Oxford Biomedica to host a free webinar showcasing its AAV expertise
August 27 2024 - 6:00AM

Oxford Biomedica to host a free webinar
showcasing its AAV expertise
Oxford, UK - 27 August
2024: On Wednesday 11 September 2024, Oxford
Biomedica, a quality and innovation-led cell and gene therapy CDMO,
will host a free 60-minute webinar on its adeno-associated virus
(AAV) development expertise and showcase their recent
breakthroughs.
The webinar titled “Advanced AAV Processing and
Potency through Characterisation of Capsid and Payload
Heterogeneity” will feature presentations by:
- Sarah
Laughlin-Toth, PhD, Senior Scientist, Analytical Development at
Oxford Biomedica
- Thomas Thiers,
Downstream Scientist, Purification Sciences at Oxford
Biomedica
- Alex Meola,
Associate Director, AAV Downstream Process Development at Oxford
Biomedica
The speakers will discuss how advanced
characterisation of AAV capsids, including their contents and
surface properties, provides crucial insights into factors
affecting product quality and potency. They will explore the
impacts of capsid modifications such as VP1 deamidation, production
conditions including bioreactor time and AAV localisation, and
capsid heterogeneity on AAV products. This knowledge is vital for
enhancing the development and manufacturing of AAV-based therapies,
with potential implications for improving product efficacy and
patient safety.
The webinar will be held at 16:00 BST /
11:00 EDT / 17:00 CET on Wednesday 11 September 2024,
followed by a live Q&A session. To register for the webinar,
please click here.
-Ends-
Enquiries:
Oxford Biomedica plc:
Sebastien Ribault, Chief Commercial Officer – T: +44 (0) 1865
509 737 / E: partnering@oxb.com
ICR Consilium:T: +44 (0)20 3709 5700 /
E: oxfordbiomedica@icrhealthcare.com
Mary-Jane Elliott / Angela Gray / Davide
Salvi
About Oxford Biomedica
Oxford Biomedica (LSE: OXB) is a quality and
innovation-led contract development and manufacturing organisation
(CDMO) in cell and gene therapy with a mission to enable its
clients to deliver life changing therapies to patients around the
world.
One of the original pioneers in cell and gene
therapy, OXB has more than 25 years of experience in viral vectors;
the driving force behind the majority of cell and gene therapies.
OXB collaborates with some of the world's most innovative
pharmaceutical and biotechnology companies, providing viral vector
development and manufacturing expertise in lentivirus,
adeno-associated virus (AAV), adenovirus, and other viral vector
types. Oxford Biomedica's world-class capabilities span from
early-stage development to commercialisation. These capabilities
are supported by robust quality-assurance systems, analytical
methods and depth of regulatory expertise.
OXB offers a vast number of unique technologies
for viral vector manufacturing, including a 4th generation
lentiviral vector system (the TetraVectaTM system), dual plasmid
system for AAV production, suspension and perfusion process using
process enhancers and stable producer and packaging cell lines.
Oxford Biomedica, a FTSE4Good constituent, is
headquartered in Oxford, UK. It has bioprocessing and
manufacturing facilities across Oxfordshire, UK, Lyon and
Strasbourg, France, and near Boston, MA, US. Learn more
at www.oxb.com, and follow us
on LinkedIn and YouTube.
Oxford Biomedica (LSE:OXB)
Historical Stock Chart
From Nov 2024 to Dec 2024
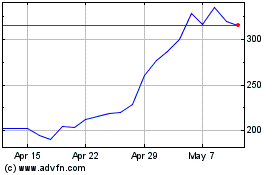
Oxford Biomedica (LSE:OXB)
Historical Stock Chart
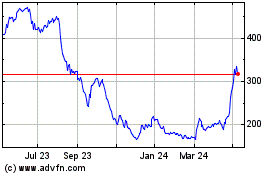
From Dec 2023 to Dec 2024