false00018332140001833214us-gaap:CommonStockMember2024-01-192024-01-190001833214sabs:WarrantsEachExercisableForOneShareOfCommonStockAtExercisePriceOf11.50PerShareMember2024-01-192024-01-1900018332142024-01-192024-01-19
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 19, 2024 |
SAB BIOTHERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39871 |
85-3899721 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
2100 East 54th Street North |
|
Sioux Falls, South Dakota |
|
57104 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 605 679-6980 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common stock, $0.0001 par value per share |
|
SABS |
|
The Nasdaq Stock Market LLC |
Warrants, each exercisable for one share of Common Stock at an exercise price of $11.50 per share |
|
SABSW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
SAB Biotherapeutics, Inc. (the "Company" or "SAB") is making available an updated corporate presentation (the “Presentation”) on the Investor Relations section of the Company’s website. A copy of the Presentation is furnished herewith as Exhibit 99.1 and is incorporated herein by reference.
Exhibit 99.1 is being furnished pursuant to Item 7.01 of Form 8-K and will not be deemed to be filed for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise be subject to the liabilities of that section, nor will it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act. The information contained in the Presentation is summary information that should be considered in the context of the Company’s filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise from time to time.
Cautionary Note Regarding Forward-Looking Statements
Certain statements made in this current report and the Release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “to be,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, including the development and efficacy of our T1D program, and other discovery programs, the closing of each tranche of the Company’s private placement offering, the timely funding to the Company by each investor in the private placement offering, financial projections and future financial and operating results (including estimated cost savings and cash runway), the outcome of and potential future government, and other third-party collaborations or funded programs.
These statements are based on the current expectations of SAB and are not predictions of actual performance, and are not intended to serve as, and must not be relied on, by any investor as a guarantee, prediction, definitive statement, or an assurance, of fact or probability. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties and other factors which may be beyond our control. Actual events and circumstances are difficult or impossible to predict, and these risks and uncertainties may cause our or our industry’s results, performance, or achievements to be materially different from those anticipated by these forward-looking statements. A further description of risks and uncertainties can be found in the sections captioned “Risk Factors” in our most recent annual report on Form 10-K, as amended, subsequent quarterly reports on Form 10-Q, as may be amended or supplemented from time to time, and other filings with or submissions to, the U.S. Securities and Exchange Commission, which are available at https://www.sec.gov/. Except as otherwise required by law, SAB disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events, or circumstances or otherwise.
Item 9.01 Financial Statements and Exhibits.
|
|
Exhibit Number |
Description |
99.1 |
Presentation |
104 |
Cover Page Interactive Data File-the cover page XBRL tags are embedded within the Inline XBRL document. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
SAB Biotherapeutics, Inc. |
|
|
|
|
Date: |
January 19, 2024 |
By: |
/s/ Eddie J. Sullivan |
|
|
|
Eddie J. Sullivan
Chief Executive Officer |

© 2024 SAB BIOTHERAPEUTICS, INC. FULLY HUMAN ANTITHYMOCYTE BIOLOGIC DEVELOPED TO DELAY ONSET OR PROGRESSION OF TYPE 1 DIABETES NASDAQ: SABS SAB BIOTHERAPEUTICS INTRODUCTION JANUARY 2024 EXHIBIT 99.1

Forward-Looking Statements 2 The material in this presentation has been prepared by SAB Biotherapeutics, Inc. (“SAB”) and is general background information about SAB’s activities current as of the date of this presentation. This information is given in summary form and is not intended to be complete. Information in this presentation, including financial forecasts, should not be considered advice or a recommendation to investors or potential investors in relation to holding, purchasing, or selling securities or other financial products or instruments and does not take into account any particular investment objectives, financial situation or needs. This presentation may contain forward-looking statements including statements regarding our intent, belief, or current expectations with respect to SAB’s businesses and operations, market conditions, results of operations and financial condition, capital adequacy, specific provisions, and risk management practices. Readers are cautioned not to place undue reliance on these forward-looking statements. SAB does not undertake any obligation to update any information herein for any reason or to publicly release the result of any revisions to these forward-looking statements to reflect events or circumstances after the date hereof to reflect the occurrence of unanticipated events unless required by law. While due care has been used in the preparation of forecast information, actual results may vary in a materially positive or negative manner and the presentation may contain errors or omissions. Forecasts and hypothetical examples are subject to uncertainty and contingencies outside SAB’s control. Past performance is not a reliable indication of future performance. The forward-looking statements contained or implied in this presentation are subject to other risks and uncertainties, including those discussed under the heading "Risk Factors" in SAB’s most recent Annual Report on Form 10-K with the Securities and Exchange Commission (the “SEC”) and in other filings that SAB makes with the SEC. Unless otherwise specified, information is current at the date hereof. The SAB logo and other trademarks of SAB appearing in this presentation are the property of SAB. All other trademarks, services marks, and trade names in this presentation are the property of their respective owners. © 2024 SAB BIOTHERAPEUTICS, INC.
Investment Thesis 3 SAB Biotherapeutics is a next generation antibody platform company with human data in > 700 patients across three indications, currently focused on prevention of Type 1 diabetes. MoA of SAB-142 in T1D is a proven therapeutic approach with support and enthusiasm from clinicians, opinion leaders and Juvenile Diabetes Research Foundation (JDRF) New onset T1D is considered an orphan condition Initiated SAB-142-101 FIM study; development plan is designed in partnership with JDRF Phase 1 data expected by YE 2024; aiming to demonstrate safety advantage over rATG (zero serum sickness and nADA) due to being fully human antibody to enable re-dosing for prevention and disease modification Strategic validation for new drugs for prevention of Type 1 diabetes is demonstrated by Sanofi's acquisition of Provention for $2.9B, another company sponsored by JDRF © 2024 SAB BIOTHERAPEUTICS, INC.

Experienced Management Team Christoph Bausch, PhD, MBA �EVP & CHIEF OPERATING OFFICER 20+ years research and discovery, biomanufacturing, business development, and platform technology commercialization MilliporeSigma (Merck KGaA) Stowers Institute for Medical Research Postdoc Eddie J. Sullivan, PhD�PRESIDENT & CEO / CO-FOUNDER 20+ years new technology development 25+ years biotech Former Japanese pharma BIO Executive Committee Reproductive physiologist Michael G. King, Jr. EVP & CHIEF FINANCIAL OFFICER 25+ years as award winning biotechnology industry analyst Entrepreneur in Residence at Fortress Biotech (FBIO) Senior Vice President and Director of Corporate Development Ziopharm Oncology (ZIOP) Samuel J. Reich�EXECUTIVE CHAIRMAN, BOD 20+ years Biopharma Executive and BOD Bioentrepreneur Co-founder Acuity Pharmaceuticals, �OPKO Health, Biscayne Neurotherapeutics Molecular Biologist Alexandra Kropotova, MD EVP & CHIEF MEDICAL OFFICER 20+ years global clinical development Biopharmaceutical R&D leader, Pfizer, Wyeth, Sanofi, Teva Specialty R&D Board member, iBio Contributed to numerous patents & compounds leading portfolios from Phase I to BLA and NDA approvals © 2024 SAB BIOTHERAPEUTICS, INC.

Only transgenic animal that carries the entire human immunoglobulin (Ig) heavy and light (κ) chain loci. HAC is subject to mitosis along with the other 60 Tc Bovine chromosomes. HAC present in the Tc Bovine allows for the highest production of human immunoglobulin repertoire most similar to humans. Tc Bovine Human Immunoglobulin G Produced in Transchromosomic Bovine Tc Bovine™ contain all the human immunoglobulin genes Human Artificial Chromosome (HAC) ~17Mb contains the entire unarranged VDJ human immunoglobulin loci (IgH + Igκ) © 2024 SAB BIOTHERAPEUTICS, INC.

SAB-142: A Human Anti-Thymocyte Globulin (hATG) – Focused Program Development in�Type 1 Diabetes © 2024 SAB BIOTHERAPEUTICS, INC.
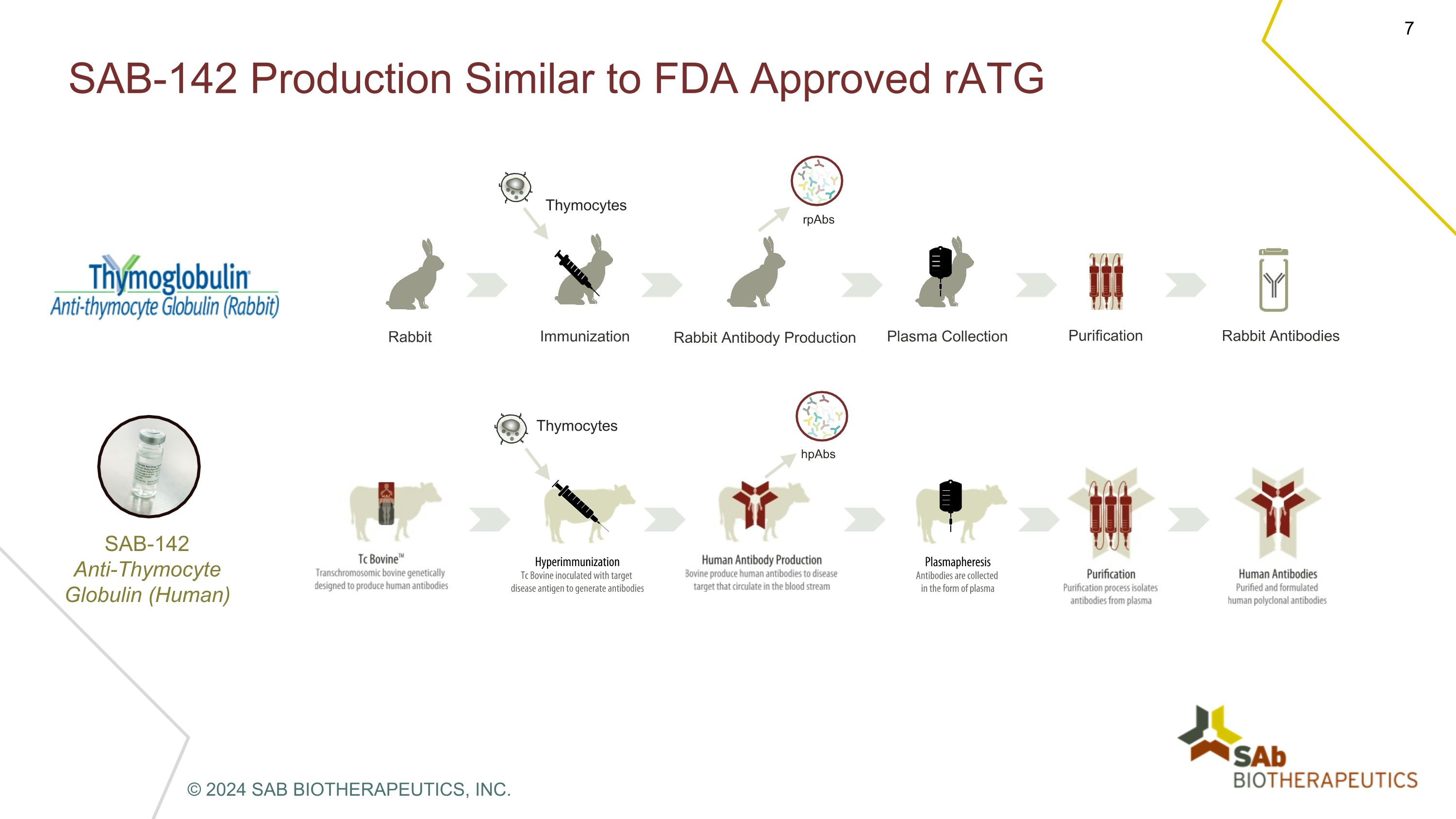
SAB-142 Production Similar to FDA Approved rATG rpAbs hpAbs Thymocytes Thymocytes Rabbit Immunization Rabbit Antibody Production Plasma Collection Purification Rabbit Antibodies SAB-142�Anti-Thymocyte Globulin (Human) © 2024 SAB BIOTHERAPEUTICS, INC.
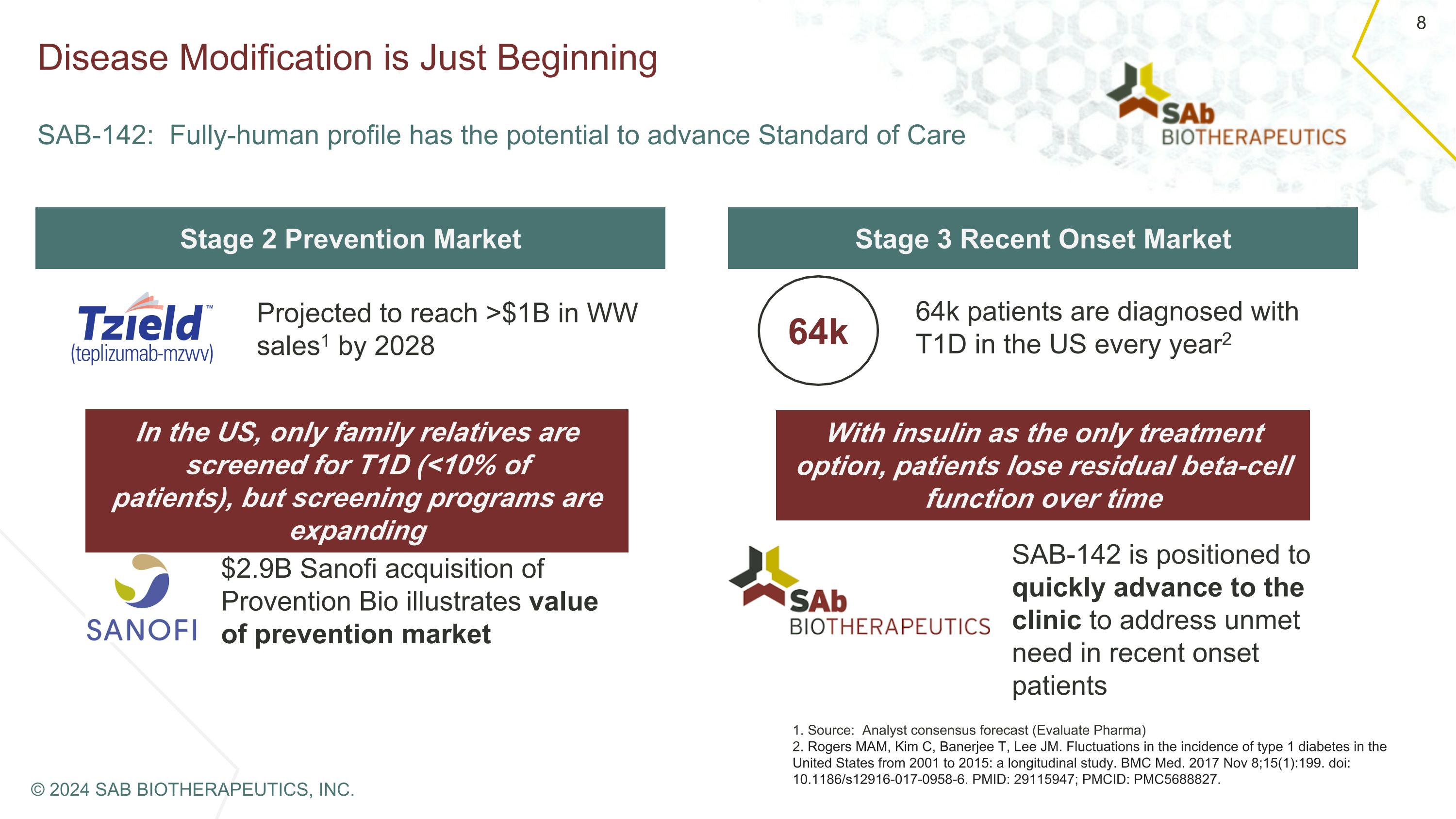
Disease Modification is Just Beginning �SAB-142: Fully-human profile has the potential to advance Standard of Care Stage 2 Prevention Market Stage 3 Recent Onset Market Projected to reach >$1B in WW sales1 by 2028 In the US, only family relatives are screened for T1D (<10% of patients), but screening programs are expanding $2.9B Sanofi acquisition of Provention Bio illustrates value of prevention market 64k 64k patients are diagnosed with T1D in the US every year2 With insulin as the only treatment option, patients lose residual beta-cell function over time SAB-142 is positioned to quickly advance to the clinic to address unmet need in recent onset patients 1. Source: Analyst consensus forecast (Evaluate Pharma) 2. Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017 Nov 8;15(1):199. doi: 10.1186/s12916-017-0958-6. PMID: 29115947; PMCID: PMC5688827. © 2024 SAB BIOTHERAPEUTICS, INC.
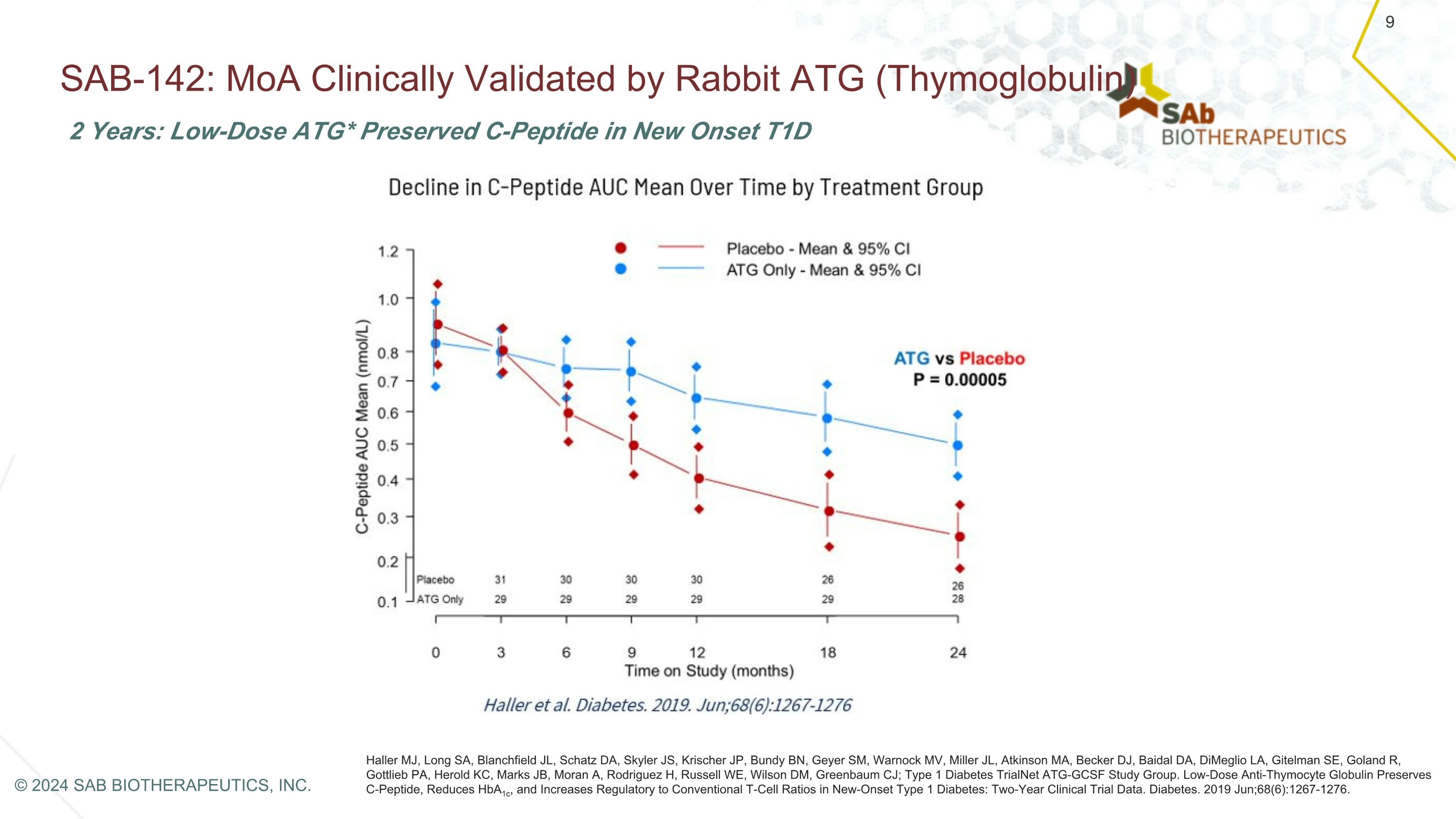
SAB-142: MoA Clinically Validated by Rabbit ATG (Thymoglobulin) Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Geyer SM, Warnock MV, Miller JL, Atkinson MA, Becker DJ, Baidal DA, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell WE, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes. 2019 Jun;68(6):1267-1276. 2 Years: Low-Dose ATG* Preserved C-Peptide in New Onset T1D 9 © 2024 SAB BIOTHERAPEUTICS, INC.
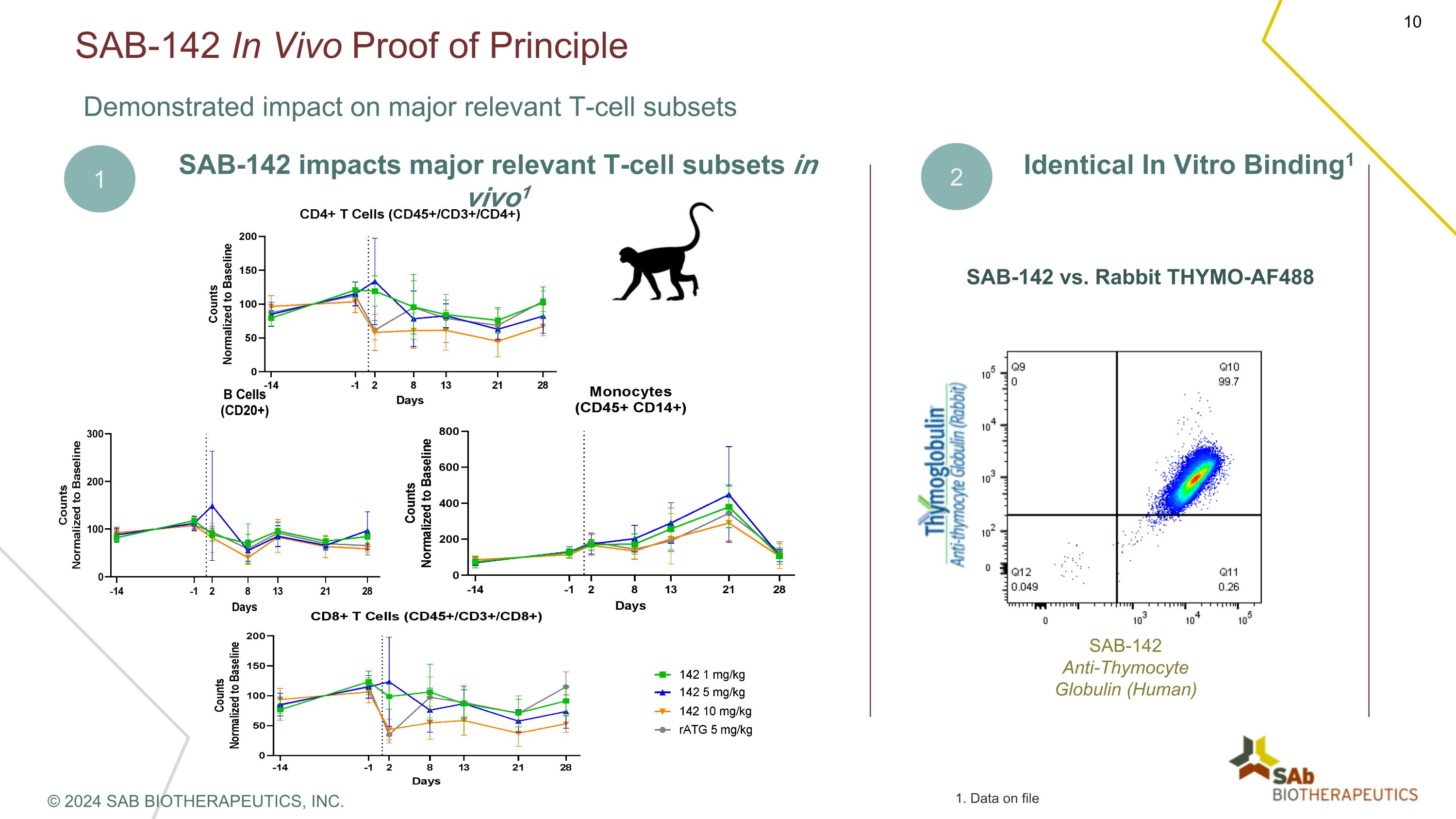
SAB-142 In Vivo Proof of Principle Demonstrated impact on major relevant T-cell subsets SAB-142 impacts major relevant T-cell subsets in vivo1 2 Identical In Vitro Binding1 SAB-142 vs. Rabbit THYMO-AF488 SAB-142�Anti-Thymocyte Globulin (Human) 1 © 2024 SAB BIOTHERAPEUTICS, INC. 1. Data on file
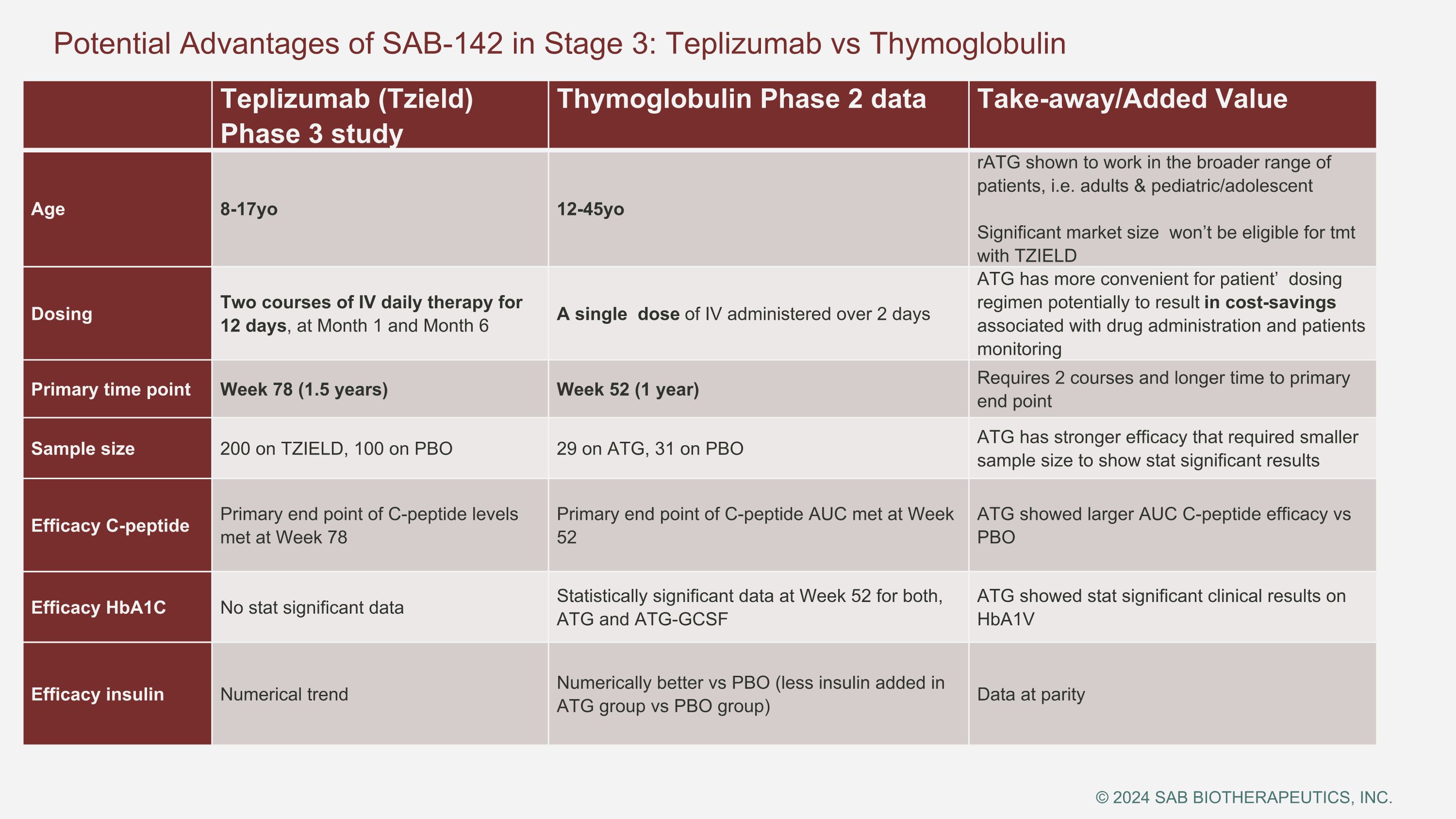
Teplizumab (Tzield) Phase 3 study Thymoglobulin Phase 2 data Take-away/Added Value Age 8-17yo 12-45yo rATG shown to work in the broader range of patients, i.e. adults & pediatric/adolescent Significant market size won’t be eligible for tmt with TZIELD Dosing Two courses of IV daily therapy for 12 days, at Month 1 and Month 6 A single dose of IV administered over 2 days ATG has more convenient for patient’ dosing regimen potentially to result in cost-savings associated with drug administration and patients monitoring Primary time point Week 78 (1.5 years) Week 52 (1 year) Requires 2 courses and longer time to primary end point Sample size 200 on TZIELD, 100 on PBO 29 on ATG, 31 on PBO ATG has stronger efficacy that required smaller sample size to show stat significant results Efficacy C-peptide Primary end point of C-peptide levels met at Week 78 Primary end point of C-peptide AUC met at Week 52 ATG showed larger AUC C-peptide efficacy vs PBO Efficacy HbA1C No stat significant data Statistically significant data at Week 52 for both, ATG and ATG-GCSF ATG showed stat significant clinical results on HbA1V Efficacy insulin Numerical trend Numerically better vs PBO (less insulin added in ATG group vs PBO group) Data at parity Potential Advantages of SAB-142 in Stage 3: Teplizumab vs Thymoglobulin © 2024 SAB BIOTHERAPEUTICS, INC.
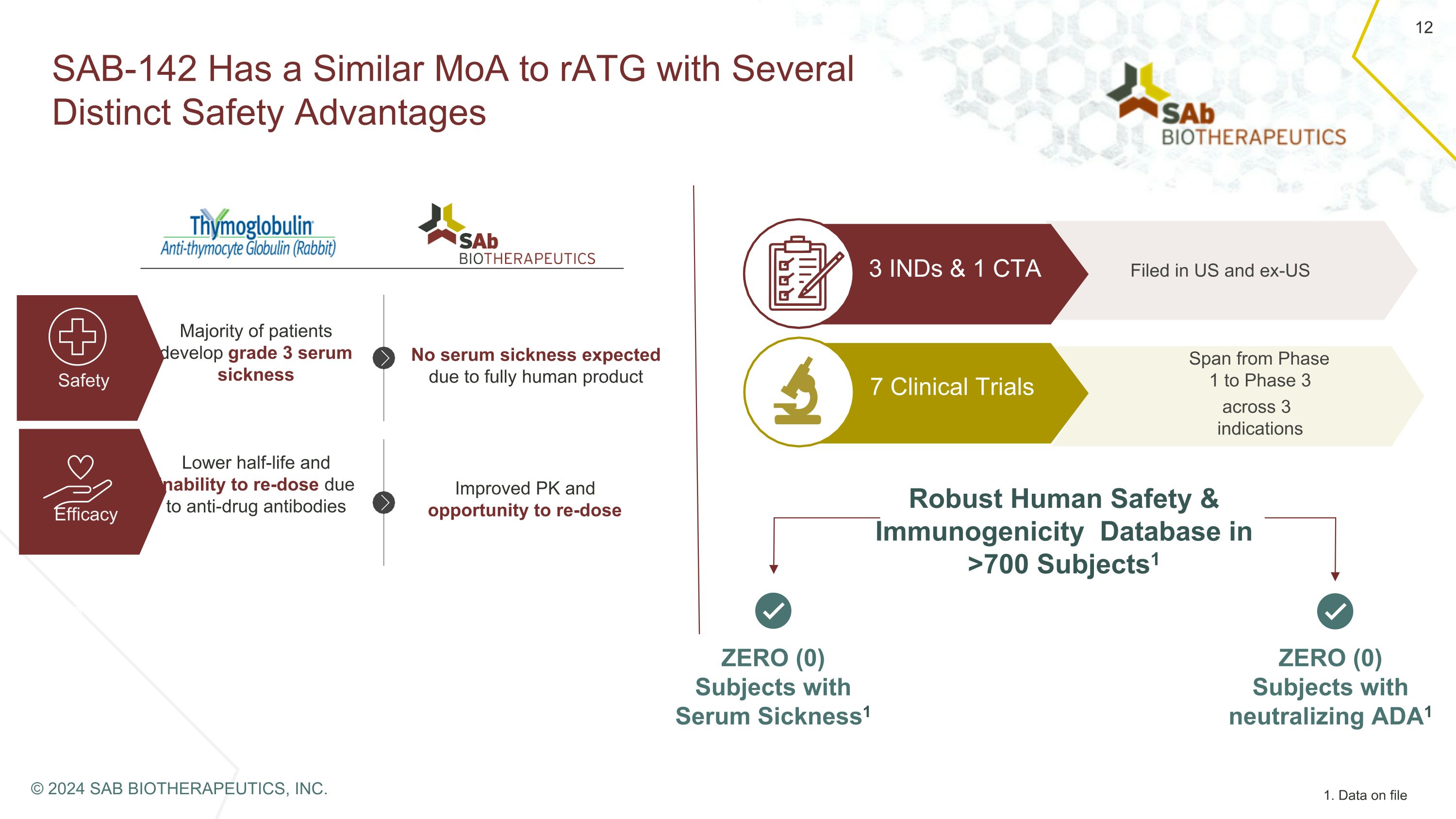
SAB-142 Has a Similar MoA to rATG with Several Distinct Safety Advantages Majority of patients develop grade 3 serum sickness Safety Efficacy No serum sickness expected due to fully human product Lower half-life and inability to re-dose due to anti-drug antibodies Improved PK and opportunity to re-dose Filed in US and ex-US 7 Clinical Trials Span from Phase 1 to Phase 3 across 3 indications 3 INDs & 1 CTA Robust Human Safety & Immunogenicity Database in >700 Subjects1 ZERO (0) Subjects with Serum Sickness1 ZERO (0) Subjects with neutralizing ADA1 1. Data on file © 2024 SAB BIOTHERAPEUTICS, INC.

SAB-142: Clinical Development Plan in T1D STUDY DESIGN "Fully HUman anti-thymocyte biologic in first-in-MAN clinical study (HUMAN trial)” Phase 1: First in Human, Randomized, Single Ascending Dose trial SAB-142 dose range: 0.03mg/kg up to 2.5mg/kg ENDPOINTS Primary end point: Acute (serum sickness, CRS) and long-term (rate of infections) safety Secondary end points: pharmacokinetics, pharmacodynamics, immunogenicity/ADA Major outcomes: Validate safety superiority based on the anticipated 0% of serum sickness and nAbs Validate MoA of SAB-142 in humans Proof of Biological Activity (POBA): change vs baseline in CD3, CD8, CD4, CD8/CD4 ratio, Tregs compared to rATG (cross study) © 2024 SAB BIOTHERAPEUTICS, INC. **Topline Data by End of Dec 2024
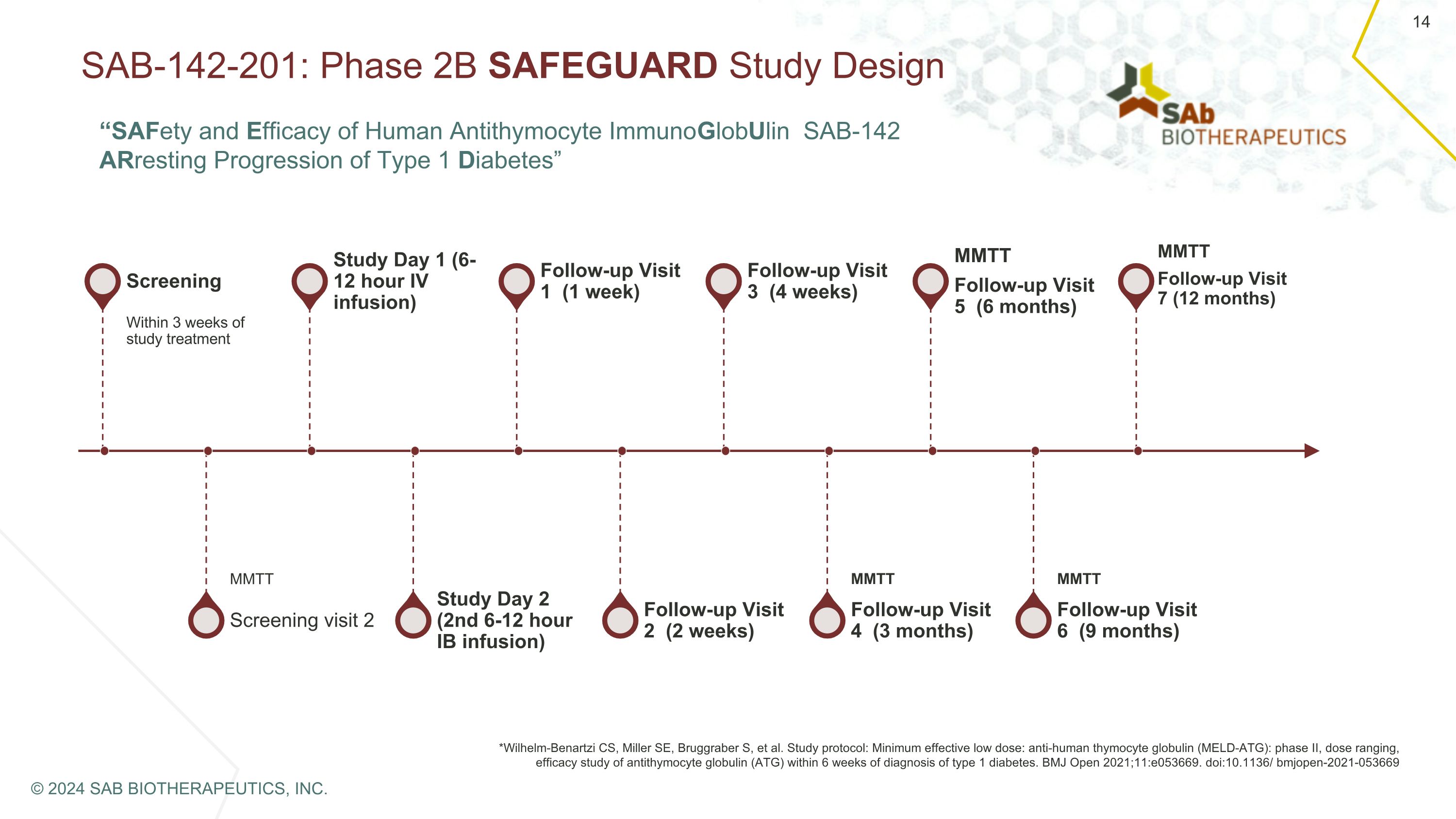
SAB-142-201: Phase 2B SAFEGUARD Study Design Screening Within 3 weeks of study treatment Screening visit 2 MMTT Study Day 1 (6-12 hour IV infusion) Study Day 2 (2nd 6-12 hour IB infusion) Follow-up Visit 1 (1 week) Follow-up Visit 2 (2 weeks) Follow-up Visit 3 (4 weeks) Follow-up Visit 4 (3 months) MMTT MMTT Follow-up Visit 5 (6 months) Follow-up Visit 6 (9 months) MMTT *Wilhelm-Benartzi CS, Miller SE, Bruggraber S, et al. Study protocol: Minimum effective low dose: anti-human thymocyte globulin (MELD-ATG): phase II, dose ranging, efficacy study of antithymocyte globulin (ATG) within 6 weeks of diagnosis of type 1 diabetes. BMJ Open 2021;11:e053669. doi:10.1136/ bmjopen-2021-053669 MMTT Follow-up Visit 7 (12 months) © 2024 SAB BIOTHERAPEUTICS, INC. “SAFety and Efficacy of Human Antithymocyte ImmunoGlobUlin SAB-142 ARresting Progression of Type 1 Diabetes”
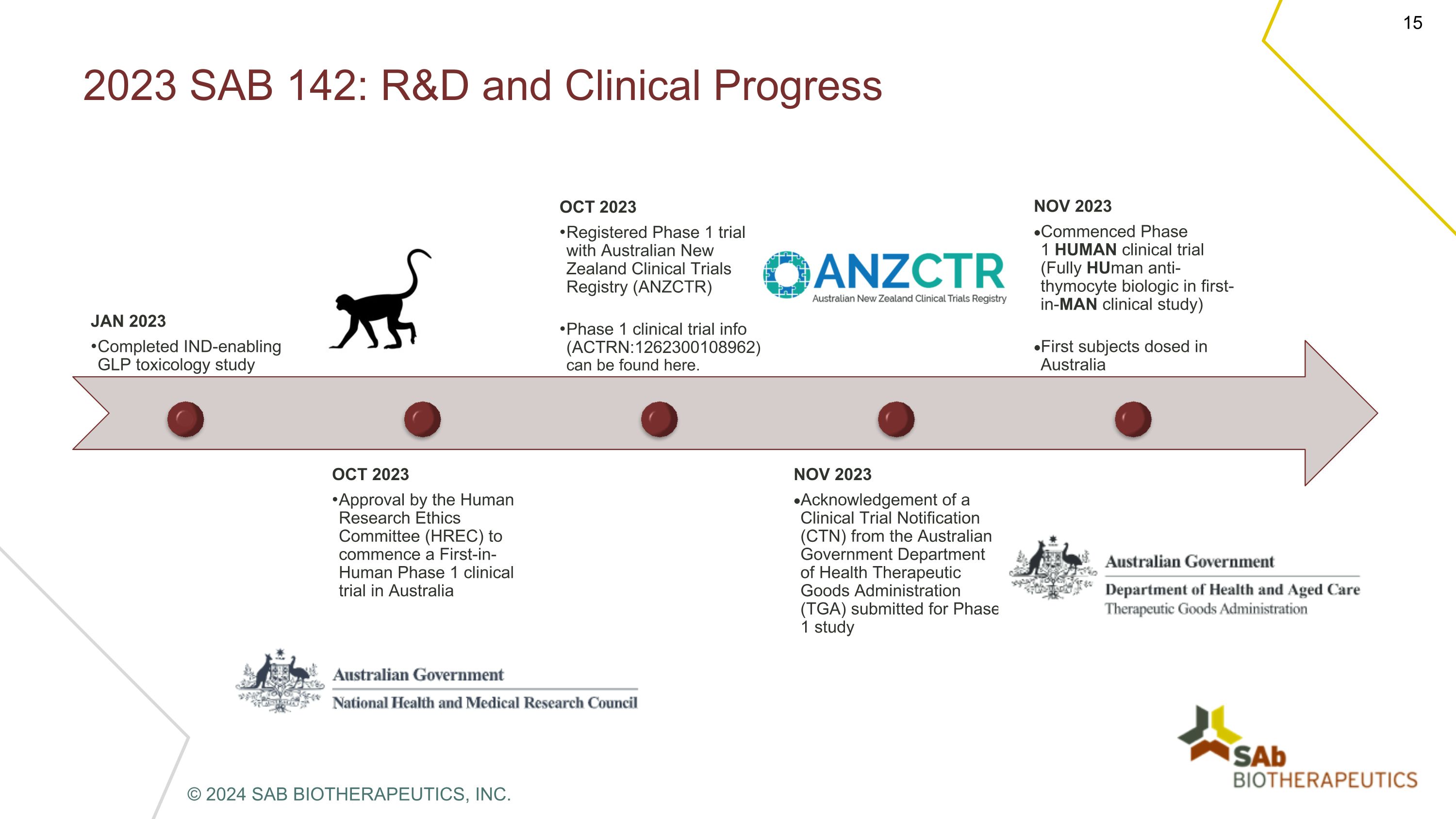
2023 SAB 142: R&D and Clinical Progress JAN 2023 Completed IND-enabling GLP toxicology study OCT 2023 Approval by the Human Research Ethics Committee (HREC) to commence a First-in-Human Phase 1 clinical trial in Australia OCT 2023 Registered Phase 1 trial with Australian New Zealand Clinical Trials Registry (ANZCTR) Phase 1 clinical trial info (ACTRN:1262300108962)can be found here. NOV 2023 Acknowledgement of a Clinical Trial Notification (CTN) from the Australian Government Department of Health Therapeutic Goods Administration (TGA) submitted for Phase 1 study NOV 2023 Commenced Phase 1 HUMAN clinical trial (Fully HUman anti-thymocyte biologic in first-in-MAN clinical study) First subjects dosed in Australia © 2024 SAB BIOTHERAPEUTICS, INC.
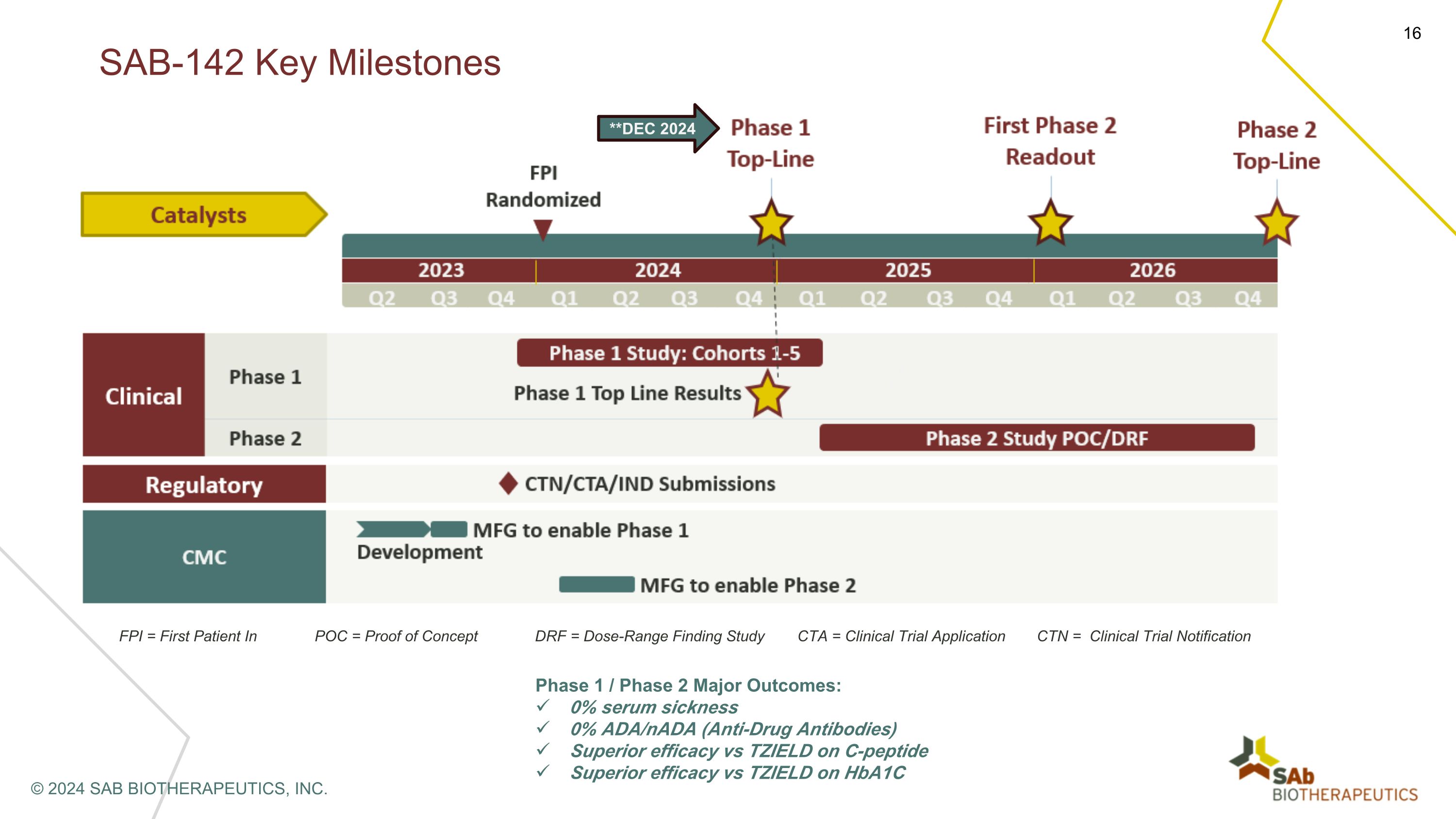
FPI = First Patient In POC = Proof of Concept DRF = Dose-Range Finding Study CTA = Clinical Trial Application CTN = Clinical Trial Notification Phase 1 / Phase 2 Major Outcomes: 0% serum sickness 0% ADA/nADA (Anti-Drug Antibodies) Superior efficacy vs TZIELD on C-peptide Superior efficacy vs TZIELD on HbA1C SAB-142 Key Milestones © 2024 SAB BIOTHERAPEUTICS, INC. **DEC 2024
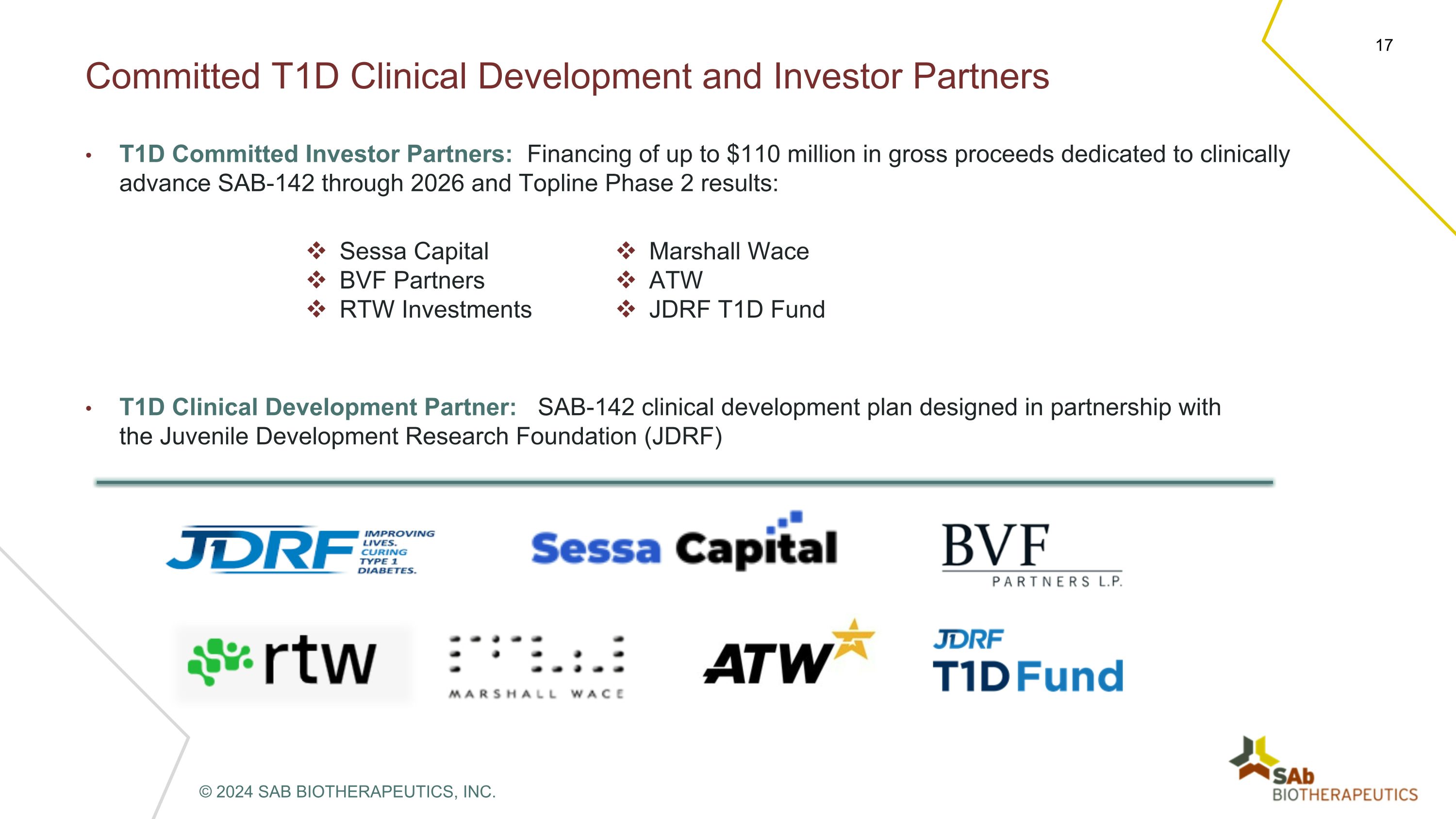
Committed T1D Clinical Development and Investor Partners 17 T1D Committed Investor Partners: Financing of up to $110 million in gross proceeds dedicated to clinically advance SAB-142 through 2026 and Topline Phase 2 results: Sessa Capital BVF Partners RTW Investments Marshall Wace ATW JDRF T1D Fund T1D Clinical Development Partner: SAB-142 clinical development plan designed in partnership with the Juvenile Development Research Foundation (JDRF) © 2024 SAB BIOTHERAPEUTICS, INC.
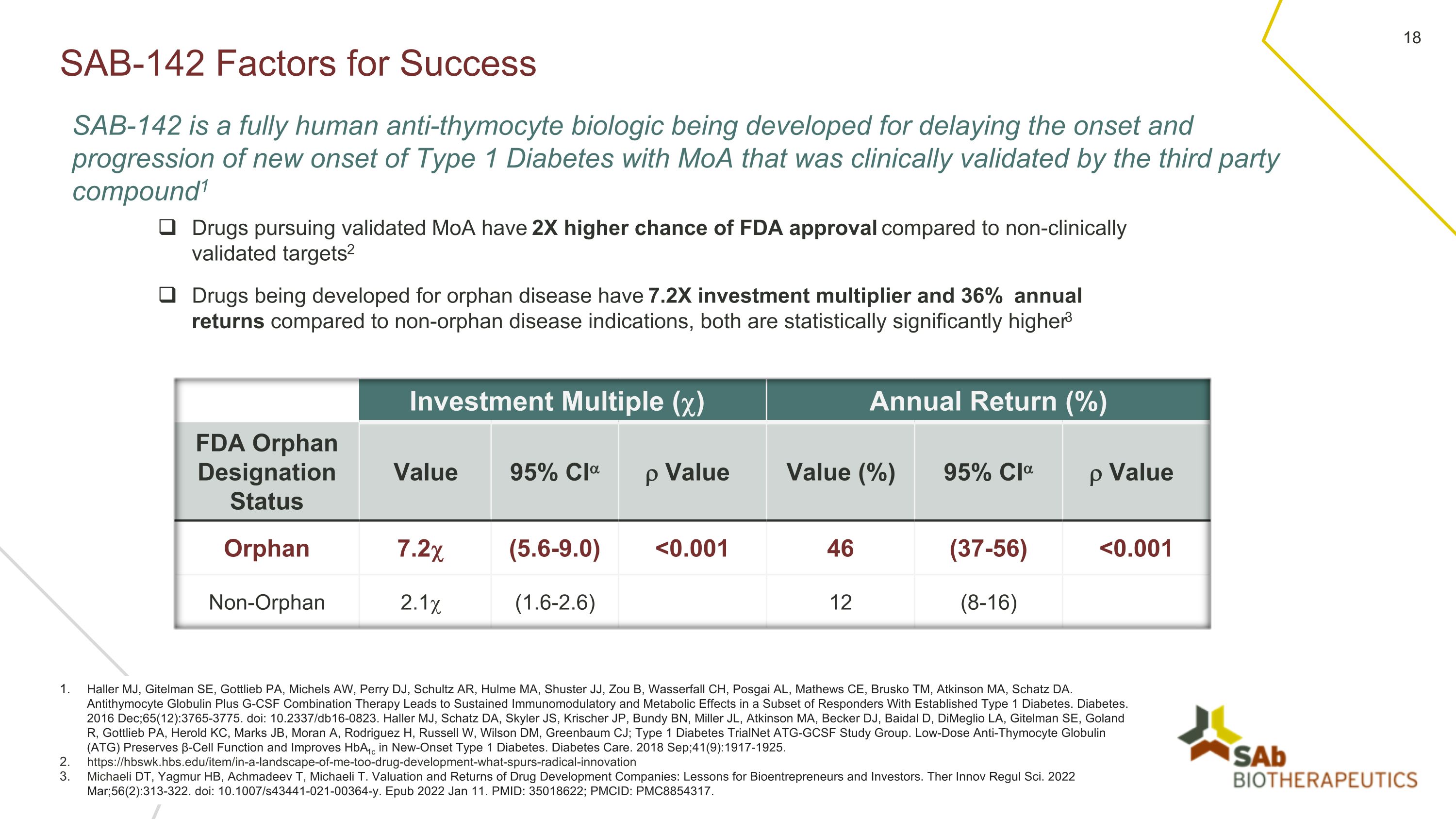
SAB-142 Factors for Success Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Perry DJ, Schultz AR, Hulme MA, Shuster JJ, Zou B, Wasserfall CH, Posgai AL, Mathews CE, Brusko TM, Atkinson MA, Schatz DA. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes. 2016 Dec;65(12):3765-3775. doi: 10.2337/db16-0823. Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, Atkinson MA, Becker DJ, Baidal D, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell W, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA1c in New-Onset Type 1 Diabetes. Diabetes Care. 2018 Sep;41(9):1917-1925. https://hbswk.hbs.edu/item/in-a-landscape-of-me-too-drug-development-what-spurs-radical-innovation Michaeli DT, Yagmur HB, Achmadeev T, Michaeli T. Valuation and Returns of Drug Development Companies: Lessons for Bioentrepreneurs and Investors. Ther Innov Regul Sci. 2022 Mar;56(2):313-322. doi: 10.1007/s43441-021-00364-y. Epub 2022 Jan 11. PMID: 35018622; PMCID: PMC8854317. SAB-142 is a fully human anti-thymocyte biologic being developed for delaying the onset and progression of new onset of Type 1 Diabetes with MoA that was clinically validated by the third party compound1 Drugs pursuing validated MoA have 2X higher chance of FDA approval compared to non-clinically validated targets2 Drugs being developed for orphan disease have 7.2X investment multiplier and 36% annual returns compared to non-orphan disease indications, both are statistically significantly higher3 Investment Multiple () Annual Return (%) FDA Orphan Designation Status Value 95% CI Value Value (%) 95% CI Value Orphan 7.2 (5.6-9.0) <0.001 46 (37-56) <0.001 Non-Orphan 2.1 (1.6-2.6) 12 (8-16)

APPENDIX © 2024 SAB BIOTHERAPEUTICS, INC.
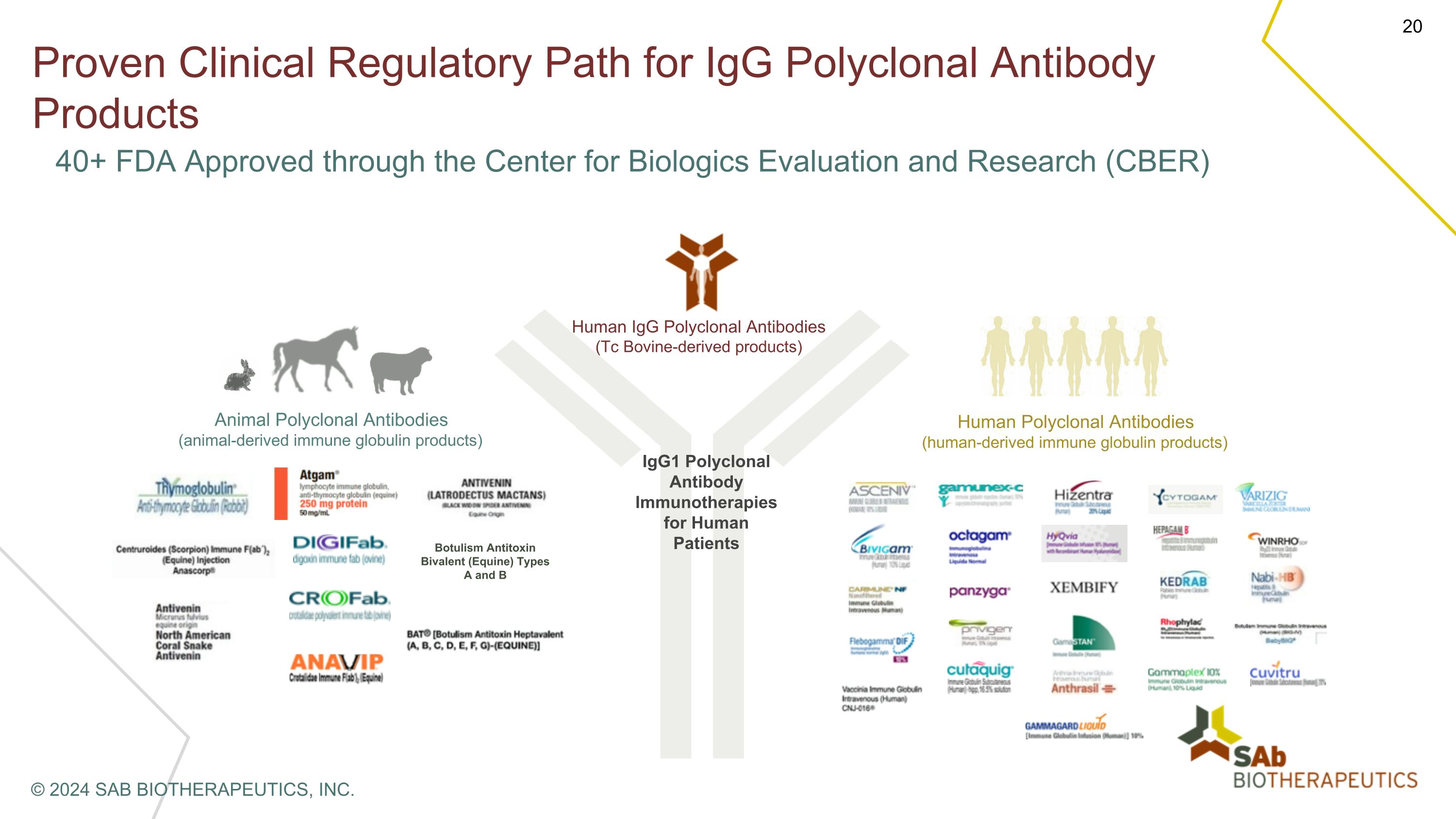
Proven Clinical Regulatory Path for IgG Polyclonal Antibody Products Human IgG Polyclonal Antibodies (Tc Bovine-derived products) Human Polyclonal Antibodies�(human-derived immune globulin products) Animal Polyclonal Antibodies (animal-derived immune globulin products) Botulism Antitoxin Bivalent (Equine) Types A and B IgG1 Polyclonal Antibody Immunotherapies �for Human �Patients 40+ FDA Approved through the Center for Biologics Evaluation and Research (CBER) © 2024 SAB BIOTHERAPEUTICS, INC.
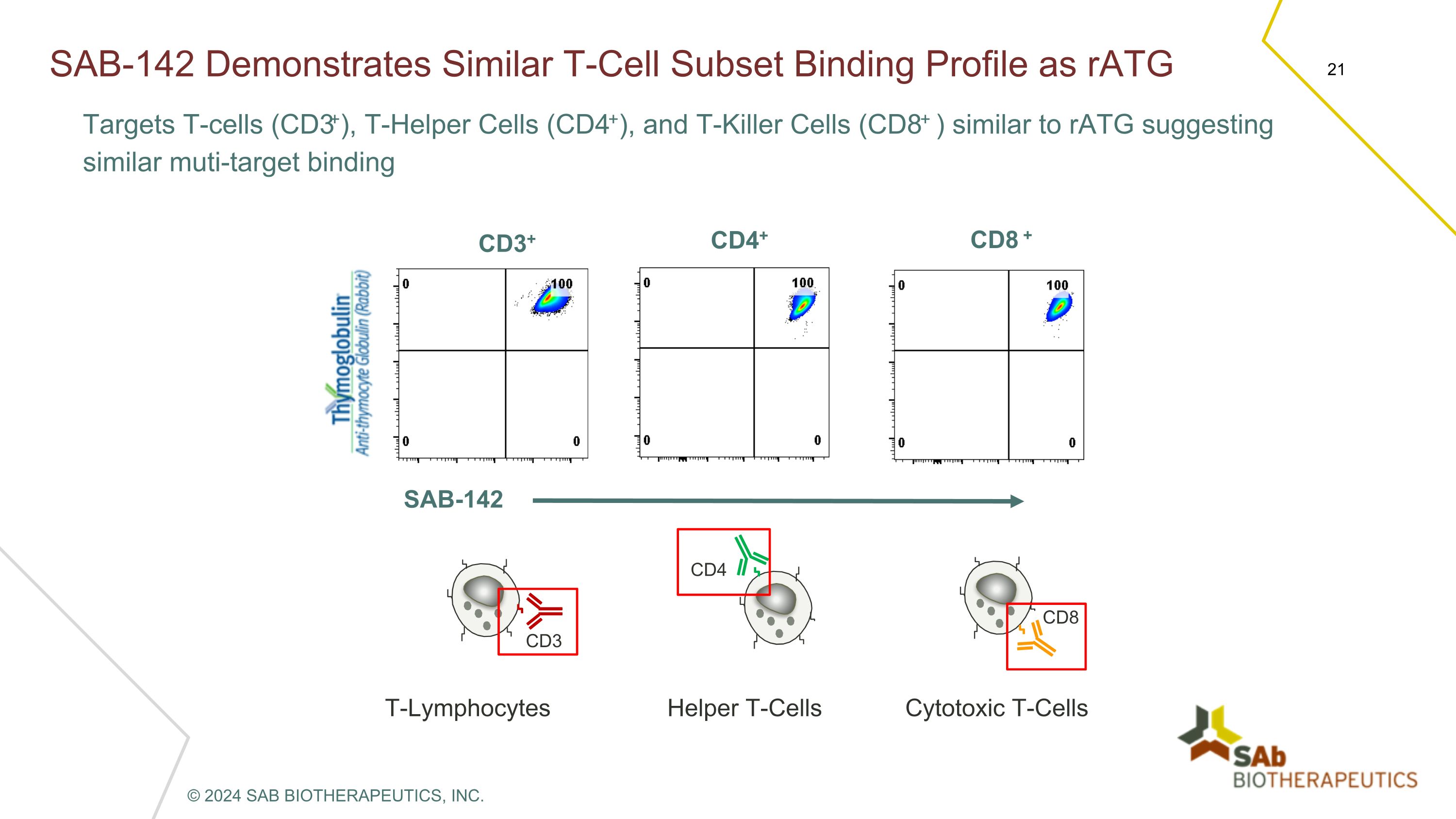
SAB-142 Demonstrates Similar T-Cell Subset Binding Profile as rATG Targets T-cells (CD3+), T-Helper Cells (CD4+), and T-Killer Cells (CD8+ ) similar to rATG suggesting similar muti-target binding CD3+ CD4+ CD8 + SAB-142 T-Lymphocytes CD3 Helper T-Cells CD4 Cytotoxic T-Cells CD8 © 2024 SAB BIOTHERAPEUTICS, INC.
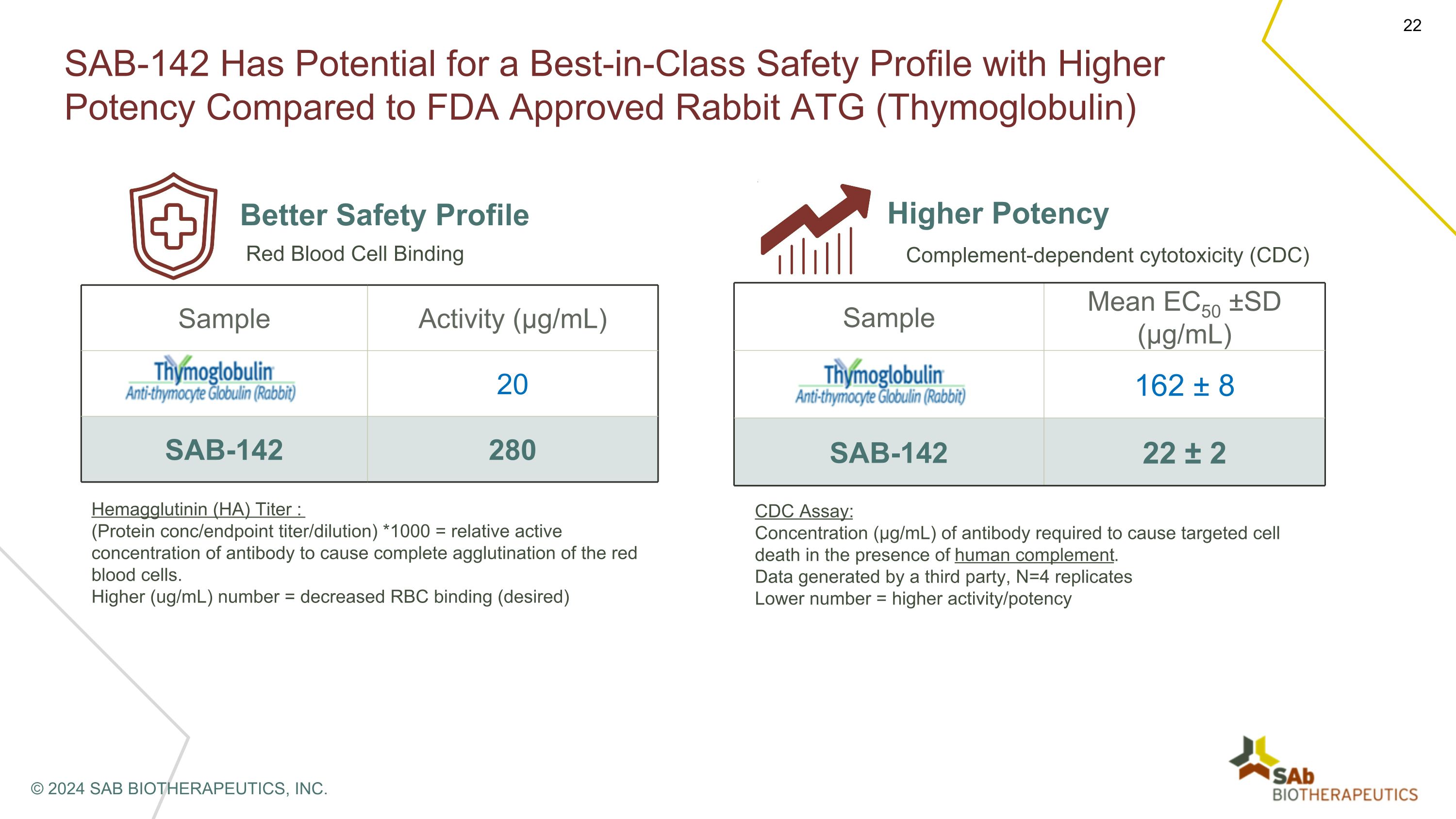
SAB-142 Has Potential for a Best-in-Class Safety Profile with Higher Potency Compared to FDA Approved Rabbit ATG (Thymoglobulin) Better Safety Profile Sample Activity (µg/mL) Thymoglobulin® 20 SAB-142 280 Red Blood Cell Binding Hemagglutinin (HA) Titer : (Protein conc/endpoint titer/dilution) *1000 = relative active concentration of antibody to cause complete agglutination of the red blood cells. Higher (ug/mL) number = decreased RBC binding (desired) Higher Potency Sample Mean EC50 ±SD (µg/mL) Thymoglobulin 162 ± 8 SAB-142 22 ± 2 Complement-dependent cytotoxicity (CDC) CDC Assay: Concentration (µg/mL) of antibody required to cause targeted cell death in the presence of human complement. Data generated by a third party, N=4 replicates Lower number = higher activity/potency © 2024 SAB BIOTHERAPEUTICS, INC.
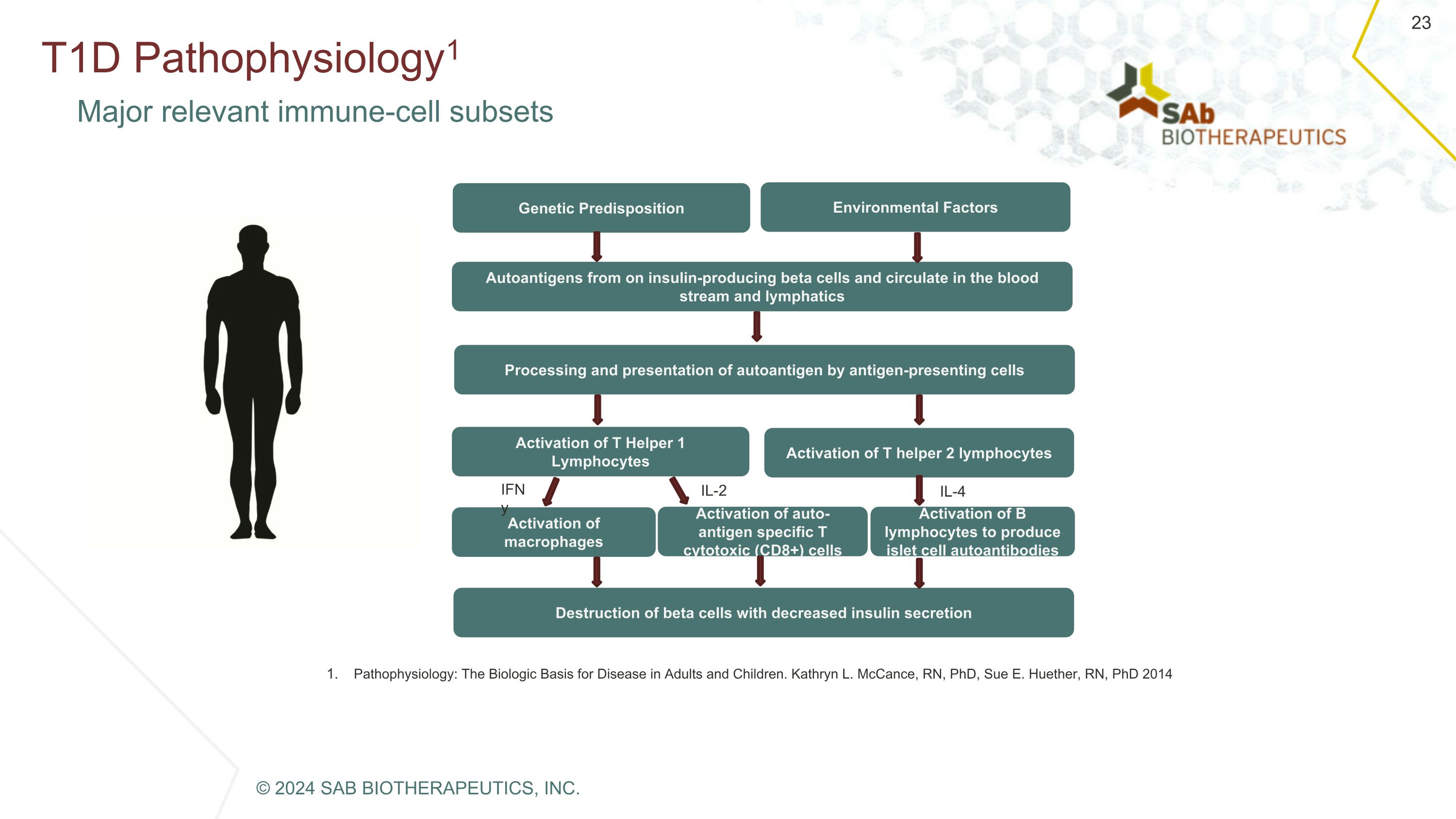
T1D Pathophysiology1 Major relevant immune-cell subsets Destruction of beta cells with decreased insulin secretion Autoantigens from on insulin-producing beta cells and circulate in the blood stream and lymphatics Processing and presentation of autoantigen by antigen-presenting cells Activation of T Helper 1 Lymphocytes Activation of T helper 2 lymphocytes Activation of macrophages Activation of B lymphocytes to produce islet cell autoantibodies Activation of auto-antigen specific T cytotoxic (CD8+) cells Environmental Factors Genetic Predisposition IFNy IL-2 IL-4 Pathophysiology: The Biologic Basis for Disease in Adults and Children. Kathryn L. McCance, RN, PhD, Sue E. Huether, RN, PhD 2014 © 2024 SAB BIOTHERAPEUTICS, INC.
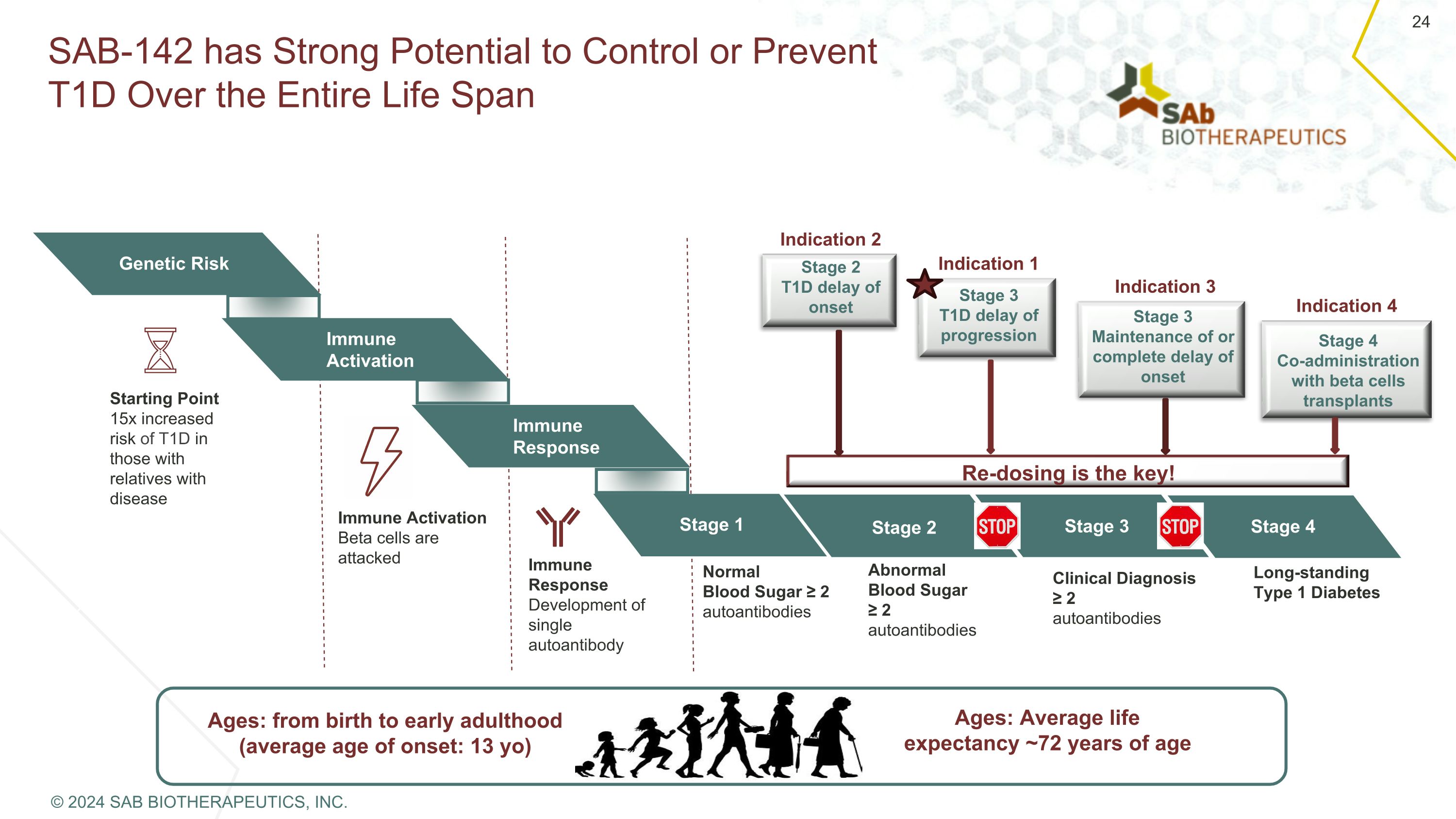
SAB-142 has Strong Potential to Control or Prevent T1D Over the Entire Life Span Ages: from birth to early adulthood (average age of onset: 13 yo) Ages: Average life expectancy ~72 years of age Genetic Risk Immune Activation Immune Response Stage 1 Starting Point 15x increased risk of T1D in those with relatives with disease Immune Activation Beta cells are attacked Immune Response Development of single autoantibody Normal Blood Sugar ≥ 2 autoantibodies Abnormal Blood Sugar ≥ 2 autoantibodies Clinical Diagnosis ≥ 2 autoantibodies Long-standing Type 1 Diabetes Stage 2 Stage 3 Stage 4 Re-dosing is the key! Stage 2 T1D delay of onset Stage 3 T1D delay of progression Stage 3 Maintenance of or complete delay of onset Stage 4 Co-administration with beta cells transplants Indication 2 Indication 1 Indication 3 Indication 4 © 2024 SAB BIOTHERAPEUTICS, INC.
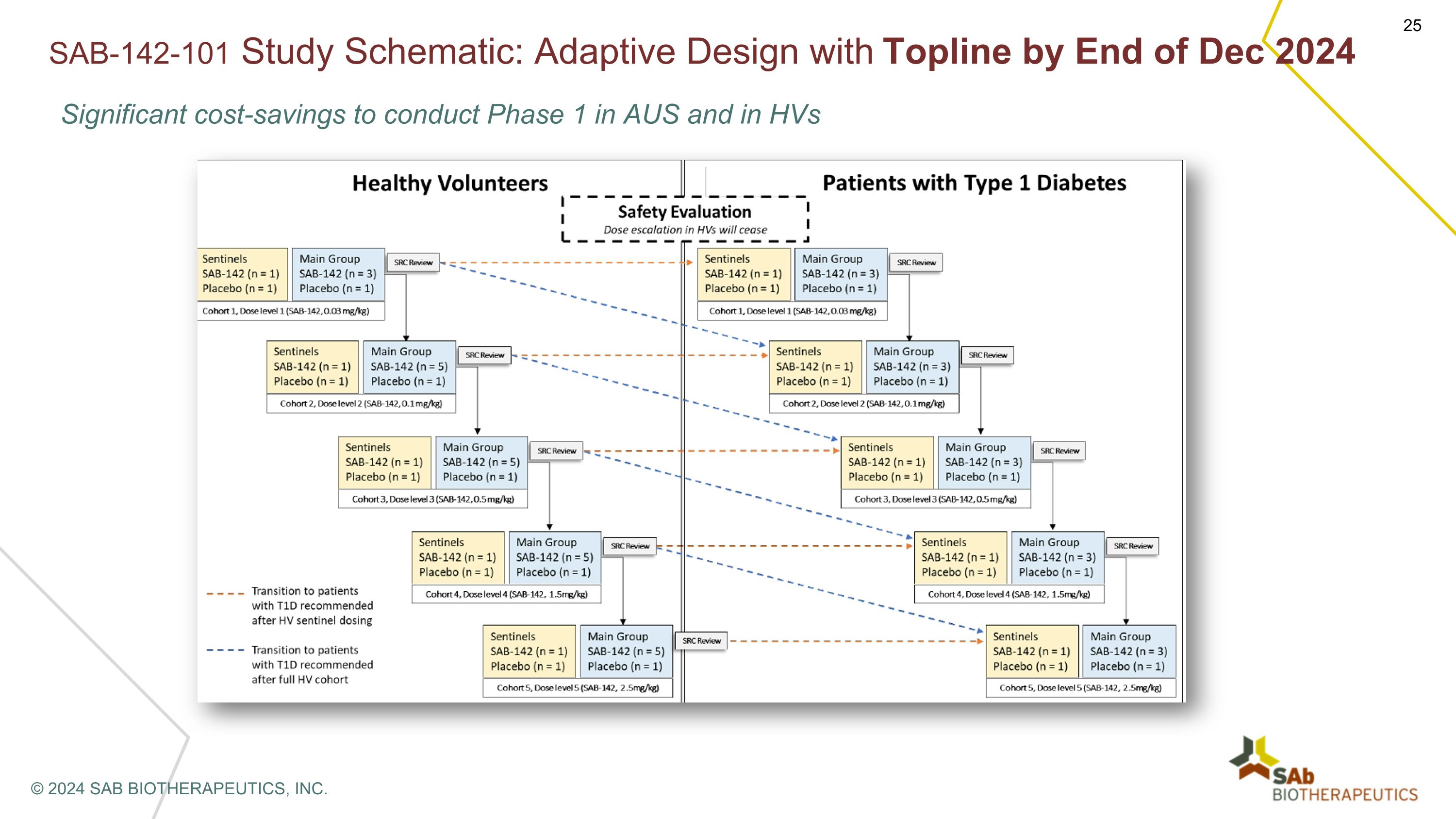
SAB-142-101 Study Schematic: Adaptive Design with Topline by End of Dec 2024 Significant cost-savings to conduct Phase 1 in AUS and in HVs © 2024 SAB BIOTHERAPEUTICS, INC.
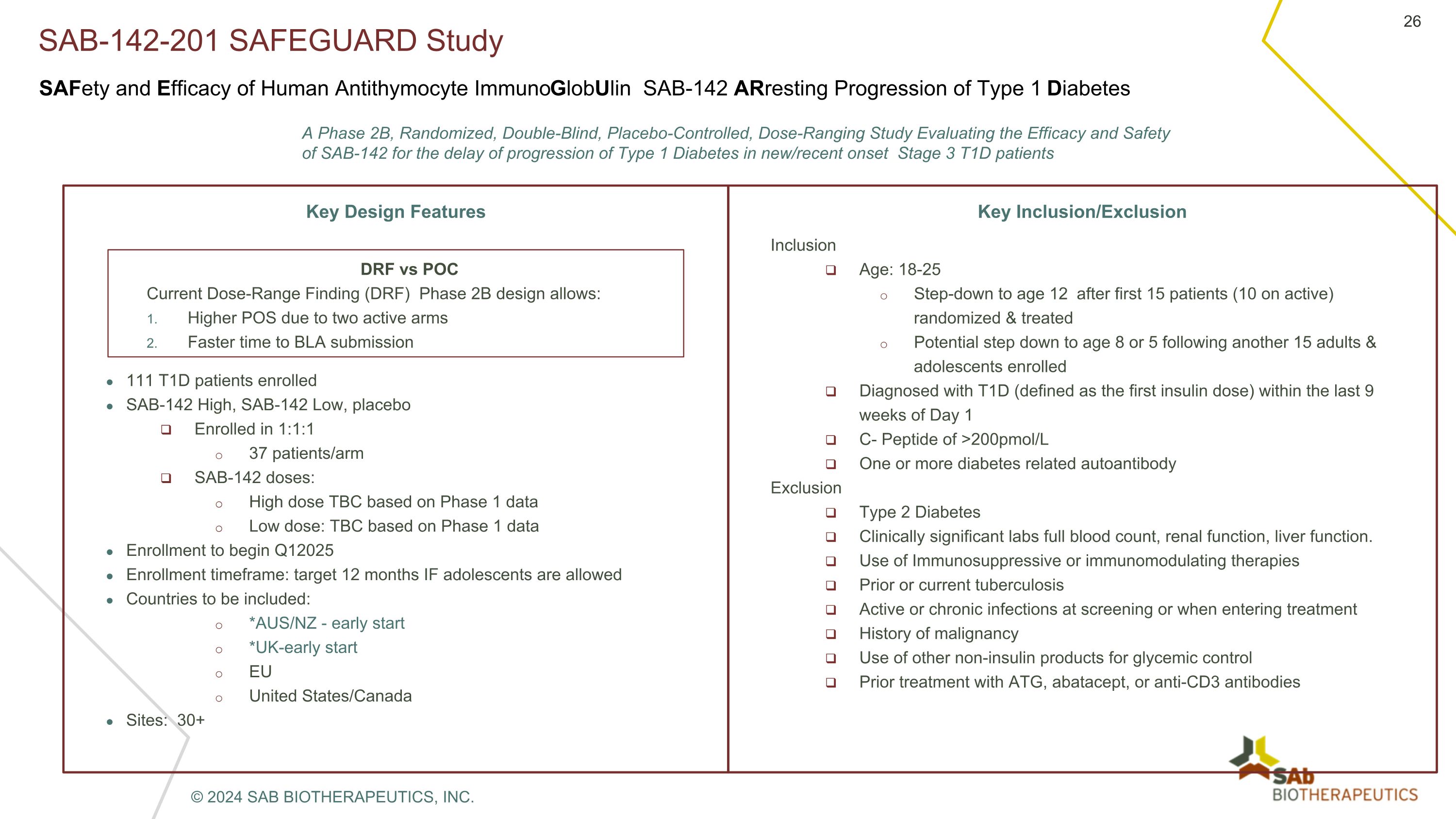
SAB-142-201 SAFEGUARD Study Key Design Features 111 T1D patients enrolled SAB-142 High, SAB-142 Low, placebo Enrolled in 1:1:1 37 patients/arm SAB-142 doses: High dose TBC based on Phase 1 data Low dose: TBC based on Phase 1 data Enrollment to begin Q12025 Enrollment timeframe: target 12 months IF adolescents are allowed Countries to be included: *AUS/NZ - early start *UK-early start EU United States/Canada Sites: 30+ Key Inclusion/Exclusion Inclusion Age: 18-25 Step-down to age 12 after first 15 patients (10 on active) randomized & treated Potential step down to age 8 or 5 following another 15 adults & adolescents enrolled Diagnosed with T1D (defined as the first insulin dose) within the last 9 weeks of Day 1 C- Peptide of >200pmol/L One or more diabetes related autoantibody Exclusion Type 2 Diabetes Clinically significant labs full blood count, renal function, liver function. Use of Immunosuppressive or immunomodulating therapies Prior or current tuberculosis Active or chronic infections at screening or when entering treatment History of malignancy Use of other non-insulin products for glycemic control Prior treatment with ATG, abatacept, or anti-CD3 antibodies A Phase 2B, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study Evaluating the Efficacy and Safety of SAB-142 for the delay of progression of Type 1 Diabetes in new/recent onset Stage 3 T1D patients DRF vs POC Current Dose-Range Finding (DRF) Phase 2B design allows: Higher POS due to two active arms Faster time to BLA submission SAFety and Efficacy of Human Antithymocyte ImmunoGlobUlin SAB-142 ARresting Progression of Type 1 Diabetes © 2024 SAB BIOTHERAPEUTICS, INC.
A Phase 2B, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study Evaluating the Efficacy and Safety of SAB-142 for the delay of progression of Type 1 Diabetes in new/recent onset Stage 3 T1D patients SAB-142-201: A Phase 2B Study 27 Primary Objective: To evaluate the change in stimulated C-Peptide response following the MMTT at Month 12 following a single dose of SAB-142 Secondary Objectives: To determine the effects of SAB-142 on time in range (TIR) To determine the effects of SAB-142 on T1D Beta score (composite end point) To determine the effects of SAB-142 on HbA1c at 3, 6, 9, and 12 months To determine the effects of SAB-142 on stimulated C-Peptide at 3, 6, 9 months. To determine the effects of SAB-142 on Insulin requirements To determine the effects of SAB-142 on the reduction of CD4+ T-cells and preservation of CD8+- T-cells To evaluate the safety parameters associated with SAB-142 To evaluate the PK and immunogenicity of SAB-142 in Stage 3 T1D patients Exploratory Objectives: To study the effects of treatment on "responder" biomarkers associated with Type 1 Diabetes © 2024 SAB BIOTHERAPEUTICS, INC.

Summary 28 SAB-142: First-in-class fully-human multi-target antibody treatment aimed to provide superior safety and efficacy for delaying onset or progression of Type 1 Diabetes. MoA of SAB-142 in T1D is clinically- validated in numerous clinical trials with rabbit ATG Safety database with human data in > 700 patients SAB antibodies produced by DIVERSITABTM platform supports anticipated zero (0) serum sickness and zero (0) neutralizing antibodies with SAB-142 in upcoming T1D studies Established Regulatory path for T1D indications and SAB-142 asset as fully human multi-epitope multi-target modality Next steps: Completion of First-in-Human Phase 1 SAB-142-101 study by the end of 2024 © 2024 SAB BIOTHERAPEUTICS, INC.
v3.23.4
Document And Entity Information
|
Jan. 19, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 19, 2024
|
| Entity Registrant Name |
SAB BIOTHERAPEUTICS, INC.
|
| Entity Central Index Key |
0001833214
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-39871
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
85-3899721
|
| Entity Address, Address Line One |
2100 East 54th Street North
|
| Entity Address, City or Town |
Sioux Falls
|
| Entity Address, State or Province |
SD
|
| Entity Address, Postal Zip Code |
57104
|
| City Area Code |
605
|
| Local Phone Number |
679-6980
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Common Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common stock, $0.0001 par value per share
|
| Trading Symbol |
SABS
|
| Security Exchange Name |
NASDAQ
|
| Warrants Each Exercisable for Common Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants, each exercisable for one share of Common Stock at an exercise price of $11.50 per share
|
| Trading Symbol |
SABSW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=sabs_WarrantsEachExercisableForOneShareOfCommonStockAtExercisePriceOf11.50PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
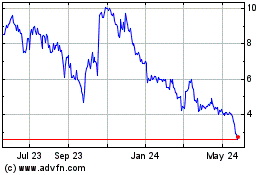
SAB Biotherapeutics (NASDAQ:SABS)
Historical Stock Chart
From Oct 2024 to Nov 2024
SAB Biotherapeutics (NASDAQ:SABS)
Historical Stock Chart
From Nov 2023 to Nov 2024