false 0001623526 0001623526 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
Stoke Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-38938 |
|
47-1144582 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 45 Wiggins Ave |
|
|
| Bedford, Massachusetts |
|
01730 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (781) 430-8200
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
STOK |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On January 8, 2024, Stoke Therapeutics, Inc., a Delaware corporation (the “Company”), posted an updated corporate presentation with additional information to its website, in advance of making a formal presentation of such information (the “Presentation”) at the J.P. Morgan Healthcare Conference on January 10, 2024. The Company is furnishing a copy of the Presentation, a full copy of which is attached hereto as Exhibit 99.1.
The information furnished with this report, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
Additionally, on January 8, 2024, the Company announced updated anticipated timing of certain milestones, including the following:
STK-001: Dravet Syndrome
| |
• |
|
In the first quarter of 2024, the Company plans to report additional clinical and modeling data from 81 patients treated in the Phase 1/2a studies of STK-001 (MONARCH and ADMIRAL) and the two ongoing open-label extension studies (OLE) (SWALLOWTAIL and LONGWING), including: |
| |
• |
|
Safety, pharmacokinetic modeling, and cerebrospinal fluid results; |
| |
• |
|
Seizure frequency data from approximately 20 patients who received 1, 2, or 3 initial doses of 70mg of STK-001 and were followed for six months; |
| |
• |
|
The effects of repeat doses of STK-001 (30mg, 45mg) on seizure frequency and cognition and behavior from patients treated in the SWALLOWTAIL and LONGWING OLE studies. |
| |
• |
|
Pending the results of the Q1 data readout, the Company plans to proceed with Phase 3 preparation activities, including discussions with global regulatory agencies, availability of chronic toxicology data, preparation of the investigator brochure, submission of a final protocol to regulatory agencies and institutional review boards. |
STK-002: Autosomal Dominant Optic Atrophy
| |
• |
|
Phase 1 study (OSPREY) of STK-002 is expected to start in the UK in 2024. |
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein that do not describe historical facts, including, but not limited to the ability of STK-001 to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in behavior or cognition at the indicated dosing levels or at all; the timing and expected progress of clinical trials, data readouts and presentations for STK-001 and STK-002; the timing of regulatory interactions or the outcome thereof; and the Company’s cash runway. Statements including words such as “anticipate,” “plan,” “will,” “continue,” “expect,” or “ongoing” and statements in the future tense are forward- looking statements. These forward-looking statements involve risks and uncertainties, as well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks and uncertainties related to: the Company’s ability to advance, obtain regulatory approval of, and ultimately commercialize its product candidates; the timing of data readouts and interim and final results of preclinical and clinical trials; positive results in a clinical trial may not be replicated in subsequent trials or successes in early stage clinical trials may not be predictive of results in later stage trials; preliminary interim data readouts of ongoing trials may show results that change when such trials are completed; the Company’s ability to fund development activities and achieve development goals into 2025; the Company’s ability to protect its intellectual property; the direct or indirect impact of global business, political and macroeconomic conditions, including inflation, interest rate volatility, cybersecurity events, uncertainty with respect to the federal budget, instability in the global banking system and volatile market conditions, and global events, including public health crises, and ongoing geopolitical conflicts, such as the conflicts in Ukraine and the Middle East; and other risks and uncertainties described under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, its quarterly reports on Form 10-Q, and the other documents the Company files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
STOKE THERAPEUTICS, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Stephen J. Tulipano |
|
|
|
|
|
|
Stephen J. Tulipano Chief Financial Officer |

Exhibit 99.1 Stoke Therapeutics NASDAQ: STOK Edward M. Kaye, M.D. Chief
Executive Officer nd 42 Annual J.P. Morgan Healthcare Conference January 10, 2024 © Copyr ©i g Ch op t 2024 yrightS 2024 toke T Sh tok era ep T e h u e tr ic as peutic1 s

Disclaimer This presentation has been prepared by Stoke Therapeutics,
Inc. ( Stoke or us ) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or Stoke or any officer, director,
employee, agent or advisor of Stoke. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provided in this presentation speaks only as of the date hereof. Stoke assumes no obligation
to publicly update any information or forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments, subsequent events, or circumstances after the date hereof, or
to reflect the occurrence of unanticipated events. This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, the
ability of STK-001 to treat the underlying causes of Dravet syndrome and reduce seizures or show improvements in behavior or cognition at the indicated dosing levels or at all; the timing and expected progress of clinical trials, data readouts and
presentations for STK-001 and STK-002; the timing of regulatory interactions or the outcomes thereof; our future operating results, financial position and cash runway; and our expectations, plans, aspirations and goals, including those related to
the goals of our collaboration with Acadia. Statements including words such as anticipate, plan, will, continue, expect, or ongoing and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and
uncertainties, as well as assumptions, which, if they prove incorrect or do not fully materialize, could cause our results to differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, risks
and uncertainties related to: Stoke's ability to advance, obtain regulatory approval of, and ultimately commercialize its produce candidates; the timing of data readouts and interim and final results of preclinical and clinical trials; positive
results in a clinical trial may not be replicated in subsequent trials or successes in early stage clinical trials may not be predictive of results in later stage trials; preliminary interim data readouts of ongoing trials may show results that
change when such trials are completed; Stoke's ability to fund development activities and achieve development goals into 2025; Stoke's ability to protect its intellectual property; global business, political and macroeconomic conditions, including
inflation, interest rate volatility, cybersecurity events, uncertainty with respect to the federal budget, instability in the global banking system and volatile market conditions, and global events, including public health crises, and ongoing
geopolitical conflicts, such as the conflicts in Ukraine and the Middle East; and other risks and uncertainties described under the heading Risk Factors in Stoke's Annual Report on Form 10-K for the year ended December 31, 2022, its quarterly
reports on Form 10-Q and the other documentation Stoke files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to revise or
update any forward-looking statements to reflect events or circumstances after the date hereof. By attending or receiving this presentation you acknowledge that you are cautioned not to place undue reliance on these forward-looking statements, which
speak only as of the date such statements are made; you will be solely responsible for your own assessment of the market and our market position; and that you will conduct your own analysis and be solely responsible for forming your own view of the
potential future performance of Stoke. © Copyright 2024 Stoke Therapeutics 2
© © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he
er ra ap pe eu ut ti ic cs s 3 3
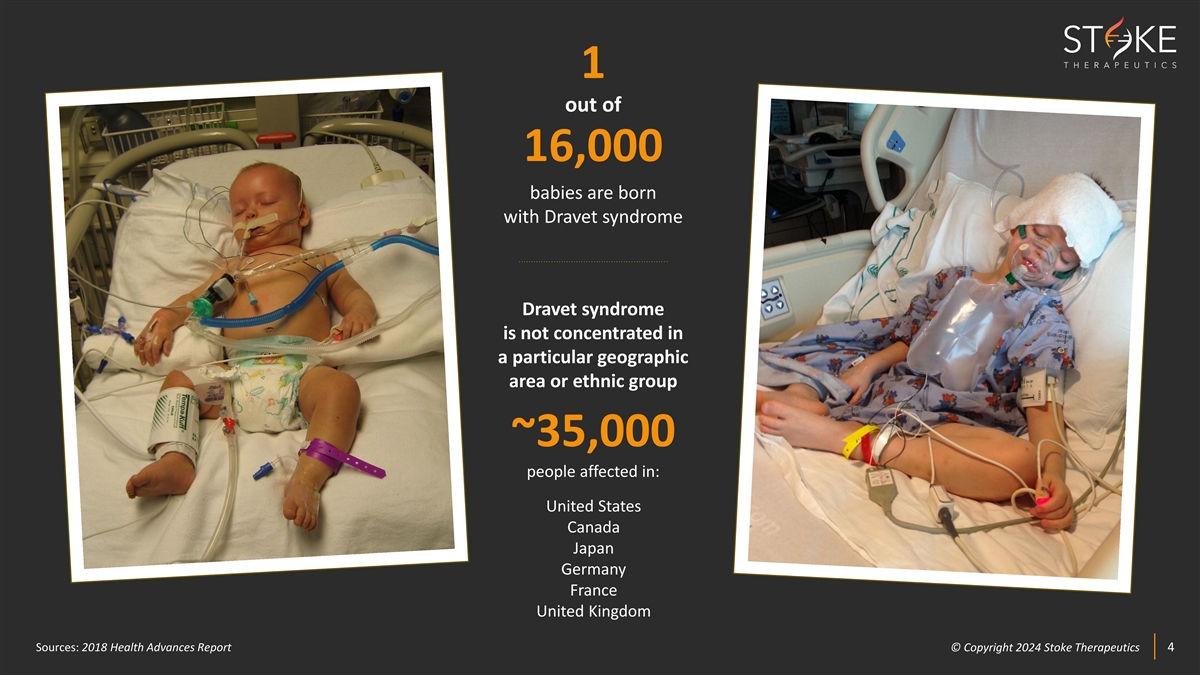
1 out of 16,000 babies are born with Dravet syndrome Dravet syndrome is
not concentrated in a particular geographic area or ethnic group ~35,000 people affected in: United States Canada Japan Germany France United Kingdom Sources: 2018 Health Advances Report © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke
e T Th he er ra ap pe eu ut ti ic cs s 4 4
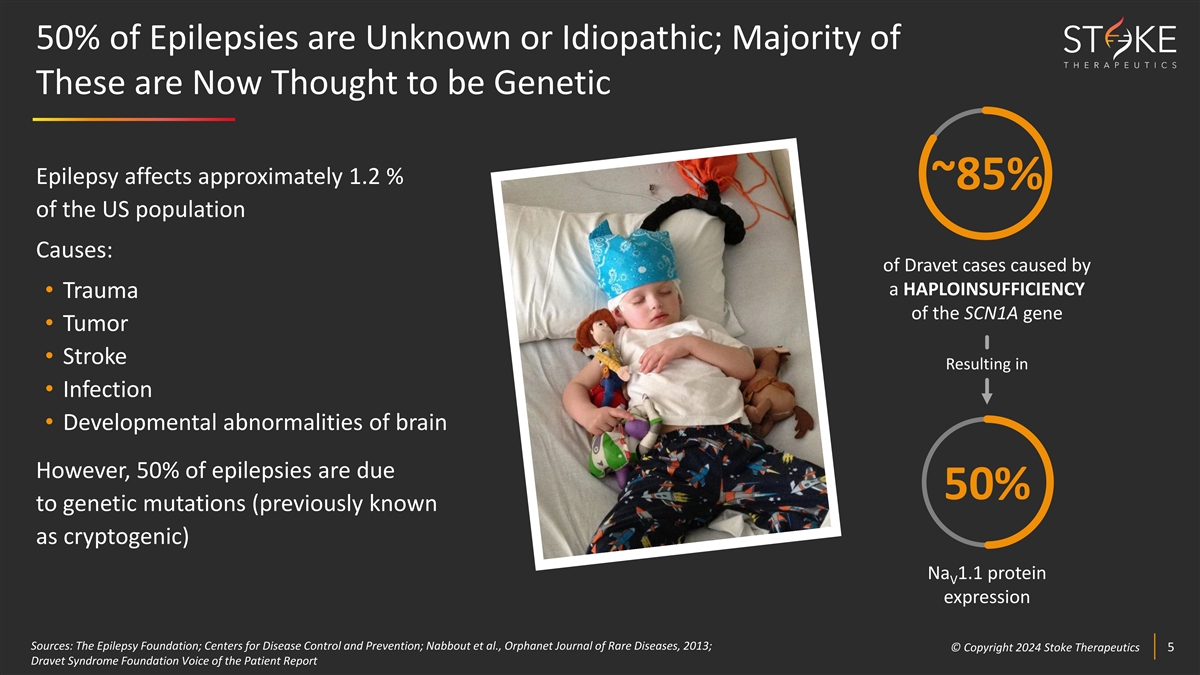
50% of Epilepsies are Unknown or Idiopathic; Majority of These are Now
Thought to be Genetic Epilepsy affects approximately 1.2 % ~85% of the US population Causes: of Dravet cases caused by a HAPLOINSUFFICIENCY • Trauma of the SCN1A gene • Tumor • Stroke Resulting in • Infection •
Developmental abnormalities of brain However, 50% of epilepsies are due 50% to genetic mutations (previously known as cryptogenic) Na 1.1 protein V expression Sources: The Epilepsy Foundation; Centers for Disease Control and Prevention; Nabbout et
al., Orphanet Journal of Rare Diseases, 2013; © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 5 5 Dravet Syndrome Foundation Voice of the Patient Report
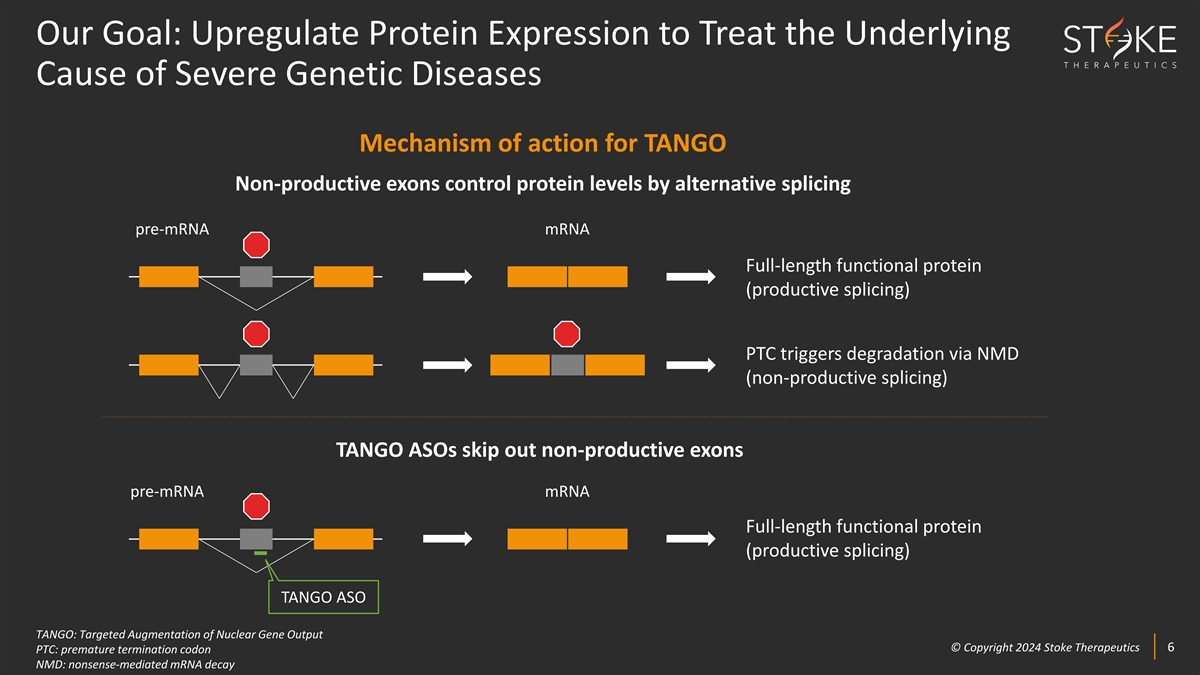
Our Goal: Upregulate Protein Expression to Treat the Underlying Cause of
Severe Genetic Diseases Mechanism of action for TANGO Non-productive exons control protein levels by alternative splicing pre-mRNA mRNA Full-length functional protein (productive splicing) PTC triggers degradation via NMD (non-productive splicing)
TANGO ASOs skip out non-productive exons pre-mRNA mRNA Full-length functional protein (productive splicing) TANGO ASO TANGO: Targeted Augmentation of Nuclear Gene Output © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra
ap pe eu ut ti ic cs s 6 6 PTC: premature termination codon NMD: nonsense-mediated mRNA decay

The Effects of Dravet Go Beyond “Just Seizures” Growth Time
Defects Loss Intellectual Disruptions Disability & Side Effects of Autonomic Developmental Nervous Delays ~20% of children and adolescents System with Dravet syndrome die before Direct Home 1 Medical adulthood, due to SUDEP , prolonged
Modifications Burden on Costs Movement seizures, seizure-related accidents or Caregiver & Balance infections Burden on Career Seizures are not Siblings Sacrifices ER Visits adequately controlled in Mood Sleep Disorders Abnormalities Language
& ~90% Speech Keto Diet Multiple Forced Disturbances Daily Retirement Medicines of people with Dravet syndrome 1 Sudden Unexpected Death in Epilepsy. © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti
ic cs s 7 7 Sources: Lagae et al., Developmental Medicine & Child Neurology, 2017; 2018 Health Advances Report; Dravet Syndrome Foundation Voice of the Patient Report
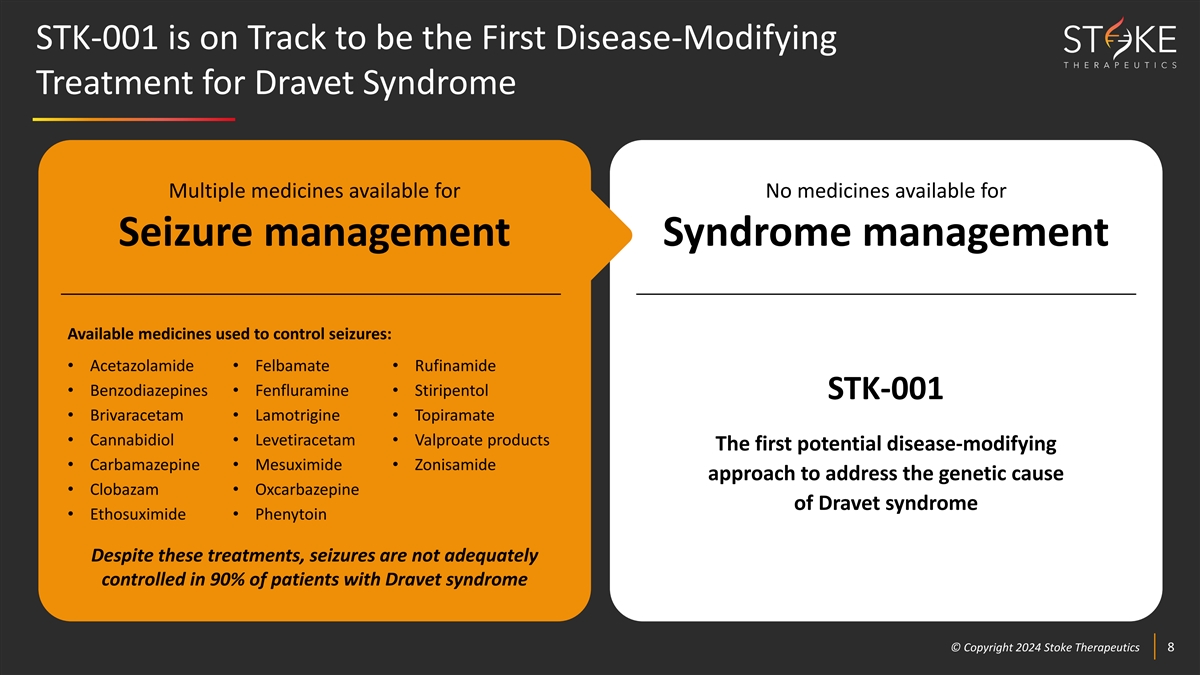
STK-001 is on Track to be the First Disease-Modifying Treatment for
Dravet Syndrome Multiple medicines available for No medicines available for Seizure management Syndrome management Available medicines used to control seizures: • Acetazolamide • Felbamate • Rufinamide • Benzodiazepines
• Fenfluramine • Stiripentol STK-001 • Brivaracetam • Lamotrigine • Topiramate • Cannabidiol • Levetiracetam • Valproate products The first potential disease-modifying • Carbamazepine •
Mesuximide • Zonisamide approach to address the genetic cause • Clobazam • Oxcarbazepine of Dravet syndrome • Ethosuximide • Phenytoin Despite these treatments, seizures are not adequately controlled in 90% of patients
with Dravet syndrome © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 8 8
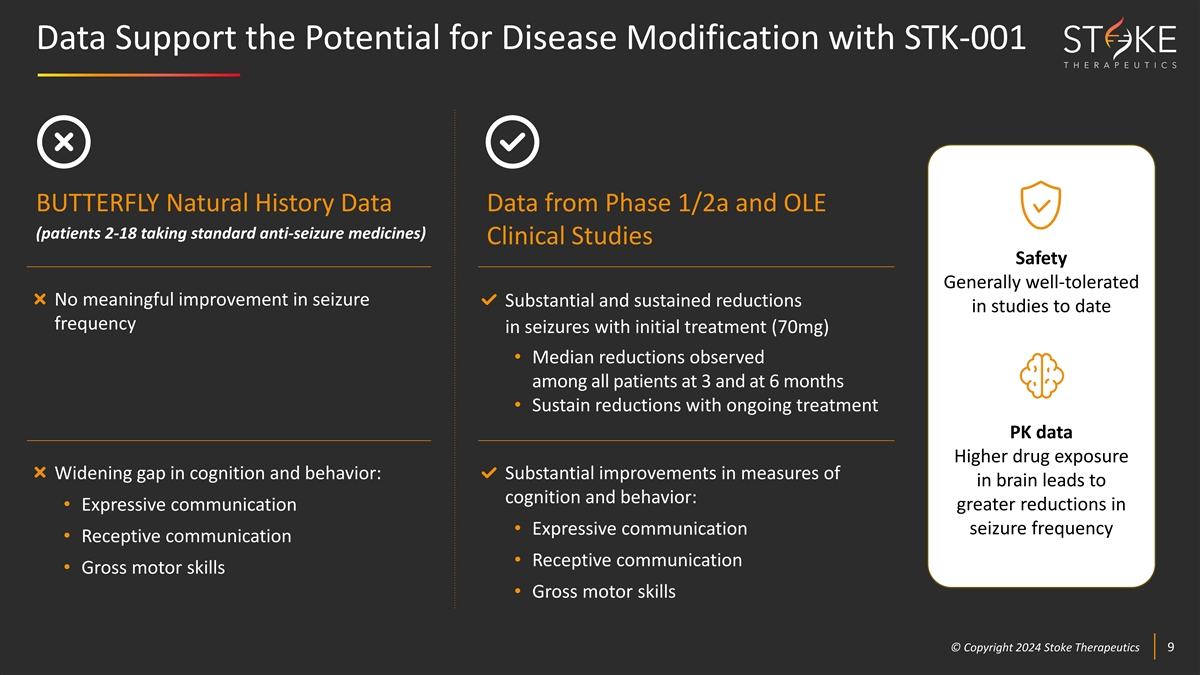
Data Support the Potential for Disease Modification with STK-001
BUTTERFLY Natural History Data Data from Phase 1/2a and OLE (patients 2-18 taking standard anti-seizure medicines) Clinical Studies Safety Generally well-tolerated No meaningful improvement in seizure Substantial and sustained reductions in studies
to date frequency in seizures with initial treatment (70mg) • Median reductions observed among all patients at 3 and at 6 months • Sustain reductions with ongoing treatment PK data Higher drug exposure Widening gap in cognition and
behavior: Substantial improvements in measures of in brain leads to cognition and behavior: greater reductions in • Expressive communication • Expressive communication seizure frequency • Receptive communication • Receptive
communication • Gross motor skills • Gross motor skills © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 9 9

No Improvement in Convulsive Seizure Frequency Despite Treatment with
Standard Anti-Seizure Medicines Over 2 Years BUTTERFLY natural history study of 2-18 years old patients with Dravet syndrome Patients were treated with the best available anti-seizure medicines Median baseline convulsive seizure frequency per 28
days (95% CI), n=26 10.0 (5.50, 15.5) Most common ongoing anti-seizure medicines, n (%) Clobazam 25 (69.4%) Fenfluramine 16 (44.4%) Stiripentol 14 (38.9%) Valproic Acid 14 (38.9%) Cannabidiol 12 (33.3%) Levetiracetam 8 (22.2%) Source: 24-Month
Analysis of BUTTERFLY: A Prospective, Observational Study to Investigate Cognition and Other Non-seizure Comorbidities in Children and Adolescents with Dravet Syndrome (DS), AES 2023. © Copyright 2024 Stoke Therapeutics 10
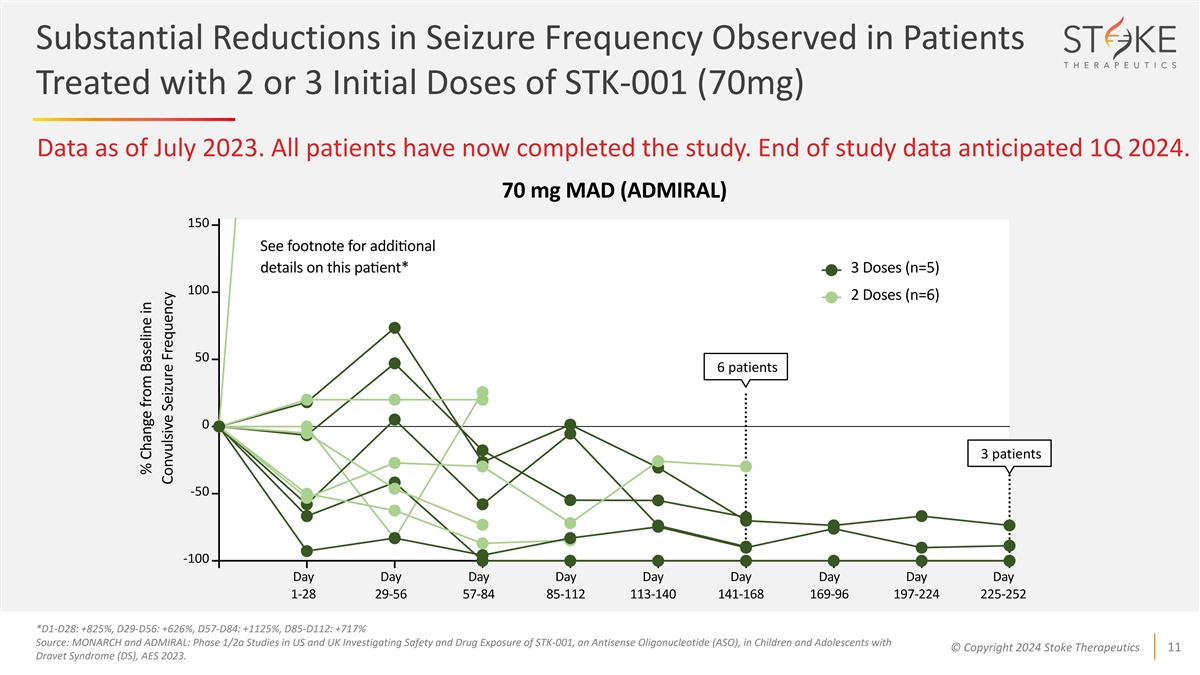
Substantial Reductions in Seizure Frequency Observed in Patients
Treated with 2 or 3 Initial Doses of STK-001 (70mg) Data as of July 2023. All patients have now completed the study. End of study data anticipated 1Q 2024. 6 patients 3 patients *D1-D28: +825%, D29-D56: +626%, D57-D84: +1125%, D85-D112: +717%
Source: MONARCH and ADMIRAL: Phase 1/2a Studies in US and UK Investigating Safety and Drug Exposure of STK-001, an Antisense Oligonucleotide (ASO), in Children and Adolescents with © Copyright 2024 Stoke Therapeutics 11 Dravet Syndrome (DS),
AES 2023.
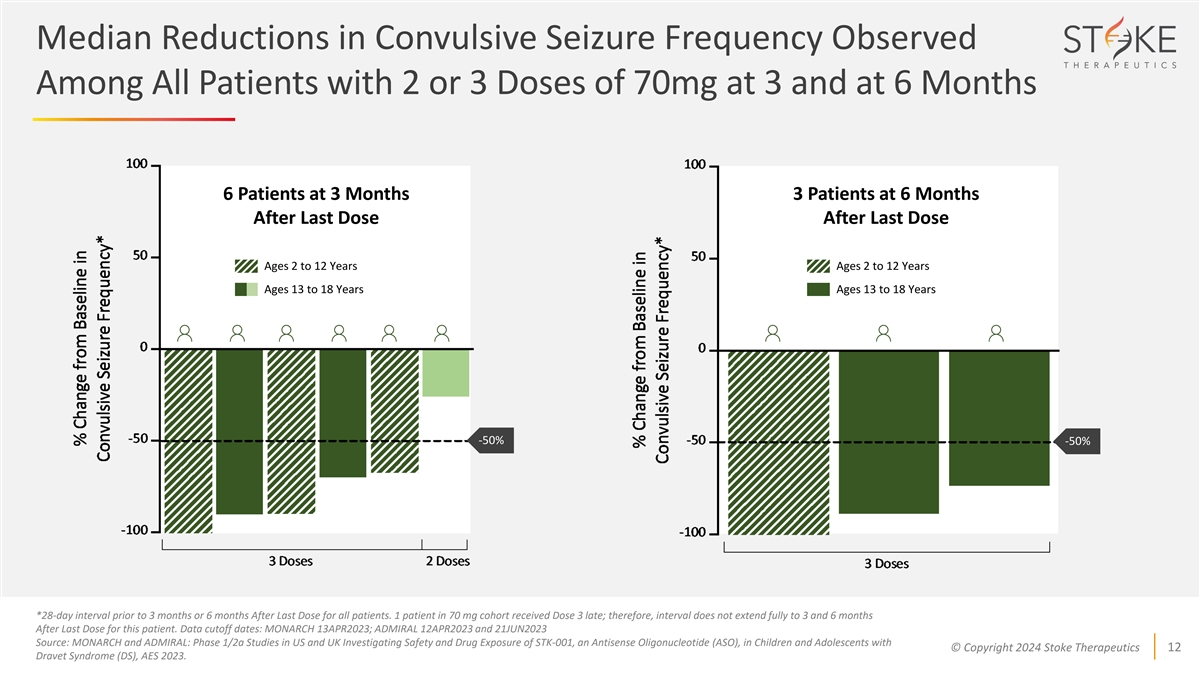
Median Reductions in Convulsive Seizure Frequency Observed Among All
Patients with 2 or 3 Doses of 70mg at 3 and at 6 Months 6 Patients at 3 Months 3 Patients at 6 Months After Last Dose After Last Dose Ages 2 to 12 Years Ages 2 to 12 Years Ages 13 to 18 Years Ages 13 to 18 Years -50% -50% *28-day interval prior to 3
months or 6 months After Last Dose for all patients. 1 patient in 70 mg cohort received Dose 3 late; therefore, interval does not extend fully to 3 and 6 months After Last Dose for this patient. Data cutoff dates: MONARCH 13APR2023; ADMIRAL
12APR2023 and 21JUN2023 Source: MONARCH and ADMIRAL: Phase 1/2a Studies in US and UK Investigating Safety and Drug Exposure of STK-001, an Antisense Oligonucleotide (ASO), in Children and Adolescents with © Copyright 2024 Stoke Therapeutics 12
Dravet Syndrome (DS), AES 2023.
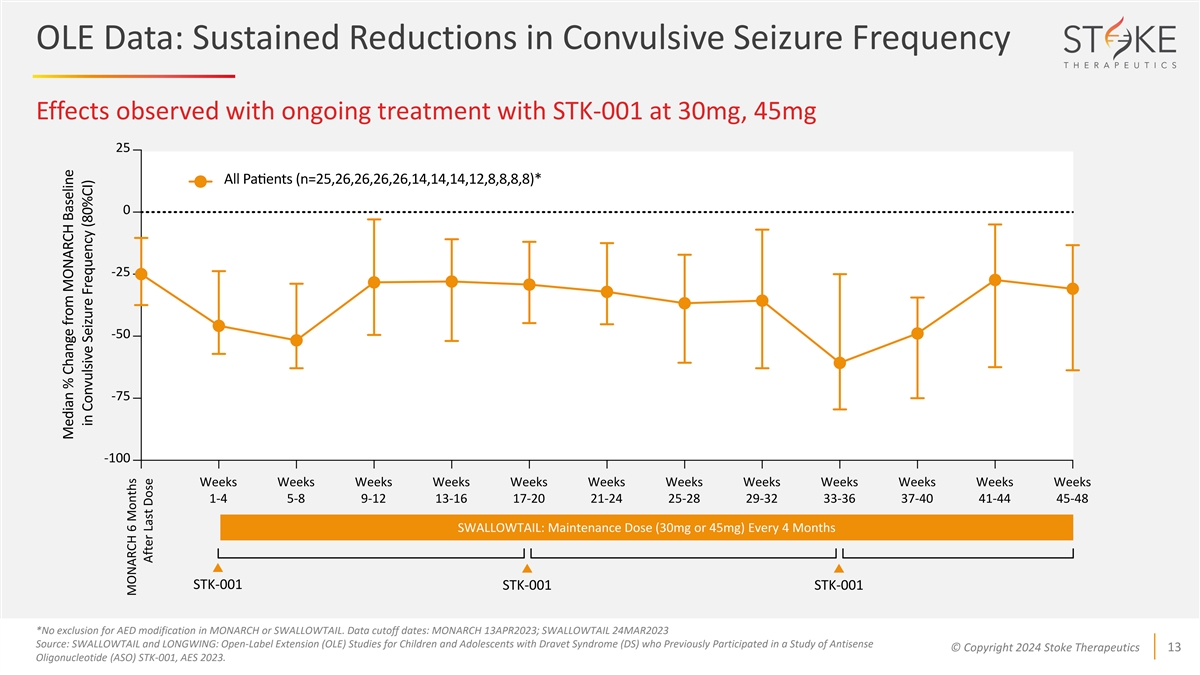
OLE Data: Sustained Reductions in Convulsive Seizure Frequency Effects
observed with ongoing treatment with STK-001 at 30mg, 45mg Weeks Weeks Weeks Weeks Weeks Weeks Weeks Weeks Weeks Weeks Weeks Weeks 1-4 5-8 9-12 13-16 17-20 21-24 25-28 29-32 33-36 37-40 41-44 45-48 SWALLOWTAIL: Maintenance Dose (30mg or 45mg) Every
4 Months STK-001 STK-001 STK-001 *No exclusion for AED modification in MONARCH or SWALLOWTAIL. Data cutoff dates: MONARCH 13APR2023; SWALLOWTAIL 24MAR2023 Source: SWALLOWTAIL and LONGWING: Open-Label Extension (OLE) Studies for Children and
Adolescents with Dravet Syndrome (DS) who Previously Participated in a Study of Antisense © Copyright 2024 Stoke Therapeutics 13 Oligonucleotide (ASO) STK-001, AES 2023. MONARCH 6 Months After Last Dose
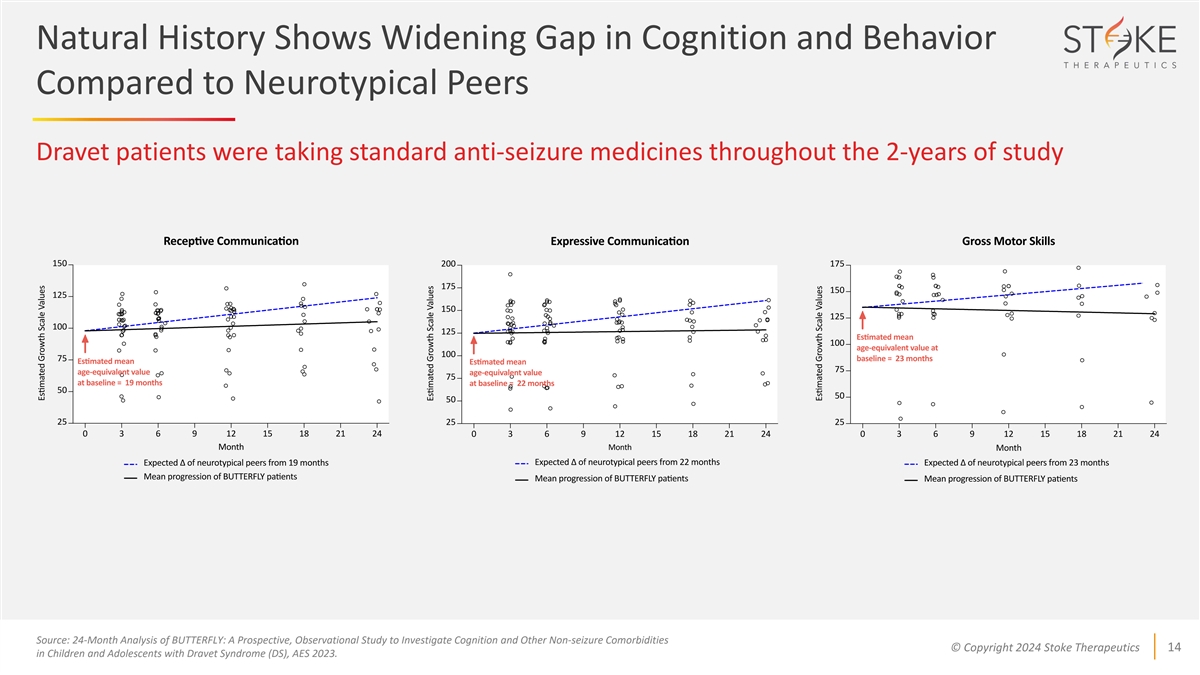
Natural History Shows Widening Gap in Cognition and Behavior Compared
to Neurotypical Peers Dravet patients were taking standard anti-seizure medicines throughout the 2-years of study Source: 24-Month Analysis of BUTTERFLY: A Prospective, Observational Study to Investigate Cognition and Other Non-seizure Comorbidities
© Copyright 2024 Stoke Therapeutics 14 in Children and Adolescents with Dravet Syndrome (DS), AES 2023.
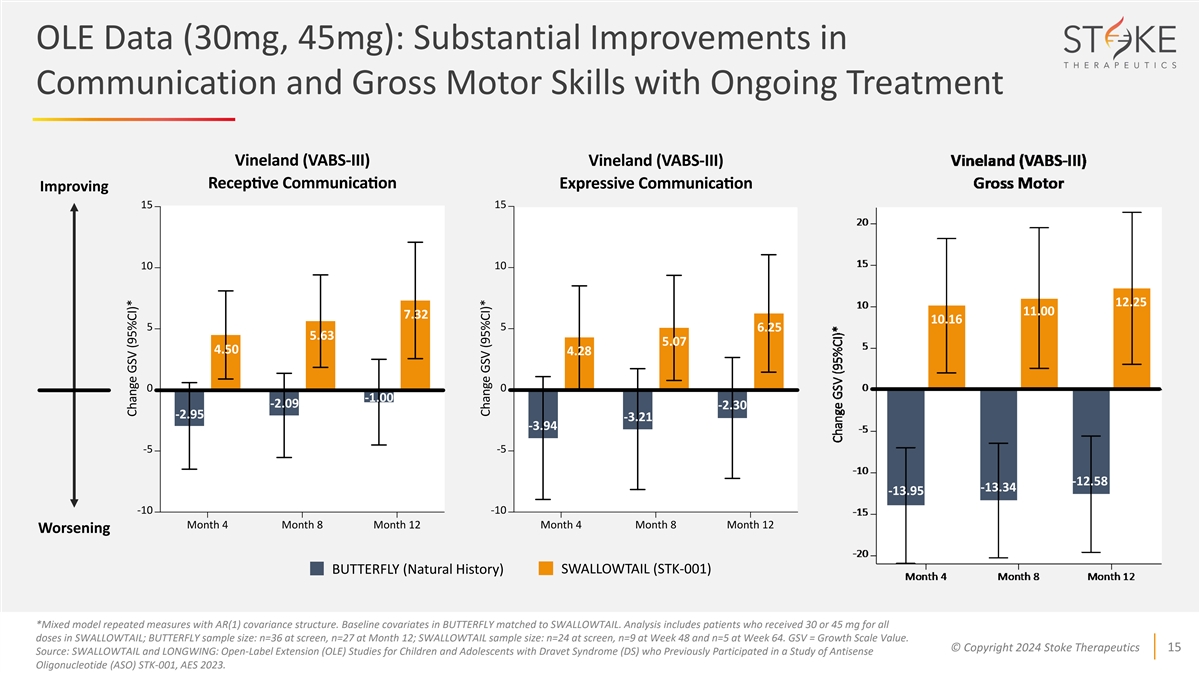
OLE Data (30mg, 45mg): Substantial Improvements in Communication and
Gross Motor Skills with Ongoing Treatment Improving 12.25 11.00 7.32 10.16 6.25 5.63 5.07 4.50 4.28 -1.00 -2.09 -2.30 -2.95 -3.21 -3.94 -12.58 -13.34 -13.95 Worsening BUTTERFLY (Natural History) SWALLOWTAIL (STK-001) *Mixed model repeated measures
with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes patients who received 30 or 45 mg for all doses in SWALLOWTAIL; BUTTERFLY sample size: n=36 at screen, n=27 at Month 12; SWALLOWTAIL sample
size: n=24 at screen, n=9 at Week 48 and n=5 at Week 64. GSV = Growth Scale Value. © Copyright 2024 Stoke Therapeutics 15 Source: SWALLOWTAIL and LONGWING: Open-Label Extension (OLE) Studies for Children and Adolescents with Dravet Syndrome
(DS) who Previously Participated in a Study of Antisense Oligonucleotide (ASO) STK-001, AES 2023.
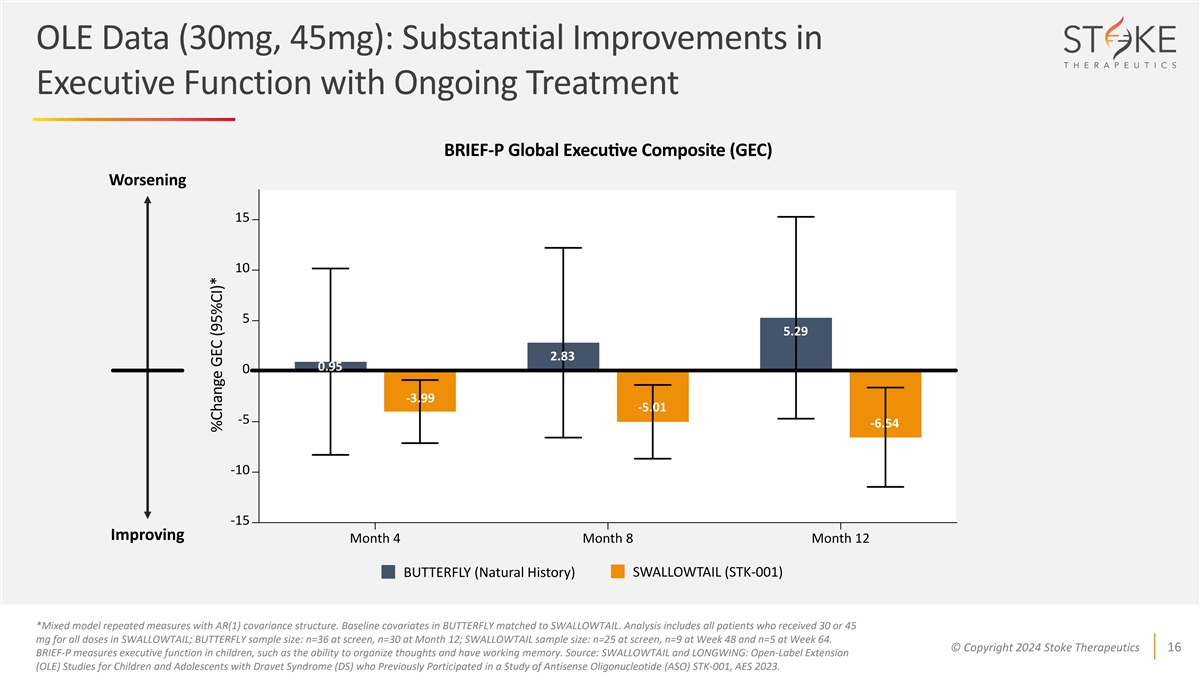
OLE Data (30mg, 45mg): Substantial Improvements in Executive Function
with Ongoing Treatment Worsening 5.29 2.83 0.95 -3.99 -5.01 -6.54 Improving SWALLOWTAIL (STK-001) BUTTERFLY (Natural History) *Mixed model repeated measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL.
Analysis includes all patients who received 30 or 45 mg for all doses in SWALLOWTAIL; BUTTERFLY sample size: n=36 at screen, n=30 at Month 12; SWALLOWTAIL sample size: n=25 at screen, n=9 at Week 48 and n=5 at Week 64. © Copyright 2024 Stoke
Therapeutics 16 BRIEF-P measures executive function in children, such as the ability to organize thoughts and have working memory. Source: SWALLOWTAIL and LONGWING: Open-Label Extension (OLE) Studies for Children and Adolescents with Dravet Syndrome
(DS) who Previously Participated in a Study of Antisense Oligonucleotide (ASO) STK-001, AES 2023.
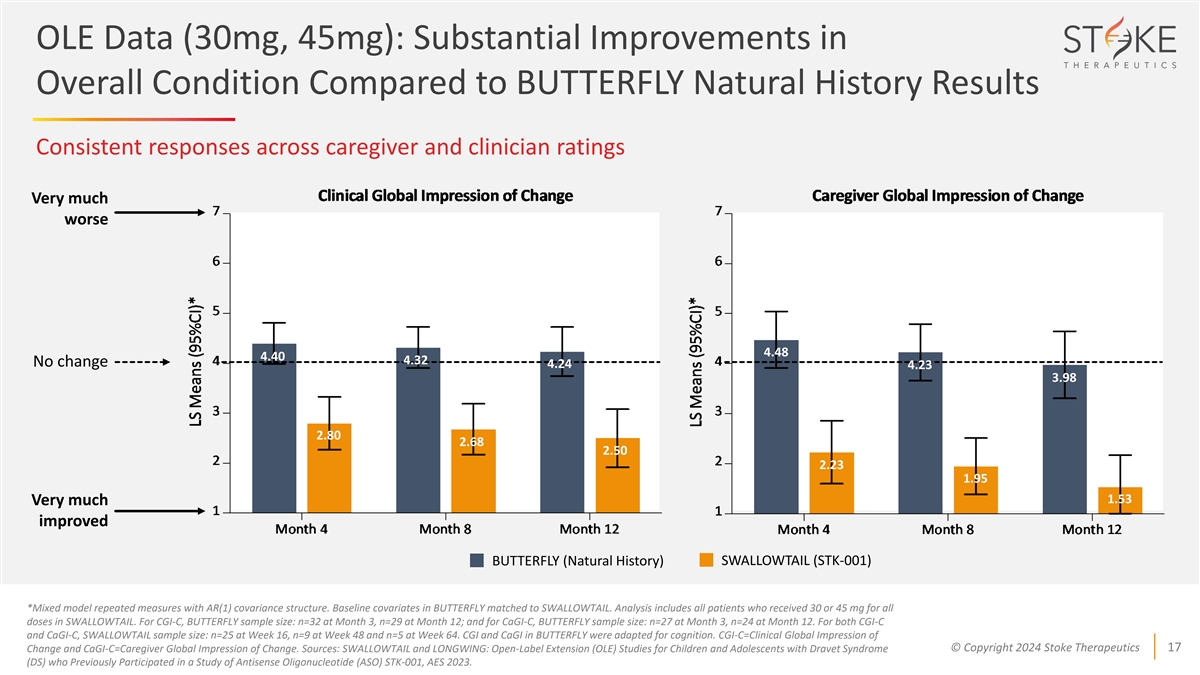
OLE Data (30mg, 45mg): Substantial Improvements in Overall Condition
Compared to BUTTERFLY Natural History Results Consistent responses across caregiver and clinician ratings Very much worse 4.48 4.40 4.32 No change 4.24 4.23 3.98 2.80 2.68 2.50 2.23 1.95 1.53 Very much improved SWALLOWTAIL (STK-001) BUTTERFLY
(Natural History) *Mixed model repeated measures with AR(1) covariance structure. Baseline covariates in BUTTERFLY matched to SWALLOWTAIL. Analysis includes all patients who received 30 or 45 mg for all doses in SWALLOWTAIL. For CGI-C, BUTTERFLY
sample size: n=32 at Month 3, n=29 at Month 12; and for CaGI-C, BUTTERFLY sample size: n=27 at Month 3, n=24 at Month 12. For both CGI-C and CaGI-C, SWALLOWTAIL sample size: n=25 at Week 16, n=9 at Week 48 and n=5 at Week 64. CGI and CaGI in
BUTTERFLY were adapted for cognition. CGI-C=Clinical Global Impression of © Copyright 2024 Stoke Therapeutics 17 Change and CaGI-C=Caregiver Global Impression of Change. Sources: SWALLOWTAIL and LONGWING: Open-Label Extension (OLE) Studies for
Children and Adolescents with Dravet Syndrome (DS) who Previously Participated in a Study of Antisense Oligonucleotide (ASO) STK-001, AES 2023.
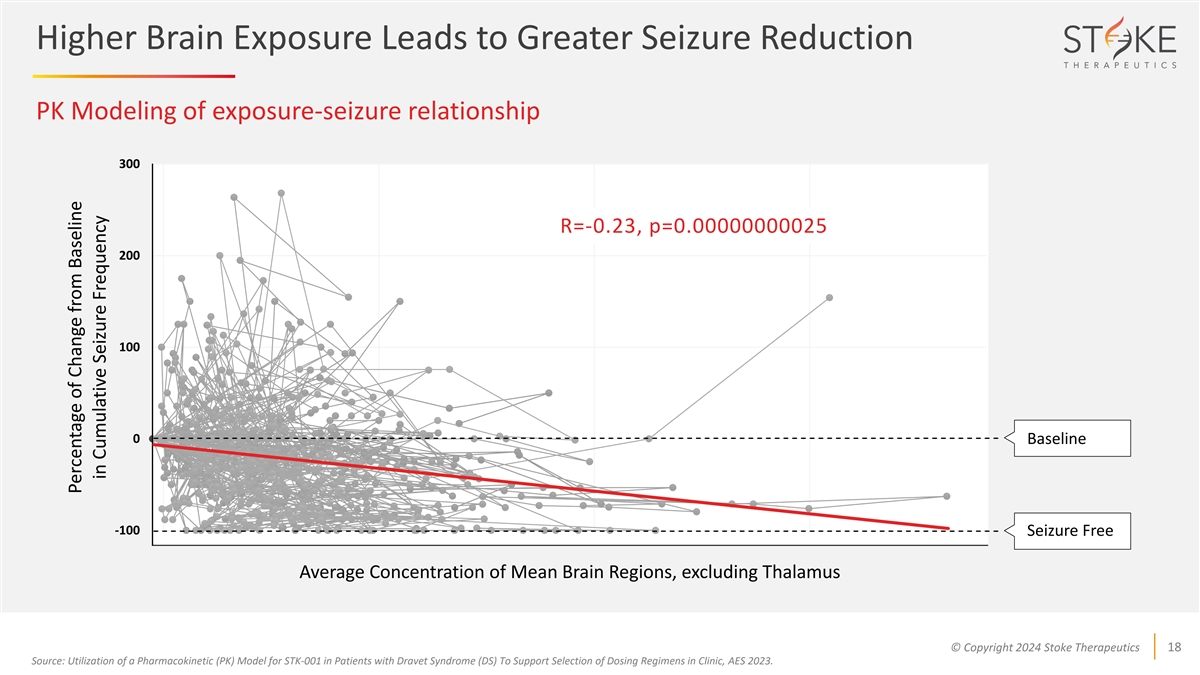
Higher Brain Exposure Leads to Greater Seizure Reduction PK Modeling of
exposure-seizure relationship 300 R=-0.23, p=0.00000000025 200 100 0 Baseline -100 Seizure Free Average Concentration of Mean Brain Regions, excluding Thalamus © Copyright 2024 Stoke Therapeutics 18 Source: Utilization of a Pharmacokinetic (PK)
Model for STK-001 in Patients with Dravet Syndrome (DS) To Support Selection of Dosing Regimens in Clinic, AES 2023. Percentage of Change from Baseline in Cumulative Seizure Frequency
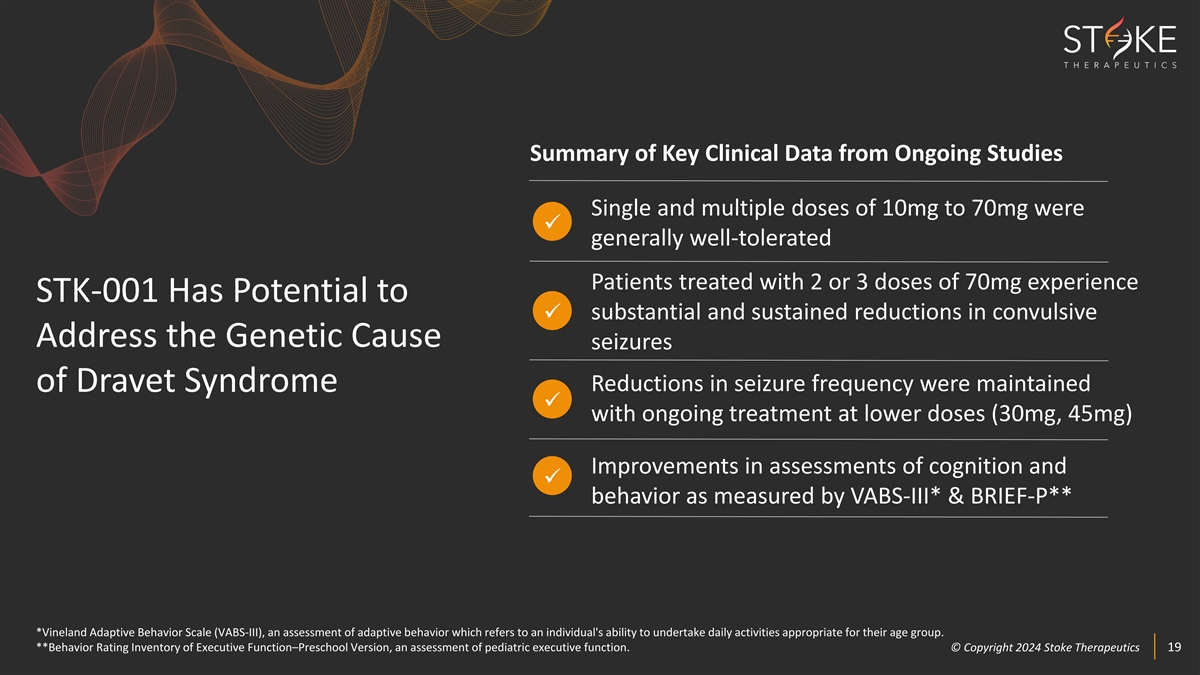
Summary of Key Clinical Data from Ongoing Studies Single and multiple
doses of 10mg to 70mg were ü generally well-tolerated Patients treated with 2 or 3 doses of 70mg experience STK-001 Has Potential to substantial and sustained reductions in convulsive ü Address the Genetic Cause seizures Reductions in
seizure frequency were maintained of Dravet Syndrome ü with ongoing treatment at lower doses (30mg, 45mg) Improvements in assessments of cognition and ü behavior as measured by VABS-III* & BRIEF-P** *Vineland Adaptive Behavior Scale
(VABS-III), an assessment of adaptive behavior which refers to an individual's ability to undertake daily activities appropriate for their age group. **Behavior Rating Inventory of Executive Function–Preschool Version, an assessment of
pediatric executive function. © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 19 19

Q1 2024 Data Readout & Next Steps Rest of Year Q1 2024 Phase 3
Preparations Key Anticipated Data qSafety, pharmacokinetic (PK) modeling, q Global regulatory interactions and cerebrospinal fluid (CSF) q Chronic toxicology data qSeizure frequency from ~20 patients who q Protocol
finalization/submission received 1, 2, or 3 initial doses of STK-001 (70mg) and were followed for six monthsq Investigator brochure qSeizure frequency, cognition and behavior q Seek IRB approvals from patients treated in the OLE
studies © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 20 20

2024 Summary of Priorities Advance STK-002 for ADOA Advance STK-001 for
Dravet • Initiate Phase 1 study (OSPREY) in 2024 Syndrome to Pivotal Develop & Expand Pipeline • Q1 Data Readout • Execute on collaboration with Acadia to advance 3 • Pending data, request Phase 3 neurodevelopmental
programs including Rett planning meetings with regulators syndrome and Syngap1 programs • Expand TANGO ASOs as a first-in-class disease- modifying approach for additional genetic diseases Current Liquidity Anticipated to Fund Operations to the
End of 2025 $214.7M in Cash, Cash Equivalents, and Marketable Securities as of 9/30/23 © © C Cop opyr yri ig gh ht t 2024 2024 S St tok oke e T Th he er ra ap pe eu ut ti ic cs s 21 21 ADOA: Autosomal dominant optic atrophy
Q&A © Copyright 2024 Stoke Therapeutics 22
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Stoke Therapeutics (NASDAQ:STOK)
Historical Stock Chart
From Oct 2024 to Oct 2024

Stoke Therapeutics (NASDAQ:STOK)
Historical Stock Chart
From Oct 2023 to Oct 2024
