0000105770false00001057702023-10-262023-10-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) – October 26, 2023

| | |
WEST PHARMACEUTICAL SERVICES, INC. |
(Exact name of registrant as specified in its charter) | | | | | | | | | | | | | | |
| | | | |
Pennsylvania | | 1-8036 | | 23-1210010 |
(State or other jurisdiction of incorporation) | | (Commission File Number) | | (IRS Employer Identification No.) |
| | | | |
530 Herman O. West Drive, Exton, PA | | | | 19341-1147 |
(Address of principal executive offices) | | | | (Zip Code) |
Registrant’s telephone number, including area code: 610-594-2900 | | |
Not Applicable |
(Former name or address, if changed since last report.) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below): | | | | | |
☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | | | |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | | | |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | | | |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Common Stock, par value $0.25 per share | WST | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On October 26, 2023, West Pharmaceutical Services, Inc. (the “Company”) issued a press release announcing its third-quarter 2023 financial results. A copy of the press release is attached hereto as Exhibit 99.1 and incorporated herein by reference.
Item 7.01 Regulation FD Disclosure.
The information set forth in “Item 2.02 Results of Operations and Financial Condition,” including the exhibit referred to therein, is incorporated herein by reference.
A copy of the Company’s presentation materials used during the call will be available through the Investors link at the Company’s website, http://www.westpharma.com, and is also attached hereto as Exhibit 99.2 and incorporated herein by reference.
The information in this report (including the exhibits attached hereto) is being furnished pursuant to Item 2.02 and Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (“Exchange Act”), or otherwise subject to the liabilities of that section, nor will it be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific referencing in such filing.
Item 9.01 Financial Statements and Exhibits.
| | | | | | | | |
(d) | Exhibit No. | Description |
| 99.1 | West Pharmaceutical Services, Inc. Press Release, dated October 26, 2023. |
| 99.2 | West Pharmaceutical Services, Inc. Presentation, dated October 26, 2023. |
| 104 | The cover page from the Company’s Current Report on Form 8-K, dated October 26, 2023, formatted in Inline XBRL. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | |
| WEST PHARMACEUTICAL SERVICES, INC. |
| |
| |
| /s/ Bernard J. Birkett |
| Bernard J. Birkett |
| Senior Vice President, Chief Financial and Operations Officer |
| |
| |
| October 26, 2023 | |
EXHIBIT INDEX
| | | | | | | | |
Exhibit No. | | Description |
99.1 | | |
99.2 | | |
104 | | The cover page from the Company’s Current Report on Form 8-K, dated October 26, 2023, formatted in Inline XBRL. |
Exhibit 99.1
West Announces Third-Quarter 2023 Results, Updates Full-Year 2023 Guidance and Declares Fourth-Quarter 2023 Dividend
- Conference Call Scheduled for 9 a.m. EDT Today -
Exton, PA, October 26, 2023 – West Pharmaceutical Services, Inc. (NYSE: WST) today announced its financial results for the third-quarter 2023, updated full-year 2023 financial guidance and declared a fourth-quarter 2023 dividend.
Third-Quarter 2023 Summary (comparisons to prior-year period)
•Net sales of $747.4 million grew 8.8%; organic net sales growth was 5.7%.
•Reported-diluted EPS of $2.14 increased 34.6%.
•Adjusted-diluted EPS of $2.16 increased 6.4%.
•Company is updating full-year 2023 net sales guidance to a new range of $2.950 billion to $2.960 billion, compared to a prior range of $2.970 billion to $2.995 billion.
•Company is updating full-year 2023 adjusted-diluted EPS guidance to a new range of $7.95 to $8.00, compared to a prior range of $7.65 to $7.80.
•The Company also announced that its Board of Directors has approved a fourth-quarter 2023 dividend of $0.20 per share, a 5.3% increase over the $0.19 per share paid in each of the four preceding quarters. This is the thirty-first consecutive annual increase in the Company's dividend. The dividend will be paid on November 15, 2023, to shareholders of record as of November 8, 2023.
“Adjusted-diluted EPS” and “organic net sales” are Non-U.S. GAAP measurements. See discussion under the heading “Non-U.S. GAAP Financial Measures” in this release.
“We had a solid quarter of organic net sales growth, driven by our Proprietary Products’ high-value product (HVP) and strong Contract Manufacturing components,” said Eric M. Green, President, Chief Executive Officer and Chair of the Board. “We are observing a slowdown in restocking trends by large Pharma and Generic customers, which is reflected in our revised guidance. As we look to the fourth-quarter 2023, we anticipate double-digit base, non-COVID-19-related organic sales growth, fueled by strong HVP component demand with certain customers and therapeutic categories.”
Proprietary Products Segment
Net sales grew by 6.3% to $602.5 million. Organic net sales growth (excluding changes in currency translation and the impact of a recent divestiture) was 3.2%, with currency translation increasing net sales growth by 250 basis points. HVP net sales represented over 75% of segment net sales and generated mid-single digit organic net sales growth, led by customer demand for HVP components such as FluroTec®, Daikyo® and Envision® as well as HVP devices such as self-injection systems and administration systems.
The Generics market unit had high-single digit organic net sales growth, and the Biologics and Pharma market units had low-single digit organic net sales growth. As expected, sales related to COVID-19 continued to decline from the same period last year. Excluding this COVID-19 impact, the Proprietary Products segment organic sales would have grown double-digits, led by Biologics and Generics market units.
Contract-Manufactured Products Segment
Net sales grew by 20.8% to $144.9 million. Organic net sales growth was 17.4% with currency translation increasing net sales growth by 340 basis points. Segment performance was led by growth in sales of components for drug-injection devices and for healthcare diagnostic devices.
Financial Highlights (first nine months of 2023)
Operating cash flow was $537.4 million, an increase of 9.0%. Capital expenditures were $253.3 million, an increase of 33.5% over the same period last year. Free cash flow (operating cash flow minus capital expenditures) was $284.1 million, a decline of 6.4%.
During the first nine months of 2023, the Company repurchased 753,399 shares for $261.3 million at an average share price of $346.86 under its share repurchase program.
Full-Year 2023 Updated Financial Guidance
•The Company is updating full-year 2023 net sales guidance to be a new range of $2.950 billion to $2.960 billion, compared to a prior range of $2.970 billion to $2.995 billion.
◦Organic net sales growth guidance is a range of 2% to 3%, compared to prior guidance of a range of 3% to 4%.
◦Net sales guidance assumes COVID-19 related sales of approximately $68 million, compared to prior guidance of $60 million.
◦Net sales guidance includes an estimated full-year 2023 tailwind of $20 million based on current foreign currency exchange rates, unchanged from prior guidance.
◦Net sales guidance also includes a reduction of $8 million resulting from a divestiture of a European facility that produced standard Proprietary Product components, unchanged from prior guidance.
•Full-year 2023 adjusted-diluted EPS is expected to be in a range of $7.95 to $8.00, compared to prior guidance range of $7.65 to $7.80.
◦Full-year adjusted-diluted EPS guidance range includes a tailwind of approximately $0.07 based on current foreign currency exchange rates, compared to prior guidance of a tailwind of $0.05.
◦The updated guidance also includes EPS of $0.41 associated with first nine-months 2023 tax benefits from stock-based compensation.
◦For the fourth-quarter 2023, our EPS guidance range assumes a tax rate of 22% and does not include potential tax benefits from stock-based compensation. Any tax benefits associated with stock-based compensation beyond those recorded in the first nine-months of 2023 would provide a positive adjustment to our full-year adjusted-diluted EPS guidance.
•Full-year 2023 capital spending guidance is unchanged and is expected to be $350 million. This includes incremental capital spending to support capacity expansions at existing HVP facilities.
Third-Quarter 2023 Conference Call
The live audio-only webcast will be made available via the Company's Investor Relations website here or by clicking here.
To participate in the conference call by asking questions to Management, please register in advance by clicking here. Upon registration, all telephone participants will receive the dial-in number along with a unique PIN number that will be used to access the call.
Management will refer to a slide presentation during the call, which will be made available on the day of the call. To view the presentation, select "Presentations" in the "Investors" section of the Company's website.
A replay of the conference call and webcast will be available on the Company's website for 30 days.
| | | | | |
| Investor Contact: | Media Contact: |
| Quintin Lai | Michele Polinsky |
| Vice President, Investor Relations | Vice President, Global Communications |
| (610) 594-3318 | (610) 594-3054 |
| Quintin.Lai@westpharma.com | Michele.Polinsky@westpharma.com |
Forward-Looking Statements
This release contains statements that constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may include such words as “unchanged,” “approved,” “will,” “be,” “expect,” “are,” “note,” “has been,” “reflected,” “unchanged,” “expected,” “to be,” “assumes,” “includes,” “does,” “would,” “provide,” and other similar terminology. These statements reflect management’s current expectations regarding future events and operating performance and speak only as of the date of this release. There is no certainty that actual results will be achieved in-line with current expectations. These forward-looking statements involve a number of risks and uncertainties. The following are some of the factors that could cause our actual results to differ materially from those expressed in or underlying our forward-looking statements: prevailing economic conditions and general uncertainties relating thereto that may be unknown and unforeseeable; customers’ changing inventory requirements and manufacturing plans and customer decisions to move forward with our new products and product categories; interruptions or weaknesses in our supply chain, illness in our workforce and access to transport for our products; disruptions or limitations in the Company’s manufacturing capacity; average profitability, or mix, of the products we sell; dependence on third-party suppliers and partners; increased raw material, energy and labor costs; fluctuations in currency exchange; the ability to meet development milestones with key customers; and the consequences of other geopolitical events, including natural disasters, acts of war, and global health crises. This list of important factors is not all inclusive. For a description of certain additional factors that could cause the Company’s future results to differ from those expressed in any such forward-looking statements, see Part I Item 1A, entitled “Risk Factors,” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and other filings with the United States Securities and Exchange Commission, including the Company’s quarterly reports on Form 10-Q and current reports on Form 8-K.
Except as required by law or regulation, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
Non-U.S. GAAP Financial Measures
This release contains certain non-GAAP financial measures, including organic net sales and adjusted-diluted EPS. For the purpose of aiding the comparison of our year-over-year results, we may refer to net sales and other financial results excluding the effects of changes in foreign currency exchange rates. Organic net sales exclude the impact from acquisitions and/or divestitures and translate the current-period reported sales of subsidiaries whose functional currency is other than the U.S. Dollar at the applicable foreign currency exchange rates in effect during the comparable prior-year period. We may also refer to financial results excluding the effects of unallocated items. The re-measured results excluding effects from currency translation and excluding the effects of unallocated items are not in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”) and should not be used as a substitute for the comparable U.S. GAAP financial measures. The non-U.S. GAAP financial measures are incorporated into our discussion and analysis as management uses them in evaluating our results of operations and believes that this information provides users a valuable insight into our overall performance and financial position. A reconciliation of these adjusted non-U.S. GAAP measures to the comparable U.S. GAAP financial measures is included in the accompanying tables.
WEST PHARMACEUTICAL SERVICES, INC.
CONSOLIDATED STATEMENTS OF INCOME
(UNAUDITED)
(in millions, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| | 2023 | | 2022 | | 2023 | | 2022 |
| Net sales | $ | 747.4 | | | 100% | | $ | 686.9 | | | 100% | | $ | 2,217.8 | | | 100% | | $ | 2,178.2 | | | 100% |
| Cost of goods and services sold | 459.1 | | | 61 | | 418.9 | | | 61 | | 1,366.8 | | | 62 | | 1,304.1 | | | 60 |
| Gross profit | 288.3 | | | 39 | | 268.0 | | | 39 | | 851.0 | | | 38 | | 874.1 | | | 40 |
| Research and development | 16.4 | | | 2 | | 13.6 | | | 2 | | 50.0 | | | 2 | | 42.6 | | | 2 |
| Selling, general and administrative expenses | 89.0 | | | 12 | | 66.3 | | | 10 | | 263.4 | | | 12 | | 231.2 | | | 11 |
| Other expense (income), net | 5.6 | | | 1 | | 1.9 | | | — | | 22.5 | | | 1 | | (4.0) | | | — |
| Operating profit | 177.3 | | | 24 | | 186.2 | | | 27 | | 515.1 | | | 23 | | 604.3 | | | 27 |
| Interest (income) expense, net | (5.9) | | | (1) | | 0.7 | | | — | | (10.8) | | | (1) | | 4.0 | | | — |
| Other nonoperating (income) expense | (3.8) | | | — | | 49.3 | | | 7 | | (3.9) | | | — | | 49.1 | | | 2 |
| Income before income taxes and equity in net income of affiliated companies | 187.0 | | | 25 | | 136.2 | | | 20 | | 529.8 | | | 24 | | 551.2 | | | 25 |
| Income tax expense | 29.4 | | | 4 | | 20.4 | | | 3 | | 87.8 | | | 4 | | 85.8 | | | 4 |
| Equity in net income of affiliated companies | (3.7) | | | (1) | | (4.8) | | | (1) | | (14.4) | | | (1) | | (17.5) | | | (1) |
| Net income | $ | 161.3 | | | 22% | | $ | 120.6 | | | 18% | | $ | 456.4 | | | 21% | | $ | 482.9 | | | 22% |
| | | | | | | | | | | | | | | |
| Net income per share: | | | | | | | | | | | | | | | |
| Basic | $ | 2.17 | | | | | $ | 1.62 | | | | | $ | 6.13 | | | | | $ | 6.49 | | | |
| Diluted | $ | 2.14 | | | | | $ | 1.59 | | | | | $ | 6.05 | | | | | $ | 6.36 | | | |
| | | | | | | | | | | | | | | |
| Average common shares outstanding | 74.3 | | | | | 74.4 | | | | | 74.4 | | | | | 74.4 | | | |
| Average shares assuming dilution | 75.3 | | | | | 75.7 | | | | | 75.5 | | | | | 75.9 | | | |
| | | | | | | | | | | | | | | |
WEST PHARMACEUTICAL SERVICES
REPORTING SEGMENT INFORMATION
(UNAUDITED)
(in millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, | | | | |
Net Sales: | 2023 | | 2022 | | 2023 | | 2022 | | | | |
| Proprietary Products | $ | 602.5 | | | $ | 567.0 | | | $ | 1,803.6 | | | $ | 1,822.0 | | | | | |
| Contract-Manufactured Products | 144.9 | | | 120.0 | | | 414.2 | | | 356.5 | | | | | |
| Eliminations | — | | | (0.1) | | | — | | | (0.3) | | | | | |
| Consolidated Total | $ | 747.4 | | | $ | 686.9 | | | $ | 2,217.8 | | | $ | 2,178.2 | | | | | |
| | | | | | | | | | | |
| Gross Profit: | | | | | | | | | | | |
| Proprietary Products | $ | 261.4 | | | $ | 247.3 | | | $ | 780.6 | | | $ | 810.3 | | | | | |
| Contract-Manufactured Products | 26.9 | | | 20.7 | | | 71.3 | | | 63.8 | | | | | |
| Unallocated | — | | | — | | | (0.9) | | | — | | | | | |
| Gross Profit | $ | 288.3 | | | $ | 268.0 | | | $ | 851.0 | | | $ | 874.1 | | | | | |
| Gross Profit Margin | 38.6 | % | | 39.0 | % | | 38.4 | % | | 40.1 | % | | | | |
| | | | | | | | | | | |
| Operating Profit (Loss): | | | | | | | | | | | |
| Proprietary Products | $ | 181.6 | | | $ | 188.6 | | | $ | 546.5 | | | $ | 615.9 | | | | | |
| Contract-Manufactured Products | 21.0 | | | 14.7 | | | 53.3 | | | 48.0 | | | | | |
| | | | | | | | | | | |
| Stock-based compensation expense | (5.9) | | | (6.0) | | | (21.9) | | | (17.0) | | | | | |
| General corporate costs | (19.4) | | | (11.1) | | | (62.8) | | | (42.6) | | | | | |
| Reported Operating Profit | $ | 177.3 | | | $ | 186.2 | | | $ | 515.1 | | | $ | 604.3 | | | | | |
| Reported Operating Profit Margin | 23.7 | % | | 27.1 | % | | 23.2 | % | | 27.7 | % | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Unallocated items | 3.5 | | | 0.2 | | | 15.6 | | | (1.0) | | | | | |
| Adjusted Operating Profit | $ | 180.8 | | | $ | 186.4 | | | $ | 530.7 | | | $ | 603.3 | | | | | |
| Adjusted Operating Profit Margin | 24.2 | % | | 27.1 | % | | 23.9 | % | | 27.7 | % | | | | |
WEST PHARMACEUTICAL SERVICES
RECONCILIATION OF NON-U.S. GAAP MEASURES (UNAUDITED)
Please refer to “Non-U.S. GAAP Financial Measures” for more information
(in millions, except per share data)
Reconciliation of Reported and Adjusted Operating Profit, Net Income and Diluted EPS
| | | | | | | | | | | | | | |
| Three Months ended September 30, 2023 | Operating
profit | Income
tax
expense | Net
income | Diluted
EPS |
| Reported (U.S. GAAP) | $177.3 | | $29.4 | | $161.3 | | $2.14 | |
| Unallocated Items: | | | | |
Cost investment impairment (2) | 3.3 | | — | | 3.3 | | 0.05 | |
Amortization of acquisition-related intangible assets (4) | 0.2 | | 0.1 | | 0.7 | | 0.01 | |
Legal settlement (5) | — | | (0.9) | | (2.9) | | (0.04) | |
| Adjusted (Non-U.S. GAAP) | $180.8 | | $28.6 | | $162.4 | | $2.16 | |
| | | | | | | | | | | | | | | |
| Nine Months ended September 30, 2023 | Operating
profit | Income
tax
expense | Net
income | Diluted
EPS |
| Reported (U.S. GAAP) | $515.1 | | $87.8 | | $456.4 | | $6.05 | |
| Unallocated Items: | | | | |
| Loss on disposal of plant (1) | 11.6 | | (0.7) | | 12.3 | | 0.16 | |
| Cost investment impairment (2) | 3.3 | | — | | 3.3 | | 0.05 | |
| Restructuring and other charges (3) | 0.1 | | (0.3) | | 0.4 | | — | |
| Amortization of acquisition-related intangible assets (4) | 0.6 | | 0.1 | | 2.1 | | 0.03 | |
| Legal settlement (5) | — | | (0.9) | | (2.9) | | (0.04) | |
| Adjusted (Non-U.S. GAAP) | $530.7 | | $86.0 | | $471.6 | | $6.25 | |
| | | | | | | | | | | | | | |
| Three Months ended September 30, 2022 | Operating
profit | Income
tax
expense | Net
income | Diluted
EPS |
| Reported (U.S. GAAP) | $186.2 | | $20.4 | | $120.6 | | $1.59 | |
| Unallocated items: | | | | |
Pension settlement (6) | — | | 20.0 | | 29.6 | | 0.39 | |
Amortization of acquisition-related intangible assets (4) | 0.2 | | 0.1 | | 0.7 | | 0.01 | |
| | | | |
Tax law changes (7) | — | | (3.2) | | 3.2 | | 0.04 | |
| Adjusted (Non-U.S. GAAP) | $186.4 | | $37.3 | | $154.1 | | $2.03 | |
| | | | | | | | | | | | | | | |
| Nine Months ended September 30, 2022 | Operating
profit | Income
tax
expense | Net
income | Diluted
EPS |
| Reported (U.S. GAAP) | $604.3 | | $85.8 | | $482.9 | | $6.36 | |
| Unallocated items: | | | | |
| Restructuring and other charges (3) | (1.6) | | (0.4) | | (1.2) | | (0.01) | |
| Pension settlement (6) | — | | 20.3 | | 30.5 | | 0.40 | |
| Amortization of acquisition-related intangible assets (4) | 0.6 | | 0.1 | | 2.1 | | 0.03 | |
| Tax law changes (7) | — | | (3.2) | | 3.2 | | 0.04 | |
| | | | | |
| Royalty acceleration (8) | — | | 1.3 | | (1.3) | | (0.02) | |
| Adjusted (Non-U.S. GAAP) | $603.3 | | $103.9 | | $516.2 | | $6.80 | |
(1)During the nine months ended September 30, 2023, the Company recorded expense of $11.6 million within other expense (income), as a result of the sale of one of the Company’s manufacturing facilities within the Proprietary Products segment. The transaction closed during the second quarter of 2023.
(2)During the three and nine months ended ended September 30, 2023, the Company recorded expense of $3.3 million within other expense (income), as a result of an impairment of one of the Company's cost investments.
(3)Restructuring and other charges of $0.1 million for the nine months ended September 30, 2023, represents the net impact of an inventory write down of $0.9 million within cost of goods and services sold and a $0.8 million benefit within other expense (income) for revised severance estimates in connection with its 2022 restructuring plan. During the nine months ended September 30, 2022, the Company recorded a benefit within other expense (income) of $1.6 million for restructuring and severance related costs in connection with its 2020 plan related to revised severance estimates.
(4)During the three and nine months ended September 30, 2023 and 2022, the Company recorded $0.2 million and $0.6 million, respectively, of amortization expense within operating profit associated with an intangible asset acquired during the second quarter of 2020. During the three and nine months ended September 30, 2023 and 2022, the Company recorded $0.6 million and $1.6 million, respectively, of amortization expense in association with an acquisition of increased ownership interest in Daikyo.
(5)During the three and nine months ended September 30, 2023, the Company recorded a benefit of $3.8 million within other nonoperating (income) expense as a result of a favorable legal settlement related to a matter not included in our normal operations.
(6)During the three and nine months ended September 30, 2022, we recorded a gross pension settlement charge of $49.6 million and $50.8 million, respectively, within other nonoperating (income) expense, that fully settled the U.S. qualified defined benefit plan (the "U.S. pension plan").
(7)During the three and nine months ended September 30, 2022, the Company incurred additional tax expense of $3.2 million due to the impact of a tax law change in the state of Pennsylvania enacted during the period.
(8)During the nine months ended September 30, 2022, the Company increased its expected tax benefit related to the prepayment of future royalties from one of its subsidiaries by $1.3 million.
WEST PHARMACEUTICAL SERVICES
RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED)
Please refer to “Non-U.S. GAAP Financial Measures” for more information
(in millions, except per share data)
Reconciliation of Net Sales to Organic Net Sales (9 and 10)
| | | | | | | | | | | | | | |
| Three Months ended September 30, 2023 | Proprietary | CM | Eliminations | Total |
| Reported net sales (U.S. GAAP) | $602.5 | | $144.9 | | $— | | $747.4 | |
| Effect of changes in currency translation rates | (21.1) | | (4.0) | | — | | (25.1) | |
Organic net sales (non-U.S. GAAP) (9) | $581.4 | | $140.9 | | $— | | $722.3 | |
| | | | | | | | | | | | | | |
| Nine Months ended September 30, 2023 | Proprietary | CM | Eliminations | Total |
| Reported net sales (U.S. GAAP) | $1,803.6 | | $414.2 | | $— | | $2,217.8 | |
| Effect of changes in currency translation rates | (6.5) | | (2.9) | | — | | (9.4) | |
Organic net sales (non-U.S. GAAP) (9) | $1,797.1 | | $411.3 | | $— | | $2,208.4 | |
| | | | | | | | | | | | | | |
| Three Months ended September 30, 2022 | Proprietary | CM | Eliminations | Total |
| Reported net sales (U.S. GAAP) | $567.0 | | $120.0 | | $(0.1) | $686.9 | |
| Effect of divestitures and/or acquisitions | (3.5) | | — | | — | | (3.5) | |
Net sales excluding divestiture (non-U.S. GAAP) (10) | $563.5 | | $120.0 | | $(0.1) | $683.4 | |
| | | | | | | | | | | | | | |
| Nine Months ended September 30, 2022 | Proprietary | CM | Eliminations | Total |
| Reported net sales (U.S. GAAP) | $1,822.0 | | $356.5 | | $(0.3) | $2,178.2 | |
| Effect of divestitures and/or acquisitions | (6.6) | | — | | — | | (6.6) | |
Net sales excluding divestiture (non-U.S. GAAP) (10) | $1,815.4 | | $356.5 | | $(0.3) | $2,171.6 | |
(9)Organic net sales exclude the impact from acquisitions and/or divestitures and translate the current-period reported sales of subsidiaries whose functional currency is other than the U.S. Dollar at the applicable foreign exchange rates in effect during the comparable prior-year period.
(10)Net sales excluding divestitures represents the 2022 comparative sales figure used in our organic sales growth calculation to eliminate the impact of our 2023 divestiture.
WEST PHARMACEUTICAL SERVICES
RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED)
Please refer to “Non-U.S. GAAP Financial Measures” for more information
(in millions, except per share data)
Reconciliation of Reported-Diluted EPS Guidance to Adjusted-Diluted EPS Guidance
| | | | | | | | | | | |
| 2022 Actual | 2023 Guidance | % Change |
| Reported-diluted EPS (U.S. GAAP) | $7.73 | $7.74 to $7.79 | 0.1% to 0.8% |
| Restructuring and other charges | 0.29 | — | — |
| Pension settlement | 0.42 | — | — |
| Amortization of acquisition-related intangible assets | 0.04 | 0.04 | — |
| Cost investment activity | 0.05 | 0.05 | — |
| Royalty acceleration | (0.02) | — | — |
| Tax law changes | 0.07 | — | — |
| Loss on disposal of plant | — | 0.16 | — |
| Legal settlement | — | (0.04) | — |
Adjusted-diluted EPS (Non-U.S. GAAP) (11) | $8.58 | $7.95 to $8.00 | (7.3%) to (6.8%) |
Notes:
See “Full-year 2023 Financial Guidance” and “Non-U.S. GAAP Financial Measures” in today’s press release for additional information regarding adjusted-diluted EPS.
(11)We have opted not to forecast 2023 tax benefits from stock-based compensation in upcoming quarters, as they are out of the Company’s control. Instead, we recognize the benefits as they occur. In the first nine months of 2023, tax benefits associated with stock-based compensation increased adjusted-diluted EPS by $0.41. Any future tax benefits associated with stock-based compensation that we receive in 2023 would provide a positive adjustment to our full-year EPS guidance. In full-year 2022, tax benefits associated with stock-based compensation increased adjusted-diluted EPS by $0.22.
WEST PHARMACEUTICAL SERVICES
CASH FLOW ITEMS
(UNAUDITED)
(in millions)
| | | | | | | | | | | |
| | Nine Months Ended
September 30, |
| | 2023 | | 2022 |
| Depreciation and amortization | $101.4 | | | $89.5 | |
| Operating cash flow | $537.4 | | | $493.2 | |
| Capital expenditures | $253.3 | | | $189.7 | |
| Free cash flow | $284.1 | | | $303.5 | |
WEST PHARMACEUTICAL SERVICES
FINANCIAL CONDITION
(UNAUDITED)
(in millions)
| | | | | | | | | | | |
| | As of September 30, 2023 | | As of
December 31, 2022 |
| Cash and cash equivalents | $898.6 | | | $894.3 | |
| Accounts receivable, net | $519.1 | | | $507.4 | |
| Inventories | $431.8 | | | $414.8 | |
| Accounts payable | $219.8 | | | $215.4 | |
| Debt | $207.3 | | | $208.9 | |
| Equity | $2,868.2 | | | $2,684.9 | |
| Working capital | $1,438.8 | | | $1,400.5 | |
Trademark Notices
Trademarks and registered trademarks are the property of West Pharmaceutical Services, Inc., in the United States and other jurisdictions, unless noted otherwise.
Daikyo®, Daikyo Crystal Zenith® and Daikyo CZ® are registered trademarks of Daikyo Seiko, Ltd. Daikyo Crystal Zenith technologies are licensed from Daikyo Seiko, Ltd.

1 Third-Quarter 2023 Third Quarter Overall Net Sales $747.4M | 8.8% Diluted Earnings Per Share: $2.14 Adjusted Diluted Earnings Per Share: $2.16 Eric M. Green President and Chief Executive Officer Chair of the Board West Pharmaceutical Services, Inc. WST Q3 2023 Earnings Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 This presentation and any accompanying management commentary contain “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about product development and operational performance. Each of these statements is based on preliminary information, and actual results could differ from any preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise. Non-U.S. GAAP Financial Measures Certain financial measures included in these presentation materials, or which may be referred to in management’s discussion of the Company’s results and outlook, have not been calculated in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”), and therefore are referred to as non- U.S. GAAP financial measures. Non-U.S. GAAP financial measures should not be considered in isolation or as an alternative to such measures determined in accordance with U.S. GAAP. Please refer to “Reconciliation of Non-U.S. GAAP Financial Measures” at the end of these materials for more information. “We had a solid quarter of organic net sales growth, driven by our Proprietary Products’ high-value product (HVP) and strong Contract Manufacturing components. We are observing a slowdown in restocking trends by large Pharma and Generic customers, which is reflected in our revised guidance. As we look to the fourth-quarter 2023, we anticipate double-digit base, non-COVID-19-related organic sales growth, fueled by strong HVP component demand with certain customers and therapeutic categories.”

West Pharmaceutical Services, Inc. Eric M. Green President & CEO, Chair of the Board Bernard J. Birkett Senior VP & Chief Financial and Operations Officer Third-Quarter 2023 Analyst Conference Call 9 a.m. Eastern Time | October 26, 2023

3 West Analyst Conference Call 9 a.m. Eastern Time October 26, 2023 A webcast of today’s call can be accessed in the “Investors” section of the Company’s website: www.westpharma.com To participate on the call by asking questions to Management, please register in advance at: https://register.vevent.com/register/BI035b5026267348e8a9e 6a05f7c90c015 Upon registration, all telephone participants will receive the dial-in number along with a unique PIN number that will be used to access the call. A replay of the conference call and webcast will be available on the Company’s website for 30 days. These presentation materials are intended to accompany today’s press release announcing the Company’s results for the third-quarter 2023 and management’s discussion of those results during today’s conference call. WST Q3 2023 Earnings

4 Safe Harbor Statement This presentation and any accompanying management commentary contain “forward- looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about product development, operational performance and expectations regarding future events. Each of these statements is based on preliminary information, and actual results could differ from any preliminary estimates. We caution investors that the risk factors listed under our “Forward Looking Statements” in our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise. Certain financial measures included in these presentation materials, or which may be referred to in management’s discussion of the Company’s results and outlook, have not been calculated in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”), and therefore are referred to as non-U.S. GAAP financial measures. Non-U.S. GAAP financial measures should not be considered in isolation or as an alternative to such measures determined in accordance with U.S. GAAP. Please refer to “Reconciliation of Non-U.S. GAAP Financial Measures” at the end of these materials for more information. Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 Non-U.S. GAAP Financial Measures Trademarks and registered trademarks used in this report are the property of West Pharmaceutical Services, Inc. or its subsidiaries, in the United States and other jurisdictions, unless noted otherwise. Daikyo Crystal Zenith® and Daikyo CZ® are registered trademarks of Daikyo Seiko, Ltd. Daikyo Crystal Zenith technologies are licensed from Daikyo Seiko, Ltd. Trademarks WST Q3 2023 Earnings

5 Financial Highlights WST Q3 2023 Earnings • Third quarter 2023 net sales of $747.4 million grew 8.8%; organic net sales increased 5.7% • Third quarter 2023 reported-diluted EPS of $2.14 compared to $1.59 in the same period last year; adjusted-diluted EPS of $2.16 compared to $2.03 in the same period last year

6 S howca s i n g ou r S c ie n t i f ic L e ade rsh i p & Te ch n ica l Exp er t i s e Glob a l Cap ac ity Exp an s ion Execute. Innovate. Grow. A commitment and focus to deliver superior value to our customers and patients WST Q3 2023 Earnings HVP Comp on e n t s & D el ive ry Syste m s

7 Third-Quarter 2023 Summary Results ($ millions, except earnings-per-share (EPS) data) Three Months Ended September 30 2023 2022 Reported Net Sales $747.4 $686.9 Gross Profit Margin 38.6% 39.0% Reported Operating Profit $177.3 $186.2 Adjusted Operating Profit (1) $180.8 $186.4 Reported Operating Profit Margin 23.7% 27.1% Adjusted Operating Profit Margin (1) 24.2% 27.1% Reported-Diluted EPS $2.14 $1.59 Adjusted-Diluted EPS (1) $2.16 $2.03 “Adjusted Operating Profit,” “Adjusted Operating Profit Margin” and “Adjusted-Diluted EPS” are Non-U.S. GAAP financial measures. See accompanying slides and the discussion under the heading “Non-U.S. GAAP Financial Measures” in today’s press release for an explanation and reconciliation of these items. (1) WST Q3 2023 Earnings

8 Overall Organic Net Sales Increase: 5.7% (Q3 2023) Proprietary Products Q3 2023 organic net sales increased 3.2% driven by growth in all three market units BIOLOGICS GENERICS PHARMA Sales led by high-value products, including Flurotec® components and self-injection delivery devices Sales led by high-value products, including Westar® components and Admin Systems Sales led by high-value products, including Westar® components and Admin Systems CONTRACT MANUFACTURING Organic sales growth of 17.4%, led by increase in sales of components associated with injection-related and healthcare diagnostic devices Low-Single Digit High-Single Digit Double DigitLow-Single Digit Third-Quarter 2023 Organic Net Sales Growth WST Q3 2023 Earnings

9 Change in Consolidated Net Sales Third-Quarter 2022 to 2023 ($ millions) WST Q3 2023 Earnings

10 Gross Profit Update ($ millions) Three Months Ended September 30, 2023 2022 Proprietary Products Gross Profit $261.4 $247.3 Proprietary Products Gross Profit Margin 43.4% 43.6% Contract-Manufactured Products Gross Profit $26.9 $20.7 Contract-Manufactured Products Gross Profit Margin 18.6% 17.3% Reported Consolidated Gross Profit $288.3 $268.0 Reported Consolidated Gross Profit Margin 38.6% 39.0% WST Q3 2023 Earnings

11 Cash Flow and Balance Sheet Metrics ($ millions) Cash Flow Items YTD Q3 2023 YTD Q3 2022 Depreciation and Amortization $101.4 $89.5 Operating Cash Flow $537.4 $493.2 Capital Expenditures $253.3 $189.7 Free Cash Flow $284.1 $303.5 Financial Condition September 30, 2023 December 31, 2022 Cash and Cash Equivalents $898.6 $894.3 Debt $207.3 $208.9 Equity $2,868.2 $2,684.9 Working Capital $1,438.8 $1,400.5 WST Q3 2023 Earnings

12 2023 Full-Year Guidance WST Q3 2023 Earnings 2023 Full-Year Guidance Consolidated Net Sales $2.950 - $2.960 billion Adjusted-Diluted EPS $7.95 to $8.00

13 Execute. Innovate. Grow. Delivering Unique Value to Customers and Patients Global Operational Effectiveness Across the Network Accelerating Investments for the Future Making a Difference to the Future of Patient Health WST Q3 2023 Earnings

14 Eric M. Green President and Chief Executive Officer, Chair of the Board Bernard J. Birkett Senior VP and Chief Financial and Operations Officer Quintin Lai VP, Corporate Strategy & Investor Relations Q & A WST Q3 2023 Earnings

15 Notes to Non-U.S. GAAP Financial Measures The Non-U.S. GAAP financial measures are incorporated into our discussion and analysis as management uses them in evaluating our results of operations and believes that this information provides users a valuable insight into our overall performance and financial position. A reconciliation of these adjusted Non-U.S. GAAP financial measures to the comparable U.S. GAAP financial measures is included in the accompanying tables. For the purpose of aiding the comparison of our year-over-year results, we may refer to net sales and other financial results excluding the effects of changes in foreign currency exchange rates. Organic net sales exclude the impact from acquisitions and/or divestitures and translate the current-period reported sales of subsidiaries whose functional currency is other than the U.S. Dollar at the applicable foreign exchange rates in effect during the comparable prior-year period. We may also refer to financial results excluding the effects of unallocated items. The re-measured results excluding effects from currency translation, the impact from acquisitions and/or divestitures, and the effects of unallocated items are not in conformity with U.S. GAAP and should not be used as a substitute for the comparable U.S. GAAP financial measures. WST Q3 2023 Earnings

16 Notes to Non-U.S. GAAP Financial Measures RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED) See “Notes to Non-U.S. GAAP Financial Measures”, “Safe Harbor Statement” (Slide 4) and today’s press release for an explanation and reconciliation of these items. Reconciliation of Reported and Adjusted Operating Profit, Net Income and Diluted EPS ($ millions, except EPS data) Three months ended September 30, 2023 Operating profit Income tax expense Net income Diluted EPS Reported (U.S. GAAP) $177.3 $29.4 $161.3 $2.14 Unallocated items: Cost investment impairment 3.3 - 3.3 0.05 Amortization of acquisition-related intangible assets 0.2 0.1 0.7 0.01 Legal settlement - (0.9) (2.9) (0.04) Adjusted (Non-U.S. GAAP) $180.8 $28.6 $162.4 $2.16 WST Q3 2023 Earnings Nine months ended September 30, 2023 Operating profit Income tax expense Net income Diluted EPS Reported (U.S. GAAP) $515.1 $87.8 $456.4 $6.05 Unallocated items: Loss on disposal of plant 11.6 (0.7) 12.3 0.16 Cost investment impairment 3.3 - 3.3 0.05 Restructuring and other charges 0.1 (0.3) 0.4 - Amortization of acquisition-related intangible assets 0.6 0.1 2.1 0.03 Legal settlement - (0.9) (2.9) (0.04) Adjusted (Non-U.S. GAAP) $530.7 $86.0 $471.6 $6.25
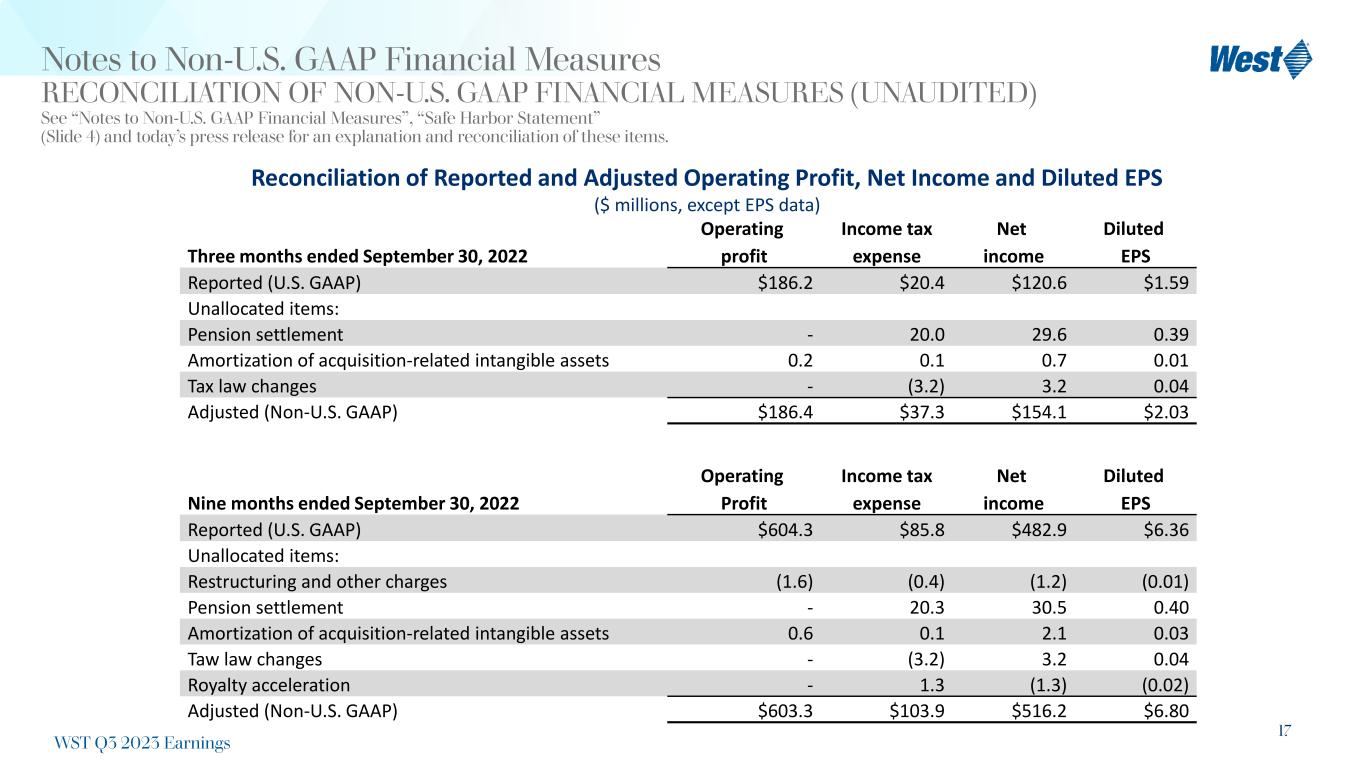
17 Notes to Non-U.S. GAAP Financial Measures RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED) See “Notes to Non-U.S. GAAP Financial Measures”, “Safe Harbor Statement” (Slide 4) and today’s press release for an explanation and reconciliation of these items. Reconciliation of Reported and Adjusted Operating Profit, Net Income and Diluted EPS ($ millions, except EPS data) Three months ended September 30, 2022 Operating profit Income tax expense Net income Diluted EPS Reported (U.S. GAAP) $186.2 $20.4 $120.6 $1.59 Unallocated items: Pension settlement - 20.0 29.6 0.39 Amortization of acquisition-related intangible assets 0.2 0.1 0.7 0.01 Tax law changes - (3.2) 3.2 0.04 Adjusted (Non-U.S. GAAP) $186.4 $37.3 $154.1 $2.03 WST Q3 2023 Earnings Nine months ended September 30, 2022 Operating Profit Income tax expense Net income Diluted EPS Reported (U.S. GAAP) $604.3 $85.8 $482.9 $6.36 Unallocated items: Restructuring and other charges (1.6) (0.4) (1.2) (0.01) Pension settlement - 20.3 30.5 0.40 Amortization of acquisition-related intangible assets 0.6 0.1 2.1 0.03 Taw law changes - (3.2) 3.2 0.04 Royalty acceleration - 1.3 (1.3) (0.02) Adjusted (Non-U.S. GAAP) $603.3 $103.9 $516.2 $6.80

18 Notes to Non-U.S. GAAP Financial Measures RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED) See “Notes to Non-U.S. GAAP Financial Measures”, “Safe Harbor Statement” (Slide 4) and today’s press release for an explanation and reconciliation of these items. Reconciliation of Net Sales to Organic Net Sales (1) ($ millions) Organic net sales exclude the impact from acquisitions and/or divestitures and translate the current-period reported sales of subsidiaries whose functional currency is other than the U.S. Dollar at the applicable foreign exchange rates in effect during the comparable prior-year period. (1) Three months ended September 30, 2023 Proprietary CM Eliminations Total Reported net sales (U.S. GAAP) $602.5 $144.9 $- $747.4 Effect of changes in currency translation rates (21.1) (4.0) - (25.1) Organic net sales (Non-U.S. GAAP) (1) $581.4 $140.9 $- $722.3 WST Q3 2023 Earnings Nine months ended September 30, 2023 Proprietary CM Eliminations Total Reported net sales (U.S. GAAP) $1,803.6 $414.2 $- $2,217.8 Effect of changes in currency translation rates (6.5) (2.9) - (9.4) Organic net sales (Non-U.S. GAAP) (1) $1,797.1 $411.3 $- $2,208.4

19 Notes to Non-U.S. GAAP Financial Measures RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED) See “Notes to Non-U.S. GAAP Financial Measures”, “Safe Harbor Statement” (Slide 4) and today’s press release for an explanation and reconciliation of these items. Reconciliation of Net Sales to Organic Net Sales (1 and 2) ($ millions) Organic net sales exclude the impact from acquisitions and/or divestitures and translate the current-period reported sales of subsidiaries whose functional currency is other than the U.S. Dollar at the applicable foreign exchange rates in effect during the comparable prior-year period. (1) Three months ended September 30, 2022 Proprietary CM Eliminations Total Reported net sales (U.S. GAAP) $567.0 $120.0 $(0.1) $686.9 Effect of divestitures and/or acquisitions (3.5) - - (3.5) Net sales excluding divestiture (Non-U.S. GAAP) (2) $563.5 $120.0 $(0.1) $683.4 WST Q3 2023 Earnings Nine months ended September 30, 2022 Proprietary CM Eliminations Total Reported net sales (U.S. GAAP) $1,822.0 $356.5 $(0.3) $2,178.2 Effect of divestitures and/or acquisitions (6.6) - - (6.6) Net sales excluding divestiture(Non-U.S. GAAP) (2) $1,815.4 $356.5 $(0.3) $2,171.6 (2) Net sales excluding divestitures represents the 2022 comparative sales figure used in our organic sales growth calculation to eliminate the impact of our 2023 divestiture.

20 Notes to Non-U.S. GAAP Financial Measures RECONCILIATION OF NON-U.S. GAAP FINANCIAL MEASURES (UNAUDITED) See “Notes to Non-U.S. GAAP Financial Measures”, “Safe Harbor Statement” (Slide 4) and today’s press release for an explanation and reconciliation of these items. Reconciliation of Reported-Diluted EPS Guidance to Adjusted-Diluted EPS Guidance 2022 Actual 2023 Guidance % Change Reported-diluted EPS (U.S. GAAP) $7.73 $7.74 to $7.79 0.1% to 0.8% Restructuring and other charges 0.29 - Pension settlement 0.42 - Amortization of acquisition-related intangible assets 0.04 0.04 Cost investment activity 0.05 0.05 Royalty acceleration (0.02) - Tax law changes 0.07 - Loss on disposal of plant - 0.16 Legal settlement - (0.04) Adjusted-diluted EPS (Non-U.S. GAAP) (1) $8.58 $7.95 to $8.00 (7.3%) to (6.8%) (1) See “Full-year 2023 Financial Guidance” and “Non-U.S. GAAP Financial Measures” in today’s press release for additional information regarding adjusted-diluted EPS. We have opted not to forecast 2023 tax benefits from stock-based compensation in upcoming quarters, as they are out of the Company’s control. Instead, we recognize the benefits as they occur. In the first nine months of 2023, tax benefits associated with stock-based compensation increased adjusted-diluted EPS by $0.41. Any future tax benefits associated with stock-based compensation that we receive in 2023 would provide a positive adjustment to our full-year EPS guidance. In 2022, tax benefits associated with stock-based compensation increased adjusted-diluted EPS by $0.22. WST Q3 2023 Earnings
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
West Pharmaceutical Serv... (NYSE:WST)
Historical Stock Chart
From Oct 2024 to Nov 2024
West Pharmaceutical Serv... (NYSE:WST)
Historical Stock Chart
From Nov 2023 to Nov 2024