemption or sinking-fund provisions applicable to
our Common Stock.
Preferred Stock
The shares of preferred stock
have such rights and preferences as our Board of Directors shall determine, from time to time, the Board of Directors may divide the
preferred stock into any number of series and shall fix the designation and number of shares of each such series. Our Board of Directors
may determine and alter the rights, powers, preferences and privileges, and qualifications, restrictions and limitations thereof, including,
but not limited to, voting rights (if any), granted to and imposed upon any wholly unissued series of preferred stock. Our Board of Directors
(within the limits and restrictions of any resolutions adopted originally fixing the number of shares of any series) may increase or
decrease the number of shares of that series; provided, that no such decrease shall reduce the number of shares of such series to a number
less than the number of shares of such series then outstanding, plus the number of shares reserved for issuance upon the exercise of
outstanding options, rights or warrants or upon the conversion of any outstanding securities issued by us convertible into shares of
such series.
Our Common Stock is subject
to the express terms of our preferred stock and any series thereof. Our Board of Directors may issue preferred stock with voting, dividend,
liquidation and other rights that could adversely affect the relative rights of the holders of our Common Stock.
Anti-Takeover Effects of Provisions of our
Certificate of Incorporation and Bylaws
Certificate of Incorporation
and Bylaw Provisions. Certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws,
which provisions are summarized in the following paragraphs, may have an anti-takeover effect and may delay, defer or prevent a takeover
attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market
price for the shares held by stockholders.
Filling Vacancies. Any
vacancy on our Board of Directors, however occurring, including a vacancy resulting from an increase in the size of the Board of Directors,
may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum.
No Written Consent of
Stockholders. Our amended and restated certificate of incorporation provides that all stockholder actions are required to be taken
by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu
of a meeting.
Advance Notice Requirements. Our
amended and restated bylaws include advance notice procedures with regard to stockholder proposals relating to the nomination of candidates
for election as directors or new business to be brought before meetings of our stockholders. These procedures provide that notice of
stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken.
Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior
to the first anniversary date of the annual meeting for the preceding year. The notice must contain certain information specified in
the amended and restated bylaws.
Amendment to Bylaws and
Certificate of Incorporation. As required by the DGCL any amendment to our amended and restated certificate of incorporation
must first be approved by a majority of our Board of Directors and, if required by law or our amended and restated certificate of incorporation,
thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment, and a majority of the outstanding shares
of each class entitled to vote thereon as a class. Our amended and restated bylaws may be amended by the affirmative vote of a majority
vote of the directors then in office, subject to any limitations set forth in the amended and restated bylaws.
Blank Check Preferred
Stock. Our amended and restated certificate of incorporation provides for 10,000,000 authorized shares of preferred stock. The existence
of authorized but unissued shares of preferred stock may enable our Board of Directors to render more difficult or to discourage an attempt
to obtain control of us by means of a merger, tender offer, proxy contest, or otherwise. In this regard, the amended and restated certificate
of incorporation grants the Board of Directors broad power to establish the rights and preferences of authorized and unissued shares
of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution
to holders of shares of Common Stock. The issuance may also adversely affect the relative rights and powers, including voting rights,
of these holders and may have the effect of delaying, deterring, or preventing a change of control of the Company.
Exclusive Forum Provision
in Certificate of Incorporation. Our amended and restated certificate of incorporation provides that the Court of Chancery of the
State of Delaware is the exclusive forum for the following types of actions or proceedings: any derivative action or proceeding brought
on behalf of the Company, any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee
of the Company to the Company or the Company’s stockholders, any action asserting a claim against the Company arising pursuant
to any provision of the DGCL or our amended and restated certificate of incorporation or our amended and restated bylaws, or any action
asserting a claim against us governed by the internal affairs doctrine. Despite the fact that our amended and restated certificate of
incorporation provides for this exclusive forum provision to be applicable to the fullest extent permitted by applicable law, Section
27 of the Exchange Act, creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the
Exchange Act or the rules and regulations thereunder and Section 22 of the Securities Act, creates concurrent jurisdiction for federal
and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
As a result, this provision of our amended and restated certificate of incorporation would not apply to claims brought to enforce a duty
or liability created by the Securities Act, Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction.
LEGAL MATTERS
Certain legal matters relating to the issuance
of the securities offered hereby will be passed upon for us by Hogan Lovells US LLP.
EXPERTS
The consolidated financial
statements of Phio Pharmaceuticals Corp. (the Company) as of December 31, 2023 and 2022 and for each of the two years in the period ended
December 31, 2023 included in this Prospectus have been so included in reliance on the report of BDO USA, P.C., an independent registered
public accounting firm, given on the authority of said firm as experts in auditing and accounting. The report on the consolidated financial
statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
Where You Can
Find More Information
We are required to file annual,
quarterly and current reports, proxy statements and other information with the SEC. Our filings with the SEC are available to the public
at the SEC’s Internet web site at http://www.sec.gov. Copies of certain information filed by us with the SEC are also available
on our website at www.phiopharma.com. Our website is not a part of this prospectus and is not incorporated by reference in this
prospectus, and you should not consider the contents of our website in making an investment decision with respect to our Common Stock.
We have filed a registration
statement, of which this prospectus is a part, covering the securities offered hereby. As allowed by SEC rules, this prospectus does
not include all of the information contained in the registration statement and the included exhibits, financial statements and schedules.
You are referred to the registration statement, the included exhibits, financial statements and schedules for further information. You
should review the information and exhibits in the registration statement for further information about us and our subsidiaries and the
securities we are offering. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement
or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should
review the complete document to evaluate these statements.
INDEX TO FINANCIAL
STATEMENTS
Annual Financial Statements (audited):
Quarterly Financial Statements (unaudited):
(b) Exhibits.
See the Exhibit Index attached hereto which is incorporated by reference.
Report of Independent Registered Public Accounting
Firm
Shareholders and Board of Directors
Phio Pharmaceuticals, Corp.
Marlborough, Massachusetts
Opinion on the Consolidated
Financial Statements
We have audited the accompanying
consolidated balance sheets of Phio Pharmaceuticals, Corp. (the “Company”) as of December 31, 2023 and 2022, the related
consolidated statements of operations, preferred stock and stockholders’ equity, and cash flows for each of the years then ended,
and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated
financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and
the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally
accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated
financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated
financial statements, the Company has suffered recurring losses from operations and negative cash flows from operations that raise substantial
doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note
1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial
statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting
Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with
the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated
financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were
we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an
understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the
Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to
assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures
that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the
consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made
by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide
a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below
is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated
to the audit committee and that: (i) relates to accounts or disclosures that are material to the consolidated financial statements and
(ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter
in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit
matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Accounting for Certain Warrants Issued in 2023
As
described in Note 9 to the consolidated financial statements, the Company completed each
of two registered direct offerings of common stock and concurrent private placement offerings
of warrants to purchase common stock in April and June 2023 (the “April 2023 Financing”
and “June 2023 Financing”, respectively). In December 2023, the Company issued
additional warrants to purchase common stock to certain holders of existing warrants in connection
with an Inducement Letter Agreement (the “December 2023 Financing”). The Company
assessed the warrants issued in the April 2023 Financing, June 2023 Financing and December
2023 Financing to determine whether the warrants should be accounted for as either liabilities
or equity instruments depending on the specific terms of the agreements. The Company determined
that the warrants issued in the April 2023 Financing, June 2023 Financing and December 2023
Financing were classified within stockholders’ equity.
We identified the assessment of the accounting
for certain warrants to purchase common stock issued in connection with the April 2023 Financing, June 2023 Financing and December 2023
Financing as a critical audit matter. Determining whether the certain warrants issued should be accounted for as either liabilities or
equity instruments requires significant judgment due to the application of complex technical accounting guidance. Auditing this element
required especially challenging and complex auditor judgement due to the nature and extent of the audit effort required to address the
matter, including the need for specialized knowledge and skill in assessing elements of the agreements.
The primary procedures we performed to address
this critical audit matter included:
| · | Reading
and analyzing agreements related to the certain warrants issued to identify relevant terms
and conditions that affect whether the certain warrants issued should be accounted for as
either liabilities or equity instruments. |
| | | |
| · | Evaluating
whether the certain warrants issued should be accounted for as either liabilities or equity
instruments. |
| | | |
| · | Utilizing
personnel with specialized knowledge and skill in the relevant technical accounting guidance
to evaluate the appropriateness of the Company’s application of the relevant technical
accounting guidance in determining whether the certain warrants issued should be accounted
for as either liabilities or equity instruments. |
/s/ BDO USA, P.C.
We have served as the Company's auditor since
2011.
Boston, Massachusetts
April 1, 2024, except for the impact of the 2024 reverse stock split
as described in Note 1, as to which the date is January 21, 2025
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share data)
| | |
| | |
| |
| | |
December 31,
2023 | | |
December 31,
2022 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 8,490 | | |
$ | 11,781 | |
| Restricted cash | |
| – | | |
| 50 | |
| Prepaid expenses and other current assets | |
| 832 | | |
| 615 | |
| Total current assets | |
| 9,322 | | |
| 12,446 | |
| Right of use asset | |
| 33 | | |
| 161 | |
| Property and equipment, net | |
| 6 | | |
| 183 | |
| Other assets | |
| 3 | | |
| 24 | |
| Total assets | |
$ | 9,364 | | |
$ | 12,814 | |
| LIABILITIES, PREFERRED STOCK AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 657 | | |
$ | 779 | |
| Accrued expenses | |
| 942 | | |
| 1,025 | |
| Lease liability | |
| 35 | | |
| 135 | |
| Total current liabilities | |
| 1,634 | | |
| 1,939 | |
| Lease liability, net of current portion | |
| – | | |
| 35 | |
| Total liabilities | |
| 1,634 | | |
| 1,974 | |
| Commitments and contingencies (Footnote 7) | |
| – | | |
| – | |
| Series D Preferred Stock, $0.0001 par value; 0 and 1 shares
authorized, issued and outstanding at December 31, 2023 and December 31, 2022, respectively | |
| – | | |
| 2 | |
| Stockholders’ equity: | |
| | | |
| | |
| Common stock, $0.0001 par value, 100,000,000 shares authorized;
416,368 and 126,558 shares issued and outstanding at December 31, 2023 and December 31, 2022, respectively | |
| – | | |
| – | |
| Additional paid-in capital | |
| 146,936 | | |
| 139,218 | |
| Accumulated deficit | |
| (139,206 | ) | |
| (128,380 | ) |
| Total stockholders’ equity | |
| 7,730 | | |
| 10,838 | |
| Total liabilities, preferred stock and
stockholders’ equity | |
$ | 9,364 | | |
$ | 12,814 | |
See accompanying notes to consolidated financial
statements.
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per
share data)
| | |
| | |
| |
| | |
Year Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Operating expenses: | |
| | | |
| | |
| Research and development | |
$ | 6,332 | | |
$ | 7,012 | |
| General and administrative | |
| 4,366 | | |
| 4,450 | |
| Loss on impairment of property and equipment | |
| 126 | | |
| – | |
| Total operating expenses | |
| 10,824 | | |
| 11,462 | |
| Operating loss | |
| (10,824 | ) | |
| (11,462 | ) |
| Total other expense, net | |
| (2 | ) | |
| (18 | ) |
| Net loss | |
$ | (10,826 | ) | |
$ | (11,480 | ) |
| Net loss per common share: | |
| | | |
| | |
| Basic and diluted | |
$ | (46.76 | ) | |
$ | (90.91 | ) |
| Weighted average number of common shares outstanding | |
| | | |
| | |
| Basic and diluted | |
| 231,508 | | |
| 126,285 | |
See accompanying notes to consolidated financial
statements.
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF PREFERRED STOCK
AND STOCKHOLDERS’ EQUITY
(Amounts in thousands, except share data)
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Series D Preferred Stock | | |
Common Stock | | |
Additional
Paid-in | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Total | |
| Balance at December 31, 2021 | |
| – | | |
$ | – | | |
| 125,324 | | |
$ | – | | |
$ | 138,832 | | |
$ | (116,900 | ) | |
$ | 21,932 | |
| Issuance of common stock upon vesting of restricted stock units | |
| – | | |
| – | | |
| 1,560 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Shares withheld for payroll taxes | |
| – | | |
| – | | |
| (326 | ) | |
| – | | |
| (28 | ) | |
| – | | |
| (28 | ) |
| Issuance of preferred stock | |
| 1 | | |
| 2 | | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 414 | | |
| – | | |
| 414 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (11,480 | ) | |
| (11,480 | ) |
| Balance at December 31, 2022 | |
| 1 | | |
$ | 2 | | |
| 126,558 | | |
$ | – | | |
$ | 139,218 | | |
$ | (128,380 | ) | |
$ | 10,838 | |
| Cash-in-lieu of fractional shares for reverse stock split | |
| – | | |
| – | | |
| (190 | ) | |
| – | | |
| (11 | ) | |
| – | | |
| (11 | ) |
| Redemption of preferred stock | |
| (1 | ) | |
| (2 | ) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Issuance of common stock and warrants, net of offering costs | |
| – | | |
| – | | |
| 218,168 | | |
| – | | |
| 7,452 | | |
| – | | |
| 7,452 | |
| Issuance of common stock upon exercise of warrants | |
| – | | |
| – | | |
| 69,881 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Issuance of common stock upon vesting of restricted stock units | |
| – | | |
| – | | |
| 2,601 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Shares withheld for payroll taxes | |
| – | | |
| – | | |
| (650 | ) | |
| – | | |
| (26 | ) | |
| – | | |
| (26 | ) |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 303 | | |
| – | | |
| 303 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (10,826 | ) | |
| (10,826 | ) |
| Balance at December 31, 2023 | |
| – | | |
$ | – | | |
| 416,368 | | |
$ | – | | |
$ | 146,936 | | |
$ | (139,206 | ) | |
$ | 7,730 | |
See accompanying notes to consolidated financial
statements.
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
| | |
| | |
| |
| | |
Year
Ended December
31, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (10,826 | ) | |
$ | (11,480 | ) |
| Adjustments to reconcile net loss to net cash used in operating
activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 56 | | |
| 71 | |
| Amortization of right of use asset | |
| 128 | | |
| 122 | |
| Impairment of property and equipment | |
| 126 | | |
| – | |
| Stock-based compensation | |
| 303 | | |
| 414 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Prepaid expenses and other assets | |
| (196 | ) | |
| 8 | |
| Accounts payable | |
| (122 | ) | |
| 496 | |
| Accrued expenses | |
| (83 | ) | |
| (1,635 | ) |
| Lease liability | |
| (135 | ) | |
| (125 | ) |
| Net cash used in operating activities | |
| (10,749 | ) | |
| (12,129 | ) |
| Cash flows from investing activities: | |
| | | |
| | |
| Cash paid for purchase of property and
equipment | |
| (5 | ) | |
| (121 | ) |
| Net cash used in investing activities | |
| (5 | ) | |
| (121 | ) |
| Cash flows from financing activities: | |
| | | |
| | |
| Net proceeds from the issuance of common stock and warrants | |
| 7,452 | | |
| – | |
| Net proceeds from the issuance of preferred stock | |
| – | | |
| 2 | |
| Cash-in-lieu of fractional shares for reverse stock split | |
| (11 | ) | |
| – | |
| Redemption of Series D Preferred Stock | |
| (2 | ) | |
| – | |
| Payment of taxes on net share settlements
of restricted stock units | |
| (26 | ) | |
| (28 | ) |
| Net cash provided by (used in) financing activities | |
| 7,413 | | |
| (26 | ) |
| Net decrease in cash and restricted cash | |
| (3,341 | ) | |
| (12,276 | ) |
| Cash and restricted cash at the beginning of period | |
| 11,831 | | |
| 24,107 | |
| Cash and restricted cash at the end of period | |
$ | 8,490 | | |
$ | 11,831 | |
The following table provides a reconciliation of cash and restricted
cash reported within the consolidated balance sheets to the totals above:
| | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash | |
$ | 8,490 | | |
$ | 11,781 | |
| Restricted cash | |
| – | | |
| 50 | |
| Total cash and restricted cash | |
$ | 8,490 | | |
$ | 11,831 | |
See accompanying notes to consolidated financial
statements.
PHIO PHARMACEUTICALS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
1. Organization and Significant Accounting Policies
Nature of Operations
Phio Pharmaceuticals Corp. (“Phio”
or the “Company”) is a clinical stage biotechnology company whose proprietary INTASYL™ self-delivering RNAi
technology platform is designed to make immune cells more effective in killing tumor cells. The Company is developing therapeutics that
are designed to leverage INTASYL to precisely target specific proteins that reduce the body’s ability to fight cancer, without
the need for specialized formulations or drug delivery systems.
Phio was incorporated in the state of Delaware
in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals Corp., to reflect
its transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology therapeutics.
Basis of Presentation
The accompanying consolidated financial statements
have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
Principles of Consolidation
The consolidated financial statements include
the accounts of the Company and its wholly-owned subsidiary, MirImmune, LLC. All material intercompany accounts have been eliminated
in consolidation.
Segments
The Company operates
as one operating segment and all assets are located in the United States.
Reverse Stock Split
Effective
January 26, 2023, the Company completed a 1-for-12 reverse stock split of the Company’s
outstanding common stock (the “Reverse Stock Split”). All share and per
share amounts in the consolidated financial statements have been retroactively adjusted for
all periods presented to give effect to the Reverse Stock Split, as applicable, including
reclassifying an amount equal to the reduction in par value to additional paid-in capital.
Additionally, the Company made adjustments to the outstanding stock option and unvested restricted
stock unit (“RSU”) balances, and related per share amounts, at each of
December 31, 2022 and December 31, 2023 to reflect final revisions to those outstanding equity
awards as a result of the Reverse Stock Split. The Reverse Stock Split did not reduce the
number of authorized shares of the Company’s common stock or preferred stock.
Subsequently, effective July 5, 2024, the Company
completed a 1-for-9 reverse stock split of the Company’s outstanding common stock (the “2024 Reverse Stock Split”).
All share and per share amounts in the consolidated financial statements have been retroactively adjusted for all periods presented to
give effect to the 2024 Reverse Stock Split, as applicable, including reclassifying an amount equal to the reduction in par value to
additional paid-in capital. Additionally, the Company made adjustments to the outstanding stock option and unvested RSU balances, and
related per share amounts, at each of December 31, 2022 and December 31, 2023 to reflect final revisions to those outstanding equity
awards as a result of the 2024 Reverse Stock Split. The 2024 Reverse Stock Split did not reduce the number of authorized shares of the
Company’s common stock or preferred stock.
Uses of Estimates in Preparation of Financial Statements
The preparation of financial statements in accordance
with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure
of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during
the reporting period. The areas subject to significant estimates and judgement include, among others, those related to the fair value
of equity awards, accruals for research and development expenses, useful lives of property and equipment, and the valuation allowance
on our deferred tax assets. On an ongoing basis the Company evaluates its estimates and bases its estimates on historical experience
and other relevant assumptions that the Company believes are reasonable under the circumstances. Actual results could differ materially
from these estimates.
Liquidity
The Company has reported recurring losses from
operations since its inception and expects to continue to have negative cash flows from operations for the foreseeable future. Historically,
the Company’s primary source of funding has been from sales of its securities. The Company’s ability to continue to fund
its operations is dependent on obtaining funding from third parties, such as proceeds from the issuance of debt, sale of equity, or strategic
opportunities, in order to maintain its operations. This is dependent on a number of factors, including the market demand or liquidity
of the Company’s common stock. There is no guarantee that debt, additional equity or other funding will be available to us on acceptable
terms, or at all. If the Company fails to obtain additional funding when needed, the Company would be forced to scale back or terminate
its operations or seek to merge with or to be acquired by another company.
The Company has limited cash resources, has reported
recurring losses from operations since inception, negative operating cash flows and has not yet received product revenues. These factors
raise substantial doubt regarding the Company’s ability to continue as a going concern, and the Company’s current cash resources
may not provide sufficient capital to fund operations for at least the next 12 months from the date of the release of these consolidated
financial statements. The continuation of the Company as a going concern depends upon the Company’s ability to raise additional
capital through an equity offering, debt offering and/or strategic opportunity to fund its operations. There can be no assurance that
the Company will be successful in accomplishing these plans in order to continue as a going concern. These consolidated financial statements
do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that
might be necessary should the Company be unable to continue as a going concern.
Restricted Cash
Restricted cash consists of certificates of deposit
held by financial institutions as collateral for the Company’s corporate credit cards.
Concentrations of Credit Risk
Financial instruments that potentially subject
the Company to concentrations of credit risk consist principally of cash. The Company maintains cash balances in several accounts with
a reputable financial institution that management believes is creditworthy, and which at times are in excess of federally insured limits.
These accounts are insured by the Federal Deposit Insurance Corporation for up to $250,000 per institution.
The Company
relies, and expects to continue to rely, on a small number of vendors to perform research activities and clinical trial activities that
continue to progress its product candidates for its development programs. These programs could be adversely affected by a significant
interruption in the related processes of these vendors.
Property and Equipment
Property and equipment are stated at cost and
depreciated using the straight-line method based on the estimated useful lives of the related assets. The Company provides for depreciation
over the assets’ estimated useful lives as follows:
| Schedule of estimated useful lives |
|
| Computer equipment |
3 years |
| Machinery & equipment |
5 years |
| Furniture & fixtures |
5 years |
| Leasehold improvements |
Lesser of lease term or 5 years |
Impairment of Long-Lived Assets
The Company reviews long-lived assets for impairment
annually or whenever an event or change in circumstance occurs in which the related carrying amounts may not be recoverable. An impairment
loss would be recognized based on the difference between the carrying value of the asset and its estimated fair value, which would be
determined based on either discounted future cash flows or other appropriate fair value methods.
Leases
At the inception of a contract, the Company determines
whether the contract is or contains a lease based on all relevant facts and circumstances. For contracts that contain a lease, the Company
identifies the lease and non-lease components, determines the consideration in the contract and recognizes the classification of the
lease as operating or financing. For leases with a term greater than one year, the Company recognizes a liability to make lease payments
and an asset representing the right to use the underlying asset during the lease term at the commencement date of the lease.
Lease liabilities and the corresponding right
of use assets are recorded based on the present value of lease payments to be made over the lease term. The discount rate used to calculate
the present value is the rate implicit in the lease, or if not readily determinable, the Company’s incremental borrowing rate.
The Company’s incremental borrowing rate is the rate of interest that the Company would have to pay to borrow on a collateralized
basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right of
use asset may be required for items such as initial direct costs or incentives received. Lease payments on operating leases, including
scheduled increases, are recognized on a straight-line basis over the expected term of the lease. Lease payments on financing leases
are recognized using the effective interest method.
Derivative Financial Instruments
Financial instruments that meet the definition
of a derivative are classified as an asset or liability and measured at fair value on the issuance date and are revalued on each subsequent
balance sheet date. The changes in fair value are recognized as current period income or loss. Financial instruments that do not meet
the definition of a derivative are classified as equity and measured at fair value and recorded as additional paid-in capital in stockholders’
equity at the date of issuance. No further adjustments to their valuation are made.
Research and Development Expenses
Research and development expenses relate to compensation
and benefits for research and development personnel, facility-related expenses, supplies, external services, costs to acquire technology
licenses, research activities under our research collaborations, expenses associated with preclinical and clinical development activities
and other operating costs. Research and development expenses are charged to expense as incurred. Payments made by the Company in advance
for research and development services not yet provided and/or for materials not yet received are recorded as prepaid expenses and expensed
when the service has been performed or when the goods have been received.
Accrued liabilities are recorded related to those
expenses for which vendors have not yet billed the Company with respect to services provided and/or materials that it has received. Accrued
liabilities for the services provided by contract research organizations are recorded during the period incurred based on such estimates
and assumptions as expected cost, passage of time, the achievement of milestones and other information available to us and are assessed
on a quarterly basis. Actual results may differ from these estimates and could have a material impact on the Company’s reported
results. The Company’s historical accrual estimates have not been materially different from its actual costs.
Collaborative Arrangements
The Company follows the provisions of the Financial
Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 808, “Collaborative
Arrangements,” (“Topic 808”) when collaboration agreements involve joint operating activities in which both
parties are active participants and that are also both exposed to significant risks and rewards. The Company also considers the guidance
in the FASB ASC Topic 606, “Revenue from Contracts with Customers,” (“Topic 606”) in determining
the appropriate treatment for activities between the Company and its collaborative partners that are more reflective of a vendor-customer
relationship and therefore, within the scope of Topic 606. Under Topic 808, the Company determines an appropriate recognition method,
either by analogy to appropriate accounting literature or by applying a reasonable accounting policy election. Generally, the classification
of transactions under the collaborative arrangements is determined based on the nature and contractual terms of the arrangement along
with the nature of the operations of the participants. The Company recognizes its share of costs arising from research and development
activities performed by collaborators in the period its collaborators incur such expense. Reimbursements that are the result of a collaborative
relationship instead of a customer relationship, such as co-development activities, are evaluated on a quarterly basis and recorded as
an offset to research and development expense incurred. Payments in excess of our collaboration expense will be recorded as revenue.
Patents and Patent Application Costs
Although the Company believes that its patents
and underlying technology have continuing value, the amount of future benefits to be derived from the patents is uncertain. Patent costs
are, therefore, expensed as general and administrative costs as incurred.
Stock-based Compensation
The Company follows the provisions of the FASB
ASC Topic 718, “Compensation — Stock Compensation” (“ASC 718”), which requires the measurement
and recognition of compensation expense for all stock-based payment awards. The fair value of RSUs is based upon the Company’s
closing stock price at the grant date. The Company uses the Black-Scholes option-pricing model to estimate the fair value of stock options
at the grant date. The Black-Scholes valuation model requires the input of valuation assumptions to calculate the value of stock options,
including expected volatility, expected term, risk-free interest rate and expected dividends. Stock-based compensation expense is recognized
on a straight-line basis over the requisite service period, which generally represents the vesting period, and commences at the date
of grant based on the fair value of the award.
Stock-based compensation expense recognized in
the consolidated financial statements is based on awards that are ultimately expected to vest. Accordingly, we are also required to estimate
forfeitures at the time of grant and to revise those estimates in subsequent periods if actual forfeitures differ from estimates. We
use historical data to estimate pre-vesting award forfeitures and record stock-based compensation expense only for those awards that
are expected to vest. Our forfeiture rate estimates are based on an analysis of our actual forfeiture experience, employee turnover behavior,
and other factors. The impact of any adjustments to our forfeiture rates or to the extent that actual forfeitures differ from our estimates,
is recorded as a cumulative adjustment in the period the estimates are revised.
Income Taxes
The Company recognizes assets or liabilities for
the deferred tax consequences of temporary differences between the tax basis of assets or liabilities and their reported amounts in the
consolidated financial statements in accordance with the FASB ASC Topic 740, “Accounting for Income Taxes” (“ASC
740”). These temporary differences will result in taxable or deductible amounts in future years when the reported amounts
of the assets or liabilities are recovered or settled. Those temporary differences referred to as deferred tax assets and liabilities
are determined at the end of each period using the tax rate expected to be in effect when taxes are actually paid or recovered. Valuation
allowances are established if, based on the weight of available evidence, it is more likely than not that all or a portion of a deferred
tax asset will not be realized. The provision for income taxes, if any, represents the tax payable for the period and the change in deferred
income tax assets and liabilities during the period.
The recognition and measurement of benefits related
to the Company’s tax positions requires significant judgment, as uncertainties often exist with respect to new laws, new interpretations
of existing laws, and rulings by taxing authorities. The Company follows a more-likely-than not threshold for financial statement recognition
and measurement of a tax position taken, or expected to be taken in a tax return. The guidance relates to, amongst other things, classification,
accounting for interest and penalties associated with tax positions, and disclosure requirements. Any interest and penalties accrued
related to uncertain tax positions are recorded as tax expense. Differences between actual results and the Company’s assumptions
or changes in the Company’s assumptions in future periods are recorded in the period they become known.
Comprehensive Loss
The Company’s comprehensive loss is equal
to its net loss for all periods presented.
Net Loss per Share
Basic net loss per share is computed by dividing
net loss by the weighted average number of common shares outstanding. Diluted net loss per share is computed by dividing the Company’s
net loss by the weighted average number of common shares outstanding and the impact of all dilutive potential common shares outstanding,
except where such dilutive potential common shares would be anti-dilutive. Dilutive potential common shares primarily consist of warrants,
RSUs and stock options.
Recent Accounting Pronouncements
In November 2023, the FASB issued Accounting Standards
Update (“ASU”) 2023-07, “Segment Reporting (Topic 280) – Improvements to Reporting Segment Disclosures”
(“ASU 2023-07”), which requires disclosure of incremental segment information on an annual and interim basis.
In addition, ASU 2023-07 clarifies circumstances in which an entity can disclose multiple segment measures of profit or loss, provides
new segment disclosure requirements for entities with a single reportable segment, and contains other disclosure requirements. The amendments
in ASU 2023-07 are effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after
December 15, 2024. Early adoption is permitted. The enhanced disclosures are required to be applied
retrospectively to all prior periods presented in the financial statements. The Company is currently evaluating the impact of
ASU 2023-07 on its consolidated financial statements and disclosures, but does not expect that it will have a material impact on its
consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09,
“Income Taxes (Topic 740) – Improvements to Income Tax Disclosures” (“ASU 2023-09”), which
requires disclosure of specific categories in the rate reconciliation table along with additional information for reconciling items that
meet a quantitative threshold, disclosure of disaggregated income taxes paid and modifies other income tax-related disclosures. The amendments
in ASU 2023-09 are effective for annual periods beginning after December 15, 2024 and allows for adoption on a prospective basis, with
a retrospective option. Early adoption is permitted. The Company is currently evaluating the impact of ASU 2023-09, but does not expect
that it will have a material impact on its consolidated financial statements.
2. Collaboration Agreement
AgonOx, Inc. (“AgonOx”)
In February 2021, the Company entered into a clinical
co-development collaboration agreement (the “Clinical Co-Development Agreement”) with AgonOx, a private company developing
a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer. Under the Clinical Co-Development
Agreement, Phio and AgonOx are working to develop a T cell-based therapy using the Company’s lead product candidate, PH-762, and
AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP TIL”) technology. Per the terms of
the Clinical Co-Development Agreement, the Company agreed to reimburse AgonOx up to $4,000,000 in expenses incurred to conduct a Phase
1 clinical trial of PH-762 treated DP TIL in patients with advanced melanoma and other advanced solid tumors.
The Company will recognize its share of costs
arising from research and development activities performed by AgonOx in the Company’s consolidated financial statements in the
period AgonOx incurs such expense. Phio will be entitled to certain future development milestones and low single-digit sales-based royalty
payments from AgonOx’s licensing of its DP TIL technology.
The Company recognized approximately $1,115,000
and $130,000 of expense in connection with these efforts during the years ended December 31, 2023 and 2022, respectively.
There is approximately $2,757,000 of remaining
costs not yet incurred under the Clinical Co-Development Agreement as of December 31, 2023.
3. Fair
Value of Financial Instruments
The Company
follows the provisions of the FASB ASC Topic 820, “Fair Value Measurement,” for the Company’s financial assets
and liabilities that are re-measured and reported at fair value each reporting period and are re-measured and reported at fair value
at least annually using a fair value hierarchy that is broken down into three levels. Level inputs are defined as follows:
Level 1
– quoted prices in active markets for identical assets or liabilities.
Level 2 – other
significant observable inputs for the assets or liabilities through corroboration with market data at the measurement date.
Level 3 – significant
unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities
at the measurement date.
At
December 31, 2022, the Company categorized its restricted cash of $50,000 as Level 2 hierarchy. Restricted cash consisted of certificates
of deposit held by financial institutions as collateral for the Company’s corporate credit cards. The
assets classified as Level 2 have initially been valued at the applicable transaction price and subsequently valued, at the end of each
reporting period, using other market observable data. Observable market data points include quoted prices, interest rates, reportable
trades and other industry and economic events.
The carrying amounts of cash,
accounts payable and accrued expenses of the Company approximate their fair values due to their short-term nature.
4. Property and Equipment
The following table summarizes the Company’s
major classes of property and equipment, in thousands:
| Schedule of property and equipment | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Computer equipment | |
$ | 62 | | |
$ | 116 | |
| Machinery & equipment | |
| 964 | | |
| 1,077 | |
| Furniture & fixtures | |
| 70 | | |
| 119 | |
| Leasehold improvements | |
| 46 | | |
| 46 | |
| Total gross fixed assets | |
| 1,142 | | |
| 1,358 | |
| Less: accumulated depreciation and amortization | |
| (1,136 | ) | |
| (1,175 | ) |
| Property and equipment, net | |
$ | 6 | | |
$ | 183 | |
Depreciation and amortization expense for the
years ended December 31, 2023 and 2022 was $56,000 and $71,000, respectively.
In
November 2023, the Company decided not to renew the lease for its corporate headquarters
and primary research facility in Marlborough, Massachusetts. Beginning in April of 2024,
we expect to continue operations as a remote business with a small laboratory facility. Based
on this evaluation, the Company determined that long-lived assets with a carrying amount
of $126,000 were no longer recoverable and an impairment charge of $126,000 was recorded
to write those assets down to their fair value. The Company did not record any impairment
charges at December 31, 2022.
5. Accrued Expenses
Accrued expenses consist of the following, in
thousands:
| Schedule of accrued expenses | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Compensation and benefits | |
$ | 222 | | |
$ | 408 | |
| Professional fees | |
| 126 | | |
| 97 | |
| Research and development costs | |
| 517 | | |
| 501 | |
| Other | |
| 77 | | |
| 19 | |
| Total accrued expenses | |
$ | 942 | | |
$ | 1,025 | |
6. Leases
In January 2019, the Company amended the lease
for its corporate headquarters and primary research facility in Marlborough, Massachusetts. The lease is for a total of 7,581 square
feet of office and laboratory space and will expire on March 31, 2024. The lease contains an option to terminate after two or three years
by providing advance written notice of termination pursuant to the terms of the agreement. The exercise of this option was not determined
to be reasonably certain and thus was not included in the lease liability on the Company’s balance sheet. The Company did not exercise
its option to terminate in either the second or third year of the lease, and the option to terminate has expired. Additionally, the lease
agreement did not contain information to determine the borrowing rate implicit in the lease. As such, the Company calculated its incremental
borrowing rate based on what the Company would have to pay to borrow on a collateralized basis over the lease term for an amount equal
to the remaining lease payments, taking into consideration such assumptions as, but not limited to, the U.S. treasury yield rate and
borrowing rates from a creditworthy financial institution using the above lease factors.
The lease for the Company’s corporate headquarters
represents all of its significant lease obligations. The amounts reported in the consolidated balance sheets for the operating lease
in which the Company is the lessee and other supplemental balance sheet information is set forth as follows, in thousands, except the
lease term (number of years) and discount rate:
| Schedule of lease amounts recorded in balance sheet | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Assets | |
| | | |
| | |
| Right of use asset | |
$ | 33 | | |
$ | 161 | |
| Liabilities | |
| | | |
| | |
| Lease liability, current | |
| 35 | | |
| 135 | |
| Lease liability, non-current | |
| – | | |
| 35 | |
| Total lease liability | |
$ | 35 | | |
$ | 170 | |
| Lease Term and Discount Rate | |
| | | |
| | |
| Weighted average remaining lease term | |
| 0.25 | | |
| 1.25 | |
| Weighted average discount rate | |
| 4.70% | | |
| 4.70% | |
Operating lease costs included in operating expense
were $132,000 for the years ended December 31, 2023 and 2022, respectively.
Cash paid for the amounts included in the measurement
of the operating lease liability on the Company’s consolidated balance sheets and included within changes in the lease liability
in the operating activities of the Company’s consolidated statements of cash flows was $139,000 and $135,000 for the years ended
December 31, 2023 and 2022, respectively.
Future lease payments for our non-cancellable
operating lease and a reconciliation to the carrying amount of the operating lease liability presented in the consolidated balance sheet
as of December 31, 2023 is as follows, in thousands:
| Schedule of future minimum lease payments | |
| |
| 2024 | |
$ | 35 | |
| Total lease payments | |
| 35 | |
| Less: Imputed interest | |
| – | |
| Total operating lease liability | |
$ | 35 | |
7. Commitments and Contingencies
Commitments
In February 2021, the Company
entered into the Clinical Co-Development Agreement with AgonOx to develop a T cell-based therapy using the Company’s lead product
candidate, PH-762, and AgonOx’s DP TIL technology. Per the terms of the Clinical Co-Development Agreement, the Company agreed to
reimburse AgonOx up to $4,000,000 in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients with
advanced melanoma and other advanced solid tumors. Refer to Note 2 for further details on the Clinical Co-Development Agreement with
AgonOx.
Refer to Note 6 for more information about the
Company’s obligations under its non-cancellable lease for its corporate headquarters.
In September 2011, the Company entered into an
agreement with Advanced RNA Technologies, LLC (“Advirna”), pursuant to which Advirna assigned to the Company its existing
patent and technology rights related to the INTASYL technology in exchange for an annual maintenance fee of $100,000, a one-time milestone
payment upon the future issuance of the first patent with valid claims covering the assigned patent and technology rights and the issuance
of shares of common stock of the Company equal to 5% of the Company’s fully-diluted shares outstanding at the time of issuance.
The one-time milestone payment and the issuance of shares of common stock of the Company were completed in 2014 and 2012, respectively.
Additionally, the Company is required to pay low single-digit royalties to Advirna on any licensing revenue received by the Company with
respect to future licensing of the assigned Advirna patent and technology rights. To date, any royalties owed to Advirna under the Advirna
agreement have been minimal.
The Company’s rights under the Advirna agreement
will expire upon the later of: (i) the expiration of the last-to-expire of the “patent rights” (as defined therein) included
in the Advirna agreement; or (ii) the abandonment of the last-to-be abandoned of such patents, unless earlier terminated in accordance
with the provisions of the Advirna agreement. Further, the Company also granted back to Advirna a license under the assigned patent and
technology rights for fields of use outside human therapeutics.
As part of its business, the Company may enter
into licensing agreements with third parties that require milestone and royalty payments based on the progress of the asset through development
stages. Milestone payments may be required, for example, upon progress through clinical trials, upon approval of the product by a regulatory
agency and/or upon a percentage of sales of the product pursuant to such agreements. The expenditures required under these arrangements
may be material individually in relation to any product candidates covered by the intellectual property licensed under any such arrangement,
and material in the aggregate in the unlikely event that milestones for multiple products covered by these arrangements were reached
in the same period. Due to the contingent nature of these payments, they are not included in the table of contractual obligations shown
below.
During the years ended December 31, 2023 and 2022,
the Company did not trigger any milestone payments.
The Company’s contractual license obligations
that will require future cash payments as of December 31, 2023, which result from payments expected in connection with annual license
fees, are as follows, in thousands:
| Schedule of future cash payments for contractual license obligations | |
| |
| Year Ending December 31, | |
| | |
| 2024 | |
$ | 100 | |
| 2025 | |
| 100 | |
| 2026 | |
| 100 | |
| 2027 | |
| 100 | |
| 2028 | |
| 100 | |
| Thereafter | |
| 100 | |
| Total | |
$ | 600 | |
The Company applies the disclosure provisions
of the FASB ASC Topic 460, “Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees
of Indebtedness of Others” (“ASC 460”), to its agreements that contain guarantee or indemnification
clauses. The Company provides: (i) indemnifications of varying scope and size to certain investors and other parties for certain
losses suffered or incurred by the indemnified party in connection with various types of third-party claims; and (ii) indemnifications
of varying scope and size to officers and directors against third-party claims arising from the services they provide to us. These indemnifications
give rise only to the disclosure provisions of ASC 460. To date, the Company has not incurred costs as a result of these obligations
and does not expect to incur material costs in the future. Accordingly, the Company has not accrued any liabilities in its consolidated
financial statements related to these indemnifications.
Litigation
From time to time, the Company may become a party
to various legal proceedings and complaints arising in the ordinary course of business. To the Company’s knowledge, it is not currently
a party to any actual or threatened material legal proceedings. Accordingly, there were no contingent liabilities recorded as of the
year ended December 31, 2023.
8. Preferred Stock
The Company has authorized up to 10,000,000 shares
of preferred stock, $0.0001 par value per share, for issuance. The Company’s Board of Directors (the “Board”)
is authorized under the Company’s Amended and Restated Certificate of Incorporation (as may be amended and/or restated from time
to time, the “Amended Certificate”), to designate the authorized preferred stock into one or more series and to fix
and determine such rights, preferences, privileges and restrictions of any series of preferred stock, including voting rights, dividend
rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by the Board upon its issuance.
In November 2022, the Company sold one share
of Series D Preferred Stock, par value $0.0001 per share (the “Series D Preferred Stock”) to Robert Bitterman, then
its interim Executive Chairman and current Chief Executive Officer, for $1,750. The Series D Preferred Stock was not convertible into,
or exchangeable for, shares of any other class or series of stock or other securities of the Company; had no rights with respect to any
distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution
or winding up of the Company, whether voluntarily or involuntarily; and was not entitled to receive dividends of any kind.
The Series D Preferred Stock was entitled to 17,500,000
votes per share exclusively with respect to any proposal to amend the Company’s Amended Certificate to effect a reverse stock split
of the Company’s common stock. The terms provided that it would be voted, without action by the holder, on any such proposal in
the same proportion as shares of the Company’s common stock were voted. The Series D Preferred Stock otherwise had no voting rights
except as otherwise required by the General Corporation Law of the State of Delaware.
Under its terms, the outstanding share of Series
D Preferred Stock was to be redeemed in whole, but not in part, at any time: (i) if such redemption was approved by the Board in its
sole discretion or (ii) automatically and effective upon the approval by the Company's stockholders of an amendment to the Amended Certificate
to effect a reverse stock split of the Company’s common stock. The Series D Preferred Stock was redeemed in whole on January 4,
2023, upon the approval by the Company’s stockholders of the Reverse Stock Split. Upon such redemption, the holder of the Series
D Preferred Stock received consideration of $1,750 in cash.
At December 31, 2023, there were no shares of
preferred stock issued or outstanding.
9. Stockholders’ Equity
April 2023 Financing
- In April 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 39,331 registered
shares of the Company’s common stock at a purchase price per share of $50.85, unregistered five and one-half year term Series A
warrants to purchase up to 39,331 shares of common stock at an exercise price of $48.60 per share and unregistered eighteen month term
Series B warrants to purchase up to 39,331 shares of common stock at an exercise price of $48.60 per share (collectively, the “April
2023 Financing”). In addition, the Company issued unregistered warrants to the placement agent, H.C. Wainwright & Co.,
LLC (“HCW”), in the April 2023 Financing to purchase a total of 2,950 shares of common stock at an exercise price
of $63.56 per share. Net proceeds to the Company from the April 2023 Financing were $1,538,000 after deducting placement agent fees and
offering expenses.
In connection with the
April 2023 Financing, the Company entered into warrant amendment agreements (the “Warrant Amendment Agreements”) with
the participating investors to amend the exercise price of certain existing warrants to purchase up to an aggregate of 21,291 shares
of common stock that were previously issued in April 2018 through January 2021, such that each of the amended warrants have an exercise
price of $48.60 per share. The Company received $24,000 as consideration in connection with the Warrant Amendment Agreements. The Company
assessed the amendments to the exercise price of the warrants under the FASB ASC Topic 815, “Derivatives and Hedging”
(“ASC 815”) and determined that the amendment to the exercise price was completed in connection with and contingent
on the close of the April 2023 Financing. The increase in fair value of $293,000 related to the Warrant Amendment Agreements was recognized
as an equity issuance cost and recorded in additional paid in capital per ASC 815.
June 2023 Financing
- In June 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 25,961 registered
shares and 8,000 unregistered shares of the Company’s common stock each at a purchase price per share of $38.52, unregistered pre-funded
warrants to purchase up to an aggregate of 69,881 shares of common stock at a purchase price per share of $38.511 and with an exercise
price of $0.009 per share, unregistered five and one-half year term Series A warrants to purchase up to an aggregate of 103,842 shares
of common stock at an exercise price of $36.27 per share and unregistered eighteen month term Series B warrants to purchase up to an
aggregate of 103,842 shares of common stock at an exercise price of $36.27 per share (collectively, the “June 2023 Financing”).
In addition, the Company issued unregistered warrants to the placement agent, HCW, in the June 2023 Financing to purchase a total of
7,788 shares of common stock at an exercise price of $48.15 per share. Net proceeds to the Company from the June 2023 Financing were
$3,510,000 after deducting placement agent fees and offering expenses.
December 2023 Financing
- In December 2023, the Company entered into an inducement letter agreement (the “Inducement Letter Agreement”)
with certain holders of the Company’s existing warrants to purchase up to an aggregate of 236,695 shares of the Company’s
common stock. The existing warrants were originally issued on dates between October 2018 and June 2023 with an exercise price of $48.60
or $36.27 per share. Pursuant to the Inducement Letter Agreement, these warrants were exercised for cash at a reduced exercise price
of $11.97 per share in consideration of the Company’s agreement to issue new five and one-half year term Series A warrants to purchase
up to 252,258 shares of common stock at an exercise price of $9.72 per share and new eighteen month term Series B warrants to purchase
up to 221,132 shares of common stock at an exercise price of $9.72 per share (collectively, the “December 2023 Financing”).
In addition, the Company issued warrants to the placement agent, HCW, in the December 2023 Financing to purchase a total of 17,752 shares
of common stock at an exercise price of $14.94 per share.
Pursuant to the terms of the Inducement Letter
Agreement, in the event that the exercise of the existing warrants in the December 2023 Financing would have otherwise caused a holder
to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued the number of shares that would
not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of shares of common stock in abeyance.
Accordingly, at December 31, 2023, an aggregate of 91,819 shares of common stock were held in abeyance (the “Abeyance Shares”)
with such Abeyance Shares evidenced through the holder’s existing warrants and which are deemed to be prepaid. The Abeyance Shares
will be held until notice is received by the holder that the balance of the shares of common stock may be issued in compliance with such
beneficial ownership limitations and may be exercised pursuant to a notice of exercise from the holder. Until such time, the Abeyance
Shares are evidenced through the holder’s existing warrants and have been included in the Company’s table of outstanding
warrants below.
Net
proceeds to the Company from the December 2023 financing were $2,404,000 after deducting placement agent fees and offering expenses.
The Company assessed the amendments to the exercise price of the warrants under the ASC 815 and determined that the amendment to the
exercise price was completed in connection with and contingent on the close of the December 2023 Financing. The increase in fair value
of $412,000 related to the modification of the terms of the warrants to induce exercise was recognized as an equity issuance cost and
recorded in additional paid in capital per ASC 815.
Warrants
The Company first assessed the warrants in the
April 2023 Financing, June 2023 Financing and December 2023 Financing under the FASB ASC Topic 480, “Distinguishing Liabilities
from Equity” (“ASC 480”) to determine whether they were within the scope of ASC 480. As there were no instances
outside of the Company’s control that could require cash settlement, the Company’s warrants issued in the April 2023 Financing,
June 2023 Financing and December 2023 Financing were determined to be outside the scope of ASC 480.
The Company then applied and followed the applicable
accounting guidance in ASC 815. Financial instruments are accounted for as either derivative liabilities or equity instruments depending
on the specific terms of the agreement. The warrants issued in the April 2023 Financing, June 2023 Financing and December 2023 Financing
did not meet the definition of a derivative instrument as they are indexed to the Company’s common stock and classified within
stockholders’ equity. Based on this determination, the warrants issued in the April 2023 Financing, June 2023 Financing and December
2023 Financing were classified within stockholders’ equity.
In addition to the December 2023 Financing, the
Company issued 69,881 shares of common stock related to exercises from the pre-funded warrants issued in the June 2023 Financing for
proceeds of $630. There were no warrants exercised during the year ended December 31, 2022.
The following table summarizes the Company’s
outstanding warrants, all of which are classified as equity instruments, at December 31, 2023:
| | |
Number
of Shares | | |
Weighted-
Average
Exercise Price
Per Share | |
| Outstanding at December 31, 2022 | |
| 60,600 | | |
$ | 490.75 | |
| Issued | |
| 858,162 | | |
| 19.55 | |
| Exercised | |
| (214,757 | ) | |
| 8.08 | |
| Expired | |
| (475 | ) | |
| 5,621.55 | |
| Outstanding at December 31, 2023 | |
| 703,530 | | |
$ | 33.09 | |
10. Stock-based Compensation
Stock Plans
The Company’s approved equity plans include
the Phio Pharmaceuticals Corp. 2020 Long Term Incentive Plan (the “2020 Plan”) and the Phio Pharmaceuticals Corp.
2012 Long Term Incentive Plan (the “2012 Plan”). These plans are administered by our Board and provide for the grant
of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock awards, RSU awards, performance
stock awards, and performance cash awards. Upon adoption of the 2020 Plan, shares that remained available for grant under the 2012 Plan
and shares that were subject to outstanding awards under the 2012 Plan were included in the authorized shares available for grant under
the 2020 Plan. Further, upon adoption of the 2020 Plan, the Company no longer grants new equity awards under the 2012 Plan. In July 2023,
the Company’s stockholders approved an amendment to the 2020 Plan to increase the number of shares authorized for issuance thereunder
to 25,712 shares of common stock.
As of December 31, 2023, there were 1,120 shares
subject to outstanding stock options, 5.520 shares subject to unvested RSUs and 14,842 shares available for future grants.
Restricted Stock Units
RSUs
are issued under the Company’s 2020 Plan or as inducement grants issued outside of
the 2020 Plan to new employees. RSUs are generally subject to graded vesting and the satisfaction
of certain service requirements. RSUs granted by the Company to employees generally vest
annually over 3 years after the grant date, (and over 1 year after the grant date for directors
of the Board of Directors. Upon vesting, each outstanding RSU will be settled for one share
of the Company’s common stock. Employee RSU recipients may elect to net share settle
upon vesting, in which case the Company pays the employee’s income taxes due upon vesting
and withholds a number of shares of equal value. The Company does not expect to repurchase
shares to satisfy RSU vests. The fair value of the RSUs awarded are based upon the Company’s
closing stock price at the grant date and are expensed over the requisite service period.
The following table summarizes the activity of
the Company’s RSUs for the year ended December 31, 2023:
| |
|
Number
of Shares |
|
|
Weighted-
Average
Grant Date Fair Value
Per Share |
|
| Unvested units at December 31, 2022 |
|
|
5,259 |
|
|
$ |
135.26 |
|
| Granted |
|
|
4,836 |
|
|
|
47.16 |
|
| Vested |
|
|
(2,599 |
) |
|
|
134.88 |
|
| Forfeited |
|
|
(1.969 |
) |
|
|
88.92 |
|
| Unvested units at December 31, 2023 |
|
|
5,527 |
|
|
$ |
74.83 |
|
The weighted-average fair value of RSUs granted
during the years ended December 31, 2023 and 2022 was $47.16 and $90.72, respectively.
Stock-based compensation expense related to RSUs
was $298,000 and $401,000 for the years ended December 31, 2023 and 2022, respectively.
The aggregate fair value of awards that vested
during the years ended December 31, 2023 and 2022 was $105,000 and $138,000, which represents the market value of the Company’s
common stock on the date that the RSUs vested.
As of December 31, 2023, the compensation
expense for all unvested RSUs in the amount of approximately $212,000 will be recognized in the Company’s results of operations
over a weighted average period of 1.30 years.
Stock Options
Stock options are available for issuance under
the 2020 Plan or as inducement grants issued outside of the 2020 Plan to new employees. Stock options are generally subject to graded
vesting and the satisfaction of service requirements. Stock options granted by the Company to employees generally vest annually over
4 years after the grant date and generally vest over 1 year after the grant date for members of the Board of Directors and expire within
ten years of grant. Upon the exercise of a stock option, the Company issues new shares and delivers them to the recipient. The Company
does not expect to repurchase shares to satisfy stock option exercises.
The Company uses the Black-Scholes option-pricing
model to determine the fair value of all its option grants. The risk-free interest rate used for each grant was based upon the yield
on zero-coupon U.S. Treasury securities with a term similar to the expected life of the related option. The Company’s expected
stock price volatility assumption is based upon the Company’s own implied volatility. As the Company has limited stock option exercise
information, the expected life assumption used for option grants is based upon the simplified method provided for under ASC 718. The
dividend yield assumption is based upon the fact that the Company has never paid cash dividends and presently has no intention of paying
cash dividends.
The Company did not grant stock options during
the year ended December 31, 2022. For valuing options granted during the year ended December 31, 2023, the following assumptions were
used:
| Schedule of assumptions | |
December 31, | |
| | |
2023 | |
| Risk-free interest rate | |
| 4.72% | |
| Expected volatility | |
| 113.74% | |
| Expected lives (in years) | |
| 5.25 | |
| Expected dividend yield | |
| 0% | |
The weighted average grant date fair value of
options granted during the year ended December 31, 2023 was $10.26 per share.
The following table summarizes the Company’s
stock option activity for the year ended December 31, 2023:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Number
of Shares |
|
|
Weighted-
Average
Exercise
Price
Per Share |
|
|
Weighted-
Average
Remaining
Contractual
Term |
|
|
Aggregate
Intrinsic
Value |
|
| Balance at December 31, 2022 |
|
|
20 |
|
|
$ |
317,082.58 |
|
|
|
|
|
|
|
|
|
| Granted |
|
|
1,136 |
|
|
|
12.33 |
|
|
|
|
|
|
|
|
|
| Exercised |
|
|
– |
|
|
|
– |
|
|
|
|
|
|
|
|
|
| Forfeited |
|
|
– |
|
|
|
– |
|
|
|
|
|
|
|
|
|
| Expired |
|
|
(10 |
) |
|
|
473,197,73 |
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2023 |
|
|
1,146 |
|
|
$ |
10,120.69 |
|
|
|
9.74 years |
|
|
$ |
– |
|
| Exercisable at December 31, 2023 |
|
|
9 |
|
|
$ |
144,240.81 |
|
|
|
2.78 years |
|
|
$ |
– |
|
Stock-based compensation expense related to stock
options for the years ended December 31, 2023 and 2022 was $5,000 and $13,000, respectively.
As of December 31, 2023, the compensation expense
for all unvested stock options in the amount of $6,000 will be recognized in the Company’s results of operations over a weighted
average period of 0.25 years.
There is no income tax benefit as the Company
is currently operating at a loss and an actual income tax benefit may not be realized.
Employee Stock Purchase Plan
The Company has 76 shares authorized for issuance
under the 2013 Employee Stock Purchase Plan (“ESPP”). The ESPP allows employees to contribute a percentage of their
cash earnings, subject to certain maximum amounts, to be used to purchase shares of the Company’s common stock on each of two semi-annual
purchase dates at a purchase price equal to 90% of the market value per share on either (a) the date of grant of a purchase right under
the ESPP or (b) the date on which such purchase right is deemed exercised, whichever is lower. As of December 31, 2023, 73 shares were
reserved for future issuance under the ESPP. There was no activity under the ESPP for the years ended December 31, 2023 and 2022.
Compensation Expense Related to Equity Awards
The following table sets forth total stock-based
compensation expense for the years ended December 31, 2023 and 2022, in thousands:
| Schedule of stock-based compensation expense | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Research and development | |
$ | 132 | | |
$ | 154 | |
| General and administrative | |
| 171 | | |
| 260 | |
| Total stock-based compensation | |
$ | 303 | | |
$ | 414 | |
11. Income Taxes
The provision for income taxes for the years ended
December 31, 2023 and 2022 are as follows, in thousands:
| Schedule of provision for income taxes | |
| | |
| |
| | |
Years Ended December 31, | |
| | |
2023 | | |
2022 | |
| Current | |
| | | |
| | |
| Federal | |
$ | – | | |
$ | – | |
| State | |
| – | | |
| – | |
| Total current | |
| – | | |
| – | |
| Deferred | |
| | | |
| | |
| Federal | |
| (1,831 | ) | |
| (1,733 | ) |
| State | |
| (718 | ) | |
| (553 | ) |
| Total deferred | |
| (2,549 | ) | |
| (2,286 | ) |
| Valuation allowance | |
| 2,549 | | |
| 2,286 | |
| Total provision for income taxes | |
$ | – | | |
$ | – | |
The following table presents a reconciliation
of the U.S. statutory tax rate to the Company’s actual effective income tax rate:
| Schedule of effective income tax reconciliation | |
| | |
| |
| | |
Years Ended December 31, | |
| | |
2023 | | |
2022 | |
| Federal statutory rate | |
| 21.0% | | |
| 21.0% | |
| State income taxes, net of federal benefit | |
| 5.9 | | |
| 7.4 | |
| Non-deductible expenses | |
| (0.5) | | |
| (0.8) | |
| Income tax credits | |
| 2.1 | | |
| 3.2 | |
| Valuation allowance | |
| (28.5) | | |
| (30.8) | |
| Effective tax rate | |
| 0.0% | | |
| 0.0% | |
The Company recognizes deferred tax assets and
liabilities to reflect the tax effects of temporary differences between the tax basis of assets or liabilities and their reported amounts
in the consolidated financial statements in accordance with ASC 740. These temporary differences will result in taxable or deductible
amounts in future years when the reported amounts of the assets or liabilities are recovered or settled.
ASC 740 requires that a valuation allowance be
established when management determines that it is more likely than not that all or a portion of a deferred asset will not be realized.
The Company evaluates the realizability of its net deferred income tax assets and valuation allowances as necessary, at least on an annual
basis. During this evaluation, the Company reviews its forecasts of income in conjunction with other positive and negative evidence surrounding
the realizability of its deferred income tax assets to determine if a valuation allowance is required. As a result of this evaluation,
the Company has recorded a full valuation allowance against its deferred tax assets as the Company believes it is more likely than not
that the benefit of all of its deferred tax assets will not be realized.
The significant components of the Company’s
deferred tax assets and liabilities are as follows, in thousands:
| Schedule of deferred tax assets and liabilities | |
| | |
| |
| | |
Years Ending December 31, | |
| | |
2023 | | |
2022 | |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 774 | | |
$ | 11,808 | |
| Tax credit carryforwards | |
| 295 | | |
| 1,227 | |
| Stock-based compensation | |
| 80 | | |
| 435 | |
| Capitalized research and development expenses | |
| 1,384 | | |
| 1,662 | |
| License fees | |
| 3 | | |
| 1,680 | |
| Lease liability | |
| 9 | | |
| 46 | |
| Other timing differences | |
| 13 | | |
| 120 | |
| Deferred tax assets | |
| 2,558 | | |
| 16,978 | |
| Deferred tax liabilities: | |
| | | |
| | |
| Right of use asset | |
| (9 | ) | |
| (43 | ) |
| Deferred tax liability | |
| (9 | ) | |
| (43 | ) |
| Valuation allowance | |
| (2,549 | ) | |
| (16,935 | ) |
| Net deferred tax asset | |
$ | – | | |
$ | – | |
Ownership changes may limit the amount of net
operating loss (“NOL”) carryforwards or tax credit carryforwards that can be utilized to offset future taxable income
or tax liability. Pursuant to Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”),
NOL and tax credit carryforwards may be subject to annual limitations in the event a cumulative change in ownership of more than 50%
occurs within a three-year period. Any limitation may result in expiration of a portion of the NOL carryforwards or tax credit carryforwards
before utilization.
During 2023, the Company completed an assessment
of the available NOL and tax credit carryforwards under Sections 382 and 383 of the Code since the last assessment completed in 2021
and concluded that the Company underwent an ownership change in 2023. As a result, NOL and tax credit carryforwards attributable to the
pre-ownership change are subject to substantial annual limitations under Sections 382 and 383 of the Code. The Company adjusted its NOL
and tax credit carryforwards to address the impact of the ownership change. For the year ended December 31, 2023, federal and state NOLs
were reduced by $52,400,000 and $25,900,000, respectively, and federal and state research and development tax credit carryforwards were
reduced by $918,000 and $517,000, respectively, as a result of the ownership change in 2023. The Company may experience ownership changes
in the future as a result of subsequent shifts in stock ownership, some of which may be outside of the Company’s control.
At December 31, 2023, the Company had federal
and state NOL carryforwards of approximately $2,900,000 and $2,475,000, respectively, to reduce future taxable income. The utilization
of the federal carryforwards as an available offset to future taxable income is subject to limitations under federal income tax laws.
Under current federal income tax law, federal NOLs generated in tax years beginning after 2017 may be carried forward indefinitely, but
are limited to offset up to 80% of future taxable income. As of December 31, 2023, all of our federal NOL carryforwards will carryforward
indefinitely. The Company’s available state NOL carryforwards will begin to expire in 2044, unless previously utilized.
At December 31, 2023, the Company also had federal
and state research and development credits of approximately $227,000 and $87,000, respectively. The federal tax credit carryforwards
will begin to expire in 2044 and the state tax credit carryforwards will begin to expire in 2039.
The Company has not recorded any uncertain tax
positions as of December 31, 2023 or 2022. The Company does not believe there will be any material changes in its unrecognized tax
positions over the next 12 months.
The Company has not incurred any interest or penalties.
In the event that the Company is assessed interest or penalties at some point in the future, they will be classified in the consolidated
financial statements as general and administrative expenses.
The Company files income tax returns in the United
States and in multiple state jurisdictions. The Company is subject to tax examinations for federal and state purposes for tax years 2015
through 2023.
12. Net Loss per Share
Basic net loss per share is computed by dividing
net loss by the weighted average number of common shares outstanding. Diluted net loss per share is computed by dividing the Company’s
net loss by the weighted average number of common shares outstanding and the impact of the dilutive effect of potential common stock
equivalents, except when the inclusion of such potential common stock equivalents would be anti-dilutive. Dilutive potential common stock
equivalents primarily consist of stock options, RSUs and warrants. Therefore, basic and diluted net loss per share applicable to common
stockholders were the same for all periods presented because the impact of these items is generally anti-dilutive during periods of net
loss.
The following table sets forth the potential common
shares excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive:
| | |
| | |
| |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Stock options | |
| 1,120 | | |
| 20 | |
| Unvested restricted stock units | |
| 5,519 | | |
| 5,258 | |
| Warrants1 | |
| 611,657 | | |
| 60,599 | |
| Total | |
| 618,296 | | |
| 65,877 | |
| |
1 |
The weighted average number of common shares outstanding as of December 31, 2023 includes the Abeyance
Shares from the December 2023 Financing, the exercise of which was prepaid and requires no further consideration for the delivery of
the shares of common stock. Therefore, these Abeyance Shares are not included in the table above. |
13. Subsequent Events
In connection with the Inducement Letter Agreement,
shares were held in abeyance in the event that the exercise of the existing warrants in the December 2023 Financing would have otherwise
caused a holder to exceed the beneficial ownership limitations set forth in the existing warrant. These Abeyance Shares will be held
until notice is received by the holder that the balance, or portion thereof, may be issued in compliance with the beneficial ownership
limitations. Subsequent to the balance sheet date, 91,819 Abeyance Shares were released and issued.
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per
share data)
(Unaudited)
| | |
| |
|
| | |
September 30,
2024 | |
December 31,
2023 |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 5,390 | | |
$ | 8,490 | |
| Prepaid expenses and other current assets | |
| 474 | | |
| 832 | |
| Total current assets | |
| 5,864 | | |
| 9,322 | |
| Right of use asset | |
| – | | |
| 33 | |
| Property and equipment, net | |
| 1 | | |
| 6 | |
| Other assets | |
| – | | |
| 3 | |
| Total assets | |
$ | 5,865 | | |
$ | 9,364 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 184 | | |
$ | 657 | |
| Accrued expenses | |
| 735 | | |
| 942 | |
| Lease liability | |
| – | | |
| 35 | |
| Total current liabilities | |
| 919 | | |
| 1,634 | |
| Commitments and contingencies (Note 2) | |
| – | | |
| – | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.0001
par value, 100,000,000 shares
authorized; no shares issued
and outstanding at September 30, 2024 and December 31, 2023 | |
| – | | |
| – | |
| Common stock, $0.0001
par value, 100,000,000 shares
authorized; 958,219 and 416,368
shares issued and outstanding at September 30, 2024 and December 31, 2023, respectively | |
| – | | |
| – | |
| Additional paid-in capital | |
| 149,676 | | |
| 146,936 | |
| Accumulated deficit | |
| (144,730 | ) | |
| (139,206 | ) |
| Total stockholders’ equity | |
| 4,946 | | |
| 7,730 | |
| Total liabilities and stockholders’ equity | |
$ | 5,865 | | |
$ | 9,364 | |
The accompanying notes are an integral part of
these condensed consolidated financial statements.
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per
share data)
(Unaudited)
| | |
| |
| |
| |
|
| | |
Three Months Ended
September 30, | |
Nine Months Ended
September 30, |
| | |
2024 | |
2023 | |
2024 | |
2023 |
| Operating expenses: | |
| |
| |
| |
|
| Research and development | |
$ | 644 | | |
$ | 1,808 | | |
$ | 2,658 | | |
$ | 5,325 | |
| General and administrative | |
| 946 | | |
| 968 | | |
| 3,055 | | |
| 3,600 | |
| Total operating expenses | |
| 1,590 | | |
| 2,776 | | |
| 5,713 | | |
| 8,925 | |
| Operating loss | |
| (1,590 | ) | |
| (2,776 | ) | |
| (5,713 | ) | |
| (8,925 | ) |
| Total other income (expense), net | |
| 66 | | |
| (4 | ) | |
| 189 | | |
| (6 | ) |
| Net loss | |
$ | (1,524 | ) | |
$ | (2,780 | ) | |
$ | (5,524 | ) | |
$ | (8,931 | ) |
| Net loss per common share: | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted | |
$ | (1.54 | ) | |
$ | (10.25 | ) | |
$ | (8.23 | ) | |
$ | (45.28 | ) |
| Weighted average number of common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted | |
| 990,033 | | |
| 271,129 | | |
| 670,875 | | |
| 197,227 | |
The accompanying notes are an integral part of
these condensed consolidated financial statements.
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED STATEMENTS OF
PREFERRED STOCK AND STOCKHOLDERS’ EQUITY
(Amounts in thousands, except share data)
(Unaudited)
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Series D Preferred Stock | |
Common Stock | |
Additional | |
| |
|
For
the Three and Nine Months Ended September
30, 2024 | |
Shares | |
Amount | |
Shares | |
Amount | |
Paid
in Capital | |
Accumulated Deficit | |
Total |
| Balance at December 31, 2023 | |
| – | | |
$ | – | | |
| 416,368 | | |
$ | – | | |
$ | 146,936 | | |
$ | (139,206 | ) | |
$ | 7,730 | |
| Issuance of common stock upon exercise of warrants | |
| – | | |
| – | | |
| 91,820 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Issuance of common stock upon vesting of restricted stock units | |
| – | | |
| – | | |
| 2,689 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Shares withheld for payroll taxes | |
| – | | |
| – | | |
| (689 | ) | |
| – | | |
| (4 | ) | |
| – | | |
| (4 | ) |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 32 | | |
| – | | |
| 32 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (2,154 | ) | |
| (2,154 | ) |
| Balance at March 31, 2024 | |
| – | | |
$ | – | | |
| 510,188 | | |
$ | – | | |
$ | 146,964 | | |
$ | (141,360 | ) | |
$ | 5,604 | |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 15 | | |
| – | | |
| 15 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (1,846 | ) | |
| (1,846 | ) |
| Balance at June 30, 2024 | |
| – | | |
$ | – | | |
| 510,188 | | |
$ | – | | |
$ | 146,979 | | |
$ | (143,206 | ) | |
$ | 3,773 | |
| Cash-in-lieu of fractional shares for reverse stock split | |
| – | | |
| – | | |
| (255 | ) | |
| – | | |
| (1 | ) | |
| – | | |
| (1 | ) |
| Issuance of common stock and warrants, net of offering costs | |
| – | | |
| – | | |
| 216,528 | | |
| – | | |
| 2,646 | | |
| – | | |
| 2,646 | |
| Issuance of common stock upon exercise of warrants | |
| – | | |
| – | | |
| 231,758 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 52 | | |
| – | | |
| 52 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (1,524 | ) | |
| (1,524 | ) |
| Balance at September 30, 2024 | |
| – | | |
$ | – | | |
| 958,219 | | |
$ | – | | |
$ | 149,676 | | |
$ | (144,730 | ) | |
$ | 4,946 | |
| | |
| |
| |
| |
| |
| |
| |
|
| | |
Series D Preferred Stock | |
Common Stock | |
Additional | |
| |
|
For the Three and Nine Months Ended
September 30, 2023 | |
Shares | |
Amount | |
Shares | |
Amount | |
Paid-in
Capital | |
Accumulated
Deficit | |
Total |
| Balance at December 31, 2022 | |
| 1 | | |
$ | 2 | | |
| 126,558 | | |
$ | – | | |
$ | 139,218 | | |
$ | (128,380 | ) | |
$ | 10,838 | |
| Cash-in-lieu of fractional shares for reverse stock split | |
| – | | |
| – | | |
| (190 | ) | |
| – | | |
| (11 | ) | |
| – | | |
| (11 | ) |
| Redemption of preferred stock | |
| (1 | ) | |
| (2 | ) | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | |
| Issuance of common stock upon vesting of restricted stock units | |
| – | | |
| – | | |
| 2,009 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Shares withheld for payroll taxes | |
| – | | |
| – | | |
| (535 | ) | |
| – | | |
| (25 | ) | |
| – | | |
| (25 | ) |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 111 | | |
| – | | |
| 111 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (3,602 | ) | |
| (3,602 | ) |
| Balance at March 31, 2023 | |
| – | | |
$ | – | | |
| 127,842 | | |
$ | – | | |
$ | 139,293 | | |
$ | (131,982 | ) | |
$ | 7,311 | |
| Issuance of common stock and warrants, net of offering costs | |
| – | | |
| – | | |
| 73,292 | | |
| – | | |
| 5,048 | | |
| – | | |
| 5,048 | |
| Issuance of common stock upon exercise of warrants | |
| – | | |
| – | | |
| 19,444 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 94 | | |
| – | | |
| 94 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (2,549 | ) | |
| (2,549 | ) |
| Balance at June 30, 2023 | |
| – | | |
$ | – | | |
| 220,578 | | |
$ | – | | |
$ | 144,435 | | |
$ | (134,531 | ) | |
$ | 9,904 | |
| Issuance of common stock upon exercise of warrants | |
| – | | |
| – | | |
| 35,588 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Issuance of common stock upon vesting of restricted stock units | |
| – | | |
| – | | |
| 222 | | |
| – | | |
| – | | |
| – | | |
| – | |
| Shares withheld for payroll taxes | |
| – | | |
| – | | |
| (13 | ) | |
| – | | |
| – | | |
| – | | |
| – | |
| Stock-based compensation expense | |
| – | | |
| – | | |
| – | | |
| – | | |
| 89 | | |
| – | | |
| 89 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (2,780 | ) | |
| (2,780 | ) |
| Balance at September 30, 2023 | |
| – | | |
$ | – | | |
| 256,375 | | |
$ | – | | |
$ | 144,524 | | |
$ | (137,311 | ) | |
$ | 7,213 | |
The accompanying notes are an integral part of
these condensed consolidated financial statements.
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
(Unaudited)
| |
|
|
|
|
|
|
|
|
| |
|
Nine Months Ended
September 30, |
| |
|
2024 |
|
2023 |
| Cash flows from operating activities: |
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(5,524 |
) |
|
$ |
(8,931 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
|
2 |
|
|
|
46 |
|
| Amortization of right of use asset |
|
|
33 |
|
|
|
95 |
|
| Loss on disposal of property and equipment |
|
|
3 |
|
|
|
– |
|
| Stock-based compensation |
|
|
99 |
|
|
|
294 |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
| Prepaid expenses and other assets |
|
|
361 |
|
|
|
(235 |
) |
| Accounts payable |
|
|
(473 |
) |
|
|
(606 |
) |
| Accrued expenses |
|
|
(207 |
) |
|
|
1,058 |
|
| Lease liability |
|
|
(35 |
) |
|
|
(100 |
) |
| Net cash used in operating activities |
|
|
(5,741 |
) |
|
|
(8,379 |
) |
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
| Cash paid for purchase of property and equipment |
|
|
– |
|
|
|
(5 |
) |
| Net cash used in investing activities |
|
|
– |
|
|
|
(5 |
) |
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
| Net proceeds from the issuance of common stock and warrants |
|
|
2,646 |
|
|
|
5,048 |
|
| Cash in lieu of fractional shares for reverse stock split |
|
|
(1 |
) |
|
|
(11 |
) |
| Redemption of Series D preferred stock |
|
|
– |
|
|
|
(2 |
) |
| Payment of taxes on net share settlements of restricted stock units |
|
|
(4 |
) |
|
|
(25 |
) |
| Net cash provided by financing activities |
|
|
2,641 |
|
|
|
5,010 |
|
| Net decrease in cash, cash equivalents and restricted cash |
|
|
(3,100 |
) |
|
|
(3,374 |
) |
| Cash, cash equivalents and restricted cash at the beginning of period |
|
|
8,490 |
|
|
|
11,831 |
|
| Cash, cash equivalents and restricted cash at the end of period |
|
$ |
5,390 |
|
|
$ |
8,457 |
|
The following table provides a reconciliation
of cash, cash equivalents and restricted cash reported within the condensed consolidated balance sheets to the totals above:
| |
|
|
|
|
| |
|
September 30, |
| |
|
2024 |
|
2023 |
| Cash and cash equivalents |
|
$ |
5,390 |
|
|
$ |
8,407 |
|
| Restricted cash |
|
|
– |
|
|
|
50 |
|
| Cash, cash equivalents and restricted cash |
|
$ |
5,390 |
|
|
$ |
8,457 |
|
The accompanying notes are an integral part of
these condensed consolidated financial statements.
PHIO PHARMACEUTICALS CORP.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1.
Organization and Significant Accounting Policies
Nature
of Operations
Phio Pharmaceuticals Corp.
(“Phio” or the “Company”) is a clinical stage biotechnology company whose proprietary INTASYL®
small interfering RNA gene silencing technology is designed to make immune cells more effective in killing tumor cells. The Company is
developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that reduce the body’s ability
to fight cancer, without the need for specialized formulations or drug delivery systems.
Phio was incorporated in
the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals
Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology
therapeutics.
Effective
July 5, 2024, the Company completed a 1-for-9
reverse stock split of the Company’s outstanding common stock, including
reclassifying an amount equal to the reduction in par value to additional paid-in capital. The reverse stock split did not reduce the
number of authorized shares of the Company’s common or preferred stock. All share and per share amounts have been adjusted to give
effect to the reverse stock split.
Basis
of Presentation
The accompanying condensed
consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in
the United States (“GAAP”). Certain information and footnote disclosures that are included in the Company’s
annual consolidated financial statements, but that are not required for interim reporting purposes, have been condensed or omitted. In
the opinion of management, all adjustments (including normal recurring accruals) considered necessary for a fair presentation of the
condensed consolidated financial statements have been included.
These statements should be
read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s most recent
Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”)
on April 1, 2024 (the “2023 Form 10-K”). Interim results are not necessarily indicative of results for a full year.
Principles
of Consolidation
The condensed consolidated
financial statements include the accounts of the Company and its wholly-owned subsidiary, MirImmune, LLC. All material intercompany accounts
have been eliminated in consolidation.
Segments
The Company operates as one operating
segment and all assets are located in the United States.
Use
of Estimates
The preparation of financial
statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of
revenues and expenses during the reporting period. The areas subject to significant estimates and judgement include, among others, those
related to the fair value of equity awards, accruals for research and development expenses, useful lives of property and equipment, and
the valuation allowance on the Company’s deferred tax assets. On an ongoing basis the Company evaluates its estimates and bases
its estimates on historical experience and other relevant assumptions that the Company believes are reasonable under the circumstances.
Actual results could differ materially from these estimates.
Liquidity
The Company has reported
recurring losses from operations since its inception and expects to continue to have negative cash flows from operations for the foreseeable
future. Historically, the Company’s primary source of funding has been from sales of its securities. The Company’s ability
to continue to fund its operations is dependent on obtaining funding from third parties, such as proceeds from the issuance of debt,
sale of equity, or strategic opportunities, in order to maintain its operations. This is dependent on a number of factors, including
the market demand or liquidity of the Company’s common stock. There is no guarantee that debt, additional equity or other funding
will be available to the Company on acceptable terms, or at all. If the Company fails to obtain additional funding when needed, the Company
would be forced to scale back or terminate its operations or seek to merge with or to be acquired by another company.
The Company has limited cash
resources, has reported recurring losses from operations since inception, has negative operating cash flows and has not yet received
product revenues. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern, and the
Company’s current cash resources may not provide sufficient capital to fund operations for at least the next 12 months from the
date of the release of these condensed consolidated financial statements. The continuation of the Company as a going concern depends
upon the Company’s ability to raise additional capital through an equity offering, debt offering and/or strategic opportunity to
fund its operations. There can be no assurance that the Company will be successful in accomplishing these plans in order to continue
as a going concern. These condensed consolidated financial statements do not include any adjustments to the recoverability and classification
of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going
concern.
Summary
of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents
include unrestricted cash accounts, money market investments and highly liquid investment instruments with original maturity of three
months or less at the date of purchase.
Other than as set forth above,
there have been no material changes to the significant accounting policies disclosed in the Company’s 2023 Form 10-K.
Recent
Accounting Pronouncements
In November 2023, the Financial
Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2023-07, “Segment
Reporting (Topic 280) – Improvements to Reporting Segment Disclosures” (“ASU 2023-07”), which requires
disclosure of incremental segment information on an annual and interim basis. In addition, ASU 2023-07 clarifies circumstances in which
an entity can disclose multiple segment measures of profit or loss, provides new segment disclosure requirements for entities with a
single reportable segment, and contains other disclosure requirements. The amendments in ASU 2023-07 are effective for fiscal years beginning
after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The
enhanced disclosures are required to be applied retrospectively to all prior periods presented in the financial statements. The Company
is currently evaluating the impact of ASU 2023-07 on its consolidated financial statements and disclosures, but does not expect that
it will have a material impact on its consolidated financial statements.
In December 2023, the FASB
issued ASU 2023-09, “Income Taxes (Topic 740) – Improvements to Income Tax Disclosures” (“ASU 2023-09”),
which requires disclosure of specific categories in the rate reconciliation table along with additional information for reconciling items
that meet a quantitative threshold, disclosure of disaggregated income taxes paid and modifies other income tax-related disclosures.
The amendments in ASU 2023-09 are effective for annual periods beginning after December 15, 2024 and allows for adoption on a prospective
basis, with a retrospective option. Early adoption is permitted. The Company is currently evaluating the impact of ASU 2023-09, but does
not expect that it will have a material impact on its consolidated financial statements.
2. Collaboration
Agreement
AgonOx, Inc. (“AgonOx”)
In
February 2021, the Company entered into a clinical co-development collaboration agreement (the “Clinical Co-Development Agreement”)
with AgonOx, a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to
cancer. On May 8, 2024, the Company terminated the Clinical Co-Development Agreement with AgonOx, which such termination was effective
immediately. Under the Clinical Co-Development Agreement, Phio and AgonOx were working to develop a T cell-based therapy using the Company’s
lead product candidate, PH-762, and AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP TIL”)
technology. Per the terms of the Clinical Co-Development Agreement, the Company had agreed to reimburse AgonOx up to $4,000,000
in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients with advanced melanoma and other
advanced solid tumors and was entitled to certain future development milestones and low single-digit sales-based royalty payments from
AgonOx licensing its DP TIL technology.
The
Company recognized its share of costs arising from research and development activities performed by AgonOx in the Company’s condensed
consolidated financial statements in the period AgonOx incurred such expense. Effective as of the date of termination, the Clinical Co-Development
Agreement and the continuing obligations of the Company and AgonOx thereunder were terminated in their entirety. As a result, the Company
is no longer required to provide financial support for the development costs incurred under the Clinical Co-Development Agreement, and
is not entitled to future development milestones or royalty payments from AgonOx’s licensing of its DP TIL technology.
The
Company will pay to AgonOx all Company payment obligations that accrued prior to the termination of the Clinical Co-Development Agreement.
Remaining payments to be made to AgonOx as of September 30, 2024 were $35,000, which primarily related to remaining accrued obligations
for patient fees and other miscellaneous costs as of the date of termination. Pursuant to the terms of the Clinical Co-Development Agreement,
the Company and AgonOx are coordinating the orderly wind-down of the Phase 1 clinical trial. Each of the Company and AgonOx shall be
responsible for its own costs and expenses incurred in connection with the wind-down of the Phase 1 clinical trial.
The
Company recognized approximately $308,000
and $414,000
in connection with the Clinical Co-Development Agreement during the three and nine months ended September 30, 2024, respectively,
which relate to the Company’s expense obligations under the Clinical Co-Development Agreement through the date of termination.
The Company recognized approximately $606,000
and $906,000
of expense in connection with the Clinical Co-Development Agreement during the three and nine months ended September 30, 2023,
respectively.
3. Fair
Value of Financial Instruments
The Company follows the provisions
of the FASB Accounting Standards Codification (“ASC”) Topic 820, “Fair Value Measurement,” for
the Company’s financial assets and liabilities that are re-measured and reported at fair value each reporting period and are re-measured
and reported at fair value at least annually using a fair value hierarchy that is broken down into three levels. Level inputs are defined
as follows:
Level 1 – quoted prices
in active markets for identical assets or liabilities.
Level 2 – other significant
observable inputs for the assets or liabilities through corroboration with market data at the measurement date.
Level 3 – significant
unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities
at the measurement date.
As of September 30, 2024,
the Company categorized its cash equivalents as Level 1 hierarchy as the carrying amounts approximate their fair value due to their short-term
nature and market rates of interest. As of December 31, 2023, the Company did not identify any financial instruments required to be presented
at fair value.
| Schedule of financial instruments at fair value | |
| | | |
| | | |
| | | |
| | |
| Description | |
September 30, 2024 | |
Quoted
Prices In
Active Markets
(Level 1) | |
Other Significant
Observable Inputs
(Level 2) | |
Unobservable
Inputs
(Level 3) |
| Assets: | |
| | | |
| | | |
| | | |
| | |
| Cash equivalents | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
| Total | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
The carrying amounts of cash,
accounts payable and accrued expenses of the Company approximate their fair values due to their short-term nature.
4.
Leases
The Company entered into
a lease for a laboratory facility located at 17 Briden Street, Worcester, Massachusetts, which covers 321
square feet of rentable space. The lease commenced on March 1, 2024 and had an original expiration date of August
31, 2024. The Company has the option to renew the lease for additional 6-month periods. On June 1, 2024, the Company elected the
option to renew the lease for an additional 6-month period, and the lease will expire on February 28, 2025. The Company made an accounting
policy election under the FASB ASC Topic 842, “Leases” not to recognize leases with a term less than one year on the
balance sheet and that do not contain a purchase option. Under the short-term lease election, the Company will recognize the lease payments
for the laboratory facility on a straight-line basis over the lease term.
The total base rent for the
premises over each 6-month term is expected to be $15,000.
During the three and nine month periods ending September 30, 2024, the Company recognized $8,129
and $18,909,
respectively, of rent expense and variable lease costs related to the laboratory facility.
The Company’s lease
for its corporate headquarters and primary research facility in Marlborough, Massachusetts was for a total of 7,581
square feet of office and laboratory space and expired on March
31, 2024. The lease agreement did not contain information to determine the borrowing rate implicit in the lease. As such, the
Company calculated its incremental borrowing rate based on what the Company would have to pay to borrow on a collateralized basis over
the lease term for an amount equal to the remaining lease payments, taking into consideration such assumptions as, but not limited to,
the U.S. treasury yield rate and borrowing rates from a creditworthy financial institution using the above lease factors. The Company
has continued operations as a primarily remote business with the expiration of the lease, but has contracted a private mailbox with an
address of 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, MA 01752 to use as its principal mailing address for SEC and other purposes.
The lease for the Company’s
former corporate headquarters represented all of the Company’s capitalized lease obligations.
The amounts reported in the
condensed consolidated balance sheets for the Company’s former corporate headquarters classified as an operating lease in which
the Company is the lessee and other supplemental balance sheet information is set forth as follows, in thousands, except the lease term
(number of years) and discount rate:
| Schedule of lease amounts recorded in balance sheet |
|
|
|
|
| |
|
September 30,
2024 |
|
December 31,
2023 |
| Assets |
|
|
|
|
|
|
|
|
| Right of use asset |
|
$ |
– |
|
|
$ |
33 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Lease liability |
|
$ |
– |
|
|
$ |
35 |
|
| Lease Term and Discount Rate |
|
|
|
|
|
|
|
|
| Weighted average remaining lease term |
|
|
– |
|
|
|
0.25 |
|
| Weighted average discount rate |
|
|
– |
|
|
|
4.70% |
|
There were no operating
lease costs for our former corporate headquarters included in operating expense for the three months ended September 30, 2024.
Operating lease costs for our former corporate headquarters included in operating expense were $33,000 for
the three months ended September 30, 2023. Operating expenses were $33,000 and
$99,000 for the nine months ended
September 30, 2024 and 2023, respectively.
The Company’s condensed
consolidated statements of cash flows for the Company’s former corporate headquarters was $35,000
and $104,000 for the nine months
ended September 30, 2024 and 2023, respectively.
5.
Stockholders’ Equity
Financings
May 2024 Financing
— On May 16, 2024, the Company entered into a purchase agreement (the “Purchase Agreement”) with Triton Funds
LP (“Triton”), pursuant to which the Company agreed to sell, and Triton agreed to purchase, upon the Company’s
request in one or more transactions, up to 95,833
shares of the Company’s common stock at a purchase price of $6.48
per share (the “Purchase Price”), for aggregate gross proceeds of up to $621,000.
The Company recorded expense of approximately $100,000, primarily related to legal fees, in connection with the execution of the Purchase
Agreement with Triton. On July 3, 2024, the Company terminated the Purchase Agreement with Triton effective immediately. No shares of
common stock were sold by the Company pursuant to the Purchase Agreement prior to termination.
July 2024 Financing
— On July 11, 2024, the Company entered into inducement letter agreements (the “July 2024 Inducement Letter Agreements”)
with certain holders of certain of the Company’s existing warrants to purchase up to an aggregate of 545,286
shares of the Company’s common stock. The existing warrants were originally issued in February 2020 through December 2023,
having exercise prices between $324.00 and $9.72 per share. Pursuant to the July 2024 Inducement Letter Agreements, these warrants were
exercised for cash at a reduced exercise of $5.45
per share in consideration of the Company’s agreement to issue new unregistered five and one-half year term Series C warrants
to purchase up to 583,098
shares of common stock at an exercise price of $5.45
and new unregistered eighteen month term Series D warrants to purchase up to 507,474
shares of common stock at an exercise price of $5.45,
both issued and sold at a price of $0.125 per warrant share (the “July 2024 Financing”). In addition, the Company
issued warrants to the placement agent, HCW, to purchase a total of 40,896 shares of common stock at an exercise price of $6.8125 per
share. The net proceeds to the Company from the July 2024 Financing were approximately $2,646,000,
after deducting placement agent fees and offering expenses. The Company incurred non-cash equity issuance cost of approximately $2.4
million for the incremental fair value of the outstanding equity classified warrants and approximately $0.2
million for placement agent warrants.
Pursuant to the terms of
the July 2024 Inducement Letter Agreements, in the event that the exercise of the existing warrants in the July 2024 Financing would
have otherwise caused a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued the
number of shares that would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of shares
of common stock in abeyance. Accordingly, an aggregate of 328,758
shares of common stock were held in abeyance (the “July 2024 Abeyance Shares”) with such July 2024 Abeyance
Shares evidenced through the holder’s existing warrants and which are deemed to be prepaid. The July 2024 Abeyance Shares were
held until notice was received by the holder that the balance of the shares of common stock could be issued in compliance with such beneficial
ownership limitations and were exercised pursuant to a notice of exercise from the holder. Until such time, the Abeyance Shares were
evidenced through the holder’s existing warrants and have been included in the Company’s table of outstanding warrants below.
During the three months ended September 30, 2024, 231,758
of the July 2024 Abeyance Shares were released. The remainder of the July 2024 Abeyance Shares were subsequently released in October
2024.
April 2023 Financing
— On April 20, 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 39,331
registered shares of the Company’s common stock at a purchase price per share of $50.85,
unregistered five and one-half year term Series A warrants to purchase up to 39,331
shares of common stock at an exercise price of $48.60
per share and unregistered eighteen month term Series B warrants to purchase up to 39,331
shares of common stock at an exercise price of $48.60
per share (collectively, the “April 2023 Financing”). In addition, the Company issued unregistered warrants
to the placement agent, H.C. Wainwright & Co., LLC (“HCW”), in the April 2023 Financing to purchase a total of
2,950
shares of common stock at an exercise price of $63.56
per share. Net proceeds to the Company from the April 2023 Financing were $1,538,000
after deducting placement agent fees and offering expenses.
In connection with the April
2023 Financing, the Company entered into warrant amendment agreements (the “Warrant Amendment Agreements”) with the
participating investors to amend the exercise price of certain existing warrants to purchase up to an aggregate of 21,291
shares of common stock that were previously issued in April 2018 through January 2021, such that each of the amended warrants
have an exercise price of $48.60
per share. The Company received $23,952
as consideration in connection with the Warrant Amendment Agreements. The Company assessed the amendments to the exercise price
of the warrants under ASC Topic 815, “Derivatives and Hedging” (“ASC 815”) and determined that
the amendment to the exercise price was completed in connection with and contingent on the close of the April 2023 Financing. The increase
in fair value of $293,000
related to the Warrant Amendment Agreements was recognized as an equity issuance cost and recorded in additional paid in capital
per ASC 815.
June 2023 Financing
— On June 2, 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 25,961
registered shares and 8,000
unregistered shares of the Company’s common stock each at a purchase price per share of $38.52,
unregistered pre-funded warrants to purchase up to an aggregate of 69,881
shares of common stock at a purchase price per share of $38.511
and with a pre-funded warrant exercise price of $0.009
per share, unregistered five and one-half year term Series A warrants to purchase up to an aggregate of 103,842
shares of common stock at an exercise price of $36.27
per share and unregistered eighteen month term Series B warrants to purchase up to an aggregate of 103,842
shares of common stock at an exercise price of $36.27
per share (collectively, the “June 2023 Financing”). In addition, the Company issued unregistered warrants
to the placement agent, HCW, in the June 2023 Financing to purchase a total of 7,788
shares of common stock at an exercise price of $48.15
per share. Net proceeds to the Company from the June 2023 Financing were $3,510,000
after deducting placement agent fees and offering expenses.
December 2023 Financing
— In December 2023, the Company entered into an inducement letter agreement (the “December 2023 Inducement Letter
Agreement”) with certain holders of the Company’s existing warrants to purchase up to an aggregate of 236,695 shares
of the Company’s common stock (the “December 2023 Financing”). Pursuant to the terms of the December 2023 Inducement
Letter Agreement, in the event that the exercise of the existing warrants in the December 2023 Financing would have otherwise caused
a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued the number of shares that
would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of shares of common stock in
abeyance. Accordingly, an aggregate of 91,820 shares of common stock were held in abeyance (the “December 2023 Abeyance Shares”)
with such December 2023 Abeyance Shares evidenced through the holder’s existing warrants and which were deemed to be prepaid. The
December 2023 Abeyance Shares were held until notice was received by the holder that the balance of the shares of common stock could
be issued in compliance with such beneficial ownership limitations and were exercised pursuant to a notice of exercise from the holder.
Until such time, the December 2023 Abeyance Shares were evidenced through the holder’s existing warrants and have been included
in the Company’s table of outstanding warrants below.
Pursuant
to the terms of the Inducement Letter Agreement, in the event that the exercise of the existing warrants in the December 2023 Financing
would have otherwise caused a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued
the number of shares that would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of
shares of common stock in abeyance. Accordingly, at December 31, 2023, an aggregate of 91,819 shares of common stock were held
in abeyance (the “Abeyance Shares”) with such Abeyance Shares evidenced through the holder’s existing warrants
and which are deemed to be prepaid. The Abeyance Shares will be held until notice is received by the holder that the balance of the shares
of common stock may be issued in compliance with such beneficial ownership limitations and may be exercised pursuant to a notice of exercise
from the holder. Until such time, the Abeyance Shares are evidenced through the holder’s existing warrants and have been included
in the Company’s table of outstanding warrants below. The remainder of the December 2023 Abeyance Shares were subsequently released
during the first quarter of 2024.
Warrants
The Company first assesses
warrants that are issued by the Company under the FASB ASC Topic 480, “Distinguishing Liabilities from Equity” (“ASC
480”) to determine whether the warrants are within the scope of ASC 480. If there are no instances outside of the Company’s
control that could require cash settlement, the Company then applies and follows the applicable accounting guidance in ASC 815. Financial
instruments are accounted for as either derivative liabilities or equity instruments depending on the specific terms of the agreement.
Based on the assessment of the warrants issued by the Company under the guidance in ASC 480 and ASC 815, the warrants issued by the Company
have been classified within stockholder’s equity.
During
the three months ended September 30, 2024, 231,758
of the July 2024 Abeyance Shares were released and issued. During the three months ended September 30, 2023, 35,588
shares of common stock were issued related to the exercise of pre-funded warrants from the June 2023 Financing.
During
the nine months ended September 30, 2024, all of the December 2023 Abeyance Shares were released and issued and 231,758
of the July 2024 Abeyance Shares were released and issued. During the nine months ended September 30, 2023, 55,032
shares of common stock were issued related to the exercise of pre-funded warrants from the June 2023 Financing.
The following table summarizes
the Company’s outstanding warrants, all of which are classified as equity instruments, at September 30, 2024:
| Schedule of outstanding warrants | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share |
| Outstanding at December 31, 2023 | |
| 703,530 | | |
$ | 35.99 | |
| Issued | |
| 1,131,468 | | |
| 5.50 | |
| Exercised | |
| (540,112 | ) | |
| 6.56 | |
| Expired | |
| – | | |
| – | |
| Outstanding at September 30, 2024 | |
| 1,294,886 | | |
$ | 21.62 | |
6.
Stock-based Compensation
Restricted Stock Units
Restricted stock units (“RSUs”)
are issued under the Company’s 2020 Long-Term Incentive Plan (the “2020 Plan”) or as inducement grants issued
outside of the 2020 Plan to new employees. RSUs are generally subject to graded vesting and the satisfaction of certain service requirements.
RSUs granted by the Company to employees and to non-employee members of the Board of Directors generally vest annually over 1
year after the grant date. Upon vesting, each outstanding RSU will be settled for one share of the Company’s common stock.
Employee RSU recipients may elect to net share settle upon vesting, in which case the Company pays the employee’s income taxes
due upon vesting and withholds a number of shares of equal value. The Company does not expect to repurchase shares to satisfy RSU vests.
The fair value of the RSUs awarded are based upon the Company’s closing stock price at the grant date and are expensed over the
requisite service period.
On September 11, 2024, the
Company modified all unvested RSUs to reduce the vesting term for employees from 3 years after the grant date to 1 year after the grant
date. No incremental expense was recognized as a result of the modification, as the fair value of the RSUs immediately before and after
the modification was unchanged.
The following table summarizes
the activity of the Company’s RSUs for the nine months ended September 30, 2024:
| Summary of RSUs activity | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Grant Date
Fair Value
Per Share |
| Unvested units at December 31, 2023 | |
| 5,527 | | |
$ | 74.83 | |
| Granted and accepted | |
| 71,000 | | |
| 2.77 | |
| Vested | |
| (3,997 | ) | |
| 71.10 | |
| Forfeited | |
| (1,530 | ) | |
| 84.55 | |
| Unvested units at September 30, 2024 | |
| 71,000 | | |
$ | 2.77 | |
The weighted-average fair
value of RSUs granted during both the three and nine months ended September 30, 2024 was $2.77.
There were no
RSUs granted during the three months ended September 30, 2023. During the nine months ended September 30, 2023, 4,833 RSUs
were granted. The weighted-average fair value of RSUs granted during the nine months ended September 30, 2023 was $47.16.
Stock-based compensation
expense related to RSUs was $52,000
and $89,000
for the three months ended September 30, 2024 and 2023, respectively. Stock-based compensation expense related to RSUs was $99,000
and $294,000
for the nine months ended September 30, 2024 and 2023, respectively.
The aggregate fair value
of awards that vested during the nine months ended September 30, 2024 and 2023 was $21,000
and $100,000,
respectively, which represents the market value of the Company’s common stock on the date that the RSUs vested.
Stock Options
Stock options are available
for issuance under the 2020 Plan or as inducement grants issued outside of the 2020 Plan to new employees. Stock options are generally
subject to graded vesting and the satisfaction of service requirements. Stock options granted by the Company to employees generally vest
annually over 4
years after the grant date and generally vest over 1
year after the grant date for non-employee members of the Board of Directors and expire within ten years of grant. Upon the exercise
of a stock option, the Company issues new shares and delivers them to the recipient. The Company does not expect to repurchase shares
to satisfy stock option exercises.
The Company uses the Black-Scholes
option-pricing model to determine the fair value of all its option grants. The risk-free interest rate used for each grant was based
upon the yield on zero-coupon U.S. Treasury securities with a term similar to the expected life of the related option. The Company’s
expected stock price volatility assumption is based upon the Company’s own implied volatility. As the Company has limited stock
option exercise information, the expected life assumption used for option grants is based upon the simplified method provided for under
the FASB ASC Topic 718, “Compensation — Stock Compensation”. The dividend yield assumption is based upon
the fact that the Company has never paid cash dividends and presently has no intention of paying cash dividends.
The Company did not grant
any stock options during the three or nine months ended September 30, 2024 and 2023.
The following table summarizes
the activity of the Company’s stock options for the nine months ended September 30, 2024:
| Summary of stock options activity | |
| |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share | |
Aggregate
Intrinsic
Value |
| Balance at December 31, 2023 | |
| 1,146 | | |
$ | 10,120.69 | | |
| | |
| Granted | |
| – | | |
| – | | |
| | |
| Exercised | |
| – | | |
| – | | |
| | |
| Forfeited | |
| – | | |
| – | | |
| | |
| Expired | |
| (13 | ) | |
| 598,313.35 | | |
| | |
| Balance at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
| Exercisable at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
Stock-based compensation
expense related to stock options for the nine months ended September 30, 2024 was $6,000.
The Company did not
have any stock-based compensation expense related to stock options for the three months ended September 30, 2024 and 2023 or the nine
months ended September 30, 2023.
Compensation Expense Related to Equity Awards
The following table sets
forth total stock-based compensation expense for the three and nine months ended September 30, 2024 and 2023, in thousands:
| Schedule of stock-based compensation expense | |
| |
| |
| |
|
| | |
Three Months Ended | |
Nine Months Ended |
| | |
September 30, | |
September 30, |
| | |
2024 | |
2023 | |
2024 | |
2023 |
| Research and development | |
$ | 25 | | |
$ | 51 | | |
$ | 14 | | |
$ | 163 | |
| General and administrative | |
| 27 | | |
| 38 | | |
| 85 | | |
| 131 | |
| Total stock-based compensation | |
$ | 52 | | |
$ | 89 | | |
$ | 99 | | |
$ | 294 | |
7.
Net Loss per Common Share
Basic net loss per share
is computed by dividing net loss by the weighted average number of common shares outstanding. Diluted net loss per share is computed
by dividing the Company’s net loss by the weighted average number of common shares outstanding and the impact of the dilutive effect
of potential common stock equivalents, except when the inclusion of such potential common stock equivalents would be anti-dilutive. Dilutive
potential common stock equivalents primarily consist of stock options, RSUs and warrants. Therefore, basic and diluted net loss per share
applicable to common stockholders were the same for all periods presented because the impact of these items is generally anti-dilutive
during periods of net loss.
The weighted average number
of common shares outstanding as of September 30, 2023 includes the pre-funded warrants issued in connection with the June 2023 Financing,
the exercise of which requires nominal consideration for the delivery of the shares of common stock. Therefore, these pre-funded warrants
are not included in the table below.
The following table sets
forth the potential common shares excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive:
| Schedule of anti dilutive shares |
|
|
|
|
| |
|
September 30, |
| |
|
2024 |
|
2023 |
| Stock options |
|
|
1,133 |
|
|
|
14 |
|
| Unvested RSUs |
|
|
71,000 |
|
|
|
7,694 |
|
| Warrants1,2 |
|
|
1,197,886 |
|
|
|
357,481 |
|
| Total |
|
|
1,270,019 |
|
|
|
365,189 |
|
8.
Subsequent Events
In accordance with ASC Topic
855, “Subsequent Events”, which establishes general standards of accounting for and disclosure of events that occur after
the consolidated balance sheet date but before the consolidated financial statements are issued, the Company has evaluated all events
or transactions that occurred after September 30, 2024, up through the date the Company issued the condensed consolidated financial
statements.
Phio Pharmaceuticals Corp.

Up to 5,930,016 Shares of Common Stock
PROSPECTUS
,
2025
PART
II
Information Not Required in Prospectus
Item 13. Other Expenses
of Issuance and Distribution
The following table sets
forth the fees and expenses payable in connection with the registration of the Common Stock hereunder. All amounts other than the SEC
registration fees are estimates.
| Item |
|
Amount
to be paid |
|
| SEC registration fees |
|
$ |
3,150.36 |
|
| Legal fees and expenses |
|
|
30,000.00 |
|
| Accounting fees and expenses |
|
|
[28,000.00 |
] |
| Printing and miscellaneous expenses |
|
|
2,000.00 |
|
| Total |
|
$ |
[___] |
|
Item 14. Indemnification
of Directors and Officers
Section 145 of the Delaware
General Corporation Law (“DGCL”) authorizes a corporation to indemnify its directors and officers against liabilities
arising out of actions, suits and proceedings to which they are made or threatened to be made a party by reason of the fact that they
have served or are currently serving as a director or officer to a corporation. The indemnity may cover expenses (including attorneys’
fees) judgments, fines and amounts paid in settlement actually and reasonably incurred by the director or officer in connection with
any such action, suit or proceeding. Section 145 permits corporations to pay expenses (including attorneys’ fees) incurred by directors
and officers in advance of the final disposition of such action, suit or proceeding. In addition, Section 145 provides that a corporation
has the power to purchase and maintain insurance on behalf of its directors and officers against any liability asserted against them
and incurred by them in their capacity as a director or officer, or arising out of their status as such, whether or not the corporation
would have the power to indemnify the director or officer against such liability under Section 145.
Our amended and restated
certificate of incorporation provides that we will indemnify to the fullest extent authorized or permitted by the DGCL or any other applicable
law as now or hereafter in effect any person made, or threatened to be made, a defendant or witness to any action, suit or proceeding
(whether civil, criminal or otherwise) by reason of the fact that he or she is or was a director of our corporation or by reason of the
fact that such director, at our request, is or was serving any other corporation, partnership, joint venture, trust, employee benefit
plan or other enterprise in any capacity. Our amended and restated certificate of incorporation also provides that no amendment or repeal
of the amended and restated certificate of incorporation will apply to or have any effect on any right to indemnification provided in
the amended and restated certificate of incorporation with respect to any acts or omissions occurring prior to such amendment or repeal.
As permitted by the DGCL,
our bylaws, as amended, provide that we will indemnify to the fullest extent authorized or permitted by applicable law as now or hereafter
in effect any person who was or is made, or is threatened to be made, a party or is otherwise involved in any action, suit or proceeding
(whether civil, criminal, administrative or investigative), by reason of the fact that he or she (or a person for whom he or she is the
legal representative) is or was a director or officer of our corporation, is or was serving at our request as a director, officer, employee,
member, trustee or agent of another corporation or of a partnership, joint venture, trust, nonprofit entity or other enterprise.
Consequently, no director
of our corporation will be personally liable to our corporation or its stockholders for monetary damages for any breach of fiduciary
duty by such a director as a director. However, notwithstanding the preceding sentence, a director will be liable to the extent provided
by the DGCL (1) for any breach of the director’s duty of loyalty to our corporation or its stockholders, (2) for acts
or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for payments of unlawful
dividends or for unlawful stock repurchases or redemption, or (4) for any transaction from which the director derived an improper
personal benefit.
We have entered into indemnification
agreements with each of our executive officers and directors. These agreements provide that, subject to limited exceptions and among
other things, we will indemnify each of our executive officers and directors to the fullest extent permitted by law and advance expenses
to each indemnitee in connection with any proceeding in which a right to indemnification is available.
We also maintain insurance
on behalf of any person who is or was our director, officer, trustee, employee or agent or serving at our request as a director, officer,
trustee, employee or agent of another corporation, partnership, joint venture, trust, non-profit entity or other enterprise against any
liability asserted against the person and incurred by the person in any such capacity, or arising out of his or her status as such.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted for directors, officers, or persons who control us, we have been informed
that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 15. Recent Sales
of Unregistered Securities
In the three years preceding
the filing of this registration statement, we have issued the following securities that were not registered under the Securities Act.
On November 16, 2022, we
entered into a subscription and investment representation agreement with Robert J. Bitterman, our President and Chief Executive Officer,
who is an accredited investor, pursuant to which we agreed to issue and sell one share of our Series D Preferred Stock, par value $0.0001
per share (the “Series D Preferred Stock”), to Mr. Bitterman for $1,750 in cash. The sale closed on November 16, 2022.
The share of our Series D Preferred Stock was entitled to at such time 17,500,000 votes per share exclusively with respect
to any proposal to amend our amended and restated certificate of incorporation to effect a reverse stock split of our Common Stock (“Reverse
Stock Split”). The terms of our Series D Preferred Stock provided that it would be voted, without action by the holder, on
any such proposal in the same proportion as shares of our Common Stock were voted. The share of our Series D Preferred Stock otherwise
had no voting rights except as otherwise required by the DGCL. Under its terms, the outstanding share of our Series D Preferred Stock
was to be redeemed in whole, but not in part, at any time: (i) if such redemption was approved by our Board of Directors in its sole
discretion or (ii) automatically and effective upon the approval by our stockholders of a Reverse Stock Split. Upon such redemption,
the holder of the share of our Series D Preferred Stock was entitled to receive consideration of $1,750 in cash. The share of our
Series D Preferred Stock was redeemed in whole on January 4, 2023, upon the approval by our stockholders of a Reverse Stock Split.
On April 18, 2023, we entered
into a securities purchase agreement relating to the registered direct offering and sale of 39,331 shares of our Common Stock at a purchase
price of $50.85 per share to certain accredited and institutional investors. In a concurrent private placement, we also issued warrants
with a five and one-half year term to purchase up to 39,331 shares of Common Stock at an exercise price of $48.60 per share and warrants
with an eighteen month term to purchase up to 39,331 shares of Common Stock at an exercise price of $48.60 per share to the same accredited
and institutional investors. In connection with this offering, we also issued warrants to purchase up to 2,950 shares of our Common Stock
at an exercise price of $63.56 to our placement agent.
On May 31, 2023, we entered
into a securities purchase agreement relating to (a) the registered direct offering and sale of 25,961 shares of our Common Stock at
a purchase price of $38.52 per share to certain accredited and institutional investors and (b) a concurrent private placement to the
same accredited and institutional investors, in which we issued 8,000 shares of unregistered Common Stock at a purchase price of $38.52
per share, unregistered pre-funded warrants to purchase up to 69,881 shares of Common Stock at a purchase price of $38.511 per share
and with an exercise price of $0.009 per share, unregistered warrants with a five and one-half year term to purchase up to 103,842 shares
of Common Stock at an exercise price of $36.27 per share and unregistered warrants with an eighteen month term to purchase up to 103,842
shares of Common Stock at an exercise price of $36.27 per share. In connection with this offering, we also issued warrants to purchase
up to 7,788 shares of our Common Stock at an exercise price of $48.15 per share to our placement agent.
On December 6, 2023, we entered
into an inducement letter agreement (the “December 2023 Inducement Letter Agreement”) with certain holders of certain
of our existing warrants to purchase up to an aggregate of 236,695 shares of our Common Stock originally issued to the holders on dates
between October 2018 and June 2023 with an exercise price of $48.60 or $36.27 per share, as applicable (the “December 2023 Existing
Warrants”). Pursuant to the December 2023 Inducement Letter Agreement, each of the holders agreed to exercise for cash
the December 2023 Existing Warrants at a reduced exercise price of $11.97 per share in consideration of our agreement to issue new unregistered
five and one-half year term warrants to purchase up to 252,258 shares of Common Stock at an exercise price of $9.72 per share and new
unregistered eighteen month term warrants to purchase up to 221,132 shares of Common Stock at an exercise price of $9.72 per share. In
connection with the December 2023 Inducement Letter Agreement, we also issued warrants to purchase up to 17,752 shares of our Common
Stock at an exercise price of $14.96 per share to our placement agent.
On July 11, 2024, we entered
into inducement letter agreements (the “July 2024 Inducement Letter Agreements”) with certain holders of certain of
our existing warrants to purchase up to an aggregate of 545,286 shares of our Common Stock, originally issued to the holders in February
2020 through December 2023, having exercise prices between $324.00 and $9.72 per share (the “July 2024 Existing Warrants”). Pursuant
to the July 2024 Inducement Letter Agreements, the holders agreed to exercise for cash the July 2024 Existing Warrants at a reduced exercise
price of $5.45 per share in consideration of our agreement to issue new unregistered five and one-half year term warrants to purchase
up to 583,098 shares of Common Stock at an exercise price of $5.45 per share and new unregistered eighteen month term warrants to purchase
up to 507,474 shares of Common Stock at an exercise price of $5.45 per share, each issued and sold at a price of $0.125 per warrant share.
In connection with the July 2024 Inducement Letter Agreements, we also issued warrants to purchase up to 40,896 shares of our Common
Stock at an exercise price of $6.8125 per share to our placement agent.
On December 19, 2024, we
entered into a securities purchase agreement relating to (a) the registered direct offering and sale of 437,192 shares of our Common
Stock at a purchase price of $2.635 per share to certain accredited and institutional investors and (b) a concurrent private placement
to the same accredited and institutional investors, in which we issued unregistered warrants with a five year term to purchase up to
437,192 shares of Common Stock at an exercise price of $2.51 per share. In connection with this offering, we also issued warrants to
purchase up to 32,789 shares of our Common Stock at an exercise price of $3.2938 per share to our placement agent.
On December 23, 2024, we entered
into a securities purchase agreement relating to (a) the registered direct offering and sale of 240,000 shares of our Common Stock at
a purchase price of $2.00 per share to certain accredited and institutional investors and (b) a concurrent private placement to the same
accredited and institutional investors, in which we issued unregistered warrants with a five year term to purchase up to 240,000 shares
of Common Stock at an exercise price of $2.00 per share. In connection with this offering, we also issued warrants to purchase up to
18,000 shares of our Common Stock at an exercise price of $2.50 per share to our placement agent.
On January 13, 2025, we entered
into a securities purchase agreement relating to (a) the registered direct offering and sale of 1,063,670 shares of our Common Stock
at a purchase price of $3.00 per share to certain accredited and institutional investors and (b) a concurrent private placement to the
same accredited and institutional investors, in which we issued unregistered warrants with a twenty-four month term to purchase up to
2,127,340 shares of Common Stock at an exercise price of $3.00 per share. In connection with this offering, we also issued warrants to
purchase up to 79,775 shares of our Common Stock at an exercise price of $3.75 per share to our placement agent.
On January 14, 2025, we entered
into a securities purchase agreement relating to (a) the registered direct offering and sale of 833,335 shares of our Common Stock at
a purchase price of $3.00 per share to certain accredited and institutional investors and (b) a concurrent private placement to the same
accredited and institutional investors, in which we issued unregistered warrants with a twenty-four month term to purchase up to 1,666,670
shares of Common Stock at an exercise price of $3.00 per share. In connection with this offering, we also issued warrants to purchase
up to 62,500 shares of our Common Stock at an exercise price of $3.75 per share to our placement agent.
On January 16, 2025, we entered
into a securities purchase agreement relating to (a) the registered direct offering and sale of 610,000 shares of our Common Stock at
a purchase price of $3.00 per share to certain accredited and institutional investors and (b) a concurrent private placement to the same
accredited and institutional investors, in which we issued unregistered warrants with a twenty-four month term to purchase up to 1,220,000
shares of Common Stock at an exercise price of $3.00 per share. In connection with this offering, we also issued warrants to purchase
up to 45,750 shares of our Common Stock at an exercise price of $3.75 per share to our placement agent.
Unless otherwise noted, all
of the transactions described in Item 15 were exempt from registration under the Securities Act pursuant to Section 4(a)(2) of the
Securities Act, or Regulation D promulgated thereunder, in that such sales did not involve a public offering.
Item 16. Exhibits
and Financial Statement Schedules
Exhibits
| Exhibit |
|
|
Incorporated by Reference Herein |
| Number |
Description |
|
Form |
|
Date |
| |
|
|
|
|
|
| 3.1 |
Amended
and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
November 19, 2018 |
| |
|
|
|
|
|
| 3.2 |
Certificate
of Amendment to the Amendment and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 14, 2020 |
| |
|
|
|
|
|
| 3.3 |
Certificate
of Amendment to the Amendment and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 25, 2023 |
| |
|
|
|
|
|
| 3.4 |
Certificate
of Designation of Series D Preferred Stock, dated November 16, 2022. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
November 16, 2022 |
| |
|
|
|
|
|
| 3.5 |
Certificate
of Amendment to the Amendment and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
July 2, 2024 |
| |
|
|
|
|
|
| 3.6 |
Amended
and Restated Bylaws of Phio Pharmaceutical Corp. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
May 2, 2022 |
| |
|
|
|
|
|
| 4.1 |
Form
of Warrant. |
|
Amendment No. 1 to the Registration Statement on Form S-1 (File No. 333-221173) |
|
September 28, 2018 |
| |
|
|
|
|
|
| 4.2 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
November 19, 2019 |
| |
|
|
|
|
|
| 4.3 |
Form
of Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
February 6, 2020 |
| |
|
|
|
|
|
| 4.4 |
Form
of Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
February 13, 2020 |
| |
|
|
|
|
|
| 4.5 |
Form
of Underwriter Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
February 13, 2020 |
| |
|
|
|
|
|
| 4.6 |
Form
of Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
April 2, 2020 |
| |
|
|
|
|
|
| 4.7 |
Form
of Common Stock Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 25, 2021 |
| |
|
|
|
|
|
| 4.8 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
February 17, 2021 |
| |
|
|
|
|
|
| 4.13 |
Form
of Series A/B Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 8, 2023 |
| |
|
|
|
|
|
| 4.14 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 8, 2023 |
| |
|
|
|
|
|
| 4.15 |
Form
of Series C/D Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
July 12, 2024 |
| |
|
|
|
|
|
| 4.16 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
July 12, 2024 |
| 4.17 |
Form
of Series E Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 20, 2024 |
| |
|
|
|
|
|
| 4.18 |
Form
of Series F Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 26, 2024 |
| 4.19 |
Form
of Series G Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 14, 2025 |
| |
|
|
|
|
|
| 4.20 |
Form
of Series H Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 15, 2025 |
| |
|
|
|
|
|
| 4.21 |
Form of Series I Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 17, 2025 |
| |
|
|
|
|
|
| 4.22 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 20, 2024 |
| |
|
|
|
|
|
| 4.23 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 26, 2024 |
| |
|
|
|
|
|
| 4.24 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 14, 2025 |
| |
|
|
|
|
|
| 4.25 |
Form
of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 15, 2025 |
| |
|
|
|
|
|
| 4.26 |
Form of Placement Agent Warrant. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 17, 2025 |
| |
|
|
|
|
|
| 4.27 |
Description
of Securities Registered Pursuant to Section 12(b) of the Securities Exchange Act of 1934. |
|
Annual Report on Form 10-K (File No. 001-36304) |
|
March 26, 2020 |
| |
|
|
|
|
|
| 5.1 |
Opinion of Hogan Lovells US LLP. * |
|
|
|
|
| |
|
|
|
|
|
| 10.1 |
Patent
and Technology Assignment Agreement between RXi Pharmaceuticals Corporation (formerly RNCS, Inc.) and Advirna, LLC, effective as
of September 24, 2011. |
|
Registration Statement on Form S-1 (File No. 333-177498) |
|
October 25, 2011 |
| |
|
|
|
|
|
| 10.2 |
Phio
Pharmaceuticals Corp. 2020 Long Term Incentive Plan, as amended and restated.# |
|
Current Report on Form 8-K (File No. 001-36304) |
|
June 21, 2024 |
| |
|
|
|
|
|
| 10.3 |
Form
of Restricted Stock Unit Award under the Company’s 2020 Long Term Incentive Plan.# |
|
Annual Report on Form 10-K (File. 001-36304) |
|
March 25, 2021 |
| |
|
|
|
|
|
| 10.4 |
Form
of Nonqualified Stock Option Award under the Company’s 2020 Long Term Incentive Plan.# |
|
Annual Report on Form 10-Q (File. 001-36304) |
|
November 9, 2023 |
| |
|
|
|
|
|
| 10.5 |
Phio
Pharmaceuticals Corp. 2012 Long Term Incentive Plan.# |
|
Quarterly Report on Form 10-Q (File No. 001-36304) |
|
November 12, 2019 |
| |
|
|
|
|
|
| 10.6 |
Form
of Incentive Stock Option Award under the Company’s 2012 Long Term Incentive Plan, as amended.# |
|
Registration Statement on Form S-1 (File No. 333-191236) |
|
September 18, 2013 |
| |
|
|
|
|
|
| 10.7 |
Form
of Non-Qualified Stock Option Award under the Company’s 2012 Long Term Incentive Plan, as amended.# |
|
Registration Statement on Form S-1 (File No. 333-191236) |
|
September 18, 2013 |
| |
|
|
|
|
|
| 10.8 |
RXi
Pharmaceuticals Corporation Employee Stock Purchase Plan.# |
|
Registration Statement on Form S-8 (File No. 333-277013) |
|
August 24, 2018 |
| |
|
|
|
|
|
| 10.9 |
Form
of Indemnification Agreement.# |
|
Amendment No. 3 to the Registration Statement on Form S-1 (File No. 333-177498) |
|
January 23, 2012 |
| 10.10 |
Employment
Agreement, dated February 20, 2023, by and between Phio Pharmaceuticals Corp. and Robert Bitterman.# |
|
Current Report on Form 8-K (File No. 001-36304) |
|
February 22, 2023 |
| |
|
|
|
|
|
| 10.11 |
Offer
Letter, executed July 16, 2024, by and between Phio Pharmaceuticals Corp. and Robert M. Infarinato. # |
|
Current Report on Form 8-K (File No. 001-36304) |
|
August 1, 2024 |
| |
|
|
|
|
|
| 10.12 |
Registration
Rights Agreement, dated January 21, 2021, by and between the Company and the Purchasers signatory therein. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 25, 2021 |
| |
|
|
|
|
|
| 10.13 |
Form
of Securities Purchase Agreement, dated April 18, 2023, by and between the Company and each of the Purchasers signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
April 20, 2023 |
| |
|
|
|
|
|
| 10.14 |
Form
of Securities Purchase Agreement, dated May 31, 2023, by and between the Company and each of the Purchasers signatory thereto (Registered
Direct Offering). |
|
Current Report on Form 8-K (File No. 001-36304) |
|
June 2, 2023 |
| |
|
|
|
|
|
| 10.15 |
Form
of Securities Purchase Agreement, dated May 31, 2023, by and between the Company and each of the Purchasers signatory thereto (PIPE
Private Placement). |
|
Current Report on Form 8-K (File No. 001-36304) |
|
June 2, 2023 |
| |
|
|
|
|
|
| 10.16 |
Form
of Registration Rights Agreement, dated May 31, 2023, by and between the Company and each of the Purchasers signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
June 2, 2023 |
| |
|
|
|
|
|
| |
|
|
|
|
|
| 10.17 |
Form
of Inducement Letter Agreement, dated July 11, 2024, by and between Phio Pharmaceuticals Corp. and the Holders. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
July 12, 2024 |
| |
|
|
|
|
|
| 10.18 |
Form
of Securities Purchase Agreement, dated December 19, 2024, by and between Phio Pharmaceuticals Corp. and the each of the Purchasers
signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 20, 2024 |
| |
|
|
|
|
|
| 10.19 |
Form
of Securities Purchase Agreement, dated December 23, 2024, by and between Phio Pharmaceuticals Corp. and the each of the Purchasers
signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
December 26, 2024 |
| |
|
|
|
|
|
| 10.20 |
Form
of Securities Purchase Agreement, dated January 13, 2025, by and between Phio Pharmaceuticals Corp. and the each of the Purchasers
signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 14, 2025 |
| |
|
|
|
|
|
| 10.21 |
Form
of Securities Purchase Agreement, dated January 14, 2025, by and between Phio Pharmaceuticals Corp. and the each of the Purchasers
signatory thereto. |
|
Current Report on Form 8-K (File No. 001-36304) |
|
January 15, 2025 |
| |
|
|
|
|
|
| * |
Filed herewith. |
| # |
Indicates a management contract or compensatory plan or arrangement. |
Financial Statement Schedules
All schedules have been omitted
because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related
notes thereto incorporated by reference herein.
Item 17. Undertakings
(a) The undersigned Registrant
hereby undertakes:
(1) To file, during any period
in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus
required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any
facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed
that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent
no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table
in the effective registration statement; and
(iii) To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information
in the registration statement;
Provided, however, that Paragraphs
(a)(1)(i), (ii), and (iii) of this section do not apply if the information required to be included in a post-effective amendment by those
paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d)
of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)) that are incorporated by reference in the registration statement.
(2) That, for the purpose
of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof;
(3) To remove from registration
by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(4) That, for the purpose
of determining liability under the Securities Act of 1933 to any purchaser:
(i) Each prospectus filed by the Registrant
pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part
of and included in the registration statement; and
(ii) Each prospectus required to be
filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering
made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities
Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus
is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus.
As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be
deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that
prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided,
however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement
will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made
in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior
to such effective date;
(6) Insofar as indemnification
for liabilities arising under the Securities Act of 1933, as amended may be permitted to directors, officers and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable. In
the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or
paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted
by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the
opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question
whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of
such issue.
(b) The undersigned Registrant
hereby undertakes that:
(i) For purposes of determining
any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration
statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4)
or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective.
(ii) For the purpose of determining
any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be
a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
Signatures
Pursuant to the requirements
of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement on Form S-1 to be signed on its
behalf by the undersigned, thereunto duly authorized, in Marlborough, Massachusetts, on January 21, 2025.
| |
PHIO PHARMACEUTICALS CORP. |
| |
|
|
| |
By: |
|
/s/ Robert Bitterman |
| |
|
|
Robert Bitterman |
| |
|
|
President and Chief Executive Officer |
Power of Attorney
KNOW ALL PERSONS BY THESE
PRESENTS, that each person whose signature appears below constitutes and appoints Robert Bitterman as attorney-in-fact, with power of
substitution, in any and all capacities, to sign any and all amendments and post-effective amendments to this registration statement,
and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission,
hereby ratifying and confirming all that said attorney-in-fact, or his substitute or substitutes, may do or cause to be done by virtue
thereof.
Pursuant to the requirements
of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates
indicated.
| Signatures |
Title |
Date |
| |
|
|
/s/ Robert
J. Bitterman
Robert J. Bitterman |
President, Chief Executive Officer and Director
(Principal Executive Officer) |
January 21, 2025 |
| |
|
|
/s/ Robert
Infarinato
Robert Infarinato |
Vice President, Chief Financial Officer
(Principal Financial Officer and Principal
Accounting Officer) |
January 21, 2025 |
| |
|
|
/s/ Patricia
Bradford
Patricia Bradford |
Director |
January 21, 2025 |
/s/ Robert
L. Ferrara
Robert L. Ferrara |
Director |
January 21, 2025 |
| |
|
|
/s/ Jonathan
E. Freeman
Jonathan E. Freeman, Ph.D. |
Director |
January 21, 2025 |
| |
|
|
/s/ Curtis
A. Lockshin
Curtis A. Lockshin, Ph.D. |
Director |
January 21, 2025 |
Exhibit 5.1
 |
Hogan Lovells US LLP
1735 Market Street, Floor 23
Philadelphia, PA 19103
T +1 267 675 4600
F +1 267 675 4601
www.hoganlovells.com |
January 21, 2025
Board of Directors
Phio Pharmaceuticals Corp.
11 Apex Drive, Suite 300A, PMB 2006
Marlborough, MA 01752
Ladies and Gentlemen:
We are acting as counsel to Phio Pharmaceuticals Corp.,
a Delaware corporation (the “Company”), in connection with its registration statement on Form S-1 (the “Registration
Statement”), filed with the Securities and Exchange Commission under the Securities Act of 1933, as amended (the “Act”),
relating to the resale or other disposition, from time to time, by the selling stockholders listed in the Registration Statement (the
“Selling Stockholders”), including their transferees, pledgees, donees or successors, of up to 5,930,016 shares of common
stock (the “Common Stock”), par value $0.0001 per share, consisting of up to 5,930,016 shares of Common Stock (the “Warrant
Shares”) issuable upon exercise of warrants to purchase 5,930,016 shares of Common Stock (the “Warrants”), issued by
the Company to the Selling Stockholders on December 20, 2024, December 24, 2024, January 14, 2025, January 15, 2025 and January 17, 2025,
as described in the prospectus that forms a part of the Registration Statement (the “Prospectus”). This opinion letter is
furnished to you at your request to enable you to fulfill the requirements of Item 601(b)(5) of Regulation S-K, 17 C.F.R. § 229.601(b)(5),
in connection with the Registration Statement.
For purposes of this opinion letter, we have examined
copies of such agreements, instruments and documents as we have deemed an appropriate basis on which to render the opinions hereinafter
expressed, including the Warrants. In our examination of the aforesaid documents, we have assumed the genuineness of all signatures, the
legal capacity of all natural persons, the accuracy and completeness of all documents submitted to us, the authenticity of all original
documents, and the conformity to authentic original documents of all documents submitted to us as copies (including pdfs). As to all matters
of fact, we have relied on the representations and statements of fact made in the documents so reviewed, and we have not independently
established the facts so relied on. This opinion letter is given, and all statements herein are made, in the context of the foregoing.
This opinion letter is based as to matters of
law solely on the Delaware General Corporation Law, as amended. We express no opinion herein as to any other statutes, rules or regulations.
Based upon, subject to and limited by the foregoing,
we are of the opinion that, as of the date hereof, (a) Warrant Shares have been duly authorized by all necessary corporate action on the
part of the Company and (b) with respect to the Warrant Shares, following (i) the exercise of the Warrants in accordance with their terms
and (ii) receipt by the Company of the consideration for the Warrant Shares specified in the Warrants, the Warrant Shares will be validly
issued, fully paid, and nonassessable.
This opinion letter has been prepared for use
in connection with the Registration Statement. We assume no obligation to advise of any changes in the foregoing subsequent to the effective
date of the Registration Statement.
We hereby consent to the filing of this opinion
letter as Exhibit 5.1 to the Registration Statement and to the reference to this firm under the caption “Legal Matters” in
the Prospectus. In giving this consent, we do not thereby admit that we are an “expert” within the meaning of the Act.
Very truly yours,
/s/ HOGAN LOVELLS US LLP
Exhibit 23.1
Consent of Independent Registered Public Accounting
Firm
Phio Pharmaceuticals Corp.
Marlborough, Massachusetts
We hereby consent to the inclusion in this Registration Statement of Phio
Pharmaceuticals Corp. (the Company) of our report dated April 1, 2024, except for the impact of the 2024 reverse stock split as described
in Note 1, as to which the date is January 21, 2025, relating to the consolidated financial statements which appears in this Annual Report
on Form 10-K for the year ended December 31, 2023. Our report contains an explanatory paragraph regarding the Company’s ability
to continue as a going concern.
We also consent to the reference to us under the caption “Experts”
in the Prospectus.
/s/ BDO USA, P.C.
Boston, Massachusetts
January 21, 2025
Exhibit 107
Calculation of
Filing Fee Tables
Form S-1
(Form Type)
Phio Pharmaceuticals
Corp.
(Exact Name of Registrant
as Specified in Its Charter)
Table 1: Newly
Registered and Carry Forward Securities
| |
|
Security
Type
|
|
Security
Class
Title
|
|
Fee
Calculation
or Carry Forward
Rule
|
|
Amount
Registered (1)
|
|
Proposed
Maximum
Offering
Price per
Share (2)
|
|
Maximum
Aggregate
Offering
Price (1)
|
|
Fee
Rate
|
|
Amount
of Registration Fee |
|
Carry
Forward Form Type |
|
Carry Forward
File
Number |
|
Carry Forward
Initial
Effective
Date |
|
Filing Fee
Previously
Paid in
Connection
with
Unsold
Securities
to be
Carried
Forward |
| Newly
Registered Securities |
| Fees
to be Paid |
|
Equity |
|
Common
Stock, par value $0.0001 per share |
|
Rule
457(c) |
|
5,930,016 |
|
$2.75 |
|
$16,307,544.00 |
|
$153.10
per million |
|
$2,496.68 |
|
- |
|
- |
|
- |
|
- |
| Fees
Previously Paid |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
| Carry
Forward Securities |
| Carry
Forward Securities |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
|
|
|
|
- |
|
- |
|
- |
|
- |
| |
|
Total
Offering Amounts |
|
|
|
$16,307,544.00 |
|
|
|
$2,496.68 |
|
|
|
|
|
|
|
|
| |
|
Total
Fees Previously Paid |
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
| |
|
Total
Fee Offsets |
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
| |
|
Net
Fee Due |
|
|
|
|
|
|
|
$2,496.68 |
|
|
|
|
|
|
|
|
| (1) |
Pursuant to Rule 416 under the Securities Act of 1933, as amended, or the Securities Act, this registration
statement also covers any additional securities that may be offered, issued or become issuable in connection with any stock split,
stock dividend or similar transaction or pursuant to anti-dilution provisions of any of the securities. |
| (2) |
Estimated solely for the purpose of calculation of the registration fee
pursuant to Rule 457(c) under the Securities Act based on a per share price of $2.75, the average of the high and low reported sales prices
of the registrant’s common stock on the Nasdaq Capital Market on January 17, 2025. |
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) - USD ($)
$ in Thousands |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 5,390
|
$ 8,490
|
| Prepaid expenses and other current assets |
474
|
832
|
| Total current assets |
5,864
|
9,322
|
| Right of use asset |
0
|
33
|
| Property and equipment, net |
1
|
6
|
| Other assets |
0
|
3
|
| Total assets |
5,865
|
9,364
|
| Current liabilities: |
|
|
| Accounts payable |
184
|
657
|
| Accrued expenses |
735
|
942
|
| Lease liability |
0
|
35
|
| Total current liabilities |
919
|
1,634
|
| Commitments and contingencies (Note 2) |
|
|
| Stockholders’ equity: |
|
|
| Preferred stock, $0.0001 par value, 100,000,000 shares authorized; no shares issued and outstanding at September 30, 2024 and December 31, 2023 |
0
|
0
|
| Common stock, $0.0001 par value, 100,000,000 shares authorized; 958,219 and 416,368 shares issued and outstanding at September 30, 2024 and December 31, 2023, respectively |
0
|
0
|
| Additional paid-in capital |
149,676
|
146,936
|
| Accumulated deficit |
(144,730)
|
(139,206)
|
| Total stockholders’ equity |
4,946
|
7,730
|
| Total liabilities and stockholders’ equity |
$ 5,865
|
$ 9,364
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (Parenthetical) - $ / shares
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred Stock, Par or Stated Value Per Share |
|
$ 0.0001
|
| Preferred Stock, Shares Authorized |
|
100,000,000
|
| Preferred Stock, Shares Outstanding |
|
0
|
| Common Stock, Par or Stated Value Per Share |
|
$ 0.0001
|
| Common Stock, Shares Authorized |
|
100,000,000
|
| Common Stock, Shares, Outstanding |
958,219
|
416,368
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Operating expenses: |
|
|
|
|
| Research and development |
$ 644
|
$ 1,808
|
$ 2,658
|
$ 5,325
|
| General and administrative |
946
|
968
|
3,055
|
3,600
|
| Total operating expenses |
1,590
|
2,776
|
5,713
|
8,925
|
| Operating loss |
(1,590)
|
(2,776)
|
(5,713)
|
(8,925)
|
| Total other income (expense), net |
66
|
(4)
|
189
|
(6)
|
| Net loss |
$ (1,524)
|
$ (2,780)
|
$ (5,524)
|
$ (8,931)
|
| Net loss per common share: |
|
|
|
|
| Earnings Per Share, Diluted |
$ (1.54)
|
$ (10.25)
|
$ (8.23)
|
$ (45.28)
|
| Weighted average number of common shares outstanding |
|
|
|
|
| Weighted Average Number of Shares Outstanding, Diluted |
990,033
|
271,129
|
670,875
|
197,227
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
CONDENSED CONSOLIDATED STATEMENTS OF PREFERRED STOCK AND STOCKHOLDERS' EQUITY (Unaudited) - USD ($)
$ in Thousands |
Preferred Stock Series D [Member] |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Total |
| Beginning balance, value at Dec. 31, 2022 |
$ 2
|
|
$ 139,218
|
$ (128,380)
|
$ 10,838
|
| Balance at beginning, shares at Dec. 31, 2022 |
1
|
126,558
|
|
|
|
| Cash-in-lieu of fractional shares for reverse stock split |
|
|
11
|
|
11
|
| Cash-in-lieu of fractional shares for reverse stock split, shares |
|
(190)
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units |
|
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units, shares |
|
2,009
|
|
|
|
| Shares withheld for payroll taxes |
|
|
(25)
|
|
(25)
|
| Shares withheld for payroll taxes, shares |
|
(535)
|
|
|
|
| Stock-based compensation expense |
|
|
111
|
|
111
|
| Net loss |
|
|
|
(3,602)
|
(3,602)
|
| Cash-in-lieu of fractional shares for reverse stock split |
|
|
(11)
|
|
(11)
|
| Redemption of preferred stock |
$ (2)
|
|
|
|
|
| Redemption of preferred stock, shares |
(1)
|
|
|
|
|
| Ending balance, value at Mar. 31, 2023 |
|
|
139,293
|
(131,982)
|
7,311
|
| Balance at ending, shares at Mar. 31, 2023 |
0
|
127,842
|
|
|
|
| Beginning balance, value at Dec. 31, 2022 |
$ 2
|
|
139,218
|
(128,380)
|
10,838
|
| Balance at beginning, shares at Dec. 31, 2022 |
1
|
126,558
|
|
|
|
| Net loss |
|
|
|
|
(8,931)
|
| Ending balance, value at Sep. 30, 2023 |
|
|
144,524
|
(137,311)
|
7,213
|
| Balance at ending, shares at Sep. 30, 2023 |
0
|
256,375
|
|
|
|
| Beginning balance, value at Mar. 31, 2023 |
|
|
139,293
|
(131,982)
|
7,311
|
| Balance at beginning, shares at Mar. 31, 2023 |
0
|
127,842
|
|
|
|
| Issuance of common stock and warrants, net of offering costs |
|
|
5,048
|
|
5,048
|
| Issuance of common stock upon exercise of warrants |
|
|
|
|
|
| Issuance of common stock upon exercise of warrants, shares |
|
19,444
|
|
|
|
| Stock-based compensation expense |
|
|
94
|
|
94
|
| Net loss |
|
|
|
(2,549)
|
(2,549)
|
| Issuance of common stock and warrants, net of offering costs, shares |
|
73,292
|
|
|
|
| Ending balance, value at Jun. 30, 2023 |
|
|
144,435
|
(134,531)
|
9,904
|
| Balance at ending, shares at Jun. 30, 2023 |
0
|
220,578
|
|
|
|
| Issuance of common stock upon exercise of warrants |
|
|
|
|
|
| Issuance of common stock upon exercise of warrants, shares |
|
35,588
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units |
|
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units, shares |
|
222
|
|
|
|
| Shares withheld for payroll taxes |
|
|
|
|
|
| Shares withheld for payroll taxes, shares |
|
(13)
|
|
|
|
| Stock-based compensation expense |
|
|
89
|
|
89
|
| Net loss |
|
|
|
(2,780)
|
(2,780)
|
| Ending balance, value at Sep. 30, 2023 |
|
|
144,524
|
(137,311)
|
7,213
|
| Balance at ending, shares at Sep. 30, 2023 |
0
|
256,375
|
|
|
|
| Beginning balance, value at Dec. 31, 2023 |
|
|
146,936
|
(139,206)
|
7,730
|
| Balance at beginning, shares at Dec. 31, 2023 |
0
|
416,368
|
|
|
|
| Issuance of common stock upon exercise of warrants |
|
|
|
|
|
| Issuance of common stock upon exercise of warrants, shares |
|
91,820
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units |
|
|
|
|
|
| Issuance of common stock upon vesting of restricted stock units, shares |
|
2,689
|
|
|
|
| Shares withheld for payroll taxes |
|
|
(4)
|
|
(4)
|
| Shares withheld for payroll taxes, shares |
|
(689)
|
|
|
|
| Stock-based compensation expense |
|
|
32
|
|
32
|
| Net loss |
|
|
|
(2,154)
|
(2,154)
|
| Ending balance, value at Mar. 31, 2024 |
|
|
146,964
|
(141,360)
|
5,604
|
| Balance at ending, shares at Mar. 31, 2024 |
0
|
510,188
|
|
|
|
| Beginning balance, value at Dec. 31, 2023 |
|
|
146,936
|
(139,206)
|
7,730
|
| Balance at beginning, shares at Dec. 31, 2023 |
0
|
416,368
|
|
|
|
| Net loss |
|
|
|
|
(5,524)
|
| Ending balance, value at Sep. 30, 2024 |
|
|
149,676
|
(144,730)
|
4,946
|
| Balance at ending, shares at Sep. 30, 2024 |
0
|
958,219
|
|
|
|
| Beginning balance, value at Mar. 31, 2024 |
|
|
146,964
|
(141,360)
|
5,604
|
| Balance at beginning, shares at Mar. 31, 2024 |
0
|
510,188
|
|
|
|
| Stock-based compensation expense |
|
|
15
|
|
15
|
| Net loss |
|
|
|
(1,846)
|
(1,846)
|
| Ending balance, value at Jun. 30, 2024 |
|
|
146,979
|
(143,206)
|
3,773
|
| Balance at ending, shares at Jun. 30, 2024 |
0
|
510,188
|
|
|
|
| Cash-in-lieu of fractional shares for reverse stock split |
|
|
(1)
|
|
(1)
|
| Cash-in-lieu of fractional shares for reverse stock split, shares |
|
(255)
|
|
|
|
| Issuance of common stock and warrants, net of offering costs |
|
|
2,646
|
|
2,646
|
| Issuance of common stock upon exercise of warrants |
|
|
|
|
|
| Issuance of common stock upon exercise of warrants, shares |
|
231,758
|
|
|
|
| Stock-based compensation expense |
|
|
52
|
|
52
|
| Net loss |
|
|
|
(1,524)
|
(1,524)
|
| Issuance of common stock and warrants, net of offering costs, shares |
|
216,528
|
|
|
|
| Cash-in-lieu of fractional shares for reverse stock split |
|
|
1
|
|
1
|
| Ending balance, value at Sep. 30, 2024 |
|
|
$ 149,676
|
$ (144,730)
|
$ 4,946
|
| Balance at ending, shares at Sep. 30, 2024 |
0
|
958,219
|
|
|
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of decrease in additional paid in capital (APIC) resulting from a stock split in which per-share par value or stated value is not changed proportionately. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares used to settle grantee's tax withholding obligation for award under share-based payment arrangement.
| Name: |
us-gaap_SharesPaidForTaxWithholdingForShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period related to Restricted Stock Awards, net of any shares forfeited. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardNetOfForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionReduction in the number of shares during the period as a result of a reverse stock split. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodSharesReverseStockSplits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock related to Restricted Stock Awards issued during the period, net of the stock value of such awards forfeited. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardNetOfForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.4
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (Unaudited) - USD ($)
$ in Thousands |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Cash flows from operating activities: |
|
|
| Net loss |
$ (5,524)
|
$ (8,931)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation and amortization |
2
|
46
|
| Amortization of right of use asset |
33
|
95
|
| Loss on disposal of property and equipment |
3
|
0
|
| Stock-based compensation |
99
|
294
|
| Changes in operating assets and liabilities: |
|
|
| Prepaid expenses and other assets |
361
|
(235)
|
| Accounts payable |
(473)
|
(606)
|
| Accrued expenses |
(207)
|
1,058
|
| Lease liability |
(35)
|
(100)
|
| Net cash used in operating activities |
(5,741)
|
(8,379)
|
| Cash flows from investing activities: |
|
|
| Cash paid for purchase of property and equipment |
0
|
(5)
|
| Net cash used in investing activities |
0
|
(5)
|
| Cash flows from financing activities: |
|
|
| Net proceeds from the issuance of common stock and warrants |
2,646
|
5,048
|
| Cash in lieu of fractional shares for reverse stock split |
(1)
|
(11)
|
| Redemption of Series D preferred stock |
0
|
(2)
|
| Payment of taxes on net share settlements of restricted stock units |
(4)
|
(25)
|
| Net cash provided by financing activities |
2,641
|
5,010
|
| Net decrease in cash, cash equivalents and restricted cash |
(3,100)
|
(3,374)
|
| Cash, cash equivalents and restricted cash at the beginning of period |
8,490
|
11,831
|
| Cash, cash equivalents and restricted cash at the end of period |
$ 5,390
|
$ 8,457
|
| X |
- References
+ Details
| Name: |
PHIO_CashInLieuOfFractionalSharesForReverseStockSplit |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, including oil and gas property and timber property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_GainLossOnSaleOfPropertyPlantEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for reacquisition of callable preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfRedeemablePreferredStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.4
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_RestrictedCash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Pay vs Performance Disclosure - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
|
|
|
| Net Income (Loss) |
$ (1,524)
|
$ (1,846)
|
$ (2,154)
|
$ (2,780)
|
$ (2,549)
|
$ (3,602)
|
$ (5,524)
|
$ (8,931)
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.4
Organization and Significant Accounting Policies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Organization and Significant Accounting Policies |
1.
Organization and Significant Accounting Policies
Nature
of Operations
Phio Pharmaceuticals Corp.
(“Phio” or the “Company”) is a clinical stage biotechnology company whose proprietary INTASYL®
small interfering RNA gene silencing technology is designed to make immune cells more effective in killing tumor cells. The Company is
developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that reduce the body’s ability
to fight cancer, without the need for specialized formulations or drug delivery systems.
Phio was incorporated in
the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals
Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology
therapeutics.
Effective
July 5, 2024, the Company completed a 1-for-9
reverse stock split of the Company’s outstanding common stock, including
reclassifying an amount equal to the reduction in par value to additional paid-in capital. The reverse stock split did not reduce the
number of authorized shares of the Company’s common or preferred stock. All share and per share amounts have been adjusted to give
effect to the reverse stock split.
Basis
of Presentation
The accompanying condensed
consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in
the United States (“GAAP”). Certain information and footnote disclosures that are included in the Company’s
annual consolidated financial statements, but that are not required for interim reporting purposes, have been condensed or omitted. In
the opinion of management, all adjustments (including normal recurring accruals) considered necessary for a fair presentation of the
condensed consolidated financial statements have been included.
These statements should be
read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s most recent
Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”)
on April 1, 2024 (the “2023 Form 10-K”). Interim results are not necessarily indicative of results for a full year.
Principles
of Consolidation
The condensed consolidated
financial statements include the accounts of the Company and its wholly-owned subsidiary, MirImmune, LLC. All material intercompany accounts
have been eliminated in consolidation.
Segments
The Company operates as one operating
segment and all assets are located in the United States.
Use
of Estimates
The preparation of financial
statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of
revenues and expenses during the reporting period. The areas subject to significant estimates and judgement include, among others, those
related to the fair value of equity awards, accruals for research and development expenses, useful lives of property and equipment, and
the valuation allowance on the Company’s deferred tax assets. On an ongoing basis the Company evaluates its estimates and bases
its estimates on historical experience and other relevant assumptions that the Company believes are reasonable under the circumstances.
Actual results could differ materially from these estimates.
Liquidity
The Company has reported
recurring losses from operations since its inception and expects to continue to have negative cash flows from operations for the foreseeable
future. Historically, the Company’s primary source of funding has been from sales of its securities. The Company’s ability
to continue to fund its operations is dependent on obtaining funding from third parties, such as proceeds from the issuance of debt,
sale of equity, or strategic opportunities, in order to maintain its operations. This is dependent on a number of factors, including
the market demand or liquidity of the Company’s common stock. There is no guarantee that debt, additional equity or other funding
will be available to the Company on acceptable terms, or at all. If the Company fails to obtain additional funding when needed, the Company
would be forced to scale back or terminate its operations or seek to merge with or to be acquired by another company.
The Company has limited cash
resources, has reported recurring losses from operations since inception, has negative operating cash flows and has not yet received
product revenues. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern, and the
Company’s current cash resources may not provide sufficient capital to fund operations for at least the next 12 months from the
date of the release of these condensed consolidated financial statements. The continuation of the Company as a going concern depends
upon the Company’s ability to raise additional capital through an equity offering, debt offering and/or strategic opportunity to
fund its operations. There can be no assurance that the Company will be successful in accomplishing these plans in order to continue
as a going concern. These condensed consolidated financial statements do not include any adjustments to the recoverability and classification
of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going
concern.
Summary
of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents
include unrestricted cash accounts, money market investments and highly liquid investment instruments with original maturity of three
months or less at the date of purchase.
Other than as set forth above,
there have been no material changes to the significant accounting policies disclosed in the Company’s 2023 Form 10-K.
Recent
Accounting Pronouncements
In November 2023, the Financial
Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2023-07, “Segment
Reporting (Topic 280) – Improvements to Reporting Segment Disclosures” (“ASU 2023-07”), which requires
disclosure of incremental segment information on an annual and interim basis. In addition, ASU 2023-07 clarifies circumstances in which
an entity can disclose multiple segment measures of profit or loss, provides new segment disclosure requirements for entities with a
single reportable segment, and contains other disclosure requirements. The amendments in ASU 2023-07 are effective for fiscal years beginning
after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The
enhanced disclosures are required to be applied retrospectively to all prior periods presented in the financial statements. The Company
is currently evaluating the impact of ASU 2023-07 on its consolidated financial statements and disclosures, but does not expect that
it will have a material impact on its consolidated financial statements.
In December 2023, the FASB
issued ASU 2023-09, “Income Taxes (Topic 740) – Improvements to Income Tax Disclosures” (“ASU 2023-09”),
which requires disclosure of specific categories in the rate reconciliation table along with additional information for reconciling items
that meet a quantitative threshold, disclosure of disaggregated income taxes paid and modifies other income tax-related disclosures.
The amendments in ASU 2023-09 are effective for annual periods beginning after December 15, 2024 and allows for adoption on a prospective
basis, with a retrospective option. Early adoption is permitted. The Company is currently evaluating the impact of ASU 2023-09, but does
not expect that it will have a material impact on its consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the general note to the financial statements for the reporting entity which may include, descriptions of the basis of presentation, business description, significant accounting policies, consolidations, reclassifications, new pronouncements not yet adopted and changes in accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 250
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/250/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationBasisOfPresentationBusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Collaboration Agreement
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Collaboration Agreement |
2. Collaboration
Agreement
AgonOx, Inc. (“AgonOx”)
In
February 2021, the Company entered into a clinical co-development collaboration agreement (the “Clinical Co-Development Agreement”)
with AgonOx, a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to
cancer. On May 8, 2024, the Company terminated the Clinical Co-Development Agreement with AgonOx, which such termination was effective
immediately. Under the Clinical Co-Development Agreement, Phio and AgonOx were working to develop a T cell-based therapy using the Company’s
lead product candidate, PH-762, and AgonOx’s “double positive” tumor infiltrating lymphocytes (“DP TIL”)
technology. Per the terms of the Clinical Co-Development Agreement, the Company had agreed to reimburse AgonOx up to $4,000,000
in expenses incurred to conduct a Phase 1 clinical trial of PH-762 treated DP TIL in patients with advanced melanoma and other
advanced solid tumors and was entitled to certain future development milestones and low single-digit sales-based royalty payments from
AgonOx licensing its DP TIL technology.
The
Company recognized its share of costs arising from research and development activities performed by AgonOx in the Company’s condensed
consolidated financial statements in the period AgonOx incurred such expense. Effective as of the date of termination, the Clinical Co-Development
Agreement and the continuing obligations of the Company and AgonOx thereunder were terminated in their entirety. As a result, the Company
is no longer required to provide financial support for the development costs incurred under the Clinical Co-Development Agreement, and
is not entitled to future development milestones or royalty payments from AgonOx’s licensing of its DP TIL technology.
The
Company will pay to AgonOx all Company payment obligations that accrued prior to the termination of the Clinical Co-Development Agreement.
Remaining payments to be made to AgonOx as of September 30, 2024 were $35,000, which primarily related to remaining accrued obligations
for patient fees and other miscellaneous costs as of the date of termination. Pursuant to the terms of the Clinical Co-Development Agreement,
the Company and AgonOx are coordinating the orderly wind-down of the Phase 1 clinical trial. Each of the Company and AgonOx shall be
responsible for its own costs and expenses incurred in connection with the wind-down of the Phase 1 clinical trial.
The
Company recognized approximately $308,000
and $414,000
in connection with the Clinical Co-Development Agreement during the three and nine months ended September 30, 2024, respectively,
which relate to the Company’s expense obligations under the Clinical Co-Development Agreement through the date of termination.
The Company recognized approximately $606,000
and $906,000
of expense in connection with the Clinical Co-Development Agreement during the three and nine months ended September 30, 2023,
respectively.
|
| X |
- DefinitionThe entire disclosure for collaborative arrangements in which the entity is a participant, including a) information about the nature and purpose of such arrangements; b) its rights and obligations thereunder; c) the accounting policy for collaborative arrangements; and d) the income statement classification and amounts attributable to transactions arising from the collaborative arrangement between participants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org/808/tableOfContent
| Name: |
us-gaap_CollaborativeArrangementDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Fair Value of Financial Instruments
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value of Financial Instruments |
3. Fair
Value of Financial Instruments
The Company follows the provisions
of the FASB Accounting Standards Codification (“ASC”) Topic 820, “Fair Value Measurement,” for
the Company’s financial assets and liabilities that are re-measured and reported at fair value each reporting period and are re-measured
and reported at fair value at least annually using a fair value hierarchy that is broken down into three levels. Level inputs are defined
as follows:
Level 1 – quoted prices
in active markets for identical assets or liabilities.
Level 2 – other significant
observable inputs for the assets or liabilities through corroboration with market data at the measurement date.
Level 3 – significant
unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities
at the measurement date.
As of September 30, 2024,
the Company categorized its cash equivalents as Level 1 hierarchy as the carrying amounts approximate their fair value due to their short-term
nature and market rates of interest. As of December 31, 2023, the Company did not identify any financial instruments required to be presented
at fair value.
| Schedule of financial instruments at fair value | |
| | | |
| | | |
| | | |
| | |
| Description | |
September 30, 2024 | |
Quoted
Prices In
Active Markets
(Level 1) | |
Other Significant
Observable Inputs
(Level 2) | |
Unobservable
Inputs
(Level 3) |
| Assets: | |
| | | |
| | | |
| | | |
| | |
| Cash equivalents | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
| Total | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
The carrying amounts of cash,
accounts payable and accrued expenses of the Company approximate their fair values due to their short-term nature.
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Leases
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Leases |
|
| Leases |
4.
Leases
The Company entered into
a lease for a laboratory facility located at 17 Briden Street, Worcester, Massachusetts, which covers 321
square feet of rentable space. The lease commenced on March 1, 2024 and had an original expiration date of August
31, 2024. The Company has the option to renew the lease for additional 6-month periods. On June 1, 2024, the Company elected the
option to renew the lease for an additional 6-month period, and the lease will expire on February 28, 2025. The Company made an accounting
policy election under the FASB ASC Topic 842, “Leases” not to recognize leases with a term less than one year on the
balance sheet and that do not contain a purchase option. Under the short-term lease election, the Company will recognize the lease payments
for the laboratory facility on a straight-line basis over the lease term.
The total base rent for the
premises over each 6-month term is expected to be $15,000.
During the three and nine month periods ending September 30, 2024, the Company recognized $8,129
and $18,909,
respectively, of rent expense and variable lease costs related to the laboratory facility.
The Company’s lease
for its corporate headquarters and primary research facility in Marlborough, Massachusetts was for a total of 7,581
square feet of office and laboratory space and expired on March
31, 2024. The lease agreement did not contain information to determine the borrowing rate implicit in the lease. As such, the
Company calculated its incremental borrowing rate based on what the Company would have to pay to borrow on a collateralized basis over
the lease term for an amount equal to the remaining lease payments, taking into consideration such assumptions as, but not limited to,
the U.S. treasury yield rate and borrowing rates from a creditworthy financial institution using the above lease factors. The Company
has continued operations as a primarily remote business with the expiration of the lease, but has contracted a private mailbox with an
address of 11 Apex Drive, Suite 300A, PMB 2006, Marlborough, MA 01752 to use as its principal mailing address for SEC and other purposes.
The lease for the Company’s
former corporate headquarters represented all of the Company’s capitalized lease obligations.
The amounts reported in the
condensed consolidated balance sheets for the Company’s former corporate headquarters classified as an operating lease in which
the Company is the lessee and other supplemental balance sheet information is set forth as follows, in thousands, except the lease term
(number of years) and discount rate:
| Schedule of lease amounts recorded in balance sheet |
|
|
|
|
| |
|
September 30,
2024 |
|
December 31,
2023 |
| Assets |
|
|
|
|
|
|
|
|
| Right of use asset |
|
$ |
– |
|
|
$ |
33 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Lease liability |
|
$ |
– |
|
|
$ |
35 |
|
| Lease Term and Discount Rate |
|
|
|
|
|
|
|
|
| Weighted average remaining lease term |
|
|
– |
|
|
|
0.25 |
|
| Weighted average discount rate |
|
|
– |
|
|
|
4.70% |
|
There were no operating
lease costs for our former corporate headquarters included in operating expense for the three months ended September 30, 2024.
Operating lease costs for our former corporate headquarters included in operating expense were $33,000 for
the three months ended September 30, 2023. Operating expenses were $33,000 and
$99,000 for the nine months ended
September 30, 2024 and 2023, respectively.
The Company’s condensed
consolidated statements of cash flows for the Company’s former corporate headquarters was $35,000
and $104,000 for the nine months
ended September 30, 2024 and 2023, respectively.
|
| X |
- References
+ Details
| Name: |
PHIO_DisclosureLeasesAbstract |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Stockholders’ Equity
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Stockholders’ Equity |
5.
Stockholders’ Equity
Financings
May 2024 Financing
— On May 16, 2024, the Company entered into a purchase agreement (the “Purchase Agreement”) with Triton Funds
LP (“Triton”), pursuant to which the Company agreed to sell, and Triton agreed to purchase, upon the Company’s
request in one or more transactions, up to 95,833
shares of the Company’s common stock at a purchase price of $6.48
per share (the “Purchase Price”), for aggregate gross proceeds of up to $621,000.
The Company recorded expense of approximately $100,000, primarily related to legal fees, in connection with the execution of the Purchase
Agreement with Triton. On July 3, 2024, the Company terminated the Purchase Agreement with Triton effective immediately. No shares of
common stock were sold by the Company pursuant to the Purchase Agreement prior to termination.
July 2024 Financing
— On July 11, 2024, the Company entered into inducement letter agreements (the “July 2024 Inducement Letter Agreements”)
with certain holders of certain of the Company’s existing warrants to purchase up to an aggregate of 545,286
shares of the Company’s common stock. The existing warrants were originally issued in February 2020 through December 2023,
having exercise prices between $324.00 and $9.72 per share. Pursuant to the July 2024 Inducement Letter Agreements, these warrants were
exercised for cash at a reduced exercise of $5.45
per share in consideration of the Company’s agreement to issue new unregistered five and one-half year term Series C warrants
to purchase up to 583,098
shares of common stock at an exercise price of $5.45
and new unregistered eighteen month term Series D warrants to purchase up to 507,474
shares of common stock at an exercise price of $5.45,
both issued and sold at a price of $0.125 per warrant share (the “July 2024 Financing”). In addition, the Company
issued warrants to the placement agent, HCW, to purchase a total of 40,896 shares of common stock at an exercise price of $6.8125 per
share. The net proceeds to the Company from the July 2024 Financing were approximately $2,646,000,
after deducting placement agent fees and offering expenses. The Company incurred non-cash equity issuance cost of approximately $2.4
million for the incremental fair value of the outstanding equity classified warrants and approximately $0.2
million for placement agent warrants.
Pursuant to the terms of
the July 2024 Inducement Letter Agreements, in the event that the exercise of the existing warrants in the July 2024 Financing would
have otherwise caused a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued the
number of shares that would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of shares
of common stock in abeyance. Accordingly, an aggregate of 328,758
shares of common stock were held in abeyance (the “July 2024 Abeyance Shares”) with such July 2024 Abeyance
Shares evidenced through the holder’s existing warrants and which are deemed to be prepaid. The July 2024 Abeyance Shares were
held until notice was received by the holder that the balance of the shares of common stock could be issued in compliance with such beneficial
ownership limitations and were exercised pursuant to a notice of exercise from the holder. Until such time, the Abeyance Shares were
evidenced through the holder’s existing warrants and have been included in the Company’s table of outstanding warrants below.
During the three months ended September 30, 2024, 231,758
of the July 2024 Abeyance Shares were released. The remainder of the July 2024 Abeyance Shares were subsequently released in October
2024.
April 2023 Financing
— On April 20, 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 39,331
registered shares of the Company’s common stock at a purchase price per share of $50.85,
unregistered five and one-half year term Series A warrants to purchase up to 39,331
shares of common stock at an exercise price of $48.60
per share and unregistered eighteen month term Series B warrants to purchase up to 39,331
shares of common stock at an exercise price of $48.60
per share (collectively, the “April 2023 Financing”). In addition, the Company issued unregistered warrants
to the placement agent, H.C. Wainwright & Co., LLC (“HCW”), in the April 2023 Financing to purchase a total of
2,950
shares of common stock at an exercise price of $63.56
per share. Net proceeds to the Company from the April 2023 Financing were $1,538,000
after deducting placement agent fees and offering expenses.
In connection with the April
2023 Financing, the Company entered into warrant amendment agreements (the “Warrant Amendment Agreements”) with the
participating investors to amend the exercise price of certain existing warrants to purchase up to an aggregate of 21,291
shares of common stock that were previously issued in April 2018 through January 2021, such that each of the amended warrants
have an exercise price of $48.60
per share. The Company received $23,952
as consideration in connection with the Warrant Amendment Agreements. The Company assessed the amendments to the exercise price
of the warrants under ASC Topic 815, “Derivatives and Hedging” (“ASC 815”) and determined that
the amendment to the exercise price was completed in connection with and contingent on the close of the April 2023 Financing. The increase
in fair value of $293,000
related to the Warrant Amendment Agreements was recognized as an equity issuance cost and recorded in additional paid in capital
per ASC 815.
June 2023 Financing
— On June 2, 2023, the Company completed a registered direct offering and a concurrent private placement of a total of: 25,961
registered shares and 8,000
unregistered shares of the Company’s common stock each at a purchase price per share of $38.52,
unregistered pre-funded warrants to purchase up to an aggregate of 69,881
shares of common stock at a purchase price per share of $38.511
and with a pre-funded warrant exercise price of $0.009
per share, unregistered five and one-half year term Series A warrants to purchase up to an aggregate of 103,842
shares of common stock at an exercise price of $36.27
per share and unregistered eighteen month term Series B warrants to purchase up to an aggregate of 103,842
shares of common stock at an exercise price of $36.27
per share (collectively, the “June 2023 Financing”). In addition, the Company issued unregistered warrants
to the placement agent, HCW, in the June 2023 Financing to purchase a total of 7,788
shares of common stock at an exercise price of $48.15
per share. Net proceeds to the Company from the June 2023 Financing were $3,510,000
after deducting placement agent fees and offering expenses.
December 2023 Financing
— In December 2023, the Company entered into an inducement letter agreement (the “December 2023 Inducement Letter
Agreement”) with certain holders of the Company’s existing warrants to purchase up to an aggregate of 236,695 shares
of the Company’s common stock (the “December 2023 Financing”). Pursuant to the terms of the December 2023 Inducement
Letter Agreement, in the event that the exercise of the existing warrants in the December 2023 Financing would have otherwise caused
a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued the number of shares that
would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of shares of common stock in
abeyance. Accordingly, an aggregate of 91,820 shares of common stock were held in abeyance (the “December 2023 Abeyance Shares”)
with such December 2023 Abeyance Shares evidenced through the holder’s existing warrants and which were deemed to be prepaid. The
December 2023 Abeyance Shares were held until notice was received by the holder that the balance of the shares of common stock could
be issued in compliance with such beneficial ownership limitations and were exercised pursuant to a notice of exercise from the holder.
Until such time, the December 2023 Abeyance Shares were evidenced through the holder’s existing warrants and have been included
in the Company’s table of outstanding warrants below.
Pursuant
to the terms of the Inducement Letter Agreement, in the event that the exercise of the existing warrants in the December 2023 Financing
would have otherwise caused a holder to exceed the beneficial ownership limitations set forth in the existing warrant, the Company issued
the number of shares that would not cause a holder to exceed such beneficial ownership limitation and agreed to hold such balance of
shares of common stock in abeyance. Accordingly, at December 31, 2023, an aggregate of 91,819 shares of common stock were held
in abeyance (the “Abeyance Shares”) with such Abeyance Shares evidenced through the holder’s existing warrants
and which are deemed to be prepaid. The Abeyance Shares will be held until notice is received by the holder that the balance of the shares
of common stock may be issued in compliance with such beneficial ownership limitations and may be exercised pursuant to a notice of exercise
from the holder. Until such time, the Abeyance Shares are evidenced through the holder’s existing warrants and have been included
in the Company’s table of outstanding warrants below. The remainder of the December 2023 Abeyance Shares were subsequently released
during the first quarter of 2024.
Warrants
The Company first assesses
warrants that are issued by the Company under the FASB ASC Topic 480, “Distinguishing Liabilities from Equity” (“ASC
480”) to determine whether the warrants are within the scope of ASC 480. If there are no instances outside of the Company’s
control that could require cash settlement, the Company then applies and follows the applicable accounting guidance in ASC 815. Financial
instruments are accounted for as either derivative liabilities or equity instruments depending on the specific terms of the agreement.
Based on the assessment of the warrants issued by the Company under the guidance in ASC 480 and ASC 815, the warrants issued by the Company
have been classified within stockholder’s equity.
During
the three months ended September 30, 2024, 231,758
of the July 2024 Abeyance Shares were released and issued. During the three months ended September 30, 2023, 35,588
shares of common stock were issued related to the exercise of pre-funded warrants from the June 2023 Financing.
During
the nine months ended September 30, 2024, all of the December 2023 Abeyance Shares were released and issued and 231,758
of the July 2024 Abeyance Shares were released and issued. During the nine months ended September 30, 2023, 55,032
shares of common stock were issued related to the exercise of pre-funded warrants from the June 2023 Financing.
The following table summarizes
the Company’s outstanding warrants, all of which are classified as equity instruments, at September 30, 2024:
| Schedule of outstanding warrants | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share |
| Outstanding at December 31, 2023 | |
| 703,530 | | |
$ | 35.99 | |
| Issued | |
| 1,131,468 | | |
| 5.50 | |
| Exercised | |
| (540,112 | ) | |
| 6.56 | |
| Expired | |
| – | | |
| – | |
| Outstanding at September 30, 2024 | |
| 1,294,886 | | |
$ | 21.62 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Stock-based Compensation
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Stock-based Compensation |
6.
Stock-based Compensation
Restricted Stock Units
Restricted stock units (“RSUs”)
are issued under the Company’s 2020 Long-Term Incentive Plan (the “2020 Plan”) or as inducement grants issued
outside of the 2020 Plan to new employees. RSUs are generally subject to graded vesting and the satisfaction of certain service requirements.
RSUs granted by the Company to employees and to non-employee members of the Board of Directors generally vest annually over 1
year after the grant date. Upon vesting, each outstanding RSU will be settled for one share of the Company’s common stock.
Employee RSU recipients may elect to net share settle upon vesting, in which case the Company pays the employee’s income taxes
due upon vesting and withholds a number of shares of equal value. The Company does not expect to repurchase shares to satisfy RSU vests.
The fair value of the RSUs awarded are based upon the Company’s closing stock price at the grant date and are expensed over the
requisite service period.
On September 11, 2024, the
Company modified all unvested RSUs to reduce the vesting term for employees from 3 years after the grant date to 1 year after the grant
date. No incremental expense was recognized as a result of the modification, as the fair value of the RSUs immediately before and after
the modification was unchanged.
The following table summarizes
the activity of the Company’s RSUs for the nine months ended September 30, 2024:
| Summary of RSUs activity | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Grant Date
Fair Value
Per Share |
| Unvested units at December 31, 2023 | |
| 5,527 | | |
$ | 74.83 | |
| Granted and accepted | |
| 71,000 | | |
| 2.77 | |
| Vested | |
| (3,997 | ) | |
| 71.10 | |
| Forfeited | |
| (1,530 | ) | |
| 84.55 | |
| Unvested units at September 30, 2024 | |
| 71,000 | | |
$ | 2.77 | |
The weighted-average fair
value of RSUs granted during both the three and nine months ended September 30, 2024 was $2.77.
There were no
RSUs granted during the three months ended September 30, 2023. During the nine months ended September 30, 2023, 4,833 RSUs
were granted. The weighted-average fair value of RSUs granted during the nine months ended September 30, 2023 was $47.16.
Stock-based compensation
expense related to RSUs was $52,000
and $89,000
for the three months ended September 30, 2024 and 2023, respectively. Stock-based compensation expense related to RSUs was $99,000
and $294,000
for the nine months ended September 30, 2024 and 2023, respectively.
The aggregate fair value
of awards that vested during the nine months ended September 30, 2024 and 2023 was $21,000
and $100,000,
respectively, which represents the market value of the Company’s common stock on the date that the RSUs vested.
Stock Options
Stock options are available
for issuance under the 2020 Plan or as inducement grants issued outside of the 2020 Plan to new employees. Stock options are generally
subject to graded vesting and the satisfaction of service requirements. Stock options granted by the Company to employees generally vest
annually over 4
years after the grant date and generally vest over 1
year after the grant date for non-employee members of the Board of Directors and expire within ten years of grant. Upon the exercise
of a stock option, the Company issues new shares and delivers them to the recipient. The Company does not expect to repurchase shares
to satisfy stock option exercises.
The Company uses the Black-Scholes
option-pricing model to determine the fair value of all its option grants. The risk-free interest rate used for each grant was based
upon the yield on zero-coupon U.S. Treasury securities with a term similar to the expected life of the related option. The Company’s
expected stock price volatility assumption is based upon the Company’s own implied volatility. As the Company has limited stock
option exercise information, the expected life assumption used for option grants is based upon the simplified method provided for under
the FASB ASC Topic 718, “Compensation — Stock Compensation”. The dividend yield assumption is based upon
the fact that the Company has never paid cash dividends and presently has no intention of paying cash dividends.
The Company did not grant
any stock options during the three or nine months ended September 30, 2024 and 2023.
The following table summarizes
the activity of the Company’s stock options for the nine months ended September 30, 2024:
| Summary of stock options activity | |
| |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share | |
Aggregate
Intrinsic
Value |
| Balance at December 31, 2023 | |
| 1,146 | | |
$ | 10,120.69 | | |
| | |
| Granted | |
| – | | |
| – | | |
| | |
| Exercised | |
| – | | |
| – | | |
| | |
| Forfeited | |
| – | | |
| – | | |
| | |
| Expired | |
| (13 | ) | |
| 598,313.35 | | |
| | |
| Balance at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
| Exercisable at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
Stock-based compensation
expense related to stock options for the nine months ended September 30, 2024 was $6,000.
The Company did not
have any stock-based compensation expense related to stock options for the three months ended September 30, 2024 and 2023 or the nine
months ended September 30, 2023.
Compensation Expense Related to Equity Awards
The following table sets
forth total stock-based compensation expense for the three and nine months ended September 30, 2024 and 2023, in thousands:
| Schedule of stock-based compensation expense | |
| |
| |
| |
|
| | |
Three Months Ended | |
Nine Months Ended |
| | |
September 30, | |
September 30, |
| | |
2024 | |
2023 | |
2024 | |
2023 |
| Research and development | |
$ | 25 | | |
$ | 51 | | |
$ | 14 | | |
$ | 163 | |
| General and administrative | |
| 27 | | |
| 38 | | |
| 85 | | |
| 131 | |
| Total stock-based compensation | |
$ | 52 | | |
$ | 89 | | |
$ | 99 | | |
$ | 294 | |
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Net Loss per Common Share
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Net loss per common share: |
|
| Net Loss per Common Share |
7.
Net Loss per Common Share
Basic net loss per share
is computed by dividing net loss by the weighted average number of common shares outstanding. Diluted net loss per share is computed
by dividing the Company’s net loss by the weighted average number of common shares outstanding and the impact of the dilutive effect
of potential common stock equivalents, except when the inclusion of such potential common stock equivalents would be anti-dilutive. Dilutive
potential common stock equivalents primarily consist of stock options, RSUs and warrants. Therefore, basic and diluted net loss per share
applicable to common stockholders were the same for all periods presented because the impact of these items is generally anti-dilutive
during periods of net loss.
The weighted average number
of common shares outstanding as of September 30, 2023 includes the pre-funded warrants issued in connection with the June 2023 Financing,
the exercise of which requires nominal consideration for the delivery of the shares of common stock. Therefore, these pre-funded warrants
are not included in the table below.
The following table sets
forth the potential common shares excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive:
| Schedule of anti dilutive shares |
|
|
|
|
| |
|
September 30, |
| |
|
2024 |
|
2023 |
| Stock options |
|
|
1,133 |
|
|
|
14 |
|
| Unvested RSUs |
|
|
71,000 |
|
|
|
7,694 |
|
| Warrants1,2 |
|
|
1,197,886 |
|
|
|
357,481 |
|
| Total |
|
|
1,270,019 |
|
|
|
365,189 |
|
| 1 |
The weighted average number of common shares outstanding as of
September 30, 2023 includes pre-funded warrants issued in the June 2023 Financing because the exercise of such warrants requires only
nominal consideration. Therefore, these pre-funded warrants are not included in the table above. |
| 2 |
The weighted average number of common shares outstanding as of
September 30, 2024 includes the Abeyance Shares from the July 2024 Financing, the exercise of which was prepaid and requires no further
consideration for the delivery of the shares of common stock. Therefore, 97,000 of these Abeyance Shares are not included in the table
above. |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Subsequent Events
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
8.
Subsequent Events
In accordance with ASC Topic
855, “Subsequent Events”, which establishes general standards of accounting for and disclosure of events that occur after
the consolidated balance sheet date but before the consolidated financial statements are issued, the Company has evaluated all events
or transactions that occurred after September 30, 2024, up through the date the Company issued the condensed consolidated financial
statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Organization and Significant Accounting Policies (Policies)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Nature of Operations |
Nature
of Operations
Phio Pharmaceuticals Corp.
(“Phio” or the “Company”) is a clinical stage biotechnology company whose proprietary INTASYL®
small interfering RNA gene silencing technology is designed to make immune cells more effective in killing tumor cells. The Company is
developing therapeutics that are designed to leverage INTASYL to precisely target specific proteins that reduce the body’s ability
to fight cancer, without the need for specialized formulations or drug delivery systems.
Phio was incorporated in
the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals
Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology
therapeutics.
Effective
July 5, 2024, the Company completed a 1-for-9
reverse stock split of the Company’s outstanding common stock, including
reclassifying an amount equal to the reduction in par value to additional paid-in capital. The reverse stock split did not reduce the
number of authorized shares of the Company’s common or preferred stock. All share and per share amounts have been adjusted to give
effect to the reverse stock split.
|
| Basis of Presentation |
Basis
of Presentation
The accompanying condensed
consolidated financial statements are unaudited and have been prepared in accordance with accounting principles generally accepted in
the United States (“GAAP”). Certain information and footnote disclosures that are included in the Company’s
annual consolidated financial statements, but that are not required for interim reporting purposes, have been condensed or omitted. In
the opinion of management, all adjustments (including normal recurring accruals) considered necessary for a fair presentation of the
condensed consolidated financial statements have been included.
These statements should be
read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s most recent
Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”)
on April 1, 2024 (the “2023 Form 10-K”). Interim results are not necessarily indicative of results for a full year.
|
| Principles of Consolidation |
Principles
of Consolidation
The condensed consolidated
financial statements include the accounts of the Company and its wholly-owned subsidiary, MirImmune, LLC. All material intercompany accounts
have been eliminated in consolidation.
|
| Segments |
Segments
The Company operates as one operating
segment and all assets are located in the United States.
|
| Use of Estimates |
Use
of Estimates
The preparation of financial
statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of
revenues and expenses during the reporting period. The areas subject to significant estimates and judgement include, among others, those
related to the fair value of equity awards, accruals for research and development expenses, useful lives of property and equipment, and
the valuation allowance on the Company’s deferred tax assets. On an ongoing basis the Company evaluates its estimates and bases
its estimates on historical experience and other relevant assumptions that the Company believes are reasonable under the circumstances.
Actual results could differ materially from these estimates.
|
| Liquidity |
Liquidity
The Company has reported
recurring losses from operations since its inception and expects to continue to have negative cash flows from operations for the foreseeable
future. Historically, the Company’s primary source of funding has been from sales of its securities. The Company’s ability
to continue to fund its operations is dependent on obtaining funding from third parties, such as proceeds from the issuance of debt,
sale of equity, or strategic opportunities, in order to maintain its operations. This is dependent on a number of factors, including
the market demand or liquidity of the Company’s common stock. There is no guarantee that debt, additional equity or other funding
will be available to the Company on acceptable terms, or at all. If the Company fails to obtain additional funding when needed, the Company
would be forced to scale back or terminate its operations or seek to merge with or to be acquired by another company.
The Company has limited cash
resources, has reported recurring losses from operations since inception, has negative operating cash flows and has not yet received
product revenues. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern, and the
Company’s current cash resources may not provide sufficient capital to fund operations for at least the next 12 months from the
date of the release of these condensed consolidated financial statements. The continuation of the Company as a going concern depends
upon the Company’s ability to raise additional capital through an equity offering, debt offering and/or strategic opportunity to
fund its operations. There can be no assurance that the Company will be successful in accomplishing these plans in order to continue
as a going concern. These condensed consolidated financial statements do not include any adjustments to the recoverability and classification
of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going
concern.
|
| Summary of Significant Accounting Policies |
Summary
of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents
include unrestricted cash accounts, money market investments and highly liquid investment instruments with original maturity of three
months or less at the date of purchase.
Other than as set forth above,
there have been no material changes to the significant accounting policies disclosed in the Company’s 2023 Form 10-K.
|
| Recent Accounting Pronouncements |
Recent
Accounting Pronouncements
In November 2023, the Financial
Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2023-07, “Segment
Reporting (Topic 280) – Improvements to Reporting Segment Disclosures” (“ASU 2023-07”), which requires
disclosure of incremental segment information on an annual and interim basis. In addition, ASU 2023-07 clarifies circumstances in which
an entity can disclose multiple segment measures of profit or loss, provides new segment disclosure requirements for entities with a
single reportable segment, and contains other disclosure requirements. The amendments in ASU 2023-07 are effective for fiscal years beginning
after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The
enhanced disclosures are required to be applied retrospectively to all prior periods presented in the financial statements. The Company
is currently evaluating the impact of ASU 2023-07 on its consolidated financial statements and disclosures, but does not expect that
it will have a material impact on its consolidated financial statements.
In December 2023, the FASB
issued ASU 2023-09, “Income Taxes (Topic 740) – Improvements to Income Tax Disclosures” (“ASU 2023-09”),
which requires disclosure of specific categories in the rate reconciliation table along with additional information for reconciling items
that meet a quantitative threshold, disclosure of disaggregated income taxes paid and modifies other income tax-related disclosures.
The amendments in ASU 2023-09 are effective for annual periods beginning after December 15, 2024 and allows for adoption on a prospective
basis, with a retrospective option. Early adoption is permitted. The Company is currently evaluating the impact of ASU 2023-09, but does
not expect that it will have a material impact on its consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
PHIO_LiquidityPolicyTextBlock |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 36
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-36
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Fair Value of Financial Instruments (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of financial instruments at fair value |
| Schedule of financial instruments at fair value | |
| | | |
| | | |
| | | |
| | |
| Description | |
September 30, 2024 | |
Quoted
Prices In
Active Markets
(Level 1) | |
Other Significant
Observable Inputs
(Level 2) | |
Unobservable
Inputs
(Level 3) |
| Assets: | |
| | | |
| | | |
| | | |
| | |
| Cash equivalents | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
| Total | |
$ | 5,390 | | |
$ | 5,390 | | |
$ | – | | |
$ | – | |
|
| X |
- DefinitionTabular disclosure of assets, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, by class that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsMeasuredOnRecurringBasisTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Leases (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Leases |
|
| Schedule of lease amounts recorded in balance sheet |
| Schedule of lease amounts recorded in balance sheet |
|
|
|
|
| |
|
September 30,
2024 |
|
December 31,
2023 |
| Assets |
|
|
|
|
|
|
|
|
| Right of use asset |
|
$ |
– |
|
|
$ |
33 |
|
| Liabilities |
|
|
|
|
|
|
|
|
| Lease liability |
|
$ |
– |
|
|
$ |
35 |
|
| Lease Term and Discount Rate |
|
|
|
|
|
|
|
|
| Weighted average remaining lease term |
|
|
– |
|
|
|
0.25 |
|
| Weighted average discount rate |
|
|
– |
|
|
|
4.70% |
|
|
| X |
- References
+ Details
| Name: |
PHIO_DisclosureLeasesAbstract |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_SupplementalBalanceSheetInformationRelatedToOperatingLeasesTableTextBlock |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Stockholders’ Equity (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Schedule of outstanding warrants |
| Schedule of outstanding warrants | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share |
| Outstanding at December 31, 2023 | |
| 703,530 | | |
$ | 35.99 | |
| Issued | |
| 1,131,468 | | |
| 5.50 | |
| Exercised | |
| (540,112 | ) | |
| 6.56 | |
| Expired | |
| – | | |
| – | |
| Outstanding at September 30, 2024 | |
| 1,294,886 | | |
$ | 21.62 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Stock-based Compensation (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Summary of RSUs activity |
| Summary of RSUs activity | |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Grant Date
Fair Value
Per Share |
| Unvested units at December 31, 2023 | |
| 5,527 | | |
$ | 74.83 | |
| Granted and accepted | |
| 71,000 | | |
| 2.77 | |
| Vested | |
| (3,997 | ) | |
| 71.10 | |
| Forfeited | |
| (1,530 | ) | |
| 84.55 | |
| Unvested units at September 30, 2024 | |
| 71,000 | | |
$ | 2.77 | |
|
| Summary of stock options activity |
| Summary of stock options activity | |
| |
| |
|
| | |
Number
of Shares | |
Weighted-
Average
Exercise
Price
Per Share | |
Aggregate
Intrinsic
Value |
| Balance at December 31, 2023 | |
| 1,146 | | |
$ | 10,120.69 | | |
| | |
| Granted | |
| – | | |
| – | | |
| | |
| Exercised | |
| – | | |
| – | | |
| | |
| Forfeited | |
| – | | |
| – | | |
| | |
| Expired | |
| (13 | ) | |
| 598,313.35 | | |
| | |
| Balance at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
| Exercisable at September 30, 2024 | |
| 1,133 | | |
$ | 3,371.79 | | |
$ | – | |
|
| Schedule of stock-based compensation expense |
| Schedule of stock-based compensation expense | |
| |
| |
| |
|
| | |
Three Months Ended | |
Nine Months Ended |
| | |
September 30, | |
September 30, |
| | |
2024 | |
2023 | |
2024 | |
2023 |
| Research and development | |
$ | 25 | | |
$ | 51 | | |
$ | 14 | | |
$ | 163 | |
| General and administrative | |
| 27 | | |
| 38 | | |
| 85 | | |
| 131 | |
| Total stock-based compensation | |
$ | 52 | | |
$ | 89 | | |
$ | 99 | | |
$ | 294 | |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the number and weighted-average grant date fair value for restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock units that were granted, vested, or forfeited during the year. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Net Loss per Common Share (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Net loss per common share: |
|
| Schedule of anti dilutive shares |
| Schedule of anti dilutive shares |
|
|
|
|
| |
|
September 30, |
| |
|
2024 |
|
2023 |
| Stock options |
|
|
1,133 |
|
|
|
14 |
|
| Unvested RSUs |
|
|
71,000 |
|
|
|
7,694 |
|
| Warrants1,2 |
|
|
1,197,886 |
|
|
|
357,481 |
|
| Total |
|
|
1,270,019 |
|
|
|
365,189 |
|
| 1 |
The weighted average number of common shares outstanding as of
September 30, 2023 includes pre-funded warrants issued in the June 2023 Financing because the exercise of such warrants requires only
nominal consideration. Therefore, these pre-funded warrants are not included in the table above. |
| 2 |
The weighted average number of common shares outstanding as of
September 30, 2024 includes the Abeyance Shares from the July 2024 Financing, the exercise of which was prepaid and requires no further
consideration for the delivery of the shares of common stock. Therefore, 97,000 of these Abeyance Shares are not included in the table
above. |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Collaboration Agreement (Details Narrative) - Clinical Co Development Agreement [Member] - Agon Ox [Member] - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Contractual Obligation |
$ 4,000,000
|
|
$ 4,000,000
|
|
| [custom:ExpenseFromContractualObligations] |
$ 308,000
|
$ 606,000
|
$ 414,000
|
$ 906,000
|
| X |
- References
+ Details
| Name: |
PHIO_ExpenseFromContractualObligations |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of contractual obligation, including, but not limited to, long-term debt, lease obligation, purchase obligation, and other commitments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
| Name: |
us-gaap_ContractualObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=PHIO_ClinicalCoDevelopmentAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=PHIO_AgonOxMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Fair Value of Financial Instruments - (Details - Financial instruments at fair value)
$ in Thousands |
Sep. 30, 2024
USD ($)
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
$ 5,390
|
| Fair Value, Inputs, Level 1 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
5,390
|
| Fair Value, Inputs, Level 2 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
0
|
| Fair Value, Inputs, Level 3 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
0
|
| Cash and Cash Equivalents [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
5,390
|
| Cash and Cash Equivalents [Member] | Fair Value, Inputs, Level 1 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
5,390
|
| Cash and Cash Equivalents [Member] | Fair Value, Inputs, Level 2 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
0
|
| Cash and Cash Equivalents [Member] | Fair Value, Inputs, Level 3 [Member] |
|
| Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation [Line Items] |
|
| Total |
$ 0
|
| X |
- DefinitionFair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_CashAndCashEquivalentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=us-gaap_CashAndCashEquivalentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- References
+ Details
| Name: |
PHIO_AssetsLesseeAbstract |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_LeaseTermAndDiscountRateAbstract |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_LiabilitiesLesseeAbstract |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.4
Leases (Details Narrative)
|
|
3 Months Ended |
9 Months Ended |
|
Mar. 01, 2024
ft²
|
Sep. 30, 2024
USD ($)
ft²
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
ft²
|
Sep. 30, 2023
USD ($)
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
| Operating Lease, Cost |
|
$ 0
|
$ 33,000
|
$ 33,000
|
$ 99,000
|
| Operating Lease, Payments |
|
|
|
$ 35,000
|
$ 104,000
|
| Property Subject to Operating Lease [Member] |
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
| Area of Real Estate Property | ft² |
321
|
7,581
|
|
7,581
|
|
| Lease Expiration Date |
Aug. 31, 2024
|
|
|
Mar. 31, 2024
|
|
| Payments for Rent |
|
|
|
$ 15,000
|
|
| Property Subject to Operating Lease [Member] | Laboratory Facility [Member] |
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
| Operating Lease, Expense |
|
$ 8,129
|
|
$ 18,909
|
|
| X |
- DefinitionArea of a real estate property.
| Name: |
us-gaap_AreaOfRealEstateProperty |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:areaItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate which lease or group of leases is set to expire, in YYYY-MM-DD format.
| Name: |
us-gaap_LeaseExpirationDate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of operating lease expense. Excludes sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-4
| Name: |
us-gaap_OperatingLeaseExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCash payments to lessor's for use of assets under operating leases. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForRent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertySubjectToOrAvailableForOperatingLeaseAxis=us-gaap_PropertySubjectToOperatingLeaseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=PHIO_LaboratoryFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stockholders' Equity (Details - Warrants outstanding) - Warrant [Member]
|
9 Months Ended |
|
Sep. 30, 2024
$ / shares
shares
|
|---|
| Class of Stock [Line Items] |
|
| Warrants outstanding, Beginning balance | shares |
703,530
|
| Warrants outstanding weighted average exercise price per share, Beginning balance | $ / shares |
$ 35.99
|
| Warrants issued, shares | shares |
1,131,468
|
| Warrants issued weighted average exercise price per share | $ / shares |
$ 5.50
|
| Warrants exercised, shares | shares |
(540,112)
|
| Warrants exercised weighted average exercise price per share | $ / shares |
$ 6.56
|
| Warrants expired, shares | shares |
0
|
| Warrants expired weighted average exercise price per share | $ / shares |
$ 0
|
| Warrants outstanding, Ending balance | shares |
1,294,886
|
| Warrants outstanding weighted average exercise price per share, Ending balance | $ / shares |
$ 21.62
|
| X |
- References
+ Details
| Name: |
PHIO_ClassOfWarrantOrRightWeightedAverageExercisePrice |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsExercisedShare |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsExercisedWeightedAverageExercisePricePerShare |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsExpiredShares |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsExpiredWeightedAverageExercisePricePerShare |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsIssuedShares |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsIssuedWeightedAverageExercisePricePerShare |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stockholders’ Equity (Details Narrative) - USD ($)
|
|
|
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
Jul. 11, 2024 |
May 16, 2024 |
Jun. 02, 2023 |
Apr. 20, 2023 |
Jul. 31, 2024 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Proceeds from Issuance or Sale of Equity |
|
|
|
|
|
|
|
$ 2,646,000
|
$ 5,048,000
|
| June 2023 Prefunded Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Stock Issued During Period, Shares, Conversion of Convertible Securities |
|
|
|
|
|
|
35,588
|
|
55,032
|
| Equity Classified Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:NoncashEquityIssuanceCost] |
|
|
|
|
$ 2,400,000
|
|
|
|
|
| Placement Agent Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:NoncashEquityIssuanceCost] |
|
|
|
|
$ 200,000
|
|
|
|
|
| May 2024 Financing [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares, Issued |
|
95,833
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
|
$ 6.48
|
|
|
|
|
|
|
|
| Proceeds from Issuance of Common Stock |
|
$ 621,000
|
|
|
|
|
|
|
|
| July 2024 Financing [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares, Issued |
545,286
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
$ 5.45
|
|
|
|
|
|
|
|
|
| Proceeds from Issuance of Common Stock |
$ 2,646,000
|
|
|
|
|
|
|
|
|
| July 2024 Financing [Member] | Series C Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares, Issued |
583,098
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
$ 5.45
|
|
|
|
|
|
|
|
|
| July 2024 Financing [Member] | Series D Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares, Issued |
507,474
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
$ 5.45
|
|
|
|
|
|
|
|
|
| July 2024 Abeyance Shares [Member] | July 2024 Inducement Letter Agreement [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Common Stock, Capital Shares Reserved for Future Issuance |
|
|
|
|
328,758
|
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Shares Issued in Period |
|
|
|
|
|
231,758
|
|
231,758
|
|
| April 2023 Financing [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
|
|
|
$ 50.85
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues |
|
|
|
39,331
|
|
|
|
|
|
| Proceeds from Issuance or Sale of Equity |
|
|
|
$ 1,538,000
|
|
|
|
|
|
| April 2023 Financing [Member] | Warrant Amendment Agreements [Member] | Previously Issued Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 48.60
|
|
|
|
|
|
| [custom:AmendedWarrants] |
|
|
|
21,291
|
|
|
|
|
|
| [custom:ProceedsFromAmendmentOfWarrants] |
|
|
|
$ 23,952
|
|
|
|
|
|
| Equity-Classified Written Call Option, Modification, Equity Issuance, Increase (Decrease) in Equity, Amount |
|
|
|
$ 293,000
|
|
|
|
|
|
| April 2023 Financing [Member] | Series A Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
|
39,331
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 48.60
|
|
|
|
|
|
| April 2023 Financing [Member] | Series B Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
|
39,331
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 48.60
|
|
|
|
|
|
| April 2023 Financing [Member] | Placement Agent Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
|
2,950
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 63.56
|
|
|
|
|
|
| June 2023 Financing [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Proceeds from Issuance or Sale of Equity |
|
|
$ 3,510,000
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Series A Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
103,842
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
$ 36.27
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Series B Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
103,842
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
$ 36.27
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Placement Agent Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
7,788
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
$ 48.15
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Registered Shares [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues |
|
|
25,961
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Unregistered Shares [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
|
|
$ 38.52
|
|
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues |
|
|
8,000
|
|
|
|
|
|
|
| June 2023 Financing [Member] | Unregistered Pre Funded Warrants [Member] |
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
| Shares Issued, Price Per Share |
|
|
$ 38.511
|
|
|
|
|
|
|
| [custom:WarrantsIssuedNewShares-0] |
|
|
69,881
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
$ 0.009
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
PHIO_AmendedWarrants |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_NoncashEquityIssuanceCost |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_ProceedsFromAmendmentOfWarrants |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
PHIO_WarrantsIssuedNewShares |
| Namespace Prefix: |
PHIO_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in equity for freestanding written call option classified as equity from modification recognized as equity issuance cost. Includes, but is not limited to, exchange by issuer and holder. Excludes share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 35
-Paragraph 17
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480299/815-40-35-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
| Name: |
us-gaap_EquityClassifiedWrittenCallOptionModificationEquityIssuanceIncreaseDecreaseInEquityAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesIssuedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConversionOfStockByUniqueDescriptionAxis=PHIO_June2023PrefundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=PHIO_EquityClassifiedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=PHIO_PlacementAgentWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=PHIO_May2024FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=PHIO_July2024FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_SeriesCWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_SeriesDWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=PHIO_July2024AbeyanceSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=PHIO_July2024InducementLetterAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=PHIO_April2023FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=PHIO_WarrantAmendmentAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=PHIO_PreviouslyIssuedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_SeriesAWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_SeriesBWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_PlacementAgentWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SecuritiesFinancingTransactionAxis=PHIO_June2023FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_RegisteredSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_UnregisteredSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=PHIO_UnregisteredPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stock-based Compensation (Details - RSU activity) - Restricted Stock Units (RSUs) [Member]
|
9 Months Ended |
|
Sep. 30, 2024
$ / shares
shares
|
|---|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
| Number of unvested restricted stock units, Beginning balance | shares |
5,527
|
| Weighted- average grant date fair value per share of unvested restricted stock units, Beginning balance | $ / shares |
$ 74.83
|
| Number of restricted stock units, Granted and accepted | shares |
71,000
|
| Weighted- average grant date fair value per share of restricted stock units, Granted and accepted | $ / shares |
$ 2.77
|
| Number of restricted stock units, Vested | shares |
(3,997)
|
| Weighted- average grand date fair value per share of restricted stock units, Vested | $ / shares |
$ 71.10
|
| Number of restricted stock units, Forfeited | shares |
(1,530)
|
| Weighted- average grant date fair value per share of restricted stock units, Forfeited | $ / shares |
$ 84.55
|
| Number of unvested restricted stock units, Ending balance | shares |
71,000
|
| Weighted- average grant date fair value per share of unvested restricted stock units, Ending balance | $ / shares |
$ 2.77
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that were forfeited during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stock-based Compensation (Details - Option activity) - Share-Based Payment Arrangement, Option [Member]
$ / shares in Units, $ in Thousands |
9 Months Ended |
|
Sep. 30, 2024
USD ($)
$ / shares
shares
|
|---|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
| Options outstanding, beginning balance | shares |
1,146
|
| Options outstanding, price per share, beginning balance | $ / shares |
$ 10,120.69
|
| Options granted | shares |
0
|
| Options granted, price per share | $ / shares |
$ 0
|
| Options exercised | shares |
0
|
| Options exercised, price per share | $ / shares |
$ 0
|
| Options forfeited | shares |
0
|
| Options forfeited, price per share | $ / shares |
$ 0
|
| Options expired | shares |
(13)
|
| Options expired, price per share | $ / shares |
$ 598,313.35
|
| Options outstanding, ending balance | shares |
1,133
|
| Options outstanding, price per share, ending balance | $ / shares |
$ 3,371.79
|
| Aggregate intrinsic value, outstanding | $ |
$ 0
|
| Options exercisable | shares |
1,133
|
| Options exercisable, price per share | $ / shares |
$ 3,371.79
|
| Aggregate intrinsic value, exercisable | $ |
$ 0
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stock-based Compensation (Details - Share-based compensation) - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
|
| Total stock-based compensation |
$ 52
|
$ 89
|
$ 99
|
$ 294
|
| Research and Development Expense [Member] |
|
|
|
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
|
| Total stock-based compensation |
25
|
51
|
14
|
163
|
| General and Administrative Expense [Member] |
|
|
|
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
|
| Total stock-based compensation |
$ 27
|
$ 38
|
$ 85
|
$ 131
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Stock-based Compensation (Details Narrative) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Payment Arrangement, Noncash Expense |
$ 52,000
|
$ 89,000
|
$ 99,000
|
$ 294,000
|
| Restricted Stock Units (RSUs) [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Net of Forfeitures |
|
0
|
|
4,833
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Weighted Average Grant Date Fair Value |
|
|
|
$ 47.16
|
| Share-Based Payment Arrangement, Noncash Expense |
$ 52,000
|
$ 89,000
|
99,000
|
$ 294,000
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Vested in Period, Fair Value |
|
|
$ 21,000
|
$ 100,000
|
| Restricted Stock Units (RSUs) [Member] | Employees [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period |
|
|
1 year
|
|
| Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Payment Arrangement, Noncash Expense |
|
$ 0
|
$ 6,000
|
|
| Share-Based Payment Arrangement, Option [Member] | Employees [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period |
|
|
4 years
|
|
| Share-Based Payment Arrangement, Option [Member] | Non Employee Members [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period |
|
|
1 year
|
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based awards for which the grantee gained the right by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodTotalFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=PHIO_EmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=PHIO_NonEmployeeMembersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Net Loss per Common Share (Details - Antidilutive shares) - shares
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
| Antidilutive shares |
|
1,270,019
|
365,189
|
| Stock Options [Member] |
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
| Antidilutive shares |
|
1,133
|
14
|
| Restricted Stock Units (RSUs) [Member] |
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
| Antidilutive shares |
|
71,000
|
7,694
|
| Warrant [Member] |
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
| Antidilutive shares |
[1],[2] |
1,197,886
|
357,481
|
|
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=PHIO_StockOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
From Dec 2024 to Jan 2025
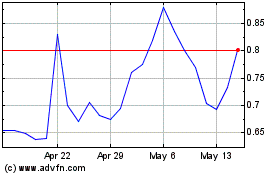
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
From Jan 2024 to Jan 2025