As
filed with the Securities and Exchange Commission on July 26, 2023
Registration
No. 333-2732333
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
| |
|
|
| |
|
|
Pre-Effective
Amendment No. 1 to
FORM S-1 |
| REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933 |
| |
|
|
| |
|
|
ADAMIS
PHARMACEUTICALS CORPORATION
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
82-0429727 |
(State or other jurisdiction
of incorporation or organization) |
|
(Primary Standard
Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
11682 El Camino Real, Suite 300
San Diego, CA 92130
(858)-997-2400
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Ebrahim
Versi, Chief Executive Officer
Adamis
Pharmaceuticals Corporation
11682
El Camino Real, Suite 300
San
Diego, CA 92130
(858)-997-2400
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
|
C. Kevin Kelso, Esq.
Weintraub Tobin
400 Capitol Mall, 11th Floor
Sacramento, CA 95814
(916) 558-6000 |
|
Ivan Blumenthal, Esq.
Mintz, Levin, Cohn, Ferris, Glovsky and Popeo P.C.
919 Third Avenue
New York, NY 10022
(212) 935-3000 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement is declared effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please
check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller
reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
☐
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on
such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement
filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities
and it is not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED July 26, 2023
PRELIMINARY
PROSPECTUS

Up to 5,263,158 Units consisting
of
5,263,158 Shares of Common
Stock or 5,263,158 Pre-Funded Warrants to purchase 5,263,158 Shares of Common Stock and
5,263,158 Warrants to purchase
up to 5,263,158 Shares of Common Stock
Up to 5,263,158 Shares of Common Stock Underlying
the Pre-Funded Warrants
Up to 5,263,158 Shares of Common Stock Underlying
the Common Warrants
We are offering on a reasonable best efforts basis up to 5,263,158 units,
each unit consisting of one share of common stock and one common warrant to purchase one share of common stock, at an assumed offering
price of $1.90 per unit, which is equal to the closing price of our common stock on the Nasdaq Capital Market on July 21, 2023. The common
warrants included in the units will have an exercise price of $ per share, will be exercisable immediately and will expire five (5) years
from the date of issuance. We are also offering the shares of our common stock that are issuable from time to time upon the exercise of
the common warrants included in the units.
We are also offering to certain
purchasers whose purchase of units in this offering would otherwise result in the purchaser, together with its affiliates and certain
related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately
following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded units, each pre-funded
unit consisting of one pre-funded warrant to purchase one share of common stock and one common warrant to purchase one share of common
stock, in lieu of units that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election
of the purchaser, 9.99%) of our outstanding common stock. The purchase price of each pre-funded unit will be equal to the price per unit
being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant included in the pre-funded
units will be $0.0001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of
the pre-funded warrants are exercised in full. Each common warrant included in the pre-funded units has an exercise price of $_________
per share, will be exercisable immediately and will expire five (5) years from the date of issuance. For each pre-funded unit we sell,
the number of units (and shares of common stock) we are offering will be decreased on a one-for-one basis. This offering also relates
to the shares of common stock issuable upon the exercise of the pre-funded warrants and the common warrants included in the pre-funded
units.
The shares of common stock or
pre-funded warrants, as the case may be, and the common warrants included in the units or the pre-funded units, can only be purchased
together in this offering, but the securities contained in the units or pre-funded units will be issued separately and will be immediately
separable upon issuance.
The securities will be
offered at a fixed price and are expected to be issued in a single closing. The offering will terminate on August 31, 2023, unless completed
sooner or unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date; however, notwithstanding
the foregoing, the shares of our common stock underlying the pre-funded warrants and the common warrants will be offered on a continuous
basis pursuant to Rule 415 under the Securities Act of 1933, as amended. We expect this offering to be completed not later than two business
days following the commencement of sales in this offering (after the effective date of the registration statement of which this prospectus
forms a part) and we will deliver all securities to be issued in connection with this offering delivery versus payment/receipt versus
payment upon receipt of investor funds received by us. Accordingly, neither we nor the placement agent have made any arrangements to
place investor funds in an escrow account or trust account since the placement agent will not receive investor funds in connection with
the sale of the securities offered hereunder.
Effective May 22, 2023, we
effected a 1-for-70 reverse stock split, or the Reverse Stock Split, of our outstanding shares of common stock. Unless specifically provided
otherwise herein, the share and per share information that follows in this prospectus, other than in the historical financial statements
and related notes included elsewhere or incorporated by reference in this prospectus and other information and documents incorporated
by reference into this prospectus, which have not been revised or restated to reflect the Reverse Stock Split, assumes the effect of
the Reverse Stock Split.
We have engaged Maxim Group
LLC, or the placement agent or Maxim, to act as our exclusive placement agent in connection with this offering. The placement agent has
agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is
not purchasing or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale
of any specific number of securities or dollar amount. We have agreed to pay to the placement agent the placement agent fees set forth
in the table below, which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to
be received in escrow, trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering.
We may sell fewer than all of the units and pre-funded units offered hereby, which may significantly reduce the amount of proceeds received
by us. Because there is no escrow account and no minimum number of securities or amount of proceeds, investors could be in a position
where they have invested in us, but we have not raised sufficient proceeds in this offering to adequately fund the intended uses of the
proceeds as described in this prospectus. See “Risk Factors” on page 14 of this prospectus. We will bear all costs associated
with the offering. See “Plan of Distribution” on page 42 of this prospectus for more information regarding these arrangements.
Our common stock is presently listed on The
Nasdaq Capital Market under the symbol “ADMP.” On July 21, 2023, the closing price of our common stock as reported on The
Nasdaq Capital Market was $1.90 per share. The public offering price per unit or pre-funded unit, as the case may be, will be determined
through negotiation among us, the placement agent and the investors in the offering based on market conditions at the time of pricing,
and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout
this prospectus may not be indicative of the final offering price. There is no established trading market for the pre-funded warrants
or the common warrants, and we do not expect a market to develop. We do not intend to apply for a listing of the units, the pre-funded
units, the pre-funded warrants or the common warrants on any securities exchange or other nationally recognized trading system. Without
an active trading market, the liquidity of the pre-funded warrants and the common warrants will be limited.
We
are a “smaller reporting company” under applicable federal securities laws and are subject to reduced public company reporting
requirements. Investing in our securities involves risks. See “Risk Factors” beginning on page 14 of this prospectus and elsewhere
in this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
| |
|
Per
Unit(1) |
|
|
Total |
|
| Public offering
price |
|
$ |
|
|
|
$ |
|
|
| Placement agent
fees(2) |
|
$ |
|
|
|
$ |
|
|
| Proceeds
to us (before expenses) |
|
$ |
|
|
|
$ |
|
|
| |
(1) |
Assumes
that all units consist of one share of common stock and one common warrant. |
| |
(2) |
We have agreed to
pay the placement agent a cash fee equal to 7.0% of the aggregate gross proceeds raised in this offering, and to reimburse
the placement agent for certain of its offering-related expenses. See “Plan of Distribution” for a description
of the compensation to be received by the placement agent. |
Delivery
of the securities offered hereby is expected to be made on or about ,
2023, subject to satisfaction of customary closing conditions.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
MAXIM
GROUP LLC
The
date of this prospectus is __________, 2023
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference
without charge by following the instructions under “Where You Can Find More Information.” You should carefully read
this prospectus as well as additional information described under “Incorporation of Certain Information by Reference,”
before deciding to invest in our securities.
We
have not, and the placement agent has not, authorized anyone to provide any information or to make any representations other than
those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred
you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may
give you. This prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions
where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current
only as of its date, regardless of its time of delivery or any sale of our securities. Our business, financial condition, results
of operations and prospects may have changed since that date. To the extent there is a conflict between the information contained
in this prospectus, on the one hand, and the information contained in any document filed with the Securities and Exchange Commission
before the date of this prospectus and incorporated by reference herein, on the other hand, you should rely on the information
in this prospectus. If any statement in a document incorporated by reference is inconsistent with a statement in another document
incorporated by reference having a later date, the statement in the document having the later date modifies or supersedes the
earlier statement.
Unless
otherwise indicated, all information contained or incorporated by reference in this prospectus concerning our industry in general
or any portion thereof, including information regarding our general expectations and market opportunity, is based on management’s
estimates using internal data, data from industry related publications, consumer research and marketing studies or other externally
obtained data.
For
investors outside the United States: We have not, and the placement agent has not, done anything that would permit this offering
or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in
the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about,
and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus outside the United
States.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to the
registration statement of which this prospectus is a part and in any document that is incorporated by reference herein were made
solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the
parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants
should not be relied on as accurately representing the current state of our affairs.
This
prospectus and the information incorporated by reference into this prospectus contain references to our trademarks and to trademarks
belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus and the information
incorporated by reference into this prospectus, including logos, artwork, and other visual displays, may appear without the ®
or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under
applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our
use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship
of us by, any other company.
The
Adamis Pharmaceuticals logo and other trademarks or service marks of Adamis Pharmaceuticals Corporation appearing in this prospectus
are the property of Adamis Pharmaceuticals Corporation. All other brand names or trademarks appearing in this prospectus are the
property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus may be referred
to without the ® or TM symbols, but such references should not be construed as any indicator that their respective owners
will not assert their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks
to imply a relationship with, or endorsement or sponsorship of us by, any other company.
CAUTIONARY
NOTE REGARDING FORWARD LOOKING STATEMENTS
The
statements contained in this prospectus, and the documents incorporated by reference in this prospectus, include forward-looking
statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and
Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that relate to future events
or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual
results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance
or achievements expressed or implied by these forward-looking statements. Words such as, but not limited to, “believe,”
“expect,” “anticipate,” “estimate,” “intend,” “may,” “plan,”
“potential,” “predict,” “project,” “targets,” “likely,” “will,”
“would,” “could,” “should,” “continue,” and similar expressions or phrases, or
the negative of those expressions or phrases, are intended to identify forward-looking statements, although not all forward-looking
statements contain these identifying words. Although we believe that we have a reasonable basis for each forward-looking statement
contained in this prospectus and incorporated by reference in this prospectus, we caution you that these statements are based
on our projections of the future that are subject to known and unknown risks and uncertainties and other factors that may cause
our actual results, level of activity, performance or achievements expressed or implied by these forward-looking statements, to
differ. The sections in our periodic reports, including our most recent Annual Report on Form 10-K, our Quarterly Reports on Form
10-Q or our Current Reports on Form 8-K, entitled “Business,” “Risk Factors,” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations,” sections in our Definitive Proxy Statement on
Schedule 14A, filed with the SEC on April 13, 2023, entitled “Risk Factors,” “Adamis Business,” “DMK
Business,” “Adamis Management’s Discussion and Analysis of Financial Condition and Results of Operations”
and “DMK Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as
other sections in this prospectus and the other documents or reports incorporated by reference in this prospectus, discuss some
of the factors that could contribute to these differences. These forward-looking statements include, among other things, statements
about:
| |
● |
our expectations regarding future revenues and
profitability; |
| |
● |
our expectations regarding future growth; |
| |
● |
our expectations concerning future product research,
development, clinical trial and commercialization activities and related costs; |
| |
● |
our expectations regarding product development
timelines; |
| |
● |
our ability to successfully commercialize and
market our product candidates in development, if approved; |
| |
● |
matters relating to the manufacture of our commercial
products; |
| |
● |
our strategies and opportunities; |
| |
● |
the potential market size, opportunity and growth
potential for our product candidates, if approved; |
| |
● |
anticipated trends in our markets; |
| |
● |
anticipated dates for commencement or completion
of clinical trials; |
| |
● |
our expectations concerning regulatory matters
concerning our product candidates, including the timing of anticipated regulatory filings; |
| |
● |
our liquidity needs and need for future funding
and working capital; |
| |
● |
our need to raise additional capital and our
ability to obtain sufficient funding to support our planned activities; |
| |
● |
our expectations regarding future expense, profit,
cash flow, or balance sheet items or any other guidance regarding future periods; |
| |
● |
the accuracy of our estimates regarding expenses,
capital requirements and needs for additional financing; |
| |
● |
our ability to continue as a going concern; |
| |
● |
the impact of the health emergencies or global
geopolitical events on our business; |
| |
● |
the success, safety and efficacy of our drug
products; |
| |
● |
the potential outcome of any litigation or legal
proceedings; |
| |
● |
the scope of protection we are able to establish
and maintain for intellectual property rights covering our product candidates and technology; |
| |
● |
the volatility of the price of our common stock; |
| |
● |
our financial performance; and |
| |
● |
other factors described from time to time in
documents that we file with the SEC. |
Such
statements are not historical facts, but are based on our current expectations and projections about future events. They are subject
to risks and uncertainties, known and unknown, that could cause actual results and developments to differ materially from those
expressed or implied in such statements.
In
addition, many forward-looking statements concerning our anticipated future business activities assume that we are able to obtain
sufficient funding to support such activities and continue our operations and planned activities. As discussed elsewhere in this
prospectus, we require additional funding to continue operations, and there are no assurances that such funding will be available.
Failure to timely obtain required funding would adversely affect and could delay or prevent our ability to realize the results
contemplated by such forward looking statements. New factors emerge from time to time, and it is not possible for us to predict
which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor,
or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
We
may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not
place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions
and expectations disclosed in the forward-looking statements we make. We have included important cautionary statements in this
prospectus and in the documents incorporated by reference in this prospectus, particularly in the “Risk Factors” section,
that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. For
a summary of such factors, please refer to the section entitled “Risk Factors” in this prospectus, as supplemented
by the discussion of risks and uncertainties under “Risk Factors” contained in our most recent Annual Report on Form
10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K, or our Definitive Proxy Statement on Schedule 14A filed
with the SEC on April 13, 2023, as well as any amendments thereto, as filed with the SEC and which are incorporated herein by
reference.
In
light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained
in this prospectus or in any document incorporated herein by reference might not occur. Investors are cautioned not to place undue
reliance on the forward-looking statements, which speak only as of the date of this prospectus or the date of the document incorporated
by reference. Further, any forward-looking statement speaks only as of the date on which it is made, and except as may be required
by applicable law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after
the date on which the statement is made or to reflect the occurrence of unanticipated events. All subsequent forward-looking statements
attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements
contained or referred to in this section.
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this prospectus. The following summary is qualified in its entirety by, and should be read together with, the more
detailed information and financial statements and related notes thereto included in this prospectus or incorporated by reference
herein. Before you decide to purchase securities in this offering, you should read the entire prospectus carefully, including the
risk factors and the financial statements and related notes included in this prospectus or incorporated by reference herein. Unless
otherwise stated or the context requires otherwise, references in this prospectus to “Adamis,” the “company,”
or the “Company,” “we,” “us,” or “our” refer to Adamis Pharmaceuticals Corporation
and our subsidiaries, taken together.
Company Overview
With the worsening of the opioid crisis due to fentanyl poisoning and our
recent merger transaction with DMK Pharmaceuticals Corporation, our focus on developing and commercializing products in the substance
use disorder space including treatment of opioid use disorder has intensified and expanded, including with a more robust product pipeline.
Our two commercial products are designed to treat opioid overdose and anaphylactic shock. The first is ZIMHI® (naloxone
HCL Injection, USP) 5 mg/0.5 mL, which was approved by the U.S. Food and Drug Administration, or FDA, for the treatment of opioid overdose,
and the second is SYMJEPI® (epinephrine) Injection 0.3mg, which was approved by the FDA for use in the emergency treatment
of acute allergic reactions, including anaphylaxis, for patients weighing 66 pounds or more, and SYMJEPI (epinephrine) Injection 0.15mg,
which was approved by the FDA for use in the treatment of anaphylaxis for patients weighing 33-65 pounds. The foundation of our development
pipeline is a proprietary portfolio of approximately 750 proprietary small molecule neuropeptide analogues. Our lead clinical-stage product
candidate is for the treatment opioid use disorder and acute and chronic pain. The library also has the potential to generate other compounds
for treatment of various substance use disorders and compounds for life cycle management and backup molecules.
Recent Developments
Merger with DMK Pharmaceuticals Corporation
On May 25, 2023, we completed a merger transaction,
or the Merger, with DMK Pharmaceuticals Corporation, or DMK, pursuant to an Agreement and Plan of Merger and Reorganization dated
as of February 24, 2023, or the Merger Agreement, by and among DMK, Aardvark Merger Sub, Inc., a wholly-owned subsidiary of Adamis,
and Adamis. Prior to the Merger, DMK was a privately-held, clinical stage biotechnology company focused on the development and
commercialization of potential products for a variety of central nervous disorders. Pursuant to the Merger, each share of common
stock of DMK was converted into the right to receive a number of shares of Adamis common stock and, in the case of certain DMK
stockholders, shares of our Series E Convertible Preferred Stock, or Series E Preferred. Upon the closing of the Merger,
Ebrahim (Eboo) Versi, M.D., Ph.D., the co-founder and chief executive officer of DMK, became the chairman and chief executive officer
of Adamis, and David J. Marguglio, formerly the President, Chief Executive Officer and a director, continued as President and was
also appointed Chief Operating Officer.
Reverse Stock Split of Common Stock
Following approval by our stockholders at
a special meeting of stockholders of the Company held on May 15, 2023, or the Special Meeting, on May 22, 2023, we effected
a 1-for-70 reverse stock split of our outstanding common stock, or the Reverse Stock Split, pursuant to which each 70 outstanding
shares of our common stock were combined into one post-reverse split share of common stock. All outstanding options, restricted
stock unit awards, and warrants were proportionately adjusted as a result of the Reverse Stock Split, pursuant to their respective
terms. Unless otherwise indicated herein, share and per share numbers and amounts in this prospectus reflect and give effect to
the Reverse Stock Split. However, the historical financial statements of the Company incorporated by reference into this prospectus,
and other information and documents incorporated by reference into this prospectus, have not been revised or restated to reflect
the Reverse Stock Split.
Financial Condition; Other
We have incurred substantial recurring losses
from continuing operations, have used, rather than provided, cash in our continuing operations, and are dependent on additional
financing to fund operations. We incurred a net loss of approximately $8.9 million and $26.5 million for the three months ended
March 31, 2023 and for the year ended December 31, 2022, respectively. As of March 31, 2023, we had cash and cash equivalents
of approximately $3.1 million, an accumulated deficit of approximately $313.5 million and total liabilities of approximately $18.6
million. These conditions raise doubt about our ability to continue as a going concern. To achieve our goals and support our overall
strategy, we will need to raise additional funding in the future and make significant investments in, among other things, product
development and working capital. Prior to the Merger, DMK obtained non-dilutive capital from government and non-government sources
to enable a significant portion of its product development efforts to date, and we intend to seek such additional funding to help
offset some of the costs of future product development. Although we anticipate that we will require additional equity or debt capital
over the next 12 months to sustain operations, satisfy our obligations and liabilities, and fund our ongoing operations, in the
longer term, if revenues from the sale of our commercial products increase sufficiently, we expect that such revenues will help
reduce the need to raise additional funding through the sale of equity securities.
On July 18, 2023, David C. Benedicto, our then-chief financial officer,
notified us that he was resigning from the Company effective July 21, 2023. His resignation was not a result of any disagreement with
the Company or its independent auditors on any matter relating to the Company’s financial statements or accounting policies or practices.
We will commence a search for a new chief financial officer. In the interim, David J. Marguglio, the Company’s President and Chief Operating
Officer, has been appointed and will assume the duties of chief financial officer of the Company on an interim basis until the Company
appoints a successor.
On July 25, 2023, we completed the sale of the building and real property
located in Conway, Arkansas, formerly utilized by our discontinued compounding pharmacy business, as well as the personal property and
equipment located at the real property and certain related assets, to an unaffiliated third party purchaser. The total aggregate consideration
for the real property and other assets was approximately $2,000,000, before estimated commissions, fees and closing costs of approximately
$232,700.
Products and Product Candidates
Opioid Overdose; ZIMHI (naloxone) Injection
Naloxone is an opioid antagonist used to treat narcotic overdoses. Naloxone,
which is generally considered the drug of choice for immediate administration for opioid overdose, blocks or reverses the effects of the
opioid, including extreme drowsiness, slowed breathing, or loss of consciousness and eventually, death. Common opioids include morphine,
heroin, tramadol, oxycodone, hydrocodone and fentanyl. Since the COVID-19 pandemic, the opioid crisis has become significantly worse,
and this increase has disproportionately affected adolescents. According to Bloomberg industry data, the U.S. naloxone market grew by
about 15% in 2022 and according to the December 31, 2022 10-K of Emergent BioSolutions, Inc. filed in March 2023, sales of Narcan®,
the leading naloxone product for treatment of opioid overdoses, were approximately $374 million for 2022.
The Centers for Disease Control and Prevention, or CDC, estimates that
between 1999 and 2020 more than 932,000 people have died of drug overdoses, with annual deaths increasing during the pandemic. More recent
statistics published by the CDC reported that drug overdoses resulted in approximately 107,081 deaths in the United States during the
12-month period ending December 2022, which was an approximately 51% increase over the approximately 71,030 deaths for the 12-month period
ending December 2019. Overdose deaths involving opioids (including both prescription and synthetic) accounted for 81,045 of the overdose
deaths in 2022 and are now the leading cause of death for Americans under age 50. More powerful synthetic opioids, like fentanyl and its
analogues, are responsible for approximately 90% of those opioid deaths. These statistics are even more stark for adolescents according
to the CDC. Comparing July-December 2019 to July-December 2021, overdose deaths among youngsters aged 10 to 19 years increased by 109%
and in that same time period, deaths involving illicitly manufactured fentanyl increased by 182% in the same age group. In June 2021,
the National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services, published the policy
brief, “Naloxone for Opioid Overdose: Life-Saving Science,” which reported that statistical modeling suggests that high rates
of naloxone distribution among laypersons and emergency personnel could avert approximately 21% of opioid deaths. The brief also stated
that overdoses involving highly potent synthetic opioids such as fentanyl or large quantities of opioids may require multiple doses of
naloxone, and if respiratory function does not improve, naloxone doses may be repeated every two to three minutes. This need for availability
of naloxone was emphasized in a CDC Morbidity and Mortality Weekly Report article in 2022 discussing drug overdose deaths among persons
aged 10-19 years, which noted that potential bystanders were present in approximately two-thirds of the overdose deaths in young people
aged 10 to 19, which suggests that at least some of the deaths could have been prevented and the number of deaths reduced if bystanders
had been equipped naloxone, knew how to use it and provided a timely overdose response.
On October 18, 2021, we announced that the FDA had approved ZIMHI (naloxone
hydrocholoride 5mg) for the treatment of opioid overdose, and it was commercially launched in the U.S. on March 31, 2022. Based on
published pharmacokinetic data from FDA approved product package insert material, we believe that ZIMHI’s intramuscular route of
administration could result in faster absorption compared to any other currently marketed naloxone products making it an ideal treatment
for overdoses caused by more potent opioids such as fentanyl.
On June 20, 2023, Dr. Versi, our Chief Executive Officer,
participated in the White House Roundtable with opioid reversal product manufacturers, hosted by White House Office of National Drug Control
Policy, or ONDCP, Director Dr. Rahul Gupta, White House Domestic Policy Council Advisor Neera Tanden, U.S. Assistant Secretary for Health
Admiral Rachel Levine, and U.S. Assistant Secretary for Mental Health and Substance Use Dr. Miriam E. Delphin-Rittmon. During the roundtable
discussion, various members of the current White House administration discussed the opioid crisis, emphasizing the extent and importance
of the opioid crisis and indicting that the administration has a directive and is seeking private-public cooperation to increase access
and affordability of naloxone, including the creation of federal guidelines to remove barriers to access of all naloxone products at state
and local levels. During this same visit to Washington, D.C., Dr. Versi met with 12 congressional offices, in both the Senate and
the House of Representatives, and Republicans and Democrats, and discussed our support of HR 4007 that is currently being drafted that
would be intended to ensure that there would be no barriers to the government purchase of any opioid reversal product. We believe these
congressional meetings were very positive and that there appeared to be bipartisan support for improving access to opioid reversal products.
This is particularly important for Adamis, as we believe some current regional guidelines should be revised and have prevented government
agencies from being able to purchase ZIMHI.
With the increasing prevalence of illicit fentanyl
on the streets, we believe the need for ZIMHI as a product that results in rapid increase in higher blood levels of naloxone is becoming
ever more important and urgent. Dr. Gupta, Director of the ONDCP, stated at the White House meeting in June that about 60% of illicit
pills imported into the U.S. contain deadly doses of fentanyl. In our opinion, this means that more naloxone is needed to counteract
this circumstance.
The results of a study sponsored by the FDA was
recently presented by Dr. David Strauss, M.D., Ph.D. Dr. Straus at a virtual public meeting of the Reagan–Udall Foundation
addressing fatal overdoses. Dr. Straus is the Director of the Division of Applied Regulatory Science at the Center for Drug
Evaluation and Research. The current standard of care is a single intranasal 4mg dose of naloxone, as contained in Narcan. Given the
fentanyl crisis, the investigators tested this single dose against two and four doses to reverse a simulated fentanyl overdose. They
showed that the most effective reversal was achieved by four administrations of 4mg intranasal naloxone given within 2.5 minutes.
However, uses of these multiple doses in such a short time, while necessary, are in fact an “off-label” use of the drug
and therefore pose a challenge for first responders and other caregivers. We believe that this data from the FDA sponsored study
suggests that rapid delivery of naloxone is the answer to a fentanyl overdose and that a single administration of ZIMHI, based on
its pharmacokinetic profile, could be the ideal agent to counter a fentanyl overdose.
Various persons with experience addressing matters relating to the opioid
crisis, including certain law enforcement officials, federal government administration officials, and parent organizations, dealing with
the opioid crisis also have voiced their concerns about the nation’s current capability of dealing with this opioid crisis, noting
the need in many instances of opioid, and particularly fentanyl, overdoses for repeat dosing and to use multiple doses of Narcan in efforts
to revive someone or achieve a recovery. Based on these experiences and other observations in the field, we believe that ZIMHI, if it
was more widely available, could aid in the nation’s efforts to treat fentanyl poisoning and inadvertent overdose, although there
can be no assurance that this will be the case.
Anaphylaxis; SYMJEPI; Epinephrine Injection Pre-Filled Single Dose
Syringe
The American Academy of Allergy Asthma and Immunology,
or AAAAI, defines anaphylaxis as a serious life-threatening allergic reaction. The most common anaphylactic reactions are to foods, insect
stings, medications and latex. According to information published by AAAAI reporting on findings from a 2009-2010 study, up to 8% of U.S.
children under the age of 18 had a food allergy, and approximately 38% of those with a food allergy had a history of severe reactions.
Anaphylaxis requires immediate medical treatment, with epinephrine as the first course of treatment to open airways and maintain blood
pressure.
We estimate that sales of prescription epinephrine
products were more than approximately $1.75 billion in 2022, based on assumptions and estimates using industry data. While we cannot provide
any assurances concerning whether annual prescription sales will decline or grow, we believe that the epinephrine market has the potential
to grow in the future, based in part on the prevalence of medical conditions, such as anaphylaxis, cardiovascular diseases, respiratory
diseases (asthma), and the increased awareness about the treatment options for the management of these diseases. The market for prescription
epinephrine products is competitive, and a number of factors have resulted in, and could continue to result in, downward pressure on the
pricing of, and revenues from sales of, our SYMJEPI (epinephrine) Injection 0.3mg and 0.15mg prescription epinephrine products. Our SYMJEPI
(epinephrine) Injection 0.15mg and 0.3mg products allow users to administer a pre-measured epinephrine dose quickly with a device that
we believe, based on human factors studies, to be intuitive to use.
On June 15, 2017, the FDA approved our SYMJEPI
(epinephrine) Injection 0.3mg product for the emergency treatment of allergic reactions (Type I) including anaphylaxis. SYMJEPI
(epinephrine) Injection 0.3mg is intended to deliver a dose of epinephrine, which is used for emergency, immediate administration
in acute anaphylactic reactions to insect stings or bites, allergic reaction to certain foods, drugs and other allergens, as well
as idiopathic or exercise-induced anaphylaxis for patients weighing 66 pounds or more. On September 27, 2018, the FDA approved
our lower dose SYMJEPI (epinephrine) Injection 0.15mg product, for the emergency treatment of allergic reactions (Type I) including
anaphylaxis in patients weighing 33 to 66 pounds. Our SYMJEPI injection products were fully launched in July 2019 by our then-commercialization
partner Sandoz Inc. Our SYMJEPI products are currently marketed and sold by USWM, LLC, or USWM or US WorldMeds, with which we entered
into an exclusive distribution and commercialization agreement, or the USWM Agreement, in May 2020 for the United States commercial
rights for the SYMJEPI products, as well as for our ZIMHI product.
SYMJEPI is manufactured and tested for us
by Catalent Belgium S.A. During Catalent’s routine testing, a small number of syringes with clogged needles were identified.
On March 21, 2022, we announced a voluntary recall of four lots of SYMJEPI (epinephrine) Injection 0.15 mg (0.15 mg/0.3 mL) and
0.3 mg (0.3 mg/0.3 mL) due to the potential clogging of the needle preventing the dispensing of epinephrine. The recall was conducted
with the knowledge of the FDA, and USWM handled the recall process for the Company, with Company oversight. As of the date of this
prospectus, neither USWM nor we have received, nor are aware of, any adverse events related to this recall and in February 2023,
the Company received notice from the FDA that the agency considers the voluntary recall of our SYMJEPI products to be terminated.
Such notice does not preclude the FDA from taking action in the future related to the recall, and we remain responsible for compliance
with applicable laws relating to the product and the recall. Catalent’s investigation determined the steel used in a specific
stainless steel needle batch as the root cause for the clogged syringes observed. The Company worked with Catalent to develop corrective
and preventive actions. However, despite the corrective actions and sourcing syringes which used a different batch of steel for
the needles, Catalent’s attempt to resume manufacturing of SYMJEPI at its Belgium facility has resulted in similar product
defects. Therefore, as of the date of this prospectus, the Company remains unable to manufacture product. While we are committed
to returning SYMJEPI to the market, we will not do so until we are satisfied that sufficient corrective actions have been implemented
to avoid a repeat of the circumstances which led to the voluntary recall. We are evaluating a range of options to restore SYMJEPI
production, including a critical assessment of Catalent.
Product Candidates
As a result of our Merger with DMK, we acquired
a library of approximately 750 novel small molecule neuropeptide analogues and a number of product candidates and technologies
in development for opioid use disorder and other neuro-based disorders. We intend to focus on developing therapies with novel mechanisms
of action to treat these important conditions where patients are currently underserved, including substance abuse disorders. We
are developing mono, bi- and tri-functional small molecules that simultaneously modulate critical networks in the nervous system
with the goal of creating treatments that are efficacious, safe, and tolerable and could address several unmet or underserved medical
needs by taking the novel approach to integrate with the body’s own efforts to regain balance of disrupted physiology. By
designing small molecule analogs of neuropeptides, one or multiple receptors can be targeted by a single molecule to support a
transition back to a balanced neurophysiological state.
Our lead clinical stage product candidate,
DPI-125, is being developed as a potential novel treatment for opioid use disorder, or OUD. We also plan to study this compound
for the treatment of moderate to severe pain, where it could potentially offer a product with competitive advantages compared to
currently marketed opioids (pain killers) and hence help prevent opioid addiction. Other product candidates include DPI-221, being
developed for treating bladder control problems, and DPI-289 being developed for treating severe end stage Parkinson’s disease.
We currently intend to focus on the development programs that target substance use disorder described above and to seek to out-license
product candidates targeting indications outside of this focus.
DPI-125
DPI-125 is a small molecule that is currently
being developed for two potential uses. The first is for the rapid stabilization of OUD patients actively using prescription or
street opioids, including deadly fentanyl and its analogues. The second potential use is as a potent, acute analgesic, with a potentially
reduced risk of respiratory depression and addiction compared to currently marketed opioids.
Most marketed opioids are pure mu agonists,
which means they bind and have their effect only through the mu receptor (Fig.1). While they do provide the desired pain relief,
they also carry the risk of respiratory depression, which can lead to death, and euphoria, which can cause addiction. In contrast,
DPI-125 binds with all three receptors, mu, kappa and delta (Fig. 2), which is intended to result in a more balanced approach that
tries to mimic the body’s own endorphins and hence reduced risk of respiratory depression, abuse liability and death.
We have completed a human Phase 1 dose escalation
study with DPI-125, and the pharmacokinetic data showed that the drug was well tolerated in the human study, with no serious adverse
events, deaths or dropouts. The next anticipated development step will be human proof of concept studies, which will attempt to
confirm what has been demonstrated in preclinical studies in terms of reduced or absent respiratory depression and abuse liability.
Following these proof-of-concept studies, assuming adequate funding and no unexpected developments, we believe that the next development
step will be to proceed into Phase 2 trials for the treatment of OUD and acute pain, where the focus of the trials will not only
be on efficacy, but also safety and tolerability. We believe that the same characteristics and mechanism
of action that may make DPI-125 a useful product in the fight against addiction could also make it a significant alternative to
all currently marketed opioids used for treating pain.

Fig. 1: Schema showing a cell surface with the transmembrane
mu, kappa and delta receptors. Opiates bind to just the mu receptors conferring analgesia, but there is the risk of death and addiction.
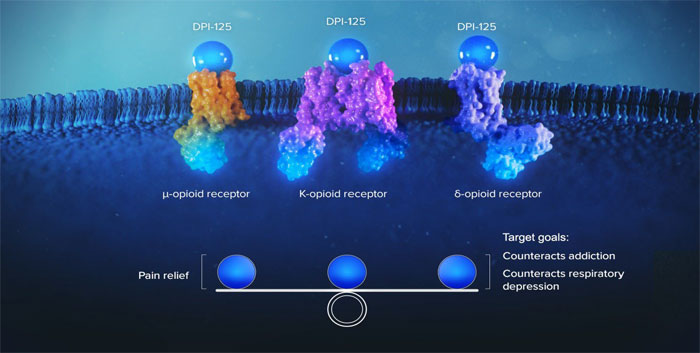
Fig. 2: DPI-125 is a ‘triple agonist’; meaning
that it readily binds and interacts positively with all three opioid receptors (Mu, Kappa & Delta). This balanced approach
more closely mimics the body’s natural endorphins and is intended to counteract respiratory depression while not providing
the euphoria that can cause addiction.
DPI-221
DPI-221 is a small molecule currently in
development as what we believe is a unique alternative to surgery for benign prostatic hyperplasia, or BPH, by reestablishing bladder
control. BPH is a common problem with approximately six million men seeking treatment annually, with an estimated market size of
approximately $5.4 billion annually in the United States. BPH is a common, chronic disease caused by an enlarged prostate. DPI-221
may offer a novel approach to the treatment of BPH by acting on the central nervous system to suppress abnormal activity without
interfering with normal bladder function. In preclinical studies, DPI-221 was effective at reestablishing neural control of the
bladder, which returns the bladder to more normal function, allowing coordinated bladder contractions and efficient voiding.
A first-in-human Phase 1 oral dose escalation
study, showed that the drug was safe and tolerable in the study. There were no serious adverse events, deaths or study dropouts.
The pharmacokinetic, or PK, characteristics have allowed planning of a proof-of-concept study, which is anticipated to be a human
urodynamic study to determine the efficacious dose that will inform dosing in a subsequent Phase 2 study. We believe that if successfully
developed, this medication could prevent or reduce the need for BPH surgery.
DPI-289
DPI-289, also a small molecule, has been
developed to treat patients suffering from severe Parkinson’s disease, or PD. Many of these patients will have been treated
with a current leading treatment product called levodopa, or L-DOPA. Unfortunately, after a few years of treatment, the duration
of effect is markedly curtailed (reduced “on-time”) and patients can exhibit severe abnormal movements called levodopa
induced dyskinesia, or LID, which make it difficult or impossible to lead a normal life. Preclinical studies have demonstrated
DPI-289’s ability to treat parkinsonian disability in rodent and non-human primate models to dramatically increase on-time
without causing dyskinesia. Our initial goal with respect to this product candidate is to target patients late in their disease
who require deep brain stimulation (DBS-brain surgery) to prevent such surgeries and also treat those patients who are not eligible
for DBS. Given this target population, we plan to seek orphan drug status from the FDA and international regulatory agencies. Initially,
we intend to develop the compound as monotherapy, but we anticipate that future studies will examine its utility in PD patients
as combination therapy with L-DOPA to increase “on-time” without increasing the debilitating side effect of dyskinesia.
We anticipate that the next step for this
program, assuming adequate funding and no unexpected developments, will be to carry out IND-enabling toxicology studies to allow
the filing of an Investigational New Drug Application, or IND, for the first in-person studies. If orphan drug status is conferred
by the FDA or other international regulatory bodies, the cost and duration of the clinical development program may be significantly
reduced, allowing for approval in an accelerated time frame.
Grant Funding
To date and prior to the Merger, development
programs for these product candidates have been largely financed by funding from government and non-governmental organization,
or NGO, awards or grants, including without limitation from the National Institute on Drug Abuse, or NIDA, a division of the National
Institutes of Health, or NIH, the New Jersey Commission on Science, Innovation and Technology, or CSIT, and the Michael J. Fox
Foundation, which has previously provided approximately $1.5 million in grant funding to support much of the preclinical work for
DPI-289. DMK was also the recipient of a grant from the National Institute on Alcohol Abuse and Alcoholism, or NIAAA, of the NIH
to support the development of a novel bifunctional small molecule for the treatment of Alcohol Use Disorder, or AUD. The grant
funding will help fund this preclinical, early-stage project to use gold-standard preclinical assays to vet our library of molecules
that possess the bi-functional attributes hypothesized to reduce excessive alcohol use. In the future, we plan to continue to seek
non-dilutive government funding from the NIH and NGOs as well as funding from other sources. Each of the NIH grants relates to
agreed-upon direct and indirect costs for specific studies or clinical trials, which may include costs such as personnel and consulting
costs, and costs paid to contract research organizations, or CROs, research institutions or other third parties involved in the
grant. We are reimbursed for our eligible direct and indirect costs over time, up to the maximum amount of each specific grant
award. Only costs that are allowable under the grant award, certain government regulations and the NIH’s supplemental policy
and procedure manual may be claimed for reimbursement, and the reimbursements are subject to routine audits from governmental agencies
from time to time. The NIH or other government agency may review our performance, cost structures and compliance with applicable
laws, regulations, policies and standards and the terms and conditions of the applicable NIH grant. If any of our expenditures
are found to be unallowable or allocated improperly or if we have otherwise violated terms of such NIH grant, the expenditures
may not be reimbursed and/or we may be required to repay funds already disbursed.
Future Development Plans
Our development
plans concerning our product candidates, including DPI-125 and the other product candidates described above, and anticipated dates
for future preclinical or clinical trials regarding our product candidates, are affected by a number of factors, including the
availability of adequate funding to support product development efforts and studies, the results of preclinical or clinical studies
that we may conduct, developments in the marketplace including the introduction of potentially competing new products by competitors,
regulatory developments including the outcome of any future discussions with the FDA concerning the regulatory approval pathway
of the applicable product candidate including the number and kind of clinical trials that the FDA will require before the FDA will
consider regulatory approval of the applicable product, and any unexpected difficulties in licensing or sublicensing intellectual
property rights that may be required for other components of the product. As a result, the timing and progress of our product development
plans could be affected by such considerations and, should we choose to seek development, out-licensing or commercialization partners
for one or more of our products or product candidates, our success in negotiating and entering into development, out-licensing
or commercialization agreements relating to our product candidates. In considering development and commercialization alternatives
for our products and product candidates and technologies, we may seek to enter into out-licensing or development agreements for
product candidates or technologies that are not within our core areas of focus.
DMK Intellectual Property
DMK has (i) four issued patents in
the United States, two divisional and one provisional United States patent applications; (ii) two pending Canadian patent applications;
and (iii) one pending European patent application. The patent portfolio comprises of utility patents and one provisional patent
for composition of matter of a transdermal drug delivery system. The patent portfolio covers inventions for the treatment of drug
addiction and Parkinson’s disease. The issued patents are expected to expire between 2026 and 2038, not taking into account
any potential patent-term extensions that may be available in the future. The pending or provisional patent applications, if granted,
are expected to expire between 2037 and 2043, not taking into account any potential patent-term extensions that may be available
in the future.
Corporate Information
We are incorporated under the laws of the
State of Delaware. Our principal executive offices are located at 11682 El Camino Real, Suite 300, San Diego, CA 92130, and our
telephone number is (858) 997-2400. Our website address is: www.adamispharmaceuticals.com. We have included our website address
as a factual reference and do not intend it to be an active link to our website. The information that can be accessed through our
website is not part of this prospectus, and investors should not rely on any such information in deciding whether to purchase our
securities.
Smaller Reporting Company
We are also currently a “smaller reporting
company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent
company that is not a smaller reporting company, and have a public float of less than $250 million or annual revenues of less than
$100 million during the most recently completed fiscal year. As a result, the disclosure that we are required to provide in our
SEC filings is less in certain respects than it would be if we were not considered a “smaller reporting company.” Specifically,
“smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are
exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting
firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased
disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited
financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting
company” may make it harder for investors to analyze our results of operations and financial prospects.
| The Offering |
| |
|
| Units to be Offered |
Up to 5,263,158 units, each unit consisting of one share of common stock
and one common warrant to purchase one share of common stock. |
| |
|
| Pre-funded Units to be Offered |
We are also offering to certain purchasers whose purchase of
units in this offering would otherwise result in the purchaser, together with its affiliates, beneficially owning more than 4.99%
of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers
so choose, pre-funded units, each pre-funded unit consisting of one pre-funded warrant to purchase one share of common stock and
one common warrant to purchase one share of common stock, in lieu of units that would otherwise result in any such purchaser’s
beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. The purchase
price of each pre-funded unit will equal the price per unit being sold to the public in this offering, minus $0.0001, and the exercise
price of each pre-funded warrant will be $0.0001 per share of common stock. For each pre-funded unit we sell, the number of units
we are offering will be decreased on a one-for-one basis. This offering also relates to the shares of common stock issuable upon
the exercise of any pre-funded warrants or common warrants comprising the pre-funded unit sold in this offering.
|
Description of
Common Warrants |
Each common warrant will have an exercise price of $______ per share, will be immediately exercisable and will expire on the five (5) year anniversary of the original issuance date. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the common warrants. |
| |
|
Description of
Pre-Funded Warrants |
Each pre-funded warrant will have an exercise price of $0.0001 per share, will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants. |
| |
|
Common Stock
Outstanding before
this Offering |
2,378,295 shares |
| |
|
Common Stock
Outstanding after
this Offering |
7,641,453 shares (assuming the sale of the maximum number of units covered
by this prospectus, no sale of pre-funded units and no exercise of the common warrants issued in this offering). |
| |
|
| Use of Proceeds |
Assuming the maximum number of units are sold in this offering at an assumed
public offering price of $1.90 per unit, which represents the closing price of our common stock on the Nasdaq Capital Market on July 21,
2023, and assuming no issuance of pre-funded warrants in connection with this offering, we estimate that the net proceeds from our sale
of shares of our common stock in this offering will be approximately $8,850,000, after deducting the placement agent fees and estimated
offering expenses payable by us and assuming no sale of any pre-funded units offered in this offering. However, this is a reasonable best
efforts offering with no minimum number of securities or amount of proceeds as a condition to closing, and we may not sell all or any
of these securities offered pursuant to this prospectus; as a result, we may receive significantly less in net proceeds. We currently
intend to use the net proceeds from this offering for working capital and general corporate purposes, which may include, without limitation,
expenditures relating to research, development and clinical trials relating to our products and product candidates, manufacturing, capital
expenditures, hiring additional personnel, the payment, repayment, refinancing, redemption or repurchase of existing or future indebtedness,
obligations or capital stock, and payment of obligations and liabilities. We may also use the proceeds to acquire or invest in complementary
products, services, technologies or other assets, although we have no agreements or understandings with respect to any acquisitions or
investments at this time. For additional information please refer to the section entitled “Use of Proceeds” on page 24
of this prospectus.
|
| Risk Factors |
An investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 14 of this prospectus and the other information included in and incorporated by reference into this prospectus for a discussion of the risk factors you should carefully consider before deciding to invest in our securities. |
| |
|
| Nasdaq Capital Market Symbol |
“ADMP.” There is no established trading market for the common warrants or the pre-funded warrants, and we do not expect a trading market to develop. We do not intend to list the common warrants or the pre-funded warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the common warrants and the pre-funded warrants will be extremely limited. |
| |
|
| Reasonable Best Efforts Offering |
We have agreed to offer and sell the securities offered hereby to the purchasers through the placement agent. The placement agent is not required to buy or sell any specific number or dollar amount of the securities offered hereby, but it will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. See “Plan of Distribution” on page 42 of this prospectus. |
Unless we indicate otherwise, all information
in this prospectus, except for our consolidated financial statements and notes thereto included or incorporated by reference herein,
gives effect to and reflects a 1-for-70 reverse stock split of our issued and outstanding shares of common stock (and proportional
adjustment of options, warrants and restricted stock units) effected May 22, 2023, and the corresponding adjustment of all common
stock price per share and stock option and warrant exercise price data.
The number of shares of our common stock to be outstanding upon completion
of this offering is based on 2,378,295 shares of common stock outstanding as March 31, 2023, and excludes, as of such date, the following:
(i) 59,277 shares of common stock issuable upon exercise of outstanding stock options, with exercise prices ranging from $43.40 to $592.20
and having a weighted average exercise price of $287.16 per share, and 9,286 shares issuable upon the vesting of restricted stock units
outstanding, awarded under our equity incentive plans, 2,143 shares of which were issued after March 31, 2023 following such vesting;
(ii) outstanding warrants and the shares issuable upon exercise of such warrants, to purchase the following numbers of shares of
common stock: 840 shares at an exercise price of $595.00 per share; 197,055 shares at an exercise price of $80.50 per share; 5,000 shares
at an exercise price of $49.00 per share; 10,714 shares at an exercise price of $32.90 per share; and 685,714 shares at an exercise price
of $9.66 per share; (iii) 202,455 shares of common stock reserved for future issuance under our 2020 Equity Incentive Plan; (iv) 1,941.2
shares of Series E Preferred convertible into approximately 1,941,200 shares of common stock subject to various beneficial ownership
and other limitations and restrictions on conversion, and 302,815 shares of common stock, issued after March 31, 2023, in connection with
the closing of the Merger transaction with DMK; (v) outstanding options to purchase 231,490 shares of common stock at an exercise
price of $2.90 per share that we assumed in connection with the Merger, and 18,005 remaining unallocated shares reserved for issuance
pursuant to the 2016 DMK Stock Plan that we assumed in connection with the Merger; (vi) approximately 9,967 shares of common stock issuable
upon conversion of 3,000 outstanding shares of Series C Convertible Preferred Stock, or Series C Preferred; (vii) options to purchase
24,962 shares of common stock that expired unexercised after March 31, 2023; and (viii) 107,142 shares of common stock (post Reverse Stock
Split) issued pursuant to the exercise of prefunded warrants after March 31, 2023.
Unless otherwise indicated, this prospectus
assumes no exercise of the pre-funded warrants and the warrants offered hereby.
RISK FACTORS
Investing in our securities involves
a high degree of risk. Before deciding to invest in our securities, you should consider carefully the risks and uncertainties described
below and under Item 1A.“Risk Factors” in our Annual Report on Form 10-K, filed with the Securities and Exchange Commission,
or SEC, on March 16, 2023, and our Definitive Proxy Statement on Schedule 14A filed with the SEC on April 13, 2023, which are each
incorporated by reference in this prospectus, together with all of the other information contained in this prospectus and documents
incorporated by reference herein, and in any free writing prospectus that we have authorized for use in connection with this offering.
If any of the matters discussed in the following risk factors were to occur, our business, financial condition, results of operations,
cash flows or prospects could be materially adversely affected, the market price of our common stock could decline and you could
lose all or part of your investment in our securities. Additional risks and uncertainties not presently known or which we consider
immaterial as of the date hereof may also have an adverse effect on our business.
Risks Related to This Offering and Ownership of Our Securities
This is a reasonable best efforts offering, with no minimum
amount of securities required to be sold, and we may sell fewer than all of the securities offered hereby.
The placement agent has agreed to use
its reasonable best efforts to solicit offers to purchase the units and pre-funded units in this offering. The placement agent
has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar
amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this
offering. As there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount,
placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set
forth above. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds
received by us, and investors in this offering will not receive a refund in the event that we do not sell all of the units or pre-funded
units offered in this offering. The success of this offering will impact our ability to use the proceeds to execute our business
plans. We may have insufficient capital to implement our business plans, potentially resulting in greater operating losses unless
we are able to raise the required capital from alternative sources. There is no assurance that alternative capital, if needed,
would be available on terms acceptable to us, or at all.
You will experience immediate and substantial dilution
in the net tangible book value per share of the common stock you purchase, and may experience additional dilution in the future.
Because the effective price per share of common stock included in the units
or issuable upon exercise of the warrants or pre-funded warrants being offered may be substantially higher than the net tangible book
value per share of our common stock, you may experience substantial dilution to the extent of the difference between the effective offering
price per share of common stock you pay in this offering and the net tangible book value per share of our common stock immediately after
this offering. Assuming the sale of 5,263,158 units at a public offering price of $1.90 per unit and our net tangible book value
as of March 31, 2023, and assuming no sale of any pre-funded units in this offering, no exercise of any of the common warrants being offered
in this offering, and after deducting the placement agent fees and estimated offering expenses payable by us, you will incur immediate
dilution in as adjusted net tangible book value of approximately $2.01 per share. As a result of the dilution to investors purchasing
securities in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the
event of the liquidation of our company. See the section entitled “Dilution” below for a more detailed discussion of the dilution
you will incur if you participate in this offering. To the extent shares are issued under outstanding options, warrants and convertible
securities at exercise prices or conversion prices lower than the public offering price of the units offered in this offering, you will
incur further dilution.
If we sell additional shares of common stock in future
financings, shareholders may experience immediate dilution and, as a result, our share price may decline.
Our charter allows us to issue up to 200,000,000
shares of our common stock and up to 10,000,000 shares of preferred stock. To raise additional capital, we may in the future sell
additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that
are lower than the prices paid by existing stockholders, and investors purchasing shares or other securities in the future could
have rights superior to existing stockholders. If we issue shares of common stock or securities convertible or exercisable into
shares of common stock, our shareholders would experience additional dilution and, as a result, our share price may decline.
Certain of our securities issued in prior offerings
include a right to receive the Black-Scholes value of the unexercised portion of those securities in the event of a fundamental transaction,
which payment could be significant.
Most of our outstanding warrants
to purchase shares of common stock issued by us in prior offerings provide that, in the event of a “fundamental transaction”
that is approved by our board of directors, including, among other things, a merger or consolidation of our company, sale of all or substantially
all of our assets or a sale of a certain percentage of our common stock, the holders of such warrants have the option to require us to
pay to such holders an amount of cash equal to the Black-Scholes value of the warrants. Such amount could be significantly more than the
warrant holders would otherwise receive if they were to exercise their warrants and receive the same consideration as the other holders
of common stock, which in turn could reduce the consideration that holders of common stock would be concurrently entitled to receive in
such fundamental transaction. Any payments we may be required to make to such holders under these provisions could also reduce the amount
of net proceeds available to us from this offering. Additionally, any future equity financing that we conduct may require us to issue
securities that have a similar feature.
We will have broad discretion in
using the proceeds of this offering, and we may not effectively spend the proceeds.
Our management will have broad discretion
in the application of the net proceeds, including for any of the purposes described in the section of this prospectus entitled
“Use of Proceeds.” You will be relying on the judgment of our management with regard to the use of these net proceeds,
and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately.
The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse
effect on our business, cause the price of our securities to decline and delay the development of our product candidates. Our management
might not be able to yield a significant return, if any, on any investment of these net proceeds, and you will not have the opportunity
to influence our decisions on how to use our net proceeds from this offering.
An active trading market for our
shares may not be sustained.
Although our shares are listed on the Nasdaq
Capital Market, the market for our shares has demonstrated varying levels of trading activity. The current level of trading may
not be sustained in the future. The lack of an active market for our shares may impair investors’ ability to sell their shares
at the time they wish to sell them or at a price that they consider reasonable, may reduce the fair market value of their shares
and may impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire
additional intellectual property assets by using our shares as consideration.
The price of our common stock may be volatile.
The market price of our common stock may
fluctuate substantially. For example, from January 2022 to June 30, 2023, the market price of our common stock has fluctuated between
$1.90 and $59.32, as adjusted by and giving effect to the Reverse Stock Split. Market prices for securities of early-stage pharmaceutical,
biotechnology and other life sciences companies have historically been particularly volatile. The price of our common stock that
will prevail in the market after this offering may be higher or lower than the price that you have paid, depending on many factors,
some of which are beyond our control and may not be related to our operating performance. Market prices for securities of early-stage
pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile. Some of the factors
that may cause the market price of our common stock to fluctuate include:
| |
● |
relatively low trading volume, which can result in significant volatility in the market price of our common stock based on a relatively smaller number of trades and dollar amount of transactions; |
| |
● |
the timing and results of our current and any future preclinical or clinical trials of our product candidates; |
| |
● |
the entry into or termination of key agreements, including, among others, key collaboration and license agreements; |
| |
● |
the results and timing of regulatory reviews relating to the approval of our product candidates; |
| |
● |
the timing of, or delay in the timing of, commercial introductions of any of our products; |
| |
● |
the initiation of, material developments in, or conclusion of, litigation or legal proceedings, including a negative outcome in any litigation or legal proceeding; |
| |
● |
failure of any of our products or product candidates to achieve commercial success; |
| |
● |
general and industry-specific economic conditions that may affect our research and development expenditures; |
| |
● |
the results of clinical trials conducted by others on products that would compete with our product candidates; |
| |
● |
the loss of key employees; |
| |
● |
the introduction of technological innovations or new commercial products by our competitors; |
| |
● |
future sales of our common stock or exercise of our outstanding warrants; |
| |
● |
publicity or announcements regarding regulatory developments relating to our products; |
| |
● |
period-to-period fluctuations in our financial results, including our cash and cash equivalents balance, operating expenses, cash burn rate or revenue levels; |
| |
● |
common stock sales in the public market by one or more of our larger stockholders, officers or directors; |
| |
● |
our filing for protection under federal bankruptcy laws; |
| |
● |
effects of public health crises, pandemics and epidemics, such as the COVID-19 outbreak; or |
| |
● |
other potentially negative financial announcements, such as a review of any of our filings by the SEC, changes in accounting treatment or restatement of previously reported financial results or delays in our filings with the SEC. |
The stock markets in general have experienced
substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations
may also adversely affect the trading price of our common stock and value of our outstanding warrants. In the past, following periods
of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation
against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention
and resources, which could significantly harm our profitability and reputation.
If our shares become subject to the
penny stock rules, it may be more difficult to sell our shares.
The SEC has adopted rules that regulate
broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price
of less than $5.00 (other than securities registered on certain national securities exchanges or authorized for quotation on certain
automated quotation systems, provided that current price and volume information with respect to transactions in such securities
is provided by the exchange or system). The OTC Bulletin Board does not meet such requirements and if the price of our shares is
less than $5.00 and our shares are no longer listed on a national securities exchange such as the Nasdaq Capital Market, our shares
may be deemed a penny stock. The penny stock rules require a broker-dealer, at least two business days prior to a transaction in
a penny stock not otherwise exempt from those rules, to deliver to the customer a standardized risk disclosure document containing
specified information and to obtain from the customer a signed and date acknowledgment of receipt of that document. In addition,
the penny stock rules require that prior to effecting any transaction in a penny stock not otherwise exempt from those rules, a
broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive:
(i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions
involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may
have the effect of reducing the trading activity in the secondary market for our shares, and therefore shareholders may have difficulty
selling their shares.
Future sales of substantial amounts of our common stock,
or the possibility that such sales could occur, could adversely affect the market price of our common stock.
Future sales in the public market of our
common stock, including shares offered by the prospectus or shares issued upon exercise of our outstanding stock options, warrants
or convertible securities, or the perception by the market that these issuances or sales could occur, could lower the market price
of our common stock and value of our outstanding warrants or make it difficult for us to raise additional capital. As of March 31,
2023, we had approximately 2,378,295 shares of common stock issued and outstanding, substantially all of which we believe may be
sold publicly, subject in some cases to volume and other limitations, provisions or limitations in registration rights agreements,
or prospectus-delivery or other requirements relating to the effectiveness and use of registration statements registering the resale
of such shares. In addition, we have a substantial number of outstanding options, warrants, restricted stock units and convertible
securities. In general, subject to applicable vesting requirements, upon exercise of these options or warrants, issuance of shares
following vesting of the restricted stock units, or issuance of shares upon conversion of outstanding convertible securities, the
underlying shares may be resold into the public market, subject in some cases to volume and other limitations or prospectus-delivery
requirements pursuant to registration statements filed or to be filed registering the resale of such shares.
This offering may cause the trading
price of our Common Stock to decrease.
The number of shares of common stock underlying
the securities we propose to issue and ultimately will issue if this offering is completed, may result in an immediate decrease
in the market price of our common stock. This decrease may continue after the completion of this offering. We cannot predict the
effect, if any, that the availability of shares for future sale represented by the pre-funded warrants or common warrants issued
in connection with the offering will have on the market price of our common stock from time to time.
Our failure to meet the continued listing requirements
of Nasdaq could result in a delisting of our common stock, which could negatively impact the market price and liquidity of our
common stock and our ability to access the capital markets.
Our common stock is currently listed on
the Nasdaq Capital Market. If we fail to satisfy the continued listing requirements of Nasdaq, such as the corporate governance
requirements, the minimum closing bid price requirement, or applicable market capitalization or shareholder equity requirements,
Nasdaq may take steps to delist our common stock. Such a delisting would have a negative effect on the price of our common stock,
impair the ability to sell or purchase our common stock when persons wish to do so, and any delisting materially adversely affect
our ability to raise capital or pursue strategic restructuring, refinancing or other transactions on acceptable terms, or at all.
Delisting from the Nasdaq Capital Market could also have other negative results, including the potential loss of institutional
investor interest and fewer business development opportunities. In the event of a delisting, we would attempt to take actions to
restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by
us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock,
prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s
listing requirements.
On December 28, 2022, we were notified by
the Listing Qualifications Department (the “Staff”) of The Nasdaq Stock Market LLC (“Nasdaq”) that, based
upon our non-compliance with the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) (the “Rule”)
as of December 27, 2022, our common stock was subject to delisting unless we timely requested a hearing before the Nasdaq Hearings
Panel (the “Panel”). We timely requested a hearing before the Panel, and a hearing was held on February 16, 2023. On
February 21, 2023, the Staff notified us that the Panel has granted our request for continued listing of our common stock on the
Nasdaq Capital Market and an extension until June 26, 2023 (the “Compliance Period”) to regain compliance with the
continued listing requirements for The Nasdaq Capital Market, including the Rule. We effected the Reverse Stock Split on May 22,
2023. On June 21, 2023, we received a communication from Nasdaq indicating that we demonstrated compliance with the requirements
to remain listed on The Nasdaq Capital Market, as required by the Panel’s February 21, 2023, decision, and that pursuant
to Listing Rule 5815(d)(4)(B), we will be subject to a Mandatory Panel Monitor for a period of one year from the date of the communication.
If, within that one-year monitoring period, the Staff finds us again out of compliance with the Rule, notwithstanding Rule 5810(c)(2)
we will not be permitted to provide the Staff with a plan of compliance with respect to that deficiency and Staff will not be permitted
to grant additional time for the Company to regain compliance with respect to that deficiency, nor will we be afforded an applicable
cure or compliance period pursuant to Rule 5810(c)(3), and the Staff will instead issue a delist determination letter and we will
have an opportunity to request a new hearing with the initial Panel or a newly convened Hearings Panel if the initial Panel is
unavailable. At any such hearing, we will have the opportunity to respond/present to the Hearings Panel as provided by Listing
Rule 5815(d)(4)(C). There can be no assurance that any such Panel or Hearing Panel would grant us additional time to
regain compliance.
On April 12, 2023, we received a notice
(the “Notice”) from the Staff of Nasdaq, notifying us that for the last 30 consecutive business days, our minimum Market
Value of Listed Securities (“MVLS”) was below the minimum of $35 million required for continued listing on the Nasdaq
Capital Market pursuant to Nasdaq Listing Rule 5550(b)(2) (the “Market Value Standard”). The Notice is only a notification
of deficiency, not of imminent delisting, and has no current effect on the listing or trading of our common stock on the Nasdaq
Capital Market. Consequently, a deficiency exists with regard to the Nasdaq listing rules. In accordance with the listing rules,
we will have 180 days, or until October 9, 2023, to either regain compliance with the Market Value Standard, or satisfy another
listing criteria such as having a minimum shareholder equity of $2.5 million. To regain compliance with the Market Value Standard,
the MVLS for our common stock must be at least $35 million for a minimum of 10 consecutive business days at any time during this
180-day period. If we regain compliance with an applicable listing standard, we anticipate that the Nasdaq Staff will provide us
with written confirmation and will close the matter. If we do not regain compliance with the applicable listing standard by October
9, 2023, Nasdaq will provide notice that our securities are subject to delisting from the Nasdaq Capital Market. In the event of
such notification, the Nasdaq rules permit us an opportunity to appeal Nasdaq’s determination and request a hearing before
a Hearing Panel. We intend to monitor both the MVLS and our shareholder equity between now and October 9, 2023, and may, if appropriate,
evaluate available options to resolve the deficiency and regain compliance with the MVLS rule. However, there can be no assurance
that we will be able to regain or maintain compliance with Nasdaq listing criteria in the future.
The rights of the holders of common stock may be impaired
by the potential issuance of preferred stock.
Our restated certificate of incorporation
gives our board of directors the right to create new series of preferred stock. As a result, the board of directors may, without
stockholder approval, issue preferred stock with voting, dividend, conversion, liquidation or other rights which could adversely
affect the voting power and equity interest of the holders of common stock. Preferred stock, which could be issued with the right
to more than one vote per share, could be utilized as a method of discouraging, delaying or preventing a change of control. The
possible impact on takeover attempts could adversely affect the price of our common stock.
We may not receive any additional funds upon the exercise of the
common warrants.
Each common warrant has an exercise price of $______
per share, and may also be exercised in certain circumstances by way of a cashless exercise, meaning that the holder may not pay a cash
purchase price upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according
to the formula set forth in the warrant. Accordingly, we may not receive any additional funds, or any significant additional funds, upon
the exercise of the warrants.
There is no public market for the common warrants or pre-funded
warrants being offered by us in this offering.
There is no established public trading
market for the common warrants or the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend
to apply to list the common warrants or the pre-funded warrants on any national securities exchange or other nationally recognized
trading system. Without an active market, the liquidity of the common warrants and the pre-funded warrants will be limited.
The common warrants included in the units and in the pre-funded
units are speculative in nature.
The common warrants represent the right
to acquire shares of common stock at a fixed price. Specifically, commencing on the date of issuance, holders of the common warrants
may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of $ per
share of common stock. Moreover, following this offering, the market value of the common warrants is uncertain and there can be
no assurance that the market value of the common warrants will equal or exceed the public offering price. There can be no assurance
that the market price of the shares of common stock will ever equal or exceed the exercise price of the common warrants, and consequently,
whether it will ever be profitable for holders of common warrants to exercise the common warrants.
Except as otherwise set forth in the common warrants and
pre-funded warrants, holders of the common warrants and the pre-funded warrants offered hereby will have no rights as stockholders
with respect to the shares of common stock underlying the common warrants and the pre-funded warrants until such holders exercise
their common warrants and pre-funded warrants and acquire our common stock.
Except as otherwise set forth in the common
warrants and pre-funded warrants, until holders of the common warrants and the pre-funded warrants acquire shares of our common
stock upon exercise thereof, such holders of the common warrants and the pre-funded warrants will have no rights with respect to
the shares of our common stock underlying such warrants, such as voting rights. Upon exercise of the common warrants or the pre-funded
warrants, as the case may be, the holder will be entitled to exercise the rights of a common stockholder only as to matters for
which the record date occurs after the exercise date.
Risks Related to our Business, Industry and Financial Condition
There is substantial doubt about our ability to continue
as a going concern.
Our consolidated financial statements are
prepared using the generally accepted accounting principles applicable to a going concern, which contemplates the realization of
assets and liquidation of liabilities in the normal course of business. However, as shown in our consolidated financial statements
for the year ended December 31, 2022, included in our Annual Report on Form 10-K for the year ended December 31, 2022, we have
sustained substantial recurring losses from operations. In addition, we have used, rather than provided, cash in our continuing
operations. As of March 31, 2023, we had cash and equivalents of approximately $3.1 million. The above conditions raise substantial
doubt about our ability to continue as a going concern within one year after such date. Our consolidated financial statements do
not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities
that might be necessary should we be unable to continue in existence. Uncertainty concerning our ability to continue as a going
concern, among other factors, may hinder our ability to obtain future financing. Continued operations and our ability to continue
as a going concern are dependent, among other factors, on our ability to successfully develop and commercialize products, the market
acceptance and success of our products and our ability to obtain additional required funding in the near term and thereafter. If
we cannot continue as a viable entity, we might be required to reduce or cease operations or seek dissolution and liquidation or
bankruptcy protection, and our stockholders would likely lose most or all of their investment in us.
Our ability to obtain required financing
will be subject to a number of factors, including without limitation market conditions, our capitalization, our operating performance
and investor sentiment. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly
delay, scale back or discontinue the development or commercialization of one or more of our product candidates, restrict our operations
or attempt to obtain funds by entering into agreements on unattractive terms, which would likely have a material adverse effect
on our business, stock price and our relationships with third parties with whom we have business relationships, and which could
result in additional dilution to our stockholders. If we do not have sufficient funds to continue operations, we could be required
to seek bankruptcy protection or other alternatives that would likely result in our stockholders losing some or all of their investment
in us.
We have incurred losses since our inception, and we anticipate
that we will continue to incur losses. We may never achieve or sustain profitability.
We incurred significant net losses for the
three months ended March 31, 2023. We expect that these losses will continue as we continue our research and development activities,
support commercialization of our approved products, and continue to conduct our business. These losses will cause, among other
things, our stockholders’ equity and working capital to decrease. Any future earnings and cash flow from operations of our
business are dependent on our ability to further develop our products and on revenue and profitability from sales of products.
There can be no assurance that we will be able to generate sufficient revenue and amounts payable to us under our commercialization
agreement relating to our SYMJEPI and ZIMHI products or other commercialization agreements that we may enter into to become profitable
at all or on a sustained basis. We expect to have quarter-to-quarter fluctuations in revenue and expenses, some of which could
be significant. If our products do not achieve market acceptance, we may never become profitable. As we commercialize and market
products, we may incur expenses for product marketing and brand awareness and conduct significant research, development, testing
and regulatory compliance activities that, together with general and administrative expenses, could result in substantial operating
losses for the foreseeable future. Even if we do achieve profitability, we may not be able to sustain or increase profitability
on a quarterly or annual basis.
We will require additional funding to continue as a going
concern.
Our continued operations and the development
of our business will require additional capital. Based on our current and anticipated level of operations, we do not believe that
our cash, cash equivalents and short-term investments, together with anticipated revenues from operations and amounts that we expect
to receive as a result of our sales of assets relating to our former U.S. Compounding, Inc. business or from other sources, will be
sufficient to meet our anticipated operating expenses, liabilities and obligations for at least 12 months from the date of this
prospectus. Even with the net proceeds from this offering, we will require additional funds to sustain operations, satisfy our
obligations and liabilities, fund our ongoing operations, or for other purposes. There are no assurances that required funding will
be available at all or will be available in sufficient amounts or on reasonable terms. In addition to product revenues, we have
historically relied upon sales of our equity or debt securities to fund our operations. We currently have no available balance in
our credit facility or committed sources of capital, and a number of factors may limit or prevent our current ability to access
capital markets to obtain any required equity or debt funding. Delays in obtaining, or the inability to obtain, required funding
from revenues relating to sales of our commercial products, debt or equity financings, sales of assets, sales or out-licenses of
intellectual property assets, products, product candidates or technologies, or other transactions or sources, would materially and
adversely affect our ability to satisfy our current and future liabilities and obligations, and would materially and adversely
affect our ability to continue operations.
Our ability to obtain required debt or equity
financing or funds from other transactions will be subject to a number of factors, including without limitation market conditions,
our capitalization, our operating performance and investor sentiment. The terms of any such funding, or the terms of any strategic
transaction that we might enter into, could result in significant dilution to our stockholders. If we are unable to raise additional
funds when required or on acceptable terms, we may have to significantly restrict our operations or seek to obtain funds by entering
into agreements on unattractive terms, which would likely have a material adverse effect on our business, stock price and our relationships
with third parties with whom we have business relationships, and which could result in additional dilution to our stockholders.
If we do not have sufficient funds to continue operations, we could be required to seek dissolution and liquidation, bankruptcy
protection or other alternatives that would likely result in our stockholders losing some or all of their investment in us.
Statements in this prospectus concerning our future plans
and operations are dependent on our ability to secure adequate funding and the absence of unexpected delays or adverse developments.
We may not be able to secure required funding.
The statements contained in this prospectus
concerning future events or developments or our future activities, such as concerning research and development activities or regulatory
matters, commercial introduction of any products that we may develop in the future, anticipated outcome of any legal proceedings
in which we are involved, and other statements concerning our future operations and activities, are forward-looking statements
that in each instance assume that we have or are able to obtain sufficient funding to support such activities and continue our
operations and satisfy our liability and obligations in a timely manner. There can be no assurance that this will be the case.
Also, such statements assume that there are no significant unexpected developments or events that delay or prevent such activities
from occurring. Failure to timely obtain any required additional funding, or unexpected developments or events, could delay the
occurrence of such events or prevent the events described in any such statements from occurring which could adversely affect our
business, financial condition and results of operations.
We have received grand jury subpoenas issued in connection
with a criminal investigation and are subject to other investigations and legal proceedings.
As we have previously disclosed, on May
11, 2021, each of the company and its USC subsidiary received a grand jury subpoena from the U.S. Attorney’s Office for the
Southern District of New York (the “USAO”) issued in connection with a criminal investigation, requesting a broad range
of documents and materials relating to, among other matters, certain veterinary products sold by the company’s USC subsidiary,
certain practices, agreements and arrangements relating to products sold by USC, including veterinary products, and certain regulatory
and other matters relating to the company and USC. The Audit Committee of the Board engaged outside counsel to conduct an independent
internal investigation to review these and other matters. The company has also received requests from the SEC that the company
voluntarily provide documents and information in connection with the SEC’s investigation relating to certain matters including
matters arising from the subject matter of the subpoenas from the USAO. The company has produced and will continue to produce and
provide documents in response to the subpoenas and requests. The company intends to continue cooperating with the USAO and the
SEC, and has continued to engage in communications with the SEC and USAO regarding their investigations. We could receive additional
requests from the USAO, SEC, or other authorities, which may require further investigation, and additional issues or facts could
arise or be determined, which could expand the scope, duration or outcome of the investigation. We are unable to predict the duration,
scope, or final outcome of the investigations by the USAO, SEC, or other agencies or what proceedings the USAO, SEC, or other federal
or state authorities may initiate if the foregoing matters are not resolved. However, in connection with resolution of these matters,
we or our USC subsidiary may be found to have violated one or more laws arising from the subject matter of the subpoenas, and to
resolve the matters and investigations with the USAO and the SEC we may be required to pay material amounts in penalties or other
payments, and to agree to other remedies or remedial measures. Payment of material amounts in connection with resolution of the
foregoing matters would reduce the amount of financial resources that we have available to support our product development programs
and commercialization activities and would adversely impact our development programs. Depending in part on the amount and timing
of any payments that we may be required to make or other remedial measures that may be implemented in connection with resolution
of these matters, a resolution of these matters with the USAO or SEC could have a material and adverse impact on the company. The
foregoing matters have diverted and will likely continue to divert management’s attention, have caused the company to suffer
reputational harm, have required and will continue to require the company to devote significant financial resources, could subject
the company, one or more of its subsidiaries, or its officers and directors to civil or criminal proceedings, and depending on
the resolution of the matters or any proceedings, could result in fines, penalties, payments, or financial remedies in amounts
that would have a material adverse effect on our financial condition, or equitable remedies, and adversely affect the company’s
business, previously reported financial results, financial results included or incorporated by reference herein, or future financial
results.
On June 8, 2021, Jerald Hammann filed a
complaint against the Company and each of its directors in the Court of Chancery of the State of Delaware, captioned Jerald
Hammann v. Adamis Pharmaceuticals Corporation et al., C.A. No. 2021-0506-PAF (the “Complaint”), seeking injunctive
and declaratory relief. The Complaint alleges, among other things, that the defendants (i) violated Rule 14a-5(f) and 14a-9(a)
of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), in connection with the Company’s 2021
annual meeting of stockholders—which was subsequently held on July 16, 2021 (the “2021 annual meeting”)—and
disseminated false and misleading information in the Company’s proxy materials relating to the 2021 annual meeting, (ii)
violated certain provisions of the Company’s bylaws relating to the 2021 annual meeting, (iii) violated section 220 of the
Delaware General Corporation Law (“DGCL”) in connection with a request for inspection of books and records submitted
by the plaintiff, and (iv) breached their fiduciary duties of disclosure and loyalty, including relating to establishing and disclosing
the date of the Company’s 2021 annual meeting and to the Company’s determination that a solicitation notice delivered
to the Company by plaintiff was not timely and was otherwise deficient. On April 4, 2022, the plaintiff filed a motion to amend
the Complaint. The proposed amended Complaint added additional allegations relating to the manner in which the defendants established
and disclosed the date of the Company’s 2021 annual meeting of stockholders and to statements the defendants made about the
plaintiff to the Company’s stockholders. On April 28, 2022, the Court granted the motion. The plaintiff has also filed various
motions with the Court, which have been resolved. The Company has filed a motion for summary judgment with respect to one of the
counts in the Complaint and a motion to dismiss certain other counts of the plaintiff’s amended Complaint. On March 13, 2023,
the Court denied the Company’s motion for summary judgment. Trial on the merits of the plaintiff’s claims was
held on March 16, 2023, and the case is under consideration by the Court. The Company believes the claims in the plaintiff’s
complaint are without merit. However, an adverse outcome in the proceeding could have a material and adverse effect on the Company’s
business, financial condition and results of operations. On January 20 and March 27, 2023, the plaintiff filed motions for sanctions
against the defendants, asserting among other things that the alleged conduct that the plaintiff argues supports his case on the
merits is sanctionable. These motions are pending before the Court. The Company believes the claims in the plaintiff’s
motions are without merit and intends to vigorously dispute them.
We may never commercialize additional product candidates
that are subject to regulatory approval or earn a profit.
Except for our SYMJEPI and ZIMHI products,
we have not received regulatory approval for any drugs or products. We may never be able to commercialize any additional product
candidates that are subject to regulatory approval or be able to generate revenue from sales of such products. Because of the risks
and uncertainties associated with developing and commercializing our specialty pharmaceuticals and other product candidates, we
are unable to predict when we may commercially introduce such products, the extent of any future losses or when we will become
profitable, if ever.
Business or economic disruptions or global health concerns,
including the COVID-19 pandemic, could harm our business.
Business or economic disruptions or global
health concerns, such as the COVID-19 pandemic, could adversely affect our business. The COVID-19 pandemic, which the World Health
Organization announced in January 2020 was a global health emergency, spread throughout most of the world including the United
States. The outbreak resulted in extended shutdowns of businesses in the United States and elsewhere and had ripple effects on
businesses and activities around the world. The COVID-19 outbreak and continued spread of COVID-19, including the identification
of novel strains of COVID-19, has affected and may continue to affect our operations, our customers and third parties on which
we rely. In addition, we could experience delays in obtaining products or services from our third-party manufacturers or suppliers
as a result of the impact of the COVID-19 pandemic or other similar outbreaks on such parties. The extent to which the COVID-19
pandemic or other health emergencies will continue to impact our business is difficult to predict and subject to change, and will
depend on future developments, which are highly uncertain and cannot be predicted, including without limitation the severity of
the disease and duration of the outbreak, travel restrictions and social distancing requirements in the United States and other
countries, future mutations and variations of the coronavirus, and the effectiveness of actions taken in the United States and
other countries to contain and treat the disease and address its impact. In addition, a severe or prolonged economic downturn or
political disruption could result in a variety of risks to our business, including our ability to raise capital when needed on
acceptable terms, if at all. A weak or declining economy or political disruption could also strain our manufacturers or suppliers,
possibly resulting in supply disruption, or cause our customers to delay making purchases or payments for our products. Any of
the foregoing could harm our business.
Even if they are approved and commercialized, if our potential
products are unable to compete effectively with current and future products targeting similar markets as our potential products,
our commercial opportunities will be reduced or eliminated.
The markets for our SYMJEPI products and
ZIMHI product, and our other product candidates, are intensely competitive and characterized by rapid technological progress. We
face competition from numerous sources, including major biotechnology and pharmaceutical companies worldwide. Many of our competitors
have substantially greater financial and technical resources, and development, production and marketing capabilities, than we do.
Our SYMJEPI product competes with a number of other currently marketed epinephrine products for use in the emergency treatment
of acute allergic reactions, including anaphylaxis. Our ZIMHI product competes with a number of other currently marketed products
utilizing naloxone for the treatment of acute opioid overdose. Certain companies have established technologies that may be competitive
with our product candidates and any future products that we may develop or acquire. Some of these products may use different approaches
or means to obtain results, which could be more effective or less expensive than our products for similar indications. In addition,
many of these companies have more experience than we do in pre-clinical testing, performance of clinical trials, manufacturing,
and obtaining FDA and foreign regulatory approvals. They may also have more brand name exposure and expertise in sales and marketing.
We also compete with academic institutions, governmental agencies and private organizations that are conducting research in the
same fields.
Competition
among these entities to recruit and retain highly qualified scientific, technical and professional personnel and consultants is
also intense. As a result, there is a risk that one or more of our competitors will develop a more effective product for the same
indications for which we are developing a product or, alternatively, bring a similar product to market before we can do so. Failure
to successfully compete will adversely impact the ability to raise additional capital and ultimately achieve profitable operations.
If
we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, identify
or discover material weaknesses in our internal control over financial reporting or fail to effectively remediate any identified
material weaknesses, our business and financial condition could be materially and adversely affected and our stock price could
decline.
Our
management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, designed
to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial
statements for external purposes in accordance with U.S. GAAP. Our management is likewise required, on a quarterly basis, to evaluate
the effectiveness of our internal controls and to disclose any material changes and weaknesses identified through such evaluation.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that
there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented
or detected on a timely basis. If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure
controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal control and accounting
procedures, our stock price could decline significantly and our business and financial condition could be adversely affected.
If material weaknesses or significant deficiencies are discovered or if we otherwise fail to achieve and maintain the adequacy
of our internal control, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal
controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls
are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot
provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose
confidence in our reported financial information, and the trading price of our common stock could decline significantly.
We
take responsive actions to address identified material weaknesses in our internal control over financial reporting. However, we
can give no assurance that such measures will remediate any material weakness that are identified or that any additional material
weaknesses or restatements of financial results will not arise in the future. In the future, our management may determine that
our disclosure controls and procedures are ineffective or that there are one or more material weaknesses in our internal controls
over financial reporting, resulting in a reasonable possibility that a material misstatement to the annual or interim financial
statements would not have been prevented or detected. Accordingly, a material weakness increases the risk that the financial information
we report contains material errors. Any system of internal controls, however well designed and operated, is based in part on certain
assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. Efforts to correct
any material weaknesses or deficiencies that may be identified could require significant financial resources to address. Moreover,
if remedial measures are insufficient to address the deficiencies that are determined to exist, we may fail to meet our future
reporting obligations on a timely basis, our consolidated financial statements could contain material misstatements, we could
be required to restate our prior period financial results, our operating results may be harmed, and we could become subject to
class action litigation or investigations or proceedings from regulatory authorities. Any of these matters could adversely affect
our business, reputation, revenues, results of operations, financial condition and stock price.
Our
principal stockholders have significant influence over us, they may have significant influence over actions requiring stockholder
approval, and your interests as a stockholder may conflict with the interests of those persons.
Based
on the number of outstanding shares of our common stock held by our stockholders as of June 30, 2023, our directors, executive
officers and their respective affiliates owned approximately 6.9% of our outstanding shares of common stock. As a result, those
stockholders have the ability to exert a significant degree of influence with respect to the outcome of matters submitted to our
stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all
of our assets. The interests of these persons may not always coincide with our interests or the interests of our other stockholders.
This concentration of ownership could harm the market price of our common stock and value of our outstanding warrants by (i) delaying,
deferring or preventing a change in corporate control, (ii) impeding a merger, consolidation, takeover or other business combination
involving us, or (iii) discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control
of us. The significant concentration of stock ownership may adversely affect the trading price of our common stock and value of
our outstanding warrants due to investors’ perception that conflicts of interest may exist or arise.
USE
OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately
$8,850,000 (assuming the sale of all units offered hereby at the assumed public offering price of $1.90 per unit, which represents the
closing sale price of our common stock on the Nasdaq Capital Market on July 21, 2023, and assuming no issuance of pre-funded warrants),
after deducting placement agent fees and estimated offering expenses payable by us, and assuming no sale of any pre-funded units offered
hereunder. However, because this is a reasonable best efforts offering with no minimum number of securities or amount of proceeds
as a condition to closing, the actual offering amount, placement agent fees and net proceeds to us are not presently determinable and
may be substantially less than the maximum amounts set forth on the cover page of this prospectus, and we may not sell all or any of the
securities we are offering. As a result, we may receive significantly less in net proceeds. Based on the assumed offering price set forth
above, we estimate that our net proceeds from the sale of 75% or 50% of the units offered in this
offering would be approximately $6.53 million and $4.20 million, respectively, after deducting placement agent fees and estimated offering
expenses payable by us.
A $0.10 increase or decrease in the assumed public offering price of $1.90
per unit would increase or decrease the net proceeds from this offering by approximately $489,474, assuming that the number of units offered
by us, as set forth on the cover page of this prospectus, remains the same and after deducting placement agent fees and estimated offering
expenses payable by us, and assuming no sale of any pre-funded units offered hereunder.
We currently intend to use the net proceeds from this offering for working
capital and general corporate purposes, which may include, without limitation, expenditures relating to research, development and clinical
trials relating to our products and product candidates, manufacturing, capital expenditures, hiring additional personnel, the payment,
repayment, refinancing, redemption or repurchase of existing or future indebtedness, obligations or capital stock, acquisitions, should
we choose to pursue any, collaborations, and payment of obligations and liabilities. We may also use the proceeds to acquire or invest
in complementary products, services, technologies or other assets, although we have no agreements or understandings with respect to any
acquisitions or investments at this time. Other than as described above, we have not yet determined the amount of net proceeds to be used
specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying
the net proceeds from this offering. Pending any use as described above, we may invest a portion of the net proceeds in high-quality,
short-term, interest-bearing securities.
DILUTION
If you purchase securities in this offering, your ownership interest will
be diluted immediately to the extent of the difference between the assumed public offering price per unit and as adjusted, net tangible
book value per share of common stock immediately after this offering. Tangible assets equal our total assets less goodwill and intangible
assets. Our historical net tangible book value per share is equal to our total tangible assets, less total liabilities, divided by the
number of outstanding shares of common stock as of March 31, 2023. As of March 31, 2023, our historical net tangible book value
was $(9,681,681), or $(4.07) per share of common stock, after giving effect to the Reverse Stock Split, and our pro forma net tangible
book value was $(9,681,681), or $(4.07) per common share.
After (i) giving effect to the sale by us in this offering of 5,263,158
units at an assumed public offering price of $1.90 per unit (the closing sale price of our common stock on the Nasdaq Capital Market on
July 21, 2023), assuming no sale of any pre-funded units in this offering and no exercise of any of the common warrants being offered
in this offering, and (ii) deducting the placement agent fees and estimated offering expenses payable by us, our pro forma as adjusted
net tangible book value as of March 31, 2023, would have been $(831,681), or $(0.11) per share of common stock. This amount represents an
immediate increase in as adjusted net tangible book value of $3.96 per share to our existing shareholders and an immediate dilution of
$2.01 per share to purchasers of our securities in this offering. We determine dilution per share to investors participating in this offering
by subtracting as adjusted net tangible book value per share after this offering from the offering price per share paid by investors participating
in this offering.
The
following table illustrates this per share dilution, which reflects the Reverse Stock Split:
| Assumed public offering price per unit |
|
|
|
|
|
$ |
1.90 |
|
| Historical net tangible book deficit value per share as of March 31, 2023 |
|
$ |
(4.07 |
) |
|
|
|
|
| Pro forma as adjusted change in historical net tangible book deficit per share attributable to this offering |
|
|
|
|
|
$ |
3.96 |
|
| As adjusted pro forma net tangible book value per share as of March 31, 2023, after giving effect to this offering |
|
|
|
|
|
$ |
(0.11 |
) |
| Dilution per share to new investors in this offering |
|
|
|
|
|
$ |
2.01 |
|
A $0.10 increase in the assumed public offering price of $1.90 per unit
(the closing sale price of our common stock on the Nasdaq Capital Market on July 21, 2023), would increase our as adjusted net tangible
book value after giving effect to this offering by approximately $489,474, or $0.06 per share, and the dilution per share to investors
purchasing securities in this offering would be approximately $2.04 per share, assuming that the maximum number of units offered by us,
as set forth on the cover page of this prospectus, remains the same and no sale of any pre-funded warrants in this offering. Similarly,
a $0.10 decrease in the assumed public offering price of $1.90 per unit would decrease our as adjusted net tangible book value after this
offering by approximately $(489,474), or $(0.06) per share, and the dilution per share to new investors in this offering would be $1.97
per share, assuming that the maximum number of units offered by us, as set forth on the cover page of this prospectus, remains the same
and after deducting the placement agent fees and estimated offering expenses payable by us.
We may also increase or decrease the number of units we are offering from
the assumed maximum number of units set forth above. An increase of 100,000 units from the assumed maximum number of units set forth on
the cover page of this prospectus would increase our as adjusted net tangible book value after this offering by approximately $176,700,
or $0.024 per share, and the dilution per share to investors purchasing securities in this offering would be approximately $1.98 per share,
assuming that the assumed public offering price remains the same and after deducting the placement agent fees and estimated offering expenses
payable by us. Similarly, a decrease of 100,000 units from the assumed maximum number units stock set forth on the cover page of this
prospectus would decrease our as adjusted net tangible book value after this offering by approximately $176,700, or $ 0.025 per share,
and the dilution per share to investors purchasing securities in this offering would be approximately $2.03 per share, assuming that the
assumed public offering price remains the same and after deducting the placement agent fees and estimated offering expenses payable by
us. A decrease in the number of units sold in the offering to approximately 75% of the assumed maximum number of units set forth on the
cover page of this prospectus, to 3,947,369 units (resulting in gross proceeds of approximately $7,500,000), would decrease our pro forma
as adjusted net tangible book value as of March 31, 2023 after this offering by approximately $2,325,000, or approximately $0.39 per share,
from the amounts presented in the table above, and would change the dilution to investors in this offering to approximately $2.40 per
share, assuming that the assumed offering price per unit as set forth on the cover page of this prospectus remains the same, after deducting
the estimated placement agent’s fees and estimated offering expenses payable by us. A decrease in the number of units sold in this
offering to approximately 50% of the assumed maximum number of units set forth on the cover page of this prospectus, to 2,631,579 units
(resulting in gross proceeds of approximately $5,000,000), would decrease our pro forma as adjusted net tangible book value as of March
31, 2023 after this offering by approximately $4,650,000, or approximately $0.99 per share, from the amounts presented in the table above,
and would change the dilution to investors in this offering to approximately $2.99 per share, assuming that the assumed offering price
per unit as set forth on the cover page of this prospectus, remains the same, after deducting the estimated placement agent’s fees
and estimated offering expenses payable by us.
The
information discussed above is illustrative only and will adjust based on the actual public offering price, the actual number
of units that we offer in this offering, and other terms of this offering determined at the time of pricing. The foregoing discussion
and table assumes no sale of pre-funded units, which if sold, would reduce the number of units that we are offering on a one-for-one
basis and does not take into account further dilution to investors in this offering that could occur upon the exercise of outstanding
options and warrants having a per share exercise price less than the public offering price per unit in this offering. In addition,
we may choose to raise additional capital due to market conditions or strategic considerations. To the extent that additional
capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in
further dilution to our stockholders.
The number of shares of our common stock outstanding after this offering
is based on 2,378,295 shares of common stock outstanding as March 31, 2023 and excludes, as of such date, the following: (i) 59,277 shares
of common stock issuable upon exercise of outstanding stock options, with exercise prices ranging from $43.40 to $592.20 and having a
weighted average exercise price of $287.16 per share, and 9,286 shares issuable upon the vesting of restricted stock units outstanding,
awarded under our equity incentive plans, 2,143 shares of which were issued after March 31, 2023 following such vesting; (ii) outstanding
warrants and the shares issuable upon exercise of such warrants, to purchase the following numbers of shares of common stock: 840 shares
at an exercise price of $595.00 per share; 197,055 shares at an exercise price of $80.50 per share; 5,000 shares at an exercise price
of $49.00 per share; 10,714 shares at an exercise price of $32.90 per share; and 685,714 shares at an exercise price of $9.66 per share;
(iii) 202,455 shares of common stock reserved for future issuance under our 2020 Equity Incentive Plan; (iv) 1,941.2 shares of Series E
Preferred convertible into approximately 1,941,200 shares of common stock subject to various beneficial ownership and other limitations
and restrictions on conversion, and 302,815 shares of common stock, issued after March 31, 2023, in connection with the closing of the
Merger transaction with DMK; (v) outstanding options to purchase 231,490 shares of common stock at an exercise price of $2.90 per
share that we assumed in connection with the Merger, and 18,005 remaining unallocated shares reserved for issuance pursuant to the 2016
DMK Stock Plan that we assumed in connection with the Merger; (vi) approximately 9,967 shares of common stock issuable upon conversion
of 3,000 outstanding shares of Series C Convertible Preferred Stock, or Series C Preferred; (vii) options to purchase 24,962 shares of
common stock that expired unexercised after March 31, 2023; and (viii) the issuance of 107,142 shares of common stock (post Reverse
Stock Split) pursuant to the exercise of prefunded warrants after March 31, 2023. Unless otherwise indicated, this prospectus assumes
no exercise of the pre-funded warrants or the common warrants.
CAPITALIZATION
The following table sets forth our capitalization as
of March 31, 2023. Such information is set forth on the following basis:
| |
● |
on a pro forma basis, reflecting the Reverse Stock Split effected on May
22, 2023; and |
|
● |
on a pro forma as adjusted basis, giving effect to the (i) pro forma adjustments
set forth in the immediately preceding bullet, and (ii) sale of the securities in this offering at the assumed public offering price of
$1.90 per unit, which represents the closing sale price of our common stock on the Nasdaq Capital Market on July 21, 2023, and assuming
no issuance of pre-funded warrants, after deducting placement agent’s fees and estimated offering expenses, and excluding
the proceeds, if any, from the subsequent exercise of the common warrants and pre-funded warrants issued pursuant to this offering. |
You
should read this table together with our financial statements and the related notes thereto, as well as “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” and the other financial information, incorporated
by reference in this prospectus from our SEC filings, including our most recent Annual Report on Form 10-K, our Quarterly Reports
on Form 10-Q and our Current Reports on Form 8-K. The information presented in the capitalization table below is unaudited.
| |
|
As of March 31, 2023 |
|
| |
|
Actual |
|
|
Pro Forma(1) (3) |
|
|
Pro Forma
as
Adjusted(2) (3) |
|
| |
|
(in thousands,
except share amounts) |
|
| Cash and cash equivalents |
|
$ |
3,100 |
|
|
$ |
3,100 |
|
|
$ |
11,950 |
|
|
Mezzanine Equity
Convertible Preferred Stock - par value
$0.0001; 10,000,000 Shares Authorized; Series C Preferred Stock 3,000 Shares Authorized, liquidation preference $110 per share; 3,000
Issued and Outstanding |
|
|
157 |
|
|
|
157 |
|
|
|
157 |
|
| Stockholders’ (Deficit) Equity |
|
|
|
|
|
|
|
|
|
|
|
|
| Common Stock, par value $0.0001; 200,000,000 Shares Authorized; 166,438,265 Issued and Outstanding (net of treasury shares), actual (1); 2,378,295 Issued and Outstanding, pro forma; and 7,641,453 Issued and Outstanding, pro forma, as adjusted |
|
|
15 |
|
|
|
0 |
|
|
|
1 |
|
| Additional paid-in capital |
|
|
303,816 |
|
|
|
303,831 |
|
|
|
312,680 |
|
| Accumulated Deficit |
|
|
(313,507) |
|
|
|
(313,507) |
|
|
|
(313,507) |
|
| Treasury Stock, 522,957 Shares, actual (1), 7,470 Shares, pro forma and 7,470 Shares, pro forma, as adjusted (at cost) |
|
|
(5) |
|
|
|
(5) |
|
|
|
(5) |
|
| Total stockholders’ deficit |
|
|
(9,681 |
) |
|
|
(9,681 |
) |
|
|
(831 |
) |
| Total capitalization (mezzanine equity and stockholders’ (deficit) equity) |
|
|
(9,524 |
) |
|
|
(9,524 |
) |
|
|
(674 |
) |
| (1) | On
May 22, 2023, the Company effected the 1-for-70 Reverse Stock Split of the outstanding shares of common stock. As a result of the
reverse stock split, every 70 shares of issued and outstanding common stock were automatically combined into one issued and
outstanding share of common stock, without any change in the par value per share. The Company did not issue any fractional shares in
the reverse stock split. The number of authorized shares of common stock under the Company’s restated certificate of
incorporation remained unchanged. After adjusting for the Reverse Stock Split, issued and outstanding shares at March 31, 2023 were
2,378,295 recast and treasury shares were 7,470 recast. |
| |
(2) |
A decrease in the number of units offered by us to 3,947,369 units (resulting in gross proceeds of approximately $7,500,000) would decrease cash, increase stockholders’ deficit, and decrease total capitalization on a pro forma as adjusted basis by approximately $2,325,000 from the amounts presented in the table above, assuming the assumed offering price of $1.90 per unit remains the same, and after deducting placement agent’s fees and estimated offering expenses payable by us. A decrease in the number of units offered by us to 2,631,579 units (resulting in gross proceeds of approximately $5,000,000) would decrease cash, increase total stockholders’ deficit, and decrease total capitalization on a pro forma as adjusted basis by approximately $4,650,000 from the amounts presented in the table above, assuming the assumed offering price of $1.90 per unit remains the same, and after deducting placement agent’s fees and commissions and estimated offering expenses payable by us. The Company has not completed its review of the accounting treatment and fair value of the common warrants and pre-funded warrants offered hereby. The table above assumes the common warrants and pre-funded warrants are accounted for within equity. If the Company determines the warrants are to be accounted for as liabilities, the fair value of the warrants will be recognized as a liability and subsequently recorded at fair value each reporting period with the change in fair value recognized within income. |
| |
(3) |
The Company has not completed accounting for the Merger transaction with
DMK, which was completed after March 31, 2023. Amounts shown in the table above do not reflect the accounting for the Merger
transaction with DMK, which could have a material effect on the amounts shown above. |
The
pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering
price and other terms of this offering determined at pricing.
Except as otherwise noted, all information in this prospectus reflects
and assumes no sale of pre-funded warrants in this offering, which, if sold, would reduce the number of shares of common stock that we
are offering on a one-for-one basis. The table above on an actual basis is based on 2,378,295 post-Reverse Stock Split shares of
common stock outstanding as of March 31, 2023, and excludes, as of that date, the following: (i) 59,277 shares of common stock issuable
upon exercise of outstanding stock options as of March 31, 2023, with exercise prices ranging from $43.40 to $592.20 and having a
weighted average exercise price of $287.16 per share, and 9,286 shares issuable upon the vesting of restricted stock units outstanding
as of March 31, 2023, awarded under our equity incentive plans, 2,143 shares of which were issued after March 31, 2023 following
such vesting; (ii) outstanding warrants as of March 31, 2023, and the shares issuable upon exercise of such warrants, to purchase
the following numbers of shares of common stock: 840 shares at an exercise price of $595.00 per share; 197,055 shares at an exercise price
of $80.50 per share; 5,000 shares at an exercise price of $49.00 per share; 10,714 shares at an exercise price of $32.90 per share; and
685,714 shares at an exercise price of $9.66 per share issued after March 31, 2023; (iii) 202,455 shares of common stock reserved
for future issuance under our 2020 Equity Incentive Plan; (i) 302,815 shares of common stock, and 1,941.2 shares of Series E
Preferred convertible into approximately 1,941,200 shares of common stock subject to various beneficial ownership and other limitations
and restrictions on conversion, issued after March 31, 2023, in connection with the closing of the Merger transaction with DMK; (v) outstanding
options to purchase 231,490 shares of common stock at an exercise price of $2.90 per share that we assumed in connection with the Merger,
and 18,005 remaining unallocated shares reserved for issuance pursuant to the 2016 DMK Stock Plan that we assumed in connection with the
Merger; (vi) options to purchase 24,962 shares of common stock that expired unexercised after March 31, 2023; (vii) approximately
9,967 shares of common stock issuable upon conversion of 3,000 outstanding shares of Series C Preferred; and (viii) the issuance of 107,142
shares of common stock (post Reverse Stock Split) pursuant to the exercise of prefunded warrants after March 31, 2023.
Unless
otherwise indicated, this prospectus assumes no exercise of the pre-funded warrants or the common warrants offered hereby.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth information, as of June 30, 2023 (the “Table Date”), regarding beneficial ownership of
all classes of our voting securities, to the extent known to us, by (i) each person who is a director; (ii) each of our named
executive officers; (iii) all directors and executive officers as a group; and (iv) each person who is known by us to be the beneficial
owner of 5% or more of any class of our voting securities. Except as otherwise noted, each person has sole voting and investment
power as to his or her shares. As of the Table Date, the applicable share numbers and percentages are based on 2,790,395 shares
of common stock issued and outstanding as of the Table Date. In computing the number of shares of common stock beneficially owned
by a person and the percentage ownership of that person, we also included and deemed outstanding shares of common stock that are
issuable upon conversion of the Series C Preferred or Series E Preferred, as applicable, upon exercise of outstanding options
and warrants, or upon vesting of restricted stock units, held by that person that are currently convertible, exercisable or issuable
within 60 days after the Table Date. We did not deem these shares outstanding, however, for the purpose of computing the percentage
ownership of any other person.
| | |
Shares Beneficially Owned (1)
Title or Class of Securities: | |
| | |
Common Stock | | |
Series C
Preferred Stock | | |
Series E
Preferred Stock | |
| Directors: | |
Shares | | |
Percent | | |
Shares | | |
Percent | | |
Shares | | |
Percent | |
Ebrahim Versi, M.D., Ph.D.
| |
| 288,824 | (2) | |
| 9.9 | % | |
| | | |
| | | |
| (2 | ) | |
| 100.0 | % |
| Jannine Versi | |
| 158,616 | (3) | |
| 5.6 | % | |
| | | |
| | | |
| | | |
| | |
| Howard C. Birndorf | |
| 840 | (4) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Meera J. Desai, Ph.D., NACD.DC | |
| 3,138 | (5) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Vickie S. Reed | |
| — | | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Named Executive Officers | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| David J. Marguglio | |
| 11,937 | (6) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| David C. Benedicto | |
| 3,486 | (7) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Dennis C. Carlo | |
| 9,892 | (8) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Ronald B. Moss | |
| 2,884 | (9) | |
| * | | |
| | | |
| | | |
| | | |
| | |
| Other Beneficial Ownership | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| All Adamis directors and executive officers as a
group (seven persons) | |
| 466,841 | (10) | |
| 16.3 | % | |
| | | |
| | | |
| | | |
| | |
| 5% or greater stockholders | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Versi Group, LLC | |
| 177,194 | (2)(11) | |
| 6.4 | % | |
| | | |
| | | |
| 1,941.2 | | |
| 100.0 | |
| Lincoln Park Capital Fund, LLC(12) | |
| 36,395 | | |
| 1.3 | % | |
| 3,000 | | |
| 100.0 | % | |
| | | |
| | |
| Armistice Capital Master Fund Ltd. | |
| 278,760 | (13) | |
| 9.9 | % | |
| | | |
| | | |
| | | |
| | |
| (1) | Based
solely upon information made available to us and our knowledge. Beneficial ownership
is determined in accordance with rules of the SEC that deem shares to be beneficially
owned by any person who has or shares voting or investment power with respect to such
shares. Unless otherwise indicated, the persons named in this table have sole voting
and sole investing power with respect to all shares shown as beneficially owned, subject
to community property laws where applicable. Shares of common stock subject to an option
or similar right that is currently exercisable or convertible within 60 days of the date
of the table are deemed to be outstanding and to be beneficially owned by the person
holding such option or right for the purpose of computing the percentage ownership of
such person but are not treated as outstanding for the purpose of computing the percentage
ownership of any other person. Except as otherwise indicated, the address of each of
the persons in this table is as follows: c/o Adamis Pharmaceuticals Corporation, 11682
El Camino Real, Suite 300, San Diego, California 92130. |
| (2) | Includes
(i) 8,745 shares of common stock held by Dr. Versi, (ii) 177,194 shares of Adamis common
stock held by Versi Group, LLC, of which Dr. Versi is the manager and a member and who
may be deemed to be the beneficial owner, and (iii) 102,885 shares of common stock issuable
upon the exercise of options held by Dr. Versi, as the result of the assumption by Adamis
of outstanding DMK stock options pursuant to the Merger and the Merger Agreement, which
options are exercisable within 60 days of the Table Date. Excludes 1,941.2 shares of
Series E Preferred held by Versi Group, LLC, convertible into approximately 1,941,200
shares of Adamis common stock, subject to 9.99% beneficial ownership limitations on conversion
and voting of shares of Series E Preferred. The address of the shareholder is c/o DMK
Pharmaceuticals Corporation, 50 Division Street, Suite 501, Somerville, New Jersey 08876.
Dr. Versi disclaims beneficial ownership of the shares held by Versi Group, LLC except
to the extent of his pecuniary interest therein. |
| (3) | Includes
options, consisting of former DMK stock options assumed by Adamis pursuant to the Merger
with DMK, to purchase 25,720 shares of Adamis common stock that are exercisable within
60 days of the Table Date. Includes 132,896 shares of Adamis common stock held by Versi
Group, LLC, of which Jannine Versi is the trustee of a member of
Versi Group, LLC that holds 75% of the membership interests of Versi Group, LLC.
Excludes 1,455.9 shares of Series E Preferred issued to Versi Group, LLC, convertible
into an estimated approximately 1,455,900 shares of Adamis common stock, subject to 9.99%
beneficial ownership limitations on conversion and voting of shares of Series E Preferred,
representing the interest of the member of Versi Group, LLC of which Ms. Versi is the
trustee in the shares held by Versi Group, LLC. Ms. Versi disclaims beneficial ownership
of the shares held by Versi Group, LLC except to the extent of her pecuniary interest
therein. The address of the shareholder is c/o DMK Pharmaceuticals Corporation, 50 Division
Street, Suite 501, Somerville, New Jersey 08876. |
| (4) | Includes
840 shares that are issuable upon the exercise of a warrant that is exercisable as of
the Table Date. |
| (5) | Includes
3,138 shares of common stock held by Dr. Desai. |
| (6) | Includes
3,341 shares of common stock owned of record, 84 shares of common stock held of record
by a family member and beneficially owned by Mr. Marguglio; 8,512 shares of common stock
underlying options which were exercisable as of the Table Date. Excludes 2,857 RSUs which
vest after such period. |
| (7) | Includes
569 shares of common stock beneficially owned, and 2,917 shares of common stock underlying
options that are exercisable as of the Table Date. |
| (8) | Not
a current executive officer. Includes 9,808 shares of common stock owned of record and
84 shares of common stock held of record by a family member and beneficially owned by
Dr. Carlo. |
| (9) | Not
a current executive officer. Includes 2,884 shares of common stock owned of record. |
| (10) | Includes
325,967 shares of common stock beneficially owned, 140,034 shares of common stock underlying
options that are exercisable as of the Table Date, and 840 shares issuable upon the exercise
of warrant that is exercisable as of Table Date. Excludes 2,857 shares issuable upon
the vesting of RSUs more than 60 days after the Table Date. |
| (11) | Includes
177,194 shares of Adamis common stock held by Versi Group, LLC. Excludes 1,941.2 shares
of Series E Preferred issued to Versi Group, LLC, convertible into approximately 1,941,200
shares of Adamis common stock, subject to 9.99% beneficial ownership limitations on conversion
and voting of shares of Series E Preferred. Dr. Versi is the manager and a member of
Versi Group LLC, and may be deemed to be the beneficial owner of the shares held by the
named shareholder. The address of the shareholder is c/o DMK Pharmaceuticals Corporation,
50 Division Street, Suite 501, Somerville, New Jersey 08876. |
| (12) | Based
on information known to the Company. Lincoln Park Capital, LLC (“LPC”) is
the Managing Member of Lincoln Park Capital Fund LLC (“Lincoln Park”). Rockledge
Capital Corporation (“RCC”) and Alex Noah Investors, LLC (“Alex Noah”)
are the managing Members of LPC. Josh Scheinfeld is the president and sole shareholder
of RCC as well as a principal of LPC. Mr. Cope is the president and sole shareholder
of Alex Noah, as well as a principal of LPC. As a result of the foregoing, Mr. Scheinfeld
and Mr. Cope have shared voting and shared investment power over shares of common stock
of the Company held directly by Lincoln Park. The address of Lincoln Park Capital Fund
LLC (“Lincoln Park”) is 440 N. Wells Street, Suite 410, Chicago, Illinois
60654. The Series C Preferred is subject to a 4.99% (or, upon prior notice by the holder,
9.99%) beneficial ownership limitation that prohibits the fund from converting any portion
of the Series C Preferred if, following such conversion, the holder’s ownership
of our common stock would exceed that ownership percentage. As of the Table Date, to
our knowledge Lincoln Park beneficially owned 15,714 shares of common stock issuable
upon exercise of warrants held by Lincoln Park. Also includes 10,714 shares of common
stock underlying warrants issued to Lincoln Park which include a beneficial ownership
cap which prohibits the issuance of any shares of common stock upon exercise of such
warrants if such issuance would cause Lincoln Park’s beneficial ownership of our
common stock to exceed 4.99% (or, upon prior notice by the holder, 9.99%) of the outstanding
common stock. Includes 9,967 shares of common stock issuable upon conversion of the outstanding
shares of Series C Preferred. |
| (13) | Based
upon the Company’s knowledge, and represents 9.99% of the outstanding shares of
common stock on the Table Date. Includes shares of common stock held by the named stockholder
and may also include warrants to purchase shares of common stock which are exercisable
within 60 days of the Table Date. Excludes additional shares of common stock issuable
upon the exercise of warrants held by the named stockholder which are not exercisable
within 60 days of the Table Date because of beneficial ownership limitations on the exercisability
of such warrants. To the Company’s knowledge, the named stockholder holds warrants
to purchase 685,714 shares of common stock, subject to beneficial ownership limitations
on the exercisability of such warrants. Such securities are directly held by Armistice
Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”),
and may be deemed to be indirectly beneficially owned by: (i) Armistice Capital, LLC
(“Armistice Capital”), as the investment manager of the Master Fund; and
(ii) Steven Boyd, as the Managing Member of Armistice Capital. Armistice Capital and
Steven Boyd disclaim beneficial ownership of the securities except to the extent of their
respective pecuniary interests therein. The business address of the Master Fund is c/o
Armistice Capital, LLC, 510 Madison Ave, 7th Floor, New York, NY 10022. Under
the terms of the warrants held by Armistice Capital, a holder may not exercise the warrants
to the extent such exercise would cause such holder, together with its affiliates and
attribution parties, to beneficially own a number of shares of common stock which would
exceed 4.99% (which percentage may be increased to up to 9.99% by a holder upon 61 days
prior notice) and 9.99%, respectively, of our then outstanding common stock following
such exercise, excluding for purposes of such determination shares of common stock issuable
upon the exercise of such warrants which have not been exercised. The address of Armistice
Capital Master Fund Ltd. Is 510 Madison Ave., 7th Floor, New York, NY 10022. |
DESCRIPTION
OF SECURITIES
We
are offering shares of our common stock, pre-funded warrants to purchase shares of our common stock, and common warrants to purchase
shares of our common stock. The following descriptions of our common stock, preferred stock, pre-funded warrants and common warrants,
and certain provisions of our restated certificate of incorporation, our bylaws and Delaware law are summaries. You should also
refer to our restated certificate of incorporation, as amended, our bylaws, which are filed as exhibits to the registration statement
of which this prospectus is a part and, with respect to preferred stock, any certificate of designation that we may file with
the SEC for a series of preferred stock we may designate.
The
shares of common stock, pre-funded warrants, and common warrants that we are offering are immediately separable and will be issued
separately.
General
Our
authorized capital stock consists of 200,000,000 shares of common stock, par value $0.0001 per share; and 10,000,000 shares of
preferred stock, par value $0.0001 per share.
Common
Stock
As
of June 30, 2023, there were 2,790,395 shares of common stock outstanding, giving effect to the Reverse Stock Split. Our board
of directors is authorized, without stockholder approval except as required by the listing standards of The Nasdaq Stock Market
LLC (or of any other stock exchange or market on which our common stock is then traded), to issue shares of our common stock from
time to time. The holders of our common stock are entitled to one vote for each share held of record on all matters submitted
to a vote of the stockholders; provided, however, that, except as otherwise required by law, holders of our common stock, as such,
shall not be entitled to vote on any amendment to our restated certificate of incorporation that relates solely to the terms of
one or more outstanding series of preferred stock if the holders of such affected series are entitled, either separately or together
with the holders of one or more other such series, to vote thereon by law or pursuant to our restated certificate of incorporation.
The holders of common stock are not entitled to cumulative voting rights with respect to the election of directors, and as a consequence,
minority stockholders will not be able to elect directors on the basis of their votes alone. As of June 30, 2023, we had approximately
61 holders of record of our common stock. Because many of our shares of common stock are held by brokers and other institutions
on behalf of stockholders, this number is not indicative of the total number of stockholders represented by these stockholders
of record.
Subject
to preferences that may be applicable to any then outstanding shares of preferred stock, holders of common stock are entitled
to receive ratably such dividends as may be declared by the board of directors out of funds legally available therefor. In the
event of a liquidation, dissolution or winding up of us, holders of the common stock are entitled to share ratably in all assets
remaining after payment of all of our debts and liabilities and the liquidation preferences of any then outstanding shares of
preferred stock. Holders of common stock have no preemptive rights and no right to convert their common stock into any other securities.
There are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of holders
of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any of our outstanding
preferred stock.
Listing
Our
common stock is currently listed under the symbol “ADMP” on the Nasdaq Capital Market.
Transfer
Agent and Registrar; Warrant Agent
The transfer agent and registrar for our common stock is Equiniti Trust
Company, LLC, which will also act as warrant agent for the common warrants.
Dividends
We
have not declared any cash dividends on our common stock and we do not anticipate paying any cash dividends on our common stock
in the foreseeable future.
Common
Warrants
The
following summary of certain terms and provisions of the common warrants included in the units and pre-funded units that are being
offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the common warrant, the
form of which will be filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors
should carefully review the terms and provisions of the form of common warrant for a complete description of the terms and conditions
of the common warrants.
Duration,
Exercise Price and Form
Each
common warrant included in the units and pre-funded units will have an exercise price equal to $ per share. The common warrants
will be immediately exercisable and may be exercised until the five-year anniversary of the original issuance date. The exercise
price and number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the event of stock
dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The common warrants
will be issued separately from the common stock or the pre-funded warrants, as the case may be, and may be transferred separately
immediately thereafter. The common warrants will be issued in electronic form.
Exercisability
The
common warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise. A holder (together
with its affiliates) may not exercise any portion of such holder’s warrants to the extent that the holder would own more
than 4.99% of the outstanding common stock (or at the election of a holder prior to the date of issuance, 9.99%) immediately after
exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership
of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the
common warrants. In certain circumstances, as more particularly set forth in the common
warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares
of common stock determined according to a formula set forth in the common warrants.
Fundamental
Transactions
In
the event of a fundamental transaction, as described in the common warrants and generally including any reorganization, recapitalization
or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties
or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common
stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock,
the holders of the common warrants will be entitled to receive upon exercise of the common warrants the kind and amount of securities,
cash or other property that the holders would have received had they exercised the common warrants immediately prior to such fundamental
transaction, and the successor entity will succeed to, and be substituted for us, and may exercise every right and power that
we may exercise and will assume all of our obligations under the common warrants with the same effect as if such successor entity
had been named in the common warrant itself. If holders of our common stock are given a choice as to the securities, cash or property
to be received in a fundamental transaction, then the holder shall be given the same choice as to the consideration it receives
upon any exercise of the common warrant following such fundamental transaction. In addition, in the event of a fundamental transaction,
we or any successor entity will be required to purchase at a holder’s option, exercisable at any time concurrently with
or within 30 days after the consummation of the fundamental transaction (or, if later, the date of the public announcement of
the applicable fundamental transaction), such holder’s common warrants for cash in an amount equal to the value of the remaining
unexercised portion of such holder’s common warrants, determined in accordance with the Black Scholes option pricing model
as more particularly set forth in the common warrants.
Warrant Agent; Global Certificate
The common warrants will be issued in registered form under a warrant agency agreement between our transfer agent or other warrant agent and us. The common warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Transferability
Subject
to applicable laws, a common warrant may be transferred at the option of the holder upon surrender of the common warrant to us
together with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the common warrants. Rather, the number of shares of common
stock to be issued will be rounded down to the nearest whole number.
Trading
Market
There
is no established trading market for the common warrants, and we do not expect a market to develop. We do not intend to apply
for a listing of the common warrants on any securities exchange or other nationally recognized trading system. Without an active
trading market, the liquidity of the common warrants will be limited. The common stock issuable upon exercise of the common warrants
is currently listed on Nasdaq.
Rights
as a Stockholder
Except
as otherwise provided in the common warrants or by virtue of the holders’ ownership of shares of common stock, the holders
of the common warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights,
until such common warrant holders exercise their common warrants.
Pre-Funded
Warrants
The
following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and
is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit
to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and
provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration,
Exercise Price and Form
Each
pre-funded unit will be sold in this offering at a purchase price equal to $_____ (equal to the purchase price per unit, minus
$0.0001). Each pre-funded warrant included in the pre-funded units offered hereby will have an initial exercise price per share
equal to $0.0001. The pre-funded warrants will be immediately exercisable and will not expire prior to exercise. The exercise
price and number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the event of stock
dividends, stock splits, reorganizations or similar events affecting our common stock.
Exercisability
The
pre-funded warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed
exercise notice and, at any time a registration statement registering the issuance of the shares of common stock underlying the
pre-funded warrants under the Securities Act is effective and available for the issuance of such shares, or an exemption from
registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available
funds for the number of shares of common stock purchased upon such exercise. A holder (together with its affiliates) may not exercise
any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder,
9.99%) of the outstanding common stock immediately after exercise, except that upon notice from the holder to us, the holder may
increase or decrease the beneficial ownership limitation in the holder’s pre-funded warrants up to 9.99% of the number of
shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined
in accordance with the terms of the pre-funded warrants provided that any increase in the beneficial ownership limitation shall
not be effective until 61 days following notice to us. If, at the time of exercise there is no effective registration statement
registering, or the prospectus contained therein is not available for the issuance of, the shares of common stock underlying the
pre-funded warrants, then the pre-funded warrants may also be exercised, in whole or in part, at such time by means of a cashless
exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according
to the formula set forth in the pre-funded warrant.
Transferability
Subject
to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant
to us together with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, the number of shares of
common stock to be issued will be rounded up to the nearest whole number.
Trading
Market
There
is no established public trading market for the pre-funded warrants, and we do not expect a market to develop. In addition, we
do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading
system. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Right
as a Stockholder
Except
as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock,
the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock with respect to the
shares of common stock underlying the pre-funded warrants, including any voting rights, until they exercise their pre-funded warrants.
The pre-funded warrants will provide that holders have the right to participate in distributions or dividends paid on our common
stock.
Fundamental
Transaction
In
the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization
or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties
or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common
stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock,
the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount
of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately
prior to such fundamental transaction, and the successor entity will succeed to, and be substituted for us, and may exercise every
right and power that we may exercise and will assume all of our obligations under the pre-funded warrants with the same effect
as if such successor entity had been named in the pre-funded warrant itself. If holders of our common stock are given a choice
as to the securities, cash or property to be received in a fundamental transaction, then the holder shall be given the same choice
as to the consideration it receives upon any exercise of the pre-funded warrant following such fundamental transaction.
Amendment
and Waiver
The
pre-funded warrants may be modified or amended or the provisions thereof waived with the written consent of our company and the
respective holder.
Preferred
Stock
The
following is a summary of the rights of our preferred stock. This summary is not complete. For more detailed information, please
see our restated certificate of incorporation and amended and restated bylaws, which are filed as exhibits to the registration
statement of which this prospectus is a part.
We
are authorized to issue a total of 10,000,000 shares of preferred stock. As of June 30, 2023, there were 3,000 shares of Series
C Convertible Preferred Stock (the “Series C Preferred”) issued and outstanding and 1,941.2 shares of Series E Convertible
Preferred Stock (the “Series E Preferred”) issued and outstanding.
Preferred
stock may be issued from time to time, in one or more series, as authorized by the board of directors, without stockholder approval.
The prospectus relating to the preferred shares offered thereby will include specific terms of any preferred shares offered, including,
if applicable:
| ● | the
title of the shares of preferred stock; |
| ● | the
number of shares of preferred stock offered, the liquidation preference per share and
the offering price of the shares of preferred stock; |
| ● | the
dividend rate(s), period(s) and/or payment date(s) or method(s) of calculation thereof
applicable to the shares of preferred stock; |
| ● | whether
the shares of preferred stock are cumulative or not and, if cumulative, the date from
which dividends on the shares of preferred stock shall accumulate; |
| ● | the
procedures for any auction and remarketing, if any, for the shares of preferred stock; |
| ● | the
provision for a sinking fund, if any, for the shares of preferred stock; |
| ● | the
provision for redemption or repurchase, if applicable, and any restrictions on our ability
to exercise those redemption and repurchase rights of the shares of preferred stock; |
| ● | any
listing of the shares of preferred stock on any securities exchange; |
| ● | the
terms and conditions, if applicable, upon which the shares of preferred stock will be
convertible into shares of common stock, including the conversion price (or manner of
calculation thereof); |
| ● | discussion
of federal income tax considerations applicable to the shares of preferred stock; |
| ● | the
relative ranking and preferences of the shares of preferred stock as to dividend rights
and rights upon liquidation, dissolution or winding up of our affairs; |
| ● | any
limitations on issuance of any series or class of shares of preferred stock ranking senior
to or on a parity with such series or class of shares of preferred stock as to dividend
rights and rights upon liquidation, dissolution or winding up of our affairs; |
| ● | any
other specific terms, preferences, rights, limitations or restrictions of the shares
of preferred stock; and |
| ● | any
voting rights of such preferred stock. |
Series
C Convertible Preferred Stock
The
preferences and rights of the Series C Preferred are as set forth in a Certificate of Designation of Preferences, Rights and Limitations
of Series C Convertible Preferred Stock (the “Series C Certificate of Designation”) filed as Exhibit 3.1 to our Current
Report on Form 8-K, filed with the SEC on July 6, 2022. The following is a summary of the material terms of our Series C Preferred
and is qualified in its entirety by the Series C Certificate of Designation. Please refer to the Series C Certificate of Designation
for more information on the preferences, rights and limitations of Series C Preferred.
Dividends.
Except for stock dividends or distributions for which adjustments are made pursuant to the Series C Certificate of Designation,
the holders of Series C Preferred will be entitled to dividends, on an as-if converted basis, equal to and in the same form as
dividends actually paid on shares of common stock, when, as and if actually paid on shares of common stock.
Voting
Rights. Except as otherwise provided in the Series C Certificate of Designation or as otherwise required by law, the Series
C Preferred will have no voting rights (other than the right to vote as a class on certain matters as provided in the Series C
Certificate of Designation). However, each share of Series C Preferred entitles the holder thereof (i) to vote exclusively on
a proposal (the “Proposal”) submitted by the board of directors of the Company to the stockholders to adopt and approve
an amendment to the Company’s restated certificate of incorporation (the “Certificate of Incorporation”) to
effect a reverse stock split of the outstanding shares of common stock at the ratio set forth in the Proposal that is to be effected
by the filing and effectiveness of a certificate of amendment to the Certificate of Incorporation with the Secretary of State
of the State of Delaware (the “Reverse Stock Split”), and any proposal to adjourn any meeting of stockholders called
for the purpose of voting on the Proposal, and (ii) to 1,000,000 votes per each share of Series C Preferred with respect only
to the foregoing matters. The Series C Preferred shall, except as required by law, vote together with the common stock and any
other issued and outstanding shares of preferred stock of the Company entitled to vote, as a single class; provided, however,
that such shares of Series C Preferred shall, to the extent cast, be automatically and without further action of the holders thereof
voted in the same proportion as shares of common stock (excluding any shares of common stock that are not voted) and any other
issued and outstanding shares of preferred stock of the Company entitled to vote (other than the Series C Preferred or shares
of such preferred stock not voted) are voted on the Proposal and any proposal to adjourn any meeting of stockholders called for
the purpose of voting on the Proposal.
Liquidation,
Dissolution or Winding Up. The Series C Preferred has a “Stated Value” of $100 per share of Series C Preferred:
(i) Upon any liquidation, dissolution or winding up of the Company (a “Liquidation”), the holders of Series C Preferred
are entitled to be paid in cash an amount per share of Series C Preferred equal to 110% of the Stated Value (the “Liquidation
Amount”), or (ii) in the event of a “Deemed Liquidation Event” as defined in the Series C Certificate of Designation,
which generally includes certain merger transactions or a sale, lease or other disposition of all or substantially all of the
assets of the Company, the holders of Series C Preferred are entitled to paid out of the consideration payable to stockholders
in such Deemed Liquidation Event or out of the “Available Proceeds” (as defined in the Series C Certificate of Designation),
in each case before any payment may be made to the holders of common stock by reason of their ownership thereof, an amount per
share of Series C Preferred equal to the Liquidation Amount. Upon certain of the Deemed Liquidation Events, if the Company does
not effect a dissolution within 90 days after such event, then the holders of Series C Preferred may require the Company to redeem
the Series C Preferred for an amount equal to the Liquidation Amount.
Conversion.
Each share of Series C Preferred is convertible at the option of the holder, at any time and from time to time after the effective
date of a Reverse Stock Split, into that number of shares (the “Conversion Shares”) of common stock (subject to the
Beneficial Ownership Limitation and the Exchange Cap described below) determined by dividing the Stated Value of such share of
Series C Preferred by the Conversion Price then in effect, rounded down to the nearest whole share (with cash paid in lieu of
any fractional shares). The “Conversion Price” for the Series C Preferred equals 90% of the lesser of (i) the closing
sale price of the common stock on the trading day immediately prior to the Closing Date, and (ii) the average of the closing sale
prices for the common stock on the five trading days immediately prior to the Closing Date, subject to adjustment as provided
in the Series C Certificate of Designation; provided, that the Conversion Price may not fall below the par value per share of
the common stock and may not exceed $42.00 per share. Based on the initial Conversion Price of $30.10 per share, the 3,000 Shares
of Series C Preferred are initially convertible into approximately 9,967 shares of common stock. The Conversion Price is subject
to adjustment as set forth in the Series C Certificate of Designation for stock dividends, stock splits, reverse stock splits,
and similar events. Upon conversion, the shares of Series C Preferred shall resume the status of authorized but unissued shares
of preferred stock of the Company.
Beneficial
Ownership Limitation. The Series C Preferred cannot be converted to common stock if the holder and its affiliates would beneficially
own more than 4.99% of the outstanding common stock (the “Beneficial Ownership Limitation”). However, any holder may
increase or decrease such percentage to any other percentage not in excess of 9.99% upon notice to us, provided that any increase
in this limitation will not be effective until 61 days after such notice from the holder to us and such increase or decrease will
apply only to the holder providing such notice.
Nasdaq
Issuance Limitation. The Company will not be obligated to issue any shares of common stock, and the holders of Series C Preferred
do not have the right to receive, upon conversion, exercise or redemption of the Series C Preferred and the warrants initially
issued to the holder (the “Purchaser”) of the Series C Preferred (the “Warrants”), taken as a whole, any
shares of common stock to the extent such issuance of shares of common stock would exceed that number of shares of common stock
which the Company may issue in the aggregate pursuant to the transactions contemplated under the Securities Purchase Agreement
entered into between the Company and the Purchaser (including pursuant to the Series C Certificate of Designation and the Warrants)
without breaching the Company’s obligations under the rules and regulations of the Nasdaq Capital Markets (the “Exchange
Cap”). In addition, no holder of Series C Preferred shall be issued, in the aggregate pursuant to the terms of the Series
C Certificate of Designation and the Warrants, shares of common stock in an amount greater than the product of the Exchange Cap
multiplied by a fraction, the numerator of which is the original Stated Value of such holder’s Series C Preferred and the
denominator of which is the aggregate Stated Value of all Series C Preferred issued on the Closing Date to all holders (with respect
to each holder, the “Exchange Cap Allocation”). In the event that the holder sells or otherwise transfers any of the
holder’s Series C Preferred, the transferee shall be allocated a pro rata portion of the holder’s Exchange Cap Allocation,
and the restrictions of the prior sentence shall apply to such transferee with respect to the portion of the Exchange Cap Allocation
allocated to such transferee. If any holder of Series C Preferred converts all of such holder’s Series C Preferred into
a number of shares of common stock which, in the aggregate, is less than such holder’s Exchange Cap Allocation, then the
difference between such holder’s Exchange Cap Allocation and the number of shares of common stock actually issued to such
holder will be allocated to the respective Exchange Cap Allocations of the remaining holders of Series C Preferred on a pro rata
basis in proportion to the shares of Series C Preferred then held by each such holder.
Redemption.
Subject to the Purchaser’s right to elect to convert all or a portion of the Series C Preferred at any time following the
effective date of the Reverse Stock Split, the Company may, with the prior notice to the holders of the Series C Preferred specified
in the Series C Certificate of Designation, redeem all or a portion of the Series C Preferred held by such holders at any time
at 105% of the Stated Value, provided, however, that a Company redemption request shall not be effective if received by a holder
of Series C Preferred before the date of the Reverse Stock Split. Each holder of Series C Preferred will have the right, with
the prior notice to the Company as specified in the Series C Certificate of Designation, to require the Company to redeem all
or a portion of the Series C Preferred held by such holder at any time at 110% of the Stated Value, provided, however, that a
holder’s request will not be effective if received by the Company less than five days after the date of a Reverse Stock
Split.
Preemptive
Rights. No holders of Series C Preferred will, as holders of Series C Preferred, have any preemptive rights to purchase or
subscribe for the common stock or any of our other securities.
Consent
Rights. In addition to the voting rights of the Series C Preferred described above, as long as any shares of Series C Preferred
are outstanding, the Company shall not, without the affirmative vote of the holders of at least a majority on voting power of
the outstanding shares of Series C Preferred: (a) alter or change adversely the powers, preferences or rights given to the Series
C Preferred or alter or amend the Series C Certificate of Designation, (b) increase the number of authorized shares of Series
C Preferred, or (c) enter into any agreement with respect to any of the foregoing.
Failure
to Deliver Conversion Shares. If the Company fails to timely deliver shares of common stock upon conversion of shares of Series
C Preferred within the time period specified in the Series C Certificate of Designation, then the holder is entitled to elect,
by notice to the Company at any time on or before its receipt of such Conversion Shares, to rescind such conversion, and the holder
shall return to the Company any Conversion Shares issued to the holder pursuant to the rescinded notice and the Company shall,
at its own expense, deliver (or cause its transfer agent to deliver) to the converting holder a new book-entry statement, registered
in the name of the holder or its designee, evidencing the number of shares of Series C Preferred owned by the holder immediately
prior to the conversion.
Compensation
for Buy-In on Failure to Timely Deliver Shares. If the Company fails to timely deliver the Conversion Shares to the holder,
and if after the required delivery date the holder is required by its broker to purchase (in an open market transaction or otherwise)
or the holder or its brokerage firm otherwise purchases, shares of common stock to deliver in satisfaction of a sale by the holder
of the Conversion Shares which the holder was entitled to receive upon such conversion, then the Company is obligated to (A) pay
in cash to the holder the amount, if any, by which (x) the holder’s total purchase price (including brokerage commissions,
if any) for the shares of common stock so purchased, exceeds (y) the amount obtained by multiplying (1) the number of Conversion
Shares that the Company was required to deliver multiplied by (2) the price at which the sell order giving rise to such purchase
obligation was executed, and (B) at the option of the holder, either reissue (if surrendered) the shares of Series C Preferred
equal to the number of shares submitted for conversion (in which case such conversion shall be deemed rescinded) or deliver to
the holder the number of shares of common stock that would have been issued had the Company timely complied with its exercise
and delivery obligations.
Series
E Convertible Preferred Stock
As
of the date of this prospectus, there are 1,941.2 shares of Series E Convertible Preferred Stock, or Series E Preferred, outstanding.
The preferences and rights of the Series E Preferred are set forth in a Certificate of Designation of Preferences, Rights and
Limitations of Series E Convertible Preferred Stock (the “Series E Certificate of Designation”), substantially in
the form filed as Exhibit 3.1 to our Current Report on Form 8-K, filed with the SEC on May 26, 2023. The following is a summary
of the material terms of our Series E Preferred and is qualified in its entirety by the Series E Certificate of Designation. Please
refer to the Series E Certificate of Designation for more information on the preferences, rights and limitations of Series E Preferred.
Dividends.
Except for stock dividends or distributions for which adjustments are made pursuant to the Series E Certificate of Designation,
the holders of Series E Preferred will be entitled to dividends, on an as-if converted basis, equal to and in the same form as
dividends actually paid on shares of common stock, when, as and if actually paid on shares of common stock.
Voting
Rights. Except as otherwise provided in the Series E Certificate of Designation or as otherwise required by law, holders of
Series E Preferred are entitled to vote with the holders of outstanding shares of common stock, voting together as a single class,
with respect to all matters presented to the stockholders of the Company. Each such holder is entitled to a number of votes equal
to the number of shares of common stock into which the Series E Preferred Stock held by such holder is convertible pursuant to
the Series E Certificate of Designation (subject to, and after giving effect to and taking into account, the Beneficial Ownership
Limitation described below and set forth in the Series E Certificate of Designation) as of the record date for such vote.
Liquidation,
Dissolution or Winding Up. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary
(a “Liquidation”), subject to the rights of the holders of any other outstanding series of preferred stock, the holders
of Series E Preferred are entitled to receive, pari passu with the holders of common stock, out of the assets of the Company an
amount equal to such amount per share as would have been payable had all shares of Series E Preferred been converted into common
stock pursuant to the Series E Certificate of Designation (without giving effect to any limitation on conversion as a result of
the Beneficial Ownership Limitation) immediately prior to such Liquidation.
Conversion.
Each share of Series E Preferred (or fraction thereof) is convertible, at any time and from time to time at the option of the
holder thereof, into the number of shares of common stock (subject to the Beneficial Ownership Limitation) at a conversion ratio
(the “Conversion Ratio”) of 1,000 shares of common stock per one whole share of Series E Preferred (and giving effect
proportionately to any conversion of a fraction of a share of Series E Preferred) (subject to adjustment). If the Company fails
to timely deliver shares of common stock upon conversion of shares of Series E Preferred within the time period specified in the
Series E Certificate of Designation, then the holder is entitled to elect, by notice to the Company at any time on or before its
receipt of such Conversion Shares, to rescind such conversion, and the holder shall return to the Company any Conversion Shares
issued to the holder pursuant to the rescinded notice. The Conversion Rate and the number of shares of common stock into which
a share of Series E Preferred is convertible is subject to proportionate adjustments in the event of stock dividends or distributions
payable in shares of common stock, stock splits or reverse stock splits, or reclassifications.
Beneficial
Ownership Limitation. Under the Series E Certificate of Designation, Adamis shall not effect any conversion of the Series
E Preferred, and a holder of Series E Preferred does not have the right to convert any portion of the Series E Preferred, to the
extent that, after giving effect to a requested conversion, such holder would beneficially own in excess of the Holder Beneficial
Ownership Limitation, or such Holder together with such Holder’s affiliates and any persons acting as a group together with
such holder or affiliates (such persons, “Attribution Parties”) would beneficially own in excess of the Affiliates
Beneficial Ownership Limitation (as defined below). For purposes of such determination, the number of shares of common stock beneficially
owned by such holder and its affiliates includes the number of shares of common stock issuable upon conversion of the Series E
Preferred with respect to which such determination is being made, but excludes the number of shares of common stock which are
issuable upon (i) conversion of the remaining, unconverted Series E Preferred beneficially owned by such holder or any of its
affiliates or Attribution Parties, and (ii) exercise or conversion of the unexercised or unconverted portion of any other securities
of Adamis subject to a limitation on conversion or exercise analogous to the limitation contained in the Series E Certificate
of Designation beneficially owned by such holder or any of its affiliates or Attribution Parties. Except as set forth in the preceding
sentence, beneficial ownership is determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations
promulgated thereunder. The “Holder Beneficial Ownership Limitation” is 9.99% of the number of shares of the common
stock outstanding immediately after giving effect to the issuance of shares of common stock issuable upon conversion of Series
E Preferred held by the applicable holder. The “Affiliates Beneficial Ownership Limitation” is 9.99% of the number
of shares of common stock outstanding immediately after giving effect to the issuance of shares of common stock issuable upon
conversion of Series E Preferred held by the applicable holder and its affiliates. The Holder Beneficial Ownership Limitation
together with the Affiliates Beneficial Ownership Limitation are sometimes referred to collectively as the “Beneficial Ownership
Limitation.”
Nasdaq
Issuance Limitation. The Company will not be obligated to issue any shares of common stock, and the holders of Series E Preferred
do not have the right to receive, upon conversion of the Series E Preferred, any shares of common stock to the extent such issuance
of shares of common stock would exceed that number of shares of common stock which the Company may issue in the aggregate pursuant
to the transactions contemplated under the Merger Agreement (including pursuant to the Certificate of Designation) without breaching
the Company’s obligations under the rules and regulations of the Nasdaq Capital Markets (the “Exchange Cap”).
In addition, no holder of Series E Preferred shall be issued, in the aggregate pursuant to the terms of the Series E Certificate
of Designation, shares of common stock in an amount greater than the product of the Exchange Cap multiplied by a fraction, the
numerator of which is the number of shares of Series E Preferred held by the holder and the denominator of which is the aggregate
number of shares of Series E Preferred originally issued to all holders in connection with the closing of the Merger (with respect
to each holder, the “Exchange Cap Allocation”). In the event that the holder sells or otherwise transfers any of the
holder’s Series E Preferred, the transferee shall be allocated a pro rata portion of the holder’s Exchange Cap Allocation,
and the restrictions of the prior sentence shall apply to such transferee with respect to the portion of the Exchange Cap Allocation
allocated to such transferee. If any holder of Series E Preferred converts all of such holder’s Series E Preferred into
a number of shares of common stock which, in the aggregate, is less than such holder’s Exchange Cap Allocation, then the
difference between such holder’s Exchange Cap Allocation and the number of shares of common stock actually issued to such
holder will be allocated to the respective Exchange Cap Allocations of the remaining holders of Series E Preferred on a pro rata
basis in proportion to the shares of Series E Preferred then held by each such holder.
Preemptive
Rights. No holders of Series E Preferred will, as holders of Series E Preferred, have any preemptive rights to purchase or
subscribe for the common stock or any of our other securities.
Consent
Rights. In addition to the voting rights of the Series E Preferred described above, as long as any shares of Series E Preferred
are outstanding, the Company shall not, without the affirmative vote of holders of a majority of the outstanding shares of Series
E Preferred, directly or indirectly, by merger, consolidation, recapitalization or otherwise, (a) alter or change adversely the
powers, preferences or rights given to the Series E Preferred or alter or amend the Series E Certificate of Designation or (b)
increase the number of authorized shares of Series E Preferred, or (c) enter into any agreement with respect to any of the foregoing.
Subsequent
Rights Offerings; Pro Rata Distributions. If the Company grants, issues or sells any common stock equivalents pro rata to
all the record holders of any class of shares of common stock (the “Purchase Rights”), then a holder of Series E Preferred
will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the holder
could have acquired if the holder had held the number of shares of common stock acquirable upon conversion of the Series E Preferred
(without regard to any limitations on conversion, including without limitation, the Beneficial Ownership Limitation) immediately
before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken,
the date as of which the record holders of shares of common stock are to be determined for the grant, issue or sale of such Purchase
Rights (provided, however, to the extent that the holder’s right to participate in any such Purchase Right would result
in the holder exceeding the Beneficial Ownership Limitation, then the holder shall not be entitled to participate in such Purchase
Right to such extent (or beneficial ownership of such shares of common stock as a result of such Purchase Right to such extent)
and such Purchase Right to such extent shall be held in abeyance for the holder until such time, if ever, as its right thereto
would not result in the holder exceeding the Beneficial Ownership Limitation). In addition, as long as the Series E Preferred
Stock is outstanding, if the Company declares or makes any dividend or other distribution of its assets (or rights to acquire
its assets) to all holders of shares of common stock (including, without limitation, any distribution of cash, stock or other
securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement
or other similar transaction) (a “Distribution”), at any time after the issuance of the Series E Preferred, then,
in each such case, the holder of Series E Preferred shall be entitled to participate in such Distribution to the same extent that
the holder would have participated therein if the holder had held the number of shares of common stock acquirable upon complete
conversion of the Series E Preferred (without regard to any limitations on conversion including without limitation, the Beneficial
Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken,
the date as of which the record holders of shares of common stock are to be determined for the participation in such Distribution
(provided, however, to the extent that the holder’s right to participate in any such Distribution would result in the holder
exceeding the Beneficial Ownership Limitation, then the holder shall not be entitled to participate in such Distribution to such
extent (or in the beneficial ownership of any shares of common stock as a result of such Distribution to such extent) and the
portion of such Distribution shall be held in abeyance for the benefit of the holder until such time, if ever, as its right thereto
would not result in the holder exceeding the Beneficial Ownership Limitation).
Merger;
Sale of Assets. If at any time while the Series E Preferred Stock is outstanding: (i) the Company effects any merger or consolidation
of the Company with or into another person pursuant to which the shares of capital stock of the Company outstanding immediately
prior to such merger or consolidation are converted into or exchanged for shares of another corporation or entity, or are converted
into or exchanged for equity securities that represent, less than a majority, by voting power, of the equity securities of (1)
the surviving or resulting party or (2) if the surviving or resulting party is a wholly owned subsidiary of another party immediately
following such merger or consolidation, the parent of such surviving or resulting party, immediately following such merger or
consolidation; or (ii) the Company sells all or substantially all of its assets in a single transaction or a series of related
transactions (each, a “Merger or Sale”), then each holder of the Series E Preferred Stock shall be entitled to receive
such number of shares of common stock of the successor or acquiring corporation and/or such other or additional consideration
as are receivable by virtue of such Merger or Sale by a holder of the number of shares of common stock for which the Series E
Preferred Stock held by the holder is convertible immediately prior to such Merger or Sale (without regard to the Beneficial Ownership
Limitation).
Possible
Anti-Takeover Effects of Delaware Law and our Charter Documents
Provisions
of the Delaware General Corporation Law, or DGCL, our restated certificate of incorporation, and our amended and restated bylaws,
could make it more difficult to acquire us by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers
and directors. These provisions, summarized below, are expected to discourage certain types of coercive takeover practices and
takeover bids that our board of directors may consider inadequate and to encourage persons seeking to acquire control of us to
first negotiate with our board of directors. We believe that the benefits of increased protection of our ability to negotiate
with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging
takeover or acquisition proposals because, among other things, negotiation of these proposals could result in an improvement of
their terms.
Delaware
Anti-Takeover Statute
We
are subject to Section 203 of the DGCL. This provision generally prohibits a Delaware corporation from engaging in any business
combination with any interested stockholder for a period of three years following the date the stockholder became an interested
stockholder, unless:
| ● | prior
to such date, the board of directors approved either the business combination or the
transaction that resulted in the stockholder becoming an interested stockholder; |
| ● | upon
consummation of the transaction that resulted in the stockholder becoming an interested
stockholder, the interested stockholder owned at least 85% of the voting stock of the
corporation outstanding at the time the transaction commenced, excluding for purposes
of determining the number of shares outstanding those shares owned by persons who are
directors and also officers and by employee stock plans in which employee participants
do not have the right to determine confidentially whether shares held subject to the
plan will be tendered in a tender or exchange offer; or |
| ● | on
or subsequent to such date, the business combination is approved by the board of directors
and authorized at an annual meeting or Special Meeting of stockholders and not by written
consent, by the affirmative vote of at least 66-2/3% of the outstanding voting stock
that is not owned by the interested stockholder. |
Section
203 defines a business combination to include:
| ● | any
merger or consolidation involving the corporation and the interested stockholder; |
| ● | any
sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation
involving the interested stockholder; |
| ● | subject
to certain exceptions, any transaction that results in the issuance or transfer by the
corporation of any stock of the corporation to the interested stockholder; |
| ● | any
transaction involving the corporation that has the effect of increasing the proportionate
share of the stock of any class or series of the corporation beneficially owned by the
interested stockholder; or |
| ● | the
receipt by the interested stockholder of the direct or indirect benefit of any loans,
advances, guarantees, pledges or other financial benefits provided by or through the
corporation. |
In
general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of
the outstanding voting stock of a corporation, or an affiliate or associate of the corporation and was the owner of 15% or more
of the outstanding voting stock of a corporation at any time within three years prior to the time of determination of interested
stockholder status; and any entity or person affiliated with or directly or indirectly controlling or controlled by such entity
or person, who presently holds the power to direct management or is in a director or officer of the corporation.
These
statutory provisions could delay or frustrate the removal of incumbent directors or a change in control of our company, and accordingly,
may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their
stock at a price above the prevailing market price.
Restated
Certificate of Incorporation and Bylaw Provisions
Our
restated certificate of incorporation, as amended, and bylaws contain provisions that could have the effect of discouraging potential
acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder
might consider favorable. In particular, the restated certificate of incorporation and bylaws, as applicable, among other things:
| ● | permit
the Board to issue up to 10,000,000 shares of preferred stock, without further action
by the stockholders, with any rights, preferences and privileges as they may designate; |
| ● | provide
that all vacancies on the Board, including newly created directorships, may, except as
otherwise required by law, or as determined otherwise by resolution of the Board, be
filled by the affirmative vote of a majority of directors then in office, even if less
than a quorum; |
| ● | do
not provide for cumulative voting rights with respect to election of directors; |
| ● | provide
that no action shall be taken by the stockholders, except at an annual or special meeting
of stockholders, and no action shall be taken by the stockholders by written consent
or by electronic transmission; |
| ● | set
forth an advance notice procedure with regard to the nomination, other than by or at
the direction of the Board, of candidates for election as directors and with regard to
business to be brought before a meeting of stockholders. Although the bylaws do not give
the Board the power to approve or disapprove of stockholder nominations of candidates
or proposals regarding other proper business to be conducted at a special or annual meeting,
the bylaws may have the effect of precluding the conduct of certain business at a meeting
if the proper procedures are not followed or may discourage or deter a potential acquirer
from conducting a solicitation of proxies to elect its own slate of directors or otherwise
attempting to obtain control of the Company; and |
| ● | provide
the Board with the ability to alter its bylaws without stockholder approval. |
Such
provisions may make it more difficult for holders of our common stock to replace our board of directors and may have the effect
of discouraging a third-party from making tender offers for our shares or acquiring us, even if doing so would be beneficial to
our stockholders. These provisions also may have the effect of preventing changes in our management.
Choice
of Forum. Our bylaws provide that unless the corporation consents in writing to the selection of an alternative forum, the
Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for
(i) any derivative action or proceeding brought on behalf of the Company; (ii) any action asserting a claim of breach of a fiduciary
duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders; (iii)
any action asserting a claim against the Company or any director or officer or other employee of the Company arising pursuant
to any provision of the DGCL, the certificate of incorporation or the bylaws of the Company, or as to which the DGCL confers jurisdiction
on the Court of Chancery of the State of Delaware; or (iv) any action asserting a claim against the Company or any director or
officer or other employee of the Company governed by the internal affairs doctrine, in all cases subject to the court’s
having personal jurisdiction over the indispensable parties named as defendants (including without limitation as a result of the
consent of such indispensable parties to the personal jurisdiction of such court). The bylaws further provide that if any action
the subject matter of which is within the scope of the preceding sentence is filed in a court other than a court located within
the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have
consented to (i) the personal jurisdiction of the state and federal courts located within the State of Delaware in connection
with any action brought in any such court to enforce the preceding sentence; and (ii) having service of process made upon such
stockholder in any such action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
The bylaws provide that the above provisions do not apply to suits brought to enforce a duty or liability created by the Securities
Act of 1933, as amended (the “Securities Act”), the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), or any other claim for which the federal courts have exclusive jurisdiction. Section 27 of the Exchange Act creates
exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules
and regulations thereunder. As a result, the exclusive forum provision will not apply to suits brought to enforce any duty or
liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our bylaws
do not relieve us of our duties to comply with federal securities laws and the rules and regulations thereunder, and our stockholders
will not be deemed to have waived our compliance with these laws, rules and regulations. The bylaws also provide that unless the
Company consents in writing to the selection of an alternative forum, the federal district courts of the United States of America
shall, to the fullest extent permitted by law, be the sole and exclusive forum for the resolution of any complaint asserting a
cause of action arising under the Securities Act, and that any person or entity purchasing or otherwise acquiring or holding any
interest in shares of capital stock of the Company shall be deemed to have notice of and consented to the provisions described
above.
Under
the Securities Act, federal and state courts have concurrent jurisdiction over all suits brought to enforce any duty or liability
created by the Securities Act. There is uncertainty as to whether a court (other than state courts in the State of Delaware, where
the Supreme Court of the State of Delaware decided in March 2020 that exclusive forum provisions for causes of action arising
under the Securities Act are facially valid under Delaware law) would enforce forum selection provisions and whether investors
can waive compliance with the federal securities laws and the rules and regulations thereunder. The forum selection provisions
in the bylaws may have the effect of discouraging lawsuits against us and/or our directors, officers and employees as it may limit
any stockholder’s ability to bring a claim in a judicial forum that such stockholder finds favorable for disputes with us
or our directors, officers or employees. In addition, stockholders who do bring a claim in the Court of Chancery in the State
of Delaware could face additional litigation costs in pursuing any such claim, particularly if they do not reside in or near Delaware.
The enforceability of similar choice of forum provisions in other companies’ charter documents has been challenged in legal
proceedings, and it is possible that, in connection with any applicable action brought against us, a future court could find the
choice of forum provisions contained in our bylaws to be inapplicable or unenforceable in such action. If a court were to find
the choice of forum provision contained in our bylaws to be inapplicable or unenforceable in an action, we may incur additional
costs associated with resolving such action in other jurisdictions, which could adversely affect our business, financial condition
or results of operations.
PLAN
OF DISTRIBUTION
We are offering on a reasonable best efforts
basis up to 5,263,158 units, based on an assumed public offering price of $1.90 per unit, which represents the closing price of our common
stock on the Nasdaq Capital Marked on July 21, 2023, for gross proceeds of up to approximately $10.0 million before deduction of placement
agent commissions and offering expenses, in a reasonable best efforts offering. There is no minimum amount of proceeds that is a condition
to closing of this offering. The actual amount of gross proceeds, if any, in this offering could vary substantially from the gross proceeds
from the sale of the maximum amount of securities being offered in this prospectus.
Pursuant
to a placement agency agreement, dated as of , 2023, we have engaged Maxim Group LLC to act as our exclusive placement agent (the
“Placement Agent”) to solicit offers to purchase the securities offered by this prospectus. The Placement Agent is
not purchasing or selling any securities, nor is it required to arrange for the purchase and sale of any specific number or dollar
amount of securities, other than to use its “reasonable best efforts” to arrange for the sale of the securities by
us. Therefore, we may not sell the entire amount of securities being offered. Investors purchasing securities offered hereby will
have the option to execute a securities purchase agreement with us. In addition to the rights and remedies available to all investors
in this offering under federal and state securities laws, the investors which enter into a securities purchase agreement will
also be able to bring claims of breach of contract against us. Investors who do not enter into a securities purchase agreement
shall rely solely on this prospectus in connection with the purchase of our securities in this offering. The Placement Agent may
engage one or more subagents or selected dealers in connection with this offering.
The
placement agency agreement provides that the Placement Agent’s obligations are subject to conditions contained in the placement
agency agreement.
We expect this offering to be completed not later than two business days
following the commencement of sales in this offering (the effective date of the registration statement of which this prospectus forms
a part) and we will deliver all securities to be issued in connection with this offering delivery versus payment/receipt versus payment
upon receipt of investor funds received by us. Accordingly, neither we nor the placement agent have made any arrangements to place investor
funds in an escrow account or trust account since the placement agent will not receive investor funds in connection with the sale of the
securities offered hereunder.
Placement
Agent Fees, Commissions and Expenses
Upon
the closing of this offering, we will pay the Placement Agent a cash transaction fee equal to 7.0% of the aggregate gross cash
proceeds to us from the sale of the securities in the offering. In addition, we will reimburse the Placement Agent for certain
of its out-of-pocket expenses incurred in connection with this offering, including up to $85,000 for the Placement Agent’s
legal fees and up to $15,000 for actual travel and reasonable out-of-pocket expenses. If this offering is not completed, we have
agreed to reimburse the Placement Agent for its actual expenses in an amount not to exceed $50,000.
The
following table shows the public offering price, placement agent fees and proceeds, before expenses, to us, assuming the sale
of all units in this offering and no sale of any pre-funded units in this offering.
| | |
Per Unit | | |
Total | |
| Public offering price | |
$ | | | |
$ | | |
| Placement agent fees (7.0%) | |
$ | | | |
$ | | |
| Proceeds to us (before expenses) | |
$ | | | |
$ | | |
We estimate that the total expenses of
the offering, including registration and filing fees, printing fees and legal and accounting expenses, but excluding the Placement
Agent commission, will be approximately $450,000, all of which are payable by us. This figure includes, among other things, the
Placement Agent’s fees and expenses that we have agreed to reimburse.
Lock-Up
Agreements
We,
each of our officers and directors and executive officers have agreed, subject to certain exceptions, not to offer, issue, sell,
contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities
convertible into or exercisable or exchangeable for our common stock for a period of 90 days after this offering is completed
without the prior written consent of the Placement Agent, subject to certain exceptions.
The
Placement Agent may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up
agreements prior to the expiration of the lock-up period. When determining whether or not to release shares from the lock-up agreements,
the Placement Agent will consider, among other factors, the security holder’s reasons for requesting the release, the number
of shares for which the release is being requested and market conditions at the time.
In
addition, we have also agreed with the purchasers of units and pre-funded units that until the six-month anniversary of the closing
date of this offering, we will not effect or enter into an agreement to effect a “Variable Rate Transaction” as defined
in the securities purchase agreement to be entered into with each purchaser.
Indemnification
We
have agreed to indemnify the Placement Agent against certain liabilities, including liabilities under the Securities Act, and
to contribute to payments that the Placement Agent may be required to make for these liabilities.
Regulation
M
The
Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to
be underwriting discounts or commissions under the Securities Act. As an underwriter, the Placement Agent would be required to
comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation
M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the Placement
Agent acting as principal. Under these rules and regulations, the Placement Agent (i) may not engage in any stabilization activity
in connection with our securities and (ii) may not bid for or purchase any of our securities or attempt to induce any person to
purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the
distribution.
Determination
of Offering Price and Warrant Exercise Price
The
actual offering price of the units and pre-funded units we are offering, and the exercise price of the common warrants included
in the units and pre-funded units that we are offering, were negotiated between us, the placement agent and the investors in the
offering based on the trading of our shares of common stock prior to the offering, among other things. Other factors considered
in determining the public offering price of the securities we are offering, as well as the exercise price of the common warrants
that we are offering, include our history and prospects, the stage of development of our business, our business plans for the
future and the extent to which they have been implemented, an assessment of our management, the general conditions of the securities
markets at the time of the offering and such other factors as were deemed relevant.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the Placement Agent or an affiliate. Other than
this prospectus, the information on the Placement Agent’s website and any information contained in any other website maintained
by the Placement Agent is not part of this prospectus or the registration statement of which this prospectus form a part, has
not been approved and/or endorsed by us or the Placement Agent, and should not be relied upon by investors. In connection with
the offering, the Placement Agent or selected dealers may distribute prospectuses electronically. No forms of electronic prospectus
other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
Other
than the prospectus in electronic format, the information on the Placement Agent’s website and any information contained
in any other website maintained by the Placement Agent is not part of the prospectus or the registration statement of which this
prospectus forms a part, has not been approved and/or endorsed by us or the placement agent in its capacity as placement agent
and should not be relied upon by investors.
Other
Relationships
The
placement agent and certain of its affiliates are full service financial institutions engaged in various activities, which may
include securities trading, commercial and investment banking, financial advisory, investment management, investment research,
principal investment, hedging, financing and brokerage activities. The placement agent and certain of its affiliates have, from
time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services
for us and our affiliates, for which they received or will receive customary fees and expenses. However, except as disclosed in
this prospectus, we have no present arrangements with the placement agent for any further services.
In
the ordinary course of their various business activities, the placement agent and certain of its affiliates may make or hold a
broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments
(including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities
may involve securities and/or instruments issued by us and our affiliates. If the placement agent or its affiliates have a lending
relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies.
The placement agent and its affiliates may hedge such exposure by entering into transactions that consist of either the purchase
of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially
the securities offered hereby. Any such short positions could adversely affect future trading prices of the securities offered
hereby. The placement agent and certain of its affiliates may also communicate independent investment recommendations, market
color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may
at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Listing
Our
common stock is traded on Nasdaq under the symbol “ADMP.”
Selling
Restrictions
Other
than in the United States, no action has been taken by us or the Placement Agent that would permit a public offering of the securities
offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus
may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in
connection with the offer and sale of any such securities be distributed or published, in any jurisdiction, except under circumstances
that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this
prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution
of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered
by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia.
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian
Securities and Investments Commission (ASIC), in relation to the offering.
This
prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations
Act 2001 (the Corporations Act) and does not purport to include the information required for a prospectus, product disclosure
statement or other disclosure document under the Corporations Act.
Any
offer in Australia of the securities may only be made to persons (the Exempt Investors) who are “sophisticated investors”
(within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section
708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations
Act so that it is lawful to offer the securities without disclosure to investors under Chapter 6D of the Corporations Act.
The
securities applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after
the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations
Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is
pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring securities must
observe such Australian on-sale restrictions.
This
prospectus contains general information only and does not take account of the investment objectives, financial situation or particular
needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making
an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives
and circumstances, and, if necessary, seek expert advice on those matters.
Brazil.
The offer of securities described in this prospectus will not be carried out by means that would constitute a public offering
in Brazil under Law No. 6,385, of December 7, 1976, as amended, under the CVM Rule (Instrução) No. 400, of December
29, 2003. The offer and sale of the securities have not been and will not be registered with the Comissão de Valores Móbilearios
in Brazil. The securities have not been offered or sold, and will not be offered or sold in Brazil, except in circumstances that
do not constitute a public offering or distribution under Brazilian laws and regulations.
Canada.
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited
investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario),
and are permitted clients, as defined in National Instrument 31 103 Registration Requirements, Exemptions and Ongoing Registrant
Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject
to, the prospectus requirements of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this
prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission
or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s
province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s
province or territory for particulars of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 of National Instrument 33 105 Underwriting Conflicts (NI 33 105), the Placement Agent is not required to comply
with the disclosure requirements of NI 33-105 regarding conflicts of interest in connection with this offering.
Cayman
Islands. No invitation, whether directly or indirectly, may be made to the public in the Cayman Islands to subscribe for
our securities.
European
Economic Area. In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive
(each, a “Relevant Member State”) an offer to the public of any securities may not be made in that Relevant Member
State, except that an offer to the public in that Relevant Member State of any securities may be made at any time under the following
exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| ● | to
any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| ● | to
fewer than 100 or, if the Relevant Member State has implemented the relevant provision
of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified
investors as defined in the Prospectus Directive), as permitted under the Prospectus
Directive, subject to obtaining the prior consent of the representatives for any such
offer; or |
| ● | in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided
that no such offer of securities shall result in a requirement for the publication by
us or any placement agent of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For
the purposes of this provision, the expression an “offer to the public” in relation to any securities in any Relevant
Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any
securities to be offered so as to enable an investor to decide to purchase any securities, as the same may be varied in that Member
State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive”
means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the
Relevant Member State), and includes any relevant implementing measure in the Relevant Member State, and the expression “2010
PD Amending Directive” means Directive 2010/73/EU.
Hong Kong.
The contents of this prospectus have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution
in relation to the offer. If you are in any doubt about any of the contents of this prospectus, you should obtain independent professional
advice. Please note that (i) our shares may not be offered or sold in Hong Kong, by means of this prospectus or any document other
than to “professional investors” within the meaning of Part I of Schedule 1 of the Securities and Futures Ordinance
(Cap.571, Laws of Hong Kong) (SFO) and any rules made thereunder, or in other circumstances which do not result in the document
being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong) (CO) or which do not
constitute an offer or invitation to the public for the purpose of the CO or the SFO, and (ii) no advertisement, invitation or
document relating to our shares may be issued or may be in the possession of any person for the purpose of issue (in each case
whether in Hong Kong or elsewhere) which is directed at, or the contents of which are likely to be accessed or read by, the public
in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to the shares which
are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within
the meaning of the SFO and any rules made thereunder.
Israel.
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not
been filed with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only
to, and is directed only at, and any offer of the shares is directed only at, investors listed in the first addendum, or the Addendum,
to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks,
portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities
with equity in excess of NIS 50 million and “qualified individuals”, each as defined in the Addendum (as it may
be amended from time to time), collectively referred to as qualified investors (in each case purchasing for their own account or,
where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors
will be required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same
and agree to it.
The People’s
Republic of China. This prospectus may not be circulated or distributed in the PRC and the shares may not be offered
or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the
PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this paragraph only, the PRC does
not include Taiwan and the special administrative regions of Hong Kong and Macau.
Switzerland.
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the SIX) or on any
other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure
standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for
listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading
facility in Switzerland. Neither this document nor any other offering or marketing material relating to the securities or the offering
may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document
nor any other offering or marketing material relating to the offering, or the securities have been or will be filed with or approved
by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be
supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of securities has not been and will not be
authorized under the Swiss Federal Act on Collective Investment Schemes (CISA). Accordingly, no public distribution, offering or
advertising, as defined in CISA, its implementing ordinances and notices, and no distribution to any non-qualified investor,
as defined in CISA, its implementing ordinances and notices, shall be undertaken in or from Switzerland, and the investor protection
afforded to acquirers of interests in collective investment schemes under CISA does not extend to acquirers of securities.
Taiwan.
The securities have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant
securities laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances
which constitutes an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval
of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice
regarding or otherwise intermediate the offering and sale of the securities in Taiwan.
United Kingdom.
This prospectus has only been communicated or caused to have been communicated and will only be communicated or caused to be communicated
as an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services
and Markets Act of 2000, or the FSMA) as received in connection with the issue or sale of our common stock in circumstances in
which Section 21(1) of the FSMA does not apply to us. All applicable provisions of the FSMA will be complied with in respect
to anything done in relation to our common stock in, from or otherwise involving the United Kingdom.
SELECTED FINANCIAL
INFORMATION
At a special meeting
of stockholders of the Company held on May 15, 2023, or the Special Meeting, our stockholders approved an amendment, or the Amendment,
to our restated certificate of incorporation, or the Restated Certificate, to effect a reverse stock split of our common stock,
or the Reverse Stock Split, at a ratio ranging from 1-for-2 to 1-for-100, with the final ratio to be determined by our Board.
Following the Special Meeting, our Board approved a one-for-seventy (1:70) reverse stock split of the outstanding shares of our
common stock. Subsequently, we filed the Amendment to our Restated Certificate with the Secretary of State of the State of Delaware
to effect the Reverse Stock Split, effective on May 22, 2022. The Amendment did not change the number of authorized shares of our
common stock.
Net loss per share
attributable to common stockholders, basic and diluted, has been derived from our audited financial statements contained in our
Annual Report on Form 10-K for the years ended December 31, 2022 and 2021, except that the net loss per share attributable
to common stockholders, basic and diluted, have been revised to reflect the new shares issued based on the reverse stock split
ratio discussed above, as shown below.
The historical
financial information set forth below may not be indicative of our future performance and should be read together with “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” and our historical financial statements and notes
to those statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, and any future filings
or other reports we may file with the SEC.
AS REPORTED
| | |
Three Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Net Loss Applicable to Common Stock | |
$ | (8,942,907 | ) | |
$ | (10,354,615 | ) |
| Basic & Diluted Loss Per Share | |
$ | (0.06 | ) | |
$ | (0.07 | ) |
| Basic & Diluted Weighted Average Shares Outstanding | |
| 152,916,598 | | |
| 149,617,429 | |
| | |
Years Ended December 31, | |
| | |
2022 | | |
2021 | |
| Net Loss Applicable to Common Stock | |
$ | (26,478,273 | ) | |
$ | (45,828,198 | ) |
| Basic & Diluted Loss Per Share | |
$ | (0.18 | ) | |
$ | (0.32 | ) |
| Basic & Diluted Weighted Average Shares Outstanding | |
| 149,851,278 | | |
| 144,157,229 | |
AS ADJUSTED FOR 1:70 REVERSE STOCK
SPLIT (UNAUDITED)
| | |
Three Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Net Loss Applicable to Common Stock | |
$ | (8,942,907 | ) | |
$ | (10,354,615 | ) |
| Basic & Diluted Loss Per Share | |
$ | (4.09 | ) | |
$ | (4.84 | ) |
| Basic & Diluted Weighted Average Shares Outstanding | |
| 2,184,523 | | |
| 2,137,392 | |
| | |
Years Ended December 31, | |
| | |
2022 | | |
2021 | |
| Net Loss Applicable to Common Stock | |
$ | (26,478,273 | ) | |
$ | (45,828,198 | ) |
| Basic & Diluted Loss Per Share | |
$ | (12.37 | ) | |
$ | (22.25 | ) |
| Basic & Diluted Weighted Average Shares Outstanding | |
| 2,140,732 | | |
| 2,059,389 | |
LEGAL MATTERS
The validity of the securities being offered
by this prospectus has been passed upon for us by Weintraub Tobin Chediak Coleman Grodin Law Corporation, 400 Capitol Mall, Suite
1100, Sacramento, CA 95814. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York, is acting as counsel for the
Placement Agent in connection with this offering.
EXPERTS
The financial statements of Adamis Pharmaceuticals
Corporation as of December 31, 2022 and 2021 and for each of the two years in the period ended December 31, 2022, incorporated
by reference in this prospectus and registration statement, have been so incorporated in reliance on the report of BDO USA, LLP (n/k/a BDO USA, P.A.),
an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The
report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going
concern.
The financial statements of DMK as of December 31,
2022 and 2021 and for the two fiscal years in the period ended December 31, 2022, incorporated by reference in this prospectus
and registration statement, have been so included in reliance on the reports of BF Borgers CPA PC, an independent registered public
accounting firm, given on the authority of said firm as experts in auditing and accounting in giving said reports.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We are a reporting company and file annual,
quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement
on Form S-1 under the Securities Act with respect to the securities being offered under this prospectus. This prospectus does not
contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further
information with respect to us and the securities being offered under this prospectus, we refer you to the registration statement
and the exhibits and schedules filed as a part of the registration statement. The SEC maintains a website that contains reports,
proxy and information statements, and other information regarding issuers that file electronically with the SEC, including Adamis.
The SEC’s website can be found at www.sec.gov.
These documents
are also available, free of charge, through the Investors section of our website. We maintain a website at www.adamispharma.com.
Information contained in or accessible through our website does not constitute a part of this prospectus.
INCORPORATION OF CERTAIN INFORMATION
BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus
from other documents that we file with it, which means that we can disclose important information to you by referring you to another
document filed separately with the SEC. The documents incorporated by reference into this prospectus contain important information
that you should read about us. The information incorporated by reference is considered to
be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with
the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of
which this prospectus is a part the information or documents listed below that we have filed with the SEC:
| |
● |
Annual Report on Form 10-K for the year ended December 31, 2022, filed on March 16, 2023; |
| |
|
|
| |
● |
Amendment No. 1 to Annual Report on Form 10-K/A, filed May 1, 2023; |
| |
|
|
| |
● |
Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, filed with the SEC on May 15, 2023; |
| |
|
|
| |
● |
Current Reports on Form 8-K filed with the SEC on February 23,
2023; February 27, 2023; March 14,
2023; March 16, 2023;
March 17, 2023; April 17,
2023; May 5, 2023; May
15, 2023; May 16, 2023; May
22, 2023; May 26, 2023; June 16,
2023; July 24, 2023; and July 24, 2023; |
| |
● |
our Definitive Proxy Statement on Schedule 14A filed with the SEC on April 13, 2023; and |
| |
|
|
| |
● |
The description of our common stock contained in our Form 8-A filed on December 11, 2013, including any amendments thereto or reports filed for the purposes of updating this description. |
We also incorporate by reference all additional
documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that
are related to such items) that we file with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act that are made
after the initial filing date of the registration statement of which this prospectus is a part (and including, without limitation,
prior to effectiveness) and after the date of this prospectus but prior to the termination of the offering. These documents include
periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well
as proxy statements. We are not, however, incorporating, in each case, any documents or information that we are deemed to furnish
and not file in accordance with SEC rules.
We will provide to each person, including
any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the
documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits which
are specifically incorporated by reference into such documents. You may request, and we will provide you with, a copy of these
filings, at no cost, by calling us at (858) 997-2400 or by writing to us at the following address:
Adamis Pharmaceuticals Corporation
11682 El Camino Real, Suite 300
San Diego, CA 92130
Attn: Corporate Secretary
Any statement contained herein or in a
document incorporated or deemed to be incorporated by reference into this document will be deemed to be modified or superseded
for purposes of the document to the extent that a statement contained in this document or any other subsequently filed document
that is deemed to be incorporated by reference into this document modifies or supersedes the statement.
Up to 5,263,158 Units consisting
of
5,263,158 Shares of Common
Stock or 5,263,158 Pre-Funded Warrants to purchase 5,263,158 Shares of Common Stock and
5,263,158 Warrants to purchase
up to 5,263,158 Shares of Common Stock
Up to 5,263,158 Shares of Common Stock Underlying
the Pre-Funded Warrants
Up to 5,263,158 Shares of Common Stock Underlying
the Common Warrants

MAXIM GROUP LLC
The date of this prospectus is __________,
2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| |
Item 13. |
Other Expenses of Issuance and Distribution |
The following table sets forth an estimate
of the fees and expenses relating to the issuance and distribution of the securities being registered hereby, other than placement
agent’s fees and expenses, all of which shall be borne by the Registrant. All of such fees and expenses, except for the SEC
registration fee, are estimated:
| SEC registration fee | |
$ | 2,204 | |
| FINRA Filing Fee | |
$ | 3,500 | |
| Legal fees and expenses | |
$ | 285,000 | |
| Accounting fees and expenses | |
$ | 100,000 | |
| Transfer Agent and Registrar Fees and Expenses | |
$ | 25,000 | |
| Printing and miscellaneous fees and expenses | |
$ | 34,296 | |
| | |
| | |
| TOTAL: | |
$ | 450,000 | |
| |
Item 14. |
Indemnification of Directors and Officers |
Section 145 of the Delaware General Corporation
Law, or the DGCL, provides that a corporation may indemnify directors and officers as well as other employees and individuals against
expenses including attorneys’ fees, judgments, fines and amounts paid in settlement in connection with various actions, suits
or proceedings, whether civil, criminal, administrative or investigative other than an action by or in the right of the corporation,
a derivative action, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests
of the corporation, and, with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct
was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification only extends to expenses
including attorneys’ fees incurred in connection with the defense or settlement of such actions, and the statute requires
court approval before there can be any indemnification where the person seeking indemnification has been found liable to the corporation.
The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s certificate
of incorporation, bylaws, agreement, a vote of stockholders or disinterested directors or otherwise. Section 145 of the Delaware
General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors
and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including
reimbursement for expenses incurred, arising under the Securities Act.
The Company’s Bylaws provide that
the Company will indemnify and hold harmless, to the fullest extent permitted by Section 145 of the DGCL, as amended from time
to time, each of its directors and officers, and may indemnify its employees and agents as set forth in the DGCL.
The DGCL permits a corporation to provide
in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its
stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
| |
● |
any breach of the director’s duty of loyalty to the corporation or its stockholders; |
| |
|
|
| |
● |
acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| |
|
|
| |
● |
payments of unlawful dividends or unlawful stock repurchases or redemptions; or |
| |
|
|
| |
● |
any transaction from which the director derived an improper personal benefit. |
The Company’s restated certificate
of incorporation and Bylaws provide that, to the fullest extent permitted by applicable law, none of our directors will be personally
liable to us or our stockholders for monetary damages. Any repeal or modification of this provision will be prospective only and
will not adversely affect any limitation, right or protection of a director of our company existing at the time of such repeal
or modification.
We have also obtained liability insurance
for our directors and officers that insures our directors and officers, within the limits and subject to the limitations of the
policy, against certain expenses in connection with the defense of actions, suits or proceedings, and certain liabilities that
might be imposed as a result of such actions, suits or proceedings, to which they are parties by reason of being or having been
directors or officers. We may apply for insurance on behalf of any director, officer, employee or other agent for liability arising
out of his or her actions, whether or not the DGCL would permit indemnification.
We have entered into indemnification agreements
with our directors and officers whereby we have agreed to indemnify our directors and officers to the fullest extent permitted by law,
including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is
threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of
the Company, provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed
to be in, or not opposed to, the best interest of the Company.
Securities and Exchange Commission Position Regarding Indemnification
Liabilities Arising Under the Securities Act
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the
foregoing provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification
is against public policy as expressed in the Securities Act and is therefore unenforceable.
| |
Item 15. |
Recent Sales of Unregistered Securities |
The shares amounts below do not give effect to the Reverse Stock Split.
On June 12, 2020,
the Company issued 1,000,000 shares of Series B Convertible Preferred Stock (“Series B Preferred”) to an accredited
investor in connection with a license agreement.
On February 15,
2022, the Company granted nonqualified stock options to two non-officer employees to purchase an aggregate of up to 130,000 shares
of common stock. The options were granted in accordance with Nasdaq Listing Rule 5635(c)(4). The stock options have a ten-year
term and have an exercise price of $0.62 per share, the closing price of the Company’s common stock on the grant date.
On July 5, 2022, the Company issued to an accredited investor in a private
placement transaction an aggregate of 3,000 shares of Series C Convertible Preferred Stock, par value $0.0001 per share (the “Series C
Preferred”), together with warrants (the “Warrants”) to purchase up to an aggregate of 750,000 shares (the “Warrant
Shares”) of common stock at an exercise price of $0.47 per share, for an aggregate subscription amount equal to $300,000.
As a result of
the Merger, effective as of the Effective Time of the Merger, the Company issued an aggregate
of 302,815 shares of Common Stock and 1,941.2 Series E Preferred, convertible into 1,941,200 shares of Common Stock subject
to certain beneficial ownership limitations, to stockholders of DMK.
All of the issuances
described above were issued in private placement transactions to a limited number of persons in reliance on the private placement
exemption provided by Section 4(a)(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering,
and/or Regulation D promulgated under the Securities Act.
| Item 16: | Exhibits, Financial Statement Schedules |
See the Exhibit Index attached to this Registration
Statement, which is incorporated by reference herein.
Schedules not listed above have been omitted
because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| |
(a) |
The
undersigned registrant hereby undertakes: |
(1) To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To
include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement; and
(iii) To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement.
Provided, however,
that paragraphs (a)(1)(i), (ii) and (iii) above do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section
13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in this registration statement.
(2) That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to
be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof.
(3) To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4) That,
for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution
of the securities, the undersigned registrant hereby undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the
securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will
be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424 (§ 230.424 of this chapter);
(ii) Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant;
(iii) The portion of any other free writing prospectus relating
to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned
registrant; and
(iv) Any other
communication that is an offer in the offering made by the undersigned registrant to the purchaser.
| |
(b) |
The undersigned registrant hereby undertakes: |
(1) That,
for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed
as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of
the time it was declared effective.
(2) For
the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar
as indemnification for liabilities arising under the Securities Act of 1933 (the “Act”) may be permitted to directors,
officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised
that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and
is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by the registrant
of expenses incurred and paid by a director, officer or controlling person of the registrant in the successful defense of any action,
suit or proceeding, is asserted by such director, officer or controlling person in connection with the securities being registered hereby,
the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act
of 1933 and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto
duly authorized, in the City of San Diego, State of California, on July 26, 2023.
| |
ADAMIS PHARMACEUTICALS CORPORATION |
| |
|
|
| |
By: |
/s/ EBRAHIM VERSI, M.D., PH.D. |
| |
|
Ebrahim Versi, M.D., Ph.D
|
| |
|
Chief Executive Officer and Director |
Pursuant to the requirements of the Securities
Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates
indicated.
| Name |
|
Title |
|
Date |
| Principal Executive Officer: |
|
|
|
|
| |
|
|
|
|
| /s/ EBRAHIM VERSI, M.D., PH.D. |
|
Chief Executive Officer and Director
|
|
July 26, 2023 |
| Ebrahim Versi, M.D., Ph.D. |
|
(Principal Executive Officer)
|
|
|
| |
|
|
|
|
| Principal Financial Officer and Principal Accounting Officer: |
|
|
|
|
| |
|
|
|
|
| /s/ DAVID J. MARGUGLIO |
|
Chief Financial Officer
(Principal Financial Officer) |
|
July 26, 2023 |
| David J. Marguglio |
|
|
|
| |
|
|
|
|
| Directors: |
|
|
|
|
| |
|
|
|
|
| /s/ JANNINE VERSI * |
|
Director |
|
July 26, 2023 |
| Jannine Versi |
|
|
|
|
| |
|
|
|
|
| /s/ HOWARD C. BIRNDORF * |
|
Director |
|
July 26, 2023 |
Howard C. Birndorf
|
|
|
|
|
| /s/ MEERA J. DESAI * |
|
Director |
|
July 26, 2023 |
Meera J. Desai
|
|
|
|
|
| /s/ VICKIE S. REED * |
|
Director |
|
July 26, 2023 |
| Vickie S. Reed |
|
|
|
|
| |
|
|
|
|
| * By: /s/ David J. Marguglio, Attorney -in-fact |
|
|
|
EXHIBIT INDEX
| |
|
|
|
|
Incorporated by Reference |
Exhibit
Number |
|
Exhibit Description |
|
Filed
Herewith |
|
Form/
File No. |
|
Date |
| 1.1 |
|
Form of Placement Agent Agreement |
|
|
|
S-1 |
|
07/13/23 |
| 2.1 |
|
Agreement and Plan of Share Exchange dated as of October 7, 2004, by and between the Company and Biosyn, Inc. |
|
|
|
8-K |
|
10/26/04 |
| 2.2 |
|
Agreement and Plan of Merger by and among the Company, US Compounding, Inc., Ursula Merger Sub Corp. and Eddie Glover dated as of March 28, 2016 |
|
|
|
8-K |
|
03/29/16 |
| 2.3 |
|
Agreement and Plan of Merger and Reorganization, dated as of February 24, 2023, by and among Adamis Pharmaceuticals, Inc., Adamis Merger Sub, Inc., and DMK Pharmaceuticals Corporation.+ |
|
|
|
8-K |
|
02/27/23 |
| 3.1 |
|
Restated Certificate of Incorporation of the Registrant |
|
|
|
S-8 |
|
03/17/14 |
| 3.2 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock dated August 19, 2014 |
|
|
|
8-K |
|
08/20//14 |
| 3.3 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series A-1 Convertible Preferred Stock |
|
|
|
8-K |
|
01/26/16 |
| 3.4 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series A-2 Convertible Preferred Stock |
|
|
|
8-K |
|
07/12/16 |
| 3.5 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series B Convertible Preferred Stock |
|
|
|
8-K |
|
06/12/20 |
| 3.6 |
|
Certificate of Amendment to Restated Certificate of Incorporation |
|
|
|
8-K |
|
09/08/20 |
| 3.7 |
|
Certificate of Designation of Preferences, Rights, and Limitations of Series C Convertible Preferred Stock |
|
|
|
8-K |
|
07/06/22 |
| 3.8 |
|
Certificate of Designation of Preferences, Rights, and Limitations of Series E Convertible Preferred Stock |
|
|
|
8-K |
|
05/26/23 |
| 4.1 |
|
Amended and Restated Bylaws of the Company |
|
|
|
8-K |
|
06/22/20 |
| 4.2 |
|
Specimen stock certificate for common stock |
|
|
|
8-K |
|
04/03/09 |
| 4.3 |
|
Form of Common Stock Purchase Warrant |
|
|
|
8-K |
|
08/01/19 |
| 4.4 |
|
Description of the Registrant’s Capital Stock |
|
|
|
10-K |
|
04/15/21 |
| 4.5 |
|
Form of Common Stock Purchase Warrant |
|
|
|
8-K |
|
02/21/20 |
| 4.6 |
|
Amended and Restated Bylaws of the Company |
|
|
|
8-K |
|
06/17/22 |
| 4.7 |
|
Form of Common Stock Purchase Warrant |
|
|
|
8-K |
|
07/06/22 |
| 4.8 |
|
Form of Common Stock Purchase Warrant |
|
|
|
8-K |
|
03/14/23 |
| 4.9 |
|
Form of Prefunded Common Stock Purchase Warrant |
|
|
|
8-K |
|
03/14/23 |
| 4.10 |
|
Common Stock Purchase Warrant dated March 16, 2023 |
|
|
|
10-Q |
|
05/15/23 |
| 4.11 |
|
Prefunded Common Stock Purchase Warrant |
|
|
|
10-Q |
|
05/15/23 |
| 4.12 |
|
Form of Common Stock Warrant |
|
|
|
S-1 |
|
07/13/23 |
| 4.13 |
|
Form of Pre-Funded Warrant |
|
|
|
S-1 |
|
07/13/23 |
| 4.14 |
|
Form of Warrant Agency Agreement |
|
|
|
S-1 |
|
07/13/23 |
| 4.15 |
|
Form of Securities Purchase Agreement |
|
|
|
S-1 |
|
07/13/23 |
| 5.1 |
|
Opinion of Weintraub Tobin Chediak Coleman Grodin, a Law Corporation |
|
|
|
S-1 |
|
07/13/23 |
| 10.1 |
|
2009 Equity Incentive Plan* |
|
|
|
S-8 |
|
07/18/18 |
| 10.2 |
|
Form of Stock Option Agreement for option awards* |
|
|
|
8-K |
|
09/16/11 |
| 10.3 |
|
Form of Option Agreement for Non-Employee Directors* |
|
|
|
8-K |
|
01/13/11 |
| 10.4 |
|
Form of Stock Appreciation Rights Agreement for Non-employee Directors |
|
|
|
10-Q |
|
11/12/19 |
| 10.5 |
|
Form of Restricted Stock Unit Agreement* |
|
|
|
10-K |
|
03/30/17 |
| 10.6 |
|
Form of Indemnity Agreement with directors and executive officers* |
|
|
|
8-K |
|
01/13/11 |
| 10.7 |
|
Funding Agreement dated October 12, 1992, by and between Ben Franklin Technology Center of Southeastern Pennsylvania and Biosyn, Inc. |
|
|
|
S-4/A 333-155322 |
|
01/12/09 |
| 10.8 |
|
Executive Employment Agreement between the Company and Dennis J. Carlo dated December 31, 2015* |
|
|
|
10-K |
|
03/23/16 |
| 10.9 |
|
Executive Employment Agreement between the Company and David J. Marguglio dated December 31, 2015* |
|
|
|
10-K |
|
03/23/16 |
| |
|
|
|
|
Incorporated by Reference |
Exhibit
Number |
|
Exhibit Description |
|
Filed
Herewith |
|
Form/
File No. |
|
Date |
| 10.10 |
|
Executive Employment Agreement between the Company and Robert O. Hopkins dated December 31, 2015* |
|
|
|
10-K |
|
03/23/16 |
| 10.11 |
|
Exclusive License and Asset Purchase Agreement dated as of August 1, 2013, by and among the Registrant, 3M Corp. and 3M Innovative Properties Company |
|
|
|
8-K |
|
08/06/13 |
| 10.12 |
|
Lease Agreement dated April 1, 2014, between the Registrant and Pacific North Court Holdings, L.P. |
|
|
|
10-KT |
|
03/26/15 |
| 10.13 |
|
First Amendment to Lease between the Registrant and Pacific North Court Holdings, L.P. |
|
|
|
10-K |
|
04/15/21 |
| 10.14 |
|
Registration Rights Agreement dated August 19, 2014, by and between the Company and Sio Partners LP, Sio Partners QP LP and Sio Partners Offshores, Ltd. |
|
|
|
8-K |
|
08/20/14 |
| 10.15 |
|
Purchase Agreement dated January 26, 2016 |
|
|
|
8-K |
|
01/26/16 |
| 10.16 |
|
Amended and Restated Registration Rights Agreement dated January 26, 2016 |
|
|
|
8-K |
|
01/26/16 |
| 10.17 |
|
Purchase Agreement dated July 11, 2016 |
|
|
|
8-K |
|
07/12/16 |
| 10.18 |
|
Registration Rights Agreement dated July 11, 2016 |
|
|
|
8-K |
|
07/12/16 |
| 10.19 |
|
Compensation Committee Authorization Regarding Discretionary Payments Ex |
|
|
|
8-K |
|
02/27/18 |
| 10.20 |
|
Offer Letter between the Company and David C. Benedicto */*** |
|
|
|
10-K |
|
03/31/22 |
| 10.21 |
|
Executive Employment Agreement between the Company and Ronald B. Moss, M.D., dated as of February 28, 2017.* |
|
|
|
10-K |
|
03/30/17 |
| 10.22 |
|
Underwriting Agreement dated August 2, 2018 |
|
|
|
8-K |
|
08/02/18 |
| 10.23 |
|
Distribution and Commercialization Agreement between the company and Sandoz, Inc.** |
|
|
|
10-Q |
|
11/09/18 |
| 10.24 |
|
Placement Agency Agreement between Maxim Group LLC and the Company dated February 20, 2020 |
|
|
|
8-K |
|
02/21/20 |
| 10.25 |
|
Form of Securities Purchase Agreement dated February 21, 2020 |
|
|
|
8-K |
|
02/21/20 |
| 10.26 |
|
Underwriting Agreement dated January 29, 2021 |
|
|
|
8-K |
|
01/29/21 |
| 10.27 |
|
Underwriting Agreement dated September 18, 2020 |
|
|
|
8-K |
|
09/18/20 |
| 10.28 |
|
August 2020 Amendment to Loan Amendment and Assumption Agreement |
|
|
|
8-K |
|
09/15/20 |
| 10.29 |
|
Amended Promissory Note |
|
|
|
8-K |
|
09/15/20 |
| 10.30 |
|
2020 Equity Incentive Plan* |
|
|
|
8-K |
|
08/24/20 |
| 10.31 |
|
Adamis Pharmaceuticals Corporation Bonus Plan* |
|
|
|
8-K |
|
06/22/20 |
| 10.32 |
|
Termination and Transfer Agreement between Sandoz Inc. and the Company ***+ |
|
|
|
10-Q |
|
08/17/20 |
| 10.33 |
|
Transition Service Agreement***+ |
|
|
|
10-Q |
|
08/17/20 |
| 10.34 |
|
License Agreement between the Company and Matrix Biomed, Inc.***+ |
|
|
|
10-Q |
|
08/17/20 |
| 10.35 |
|
Distribution and Commercialization Agreement between the Company and USWM, LLC*** |
|
|
|
10-Q |
|
08/17/20 |
| 10.36 |
|
Lease Agreement between the Company and Oil States Energy Services, LLC, as amended+ |
|
|
|
10-K |
|
04/15/21 |
| 10.37 |
|
Promissory Note dated March 15, 2021 |
|
|
|
10-K |
|
04/15/21 |
| 10.38 |
|
Underwriting Agreement |
|
|
|
8-K |
|
01/29/21 |
| 10.39 |
|
Asset Purchase Agreement effective as of July 30, 2021, by and among the Registrant, US Compounding, Inc. and Fagron Compounding Services, LLC.+*** |
|
|
|
8-K |
|
08/05/21 |
| 10.40 |
|
Supply Agreement Addendum by and among the Registrant, US Compounding Inc. and Fagron Compounding, LLC*** |
|
|
|
8-K |
|
08/05/21 |
| 10.41 |
|
Settlement Agreement between the Company, US Compounding Inc., Nephron Pharmaceuticals Corporation, Nephron S.C., Inc., Nephron Sterile Compounding Center, LLC and certain other parties.+*** |
|
|
|
10-Q |
|
11/22/21 |
| 10.42 |
|
First Amendment to Exclusive License Agreement dated November 9, 2021 between the Company and Matrix Biomed, Inc.*** |
|
|
|
10-K |
|
03/31/22 |
| |
|
|
|
|
Incorporated by Reference |
Exhibit
Number |
|
Exhibit Description |
|
Filed
Herewith |
|
Form/
File No. |
|
Date |
| 10.43 |
|
Executive Employment Agreement between the Company and David J. Marguglio dated as of May 18, 2022 |
|
|
|
8-K |
|
05/19/22 |
| 10.44 |
|
Executive Employment Agreement between the Company and David C. Benedicto dated as of June 22, 2022 |
|
|
|
8-K |
|
06/24/22 |
| 10.45 |
|
Securities Purchase Agreement dated July 5, 2022, between the Company and the parties thereto. |
|
|
|
8-K |
|
07/06/22 |
| 10.46 |
|
Registration Rights Agreement dated July 5, 2022, between the Company and the parties thereto. |
|
|
|
8-K |
|
07/06/22 |
| 10.47 |
|
Forms of Securities Purchase Agreement |
|
|
|
8-K |
|
03/14/23 |
| 10.48 |
|
Form of Support Agreement, dated February 24, 2023, by and among Adamis Pharmaceuticals, Inc., Aardvark Merger Sub, Inc., DMK Pharmaceuticals Corporation, and certain stockholders of DMK Pharmaceuticals Corporation |
|
|
|
8-K |
|
02/27/23 |
| 10.49 |
|
DMK 2016 Stock Plan |
|
|
|
8-K |
|
05/26/2023 |
| 10.50 |
|
Securities Purchase Agreement |
|
|
|
10-Q |
|
05/15/23 |
| 10.51 |
|
Support Agreement |
|
|
|
10-Q |
|
05/15/23 |
| 10.52 |
|
Form of Indemnity Agreement* |
|
|
|
10-Q |
|
05/15/23 |
| 10.53 |
|
Offer Letter dated May 24, 2023 |
|
|
|
8-K |
|
05/26/23 |
| 10.54 |
|
Purchase and Sale Agreement +/**** |
|
|
|
8-K |
|
07/24/23 |
| 10.55 |
|
Sales Agreement. + |
|
|
|
8-K |
|
07/24/23 |
| 21.1 |
|
Subsidiaries of the Registrant |
|
X |
|
|
|
|
| 23.1 |
|
Consent of BDO USA, P.A. Independent Registered Public Accounting Firm |
|
X |
|
|
|
|
| 23.2 |
|
Consent of BF Borger CPA PC, Independent Registered Public Accounting Firm |
|
X |
|
|
|
|
| 23.3 |
|
Consent of Weintraub Tobin Chediak Coleman Grodin, A Law Corporation (included in Exhibit 5.1) (1) |
|
|
|
|
|
|
| 24.1 |
|
Power of Attorney (See signature page) |
|
|
|
S-1 |
|
07/13/23 |
| |
|
|
|
|
|
|
|
|
| 107 |
|
Filing Fee Table |
|
|
|
S-1 |
|
07/13/23 |
| |
|
|
|
|
|
|
|
|
| + |
Non-material schedules and exhibits have been
omitted pursuant to Item 601(a)(5) of Regulation S-K. The Registrant hereby undertakes to furnish supplemental copies of any
of the omitted schedules and exhibits upon request by SEC. |
| * |
Represents a compensatory plan or arrangement. |
| ** |
We have received confidential treatment for certain portions of this exhibit. |
| *** |
Certain marked information (indicated by “[***]”)
has been omitted from this exhibit as the registrant has determined it is both not material and is the type that the registrant
customarily and actually treats as private or confidential. |
| **** |
Certain marked information has been omitted
from this exhibit because it is both not material and would be competitively harmful if publicly disclosed.
|
We hereby consent to the incorporation in this Registration Statement on
Form S-1-A1 of our report dated March 28, 2023 relating to the financial statements of DMK Pharmaceuticals Corporation as of December
31, 2022 and 2021 and to all references to our firm included in this Registration Statement.